CHAPTER 8 Clinical Features of Gingivitis
Changes in Gingival Contour
Experimental gingivitis studies provided the first empiric evidence that accumulation of microbial biofilm on clean tooth surfaces results in the development of an inflammatory process around gingival tissue.41,69 Research has also shown that the local inflammation will persist as long as the microbial biofilm is present adjacent to the gingival tissues and that the inflammation may resolve subsequent to a meticulous removal of the biofilm.69
The prevalence of gingivitis is evident worldwide. For example, epidemiologic studies indicate that more than 82% of adolescents in the United States (US) have overt gingivitis and signs of gingival bleeding. A similar or higher prevalence of gingivitis is reported for children and adolescents in other parts of the world.7 A significant percentage of adults also show signs of gingivitis; more than half the US adult population is estimated to exhibit gingival bleeding, and other populations show even higher levels of gingival inflammation.3,5,6,8,66 Whereas plaque remains the primary etiological factor causing gingivitis, other factors may affect the development of periodontal diseases. Recent findings suggest that distinct biologic phenotypes may play a role in the development of gingivitis.59
In general, clinical features of gingivitis may be characterized by the presence of any of the following clinical signs: redness and sponginess of the gingival tissue, bleeding on provocation, changes in contour, and presence of calculus or plaque with no radiographic evidence of crestal bone loss.10 Histologic examination of inflamed gingival tissue reveals ulcerated epithelium. The presence of inflammatory mediators negatively affects epithelial function as a protective barrier. Repair of this ulcerated epithelium depends on the proliferative or regenerative activity of the epithelial cells. Removal of the etiologic agents triggering the gingival breakdown is essential.
Course and Duration
Gingivitis can occur with sudden onset and short duration and can be painful. A less severe phase of this condition can also occur.
Recurrent gingivitis reappears after having been eliminated by treatment or disappeared spontaneously.
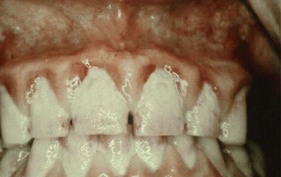
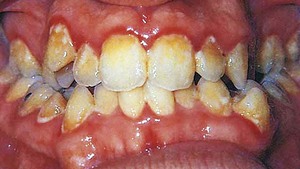
Chronic gingivitis is slow in onset and of long duration. It is painless, unless complicated by acute or subacute exacerbations, and is the type most often encountered (Figure 8-1). Chronic gingivitis is a fluctuating disease in which inflammation persists or resolves and normal areas become inflamed.30,36
Description
Localized gingivitis is confined to the gingiva of a single tooth or group of teeth, whereas generalized gingivitis involves the entire mouth.
Marginal gingivitis involves the gingival margin and may include a portion of the contiguous attached gingiva. Papillary gingivitis involves the interdental papillae and often extends into the adjacent portion of the gingival margin. Papillae are involved more frequently than the gingival margin, and the earliest signs of gingivitis often occur in the papillae. Diffuse gingivitis affects the gingival margin, the attached gingiva, and the interdental papillae. Gingival disease in individual cases is described by combining the preceding terms as follows:
Clinical Findings
A systematic approach is required in evaluation of the clinical features of gingivitis. The clinician should focus on subtle tissue alterations because these may be of diagnostic significance. A systematic clinical approach requires an orderly examination of the gingiva for color, contour, consistency, position, and ease and severity of bleeding and pain. This section discusses these clinical characteristics and the microscopic changes responsible for each.
Gingival Bleeding on Probing
The two earliest signs of gingival inflammation preceding established gingivitis are (1) increased gingival crevicular fluid production rate and (2) bleeding from the gingival sulcus on gentle probing (Figure 8-7). Chapter 6 discusses gingival crevicular fluid in detail.
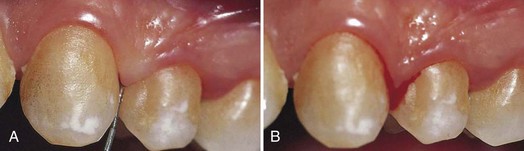
Figure 8-7 Bleeding on probing. A, Mild edematous gingivitis; a probe has been introduced to the bottom of the gingival sulcus. B, Bleeding appears after a few seconds.
Gingival bleeding varies in severity, duration, and ease of provocation. Bleeding on probing is easily detected clinically and therefore is of value for the early diagnosis and prevention of more advanced gingivitis. It has been shown that bleeding on probing appears earlier than a change in color or other visual signs of inflammation36,37,46; in addition, the use of bleeding rather than color changes to diagnose early gingival inflammation is advantageous in that bleeding is a more objective sign that requires less subjective estimation by the examiner. For example, it is estimated that 53.2 million (50.3%) of US adults 30 years and older exhibit gingival bleeding.6 Probing pocket depth measurements by themselves are of limited value for the assessment of the extent and severity of gingivitis. For example, gingival recession may result in reduction of the probing depth and thus cause an inaccurate assessment of the periodontal status.4 Therefore bleeding on probing is widely used by clinicians and epidemiologists to measure disease prevalence and progression, to measure outcomes of treatment, and to motivate patients with their home care.24 Several gingival indices based on bleeding have been developed,2,16,53 as described in Chapter 5. (For further considerations on probing, see Chapter 30.)
In general, gingival bleeding on probing indicates an inflammatory lesion both in the epithelium and in the connective tissue that exhibits specific histologic differences compared with healthy gingiva.25 Even though gingival bleeding on probing may not be a good diagnostic indicator for clinical attachment loss, its absence is an excellent negative predictor of future attachment loss.34 Therefore the absence of gingival bleeding on probing is desirable and implies a low risk of future clinical attachment loss. Longitudinal findings revealed that sites with consistent bleeding (gingival index [GI] = 2) had 70% more attachment loss than sites that were noninflamed over a 26-year period in 565 males. Thus persistent gingivitis can be considered as a risk factor for periodontal attachment loss that may lead to tooth loss.35
Interestingly, numerous studies show that current cigarette smoking suppresses the gingival inflammatory response, and smoking was found to exert a strong, chronic, dose-dependent suppressive effect on gingival bleeding on probing in the third National Health and Nutrition Examination Survey (NHANES III).19 In addition, recent research reveals an increase in gingival bleeding on probing in patients who quit smoking.54 Thus people who are committed to a smoking cessation program should be informed about the possibility of an increase in gingival bleeding associated with smoking cessation.
Gingival Bleeding Caused by Local Factors
Contributing factors to plaque retention that may lead to gingivitis include anatomic and developmental tooth variations, caries, frenum pull, iatrogenic factors, malpositioned teeth, mouth breathing, overhangs, partial dentures, lack of attached gingiva, and recession. In addition, orthodontic treatment and fixed retainers were associated with increased plaque retention and increased bleeding on probing.38,78
Chronic and Recurrent Bleeding
The most common cause of abnormal gingival bleeding on probing is chronic inflammation.50 The bleeding is chronic or recurrent and is provoked by mechanical trauma (e.g., from toothbrushing, toothpicks, or food impaction) or by biting into solid foods such as apples.
In gingival inflammation, histopathologic alterations that result in abnormal gingival bleeding include dilation and engorgement of the capillaries and thinning or ulceration of the sulcular epithelium (Figure 8-8). Because the capillaries are engorged and closer to the surface, and the thinned, degenerated epithelium is less protective, stimuli that are normally innocuous cause rupture of the capillaries and gingival bleeding. Sites that bleed on probing have a greater area of inflamed connective tissue (i.e., cell-rich, collagen-poor tissue) than sites that do not bleed. In most cases the cellular infiltrate of sites that bleed on probing is predominantly lymphocytic (a characteristic of stage II, or early, gingivitis).9,17,25
Histologic evaluations on animal specimens revealed that in early stages of gingivitis, expression of cytokines responsible for connective tissue breakdown, matrix metalloproteinases (MMPs), is ubiquitous. Different MMPs play a role in this breakdown at different stages (e.g., a decrease of MMP-14 activity at 7 days of inflammation and an immediate increase in MMP-2, especially with fibroblastic stimulation). In addition, MMP-9 expression peaked 5 days after gingivitis occurrence, which was also regulated by macrophages and neutrophils. Thus extracellular matrix remodeling was regulated with MMP-2 and -9 production and activation by host inflammatory response.42
The severity of bleeding and the ease of its provocation depend on the intensity of the inflammation. After the vessels are damaged and ruptured, interrelated mechanisms induce hemostasis.73 The vessel walls contract, blood flow diminishes, blood platelets adhere to the edges of the tissue, and a fibrous clot is formed, which contracts and results in approximation of the edges of the injured area. Bleeding recurs when the area is irritated.
In cases of moderate or advanced periodontitis, the presence of bleeding on probing is considered a sign of active tissue destruction.
Acute episodes of gingival bleeding are caused by injury and can occur spontaneously in gingival disease. Laceration of the gingiva by toothbrush bristles during aggressive toothbrushing or by sharp pieces of hard food can cause gingival bleeding even in the absence of gingival disease. Gingival burns from hot foods or chemicals increase the ease of gingival bleeding.
Spontaneous bleeding or bleeding on slight provocation can occur in acute necrotizing ulcerative gingivitis. In this condition, engorged blood vessels in the inflamed connective tissue are exposed by ulceration of the necrotic surface epithelium.
![]() Science Transfer
Science Transfer
Gingivitis is diagnosed by the presence of redness, swelling, and increased edema of the gingival tissues. There may be increased pocket depth without attachment loss caused by gingival enlargement, and bleeding on probing is a hallmark of gingivitis and periodontitis. Early diagnosis, simple nonsurgical therapy, and improvements in the patient’s oral hygiene are all that is required to control gingivitis in most patients. Careful, detailed monitoring of the gingival status around each tooth, with recordings of pocket depths, bleeding on probing, and position of the gingival margin, is essential so that patients with gingivitis do not progress to periodontal bone loss.
Gingival recession must also be documented to see if it is progressive and to determine if mucogingival surgery is required to enhance the amount of attached keratinized gingiva and to treat unaesthetic problems.
Gingival Bleeding Associated with Systemic Changes
In some systemic disorders, gingival hemorrhage occurs spontaneously or after irritation and is excessive and difficult to control. These hemorrhagic diseases represent a wide variety of conditions that vary in etiologic factors and clinical manifestations. Such conditions have the common feature of a hemostatic mechanism failure and result in abnormal bleeding in the skin, internal organs, and other tissues, including the oral mucosa.71
Hemorrhagic disorders in which abnormal gingival bleeding is encountered include vascular abnormalities (vitamin C deficiency or allergy [e.g., Schönlein-Henoch purpura]), platelet disorders27 (thrombocytopenic purpura), hypoprothrombinemia (vitamin K deficiency), other coagulation defects (hemophilia, leukemia, Christmas disease), deficient platelet thromboplastic factor (PF3) resulting from uremia,47 multiple myeloma12 and postrubella purpura.30 The effects of hormonal replacement therapy, oral contraceptives, pregnancy, and the menstrual cycle are also reported to affect gingival bleeding.43,65,79,80 In addition, in women, long-term depression-related stress exposure may increase concentrations of interleukin-6 (IL-6) in gingival crevicular fluid and may worsen periodontal conditions with elevated gingival inflammation and increased pocket depths.32
In addition, changes in androgenic hormones have long been established as significant modifying factors in gingivitis, especially among adolescents. Several reports have shown notable effects of fluctuating estrogen/progesterone levels on the periodontium, starting as early as puberty.1,55 Among pathologic endocrine changes, diabetes is an endocrine condition with a well-characterized effect on gingivitis.78 In diabetes, marked inflammation affects both the epithelial and connective tissues, which leads to degeneration of the dermal papilla, increase in the number of inflammatory cells, destruction of reticulin fibers, and accumulation of dense collagen fibers that causes fibrosis.70
Several medications have also been found to have adverse effects on the gingiva. For example, anticonvulsants, antihypertensive calcium channel blockers, and immunosuppressant drugs are known to cause gingival enlargement (see Chapter 9), which secondarily can cause gingival bleeding. The American Heart Association has recommended over-the-counter aspirin as a therapeutic agent for cardiovascular disease, and aspirin is often prescribed for rheumatoid arthritis, osteoarthritis, rheumatic fever, and other inflammatory joint diseases.28 Thus it is important to consider aspirin’s effect on bleeding during a routine dental examination to avoid false-positive readings, which could result in an inaccurate patient diagnosis.67 (Chapter 27 discusses periodontal involvement in hematologic disorders.)
Color Changes in the Gingiva
The color of the gingiva is determined by several factors, including the number and size of blood vessels, epithelial thickness, quantity of keratinization, and pigments within the epithelium.
Color Changes in Gingivitis
Change in color is an important clinical sign of gingival disease. The normal gingival color is “coral pink” and is produced by the tissue’s vascularity and modified by the overlying epithelial layers. For this reason, the gingiva becomes red when vascularization increases or the degree of epithelial keratinization is reduced or disappears. The color becomes pale when vascularization is reduced (in association with fibrosis of the corium) or epithelial keratinization increases. Thus chronic inflammation intensifies the red or bluish red color because of vascular proliferation and reduction of keratinization. Additionally, venous stasis will contribute a bluish hue. The gingival color changes with increasing chronicity of the inflammatory process. The changes start in the interdental papillae and gingival margin and spread to the attached gingiva (see Figure 8-1).
Proper diagnosis and treatment require an understanding of the tissue changes that alter the color of the gingiva at the clinical level.
Color changes in acute gingival inflammation differ in both nature and distribution from those in chronic gingivitis. The color changes may be marginal, diffuse, or patchlike, depending on the underlying acute condition. In acute necrotizing ulcerative gingivitis, the involvement is marginal; in herpetic gingivostomatitis, it is diffuse; and in acute reactions to chemical irritation, it is patchlike or diffuse.
Color changes vary with the intensity of the inflammation. Initially, there is an increase in erythema. If the condition does not worsen, this is the only color change until the gingiva reverts to normal. In severe acute inflammation, the red color gradually becomes a dull, whitish gray. The gray discoloration produced by tissue necrosis is demarcated from the adjacent gingiva by a thin, sharply defined erythematous zone. Chapter 10 provides detailed descriptions of the clinical and pathologic features of the various forms of acute gingivitis.
Metallic Pigmentation
Heavy metals (bismuth, arsenic, mercury, lead, and silver) absorbed systemically from therapeutic use or occupational or household environments may discolor the gingiva and other areas of the oral mucosa.45 These changes are rare but still should be ruled out in suspected cases.
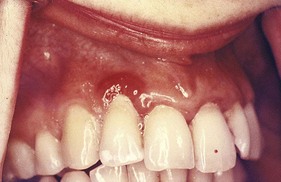
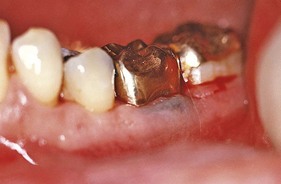
Typically, metals produce a black or bluish line in the gingiva that follows the contour of the margin (Figure 8-9). The pigmentation may also appear as isolated black blotches involving the interdental marginal and attached gingiva. This differs from the tattooing produced by the accidental embedding of amalgam or other metal fragments15 (Figure 8-10).
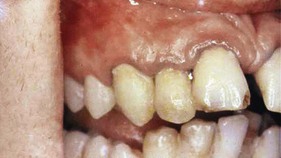
Figure 8-9 Bismuth gingivitis. Note linear black discoloration of the gingiva in a patient receiving bismuth therapy.
Gingival pigmentation from systemically absorbed metals results from perivascular precipitation of metallic sulfides in the subepithelial connective tissue. Gingival pigmentation is not a result of systemic toxicity. It occurs only in areas of inflammation in which the increased permeability of irritated blood vessels permits seepage of the metal into the surrounding tissue. In addition to inflamed gingiva, mucosal areas irritated by biting or abnormal chewing habits (e.g., inner surface of lips, cheek at level of occlusal line, lateral border of tongue) are common sites of pigmentation. Pigmentation can be eliminated by treating the inflammatory changes without necessarily discontinuing the metal-containing medication.
Color Changes Associated with Systemic Factors
Many systemic diseases may cause color changes in the oral mucosa, including the gingiva.22 In general, these abnormal pigmentations are nonspecific and should stimulate further diagnostic efforts or referral to the appropriate specialist.68
Endogenous oral pigmentations can be caused by melanin, bilirubin, or iron.45 Melanin oral pigmentations can be normal physiologic pigmentations and are often found in highly pigmented ethnic groups (see Chapter 2). Diseases that increase melanin pigmentation include the following:
Skin and mucous membranes can also be stained by bile pigments. Jaundice is best detected by examination of the sclera, but the oral mucosa may also acquire a yellowish color. The deposition of iron in hemochromatosis may produce a blue-gray pigmentation of the oral mucosa. Several endocrine and metabolic disturbances, including diabetes and pregnancy, may result in color changes. Blood dyscrasias, such as anemia, polycythemia, and leukemia, may also induce color changes.
Exogenous factors capable of producing color changes in the gingiva include atmospheric irritants, such as coal and metal dust, and coloring agents in food or lozenges. Tobacco causes hyperkeratosis of the gingiva and also may induce a significant increase in melanin pigmentation of the oral mucosa.56 Localized bluish black areas of pigment are often caused by amalgam implanted in the mucosa (see Figure 8-10).
In recent years the need for esthetics in dentistry has increased, with a growing demand for a pleasing smile. This has made many individuals more aware of their gingival pigmentation that may be apparent during smiling and speech.20,21 Traditionally, gingival depigmentation has been carried out using nonsurgical and surgical procedures such as chemical, cryosurgical, and electrosurgical techniques. However, those techniques were met with skepticism because of varying degrees of success. More recently, lasers have been used to ablate cells producing the melanin pigment; a nonspecific laser beam destroys the epithelial cells, including those at the basal layer. In addition, selective ablation using a laser beam with a wavelength that is specifically absorbed in melanin effectively destroys the pigmented cells without damaging the nonpigmented cells. In both cases, radiation energy is transformed into ablation energy, resulting in cellular rupture and vaporization with minimal heating of the surrounding tissue.76
Changes in Consistency of the Gingiva
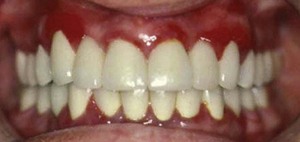
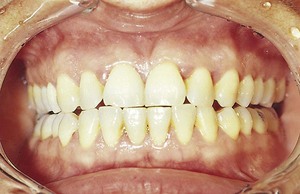
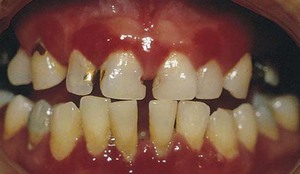
Both chronic and acute inflammations produce changes in the normal firm and resilient consistency of the gingiva. As previously noted, in chronic gingivitis, both destructive (edematous) and reparative (fibrotic) changes coexist, and the consistency of the gingiva is determined by their relative predominance (Figures 8-11 and 8-12). Table 8-1 summarizes the clinical alterations in the consistency of the gingiva and the microscopic changes that produce them.
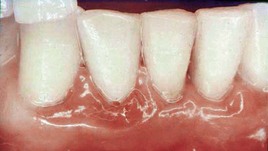
Figure 8-11 Chronic gingivitis. Swelling, loss of stippling, and discoloration occur when inflammatory exudate and edema are the predominant microscopic changes. The gingiva is soft and friable and bleeds easily.
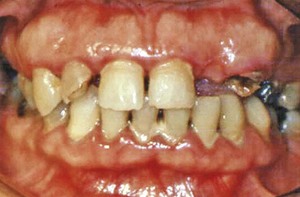
Figure 8-12 Chronic gingivitis. Firm gingiva is produced when fibrosis predominates in the inflammatory process.
TABLE 8-1 Clinical and Histopathologic Changes in Gingival Consistency
Calcified Masses in the Gingiva
Calcified microscopic masses may be found in the gingiva.14,52 They can occur alone or in groups and vary in size, location, shape, and structure. Such masses may be calcified material removed from the tooth and traumatically displaced into the gingiva during scaling,52 root remnants, cementum fragments, or cementicles. Chronic inflammation and fibrosis, an occasional foreign body, and giant cell activity occur in relation to these masses.
They are sometimes enclosed in an osteoidlike matrix. Crystalline foreign bodies have also been described in the gingiva, but their origin has not been determined.60
Toothbrushing
Toothbrushing has various effects on the consistency of the gingiva such as promoting keratinization of the oral epithelium, enhancing capillary gingival circulation, and thickening alveolar bone.44,49,77 In animal studies, mechanical stimulation by toothbrushing was found to increase the proliferative activity of the junctional basal cells in dog gingiva by 2.5 times compared with using a scaler.31 These findings may indicate that toothbrushing causes an increased turnover rate and desquamation of the junctional epithelial surfaces. This process may repair small breaks in the junctional epithelium and prevent direct access to the underlying tissue by periodontal pathogens.81
Changes in Surface Texture of the Gingiva
The surface of normal gingiva usually exhibits numerous small depressions and elevations, giving the tissue an orange-peel appearance referred as stippling.13 Stippling is restricted to the attached gingiva and is predominantly localized to the subpapillary area, but it extends to a variable degree into the interdental papilla.61 Although the biologic significance of gingival stippling is not known, some investigators conclude that loss of stippling is an early sign of gingivitis.33,61 However, clinicians must take into consideration that its pattern and extent vary in different mouth areas, among patients, and with age.
In chronic inflammation the gingival surface is either smooth and shiny or firm and nodular, depending on whether the dominant changes are exudative or fibrotic. Smooth surface texture is also produced by epithelial atrophy in atrophic gingivitis, and peeling of the surface occurs in chronic desquamative gingivitis. Hyperkeratosis results in a leathery texture, and drug-induced gingival overgrowth produces a nodular surface.
Changes in Position of the Gingiva
Traumatic Lesions
One of the unique features of the most recent gingival disease classification is the recognition of non–plaque-induced traumatic gingival lesions as distinct gingival conditions.11 Traumatic lesions, whether chemical, physical, or thermal, are among the most common lesions in the mouth. Sources of chemical injuries include aspirin, hydrogen peroxide, silver nitrate, phenol, and endodontic materials. Physical injuries can include lip, oral, and tongue piercing, which can result in gingival recession. Thermal injuries can result from hot drinks and foods. In acute cases, the appearance of slough (necrotizing epithelium), erosion, or ulceration and the accompanying erythema are common features. In chronic cases, permanent gingival defects are usually present in the form of gingival recession. Typically, the localized nature of the lesions and the lack of symptoms readily eliminate them from the differential diagnosis of systemic conditions that may be present with erosive or ulcerative oral lesions.64
Gingival Recession
Gingival recession is a common finding. The prevalence, extent, and severity of gingival recession increase with age and are more prevalent in males.6
Positions of the Gingiva
By clinical definition, recession is exposure of the root surface by an apical shift in the position of the gingiva. To understand recession, it helps to distinguish between the actual and apparent positions of the gingiva. The actual position is the level of the coronal end of the epithelial attachment on the tooth, whereas the apparent position is the level of the crest of the gingival margin (Figure 8-13). The severity of recession is determined by the actual position of the gingiva, not its apparent position. For example, in periodontal disease, the inflamed pocket wall covers part of the denuded root; thus some of the recession is hidden and some may be visible. The total amount of recession is the sum of the two.
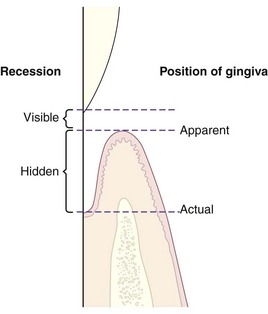
Figure 8-13 Diagram of apparent and actual positions of the gingiva and visible and hidden recession.
Recession refers to the location of the gingiva not its condition. Receded gingiva can be inflamed but may be normal except for its position (Figure 8-14). Recession may be localized to one tooth or a group of teeth, or it may be generalized throughout the mouth.
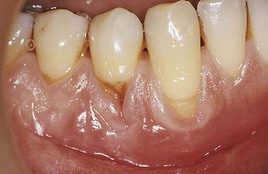
Figure 8-14 Different degrees of recession. Recession is slight in teeth #26 and #29 and marked in #27 and #28. The change in gingival contour and the recession, as seen in tooth #28, are referred to as Stillman’s clefts.
Gingival recession increases with age; the incidence varies from 8% in children to 100% after age 50 years.83 This has led some investigators to assume that recession may be a physiologic process related to aging. However, no convincing evidence has been presented for a physiologic shift of the gingival attachment.39 The gradual apical shift is most likely the result of the cumulative effect of minor pathologic involvement and repeated minor direct trauma to the gingiva. In some populations without access to dental care, however, recession may be the result of increasing periodontal disease.29,40
The following etiologic factors have been implicated in gingival recession: faulty toothbrushing technique (gingival abrasion), tooth malposition, friction from soft tissues (gingival ablation),72 gingival inflammation, abnormal frenum attachment, and iatrogenic dentistry. Trauma from occlusion has been suggested in the past, but its mechanism of action has never been demonstrated. For example, a deep overbite has been associated with gingival inflammation and recession. Excessive incisal overlap may result in a traumatic injury to the gingiva. Orthodontic movement in a labial direction in monkeys has been shown to result in loss of marginal bone and connective tissue attachment, as well as in gingival recession.74
Standard oral hygiene procedures, whether toothbrushing or flossing, may lead to a frequent transient and minimal gingival injury.18,62 Although toothbrushing is important for gingival health, faulty toothbrushing technique or brushing with hard bristles may cause significant injury. This type of injury may present as lacerations, abrasions, keratosis, and recession, with the facial marginal gingiva most affected.59 Thus, in these cases, recession tends to be more frequent and severe in patients with clinically healthy gingiva, little bacterial plaque, and good oral hygiene.23,57,58
Susceptibility to recession is also influenced by the position of teeth in the arch,82 the root-bone angle, and the mesiodistal curvature of the tooth surface.51 On rotated, tilted, or facially displaced teeth, the bony plate is thinned or reduced in height. Pressure from mastication or moderate toothbrushing damages the unsupported gingiva and produces recession. The effect of the angle of the root in the bone on recession is often observed in the maxillary molar area. If the lingual inclination of the palatal root is prominent or the buccal roots flare outward, the bone in the cervical area is thinned or shortened, and recession results from repeated trauma of the thin, marginal gingiva.
The health of the gingival tissue also depends on properly designed and placed restorative materials. Pressure from a poorly designed partial denture, such as an ill-fitting denture clasp, can cause gingival trauma and recession.84 Overhanging dental restorations have long been viewed as a contributing factor to gingivitis because of plaque retention. In addition, there is general agreement that placing restorative margins within the biologic width frequently leads to gingival inflammation, clinical attachment loss, and eventually, bone loss. Clinically, the violation of biologic width typically manifests as gingival inflammation, deepened periodontal pockets, and gingival recession.
A relationship may exist between smoking and gingival recession (see Chapter 26). The multifactorial mechanisms may include reduced gingival blood flow and altered immune response but are not as yet conclusive.26,63
Clinical Significance
Several aspects of gingival recession make it clinically significant. Exposed root surfaces are susceptible to caries. Abrasion or erosion of the cementum exposed by recession leaves an underlying dentinal surface that can be sensitive. Hyperemia of the pulp and associated symptoms may also result from excessive exposure of the root surface.48 Interproximal recession creates oral hygiene problems and resulting plaque accumulation.
Changes in Gingival Contour
Changes in gingival contour are primarily associated with gingival enlargement (see Chapter 9), but such changes may also occur in other conditions. Of historical interest are the descriptions of indentations of the gingival margin referred to as Stillman’s clefts (see Figure 8-14)75 and the McCall festoons. The term “Stillman’s clefts” has been used to describe a specific type of gingival recession consisting of a narrow, triangular-shaped gingival recession. As the recession progresses apically, the cleft becomes broader, exposing the cementum of the root surface. When the lesion reaches the mucogingival junction, the apical border of oral mucosa is usually inflamed because of the difficulty in maintaining adequate plaque control at this site.
The term “McCall festoons” has been used to describe a rolled, thickened band of gingiva usually seen adjacent to the cuspids when recession approaches the mucogingival junction. Initially, Stillman’s clefts and McCall festoons were attributed to traumatic occlusion, and the recommended treatment was occlusal adjustment. However, this association was never proved, and these indentations merely represent peculiar inflammatory changes of the marginal gingiva.14
1 Addy M, Hunter ML, Kingdon A, et al. An 8-year study of changes in oral hygiene and periodontal health during adolescence. Int J Paediatr Dent. 1994;4:75.
2 Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. 1975;25:229.
3 Albandar JM. Periodontal diseases in North America. Periodontol 2000. 2002;29:31.
4 Albandar JM, Rams TE. Global epidemiology of periodontal diseases: an overview. Periodontol 2000. 2002;29:7.
5 Albandar JM, Tinoco EM. Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000. 2002;29:153.
6 Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:13.
7 Albandar JM, Brown LJ, Brunelle JA, et al. Gingival state and dental calculus in early-onset periodontitis. J Periodontol. 1996;67:953.
8 Albandar JM, Kingman A, Brown LJ, et al. Gingival inflammation and subgingival calculus as determinants of disease progression in early-onset periodontitis. J Clin Periodontol. 1998;25:231.
9 Amato R, Caton J, Polson A, et al. Interproximal gingival inflammation related to the conversion of a bleeding to a nonbleeding state. J Periodontol. 1986;57:63.
10 American Academy of Periodontology. Parameter on plaque-induced gingivitis. J Periodontol. 2000;71:851.
11 Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1.
12 Bennett JH, Shankar S. Gingival bleeding as the presenting feature of multiple myeloma. Br Dent J. 1984;157:101.
13 Bergström J. The topography of papillary gingiva in health and early gingivitis. J Clin Periodontol. 1984;11:423.
14 Box HK. Gingival clefts and associated tracts. NY State Dent J. 1950;16:3.
15 Buchner A, Hansen LS. Amalgam pigmentation (amalgam tattoo) of the oral mucosa: a clinicopathologic study of 268 cases. Oral Surg Oral Med Oral Pathol. 1980;49:139.
16 Carter HG, Barnes GP. The gingival bleeding index. J Periodontol. 1974;45:801.
17 Cooper PG, Caton JG, Polson AM. Cell populations associated with gingival bleeding. J Periodontol. 1983;54:497.
18 Danser MM, Timmerman MF, IJzerman Y, et al. Evaluation of the incidence of gingival abrasion as a result of toothbrushing. J Clin Periodontol. 1998;25:701.
19 Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J Periodontol. 2004;75:16.
20 Dummett CO. Oral pigmentation: first symposium on oral pigmentation. J Periodontol. 1960;31:356.
21 Dummett CO. Overview of normal oral pigmentations. Ala J Med Sci. 1979;16:262.
22 Dummett CO. Oral tissue color changes. Ala J Med Sci. 1979;16:274.
23 Gorman WJ. Prevalence and etiology of gingival recession. J Periodontol. 1967;38:316.
24 Greenstein G. The role of bleeding upon probing in the diagnosis of periodontal disease: a literature review. J Periodontol. 1984;55:684.
25 Greenstein G, Caton J, Polson AM. Histologic characteristics associated with bleeding after probing and visual signs of inflammation. J Periodontol. 1981;52:420.
26 Gunsolley JC, Quinn SM, Tew J, et al. The effect of smoking on individuals with minimal periodontal destruction. J Periodontol. 1998;69:165.
27 Haeb HP. Postrubella thrombocytopenic purpura: a report of eight cases with discussion of hemorrhagic manifestations of rubella. Clin Pediatr. 1968;7:350.
28 Hennekens CH, Dyken ML, Fuster V. Aspirin as a therapeutic agent in cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1997;96:2751.
29 Hirschfeld I. A study of skulls in the American Museum of Natural History in relation to periodontal disease. J Dent Res. 1923;5:241.
30 Hoover DR, Lefkowitz W. Fluctuation in marginal gingivitis. J Periodontol. 1965;36:310.
31 Horiuchi M, Yamamoto T, Tomofuji T, et al. Toothbrushing promotes gingival fibroblast proliferation more effectively than removal of dental plaque. J Clin Periodontol. 2002;29:791.
32 Johannsen A, Rydmark I, Söder B, Asberg M. Gingival inflammation, increased periodontal pocket depth and elevated interleukin-6 in gingival crevicular fluid of depressed women on long-term sick leave. J Periodontal Res. 2007 Dec;42(6):546-552.
33 King JD. Gingival disease in Dundee. Dent Rec. 1945;56:9.
34 Lang NP, Adler R, Joss A, et al. Absence of bleeding upon probing: an indicator of periodontal stability. J Clin Periodontol. 1990;17:714.
35 Lang NP, Schätzle MA, Löe H. Gingivitis as a risk factor in periodontal disease. J Clin Periodontol. 2009 Jul;36(Suppl 10)):3-8.
36 Larato DC, Stahl SS, Brown RJr, et al. The effect of a prescribed method of toothbrushing on the fluctuation of marginal gingivitis. J Periodontol. 1969;40:142.
37 Lenox JA, Kopczyk RA. A clinical system for scoring a patient’s oral hygiene performance. J Am Dent Assoc. 1973;86:849.
38 Levin L, Samorodnitzky-Naveh GR, Machtei EE. The association of orthodontic treatment and fixed retainers with gingival health. J Periodontol. 2008 Nov;79(11):2087-2092.
39 Löe H. The structure and physiology of the dentogingival junction. In: Miles AE, editor. Structural and chemical organization of teeth, vol 2. New York: Academic Press; 1967.
40 Löe H, Anerud A, Boysen H. The natural history of periodontal disease in man: prevalence, severity and extent of gingival recession. J Periodontol. 1992;63:489.
41 Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177.
42 Lorencini M, Silva JA, de la Hoz CL, et al. Changes in MMPs and inflammatory cells in experimental gingivitis. Histol Histopathol. 2009 Feb;24(2):157-166.
43 Machtei EE, Mahler D, Sanduri H, et al. The effect of menstrual cycle on periodontal health. J Periodontol. 2004;75:408.
44 Mackenzie IC. Does toothbrushing affect gingival keratinization? Proc R Soc Med. 1972;65:1127.
45 McCarthy FP, Shklar G. Diseases of the oral mucosa. New York: McGraw-Hill; 1964.
46 Meitner SW, Zander H, Iker HP, et al. Identification of inflamed gingival surfaces. J Clin Periodontol. 1979;6:93.
47 Merril A, Peterson LJ. Gingival hemorrhage secondary to uremia: review and report of a case. Oral Surg Oral Med Oral Pathol. 1970;29:530.
48 Merritt AA. Hyperemia of the dental pulp caused by gingival recession. J Periodontol. 1933;4:30.
49 Miake K, Agematsu H, Fukayama M, et al. An experimental study on histological changes occurring in rat-incisor alveolar bone during toothbrushing. In: Ishikawa J, Kawasaki H, Ikeda K, et al, editors. Recent advances in clinical periodontology. Amsterdam: Elsevier, 1988.
50 Milne AM. Gingival bleeding in 848 army recruits: an assessment. Br Dent J. 1967;122:111.
51 Morris ML. The position of the margin of the gingiva. J Oral Surg. 1958;11:969.
52 Moskow BS. Calcified material in human gingival tissue. J Dent Res. 1961;40:644.
53 Muhlemann HR, Son S. Gingival sulcus bleeding: a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107.
54 Nair P, Sutherland G, Palmer RM, et al. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. 2003;30:435.
55 Nakagawa S, Fujii H, Machida Y, et al. A longitudinal study from prepuberty to puberty of gingivitis: correlation between the occurrence of Prevotella intermedia and sex hormones. J Clin Periodontol. 1994;21:658.
56 Neville BW, Damm DD, Allen CM, et al. Oral and maxillofacial pathology. Philadelphia: Saunders; 1995.
57 O’Leary TJ, Drake RB, Crump PP, et al. The incidence of recession in young males: further study. J Periodontol. 1971;42:264.
58 O’Leary TJ, Drake RV, Jividen GJ, et al. The incidence of recession in young males: relationship to gingival and plaque scores, SAM-TR-67-97. Tech Rep SAM-TR. 1967:1.
59 Offenbacher S, Barros SP, Singer RE, Moss K, et al. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78(10):1911-1925.
60 Orban B. Gingival inclusions. J Periodontol. 1945;16:16.
61 Orban B. Clinical and histological study of the surface characteristics of the gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1948;1:827.
62 Pearlman BA. A mistaken health belief resulting in gingival injury: a case report. J Periodontol. 1994;65:284.
63 Preber H, Bergstrom J. Occurrence of gingival bleeding in smokers and non-smoker patients. Acta Odontol Scand. 1985;43:315.
64 Rawal SY, Claman LJ, Kalmar JR, et al. Traumatic lesions of the gingiva: a case series. J Periodontol. 2004;75:762.
65 Reinhardt RA, Payne JB, Maze CA, et al. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J Periodontol. 1999;70:823.
66 Rose G. The strategy of preventive medicine. Oxford: Oxford University Press; 1992.
67 Royzman D, Recio L, Badovinac RL, et al. The effect of aspirin intake on bleeding on probing in patients with gingivitis. J Periodontol. 2004;75:679.
68 Shklar G, McCarthy PL. The oral manifestations of systemic disease. Boston: Butterworth; 1976.
69 Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121.
70 Silva JA, Lorencini M, Reis JR, et al. The influence of type I diabetes mellitus in periodontal disease induced changes of the gingival epithelium and connective tissue. Tissue Cell. 2008 Aug;40(4):283-292.
71 Sodeman WAJr, Sodeman WA. Pathologic physiology: mechanisms of disease, ed 7. Philadelphia: Saunders; 1985.
72 Sognnaes RF. Periodontal significance of intraoral frictional ablation. J West Soc Periodontol Periodontal Abstr. 1977;25:112.
73 Stefanini M, Dameshek W. The hemorrhagic disorders, ed 2. New York: Grune & Stratton; 1962.
74 Steiner GG, Pearson JK, Ainamo J. Changes of the marginal periodontium as a result of labial tooth movement in monkeys. J Periodontol. 1981;52:314.
75 Stillman PR. Early clinical evidences of disease in the gingiva and pericementum. J Dent Res. 1921;3:25.
76 Tal H, Oegiesser D, Tal M. Gingival depigmentation by erbium:YAG laser: clinical observations and patient responses. J Periodontol. 2003;74:1660.
77 Tanaka M, Hanioka T, Kishimoto M, et al. Effect of mechanical toothbrush stimulation on microcirculatory functions in inflamed gingiva of dogs. J Clin Periodontol. 1998;25:561.
78 Tatakis DN, Trombelli L. Modulation of clinical expression of plaque-induced gingivitis. I. Background review and rationale. J Clin Periodontol. 2004;31:229.
79 Tilakaratne A, Soory M, Ranasinghe AW, et al. Effects of hormonal contraceptives on the periodontium, in a population of rural Sri-Lankan women. J Clin Periodontol. 2000;27:753.
80 Tilakaratne A, Soory M, Ranasinghe AW, et al. Periodontal disease status during pregnancy and 3 months post-partum, in a rural population of Sri-Lankan women. J Clin Periodontol. 2000;27:787.
81 Tomofuji T, Morita M, Horiuchi M, et al. The effect of duration and force of mechanical toothbrushing stimulation on proliferative activity of the junctional epithelium. J Periodontol. 2002;73:1149.
82 Trott JR, Love B. An analysis of localized gingival recession in 766 Winnipeg High School students. Dent Pract Dent Rec. 1966;16:209.
83 Woofter C. The prevalence and etiology of gingival recession. Periodontal Abstr. 1969;17:45.
84 Zlataric DK, Celebic A, Valentic-Peruzovic M. The effect of removable partial dentures on periodontal health of abutment and non-abutment teeth. J Periodontol. 2002;73:137.