Nutrition for Oral and Dental Health
Sections of this chapter were written by Riva Touger-Decker, PhD, RD, FADA for the previous edition of this text.
Diet and nutrition play key roles in tooth development, integrity of the gingiva (gums) and mucosa, bone strength, and the prevention and management of diseases of the oral cavity. Diet has a local effect on tooth integrity; the type, form, and frequency of foods and beverages consumed have a direct effect on the oral pH and microbial activity, which may promote dental decay. Nutrition systemically affects the development, maintenance, and repair of teeth and oral tissues.
Nutrition and diet affect the oral cavity, but the reverse is also true; that is, the status of the oral cavity may affect one’s ability to consume an adequate diet and achieve nutritional balance. Indeed, there is a lifelong synergy between nutrition and the integrity of the oral cavity in health and disease related to the known roles of diet and nutrients in the growth, development, and maintenance of the oral cavity structure, bones, and tissues (Touger-Decker et al., 2007).
Nutrition For Tooth Development
Primary tooth development begins at 2 to 3 months’ gestation. Mineralization begins at approximately 4 months’ gestation and continues through the preteen years. Therefore maternal nutrition must supply the preeruptive teeth with the appropriate building materials. Inadequate maternal nutrition consequently affects tooth development.
Teeth are formed by the mineralization of a protein matrix. In dentin, protein is present as collagen, which depends on vitamin C for normal synthesis. Vitamin D is essential to the process by which calcium and phosphorus are deposited in crystals of hydroxyapatite, a naturally occurring form of calcium and phosphorus that is the mineral component of enamel and dentin. Fluoride added to the hydroxyapatite provides unique caries-resistant properties to teeth in both prenatal and postnatal developmental periods.
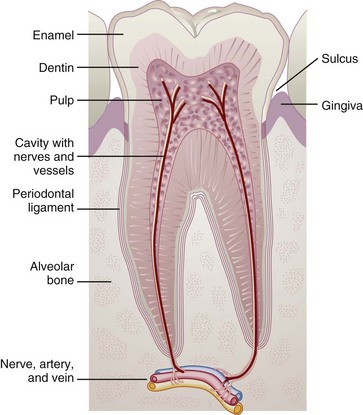
Diet and nutrition are important in all phases of tooth development, eruption, and maintenance (Figure 26-1). Posteruption, diet and nutrient intake continue to affect tooth development and mineralization, enamel development and strength, and eruption patterns of the remaining teeth. The local effects of diet, particularly fermentable carbohydrates and eating frequency, affect the production of organic acids by oral bacteria and the rate of tooth decay as described later in this chapter.
Dental Caries
Dental caries is one of the most common infectious diseases. According to a Surgeon General’s report on oral health in 2000, dental caries is seven times more common than hay fever and five times more common than asthma. Unfortunately, differences are evident in caries prevalence; approximately 20% to 25% of U.S. children have 80% of the dental caries. Trends in dental caries have demonstrated that children who come from homes in which parents have at least a college education have fewer caries than children from homes in which parents have less than a college education (Centers for Disease Control and Prevention [CDC], 2010). These differences, or health disparities, may happen as a result of lack of access to care, cost of care not reimbursed by third-party payors (e.g., insurance, Medicaid), lack of knowledge of preventive dental care, or a combination of factors.
Pathophysiology
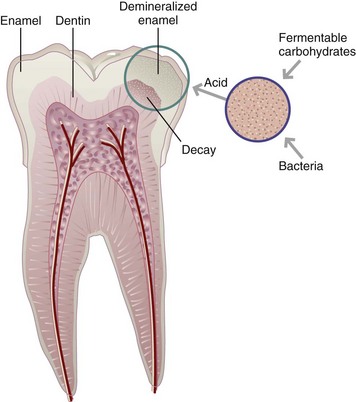
Dental caries is an oral infectious disease in which organic acid metabolites lead to gradual demineralization of tooth enamel, followed by rapid proteolytic destruction of the tooth structure. Caries can occur on any tooth surface. The cause of dental caries involves many factors. Four factors must be present simultaneously: (1) a susceptible host or tooth surface; (2) microorganisms such as Streptococcus or Lactobacillus in the dental plaque or oral cavity; (3) fermentable carbohydrates in the diet, which serve as the substrate for bacteria; and (4) time (duration) in the mouth for bacteria to metabolize the fermentable carbohydrates, produce acids, and cause a drop in salivary pH to less than 5.5. Once the pH is acidic, which can occur within minutes, oral bacteria can initiate the demineralization process. Figure 26-2 shows the formation of dental caries.
Susceptible Tooth
The development of dental caries requires the presence of a tooth that is vulnerable to attack. The composition of enamel and dentin, the location of teeth, the quality and quantity of saliva, and the presence and extent of pits and fissures in the tooth crown are some of the factors that govern susceptibility. Alkaline saliva may have a protective effect, whereas acidic saliva increases susceptibility to decay.
Microorganisms
Bacteria are an essential part of the decay process. Streptococcus mutans is the most prevalent, followed by Lactobacillus casein and Streptococcus sanguis. All three contribute to the process because they metabolize carbohydrates in the mouth, producing acid as a byproduct, which is sufficient to cause decay. Genetic variations of the type and quantity of bacteria present in the oral cavity may put someone at an increased risk for caries and periodontal disease, but the quantity and quality of oral hygiene contributes directly to the risk of oral infectious disease.
Substrate
Fermentable carbohydrates, those carbohydrates susceptible to the actions of salivary amylase, are the ideal substrate for bacterial metabolism. The acids produced by their metabolism cause a drop in salivary pH to less than 5.5, creating the environment for decay. Bacteria are always present and begin to reduce pH when they have exposure to fermentable carbohydrates.
Although both the Dietary Guidelines for Americans and the MyPlate Food Guidance system support a diet high in carbohydrates, it is important to be aware of the cariogenicity of foods. Cariogenicity refers to the caries-promoting properties of a diet or food. The cariogenicity of a food varies, depending on the form in which it occurs, its nutrient composition, when it is eaten in relation to other foods and fluids, the duration of its exposure to the tooth, and the frequency with which it is eaten (Box 26-1).
Individuals should be aware of the form of food consumed and the frequency of intake to integrate positive diet and oral hygiene habits to reduce risk of oral disease.
Fermentable carbohydrates are found in three of the five MyPlate food groups: (1) grains, (2) fruits, and (3) dairy. Although some vegetables may contain fermentable carbohydrates, little has been reported about the cariogenicity, or caries-promoting properties, of vegetables. Examples of grains and starches that are cariogenic by nature of their fermentable carbohydrate composition include crackers, chips, pretzels, hot and cold cereals, and breads.
All fruits (fresh, dried, and canned) and fruit juices may be cariogenic. Fruits with high water content, such as melons, have a lower cariogenicity than others such as bananas and dried fruits. Fruit drinks, sodas, ice teas, and other sugar-sweetened beverages; desserts; cookies; candies; and cake products may be cariogenic. Dairy products sweetened with fructose, sucrose, or other sugars can also be cariogenic because of the added sugars; however, dairy products are rich in calcium, and their alkaline nature may have a positive influence, reducing the cariogenic potential of the food.
Like other sugars (glucose, fructose, maltose, and lactose), sucrose stimulates bacterial activity. The causal relationship between sucrose and dental caries has been established (Marshall, 2007; Moynihan, 2005). All dietary forms of sugar, including honey, molasses, brown sugar, and corn syrup solids, have cariogenic potential and can be used by bacteria to produce organic acid.
Caries Promotion by Individual Foods
It is important to differentiate between cariogenic, cariostatic, and anticariogenic foods. Cariogenic foods are those that contain fermentable carbohydrates, which, when in contact with microorganisms in the mouth, can cause a drop in salivary pH to 5.5 or less and stimulate the caries process.
Cariostatic foods do not contribute to decay, are not metabolized by microorganisms, and do not cause a drop in salivary pH to 5.5 or less within 30 minutes. Examples of cariostatic foods are protein foods such as eggs, fish, meat, and poultry; most vegetables; fats; and sugarless gums. Sugarless gum may help to reduce decay potential because of its ability to increase saliva flow and because it uses noncarbohydrate sweeteners (Deshpande, 2008; Splieth, 2009).
Anticariogenic foods are those that, when eaten before an acidogenic food, prevent plaque from recognizing the acidogenic food. Examples are aged cheddar, Monterey Jack, and Swiss because of the casein, calcium, and phosphate in the cheese. The five-carbon sugar alcohol, xylitol, is considered anticariogenic because bacteria cannot metabolize five-carbon sugars in the same way as six-carbon sugars like glucose, sucrose, and fructose. It is not broken down by salivary amylase and is not subject to bacterial degradation. Salivary stimulation leads to increased buffering activity of the saliva and subsequent increased clearance of fermentable carbohydrates from tooth surfaces. Another anticariogenic mechanism of xylitol gum is that it replaces fermentable carbohydrates in the diet. S. mutans cannot metabolize xylitol and is inhibited by it. Both the antimicrobial activity against S. mutans and the effect of gum chewing on salivary stimulation are protective. Brands of chewing gum that include xylitol are Arm and Hammer Advance White, Dentyne Ice, Spry, and Trident.
Remineralization is mineral restoration of the hydroxyapatite in dental enamel. Casein phosphopeptide-amorphous calcium phosphate (CPP-ACP; Recaldent) is a substance that promotes remineralization of enamel surfaces. It is currently available as an ingredient in Trident White brand chewing gums (Ramalingam, 2005). CPP-ACP has also exhibited anticariogenic activity in randomized, controlled clinical trials of sugar-free gum and a tooth cream (Walker et al., 2010). Its use for that purpose is not suggested at this time.
Factors Affecting Cariogenicity of Food
Cariogenicity also is influenced by the volume and quality of saliva; the sequence, consistency, and nutrient composition of the foods eaten; dental plaque buildup; and the genetic predisposition of the host to decay.
Form and Consistency
The form and consistency of a food have a significant effect on its cariogenic potential and pH-reducing or buffering capacity. Food form determines the duration of exposure or retention time of a food in the mouth, which, in turn, affects how long the decrease in pH or the acid-producing activity will last. Liquids are rapidly cleared from the mouth and have low adherence (or retentiveness) capabilities. Solid foods such as crackers, chips, pretzels, dry cereals, and cookies can stick between the teeth (referred to as the interproximal spaces) and have high adherence (or retention) capability.
Consistency also affects adherence. Chewy foods such as gum drops and marshmallows, although high in sugar content, stimulate saliva production and have a lower adherence potential than solid, sticky foods such as pretzels, bagels, or bananas. High-fiber foods with few or no fermentable carbohydrates, such as popcorn and raw vegetables, are cariostatic.
Exposure
The duration of exposure may be best explained with starchy foods, which are fermentable carbohydrates subject to the action of salivary amylase. The longer starches are retained in the mouth, the greater their cariogenicity (Fontana, 2006). Given sufficient time, such as when food particles become lodged between the teeth, salivary amylase makes additional substrate available as it hydrolyzes starch to simple sugars. Processing techniques, either by partial hydrolysis or by reducing particle size, make some starches rapidly fermentable by increasing their availability for enzyme action.
Sugar-containing candies rapidly increase the amount of sugar available in the oral cavity to be hydrolyzed by bacteria. Sucking on hard candies such as lollipops or sugared breath mints results in prolonged sugar exposure in the mouth. Simple carbohydrate-based snacks and dessert foods (e.g., potato chips, pretzels, cookies, cakes, and doughnuts) provide gradually increasing oral sugar concentrations for a longer duration because these foods often adhere to the tooth surfaces and are retained for longer periods than candies (Fontana, 2006).
Nutrient Composition
Nutrient composition contributes to the ability of a substrate to produce acid and to the duration of acid exposure. Dairy products, by virtue of their calcium- and phosphorus-buffering potential, are considered to have low cariogenic potential. Evidence suggests that cheese and milk, when consumed with cariogenic foods, help to buffer the acid pH produced by the cariogenic foods. Because of the anticariogenic properties of cheese, eating cheese with a fermentable carbohydrate, such as dessert at the end of a meal, may decrease the cariogenicity of the meal and dessert (Moynihan, 2005).
Nuts, which do not contain a significant amount of fermentable carbohydrates and are high in fat and dietary fiber, are cariostatic. Protein foods such as seafood, meats, eggs, and poultry, along with other fats such as oils, margarine, butter, and seeds, are also cariostatic.
Sequence and Frequency of Eating
Eating sequence and combination of foods also affect the caries potential of the substrate. Bananas, which are cariogenic because of their fermentable carbohydrate content and adherence capability, have less potential to contribute to decay when eaten with cereal and milk than when eaten alone as a snack. Milk, as a liquid, reduces the adherence capability of the fruit. Crackers eaten with cheese are less cariogenic than when eaten alone.
The frequency with which a cariogenic food or beverage is consumed determines the number of opportunities for acid production. Every time a fermentable carbohydrate is consumed, a decline in pH is initiated within 5 to 15 minutes, causing caries-promoting activity. Small, frequent meals and snacks, often high in fermentable carbohydrate, increase the cariogenicity of a diet more than a diet consisting of three meals and minimal snacks. Eating several cookies at once, followed by brushing the teeth or rinsing the mouth with water, is less cariogenic than eating a cookie several times throughout a day. Table 26-1 lists messages that can be given to children to prevent caries.
TABLE 26-1
Nutrition Messages Related to Oral Health for 3- to 10-Year-Old Children and Their Caregivers
| Message | Rationale |
| Starchy, sticky, or sugary foods should be eaten with nonsugary foods. | The pH will rise if a nonsugary item that stimulates saliva is eaten immediately before, during, or after a challenge. |
| Combine dairy products with a meal or snack. | Dairy products (nonfat milk, yogurt) enhance remineralization and contain calcium. |
| Combine chewy foods such as fresh fruits and vegetables with fermentable carbohydrates. | Chewy, fibrous foods induce saliva production and buffering capacity. |
| Space eating occasions at least 2 hours apart and limit snack time to 15-30 minutes. | Fermentable carbohydrates eaten sequentially one after another promote demineralization. |
| Limit bedtime snacks. | Saliva production declines during sleep. |
| Limit consumption of acidic foods such as sports drinks, juices, and sodas. | Acidic foods promote tooth erosion that increases risk for caries. |
| Combine proteins with carbohydrates in snacks: Examples: tuna and crackers, apples and cheese |
Proteins act as buffers and are cariostatic. |
| Combine raw and cooked or processed foods in a snack. | Raw foods encourage mastication and saliva production, whereas cooked or processed foods may be more available for bacterial metabolism if eaten alone. |
| Encourage use of xylitol- or sorbitol-based chewing gum and candies immediately following a meal or snack.* | Five minutes of exposure is effective in increasing saliva production and dental plaque pH. |
| Sugar-free chewable vitamin and mineral supplements and syrup-based medication should be recommended. | Sugar-free varieties are available and should be suggested for high–caries risk groups. |
| Encourage children with pediatric GERD to adhere to dietary guidelines. | GERD increases risk for dental erosion and thus increases risk for caries. |
GERD, Gastroesophageal reflux disease.
*Gum is not recommended for children younger than 6 years old.
Modified from Mobley C: Frequent dietary intake and oral health in children 3 to 10 years of age, Building Blocks 25(1):17-20, 2001.
The Decay Process
The carious process begins with the production of acids as a by-product of bacterial metabolism taking place in the dental plaque. Decalcification of the surface enamel continues until the buffering action of the saliva is able to raise the pH above the critical level. See Box 26-2 for prevention guidelines.
Plaque is a sticky, colorless mass of microorganisms and polysaccharides that forms around the tooth and adheres to teeth and gums. It harbors acid-forming bacteria and keeps the organic products of their metabolism in close contact with the enamel surface. As a cavity develops, the plaque blocks the tooth, to some extent, from the buffering and remineralization action of the saliva. In time the plaque combines with calcium and hardens to form calculus.
An acidic pH is also required for plaque formation. Soft drinks (diet and regular), sports beverages, citrus juices and “ades,” and vitamin C supplements have high acid content. Research using National Health and Nutrition Examination Survey III data reported significantly more dental caries in children (ages 2 to 10 years) who consumed large amounts of carbonated soft drinks or juices when compared with children who had high consumption of water or milk (Sohn, 2006). Other beverages and foods contribute to dental erosion, a loss of minerals from tooth surfaces by a chemical process in the presence of acid (Wongkhantee, 2006). For example, diet soft drinks, which may not contain sugar, also are acidic by nature and therefore cause a drop in pH. Chewable vitamin C supplements provide an acidic substance that directly contacts tooth surfaces and causes a drop in pH of the oral cavity, making teeth susceptible to erosion.
Roles of Saliva
Salivary flow clears food from around the teeth. By means of the bicarbonate-carbonic acid and phosphate buffer system, it also provides buffering action to neutralize bacterial acid metabolism. Chewing promotes saliva production and may account for the reduced cariogenicity of fermentable carbohydrates consumed with a meal.
Saliva is supersaturated with calcium and phosphorus. Once buffering action has restored pH above the critical point, remineralization can occur. If fluoride is present in the saliva, the minerals are deposited in the form of fluoroapatite, which is resistant to erosion. It should be noted that salivary production decreases as a result of diseases affecting salivary gland function (e.g., Sjögren’s syndrome); as a side-effect of fasting; as a result of radiation therapy to the head and neck involving the parotid gland; normally during sleep; with the use of medications associated with reduced salivary flow; or with xerostomia, dry mouth caused by inadequate saliva production. There are estimates that between 400 and 500 medications currently available by prescription or over the counter may cause dry mouth. The degree of xerostomia may vary but may be caused by medications such as those to treat depression, hypertension, anxiety, human immunodeficiency virus (HIV), and allergies, to name a few.
Caries Patterns
Caries patterns describe the location and surfaces of the teeth affected. Coronal caries affect the crown of the tooth, the part of the tooth visible above the gum line, and may occur on any tooth surface. Although the overall incidence of decay in the United States has declined, as many as 17% of children between 2 and 4 years of age have tooth decay. According to the National Oral Health Surveillance System, many states report 40% to 70% of children having some decay by age 8.
Root caries, occurring on the root surfaces of teeth secondary to gingival recession, affect a large portion of the older population. Root caries is a dental infection that is increasing in older adults, partly because this population is retaining their natural teeth longer. The gums recede in older age, exposing the root surface. Other factors related to the increased incidence of this decay pattern are lack of fluoridated water, poor oral hygiene practices, decreased saliva, frequent eating of fermentable carbohydrates, and dementia (Chalmers, 2008;). Management of root caries includes dental restoration and nutrition counseling. Poor oral health from caries, pain, or edentulism may adversely affect dietary intake and nutritional status in the older adult (Quandt, 2009).
Lingual caries, or caries on the lingual side (surface next to or toward the tongue) of the anterior teeth, are seen in persons with gastrointestinal reflux, bulimia, or anorexia-bulimia (see Chapter 23). Frequent intake of fermentable carbohydrates, combined with regurgitation or induced vomiting of acidic stomach contents, results in a constant influx of acid into the oral cavity. The caries are the end result of tooth erosion characterized by erosion of the palatal and buccal surfaces of the maxillary anterior teeth and the lingual surfaces of the palatal surface of the maxillary posterior teeth (Holbrook, 2009).
Fluoride
Fluoride is a primary anticaries agent. Used systemically and locally, it is a safe, effective public health measure to reduce the incidence and prevalence of dental caries (Palmer and Wolfe, 2005). Water fluoridation began in 1940; by 1999 the Centers for Disease Control and Prevention listed water fluoridation as one of the top 10 greatest public health achievements of the 20th century because of its influence on decreasing the rate of dental caries (CDC, 2006). The effect of fluoride on caries prevention continues with water fluoridation, fluoridated toothpastes, oral rinses, and dentifrices, as well as beverages made with fluoridated water. Optimal water fluoridation concentrations (0.7 to 1.2 ppm) can provide protection against caries development without causing tooth staining (Palmer and Wolfe, 2005). See Focus On: Water Fluoridation.
Mechanism of Action
There are four primary mechanisms of fluoride action on teeth: (1) when incorporated into enamel and dentin along with calcium and phosphorus, it forms fluoroapatite, a compound more resistant to acid challenge than hydroxyapatite; (2) it promotes repair and remineralization of tooth surfaces with early signs of decay (incipient carious lesions); (3) it helps to reverse the decay process while promoting the development of a tooth surface that has increased resistance to decay; and (4) helps to deter the harmful effects of bacteria in the oral cavity by interfering with the formation and function of microorganisms.
Food Sources
Most foods, unless prepared with fluoridated water, contain minimal amounts of fluoride, except for brewed tea, which has approximately 1.4 ppm (Morin, 2006). Fluoride may be unintentionally added to the diet in a number of ways, including through the use of fluoridated water in the processing of foods and beverages. Fruit juices and drinks, particularly white grape juice produced in cities with fluoridated water, may have increased fluoride content; however, because of the wide variation in fluoride content, it is difficult to estimate amounts consumed.
Supplementation
It is prudent for health professionals to consider a child’s fluid intake as well as food sources and the availability of fluoridated water in the community before prescribing fluoride supplements. Because bones are repositories of fluoride, bone meal, fish meal, and gelatin made from bones are potent sources of the mineral. In communities without fluoridated water, dietary fluoride supplements may be recommended for children ages 6 months to 16 years.
Fluoride can be used topically and systemically. When consumed in food and drink, it enters the systemic circulation and is deposited in bones and teeth. Systemic sources have a topical benefit as well by providing fluoride to the saliva. A small amount of fluoride enters the soft tissues; the remainder is excreted. The primary source of systemic fluoride is fluoridated water; food and beverages supply a smaller amount. Table 26-2 contains a schedule of fluoride supplementation for the public through age 16.
TABLE 26-2
Dietary Fluoride Supplement Schedule
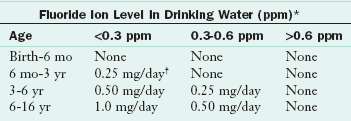
*1 ppm = 1 mg/L.
†2.2 mg of sodium fluoride contains 1 mg of fluoride ion.
Approved by The American Dental Association, The American Academy of Pediatrics, and The American Academy of Pediatric Dentistry, 1994.
Fluoride supplements are not recommended for formula-fed infants or for breast-fed infants living in fluoridated communities if these infants receive drinking water between feedings. If the infant does not drink water between feedings or drinks bottled water when on a diet of only breast milk, he or she should be supplemented according to the fluoride supplement guidelines. Fluoride supplements must be prescribed by the child’s doctor; they are not available as over-the-counter supplements (American Dental Association, 2005).
Topical fluoride sources include toothpastes, gels, and rinses used by consumers daily, along with more concentrated forms applied by dental professionals in the form of gels, foams, and rinses. Frequent fluoride exposure via topical fluorides, fluoridated toothpastes, rinses, and fluoridated water is important in maintaining an optimal concentration of fluoride, but excess intake should be avoided. (See Chapter 3.)
Excess Fluoride
Fluorosis occurs when too much fluoride is provided during tooth development and can range from mild to severe and present on teeth from unnoticeable to very apparent dark spots on teeth (Alvarez, 2009). Causes of mild fluorosis from excessive fluoride intake include misuse of dietary fluoride supplements, ingestion of fluoridated toothpastes and rinses, or excessive fluoride intake secondary to fluoride in foods and beverages processed in fluoridated areas and transported to other areas (Palmer and Wolfe, 2005). Topical fluorides, available as fluoridated toothpaste and mouthwashes, are effective sources of fluoride that can be used in the home, school, or dental office. Caries prevention efforts in preschool children include diet modification, water fluoridation or supplements in nonfluoridated areas, and supervised toothbrushing with fluoridated toothpaste (Alvarez, 2009).
Children younger than 6 years of age should not use fluoridated mouthwashes, and older children should be instructed to rinse, but not swallow, mouthwash. No more than a pea-size amount of toothpaste should be placed on a child’s toothbrush to reduce the risk of accidental fluoride ingestion. Topical fluorides may be administered in the dental office.
Fluoride gels often are prescribed for adults and older adults. Such gels are effective in reducing the risk of coronal and root decay and tooth loss (Weintraub, 2006). Fluoride is most effective when given from birth through ages 12 to 13, the period when mineralization of unerupted permanent teeth occurs.
Early Childhood Caries
Early childhood caries (ECC), often called “baby-bottle tooth decay,” describes a caries pattern in the maxillary anterior teeth of infants and young children. Characteristics include rapidly developing carious lesions in the primary anterior teeth and the presence of lesions on tooth surfaces not usually associated with a high caries risk. Because tooth decay remains a common oral disease of childhood, caries are a primary marker for a child’s oral health. Good behavioral habits and child nutrition patterns must be encouraged, beginning in infancy.
Pathophysiology and Incidence
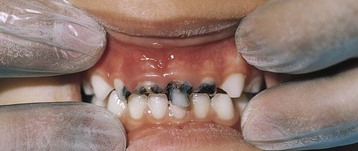
Often ECC follows prolonged bottle-feeding, especially at night, of juice, milk, formula, or other sweetened beverages. The extended contact time with the fermentable carbohydrate–containing beverages, coupled with the position of the tongue against the nipple, which causes pooling of the liquid around the maxillary incisors, particularly during sleep, contributes to the decay process. The mandibular anterior teeth are usually spared (Figure 26-3) because of the protective position of the lip and tongue and the presence of a salivary duct in the floor of the mouth. In general, children from low-income families and minority populations experience the greatest amount of oral disease, the most extensive disease, and the most frequent use of dental services for pain relief; yet these children have the fewest overall dental visits (CDC, 2010).
Nutrition Care
Management of ECC includes diet and oral hygiene education for parents, guardians, and caregivers (Zero, 2010). Messages should be targeted to counter the health habits that contribute to this problem: poor oral hygiene, failure to brush a child’s teeth at least daily, frequent use of bottles filled with sweetened beverages, and lack of fluoridated water. Dietary guidelines include removal of the bedtime bottle and modification of the frequency and content of the daytime bottles. Bottle contents should be limited to water, formula, or milk. Infants and young children should not be put to bed with a bottle. Teeth and gums should be cleaned with a gauze pad or washcloth after all bottle feedings. All efforts should be made to wean children from a bottle by 1 year of age. Educational efforts should be positive and simple, focusing on oral hygiene habits and promotion of a balanced, healthy diet. Between-meal snacks should include cariostatic foods. When foods are cariogenic, they should be followed by tooth brushing or rinsing the mouth. Parents and caregivers need to understand the causes and consequences of ECC and how they can be avoided.
Caries Prevention
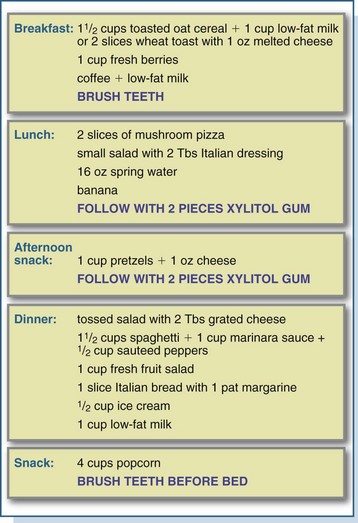
Caries prevention programs focus on a balanced diet, modification of the sources and quantities of fermentable carbohydrates, and the integration of oral hygiene practices into individual lifestyles (Zero, 2010). Meals and snacks should be followed with brushing, rinsing the mouth vigorously with water, or chewing sugarless gum for 15 to 20 minutes, preferably gum that contains xylitol (Splieth, 2009). Positive habits should be encouraged, including snacking on anticariogenic or cariostatic foods, chewing sugarless gum after eating or drinking cariogenic items, and having sweets with meals rather than as snacks. Despite the potential for a diet that is based on the dietary guidelines to be cariogenic, with proper planning and good oral hygiene a balanced diet low in cariogenic risk can be planned. See Figure 26-4 for a sample diet.
Practices to avoid include sipping carbonated beverages for extended periods; frequent snacking; and harboring candy, sugared breath mints, or hard candies in the mouth for extended periods. Over-the-counter chewable or liquid medications and vitamin preparations, such as chewable vitamin C or liquid cough syrup, may contain sugar and contribute to caries risk.
Fermentable carbohydrates such as candy, crackers, cookies, pastries, pretzels, snack crackers, chips, and even fruits should be eaten with meals. Notably, “fat-free” snack and dessert items and “baked” chips and snack crackers tend to have a higher simple sugar concentration than their higher fat-containing counterparts.
Tooth Loss and Dentures
Tooth loss (edentulism) and removable prostheses (dentures) can have a significant effect on dietary habits, masticatory function, olfaction, and nutritional adequacy. As dentition status declines, masticatory performance is compromised. Compromised masticatory function, from partial or complete edentulism or complete dentures, may have a negative effect on food choices, resulting in decreased intake of whole grains, fruits, and vegetables (Tsakos, 2010). This problem is more pronounced in older adults, whose appetite and intake may be compromised further by chronic disease, social isolation, and the use of multiple medications (see Chapter 9).
Dentures need to be checked periodically by a dental professional for appropriate fit. Changes in body weight or changes in alveolar bone over time possibly may alter the fit of the dentures. Counseling on appropriate food choices and textures is advocated.
Nutrition Care
Full dentures replace missing teeth but are not a perfect substitute for natural dentition. Both before and after denture placement, many individuals may experience difficulty biting and chewing, even after denture insertion. The foods found to cause the greatest difficulty for persons with complete dentures include fresh whole fruits and vegetables (e.g., apples and carrots), hard-crusted breads, and steak. Therefore dietary assessment and counseling related to oral health should be provided to the denture wearer. Simple guidelines should be provided for cutting and preparing fruits and vegetables to minimize the need for biting and reduce the amount of chewing. The importance of positive eating habits needs to be stressed as a component of total health. Overall, guidelines that reinforce the importance of a balanced diet should be part of the routine counseling given to all patients.
Other Oral Disorders
Oral diseases extend beyond dental caries. Deficiencies of several vitamins (riboflavin, folate, B12, and C) and minerals (iron and zinc) may be first detected in the oral cavity because of the rapid tissue turnover of the oral mucosa (see Appendix 30). Periodontal disease is a local and systemic disease. Select nutrients play a role, including vitamins A, C, E; folate; β-carotene; and the minerals calcium, phosphorus, and zinc. Oral cancer, often a result of tobacco and alcohol abuse, can have a significant effect on eating ability and nutritional status. This problem is compounded by the increased caloric and nutrient needs of persons with oral carcinomas. In addition, surgery, radiation therapy, and chemotherapy are modalities used to treat oral cancer that also can affect dietary intake, appetite, and the integrity of the oral cavity. Some but not all problems affecting the oral cavity are discussed here.
Periodontal Disease
Periodontal disease is an inflammation of the gingiva with infection caused by oral bacteria and subsequent destruction of the tooth attachment apparatus. Untreated disease results in a gradual loss of tooth attachment to the bone. Progression is influenced by the overall health of the host and the integrity of the immune system. The primary causal factor in the development of periodontal disease is plaque. Plaque in the gingival sulcus, a shallow, V-shaped space around the tooth, produces toxins that destroy tissue and permit loosening of the teeth. Important factors in the defense of the gingiva to bacterial invasion are (1) oral hygiene, (2) integrity of the immune system, and (3) optimal nutrition. The defense mechanisms of the gingival tissue, epithelial barrier, and saliva are affected by nutritional intake and status. Healthy epithelial tissue prevents the penetration of bacterial endotoxins into subgingival tissue.
Nutritional Care
Deficiencies of vitamin C, folate, and zinc increase the permeability of the gingival barrier at the gingival sulcus, increasing susceptibility to periodontal disease. Severe deterioration of the gingiva is seen in individuals with scurvy or vitamin C deficiency. Although other nutrients, including vitamins A, E, β-carotene, and protein, have a role in maintaining gingival and immune system integrity, there are no scientific data to support supplemental uses of any of these nutrients to treat periodontal disease.
Although optimal nutrition may play a role in positive outcomes of periodontal treatment, nutrients alone are not a cure for the disease (Schifferle, 2005). In societies in which malnutrition and periodontal disease are prevalent, poor oral hygiene is also usually evident. In such instances it is difficult to determine whether malnutrition is the cause of the disease or one of many contributing factors, including poor oral hygiene, heavy plaque buildup, insufficient saliva, or coexisting illness.
The roles of calcium and vitamin D relate to the link between osteoporosis and periodontal disease, in which bone loss is the common denominator. The association between periodontal disease and systemic osteopenia and osteoporosis has been documented (Jeffcoat, 2005) (see Chapter 25). Because dairy foods are a rich source of calcium and vitamin D, researchers documented an inverse relationship between increased dairy food intake and decreased incidence of periodontal disease (Al-Zahrani, 2006). Although causal relationships have not been determined, the association of calcium and dairy foods with periodontal disease warrants advocating a sufficient intake of dairy foods in those who tolerate them. Management strategies for the patient or client with periodontal disease follow many of the same guidelines as for caries prevention listed in Box 26-2.
Severe periodontal disease may be treated surgically. Diet adequacy is particularly important both before and after periodontal surgery, when adequate nutrients are needed to regenerate tissue and support immunity to prevent infection. Adequacy of calories, protein, and micronutrients should be ensured. If the ability to consume one’s regular diet is altered, a diet modified in consistency can be individually designed. Oral supplements can be used when necessary to attain adequate nutrient intake.
Oral Manifestations of Systemic Disease
Acute systemic diseases such as cancer and infections, as well as chronic diseases such as diabetes mellitus, autoimmune diseases, and chronic kidney disease, are characterized by oral manifestations that may alter the diet and nutritional status. Cancer therapies, including irradiation of the head and neck region, chemotherapy, and surgeries to the oral cavity, have a significant effect on the integrity of the oral cavity and on an individual’s eating ability, which may consequently affect nutrition status (see Chapter 37).
If the condition of the mouth adversely affects one’s food choices, the person with chronic disease may not be able to follow the optimal diet for medical nutrition therapy. For example, poorly controlled diabetes may manifest in xerostomia or candidiasis, which may then affect the ability to consume a diet to appropriately control blood sugar, further deteriorating glucose control.
In addition, many medications alter the integrity of the oral mucosa, taste sensation, or salivary production (see Chapter 9). Phenytoin (Dilantin) may cause severe gingivitis. Many of the protease inhibitor drugs used to treat HIV and acquired immune deficiency syndrome (AIDS) are associated with altered taste and dry mouth. Care should be taken to assess the effects of medication on the oral cavity and minimize these effects using alterations in diet or drug therapy.
Diabetes Mellitus
Diabetes is associated with several oral manifestations, many of which occur only in periods of poor glucose control. These include burning mouth syndrome, periodontal disease, candidiasis, dental caries, and xerostomia (Lamster, 2008). The microangiopathic conditions seen in diabetes, along with altered responses to infection, contribute to risk of periodontal disease in affected persons. Tooth infection, more common in those with diabetes, leads to deterioration of diabetes control (Bender and Bender, 2003). Besides blood glucose control, dietary management for people with diabetes after any oral surgery procedures or placement of dentures should include modifications in the consistency, temperature, and texture of food to increase eating comfort, reduce oral pain, and prevent infections or decay while managing glucose control (see Chapter 31).
Fungal Infections
Oropharyngeal fungal infections may cause a burning, painful mouth and dysphagia. The ulcers that accompany viral infections such as herpes simplex and cytomegalovirus cause pain and can lead to reduced oral intake. Very hot and cold foods or beverages, spices, and sour or tart foods also may cause pain and should be avoided. Consumption of temperate, moist foods without added spices should be encouraged. Small, frequent meals followed by rinsing with lukewarm water or brushing to reduce the risk of dental caries are helpful. Once the type and extent of oral manifestations are identified, a nutrition care plan can be developed. Oral high calorie–high protein supplements in liquid or pudding form may be needed to meet nutrient needs and optimize healing.
Head and Neck Cancers
Head, neck, and oral cancers can alter eating ability and nutrition status because of the surgeries and therapies used to treat these cancers. Surgery, depending on the location and extent, may alter eating or swallowing ability, as well as the capacity to produce saliva. Radiation therapy of the head and neck area and chemotherapeutic agents can affect the quantity and quality of saliva and the integrity of the oral mucosa. Thick, ropey saliva is often the result of radiation therapy to the head and neck area, causing xerostomia. Dietary management focuses on the recommendations described earlier for xerostomia, along with modifications in food consistency following surgery (see Chapter 37).
HIV Infection and AIDS
Viral and fungal infections, stomatitis, xerostomia, periodontal disease, and Kaposi sarcoma are oral manifestations of HIV that can cause limitations in nutrient intake and result in weight loss and compromised nutrition status. These infections often are compounded by a compromised immune response, preexisting malnutrition, and gastrointestinal consequences of HIV infection (see Chapter 38). Viral diseases, including herpes simplex and cytomegalovirus, result in painful ulcerations of the mucosa.
Stomatitis, or inflammation of the oral mucosa, causes severe pain and ulceration of the gingiva, oral mucosa, and palate, which makes eating painful. Candidiasis on the tongue, palate, or esophagus can make chewing, sucking, and swallowing painful (odynophagia), thus compromising intake. Table 26-3 outlines the effects of associated oral infections.
Xerostomia
Xerostomia (dry mouth) is seen in poorly controlled diabetes mellitus, Sjögren’s syndrome, other autoimmune diseases, and as a consequence of radiation therapy and certain medications (Box 26-3). Xerostomia from radiation therapy may be more permanent than that from other causes (Kielbassa et al., 2006). Radiation therapy procedures to spare the parotid gland should be implemented when possible to reduce the damage to the salivary gland. Efforts to stimulate saliva production using pilocarpine and citrus-flavored, sugar-free candies may ease eating difficulty.
Individuals without any saliva at all have the most difficulty eating; artificial salivary agents may not offer sufficient relief. Lack of saliva impedes all aspects of eating, including chewing, forming a bolus, swallowing, and sensing taste; causes pain; and increases the risk of dental caries and infections. Dietary guidelines focus on the use of moist foods without added spices, increased fluid consumption with and between all meals and snacks, and judicious food choices.
Problems with chewy (steak), crumbly (cake, crackers, rice), dry (chips, crackers), and sticky (peanut butter) foods are common in persons with severe xerostomia. Alternatives should be suggested, or the foods should be avoided to avert dysphagia risk. Drinking water with a lemon or lime twist or citrus-flavored seltzers or sucking on frozen tart grapes, berries, or sugar-free candies may help. Good oral hygiene habits are important in reducing the risk of tooth decay and should be practiced after all meals and snacks. Flavored gums and mints containing xylitol may help to reduce the risk of associated decay.
American Academy of Pediatric Dentistry
American Dental Hygienists Association
American Academy of Periodontology
http://www.nidcr.nih.gov/HealthInformation/DiseasesAndConditions/DiabetesAndOralHealth/default.htm
http://www.diabetes.org/living-with-diabetes/treatment-and-care/oral-health-and-hygiene/
National Institute of Dental and Craniofacial Research
Surgeon General Report on Oral Health
http://www.surgeongeneral.gov/library/oralhealth/
References
Alvarez, JA, et al. Dental fluorosis: exposure, prevention and management. Med Oral Patol Oral Cir Bucal. 2009;14:E103.
Al-Zahrani, MS. Increased intake of dairy products is related to lower periodontitis prevalence. J Periodontol. 2006;77:289.
American Dental Association. Council on Access Prevention and Interprofessional Relations: fluoridation facts. http://www.ada.org/sections/newsAndEvents/pdfs/fluoridation_facts.pdf, 2005. [Accessed 22 April 2010 from].
Bender, IB, Bender, AB. Diabetes mellitus and the dental pulp. Journal of Endodontics. 2003;29:383.
Centers for Disease Control and Prevention (CDC). National Oral Health Surveillance System. http://apps.nccd.cdc.gov/nohss/IndicatorV.asp?Indicator=2, 2006. [Accessed 22 April 2010 from].
Centers for Disease Control and Prevention (CDC). Improving oral health: preventing cavities, gum disease, tooth loss, and oral cancer. http://www.cdc.gov/chronicdisease/resources/publications/AAG/doh.htm, 2010. [Accessed 22 April 2010 from].
Chalmers, JM, Ettinger, RL. Public health issues in geriatric dentistry in the United States. Dental Clinics of North America. 2008;52:423.
Deshpande, A, Jadad, AR. The impact of polyol-containing chewing gums on dental caries: asystematic review of original randomized trials and observational studies. J Am Dent Assoc. 2008;139:1602.
Fontana, M, Zero, DT. Assessing patients’ caries risk. J Am Dent Assoc. 2006;137:1231.
Holbrook, WP, et al. Gastric reflux is a significant causative factor of tooth erosion. J Dent Res. 2009;88:422.
Jeffcoat, M. The association between osteoporosis and oral bone loss. J Periodontol. 2005;76:2125S.
Kielbassa, AM, et al. Radiation-related damage to dentition. Lancet Oncol. 2006;7:326.
Lamster, IB, et al. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139:19S.
Marshall, TA, et al. Comparison of the intakes of sugars by young children with and without dental caries experience. J Am Dent Assoc. 2007;138:39.
Morin, K. Fluoride: action and use. MCN Am J Matern Child Nurs. 2006;31:127.
Moynihan, P. The interrelationship between diet and oral health. Proc Nutr Soc. 2005;64:571.
Palmer, C, Wolfe, SH. Position of the American Dietetic Association: the impact of fluoride on health. J Am Diet Assoc. 2005;105:1620.
Quandt, SA, et al. Food avoidance and food modification practices of older rural adults: association with oral health status and implications for service provision. Gerontologist. 2009;50:100.
Ramalingam, L, et al. Adding casein phosphopeptide-amorphous calcium phosphate to sports drinks to eliminate in vitro erosion. Pediatr Dent. 2005;27:61.
Schifferle, RE. Nutrition and periodontal disease. Dent Clin North Am. 2005;49:595.
Sohn, WB, et al. Carbonated soft drinks and dental caries in the primary dentition. J Dent Res. 2006;85:262.
Splieth, CH, et al. Effect of xylitol and sorbitol on plaque acidogenesis. Quintessence Int. 2009;40:279.
Touger-Decker, R, et al. Position of the American Dietetic Association: oral health and nutrition. J Am Diet Assoc. 2007;107:1418.
Tsakos, GK, et al. Edentulism and fruit and vegetable intake in low-income adults. J Dent Res. 2010;89:462.
Walker, GD, et al. Casein phosphopeptide-amorphous calcium phosphate incorporated into sugar confections inhibits the progression of enamel subsurface lesions in situ. Caries Res. 2010;44:33.
Weintraub, JA, et al. Fluoride varnish efficacy in preventing early childhood caries. J Dent Res. 2006;85:172.
Wongkhantee, SV, et al. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006;34:214.
Zero, DT, et al. The biology, prevention, diagnosis and treatment of dental caries: scientific advances in the United States. J Am Dental Assoc. 2010;1:25S.