Cardiovascular System and Lymphatic Vessels
Lisa M. Miller, John F. Van Vleet and Arnon Gal*
Structure
Development of the Heart and Great Vessels
The heart is a conical, muscular organ that in mammals has evolved into a four-chambered pump with four valves. During early fetal development, it is converted from an elongated muscular tube into a C-shaped structure by a process termed looping (Fig. 10-1). Subsequently, septation occurs to produce the right and left atrial and ventricular chambers and separation of the common truncus arteriosus into the aorta and pulmonary artery, respectively. The heart is interposed as a pump into the vascular system, with the right side supplying the pulmonary circulation and the left side the systemic circulation (Web Fig. 10-1; also see Chapter 2). The vascular system is subdivided into arterial, capillary, venous, and lymphatic segments. The arteries are classified into three types: elastic arteries, muscular arteries, and arterioles. The venous vessels are termed venules and veins. The lymphatic vasculature includes lymphatic capillaries and lymphatic vessels. Interposed between the arterial and venous segments are the capillary beds. A vascular segment termed the microcirculation (systemic capillary beds) includes arterioles, capillaries, and venules and is the major area of exchange between the circulating blood and the peripheral tissue (see Web Fig. 10-1; also see Chapter 2).
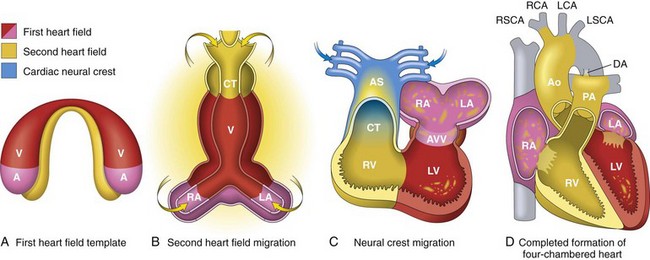
Fig. 10-1 Heart development, emphasizing the three sources of cells.
A, Day 15. First heart field (FHF) cells (shown in red) form a crescent shape in the anterior embryo with second heart field (SHF) cells (shown in yellow) near the FHF. B, Day 21. SHF cells lie dorsal to the straight heart tube and begin to migrate (arrows) into the anterior and posterior ends of the tube to form the right ventricle (RV), conotruncus (CT), and part of the atria (A). C, Day 28. Following rightward looping of the heart tube, cardiac neural crest cells (shown in blue) also migrate (arrow) into the outflow tract from the neural folds to septate the outflow tract and pattern the bilaterally symmetric aortic arch arteries (III, IV, and VI). D, Day 50. Septation of the ventricles, atria, and atrioventricular valves (AVV) results in the appropriately configured four-chambered heart. Ao, Aorta; AS, aortic sac; DA, ductus arteriosus; LA, left atrium; LCA, left carotid artery; LSCA, left subclavian artery; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RCA, right carotid artery; RSCA, right subclavian artery; V, ventricle. (Modified from Srivastava D: Making or breaking the heart: from lineage determination to morphogenesis, Cell 126:1037, 2006. In Kumar V, Abbas A, Fausto N et al: Robbins & Cotran pathologic basis of disease, ed. 8, Philadelphia, 2009, Saunders.)
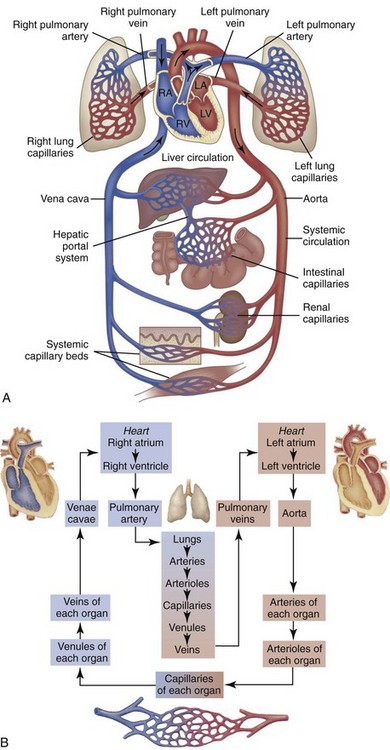
Web Fig. 10-1 Schematic diagram showing serially connected pulmonary and systemic circulatory systems and how to trace the flow of blood.
A, Right heart chambers propel unoxygenated blood through the pulmonary circulation, and the left heart propels oxygenated blood through the systemic circulation. B, The direction of blood flow begins at the left ventricle of the heart, flows to the arteries, arterioles, capillaries of each body organ, venules, veins, right atrium, right ventricle, pulmonary artery, lung capillaries, pulmonary veins, left atrium, and then goes back to the left ventricle. RA, Right atrium; RV, right ventricle; LA, left atrium, LV, left ventricle. (From McCance, K: Pathophysiology: The biologic basis for disease in adults and children, ed 6, St Louis, 2009, Mosby.)
Macroscopic Structure
The heart lies within a fibroelastic sac called the pericardium, and the wall of the heart is composed of three layers: the epicardium, the myocardium, and the endocardium (Fig. 10-2). Structurally, the heart contains in order of blood flow four major blood vessels (vena cava, pulmonary artery, pulmonary vein, and aorta), four chambers (right atrium/auricle, right ventricle, left atrium/auricle, and left ventricle), and four valves (tricuspid, pulmonic semilunar, mitral, and aortic semilunar) (Fig. 10-3).
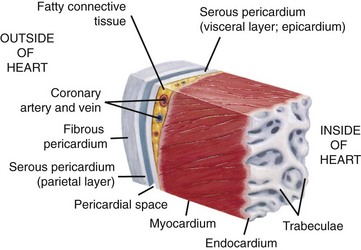
Fig. 10-2 Wall of the heart.
This section of the heart wall shows the fibrous pericardium, the parietal and visceral layers of the serous pericardium (with the pericardial space between them), the myocardium, and the endocardium. Note the fatty connective tissue between the visceral layer of the serous pericardium (epicardium) and the myocardium. Note also that the endocardium covers beamlike projections of myocardial muscle tissue called trabeculae. (From Huether SE, McCance KL: Understanding pathophysiology, ed 4, St Louis, 2007, Mosby.)
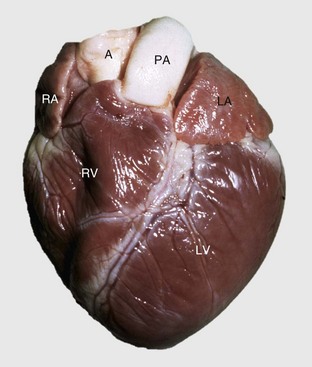
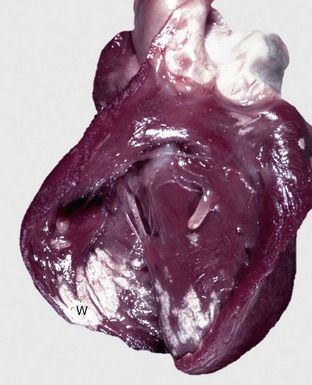
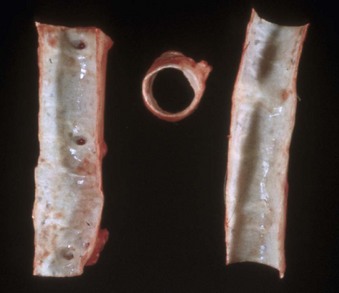
Fig. 10-3 Normal heart, pig.
A, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle. (Courtesy School of Veterinary Medicine, Purdue University.)
Pericardium and Epicardium
The pericardium, which normally contains a small amount of clear, serous fluid (see Fig. 10-2), is composed of an outer fibrous component and an inner serous layer, which form the sac surrounding the heart. The outer component is continuous with the mediastinal pleura. The base of the fibrous pericardium surrounds and blends with the adventitia of the greater arteries and veins exiting and entering the heart. The serous pericardium forms a closed sac surrounding the heart.
The epicardium (also known as visceral pericardium), the outermost layer of the heart, is continuous at the cardiac base with the parietal pericardium. The parietal pericardium is fused with the fibrous pericardium. The entire inner surface of the pericardial cavity is covered by mesothelium. The subepicardial layer is attached to the myocardium and consists of a thin layer of fibrous connective tissue, variable but generally abundant amounts (in well-nourished animals) of adipose tissue, and numerous blood vessels, lymphatic vessels, and nerves.
Myocardium
The myocardium is the muscular layer of the heart. It consists of cardiac muscle cells (cardiac myocytes [also known as cardiac rhabdomyocytes]) arranged in overlapping spiral patterns. These sheets of cells are anchored to the fibrous skeleton of the heart, which surrounds the atrioventricular valves and the origins of the aorta and pulmonary artery. The myocardial thickness is related to the pressure present in each chamber; thus the atria are thin walled and the ventricles are thicker. In adult animals, the thickness of the left ventricular free wall is approximately threefold that of the right ventricle, measured in a transverse section across the middle of the ventricles, because the pressure is greater in the systemic circulation than in the pulmonary circuit.
The arterial supply to the heart is the left and right coronary arteries, which arise from the aorta at the sinus of Valsalva behind the left and right cusps of the aortic valves. The arteries course over the heart in the subepicardium and give off perforating intramyocardial arteries that supply a rich capillary bed throughout the myocardium. Extensive anastomoses occur between the capillaries that tend to run parallel to the elongated cardiac muscle cells. The ratio of the area of capillaries to that of muscle cells is approximately 1 : 1, a fact evident when the myocardium is viewed histologically in cross-section.
Cardiac Conduction System
The heart is a muscular four chamber pump that simultaneously supplies blood to the pulmonary and systemic circulatory beds (see Web Fig. 10-1 and Fig. 2-1). Mechanical pumping is composed of sequential contraction (systole) and relaxation (diastole) that must be preceded by an electrophysiologic process that triggers a coordinated chronologic sequence of electrical events that result in muscle contractions. This electrophysiologic process is made possible by a network of special conducting fibers that are collectively referred to as the cardiac conduction system.
The cardiac conduction system is infrequently examined in animals because it is a labor-intensive process. Exceptions are cases with documented electrocardiographic alterations of undetermined origin. Components include (1) the sinoatrial node (SAN) at the junction of the cranial vena cava and the right atrium, (2) the atrioventricular node (AVN) located above the septal leaflet of the tricuspid valve and the atrioventricular (AV) bundle traversing the lower atrial septum onto the dorsal portion of the muscular interventricular septum, and (3) the right and left bundle branches that descend on each side of the muscular interventricular septum and eventually ramify in the ventricular myocardium as the Purkinje fiber network.
The major pacemaker of the cardiac conduction system is the SAN. This disk-shaped structure lies between the wall of the cranial vena cava and the external wall of the right auricular appendage. Four internodal pathways connect the SAN with the AVN. The AVN is present in the wall of the right atrium dorsal to the septal cusp of the tricuspid valve. For the atria to be electrically insulated from the ventricles, so that an unwarranted ectopic conduction wave will not activate the ventricles (or vice versa) and disrupt the synchronous events of the cardiac cycle, a fibrous cardiac skeleton composed of a layer of dense collagen (central fibrous body [CFB]), as well as occasional plates of chondroid and osseous metaplasia, separates the atrial from the ventricular myocardium. This skeleton forms two fibrous rings around the AV orifices and the aortic and pulmonic orifices. Conduction fibers arising from the AVN, known as the bundle of His or AV bundle, pierce through the CFB into the ventricles and continue along the subendocardium of the interventricular septum. The AV bundle then splits into the right and left bundle branches that further split and ramify into many other smaller branches that blend into the ventricular myocardium. Purkinje fibers constitute the AV bundle and downstream conduction pathways.
Endocardium and Heart Valves
The endocardium is the innermost layer of the heart and lines the chambers and extends over projecting structures such as the valves, chordae tendineae, and papillary muscles. The endocardium of the atria is thicker than that of the ventricles and thus normally appears white to gray on gross examination. The surface of the endocardium is endothelium that lies on a thin layer of vascularized connective tissue; the subendocardial layer contains blood vessels, nerves, and connective tissue. Purkinje fibers are distributed in the subendocardium throughout both ventricles. The heart valves (tricuspid valve [right AV valve], mitral valve [left AV valve], aortic valve, and pulmonary valve) are attached to fibrous rings and have thin avascular cusps. The valves open and close to regulate blood flow through the heart. During embryogenesis, endocardial cushions (mesenchymal tissue covered by endothelium) are precursors of the valve cusps. By remodeling, growth, and elongation, the cushions become thin mature cusps composed of connective tissue with an endothelial covering.
Blood and Lymphatic Vascular Systems
Blood Vessels: The aorta originates from the left ventricle and provides oxygenated blood to the entire body via arteries. In a treelike manner, arteries branch and become smaller arterioles as they approach capillary beds (see Web Fig. 10-1; also see Chapter 2). These beds and postcapillary venules provide the site for exchange of oxygen, carbon dioxide, nutrients, and waste. Small venules return the exchanged fluid and blood to larger veins and eventually the postcava and precava drain into the right atrium. The poorly oxygenated blood enters the pulmonary artery from the right ventricle. Oxygen exchange occurs in the capillaries of the lung, and oxygenated blood is returned to the heart via the pulmonary veins into the left atrium.
Lymphatic Vessels: Lymphatic vessels are thin walled, endothelial-lined channels that originate near the capillary beds and serve as a drainage system for returning interstitial tissue fluid and inflammatory cells to the blood. Afferent lymphatic vessels drain lymph into regional lymph nodes, which then filter and provide immunologic surveillance of the lymph, its cells, and the foreign matter it contains. The filtered lymph continues into larger efferent lymphatic vessels, which eventually drain into the caval blood via the thoracic duct. Both lymphatic vessels and veins have valves to prevent backflow of fluid. A more complete description can be found in Chapter 2.
Microscopic Structure
The pericardial sac is composed of parietal and visceral pericardium, both of which are covered by mesothelium. Beneath the visceral mesothelium is a thin layer of fibrous connective tissue, adipocytes, and vascular structures. This organization forms the subepicardium and interdigitates with the myocardium. The parietal pericardium is composed of an inner layer of mesothelium that interdigitates with the dense connective tissue forming the outer pericardial sac.
Myocardium
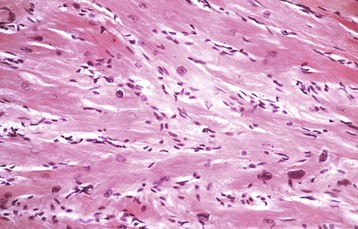
The myocardium consists of cardiac muscle cells surrounded by interstitial components that include blood and lymphatic vessels, nerves, and connective tissue cells, such as fibroblasts, histiocytes, mast cells, pericytes, primitive mesenchymal stem cells, and extracellular matrix elements of connective tissue, including collagen fibrils, elastic fibers, and acid mucopolysaccharides. Cardiac muscle cells can be divided into two populations: the contracting myocytes and the specialized fibers of the conduction system. The contracting myocyte is a cross-striated branching fiber of an irregular cylindric shape that measures 60 to 100 µm in length and 10 to 20 µm in diameter, with centrally located, elongated nuclei. Myocytes in young animals are smaller and have small amounts of sarcoplasm. Atrial myocytes are smaller than ventricular myocytes. Adjacent myocytes are joined end-to-end by specialized junctions known as intercalated disks and less frequently by side-to-side connections termed lateral junctions. Multinucleated fibers with nuclei arranged in central rows are frequently seen in hearts of growing pigs (Fig. 10-4). The myocytes of old animals commonly have large polyploid nuclei. The cytoplasm (sarcoplasm) of myocytes is largely occupied by the contractile proteins that are highly organized into sarcomeres, the repeating contractile units of the myofibril (see Figs. 15-3 and 15-8). Myofibrils are formed by end-to-end attachment of many sarcomeres. The cross-striated or banded appearance of myocytes is the result of sarcomere organization into A bands composed of myosin in the form of “thick” filaments (12 to 16 nm in diameter), I bands composed of actin in the form of “thin” filaments (5 to 8 nm in diameter), and dense Z bands at the end of each sarcomere. Thick and thin filaments interdigitate and provide the basis for the sliding mechanism of muscle contraction. Myocytes are enclosed by the sarcolemma, which consists of the plasma membrane and the covering basal lamina (external lamina). Other important components of cardiac muscle cells are generally only apparent in electron micrographs and include abundant mitochondria, a highly organized network of intracellular tubules termed the sarcoplasmic reticulum, cylindric invaginations of the plasma membrane called T tubules, ribosomes, cytoskeletal filaments, glycogen particles, lipid droplets, Golgi complexes, atrial granules (contain atrial natriuretic factor), lysosomes, and residual bodies (Web Fig. 10-2).
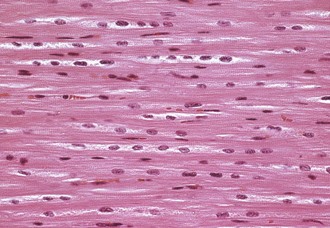
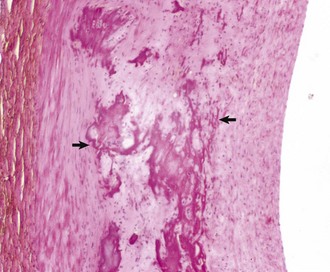
Fig. 10-4 Normal cardiac muscle.
Left ventricular myocardium, longitudinal section, normal young pig. The multiple nuclei in a myocyte are readily seen and evaluated in a longitudinal section. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
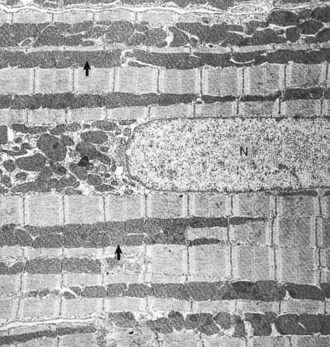
Web Fig. 10-2 Normal cardiac muscle.
Heart, left ventricular myocytes, longitudinal section, normal rat. Numerous dense mitochondria (arrows) lie between myofibrils, which have prominent bands. N, Nucleus. TEM. Uranyl acetate and lead citrate stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Cardiac Conduction System
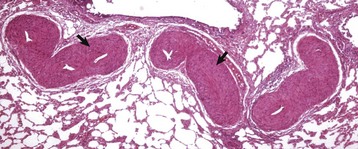
The morphologic features of the cardiac muscle cells that form specialized conduction tissues, including the SAN, AVN, AV bundle (bundle of His), and bundle branches, vary greatly at different sites and among animal species but generally are thin, branching nodal muscle cells with scarce myofibrils separated by highly vascularized connective tissue (Fig. 10-5; Web Fig. 10-3). Autonomic nerve fibers are contained within the SAN. The Purkinje fibers (cardiac conduction fibers) are distinguished by their large diameters (in horse, ox) and abundant pale eosinophilic sarcoplasm rich in glycogen and poor in myofibrils.
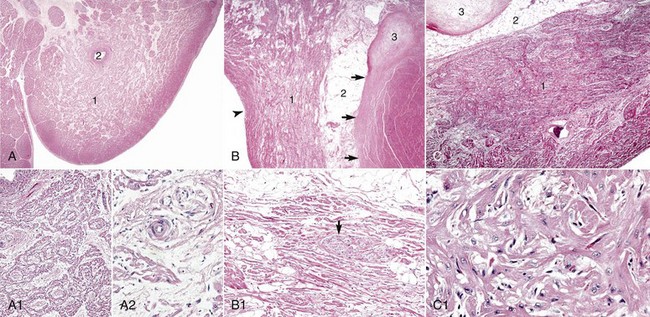
Fig. 10-5 Cardiac conduction system.
A, Sinoatrial (SA) node, foal. The center of the SA node (1) contains a nodal artery (2). H&E stain. A1, Higher magnification. Haphazardly oriented myofibers are embedded within abundant loose collagenous and elastic connective tissue. H&E stain. A2, Higher magnification. Nodal myofibers have discrete cell borders, a moderate amount of wavy sarcoplasm and an elongated nucleus. H&E stain. B, Atrioventricular (AV) node, goat. The AV node (1) is composed of interconnecting nodal myofibers that are supported by loose collagenous and elastic fibrous stroma. The node is embedded in adipose tissue (2). Note that in this illustration the AV node (1) is a poorly demarcated region (see B1 for greater detail) that is elongated (flattened) from top to bottom and that it and its surrounding adipose tissue are positioned adjacent to the cardiac fibrous skeleton (arrows) that has undergone focal chondroid metaplasia (3). The position and overall shape of the AV node in a histologic section is dependent upon the plane of section. Endocardium (arrowhead). H&E stain. B1, AV node, goat. In this higher magnification of B, AV nodal myofibers have a characteristic pale eosinophilic and thin sarcoplasm, with abundance of distinct striations and a short oval to elongated nucleus with dispersed chromatin. An autonomic myelinated nerve is present in the AV node (arrow). H&E stain. C, AV bundle, goat. The AV bundle (1) travels diagonally through the center of the figure from the lower left to the upper right margins. It is formed by an interweaving pseudosyncytium of cardiac myofibers supported by a loose to dense intervening collagenous stroma (see C1 for greater detail) and may be surrounded by adipose tissue (2). Cardiac cartilaginous skeleton (3). H&E stain. C1, Higher magnification. The AV bundle myofibers of the pseudosyncytium have moderate to large, pale eosinophilic sarcoplasm with prominent striations and large nuclei with fine stippled chromatin. H&E stain. (Courtesy Drs. A. Gal and J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
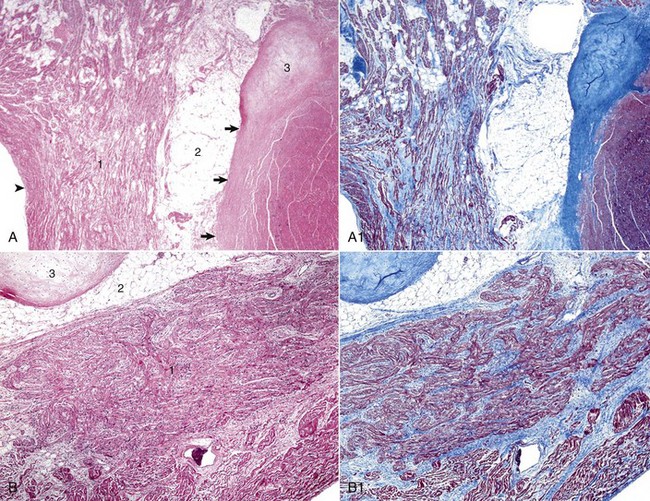
Web Fig. 10-3 Heart, fibrous stroma of the cardiac conduction system, goat.
A, Atrioventricular (AV) node. The AV node (1) is composed of interconnecting nodal myofibers that are supported by loose collagenous and elastic fibrous stroma. The node is embedded in adipose tissue (2) that is adjacent to the cardiac fibrous skeleton (arrows) that has undergone focal chondroid metaplasia (3). Endocardium (arrowhead). H&E stain. A1, AV node, goat. Serial section of A. Note the overall deposition of collagen fibers (blue) in the structures labeled 1 and 3 in Web Fig. 10-3, A. Masson’s trichrome stain. B, AV bundle. In this illustration, the AV bundle (1) travels diagonally through the center of the image from the lower left to the upper right margins. It is supported by a loose to dense intervening interstitial collagenous stroma and is surrounded by adipose tissue (2). Cardiac cartilaginous skeleton (3). H&E stain. B1, AV bundle, goat. Serial section of B. Note the overall deposition of collagen fibers (blue) in the structures labeled in Web Fig. 3, B. Masson’s trichrome stain. (Courtesy Drs. A. Gal and J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
Sinoatrial Node: The SAN is positioned adjacent to the epicardial adipose tissue and is often centered around a branch of the right coronary artery (Fig. 10-5, A). Several large autonomic nervous system ganglions can occasionally be seen clustering in the epicardium adjacent to the node. The SAN lacks discrete structure, and its ill-defined borders merge with the adjacent atrial wall. It structurally consists of a collection of haphazardly oriented myofibers that appear as a pseudosyncytium and are embedded within abundant loose collagenous and elastic connective tissue, with rare cores of epicardially oriented dense collagen fibers (Fig. 10-5, A1). The nodal myofibers have discrete cell borders, a moderate amount of wavy sarcoplasm with sparse myofibrils, and an elongated nucleus that contains clumps of coarse chromatin (Fig. 10-5, A2).
Atrioventricular Node, Atrioventricular Bundle, and Bundle Branches: The AVN lies within the right atrial subendocardium and consists of a discrete, compact to loose mass of interconnecting myofibers that are often embedded within adipose tissue. A small nodal artery, parasympathetic ganglia, and large myelinated autonomic nerves are often present adjacent to the AVN. The nodal myofibers that have characteristic pale eosinophilic and thin sarcoplasm generally run parallel to each other but occasionally have an interweaving pattern with intervening loose collagen fibers. These myofibers contain a moderate amount of sarcoplasm with abundance of distinct striations and a short oval to elongated nucleus with dispersed chromatin.
The AV bundle (Fig. 10-5, B) emerges from the cranial pole of the AVN (Fig. 10-5, B1) and pierces through the CFB, approximately at the level of the annuli of the aortic and mitral valves (Fig. 10-5, B2), to become the left and right bundle branches. The size of an AV bundle myofiber progressively enlarges and its cytomorphology transitions from a pale eosinophilic, small and thin (AVN-like morphology) myofiber to a pale eosinophilic, foamy to waxy, large, and somewhat rectangular myofiber that lacks cross striations (a Purkinje-like cellular morphology) (Fig. 10-5, C).
Autonomic Nervous System: The nerve supply to the heart is autonomic and includes sympathetic, parasympathetic, and nonadrenergic noncholinergic innervation. Histologically, large nerves can be seen in the epicardium and adjacent to the coronary blood vessels, whereas special staining techniques are required for demonstration of neural tissue elsewhere. Electron microscopy and immunohistochemistry allow differentiation between sympathetic and parasympathetic nerves that are otherwise indistinguishable with H&E stain. Preganglionic parasympathetic fibers pass to the heart through the cardiac branch of the vagal nerve and synapse with parasympathetic ganglionic neurons. Postganglionic neurons are distributed to the SAN and AVN, as well as to atrial and, to a much lesser extent, ventricular myocardium (however, the ventricular conduction system is well supplied by cholinergic innervation). Postganglionic sympathetic fibers arising from the cervicothoracic and middle cervical ganglia intensely innervate the SAN and AVN and to lesser extent the AV bundle. The atrial endocardium, myocardium, and epicardium are evenly innervated, whereas the ventricles are considerably less innervated with epicardium more densely populated by neural tissue than the endocardium.
Endocardium and Heart Valves
The endocardium, lining the atrium and ventricles, consists of a continuous endothelium, subendothelium, and subendocardium. The subendothelial layer contains dense irregular fibroblasts intermixed with collagen and elastic fibers and occasional smooth muscle cells. Elastic fibers are abundant within the subendocardium of the atria. The subendocardial layer contains vascular structures, elastic and collagen bands, and fibroblasts and is continuous with the myocardium. Purkinje fibers are located in the subendocardium. The heart valves are poorly vascularized, endocardial folds covered by endothelium. The subendothelial layer is composed of fibroblasts with abundant elastic and collagen fibers. The AV valves (AVVs) consist of a layer of stratum spongiosum and stratum fibrosum. The stratum spongiosum consists of loosely arranged fibroblasts with moderate amounts of collagen and elastin fibers and vascular structures. The stratum fibrosis contains fibroblasts and collagen, which are continuous with the annulus fibrosis and chordae tendineae.
Blood and Lymphatic Vascular Systems
The overall design of the blood and lymphatic vessels is similar, except that luminal diameter, wall thickness, and the presence of other anatomic features, such as valves, vary between the different segments. The luminal surface of all vessels is lined by longitudinally aligned endothelial cells covering a basal lamina. Vessel walls are divided into three layers or tunics: intima, media, and adventitia. However, some of the layers can be absent or all of the layers can be thinned in some segments of the vascular system, depending on the intravascular pressures. The large elastic arteries, such as the aorta, have (1) an intima composed of endothelium and subendothelial connective tissue, (2) a very thick tunica media composed of fenestrated elastic laminae with interposed smooth muscle cells and ground substance and bordered internally by the internal elastic lamina and externally by the external elastic lamina, and (3) an outer tunica adventitia layer composed of collagen and elastic fibers and connective tissue cells with penetrating blood vessels, termed the vasa vasorum, supplying nutrients to the adventitia and the outer half of the media. In muscular arteries and arterioles, the tunica media is composed largely of smooth muscle cells arranged in a circumferential pattern. Arterioles are the smallest arterial channels and are generally less than 100 µm in diameter and with one to three layers of smooth muscle cells in the tunica media.
Capillaries are 5 to 10 µm in diameter, and their endothelium is one of three types: (1) continuous, (2) fenestrated (as in the endocrine glands), or (3) porous (as in renal glomeruli). The endothelium rests on an external lamina surrounded by pericytes. Pericytes are located abluminally to capillaries and postcapillary venules and because of their location, contractility, and cytoskeletal proteins may play a role in regulating capillary and venular blood flow. Lesions of the endothelium might not be evident by light microscopy, and electron microscopy is required for characterization.
Veins have thin walls in relation to their luminal size when compared with those of arteries, in which blood pressure is greater. The adventitia is the thickest layer. Valves are present to prevent retrograde blood flow (i.e., away from the heart).
Lymphatic capillaries lack a basal lamina. Large lymphatic vessels are similar in structure to veins and generally have large lumina, thin walls, and intimal valves but contain lymph.
The morphology of large arteries, veins, microvasculature, and lymphatic vessels is described in Chapter 2 and is not discussed further in this chapter.
Necropsy Assessment of Heart and Vascular Structures
Information on this topic including Web Appendix 10-1 and Web Figs. 10-4 and 10-5 is available at evolve.elsevier.com/Zachary/McGavin/.
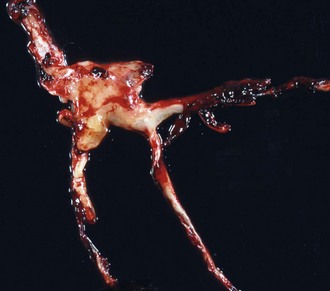
Web Fig. 10-4 Postmortem “chicken fat” arterial cast, dog.
Note how the clot conforms to the shape of the lumens of the vessels from which it was removed. Chicken fat clots consist primarily of clotted plasma and fibrin and other proteins of the coagulation cascade. They are often indicative of anemia; however, in all animals their formation by the separation of the red blood cells from the rest of the components of blood depends on the erythrocyte sedimentation rate (ESR). Separation can occur in all animals in response to systemic inflammation, which increases the ESR, but the horse normally has a high ESR because equine erythrocytes clump together in rouleau formation, which increases the ESR. Thus depending on the ESR, postmortem clots may be pale white to yellow (“chicken fat” clot) or shiny red (“currant jelly” clot) or sometimes a mixture. (Courtesy Dr. R.K. Myers, College of Veterinary Medicine, Iowa State University.)
Web Fig. 10-5 Focal hemorrhage and discoloration, intracardiac injection, euthanasia solution, left ventricle, dog.
Euthanasia solution is often injected into the left ventricle. In this case, solution was injected into the myocardium and caused a localized area of hemorrhage and discoloration. A mixture of euthanasia solution and blood often form a brownish sludge in the ventricle. (Courtesy College of Veterinary Medicine, University of Illinois.)
Examination of the Cardiovascular System and Lymphatic Vessels at Necropsy and Tissue Sampling for Histopathologic Evaluation
Information on this topic including Web Appendix 10-2 and Web Fig. 10-6 is available at evolve.elsevier.com/Zachary/McGavin/.
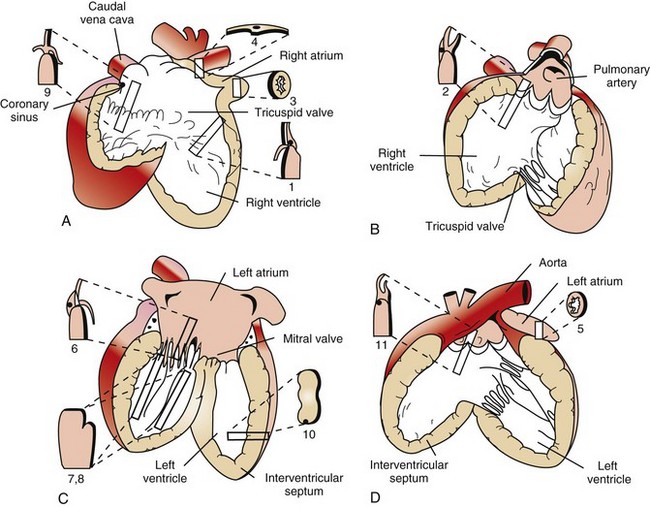
Web Fig. 10-6 Schematic diagram of the gross and microscopic examination of the heart.
Diagrams A to D illustrate the heart opened. The numbers indicate the area and the shape of the blocks of tissue removed for histopathology. A, Right ventricle and right atrium. B, Right ventricular cavity and pulmonary outflow tract. C, Left ventricle and left atrium. D, Left ventricle and aortic outflow tract. 1, Right ventricular free wall, atrioventricular valve, and atrium. 2, Pulmonic valve, right ventricular outflow tract, and pulmonary artery. 3, Right auricular appendage. 4, Sinoatrial node. 5, Left auricular appendage. 6, Left atrioventricular valve, ventricle, and atrium. 7 and 8, Left ventricular free wall and papillary muscles. 9, Atrioventricular node, right atrioventricular valve, and atrium. 10, Interventricular septum. 11, Aortic valve, left aortic outflow tract, and aorta. (From Bishop SP: Necropsy techniques for the heart and great vessels. In Fox P, Sisson D, Moise N, eds: Textbook of canine and feline cardiology, ed 2, Philadelphia, 1999, Saunders.)
Function
The primary function of the cardiovascular and lymphatic systems is to maintain an adequate and steady supply of nutrients to and facilitate the removal of waste products from all organs and tissues of the body. Cardiac myocytes provide the force of contraction; the conduction system and the nervous system control the flow and volume.
Pericardium and Epicardium
The pericardium contains a small amount of serous fluid, which allows frictionless cardiac movement of the mesothelial surfaces of the pericardium and epicardium on each other. The pericardial sac can adapt to changes in the heart size provided adequate time. The pericardium functions to provide a protective environment for cardiac function. Rapid, abnormal filling with blood (hemopericardium), fluid (hydropericardium), or exudate (pyopericardium) can result in compression of the heart (cardiac tamponade), particularly the large veins, right atrium, and right ventricle. Animals can survive without a pericardial sac.
Myocardium
The results of normal cardiac function include the maintenance of adequate blood flow, called cardiac output, to peripheral tissues that provide delivery of oxygen and nutrients, the removal of carbon dioxide and other metabolic waste products, the distribution of hormones and other cellular regulators, and the maintenance of adequate thermoregulation and glomerular filtration pressure (urine output). The normal heart has a threefold to fivefold functional reserve capacity, but this capacity can eventually be lost in cardiac disease and the result is impaired function.
Cardiac Pathophysiology: Myocardial Dysfunction
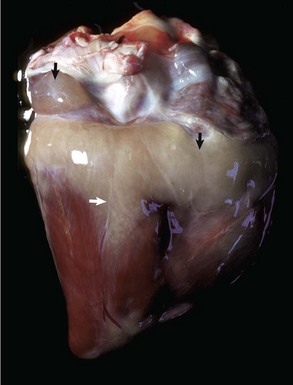
Cardiovascular dysfunction is the result of one or more of six basic pathophysiologic mechanisms (Box 10-1). Compensatory mechanisms operate in both normal and diseased hearts in an attempt to meet both the short- and long-term demands for adequate cardiac output. These mechanisms include cardiac dilation, myocardial hypertrophy, increase in heart rate, increase in peripheral resistance, increase in blood volume, and redistribution of blood flow. These compensatory mechanisms can maintain cardiac output that is adequate for some time, even in animals with severe cardiac disease sufficient to compromise cardiac function from loss of myocardial contractility, sustained pressure overload, or sustained volume overload. Cardiac dilation can occur as a terminal lesion in many cardiac diseases (Fig. 10-6, A). As a compensatory response to achieve increased cardiac output, dilation allows stretching of cardiac muscle cells to increase contractile force according to the Frank-Starling phenomenon and increased stroke volume is the result. However, stretching beyond certain limits decreases contractile strength. Myocardial hypertrophy is an important long-term compensatory response of the heart to maintain adequate cardiac output in the face of increased pressure or volume overload (see the discussion on the myocardium in the section on Responses to Injury) (Fig. 10-6, B).
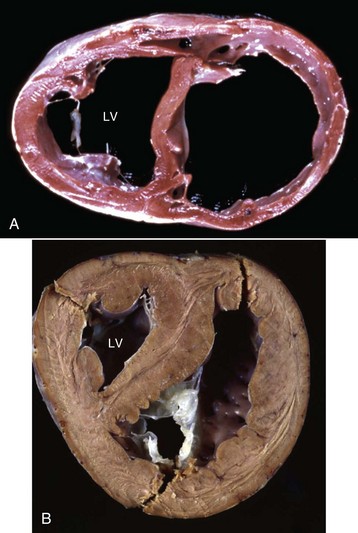
Fig. 10-6 Cardiac dilation and hypertrophy, heart, transected ventricles, dog.
A, Cardiac dilation. Note the thin walls of both dilated ventricles. LV, Left ventricle. B, Cardiac hypertrophy (fixed tissue). Note that the right ventricular and left ventricular (LV) walls are approximately the same thickness, indicating that there is right ventricular hypertrophy. (A courtesy Dr. Y. Niyo, College of Veterinary Medicine, Iowa State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia. B courtesy College of Veterinary Medicine, University of Florida; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Syndromes of Cardiac Failure or Decompensation
Cardiac Syncope
Cardiac syncope, an acute expression of cardiac disease, is characterized clinically by collapse, loss of consciousness, and extreme changes in heart rate and blood pressure, and with or without demonstrable lesions. Syncope can be caused by massive myocardial necrosis, ventricular fibrillation, heart block, arrhythmias, and reflex cardiac inhibition (e.g., that associated with high intestinal blockage).
Types of Heart Failure
A wide variety of experimental animal models of heart failure exist (see Web Table 10-1). The models have been used to develop an understanding of human cardiac diseases.
Congestive Heart Failure: Congestive heart failure usually develops slowly from gradual loss of cardiac pumping efficiency coupled with either pressure or volume overload or myocardial damage (Figs. 10-7 and 10-8; also see Figs. 8-29, 8-30, and 9-39). Pathophysiologically, congestive heart failure is initiated by development of cardiac disease (e.g., myocardial, valvular, congenital) or increased workload associated with pulmonary, renal, or vascular disease leading to loss of cardiac reserve and development of decreased blood flow to peripheral tissue (forward failure) and accumulation of blood behind the failing chamber (backward failure). Reduced renal blood flow creates hypoxia in the kidneys and increases renin release from the juxtaglomerular apparatus, resulting in stimulation of aldosterone release from the zona glomerulosa of the adrenal cortex. Sodium and water retention are the results of the action of aldosterone on the renal tubules; increased plasma volume follows, as does accumulation of edema fluid (mainly in body cavities). Hypoxia also stimulates increased erythropoiesis in bone marrow and extramedullary organs, such as the spleen, causing polycythemia and thus increased viscosity of the blood. The hypervolemia from aldosterone-induced water retention increases the workload on the already failing heart. Thus a vicious cycle of cardiac decompensation is initiated that eventually leads to death from cardiac failure unless therapeutic intervention occurs. Cardiac dilation, hypertrophy, and increased heart rate can provide some compensation for the increased workload.
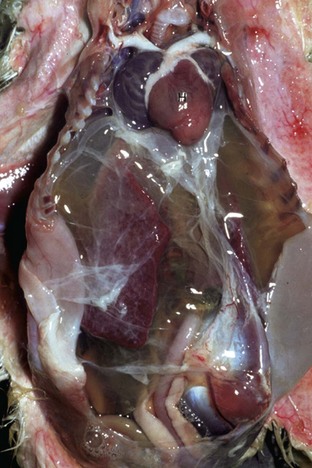
Fig. 10-7 Ascites, congestive heart failure, furazolidone cardiotoxicity, heart and liver, duckling.
Note prominent accumulations of serous fluid in the abdomen and fibrin deposits over the surface of the liver. The heart (H) is dilated. (Courtesy School of Veterinary Medicine, Purdue University.)
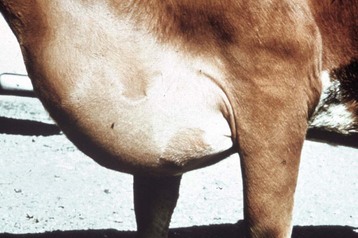
Fig. 10-8 Subcutaneous edema, high altitude disease with congestive heart failure (“brisket disease”), presternal, sternal, and caudal sternocephalic regions (brisket), cow.
The extensive subcutaneous edema is the result of chronic congestive heart failure. (Courtesy School of Veterinary Medicine, Purdue University.)
Acute and Chronic Left-Sided Heart Failure: Acute left-sided heart failure is manifested by pulmonary congestion and edema, whereas chronic left-sided heart failure is manifested by chronic passive pulmonary congestion, chronic pulmonary edema, hemosiderosis (“heart failure cells”; see Fig. 9-39), and fibrosis. The most common causes are (1) myocardial contractility loss associated with myocarditis, myocardial necrosis, or cardiomyopathy; (2) dysfunction of the mitral or aortic valves; and (3) several congenital heart diseases.
Acute and Chronic Right-Sided Heart Failure: Acute right-sided heart failure results in acute passive congestion (see Fig. 2-35) leading to hepatomegaly and splenomegaly (see Figs. 2-35 and 13-50, A), whereas chronic right-sided heart failure (see Fig. 2-36) results in hepatic congestion (nutmeg liver) (see Fig. 8-30) and more severe sodium and water retention than in left-sided heart failure. Edema is evident predominantly as ventral subcutaneous edema in horses and ruminants (see Fig. 10-8), ascites in dogs, and hydrothorax in cats. Causes of right-sided failure include (1) pulmonary hypertension, (2) cardiomyopathy, and (3) disease of the tricuspid and pulmonary valves. (See Chapters 8 and 9 for further details on hepatic and pulmonary lesions, respectively, associated with congestive heart failure.)
Clinical Diagnostic Procedures:
Information on this topic including Web Appendix 10-3 is available at evolve.elsevier.com/Zachary/McGavin/.
Cardiac Conduction System
Cells of the cardiac conduction system are modified cardiac myofibers that are able to spontaneously depolarize, which is also called autoexcitation, and function to (1) coordinate the sequence of events required for efficient ventricular filling during diastole and ejection during systole and (2) maintain the pressure in the pulmonary and systemic circuits (see Web Fig. 10-1).
Depolarization of the membrane of these pacemaker cells is due to a transiently increased rapid permeability to sodium ions and a slightly longer lasting increase in permeability to calcium ions that results in their influx into the myofiber sarcoplasm, thus changing the membrane potential (see Chapter 14). As this transient permeability is lost, membranal potassium channels open, and rapid outward potassium flux results in myofiber membranal hyperpolarization (along with sodium and calcium efflux), ultimately bringing the membrane potential back to a “normal” steady state (resting potential). During this period of time (refractory period), the myocyte cannot depolarize again because of a special conformation of membrane sodium channels that is transiently lost with depolarization and regained only with hyperpolarization. Intrinsically, the resting membrane potential of pacemaker cells is more positive than that of contracting cardiomyocytes and slightly more negative than the membrane threshold potential. This difference is due to leakiness of the pacemaker cell membrane to sodium and calcium ions and a steady low-grade influx of these ions.
Once one pacemaker cell depolarizes, a wave of depolarization propagates through the surrounding myocytes because these cells are connected to each other by special membranal pores (gap junctions), which allow for ionic exchange between adjacent cells. The number of gap junctions between cells of the conduction system is therefore a property that affects conduction velocity. Because the heart cycle must be strictly coordinated so that both atria and both ventricles contract and relax at the same time, and atrial contraction occurs simultaneous to the late ventricular relaxation, the depolarization wave has to be conducted fast at certain points along the conduction pathway and slower at others. Therefore, at different anatomic regions along the conduction pathway, the degree of cell-to-cell interaction dictates a slightly different cellular morphology.
When an electric signal (a propagating depolarization front) is generated in the SAN and spreads throughout the atria from cell to cell and eventually reaches the AVN, it is also conveyed through the specialized internodal conducting fibers. The conduction in these fibers is approximately three times faster than that of the atrial myofibers. By conveying the signal through these fibers, both atria can contract simultaneously and in a coordinated manner that allows the process of pushing of blood into the ventricles. Correlating with their special fast conducting function, these cells have a Purkinje-like morphology. The conduction velocity is then slowed down when propagating through the AVN. The time it takes to conduct the signal through the AVN and penetrating AV bundles is approximately four times longer than the time it takes for it to be conducted from the SAN to the AVN. This delay in conduction serves to empty the atria from blood before the ventricles start to contract. It also contributes to the unidirectional blood flow between the atria and the ventricles, above and beyond the similar role played by the AVVs.
Finally, the signal is delivered through the AV bundle, bundle branches, and Purkinje fibers in a velocity approximately 150 times faster than that of the AVN. The bundle branches run along the subendocardium of the interventricular septum and free ventricular walls and give rise to Purkinje fibers that supply the myocardium in a subendocardial-to-epicardial direction. This organization allows for a rapid and synchronous contraction of both ventricles at an order that ultimately enables blood to be “squeezed” from apex-to-base, toward both outflow tracts.
Cardiac myofibers have a unique property of intrinsic coupling of the electrical stimulation with mechanical contraction, which is fundamental for cardiac function. Diastole starts immediately at the end of ventricular contraction, as the cardiac muscle (myocardium) starts to relax. For a brief moment, relaxation leads to a rapid fall in ventricular pressure, without change in ventricular volume as the AVVs are still sealed off (isovolumetric relaxation). Because of the fall in ventricular pressure, the blood that has pooled in the atria during systole, along with incoming blood that constantly flows from systemic and pulmonary veins through the atria, pushes the AVV open and rapidly and passively fills the ventricles (rapid-filling phase). Next, because of the resultant pressure rise in the ventricles, the flow of blood that continues to enter the ventricles through the atria abruptly ends (diastasis phase). The last phase of diastole is active contraction of the atria, which pushes blood into the (now somewhat less compliant) ventricles and further raises their pressure (“atrial kick”).
In systole, the myocardium contracts, leading to a rapid increase in intraventricular pressure. Since the aortic and pulmonary valves are shut during diastole, the sudden rise in pressure leads to closure of the AVVs. In this brief period, which is termed isovolumetric contraction, the change in ventricular pressure has not led to a change in ventricular volume, since the blood had not yet been ejected into the aorta and pulmonary arteries. When the pressure in the ventricles exceeds the pressure in the great arteries, the aortic and pulmonary valves (semilunar valves) open and the ejection of the blood through the right and left side outflow tracts ensues (ejection phase). Simultaneously with the ejection phase, the atria relax, atrial pressure falls, and blood enters the atria and pools within it passively.
An additional level of complexity is brought about by the innervation of the autonomous nervous system (ANS). In general, the ANS influences heart rate (chronotropy), alters the rate of conduction (dromotropy), and controls myocardial contractility (inotropy) and the rate of mechanical relaxation (lusitropy). Parasympathetic postganglionic nerve terminals secrete acetylcholine that affects muscarinic (M2) receptors, whereas sympathetic postganglionic nerve terminals secrete norepinephrine that predominantly acts on the β1-adrenergic receptors. The latter, when stimulated by catecholamines, lead to a chain of intracellular events that increases calcium influx, increases the magnitude of potassium and chloride repolarization, and shortens the refractory period. Therefore adrenergic agonists are said to be positive chronotropes, inotropes, dromotropes, and lusitropes, whereas parasympathetic agonists have the opposite effects. Heart rate is primarily regulated by opposing effects of adrenergic and cholinergic nerve terminals on the SAN and at the same time by modulation of conduction velocity of the AVN and AV bundle. Physiologically, the force of contraction represents the sum of interactions between myocardial contractility, which is positively modulated by adrenergic nerve terminals that act on β1 receptors on ventricular myocardial myofibers (positive inotropic effect), and by the volume of blood that is present in the ventricles just before contraction (preload), as well as by the resistance in front of which contraction actually takes place (afterload).
Endocardium and Heart Valves
The endocardium lines the myocardium and contains Purkinje nerve fibers, which transmit a rhythmic action potential throughout the myocardium leading to contraction. The endocardium is lined by endothelial cells, which modulate many aspects of normal hemostasis. In normal states, the endothelial cells are antithrombotic preventing circulating cells from attaching and thus allowing normal flow of blood through the heart and blood vessels. The endocardium is continuous with the endothelium of blood and lymphatic vessels. Normal flow of blood through the heart depends on functional valves (see Chapter 2). Properly functioning valves serve as one-way valves, allowing blood to flow either from one chamber to another (AVVs) or exiting from the heart to either the pulmonary circulation (pulmonic valve) or the systemic circulation (aortic valve).
Blood and Lymphatic Vascular Systems
Blood and lymphatic vessels have several important functions. Blood vessels regulate the differential distribution of blood flow to tissues. Blood vessels actively synthesize and secrete vasoactive substances that regulate vascular tone and antithrombotic substances, which maintain the fluidity of the blood. Blood and lymphatic vessels play an important role in transporting and controlling inflammation and thrombosis. Blood and lymphatic vessels also constitute an important pathway for disease dissemination through transport of bacteria and tumor cells to distant sites.
Portals of Entry
Routes used to enter the cardiovascular system and lymphatic vessels are numerous and listed in Box 10-2. Toxic chemicals and pathogenic organisms can enter via ingestion, inhalation, cutaneous contact, trauma, or iatrogenic injection and gain access to the cardiovascular system. Microorganisms and toxins penetrate and enter deeper tissues, dermis, lamina propria, subcutis, or submucosa, triggering an acute inflammatory reaction. All three of the major components of acute inflammation may be responsible for entry of the toxin or organism into the vascular or lymphatic system. The increase in the vascular caliber increasing blood flow increases the number of capillary beds exposed to the agent. Changes in the microvasculature that allow the exit of plasma proteins and leukocytes also increase the entry of an agent. Finally, the increase in leukocytes can result in vascular injury and phagocytosis of material. Organisms that are not diluted, but are denatured by molecules from lysosomes of neutrophils, or restricted in movement by being trapped in fibrin at the site of inflammation can gain entry into the lymphatic vessels, thin-walled capillaries, or venules. Entry into lymphatic vessels allows invading microorganisms to be carried in lymph to draining regional and systemic lymph nodes and eventually via the thoracic duct to the circulatory system. Microorganisms that gain access to veins spread with the circulation and can localize in the lungs. In occurrences in which severe pulmonary inflammation leads to the formation of AV fistulas, microorganisms can gain access to pulmonary veins, be pumped through the left heart, and enter the systemic arterial circulation. The circulatory system can distribute organisms and materials to other organs and tissues (see Web Fig. 10-1, also see Chapter 2).
Epicardium and Pericardium
Bacteria and viral agents can enter the pericardial sac via endothelial damage to capillaries on visceral (epicardium) and parietal (pericardium) surface. Bacteria can enter by direct penetration. In cattle, foreign bodies exiting the reticulum and penetrating the diaphragm carry bacterial pathogens into the pericardial cavity. Direct entry from bacterial or viral infections in the pleura or mediastinum can also occur.
Myocardium
Pathogens gain entry to the myocardium through the vascular system from the coronary arteries, which provide blood flow to the myocardium. Coronary arteries originate in the sinus of Valsalva at the origin of the aorta and travel in the coronary grooves to the apex of the heart supplying blood to both ventricles. Branches of the coronary arteries bifurcate and send smaller arteries on the outer surface of the heart within the visceral pericardium. These smaller arteries then penetrate the myocardium, becoming arterioles and finally a rich network of capillaries in which there is nearly one vessel adjacent to each cardiac muscle cell. This rich network of capillaries provides an opportunity for bacteria or virus to gain entrance into the myocardium once they have entered the circulatory system. As a result many bacterial and viral infections may result in myocarditis. Bacteria within fibrin and inflammatory debris loosely attached to affected valves (bacterial valvular endocarditis) may detach and lodge in coronary arteries. This septic emboli damages endothelial cells and initiates acute inflammation resulting in myocarditis. Toxins or toxic byproducts can directly damage endothelial cells or diffuse through the endothelium to affect myocardial fibers. In addition, the myocardium is susceptible to direct extension of pathogens located within the endocardium or pericardium.
Endocardium and Heart Valves
The endocardium and the cardiac valves are in direct contact with any pathogen that enters the circulatory system, including parasites, bacterial and viral pathogens, and toxins. The endocardium, particularly the left atrial endocardium, is particularly susceptible to toxins resulting from renal failure in the dog.
Blood and Lymphatic Vascular Systems
The circulatory systems are intrinsically susceptible to microbial organisms and toxins because of the primary role of providing oxygen and nutrients to and removing waste from tissues. Hematogenous, lymphogenous dissemination of microbial pathogens and toxins directly exposes the corresponding vasculature to these hazards. Parasitic migration and local extension of an inflammatory process can directly result in entry into the circulatory or lymphatic system and directly damage these tissues.
Defense Mechanisms
Defense mechanisms used by the cardiovascular system and lymphatic vessels are listed in Box 10-3. These structures are fortunate in that most of the components of the innate and humoral immunity are present within the lumen. A review of inflammation (see Chapter 3) and circulatory disturbances (see Chapter 2) is invaluable in understanding the defense mechanisms of the cardiovascular and lymphatic systems. The constant flow of blood and lymph through intact circulatory and lymphatic systems, respectively, provides the surface endothelium of the chambers and vessels with constant exposure to nutrients, plasma proteins such as immunoglobulins, preformed chemical mediators, and circulating leukocytes.
Responses to Injury
Common responses of the cardiovascular system and lymphatic vessels to injury are listed in Box 10-4. The key characteristics of these responses are summarized next. The specific diseases that result from these responses are discussed in greater detail in the sections covering diseases that occur in all domestic animal species or are unique to one species.
Pericardium and Epicardium
Hemorrhage: Hemorrhage involving the pericardium and epicardium (Fig. 10-9; Web Fig. 10-7) results from stretching, tearing, lacerating, or crushing blood vessels in these structures and may be caused by penetrating wounds (foreign objects, bullets, or knives), lacerations from fractured bone, shear forces that stretch and ultimately tear vessels in tissues (blunt force trauma), and tears and rupture of tissue resulting from loss of structural integrity attributable to invasive and destructive properties of neoplasms such as hemangiosarcomas (see Chapter 2).
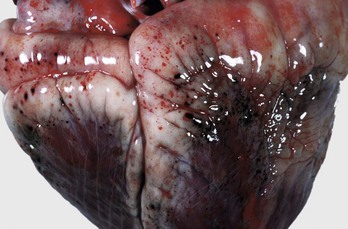
Fig. 10-9 Epicardial hemorrhage, petechiae and ecchymoses, endotoxemia, heart, cow.
Note the epicardial and subepicardial hemorrhages in the fat of the coronary groove (a common site). Petechiae and ecchymoses are often attributable to severe septicemia, endotoxemia, anoxia, or electrocution. In this case, the hemorrhage resulted from injury to the endothelium from endotoxin (component of the cell wall of Gram-negative bacteria). The smaller, pinpoint hemorrhages (1 to 2 mm) are petechiae. The larger hemorrhages (3 to 5 mm) are ecchymoses. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
Effusions: See the discussions on the mechanisms of edema formation and Fig. 2-6 in Chapter 2 and on the formation of transudates and exudates and Figure 3-3 in Chapter 3.
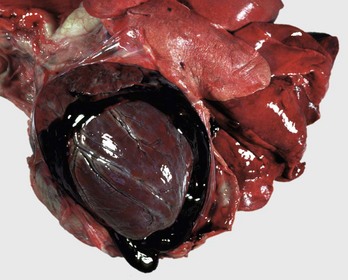
Hemopericardium: Hemopericardium is an accumulation of whole blood in the pericardial sac (Figs. 10-10 and 10-11; also see the section on Disorders of Domestic Animals). Death often occurs unexpectedly from cardiac tamponade, a condition in which the compression of the heart caused by the accumulation of blood in the pericardial sac leads to reduced cardiac output and poor perfusion of vascular beds in all organ systems. As examples, hemopericardium may be caused by blunt force trauma (impact with an automobile) or from rupture of the wall of the right atrium after invasion by a hemangiosarcoma.
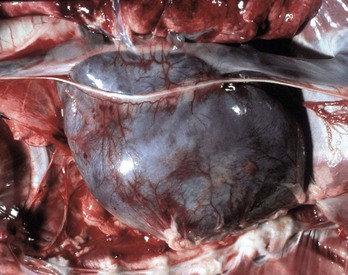
Fig. 10-10 Hemopericardium (cardiac tamponade), right atrium, hemangiosarcoma, heart, dog.
The pericardium is distended and dark blue because it contains whole blood secondary to rupture of an atrial hemangiosarcoma. Hemopericardium can cause death if it is sudden and is of sufficient volume to compress the heart and thus reduce cardiac output, a condition known as cardiac tamponade. On clinical examination, heart sounds are muffled. (Courtesy College of Veterinary Medicine, University of Illinois.)
Hydropericardium: Hydropericardium is the accumulation of clear, light yellow, watery, serous fluid (i.e., transudate) in the pericardial sac (Fig. 10-12; Web Fig. 10-8; also see the section on Disorders of Domestic Animals). As examples, hydropericardium can occur in animals with (1) hypoproteinemia (decreased colloid osmotic pressure) caused by liver disease or protein-losing nephropathy/enteropathy, (2) heart failure (increased hydrostatic pressure) where there is poor venous return to the heart, and (3) vascular injury, where damage to the barrier function of the vascular wall can result in leakage of small quantities of plasma proteins. In this latter example, a few fibrin strands can be present and the fluid could clot after exposure to air. The pericardial surfaces are smooth and glistening in acute cases, but in chronic cases, the epicardium becomes opaque because of mild fibrous thickening and can appear roughened and granular when there is villous proliferation of fibrous tissue, especially over the atria. The mechanisms involved in these fluid shifts are discussed in Chapters 2 and 3.
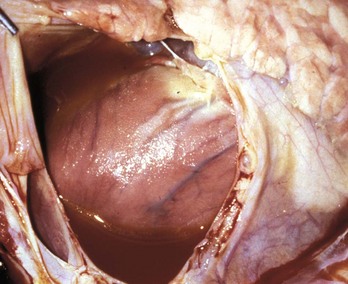
Fig. 10-12 Hydropericardium, pericardial sac, pig.
The thin-walled dilated pericardial sac contains serous fluid that has accumulated secondary to alterations in hydrostatic pressure between the pericardial cavity, circulatory system, and lymphatic system. (Courtesy College of Veterinary Medicine, University of Illinois.)
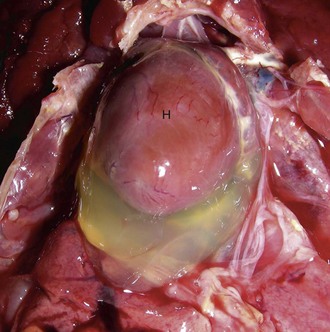
Web Fig. 10-8 Hydropericardium, chicken.
Note the accumulation of clear yellow fluid (transudate) in the pericardial cavity. This fluid may occur as a result of hypoproteinemia, heart failure, and vascular injury. If this accumulation is rapid, it compresses the heart (H) and great vessels, leading to a condition known as “cardiac tamponade,” which restricts venous return to the heart and reduces the end diastolic pumping volume of the heart. (Courtesy College of Veterinary Medicine, University of Illinois.)
Disturbances of Growth
Serous Atrophy: Serous atrophy of fat is readily identified by the gray gelatinous appearance of epicardial fat deposits (Fig. 10-13). Healthy animals normally have abundant white or yellow epicardial fat deposits, especially along the AV junction. Microscopically, lipocytes are atrophic and edema is present in the interstitial tissue. Serous atrophy of epicardial fat occurs rapidly during anorexia, starvation, or cachexia because fat is catabolized to maintain energy balance.
Pericardial Dilatation: The pericardium responds to excess fluid in the pericardial space by dilation. However, this outcome requires adequate time to allow adjustments in size. In hemopericardium, blood rapidly fills the pericardial cavity and death often occurs unexpectedly from cardiac tamponade, a condition with compression of the heart caused by blood accumulation leading to reduced cardiac output. Hydropericardium is the accumulation of clear, light yellow, watery, serous fluid (i.e., transudate) in the pericardial sac (see Fig. 10-12; see Web Fig. 10-8). In cases associated with vascular injury, a few fibrin strands are present, and the fluid could clot after exposure to air.
Cell Degeneration and Death
Epicardial Calcification: Epicardial calcification is a feature in hereditary calcinosis in mice and cardiomyopathy in hamsters (Web Fig. 10-9). Myocardial mineralization (discussed later) may be visible on the epicardial surface in vitamin E–selenium deficiency in sheep and cattle, vitamin D toxicity in several species, calcinogenic plant toxicosis in cattle (“Manchester wasting disease”), and spontaneous myocardial calcification in aged rats and guinea pigs.
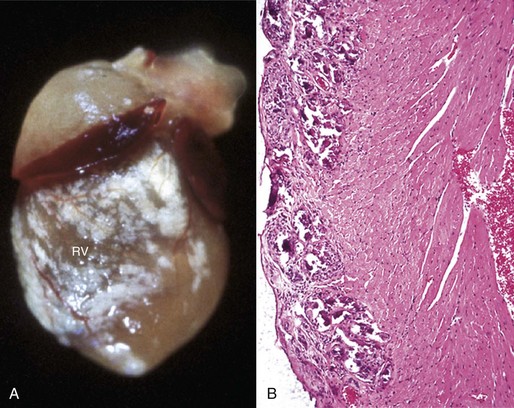
Web Fig. 10-9 Epicardial calcification, heart, right ventricle, mouse.
A, Note the prominent white mineral deposits over the right ventricle (RV). B, The basophilic mineral deposits are present epicardially and in the outer myocardium (left). H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Gout: Visceral gout has not been reported in domestic animals but does occur in birds and reptiles (Web Fig. 10-10; see Chapter 1).
Inflammation
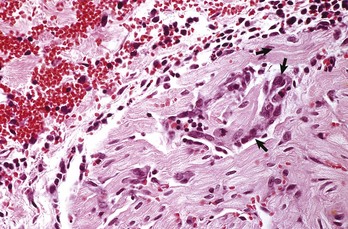
Pericarditis: Inflammation of the pericardium is frequently seen with bacterial septicemias and typically results in fibrinous pericarditis. Grossly, both the visceral and parietal pericardial surfaces are covered by variable amounts of yellow fibrin deposits, which can result in adherence between the parietal and visceral layers. When the pericardial sac is opened the attachments are torn away (so-called bread-and-butter heart) (Fig. 10-14). Microscopically, an eosinophilic layer of fibrin with admixed neutrophils lies over a congested epicardium (Fig. 10-15).
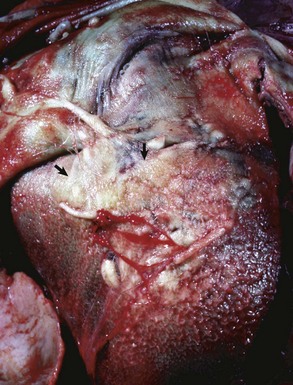
Fig. 10-14 Fibrinous pericarditis, heart, epicardium, horse.
The epicardium is covered dorsally by a thick, yellow layer of fibrin (arrows) and ventrally by granulation tissue (finely granular surface), thus indicating the chronicity of the inflammatory process. The apposing parietal pericardium (not shown) was also covered with fibrin. This lesion commonly occurs in horses with Streptococcus equi ssp. zooepidemicus septicemia causing vasculitis. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
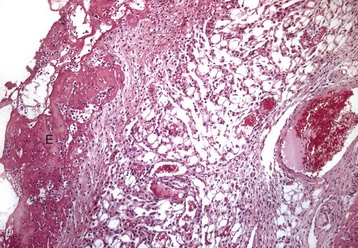
Fig. 10-15 Fibrinous pericarditis, heart, epicardium, pig.
Note eosinophilic fibrin deposits (E) (left) on the epicardial surface. This lesion commonly occurs with septicemias by bacteria that cause vasculitis. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
The outcome of fibrinous pericarditis varies. Early death is frequent because many of these lesions result from infection by highly virulent bacteria and concurrent septicemia. When survival is prolonged, adhesions form between the pericardial surfaces after fibrous organization of the exudate.
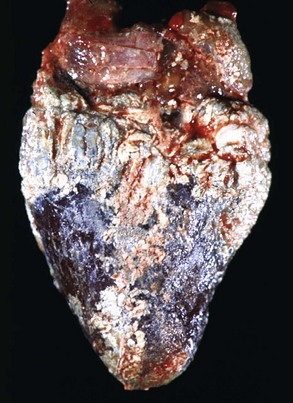
Suppurative pericarditis is seen mainly in cattle as a complication of traumatic reticuloperitonitis (“hardware disease”). Foreign bodies, such as nails or pieces of wire that accumulate in the reticulum, occasionally penetrate the reticular wall and diaphragm, enter the adjacent pericardial sac, and introduce infection. Some affected cattle survive for weeks to months until death ensues from congestive heart failure or septicemia. Grossly, the pericardial surfaces are notably thickened by white, often rough, shaggy-appearing masses of fibrous connective tissue that enclose an accumulation of white to gray, thick, foul-smelling, purulent exudate (Fig. 10-16).
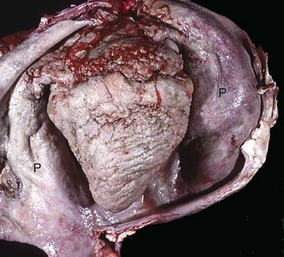
Fig. 10-16 Chronic suppurative (active) pericarditis, traumatic reticuloperitonitis (“hardware disease”), heart, pericardial sac (opened), cow.
The exposed epicardial and parietal surfaces are notably thickened by fibrous connective tissue and covered by a fibrinopurulent exudate. On clinical examination, heart sounds are muffled. P, Reflected parietal pericardium. (Courtesy Dr. J. King, College of Veterinary Medicine, Cornell University.)
Constrictive pericarditis is a chronic inflammatory lesion of the pericardium accompanied by extensive fibrous proliferation and eventual formation of fibrous adhesions between the surfaces of the visceral and parietal pericardium. The condition is seen in some cases of suppurative pericarditis in cattle and pigs with chronic fibrinous pericarditis. Severe lesions obliterate the pericardial sac and constrict the heart with fibrous tissue and can interfere with cardiac filling and thus cardiac output. Compensatory myocardial hypertrophy can result in diminished ventricular chamber volumes and contribute to the eventual development of heart failure.
Myocardium
The involvement of specific areas of the heart with major myocardial diseases is illustrated in Fig. 10-17.
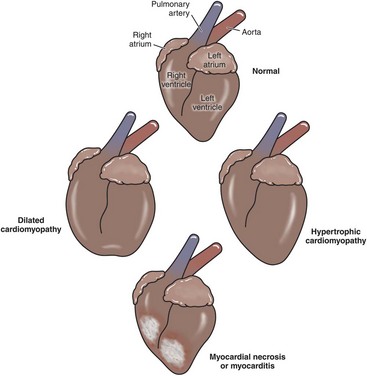
Fig. 10-17 Schematic diagram of the major myocardial diseases. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Disturbances of Circulation
Hemorrhage: Trauma (Physical Injury): See the discussion on hemorrhage in the section on Pericardium and Epicardium, Disturbances of Circulation, and the discussion on hemorrhage in Chapter 2.
Disturbances of Growth
See the discussion on hypertrophy in Chapter 1.
Hypertrophy: Hypertrophy of the myocardium represents an increase in muscle mass, which is the result of an increase in the size of cardiac muscle cells (Fig. 10-18; see Fig. 10-6, B). Hypertrophy is generally secondary and is the result of a compensatory response to increased workload; it is usually reversible on removal of the cause. However, primary hypertrophy also occurs, as in cats and dogs with idiopathic hypertrophic cardiomyopathy (see later discussion), and is not reversible. Two anatomic forms of hypertrophy are recognized. Eccentric hypertrophy results in a heart with enlarged ventricular chambers and walls of normal to somewhat decreased thickness; it is produced by lesions that increase blood volume load such as valvular insufficiencies and septal defects. In concentric hypertrophy the heart is characterized by small ventricular chambers and thick walls; it results from lesions that increase pressure load such as valvular stenosis, systemic hypertension, and pulmonary disease. Some cats with hyperthyroidism have a cardiac hypertrophy that is mediated by enhanced production of myocardial contractile proteins under the influence of increased concentration of circulating thyroid hormones (Fig. 10-19). The hypertrophy is reversible on return to euthyroidism.
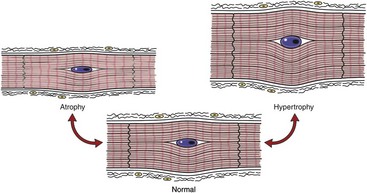
Fig. 10-18 Schematic diagram of cardiac muscle cells.
Growth disturbances of atrophy and hypertrophy. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
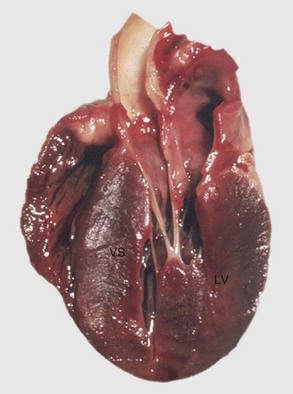
Fig. 10-19 Left ventricular hypertrophy, hyperthyroidism, heart, bisected, cat.
Note prominent thickening of the left ventricular (LV) free wall. The ventricular septum (VS) is also thickened. (Courtesy School of Veterinary Medicine, Purdue University.)
Stages of Myocardial Hypertrophy: Three stages of myocardial hypertrophy are recognizable: (1) initiation, (2) stable hyperfunction, and (3) deterioration of function associated with degeneration of hypertrophied myocytes. Microscopically, in myocardial hypertrophy, the myocytes are enlarged and have large nuclei (Fig. 10-20).
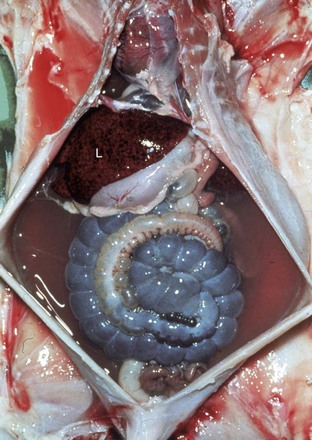
Right Ventricular Hypertrophy: Right ventricular hypertrophy is structurally or functionally caused by pulmonary stenosis or pulmonary hypertension, respectively. The former diseases include congenital pulmonic stenosis in dogs (see Fig. 10-59); the latter include dirofilariasis in dogs and cats (Fig. 10-21), “brisket disease” (“high-altitude disease”) in cattle (see Fig. 10-8), and chronic alveolar emphysema (“heaves”) in horses (see Fig. 9-53). Dirofilariasis (heartworm disease) may occur in 35% to 45% of dogs and 2% of cats in areas of high infection rates such as within 150 miles of the Atlantic and Gulf coasts from Texas to New Jersey and along the Mississippi River and its major tributaries. The extent of cardiac alterations is related to the numbers of adult parasites present. Initially, the parasites accumulate in the pulmonary arteries (see Figs. 10-21 and 10-90), and as the numbers increase, they are present in the right ventricle, then in the right atrium, and finally may occupy the vena cavae. Pulmonary hypertension results from vascular blockage and pulmonary vascular lesions produced by the parasites and right ventricular hypertrophy follows. Right-sided heart failure may eventually develop. Dogs with massive adult parasite loads may suffer acute collapse from caval syndrome, characterized by shock, intravascular hemolysis, and hepatic and renal failure. In “brisket disease,” cattle maintained under the hypoxic conditions that exist at altitudes above 7000 feet above sea level develop pulmonary hypertension and subsequent right-sided heart failure with subcutaneous edema, chronic passive congestion of the liver (nutmeg liver), and right ventricular hypertrophy. Exposure to certain poisonous plants (Oxytropis spp. and Astragalus spp.) increases the severity of the disease.
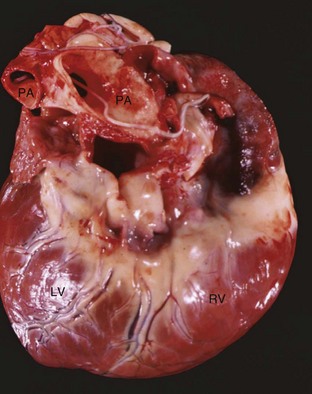
Fig. 10-21 Dirofilariasis, heart, dog.
Note the hypertrophy of the right ventricle (RV) and adult Dirofilaria immitis in the pulmonary artery and its branches (PA). LV, Left ventricle. (Courtesy Dr. K. Read, College of Veterinary Medicine, Texas A&M University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Left Ventricular Hypertrophy: Left ventricular hypertrophy occurs in dogs with congenital subaortic stenosis, in cats with hyperthyroidism, and in dogs and cats with systemic hypertension. Systemic hypertension tends to be associated with hyperthyroidism and chronic renal failure in cats, and with chronic renal failure, hyperadrenocorticism, and pheochromocytomas in dogs. Affected animals may have ocular lesions secondary to damage to retinal vessels.
Biventricular Hypertrophy: Biventricular hypertrophy can occur with hypertrophic cardiomyopathy and various congenital cardiac anomalies. Eccentric hypertrophy develops in the late stages of diseases that initially cause concentric hypertrophy as cardiac dilation is superimposed.
The appearance of cardiac hypertrophy and dilation is summarized in Fig. 10-22.
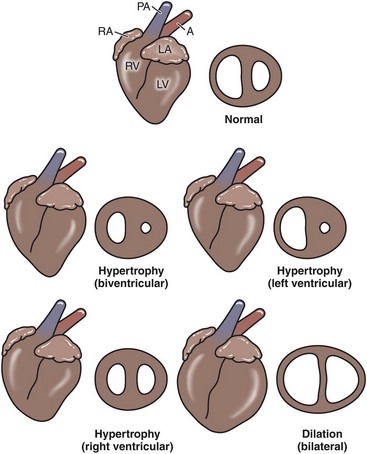
Fig. 10-22 Schematic diagram of the types of myocardial hypertrophy and dilation.
Left lateral view and midventricular cross section (not drawn to same scale). A, Aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Physiologic Atrophy: Physiologic atrophy of heart muscle may occur in confined animals and also occurs as a result of decompensation of cardiac myocytes in chronic congestive heart failure (see Fig. 10-6, A). Initially, these myocytes respond through hypertrophy with increased contractile force according to the Frank-Starling phenomenon. However, stretching beyond certain limits decreases contractile strength and eventually leads to loss of contractile proteins within these cells or loss of these cells resulting in atrophy of the affected myocardium (see Fig. 10-18).
Neoplastic Transformation: Rhabdomyomas and rhabdomyosarcomas are primary tumors that originate from the myocardium in domestic animals (see Chapter 6). Fibrosarcomas also occur rarely. Numerous types of secondary tumors, such as lymphosarcomas, metastasize to the myocardium and are discussed in this chapter and other chapters of this book. Hemangiosarcomas are tumors of blood vessels of the myocardium of the right atrium and are discussed later.
Cell Degeneration and Death
Histopathologic study of sections of the myocardium is substantially limited in respect to specific diagnoses and only rarely can an etiologic diagnosis be made from the morphologic alterations. This inadequacy exists because the spectrum of pathologic reactions is limited, and many agents that damage the heart produce similar lesions. Myocardial necrosis can be confused with myocardial inflammation with secondary necrosis because both lesions have substantial leukocytic infiltration. Some animals that die peracutely from cardiac failure lack detectable microscopic alterations and are presumed to have suffered from an arrhythmic episode resulting in syncope. Hearts with long-standing myocardial damage have foci of fibrosis, regardless of the cause of the loss of myocytes. Correlation between the severity of clinical cardiac disease and the severity of myocardial injury can be poor: a small lesion at a critical site, such as a portion of the conduction system, can be fatal, whereas a widespread myocardial lesion, such as myocarditis, can be asymptomatic (Fig. 10-23; see Chapter 1).
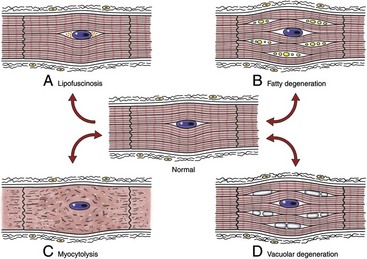
Fig. 10-23 Schematic diagram of various sublethal cardiac muscle cell injuries.
A, Lipofuscinosis. B, Fatty degeneration. C, Myocytolysis. D, Vacuolar degeneration. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Cardiac myofibers can respond to toxins in a variety of ways—apoptosis, myofibrillar lysis, and coagulation necrosis are but a few mechanisms that may result from toxic exposure. A long list of toxins (included in Box 10-5) are responsible for causing myocardial injury; some of the most frequently observed current examples are ionophore toxicity in horses and ruminants, vitamin E–selenium deficiency in the young of many species, “heart-brain syndrome” of dogs (see Fig. 10-83), anthracycline toxicity in dogs, and gossypol toxicosis in pigs. In various localized areas throughout the world, numerous deaths in ruminants have resulted from consumption of poisonous plants such as Acacia georginae and Dichapetalum cymosum.
Cardiotoxicity has emerged as a significant clinical entity in veterinary medicine in recent years with the growing use of antineoplastic drugs in small animal practice and the widespread use of growth promotants in ruminants (see Fig. 10-69). The mechanisms of cardiotoxicities include (1) exaggerated pharmacologic action of drugs acting on cardiovascular tissues, (2) exposure to substances that depress myocardial function, (3) direct injury of cardiac muscle cells by chemicals, and (4) hypersensitivity reactions.
Oncotic Necrosis: Necrosis of cardiac muscle cells is generally followed by leukocytic invasion and phagocytosis of sarcoplasmic debris (Fig. 10-24; see Figs. 15-15 and 15-16). The end result is persistence of collapsed sarcolemmal “tubes” of basal lamina surrounded by condensed interstitial stroma and vessels. Lesions with severe disruption of the myocardium have residual changes of fibroblastic proliferation and collagen deposition to form scar tissue. Regeneration of cardiac muscle cells generally does not occur, except in less evolved animals, such as amphibians and fish, and in certain inbred mouse strains. The continual contraction of intact cardiac muscle cells impairs the mechanisms for regeneration. Also, in hearts of neonatal animals and more often in avian hearts, a limited amount of myocyte regeneration has been reported. Recent studies indicate that stem cells exist in adult animal and human hearts, and with myocardial injury, these cells may differentiate into cardiac muscle cells. However, the extent of myocyte regeneration is probably minimal. Hyperplasia of myocytes is a normal component of cardiac growth in the first several months of life. Then proliferation ceases. Normal growth then is the result of hypertrophy of myocytes until cell sizes normal for the species are reached.
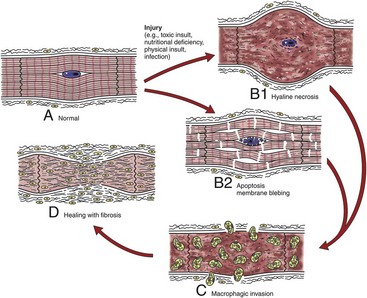
Fig. 10-24 Schematic diagram of the sequential events in myocardial necrosis.
A, Various injuries lead to (B1) hyaline necrosis or (B2) apoptosis membrane blebbing. C, Healing with phagocytosis of cellular debris by macrophages, and (D) subsequent healing with fibrosis, rather than by regeneration. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Apoptosis: Apoptosis (programmed cell death of cardiac myocytes) is increasingly recognized for its role in the development of various myocardial lesions and cardiac diseases (see Chapter 1). These conditions include cardiac development, ischemic injury, several types of experimentally induced heart failure (ischemia-reperfusion, hypoxia, pressure-overload hypertrophy), and cardiotoxicity. In some cell systems, apoptosis can be triggered by the presence of excessive amounts of oxygen free radicals. Cells dying by apoptosis shrink and form apoptotic bodies. In contrast to cell death by necrosis, apoptosis is not accompanied by an inflammatory reaction and fibrosis.
Fatty Infiltration: Fatty infiltration is the presence of increased numbers of lipocytes interposed between myocardial fibers. The lesion is associated with obesity and age and appears as abundant epicardial and myocardial deposits of adipose tissue. Grossly, the myocardium has irregular layers of adipose tissue infiltrating normal myocardium. The atria and right ventricle are most often affected.
Fatty Degeneration: Fatty degeneration (fatty change) is the accumulation of abundant lipid droplets in the sarcoplasm of myocytes. Microscopically, affected myocytes have numerous variably sized spherical droplets that appear as empty vacuoles in paraffin sections but stain positively for lipids with lipid-soluble stains in frozen sections. This lesion occurs with systemic disorders, such as severe anemia, toxemia, and copper deficiency, but is seen much less often in the heart than in the liver and kidneys (also see Chapter 1).
Hydropic Degeneration: Hydropic degeneration, a distinctive microscopic alteration in cardiac muscle cells, is associated with chronic administration of anthracyclines, a group of antineoplastic drugs. Chronic passive congestion with ascites and cardiac dilation may result (Web Figs. 10-11 and 10-12; see Figs. 10-6, A and 10-7). Affected fibers have extensive vacuolization of sarcoplasm that is initiated by distention of elements of sarcoplasmic reticulum and eventually ends in lysis of contractile material (Web Figs. 10-13 and 10-14).
Web Fig. 10-11 Chronic passive congestion, doxorubicin cardiotoxicity, congestive heart failure, liver, ascites, rabbit.
Note the light-red-stained transparent fluid in the peritoneal cavity (ascites) and the mottled liver (L) characteristic of chronic passive congestion. (Courtesy School of Veterinary Medicine, Purdue University.)
Web Fig. 10-12 Cardiac dilation, doxorubicin cardiotoxicity, heart, rabbit.
All cardiac chambers are dilated. (Courtesy School of Veterinary Medicine, Purdue University.)
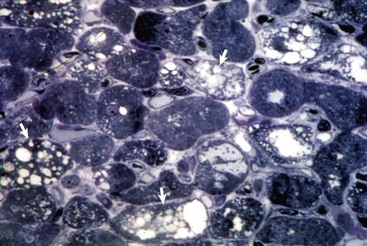
Web Fig. 10-13 Myocardial vacuolar degeneration, chronic doxorubicin cardiotoxicity, heart, section of myocardium, dog.
The affected myocytes have prominent sarcoplasmic vacuolation (arrows). Plastic-embedded, toluidine blue–stained section. (Courtesy School of Veterinary Medicine, Purdue University.)
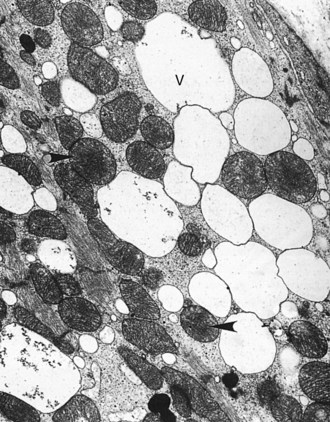
Web Fig. 10-14 Sarcoplasmic vacuolation, chronic doxorubicin cardiotoxicity, heart, section of myocardium, dog.
The prominent sarcoplasmic vacuolation (V) is produced by distention of elements of sarcoplasmic reticulum. Even though the myofibrils have extensive lysis, mitochondria (arrowheads) are intact. TEM. Uranyl acetate and lead citrate stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Hydropic degeneration of cardiac muscle cells may also result from drug-induced injury to mitochondria. Antiretroviral drugs, such as nucleoside reverse transcriptase inhibitors (zidovudine or AZT), are linked to this distinctive lesion. Scattered cardiac muscle cells appear swollen with pale sarcoplasm by light microscopy. Electron microscopy reveals extensive mitochondrial swelling and disruption of cristae with subsequent formation of myelin figures from the membrane debris of damaged mitochondria.
Myofibrillar Degeneration: Myofibrillar degeneration (myocytolysis) represents a distinctive sublethal injury of cardiac muscle cells. Affected fibers have pale eosinophilic sarcoplasm and lack cross-striations. Ultrastructurally, myofibrils have a variable extent of dissolution (myofibrillar lysis). This lesion has been described in furazolidone cardiotoxicity in birds (Fig. 10-25; Web Fig. 10-15) and potassium deficiency in rats.
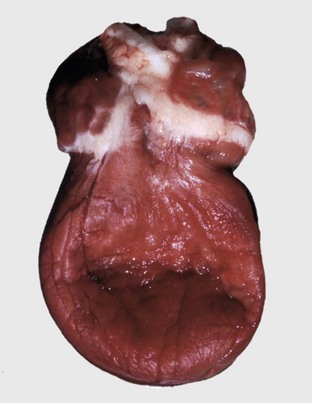
Fig. 10-25 Ventricular dilation, furazolidone cardiotoxicity, heart, duckling.
Note that the dilated ventricles have collapsed once the blood was removed. (Courtesy School of Veterinary Medicine, Purdue University.)
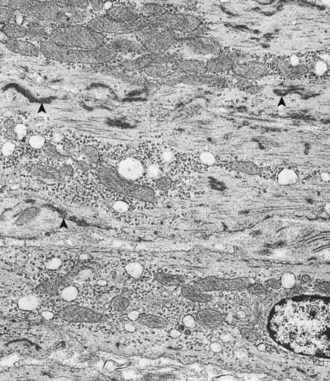
Web Fig. 10-15 Myofibrillar lysis, furazolidone cardiotoxicity, heart, ventricular myocardium, duckling.
Affected myocytes have extensive dissolution of myofibrils with scattered free myofilaments and dense clumps of Z-band material (arrowheads). Other organelles appear normal. TEM. Uranyl acetate and lead citrate stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Lipofuscinosis: Lipofuscinosis (brown atrophy) of the myocardium occurs in aged animals and in animals with severe cachexia, but it also has been described as a hereditary lesion in healthy Ayrshire cattle. Severely affected hearts appear brown and microscopically have clusters of yellow-brown granules at the nuclear poles of myocytes. These granules represent intralysosomal accumulation of membranous and amorphous debris (residual bodies).
Mineralization: Myocardial mineralization (calcium) is a prominent feature in several diseases, such as hereditary calcinosis in mice, cardiomyopathy in hamsters, vitamin E–selenium deficiency in sheep and cattle (Fig. 10-26; see Web Fig. 10-9), vitamin D toxicity in several species, calcinogenic plant toxicosis in cattle (“Manchester wasting disease”), and spontaneous myocardial calcification in aged rats and guinea pigs.
Myocardial Necrosis: Myocardial necrosis can result from a number of causes, including nutritional deficiencies, chemical and plant toxins, ischemia, metabolic disorders, heritable diseases, and physical injuries (see Box 10-5). Grossly, affected areas appear pale initially, and some progress to prominent yellow to white (see Fig. 10-69), dry areas made gritty by dystrophic mineralization. The lesions are focal, multifocal, or diffuse. The most frequent sites of focal lesions are the left ventricular papillary muscles and the subendocardial myocardium, especially when such lesions are related to transient reduction of vascular perfusion. These lesions can be overlooked at necropsy unless multiple incisions are made in the ventricular myocardium. In diseases with diffuse cardiac necrosis, such as white muscle disease of calves and lambs due to vitamin E–selenium deficiency, the discrete white lesions can be readily observed beneath the epicardial and endocardial surfaces (Fig. 10-27).
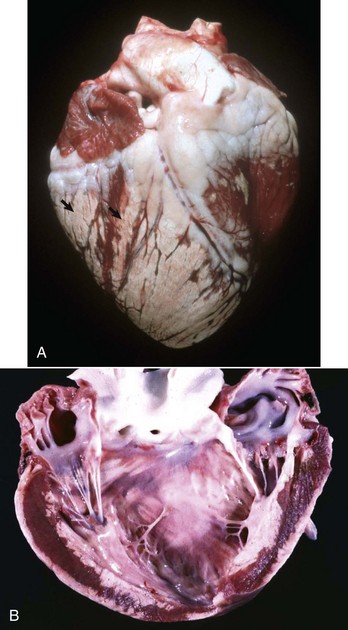
Fig. 10-27 Myocardial necrosis, vitamin E–selenium deficiency, heart, left ventricular myocardium, calf.
A, Note the prominent white chalky areas of necrosis with mineralization (arrows) of the myocardium. B, Similar necrosis is subepicardially and subendocardially in the sectioned free walls of the left ventricle and subendocardially in the myocardium of the ventricular septum (center). (A courtesy School of Veterinary Medicine, Purdue University. B courtesy Dr. P.N. Nation, Animal Pathology Services; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
Microscopically, the appearance depends on the age of the lesions. Fibers in areas of recent necrosis often appear swollen and hypereosinophilic (hyaline necrosis). Striations are indistinct, and nuclei are pyknotic. Necrotic fibers often have scattered basophilic granules (Fig. 10-28) that represent calcified mitochondria and can be confirmed by electron microscopy (Web Fig. 10-16). In a second pattern of necrosis, affected myocytes have a “shredded” appearance because of hypercontraction and the formation of multiple transversely oriented bars of disrupted contractile material (often termed contraction band necrosis) (Web Fig. 10-17). A third pattern is seen in necrotic myocytes in large areas of ischemic necrosis (infarcts). These myocytes have features of coagulation necrosis and have relaxed rather than hypercontracted contractile elements.
Fig. 10-28 Acute myocardial necrosis with mineralization, minoxidil cardiotoxicity, heart, ventricular myocardium, pig.
The darker red myocytes are necrotic, and some are mineralized (purplish areas). H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
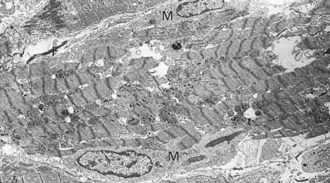
Web Fig. 10-16 Myocardial necrosis, monensin toxicosis, necrotic myocyte, heart, longitudinal section, calf.
The necrotic myocyte (center) has disrupted myofibrils, damaged mitochondria with matrical densities, and several invading macrophages (M). F, Fibrin. TEM. Uranyl acetate and lead citrate stain. (Courtesy School of Veterinary Medicine, Purdue University.)
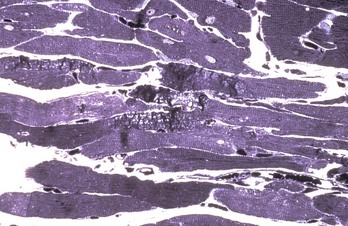
Web Fig. 10-17 Myocardial necrosis, electric shock overdose by defibrillator, heart, dog.
The dark shredded segments of myocytes are due to acute contraction band necrosis. The time interval between defibrillation and the fixation of the heart was 24 hours. Plastic-embedded, toluidine blue–stained section. (Courtesy School of Veterinary Medicine, Purdue University.)
Within 24 to 48 hours after injury, necrotic areas are infiltrated by inflammatory cells, mainly macrophages and a few neutrophils; these phagocytose and lyse the necrotic cellular debris (Web Fig. 10-18). In early stages of healing of necrosis, it is often difficult to distinguish the lesions from those found in some types of myocarditis (see later discussion). Later, when necrosis has progressed somewhat, lesions consist of persistent stromal tissue (interstitial fibroblasts, collagen, and capillaries) and empty “tubes” of basal laminae formally occupied by necrotic myocytes (see Chapter 15). The healing phase is characterized by proliferation of connective tissue cells (fibroblasts) (Fig. 10-29) and by deposition of connective tissue products (collagen and elastic tissue and acid mucopolysaccharides). Grossly, these areas with healing of myocardial necrosis appear as white, firm, contracted scars.
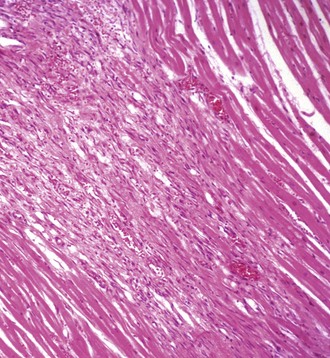
Fig. 10-29 Healing, postmyocardial necrosis, heart, ventricle, dog.
The necrotic myocytes have been removed by phagocytosis by macrophages (not seen here), and the area is now undergoing fibrosis. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
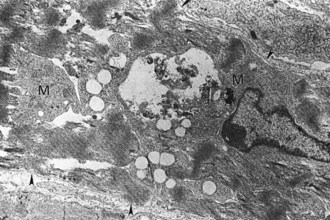
Web Fig. 10-18 Myocardial necrosis, monensin toxicosis, heart, section of myocardium, calf.
Necrotic myocyte has disrupted contractile material invaded by a macrophage (M). The basal lamina of the necrotic myocyte is noted by arrowheads. TEM. Uranyl acetate and lead citrate stain. (Courtesy School of Veterinary Medicine, Purdue University.)
The outcome of myocardial necrosis varies, depending on the extent of the damage:
• Many animals die unexpectedly of acute cardiac failure if the myocardial damage is extensive.
• Early deaths from necrosis-related arrhythmias also occur when cardiac conduction is disrupted.
• Some cases eventually develop cardiac decompensation and die with cardiac dilation, scarring, and lesions of chronic congestive failure.
Hearts with minimal damage have only microscopically detectable myocardial fibrosis when death eventually occurs from other diseases.
Inflammation
Myocarditis: Myocarditis generally is the result of infections spread hematogenously to the myocardium and occurs in various systemic diseases. Infrequently, the heart is the primary location in affected animals and responsible for death. Types of inflammation provoked by infectious agents that produce myocarditis include suppurative, necrotizing, hemorrhagic, lymphocytic, and eosinophilic. Suppurative myocarditis results from localization of pyogenic bacteria in the myocardium (Fig. 10-30) that are trapped in thromboemboli originating from vegetative valvular endocarditis on the mitral and aortic valves. Septic infarcts with pale, disseminated lesions may be grossly evident in the myocardium. These foci consist of neutrophils and necrotic myocytes that form abscesses. The various infectious diseases that cause myocarditis in animals are summarized in Box 10-6.
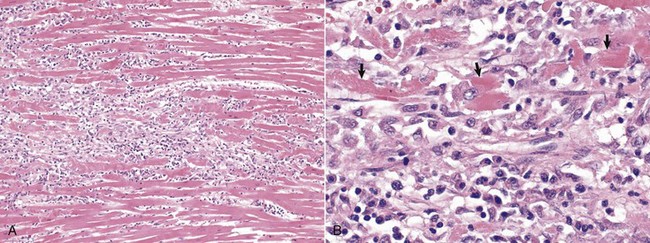
Fig. 10-30 Acute myocarditis, horse.
A, The parallel arrays of myofibers are disrupted by acute inflammatory cells, edema fluid, and fibrin. B, Higher magnification of A. Note the myocardial fiber degeneration and necrosis with loss of cross striations (arrows); fragmentation of cardiac rhabdomyocytes (cardiomyocytes); and hypereosinophilia, coagulation, and clumping of the sarcoplasm. Neutrophils are the predominant inflammatory cell in the exudate. H&E stain. (Courtesy Dr. J.F. Zachary, College of Veterinary Medicine, University of Illinois.)
The pathogenesis and expected outcome of cases of myocarditis remain an important area of research because of the severity of this lesion in cardiac failure in humans. The sequelae to myocarditis include (1) complete resolution of lesions, (2) scattered residual myocardial scars, or (3) progressive myocardial damage with acute or, in some cases, chronic cardiac failure as secondary dilated (congestive) cardiomyopathy. In experimental studies of myocarditis induced in mice by coxsackie B virus, the severity of myocarditis was influenced by the virulence of the virus and mouse strain and was enhanced by host factors such as young age, male sex, pregnancy, poor nutrition, whole-body ionizing radiation, cold environmental temperatures, alcohol ingestion, exercise, and cortisone administration. Much of the myocardial damage in coxsackie B virus infection is induced by immunologic reactions (with T lymphocyte involvement) rather than by direct viral injury.
Cardiac Conduction System
In domestic animals, the responses to injury of the cardiac conduction system are poorly understood but likely involve forms of cell degeneration and death, as well as inflammation as discussed in other sections.
Endocardium and Heart Valves
Hemorrhage: Endocardial hemorrhages are commonly seen and may be the result of trauma or septicemias, especially those with endotoxins, or can occur agonally at death (Fig. 10-31). See the previous sections on the Pericardium and Epicardium and the Myocardium and also Chapter 2.
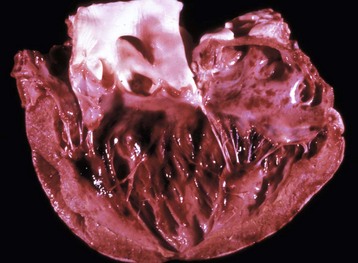
Fig. 10-31 Endocardial suffusive hemorrhage, heart, left ventricle, calf.
A red to dark-red sheet of suffusive hemorrhage is present in the endocardium of the left ventricle. Suffusive hemorrhage is often attributed to severe septicemia, endotoxemia, anoxia, or electrocution. (Courtesy College of Veterinary Medicine, University of Illinois.)
Disturbances of Growth
Valvular Anomalies and Dysplasia: See the discussion on valvular anomalies and dysplasia in the section on Endocardium and Heart Valves, Disturbances of Growth, Disorders of Domestic Animals.
Cellular Degeneration and Death
See Chapter 1.
Myxomatous Valvular Degeneration (Endocardiosis): See the discussion on myxomatous valvular degeneration (endocardiosis) (see Fig. 10-84) in the section on Disorders of Dogs.
Mineralization: Mineralization of the endocardium is seen with Vitamin D toxicity, calcinogenic plant toxicosis in cattle, and calcium phosphorus imbalance, as well as in Johne’s disease (Fig. 10-32; see Chapter 7). The endocardium and large elastic arteries are prone to mineralization because of their abundant elastic fibers.
Inflammation
See Chapter 3.
Vegetative Valvular and Mural Endocarditis: Endocarditis is usually the result of bacterial infections, except for lesions produced by migrating Strongylus vulgaris larvae in horses and rarely in mycotic infections. The lesions are often very large by the time of death and are present on the valves (valvular endocarditis), although some lesions originate from the underlying myocardium or extend from the affected valve to the adjacent wall (mural endocarditis). Grossly, the affected valves have large, adhering, friable, yellow-to-gray masses of fibrin termed vegetations, which can occlude the valvular orifice (see Figs. 10-73, A, and 10-76). In chronic lesions, the fibrin deposits are organized by fibrous connective tissue to produce irregular nodular masses termed verrucae (wartlike lesions). Microscopically, the lesion consists of accumulated layers of fibrin and numerous embedded bacterial colonies underlain by a zone of infiltrated leukocytes and granulation tissue (see Fig. 10-73, B). Relative frequency of valvular involvement with endocarditis in animals is mitral > aortic > tricuspid > pulmonary.
The pathogenesis of endocarditis is complicated and incompletely understood, but the components of Virchow’s triad in thrombogenesis—endothelial injury, turbulence, and hypercoagulability—are involved. Affected animals often have preexisting extracardiac infections, such as gingivitis, mastitis, hepatic abscesses or dermatitis, that have resulted in one or more bouts of bacteremia. Turbulent intracardiac blood flow associated with congenital anomalies or the presence of intracardiac and vasculature devices, such as catheters, may contribute to initiation of the lesion. Focal trauma–induced endothelial disruption on the surface of the normally avascular valves allows bacteria to adhere, proliferate, and initiate an inflammatory reaction that results in subsequent deposition of masses of fibrin. Death is the result of cardiac failure from valvular dysfunction or the effects of bacteremia. In some animals, septic emboli lodge in organs such as the heart and kidneys, leading to infarction and localized inflammation or abscess formation. Also see the discussion of vegetative valvular and mural endocarditis in the section on Endocardium, Disorders of Domestic Animals.
Blood and Lymphatic Vascular Systems
The response of blood vessels to injury involves a complex interaction among the cellular and noncellular elements of the vessel wall and the cellular and noncellular elements of the blood. The key cells of vessels in these reactions are endothelial cells and smooth muscle cells. Endothelial cells are metabolically active and provide a thromboresistant monolayer at the interface of blood and the vessel wall unless damaged. Endothelial cells play an important role in fluid distribution, inflammation, angiogenesis, and hemostasis (see Chapter 2). Responses of blood vessels to a variety of toxins are listed in Web Box 10-1.
Key functions of endothelial cells include prostacyclin production, macromolecular transport, and recruitment of inflammatory cells. Injury of endothelial cells is followed by separation from the underlying basement membrane and increased permeability to movement of plasma proteins into the subendothelium. Necrosis of endothelium exposes subendothelial collagen and elicits thrombus formation. Endothelial cells at the margin of denuded areas proliferate and re-endothelialize the damaged area. The arterial intima has regional differences in the uptake of macromolecules, as well as other unique structural and functional features that result in lesion-prone areas of the vasculature. Bilirubin staining of the intima results in yellow discoloration in jaundiced animals (Fig. 10-33).
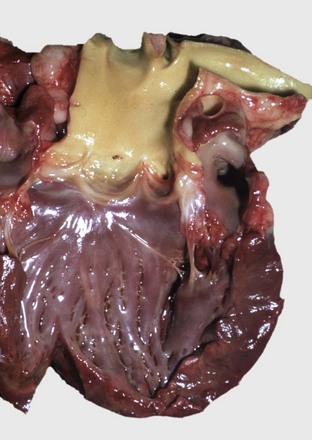
Fig. 10-33 Jaundice, heart, aorta, dog.
Note yellow discoloration of the aortic intima. (Courtesy School of Veterinary Medicine, Purdue University.)
The other major cellular component of vessels involved in reaction to injury is the smooth muscle cell. These cells have important functions, including production of extracellular components, such as collagen, elastin, and proteoglycans; maintenance of vascular tone; monocyte recruitment; lipoprotein metabolism; production of bioactive lipids, such as prostaglandins; and formation of oxygen-free radicals. These functions are regulated by a wide variety of biochemical mediators such as various growth factors, cytokines, and inflammatory mediators.
Disturbances of Circulation
Hemorrhage: Hemorrhage resulting from vascular injury is a frequent lesion of the epicardium, endocardium, and myocardium. Hemorrhages vary in size from petechiae (1- to 2-mm diameter), to ecchymoses (2- to 10-mm diameter), to suffusive (diffuse). Animals dying from septicemia, endotoxemia, anoxia, or electrocution often have prominent epicardial (see Fig. 10-9) and endocardial (see Fig. 10-31) hemorrhages. Horses dying of any cause usually have agonal hemorrhages on the epicardial and endocardial surfaces. A distinctive example of a specific disease with cardiac hemorrhage is mulberry heart disease, associated with vitamin E–selenium deficiency in growing pigs. In these pigs, hydropericardium accompanies severe myocardial hemorrhage that results in a red, mottled (mulberry-like) appearance of the heart. Also see the previous discussions on disturbances of circulation in the sections on the Pericardium and Epicardium and the Myocardium in the Responses to Injury section.
Effusions: See the previous discussion on disturbances of circulation in the section on the Pericardium and Epicardium.
Hemopericardium: See the previous discussion on hemopericardium under Effusions in the section on Disturbances of Circulation, Pericardium and Epicardium, Responses to Injury and the later discussion on the hemopericardium under Effusions in the section on Disturbances of Circulation, Pericardium and Epicardium, Disorders of Domestic Animals.
Hemothorax and Hemoabdomen: Hemothorax and hemoabdomen arise from spontaneous or traumatic rupture of large arteries or veins or from rupture of aneurysms located in the thoracic or abdominal cavity, respectively. An aneurysm is a localized dilation or outpouching of a thinned and weakened portion of a vessel. Usually, arteries are affected, especially large elastic arteries, but the lesion can also occur in veins. Known causes include copper deficiency in pigs (Fig. 10-34) because copper is necessary for normal development of elastic tissue and damage from infection with Spirocerca lupi in dogs or Strongylus vulgaris in horses. Most cases are idiopathic. Dissecting aneurysms are infrequent but have been seen in birds, most notably turkeys (Fig. 10-35). They result from disruption of the intima, which allows entry of blood into the media, and this dissects along the wall. Aneurysms can rupture. Usually the consequences are rapidly fatal, as rather large arteries typically are involved. See the later discussion on hemopericardium under Effusions in the section on Disturbances of Circulation, Pericardium and Epicardium, Disorders of Domestic Animals.
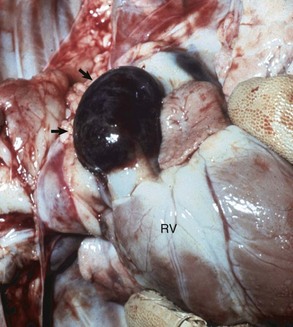
Fig. 10-34 Dissecting aneurysm, copper deficiency, heart, pulmonary artery, right ventricle (RV), pig.
The dark, blood-filled, bulging segment of the wall of the pulmonary artery (arrows) has resulted from disruption of elastic fibers. (Courtesy School of Veterinary Medicine, Purdue University.)
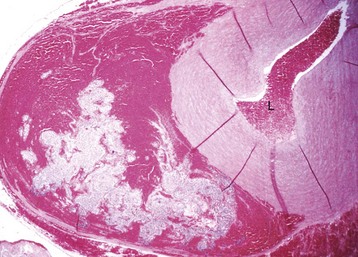
Fig. 10-35 Dissecting aneurysm, aorta, turkey.
Blood has dissected through the tunica media (in a nearby section of the aorta) and in this section has come to lie in the outer layers of the tunica media and adventitia. L, Vessel lumen. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Disturbances of Growth
See Chapter 1.
Hypertrophy: Arterial hypertrophy is a response to sustained increases in pressure or volume loads. Affected vessels are generally muscular arteries, and the increase in wall thickness is predominantly caused by hypertrophy (and to some degree, hyperplasia) of smooth muscle cells of the tunica media. Muscular pulmonary arteries of cats are frequently affected, and the lesion has been associated with infection by several parasites, including Aelurostrongylus abstrusus (the lungworm of cats), Toxocara sp., and Dirofilaria immitis (Fig. 10-36). However, the lesions often occur in the absence of parasitic infections (Fig. 10-37). Often, no clinical disease is associated with the lesion in cats, but asthmatic signs have been seen in cats with these parasitic infections. Similar hypertrophy of muscular pulmonary arteries occurs in cattle with hypoxia-induced pulmonary arterial vasoconstriction and subsequent pulmonary hypertension associated with right-sided heart failure from exposure to high altitudes (so-called high-altitude disease or “brisket disease”) (see the previous section on Stages of Myocardial Hypertrophy). Also, animals with cardiovascular anomalies that shunt blood left to right result in pulmonary hypertension and have hypertrophy of the muscular pulmonary arteries and can result in plexogenic pulmonary arteriopathy. Uterine arteries in pregnant animals also are hypertrophic.
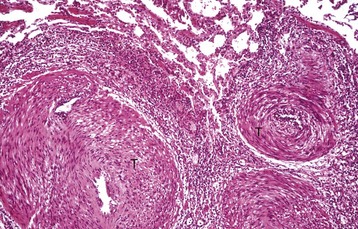
Fig. 10-36 Medial hypertrophy, periarteritis, dirofilariasis, lung, small pulmonary arteries, cat.
Note the massively thickened tunica media (T) of the small branches of the pulmonary arteries and their periarterial cuff of chronic inflammatory cells and some eosinophils. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Agenesis (Aplasia), Hypoplasia, and Dysplasia (Dysgenesis): See the discussion on Developmental Errors in the section on Endocardium and Heart Valves, Disorders of Domestic Animals.
Neoplastic Transformation: See Chapter 6; also see the section on Disorders of Dogs.
Hemangiosarcoma: Cardiac hemangiosarcoma is an important neoplasm of dogs and can arise either in the heart (primary) or by metastasis (secondary) from sites such as the spleen. This neoplasm is usually seen in the wall of the right atrium and only occasionally involves the right ventricle (see the section on Disorders of Dogs).
Cell Degeneration and Death
See Chapter 1. Generalized vascular degenerative diseases in animals are classified into the following three principal groups:
Hyaline degeneration, fibrinoid necrosis, and amyloidosis also occur in all animal species.
Arteriosclerosis: Arteriosclerosis is characterized by intimal fibrosis of large elastic arteries, atherosclerosis is characterized by intimal and medial lipid deposits in elastic and muscular arteries, and arterial medial calcification has characteristic mineralization of the walls of elastic and muscular arteries.
Arteriosclerosis is an age-related disease that occurs frequently in many animal species but rarely causes clinical signs. The disease develops as chronic degenerative and proliferative responses in the arterial wall and results in loss of elasticity (“hardening of the arteries”) and less often luminal narrowing. The abdominal aorta is most frequently affected, but other elastic arteries and peripheral large muscular vessels may be involved. Lesions are often localized around the orifices of arterial branches. Etiologic factors in the development of arteriosclerosis are not well defined, but the significant role of hemodynamic influences is suggested by the frequent involvement at arterial branching sites, in which blood flow is turbulent. Grossly, the lesions are seen as slightly raised, firm, white plaques. Microscopically, initially the intima is thickened by accumulation of mucopolysaccharides and later by the proliferation of smooth muscle cells in the tunica media and fibrous tissue infiltration into the intima. Splitting and fragmentation of the internal elastic lamina are common.
Atherosclerosis: Atherosclerosis, the vascular disease of greatest importance in humans, occurs only infrequently in animals and rarely leads to clinical disease such as infarction of the heart or brain. The principal alteration is accumulation of deposits (atheroma) of lipid, fibrous tissue, and calcium in vessel walls, which eventually results in luminal narrowing. Many studies have established that the pig, rabbit, and chicken are susceptible to the experimental disease produced by the feeding of a high-cholesterol diet; the dog, cat, cow, goat, and rat are resistant. Lesions of the naturally occurring disease have been detected in aged pigs and birds (especially parrots) and in dogs with hypothyroidism and diabetes mellitus that develop an accompanying hypercholesterolemia. Arteries of the heart, mesentery, and kidneys are prominently thickened, firm, and yellow-white (Fig. 10-38, A). Microscopically, lipid globules accumulate in the cytoplasm of smooth muscle cells and macrophages, often termed foam cells, in the media and intima (Fig. 10-38, B and C; also see Fig. 3-10). Necrosis and fibrosis develop in some arterial lesions.
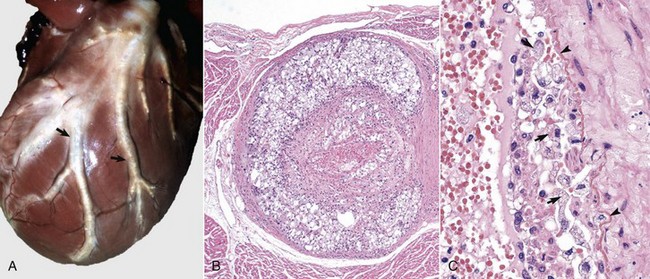
Fig. 10-38 Coronary atherosclerosis, hypothyroidism, heart, left ventricle, dog.
A, The affected coronary arteries are prominent and cordlike (arrows) with thickened walls. The diffuse and focal yellow areas in the walls of the arteries are the sites of atheromatous deposits. B, Note the extensive accumulation of lipid-laden (clear vacuoles) macrophages termed “foam cells” throughout the thickened tunica intima and media of this branch of the coronary artery. H&E stain. C, Higher magnification of B. The tunica intima contains abundant lipid-laden macrophages (arrows). Note the disruption of the endothelium and the plasma proteins and fibrin on their surfaces. This condition is highly unstable and is prone to activation of Virchow’s triad and the coagulation cascade with the formation of mural thrombi and infarction of myocardium supplied by this artery. The arrowheads identify the internal elastic lamina of the tunica intima. H&E stain. (A courtesy School of Veterinary Medicine, Purdue University. B and C courtesy College of Veterinary Medicine, University of Illinois.)
Arterial Medial Calcification: Arterial medial calcification is a frequent lesion in animals that often have concurrent endocardial mineralization and involves both elastic and muscular arteries. The causes of arterial medial calcification include calcinogenic plant toxicosis, vitamin D toxicosis, renal insufficiency, and severe debilitation, as seen in cattle with Johne’s disease (Fig. 10-39). Medial calcification occurs spontaneously in horses, rabbits, aged guinea pigs, and in rats with chronic renal disease. Affected arteries, such as the aorta, have a unique gross appearance; they appear as solid, dense, pipelike structures with raised, white, solid intimal plaques (Fig. 10-40). Microscopically, in elastic arteries, prominent basophilic granular mineral deposits are present on elastic fibers of the media, but in muscular arteries, they can form a complete ring of mineralization in the tunica media (Fig. 10-41). Siderocalcinosis (so-called iron rings), the result of deposition of both iron and calcium salts, occurs in the cerebral arteries of aged horses. Lesions in the surrounding brain tissue are generally absent. Siderocalcinosis lesions are considered incidental.
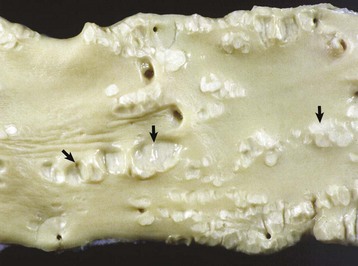
Fig. 10-39 Johne’s disease, arteriosclerosis, aorta, cow.
Multiple prominent, white, mineralized foci are in the tunica intima and media (arrows). (Courtesy College of Veterinary Medicine, University of Illinois.)
Hyaline Degeneration, Fibrinoid Necrosis, and Amyloidosis: Hyaline degeneration, fibrinoid necrosis, and amyloidosis are vascular lesions of small muscular arteries and arterioles and occur in all animal species. These lesions are generally not detected grossly, but in some diseases with fibrinoid necrosis of vessels, hemorrhages and edema are seen in affected organs at necropsy. The microscopic feature shared by these lesions is the formation of a homogeneous eosinophilic zone in the vessel wall (Fig. 10-42; also see Fig. 10-79). Special stains allow differentiation into three types: (1) amyloid confirmed by Congo red and methyl violet; (2) fibrinoid deposits, positive by the periodic acid–Schiff technique; and (3) negative staining of hyaline deposits by these stains. Amyloidosis and hyaline degeneration are often observed in small muscular arteries of the myocardium, lungs, and spleen of old dogs. Lesions in the intramyocardial arteries can cause small foci of myocardial infarction.
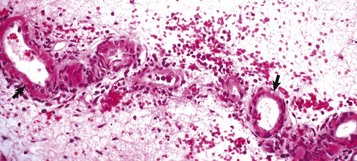
Fig. 10-42 Fibrinoid necrosis of small arteries, edema disease, stomach, submucosa, pig.
Note the circumferential eosinophilic (arrows) material in the walls of the arterioles and the extensive edema and mild hemorrhage in surrounding submucosa. H&E stain. (Courtesy School of Veterinary Medicine, Purdue University.)
Fibrinoid necrosis of arteries is associated with endothelial damage and is characterized by entry and accumulation of serum proteins followed by fibrin polymerization in the vessel wall. These materials form an intensely eosinophilic collar that obliterates cellular detail. This lesion is frequent in many acute degenerative and inflammatory diseases of small arteries and arterioles. Fibrinoid necrosis is seen frequently in dogs with uremia and in dogs with hypertension, although hypertension is an uncommon finding in animals.
Inflammation
See Chapter 3.
Arteritis and Vasculitis: Arteritis occurs as a feature of many infectious and immune-mediated diseases (Box 10-7). Often, all types of vessels are affected rather than only arteries, and then vasculitis or angiitis (a term that includes blood and lymphatic vessels) is the term applied to the lesions. The vascular system serves as the major mechanisms for transport of organisms, e.g. Bacillus anthracis. In inflamed vessels, leukocytes are present within and surrounding the walls, and damage to the vessel wall is evident as fibrin deposits or necrotic endothelial and smooth muscle cells. As a result of endothelial damage, thrombosis, which can result in ischemic injury or infarction in the circulatory field, may be present. Arteritis and vasculitis can develop from endothelial injury caused by either infectious agents or immune-mediated mechanisms or may be caused by local extension of suppurative and necrotizing inflammatory processes in adjacent tissues. Arteritis is a prominent feature of several parasitic diseases.
Systemic infections with phlebitis as a lesion include salmonellosis in several species and feline infectious peritonitis. In pigs with various septicemias, such as salmonellosis and colibacillosis, the gastric fundic mucosa is often severely congested and hemorrhagic because of venous endothelial damage and thrombosis. In severe local infections, such as in metritis or hepatic abscesses, inflammation extends into the walls of adjacent veins and produces phlebitis, with or without thrombosis. Intravenous injections of irritant solutions, injecting solutions into the vascular wall, or intimal trauma produced by indwelling venous catheters result in vascular damage and create an opportunity for localization and proliferation of infectious agents and development of phlebitis and thrombosis (Fig. 10-43). Animals with phlebitis complicated by thrombosis have the additional risk of septic embolism, which can cause endocarditis and pulmonary abscesses or pulmonary infarcts.
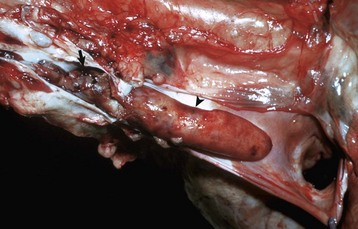
Fig. 10-43 Thrombus (mural), jugular vein (opened), dog.
Note the nodular mural thrombus (left [arrow]) in the jugular vein. This thrombus likely occurred at a site of venipuncture and a subsequent phlebitis. The smooth-surfaced red-tan thrombus (right [arrowhead]) extending toward the heart is a trailing thrombus, a continuation of the mural thrombus. (Courtesy School of Veterinary Medicine, Purdue University.)
Many reportable foreign animal diseases are viral diseases, which are endotheliotropic and result in vasculitis; examples include classic swine fever (hog cholera), African horse sickness, and African swine fever (see the sections on disorders of specific animal species).
Coronary and Other Arteries: Thrombosis or embolism of the coronary arteries can result in myocardial infarction (Fig. 10-44) and cardiac failure. These lesions are much less common in animals than in humans. Affected animals generally have one of several types of coronary arterial disease, including atherosclerosis, arteriosclerosis, or periarteritis. In atherosclerosis associated with hypothyroidism or diabetes mellitus (discussed previously), severe lesions are present in the extramural (epicardial) coronary arteries of dogs, but this only rarely leads to thrombosis and myocardial infarction. In contrast, severe arteriosclerosis of intramural cardiac arteries in aged dogs can cause small multifocal myocardial infarcts (see Fig. 10-44). Affected dogs often also have myxomatous valvular degeneration (valvular endocardiosis), which is also an age-related disease. Thrombosis of or embolism to other large arteries, such as the interlobular artery of the kidney, can lead to infarction of the tissue supplied by the artery (Web Fig. 10-19; also see Chapter 2).
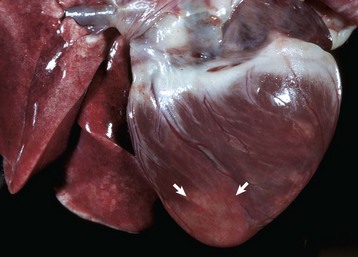
Fig. 10-44 Myocardial infarction, heart, left and right ventricles, dog.
Pale, necrotic, circumscribed areas (arrows) are present in the ventricular walls and are most prominent at the apex. (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
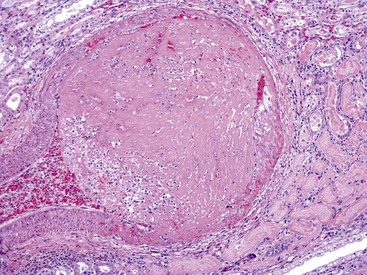
Web Fig. 10-19 Arterial thrombosis, subacute, interlobular artery, kidney, dog.
An interlobular artery contains a thromboembolus, which has firmly attached to the vessel wall. Note the large size of the thromboembolus when compared to the luminal diameter (see bottom left corner) of the artery. This finding suggests that it is “growing” in size via the mechanisms of Virchow’s triad and the adherence of platelets and fibrin to the initial embolus. (Courtesy College of Veterinary Medicine, University of Illinois.)
Lymphatic Vessels
Rupture of the Thoracic Duct: Rupture of the thoracic duct, either as a result of trauma or from spontaneous disruption, causes chylothorax in dogs and cats (see Fig. 9-101). However, many cases of chylothorax occur without injury to the thoracic duct and have been attributed to lesions that interfere with central venous return or produce obstruction of the thoracic duct (right-sided heart failure, neoplasms, granulomas, cranial vena cava thrombosis, or dirofilariasis) or that are idiopathic.
Disturbances of Growth
Agenesis (Aplasia), Hypoplasia, Dysplasia (Dysgenesis): See the discussion on Developmental Errors: Congenital Anomalies in the section on Disturbances of Growth, Lymphatic Vessels, Disorders of Domestic Animals.
Neoplastic Transformation: Tumors arising from lymphatic vessels are benign (lymphangioma) and malignant (lymphangiosarcoma). See Chapter 6 and also the section on Disorders of Dogs.
Inflammation
The endothelial cells lining the lymphatic vessels are subject to the same reactions to injury and inflammation as the vascular system. Inflammation of the lymphatic vessels is called lymphangitis and may be seen with specific diseases such as septicemias caused by bacteria like Salmonella spp. (Box 10-8). Lymphangitis may be acute, subacute, granulomatous, or chronic resulting in lymphedema. See Chapter 3 and also see the discussion on Glanders disease and other cutaneous lymphangitides in the section on Disorders of Horses.
Disorders of Domestic Animals
The most common cardiac diseases in horses, ruminants, pigs, dogs, and cats are summarized in Boxes 10-9 and 10-10. The appearance of the heart with major neoplastic diseases is illustrated in Fig. 10-45. Cardiovascular diseases with known or suspected heritability are listed in Web Box 10-2.
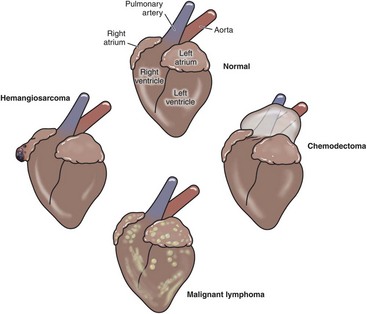
Fig. 10-45 Schematic diagram of the locations of the major cardiac neoplasms. (Redrawn with permission from School of Veterinary Medicine, Purdue University.)
Pericardium and Epicardium
See the discussion on the pericardium and epicardium in the section on Responses to Injury.
Disturbances of Circulation
Hydropericardium: Hydropericardium occurs in those diseases that have generalized edema (see Fig. 10-12). Thus ascites and hydrothorax often occur concurrently with hydropericardium. Congestive heart failure is an important mechanism of hydropericardium and is usually the result of primary myocardial, valvular, congenital, or neoplastic diseases. Common specific diseases include dilated cardiomyopathy of dogs and cats and “ascites syndrome” of poultry. Hydropericardium can also accompany pulmonary hypertension (e.g., “brisket disease” or “high-altitude disease” of cattle), renal failure, and hypoproteinemia from various chronic debilitating diseases. Hydropericardium can also occur in various systemic diseases with vascular injury, such as septicemia in pigs, “heartwater” (Cowdria ruminantium infection) in small ruminants, African horse sickness, and bovine ephemeral fever.
Hemopericardium: Bleeding into the pericardial sac can result from spontaneous atrial rupture in dogs, atrial rupture in dogs with hemangiosarcoma, rupture of the intrapericardial aorta or pulmonary artery in horses, or as a complication of intracardiac injections (see Figs. 10-10 and 10-11).
Disturbances of Growth
Atrophy: See the previous discussion on serous atrophy of fat in the section on the Pericardium and Epicardium, in the Responses to Injury section.
Inflammation
Pericarditis: See the discussion on pericarditis in the section on the Pericardium and Epicardium, Responses to Injury.
Fibrinous Pericarditis: Hematogenous spread of specific organisms may result in fibrinous pericarditis. Mannheimiosis, blackleg, coliform septicemias, contagious bovine pleuropneumonia, sporadic bovine encephalomyelitis, and in utero Brucella spp. and Arcanobacter pyogenesis fetal septicemias may all produce fibrinous pericarditis. In sheep, Mannheimiosis and streptococcal infections most commonly result in fibrinous pericarditis. Sterile swabs of the pericardial exudates are recommended to identify the causative organism.