Medical Nutrition Therapy for Cancer Prevention, Treatment, and Recovery
Cancer involves the abnormal division and reproduction of cells that can spread throughout the body. Usually thought of as a single disease, cancer actually consists of more than 100 distinct types. The American Cancer Society (ACS) predicts the lifetime risk for developing cancer in the United States is slightly less than half of men and a little more than one third of women (American Cancer Society [ACS], 2009a). Annually in the United States, cancer is responsible for almost one out of every four deaths (ACS, 2009a). Evidence suggests that one third of the more than 560,000 cancer deaths may be attributed to nutrition and lifestyle behaviors such as poor diet, physical inactivity, alcohol use, and overweight and obesity. Almost an additional 171,000 cancer deaths are caused by tobacco use (ACS, 2010). It is estimated that 50% to 70% of cancer deaths are potentially preventable by decreasing high-risk behaviors; with approximately 30% of cancer deaths attributed to tobacco use and at least an additional 30% to poor nutrition (Brawer et al, 2009).
The cost of cancer care in the United States has doubled in the past 20 years to more than $48 billion annually (NCI, 2010a). Private insurance pays for 50% of the cost, Medicare coverage accounts for 34%, and Medicaid payment and other public programs cover the difference. Most medical care spending for cancer has shifted away from an inpatient care setting to outpatient care and treatment.
For dietetic professionals with interest in practicing in the area of oncology, the Standards of Practice and Standards of Professional Performance for Oncology Nutrition Practice provide guidance (Robien et al., 2010).
Etiology
The most prevalent types of cancer diagnosed in the United States are prostate, lung and bronchus, colorectal, and urinary bladder cancers for men; and breast, lung and bronchus, colorectal, and uterine cancers for women. The ACS established 2015 Challenge Goals to improve cancer prevention and early detection efforts for lowering cancer incidence and mortality rates. These national recommendations outline specific measures to expand the use of established screening guidelines for the early detection of cancer, and ways to influence individual health behaviors such as protection from the sun, reducing tobacco use, maintaining a healthy body weight, improving diet, and increasing regular physical activity (ACS, 2010).
Overall, fewer Americans are dying from cancer, a trend that began more than 15 years ago. For many, cancer is now a chronic disease, like heart disease and diabetes. According to the National Cancer Institute (NCI), there are 11.7 million Americans living with a history of cancer; this means they are cancer free, are living with evidence of disease, or are undergoing cancer treatment (National Cancer Institute [NCI], 2010g). As a result of improvements in early detection of cancer and the development of new anticancer therapies, the relative survival rate for all cancers is now 66%, up from 50% in the 1970s (ACS, 2009a; NCI, 2010g). The Annual Report to the Nation on the Status of Cancer, 1975-2006, released in December 2009, found that rates of new diagnoses and rates of death from common cancers have declined significantly for men and women overall, as well as for most racial and ethnic populations. Although cancer rates continue to be higher for men than for women, men have experienced greater declines in cancer mortality. For colorectal cancer, the third most frequently diagnosed cancer in both men and women, overall rates have declined. Unfortunately, the incidence in men and women younger than 50 years old remains a concern.
Pathophysiology
Carcinogenesis is the origin or development of cancer. Oncology is the study of all forms of cancer, and an oncologist is the medical doctor who specializes in cancer. Researchers believe changes in gene function cause normal cells to transform into cancerous cells. Thus the study of genetic material and its function (genomics) is of great scientific interest in cancer and its treatment. See Pathophysiology and Care Management Algorithm: Cancer.
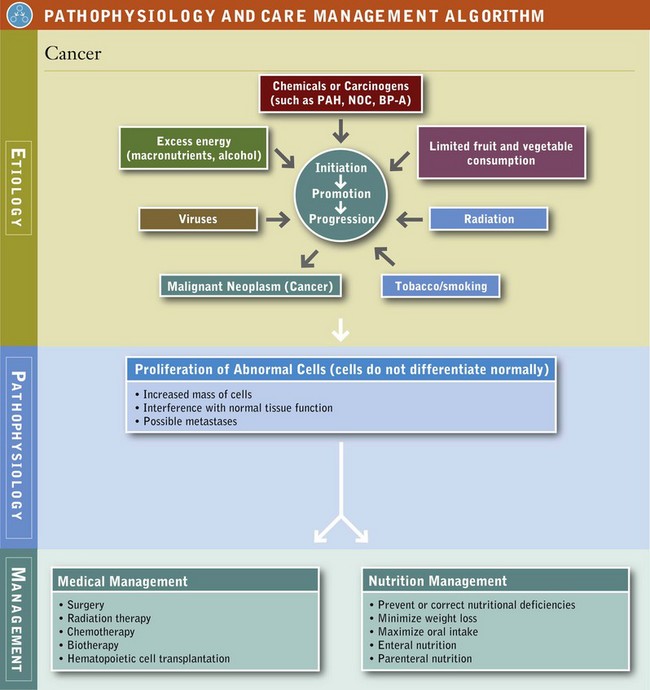
Oncogenes are altered genes that promote tumor growth and change programmed cell death (apoptosis). Tumor suppressor genes are the opposite of oncogenes; these genes become deactivated in cancer cells. This loss in function can lead to unregulated cell growth and, ultimately, cancer. Examples of tumor suppressor genes include adenomatosis polyposis coli (APC), breast cancer types BRCA1 and BCRA2; and tumor suppressor p53, a protein that is involved in preventing cancer. Only approximately 5% of all cancers have been shown to occur as result of inherited genetic alterations. Factors observed in families with hereditary cancers include:
• A cancer diagnosis at an earlier age than normal for certain kinds of cancer
• Individuals with one type of cancer being diagnosed with a second type of cancer
• Certain types of cancers observed in specific ethnic populations (e.g., individuals of Ashkenazi Jewish ancestry with breast and ovarian cancer)
• Recognized cancer syndromes such as hereditary nonpolyposis colorectal cancer or Lynch syndrome, which cause individuals to be at greater risk for developing gastrointestinal (GI), ovarian, uterine, brain, or skin cancer (NCI, 2010b)
Genetic counselors assist individuals and their families to evaluate their risk of hereditary predisposition, that is, testing positive for gene mutations.
Phases of Carcinogenesis
A carcinogen is a physical, chemical, or viral agent that induces cancer. Carcinogenesis is a biologic, multistage process that proceeds on a continuum in three distinct phases: initiation, promotion, and progression. Initiation involves transformation of cells produced by the interaction of chemicals, radiation, or viruses with cellular deoxyribonucleic acid (DNA). Transformation occurs rapidly, but cells can remain dormant for a variable period until they are activated by a promoting agent. After the initial cellular damage has occurred, transformation from normal cells to a detectable cancer can take many years or even decades. During promotion, initiated cells multiply and escape the mechanisms set in place to protect the body from the growth and spread of such cells. A neoplasm, new and abnormal tissue with no useful function, is established. In the third phase (progression), tumor cells aggregate and grow into a fully malignant neoplasm or a tumor.
In the process known as metastasis, the neoplasm has the capacity for tissue invasion that can spread to distant tissues and organs. For a cancer to metastasize, it must develop its own blood supply to sustain its growth of rapidly dividing abnormal cells. In normal cells, angiogenesis promotes the formation of new blood vessels that are essential to supply the body’s tissues with oxygen and nutrients. In cancer cells, tumor angiogenesis occurs when tumors release substances that aid in the development of new blood vessels needed for their growth and metastasis.
Nutrition and Carcinogenesis
Nutrition may modify the carcinogenic process at any stage, including carcinogen metabolism, cellular and host defense, cell differentiation, and tumor growth. Gene expression can be promoted or altered by nutrients during pregnancy, childhood, and throughout a lifetime. Thus nutrition and diet contribute approximately 35% to causal factors for cancer (Greenwald et al., 2006). The strong influence of diet and nutrients is readily seen in studies of migration between cultures. Patterns of cancer occurrence often change over time to resemble that of the new country. For example, in Japan mortality from breast and colon cancer is low, and mortality from stomach cancer is high; the reverse is true among Japanese individuals living in the United States. After two or three generations, the cancer patterns become similar.
Studies looking at the role of nutrition and diet as causal factors of cancer seek to identify relationships between the diets of population groups and categories of individuals and the incidence of specific cancers. Sets of individuals are compared in case control, cohort, or cross-sectional studies. The strongest evidence comes from consistent findings of these different types of epidemiologic studies in diverse populations. In cancer research, epidemiologists look at human populations and evaluate how many people are diagnosed with cancer, what types of cancer occur in different populations and cultures, and what factors such as diet and lifestyle play a role in the development of the cancers.
The sheer complexity of diet presents a difficult challenge. There are literally thousands of chemicals in a normal diet; some are well known, and others are little known and unmeasured. Some naturally occurring dietary carcinogens are natural pesticides produced by plants for protection against fungi, insects, or animal predators, or mycotoxins that are secondary metabolites produced by molds in foods (e.g., aflatoxins, fumonisins, or ochratoxin). Food preparation and preservation methods may also provide dietary carcinogens. Thus diets contain both inhibitors and enhancers of carcinogenesis. Dietary carcinogen inhibitors include antioxidants (e.g., vitamin C, vitamin A and the carotenoids, vitamin E, selenium, zinc) and phytochemicals. See Table 37-1 and Table 12-5. Dietary enhancers of carcinogenesis may be the fat in red meat or the polycyclic aromatic hydrocarbons (PAHs) that form with the grilling of meat at high heat. Complicating the study of nutrition, diet, and cancer is the fact that when one major component of the diet is altered, other changes take place simultaneously. For example, decreasing animal protein also decreases animal fat. This makes the interpretation of research findings difficult because the effects cannot be clearly associated with a single factor.
TABLE 37-1
Cancer-Protective Phytochemicals in Vegetables and Fruits
| Color | Phytochemical | Vegetables and Fruits |
| Red | Lycopene | Tomatoes and tomato products, pink grapefruit, watermelon |
| Red and purple | Anthocyanins, polyphenols | Berries, grapes, red wine, plums |
| Orange | α- and β-carotene | Carrots, mangos, pumpkin |
| Orange and yellow | β-cryptoxanthin, flavonoids | Cantaloupe, peaches, oranges, papaya, nectarines |
| Yellow and green | Lutein, zeaxanthin | Spinach, avocado, honeydew, collard and turnip greens |
| Green | Sulforaphanes, indoles | Cabbage, broccoli, Brussels sprouts, cauliflower |
| White and green | Allyl sulphides | Leeks, onion, garlic, chives |
Cancer cells can have a long latency or dormant period. This makes the diet at the time of cancer cell initiation or promotion—not at the time of diagnosis—most important. Some prospective epidemiologic studies attempt to deal with this challenge by measuring diet at one point in time and following the same subjects for several years. Studies done with laboratory animals test this effect, and since the early part of the last century, laboratory scientists have shown that various nutritional manipulations influence the occurrence of cancerous tumors in animals. Epidemiologic research, together with animal studies, provide a viable method for discovering the links between nutrition and cancer in humans.
Alcohol
Alcohol consumption is associated with increased cancer risk for cancers of the mouth, pharynx, larynx, esophagus, lung, colon, rectum, liver, and breast (both pre- and postmenopausal women) (World Cancer Research Fund [WCRF] and American Institute for Cancer Research [AICR], 2007). For cancers of the mouth, pharynx, larynx, and esophagus, daily consumption of two to three drinks increases risk two to three times compared with nondrinkers. When combined with tobacco use, the sum total risk is higher than the risk for just alcohol or tobacco alone, again compared with nondrinkers (Baan et al., 2007; WCRF and AICR, 2007). In addition, malnutrition associated with alcoholism is also likely to be important in the increased risk for certain cancers. In the United States, if people choose to drink, men are recommended to limit alcohol intake to two drinks per day and women to one drink per day. Serving sizes of popular alcoholic drinks include beer (12 oz), wine (5 oz), spirits/liquors (1.5 oz of 80-proof spirits). Three to four alcoholic drinks or more per week after breast cancer diagnosis may increase recurrence risk, especially among postmenopausal and overweight or obese women (Kwan, 2010).
Energy Intake and Body Weight
In animal studies chronic restriction of food inhibited the growth of most experimentally induced cancers and the occurrence of many spontaneous cancers. This effect was observed even when underfed animals ingested more dietary fat than control animals ingested. Caloric restriction, without malnutrition, appears to have a positive effect on cancer prevention in animals; it is unclear whether that effect translates to humans (Longo et al., 2010).
Obesity is a risk factor for cancer and may account for 6% of all cancers (Polednak, 2008). Currently 68% of all American adults are overweight or obese (Flegal et al., 2010). See Chapter 22. Obesity increases the risk for developing and dying from cancer (Schelbert, 2009). The relationship between body weight, body mass index (BMI), or relative body weight and site-specific cancer has been widely investigated; a positive association has been seen with cancers of the esophagus, pancreas, gallbladder, liver, breast (postmenopausal), endometrium, kidney, colon, and rectum (Toles et al., 2008; WCRF and AICR, 2007). For men, increased BMI correlates with esophageal, thyroid, colon, and kidney cancers; a weaker association exists between BMI and malignant melanoma, multiple myeloma, rectal cancer, leukemia, and non-Hodgkin’s lymphoma (NHL) (Brawer et al., 2009). In women, stronger correlations are found between increased BMI and endometrium, gallbladder, kidney, and esophageal cancers; and a weaker association between increased BMI and leukemia, thyroid, postmenopausal breast, pancreas, and colon cancers and NHL. Bariatric surgery using gastric bypass may reduce cancer mortality by as much as 60% (Brawer et al., 2009).
The median adult BMI should be between 21 and 23 depending on the normal range with different populations (WCRF and AICR, 2007). Body weight throughout childhood should be at the lower end of normal BMI, because excessive weight in adolescence has been correlated with twofold increased risk of death for colon cancer in adulthood (Anderson et al., 2009). Being overweight or obese also appears to increase risk of cancer recurrence and decrease cancer survival (Anderson et al., 2009).
Obesity, age, hyperglycemia, and the incidence of metabolic syndrome play a role in the circulating levels of insulin-like growth factor-1 (IGF-1), a potentially cancer-causing compound. IGF-1 is a polypeptide secreted primarily by the liver and plays a key role in normal growth and development. It can promote the development and progression of prostate, breast, lung, and colon cancer. It has been hypothesized to stimulate the growth of cancer cells and inhibit their death (Blackburn, 2007; Pollack, 2008). IGF-1 secretion is increased when insulin levels are elevated. Obesity and high simple carbohydrate intakes potentially increase insulin resistance and raise circulating insulin levels. This area of research connects several known risk factors between nutrition, diet, and cancer (Parekh et al., 2010).
Overweight and obese cancer survivors are at risk for recurrence and for developing additional problems after surgery, including impaired wound healing, lymphedema following lymph node dissections, second cancers, heart disease, and diabetes mellitus (Anderson et al., 2009). Regular physical activity reduces mortality, especially in women and older individuals (Teucher, 2010). All types of physical activity help to protect against colon cancer, postmenopausal breast cancer, and endometrial cancer (Teucher, 2010). Therefore the ACS encourages all Americans to strive for 45-60 minutes of moderate to vigorous physical activity most days of the week (Doyle et al., 2006). Achieving and maintaining a reasonable weight should be a primary health goal for all cancer survivors (Doyle et al., 2006).
Fat
Research shows a link between some types of cancers and the amount of fat in the diet. Diets high in fat often contain significant amounts of meat. The link between meat and colorectal cancer risk results from a number of possible mechanisms: production of carcinogens from a high-fat diet, from heterocyclic amines (HCAs) and or polycyclic aromatic hydrocarbons (PAHs) from cooking; formation of carcinogenic N-nitroso compounds (NOCs) from processing and the influence of heme-iron are also suspected (WCRF and AICR, 2007).
Diets high in fat also tend to be high in calories, and contribute to overweight and obesity conditions. Because dietary fat intake is correlated with intake of other nutrients and dietary components, it is difficult to distinguish between the effects of dietary fats and protein, total calories, and fiber. Saturated fat in red meats may be associated with an increased risk of colorectal, endometrial, and possibly lymphoid and hematologic cancers (Ferguson, 2010; WCRF and AICR, 2007). Two large prospective randomized studies in the area of diet and breast cancer survival showed mixed results. The Women’s Intervention Nutrition Study (WINS) found that an intervention that reduced dietary fat to 20% of total calories and caused a modest reduction in body weight may favorably influence breast cancer prognosis. However, the Women’s Healthy Eating and Living (WHEL) study, which was very high in vegetables, fruit, and fiber and low in fat, demonstrated no significant survival benefit (Pierce et al., 2007).
Eating more ω-3 fatty acids (foods such as fatty fish, flaxseed oil, walnuts, certain algae) in relation to ω-6 fatty acids (polyunsaturated fats like corn oil, safflower oil, and sunflower oil) may potentially reduce risk of colon and prostate cancers (Berquin et al., 2008). Fish is a healthier protein selection than red meat, is lower in fat, and is a potentially rich source of ω-3 fatty acids. If choosing red meat, it is recommended to select leaner cuts and smaller portions. Poultry or legumes are also desirable alternatives to beef, veal, pork, and lamb.
Fiber, Carbohydrates, and Glycemic Index
Higher-fiber foods such as complex carbohydrates and whole grains are an important part of a healthy diet. The intake of dietary fiber can influence the intake of meat, dietary fat, and simple carbohydrates. Unfortunately, the studies on dietary fiber and cancer have been inconsistent, so dietary fiber was not included in the oncology section of the Evidence Analysis Library of the American Dietetic Association (American Dietetic Association [ADA], 2010a). Epidemiologic studies looking at the possible relationship between dietary fiber and large-bowel cancer showed no effect of a low-fat, high-fiber, high-fruits, and high-vegetables diet on adenoma recurrence years after randomization (ADA, 2008.) Dietary fiber may play a role in preventing breast cancer through nonestrogen pathways among postmenopausal women, but more research needs to be done (Park et al., 2009). However, fiber-rich fruits and vegetables are excellent sources of vitamins, minerals, and phytochemicals. Legumes and lentils have both fiber and additional nutrients worth consuming.
Nonnutritive and Nutritive Sweeteners
The Food and Drug Administration (FDA) has approved five nonnutritive sweeteners (acesulfame-K, aspartame, neotame, saccharin, and sucralose) for use in the food supply, and regulates them as food additives. They appear to be safe when used in moderation. Described as “high-intensity” sweeteners, nonnutritive sweeteners provide little or no energy because they sweeten in minute amounts. Nonnutritive sweeteners have been investigated primarily in relation to potential adverse health concerns, including long-term safety and carcinogenicity, but multiple studies during the past 20 years have indicated that when consumed in reasonable amounts, they are safe. Newer sugar substitutes on the market include Stevia, sugar alcohols (e.g., mannitol, sorbitol, xylitol), and blue agave. Stevia, a nonnutritive sweetener, is considered a dietary supplement but has approval from the FDA. Sugar alcohols are not considered nonnutritive sweeteners even though they are used in a similar way. Blue agave is the juice from the Agave tequiliana plant; the jury is still out on this sweetening option.
Protein
Most diets that contain significant amounts of protein also contain significant amounts of meat and fat, and insignificant amounts of fiber. The effect of protein on carcinogenesis depends on the tissue of origin and the type of tumor, as well as on the type of protein and the calorie content of the diet. In general, tumor development is suppressed by diets that contain levels of protein below that required for optimal growth and development; whereas it is enhanced by protein levels two to three times the amount that is required. The effects may be attributable to specific amino acids, a general effect of protein, or, in the case of low-protein diets, depressed food intake. Epidemiologic studies have found limited and conflicting results. Recommendations for lowering cancer risk and improving overall health encourage intake of plant foods and limiting foods from animal sources, including red meat and processed meats and poultry (WCRF and AICR, 2007).
Smoked, Grilled, and Preserved Foods
Nitrates are added as preservatives to processed meats. Nitrates can be readily reduced to form nitrites, which in turn can interact with dietary substrates such as amines and amides to produce N-nitroso compounds (NOCs): nitrosamines and nitrosamides, which are known mutagens and carcinogens. Nitrates or nitrites are used in smoked, salted, and pickled foods. Sodium and potassium nitrates are present in a variety of foods, and give hot dogs and processed deli meats their pink color, but the main dietary sources are vegetables and drinking water.
NOCs are also produced endogenously in the stomach and colon of people who eat large amounts of red meat. Studies looking at the detrimental effects of smoked foods have not demonstrated a clear, consistent connection between these foods and stomach cancer (WCRF and AICR, 2007). Diets with high amounts of fruits and vegetables that contain vitamin C and phytochemicals that can retard the conversion of nitrites to NOCs should be encouraged (Kushi et al., 2006).
Charring or cooking meat at high temperatures over an open flame (400° F or more) can cause the formation of polycyclic aromatic hydrocarbons (PAHs) and heterocyclic amines. PAHs have shown clear indications of mutagenicity and carcinogenicity. Normal roasting or frying food does not produce large amounts of PAHs compared with the amount produced when cooking over open flames. Animal proteins that produce the greatest dripping of fat on to the flames register the highest PAH formation. For example, grilled beef produces larger amounts of PAHs than grilled chicken, which produces higher amounts than oven-grilled chicken. The source of the flame can also influence PAH production; charcoal grilling promotes the most, followed by flame gas, and finally oven grilling (Farhadian et al., 2010).
Toxic Environments
The Environmental Protection Agency (EPA) was established in 1970, for the purpose of overseeing the acute and long-term health threats caused by substances in the environment. As part of this protection, the Toxic Substances Control Act passed in 1976 required manufacturers to submit health and safety information on all new chemicals. However, many were grandfathered in with the passage of this law and are still untested.
Everyday activities expose people to a myriad of chemicals through the air, and water, food, and beverages. In fact, 740 cancers per million people are estimated to be caused by these very common exposures (Chey, 2008). Health care practitioners grapple with assessing exposure to so many different agents. A good environmental history can be performed at clinical visits and then quickly reviewed for outdoor air pollutants such as nitrogen dioxide, ozone, and carbon monoxide which pose health risks. Exposure to heavy metals, pesticides, herbicides, and occupational exposures may also be noted. In addition to determining the patient’s environmental exposure, practitioners must also determine exposure of family members or others living in the same household. Oxidative stress caused by these environmental exposures can be alleviated by changes in lifestyle, including smoking and diet. High intakes of antioxidant-rich foods and a nutrient-rich diet (not supplementation) is suggested (Kushi et al., 2006).
Toxicity from Bisphenol A
Bisphenol A (BPA) is an industrial chemical used since the 1960s in the manufacturing of many hard plastic bottles and the epoxy linings of metal-based food and beverage cans. It is also an ingredient in the production of epoxy resin used in paints and adhesives. Studies done when the product was developed indicated it was safe to use in food and beverage containers. However, recent studies employing novel approaches to test for subtle effects raised health concerns especially in developing fetuses, infants, and children (Layton, 2010). The National Toxicology Program at the National Institutes of Health (NIH) and the FDA are responding to growing evidence that BPA might increase the risk for cancer (FDA, 2010).
The shift in opinion likely resulted from a change in organizations evaluating the safety and the evaluative tools used (Beronius, 2010). The current goal is to reduce the use and exposure to BPA through several actions: encouraging the production of BPA-free baby bottles, using alternatives to the glue used in food containers, and increasing the oversight on the use of BPA in manufacturing and testing. The U.S. Department of Health and Human Services (USDHHS) supports eliminating it from infant and all food-related product production. Originally it was thought to leach from the plastic only when exposed to heat; now it is believed to leach even with cold temperatures. Exposure to BPA is so widespread in the food and beverage supply that it is estimated that 90% of Americans have traces of it in their urine (Layton, 2010).
Nutrients for Cancer Prevention
Eating behaviors play a very important role in health promotion and disease prevention. Chemoprevention involves specific compounds or drugs used to prevent, delay, or retard the development of cancer (Kashfi, 2009). Nonsteroidal antiinflammatory drugs may protect against colon cancer. Other natural products currently being investigated include the hundreds of polyphenols in fruits and vegetables, green tea, curcumin from curry, and resveratrol from grapes and berries. Phenolic acid, flavonoids, stilbenes, and lignans are the most abundant polyphenols; the chemopreventive potential of these compounds comes from their ability to modulate epigenetic alterations in cancer cells. The epigenetic modification step occurs early in the development of a cancer cell, at a time when it is potentially reversible. Scientists are not completely clear how this process works. Yet it is reasonable to recommend a health-promoting diet rich in fruits, vegetables, soy, therapeutic culinary herbs such as turmeric and cinnamon, green tea, and coffee (Link et al., 2010). For this reason, health organizations have diet and lifestyle recommendations to reduce cancer risk. See Box 37-1.
Calcium and Vitamin D
Calcium supplementation and dairy, especially milk, may be associated with lower colorectal cancer risk. However, other studies suggest an increased risk for aggressive forms of prostate cancer with significant dairy intake or calcium supplementation (Chung et al., 2009; Huncharek et al., 2009). The relationships need to be explored further before clear recommendations can be made.
Vitamin D deficiencies are being detected in all age groups prompting exploration of the role of vitamin D in cancer prevention. For years, public health messages have encouraged use of sun screens and less direct exposure to the sun. Because of this there is reduced conversion of vitamin D on the skin surface and this may be responsible for the increase in deficiencies. Studies have reported that higher serum 25-hydroxyvitamin D (25 (OH) D) levels are associated with lower incidence rates of colon, breast, ovarian, renal, pancreatic, aggressive prostate, and others cancers. Until more is learned about the interaction between vitamin D3 and cancer prevention, taking 2000 IU of vitamin D per day to achieve serum 25(OH)D levels of 40-60 ng/mL is considered safe (Garland, 2009; Garland et al, 2011).
Coffee and Tea
Coffee contains various antioxidant and phenolic compounds, some of which have been shown to have anticancer properties. Coffee also contains caffeine, a compound in the alkaloid phytochemical family. Coffee as a major source of antioxidants in the American diet may have a protective effect against cancer.
Tea is also a good source of phenols and antioxidants. Green tea is made from leaves that have been cooked, pressed, dried and not roasted, and because of this green tea, more so than black tea contains catechins that possess biologic activity with antioxidant, antiangiogenesis, and antiproliferative properties that are relevant to cancer prevention (Kuzuhara, 2008).
Folate and Folic Acid
Folate, from foods, affects DNA methylation, synthesis, and repair. Folate-associated one-carbon metabolism may play an important role in colorectal carcinogenesis because of gene variations. (Levine et al., 2010). Several epidemiologic studies suggest that higher folate intake is also associated with decreased pancreatic cancer risk (Oaks et al., 2010). However, excessive folate may also contribute to deleterious effects in certain cancers (Bailey et al., 2010). More research is needed to evaluate variables such as genetic polymorphisms, and folate from food versus folic acid from supplements.
Fruits and Vegetables
Fruit intake is protective against cancers of the mouth, pharynx, larynx, esophagus, cervix, lung and stomach (WCRF and AICR, 2007). Health benefits from vegetables are more difficult to quantify. Nonstarchy vegetables such as spinach, tomatoes, and peppers probably provide protection against mouth, pharynx, larynx, and esophageal cancers; all vegetables, but particularly green and yellow ones, probably protect against stomach cancer (WCRF and AICR, 2007). Most countries have recommendations for the consumption of vegetables and fruits that vary, but generally are for three or more servings of vegetables and two or more servings of fruit daily with a serving being approximately 80 g or 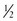 cup (WCRF and AICR, 2007).
cup (WCRF and AICR, 2007).
Anticarcinogenic agents found in fruits and vegetables include antioxidants such as vitamins C and E, selenium, and phytochemicals. Phytochemicals include carotenoids, flavonoids, isoflavones, lignans, organosulfides, phenolic compounds, and monoterpenes. It is still unclear which specific substances of fruits and vegetables are the most protective against cancer (Russo, 2007). These substances have both complementary and overlapping mechanisms, including the induction of detoxification enzymes, inhibition of nitrosamine formation, provision of substrate for formation of chemotherapy agents, dilution and binding of carcinogens in the digestive tract, alteration of hormone metabolism, and antioxidant effects. It appears extremely unlikely that any one substance is responsible for all of the observed associations. See Table 12-5 and Table 37-1 for discussion of chemoprotective agents in fruits and vegetables.
Soy and Phytoestrogens
Soy is a plant-based protein, and it contains phytoestrogens (very weak plant-based estrogens) and isoflavones such as genistein and daidzein. Diets containing modest amounts of soy protect against breast cancer (Lee et al., 2010,) especially if the soy foods have been consumed before reaching adulthood apparently due to exposure to the weak estrogenic effects of isoflavones early in life (Lee et al., 2010). However, the use of soy remains controversial for women already diagnosed with hormone-sensitive cancers (e.g., breast, endometrium) and for postmenopausal women.
Commercially prepared soy supplement powders and foods made from soy products can, but may not always contain isoflavones at much higher concentrations than traditional whole soy foods such as edamame beans, tofu, or soy milk (Gardner et al., 2008; U.S. Department of Agriculture (USDA), 2010). The ACS advises breast cancer survivors to limit the consumption of soy foods to no more than three servings daily, and to avoid using prepared soy supplement powders and products (Doyle et al., 2006). Unlike the advice for women, men with hormone-sensitive cancer such as prostate cancer may benefit from regular consumption of soy foods. Prostate cancer is a testosterone driven cancer and estrogens (or phytoestrogens) are agonists.
Medical Diagnosis and Staging of Cancer
Assessing symptoms of cancer at the earliest stage is critical for treatment effectiveness and survival. Table 37-2 summarizes constitutional or systemic symptoms of cancer and metastatic disease. Many symptoms of early or metastatic cancer affect an individual’s ability to eat, digest, or absorb. According to the ACS, the following early warning signs and symptoms of cancer are described using the acronym “CAUTION”
TABLE 37-2
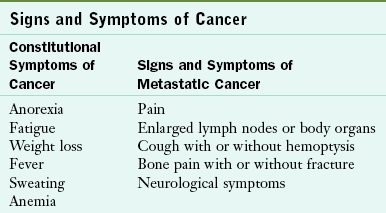
Data from National Cancer Institute (NCI): Dictionary of terms, 2010d. Accessed 23 October 2010 from http://www.cancer.gov/dictionary/.
Change in bowel or bladder habits
Thickening or lump in breast or elsewhere
Indigestion or difficulty in swallowing or chewing
When symptoms or screening tests suggest cancer, physicians use the following to establish a definitive diagnosis: evaluation of an individual’s medical, social and family histories, physical examination, laboratory tests, imaging procedures, and tissue biopsy. Laboratory evaluation is composed of analysis of blood, urine, and other body fluids. In particular, oncologists evaluate tumor markers (e.g., α-fetoprotein [AFP], cancer antigen [CA] 125, CA 19-9, carcinoembryonic antigen [CEA], prostate-specific antigen [PSA]) and other substances in blood or body fluids that can be elevated in cancer. Imaging procedures and studies help determine a diagnosis (Table 37-3). Pathologists perform cytologic examinations by analyzing body fluids, sputum, urine, or tissue under a microscope. To detect malignant cells, they use a histopathologic examination to review specially stained tissue, flow cytometry to count and examine cells and chromosomes, immunohistochemistry to review antibodies for specific cell proteins, and cytogenetics to visualize genetic defects.
TABLE 37-3
Imaging Studies for Cancer Diagnosis and Disease Monitoring
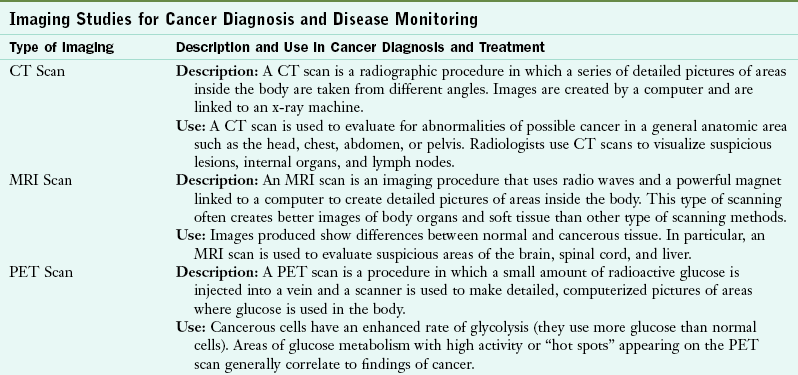
CT, Computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Data from American Cancer Society: Cancer glossary, 2010a. Accessed 10 June 2010 from http://www.cancer.org/CancerGlossary/index; National Cancer Institute (NCI): Dictionary of terms, 2010d. Accessed 23 October 2010 from http://www.cancer.gov/dictionary/.
Oxidative damage to lipids in cellular membranes, proteins, and DNA is often permanent. Thus biomarkers may be used to estimate the DNA damage after exposure to cancer-causing agents such as tobacco smoke, asbestos fibers, heavy metals, andPAHs. 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a new biomarker for the measurement of endogenous oxidative DNA damage and risk for cancer (Valavanidis et al., 2009). See Table 8-5 in Chapter 8.
Staging is used to identify how much a cancer has spread throughout the body. The stage of the cancer at the time of diagnosis is a strong predictor of survival and it directs oncologists to the most effective treatment plan. Cancer staging is most frequently described as stage I, II, III, or IV—stage I being the least amount of disease and stage IV being the most advanced. The tumor-node-metastasis (TNM) staging system is also commonly used by oncologists. T stands for the size of the tumor, N stands for nodes or whether it has spread into lymph nodes, and M stands for metastasis, or whether the cancer has spread to distant organs.
For classification, tumors are often referred to as solid cancers, and hematologic-related cancers of the blood are frequently called liquid cancers. The classification of tumors is based on their tissue of origin, their growth properties, and their invasion of other tissues. Tumors that are not malignant are typically described as benign.
Because cancer occurs in cells that are replicating, the patterns of cancer are quite different in children and adults. In early life the brain, nervous system, bones, muscles, and connective tissues are still growing; therefore cancers involving these tissues are more prevalent in children than in adults. Common childhood cancers include neuroblastoma; medulloblastoma; osteosarcoma; and soft tissue sarcomas such as rhabdomyosarcoma, schwannoma, and germ cell tumors. Conversely, adult cancers frequently involve epithelial tissues that cover and line the body’s internal and external surfaces. Cancers of the epithelial tissues include cancers of the skin, and circulatory, digestive, endocrine, reproductive, respiratory, and urinary systems. Cancers arising from these tissues are referred to as carcinomas and common types are classified as adenocarcinomas, basal cell carcinomas, papillomas, and squamous cell carcinomas.
Leukemias, lymphomas, and myelomas are cancers of the immune system and can occur in either children or adults. Leukemias arise most frequently from white blood cells of the bone marrow. Lymphomas are cancers that develop in the lymphatic system—its nodes, glands, and organs. Myeloma is cancer that originates in the plasma cells of the bone marrow and most frequently occurs in older adults.
Other types of cancer are related to infectious causes and cancer experts recommend antibiotics, vaccines, and changes in behavior for their prevention (ACS, 2009a). Examples include hepatocellular carcinoma linked to hepatitis B virus (HBV) exposure and alcoholic-related cirrhosis, oropharyngeal and cervical cancer incidence linked to human papillomavirus infection (HPV), and stomach cancer caused by chronic inflammation by Helicobacter pylori. See Chapter 28.
Medical Treatment
Cancer treatment in the United States and in more than 115 countries is guided by evidence-based standards known as the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (2010). The NCCN Guidelines encompass evidenced-based care for 97% of all cancers treated in oncology practice. Also listed with these guidelines are evidence-based recommendations for providing supportive care (e.g., management for cancer and cancer treatment–related pain, fatigue, and nausea).
Conventional modalities include antineoplastic therapy (e.g., chemotherapy, biotherapy, antiangiogenic agents, or hormonal agents), radiation therapy, and surgery used alone or in combination with other cancer therapies. Solid tumors and hematologic malignant diseases such as leukemias, lymphomas, and multiple myelomas may be treated with hematopoietic cell transplantation (HCT).
Chemotherapy is the use of chemical agents or medications to systematically treat cancer. Biotherapy is the use of biologic agents to produce anticancer effects indirectly by inducing, enhancing, or suppressing an individual’s own immune response. Antiangiogenic agents are used to inhibit the development of new blood vessels needed by cancers (tumor vasculature) and thus prevent their growth, invasion, and spread. Hormonal therapy is systemic therapy used for the treatment of hormone-sensitive cancers (e.g., breast, ovarian, prostate) by blocking or reducing the source of a hormone or its receptor site.
Radiation oncologists work in the area of therapeutic radiation therapy, which uses high-energy (ionizing radiation) in multiple fractionated doses, or radioactive chemicals to treat cancer. Surgery involves the surgical removal of cancerous tissue.
Response to cancer treatment is defined as complete or partial response (improvement), stable disease (same), or disease progression (worsening). Factors that affect an individual’s response to treatment include tumor burden (the larger the tumor, the greater risk of metastatic disease), rate of tumor growth (rapidly growing tumors are usually more responsive to therapy), and drug resistance (tumors mutate as they grow, and with successive mutations new cancer cells become more likely to be resistant to therapy). Other factors contributing to an individual’s response to cancer treatment include comorbid diseases (e.g., diabetes, renal disease, cardiopulmonary disease), age, performance status, support system, bone marrow reserve, and overall general health (NCI, 2010d; Polovich et al., 2009).
Goals of Treatment
The goal of cancer treatment may be to cure, control, or palliate. Cure is a complete response to treatment. Even if a treatment cannot cure a cancer, often there can be cancer control that extends life when a cure is not possible. Control measures may obscure microscopic metastases after tumors are surgically removed, reduce the size of tumors before surgery or radiation therapy, or alleviate symptoms and side effects of cancer. If a cancer cannot be cured or controlled, palliative care is offered. Palliative care helps individuals be as comfortable as possible. Palliation is designed to relieve pain and manage symptoms of illness; lessen isolation, anxiety and fear; and help maintain independence as long as possible (National Hospice and Palliative Care Organization [NHPCO], 2010). Hospice is care for individuals with a life expectancy of months and focuses on relieving symptoms, controlling pain, and providing support to patients and their families. Patients are made as comfortable as possible through the end of their lives.
Medical Nutrition Therapy
Dietetic professionals should use the nutrition care process steps when providing medical nutrition therapy (MNT) (ADA, 2011). To further assist clinicians working in the cancer care setting the American Dietetic Association has developed the Oncology Toolkit with MNT protocols for breast, colorectal, esophageal, gastric, head and neck, hematologic, lung, and pancreatic cancers (ADA, 2010b). It also includes instructional and documentation forms using the nutrition care process (NCP) and standardized language to help individualize nutrition care with recommendations based on the current state of science.
Nutrition Screening and Assessment
With the recent shift of cancer care from the hospital setting to outpatient settings, nutrition screening and assessment should continue throughout the continuum of care. Ideally, nutrition screening and assessment for risk of nutrition problems should be interdisciplinary, instituted at the time of diagnosis, and reevaluated and monitored throughout treatment and recovery. The patient-generated Subjective Global Assessment has been adapted for use with cancer patients and incorporates sections completed by the patient or caregiver on weight history, food intake, symptoms, and functioning. Sections completed by a health care member (e.g., physician, nurse, registered dietitian, social worker) evaluate weight loss, disease, metabolic stress, and include a nutrition-focused physical examination. Nutritional risk and intervention are then determined by a scoring system (Charney and Cranganu, 2010).
Other tools are the Activities of Daily Living (ADL) tool, the Common Toxicity Criteria (CTC), and the Karnofsky Performance Scale (KPS) Index. The ADL tool assesses routine activities that people do each day without assistance such as bathing, dressing, and walking. CTC is an outcome measure used in cancer centers that compares acute toxicities of cancer treatment, and KPS is a scoring index that associates an individual’s functional status with disease status and survival (McCallum, 2006).
In-depth assessment is undertaken to obtain more information and to identify nutrition problems. Careful review of the individual’s appetite and oral intake is required, with an assessment of symptoms (e.g., nausea, vomiting, and diarrhea), weight status, comorbidities, and laboratory studies. A nutrition-focused physical examination is recommended to fully evaluate nutrition status and degree of risk (Fuhrman, 2009). Components of this type of assessment include a general survey of the body, review of vital signs and anthropometrics, and an evaluation of subcutaneous fat stores, muscle mass, and fluid status.
Energy
Determining individualized energy needs is vital to helping people maintain their weight and prevent weight loss associated with cancer. Methods used to estimate energy requirements for adults include using standardized equations or measuring resting metabolic rate using indirect calorimetry (Russell and Malone, 2009). See Chapter 2 for methods for determining energy requirements such as the Mifflin-St. Jeor and Ireton-Jones equations. To ensure that adequate energy is being provided. the individual’s diagnosis, presence of other diseases, intent of treatment (e.g., curative, control, or palliation), anticancer therapies (e.g., surgery, chemotherapy, biotherapy, or radiation therapy), presence of fever or infection, and other metabolic complications need consideration. Established guidelines for quickly estimating energy needs of people with cancer based on body weight are shown in Table 37-4.
TABLE 37-4
Estimating Energy Needs of People with Cancer
| Condition | Energy Needs |
| Cancer, nutritional repletion, weight gain | 30-40 kcal/kg/day |
| Cancer, normometabolic | 25-30 kcal/kg/day |
| Cancer, hypermetabolic, stressed | 35 kcal/kg/day |
| Hematopoietic cell transplant | 30-35 kcal/kg/day |
| Sepsis | 25-30 kcal/kg/day |
| Obese | 21-25 kcal/kg/day |
Data from Gottschlich MM, editor: The A.S.P.E.N. nutrition support core curriculum: a case-based approach—the adult patient. Silver Spring, MD, 2007, American Society for Parenteral and Enteral Nutrition; Hurst JD, Gallagher AL: Energy, protein, micronutrient, and fluid requirement. In Elliott L et al., editors: The clinical guide to oncology nutrition, ed 2, Chicago, 2006, American Dietetic Association.
Protein
An individual’s need for protein is increased during times of illness and stress. Additional protein is required by the body to repair and rebuild tissues affected by cancer therapy, and to maintain a healthy immune system (Hurst and Gallagher, 2006). Adequate energy should be provided, or the body will use its lean body mass as a fuel source. When determining protein requirements, dietetic professionals need to consider the degree of malnutrition, extent of disease, degree of stress, and ability to metabolize and use protein (Russell and Malone, 2009). For example, a cancer patient with a hematopoietic cell transplant may require 1.5-2 g/kg/day. A patient with severe stress may need 1.5-2.5 g/kg/day. Daily protein requirements are generally calculated using actual body weight.
Fluid
Fluid management in cancer care must ensure adequate hydration and electrolyte balance, and prevent dehydration and hypovolemia. Altered fluid balance may occur with fever, ascites, edema, fistulas, profuse vomiting or diarrhea, multiple concurrent intravenous (IV) therapies, impaired renal function, or medications such as diuretics. Individuals need close monitoring for dehydration (e.g., intracellular fluid losses caused by inadequate intake of fluid because of mucositis or anorexia) and hypovolemia (e.g., extracellular fluid losses from fever or GI fluids such as vomiting, diarrhea or malabsorption). Signs and symptoms of dehydration include fatigue, acute weight loss, hypernatremia, poor skin turgor, dry oral mucosa, dark or strong smelling urine, and decreased urine output. To carefully assess for hypovolemia, levels of serum electrolytes, blood urea nitrogen and creatinine should also be evaluated. A general guideline for estimating fluid needs for all adults without renal concerns is 30-35 mL/kg/day (Hurst and Gallagher, 2006). Another guideline is 1 mL fluid per 1 kcal of estimated calorie needs (Russell and Malone, 2009). In some instances, individuals undergoing cancer therapy may require IV fluid hydration to meet their treatment-related fluid needs.
Vitamins and Minerals
Individuals diagnosed with cancer often take large amounts of vitamin and mineral supplements because they believe that these products can enhance their immune system or even reverse the course of their disease. Others may see dietary supplementation as a way to make up for existing nutritional deficiencies at the time of diagnosis caused by poor diet and lifestyle choices. If individuals are experiencing difficulty with eating and treatment-related side effects, a multivitamin and mineral supplement that provides no more than 100% of the dietary reference intakes (DRIs) is considered safe (Doyle et al., 2006). In contrast, the American Institute for Cancer Research (AICR) encourages all people (including cancer survivors) not to use dietary supplements for cancer prevention, citing evidence that high-dose dietary supplementation can have cancer-promoting effects (WCRF and AICR, 2007). Whether for primary or secondary prevention, all individuals should consume vitamin and minerals from the foods they eat rather than use dietary supplements. In some instances during and after a cancer diagnosis, supplementation or restriction of specific micronutrients may be required above or below DRI levels, depending on medical diagnosis and laboratory analysis (e.g., iron supplementation for iron-deficiency anemia).
Supplement Use
The majority of cancer survivors use dietary supplements during all phases of anticancer treatment (Hardy, 2008). Despite increased use, oncology practitioners ask that patients avoid use of dietary supplements during treatment. Specifically, controversy continues over whether the use of antioxidant dietary supplements such as vitamins A, C, E, β-carotene, zinc, and selenium actually inhibits or enhances the antitumor effects of radiation therapy and chemotherapy (ACS, 2009b). Several randomized trials showed some potential for reducing treatment dose-limiting toxicities (Block et al., 2008). However, well-designed studies evaluating larger numbers of individuals are needed.
Cancer survivors should carefully evaluate the need and wisdom of taking dietary supplements both during and after treatment (Miller, 2008) and should avoid using antioxidant supplements while undergoing treatment until further research supports their use (ACS, 2009b; Hardy, 2008; WCRF and AICR, 2007). Individuals diagnosed with cancer should be encouraged to consume antioxidants from a variety of colorful food sources such as fruits, vegetables, and whole grains as a way to safely consume these naturally occurring, health-promoting phytonutrients, vitamins, and minerals (Grant et al., 2010).
Nutrition Diagnosis
Nutrition diagnosis identifies the specific nutrition problems that can be resolved or improved through nutrition intervention. (ADA, 2011). See Chapter 11. The following are examples of nutrition diagnoses using the “problem, etiology, and signs and symptoms” system developed for the cancer care setting:
Intake Domain
• Inadequate oral intake related to pelvic radiation therapy as evidenced by diarrhea and 2.5-pound weight loss in the preceding week
• Inadequate enteral nutrition (EN) infusion related to intolerance of EN as evidenced by nausea, abdominal distention, and weight loss of 3 pounds in the preceding 5 days
• Malnutrition related to cancer cachexia as evidenced by wasting of temporalis and interosseous muscles, and weight loss of more than 7.5% in 3 months
Clinical Domain
• Altered GI function related to recent ileostomy surgery as evidenced by 2 L/day ostomy diarrhea output, and the need for daily IV hydration during the preceding week
• Altered GI function related to biweekly chemotherapy as evidenced by nausea, vomiting, and anorexia in the preceding 4 days
• Swallowing difficulty related to an obstructing esophageal tumor as evidenced by dysphagia, odynophagia, and 10-pound weight loss in the preceding month
Behavioral-Environmental Domain
• Limited access to nutrition-related supplies related to lack of insurance and financial resources as evidenced by not using the prescribed amount of tube feeding formula and continued weight loss to 80% of usual weight during the preceding month
• Intake of unsafe food related to exposure to contaminated food while neutropenic as evidenced by hospitalization, diarrhea, and positive stool culture for salmonella
• Undesirable food choices related to an unwillingness to apply nutrition information as evidenced by ongoing diarrhea and diet history of continued high fiber food intake while undergoing pelvic radiation therapy
Nutrition Intervention
Nutrition intervention outlines specific actions to manage a nutrition diagnosis. It includes two distinct, interrelated components—planning and implementing nutrition interventions (ADA, 2011). The Oncology Toolkit recommends careful appraisal if the planned nutrition intervention will negatively affect patient safety or possibly interfere with the cancer treatment (ADA, 2010b). The Toolkit also advises evaluation of the nutrition intervention’s likely effectiveness for improving nutrition status, possible financial burden, and patient acceptance
Intervention goals should be specific, achievable, and individualized to encourage cooperation. Goals need to be directed toward an objective measure such as body weight or some other meaningful index. Another goal is to minimize the effects of “nutrition impact symptoms” and to maximize the individual’s nutritional parameters. Nutrition impact symptoms can be defined as symptoms and side effects of cancer and cancer treatment that directly affect the nutrition status. Consultation with the individual, caregivers, or family members regarding expected problems and their possible solutions should be initiated early in the course of cancer therapy and should continue in conjunction with follow-up nutrition assessment and care.
The adverse nutritional effects of cancer can be severe and may be compounded by the effects of the treatment regimens and the psychological effects of cancer. The result is often a profound depletion of nutrient stores and deterioration in nutrition status. Malnutrition, anorexia (loss of appetite), and weight loss are all significant issues in cancer care and are often present in many individuals at the time of diagnosis, even in children (Goldman et al., 2006). More than 50% of people with cancer lose body weight and more than one third lose more than 5% of their usual body weight (Skipworth, 2007). Studies consistently show that even small amounts of weight loss (less than 5% of body weight) before treatment is associated with a poorer prognosis and decreased quality of life, thus reinforcing the importance of early MNT (Fearon, 2008).
Oral Nutrition Management Strategies
Ideally the route of feeding is oral, although individuals may experience symptoms that affect this. Strategies for modifying dietary intake may be necessary, and depend on the specific eating problem and the individual’s nutritional status. Food and its presentation may need modification. Liquid medical food supplements may be recommended for those unable to consume enough energy and protein to maintain weight and nutrition status (see Chapter 14). Education materials with suggestions for improving oral intake and managing treatment-related side effects include Eating Hints (NCI, 2010e), Chemotherapy and You (NCI, 2010c), and Radiation Therapy and You (NCI, 2010f). Table 37-5 outlines examples of nutrition intervention strategies.
TABLE 37-5
Nutrition Intervention Strategies for Patients with Cancer


Data from Elliott L et al., editors: The clinical guide to oncology nutrition, ed 2, Chicago, 2006, American Dietetic Association; Grant BL et al., editors: American Cancer Society’s complete guide to nutrition for cancer survivors, ed 2, Atlanta, 2010, American Cancer Society; Grant BL, Hamilton KK, editors: Management of nutrition impact symptoms in cancer and educational handouts, Chicago, 2005, American Dietetic Association; National Cancer Institute (NCI): Eating hints, 2010e. Accessed 20 October 2010 from http://www.cancer.gov/publications/.
Managing Anorexia and Alterations in Taste and Smell
Sometimes even before diagnosis, and then throughout cancer treatment, individuals may report anorexia, early satiety, and decreased food intake. Alterations in taste and smell are commonly experienced as well. Taste alterations can be associated with the disease itself, certain chemotherapy agents, radiation therapy, or surgery to the head and neck. Chemotherapy-induced, learned taste aversions have been reported in both adults and children. Individuals may also develop a heightened sense of smell that results in sensitivity to food preparation odors and aversions to nonfood items such as soaps or perfumes. These sensation abnormalities do not consistently correlate with the tumor site, extent of tumor involvement, tumor response to therapy, or food preferences and intake. Nutrition interventions that decrease the aroma of foods, such as serving foods cold instead of hot, may be helpful (NCI, 2010e).
Managing Alterations in Energy Metabolism
Energy metabolism is intimately related to carbohydrate, protein, and lipid metabolism, all of which are altered by tumor growth. Tumors exert a consistent demand for glucose, exhibit a characteristically high rate of anaerobic metabolism, and yield lactate as the end product. This expanded lactic acid pool requires an increased rate of host gluconeogenesis via Cori cycle activity, which is increased in some people with cancer but not in others. Both protein breakdown and lipolysis take place at increasing rates to maintain high rates of glucose synthesis. There is glucose intolerance and insulin resistance, characterized by excess fatty acid oxidation and decreased uptake and use of glucose by muscle.
Alterations in protein metabolism appear to be directed toward providing adequate amino acids for tumor growth. Most notable is the loss of skeletal muscle protein caused by increased protein breakdown, as well as decreased protein synthesis.
Managing Cancer Cachexia
A common secondary diagnosis in people with advanced cancer is a variant of protein-energy malnutrition. This syndrome is termed cancer cachexia and is characterized by progressive weight loss, anorexia, generalized wasting and weakness, immunosuppression, altered basal metabolic rate, and abnormalities in fluid and energy metabolism. There is also increased loss of adipose tissue, which is related to an increased rate of lipolysis, rather than a decrease in lipogenesis. Increased levels of lipid-mobilizing factor and proteolysis-inducing factor secreted by tumor cells will lead to increased loss of both fat and muscle mass. Individuals at the time of diagnosis with breast or hematologic cancers rarely present with significant weight loss, whereas individuals with lung, esophageal, or head and neck cancers often exhibit substantial weight loss. Cancer cachexia is caused in part by cytokines (immune-modulating agents), produced by the cancer itself or by the immune system in response to the cancer. Cytokines can cause metabolic changes and wasting that is similar to changes seen in inflammation. Proinflammatory cytokines include tumor necrosis factor (TNF)-α (cachectin) and TNF-β, interleukin (IL)-1, IL-6, and interferon-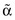 . These cytokines have overlapping physiologic activities, which makes it likely that no single substance is the sole cause. Resting energy expenditure (REE) is elevated, which is in contrast to the REE in chronic starvation wherein the body adapts to conserve energy and preserve body tissue. Cancer cachexia often increases closer to the time of death.
. These cytokines have overlapping physiologic activities, which makes it likely that no single substance is the sole cause. Resting energy expenditure (REE) is elevated, which is in contrast to the REE in chronic starvation wherein the body adapts to conserve energy and preserve body tissue. Cancer cachexia often increases closer to the time of death.
Pharmacotherapy
The pharmacologic management of cachexia and anorexia requires careful evaluation based on the patient’s treatment goals and prognosis, and on close monitoring of symptoms. Prescribed medications sometimes prevent adequate intake. Ideally these agents are prescribed in combination with nutrition counseling and physical activity. A number of pharmacologic agents are under investigation, including appetite stimulants, metabolic agents, cytokine blockers, prokinetic agents, and anabolic agents. Several trials have shown improved appetite and increased energy intake and body weight in cancer patients treated with megestrol acetate, a progestational agent. Prolonged use of corticosteroids is associated with negative side effects such as osteoporosis, fluid retention, adrenal suppression, glucose intolerance, electrolyte imbalance, or even arm- and leg-muscle wasting. Oxandrolone, a synthetic anabolic steroid, combined with a resistance exercise program, may increase total body weight and lean tissue weight. Growth hormones have been studied in patients with wasting associated with human immunodeficiency virus, but few data are available regarding their use with cancer.
Managing Other Cancer-Related Metabolic Abnormalities
Metabolic alterations vary by tumor type. An individual’s immunologic function can be impaired, apparently as the result of the disease, cancer treatment, or progressive malnutrition. In addition to the cancer-induced metabolic effects, the mass of the tumor may anatomically alter the physiology of specific organ systems. The activities of several enzyme systems involved with digestion and absorption can be affected, as can certain endocrine functions.
Critical imbalances in fluid and electrolyte status can occur in people who have cancers or are undergoing cancer treatments that promote excessive diarrhea, vomiting, or malabsorption. Profuse and often severe diarrhea can result from partial bowel obstructions and endocrine-secreting tumors such as those secreting serotonin (carcinoid tumors), calcitonin, or gastrin (Zollinger-Ellison syndrome). The use of antimetabolites, alkylating agents, and antibiotics may also lead to the development of severe diarrhea. In some instances, people who are immunocompromised or have undergone GI surgery may experience profuse diarrhea that is caused by intestinal pathogens such as Clostridium difficile.
Persistent vomiting is associated with intestinal obstruction, radiation therapy to the stomach and abdomen or brain, highly emetogenic (nausea-causing) chemotherapy agents, intracranial tumors, and advanced cancer (Grant, 2006). Careful assessment and evaluation of the cause of the diarrhea or vomiting is critical for effective management. Malabsorption may be caused by treatment-related pancreatic dysfunction, postsurgical short gut syndrome, acute or chronic radiation enteritis (inflammation of the GI tract tissues secondary to radiation), excess serotonin, steatorrhea, or chronic diarrhea.
Hypercalcemia may occur in individuals with bone metastases, caused by the osteolytic activity of tumor cells releasing calcium into the extracellular fluid. Hypercalcemia is potentially fatal, and is associated most commonly with multiple myeloma, lung cancer, and advanced breast and prostate cancer. Nausea, weakness, fatigue, lethargy, and confusion occur. Medical management of hypercalcemia includes rehydration and use of antihypercalcemic agents. Calcium supplementation from dietary supplements and antacids should be avoided. Restricting the intake of foods containing calcium is not indicated because the consumption of these foods has little effect in the overall management of hypercalcemia.
Nutritional Impact of Cancer Treatments
Chemotherapy uses chemical agents or medications to treat cancer. Classifications of chemotherapy cytotoxic agents include alkylating agents, antimetabolites, antitumor antibiotics, miscellaneous agents, nitrosoureas, and plant alkaloids (Wilkes and Barton-Burke, 2010). Once in the bloodstream, these agents are carried through the body to reach as many cancer cells as possible. Routes of administration for chemotherapy include:
• Oral: capsule, pill, or liquid
• Intravenous (IV): delivery of medication via an injection or an indwelling catheter into a vein
• Intraperitoneal: delivery of medication via a catheter directly into the abdominal cavity
• Intravesicular: delivery of medication via a Foley catheter directly into the bladder
• Intrathecal: delivery of medication via an injection into the central nervous system using an Ommaya reservoir or a lumbar puncture (Polovich et al., 2009)
Whereas surgery and radiation therapy are used to treat localized tumors, chemotherapy is a systemic therapy that affects the malignant tissue and normal cells as well. Cells of the body with a rapid turnover such as bone marrow, hair follicles, and the mucosa of the alimentary tract are the most affected. As a result, nutrition intake and nutrition status can be adversely affected. Nutrition-related symptoms include myelosuppression (suppression of bone marrow production of neutrophils, platelets, and red blood cells), anemia, fatigue, nausea and vomiting, loss of appetite, mucositis, changes in taste and smell, xerostomia (mouth dryness), dysphagia, and altered bowel function such as diarrhea or constipation (Table 37-6).
TABLE 37-6
Nutrition-Related Effects of Antineoplastic Agents: Chemotherapy, Biotherapy, Hormone Therapy, and Anti-angiogenic Agents
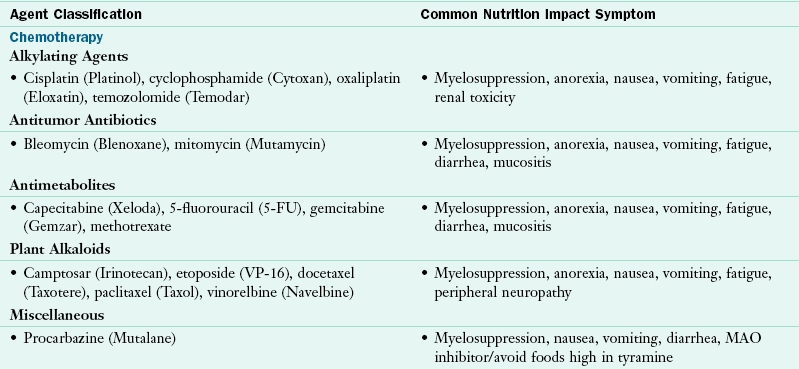

Data from Polovich M et al: Chemotherapy and biotherapy guidelines and recommendations for practice, Pittsburgh, 2009, Oncology Nursing Society; Wilkes GM, Barton-Burke M: 2010 oncology nursing drug handbook, Boston, 2010, Jones and Bartlett.
The severity of the side effects depends on the specific agents used, dosage, duration of treatment, number of treatment cycles, accompanying drugs, individual response, and current health status. The timely and appropriate use of supportive therapies such as antiemetics, antidiarrheals, hematopoietic agents, and antibiotics, as well as dietary changes, is important. Many people experience significant side effects, especially in “dose-intensive” multiple-agent chemotherapy regimens; neutropenia (reduced white blood cells or neutrophils) and myelosuppression are the primary factors limiting chemotherapy administration. Commonly experienced chemotherapy induced toxicities affecting the GI system include mucositis, nausea, vomiting, diarrhea, and constipation. Chemotherapy related taste abnormalities can lead to anorexia and decreased oral intake. Symptoms of GI toxicity are usually temporary; however, some multiagent chemotherapy regimens may lead to lasting GI side effects.
Diarrhea
Diarrhea is a common side effect of certain chemotherapy agents. Left unmanaged, it can lead to depletion of fluids, electrolytes, malnutrition, and even hospitalization (Muehlbauer et al., 2009). The intestinal mucosa and digestive processes can be affected, thus altering digestion and absorption to some degree. Protein, energy, and vitamin metabolism may be impaired. Total lymphocyte count is often depressed and does not accurately reflect nutrition status after chemotherapy administration.
Nausea and Vomiting
Chemotherapy induced nausea and vomiting are commonly classified as anticipatory (occurs before receiving treatment), acute (occurs within the first 24 hours after receiving treatment), or delayed (occurs 1 to 4 days after treatment), each of which is characterized by distinct pathophysiologic events and requires different therapeutic interventions (NCCN, 2010). Effective agents for treatment-related nausea and vomiting are the serotonin antagonists (e.g., ondansetron, granisetron, and dolasetron), neurokinin-1 (NK-1) receptor antagonists (e.g., aprepitant), dopamine antagonists (e.g., metoclopramide, prochlorperazine), and corticosteroids such as dexamethasone (Polovich et al., 2009; Tipton et al., 2007). Other antiemetic agents include cannabinoids (e.g., dronabinol, nabilone) and anxiolytics (e.g., lorazepam).
Food-Drug Interactions
Dietetic professionals can gain valuable insights regarding possible drug-nutrient interactions and contraindications by reviewing product medication inserts, pharmacy resource books, and medication databases or by consulting with pharmacy personnel (see Chapter 9 and Appendix 31). Some chemotherapy agents can cause potentially severe adverse events (Grant and Byron, 2006); for example:
• Individuals with certain types of lung cancer who are being treated with pemetrexed (Alimta) require vitamin B12 and folic acid supplementation throughout the duration of their therapy to avoid significant anemia associated with this chemotherapy agent.
• A severe hypertensive event is possible when tyramine-rich foods and beverages are consumed while taking procarbazine (Mutalane), a chemotherapy agent commonly used to treat brain cancer (see Chapter 9).
• Individuals with colon cancer receiving oxaliplatin (Eloxatin) should not drink, eat, or handle cold drinks or foods for up to 5 days because of treatment-related dysesthesias or transient paresthesias of the hands, feet, and throat.
• In order to prevent unnecessary gastric upset, individuals taking the medication, capecitabine (Xeloda), must take the medication within 30 minutes of eating food or a meal. Conversely, medications such as erlotinib (Tarceva) should not be taken with food and it can cause a rash and profound diarrhea unless taken on an empty stomach.
Oral Changes
People with altered taste acuity (dysgeusia, hypogeusia, ageusia) may benefit from increased use of flavorings and seasonings during food preparation. Meat aversions may require the elimination of red meats, which tend to be strong in flavor, or the substitution of alternative protein sources. Herpes simplex virus and Candida albicans (thrush) account for most oral infections. In addition to causing oral infections, some agents, especially corticosteroids, can cause hyperglycemia and can lead to excessive losses of urinary protein, potassium, and calcium.
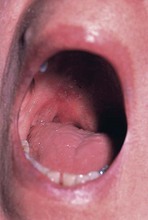
Mucositis
Oral mucositis, an inflammation of the mucous membranes lining the oropharynx and esophagus, is a common side effect of some types of chemotherapy (Figure 37-1). Although many interventions exist, most strategies lack scientific evidence (Harris et al., 2008). General care guidelines include recommending daily oral care (e.g., keeping the mouth clean, avoidance of tobacco, alcohol, and irritating foods) and the use of bland rinses (e.g., baking soda or saline rinses). Bland liquids and soft solids are usually better tolerated in individuals with oral or esophageal mucositis and strong-flavored, acidic, or spicy foods should also be avoided. Commercially prepared liquid medical food supplements can be useful.
Biotherapy
Biotherapy is immunotherapy, a group of cancer treatment drugs prescribed to stimulate the body’s own immune system and natural defenses to treat cancer. Biotherapy is sometimes used by itself, but it is most often given in combination with chemotherapy drugs. Different kinds of biotherapy drugs used to help the immune system recognize cancer cells and strengthen its ability to destroy them include:
• Cytokines such as interferon and IL-2 for treatment of malignant melanoma and metastatic melanoma
• Monoclonal antibodies such as trastuzumab (Herceptin) for treatment of specific types of breast cancer, and rituximab (Rituxan) for treatment of NHL
• Cancer vaccines made from an individual’s own cancer or substances from tumor cells are currently under investigation in clinical cancer trials (Wilkes and Barton-Burke, 2010).
Other types of biotherapy drugs are groups of proteins that cause blood cells to grow and mature (NCI, 2010d). These drugs are called hematopoietic growth factors and they include supportive care medications such as darbepoetin (Aranesp) or epoetin alfa (Procrit) to stimulate red blood cell production, and filgrastim (Neupogen) or pegfilgrastim (Neulasta) to stimulate the production of neutrophils in the bone marrow (Polovich et al., 2009). Individuals receiving these agents may experience fatigue, chills, fever, and flulike symptoms.
Hormone Therapy
Hormone therapy adds, blocks, or removes hormones to slow or stop the growth of hormone-sensitive breast or prostate cancer (NCI, 2010d). Examples of these agents include tamoxifen (Nolvadex) and anastrozole (Arimidex) for breast cancer and leuprolide (Lupron) or bicalutamide (Casodex) for prostate cancer (Wilkes and Barton-Burke, 2010). Side effects commonly include hot flashes, decreased libido, and bone pain.
Antiangiogenic Therapy
Antiangiongenic therapy prevents or reduces the growth of new blood vessels, and prevents tumor invasion. These agents are most frequently used in combination with other chemotherapy agents to maximize their effectiveness. An example of an antiangoiogenic agent used to treat colon or breast cancer is bevacizumab (Avastin).
Radiation Therapy
Radiation therapy, ionizing radiation used in multiple fractionated doses, is used to cure, control, or palliate cancer. Radiation therapy can be delivered externally into the body from a megavoltage machine or with brachytherapy by placing a radioactive source (implant) in or near the tumor to deliver a highly localized dose. Advances in technology to deliver radiation therapy with precise accuracy include radiation surgery (e.g., stereotactic radiosurgery) and intensity-modulated radiation therapy (IMRT). Whereas chemotherapy is a systemic therapy, radiation therapy affects only the tumor and the surrounding area. The side effects of radiation therapy are usually limited to the specific site being irradiated. Chemotherapy agents may also be given in combination with radiation therapy to produce a radiation-enhancing effect. People receiving multimodality therapy often experience side effects sooner and with greater intensity.
The acute side effects of radiation therapy when used alone generally occur around the second or third week of treatment, and usually resolve within 2 to 4 weeks after the radiation therapy has been completed. Late effects of radiation therapy may happen several weeks, months, or even years after treatment. Commonly experienced nutrition-related symptoms include fatigue, loss of appetite, skin changes, and hair loss in the area being treated (Table 37-7).
TABLE 37-7
Nutrition-Related Effects of Radiation Therapy
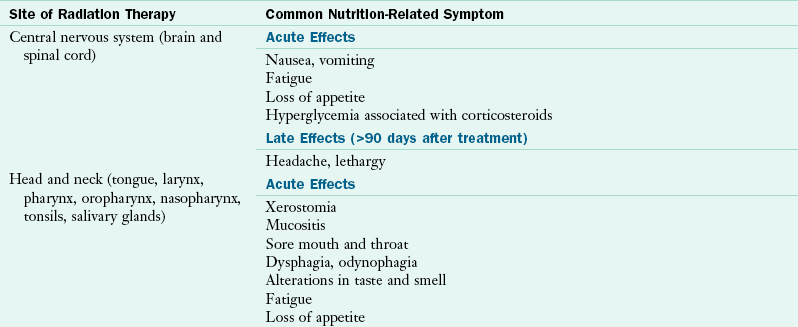
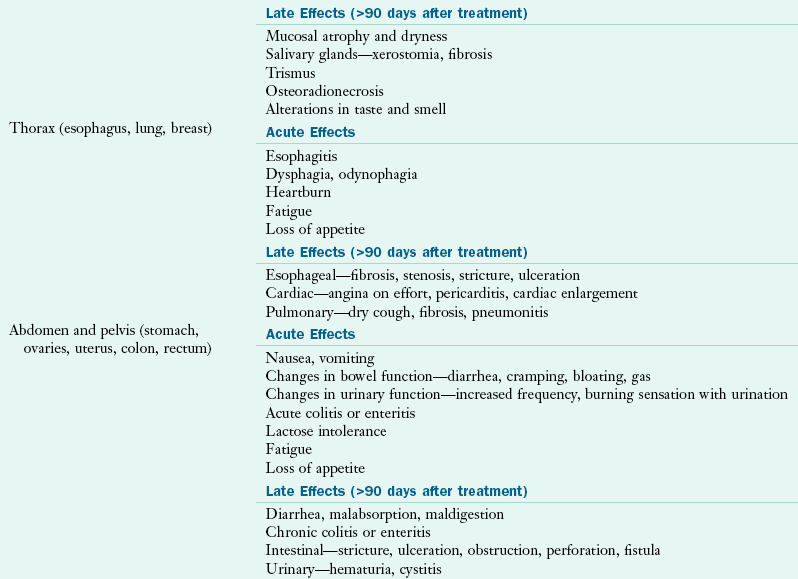
Data from: Bruner DW et al: Manual for radiation oncology and nursing practice and education, ed 3, Pittsburgh, 2005, Oncology Nursing Society; Havrila C et al: Medical and radiation oncology. In Marian M, Roberts S, editors: Clinical nutrition for oncology patients, Sudbury, MA, 2010, Jones and Bartlett.
Radiation to the Head and Neck
Treatment for head and neck cancer usually includes a multimodality approach with aggressive chemotherapy, radiation therapy, and often surgery. Radiation therapy to the head and neck can cause acute nutrition-related symptoms: sore mouth, altered taste and smell, dysphagia and odynophagia, mucositis, xerostomia, anorexia, fatigue, and weight loss (Havrila et al., 2010). Prophylactic placement of percutaneous endoscopic gastrostomy (PEG) feeding tubes can help to reduce treatment-associated weight loss and malnutrition (Cady, 2007).
Salivary stimulants and substitutes or oral lubricants are beneficial for temporary relief of xerostomia (diminished salivation or loss of salivation) caused by head and neck radiation therapy or certain types of medications (e.g., pain medications). In addition, liquids and foods with sauces and gravies are usually well tolerated. Late effects of radiation therapy may include dental caries, permanent xerostomia, trismus (an inability to fully open the mouth), and osteoradionecrosis of the jaw (necrosis of the bone caused by exposure to radiation therapy).
Before beginning therapy, individuals should undergo a dental evaluation and thorough teeth cleaning and receive instruction in good oral hygiene and care, including daily brushing and rinsing (National Institute of Dental and Craniofacial Research, 2010). After therapy has been completed, individuals should continue to have close dental monitoring and follow-up. Individuals may also benefit from a referral to a speech therapist for assessment and evaluation of swallowing function.
Radiation to the Thorax
Nutrition-related symptoms of radiation therapy to the thorax (chest) can include heartburn and acute esophagitis, accompanied by dysphagia and odynophagia. Late effects include possible esophageal fibrosis and stenosis. When this occurs, individuals are generally only able to swallow liquids, and the use of medical food supplements and nutrition support enteral nutrition (EN) may be necessary to meet nutritional needs. Often, individuals undergo esophageal dilations or swallowing therapy and rehabilitation to improve swallowing function.
Radiation to the Abdomen or Pelvis
Radiation therapy to the abdomen or pelvis may cause gastritis or enteritis that can be accompanied by nausea, vomiting, diarrhea, and anorexia (Muehlbauer et al., 2009. Late effects can include lasting GI damage such as malabsorption of disaccharides (e.g., lactose), fats, vitamins, minerals, and electrolytes. Proactive management includes encouraging affected individuals to consume soluble fiber, to increase intake of hydrating liquids, and to avoid eating high non-soluble fiber or lactose containing foods. To alleviate symptoms, medications such as antidiarrheals like loperamide and antimotility agents (e.g., metoclopramide) may be given to reduce intestinal motility.
Chronic radiation enteritis can develop with diarrhea, ulceration, or obstruction, intensifying the risk of malnutrition. Chronic radiation enteritis combined with or without significant bowel resection can result in bowel dysfunction (see Chapter 29 regarding short bowel syndrome [SBS]). The severity of this condition depends on the length and location of the nonfunctional or resected bowel, and generally is diagnosed when the individual has less than 150 cm of small intestine remaining. The sequelae of SBS include malabsorption, malnutrition, dehydration, weight loss, fatigue, and lactose intolerance (Havrila et al., 2010).
Initially parenteral nutrition (PN) may be required, and frequent monitoring of fluids and electrolytes may be necessary for weeks or months. Individuals with SBS may require an oral diet restricted to defined formula tube feedings or to frequent small meals high in protein, low in fat and fiber, and lactose-free. Dietary supplements that contain vitamin B12; folic acid; thiamin; calcium; and vitamins A, E, and K are often indicated to prevent deficiencies. Serum concentrations of various minerals should also be monitored and adjusted as needed.
Total-Body Irradiation
Total-body irradiation (TBI) is a technique of radiation therapy that is used in hematopoietic cell transplantation (HCT) to eliminate malignant cells, to ablate the bone marrow and make room for the engraftment of the infused hematopoietic cells, and to suppress the immune system to decrease the risk of rejection. Commonly encountered side effects are fever, nausea, vomiting, headache, mucositis, parotitis (inflammation of the parotid glands), xerostomia, diarrhea, anorexia, fatigue, and associated weight loss.
Surgery
The surgical resection or removal of any part of the alimentary tract (mouth to anus), as well as the malignant disease process, can potentially impair normal digestion and absorption (Huhmann and August, 2010). Surgery may be used as a single mode of cancer treatment, or it may be combined with preoperative or postoperative adjuvant chemotherapy or radiation therapy. After surgery, individuals commonly experience fatigue, temporary changes in appetite and bowel function caused by anesthesia, and pain. They often require additional energy and protein for wound healing and recovery. Most side effects are temporary and dissipate after a few days following surgery. However, some surgical interventions have long-lasting nutritional implications (Table 37-8). When performing a nutrition assessment, it is very important to understand which part of the alimentary tract has been affected or surgically removed so the appropriate nutrition intervention can be recommended. Refer to Chapter 1 for a review gastrointestinal physiology.
TABLE 37-8
Nutrition-Related Effects of Surgery in Cancer Treatment


Data from Elliott L et al., editors: The clinical guide to oncology nutrition, ed 2, Chicago, 2006, American Dietetic Association; Huhmann MB, August D: Surgical oncology. In Marian M, Roberts S, editors: Clinical nutrition for oncology patients, Sudbury, MA, 2010, Jones and Bartlett.
Head and Neck Cancer
Individuals with head and neck cancer often have difficulty with chewing and swallowing caused by the cancer itself or the specific surgical intervention required to remove cancerous tissues. There can be additional problems because of history of smoking and alcohol abuse, illicit drug use, and subsequent poor nutrition intake, which place them at high risk for malnutrition and postoperative complications. Surgery often necessitates temporary or long-term reliance on EN support (e.g., PEG tube feedings). See Chapter 14. Individuals who resume oral intake often have prolonged dysphagia and require modifications of food consistency and extensive training in chewing and swallowing. Referrals to a speech therapist can yield dramatic positive results through evaluation and individualized instruction in swallowing and positioning techniques, as well as evaluation for aspiration risk.
Esophageal Cancer
Surgical intervention for treatment of esophageal cancer often requires partial or total removal of the esophagus. The stomach is commonly used for esophageal reconstruction. A feeding jejunostomy tube, which allows for early postoperative tube feedings, can be placed before an individual undergoes surgery or at the time of surgery. Usually the individual is able to progress to oral intake with specific dietary recommendations to minimize nutrition-related symptoms, which include reflux, dumping syndrome (discussed later in this chapter), dysmotility, gastroparesis, early satiety, vomiting, and fluid and electrolyte imbalances (Huhmann and August, 2010). Postsurgical recommendations include a low-fat diet with small, frequent feedings of energy-dense foods and avoidance of large amounts of fluids at any one time (see Chapter 28).
Gastric Cancer
Surgery is the most common treatment for cancer of the stomach, although chemotherapy and radiation therapy can be used before or after surgery to improve survival. Surgical interventions include partial, subtotal, or total gastrectomy. Placement of a jejunostomy feeding tube at surgery is advisable, and enteral nutrition (EN) support using a jejunal feeding tube is generally feasible within a few days after surgery.
Postgastrectomy syndrome encompasses a myriad of symptoms, including dumping syndrome, fat malabsorption, gastric stasis, lactose intolerance, anemias, and metabolic bone disease (osteoporosis, osteopenia, osteomalacia). Dumping syndrome is a common complication of gastric surgery, manifested by the rapid transit of foods or liquids, and the dilutional response of the small remaining stomach to highly osmotic bolus feedings. Individuals may experience GI and vasomotor symptoms such as abdominal cramps, diarrhea, nausea, vomiting, flushing, faintness, diaphoresis, and tachycardia (Huhmann and August, 2010). Individuals experiencing dumping syndrome should limit simple carbohydrates and liquids at meal times. See Chapter 28 for further recommendations for managing dumping syndrome.
Malabsorption is another complication of gastric surgery; deficiency of iron, folic acid, and less commonly vitamin B12 can lead to anemia. Micronutrient deficiencies of calcium and fat-soluble vitamins are also common (Huhmann and August, 2010). Individuals benefit from consumption of six to eight small meals per day, with fluids taken between meals. There may be fat intolerance, especially if the vagal nerve is severed. Administration of pancreatic enzymes with meals may help when the duodenal mixing of food and pancreatic juices is inadequate.
Pancreatic Cancer
Cancer of the pancreas, with or without surgical resection, can have significant nutritional consequences. The Whipple procedure and the pylorus-sparing pancreatic duodenectomy are the most common pancreatic cancer surgeries. Postsurgical complications include delayed gastric emptying, early satiety, glucose intolerance, bile acid insufficiency, diarrhea, and fat malabsorption. Pancreatic enzyme replacement, the use of small, more frequent low-fat meals and snacks, and avoidance of simple carbohydrates aid digestion and absorption.
Cancers of the Intestinal Tract
Partial or total resections of the intestinal tract because of colorectal cancer or carcinoid syndrome may induce profound losses of fluid and electrolytes secondary to decreased transit time and diarrhea, the severity of which is related to the length and site of the resection. Resections of as little as 15 cm of the terminal ileum can result in bile salt losses that exceed the liver’s capacity for resynthesis, and vitamin B12 absorption is affected. With depletion of the bile salt pool, steatorrhea develops. Nutrition intervention strategies consist of a diet low in fat, osmolality, lactose, and oxalates (see Chapters 29).
Hematopoietic Cell Transplantation (HCT)
HCT is performed for the treatment of certain hematologic cancers such as leukemia, lymphoma, and multiple myeloma. The stem cells used for HCT arise from bone marrow, peripheral blood, or umbilical cord blood. The preparative regimen includes cytotoxic chemotherapy, with or without total-body irradiation (TBI). This treatment regimen is followed by intravenous (IV) infusion of hematopoietic cells from the individual (autologous) or from a histocompatible related or unrelated donor (allogeneic) or from an identical twin (syngeneic) (National Marrow Donor Program, 2010).
HCT procedures can significantly affect nutrition status. Dietetic professionals should conduct a thorough nutrition assessment of the individual before the initiation of therapy and reassessments and monitoring throughout the entire transplant course. The acute toxicities of immunosuppression that can last for 2 to 4 weeks after the transplant include nausea, vomiting, anorexia, dysgeusia, stomatitis, oral and esophageal mucositis, fatigue, and diarrhea. In addition, immunosuppressive medications can also adversely affect nutrition status.
Individuals typically have little or no oral intake and the GI tract is compromised during the first few weeks following transplant. Parenteral nutrition (PN) has become a standard component of care (Robien, 2010). Gastrostomy tubes are useful for long-term nutrition support; the PN should be reserved for individuals who are unable to tolerate oral or enteral feeding (ADA, 2010a). In addition, administration of optimal levels of PN is often complicated by the frequent need to interrupt it for the infusion of antibiotics, blood products, and IV medications. Careful monitoring and the use of more concentrated nutrient solutions, increased flow rates and volumes, and double- or triple-lumen catheters are needed.
Autologous HCT involves the use of the individual’s own stem cells to reestablish hematopoietic stem cell function after the administration of high-dose chemotherapy. In some cases the use of mobilized stem cell progenitors has replaced autologous bone marrow as the source of hematopoietic progenitors for transplantation. Their use has shortened the period of pancytopenia (reduction in the cellular components of the blood), when individuals are at risk for bleeding, serious infections, or sepsis. These advances, along with improved prophylactic antibiotic regimens that are relatively easy to administer, have allowed individuals to receive autologous marrow transplantation in the outpatient setting. The reduced cost of transplantation has made it available to an increased number of people.
Because a majority of people receive much of their care outside the hospital, regular nutrition assessment and monitoring are important (Robien, 2010). The HCT procedure is associated with severe nutritional consequences that require prompt, proactive intervention. Nausea, vomiting, and diarrhea are caused by the cytotoxic conditioning regimen and may later accompany antibiotic administration. Complications of delayed-onset nutrition-related symptoms include varying degrees of mucositis, xerostomia, and dysgeusia. Mucositis, which is often severe and extremely painful, develops in more than 75% of transplant patients (see Figure 37-1).
Nutritional Precautions with Neutropenia
Individuals receiving HCT become immunocompromised and require supportive therapy, including medications and dietary changes to prevent infection. Of note, some cancer centers continue to prescribe a low-microbial or low-bacteria diet for people with low white blood cell counts (neutropenia). However, there is no clear evidence to support a strict “neutropenic” diet (only cooked foods) to reduce overall rates of infection or death (Gardner et al., 2008). Thus individuals should be instructed on food safety practices (Grant et al., 2010; Seattle Cancer Care Alliance [SCCA], 2010) that include:
• Avoidance of foods that contain unsafe levels of bacteria (raw meats, spoiled or moldy foods, and unpasteurized beverages)
• Special handling of raw meats, game, poultry, and eggs, utensils, cutting boards, and countertops
• Avoidance of untested well water
• Storage of foods at appropriate temperatures (below 40° F and above 140° F).
Graft-versus-Host Disease (GVHD)
Graft-versus-host disease (GVHD) is a major complication seen primarily after allogeneic transplants, in which the donated “donor” stem cells react against the tissues of the transplant recipient “host.” The functions of several target organs (skin, liver, gut, lymphoid cells) are disrupted and are susceptible to infection. Acute GVHD can occur within the first 100 days after the transplant and may be seen as early as 7 to 10 days posttransplant. It may resolve, or it may develop into a chronic form that requires long-term treatment and dietary management. Skin GVHD is characterized by a maculopapular rash. GVHD of the liver, evidenced by jaundice and abnormal liver function tests, often accompanies GI GVHD and further complicates nutrition management.
The symptoms of acute GI GVHD can be severe; individuals may experience gastroenteritis, abdominal pain, nausea, vomiting, and large volumes of secretory diarrhea. Immunosuppressive medications and a phased dietary regimen should be instituted (Charuhas, 2006; SCCA, 2010). The first phase consists of total bowel rest and the use of PN until diarrhea subsides. Nitrogen losses associated with diarrhea can be severe and are compounded by the high-dose corticosteroids used to treat GVHD. The second phase reintroduces oral feedings of beverages that are isosmotic, low-residue, and lactose-free so as to compensate for the loss of intestinal enzymes secondary to alterations in the intestinal villi and mucosa. If these beverages are tolerated, phase three includes the reintroduction of solids that contain low levels of lactose, fiber, fat, and total acidity and no gastric irritants. In phase four dietary restrictions are progressively reduced as foods are gradually introduced and tolerance is established. Phase five includes the resumption of the individual’s regular diet.
Chronic GVHD can develop up to 3 months after transplant and is observed with increased frequency in nonidentical related donors and unrelated donors. Chronic GVHD can affect the skin, oral mucosa (ulcerations, stomatitis, xerostomia), and the GI tract (anorexia, reflux symptoms, diarrhea) and can cause changes in body weight.
Another transplant-related complication is sinusoidal obstructive syndrome (SOS) (also known as venoocclusive disease), characterized by chemotherapy- or radiation therapy–induced damage to the hepatic venules. It can develop 1 to 3 weeks after transplant. Symptoms of right upper quadrant discomfort, hepatomegaly, fluid retention, and jaundice can occur; in severe cases individuals may experience progressive hepatic failure leading to encephalopathy and multiple-organ system failure. Nutrition support requires concentrated parenteral nutrients, careful fluid and electrolyte management, close monitoring, and adjustment of macronutrients and micronutrients based on the tolerance and response of each individual. The use of branched-chain amino acid formula is controversial. Serum ammonia level may not be a reliable indicator of protein tolerance, or of the development of encephalopathy (see Chapter 30.
Other acute or chronic complications of HCT include osteoporosis, pulmonary disease, impaired renal function, rejection of the graft, growth abnormalities in children, sepsis, and infection. Nutrition-related symptoms associated with HCT may persist; individuals receiving outpatient marrow transplantation require frequent monitoring and intervention.
Nutrition Monitoring and Evaluation
Dietetic professionals must determine and quantify their patients’ nutrition care goals by monitoring progress, measuring and evaluating outcomes and changes, and documenting this information throughout the process. See Chapter 11.
Physical Activity
Physical activity is an important part of cancer care. The effect of cancer and cancer treatment on the individual’s quality of life should be addressed throughout cancer treatment and continue until the individual is able to successfully resume activities of daily living. Recovery from cancer treatment also requires physical activity to rebuild muscle; regain strength, energy and flexibility; and help relieve symptoms of stress, anxiety, and even depression. Physical activity and exercise may be helpful in strengthening the immune system. However, before participating in any type of physical activity and exercise program, individuals should be advised to undergo evaluation by qualified professionals, who can then design an individualized physical assessment and activity plan. The American College of Sports Medicine (ACSM) now offers a certification program for trainers working with people diagnosed with cancer (a Certified Cancer Exercise Trainer) (ACSM, 2010). In addition, community-based programs such as the YMCA’s LiveStrong are available across the United States and offer physical activity and exercise opportunities to support cancer survivors; see www.livestrong.org/ymca.
Pediatric Cancer
Like adults, children with cancer can experience malnutrition and nutrition-related symptoms as a result of their cancer and its treatment. The incidence of malnutrition ranges from 6% to 50% in the pediatric population, depending on the type, stage, and location of the cancer. It usually has greater severity in the presence of more aggressive cancers in later stages of the disease.
Psychogenic food refusal in children requires interventions that address underlying psychological issues. Families and caregivers often express their fears of dying through an extreme preoccupation with eating and maintaining weight. Creative efforts are required to minimize the psychological effects of fear, unpleasant hospital routines, unfamiliar foods, learned food aversions, and pain. Nutrition intervention strategies that use oral intake should stress the maximum use of favorite, nutrient-dense foods during times when intake is likely to be best and food aversions are least likely to occur. Oral medical foods can be useful, but their acceptance is often a problem; thus children should be offered a selection from which to choose.
EN support by nasogastric tube is indicated for selected children who are able to cooperate and who have functional GI tracts. Some children have even been taught to pass their own nasogastric tube for intermittent or nighttime feedings. It should be remembered, however, that aspiration is always a potential risk. PN is indicated for children who are receiving intense treatment associated with severe GI toxicity, and for children with favorable prognoses who are malnourished or have a high risk of developing malnutrition. PN is seldom indicated for children with advanced cancer associated with significant deterioration or with diseases that are unresponsive to therapy.
Universally accepted, evidenced-based guidelines for children diagnosed with cancer do not exist. However, the American Society for Parenteral and Enteral Nutrition (ASPEN) has established standards for the nutrition screening and specialized nutrition support for all hospitalized pediatric patients (Wessel et al., 2005). The nutritional requirements of pediatric patients with cancer are similar, with an adjustment for activity, to those of normal growing children. Often pediatric patients with cancer are not bedridden, but are as active as their healthy peers. Factors that may alter nutrient requirements in cancer include the effect of the disease on host metabolism; the catabolic effects of cancer therapy; and physiologic stress from surgery, fever, malabsorption, and infection. Fluid requirements are increased during anticancer therapy or in the presence of fever, diarrhea, or renal failure. Micronutrients may require supplementation during periods of poor intake, stress, or malabsorption.
The best long-term indicator of adequate nutrient intake is growth. Children have increased nutritional requirements for growth and development that must be met despite extended periods of cancer treatment (see Chapters 17 through 19). A special vulnerability exists during the adolescent growth spurt. Ewing sarcoma is frequently associated with malnutrition.
Another reason why children with advanced cancer are at greater risk of severe nutritional depletion than adults is the frequent use of more aggressive, multimodality treatment. The long-term nutritional effects of cancer and its treatment in children are not well documented. Deficiencies in energy and protein can be expected to affect growth adversely, although the effects may be temporary, and catch-up growth depends on how much energy children are able to consistently consume (Corrales and Utter, 2005; Ringwald-Smith et al., 2006). However, some cancer treatment regimens may have an effect on growth and development that is independent of nutritional deprivation. HCT is now an accepted and increasingly successful intensive therapy for a wide range of disorders in children. Many supportive therapies may be safely managed in the outpatient arena, thus reducing the period of hospitalization.
Nutrition Recommendations for Cancer Survivors
From the time of diagnosis through the balance of life, the ACS defines anyone living with a cancer diagnosis as a cancer survivor (Doyle et al., 2006). The ACS guidelines as well as the WCRF and AICR recommendations provide sound diet, nutrition, and physical activity advice for primary cancer prevention and health for all individuals, including cancer survivors. In addition, the ACS has published a Guide for Informed Choices for Nutrition and Physical Activity for cancer survivors. The ACS specifically declined to call these a set of “guidelines” or “recommendations” because the evidence in this area of study is not as plentiful as in the primary prevention realm.
Cancer survivors represent one of the largest groups of people living with a chronic disease. It is estimated that there were 11 million survivors in the United States in 2009, and a projected number of 20 million in 2020 (Cancer Facts and Figures, 2009). The majority of individuals with cancer are able to return to full function and regain quality of life. This trend is expected to continue because of recent awareness in cancer prevention, advances in cancer detection, development of more effective cancer treatments, and advancements in determining the genetic causes of cancer. Nutrition can be a very important component in the long-term survival plan.
Complementary and Integrative Oncology
Integrative, complementary, and alternative medicine describe therapies used by persons interested in the promotion of health or symptom management. Complementary therapies are typically noninvasive, inexpensive, and useful in controlling symptoms and improving quality of life during and after cancer treatment; they are used in addition to conventional medicine. Conversely, alternative medicine is used instead of conventional anticancer treatment; it can be expensive, possibly harmful, and may interfere with treatments or medications. Integrative medicine or integrative oncology is emerging as the preferred term to differentiate between alternative therapies that are unproven and potentially unsafe, and therapies that are more evidenced-based (Belk, 2006; Wesa et al., 2008). Integrative medicine works to integrate evidence-based complementary therapies into conventional cancer treatment. Integrative medicine uses strategies to promote self-empowerment, individual responsibility, and lifestyle changes that can potentially reduce risk for both cancer recurrence and second primary tumors (Sagar, 2009). Most experts agree that a large proportion of cancer survivors participate in these modalities; several studies report more than 90% participate in some form of CAM during and after treatment (Hardy, 2008).
The health care team working in oncology needs to be informed on the different therapies and knowledgeable regarding resources used to evaluate and educate the individuals in their care. Some cancer centers are fortunate; increasing consumer demand has encouraged health care institutions to create “Integrative Medicine” departments with onsite complementary services. Cancer survivors look for open, honest discussions or recommendations from their health care team. Medical, nursing, and nutrition assessments should include open-ended questions on dietary supplement use, such as, “What vitamins, minerals, herbs, or other dietary supplements are you currently taking?” and questions regarding the additional integrative or complementary therapies they are following at this time. The core components to discuss CAM therapies involve understanding and respecting the need people have to do something for themselves; having a willingness to listen to, explore, and respond frankly to questions; taking the time to discuss the options and offer advice; summarizing the discussion; documenting the dialog; and monitoring the progress of the therapy.
The NIH established a National Center for Complementary and Alternative Medicine (NCCAM) in 1999 and works to create a framework in which to evaluate and research CAM. See Box 37-2.
Dietary Supplements
The most common form of CAM practiced in the United States is the use of dietary supplements. Consumers spend in excess of $23 billion every year on natural products marketed to maintain or enhance health (Ashar, 2008). The largest percentage (18%) use nonvitamin, nonmineral natural products such as fish oil supplements and ginseng (Barnes, 2008). That number jumps significantly when surveying cancer survivors, in whom significant use occurs (Hardy, 2008). A primary motivation is symptom management but most also hope for tumor suppression (Wesa et al., 2008).
Nondisclosure of use is a common occurrence, with a reported 53% of individuals receiving chemotherapy not discussing use of dietary supplements with their health care team (Hardy, 2008). Unfortunately, many people view dietary supplements as natural, inexpensive alternatives for prescription medications or a quick, easy remedy to an underlying medical problem. Distrust of the medical system, fear of being “fired” or dismissed by their doctor, or anticipated ignorance from the health care team may prevent cancer survivors from discussing use of dietary supplements. Ashar and Lee outline five steps: 1) inquire about use, 2)evaluate the supplement, 3) discuss any relevant regulatory issues, 4) discuss available safety and efficacy data, and 5) compare risks and benefits of use to available conventional therapies (Ashar et al., 2008). Although time consuming, conversations that include most or all of these steps will not only help open lines of communication between health care provider and survivor, but also help prevent an adverse event or poor treatment decisions. Table 37-9 lists some of the commonly used supplements.
TABLE 37-9
Potential Adverse Events with Integrative Therapies Commonly Used by Cancer Survivors
| Dietary Supplement | Claim and Common Uses | Potential Adverse Event |
| Echinacea | Boosts the immune system. | May cause inflammation of the liver if used in conjunction with other medications such as anabolic steroids, methotrexate (chemotherapy), or others. |
| Garlic | Helps lower cholesterol. | May increase risk for excessive bleeding, especially when used with certain anticlotting agents. |
| Ginger | Helps with nausea. | May increase risk for excessive bleeding, especially when used with certain anticlotting agents. |
| Ginkgo | Helps increase blood circulation and oxygenation. Enhances memory and mental concentration. |
May increase risk for excessive, bleeding especially when used with certain anticlotting agents. |
| Ginseng | Increases physical stamina and mental concentration. | May increase risk for excessive bleeding, especially when used with certain anticlotting agents; may increase heart rate and high blood pressure; may increase bleeding in women after menopause. |
| Goldenseal | Helps reduce inflammation and promote good bowel function. | May worsen swelling or high blood pressure. |
| Licorice | Helps soothe the stomach. | Certain licorice mixtures may cause high blood pressure, increase swelling, and cause electrolyte imbalances. |
| Saw palmetto | Helps with enlarged prostate and urinary inflammation. | May interact with other hormone therapies. |
| St. John’s wort | Helps with mild to moderate depression, anxiety, or sleep disorders. | May decrease effectiveness of all currently marketed medication using cytochrome P450 pathway in the liver: HIV and AIDS medications (NNRTIs and PIs), carbamazepine, cyclosporine, irinotecan (Camptosar) for chemotherapy, midazolam (Versed), nifedipine (Procardia), simvastatin (Zocor), theophylline, warfarin (Coumadin). |
| Valerian | Helps as mild sedative or sleep aid, or muscle relaxant. | May increase effects of certain antiseizure medications or prolong effects of anesthetic agents. |
AIDS, Acquired immune deficiency syndrome; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Data from Natural Standards, 2010.
Diet Therapies
Metabolic therapy is a term used for a variety of cancer management methods, including unproven and disproved diagnostic methods and treatments (ACS, 2009b). Metabolic practitioners generally claim that diseases, including cancer, are caused by an accumulation of toxic substances in the body. They allege that, if these toxins are removed, the body can heal itself naturally. Three basic steps are common to metabolic therapy: detoxification, strengthening of the immune system, and the use of special modalities to attack cancer. These therapy regimens generally include colonic cleansing with coffee, wheat grass, or other substances; special diets; and vitamin and mineral supplementation. Complications of colonic irrigation include electrolyte imbalance, toxic colitis, bowel perforation, and sepsis. Most regimens promote “natural” and “organic” foods and recommend restriction of animal products, refined flours and sugars, and foods that are processed or contain artificial ingredients. Examples of metabolic therapies include the Gerson therapy, the Gonzalez regimen, the Livingston-Wheeler therapy and the Issels treatment. The special diets associated with cancer care promote the idea that food is medicine. Diet plans are individualized and food specifically chosen and prepared.
The macrobiotic diet and lifestyle is a program promoting natural healing, popularized in the United States by Michio Kushi in the late 1970s. This macrobiotic diet derives 40% to 60% of its calories from whole grains; 20% to 30% from vegetables; and the remainder from beans, bean products, sea vegetables, fruit, seeds, nuts, white-meat fish, and very occasionally seafood, poultry, red meat, eggs, and dairy (Kushi et al., 2006) (Figure 37-2). Research has determined that the diet is naturally deficient in calcium and vitamin B12. The macrobiotic diet has not been scientifically proven to treat or cure cancer.
Orthomolecular Medicine
Orthomolecular medicine (OM) is the practice of restoring an optimal environment in the body by correcting imbalances and deficiencies, another alternative medicine practice in cancer care. The treatment is based on the theory that by correcting imbalances and deficiencies, the body will regain health. This has not been proven in clinical trials, and instead is extrapolated from basic science. Infusions or supplementation may involve large doses of vitamins, minerals, essential fatty acids, fiber, amino acids, or enzymes. Orthomolecular practitioners (often medical doctors) consider a number of CAM practices to be consistent with OM philosophy and may incorporate parts of naturopathic medicine, nutrition, acupuncture, mind-body therapies, and manipulative and body-based practices such as massage into their treatments.
Advanced Cancer and Palliative Care
Palliative care is the active total care of an individual when curative measures are no longer considered an option by either the medical team or the individual. Hospice care focuses on relieving symptoms and supporting individuals with a life expectancy of months, not years (NHPCO, 2010). The objectives are to provide for optimal quality of life; relieve physical symptoms; alleviate isolation, anxiety, and fear associated with advanced disease; and to help patients maintain independence as long as possible (McCallum and Fornari, 2006). The goals of nutrition intervention should focus on managing nutrition-related symptoms such as pain, weakness, loss of appetite, early satiety, constipation, weakness, dry mouth, and dyspnea (McCallum and Fornari, 2006). Another important goal is maintaining strength and energy to enhance quality of life, independence, and ability to perform activities of daily living. Nutrition should be provided “as tolerated or as desired” along with emotional support and awareness of and respect for individual needs and wishes. Thus the pleasurable aspects of eating should be emphasized, without concern for quantity or nutrient and energy content.
The use of nutrition support and hydration in individuals with advanced, incurable cancer is a difficult and often controversial issue and should be determined on a case-by-case basis. Advance directives are legal documents that guide health care providers regarding the specific wishes of individuals, outlining the extent of their desired medical care, including the provision of artificial nutrition and hydration. Whenever providing nutrition care, consideration should be given to advanced directives that may be in place.
American Institute for Cancer Research
National Center for Complementary and Alternative Medicine (NCCAM)
Oncology Nutrition Practice Group
http://www.iom/edu/en/Reports/2005/From-Cancer-Patient-to-Cancer-Survivor-Lost-in-Transition.aspx
References
American Cancer Society. Cancer facts & figures, 2009. Atlanta: American Cancer Society; 2009.
American Cancer Society. Cancer glossary. Accessed 10 June 2010 from http://www.cancer.org/CancerGlossary/index, 2010.
American Cancer Society. Cancer prevention & early detection facts & figures, 2010. Atlanta: American Cancer Society; 2010.
American Cancer Society. Complete guide to complementary and alternative cancer therapies, ed 2. Atlanta: American Cancer Society; 2009.
American College of Sports Medicine (ACSM). Certified cancer exercise trainer. Accessed 26 October 2010 from www.acsm.org, 2010.
American Dietetic Association(ADA). Evidence analysis library: oncology evidence-based nutrition practice guidelines. Chicago: American Dietetic Association; 2010.
American Dietetic Association (ADA). International dietetics & terminology: reference manual, standardized language for the nutrition care process. Chicago: American Dietetic Association; 2011.
American Dietetic Association (ADA). Oncology toolkit. Chicago: American Dietetic Association; 2010.
American Dietetic Association (ADA). Position of the American Dietetic Association: Health Implications of Dietary Fiber. J American Dietetic Association. 108, 2008.
Anderson, AS, et al. Obesity Management-An Opportunity for Cancer Prevention. Surgeon. 2009;7:5.
Ashar, BH, et al. Advising patients who use dietary supplements. Am J Med. 121, 2008.
Bailey, RL, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr. 2010;91:231.
Baan, R, et al. Carcinogenicity of alcoholic beverages. Lancet Oncology. 8, 2007.
Barnes PM, et al: Complementary and alternative medicine use among adults and children: United States, 2007, National Health Statistics Reports, US Department of Health and Human Services, Centers for Disease Control, 10, 2008
Belk, LB. Primer on integrative oncology. Hematol Oncol Clin N Am. 20, 2006.
Beronius, A, et al. Risk to all or none? A comparative analysis of controversies in the health risk assessment of bisphenol A. Reprod Toxicol. 2010;29:1.
Berquin, IM, et al. Multi-targeted therapy of cancer by omega-3 fatty acids. Science Direct. 269, 2008.
Blackburn, GL, et al. Metabolic Syndrome and the Onset of Cancer. American Journal of Clinical Nutrition. 2007;86:3.
Block, KI, et al. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2008;123:1227.
Brawer, R, et al. Obesity and Cancer. Primary Care Clinical Office Practice. 36, 2009.
Bruner, DW, et al. Manual for radiation oncology and nursing practice and education, ed 3. Pittsburgh: Oncology Nursing Society; 2005.
Cady, J. Nutritional support during radiotherapy for head and neck: the role of prophylactic feeding tube placement. J Clin Onc Nurs. 2007;11:875.
2009. Cancer facts and figures. Accessed 12 December 2009 from www.cancer.org, 2009.
Charney, P, Cranganu, A. Nutrition screening and assessment in oncology. In: Marian M, Roberts S, eds. Clinical nutrition for oncology patients. Sudbury, MA: Jones and Bartlett, 2010.
Charuhas, PM. Medical nutrition therapy in bone marrow transplantation. In Elliott L, et al, eds.: The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
Chey, H, et al. Toxins in everyday life. Prim Care Clin Office Pract. 35, 2008.
Chung, M, et al. Vitamin D and Calcium: systematic review of health outcomes. Evid Rep Tech Assess. 183, 2009.
Corrales, KM, Utter, SL. Growth failure. In Samour PQ, King K, eds.: Handbook of pediatric nutrition, ed 3, Sudbury, MA: Jones and Bartlett, 2005.
Doyle, C, et al. The 2006 Nutrition, Physical Activity and Cancer Survivorship Advisory Committee. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353.
Elliott L, et al, eds. The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
Farhadian, A, et al. Determination of polycyclic aromatic hydrocarbons in grilled meat. Food Control. 21, 2010.
Fearon, KC. Cancer cachexia: developing multimodal therapy for a multidimensional problem. Eur J Cancer. 2008;44:1124.
Ferguson, LR. Meat and Cancer. Meat Science. 2010;84:308.
Flegel, KM, et al. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:3.
Food and Drug Administration (FDA). News and events: bisphenol A (BPA): update on bisphenol A (BPA) for use in food. Accessed 3 November 2010 from www.fda.gov, January 2010.
Fuhrman, MP. Nutrition-focused physical assessment. In: Charney P, Malone A, eds. Nutrition assessment. Chicago: American Dietetic Association, 2009.
Gardner, A, et al. Randomized comparison of cooked and non-cooked diet in patient undergoing remission induction therapy for acute myeloid leukemia. J Clin Oncol. 2008;26:5684.
Garland, CF, et al. Vitamin D for cancer prevention: global perspective. Annual of Epidemiology. 2009;19:468.
Garland, CF, et al. Vitamin d supplement doses and serum 25 hydroxy d in the range associated with cancer prevention. Anticancer Res. 2011;31:607.
Goldman, A, et al. Symptoms in children/young people with progressive malignant disease: United Kingdom Children’s Cancer Study Group/Paediatric Oncology Nurses Forum survey. Pediatrics. 2006;117:1179.
Gottschlich MM, ed. The A.S.P.E.N. nutrition support core curriculum: a case-based approach—the adult patient. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition, 2007.
Grant, B, Byron, J. Nutritional implications of chemotherapy. In Elliott L, et al, eds.: The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
Grant BL, et al, eds. American Cancer Society’s complete guide to nutrition for cancer survivors, ed 2, Atlanta: American Cancer Society, 2010.
Grant BL, Hamilton KK, eds. Management of nutrition impact symptoms in cancer and educational handouts. Chicago: American Dietetic Association, 2005.
Greenwald, P, et al. The challenge of nutrition in cancer. In: Blackburn V, et al, eds. Nutritional oncology. St Louis: Elsevier, 2006.
Hardy, ML. Dietary supplement use in cancer care: help or harm. Hematol Oncol Clin N Am. 22, 2008.
Harris, DJ, et al. Putting evidence into practice: evidence-based interventions for the management of oral mucositis. J Clin Onc Nurs. 2008;12:141.
Havrila, C, et al. Medical and radiation oncology. In: Marian M, Roberts S, eds. Clinical nutrition for oncology patients. Sudbury, MA: Jones and Bartlett, 2010.
Huhmann, MB, August, D. Surgical oncology. In: Marian M, Roberts S, eds. Clinical nutrition for oncology patients. Sudbury, MA: Jones and Bartlett, 2010.
Huncharek, M, et al. Colorectal cancer risk and dietary intake of calcium, vitamin D and dairy products: a meta-analysis of 26,355 cases from 60 observational studies. Nutr Cancer. 2009;61:1.
Hurst, JD, Gallagher, AL. Energy, protein, micronutrient, and fluid requirement. In Elliott L, et al, eds.: The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
Kashfi, K. Anti-inflammatory agents as cancer therapeutics. Adv Pharmacol. 57, 2009.
Kushi, LH, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:310.
Kuzuhara, T, et al. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Letter. 261, 2008.
Kwan, M, et al. Alcohol Consumption and Breast Cancer Recurrence and Survival Among Women with Early–State Breast Cancer: The Life After Cancer Epidemiology Study. J Clin Oncol. 2010;10:1200.
Layton L: Reversing itself, FDA expresses concerns over health risks from BPA, Washington Post Saturday, 16 January 2010.
Lee, SA, et al. Adolescent and adult soy food intake and breast cancer risk: results from the Shanghai women’s health study. Breast Dis. 2010;21:2.
Levine, AJ, et al. A candidate gene study of folate-associated one carbon metabolism genes and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19:1812.
Link, A, et al. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80:1.
Longo, V, et al. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends in Pharmacological Sciences. 2010;31:2.
McCallum, PD. Nutrition screening and assessment in oncology. In Elliott L, et al, eds.: The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
McCallum, PD, Fornari, A. Nutrition therapy in palliative care. In Elliott L, et al, eds.: The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
Miller, M, et al. Dietary supplement use in individuals living with cancer and other chronic conditions: a population-based study. J Am Diet Assoc. 2008;108:3.
Muehlbauer, PM, et al. Putting evidence into practice: evidence-based interventions to prevent, manage and treat chemotherapy and radiotherapy-induced diarrhea. J Clin Onc Nurs. 2009;13:336.
National Cancer Institute (NCI). Cancer bulletin—cost of cancer care has doubled in the past 20 years. Accessed 23 October 2010 from http://www.cancer.gov/ncicancerbulletin/051810/page10, 2010.
National Cancer Institute (NCI). Cancer genetics overview PDQ (Health Professional Version). Accessed 23 October 2010 from http://www.cancer.gov/cancertopics/pdq/genetics/overview/healthprofessional, 2010.
National Cancer Institute (NCI). Chemotherapy and you. Accessed 18 October 2010 from http://www.cancer.gov/publications/, 2010.
National Cancer Institute (NCI). Dictionary of terms. Accessed 23 October 2010 from http://www.cancer.gov/dictionary/, 2010.
National Cancer Institute (NCI). Eating hints. Accessed 20 October 2010 from http://www.cancer.gov/publications/, 2010.
National Cancer Institute (NCI). Radiation therapy and you. Accessed 23 October 2010 from http://cancer.gov/publications/, 2010.
National Cancer Institute (NCI). SEER stat fact sheets—cancer of all sites. Accessed 23 October 2010 from http://seer.cancer.gov/statfacts/html/all.print.html#incidence-mortality, 2010.
National Center for Complementary and Alternative Medicine (NCCAM). Main page. Accessed 23 October 2010 from http://nccam.nih.gov/, 2010.
National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology (NCCN guidelines). Accessed 24 October 2010 from http://www.nccn.org/clinical.asp, 2010.
Natural Standards Database. subscription www.naturalstandard.com. [accessed 11.10].
National Hospice and Palliative Care Organization (NHPCO). How can palliative care help? Accessed 23 October 2010 from http://www.nhpco.org, 2010.
National Institute of Dental and Craniofacial Research (NIDCR). Cancer treatment and oral health. Accessed 23 October 2010 from http://www.nidcr.nih.gov/OralHealth/Topics/CancerTreatment/, 2010.
National Marrow Donor Program (NMDP). Types of transplants. Accessed 23 October 2010 from http://www.marrow.org, 2010.
Oaks, BM, et al. Folate intake, post-folic acid grain fortification, and pancreatic cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2010;91:449.
Park, Y, et al. Dietary Fiber intake and risk of breast cancer in postmenopausal women: the National Institutes of Health-AARP Diet and Health Study. American Journal of Clinical Nutrition. 90, 2009.
Parekh, N, et al. Lifestyle, Anthropometric, and Obesity-Related Physiologic Determinants of Insulin-like Growth Factor-1 in the Third National Health and Nutrition Examination Survey (1988-1994). Annuals of Epidemiology. 2010;20:3.
Polednak, AP. Estimating the number of US incident cancers attributable to obesity and the impact on temporal trends in incidence rates for obesity-related cancers. Cancer Detection and Prevention. 2008;32:190.
Pierce, JP, et al. Influence of a diet very high in vegetables, fruit and fiber and low in fat following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298:3.
Pollack, M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. 2008;22:4.
Polovich, M, et al. Chemotherapy and biotherapy guidelines and recommendations for practice. Pittsburgh: Oncology Nursing Society; 2009.
Ringwald-Smith, K, et al. Medical nutrition therapy in pediatric oncology. In Elliott L, et al, eds.: The clinical guide to oncology nutrition, ed 2, Chicago: American Dietetic Association, 2006.
Robien, K. Hematological malignancies. In: Marian M, Roberts S, eds. Clinical nutrition for oncology patients. Sudbury, MA: Jones and Bartlett, 2010.
Robien, K, et al. American Dietetic Association: revised standards of practice and standards of professional performance for registered dietitians (generalist, specialty, and advanced) in oncology nutrition care. J Am Diet Assoc. 2010;110:310.
Russo, GL. Ins and Outs of Dietary Phytochemical in Cancer Prevention. Biochemical Pharmacology. 74, 2007.
Russell, M, Malone, A. Nutrient requirements. In: Charney P, Malone A, eds. Nutrition assessment. Chicago: American Dietetic Association, 2009.
Sagar, SM. The role of integrative medicine in a tertiary prevention survivorship program. Prev Med. 40, 2009.
Schelbert, KB. Comorbities of obesity. Prim Care. 2009;36:271.
Seattle Cancer Care Alliance (SCCA). Diet guidelines for immunosuppressed patients. Accessed 30 September 2010 from http://www.seattlecca.org/general-oncology-diet-guidelines.cfm, 2010.
Skipworth, RJ, et al. Pathophysiology of cancer cachexia: much more than host-tumour interaction? Clin Nutr. 2007;266:667.
Teucher, B, et al. Obesity: Focus on all-cause mortality and cancer. Maturitas. 65, 2010.
Toles, M, et al. Nutrition and the cancer survivor: evidence to guide oncology nursing practice. Semin Oncol Nurs. 2008;24:3.
Tipton, JM, et al. Putting evidence into practice: evidence-based interventions to prevent, manage and treat chemotherapy-induced nausea and vomiting. J Clin Onc Nurs. 2007;11:70.
U.S. Department of Agriculture (USDA). USDA-Iowa State University database on the isoflavone content of foods. Accessed 23 October 2010 from http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isfl_doc.pdf, 2010.
Valavanidis, A, et al. 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120.
Wesa, K, et al. Integrative oncology: complementary therapies for cancer survivors. Hematol Oncol Clin N Am. 22, 2008.
Wessel, J, et al. American Society for Parenteral and Enteral Nutrition: task force on standards for specialized nutrition support for hospitalized pediatric patients. Nutr Clin Pract. 2005;20:103.
Wilkes, GM, Barton-Burke, M. 2010 oncology nursing drug handbook. Boston: Jones and Bartlett; 2010.
World Cancer Research Fund (WCRF), American Institute for Cancer Research (AICR). Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: WCRF and AICR; 2007.