Section B
Section B
Feline Lymphoma and Leukemia
Lymphoma
The lymphomas (malignant lymphoma or lymphosarcoma) are a diverse group of neoplasms that have in common their origin from lymphoreticular cells. They usually arise in lymphoid tissues such as lymph nodes, spleen, and bone marrow; however, they may arise in almost any tissue in the body. Lymphoma is one of the most common neoplasms seen in the cat.
Incidence
Epidemiologic reports prior to 1990 suggested that lymphoma accounted for 50% to 90% of all hematopoietic tumors in the cat,1,2 and since hematopoietic tumors (lymphoid and myeloid) represent approximately one-third of all feline tumors, it was estimated lymphoid neoplasia accounted for an incidence of 200 per 100,000 cats at risk.3 In one series of 400 cats with hematopoietic tumors, 61% had lymphoma and 39% had leukemias and MPDs, of which 21% were categorized as undifferentiated leukemias, most likely myeloid in origin.4 However, a significant change in the epidemiology and characteristics of lymphoma in cats coincides with the widespread integration of clinically relevant feline leukemia virus (FeLV) diagnostic assays and affected animal elimination regimens of the late 1970s and 1980s and was further enhanced by the commercially available FeLV vaccines appearing in the late 1980s (see the later section on viral etiology). The decline in FeLV-associated lymphoma was mirrored by a decline in the overall prevalence per year of FeLV positivity in cats tested as characterized by reports, including the Tufts Veterinary Diagnostic Laboratory from 1989 to 1997,5,6 and by the Louwerens group, who reported a decline in FeLV association in over 500 cases of lymphoma in cats presenting to the University of California at Davis veterinary teaching hospital.7 In these reports, FeLV antigenicity declined to represent only 14% to 25% of cats presenting with lymphoma. Importantly, Louwerens’ study revealed that despite a sharp drop in FeLV-associated lymphoma, the overall prevalence of lymphoma in cats is increasing. The increased prevalence appears due to an increase in the number and relative frequency of the alimentary (and in particular the intestinal) anatomic form of lymphoma in the species. This is supported by an epidemiologic survey of 619 cases of feline intestinal lymphoma; 534 (86%) were from the 20 years following 1985 and only 14% were from cases diagnosed in the 20 years prior to 1985.8 The true annual incidence rate for lymphoma in cats is currently unknown. With respect to feline pediatric tumors, a study in the United Kingdom (n = 233 pathology specimens) found that 73 (31%) represented hematopoietic tumors, of which 51 (70%) were lymphoma—note that FeLV status was unavailable for this compilation of cases.9
The typical signalment for cats with lymphoma cannot be uniformly stated as it varies widely based on anatomic site and FeLV status and therefore will be discussed individually under site-specific discussions. In general, based on two large compilations (n = 700) of cases in North America,5,7 Siamese cats appear overrepresented and although a 1.5 : 1 male to female ratio was observed in one, no association with sex or neutering status was observed in the other. In a large compilation of Australian cases, male cats and the Siamese/oriental breeds were overrepresented,10 and similar breed findings have been observed in North America, although similar sex predilections have not been found. Within the Siamese/oriental breeds, there appears to be a predisposition for a mediastinal form that is not FeLV associated and represents a younger population (median of 2 years).
Etiology
Viral Factors
FeLV was the most common cause of hematopoietic tumors in the cat in the so-called “FeLV era” of the 1960s through the 1980s when approximately two-thirds of lymphoma cases were associated with FeLV antigenemia. Several studies have documented the potential molecular means by which FeLV can result in lymphoid neoplasia (see Chapter 1, Section C). As one would predict, along with a shift away from FeLV-associated tumors came a shift away from traditional signalment and relative frequency of anatomic sites. This is also supported outside of North America by similar signalment and anatomic frequency data observed in Australia where FeLV infection is quite rare.10,11 The median age of approximately 11 years now reported in North America is considerably higher than the median ages of 4 to 6 years reported in the FeLV era.1,2,5-7 The median age of cats within various anatomic tumor groupings has not changed, and anatomic forms traditionally associated with FeLV such as the mediastinal form still occur in younger, FeLV antigenemic cats. Similarly, the alimentary form occurs most often in older, FeLV-negative cats. Table 32-8 presents an overview of the characteristics, including FeLV antigenemic status, of the various anatomic sites of lymphoma in cats. As our ability to interrogate FeLV associations on a molecular basis has improved (e.g., PCR amplification and fluorescent in-situ hybridization), several reports exist defining the role or potential role of FeLV in cats with and without FeLV antigenemia.12-17 Collectively, these studies indicate FeLV proviral insertion exists in a significant proportion of feline lymphoma tissues and is more common in those of T-cell origin, particularly the thymic and peripheral lymph node anatomic forms. They also suggest that several common FeLV integration sites exist.
Table 32-8
General Characteristics of the Most Commonly Encountered Anatomic Forms of Lymphoma in Cats*
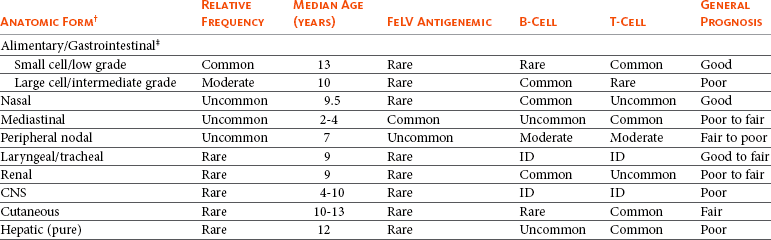
FeLV, Feline leukemia virus; ID, insufficient data; CNS, central nervous system.
Common = >50% of clinical presentations; moderate = 20%-50% of clinical presentations; uncommon = 5%-20% of clinical presentation; rare = <5% of clinical presentations.
*Data may include overlap or mixing of sites and represents the post-FeLV era.
†As the primary site of presentation, rather than extension or progression.
‡Includes those reported as “intraabdominal” in which intestinal is a documented component.
There is also evidence that feline immunodeficiency virus (FIV) infection can increase the incidence of lymphoma in cats.18-27 In contrast to the direct role of FeLV in tumorigenesis, most evidence points toward an indirect role for FIV secondary to the immunosuppressive effects of the virus. Shelton18 determined that FIV infection alone in cats was associated with a fivefold increased risk for development of lymphoma. Coinfection with FeLV will further potentiate the development of lymphoproliferative disorders. Experimentally, cats infected with FIV have developed lymphoma in the kidney, alimentary tract, liver, and multicentric sites. FIV-associated lymphoma is more likely that of the B-cell immunophenotype rather than the T-cell predominance associated with FeLV. It has been suggested that FIV infection may be associated more commonly with alimentary lymphoma of B-cell origin,28,29 and this may be related to chronic dysregulation of the immune system or the activation of oncogenic pathways; however, FIV antigenemia was only rarely associated with alimentary lymphoma in other large compilations of cases.5,30-33
Genetic and Molecular Factors
As discussed earlier in Section A, recent advances in molecular cytogenetics (see Chapter 1, Section A, and Chapter 8), including gene microarray techniques, have and are currently being applied to investigations of chromosomal aberrations in veterinary species with lymphoma. Indeed a predisposition of the oriental cat breeds to develop lymphoma suggests a genetic predisposition and indicates heritable risk.7,10 Altered oncogene/tumor suppressor gene expression, epigenetic changes, signal transduction, and cell death-pathway alterations are common in lymphomas of humans and are likely also involved in the cat. Several genetic factors have already been discussed as they relate to FeLV associations. Additionally, N-ras aberrations have been implicated, although they are rare in cats.34 Furthermore, telomerase activity (see Chapter 2) has been documented in feline lymphoma tissues.35,36 Alterations in cellular proliferation and in cell-cycle and death (apoptosis) pathways, in particular the cyclin-dependent kinase cell-cycle regulators and the Bcl-2 family of proapoptotic and antiapoptotic governing molecules, have also been implicated in feline lymphoma.37-39
Environmental Factors
Evidence for exposure to environmental tobacco smoke (ETS) as a risk factor for lymphoma in humans has prompted investigations in cats. In one report, the relative risk of developing lymphoma in cats with any exposure to ETS and with 5 or more years of exposure to ETS was 2.4 and 3.2, respectively.40 A large European study documenting an association between proximity of waste management and cancer in dogs failed to show increased risk in cats.41
Immunosuppression
Immune system alterations in the cat such as those accompanying FIV infection has been implicated in the development of lymphoma.18-20,25 As is the case in immunosuppressed human organ transplantation patients, two reports of immunosuppressed feline renal transplant recipients document increased risk of lymphoma following transplant and associated immunosuppressive therapy.42,43 In both studies, nearly 10% of transplanted cats developed de novo malignant lymphoma.
Chronic Inflammation
Although definitive proof is lacking, there is a growing body of indirect evidence to suggest that lymphoma can be associated with the presence of chronic inflammation, which theoretically could be the case with intestinal and nasal lymphoma. In particular, an association has been suggested between intestinal lymphoma and inflammatory bowel disease7,44-46; however, others have not found support for this concept.47 Additionally, an association between gastric Helicobacter infection and gastric MALT lymphoma in cats is suggested in one study, and because this is a recognized syndrome in humans, it warrants further investigation.47a
Diet and Intestinal Lymphoma
Although no direct evidence exists, a link between diet and the development of intestinal lymphoma in cats has been suggested.7 Support is offered by the relative and absolute increase in the alimentary form of lymphoma in the past 20 years and the fact that several dietary modifications in cat food have occurred in a similar timeframe in response to diseases, such as urinary tract disease. Further investigation is warranted to prove or disprove such assertions.
Pathology and Natural Behavior
Lymphoma can be classified based on anatomic location and histologic and immunophenotypic criteria; often, the two are intimately associated because certain histologic and immunophenotypic types are commonly associated with specific anatomic locations necessitating discussions within the individual anatomic categories that follow. The largest compilation of feline cases subjected to rigorous histologic classification was reported by Valli and others48 using the NCI WF. WHO has also published a histologic classification system that uses the REAL system as a basis for defining histologic categories of hematopoietic tumors of domestic animals.49 This system incorporates both histologic criteria and immunohistologic criteria (e.g., B- and T-cell immunophenotype). Regarding anatomic location, as discussed previously, a profound change in presentation, signalment, FeLV antigenemia, immunophenotype, and frequency of anatomic sites has occurred in cats with lymphoma in the “post-FeLV” era (see Table 32-8). Because of this shift, characteristics of feline lymphoma discussed in this chapter will be primarily limited to reports collected from cases presenting after 1995.
Several anatomic classifications exist for lymphoma in the cat, and some categorize the disease as mediastinal, alimentary, multicentric, nodal, leukemic, and individual extranodal forms. Others have combined various nodal and extranodal forms into categories of atypical, unclassified, and mixed, and others have combined intestinal, splenic, hepatic, and mesenteric nodal forms into one category termed intraabdominal. Some discrepancies in the discussion of frequency will inevitably result from the variations in classification used in the literature. The relative frequency of anatomic forms and their associated immunophenotype may also vary with geographic distribution and may be related to genetic and FeLV strain differences, as well as prevalence of FeLV vaccine use.
Alimentary/Gastrointestinal Lymphoma
Alimentary/GI lymphoma can present as a purely intestinal infiltration or a combination of intestinal, mesenteric lymph nodes and liver involvement. The tumors can be solitary but more commonly diffuse throughout the intestines. Some reports limit the alimentary form to GI involvement with or without extension to the liver. Lymphoma is the most common tumor type found in the intestines of cats, representing 55% of cases in an epidemiologic survey of 1129 intestinal tumors in the species.8 The Siamese breed is reported at increased risk.7,8 While lymphoma may occur in cats of any age, it is primarily a disease of aged cats with a mean age of approximately 13 years for T-cell alimentary lymphoma and 12 years for B-cell lymphoma.7,8,50-52 No consistent sex bias is noted. Anatomically, alimentary lymphoma is nearly 4 times more likely to occur in the small intestine than the large intestine.52 In a series of colonic neoplasia in cats, lymphoma was the second most common malignancy (41%), second only to adenocarcinoma.33
There is some discordance in the literature regarding the histologic type (primarily cell size: small versus large), immunophenotype, and architecture involved with GI lymphoma. While studies (often older or smaller reports) suggest a majority of B-cell immunophenotypes,5,53 larger, more recent reports51,54,55 indicate the majority represent mucosal low-grade T-cell immunophenotypes. Conversely, the vast majority of B-cell GI lymphomas in cats are large cells and intermediate or high grade.51,53 The largest compilation to date (n = 120), by Moore and others,51 classified GI lymphomas based on immunophenotype, then as either mucosal (infiltrate confined to mucosa and lamina propria with minimal submucosal extension) or transmural (significant extension into submucosa and muscularis propria). They then compared infiltration patterns with the WHO classification scheme,56 as well as documenting anatomic location, cell size, presence of epitheliotropism, clonality, and outcome data. This information is summarized in Table 32-9. Of the 120 cases, none tested serologically positive for FeLV and only 3 for FIV. Four cats had concurrent large B-cell lymphoma (stomach, cecum, or colon) and small T-cell lymphoma of the small intestine. Topographically, T-cell variants are much more likely to occur in the small intestine (94%) and rarely in the stomach or large intestine. Conversely, B-cell variants were often multiple and often occurred simultaneously within the stomach, small intestine, and ileocecocolic junction. The vast majority of T-cell variants were mucosal (equivalent to WHO enteropathy-associated T-cell lymphoma [WHO EATCL] type II), and the vast majority of B-cell tumors were transmural (equivalent to WHO EATCL type I classification). Regarding cell size, nearly all mucosal T-cell tumors were composed of small lymphocytes, and slightly more than half of transmural T-cell and all B-cell variants were composed of larger cells. Epitheliotropism is present in approximately 40% of T-cell tumors but is rare in B-cell tumors. Other abdominal organ involvement is common, and in one report of 29 cases of low-grade T-cell intestinal lymphoma, liver and mesenteric involvement was documented in 53% and 33% of cases, respectively.57 Hepatic lymphoma can occur concurrently with GI lymphoma or be confined solely to the liver.52,58 Most are T-cell and clonal or oligoclonal based on PCR analysis.
Table 32-9
Characteristics of Feline Gastrointestinal Lymphoma
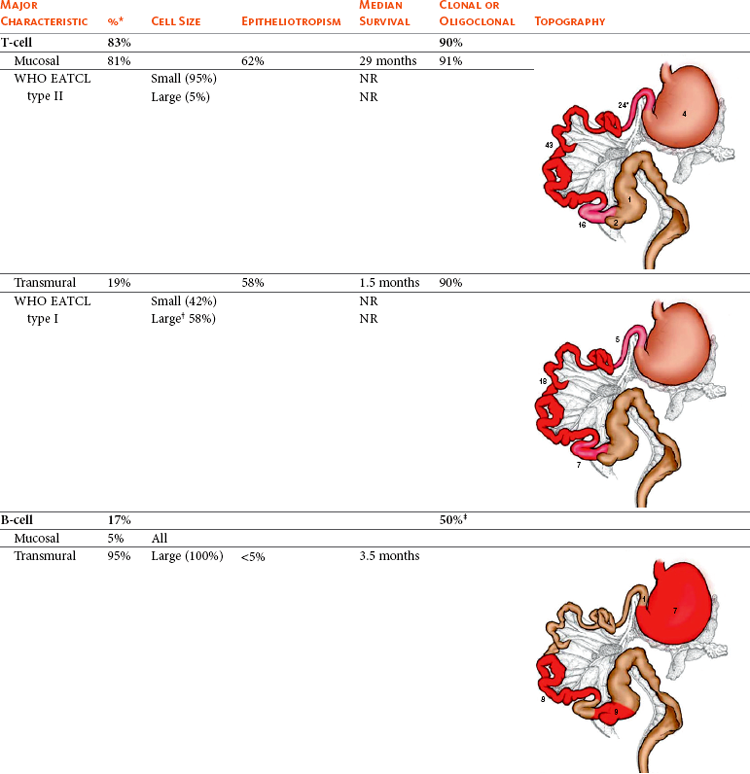
WHO EATCL, World Health Organization enteropathy-associated T-cell lymphoma; NR, not report
Numbers in figures indicate case incidence out of 103 reported cases.
*3% of cats had both B- and T-cell immunophenotypes within the gastrointestinal tract.
†82% of transmural large T-cell lymphomas are large granular lymphoma subtype.
‡39% pseudo-clonal.
Data modified from Moore PF, Rodriguez-Bertos A, Kass PH: Feline gastrointestinal lymphoma: Mucosal architecture, immunophenotype and molecular clonality, Vet Pathol April 19, 2011. Epub ahead of print.
A less common, distinct form of alimentary lymphoma, large granular lymphoma (LGL), also occurs in older (median age 9 to 10 years) cats.51,53,59-61 These granulated round cell tumors have been termed globule leukocyte tumors, although they are likely variations of the same disease. LGL is characterized by lymphoblasts described as 12 to 20 µm in diameter with a round, clefted, or cerebriform nucleus; variably distinct nucleoli; finely granular to lacey chromatin; and a moderate amount of basophilic granular cytoplasm that was occasionally vacuolated.59 Prominent magenta or azurophilic granules are characteristic (see Figure 7-34, Chapter 7). They are granzyme B positive by immunohistochemistry.51 This population of cells includes cytotoxic T-cells and occasionally NK cell immunophenotypes—most are CD3+, CD8+, and CD20− and have T-cell receptor gene rearrangement.51,60 In one report, nearly 60% expressed CD103 (integrin).60 Approximately 10% express neither B- or T-cell markers and are thus classified as NK cells. These NK tumors commonly originate in the small intestine, especially the jejunum, are transmural, often exhibit epitheliotropism, and at least two-thirds present with other organs involved—most with mesenteric lymph node involvement and many with liver, spleen, kidney, peritoneal malignant effusions, and bone marrow infiltration. Also, thoracic involvement may occur with malignant pleural effusion and a mediastinal mass present. Peripheral blood involvement was present in 10% of cases in one report59 and 86% in another.60 Affected cats are generally FeLV/FIV negative.
Mediastinal Form
The mediastinal form can involve the thymus, mediastinal, and sternal lymph nodes. Pleural effusion is common. In two large compilations, 63% of cats with thymic disease and 17% of cats with pleural effusion were documented as having lymphoma.62,63 Hypercalcemia occurs frequently with mediastinal lymphoma in dogs but is rare in cats. The majority of cats with mediastinal lymphoma are young (median age 2 to 4 years), FeLV positive, and the T-cell immunophenotype.5,7,9-11 The disease is confined to the mediastinum in most cases.7 There also exists a form of mediastinal lymphoma occurring primarily in young, FeLV-negative Siamese cats that appears to be less biologically aggressive and more responsive to chemotherapy than FeLV-associated forms.64
Nodal Lymphoma
Involvement limited to peripheral lymph nodes is unusual in cats with lymphoma, representing approximately 4% to 10% of cases.5,7 In contrast, approximately one-quarter of all other anatomic forms of lymphoma have some component of lymph node involvement. One-third of cats with nodal lymphoma are T-cell immunophenotype and FeLV antigenemic; however, complete categorizations have not occurred in the post-FeLV era and this may no longer be true.5,7,11,55 Peripheral nodal lymphoma was the most common anatomic form of lymphoma reported in a recent compilation of cases in cats under the age of 1 year, representing a full third of cases in this age group.9 As lymphoma progresses, bone marrow and hepatic infiltration may develop.
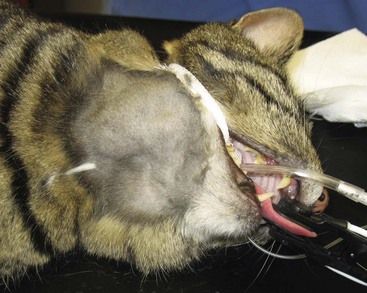
An uncommon and distinct form of nodal lymphoma in cats referred to as “Hodgkin’s-like” lymphoma has been reported.65,66 This form typically involves solitary or regional nodes of the head and neck (Figure 32-12) and histologically resembles Hodgkin’s lymphoma in humans. Affected cats generally present with enlargement of one or two mandibular or cervical nodes initially, and tumors are immunophenotypically classified as T-cell–rich, B-cell lymphoma. One case each of inguinal node, multicentric nodal, and conjunctival involvement have been reported.66,67 Histologically, lymph nodes can be effaced by either nodular or diffuse small to blastic lymphocytes with characteristic bizarre or multinucleated cells (Reed-Sternberg–like cells) (Figure 32-13). No association with FeLV or FIV has been documented.
Extranodal Lymphoma
The most common extranodal sites for lymphoma in cats include nasal (including nasopharyngeal and sinonasal), kidney, CNS, laryngeal and tracheal, ocular, retrobulbar, and skin.
Nasal lymphoma is the most common extranodal lymphoma in cats.68 It is usually a localized disease; however, 20% have local extension or distant metastasis at necropsy.69 The majority of nonviral nasal/paranasal disease in cats are neoplasias, and lymphoma represents nearly one-third to half of these cases.70-72 It occurs primarily in older (median age 9 to 10 years; range 3 to 17 years) FeLV/FIV-negative cats and at least three-quarters are B-cell in origin, although T-cell and mixed B-cell/T-cell immunophenotypes can be seen in approximately 10% to 15% of cases.5,68,69,73 Siamese cats appear overrepresented, and one report73 observed a 2 : 1 male-to-female ratio. Most are of intermediate- or high-grade histology.69,73 Epitheliotropism is common if the epithelium is present in the biopsy.
Renal lymphoma is the second most common form of extranodal lymphoma after the nasal form, occurring in approximately one-third of cases.68 It can present as primary to kidney lymphoma or occur concurrent with alimentary lymphoma. In more contemporary reports, the median age at presentation is 9 years, although 6% occurred in cats under 1 year of age.68,69 The vast majority of cases are not associated with either FeLV or FIV. The greater median age and lack of FeLV/FIV association are in contrast to reports compiled prior to the post-FeLV era; in earlier studies, the median age was approximately 7.5 years, 25% of cases were FeLV antigenemic, and the majority constituted a B-cell immunophenotype. Little contemporary information exists on the immunohistologic classification of renal lymphoma; however, in Australia where FeLV is not a significant problem, most renal lymphoma is B-cell and intermediate to high grade.11 Extension to the CNS is a frequent sequela to renal lymphoma and occurs in 40% to 50% of treated cats.74
CNS lymphoma can be intracranial, spinal, or both. CNS lymphoma made up 14% of 110 reported cases of extranodal lymphoma,68 15% to 31% of intracranial tumors,75,76 and 39% of spinal cord tumors,77 making it one of the most common malignancies encountered in the CNS in cats. Although some discordance exists in the literature, cats with CNS lymphoma are younger (median ages of 4 to 10.5 years reported), and 17% to 50% of cases are FeLV antigenemic.76-78 Approximately two-thirds of intracranial cases are part of a multicentric, extracranial process, and approximately 40% of spinal lymphoma cases occur in multiple spinal cord sites with one-third also involving intracranial locations.76-78 In a compilation of 160 cases of intracranial tumors in cats, diffuse cerebral and diffuse brainstem involvement was most common for lymphoid malignancies.76 Spinal lesions are usually both extradural and intradural, although they can be limited to one or the other compartment.77 Feline CNS lymphoma may be primary but more commonly (approximately 80%) represents a multicentric process (especially renal or bone marrow).76,78 A paucity of information exists on the immunophenotype of CNS lymphoma.
Laryngeal lymphoma made up 10% of 110 cases of extranodal forms in one report and represented 11% of all laryngeal disease in the species.68,79 It occurs in older cats (median age 9 years), is not associated with FeLV, and may be a solitary lesion or occur in the presence of other multicentric sites. No information on immunophenotype is available.
Cutaneous lymphoma is a rarely encountered anatomic form in the cat. It is usually seen in older cats (median age 10 to 13.5 years), with no sex or breed predominance, and is not associated with FeLV/FIV.80,81 It can be solitary or generalized, often affecting the head and face and is generally a slow chronic disease. Two forms of cutaneous lymphoma have been distinguished histologically and immunohistochemically. Most reports in the cat are epitheliotropic and consist of T-cells, although unlike the disease in dogs, adnexal structures are often spared. A report of nonepitheliotropic cutaneous lymphoma in cats also found five of six cases to be of T-cell derivation.82 Cutaneous “lymphocytosis,” an uncommon disease histologically resembling well-differentiated lymphoma, was characterized in 23 cats.83 Solitary lesions were most common, and all were composed primarily of T-cells, with two-thirds having some B-cell aggregates. Cutaneous lymphocytosis was characterized as a slowly progressive disorder; however, a few cases went on to develop internal organ infiltration. Two case reports exist of cats with cutaneous T-cell lymphoma and circulating atypical lymphocytes.84,85 The circulating cells were lymphocytes with large, hyperchromatic, grooved nuclei, and one case was immunophenotyped as a CD3/CD8 population. In humans, cutaneous T-cell lymphoma with circulating malignant cells is termed Sézary syndrome.
Ocular lymphoma was identified in 5 of 110 cases of extranodal lymphoma in one report.68 In a compilation of 75 cases of intraocular tumors, 15 (20%) were lymphoma (7 B-cell and 4 T-cell).86 It was presumed but not proved that the majority were part of a systemic multicentric process.
History, Clinical Signs, and Physical Examination Findings
The clinical signs associated with feline lymphoma are variable and depend on anatomic location and extent of disease.
The alimentary form is most commonly associated with nonspecific signs associated with the intestinal tract. In the more common low-grade small cell forms, weight loss (83% to 100%), vomiting and/or diarrhea (73% to 88%), and anorexia (66%) are the most common findings, and icterus is uncommon (7%).50,52,87 Abdominal palpation is abnormal in approximately 70% of cases, with half consisting of intestinal wall thickening and one-third having a palpable mass. Clinical signs are usually present for several months (median: 6 months).87 In contrast, although the lymphoblastic high-grade forms tend to cause similar clinical signs, they progress more rapidly with signs present for days or weeks and are more likely to present with a palpable abdominal mass originating from the GI tract, enlarged mesenteric lymph nodes, or liver.31,50,88 Icterus is also more common in large cell forms. Hematochezia and tenesmus may also be present if the colon is involved.33 Rarely, cats may present with signs consistent with an acute abdomen due to intestinal obstruction or perforation and concurrent peritonitis. Cats with intestinal LGL are presented with anorexia, weight loss, lethargy, and vomiting.59,60 A palpable abdominal mass is present in approximately half of LGL cases, and hepatomegaly, splenomegaly, and renomegaly are common. Abdominal effusions, pleural effusions, and icterus are observed in less than 10% of cases.
The clinical signs associated with the mediastinal form of lymphoma include dyspnea, tachypnea, and a noncompressible anterior mediastinum with dull heart and lung sounds.89 Rarely, a Horner’s syndrome and precaval syndrome may be observed. Pleural effusion is common and characterized by serohemorrhagic to chylous effusion, and in most cases, neoplastic cells (lymphoblasts) are identified.63,90
Cats with the nodal form of lymphoma present with variable clinical signs depending on the extent of disease; however, they are often depressed and lethargic. Peripheral lymphadenopathy, as the only physical finding, is an uncommon presentation. Cats with Hodgkin’s-like nodal lymphoma usually present without overt clinical signs.65,66
Cats with nasal lymphoma are typically presented with nasal discharge (60% to 85%), sneezing (20% to 70%), upper respiratory noise (stridor, stertor, wheezing; 20% to 60%), facial deformity (0% to 20%), anorexia (10% to 60%), epiphora (10% to 30%), and occasionally increased respiratory effort and coughing.68,69,73 The nasal discharge is usually mucopurulent, although epistaxis is present in up to one-third of cases. Regional lymphadenopathy can also occur. The median duration of clinical signs prior to diagnosis is 2 months (range of 1 to 1800 days).
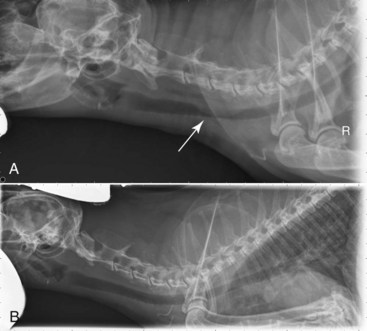
Cats with renal lymphoma present with signs consistent with renal insufficiency: inappetence, weight loss, and polyuria/polydipsia.68,74 On physical examination, renomegaly (usually bilateral, lumpy, and irregular) is palpated in the majority of cases (Figure 32-14).
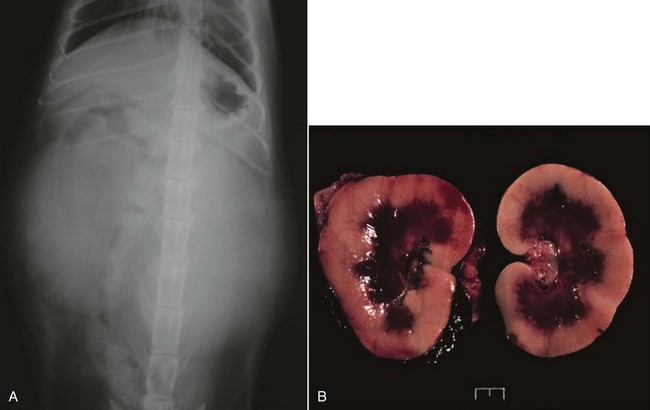
Figure 32-14 A, Ventrodorsal projection of a cat with renal lymphoma. Massive, bilateral renomegaly is observed. B, Necropsy specimens of a cat with bilateral renal lymphoma illustrating the diffuse cortical nature of the disease that is most common.
Cats with CNS lymphoma can present with constitutional signs (anorexia, lethargy) and signs referring to intracranial lesions, spinal lesions, or both.68,75-77,91,92 Intracranial signs may include ataxia, altered consciousness, aggression, central blindness, and vestibular abnormalities. In a study of cats with seizures, of those diagnosed with intracranial lesions, 8% were due to lymphoma.75 Clinical signs referring to spinal cord involvement may include paresis or paraplegia (>80%; tetraparesis in 20%), ataxia, pain, and constipation, and nonspecific constitutional signs (e.g., anorexia, lethargy, weight loss) are also common.77,92 In cats with spinal cord involvement, neurologic examination may further reveal tetraparesis, lower or upper motor neuron bladder, tail flaccidity, and absent deep pain; approximately one-third of signs will be asymmetric and most refer to thoracolumbar involvement. The neurologic dysfunction may be insidious or progress rapidly.
Signs associated with laryngeal lymphoma in cats most commonly include dyspnea, dysphonia, stridor, gagging or retching, and rarely, coughing.68,79
Cutaneous lymphoma may be solitary or diffuse with a varied presentation.80,83 In decreasing order of likelihood, lesions may include erythematous patches, alopecia, scaling, dermal nodules, or ulcerative plaques. Nasal hypopigmentation, miliary dermatitis, and mucosal lesions are rarely observed. Peripheral lymphadenopathy may also be present. In most cats, the duration of signs will be prolonged, lasting several months.
Cats with ocular lymphoma are presented with uveitis or iridial masses, as well as signs related to systemic involvement of disease.68
Nonspecific Signs
All cats with lymphoma, regardless of site, may be presented with nonspecific constitutional signs that may include anorexia, weight loss, lethargy, or depression. Secondary bone marrow infiltration may lead to anemia—at least 50% of affected cats have moderate-to-severe nonregenerative anemia. Signs related to paraneoplastic hypercalcemia (PU/PD) can occur in cats, however, much less commonly than in the dog. In one survey of hypercalcemia in cats, approximately 10% were diagnosed with lymphoma of various anatomic types.93
Diagnosis and Clinical Staging
For most cats with suspect lymphoma, the diagnostic evaluation should include a baseline assessment consisting of a CBC with differential cell count, platelet count, serum chemistry profile, urinalysis, and retroviral (FeLV/FIV) screen. Serum chemistry profiles can help establish the overall health of the animal, as well as, in some cases, suggest site-specific tumor involvement; for example, increased activities of liver enzymes may indicate hepatic infiltration and increased blood urea nitrogen (BUN) and creatinine may indicate renal lymphoma. For cats with alimentary lymphoma, hypoproteinemia and anemia are reported to occur in up to 23% and 76% of cases, respectively.31,52,94 Hypercalcemia is rarely seen in cats but has been reported in cats with lymphoma at various anatomic sites. Hypoglycemia was reported in approximately one-third of cats with lymphoma in one Australian study.94 In a series of cats with various anatomic forms of lymphoma, serum albumin concentrations were significantly lower and β-globulin concentrations (as measured by protein electrophoresis) were significantly higher than a healthy control population.95
The use of various imaging modalities in cats with lymphoma depends on the anatomic site and will be discussed in site-specific discussions to follow.
Cytopathologic or histopathologic evaluation of lymph node or involved organ tissue, procured via needle aspirate cytology (see Chapter 7), surgical, endoscopic, or needle-core biopsy (see Chapter 9) is required for a definitive diagnosis. FNA cytology alone may not be sufficient in some cases, owing to difficulties encountered in distinguishing lymphoma from benign hyperplastic or reactive lymphoid conditions. In such cases, whole lymph node excision and/or involved organ biopsy is preferred because orientation and information regarding invasiveness and architectural abnormalities may be necessary for diagnosis. Additionally, involved tissue, needle aspirate, and fluid samples can be further interrogated by various histochemical, immunohistochemical, flow cytometric analysis (e.g., size and immunophenotypic assessment), and molecular techniques (e.g., PARR to assess clonality) to further characterize the disease process and refine the diagnosis in equivocal cases.
The reader is referred to Chapter 8 for a general discussion of flow cytometric analysis and molecular diagnostic techniques, as well as the molecular diagnostic techniques section in Section A of this chapter for specific applications to lymphoma. PARR applications in cats have been described as being approximately 80% sensitive for the diagnosis of feline lymphoma96; however, assessment of specificity has not been clearly established. Clonality assessment tools (e.g., primers) for both Ig and T-cell receptor variable region genes have been developed in cats.97-100
Assessments of tumor proliferation rates (e.g., Ki67, PCNA, AgNOR), telomerase activity, and serum protein electrophoresis can also be performed on involved tissues in cats; however, consistent prognostic value across the anatomic, histopathologic, and immunophenotypic variants of lymphoma in cats is not well characterized. If these ancillary assays are helpful with respect to prognosis or diagnosis, they will be discussed in site-specific discussions to follow.
Thorough staging, including a bone marrow aspiration or biopsy, peripheral lymph node assessment (clinically normal or abnormal nodes), and thoracic and/or abdominal imaging, is indicated when (1) solitary site disease is suspected (in particular, extranodal sites) and a decision between locoregional therapy (i.e., surgery and/or RT) versus systemic therapy (i.e., chemotherapy) is being considered; (2) it provides prognostic information that will help a caregiver make treatment decisions; and (3) complete staging of the extent of disease is required as part of a clinical trial. Bone marrow evaluation may be of particular interest if anemia, cellular atypia, and leukopenia are present. A WHO staging system exists for the cat that is similar to that used in the dog (see Box 32-1); however, because of the high incidence of visceral/extranodal involvement in the feline species, a separate staging system has been evaluated and is often used (Box 32-3).101 Because lymphoma in cats is more varied with respect to anatomic locations, staging systems are generally less helpful for predicting response.
Anatomic Site-Specific Diagnostics
Alimentary/Gastrointestinal Lymphoma: The diagnosis of large cell, high-grade alimentary/GI lymphoma is generally less complicated than for the more common low-grade GI type. The former (including LGL) is often diagnosed with physical examination, abdominal imaging (e.g., ultrasound), and cytologic or histologic assessment of needle aspirate or needle biopsy samples from intestinal masses, enlarged mesenteric lymph nodes, or liver because mass lesions and gross lymphadenopathy are more commonly present. If obvious abdominal masses are present on physical examination, transabdominal needle aspiration may be possible without the aid of abdominal imaging. Less commonly, abdominal exploration is necessary if lesions are more subtle or not amenable to transabdominal sampling. Further staging via thoracic imaging, peripheral lymph node aspiration, and bone marrow assessment may be performed, but rarely contributes prognostic information or alters treatment decisions because the disease is already widespread and systemic therapy is required.
In contrast, low-grade, small cell GI lymphoma is more commonly associated with modest (or palpably absent) intestinal thickening without mass effect and is clinically similar if not identical in presentation to benign inflammatory bowel disease (IBD). Cytologic assessment alone is often not sufficient for diagnosis; in one study, eight of nine cases in which mesenteric lymph nodes were confirmed histologically as lymphoma, cytologic assessment incorrectly indicated benign lymphoid hyperplasia.52 The key elements necessary for the diagnosis of low-grade, small cell GI lymphoma (and differentiation from IBD) include abdominal imaging (usually ultrasound), procurement of tissue for histopathology, and if necessary, assessment of immunophenotype and clonality.
Abdominal ultrasound will be abnormal in approximately 60% to 90% of cats with low-grade, small cell GI lymphoma.31,52,102,103 Diffuse small intestinal wall thickening is the most common finding; 50% to 70% of cats with lymphoma will have ultrasonic evidence of wall thickening, which predominantly involves the muscularis propria, and submucosa, although mucosal thickening can also occur. Focal mural masses are uncommon. Mesenteric lymphadenopathy is also common and reported in 45% to 80% of affected cats. These ultrasonographic findings are by no means pathognomonic for lymphoma, however, because 10% to 50% and 15% to 20% of cats with IBD also have ultrasonographic evidence of intestinal wall thickening and lymphadenopathy, respectively.102,103 Mucosal thickening is more common, and muscularis propria thickening is less common in IBD than lymphoma. Less commonly, cats with low-grade intestinal lymphoma will have ultrasonographic abnormalities in other abdominal organs such as the stomach, liver, spleen, colon, and pancreas, and occasionally, mild effusions are observed.
As mentioned previously, although aspirate cytology may be sufficient for diagnosis of large cell intermediate- or high-grade alimentary lymphoma in cats, it is rarely diagnostic for low-grade, small cell intestinal lymphoma, and tissue procurement for histologic and ancillary assessment is required for diagnosis (and differentiation from IBD). The debate still rages as to whether endoscopically obtained tissue is sufficient for diagnosis or if full-thickness tissue procured during laparotomy or laparoscopy is necessary in light of similarities with IBD.51,54,103,104 As previously discussed, histologic features that help differentiate intestinal lymphoma from IBD include lymphoid infiltration of the intestinal wall beyond the mucosa, epitheliotropism (especially intraepithelial nests and plaques), heterogeneity, and nuclear size of lymphocytes.51,54 Although the presence of transmural involvement is highly suggestive of lymphoma, the lack of transmural infiltration is not pathognomonic for IBD; transmural infiltration is common with B-cell and large T-cell (including LGL) intestinal lymphoma but is observed in the minority of low-grade T-cell intestinal lymphomas that represent the largest group in cats (see Table 32-9).51 For these reasons, if the differentiation of lymphoma and IBD is equivocal after standard histopathologic assessment, the addition of immunophenotypic and PARR analysis in a stepwise fashion, as proposed by Kiupel and others,54 may be ultimately necessary for a definitive diagnosis. Their study of 63 cats with either lymphoma or IBD found that, although standard histopathology was highly specific for diagnosis of lymphoma (99% specific, 72% sensitive), sensitivity was enhanced by the addition of immunophenotypic analysis (99% specific, 78% sensitive) and further enhanced by PARR analysis (99% specific, 83% sensitive).
Mediastinal Lymphoma: For cats with mediastinal lymphoma, diagnostic suspicion may begin with a noncompressible cranial thorax on physical examination and confirmation of a mediastinal mass/pleural effusion on thoracic radiograph. FNA of the mass or cytologic evaluation of pleural fluid may be sufficient to establish a diagnosis. In most cats, the finding of a monotonous population of intermediate- or high-grade cells will establish a diagnosis. However, definitive diagnosis of lymphoma in cats with a mediastinal mass and concurrent chylothorax can be challenging. CT appearance may be helpful but generally does not contribute to a definitive diagnosis. If lymphoblasts are not identified in the pleural chylous effusion, then cholesterol and triglyceride concentrations can be measured.105 In chylous effusions, the pleural fluid triglyceride concentration will be greater than in the serum; however, anorectic cats will have lower triglyceride levels in the pleural fluid. A major differential for mediastinal lymphoma is thymoma. The cytologic features of thymoma can be distinct from lymphoma in many cases, but the diagnosis can be challenging because of a preponderance of small lymphocytes in thymoma. Mast cells can also be seen in up to 50% of aspirations from thymomas. The addition of immunophenotypic and clonality assessment may be helpful in equivocal cases.
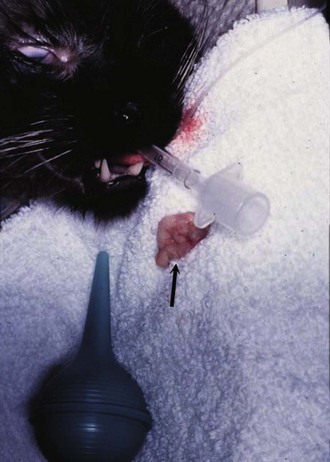
Nasal Lymphoma: If nasal lymphoma is suspected, advanced imaging (CT, MRI), rhinoscopy, and biopsy are usually necessary for diagnosis (see Chapter 23, Section B). CT or MRI is useful to determine the extent of involvement and to help plan biopsy procurement and RT if that treatment option is pursued. CT characteristics associated with sinonasal tumors in cats include the presence of a unilateral or bilateral nasal/sinus mass or fluid, bulla effusion, and lysis of associated bony structures.106,107 A biopsy can be procured either by intranasal procurement (with or without rhinoscopy) or by flushing one hemicavity with a bulb syringe and saline while occluding the contralateral cavity and collecting samples flushed out of the nasopharynx (Figure 32-15). Thorough staging (i.e., regional node assessment, thoracic and abdominal staging, and bone marrow assessment) to ensure the disease is confined to the nasal passages is recommended, if local RT without systemic chemotherapy is being considered.
Renal Lymphoma: In the case of renal lymphoma, physical examination findings of massive and often bilateral renomegaly will raise the index of suspicion. Radiographic appearance is smooth-to-irregular renomegaly (see Figure 32-14, A). Ultrasonographic imaging usually reveals bilateral (>80%), irregular renomegaly with hypoechoic subcapsular thickening.108 Approximately one-third of cases will have ultrasonographic evidence of other abdominal organ involvement. The disease is usually diffuse throughout the renal cortex (see Figure 32-14, B) and transabdominal needle aspirate or core biopsy is diagnostic in most cases.
Central Nervous System Lymphoma: In cats with suspected spinal lymphoma, survey radiographs of the spine will rarely reveal osseous lesions. Myelograms, CT, or MRI are indicated, and in approximately 75% of the cases, an extradural or intradural mass will be detected.76,77,91,92 Most lesions occur at a thoracolumbar or lumbosacral location, and they are often found in more than one location. Image-guided needle aspiration of epidural lesions may yield enough tissue for a cytologic diagnosis. CT or MR also reveals multifocal disease in the majority of cats with intracranial lymphoma.75,76 CSF analysis may be helpful but is rarely definitive for lymphoma. One of 11 cats with confirmed spinal lymphoma in one study77 and 6 of 17 with confirmed intracranial lymphoma in another76 had evidence of lymphoblasts in the CNS, and an increased protein content was commonly found. In cats suspected of CNS lymphoma, bone marrow and renal involvement are often present, and cytologic assessment of these or other more accessible organs is generally more easily attainable than from spinal sites.
For cats suspected of cutaneous lymphoma, punch biopsies (4 to 8 mm) should be taken from the most representative and infiltrative sites, while avoiding overtly infected skin lesions. Immunophenotypic and PARR analysis often are helpful in definitive diagnosis. Complete staging to rule out systemic disease is also recommended for cats with cutaneous lymphoma because local therapies can be applied in cases of solitary disease.
Treatment and Prognosis
Our knowledge base for treating cats with lymphoma is less well established, and outcomes are less predictable than that in dogs, primarily due to the greater variation in histologic type and anatomic location observed in the species. This is further complicated by the plethora of papers that “lump” very small numbers of cases representing multiple anatomic/immunophenotypic and histologic subtypes (e.g., small cell versus large cell variants) together when reporting survival analysis following chemotherapy. This provides only general observations rather than important specific outcome information (i.e., response rate and durability of response) that can vary significantly with respect to anatomic and histologic subtype. In general, canine lymphoma is most commonly intermediate-high grade and nodal, whereas cats more commonly present with GI or extranodal (±nodal extension), small cell, low-grade, and/or indolent forms. As will be discussed subsequently, the author bases most treatment decisions on assessment of whether the individual case represents a low-grade (e.g., indolent, small cell variants) versus an intermediate- or high-grade (e.g., large cell) lymphoma. Finally, much of the early work on chemotherapy protocol development for cats with lymphoma occurred during the FeLV era, and care should be exercised when applying this information in the post-FeLV era.
In general, cats tolerate chemotherapy for lymphoma quite well, most clients are happy with their choice to initiate treatment, and quality of life generally improves following commencement of therapy.109,110 The chemotherapeutic agents used most commonly to treat intermediate- or high-grade lymphoma in cats are similar to those used for dogs and humans with lymphoma (see Section A in this chapter) and include doxorubicin, vincristine, cyclophosphamide, methotrexate, L-asparaginase, CCNU (lomustine), and prednisone. Most combination induction protocols currently employed in cats are modifications of CHOP protocols initially designed for human oncologic use.5,110-116 CHOP represents combinations of cyclophosphamide (C), doxorubicin (H, hydroxydaunorubicin), vincristine (O, Oncovin) and prednisone (P). In general, CHOP-based protocols are appropriate for cats with large cell, intermediate- and high-grade lymphoma involving any anatomic site (e.g., peripheral nodal, mediastinal, and renal forms) but should not be first-line therapy for small cell, low-grade variants. As in the dog (see Section A in this chapter), a plethora of modifications are used with CHOP-based protocols, although virtually no quality comparative data exist to compare outcomes, and as such, the protocol used should be based on cost, ease, client/veterinarian preference, and level of comfort. The current CHOP-based protocol in use by the author is presented in Table 32-10. This protocol has been used in many cats with various forms of intermediate- and high-grade lymphoma and is generally well tolerated. At present, most canine lymphoma protocols (see Section A in this chapter) discontinue chemotherapy by the 25th week, and we have sufficient data to show shorter, maintenance-free protocols are as good if not superior to longer maintenance protocols; however, similar comparative data do not exist in the cat. Until such time as evidence to the contrary exists, the author presently recommends discontinuation of chemotherapy at week 25 in cats who have attained a complete remission. Doxorubicin alone (25 mg/m2, every 3 weeks for 5 total treatments) or palliative prednisone therapy is offered if clients decline more aggressive CHOP-based therapy. Cats are generally less tolerant of doxorubicin than are dogs; therefore a lower dosage (25 mg/m2 IV or 1 mg/kg IV) is used (see Chapter 11). Cardiac toxicity does not appear to be a clinically significant problem in cats, although renal toxicity is more commonly encountered in the species117 and renal function should be monitored (i.e., serial BUN, creatinine, and urine specific gravity) closely prior to and during therapy. The use of COP (i.e., CHOP without the addition of doxorubicin) is often used in cats in Europe, and one compilation reported similar results to CHOP.64 A COP protocol commonly employed in cats is presented in Table 32-11. Some studies with relatively few case entries have reported limited activity for doxorubicin as a single agent in cats with lymphoma118,119; however, larger studies using combination protocols have more consistently reported the addition of doxorubicin as necessary for the attainment of more durable responses.5,114 Interestingly, in a report of 23 cats having relapsed following COP-based protocols (without doxorubicin), only 22% responded subsequently to doxorubicin-containing rescue therapy.120 A small number of cats with lymphoma have been treated with single-agent oral CCNU (lomustine) at a dosage range of 30 to 60 mg/m2 every 3 to 6 weeks.121,122 Whereas activity was noted, only partial responses were reported. L-asparaginase, which is often included in protocols for lymphoma in cats, has a much shorter asparagine-depleting effect in cats (lost by 7 days) than in dogs and in one study in 13 cats with lymphoma resulted in only a 30% response rate.123
Table 32-10
The CHOP-Based Chemotherapy Protocol for Cats with Lymphoma Employed by the Author
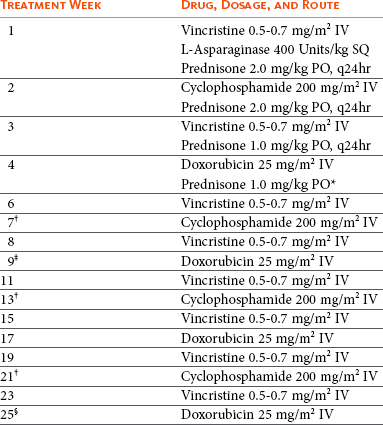
IV, Intravenous; SQ, subcutaneous; PO, by mouth.
note: A complete blood count (CBC) should be performed prior to each chemotherapy. If neutrophils are <1500 cells/UL, wait 5 to 7 days, repeat CBC, then administer the drug if neutrophils have risen above the 1500 cell/UL cutoff.
*Prednisone is continued (1 mg/kg PO) every other day from this point on.
†If renal lymphoma or central nervous system (CNS) lymphoma is present, substitute cytosine arabinoside (Ara-C) at 600 mg/m2 divided SQ twice a day (BID) over 2 days at these treatments.
‡If in complete remission at week 9, continue to week 11.
§If in complete remission at week 25, therapy is discontinued and cat is rechecked monthly for recurrence.
Table 32-11
COP Protocol for Lymphoma in Cats
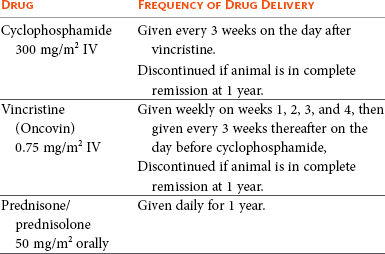
note: A complete blood count (CBC) should be performed prior to each treatment. If neutrophils are <1.5 × 109/L, wait 5 to 7 days and repeat the CBC. Treat if neutrophils are ≥1.5 × 109/L.
In general, cats with intermediate- and high-grade lymphoma treated with CHOP-based or COP protocols do not enjoy the same level of success as dogs. Bearing in mind that these reports group together a wide variety of subtypes having dissimilar prognoses (see subsequent site-specific treatment sections), the overall response rates tend to be in the 50% to 80% range with median remission and survival durations of 4 and 6 months, respectively.5,64,110-116,124
Anatomic Site-Specific Treatment
Alimentary/Gastrointestinal Lymphoma: Representing the most common presentation for cats with lymphoma, the large majority have the small cell, mucosal, T-cell variant that carries a good prognosis, often with less aggressive chemotherapy protocols (e.g., oral chlorambucil and prednisone).51,52,87,125 Chlorambucil (20 mg/m2 PO, every 2 weeks [preferred by the author] or 2 mg PO every other day) and prednisone or prednisolone (initially 1 to 2 mg/kg PO daily, reduced to 0.5 to 1.0 mg/kg every other day over several weeks) results in response rates (i.e., resolution of clinical signs) of greater than 90% and median survivals of approximately 2 years or longer.52,87,125 Cats who relapse with this protocol often will subsequently respond to alternative alkylators, such as cyclophosphamide or lomustine.125 Anecdotally, many will also respond to vinblastine chemotherapy if they no longer are responsive to alkylators.
In contrast, cats with B-cell or large T-cell (including LGL) or small T-cell lymphoma that is transmural typically do not enjoy a durable response to therapy and survivals are much shorter.31,51,59,60 Median survivals range from 45 to 100 days, even in cats treated with more aggressive COP-based protocols. In the author’s experience, these variants are more likely to respond to CHOP-based protocols than chlorambucil/prednisone; however, durable responses occur only in a minority of cases. In particular, LGL appears to carry a grave prognosis59,60; in 2 compilations of 66 cats with LGL, median survivals of approximately 2 months were reported, including 23 cats receiving either COP or CHOP-based protocols, which resulted in only a 30% response rate.
Nutritional support is especially important for cats with GI lymphomas. It may be necessary to place a feeding tube in cats undergoing chemotherapy, particularly if prolonged anorexia is present (see Chapter 15, Section B).
Recently, two preliminary studies evaluated RT, either as rescue following recurrence or in addition to chemotherapy for the treatment of intestinal lymphoma in cats.126,127 Eleven cats (6 small cell, 4 large cell, and 1 LGL) that progressed following chemotherapy received abdominal radiation (8 Gy in 2 fractions over 2 days) and resulted in a median survival of 7 months, although numbers were small and 40% were lost to follow-up.127 A second report of eight cats (seven with large cell lymphoma) underwent 6 weeks of CHOP-based combination chemotherapy, followed 2 weeks later by whole abdomen radiation consisting of 10 daily 1.5 Gy fractions.126 Although three cats died within 3 weeks of RT, five enjoyed durable remissions. These preliminary promising outcomes warrant further investigation.
Mediastinal Lymphoma: Mediastinal lymphoma in young FeLV-positive cats is generally associated with a poor prognosis, and survival times of approximately 2 to 3 months are expected following CHOP- or COP-based protocols .5,116 In contrast, young FeLV-negative Siamese cats with mediastinal lymphoma experience remission rates approaching 90%, and responses tend to be more durable (median ≈9 months).64
Nodal Lymphoma: The treatment choice for peripheral nodal lymphoma in cats depends on whether the individual case represents a low-grade (e.g., indolent, small cell variants) versus an intermediate- or high-grade (e.g., large cell) lymphoma; the latter are best treated with CHOP- or COP-based protocols and carry a less favorable prognosis, whereas the former generally respond to less aggressive chlorambucil/corticosteroid protocols and enjoy durable responses. Less is known regarding the treatment of Hodgkin’s-like lymphoma involving solitary or regional nodes of the head and neck.65,66 Clinical outcome following surgical extirpation of the affected node (or nodes if a reasonable number) is often associated with long-term, disease-free intervals and survivals of approximately 1 year, suggesting it is a more indolent form of lymphoma. Eventual recurrence in distal nodes following surgical excision is common, and the author currently offers clients the option of adjuvant chlorambucil/corticosteroids following surgery—this theoretically may have benefit; however, insufficient data exist to document a survival advantage with this approach.
Nasal Lymphoma: Cats with nasal lymphoma generally enjoy durable remissions following therapy and lengthy overall survival durations.5,68,73,128,129 If disease is documented as confined to the nasal cavity following thorough staging (node cytology, thoracic and abdominal imaging, bone marrow aspiration), then RT is the treatment of choice. CRs in the order of 75% to 95% are reported, with reports of median survivals following RT of 1.5 to 3 years.73,129 Cats that do not achieve a CR with RT have a median survival of approximately 4.5 months. Total radiation dosage does affect survival durations, and a total dose greater than 32 Gy is recommended.73 The addition of chemotherapy has not been shown to enhance survival for cats with locally confined disease; combinations of RT and chemotherapy result in similar response rates and survival times.73,128,129 Chemotherapy (COP- or CHOP-based protocols) used in the absence of RT is a reasonable alternative, with complete response rates of approximately 75% and median survivals of approximately 2 years reported for cats achieving CR.68 The author’s preference is to initiate systemic chemotherapy only for (1) cases that have confirmed disease beyond the nasal passage, (2) cases that relapsed following RT, or (3) cases in which RT is unavailable or declined.
Central Nervous System Lymphoma: Very few cases involving treatment for CNS lymphoma exist, and although an occasional case experienced durable response to systemic chemotherapy, generally less than 50% will respond and median survivals of 1 to 2 months can be expected.68,76,77
Laryngeal/Tracheal Lymphoma: The vast majority of cats with laryngeal or tracheal lymphoma respond to either RT (if localized) or systemic chemotherapy (90% CR to COP- or CHOP-based protocols) (Figure 32-16).68 Whereas the authors experience is that most have durable responses and survival durations typically approach or exceed 1 year, the only case series (n = 8) reported a median survival of 5.5 months following achievement of a CR.
Cutaneous Lymphoma: Very little has been published regarding the treatment of cutaneous lymphoma or mycosis fungoides in cats80; however, a report of a CR to lomustine exists.130 Cats with a solitary disease could theoretically be treated with surgical excision or RT, although clinical staging is necessary to rule out possible further systemic involvement. For multiple sites, combination chemotherapy may be considered.
General Summary of Prognosis for Cats with Lymphoma
As previously discussed, the prediction of outcomes in cats with lymphoma is not generalizable due to the wide spectrum of histologic and anatomic subtypes encountered. Much has been mentioned in the previous treatment sections, and Tables 32-8 and Table 32-9 summarize prognostic parameters for lymphoma in cats.
Feline Leukemias, Myeloproliferative Disorders, and Myelodysplasia
For a complete discussion of leukemias and MPDs, including a general discussion of hematopoiesis, etiologies, lineage classification and descriptions, see Section C of this chapter. The classification of leukemias in cats is difficult because of the similarity of clinical and pathologic features and the transition, overlap, or mixture of cell types involved.131-135 Most case-series reports are from the FeLV era and generally only single case reports exist from the more contemporary post-FeLV era, which further confuses our understanding of the biology and outcome. For this reason, only a simplistic discussion, primarily relating to the lymphoid leukemias will be presented here and the interested reader is again referred to Section C for a general discussion of nonlymphoid leukemia.
For cats with suspected leukemia, peripheral blood assessment (e.g., CBC with differential, flow cytometric analysis for size and immunophenotype, and PARR [for lymphoid leukemias]), and bone marrow aspiration or biopsy may contribute to a diagnosis. The preferred sites for bone marrow aspiration are the proximal humerus or iliac crest. Cats with acute leukemia are likely to have malignant cellular infiltrates in organs other than bone marrow.134 A bone marrow aspirate with greater than 30% abnormal blast cells is sufficient to make a diagnosis of an acute leukemia. In cats with suspected CLL, infiltration of the bone marrow with more than 20% mature lymphocytes helps confirm the diagnosis. All cats with leukemia should be tested for FeLV/FIV. Determining the lineage of some leukemias can be challenging; most can be distinguished from one another by histologic appearance, histochemical stains, or immunohistochemical or flow cytometric analysis of the leukemic cells for cellular antigens that identify their lineage (see Chapter 8 and Section C in this chapter).131,133,136 In addition, examination of blast cells by electron microscopy may reveal characteristic ultrastructural features. The French-American-British (FAB) classification system is considered useful in cats with myelodysplastic syndromes and almost all will be FeLV antigenemic.136,137
Lymphoid Leukemia
ALL was the most commonly encountered type of leukemia in cats in the FeLV era; however, it is much less common today. ALL is characterized by poorly differentiated lymphoblasts and prolymphocytes in blood and bone marrow. Approximately 60% to 80% of cats with ALL were FeLV positive, and most malignant cells have T-cell immunophenotypes138; however, little information is available in the contemporary literature.
CLL is rarely reported in cats and is characterized by well-differentiated, small, mature lymphocytes in peripheral blood and bone marrow. Whereas most are of the T-cell lineage, B-cell CLL has also been reported.136,139,140 Most cats have increased WBC counts greater than 50,000/µl, and most are FeLV negative.
Treatment of Leukemias
The use of chemotherapy to treat ALL has been disappointing. Using COP-based protocols, Cotter124 reported a 27% CR rate. CLL can be treated with chlorambucil (0.2 mg/kg PO or 2 mg/cat QOD) and prednisone (1 mg/kg PO daily); however, little information exists regarding outcome. As in humans and dogs, if significant clinical signs or profound cytopenias are not present, treatment can be withheld—one cat with CLL remained stable without chemotherapy for over a year.140 The prognoses for acute nonlymphoblastic leukemias are generally very poor, although some exceptions exist in case report form in the historic literature.