Cushing Syndrome
Cushing syndrome is a characteristic group of manifestations caused by excessive circulating free cortisol. It can result from a variety of causes, which generally fall into one of five categories (Box 38-12 and Table 38-2). Cushing syndrome in young children may be due to an adrenal tumor (Moshang, 2003).
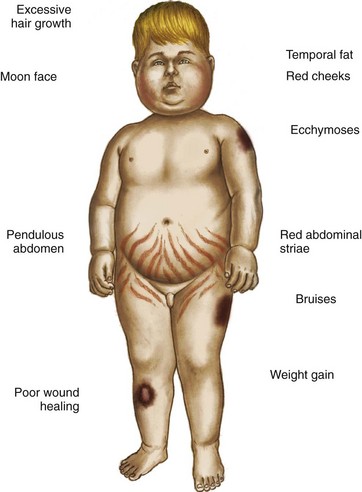
Cushing syndrome is uncommon in children. When seen, it is often caused by excessive or prolonged steroid therapy that produces a cushingoid appearance (see Fig. 38-4). This condition is reversible once the steroids are gradually discontinued. Abrupt withdrawal precipitates acute adrenal insufficiency. Gradual withdrawal of exogenous supplies is necessary to allow the anterior pituitary an opportunity to secrete increasing amounts of ACTH to stimulate the adrenals to produce cortisol.
Clinical Manifestations
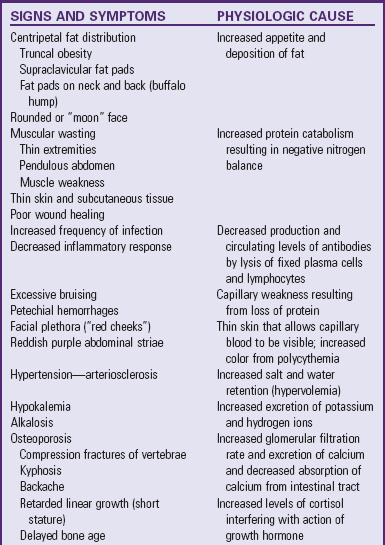
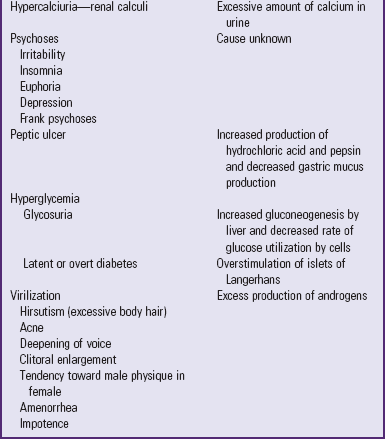
Because the actions of cortisol are widespread, clinical manifestations are equally profound and diverse (see Table 38-2). Those symptoms that produce changes in physical appearance occur early in the disorder and are of considerable concern to school-age and older children (Fig. 38-5). The physiologic disturbances, such as hyperglycemia, susceptibility to infection, hypertension, and hypokalemia, may have life-threatening consequences unless recognized early and treated successfully. Children with short stature may be responding to increased cortisol levels, resulting in Cushing syndrome. Cortisol inhibits the action of GH.
Diagnostic Evaluation
Several tests are helpful in confirming Cushing syndrome. Serum cortisol levels should be measured at midnight and in the morning, along with corticotropin hormone, urinary free cortisol, fasting blood glucose levels for hyperglycemia, serum electrolyte levels for hypokalemia and alkalosis, and 24-hour urinary levels of elevated 17-hydroxycorticoids and 17-ketosteroids. Imaging of the pituitary and adrenal glands to assess for tumors, bone density studies for evidence of osteoporosis, and skull x-rays examination to determine enlargement of the sella turcica may also aid in the diagnosis. Another procedure used to establish a more definitive diagnosis is the dexamethasone (cortisone) suppression test (Batista, Riar, Keil, et al, 2007). Administration of an exogenous supply of cortisone normally suppresses ACTH production. However, in individuals with Cushing syndrome, cortisol levels remain elevated. This test is helpful in differentiating between children who are obese and those who appear to have cushingoid features.
Therapeutic Management
Treatment depends on the cause. In most cases surgical intervention involves bilateral adrenalectomy and postoperative replacement of the cortical hormones (the therapy for this is the same as that outlined for chronic adrenal insufficiency). If a pituitary tumor is found, surgical extirpation or irradiation may be chosen. In either of these instances, treatment of panhypopituitarism with replacement of GH, thyroid extract, ADH, gonadotropins, and steroids may be necessary for an indefinite period.
Nursing Care Management
Nursing care also depends on the cause. When cushingoid features are caused by steroid therapy, the effects may be lessened with administration of the drug early in the morning and on an alternate-day basis. Giving the drug early in the day maintains the normal diurnal pattern of cortisol secretion. If given during the evening, it is more likely to produce symptoms because endogenous cortisol levels are normally low and the additional supply exerts more pronounced effects. An alternate-day schedule allows the anterior pituitary an opportunity to maintain more normal hypothalamic-pituitary-adrenal control mechanisms.
If an organic cause is found, nursing care is related to the treatment regimen. Although a bilateral adrenalectomy permanently solves one condition, it reciprocally produces another syndrome. Before surgery, parents need to be adequately informed of the operative benefits and disadvantages. Postoperative teaching regarding drug replacement is the same as discussed in the previous section.
Anorexia and nausea and vomiting are common and may be improved with the use of nasogastric decompression. Muscle and joint pain may be severe, requiring use of analgesics. The psychologic depression can be profound and may not improve for months. Parents should be aware of the physiologic reasons behind these symptoms in order to be supportive of the child.
Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is a family of disorders caused by decreased enzyme activity required for cortisol production in the adrenal cortex. The most common defect (accounting for >90% of cases) is 21-hydroxylase deficiency (American Academy of Pediatrics, 2000 [reaffirmed 2005]). This deficiency is an autosomal recessive disorder that results in improper steroid hormone synthesis. It occurs in approximately 1 per 12,000 to 15,000 births. In its most severe form, it can be life threatening (Glatt, Garzon, and Popovic, 2005).
Pathophysiology
Interference in the biosynthesis of cortisol during fetal life results in an increased production of ACTH, which stimulates hyperplasia of the adrenal gland. Depending on the enzymatic defect, increased quantities of cortisol precursors and androgens are secreted. There are six major types of biochemical defects. The most common is partial or complete 21-hydroxylase deficiency. With partial deficiency, enough aldosterone is produced to preserve sodium, and adequate cortisol is produced to prevent signs of adrenocortical insufficiency.
In the complete, or salt-losing, form, insufficient amounts of aldosterone and cortisol are produced. If salt-losing CAH is not diagnosed and treated at birth, infants will exhibit symptoms of failure to thrive, weakness, vomiting, and dehydration, and a salt-losing crisis will ensue (Behrman, Kliegman, Jenson, et al, 2009). In 11-hydroxylase deficiency there is an increase in the mineralocorticoid 11-desoxycorticosterone, which leads to hypertension. In each of these types excess production of androgens causes ambiguous genitalia in females and precocious genital development in males. Other forms of CAH do not result in excess production of androgens but cause various degrees of hypoaldosteronism or hyperaldosteronism.
Clinical Manifestations
Excessive androgens cause masculinization of the urogenital system at approximately the tenth week of fetal development. The most pronounced abnormalities occur in the female, who is born with varying degrees of ambiguous genitalia. Masculinization of external genitalia causes the clitoris to enlarge so that it appears as a small phallus. Fusion of the labia produces a saclike structure resembling the scrotum without testes. However, no abnormal changes occur in the internal sexual organs, although the vaginal orifice is usually closed by the fused labia. (See also Ambiguous Genitalia, Chapter 11.) The label ambiguous genitalia should be applied to any infant with hypospadias or micropenis and no palpable gonads, and a diagnostic evaluation for CAH should be contemplated. Males do not display genital abnormalities at birth (New and Ghizzoni, 2003), so it may go undetected.
Increased pigmentation of skin creases and genitalia caused by increased ACTH may be a subtle sign of adrenal insufficiency. A salt-wasting crisis frequently occurs, usually within the first few weeks of life (Behrman, Kliegman, Jenson, et al, 2009). Infants fail to gain weight, and hyponatremia and hyperkalemia may be significant. Cardiac arrest can occur.
Untreated CAH results in early sexual maturation, with enlargement of the external sexual organs; development of axillary, pubic, and facial hair; deepening of the voice; acne; and marked increase in musculature with changes toward an adult male physique. However, in contrast to precocious puberty, breasts do not develop in the female, and she remains amenorrheic and infertile. In the male the testes remain small, and spermatogenesis does not occur. In both sexes linear growth is accelerated, and epiphyseal closure is premature, resulting in short stature by the end of puberty.
Diagnostic Evaluation
Clinical diagnosis is initially based on congenital abnormalities that lead to difficulty in assigning sex to the newborn and on signs and symptoms of adrenal insufficiency. Newborn screening is currently done in all 50 U.S. states by measurement of the cortisol precursor 17-hydroxyprogesterone. Definitive diagnosis is confirmed by evidence of increased 17-ketosteroid levels in most types of CAH (American Academy of Pediatrics, 2000 [reaffirmed 2005]). In complete 21-hydroxylase deficiency, blood electrolytes demonstrate loss of sodium and chloride and elevation of potassium. In older children bone age is advanced, and linear growth is increased. DNA analysis for positive sex determination and to rule out any other genetic abnormality (e.g., Turner syndrome) is done in any case of ambiguous genitalia.
Another test that can be used to visualize the presence of pelvic structures is ultrasonography, a noninvasive imaging technique that does not require anesthesia or sedation. It is especially useful in CAH because it readily identifies the presence of female reproductive organs or male testes in a newborn or child with ambiguous genitalia. Because ultrasonography yields immediate results, it has the advantage of determining the child’s gender before the more complex laboratory results for chromosome analysis or steroid levels are available.
Therapeutic Management
After diagnosis is confirmed, medical management includes administration of glucocorticoids to suppress the abnormally high secretions of ACTH and adrenal androgens (Glatt, Garzon, and Popovic, 2005). If cortisone begins early enough, it is very effective. Cortisone depresses the secretion of ACTH by the adenohypophysis, which in turn inhibits the secretion of adrenocorticosteroids, which stems the progressive virilization. The signs and symptoms of masculinization in the female gradually disappear, and excessive early linear growth is slowed. Puberty occurs normally at the appropriate age.
The recommended oral dosage is divided to simulate the normal diurnal pattern of ACTH secretion. Because these children are unable to produce cortisol in response to stress, it is necessary to increase the dosage during episodes of infection, fever, or other stresses. Acute emergencies require immediate IV or intramuscular administration. Emergency situations include bacterial and viral infections, vomiting, surgery, fractures, major injuries, and sometimes insect stings.
Children with the salt-losing type of CAH require aldosterone replacement, as outlined under chronic adrenal insufficiency, and supplementary dietary salt. Frequent laboratory tests are conducted to assess the effects on electrolytes, hormonal profiles, and renin levels. The frequency of testing is individualized to the child.
Gender assignment and surgical intervention in the newborn with ambiguous genitalia is complex and controversial. It is a significant stress for families, who need support and education from a multidisciplinary team of experienced specialists. Factors that influence gender assignment include genetic diagnosis, genital appearance, surgical options, fertility, and family and cultural preferences. Generally, genetically female (46XX) infants should be raised as girls. Early reconstructive surgery should be considered only in the case of severe virilization (Lee, Houk, Ahmed, et al, 2006). Emphasis is on functional rather than cosmetic outcomes, and surgery can often be delayed. Reports concerning sexual satisfaction after partial clitoridectomy indicate that the capacity for orgasm and sexual gratification is not necessarily impaired. Male infants may require phallic reconstruction by an experienced surgeon.
Unfortunately, not all children with CAH are diagnosed at birth and raised in accordance with their genetic sex. Particularly in the case of affected females, masculinization of the external genitalia may have led to sex assignment as a male. In children with milder forms of CAH, especially males, diagnosis may be delayed until early childhood, when signs of virilism appear. In these situations it is usually advisable to continue rearing the child as a male in accordance with assigned sex and phenotype. Hormone replacement may be required to permit linear growth and to initiate male pubertal changes. Surgery is usually indicated to remove the female organs and reconstruct the phallus for satisfactory sexual relations. These individuals are not fertile.
Nursing Care Management
Of major importance is early recognition of ambiguous genitalia and diagnostic confirmation in newborns. As with any congenital defect, the parents require an adequate explanation of the condition and time to grieve for the loss of perfection. In this instance they may also need to grieve for the loss of the desired-sex child. For example, the birth of a phenotypically male infant may fulfill their wish for a son. Knowledge of the child’s actual sex may leave them disappointed. Such situations may also lead them to discuss the possibility of raising the child as a boy despite the actual sex. This is a difficult question that requires thoughtful discussion among the parents and members of the health team.
In general, rearing the genetically female child as a girl is preferred because of the success of surgical intervention and the satisfactory results with hormones in reversing virilism and providing a prospect of normal puberty and the ability to conceive. This is in contrast to the choice of rearing the child as a boy, in which case the child is sterile and may never be able to function satisfactorily in heterosexual relationships. If the parents persist in their decision to assign a male sex to a genetically female child, request a psychologic consultation to explore their motivations and ensure their understanding of the future consequences for the child.
Parents need an explanation regarding this disorder that helps them explain it to others. When referring to the external genitalia, it is preferable to refer to them as sex organs and to emphasize the similarity between the penis-clitoris and scrotum-labia during fetal development. Explain that the sex organs were overdeveloped because of too much male hormone secretion. Using a correct vocabulary allows parents to explain the abnormalities to others in a straightforward manner, just as if the defect involved the heart or an extremity.
Parents often fear that the infant will retain “male behavioral characteristics” because of prenatal masculinization and will not be able to develop female characteristics. It is important to stress that gender identity and psychosexual development depend on multiple influences. Because the prognosis for normal sexual development is excellent after early treatment, the nurse should foster identification with the child as one sex only. Ambiguous genitalia have no relationship to sexual preference for partners later in life.
As soon as the sex is determined, inform parents of the findings and encourage them to choose an appropriate name and identify the child as a male or female, with no reference to ambiguous sex. If the appearance of the enlarged genitalia in a girl concerns the parents, encourage them to discuss their feelings. Suggesting ways to avoid questioning remarks from visitors, such as diapering the child in a separate room, is also helpful. If surgery is anticipated, showing parents before-and-after photographs of reconstruction helps to reinforce the expected cosmetic benefits.
Nursing care management regarding cortisol and aldosterone replacement are the same as those discussed for chronic adrenocortical insufficiency. A follow-up visit by a home health nurse may be desirable to ensure that parents understand and comply with the treatment regimen. Nurses in well-child facilities should assume responsibility for guidance and supervision regarding this aspect of care during each visit. Monitor vital signs closely for early signs of hypertension due to cortisol therapy.
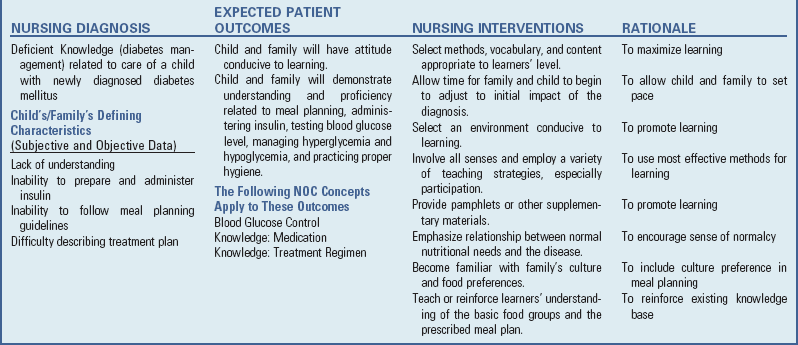
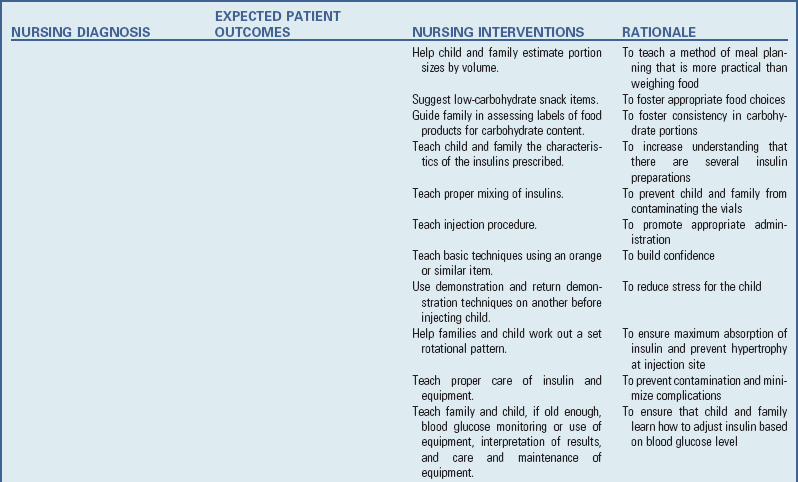
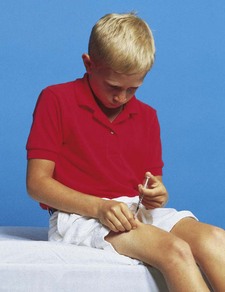
Because infants are especially prone to dehydration and salt-losing crises, parents need to be aware of signs of dehydration and the urgency of immediate medical intervention to stabilize the child’s condition. Parents should have injectable hydrocortisone available and know how to prepare and administer the intramuscular injection. (See Chapter 27.) Parents, and later the child, need to understand that the medical regimen must be a lifelong commitment; therefore provide them with the education and counseling that is most likely to ensure informed and willing compliance. They also need to know that growth retardation that may have occurred before therapy cannot be overcome and that normal stature is not a realistic expectation, even though growth velocity may improve with medication. Assess males for development of testicular tumors.
In the unfortunate situation in which the sex is erroneously assigned and the correct sex determined later, parents need a great deal of help in understanding the reason for the incorrect sex identification and the options for sex reassignment or medical-surgical intervention.
Because the hereditary form of adrenal hyperplasia is an autosomal recessive disorder, refer parents for genetic counseling before they conceive another child. The nurse’s role is to ensure that parents understand the probability of transmitting the trait or disorder with each pregnancy. Prenatal diagnosis and treatment for CAH are available. Affected offspring also require genetic counseling, since both sexes are generally able to reproduce. (See Chapter 5 for recurrence risks and genetic counseling.)
Hyperaldosteronism
Excessive secretion of aldosterone may be caused by an adrenal tumor or, in some types of adrenogenital syndromes, result from enzymatic deficiency. The signs and symptoms are caused by increased sodium levels, water retention, and potassium loss. Hypervolemia causes hypertension and resultant headaches. Paradoxically, funduscopic changes resulting from increased blood pressure and edema from water retention are minimal. Hypokalemia results in muscular weakness, paresthesia, episodes of paralysis, and tetany and may be responsible for polyuria and consequent polydipsia.
The clinical diagnosis is suspected when there are findings of hypertension, hypokalemia, and polyuria that fail to respond to ADH administration. Renin and angiotensin titers are abnormally low. Urinary levels of 17-hydroxycorticosteroids and 17-ketosteroids are normal in primary hyperaldosteronism caused by an aldosterone-secreting tumor but are usually abnormal in adrenogenital syndrome.
Therapeutic Management
Temporary treatment of the disorder involves replacement of potassium and administration of spironolactone (Aldactone), a diuretic that blocks the effects of aldosterone, thereby promoting excretion of sodium and water while preserving potassium. Definitive treatment is similar to that for chronic adrenocortical insufficiency.
Nursing Care Management
An important nursing consideration is recognition of the syndrome, particularly in children with high blood pressure. Other clues include bed-wetting, excessive thirst, and unexplained weakness. After the diagnosis, nursing care is related to the treatment regimen. If diuretics are used, they should be administered in the morning to avoid accidents during the night. Children need unrestricted restroom privileges at school. Potassium supplements should be mixed with fruit juice such as grape juice to increase their acceptability, and potassium-rich foods should be encouraged. Parents need to be aware of the signs of hypokalemia and hyperkalemia. After an adrenalectomy, nursing care is similar to that for chronic adrenocortical insufficiency.
Pheochromocytoma
Pheochromocytoma is a rare tumor characterized by secretion of catecholamines. The tumor most commonly arises from the chromaffin cells of the adrenal medulla but may occur wherever these cells are found, such as along the paraganglia of the aorta or thoracolumbar sympathetic chain. Approximately 10% of these tumors are located in extraadrenal sites. In children they are frequently bilateral or multiple and are generally benign. Often there is a familial transmission of the condition as an autosomal dominant trait (Behrman, Kliegman, Jenson, et al, 2009).
Clinical Manifestations
The clinical manifestations of pheochromocytoma are caused by an increased production of catecholamines, producing hypertension, tachycardia, headache, decreased gastrointestinal activity with resultant constipation, increased metabolism with anorexia, weight loss, hyperglycemia, polyuria, polydipsia, hyperventilation, nervousness, heat intolerance, and diaphoresis. In severe cases, signs of congestive heart failure are evident.
Diagnostic Evaluation
The clinical manifestations mimic those of other disorders, such as hyperthyroidism or DM. Tests specific to these conditions may be performed as part of the differential diagnosis. In a small number of instances a palpable tumor suggests the diagnosis. Usually the tumor is identified by a CT scan or MRI. Definitive tests include 24-hour measurement of urinary levels of the catecholamine metabolites; histamine stimulation, which provokes a hypertensive attack from sudden release of large amounts of catecholamines; and α-adrenergic blocking agents, which produce a hypotensive episode by inhibiting the action of circulating catecholamines.
Therapeutic Management
Definitive treatment consists of surgical removal of the tumor. In children the tumors may be bilateral, requiring a bilateral adrenalectomy and lifelong glucocorticoid and mineralocorticoid therapy. The major complications that can occur during surgery are severe hypertension, tachyarrhythmias, and hypotension. The first two are caused by excessive release of catecholamines during manipulation of the tumor, and the latter results from catecholamine withdrawal and hypovolemic shock.
Preoperative medication to inhibit the effects of catecholamines is begun 1 to 3 weeks before surgery to prevent these complications. The major group of drugs used is the α-adrenergic blocking agents with or without β-adrenergic blocking agents. The most commonly used α-adrenergic blocker is phenoxybenzamine (Dibenzyline), a longer-acting medication given orally every 12 hours. The shorter-acting phentolamine (Regitine) is equally effective but less satisfactory for long-term use, although it is useful for acute hypertension. To control catecholamine release when α-adrenergic blocking agents are inadequate, the child is given β-adrenergic blocking agents.
Success of therapy is judged by lowering of blood pressure to normal, absence of hypertensive attacks (flushing or blanching, fainting, headache, palpitations, tachycardia, nausea and vomiting, profuse sweating), heat tolerance, decrease in perspiration, and disappearance of hyperglycemia. A disadvantage of these drugs is their inability to block the effects of catecholamines on β-receptors.
Nursing Care Management
An initial nursing objective is identification of children with this disorder. Outstanding clues are hypertension and hypertensive attacks. Because of behavioral changes (nervousness, excitability, overactivity, even psychosis), increased cardiac and respiratory activity may appear to be related to an acute anxiety attack. Therefore a careful history of the onset of symptoms and association with stressful events is helpful in distinguishing between an organic and a psychologic cause for the symptoms.
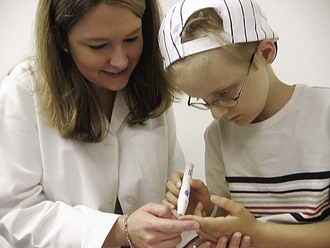
Preoperative nursing care involves frequent monitoring of vital signs and observation for evidence of hypertensive attacks and congestive heart failure. Therapeutic effects are evidenced by normal vital signs and absence of glycosuria. Note daily blood glucose levels and urine acetone; report any signs of hyperglycemia immediately.
The environment is made conducive to rest and free of emotional stress. This requires adequate preparation during hospital admission and before surgery. Encourage parents to room-in with their child and to participate in care. Play activities need to be tailored to the child’s energy level without being overly strenuous or challenging because these can increase metabolic rate and promote frustration and anxiety.
After surgery observe the child for signs of shock from removal of excess catecholamines. If a bilateral adrenalectomy was performed, the nursing interventions are those discussed for chronic adrenocortical insufficiency.
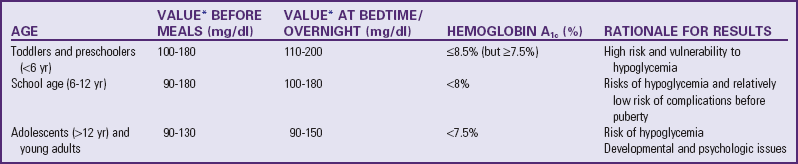
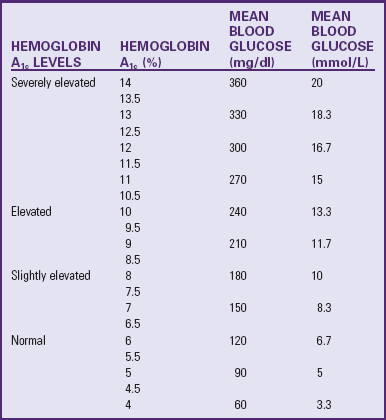
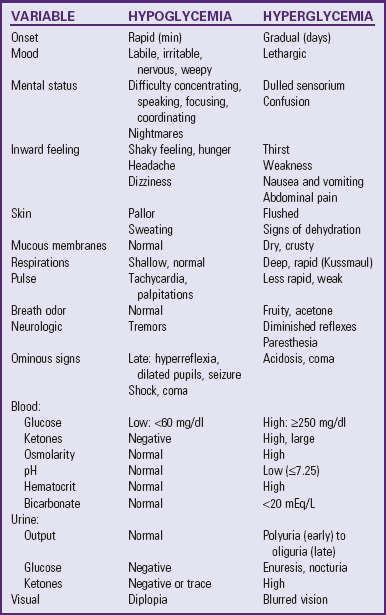
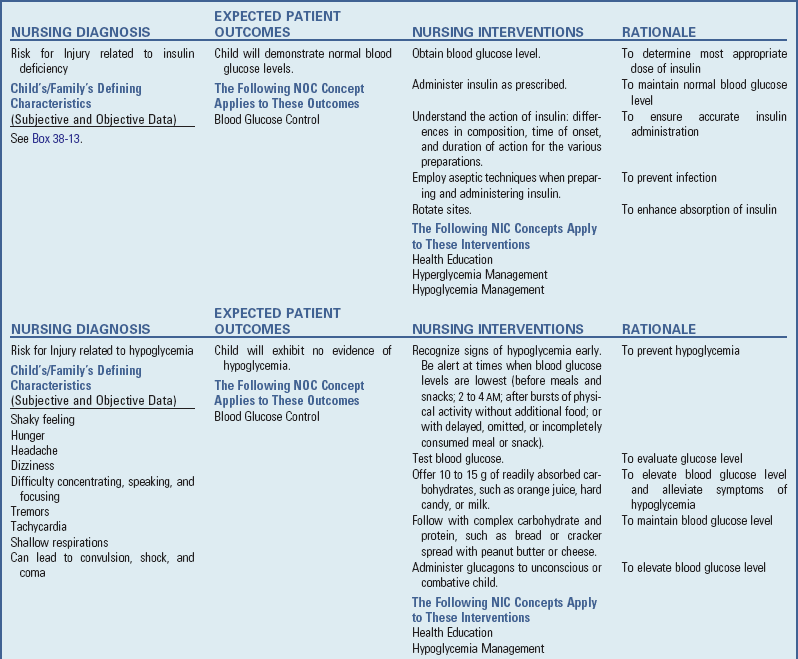
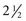 to 3 years after diagnosis; however, with good to excellent control, changes can be postponed for 20 or more years. Intensive insulin therapy appears to delay the onset and slow the progression of clinically important retinopathy, including vision-threatening lesions, nephropathy, and neuropathy, by 35% to more than 70%, according to studies on treatment and complications of type 1 DM (
to 3 years after diagnosis; however, with good to excellent control, changes can be postponed for 20 or more years. Intensive insulin therapy appears to delay the onset and slow the progression of clinically important retinopathy, including vision-threatening lesions, nephropathy, and neuropathy, by 35% to more than 70%, according to studies on treatment and complications of type 1 DM (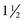 hours. Because of its rapid onset, each of the analogs must be injected within 15 minutes before eating.
hours. Because of its rapid onset, each of the analogs must be injected within 15 minutes before eating.