1 What is pharmacology?
Overview
In this introductory chapter, we explain how pharmacology came into being and evolved as a scientific discipline, and describe the present day structure of the subject and its links to other biomedical sciences. The structure that has emerged forms the basis of the organisation of the rest of the book. Readers in a hurry to get to the here-and-now of pharmacology can safely skip this chapter.
What Is A Drug?
For the purposes of this book, a drug can be defined as a chemical substance of known structure, other than a nutrient or an essential dietary ingredient,1 which, when administered to a living organism, produces a biological effect.
A few points are worth noting. Drugs may be synthetic chemicals, chemicals obtained from plants or animals, or products of genetic engineering. A medicine is a chemical preparation, which usually but not necessarily contains one or more drugs, administered with the intention of producing a therapeutic effect. Medicines usually contain other substances (excipients, stabilisers, solvents, etc.) besides the active drug, to make them more convenient to use. To count as a drug, the substance must be administered as such, rather than released by physiological mechanisms. Many substances, such as insulin or thyroxine, are endogenous hormones but are also drugs when they are administered intentionally. Many drugs are not used in medicines but are nevertheless useful research tools. In everyday parlance, the word drug is often associated with addictive, narcotic or mind-altering substances—an unfortunate negative connotation that tends to bias uninformed opinion against any form of chemical therapy. In this book, we focus mainly on drugs used for therapeutic purposes but also describe important examples of drugs used as experimental tools. Although poisons fall strictly within the definition of drugs, they are not covered in this book.
Origins and Antecedents
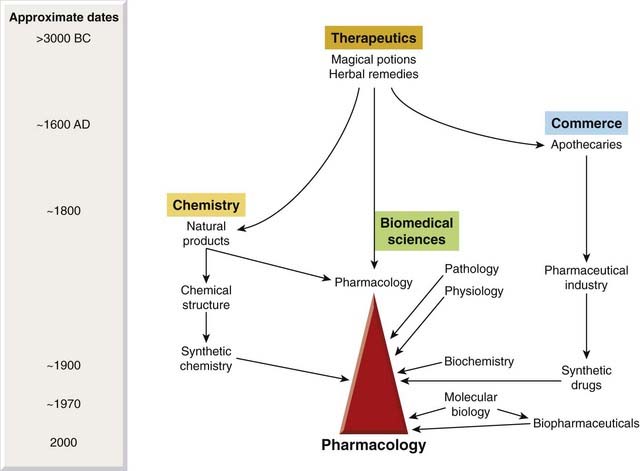
Pharmacology can be defined as the study of the effects of drugs on the function of living systems. As a science, it was born in the mid-19th century, one of a host of new biomedical sciences based on principles of experimentation rather than dogma that came into being in that remarkable period. Long before that—indeed from the dawn of civilisation—herbal remedies were widely used, pharmacopoeias were written, and the apothecaries’ trade flourished, but nothing resembling scientific principles was applied to therapeutics. Even Robert Boyle, who laid the scientific foundations of chemistry in the middle of the 17th century, was content, when dealing with therapeutics (A Collection of Choice Remedies, 1692), to recommend concoctions of worms, dung, urine and the moss from a dead man’s skull. The impetus for pharmacology came from the need to improve the outcome of therapeutic intervention by doctors, who were at that time skilled at clinical observation and diagnosis but broadly ineffectual when it came to treatment.2 Until the late 19th century, knowledge of the normal and abnormal functioning of the body was too rudimentary to provide even a rough basis for understanding drug effects; at the same time, disease and death were regarded as semisacred subjects, appropriately dealt with by authoritarian, rather than scientific, doctrines. Clinical practice often displayed an obedience to authority and ignored what appear to be easily ascertainable facts. For example, cinchona bark was recognised as a specific and effective treatment for malaria, and a sound protocol for its use was laid down by Lind in 1765. In 1804, however, Johnson declared it to be unsafe until the fever had subsided, and he recommended instead the use of large doses of calomel (mercurous chloride) in the early stages—a murderous piece of advice which was slavishly followed for the next 40 years.
The motivation for understanding what drugs can and cannot do came from clinical practice, but the science could be built only on the basis of secure foundations in physiology, pathology and chemistry. It was not until 1858 that Virchow proposed the cell theory. The first use of a structural formula to describe a chemical compound was in 1868. Bacteria as a cause of disease were discovered by Pasteur in 1878. Previously, pharmacology hardly had the legs to stand on, and we may wonder at the bold vision of Rudolf Buchheim, who created the first pharmacology institute (in his own house) in Estonia in 1847.
In its beginnings, before the advent of synthetic organic chemistry, pharmacology concerned itself exclusively with understanding the effects of natural substances, mainly plant extracts—and a few (mainly toxic) chemicals such as mercury and arsenic. An early development in chemistry was the purification of active compounds from plants. Friedrich Sertürner, a young German apothecary, purified morphine from opium in 1805. Other substances quickly followed, and, even though their structures were unknown, these compounds showed that chemicals, not magic or vital forces, were responsible for the effects that plant extracts produced on living organisms. Early pharmacologists focused most of their attention on such plant-derived drugs as quinine, digitalis, atropine, ephedrine, strychnine and others (many of which are still used today and will have become old friends by the time you have finished reading this book).3
Pharmacology in the 20th and 21st Centuries
Beginning in the 20th century, the fresh wind of synthetic chemistry began to revolutionise the pharmaceutical industry, and with it the science of pharmacology. New synthetic drugs, such as barbiturates and local anaesthetics, began to appear, and the era of antimicrobial chemotherapy began with the discovery by Paul Ehrlich in 1909 of arsenical compounds for treating syphilis. Further breakthroughs came when the sulfonamides, the first antibacterial drugs, were discovered by Gerhard Domagk in 1935, and with the development of penicillin by Chain and Florey during the Second World War, based on the earlier work of Fleming.
These few well-known examples show how the growth of synthetic chemistry, and the resurgence of natural product chemistry, caused a dramatic revitalisation of therapeutics in the first half of the 20th century. Each new drug class that emerged gave pharmacologists a new challenge, and it was then that pharmacology really established its identity and its status among the biomedical sciences.
In parallel with the exuberant proliferation of therapeutic molecules—driven mainly by chemistry—which gave pharmacologists so much to think about, physiology was also making rapid progress, particularly in relation to chemical mediators, which are discussed in depth elsewhere in this book. Many hormones, neurotransmitters and inflammatory mediators were discovered in this period, and the realisation that chemical communication plays a central role in almost every regulatory mechanism that our bodies possess immediately established a large area of common ground between physiology and pharmacology, for interactions between chemical substances and living systems were exactly what pharmacologists had been preoccupied with from the outset. The concept of ‘receptors’ for chemical mediators, first proposed by Langley in 1905, was quickly taken up by pharmacologists such as Clark, Gaddum, Schild and others and is a constant theme in present day pharmacology (as you will soon discover as you plough through the next two chapters). The receptor concept, and the technologies developed from it, have had a massive impact on drug discovery and therapeutics. Biochemistry also emerged as a distinct science early in the 20th century, and the discovery of enzymes and the delineation of biochemical pathways provided yet another framework for understanding drug effects. The picture of pharmacology that emerges from this brief glance at history (Fig. 1.1) is of a subject evolved from ancient prescientific therapeutics, involved in commerce from the 17th century onwards, and which gained respectability by donning the trappings of science as soon as this became possible in the mid-19th century. Signs of its carpetbagger past still cling to pharmacology, for the pharmaceutical industry has become very big business and much pharmacological research nowadays takes place in a commercial environment, a rougher and more pragmatic place than the glades of academia.4 No other biomedical ‘ology’ is so close to Mammon.
Alternative Therapeutic Principles
Modern medicine relies heavily on drugs as the main tool of therapeutics. Other therapeutic procedures such as surgery, diet, exercise, etc. are also important, of course, as is deliberate non-intervention, but none is so widely applied as drug-based therapeutics.
Before the advent of science-based approaches, repeated attempts were made to construct systems of therapeutics, many of which produced even worse results than pure empiricism. One of these was allopathy, espoused by James Gregory (1735–1821). The favoured remedies included blood letting, emetics and purgatives, which were used until the dominant symptoms of the disease were suppressed. Many patients died from such treatment, and it was in reaction against it that Hahnemann introduced the practice of homeopathy in the early 19th century. The guiding principles of homeopathy are:
The system rapidly drifted into absurdity: for example, Hahnemann recommended the use of drugs at dilutions of 1 : 1060, equivalent to one molecule in a sphere the size of the orbit of Neptune.
Many other systems of therapeutics have come and gone, and the variety of dogmatic principles that they embodied have tended to hinder rather than advance scientific progress. Currently, therapeutic systems that have a basis which lies outside the domain of science are actually gaining ground under the general banner of ‘alternative’ or ‘complementary’ medicine. Mostly, they reject the ‘medical model’, which attributes disease to an underlying derangement of normal function that can be defined in biochemical or structural terms, detected by objective means, and influenced beneficially by appropriate chemical or physical interventions. They focus instead mainly on subjective malaise, which may be disease-associated or not. Abandoning objectivity in defining and measuring disease goes along with a similar departure from scientific principles in assessing therapeutic efficacy and risk, with the result that principles and practices can gain acceptance without satisfying any of the criteria of validity that would convince a critical scientist, and that are required by law to be satisfied before a new drug can be introduced into therapy. Public acceptance, alas, has little to do with demonstrable efficacy.5
The Emergence of Biotechnology
Since the 1980s, biotechnology has emerged as a major source of new therapeutic agents in the form of antibodies, enzymes and various regulatory proteins, including hormones, growth factors and cytokines (see Buckel, 1996; Walsh, 2003). Although such products (known as biopharmaceuticals) are generally produced by genetic engineering rather than by synthetic chemistry, the pharmacological principles are essentially the same as for conventional drugs. Looking further ahead, gene- and cell-based therapies (Ch. 59), although still in their infancy, will take therapeutics into a new domain. The principles governing the design, delivery and control of functioning artificial genes introduced into cells, or of engineered cells introduced into the body, are very different from those of drug-based therapeutics and will require a different conceptual framework, which texts such as this will increasingly need to embrace if they are to stay abreast of modern medical treatment.
Pharmacology Today
As with other biomedical disciplines, the boundaries of pharmacology are not sharply defined, nor are they constant. Its exponents are, as befits pragmatists, ever ready to poach on the territory and techniques of other disciplines. If it ever had a conceptual and technical core that it could really call its own, this has now dwindled almost to the point of extinction, and the subject is defined by its purpose—to understand what drugs do to living organisms, and more particularly how their effects can be applied to therapeutics—rather than by its scientific coherence.
Figure 1.2 shows the structure of pharmacology as it appears today. Within the main subject fall a number of compartments (neuropharmacology, immunopharmacology, pharmacokinetics, etc.), which are convenient, if not watertight, subdivisions. These topics form the main subject matter of this book. Around the edges are several interface disciplines, not covered in this book, which form important two-way bridges between pharmacology and other fields of biomedicine. Pharmacology tends to have more of these than other disciplines. Recent arrivals on the fringe are subjects such as pharmacogenomics, pharmacoepidemiology and pharmacoeconomics.
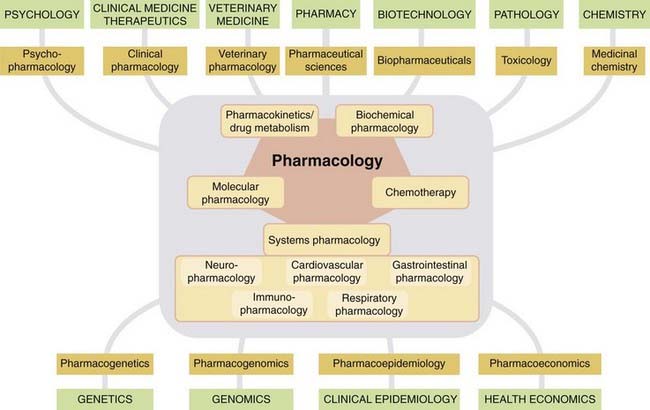
Fig. 1.2 Pharmacology today with its various subdivisions.
Interface disciplines (brown boxes) link pharmacology to other mainstream biomedical disciplines (green boxes).
Biotechnology
Originally, this was the production of drugs or other useful products by biological means (e.g. antibiotic production from microorganisms or production of monoclonal antibodies). Currently in the biomedical sphere, biotechnology refers mainly to the use of recombinant DNA technology for a wide variety of purposes, including the manufacture of therapeutic proteins, diagnostics, genotyping, production of transgenic animals, etc. The many non-medical applications include agriculture, forensics, environmental sciences, etc.
Pharmacogenetics
This is the study of genetic influences on responses to drugs. Originally, pharmacogenetics focused on familial idiosyncratic drug reactions, where affected individuals show an abnormal—usually adverse—response to a class of drug (see Nebert & Weber, 1990). It now covers broader variations in drug response, where the genetic basis is more complex.
Pharmacogenomics
This recent term overlaps with pharmacogenetics, describing the use of genetic information to guide the choice of drug therapy on an individual basis. The underlying principle is that differences between individuals in their response to therapeutic drugs can be predicted from their genetic make-up. Examples that confirm this are steadily accumulating (see Ch. 11). So far, they mainly involve genetic polymorphism of drug-metabolising enzymes or receptors (see Weinshilboum & Wang, 2004; Swen et al., 2007). Ultimately, linking specific gene variations with variations in therapeutic or unwanted effects of a particular drug should enable the tailoring of therapeutic choices on the basis of an individual’s genotype. Steady improvements in the cost and feasibility of individual genotyping will increase its applicability, with far-reaching consequences for therapeutics.6
Pharmacoepidemiology
This is the study of drug effects at the population level (see Strom, 2000). It is concerned with the variability of drug effects between individuals in a population, and between populations. It is an increasingly important topic in the eyes of the regulatory authorities who decide whether or not new drugs can be licensed for therapeutic use. Variability between individuals or populations has an adverse effect on the utility of a drug, even though its mean effect level may be satisfactory. Pharmacoepidemiological studies also take into account patient compliance and other factors that apply when the drug is used under real-life conditions.
Pharmacoeconomics
This branch of health economics aims to quantify in economic terms the cost and benefit of drugs used therapeutically. It arose from the concern of many governments to provide for healthcare from tax revenues, raising questions of what therapeutic procedures represent the best value for money. This, of course, raises fierce controversy, because it ultimately comes down to putting monetary value on health and longevity. As with pharmacoepidemiology, regulatory authorities are increasingly requiring economic analysis, as well as evidence of individual benefit, when making decisions on licensing. For more information on this complex subject, see Drummond et al. (1997), Rascati (2009).
References and Further Reading
Buckel P. Recombinant proteins for therapy. Trends Pharmacol. Sci.. 1996;17:450-456. (Thoughtful review of the status of, and prospects for, protein-based therapeutics)
Drews J. In quest of tomorrow’s medicines. New York: Springer-Verlag; 1998. (An excellent account of the past, present and future of the drug discovery process, emphasising the growing role of biotechnology)
Drummond M.F., O’Brien B., Stoddart G.I., Torrance G.W. Methods for the economic evaluation of healthcare programmes. Oxford: Oxford University Press; 1997. (Coverage of the general principles of evaluating the economic costs and benefits of healthcare, including drug-based therapeutics)
Evans W.E., Relling M.V. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487-501. (A general overview of pharmacogenomics)
Lynch T.J., Bell D.W., Sordella R., et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med.. 2004;350:2129-2139. (An important early example of a genetic determinant of therapeutic efficacy depending on mutations affecting the drug target—a likely pointer to what is to come)
Nebert D.W., Weber W.W. Pharmacogenetics. In Pratt W.B., Taylor P., editors: Principles of Drug Action, third ed, New York: Churchill-Livingstone, 1990. (A detailed account of genetic factors that affect responses to drugs, with many examples from the pregenomic literature)
Porter R. The greatest benefit to mankind. London: Harper-Collins; 1997. (An excellent and readable account of the history of medicine, with good coverage of the early development of pharmacology and the pharmaceutical industry)
Rascati K.L. Essentials of pharmcoaeconomics. Philadelphia: Lippincott Williams & Wilkins; 2009.
Strom B.L., editor. Pharmacoepidemiology, fourth ed, Chichester: Wiley, 2005. (A multiauthor book covering all aspects of a newly emerged discipline, including aspects of pharmacoeconomics)
Swen J.J., Huizinga T.W., Gelderblom H., et al. Translating pharmacogenomics: challenges on the road to the clinic. PLoS. Med.. 2007;4:e209.
Walsh G. Biopharmaceuticals: biochemistry and biotechnology. Chichester: Wiley; 2003. (Good introductory textbook covering many aspects of biotechnology-based therapeutics)
Weinshilboum R., Wang L. Pharmacogenomics: bench to bedside. Nat. Rev. Drug Discov.. 2004;3:739-748. (Discusses, with examples, the growing importance of the correlation between genetic make-up and response to therapeutic drugs)
1Like most definitions, this one has its limits. For example, there are a number of essential dietary constituents, such as iron and various vitamins, that are used as medicines.
2Oliver Wendell Holmes, an eminent physician, wrote in 1860: ‘… firmly believe that if the whole materia medica, as now used, could be sunk to the bottom of the sea, it would be all the better for mankind and the worse for the fishes.’ (See Porter, 1997.)
3A handful of synthetic substances achieved pharmacological prominence long before the era of synthetic chemistry began. Diethyl ether, first prepared as ‘sweet oil of vitriol’ in the 16th century, and nitrous oxide, prepared by Humphrey Davy in 1799, were used to liven up parties before being introduced as anaesthetic agents in the mid-19th century (see Ch. 40). Amyl nitrite (see Ch. 21) was made in 1859 and can claim to be the first ‘rational’ therapeutic drug; its therapeutic effect in angina was predicted on the basis of its physiological effects—a true ‘pharmacologist’s drug’ and the smelly forerunner of the nitrovasodilators that are widely used today. Aspirin (Ch. 26), the most widely used therapeutic drug in history, was first synthesised in 1853, with no therapeutic application in mind. It was rediscovered in 1897 in the laboratories of the German company Bayer, who were seeking a less toxic derivative of salicylic acid. Bayer commercialised aspirin in 1899 and made a fortune.
4Some of our most distinguished pharmacological pioneers made their careers in industry: for example, Henry Dale, who laid the foundations of our knowledge of chemical transmission and the autonomic nervous system (Ch. 11); George Hitchings and Gertrude Elion, who described the antimetabolite principle and produced the first effective anticancer drugs (Ch. 54); and James Black, who introduced the first β-adrenoceptor and histamine H2-receptor antagonists (Chs 13 and 17). It is no accident that in this book, where we focus on the scientific principles of pharmacology, most of our examples are products of industry, not of nature.
5Antiscientific populism and commercial pressures recently caused the UK Medicines and Healthcare Regulatory Agency (MHRA) to approve a homeopathic product, despite the lack of evidence that it worked.
6An interesting recent example concerns a newly introduced anticancer drug, gefitinib, which is highly effective in treating lung cancer but works in only about 10% of cases. Responders have mutations in the receptor tyrosine kinase (see Ch. 3) that is the target of this drug, and can be identified in advance by genotyping (see Lynch et al., 2004).