Chapter 100Pathophysiology and Clinical Diagnosis of Cortical and Subchondral Bone Injury
Pathophysiology
Bone is an amazing living tissue that is able to conform and adapt to its environment. When bone is exposed to a load, it deforms. This deformation is elastic and within certain limits; bone returns to its original state once the load is removed. If the degree of deformation is beyond bone’s upper limit, complete failure occurs. Under other conditions, bone may be subject to loads that are persistently different. Alterations in bone strain patterns develop, and the bone responds accordingly. Bone will then counteract the variation in load by changing its inertial properties via the processes of modeling and remodeling. In time, bone will modify its shape and structure, effectively adapting to the load under which it is placed (Wolff’s law).
The term modeling refers to the geometric sculpting of bone by formation or resorption.1 With modeling, osteoblasts and osteoclasts work independently, resulting in increased or decreased bone density. Resorption occurs when strain patterns are below the minimum threshold, and bone formation occurs when bone strain is above the maximum strain threshold.1 In cortical bone, modeling adaption results in periosteal thickening (bone formation) or thinning (bone resorption). In trabecular bone, subchondral sclerosis or osteoporosis ensues. Eventually, modeling alters the size, shape, and density of bone. Remodeling refers to the coordinated action of osteoclasts and osteoblasts resulting in the removal of biomechanically inferior bone and replacement with new bone.1 Osteoclasts move through bone at a much quicker rate than osteoblasts; therefore bone resorption (within weeks) is followed by bone formation (months).
Bone modeling and remodeling are active ongoing processes and can occur in the same bone at the same time. These processes are driven by site-specific bone strain patterns, although the exact mechanism by which strain induces bone change is unknown. Throughout life, bone continually adapts to its physiological loads by modeling and/or remodeling until its mechanical stimulus is normalized. These processes ensure the mechanical integrity of the skeleton. For example, when a horse undergoes athletic training, its bones are exposed to increasing and varying loads at specific locations. In turn, bone responds by adding more bone to regions of higher load. An adaptive site-specific response to race training in Thoroughbred (TB) horses is the dramatic thickening that occurs in the dorsomedial aspect of the third metacarpal bone (McIII).
In ideal situations, a balance exists between load and the rate of bone adaptation. Repetitive cyclic loading produces an accumulation of focal microdamage at sites that are maximally stressed. Microdamage incites a bone response, followed by successful repair and remodeling. If there is imbalance between removal and replacement, the very process of bone repair may contribute to a bone’s failure. Because osteoclasts act more quickly than osteoblasts, transient weakness occurs after damaged bone has been removed and before completion of bone replacement. Prolonged or rapid increases in load, such as those during high-speed workouts and racing, result in an accumulation of microdamage. The resorptive phase of remodeling may then exceed replacement capacity and transiently weaken the bone.2,3 This focal weakness can function as a stress riser and allow initiation of a complete fracture or fragmentation under otherwise normal physiological loading.4
Failure of the bone to adapt in a timely fashion combined with continual and compounded microdamage results in stress-related bone injury.5 Horses undergoing race training and racing are particularly susceptible to injury because their bones are constantly subjected to large repetitive loads. Risk for injury is high until a satisfactory adaptive bone response is completed.3 Evaluation of postmortem specimens indicates that most musculoskeletal injuries are overuse injuries. Evidence of stress remodeling has been observed in equine long bones (the McIII, humerus, scapula, or tibia), the third carpal bone (C3), the pelvis, and vertebrae.6-14 Site-specific pathology includes periosteal callus, sclerosis of endosteal or subchondral bone, and focal osteoporosis.
Stress-related cortical bone injury is commonly referred to as a stress or fatigue fracture. Stress fractures occur in horses undergoing intense exercise (repetitive high-strain loading) and are not correlated with a specific traumatic event.3,8,15-17 They are associated with activities that produce repetitive loading of the involved bone. Repeated cyclic loading of cortical bone results in loss of stiffness, reduction in strength, and development of microcracks. With continued loading, microcracks propagate and coalesce into macrocracks (i.e., stress fractures). Stress fractures are regularly identified, clinically and at postmortem examination, in consistent locations presumably at sites of maximal load. They are very common in racehorses and are a well-recognized cause of lameness. If the stress remodeling continues in an unbalanced fashion, trauma to an already fatigued bone can lead to catastrophic fracture. Periosteal callus, indicative of preexisting reparative response, at equine cortical fracture interfaces6,13 is common and provides evidence of a continuum of stress-related bone injury.
Like cortical bone, subchondral bone is susceptible to stress-related bone injury. Although features of bone modeling and remodeling are similarly induced, depending on the mechanical impact periosteal callus is not seen. Normally, the compliant subchondral plate acts as a shock absorber between articular cartilage and subchondral bone, thereby dissipating the impact to the articular surface. Repeated loading results in subchondral bone mineralization and subsequent stiffening to combat the increased bone strain. The progressive increased bone density of the trabecular bone adjacent to the subchondral plate of the radial fossa of the C3 and the palmar condyles of the McIII or plantar condyles of the third metatarsal bone (MtIII) are examples of a subchondral response in race-trained horses.12,18,19 As with cortical bone, repetitive loading of the subchondral bone may result in a repair process dominated by the resorptive phase of remodeling. Associated focal subchondral osteoporosis and microcracks may result. Small cracks may develop into larger fractures, resulting in fragmentation and overt fracture along articular margins.20 Preexisting subchondral sclerosis is commonly seen in racehorses with distal McIII condylar fractures21 and is a proposed precursor to C3 slab fractures.12,22 Sclerotic subchondral bone may result in articular cartilage damage. Progressive cartilaginous erosion and ulceration has been identified at areas of increased subchondral thickness.9,23,24 Collapse of subchondral bone resulted in flattening and indentation of cartilage, osteochondral fragmentation, and ultimately osteoarthritis (OA).7,9,20
Clinical Examination
A pathological continuum of cortical and subchondral stress-related bone injury causes lameness, subchondral sclerosis, subchondral lucency, incomplete (stress or fatigue) and complete fracture, and, in horses with subchondral damage, the eventual development of OA. Clearly, early and accurate diagnosis of stress-related bone injury in racehorses is important for the well-being of the horse and the safety of the industry. However, clinical recognition of these injuries can be challenging, and, although stress fractures are a well-recognized cause of lameness in racehorses,11,15,16,25-34 most of these injuries occur in the absence of a specific traumatic event.3,8,15-17,30 Characteristic history includes acute onset of lameness after racing or training that responds to rest.25-27,30,35 Over time, lameness may worsen or linger, and some horses may have a history of weeks to months of poor performance, intermittent unilateral lameness or lameness in numerous limbs, or reports from exercise riders or drivers that horses are not “right.”27,36,37 Lameness scores at the time of diagnosis are quite variable, with some horses exhibiting no lameness and others being severely lame. Physical findings such as swelling or sensitivity to palpation are often subtle or absent. This is especially true in horses with upper limb long bone stress fractures, in which palpation may be at best difficult.
Clinical signs of cortical bone injury include periosteal thickening and local sensitivity. This is easily recognized in horses with the bucked-shin complex38-40 (see Chapter 102). However, in the majority of long bones, overlying soft tissue and muscle prohibit clear identification. Localized signs of cortical pain in the tibia or humerus are uncommon and frequently absent.16,25,28,31 When noted, pain during palpation and percussion of the medial diaphysis of the tibia occurs in as few as 30% of affected horses.25 Careful palpation may reveal focal pain in horses with avulsion fracture of the proximal palmar or plantar aspect of the McIII or the MtIII, respectively.41 Pain on palpation or profound muscle spasm of the affected side and ventral displacement of a tuber sacrale are consistent but subtle physical abnormalities in horses with stress fractures of the wing of the ilium.15,30 Flexion tests, time-honored clinical tools to help exacerbate and localize lameness, often have inconclusive results, and findings may even be absent in horses with cortical bone injury.24,42
Clinical signs of subchondral bone injury are also variable and often subtle. Injuries are frequently bilateral, and overt unilateral limb lameness is unusual. Affected horses may simply be performing at a lower level than expected. Overlying cartilage is minimally affected early in the disease process, and joint effusion is absent. Early subchondral bone injury of the distal aspects of the McIII and the MtIII rarely causes fetlock effusion,36,43-45 and carpal effusion is often less than expected based on severity of lameness in Standardbreds (STBs) with subchondral lucency of the C3.46,47 Diagnostic analgesia is essential for accurate diagnosis, but perineural techniques are consistently more effective than intraarticular analgesia, presumably because pain is associated with subchondral bone and not synovitis, capsulitis, and overt cartilage damage (see Chapter 42). Veterinarians and trainers are often incredulous when a diagnosis of subchondral bone injury is made by use of diagnostic analgesia and scintigraphic examination when clinical signs are lacking. As with cortical bone injury, flexion test results are inconsistent and often negative in horses with subchondral bone injury.36,46 Subchondral increased radiopacity may be radiologically apparent, but it is often difficult to determine whether this is successful adaptive change or a maladaptive or nonadaptive response. In those horses with severe or chronic subchondral bone injury, clinical signs are often more obvious. Synovitis, lameness, and OA may be noted.
Diagnostic Analgesia
Although diagnostic analgesia is one of the most valuable tools used to localize the authentic source of pain, there are several considerations in horses with stress fractures and subchondral bone injury. At the time of evaluation, horses must be lame enough to visually assess response to diagnostic analgesia. Horses with stress fractures are notorious for being lame immediately after intense exercise but subtly lame or sound at the time of lameness examination. Neither perineural nor intraarticular analgesia can be used to diagnose upper limb stress fractures; however, such fractures should be suspected when lameness cannot be improved by routine distal limb diagnostic analgesia.* In addition, response to diagnostic analgesia is often variable. Lameness associated with cortical or subchondral injury of the proximal palmar aspect of the McIII may improve with high palmar analgesia or intraarticular analgesia of the middle carpal joint.27,41,42,50 Pain associated with nonadaptive subchondral remodeling of the distal aspect of the McIII or the MtIII may be alleviated by using a low palmar or plantar or the lateral palmar metacarpal or plantar metatarsal block.51 Often these horses will not improve with intraarticular analgesia and are similarly unresponsive to intraarticular medications.36,43,44 Diagnostic analgesia is a good starting point but must not be used alone.
Diagnostic Imaging
Radiography and Radiology
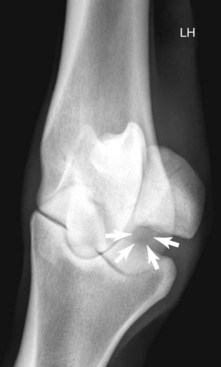
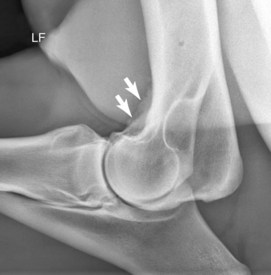
Radiography remains hugely important in the diagnosis of bone injury in racehorses; however, it is insensitive during early phases of stress-related bone injury and often inadequate for subtle subchondral changes. Radiology provides structural information, much of which must be several weeks old before it can be seen. Lag time for enough structural change to occur to be seen radiologically is an obviously serious limitation of radiography, especially when one is attempting to localize and identify early or subtle bone injury. It takes 2 to 3 weeks or more before radiological changes of periostitis are apparent (see Chapter 15).52-55 Even frank fracture lines or compression injury of subchondral bone can take days to weeks to be recognized. Supplementary radiographic images of site-specific regions may enhance detection of injury. For example, the dorsal 30° proximal 45° lateral-palmar (or plantar) distal medial oblique and dorsal 30° proximal medial-palmar or plantar distal lateral oblique (“down-angled”) images (Figure 100-1) are useful for evaluation of subchondral injury of the condyles of the McIII or the MtIII.51,55 A dorsal 60° proximal 45° lateral-palmar distal medial oblique image or dorsal 60° proximal 45° medial-palmar distal lateral oblique image of the distal phalanx is necessary for identification of palmar process fractures.56-58 Even with additional images, subtle subchondral radiolucency and increased radiopacity are often difficult to recognize because density differences must be at least 30% to 50% and lesions 1 to 1.5 cm in diameter before recognition is possible.52 Another obvious limitation of radiography is the inability to distinguish whether the radiologically apparent lesion is an active process or simply the result of an adaptive bone response.
Nuclear Scintigraphy
Increased awareness of location and prevalence of stress-related bone injuries and scintigraphic examination of affected racehorses enhance early detection of bone pathology before catastrophic fracture. Nuclear scintigraphy is an extremely sensitive method of detecting exercise-induced bone remodeling (see Chapter 19). Normal bone response in the dorsal aspect of the McIII, the proximal sesamoid bones (PSBs), and the metacarpal condyles is easily identified and has typical patterns of increased radiopharmaceutical uptake (IRU) in horses undergoing high-speed exercise.59,60 Scintigraphy is useful to detect abnormal alterations in local bone metabolism such as increased activity from cyclic loading and provides a foundation for the diagnosis of stress fractures, stress remodeling, and subchondral trauma.* A focal, moderate-to-intense area of IRU is the scintigraphic hallmark of stress fracture.
Several circumstances exist in which scintigraphy is invaluable for the diagnosis of cortical or subchondral bone injury. Racehorses with stress fractures often have a history of acute severe lameness that abates with rest. These horses have few localizing clinical signs and are diagnostic challenges. Scintigraphy can be used as a screening tool and obviates the need for numerous radiographic images. In addition, a negative bone scan is invaluable because it rules out active bone disease and virtually eliminates the possibility of a stress fracture.52,54 Another scenario is when a racehorse fails to respond to diagnostic analgesia. This is especially true in horses with upper limb stress fractures. Scintigraphy is also helpful for horses with bone injury in numerous areas or multiple limbs. Racehorses with stress remodeling of the distal aspects of the McIII or the MtIII are often in this category.
Scintigraphy is more sensitive than radiography for the identification of stress-related bone injury, and a positive bone scan may precede conclusive radiological change by at least 2 to 3 weeks.52-54 In reports of scintigraphic examinations of racehorses, nearly half of areas of IRU were not associated with radiologically detectable abnormalities.61,63,64 A positive bone scan may also enhance the recognition of subtle radiological changes41 and assist in determination of a structural abnormality as an active process or a reflection of past change. For example, radiographs of a 3-year-old TB racehorse reveal extensive thickening of the dorsal cortex of the McIII, but the bone scan is negative. Gradual resolution of IRU of the dorsal cortex of the McIII can be interpreted as evidence of satisfactory healing of a previously identified dorsal cortical fracture. On the other hand, a persistent fracture line without concurrent moderate-to-intense IRU may indicate the development of a nonunion, because the amount of radiopharmaceutical uptake reflects the rate of bony repair.29 Carpal pain is not likely in a STB with radiological evidence of extensive sclerosis of the C3 but with only mild or no IRU seen scintigraphically.
Ultrasonography
Ilial wing fractures can be detected ultrasonographically.15,17,30,66,67 Hematoma formation, irregular bone contour (indicative of callus formation), and breaks in the normal hyperechogenic contour of bone may be seen if the fracture involves the dorsal surface of the bone (see Figure 49-3, page 566). A displaced fracture is seen as hyperechogenic bony structure distracted from adjacent bone (“stair-step” sign). Ultrasonographic abnormalities are usually most severe at the caudal margins of the fracture.66,67 These findings are supported by postmortem studies in horses with ilial wing fractures.11,13 Serial ultrasonographic examinations can be used to determine if the fracture has healed but should not be the single means of diagnosis. Because of variability in conformation of the ilium and other factors, ultrasonographic findings should be combined with a thorough clinical examination and, if necessary, scintigraphic and occasionally radiographic examinations.15,66
Magnetic Resonance Imaging and Computed Tomography
Slice-by-slice high-resolution images obtained during magnetic resonance imaging (MRI) detect alterations within bone early in a disease process before they are detectable by most other imaging modalities. In people, MRI is frequently used when conventional radiological findings are unremarkable, because MRI has better spatial resolution and specificity. Low signal intensity on T1- and T2-weighted gradient echo (GRE) or proton density (PD) images is the classic appearance of stress-related bone injury. Increased signal intensity in the trabecular and/or cortical bone on short tau inversion recovery (STIR) sequences or fat-suppressed T2-weighted fast spin echo sequences may reflect a bone contusion or fracture, depending on the distribution of altered signal intensity. Computed tomography (CT) may identify linear alteration in signal intensity in the cortex and endosteal and periosteal callus and is more sensitive than plain radiography. As with conventional radiography, false-negative findings are not uncommon. Obvious limitations of MRI and CT acquisition include costly equipment, often the need for general anesthesia, and the inability to image equine upper limbs and pelvis.
Specific Locations of Cortical and Subchondral Bone Injury
Knowledge of the prevalence of stress-related bone injuries provides insight into the likelihood of an area causing clinically important problems, especially when diagnostic analgesia fails to localize the lameness, or radiological and ultrasonographic examination findings are unremarkable. The type of training and racing largely determines the location of injury and whether the injury involves cortical or subchondral bone (Tables 100-1 and 100-2). In lame STBs, IRU is most commonly associated with exercise-induced subchondral bone remodeling, and prevalent sites include the PSBs followed by the C3 and the tarsus.63 The distal palmar or plantar aspect of the McIII or the MtIII is also a frequent location of IRU and, when combined with IRU of the PSB, is the most common location of stress remodeling.55,63 In TBs, IRU of the distal palmar or plantar aspects of the McIII or the MtIII is the most common abnormal scintigraphic finding.62 In the forelimbs, sites of prevalence are the distal palmar aspect of the McIII, followed by the carpus and the dorsal cortex of the McIII.62 In the hindlimbs, the distal plantar aspect of the MtIII is the most common abnormal area, followed by the tarsus and tibia.62
TABLE 100-1 Prevalences of Sites of Increased Radiopharmaceutical Uptake in the Forelimbs of Racehorses
| SITE | STANDARDBRED (%) | THOROUGHBRED (%) |
|---|---|---|
| Phalanges | 14 | 10 |
| Proximal phalanx | 7 | — |
| Middle phalanx | 0 | — |
| Distal phalanx | 7 | — |
| Proximal sesamoid bone | 32 | 6 |
| Third metacarpal bone | ||
| Distal palmar | 21 | 50 |
| Dorsal | 0 | 15 |
| Proximal palmar | 3 | 4.5 |
| Carpus (C3) | 43 | 17 |
| Radius | 2 | 4 |
| Humerus | 0 | 10 |
| Scapula | 0 | 2 |
C3, Third carpal bone.
From Arthur RM, Constantinide D: Results of 428 nuclear scintigraphic examinations of the musculoskeletal system at a Thoroughbred racetrack, Proc Am Assoc Equine Pract 41:84, 1995; and Ehrlich PJ, Dohoo IR, O’Callaghan MW: Results of bone scintigraphy in racing Standardbred horses: 64 cases (1992-1994), J Am Vet Med Assoc 215:982, 1999.
TABLE 100-2 Prevalences of Sites of Increased Radiopharmaceutical Uptake in the Hindlimbs of Racehorses
| SITE | STANDARDBRED (%) | THOROUGHBRED (%) |
|---|---|---|
| Phalanges | — | 3 |
| Proximal phalanx | 11 | — |
| Middle phalanx | 0 | — |
| Distal phalanx | 1 | — |
| Proximal sesamoid bone | 35 | 6 |
| Third metatarsal bone | ||
| Distal plantar | 19 | 28 |
| Dorsal | 2 | 3 |
| Proximal plantar | 4.5 | <1 |
| Tarsus | 33 | 19 |
| Tibia | 0 | 11 |
| Femur | 4.5 | 2 |
| Pelvis | — | 6 |
From Arthur RM, Constantinide D: Results of 428 nuclear scintigraphic examinations of the musculoskeletal system at a Thoroughbred racetrack, Proc Am Assoc Equine Pract 41:84, 1995; and Ehrlich PJ, Dohoo IR, O’Callaghan MW: Results of bone scintigraphy in racing Standardbred horses: 64 cases (1992-1994), J Am Vet Med Assoc 215:982, 1999.
Distal Phalanx
IRU associated with the distal phalanx in the absence of radiological abnormalities in racehorses that subsequently developed distal phalanx fractures suggests that these fractures are not single-event injuries, but rather the result of repetitive stress.56,57 Clinical examination findings include unilateral lameness localized to the digit using palmar digital analgesia. A fracture usually results in acute, severe lameness. If the fracture is articular, distal interphalangeal joint distention may be noted, and lameness is often alleviated by intraarticular analgesia.58 Response to hoof tester application is variable and often unreliable. Distal phalangeal fractures occur more often in the forelimbs than the hindlimbs, and STBs may be overrepresented.56,57 The lateral aspect of the left front and the medial aspect of the right front distal phalanges are most commonly affected, a finding that may reflect compression of these sides of the distal phalanx during counterclockwise racing in the United States.56-58 The distribution of distal phalangeal fractures in racehorses from the United Kingdom or continental Europe is unknown.
Numerous scintigraphic images including lateral, dorsal, and solar images are recommended for complete and accurate diagnosis.59 Oblique radiographic images of the palmar processes of the distal phalanx should be obtained to assist in identification of subtle radiolucent lines, particularly in horses with IRU identified scintigraphically.59,61
Palmar or Plantar Aspects of the Metacarpophalangeal/Metatarsophalangeal Joints
The true incidence of distal palmar or plantar subchondral injury of the McIII or the MtIII is unknown (see Chapters 36 and 42). Scintigraphic studies have indicated as many as 50% of TBs62 and 20% of lame STBs may be affected.63 Subchondral bone injury of the MtIII was identified as a major cause of hindlimb lameness in STB racehorses.55 The proposed pathogenesis of subchondral injury to the distal palmar or plantar aspects of the McIII or the MtIII includes a progression of subchondral remodeling in response to repetitive stress including trabecular thickening (sclerosis). Under normal circumstances, this functional adaptation attenuates the load and spares stress on the articular cartilage. Under pathological conditions, this adaptation fails to protect the joint. Microcracking of the subchondral bone and cartilage68-70 and/or focal necrosis7,9 ensue, promoting the development of OA.
Subchondral bone changes in the distal aspect of the McIII or the MtIII may precede and predispose to condylar fractures.71 The adapted and thicker subchondral bone of the condyles is significantly stiffer than adjacent bone, which results in a gradient between the relatively less dense sagittal ridge and the condylar bone. Strain accumulation at this interface may lead to an increase of localized fatigue damage, predisposing it to catastrophic failure.71 Alternatively, larger cracks may be a manifestation of smaller microcracks, which originate in the sclerotic zone as part of the resorption phase of the remodeling response.20 Regardless of the underlying chain of events, preexisting pathology such as focal regions of osteoporosis and microdamage in the surrounding bone have been identified in bones of horses with lateral condylar fracture of the McIII/MtIII.21,70,71 Fracture lines pass through areas of localized porosity.21,71
Early in the disease process, when lameness is thought to be related to subchondral bone pain, abnormal physical examination findings are often lacking and clinical signs can be easily overlooked. Lameness is mild or apparent only at high speeds, joint effusion is uncommon, and most horses do not manifest a positive response to flexion tests.36,55 When the hindlimbs are affected a bunny-hopping type of gait or lack of impulsion may be noted when horses canter.45 In horses affected bilaterally, a short, choppy, uncomfortable gait or an intermittent, shifting hindlimb lameness may be present. Perineural analgesia is more effective than intraarticular analgesia in abolishing pain at this stage of the disease process. Either a low palmar or plantar or lateral (medial) palmar metacarpal or plantar metatarsal nerve block is recommended,51 because intraarticular analgesia of the fetlock joint inconsistently abolishes subchondral pain.36,43 Perineural analgesia in one limb will often result in horses then exhibiting contralateral limb lameness. Radiological evaluation is frequently unremarkable; however, IRU in the distal palmar or plantar aspects of the McIII or the MtIII is diagnostic if lameness has been localized using diagnostic analgesia. As the disease progresses, lameness worsens, and horses are more likely to have a positive response to a lower limb flexion test. Both a positive bone scan and abnormal radiological findings (increased subchondral radiopacity and radiolucency) are apparent. Later, joint effusion, more prominent lameness, and a positive response to intraarticular analgesia may be noted.
Scintigraphy is the method of choice for diagnosis as radiographs are often negative or equivocal, especially in the early stages of disease. Standing lateral, plantar, and flexed lateral scintigraphic images are recommended (Figure 100-2). The most common scintigraphic abnormality is focal IRU of the distal palmar or plantar aspects of the McIII or the MtIII, but the PSBs may be involved. Flexed lateral images are especially helpful to differentiate IRU of the distal aspect of the McIII or the MtIII from that involving the PSBs.55 In a retrospective study of lame STBs undergoing scintigraphic evaluation, 20% of images had IRU associated with the palmar or plantar aspect of the fetlock joints.63 It is not uncommon for more than one limb to be affected62 or for IRU to be identified in all four fetlock joints.61 Front and hind fetlock joints are commonly concomitantly affected.61,63 Metatarsophalangeal joint lameness may be more common in STBs because of gait and load distribution, and IRU of the distal plantarolateral aspect of the MtIII predominates.55 In TBs, IRU in the distal palmar aspect of the McIII is more common than IRU in the distal plantar aspect of the MtIII, and bilateral IRU is prevalent.61,62
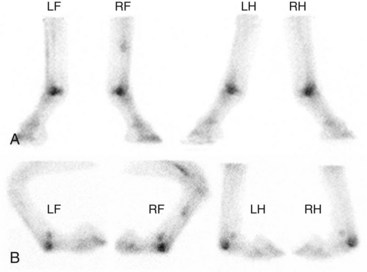
Fig. 100-2 A, Delayed phase lateral scintigraphic images of the distal aspect of the limbs of a 4-year-old Thoroughbred with focal, intense increased radiopharmaceutical uptake (IRU) in the palmar and plantar aspects of the metacarpophalangeal and metacarpophalangeal joints. B, The flexed lateral images confirm that IRU is confined to the distal palmar or plantar aspect of the third metacarpal and metatarsal bones, not the proximal sesamoid bones or the proximal phalanx.
In addition to routine radiographic images of the metacarpophalangeal and metatarsophalangeal joints, “down-angled” oblique images are recommended for complete evaluation of the distal palmar or plantar aspects of the McIII or the MtIII.51,55 Less than half of horses with IRU will have radiological abnormalities.55,61,63 When apparent, bony changes include subchondral lucency and increased radiopacity (see Figure 100-1).45,55 Radiological evidence of lucency may represent a later stage of the remodeling process and may be indicative of necrotic subchondral bone.7,55
Advanced imaging (MRI and CT) has improved our understanding of subchondral bone damage in fetlock joints and has great potential for early detection and implementation of management techniques directed toward injury prevention. Bony changes identified during MRI include small areas of high signal intensity within dense, sclerotic subchondral bone on STIR and T2-weighted images consistent with fluid, fibrosis, or bone necrosis within the subchondral bone and hypointense regions within the condyle on PD images consistent with increased bone density (Figure 100-3).72 Through the use of CT, subchondral increased radiopacity in the condyles59,69,71 and bone porosity at the site of condylar fracture69,71 have been identified in cadaver specimens.
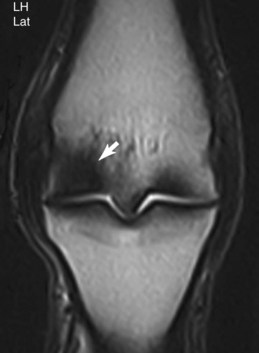
Fig. 100-3 Dorsal T2-weighted gradient echo magnetic resonance image of the right metatarsophalangeal joint of a 3-year-old Standardbred racehorse with moderate right hindlimb lameness, which subsequently improved with lateral plantar metatarsal analgesia. There is a hypointense signal (arrow) within the lateral condyle of the distal aspect of the third metatarsal bone, consistent with increased bone density.
Dorsal Cortex of the Third Metacarpal Bone
Stress-related bone injury of the dorsal cortex of the McIII (bucked-shin complex) is a well-recognized disease of racehorses, particularly young TBs, in which incidence has been reported to be as high as 70%.38 There are two clinical syndromes, diffuse periostitis (bucked shins) and dorsal cortical fracture (saucer, fatigue, stress fracture), thought to be closely related and representing a continuum of stress-related bone injury (see Chapter 102).
Race training in TBs results in repetitive loading on the McIII. The McIII then responds by modeling and remodeling, adding to and increasing the density of the dorsal cortex. Under balanced conditions, the dorsal cortex thickens, the minimum moment of inertia changes significantly, and the bone is then adapted.3 Histological examination of classically trained TBs shows that bone remodeling is confined to the dorsomedial surface of the bone.73 If the modeling process is unable to keep up with bone strain, more bone stiffness is lost, and more periosteal new bone is formed. This resulting microdamage manifests itself clinically as “bucked shin.”73 The relatively thinner dorsolateral cortex is subject to lower strains during race training, never responding with clinically significant modeling and remodeling. As the speed of training increases and the high-strain cycles accumulate, this thinner poorly adapted dorsolateral cortex may sustain incomplete cortical stress fractures.73
Classically, TBs develop bucked shins early in training, at or about the time of high-speed work, usually in the 2-year-old year. Clinical signs are usually sufficient for diagnosis. The condition is characterized by pain, heat, and swelling on palpation of the dorsal aspect of the McIII.29,38,44,60 Frank lameness may be present, but subtle signs such as unwillingness to train at high speeds may be apparent.29,60 In North American TBs the left forelimb may be more commonly affected than the right, although the disease occurs bilaterally. This injury is rarely seen in older horses, especially those that have raced successfully. Racehorses trained and raced in Europe may develop bucked shins when they relocate to North American dirt tracks.40 Once the condition is recognized and the horse recovers, it rarely recurs. Clinical signs of dorsal cortical fractures include firm protuberance on the dorsal aspect of the McIII, usually in the middle third of the diaphysis. Application of firm digital pressure often elicits pain. Dorsal cortical fractures usually develop some months after the acute phase of bucked shins and most commonly in 3-year-old TB racehorses. About 10% to 12% of horses with bucked shins will also have dorsal cortical stress fracture.38,40 Dorsal cortical fractures can occur along the proximal, middle, or distal dorsal aspects of the McIII but are most common in the middle third of the bone.
Although signs of dorsal McIII pain are common in TBs, STBs seldom exhibit it. In fact, TBs are 8.6 times more likely to develop dorsal metacarpal disease than STBs.60 Bucked shins occasionally occur in pacers in June or July of the 2-year-old year and are unusual in trotters. Dorsal cortical fractures occur rarely, usually in 3-year-old pacers in the midportion of the racing year. Because mechanical properties of the McIII are not significantly different between STBs and TBs,39 the disparity in prevalence is most likely the result of differences in training regimens between the breeds. STBs race at slower speeds than do TBs and in a different gait. In addition, STBs typically train long, slow jogging miles before introduction to speed work, unlike TBs, which typically have an earlier introduction of speed work during training. When compared with the TB McIII, STB McIIIs are significantly more developed before onset of racing,39 a finding that would explain an apparent resistance to the development of bucked shins in young STBs.
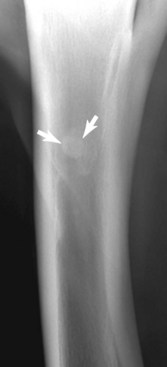
Radiological diagnosis of stress-related bone injury of the dorsal aspect of the McIII is often delayed after clinical diagnosis. When apparent, periostitis is identified by the presence of periosteal new bone (bony thickening or roughening) over the dorsal or dorsomedial aspect of the McIII. A diagnosis of dorsal cortical stress fracture is confirmed by the presence of a short, oblique, linear radiolucency at 30- to 45-degree angle to the dorsal cortex and is often noted in conjunction with periostitis. Well-exposed and well-positioned plain, digital, or computed radiographic views are required.
Mild, diffuse IRU in the dorsal cortex of the McIII is common in young TBs in training and is attributed to the rate of accelerated modeling that occurs in the McIII during training. Patterns of uptake considered a normal finding in these horses include mild, uniform, and diffuse IRU in the dorsal cortex of the McIII relative to the palmar cortex, but activity should be less than in the metaphyses (Figure 100-4).29 Scintigraphic studies of racehorses evaluated for lameness indicated that 45% to 68% of TBs29,60 and 95% to 97% of STBs60 have some IRU in the dorsal aspect of the McIII, but most of these horses do not have clinical signs of metacarpal pain. Periostitis (bucked shins) is evident by uniform and diffuse IRU in the dorsal cortex of the McIII relative to the palmar cortex and metaphyses (Figure 100-5).29 IRU consistent with periostitis may be noted in as many as 12% to 34% of TBs29,60,62 and 1% to 3% of STBs.60 Clinical signs of periostitis correlate well with scintigraphy; most horses with abnormal IRU also have metacarpal pain. Dorsal cortical fractures are evident as focal moderate-to-intense IRU in the dorsal cortex of the McIII29,60 (Figure 100-6) and are occasionally identified in combination with periostitis.29 Focal IRU is a much more important finding in establishing the diagnosis of fracture than is intensity of IRU. In STBs, dorsal cortical stress fractures are exceedingly uncommon, reported in only 1% of the population of lame horses undergoing scintigraphic examination.60

Fig. 100-4 Delayed phase standing lateral scintigraphic images of the distal forelimbs of a young Thoroughbred in active race training with normal, adaptive uniform and diffuse radiopharmaceutical uptake in the dorsal cortex of the third metacarpal bones (arrows).
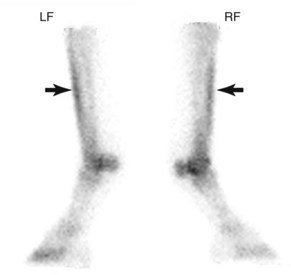
Fig. 100-5 Delayed phase lateral scintigraphic images of the distal forelimbs of a 3-year-old Thoroughbred with diffuse moderate increased radiopharmaceutical uptake (IRU) in the dorsal cortex of the third metacarpal bones (arrows). This pattern of uptake is consistent with a diagnosis of bilateral periostitis (bucked shins). The distal palmar aspects of both third metacarpal bones exhibit mild IRU as well.
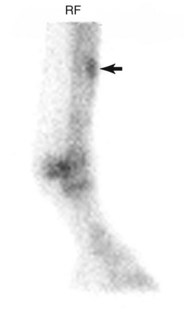
Fig. 100-6 Delayed phase lateral scintigraphic image of the distal right forelimb of a 3-year-old Thoroughbred with focal, intense increased radiopharmaceutical uptake in the dorsal cortex of the right third metacarpal bone. This pattern of uptake is consistent with a diagnosis of a dorsal cortical fracture.
Proximal Palmar Aspect of the Metacarpal Region
Proximal palmar metacarpal region pain is a common cause of forelimb lameness in athletic horses (see Chapter 37).27,35,41,42,50 Many pathological bony processes have been described, including avulsion fracture of the McIII at the origin of the suspensory ligament, palmar proximal cortical incomplete fracture of the McIII, and stress reaction or stress fracture of the McIII.27,35,41,42 The differential diagnosis is based on clinical findings, perineural analgesia, and diagnostic imaging. Scintigraphic, ultrasonographic, radiographic, and/or MRI examinations are often required to confirm and categorize a diagnosis. Making a clear distinction between primary bony injury and secondary bone modeling that occurs in some horses with proximal suspensory desmitis can be challenging without MRI. Physical examination findings are often lacking.27,35,42,50,74 Moderate-to-severe lameness is the most consistently observed clinical sign* and typically occurs at or near racing speeds.27,42,50 Some horses respond to focal digital pressure on the proximal palmar aspect of the McIII,27,41,42,50,74 and few have evidence of heat or swelling.74 Proximal palmar metacarpal region pain is confirmed by improvement in lameness severity after diagnostic analgesia. Multiple techniques for analgesia of this region include lateral palmar, high palmar, and subcarpal analgesia, local infiltration of the proximal palmar aspect of the McIII, and middle carpal analgesia.†
†References 27, 35, 41, 42, 50, 74.
Radiological evaluation may reveal a plethora of bony abnormalities. The classic appearance of an avulsion fracture of the suspensory origin consists of a crescent-shaped radiolucent defect of the proximal aspect of the McIII seen on a dorsopalmar image. Incomplete longitudinal fracture is seen as a linear or slightly oblique radiolucency in the proximal aspect of the McIII, medial to the midline.35,42 Increased radiopacity of the subchondral bone is best identified on a dorsopalmar image and may be seen alone, in conjunction with fracture, or in association with bony modeling secondary to proximal suspensory desmitis. More subtle radiological findings such as periosteal reaction, enthesophyte formation, and small radiolucent defects in combination with positive scintigraphic findings are also diagnostic.
Focal moderate or intense IRU in the proximal palmar aspect of the McIII confirms the presence of stress-induced bone injury and may represent avulsion fracture,41 incomplete fracture,35 stress reaction, or stress fracture of the McIII,27 but can also be seen in association with acute, severe proximal suspensory desmitis. Radiological abnormalities are inconsistently identifiable in horses with IRU,27,35,41 and the clinical importance of subtle or questionable radiological abnormalities is enhanced with positive bone scan findings.41 Scintigraphy is quite useful for differentiation of middle carpal and carpometacarpal joint lesions, particularly in horses with lameness abolished by middle carpal analgesia.
Ultrasonographic examination is recommended for identification of confounding and/or contributing suspensory desmitis. Individual variation in size and shape of the proximal aspect of the suspensory ligament, artifacts, and variable amounts of muscular tissue complicate ultrasonographic interpretation. Ultrasonographic images underestimate ligament size because the medial and lateral margins are difficult to evaluate; they are less anatomically detailed than MRI images,75 and low-grade lesions may be missed.
Recent experience with MRI has demonstrated superior ability to assess both soft tissue and bone injury in the proximal palmar aspect of the McIII.74,75 High signal intensity on STIR sequences is indicative of fluid or edema (bone contusion), fibrosis, or necrosis and may be caused by the bone’s response to tearing of the origin of the proximal aspect of the suspensory ligament, but may also reflect primary osseous injury.74 Low signal intensity on PD and T2-weighted GRE images is indicative of increased bone density and may be a sign of chronic injury.74
Subchondral Injury of the Third Carpal Bone
The C3 adapts to high-intensity exercise by increasing its subchondral density by bone modeling.22,76-80 The radial facet of the C3 is particularly vulnerable to repetitive loads encountered during race training (see Chapter 38).76,77,79 Histological changes include progressive thickening of the subchondral bone plate at the expense of medullary space that ultimately produces a bony bridge between the proximal and distal cortices.12,22 Although some degree of sclerosis is a normal adaptive response,22 continued modeling in response to loading may cause excessive increase in bone density. In horses with extreme sclerosis, cancellous bone appears to have been almost entirely converted into compact cortical bone, and abnormal dissipation of forces between articular cartilage and subchondral bone may ensue. With subchondral bone density increases, the shock-absorptive capacity decreases, predisposing to cartilage damage81 and OA. Cartilaginous lesions18,79 and reduced cartilage stiffness79 have been noted in sites of maximal subchondral density; however, the interactive mechanism by which bone changes may affect cartilage, and vice versa, is disputed and the subject of intense study.
Modeling of the subchondral bone at the expense of the marrow and vascular spaces may also lead to ischemia of the subchondral bone,9,12,22 although the role of ischemia has not been clearly delineated and remains controversial. Further mechanical abuse to ischemic regions may cause progression to focal osteolysis and gross subchondral loss, predisposing to chip or slab fracture.9,12,22 Histological and/or gross sections of C3 slab fractures indicate that these injuries are pathological fractures, occurring in chronically damaged necrotic bony tissue.9
The localized increase in bone density is evident radiologically as increased radiopacity and loss of trabecular structure (sclerosis) on a dorsoproximal-dorsodistal oblique (skyline) image of the distal row of carpal bones. Histological evidence of increased subchondral bone density corresponds to increased radiopacity noted radiologically22,77,82 and is usually localized to the radial fossa of the C3.82,83 C3 sclerosis and associated subchondral bone pain were implicated as an important cause of carpal lameness in STB and TB racehorses.46,63,83-85 In addition, increased radiopacity is seen in most horses with fractures of the C3.83,86 However, some degree of increased radiopacity represents a normal physiological adaptation to exercise, and not all horses with severe increased radiopacity are lame.22,84,85 Therefore the value of radiology and the clinical significance of increased radiopacity of the C3 can be questioned. The presence of IRU supports the clinical significance of radiologically apparent increased radiopacity of the C3.
Subchondral lucency of the C3, commonly in combination with increased radiopacity of the radial fossa, causes lameness in STBs.46,47,63,85 The pathogenesis is not clearly understood, but it may result from osteochondral collapse and cartilage damage because of chronic degenerative subchondral bone.46,82 Subchondral lucency is not a disease of racehorses in early training and may be the end result of a continuum of compression injury of the sclerotic C3.46
The amount of sclerosis that can be considered physiological and how the transition to pathology can be detected or prevented remain a major diagnostic dilemma. Therefore the clinical diagnosis of C3 subchondral bone injury can be challenging. The majority of horses with authentic subchondral C3 pain are lame, and often the degree of lameness is more pronounced than other clinical signs. As with other subchondral bone injuries, joint effusion, positive response to a carpal flexion test, and pain on palpation are often lacking.44,46,47,85 Horses with carpal pain tend to place the limb more widely than expected during protraction and may abduct the limb during advancement.44,46 Diagnostic analgesia in combination with well-exposed, well-positioned radiographic images and positive bone scan findings are recommended for diagnosis.
Scintigraphic examination reveals focal IRU of the C3 or the medial aspect of the subchondral bone of the middle carpal joint (Figure 100-7). Flexed dorsal images of the carpus help to differentiate IRU of the C3 from the radial carpal bone. In retrospective studies IRU of the C3 is common in lame STBs and frequently bilateral.63 IRU in the C3 is also noted in TBs, and about one third of horses are bilaterally affected.62 Radiological evidence of increased radiopacity accompanies IRU in about half of injured horses.63 Horses with focal, moderate-to-intense IRU are more likely to have radiological abnormalities (Figure 100-8).63
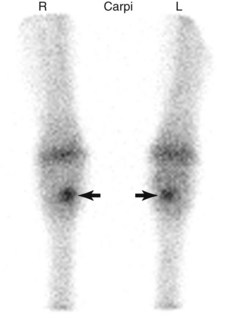
Fig. 100-7 Dorsal delayed phase scintigraphic image of the carpal region in a 2-year-old Standardbred with focal intense increased radiopharmaceutical uptake in the third carpal bones (arrows).
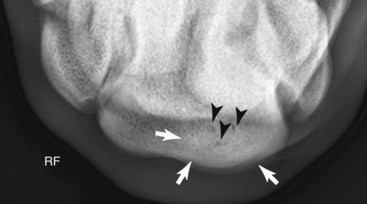
Fig. 100-8 Dorsoproximal-,dorsodistal (skyline) radiographic image of the distal row of carpal bones of the right carpus of the horse in Figure 100-7 showing increased radiopacity (sclerosis) (white arrows) and small radiolucent defects (black arrows) of the radial fossa of the third carpal bone.
Radius
Stress fractures of the radius are uncommon.26,62,63 They are usually unilateral and midshaft,26 although bilateral fractures are seen occasionally.26,62 Enostosis-like lesions can mimic stress fractures and are seen more commonly in the radius than are stress fractures during scintigraphic evaluation (see Chapter 39).87 Enostosis-like lesions are characterized by focal areas of IRU often close to the nutrient foramen and not involving the cortex.87 Correct and careful positioning and/or additional images assist in differentiation.87 Radiological abnormalities are typical for stress remodeling; periosteal bone reaction and fracture lines are identified infrequently. Enostosis-like lesions appear as focal intramedullary radiopacities corresponding to areas of IRU (Figure 100-9).87
Humerus
Complete, catastrophic humeral fractures in TB racehorses are now known to be associated with preexisting pathology (periosteal callus and incomplete fracture).6 Increased awareness of incomplete humeral stress fractures and scintigraphic examination of horses with undiagnosed forelimb lameness have enhanced early detection.
Stress fractures of the humerus are relatively common in young TB racehorses in early or mid training,2,6,25,26 and half of affected horses are unraced before diagnosis.25 Race training after a lay-up period may be a predisposing factor, because return from lay-up has been strongly associated with increased risk of complete humeral facture.2 Initially, stress fractures of the humerus were reported more commonly in the left forelimb,26 but more recent studies showed no difference between left and right forelimbs.6,25 Bilateral humeral stress fractures and recurrence are uncommon.25,26 The prevalence of humeral stress fractures in STBs is low and the location and conformation of stress-related bone injury of the humerus in STBs may be different than that of the TB.34,63 Differences in training and racing regimens may be responsible for the low prevalence of humeral stress fractures in STBs.
Most affected horses are unilaterally lame.25,26,34 Typical of horses with stress fractures, lameness is often severe immediately after exercise and improves quickly with box rest.25,26 Lameness may be more severe than with stress fractures elsewhere in the forelimbs. Physical examination findings are generally unremarkable, and few affected horses show pain during upper limb manipulation.25,34 Lameness is not abolished with distal limb analgesia25,26,34 and may become worse.25 Intrathecal analgesia of the bicipital bursa may partially improve lameness severity in horses with stress fractures of the proximal aspect of the humerus because of diffusion of local anesthetic solution.34
Nuclear scintigraphy is the most sensitive method to diagnose humeral stress fractures; there is focal IRU in the humeral cortex.25,26,34 The caudoproximal25,26 and the craniodistal25,34 aspects of the humerus and usually the medial cortex are the most common sites (Figure 100-10). Rarely, areas of IRU that are seen extending from the proximal caudal cortex to the distal cranial cortex of the humerus are highly suggestive of an incomplete spiral fracture. The scintigraphic pattern generally follows the same pattern as complete humeral fractures.6 Radiological examination is less sensitive, positive in only half of horses with focal IRU associated with the humerus, but is more specific than scintigraphy.25 Callus formation and less commonly a distinct fracture line may be noted (Figure 100-11).
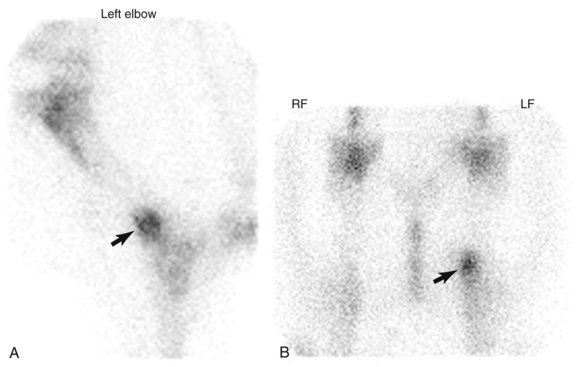
Fig. 100-10 A, Lateral delayed phase scintigraphic image of the left elbow region of a 3-year-old Thoroughbred with focal, intense increased radiopharmaceutical uptake in the distal cranial aspect of the humerus (arrow). B, In merged cranial delayed phase scintigraphic images of the right and left shoulder regions, the increased radiopharmaceutical uptake is more prominent on the distal medial aspect of the left humerus (arrow).
Scapula
Postmortem examination has identified scapular stress fractures as singular and focal and occurring along the distolateral aspect of the scapular spine.13 Periosteal callus, indicative of preexisting bone stress, is also found in association with complete fractures.13 Although rare, stress fractures of the scapula should be considered when an upper forelimb stress fracture is suspected clinically.48,49 Affected TB racehorses have a history typical of other stress-related bone injuries—acute unilateral forelimb lameness after racing or speed work.48,49 To date, scapular stress fractures remain unreported in STBs. Few localizing clinical examination findings are apparent. Injured horses have a negative response to diagnostic analgesia of the distal limb and infrequently a positive response to upper limb manipulation.48,49 Diagnosis is confirmed scintigraphically with focal, intense IRU in the scapula along the caudal ventral aspect,48 the middistal spine of the scapula, or the supraspinous fossa.49 Radiological abnormalities are difficult to detect in horses with proximally located stress fracture but may be apparent in those with distal fractures. Ultrasonographic examination may reveal bony irregularity and/or a “stair-step” appearance of the site of injury.
Tibia
Tibial stress fractures occur predominately in TBs and are one of the more common causes of acute hindlimb lameness in the racehorse.25,26,88 Tibial stress fractures occur infrequently in STBs and Quarter Horses.16,26,28,31,89 Most tibial stress fractures occur during race training, and in one study, more than half of affected horses had had at least one 60-day lay-off period within the last three starts before injury.32,33 Most affected horses are 2- or 3-year-olds,25,28,32,33 but fractures occur in older TBs and STBs as well.26,28,89 Fractures may occur unilaterally and are seen equally in the right and left hindlimbs. However, unilateral lameness may be associated with bilateral fractures. Fractures occur in a variety of locations, including proximocaudal, middiaphyseal, and distomedial.* Bilateral lameness and bilateral fractures may occur,25,31,32,88 and fractures can occur in the contralateral tibia at a later date.25 Tibial stress fracture can propagate to a complete fracture even with enforced stall confinement.32
Typically, horses have moderate-to-severe acute lameness after racing or training.† Affected horses may be more lame than horses with stress fractures in other locations and should be suspected of having a tibial stress or spiral fracture if acute non–weight-bearing hindlimb lameness is present. There are few localizing signs on physical examination. Some affected horses have a positive response to firm pressure and percussion of the medial diaphysis of the tibia.25 Horses may have a positive response to upper limb flexion16,25,26,31 and pain when torsion is placed on the tibia.45 History and a negative response to diagnostic analgesia in the remainder of the limb are important.25,26,28
†References 25, 26, 28, 32, 89, 90.
Tibial stress fractures are identified as focal areas of IRU, and numerous sites of injury have been described.§ The thickness of the soft tissue overlying the lateral aspect of the midcrus may impede detection in all affected horses in lateral images; therefore additional caudal or oblique images are recommended for horses with subtle areas of IRU in the tibia and in those in which there is a high index of suspicion but no convincing area of IRU is seen in standard images. The most common site of IRU is in the caudolateral aspect of the middiaphysis (Figure 100-12) or the proximocaudal aspect of the tibia.| Stress fractures of the caudomedial cortex may occur but are uncommon. Occasionally, diffuse areas of IRU in the distal aspect of the tibia are seen with spiral tibial stress fractures (Figure 100-13). Postmortem studies correlate with scintigraphic findings and have identified periosteal callus in a variety of locations.13 Radiological examination can confirm diagnosis in the majority of but not in all horses.25,28,31,32 Radiological abnormalities include cortical thickening, periosteal or endosteal bony reaction, and an oblique cortical fracture line.*
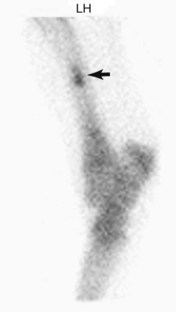
Fig. 100-12 Lateral delayed phase scintigraphic image of the left tibia of a Thoroughbred racehorse with left hindlimb lameness. There is focal moderate-intense increased radiopharmaceutical uptake (arrow) in the caudal, lateral aspect of the tibial diaphysis consistent with a tibial stress fracture.
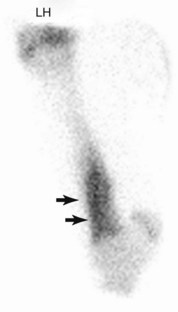
Fig. 100-13 Lateral delayed phase scintigraphic image of the left tibia of a Thoroughbred racehorse with severe left hindlimb lameness. There is diffuse intense increased radiopharmaceutical uptake (arrows) in the distal aspect of the tibial diaphysis consistent with a spiral tibial stress fracture. Despite enforced stall confinement, the horse sustained a catastrophic fracture 30 days after diagnosis.
Ilium
The ilium is the most common site of stress-related bone injury in the pelvis (see Chapter 49).11,13,15,17,67 Within the ilium the most frequent site is 10 to 15 cm lateral to the tuber sacrale.15 Fractures usually originate at the caudal border of the ilium near the sacroiliac joint and course in a craniodorsal or craniolateral direction.11 They may be unilateral and incomplete; however, bilateral injury is frequently seen.11,15,67 Horses usually sustain an ilial stress fracture during race training,2,33 and female and older racehorses are more likely to be affected, although this prevalence is derived from postmortem specimen data.2,11 Fractures extending into the ilial shaft are occasionally seen, especially in National Hunt racehorses in Europe, and horses with this type of ilial stress fracture have a more guarded prognosis than those with fractures limited to the ilial wing.
Initially, most affected horses are lame, although lameness severity varies and lameness often resolves rapidly within 24 to 48 hours.15,30,67 Shortened stride, hunched up back, and plaiting may be apparent.15,67 Mildly affected horses may lack propulsion and exhibit poor action behind without overt lameness.15 Horses may exhibit pain on palpation of the tuber sacrale on the affected side.15,30,67 Subtle, ventral displacement of the ipsilateral tuber sacrale may be noted when fracture is complete, but this clinical finding is easily missed.15,30,67 Muscle atrophy and resultant asymmetry of the sacral region may also be noted in horses with chronic fractures. Rectal palpation is useful if the fracture involves the ilial shaft and is complete. Gentle rocking of the pelvis while palpating may reveal the presence of crepitus or hematoma with complete fractures.67
Scintigraphic evaluation reveals marked, focal IRU associated with the ilial wing, just lateral to midline (Figure 100-14). Right and left dorsal oblique images of the pelvis enhance the diagnosis by decreasing superimposition and improving image geometry of surrounding structures (Figure 100-15).12,91 Less commonly there is linear IRU extending from the tuber coxae axially along the caudal cortex of the ilial wing. Fractures of the ilial shaft may be more difficult to detect because of the greater overlying muscle mass resulting in shielding; subtle IRU consistent with a fracture is easily overlooked.
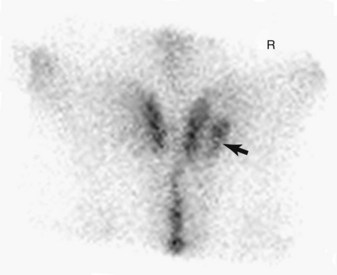
Fig. 100-14 Dorsal delayed phase scintigraphic image of a 3-year-old Thoroughbred with moderate, focal increased radiopharmaceutical uptake (arrow) in the right ilium. This is a common area of uptake seen in racehorses with stress fractures of the ilium. Cranial is to the top of the image.
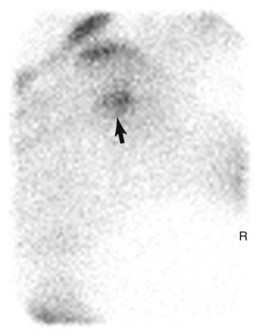
Fig. 100-15 Right oblique delayed phase scintigraphic image (cranial is to the right) of the same horse as shown in Figure 100-14. Increased radiopharmaceutical uptake consistant with an ilial stress fracture (arrow) can readily be seen.
Ultrasonographic examination has been discussed previously (see page 937). Pelvic radiography is useful only for diagnosis of complete ilial stress fractures and requires general anesthesia in most horses. However, diagnostic images of the tuber coxae, the ilial shaft, the acetabulum of the pelvis, and the ischium can be obtained in a standing, sedated horse.
Lumbar Vertebrae
A postmortem study of Californian TBs identified lumbar vertebral stress fractures in 50% of specimens.11 Lesions were characterized by incomplete fracture and focal periosteal proliferation. Vertebral laminar stress fractures were mostly unilateral, and affected specimens tended to be from older horses. All vertebral stress fractures were continuous with vertical articular clefts of the cranial articular facets, near the junction of the dorsal spinous process in most. They were positively associated with the severity of both impingement of dorsal spinous processes and OA of synovial articulations. Although they were a common postmortem finding, the clinical prevalence is less in a normal racing population. Antemortem physical examination findings of affected horses are vague at best. Nonspecific signs of back pain such as poor performance, hunched-up lumbar spine, and poor hindlimb propulsion may be noted. Scintigraphic abnormalities of the lumbar vertebrae are not common.92 There is focal intense IRU in the affected vertebra; a dorsal image can help to determine if the lesion is on the left or the right. Radiological examination may reveal marked enlargement of the affected synovial articulation and a generalized increase in radiopacity.