Henry J. Binder
The small intestine and large intestine have many similarities in structure and function. In some cases, different regions of the intestinal tract carry out certain functions in much the same manner. In other cases, however, substantial heterogeneity exists between different intestinal segments (e.g., ileum versus jejunum) or between different mucosal areas (i.e., villus versus crypt) in one intestinal segment.
As discussed in Chapter 41, the basic structure of the intestine is a hollow cylinder with columnar epithelial cells lining the lumen, with circular and longitudinal layers of smooth muscle in the wall, and with endocrine and neural elements (see Fig. 41-2). Enteric neurons, as well as endocrine and paracrine agonists, regulate both epithelial transport and motor activity during both the interdigestive and the postprandial periods. As a result, the intestines propagate their contents in a caudad direction while either removing fluid and electrolytes from the intestinal lumen (i.e., absorption) or adding these substances to the lumen (i.e., secretion).
Among mammals, absorption of dietary nutrients is an exclusive function of the small intestine. Only during the neonatal period does significant nutrient absorption take place in the large intestine. The small intestine absorbs nonelectrolytes after extensive digestion of dietary nutrients by both luminal and brush border enzymes, as discussed in Chapter 45. In contrast, both the small intestine and the large intestine absorb fluid and electrolytes by several different cellular transport processes, which may differ between the small intestine and the large intestine and are the subject of this chapter.
Another vitally important function of the intestinal epithelium is the secretion of intestinal fluid and electrolytes. Teleologically, fluid secretion may be considered an adaptive mechanism used by the intestinal tract to protect itself from noxious agents, such as bacteria and bacterial toxins. In general, the cellular mechanisms of intestinal electrolyte secretion in the small intestine and colon are similar, if not identical. Frequently, the adaptive signal that induces the secretory response also stimulates a simultaneous motor response from the intestinal muscle; together, these factors result in a propagated propulsive response in an attempt to dilute and eliminate the offending toxin.
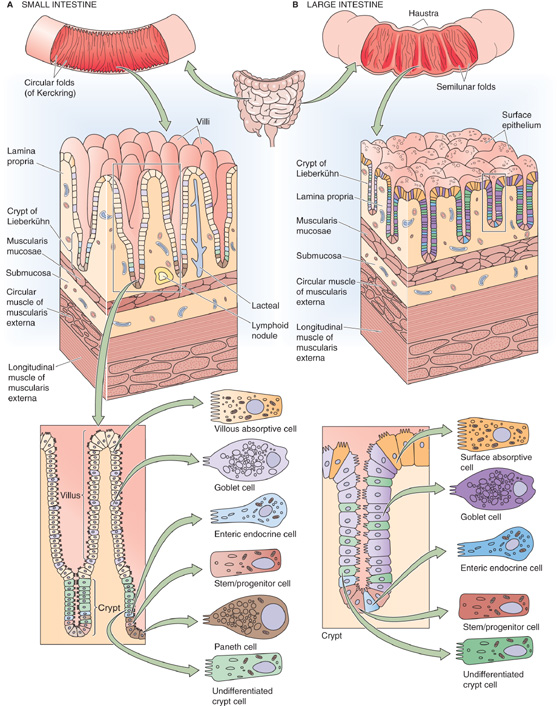
Both the small intestine and the large intestine have a specialized epithelial structure that correlates well with epithelial transport function. The small intestine (Fig. 44-1A) consists of finger-like projections—villi—surrounded by the openings of glandular structures called crypts of Lieberkühn, or simply crypts. Both villi and crypts are covered by columnar epithelial cells. The cells lining the villi are considered to be the primary cells responsible for both nutrient and electrolyte absorption, whereas the crypt cells primarily participate in secretion.

Figure 44-1 Microscopic view of the anatomy of small and large intestine. A, The surface area of the small intestine is amplified at three levels: (1) macroscopic folds of Kerckring, (2) microscopic villi and crypts of Lieberkühn, and (3) submicroscopic microvilli. B, The surface area of the colon is amplified at the same three levels as the small intestine: (1) macroscopic semilunar folds, (2) crypts (but not villi), and (3) microvilli.
The colon (Fig. 44-1B) does not have villi. Instead, the cells lining the large intestine are surface epithelial cells, and interspersed over the colonic surface are numerous apertures of colonic crypts (or glands) that are similar in function and structure to the small intestinal crypts. Not surprisingly, the surface epithelial cells of the colon are the primary cells responsible for colonic electrolyte absorption, whereas colonic gland cells are generally believed to mediate ion secretion.
The intestinal mucosa is a dynamic organ with continuous cell proliferation and migration. The zone of cell proliferation is at the base of the crypt in both the small and large intestine, and the program of events is similar in both organs. The progenitor cell is a stem cell that differentiates into several specialized cells (e.g., vacuolated, goblet, and Paneth cells) that line the villi and crypts in the small intestine and the surface and glands in the colon. The vacuolated cell migrates along the crypt-villus axis and becomes a villous absorptive cell after undergoing substantial changes in its morphologic and functional characteristics. In the small intestine, these villous cells migrate until they reach the tips of the villi and then slough into the lumen of the intestine. The overall period from the initiation of cell proliferation to sloughing is ~48 to 96 hours. The overall rate of cell migration may increase or decrease: decreased cell turnover occurs during starvation, whereas increased cell turnover occurs during feeding and lactation, as well as after intestinal resection. The compensatory response that follows intestinal resection involves both luminal and hormonal factors.
An additional hallmark of both the small and large intestine is the presence of structures that amplify function by increasing the luminal surface area. These structures exist at three levels. In the small intestine, the first level consists of the macroscopic folds of Kerckring. The second level consists of the microscopic villi and crypts that we have already discussed. The third level is the submicroscopic microvilli on the apical surfaces of the epithelial cells. Thus, if the small intestine is thought of as a hollow cylinder, the net increase in total surface area of the small intestine (versus that of a smooth cylinder) is 600-fold. The total surface area of the human small intestine is ~200 m2, or the surface area of a doubles tennis court (Table 44-1). The colonic surface area is also amplified, but to a more limited extent. Because the colon lacks villi, amplification is a result of only the presence of colonic folds, crypts, and microvilli. Amplification is an effective means of increasing the surface area that is available for intestinal absorption, the primary function of the small and large intestine.
Table 44-1 Structural and Functional Differences Between the Small and the Large Intestine
|
Small Intestine |
Large Intestine |
Length (m) |
6 |
2.4 |
Area of apical plasma membrane (m2) |
~200 |
~25 |
Folds |
Yes |
Yes |
Villi |
Yes |
No |
Crypts or glands |
Yes |
Yes |
Microvilli |
Yes |
Yes |
Nutrient absorption |
Yes |
No |
Active Na+ absorption |
Yes |
Yes |
Active K+ secretion |
No |
Yes |
The fluid content of the average diet is typically 1.5 to 2.5 L/day. However, the fluid load to the small intestine is considerably greater—8 to 9 L/day. The difference between these two sets of figures is accounted for by salivary, gastric, pancreatic, and biliary secretions, as well as the secretions of the small intestine itself (Fig. 44-2). Similarly, the total quantity of electrolytes (Na+, K+, Cl−, and HCO−3) that enter the lumen of the small intestine also consists of dietary sources in addition to endogenous secretions from the salivary glands, stomach, pancreas, liver, and small intestine.
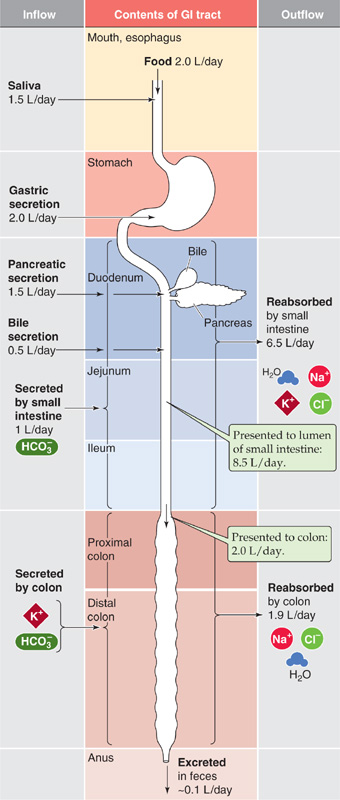
Figure 44-2 Fluid balance in the gastrointestinal (GI) tract. For each segment of the GI tract, the figure shows substances flowing into the lumen on the left and substances flowing out of the lumen on the right. Of the ~8.5 L/day presented to the small intestine, the small intestine removes ~6.5 L/day, delivering ~2 L/day to the colon. The large intestine removes ~1.9 L/day, leaving ~0.1 L/day in the feces.
We can calculate the absorption of water and electrolytes from the small intestine by comparing the total load that is presented to the lumen of the small intestine (i.e., 7.5 L/day entering from other organs +1.0 L/day secreted by the small intestine = 8.5 L/day) with that leaving the small intestine (i.e., ileocecal flow). The latter is ~2.0 L/day in normal subjects. Thus, overall small intestinal water absorption is 8.5 to 2.0, or ~6.5 L/day. Na+ absorption is ~600 mEq/day. Maximal small intestinal fluid absorption has not been directly determined but has been estimated to be as great as 15 to 20 L/day.
Colonic fluid absorption is the difference between ileocecal flow (~2.0 L/day) and stool water, which is usually less than 0.2 L/day (~0.1 L/day). Thus, colonic water absorption is ~2.0 to 0.1, or 1.9 L/day. In contrast, the maximal colonic water absorptive capacity is between 4 and 5 L/day. As a result, a significant increase in ileocecal flow (e.g., up to perhaps 5 L/day, as occurs with a decrease in small intestinal fluid absorption) will not exceed the absorptive capacity of the large intestine. Thus, a compensatory increase in colonic fluid absorption can prevent an increase in stool water (i.e., diarrhea) despite substantial decreases in fluid absorption by the small intestine.
Net ion movement represents the summation of several events. At the level of the entire small or large intestine, substantial movement of ions occurs from the intestinal lumen into the blood and from the blood into the lumen. The net ion movement across the entire epithelium is the difference between these two unidirectional fluxes.
Fluid and electrolyte transport in the intestine varies considerably in two different axes, both along the length of the intestines (segmental heterogeneity) and from the bottom of a crypt to the top of a villus or to the surface cells (crypt-villus/surface heterogeneity). A comparison of two different segments of intestine (e.g., duodenum versus ileum) shows that they differ substantially in function. These differences in function reflect segmental heterogeneity of ion transport processes along the longitudinal axis of the intestine in different macroscopic regions of both the small and the large intestine; these differences are both qualitative and quantitative. For example, HCO−3 stimulation of Na+ absorption occurs only in the proximal part of the small intestine. In contrast, the so-called electrogenic Na+ absorption (i.e., absorption associated with the development of a transepithelial potential difference) is restricted to the rectosigmoid segment of the colon.
Within an intestinal segment (e.g., a piece of ileum), crypt-villus/surface heterogeneity leads to differences in transport function along the radial axis of the intestine wall. For example, it is generally believed that absorptive function is located in villous cells in the small intestine (and surface epithelial cells in the large intestine), whereas secretory processes reside in the crypt cells. Finally, at a certain level within a single villus or crypt—or within a very small area of the colonic surface epithelium—individual cells may demonstrate further heterogeneity (cellular heterogeneity), with specific transport mechanisms restricted to different cells.
Overall ion movement in any segment of the intestine represents the summation of these various absorptive and secretory events. These events may be paracellular or transcellular, may occur in the villus or crypt, and may be mediated by a goblet cell or an absorptive cell.
Despite the segmental heterogeneity of small intestinal electrolyte transport, overall water and ion movement in the proximal and distal portions of the small intestine is similar: in health, the small intestine is a net absorber of water, Na+, Cl−, and K+, but it is a net secretor of HCO−3 (Fig. 44-2). Fluid absorption is isosmotic in the small intestine, similar to that observed in the renal proximal tubule (see Chapter 35). In general, absorptive processes in the small intestine are enhanced in the postprandial state. The human colon carries out net absorption of water, Na+, and Cl− with few exceptions, but it carries out net secretion of K+ and HCO−3.
As discussed in Chapter 5, intestinal epithelial cells are polar; that is, they have two very different membranes—an apical membrane and a basolateral membrane—separated from one another by tight junctions. The transport processes present in the small and large intestine are quite similar to those present in other epithelia, such as the renal tubules, with only some organ-specific specialization to distinguish them. The transepithelial movement of a solute across the entire epithelium can be either absorptive or secretory. In each case, the movement can be either transcellular or paracellular. In transcellular movement, the solute must cross the two cell membranes in series. In general, movement of the solute across at least one of these membranes must be active (i.e., against an electrochemical gradient). In paracellular movement, the solute moves passively between adjacent epithelial cells through the tight junctions.
All transcellular Na+ absorption is mediated by the Na-K pump (i.e., Na, K-ATPase) located at the basolateral membrane. This enzyme is responsible for Na+ extrusion across the basolateral membrane and results in a relatively low [Na+]i (~15 mM) and an intracellular-negative membrane potential. This Na+ gradient serves as the driving force, in large part, for Na+ entry into the epithelial cell across the luminal (apical) membrane, a process mediated either by Na+ channels or by Na+-coupled transporters (e.g., Na/glucose cotransport, Na-H exchange). The epithelial cell may also use this Na+ gradient to energize other transport processes at the apical or basolateral membrane.
Fluid movement is always coupled to active solute movement. The model of the osmotic coupling of fluid movement to solute movement in the intestine is similar to that in all or most epithelial cells (see Chapter 5). It is likely that the water movement occurs predominantly by a paracellular route rather than by a transcellular route. (See Note: Pathways of Intestinal Water Movement; Aquaporins in the Apical Membranes of the GI tract)
Solute movement is the driving force for fluid movement. However, the converse may also be true: solute movement may be coupled to fluid movement by solvent drag, a phenomenon in which the dissolved solute is swept along by bulk movement of the solvent (i.e., water). Solvent drag accounts for a significant fraction of the Na+ and urea absorbed in the human jejunum (but not in the more distal segments of the small intestine or the large intestine). For all intents and purposes, solvent drag occurs through the paracellular route, and it depends on the permeability properties of the tight junctions (reflection coefficient; see Chapter 20) and the magnitude of the convective water flow. Thus, solvent drag contributes primarily to the absorption of relatively small, water-soluble molecules, such as urea and Na+, and it does so mainly in epithelia with relatively high permeability. The transepithelial permeability of the jejunum is considerably greater than that of the ileum or colon, as evidenced by its lower spontaneous transepithelial voltage difference (VTE), higher passive movement of NaCl, and larger apparent pore size.
Epithelial permeability is an inverse function of transepithelial resistance. In epithelial structures such as the small and large intestine, transepithelial resistance is determined by cellular resistance and paracellular resistance, which are arranged in parallel (see Chapter 5). Paracellular resistance is considerably lower than transcellular resistance; therefore, overall mucosal resistance depends mainly on paracellular resistance, which, in turn, depends primarily on the properties of the tight junctions. Therefore, intestinal permeability is essentially a function of tight junction structure. Just as transport function varies greatly throughout the intestine, major differences in transepithelial permeability and the properties of tight junctions are also present throughout the intestinal tract. In general, resistance increases in the aboral direction (i.e., moving away from the mouth). Thus, the resistance of the jejunum is considerably lower than that of the distal end of the colon. Evidence also indicates that the permeability of the tight junctions in the crypt is greater than that in the villus.
Both the small intestine and the large intestine absorb large amounts of Na+ and Cl− daily, but different mechanisms are responsible for this extremely important physiological process in different segments of the intestine. The villous epithelial cells in the small intestine and the surface epithelial cells in the colon are responsible for absorbing most of the Na+. Absorption of Na+ is the result of a complex interplay of both apical and basolateral membrane transport processes. Figure 44-3 summarizes the four fundamental mechanisms by which Na+ may enter the cell across the apical membrane. In each case, the Na-K pump is responsible, at least in part, for the movement of Na+ from cell to blood. Also in each case, the driving force for apical Na+ entry is provided by the large, inwardly directed electrochemical gradient for Na+, which, in turn, is provided by the Na-K pump. The following four sections describe these four apical membrane transport processes.
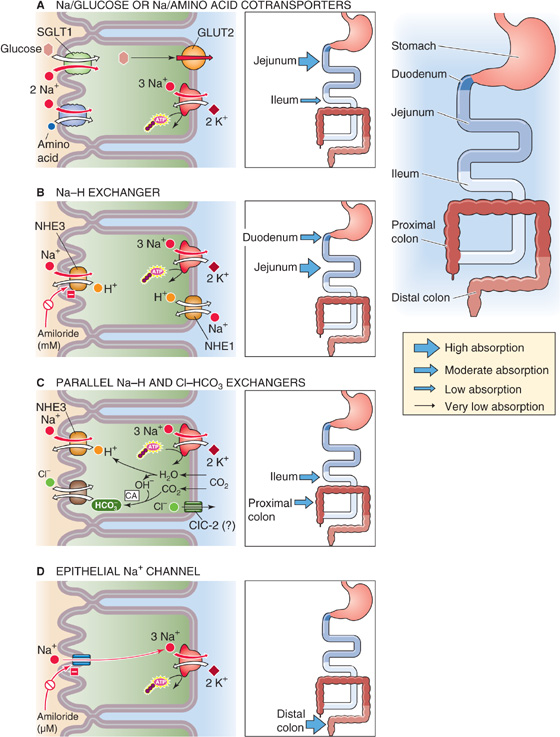
Figure 44-3 Modes of active Na+ absorption by the intestine. A, Nutrient-coupled Na+ absorption occurs in the villous cells of the jejunum and ileum and is the primary mechanism for postprandial Na+ absorption. The thickness of the arrows in the inset indicates the relative magnitude of the Na+ absorptive flux through this pathway. B, Electroneutral Na-H exchange at the apical membrane, in the absence of Cl-HCO3 exchange, is stimulated by the high pH of the HCO−3-rich luminal contents. C, Na-H and Cl-HCO3 exchange is coupled by a change in intracellular pH that results in electroneutral NaCl absorption, which is the primary mechanism for interdigestive Na+ absorption. D, In electrogenic Na+ absorption, the apical step of Na+ movement occurs through the ENaC. CA, carbonic anhydrase.
Nutrient-coupled Na+ absorption (Fig. 44-3A) occurs throughout the small intestine. Although glucose- and amino acid– coupled Na+ absorption also takes place in the colon of the newborn, it disappears during the neonatal period. Glucose- and amino acid–coupled Na+ absorption occurs only in villous epithelial cells and not in crypt epithelial cells (Fig. 44-3A). This process is the primary mechanism for Na+ absorption after a meal, but it makes little contribution during the interdigestive period, when only limited amounts of glucose and amino acids are present in the intestinal lumen.
Glucose-and amino acid–coupled Na+ absorption is mediated by specific apical membrane transport proteins. The Na/glucose cotransporter SGLT1 (see Chapter 5) is responsible for glucose uptake across the apical membrane, as discussed in Chapter 45. Several distinct Na/amino acid cotransporters, each specific for a different class of amino acids (see Table 36-1), are responsible for the Na+-coupled uptake of amino acids across the apical membrane. Because these transporters couple the energetically downhill movement of Na+ to the uphill movement of glucose or an amino acid, the transporter processes are examples of secondary active transport (see Chapter 5). The glucose-and amino acid–coupled uptake of Na+ entry across the apical membrane increases [Na+]i, which, in turn, increases Na+ extrusion across the basolateral membrane through the Na-K pump. Because the apical Na/glucose and Na/amino acid cotransporters are electrogenic, as is the Na-K pump, the overall transport of Na+ carries net charge and makes VTE more lumen negative. Thus, glucose-and amino acid–stimulated Na+ absorption is an electrogenic process. As discussed later, the increase in the lumen-negative VTE provides the driving force for the parallel absorption of Cl−.
Nutrient-coupled Na+ transporters, unlike other small intestinal Na+ absorptive mechanisms, are not inhibited by either cAMP or [Ca2+]i. Thus, agonists that increase [cAMP]i (i.e., Escherichia coli or cholera enterotoxin) or [Ca2+]i (i.e., serotonin) do not inhibit glucose-or amino acid–stimulated Na+ absorption.
Luminal HCO−3—the result of pancreatic, biliary, and duodenal secretion—increases Na+ absorption in the proximal portion of the small intestine by stimulating apical membrane Na-H exchange (Fig. 44-3B). The Na-H exchanger couples Na+ uptake across the apical membrane to proton extrusion into the intestinal lumen, a process that is enhanced by both decreases in intracellular pH (pHi) and increases in luminal pH. The energy for Na-H exchange comes from the Na+ gradient, a consequence of the ability of the Na-K pump to extrude Na+, thereby lowering [Na+]i. This process is characteristically inhibited by millimolar concentrations of the diuretic amiloride.
Several isoforms of the Na-H exchanger exist (see Chapter 5), and different isoforms are present on the apical and basolateral membranes. Intestinal epithelial cells also have Na-H exchangers on their basolateral membranes. However, this NHE1 isoform, like its counterpart in nonepithelial cells, regulates pHi (a “housekeeping” function) and does not contribute to the transepithelial movement of Na+. In contrast, both the NHE2 and NHE3 exchanger isoforms present on the apical membrane are responsible for both transepithelial Na+ movement and pHi regulation. Although Na-H exchangers are present on the apical membrane of villous epithelial cells throughout the entire intestine, only in the duodenum and jejunum (i.e., the proximal part of the small intestine) is Na-H exchange present without the parallel presence of Cl-HCO3 exchangers (see next section). Thus, in the proximal portion of the small intestine, the Na-H exchanger solely mediates the Na+ absorption that is stimulated by the alkalinity of the HCO−3-rich intraluminal contents.
Electroneutral NaCl absorption occurs in portions of both the small and large intestine (Fig. 44-3C). Electroneutral NaCl absorption is not the result of an Na/Cl cotransporter, but rather of parallel apical membrane Na-H and Cl-HCO3 exchangers that are closely linked by small changes in pHi. In the human colon, DRA (downregulated-in-adenoma; SLC26A3; see Chapter 5) mediates this Cl-HCO3 exchange. This mechanism of NaCl absorption is the primary method of Na+ absorption between meals (i.e., the interdigestive period), but it does not contribute greatly to postprandial Na+ absorption, which is mediated primarily by the nutrient-coupled transporters described previously. Electroneutral NaCl absorption occurs in the ileum and throughout the large intestine, with the exception of the most distal segment. It is not affected by either luminal glucose or luminal HCO−3. However, aldosterone inhibits electroneutral NaCl absorption.
Oral Rehydration Solution
The therapeutic use of oral rehydration solution (ORS) provides an excellent demonstration of applied physiology. Many diarrheal illnesses (see the box titled Secretory Diarrhea) are caused by bacterial exotoxins that induce fluid and electrolyte secretion by the intestine. Hence such a toxin is referred to as an enterotoxin. Despite the massive toxin-induced fluid secretion, both intestinal morphology and nutrient-coupled Na+ absorption are normal. Because nutrient-coupled (e.g., glucose or amino acid) fluid absorption is intact, therapeutically increasing the concentration of glucose or amino acids in the intestinal lumen can enhance absorption. ORS contains varying concentrations of glucose, Na+, Cl−, and HCO−3 and is extremely effective in enhancing fluid and electrolyte absorption in secretory diarrhea when the intestine secretes massive amounts of fluid. Administration of ORS can reverse the dehydration and metabolic acidosis that may occur in severe diarrhea and that are often the primary cause of morbidity and mortality, especially in children younger than 5 years. ORS is the major advance of the past half century in the treatment of diarrheal disease, especially in developing countries. The development of ORS was a direct consequence of research on the physiology of glucose- and amino acid–stimulated Na+ absorption.
The overall electroneutral NaCl absorptive process is regulated by both cAMP and cGMP, as well as by intracellular Ca2+. Increases in each of these three intracellular messengers reduce NaCl absorption. Conversely, decreases in [Ca2+]i increase NaCl absorption. Decreased NaCl absorption is important in the pathogenesis of most diarrheal disorders. For example, one of the common causes of traveler’s diarrhea (see the box titled Secretory Diarrhea) is the heat-labile enterotoxin produced by the bacterium E. coli. This toxin activates adenylyl cyclase and increases [cAMP]i, which, in turn, decreases NaCl absorption and stimulates active Cl− secretion, as discussed later. This toxin does not affect glucose-stimulated Na+ absorption.
In electrogenic Na+ absorption (Fig. 44-3D), Na+ entry across the apical membrane occurs through epithelial Na+ channels (ENaCs) that are highly specific for Na+ (see Chapter 5). Like the Na-H exchanger, these ENaCs are blocked by the diuretic amiloride, but at micromolar rather than millimolar concentrations. Na+ absorption in the distal part of the colon is highly efficient. Because this segment of the colon is capable of absorbing Na+ against large concentration gradients, it plays an important role in Na+ conservation. Na+ movement through electrogenic Na+ absorption is not affected by luminal glucose or by HCO−3, nor is it regulated by cyclic nucleotides. However, it is markedly enhanced by mineralocorticoids (e.g., aldosterone).
Mineralocorticoids increase Na+ absorption in the colon—as in other aldosterone-responsive epithelia, notably the renal collecting duct (see Chapter 35)—through multiple mechanisms. Aldosterone increases electrogenic Na+ absorption by increasing Na+ entry through the apical Na+ channel and by stimulating activity of the Na-K pump. The increase in apical Na+ uptake can occur (1) rapidly (i.e., within seconds) as a consequence of an increase in the opening of apical Na+ channels, (2) more gradually (within minutes) because of the insertion of preformed Na+ channels from subapical epithelial vesicle pools into the apical membrane, or (3) very slowly (within hours) as a result of an increase in the synthesis of both new apical Na+ channels and Na-K pumps.
Cl− absorption occurs throughout the small and large intestine and is often closely linked to Na+ absorption. Cl− and Na+ absorption may be coupled through either an electrical potential difference or by pHi. However, sometimes no coupling takes place, and the route of Cl− movement may be either paracellular or transcellular.
Cl− absorption can be a purely passive process (Fig. 44-4A), driven by the electrochemical gradient for Cl− either across the tight junctions (paracellular route) or across the individual membranes of the epithelial cell (transcellular route). In either case, the driving force for Cl− absorption derives from either of the two electrogenic mechanisms of Na+ absorption described previously (namely, nutrient-coupled transport in the small intestine and the ENaCs in the distal end of the colon), which, in turn, are energized by the Na-K pump. This process is referred to as voltage-dependent Cl− absorption; it is not an active transport process.
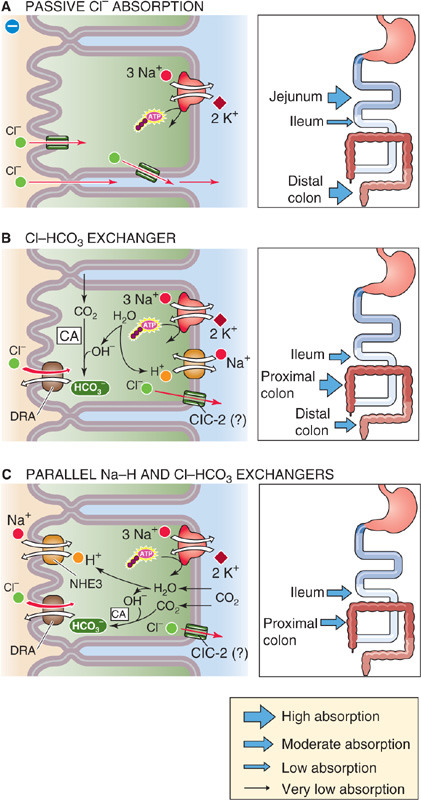
Figure 44-4 Modes of Cl− absorption by the intestine. A, In voltage-dependent Cl− absorption, Cl− may passively diffuse from lumen to blood across the tight junctions, driven by the lumen-negative transepithelial voltage (paracellular route). Alternatively, Cl− may diffuse through apical and basolateral Cl− channels. The thickness of the arrows in the inset indicates the relative magnitude of the Cl− absorptive flux through this pathway. B, In the absence of a parallel Na-H exchanger, electroneutral Cl-HCO3 exchange at the apical membrane results in Cl− absorption and HCO−3 secretion. C, Electroneutral NaCl absorption (see Fig. 44-3C) can mediate Cl− absorption in the interdigestive period. pHi couples the two exchangers. CA, carbonic anhydrase.
Within the small intestine, induction of a lumen-negative potential difference by glucose- and amino acid–induced Na+ absorption (Fig. 44-3A) provides the driving force for Cl− absorption that occurs following a meal. As noted earlier, nutrient-coupled Na+ absorption primarily represents a villous cell process that occurs in the postprandial period and is insensitive to cyclic nucleotides and changes in [Ca2+]i. Voltage-dependent Cl− absorption shares these properties. It is most likely that the route of voltage-dependent Cl− absorption is paracellular.
In the large intestine, especially in the distal segment, electrogenic Na+ absorption through the ENaC (Fig. 44-3D) also induces a lumen-negative potential difference that provides the driving force for colonic voltage-dependent Cl− absorption. Factors that increase or decrease the voltage difference similarly affect Cl− absorption.
Congenital Chloridorrhea
The congenital absence of an apical Cl-HCO3 exchanger (which mediates the Cl-HCO3 involved in electroneutral NaCl absorption) is an autosomal recessive disorder known as congenital chloridorrhea or congenital Cl− diarrhea (CLD). Affected children have diarrhea with an extremely high stool [Cl−], a direct consequence of absence of the apical membrane Cl-HCO3 exchanger. In addition, because HCO−3 secretion is reduced, patients are alkalotic (i.e., have an increased plasma [HCO−3]). The gene for congenital chloridorrhea is located on chromosome 7q31. The gene product is the same as that of the DRA gene. DRA (SLC26A3; see Chapter 5) •• and mediates Cl-HCO3 exchange. In addition, DRA transports sulfate and other anions. However, DRA is distinct from the AE (anion exchanger) gene family that encodes the Cl-HCO3 exchangers in erythrocytes and several other tissues. Indeed, Cl-HCO3 exchange in the renal tubule, erythrocytes, and other cells is unaffected in individuals with CLD, as are other intestinal transport processes.
Electroneutral Cl-HCO3 exchange, in the absence of parallel Na-H exchange, occurs in villous cells in the ileum and in surface epithelial cells in the large intestine (Fig. 44-4B). It is not known whether this process occurs in the cells lining the crypts. A Cl-HCO3 exchanger in the apical membrane is responsible for the 1 : 1 exchange of apical Cl− for intracellular HCO−3. In humans, this Cl-HCO3 exchanger is DRA (see Chapter 5). The details of Cl− movement across the basolateral membrane are not well understood, but the process may involve a ClC-2 Cl− channel (see Chapter 6).
Electroneutral NaCl absorption, discussed in connection with Na+ absorption (Fig. 44-3C), also mediates Cl− absorption in the ileum and proximal part of the colon (Fig. 44-4C). The apical step of Cl− absorption by this mechanism is mediated by parallel Na-H exchange (NHE3 or SLC9A3) and Cl-HCO3 exchange (DRA or SLC26A3), which are coupled through pHi.
In the previous three sections, we saw that intestinal Cl− absorption occurs through three mechanisms. The small intestine and the large intestine are also capable of active Cl− secretion, although Cl− secretion is believed to occur mainly in the crypts rather than in either the villi or surface cells. (See Note: Spatial Distribution of Cl− Secretion)
A small amount of Cl− secretion probably occurs in the “basal” state but is masked by the higher rate of the three Cl− absorptive processes that are discussed earlier in this subchapter. However, Cl− secretion is markedly stimulated by secretagogues such as acetylcholine and other neurotransmitters. Moreover, Cl− secretion is the major component of the ion transport events that occur during most clinical and experimental diarrheal disorders.
The cellular model of active Cl− secretion is outlined in Figure 44-5 and includes three transport pathways on the basolateral membrane: (1) an Na-K pump, (2) an Na/K/Cl cotransporter (NKCC1 or SLC12A2), and (3) two types of K+ channels (IK1 and BK). In addition, a Cl− channel (cystic fibrosis transmembrane regulator [CFTR]) is present on the apical membrane. This complex Cl− secretory system is energized by the Na-K pump, which generates a low [Na+]i and provides the driving force for Cl− entry across the basolateral membrane through Na/K/Cl cotransport. As a result, [Cl−]i is raised sufficiently that the Cl− electrochemical gradient favors the passive efflux of Cl− across the apical membrane. One consequence of these many transport processes is that the transepithelial voltage becomes more lumen negative, thereby promoting voltage-dependent Na+ secretion. This Na+ secretion that accompanies active Cl− secretion presumably occurs through the tight junctions (paracellular pathway). Thus, the net result is stimulation of NaCl and fluid secretion.
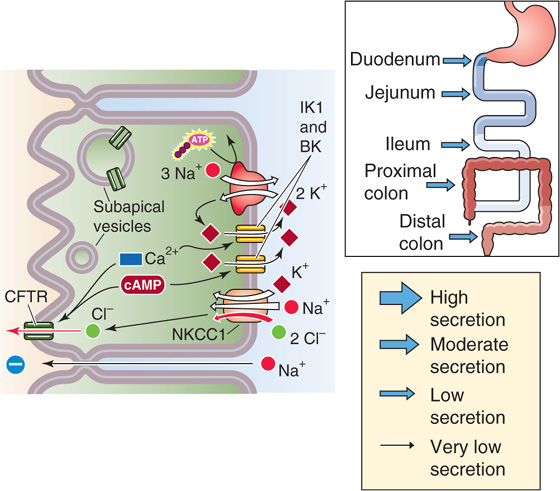
Figure 44-5 Cellular mechanism of electrogenic Cl− secretion by crypt cells. The basolateral Na/K/Cl cotransporter brings Cl− into the crypt cell; the Cl− exits across the apical Cl− channel. Secretagogues may open preexisting Cl− channels or may cause subapical vesicles to fuse with the apical membrane, thus delivering new Cl− channels. The paracellular pathway allows Na+ movement from blood to lumen, driven by the lumen-negative transepithelial voltage. The thickness of the arrows in the inset indicates that the magnitude of the Cl− secretory flux through this pathway is the same throughout the intestine.
Normally (i.e., in the unstimulated state), the crypts secrete little Cl− because the apical membrane Cl− channels are either closed or not present. Cl− secretion requires activation by cyclic nucleotides or [Ca2+], which are increased by any of several secretagogues, including (1) bacterial exotoxins (i.e., enterotoxins), (2) hormones and neurotransmitters, (3) products of cells of the immune system (e.g., histamine), and (4) laxatives (Table 44-2).
Table 44-2 Mode of Action of Secretagogues
Category |
Secretagogue |
Second Messenger |
Bacterial enterotoxins |
Cholera toxin |
cAMP |
|
Escherichia coli toxins: heat labile |
cAMP |
|
E. coli toxins: heat stable |
cGMP |
|
Yersinia toxin |
cGMP |
|
Clostridium difficile toxin |
Ca2+ |
Hormones and neurotransmitters |
VIP |
cAMP |
|
Guanylin |
cGMP |
|
Acetylcholine |
Ca2+ |
|
Bradykinin |
Ca2+ |
|
Serotonin (5-HT) |
Ca2+ |
Immune cell products |
Histamine |
cAMP |
Prostaglandins |
cAMP | |
Laxatives |
Bile acids |
Ca2+ |
|
Ricinoleic acid |
? |
Some secretagogues initially bind to membrane receptors and stimulate the activation of adenylyl cyclase (vasoactive intestinal peptide [VIP]), guanylyl cyclase (the heat-stable toxin of E. coli), or phospholipase C (acetylcholine). Others increase [Ca2+]i by opening Ca2+ channels at the basolateral membrane. The resulting activation of one or more protein kinases—by any of the aforementioned pathways—increases the Cl− conductance of the apical membrane either by activating preexisting Cl− channels or by inserting into the apical membrane Cl− channels that—in the unstimulated state—are stored in subapical membrane vesicles. In either case, Cl− is now able to exit the cell through apical Cl− channels. The resulting decrease in [Cl−]i leads to increased uptake of Na+, Cl−, and K+ across the basolateral membrane through the Na/K/Cl cotransporter (NKCC1). The Na+ is recycled out of the cell through the Na-K pump. The K+ is recycled through basolateral K+ channels that are opened by the same protein kinases that increase Cl− conductance. The net result of all these changes is the initiation of active Cl− secretion across the epithelial cell.
The induction of apical membrane Cl− channels is extremely important in the pathophysiology of many diarrheal disorders. The box titled Secretory Diarrhea discusses the changes in ion transport that occur in secretory diarrheas such as cholera. A central role in cystic fibrosis has been posited for the CFTR Cl− channel in the apical membrane (see Chapter 43). However, more than one (and possibly several) Cl− channels are present in the intestine, and CFTR may not be the only Cl− channel associated with active Cl− secretion.
The gastrointestinal tract participates in overall K+ balance, although when compared with the role of the kidneys, the small intestine and large intestine play relatively modest roles, especially in healthy individuals. The pattern of intestinal K+ movement parallels that of the kidney: (1) the intestines have the capacity for both K+ absorption and secretion, and (2) the intestines absorb K+ in the proximal segments but secrete it in the distal segments.
Dietary K+ furnishes 80 to 120 mmol/day, whereas stool K+ output is only ~10 mmol/day. The kidney is responsible for disposal of the remainder of the daily K+ intake (see Chapter 37). Substantial quantities of K+ are secreted in gastric, pancreatic, and biliary fluid. Therefore, the total K+ load presented to the small intestine is considerably greater than that represented by the diet. The concentration of K+ in stool is frequently more than 100 mM. This high stool [K+] is the result of several factors, including both colonic K+ secretion and water absorption, especially in the distal part of the colon.
Studies in which a plasma-like solution is perfused through segments of the intestine established that K+ is absorbed in the jejunum and ileum of the small intestine and is secreted in the large intestine. Although the small intestine absorbs substantial amounts of K+, no evidence has been presented to suggest that K+ absorption in the jejunum and ileum is an active transport process or even carrier mediated. Thus, K+ absorption in the small intestine is probably passive, most likely a result of solvent drag (i.e., pulled along by bulk water movement), as illustrated in Figure 44-6A. Although changes in dietary Na+ and K+ and alterations in hydration influence K+ movement in the colon, similar physiological events do not appear to affect K+ absorption in the small intestine.
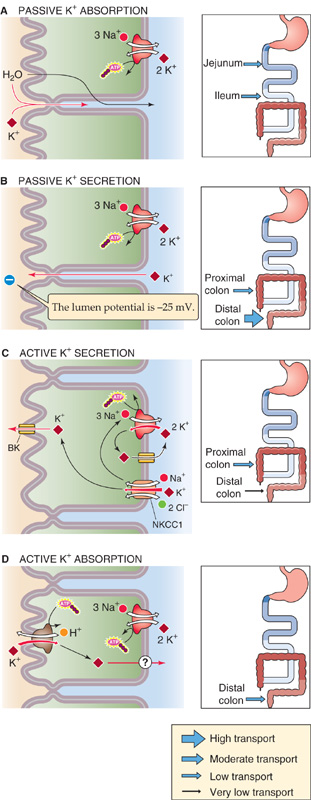
Figure 44-6 Cellular mechanisms of K+ secretion and absorption. A, This mechanism pertains only to the small intestine, which is a net absorber of K+ through solvent drag across tight junctions. The thickness of the arrows in the inset indicates the relative magnitude of the K+ flux through this pathway. B, The colon is a net secretor of K+. The primary mechanism is passive K+ secretion through tight junctions, which occurs throughout the colon. The driving force is a lumen-negative transepithelial voltage. C, Another mechanism of K+ secretion throughout the colon is a transcellular process that involves the basolateral uptake of K+ through the Na-K pump and the Na/K/Cl cotransporter, followed by the efflux of K+ through apical K+ channels. D, Confined to the distal colon is a transcellular mechanism of K+ absorption that is mediated by an apical H-K pump.
In contrast to the small intestine, the human colon is a net secretor of K+. This secretion occurs by two mechanisms: a passive transport process that is discussed in this section and an active process that is discussed in the next. Together, these two K+ secretory pathways are greater than a modest component of active K+ absorption in the distal part of the colon and thus account for the overall secretion of K+ by the colon.
Passive K+ secretion, which is the pathway that is primarily responsible for overall net colonic K+ secretion, is driven by the lumen-negative VTE of 15 to 25 mV The route of passive K+ secretion is predominantly paracellular, not transcellular (Fig. 44-6B). Because VTE is the primary determinant of passive K+ secretion, it is not surprising that passive K+ secretion is greatest in the distal end of the colon, where VTE difference is most negative. Similarly, increases in the lumen-negative VTE that occur as an adaptive response to dehydration—secondary to an elevation in aldosterone secretion (see the next section)—result in an enhanced rate of passive K+ secretion. Information is not available regarding the distribution of passive K+ secretion between surface epithelial and crypt cells.
In addition to passive K+ secretion, active K+ transport processes—both secretory and absorptive—are also present in the colon. However, active transport of K+ is subject to considerable segmental variation in the colon. Whereas active K+ secretion occurs throughout the colon, active K+ absorption is present only in the distal segments of the large intestine. Thus, in the rectosigmoid colon, active K+ absorption and active K+ secretion are both operative and appear to contribute to total body homeostasis.
The model of active K+ secretion in the colon is quite similar to that of active Cl− secretion (Fig. 44-5) and is also parallel to that of active K+ secretion in the renal distal nephron (see Chapter 37). The general paradigm of active K+ transport in the colon is a pump-leak model (Fig. 44-6C). Uptake of K+ across the basolateral membrane is a result of both the Na-K pump and the Na/K/Cl cotransporter (NKCC1), which is energized by the low [Na+]i that is created by the Na-K pump. Once K+ enters the cell across the basolateral membrane, it may exit either across the apical membrane (K+ secretion) or across the basolateral membrane (K+ recycling). The cell controls the extent to which secretion occurs, in part by K+ channels present in both the apical and the basolateral membranes. When apical K+ channel activity is less than basolateral channel activity, K+ recycling dominates. Indeed, in the basal state, the rate of active K+ secretion is low because the apical K+ channel activity is minimal in comparison with the K+ channel activity in the basolateral membrane.
It is likely that aldosterone stimulates active K+ secretion in surface epithelial cells of the large intestine, whereas cAMP enhances active K+ secretion in crypt cells. In both cases, the rate-limiting step is the apical BK K+ channel, and both secretagogues act by increasing K+ channel activity.
Aldosterone This mineralocorticoid enhances overall net K+ secretion by two mechanisms. First, it increases passive K+ secretion by increasing Na-K pump activity and thus increasing electrogenic Na+ absorption (Fig. 44-3D). The net effects are to increase the lumen-negative VTE and to enhance passive K+ secretion (Fig. 44-6B). Second, aldosterone stimulates active K+ secretion by increasing the activity of both apical K+ channels and basolateral Na-K pumps (Fig. 44-6C).
cAMP and Ca2+ VIP and cholera enterotoxin both increase [cAMP]i and thus stimulate K+ secretion. Increases in [Ca2+]i—induced, for example, by serotonin (or 5-hydroxytryptamine [5-HT])—also stimulate active K+ secretion. In contrast to aldosterone, neither of these second messengers has an effect on the Na-K pump; rather, they increase the activity of both the apical and the basolateral K+ channels. Because the stimulation of K+ channels is greater at the apical than at the basolateral membrane, the result is an increase in K+ exit from the epithelial cell across the apical membrane (i.e., secretion). Stimulation of K+ secretion by cAMP and Ca2+, both of which also induce active Cl− secretion (Fig. 44-5), contributes to the significant fecal K+ losses that occur in many diarrheal diseases.
As noted earlier, not only does the distal end of the colon actively secrete K+, but also it actively absorbs K+. The balance between the two processes plays a role in overall K+ homeostasis. Increases in dietary K+ enhance both passive and active K+ secretion (Fig. 44-6B, C). However, dietary K+ depletion enhances active K+ absorption (Fig. 44-6D). The mechanism of active K+ absorption appears to be an exchange of luminal K+ for intracellular H+ across the apical membrane, mediated by an H-K pump (see Chapter 5). The colonic H-K pump is ~60% identical at the amino acid level to both the Na-K pump and the gastric parietal cell H-K pump. Thus, colonic K+ movement through the active K+ absorption process occurs through a transcellular route, in contrast to the paracellular route that characterizes K+ absorption in the small intestine (Fig. 44-6A). The mechanism of K+ exit across the basolateral membrane may involve K/Cl cotransport. Not known is whether active K+ secretion (Fig. 44-6C) and active K+ absorption (Fig. 44-6D) occur in the same cell or in different cells.
Numerous chemical mediators from several different sources regulate intestinal electrolyte transport. Some of these agonists are important both in health and in diarrheal disorders, and at times only quantitative differences separate normal regulatory control from the pathophysiology of diarrhea. These mediators may function in one or more modes: neural, endocrine, paracrine, and perhaps autocrine (see Chapter 3). Most of these agonists (i.e., secretagogues) promote secretion, whereas some others (i.e., absorptagogues) enhance absorption.
The enteric nervous system (ENS), discussed in Chapters 14 and 41, is important in the normal regulation of intestinal epithelial electrolyte transport. Activation of enteric secretomotor neurons results in the release of acetylcholine from mucosal neurons and in the induction of active Cl− secretion (Fig. 44-5). Additional neurotransmitters, including VIP, 5-HT, and histamine, mediate ENS regulation of epithelial ion transport.
An example of regulation mediated by the endocrine system is the release of aldosterone from the adrenal cortex and the subsequent formation of angiotensin II; both dehydration and volume contraction stimulate this renin-angiotensin-aldosterone axis (see Chapter 40). Both angiotensin and aldosterone regulate total body Na+ homeostasis by stimulating Na+ absorption, angiotensin in the small intestine and aldosterone in the colon. Their effects on cellular Na+ absorption differ. In the small intestine, angiotensin enhances electroneutral NaCl absorption (Fig. 44-3C), probably by upregulating apical membrane Na-H exchange. In the colon, aldosterone stimulates electrogenic Na+ absorption (Fig. 44-3D).
The response of the intestine to angiotensin and aldosterone represents a classic endocrine feedback loop: dehydration results in increased levels of angiotensin and aldosterone, the primary effects of which are to stimulate fluid and Na+ absorption by both the renal tubules (see Chapter 35) and the intestines, thus restoring total body fluid and Na+ content.
Regulation of intestinal transport also occurs by paracrine effects. Endocrine cells constitute a small fraction of the total population of mucosal cells in the intestines. These endocrine cells contain several peptides and bioactive amines that are released in response to various stimuli. Relatively little is known about the biology of these cells, but gut distention can induce the release of one or more of these agonists (e.g., 5-HT). The effect of these agonists on adjacent surface epithelial cells represents a paracrine action.
Another example of paracrine regulation of intestinal fluid and electrolyte transport is the influence of immune cells in the lamina propria (Fig. 44-1). Table 44-3 presents these immune cells and a partial list of the agonists that they release. The same agonist may be released from more than one cell, and individual cells produce multiple agonists. These agonists may activate epithelial cells directly or may activate other immune cells or enteric neurons. For example, reactive oxygen radicals released by mast cells affect epithelial cell function by acting on enteric neurons and fibroblasts, and they also have direct action on surface and crypt epithelial cells.
Table 44-3 Products of Lamina Propria Cells That Affect Intestinal Ion Transport
Cell |
Product |
Macrophages |
Prostaglandins |
|
O2 radicals |
Mast cells |
Histamine |
Neutrophils |
Eicosanoids |
|
Platelet-activating factor |
Fibroblasts |
Eicosanoids |
|
Bradykinin |
A single agonist usually has multiple sites of action. For example, the histamine released from mast cells can induce fluid secretion as a result of its interaction with receptors on surface epithelial cells (Fig. 44-7). However, histamine can also activate ENS motor neurons, which can, in turn, alter epithelial cell ion transport, as well as intestinal smooth muscle tone and blood flow. As a consequence, the effects of histamine on intestinal ion transport are multiple and amplified.
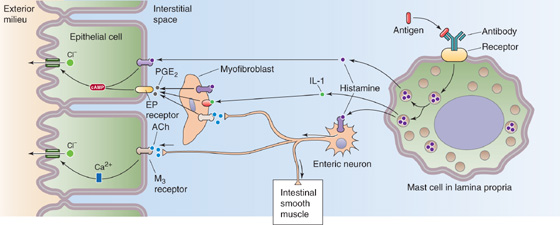
Figure 44-7 Mast cell activation. Activation of mast cells in the lamina propria triggers the release of histamine, which directly affects epithelial cells, or which stimulates an enteric neuron and thus has an indirect effect. The neuron modulates the epithelium (secretion), intestinal smooth muscle (motility), or vascular smooth muscle (blood flow). ACh, acetylcholine.
Several agonists induce the accumulation of fluid and electrolytes in the intestinal lumen (i.e., net secretion). These secretagogues are a diverse, heterogeneous group of compounds, but they can be effectively classified in two different ways: by the type of secretagogue and by the intracellular second messenger that these agonists activate.
Grouped according to type, the secretagogues fall into four categories: (1) bacterial exotoxins (i.e., enterotoxins), (2) hormones and neurotransmitters, (3) products of cells of the immune system, and (4) laxatives. Table 44-2 provides a partial list of these secretagogues. A bacterial exotoxin is a peptide that is produced and excreted by bacteria that can produce effects independently of the bacteria. An enterotoxin is an exotoxin that induces changes in intestinal fluid and electrolyte movement. For example, E. coli produces two distinct enterotoxins (the so-called heat-labile and heat-stable toxins) that induce fluid and electrolyte secretion through two distinct receptors and second-messenger systems.
We can also classify secretagogues according to the signal transduction system that they activate after binding to a specific membrane receptor. As summarized in Table 44-2, the second messengers of these signal transduction systems include cAMP, cGMP, and Ca2+. For example, the heat-labile toxin of E. coli binds to apical membrane receptors, becomes internalized, and then activates basolateral adenylyl cyclase. The resulting increase in [cAMP]i activates protein kinase A. VIP also acts by this route (Fig. 44-8). The heat-stable toxin of E. coli binds to and activates an apical receptor guanylyl cyclase, similar to the atrial natriuretic peptide (ANP) receptor (see Chapter 3). The newly produced cGMP activates protein kinase G and may also activate protein kinase A. The natural agonist for this pathway is guanylin, a 15–amino acid peptide secreted by mucosal cells of the small and large intestine. Still other secretory agonists (e.g., 5-HT) produce their effects by increasing [Ca2+]i and thus activating protein kinase C or Ca2+-calmodulin–dependent protein kinases. One way that secretagogues can increase [Ca2+]i is by stimulating phospholipase C, which leads to the production of inositol 1, 4, 5-triphosphate (IP3) and the release of Ca2+ from intracellular stores (see Chapter 3). Secretagogues can also increase [Ca2+]i by activating protein kinases, which may stimulate basolateral Ca2+ channels.
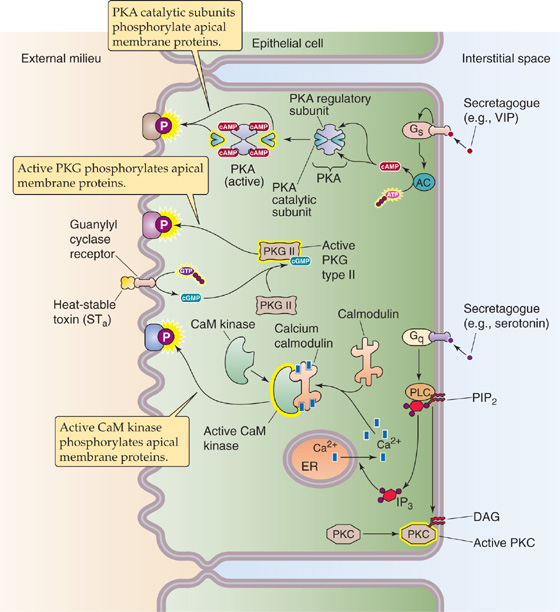
Figure 44-8 Action of secretagogues. Secretagogues (agents that stimulate the net secretion of fluid and electrolytes into the intestinal lumen) act by any of three mechanisms. Some (e.g., VIP, heat-labile toxin) activate adenylyl cyclase, which, in turn, generates cAMP and thus stimulates protein kinase A (PKA). Others (e.g., a heat-stable toxin, also known as STa) bind to the guanylin receptor, which is a receptor guanylyl cyclase that generates cGMP and results in the stimulation of protein kinase G (PKG). Others (e.g., serotonin) stimulate the phospholipase C (PLC) pathway, which leads to the generation of IP3 and diacylglycerol (DAG). The DAG activates protein kinase C (PKC). The increased [Ca2+]i stimulates PKC and Ca2+-calmodulin–dependent protein kinase (CaM kinase). These activated kinases stimulate net secretion by phosphorylating apical membrane transporters or other proteins. AC, adenylyl cyclase; Gq and Gs, a-subunit types of G proteins; PIP2, phosphatidylinositol 4, 5-biphosphate.
Although the secretagogues listed in Table 44-2 stimulate fluid and electrolyte secretion through one of three distinct second messengers (i.e., cAMP, cGMP, and Ca2+), the end effects are quite similar. As summarized in Table 44-4, all three second-messenger systems stimulate active Cl− secretion (Fig. 44-5) and inhibit electroneutral NaCl absorption (Fig. 44-3C). The abilities of cAMP and Ca2+ to stimulate Cl− secretion and to inhibit electroneutral NaCl absorption are almost identical. In contrast, cGMP’s ability to stimulate Cl− secretion is somewhat less, although its effects on electroneutral NaCl absorption are quantitatively similar to those of cAMP and Ca2+. Both stimulation of Cl− secretion and inhibition of electroneutral NaCl absorption have the same overall effect: net secretion of fluid and electrolytes. It is uncertain whether the observed decrease in electroneutral NaCl absorption is the result of inhibiting Na-H exchange, Cl-HCO3 exchange, or both, inasmuch as electroneutral NaCl absorption represents the coupling of separate Na-H and Cl-HCO3 exchange processes through pHi (Fig. 44-3C).
Table 44-4 End Effects of Second Messengers on Intestinal Transport
Second Messenger |
Increased Anion Secretion |
Inhibited NaCl Absorption |
cAMP |
+ + + |
+ + + |
cGMP |
+ |
+ + + |
Ca2+ |
+ + + |
+ + + |
Although multiple secretagogues exist, relatively few agonists can be found that enhance fluid and electrolyte absorption. The cellular effects of these absorptagogues are less well understood than those of the secretagogues. Those few absorptagogues that have been identified increase intestinal fluid and electrolyte absorption by either a paracrine or an endocrine mechanism.
Secretory Diarrhea
Diarrhea is a common medical problem and can be defined as a symptom (i.e., an increase in the number of bowel movements or a decrease in stool consistency) or as a sign (i.e., an increase in stool volume of more than 0.2 L/24 hours). Diarrhea has many causes and can be classified in various ways. One classification divides diarrheas by the causative factor. The causative factor can be a dietary nutrient that is not absorbed, in which case the result is osmotic diarrhea. An example of osmotic diarrhea is primary lactase deficiency. Alternatively, the causative factor may not be a dietary nutrient, but rather endogenous secretions of fluid and electrolytes from the intestine, in which case the result is secretory diarrhea.
The leading causes of secretory diarrhea include infections with E. coli (the major cause of traveler’s diarrhea) and cholera (a substantial cause of morbidity and mortality in developing countries). In these infectious diarrheas, an enterotoxin produced by one of many bacterial organisms raises [cAMP]i, [cGMP]i, or [Ca2+]i (see Table 44-2).
A second group of secretory diarrheas includes those produced by different, although relatively uncommon, hormone-producing tumors. Examples include tumors that produce VIP (the Verner-Morrison syndrome), glucagon (glucagonomas), and serotonin (the carcinoid syndrome). These secretagogues act by raising either [cAMP]i or [Ca2+]i (Table 44-2). When a tumor produces these secretagogues in abundance, the resulting diarrhea can be copious and explosive.
As we have seen, the secretory diarrheas have in common their ability to increase [cAMP]i, [cGMP]i, or [Ca2+]i. Table 44-4 summarizes the mechanisms by which these second messengers produce the secretory diarrhea. Because the second messengers do not alter the function of nutrient-coupled Na+ absorption, administration of an ORS containing glucose and Na+ is effective in the treatment of enterotoxin-mediated diarrhea (see the earlier box titled Oral Rehydration Solution).
Corticosteroids are the primary hormones that enhance intestinal fluid and electrolyte absorption. Mineralocorticoids (e.g., aldosterone) stimulate Na+ absorption and K+ secretion in the distal end of the colon; they do not affect ion transport in the small intestine. Their cellular actions are outlined in Chapter 50. Aldosterone induces both apical membrane Na+ channels (a process that is inhibited by the diuretic amiloride) and basolateral Na-K pumps; this action results in substantial enhancement of colonic electrogenic Na+ absorption. Although the effects of glucocorticoids on ion transport have most often been considered a result of crossover binding to the mineralocorticoid receptor (see Chapter 35), it is now evident that glucocorticoids also have potent actions on ion transport through their own receptor and that these changes in ion transport are distinct from those of the mineralocorticoids. Glucocorticoids stimulate electroneutral NaCl absorption (Fig. 44-3C) throughout the large and small intestine without any effect on either K+ secretion or electrogenic Na+ absorption. Both corticosteroids act, at least in part, by genomic mechanisms (see Chapter 4).
Other agonists appear to stimulate fluid and electrolyte absorption by stimulating electroneutral NaCl absorption and inhibiting electrogenic HCO−3 secretion; both these changes enhance fluid absorption. Among these absorptagogues are somatostatin, which is released from endocrine cells in the intestinal mucosa (see Chapter 42), and the enkephalins and norepinephrine, which are neurotransmitters of enteric neurons. The limited information available suggests that these agonists affect ion transport by decreasing [Ca2+]i, probably by blocking Ca2+ channels. Thus, it appears that fluctuations in [Ca2+]i regulate Na+ and Cl− transport in both the absorptive (low [Ca2+]i) and secretory (high [Ca2+]i) directions. Therefore, Ca2+ is clearly a critical modulator of intestinal ion transport.
Books and Reviews
Binder HJ, Sandle GI: Electrolyte transport in the mammalian colon. In Johnson LR (ed): Physiology of the Gastrointestinal Tract, 3rd ed, pp 2133-2172. New York: Raven Press, 1994.
Greger R, Bleich M, Leipziger J, et al: Regulation of ion transport in colonic crypts. News Physiol Sci 1997; 12:62-66.
Montrose MH, Keely SJ, Barrett KE: Electrolyte secretion and absorption: Small intestine and colon. In Yamada T (ed): Textbook of Gastroenterology, vol 1, 4th ed, Philadelphia: Lippincott Williams & Wilkins, 2003 308-340.
Palacin M, Estevez R, Bertran J, Zorzano A: Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev 1998; 78:969-1054.
Rao MC. Oral rehydration therapy: New explanations for an old remedy. Annu Rev Physiol 2004; 66: 385-417.
Zachos NC, Tse M, Donowitz M: Molecular physiology of intestinal Na/H exchange. Annu Rev Physiol 2005; 67: 411-443.
Journal Articles
Canessa CM, Horisberger J-D, Rossier BC: Epithelial sodium channel related to proteins involved in neurodegeneration. Nature 1993; 361:467-470.
Knickelbein RG, Aronson PS, Schron CM, et al: Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol 1985; 249:G236-G245.
Moseley RH, Hoglund P, Wu GD, et al: Downregulated in adenoma gene encodes a chloride transporter defective in congenital chloride diarrhea. Am J Physiol 1999; 276:G185-G192.
Schulz S, Green CK, Yuen PST, Garbers DL: Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 1990; 63:941-948.
Singh SK, Binder HJ, Boron WF, Geibel JP: Fluid absorption in isolated perfused colonic crypts. J Clin Invest 1995; 96:2373-2379.