40 General anaesthetic agents
Overview
General anaesthetics are used to render patients unaware of, and unresponsive to, painful stimulation during surgical procedures. They are given systemically and exert their main effects on the central nervous system (CNS), in contrast to local anaesthetics (Ch. 42). Although we now take them for granted, general anaesthetics are the drugs that paved the way for modern surgery. Without them, much of modern medicine would be impossible.
In this chapter, we describe the pharmacology of the main agents in current use, which fall into two main groups: intravenous agents and inhalation agents (gases and volatile liquids). Detailed information on the clinical pharmacology and use of anaesthetic agents can be found in specialised textbooks (e.g. Aitkinhead et al., 2006).
Introduction
It was only when inhalation anaesthetics were first discovered, in 1846, that most surgical operations became a practical possibility. Until that time, surgeons relied on being able to operate on struggling patients at lightning speed, and most operations were amputations.
 The use of nitrous oxide to relieve the pain of surgery was suggested by Humphrey Davy in 1800. He was the first person to make nitrous oxide, and he tested its effects on several people, including himself and the Prime Minister, noting that it caused euphoria, analgesia and loss of consciousness. The use of nitrous oxide, billed as ‘laughing gas’, became a popular fairground entertainment and came to the notice of an American dentist, Horace Wells, who had a tooth extracted under its influence, while he himself squeezed the inhalation bag. Ether also first gained publicity in a disreputable way, through the spread of ‘ether frolics’, at which it was used to produce euphoria among the guests. William Morton, also a dentist and a student at Harvard Medical School, used it successfully to extract a tooth in 1846 and then suggested to Warren, the illustrious chief surgeon at Massachusetts General Hospital, that he should administer it for one of Warren’s operations. Warren grudgingly agreed, and on 16 October 1846 a large audience was gathered in the main operating theatre;1 after some preliminary fumbling, Morton’s demonstration was a spectacular success. ‘Gentlemen, this is no humbug’, was the most gracious comment that Warren could bring himself to make to the assembled audience.
The use of nitrous oxide to relieve the pain of surgery was suggested by Humphrey Davy in 1800. He was the first person to make nitrous oxide, and he tested its effects on several people, including himself and the Prime Minister, noting that it caused euphoria, analgesia and loss of consciousness. The use of nitrous oxide, billed as ‘laughing gas’, became a popular fairground entertainment and came to the notice of an American dentist, Horace Wells, who had a tooth extracted under its influence, while he himself squeezed the inhalation bag. Ether also first gained publicity in a disreputable way, through the spread of ‘ether frolics’, at which it was used to produce euphoria among the guests. William Morton, also a dentist and a student at Harvard Medical School, used it successfully to extract a tooth in 1846 and then suggested to Warren, the illustrious chief surgeon at Massachusetts General Hospital, that he should administer it for one of Warren’s operations. Warren grudgingly agreed, and on 16 October 1846 a large audience was gathered in the main operating theatre;1 after some preliminary fumbling, Morton’s demonstration was a spectacular success. ‘Gentlemen, this is no humbug’, was the most gracious comment that Warren could bring himself to make to the assembled audience.
In the same year, James Simpson, Professor of Midwifery at Edinburgh University, used chloroform to relieve the pain of childbirth, bringing on himself fierce denunciation from the clergy, one of whom wrote:
‘Chloroform is a decoy of Satan, apparently offering itself to bless women; but in the end it will harden society and rob God of the deep, earnest cries which arise in time of trouble, for help.’
Opposition was effectively silenced in 1853, when Queen Victoria gave birth to her seventh child under the influence of chloroform, and the procedure became known as anaesthésie à la reine.
Mechanism of Action of Anaesthetic Drugs
Unlike most drugs, anaesthetics, which include substances as diverse as simple gases (e.g. nitrous oxide and xenon), halogenated hydrocarbons (e.g. isoflurane), barbiturates (e.g. thiopental) and steroids (e.g. alphaxalone), belong to no recognisable chemical class. At one time it appeared that the shape and electronic configuration of the molecule were relatively unimportant, and the pharmacological action required only that the molecule had certain physicochemical properties. We now know much more about how different anaesthetics interact with neuronal membrane proteins and have come to realise that there are multiple mechanisms by which anaesthesia can be produced and that different anaesthetics work by different mechanisms.
As the concentration of an anaesthetic is increased, the switch from being conscious to unconscious occurs over a very narrow concentration range (approximately 0.2 of a log unit). This is a much steeper concentration–response curve than that seen with drugs that interact as agonists or antagonists at classical receptors (see Ch. 2).
Lipid Solubility
Overton and Meyer, at the turn of the 20th century, showed a close correlation between anaesthetic potency and lipid solubility in a diverse group of simple and unreactive organic compounds that were tested for their ability to immobilise tadpoles. This led to a bold theory, formulated by Meyer in 1937: ‘Narcosis commences when any chemically indifferent substance has attained a certain molar concentration in the lipids of the cell.’
The relationship between anaesthetic activity and lipid solubility has been repeatedly confirmed. Anaesthetic potency in humans is usually expressed as the minimal alveolar concentration (MAC) required to abolish the response to surgical incision in 50% of subjects. Figure 40.1 shows the correlation between MAC (inversely proportional to potency) and lipid solubility, expressed as oil:water partition coefficient, for a wide range of inhalation anaesthetics. The Overton–Meyer studies did not suggest any particular mechanism, but revealed an impressive correlation, for which any theory of anaesthesia needs to account. Oil:water partition was assumed to predict partition into membrane lipids, consistent with the suggestion that anaesthesia results from an alteration of membrane function.
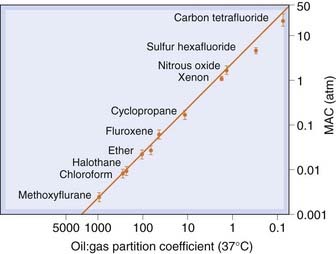
Fig. 40.1 Correlation of anaesthetic potency with oil:gas partition coefficient.
Anaesthetic potency in humans is expressed as minimum alveolar partial pressure (MAC) required to produce surgical anaesthesia. There is a close correlation with lipid solubility, expressed as the oil:gas partition coefficient.
(From: Halsey, 1989.)
How the simple introduction of inert foreign molecules into the lipid bilayer could cause a functional disturbance was not explained. Two possible mechanisms, namely volume expansion and increased membrane fluidity, have been suggested and tested experimentally, but both are now largely discredited (see Halsey, 1989; Little, 1996), and attention has swung from lipids to proteins, the correlation of potency with lipid solubility being explained by molecules of anaesthetic binding to hydrophobic pockets within specific membrane protein targets.
Effects on ION Channels
Following early studies that showed that anaesthetics can bind to various proteins as well as lipids, it was found that anaesthetics affect several different types of ion channels (see Rudolph & Antkowiak, 2004; Franks, 2008). For most anaesthetics, there are no known competitive antagonists, so this approach to identify sites of action is denied. Therefore the main criterion for identifying putative mechanisms of action of general anaesthetics is that, for an effect to be relevant to the anaesthetic or analgesic actions of these agents, it must occur at therapeutically relevant concentrations.
GABAA receptors.
Almost all anaesthetics (with the exceptions of cyclopropane, ketamine and xenon) potentiate the action of GABA at the GABAA receptor. As described in detail in Chapter 37, GABAA receptors are ligand-gated Cl− channels made up of five subunits (generally comprising two α, two β and one γ or δ subunit). Anaesthetics can bind to hydrophobic pockets within different GABAA receptor subunits (see Fig. 40.2).
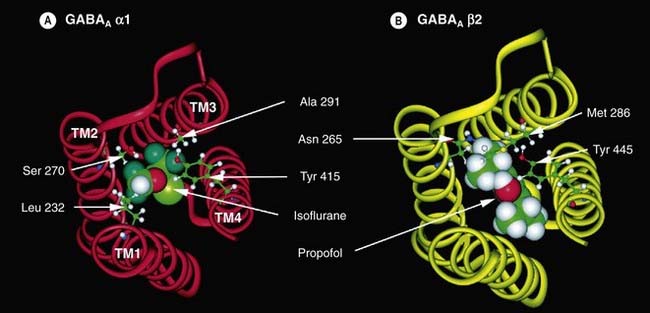
Fig. 40.2 Putative anaesthetic binding sites on GABAA receptor subunits.
[A] A model of the α1 subunit of the GABAA receptor with the amino acids that form the binding site (Leu 232, Ser 270, Ala 291 and Tyr 415) illustrated in ball-and-stick mode. A molecule of isoflurane is shown sitting in the putative binding site. The transmembrane α-helices (TM) are numbered 1–4. [B] A model of the β2 subunit of the GABAA receptor with Asn 265, Met 286 and Tyr 445 illustrated in ball-and-stick mode. A molecule of propofol is shown sitting in the putative binding site.
(Reproduced with permission from Hemmings H C et al. 2005 Trends Pharmacol Sci 26: 503–510.)
Specific mutations of the amino acid sequence of the α subunit inhibit the actions of volatile anaesthetics but not those of intravenous anaesthetics, whereas mutations of the β subunit inhibit both volatile and intravenous anaesthetics (see Franks, 2008). This suggest that volatile anaesthetics may bind at the interface between α and β subunits (analogous to benzodiazepines that bind at the interface between α and γ/δ subunits, see Ch. 37), whereas the intravenous anaesthetics bind only on the β subunit. A further level of complexity arises because there are different subtypes of each subunit (see Ch. 37). Different subunit compositions give rise to subtly different subtypes of GABAA receptor. It has recently been shown that the GABAA receptors clustered at the synapse have different pharmacological and kinetic properties from those that are distributed elsewhere across the cell (extrasynaptic receptors; see Ch. 4). Anaesthetics appear to have a greater potentiating effect on these extrasynaptic GABAA receptors.
Two-pore domain K+ channels.
These belong to a family of ‘background’ K+ channels that modulate neuronal excitability. They are homomeric or heteromeric assemblies of a family of structurally related subunits (see Ch. 4 and Bayliss & Barrett, 2008). Channels made up of TREK1, TREK2, TASK1, TASK3 or TRESK subunits can be directly activated by low concentrations of volatile and gaseous anaesthetics, thus reducing membrane excitability (see Franks, 2008). This may contribute to the analgesic, hypnotic and immobilising effects of these agents. Two-pore domain K+ channels do not appear to be affected by intravenous anaesthetics.
NMDA receptors.
Glutamate, the major excitatory neurotransmitter in the CNS, activates three main classes of ionotropic receptor—AMPA, kainate and NMDA receptors (see Ch. 37). NMDA receptors are an important site of action for anaesthetics such as nitrous oxide, xenon and ketamine which act, in different ways, to reduce NMDA receptor-mediated responses. Xenon appears to inhibit NMDA receptors by competing with glycine for its regulatory site on this receptor whereas ketamine blocks the pore of the channel (see Ch. 37). Other inhalation anaesthetics may also exert effects on the NMDA receptor in addition to their effects on other proteins such as the GABAA receptor.
Other ion channels.
Anaesthetics may also exert actions at other neuronal ligand-gated channels including glycine, nicotinic and 5-hydroxytryptamine receptors as well as at cyclic nucleotide-gated K+ channels. Some general anaesthetics inhibit certain subtypes of voltage-gated Na+ channels. Inhibition of presynaptic Na+ channels may give rise to the inhibition of transmitter release at excitatory synapses. For further reading, see Hemmings et al. (2005) and Franks (2008).
It may be overly simplistic to think of each anaesthetic as having only one mechanism of action: as Little (1996) emphasises, individual anaesthetics differ in their actions and affect cellular function in several different ways, so a single mechanism is unlikely to be sufficient.
Comprehensive reviews of the molecular and cellular actions of general anaesthetics can be found in Schüttler & Schwilden, 2008.
Theories of anaesthesia ![]()
Effects on the Nervous System
At the cellular level, the effects of anaesthetics are to enhance tonic inhibition (through enhancing the actions of GABA), reduce excitation (opening K+ channels) and to inhibit excitatory synaptic transmission (by depressing transmitter release and inhibiting ligand-gated ion channels). Effects on axonal conduction are relatively unimportant.
The anaesthetised state comprises several components, including unconsciousness, loss of reflexes (muscle relaxation) and analgesia. Much effort has gone into identifying the brain regions on which anaesthetics act to produce these effects. The most sensitive regions appear to be the midbrain reticular formation, thalamic sensory relay nuclei and, to a lesser extent, parts of the cortex. Inhibition of these regions results in unconsciousness and analgesia. Some anaesthetics—particularly volatile anaesthetics—cause inhibition at the spinal level, producing a loss of reflex responses to painful stimuli, although, in practice, neuromuscular-blocking drugs (Ch. 13) are used as an adjunct to produce muscle relaxation rather than relying on the anaesthetic alone. Anaesthetics, even in low concentrations, cause short-term amnesia. It is likely that interference with hippocampal function produces this effect, because the hippocampus is involved in short-term memory, and certain hippocampal synapses are highly susceptible to inhibition by anaesthetics.
As the anaesthetic concentration is increased, all brain functions are progressively affected, including motor control and reflex activity, respiration and autonomic regulation. Therefore it is not possible to identify a critical ‘target site’ in the brain responsible for all the phenomena of anaesthesia.
High concentrations of any general anaesthetic affect all parts of the CNS, causing profound inhibition which, in the absence of artificial respiration, leads to death from respiratory failure. The margin between surgical anaesthesia and potentially fatal respiratory and circulatory depression is quite narrow, requiring careful monitoring by the anaesthetist and adjustment of the level of anaesthesia.
Effects on the Cardiovascular and Respiratory Systems
Most anaesthetics decrease cardiac contractility, but their effects on cardiac output and blood pressure vary because of concomitant actions on the sympathetic nervous system and vascular smooth muscle. Isoflurane and other halogenated anaesthetics inhibit sympathetic outflow, reduce arterial and venous tone and thus decrease arterial pressure and venous pressure. By contrast, nitrous oxide and ketamine increase sympathetic discharge and plasma noradrenaline concentration and, if used alone, increase heart rate and maintain blood pressure.
Many anaesthetics, especially halothane, cause ventricular extrasystoles. The mechanism involves sensitisation to adrenaline. Electrocardiogram monitoring shows that extrasystolic beats occur commonly in patients under anaesthesia, with no harm coming to the patient. If catecholamine secretion is excessive, however (par excellence in phaeochromocytoma; see Ch. 14), there is a risk of precipitating ventricular fibrillation.
With the exception of nitrous oxide, ketamine and xenon, all anaesthetics depress respiration markedly and increase arterial PCO2. Nitrous oxide has much less effect, in part because its low potency prevents very deep anaesthesia from being produced with this drug. Some inhalation anaesthetics are pungent, particularly desflurane which is liable to cause coughing, laryngospasm and bronchospasm, so desflurane is not used for induction of anaesthesia but only for maintenance.
Pharmacological effects of anaesthetic agents ![]()
Intravenous Anaesthetic Agents
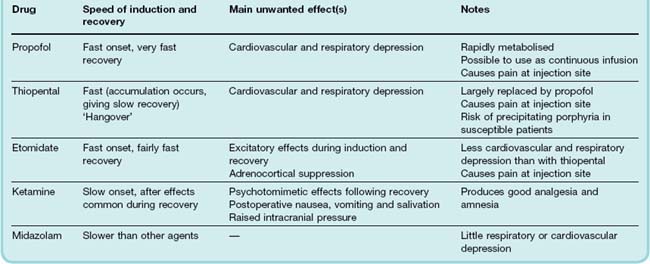
Even the fastest-acting inhalation anaesthetics, such as nitrous oxide, take a few minutes to act and cause a period of excitement before anaesthesia is induced. Intravenous anaesthetics act more rapidly, producing unconsciousness in about 20 s, as soon as the drug reaches the brain from its site of injection. These drugs (e.g. propofol, thiopental and etomidate) are normally used for induction of anaesthesia. They are preferred by many patients because injection generally lacks the menacing quality associated with a face mask in an apprehensive individual. With propofol, recovery is also fast due to rapid metabolism.
Although many intravenous anaesthetics are not suitable for maintaining anaesthesia because their elimination from the body is relatively slow compared with that of inhalation agents, propofol can be used as a continuous infusion, and the duration of action of ketamine is sufficient that it can be used as a single bolus for short operations without the need for an inhalation agent.
The properties of the main intravenous anaesthetics are summarised in Table 40.1.2
Propofol
Propofol, introduced in 1983, has now largely replaced thiopental as an induction agent. It has a rapid onset of action (approximately 30 s) and rapid rate of distribution (t1/2 2–4 min). It has the advantage over thiopental of being very rapidly metabolised to inactive conjugates and quinols; therefore giving rapid recovery with less hangover effect. It has a cardiovascular depressant effect that may lead to hypotension and bradycardia. Respiratory depression and pain with injection may also occur. Propofol has less tendency to cause involuntary movement and adrenocortical suppression seen with etomidate. It is particularly useful for day-case surgery especially as its use is associated with less nausea and vomiting when compared with inhalation anaesthetics.
Propofol can also be given as a continuous infusion to maintain surgical anaesthesia without the need for any inhalation agent. However, there have been reports of a propofol infusion syndrome occurring in approximately 1 in 300 patients when high doses have been given for a prolonged period, particularly to sick patients—especially children—in intensive care units. This is characterised by severe metabolic acidosis, skeletal muscle necrosis (rhabdomyolysis), hyperkalaemia, lipaemia, hepatomegaly, renal failure, arrhythmia and cardiovascular collapse.
Thiopental
Thiopental is the only remaining barbiturate in common use as an anaesthetic. It has very high lipid solubility, and this accounts for the speed of onset and transience of its effect when it is injected intravenously. The free acid is insoluble in water, so thiopental is given as the sodium salt. On intravenous injection, thiopental causes unconsciousness within about 20 s, lasting for 5–10 min. The anaesthetic effect closely parallels the concentration of thiopental in the blood reaching the brain, because its high lipid solubility allows it to cross the blood–brain barrier without noticeable delay.
The blood concentration of thiopental declines rapidly, by about 80% within 1–2 min, following the initial peak after intravenous injection, because the drug is redistributed, first to tissues with a large blood flow (liver, kidneys, brain, etc.) and more slowly to muscle. Uptake into body fat, although favoured by the high lipid solubility of thiopental, occurs only slowly, because of the low blood flow to this tissue. After several hours, however, most of the thiopental present in the body will have accumulated in body fat, the rest having been metabolised. Recovery from the anaesthetic effect of a bolus dose occurs within about 5 min, governed entirely by redistribution of the drug to well-perfused tissues; very little is metabolised in this time. After the initial rapid decline, the blood concentration drops more slowly, over several hours, as the drug is taken up by body fat and metabolised. Consequently, thiopental produces a long-lasting hangover. Repeated intravenous doses cause progressively longer periods of anaesthesia, because the plateau in blood concentration becomes progressively more elevated as more drug accumulates in the body. For this reason, thiopental is not used to maintain surgical anaesthesia but only as an induction agent.
Thiopental binds to plasma albumin (roughly 85% of the blood content normally being bound). The fraction bound is less in states of malnutrition, liver disease or renal disease, which affect the concentration and drug-binding properties of plasma albumin, and this can appreciably reduce the dose needed for induction of anaesthesia.
Accidental injection of intravenous thiopental—a strongly alkaline solution—around rather than into the vein, or into an artery, can cause pain, local tissue necrosis and ulceration or severe arterial spasm that can result in gangrene. If the injection is into an artery then immediate injection of procaine, through the same needle, is the recommended procedure to encourage vasodilatation.
The actions of thiopental on the nervous system are very similar to those of inhalation anaesthetics, although it has little analgesic effect and can cause profound respiratory depression even in amounts that fail to abolish reflex responses to painful stimuli. Its long after-effect, associated with a slowly declining plasma concentration, means that drowsiness and some degree of respiratory depression persist for some hours.
Etomidate
Etomidate has gained favour over thiopental on account of the larger margin between the anaesthetic dose and the dose needed to produce cardiovascular depression. It is more rapidly metabolised than thiopental, and thus less likely to cause a prolonged hangover. It causes less hypotension than propofol or thiopental. In other respects, etomidate is very similar to thiopental, although it appears more likely to cause involuntary movements during induction, postoperative nausea and vomiting, and pain at the injection site. Etomidate, particularly with prolonged infusion, suppresses the production of adrenal steroids, an effect that has been associated with an increase in mortality in severely ill patients. It should be avoided in patients at risk of having adrenal insufficiency, e.g. in sepsis. It is preferable to thiopental in patients at risk of circulatory failure.
Other Intravenous Agents
Ketamine
 Ketamine closely resembles, both chemically and pharmacologically, phencyclidine, which is a ‘street drug’ with a pronounced effect on sensory perception (see Ch. 47). Both drugs produce a similar anaesthesia-like state and profound analgesia, but ketamine produces less euphoria and sensory distortion than phencyclidine and is thus more useful in anaesthesia. Both drugs are believed to act by blocking activation of the NMDA receptor (see Ch. 37).
Ketamine closely resembles, both chemically and pharmacologically, phencyclidine, which is a ‘street drug’ with a pronounced effect on sensory perception (see Ch. 47). Both drugs produce a similar anaesthesia-like state and profound analgesia, but ketamine produces less euphoria and sensory distortion than phencyclidine and is thus more useful in anaesthesia. Both drugs are believed to act by blocking activation of the NMDA receptor (see Ch. 37).
Given intravenously, ketamine takes effect more slowly (1–2 min) than thiopental, and produces a different effect, known as ‘dissociative anaesthesia’, in which there is a marked sensory loss and analgesia, as well as amnesia, without complete loss of consciousness. During induction and recovery, involuntary movements and peculiar sensory experiences often occur. Ketamine does not act simply as a CNS depressant, and it produces cardiovascular and respiratory effects quite different from those of most anaesthetics. Blood pressure and heart rate are usually increased, and respiration is unaffected by effective anaesthetic doses. This makes it relatively safe to use in low-technology healthcare situations or in emergencies in the field. However, ketamine, unlike other intravenous anaesthetic drugs, can increase intracranial pressure, so it should not be given to patients with raised intracranial pressure or at risk of cerebral ischaemia. The other main drawback of ketamine is that hallucinations, and sometimes delirium and irrational behaviour, are common during recovery. These after-effects limit the usefulness of ketamine but are said to be less marked in children,3 and ketamine, often in conjunction with a benzodiazepine, is sometimes still used for minor procedures in paediatrics.
Midazolam
Midazolam, a benzodiazepine (Ch. 43), is slower in onset and offset than the drugs discussed above but, like ketamine, causes less respiratory or cardiovascular depression. Midazolam (or diazepam) is often used as a preoperative sedative and during procedures such as endoscopy, where full anaesthesia is not required. It can be administered in combination with an analgesic such as alfentanyl. In the event of overdose it can be reversed by flumazenil (see Ch. 43).
Neuroleptanalgesia
The combined use of a sedative (e.g. the dopamine antagonist droperidol) related to antipsychotic drugs (Ch. 45) and an opiate analgesic such as fentanyl (Ch. 41) can produce a state of deep sedation and analgesia (known as neuroleptanalgesia) in which the patient remains responsive to simple commands and questions, but does not respond to painful stimuli or retain any memory of the procedure. This can be used for minor procedures such as endoscopy but is less used since the advent of midazolam which has a shorter duration of action. Use of neuroleptanalgesics is more common in veterinary medicine; they are the pharmacological component in chemical darts used to immobilise wild animals.
Intravenous anaesthetic agents ![]()
Inhalation Anaesthetics
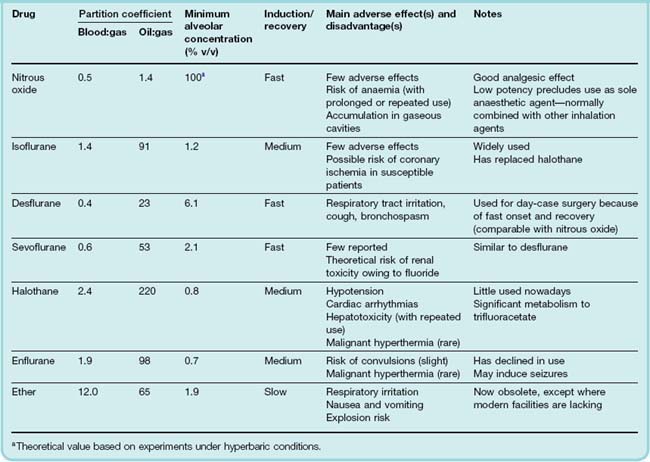
Many inhalation anaesthetics that were once widely used, such as ether, chloroform, trichloroethylene, cyclopropane, methoxyflurane and enflurane, have now been replaced in clinical practice, particularly by isoflurane, sevoflurane and desflurane which have improved pharmacokinetic properties, fewer side effects and are non-flammable. Of the older agents, nitrous oxide is still used widely (especially in obstetric practice), and halothane now only occasionally. Inhalation anaesthetics are most commonly used for the maintenance of anaesthesia.
Pharmacokinetic Aspects
An important characteristic of an inhalation anaesthetic is the speed at which the arterial blood concentration, which governs the pharmacological effect in the brain, follows changes in the partial pressure of the drug in the inspired air. Ideally, the blood concentration should follow as quickly as possible, so that the depth of anaesthesia can be controlled rapidly. In particular, the blood concentration should fall to a subanaesthetic level rapidly when administration is stopped, so that the patient recovers consciousness with minimal delay. A prolonged semicomatose state, in which respiratory reflexes are weak or absent, is particularly hazardous.
The lungs are the only quantitatively important route by which inhalation anaesthetics enter and leave the body. For modern inhalation anaesthetics, metabolic degradation is generally insignificant in determining their duration of action. Inhalation anaesthetics are all small, lipid-soluble molecules that readily cross alveolar membranes. It is therefore the rates of delivery of drug to and from the lungs, via (respectively) the inspired air and bloodstream, that determine the overall kinetic behaviour of an anaesthetic. The reason that anaesthetics vary in their kinetic behaviour is that their relative solubilities in blood, and in body fat, vary between one drug and another.
The main factors that determine the speed of induction and recovery can be summarised as follows:
Solubility of Inhalation Anaesthetics
Inhalation anaesthetics can be regarded physicochemically as ideal gases: their solubility in different media is expressed as partition coefficients, defined as the ratio of the concentration of the agent in two phases at equilibrium.
The blood:gas partition coefficient is the main factor that determines the rate of induction and recovery of an inhalation anaesthetic, and the lower the blood:gas partition coefficient, the faster is induction and recovery (Table 40.2). This is because it is the partial pressure of the gas in the alveolar space that governs the concentration in the blood. The lower the blood:gas partition coefficient, the more rapidly the partial pressure of the gas in the alveolar space will equal that being administered in the inspired air (see below).
The oil:gas partition coefficient, a measure of fat solubility, determines the potency of an anaesthetic (as already discussed) and also influences the kinetics of its distribution in the body, the main effect being that high lipid solubility delays recovery from anaesthesia. Values of blood:gas and oil:gas partition coefficients for some anaesthetics are given in Table 40.2.
Induction and Recovery
Cerebral blood flow is a substantial fraction of cardiac output (~15%), and the blood–brain barrier is freely permeable to anaesthetics, so the concentration of anaesthetic in the brain closely tracks that in the arterial blood. The kinetics of transfer of anaesthetic between the inspired air and the arterial blood therefore determine the kinetics of the pharmacological effect.
When a volatile anaesthetic is first administered, the initial breaths are diluted into the residual gas volume in the lungs resulting in a reduction in the alveolar partial pressure of the anaesthetic as compared with the inspired gas mixture. With subsequent breaths, the alveolar partial pressure rises towards equilibrium. For an anaesthetic with a low blood:gas partition coefficient, the absorption into the blood will be slower, so with repeated breaths the partial pressure in the alveolar space will rise faster than with an agent of high blood:gas partition coefficient. Thus a smaller number of breaths (i.e. a shorter time) will be needed to reach equilibrium. Therefore, contrary to what one might intuitively suppose, the lower the solubility in blood, the faster is the process of equilibration. Figure 40.3 shows the much faster equilibration for nitrous oxide, a low-solubility agent, than for ether, a high-solubility agent.
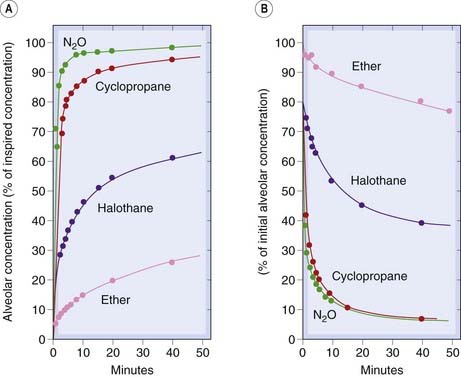
Fig. 40.3 Rate of equilibration of inhalation anaesthetics in humans.
The curves show alveolar concentration (which closely reflects arterial blood concentration) as a function of time during induction and recovery. The initial rate of equilibration reflects solubility in blood. There is also a slow phase of equilibration, most marked with highly lipid-soluble drugs (ether and halothane), owing to the slow transfer between blood and fat (Fig. 40.4). [A] Induction. [B] Recovery.
(From Papper E M, Kitz R (eds) 1963 Uptake and distribution of anaesthetic agents. McGraw-Hill, New York.)
The transfer of anaesthetic between blood and tissues also affects the kinetics of equilibration. Figure 40.4 shows a very simple model of the circulation, in which two tissue compartments are included. Body fat has a low blood flow but has a high capacity to take up anaesthetics, and constitutes about 20% of the volume of a representative man. Therefore, for a drug such as halothane, which is about 100 times more soluble in fat than in water, the amount present in fat after complete equilibration would be roughly 95% of the total amount in the body. Because of the low blood flow to adipose tissue, it takes many hours for the drug to enter and leave the fat, which results in a pronounced slow phase of equilibration following the rapid phase associated with the blood–gas exchanges (Fig. 40.3). The more fat-soluble the anaesthetic and the fatter the patient, the more pronounced this slow phase becomes and recovery will also be delayed.
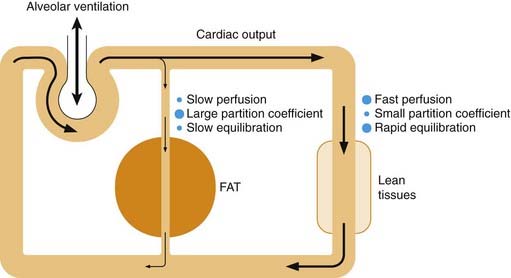
Fig. 40.4 Factors affecting the rate of equilibration of inhalation anaesthetics in the body.
The body is represented as two compartments. Lean tissues, including the brain, have a large blood flow and low partition coefficient for anaesthetics, and therefore equilibrate rapidly with the blood. Fat tissues have a small blood flow and large partition coefficient, and therefore equilibrate slowly, acting as a reservoir of drug during the recovery phase.
Of the physiological factors affecting the rate of equilibration of inhalation anaesthetics, alveolar ventilation is the most important. The greater the minute volume (respiration rate × tidal volume), the faster is equilibration, particularly for drugs that have high blood:gas partition coefficients. Respiratory depressant drugs such as morphine (see Ch. 41) can thus retard recovery from anaesthesia.
Recovery from anaesthesia involves the same processes as induction but in reverse (Fig. 40.3), the rapid phase of recovery being followed by a slow ‘hangover’. Because of these kinetic factors, the search for improved inhalation anaesthetics has focused on agents with low blood and tissue solubility. Newer drugs, which show kinetic properties similar to those of nitrous oxide but have higher potency, include sevoflurane and desflurane (Table 40.2).
Metabolism and Toxicity
Metabolism, although not quantitatively important as a route of elimination of inhalation anaesthetics, can generate toxic metabolites.4 Chloroform (now obsolete) causes hepatotoxicity associated with free radical formation in liver cells. Methoxyflurane, a halogenated ether, is no longer used because about 50% is metabolised to fluoride and oxalate, which cause renal toxicity. Halothane is less used nowadays because it undergoes substantial metabolism, about 30% being converted to bromide, trifluoroacetic acid and other metabolites that are implicated in rare instances of liver toxicity (see below). Enflurane and sevoflurane also generate fluoride, but at much lower (non-toxic) concentrations (Table 40.2).
Malignant hyperthermia is caused by heat production in skeletal muscle, due to excessive release of Ca2+ from the sarcoplasmic reticulum. The result is muscle contracture, acidosis, increased metabolism and an associated dramatic rise in body temperature that can be fatal unless treated promptly. Triggers include halogenated anaesthetics and depolarising neuromuscular-blocking drugs (see Ch. 13). Susceptibility has a genetic basis, being associated with mutations in the gene encoding the ryanodine receptor, which controls Ca2+ release from the sarcoplasmic reticulum (Ch. 4). Malignant hyperthermia is treated with dantrolene, a muscle relaxant drug that blocks these calcium release channels.
Pharmacokinetic properties of inhalation anaesthetics ![]()
Individual Inhalation Anaesthetics
The main inhalation anaesthetics currently used in developed countries are isoflurane, desflurane and sevoflurane sometimes used in combination with nitrous oxide. Due to its relatively rapid onset of action sevoflurane can, under some circumstances, be used on its own to induce anaesthesia. Xenon, an inert gas shown many years ago to have anaesthetic properties, is making something of a comeback in the clinic because—not surprisingly for an inert gas—it lacks toxicity, but its relatively low potency and high cost are disadvantages.
Halothane is still used in veterinary medicine in species that do not metabolise it to toxic products, and is occasionally used in human medicine when a slow recovery from anaesthesia is desirable. Enflurane has decreased in use because of its propensity to induce seizures.
Isoflurane, Desflurane, Sevoflurane, Enflurane and Halothane
Isoflurane is now the most widely used volatile anaesthetic. It is not appreciably metabolised and lacks the proconvulsive property of enflurane. It can cause hypotension and is a powerful coronary vasodilator. This can exacerbate cardiac ischaemia in patients with coronary disease, because of the ‘steal’ phenomenon (see Ch. 21).
Desflurane is chemically similar to isoflurane, but its lower solubility in blood and fat means that titration of anaesthetic depth and recovery are faster, so it is increasingly used as an anaesthetic for day-case surgery. It is not appreciably metabolised. It is less potent than the drugs described above. At the concentrations used for induction of anaesthesia (about 10%), desflurane causes some respiratory tract irritation, which can lead to coughing and bronchospasm. Rapid increases in the depth of desflurane anaesthesia can be associated with a striking increase in sympathetic activity which is undesirable in patients with ischaemic heart disease.
Sevoflurane resembles desflurane but is more potent and does not cause the same degree of respiratory irritation. It is partially (about 3%) metabolised, and detectable levels of fluoride are produced, although this does not appear to be sufficient to cause toxicity.
Enflurane has a moderate speed of induction but is little used nowadays. It was originally introduced as an alternative to methoxyflurane. It can cause seizures, either during induction or following recovery from anaesthesia, especially in patients suffering from epilepsy. In this connection, it is interesting that a related substance, the fluorine-substituted diethyl-ether hexafluoroether, is a powerful convulsant agent, although the mechanism is not understood.
Halothane was an important drug in the development of volatile inhalation anaesthetics, but its use has declined in favour of isoflurane due to the potential for accumulation of toxic metabolites. Halothane has a marked relaxant effect on the uterus which can cause postpartum bleeding and limits its usefulness for obstetric purposes.
Nitrous Oxide
Nitrous oxide (N2O, not to be confused with nitric oxide, NO) is an odourless gas with many advantageous features for anaesthesia. It is rapid in onset of action because of its low blood:gas partition coefficient (Table 40.2), and is an effective analgesic in concentrations too low to cause unconsciousness. Its potency is low. It is used as a 50:50 mixture with O2 to reduce pain during childbirth. It must never be given as 100% of the inspired gas as patients do need to breathe oxygen! Even at 80% in the inspired gas mixture, nitrous oxide does not produce surgical anaesthesia. It is not therefore used on its own as an anaesthetic, but is used (as 70% nitrous oxide in oxygen) as an adjunct to volatile anaesthetics, allowing them to be used at lower concentrations. During recovery from nitrous oxide anaesthesia, the transfer of the gas from the blood into the alveoli can be sufficient to reduce, by dilution, the alveolar partial pressure of oxygen, producing transient hypoxia (known as diffusional hypoxia). This is important for patients with respiratory disease.
Nitrous oxide tends to enter gaseous cavities in the body causing them to expand. This can be dangerous if a pneumothorax or vascular air embolus is present, or if the intestine is obstructed.
Given for brief periods, nitrous oxide is devoid of any serious toxic effects, but prolonged exposure (> 6 h) causes inactivation of methionine synthase, an enzyme required for DNA and protein synthesis, resulting in bone marrow depression that may cause anaemia and leucopenia, so its use should be avoided in patients with anaemia related to vitamin B12 deficiency. Bone marrow depression does not occur with brief exposure to nitrous oxide, but prolonged or repeated use (for example, in intermittently painful conditions such as sickle cell anaemia) should be avoided. Nitrous oxide ‘sniffers’ are subject to this danger.
Individual inhalation anaesthetics ![]()
Clinical uses of general anaesthetics ![]()
Use of Anaesthetics in Combination with Other Drugs
Only in simple, short surgical procedures would a single anaesthetic be used on its own. In complex surgery, an array of drugs will be given at various times throughout the procedure. These may include a sedative or anxiolytic premedication (e.g. a benzodiazepine, see Ch. 43), an intravenous anaesthetic for rapid induction (e.g. propofol), a perioperative opioid analgesic (e.g. remifentanyl, see Ch. 41), an inhalation anaesthetic to maintain anaesthesia during surgery (e.g. nitrous oxide and isoflurane), a neuromuscular blocking agent to produce adequate muscle relaxation (e.g. vecuronium, see Ch. 13), an antiemetic agent (e.g. ondansetron, see Ch. 29) and a muscarinic antagonist to prevent or treat bradycardia or to reduce bronchial and salivary secretions (e.g. atropine or glycopyrrolate, see Ch. 13) and, towards the end of the procedure, an anticholinesterase agent (e.g. neostigmine, see Ch. 13) to reverse the neuromuscular blockade and an analgesic for postoperative pain relief (e.g. an opioid such as morphine and/or a non-steroidal anti-inflammatory drug such as diclofenac, see Ch. 41). Such combinations of drugs result in much faster induction and recovery, avoiding long (and potentially hazardous) periods of semiconsciousness, and it enables surgery to be carried out with less undesirable cardiorespiratory depression.
References and Further Reading
Aitkinhead A.R., Smith G.B., Rowbotham D.J. Textbook of anaesthesia, fifth ed. London: Churchill Livingstone; 2006. (The title says it all!)
Bayliss D.A., Barrett P.Q. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol. Sci.. 2008;29:566-575.
Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci.. 2008;9:370-386. (Detailed discussion of the sites of action of general anaesthetics on specific ion channels)
Halsey M.J. Physicochemical properties of inhalation anaesthetics. In: Nunn J.F., Utting J.E., Brown B.R., editors. General anaesthesia. London: Butterworth, 1989. (Good summary of evidence supporting lipid theories of anaesthesia)
Hemmings H.C.Jr, Akabas M.H., Goldstein P.A., et al. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol. Sci.. 2005;26:503-510.
Little H.J. How has molecular pharmacology contributed to our understanding of the molecular mechanism(s) of general anaesthesia? Pharmacol. Ther.. 1996;69:37-58. (Balanced account of the strengths and shortcomings of current theories)
Rudolph U., Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat. Rev. Neurosci.. 2004;5:709-720. (Useful review article covering both the interaction of general anaesthetic agents with different ion channels, and the neuronal pathways that are affected)
Schüttler J., Schwilden H. Modern anesthetics. Handb. Exp. Pharmacol.. 182, 2008. (Entire volume given over to multiauthor reviews of the mechanisms of action of general anaesthetics)
1Now preserved as the Ether Dome, a museum piece at Massachusetts General Hospital.
2Propanidid and alphaxalone were withdrawn because of allergic reactions including hypotension and bronchoconstriction—probably attributable to the solvent Cremophor—but a new formulation of alphaxalone has been reintroduced to veterinary medicine and is thought to be less allergenic.
3A cautionary note: many adverse effects are claimed to be less marked in children, perhaps because they cannot verbalise their experiences. At one time, muscle relaxants alone were used without anaesthesia during cardiac surgery in neonates. The babies did not complain of pain, but their circulating catecholamines reached extreme levels.
4The problem of toxicity of low concentrations of anaesthetics inhaled over long periods by operating theatre staff has been a cause for concern. Strict measures are now used to minimise the escape of anaesthetics into the air of operating theatres.