CHAPTER 35 Rationale for Periodontal Treatment
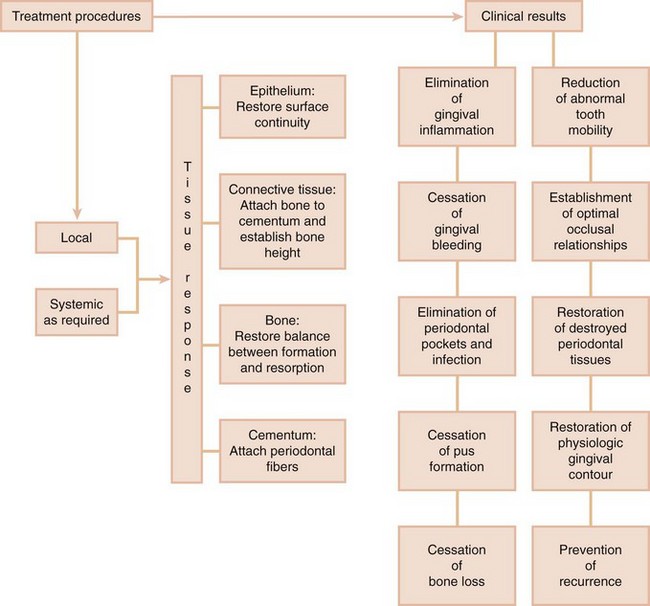
What does Periodontal Therapy Accomplish?
The effectiveness of periodontal therapy is made possible by the remarkable healing capacity of the periodontal tissues. Periodontal therapy can restore chronically inflamed gingiva so that, from a clinical and structural point of view, it is almost identical with gingiva that has never been exposed to excessive plaque accumulation20 (see Part 8).
Properly performed, periodontal treatment can eliminate pain, exudate, gingival inflammation,27 and bleeding. It can also reduce periodontal pockets, eliminate infection, arrest the destruction of soft tissue and bone,28 and reduce abnormal tooth mobility.7 Other benefits are to establish optimal occlusal function, restore tissue destroyed by disease, reestablish physiologic gingival contour, and prevent the recurrence of disease, which will all help to maintain the natural dentition24 (Figure 35-1).
Local Therapy
The cause of periodontitis and gingivitis is bacterial plaque accumulation on the tooth surface close to the gingival tissue. The accumulation of plaque can be favored by a variety of local factors, such as calculus, overhanging margins of restorations, and food impaction. The removal of plaque and all of the factors that favor its accumulation is the primary goal in local therapy.
Abnormal forces on the tooth can increase tooth mobility. The thorough elimination of plaque and the prevention of its formation can help maintain periodontal health, even if traumatic forces are allowed to persist.18,19 However, the elimination of trauma may increase the chances for bone regeneration and the gain of attachment.15 Although this point is not widely accepted,25 it appears that creating occlusal relationships that are more tolerable to the periodontal tissues increases the margin of safety of the periodontium to the buildup of plaque, in addition to reducing tooth mobility. It should be remembered that total plaque elimination as obtained in experimental studies may not be possible in human subjects.
Systemic Therapy
Systemic therapy may be employed as an adjunct to local measures for specific purposes such as controlling systemic complications from acute infections or chemotherapy and preventing harmful effects of posttreatment bacteremia. The control of systemic diseases that aggravate the patient’s periodontal condition is always a consideration so proper precautions can be instituted during therapy (see Chapters 37, 38, and 39).
Systemic therapy for treatment of the periodontal condition in conjunction with local therapy is indicated in patients with aggressive periodontitis. In these diseases, systemic antibiotics are used to eliminate the bacteria that invade the gingival tissues and can repopulate the pocket after scaling and root planing (see Chapters 40 and 47).
In addition, periodontal manifestations of systemic diseases (see Chapter 28) are treated primarily by other local measures.
In the late twentieth century, the concept of host modulation was introduced as a medical approach to periodontal treatment. The classic 1979 paper by Nyman, Schroeder, and Lindhe23 reported that it was possible to block periodontal bone loss in animals with the aspirinlike drug indomethacin. Evidence was then presented that some nonsteroidal antiinflammatory drugs (NSAIDs), such as flurbiprofen and ibuprofen, can reduce the development of experimental gingivitis,9 as well as the loss of alveolar bone in periodontitis.11,33-35 These drugs are propionic acid derivatives and act by inhibiting the cyclooxygenase pathway of arachidonic acid metabolism, thereby reducing prostaglandin formation. These NSAIDs can be administered by mouth13 or applied topically.33
Another drug that has a strong inhibitory effect on bone resorption is alendronate, a bisphosphonate, which is currently used to treat metabolic diseases in humans, such as Paget’s disease or hypercalcemia of malignancy, which result in bone resorption. Experimental studies in monkeys have shown that alendronate reduced the bone loss associated with periodontitis.3,32
Host modulation is still in its experimental stages, and protocols for its clinical use have not been established. However, current studies indicates that future treatment modalities may attempt not only to control the bacterial cause of the disease but also to suppress the self-destructive components of the host inflammatory response11 (see Chapter 48).
Factors That Affect Healing
In the periodontium, as elsewhere in the body, healing is affected by local and systemic factors.
Local Factors
Systemic conditions that impair healing may reduce the effectiveness of local periodontal treatment and should be corrected before or during local procedures. However, local factors, particularly plaque microorganisms, are the most common deterrents to healing after periodontal treatment.
Healing is also delayed by (1) excessive tissue manipulation during treatment, (2) trauma to the tissues, (3) the presence of foreign bodies, and (4) repetitive treatment procedures that disrupt the orderly cellular activity in the healing process. An adequate blood supply is needed for the increased cellular activity during healing. If the blood supply is impaired or insufficient, areas of necrosis will develop and delay the healing process.
Healing is improved by debridement (removal of degenerated and necrotic tissue), immobilization of the healing area, and pressure on the wound. The cellular activity in healing entails an increase in oxygen consumption, but healing of the gingiva is not accelerated by artificially increasing the oxygen supply beyond the normal requirements.8
Systemic Factors
The effects of systemic conditions on healing have been extensively documented in animal experiments but are less clearly defined in humans. Healing capacity diminishes with age,4,10 probably because of the atherosclerotic vascular changes common in aging and the resulting reduction in blood circulation. Healing is delayed in patients with generalized infections and in those with diabetes and other debilitating diseases.
Healing is impaired by insufficient food intake; bodily conditions that interfere with the use of nutrients; and deficiencies in vitamin C,1,31 proteins,30 and other nutrients. However, the nutrient requirements of the healing tissues in minor wounds, such as those created by periodontal surgical procedures, are usually satisfied by a well-balanced diet.
Healing is also affected by hormones. Systemically administered glucocorticoids such as cortisone hinder repair by depressing the inflammatory reaction or by inhibiting the growth of fibroblasts, the production of collagen, and the formation of endothelial cells. Systemic stress,29 thyroidectomy, testosterone, adrenocorticotropic hormone (ACTH), and large doses of estrogen suppress the formation of granulation tissue and impair healing.4 Progesterone increases and accelerates the vascularization of immature granulation tissue17 and appears to increase the susceptibility of the gingiva to mechanical injury by causing dilation of the marginal vessels.12
Healing After Periodontal Therapy
The basic healing processes are the same after all forms of periodontal therapy. These processes consist of the removal of degenerated tissue debris and the replacement of tissues destroyed by disease. This implies regeneration and repair of the periodontal structures but not necessarily a gain in attachment. Techniques to gain attachment and bone level are discussed in the sections on new attachment and periodontal reconstruction.
Regeneration
Regeneration is the natural renewal of a structure, produced by growth and differentiation of new cells and intercellular substances to form new tissues or parts. Regeneration occurs through growth from the same type of tissue that has been destroyed or from its precursor. In the periodontium, gingival epithelium is replaced by epithelium, and the underlying connective tissue and periodontal ligament are derived from connective tissue. Bone and cementum are replaced by connective tissue, which is the precursor of both. Undifferentiated connective tissue cells develop into osteoblasts and cementoblasts, which form bone and cementum.
Regeneration of the periodontium is a continuous physiologic process. Under normal conditions, new cells and tissues are constantly being formed to replace those that mature and die; this is termed wear and tear repair.16 It is manifested by (1) mitotic activity in the epithelium of the gingiva and the connective tissue of the periodontal ligament, (2) the formation of new bone, and (3) the continuous deposition of cementum.
Regeneration is occurring even during destructive periodontal disease. Most gingival and periodontal diseases are chronic inflammatory processes and as such are healing lesions. Regeneration is part of healing. However, bacteria and bacterial products that perpetuate the disease process, along with the resulting inflammatory exudate, are injurious to the regenerating cells and tissues, thus preventing completion of the healing process.
By removing bacterial plaque and creating the conditions to prevent its new formation, periodontal treatment removes the obstacles to regeneration and enables the patient to benefit from the inherent regenerative capacity of the tissues. A brief “spurt” in regenerative activity occurs immediately after periodontal treatment, but no local treatment procedures promote or accelerate regeneration.
Repair
Repair simply restores the continuity of the diseased marginal gingiva and reestablishes a normal gingival sulcus at the same level on the root as the base of the preexisting periodontal pocket (Figure 35-2, B). This process, called healing by scar,26 arrests bone destruction but does not result in gain of gingival attachment or bone height. This return of the destroyed periodontium to health involves regeneration and mobilization of epithelial and connective tissue cells into the damaged area and increased local mitotic divisions to provide sufficient numbers of cells (Figure 35-3).
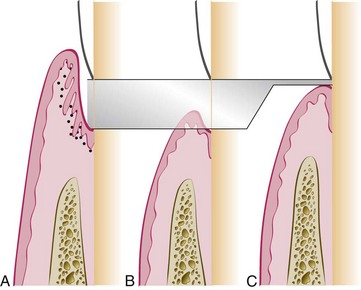
Figure 35-2 Two possible outcomes of pocket elimination. A, Periodontal pocket before treatment. B, Normal sulcus reestablished at the level of the base of the pocket. C, Periodontium restored on the root surface previously denuded by disease; this is called new attachment. Shaded areas show denudation caused by periodontal disease.
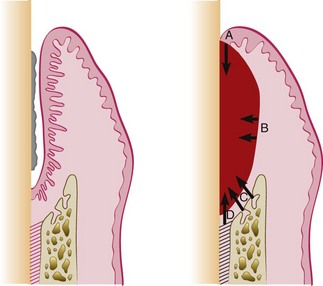
Figure 35-3 Sources of regenerating cells in the healing stages of a periodontal pocket. Left, Intrabony pocket. Right, After therapy the clot formed is invaded by cells from A, the marginal epithelium; B, the gingival connective tissue; C, the bone marrow; and D, the periodontal ligament.
For the diseased gingiva and attachment apparatus to regain (totally or partially) their level on the root (Figure 35-2, C), therapy must include special materials and techniques. If these are not used or are not successful, tissues undergo repair only, which involves regeneration of tissue to remodel the attachment apparatus but does not include regaining attachment level or new bone height. For this reason, we prefer to use the term reconstruction of the periodontium to refer to the crucial therapeutic techniques that seek to rebuild the periodontium and result in a significant gain of attachment and bone height (see Chapter 61).
New Attachment
New attachment is the embedding of new periodontal ligament fibers into new cementum and the attachment of the gingival epithelium to a tooth surface previously denuded by disease.
The critical phrase in this definition is “tooth surface previously denuded by disease” (Figure 35-4, area B). The attachment of the gingiva or the periodontal ligament to areas of the tooth from which they have been removed in the course of treatment (or during preparation of teeth for restorations) represents simple healing or reattachment of the periodontium, not new attachment.14 The term reattachment refers to repair in areas of the root not previously exposed to the pocket such as after surgical detachment of the tissues or following traumatic tears in the cementum, tooth fractures, or the treatment of periapical lesions (Figure 35-4, area D).
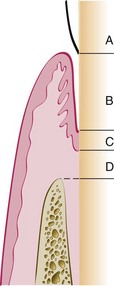
Figure 35-4 Enamel surface (A). Area of cementum denuded by pocket formation (B). Area of cementum covered by junctional epithelium (C). Area of cementum apical to junctional epithelium (D). The term new attachment refers to a new junctional epithelium and attached connective tissue fibers formed on zone B.
Epithelial adaptation differs from new attachment in that it is the close apposition of the gingival epithelium to the tooth surface, with no gain in height of gingival fiber attachment. The pocket is not completely obliterated, although it may not permit passage of a probe (Figure 35-5).
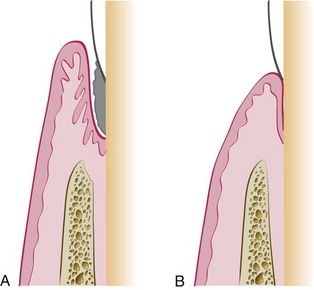
Figure 35-5 Epithelial adaptation after periodontal treatment. A, Periodontal pocket. B, After treatment. The pocket epithelium is closely adapted to but not attached to the root.
However, studies have shown that these deep sulci lined by long, thin epithelium may be as resistant to disease as true connective tissue attachments.2,21 The absence of bleeding or secretion on probing, the absence of clinically visible inflammation, and the absence of stainable plaque on the root surface when the pocket wall is deflected from the tooth may indicate that the “deep sulcus” persists in an inactive state, causing no further loss of attachment.5,35 A posttherapy depth of 4 or even 5 mm may therefore be acceptable in these cases.
Periodontal Reconstruction
As previously discussed, the term periodontal reconstruction refers to the process of regeneration of cells and fibers and remodeling of the lost periodontal structures that results in (1) gain of attachment level, (2) formation of new periodontal ligament fibers, and (3) a level of alveolar bone significantly coronal to that present before treatment.
A technique to attain these ideal results has been a constant but elusive goal of periodontal therapy for centuries.6 Since the 1970s, renewed laboratory and clinical research efforts have resulted in new concepts and techniques that have moved us much closer to attaining this ideal result of therapy. Melcher22 pointed out that the regeneration of the periodontal ligament is the key to periodontal reconstruction because it “provides continuity between the alveolar bone and the cementum and also because it contains cells that can synthesize and remodel the three connective tissues of the alveolar part of the periodontium.” Chapter 61 discusses growth factors that help the maturation of these tissues.
![]() Science Transfer
Science Transfer
The successful treatment of periodontal disease leads to enhancement of the patients quality of life by ensuring a healthy dentition that is functional and has good esthetic qualities. There is also increasing evidence that control of active periodontal disease leads to reduction in specific systemic diseases, such as arteriosclerosis, stroke, pulmonary infections, and diabetes, and the incidence of premature low-birth-weight babies. Clinicians can now advise patients that periodontal treatment has benefits beyond providing stable oral health and that there is a need for closer working relationships between dentists and physicians to expand the availability of periodontal therapy. This applies particularly to patients that have documented risk factors from these systemic health problems.
Surgical and nonsurgical periodontal procedures have a high success rate in obtaining periodontal health by a variety of tissue reactions, including repair and new attachment. There is a continuing improvement in success rates as new procedures and new biologic approaches are instituted, so that clinicians can have increasing confidence in therapeutic success. Maintenance of low levels of bacterial plaque after successful periodontal therapy is the essential element for long-term periodontal stability.
During the healing stages of a periodontal pocket, the area is invaded by cells from four different sources (see Figure 35-3): oral epithelium, gingival connective tissue, bone, and periodontal ligament.
The final outcome of periodontal pocket healing depends on the sequence of events during the healing stages.22 If the epithelium proliferates along the tooth surface before the other tissues reach the area, the result will be a long junctional epithelium. If the cells from the gingival connective tissue are the first to populate the area, the result will be fibers parallel to the tooth surface and remodeling of the alveolar bone with no attachment to the cementum. If bone cells arrive first, root resorption and ankylosis may occur. Finally, only when cells from the periodontal ligament proliferate coronally is there new formation of cementum and periodontal ligament.22
Several methods, based on different concepts and resulting in various techniques, have been recommended to improve the likelihood of gaining new attachment and increased bone levels, as discussed in Chapter 61.
1 Barr CE. Oral healing in ascorbic acid deficiency. Periodontics. 1965;3:286.
2 Beaumont RH, O’Leary TJ, Kafrawy AH. Relative resistance of long junctional epithelial adhesions and connective tissue attachments to plaque-induced inflammation. J Periodontol. 1984;55:213.
3 Brunsvold MA, Chaves ES, Kornman KS, et al. Effects of a bisphosphonate on experimental periodontitis in monkeys. J Periodontol. 1992;63:825.
4 Butcher EO, Klingsberg J. Age, gonadectomy, and wound healing in the palatal mucosa. J Dent Res. 1961;40:694.
5 Caffesse RG, Ramfjord SP, Nasjleti CE. Reverse bevel periodontal flaps in monkeys. J Periodontol. 1968;39:219.
6 Carranza F, Shklar G. History of periodontology. Chicago: Quintessence; 2003.
7 Ferris RT. Quantitative evaluation of tooth mobility following initial periodontal therapy. J Periodontol. 1966;37:190.
8 Glickman I, Turesky SS, Manhold J. The oxygen consumption of healing gingiva. J Dent Res. 1950;29:429.
9 Heasman PA, Seymour RA. The effect of a systemically administered non-steroidal anti-inflammatory drug (flurbiprofen) on experimental gingivitis in humans. J Clin Periodontol. 1989;16:551.
10 Holm-Pedersen P, Löe H. Wound healing in the gingiva of young and old individuals. Scand J Dent Res. 1971;79:40.
11 Howell TH, Williams RC. Nonsteroidal antiinflammatory drugs as inhibitors of periodontal disease progression. Crit Rev Oral Biol Med. 1993;4:177.
12 Hugoson A. Gingival inflammation and female sex hormones. J Periodontal Res. 1970;5(suppl):1.
13 Jeffcoat MK, Williams RC, Reddy MS, et al. Flurbiprofen treatment of human periodontitis: effect on alveolar bone height and metabolism. J Periodontal Res. 1988;23:381.
14 Kalkwarf KL. Periodontal new attachment without the placement of osseous potentiating grafts. Periodont Abstr. 1974;22:53.
15 Kantor M, Polson AM, Zander HA. Alveolar bone regeneration after removal of inflammatory and traumatic factors. J Periodontol. 1976;47:687.
16 Leblond CP, Walker BE. Renewal of cell populations. Physiol Rev. 1956;36:255.
17 Lindhe J, Branemark PI. The effect of sex hormones on vascularization of a granulation tissue. J Periodontal Res. 1968;3:6.
18 Lindhe J, Ericsson I. The influence of trauma from occlusion on reduced but healthy periodontal tissues in dogs. J Clin Periodontol. 1976;3:110.
19 Lindhe J, Nyman S. The effect of plaque control and surgical pocket elimination on the establishment and maintenance of periodontal health: a longitudinal study of periodontal therapy in cases of advanced periodontal disease. J Clin Periodontol. 1975;2:67.
20 Lindhe J, Parodi R, Liljenberg B, et al. Clinical and structural alterations characterizing healing gingiva. J Periodontal Res. 1978;13:410.
21 Magnusson I, Runstad L, Nyman S, et al. A long junctional epithelium: a locus minoris resistentiae in plaque infection? J Clin Periodontol. 1983;10:33.
22 Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256.
23 Nyman S, Schroeder HE, Lindhe J. Suppression of inflammation and bone resorption by indomethacin during experimental periodontitis in dogs. J Periodontol. 1979;50:450.
24 Oliver RC. Tooth loss with and without periodontal therapy. Periodont Abstr. 1969;17:8.
25 Polson AM. Interrelationship of inflammation and tooth mobility (trauma) in pathogenesis of periodontal disease. J Clin Periodontol. 1980;7:351.
26 Ratcliff PA. An analysis of repair systems in periodontal therapy. Periodont Abstr. 1966;14:57.
27 Rateitschak K. The therapeutic effect of local treatment on periodontal disease assessed upon evaluation of different diagnostic criteria. 2. Changes in gingival inflammation. J Periodontol. 1964;35:155.
28 Rateitschak K, Engelberger A, Marthaler TM. The therapeutic effect of local treatment on periodontal disease assessed upon evaluation of different diagnostic criteria. 3. Radiographic changes in appearance of bone. J Periodontol. 1964;35:263.
29 Stahl SS. Healing gingival injury in normal and systemically stressed young adult male rats. J Periodontol. 1961;32:63.
30 Stahl SS. The effect of a protein-free diet on the healing of gingival wounds in rats. Arch Oral Biol. 1962;7:551.
31 Turesky SS, Glickman I. Histochemical evaluation of gingival healing in experimental animals on adequate and vitamin C deficient diets. J Dent Res. 1954;33:273.
32 Weinreb M, Quartuccio H, Seedor JG, et al. Histomorphometrical analysis of the effects of the bisphosphonate alendronate on bone loss caused by experimental periodontitis in monkeys. J Periodontal Res. 1994;29:35.
33 Williams RC, Jeffcoat MK, Howell TH, et al. Topical flurbiprofen treatment of periodontitis in beagles. J Periodontal Res. 1988;23:166.
34 Williams RC, Jeffcoat MK, Howell H, et al. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol. 1989;60:485.
35 Williams RC, Jeffcoat MK, Kaplan ML, et al. Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science. 1985;227:640.