Impairments to consider during evaluation and intervention
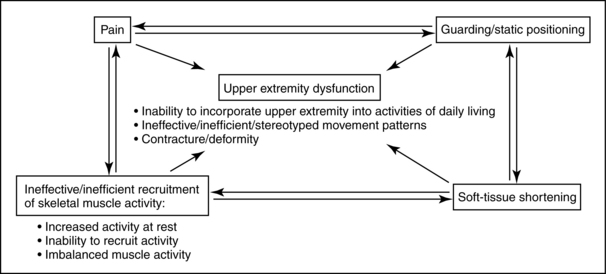
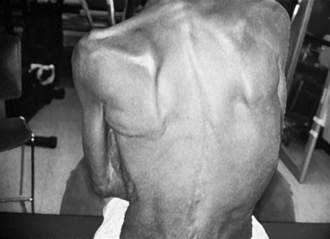
Evaluation and intervention of the upper extremity are complex tasks that require an understanding of multiple systems. Therapists need to remain open-minded about their interventions and consider the complexity of causes that interfere with upper extremity use (Fig. 10-10). Many of the various problems associated with upper extremity function overlap and build on each other. The following paragraphs review common impairments in stroke survivors that may or may not interfere with integrating the upper extremity into daily occupations.
Impaired postural control
Improving proximal stability to enhance distal mobility has long been a tenet of occupational therapy interventions. Postural adjustments stabilize supporting body parts while other parts (e.g., the upper extremities) are being moved.73 The following studies describe the effect of postural adjustments on arm function.
In the classic study by Belenkii, Gurfinkle, and Paltsev,16 unimpaired subjects who were evaluated while standing were asked to raise their arm to a horizontal position after they heard an external signal. Various EMG studies were performed to trace the pattern of muscle activation. The results demonstrated that the postural muscle synergies of the trunk and lower extremities were activated before (by 90 milliseconds) the anterior deltoid, the primary muscle used to perform this motion. The subjects then were evaluated in the supine position while performing the same task. No lower extremity activation was detected in this position (i.e., a different pattern of postural adjustments). The following conclusions can be inferred from this study:
1. Postural adjustments are task-specific.
2. Training of the upper extremity in the supine position does not automatically carry over to activities performed while sitting or standing (if postural control is a limiting factor).
3. Having different disciplines treat one particular half of the body is detrimental to patients’ progress because upper extremity function depends on postural support from the lower extremities and trunk.
Bouisset and Zattara29 replicated the previous study and demonstrated that an upward and forward trunk movement resulting from spine and/or lower limb extension precedes upper limb movement. This movement pattern is familiar to therapists who cue their patients to focus on spinal extension and the associated anterior pelvic tilt while treating arm function.
Horak and colleagues88 compared postural adjustments of subjects with and without hemiplegia during a variety of tasks with different parameters. The hemiplegic subjects demonstrated the same sequence of muscle activation as the subjects without hemiplegia, although activity on the hemiparetic side was delayed. In addition, the hemiparetic individuals were not capable of making rapid movements with the unimpaired arm. This was hypothesized to result from a delay in the anticipatory activity of the contralateral hemiplegic muscles. This study dispels the myth of “good” and “bad” sides after a stroke, especially when postural control is compromised.
In their study of postural adjustments during arm movements, Cordo and Nashner52 were able to demonstrate that when subjects’ postural stability was increased (e.g., by outside shoulder support or placing a finger lightly on a support rail), postural activity was reduced, and voluntary movement enhanced. This concept is crucial to understand when treating upper extremity dysfunction. As support is increased, the postural demands of the task are decreased and vice versa. The therapist can control the patient’s level of postural stability by manipulating the following treatment environment factors: positioning—supine to sitting to standing; type of support surface—stationary or unstable surfaces; positioning of objects used in activities—near or far, base of support, and amount of external stability.
Cordo and Nashner52 also made a critical distinction between associated postural adjustments that precede voluntary movements (e.g., reaching) and automatic postural adjustments that follow external perturbations (e.g., standing on a bus that stops at a light or being moved by the therapist). Training in one type of adjustment cannot be assumed to carry over into other types of adjustments.
Woollacott, Bonnet, and Yabe194 demonstrated that their subjects’ postural activity varied depending on the task being performed (pushing, pulling) and whether they received information in advance regarding the goal of the task.
Massion119 points out that voluntary movements are “accompanied by postural adjustments which show three main characteristics: (1) they are ‘anticipatory’ with respect to movement and minimize the perturbations of posture and equilibrium due to the movement, (2) they are adaptable to the conditions in which the movement is executed, and (3) they are influenced by the instructions given to the subject concerning the task to be performed.”
Postural control disorders in stroke patients have been well-documented. Lee109 emphasized the detrimental impact that postural dysfunction has on free arm movements and therefore ADL. Although a variety of muscles can serve as postural stabilizers, postural control of the trunk is critical for upper extremity function.19 See Chapter 7.
Occupational therapists must use their activity analysis skills to help patients develop the missing trunk control components. (See Table 7-9 for examples of the effects of object positioning on trunk control and weight shifting during reaching activities.) Functional mobility patterns requiring increased trunk control (e.g., scooting) should be incorporated into treatment plans for upper extremity function. See Chapter 14.
Postural control evaluations should be performed within the context of upper extremity tasks such as reaching or performing ADL and IADL. Evaluating postural control separately does not provide the therapist with sufficient information for intervention. (See Chapters 7 and 8 for more information related to postural control.)
Weakness
Until relatively recently, the impact of weakness (a negative symptom) on stroke patients’ functional status has long been ignored. The motor control deficits in patients previously were attributed exclusively to spasticity, which resulted in treatment focused on inhibiting the spasticity. Many therapists considered upper extremity muscle tests for strength difficult to interpret because of common “synergy patterns.” Bourbonnais and colleagues31 demonstrated that the patterns of activity in the elbow flexor muscles were not consistent with established synergistic patterns. Weakness of the upper extremity musculature plays a major role in upper extremity dysfunction, most likely more than the positive symptoms after stroke. Muscle weakness is reflected by the inability of patients to generate normal levels of muscle force.30 Stroke survivors who have written about their experiences focus on the difficulty in force production. Brodal35 reflected on his own stroke: “It was a striking and repeatedly made observation that the force needed to make a severely paretic muscle contract is considerable. . . . Subjectively this is experienced as a kind of mental force, a power of will. In the case of a muscle just capable of being actively moved the mental effort needed was very great.”
Bourbonnais and Vanden Noven30 reviewed the physiological changes in the nervous system that contribute to muscle weakness in patients with hemiparesis. They summarized specific changes at the motor neuron and muscle levels that decrease a patient’s ability to produce force. Box 10-7 summarizes these changes.
Box 10-7 Physiological Changes Contributing to Weakness
 Motor neuron changes: loss of agonist motor units, changes in recruitment order of motor units, and changes in the firing rates of motor units
Motor neuron changes: loss of agonist motor units, changes in recruitment order of motor units, and changes in the firing rates of motor units
 Nerve changes: changes in peripheral nerve conduction
Nerve changes: changes in peripheral nerve conduction
 Muscle changes: changes in the morphological and contractile properties of motor units and in the mechanical properties of muscles
Muscle changes: changes in the morphological and contractile properties of motor units and in the mechanical properties of muscles
Bohannon and colleagues21 found that static strength deficits of the shoulder medial rotator and elbow flexor muscles did not correlate with antagonist muscle spasticity. They concluded that therapists might determine the capacity for force production for an agonist muscle based on its own tone rather than that of its antagonist.
Gowland and colleagues79 studied agonist and antagonist activity during upper limb movements in stroke patients and concluded that treatment should be aimed at improving motor neuron recruitment rather than reducing antagonist activity. In their study, patients who could not perform select upper extremity tasks had EMG values significantly and consistently lower than those of patients who were successful at the task.
Indeed recent empirical evidence highlights the relationship between weakness and loss of function. Findings include:
 In a study of 93 community dwelling stroke survivors, Harris and Eng85 concluded that paretic upper limb strength had the strongest relationship with variables of activity and best explained upper limb performance in ADL. Grip strength was also a factor.
In a study of 93 community dwelling stroke survivors, Harris and Eng85 concluded that paretic upper limb strength had the strongest relationship with variables of activity and best explained upper limb performance in ADL. Grip strength was also a factor.
 A longitudinal study of 27 stroke survivors found that weakness was the main and only contributor to activity limitations as opposed to spasticity or contracture.6
A longitudinal study of 27 stroke survivors found that weakness was the main and only contributor to activity limitations as opposed to spasticity or contracture.6
 Chae and colleagues46 described the relationship between poststroke upper limb muscle weakness and cocontraction, and clinical measures of upper limb motor impairment and physical disability. The authors measured EMG activity of the paretic and nonparetic wrist flexors and extensors of 26 chronic stroke survivors. Upper limb motor impairment and physical disability were assessed with the Fugl-Meyer motor assessment and the arm motor ability test. They concluded that muscle weakness and degree of cocontraction correlate significantly with motor impairment and physical disability in upper limb hemiplegia.
Chae and colleagues46 described the relationship between poststroke upper limb muscle weakness and cocontraction, and clinical measures of upper limb motor impairment and physical disability. The authors measured EMG activity of the paretic and nonparetic wrist flexors and extensors of 26 chronic stroke survivors. Upper limb motor impairment and physical disability were assessed with the Fugl-Meyer motor assessment and the arm motor ability test. They concluded that muscle weakness and degree of cocontraction correlate significantly with motor impairment and physical disability in upper limb hemiplegia.
 Mercier and Bourbonnais123 compared the relative strength of different muscle groups of the paretic upper limb and assess the relationship with motor performance. The maximal active torques of five muscle groups were measured in both upper limbs. Upper limb function was assessed using the Box and Block Test, the Finger-to-Nose Test, the Fugl-Meyer Test, and the TEMPA. They concluded that “the relative forces for shoulder flexion and handgrip are the best predictors of the upper limb function.” Additionally, they concluded that the results “do not confirm classical clinical teaching regarding the distribution of weakness following stroke (e.g., proximal to distal gradient; extensors more affected than flexors) but support the hypothesis that strength is related to the function of the paretic upper limb.”
Mercier and Bourbonnais123 compared the relative strength of different muscle groups of the paretic upper limb and assess the relationship with motor performance. The maximal active torques of five muscle groups were measured in both upper limbs. Upper limb function was assessed using the Box and Block Test, the Finger-to-Nose Test, the Fugl-Meyer Test, and the TEMPA. They concluded that “the relative forces for shoulder flexion and handgrip are the best predictors of the upper limb function.” Additionally, they concluded that the results “do not confirm classical clinical teaching regarding the distribution of weakness following stroke (e.g., proximal to distal gradient; extensors more affected than flexors) but support the hypothesis that strength is related to the function of the paretic upper limb.”
From a treatment planning perspective, integrating strengthening interventions is imperative in efforts to regain limb function. Bohannon and Smith23 analyzed strength deficits in stroke patients and verified that muscle strength improves in stroke patients with hemiplegia who are undergoing rehabilitation. Empirical evidence supports the use of strengthening interventions in this population without deleterious effects:
 Flinn70 presented a case study of a young female with left-sided hemiplegia. Her treatment program focused on participating in graded functional tasks that systematically increased the motor demands on the more affected upper extremity. Her task-oriented treatment program was augmented by resistive exercises using elastic tubing. Substantial results after six months of therapy included improved level of occupational performance in ADL and IADL, improved manual muscle test scores (which increased from 2/5 to the 4/5 and 5/5 ranges), improved hand function, and improved grip strength scores. Identifying the underlying problems (in this case, weakness and an inability to control excess degrees of freedom) is of utmost importance when planning treatment strategies.
Flinn70 presented a case study of a young female with left-sided hemiplegia. Her treatment program focused on participating in graded functional tasks that systematically increased the motor demands on the more affected upper extremity. Her task-oriented treatment program was augmented by resistive exercises using elastic tubing. Substantial results after six months of therapy included improved level of occupational performance in ADL and IADL, improved manual muscle test scores (which increased from 2/5 to the 4/5 and 5/5 ranges), improved hand function, and improved grip strength scores. Identifying the underlying problems (in this case, weakness and an inability to control excess degrees of freedom) is of utmost importance when planning treatment strategies.
 Bütefisch and colleagues39 examined the effect of a standardized training on movements of the affected hand in 27 hemiparetic patients using a multiple baseline approach. The training consisted of repetitive hand and finger flexions and extensions against various loads and was carried out twice daily during 15-minute periods. Grip strength, peak force of isometric hand extensions, peak acceleration of isotonic hand extensions, and contraction velocities as indicators of motor performance significantly improved during the training period. Additionally, 24 out of 27 patients improved on the Rivermead Motor Assessment. The authors further challenged traditional therapy (the Bobath concept) aimed at reducing enhanced muscle tone without reinforcing the activity in centrally paretic hand. In the study, patients undergoing this treatment approach alone did not experience a significant improvement in the motor capacity of the hand. The authors emphasized the importance of frequent movement repetition for the motor rehabilitation of the centrally paretic hand and challenge conventional therapeutic strategies that focus on spasticity reduction instead of early initiation of active movements.
Bütefisch and colleagues39 examined the effect of a standardized training on movements of the affected hand in 27 hemiparetic patients using a multiple baseline approach. The training consisted of repetitive hand and finger flexions and extensions against various loads and was carried out twice daily during 15-minute periods. Grip strength, peak force of isometric hand extensions, peak acceleration of isotonic hand extensions, and contraction velocities as indicators of motor performance significantly improved during the training period. Additionally, 24 out of 27 patients improved on the Rivermead Motor Assessment. The authors further challenged traditional therapy (the Bobath concept) aimed at reducing enhanced muscle tone without reinforcing the activity in centrally paretic hand. In the study, patients undergoing this treatment approach alone did not experience a significant improvement in the motor capacity of the hand. The authors emphasized the importance of frequent movement repetition for the motor rehabilitation of the centrally paretic hand and challenge conventional therapeutic strategies that focus on spasticity reduction instead of early initiation of active movements.
 Sterr and Freivogel166 “assessed whether intensive training increases spasticity and leads to the development of ‘pathologic movement patterns,’ a concern often raised by Bobath-trained therapists. The authors used a baseline-control repeated-measures test to study 29 patients with chronic upper limb hemiparesis who received daily shaping training. Their results suggest that training has no adverse effects on muscle tone and movement quality.”2
Sterr and Freivogel166 “assessed whether intensive training increases spasticity and leads to the development of ‘pathologic movement patterns,’ a concern often raised by Bobath-trained therapists. The authors used a baseline-control repeated-measures test to study 29 patients with chronic upper limb hemiparesis who received daily shaping training. Their results suggest that training has no adverse effects on muscle tone and movement quality.”2
 A systematic review of multiple studies concluded that “Strengthening interventions increase strength, improve activity, and do not increase spasticity. These findings suggest that strengthening programs should be part of rehabilitation after stroke.”
A systematic review of multiple studies concluded that “Strengthening interventions increase strength, improve activity, and do not increase spasticity. These findings suggest that strengthening programs should be part of rehabilitation after stroke.”
 In their strength training study after stroke, Badics and colleagues10 concluded that “The extent of strength gain was positively correlated with the intensity and the number of exercising units. Muscle tone, which was abnormally high at baseline, did not further increase in any one case. The results of this study showed that targeted strength training significantly increased muscle power in patients with muscle weakness of central origin without any negative effects on spasticity.”
In their strength training study after stroke, Badics and colleagues10 concluded that “The extent of strength gain was positively correlated with the intensity and the number of exercising units. Muscle tone, which was abnormally high at baseline, did not further increase in any one case. The results of this study showed that targeted strength training significantly increased muscle power in patients with muscle weakness of central origin without any negative effects on spasticity.”
 In their review of weakness and strengthening post stroke, Patten and colleagues141 identified nine trials of progressive resistive training after stroke. They concluded “All of these studies reported positive adaptations to strength training . . . With one exception, all studies strongly suggest positive effects of strength training on various indices of functional outcome . . .” They further concluded that “while insufficient data exist to draw firm conclusions at this time, functional effects of strengthening appear persistent. Four of the available studies evaluated effects of strength training on spasticity and found no deleterious effects.”
In their review of weakness and strengthening post stroke, Patten and colleagues141 identified nine trials of progressive resistive training after stroke. They concluded “All of these studies reported positive adaptations to strength training . . . With one exception, all studies strongly suggest positive effects of strength training on various indices of functional outcome . . .” They further concluded that “while insufficient data exist to draw firm conclusions at this time, functional effects of strengthening appear persistent. Four of the available studies evaluated effects of strength training on spasticity and found no deleterious effects.”
The debate about which type of muscle contraction (eccentric, concentric, or isometric) is the most effective in strengthening patients has been long-standing. Muscle groups need to contract in a variety of ways to complete functional tasks successfully. For example, when a person reaches for a can of soup on a high shelf, the shoulder musculature must contract (concentrically) to bring the hand to the level of the shelf, maintain the contraction (isometrically) to locate the correct item, and control the weight of the arm and item in gravity (eccentrically) as the can is placed with control on the countertop.
In a study of dynamical muscle strength training in stroke patients, Engardt and colleagues65 found that eccentric contractions were more effective than concentric contractions. Twenty patients with hemiparesis resulting from strokes participated in activities that elicited concentric or eccentric contractions. After the treatment, significant improvements resulted in the relative strength of paretic muscles during eccentric and concentric actions in the group that was trained solely with eccentric contractions (i.e., eccentric training increased the strength of both types of contractions); this was not true for the group that only received concentric contraction training. Therefore, the authors determined eccentric contraction training to be more advantageous and efficient (Box 10-8). See Table 10-1 for a review of evidence-based interventions related to strengthening.
Spasticity
Spasticity, which is a positive symptom according to Jackson classification system, has been a subject of debate by various authors. Although an abundance of research has been done on spasticity, disagreements still exist about its definition, physiological basis, treatment, and evaluation. Glenn and Whyte75 define spasticity as “a motor disorder with persistent increase in the involuntary reflex activity of a muscle in response to stretch. Four specific phenomena may be variably observed in the constellation of spasticity: hypertonia (frequently velocity dependent and demonstrating the clasp-knife phenomenon), hyperactive (phasic) deep tendon reflexes, clonus, and spread of reflex responses beyond the muscle stimulated.” In addition, Babinski sign is characteristic, and hyperactive tonic neck or vestibular reflexes may be present.116
Several different phenomena commonly observed in stroke rehabilitation including hyperactive stretch reflexes, increased resistance to passive movement, posturing of the extremities, excessive cocontraction, and stereotypical movement synergies are clumped together in the category of spasticity. Spasticity has become a catchall term for a variety of problems. Rather than being a specific symptom, spasticity is related to a variety of neural and nonneural factors. Therefore, spasticity cannot be treated uniformly by surgical, physical, or pharmacological procedures. Spastic paresis is a commonly used term that implies a cause-and-effect relationship (i.e., a cause-and-effect relationship between positive and negative symptoms). This belief has been challenged recently.
Preston and Hecht147 provide further information regarding the clinical presentation of spasticity to include the following:
 Patients having difficulty initiating rapid alternating movements
Patients having difficulty initiating rapid alternating movements
 Abnormally timed EMG activation of the agonist and antagonist
Abnormally timed EMG activation of the agonist and antagonist
 Fluctuation of spasticity as a result of a change in position
Fluctuation of spasticity as a result of a change in position
 Usual patterns include upper extremity flexion and lower extremity extension
Usual patterns include upper extremity flexion and lower extremity extension
Bobath18 stated that there is “An intimate relationship between spasticity and movement . . . spasticity must be held responsible for much of the patient’s motor deficit.” Treatment techniques were based on “helping the patient gain control over the released patterns of spasticity by their inhibition.” Patients were treated under the assumption that “Weakness of muscles may not be real, but relative to the opposition by spastic antagonists.” A variety of studies have been published that refute these assumptions. See Chapter 6.
Sahrmann and Norton159 studied normal subjects and subjects with upper motor neuron symptoms. The movement pattern studied was alternating flexion and extension of the elbow. The analysis of their EMG findings showed that the primary cause of impaired movement was not antagonist stretch reflexes but was limited and prolonged agonist contraction recruitment and delayed cessation of agonist contractions after movement had stopped. Rather than focusing treatment on inhibiting spasticity, therapists should train patients to perform alternating movement patterns (e.g., hand-to-mouth patterns) efficiently.
Fellows, Kaus, and Thilmann67 studied the importance of hyperreflexia and paresis on voluntary arm movements in normal subjects and subjects with spasticity resulting from a unilateral ischemic cerebral lesion. The subjects with spasticity showed a lower maximum movement velocity; the more marked the paresis, the greater the reduction in maximum velocity. No relationship was found between the degree of voluntary movement impairment and level of passive muscle hypertonia in the antagonist. The conclusion was that agonist muscle paresis, rather than antagonist muscle hypertonia, had the most significant effect on impaired voluntary movement.
In their study on overcoming limited elbow movement in the presence of antagonist hyperactivity, Wolf and colleagues190 concluded that functional elbow improvements could be made without first training the patient specifically to inhibit hyperactivity.
Landau108 performed pharmacological interventions that effectively abolished the hyperactive stretch reflexes in his patients. This intervention did not result in a corresponding improvement in motor behavior.
Ada, O’Dwyer, and O’Neill6 examined the relationship between the motor impairments (spasticity and weakness) and their impact on physical activity. They specifically aimed to study the contribution of weakness and spasticity to contracture, and the contribution of all three impairments to limitations in physical activity during the first 12 months after stroke. The authors followed 27 stroke survivors for one year. They found that “the major independent contributors to contracture were spasticity for the first four months after stroke (p = 0.0001-0.10) and weakness thereafter (p = 0.01-0.05). However, the major and only independent contributor to limitations in physical activity throughout the year was weakness (p = 0.0001-0.05).” “For the first time, from a longitudinal study, the findings show that spasticity can cause contracture after stroke, consistent with the prevailing clinical view. However, weakness is the main contributor to activity limitations.”
In the traditional evaluation of spasticity, the therapist moves the patient’s limb quickly in a direction opposite to the pull of the muscle group being tested, and the examiner feels for a resistance to the movement. The gold standard for rating resistance is the Ashworth Scale9 or the Modified Ashworth Scale24 (Box 10-9).
Ashworth scale*
2 Slight hypertonus; noticeable catch when limb is moved
3 More significant hypertonus, but affected limb still moves easily
Modified ashworth scale†
1 Slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part is moved in flexion or extension
1+ Slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of range of motion
2 More significant increase in muscle tone throughout most of the range of motion but affected part is moved easily.
3 Considerable increase in muscle tone; difficult passive movement
The response of a spastic muscle to stretch has been argued not to be the same during passive and active movement. In addition, spasticity is a multidimensional problem that incorporates neural and nonneural components (e.g., altered soft-tissue compliance). Therefore, some authors have questioned the usefulness of test measures such as the Ashworth Scale and are investigating a more comprehensive evaluation of spasticity.
Although the research on spasticity does not support focusing treatment on suppressing stretch reflexes, it does support treatment focusing on preventing secondary structural muscle changes in patients with spasticity.
Hufschmidt and Mauritz’s study90 suggested that spastic contracture is the result of degenerative changes (e.g., atrophy and fibrosis) and changes of the passive and contractile muscle properties.
In their study on spastic and rigid muscles, Dietz, Quintern, and Berger61 concluded that the actual muscle fibers undergo changes, which explains the increased muscle tone in spastic patients.
For treating patients with spasticity, Perry142 emphasized early mobilization and assistance with developing evolving motor control into effective function. These two interventions result in minimal contractures and prevent improper use of patients’ available control mechanisms. Hummelsheim and colleagues91 studied the results of sustained stretch in spastic patients. They found that sustained muscle stretch of approximately 10 minutes led to significant reduction in the spastic hypertonus in the elbow, hand, and finger flexors. They hypothesized that this benefit is due to stretch receptor fatigue or adaptation to the new extended position.
Little and Massagli116 also emphasized using a stretching program incorporating pain prevention and patient education focusing on the adverse effects of spasticity (contracture), use of slow movements, and importance of daily stretching.
In addition to the mentioned techniques, specific modalities and their physiological bases have been described in the literature and include local cooling, vibration therapy, and electrical stimulation.
Perry142 summarized the effective rehabilitation of a patient with spasticity by using five categories: contracture minimization, realistic planning, muscle strength preservation and restoration, enhancement of returning control, and substitution for permanent functional loss.
Carr, Shepherd, and Ada43 summarized their treatment approach based on the assumption that clinical spasticity is a manifestation of length-associated muscle changes and disordered motor control: “The development of spasticity will be less severe if soft-tissue length can be maintained and if motor training emphasizes elimination of unnecessary muscle force and training muscle synergies as part of specific actions.”
Again, the point must be emphasized that many of the observed phenomena that occur during treatment should not be attributed automatically to spasticity and require more in-depth evaluations and treatment plans (Box 10-10; Table 10-6).
Box 10-10 Treatment of Spasticity
 Guide appropriate use of available motor control.
Guide appropriate use of available motor control.
 Avoid using excessive effort during movement.
Avoid using excessive effort during movement.
 Encourage slow and controlled movements.
Encourage slow and controlled movements.
 Teach specific functional synergies during tasks.
Teach specific functional synergies during tasks.
 Avoid use of repetitive compensatory movement patterns.
Avoid use of repetitive compensatory movement patterns.
 Keep spastic muscles on stretch via positioning or orthotics to prevent contracture.
Keep spastic muscles on stretch via positioning or orthotics to prevent contracture.
 Teach the patient or caretaker specific stretching techniques targeted at the spastic muscles.
Teach the patient or caretaker specific stretching techniques targeted at the spastic muscles.
 Use activities to enhance the agonist/antagonist relationship.
Use activities to enhance the agonist/antagonist relationship.
 Refer when appropriate for pharmacological or surgical interventions.147
Refer when appropriate for pharmacological or surgical interventions.147
Table 10-6 Suggested Interventions for Problems Commonly Thought to Be Caused by Spasticity*
| OBSERVATIONS DURING TREATMENT | SUGGESTED INTERVENTIONS |
|---|---|
| Posturing of upper extremity—usually consisting of retraction, posterior trunk rotation, internal rotation, elbow flexion, and wrist and digit flexion—during difficult tasks (e.g., gait, transfers, and dressing) | Upper extremity posturing indicates that the task is difficult for the patient. Treatment should include increasing the efficiency of task performance by building in trunk and lower extremity control, incorporating the upper extremity into the task (e.g., by bilateral ironing or using arm as postural support), and teaching the patient to relax the upper extremity after difficult tasks. |
| Stereotypical flexor patterns when attempting to move arm against gravity | Evaluate components of movement pattern and identify factors that limit efficient movement (e.g., weakness, postural dysfunction, malalignment, and inappropriate task choice). Provide activities that elicit the missing components of movement pattern. |
| Flexion posture when resting | Implement a contracture prevention program. Provide adequate positioning and teach safe, self range-of-motion exercises. |
| “Catch” felt during quick-stretch evaluation of upper extremity | Do not assume that this phenomenon is resulting in observed movement dysfunction. Instead, interpret it as a red flag warning that soft-tissue shortening may be present or developing. |
* This table represents a variety of functional limitations and problems traditionally considered to be the direct result of spasticity. Although sometimes interconnected, these problems stem from different sources and must be treated accordingly.
Preston and Hecht147 have comprehensively reviewed the literature related to spasticity management, including topics such as oral and intrathecal medications, nerve blocks, orthopedic surgery, and neurosurgical interventions.
Nerve blocks are being used increasingly as an adjunct therapy in the rehabilitation process. Preston and Hecht147 differentiated between short-term blocks such as procaine and bupivacaine used to diagnose and assist in the evaluation process and long-term blocks such as phenol and botulinum toxin type A (Botox).
Rousseaux, Kozlowski, and Froger154 assessed the efficacy of Botox treatment on disability, especially in manual activities, and attempted to identify predictive factors of improvement in 20 patients with stroke. They concluded that botulinum toxin A is efficient in improving hand use in patients with relatively preserved distal motricity and in increasing comfort in patients with severe global disorders. Similarly, Bakheit and colleagues11 completed a randomized controlled trial to assess the efficacy of Botox in decreasing spasticity in stroke survivors. They concluded that treatment with Botox reduced muscle tone in patients with poststroke upper limb spasticity.
While the positive effect at the impairment level (i.e., reduction of spasticity) has been well-documented, the effect on functional limitations is not as clear.164,183 As spasticity increases, the risk for soft-tissue shortening is heightened, which in fact may lead to a vicious circle of problems such as spasticity, soft-tissue shortening, overrecruitment of shortened muscles, and increased stretch reflexes. Secondary problems that may occur if the spasticity is not managed in a therapy program include the following:
 Deformity of the limbs, specifically the distal upper limb (elbow to digits)
Deformity of the limbs, specifically the distal upper limb (elbow to digits)
 Impaired upright function caused by soft-tissue contracture (e.g., plantar flexion contractures resulting in a loss of the ankle strategies required to maintain upright stance)
Impaired upright function caused by soft-tissue contracture (e.g., plantar flexion contractures resulting in a loss of the ankle strategies required to maintain upright stance)
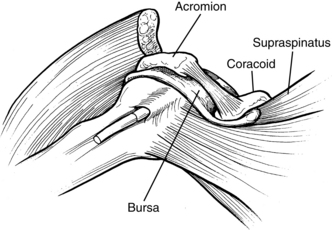
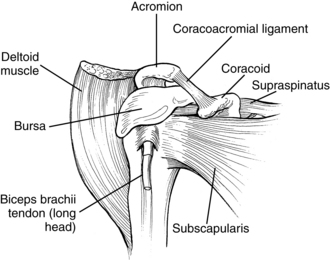
 Pain syndromes resulting from loss of normal joint kinematics. These syndromes are usually related to soft-tissue contracture blocking full joint excursion. A typical example of this issue is the loss of full passive external rotation of the glenohumeral joint. Attempts at forced abduction in these cases results in a painful impingement syndrome of the tissues in the subacromial space.
Pain syndromes resulting from loss of normal joint kinematics. These syndromes are usually related to soft-tissue contracture blocking full joint excursion. A typical example of this issue is the loss of full passive external rotation of the glenohumeral joint. Attempts at forced abduction in these cases results in a painful impingement syndrome of the tissues in the subacromial space.
 Impaired ability to manage basic ADL tasks, specifically upper extremity dressing and bathing of the affected hand and axilla when flexor posturing is present
Impaired ability to manage basic ADL tasks, specifically upper extremity dressing and bathing of the affected hand and axilla when flexor posturing is present
 Loss of reciprocal arm swing during gait activities
Loss of reciprocal arm swing during gait activities
 Risk for falls because of postural malalignment78
Risk for falls because of postural malalignment78
In summary, although reducing spasticity does not appear to result in automatic improvements related to function, therapists must manage spasticity to prevent soft-tissue contracture, prevent deformity, and maintain a flexible and mobile arm.
Loss of soft-tissue elasticity (contractures and deformities)
Contracture in stroke patients results from immobilization and may be attributed to spasticity (particularly during the first four months after stroke) and weakness thereafter,6 improper positioning, postural malalignment, a lack of variation in limb postures (e.g., prolonged sling use), or a combination of various factors. The formation of contractures indicates a poor prognosis for limb function. Perry142 discussed the vicious circle of contracture and spasticity: “contractures stiffen tissues, immobility creates contractures. Spasticity preserves the contracture by excluding the intramuscular fibrous tissues from the stretching force.”
Botte, Nickel, and Akeson28 have reviewed the literature correlating spasticity and contracture. As the stroke patient progresses to a state of spasticity, the increased activity of the spastic muscles may result in characteristic posturing of the limb, resulting in increased stiffness of the soft-tissue surrounding the joint and the eventual formation of fixed contracture. The authors further pointed out that contracture is associated with loss of elasticity and fixed shortening of involved tissues. Contracture may occur in a variety of soft-tissues including the following: skin, subcutaneous tissue, muscle, tendon, ligament, joint capsule, vessels, and nerves.
Halar and Bell82 categorized contracture as arthrogenic (resulting from cartilage damage, joint incongruency, or capsular fibrosis), soft-tissue related (skin, tendons, ligaments, subcutaneous tissue), and myogenic (shortening of the muscle by intrinsic or extrinsic factors). The therapist must consider the difference between myogenic and joint contracture, especially if the muscle spans two or more joints (e.g., the wrist and hand). The therapist can differentiate contractures by flexing the proximal joint and noting the resulting position of the distal joints. Joint contracture is not affected by changes in proximal joint position. See Chapter 13.
Booth27 reviewed the physiological and biochemical effects of immobilization on muscle. His findings indicate that muscle strength rapidly declines during limb immobilization because of a decrease in muscle size; muscle fatigability increases rapidly after immobilization. His observations also indicate that muscle atrophy in immobilized limbs begins rapidly, and a decrease in muscle size is greatest in the early phases of immobilization.
Passive range of motion.
Soft-tissue and joint mobilization are the treatments of choice for preventing contracture. The benefits of mobilization include maintenance of joint lubrication,28 prevention of secondary orthopedic problems (impingements), maintenance of soft-tissue length, and possible reduction of spasticity by acting on the nonneural components of spasticity.
Contracture is prevented by deliberate and frequent limb movement, with active movement being preferred over passive when possible. Perry142 pointed out that it is essential to move the patient through complete ROM and not just the middle ranges. Therapists must determine what a full ROM is for each patient and must consider age-related factors. Determining the full ROM on the less affected side may be helpful. A joint that moves or is moved through its full ROM several times daily develops almost no deformities. Although the therapist should maintain the patient’s ability to participate in all ranges of trunk and upper extremity activities, the therapist should pay particular attention to the following ranges:
 The mobility of the scapula on the thoracic wall with emphasis on protraction and upward rotation should be maintained because this range is critical in the prevention of soft-tissue impingement in the subacromial space during overhead movements of the arm and in preparation for forward reach patterns. Overhead ranges should not be attempted unless the scapula is freely gliding in upward rotation.
The mobility of the scapula on the thoracic wall with emphasis on protraction and upward rotation should be maintained because this range is critical in the prevention of soft-tissue impingement in the subacromial space during overhead movements of the arm and in preparation for forward reach patterns. Overhead ranges should not be attempted unless the scapula is freely gliding in upward rotation.
 Maintaining external (lateral) rotation of the glenohumeral joint allows abduction of the arm as the humerus rotates laterally to permit the greater tuberosity of the humerus to clear the acromial process. Bohannon and colleagues,22 Ikai and colleagues,93 and Zorowitz and colleagues203 concluded that loss of external rotation ROM was the factor most significantly correlated to shoulder pain.
Maintaining external (lateral) rotation of the glenohumeral joint allows abduction of the arm as the humerus rotates laterally to permit the greater tuberosity of the humerus to clear the acromial process. Bohannon and colleagues,22 Ikai and colleagues,93 and Zorowitz and colleagues203 concluded that loss of external rotation ROM was the factor most significantly correlated to shoulder pain.
 Elbow extension is important because the majority of stroke patients favor elbow flexion as a rest posture.
Elbow extension is important because the majority of stroke patients favor elbow flexion as a rest posture.
 The therapists also should maintain wrist extension with concurrent radial deviation. During wrist ROM exercises, therapists must realize that the range of wrist deviation is at a maximum when the wrist is slightly flexed and a minimum when the wrist is fully flexed. Wrist extension is at a maximum during neutral deviation and a minimum during ulnar deviation.100
The therapists also should maintain wrist extension with concurrent radial deviation. During wrist ROM exercises, therapists must realize that the range of wrist deviation is at a maximum when the wrist is slightly flexed and a minimum when the wrist is fully flexed. Wrist extension is at a maximum during neutral deviation and a minimum during ulnar deviation.100
 Composite flexion of the digits leads to collateral ligament elongation. Therapists must maintain this length to prevent deformity and prepare the hand for return of motor function.
Composite flexion of the digits leads to collateral ligament elongation. Therapists must maintain this length to prevent deformity and prepare the hand for return of motor function.
 Composite extension of the wrist and digits results in long flexor elongation.
Composite extension of the wrist and digits results in long flexor elongation.
 Digits ranged in intrinsic plus (metacarpophalangeal flexion and interphalangeal extension) and intrinsic minus (metacarpophalangeal extension and interphalangeal flexion).
Digits ranged in intrinsic plus (metacarpophalangeal flexion and interphalangeal extension) and intrinsic minus (metacarpophalangeal extension and interphalangeal flexion).
Halar and Bell82 recommended active ROM and passive ROM combined with a terminal stretch at least twice per day if contracture is beginning to develop. Therapists must use low-load prolonged stretch if a contracture has developed (see Chapter 13). During the terminal stretch, the therapist should stabilize the proximal body part well. The therapist may distract the joint slightly during the stretch to prevent soft-tissue impingement. The therapist must monitor scapula position during passive ROM activities. If necessary, the therapist should support the scapula in a position of protraction and upward rotation. In addition, the therapist must support the humerus in an external rotation position. The elbow crease should be facing up (not medially toward the trunk) to ensure proper alignment (Fig. 10-12).
Positioning.
Positioning is another effective means of maintaining soft-tissue length and can be used to promote low-load, prolonged stretch. Therapists must address positioning needs of patients while they are in bed or wheelchairs/armchairs (see Chapter 26) and anytime they are in a recumbent position. Effective positioning encourages proper joint alignment, variations in joint position, comfort, and the maintenance of stretch in areas at risk for contracture. Common areas of concern during patient positioning include head and neck alignment, trunk alignment, glenohumeral joint alignment, scapula alignment, maintenance of abduction, external rotation, elbow extension, and maintenance of long flexor length.
A thorough literature review comparing authors’ strategies on bed positioning has been published.41 This review found no consensus on some issues and multiple discrepancies on strategies. Many of the positioning protocols are based on the principle of inhibiting primitive reflexes, a topic of considerable debate.
Patients are engaged in therapy only a portion of the day. Studies have shown that patients in rehabilitation units spend almost half of their days engaged in passive pursuits including sitting unoccupied and lying in bed.15 Therefore, patients at risk for developing contracture because of limb immobilization are good candidates for participation in a positioning program in addition to therapy.
The positioning suggestions in Box 10-11 are based on Carr and Kenney’s review41 of the positioning literature and highlights the consensus of reviewed authors.
Box 10-11 Suggested Bed Positioning
| POSITIONING OF PATIENT | |
| On unaffected side | |
| On affected side | |
| In supine | |
Although the positioning suggestions in Box 10-11 represent the consensus of many authors, major areas of intervention are missing, which result in the controversies surrounding this area of intervention. For example, glenohumeral joint support remains controversial. Although most authors agree that the scapula should be protracted with a pillow, no consensus exists about support of the humerus. If only the scapula is protracted with a pillow, the humerus takes on a position of relative extension. Therefore, only support of both the scapula and humerus achieves the original goal of proper joint alignment (Fig. 10-13).
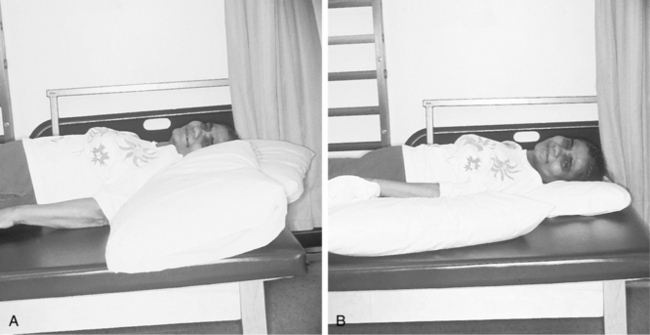
Figure 10-13 A, Bed positioning with only the scapula supported. The humerus takes on a position of relative extension, with the head of the humerus migrating anteriorly. B, Proper support of scapula and humerus ensures proper biomechanical alignment of shoulder joint.
At this point, no definitive studies support one type of positioning more than another with few exceptions. Ada and colleagues5 determined that positioning patients in supine with the affected shoulder abducted to 45-degrees and the elbow flexed to 90-degrees and placed in maximum comfortable external rotation, with towels or pillows to support the forearm 30 minutes per day prevented contracture of the internal rotators. Occupational therapists must decide what their intervention goals are and critically analyze their effectiveness. Therapists should not use general, generic strategies for bed positioning; instead, they should evaluate each patient’s positioning needs individually.
Patient management of the extremity.
Strategies to teach patients safe ROM activities they can perform themselves need to be initiated as soon as patients are medically stable. Although the clasped-hand position followed by overhead movements of both extremities has been advocated by some authors, this position may not be the most effective, especially for trauma prevention. This movement pattern does not account for factors such as scapula-humeral rhythm (especially if weakness, malalignment, or tightness around the scapula exists), overzealous patients who do not or cannot respect their pain, or critical shoulder biomechanics. Many patients observed performing this type of ROM activity have their trunk hyperextended, scapula retracted, and humerus internally rotated. This type of alignment does not correspond with an ROM pattern that emphasizes forward flexion of the humerus; it promotes proximal patterns (e.g., retraction) that should be discouraged (Fig. 10-14). Recommended techniques for patients performing ROM activities by themselves safely include the following:
1. “Towel on table”: The patient is seated at a table with both arms on top of a towel. The less affected arm guides the towel around the table, with the majority of movement occurring in the trunk and from hip flexion. The patient’s goal is to “polish the table” while holding positions at the end of desired ROM. The farther the patient’s chair is positioned from the table, the greater the ROM. This technique not only enhances the range of the glenohumeral and elbow joint but also encourages scapula protraction and weight-shifting. Excessive effort is minimized because the towel assists the movement (Fig. 10-15).
2. “Rock the baby”: The patient’s less affected arm cradles the more affected arm, lifts it to 90 degrees, and places it into positions of horizontal abduction and adduction. Increased horizontal adduction on the more affected side encourages scapula protraction. This technique also encourages trunk rotation (Fig. 10-16).
3. While seated or standing, the patient reaches down to the floor and allows both arms to dangle. This position encourages extension of the elbow, wrist, and digits and forward flexion of the humerus with scapula protraction. The activity is an especially useful technique for patients after they have performed an excessively difficult activity (e.g., gait, transfer, or dressing) that results in stereotypical arm posturing (Fig. 10-17).
4. While seated or standing, the patient places the more affected extremity onto a table or counter so that the forearm is bearing the weight. With the extremity in this position, the patient turns the trunk away from the supported extremity. As the trunk turns farther away and is enhanced by the posterior reach of the less affected arm, the external rotation of the more affected shoulder increases (Fig. 10-18).
5. Davis57 has advocated rolling over the protracted scapula (from supine to side lying) several times to mobilize the scapula.
6. If the scapula of a patient is mobile and stays mobile, the range of abduction and external rotation may be increased by having the patient lie supine, placing the hands behind the head, and allowing the elbows to fall toward the bed (Fig. 10-19). This is a common resting position for an individual who has unimpaired upper extremity function. This technique should be used judiciously and only for patients who move slowly, respect pain, and have a mobile scapula. Therapists may use the five techniques outlined previously for almost all patients because they inherently follow biomechanical principles.
7. Avoid the use of overhead pulleys.104

Figure 10-14 Because of multiple biomechanical concerns (e.g., impingement), self-overhead range of motion is discouraged.

Figure 10-15 “Towel on table.” Therapist is training patient to perform safe self range-of-motion activity. As the patient pushes the towel toward bottle, range of motion is gained in humeral flexion, scapular protraction, and elbow extension (which are ranges required for functional reach). Much of the range is gained by hip and trunk flexion.



Figure 10-16 “Rock the baby.” The patient lifts upper extremity to chest level (A) and abducts (B) and adducts (C) horizontally, allowing trunk rotation.

Figure 10-17 Patient performs self range-of-motion activity by reaching to floor. This pattern is especially effective after a difficult task that results in stereotypical posturing.
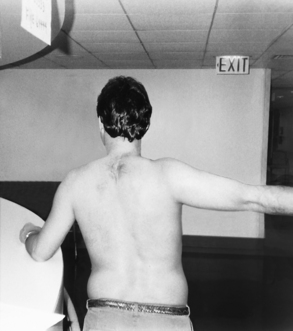
Figure 10-18 External rotation of the left glenohumeral joint is achieved by reaching to side and behind with opposite arm.

Figure 10-19 Internal rotators stretch to be used judiciously for patients who respect their own pain. This rest posture is effective at maintaining external rotation and abduction of the glenohumeral joint. If range is lacking, the humerus can be supported with a towel until patient gains increased external rotation and horizontal abduction.
The ultimate strategy used to decrease contracture and maintain ROM is encouraging functional use of the trunk and upper extremity. A person who has never had a stroke maintains ROM of an extremity by incorporating it into ADL. Activities that eliminate maladaptive positions during activities, improve balanced muscle activity on both sides of the joints, and focus on activities that encourage ROMs that are commonly decreased in stroke patients (e.g., external rotation, forward flexion, abduction, and protraction) should be incorporated into a comprehensive upper extremity program. (See Chapter 13 for other adjunct treatments to prevent or correct soft-tissue shortening.)