Chapter 39 The baby at birth
A newborn baby’s survival is dependent on his ability to adapt to an extrauterine environment. This involves adaptations in cardiopulmonary circulation and other physiological adjustments to replace placental function and maintain homeostasis. It is also the commencement of the early parent–baby relationship.
Introduction
The transition from intrauterine to extrauterine life is a dramatic one and demands considerable and effective physiological alterations by the baby in order to ensure survival. The fetus leaves the uterine environment, which has been completely life sustaining for oxygenation, nutrition, excretion and thermoregulation. The aquatic amniotic sac has permitted movement but freedom to extend the limbs has been limited towards the end of pregnancy as the size of the fetus has increased in relation to the capacity of the uterus. Though the fetus is sensitive to sound, the dim uterine environment has dulled the impact of the noise of the outside world.
Subjected to intermittent diminution of the oxygen supply during uterine contractions, compression followed by decompression of the head and chest, and extension of the limbs, hips and spine during birth, the baby emerges from the mother to encounter light, noises, cool air, gravity and tactile stimuli for the first time. Simultaneously, the baby has to make major adjustments in the respiratory and circulatory systems as well as controlling body temperature. These initial adaptations are crucial to the baby’s subsequent well-being and should be understood and facilitated by the midwife at the time of birth.
Respiratory and cardiovascular changes are interdependent and concurrent.
Adaptation to extrauterine life
Onset of respiration
At birth, a baby is transposed from the warm contentment of the uterine environment to the outside world, where the role of independent existence is assumed. The baby must be able to make this sharp transition swiftly, and in order to achieve this, a series of adaptive functions have been developed to accommodate the dramatic change from the intrauterine to extrauterine environment.
Pulmonary adaptation
Until the time of birth, the fetus depends upon maternal blood gas exchange via the maternal lungs and the placenta. Following the sudden removal of the placenta after delivery, very rapid adaptation takes place to ensure continued survival. Prior to birth, the fetus makes breathing movements and the lungs will have matured both biochemically and anatomically to produce surfactant and have adequate numbers of alveoli for gas exchange. Before birth, the fetal lung is full of fluid, which is excreted by the lung itself. During birth, this fluid leaves the alveoli either by being squeezed up the airway and out of the mouth and nose, or by moving across the alveolar walls into the pulmonary lymphatic vessels and into the thoracic duct or to the lung capillaries.
The stimuli of respiration include mild hypercapnia, hypoxia and acidosis, which result from normal labour, due partially to the intermittent cessation of maternal-placental perfusion with contractions. The rhythm of respiration changes from episodic shallow fetal respiration to regular deeper breathing as a result of a combination of chemical and neural stimuli, notably a fall in pH and PaO2 and a rise in PaCO2. Other stimuli include cold, light, noise, touch and pain. Considerable negative intrathoracic pressure of up to 9.8 kPa (100 cm water) is exerted as the first breath is taken. The pressure exerted to effect inhalation diminishes with each breath taken until only 5 cm water pressure is required to inflate the lungs. This effect is caused by surfactant, which lines the alveoli, lowering the surface tension thus permitting residual air to remain in the alveoli between breaths. Surfactant is a complex of lipoproteins and proteins produced by the alveolar type 2 cells in the lungs, and is primarily concerned with the reduction in surface tension at the alveolar surface, thus reducing the work of breathing (Halliday et al 1998).
Cardiovascular adaptation
Prior to birth, the fetus relies solely on the placenta for all gas exchanges and excretion of metabolic waste. Separated from the placenta at birth, the baby’s circulatory system must make major adjustments in order to divert deoxygenated blood to the lungs for reoxygenation. This involves several mechanisms, which are influenced by the clamping of the umbilical cord and also by the lowered resistance in the pulmonary vascular bed.
During fetal life (see Ch. 12) only approximately 10% of the cardiac output is circulated to the lungs through the pulmonary artery. With the expansion of the lungs and lowered pulmonary vascular resistance, virtually all of the cardiac output is sent to the lungs. Oxygenated blood returning to the heart from the lungs increases the pressure within the left atrium. At almost the same time, pressure in the right atrium is lowered because blood ceases to flow through the cord. As a result, a functional closure of the foramen ovale is achieved. During the first days of life, this closure is reversible; re-opening may occur if pulmonary vascular resistance is high, for example when crying, resulting in transient cyanotic episodes in the baby (Perry 2002). The septa usually fuse within the first year of life, forming the interatrial septum, though in some individuals perfect anatomical closure may never be achieved.
The ductus arteriosus, which is nearly as wide as the aorta, provides a diversionary route to bypass the lungs of the fetus. Contraction of its muscular walls occurs almost immediately after birth. This is thought to occur because of sensitivity of the muscle of the ductus arteriosus to increased oxygen tension and reduction in circulating prostaglandin (Tannenbaum et al 1996). As a result of altered pressure gradients between the aorta and pulmonary artery, a temporary reverse left-to-right shunt through the ductus may persist for a few hours, although there is usually functional closure of the ductus within 8–10 hrs of birth. Intermittent patency has been demonstrated in most healthy infants in the first 3 days of life, but complete closure takes several months. Persistence or reopening of the ductus, with associated cyanosis or cyanotic attacks, may occur if pulmonary vascular resistance is high or hypoxia is present (Linh et al 2007). This is a common problem in pre-term infants with respiratory distress syndrome (see Ch. 44). Persistence of the foramen ovale or ductus arteriosus, or both, may be lifesaving in some forms of congenital heart abnormality (see Ch. 46).
The remaining temporary structures of the fetal circulation – the umbilical vein, ductus venosus and hypogastric arteries – close functionally within a few minutes after birth and constriction of the cord. Anatomical closure by fibrous tissue occurs within 2–3 months, resulting in the formation of the ligamentum teres, ligamentum venosum and the obliterated hypogastric arteries. The proximal portions of the hypogastric arteries persist as the superior vesical arteries.
Thermal adaptation
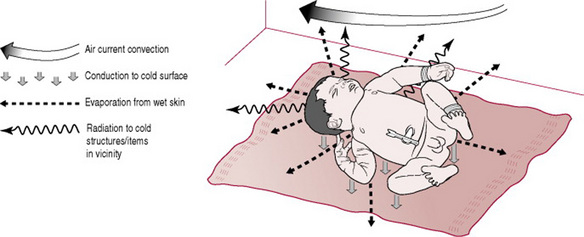
The baby enters a much cooler atmosphere at birth, with a birthing room temperature of at least 21 °C contrasting sharply with an intrauterine temperature of 37.7 °C. This causes rapid cooling of the baby as amniotic fluid evaporates from the skin. Each millilitre that evaporates removes 560 calories of heat (Rutter 2005). The baby’s large surface area:body mass ratio potentiates heat loss, especially from the head, which comprises 25% of body mass. The subcutaneous fat layer is thin and provides poor insulation, allowing rapid transfer of core heat to the skin, then to the environment, and it also affects blood cooling. In addition to heat loss by evaporation, further heat will be lost by conduction when the baby is in contact with cold surfaces, by radiation to cold objects in the environment, and by convection caused by currents of cool air passing over the surface of the body (Fig. 39.1) (Brueggemeyer 1993, Rutter 2005, Thomas 1994).
The heat-regulating centre in the baby’s brain has the capacity to promote heat production in response to stimuli received from thermoreceptors. However, this is dependent on increased metabolic activity, compromising the baby’s ability to control body temperature especially in adverse environmental conditions. The baby has a limited ability to shiver and is unable to increase muscle activity voluntarily in order to generate heat. This means that the baby must depend on his ability to produce heat by metabolism.
The neonate is endowed with brown adipose tissue, which assists in the rapid mobilization of heat resources (namely free fatty acids and glycerol) in times of cold stress. This mechanism is called non-shivering thermogenesis (Sheldon & Korones 2004). Babies derive most of their heat production from the metabolism of brown fat. The term ‘brown fat’ refers to the reddish-brown colouring of the fat, which is caused by the high degree of vascularization of the tissue. Brown fat is stored in pockets throughout the baby’s body. The majority of brown fat is located around the neck, along the line of the spinal column between the scapulae, across the clavicle line and down the sternum (Fig. 39.2). It also surrounds the major thoracic vessels and pads the kidneys (Merkin 2005). The term baby has sufficient brown fat to meet minimum heat needs for 2–4 days after birth, but cold stress results in increased oxygen consumption as the baby strives to maintain sufficient heat for survival. Brown fat uses up to three times as much oxygen as other tissue (Wong 1995), with the undesired effect of diverting oxygen and glucose from vital centres such as the brain and cardiac muscle. In addition, cold stress causes vasoconstriction, thus reducing pulmonary perfusion, and respiratory acidosis develops as the pH and PaO2 of the blood decrease and the PaCO2 increases leading to respiratory distress, exhibited by tachypnoea (Box 39.1), and grunting respirations (see Ch. 44). This, together with the reduction in pulmonary perfusion, may result in the re-opening or maintenance of the right-to-left shunt across the ductus arteriosus. Anaerobic glycolysis (i.e. the metabolism of glucose in the absence of oxygen) results in the production of acid compounding the situation by adding a metabolic acidosis. Protraction of cold stress should therefore be avoided. The peripheral vasoconstrictor mechanisms of the baby are unable to prevent the fall in core body temperature, which occurs within the first few hours after birth. It is important, therefore, for the midwife to ensure that she employs measures to minimize heat loss at birth (Rutter 2005).
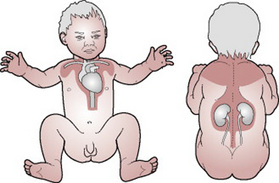
Figure 39.2 Sites of brown fat.
(From Brendan Ellis, medical illustrator, Royal Group of Hospitals, Belfast, with permission.)
Box 39.1 Transient tachypnoea of the newborn
This condition is characterized by rapid respirations of up to 120/min; it is especially common after a caesarian section. The baby may be cyanosed but maintains normal blood gases apart from PaO2. Little or no recession of the rib cage is evident and there is minimal, if any, grunt on expiration. The respiratory rate may remain elevated for up to 5 days. Treatment consists of oxygen therapy to maintain adequate oxygenation. It is essential that other causes of respiratory distress are excluded, especially infective causes (which mimic this condition) and respiratory distress syndrome (see also Ch. 44).
Intrauterine hypoxia
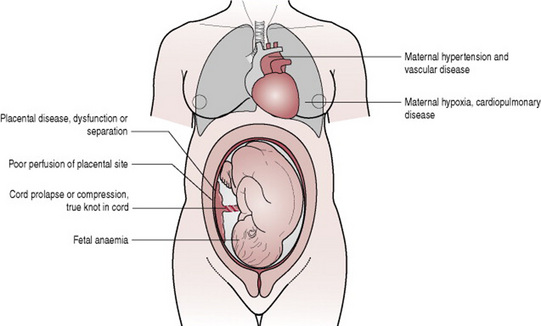
Oxygenation of the fetus is dependent on oxygenation of the mother, adequate perfusion of the placental site, placental function, fetoplacental circulation and adequate fetal haemoglobin. Absence or impairment of any of these factors will result in a reduction of oxygen supply to the fetus (Fig. 39.3).
Oxygenation of the mother may be impaired as a result of cardiac or respiratory disease, an eclamptic fit or during induction of general anaesthesia if difficulties arise during intubation. Perfusion of the placental site is dependent on satisfactory blood supply. This may be reduced in the presence of maternal hypertension or if hypotension occurs in response to haemorrhage, shock or aortocaval occlusion. Hypertonic uterine action, when uterine resting tone is elevated, will impede the blood supply to the placental site. This is sometimes due to hyperstimulation of the uterus by oxytocin and necessitates discontinuation of the oxytocic agent to allow the uterus to relax, thus restoring circulation to the placental bed. The umbilical cord transports oxygenated blood to the fetus. If prolapsed or compressed, fetal oxygenation will be reduced. The transport of oxygen within the fetus necessitates the availability of adequate haemoglobin, which may be reduced if Rhesus incompatibility is present. Abnormal fetal cardiac function may also diminish the supply of oxygen to the fetal brain.
The fetus responds to hypoxia by accelerating the heart rate in an effort to maintain supplies of oxygen to the brain. If hypoxia persists, glucose depletion will stimulate anaerobic glycolysis resulting in a metabolic acidosis. Cerebral vessels will dilate and some brain swelling may occur. Peripheral circulation will be reduced. As the fetus becomes acidotic and cardiac glycogen reserves are depleted, bradycardia develops, the anal sphincter relaxes and the fetus may pass meconium into the liquor. Gasping breathing movements triggered by hypoxia may result in the aspiration of meconium-stained liquor into the lungs, which presents an additional problem after birth.
Auscultation of the fetal heart, use of cardiotocography and observation of meconium staining of the liquor draining from the vagina should alert the midwife to fetal compromise (see Ch. 26). Subsequent fetal blood sampling may confirm a compromised fetus by revealing acidosis. However, Apgar scores do not always correlate with these findings (Fox 1993, Silverman et al 1985).
The length of time during which the fetus or neonate is subjected to hypoxia determines the outcome. It is considered that the human neonate responds to hypoxia in a similar manner to other young mammals (Roberton 2005). This involves an initial response of gasping respirations followed by a period of apnoea lasting 1½ min – primary apnoea – which, if not resolved by intervention techniques, is followed by a further episode of gasping respirations, which accelerate while diminishing in depth until, approximately 8 min after birth, respirations cease completely – secondary (terminal) apnoea. The essential difference between primary and secondary apnoea is the baby’s circulatory status. During primary apnoea, the circulation and heart rate are maintained and such babies respond quickly to simple resuscitation measures. In terminal apnoea the circulation is impaired, the heart rate is slow and the baby looks shocked (Table 39.1).
Table 39.1 Degrees of respiratory depression
| Mildly depressed | Severely depressed |
|---|---|
| Heart rate not severely reduced (60–80 b.p.m.) | Slow feeble heart rate (<40 b.p.m.) |
| Short delay in onset of respiration | No attempt to breathe |
| Good muscle tone | Poor muscle tone |
| Responsive to stimuli | Limp, unresponsive to stimuli |
| Deeply cyanosed | Pale, grey |
| Apgar score 5–7 | Apgar score <5 |
|---|---|
| No significant deprivation of oxygen during labour (primary apnoea) | Oxygen lack has been prolonged before or after delivery, circulatory failure is present, baby is shocked (secondary (terminal) apnoea) |
Failure to establish respiration at birth
The first few breaths overcome the surface tension within the lungs, drive any residual fluid from the alveoli into the circulation, and fill the lungs with air. Once the initial opening pressure has been achieved, subsequent breaths need not be so forceful (Hamilton 1999). The majority of babies gasp and establish respirations within 60 s of birth, however if they fail to initiate and sustain respiration at birth, prompt and effective intervention by the midwife is essential.
The principles of resuscitation of the newborn are applicable wherever and whenever apnoea occurs.
The midwife must therefore be aware of the predisposing factors and causes of respiratory depression and be proficient in the resuscitative measures, to be able to implement emergency care while awaiting medical assistance (Box 39.2).
Box 39.2 Resuscitation action plan
(Resuscitation Council (UK) 2006)
Don’t let the baby get cold and observe and record the sequence of events during resuscitation accurately.
Causes of respiratory depression
Obstruction of the baby’s airway by mucus, blood, liquor or meconium is one of the most common reasons for a baby failing to establish respirations. Depression of the respiratory centre may be due to:
Methods of resuscitation
Though the need for resuscitation can be anticipated in many situations, in some instances a baby is born in poor condition without forewarning. It is essential that resuscitation equipment (Box 39.3) is always available and in working order and that personnel in attendance at the birth of a baby are familiar with the equipment, resuscitation techniques and local policies regarding the provision of medical aid.
Box 39.3 Resuscitation equipment
In some units resuscitation of babies is undertaken in a specific area, whereas in others each birthing room is equipped to deal with this emergency. Whenever problems are anticipated, such as pre-term delivery, instrumental or breech delivery or fetal compromise, it is desirable that a paediatrician, neonatal nurse or midwife experienced in resuscitation techniques is present at the delivery. (In some centres an anaesthetist may be the person responsible for neonatal resuscitation.) At a home birth the midwife is the responsible person.
Aims of resuscitation
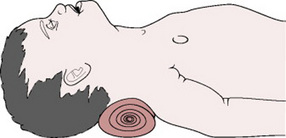
As soon as the baby is born, the clock timer should be started. The Apgar score is assessed in the normal manner at 1 min and 5 min. In the absence of any respiratory effort, resuscitation measures are commenced. The baby’s upper airways should be cleared by gentle suction of the oro- and nasopharynx and the presence of a heartbeat verified. The baby is dried quickly and transferred to a well-lit resuscitaire and placed on a flat, firm surface at a comfortable working height and under a radiant heat source to prevent hypothermia. The baby’s shoulders may be elevated on a small towel, which causes slight extension of the head and straightens the trachea (Fig. 39.4). Hyperextension may cause airway obstruction owing to the short neck of the neonate and large, ill-supported tongue.
Airway management
Most babies require no airway clearance at birth; however, if there is obvious respiratory difficulty (absence of chest wall movement) a suction catheter may be used; size 10 FG (8 FG in pre-term babies). It is recommended that the catheter tip should be inserted not further than 5 cm and that each suction attempt should last not longer than 5 s (Resuscitation Council 2005). Even with a soft catheter it is still possible to traumatize the delicate mucosa, especially in the premature baby.
Most babies born through meconium-stained liquor have not inhaled any particulate material into the lower respiratory tract. If they have not done so as a result of a period of anoxic gasping before birth, they will only very rarely do so at birth. There is now good evidence that the long recommended practice of ‘prophylactic’ suctioning of the oropharynx and nasopharynx of the emerging baby before delivery of the shoulders is of no benefit (Vain et al 2004).
If thick meconium is present and obstructing the airway, suction under direct vision should be performed by passing a laryngoscope and visualizing the larynx (Roberton 2005). Care should be taken to avoid touching the vocal cords as this may induce laryngospasm, apnoea and bradycardia. Thick meconium may need to be aspirated out of the trachea through an endotracheal tube.
Ventilation and oxygenation
If the baby fails to respond to these simple measures, assisted ventilation is necessary. This can be achieved in a variety of ways. Face-mask ventilation is the most commonly used method of inflating the baby’s lungs. It is effective and relatively safe in experienced hands. Choose an appropriately sized mask (usually 00 or 0/1) (Fig. 39.5) and position it on the face so that it covers the nose and mouth and ensures a good seal. Use a T Piece with controlled pressure (e.g. Neopuff) or a 500 mL self-inflating bag (Fig. 39.5) (do not use a 250 mL bag as it does not permit sustained inflation) (Hussey et al 2004). Care should be taken not to apply pressure on the soft tissue under the jaw as this may obstruct the airway. To aerate the lungs, deliver five sustained inflations using oxygen or air, or a combination of both, with a pressure of 30 cm H2O applied for 2–3 s and repeated five times, then continue to ventilate at a rate of 40 respirations/min (Drew et al 2000). Insertion of a neonatal airway helps to prevent obstruction by the baby’s tongue. Note that overextension of the baby’s head causes airway obstruction. A longer inspiration phase improves oxygenation. Higher inflation pressures may be required to produce chest movement. Bag and mask technique and T Piece with controlled pressure therefore require a skilled operator to achieve success (Fig. 39.6). Used correctly these methods can avoid the need for endotracheal intubation.

Figure 39.5 Face-mask sizes, self-inflating bag and face mask.
(From Medical Photography Department, Royal Group of Hospitals, Belfast, with permission.)
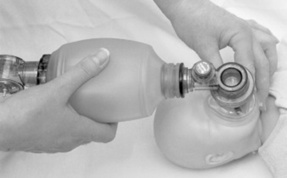
Figure 39.6 Bagging demonstration.
(From Medical Photography Department, Royal Group of Hospitals, Belfast, with permission.)
Endotracheal intubation
If the baby fails to respond to intermittent positive pressure ventilation (IPPV) by bag and mask, or if bradycardia is present, an endotracheal tube should be passed without delay. Intubating a baby requires special skill that, once acquired, must be practised to be retained.
Practicalities of intubation
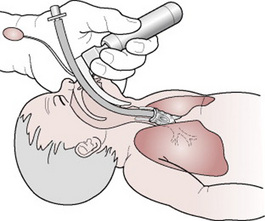
Ensure that all the equipment listed in Box 39.3 is available and in working order. Position the baby on a flat surface, preferably a resuscitaire, and extend the neck into the ‘neutral position’. Place a rolled-up towel under the shoulders, which will help maintain proper alignment. Introduce the blade of the laryngoscope over the baby’s tongue into the pharynx until the epiglottis is visualized. Elevation of the epiglottis by the tip of the laryngoscope reveals the vocal cords. Any mucus, blood or meconium obstructing the trachea should be cleared by careful suction prior to passing the endotracheal tube a distance of 1.5–2 cm into the trachea (Fig. 39.7). (Pressure on the cricoid cartilage may facilitate visualization of the larynx.) Intubation may be easier if a tracheal introducer made of plastic-covered soft metal wire is used. This will increase the stiffness and curvature of the tube. After the laryngoscope is removed, oxygen is administered by intermittent positive pressure ventilation (IPPV) to the endotracheal tube via the ‘Neopuff’ or the self inflating bag. A maximum of 30 cm water pressure should be applied, as there is risk of rupture of alveoli or tension pneumothorax if higher pressure is applied. The rise and fall of the chest wall should indicate whether the tube is in the trachea. This can be confirmed by auscultation of the chest listening for air entry to be equal on both sides. Distension of the stomach indicates oesophageal intubation necessitating removing and re-siting of the tube.
Mouth-to-face/nose resuscitation
In the absence of a bag and mask, assisted ventilation can be achieved by mouth-to-face resuscitation. With the baby’s head in the ‘sniffing’ position (Fig. 39.4) the operator places his/her mouth over the baby’s mouth and nose and, using as much air as he/she can easily keep in their cheeks inflate the babies chest with each breath, at a rate of 20–30 breaths/min, allowing the infant to exhale between breaths (Resuscitation Council 2006). Findings indicate that the nasal route of air entry is more effective than the combined nose and mouth or mouth routes and that neck flexion impedes air entry (Wilson-Davis et al 1997). It may be easier with larger babies to use mouth-to-nose resuscitation (Tonkin et al 1995).
External cardiac massage
Chest compressions should be performed if the heart rate is <60, or between 60 and 100 and falling despite adequate ventilation. The most effective way of achieving this is by encircling the baby’s chest with the fingers on the spine and thumbs on the lower third of the sternum (Fig. 39.8) (Milner 1991, Roberton 2005). Research undertaken by Houri et al 1997 concluded that the two thumbs method of chest compression proved haemodynamically more effective. The chest is depressed at a rate of 100–120 times/min, at a ratio of three compressions to one ventilation, and at a depth of 2–3 cm of the baby’s chest. (Excessive pressure over the lower end of the sternum may cause rib, lung or liver damage).
Use of drugs for resuscitation
If the baby’s response is slow or he remains hypotonic after ventilation is achieved, consideration will be given to the use of drugs. In specialist obstetric units, pulse oximetry may be employed to monitor hypoxia (Letko 1996) and blood obtained through the umbilical artery or vein to ascertain biochemical status (Harris et al 1996). Results will enable appropriate administration of resuscitation drugs, as discussed below.
Sodium bicarbonate
This is not recommended for brief periods of cardiopulmonary resuscitation. Once tissues are oxygenated by lung inflation with 100% oxygen and cardiac compression, the acidosis will self-correct unless asphyxia is very severe. If the heart rate is <60, despite effective ventilation, chest compression and two intravenous doses of adrenaline (epinephrine), then sodium bicarbonate 4.2% solution (0.5 mmol/mL), can be administered using 2–4 mL/kg (1–2 mmol/kg) by slow intravenous injection at a rate of 1 mL/min in order to avoid rapid elevation of serum osmolality with the attendant risk of intracranial haemorrhage (Drew et al 2000). It should not be given prior to ventilation being established. THAM 7% (tris-hydroxymethyl-amino-methane), 0.5 mmol/kg may be used in preference to sodium bicarbonate (Roberton 2005).
Adrenaline (epinephrine)
This is indicated if the heart rate is <60, despite 1 min of effective ventilation and chest compression. An initial dose of 0.1–0.3 mL/kg of 1:10 000 solution (10–30 μg/kg) can be given intravenously; this can be repeated after 3 min for a further two doses. The Royal College of Paediatrics and Child Health (1997) recommends a higher dose of 100 μg/kg i.v. if there is no response to the boluses. It is reasonable to try giving one dose via the endotracheal tube of adrenaline (epinephrine) 0.1 mL/kg of 1:1000 as this sometimes has an immediate effect (Halliday et al 1998).
10% dextrose
Hypoglycaemia is not usually a problem unless resuscitation has been prolonged. A solution of 10% dextrose 3 mL/kg may be given intravenously via the umbilical vein to correct a blood sugar of <2.5 mmol/L.
Volume replacement
On rare occasions, bradycardia will only respond to volume expansion and a bradycardia (as opposed to asystole) that does not respond to chest compressions or drugs is very suggestive of hypovolaemia. Preparation is 0.9% normal saline 10 mL/kg initially via the umbilical vein is given and can be repeated once if necessary (Resuscitation Council 2006).
In rare incidences where the baby has lost blood, e.g. acute feto–maternal transfusion or vasa praevia, replace with emergency 0 Rh neg blood 10 mL/kg.
Naloxone hydrochloride
This is not an emergency drug per se and should be used with caution and only in specific circumstances. It is a powerful anti-opioid drug used to reverse the effects of maternal narcotic drugs given in the preceding 3 hrs. Ventilation should be established prior to its use. It must not be given to apnoeic babies. A dose of up to 100 μg/kg body weight may be administered intramuscularly for prolonged action (Drew et al 2000). As opioid action may persist for some hours, the midwife must be alert for signs of relapse when a repeat dose may be required. Note: It should not be administered to babies of narcotic-addicted mothers as this may precipitate acute withdrawal (Zelon et al 1998). Policies relating to dosage and route of administration may vary in different hospitals.
Immediate care of the baby at birth by the attending midwife
Observations during and after resuscitation
Throughout the resuscitation procedure, the baby’s response should be monitored and recorded. An accurate written record detailing the resuscitation events is essential, not only because it forms an integral part of the medical and midwifery management of the baby, but also because it can help to protect the practitioner if defence of his/her practice is required. The endotracheal tube may be left in place for a few minutes after the baby starts to breathe spontaneously. Suction may be applied through the endotracheal tube as it is removed. There are some babies who at birth may be somewhat distressed but improve with good resuscitation, and require observations for a few hours before a decision is made whether to admit to a postnatal ward or the neonatal unit. The labour suite is an ideal place for this form of transitional care to take place. A baby whose Apgar score was less than 6 at 5 min, or who was slow to respond to resuscitation, or who requires continued ventilatory assistance, should be transferred to the neonatal unit for a period of observation in order to monitor behaviour and detect early signs of hypoxic ischaemic encephalopathy (HIE) (see Ch. 45).
Explanation about the resuscitation and the need for transfer to hospital (if born at home) or to the neonatal unit must be given to the parents and, if the baby’s condition permits, the mother should have the opportunity to see and hold her baby prior to separation. This assists the attachment process described later in this chapter. Babies who respond quickly to resuscitation can be reunited with their parents and remain with them in the labour room until transfer to the postnatal ward is organized.
It can be seen that the midwife’s role at the time of birth is one both of privilege and of immense responsibility. Her wish to meet the psychological needs of the parents and the baby must be tempered with the need to accommodate the baby’s necessary adaptations at the time of birth and to institute emergency care when required. Continued care of the newborn takes the history of the baby’s condition at birth into account and is discussed in Chapter 40.
Assessment of the baby’s condition
As soon as the baby has been born, the midwife can proceed with drying the skin, which will help minimize heat loss (Bauer & Versmold 1995). In the vast majority of cases, babies are well and can be handed directly to their parents. Whether a home or hospital birth, the midwife at 1 min and 5 min after the birth will make an assessment of the baby’s general condition using the Apgar score (Apgar 1953) (Table 39.2). The assessment at 1 min is important for the further management of resuscitation. However, it has been shown that an assessment at 5 min is more reliable as a predictor of the risk of death during the first 28 days of life, and of the child’s neurological state and risk of major disability at the age of 1 year. The higher the score, the better the outcome for the baby. The Apgar score must be fully documented in the baby’s records.
Prevention of heat loss
With the midwife’s knowledge of the baby’s transitional requirements it is her responsibility to ensure appropriate preparations are made for the birth of the baby. Whether the baby is born at home or in hospital, it is important that the midwife endeavours to provide an ambient temperature in the range 21–25 °C. It is accepted that in some remote parts of the world and in emergency situations, this may not be possible. However, within controlled circumstances, provision of an optimal thermal environment is paramount in facilitating a successful transition to extrauterine life. Switching off fans prior to birth helps to minimize heat loss by convection, and closing curtains reduces the radiant heat loss to windows (Karlsson 1996). The baby’s temperature can drop by as much as 3–4 °C within the first minute (Sinclair 1992, Thomas 1994). After birth, the baby’s body and head should be dried immediately thus helping to minimize heat loss by evaporation. It is important to ensure that the wet towel is then removed and the baby wrapped in another dry pre-warmed towel. Skin-to-skin contact is an effective method of preventing heat loss, the mother’s chest or abdomen is the ideal surface, clean and just at the right temperature. If skin-to-skin is not acceptable the baby should be wrapped or swaddled, a hat put on its head and placed in its mother’s arms (WHO 1997). However, considerable heat loss continues by convection, conduction and radiation, particularly from exposed areas of the baby’s skin (Karlsson 1996). The midwife’s role is to protect and keep the baby warm and she can do this by: keeping the labour room warm, drying the baby at birth, encouraging skin-to-skin, wrapping the baby and encourage breastfeeding (WHO 1997). Pre-term babies in particular can lose heat very rapidly after birth. Research has shown that placing the pre-term baby in a plastic bag up to its neck will prevent heat loss. This method has now been adopted by many labour wards (Knobel & Holditch-Davis 2007).
Clearing the airway
As the baby’s head is born, excess mucus may be wiped gently from the mouth. However, care must be taken to avoid touching the nares, as such action may stimulate reflex inhalation of debris in the trachea. Although fetal pulmonary fluid is present in the mouth, most babies will achieve a clear airway unaided. If you are not achieving chest expansion, the airway can be cleared with the aid of a soft suction catheter attached to low pressure (10 cm water) mechanical suction. It is important to aspirate the oropharynx prior to the nasopharynx so that, if the baby gasps as his nasal passages are aspirated, mucus or other material is not drawn down into the respiratory tract. Excess suction can result in vagal stimulation, with laryngospasm and bradycardia.
Stimulation
Rough handling of the baby merely serves to increase shock and is unnecessary. Gentle stimulation by drying the baby and clearing the airway may initiate breathing, but there are a number of different methods that can be used, for example a single finger flick to the sole of the foot, or gentle back rubbing; this will give an indication as to whether tactile stimulation is likely to be effective. Under no circumstances should the method used cause pain or bruising.
Cutting the cord
The umbilical cord is the lifeline of the fetus and of the baby in the first few minutes after birth. Separation of the baby from the placenta is achieved by dividing the umbilical cord between two clamps, which should be applied approximately 8–10 cm from the umbilicus. Application of a gauze swab over the cord while cutting it with scissors will prevent blood spraying the delivery field. The cord should not be cut until it has been clamped securely. Failure to comply with this procedure may result in excessive blood loss from the baby. In some labour suites, it is now common practice for the father of the baby to assist the midwife and cut the umbilical cord. Care of the umbilical cord and stump in the immediate period varies according to social, cultural and geographic factors. The optimal time for umbilical cord clamping after birth remains unknown. Research continues into cord clamping (Mercer et al 2000). In 2007 a controlled trial comparing late versus early cord clamping following birth in infants born at 37 or more weeks’ gestation was undertaken and concluded that delaying clamping of the umbilical cord in full-term neonates for a minimum of 2 min following birth is beneficial to the newborn. Although there was an increase in polycythaemia among babies in whom cord clamping was delayed this condition appeared to be benign (Hutton & Hassan 2007).
Some centres advocate delay in cutting until respirations are established and cord pulsation has ceased, thus ensuring that the infant receives a placental transfusion of some 70 mL of blood (Nelle et al 1993). This view is countered by those who maintain that the placental transfusion so acquired may predispose to neonatal jaundice (see Ch. 47). What is agreed is that a term baby at birth can be drawn up on to the mother’s abdomen, but raised no higher, and a pre-term baby should be kept at the level of the placenta. This is because, if a pre-term baby is held above the placenta then blood can drain from the baby to the placenta, resulting in anaemia, and if held below can cause the baby to receive a blood transfusion.
Identification of baby
The time of birth and sex of the baby are noted and recorded once the baby has been completely expelled from its mother. It is the practice in many units to apply name-bands to the baby before the cord is cut. Within the baby’s own home, unless a twin delivery, identification does not present a problem. However, when babies are born in hospital it is essential that they are readily identifiable from one another. Various methods of indicating identity can be employed, for example name-bands or identity tags. In the UK, two name-bands are applied, each of which should indicate legibly in indelible pen the family name, sex of the baby, date and time of birth. In some centres, the name-bands are number coded with the baby’s case records; in others the number coding corresponds with that of the mother. The amount of information written on the name-bands may vary slightly according to local policy. The mother or father, or both, should verify that the information on the bands or tags is correct prior to these being attached to the baby. The midwife should ensure that the name-bands are fastened securely and are neither too tight, impeding circulation or likely to excoriate the skin, nor too loose, risking loss of the means of identification. Name-bands or tags should remain on the baby until discharged from hospital. The midwives must advise the mother to inform a member of the midwifery or nursing staff if the name-band or tag becomes detached or is removed for any reason. At birth, the midwife will issue the mother with a Personal Child Health Record (Red Book), which will be the main record of their child’s health, growth and development. The examination of their baby at birth will be recorded in this and parents will be instructed to bring the book with them when their baby attends for a general practitioner, health visitor, or hospital appointment.
First examination by midwife
Prior to leaving the mother’s home, or transferring the baby to the ward, the midwife undertakes a detailed examination of the baby checking for obvious abnormalities, such as spina bifida, imperforate anus, cleft lip or palate, abrasions, fractures or haemorrhage due to trauma. The initial cord clamp is replaced with another method of securing haemostasis by applying a disposable plastic clamp (or a secure ligature) approximately 2–3 cm from the umbilicus and cutting off the redundant cord. The baby’s temperature is now recorded. The midwife should ensure that the environmental temperature remains warm, between 21–25 °C, as previously discussed. Early transfer of the baby to a postnatal ward has been advocated as a means of minimizing heat loss. The baby should be transferred with its mother, in her arms, to avoid heat loss and to promote mother–baby attachment.
Instillation of eyedrops as prophylaxis against gonococcal infection is not practised in the UK. In other parts of the world, drops or antibiotic ointment may be instilled (Wong 1995). It is suggested that, to be effective, such treatments should be administered within 1 hrs of birth (Tyson 1992). The first bath and other non-urgent procedures may be deferred in order to minimize heat loss. (Further care is discussed in Ch. 40.) All babies should remain with their mother during the first few hours of life, providing both mother and baby are in good condition, as this is the time when parent–baby interactions are initiated and the reality of parenthood begins.
Vitamin K
Depending on local policy, vitamin K, intramuscular or orally may be given as prophylaxis against bleeding disorders (see Ch. 45). Vitamin K is fat soluble; it can only be absorbed from the intestine in the presence of bile salts. The body’s capacity to store vitamin K is very low and the half-life of the vitamin-K-dependent coagulation factors is short (Zipursky 1999). Midwives must be aware of and follow their local hospital policy regarding consent and administration of vitamin K (DoH 1998).
Early parent–baby relationships
The safe birth of a healthy baby engenders considerable emotion in most parents and indeed in attendants at the birth. The efforts of the preceding hours are temporarily forgotten as the mother sees her baby for the first time. Characteristically, her first query often relates to the sex of the baby, speedily followed by an anxious enquiry about the baby’s state of health: ‘is he/she all right?’ Reassured on these points, a mother progresses to an examination of her baby, which follows a fairly predictable pattern unless the condition of either the mother or the baby is impaired by the process of labour or by narcotic drugs. Fathers too are involved in this early exploration of the newborn baby. The response of both parents is influenced not only by their prenatal understanding and previous experiences with babies but also by the appearance, behaviour and responses of their baby, who takes an active part in the proceedings (Salariya 1990, Williams 1995). Cultural background may also play a part in parental behaviours at this time (Callister 1995).
The first hour after birth is a time of particular sensitivity for the parents and is a time when the baby has a long period (60%) of ‘quiet alert state’ (Saigal et al 1981). In this state and while acclimatizing to the outside world, the baby is especially interested in the faces of its mother and father, the sound of their voices, and their smell and touch. Parents can also learn about their baby’s natural abilities in this state, in which they can follow the parents’ faces or an object, turn to the mother’s voice and stop crying when held by the mother or father (Klaus & Klaus 2004).
Regardless of age, parity or marital status, mothers are likely to display a similar behavioural pattern when touching their babies for the first time. This sequence of touching behaviour is enhanced if the baby is naked. The mother begins her examination of her baby by exploring the extremities and head with her fingertips. Thereafter, she caresses her baby’s body with her entire hand before gathering her baby in her arms often in the en face position where eye-to-eye contact can be established. She talks to her baby with great emotion, looking for positive reinforcement from her partner and other birth attendants (Klaus et al 1975, Sander 1993).
Her emotions at this time may be mixed. She may display great excitement and happiness – laughing, talking or even crying with joy, or she may feel too tired to react positively towards her baby. Factors which may predispose to this latter reaction include prolonged labour, instrumental birth, and baby of the ‘wrong’ sex or congenital abnormality. Lack of support from partner or parents may influence the behaviour of an unmarried mother and, for some mothers, high parity may dampen their response. Childhood deprivation can inhibit some women from reacting in the anticipated manner.
For some mothers, the sight of an unwashed, wet and sometimes bloody baby is profoundly distasteful and they are not appreciative of skin-to-skin contact with a baby in this condition. A good midwife will ascertain the mother’s attitude during pregnancy or early labour. This will allow her to modify her technique in assisting the birth and immediate care of the baby to meet the mother’s wishes and so assist the mother to feel comfortable at her first meeting with her baby. Early skin-to-skin contact and breastfeeding are significantly associated with exclusive breastfeeding at discharge and also promote good mother–baby relationships and stimulate lactation (Catlaneo & Buzzetti 2001) (see Ch. 41).
While midwives are providing women-centred care it is important for them to remember to involve the fathers in decision-making and acknowledge their role and needs in one of the most important events in their lifetime (Ratnaike 2007, Tiedje & Darling-Fisher 2003).
Many fathers are surprised at their profound emotional response to the birth of their baby. Sometimes a man’s reactions are stronger than those of his partner, who may be rather tired initially. The father feels a sense of deep satisfaction and self-esteem, and is elated and keen to touch and hold his baby and his partner and share his excitement (Barclay & Lupton 1999, Wildman 1995). Intimacy shared between the father and mother at this time is extended to include the baby within an exclusive family group, often oblivious to their surroundings.
Having accomplished the immediate physiological adaptations of respiration and circulation, the baby displays interest in sound, light and nutrition, responding to the mother’s voice by moving limbs in synchrony. The response to touch is illustrated in the grasp reflex and in suckling of the breast if offered – or the fist. Newborn babies appear to focus on their mother’s face at a distance of 20 cm and respond to movement of bright shiny objects, such as their mother’s eyes, by tracking them visually. These responsive behaviours evoke reinforcing responses from parents, thus promoting the interactions essential for survival, which is dependent on good parenting. A slightly darkened birthing room encourages babies to open their eyes widely and look around them, whereas bright lights cause frowning. The midwife’s understanding of these responses allows her to create optimal conditions for interaction to occur.
Promotion of parent–baby interaction
The term ‘bonding’ has been used to describe the establishment of parent–baby relationships in the early neonatal period. The implication of the desirability to feel instant love for one’s child can lead to feelings of guilt in some parents who do not identify a strong emotional tie with their baby at birth. It is important to recognize that, as individuals, parents develop a loving relationship with their child at their own pace, some taking longer than others – days, weeks or months. Parents should feel able to express their disappointments as well as their joys without fear of being thought a ‘bad’ parent (Barclay & Lupton 1999).
A good rapport between the parents and the midwife should enable the development of their love for their baby to progress happily and at its own speed. However, the midwife must be alert to report and document marked negative reactions from either or both parents, as this may be an early sign of future parenting difficulties. Adverse behaviours of note include hostile verbal or non-verbal attitude, lack of supportive interaction between the parents, disinclination to touch or hold their baby, disparaging remarks about the baby or marked disappointment about the sex of the baby.
Involvement of the father in the birth of the baby’s body, clamping of the cord and early bathing have been introduced in some centres to help to promote father–baby relationships. The midwife can do much to promote the beginnings of loving relationships by encouraging both parents to handle and examine their baby, by her positive comments, and by undertaking the examination of the baby beside the parents.
Privacy to talk, touch and be alone together with their baby is important for parents and opportunities to do so should be provided whether their baby is born at home or in hospital. The midwife should be sensitive to this often-unexpressed need and leave the family together for some time before progressing with further care of the baby.
The opportunity for this initial intimate family moment is dependent on the baby’s condition. A baby whose Apgar score is below 7 requires some form of resuscitation, which may necessitate speedy removal to a special resuscitation area. As parents have entered an entirely new phase of their lives it is essential that they receive a reassuring explanation and adequate information about the need to be separated from their baby, which can be totally unexpected and therefore very frightening.
Apgar V. A proposal for a new method of evaluation of the newborn infant. Current Research in Anaesthesiology and Analgesics. 1953;40:340.
Barclay L, Lupton D. The experiences of new fatherhood: a socio-cultural analysis. Journal of Advanced Nursing. 1999;29(4):1013-1020.
Bauer K, Versmold H. Prevention of neonatal hypothermia in the delivery room. In: Okken A, Koch J, editors. Thermoregulation of sick and low birth neonates. Berlin: Springer; 1995:219.
Brueggemeyer A. Neonatal thermoregulation. In: Kenner C, Brueggemeyer A, Gunderson LP, editors. Comprehensive neonatal nursing: a physiologic perspective. Philadelphia: W B Saunders, 1993.
Callister LC. Cultural meanings of childbirth. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1995;24(4):327-331.
Catlaneo A, Buzzetti P. Effects on rates of breastfeeding in training for the baby friendly hospital initiative. British Medical Journal. 2001;323:1358-1362.
Department of Health. Vitamin K for babies. London: DoH, 1998. PLO/CNO/998/4
Drew D, Jevon P, Ravy M. Resuscitation of the newborn: a practical approach. Oxford: Butterworth-Heinemann, 2000.
Fox HE. Apgar: A commentary. P&S Medical Review. 1993;1(1):1-3.
Halliday HL, McClure BG, Reid M. Handbook of neonatal intensive care, 4th edn. W B Saunders, London, 1998.
Hamilton P. ABC of labour care, care of the newborn in the delivery room. British Medical Journal. 1999;318:1403-1406.
Harris M, Beckley SL, Garibaldi JM, et al. Umbilical cord blood analysis at the time of delivery. Midwifery. 1996;12(3):146-150.
Houri PK, Frank LR, Menegazzi JJ, et al. A randomized, controlled trial of two-thumbs vs. two finger chest compression in a swine infant model of cardiac arrest. Prehospital Emergency Care. 1997;1(2):65-67.
Hussey SG, Ryan CA, Murphy BP. Comparison of three manual ventilation devices using an intubated mannequin. Archives of Disease in Childhood Fetal and Neonatal Edition. 2004;89:F490-F493.
Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates. Journal of the American Medical Association. 2007;297(11):1241-1252.
Karlsson H. Skin to skin care: heat balance. Archives of Disease in Childhood. 1996;75(2):F130-F132.
Klaus P, Klaus M. No separation of mother and baby with unlimited opportunity for breastfeeding. Journal of Perinatal Education. 2004;13(2):35-41.
Klaus MH, Trause MA, Kennell JH. Does human maternal behaviour after delivery show a characteristic pattern? In: CIBA Foundation Symposium 33, parent–infant interaction. Amsterdam: Associated Scientific; 1975.
Knobel R, Holditch-Davis D. Thermoregulation and heat loss prevention after birth. Journal of Obstetrics, Gynecology, and Neonatal Nursing. 2007;36(3):280-287.
Letko MD. Understanding the Apgar score. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1996;25(4):299-303.
Linh GLy, Hawes J, Whyte HE, et al. The hemodynamically significant ductus arteriosus in critically ill full-term neonates. Neonatology. 2007;91(4):260-265.
Mercer JS, Nelson CC, Skovgaard RL. Umbilical cord clamping: beliefs and practices of American nurse-midwives. Journal of Midwifery and Women’s Health. 2000;45(1):58-65.
Merkin RJ. Growth and distribution of human brown fat. The Anatomical Record. 2005;178(3):637-645.
Milner AD. Resuscitation of the newborn. Archives of Diseases in Childhood. 1991;66(1):66-69.
Nelle M, Zilow EP, Kraus M. The effect of Leboyer delivery on blood viscosity and other hemorheologic parameters in term neonates. American Journal of Obstetrics and Gynecology. 1993;169(1):189-193.
Perry SE. Nursing care of the newborn. In Bobak IM, Lowdermilk DL, Jensen MD, et al, editors: Maternity nursing, 6th edn, Edinburgh: Elsevier Health Sciences, 2002. Ch 18
Puckett RM, Offringa M. The Dutch Cochrane Centre, The Netherlands, 2000.
Ratnaike D. Father’s present or just in the room? Midwives. 2007;10(3):106.
Resuscitation Council (UK). Resuscitation guidelines, newborn life support. 2005. Online. Available: www.resus.org.uk.
Resuscitation Council UK. Newborn Life Support Resuscitation at birth, 2nd edn. Kent: TT Litho Printers, 2006.
Roberton NRC. Resuscitation of the newborn. In Rennie JM, editor: Roberton’s textbook of neonatology, 4th edn, Edinburgh: Churchill Livingstone, 2005.
Royal College of Paediatrics and Child Health. Resuscitation of babies at birth. London: BMJ Publishing, 1997.
Rutter N. Temperature control and disorders. In Rennie JM, editor: Roberton’s textbook of neonatology, 4th edn, Edinburgh: Churchill Livingstone, 2005.
Saigal S, Nelson N, Bennett K, et al. Observation on the behavioral state of newborn infants during the first hours of life. American Journal of Obstetrics and gynecology. 1981;139:715.
Sander LW. The earliest relationship. Journal of the American Psychoanalytic Association. 1993;41:281-284.
Salariya E. Parental-infant attachment. In: Alexander J, Levy V, Roch S, editors. Postnatal care – a research based approach. Basingstoke: Macmillan, 1990.
Sheldon B, Korones MD. An encapsulated history of thermoregulation in the neonate. NeoReviews. 2004;5(3):e78.
Silverman F, Suidan J, Wasserman J, et al. The Apgar score: is it enough? Obstetrics and Gynaecology. 1985;66:331-336.
Sinclair JC. Management of the thermal environment. In: Sinclair JC, Bracken MB, editors. Effective care of the newborn infant. Oxford: Oxford University Press, 1992.
Tannenbaum JE, Waleh NS, Mauray F, et al. ransforming growth factor-β protein and messenger RND expression is increased in the closing ductus arteriosus. Paediatric Research. 1996;39(3):427-434.
Thomas K. Thermoregulation in neonates. Neonatal Network. 1994;13(2):15-22.
Tiedje LB, Darling-Fisher C. Promoting father-friendly healthcare. American Journal of Maternal Child Nursing. 2003;28(6):350-357.
Tonkin SL, Davis SL, Gunn TR. Nasal route for infant resuscitation by mothers. Lancet. 1995;345(8961):1353-1354.
Tyson JE. Immediate care of the newborn infant. In: Sinclair JE, Bracken MB, editors. Effective care of the newborn infant. Oxford: Oxford University Press, 1992.
Vain NE, Szyld EG, Prudent LM, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: multicentre, randomized controlled trial. Lancet. 2004;364:597-602.
Wildman J. Is this finally it? New Generation. 1995;14(2):5.
Williams RP. Family dynamics after childbirth. In Bobak IM, Lowdermilk DL, Jensen MD, editors: Maternity nursing, 4th edn, St Louis: CV Mosby, 1995.
Wilson-Davis SL, Tonkin SL, Gunn TR. Air entry in infant resuscitation: oral or nasal routes? Journal of Applied Physiology. 1997;82(1):152-155.
Wong DL, editor. Whaley and Wong’s nursing care of infants and children, 5th edn, St Louis: CV Mosby, 1995.
World Health Organization. Thermal control of the newborn: a practical guide. World Health Organization, 1997;5-14. WHO/RHT/MSM/97.2 Ch 1
Zelon C, Rubio E, Wasserman E. Neonatal drug withdrawal. American Academy of Pediatrics. 1998;101(6):1079-1088.
Zipursky A. Prevention of vitamin K deficiency bleeding in newborns. British Journal of Haematology. 1999;104:430-437.
Beachy P, Deacon J. The core curriculum for neonatal intensive care nursing. Philadelphia: W B Saunders, 1993.
This book is written for nurses caring for high risk babies. As well as providing information regarding the physiological problems of the sick newborn, it also deals with problems concerning the family, legal and ethical issues.
Bracken M, Sinclair J. Effective care of the newborn infant. Oxford: Oxford University Press, 1992.
This informative textbook covers all aspects of neonatal conditions with contributions from many neonatal specialists. It should be used as a reference to complement conditions discussed in the chapter.
Brazelton TB, Cramer B. The earliest relationship. New York: Addison Wesley, 1990.
This book is a collaboration between a paediatrician and a psychoanalyst and weaves together the fundamentals of new and empirical research in earliest infancy. It is an easy-to-read book which contains a wealth of information and clinical expertise.
Kenner C, Wright Lott J, Flandermeyer A. Comprehensive neonatal nursing: a physiologic perspective, 2nd edn. Philadelphia: W B Saunders, 1998.
This book provides a comprehensive approach to neonatal care, and is an essential text for those caring for newborn babies and their families.
Rennie JM, editor. Textbook of neonatology, 4th edn, Edinburgh: Churchill Livingstone, 2005.
This is a valuable book for resource material; as well as covering all the conditions that effect the baby it covers topics such as litigation and how the law relates to the baby. It also looks at ethical and moral questions raised because of modern intensive care.
Resuscitation Council (UK). Newborn life support, 2nd edn. Kent: TT Litho Printers, 2006.
This informative book covers all aspects of newborn life support, and can be used as a reference source in newborn resuscitation training.