CHAPTER 69 Clinical Evaluation of the Implant Patient
Endosseous dental implants and their retained prostheses have had great success over the past few decades following the landmark research and development of osseointegrated implants by Brånemark et al.18-20 Initially, most prosthetic reconstructions with osseointegrated implants were limited to use in the edentulous patient, with many reports documenting excellent long-term success of implant-retained prostheses for edentulous patients.1,2,32
Following much success with implants in edentulous patients, the original implant treatment protocols were adapted for use in partially edentulous patients. Although some transitional problems were associated with the early use of dental implants in the partially edentulous patient, successes were achieved for this population as well. Subsequently, modifications in implant design, procedural techniques, and treatment planning greatly improved implant therapy for the partially edentulous patient. Currently, the long-term success of dental implants used to replace single and multiple missing teeth in the partially edentulous patient is very good37,51,53,63,70 (see Chapter 80). Additionally, with the implementation of bone augmentation procedures, even patients with inadequate bone volume have a good opportunity to be successfully restored with implant-retained prostheses.34,43,72 Virtually any patient with an edentulous space is a candidate for endosseous implants, and studies suggest that greater than 90% to 95% success rates can be expected in healthy patients with good bone and normal healing capacity.30
The ultimate goal of dental implant therapy is to satisfy the patient’s desire to replace one or more missing teeth in an esthetic, secure, functional, and long-lasting manner. To achieve this goal, clinicians must accurately diagnose the current dentoalveolar condition, as well as the overall mental and physical well-being of the patient to determine whether implant therapy is possible or practical and perhaps most importantly, whether it is indicated for a particular patient. Local evaluation of potential jaw sites for implant placement (e.g., measuring available alveolar bone height, width, and spatial relationship) and prosthetic restorability are an essential part of determining whether an implant(s) is possible. However, determining whether the patient is a good candidate for implants is an equally important aspect of the evaluation process. This aspect of the patient evaluation includes identifying factors that might increase the risk of failure or complications, as well as determining whether the patient’s expectations are reasonable.
This chapter presents an overview of the clinical aspects of dental implant therapy, including an assessment of possible risk factors and contraindications. It also provides guidelines for the pretreatment evaluation of potential implant patients, posttreatment evaluation of patients with implants, and implant maintenance.
Case Types and Indications
Edentulous Patients
The patients who seem to benefit most from dental implants are those with fully edentulous arches. These patients can be effectively restored, both esthetically and functionally, with an implant-assisted removable prosthesis or an implant-supported fixed prosthesis.
The original design for the edentulous arch was a fixed-bone–anchored bridge that used five to six implants in the anterior area of the mandible or the maxilla to support a fixed, hybrid prosthesis. The design is a denture-like complete arch of teeth attached to a substructure (metal framework), which in turn is attached to the implants with cylindrical titanium abutments (Figure 69-1). The prosthesis is fabricated without flange extensions and does not rely on any soft tissue support. It is entirely implant supported (see Figure 76-13). Usually, the prosthesis includes bilateral distal cantilevers, which extend to replace posterior teeth (back to premolars or first molars).
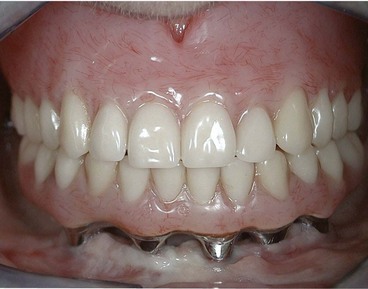
Figure 69-1 Clinical photograph of patient with a complete maxillary denture opposing a full-arch implant-supported fixed prosthesis in the mandibular arch.
Another implant-supported design used to restore an edentulous arch is the ceramic-metal fixed bridge (Figure 69-2). Some patients prefer this design because the ceramic restoration emerges directly from the gingival tissues in a manner similar to the appearance of natural teeth. One limitation of both hybrid and ceramometal implant-supported fixed prostheses is that they provide very little lip support and thus may not be indicated for patients who have lost significant alveolar dimension. This is often more problematic for maxillary reconstructions because lip support is more critical in the upper arch. For some patients, the lack of a complete seal (i.e., spaces under the framework) allows air to escape during speech, thus creating phonetic problems.
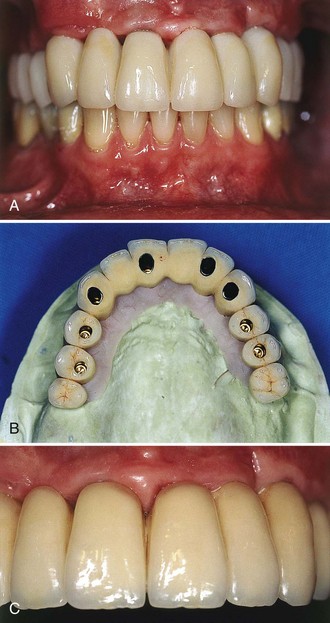
Figure 69-2 A, Clinical photograph of acrylic provisional fixed full-arch prosthesis in the maxilla. B, Clinical photograph of the final ceramometal restoration, anterior view. C, Occlusal view of final restoration on master cast.
(Courtesy Dr. Russell Nishimura, Westlake Village, CA.)
Depending on the volume of existing bone, the jaw relationship, the amount of lip support, and phonetics, some patients may not be able to be rehabilitated with an implant-supported fixed prosthesis. For these patients, a removable, complete-denture type of prosthesis is a better choice because it provides a flange extension that can be adjusted and contoured to support the lip, and there are no spaces for unwanted air escape during speech. This type of prosthesis can be retained and stabilized by two or more implants placed in the anterior region of the maxilla or mandible. Methods used to secure the denture to the implants vary from separate attachments on each individual implant to clips or other attachments that connect to a bar, which splints the implants together (Figure 69-3). Advantages and disadvantages of these attachment designs are discussed in Chapter 76.
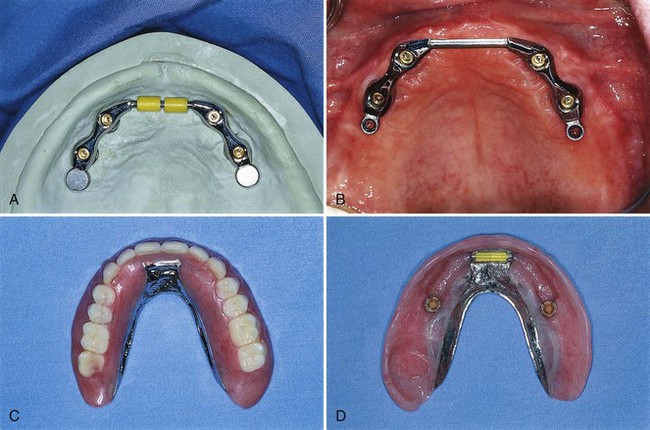
Figure 69-3 A, Laboratory view of maxillary overdenture bar attached to four implants with anterior clips and posterior extracoronal resilient attachments (ERAs). B, Clinical view of maxillary overdenture bar. C, Palateless maxillary complete overdenture. D, Tissue surface of same maxillary implant-assisted overdenture showing clips and ERAs.
(Courtesy Dr. John Beumer, UCLA Maxillofacial Prosthodontics, Los Angeles.)
Although the stability of the implant-retained overdenture does not compare to the rigidly attached, implant-supported fixed prosthesis, the increased retention and stability over conventional complete dentures is an important advantage for denture wearers.74 Additionally, implant-assisted and implant-supported prostheses are thought to protect alveolar bone from additional bone loss caused by long-term use of removable prostheses that are bearing directly on the alveolar ridges.
Partially Edentulous Patients
Multiple Teeth
Partially edentulous patients with multiple missing teeth represent another viable treatment population for osseointegrated implants, but the remaining natural dentition (occlusal schemes, periodontal health status, spatial relationships, and esthetics) introduces additional challenges for successful rehabilitation.52 The juxtaposition of implants with natural teeth in the partially edentulous patient presents the clinician with challenges not encountered with implants in the edentulous patient. As a result of distinct differences in the biology and function of implants compared with natural teeth, clinicians must educate themselves and use a prescribed approach to the evaluation and treatment planning of implants for partially edentulous patients (see Chapter 76). In general, endosseous dental implants can support a freestanding fixed partial denture. Adjacent natural teeth are not necessary for support, but their close proximity requires special attention and planning.12 The major advantage of implant-supported restorations in partially edentulous patients is that they replace missing teeth without invasion or alteration of adjacent teeth. Preparation of natural teeth becomes unnecessary, and larger edentulous spans can be restored with implant-supported fixed bridges.65 Moreover, patients who previously did not have a fixed option, such as those with Kennedy Class I and II partially edentulous situations, can be restored with an implant-supported fixed restoration (Figure 69-4).
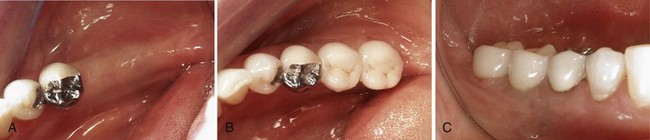
Figure 69-4 A, Clinical view of partially edentulous posterior mandible (Kennedy Class II distal extension). B, Occlusal view of same patient in A restored with implant-supported fixed restoration replacing teeth #18 and 19. Notice the dimensions of the crowns are smaller than typical mandibular molars (i.e., closer to bicuspid size). C, Buccal view of same restorations.
Early attempts to use endosseous implants to replace missing teeth in the partially edentulous patient were a challenge partly because the implants and armamentarium were designed for the edentulous patient and did not have much flexibility for adaptation and use in the partially edentulous patient (i.e., one standard-diameter implant, one type of abutment, and one surgical kit with instrumentation that was difficult to use adjacent to teeth). As the demand for implants in the partially edentulous patient increased, implant manufacturers responded with the development of wide-diameter and narrow-diameter implants and a variety of abutment choices. They also developed instrumentation that was better suited for the placement of implants adjacent to natural teeth. Currently, clinicians have many choices in terms of implant length, diameter, and abutment connection to choose for the optimal replacement of any missing tooth, large or small (Figure 69-5).
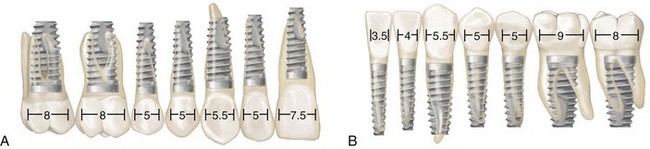
Figure 69-5 Diagram representing the use of wide-, narrow-, and standard-diameter implants for molars, mandibular incisors, and other teeth (different-sized implants superimposed over various teeth). A, Maxillary teeth. B, Mandibular teeth.
Another difficulty with partially edentulous cases is an underestimation of the importance of treatment planning for implant-retained restorations with an adequate number of implants to withstand occlusal loads. For example, one problem that required correction was the misconception that two implants could be used to support a multiunit fixed bridge in the posterior area. Multiunit fixed restorations in the posterior jaw are more likely to experience complications or failures (mechanical or biologic) when they are inadequately supported either in terms of the number of implants, quality of bone, or strength of the implant material (see Chapter 76). The use of stronger implants and better treatment planning (more implants used to support more restorative units), particularly in areas of poor-quality bone, has solved many of these problems.
Single Tooth
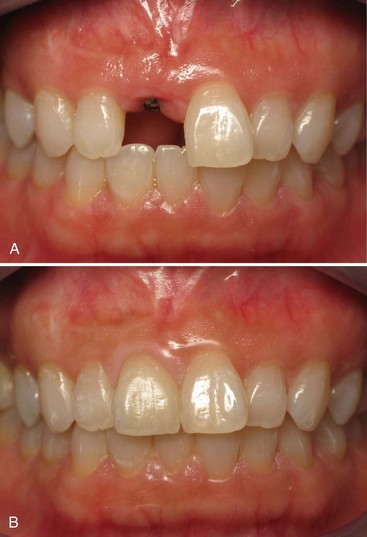
Patients with a missing single tooth (anterior or posterior) represent another type of patient who benefits greatly from the success and predictability of endosseous dental implants. Replacement of a single missing tooth with an implant-supported crown is a much more conservative approach than preparing two adjacent teeth for the fabrication of a tooth-supported fixed partial denture. It is no longer necessary to “cut” healthy or minimally restored adjacent teeth to replace a missing tooth with a nonremovable prosthetic replacement (Figure 69-6). Reported success rates for single-tooth implants are excellent.29
Replacement of an individual missing posterior tooth with an implant-supported restoration has been successful as well. The greatest challenges to overcome with the single-tooth implant restorations were screw loosening and implant or component fracture. Because of increased potential to generate forces in the posterior area, the implants, components, and screws often failed. Both these problems have been addressed with the use of wider-diameter implants and internal fixation of components (Figure 69-7). Wide-diameter implants often have a wider platform (restorative interface) that resists tipping forces and thus reduces screw loosening. The wide-diameter implant also provides greater strength and resistance to fracture as a result of increased wall thickness (the thickness of the implant between the inner screw thread and the outer screw thread). Implants with an internal connection are inherently more resistant to screw loosening and thus have an added advantage for single-tooth applications. Most dental implant manufacturers now sell implants with internal component fixation.
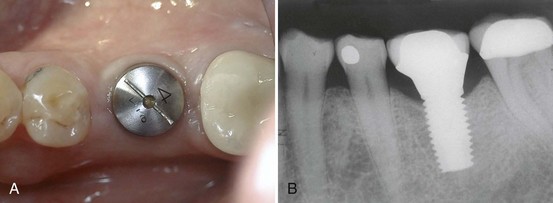
Figure 69-7 A, Occlusal view of healing abutment, which is attached to a wide-diameter implant used to replace a single missing molar. B, Radiograph of same patient depicted in A, showing the wide-diameter implant supporting the final restoration (molar replaced with a single-tooth implant-supported crown).
Esthetic Considerations
Anterior single-tooth implants present some of the same challenges as the single posterior tooth supported by an implant, but they also are an esthetic concern for patients. Some cases are more esthetically challenging than others because of the nature of each individual’s smile and display of teeth. The prominence and occlusal relationship of existing teeth, the thickness and health of periodontal tissues, and the patient’s own psychologic perception of esthetics all play a role in the esthetic challenge of the case. Cases with good bone volume, bone height, and tissue thickness can be predictable in terms of achieving satisfactory esthetic results (see Figure 69-6). However, achieving esthetic results for patients with less-than-ideal tissue qualities poses difficult challenges for the restorative and surgical team.12 Replacing a single tooth with an implant-supported crown in a patient with a high smile line, compromised or thin periodontium, inadequate hard or soft tissues, and high expectations is probably one of the most difficult challenges in implant dentistry and should not be attempted by novice clinicians.
Pretreatment Evaluation
A comprehensive evaluation is indicated for any patient who is being considered for dental implant therapy. The evaluation should assess all aspects of the patient’s current health status, including a review the patient’s past medical history, medications, and medical treatments. Patients should be questioned about parafunctional habits, such as clenching or grinding teeth, as well as any substance use or abuse, including tobacco, alcohol, and drugs. The assessment should also include an evaluation of the patient’s motivations, level of understanding, compliance, and overall behavior. For most patients, this involves simply observing their demeanor and listening to their comments for an impression of their overall sensibility and coherence with other patient norms. for some individuals with questionable behavior, however, a professional psychologic assessment of their mental health status may be indicated.
An intraoral and radiographic examination must be done to determine whether it is possible to place implant(s) in the desired location(s). Properly mounted diagnostic study models and intraoral clinical photographs are a useful part of the clinical examination and treatment-planning process to aid in assessment of spatial and occlusal relationships. Once the data collection is completed, the clinician will be able to determine whether implant therapy is possible, practical, and indicated for the patient.
Conducting an organized, systematic history and examination is essential to obtaining an accurate diagnosis and creating a treatment plan that is appropriate for the patient. Each treatment plan should be comprehensive and provide several treatment options for the patient, including periodontal and restorative therapies. Then, in consultation, the clinician can agree on the final treatment plan with the patient. Information gathered throughout the process will help the clinician’s decision making and determination of whether a patient is a good candidate for dental implants. A thoughtful and well-executed evaluation can also reveal deficiencies and indicate what additional surgical procedures may be necessary to accomplish the desired goals of therapy (e.g., localized ridge augmentation, sinus bone augmentation). Each part of the pretreatment evaluation is briefly discussed here.
Chief Complaint
What is the problem or concern in the patient’s own words? What is the patient’s goal of treatment, and how realistic are the patient’s expectations? The patient’s chief concern, desires for treatment, and vision of the successful outcome must be taken into consideration. The patient will measure implant success according to personal criteria. The overall comfort and function of the implant restoration are often the most important factors, but satisfaction with the appearance of the final restoration will also influence the patient’s perception of success. Furthermore, patient satisfaction may be influenced simply by the impact that the treatment has on the patient’s perceived quality of life. Whether the clinician inquires about quality-of-life changes or not, the patient most likely will measure the success of treatment by comparison to the pretreatment condition. Patients will evaluate for themselves whether the treatment helped them to eat better, look better, or feel better about themselves.
The clinician could consider an implant(s) and the retained prosthesis a success using standard criteria of symptom-free implant function, implant stability, and lack of periimplant infection or bone loss. at the same time, however, the patient who does not like the esthetic result or does not think the condition has improved could consider the treatment a failure. Therefore it is critical to inquire, as specifically as possible, about the patient’s expectations before initiating implant therapy and to appreciate the patient’s desires and values. With this goal in mind, it is often helpful and advisable to invite patients to bring their spouse or a family member to the consultation and treatment-planning visits to add an independent “trusted” observer to the discussion of treatment options. Ultimately, it is the clinician’s responsibility to determine if the patient has realistic expectations for the outcome of therapy and to educate the patient about realistic outcomes for each treatment option.
Medical History
A thorough medical history is required for any patient in need of dental treatment, regardless of whether implants are part of the plan. This history should be documented in writing by the patient’s completion of a standard health history form and verbally through an interview with the treating clinician. The patient’s health history should be reviewed for any condition that might put the patient at risk for adverse reactions or complications.
Patients must be in reasonably good health to undergo surgical therapy for the placement of dental implants. Any disorder that may impair the normal wound-healing process, especially as it relates to bone metabolism, should be carefully considered as a possible risk factor or contraindication to implant therapy (see later discussion).
A thorough physical examination is warranted if any questions arise about the health status of the patient.18 Appropriate laboratory tests (e.g., coagulation tests for a patient receiving anticoagulant therapy) should be requested to evaluate further any conditions that may affect the patient’s ability to undergo the planned surgical and restorative procedures safely and effectively. If any questions remain about the patient’s health status, a medical clearance for surgery should be obtained from the patient’s treating physician.
Dental History
A review of a patient’s past dental experiences can be a valuable part of the overall evaluation. Does the patient report a history of recurrent or frequent abscesses, which may indicate a susceptibility to infections or diabetes? Does the patient have many restorations? How compliant has the patient been with previous dental recommendations? What are the patient’s current oral hygiene practices?
The individual’s previous experiences with surgery and prosthetics should be discussed. If a patient reports numerous problems and difficulties with past dental care, including a history of dissatisfaction with past treatment, the patient may have similar difficulties with implant therapy. It is essential to identify past problems and to elucidate any contributing factors. The clinician must also assess the patient’s dental knowledge and understanding of the proposed treatment, as well as the patient’s attitude and motivation toward implants.
Intraoral Examination
The oral examination is performed to assess the current health and condition of existing teeth, as well as to evaluate the condition of the oral hard and soft tissues. It is imperative that no pathologic conditions are present in any of the hard or soft tissues in the maxillofacial region. All oral lesions, especially infections, should be diagnosed and appropriately treated before implant therapy. Additional criteria to consider include the patient’s habits, level of oral hygiene, overall dental and periodontal health, occlusion, jaw relationship, temporomandibular joint condition, and ability to open wide.
After a thorough intraoral examination, the clinician can evaluate potential implant sites. All sites should be clinically evaluated to measure the available space in the bone for the placement of implants and in the dental space for prosthetic tooth replacement (Box 69-1). The mesial-distal and buccal-lingual dimensions of edentulous spaces can be approximated with a periodontal probe or other measuring instrument. The orientation or tilt of adjacent teeth and their roots should be noted as well. There may be enough space in the coronal area for the restoration but not enough space in the apical region for the implant if roots are directed into the area of interest (Figure 69-8). Conversely, there may be adequate space between roots, but the coronal aspects of the teeth may be too close for emergence and restoration of the implant. If either of these conditions is discovered, orthodontic tooth movement may be indicated. Ultimately, edentulous areas need to be precisely measured using diagnostic study models and imaging techniques to determine whether space is available and whether adequate bone volume exists to replace missing teeth with implants and implant restorations. Figure 69-9 diagrams the minimal space requirements for standard-, wide-, and narrow-diameter implants placed between natural teeth, and the minimal interocclusal space needed to restore implants.
BOX 69-1 How Much Space Is Required for Placement of One or More Implants?*
Alveolar Bone
Assuming an implant that is 4 mm in diameter and 10 mm long, the minimal width of the jawbone needs to be 6 to 7 mm, and the minimal height should be 10 mm (minimum of 12 mm in the posterior mandible, where an additional margin of safety is required over the mandibular nerve). This dimension is desired to maintain at least 1.0 to 1.5 mm of bone around all surfaces of the implant after preparation and placement.
Interdental Space
Edentulous spaces need to be measured to determine whether enough space exists for the placement and restoration with one or more implant crowns. The minimal space requirements for the placement of one, two, or more implants are illustrated diagrammatically in Figure 69-9. The minimal mesial-distal space for an implant placed between two teeth is 7 mm. The minimal mesial-distal space required for the placement of two standard-diameter implants (4.0-mm diameter) between teeth is 14 mm. The required minimal dimensions for wide-diameter or narrow-diameter implants will increase or decrease incrementally according to the size of the implant. For example, the minimal space needed for the placement of an implant 6 mm in diameter is 9 mm (= 7 mm + 2 mm). Whenever the available space between teeth is greater than 7 mm and less than 14 mm, only one implant, such as placement of a wide-diameter implant, should be considered. The placement of a wide-diameter implant should be considered. Two narrow-diameter implants could be positioned in a space that is 12 mm. However, the smaller implant may be more vulnerable to implant fracture.
Interocclusal Space
The restoration consists of the abutment, the abutment screw, and the crown (it may also include a screw to secure the crown to the abutment if it is not cemented). This restorative “stack” is the total of all the components used to attach the crown to the implant. The dimensions of the restorative stack vary slightly depending on the type of abutment and the implant-restorative interface (i.e., internal or external connection). The minimum amount of interocclusal space required for the restorative “stack” on an external hex-type implant is 7 mm.
* All the minimal space requirements discussed here are generic averages. The actual space limitations for any particular implant system must be determined according to the manufacturer’s specifications.
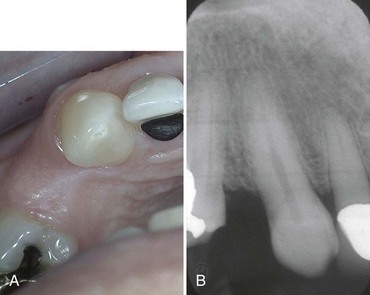
Figure 69-8 A, Clinical photograph of maxillary premolar space with apparently adequate space between the remaining teeth for an implant-supported crown. B, Radiograph clearly shows a lack of space between the roots of the adjacent teeth as a result of convergence into the space (same patient as in A).
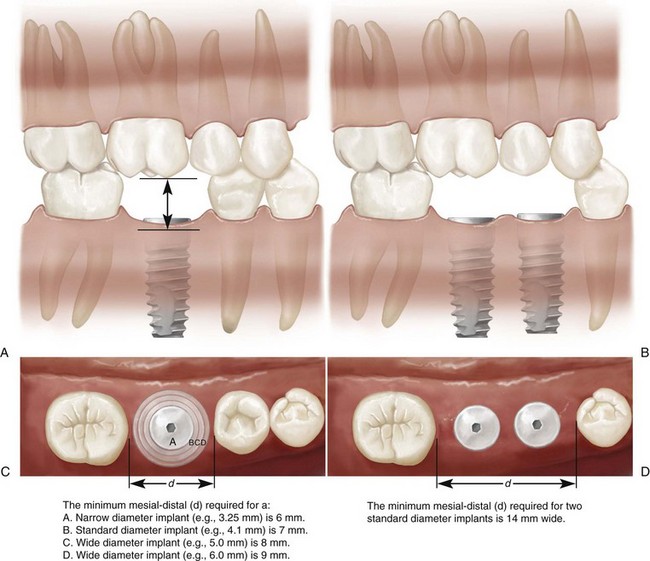
Figure 69-9 A, Minimum amount of mesial-distal space (d) required for placement of single-tooth implant between natural teeth: A, 6 mm for narrow-diameter implant (3.25 mm); B, 7 mm for standard-diameter implant (4.1 mm); C and D, 8 mm and 9 mm, respectively, for wide-diameter implants (5.0 mm and 6.0 mm). B, minimum amount of mesial-distal space (d) required for placement of two standard-diameter (4.1 mm) implants between natural teeth is 14 mm. This allows approximately 2 mm between teeth/implants and between implant/implant. Minimum amount of space required between implant/restoration interface and opposing occlusal surfaces for restoration of an implant. This dimension will vary, depending on implant design and manufacturer component dimensions. The minimal dimension of 7 mm is based on an externally hexed implant and UCLA abutment.
Diagnostic Study Models
Mounted study models are an excellent means of assessing potential sites for dental implants. Properly articulated models with diagnostic wax-up of the proposed restorations allow the clinician to evaluate the available space and to determine potential limitations of the planned treatment (Figure 69-10 online). This is particularly useful when multiple teeth are to be replaced with implants or when a malocclusion is present.
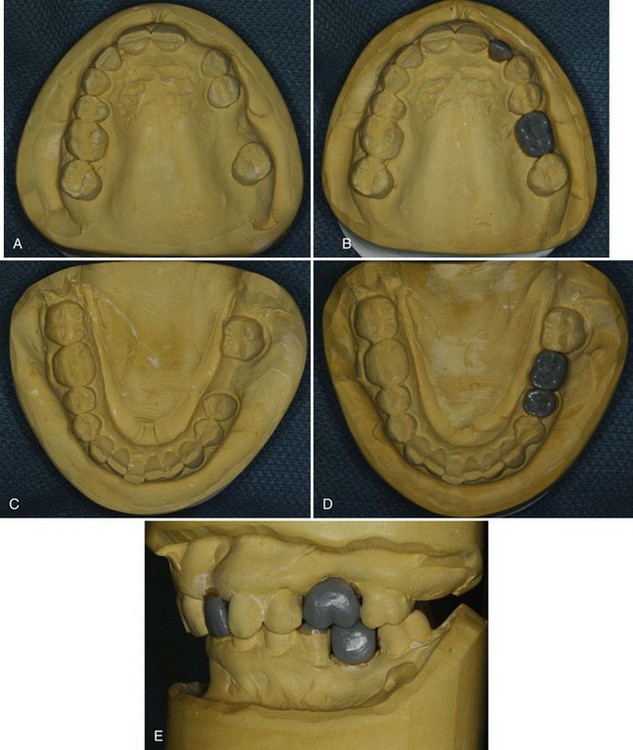
Figure 69-10 Photographs of a diagnostic models with proposed lateral incisor and first molar tooth replacement waxed-up to evaluate the amount of space and contours. A, Diagnostic cast of maxillary arch with missing left lateral incisor and left first molar. B, Diagnostic wax-up of lateral incisor and first molar in the maxillary arch. C, Diagnostic cast of mandibular arch with missing left first molar. D, Diagnostic wax-up of first molar. E, Articulated maxillary and mandibular diagnostic models with wax-up of lateral incisor and first molars to evaluate dimensions and contours.
(Courtesy Dr. Stacy Yu, University of California, Los Angeles).
Hard Tissue Evaluation
The amount of available bone is the next criterion to evaluate. Wide variations in jaw anatomy are encountered, and it is therefore important to analyze the anatomy of the dentoalveolar region of interest both clinically and radiographically.
A visual examination can immediately identify deficient areas (Figure 69-11), whereas other areas that appear to have good ridge width will require further evaluation (Figure 69-12). Clinical examination of the jawbone consists of palpation to feel for anatomic defects and variations in the jaw anatomy, such as concavities and undercuts. If desired, it is possible with local anesthesia to probe through the soft tissue (intraoral bone mapping) to assess the thickness of the soft tissues and measure the bone dimensions at the proposed surgical site.
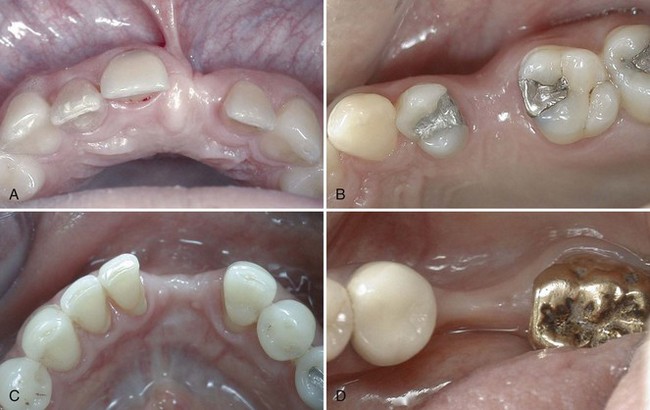
Figure 69-11 Clinical photographs of edentulous areas with obvious deficient areas of alveolar dimension noted on visual examination: A, anterior maxilla; B, posterior maxilla; C, anterior mandible; D, posterior mandible. These clinical images all represent buccal-lingual deficiencies in the alveolar dimensions.
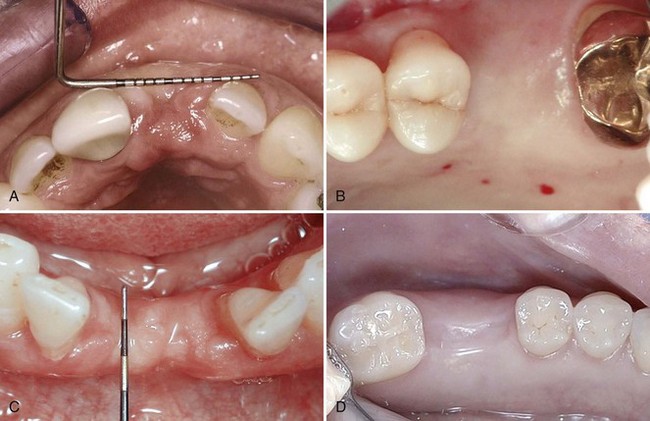
Figure 69-12 Clinical photographs of edentulous areas with apparent good alveolar dimension noted on visual examination: A, anterior maxilla; B, posterior maxilla; C, anterior mandible; D, posterior mandible. It is likely that these sites have adequate bone volume for implant placement. However, it is also possible to find alveolar deficiencies despite the appearance of wide ridges.
The spatial relationship of the bone must be evaluated in a three-dimensional view because the implant must be placed in the appropriate position relative to the prosthesis. it is possible that an adequate dimension of bone is available in the anticipated implant site (see Box 69-1), but that the bone and thus the implant placement might be located too lingual or too buccal for the desired prosthetic tooth replacement.38 Bone augmentation procedures may be necessary to facilitate the placement of an implant in an acceptable prosthetic position despite the availability of an adequate quantity of bone (i.e., the bone is in the wrong location). Indications for the use of bone augmentation procedures are discussed in Chapter 72.
Radiographic Examination
Radiographic assessment of the quantity, quality, and location of available alveolar bone in potential implant sites ultimately determines whether a patient is a candidate for implants and if a particular implant site needs bone augmentation. Appropriate radiographic procedures, including periapical radiographs, panoramic projections, and tomographic cross-sectional imaging, can help identify vital structures such as the floor of the nasal cavity, maxillary sinus, mandibular canal, and mental foramen (see Chapter 70). In addition to the absolute dimensional measurement of the alveolar bone, it is important to determine whether the volume of bone radiographically (as well as clinically) is located in a position to allow for the proper position of the implant to facilitate restoration of the tooth/teeth in proper esthetic and functional relationship with the adjacent and opposing dentition. The best way to evaluate the relationship of available bone to the dentition is to image the patient with a diagnostically accurate guide using radiopaque markers that accurately represent the proposed prosthetic contours (see Figure 70-3).
Soft Tissue Evaluation
Evaluation of the quality, quantity, and location of soft tissue present in the anticipated implant site helps to anticipate the type of tissue that will surround the implant(s) after treatment is completed (keratinized versus nonkeratinized mucosa). For some cases, depending on the clinician’s view of keratinized tissue, evaluation may reveal a need for soft tissue augmentation (Box 69-2). Areas with minimal or no existing keratinized mucosa may be augmented with gingival or connective tissue grafts. Additionally, any mucogingival concerns, such as frenum attachments or pulls, should be thoroughly evaluated.
BOX 69-2 How Much Keratinized Tissue Is Required for Health and Maintenance of Implants?
Debate continues about whether it is necessary to have a zone of keratinized tissue surrounding implants. Despite strong opinions and beliefs about the need for keratinized mucosa around implants versus this mucosa being unnecessary, neither argument has been proved.
Some studies have concluded that, in the presence of good oral hygiene, a lack of keratinized tissue does not impair the health or function of implants.73 Others strongly believe that keratinized mucosa has better functional and esthetic results for implant restorations. Keratinized mucosa is typically thicker and denser than alveolar mucosa (nonkeratinized). It forms a strong seal around the implant with a cuff of circular (parallel) fibers around the implant, abutment, or restoration that is resistant to retracting with mastication forces and oral hygiene procedures. Implants with coated surfaces (i.e., hydroxyapatite [H] or titanium plasma spray [TPS] coating) demonstrate greater periimplant bone loss and failures in the absence of keratinized mucosa.15,47
Risk Factors and Contraindications
Clearly, there are numerous indications for the use of endosseous dental implants to replace missing teeth. Most patients who are missing one or more teeth can benefit from the application of an implant-retained prosthesis provided they meet the requirements for surgical and prosthetic rehabilitation. Edentulous patients who are unable to function with complete dentures and who have adequate bone for the placement of dental implants can be especially good dental implant candidates. More and more partially edentulous patients are also being treated with dental implant restorations. Many patients, whether they are missing one, several, or all of their teeth, can be predictably restored with implant-retained prostheses.
In this era of high implant success and predictability and thus possible complacency, it is imperative for clinicians to recognize risk factors and contraindications to implant therapy so that problems can be minimized and patients can be accurately informed about risks. As such, the clinician must be knowledgeable in this area and inform patients about risk factors and contraindications before initiating treatment. Contraindications for the use of dental implants, although relatively few and often not well defined, do exist. Some conditions are probably best described as “risk factors” rather than “contraindications” to treatment because implants can be successful in almost all patients; implants may be less predictable in some situations, and this distinction should be recognized. Ultimately, it is the clinician’s responsibility with the patient to make decisions as to when implant therapy is not indicated.
Table 69-1 lists some conditions and factors that are thought to increase the risk for implant failure or otherwise deem the patient a poor candidate for implant therapy. Some of these conditions are briefly discussed here.
TABLE 69-1 Risk Factors and Contraindications for Implant Therapy
| Risk Factor | Contraindication | |
|---|---|---|
| Medical and Systemic Health–Related Issues | ||
| Diabetes (poorly controlled) | ??—Possibly | Relative |
| Bone metabolic disease (e.g., osteoporosis) | ??—Probably | Relative |
| Radiation therapy (head and neck) | Yes | Relative/Absolute |
| Bisphosphonate therapy (intravenous) | ??—Probably | Relative/Absolute |
| Bisphosphonate therapy (oral) | ??—Possibly | Relative |
| Immunosuppressive medication | ??—Probably | Relative |
| Immunocompromising disease (e.g., HIV, AIDS) | ??—Possibly | Relative |
| Psychologic and Mental Conditions | ||
| Psychiatric syndromes (e.g., schizophrenia, paranoia) | No | Absolute |
| Mental instability (e.g., neurotic, hysteric) | No | Absolute |
| Mentally impaired; uncooperative | No | Absolute |
| Irrational fears; phobias | No | Absolute |
| Unrealistic expectations | No | Absolute |
| Habits and Behavioral Considerations | ||
| Smoking; tobacco use | Yes | Relative |
| Parafunctional habits | Yes | Relative |
| Substance abuse (e.g., alcohol, drugs) | ??—Possibly | Absolute |
| Intraoral Examination Findings | ||
| Atrophic maxilla | Yes | Relative |
| Current infection (e.g., endodontic) | Yes | Relative |
| Periodontal disease | ??—Possibly | Relative |
HIV, Human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome.
Medical and Systemic Health–Related Issues
Although few absolute medical contraindications to implant therapy exist, some relative contraindications are important to consider. The clinician must consider medical and health-related conditions that affect bone metabolism or any aspect of the patient’s capacity to heal normally.11 This category includes conditions such as diabetes, osteoporosis, and immune compromise, medications, and medical treatments such as chemotherapy and irradiation.
Diabetes Mellitus
Diabetes is a metabolic disease that can have significant effects on the patient’s ability to heal normally and resist infections. This is particularly true for patients whose diabetes is not well controlled. Poorly controlled diabetics often have impaired wound healing and a predisposition to infections, whereas diabetic patients whose disease is well controlled experience few, if any, problems (see Chapter 27).
There is concern about the success and predictability of implants in patients with diabetes. Several studies have reported moderate failure rates in diabetic patients, with implant success ranging from 85.6% to 94.3%.9,33,45,48 A prospective study demonstrated 2.2% early failures and 7.3% late failures in diabetic patients.69 After 5 years, the overall success rate for this group of diabetic patients was 90%.62 None of these studies was able to correlate gender, age, smoking, diabetes type, or level of diabetic control with implant failure. In a metaanalytical review of implant failures in patients who were not diabetics, the early implant failure rate was 3.2% and the late implant failure rate 5.2%.28 The finding that diabetic patients experience slightly more late failures may be related to less tissue integrity caused by reduced tissue turnover and impaired tissue perfusion. These results suggest that diabetes may be a risk factor for implants, particularly for late failures. However, the risk does not appear to be particularly high.
Bone Metabolic Disease
Osteoporosis is a skeletal condition characterized by decreased mineral density. The two main classifications are primary (three types) and secondary (many types) osteoporosis. Primary osteoporosis has been attributed to menopausal changes (type I), age-related changes (type II), or idiopathic causes (type III). Secondary osteoporosis has been attributed to many different diseases and conditions, including diabetes, alcoholism, malnutrition, and smoking.39
All the various types of osteoporosis share the same fundamental problem of decreased bone mineral density, and the concern that this condition may impair the patient’s ability to achieve and maintain implant osseointegration. The premise that implants will not perform as well in a patient with osteoporosis is reasonable given that osseointegration depends on bone formation adjacent to the implant surface and that success rates are highest in dense bone and lowest in poor-quality, loose trabecular bone. However, to date, there is no clear evidence to suggest that implants will not be successful in patients with osteoporosis, so the issue continues to be debated.10,24 On the positive side, although the evidence is weak, case reports have demonstrated successful implant treatment in patients with osteoporosis.38 Some investigators advocate the use of longer healing times for osseointegration to occur before loading the implants in patients with osteoporosis.35 Conversely, in a retrospective analysis of 49 patients who received sinus bone augmentation, individuals (11 patients) with lower bone mass density had significantly lower implant success rates as compared to age- and sex-matched controls.16 Other parameters evaluated in this study did not demonstrate any significant differences.
Interestingly, there is a trend in aging adults (men over 50 years and postmenopausal women) for bone mass to decrease progressively through bone demineralization at a rate of 1% to 2% per year and in some individuals as much as 5% to 8% per year throughout their later life.26,44 If one considers this decline in bone mass with aging along with a continually increasing life expectancy in the population, the number of individuals with osteopenia or osteoporosis will continue to increase, and the concern about this condition’s influence on implant success will become increasingly important for clinicians.
Medications
Some prescribed medications, including steroids and bisphosphonates, may be cause for concern relative to the potential implant patient. Corticosteroid therapy, whether used for hormone replacement, cancer treatment, immune suppression or other chronic condition may suppress the immune response, impair wound healing or compromise the normal adrenal response to stress. See the later section on immune compromise and immune suppression, as well as Chapters 27 and 37 for more information on the treatment of patients taking corticosteroids and bisphosphonate medications. Only a brief statement regarding the risk of bisphosphonate therapy is offered here. Readers are encouraged to review more detailed explanations in Chapters 27 and 37 and to consult online information, as well as other resources to get updated information about this important subject as more is learned and recommendations are developed.
Although there is heightened awareness and great concern about risk of bisphosphonate-related osteonecrosis of the jaw (BRONJ), the causal relationship and pathogenesis of the problem has not been defined. A review of available literature offers information that will help to guide clinicians in their decision-making but it is far from definitive. The prevalence and incidence remains uncertain. In general, the risk of BRONJ is between 1 in 10,000 and 1 in 100,000 but may increase to 1 in 300 after an oral surgical procedure. The great majority of BRONJ cases will likely remain in the intravenous population. Co-factors, such as smoking, steroid use, anemia, hypoxemia, diabetes, infection, and immune deficiency, have not been firmly established but may be important.56 Rarely does BRONJ in the oral bisphosphonate patient appear to progress beyond stage 2, and many cases reverse with discontinuation of oral medication. Procedures reported to have contributed to the development of BRONJ include extractions, periodontal surgery, root canal treatment, and dental implant surgery.57 Dental implant therapy, as well as other surgical procedures, should be avoided in individuals who have been treated with intravenous (IV) bisphosphonate therapy and carefully considered with caution in patients treated with oral bisphosphonate therapy, particularly those with a history of more than 3 years of use.4
Immune Compromise and Immune Suppression
Individuals undergoing chemotherapy or taking medications that impair healing potential (e.g., steroids) are probably not good candidates for implant therapy because of the effects these agents have on normal healing. This is especially true for cancer chemotherapy. A lowered resistance to infection may also be problematic for these patients. Patients with very low or undetectable viral loads and normal (T cell counts) immune function may be candidates for implant therapy (see Chapter 19). Past history of chemotherapy or immunosuppressive therapy may not be problematic if the patient has recovered from the side effects of treatment.
Patients with an immunocompromising disease, such as human immunodeficiency virus (HIV) infection or acquired immunodeficiency syndrome (AIDS), are probably not good candidates for implants, especially when their immune system is seriously impaired.
Radiation Therapy
Patients with a history of radiation treatment to the head and neck region may not heal well after surgery. Soft tissue dehiscence may follow surgical manipulation, which may lead to osteoradionecrosis (ORN), a serious condition of nonhealing exposure and infection of bone. This is especially problematic for patients who have received radiation dosages greater than 60 Gy. Surgical procedures, or any procedure that may initiate a wound, are generally avoided in patients with a history of radiation therapy. If deemed necessary, surgical procedures can be done in conjunction with hyperbaric oxygen (HBO) therapy to reduce the risk of ORN.
Several studies have documented poor success rates for implants in patients with a history of radiation therapy.40,41,55 In a literature review, Sennerby and Roos68 found irradiation to be associated with high failure rates, as did Esposito et al30 in their review. Beumer et al14 reported success rates as low as 60.4% in the irradiated maxilla. Granstrom et al40 reported a significant improvement in survival rates for implants in patients treated with HBO. However, in a systematic review, Coulthard et al23 concluded that the evidence is lacking to support the clinical effectiveness of HBO in irradiated patients receiving implants. The application of implants in patients with a history of irradiation, with or without the use of HBO, is not resolved and continues to be debated. Clearly, irradiation is a risk factor for implant success and may be a contraindication.
Psychologic and Mental Conditions
In general, any type of psychologic abnormality can be considered a contraindication to dental implant treatment because of the patient’s uncooperativeness, lack of understanding, or behavioral problems. Physiologically, there is no reason to suspect that implants could not become osseointegrated in these patients. However, the patient’s ability to tolerate the number and type of treatment appointments required for implant placement, restoration, and maintenance could be problematic. All psychologic conditions have the potential to be absolute contraindications to implant treatment depending on the severity of the condition. The exception might be individuals who demonstrate good cooperative behavior with only mild psychologic or mental impairment. The clinician should take great care before accepting a mentally or psychologically impaired individual for treatment with implants.
Habits and Behavioral Considerations
Patients have a variety of habits and behaviors that may increase the risk of failure for dental implants. Smoking, clenching or grinding of teeth, and drug or alcohol abuse are among the most well-known habits that should be identified because of the increased risk for implant failure or complications.
Smoking and Tobacco Use
Moderate to heavy smoking has been documented to result in higher rates of early implant failure and adversely affect the long-term prognosis of dental implant restorations.7,25,54 This is particularly true for implants placed in poor quality bone such as the posterior maxilla.48 The mechanisms of action responsible for higher implant failures associated with smoking are not understood. Plausible explanations include the effect of smoking on white blood cells, vasoconstriction, wound healing, and osteoporosis.5,46 Smoking is a known risk factor for osteoporosis and thus may adversely affect implant success through its effect on bone metabolism. Smoking cessation may improve the success rate of implants.6 In a metaanalytical review, Bain et al8 found that implants with an altered surface microtopography (Osseotite, acid-etched surface) seemed to significantly lessen the adverse affects of smoking on implant success.
Parafunctional Habits
Parafunctional habits, such as clenching or grinding of teeth (consciously or unconsciously), has been associated with an increased rate of implant failure (e.g., failure to integrate, loss of integration, implant fracture). Repeated lateral forces (i.e., parafunctional habits) applied to implants can be detrimental to the osseointegration process, especially during the early healing period. Patients with known parafunctional habits should be advised of an increased risk of complications or failures as a result of their clenching or grinding. Many consider bruxism to be a contraindication to implant treatment, especially in the case of a short-span, fixed partial denture or a single-tooth implant. If implants are planned for a patient with parafunctional habits, protective measures should be employed, such as creating a narrow occlusal table with flat cusp angles, protected occlusion, and the regular use of occlusal guards (see Chapter 76).
Substance Abuse
Drug and alcohol abuse should be considered a contraindication for implant therapy for reasons similar to the psychologic problems discussed earlier. Patients with drug or alcohol addictions can be irresponsible and noncompliant with treatment recommendations. Depending on the severity and duration of an individual’s addiction, some patients may be malnourished or may even have impaired organ function and therefore may not be a good surgical candidate because of poor healing capacity. All elective treatments, including implant therapy, should be refused until addictions are treated and controlled.
Posttreatment Evaluation
Periodic posttreatment examination of implants, the retained prosthesis, and the condition of the surrounding periimplant tissue is an important part of successful treatment. Aberrations and complications can often be treated if discovered early, but many problems will go unnoticed by the patient. Thus periodic examination is essential to discovering problems early. Several parameters are available to evaluate the condition of the prosthesis, the stability of the implant(s), and the health of surrounding periimplant tissues after implant integration and prosthetic restoration. Many of these clinical measures are adaptations from dental and periodontal examination methods, such as clinical inspection, probing, and radiographic examination.
Clinical Examination
The clinical examination includes visual inspection and probing. Visual evaluation of the tissue color contour and consistency, periimplant probing, and radiographic images are some of the ways to evaluate implants in the posttreatment phase. Soft tissues can be visually inspected for signs of inflammation or swelling. They can also be palpated to detect areas of edema, tenderness, exudate, or suppuration. Periimplant probing can be used to assess the condition and level of hard and soft tissues surrounding implants.
Periimplant Probing
Periodontal probing around natural teeth is very useful to assess the health of periodontal tissues, the sulcus or pocket depth, and the level of attachment. However, using a periodontal probe around implants may not provide comparable results.17 Clinicians should use caution when evaluating periimplant probing because these measures cannot be interpreted the same as probing depths around teeth. Because of distinct differences in the surrounding tissues that support teeth compared to those that support implants, the probe inserts and penetrates differently. Around teeth, the periodontal probe is resisted by the health of the periodontal tissues and, perhaps most importantly, by the insertion of supracrestal connective tissue fibers into the cementum of the root surface.
These fibers, unique to teeth, are the primary source of resistance to the probe.3 There is no equivalent fiber attachment around implants. Connective tissue fibers around implants generally run parallel to the implant or restorative surface and do not have perpendicular or inserting fibers (see Chapter 68). The primary source of resistance to the probe around an implant will differ, depending on the conditions surrounding the implant.27,50 At noninflamed sites, the probe will be resisted by the most coronal aspect of connective tissue adhesion to the implant. At inflamed sites, the probe tip consistently penetrates farther into the connective tissue until less inflamed connective tissue is encountered, which is often close to or at the level of bone.
The value of periimplant probing is different than periodontal probing and offers very limited information by comparison. Probing around implants can measure the level of the mucosal margin relative to a fixed position on the implant or restoration and can also measure the depth of tissue around the implant. The periimplant probing depth is often a measure of the thickness of the surrounding connective tissues and correlates most consistently with the level of surrounding bone. However, periimplant probing is affected by several conditions, including the size of the probe, the force and direction of insertion, the health and resistance of periimplant tissues, the level of bone support, and the features of the implant, abutment, and prosthesis design. In other words, the probe can be an accurate measure of soft tissue thickness around an implant (i.e., periimplant soft tissue above the bone level), but in some cases or sites, it may not be an accurate assessment of soft tissue thickness caused by an inability to properly angle and direct the probe alongside of the implant. In these situations, the area should not be measured or recorded since the as a result will be erroneous. Furthermore, probing around implants is likely to be more variable than around teeth; studies have shown that a change in probing force around implants results in more dramatic changes than a similar change in probing force around teeth.60 The probing depth around implants presumed to be “healthy” (and without bleeding) has been documented to be about 3 mm around all surfaces.2,21 The absence of bleeding on probing around teeth has been established as an indicator of health and a predictor of periodontal stability.49 Studies comparing bleeding on probing around teeth and implants in the same patient have reported that bleeding around implants occurs more frequently. However, the ability to use bleeding as an indicator of assessing diseased versus healthy sites around implants has not been established. Microbiologic studies suggest that greater probing depth or “pockets” around implants harbor higher levels of pathogenic microorganisms.61,66,67
Microbial Testing
Studies in animals and humans have demonstrated the development of periimplant mucosal inflammation in response to the accumulation of bacterial plaque.13,64 Studies have also documented similarities in the microbial composition of plaque in healthy periodontal sites compared with healthy periimplant sites.59 Likewise, evidence indicates that the microbiota of inflamed periimplant sites (periimplantitis) harbors the same periodontal pathogenic microorganisms as those observed in diseased periodontal pockets.59,67 However, there is no evidence to prove that periodontal pathogens cause periimplant disease, and the pathogenesis of inflammatory disease around implants has not been defined.22 No convincing evidence indicates that laboratory tests for the identification of suspected periodontal pathogens are of any use in the evaluation of implants.30 The usefulness of microbial testing may be limited to the evaluation of periimplant sites that are showing signs of infection and bone loss, so the clinician can prescribe appropriate antibiotics.
Stability Measures
The assessment of implant stability (or mobility) is an important measure for determining whether osseointegration is being maintained. Importantly, however, this measure has extremely low sensitivity but high specificity. That is, a large amount of bone loss can occur around an implant, but the implant remains stable (stability measure in this case has a low sensitivity for the detection of an implant that has lost much of its bone support). On the other hand, if significant mobility is detected, the implant has likely failed (mobility is highly specificity for the detection of implant failure). There is great interest in evaluating the stability of the bone-to-implant contact in a non invasive manner. Two techniques that have been used as non invasive ways of evaluating implant stability are impact resistance (e.g., Periotest) and resonance frequency analysis (RFA).
Originally designed to evaluate tooth mobility quantitatively, the Periotest (Gulden, Bensheim, Germany) is a noninvasive, electronic device that provides an objective measurement of the reaction of the periodontium to a defined impact load applied to the tooth crown. The Periotest value depends to some extent on tooth mobility but mainly on the damping characteristics of the periodontium. Despite the dependence on the periodontium, the Periotest has been used to evaluate implant stability as well. However, unlike teeth, the movement of implants and the surrounding bone is minuscule, and therefore the Periotest values fall within a much smaller range compared to the range found with teeth. Detection of horizontal mobility may be a significant advantage for the use of the Periotest because it is much more sensitive to horizontal movement than similar detection by other means, such as manual assessment.30 Additionally, many variables have been associated with the use of the Periotest related to positioning of the device.
Resonance frequency analysis (RFA) is another noninvasive method used to measure the stability of implants.58 This method uses a transducer that is attached to the implant or abutment. A steady-state signal is applied to the implant through the transducer, and a response is measured. The RFA value is a function of the stiffness of the implant in the surrounding tissues. The stiffness is influenced by the implant, the interface between the implant and bone, and soft tissues as well as the surrounding bone itself. Additionally, the height of the implant or abutment above the bone will influence the RFA value. Unlike the Periotest, however, the RFA is not dependent on movement in only one direction. Thus the absolute RFA values vary from one implant design to another and from one site to another, but there is high consistency for any one implant or location. The value of RFA is most appreciated with repeated measures of the same implant over time because it is very sensitive to changes in the bone-implant interface. Small changes in tissue support can be detected using RFA. An increase in RFA value indicates increased implant stability, whereas a decrease indicates loss of stability. However, this is a relative measure and it has not been determined whether RFA is capable of detecting impending failure before the implant actually fails. Currently, much interest and research have focused on the use of noninvasive methods to evaluate implant stability. Mobility remains the cardinal sign of implant failure, and detecting mobility is therefore an important parameter.
Radiographic Examination
Intraoral radiographs should be taken at the time of placement (baseline), at the time of abutment connection (confirm seating and serve as another baseline), at the time of final restoration delivery (loading), and subsequently to monitor marginal or periimplant bone changes. Periapical radiographs have excellent resolution and provide adequate details for evaluating bone support around implants if taken at a perpendicular direction. The limitation of periapical radiographs is that they are difficult to standardize, and great variability is inherent in the acquisition process. However, periapical films are relatively simple, inexpensive, and readily available in the dental office.
The objective of the radiographic examination is to measure the height of bone adjacent to the implant(s) and to evaluate the presence and quality of bone along the length of the implant. Finally, the periimplant areas are assessed for any radiolucent lesions around the implant. Although the predictive value of assessing implant stability with radiographs is low, films do offer a reasonable method to measure changes in bone levels.71 The predictive value of detecting implant failure or loss of stability is good when radiolucent lesions are discovered with periapical radiographs. Radiographic identification of unstable implants is reliable when performed as part of annual examinations and when examining patients on a routine, long-term basis.42
The radiographic examination remains one of the primary tools for detection of failed implants in routine clinical evaluation, even though it is not as accurate as mobility tests. In one study designed to evaluate the accuracy and precision of radiographic diagnosis of mobility, the probability of predicting implant mobility in a population with a low prevalence of implant failures was found to be low.71 Other studies, however, have demonstrated much higher predictive value for radiographic diagnosis of implant mobility.36,42 The authors concluded that the most important factors for making an accurate radiographic diagnosis are the quality of the radiograph and the experience of the clinician.42,71
Oral Hygiene and Implant Maintenance
The long-term success of dental implants likely requires the maintenance of healthy periimplant tissues because the soft tissue “seal” around implants is best when the surrounding mucosa is not inflamed. For this reason, good oral hygiene and regular professional care are essential to maintaining implants. The importance of good oral hygiene should be stressed even before implants are placed, and oral hygiene instructions for plaque control should begin as early as possible. The patient’s ability to maintain good oral hygiene should be monitored and reinforced at each visit, and the patient should be given instructions specific to individual needs. Interestingly, despite many years of experience with implants, a recent systematic review revealed that no well-designed studies with a high level of evidence (i.e., randomized controlled trials) have defined the most effective regimens for long-term implant maintenance.31
Professionally, several actions can be taken to enhance the patient’s ability to perform good oral hygiene. For example, implant superstructures, frameworks, and restorations should be fabricated to accommodate and facilitate oral hygiene (e.g., embrasure spaces should be made to allow the passage of a proxy brush). Specific oral hygiene procedures with appropriate hygiene aids should be demonstrated for each implant area for every patient. Initially, for the first year after treatment is completed, recall maintenance visits should be scheduled at 3-month intervals and then adjusted to suit the patient’s individual needs. Some patients, with good oral hygiene and minimal deposits, will require infrequent professional hygiene maintenance, whereas others, with poor oral hygiene and heavy deposits, will require more frequent follow-up care.
The use of plastic and gold-coated curettes has been advocated to protect the titanium implant surface and the titanium abutment from contamination by other metals. These curettes were also used to reduce the likelihood of scratching the surface. Unfortunately, plastic curettes do not work very well, and gold-coated curettes cannot be sharpened. Most current implant prostheses are made with gold alloys or ceramic materials, which are usually identical to the materials used in restorations for the natural dentition. Furthermore, the location of the connection between these restorative materials and the implant is typically below the mucosa and often near the crest of bone; most calculus removal will be above this level. Thus the fear of contaminating the titanium implant is unwarranted. The gold alloy or ceramic surfaces can be debrided with most scalers and curettes (plastic, gold coated, stainless steel) without damaging the surface. Rotary instruments (e.g., prophy cup) can be used to remove plaque or biofilms and polish surfaces. The use and sonic instruments (e.g., Cavitron) should be avoided because of irregularities that can easily be created in the surface, which can contribute to plaque and calculus accumulation. One advantage of using screw-retained implant restorations (e.g., super structures, overdenture bars, fixed prosthesis, etc.) is that they can be removed, cleaned outside the mouth, and replaced.
Recall maintenance visits should include an evaluation of soft and hard tissue health, the patient’s level of oral hygiene compliance and plaque control, prosthesis integrity and stability, and implant stability. Implant stability can be evaluated with a combination of mobility testing and radiographic assessment.
Conclusion
Dental clinicians can now predictably replace missing teeth with endosseous dental implants. Most patients, whether missing a single tooth, several teeth, or all their teeth, can be candidates for dental implant therapy. However, many factors influence the outcome; the clinician must consider the quantity, quality, and location of available bone; the patient’s mental and physical health; and risk factors and contraindications. Patients should be advised about risk factors and provided treatment options both with and without dental implants. Periodic evaluation, good oral hygiene, and regular maintenance are important aspects of care for the long-term success and the prevention of complications with dental implants. Radiographic examination and mobility tests appear to be some of the most reliable parameters in the assessment of endosseous dental implants.
![]() Science Transfer
Science Transfer
All implant patients need to have a comprehensive evaluation before treatment planning. This includes a thorough medical history and documented detailed clinical examinations. Three-dimensional radiographs should be used for all patients. Edentulous patients can be restored to function with either implant supported fixed restorations or implant supported removable overdentures. If the clinician is concerned with lip support for esthetics then the overdenture is advised. Also, this approach prevents problems of saliva and air passage through fixed restorations that can affect speech and cause uncontrolled spitting of saliva.
Some patients have definite contraindications to implant treatment. These include patients with a history of 60 Gy or more therapeutic radiation exposure, patients who are immunocompromised (e.g., undergoing immunosuppressive therapy, patients with active HIV infections with lowered T cell counts), patients who are unable to maintain adequate levels of oral hygiene (e.g., some physically or mentally compromised patients), or patients with substance abuse who are oral hygiene incompetent. Patients with uncontrolled diabetes should not have implant surgery nor should patients who have undergone intravenous bisphosphonate therapy. Controlled diabetic patients can be treated and may have a small risk of increased implant complications. Patients who have a history of treatment with oral bisphosphonate therapy are at risk for bisphosphonate-related osteonecrosis, particularly if they have 3 or more years of treatment and have associated corticosteroid usage. These patients can be assessed using a blood test for C-terminal cross linked teleopeptide type I collagen (CTX), which measures the ability of bone to heal. Levels of 150 pg/ml or higher are generally not at risk and so can be successfully treated with implant surgery.
Smokers and patients with parafunctional occlusal abnormalities have increased risk of complications and failure following implant surgery. Osteoporotic patients can be successfully treated with implants, but it is wise to lengthen the time for osseointegration before functional loading. HIV-positive patients with undetectable viral loads and normal T cell counts can be treated with implants as well as patients with a history of chemotherapy who currently have normal immunological function.
1 Adell R, Eriksson B, Lekholm U, Branemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347-359.
2 Adell R, Lekholm U, Rockler B, Branemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387-416.
3 Armitage GC, Svanberg GK, Loe H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J Clin Periodontol. 1977;4:173-190.
4 Assael LA. Oral bisphosphonates as a cause of bisphosphonate-related osteonecrosis of the jaws: clinical findings, assessment of risks, and preventive strategies. J Oral Maxillofac Surg. 2009;67:35-43.
5 Baig MR, Rajan M. Effects of smoking on the outcome of implant treatment: a literature review. Indian J Dent Res. 2007;18:190-195.
6 Bain CA. Smoking and implant failure–benefits of a smoking cessation protocol. Int J Oral Maxillofac Implants. 1996;11:756-759.
7 Bain CA, Moy PK. The association between the failure of dental implants and cigarette smoking. Int J Oral Maxillofac Implants. 1993;8:609-615.
8 Bain CA, Weng D, Meltzer A, et al. A meta-analysis evaluating the risk for implant failure in patients who smoke. Compend Contin Educ Dent. 2002;23:695-699. 702, 704 passim; quiz 708
9 Balshi TJ, Wolfinger GJ. Dental implants in the diabetic patient: a retrospective study. Implant Dent. 1999;8:355-359.
10 Baxter JC, Fattore L. Osteoporosis and osseointegration of implants. J Prosthodont. 1993;2:120-125.
11 Beikler T, Flemmig TF. Implants in the medically compromised patient. Crit Rev Oral Biol Med. 2003;14:305-316.
12 Belser UC, Buser D, Hess D, et al. Aesthetic implant restorations in partially edentulous patients—a critical appraisal. Periodontol 2000. 1998;17:132-150.
13 Berglundh T, Lindhe J, Marinello C, Ericsson I, Liljenberg B. Soft tissue reaction to de novo plaque formation on implants and teeth. An experimental study in the dog. Clin Oral Implants Res. 1992;3:1-8.
14 Beumer J3rd, Roumanas E, Nishimura R. Advances in osseointegrated implants for dental and facial rehabilitation following major head and neck surgery. Semin Surg Oncol. 1995;11:200-207.
15 Block MS, Gardiner D, Kent JN, et al. Hydroxyapatite-coated cylindrical implants in the posterior mandible: 10-year observations. Int J Oral Maxillofac Implants. 1996;11:626-633.
16 Blomqvist JE, Alberius P, Isaksson S, et al. Factors in implant integration failure after bone grafting: an osteometric and endocrinologic matched analysis. Int J Oral Maxillofac Surg. 1996;25:63-68.
17 Bragger U, Burgin WB, Hammerle CH, et al. Associations between clinical parameters assessed around implants and teeth. Clin Oral Implants Res. 1997;8:412-421.
18 Branemark P, Zarb GA, Albrektsson T. Tissue-integrated prostheses. Chicago: Quitessence; 1985.
19 Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399-410.
20 Branemark PI, Adell R, Breine U, et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3:81-100.
21 Buser D, Weber HP, Bragger U. The treatment of partially edentulous patients with ITI hollow-screw implants: presurgical evaluation and surgical procedures. Int J Oral Maxillofac Implants. 1990;5:165-175.
22 Cooper L, Moriarty J. Prosthodontic and periodontal considerations for implant-supported dental restorations. Curr Opin Periodontol. 1997;4:119-126.
23 Coulthard P, Esposito M, Worthington HV, et al. Therapeutic use of hyperbaric oxygen for irradiated dental implant patients: a systematic review. J Dent Educ. 2003;67:64-68.
24 Dao TT, Anderson JD, Zarb GA. Is osteoporosis a risk factor for osseointegration of dental implants? Int J Oral Maxillofac Implants. 1993;8:137-144.
25 DeBruyn H, Collaert B. The effect of smoking on ealry implant failure. Clin Oral Implants Res. 1994;5:260.
26 Elders PJ, Netelenbos JC, Lips P, et al. Accelerated vertebral bone loss in relation to the menopause: a cross-sectional study on lumbar bone density in 286 women of 46 to 55 years of age. Bone Miner. 1988;5:11-19.
27 Ericsson I, Lindhe J. Probing depth at implants and teeth. An experimental study in the dog. J Clin Periodontol. 1993;20:623-627.
28 Esposito M, Hirsch JM, Lekholm U, et al. Failure patterns of four osseointegrated oral implant systems. J Mater Sci Mater Med. 1997;8:843-847.
29 Esposito M, Hirsch JM, Lekholm U, et al. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106:527-551.
30 Esposito M, Hirsch JM, Lekholm U, et al. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci. 1998;106:721-764.
31 Esposito M, Worthington HV, Thomsen P, et al. Interventions for replacing missing teeth: maintaining health around dental implants. Cochrane Database Syst Rev. 2004:CD003069.
32 Ferrigno N, Laureti M, Fanali S, et al. A long-term follow-up study of non-submerged ITI implants in the treatment of totally edentulous jaws. Part I: Ten-year life table analysis of a prospective multicenter study with 1286 implants. Clin Oral Implants Res. 2002;13:260-273.
33 Fiorellini JP, Chen PK, Nevins M, et al. A retrospective study of dental implants in diabetic patients. Int J Periodontics Restorative Dent. 2000;20:366-373.
34 Fiorellini JP, Nevins ML. Localized ridge augmentation/preservation. A systematic review. Ann Periodontol. 2003;8:321-327.
35 Friberg B, Ekestubbe A, Mellstrom D, et al. Branemark implants and osteoporosis: a clinical exploratory study. Clin Implant Dent Relat Res. 2001;3:50-56.
36 Friberg B, Jemt T, Lekholm U. Early failures in 4,641 consecutively placed Branemark dental implants: a study from stage 1 surgery to the connection of completed prostheses. Int J Oral Maxillofac Implants. 1991;6:142-146.
37 Fugazzotto PA, Gulbransen HJ, Wheeler SL, et al. The use of IMZ osseointegrated implants in partially and completely edentulous patients: success and failure rates of 2,023 implant cylinders up to 60+ months in function. Int J Oral Maxillofac Implants. 1993;8:617-621.
38 Fujimoto T, Niimi A, Nakai H, et al. Osseointegrated implants in a patient with osteoporosis: a case report. Int J Oral Maxillofac Implants. 1996;11:539-542.
39 Glaser DL, Kaplan FS. Osteoporosis. Definition and clinical presentation. Spine. 1997;22:12S-16S.
40 Granstrom G, Tjellstrom A, Branemark PI. Osseointegrated implants in irradiated bone: a case-controlled study using adjunctive hyperbaric oxygen therapy. J Oral Maxillofac Surg. 1999;57:493-499.
41 Granstrom G, Tjellstrom A, Branemark PI, et al. Bone-anchored reconstruction of the irradiated head and neck cancer patient. Otolaryngol Head Neck Surg. 1993;108:334-343.
42 Grondahl K, Lekholm U. The predictive value of radiographic diagnosis of implant instability. Int J Oral Maxillofac Implants. 1997;12:59-64.
43 Hammerle CH, Jung RE, Feloutzis A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J Clin Periodontol. 2002;29(Suppl 3):226-231. discussion 232-223
44 Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol. 1997;26:3-15.
45 Kapur KK, Garrett NR, Hamada MO, et al. A randomized clinical trial comparing the efficacy of mandibular implant-supported overdentures and conventional dentures in diabetic patients. Part I: Methodology and clinical outcomes. J Prosthet Dent. 1998;79:555-569.
46 Kenney EB, Kraal JH, Saxe SR, et al. The effect of cigarette smoke on human oral polymorphonuclear leukocytes. J Periodontal Res. 1977;12:227-234.
47 Kirsch A, Ackermann KL. The IMZ osteointegrated implant system. Dent Clin North Am. 1989;33:733.
48 Klokkevold PR, Han TJ. How do smoking, diabetes, and periodontitis affect outcomes of implant treatment? Int J Oral Maxillofac Implants. 2007;22(Suppl):173-202.
49 Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990;17:714-721.
50 Lang NP, Wetzel AC, Stich H, et al. Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin Oral Implants Res. 1994;5:191-201.
51 Lekholm U, Gunne J, Henry P, et al. Survival of the Branemark implant in partially edentulous jaws: a 10-year prospective multicenter study. Int J Oral Maxillofac Implants. 1999;14:639-645.
52 Lekholm U, Van Steenberghe D, Herrmann I, et al. Osseointegrated implants in the treatment of partially endentulous jaws: a prospective 5-year multicenter study. Int J Oral Maxillofac Implants. 1994;9:627.
53 Lindh T, Gunne J, Tillberg A, et al. A meta-analysis of implants in partial edentulism. Clin Oral Implants Res. 1998;9:80-90.
54 Lindquist LW, Carlsson GE, Jemt T. Association between marginal bone loss around osseointegrated mandibular implants and smoking habits: a 10-year follow-up study. J Dent Res. 1997;76:1667-1674.
55 Lindquist LW, Rockler B, Carlsson GE. Bone resorption around fixtures in edentulous patients treated with mandibular fixed tissue-integrated prostheses. J Prosthet Dent. 1988;59:59-63.
56 Marx RE. Oral & intravenous bisphosphonate-induced osteonecrosis of the jaws: history, etiology, prevention, and treatment. Chicago: Quintessence Pub. Co; 2007. pp viii, 150 p.
57 Marx RE, Sawatari Y, Fortin M, et al. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567-1575.
58 Meredith N, Alleyne D, Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261-267.
59 Mombelli A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000. 2002;28:177-189.
60 Mombelli A, Muhle T, Bragger U, et al. Comparison of periodontal and peri-implant probing by depth-force pattern analysis. Clin Oral Implants Res. 1997;8:448-454.
61 Mombelli A, van Oosten MA, Schurch EJr, et al. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 1987;2:145-151.
62 Olson JW, Shernoff AF, Tarlow JL, et al. Dental endosseous implant assessments in a type 2 diabetic population: a prospective study. Int J Oral Maxillofac Implants. 2000;15:811-818.
63 Pjetursson BE, Tan K, Lang NP, et al. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin Oral Implants Res. 2004;15:667-676.
64 Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5:254-259.
65 Pylant T, Triplett RG, Key MC, et al. A retrospective evaluation of endosseous titanium implants in the partially edentulous patient. Int J Oral Maxillofac Implants. 1992;7:195-202.
66 Rams TE, Link CCJr. Microbiology of failing dental implants in humans: electron microscopic observations. J Oral Implantol. 1983;11:93-100.
67 Sanz M, Newman MG, Nachnani S, et al. Characterization of the subgingival microbial flora around endosteal sapphire dental implants in partially edentulous patients. Int J Oral Maxillofac Implants. 1990;5:247-253.
68 Sennerby L, Roos J. Surgical determinants of clinical success of osseointegrated oral implants: a review of the literature. Int J Prosthodont. 1998;11:408-420.
69 Shernoff AF, Colwell JA, Bingham SF. Implants for type II diabetic patients: interim report. VA Implants in Diabetes Study Group. Implant Dent. 1994;3:183-185.
70 Sullivan DY, Sherwood RL, Porter SS. Long-term performance of Osseotite implants: a 6-year clinical follow-up. Compend Contin Educ Dent. 2001;22:326-328. 330, 332-324
71 Sunden S, Grondahl K, Grondahl HG. Accuracy and precision in the radiographic diagnosis of clinical instability in Branemark dental implants. Clin Oral Implants Res. 1995;6:220-226.
72 Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003;8:328-343.
73 Wennstrom JL, Bengazi F, Lekholm U. The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin Oral Implants Res. 1994;5:1-8.
74 Zitzmann NU, Marinello CP. Treatment plan for restoring the edentulous maxilla with implant-supported restorations: removable overdenture versus fixed partial denture design. J Prosthet Dent. 1999;82:188-196.