Henry J. Binder
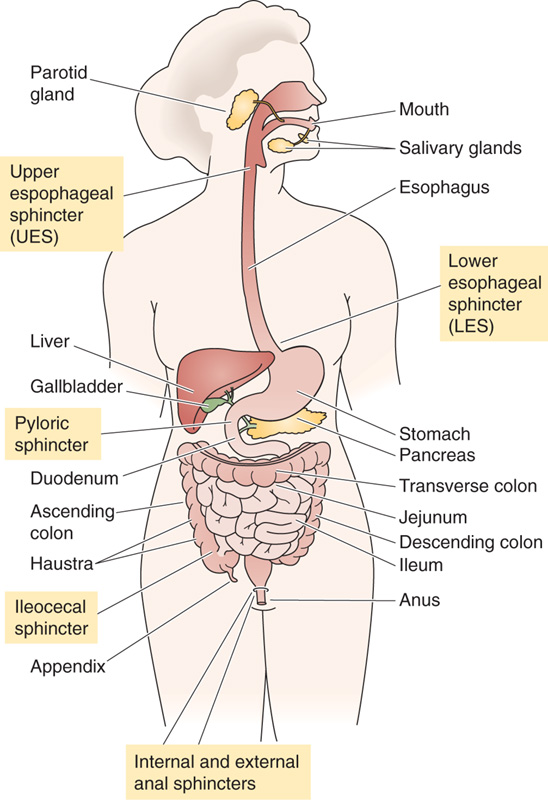
The gastrointestinal (GI) tract consists of both the series of hollow organs stretching from the mouth to the anus and the several accessory glands and organs that add secretions to these hollow organs (Fig. 41-1). Each of these hollow organs, separated from each other at key locations by sphincters, has evolved to serve a specialized function. The mouth and oropharynx are responsible for chopping food into small pieces, lubricating it, initiating carbohydrate and fat digestion, and propelling the food into the esophagus. The esophagus acts as a conduit to the stomach. The stomach (see Chapter 42) temporarily stores food and also initiates digestion by churning and by secreting proteases and acid. The small intestine (see Chapters 44 and 45) continues the work of digestion and is the primary site for the absorption of nutrients. The large intestine (see Chapters 44 and 45) reabsorbs fluids and electrolytes and also stores the fecal matter before expulsion from the body. The accessory glands and organs include the salivary glands, pancreas, and liver. The pancreas (see Chapter 43) secretes digestive enzymes into the duodenum, in addition to secreting HCO−3 to neutralize gastric acid. The liver secretes bile (see Chapter 46), which the gallbladder stores for future delivery to the duodenum during a meal. Bile contains bile acids, which play a key role in the digestion of fats.

Figure 41-1 The major components of the human digestive system.
Although the anatomy of the wall of the GI tract varies along its length, certain organizational themes are common to all segments. Figure 41-2, a cross section through a generic piece of stomach or intestine, shows the characteristic layered structure of mucosa, submucosa, muscle, and serosa.
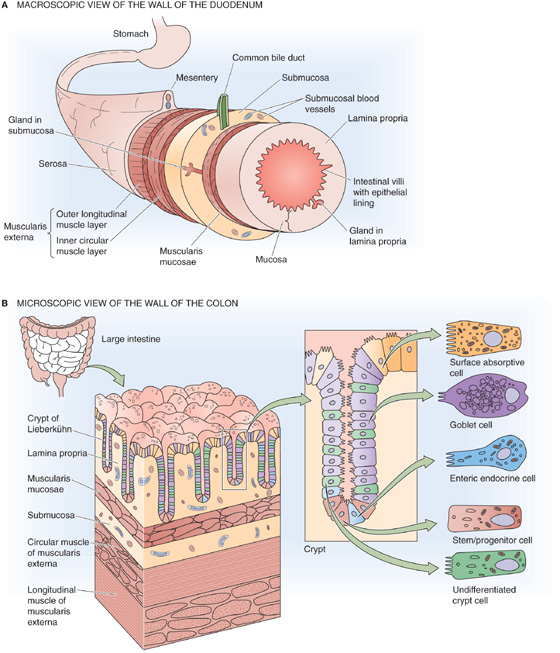
Figure 41-2 The wall of the gastrointestinal tract. A, The wall of a generic segment of the gastrointestinal tract consists of the following structures, from inside to outside: an epithelial layer with crypts, lamina propria, muscularis mucosae, submucosa, the circular and then the longitudinal layer of the muscularis externa, and serosa. B, The colon has the same basic structure as the small intestine. Some of the epithelial cells are on the surface, and others are in the crypts that penetrate into the wall of the colon.
The mucosa consists of the epithelial layer, as well as an underlying layer of loose connective tissue known as the lamina propria, which contains capillaries, enteric neurons, and immune cells (e.g., mast cells), as well as a thin layer of smooth muscle known as the lamina muscularis mucosae (literally, the muscle layer of the mucosa). The surface area of the epithelial layer is amplified by several mechanisms. Most cells have microvilli on their apical surfaces. In addition, the layer of epithelial cells can be evaginated to form villi or invaginated to form glands (or crypts). Finally, on a macroscopic scale, the mucosa is organized into large folds.
The submucosa consists of loose connective tissue and larger blood vessels. The submucosa may also contain glands that secrete material into the GI lumen.
The muscle layer, the muscularis externa, includes two layers of smooth muscle. The inner layer is circular, whereas the outer layer is longitudinal. Enteric neurons are present between these two muscle layers.
The serosa is an enveloping layer of connective tissue that is covered with squamous epithelial cells.
The sedentary human body requires ~30 kcal/kg body weight each day (see Chapter 58). This nutrient requirement is normally acquired by the oral intake of multiple food substances that the GI tract then assimilates. Although antigenic amounts of protein enter the body through the skin and across the pulmonary epithelium, caloric uptake by routes other than the GI tract is not thought to occur. Both the small and large intestines absorb water and electrolytes, but only the small intestine absorbs lipids, carbohydrates, and amino acids. However, even without effective GI function, parenteral (i.e., intravenous) alimentation can provide sufficient calories to sustain adults and to support growth in premature infants. Total parenteral nutrition has been used successfully on a long-term basis in many clinical settings in which oral intake is impossible or undesirable.
Food substances are not necessarily—and often are—consumed in a chemical form that the small intestine can directly absorb. To facilitate absorption, the GI tract digests the food by both mechanical and chemical processes.
Mechanical disruption of ingested food begins in the mouth with chewing (mastication). Individuals without teeth usually require their solid food to be cut into smaller pieces before eating. The mechanical processes that alter food composition to facilitate absorption continue in the stomach (see Chapter 42), both to initiate protein and lipid enzymatic digestion and to allow passage of gastric contents through the pylorus into the duodenum. This change in the size and consistency of gastric contents is necessary because solids that are greater than 2 mm in diameter do not pass through the pylorus.
The chemical form in which different nutrients are ingested and absorbed varies according to the specific nutrient in question. For example, although most lipids are consumed in the form of triglycerides, it is fatty acids and monoglycerides, not triglycerides, that are absorbed by the small intestine. Thus, complex series of chemical reactions (i.e., lipid digestion) are required to convert dietary triglycerides to these smaller lipid forms (see Chapter 45). Similarly, amino acids are present in food as proteins and large peptides, but only amino acids and small peptides—primarily dipeptides and tripeptides—are absorbed by the small intestine. Carbohydrates are present in the diet as starch, disaccharides, and monosaccharides (e.g., glucose). However, because the small intestine absorbs all carbohydrates as monosaccharides, most dietary carbohydrates require chemical digestion before their absorption.
Digestion involves the conversion of dietary food nutrients to a form that the small intestine can absorb. For carbohydrates and lipids, these digestive processes are initiated in the mouth by salivary and lingual enzymes: amylase for carbohydrates and lipase for lipids. Protein digestion is initiated in the stomach by gastric proteases (i.e., pepsins), whereas additional lipid digestion in the stomach occurs primarily as a result of the lingual lipase that is swallowed, although some gastric lipase is also secreted. Carbohydrate digestion does not involve any secreted gastric enzymes.
Digestion is completed in the small intestine by the action of both pancreatic enzymes and enzymes at the brush border of the small intestine. Pancreatic enzymes, which include lipase, chymotrypsin, and amylase, are critical for the digestion of lipids, protein, and carbohydrates, respectively. The enzymes on the luminal surface of the small intestine (e.g., brush border disaccharidases and dipeptidases) complete the digestion of carbohydrates and proteins. Digestion by these brush border enzymes is referred to as membrane digestion.
The material presented to the small intestine includes both dietary intake and secretory products. The food material entering the small intestine differs considerably from that of the ingested material because of the mechanical and chemical changes just discussed. The load to the small intestine is also significantly greater than that of the ingested material. Dietary fluid intake is 1.5 to 2.5 L/day, whereas the fluid load presented to the small intestine is 8 to 9 L/day. The increased volume results from substantial quantities of salivary, gastric, biliary, pancreatic, and small intestinal secretions. These secretions contain large amounts of protein, primarily in the form of the digestive enzymes discussed earlier.
Digestion of food involves multiple secretory, enzymatic, and motor processes that are closely coordinated with one another. The necessary control is achieved by neural and hormonal processes that are initiated by dietary food substances; the result is a coordinated series of motor and secretory responses. For example, chemoreceptors, osmoreceptors, and mechanical receptors in the mucosa in large part generate the afferent stimuli that induce gastric and pancreatic secretions. These receptors sense the luminal contents and initiate a neurohumoral response.
Endocrine, neural, and paracrine mechanisms all contribute to digestion. All three include sensor and transmitter processes. An endocrine mechanism (see Chapter 3) involves the release of a transmitter (e.g., peptide) into the blood. For example, protein in the stomach stimulates the release of gastrin from antral G cells. Gastrin then enters the blood and stimulates H+ release from parietal cells in the body of the stomach. A neural mechanism involves the activation of nerves and neurotransmitters that influence either secretory or motor activity. Neural transmission of these responses may involve the enteric nervous system (ENS; see later) or the central nervous system (CNS). An example of neural control is activation of the vagus nerve in response to the smell of food. The resultant release of the neurotransmitter acetylcholine (ACh) also releases H+ from parietal cells in the stomach.
The third mechanism of neurohumoral control is paracrine (see Chapter 3). In this mechanism, a transmitter is released from a sensor cell, and it affects adjacent cells without either entering the blood or activating neurons. For example, paracrine mechanisms help to regulate gastric acid secretion by parietal cells: the histamine released from so-called enterochromaffin-like (ECL) cells in the body of the stomach stimulates H+ release from neighboring parietal cells.
In addition to the primary response that leads to the release of one or more digestive enzymes, other signals terminate these secretory responses. Enteric neurons are important throughout the initiation and termination of the responses.
Although the endocrine, neural, and paracrine responses are most often studied separately, with considerable effort made to isolate individual events, these responses do not occur as isolated events. Rather, each type is part of an integrated response to a meal that results in the digestion and absorption of food. This entire series of events that results from the ingestion of food can best be described as an integrated response that includes both afferent and efferent limbs.
Although its primary roles are absorbing and digesting nutrients, the GI tract also excretes waste material. Fecal material includes nondigested and nonabsorbed dietary food products, colonic bacteria and their metabolic products, and several excretory products. These excretory products include the following: (1) heavy metals such as iron and copper, whose major route of excretion is in bile; and (2) several organic anions and cations, including drugs, that are excreted in bile but are either poorly or not at all reabsorbed by either the small or large intestine.
As noted earlier, the small intestine is presented with 8 to 9 L/day of fluid, an amount that includes ~1 L/day that the intestine itself secretes. Almost all this water is reabsorbed in the small and large intestine; therefore, stool has relatively small amounts of water (~0.1 L/day). Diarrhea (an increase in stool liquidity and weight, >200 g/day) results from either increased fluid secretion by the small or large intestine or decreased fluid reabsorption by the intestines. An important clinical example of diarrhea is cholera, especially in developing countries. Cholera can be fatal because of the water and electrolyte imbalance that it creates. Thus, the GI tract plays a crucial role in maintaining overall fluid and electrolyte balance (see Chapter 44).
The GI tract also contributes to immune function. The mucosal immune system, or gut-associated lymphoid tissue (GALT), consists of both organized aggregates of lymphoid tissue (e.g., Peyer’s patches; Fig. 41-2B) and diffuse populations of immune cells. These immune cells include lymphocytes that reside between the epithelial cells lining the gut, as well as lymphocytes and mast cells in the lamina propria. GALT has two primary functions: (1) to protect against potential microbial pathogens, including bacteria, protozoans, and viruses; and (2) to permit immunologic tolerance to both the potentially immunogenic dietary substances and bacteria that normally reside primarily in the lumen of the large intestine.
The mucosal immune system is important because the GI tract has the largest area of the body in potential direct contact with infectious, toxic, and immunogenic material. Approximately 80% of the immunoglobulin-producing cells are found in the small intestine. Although GALT has some interaction with the systemic immune system, GALT is operationally distinct. Finally, evidence indicates communication between the GALT and mucosal immune systems at other mucosal surfaces, such as the pulmonary epithelia.
Certain nonimmunologic defense processes are also important in protecting against potential luminal pathogens and in limiting the uptake of macromolecules from the GI tract. The nonimmunologic mechanisms that are critical in maintaining the ecology of intestinal flora include gastric acid secretion, intestinal mucin, peristalsis, and the epithelial cell permeability barrier. Thus, whereas relatively low levels of aerobic bacteria are present in the lumen of the small intestine of physiologically normal subjects, individuals with impaired small intestinal peristalsis often have substantially higher levels of both aerobic and anaerobic bacteria in their small intestine. A consequence may be diarrhea or steatorrhea (i.e., increased fecal fat excretion). The clinical manifestation of impaired intestinal peristalsis is referred to as either blind loop syndrome or stagnant bowel syndrome.
The ENS is the primary neural mechanism that controls GI function and, as described in Chapter 14, is one of the three divisions of the autonomic nervous system (ANS), along with the sympathetic and parasympathetic divisions. One indication of the importance of the ENS is the number of neurons consigned to it. The ENS consists of ~100 million neurons, roughly the number in the spinal cord or in the rest of the entire ANS. The ENS is located solely within GI tissue, but it can be modified by input from the brain. Neurons of the ENS are primarily, but not exclusively, clustered in one of two collections of neurons (Fig. 41-3A): the submucosal plexus and the myenteric plexus. The submucosal (or Meissner’s) plexus is found in the submucosa only in the small and large intestine. The myenteric (or Auerbach’s) plexus is located between the circular and longitudinal muscle layers throughout the GI tract from the proximal end of the esophagus to the rectum.
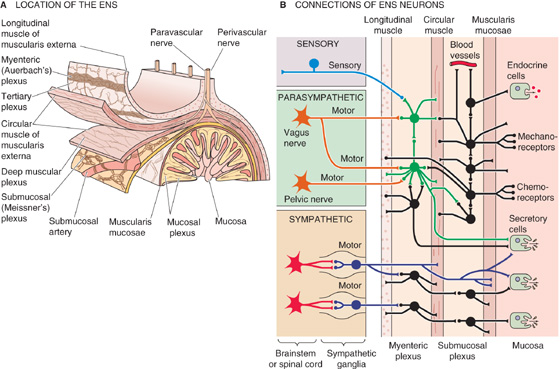
Figure 41-3 Schematic representation of the ENS. A, The submucosal (or Meissner’s) plexus is located between the muscularis mucosae and the circular muscle of the muscularis externa. The myenteric (or Auerbach’s) plexus is located between the circular and longitudinal layers of the muscularis externa. In addition to these two plexuses that have ganglia, three others—mucosal, deep muscular, and tertiary plexus—are also present. B, The ENS consists of sensory neurons, interneurons, and motor neurons. Some sensory signals travel centrally from the ENS. Both the parasympathetic and the sympathetic divisions of the ANS modulate the ENS. This figure illustrates some of the typical circuitry of ENS neurons.
The ENS is a complete reflex circuit and can operate totally within the GI tract, without the participation of either the spinal cord or the cephalic brain. As with other neurons, the activity of the ENS is the result of the generation of action potentials by single neurons and the release of chemical neurotransmitters that affect either other neurons or effector cells (i.e., epithelial or muscle cells). The ENS consists of sensory circuits, interneuronal connections, and secretomotor neurons (Fig. 41-3B). Sensory (or afferent) neurons monitor changes in luminal activity, including distention (i.e., smooth muscle tension), chemistry (e.g., pH, osmolality, specific nutrients), and mechanical stimulation. These sensory neurons, in turn, activate interneurons, which relay signals that activate efferent secretomotor neurons. These efferent secretomotor neurons stimulate or inhibit a wide range of effector cells: smooth muscle cells, epithelial cells that secrete or absorb fluid and electrolytes, submucosal blood vessels, and enteric endocrine cells.
The largely independent function of the ENS has given rise to the concept of a GI “minibrain.” Because the efferent response to several different stimuli is often quite similar, a generalized concept has developed that the ENS possesses multiple preprogrammed responses. For example, both mechanical distention of the jejunum and the presence of a bacterial enterotoxin in the jejunum can elicit identical responses: stimulation of profuse fluid and electrolyte secretion, together with propagated, propulsive, coordinated smooth muscle contractions. Such preprogrammed efferent responses are probably initiated by sensory input to the enteric interneuronal connections. However, efferent responses controlled by the ENS may also be modified by input from autonomic ganglia, which are, in turn, under the influence of the spinal cord and brain (see Chapter 14). In addition, the ENS receives input directly from the brain through parasympathetic nerves (i.e., the vagus nerve). (See Note: Hierarchical Reflex Loops in the ANS)
ACh is the primary preganglionic and postganglionic neurotransmitter regulating both secretory function and smooth muscle activity in the GI tract. In addition, many other neurotransmitters are present in enteric neurons. Among the peptides, vasoactive intestinal peptide (VIP) has an important role in both inhibition of intestinal smooth muscle and stimulation of intestinal fluid and electrolyte secretion. Although VIP was first identified in the GI tract, it is now appreciated that VIP is also an important neurotransmitter in the brain (see Table 13-1). Other substances probably also play an important role in GI regulation. These substances include the following: other peptides, such as the enkephalins, somatostatin, and substance P; amines such as serotonin (5-hydroxytryptamine [5-HT]); and nitric oxide (NO).
The field of ENS neurotransmitters is rapidly evolving, and the list of agonists grows ever longer. In addition, substantial species differences exist. Frequently, chemical neurotransmitters are identified in neurons without a clear-cut demonstration of their physiological role in the regulation of organ function. More than one neurotransmitter has been identified within single neurons, a finding suggesting that regulation of some cell functions may require more than one neurotransmitter.
Well recognized, but poorly understood, is the modification of several different aspects of GI function by the brain. In other words, neural control of the GI tract is a function not only of intrinsic nerves (i.e., the ENS), but also of nerves that are extrinsic to the GI tract. These extrinsic pathways are composed of both the parasympathetic—and, to a lesser extent, the sympathetic—nervous system and are under the control of autonomic centers in the brainstem (see Chapter 14).
Parasympathetic innervation of the GI tract from the pharynx to the distal colon is through the vagus nerve; the distal third of the colon receives its parasympathetic innervation from the pelvic nerves (see Fig. 14-4). The preganglionic fibers of the parasympathetic nerves use ACh as their neurotransmitter and synapse on some neurons of the ENS (Fig. 41-3B). These ENS neurons are thus postganglionic parasympathetic fibers, and their cell bodies are, in a sense, the parasympathetic ganglion. These postganglionic parasympathetic fibers use mainly ACh as their neurotransmitter; however, as noted in the previous section, many other neurotransmitters are also present. The results of parasympathetic stimulation are—after one or more synapses in a very complex ENS network—increased secretion and motility. The parasympathetic nerves also contain afferent fibers (see Chapter 14) that carry information to autonomic centers in the medulla from chemoreceptors, osmoreceptors, and mechanical receptors in the mucosa. The loop that is initiated by these afferents, integrated by central autonomic centers, and completed by the aforementioned parasympathetic efferents is known as a vagovagal reflex.
The preganglionic sympathetic fibers to the GI tract synapse on postganglionic neurons in the prevertebral ganglia (see Fig. 14-4); the neurotransmitter at this synapse is ACh (see Chapter 14). The postganglionic sympathetic fibers either synapse in the ENS or directly innervate effector cells (Fig. 41-3B).
In addition to the control that is entirely within the ENS, as well as control by autonomic centers in the medulla, the GI tract is also under the control of higher CNS centers. Examples of cerebral function that affects GI behavior include the flight-or-fight response, which reduces blood flow to the GI tract, and the sight and smell of food, which increase gastric acid secretion.
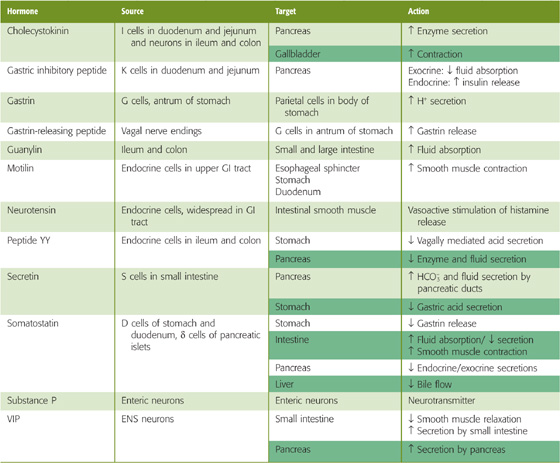
Communication between the GI tract and the higher CNS centers is bidirectional. For example, cholecystokinin from the GI tract mediates, in part, the development of food satiety in the brain. In addition, gastrin-releasing peptide, a neurotransmitter made in ENS cells (see Chapter 42), inhibits gastric acid secretion when it is experimentally injected into the ventricles of the brain. Table 41-1 summarizes peptide hormones made by the GI tract, as well as their major actions.
Table 41-1 Gastrointestinal Peptide Hormones (See Note: Gastrointestinal Peptide Hormones)
In addition to the “hard-wired” communications involved in sensory input and motor output, communication through the gut-brain axis also requires significant participation of the immune system. Neuroimmune regulation of both epithelial and motor function in the small and large intestine primarily involves mast cells in the lamina propria of the intestine. Because the mast cells are sensitive to neurotransmitters, they can process information from the brain to the ENS and can also respond to signals from interneurons of the ENS. In addition, mast cells monitor sensory input from the intestinal lumen by participating in the immune response to foreign antigens. In turn, chemical mediators released by mast cells (e.g., histamine) directly affect both intestinal smooth muscle cells and epithelial cells. Our understanding of how the immune system modulates the neural control of GI function is rapidly evolving.
In conclusion, three parallel components of the gut-brain axis—the ENS, GI hormones, and the immune system—control GI function, an arrangement that provides substantial redundancy. Such redundancy permits refinement of the regulation of digestive processes and provides backup or “fail-safe” mechanisms that ensure the integrity of GI function, especially at times of impaired function (i.e., during disease).
The motor activity of the GI tract performs three primary functions. First, it produces segmental contractions that are associated with nonpropulsive movement of the luminal contents. The result is the increased mixing (or churning) that enhances the digestion and absorption of dietary nutrients. Second, GI motor activity produces propulsion, which is a progressive wave of relaxation, followed by contraction. Peristaltic contractions cause propulsion, or the propagated movement of food and its digestive products in a caudal direction. The result is elimination of nondigested, nonabsorbed material. We discuss churning and propulsion later in this chapter. Third, motor activity allows some hollow organs—particularly the stomach and large intestine—to act as reservoirs for holding the luminal content. This reservoir function is made possible by sphincters that separate the organs of the GI tract. All these functions are primarily accomplished by the coordinated activity of smooth muscle (see Chapter 9).
The electrical and mechanical properties of intestinal smooth muscle needed for these functions include both tonic (i.e., sustained) contractions and rhythmic contractions (i.e., alternating contraction and relaxation) of individual muscle cells. The intrinsic rhythmic contractility is a function of the membrane voltage (Vm) of the smooth muscle cell. Vm can either oscillate in a subthreshold range at a low frequency (several per minute), referred to as slow-wave activity, or reach a threshold for initiating a true action potential (see Fig. 9-15). The integrated effect of the slow waves and action potentials determines the smooth muscle activity of the GI tract. Slow-wave activity apparently occurs as voltage-gated Ca2+ channels depolarize the cell and increase [Ca2+]i, followed by the opening of Ca2+-activated K+ channels, which repolarize the cell (see Chapter 9).
These activities are regulated, in large part, by both neural and hormonal stimuli. Modulation of intestinal smooth muscle contraction is largely a function of [Ca2+]i (see Chapter 9). Several agonists regulate [Ca2+]i by one of the following two mechanisms: (1) activation of G protein–linked receptors, resulting in the formation of inositol 1, 4, 5-triphosphate (IP3) and the release of Ca2+ from intracellular stores; or (2) opening and closing of plasma membrane Ca2+ channels. Both excitatory and inhibitory neurotransmitters can modulate smooth muscle [Ca2+]i and thus contractility. In general, ACh is the predominant neurotransmitter of excitatory motor neurons, whereas VIP and NO are the neurotransmitters of inhibitory motor neurons. Different neural or hormonal inputs probably increase (or decrease) the frequency with which Vm exceeds threshold and produces an action potential and thus increases (or decreases) muscle contractility.
An additional, unique factor in the aforementioned regulatory control is that luminal food and digestive products activate mucosal chemical and mechanical receptors, as discussed earlier, thus inducing hormone release or stimulating the ENS and controlling smooth muscle function. For example, gastric contents with elevated osmolality or a high lipid content entering the duodenum activate mucosal osmoreceptors and chemoreceptors that increase the release of cholecystokinin and thus delay gastric emptying (see Chapter 42).
The muscle layers of the GI tract consist almost entirely of smooth muscle. Exceptions are the striated muscle of the upper esophageal sphincter ([UES] which separates the hypopharynx from the esophagus), the upper third of the esophagus, and the external anal sphincter. As shown earlier in Figure 41-2, the two smooth muscle layers are arranged as an inner circular layer and an outer longitudinal layer. The myenteric ganglia of the ENS are located between the two muscle layers.
The segments of the GI tract through which food products pass are hollow, low-pressure organs that are separated by specialized circular muscles or sphincters. These sphincters function as barriers to flow by maintaining a positive resting pressure that serves to separate the two adjacent organs, in which lower pressures prevail. Sphincters thus regulate both antegrade (forward) and retrograde (reverse) movement. For example, the resting pressure of the pyloric sphincter controls, in part, the emptying of gastric contents into the duodenum. Conversely, the resting pressure of the lower esophageal sphincter (LES) serves to prevent gastric contents from refluxing back into the esophagus and causing gastroesophageal reflux disease (GERD). As a general rule, stimuli proximal to a sphincter cause sphincteric relaxation, whereas stimuli distal to a sphincter induce sphincteric contraction. Changes in sphincter pressure are coordinated with the smooth muscle contractions in the organs on either side. This coordination depends on both the intrinsic properties of sphincteric smooth muscle and neurohumoral stimuli.
Sphincters effectively serve as one-way valves. Thus, the act of deglutition (or swallowing) induces relaxation of the UES, whereas the LES remains contracted. Only when the UES returns to its initial pressure does the LES begin to relax, ~3 seconds after the start of deglutition. Disturbances in sphincter activity are often associated with alterations in one or more of these regulatory processes.
Six sphincters are present in the GI tract (Fig. 41-1), each with different resting pressures and different responses to various stimuli. An additional sphincter, the sphincter of Oddi, regulates movement of the contents of the common bile duct into the duodenum.
Achalasia
Achalasia is a relatively uncommon condition associated with difficulty swallowing (dysphagia) and a dilated esophagus proximal to a narrowed, tapered area at the gastroesophageal junction. The term achalasia is derived from Greek words meaning “absence of relaxation.” The distal narrowed area of the esophagus suggests the presence of a stricture. However, it is easy to introduce an esophagoscope into the stomach through the narrowed area. Subsequent studies of esophageal motility in which investigators measured intraesophageal pressure demonstrated the presence of two defects in patients with achalasia: (1) failure of the LES to relax and (2) impaired peristalsis in the distal two thirds of the body of the esophagus (i.e., the portion that consists of smooth muscle). Peristalsis is intact in the proximal third of the esophagus, which consists of striated muscle. In essence, the smooth muscle portions of the esophagus behave as a denervated structure. The fundamental defect in achalasia is unknown but is probably related to selective loss of inhibitory neurons that regulate the LES, the neurotransmitters of which are VIP and NO. Treatment is either physical distention (or stretching) of the LES with a pneumatic bag dilator or surgical cutting of the LES (i.e., an esophageal myotomy or Heller procedure).
Upper Esophageal Sphincter Separating the pharynx and the upper part of the esophagus is the UES, which consists of striated muscle and has the highest resting pressure of all the GI sphincters. Control of the swallowing mechanism, including the oropharynx and the UES, is largely under the control of the swallowing center in the medulla through cranial nerves V (trigeminal), IX (glossopharyngeal), X (vagus), and XII (hypoglossal). Respiration and deglutition are closely integrated (see Chapter 32). The UES is closed during inspiration, thereby diverting atmospheric air to the glottis and away from the esophagus. During swallowing, the situation reverses, with closure of the glottis and inhibition of respiration, but with relaxation of the UES (Fig. 41-4). These changes permit the entry of food contents into the esophagus and not into the airways of the respiratory tract.
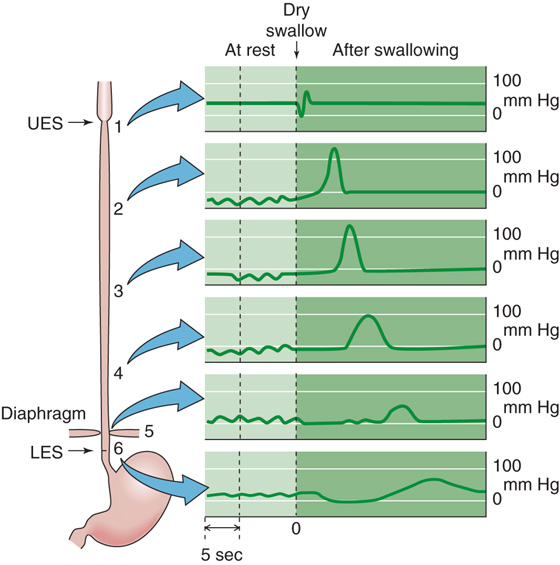
Figure 41-4 Esophageal pressures during swallowing. The swallowing center in the medulla that initiates deglutition includes the nucleus ambiguus (cranial nerves [CN] IX and X), the dorsal motor nucleus of the vagus (CN X), and others. Shown are recordings of intraluminal pressures at different sites along the esophagus, from the UES (record 1) to the LES (record 6). The left side of the graph shows the pressures at rest. As shown on the right side, after a dry swallow, the pressure wave of primary peristalsis moves sequentially down the esophagus. (Data from Conklin JL, Christensen J: In Johnson LR [ed]: Physiology of the Gastrointestinal Tract, 3rd ed, pp 903-928. New York: Lippincott-Raven, 1994.)
Lower Esophageal Sphincter The esophagus is separated from the stomach by the LES, which is composed of specialized smooth muscle that is both anatomically and pharmacologically distinct from adjacent smooth muscle in the distal end of the esophagus and proximal portion of the stomach. The primary functions of the LES are (1) to permit coordinated movement of ingested food into the stomach from the esophagus after swallowing or deglutition and (2) to prevent reflux of gastric contents into the esophagus. Either deglutition or distention of the esophagus results in a reduction in LES pressure (Fig. 41-4) to that of intragastric pressure, thereby permitting entry of food into the stomach. Relaxation of the LES occurs after the UES has already returned to its resting pressure. The LES maintains a resting tone that is the result of both intrinsic myogenic properties of the sphincteric muscle and cholinergic regulation. Relaxation of the LES is mediated both by the vagus nerve and by intrinsic properties of the smooth muscle, including important inhibitory effects by VIP and by NO.
Abnormalities of both resting LES pressure and its relaxation in response to deglutition are often associated with significant symptoms. Thus, a reduced resting LES pressure often results in gastroesophageal reflux, which may cause esophagitis (i.e., inflammation of the esophageal mucosa). A defect in LES relaxation is a major component of a condition called achalasia (see the box titled Achalasia), which often results in dilatation of the esophagus (megaesophagus) and is associated with difficulty in swallowing (dysphagia).
Swallowing and the function of the UES and the LES are closely integrated into the function of the esophagus. Under normal circumstances, esophageal muscle contractions are almost exclusively peristaltic and are initiated by swallowing. Deglutition initiates relaxation of the UES and propagated contractions, first of the UES and then of the muscles along the esophagus (Fig. 41-4). In the meantime, the LES has already relaxed. The result of the advancing peristaltic wave is the caudad propulsion of a bolus toward the stomach.
Distention of the esophagus (in the absence of swallowing) also initiates propulsive esophageal contractions that are distal to the site of distention, as well as relaxation of the LES. Reflux of gastric contents into the lower part of the esophagus also produces such a local distention, without a swallow, and elicits the same response: peristaltic contractions that clear the esophagus of refluxed gastric material. Peristalsis that is initiated by swallowing is called primary peristalsis, whereas that elicited by distention of the esophagus is referred to as secondary peristalsis. Esophageal contractions after a swallow are regulated by the medullary swallowing center, intramural esophageal plexuses, the vagus nerve, and intrinsic myogenic processes.
Pyloric Sphincter The pylorus is the sphincter that separates the stomach from the duodenum. The pressure of the pyloric sphincter regulates, in part, gastric emptying and prevents duodenal-gastric reflux. However, although a specific pyloric sphincter is present, it is quite short and is a relatively poor barrier (i.e., it can resist only a small pressure gradient). The stomach, duodenum, biliary tract, and pancreas—which are closely related embryologically—function as a unit. Indeed, coordinated contraction and relaxation of the antrum, pylorus, and duodenum (which is sometimes referred to as the antroduodenal cluster unit) are probably more important than simply the pressure produced by the pyloric smooth muscle per se. Regulation of gastric emptying is discussed further in Chapter 42.
Ileocecal Sphincter The valve-like structure that separates the ileum and cecum is called the ileocecal sphincter. Similar to other GI sphincters, the ileocecal sphincter maintains a positive resting pressure and is under the control of the vagus nerve, sympathetic nerves, and the ENS. Distention of the ileum results in relaxation of the sphincter, whereas distention of the proximal (ascending) colon causes contraction of the ileocecal sphincter. As a consequence, ileal flow into the colon is regulated by luminal contents and pressure, both proximal and distal to the ileocecal sphincter.
Internal and External Anal Sphincters The “anal sphincter” actually consists of both an internal and an external sphincter. The internal sphincter has both circular and longitudinal smooth muscle and is under involuntary control. The external sphincter, which encircles the rectum, contains only striated muscle but is controlled by both voluntary and involuntary mechanisms. The high resting pressure of the overall anal sphincter predominantly reflects the resting tone of the internal anal sphincter. Distention of the rectum (Fig. 41-5A), either by colonic contents (i.e., stool) or experimentally by balloon inflation, initiates the rectosphincteric reflex by relaxing the internal sphincter (Fig. 41-5B). If defecation is not desired, continence is maintained by an involuntary reflex—orchestrated by the sacral spinal cord—that contracts the external anal sphincter (Fig. 41-5C). If defecation is desired, a series of both voluntary and involuntary events occur that include relaxation of the external anal sphincter, contraction of abdominal wall muscles, and relaxation of pelvic wall muscles. Flexure of the hips and descent of the pelvic floor then facilitate defecation by minimizing the angle between the rectum and anus. In contrast, if a delay in defecation is needed or desired, voluntary contraction of the external anal sphincter is usually sufficient to override the series of reflexes initiated by rectal distention.
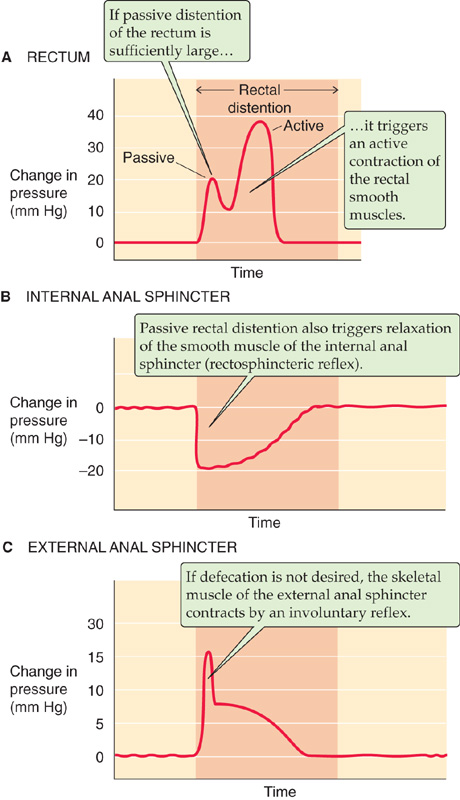
Figure 41-5 A to C, Pressure changes initiated by rectal distention. (Data from Schuster MM: Johns Hopkins Med J 1965; 116:70-88.)
Digestion and absorption of dietary nutrients are the primary functions of the small intestine, and the motor activity of the small intestine is closely integrated with its digestive and absorptive roles. The two classes of small intestine motor activity are churning (or mixing) and propulsion of the bolus of luminal contents. Churning—which is accomplished by segmental, nonpropulsive contractions—mixes the luminal contents with pancreatic, biliary, and small intestinal secretions, thus enhancing the digestion of dietary nutrients in the lumen. These segmental contractions also decrease the unstirred water layer that is adjacent to the apical membranes of the small intestine cells, thus promoting absorption. Churning or mixing movements occur following eating and are the result of contractions of circular muscle in segments flanked at either end by receiving segments that relax. Churning, however, does not advance the luminal contents along the small intestine. In contrast, propulsion—which is accomplished by propagated, peristaltic contractions—results in caudad movement of the intestinal luminal contents, either for absorption at more distal sites of the small or large intestine or for elimination in stool. Peristaltic propulsion occurs as a result of contraction of the circular muscle and relaxation of the longitudinal muscle in the propulsive or upstream segment, together with relaxation of the circular muscle and contraction of the longitudinal muscle in the downstream receiving segment. Thus, circular smooth muscle in the small intestine participates in both churning and propulsion.
Hirschsprung Disease
The anal sphincter controls defecation and consists of a smooth muscle internal sphincter and a striated muscle external sphincter. Distention of the rectum by inflation of a balloon—which simulates the effect of the presence of solid feces in the rectum—results in relaxation of the internal sphincter and contraction of the external sphincter (Fig. 41-5). Voluntary control of the external sphincter regulates the timing of defecation.
Hirschsprung disease is a congenital polygenic disorder. At least eight genes have been associated with Hirschsprung disease, including mutations in the endothelin-B receptor. Variable penetrance leads to variable manifestations of the disease. At the cellular level, the fundamental defect is arrest of the caudad migration of neural crest cells, which are the precursors of ganglion cells. Symptoms include constipation, megacolon, and a narrowed segment of colon in the rectum. Histologic examination of this narrowed segment reveals an absence of ganglion cells from both the submucosal (or Meissner’s) and myenteric (or Auerbach’s) plexuses (Fig. 41-3A). The patient’s constipation and resulting megacolon are secondary to failure of this “aganglionic” segment to relax in response to proximal distention. Manometric assessment of the internal and external anal sphincters reveals that the smooth muscle internal sphincter does not relax after rectal distention (Fig. 41-5), but the external anal sphincter functions normally. Treatment of this condition is usually surgical, with removal of the narrowed segment that is missing the ganglia that normally regulate relaxation of the smooth muscle of the internal anal sphincter.
As noted earlier and in Chapter 9, the Vm changes of intestinal smooth muscle cells consist of both slow-wave activity and action potentials. The patterns of electrical and mechanical activity differ in the fasting and fed states. In the fasting state, the small intestine is relatively quiescent but exhibits synchronized, rhythmic changes in both electrical and motor activity (Fig. 41-6). The interdigestive myoelectric or migrating motor complex (MMC) is the term used to describe these rhythmic contractions of the small intestine that are observed in the fasting state. MMCs in humans occur at intervals of 90 to 120 minutes and consist of four distinct phases: (1) a prolonged quiescent period, (2) a period of increasing action potential frequency and contractility, (3) a period of peak electrical and mechanical activity that lasts a few minutes, and (4) a period of declining activity that merges into the next quiescent period. During the interdigestive period, particles greater than 2 mm in diameter can pass from the stomach into the duodenum, thus permitting emptying of ingested material from the stomach (e.g., bones, coins) that could not be reduced in size to less than 2 mm. The slow propulsive contractions that characterize phases 2 to 4 of the MMCs clear the small intestine of its residual content, including undigested food, bacteria, desquamated cells, and intestinal and pancreatic biliary secretions. MMCs usually originate in the stomach and often travel to the distal end of the ileum, but ~25% are initiated in the duodenum and proximal part of the jejunum.
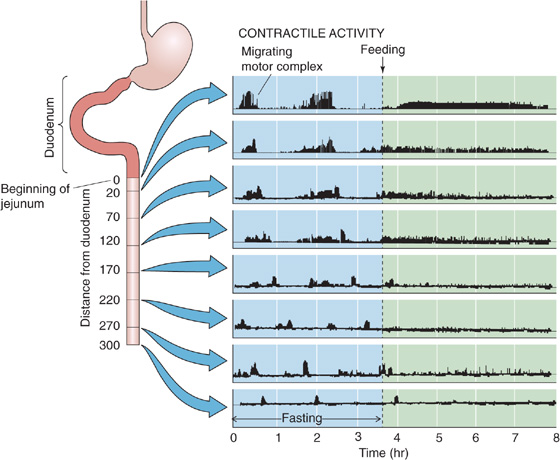
Figure 41-6 Mechanical activity in the fasting and fed states. Shown are records of intraluminal pressure along the small intestine of a conscious dog. Before feeding (left side), the pattern is one of MMCs. Feeding triggers a switch to a different pattern, characterized by both segmental contractions that churn the contents and peristaltic contractions that propel the contents along the small intestine. (Data from Itoh Z, Sekiguchi T: Scand J Gastroenteral Suppl 1983; 82:121-134.)
Feeding terminates MMCs and initiates the appearance of the fed motor pattern (Fig. 41-6). The latter is less well characterized than MMCs but, as noted earlier, consists of both segmental contractions (churning), which enhance digestion and absorption, and peristaltic contractions (propulsion).
Determination of the primary factors that regulate both MMCs and transition to the fed pattern has been hampered by both species differences and complex interactions among the multiple probable mediators. Nonetheless, clear evidence has been presented for a role of the ENS, one or more humoral factors, and extrinsic innervation. A major determinant of the MMC pattern is the hormone motilin, a 22–amino acid peptide that is synthesized in the duodenal mucosa and is released just before the initiation of phase 3 of the MMC cycle. Motilin does not appear to have a role in the motor pattern that is observed in the fed state. Factors important in induction of the fed pattern include the vagus nerve (because sham feeding also both terminates MMCs and initiates a fed pattern) and the caloric content, as well as the type of food (e.g., fat more than protein) in the meal.
The human large intestine has four primary functions. First, the colon absorbs large quantities of fluid and electrolytes and converts the liquid content of ileocecal material to solid or semisolid stool. Second, the colon avidly absorbs the short-chain fatty acids formed by the catabolism (or fermentation) of dietary carbohydrates that are not absorbed in the small intestine. The abundant colonic microflora accomplish this fermentation. Third, the storage of colonic content represents a reservoir function of the large intestine. Fourth, the colon eliminates its contents in a regulated and controlled fashion, largely under voluntary control. To accomplish these important activities, the large intestine functionally acts as two distinct organs. The proximal (or ascending and transverse) part of the colon is the site where most of the fluid and electrolyte absorption occurs and where bacterial fermentation takes place. The distal (or descending and rectosigmoid) portion of the colon provides final desiccation, as well as reservoir function, and serves as a storage organ for colonic material before defecation.
In contrast to the motor pattern in the small intestine, no distinct fasting and fed patterns of contractions are seen in the colon. Similar to small intestinal motor activity, colonic contractions are regulated by myogenic, neurogenic, and hormonal factors. Parasympathetic control of the proximal two thirds of the colon is mediated by the vagus nerve, whereas parasympathetic control of the descending and rectosigmoid colon is mediated by pelvic nerves originating from the sacral spinal cord.
The proximal colon has two types of motor activity: nonpropulsive segmentation and mass peristalsis. Nonpropulsive segmentation is generated by slow-wave activity that produces circular muscle contractions that churn the colonic contents and move them in an orad direction (i.e., toward the cecum). The segmental contractions that produce the churning give the colon its typical appearance of segments or haustra (Fig. 41-1). During this mixing phase, material is retained in the proximal portion of the large intestine for relatively long periods, and fluid and electrolyte absorption continues. One to three times a day, a so-called mass peristalsis occurs in which a portion of the colonic contents is propelled distally 20 cm or more. Such mass peristaltic contractions are the primary form of propulsive motility in the colon and may be initiated by eating. During mass peristalsis, the haustra disappear; they reappear after the completion of mass peristalsis.
In the distal colon, the primary motor activity is nonpropulsive segmentation that is produced by annular or segmental contractions. It is in the distal part of the colon that the final desiccation of colonic contents occurs. It is also here that these contents are stored before an occasional mass peristalsis that propels them into the rectum. The rectum itself is kept nearly empty by nonpropulsive segmentation until it is filled by mass peristalsis of the distal end of the colon. As described in Figure 41-5, filling of the rectum triggers a series of reflexes in the internal and external anal sphincters that lead to defecation.
Books and Reviews
Andrews JM, Dent J: Small intestinal motor physiology. In Feldman J, Friedman LS, Sleisenger MH (eds): Gastrointestinal and Liver Disease, vol 2, 7th ed, pp 1665-1678. Philadelphia: WB Saunders, 2002.
Biancani P, Hartnett KM, Behar J: Esophageal motor function. In Yamada T (ed): Textbook of Gastroenterology, vol 1, 4th ed, pp 166-194. Philadelphia: Lippincott Williams & Wilkins, 2003.
Conklin JL, Christensen J: Motor functions of the pharynx and esophagus. In Johnson LR (ed): Physiology of the Gastrointestinal Tract, 3rd ed, pp 903-928. New York: Lippincott-Raven, 1994.
Cook IJ, Brookes SJ: Motility of the large intestine. In Feldman J, Friedman LS, Sleisenger MH (eds): Gastrointestinal and Liver Disease. vol 2, 7th ed, pp 1679-1691. Philadelphia: WB Saunders, 2002.
Maklouf GM: Smooth muscle of the gut. In Yamada T (ed): Textbook of Gastroenterology, vol 1, 4th ed, pp 92-116. Philadelphia: Lippincott Williams & Wilkins, 2003.
Rehfeld JF: The new biology of gastrointestinal hormones. Physiol Rev 1998; 78:1087-1108.
Surprenant A: Control of the gastrointestinal tract by enteric neurons. Annu Rev Physiol 1994; 56:117-140.
Wood JD: Enteric neuroimmunophysiology and pathophysiology. Gastroenterology 2004; 127:635-657.
Wood JD: The first Nobel prize for integrated systems physiology: Ivan Petrovich Pavlov, 1904. Physiology 2004; 19:326-330.
Journal Articles
Itoh Z, Sekiguchi T: Interdigestive motor activity in health and disease. Scand J Gastroenterol Suppl 1983; 82:121-134.
Schuster MM: Simultaneous manometric recording of internal and external anal sphincteric reflexes. Johns Hopkins Med J 1965; 116:70-88.