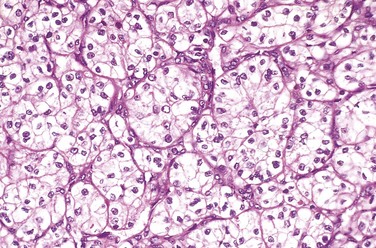
Chapter 13 Kidney and Its Collecting System
See Targeted Therapy available online at studentconsult.com
The kidney is a structurally complex organ that has evolved to carry out a number of important functions: excretion of the waste products of metabolism, regulation of body water and salt, maintenance of acid balance, and secretion of a variety of hormones and prostaglandins. Diseases of the kidney are as complex as its structure, but their study is facilitated by dividing them into those that affect its four components: glomeruli, tubules, interstitium, and blood vessels. This traditional approach is useful because the early manifestations of diseases that affect each of these components tend to be distinctive. Furthermore, some structures seem to be more vulnerable to specific forms of renal injury; for example, glomerular diseases are often immunologically mediated, whereas tubular and interstitial disorders are more likely to be caused by toxic or infectious agents. However, some disorders affect more than one structure, and functional interdependence of structures in the kidney means that damage to one component almost always secondarily affects the others. Thus, severe glomerular damage impairs the flow through the peritubular vascular system; conversely, tubular destruction, by increasing intraglomerular pressure and inducing cytokines and chemokines, may induce glomerular sclerosis. Whatever the origin, there is a tendency for chronic renal disease ultimately to damage all four components of the kidney, culminating in end-stage kidney disease. For these reasons, the early signs and symptoms of renal disease are particularly important in discerning the initiating cause of the disease, and therefore are referred to in the discussion of individual diseases. The functional reserve of the kidney is large, and much damage may occur before renal dysfunction becomes evident.
Clinical Manifestations Of Renal Diseases
The clinical manifestations of renal disease can be grouped into reasonably well-defined syndromes. Some are peculiar to glomerular diseases and others are shared by several renal disorders. Before we list the syndromes, a few terms must be defined.
Azotemia is an elevation of blood urea nitrogen and creatinine levels and usually reflects a decreased glomerular filtration rate (GFR). GFR may be decreased as a consequence of intrinsic renal disease or extrarenal causes. Prerenal azotemia is encountered when there is hypoperfusion of the kidneys, which decreases GFR in the absence of parenchymal damage. Postrenal azotemia results when urine flow is obstructed below the level of the kidney. Relief of the obstruction is followed by correction of the azotemia.
When azotemia gives rise to clinical manifestations and systemic biochemical abnormalities, it is termed uremia. Uremia is characterized not only by failure of renal excretory function but also by a host of metabolic and endocrine alterations incident to renal damage. There is, in addition, secondary gastrointestinal (e.g., uremic gastroenteritis); neuromuscular (e.g., peripheral neuropathy); and cardiovascular (e.g., uremic fibrinous pericarditis) involvement.
We now turn to a brief description of the major renal syndromes:
• Nephritic syndrome results from glomerular injury and is dominated by the acute onset of usually grossly visible hematuria (red blood cells and red cell casts in urine), proteinuria of mild to moderate degree, azotemia, edema, and hypertension; it is the classic presentation of acute poststreptococcal glomerulonephritis.
• Nephrotic syndrome is a glomerular syndrome characterized by heavy proteinuria (excretion of greater than 3.5 g of protein/day in adults), hypoalbuminemia, severe edema, hyperlipidemia, and lipiduria (lipid in the urine).
• Asymptomatic hematuria or non-nephrotic proteinuria, or a combination of these two, is usually a manifestation of subtle or mild glomerular abnormalities.
• Rapidly progressive glomerulonephritis is associated with severe glomerular injury and results in loss of renal function in a few days or weeks. It is manifested by microscopic hematuria, dysmorphic red blood cells and red cell casts in the urine sediment, and mild to moderate proteinuria.
• Acute kidney injury is dominated by oliguria or anuria (no urine flow), and recent onset of azotemia. It can result from glomerular injury (such as rapidly progessive glomerulonephritis), interstitial injury, vascular injury (such as thrombotic microangiopathy), or acute tubular injury.
• Chronic kidney disease, characterized by prolonged symptoms and signs of uremia, is the result of progressive scarring in the kidney from any cause and may culminate in end-stage kidney disease, requiring dialysis or transplantation.
• Urinary tract infection is characterized by bacteriuria and pyuria (bacteria and leukocytes in the urine). The infection may be symptomatic or asymptomatic, and it may affect the kidney (pyelonephritis) or the bladder (cystitis) only.
• Nephrolithiasis (renal stones) is manifested by renal colic, hematuria (without red cell casts), and recurrent stone formation.
In addition to these renal syndromes, urinary tract obstruction and renal tumors also commonly present with signs and symptoms related to renal dysfunction and are discussed later.
Glomerular Diseases
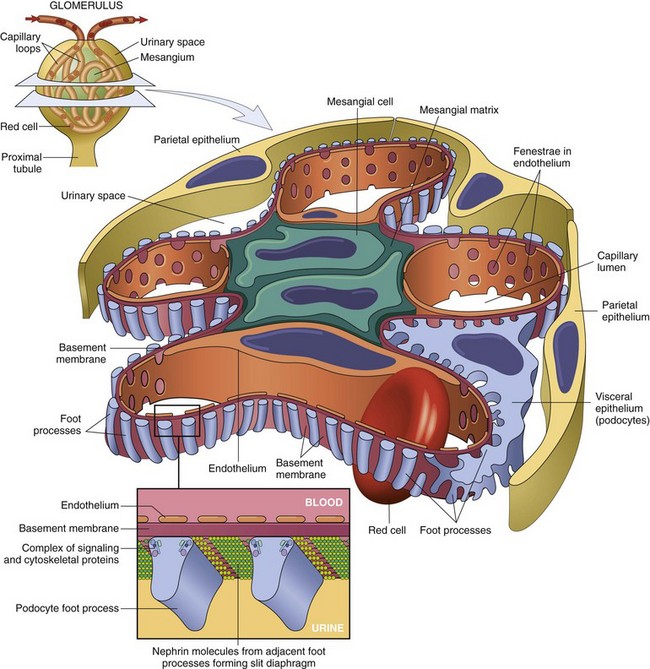
Disorders affecting the glomerulus encompass a clinically important category of renal disease. The glomerulus consists of an anastomosing network of capillaries invested by two layers of epithelium. The visceral epithelium (composed of podocytes) is an intrinsic part of the capillary wall, whereas the parietal epithelium lines Bowman space (urinary space), the cavity in which plasma ultrafiltrate first collects. The glomerular capillary wall is the filtration unit and consists of the following structures (Figs. 13-1 and 13-2):
• A thin layer of fenestrated endothelial cells, each fenestra being 70 to 100 nm in diameter.
• A glomerular basement membrane (GBM) with a thick, electron-dense central layer, the lamina densa, and thinner, electron-lucent peripheral layers, the lamina rara interna and lamina rara externa. The GBM consists of collagen (mostly type IV), laminin, polyanionic proteoglycans, fibronectin, and several other glycoproteins.
• Podocytes, which are structurally complex cells that possess interdigitating processes embedded in and adherent to the lamina rara externa of the basement membrane. Adjacent foot processes are separated by 20- to 30-nm-wide filtration slits, which are bridged by a thin slit diaphragm composed in large part of nephrin (see further on).
• The glomerular tuft is supported by mesangial cells lying between the capillaries. Basement membrane–like mesangial matrix forms a meshwork through which the mesangial cells are scattered. These cells, of mesenchymal origin, are contractile and are capable of proliferation, of laying down collagen and other matrix components, and of secreting a number of biologically active mediators.
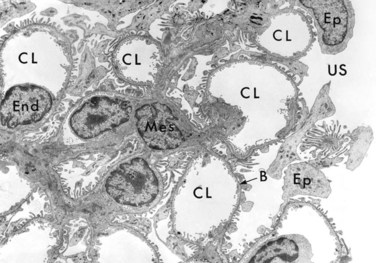
Figure 13–2 Low-power electron micrograph of rat glomerulus. B, basement membrane; CL, capillary lumen; End, endothelium; Ep, visceral epithelial cells (podocytes) with foot processes; Mes, mesangium; US, urinary space.
Normally, the glomerular filtration system is extraordinarily permeable to water and small solutes and almost completely impermeable to molecules of the size and molecular charge of albumin (a 70,000-kDa protein). This selective permeability, called glomerular barrier function, discriminates among protein molecules according to their size (the larger, the less permeable), their charge (the more cationic, the more permeable), and their configuration. The characteristics of the normal barrier depend on the complex structure of the capillary wall, the integrity of the GBM, and the many anionic molecules present within the wall, including the acidic proteoglycans of the GBM and the sialoglycoproteins of epithelial and endothelial cell coats. The podocyte is also crucial to the maintenance of glomerular barrier function. Podocyte slit diaphragms are important diffusion barriers for plasma proteins, and podocytes are also largely responsible for synthesis of GBM components.
In the past few years, much has been learned about the molecular architecture of the glomerular filtration barrier. Nephrin, a transmembrane glycoprotein, is the major component of the slit diaphragms between adjacent foot processes. Nephrin molecules from adjacent foot processes bind to each other through disulfide bridges at the center of the slit diaphragm. The intracellular part of nephrin interacts with several cytoskeletal and signaling proteins (Fig. 13–1). Nephrin and its associated proteins, including podocin, have a crucial role in maintaining the selective permeability of the glomerular filtration barrier. This role is dramatically illustrated by rare hereditary diseases in which mutations of nephrin or its partner proteins are associated with abnormal leakage into the urine of plasma proteins, giving rise to the nephrotic syndrome (discussed later). This observation suggests that acquired defects in the function or structure of slit diaphragms constitute an important mechanism of proteinuria, the hallmark of the nephrotic syndrome.
Glomeruli may be injured by diverse mechanisms and in the course of a number of systemic diseases (Table 13–1). Immunologically mediated diseases such as systemic lupus erythematosus, vascular disorders such as hypertension and hemolytic uremic syndrome, metabolic diseases such as diabetes mellitus, and some purely hereditary conditions such as Alport syndrome often affect the glomerulus. These are termed secondary glomerular diseases to differentiate them from those in which the kidney is the only or predominant organ involved. The latter constitute the various types of primary glomerular diseases, which are discussed later in this section. The glomerular alterations in systemic diseases are discussed elsewhere.
Table 13–1 Glomerular Diseases
| Primary Glomerular Diseases |
| Glomerulopathies Secondary to Systemic Diseases |
| Hereditary Disorders |
GN, glomerulonephritis; IgA, immunoglobulin A.
Mechanisms of Glomerular Injury and Disease
Although little is known about the etiologic agents or triggering events, it is clear that immune mechanisms underlie most types of primary glomerular diseases and many of the secondary glomerular diseases. Under experimental conditions, glomerulonephritis (GN) can be readily induced by antibodies, and deposits of immunoglobulins, often with various components of complement, are found frequently in patients with GN. Cell-mediated immune mechanisms may also play a role in certain glomerular diseases.
Two forms of antibody-associated injury have been established: (1) injury resulting from deposition of soluble circulating antigen-antibody complexes in the glomerulus and (2) injury by antibodies reacting in situ within the glomerulus, either with insoluble fixed (intrinsic) glomerular antigens or with molecules planted within the glomerulus (Fig. 13–3). In addition, antibodies directed against glomerular cell components may cause glomerular injury. These pathways are not mutually exclusive, and in humans all may contribute to injury.
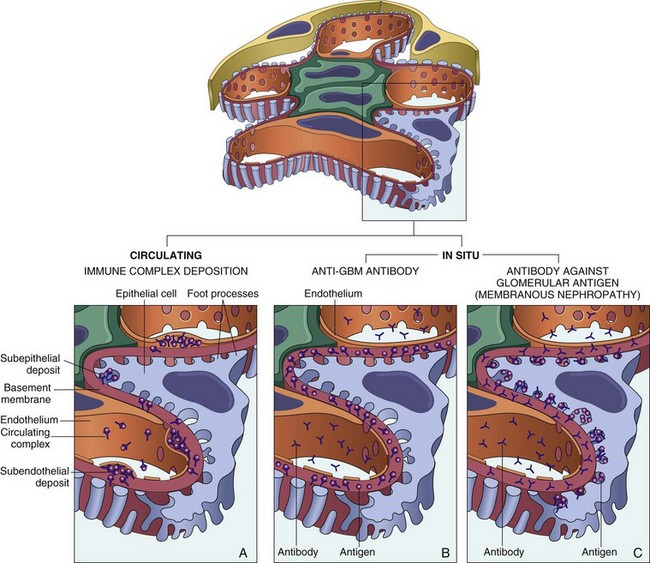
Figure 13–3 Antibody-mediated glomerular injury. Injury can result either from the deposition of circulating immune complexes or from formation of complexes in situ. A, Deposition of circulating immune complexes gives a granular immunofluorescence pattern. B, Anti-glomerular basement membrane (anti-GBM) antibody glomerulonephritis is characterized by a linear immunofluorescence pattern. C, Antibodies against some glomerular components deposit in a granular pattern.
Glomerulonephritis Caused by Circulating Immune Complexes
The pathogenesis of immune complex diseases is discussed in detail in Chapter 4. Presented here is a brief review of the salient features that relate to glomerular injury in GN.
With circulating immune complex–mediated disease, the glomerulus may be considered an “innocent bystander” because it does not incite the reaction. The antigen is not of glomerular origin. It may be endogenous, as in the GN associated with systemic lupus erythematosus, or it may be exogenous, as is probable in the GN that follows certain bacterial (streptococcal), viral (hepatitis B), parasitic (Plasmodium falciparum malaria), and spirochetal (Treponema pallidum) infections. Often the inciting antigen is unknown, as in most cases of membranoproliferative GN (MPGN).
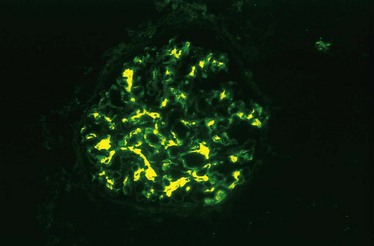
Whatever the antigen may be, antigen–antibody complexes are formed in situ or in the circulation and are then trapped in the glomeruli, where they produce injury, in large part through the activation of complement and the recruitment of leukocytes. Injury also may occur through the engagement of Fc receptors on leukocytes independent of complement activation, as cross-linking of Fc receptors by IgG antibodies also results in leukocyte activation and degranulation. Regardless of the mechanism, the glomerular lesions usually consist of leukocytic infiltration (exudation) into glomeruli and variable proliferation of endothelial, mesangial, and parietal epithelial cells. Electron microscopy reveals the immune complexes as electron-dense deposits or clumps that lie at one of three sites: in the mesangium, between the endothelial cells and the GBM (subendothelial deposits), or between the outer surface of the GBM and the podocytes (subepithelial deposits). Deposits may be located at more than one site in a given case. The presence of immunoglobulins and complement in these deposits can be demonstrated by immunofluorescence microscopy (Fig. 13–4, A). The pattern and location of immune complex deposition are helpful in distinguishing among various types of GN.
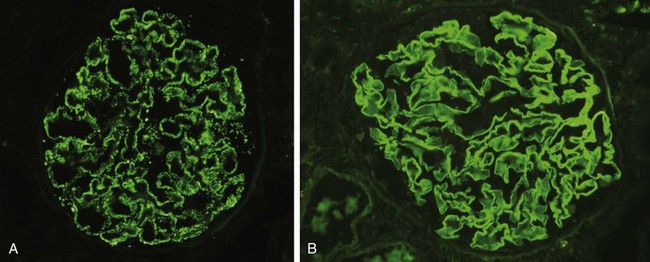
Figure 13–4 Two patterns of deposition of immune complexes as seen by immunofluorescence microscopy. A, Granular, characteristic of circulating and in situ immune complex deposition. B, Linear, characteristic of classic anti-glomerular basement membrane (anti-GBM) antibody glomerulonephritis.
(A, Courtesy of Dr. J. Kowalewska, Department of Pathology, University of Washington, Seattle, Washington.)
Once deposited in the kidney, immune complexes may eventually be degraded or phagocytosed, mostly by infiltrating leukocytes and mesangial cells, and the inflammatory changes may then subside. Such a course occurs when the exposure to the inciting antigen is short-lived and limited, as in most cases of poststreptococcal or acute infection-related GN. However, if exposure to antigen is sustained over time, repeated cycles of immune complex formation, deposition, and injury may occur, leading to chronic GN. In some cases the source of chronic antigenic exposure is clear, such as in hepatitis B virus infection and self nuclear antigens in systemic lupus erythematosus. In other cases, however, the antigen is unknown. Circulating immune complex deposition as a mechanism of injury is well studied in animal models but is uncommonly identified in human disease.
Glomerulonephritis Caused by In Situ Immune Complexes
Antibody deposition in the glomerulus is a major pathway of glomerular injury. As noted, antibodies in this form of injury react directly with fixed or planted antigens in the glomerulus. Immune reactions in situ, trapping of circulating complexes, interactions between these two events, and local hemodynamic and structural determinants in the glomerulus all contribute to the morphologic and functional alterations in GN. Antibodies also may react in situ with previously “planted” nonglomerular antigens, which may localize in the kidney by interacting with various intrinsic components of the glomerulus. Planted antigens include nucleosomal complexes (in patients with systemic lupus erythematosus); bacterial products, such as endostroptosin, a protein expressed by group A streptococci; large aggregated proteins (e.g., aggregated immunoglobulin G [IgG]), which tend to deposit in the mesangium; and immune complexes themselves, because they contain reactive sites for further interactions with free antibody, free antigen, or complement. Most of these planted antigens induce a granular pattern of immunoglobulin deposition as seen by immunofluorescence microscopy.
The following factors affect glomerular localization of antigen, antibody, or immune complexes: the molecular charge and size of the reactants; glomerular hemodynamics; mesangial function; and the integrity of the charge-selective glomerular barrier. The localization of antigen, antibody, or immune complexes in turn determines the glomerular injury response. Studies in experimental models have shown that complexes deposited in the endothelium or subendothelium elicit an inflammatory reaction in the glomerulus with infiltration of leukocytes and exuberant proliferation of glomerular resident cells. By contrast, antibodies directed to the subepithelial region of glomerular capillaries are largely noninflammatory and elicit lesions similar to those of Heymann nephritis or membranous nephropathy (discussed later).
Anti-Glomerular Basement Membrane Antibody–Mediated Glomerulonephritis
The best-characterized disease in this group is classic anti-GBM antibody–mediated crescentic GN (Fig. 13–3, B). In this type of injury, antibodies are directed against fixed antigens in the GBM. It has its experimental counterpart in the nephritis of rodents called nephrotoxic serum nephritis. This is produced by injecting rats with anti-GBM antibodies produced by immunization of rabbits or other species with rat kidney. Antibody–mediated GN in humans results from the formation of autoantibodies directed against the GBM. Deposition of these antibodies creates a linear pattern of staining when the bound antibodies are visualized with immunofluorescence microscopy, in contrast with the granular pattern described for other forms of immune complex–mediated nephritis (Fig. 13–4, B). This distinction is useful in the diagnosis of glomerular disease. A conformational change in the α3 chain of the type IV collagen of the GBM appears to be key in inciting autoimmunity. Sometimes the anti-GBM antibodies cross-react with basement membranes of lung alveoli, resulting in simultaneous lung and kidney lesions (Goodpasture syndrome). Although anti-GBM antibody–mediated GN accounts for less than 1% of human GN cases, the resulting disease can be very serious. Many instances of anti-GBM antibody–mediated crescentic GN are characterized by very severe glomerular damage with necrosis and crescents and the development of the clinical syndrome of rapidly progressive GN (see below).
Mediators of Immune Injury
Once immune reactants are localized in the glomerulus, how does glomerular damage ensue? A major pathway of antibody-initiated injury involves complement activation and recruitment of leukocytes (Fig. 13–5). Activation of complement via the classical pathway leads to the generation of chemotactic agents (mainly C5a) for neutrophils and monocytes. Neutrophils release proteases, which cause GBM degradation; oxygen-derived free radicals, which cause cell damage; and arachidonic acid metabolites, which contribute to reduction in GFR. This mechanism applies only to some types of GN, however, because many types show few neutrophils in the damaged glomeruli. In these cases neutrophil-independent but complement-dependent injury may occur, possibly caused by the C5b-C9 membrane attack complex, which is formed on the GBM and may induce sublytic epithelial cell injury and stimulate the secretion of various inflammatory mediators from mesangial and epithelial cells. The alternative and mannose-binding lectin pathways of complement can be activated by cell injury or apoptosis, also leading to glomerular injury (Fig. 13–5).
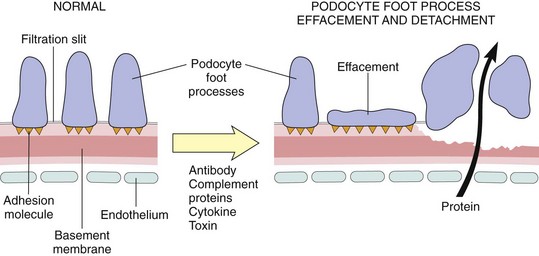
Figure 13–5 Podocyte injury. The postulated sequence may be initiated by antibodies to podocyte antigens, toxins, cytokines, or other factors. The common features are podocyte injury leading to foot process effacement and variable degrees of podocyte detachment, and degradation of the basement membrane. These defects permit plasma proteins to be lost into the urinary space.
Antibodies against glomerular cell antigens also may directly damage glomerular cells or slit diaphragms. Such antibodies are suspected of being involved in certain disorders in which immune complexes are not found. Other mediators of glomerular damage include the following:
• Monocytes and macrophages, which infiltrate the glomerulus in antibody- and cell-mediated reactions and, when activated, release diverse mediators
• Sensitized T cells, formed during the course of a cell-mediated immune reaction, can cause experimental glomerular injury. In some forms of experimental GN, the disease can be induced by transfer of sensitized T cells. T cell–mediated injury may account for the instances of GN in which either there are no deposits of antibodies or immune complexes or the deposits do not correlate with the severity of damage. However, it has been difficult to establish a causal role for T cells or cell-mediated immune responses in human GN.
• Platelets, which aggregate in the glomerulus during immune-mediated injury and release prostaglandins and growth factors
• Resident glomerular cells (epithelial, mesangial, and endothelial), which can be stimulated to secrete mediators such as cytokines (interleukin-1), arachidonic acid metabolites, growth factors, nitric oxide, and endothelin
• Thrombin, produced as a consequence of intraglomerular thrombosis, which causes leukocyte infiltration and glomerular cell proliferation by triggering protease-activated receptors (PARs)
In essence, virtually all of the mediators described in the discussion of inflammation in Chapter 2 may contribute to glomerular injury.
Other Mechanisms of Glomerular Injury
Other mechanisms contribute to glomerular damage in certain primary renal disorders. Two that deserve special mention due to their importance are podocyte injury and nephron loss.
Podocyte Injury
Podocyte injury can be induced by antibodies to podocyte antigens; by toxins, as in an experimental model of proteinuria induced by the ribosome poison puromycin; conceivably by certain cytokines; or by still poorly characterized circulating factors, as in some cases of focal segmental glomerulosclerosis (see later). Podocyte injury is reflected by morphologic changes, which include effacement of foot processes, vacuolization, and retraction and detachment of cells from the GBM, and clinically by proteinuria. In most forms of glomerular injury, loss of normal slit diaphragms is key in the development of proteinuria (Fig. 13–5). Functional abnormalities of the slit diaphragm also may result from mutations in its structural components, such as nephrin and the associated podocin. Such mutations cause rare hereditary forms of the nephrotic syndrome.
Nephron Loss
Once renal disease, glomerular or otherwise, destroys sufficient nephrons to reduce the GFR to 30% to 50% of normal, progression to end-stage kidney disease proceeds inexorably at varying rates. Affected persons have proteinuria, and their kidneys show widespread glomerulosclerosis. Such progressive sclerosis may be initiated, at least in part, by the adaptive changes that occur in the remaining glomeruli not destroyed by the initial disease. These remaining glomeruli undergo hypertrophy to maintain renal function. This hypertrophy is associated with hemodynamic changes, including increases in single-nephron GFR, blood flow, and transcapillary pressure (capillary hypertension). These alterations ultimately become “maladaptive” and lead to further endothelial and podocyte injury, increased glomerular permeability to proteins, and accumulation of proteins and lipids in the mesangial matrix. This is followed by capillary obliteration, increased deposition of mesangial matrix and plasma proteins, and ultimately by segmental (affecting a portion) or global (complete) sclerosis of glomeruli. The latter results in further reductions in nephron mass and a vicious circle of progressive glomerulosclerosis.
![]() Summary
Summary
Glomerular Injury
• Antibody-mediated immune injury is an important mechanism of glomerular damage, mainly by way of complement- and leukocyte-mediated pathways. Antibodies also may be directly cytotoxic to cells in the glomerulus.
• The most common forms of antibody-mediated GN are caused by the formation of immune complexes, whether occurring in situ or by deposition of circulating immune complexes. These immune complexes may contain exogenous (e.g. microbial) circulating antigens or endogenous antigens (e.g. in membranous nephropathy). Immune complexes show a granular pattern of deposition.
• Autoantibodies against components of the GBM are the cause of anti-GBM-antibody–mediated disease, often associated with severe injury. The pattern of antibody deposition is linear.
• Immune complexes and antibodies cause injury by complement activation and leukocyte recruitment, with release of various mediators, and sometimes by direct podocyte damage.
We now turn to a consideration of specific types of GN and the glomerular syndromes they produce.
The Nephrotic Syndrome
The nephrotic syndrome refers to a clinical complex that includes
• Massive proteinuria, with daily protein loss in the urine of 3.5 g or more in adults
• Hypoalbuminemia, with plasma albumin levels less than 3 g/dL
• Generalized edema, the most obvious clinical manifestation
The nephrotic syndrome has diverse causes that share a common pathophysiology (Table 13–2). In all there is a derangement in the capillary walls of the glomeruli that results in increased permeability to plasma proteins. Any increased permeability resulting from either structural or physicochemical alterations in the GBM allows protein to escape from the plasma into the glomerular filtrate. With long-standing or extremely heavy proteinuria, serum albumin is decreased, resulting in hypoalbuminemia and a drop in plasma colloid osmotic pressure. As discussed in Chapter 3, the resulting decrease in intravascular volume and renal blood flow triggers increased release of renin from renal juxtaglomerular cells. Renin in turn stimulates the angiotensin-aldosterone axis, which promotes the retention of salt and water by the kidney. This tendency is exacerbated by reductions in the cardiac secretion of natriuretic factors. In the face of continuing proteinuria, these alterations further aggravate the edema and if unchecked may lead to the development of generalized edema (termed anasarca). At the onset, there is little or no azotemia, hematuria, or hypertension.
Table 13–2 Causes of Nephrotic Syndrome
| Cause | Prevalence (%)* | |
|---|---|---|
| Children | Adults | |
| Primary Glomerular Disease | ||
| Membranous nephropathy | 5 | 30 |
| Minimal-change disease | 65 | 10 |
| Focal segmental glomerulosclerosis | 10 | 35 |
| Membranoproliferative glomerulonephritis | 10 | 10 |
| IgA nephropathy and others | 10 | 15 |
| Systemic Diseases with Renal Manifestations | ||
| Diabetes mellitus | ||
| Amyloidosis | ||
| Systemic lupus erythematosus | ||
| Ingestion of drugs (gold, penicillamine, “street heroin”) | ||
| Infections (malaria, syphilis, hepatitis B, HIV infection) | ||
| Malignancy (carcinoma, melanoma) | ||
| Miscellaneous (bee sting allergy, hereditary nephritis) | ||
HIV, human immunodeficiency virus.
* Approximate prevalence of primary disease is 95% of the cases in children, 60% in adults. Approximate prevalence of systemic disease is 5% of the cases in children, 40% in adults.
The genesis of the hyperlipidemia is more obscure. Presumably, hypoalbuminemia triggers increased synthesis of lipoproteins in the liver or massive proteinuria causes loss of an inhibitor of their synthesis. There is also abnormal transport of circulating lipid particles and impairment of peripheral breakdown of lipoproteins. The lipiduria, in turn, reflects the increased permeability of the GBM to lipoproteins.
The relative frequencies of the several causes of the nephrotic syndrome vary according to age (Table 13–2). In children 1 to 7 years of age, for example, the nephrotic syndrome is almost always caused by a lesion primary to the kidney, whereas among adults it often is due to renal manifestations of a systemic disease. The most frequent systemic causes of the nephrotic syndrome in adults are diabetes, amyloidosis, and systemic lupus erythematosus. The renal lesions produced by these disorders are described in Chapter 4. The most important of the primary glomerular lesions that characteristically lead to the nephrotic syndrome are focal and segmental glomerulosclerosis and minimal-change disease. The latter is more important in children; the former, in adults. Two other primary lesions, membranous nephropathy and membranoproliferative glomerulonephritis, also commonly produce the nephrotic syndrome. These four lesions are discussed individually next.
Minimal-Change Disease
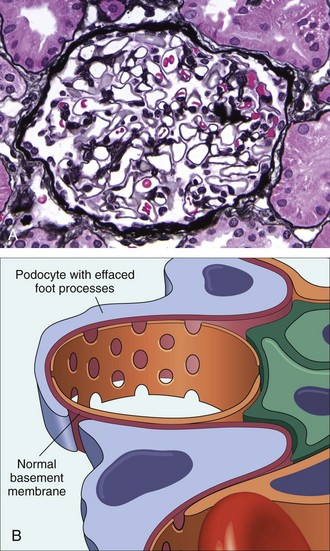
Minimal-change disease, a relatively benign disorder, is the most frequent cause of the nephrotic syndrome in children. Characteristically, the glomeruli have a normal appearance by light microscopy but show diffuse effacement of podocyte foot processes when viewed with the electron microscope. Although it may develop at any age, this condition is most common between the ages of 1 and 7 years.
The pathogenesis of proteinuria in minimal-change disease remains to be elucidated. On the basis of some experimental studies, the proteinuria has been attributed to a circulating, possibly T cell–derived, factor that causes podocyte damage and effacement of foot processes. Neither the nature of such a putative factor nor a causal role of T cells, however, is established in the human disease.
![]() Morphology
Morphology
Under the light microscope, the glomeruli appear normal, thus giving rise to the name “minimal-change disease” (Fig. 13–6, A). The cells of the proximal convoluted tubules often are heavily laden with protein droplets and lipids, but this feature is secondary to tubular reabsorption of the lipoproteins passing through the diseased glomeruli. Even under the electron microscope, the GBM appears normal. The only obvious glomerular abnormality is the uniform and diffuse effacement of the foot processes of the podocytes (Fig. 13–6, B). The cytoplasm of the podocytes thus appears flattened over the external aspect of the GBM, obliterating the network of arcades between the podocytes and the GBM. There are also epithelial cell vacuolization, microvillus formation, and occasional focal detachments, suggesting some form of podocyte injury. With reversal of the changes in the podocytes (e.g., in response to corticosteroids), the proteinuria remits.
Clinical Course
The disease manifests with the insidious development of the nephrotic syndrome in an otherwise healthy child. There is no hypertension, and renal function is preserved in most of these patients. The protein loss usually is confined to the smaller plasma proteins, chiefly albumin (selective proteinuria). The prognosis for children with this disorder is good. More than 90% of children respond to a short course of corticosteroid therapy; however, proteinuria recurs in more than two thirds of the initial responders, some of whom become steroid-dependent. Less than 5% develop chronic kidney disease after 25 years, and it is likely that most persons in this subgroup had nephrotic syndrome caused by focal and segmental glomerulosclerosis not detected by biopsy. Because of its responsiveness to therapy in children, minimal-change disease must be differentiated from other causes of the nephrotic syndrome in nonresponders. Adults with this disease also respond to steroid therapy, but the response is slower and relapses are more common.
Focal Segmental Glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS) is characterized histologically by sclerosis affecting some but not all glomeruli (focal involvement) and involving only segments of each affected glomerulus (segmental involvement). This histologic picture often is associated with the nephrotic syndrome. FSGS may be primary (idiopathic) or secondary to one of the following conditions:
• In association with other conditions, such as HIV infection (HIV nephropathy) or heroin abuse (heroin nephropathy)
• As a secondary event in other forms of GN (e.g., IgA nephropathy)
• As a maladaptation to nephron loss (as described earlier)
• In inherited or congenital forms. Autosomal dominant forms are associated with mutations in cytoskeletal proteins and podocin, both of which are required for the integrity of podocytes. In addition, a sequence variant in the apolipoprotein L1 gene (APOL1) on chromosome 22 appears to be strongly associated with an increased risk of FSGS and renal failure in individuals of African descent.
Primary FSGS accounts for approximately 20% to 30% of all cases of the nephrotic syndrome. It is an increasingly common cause of nephrotic syndrome in adults and remains a frequent cause in children.
![]() Pathogenesis
Pathogenesis
The pathogenesis of primary FSGS is unknown. Some investigators have suggested that FSGS and minimal-change disease are part of a continuum and that minimal-change disease may transform into FSGS. Others believe them to be distinct clinicopathologic entities from the outset. In any case, injury to the podocytes is thought to represent the initiating event of primary FSGS. As with minimal-change disease, permeability-increasing factors produced by lymphocytes have been proposed. The deposition of hyaline masses in the glomeruli represents the entrapment of plasma proteins and lipids in foci of injury where sclerosis develops. IgM and complement proteins commonly seen in the lesion are also believed to result from nonspecific entrapment in damaged glomeruli. The recurrence of proteinuria and subsequent FSGS in a renal transplant in some patients who had FSGS, sometimes within 24 hours of transplantation, supports the idea that a circulating mediator is the cause of the podocyte damage in some cases.
![]() Morphology
Morphology
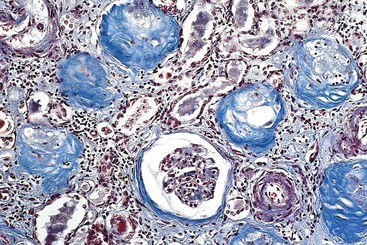
In FSGS, the disease first affects only some of the glomeruli (hence the term focal) and, in the case of primary FSGS, initially only the juxtamedullary glomeruli. With progression, eventually all levels of the cortex are affected. On histologic examination, FSGS is characterized by lesions occurring in some tufts within a glomerulus and sparing of the others (hence the term segmental). Thus, the involvement is both focal and segmental (Fig. 13–7). The affected glomeruli exhibit increased mesangial matrix, obliterated capillary lumina, and deposition of hyaline masses (hyalinosis) and lipid droplets. In affected glomeruli, immunofluorescence microscopy often reveals nonspecific trapping of immunoglobulins, usually IgM, and complement in the areas of hyalinosis. On electron microscopy, the podocytes exhibit effacement of foot processes, as in minimal-change disease.
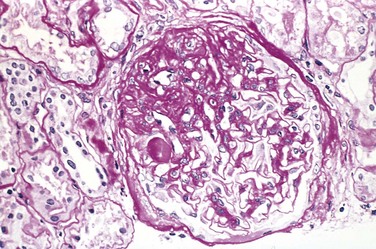
Figure 13–7 High-power view of focal and segmental glomerulosclerosis (periodic acid–Schiff stain), seen as a mass of scarred, obliterated capillary lumens with accumulations of matrix material that has replaced a portion of the glomerulus.
(Courtesy of Dr. H. Rennke, Department of Pathology, Brigham and Women’s Hospital, Boston, Massachusetts.)
In time, progression of the disease leads to global sclerosis of the glomeruli with pronounced tubular atrophy and interstitial fibrosis. This advanced picture is difficult to differentiate from other forms of chronic glomerular disease, described later on.
A morphologic variant called collapsing glomerulopathy is being increasingly reported. It is characterized by collapse of the glomerular tuft and podocyte hyperplasia. This is a more severe manifestation of FSGS that may be idiopathic or associated with HIV infection, drug-induced toxicities, and some microvascular injuries. It carries a particularly poor prognosis.
Clinical Course
In children it is important to distinguish FSGS as a cause of the nephrotic syndrome from minimal-change disease, because the clinical courses are markedly different. The incidence of hematuria and hypertension is higher in persons with FSGS than in those with minimal-change disease; the FSGS-associated proteinuria is nonselective; and in general the response to corticosteroid therapy is poor. At least 50% of patients with FSGS develop end-stage kidney disease within 10 years of diagnosis. Adults typically fare even less well than children.
Membranous Nephropathy
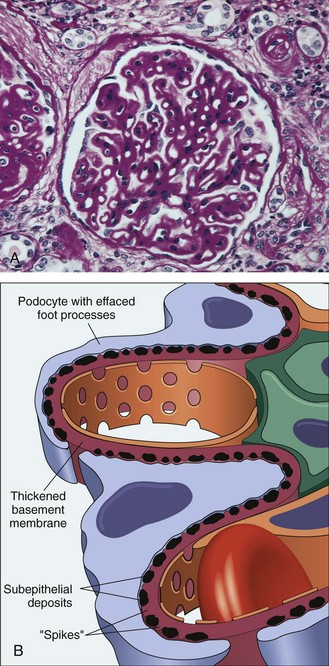
Membranous nephropathy is a slowly progressive disease, most common between 30 and 60 years of age. It is characterized morphologically by the presence of subepithelial immunoglobulin-containing deposits along the GBM. Early in the disease, the glomeruli may appear normal by light microscopy, but well-developed cases show diffuse thickening of the capillary wall.
In about 85% of cases, membranous nephropathy is caused by autoantibodies that cross-react with antigens expressed by podocytes. In the remainder (secondary membranous nephropathy), it occurs secondary to other disorders, including
• Infections (chronic hepatitis B, syphilis, schistosomiasis, malaria)
• Malignant tumors, particularly carcinoma of the lung and colon and melanoma
• Systemic lupus erythematosus and other autoimmune conditions
• Exposure to inorganic salts (gold, mercury)
• Drugs (penicillamine, captopril, nonsteroidal anti-inflammatory agents)
![]() Pathogenesis
Pathogenesis
Membranous nephropathy is a form of chronic immune complex glomerulonephritis induced by antibodies reacting in situ to endogenous or planted glomerular antigens. An endogenous podocyte antigen, the phospholipase A2 receptor, is the antigen that is most often recognized by the causative autoantibodies.
The experimental model of membranous nephropathy is Heymann nephritis, which is induced in animals by immunization with renal tubular brush border proteins that also are present on podocytes. The antibodies that are produced react with an antigen located in the glomerular capillary wall, resulting in granular deposits (in situ immune complex formation) and proteinuria without severe inflammation.
A puzzling aspect of the disease is how antigen-antibody complexes cause capillary damage despite the absence of inflammatory cells. The likely answer is by activating complement, which is uniformly present in the lesions of membranous nephropathy. It is hypothesized that complement activation leads to assembly of the C5b-C9 membrane attack complex, which damages mesangial cells and podocytes directly, setting in motion events that cause the loss of slit filter integrity and proteinuria.
![]() Morphology
Morphology
Histologically, the main feature in membranous nephropathy is diffuse thickening of the capillary wall (Fig. 13–8, A). Electron microscopy reveals that this thickening is caused in part by subepithelial deposits, which nestle against the GBM and are separated from each other by small, spikelike protrusions of GBM matrix that form in reaction to the deposits (spike and dome pattern) (Fig. 13–8, B). As the disease progresses, these spikes close over the deposits, incorporating them into the GBM. In addition, as in other causes of nephrotic syndrome, the podocytes show effacement of foot processes. Later in the disease, the incorporated deposits may be broken down and eventually disappear, leaving cavities within the GBM. Continued deposition of basement membrane matrix leads to progressive thickening of basement membranes. With further progression, the glomeruli can become sclerosed. Immunofluorescence microscopy shows typical granular deposits of immunoglobulins and complement along the GBM (Fig. 13–4, A).
Clinical Course
Most cases of membranous nephropathy present as full-blown nephrotic syndrome, usually without antecedent illness; other individuals may have lesser degrees of proteinuria. In contrast with minimal-change disease, the proteinuria is nonselective, with urinary loss of globulins as well as smaller albumin molecules, and does not usually respond to corticosteroid therapy. Secondary causes of membranous nephropathy should be ruled out. Membranous nephropathy follows a notoriously variable and often indolent course. Overall, although proteinuria persists in greater than 60% of patients with membranous nephropathy, only about 40% suffer progressive disease terminating in renal failure after 2 to 20 years. An additional 10% to 30% have a more benign course with partial or complete remission of proteinuria.
Membranoproliferative Glomerulonephritis and Dense Deposit Disease
Membranoproliferative GN (MPGN) is manifested histologically by alterations in the GBM and mesangium and by proliferation of glomerular cells. It accounts for 5% to 10% of cases of idiopathic nephrotic syndrome in children and adults. Some patients present only with hematuria or proteinuria in the non-nephrotic range; others exhibit a combined nephrotic–nephritic picture. Two major types of MPGN (I and II) have traditionally been recognized on the basis of distinct ultrastructural, immunofluorescence, microscopic, and pathogenic findings, but these are now recognized to be separate entities, termed MPGN type I and dense deposit disease (formerly MPGN type II). Of the two types of disease, MPGN type I is far more common (about 80% of cases).
![]() Pathogenesis
Pathogenesis
Different pathogenic mechanisms are involved in the development of MPGN and dense deposit disease.
• Some cases of type I MPGN may be caused by circulating immune complexes, akin to chronic serum sickness, or may be due to a planted antigen with subsequent in situ immune complex formation. In either case, the inciting antigen is not known. Type I MPGN also occurs in association with hepatitis B and C antigenemia, systemic lupus erythematosus, infected atrioventricular shunts, and extrarenal infections with persistent or episodic antigenemia.
• The pathogenesis of dense deposit disease is less clear. The fundamental abnormality in dense deposit disease appears to be excessive complement activation. Some patients have an autoantibody against C3 convertase, called C3 nephritic factor, which is believed to stabilize the enzyme and lead to uncontrolled cleavage of C3 and activation of the alternative complement pathway. Mutations in the gene encoding the complement regulatory protein factor H or autoantibodies to factor H have been described in some patients. These abnormalities result in excessive complement activation. Hypocomplementemia, more marked in dense deposit disease, is produced in part by excessive consumption of C3 and in part by reduced synthesis of C3 by the liver. It is still not clear how the complement abnormality induces the glomerular changes.
![]() Morphology
Morphology
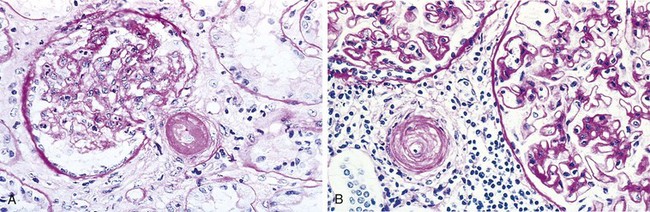
By light microscopy, type I MPGN and many cases of dense deposit disease are similar. The glomeruli are large, with an accentuated lobular appearance, and show proliferation of mesangial and endothelial cells as well as infiltrating leukocytes (Fig. 13–9, A). The GBM is thickened, and the glomerular capillary wall often shows a double contour, or “tram track,” appearance, especially evident with use of silver or periodic acid–Schiff (PAS) stains. This “splitting” of the GBM is due to extension of processes of mesangial and inflammatory cells into the peripheral capillary loops and deposition of mesangial matrix (Fig. 13–9, B).
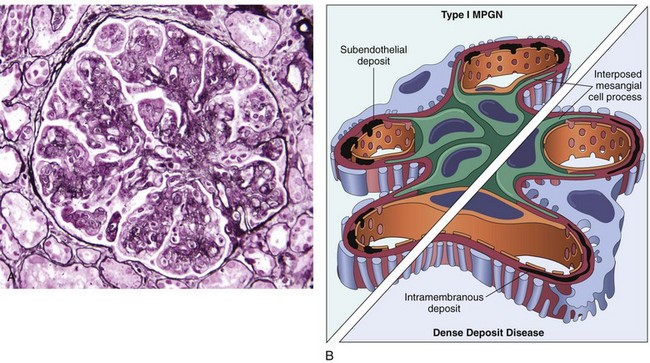
Figure 13–9 A, Membranoproliferative glomerulonephritis (MPGN), showing mesangial cell proliferation, basement membrane thickening, leukocyte infiltration, and accentuation of lobular architecture. B, Schematic representation of patterns in the two types of MPGN. In type I there are subendothelial deposits; in type II, now called dense deposit disease, intramembranous characteristically dense deposits are seen. In both types, mesangial interposition gives the appearance of split basement membranes when viewed by light microscopy.
Type I MPGN is characterized by discrete subendothelial electron-dense deposits (Fig. 13–9, B). By immunofluorescence microscopy, C3 is deposited in an irregular granular pattern, and IgG and early complement components (C1q and C4) often are also present, indicative of an immune complex pathogenesis.
By contrast, in the aptly named dense deposit disease the lamina densa and the subendothelial space of the GBM are transformed into an irregular, ribbon-like, extremely electron-dense structure, resulting from the deposition of material of unknown composition. C3 is present in irregular chunky and segmental linear foci in the basement membranes and in the mesangium. IgG and the early components of the classical complement pathway (C1q and C4) are usually absent.
Clinical Course
The principal mode of presentation (in approximately 50% of cases) is the nephrotic syndrome, although MPGN or dense deposit disease may begin as acute nephritis or mild proteinuria. The prognosis of MPGN type I generally is poor. In one study, none of the 60 patients followed for 1 to 20 years showed complete remission. Forty percent progressed to end-stage renal failure, 30% had variable degrees of renal insufficiency, and the remaining 30% had persistent nephrotic syndrome without renal failure. Dense deposit disease carries an even worse prognosis, and it tends to recur more frequently in renal transplant recipients. MPGN type I may occur in association with other disorders (secondary MPGN), such as systemic lupus erythematosus, hepatitis B and C, chronic liver disease, and chronic bacterial infections. Indeed, many so-called idiopathic cases are believed to be associated with hepatitis C and related cryoglobulinemia.
![]() Summary
Summary
The Nephrotic Syndrome
• The nephrotic syndrome is characterized by proteinuria, which results in hypoalbuminemia and edema.
• Podocyte injury is an underlying mechanism of proteinuria, and may be the result of nonimmune causes (as in minimal-change disease and FSGS) or immune mechanisms (as in membranous nephropathy).
• Minimal-change disease is the most frequent cause of nephrotic syndrome in children; it is manifested by proteinuria and effacement of glomerular foot processes without antibody deposits; the pathogenesis is unknown; the disease responds well to steroid therapy.
• FSGS may be primary (podocyte injury by unknown mechanisms) or secondary (e.g., as a consequence of previous glomerulonephritis, hypertension, or infection such as with HIV); glomeruli show focal and segmental obliteration of capillary lumina, and loss of foot processes; the disease often is resistant to therapy and may progress to end-stage renal disease.
• Membranous nephropathy is caused by an autoimmune response, most often directed against the phospholipase A2 receptor on podocytes; it is characterized by granular subepithelial deposits of antibodies with GBM thickening and loss of foot processes but little or no inflammation; the disease often is resistant to steroid therapy.
• MPGN and dense deposit disease are now recognized to be distinct entities. MPGN is caused by immune complex deposition; dense deposit disease is a consequence of complement dysregulation. Both may present with nephrotic and/or nephritic features.
The Nephritic Syndrome
The nephritic syndrome is a clinical complex, usually of acute onset, characterized by (1) hematuria with dysmorphic red cells and red cell casts in the urine; (2) some degree of oliguria and azotemia; and (3) hypertension.
Although proteinuria and even edema also may be present, these usually are not as severe as in the nephrotic syndrome. The lesions that cause the nephritic syndrome have in common proliferation of the cells within the glomeruli, often accompanied by an inflammatory leukocytic infiltrate. This inflammatory reaction severely injures the capillary walls, permitting blood to pass into the urine and inducing hemodynamic changes that lead to a reduction in the GFR. The reduced GFR is manifested clinically by oliguria, fluid retention, and azotemia. Hypertension probably is a result of both the fluid retention and some augmented renin release from the ischemic kidneys.
The acute nephritic syndrome may be produced by systemic disorders such as systemic lupus erythematosus, or it may be secondary to primary glomerular disease. The latter is exemplified by acute postinfectious GN.
Acute Postinfectious (Poststreptococcal) Glomerulonephritis
Acute postinfectious GN, one of the more frequently occurring glomerular disorders, is caused by glomerular deposition of immune complexes resulting in proliferation of and damage to glomerular cells and infiltration of leukocytes, especially neutrophils. The inciting antigen may be exogenous or endogenous. The prototypic exogenous pattern is seen in poststreptococcal GN. Infections by organisms other than streptococci may also be associated with postinfectious GN. These include certain pneumococcal and staphylococcal infections as well as several common viral diseases such as mumps, measles, chickenpox, and hepatitis B and C. Endogenous antigens, as occur in systemic lupus erythematosus, also may cause a proliferative GN but more commonly result in a membranous nephropathy (see earlier) lacking the neutrophil infiltrates that are characteristic of postinfectious GN.
The classic case of poststreptococcal GN develops in a child 1 to 4 weeks after they recover from a group A streptococcal infection. Only certain “nephritogenic” strains of β-hemolytic streptococci evoke glomerular disease. In most cases, the initial infection is localized to the pharynx or skin.
![]() Pathogenesis
Pathogenesis
Poststreptococcal GN is an immune complex disease in which tissue injury is primarily caused by complement activation by the classical pathway. Typical features of immune complex disease, such as hypocomplementemia and granular deposits of IgG and complement on the GBM, are seen. The relevant antigens probably are streptococcal proteins. Specific antigens implicated in pathogenesis include streptococcal exotoxin B (Spe B) and streptococcal GAPDH. Both activate the alternative complement pathway and have affinity for glomerular proteins and plasmin. It is not clear if immune complexes are formed mainly in the circulation or in situ (the latter by binding of antibodies to bacterial antigens “planted” in the GBM).
![]() Morphology
Morphology
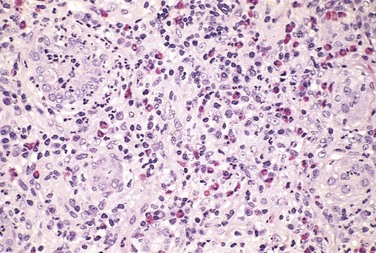
By light microscopy, the most characteristic change in postinfectious GN is increased cellularity of the glomerular tufts that affects nearly all glomeruli—hence the term diffuse (Fig. 13–10, A). The increased cellularity is caused both by proliferation and swelling of endothelial and mesangial cells and by infiltrating neutrophils and monocytes. Sometimes there is necrosis of the capillary walls. In a few cases, “crescents” (described later) may be observed within the urinary space, formed in response to the severe inflammatory injury. Electron microscopy shows deposited immune complexes arrayed as subendothelial, intramembranous, or, most often, subepithelial “humps” nestled against the GBM (Fig. 13–10, B). Mesangial deposits also are occasionally present. Immunofluorescence studies reveal scattered granular deposits of IgG and complement within the capillary walls and some mesangial areas, corresponding to the deposits visualized by electron microscopy. These deposits usually are cleared over a period of about 2 months.
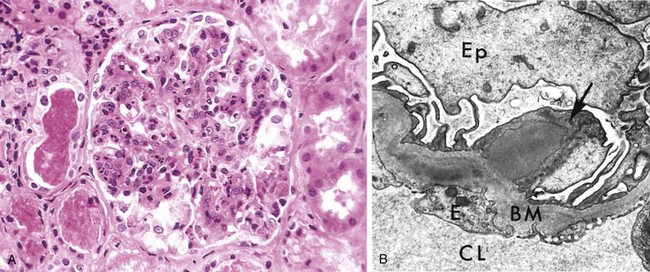
Figure 13–10 Poststreptococcal glomerulonephritis. A, Glomerular hypercellularity is caused by intracapillary leukocytes and proliferation of intrinsic glomerular cells. Note the red cell casts in the tubules. B, Typical electron-dense subepithelial “hump” (arrow) and intramembranous deposits. BM, basement membrane; CL, capillary lumen; E, endothelial cell; Ep, visceral epithelial cells (podocytes).
Clinical Course
The onset of the kidney disease tends to be abrupt, heralded by malaise, a slight fever, nausea, and the nephritic syndrome. In the usual case, oliguria, azotemia, and hypertension are only mild to moderate. Characteristically, there is gross hematuria, the urine appearing smoky brown rather than bright red. Some degree of proteinuria is a constant feature of the disease, and as mentioned earlier it occasionally may be severe enough to produce the nephrotic syndrome. Serum complement levels are low during the active phase of the disease, and serum anti–streptolysin O antibody titers are elevated in poststreptococcal cases.
Recovery occurs in most children in epidemic cases. Some children develop rapidly progressive GN owing to severe injury with formation of crescents, or chronic renal disease from secondary scarring. The prognosis in sporadic cases is less clear. In adults, 15% to 50% of affected persons develop end-stage renal disease over the ensuing few years or 1 to 2 decades, depending on the clinical and histologic severity. By contrast, in children, the prevalence of chronicity after sporadic cases of acute postinfectious GN is much lower.
IgA Nephropathy
This condition usually affects children and young adults and begins as an episode of gross hematuria that occurs within 1 or 2 days of a nonspecific upper respiratory tract infection. Typically, the hematuria lasts several days and then subsides, only to recur every few months. It may be associated with local pain. IgA nephropathy is one of the most common causes of recurrent microscopic or gross hematuria and is the most common glomerular disease revealed by renal biopsy worldwide.
The hallmark of the disease is the deposition of IgA in the mesangium. Some workers have considered IgA nephropathy to be a localized variant of Henoch-Schönlein purpura, also characterized by IgA deposition in the mesangium. In contrast with IgA nephropathy, which is purely a renal disorder, Henoch-Schönlein purpura is a systemic syndrome involving the skin (purpuric rash), gastrointestinal tract (abdominal pain), joints (arthritis), and kidneys.
![]() Pathogenesis
Pathogenesis
Accumulating evidence suggests that IgA nephropathy is associated with an abnormality in IgA production and clearance, as well as antibodies against abnormally glycosylated IgA. IgA, the main immunoglobulin in mucosal secretions, is increased in 50% of patients with IgA nephropathy owing to increased production of the IgA1 subtype by plasma cells in the bone marrow. In addition, circulating IgA-containing immune complexes are present in some cases. A genetic influence is suggested by the occurrence of this condition in families and in HLA–identical siblings, and by the increased frequency of certain HLA and complement genotypes in some populations. Studies also suggest an abnormality in glycosylation of the IgA1 immunoglobulin that reduces plasma clearance and favors deposition in the mesangium. This abnormal IgA1 may also elicit glycan-specific IgG antibodies. The prominent mesangial deposition of IgA may stem from entrapment of IgA immune complexes, and the absence of C1q and C4 in glomeruli points to activation of the alternative complement pathway. Taken together, these clues suggest that in genetically susceptible individuals, respiratory or gastrointestinal exposure to microbial or other antigens (e.g., viruses, bacteria, food proteins) may lead to increased IgA synthesis, some of which is abnormally glycosylated, and deposition of IgA and IgA-containing immune complexes in the mesangium, where they activate the alternative complement pathway and initiate glomerular injury. In support of this scenario, IgA nephropathy occurs with increased frequency in individuals with celiac disease, in whom intestinal mucosal defects are seen, and in liver disease, in which there is defective hepatobiliary clearance of IgA complexes (secondary IgA nephropathy).
![]() Morphology
Morphology
Histologically, the lesions in IgA nephropathy vary considerably. The glomeruli may be normal or may show mesangial widening and segmental inflammation confined to some glomeruli (focal proliferative GN); diffuse mesangial proliferation (mesangioproliferative GN); or (rarely) overt crescentic GN. The characteristic immunofluorescence picture is of mesangial deposition of IgA, often with C3 and properdin and smaller amounts of IgG or IgM (Fig. 13–11). Early components of the classical complement pathway usually are absent. Electron microscopy confirms the presence of electron-dense deposits in the mesangium. The deposits may extend to the subendothelial area of adjacent capillary walls in a minority of cases, usually those with focal proliferation. Biopsy findings may help predict whether progression or response to intervention is likely.
Clinical Course
The disease most often affects children and young adults. More than half of those with IgA nephropathy present with gross hematuria after an infection of the respiratory or, less commonly, gastrointestinal or urinary tract; 30% to 40% have only microscopic hematuria, with or without proteinuria, and 5% to 10% develop a typical acute nephritic syndrome. The hematuria typically lasts for several days and then subsides, only to return every few months. The subsequent course is highly variable. Many patients maintain normal renal function for decades. Slow progression to chronic renal failure occurs in 25% to 50% of cases over a period of 20 years. Renal biopsy findings may help identify those with worse prognosis, as indicated by diffuse mesangial proliferation, segmental sclerosis, endocapillary proliferation, or tubulointerstitial fibrosis.
Hereditary Nephritis
Hereditary nephritis refers to a group of hereditary glomerular diseases caused by mutations in genes encoding GBM proteins. The best-studied entity is Alport syndrome, in which nephritis is accompanied by nerve deafness and various eye disorders, including lens dislocation, posterior cataracts, and corneal dystrophy.
![]() Pathogenesis
Pathogenesis
The GBM is composed largely of type IV collagen, which is made up of heterotrimers of α3, α4, and α5 type IV collagen. This form of type IV collagen is crucial for normal function of the lens, cochlea, and glomerulus. Mutation of any one of the α chains results in defective heterotrimer assembly and, consequently, the disease manifestations of Alport syndrome.
![]() Morphology
Morphology
On histologic examination, glomeruli in hereditary nephritis appear unremarkable until late in the course, when secondary sclerosis may occur. In some kidneys, interstitial cells take on a foamy appearance as a result of accumulation of neutral fats and mucopolysaccharides (foam cells) as a reaction to marked proteinuria. With progression, increasing glomerulosclerosis, vascular sclerosis, tubular atrophy, and interstitial fibrosis are typical changes. Under the electron microscope, the basement membrane of glomeruli is thin and attenuated early in the course. Late in the course, the GBM develops irregular foci of thickening or attenuation with pronounced splitting and lamination of the lamina densa, yielding a “basketweave” appearance.
Clinical Course
The inheritance is heterogeneous, being most commonly X-linked as a result of mutation of the gene encoding α5 type IV collagen. Males therefore tend to be affected more frequently and more severely than females and are more likely to develop renal failure. Rarely, inheritance is autosomal recessive or dominant, linked to defects in the genes that encode α3 or α4 type IV collagen. Persons with hereditary nephritis present at age 5 to 20 years with gross or microscopic hematuria and proteinuria, and overt renal failure occurs between 20 and 50 years of age.
Female carriers of X-linked Alport syndrome or carriers of either gender of the autosomal forms usually present with persistent hematuria, which most often is asymptomatic and is associated with a benign clinical course. In these patients, biopsy specimens show only thinning of the GBM.
![]() Summary
Summary
The Nephritic Syndrome
• The nephritic syndrome is characterized by hematuria, oliguria with azotemia, proteinuria, and hypertension.
• The most common cause is immunologically mediated glomerular injury; lesions are characterized by proliferative changes and leukocyte infiltration.
• Acute postinfectious glomerulonephritis typically occurs after streptococcal infection in children and young adults but may occur following infection with many other organisms; it is caused by deposition of immune complexes, mainly in the subepithelial spaces, with abundant neutrophils and proliferation of glomerular cells. Most affected children recover; the prognosis is worse in adults.
• IgA nephropathy, characterized by mesangial deposits of IgA-containing immune complexes, is the most common cause of the nephritic syndrome worldwide; it is also a common cause of recurrent hematuria; it commonly affects children and young adults and has a variable course.
• Hereditary nephritis (Alport syndrome) is caused by mutations in genes encoding GBM collagen; it manifests as hematuria and slowly progressing proteinuria and declining renal function; glomeruli appear normal by light microscopy until late in the disease course.
Rapidly Progressive Glomerulonephritis
Rapidly progressive glomerulonephritis (RPGN) is a clinical syndrome and not a specific etiologic form of GN. It is characterized by progressive loss of renal function, laboratory findings typical of the nephritic syndrome, and often severe oliguria. If untreated, it leads to death from renal failure within a period of weeks to months. The characteristic histologic finding associated with RPGN is the presence of crescents (crescentic GN).
![]() Pathogenesis
Pathogenesis
Crescentic GN may be caused by a number of different diseases, some restricted to the kidney and others systemic. Although no single mechanism can explain all cases, there is little doubt that in most cases the glomerular injury is immunologically mediated. The diseases causing crescentic GN may be associated with a known disorder or it may be idiopathic. When the cause can be identified, about 12% of the patients have anti-GBM antibody–mediated crescentic GN with or without lung involvement; 44% have immune complex GN with crescents; and the remaining 44% have pauci-immune crescentic GN. All have severe glomerular injury.
Anti-Glomerular Basement Membrane Antibody–Mediated Crescentic Glomerulonephritis
Anti-GBM antibody–mediated crescentic GN is characterized by linear deposits of IgG and, in many cases, C3 on the GBM, as described earlier. In some patients, the anti-GBM antibodies also bind to pulmonary alveolar capillary basement membranes to produce the clinical picture of pulmonary hemorrhages associated with renal failure. These patients are said to have Goodpasture syndrome, to distinguish their condition from so-called idiopathic cases, in which renal involvement occurs in the absence of pulmonary disease. Anti-GBM antibodies are present in the serum and are helpful in diagnosis. It is important to recognize anti-GBM antibody–mediated crescentic GN, because affected persons benefit from plasmapheresis, which removes pathogenic antibodies from the circulation.
![]() Morphology
Morphology
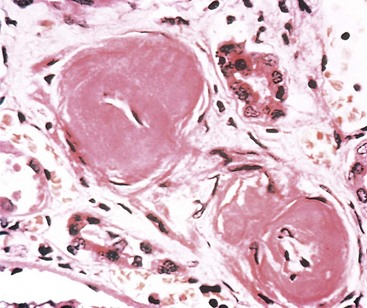
The kidneys are enlarged and pale, often with petechial hemorrhages on the cortical surfaces. Glomeruli show segmental necrosis and GBM breaks, with resulting proliferation of the parietal epithelial cells in response to the exudation of plasma proteins and the deposition of fibrin in Bowman’s space. These distinctive lesions of proliferation are called crescents owing to their shape as they fill Bowman’s space. Crescents are formed both by proliferation of parietal cells and by migration of monocytes/macrophages into Bowman’s space (Fig. 13–12). Smaller numbers of other types of leukocytes also may be present. The uninvolved portion of the glomerulus shows no proliferation. Immunofluorescence studies characteristically show strong staining of linear IgG and C3 deposits along the GBM (Fig. 13–4, B). These antibodies typically recognize type IV collagen. Because of the diffuse distribution of type IV collagen in the glomerulus, the density of antibody : antigen complexes is not high enough for them to be seen by electron microscopy. Electron microscopy may show distinct ruptures in the GBM. The crescents eventually obliterate Bowman’s space and compress the glomeruli. In time, crescents may undergo scarring, and glomerulosclerosis develops.
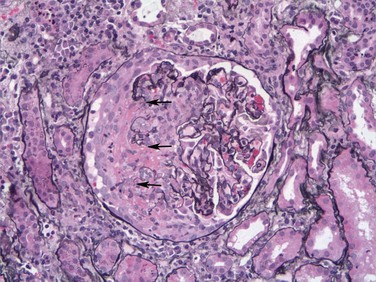
Figure 13–12 Crescentic glomerulonephritis (GN) (Jones silver methenamine stain). Note the areas of necrosis with rupture of capillary loops (arrows) and destruction of normal glomerular structures, and the adjacent crescent-shaped mass of proliferating cells and leukocytes filling the urinary space. The segmental distribution of the necrotizing and crescentic GN is typical of ANCA (antineutrophil cytoplasmic antibody)-associated crescentic GN.
Immune Complex–Mediated Crescentic Glomerulonephritis
Crescents can be a complication of any of the immune complex nephritides, including poststreptococcal GN, systemic lupus erythematosus, IgA nephropathy, and Henoch-Schönlein purpura. In some cases, immune complexes can be demonstrated but the underlying cause is undetermined. A consistent finding in this form of GN of any cause is the characteristic granular (“lumpy bumpy”) pattern of staining of the GBM and/or mesangium for immunoglobulin and/or complement on immunofluorescence studies. This disorder usually does not respond to plasmapheresis.
![]() Morphology
Morphology
There is severe injury in the form of segmental necrosis and GBM breaks with resultant crescent formation, as described earlier. However, in contrast with crescentic GN associated with anti-GBM antibodies, segments of glomeruli without necrosis show evidence of the underlying immune complex GN (e.g., diffuse proliferation and leukocyte exudation in postinfectious GN or systemic lupus erythematosus; mesangial proliferation in IgA nephropathy or Henoch-Schönlein purpura). Immunofluorescence shows the characteristic granular pattern of immune complex disease, and electron microscopy demonstrates discrete deposits.
Pauci-Immune Crescentic Glomerulonephritis
Pauci-immune type crescentic GN is defined by the lack of anti-GBM antibodies or of significant immune complex deposition detectable by immunofluorescence and electron microscopy. Antineutrophil cytoplasmic antibodies (ANCA) typically are found in the serum, which, as described in Chapter 9, have an etiopathogenic role in some vasculitides. In some instances, therefore, crescentic GN is a component of a systemic vasculitis such as microscopic polyangiitis or Wegener granulomatosis. In many cases, however, pauci-immune crescentic GN is limited to the kidney and is thus called idiopathic.
![]() Morphology
Morphology
Glomeruli show segmental necrosis and GBM breaks with resulting crescent formation (see earlier). Uninvolved segments of glomeruli appear normal without proliferation or prominent inflammatory cell influx. In contrast with anti-GBM antibody disease, however, results of immunofluorescence studies for immunoglobulin and complement are negative or nearly so, and no deposits are detectable by electron microscopy.
Clinical Course
The onset of RPGN is much like that of the nephritic syndrome, except that the oliguria and azotemia are more pronounced. Proteinuria sometimes approaching nephrotic range may occur. Some affected persons become anuric and require long-term dialysis or transplantation. The prognosis can be roughly related to the fraction of involved glomeruli: Patients in whom crescents are present in less than 80% of the glomeruli have a better prognosis than those in whom the percentages of crescents are higher. Plasma exchange is of benefit in those with anti-GBM antibody GN and Goodpasture disease, as well as in some patients with ANCA-related pauci-immune crescentic GN.
![]() Summary
Summary
Rapidly Progressive Glomerulonephritis
• RPGN is a clinical entity with features of the nephritic syndrome and rapid loss of renal function.
• RPGN is commonly associated with severe glomerular injury with necrosis and GBM breaks and subsequent proliferation of parietal epithelium (crescents).
• RPGN may be immune-mediated, as when autoantibodies to the GBM develop in anti-GBM antibody disease or when it arises consequent to immune complex deposition; it also can be pauci-immune, associated with antineutrophil cytoplasmic antibodies.
Diseases Affecting Tubules and Interstitium
Most forms of tubular injury also involve the interstitium, so the two are discussed together. Presented under this heading are diseases characterized by (1) inflammatory involvement of the tubules and interstitium (interstitial nephritis) or (2) ischemic or toxic tubular injury, leading to the morphologic appearance of acute tubular injury and the clinical syndrome of acute kidney injury.
Tubulointerstitial Nephritis
Tubulointerstitial nephritis (TIN) refers to a group of inflammatory diseases of the kidneys that primarily involve the interstitium and tubules. The glomeruli may be spared altogether or affected only late in the course. In most cases of TIN caused by bacterial infection, the renal pelvis is prominently involved—hence the more descriptive term pyelonephritis (from pyelo, “pelvis”). The term interstitial nephritis generally is reserved for cases of TIN that are nonbacterial in origin. These include tubular injury resulting from drugs, metabolic disorders such as hypokalemia, physical injury such as irradiation, viral infections, and immune reactions. On the basis of clinical features and the character of the inflammatory exudate, TIN, regardless of the etiologic agent, can be divided into acute and chronic categories. Discussed next is acute pyelonephritis, which is always of bacterial origin, followed by consideration of other, nonbacterial forms of interstitial nephritis.
Acute Pyelonephritis
Acute pyelonephritis, a common suppurative inflammation of the kidney and the renal pelvis, is caused by bacterial infection. It is an important manifestation of urinary tract infection (UTI), which can involve the lower (cystitis, prostatitis, urethritis) or upper (pyelonephritis) urinary tract, or both. As we shall see, the great majority of cases of pyelonephritis are associated with infection of the lower urinary tract. Such infection, however, may remain localized without extending to involve the kidney. UTIs constitute an extremely common clinical problem.
![]() Pathogenesis
Pathogenesis
The principal causative organisms in acute pyelonephritis are the enteric gram-negative rods. Escherichia coli is by far the most common one. Other important organisms are Proteus, Klebsiella, Enterobacter, and Pseudomonas; these usually are associated with recurrent infections, especially in persons who undergo urinary tract manipulations or have congenital or acquired anomalies of the lower urinary tract (see later). Staphylococci and Streptococcus faecalis also may cause pyelonephritis, but they are uncommon pathogens in this setting.
Bacteria can reach the kidneys from the lower urinary tract (ascending infection) or through the bloodstream (hematogenous infection) (Fig. 13–13). Ascending infection from the lower urinary tract is the most important and common route by which the bacteria reach the kidney. Adhesion of bacteria to mucosal surfaces is followed by colonization of the distal urethra (and the introitus in females). Genetically determined properties of both the urothelium and the bacterial pathogens may facilitate adhesion to the urothelial lining by bacterial fimbriae (proteins that attach to receptors on the surface of urothelial cells), conferring susceptibility to infection. The organisms then reach the bladder, by expansive growth of the colonies and by moving against the flow of urine. This may occur during urethral instrumentation, including catheterization and cystoscopy. Although hematogenous spread is the far less common of the two, acute pyelonephritis may result from seeding of the kidneys by bacteria in the course of septicemia or infective endocarditis.
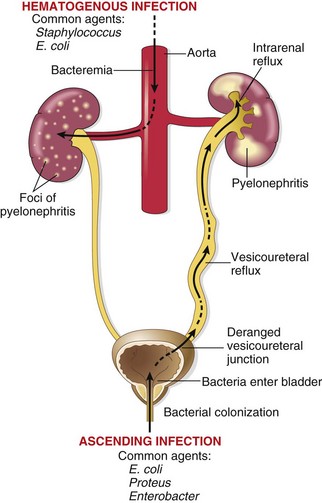
Figure 13–13 Pathways of renal infection. Hematogenous infection results from bacteremic spread. More common is ascending infection, which results from a combination of urinary bladder infection, vesicoureteral reflux, and intrarenal reflux.
In the absence of instrumentation, UTI most commonly affects females. Because of the close proximity of the female urethra to the rectum, colonization by enteric bacteria is favored. Furthermore, the short urethra, and trauma to the urethra during sexual intercourse, facilitate the entry of bacteria into the urinary bladder. Ordinarily, bladder urine is sterile, as a result of the antimicrobial properties of the bladder mucosa and the flushing mechanism associated with periodic voiding of urine. With outflow obstruction or bladder dysfunction, however, the natural defense mechanisms of the bladder are overwhelmed, setting the stage for UTI. In the presence of stasis, bacteria introduced into the bladder can multiply undisturbed, without being flushed out or destroyed by the bladder wall. From the contaminated bladder urine, the bacteria ascend along the ureters to infect the renal pelvis and parenchyma. Accordingly, UTI is particularly frequent among patients with urinary tract obstruction, as may occur with benign prostatic hyperplasia and uterine prolapse. UTI frequency also is increased in diabetes because of the increased susceptibility to infection and neurogenic bladder dysfunction, which in turn predisposes to stasis.
Incompetence of the vesicoureteral orifice, resulting in vesicoureteral reflux (VUR), is an important cause of ascending infection. The reflux allows bacteria to ascend the ureter into the pelvis. VUR is present in 20% to 40% of young children with UTI, usually as a consequence of a congenital defect that results in incompetence of the ureterovesical valve. VUR also can be acquired in persons with a flaccid bladder resulting from spinal cord injury or with neurogenic bladder dysfunction secondary to diabetes. VUR results in residual urine after voiding in the urinary tract, which favors bacterial growth. Furthermore, VUR affords a ready mechanism by which the infected bladder urine can be propelled up to the renal pelvis and farther into the renal parenchyma through open ducts at the tips of the papillae (intrarenal reflux).
![]() Morphology
Morphology
One or both kidneys may be involved. The affected kidney may be normal in size or enlarged. Characteristically, discrete, yellowish, raised abscesses are grossly apparent on the renal surface (Fig. 13–14). They may be widely scattered or limited to one region of the kidney, or they may coalesce to form a single large area of suppuration.
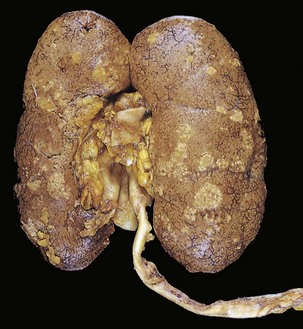
Figure 13–14 Acute pyelonephritis. The cortical surface is studded with focal pale abscesses, more numerous in the upper pole and middle region of the kidney; the lower pole is relatively unaffected. Between the abscesses there is dark congestion of the renal surface.
The characteristic histologic feature of acute pyelonephritis is liquefactive necrosis with abscess formation within the renal parenchyma. In the early stages pus formation (suppuration) is limited to the interstitial tissue, but later abscesses rupture into tubules. Large masses of intratubular neutrophils frequently extend within involved nephrons into the collecting ducts, giving rise to the characteristic white cell casts found in the urine. Typically, the glomeruli are not affected.
When obstruction is prominent, the pus may not drain and then fills the renal pelvis, calyces, and ureter, producing pyonephrosis.
A second (and fortunately infrequent) form of pyelonephritis is necrosis of the renal papillae, known as papillary necrosis. There are three predisposing conditions for this: diabetes, urinary tract obstruction, and analgesic abuse. This lesion consists of a combination of ischemic and suppurative necrosis of the tips of the renal pyramids (renal papillae). The pathognomonic gross feature of papillary necrosis is sharply defined gray-white to yellow necrosis of the apical two thirds of the pyramids. One papilla or several or all papillae may be affected. Microscopically, the papillary tips show characteristic coagulative necrosis, with surrounding neutrophilic infiltrate.
When the bladder is involved in a UTI, as is often the case, acute or chronic cystitis results. In long-standing cases associated with obstruction, the bladder may be grossly hypertrophic, with trabeculation of its walls, or it may be thinned and markedly distended from retention of urine.
Clinical Course
Acute pyelonephritis often is associated with predisposing conditions, as described previously in the discussion of pathogenetic mechanisms. These factors include
• Urinary obstruction, either congenital or acquired
• Instrumentation of the urinary tract, most commonly catheterization
• Pregnancy—4% to 6% of pregnant women develop bacteriuria sometime during pregnancy, and 20% to 40% of these eventually develop symptomatic urinary infection if not treated.
• Female gender and patient age. After the first year of life (an age by which congenital anomalies in males commonly become evident) and up to approximate age 40 years, infections are much more frequent in females. With increasing age, the incidence in males rises as a result of the development of prostatic hyperplasia, which causes urinary outflow obstruction.
• Preexisting renal lesions, causing intrarenal scarring and obstruction
• Diabetes mellitus, in which common predisposing factors are infection and bladder dysfunction
The onset of uncomplicated acute pyelonephritis usually is sudden, with pain at the costovertebral angle and systemic evidence of infection, such as chills, fever, and malaise, and localizing urinary tract signs of dysuria, frequency, and urgency. The urine appears turgid due to the contained pus (pyuria). Even without antibiotic treatment, the disease tends to be benign and self-limited. The symptomatic phase of the disease typically lasts no longer than a week, although bacteriuria may persist much longer. The disease usually is unilateral, and affected persons thus do not develop renal failure because they still have one unaffected kidney. In cases in which predisposing factors are present, the disease may become recurrent or chronic, particularly when involvement is bilateral. The development of papillary necrosis is associated with a much poorer prognosis.
Chronic Pyelonephritis and Reflux Nephropathy
Chronic pyelonephritis is defined here as a morphologic entity in which predominantly interstitial inflammation and scarring of the renal parenchyma are associated with grossly visible scarring and deformity of the pelvicalyceal system. Chronic pyelonephritis is an important cause of chronic renal failure. It can be divided into two forms: chronic obstructive pyelonephritis and chronic reflux–associated pyelonephritis.
Chronic Obstructive Pyelonephritis
As noted, obstruction predisposes the kidney to infection. Recurrent infections superimposed on diffuse or localized obstructive lesions lead to recurrent bouts of renal inflammation and scarring, which eventually cause chronic pyelonephritis. The disease can be bilateral, as with congenital anomalies of the urethra (e.g., posterior urethral valves), resulting in fatal renal insufficiency unless the anomaly is corrected, or unilateral, such as occurs with calculi and unilateral obstructive lesions of the ureter.
Chronic Reflux–Associated Pyelonephritis (Reflux Nephropathy)
This is the more common form of chronic pyelonephritic scarring and results from superimposition of a UTI on congenital vesicoureteral reflux and intrarenal reflux. Reflux may be unilateral or bilateral; thus, the resultant renal damage either may cause scarring and atrophy of one kidney or may involve both, potentially leading to chronic renal insufficiency.
![]() Morphology
Morphology
One or both kidneys may be involved, either diffusely or in patches. Even when involvement is bilateral, the kidneys are not equally damaged and therefore are not equally contracted. This uneven scarring is useful in differentiating chronic pyelonephritis from the more symmetrically contracted kidneys associated with vascular sclerosis (often referred to as “benign nephrosclerosis”) and chronic GN. The hallmark of chronic pyelonephritis is scarring involving the pelvis or calyces, or both, leading to papillary blunting and marked calyceal deformities (Fig. 13–15).
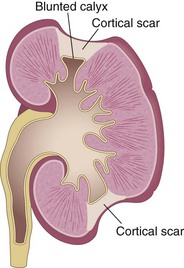
Figure 13–15 Typical coarse scars of chronic pyelonephritis associated with vesicoureteral reflux. The scars are usually located at the upper or lower poles of the kidney and are associated with underlying blunted calyces.
The microscopic changes are largely nonspecific, and similar alterations may be seen with other chronic tubulointerstitial disorders such as analgesic nephropathy. The parenchyma shows the following features:
• Uneven interstitial fibrosis and an inflammatory infiltrate of lymphocytes, plasma cells, and occasionally neutrophils
• Dilation or contraction of tubules, with atrophy of the lining epithelium. Many of the dilated tubules contain pink to blue, glassy-appearing PAS-positive casts, known as colloid casts, that suggest the appearance of thyroid tissue—hence the descriptive term thyroidization. Often, neutrophils are seen within tubules.
• Chronic inflammatory cell infiltration and fibrosis involving the calyceal mucosa and wall
• Arteriolosclerosis caused by the frequently associated hypertension
• Glomerulosclerosis that usually develops as a secondary process caused by nephron loss (a maladaptation discussed earlier).
Clinical Course
Many persons with chronic pyelonephritis come to medical attention relatively late in the course of the disease, because of the gradual onset of renal insufficiency or because signs of kidney disease are noticed on routine laboratory tests. In other cases, the renal disease is heralded by the development of hypertension. The radiologic image is characteristic: The affected kidney is asymmetrically contracted, with some degree of blunting and deformity of the calyceal system (caliectasis). The presence or absence of significant bacteriuria is not particularly helpful diagnostically; its absence certainly should not rule out chronic pyelonephritis. If the disease is bilateral and progressive, tubular dysfunction occurs with loss of concentrating ability, manifested by polyuria and nocturia.
As noted earlier, some persons with chronic pyelonephritis or reflux nephropathy ultimately develop secondary glomerulosclerosis, associated with proteinuria; eventually, these injuries all contribute to progressive chronic kidney disease.
Drug-Induced Interstitial Nephritis
In this era of widespread antibiotic and analgesic use, drugs have emerged as an important cause of renal injury. Acute drug-induced tubulointerstitial nephritis (TIN) occurs as an adverse reaction to any of an increasing number of drugs. Acute drug-induced TIN is associated most frequently with synthetic penicillins (methicillin, ampicillin), other synthetic antibiotics (rifampin), diuretics (thiazides), nonsteroidal anti-inflammatory agents, and numerous other drugs (phenindione, cimetidine).
![]() Pathogenesis
Pathogenesis
Many features of the disease suggest an immune mechanism. Clinical evidence of hypersensitivity includes latent period, eosinophilia and rash, the idiosyncratic nature of the drug reaction (i.e., the lack of dose dependency), and the recurrence of hypersensitivity after reexposure to the same drug or others that are similar in structure. Serum IgE levels are increased in some persons, suggesting type I hypersensitivity. In other cases the nature of the inflammatory infiltrate (discussed below) and the presence of positive skin tests to drugs suggest a T cell–mediated (type IV) hypersensitivity reaction.
The most likely sequence of pathogenic events is as follows: The drugs act as haptens that, during secretion by tubules, covalently bind to some cytoplasmic or extracellular component of tubular cells and become immunogenic. The resultant tubulointerstitial injury is then caused by IgE- and cell-mediated immune reactions to tubular cells or their basement membranes.
![]() Morphology
Morphology
The abnormalities in acute drug-induced nephritis are in the interstitium, which shows pronounced edema and infiltration by mononuclear cells, principally lymphocytes and macrophages (Fig. 13–16). Eosinophils and neutrophils may be present, often in large numbers. With some drugs (e.g., methicillin, thiazides, rifampin), interstitial non-necrotizing granulomas with giant cells may be seen. The glomeruli are normal except in some cases caused by nonsteroidal anti-inflammatory agents, in which the hypersensitivity reaction also leads to podocyte foot process effacement and the nephrotic syndrome.
Clinical Course
The disease begins about 15 days (range, 2 to 40 days) after exposure to the drug and is characterized by fever, eosinophilia (which may be transient), a rash (in about 25% of persons), and renal abnormalities. Urinary findings include hematuria, minimal or no proteinuria, and leukocyturia (sometimes including eosinophils). A rising serum creatinine or acute kidney injury with oliguria develops in about 50% of cases, particularly in older patients. Clinical recognition of drug-induced kidney injury is imperative, because withdrawal of the offending drug is followed by recovery, although it may take several months for renal function to return to normal.
![]() Summary
Summary
Tubulointerstitial Nephritis
• TIN consists of inflammatory disease primarily involving the renal tubules and interstitium.
• Acute pyelonephritis is a bacterial infection caused either by ascending infection as a result of reflux, obstruction, or other abnormality of the urinary tract, or by hematogenous spread of bacteria; characterized by abscess formation in the kidneys, sometimes with papillary necrosis.
• Chronic pyelonephritis usually is associated with urinary obstruction or reflux; results in scarring of the involved kidney, and gradual renal insufficiency.
• Drug-induced interstitial nephritis is an IgE- and T cell–mediated immune reaction to a drug; characterized by interstitial inflammation, often with abundant eosinophils, and edema.
Acute Tubular Injury
Acute tubular injury (ATI) is a clinicopathologic entity characterized morphologically by damaged tubular epithelial cells and clinically by acute decline of renal function, with granular casts and tubular cells observed in the urine. This constellation of changes, termed acute kidney injury, manifests clinically as decreased GFR. When ATI is caused by acute kidney injury, there may be oliguria (defined as urine output of less than 400 mL/day). Other causes of acute kidney injury include (1) severe glomerular diseases manifesting clinically as RPGN; (2) acute tubular injury caused by diffuse renal vascular diseases, such as microscopic polyangiitis and thrombotic microangiopathies; and (3) acute drug-induced allergic interstitial nephritis, which often is not associated with tubular injury. These other disorders involving acute kidney injury are discussed elsewhere in this chapter.
ATI arises in a variety of clinical settings, so it occurs relatively frequently. Most of these clinical conditions, ranging from severe trauma to acute pancreatitis to septicemia, have in common a period of inadequate blood flow to all or regions of peripheral organs such as the kidney, sometimes in the setting of marked hypotension and shock. The pattern of ATI associated with generalized or localized reduction in blood flow is called ischemic ATI. Mismatched blood transfusions and other hemolytic crises, as well as myoglobinuria, also produce a clinical picture resembling that in ischemic ATI. A second pattern, called nephrotoxic ATI, is caused by a variety of poisons, including heavy metals (e.g., mercury); organic solvents (e.g., carbon tetrachloride); and a multitude of drugs such as gentamicin and other antibiotics, and radiographic contrast agents. ATI is often reversible, and proper recognition and management can mean the difference between full recovery and death.
![]() Pathogenesis
Pathogenesis
The decisive events in both ischemic and nephrotoxic ATI are believed to be
• Tubular injury. Tubular epithelial cells are particularly sensitive to anoxia and are also vulnerable to toxins (Fig. 13–17). Several factors predispose the tubules to toxic injury, including elevated intracellular concentrations of various molecules that are resorbed or secreted across the proximal tubule, as well as exposure to high concentrations of luminal solutes that are concentrated by the resorption of water from the glomerular filtrate.
• Persistent and severe disturbances in blood flow resulting in diminished oxygen and substrate delivery to tubular cells. Ischemia causes numerous structural alterations in epithelial cells. Loss of cell polarity is an early reversible event. It leads to the redistribution of membrane proteins (e.g., Na+,K+-ATPase) from the basolateral to the luminal surface of tubular cells, resulting in decreased sodium reabsorption by proximal tubules and hence increased sodium delivery to distal tubules. The latter, through a tubuloglomerular feedback system, contributes to preglomerular arteriolar vasoconstriction. Redistribution or alteration of integrins that anchor tubular cells results in their detachment from the basement membranes and their shedding into the urine. If sufficient tubular debris builds up it can block the outflow of urine (obstruction by casts), increasing intratubular pressure and thereby decreasing the GFR. Additionally, fluid from the damaged tubules may leak into the interstitium (backleak), resulting in increased interstitial pressure and collapse of the tubules. Ischemic tubular cells also express chemokines, cytokines, and adhesion molecules such as P-selectin that recruit leukocytes and can participate in tissue injury (interstitial inflammation).
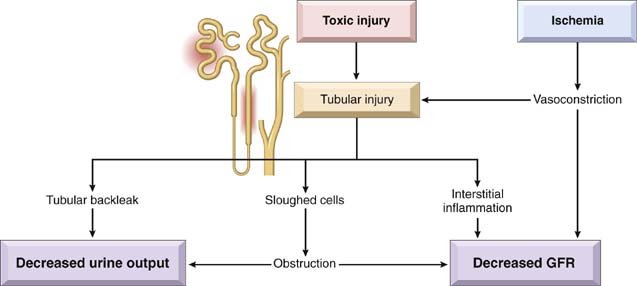
Figure 13–17 Pathophysiologic mechanisms of acute kidney injury. Various toxic injuries can directly damage tubules, which in turn directly decreases GFR and lowers urine output through multiple mechanisms. Ischemia and consequent vasoconstriction contribute directly to diminished GFR, and further contribute indirectly through injury to the tubules. Tubular cells, which are highly metabolically active and uniquely sensitive to diminished blood supply within the kidney, release several vasoconstrictor substances as part of the response to hypoxia, which then further exacerbates the overall injury.
(Modified from Lameire N, et al: JASN 12:S20-S32, 2001.)
Ischemic renal injury also is characterized by severe hemodynamic alterations that cause reduced GFR. The major one is intrarenal vasoconstriction, which results in both reduced glomerular plasma flow and reduced oxygen delivery to the tubules in the outer medulla (thick ascending limb and straight segment of the proximal tubule) (Fig. 13–17). Although a number of vasoconstrictor pathways have been implicated in this phenomenon (e.g., renin-angiotensin, thromboxane A2, sympathetic nerve activity), the current opinion is that vasoconstriction is mediated by sublethal endothelial injury, leading to increased release of the endothelial vasoconstrictor endothelin and decreased production of vasodilatory nitric oxide and prostaglandins. Finally, some evidence points to a direct effect of ischemia or toxins on the glomerulus, causing a reduced effective glomerular filtration surface.
In addition to vasoconstriction, the pathogenesis of ATI may involve apoptosis and necrosis of tubular cells. Dead cells may elicit an inflammatory reaction (Chapter 2) that exacerbates the tubular injury and functional derangements.
![]() Morphology
Morphology
Ischemic ATI is characterized by lesions in the straight portions of the proximal tubule and the ascending thick limbs, but no segment of the proximal or distal tubules is spared. There is often a variety of tubular injuries, including attenuation of proximal tubular brush borders, blebbing and sloughing of brush borders, vacuolization of cells, and detachment of tubular cells from their underlying basement membranes with sloughing of cells into the urine. A striking additional finding is the presence of proteinaceous casts in the distal tubules and collecting ducts, which consist of Tamm-Horsfall protein (normally secreted by tubular epithelium) along with hemoglobin and other plasma proteins. When crush injuries have produced ATI, the casts also contain myoglobin. The interstitium usually shows generalized edema along with a mild inflammatory infiltrate consisting of polymorphonuclear leukocytes, lymphocytes, and plasma cells. The histologic picture in toxic ATI is basically similar, with some differences. Overt necrosis is most prominent in the proximal tubule, and the tubular basement membranes generally are spared.
If the patient survives for a week, epithelial regeneration becomes apparent in the form of a low cuboidal epithelial covering and mitotic activity in the surviving tubular epithelial cells. Acute kidney injury with underlying acute tubular injury as its cause may result in fibrosis rather than repair if the proximal tubular cells are arrested at G2/M stage of the cell cycle after injury, as this arrest amplifies profibrotic mediators.
Clinical Course
The clinical course of ischemic ATI initially is dominated by the inciting medical, surgical or obstetric event. Affected patients often present with manifestations of acute kidney injury, including oliguria and decreased GFR. Not all patients may manifest oliguria; some will have anuria, while in others, particularly if the injury is milder, the ATI may be nonoliguric. During acute kidney injury, the clinical picture is dominated by electrolyte abnormalities, acidosis and the signs and symptoms of uremia and fluid overload. Depending upon the severity and nature of the underlying injury and comorbid conditions, the prognosis varies. In the absence of careful supportive treatment or dialysis, patients may die. When the cause of acute kidney injury is ATI, repair and tubular regeneration lead to gradual clinical improvement. With supportive care, patients who do not die from the underlying precipitating problem have a good chance of recovering renal function unless kidney disease was present at the time of the acute insult. In those with preexisting kidney disease complete recovery is less certain, and progression over time to end-stage renal disease is unfortunately too frequent.
![]() Summary
Summary
Acute Tubular Injury
• ATI is the most common cause of acute kidney injury; its clinical manifestations are electrolyte abnormalities, acidosis, uremia, and signs of fluid overload, often with oliguria.
• ATI results from ischemic or toxic injury to renal tubules, and is associated with intrarenal vasoconstriction resulting in reduced GFR and diminished delivery of oxygen and nutrients to tubular epithelial cells.
• ATI is characterized morphologically by injury or necrosis of segments of the tubules (typically the proximal tubules), proteinaceous casts in distal tubules, and interstitial edema.
Diseases Involving Blood Vessels
Nearly all diseases of the kidney involve the renal blood vessels secondarily. Systemic vascular diseases, such as various forms of vasculitis, also involve renal blood vessels, and often the effects on the kidney are clinically important (Chapter 9). The kidney is intimately involved in the pathogenesis of both essential and secondary hypertension. This section covers the renal lesions associated with benign and malignant hypertension.
Arterionephrosclerosis
Arterionephrosclerosis is the term used for the thickening and sclerosis of arterial walls and the renal changes associated with benign hypertension. The characteristic morphologic alterations involve small arterioles and are called hyaline arteriolosclerosis. Some degree of arterionephrosclerosis, albeit mild, is present at autopsy in many persons older than 60 years of age. The frequency and severity of the lesions are increased at any age when hypertension is present.
![]() Pathogenesis
Pathogenesis
Of note, many renal diseases cause hypertension, which in turn is associated with arterionephrosclerosis. Thus, this renal lesion often is superimposed on other primary kidney diseases. Similar changes in arteries and arterioles are seen in individuals with chronic thrombotic microangiopathies. Whether hypertension causes the arterionephrosclerosis, or a subtle primary microvascular renal injury causes the hypertension, which in turn accelerates the sclerosis, is unknown. Recent studies implicate mutation in the apolipoprotein L1 gene (the same gene implicated in increased risk for FSGS) as tightly linked to the high incidence of arterionephrosclerosis observed in African Americans. The mechanisms of increased risk of kidney disease are unknown, but this mutation confers protection against trypanosomal disease, so its prevalence may have been influenced by natural selection.
![]() Morphology
Morphology
Grossly, the kidneys are symmetrically atrophic, each weighing 110 to 130 g. Typically the renal surface shows diffuse, fine granularity that resembles grain leather. Microscopically, the basic anatomic change is hyaline thickening of the walls of the small arteries and arterioles, known as hyaline arteriolosclerosis. This appears as a homogeneous, pink hyaline thickening, at the expense of the vessel lumina, with loss of underlying cellular detail (Fig. 13–18). The narrowing of the lumen results in markedly decreased blood flow through the affected vessels, with consequent ischemia in the organ served. All structures of the kidney show ischemic atrophy. In advanced cases of arterionephrosclerosis, the glomerular tufts may become sclerosed. Diffuse tubular atrophy and interstitial fibrosis are present. Often there is a scant interstitial lymphocytic infiltrate. The larger blood vessels (interlobar and arcuate arteries) show reduplication of internal elastic lamina along with fibrous thickening of the media (fibroelastic hyperplasia) and the subintima.
Clinical Course
This renal lesion alone rarely causes severe damage to the kidney except in persons with genetic susceptibility, such as African Americans, in whom it may lead to uremia and death. However, all patients with this lesion usually show some functional impairment, such as loss of concentrating ability or a variably diminished GFR. A mild degree of proteinuria is a frequent finding.
Malignant Hypertension
Malignant hypertension, defined as blood pressure usually greater than 200/120 mm Hg, is far less common in the United States than so-called “benign” hypertension and occurs in only about 5% of persons with elevated blood pressure. It may arise de novo (i.e., without preexisting hypertension), or it may appear suddenly in a person who had mild hypertension. The prevalence of malignant hypertension is higher in less developed countries.
![]() Pathogenesis
Pathogenesis
The basis for this turn for the worse in hypertensive subjects is unclear, but the following sequence is suggested: The initial event seems to be some form of vascular damage to the kidneys. This most commonly results from long-standing hypertension, with eventual injury to the arteriolar walls. The result is increased permeability of the small vessels to fibrinogen and other plasma proteins, endothelial injury, and platelet deposition. This leads to the appearance of fibrinoid necrosis of arterioles and small arteries and intravascular thrombosis. Mitogenic factors from platelets (e.g., platelet-derived growth factor) and plasma cause intimal hyperplasia of vessels, resulting in the hyperplastic arteriolosclerosis typical of organizing injury of malignant hypertension and of morphologically similar thrombotic microangiopathies (see later) and further narrowing of the lumina. The kidneys become markedly ischemic. With severe involvement of the renal afferent arterioles, the renin-angiotensin system receives a powerful stimulus. This then sets up a self-perpetuating cycle in which angiotensin II causes intrarenal vasoconstriction and the attendant renal ischemia perpetuates renin secretion. Aldosterone levels also are elevated, and the resultant salt retention exacerbates the elevation of blood pressure.
![]() Morphology
Morphology
The kidney may be essentially normal in size or slightly shrunken, depending on the duration and severity of the hypertensive disease. Small, pinpoint petechial hemorrhages may appear on the cortical surface from rupture of arterioles or glomerular capillaries, giving the kidney a peculiar, flea-bitten appearance.
The microscopic changes reflect the pathogenetic events described earlier. Damage to the small vessels is manifested as fibrinoid necrosis of the arterioles (Fig. 13–19, A). The vessel walls show a homogeneous, granular eosinophilic appearance masking underlying detail. In the interlobular arteries and larger arterioles, proliferation of intimal cells after acute injury produces an onion-skin appearance (Fig. 13–19, B). This name is derived from the concentric arrangement of cells whose origin is believed to be intimal smooth muscle, although this issue has not been finally settled. This lesion, called hyperplastic arteriolosclerosis, causes marked narrowing of arterioles and small arteries, to the point of total obliteration. Necrosis also may involve glomeruli, with microthrombi within the glomeruli as well as necrotic arterioles. Similar lesions are seen in persons with acute thrombotic microangiopathies (described later), and in patients with scleroderma in renal crisis.
Clinical Course
The full-blown syndrome of malignant hypertension is characterized by papilledema, encephalopathy, cardiovascular abnormalities, and renal failure. Most often, the early symptoms are related to increased intracranial pressure and include headache, nausea, vomiting, and visual impairment, particularly the development of scotomas, or “spots” before the eyes. At the onset of rapidly mounting blood pressure there is marked proteinuria and microscopic, or sometimes macroscopic, hematuria but no significant alteration in renal function. Soon, however, acute kidney injury develops. The syndrome represents a true medical emergency that requires prompt and aggressive antihypertensive therapy before irreversible renal lesions develop. About 50% of patients survive at least 5 years, and further progress is still being made. Ninety percent of deaths are caused by uremia and the other 10% by cerebral hemorrhage or cardiac failure.
Thrombotic Microangiopathies
As described in Chapter 11, the term thrombotic microangiopathy refers to lesions seen in various clinical syndromes characterized morphologically by widespread thrombosis in the microcirculation and clinically by microangiopathic hemolytic anemia, thrombocytopenia, and, in certain instances, renal failure. Common causes of thrombotic microangiopathy include
![]() Pathogenesis
Pathogenesis
The major pathogenetic factors in the thrombotic microangiopathies are endothelial activation (the dominant abnormality in HUS) and platelet activation and aggregation (which is dominant in TTP). Both may be caused by a number of external insults and inherited mutations, and together they lead to excessive small vessel thrombosis, the hallmark of these diseases.
• Childhood HUS is the best-characterized of the renal syndromes associated with thrombotic microangiopathy. As many as 75% of cases follow intestinal infection with Shiga toxin–producing E. coli, such as occurs in epidemics caused by ingestion of infected ground meat (e.g., in hamburgers) and infections with Shigella dysenteriae type I. The pathogenesis of this syndrome is related to the effects of Shiga toxin, which is carried by neutrophils in the circulation. Renal glomerular endothelial cells are targets because the cells express the membrane receptor for the toxin. The toxin has multiple effects on the endothelium, including increased adhesion of leukocytes, increased endothelin production, and loss of endothelial nitric oxide (both favoring vasoconstriction), and (in the presence of cytokines, such as tumor necrosis factor) endothelial damage. The toxin also gains entry to the cells and directly causes cell death. The resultant endothelial damage leads to thrombosis, which is most prominent in glomerular capillaries, afferent arterioles, and interlobular arteries, as well as vasoconstriction, resulting in the characteristic thrombotic microangiopathy.
• Adult HUS. In typical (epidemic, classic, diarrhea-positive) HUS, the trigger for endothelial injury and activation usually is a Shiga-like toxin, while in inherited forms of atypical HUS, the cause of endothelial injury appears to be excessive, inappropriate activation of complement. Many other forms of exposures and conditions, including drug toxicities, can occasionally precipitate a HUS-like picture, presumably also by injuring the endothelium.
• TTP often is caused by an acquired defect in proteolytic cleavage of von Willebrand factor (vWF) multimers due to autoantibodies, or more rarely, an inherited defect as seen in familial TTP (Chapter 11). Pathogenic autoantibodies, whether arising in a context of autoimmunity or drug-induced, typically are directed against ADAMTS 13 (a disintegrin and metalloprotease with thrombospondin-like motifs), a plasma protease that cleaves vWF multimers into smaller sizes. Autoantibody binding to ADAMTS 13 results in loss of function and increased levels of large vWF multimers in the circulation, which in turn can activate platelets spontaneously, leading to platelet aggregation and thrombosis. Genetic defects in ADAMTS 13 lead to a similar pattern of disease.
![]() Morphology
Morphology
In childhood HUS, there are lesions of classic thrombotic microangiopathy with fibrin thrombi predominantly involving glomeruli, and extending into arterioles and larger arteries in severe cases. Cortical necrosis may be present. Morphologic changes in glomeruli resulting from endothelial injury include widening of the subendothelial space in glomerular capillaries, duplication or splitting of GBMs, and lysis of mesangial cells with mesangial disintegration. Chronically, scarring of glomeruli may develop.
Clinical Course
Typically, childhood HUS is characterized by the sudden onset, usually after a gastrointestinal or flulike prodromal episode, of bleeding manifestations (especially hematemesis and melena), severe oliguria, hematuria, microangiopathic hemolytic anemia, and (in some persons) prominent neurologic changes. This disease is one of the main causes of acute kidney injury in children. If the acute kidney injury is managed properly with dialysis, most patients recover in a matter of weeks. The long-term prognosis (over 15 to 25 years), however, is not uniformly favorable, because in about 25% of these children, renal insufficiency eventually develops as a consequence of the secondary scarring. Although HUS and TTP have some overlapping clinical features, such as microangiopathic hemolytic anemia and thrombocytopenia, TTP more often has dominant involvement of the central nervous system and the kidneys are less commonly involved compared to HUS.
![]() Summary
Summary
Vascular Diseases of the Kidney
• Arterionephrosclerosis: Progressive, chronic renal damage associated with hypertension. Characteristic features are hyaline arteriolosclerosis and narrowing of vascular lumina with resultant cortical atrophy.
• Malignant hypertension: Acute kidney injury associated with severe elevation of blood pressure. Arteries and arterioles show fibrinoid necrosis and hyperplasia of smooth muscle cells; petechial hemorrhages on the cortical surface.
• Thrombotic microangiopathies: Disorders characterized by fibrin thrombi in glomeruli and small vessels resulting in acute kidney injury. Childhood HUS is usually caused by endothelial injury by an E. coli toxin; TTP is often caused by defects in von Willebrand factor leading to excessive thrombosis, with platelet consumption.
Chronic Kidney Disease
Chronic kidney disease is the result of progressive scarring resulting from any type of kidney disease. Alterations in the function of remaining initially intact nephrons are ultimately maladaptive and cause further scarring. This eventually results in an end-stage kidney where glomeruli, tubules, interstitium and vessels are sclerosed, regardless of the primary site of injury. Unless the disorder is treated with dialysis or transplantation, death from uremia results.
![]() Morphology
Morphology
Classically, the kidneys are symmetrically contracted, and their surfaces are red-brown and diffusely granular when the underlying disorder affects blood vessels or glomeruli. Kidneys damaged by chronic pyelonephritis are typically unevenly involved and have deep scars. Microscopically, the feature common to all cases is advanced scarring of the glomeruli, sometimes to the point of complete sclerosis (Fig. 13–20). This obliteration of the glomeruli is the end point of many diseases, and it is impossible to ascertain from such kidneys the nature of the initial lesion. There is also marked interstitial fibrosis, associated with atrophy and dropout of many of the tubules in the cortex, and diminution and loss of portions of the peritubular capillary network. The small and medium-sized arteries frequently are thick-walled, with narrowed lumina, secondary to hypertension. Lymphocytic (and, rarely, plasma cell) infiltrates are present in the fibrotic interstitial tissue. As damage to all structures progresses, it may become difficult to ascertain whether the primary lesion was glomerular, vascular, tubular, or interstitial. Such markedly damaged kidneys have been designated end-stage kidneys.
Clinical Course
Chronic kidney disease may sometimes develop insidiously and be discovered only late in its course, after the onset of renal insufficiency. Frequently, renal disease is first detected with the discovery of proteinuria, hypertension, or azotemia on routine medical examination. Disease-specific findings may precede development of chronic kidney disease. In patients with glomerular disease resulting in nephrotic syndrome, as the glomeruli undergo sclerotic changes, the avenue for protein loss is progressively closed, and the nephrotic syndrome thus becomes less severe with more advanced disease. Some degree of proteinuria, however, is present in almost all cases. Hypertension is very common, and its effects may dominate the clinical picture. Although microscopic hematuria is usually present, grossly bloody urine is infrequent at this late stage.
Without treatment, the prognosis is poor; relentless progression to uremia and death is the rule. The rate of progression is extremely variable.
Cystic Diseases of the Kidney
Cystic diseases of the kidney are a heterogeneous group comprising hereditary, developmental, and acquired disorders. These diseases are important for several reasons:
• They are reasonably common and often present diagnostic problems for clinicians, radiologists, and pathologists.
• Some forms, such as adult polycystic disease, constitute major causes of chronic renal failure.
• Simple cysts can occasionally be confused with malignant tumors.
An emerging theme in the pathophysiology of the hereditary cystic diseases is that the underlying defect is in the cilia–centrosome complex of tubular epithelial cells. Such defects may interfere with fluid absorption or cellular maturation, resulting in cyst formation. A brief overview of simple cysts, the most common form, is presented next, followed by a more detailed discussion of polycystic kidney disease.
Simple Cysts
Simple cysts are generally innocuous lesions that occur as multiple or single cystic spaces of variable size. Commonly, they are 1 to 5 cm in diameter; translucent; lined by a gray, glistening, smooth membrane; and filled with clear fluid. On microscopic examination, these membranes are seen to be composed of a single layer of cuboidal or flattened cuboidal epithelium, which in many instances may be completely atrophic. The cysts usually are confined to the cortex. Rarely, massive cysts as large as 10 cm in diameter are encountered.
Simple cysts constitute a common postmortem finding that has no clinical significance. The main importance of cysts lies in their differentiation from kidney tumors, when they are discovered either incidentally or during evaluation of hemorrhage and pain. Radiographic studies show that in contrast with renal tumors, renal cysts have smooth contours, are almost always avascular, and produce fluid rather than solid tissue signals on ultrasonography.
Dialysis-associated acquired cysts occur in the kidneys of patients with end-stage kidney disease who have undergone prolonged dialysis. They are present in both the cortex and the medulla and may bleed, causing hematuria. Occasionally, renal adenomas or even papillary adenocarcinomas arise in the walls of these cysts.
Autosomal Dominant (Adult) Polycystic Kidney Disease
Adult polycystic kidney disease is characterized by multiple expanding cysts affecting both kidneys that ultimately destroy the intervening parenchyma. It is seen in approximately 1 in 500 to 1000 persons and accounts for 10% of cases of chronic kidney disease. This disease is genetically heterogeneous. It can be caused by inheritance of one of at least two autosomal dominant genes of very high penetrance. In 85% to 90% of families, PKD1, on the short arm of chromosome 16, is the defective gene. This gene encodes a large (460-kDa) and complex cell membrane–associated protein, called polycystin-1.
![]() Pathogenesis
Pathogenesis
The polycystin molecule is mainly extracellular and has regions of homology with proteins involved in cell–cell or cell–matrix adhesion (e.g., domains that bind collagen, laminin, and fibronectin). It also has several other domains including those that can bind receptor tyrosine phosphatases. The polycystins have been localized to the primary cilium of tubular cells, like the nephrocystins linked to medullary cystic disease that are discussed later on, giving rise to the concept of renal cystic diseases as a type of ciliopathy. Cilia are hairlike organelles that project into the lumina from the apical surface of tubular cells, where they serve as mechanosensors of fluid flow. Current evidence suggests that polycystin mutations produce defects in mechanosensing. This in turn alters downstream signaling events involving calcium influx, leading to dysregulation of cell polarity, proliferation, and cell-cell and cell-matrix adhesion. It is interesting to note that whereas germline mutations of the PKD1 gene are present in all renal tubular cells of affected persons, cysts develop in only some tubules. This is most likely due to loss of both alleles of PKD1. Thus, as with tumor suppressor genes, a second “somatic hit” is required for expression of the disease. The PKD2 gene, implicated in 10% to 15% of cases, resides on chromosome 4 and encodes polycystin 2, a smaller, 110-kD protein. Polycystin 2 is thought to function as a calcium-permeable membrane channel, and is also expressed in cilia. Although structurally distinct, polycystins 1 and 2 are believed to act together by forming heterodimers. Thus, mutation in either gene gives rise to essentially the same phenotype, although patients with PKD2 mutations have a slower rate of disease progression as compared with patients with PKD1 mutations.
![]() Morphology
Morphology
In autosomal dominant adult polycystic kidney disease, the kidney may reach enormous size, and weights of up to 4 kg for each kidney have been recorded. These very large kidneys are readily palpable abdominally as masses extending into the pelvis. On gross examination the kidney seems to be composed solely of a mass of cysts of various sizes up to 3 or 4 cm in diameter with no intervening parenchyma. The cysts are filled with fluid, which may be clear, turbid, or hemorrhagic (Fig. 13–21).
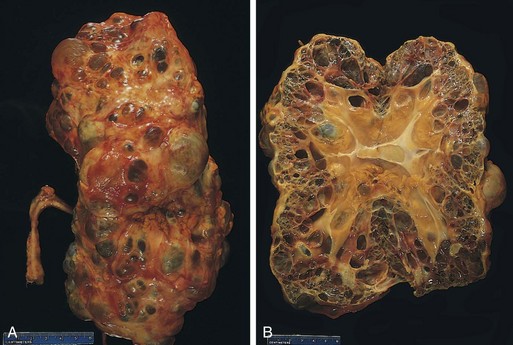
Figure 13–21 Autosomal dominant adult polycystic kidney, viewed from the external surface (A) and bisected (B). The kidney is markedly enlarged (centimeter rule is shown for scale), with numerous dilated cysts.
Cysts may arise at any level of the nephron, from tubules to collecting ducts, and therefore they have a variable, often atrophic, lining. Occasionally, Bowman’s capsules are involved in the cyst formation, and in these cases glomerular tufts may be seen within the cystic space. The pressure of the expanding cysts leads to ischemic atrophy of the intervening renal substance. Some normal parenchyma may be dispersed among the cysts. Evidence of superimposed hypertension or infection is common. Asymptomatic liver cysts occur in one third of the patients.
Clinical Course
Polycystic kidney disease in adults usually does not produce symptoms until the fourth decade of life, by which time the kidneys are quite large, although small cysts start to develop in adolescence. The most common presenting complaint is flank pain or a heavy, dragging sensation. Acute distention of a cyst, either by intracystic hemorrhage or by obstruction, may cause excruciating pain. Sometimes attention is first drawn to the lesion on palpation of an abdominal mass. Intermittent gross hematuria commonly occurs. The most important complications, because of their deleterious effect on already marginal renal function, are hypertension and urinary infection. Hypertension of variable severity develops in about 75% of persons with this disorder. Saccular aneurysms of the circle of Willis (Chapter 22) are present in 10% to 30% of patients and are associated with a high incidence of subarachnoid hemorrhage.
Although the disease is ultimately fatal, the outlook is generally better than with most chronic kidney diseases. The condition tends to be relatively stable and progresses very slowly. End-stage kidney disease occurs at about age 50, but there is wide variation in the course of this disorder, and nearly normal life spans are reported. Patients in whom the disease progresses to renal failure are treated by renal transplantation. Death usually results from uremia or hypertensive complications.
Autosomal Recessive (Childhood) Polycystic Kidney Disease
The childhood form of polycystic kidney disease is a rare autosomal recessive disorder that is genetically distinct from adult polycystic kidney disease. It occurs in approximately 1 in 20,000 live births. Perinatal, neonatal, infantile, and juvenile subcategories have been defined, depending on age at presentation and the presence of associated hepatic lesions. All types result from mutations in the PKHD1 gene, coding for a putative membrane receptor protein called fibrocystin, localized to the short arm of chromosome 6 (6p). Fibrocystin is found in cilia in tubular epithelial cells, but its function remains unknown.
![]() Morphology
Morphology
In autosomal recessive polycystic kidney disease, numerous small cysts in the cortex and medulla give the kidney a spongelike appearance. Dilated, elongated channels at right angles to the cortical surface completely replace the medulla and cortex. The cysts have a uniform lining of cuboidal cells, reflecting their origin from the collecting tubules. The disease is invariably bilateral. In almost all cases, findings include multiple epithelium-lined cysts in the liver and proliferation of portal bile ducts.
Medullary Diseases with Cysts
There are two major types of cystic disease affecting the medulla: medullary sponge kidney, a relatively common and usually innocuous condition, occasionally associated with nephrolithiasis, which will not be discussed further, and nephronophthisis-medullary cystic disease complex, which is almost always associated with renal dysfunction.
Nephronophthisis–medullary cystic disease complex is an under-appreciated cause of chronic kidney disease that usually begins in childhood. Four variants of this disease complex are recognized on the basis of the timing of onset: infantile, juvenile, and adolescent nephronophthisis and medullary cystic disease developing later in adult life. The juvenile form is the most common. Approximately 15% to 20% of children with juvenile nephronophthisis have extrarenal manifestations, which most often appear as retinal abnormalities, including retinitis pigmentosa, and even early-onset blindness in the most severe form. Other abnormalities found in some persons include oculomotor apraxia, mental retardation, cerebellar malformations, and liver fibrosis. In aggregate, the various forms of nephronophthisis are now thought to be the most common genetic cause of end-stage renal disease in children and young adults.
At least nine gene loci (NHP1-NHP9) have been identified for the autosomal recessive forms of the nephronophthisis complex. The majority of these genes encode proteins that are components of epithelial cilia, as is the case with other types of polycystic disease. Two autosomal forms cause disease in adults; these are far less common.
![]() Morphology
Morphology
Pathologic features of medullary cystic disease include small contracted kidneys. Numerous small cysts lined by flattened or cuboidal epithelium are present, typically at the corticomedullary junction. Other pathologic changes are nonspecific, but most notably they include a chronic tubulointerstitial nephritis with tubular atrophy and thickened tubular basement membranes and progressive interstitial fibrosis.
Clinical Course
The initial manifestations are usually polyuria and polydipsia, a consequence of diminished tubular function. Progression to end-stage kidney disease ensues over a 5- to 10-year period. The disease is difficult to diagnose, since there are no serologic markers and the cysts may be too small to be seen with radiologic imaging. Adding to this difficulty, cysts may not be apparent on renal biopsy if the corticomedullary junction is not well sampled. A positive family history and unexplained chronic renal failure in young patients should lead to suspicion of nephronophthisis.
![]() Summary
Summary
Cystic Diseases
• Adult polycystic kidney disease is a disease of autosomal dominant inheritance caused by mutations in the genes encoding polycystin-1 or -2. It accounts for about 10% of cases of chronic renal failure; kidneys may be very large and contain many cysts.
• Autosomal recessive (childhood) polycystic kidney disease is caused by mutations in the gene encoding fibrocystin. It is less common than the adult form and strongly associated with liver abnormalities; kidneys contain numerous small cysts.
• Nephronophthisis–medullary cystic disease complex is being increasingly recognized as a cause of chronic kidney disease in children and young adults. Of autosomal recessive inheritance, it is associated with mutations in several genes that encode epithelial cell proteins called nephrocystins that may be involved in ciliary function; kidneys are contracted and contain multiple small cysts.
Urinary Outflow Obstruction
Renal Stones
Urolithiasis is calculus formation at any level in the urinary collecting system, but most often the calculi arise in the kidney. They occur frequently, and it is estimated that by the age of 70 years, 11% of men and 5.6% of women in the United States will have experienced a symptomatic kidney stone. Symptomatic urolithiasis is more common in men than in women. A familial tendency toward stone formation has long been recognized.
![]() Pathogenesis
Pathogenesis
There are three major types of stones.
• About 80% of renal stones are composed of either calcium oxalate or calcium oxalate mixed with calcium phosphate.
In all cases, an organic matrix of mucoprotein is present that makes up about 2.5% of the stone by weight (Table 13–3).
Table 13–3 Prevalence of Various Types of Renal Stones
| Stone | Distribution (%) |
|---|---|
| 80 | |
| 10 | |
| 6–7 | |
| 1–2 | |
| ±1–2 |
The cause of stone formation is often obscure, particularly in the case of calcium-containing stones. Probably involved is a confluence of predisposing conditions, including the concentration of the solute, changes in urine pH, and bacterial infections. The most important cause is increased urinary concentration of the stone’s constituents, so that it exceeds their solubility in urine (supersaturation). As shown in Table 13–3, 50% of patients who develop calcium stones have hypercalciuria that is not associated with hypercalcemia. Most in this group absorb calcium from the gut in excessive amounts (absorptive hypercalciuria) and promptly excrete it in the urine, and some have a primary renal defect of calcium reabsorption (renal hypercalciuria).
The causes of the other types of renal stones are better understood. Magnesium ammonium phosphate (struvite) stones almost always occur in persons with a persistently alkaline urine resulting from UTIs. In particular, infections with urea-splitting bacteria, such as Proteus vulgaris and staphylococci, predispose individuals to urolithiasis. Moreover, bacteria may serve as particulate nidi for the formation of any kind of stone. In avitaminosis A, desquamated cells from the metaplastic epithelium of the collecting system act as nidi.
Gout and diseases involving rapid cell turnover, such as the leukemias, lead to high uric acid levels in the urine and the possibility of uric acid stones. About half of people with uric acid stones, however, have neither hyperuricemia nor increased urine urate but demonstrate an unexplained tendency to excrete a persistently acid urine (with a pH less than 5.5). This low pH favors uric acid stone formation—in contrast with the high pH that favors formation of stones containing calcium phosphate. Cystine stones are almost invariably associated with a genetically determined defect in the renal transport of certain amino acids, including cystine. Like uric acid stones, cystine stones are more likely to form when the urine is relatively acidic.
Urolithiasis also may result from the lack of substances that normally inhibit mineral precipitation. Inhibitors of crystal formation in urine include Tamm-Horsfall protein, osteopontin, pyrophosphate, mucopolysaccharides, diphosphonates, and a glycoprotein called nephrocalcin, but no deficiency of any of these substances has been consistently demonstrated in persons with urolithiasis.
![]() Morphology
Morphology
Stones are unilateral in about 80% of patients. Common sites of formation are renal pelves and calyces and the bladder. Often, many stones are found in one kidney. They tend to be small (average diameter, 2 to 3 mm) and may be smooth or jagged. Occasionally, progressive accretion of salts leads to the development of branching structures known as staghorn calculi, which create a cast of the renal pelvis and calyceal system. These massive stones usually are composed of magnesium ammonium phosphate.
Clinical Course
Stones may be present without producing either symptoms or significant renal damage. This is particularly true with large stones lodged in the renal pelvis. Smaller stones may pass into the ureter, where they may lodge, producing a typical intense pain known as renal or ureteral colic, characterized by paroxysms of flank pain radiating toward the groin. Often at this time there is gross hematuria. The clinical significance of stones lies in their capacity to obstruct urine flow or to produce sufficient trauma to cause ulceration and bleeding. In either case, they predispose the sufferer to bacterial infection. Fortunately, in most cases the diagnosis is readily made radiologically.
Hydronephrosis
Hydronephrosis refers to dilation of the renal pelvis and calyces, with accompanying atrophy of the parenchyma, caused by obstruction to the outflow of urine. The obstruction may be sudden or insidious, and it may occur at any level of the urinary tract, from the urethra to the renal pelvis. The most common causes are categorized as follows:
• Congenital: atresia of the urethra, valve formations in either ureter or urethra, aberrant renal artery compressing the ureter, renal ptosis with torsion, or kinking of the ureter
Bilateral hydronephrosis occurs only when the obstruction is below the level of the ureters. If blockage is at the ureters or above, the lesion is unilateral. Sometimes obstruction is complete, allowing no urine to pass; usually it is only partial.
![]() Pathogenesis
Pathogenesis
Even with complete obstruction, glomerular filtration persists for some time, and the filtrate subsequently diffuses back into the renal interstitium and perirenal spaces, whence it ultimately returns to the lymphatic and venous systems. Because of the continued filtration, the affected calyces and pelvis become dilated, often markedly so. The unusually high pressure thus generated in the renal pelvis, as well as that transmitted back through the collecting ducts, causes compression of the renal vasculature. Both arterial insufficiency and venous stasis result, although the latter probably is more important. The most severe effects are seen in the papillae, because they are subjected to the greatest increases in pressure. Accordingly, the initial functional disturbances are largely tubular, manifested primarily by impaired concentrating ability. Only later does glomerular filtration begin to diminish. Experimental studies indicate that serious irreversible damage occurs in about 3 weeks with complete obstruction, and in 3 months with incomplete obstruction. In addition to functional changes, the obstruction also triggers an interstitial inflammatory reaction, leading eventually to interstitial fibrosis.
![]() Morphology
Morphology
Bilateral hydronephrosis (as well as unilateral hydronephrosis when the other kidney is already damaged or absent) leads to renal failure, and the onset of uremia tends to abort the natural course of the lesion. By contrast, unilateral involvement is associated with the full range of morphologic changes, which vary with the degree and speed of obstruction. With subtotal or intermittent obstruction, the kidney may be massively enlarged (lengths in the range of 20 cm), and the organ may consist almost entirely of the greatly distended pelvicalyceal system. The renal parenchyma itself is compressed and atrophied, with obliteration of the papillae and flattening of the pyramids (Fig. 13–22). On the other hand, when obstruction is sudden and complete, glomerular filtration is compromised relatively early, and as a consequence, renal function may cease while dilation is still comparatively slight. Depending on the level of the obstruction, one or both ureters may be dilated (hydroureter).
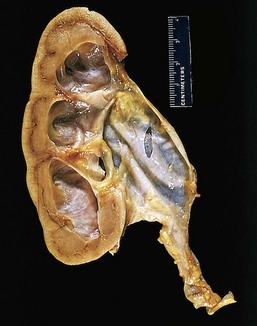
Figure 13–22 Hydronephrosis of the kidney, with marked dilation of the pelvis and calyces and thinning of renal parenchyma.
On microscopic examination the early lesions show tubular dilation, followed by atrophy and fibrous replacement of the tubular epithelium with relative sparing of the glomeruli. Eventually, in severe cases the glomeruli also become atrophic and disappear, converting the entire kidney into a thin shell of fibrous tissue. With sudden and complete obstruction, there may be coagulative necrosis of the renal papillae, similar to the changes of papillary necrosis. In uncomplicated cases the accompanying inflammatory reaction is minimal. Superimposed pyelonephritis, however, is common.
Clinical Course
Bilateral complete obstruction produces anuria, which is soon brought to medical attention. When the obstruction is below the bladder, the dominant symptoms are those of bladder distention. Paradoxically, incomplete bilateral obstruction causes polyuria rather than oliguria, as a result of defects in tubular concentrating mechanisms, and this may obscure the true nature of the disturbance. Unfortunately, unilateral hydronephrosis may remain completely silent for long periods unless the other kidney is for some reason not functioning. Often the enlarged kidney is discovered on routine physical examination. Sometimes the basic cause of the hydronephrosis, such as renal calculi or an obstructing tumor, produces symptoms that indirectly draw attention to the hydronephrosis. Removal of obstruction within a few weeks usually permits full return of function; however, with time the changes become irreversible.
Tumors
Many types of benign and malignant tumors occur in the urinary tract. In general, benign tumors such as small (less than 0.5 cm in diameter) cortical papillary adenomas, which are found in 40% of adults, have no clinical significance. The most common malignant tumor of the kidney is renal cell carcinoma, followed in frequency by nephroblastoma (Wilms tumor) and by primary tumors of the calyces and pelvis. Other types of renal cancer are rare and need not be discussed here. Tumors of the lower urinary tract are about twice as common as renal cell carcinomas. They are described at the end of this section.
Tumors of the Kidney
Oncocytoma
Oncocytoma, a benign tumor that arises from the intercalated cells of collecting ducts, represents about 10% of renal tumors. These tumors are associated with genetic changes—loss of chromosomes 1, 14, and Y—that distinguish them from other renal neoplasms. Oncocytomas are histologically characterized by a plethora of mitochondria, providing the basis for their tan color and their finely granular eosinophilic cytoplasm that is seen histologically. A central stellate scar, which is another feature of oncocytomas, provides a characteristic appearance on imaging studies. Owing to their large size and clinical and radiologic similarity to some renal cell carcinomas, however, they are removed by nephrectomy, both to prevent such complications as spontaneous hemorrhage and to make a definitive diagnosis.
Renal Cell Carcinoma
Renal cell carcinomas are derived from the renal tubular epithelium and hence they are located predominantly in the cortex. These tumors represent 80% to 85% of all primary malignant tumors of the kidney and 2% to 3% of all cancers in adults. These data translate into about 58,000 cases per year in the United States; 40% of patients die of the disease. Carcinomas of the kidney are most common from the sixth to seventh decades, and men are affected about twice as commonly as women. The risk of developing these tumors is higher in smokers, hypertensive or obese patients, and those who have had occupational exposure to cadmium. The risk of developing renal cell cancer is increased 30-fold in persons who acquire polycystic disease as a complication of chronic dialysis. The role of genetic factors in the causation of these cancers is discussed later on.
Renal cell cancers are classified on the basis of morphology and growth patterns. However, recent advances in the understanding of the genetic basis of renal carcinomas have led to a new classification that takes into account the molecular origins of these tumors. The three most common forms, discussed next, are clear cell carcinoma, papillary renal cell carcinoma, and chromophobe renal carcinoma.
Clear Cell Carcinomas
Clear cell carcinomas are the most common type, accounting for 65% of renal cell cancers. Histologically, they are composed of cells with clear cytoplasm. Although most are sporadic, they also occur in familial forms or in association with von Hippel-Lindau (VHL) disease. It is the study of VHL disease that has provided molecular insights into the causation of clear cell carcinomas. VHL disease is inherited as an autosomal dominant trait and is characterized by predisposition to a variety of neoplasms, but particularly to hemangioblastomas of the cerebellum and retina. Hundreds of bilateral renal cysts and bilateral, often multiple, clear cell carcinomas develop in 40% to 60% of affected persons. Those with VHL syndrome inherit a germline mutation of the VHL gene on chromosomal band 3p25 and lose the second allele by somatic mutation. Thus, the loss of both copies of this tumor suppressor gene is a key step in the development of clear cell carcinoma. The VHL gene is also involved in the majority of sporadic clear cell carcinomas. Cytogenetic abnormalities giving rise to loss of chromosomal segment 3p14 to 3p26 are often seen in sporadic renal cell cancers. This region harbors the VHL gene (3p25.3). The second, nondeleted allele is inactivated by a somatic mutation or hypermethylation in 60% of sporadic cases. Thus, homozygous loss of the VHL gene seems to be the common underlying molecular abnormality in both sporadic and familial forms of clear cell carcinomas. The VHL protein causes the degradation of hypoxia-induced factors (HIFs), and in the absence of VHL, HIFs are stabilized. HIFs are transcription factors that contribute to carcinogenesis by stimulating the expression of vascular endothelial growth factor (VEGF), an important angiogenic factor, as well as a number of other genes that drive tumor cell growth (Chapter 5). An uncommon familial form of clear cell carcinoma unrelated to VHL disease also is associated with cytogenetic abnormalities involving the short arm of chromosome 3 (3p). In addition, recent deep sequencing of clear cell carcinoma genomes has revealed frequent loss-of-function mutations in SETD2, JARID1C, and UTX, all of which encode proteins that regulate histone methylation, suggesting that changes in the “epigenome” have a central role in the genesis of this subtype of renal carcinoma.
Papillary Renal Cell Carcinomas
Papillary renal cell carcinomas account for 10% to 15% of all renal cancers. As the name indicates, they show a papillary growth pattern. These tumors are frequently multifocal and bilateral and appear as early-stage tumors. Like clear cell carcinomas, they occur in familial and sporadic forms, but unlike these tumors, papillary renal cancers are not associated with abnormalities of chromosome 3. The culprit in most cases of hereditary papillary renal cell cancers is the MET proto-oncogene, located on chromosomal sub-band 7q31. The MET gene is a tyrosine kinase receptor for the growth factor called hepatocyte growth factor. The increased dosage of the MET gene due to duplications of chromosome 7 seems to spur abnormal growth in the proximal tubular epithelial cell precursors of papillary carcinomas. In familial cases, genetic analysis shows activating mutations of MET in the germline, along with increased gene dosage in the cancers. Activating mutations of the MET gene also are found in a subset of patients with sporadic forms of papillary renal cell carcinoma.
Chromophobe Renal Carcinomas
Chromophobe renal carcinomas are the least common, representing 5% of all renal cell carcinomas. They arise from intercalated cells of collecting ducts. Their name derives from the observation that the tumor cells stain more darkly (i.e., they are less clear) than cells in clear cell carcinomas. These tumors are unique in having multiple losses of entire chromosomes, including chromosomes 1, 2, 6, 10, 13, 17, and 21. Thus, they show extreme hypodiploidy. Because of multiple losses, the “critical hit” has not been determined. In general, chromophobe renal cancers have a good prognosis.
![]() Morphology
Morphology
Clear cell cancers (the most common form of these renal carcinomas) usually are solitary and large when symptomatic (spherical masses 3 to 15 cm in diameter), but high-resolution radiographic techniques for investigation of unrelated problems sometimes detect smaller lesions incidentally. They may arise anywhere in the cortex. The cut surface of clear cell renal cell carcinomas is yellow to orange to gray-white, with prominent areas of cystic softening or of hemorrhage, either fresh or old (Fig. 13–23). The margins of the tumor are well defined. However, at times small processes project into the surrounding parenchyma and small satellite nodules are found, providing clear evidence of the aggressiveness of these lesions. As the tumor enlarges, it may fungate through the walls of the collecting system, extending through the calyces and pelvis as far as the ureter. Even more frequently, the tumor invades the renal vein and grows as a solid column within this vessel, sometimes extending in serpentine fashion as far as the inferior vena cava and even into the right side of the heart. Occasionally, direct invasion into the perinephric fat and adrenal gland may be seen.
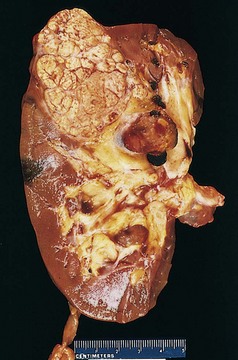
Figure 13–23 Renal cell carcinoma: Representative cross-section showing yellowish, spherical neoplasm in one pole of the kidney. Note the tumor in the dilated, thrombosed renal vein.
Depending on the amounts of lipid and glycogen present, the tumor cells of clear cell renal cell carcinoma may appear almost vacuolated or may be solid. The classic vacuolated (lipid-laden), or clear cells are demarcated only by their cell membranes. The nuclei are usually small and round (Fig. 13–24). At the other extreme are granular cells, resembling the tubular epithelium, which have small, round, regular nuclei enclosed within granular pink cytoplasm. Some tumors are highly anaplastic, with numerous mitotic figures and markedly enlarged, hyperchromatic, pleomorphic nuclei. Between the extremes of clear cells and solid, granular cells, all intergradations may be found. The cellular arrangement, too, varies widely. The cells may form abortive tubules or may cluster in cords or disorganized masses. The stroma is usually scant but highly vascularized.
Papillary renal cell carcinomas exhibit various degrees of papilla formation with fibrovascular cores. They tend to be bilateral and multiple. They also may show gross evidence of necrosis, hemorrhage, and cystic degeneration, but they are less vibrantly orange-yellow because of their lower lipid content. The cells may have clear or, more commonly, pink cytoplasm. Chromophobe-type renal cell carcinoma tends to be grossly tan-brown. The cells usually have clear, flocculent cytoplasm with very prominent, distinct cell membranes. The nuclei are surrounded by halos of clear cytoplasm. Ultrastructurally, large numbers of characteristic macrovesicles are seen.
Clinical Course
Renal cell carcinomas have several peculiar clinical characteristics that create especially difficult and challenging diagnostic problems. The signs and symptoms vary, but the most frequent presenting manifestation is hematuria, occurring in more than 50% of cases. Macroscopic hematuria tends to be intermittent and fleeting, superimposed on a steady microscopic hematuria. Less commonly the tumor may declare itself simply by virtue of its size, when it has grown large enough to produce flank pain and a palpable mass. Because of the widespread use of imaging studies for unrelated conditions, even smaller tumors are detected. Extra-renal effects are fever and polycythemia, which, because they are nonspecific, may be misinterpreted for some time before their association with the renal tumor is appreciated. Polycythemia affects 5% to 10% of persons with this disease. It results from elaboration of erythropoietin by the cancer cells. Uncommonly, these tumors produce other hormone-like substances, resulting in hypercalcemia, hypertension, Cushing syndrome, or feminization or masculinization. These, as noted in Chapter 5, are paraneoplastic syndromes. In many patients, the primary tumor remains silent and is discovered only after its metastases have produced symptoms. The prevalent locations for metastases are the lungs and the bones. It must be apparent that renal cell carcinoma manifests in many ways, some quite devious, but the triad of painless hematuria, a palpable abdominal mass, and dull flank pain is characteristic.
![]() Summary
Summary
Renal Cell Carcinoma
Renal cell carcinomas account for 2% to 3% of all cancers in adults and are classified into three types:
• Clear cell carcinomas are the most common and are associated with homozygous loss of the VHL tumor suppressor protein; tumors frequently invade the renal vein.
• Papillary renal cell carcinomas frequently are associated with increased expression and activating mutations of the MET oncogene; they tend to be bilateral and multiple and show variable papilla formation.
• Chromophobe renal cell carcinomas are less common; tumor cells are not as clear as in the other renal cell carcinomas.
Wilms Tumor
Although Wilms tumor occurs infrequently in adults, it is the third most common organ cancer in children younger than 10 years of age. These tumors contain a variety of cell and tissue components, all derived from the mesoderm. Wilms tumor, like retinoblastoma, may arise sporadically or be familial, with the susceptibility to tumorigenesis inherited as an autosomal dominant trait. This tumor is discussed in greater detail in Chapter 6 along with other tumors of childhood.
Tumors and other lesions of the lower urinary tract (ureters, bladder, and urethra) are described in Chapter 17.
Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088. [A comprehensive update on the pathogenesis, clinical manifestations, and treatment of this disease.]
Beck LHJr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11. [A landmark study describing the discovery of the antigen in idiopathic membranous nephropathy.]
D’Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008;17:271. [A comprehensive review of mechanisms contributing to various forms of FSGS.]
Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841. [A landmark study of natural selection, linking a genetic variant of apolipoprotein L1 in African Americans to protection against sleeping sickness, and risk for kidney disease.]
Guay-Woodford LM. Renal cystic diseases: diverse phenotypes converge on the cilium/centrosome complex. Pediatr Nephrol. 2006;21:1369. [An excellent review on the pathophysiology of renal cystic diseases, with emphasis on the role of ciliary dysfunction in tubular epithelial cells.]
Gubler MC. Inherited diseases of the glomerular basement membrane. Nat Clin Pract Nephrol. 2008;4:24. [A superb review of the pathophysiology, clinical presentations and diagnostic testing strategies for Alport syndrome, thin basement membrane disease, and other types of hereditary nephritis.]
Harris PC. 2008 Homer W. Smith Award: Insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol. 2009;20:1188. [A review of the discovery of the major genes leading to polycystic kidney disease, along with their phenotypic manifestations.]
Knowles MA. Molecular subtypes of bladder cancer: Jekyll and Hyde or chalk and cheese. Carcinogenesis. 2006;27:371. [Comprehensive review of molecular changes in different types of bladder cancer.]
Lionaki S, Jennette JC, Falk RJ. Anti-neutrophil cytoplasmic (ANCA) and anti-glomerular basement membrane (GBM) autoantibodies in necrotizing and crescentic glomerulonephritis. Semin Immunopathol. 2007;29:459. [A good summary of mechanisms of injury and clinical manifestations in ANCA and anti-GBM antibody–mediated disease.]
Mathieson PW. Minimal change nephropathy and focal segmental glomerulosclerosis. Semin Immunopathol. 2007;29:415. [An excellent overview of new insights into the pathogenesis and diagnosis of MCD versus FSGS.]
Miller O, Hemphill RR. Urinary tract infection and pyelonephritis. Emerg Med Clin North Am. 2001;19:655. [An excellent review of acute urinary tract infections.]
Murray PT, Devarajan P, Levey AS, et al. A framework and key research questions in AKI diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008;3:864. [An excellent review outlining recent advances in early diagnosis and consequences of acute kidney injury.]
Nsar SH, Markowitz GS, Stokes MB, et al. Acute postinfectious glomerulonephritis in modern era: experience with 86 adults and review of the literature. Medicine. 2008;87:21. [A contemporary review of postinfectious glomerulonephritis with an emphasis on clinicopathologic correlations and epidemiologic associations.]
Ronco P, Debiec H. Membranous glomerulopathy: the evolving story. Curr Opin Nephrol Hypertens. 2010;19:254. [An excellent review of recent insights into the etiology of membranous nephropathy.]
Schrier RW, Wang W, Poole B, et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5. [An insightful review covering all aspects of acute renal failure.]
Tryggvason K, Patrakka J, Wartiovaava J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387. [An excellent review of the pathophysiology of defects in glomerular permeability.]
Tsai HM. The molecular biology of thrombotic microangiopathy. Kidney Int. 2006;70:16. [An excellent review of the pathogenesis of HUS and TTP.]
Wilson PD, Goilav B. Cystic disease of the kidney. Annu Rev Pathol. 2007;2:341. [Pathobiology of a common condition affecting the kidney.]
Worcester EM, Coe FL. Calcium kidney stones. N Engl J Med. 2010;363:954. [A comprehensive review of the pathophysiology and management of the most common types of kidney stones.]