Chapter 9 Blood Vessels
See Targeted Therapy available online at studentconsult.com
Vascular maladies are of central importance in medicine, as they are responsible for some of the most common and lethal diseases afflicting mankind. Although most clinically significant vascular diseases are caused by arterial lesions, venous disorders also can wreak havoc. Vascular disease develops through two principal mechanisms:
• Narrowing or complete obstruction of vessel lumina, occurring either progressively (e.g., by atherosclerosis) or acutely (e.g., by thrombosis or embolism)
• Weakening of vessel walls, causing dilation and/or rupture
Presented next is an overview of vascular structure and function, as background for the diseases of blood vessels discussed later in the chapter.
Structure and Function of Blood Vessels
In essence, all blood vessels consist of a tube with a luminal lining of endothelial cells surrounded by varying amounts of smooth muscle cells and extracellular matrix (ECM). However, the structure of each of these components varies in different parts of the vasculature according to functional needs (Fig. 9–1). To accommodate pulsatile flow and higher blood pressures, arterial walls are thicker than veins and invested with reinforcing layers of smooth muscle cells. As arteries narrow to arterioles, the ratio of wall thickness to lumen diameter increases, to allow more precise regulation of intravascular pressures. Veins, on the other hand, are distensible thin-walled vessels with high capacitance. In keeping with these specializations, certain pathologic lesions characteristically involve particular kinds of vessels. For example, atherosclerosis occurs mainly in larger, muscular arteries, while hypertension affects small arterioles, and specific forms of vasculitis selectively involve vessels of only a certain caliber.
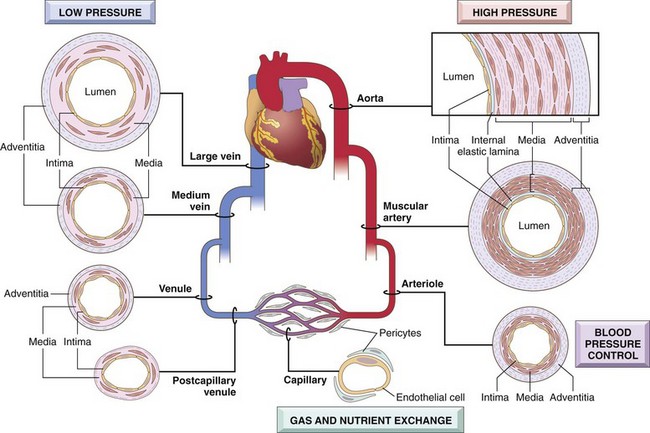
Figure 9–1 Regional vascular specializations. Although all vessels share the same general constituents, the thickness and composition of the various layers differ as a function of hemodynamic forces and tissue requirements.
Vessel walls are organized into three concentric layers: intima, media, and adventitia (see Fig. 9–1). These layers are present in all vessels but are most apparent in larger vessels and particularly arteries. The intima consists of an endothelial cell monolayer on a basement membrane with minimal underlying ECM; it is separated from the media by a dense elastic membrane called the internal elastic lamina. The media is composed predominantly of smooth muscle cells and ECM, surrounded by loose connective tissue, nerve fibers, and smaller vessels of the adventitia. An external elastic lamina is present in some arteries and defines the transition between media and adventitia. Diffusion of oxygen and nutrients from the lumen is adequate to sustain thin-walled vessels and the innermost smooth muscle cells of all vessels. In large and medium-sized vessels, however, small arterioles within the adventitia (called vasa vasorum—literally, “vessels of the vessels”) supply the outer half to two thirds of the media.
Vascular Organization
Arteries are divided into three types based on their size and structure:
• Large elastic arteries (e.g., aorta, arch vessels, iliac and pulmonary arteries). In these vessels, elastic fibers alternate with smooth muscle cells throughout the media, which expands during systole (storing some of the energy of each cardiac contraction), and recoils during diastole to propel blood distally. With age, the elasticity is lost, and vessels become “stiff pipes” that transmit high arterial pressures to distal organs, or dilated and tortuous (ectatic) conduits prone to rupture.
• Medium-sized muscular arteries (e.g., coronary and renal arteries). Here, the media is composed primarily of smooth muscle cells, with elastin limited to the internal and external elastic lamina. The medial smooth muscle cells are circularly or spirally arranged around the lumen, and regional blood flow is regulated by smooth muscle cell contraction (vasoconstriction) and relaxation (vasodilation) controlled by the autonomic nervous system and local metabolic factors (e.g., acidosis).
• Small arteries (2 mm or less in diameter) and arterioles (20 to 100 µm in diameter) that lie within the connective tissue of organs. The media in these vessels is mostly composed of smooth muscle cells. Arterioles are where blood flow resistance is regulated. As pressures drop during passage through arterioles, the velocity of blood flow is sharply reduced, and flow becomes steady rather than pulsatile. Because the resistance to fluid flow is inversely proportional to the fourth power of the diameter (i.e., halving the diameter increases resistance 16-fold), small changes in arteriolar lumen size have profound effects on blood pressure.
Capillaries have lumen diameters that approximate those of red cells (7 to 8 µm). These vessels are lined by endothelial cells and partially surrounded by smooth muscle cell–like cells called pericytes. Collectively, capillary beds have a very large total cross-sectional area and a low rate of blood flow. With their thin walls and slow flow, capillaries are ideally suited to the rapid exchange of diffusible substances between blood and tissue. The capillary network of most tissues is necessarily very rich, because diffusion of oxygen and nutrients is not efficient beyond 100 µm; metabolically active tissues (e.g., heart) have the highest capillary density.
Veins receive blood from the capillary beds as postcapillary venules, which anastomose to form collecting venules and progressively larger veins. The vascular leakage (edema) and leukocyte emigration characteristic of inflammation occurs preferentially in postcapillary venules (Chapter 2).
Compared with arteries at the same level of branching, veins have larger diameters, larger lumina, and thinner walls with less distinct layers, all adaptations to the low pressures found on the venous side of the circulation (see Fig. 9–1). Thus, veins are more prone to dilation, external compression, and penetration by tumors or inflammatory processes. In veins in which blood flows against gravity (e.g., those of the lower extremities), backflow is prevented by valves. Collectively, the venous system has a huge capacitance and normally contains approximately two thirds of the blood.
Lymphatics are thin-walled, endothelium-lined channels that drain fluid (lymph) from the interstitium of tissues, eventually returning it to the blood via the thoracic duct. Lymph also contains mononuclear inflammatory cells and a host of proteins. By delivering interstitial fluid to lymph nodes, lymphatics enable continuous monitoring of peripheral tissues for infection. These channels can also disseminate disease by transporting microbes or tumor cells to distant sites.
Endothelial Cells
Endothelium is a continuous sheet of cells lining the entire vascular tree that regulates many aspects of blood and blood vessel function (Table 9–1). Resting endothelial cells maintain a nonthrombogenic blood-tissue interface (Chapter 3), modulate inflammation (Chapter 2), and affect the growth of other cell types, particularly smooth muscle cells. Endothelial cells influence the vasoreactivity of the underlying smooth muscle cells by producing both relaxing factors (e.g., nitric oxide [NO]) and contracting factors (e.g., endothelin). In most regions, the interendothelial junctions normally are impermeable. However, these junctions open under the influence of hemodynamic stress (e.g., high blood pressure) and/or vasoactive agents (e.g., histamine in inflammation), flooding the adjacent tissues with electrolytes and protein. Vacuolar transcytosis also permits the movement of large amounts of solutes across intact endothelium. Endothelial cells also are active participants in the egress of leukocytes during inflammatory cell recruitment (Chapter 2).
Table 9–1 Endothelial Cell Properties and Functions
| Property/Function | Mediators/Products |
|---|---|
| Maintenance of permeability barrier | |
| Elaboration of anticoagulant, antithrombotic, fibrinolytic regulators | |
| Elaboration of prothrombotic molecules | |
| Extracellular matrix production | |
| Modulation of blood flow and vascular reactivity | |
| Regulation of inflammation and immunity | |
| Regulation of cell growth | |
| Oxidation of LDL |
ACE, angiotensin-converting enzyme; CSF, colony-stimulating factor; FGF, fibroblast growth factor; ICAM, intercelluar adhesion molecule; IL, interleukin; LDL, low-density lipoprotein; NO, nitric oxide; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β; VCAM, vascular cell adhesion molecule.
Although endothelial cells throughout the vasculature share many attributes, they also show phenotypic variability depending on the anatomic site and adaptations to local environmental cues. Thus, endothelial cell populations from different parts of the vasculature (e.g., large vessels versus capillaries, or arteries versus veins) have distinct transcriptional programs and behaviors. Fenestrations (holes) in endothelial cells lining hepatocyte cords or renal glomeruli are specializations that facilitate filtration. Conversely, in the central nervous system, endothelial cells—in conjunction with astrocytes—collaborate to generate an impermeable blood–brain barrier.
Maintenance of a “normal,” nonthrombogenic endothelial cell lining requires laminar flow, certain growth factors (e.g., vascular endothelial growth factor [VEGF]), and firm adhesion to the underlying basement membrane (Fig. 9–2). Trauma or other injuries that denude vessel walls of endothelial cells understandably tip the scales towards thrombosis and vasoconstriction. However, endothelial cells also respond to various physiologic and pathologic stimuli by modulating their usual (constitutive) functions and by expressing new (inducible) properties—a process called endothelial activation.
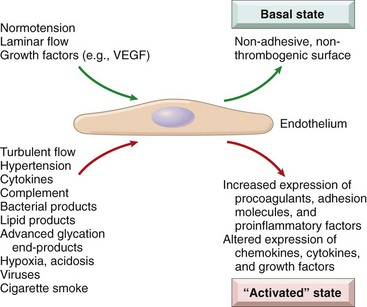
Figure 9–2 Basal and activated endothelial cell states. Normal blood pressure, laminar flow, and stable growth factor levels promote a basal endothelial cell state that maintains a nonthrombotic surface and appropriate vascular wall smooth muscle tone. Injury or exposure to certain mediators results in endothelial activation, a state in which endothelial cells have adhesive, procoagulant surfaces and release factors that lead to smooth muscle contraction and/or proliferation and matrix synthesis.
Inducers of endothelial activation include bacterial products, inflammatory cytokines, hemodynamic stresses and lipid products (relevant to atherosclerosis, described later), advanced glycation end products (important in diabetic vascular injury), viruses, complement, and various metabolic insults (e.g., hypoxia) (see Fig. 9–2). Activated endothelial cells undergo shape changes, express adhesion molecules, and produce cytokines, chemokines, growth factors, pro- and anticoagulant factors, and a host of other biologically active products—all presumably intended to respond to the original stimulus. Some of these responses are rapid (occurring within minutes), reversible, and independent of new protein synthesis (e.g., endothelial contraction induced by histamines); others involve alterations in gene and protein expression, and may take days to develop or abate. Exposure of endothelial cells to inducers of activation in high amounts or for sustained periods may result in endothelial dysfunction, characterized by impaired endothelium-dependent vasodilation, hypercoagulable states, and increased oxygen free radical production. Dysfunctional endothelium can initiate thrombosis, promote atherosclerosis, or contribute to formation of the vascular lesions of hypertension and diabetes.
Vascular Smooth Muscle Cells
Smooth muscle cells participate in both normal vascular repair and pathologic processes such as atherosclerosis. When stimulated by various factors, smooth muscle cells can proliferate; upregulate ECM collagen, elastin, and proteoglycan production; and elaborate growth factors and cytokines. Smooth muscle cells also mediate the vasoconstriction or vasodilation that occurs in response to physiologic or pharmacologic stimuli.
The migratory and proliferative activities of smooth muscle cells are regulated by numerous factors. Among the most important pro-growth factors are platelet-derived growth factor (PDGF), endothelin, thrombin, fibroblast growth factors, and inflammatory mediators such as interferon-γ (IFN-γ) and interleukin-1 (IL-1). Factors that maintain smooth muscle cells in a quiescent state include heparan sulfate, NO, and transforming growth factor-α (TGF-α).
![]() Summary
Summary
Vascular Structure and Function
• All vessels are lined by endothelium; although all endothelial cells share certain homeostatic properties, endothelial cells in specific vascular beds have special features that allow for tissue-specific functions (e.g., fenestrated endothelial cells in renal glomeruli).
• The relative smooth muscle cell and matrix content of vessel walls (e.g., in arteries, veins, and capillaries) vary according to hemodynamic demands (e.g., pressure, pulsatility) and functional requirements.
• Endothelial cell function is tightly regulated in both the basal and activated states. Various physiologic and pathophysiologic stimuli induce endothelial activation and dysfunction that alter the endothelial cell phenotype (e.g., pro- versus anticoagulative, pro- versus anti-inflammatory, nonadhesive versus adhesive).
Congenital Anomalies
Although rarely symptomatic, unusual anatomic variants in the vascular supply can cause complications during surgery, such as when a vessel in an unexpected location is injured. Cardiac surgeons and interventional cardiologists also must be familiar with coronary artery variants. Among the other congenital vascular anomalies, three deserve further mention:
• Berry aneurysms are thin-walled arterial outpouchings in cerebral vessels, classically at branch points around the circle of Willis; they occur where the arterial media is congenitally attenuated and can spontaneously rupture causing fatal intracerebral hemorrhage (see Chapter 22).
• Arteriovenous (AV) fistulas are abnormal connections between arteries and veins without an intervening capillary bed. They occur most commonly as developmental defects but can also result from rupture of arterial aneurysms into adjacent veins, from penetrating injuries that pierce arteries and veins, or from inflammatory necrosis of adjacent vessels. AV fistulas also are created surgically to provide vascular access for hemodialysis. Extensive AV fistulas can cause high-output cardiac failure by shunting large volumes of blood from the arterial to the venous circulation.
• Fibromuscular dysplasia is a focal irregular thickening of the walls of medium-sized and large muscular arteries due to a combination of medial and intimal hyperplasia and fibrosis. It can manifest at any age but occurs most frequently in young women. The focal wall thickening results in luminal stenosis or can be associated with abnormal vessel spasm that reduces vascular flow; in the renal arteries, it can lead to renovascular hypertension. Between the focal segments of thickened wall, the artery often also exhibits medial attenuation; vascular outpouchings can develop in these portions of the vessel and sometimes rupture.
Blood Pressure Regulation
Systemic and local blood pressure must be maintained within a narrow range to prevent adverse outcomes. Low blood pressure (hypotension) results in inadequate organ perfusion, organ dysfunction, and sometimes tissue death. Conversely, high blood pressure (hypertension) causes vessel and end-organ damage and is one of the major risk factors for atherosclerosis (see later on).
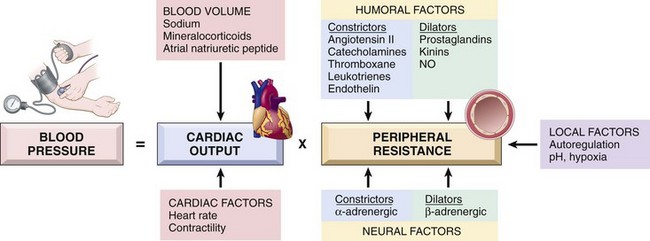
Blood pressure is a function of cardiac output and peripheral vascular resistance, both of which are influenced by multiple genetic and environmental factors (Fig. 9–3). The integration of the various inputs ensures adequate systemic perfusion, despite regional demand differences.
• Cardiac output is a function of stroke volume and heart rate. The most important determinant of stroke volume is the filling pressure, which is regulated through sodium homeostasis and its effect on blood volume. Heart rate and myocardial contractility (a second factor affecting stroke volume) are both regulated by the α- and β-adrenergic systems (in addition to their effects on vascular tone).
• Peripheral resistance is regulated predominantly at the level of the arterioles by neural and hormonal inputs. Vascular tone reflects a balance between vasoconstrictors (including angiotensin II, catecholamines, and endothelin) and vasodilators (including kinins, prostaglandins, and NO). Resistance vessels also exhibit autoregulation, whereby increased blood flow induces vasoconstriction to protect tissues against hyperperfusion. Finally, blood pressure is fine-tuned by tissue pH and hypoxia to accommodate local metabolic demands.
Factors released from the kidneys, adrenals, and myocardium interact to influence vascular tone and to regulate blood volume by adjusting sodium balance (Fig. 9–4). The kidneys filter 170 liters of plasma containing 23 moles of salt daily. Thus, with a typical diet containing 100 mEq of sodium, 99.5% of the filtered salt must be reabsorbed to maintain total body sodium levels. About 98% of the filtered sodium is reabsorbed by several constitutively active transporters. Recovery of the remaining 2% of sodium occurs by way of the epithelial sodium channel (ENaC), which is tightly regulated by the renin–angiotensin system; it is this pathway that determines net sodium balance.
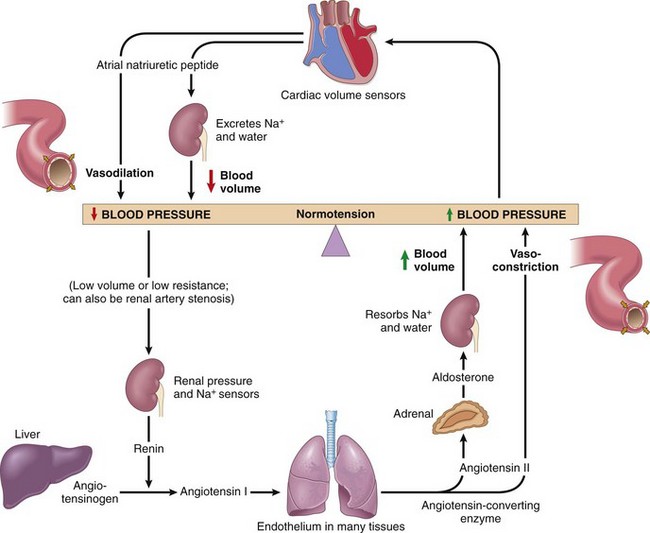
Figure 9–4 Interplay of renin, angiotensin, aldosterone, and atrial natriuretic peptide in blood pressure regulation (see text).
Kidneys influence peripheral resistance and sodium excretion/retention primarily through the renin–angiotensin system. The kidneys and heart contain cells that sense changes in blood pressure or blood volume. In response, these cells release several important regulators that act in concert to maintain normal blood pressure, as follows:
• Renin is a proteolytic enzyme produced by renal juxtaglomerular cells, myoepithelial cells that surround the glomerular afferent arterioles. Renin is released in response to low blood pressure in afferent arterioles, elevated levels of circulating catecholamines, or low sodium levels in the distal convoluted renal tubules. The latter occurs when the glomerular filtration rate falls (e.g., when the cardiac output is low), leading to increased sodium resorption by the proximal tubules and lower sodium levels more distally.
• Renin cleaves plasma angiotensinogen to angiotensin I, which in turn is converted to angiotensin II by angiotensin-converting enzyme (ACE) in the periphery. Angiotensin II raises blood pressure by (1) inducing vascular smooth muscle cell contraction, (2) stimulating aldosterone secretion by the adrenal gland, and (3) increasing tubular sodium resorption.
• The kidney also produces a variety of vascular relaxing substances (including prostaglandins and NO) that presumably counterbalance the vasopressor effects of angiotensin.
• Adrenal aldosterone increases blood pressure by its effect on blood volume; aldosterone increases sodium resorption (and thus water) in the distal convoluted tubule while also driving potassium excretion into the urine.
• Myocardial natriuretic peptides are released from atrial and ventricular myocardium in response to volume expansion; these inhibit sodium resorption in the distal renal tubules, thus leading to sodium excretion and diuresis. They also induce systemic vasodilation.
![]() Summary
Summary
Blood Pressure Regulation
• Blood pressure is determined by vascular resistance and cardiac output.
• Vascular resistance is regulated at the level of the arterioles, influenced by neural and hormonal inputs.
• Cardiac output is determined by heart rate and stroke volume, which is strongly influenced by blood volume. Blood volume in turn is regulated mainly by renal sodium excretion or resorption.
• Renin, a major regulator of blood pressure, is secreted by the kidneys in response to decreased blood pressure in afferent arterioles. In turn, renin cleaves angiotensinogen to angiotensin I; subsequent peripheral catabolism produces angiotensin II, which regulates blood pressure by increasing vascular smooth muscle cell tone and by increasing adrenal aldosterone secretion and, consequently, renal sodium resorption.
Hypertensive Vascular Disease
Hypertension is a major health problem in the developed world. Although it occasionally manifests in an acute aggressive form, high blood pressure is much more often asymptomatic for many years. This insidious condition is sometimes referred to as benign hypertension, but it is in fact far from harmless. Besides increasing the risk of stroke and atherosclerotic coronary heart disease, hypertension can lead to cardiac hypertrophy and heart failure (hypertensive heart disease), aortic dissection, multi-infarct dementia, and renal failure. While the molecular pathways of blood pressure regulation are reasonably well understood, the mechanisms leading to hypertension in the vast majority of affected persons remain unknown. The accepted wisdom is that such “essential hypertension” results from the interplay of genetic polymorphisms (which individually might be inconsequential) and environmental factors, which conspire to increase blood volume and/or peripheral resistance.
Epidemiology of Hypertension
Like height and weight, blood pressure is a continuously distributed variable, and the detrimental effects increase continuously as the pressure rises; no rigidly defined threshold reliably predicts who will suffer ill effects. Nevertheless, sustained diastolic pressures greater than 90 mm Hg, or sustained systolic pressures in excess of 140 mm Hg, are associated with an increased risk of atherosclerosis and are therefore used as cutoffs in diagnosing hypertension in clinical practice. By these criteria, some 25% of persons in the general population are hypertensive. As noted however, these values are somewhat arbitrary, and in patients with other cardiovascular risk factors (e.g., diabetes), lower thresholds may be applicable. The prevalence of pathologic effects of high blood pressure increases with age and is also higher in African Americans. Without appropriate treatment, some 50% of hypertensive patients die of ischemic heart disease (IHD) or congestive heart failure, and another third succumb to stroke. Reduction of blood pressure dramatically reduces the incidence and clinical sequelae (including death) of all forms of hypertension-related disease. Indeed, detection and treatment of asymptomatic hypertension constitute one of the few instances in which “preventive medicine” has a major demonstrated health benefit.
A small percentage of hypertensive patients (approximately 5%) present with a rapidly rising blood pressure that, if untreated, leads to death in within 1 to 2 years. Such malignant hypertension usually is severe (i.e., systolic pressures over 200 mm Hg or diastolic pressures over 120 mm Hg) and associated with renal failure and retinal hemorrhages, with or without papilledema. It can arise de novo but most commonly is superimposed on preexisting benign hypertension.
![]() Pathogenesis
Pathogenesis
Table 9–2 lists the major causes of hypertension, but most cases (95%) are idiopathic (essential hypertension). This form is compatible with long life unless a myocardial infarction, stroke, or another complication supervenes. Most of the remaining cases (secondary hypertension) are due to primary renal disease, renal artery narrowing (renovascular hypertension), or adrenal disorders. Several relatively rare single-gene disorders cause hypertension (and hypotension) by affecting renal sodium resorption. Such disorders include
• Gene defects in enzymes involved in aldosterone metabolism (e.g., aldosterone synthase, 11β-hydroxylase, 17α-hydroxylase), leading to increased aldosterone secretion, increased salt and water resorption, and plasma volume expansion
• Mutations in proteins that affect sodium resorption (as in Liddle syndrome, which is caused by mutations in ENaC, leading to increased distal tubular resorption of sodium induced by aldosterone)
Table 9–2 Types and Causes of Hypertension (Systolic and Diastolic)
Mechanisms of Essential Hypertension
Although the specific triggers are unknown, it appears that both altered renal sodium handling and increased vascular resistance contribute to essential hypertension.
• Reduced renal sodium excretion in the presence of normal arterial pressure probably is a key pathogenic feature; indeed, this is a common etiologic factor in most forms of hypertension. Decreased sodium excretion causes an obligatory increase in fluid volume and increased cardiac output, thereby elevating blood pressure (Fig. 9–3). At the new higher blood pressure, the kidneys excrete additional sodium. Thus, a new steady state of sodium excretion is achieved, but at the expense of an elevated blood pressure.
• Increased vascular resistance may stem from vasoconstriction or structural changes in vessel walls. These are not necessarily independent factors, as chronic vasoconstriction may result in permanent thickening of the walls of affected vessels.
• Genetic factors play an important role in determining blood pressure, as shown by familial clustering of hypertension and by studies of monozygotic and dizygotic twins. Hypertension has been linked to specific angiotensinogen polymorphisms and angiotensin II receptor variants; polymorphisms of the renin-angiotensin system also may contribute to the known racial differences in blood pressure regulation. Susceptibility genes for essential hypertension in the larger population are currently unknown but probably include those that govern renal sodium handling, pressors, and smooth muscle cell growth.
• Environmental factors, such as stress, obesity, smoking, physical inactivity, and high levels of salt consumption, modify the impact of genetic determinants. Evidence linking dietary sodium intake with the prevalence of hypertension in different population groups is particularly strong.
![]() Morphology
Morphology
Hypertension not only accelerates atherogenesis but also causes degenerative changes in the walls of large and medium-sized arteries that can lead to aortic dissection and cerebrovascular hemorrhage. Two forms of small blood vessel disease are hypertension-related: hyaline arteriolosclerosis and hyperplastic arteriolosclerosis (Fig. 9–5).
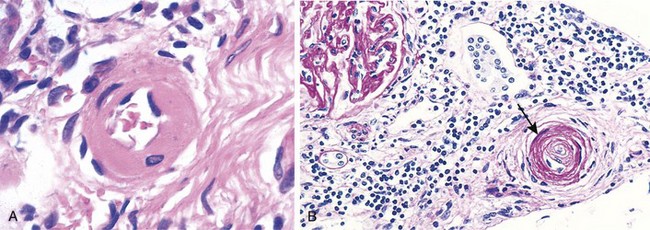
Figure 9–5 Hypertensive vascular disease. A, Hyaline arteriolosclerosis. The arteriolar wall is thickened with the deposition of amorphous proteinaceous material (hyalinized), and the lumen is markedly narrowed. B, Hyperplastic arteriolosclerosis (“onion-skinning”) (arrow) causing luminal obliteration (periodic acid–Schiff stain).
(Courtesy of Helmut Rennke, MD, Brigham and Women’s Hospital, Boston, Massachusetts.)
Hyaline arteriolosclerosis is associated with benign hypertension. It is marked by homogeneous, pink hyaline thickening of the arteriolar walls, with loss of underlying structural detail, and luminal narrowing (Fig. 9–5, A). The lesions stem from leakage of plasma components across injured endothelial cells, into vessel walls and increased ECM production by smooth muscle cells in response to chronic hemodynamic stress. In the kidneys, the arteriolar narrowing caused by hyaline arteriosclerosis leads to diffuse vascular compromise and nephrosclerosis (glomerular scarring). Although the vessels of elderly patients (normo- or hypertensive) show the same changes, hyaline arteriolosclerosis is more generalized and severe in patients with hypertension. The same lesions also are common in diabetic microangiopathy; in this disorder, the underlying etiology is hyperglycemia-associated endothelial cell dysfunction.
Hyperplastic arteriolosclerosis is more typical of severe hypertension. Vessels exhibit “onionskin,” concentric, laminated thickening of arteriolar walls and luminal narrowing (Fig. 9–5, B). The laminations consist of smooth muscle cells and thickened, reduplicated basement membrane. In malignant hypertension these changes are accompanied by fibrinoid deposits and vessel wall necrosis (necrotizing arteriolitis), which are particularly prominent in the kidney.
![]() Summary
Summary
Hypertension
• Hypertension is a common disorder affecting 25% of the population; it is a major risk factor for atherosclerosis, congestive heart failure, and renal failure.
• Essential hypertension represents 95% of cases and is a complex, multifactorial disorder, involving both environmental influences and genetic polymorphisms that may influence sodium resorption, aldosterone pathways, and the renin–angiotensin system.
• Hypertension occasionally is caused by single-gene disorders or is secondary to diseases of the kidney, adrenal, or other endocrine organs.
Vascular Wall Response to Injury
Fundamental to a wide variety of vascular disorders is injury to the vessel wall, in particular endothelial cells. Such injurious stimuli may be biochemical, immunologic, or hemodynamic. As the main cellular components of the blood vessel walls, endothelial cells and smooth muscle cells play central roles in vascular pathology. The integrated function of these cells is critical for the vasculature to respond to various stimuli, and its responses can be adaptive or lead to pathologic lesions. Thus, endothelial injury or dysfunction (see earlier discussion) contributes to a host of pathologic processes including thrombosis, atherosclerosis, and hypertensive vascular lesions. Smooth muscle cell proliferation and matrix synthesis can help to repair a damaged vessel wall but also can lead to luminal occlusion.
Intimal Thickening: A Stereotypical Response to Vascular Injury
Vascular injury leading to endothelial cell loss or dysfunction stimulates smooth muscle cell growth and associated matrix synthesis. Healing of injured vessels involves the migration of smooth muscle cells or smooth muscle cell precursor cells into the intima. Here these cells proliferate, and synthesize ECM in much the same way that fibroblasts fill in a wound (Fig. 9–6), forming a neointima that typically is covered by an intact endothelial cell layer. This neointimal response occurs with any form of vascular damage or dysfunction, including infection, inflammation, immune injury, physical trauma (e.g., from a balloon catheter or hypertension), or toxic exposure (e.g. oxidized lipids or cigarette smoke). Thus, intimal thickening is a stereotypical response of the vessel wall to any insult.
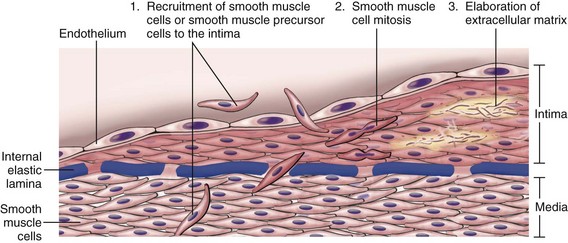
Figure 9–6 Stereotypical response to vascular injury. Schematic diagram of intimal thickening, emphasizing intimal smooth muscle cell migration and proliferation associated with extracellular matrix synthesis. Intimal smooth muscle cells may derive from the underlying media or may be recruited from circulating precursors; they are depicted in a color different from that of the medial smooth muscle cells, to emphasize their distinct phenotype.
Of note, the phenotype of neointimal smooth muscle cells is distinct from medial smooth muscle cells; neointimal smooth muscle cells lack the capacity to contract like medial smooth muscle cells, but do have the capacity to divide and have a considerably greater synthetic capacity than their medial colleagues. Although neointimal cells were previously thought to arise from dedifferentiated medial smooth muscle cells, increasing evidence suggests that at least a subset is derived from circulating precursor cells. The migratory, proliferative, and synthetic activities of the intimal smooth muscle cells are regulated by growth factors and cytokines produced by platelets, endothelial cells, and macrophages, as well as by activated coagulation and complement factors (as described previously).
With restoration and/or normalization of the endothelial cell layer, intimal smooth muscle cells can return to a nonproliferative state, but not before the healing response produces irreversible intimal thickening. With persistent or recurrent insults, further thickening can occur that leads to the stenosis of small and medium-sized blood vessels (e.g., as in atherosclerosis, discussed later). As a final note, it is also important to recognize that intimal thickening appears to be a part of normal aging. Such age-related intimal change typically is of no consequence, in part because compensatory outward remodeling of the vessel results in little net change in the luminal diameter.
Arteriosclerosis
Arteriosclerosis literally means “hardening of the arteries”; it is a generic term reflecting arterial wall thickening and loss of elasticity. Three distinct types are recognized, each with different clinical and pathologic consequences:
• Arteriolosclerosis affects small arteries and arterioles and may cause downstream ischemic injury. The two variants, hyaline and hyperplastic arteriolosclerosis, were described above in relation to hypertension.
• Mönckeberg medial sclerosis is characterized by the presence of calcific deposits in muscular arteries, typically in persons older than 50. The lesions do not encroach on the vessel lumen and usually are not clinically significant.
• Atherosclerosis, from Greek root words for “gruel” and “hardening,” is the most frequent and clinically important pattern and is the subject of the next section.
Atherosclerosis
Atherosclerosis is characterized by the presence of intimal lesions called atheromas (or atheromatous or atherosclerotic plaques). Atheromatous plaques are raised lesions composed of soft grumous lipid cores (mainly cholesterol and cholesterol esters, with necrotic debris) covered by fibrous caps (Fig. 9–7). Atherosclerotic plaques can mechanically obstruct vascular lumina and are prone to rupture, resulting in catastrophic vessel thrombosis. Plaques also weaken the underlying media, sometimes leading to aneurysm formation. In the Western world, morbidity and mortality rates for atherosclerosis are higher than for any other disorder, with roughly half of all deaths attributable to this entity. Because coronary artery disease is an important manifestation of atherosclerosis, epidemiologic data related to atherosclerosis mortality typically reflect deaths caused by ischemic heart disease (IHD) (Chapter 10); indeed, myocardial infarction is responsible for almost one fourth of all deaths in the United States.
Epidemiology of Atherosclerosis
Atherosclerosis is virtually ubiquitous among most developed nations but is much less prevalent in Central and South America, Africa, and parts of Asia. The mortality rate for IHD in the United States is among the highest in the world, approximately five times higher than that in Japan. However, IHD is increasing in Japan, where it is now the second leading cause of death. Furthermore, Japanese emigrants who come to the United States and adopt American life styles and dietary customs acquire the same atherosclerosis risk as for U.S.-born persons, emphasizing the important etiologic role of environmental factors.
The prevalence and severity of atherosclerosis and IHD have been correlated with a number of risk factors in several prospective analyses (e.g., the Framingham Heart Study); some of these risk factors are constitutional (and therefore less controllable) but others are acquired or related to modifiable behaviors (Table 9–3). These risk factors have roughly multiplicative effects. Thus, two factors increase the risk of myocardial infarction approximately four-fold, and three (i.e., hyperlipidemia, hypertension, and smoking), increase the rate by a factor of 7 (Fig. 9–8).
Table 9–3 Major Risk Factors for Atherosclerosis
| Nonmodifiable (Constitutional) |
| Modifiable |
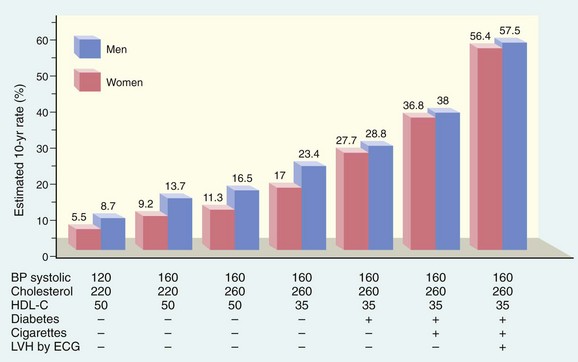
Figure 9–8 Estimated 10-year risk of coronary artery disease in 55-year-old men and women as a function of established risk factors—hyperlipidemia, hypertension, smoking, and diabetes. BP, blood pressure; ECG, electrocardiogram; HDL-C, high-density lipoprotein cholesterol; LVH, left ventricular hypertrophy.
(Data from O’Donnell CJ, Kannel WB: Cardiovascular risks of hypertension: lessons from observational studies. J Hypertension 16[Suppl 6]:3, 1998.)
Constitutional Risk Factors
• Genetics. Family history is the most important independent risk factor for atherosclerosis. Certain mendelian disorders are strongly associated with atherosclerosis (e.g., familial hypercholesterolemia) (Chapter 6), but these account for only a small percentage of cases. Most familial risk is related to polygenic traits that go hand-in-hand with atherosclerosis, such as hypertension and diabetes, as well as other genetic polymorphisms.
• Age. Atherosclerosis usually remains clinically silent until lesions reach a critical threshold in middle age or later. Thus, the incidence of myocardial infarction increases five-fold between the ages of 40 and 60. Death rates from IHD continue to rise with each successive decade.
• Gender. All other factors being equal, premenopausal women are relatively protected against atherosclerosis (and its consequences) compared with age-matched men. Thus, myocardial infarction and other complications of atherosclerosis are uncommon in premenopausal women in the absence of other predisposing factors such as diabetes, hyperlipidemia, or severe hypertension. After menopause, however, the incidence of atherosclerosis-related diseases increases and, in old age, even exceeds that in men. Although a salutary effect of estrogen has long been proposed to explain this gender difference, clinical trials have shown no benefit of hormonal therapy for prevention of vascular disease. Indeed, postmenopausal estrogen replacement appears to increase cardiovascular risk. In addition to atherosclerosis, gender also influences other factors that can affect outcome in patients with IHD, such as hemostasis, infarct healing, and myocardial remodeling.
Modifiable Major Risk Factors
• Hyperlipidemia—and, more specifically, hypercholesterolemia—is a major risk factor for development of atherosclerosis and is sufficient to induce lesions in the absence of other risk factors. The main cholesterol component associated with increased risk is low-density lipoprotein (LDL) cholesterol (“bad cholesterol”); LDL distributes cholesterol to peripheral tissues. By contrast, high-density lipoprotein (HDL) (“good cholesterol”) mobilizes cholesterol from developing and existing vascular plaques and transports it to the liver for biliary excretion. Consequently, higher levels of HDL correlate with reduced risk.
Recognition of these relationships has spurred the development of dietary and pharmacologic interventions that lower total serum cholesterol or LDL, and/or raise serum HDL, as follows:
 High dietary intake of cholesterol and saturated fats (present in egg yolks, animal fats, and butter, for example) raises plasma cholesterol levels. Conversely, diets low in cholesterol, and/or containing higher ratios of polyunsaturated fats, lower plasma cholesterol levels.
High dietary intake of cholesterol and saturated fats (present in egg yolks, animal fats, and butter, for example) raises plasma cholesterol levels. Conversely, diets low in cholesterol, and/or containing higher ratios of polyunsaturated fats, lower plasma cholesterol levels. Omega-3 fatty acids (abundant in fish oils) are beneficial, whereas (trans)-unsaturated fats produced by artificial hydrogenation of polyunsaturated oils (used in baked goods and margarine) adversely affect cholesterol profiles.
Omega-3 fatty acids (abundant in fish oils) are beneficial, whereas (trans)-unsaturated fats produced by artificial hydrogenation of polyunsaturated oils (used in baked goods and margarine) adversely affect cholesterol profiles. Exercise and moderate consumption of ethanol raise HDL levels, whereas obesity and smoking lower them.
Exercise and moderate consumption of ethanol raise HDL levels, whereas obesity and smoking lower them. Statins are a widely used class of drugs that lower circulating cholesterol levels by inhibiting hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in hepatic cholesterol biosynthesis.
Statins are a widely used class of drugs that lower circulating cholesterol levels by inhibiting hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in hepatic cholesterol biosynthesis.• Hypertension (see earlier discussion) is another major risk factor for development of atherosclerosis. On its own, hypertension can increase the risk of IHD by approximately 60% (see Fig. 9–8). Hypertension also is the major cause of left ventricular hypertrophy (LVH), which also can contribute to myocardial ischemia (see Fig. 9–8).
• Cigarette smoking is a well-established risk factor in men and probably accounts for the increasing incidence and severity of atherosclerosis in women. Prolonged (years) smoking of one or more packs of cigarettes a day doubles the rate of IHD-related mortality, while smoking cessation reduces the risk.
• Diabetes mellitus is associated with raised circulating cholesterol levels and markedly increases the risk of atherosclerosis. Other factors being equal, the incidence of myocardial infarction is twice as high in diabetics as in nondiabetics. In addition, this disorder is associated with an increased risk of stroke and a 100-fold increase in atherosclerosis-induced gangrene of the lower extremities.
Additional Risk Factors
Roughly 20% of cardiovascular events occur in the absence of identifiable risk factors. For example, in previously healthy women more than 75% of cardiovascular events occur in those with LDL cholesterol levels below 160 mg/dL (a cut-off value generally considered to connote low risk). Other factors that contribute to risk include the following:
• Inflammation. Inflammatory cells are present during all stages of atheromatous plaque formation and are intimately linked with plaque progression and rupture (see following discussion). With increasing recognition of the role of inflammation, measures of systemic inflammation have become important in risk stratification. While several systemic markers of inflammation correlate with IHD risk, determination of C-reactive protein (CRP) has emerged as one of the simplest and most sensitive.
• CRP levels. CRP, a member of pentraxin family, is an acute-phase reactant synthesized primarily by the liver in response to a variety of inflammatory cytokines. Locally, CRP secreted by cells within atherosclerotic plaques can activate endothelial cells, increasing adhesiveness and inducing a prothrombotic state. Its clinical importance lies in its value as a circulating biomarker: CRP levels strongly and independently predict the risk of myocardial infarction, stroke, peripheral arterial disease, and sudden cardiac death, even among apparently healthy persons (Fig. 9–9). While there is no direct evidence that lowering CRP diminishes cardiovascular risk, it is of interest that CRP is reduced by smoking cessation, weight loss, and exercise. Moreover, statins reduce CRP levels independent of their LDL cholesterol-lowering effects, suggesting a possible anti-inflammatory action of these agents.
• Hyperhomocysteinemia. Serum homocysteine levels correlate with coronary atherosclerosis, peripheral vascular disease, stroke, and venous thrombosis. Homocystinuria, due to rare inborn errors of metabolism, causes elevated circulating homocysteine (greater than 100 µmol/L) and is associated with early-onset vascular disease. Although low folate and vitamin B12 levels can increase homocysteine levels, supplemental vitamin ingestion does not affect the incidence of cardiovascular disease.
• Metabolic syndrome. Associated with central obesity (Chapter 7), this clinical entity is characterized by insulin resistance, hypertension, dyslipidemia (elevated LDL and depressed HDL), hypercoagulability, and a pro-inflammatory state, which may be triggered by cytokines released from adipocytes. The dyslipidemia, hyperglycemia, and hypertension are all cardiac risk factors, while the systemic hypercoagulable and pro-inflammatory state may contribute to endothelial dysfunction and/or thrombosis.
• Lipoprotein(a) levels. Lipoprotein(a) is an LDL-like particle that contains apolipoprotein B-100 linked to apolipoprotein A. Lipoprotein(a) levels are correlated with coronary and cerebrovascular disease risk, independent of total cholesterol or LDL levels.
• Elevated levels of procoagulants are potent predictors of risk for major cardiovascular events. Excessive activation of thrombin, which you will recall initiates inflammation through cleavage of protease-activated receptors (PARs) on leukocytes, endothelium, and other cells, may be particularly atherogenic.
• Other factors associated with difficult-to-quantify risks include lack of exercise and living a competitive, stressful life style (“type A personality”).
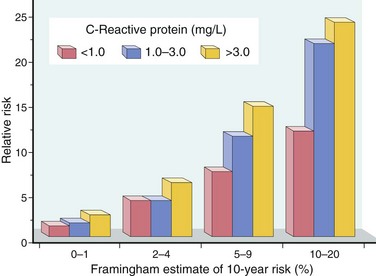
Figure 9–9 Prognostic value of C-reactive protein (CRP) in coronary artery disease. Relative risk (y-axis) reflects the risk of a cardiovascular event (e.g., myocardial infarction). The x-axis shows the 10-year risk of a cardiovascular event calculated from the traditional risk factors identified in the Framingham Study. In each risk group, CRP levels further stratify the patients.
(Data from Ridker PM, et al: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347:1557, 2002.)
![]() Pathogenesis
Pathogenesis
Historically, there have been two dominant theories regarding atherogenesis; one emphasizing intimal cellular proliferation in response to endothelial injury, and the other focusing on repeated formation and organization of thrombi. The contemporary view of atherogenesis incorporates elements of both theories and also integrates the risk factors previously discussed. Called the response-to-injury hypothesis, the model views atherosclerosis as a chronic inflammatory response of the arterial wall to endothelial injury. Lesion progression involves interaction of modified lipoproteins, monocyte-derived macrophages, T lymphocytes, and the cellular constituents of the arterial wall (Fig. 9–10). According to this model, atherosclerosis results from the following pathogenic events:
• Endothelial injury—and resultant endothelial dysfunction—leading to increased permeability, leukocyte adhesion, and thrombosis
• Accumulation of lipoproteins (mainly oxidized LDL and cholesterol crystals) in the vessel wall
• Monocyte adhesion to the endothelium, migration into the intima, and differentiation into macrophages and foam cells
• Lipid accumulation within macrophages, which release inflammatory cytokines
• Smooth muscle cell recruitment due to factors released from activated platelets, macrophages, and vascular wall cells
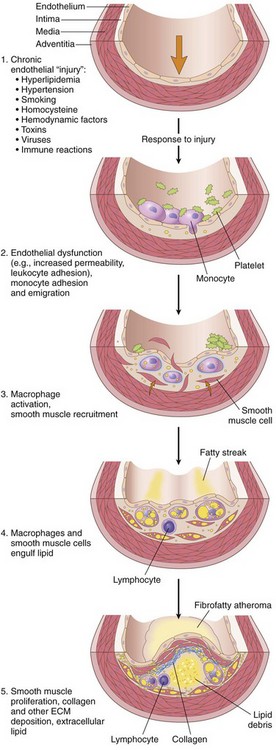
Figure 9–10 Response to injury in atherogenesis: 1, Normal. 2, Endothelial injury with monocyte and platelet adhesion. 3, Monocyte and smooth muscle cell migration into the intima, with macrophage activation. 4, Macrophage and smooth muscle cell uptake of modified lipids and further activation. 5, Intimal smooth muscle cell proliferation with ECM elaboration, forming a well-developed plaque.
Some details of these steps are presented next.
Endothelial Injury
Endothelial cell injury is the cornerstone of the response to injury hypothesis. Endothelial cell loss due to any kind of injury—induced experimentally by mechanical denudation, hemodynamic forces, immune complex deposition, irradiation, or chemicals—results in intimal thickening; in the presence of high-lipid diets, typical atheromas ensue. However, early human atherosclerotic lesions begin at sites of intact, but dysfunctional, endothelium. These dysfunctional endothelial cells exhibit increased permeability, enhanced leukocyte adhesion, and altered gene expression, all of which may contribute to the development of atherosclerosis.
Suspected triggers of early atheromatous lesions include hypertension, hyperlipidemia, toxins from cigarette smoke, homocysteine, and even infectious agents. Inflammatory cytokines (e.g., tumor necrosis factor [TNF]) also can stimulate proatherogenic patterns of endothelial cell gene expression. Nevertheless, the two most important causes of endothelial dysfunction are hemodynamic disturbances and hypercholesterolemia.
Hemodynamic Disturbances
The importance of hemodynamic factors in atherogenesis is illustrated by the observation that plaques tend to occur at ostia of exiting vessels, at branch points, and along the posterior wall of the abdominal aorta, where there is turbulent blood flow. In vitro studies further demonstrate that nonturbulent laminar flow leads to the induction of endothelial genes whose products protect against atherosclerosis. Such “atheroprotective” genes could explain the nonrandom localization of early atherosclerotic lesions.
Lipids
Lipids typically are transported in the bloodstream bound to specific apoproteins (forming lipoprotein complexes). Dyslipoproteinemias can result from mutations in genes that encode apoproteins or lipoprotein receptors, or from disorders that derange lipid metabolism, e.g., nephrotic syndrome, alcoholism, hypothyroidism, or diabetes mellitus. Common lipoprotein abnormalities in the general population (and indeed, present in many myocardial infarction survivors) include (1) increased LDL cholesterol levels, (2) decreased HDL cholesterol levels, and (3) increased levels of lipoprotein(a).
Several lines of evidence implicate hypercholesterolemia in atherogenesis:
• The dominant lipids in atheromatous plaques are cholesterol and cholesterol esters.
• Genetic defects in lipoprotein uptake and metabolism that cause hyperlipoproteinemia are associated with accelerated atherosclerosis. Thus, homozygous familial hypercholesterolemia, caused by defective LDL receptors and inadequate hepatic LDL uptake, can lead to myocardial infarction by age 20.
• Other genetic or acquired disorders (e.g., diabetes mellitus, hypothyroidism) that cause hypercholesterolemia lead to premature atherosclerosis.
• Epidemiologic analyses such as the famous Framingham study demonstrate a significant correlation between the severity of atherosclerosis and the levels of total plasma cholesterol or LDL.
• Lowering serum cholesterol by diet or drugs slows the rate of progression of atherosclerosis, causes regression of some plaques, and reduces the risk of cardiovascular events.
The mechanisms by which dyslipidemia contributes to atherogenesis include the following:
• Chronic hyperlipidemia, particularly hypercholesterolemia, can directly impair endothelial cell function by increasing local oxygen free radical production; among other things, oxygen free radicals accelerate NO decay, damping its vasodilator activity.
• With chronic hyperlipidemia, lipoproteins accumulate within the intima, where they are hypothesized to generate two pathogenic derivatives, oxidized LDL and cholesterol crystals. LDL is oxidized through the action of oxygen free radicals generated locally by macrophages or endothelial cells and ingested by macrophages through the scavenger receptor, resulting in foam cell formation. Oxidized LDL stimulates the local release of growth factors, cytokines, and chemokines, increasing monocyte recruitment, and also is cytotoxic to endothelial cells and smooth muscle cells. More recently, it has been shown that minute extracellular cholesterol crystals found in early atherosclerotic lesions serve as “danger” signals that activate innate immune cells such as monocytes and macrophages.
Inflammation
Inflammation contributes to the initiation, progression, and complications of atherosclerotic lesions. Normal vessels do not bind inflammatory cells. Early in atherogenesis, however, dysfunctional endothelial cells express adhesion molecules that promote leukocyte adhesion; vascular cell adhesion molecule-1 (VCAM-1), in particular, binds monocytes and T cells. After these cells adhere to the endothelium, they migrate into the intima under the influence of locally produced chemokines.
• Monocytes differentiate into macrophages and avidly engulf lipoproteins, including oxidized LDL and small cholesterol crystals. Cholesterol crystals appear to be particularly important instigators of inflammation through activation of the inflammasome and subsequent release of IL-1 (Chapter 2). Activated macrophages also produce toxic oxygen species that drive LDL oxidation and elaborate growth factors that stimulate smooth muscle cell proliferation.
• T lymphocytes recruited to the intima interact with the macrophages and also contribute to a state of chronic inflammation. It is not clear whether the T cells are responding to specific antigens (e.g., bacterial or viral antigens, heat-shock proteins [see further on], or modified arterial wall constituents and lipoproteins) or are nonspecifically activated by the local inflammatory milieu. Nevertheless, activated T cells in the growing intimal lesions elaborate inflammatory cytokines (e.g., IFN-γ), which stimulate macrophages, endothelial cells, and smooth muscle cells.
• As a consequence of the chronic inflammatory state, activated leukocytes and vascular wall cells release growth factors that promote smooth muscle cell proliferation and matrix synthesis.
Infection
There is circumstantial evidence linking infections to atherosclerosis. Herpesvirus, cytomegalovirus, and Chlamydia pneumoniae all have been found in atherosclerotic plaque, and seroepidemiologic studies show increased antibody titers to Chlamydia pneumoniae in patients with more severe atherosclerosis. Infections with these organisms, however, are exceedingly common (as is atherosclerosis), making it difficult to draw conclusions about causality. It also is important to recognize that atherosclerosis can be induced in germ-free mice, indicating that there is no obligate role for infection in the disease process.
Smooth Muscle Proliferation and Matrix Synthesis
Intimal smooth muscle cell proliferation and ECM deposition lead to conversion of the earliest lesion, a fatty streak, into a mature atheroma, thus contributing to the progressive growth of atherosclerotic lesions (Fig. 9–10). Intimal smooth muscle cells can originate from the media or from circulating precursors; regardless of their source, they have a proliferative and synthetic phenotype distinct from that of the underlying medial smooth muscle cells. Several growth factors are implicated in smooth muscle cell proliferation and matrix synthesis, including platelet-derived growth factor (released by locally adherent platelets, macrophages, endothelial cells, and smooth muscle cells), fibroblast growth factor, and TGF-α. The recruited smooth muscle cells synthesize ECM (most notably collagen), which stabilizes atherosclerotic plaques. However, activated inflammatory cells in atheromas also can cause intimal smooth muscle cell apoptosis and breakdown of matrix, leading to the development of unstable plaques (see later).
![]() Morphology
Morphology
Fatty Streaks
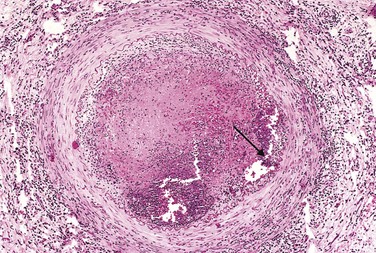
Fatty streaks begin as minute yellow, flat macules that coalesce into elongated lesions, 1 cm or more in length (Fig. 9–11). They are composed of lipid-filled foamy macrophages but are only minimally raised and do not cause any significant flow disturbance. Fatty streaks can appear in the aortas of infants younger than 1 year of age and are present in virtually all children older than 10 years, regardless of genetic, clinical, or dietary risk factors. The relationship of fatty streaks to atherosclerotic plaques is uncertain; although fatty streaks may evolve into plaques, not all are destined to progress. Nevertheless, it is notable that coronary fatty streaks form during adolescence at the same anatomic sites that are prone to plaques later in life.
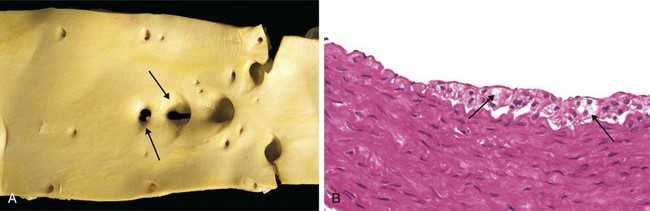
Figure 9–11 Fatty streaks. A, Aorta with fatty streaks (arrows), mainly near the ostia of branch vessels. B, Fatty streak in an experimental hypercholesterolemic rabbit, demonstrating intimal, macrophage-derived foam cells (arrow).
(B, Courtesy of Myron I. Cybulsky, MD, University of Toronto, Toronto, Ontario, Canada.)
Atherosclerotic Plaque
The key features of these lesions are intimal thickening and lipid accumulation (Fig. 9–7). Atheromatous plaques are white to yellow raised lesions; they range from 0.3 to 1.5 cm in diameter but can coalesce to form larger masses. Thrombus superimposed on ulcerated plaques imparts a red-brown color (Fig. 9–12).
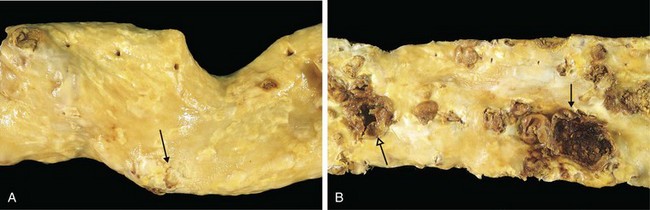
Figure 9–12 Atherosclerotic lesions. A, Aorta with mild atherosclerosis composed of fibrous plaques, one denoted by the arrow. B, Aorta with severe diffuse complicated lesions, including an ulcerated plaque (open arrow), and a lesion with overlying thrombus (closed arrow).
Atherosclerotic plaques are patchy, usually involving only a portion of any given arterial wall; on cross-section, therefore, the lesions appear “eccentric” (Fig. 9–13, A). The focal nature of atherosclerotic lesions may be related to the vagaries of vascular hemodynamics. Local flow disturbances, such as turbulence at branch points, make certain parts of a vessel wall especially susceptible to plaque formation.
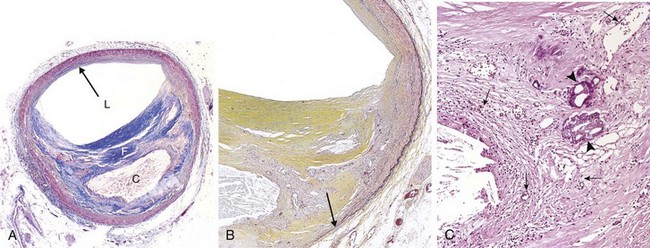
Figure 9–13 Atherosclerotic plaque in the coronary artery. A, Overall architecture demonstrating fibrous cap (F) and a central necrotic (largely lipid) core (C); collagen (blue) is stained with Masson trichrome. The lumen (L) is moderately narrowed by this eccentric lesion, which leaves part of the vessel wall unaffected (arrow). B, Moderate-power view of the plaque shown in A, stained for elastin (black); the internal and external elastic membranes are attenuated and the media of the artery is thinned under the most advanced plaque (arrow). C, High-power view of the junction of the fibrous cap and core, showing scattered inflammatory cells, calcification (arrowheads), and neovascularization (small arrows).
In descending order, the most extensively involved vessels are the infrarenal abdominal aorta, the coronary arteries, the popliteal arteries, the internal carotid arteries, and the vessels of the circle of Willis. Even in the same patient, atherosclerosis typically is more severe in the abdominal aorta than in the thoracic aorta. Vessels of the upper extremities usually are spared, as are the mesenteric and renal arteries, except at their ostia. Nevertheless, in any individual case, the severity of atherosclerosis in one artery does not predict its severity in another. Moreover, in any given vessel, lesions at various stages often coexist.
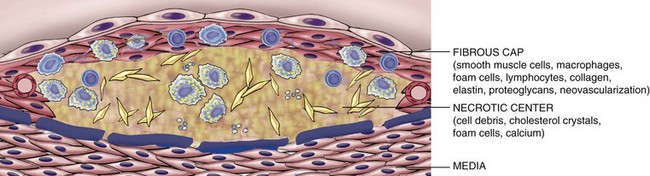
Atherosclerotic plaques have three principal components: (1) cells, including smooth muscle cells, macrophages, and T cells; (2) extracellular matrix, including collagen, elastic fibers, and proteoglycans; and (3) intracellular and extracellular lipid (Fig. 9–13, A and B). The proportion and configuration of each component varies from lesion to lesion. Most commonly plaques have a superficial fibrous cap composed of smooth muscle cells and relatively dense collagen. Where the cap meets the vessel wall (the “shoulder”) is a more cellular area containing macrophages, T cells, and smooth muscle cells. Deep to the fibrous cap is a necrotic core, containing lipid (primarily cholesterol and cholesterol esters), necrotic debris, lipid-laden macrophages and smooth muscle cells (foam cells), fibrin, variably organized thrombus, and other plasma proteins. The extracellular cholesterol frequently takes the forms of crystalline aggregates that are washed out during routine tissue processing, leaving behind empty “cholesterol clefts.” The periphery of the lesions shows neovascularization (proliferating small blood vessels) (Fig. 9–13, C). The media deep to the plaque may be attenuated and exhibit fibrosis secondary to smooth muscle atrophy and loss. Typical atheromas contain relatively abundant lipid, but some so-called fibrous plaques are composed almost exclusively of smooth muscle cells and fibrous tissue.
Plaques generally continue to change and progressively enlarge through cell death and degeneration, synthesis and degradation of ECM (remodeling), and thrombus organization. Atheromas also often undergo calcification (Fig. 9–10, C).
Clinical Consequences of Atherosclerotic Disease
Large elastic arteries (e.g., aorta, carotid, and iliac arteries) and large and medium-sized muscular arteries (e.g., coronary, renal, and popliteal arteries) are the vessels most commonly involved by atherosclerosis. Accordingly, atherosclerosis is most likely to present with signs and symptoms related to ischemia in the heart, brain, kidneys, and lower extremities. Myocardial infarction (heart attack), cerebral infarction (stroke), aortic aneurysms, and peripheral vascular disease (gangrene of extremities) are the major clinical consequences of atherosclerosis.
The natural history, principal morphologic features, and main pathogenic events are schematized in Figure 9–14. The principal pathophysiologic outcomes depend on the size of the affected vessel, the size and stability of the plaques, and the degree to which plaques disrupt the vessel wall:
• Occlusion of smaller vessels can compromise tissue perfusion.
• Plaque rupture can expose atherosclerotic debris, leading to acute (and frequently catastrophic) vascular thrombosis or (with shedding of debris) distal embolization.
• Destruction of the underlying vessel wall can lead to aneurysm formation, with secondary rupture and/or thrombosis.
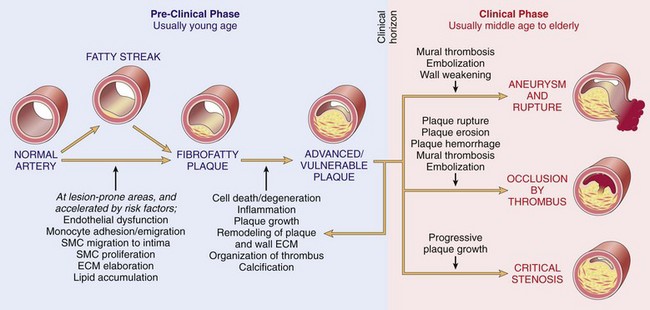
Figure 9–14 Summary of the natural history, morphologic features, main pathogenic events, and clinical complications of atherosclerosis.
Atherosclerotic Stenosis
At early stages, remodeling of the media tends to preserve the luminal diameter by increasing the vessel circumference. Owing to limits on remodeling, however, eventually the expanding atheroma may impinge on blood flow. Critical stenosis is the tipping point at which chronic occlusion limits flow so severely that tissue demand exceeds supply. In the coronary artery (and other) circulations, this typically occurs at approximately 70% fixed occlusion. At rest, affected patients have adequate cardiac perfusion, but with even modest exertion demand exceeds supply, and chest pain develops because of cardiac ischemia (stable angina) (see Chapter 10). The toll of chronic arterial hypoperfusion due to atherosclerosis in various vascular beds includes bowel ischemia, sudden cardiac death, chronic IHD, ischemic encephalopathy, and intermittent claudication (ischemic leg pain).
Acute Plaque Change
Plaque erosion or rupture typically triggers thrombosis, leading to partial or complete vascular obstruction and often tissue infarction (see Fig. 9–14). Plaque changes fall into three general categories:
• Rupture/fissuring, exposing highly thrombogenic plaque constituents
• Erosion/ulceration, exposing the thrombogenic subendothelial basement membrane to blood
It is now recognized that plaques responsible for myocardial infarctions and other acute coronary syndromes often are asymptomatic before the acute event, which superimposes thrombosis on a lesion that previously did not produce significant luminal occlusion. The worrisome conclusion is that large numbers of asymptomatic persons are at risk for a catastrophic coronary event. The causes of acute plaque change are complex and include both intrinsic (e.g., plaque structure and composition) and extrinsic factors (e.g., blood pressure). These factors combine to weaken the integrity of the plaque, making it unable to withstand vascular shear forces.
Certain types of plaques are believed to be at particularly high risk of rupturing. These include plaques that contain large numbers of foam cells and abundant extracellular lipid, plaques that have thin fibrous caps containing few smooth muscle cells, and plaques that contain clusters of inflammatory cells. Plaques at high risk for rupture are referred to as “vulnerable plaques” (Fig. 9–15). The fibrous cap also undergoes continuous remodeling; its mechanical strength and stability is proportional to its collagen content, so the balance of collagen synthesis and degradation affects cap integrity. Collagen in atherosclerotic plaques is synthesized primarily by smooth muscle cells, and loss of smooth muscle cells understandably results in cap weakening. Collagen is degraded by matrix metalloproteinases (MMPs), enzymes elaborated by macrophages within the atheromatous plaque; conversely, tissue inhibitors of metalloproteinases (TIMPs) produced by endothelial cells, smooth muscle cells, and macrophages, all act to dampen MMP activity.
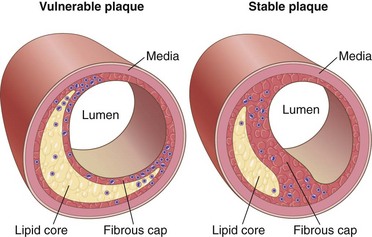
Figure 9–15 Vulnerable and stable atherosclerotic plaque. Stable plaques have densely collagenized and thickened fibrous caps with minimal inflammation and negligible underlying atheromatous cores, whereas vulnerable plaques have thin fibrous caps, large lipid cores, and increased inflammation.
(Adapted from Libby P: Circulation 91:2844, 1995.)
In general, plaque inflammation increases collagen degradation and reduces collagen synthesis, thereby destabilizing the mechanical integrity of the cap. Of interest, statins may have a beneficial effect not only by reducing circulating cholesterol levels but also by stabilizing plaques through a reduction in plaque inflammation.
Factors extrinsic to plaques also are important. Thus, adrenergic stimulation (as with intense emotions) can increase systemic blood pressure or induce local vasoconstriction, thereby increasing the mechanical stress on a given plaque. Indeed, one explanation for the pronounced circadian periodicity in the onset of heart attacks (peak incidence between 6 am and 12 noon) is the adrenergic surge associated with waking and rising, which is sufficient to cause blood pressure spikes and heightened platelet reactivity.
Fortunately, not all plaque ruptures result in occlusive thromboses with catastrophic consequences. In fact, silent plaque disruption and ensuing superficial platelet aggregation and thrombosis probably occur frequently and repeatedly in those with atherosclerosis. Healing of these subclinical plaque disruptions—and their overlying thromboses—is an important mechanism for atheroma enlargement.
![]() Morphology
Morphology
Atherosclerotic plaques are susceptible to several clinically important changes:
• Rupture, ulceration, or erosion of the luminal surface of atheromatous plaques exposes highly thrombogenic substances and induces thrombus formation. Thrombi may partially or completely occlude the lumen, leading to tissue ischemia (e.g., in the heart) (Chapter 10) (Fig. 9–16). If the patient survives, thrombi become organized and incorporated into the growing plaque.
• Hemorrhage into a plaque. Rupture of the overlying fibrous cap or of the thin-walled vessels in the areas of neovascularization can cause intra-plaque hemorrhage; the resulting hematoma may cause rapid plaque expansion or plaque rupture.
• Atheroembolism. Ruptured plaque can discharge debris into the blood, producing microemboli composed of plaque contents.
• Aneurysm formation. Atherosclerosis-induced pressure or ischemic atrophy of the underlying media, with loss of elastic tissue, causes structural weakening that can lead to aneurysmal dilation and rupture.
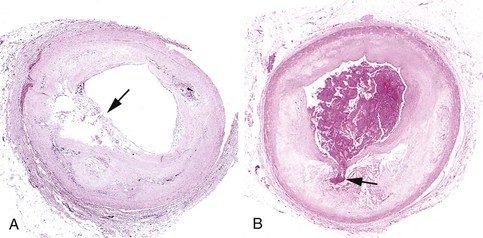
Figure 9–16 Atherosclerotic plaque rupture. A, Plaque rupture without superimposed thrombus, in a patient who died suddenly. B, Acute coronary thrombosis superimposed on an atherosclerotic plaque with focal disruption of the fibrous cap, triggering fatal myocardial infarction. In both A and B, an arrow points to the site of plaque rupture.
(B, Reproduced from Schoen FJ: Interventional and Surgical Cardiovascular Pathology: Clinical Correlations and Basic Principles. Philadelphia, WB Saunders, 1989, p 61.)
![]() Summary
Summary
Atherosclerosis
• Atherosclerosis is an intima-based lesion composed of a fibrous cap and an atheromatous (literally, “gruel-like”) core; the constituents of the plaque include smooth muscle cells, ECMs, inflammatory cells, lipids, and necrotic debris.
• Atherogenesis is driven by an interplay of vessel wall injury and inflammation. The multiple risk factors for atherosclerosis all cause endothelial cell dysfunction and influence smooth muscle cell recruitment and stimulation.
• Atherosclerotic plaques develop and grow slowly over decades. Stable plaques can produce symptoms related to chronic ischemia by narrowing vessels, whereas unstable plaques can cause dramatic and potentially fatal ischemic complications related to acute plaque rupture, thrombosis, or embolization.
• Stable plaques tend to have a dense fibrous cap, minimal lipid accumulation, and little inflammation, whereas “vulnerable” unstable plaques have thin caps, large lipid cores, and relatively dense inflammatory infiltrates.
Aneurysms and Dissections
Aneurysms are congenital or acquired dilations of blood vessels or the heart (Fig. 9–17). “True” aneurysms involve all three layers of the artery (intima, media, and adventitia) or the attenuated wall of the heart; these include atherosclerotic and congenital vascular aneurysms, as well as ventricular aneurysms resulting from transmural myocardial infarctions. By comparison, a false aneurysm (pseudoaneurysm) results when a wall defect leads to the formation of an extravascular hematoma that communicates with the intravascular space (“pulsating hematoma”). Examples are ventricular ruptures contained by pericardial adhesions and leaks at the junction of a vascular graft with a natural artery. In arterial dissections, pressurized blood gains entry to the arterial wall through a surface defect and then pushes apart the underlying layers. Aneurysms and dissections are important causes of stasis and subsequent thrombosis; they also have a propensity to rupture—often with catastrophic results.
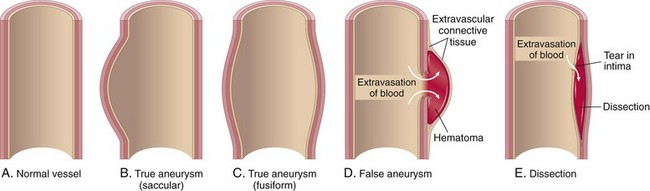
Figure 9–17 Aneurysms. A, Normal vessel. B, True aneurysm, saccular type. The wall bulges outward and may be attenuated but is otherwise intact. C, True aneurysm, fusiform type. There is circumferential dilation of the vessel. D, False aneurysm. The wall is ruptured, creating a collection of blood (hematoma) bounded externally by adherent extravascular tissues. E, Dissection. Blood has entered the wall of the vessel and separated (dissected) the layers.
Aneurysms can be classified by shape (see Fig. 9–17). Saccular aneurysms are discrete outpouchings ranging from 5 to 20 cm in diameter, often with a contained thrombus. Fusiform aneurysms are circumferential dilations up to 20 cm in diameter; these most commonly involve the aortic arch, the abdominal aorta, or the iliac arteries.
![]() Pathogenesis
Pathogenesis
Arteries are dynamic tissues that maintain their integrity through the ongoing synthesis, degradation, and repair of their extracellular matrix. Aneurysms occur when the structure or function of the connective tissue is compromised by any of the following factors:
• Inadequate or abnormal connective tissue synthesis. Several rare inherited diseases provide insight into the types of molecular abnormalities that can lead to aneurysm formation. As described previously, TGF-β regulates smooth muscle cell proliferation and matrix synthesis. Thus, mutations in TGF-β receptors or downstream signaling pathways result in defective elastin and collagen synthesis; aneurysms in affected persons often rupture, even when small. In Marfan syndrome (Chapter 6), defective synthesis of the scaffolding protein fibrillin leads to abnormal sequestration of TGF-β in the aortic wall, with subsequent dilation due to dysregulated signaling and progressive loss of elastic tissue. Defective type III collagen synthesis with aneurysm formation is a hallmark of the type IV Ehlers-Danlos syndrome (Chapter 6).
• Excessive connective tissue degradation. Increased MMP expression, such as by macrophages in atherosclerotic plaque, can contribute to aneurysm development by degrading arterial ECM in the arterial wall; similarly, decreased TIMP expression can also tip the balance toward net ECM degradation. A genetic predisposition to aneurysm formation in the setting of inflammation may be related to MMP and/or TIMP polymorphisms, or to the nature of the local inflammatory response that drives MMP or TIMP production.
• Loss of smooth muscle cells or change in the smooth muscle cell synthetic phenotype. Atherosclerotic thickening of the intima can cause ischemia of the inner media by increasing the diffusion distance from the lumen. Conversely, systemic hypertension can cause luminal narrowing of the aortic vasa vasorum, leading to ischemia of the outer media. Such ischemia results in smooth muscle cell loss as well as aortic “degenerative changes,” which include fibrosis (replacing distensible elastic tissue), inadequate ECM synthesis, and accumulation of increasing amounts of amorphous proteoglycans. Histologically, these changes are collectively called cystic medial degeneration (Fig. 9–18), although no true cysts are formed. Such changes are nonspecific; they can occur whenever ECM synthesis is defective, including in genetic disorders such as Marfan syndrome and metabolic syndrome such as scurvy.

Figure 9–18 Cystic medial degeneration. A, Cross-section of aortic media from a patient with Marfan syndrome, showing marked elastin fragmentation and areas devoid of elastin that resemble cystic spaces (asterisks). B, Normal media for comparison, showing the regular layered pattern of elastic tissue. In both A and B, elastin is stained black.
The two most important causes of aortic aneurysms are atherosclerosis and hypertension. Atherosclerosis is the more dominant factor in abdominal aortic aneurysms, while hypertension is associated with ascending aortic aneurysms. Other conditions that weaken vessel walls and lead to aneurysms include trauma, vasculitis (see later), congenital defects, and infections, so-called mycotic aneurysms. Mycotic aneurysms result from (1) embolization of a septic embolus, usually as a complication of infective endocarditis; (2) extension of an adjacent suppurative process; or (3) direct infection of an arterial wall by circulating organisms. Tertiary syphilis is a rare cause of aortic aneurysms. A predilection of the spirochetes for the vasa vasorum of the ascending thoracic aorta—and the subsequent immune response to them—results in an obliterative endarteritis that compromises blood flow to the media; the ensuing ischemic injury leads to aneurysmal dilation that occasionally also can involve the aortic valve annulus.
Abdominal Aortic Aneurysm
Atherosclerotic aneurysms occur most frequently in the abdominal aorta, but the common iliac arteries, aortic arch, and descending thoracic aorta can also be involved. Abdominal aortic aneurysm (AAA) occurs more frequently in men and in smokers and rarely develops before the age of 50 years. Atherosclerosis is a major cause of AAA, but other factors clearly contribute, since the incidence is less than 5% in men older than 60 despite almost universal abdominal aortic atherosclerosis in that population.
In the majority of cases, AAA results from excess ECM degradation mediated by local inflammatory infiltrates in atherosclerotic arteries and the destructive proteolytic enzymes produced at these sites. Atherosclerotic plaques compromise the diffusion of nutrients and wastes between the vascular lumen and the arterial wall, and also directly compress the underlying media. As a result, the media undergoes degeneration and necrosis, which results in arterial wall thinning. A familial predisposition to AAA, independent of genetic predilection to atherosclerosis or hypertension, may be a factor in some persons; thus, hereditary defects in structural components of the aorta can produce aneurysms (e.g., Marfan syndrome). Of note, the risks of AAA and smoking-related emphysema are associated, suggesting that some affected patients have a systemic dysregulation of ECM degradation.
![]() Morphology
Morphology
AAAs typically occur between the renal arteries and the aortic bifurcation; they can be saccular or fusiform and up to 15 cm in diameter and 25 cm in length (Fig. 9–19). In the vast majority of cases, extensive atherosclerosis is present, with thinning and focal destruction of the underlying media. The aneurysm sac usually contains bland, laminated, poorly organized mural thrombus, which can fill much of the dilated segment. Not infrequently, AAAs are accompanied by smaller iliac artery aneurysms.
• Inflammatory AAAs are a distinct subtype characterized by dense periaortic fibrosis containing abundant lymphoplasmacytic inflammation with many macrophages and giant cells.
• Mycotic AAAs occur when circulating microorganisms (as in bacteremia from a Salmonella gastroenteritis) seed the aneurysm wall or the associated thrombus; the resulting suppuration accelerates the medial destruction and may lead to rapid dilation and rupture.
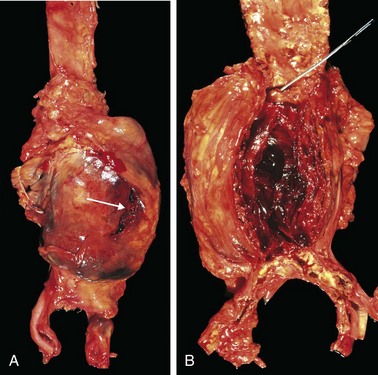
Figure 9–19 Abdominal aortic aneurysm. A, External view of a large aortic aneurysm that ruptured at the site is indicated by the arrow. B, Opened view, with the location of the rupture tract indicated by a probe. The wall of the aneurysm is attenuated, and the lumen is filled by a large, layered thrombus.
Clinical Consequences
The clinical consequences of AAA may include
• Obstruction of a vessel branching off the aorta (e.g., the renal, iliac, vertebral, or mesenteric arteries), resulting in distal ischemia of the kidneys, legs, spinal cord, or gastrointestinal tract, respectively
• Embolism from atheroma or mural thrombus
• Impingement on adjacent structures, e.g., compression of a ureter or erosion of vertebrae by the expanding aneurysm
• An abdominal mass (often palpably pulsating) that simulates a tumor
• Rupture into the peritoneal cavity or retroperitoneal tissues, leading to massive, often fatal hemorrhage
The risk of rupture is determined by size. AAAs 4 cm or less in diameter almost never burst, while those between 4 and 5 cm do so at a rate of 1% per year. The risk rises to 11% per year for AAAs 5 to 6 cm in diameter, and to 25% per year for aneurysms greater than 6 cm in diameter. Thus, aneurysms 5 cm in diameter or larger are managed surgically, either by open placement of tubular prosthetic grafts or with endoluminal insertion of stented grafts (expandable wire frames covered by a cloth sleeve). Timely intervention is critical, because the mortality rate for elective procedures is approximately 5%, whereas the rate for emergency surgery after rupture is roughly 50%.
A point worthy of emphasis is that because atherosclerosis is a systemic disease, a patient with AAA also is very likely to have atherosclerosis in other vascular beds and is at a significantly increased risk for IHD and stroke.
Thoracic Aortic Aneurysm
Thoracic aortic aneurysms most commonly are associated with hypertension and Marfan syndrome, with other disorders caused by mutations in TGF-β signaling pathway components also being increasingly recognized as etiologies. These aneurysms manifest with signs and symptoms referable to (1) encroachment on mediastinal structures (e.g., respiratory or feeding difficulties due to airway or esophageal compression, respectively); (2) persistent cough from irritation of the recurrent laryngeal nerves; (3) pain caused by erosion of bone (i.e., ribs and vertebral bodies); (4) cardiac disease due to valvular insufficiency or narrowing of the coronary ostia; and (5) aortic rupture. Rare patients with syphilitic aneurysms commonly die of heart failure induced by aortic valvular incompetence.
Aortic Dissection
Aortic dissection occurs when blood splays apart the laminar planes of the media to form a blood-filled channel within the aortic wall (Fig. 9–20); this development can be catastrophic if the dissecting blood ruptures through the adventitia and escapes into adjacent spaces. Aortic dissection need not be associated with aortic dilation, and the older term “dissecting aneurysm” should be avoided.
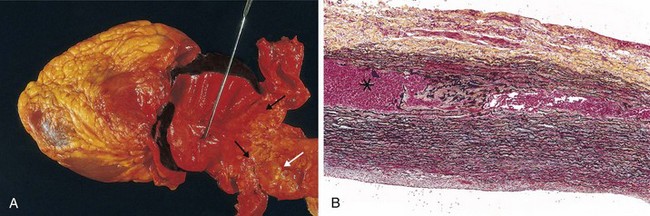
Figure 9–20 Aortic dissection. A, An opened aorta with a proximal dissection originating from a small, oblique intimal tear (identified by the probe) associated with an intramural hematoma. Note that the intimal tear occurred in a region largely free of atherosclerotic plaque. The distal edge of the intramural hematoma (black arrows) lies at the edge of a large area of atherosclerosis (white arrow), which arrested the propagation of the dissection. B, Histologic preparation showing the dissection and intramural hematoma (asterisk). Aortic elastic layers are black and blood is red in this section, stained with the Movat stain.
Aortic dissection occurs mainly in two age groups: (1) men aged 40 to 60 with antecedent hypertension (more than 90% of cases); and (2) younger patients with connective tissue abnormalities that affect the aorta (e.g., Marfan syndrome). Dissections also can be iatrogenic (e.g., complicating arterial cannulation during diagnostic catheterization or cardiopulmonary bypass). Rarely, for unknown reasons, pregnant women develop dissection of the aorta or its branches, including the coronary arteries. Dissection is unusual in the presence of substantial atherosclerosis or other causes of medial scarring, presumably because the medial fibrosis inhibits propagation of the dissecting hematoma (see Fig. 9–20).
![]() Pathogenesis
Pathogenesis
Hypertension is the major risk factor for aortic dissection. Aortas in hypertensive patients show medial hypertrophy of the vasa vasorum associated with degenerative changes in ECM and variable loss of medial smooth muscle cells, suggesting that diminished flow through the vasa vasorum is contributory. Most other dissections are related to heritable or acquired connective tissue disorders that give rise to abnormal aortic ECM, including Marfan syndrome, Ehlers-Danlos syndrome type IV, and defects in copper metabolism.
The trigger for the intimal tear and subsequent intramural hemorrhage is not known in most cases. Nevertheless, once the tear has occurred, blood under systemic pressure dissects through the media along laminar planes. Accordingly, aggressive pressure-reducing therapy may be effective in limiting an evolving dissection. In rare cases, disruption of the vasa vasorum can give rise to an intramural hematoma without an intimal tear.
![]() Morphology
Morphology
In most dissections, the intimal tear marking the point of origin is found in the ascending aorta within 10 cm of the aortic valve (Fig. 9–20, A). Such tears usually are transverse or oblique in orientation and 1 to 5 cm long, with sharp, jagged edges. The dissection plane can extend retrograde toward the heart or distally, occasionally as far as the iliac and femoral arteries, and usually lies between the middle and outer thirds of the media (Fig. 9–20, B).
External rupture causes massive hemorrhage, or results in cardiac tamponade if it occurs into the pericardial sac. In some (fortunate) instances, the dissecting hematoma reenters the lumen of the aorta through a second distal intimal tear, creating a second vascular channel within the media (so-called double-barreled aorta). Over time, such false channels become endothelialized to give rise to chronic dissections.
In most instances, no specific underlying causal pathology is identified in the aortic wall. The most frequent preexisting histologically detectable lesion is the cystic medial degeneration described previously; this is characterized by smooth muscle layer dropout and necrosis, elastic tissue fragmentation, and accumulations of amorphous proteoglycan-rich ECM (Fig. 9–18). Inflammation is characteristically absent. Recognizable medial damage appears to be neither a prerequisite for dissection nor a guarantee that dissection is imminent. Occasionally, dissections occur in the setting of rather trivial medial degeneration, while marked degenerative changes frequently are seen at autopsy in persons who exhibited no clinical manifestations in life.
Clinical Consequences
The clinical manifestations of dissection depend primarily on the portion of the aorta affected; the most serious complications occur with dissections involving the proximal aorta and arch. Thus, aortic dissections generally are classified into two types (Fig. 9–21):
• Proximal lesions: type A dissections, involving the ascending aorta, with or without involvement of the descending aorta (DeBakey type I or II, respectively)
• Distal lesions, usually beginning beyond the subclavian artery: type B dissections (DeBakey type III)
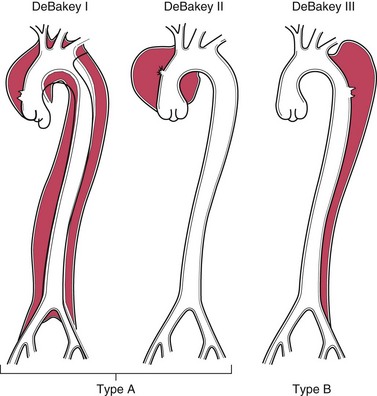
Figure 9–21 Classification of dissections. Type A (proximal) involves the ascending aorta, either as part of a more extensive dissection (DeBakey type I), or in isolation (DeBakey type II). Type B (distal, or DeBakey type III) dissections arise after the takeoff of the great vessels.
The classic clinical symptom of aortic dissection is the sudden onset of excruciating tearing or stabbing pain, usually beginning in the anterior chest, radiating to the back between the scapulae, and moving downward as the dissection progresses. The most common cause of death is rupture of the dissection into the pericardial, pleural, or peritoneal cavity. Retrograde dissection into the aortic root also can cause fatal disruption of the aortic valvular apparatus or compression of the coronary arteries. Common clinical presentations with cardiac involvement include tamponade, aortic insufficiency, and myocardial infarction. Other complications are related to extension of the dissection to the great arteries of the neck and the renal, mesenteric, or iliac arteries, any of which may become obstructed. Similarly, compression of spinal arteries can cause transverse myelitis.
In type A dissections, rapid diagnosis and institution of intensive antihypertensive therapy coupled with surgical plication of the aortic intimal tear can save 65% to 85% of the patients. However, the mortality rate approaches 70% in patients who present with hemorrhage or symptoms related to distal ischemia, and the overall 10-year survival rate is only 40% to 60%. Most type B dissections can be managed conservatively; patients have a 75% survival rate whether they are treated with surgery or with antihypertensive medication only.
![]() Summary
Summary
Aneurysms and Dissections
• Aneurysms are congenital or acquired dilations of the heart or blood vessels that involve the entire wall thickness. Complications are related to rupture, thrombosis, and embolization.
• Dissections occur when blood enters the wall of a vessel and separates the various layers. Complications arise as a result of rupture or obstruction of vessels branching off the aorta.
• Aneurysms and dissections result from structural weakness of the vessel wall caused by loss of smooth muscle cells or insufficient extracellular matrix, which can be a consequence of ischemia, genetic defects, or defective matrix remodeling.
Vasculitis
Vasculitis is a general term for vessel wall inflammation. The possible clinical manifestations are protean, but largely depend on the specific vascular bed that is affected. Besides findings referable to the involved tissue(s), there are usually also signs and symptoms of systemic inflammation, such as fever, myalgia, arthralgias, and malaise.
Although several forms of vasculitis have a predilection for relatively large vessels (e.g., large or medium-sized muscular arteries), most affect small vessels (arterioles, capillaries, and venules). Some 20 primary forms of vasculitis are recognized, and classification schemes attempt (with varying success) to group them according to vessel size, role of immune complexes, presence of specific autoantibodies, granuloma formation, tissue tropism, and other, poorly defined criteria (Fig. 9–22). As we shall see, there is considerable clinical and pathologic overlap among many of these disorders.
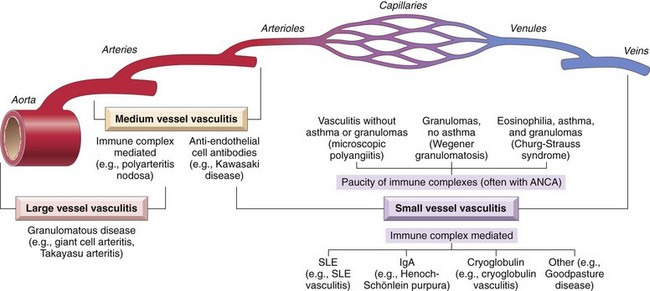
Figure 9–22 Vascular sites involved in the more common vasculitides and their presumptive etiology. Note the considerable overlap in distributions. ANCA, anti-neutrophil cytoplasmic antibody; SLE, systemic lupus erythematosus.
(Data from Jennette JC, Falk RJ: Nosology of primary vasculitis. Curr Opin Rheumatol 19:17, 2007.)
The two most common pathogenic mechanisms of vasculitis are immune-mediated inflammation and direct vascular invasion by infectious pathogens. Infections also can indirectly precipitate immune-mediated vasculitis (e.g., by generating immune complexes or triggering cross-reactivity). In any given patient, it is critical to distinguish between infectious and immunologic mechanisms because immunosuppressive therapy is appropriate for immune-mediated vasculitis but could exacerbate infectious vasculitis. Physical and chemical injury, including that due to radiation, mechanical trauma, and toxins, also can cause vasculitis.
Noninfectious Vasculitis
The main immunologic mechanisms underlying noninfectious vasculitis are
Immune Complex–Associated Vasculitis
This form of vasculitis is seen in immunologic disorders such as systemic lupus erythematosus (Chapter 4) that are associated with autoantibody production. The vascular lesions resemble those found in experimental immune complex–mediated disorders, such as the Arthus phenomenon and serum sickness, and in many cases contain readily identifiable antibody and complement. Often, however, this type of vasculitis is a diagnostic challenge. Only rarely is the specific antigen responsible for immune complex formation known. While immune complexes are occasionally detected in the blood, in most instances it is not clear whether the pathogenic antigen-antibody complexes are deposited from the circulation or form in situ. In fact, in many suspected cases, even the antigen-antibody deposits are scarce, perhaps because the immune complexes have been degraded by the time of biopsy.
Immune complex deposition is implicated in the following vasculitides:
• Drug hypersensitivity vasculitis. In some cases drugs (e.g., penicillin) act as haptens by binding to host proteins; other agents are themselves foreign proteins (e.g., streptokinase). Regardless, antibodies directed against the drug-modified proteins or foreign molecules result in immune complex formation. The clinical manifestations can be mild and self-limiting, or severe and even fatal; skin lesions are most common. It is always important to consider drug hypersensitivity as a cause of vasculitis, since discontinuation of the offending agent usually leads to resolution.
• Vasculitis secondary to infections. Antibody to microbial constituents can form immune complexes that circulate and deposit in vascular lesions. In up to 30% of patients with polyarteritis nodosa (see further on), the vasculitis is attributable to immune complexes composed of hepatitis B surface antigen (HBsAg) and anti-HBsAg antibody.
Anti-Neutrophil Cytoplasmic Antibodies
Many patients with vasculitis have circulating antibodies that react with neutrophil cytoplasmic antigens, so-called anti-neutrophil cytoplasmic antibodies (ANCAs). ANCAs are a heterogeneous group of autoantibodies directed against constituents (mainly enzymes) of neutrophil primary granules, monocyte lysosomes, and endothelial cells. ANCAs are very useful diagnostic markers; their titers generally mirror clinical severity, and a rise in titers after periods of quiescence is predictive of disease recurrence. Although a number of ANCAs have been described, two are most important:
• Antiproteinase-3 (PR3-ANCA), previously called c-ANCA. PR3 is a neutrophil azurophilic granule constituent that shares homology with numerous microbial peptides, possibly explaining the generation of PR3-ANCAs. PR3-ANCAs are associated with Wegener granulomatosis (see below).
• Anti-myeloperoxidase (MPO-ANCA), previously called p-ANCA. MPO is a lysosomal granule constituent involved in oxygen free radical generation (Chapter 2). MPO-ANCAs are induced by several therapeutic agents, particularly propylthiouracil. MPO-ANCAs are associated with microscopic polyangiitis and Churg-Strauss syndrome (see later).
The close association between ANCA titers and disease activity suggests a pathogenic role for these antibodies. Of note, ANCAs can directly activate neutrophils, stimulating the release of reactive oxygen species and proteolytic enzymes; in vascular beds, this may lead to endothelial cell injury. While the antigenic targets of ANCA are primarily intracellular (and therefore not usually accessible to circulating antibodies), it is now clear that ANCA antigens (especially PR3) either are constitutively expressed at low levels on the plasma membrane or are translocated to the cell surface in activated and apoptotic leukocytes.
A plausible mechanism for ANCA vasculitis involves the following sequence:
• Drugs or cross-reactive microbial antigens induce ANCA formation; alternatively, leukocyte surface expression or release of PR3 and MPO (in the setting of infections) incites ANCA development in a susceptible host.
• Subsequent infection, endotoxin exposure, or inflammatory stimulus elicits cytokines such as TNF that upregulate the surface expression of PR3 and MPO on neutrophils and other cell types.
• ANCAs bind these cytokine-activated cells, causing further activation of neutrophils.
• ANCA-activated neutrophils cause endothelial cell injury by releasing granule contents and reactive oxygen species.
The ANCA autoantibodies are directed against cellular constituents and do not form circulating immune complexes. The vascular lesions do not typically contain demonstrable antibody and complement; therefore ANCA-associated vasculitides are often described as “pauci-immune.” Of interest, ANCAs directed against proteins other than PR3 and MPO are sometimes seen in patients with nonvasculitic inflammatory disorders (e.g., inflammatory bowel disease, sclerosing cholangitis, and rheumatoid arthritis).
Anti-Endothelial Cell Antibodies
Antibodies to endothelial cells, underlie certain vasculitides, such as Kawasaki disease (discussed later).
Presented next is a brief overview of several of the best-characterized vasculitides, with emphasis on the substantial overlap among the different entities. Of note, many cases lack a classic constellation of findings and are difficult to fit into one specific diagnostic category.
Giant Cell (Temporal) Arteritis
Giant cell (temporal) arteritis is the most common form of vasculitis among the elderly in developed countries. It takes the form of chronic, typically granulomatous, inflammation of large to small size arteries, mainly those supplying the head—especially the temporal arteries. Vertebral and ophthalmic arteries, as well as the aorta (giant cell aortitis), also can be involved. Because ophthalmic artery involvement can lead to sudden and permanent blindness, affected persons must be diagnosed and treated promptly.
![]() Pathogenesis
Pathogenesis
The bulk of evidence suggests the culprit is a T cell–mediated immune response to an as-yet uncharacterized vessel wall antigen. Pro-inflammatory cytokines (especially TNF) and anti-endothelial cell antibodies also contribute. The characteristic granulomatous inflammation, the association with certain MHC class II haplotypes, and the excellent therapeutic response to steroids all are in favor of an immune etiology. The extraordinary predilection for the temporal artery remains unexplained, although one hypothesis is that vessels in various parts of the body develop from distinct anlagen and may, therefore, express unique antigens.
![]() Morphology
Morphology
In giant cell arteritis, the pathologic changes are notoriously patchy along the length of affected vessels. Involved arterial segments exhibit nodular intimal thickening (and occasional thromboses) that reduce the lumen diameter and cause distal ischemia. Classic lesions exhibit granulomatous inflammation within the inner media centered on the internal elastic membrane; there is an infiltrate of lymphocytes and macrophages, with multinucleate giant cells, and fragmentation of the internal elastic lamina (Fig. 9–23). In up to 25% of cases, granulomas and giant cells are absent, and lesions exhibit only a nonspecific panarteritis with a mixed infiltrate of acute and chronic inflammation. Healing is marked by medial and adventitial fibrosis and intimal thickening. Characteristically, lesions at different stages of development are seen within the same artery.
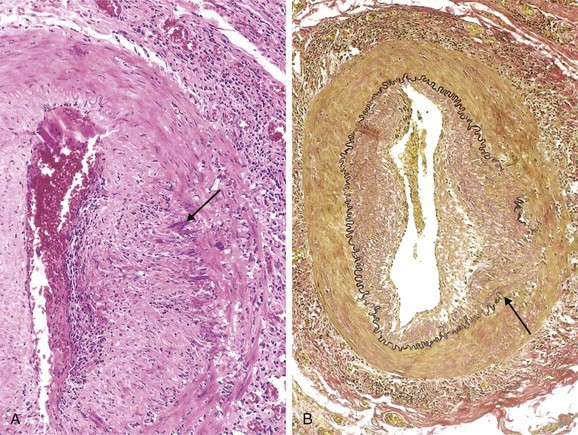
Figure 9–23 Temporal (giant cell) arteritis. A, H&E-stained section of temporal artery showing giant cells near the fragmented internal elastic membrane (arrow), along with medial and adventitial inflammation. B, Elastic tissue stain demonstrating focal destruction of the internal elastic membrane (arrow) and associated medial attenuation and scarring. H&E, hematoxylin-eosin.
Clinical Features of Giant Cell Arteritis
Temporal arteritis is rare before the age of 50. Signs and symptoms may be vague and constitutional—fever, fatigue, weight loss—or take the form of facial pain or headache, most intense along the course of the superficial temporal artery, which is painful to palpation. Ocular symptoms (associated with involvement of the ophthalmic artery) abruptly appear in about 50% of patients; these range from diplopia to complete vision loss. Diagnosis depends on biopsy and histology; however, because involvement in temporal arteritis is patchy a negative biopsy result does not exclude the diagnosis. Corticosteroid or anti-TNF therapies are effective treatments.
Takayasu Arteritis
Takayasu arteritis is a granulomatous vasculitis of medium-sized and larger arteries characterized principally by ocular disturbances and marked weakening of the pulses in the upper extremities (hence the alternate name, pulseless disease). This disorder manifests with transmural scarring and thickening of the aorta—particularly the aortic arch and great vessels—with severe luminal narrowing of the major branch vessels (Fig. 9–24). Aortic lesions share many of the clinical and histologic features of giant cell aortitis. Indeed, the distinction between the two entities is made largely on the basis of a patient’s age; those older than 50 years are designated giant cell aortitis, and those younger than 50 years, Takayasu aortitis. Although historically associated with Japanese ethnicity and certain HLA haplotypes, Takayasu aortitis has a global distribution. An autoimmune etiology is likely.
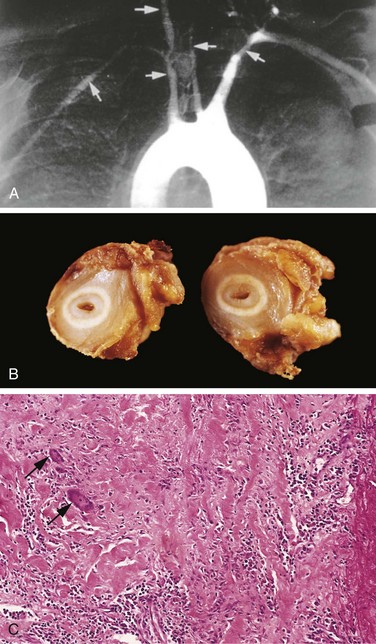
Figure 9–24 Takayasu arteritis. A, Aortic arch angiogram showing reduced flow of contrast material into the great vessels and narrowing of the brachiocephalic, carotid, and subclavian arteries (arrows). B, Cross-sections of the right carotid artery from the patient shown in A demonstrating marked intimal thickening and luminal narrowing. The white circles correspond to the original vessel wall; the inner core of tan tissue is the area of intimal hyperplasia. C, Histologic appearance in active Takayasu aortitis illustrating destruction and fibrosis of the arterial media associated with mononuclear infiltrates and inflammatory giant cells (arrows).
![]() Morphology
Morphology
Takayasu arteritis classically affects the aortic arch and arch vessels; a third of cases also involve the remainder of the aorta and its branches. Occasionally, aortic root involvement causes dilation and aortic valve insufficiency. Pulmonary arteries are involved in 50% of patients, and renal and coronary arteries also can be affected. The takeoffs of the great vessels can be markedly narrowed and even obliterated (Fig. 9–24, A and B), explaining the upper extremity weakness and faint carotid pulses. The histologic picture (Fig. 9–24, C) encompasses a spectrum ranging from adventitial mononuclear infiltrates and perivascular cuffing of the vasa vasorum, to intense transmural mononuclear inflammation, to granulomatous inflammation, replete with giant cells and patchy medial necrosis. The inflammation is associated with irregular thickening of the vessel wall, intimal hyperplasia, and adventitial fibrosis.
Clinical Features of Takayasu Aortitis
Initial signs and symptoms usually are nonspecific, including fatigue, weight loss, and fever. With progression, vascular signs and symptoms appear and dominate the clinical picture. These include reduced upper extremity blood pressure and pulse strength; neurologic deficits; and ocular disturbances, including visual field defects, retinal hemorrhages, and total blindness. Distal aorta disease can manifest as leg claudication, and pulmonary artery involvement can cause pulmonary hypertension. Narrowing of the coronary ostia can lead to myocardial infarction, and involvement of the renal arteries causes systemic hypertension in roughly half of the patients. The evolution of the disease is variable. Some cases rapidly progress, while others become quiescent after 1 to 2 years. In the latter scenario, long-term survival, albeit with visual or neurologic deficits, is possible.
Polyarteritis Nodosa
Polyarteritis nodosa (PAN) is a systemic vasculitis of small or medium-sized muscular arteries that typically involves the renal and visceral vessels and spares the pulmonary circulation. There is no association with ANCAs, but a third of the patients have chronic hepatitis B infection, which leads to the formation of immune complexes containing hepatitis B antigens that deposit in affected vessels. The cause is unknown in the remaining cases.
![]() Morphology
Morphology
Classic PAN is a segmental transmural necrotizing inflammation of small to medium-sized arteries, often with superimposed thrombosis. Kidney, heart, liver, and gastrointestinal tract vessels are affected in descending order of frequency. Lesions usually involve only part of the vessel circumference and have a predilection for branch points. Impaired perfusion may lead to ulcerations, infarcts, ischemic atrophy, or hemorrhages in the distribution of affected vessels. The inflammatory process also weakens the arterial wall, leading to aneurysms and rupture.
In the acute phase, there is transmural mixed inflammatory infiltrate composed of neutrophils and mononuclear cells, frequently accompanied by fibrinoid necrosis and luminal thrombosis (Fig. 9–25). Older lesions show fibrous thickening of the vessel wall extending into the adventitia. Characteristically, all stages of activity (from early to late) coexist in different vessels or even within the same vessel, suggesting ongoing and recurrent pathogenic insults.
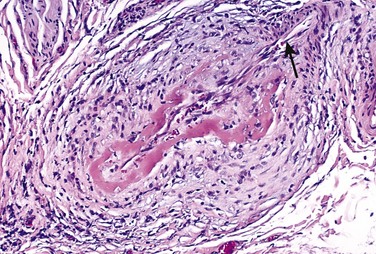
Figure 9–25 Polyarteritis nodosa, associated with segmental fibrinoid necrosis and thrombotic occlusion of a small artery. Note that part of the vessel (upper right, arrow) is uninvolved.
(Courtesy of Sidney Murphree, MD, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
Clinical Features of PAN
PAN is primarily a disease of young adults but can occur in all age groups. The clinical course may range from acute to chronic but typically is episodic, with long symptom-free intervals. The systemic findings—malaise, fever, and weight loss—are nonspecific, and the vascular involvement is widely scattered, so that the clinical manifestations can be varied and puzzling. A “classic” presentation can involve some combination of rapidly accelerating hypertension due to renal artery involvement; abdominal pain and bloody stools caused by vascular gastrointestinal lesions; diffuse muscular aches and pains; and peripheral neuritis, predominantly affecting motor nerves. Renal involvement often is prominent and constitutes a major cause of death in these patients. Untreated, PAN typically is fatal; however, immunosuppression can yield remission or cure in 90% of the cases.
Kawasaki Disease
Kawasaki disease is an acute, febrile, usually self-limited illness of infancy and childhood (80% of the patients are younger than 4 years of age) associated with an arteritis of mainly large to medium-sized vessels. Its clinical significance stems from the involvement of coronary arteries. Coronary arteritis can cause aneurysms that rupture or thrombose, resulting in myocardial infarction. Originally described in Japan, the disease is now recognized in the United States and elsewhere.
In genetically susceptible persons, a variety of infectious agents (mostly viral) have been posited to trigger the disease. The vasculitis may result from a delayed-type hypersensitivity response directed against cross-reactive or newly uncovered vascular antigen(s). Subsequent cytokine production and polyclonal B cell activation result in autoantibodies to endothelial cells and smooth muscle cells that precipitate the vasculitis.
![]() Morphology
Morphology
The vasculitis resembles that seen in polyarteritis nodosa. There is a dense transmural inflammatory infiltrate, although the fibrinoid necrosis usually is less prominent than in polyarteritis nodosa. The acute vasculitis typically subsides spontaneously or in response to treatment, but aneurysm formation due to wall damage can supervene. As with other arteritides, healed lesions also can exhibit obstructive intimal thickening. Pathologic changes outside the cardiovascular system are rarely significant.
Clinical Features of Kawasaki Disease
Kawasaki disease typically manifests with conjunctival and oral erythema and blistering, edema of the hands and feet, erythema of the palms and soles, a desquamative rash, and cervical lymph node enlargement (hence its other name, mucocutaneous lymph node syndrome). Approximately 20% of untreated patients develop cardiovascular sequelae, ranging from asymptomatic coronary arteritis, to coronary artery ectasia, to large coronary artery aneurysms (7 to 8 mm in diameter) with rupture or thrombosis, myocardial infarction, and sudden death. With intravenous immunoglobulin therapy and aspirin, the rate of symptomatic coronary artery disease is reduced to about 4%.
Microscopic Polyangiitis
Microscopic polyangiitis is a necrotizing vasculitis that generally affects capillaries, as well as small arterioles and venules. It also is called hypersensitivity vasculitis or leukocytoclastic vasculitis. Unlike in polyarteritis nodosa, all lesions of microscopic polyangiitis tend to be of the same age in any given patient. The skin, mucous membranes, lungs, brain, heart, gastrointestinal tract, kidneys, and muscle all can be involved; necrotizing glomerulonephritis (seen in 90% of patients) and pulmonary capillaritis are particularly common. Microscopic angiitis can be a feature of a number of immune disorders, such as Henoch-Schönlein purpura, essential mixed cryoglobulinemia, or the vasculitis associated with connective tissue disorders.
In some cases, antibody responses to antigens such as drugs (e.g., penicillin), microorganisms (e.g., streptococci), heterologous proteins, or tumor proteins have been implicated. These reactions can either lead to immune complex deposition or trigger secondary immune responses (e.g., the development of ANCAs) that are pathogenic. Indeed, most cases are associated with MPO-ANCA. Recruitment and activation of neutrophils within affected vascular beds probably are responsible for the disease manifestations.
![]() Morphology
Morphology
Microscopic polyangiitis is characterized by segmental fibrinoid necrosis of the media with focal transmural necrotizing lesions; granulomatous inflammation is absent. These lesions resemble those of polyarteritis nodosa but spare medium-sized and larger arteries, so that macroscopic infarcts are uncommon. In some areas (typically postcapillary venules), only infiltrating neutrophils that frequently undergo fragmentation are seen, giving rise to the term leukocytoclastic vasculitis (Fig. 9–26, A). Although immunoglobulins and complement components can be demonstrated in early skin lesions, most lesions are “pauci-immune” (i.e., show little or no antibody).
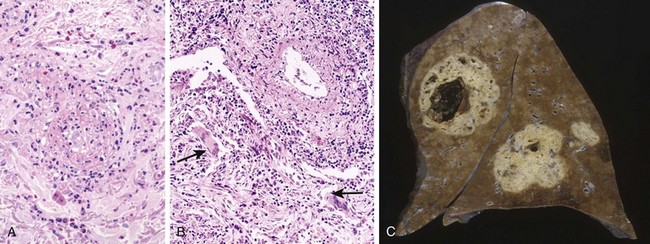
Figure 9–26 ANCA-associated small vessel vasculitis. A, Microscopic polyangiitis (leukocytoclastic vasculitis) with fragmented neutrophils in the thickened vessel wall. B and C, Wegener granulomatosis. B, Vasculitis of a small artery with adjacent granulomatous inflammation including giant cells (arrows). C, Lung from a patient with Wegener granulomatosis, demonstrating large nodular cavitating lesions.
(A, Courtesy of Scott Granter, MD, Brigham and Women’s Hospital, Boston, Massachusetts. C, Courtesy of Sidney Murphree, MD, Department of Pathology, University of Texas Southwestern Medical School, Dallas, Texas.)
Clinical Features of Microscopic Polyangiitis
Depending on the vascular bed involved, major features include hemoptysis, hematuria, proteinuria, abdominal pain or bleeding, muscle pain or weakness, and palpable cutaneous purpura. With the exception of patients with widespread renal or CNS involvement, immunosuppression and removal of the offending agent induce durable remissions.
Wegener Granulomatosis
Wegener granulomatosis is a necrotizing vasculitis characterized by a specific triad of findings:
• Granulomas of the lung and/or the upper respiratory tract (ear, nose, sinuses, throat)
• Vasculitis of small to medium-sized vessels (capillaries, venules, arterioles, and arteries), most prominently in the lungs and upper respiratory tract
“Limited” forms of disease can be restricted to the respiratory tract. Conversely, a widespread form of the disease can affect eyes, skin, and other organs, notably the heart; clinically, this resembles polyarteritis nodosa with the additional feature of respiratory involvement.
Wegener granulomatosis is likely to be initiated as a cell-mediated hypersensitivity response directed against inhaled infectious or environmental antigens. PR3-ANCAs are present in almost 95% of cases and probably drive the subsequent tissue injury; they also are useful markers of disease activity. After immunosuppressive therapy, ANCA levels fall dramatically, while rising titers are predictive of relapse.
![]() Morphology
Morphology
Upper respiratory tract lesions range from granulomatous sinusitis to ulcerative lesions of the nose, palate, or pharynx; lung findings also vary, ranging from diffuse parenchymal infiltrates to granulomatous nodules. There is multifocal necrotizing granulomatous vasculitis with a surrounding fibroblastic proliferation (see Fig. 9–26, B). Multiple granulomata can coalesce to produce radiographically visible nodules with central cavitation (see Fig. 9–26, B). Destruction of vessels can lead to hemorrhage and hemoptysis. Lesions can ultimately undergo progressive fibrosis and organization.
The renal lesions range from mild, focal glomerular necrosis with thrombosis of isolated glomerular capillary loops (focal and segmental necrotizing glomerulonephritis) to more advanced glomerular lesions with diffuse necrosis and parietal cell proliferation forming epithelial crescents (crescentic glomerulonephritis) (Chapter 13).
Clinical Features of Wegener Granulomatosis
The typical patient is a 40-year old man, although women and persons of other ages can be affected. Classic presentations include bilateral pneumonitis with nodules and cavitary lesions (95%), chronic sinusitis (90%), mucosal ulcerations of the nasopharynx (75%), and renal disease (80%); patients with low-grade renal involvement may demonstrate only hematuria and proteinuria responsive to therapy, whereas more severe disease can portend rapidly progressive renal failure. Rash, myalgias, articular involvement, neuritis, and fever can also occur. If untreated, the mortality rate at 1 year is 80%. Treatment with steroids, cyclophosphamide, TNF inhibitors and anti–B cell antibodies (Rituximab) has improved this picture considerably. Most patients with Wegener granulomatosis now survive, but remain at high risk for relapses that can ultimately lead to renal failure.
Churg-Strauss Syndrome
Churg-Strauss syndrome (also called allergic granulomatosis and angiitis) is a small vessel necrotizing vasculitis classically associated with asthma, allergic rhinitis, lung infiltrates, peripheral eosinophilia, extravascular necrotizing granulomas, and a striking infiltration of vessels and perivascular tissues by eosinophils. It is a rare disorder, affecting 1 in 1 million people. Cutaneous involvement (with palpable purpura), gastrointestinal bleeding, and renal disease (primarily as focal and segmental glomerulosclerosis) are the major associations. Cytotoxicity produced by the myocardial eosinophilic infiltrates often leads to cardiomyopathy; cardiac involvement is seen in 60% of patients and is a major cause of morbidity and death.
Churg-Strauss syndrome may stem from “hyperresponsiveness” to some normally innocuous allergic stimulus. MPO-ANCAs are present in a minority of cases, suggesting that the disorder is pathogenically heterogeneous. The vascular lesions differ from those of polyarteritis nodosa or microscopic polyangiitis by virtue of the presence of granulomas and eosinophils.
Thromboangiitis Obliterans (Buerger Disease)
Thromboangiitis obliterans (Buerger disease) is a distinctive disorder that frequently results in severe vascular insufficiency and gangrene of the extremities. It is characterized by focal acute and chronic inflammation of medium-sized and small arteries, especially the tibial and radial arteries, associated with thrombosis; occasionally, secondary extension into adjacent veins and nerves may be seen. Buerger disease occurs almost exclusively in heavy tobacco smokers and usually develops before age 35.
The etiology is unknown. Direct endothelial cell toxicity caused by some component of tobacco is suspected; alternatively, a reactive compound in tobacco may modify vessel wall components and induce an immune response. Indeed, most patients with Buerger disease are hypersensitive to tobacco extracts. A genetic predilection is suggested by an increased prevalence in certain ethnic groups (Israeli, Indian subcontinent, Japanese) and an association with certain HLA haplotypes.
![]() Morphology
Morphology
In thromboangiitis obliterans, there is a sharply segmental acute and chronic transmural vasculitis of medium-sized and small arteries, predominantly those of the extremities. In early stages, mixed inflammatory infiltrates are accompanied by luminal thrombosis; small microabscesses, occasionally rimmed by granulomatous inflammation, also may be present (Fig. 9–27). The inflammation often extends into contiguous veins and nerves (a feature that is rare in other forms of vasculitis). With time, thrombi can organize and recanalize, and eventually the artery and adjacent structures become encased in fibrous tissue.
Clinical Features of Buerger Disease
Early manifestations include cold-induced Raynaud phenomenon, instep foot pain induced by exercise (instep claudication), and a superficial nodular phlebitis (venous inflammation). The vascular insufficiency of Buerger disease tends to be accompanied by severe pain—even at rest—undoubtedly from the neural involvement. Chronic extremity ulcerations can develop, progressing over time (occasionally precipitously) to frank gangrene. Smoking abstinence in the early stages of the disease often can ameliorate further attacks; however, once established, the vascular lesions do not respond to smoking abstinence.
Vasculitis Associated with Other Noninfectious Disorders
Vasculitis resembling hypersensitivity angiitis or classic PAN can be associated with many other diseases, including malignancies and immunologic disorders such as rheumatoid arthritis, systemic lupus erythematosus, antiphospholipid antibody syndrome, and Henoch-Schönlein purpura. Rheumatoid vasculitis can occur in patients with severe, long-standing rheumatoid arthritis; it can cause a clinically significant aortitis but typically affects small and medium-sized arteries, leading to visceral infarction. Linking vasculitis to specific disorders may have important therapeutic implications. For example, although classic immune complex lupus vasculitis and antiphospholipid antibody syndrome can share morphologic features, the former requires anti-inflammatory therapy while anticoagulation is indicated in the latter.
Infectious Vasculitis
Localized arteritis may be caused by the direct invasion of arteries by infectious agents, usually bacteria or fungi, and in particular Aspergillus and Mucor spp. Vascular invasion can be part of a more general tissue infection (e.g., bacterial pneumonia or adjacent to abscesses), or—less commonly—arise from hematogenous spread of bacteria during septicemia or embolization from infective endocarditis.
Vascular infections can weaken arterial walls and culminate in mycotic aneurysms (see earlier), or can induce thrombosis and infarction. Thus, inflammation of vessels in bacterial meningitis can cause thrombosis and infarction, leading ultimately to extension of a subarachnoid infection into the brain parenchyma.
![]() Summary
Summary
Vasculitis
• Vasculitis is defined as inflammation of vessel walls; it frequently is associated with systemic manifestations (including fever, malaise, myalgias, and arthralgias) and organ dysfunction that depends on the pattern of vascular involvement.
• Vasculitis can result from infections but more commonly has an immunologic basis such as immune complex deposition, anti-neutrophil antibodies (ANCAs), or anti–endothelial cell antibodies.
• Different forms of vasculitis tend to specifically affect vessels of a particular caliber and location (see Fig. 9–22).
Disorders of Blood Vessel Hyperreactivity
Several disorders are characterized by inappropriate or exaggerated vasoconstriction of blood vessels.
Raynaud Phenomenon
Raynaud phenomenon results from exaggerated vasoconstriction of arteries and arterioles in the extremities, particularly the fingers and toes, but also sometimes the nose, earlobes, or lips. The restricted blood flow induces paroxysmal pallor or cyanosis; involved digits characteristically show “red-white-and-blue” color changes from most proximal to most distal, reflecting proximal vasodilation, central vasoconstriction, and more distal cyanosis, respectively. Raynaud phenomenon can be a primary entity or may be secondary to other disorders.
Primary Raynaud phenomenon (previously called Raynaud disease) is caused by exaggerated central and local vasomotor responses to cold or emotion; it affects 3% to 5% of the general population and has a predilection for young women. Structural changes in the arterial walls are absent except late in the course, when intimal thickening may appear. The course usually is benign, but in chronic cases, atrophy of the skin, subcutaneous tissues, and muscles may occur. Ulceration and ischemic gangrene are rare.
Secondary Raynaud phenomenon refers to vascular insufficiency due to arterial disease caused by other entities including systemic lupus erythematosus, scleroderma, Buerger disease, or even atherosclerosis (see later). Indeed, since Raynaud phenomenon may be the first manifestation of such conditions, every patient with Raynaud phenomenon should be evaluated for these secondary causes.
Myocardial Vessel Vasospasm
Excessive constriction of arteries or arterioles may cause ischemia, and persistent vasospasm can even lead to tissue infarction. In addition to intrinsic hyperreactivity of medial smooth muscle cells, as described earlier for primary Raynaud disease, high levels of vasoactive mediators can precipitate prolonged vascular contraction. Such agents can be endogenous (e.g., epinephrine released by pheochromocytomas) or exogenous (cocaine or phenylephrine). Elevated thyroid hormone causes a similar effect by increasing the sensitivity of vessels to circulating catecholamines, while autoantibodies and T cells in scleroderma (Chapter 4) can cause vascular instability and vasospasm. In some susceptible persons, extreme psychological stress and the attendant release of catecholamines can lead to pathologic vasospasm.
When vasospasm of cardiac arterial or arteriolar beds (so-called cardiac Raynaud) is of sufficient duration (20 to 30 minutes), myocardial infarction occurs. Elevated levels of catechols also increase heart rate and myocardial contractility, exacerbating ischemia caused by the vasospasm. The outcome can be sudden cardiac death (probably caused by a fatal arrhythmia) or an ischemic dilated cardiomyopathy—so-called Takotsubo cardiomyopathy (also called “broken heart syndrome,” because of the association with emotional duress). Histologic findings in acute cases may include microscopic areas of necrosis characterized by myocyte hypercontraction (contraction band necrosis) (Chapter 10); in subacute and chronic cases, microscopic foci of granulation tissue and/or scar may be present.
Veins and Lymphatics
Varicose veins and phlebothrombosis/thrombophlebitis account for at least 90% of cases of clinically relevant venous disease.
Varicose Veins of the Extremities
Varicose veins are abnormally dilated tortuous veins produced by chronically increased intraluminal pressures and weakened vessel wall support. The superficial veins of the upper and lower leg typically are involved. Up to 20% of men and a third of women develop lower extremity varicose veins. Obesity increases the risk, and the higher incidence in women probably reflects the prolonged elevation in venous pressure caused by compression of the inferior vena cava by the gravid uterus during pregnancy. There is also a familial tendency toward premature varicosities.
Clinical Features of Varicose Veins
Varicose dilation renders the venous valves incompetent and leads to lower extremity stasis, congestion, edema, pain, and thrombosis. The most disabling sequelae include persistent edema in the extremity and secondary ischemic skin changes, including stasis dermatitis and ulcerations. The latter can become chronic varicose ulcers as a consequence of poor wound healing and superimposed infections. Of note, embolism from these superficial veins is very rare, in contrast with the relatively frequent emboli that arise from thrombosed deep veins (Chapter 3).
Varicosities of Other Sites
Venous dilations in two other sites merit special attention:
• Esophageal varices. Liver cirrhosis (less frequently, portal vein obstruction or hepatic vein thrombosis) causes portal vein hypertension (Chapter 15). This in turn leads to the opening of porto-systemic shunts and increased blood flow into veins at the gastro-esophageal junction (forming esophageal varices), rectum (forming hemorrhoids), and periumbilical veins of the abdominal wall (forming a caput medusae). Esophageal varices are most important since they are prone to ruptures that can lead to massive (even fatal) upper gastrointestinal hemorrhage.
• Hemorrhoids are varicose dilations of the venous plexus at the anorectal junction that result from prolonged pelvic vascular congestion associated with pregnancy or straining to defecate. Hemorrhoids are a source of bleeding and prone to thrombosis and painful ulceration.
Thrombophlebitis and Phlebothrombosis
Thrombosis of deep leg veins accounts for more than 90% of cases of thrombophlebitis and phlebothrombosis. These two terms are largely interchangeable designations for venous thrombosis and inflammation. Other sites where venous thrombi may form are the periprostatic venous plexus in males and the pelvic venous plexus in females, as well as the large veins in the skull and the dural sinuses (especially in the setting of infection or inflammation). Peritoneal infections, including peritonitis, appendicitis, salpingitis, and pelvic abscesses, as well as certain conditions associated with hypercoagulability (e.g., polycythemia vera) (Chapter 11) can lead to portal vein thrombosis.
In deep venous thrombosis (DVT) of the legs, prolonged immobilization resulting in venous stasis is the most important risk factor. This can occur with extended bed rest or even just sitting during long plane or automobile trips. The postoperative state is another independent risk factor for DVT, as are congestive heart failure, pregnancy, oral contraceptive use, and obesity. Inherited defects in coagulation factors (Chapter 3) often predispose affected persons to development of thrombophlebitis. Venous thrombi may result from elaboration of procoagulant factors from malignant tumors (Chapter 5). The resulting hypercoagulable state can manifest as evanescent thromboses in different vascular beds at different times, resulting in so-called migratory thrombophlebitis or Trousseau syndrome.
Thrombi in the legs tend to produce few, if any, reliable signs or symptoms. When present, local manifestations include distal edema, cyanosis, superficial vein dilation, heat, tenderness, redness, swelling, and pain. In some cases, pain can be elicited by pressure over affected veins, squeezing the calf muscles, or forced dorsiflexion of the foot (Homan sign). However, symptoms often are absent, especially in bedridden patients, and the absence of findings does not exclude DVT.
Pulmonary embolism is a common and serious clinical complication of DVT (Chapter 3), resulting from fragmentation or detachment of the venous thrombus. In many cases, the first manifestation of thrombophlebitis is a pulmonary embolus. Depending on the size and number of emboli, the outcome can range from resolution with no symptoms to death.
Superior and Inferior Vena Cava Syndromes
The superior vena cava syndrome usually is caused by neoplasms that compress or invade the superior vena cava, such as bronchogenic carcinoma or mediastinal lymphoma. The resulting obstruction produces a characteristic clinical complex consisting of marked dilation of the veins of the head, neck, and arms associated with cyanosis. Pulmonary vessels also can be compressed, causing respiratory distress.
The inferior vena cava syndrome can be caused by neoplasms that compress or invade the inferior vena cava or by a thrombus from the hepatic, renal, or lower extremity veins that propagates upward. Certain neoplasms—particularly hepatocellular carcinoma and renal cell carcinoma—show a striking tendency to grow within veins, and these tumors may ultimately occlude the inferior vena cava. Obstruction of the inferior vena cava induces marked lower extremity edema, distention of the superficial collateral veins of the lower abdomen, and—with renal vein involvement—proteinuria of marked degree.
Lymphangitis and Lymphedema
Primary disorders of lymphatic vessels are extremely uncommon. Much more commonly, lymphatic vessels are involved by inflammatory, infectious, or malignant processes secondarily.
Lymphangitis refers to an acute inflammatory process caused by bacterial seeding of the lymphatic vessels and was discussed in Chapter 2. Clinically, the inflamed lymphatics appear as red, painful subcutaneous streaks, usually associated with tender enlargement of draining lymph nodes (acute lymphadenitis). If bacteria are not contained within the lymph nodes, they can pass into the venous circulation and cause bacteremia or sepsis.
Primary lymphedema can occur as an isolated congenital defect (simple congenital lymphedema) or as the familial Milroy disease (heredofamilial congenital lymphedema), resulting from agenesis or hypoplasia of lymphatics. Secondary or obstructive lymphedema stems from the accumulation of interstitial fluid behind an obstructed, previously normal lymphatic; such obstruction can result from various disorders or conditions:
• Tumors involving either the lymphatic channels or the regional lymph nodes
• Surgical procedures that sever lymphatic connections (e.g., axillary lymph nodes in radical mastectomy)
Regardless of the cause, lymphedema increases the hydrostatic pressure in the lymphatics distal to the obstruction and causes edema. Chronic edema in turn may lead to deposition of ECM and fibrosis, producing brawny induration or a peau d’orange appearance of the overlying skin. Eventually, inadequate tissue perfusion can lead to skin ulceration. Rupture of dilated lymphatics, typically following obstruction by an infiltrating tumor mass, can lead to milky accumulations of lymph in various spaces designated chylous ascites (abdomen), chylothorax, and chylopericardium.
Tumors
Tumors of blood vessels and lymphatics include common and benign hemangiomas, locally aggressive neoplasms that metastasize infrequently, and rare, highly malignant angiosarcomas (Table 9–4). Primary tumors of large vessels (aorta, pulmonary artery, and vena cava) are extremely rare and are mostly sarcomas. Congenital or developmental malformations and non-neoplastic reactive vascular proliferations (e.g., bacillary angiomatosis) also can manifest as tumor-like lesions.
Table 9–4 Classification of Vascular Tumors and Tumor-like Conditions
| Benign Neoplasms, Developmental and Acquired Conditions |
|
|
| Intermediate-Grade Neoplasms |
| Malignant Neoplasms |
Vascular neoplasms can arise from endothelium (e.g., hemangioma, lymphangioma, angiosarcoma) or cells that support or surround blood vessels (e.g., glomus tumor). Although a benign hemangioma usually can be distinguished with ease from an anaplastic high-grade angiosarcoma, on occasion the distinction between benign and malignant can be difficult. General rules of thumb are as follows:
• Benign tumors usually contain obvious vascular channels filled with blood cells or lymph that are lined by a monolayer of normal-appearing endothelial cells.
• Malignant tumors are more cellular, show cytologic atypia, are proliferative, and usually do not form well-organized vessels; confirmation of the endothelial derivation of such proliferations may require immunohistochemical detection of endothelial cell–specific markers, such as CD31 or von Willebrand factor.
Because these are tumors of dysregulated endothelial cells, the possibility of controlling their growth with inhibitors of blood vessel formation (antiangiogenic factors) is being explored.
Benign Tumors and Tumor-Like Conditions
Vascular Ectasias
Ectasia is a generic term for any local dilation of a structure, while telangiectasia is used to describe a permanent dilation of preexisting small vessels (capillaries, venules, and arterioles, usually in the skin or mucous membranes) that forms a discrete red lesion. These lesions can be congenital or acquired and are not true neoplasms.
• Nevus flammeus (a “birthmark”), the most common form of vascular ectasia, is a light pink to deep purple flat lesion on the head or neck composed of dilated vessels. Most ultimately regress spontaneously.
• The so-called port wine stain is a special form of nevus flammeus. These lesions tend to grow during childhood, thicken the skin surface, and do not fade with time. Such lesions occurring in the distribution of the trigeminal nerve are associated with the Sturge-Weber syndrome (also called encephalotrigeminal angiomatosis). This uncommon congenital disorder is associated with facial port wine nevi, ipsilateral venous angiomas in the cortical leptomeninges, mental retardation, seizures, hemiplegia, and radiopacities of the skull. Thus, a large facial telangiectasia in a child with mental deficiency may indicate the presence of additional vascular malformations.
• Spider telangiectasias are non-neoplastic vascular lesions with a general shape resembling that of a spider. These lesions manifest as radial, often pulsatile arrays of dilated subcutaneous arteries or arterioles (the “legs” of the spider) about a central core (the spider’s “body”) that blanch with pressure. Spider telangiectasias commonly occur on the face, neck, or upper chest and most frequently are associated with hyperestrogenic states (e.g., in pregnant women or patients with cirrhosis).
• Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease) is an autosomal dominant disorder caused by mutations in genes that encode components of the TGF-β signaling pathway in endothelial cells. The telangiectasias are malformations composed of dilated capillaries and veins that are present at birth. They are widely distributed over the skin and oral mucous membranes, as well as in the respiratory, gastrointestinal, and urinary tracts. The lesions can spontaneously rupture, causing serious epistaxis (nosebleed), gastrointestinal bleeding, or hematuria.
Hemangiomas
Hemangiomas are very common tumors composed of blood-filled vessels (Fig. 9–28). These lesions constitute 7% of all benign tumors of infancy and childhood; most are present from birth and initially increase in size, but many eventually regress spontaneously. While hemangiomas typically are localized lesions confined to the head and neck, they occasionally may be more extensive (angiomatosis) and can arise internally. Nearly one third of these internal lesions are found in the liver. Malignant transformation is rare. Several histologic and clinical variants have been described:
• Capillary hemangiomas are the most common type; these occur in the skin, subcutaneous tissues, and mucous membranes of the oral cavities and lips, as well as in the liver, spleen, and kidneys (Fig. 9–28, A). Histologically, they are comprised of thin-walled capillaries with scant stroma (Fig. 9–28, B).
• Juvenile hemangiomas (so-called strawberry hemangiomas) of the newborn skin are extremely common (1 in 200 births) and can be multiple. These grow rapidly for a few months but then fade by the age of 1 to 3 years, with complete regression by age 7 in the vast majority of cases.
• Pyogenic granulomas are capillary hemangiomas that manifest as rapidly growing red pedunculated lesions on the skin, gingival, or oral mucosa. Microscopically they resemble exuberant granulation tissue. They bleed easily and are often ulcerated (Fig. 9–28, C). Roughly a quarter of the lesions develop after trauma, reaching a size of 1 to 2 cm within a few weeks. Curettage and cautery usually are curative. Pregnancy tumor (granuloma gravidarum) is a pyogenic granuloma that occurs infrequently (1% of patients) in the gingiva of pregnant women. These lesions may spontaneously regress (especially after pregnancy) or undergo fibrosis, but occasionally require surgical excision.
• Cavernous hemangiomas are composed of large, dilated vascular channels. Compared with capillary hemangiomas, cavernous hemangiomas are more infiltrative, frequently involve deep structures, and do not spontaneously regress. On histologic examination, the mass is sharply defined but unencapsulated and is composed of large, cavernous blood-filled vascular spaces, separated by connective tissue stroma (see Fig. 9–28, D). Intravascular thrombosis with associated dystrophic calcification is common. They may be locally destructive, so surgical excision may be required in some cases. More often the tumors are of little clinical significance, but they can be cosmetically troublesome and are vulnerable to traumatic ulceration and bleeding. Moreover, cavernous hemangiomas detected by imaging studies may be difficult to distinguish from their malignant counterparts. Brain hemangiomas also are problematic, as they can cause symptoms related to compression of adjacent tissue or rupture. Cavernous hemangiomas constitute one component of von Hippel-Lindau disease (Chapter 22), in which vascular lesions are commonly found in the cerebellum, brain stem, retina, pancreas, and liver.
Figure 9–28 Hemangiomas. A, Hemangioma of the tongue. B, Histologic appearance in juvenile capillary hemangioma. C, Pyogenic granuloma of the lip. D, Histologic appearance in cavernous hemangioma.
(A and D, Courtesy of John Sexton, MD, Beth Israel Hospital, Boston, Massachusetts. B, Courtesy of Christopher D.M. Fletcher, MD, Brigham and Women’s Hospital, Boston, Massachusetts. C, Courtesy of Thomas Rogers, MD, University of Texas Southwestern Medical School, Dallas, Texas.)
Lymphangiomas
Lymphangiomas are the benign lymphatic counterpart of hemangiomas.
• Simple (capillary) lymphangiomas are slightly elevated or sometimes pedunculated lesions up to 1 to 2 cm in diameter that occur predominantly in the head, neck, and axillary subcutaneous tissues. Histologically, lymphangiomas are composed of networks of endothelium-lined spaces that can be distinguished from capillary channels only by the absence of blood cells.
• Cavernous lymphangiomas (cystic hygromas) typically are found in the neck or axilla of children, and more rarely in the retroperitoneum. Cavernous lymphangiomas can be large (up to 15 cm), filling the axilla or producing gross deformities of the neck. Of note, cavernous lymphangiomas of the neck are common in Turner syndrome. These lesions are composed of massively dilated lymphatic spaces lined by endothelial cells and separated by intervening connective tissue stroma containing lymphoid aggregates. The tumor margins are indistinct and unencapsulated, making definitive resection difficult.
Glomus Tumors (Glomangiomas)
Glomus tumors are benign, exquisitely painful tumors arising from specialized smooth muscle cells of glomus bodies, arteriovenous structures involved in thermoregulation. Although they may superficially resemble cavernous hemangiomas, glomangiomas arise from smooth muscle cells, rather than endothelial cells. They most commonly are found in the distal portion of the digits, especially under the fingernails. Excision is curative.
Bacillary Angiomatosis
Bacillary angiomatosis is a vascular proliferation in immunocompromised hosts (e.g., patients with AIDS) caused by opportunistic gram-negative bacilli of the Bartonella family. The lesions can involve the skin, bone, brain, and other organs. Two species have been implicated:
• Bartonella henselae, whose principal reservoir is the domestic cat; this organism causes cat-scratch disease (a necrotizing granulomatous disorder of lymph nodes) in immunocompetent hosts.
• Bartonella quintana, which is transmitted by human body lice; this microbe was the cause of “trench fever” in World War I.
Skin lesions are red papules and nodules, or rounded subcutaneous masses. Histologically, there is a proliferation of capillaries lined by prominent epithelioid endothelial cells, which exhibit nuclear atypia and mitoses (Fig. 9–29). Other features include infiltrating neutrophils, nuclear debris, and purplish granular collections of the causative bacteria.
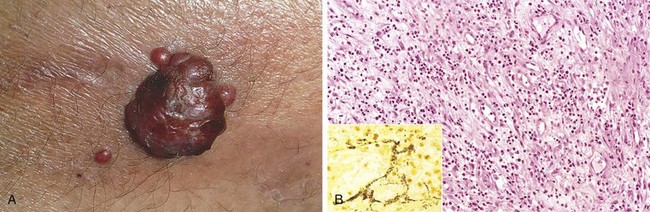
Figure 9–29 Bacillary angiomatosis.
A, Characteristic cutaneous lesion. B, Histologic features are those of acute inflammation and capillary proliferation. Inset, Modified silver (Warthin-Starry) stain demonstrates clusters of tangled bacilli (black).
(A, Courtesy of Richard Johnson, MD, Beth Israel Deaconess Medical Center, Boston, Massachusetts. B and inset, courtesy of Scott Granter, MD, Brigham and Women’s Hospital, Boston, Massachusetts.)
The bacteria induce host tissues to produce hypoxia-inducible factor-1α (HIF-1α), which drives vascular endothelial growth factor (VEGF) production and vascular proliferation. The infections (and lesions) are cured by antibiotic treatment.
Intermediate-Grade (Borderline) Tumors
Kaposi Sarcoma
Kaposi sarcoma (KS) is a vascular neoplasm caused by Kaposi sarcoma herpesvirus (KSHV, also known as human herpesvirus-8, or HHV-8). Although it occurs in a number of contexts, it is by far most common in patients with AIDS; indeed, its presence is used as a criterion for the diagnosis. Four forms of KS, based on population demographics and risks, are recognized:
• Classic KS is a disorder of older men of Mediterranean, Middle Eastern, or Eastern European descent (especially Ashkenazic Jews); it is uncommon in the United States. It can be associated with malignancy or altered immunity but is not associated with HIV infection. Classic KS manifests as multiple red-purple skin plaques or nodules, usually on the distal lower extremities; these progressively increase in size and number and spread proximally. Although persistent, the tumors typically are asymptomatic and remain localized to the skin and subcutaneous tissue.
• Endemic African KS typically occurs in younger (under age 40) HIV-seronegative persons and can follow an indolent or aggressive course; it involves lymph nodes much more frequently than in the classic variant. In combination with AIDS-associated KS (see later), KS is now the most common tumor in central Africa. A particularly severe form, with prominent lymph node and visceral involvement, occurs in prepubertal children; the prognosis is poor, with an almost 100% mortality rate within 3 years.
• Transplantation-associated KS occurs in solid organ transplant recipients in the setting of T cell immunosuppression. The risk of KS is increased 100-fold in transplant recipients, in whom it pursues an aggressive course and often involves lymph nodes, mucosa, and viscera; cutaneous lesions may be absent. Lesions often regress with attenuation of immunosuppression, but at the risk of organ rejection.
• The incidence of KS has fallen more than 80% with the advent of antiretroviral therapy, but it continues to occur in HIV-infected persons with an incidence that is greater than 1000-fold higher than in the general population. Worldwide, KS is the most common HIV-related malignancy. AIDS-associated KS often involves lymph nodes and disseminates widely to viscera early in its course. Most patients eventually die of opportunistic infections rather than from KS.
![]() Pathogenesis
Pathogenesis
Virtually all KS lesions are infected by KSHV. Like Epstein-Barr virus, KSHV is a γ-herpesvirus. It is transmitted both through sexual contact and by poorly understood nonsexual routes potentially including oral secretions and cutaneous exposures (of note, the prevalence of endemic African KS is inversely related to the wearing of shoes). KSHV and altered T cell immunity probably are required for KS development; in the elderly, diminished T cell immunity may be related to aging. It also is probable that acquired somatic mutations in the cells of origin contribute to tumor development and progression.
KSHV causes lytic and latent infections in endothelial cells, both of which probably are important in KS pathogenesis. A virally encoded G protein induces VEGF production, stimulating endothelial growth, and cytokines produced by inflammatory cells recruited to sites of lytic infection also create a local proliferative milieu. In latently infected cells, KSHV-encoded proteins disrupt normal cellular proliferation controls (e.g., through synthesis of a viral homologue of cyclin D) and prevent apoptosis by inhibiting p53. Thus, the local inflammatory environment favors cellular proliferation, and latently infected cells have a growth advantage. In its early stages, only a few cells are KSHV-infected, but with time, virtually all of the proliferating cells carry the virus.
![]() Morphology
Morphology
In classic KS (and sometimes in other variants), the cutaneous lesions progress through three stages: patch, plaque, and nodule.
• Patches are pink, red, or purple macules, typically confined to the distal lower extremities (Fig. 9–30, A). Microscopic examination reveals dilated, irregular, and angulated blood vessels lined by endothelial cells and an interspersed infiltrate of chronic inflammatory cells, sometimes containing hemosiderin. These lesions can be difficult to distinguish from granulation tissue.
• With time, lesions spread proximally and become larger, violaceous, raised plaques (see Fig. 9–30, A) composed of dilated, jagged dermal vascular channels lined and surrounded by plump spindle cells. Other prominent features include extravasated erythrocytes, hemosiderin-laden macrophages, and other mononuclear cells.
• Eventually nodular, more overtly neoplastic, lesions appear. These are composed of plump, proliferating spindle cells, mostly located in the dermis or subcutaneous tissues (Fig. 9–30, B), often with interspersed slitlike spaces. The spindle cells express both endothelial cell and smooth muscle cell markers and often contain round, pink cytoplasmic globules that represent degenerating red blood cells within phagolysosomes. Hemorrhage and hemosiderin deposition is more pronounced, and mitotic figures are common. The nodular stage often is accompanied by nodal and visceral involvement, particularly in the African and AIDS-associated variants.
Clinical Features of KS
The course of disease varies widely according to the clinical setting. Most primary HHV-8 infections are asymptomatic. Classic KS is—at least initially—largely restricted to the surface of the body, and surgical resection usually is adequate for an excellent prognosis. Radiation therapy can be used for multiple lesions in a restricted area, and chemotherapy yields satisfactory results for more disseminated disease, including nodal involvement. In KS associated with immunosuppression, withdrawal of therapy (with or without adjunct chemotherapy or radiotherapy) often is effective. For AIDS-associated KS, HIV antiretroviral therapy generally is beneficial, with or without additional therapy. Interferon-γ and angiogenesis inhibitors also have proved somewhat effective.
Hemangioendotheliomas
Hemangioendotheliomas comprise a wide spectrum of borderline vascular neoplasms with clinical behaviors intermediate between those of benign, well-differentiated hemangiomas and aggressively malignant angiosarcomas.
As an example, epithelioid hemangioendothelioma is a vascular tumor of adults arising in association with medium-sized to large veins. The clinical course is highly variable; while excision is curative in a majority of the cases, up to 40% of the tumors recur, and 20% to 30% eventually metastasize; perhaps 15% of patients die of their tumors. The tumor cells are plump and cuboidal and do not form well-defined vascular channels, so that they can be mistaken for metastatic epithelioid tumors or melanomas.
Malignant Tumors
Angiosarcomas
Angiosarcomas are malignant endothelial neoplasms (Fig. 9–31) that range from highly differentiated tumors resembling hemangiomas to wildly anaplastic lesions difficult to distinguish from carcinomas or melanomas. Older adults are more commonly affected. There is no gender bias, and lesions can occur at any site, but most often involve the skin, soft tissue, breast, and liver.
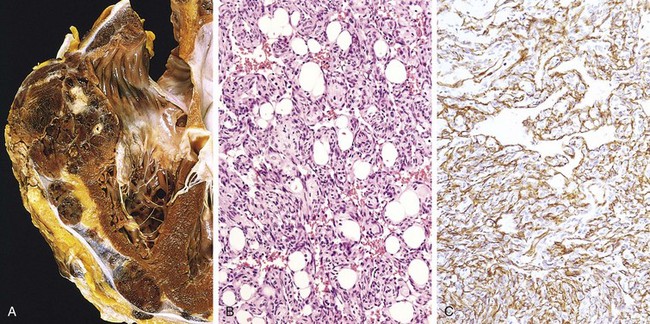
A, Angiosarcoma of the right ventricle. B, Moderately differentiated angiosarcoma with dense clumps of atypical cells lining distinct vascular lumina. C, Immunohistochemical staining of angiosarcoma for the endothelial cell marker CD31.
Hepatic angiosarcomas are associated with certain carcinogens, including arsenical pesticides, Thorotrast (a radioactive contrast agent formerly used for radiologic imaging), and polyvinyl chloride (a widely used plastic, and one of the best known examples of human chemical carcinogenesis). A latent period of years between exposure and subsequent tumor development is typical.
Angiosarcomas also can arise in the setting of lymphedema, classically in the ipsilateral upper extremity several years after radical mastectomy (i.e., with lymph node resection) for breast cancer. In such instances, the tumor presumably arises from lymphatic vessels (lymphangiosarcoma). Angiosarcomas also can be induced by radiation and rarely are associated with long-term (years) indwelling foreign bodies (e.g., catheters).
![]() Morphology
Morphology
In the skin, angiosarcomas begin as small, sharply demarcated, asymptomatic red nodules. More advanced lesions are large, fleshy red-tan to gray-white masses (Fig. 9–31, A) with margins that blend imperceptibly with surrounding structures. Necrosis and hemorrhage are common.
On microscopic examination, the extent of differentiation is extremely variable, ranging from plump atypical endothelial cells that form vascular channels (Fig. 9–31, B) to undifferentiated spindle cell tumors without discernible blood vessels. The endothelial cell origin can be demonstrated in the poorly differentiated tumors by staining for the endothelial cell markers CD31 and von Willebrand factor (Fig. 9–31, C).
Clinically, angiosarcomas are aggressive tumors that invade locally and metastasize. Current 5-year survival rates are only about 30%.
Hemangiopericytomas
These tumors derive their name from the cells of origin, the pericytes, myofibroblast-like cells that surround capillaries and venules. Recent studies indicate that tumors of pericytes are exceedingly rare and that most tumors previously assigned to this group have other cellular origins (e.g., fibroblasts). Accordingly, many are now placed in other diagnostic categories, such as solitary fibrous tumor, which often arises on the surface of the pleura.
![]() Summary
Summary
Vascular Tumors
• Vascular ectasias are not neoplasms, but rather dilations of existing vessels.
• Vascular neoplasms can derive from either blood vessels or lymphatics, and can be composed of endothelial cells (hemangioma, lymphangioma, angiosarcoma) or other cells of the vascular wall (e.g., glomus tumor)
• Most vascular tumors are benign (e.g., hemangiomas), some have an intermediate, locally aggressive behavior (e.g., Kaposi sarcoma), and others are highly malignant (e.g., angiosarcoma).
• Benign tumors typically form obvious vascular channels lined by normal-appearing endothelial cells. Malignant tumors more often are solid and cellular, exhibit cytologic aytpia, and lack well-defined vessels.
Pathology of Vascular Intervention
The morphologic changes that occur in vessels following therapeutic intervention—balloon angioplasty, stenting, or bypass surgery—recapitulate many of the changes that occur in the setting of other forms of vascular injury. Local trauma (due to stenting), vascular thrombosis (after angioplasty), and abnormal mechanical forces (e.g., a saphenous vein inserted into the arterial circulation as a coronary artery bypass graft) all induce the same stereotypical healing responses. Thus, just as with several risk factors for atherosclerosis, interventions that injure the endothelium also tend to induce intimal thickening by recruiting smooth muscle cells and promoting ECM deposition.
Endovascular Stenting
Arterial stenoses (especially those in coronary and carotid arteries) can be dilated by transiently inflating a balloon catheter to pressures sufficient to rupture the occluding plaque (balloon angioplasty); in doing so, a (hopefully) limited arterial dissection also is induced. Although most patients experience lessening of clinical signs and symptoms after angioplasty alone, abrupt reclosure can occur as a result of compression of the lumen by an extensive circumferential or longitudinal dissection, by vessel wall spasm, or by thrombosis. Thus, greater than 90% of endovascular coronary procedures now involve both angioplasty and concurrent coronary stent placement.
Coronary stents are expandable tubes of metallic mesh. They provide a larger and more regular lumen, “tack down” the intimal flaps and dissections that occur during angioplasty, and mechanically limit vascular spasm. Nevertheless, as a consequence of endothelial injury, thrombosis is an important immediate post-stenting complication, and patients must receive potent antithrombotic agents (primarily platelet antagonists) to prevent acute catastrophic thrombotic occlusions. The long-term success of angioplasty is limited by the development of proliferative in-stent restenosis. This intimal thickening is due to smooth muscle cell ingrowth, proliferation, and matrix synthesis, all driven by the initial vascular wall injury; it results in clinically significant luminal occlusion in 5% to 35% of patients within 6 to 12 months of stenting (Fig. 9–32).
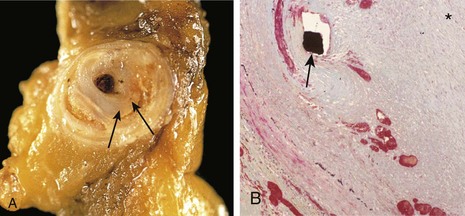
Figure 9–32 Restenosis after angioplasty and stenting. A, Gross view demonstrating residual atherosclerotic plaque (arrows) and a new, glistening intimal proliferative lesion. B, Histologic view shows a thickened neointima separating and overlying the stent wires (the black diamond indicated by the arrow), which encroaches on the lumen (indicated by the asterisk).
(B, Reproduced from Schoen FJ, Edwards WD: Pathology of cardiovascular interventions, including endovascular therapies, revascularization, vascular replacement, cardiac assist/replacement, arrhythmia control, and repaired congenital heart disease. In Silver MD, et al [eds]: Cardiovascular Pathology, 3rd ed. Philadelphia, Churchill Livingstone, 2001.)
The newest generation of drug-eluting stents is designed to avoid this complication by leaching antiproliferative drugs (e.g., paclitaxel, sirolimus) into the adjacent vessel wall to block smooth muscle cell activation. Although the duration of drug elution is short (on the order of days), use of these stents nevertheless reduces the incidence of restenosis at 1 year by 50% to 80%.
Vascular Replacement
Synthetic or autologous vascular grafts commonly are used to replace damaged vessels or bypass diseased arteries. Of the synthetic grafts, large-bore (12- to 18-mm-diameter) conduits function well in high-flow locations such as the aorta, while small-diameter artificial grafts (8 mm or less in diameter) generally fail as a result of acute thrombosis or late intimal hyperplasia, primarily at the junction of the graft with the native vasculature.
Consequently, when small-bore vessel replacement is needed (e.g., in the more than 400,000 coronary artery bypass surgeries per year), grafts generally are composed of either autologous saphenous vein (taken from the patient’s own leg) or left internal mammary artery (owing to its proximity to the heart). The long-term patency of saphenous vein grafts is only 50% at 10 years. These grafts occlude as a consequence of thrombosis (typically early), intimal thickening (months to years postoperatively), and vein graft atherosclerosis—sometimes with superimposed plaque rupture, thrombus formation, or aneurysms (usually more than 2 to 3 years later). By contrast, more than 90% of internal mammary artery grafts are patent after 10 years.
Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357. [A fascinating paper linking cholesterol microcrystals to phagocyte activation, cytokine production, and atherogenesis.]
Finn AV, Nakano M, Narula J, et al. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282. [Good overview of the evolving concepts regarding plaque stability.]
Ganem D. KSHV infection and pathogenesis of Kaposi’s sarcoma. Annu Rev Pathol. 2006;1:273. [Excellent scholarly review of the pathologic mechanisms underlying Kaposi sarcoma.]
Jaffe R, Strauss B. Late and very late thrombosis of drug-eluting stents: evolving concepts and perspectives. J Am Coll Cardiol. 2007;50:119. [Even-handed discussion of the complications following coronary artery stent placement.]
Jennette J, Falk R. Nosology of primary vasculitis. Curr Opin Rheumatol. 2007;19:10. [Classification of vasculitis based on pathogenic pathways and the vessels involved; provides a good organization to a complex and potentially confusing aspect of vascular pathology.]
Kallenberg C. Antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Curr Opin Rheumatol. 2007;19:17. [Up-to-date overview of the pathogenesis of ANCA-associated vasculitides.]
Libby P, Ridker PM, Hansson GK, et al. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129. [Excellent overview of the role of inflammation in atherosclerotic disease.]
Michel JB, Martin-Ventura JL, Egido J, et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18. [Good discussion of the molecular pathways underlying human abdominal aortic aneurysm formation.]
Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol. 2009;31:5. [Well-written review concerning the roles of innate and adaptive immune responses in the pathogenesis of atherosclerosis.]
Penel N, Marréaud S, Robin YM, Hohenberger P. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol. 2010 Nov 3. (Epub ahead of print.) [Extensive clinical summary of this aggressively malignant vascular tumor.]
Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol Mech Dis. 2009;4:71. [Well-written and scholarly review of the etiology and outcomes of endothelial injury.]
Ramirez F, Dietz H. Marfan syndrome: from molecular pathogenesis to clinical treatment. Curr Opin Genet Dev. 2007;17:252. [Excellent summary of the mechanisms and therapeutic targets in Marfan syndrome.]
Ridker P. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis towards consensus. J Am Coll Cardiol. 2007;49:2129. [A solid opinion piece regarding the utility of inflammatory markers in providing additional independent information for predicting cardiovascular events.]
Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009;6:399. [Good review of the interaction of risk factors, including the potential role of the metabolic syndrome in atherosclerotic disease.]
Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365:1577. [A good review of diagnosis, treatment, and pathogenesis.]
Singh M, Mensah GA, Bakris G. Pathogenesis and clinical physiology of hypertension. Cardiol Clin. 2010;28:545. [Excellent and up-to-date overview of normal blood pressure regulation and the interaction of genetics and environment in the pathophysiology of hypertension.]
Yasue H, Nakagawa H, Itoh T, et al. Coronary artery spasm—clinical features, diagnosis, pathogenesis, and treatment. J Cardiol. 2008;51:2. [Good clinical overview of coronary spasm and its sequelae.]