CHAPTER 21 Disorders of the Trachea and Bronchi
GENERAL CONSIDERATIONS
Common diseases of the trachea and bronchi include canine infectious tracheobronchitis, canine chronic bronchitis, feline bronchitis, collapsing trachea, and allergic bronchitis. Oslerus osleri infection is an important consideration in young dogs.
Other diseases may involve the airways, either primarily or concurrently with pulmonary parenchymal disease. These diseases, such as viral, mycoplasmal, and bacterial infection; other parasitic infections; and neoplasia are discussed in Chapter 22. Feline bordetellosis can cause signs of bronchitis (e.g., cough) but is more often associated with signs of upper respiratory disease (see the section on feline upper respiratory infection, in Chapter 15) or bacterial pneumonia (see the section on bacterial pneumonia, in Chapter 22). Dogs with mild canine influenza virus infections have acute cough and often nasal discharge. This form of the disease is similar to canine infectious tracheobronchitis and is self-limiting. The severe form of the disease is characterized by pneumonia. Canine influenza is discussed in Chapter 22.
CANINE INFECTIOUS TRACHEOBRONCHITIS
Etiology
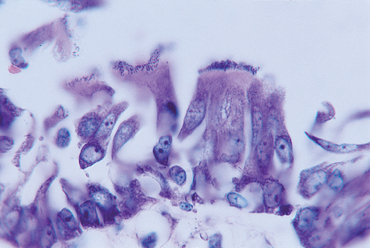
Canine infectious tracheobronchitis, or “kennel cough,” is a highly contagious, acute disease that is localized in the airways. One or more infectious agents cause it, including canine adenovirus 2 (CAV2), parainfluenza virus (PIV), canine respiratory coronavirus and Bordetella bronchiseptica. Bordetella organisms infect ciliated respiratory epithelium (Fig. 21-1) and can decrease mucociliary clearance. Other organisms may become involved as secondary pathogens. In most dogs the disease is self-limiting, with resolution of clinical signs in approximately 2 weeks.
Clinical Features
Affected dogs are first seen because of the sudden onset of a severe productive or nonproductive cough, which is often exacerbated by exercise, excitement, or the pressure of the collar on the neck. Palpating the trachea easily induces the cough. Gagging, retching, or nasal discharge can also occur. A recent history (i.e., within 2 weeks) of boarding, hospitalization, or exposure to a puppy or dog that has similar signs is common. Puppies recently obtained from pet stores, kennels, or shelters have often been exposed to the pathogens.
The majority of dogs with infectious tracheobronchitis are considered to have “uncomplicated,” self-limiting disease and do not show signs of systemic illness. Therefore dogs showing respiratory distress, weight loss, persistent anorexia, or signs of involvement of other organ systems, such as diarrhea, chorioretinitis, or seizures, may have some other, more serious disease, such as canine distemper, severe canine influenza, or a mycotic infection. Although uncommon, serious respiratory complications can result from infectious tracheobronchitis. Secondary bacterial pneumonia can develop, particularly in puppies, immunocompromised dogs, and dogs that have preexisting lung abnormalities such as chronic bronchitis. Dogs with chronic airway disease or tracheal collapse can experience an acute, severe exacerbation of their chronic problems, and extended management may be necessary to resolve the signs associated with infection in these animals. Bordetella infection has been associated with canine chronic bronchitis.
Diagnosis
Uncomplicated cases of kennel cough are diagnosed on the basis of the presenting signs. However, differential diagnoses should also include the early presentation of a more serious disease and the mild form of canine influenza. Diagnostic testing is indicated for dogs with systemic, progressive, or unresolving signs. Tests to be considered include thoracic radiographs, a complete blood count (CBC), tracheal wash fluid analysis, and polymerase chain reaction (PCR) testing, paired serology, or other tests for canine influenza (see p. 302) and other respiratory pathogens. Tracheal wash fluid cytology shows acute inflammation, and bacterial culture of the fluid can be useful for identifying any bacteria involved in the disease. Concurrent antibiotic sensitivity information is helpful in selecting antibiotics.
Treatment
Uncomplicated infectious tracheobronchitis is a self-limiting disease. Rest for at least 7 days, specifically avoiding exercise and excitement, is indicated to minimize the continual irritation of the airways caused by excessive coughing. Cough suppressants are valuable for the same reason but should not be given if the cough is productive or if exudate is suspected to be accumulating in the lungs on the basis of auscultation or thoracic radiograph findings. As discussed in Chapter 19 it is not always possible to recognize a productive cough in dogs. Therefore cough suppressants should be used judiciously to treat frequent or severe cough, allow for restful sleep, and prevent exhaustion.
A variety of cough suppressants can be used in dogs (Table 21-1). Dextromethorphan is available in over-the-counter preparations; however, it has questionable efficacy in dogs. Cold remedies with additional ingredients such as antihistamines and decongestants should be avoided. Pediatric liquid preparations are palatable for most dogs, and the alcohol contained in them may also have a mild tranquilizing effect. Narcotic cough suppressants are more likely to be effective. Butorphanol is available as a veterinary labeled product (Torbutrol, Fort Dodge Animal Health). Hydrocodone bitartrate is a potent alternative for dogs with refractory cough.
 TABLE 21-1 Common Cough Suppressants for Use in Dogs*
TABLE 21-1 Common Cough Suppressants for Use in Dogs*
| AGENT | DOSAGE |
|---|---|
| Dextromethorphan† | 1 to 2 mg/kg, q6-8h orally |
| Butorphanol | 0.5 mg/kg, q6-12h orally |
| Hydrocodone bitartrate | 0.25 mg/kg, q6-12h orally |
* Centrally acting cough suppressants are rarely, if ever, indicated for use in cats and can result in adverse reactions. The preceding dosages are for dogs only.
† Efficacy is questionable in dogs.
In theory, antibiotics are not indicated for most dogs with infectious tracheobronchitis for two reasons: (1) The disease is usually self-limiting and tends to resolve spontaneously, regardless of any specific treatment that is implemented, and (2) no antibiotic protocol has been proven to eliminate Bordetella organisms from the airways. In practice, however, antibiotics are often prescribed, and their use is justified because of the potential role of Bordetella in the disease. Fluoroquinolones have the advantage of reaching high concentrations in the airway secretions, but their use is ideally reserved for more serious infections. Other antibiotics that are effective against many Bordetella isolates include amoxicillin with clavulanate (20 to 25 mg/kg q8h), doxycycline (5 to 10 mg/kg q12h, followed by a bolus of water), and chloramphenicol (50 mg/kg q8h). Beta-lactam antibiotics do not generally reach therapeutic concentrations in airway secretions of healthy (not inflamed) subjects. If such an antibiotic is used for bronchial infections, the high end of the dosage range should be used and the drug administered every 8 hours. The ability of doxycycline to reach therapeutic concentration within the airways is questionable because in the dog it is highly protein bound, although the presence of inflammatory cells may increase locally available concentrations of the drug. Bacterial susceptibility data from tracheal wash fluid can be used to guide the selection of an appropriate antibiotic. Antibiotics are administered for 5 days beyond the time the clinical signs resolve or for at least 14 days.
Administration of gentamicin by nebulization can be considered for refractory cases or in outbreaks of infection involving dogs housed together, although no controlled studies have been published. An early study by Bemis et al. (1997) showed that bacterial populations of Bordetella in the trachea and bronchi were reduced for up to 3 days after treatment with nebulized gentamicin but not orally administered antibiotics, and clinical signs were reduced. Note that the numbers of organisms returned to pretreatment values within 7 days. Some clinicians have since reported success in managing difficult cases and outbreaks with this treatment (Miller et al., 2003). The protocol used by Bemis et al. (1997) is 50 mg of gentamicin sulfate in 3 ml of sterile water, delivered by nebulizer and face mask (see Fig. 22-1) for 10 minutes every 12 hours for 3 days. Sterile technique must be maintained to keep from delivering additional bacteria to the airways. Nebulization of drugs has the potential to induce bronchospasms, so dogs should be carefully observed during the procedure. Pretreatment with bronchodilators should be considered, and additional bronchodilators (metered dose inhaler and/or injectable) should be at hand for use as needed.
Glucocorticoids should not be used. A field trial conducted by Thrusfield et al. (1991) failed to demonstrate any benefit of steroid therapy, either alone or in combination with antibiotics.
If clinical signs have not resolved within 2 weeks, further diagnostic evaluation is indicated. See Chapter 22 for the management of complicated cases of infectious tracheobronchitis with bacterial pneumonia.
Prevention
Canine infectious tracheobronchitis can be prevented by minimizing an animal’s exposure to organisms and through vaccination programs. Excellent nutrition, routine deworming, and avoidance of stress improve the dog’s ability to respond appropriately to infection without showing serious signs.
Bordetella may persist in the airways of dogs for up to 3 months after infection. To minimize exposure to Bordetella or respiratory viruses, dogs are kept isolated from puppies or dogs that have been recently boarded. Careful sanitation should be practiced in kenneling facilities. Caretakers should be instructed in the disinfection of cages, bowls, and runs, and anyone working with the dogs must wash their hands after handling each animal. Dogs should not be allowed to have face-to-face contact. Adequate air exchange and humidity control are necessary in rooms housing several dogs. Recommended goals are at least 10 to 15 air exchanges per hour and less than 50% humidity. An isolation area is essential for the housing of dogs with clinical signs of infectious tracheobronchitis.
Injectable and intranasal vaccines are available for the three major pathogens involved in canine infectious tracheobronchitis (i.e., CAV2, PIV, B. bronchiseptica). Injectable modified-live virus vaccines against CAV2 and PIV are adequate for most pet dogs. They are conveniently included in most combination distemper vaccines. Because maternal antibodies interfere with the response to the vaccines, puppies must be vaccinated every 2 to 4 weeks, beginning at 6 to 8 weeks of age and through 14 to 16 weeks of age. At least two vaccines must be given initially. For most healthy dogs, a booster is recommended after 1 year, followed by subsequent vaccinations every 3 years (see Chapter 94).
Dogs at high risk for disease, such as those in kennels where the disease is endemic or those that are frequently boarded, may benefit from vaccines incorporating B. bronchiseptica. These vaccines do not prevent infection but aim to decrease clinical signs if infection occurs. They may also reduce the duration of shedding of organisms after infection. A study by Ellis et al. (2001) indicated that both intranasal and parenteral Bordetella vaccines afford similar protection based on antibody titers, clinical signs, upper airway cultures, and histopathologic examination of tissues after exposure to organisms. The greatest benefit was achieved by administering both forms of vaccine sequentially at a 2-week interval. Unfortunately, the parenteral vaccine used in the study was a killed bacterin that is no longer available. The dogs in this study were vaccinated between 14 to 18 weeks of age. Also in experimental settings, protection against challenge following intranasal vaccination against B. bronchiseptica and PIV began by 72 hours after vaccination and persisted for at least 13 months (Gore et al., 2005; Jacobs et al., 2005). Intranasal Bordetella vaccines occasionally cause clinical signs, predominantly cough. The signs are generally self-limiting but are disturbing to most owners.
CANINE CHRONIC BRONCHITIS
Etiology
Canine chronic bronchitis is a disease syndrome defined as cough occurring on most days of 2 or more consecutive months in the past year in the absence of other active disease. Histologic changes of the airways are those of long-term inflammation and include fibrosis, epithelial hyperplasia, glandular hypertrophy, and inflammatory infiltrates. Some of these changes are irreversible. Excessive mucus is present within the airways, and small airway obstruction occurs. In people chronic bronchitis is strongly associated with smoking. It is presumed that canine chronic bronchitis is a consequence of a long-standing inflammatory process initiated by infection, allergy, or inhaled irritants or toxins. A continuing cycle of inflammation likely occurs as mucosal damage, mucus hypersecretion, and airway obstruction impairs normal mucociliary clearance, and inflammatory mediators amplify the response to irritants and organisms.
Clinical Features
Chronic bronchitis occurs most often in middle-aged or older, small-breed dogs. Breeds commonly affected include Terriers, Poodles, and Cocker Spaniels. Small-breed dogs are also predisposed to the development of collapsing trachea and mitral insufficiency with left atrial enlargement causing compression of the mainstem bronchi. These causes for cough must be differentiated, and their contribution to the development of the current clinical features determined, for appropriate management to be implemented.
Dogs with chronic bronchitis are evaluated because of loud, harsh cough. Mucus hypersecretion is a component of the disease, but the cough may sound productive or nonproductive. The cough has usually progressed slowly over months to years, although clients usually report the initial onset as acute. There should be no systemic signs of illness such as anorexia or weight loss. As the disease progresses, exercise intolerance becomes evident; then incessant coughing or overt respiratory distress is seen.
Potential complications of chronic bronchitis include bacterial or mycoplasmal infection, tracheobronchomalacia (see p. 297), pulmonary hypertension (Chapter 22), and bronchiectasis. Bronchiectasis is the term for permanent dilation of the airways (Fig. 21-2; see also Fig. 20-4). Bronchiectasis can be present secondary to other causes of chronic airway inflammation, airway obstruction, and in association with certain congenital disorders such as ciliary dyskinesia (i.e., immotile cilia syndrome). Bronchiectasis caused by traction on the airways, rather than bronchial disease, can be seen with idiopathic pulmonary fibrosis. Generally, all the major airways are dilated in dogs with bronchiectasis, but occasionally it is localized. Recurrent bacterial infections and overt bacterial pneumonia are common complications in dogs with bronchiectasis.
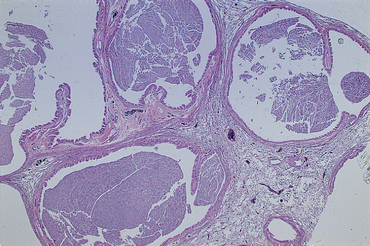
FIG 21-2 Photomicrograph of a lung biopsy from a dog with severe bronchiectasis. The airways are filled with exudate and are greatly dilated (H&E stain).
Dogs with chronic bronchitis are often brought to a veterinarian because of sudden exacerbation of signs. The change in signs may result from transient worsening of the chronic bronchitis, perhaps after a period of unusual excitement, stress, or exposure to irritants or allergens; from a secondary complication, such as bacterial infection; or from the development of a concurrent disease, such as left atrial enlargement and bronchial compression or heart failure (Box 21-1). In addition to obtaining a routine complete history, the client should be carefully questioned about the character of the cough and the progression of signs. Detailed information should be obtained regarding the following: environmental conditions, particularly exposure to smoke, other potential irritants and toxins, or allergens; exposure to infectious agents, such as boarding or exposure to puppies; and all previous and current medications and the response to treatment.
 BOX 21-1 Diagnostic Considerations for Dogs with Signs Consistent with Canine Chronic Bronchitis
BOX 21-1 Diagnostic Considerations for Dogs with Signs Consistent with Canine Chronic Bronchitis
* Gastroesophageal reflux is a common cause of chronic cough in people. Documentation in dogs and cats is limited.
On physical examination, increased breath sounds, crackles, or occasionally wheezes are auscultated in animals with chronic bronchitis. End-expiratory clicks caused by mainstem bronchial or intrathoracic tracheal collapse may be heard in animals with advanced disease. A prominent or split second heart sound occurs in animals with secondary pulmonary hypertension. Dogs with respiratory distress (end-stage disease) characteristically show marked expiratory efforts because of the narrowing and collapse of the intrathoracic large airways. The presence of a fever or other systemic signs is suggestive of other disease, such as bacterial pneumonia.
Diagnosis
Canine chronic bronchitis is defined as a cough occurring on most days of 2 or more consecutive months in the past year in the absence of other active disease. Therefore chronic bronchitis is diagnosed on the basis of not only clinical signs but also the elimination of other diseases from the list of differential diagnoses (see Box 21-1). The possibility of secondary disease complicates this simple definition.
A bronchial pattern with increased interstitial markings is typically seen on thoracic radiographs, but changes are often mild and difficult to distinguish from clinically insignificant changes associated with aging. In a study by Mantis et al. (1998), thoracic radiographs had a sensitivity of 50% to 65% for the diagnosis of chronic bronchitis. Thoracic radiographs are most useful for ruling out other active disease and identifying concurrent or secondary disease.
Tracheal wash or bronchoalveolar lavage (BAL) fluid should be collected at the time of the initial presentation and after a persistent exacerbation of signs. Neutrophilic or mixed inflammation and increased amounts of mucus are usually present. The finding of degenerative neutrophils indicates the possibility of a bacterial infection. Although not a specific finding, airway eosinophilia is suggestive of a hypersensitivity reaction, as can occur with allergy, parasitism, or heartworm disease. Slides should be carefully examined for organisms. Bacterial cultures are performed and the results interpreted as discussed in Chapter 20. Although the role of Mycoplasma infections in these cases is not well understood, Mycoplasma cultures are also considered.
Bronchoscopy, with specimen collection, is performed in selected cases, primarily to help rule out other diseases. The maximal benefit of bronchoscopy is obtained early in the course of disease, before severe permanent damage has occurred and while the risk of the procedure is minimal. Gross abnormalities visualized by bronchoscopy include an increased amount of mucus, roughened mucosa, and hyperemia. Major airways may collapse during expiration as a result of weakened walls (Fig. 21-3), and polypoid mucosal proliferation may be present. Bronchial dilatation is seen in animals with bronchiectasis.
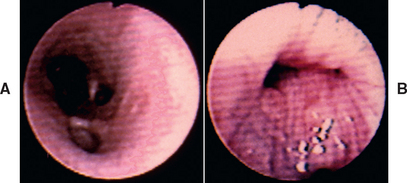
FIG 21-3 Bronchoscopic view of the right caudal bronchus of a dog with chronic bronchitis and severe bronchomalacia. The airways appear normal during inspiration (A) but completely collapse during expiration, obliterating the lumen of the airway (B).
Further diagnostic procedures are indicated to rule out other potential causes of chronic cough, and the selection of these depends on the presenting signs and the results of the previously discussed diagnostic tests. Diagnostic tests to be considered include heartworm tests, fecal examinations for pulmonary parasites, echocardiography, and systemic evaluation (i.e., CBC, serum biochemical panel, urinalysis). Echocardiography may reveal evidence of secondary pulmonary hypertension, including right heart enlargement (i.e., cor pulmonale).
Ciliary dyskinesia, in which ciliary motion is abnormal, is uncommon but should be considered in young dogs with bronchiectasis or recurrent bacterial infection. Abnormalities exist in all ciliated tissues, and situs inversus (i.e., lateral transposition of the abdominal and thoracic organs, such that left-sided structures are found on the right and vice versa) is seen in 50% of such dogs. Dextrocardia occurring in association with chronic bronchitis is extremely suggestive of this disease. Sperm motility can be evaluated in intact male dogs. The finding of normal sperm motility rules out a diagnosis of ciliary dyskinesia. The disease is diagnosed on the basis of the rate at which radioisotopes deposited at the carina are cleared and the findings from electron microscopic examination of bronchial biopsy, nasal biopsy, or sperm specimens.
Treatment
Chronic bronchitis is managed symptomatically, with specific treatment possible only for concurrent or complicating diseases that are identified. Each dog with chronic bronchitis is presented at a different stage of the disease, with or without concurrent or secondary cardiopulmonary disease (see Box 21-1). Hence each dog must be managed individually. Ideally, medications are initiated one at a time to assess the most effective combination. It will likely be necessary to modify treatment over time.
GENERAL MANAGEMENT
Exacerbating factors, either possible or proven, are avoided. Potential allergens are considered in dogs with eosinophilic inflammation and trial elimination pursued (see the section on allergic bronchitis, p. 299). Exposure to irritants such as smoke (from tobacco or fireplace) and perfumed products should be avoided in all dogs. Motivated clients can take steps to improve the air quality in their home, such as carpet, furniture, and drapery cleaning; cleaning of the furnace and the frequent replacement of air filters; and the use of an air cleaner. The American Lung Association has a useful Web site with nonproprietary recommendations for improving indoor air quality (www.lungusa.org). Excitement or stress can cause an acute worsening of signs in some animals, and short-term tranquilization with acepromazine or sedation with phenobarbital can be helpful in relieving the signs.
It is normal for flora from the oropharynx to be aspirated into the airways. Routine dental prophylaxis and teeth brushing will help maintain a healthy oral flora and may decrease any contributions of normal aspiration to ongoing airway inflammation in these patients with reduced mucociliary clearance.
Airway hydration should be maintained to facilitate mucociliary clearance. Adequate airway hydration is best achieved by maintaining systemic hydration. Therefore diuretic therapy is not recommended in these patients. For severely affected dogs, placing the animal in a steamy bathroom or in a room with a vaporizer daily may provide symptomatic relief, although the moisture does not penetrate very deeply into the airways. Nebulization of saline will allow moisture to go more deeply in the lungs. This technique is discussed further in the section on bacterial pneumonia in Chapter 22.
Patients that are overweight and/or unfit may benefit from weight loss (Chapter 54) and exercise. Exercise should be tailored to the dog’s current fitness level and degree of pulmonary dysfunction to keep from causing excessive respiratory efforts or even death. Observing the dog during specific exercise, such as a short walk, while in the client’s presence may be necessary to make initial recommendations. Instructing clients in the measurement of respiratory rate, observation of mucous membrane color, and signs of increased respiratory effort will improve their ability to assess their dog’s status during exercise.
DRUG THERAPIES
Medications to control clinical signs include bronchodilators, glucocorticoids, and cough suppressants.
Theophylline, a methylxanthine bronchodilator, has been used for years for the treatment of chronic bronchitis in people and dogs. This drug became unpopular with physicians when newer bronchodilators with fewer side effects became available. However, recent research in people suggests that theophylline is effective in treating the underlying inflammation of chronic bronchitis, even at concentrations below those resulting in bronchodilation (hence, reducing side effects), and that the antiinflammatory effects may be synergistic with those of glucocorticoids. Theophylline may also improve mucociliary clearance, decrease fatigue of respiratory muscles, and inhibit the release of mast cell mediators of inflammation. The potential beneficial effects of theophylline beyond bronchodilation may be of particular importance in dogs because their airways are not as reactive (i.e., likely to bronchospasm) as those of cats and people. However, theophylline alone is rarely sufficient to control the clinical signs of chronic bronchitis.
Other advantages of theophylline are the availability of long-acting preparations that can be administered twice daily to dogs and the fact that plasma concentrations of drug can be easily measured by commercial diagnostic laboratories. A disadvantage of theophylline is that other drugs, such as fluoroquinolones and chloramphenicol, can delay its clearance and cause signs of theophylline toxicity if the dosage is not reduced by one third to one half. Potential adverse effects include gastrointestinal signs, cardiac arrhythmias, nervousness, and seizures. Serious adverse effects are extremely rare at therapeutic concentrations.
Variability in sustained plasma concentrations has been found for different long-acting theophylline products. Dosage recommendations are currently available for a generic product from a specific manufacturer (Box 21-2). If beneficial effects are not seen, the patient is predisposed to adverse effects, or adverse effects occur, plasma theophylline concentrations should be measured. Therapeutic peak concentrations for bronchodilation, based on data from people, are 5 to 20 μg/ml. Plasma is collected during peak concentrations, generally 4 to 5 hours after administration of a long-acting product or 1.5 to 2 hours after administration of immediate release products. Measurement of concentrations immediately before the next scheduled dose might provide useful information concerning duration of therapeutic concentrations.
 BOX 21-2 Common Bronchodilators for Use in Dogs and Cats
BOX 21-2 Common Bronchodilators for Use in Dogs and Cats
Methylxanthines
Oxtriphylline elixir (Choledyl, Parke-Davis)
Theophylline base (immediate release)
Long-acting theophylline (Theochron or TheoCap, Inwood Laboratories, Inwood, NY)*
* Canine dosage for these products from Inwood Laboratories from Bach JF et al: Evaluation of the bioavailability and pharmacokinetics of two extended-release theophylline formulations in dogs, J Am Vet Med Assoc 224:1113, 2004. Feline dosage from Guenther-Yenke CL et al: Pharmacokinetics of an extended-release theophylline product in cats. J Am Vet Med Assoc 231:900, 2007. Monitoring of plasma concentrations is recommended in patients at risk for or with signs of toxicity and in patients that fail to respond to treatment.
Theophylline and related drugs that are not long acting are useful in specific circumstances but must be administered three times daily (see Box 21-2). Palatable elixirs of theophylline derivatives (e.g., oxtriphylline) are convenient for administration to toy breeds. Therapeutic blood concentrations are reached more quickly after the administration of liquids, or tablets or capsules that are not long acting.
Sympathomimetic drugs are preferred by some clinicians as bronchodilators (see Box 21-2). Terbutaline and albuterol are selective for β2-adrenergic receptors, lessening their cardiac effects. Potential adverse effects include nervousness, tremors, hypotension, and tachycardia. The clinical use of bronchodilators delivered by metered-dose inhaler, such as albuterol and ipatropium (a parasympatholytic), has not been reported in dogs with chronic bronchitis.
Glucocorticoids are often effective in controlling the signs of chronic bronchitis and may slow the development of permanent airway damage by decreasing inflammation. They may be particularly helpful in dogs with eosinophilic airway inflammation. Potential negative effects include an increased susceptibility to infection in dogs already impaired by decreased airway clearance; a tendency toward obesity, hepatomegaly, and muscle weakness that may adversely affect ventilation; and pulmonary thromboembolism. Therefore short-acting products are used, the dose is tapered to the lowest effective one (when possible, 0.5 mg/kg q48h or less), and the drug is discontinued if no beneficial effect is seen. Prednisone is initially given at a dose of 0.5 to 1.0 mg/kg every 12 hours, with a positive response expected within 1 week.
Dogs that require relatively high dosages of prednisone, have unacceptable adverse effects, or have conditions for which glucocorticoids are relatively contraindicated (e.g., diabetes mellitus) may benefit from local treatment with metered-dose inhalers. This route of administration is discussed in more detail later in this chapter, in the section on feline bronchitis (p. 295).
Cough suppressants are used cautiously because cough is an important mechanism to clear airway secretions. In some dogs, however, the cough is incessant and exhausting, or ineffective because of marked tracheobronchomalacia and airway collapse. Cough suppressants can provide significant relief in such animals and may even facilitate ventilation and decrease anxiety.
Although the doses given in Table 21-1 are the ones that provide prolonged effectiveness, less frequent administration (i.e., only during times of the day when coughing is most severe) may preserve some beneficial effect of cough. For dogs with severe cough, hydrocodone may provide the greatest relief.
MANAGEMENT OF COMPLICATIONS
Antibiotics are often prescribed for dogs with chronic bronchitis. If possible, confirmation of infection and antibiotic sensitivity information should be obtained by culture of an airway specimen (e.g., tracheal wash fluid). Because cough in dogs with chronic bronchitis often waxes and wanes in severity, it is difficult to make a diagnosis of infection on the basis of the patient’s response to therapy. Furthermore, organisms involved in bronchial infections generally originate from the oropharynx. They are frequently gramnegative with unpredictable antibiotic sensitivity patterns. The role of Mycoplasma organisms in canine chronic bronchitis is not well understood. They may be an incidental finding or pathogenic. Ideally, antibiotic selection is based on results of culture. Antibiotics that are generally effective against Mycoplasma include doxycycline, azithromycin, chloramphenicol, and fluoroquinolones.
In addition to the susceptibility of identified organisms, the ability of selected antibiotics to penetrate the airway secretions to the site of infection should be considered when selecting an antibiotic. Antibiotics that are likely to reach concentrations effective against susceptible organisms include chloramphenicol, fluoroquinolones, azithromycin, and possibly amoxicillin with clavulanate. Beta-lactam antibiotics do not generally reach therapeutic concentrations in airway secretions of healthy (not inflamed) subjects. If used for bronchial infections, the high end of the dosage range should be used and the drug administered every 8 hours (20 to 25 mg/kg q8h). Doxycycline has often been recommended because Mycoplasma and many Bordetella isolates are susceptible to this drug. However, the ability of doxycycline to reach therapeutic concentration within the airways is questionable because in the dog it is highly protein bound, although the presence of inflammatory cells may increase locally available concentrations of the drug. It is preferable to reserve fluoroquinolones for serious infections.
If an antibiotic is effective, a positive response is generally seen within 1 week. Treatment is then continued for at least 1 week beyond the time when the clinical signs stabilize because complete resolution is unlikely in these animals. Antibiotic treatment usually is necessary for 3 to 4 weeks. Even longer treatment may be necessary in some cases, particularly if bronchiectasis or overt pneumonia is present. The use of antibiotics for the treatment of respiratory tract infections is also discussed in the section on canine infectious tracheobronchitis in this chapter (p. 285) and in the section on bacterial pneumonia in Chapter 22.
Tracheobronchomalacia is discussed on p. 297, and pulmonary hypertension is discussed in Chapter 22.
Prognosis
Canine chronic bronchitis cannot be completely cured. The prognosis for the control of signs and a satisfactory quality of life in animals is good if the owners are conscientious about performing the medical management aspects of care, are willing to adjust treatment over time, and treat secondary problems as they occur.
FELINE BRONCHITIS (IDIOPATHIC)
Etiology
Cats with respiratory disease of many etiologies present with signs of bronchitis or asthma. Cat airways are much more reac tive, prone to bronchoconstriction, than dogs. The common presenting signs of bronchitis (i.e., cough, wheezing, and/or respiratory distress) can occur in cats with diseases as varied as lung parasites, heartworm disease, allergic bronchitis, bacterial or viral bronchitis, toxoplasmosis, idiopathic pulmonary fibrosis, carcinoma, and aspiration pneumonia (Table 21-2). Veterinarians often assume that cats with presenting signs of bronchitis or asthma have idiopathic disease because in most cats an underlying etiology cannot be found. However, as with canine chronic bronchitis, a diagnosis of idiopathic feline bronchitis can be made only by ruling out other active disease. Care should be taken when using the terms feline bronchitis or feline asthma to distinguish between a presentation consistent with bronchitis in a broad sense and a clinical diagnosis of idiopathic disease. Cats with idiopathic bronchitis often have some degree of airway eosinophilia, typical of an allergic reaction. This author prefers to reserve the diagnosis of allergic bronchitis to patients who respond dramatically to the elimination of a suspected allergen (see p. 299).
 TABLE 21-2 Differential Diagnoses (Etiologic) for Cats with Presenting Signs of Bronchitis
TABLE 21-2 Differential Diagnoses (Etiologic) for Cats with Presenting Signs of Bronchitis
| DIAGNOSIS | DISTINGUISHING FEATURES COMPARED WITH IDIOPATHIC FELINE BRONCHITIS |
|---|---|
| Allergic bronchitis | Dramatic clinical response to elimination of suspected allergen(s) from environment or diet. |
| Pulmonary parasites (Aelurostrongylus abstrusus; Capillaria aerophila; Paragonimus kellicotti) | Thoracic radiographs may have a nodular pattern; Larvae (Aelurostongylus) or eggs identified in tracheal wash or BAL fluid or in the feces. See Chapter 20 for appropriate procedures for fecal testing. |
| Heartworm disease | Pulmonary artery enlargement may be present on thoracic radiographs; positive heartworm antigen test or identification of adult worm(s) on echocardiography (see Chapter 10). |
| Bacterial bronchitis | Intracellular bacteria in tracheal wash or BAL fluid and significant growth on culture (see Chapter 20). |
| Mycoplasmal bronchitis | Growth of Mycoplasma on specific culture of tracheal wash or BAL fluid (presence may indicate primary infection, secondary infection, or be incidental). |
| Idiopathic pulmonary fibrosis | Radiographs may show more severe infiltrates than expected in cats with idiopathic bronchitis; diagnosis requires lung biopsy (see Chapter 22). |
| Carcinoma | Radiographs may show more severe infiltrates than expected in cats with idiopathic bronchitis. Cytologic or histologic identification of malignant cells in tracheal wash or BAL fluid, lung aspirates, or lung biopsy. Histologic confirmation is ideal. |
| Toxoplasmosis | Systemic signs usually present (fever, anorexia, depression). Radiographs may show more severe infiltrates than expected in cats with idiopathic bronchitis, possibly with a nodular pattern. Diagnosis is confirmed by identification of organisms (tachyzoites) in tracheal wash or BAL fluid. Rising serum antibody titers or elevated IgM concentrations are supportive of the diagnosis (see Chapter 99). |
| Aspiration pneumonia | Unusual in cats. History supportive of a predisposing event or condition. Radiographs typically show an alveolar pattern, worse in the dependent (cranial and middle) lung lobes. Neutrophilic inflammation, usually with bacteria, in tracheal wash fluid. |
| Idiopathic feline bronchitis | Elimination of other diseases from the differential diagnoses. |
BAL, bronchoalveolar lavage.
A wide variety of pathologic processes can affect individual cats with idiopathic bronchitis. Clinically, the range in the severity of signs and the response to therapy shows this diversity. Different combinations of factors that result in small airway obstruction, a consistent feature of feline bronchial disease, are present in each animal (Box 21-3). Some of these factors are reversible (e.g., bronchospasm, inflammation), and some are permanent (e.g., fibrosis, emphysema). The classification proposed by Moise et al. (1989), which was formulated on the basis of similar pathologic processes that occur in people, is recommended as a way to better define bronchial disease in individual cats for the purpose of treatment recommendations and prognostication (Box 21-4). A cat can also have more than one type of bronchitis. Although it is not always possible to absolutely determine the type or types of bronchial disease present without sophisticated pulmonary function testing, routine clinical data (i.e., history and physical examination findings, thoracic radiographs, analysis of airway specimens, progression of signs) can be used to classify the disease in most cats.
 BOX 21-3 Factors that Can Contribute to Small Airway Obstruction in Cats with Bronchial Disease
BOX 21-3 Factors that Can Contribute to Small Airway Obstruction in Cats with Bronchial Disease
Bronchial smooth muscle hypertrophy
Inflammatory exudate in airway lumens
 BOX 21-4 Classification of Feline Bronchial Disease
BOX 21-4 Classification of Feline Bronchial Disease
Adapted from Moise NS et al: Bronchopulmonary disease. In Sherding RG, editor: The cat: diseases and clinical management, New York, 1989, Churchill Livingstone.
Bronchial Asthma
Predominant feature: reversible airway obstruction primarily resulting from bronchoconstriction
Other common features: hypertrophy of smooth muscle, increased mucus production, eosinophilic inflammation
Acute Bronchitis
Predominant feature: reversible airway inflammation of short duration (<1-3 months)
Other common features: increased mucus production, neutrophilic or macrophagic inflammation
Chronic Bronchitis
Predominant feature: chronic airway inflammation (>2-3 months) resulting in irreversible damage (e.g., fibrosis)
Other common features: increased mucus production; neutrophilic, eosinophilic, or mixed inflammation; isolation of bacteria or Mycoplasma organisms causing infection or as nonpathogenic inhabitants; concurrent bronchial asthma
Clinical Features
Idiopathic bronchitis can develop in cats of any age, although it most commonly develops in young adult and middle-aged animals. The major clinical feature is cough or episodic respiratory distress or both. The owners may report audible wheezing during an episode. The signs are often slowly progressive. Weight loss, anorexia, depression, or other systemic signs are not present. If systemic signs are identified, another diagnosis should be aggressively pursued.
Owners should be carefully questioned regarding an association with exposure to potential allergens or irritants. Irritants in the environment can cause worsening of signs of bronchitis regardless of the underlying etiology. Environmental considerations include exposure to new litter (usually perfumed), cigarette or fireplace smoke, carpet cleaners, and household items containing perfumes such as deodorant or hair spray. Clients should also be questioned about whether there has been any recent remodeling or any other change in the cat’s environment. Seasonal exacerbations are suggestive of potential allergen exposure.
Physical examination abnormalities result from small airway obstruction. Cats that are in distress show tachypnea. Typically the increased respiratory efforts are more pronounced during expiration, and auscultation reveals expiratory wheezes. Crackles are occasionally present. In some patients in distress, hyperinflation of the lungs due to air trapping may result in increased inspiratory efforts and decreased lung sounds. Physical examination findings may be unremarkable between episodes.
Diagnosis
A diagnosis of idiopathic feline bronchitis is made on the basis of typical historical, physical examination, and thoracic radiographic findings and the elimination of other possible differential diagnoses (see Table 21-2). A thorough search for other diagnoses is highly recommended, even though a specific diagnosis is not commonly found, because identifying an etiology for the clinical signs may allow for specific treatment and even cure of an individual cat. Factors to consider when developing a diagnostic plan include the clinical condition of the cat and the client’s tolerance for expense and risk. Cats that are in respiratory distress or are otherwise in critical condition should not undergo any stressful testing until their condition has stabilized. Sufficiently stable cats that have any indication of a diagnosis other than idiopathic disease on the basis of presenting signs and thoracic radiographs or any subsequent test results require a thorough evaluation. Certain tests are completely safe, such as fecal testing for pulmonary parasites, and their inclusion in the diagnostic plan is largely based on financial considerations. In most cats with signs of bronchitis, collection of tracheal wash fluid for cytology and culture and tests for pulmonary parasitism and heartworm disease are recommended.
A CBC is often performed as a routine screening test. Cats with idiopathic bronchitis are often thought to have peripheral eosinophilia. However, this finding is neither specific nor sensitive and cannot be used to rule out or definitively diagnose feline bronchitis.
Thoracic radiographs from cats with bronchitis generally show a bronchial pattern (see Fig. 20-3). Increased reticular interstitial markings and patchy alveolar opacities may also be present. The lungs may be seen to be overinflated as a result of the trapping of air, and occasionally collapse (i.e., atelectasis) of the right middle lung lobe is seen (see Fig. 20-9). However, because clinical signs can precede radiographic changes and because radiographs cannot detect mild airway changes, thoracic radiographs may be normal in cats with bronchitis. Radiographs are also scrutinized for signs of specific diseases (see Table 21-2).
The tracheal wash or BAL fluid cytologic findings are generally representative of the airway inflammation and consist of increased numbers of inflammatory cells and an increased amount of mucus. Inflammation can be eosinophilic, neutrophilic, or mixed. Although not a specific finding, eosinophilic inflammation is suggestive of a hypersensitivity response to allergens or parasites. Neutrophils should be examined for signs of the degeneration suggestive of bacterial infection. Slides should be carefully scrutinized for the presence of organisms, particularly bacteria and parasitic larvae or ova. Fluid should be cultured for bacteria, although it is important to note that the growth of organisms may or may not indicate the existence of true infection (see Chapter 20). Cultures for Mycoplasma spp. may also be helpful.
Testing for heartworm disease is described in Chapter 10. Multiple fecal examinations using special concentrating techniques are performed to identify pulmonary parasites, particularly in young cats and cats with airway eosinophilia (see Chapter 20). Other tests may be indicated for individual cats.
EMERGENCY STABILIZATION
The condition of cats in acute respiratory distress should be stabilized before diagnostic tests are performed. Successful treatment includes administration of a bronchodilator, rapid-acting glucocorticoids, and oxygen supplementation. Terbutaline can be administered subcutaneously, a route that avoids additional patient stress (see Box 21-2). Prednisolone sodium succinate is the recommended glucocorticoid for a life-threatening crisis (up to 10 mg/kg, administered intravenously). If intravenous administration is too stressful, the drug can be given intramuscularly. Alternatively, dexamethasone sodium phosphate (up to 2 mg/kg, administered intravenously) can be given. After the drugs are administered, the cat is placed in a cool, stress-free, oxygen-enriched environment. If additional bronchodilation is desired, albuterol can be administered by nebulization or metered-dose inhaler (MDI). Administration of drugs by MDI is described later in this section. (See Chapter 26 for further discussion of cats with respiratory distress.)
ENVIRONMENT
The potential influence of the environment on clinical signs should be investigated. Allergic bronchitis is diagnosed through the elimination of potential allergens from the environment (see the section on allergic bronchitis). However, even cats with idiopathic bronchitis can benefit from improvement in indoor air quality through the reduction of irritants or unidentified allergens. Potential sources of allergens or irritants are determined through careful owner questioning as described in the section on clinical features. Smoke can often aggravate signs because of its local irritating effects. The effect of litter perfumes can be evaluated by replacing the litter with sandbox sand or plain clay litter. Indoor cats may show improvement in response to measures taken to decrease the level of dusts, molds, and mildew in the home. Such measures include carpet, furniture, and drapery cleaning; cleaning of the furnace and the frequent replacement of air filters; and the use of an air cleaner. The American Lung Association has a useful website with nonproprietary recommendations for improving indoor air quality (www.lungusa.org). Any beneficial response to an environmental change is usually seen within 1 to 2 weeks.
GLUCOCORTICOIDS
Therapy with glucocorticoids, with or without bronchodilators, is necessary for most cats with idiopathic bronchitis. Results can be dramatic. However, drug therapy can interfere with environmental testing; therefore the ability of the animal to tolerate a delay in the start of drug therapy must be assessed on an animal-by-animal basis. Glucocorticoids can relieve the clinical signs in most cats and may protect the airways from the detrimental effects of chronic inflammation. Short-acting products such as prednisolone are recommended because the dose can be tapered to the lowest effective amount. Anecdotal experience and a preliminary study suggest that prednisolone may be more effective in cats than prednisone (Graham-Mize et al., 2004). A dose of 0.5 to 1 mg/kg is administered every 12 hours initially, with the dose doubled if signs are not controlled within 1 week. Once the signs are well controlled, the dose is tapered. A reasonable goal is to administer 0.5 mg/kg or less every other day. Outdoor cats that cannot be treated frequently can be administered depot steroid products, such as methylprednisolone acetate (10 mg/cat intramuscularly may be effective for up to 4 weeks).
Glucocorticoids, such as fluticasone propionate (Flovent, GlaxoSmithKline), can also be administered locally to the airways by MDI, as is routine for treating asthma in people. The advantages are minimal systemic side effects and relative ease of administration in some cats compared with pilling. To date, however, it is still not known how much drug is deposited in the lower airways, how much remains in the oral and nasal cavities, and how much is absorbed systemically in cats. Theoretical concerns about the oronasal deposition of the potent glucocorticoid in cats, compared with people, include the high incidence of periodontal disease and latent herpesvirus infections and the inability to effectively rinse the mouth with water after use. Local dermatitis because of mites, dermatophytes, or bacteria can occur. However, some veterinarians have been using glucocorticoid MDIs to treat idiopathic feline bronchitis for many years without frequent, obvious adverse effects.
This author prefers to obtain a clinical remission of signs using orally administered drug first, except in cats with relative contraindications for systemic glucocorticoid therapy, such as diabetes mellitus. Cats that require a relatively low dose of oral glucocorticoids to control clinical signs, have no noticeable adverse effects, and can be pilled without difficulty are often well maintained with oral therapy. Otherwise, once signs are in remission, treatment by MDI is initiated and the dosage of oral prednisolone gradually reduced.
A spacer must be used for effectively administering drugs by MDI to cats, and the airflow generated by the cat must be sufficient to activate the spacer valve. Padrid (2000) has found the OptiChamber (Respironics, Inc) to be effective (Fig. 21-4). A small anesthetic mask, with rubber diaphragm, is attached to the spacer. Widening of the adapter of the anesthetic mask that is inserted into the spacer is necessary to create a snug fit. This is achieved by wrapping adhesive tape around the adapter. Alternatively, a mask with spacer specifically designed for use in cats is available (Aerokat, Trudell Medical International). The cat is allowed to rest comfortably on a table or in the client’s lap. The client places his or her arms on either side of the cat or gently steadies the cat’s neck and head to provide restraint (Fig. 21-5). The MDI, attached to the spacer, is actuated (i.e., pressed) twice. The mask is placed immediately on the cat’s face, covering the mouth and nose completely, and is held in place while the cat takes 7 to 10 breaths, inhaling the drug into its airways.
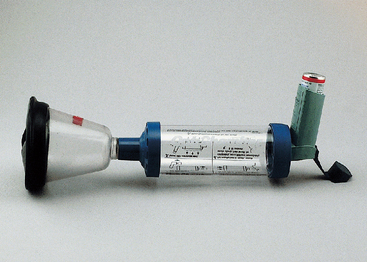
FIG 21-4 Apparatus for administering drugs by metered dose inhaler (MDI) to cats consisting of an anesthetic mask, spacer (OptiChamber, Respironics, Inc., Pittsburgh, Pa.), and MDI (Ventolin, GlaxoSmithKline, Research Triangle Park, N.C.).
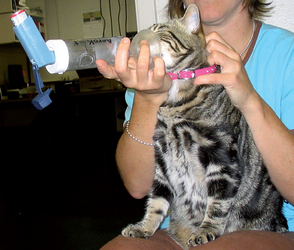
FIG 21-5 Administering drugs by metered-dose inhaler (MDI) to a cat. The mask and chamber apparatus is the Aerokat (Trudell Medical International, London, Ontario, Canada).
The following treatment schedule has been recommended (Padrid, 2000): Cats with mild daily symptoms should receive 220 μg of fluticasone propionate by MDI twice daily and albuterol by MDI as needed. The maximal effect of fluticasone is not expected until 7 to 10 days of treatment. Cats with moderate daily symptoms should receive treatments with MDI as described for mild symptoms; in addition, prednisolone is administered orally for 10 days (1 mg/kg every 12 hours for 5 days, then every 24 hours for 5 days). For cats with severe symptoms, dexamethasone is administered once (2 mg/kg, intravenously), albuterol is administered by MDI every 30 minutes for up to 4 hours, and oxygen is administered. Once stabilized, these cats are prescribed 220 μg of fluticasone propionate by MDI every 12 hours and albuterol by MDI every 6 hours as needed. Oral prednisolone is administered as needed.
BRONCHODILATORS
Cats that require relatively large amounts of glucocorticoids to control clinical signs, react unfavorably to glucocorticoid therapy, or suffer from periodic exacerbations of signs can benefit from bronchodilator therapy. Recommended doses of these drugs are listed in Box 21-2.
This author prefers to use theophylline because it is effective and inexpensive and can be given to cats once daily; moreover, the plasma concentrations can be easily measured for the monitoring of difficult cases. Additional properties of theophylline, potential drug interactions, and adverse effects are described in the section on canine chronic bronchitis (p. 290).
The pharmacokinetics of theophylline products are different in cats compared with dogs, resulting in different dosages (see Box 21-2). Variability in sustained plasma concentrations in both species has been found for different long-acting theophylline products. Dosage recommendations are currently available for a generic product from a specific manufacturer (Box 21-2). However, the individual metabolism of all of the methylxanthines is variable. If beneficial effects are not seen, the patient is predisposed to adverse effects, or adverse effects occur, plasma theophylline concentrations should be measured. Therapeutic peak concentrations, based on data from human subjects, are 5 to 20 μg/ml. Plasma for the determination of these concentrations should be collected 12 hours after the evening dosing of the long-acting products and 2 hours after short-acting products. Measurement of concentrations immediately before the next scheduled dose might provide useful information concerning duration of therapeutic concentrations.
Sympathomimetic drugs can also be effective bronchodilators. Terbutaline is selective for β2-adrenergic receptors, lessening its cardiac effects. Potential adverse effects include nervousness, tremors, hypotension, and tachycardia. It can be administered subcutaneously for the treatment of respiratory emergencies; it can also be administered orally. Note that the recommended oral dose for cats (one eighth to one fourth of a 2.5-mg tablet; see Box 21-2) is lower than the commonly cited dose of 1.25 mg/cat. The subcutaneous dose is lower still: 0.01 mg/kg, repeated once in 5 to 10 minutes if necessary.
Bronchodilators can be administered to cats by MDI for the immediate treatment of acute respiratory distress (asthma attack). Cats with idiopathic bronchitis are routinely prescribed an albuterol MDI, spacer, and mask (see the section on glucocorticoids for details) to be kept at home for emergencies.
OTHER POTENTIAL TREATMENTS
A therapeutic trial with an antibiotic effective against Mycoplasma is considered because of the difficulty in documenting infection with this organism. Either doxycycline (5 to 10 mg/kg q12h) or chloramphenicol (10 to 15 mg/kg q12h) is administered for 14 days. For cats that are difficult to medicate, azithromycin (5 to 10 mg/kg q24h for 3 days, then q72h) can be tried. Remember that administration of doxycycline should always be followed with a bolus of water to minimize the incidence of esophageal stricture.
Antihistamines are not recommended for treating feline bronchitis because histamine in some cats produces bronchodilation. However, work done by Padrid et al. (1995) has shown that the serotonin antagonist, cyproheptadine, has a bronchodilatory effect in vitro. A dose of 2 mg/cat orally every 12 hours can be tried in cats with signs that cannot be controlled with routine bronchodilator and glucocorticoid therapy. This treatment is not consistently effective.
Much interest has been shown among clients and veterinarians in the use of oral leukotriene inhibitors in cats (e.g., Accolate, Singulair, and Zyflo). However, the clinician should be aware that in people, leukotriene inhibitors are less effective in the management of asthma than glucocorticoids, and they are not used in the emergency management of the disease or for refractory cases. Their advantage for people lies in decreased side effects, compared with glucocorticoids, and ease of administration. To date, toxicity studies have not been performed on these drugs in cats. Furthermore, several preliminary studies suggest that leukotriene inhibition in cats would not be expected to have efficacy comparable to that in people. Therefore their routine use in cats is not currently advocated. Further investigation into their potential role in treating feline bronchitis is certainly indicated.
FAILURE TO RESPOND
The clinician should ask himself or herself the questions listed in Box 21-5 if cats fail to respond to glucocorticoid and bronchodilator therapy or if exacerbation of signs occurs during chronic treatment.
 BOX 21-5 Considerations for Cats with Bronchitis that Fail to Respond to Glucocorticoid and Bronchodilator Therapy
BOX 21-5 Considerations for Cats with Bronchitis that Fail to Respond to Glucocorticoid and Bronchodilator Therapy
Was an Underlying Disease Missed on Initial Evaluation?
Repeat diagnostic evaluation, including complete history for potential allergens, thoracic radiographs, tracheal wash fluid analysis, heartworm tests, and fecal examinations for parasites. In addition, perform complete blood count, serum biochemical analysis, and urinalysis.
Initiate trial therapy with anti-Mycoplasma drug.
Initiate trial environmental manipulations to minimize potential allergen and irritant exposure.
Prognosis
The prognosis for the control of clinical signs of idiopathic feline bronchitis is good for most cats, particularly if extensive permanent damage has not yet occurred. Complete cure is unlikely, and most cats require continued medication. Cats that have severe, acute asthmatic attacks are at risk for sudden death. Cats with persistent, untreated airway inflammation can develop the permanent changes of chronic bronchitis and emphysema.
COLLAPSING TRACHEA AND TRACHEOBRONCHOMALACIA
Etiology
The normal trachea is seen to be circular on cross section (see Fig. 21-8, B, and Fig. 20-27, A). An open lumen is maintained during all phases of quiet respiration by the cartilaginous tracheal rings, which are connected by fibroelastic annular ligaments to maintain flexibility, thereby allowing movement of the neck without compromising the airway. The cartilaginous rings are incomplete dorsally. The dorsal tracheal membrane, consisting of the longitudinal tracheal muscle and connective tissue, completes the rings. The term tracheal collapse refers to the narrowing of the tracheal lumen resulting from weakening of the cartilaginous rings, a redundancy of the dorsal tracheal membrane, or both. The condition can affect the extrathoracic trachea, the intrathoracic trachea, or both.
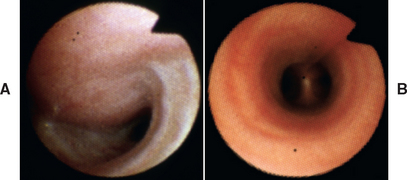
FIG 21-8 Bronchoscopic images from a dog with tracheal collapse (A). The dorsal tracheal membrane is much wider than that of a normal dog (B). The airway lumen is greatly compromised.
A credible theory of the pathogenesis of tracheal collapse is that certain dogs are predisposed to collapse because of inherent abnormalities in their cartilage but are initially asymptomatic. An exacerbating problem develops that results in increased respiratory efforts, airway inflammation, and/or cough. Changes in intrathoracic and airway pressures during increased respiratory efforts or cough likely contribute to narrowing of the trachea, and the chronic presence of inflammatory mediators (e.g., collagenases and proteases) within the trachea likely further weaken its structure. Any narrowing of the trachea results in greatly increased resistance to air flow and local turbulence because the resistance to airflow is proportional to the reciprocal of the radius of the lumen to the fourth power. This increased resistance may further contribute to a cycle of increased respiratory efforts, cough, and inflammation. In addition, as described for canine chronic bronchitis, a continuing cycle of inflammation is also plausible as a result of mucosal damage. Mucus hypersecretion and airway obstruction impair normal mucociliary clearance, and inflammatory mediators amplify the response to irritants and organisms.
Clinically, tracheal collapse often occurs in conjunction with canine chronic bronchitis. In dogs with chronic bronchitis, the intrathoracic trachea is most often affected. Dogs with chronic bronchitis may initially demonstrate collapse of their major (mainstem and/or lobar) bronchi. The lumen of these airways is normally maintained by rafts of cartilage within their walls, rather than rings. Chronic exposure to inflammatory mediators presumably plays a role in the resultant loss of normal airway structure. In addition, obstruction of smaller airways because of excess mucus and mucosal alterations may decrease the intraluminal airway pressures in the larger airways during expiration and contribute to airway collapse. The general term for weakening of the normal tracheal and bronchial structure is tracheobronchomalacia.
As a result of intrathoracic and airway pressures, the extrathoracic trachea tends to collapse during inspiration. The intrathoracic trachea and mainstem and lobar bronchi tend to collapse during expiration.
Clinical Features
Tracheal collapse is common in middle-aged toy and miniature dogs, although it also can occur early in life and in large-breed dogs. Signs may occur acutely but then slowly progress over months to years. The primary clinical feature in most dogs is a nonproductive cough, described as a “goose honk.” The cough is worse during excitement or exercise or when the collar exerts pressure on the neck. Eventually (usually after years of chronic cough), respiratory distress caused by obstruction to airflow may be brought on by excitement, exercise, or overheating. Systemic signs such as weight loss, anorexia, and depression are not expected. Occasionally, dogs are presented primarily for signs of upper airway obstruction without cough, also exacerbated during excitement, exercise, or hot weather. Stertorous sounds may be heard during periods of increased respiratory efforts. Such signs are usually the result of extrathoracic tracheal collapse. Tracheal collapse is rare in cats, and most often it occurs secondary to a tracheal obstruction such as a tumor or traumatic injury.
On physical examination a cough can usually be elicited by palpation of the trachea. An end-expiratory snap or click may be heard during auscultation if intrathoracic collapse is present. In advanced cases or after exercise, increased inspiratory effort may be observed in dogs with extrathoracic collapse and increased expiratory effort observed in those with intrathoracic collapse, often accompanied by audible sounds.
History and physical examination should also emphasize a search for exacerbating or complicating disease. The frequent association with canine chronic bronchitis has been mentioned. Other possibilities include cardiac disease causing left atrial enlargement with bronchial compression or pulmonary edema; airway inflammation caused by bacterial infection, allergic bronchitis, exposure to smoke (e.g., from cigarettes, fireplaces), or recent intubation; upper airway obstruction caused by elongated soft palate, stenotic nares, or laryngeal paralysis; and systemic disorders such as obesity or hyperadrenocorticism.
Diagnosis
Collapsing trachea is most often diagnosed on the basis of clinical signs and the findings from cervical and thoracic radiography. Radiographs of the neck to evaluate the size of the lumen of the extrathoracic trachea are taken during inspiration (Fig. 21-6), when narrowing caused by tracheal collapse is more evident because of negative airway pressure. Conversely, the size of the lumen of the intrathoracic trachea is evaluated on thoracic radiographs taken during expiration, when increased intrathoracic pressures make collapse more apparent (Fig. 21-7). Radiographs of the thorax should also be taken during inspiration to detect concurrent bronchial or parenchymal abnormalities. (See Chapter 20 for further discussion of radiography.)
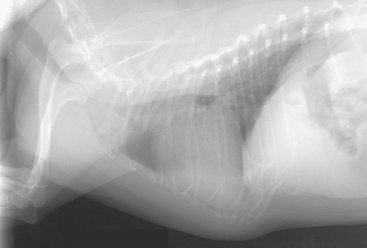
FIG 21-6 Lateral radiograph of the thorax and neck of a dog with collapsing trachea taken during inspiration. The extrathoracic airway stripe is severely narrowed cranial to the thoracic inlet.
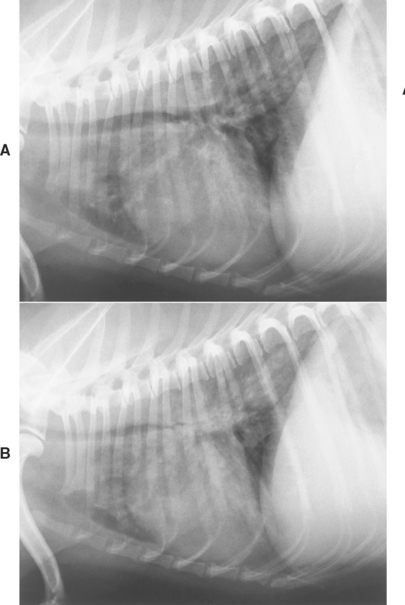
FIG 21-7 Lateral radiographs of a dog with tracheobronchomalacia. During inspiration (A) the trachea and mainstem bronchi are nearly normal. During expiration (B) the intrathoracic trachea and mainstem bronchi are markedly narrowed. Evaluation of the pulmonary parenchyma should not be attempted using films exposed during expiration.
Fluoroscopic evaluation provides a “motion picture” view of large airway dynamics, making changes in luminal diam eter easier to identify than by routine radiography. The sensitivity of fluoroscopy in detecting airway collapse is enhanced if the patient can be induced to cough during the evaluation by applying pressure to the trachea. Some degree of collapse is probably normal during cough, and in people a diagnosis of tracheobronchomalacia is generally made if the luminal diameter decreases by greater than 50% during forced exhalation.
Bronchoscopy is also useful in the diagnosis of airway collapse (Fig. 21-8; see also Fig. 21-3). The bronchi of smaller dogs may be difficult to evaluate by radiography or fluoroscopy but are easily examined bronchoscopically. Bronchoscopy and the collection of airway specimens (such as by BAL) is useful for identifying exacerbating or concurrent conditions.
Bronchoscopy is performed with the patient under general anesthesia, which interferes with the ability to induce cough. However, allowing the patient to reach a light plane of anesthesia combined with the manipulation of the airways will often cause more forceful respirations that increase the likelihood of identifying airway collapse.
Additional tests are performed to identify exacerbating or concurrent conditions. Tracheal wash fluid is analyzed by cytology and culture if bronchoscopy and BAL are not done. Other considerations include an upper airway examination, cardiac evaluation, and screening for systemic disease.
Treatment
Medical therapy is adequate treatment for most animals. In a study of 100 dogs by White et al. (1994), medical therapy resulted in resolution of signs for at least 1 year in 71% of cases. Dogs that are overweight are placed on a weight-reducing diet. Harnesses should be used instead of collars, and owners should be counseled to keep their dogs from becoming overheated (e.g., they should not be left in a car). Excessive excitement should also be avoided. Sedatives such as phenobarbital are prescribed for some animals, and these can be administered before known stressful events.
Cough suppressants are used to control signs and disrupt the potential cycle of perpetuating cough (see Table 21-1). The dose and frequency of administration of cough suppressants are adjusted as needed. Initially, high, frequent dosing may be needed to break the cycle of coughing. Subsequently, it is often possible to decrease frequency of administration and dose. Bronchodilators may be beneficial in dogs with signs of chronic bronchitis (see p. 290). Antiinflammatory doses of glucocorticoids can be given for a short period during exacerbation of signs (prednisone, 0.5 to 1 mg/kg q12h for 3 to 5 days, then tapered and discontinued over 3 to 4 weeks). Long-term use is not recommended because of potential detrimental side effects such as obesity, but this is often necessary to control signs in patients with chronic bronchitis. Dogs with signs referable to mitral insufficiency are managed for this disease (see Chapter 8). Dogs with abnormalities causing upper airway obstruction are treated with corrective surgical procedures.
Antibiotics are not indicated for the routine management of a collapsing trachea. Dogs in which tracheal wash or BAL fluid analysis has revealed evidence of infection should be treated with appropriate antibiotics (selected on the basis of the results of sensitivity testing). Because most antibiotics do not reach high concentrations in the airways, relatively high doses of antibiotics should be administered for several weeks, as described for canine chronic bronchitis (p. 291). Any other potential related problems identified during the diagnostic evaluation are addressed.
Management of dogs in acute distress with signs of either extrathoracic airway obstruction or intrathoracic large airway obstruction is discussed in Chapter 26.
Surgical treatment of a collapsing trachea should be considered for animals that are no longer responsive to medical management, usually because of respiratory difficulty. The introduction of intraluminal stents has greatly reduced the morbidity and improved the success of surgical intervention. The most commonly used stents are self-expanding and made of nickel-titanium alloys (Fig. 21-9). In experienced hands, these stents are simple to place during a short period of anesthesia using fluoroscopic or bronchoscopic guidance. There is minimal morbidity associated with stent placement, and response is immediate and often dramatic. However, clinical signs (particularly cough) may not completely resolve, collapse of airways beyond the trachea and concurrent conditions are not directly addressed (often resulting in the continued need for medical management), and complications such as granuloma formation and stent fracture can occur. Results from stent placement are sufficiently encouraging that motivated clients with a dog that is failing medical management of tracheal collapse should be referred to someone experienced in stent placement for further consideration of this option.
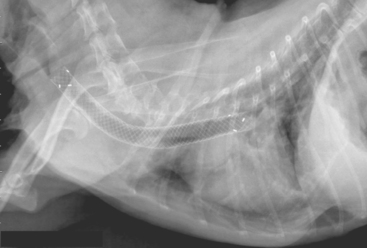
FIG 21-9 Lateral radiograph of the dog with tracheal collapse shown in Fig. 21-6 after placement of an intraluminal stent. The stent is has a meshlike structure and extends nearly the entire length of the trachea.
Prognosis
In most dogs clinical signs can be controlled with conscientiously performed medical management, with diagnostic evaluations performed during episodes of persistent exacerbations of signs. Animals in which severe signs develop despite appropriate medical care have a guarded prognosis, and motivated clients should be referred for possible stent placement.
ALLERGIC BRONCHITIS
Allergic bronchitis is a hypersensitivity response of the airways to an allergen or allergens. The offending allergens are presumably inhaled, although food allergens could also be involved. A definitive diagnosis requires identification of allergen(s) and resolution of signs after elimination of the allergen(s). Large controlled studies describing allergic bronchitis in dogs or cats are lacking. A study by Prost (2004) presented as an abstract found that 15 of 20 cats had positive intradermal skin tests to aeroallergens. For cats that reacted to storage mites or cockroach antigen, discontinuation of any dry food was recommended (i.e., only canned food was provided). Remission of signs occurred in 3 cats with only this treatment. Immunotherapy (desensitization) appeared to reduce or eliminate signs in some of the other cats. As a preliminary study, other treatments were also given to the study cats, and a control population was not described.
It is likely that some patients with allergic bronchitis are misdiagnosed because of difficulty in identifying spe-cific allergens. In dogs long-standing allergic bronchitis may result in the permanent changes recognized as canine chronic bronchitis. In cats failure to identify specific allergen(s) results in a diagnosis of idiopathic feline bronchitis.
Allergic bronchitis in dogs may result in acute or chronic cough. Rarely, respiratory distress and wheezing occur. The physical examination and radiographic findings reflect the presence of bronchial disease, as described in the section on canine chronic bronchitis. Eosinophilic inflammation is expected in tracheal wash or BAL fluid. Heartworm tests and fecal examinations for pulmonary parasites are performed to eliminate parasitism as the cause of the eosinophilic inflammation. In dogs younger than than 2 years of age, bronchoscopic evaluation for Oslerus osleri also should be considered (see the following section). Allergic bronchitis in cats has the same presentation and results of diagnostic testing as described for idiopathic feline bronchitis, with eosinophilia expected in airway specimens.
Management of allergic bronchitis is initially focused on identifying and eliminating potential allergens from the environment (see the section on feline bronchitis). Diet trials with novel protein and carbohydrate sources also can be considered. According to the preliminary study previously described, a change in diet to canned food may be beneficial in some cases. Such experimentation with environment and diet is possible only in patients with clinical signs that are sufficiently mild to delay the administration of glucocorticoids and bronchodilators, as described in the sections on canine chronic bronchitis and feline bronchitis (idiopathic). Elimination trials can still be pursued once clinical signs are controlled with medications, but confirmation of a beneficial effect will require discontinuation of the medication and, for a definitive diagnosis to be made, reintroduction of the allergen. The latter may not be necessary or practical in all cases.
OSLERUS OSLERI
Etiology
Oslerus osleri is an uncommon parasite of young dogs, usually those younger than 2 years of age. The adult worms live at the carina and mainstem bronchi and cause a local, nodular inflammatory reaction with fibrosis. First-stage larvae are coughed up and swallowed. The main cause of infection in dogs appears to be through intimate contact with their dam as puppies.
Clinical Features
Young affected dogs have an acute, loud, nonproductive cough and occasionally wheezing. The dogs appear otherwise healthy, making the initial presentation indistinguishable from that of canine infectious tracheobronchitis. However, the cough persists, and eventually airway obstruction occurs as a result of the formation of reactive nodules.
Diagnosis
Nodules at the carina occasionally can be recognized radiographically. Cytologic examination of tracheal wash fluid in some dogs shows the characteristic ova or larvae, providing the basis for a definitive diagnosis (see Table 20-1). Rarely, larvae are found in fecal specimens using zinc sulfate (s.g. 1.18) flotation (preferred) or the Baermann technique (see Box 20-8).
The most sensitive diagnostic method is bronchoscopy, which enables the nodules to be readily seen (Fig. 21-10). Brushings of the nodules are obtained and immediately evaluated cytologically to detect the larvae. Material can be examined directly in saline solution or stained with new methylene blue. If a definitive diagnosis is not obtained from analysis of the brushings, biopsy specimens are obtained.
Treatment
Treatment with ivermectin (400 μg/kg orally or subcutaneously) is recommended. The same dose is administered again every 3 weeks for four treatments. This treatment has not been extensively investigated, however, and is not an approved use of this drug. It cannot be administered to Collies or related breeds. An alternative treatment is fenbendazole (50 mg/kg q24h for 7 to 14 days).
Bach JF, et al. Evaluation of the bioavailability and pharmacokinetics of two extended-release theophylline formulations in dogs. J Am Vet Med Assoc. 2004;224:1113.
Bemis DA, et al. Aerosol, parenteral, and oral antibiotic treatment of Bordetella bronchiseptica infections in dogs. J Am Vet Med Assoc. 1977;170:1082.
Bidgood T, et al. Comparison of plasma and interstitial fluid concentrations of doxycycline and meropenem following constant rate intravenous infusion in dogs. Am J Vet Res. 2003;64:1040.
Buonavoglia, et al. Canine respiratory viruses. Vet Res. 2007;38:455.
Dye JA, et al. Chronopharmacokinetics of theophylline in the cat. J Vet Pharmacol Ther. 1990;13:278.
Ellis JA, et al. Effect of vaccination on experimental infection with Bordetella bronchiseptica in dogs. J Am Vet Med Assoc. 2001;218:367.
Gore T. Intranasal kennel cough vaccine protecting dogs from experimental Bordetella bronchiseptica challenge within 72 hours. Vet Record. 2005;156:482.
Graham-Mize CA, et al. Bioavailability and activity of prednisone and prednisolone in the feline patient. Abstr. Vet Dermatol. 2004;15(Suppl 1):9.
Guenther-Yenke CL, et al. Pharmacokinetics of an extended-release theophylline product in cats. J Am Vet Med Assoc. 2007;231:900.
Jacobs AAC, et al. Protection of dogs for 13 months against Bordetella bronchiseptica and canine parainfluenza virus with a modified live vaccine. Vet Record. 2005;157:19.
Johnson LR. Tracheal collapse: diagnosis and medical and surgical treatment. Vet Clin North Am Small Anim Pract. 2000;30:1253.
Johnson LR, et al. Clinical and microbiologic findings in dogs with bronchoscopically diagnosed tracheal collapse: 37 cases (1990–1995). J Am Vet Med Assoc. 2001;219:1247.
Mantis P, et al. Assessment of the accuracy of thoracic radiography in the diagnosis of canine chronic bronchitis. J Small Anim Pract. 1998;39:518.
McKiernan BC. Current uses and hazards of bronchodilator therapy. In: Kirk RW, et al, editors. Current veterinary therapy XI. Philadelphia: WB Saunders, 1992.
McKiernan BC. Diagnosis and treatment of chronic bronchitis: twenty years of experience. Vet Clin North Am Small Anim Pract. 2001;30:1267.
Miller DJM, et al. Gentamicin aerosolization for the treatment of infectious tracheobronchitis. Abstr. Proceed Am Coll Vet Intern Med. 2003.
Moise NS, et al. Bronchopulmonary disease. In: Sherding RG, editor. The cat: diseases and clinical management. New York: Churchill Livingstone, 1989.
Moritz A, et al. Management of advanced tracheal collapse in dogs using intraluminal self-expanding biliary wall stents. J Vet Intern Med. 2004;18:31.
Outerbridge CA, et al. Oslerus osleri tracheobronchitis: treatment with ivermectin in 4 dogs. Can J Vet. 1998;39:238.
Padrid PA, et al. Cyproheptadine-induced attenuation of type-I immediate hypersensitivity reactions of airway smooth muscle from immune-sensitized cats. Am J Vet Res. 1995;56:109.
Padrid P. Feline asthma: diagnosis and treatment. Vet Clin North Am Small Anim Pract. 2000;30:1279.
Prost C. Treatment of allergic feline asthma with allergen avoidance and specific immunotherapy. Abstr. Vet Dermatol. 2004;13(Suppl 1):55.
Randolf JF, et al. Prevalence of mycoplasmal and ureaplasmal recovery from tracheobronchial lavages and of mycoplasmal recovery from pharyngeal swab specimens in cats with or without pulmonary disease. Am J Vet Res. 1993;54:897.
Ridyard A. Heartworm and lungworm in dogs and cats in the UK. In Practice. 2005;27:147.
Speakman AJ, et al. Antibiotic susceptibility of canine Bordetella bronchiseptica isolates. Vet Microbiol. 2000;71:193.
Thrusfield MV, et al. A field investigation of kennel cough: efficacy of different treatments. J Small Anim Pract. 1991;32:455.
Wheeldon EB, et al. Chronic respiratory disease in the dog. J Small Anim Pract. 1977;18:229.
White RAS, et al. Tracheal collapse in the dog: is there really a role for surgery? A survey of 100 cases. J Small Anim Pract. 1994;35:191.