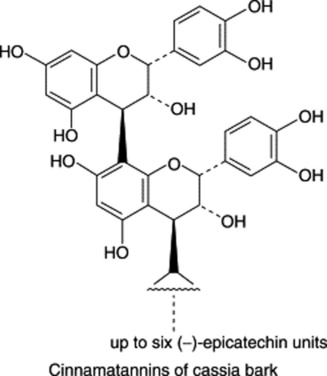
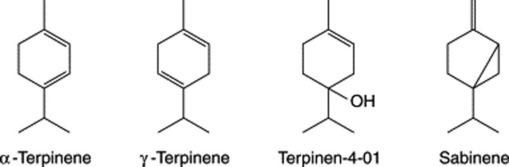
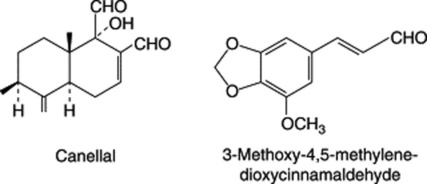
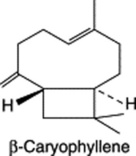
Chapter 22 Volatile oils and resins
VOLATILE OILS
Volatile or essential oils, as their name implies, are volatile in steam. They differ entirely in both chemical and physical properties from fixed oils. With the exception of oils such as oil of bitter almonds, which are produced by the hydrolysis of glycosides, these oils are contained largely as such in the plant. They are secreted in oil cells, in secretion ducts or cavities or in glandular hairs (see Chapter 42). They are frequently associated with other substances such as gums and resins and themselves tend to resinify on exposure to air.
Production and uses of volatile oils
Large quantities of volatile oil are produced annually; as examples, for lemon oil, eucalyptus oil, clove leaf oil and peppermint oil world production annually runs into several thousand metric tons each.
Although the production of major oils is highly organized, a number of developing countries have volatile oil-rich flora not fully utilized or cultivated and the United Nations Industrial Development Organisation has taken steps to inform on the setting-up of rural based small-scale essential oil industries (see ‘Further reading’). India and China now produce large quantities of oil for export.
Volatile oils are used for their therapeutic action, for flavouring (e.g. oil of lemon), in perfumery (e.g. oil of rose) or as starting materials for the synthesis of other compounds (e.g. oil of turpentine). For therapeutic purposes they are administered as inhalations (e.g. eucalyptus oil), orally (e.g. peppermint oil), as gargles and mouthwashes (e.g. thymol) and transdermally (many essential oils including those of lavender, rosemary and bergamot are employed in the practice of aromatherapy.
Those oils with a high phenol content, e.g. clove and thyme, have antiseptic properties, whereas others are used as carminatives. Oils showing antispasmodic activity, and much used in popular medicine, are those of Melissa officinalis, Rosmarinus officinalis, Mentha piperita, Matricaria chamomilla, Foeniculum vulgare, Carum carvi and Citrus aurantium. The antispasmodic activity of some of these oils has also been demonstrated experimentally. The constituents of many volatile oils are stated to interfere with respiration and electron transport in a variety of bacteria, hence accounting for their use in food preservation and in cosmetic preparations.
Composition of volatile oils
With the exception of oils derived from glycosides (e.g. bitter almond oil and mustard oil) volatile oils are generally mixtures of hydrocarbons and oxygenated compounds derived from these hydrocarbons. In some oils (e.g. oil of turpentine) the hydrocarbons predominate and only limited amounts of oxygenated constituents are present; in others (e.g. oil of cloves) the bulk of the oil consists of oxygenated compounds. The odour and taste of volatile oils is mainly determined by these oxygenated constituents, which are to some extent soluble in water (note orange-flower water, rose water, etc.) but more soluble in alcohol (note tinctures or essences of lemon, etc.). Many oils are terpenoid in origin; a smaller number such as those of cinnamon and clove contain principally aromatic (benzene) derivatives mixed with the terpenes. A few compounds (e.g. thymol, carvacrol), although aromatic in structure, are terpenoid in origin.
Evaluation
Various pharmacopoeial procedures are given for the evaluation of volatile oils. Odour and taste are obviously important in the preliminary examination; however oils should not be tasted neat but only after dilution with a sugar solution in ethanol as prescribed in the BP. Physical measurements including optical rotation, relative density and refractive index are regularly employed for identification and
assessment of purity; similarly thin-layer chromatograms. Capillary gas chromatographic profiles are used to determine the proportions of individual components of certain oils. Advances in gas chromatography have now made possible the ready determination of the chirality of particular components of volatile oils thus detecting adulteration with synthetic material or unwanted other oils; examples of its use are carvone in caraway oil, linalol in coriander oil and linalol and linalyl acetate in neroli oil. The ketone and aldehyde contents of oils such as caraway and lemon respectively are determined by reaction with hydroxylamine hydrochloride (oxime formation) and titration of the liberated acid. Other general tests described in the BP include examination for fixed and resinified oils (residue after evaporation), foreign esters (conversion to a crystalline deposit) and presence of water (turbidity of a carbon disulphide solution).
There have been a number of recent problems (1999) with the occurrence of tetrachloromethane in essential oils, particularly spearmint oil. This is probably not due to deliberate adulteration (the adulterant is present at low ppm levels) but a consequence of cleaning the drums before use with offending solvent and inefficient removal of it before filling the drums. Unfortunately on detection it renders the oil useless, as in food tetrachloromethane is prohibited.
The volatile oil content of crude drugs is commonly determined by distillation (Chapter 16).
Biogenesis
The origin of metabolites with phenylpropane and terpenoid structures has been discussed in Chapter 18. In medicinal essential oils the number of the former is limited but for the monoterpenes which arise at the geranyl pyrophosphate (GPP) level of terpenoid synthesis there are numerous examples. Analyses show that these oils commonly contain 40–80 monoterpenoids, many in relatively small proportion. A major constituent of one oil may be a minor one in another.
Three groups of monoterpenoid structures are involved: (1) acyclic or linear; (2) monocyclic; and (3) bicyclic. In the plant they are sequentially derived from limonene in this order as illustrated in Fig. 22.1. Relatively few enzymes, termed cyclases, appear to determine the skeletal class (e.g. menthanes, pinanes, thujanes etc.) and it is possible that these serve as rate-limiting enzymes. However, regulatory factors for the control of synthesis operate not only at the biosynthetic enzyme level per se but in a hierarchical manner up to the whole-organism level.
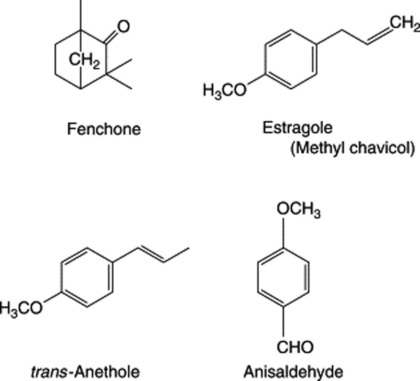
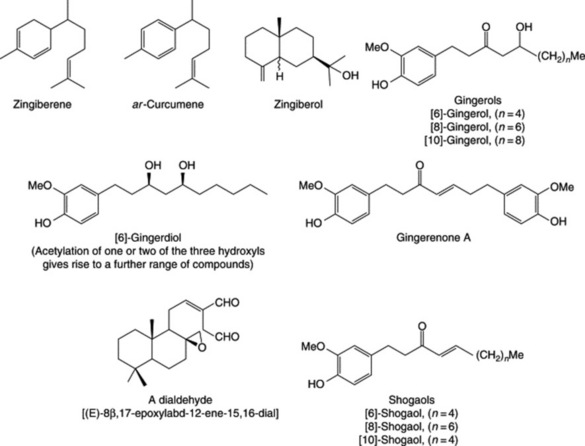
Because of the trans geometry of the double bond at the C-2 of GPP, direct cyclization to limonene is not possible and for Mentha spp. it has been shown that (−)-limonene synthase located within the oil glands catalyses the isomerization of GPP to enzyme-bound (+)-3S-linalyl pyrophosphate with subsequent cyclization to (−)-limonene. However, many enzymatic steps are involved in the subsequent modifications and interconversions of these monoterpenes. Some components of volatile oils are sesquiterpenes (C15H24) (q.v.) and they include cadinene, zingiberene (structure, Fig. 18.17) and caryophyllene. The formulae of some of the more common constituents of pharmaceutical volatile oils are given in Fig. 22.2.
It is increasingly evident that some monoterpenes and other components of volatile oils also occur in plants in the glycosidic form. Thus, geraniol, nerol and citronellol occur as glycosides in the petals of Rosa dilecta, thymol and carvacrol as glucosides and galactosides in Thymus vulgaris and eugenol, benzyl alcohol, β-phenylethyl alcohol, nerol, geraniol and geranic acid as glucosides in Melissa officinalis. It is considered that these glucosides of monoterpenols and of 2-phenylethanol are translocated from leaves to flowers as aroma precursors.
Table 22.1 may be used to compare the compositions of important volatile oils. The classification is arbitrary, since an oil may contain a number of compounds all about equally important but belonging to different chemical classes. The substance used for classification is not necessarily the one present in greatest amount. Thus, nutmeg is classified on its myristicin and lemon on citral, although these constituents form only a small percentage of these oils.
Table 22.1 Composition of volatile oils.
| Name | Botanical name | Important constituents |
|---|---|---|
| Terpenes or sesquiterpenes | ||
| Tea-tree | Melaleuca alternifolia | Cyclic monoterpenes |
| Turpentine | Pinus spp. | Terpenes (pinenes, camphene) |
| Juniper | Juniperus communis | Terpenes (pinene, camphene); sesquiterpene (cadinene); alcohols |
| Cade (Juniper Tar Oil) | Juniperus oxycedrus | Sesquiterpenes (cadinene); phenols (guaiacol, cresol) |
| Alcohols | ||
| Coriander | Coriandrum sativum | Linalol (65–80% alcohols); terpenes |
| Otto of rose | Rosa spp. | Geraniol, citronellol (70–75% alcohols); esters |
| Geranium | Pelargonium spp. | Geraniol; citronellol; esters |
| Indian or Turkish geranium (Palmarosa) | Cymbopogon spp. | Geraniol (85–90%) |
| Sandalwood | Santalum album | Santalols (sesquiterpene alcohols), esters, aldehydes |
| Esters and alcohols | ||
| Bergamot | Citrus bergamia | Linalyl acetate, linalol |
| Lavender | Lavandula officinalis | Linalol; linalyl acetate (much); ethyl-pentyl ketone |
| Rosemary | Rosmarinus officinalis | Borneol and linalol (10–18%); bornyl acetate, etc. (2–5%); terpenes; cineole |
| Pumilio pine | Pinus mugo var. pumilio | Bornyl acetate (about 10%); terpenes; sesquiterpenes |
| Peppermint | Mentha piperita | Menthol (about 45%); menthyl acetate (4–9%) |
| Aldehydes | ||
| Cinnamon bark | Cinnamomum verum Presl. | Cinnamaic aldehyde (60–75%); eugenol; terpenes |
| Cassia | Cinnamomum cassia | Cinnamic aldehyde (80%) |
| Lemon | Citrus limon | Citral (over 3.5%); limonene (about 90%) |
| Lemon grass | Cymbopogon spp. | Citral and citronellal (75–85%); terpenes |
| ‘Citron-scented’ eucalyptus | Eucalyptus citriodora | Citronellal (about 70%) |
| Ketones | ||
| Spearmint | Mentha spicata and M. cardiaca | Carvone (55–70%); limonene, esters |
| Caraway | Carum carvi | Carvone (60%); limonene, etc. |
| Dill | Anethum graveolens | Carvone (50%); limonene, etc. |
| Sage | Salvia officinalis | Thujone (about 50%); camphor; cineole etc. |
| Wormwood | Artemisia absinthium | Thujone (up to 35%); thujyl alcohol; azulenes |
| Phenols | ||
| Cinnamon leaf | Cinnamomum verum Presl. | Eugenol (up to 80%) |
| Clove | Syzygium aromaticum (L.) Merr & L. M. Perry | Eugenol (85–90%); acetyl eugenol, methylpentyl ketone, vanillin |
| Thyme | Thymus vulgaris | Thymol (20–30%) |
| Horsemint | Monarda punctata | Thymol (about 60%) |
| Ajowan | Trachyspermum ammi | Thymol (4–55%) |
| Ethers | ||
| Anise and Star-anise | Pimpinella anisum and Illicium verum | Anethole (80–90%); chavicol methyl ether, etc. |
| Fennel | Foeniculum vulgare | Anethole (60%); fenchone, a ketone (20%) |
| Eucalyptus | Eucalyptus globulus | Cineole (over 70%); terpenes, etc. |
| Cajuput | Melaleuca spp. | Cineole (50–60%); terpenes, alcohols and esters |
| Camphor | Cinnamomum camphora | After removal of the ketone camphor contains safrole; terpenes, etc. |
| Parsley | Petroselinum sativum | Apiole (dimethoxysafrole) |
| Indian dill | Peucedanum soja | Dill-apiole (dimethoxysafrole) |
| Nutmeg | Myristica fragrans | Myristicin (methoxysafrole) up to 4%; terpenes (60–85%); alcohols, phenols |
| Peroxides | ||
| Chenopodium | Chenopodium ambrosioides var. anthelmintica | Ascaridole (60–77%), an unsaturated terpene peroxide |
| Non-terpenoid and derived from glycosides | ||
| Mustard | Brassica spp. | Glucosinolates |
| Wintergreen | Gaultheria procumbens | Methyl salicylate |
| Bitter almond | Prunus communis var. amara | Benzaldehyde and HCN (from amygdalin) |
C20 or diterpenoid compounds include such resin acids as (+)- and (−)-pimaric acid and their isomer, the abietic acid of pine resin. The abietane acids have antimicrobial, antiulcer and cardiovascular properties (for a review covering some 56 acids over the period 1967–92 see A. S. Feliciano et al., Planta Med., 1993, 59, 485). Many diterpenoids (e.g. vitamin A and gibberellic acid) do not belong to the volatile oil–resin group. Similarly, only a small proportion of triterpenoid compounds (C30) are found as resin constituents (e.g. in Euphorbia resinifera).
Preparation of volatile oils
All the official volatile oils are extracted by distillation with the exception of oil of lemon and oil of cade. The distillation of volatile oils by means of water or steam has long been practised, but modern plants possess many advantages over the older stills, in which charring and undesirable decomposition of the oil often took place. Modern volatile oil stills contain the raw material on perforated trays or in perforated baskets. The still contains water at the base which is heated by steam coils, and free steam under pressure may also be passed in. Tough material such as barks, seeds and roots may be comminuted tofacilitate extraction but flowers are usually placed in the still without further treatment as soon as possible after collection. Distillation is frequently performed in the field.
The distillate, which consists of a mixture of oil and water, is condensed and collected in a suitable receiver which is usually a Florentine flask or a large glass jar with one outlet near the base and another near the top. The distillate separates into two layers, the oil being withdrawn through the upper outlet and the water from the lower outlet, or vice versa in the case of oils, such as oil of cloves, which are heavier than water. The oil-saturated aqueous layer may be returned to the still or may form an article of commerce, as in the case of rose water and orange-flower water.
Certain oils (e.g. oil of cajuput, oil of caraway, oil of turpentine and oil of Australian sandalwood) are rectified. Rectification usually takes the form of a second distillation in steam, which frees the oil from resinous and other impurities. Light and atmospheric oxygen appear to have an adverse effect on most volatile oils and the official directions with regard to storage should be rigidly followed. The distillation of oil of chenopodium must be done as rapidly as possible, as the chief constituent, ascaridole, gradually decomposes on boiling with water.
Extraction of oils used in perfumery
Certain oils used in perfumery, such as oil of rose, are prepared by steam distillation as described above but many of the flower perfumes require other treatment. An important centre for the extraction of flower oils is Grasse, in the south of France. Here the oils are extracted by enfleurage, by digestion in melted fats, by pneumatic methods or by means of solvents. In the enfleurage process glass plates are covered with a thin layer of fixed oil or fat upon which the fresh flowers are spread. The volatile oil gradually passes into the fat and the exhausted flowers are removed and replaced by a fresh supply. Formerly the flowers had to be picked off by hand but this is now done mechanically. Only a small percentage of the flowers, which resist the action of the machine, require removal by the fingers or by means of a vacuum cleaner. The pneumatic method, which is similar in principles to the enfleurage process, involves the passage of a current of warm air through the flowers. The air, laden with suspended volatile oil, is then passed through a spray of melted fat in which the volatile oil is absorbed. In the digestion process the flowers are gently heated in melted fat until exhausted, when they are strained out and the perfume-containing fat is allowed to cool. It will be seen that in each of the above processes the volatile oil has now been obtained in a fatty base. The volatile oil is obtained from this by three successive extractions with alcohol. The alcoholic solutions may be put on the market as flower perfumes or the oil may be obtained in a pure form by recovery of the alcohol. Solvent extraction is based on the Soxhlet principle (see Chapter 16).
Oil of rose
Oil of rose (Otto or Attar of Rose, Oleum Rosae) is a volatile oil obtained by distillation from the fresh flowers of Rosa damascena, R. gallica, R. alba and R. centifolia (Rosaceae). It is included in the USP/NF, 1995. The chief producing countries are Bulgaria, Turkey and Morocco but smaller quantities are prepared elsewhere.
The oil is prepared in copper alembic stills by the peasants or in large factories under careful scientific control. Some 3000 parts of flowers yield only one part of oil. The oil is very expensive and very liable to adulteration. The ‘peasant distilled’ oil usually fetches a lower price than that produced in the larger works.
The oil is a pale yellow semisolid. The portion which is solid at ordinary temperatures forms about 15–20% and consists of odourless stearoptene containing principally saturated aliphatic hydrocarbons (C14–C23 normal paraffins). The liquid portion forms a clear solution with 70% alcohol. It consists of the alcohols geraniol, citronellol, nerol and 2-phenylethanol with smaller quantities of esters and other odorous principles. Although the alcohols form about 70–75% of the oil, the odour is so modified by the other constituents, such as sulphur containing compounds, that no artificial mixture of the known constituents can be made to reproduce the odour of the natural oil. Phenylalanine has been shown to act as a precursor of the 2-phenylethanol; acetate and mevalonate are incorporated into the terpene alcohols. A citronellyl disaccharide glycoside has been identified as an aroma precursor of citronellol in flowers of R. damascena var. bulgaria (N. Oka et al., Phytochemistry, 1998, 47, 1527). Among a number of lines of callus derived from the leaf-bud of R. damascena a few have been shown to produce 2-phenylethanol.
From Rosa rugosa var. plena growing in central China some 108 compounds have been identified in the flower oil; these include citronellol (60%), geraniol (8.6%), nerol (2.8%), citronellyl acetate (2.7%) and E,E-farnesol (2.5%). For a review see Y. Hashidoko, Phytochemistry, 1996, 43, 535.
Oil of rose is of great importance in perfumery (for a fuller account of its history and utilization see Widrlechner, Econ. Bot., 1981, 35, 42).
PEPPERMINT LEAF AND PEPPERMINT OIL
Peppermint Leaf as defined in the BP and EP is the dried leaves of Mentha × piperita L. (Labiatae). It is required to contain not less than 1.2% of volatile oil. The oil is obtained from the same plant by steam distillation using the flowering tops. The European and American oil appears to be derived to a large extent from the two varieties M. piperita var. vulgaris Sole (‘black mint’) and M. piperita var. officinalis Sole (‘white mint’).
Mentha × piperita is, as implied by the written botanical name, a hybrid species from the two parents, M. spicata (2n = 36 or 48) and M. aquatica (2n = 96). M. × piperita strains commonly have a somatic number of 72, a smaller proportion 66, but other figures have also been reported.
Macroscopical characters
All the mints have square stems and creeping rhizomes. The flowers are arranged in verticillasters and have the floral formula K(5),C(5),A4,G(2). The black mint, which is the one most commonly cultivated in England, has purple stems and dark green petiolate leaves which are tinged with purple. The leaf blades are 3–9 cm long and have a grooved petiole up to 1 cm long. They have a pinnate venation with lateral veins leaving the midrib at about a 45° angle, acuminate apex and sharply dentate margin. Glandular trichomes can be seen as bright yellowish points when the lower surface is examined with a hand lens. The leaves are broader than those of M. spicata (spearmint), but narrower than those of M. aquatica (water mint). The small, purple flowers appear in late summer.
Microscopical characters
The microscopy of peppermint leaves is typical of the family, showing numerous diacytic stomata on the lower surface (Fig. 42.2G), three- to eight-celled clothing trichomes with a striated cuticle (Fig. 42.4C), and two types of glandular trichome, one with a unicellular base and small single-celled head and the other with a multicellular head characteristic of the family (Fig. 42.5E). Calcium oxalate is absent.
There is a 5% limit of stems over 1 mm in diameter for the official leaves, and as mints are very susceptible to most diseases, there is a 10% limit of leaves infected by Puccinia menthae.
Of commercial importance has been the development by mutation breeding at the A. M. Todd Co. of a strain of Mitcham peppermint resistant to the wilt disease Verticillium albo-duram var. menthae. The strain retains the Mitcham cultivar organoleptic characteristics and gives a good oil production in verticillium-prone soils where cultivation with the ordinary varieties is impossible.
OIL OF PEPPERMINT
The oil of the BP (1993) was required to contain 4.5–10% of esters calculated as menthyl acetate, not less than 44% of free alcohols calculated as menthol and 15–32% of ketones calculated as menthone. However, these chemical evaluations are now replaced by a capillary GC profile; limits for individual compounds are limonene 1.0–5.0%, cineole 3.5–14.0%, menthone 14–32%, menthofuran 1.0–9.0%, isomenthone 1.5–10.0%, menthylacetate 2.8–10.0%, menthol 30.0–55.0%, pulegone 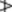 4.0%, carvone
4.0%, carvone 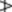 1.0%. The ratio of the cineole to limonene contents exceeds 2.
1.0%. The ratio of the cineole to limonene contents exceeds 2.
Small quantities of the sesquiterpene viridoflorol form a useful identification characteristic of the oil. A basic fraction of the oil contains a number of pyridine derivatives such as 2-acetyl-4-isopropenyl pyridine which has a powerful grass-like minty odour. High-resolution GC has been used to identify over 85 components of the oil.
As with other cultivated Labiatae, the oil composition of M. × piperita is greatly influenced by genetic factors and seasonal variations (see relevant chapters). The task of elucidating the nature of the genetic control for the formation of various constituents has been rendered difficult by the hybrid and polyploid nature of the genus. Much progress in this area was achieved by M. J. Murray, a mint breeder with the A. M. Todd Company, Kalamazoo, Michigan. His collection of over 600 accessions of mint species, which has continued to be researched and added to, now forms the basis of the collection of the USDA-ARS-National Clonal Germplasm Repository in Cornvallis, Oregon.
Biogenesis of peppermint monoterpenoids
The biosynthetic isoprenoid origin of monoterpenes was mentioned at the beginning of this chapter. As the essential oils of the Labiatae are synthesized in the cells of the glandular trichomes, techniques such as cell and root culture are of little value as experimental tools. However, new procedures, using gentle abrasion of leaf surfaces with glass beads, have been developed for isolating in high purity and excellent yield, peltate glandular trichomes of peppermint which retain their biosynthetic activity.
Developmental changes in the oil composition of the leaves include the disappearance of limonene, the accumulation of 1,8-cineole, the conversion of menthone to menthol and the acetylation of menthol. All these processes begin at the distal extremity of the leaf and shift progressively to the leaf base (B. Voirin and C. Bayer, Phytochemistry, 1996, 43, 573).
A proposed pathway for the formation of monoterpenes in peppermint is given in Fig. 22.3. A number of enzymes involved in the reactions have been characterized. The hydrolase system involving (−)-limonene-3-hydroxylase in the formation of the alcohol (−)-trans-isopiperitenol is cytochrome-P450-dependent and is associated with the oil gland microsomal fraction. The remaining steps are catalysed by operationally soluble enzymes of the oil cells. It will be noted that (+)-pulegone is a branching point for the formation of menthol stereoisomers.
Japanese peppermint oil is derived from Mentha canadenis var. piperascens; it contains 70–90% menthol, for the extraction of which it is largely used. The commercial dementholized Japanese oil contains approximately the same amount of menthol and its esters as the American oil.
DEMENTHOLIZED MINT OIL BP
This is cited as the volatile oil from Mentha arvensis var. piperascens from which the menthol has been partially removed. The two commercial oils, Brazilian and Chinese, differ somewhat in their ranges of ester and alcohol contents; standards are given for each. For both, the cineole:limonene ratio, as determined by GC, is less than unity.
SPEARMINT OIL
Spearmint or ordinary garden mint consists of the dried leaf and flowering top of Mentha spicata L. (M. viridis Linn.) and Mentha × cardiaca (Labiatae). The BP oil is prepared by steam distillation and should contain not less than 55% of carvone, 2–25% limonene with upper limits for a number of other constituents as determined by gas chromatography. The commercial oil was originally produced in North America but the industry has now largely transferred to India.
Characters
Mint has more or less crumpled, opposite, ovate-lanceolate leaves, 3–7 cm long. The apex is acute or acuminate, and the margin unequally serrate. The leaves differ from those of peppermint in that they are almost sessile and have a bright green colour free from purple.
Constituents
Oil of spearmint contains (−)-carvone, (−)-limonene, phellandrene and esters. As with M. × piperita limonene is the precursor of the monoterpenoids and in this case the action of a (−)-limonene-6-hydroxylase predominates to give the alcohol (−)-trans-carveol which is oxidized to carvone (Fig. 22.4). Dihydrocarvone is formed later in the season and is absent from plantlets produced by shoot-tip culture. Again like peppermint, oil production is influenced by the age of plant, time of collection, chemical varieties and hybridization.
SAGE LEAF
The official drug consists of whole or cut leaves of Salvia officinalis (Labiatae) containing not less than 1.5% (whole leaf) or 1.0% (cut leaf) of essential oil which is determined by steam distillation. The plant isindigenous to Mediterranean areas but is now cultivated world-wide, principally for its use as a culinary herb.
Macroscopical characters
The petiolate oblong-lanceolate leaves are up to 10 cm length and 2 cm in breadth, greenish-grey on the upper surface and tomentose on the lower with a markedly reticulate venation. The leaf apex is rounded, the base rounded or cordate and the margin crenulate. The odour and taste are characteristically pungent.
Microscopical characters
The upper epidermal cells have beaded anticlinal walls, the lower ones are thin-walled and sinuous; both epidermi possess diacytic stomata. Glandular trichomes of the typical labiate type occur on both surfaces with rarer uniseriate glandular trichomes having a double- or single-celled head. Clothing trichomes are numerous, particularly on the lower surface, composed of a short thickened basal cell with articulated and bent terminal cells. A few single-celled warty-walled trichomes are present. The long protective trichomes serve to distinguish S. officinalis from S. sclarea and S. pratensis (M. Then et al., Acta Pharm. Hung., 1998, 63, 163).
Constituents
The volatile oil of sage contains about 50% of α- and β-thujone together with cineole, borneol and other constituents (Fig. 22.2). Varieties and other species of sage contain differing amounts of thujone.
Non-volatile components of the leaf include diterpenes, phenolic glycosides based on caffeic and p-hydroxybenzoic acids (for recent isolations see M. Wang et al., J. Nat. Prod., 1999, 62, 454), and tannins.
Action and uses
Sage as an infusion is used as a mouthwash and gargle for its antiseptic and astringent action. Recent attention has focused on the cholinergic activity of the drug and its possible role in the treatment of Alzheimer’s disease and memory loss. It is interesting to note that long before recent advances in the understanding of the neurobiology of Alzheimer’s disease, plant materials including sage and balm (Melissa officinalis) were recommended in old reference books as possessing memory-improving properties (see E. K. Perry et al., J. Pharm. Pharmacol., 1999, 51, 527). The phenolic glycosides of sage together with those of Melissa officinalis and Lavandula angustifolia possess antioxidant properties (J. Hohmann et al., Planta Medica, 1999, 65, 576).
THREE-LOBED SAGE LEAF
Three-lobed sage leaf BP/EP also known as Greek sage, consists of the whole or cut, dried leaves of Salvia fructicosa Mill. (S. triloba L. fill) containing not less than 1.8% essential oil in the whole drug and not less than 1.2% in the cut drug. The leaves have a grey–green upper surface and conspicuously downy underside. They are somewhat larger (8–50 mm in length, 4–20 mm in width) than those of common sage and are considerably more pubescent. The clothing trichomes and odour (spicy and similar to that of eucalyptus oil) constitute features of the powder, which otherwise resembles S. officinalis.
An alcoholic extract of the drug subjected to TLC is used by the BP to detect the presence of cineole and the almost complete absence of thujone in the sample.
SAGE OIL
Sage oils are produced commercially by steam distillation from a number of Salvia species but the oil composition is not uniform, as illustrated by the three species considered here. For this reason, the BP/EP specifies one species, S. sclarea L., the clary sage, as the source of the official oil. The plant is a native of Mediterranean regions and had been introduced into England by the the 14th century.
The pharmacopoeia specifies the acceptable concentration limits for constituents of the oil as follows: α- and β-thujone (less than 0.2%), linalol (6.5–24.0%), linalyl acetate (50–80%); α-terpineol (less than 5.0%), germacrene-D (1.0–12%) and sclareol (0.4–2.6%).
The oil is widely used for flavouring and perfumery purposes.
ROSEMARY LEAF
Rosemary leaf BP/EP, BHP is the whole dried leaf of Rosmarinus officinalis L., family Labiatae. The plant is native to Mediterranean regions and is widely cultivated elsewhere in herb gardens and as an aromatic ornamental. Many horticultural varieties varying in habit and flower colour exist. Commercial supplies of the leaf come principally from Spain, Morocco and Tunisia.
R. officinalis is an aromatic evergreen shrub, variable in its form, but mostly with stems reaching a height of over 1 m. The bilobed corollas of the flowers are pale to dark blue and occur clustered in spikes at the ends of the branches; they are larger than those of either lavender or the mints. The leathery, opposite leaves are up to 4 cm long and up to 4 mm wide with entire strongly recurved margins and prominent midribs. The upper surfaces are green, the lower ones grey and somewhat woolly due to numerous branched trichomes. Typical labiate hairs contain the volatile oil, of which the BP specifies a minimum content of 1.2% calculated on the anhydrous drug.
Constituents
The compositon of the essential oil is considered under ‘Rosemary Oil’, below. Hydroxycinnamic acids include caffeic acid and a dimer rosmarinic acid (a characteristic metabolite of the subfamily Saturejoidae to which Rosmarinus belongs). For the dried leaf, the BP sets a minimum requirement of 3.0% for total hydroxycinnamic acids expressed as rosmarinic acid.
In recent years, a large number of phenolic abietane diterpenoids have been recorded for the leaves including the potent antioxidant carnosic acid together with its degradation products carnosol, rosmanol, epirosmanol and 7-methylepirosmanol. For new recently isolated diterpenes, see A. A. Mahmoud et al., Phytochemistry, 2005, 66, 1685; C. L. Cantrell et al., J. Nat. Prod., 2005, 68, 98. Triterpenes include the alcohols α- and β-amyrin, ursolic acid and oleanolic acid.
In vitro cell cultures of rosemary have been produced which biosynthesise carnosic acid, carnosol and rosmarinic acid (A. Kuhlmann and C. Rohl, Pharm. Biol., 2006, 44, 401).
ROSEMARY OIL
Rosemary oil is steam distilled from the flowering aerial parts of Rosmarinus officinalis L. The fresh material yields about 1–2% of a colourless to pale yellow volatile oil with a very characteristic odour. It contains 0.8–6% of esters and 8–20% of alcohols. The principal constituents are 1,8-cineole, borneol, camphor, bornyl acetate and monoterpene hydrocarbons, principally α-pinene and camphene. Many minor components have been identified. Chemical races (G. Flamini et al., J. Agric. Food Chem., 2002, 50, 1512) and geographical variants concerning the proportions of constituents in the oils are known. The BP/EP accordingly gives the limits for the percentage content of 12 constituents for oils of the Spanish type and for those of the Moroccan and Tunisian type. These are determined by gas chromatography.
The oil is frequently used in aromatherapy, in the perfumery industry and for the preparation of spirits and liniments for medical use; it has antibacterial and antispasmodic properties.
LEMON BALM
Lemon balm BP/EP consists of the dried leaf of Melissa officinalis L. family Labiatae/Lamiaceae; Balm leaf BHP 1990 is from the same source but also includes the flowering tops. The plant is a perennial herb native to southern and eastern Mediterranean regions but now widely grown in gardens for its aroma or for culinary purposes and cultivated commercially in Eastern Europe and Spain.
The leaves are opposite on a hairy quadangular stem; flowering branches arise in the axils of the lower leaves and flowers in clusters at the upper ends of the stems. The two-lipped corollas are initially white, changing later to pale blue or pink; the calyx is toothed with long spreading hairs. Leaf blades are 3–7 cm in length, longly petiolate on the lower parts of stems but shortly so on the upper parts, margins are deeply crenate or serrate, veins are prominent on the lower pale-green surface. Microscopical features are characteristic of the Lamiaceae and include eight-celled glandular trichomes, clothing trichomes and diacytic stomata on the lower epidermis.
Lemon balm yields only a small quantity of volatile oil (0.06–0.4%), which none the less gives the plant, when crushed, its strong lemon-like odour. Principal components of the oil are the aldehydes citral (composed of the isomers geranial and neral) and citronellal. Other components in smaller proportions are citronellol, nerol and the sesquiterpene β-caryophyllene; in all, over 70 constituents have been reported. Due to the low yield of oil from the plant, lemon balm oil is subject to adulteration with lemon-grass oil (Cymbopogon citratus), lemon-scented verbena oil (Aloysia triphylla) or various citrus products.
The BP/EP drug is assayed on its total content (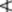 4.0%) of hydroxycinnamic acid derivatives expressed as rosmarinic acid (p. 270); these are mainly structurally related to caffeic acid. Other constituents are flavonoids, principally luteolin glycosides (Table 21.5).
4.0%) of hydroxycinnamic acid derivatives expressed as rosmarinic acid (p. 270); these are mainly structurally related to caffeic acid. Other constituents are flavonoids, principally luteolin glycosides (Table 21.5).
For over 2000 years lemon balm has been used for medicinal and culinary purposes. It is used traditionally for its sedative, spasmolytic and antibacterial properties; more recently it has been investigated by a number of researchers for its topical use in the treatment of Herpes labialis.
THYME
A number of Thymus species have been used traditionally for their medicinal and culinary properties. Under the above heading, the BP/EP recognizes the leaves and flowers separated from the dried aerial parts of T. vulgaris L. and T. zygis L., family Labiatae, or mixtures of the two. The former is the garden thyme or common thyme, native to Mediterranean regions, and possibly introduced into Britain by the Romans; the latter is also known as Spanish thyme.
Both species have similar morphological and microscopical characteristics and can be difficult to distinguish in the dried state. Stems above 15 mm in length and over 1 mm in diameter are limited to 10% by the pharmacopoeia. The grey–green leaves are slightly hairy on the upper surface and densely so on the lower surface, up to 12 mm long, and 3 mm wide, opposite, sessile and ovate to lanceolate in shape with slightly rolled edges. Under the microscope, both species show on the lower surface volatile oil-containing glandular trichomes typical of the Labiatae and numerous warty-walled clothing trichomes. The characteristic elbow-shaped trichomes of T. vulgaris are illustrated in Fig. 42.4. Numerous thick bundles of fibres are apparent in the powder of T. zygis.
Constituents
The volatile oil composition of thyme can vary enormously and various chemotypes have been recorded, particularly regarding thymol and carvacrol. The official drug is required to contain not less than 1.2% volatile oil, of which not less than 40% consists of thymol and carvacrol. It is these phenols that are largely responsible for the antiseptic, antitussive and expectorant properties of the drug. Other common variables of the oil are cymene (10–24%) and γ-terpinene (4–18%). Two extreme variations recorded are an almost complete lack of thymol and carvacrol in T. vulgaris and an oil containing 74% thymol from T. zygis.
A number of monoterpenoid glycosides occur in the leaves, particularly glucosides and galactosides of thymol and terpineol; seven new such constituents have recently been described (J. Katajima et al., Phytochemistry, 2004, 65, 3279; Chem. Pharm. Bull., 2004, 52, 1013). Flavones, highly oxygenated flavones, flavanones and dihydroflavonals may be responsible for the spasmolytic effect of the leaves, and the biphenyls reported in 1989 may have deodorant properties. Other constituents include rosmarinic acid (see ‘Rosemary’) up to 2.6%, various acids, tannins and resins. Rosmarinic acid and 3′-O-(8″-Z-caffeoyl) rosmarinic acid have been reported as the most important radical scavengers of the leaves (A. Dapkevicins et al., J. Nat. Prod., 2002, 65(6), 892).
THYME OIL
Thyme Oil BP/EP is obtained by steam distillation from the fresh flowering aerial parts of Thymus vulgaris L., T. zygis Loefl. ex L., or a mixture of both species. The oil therefore resembles that obtained from the official drug described above but reflects any changes that occur during drying and storage. The oil may vary in colour from yellow to dark reddish-brown; it has an aromatic spicy odour suggesting that of thymol.
As with the whole drug, the constituents are subject to variation due to geographic and genetic factors. The BP therefore requires a gas chromatographic profile and provides limits for the proportions of major constituents, which are: β-myrcene (1–3%), γ-terpinene (5–10%), p-cymene (15–28%), linalol (4.0–6.5%), terpinen-4-ol (0.2–2.5%), thymol (36–55%) and carvacrol (1.0 and 4.0%).
WILD THYME
The BP/EP drug is defined as the whole or cut, dried, flowering aerial parts of Thymus serpyllum L.s.l. It is required to contain a minimum of 0.3% essential oil (dried drug).
The species is an extremely variable aggregate, differing in its forms and chemical constituents both locally and across its wider geographical distribution. It grows on heaths, dry grasslands, dunes and in rocky environments extending from coasts to the lower mountain slopes of central and northern Europe, including the UK.
Wild thyme is used both medicinally and as a flavour in a similar manner to the common thyme, but is less powerful in its actions. The principal constituents are again thymol and carvacrol, which, however, vary appreciably according to source; some chemotypes contain neither of these phenolic compounds. For quality control, the BP/EP relies on the minimum oil content (as above) and TLC to indicate the presence of thymol and carvacrol and to give an indication of their respective concentrations.
For those essential oils having a low phenol content, major constituents have been variously reported as cineole, β-caryophyllene, nerolidol, myrcene, geranyl acetate and linalyl acetate. Other constituents of the drug (flavonoids, various acids, triterpenes) again resemble those of garden thyme.
The drug has been used traditionally for the treatment of respiratory infections, gastrointestinal problems and skin conditions requiring an antiseptic.
For an elaboration of the chemical constituents, pharmacological actions, therapeutics and research references concerning the thymes, see P. Bradley, 2006, British Herbal Compendium, Vol. 2, pp. 369–375; 389–392. British Herbal Medical Association, Bournemouth, UK. For a complete overview of all aspects of the genus, see Stahl-Biskup, E. (ed.), Hardman, R. (series ed.) 2002 Thyme: the genus Thymus. Taylor and Francis, New York. 230 pp., 956 references.
OREGANO
There is a large number of marjorams, and various varieties are cultivated extensively for ornamental and culinary purposes. Two medicinally used species described in the BP/EP are Origanum onites L. (syn. Majoram onites) the pot marjoram or Greek oregano, and O. vulgare L. subsp. hirtum (Link) Ietsw., a subspecies of the wild marjoram, or oregano, family Labiatae. The dried leaves and flowers are separated from the stems; a mixture of both species may be used. Both have a strong, thyme-like odour. Both appear similar in the dried state but the leaves of O. onites are yellowish–green whereas those of O. vulgare are more distinctly green. In the powdered form, both show typical laminaceous characteristics.
In view of the diverse nature of the genus, with many varieties of the above and the fact that other plants may be sold commercially under the name ‘oregano’, the characteristics of the oil are important. The BP/EP requires a minimum of 2.5% oil in the drug and a minimum 1.5% carvacol and thymol. Other constituents of the oil include caryophyllene, β-bisabolene, cymene, linalool and borneol. Plants grown near the Mediterranean coast are stated to be the most fragrant of all. Tannins, sterols, flavonoids and resin have been reported in the drug. A reddish dye can be obtained from the aerial parts of O. vulgare.
LAVENDER FLOWER
The general term ‘lavender’ applies to a number of species and numerous hybrids and varieties of the genus Lavandula, plants used from classical times for their aromatic and medicinal properties. The generic name derives from the Latin lavare, referring to the use of lavender by the Romans as a bath perfume. The numerous cultivated varieties vary in their flower colour (blue through purple to white), habit, foliage and, importantly, their oil composition as indicated under ‘Lavender Oil’ below; many are hybrids and do not breed true.
Lavender flower BP/EP 2007, BHP 1983 consists of the dried flowers of L. angustifolia P.Mill. (L. officinalis Chaix). It is required (BP) to contain a minimum volatile oil content of 1.3% expressed on a dry weight basis.
The flowers, up to 5 mm in length, are packed closely together in verticillasters on a quadrangular stem forming a compact terminal spike. Each verticillaster consists of six to ten shortly stalked flowers. In the fresh condition, oily glandular trichomes can be discerned among the numerous covering trichomes on the surface of the five-lobed calyx. The blue corolla is bilabiate with an upper bifid lip and a lower three-lobed lip.
Microscopical features of the powder include fragments of the calyx and corolla with numerous associated glandular and clothing trichomes; prismatic crystals of calcium oxalate in cells of the calyx; pollen grains up to about 35 μm in diameter with six pores and six pitted lines radiating from the poles; vascular tissue from the pedicel.
Gas chromatographic analysis of the oil isolated in the volatile oil determination above is employed by the BP/EP to establish the absence in the sample of species and varieties other than L. angustifolia. The chromatogram obtained is compared with that of a reference solution containing five compounds expected to be found in the oil of a genuine sample; the peak for camphor should not exceed 1% of the total area of the peaks thus excluding camphoraceous species such as L. latifolia.
Uses
Although it is the volatile oil of lavender that is principally used for medicinal purposes the BHP 1983 cites flatulent dyspepsia, colic and depressive headache as indications for use of the flowers. It may be used in combination with other drugs such as rosemary, valerian, meadow-sweet and others.
LAVENDER OIL
The botanical source of lavender oil is Lavandula angustifolia Miller (Lavandula officinalis Chaix), family Labiatae. Originally (BP 1980) oil from this species was referred to as ‘foreign oil’ to distinguish it from that of L. intermedia Loisel, which was termed ‘English oil’. The latter has a much finer fragrance than the Continental oil and there were separate pharmacopoeial standards for the two oils. Unfortunately the straggly habit of the English lavender does not lend itself to mechanical harvesting and oil produced commercially is now of the Continental type. France, once the principal producer, has been superseded by Bulgaria, with smaller quantities of oil coming from the former USSR, Australia and other countries.
Lavender oil types
The taxonomy of the lavenders is confusing and Continental oils differ among themselves owing to the fact that a number of different species, varieties and hybrids are distilled. The true lavender, L. officinalis, yields the best oil when grown at a fairly high altitude, the variety growing under these conditions being known as ‘petite lavande’. At a lower altitude the ‘lavande moyenne’ yields a somewhat less esteemed oil. ‘Grande lavande’, L. latifolia Villers (L. spica DC), yields a much coarser oil, which is sold as oil of spike. The above plant readily hybridizes with L. officinalis yielding a plant known as ‘grosse lavande’ or ‘lavandin’, the oil of which is intermediate in character between that of the parent forms. According to Tucker (Baileya, 1981, 21, 131), of the many names applied to this hybrid species, the correct one is L. × intermedia Emeric ex Loiseleur. As hybrids the plants do not breed true and are normally propagated vegetatively; a simple efficient method for the in vitro shoot regeneration from the leaves has offered possibilities for future breeding (S. Dronne et al., Plant Cell Reports, 1999, 18, 429). The lavandin oil market is controlled by the French with Spain the principal producer.
The evergreen plant flowers from July to September and the fresh flowering spikes yield about 0.5% of volatile oil. The amount varies according to variety, season and method of distillation; modern steam stills give a rather larger yield than those in which the flowers are boiled with water. Genuine Continental lavender oil normally contains over 35% of esters. Oil of spike, which is largely used in cheap perfumery, contains little ester but a high proportion of free alcohols (about 23–41% calculated as borneol); 30 components have been identified. The nature of the alcohols also varies from a mixture of linalol and geraniol in the best lavender oil to borneol in oil of spike. Hybrids are of intermediate character (e.g. ‘lavandin oil’) and contain about 6–9% of esters and about 35% of alcohols. An analysis of the Spanish oil (J. de Pascual Teresa, Planta Medica, 1989, 55, 398) enabled the identification of 50 compounds, the principal ones being 1:8-cineole, linalol and camphor; in contrast to the oil of L. angustifolia, linalyl acetate was not present.
A GC profile together with prescribed percentage ranges of 10 components of the pharmaceutical oil is given in the BP/EP; linalol (20–45%) and linalyl acetate (25–46%) are the principal constituents with a maximum limit for camphor of 1.2%. Chiral chromatography is used to determine the chiral purity of the linalol and linalyl acetate contents.
As with other Labiatae, Lavandula cell cultures do not produce essential oil and for L. vera rosmarinic acid is the principal phenolic component together with caffeic acid and traces of others. An enol ester of caffeic acid is a blue pigment also found in cell cultures (see E. Kovatcheva et al., Phytochemistry, 1996, 43, 1243).
Species of Lavandula other than the above are also cultivated. L. stoechas has a markedly different odour and of its 51 volatile components, fenchone, pinocaryl acetate, camphor, eucalyptol and myrthenol predominate. Large producers are Spain and France. Oil from wild plants growing in the Algiers region of Algeria contained as significant constituents fenchone (31.6%), camphor (22.4%), p-cymene (6.5%) lavandulyl acetate (3.0%) and α-pinene (1.0%). Fifty-four components amounting to ca 73% of the oil were identified (T. Dob et al., Pharm Biol., 2006; 44, 60).
Uses
Lavender oil is principally used in the toiletry and perfumery industries and occasionally in ointments, etc., to mask disagreeable odours. It is employed pharmaceutically in the antiarthropod preparation Gamma Benzene Hexachloride Application. Lavender flowers are included in the BHP and are indicated for the treatment of flatulent dyspepsia and topically, as the oil, for rheumatic pain. The oil is extensively used in aromatherapy (q.v.).
CARAWAY FRUIT
Caraway (Caraway Fruit) consists of the dried, ripe fruits of Carum carvi (Umbelliferae), a biennial herb about 1 m high. It occurs both wild and cultivated in central and northern Europe (The Netherlands, Denmark, Germany, Russia, Finland, Poland, Hungary and Britain) and in Egypt, Morocco, Australia and China.
History
Caraway fruits were known to the Arabian physicians and probably came into use in Europe in the thirteenth century.
Macroscopical characters
The commercial drug (Fig. 22.5) usually consists of mericarps separated from the pedicels. The fruits are slightly curved, brown and glabrous, about 4–7 mm long, 1–2.3 mm wide and tapered at both ends; they are crowned with a stylopod often with style and stigma attached. Each mericarp shows five almost equal sides, five narrow primary ridges, and, when cut transversely, four dorsal and two commissural vittae. They have a characteristic aromatic odour and taste.
Fig. 22.5 Caraway. A, mericarps showing attachments to carpophore; A1, mericarp sectioned longitudinally to show position of embryo; A2, mericarp side view (×8). B, transverse section of mericarp (×50); C, portion of vitta isolated by alkali maceration (×25); D, sclereids of mesocarp; E, endosperm cells with micro-rosette crystals of calcium oxalate; F, endocarp layer in surface view (all ×200). c.m, commissural meristeles; em, embryo; en, endosperm; end, endocarp; mc, mesocarp; r, three of five primary ridges; ra, position of raphe; s, stylopod; s.c, secretory canal; t, testa; v, vitta; v.b, vascular bundle with associated finely pitted sclerenchyma.
Microscopical characters
A transverse section of a caraway mericarp (Fig. 22.5) shows five primary ridges, in each of which is a vascular strand with associated pitted sclerenchyma and having a single secretory canal at the outer margin of each. The six vittae which appear somewhat flattened and elliptical in transverse section may attain a width of 350 μm; they extend from the base of the fruit to the base of the stylopod. They are lined with small, dark reddish-brown cells and contain a pale yellow or colourless oleoresin (Fig. 22.5B, C). The raphe lies on the inner side of the endosperm, which is non-grooved. Occupying the majority of the transverse section is the endosperm, with thickened cellulose walls (having also deposits of a β-(1,4)-mannan as a reserve polysaccharide) and containing fixed oil and aleurone grains having one or two microrosettes of calcium oxalate. The embryo, which is situated near the apex of the mericarp, will only be seen in sections passing through that region.
More detailed examination shows that the outer epidermis of the pericarp is glabrous (cf. aniseed) and has a striated cuticle (cf. fennel). The mesocarp consists of more or less collapsed parenchyma and lacks the reticulated cells of fennel. The endodermis (or inner epidermis of the pericarp) (Fig. 22.5F) consists of a single layer of elongated cells, arranged more or less parallel to one another and not showing the ‘parquetry’ arrangement of coriander.
CARAWAY OIL
The volatile oil (Caraway Oil BP/EP) consists largely of the ketone carvone and the terpene limonene (formulae, Fig. 22.4) with small quantities of dihydrocarvone, carveol and dihydrocarveol. As there is a demand for pure carvone, there is a considerable amount of decarvonized oil available for adulteration.
Official tests include a TLC examination to ascertain the presence of carvone and carveol and the measurement of optical rotation (+65° to +81°), refractive index (1.484–1.490) and acid value (maximum 1.0). The proportions of individual components are required to fall within certain limits as determined by gas chromatography: limonene (30–45%), carvone (50–65%), β-myrcene (0.1–1.0%) with maximum limits for trans-dihydrocarvone and trans-carveol (both 2.5%). The gas chromatographic chirality assay limits (−)-carvone to 1.0%.
DILL AND DILL OIL
Dill (Dill Fruit) consists of the dried, ripe fruits of Anethum graveolens (Umbelliferae), a small annual indigenous to southern Europe. It is cultivated in Central and Eastern Europe and Egypt. Dill was known to Dioskurides and was employed in England in Anglo-Saxon times.
Macroscopical characters
The drug usually consists of separate, broadly oval mericarps, about 4 mm long and 2–3 mm broad. The fruits are very much compressed dorsally, the two central ridges being prolonged into membranous wings, while the dorsal ones are inconspicuous. The fruits have an aromatic odour and taste similar to those of caraway.
Microscopical characters
Each mericarp has four vittae on the dorsal surface and two on the commissural. The outer epidermis has a striated cuticle (distinction from fennel), and the mesocarp contains lignified, reticulate parenchyma (distinction from caraway). The endosperm is much flattened but otherwise resembles that of caraway.
Constituents
The volatile oil (Dill Oil BP/EP) resembles oil of caraway in containing carvone and limonene. The European fruits yield about 3–4% of volatile oil, which should contain from 43 to 63% of carvone; the carvone content is determined by reaction with hydroxylamine hydrochloride (oxime formation) and titration of the liberated acid. Other constituents reported for the oil include trans- and cis-dihydrocarvone, trans- and cis-carveol, limonene, D- and L-dihydrocarveol, α- and γ-terpinene, α-phellandrene and others. Chemical types based on the proportion of carvone present, and the presence or absence of dillapiole and myristicin have been distinguished.
Monoterpene glycosides have been isolated from the water-soluble fraction of the fruits.
For further details on constituents, see T. Ishikawa et al., Chem. Pharm. Bull., 2002, 50, 501; M. Kosar et al., Pharm. Biol., 2005, 41, 491.
CORIANDER AND CORIANDER OIL
Coriander (Coriander Fruit) of the BP is the dried, nearly ripe fruit of Coriandrum sativum (Umbelliferae), an annual about 0.7 m high with white or pinkish flowers. It is indigenous to Italy, but is widely cultivated in The Netherlands, Central and Eastern Europe, the Mediterranean (Morocco, Malta, Egypt), China, India and Bangladesh. Coriander is mentioned in the papyrus of Ebers (c. 1550 BC), and in the writings of Cato and Pliny. It was well known in England before the Norman Conquest. Ukraine is the major producer of oil and controls the world price on a supply and demand basis; in one large factory continuous distillation has replaced the batch process.
Macroscopical characters
The drug (Fig. 22.6A) usually consists of the whole cremocarps, which, when ripe, are about 2.3–4.3 mm diameter and straw-yellow. Each consists of two hemispherical mericarps united by their margins. Considerable variation exists in coriander. The Indian variety is oval, but the more widely distributed spherical varieties vary in size from the Ukrainian 2.3–3.7 mm to the Moroccan 4.0–4.3 mm. The apex bears two divergent styles. The 10 primary ridges are wavy and inconspicuous; alternating with these are eight more prominent, straight, secondary ridges. The fruits have an aromatic odour and a spicy taste. They are somewhat liable to insect attacks.
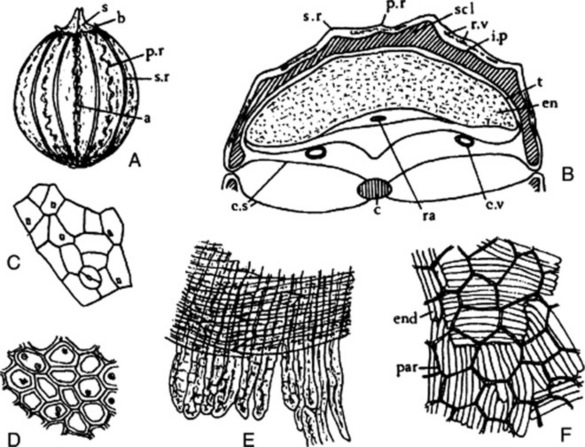
Fig. 22.6 Coriander. A, Whole fruit (×8); B, transverse section of fruit (×16); C, fragment of epicarp in surface view with stoma and small prismatic crystals of calcium oxalate; D, endosperm cells with microrosette crystals of calcium oxalate; E, layers of sclerenchyma from the mesocarp; F, lignified parenchyma of the mesocarp and underlying endodermis showing ‘parquetry’ arrangement (all ×200). a, line of attachment of mericarps; b, sepal; c, carpophore, c.s, commissural surfaces; c.v, commissural vitta; en, endosperm; end, endodermis; par, lignified parenchyma of mesocarp; p.r, primary ridge; ra, raphe; r.v, remains of dorsal vittae; s, stylopod; scl, sclerenchyma; s.r, secondary ridge; t, testa.
Microscopical characters
A transverse section of a fully ripe fruit shows only two mature vittae in each mericarp, both on the commissural surface (Fig. 22.6B). The numerous vittae present in the immature fruit on the dorsal surface of each mericarp gradually join and are eventually compressed into slits. The outer part of the pericarp, which possesses stomata and prisms of calcium oxalate, is more or less completely thrown off. Within the vittae-bearing region of the mesocarp a thick layer of sclerenchyma is formed, which consists of pitted, fusiform cells. These sclerenchymatous fibres tend in the outer layers to be longitudinally directed and in the inner layers to be tangentially directed. In the region of the primary ridges more of the fibres are longitudinally directed; in the secondary ridges nearly all are tangentially directed. Traversing the band of sclerenchyma and corresponding in position to the primary ridges are small vascular strands composed of a small group of spiral vessels. The mesocarp within the sclerenchymatous band is composed of irregular polygonal cells with lignified walls. The inner epidermis of the pericarp is composed of ‘parquetry’ cells, which in the powder are often seen united to the cells of the inner mesocarp. The testa is composed of brown flattened cells. The endosperm is curved and consists of parenchymatous cells containing fixed oil and aleurone grains. The latter contain rosettes of calcium oxalate 3–10 mm diameter (see Fig. 22.6 C–F).
Constituents
Coriander fruits contain up to 1.8% of volatile oil according to origin (BP/EP standard not less than 0.2%). The distilled oil (Coriander Oil BP/EP) contains 65–70% of (+)-linalool (coriandrol), depending on the source, and smaller amounts of α-pinene, γ-terpinene, limonene and p-cymene together with various non-linalool alcohols and esters. Some 40 constituents have been identified. The BP/EP uses GC for the evaluation of the oil with linalool and geraniol as internal standards; there is also a test for chiral purity ((R)-linalool, maximum 14%). Other constituents isolated from the fruits include flavonoids, coumarins, isocoumarins, phthalides and phenolic acids. T. Ishikawa et al. (Chem. Pharm. Bull., 2003, 51, 32) obtained 33 compounds from the water-soluble fraction of a methanolic extract of the fruits; new constituents included monoterpenoids, monoterpenoid glycosides, monoterpenoid glucoside sulphates and aromatic compound glycosides. The high content of fats (16–28%) and protein (11–17%) in the fruits make distillation residues suitable for animal feed. The fruits yield 5–7% of ash.
The unripe plant has an unpleasant, mousy odour, which is also present in oil distilled from unripe fruits (mainly aldehydes such as n-decanal contained in peripheral vittae). Marked changes occur in volatile oil composition during ontogenesis; the peripheral vittae flatten and lose their oil, all of which is then produced by the commissural vittae. During ordinary storage of the fruits, the oil composition undergoes considerable alteration.
Uses
Very large quantities of the spice are produced in many countries for domestic purposes, such as for use in curries. In the former USSR linalool is isolated from the oil as starting material for other derivatives. Pharmaceutically coriander and its oils are used as a flavouring agent and carminative.
ANISEED AND ANISEED OIL
Aniseed (Anise Fruit) of the BP and EP consists of the dried, ripe fruits of Pimpinella anisum (Umbelliferae), an annual plant indigenous to the Levant but widely cultivated both in Europe (Spain, Germany, Italy, Russia, Bulgaria), Egypt and America (Chile, Mexico). Anise is mentioned in the writings of Theophrastus, Dioskurides and Pliny. It was cultivated in Germany in the ninth century. Spain and Egypt are the principal producers of the oil.
Macroscopical characters
The drug (Fig. 22.7A) consists of greyish-brown, pear-shaped, somewhat compressed cremocarps, which are usually attached to pedicels 2–12 mm in length. The cremocarps are 3–6 mm long and 2–3 mm broad. The Spanish (Alicante) and Italian are distinguished by their large size and light colour, while the German and ‘Russian’ are smaller, more ovoid and darker. Each mericarp has five somewhat wavy ridges and is slightly pubescent on the dorsal surface. They have an aromatic odour and a sweet, aromatic taste.
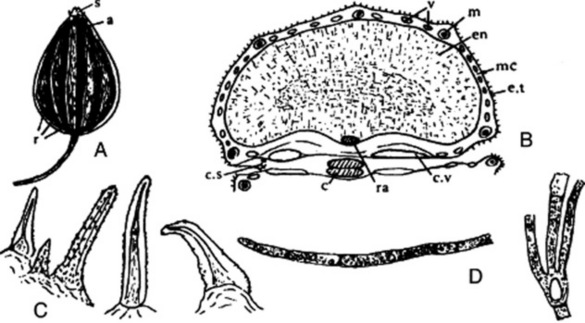
Fig. 22.7 Aniseed. A, Side view of cremocarp showing line of attachment to the two mericarps (×8); B, transverse section of mericarp (×25); C, covering trichomes of epicarp (×200); D, branched and unbranched vittae isolated by alkali maceration (×25). a, Line of attachment of mericarps; c, carpophore; c.s, commissural surfaces; c.v, commissural vitta; en, endosperm; e.t, epicarp bearing trichomes; m, meristele; mc, mesocarp; r, three of five primary ridges of one mericarp; ra, raphe; s, stylopod; v, vittae.
Microscopical characters
Microscopical examination shows that the epidermis bears numerous papillae and unicellular hairs. On the dorsal surface of each mericarp are from 15 to 45 branched vittae. A small amount of vascular tissue and reticulated parenchyma is present. The endosperm is slightly concave on the commissural surface and contains protein and fixed oil (see Fig. 22.7B–D).
STAR ANISE FRUIT AND OIL
The star-anise Illicium verum Hook, f., family Illiciaceae, is an evergreen tree about 4–5 m in height, indigenous to the south-west provinces of China. The fruits are collected and the oil distilled locally in China and Vietnam.
The fruits consist of eight (rarely seven or nine) one-seeded follicles. Each follicle is about 12–17 mm long. The pericarp is reddish-brown, woody and only slightly wrinkled. Each carpel has, as a rule, partly dehisced to expose the seed. The latter has a brittle, shining testa and an oily kernel. The beak of each carpel is not turned upwards and the fruit stalk, which is about 3 cm long, is curved (distinction from I. religiosum). The oil, which is present in both seed and pericarp, gives the drug an aromatic odour and spicy taste.
The genuine fruits of I. verum should yield a minimum of 7.0% volatile oil containing not less than 86.0% of trans-anethole. More recently, they have been employed for the extraction of shikimic acid (see Fig. 19.5), which is the starting material for the synthesis of the antiviral drug Tamiflu (Roche). As a consequence, the plant is in some danger of overexploitation and other sources of the acid are being investigated (q.v.).
Bastard star-anise or shikimi fruits occur in Eastern commerce and are occasionally exported. They are derived from I. religiosum (I. anisatum), a species cultivated near the Buddhist temples in Japan and also on the mainland. The carpels are equal in number to those of I. verum but are smaller, are much wrinkled and have a curved-up apex. The stalk is shorter than the genuine fruit, and straight. These fruits, which contain shikimic acid, are poisonous, as they contain an amorphous toxic substance sikimitoxin, and a crystalline toxic substance sikimin. New phenylpropanoid glycosides have been recently reported (Z.-H. Jiang et al., Chem. Pharm. Bull., 1999, 47, 421). In recent years, Japanese workers have isolated a number of novel sesquiterpene lactones (anisatin-like compounds) from the pericarps of various Illicium species including I. verum; a number of these compounds are convulsants. T. Nakamura et al. (Chem. Pharm. Bull., 1996, 44, 1908) describe the isolation of three sesquiterpenoid compounds (veranistans, A,B,C) which are neurotoxins. For additional isolations, see J.-M. Huang et al., Chem. Pharm. Bull., 2000, 48, 657.
Star anise oil
The essential oil should contain 87–94% trans-anethole, 0.5–5.0% of estragole and smaller amounts of anisaldehyde (0.1–0.5%) and foeniculin (0.1–3.0%); other minor components include chavicol methyl ether (an isomeride of anethole), p-methoxyphenylacetone, safrole and other minor components. The oil is, for all ordinary purposes, indistinguishable from that of P. anisum but differences in the gas chromatographic profiles can be seen. The oil is liable to atmospheric oxidation and both anisic aldehyde and anisic acid are normally present. This change is said to diminish the tendency of the oil to solidify, which it normally does on cooling to about 15°C. In the past, the oil was imported in lead containers and some pharmacopoeias give a limit test for heavy metals.
Both aniseed oil and star anise oil are used as flavouring agents and as carminatives. Anethole (a colourless crystalline solid m.p. 21°C) may be prepared from the oil or manufactured synthetically.
BITTER FENNEL AND SWEET FENNEL
Bitter Fennel consists of the dried ripe fruits of Foeniculum vulgare, subsp. vulgare, var. vulgare (Umbelliferae). It is cultivated in many parts of Europe and much is imported from India, China and Egypt. The commercial drug consists partly of whole cremocarps and partly of isolated mericarps. Bitter fennel, now little used in British medicine, is more fully described in the 11th edition of this book. The drug has, however, been re-introduced into the BP on account of its EP status.
The fruits contain 1–4% of volatile oil with higher yields recorded.
The principal constituents of bitter fennel oil, with BP/EP prescribed limits, are fenchone (12–25%), trans-anethole (55–75%) together with anisaldehyde (maximum 2.0%) and estragole (methyl chavicol) (maximum 5.0%). Minor components include limonene and other monoterpene hydrocarbons.
Anethole is derived via the shikimic acid pathway and fenchone (a bicyclic monoterpene) is formed from fenchol by the action of a dehydrogenase. Other components of the fruits include flavonoids, coumarins and glycosides. The latter, which may have a biogenetic relationship with the volatile oil constituents, have been actively investigated by Japanese workers. Thus M. Ono et al. (Chem. Pharm. Bull., 1995, 43, 868; 1996, 44, 337) describe a number of monoterpene glycosides based on 1,8-cineole and cis-miyabenol C which they have termed foeniculosides I–IX. J. Kitajima, T. Ishikawa and co-workers in nine studies on the water-soluble glycosides and sugars of fennel fruit (Chem. Pharm. Bull., 1998, 46, 1587, 1591, 1599, 1603, 1643, 1738; 1999, 47, 805, 988) have recorded alkyl-, erythro-anethol-, p-hydroxyphenylpropylene glycol-, fenchane-, menthane-, aromatic (phenylpropane etc)- and 1,8-cineole-type glycosides. It is of further interest that of the cineole-type glycosides
one had previously been isolated from Cunila spicata (Lamiaceae), a plant used in Brazilian traditional medicine, one from the peel and flower buds of Citrus unshiu and two were biotransformation products from a Eucalyptus cell suspension culture following administration of 1,8-cineole. Annual world production of the oil is less than 5 tonnes.
Fennel and its volatile oil are used as an aromatic and carminative.
Sweet Fennel is derived from F. vulgare, subsp. vulgare, var. dulce and is also included in the BP/EP. The fruits resemble those of the bitter variety but have a sweet taste and lower volatile oil content (not less than 2.0%) of different quantitative composition. Not less than 80% of the oil is required to be anethole, not more than 7.5% fenchone, and not more than 10% estragole.
Cumin
Cumin consists of the dried ripe fruits of Cuminum cyminum (Umbelliferae), a small, annual plant indigenous to Egypt. It is widely cultivated and UK supplies are obtained from Sicily, Malta, Mogadore and India. Spain and Egypt are the major cumin oil producers.
Cumin fruits are about 6 mm long and resemble caraway at first glance. The mericarps, however, are straighter than those of caraway and are densely covered with short, bristly hairs. Whole cremocarps attached to short pedicels occur, as well as isolated mericarps. Each mericarp has four dorsal vittae and two commissural ones. The odour and taste are coarser than those of caraway.
Cumin yields 2.5–4.0% of volatile oil. This contains 25–35% of aldehydes (cuminic aldehyde), pinene and α-terpinol.
As with other umbelliferous fruits the water-soluble constituents of cumin have been recently investigated. Compounds isolated include flavonoid glycosides such as the 7-O-β-D-glycopyranosides of apigenin and luteolin, some 16 monoterpenoid glycosides and new sesquiterpenoid glucosides, e.g. cuminosides A and B (T. Ishikawa et al., Chem. Pharm. Bull., 2002, 50, 1471; T. Takayanagi et al., Phytochemistry, 2003, 63, 479).
Cumin was one of the commonest spices in the Middle Ages. It is employed in Indian medicine (for a study of its activity see S. C. Jain et al., Fitoterapia, 1992, 63, 291).
TURPENTINE OIL
Pharmaceutical turpentine oil is obtained by distillation and rectification from the oleoresin produced by various species of Pinus. The unrectified oil is the turpentine of commerce. The resin remaining in the still is the source of colophony (q.v. under ‘Resins’).
Rectification of the commercial oil consists of treatment with aqueous alkali to remove traces of phenols, cresols, resin acids etc. and possible redistillation.
The genus Pinus is widely distributed and many countries have considerable reserves of pine forest. The principal species employed are (1) Pinus palustris (longleaf pine) and P. elliottii (slash pine) in the S. and S.E. United States; (2) P. pinaster (P. maritima) in France, Italy, Portugal and Spain; (3) P. halepensis in Greece and Spain; (4) P. roxburghii (P. longifolia) in India and Pakistan; (5) P. massoniana and P. tabuliformis in China; (6) P. carribaea var. hondurensis and P. oocarpa in Central America and (7) P. radiata in New Zealand.
The collection of the oleoresin is very labour-intensive and for this reason output in the USA has declined considerably. Principal world producers are now Portugal and China; other contributors, in addition to the USA, include Spain, Greece, Morocco, France, India, the former USSR, Honduras and Poland. Many other countries produce smaller quantities for their own use. It is considered that about 250 000 trees are required to sustain a small commercial processing plant.
Oil of turpentine is a colourless liquid with a characteristic odour and a pungent taste. It is soluble in alcohol, ether, chloroform and glacial acetic acid. Oil of turpentine is optically active, but the rotation varies not only with the species of pine from which it has been obtained, but also
in samples taken from the same tree at different periods. Samples taken from the same tree at different times have given rotations varying from −7° 27′ to +18° 18′ in the case of Pinus palustris, and −28° 26′ to +1° 23′ in the case of Pinus heterophylla. The French oils from Pinus pinaster are strongly laevorotatory (−20° to −38°). Over forty components have been identified in French turpentine oil derived from P. pinaster.
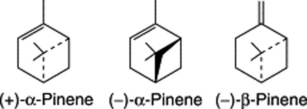
Oil of turpentine consists chiefly of the terpenes (+)- and (−)-α-pinene, (−)-β-pinene and camphene. These tend to undergo atmospheric oxidation, with the formation of complex resinous substances, the removal of which is accomplished by the process of rectification mentioned above. The varying optical rotations of differing turpentines are mainly due to the varying proportions of the (+)- and (−)-α-pinenes present; (−)-β-pinene is found in almost all Pinus spp. in a high state of optical purity and typically occurs with the predominantly (+)-α-pinene. These two isomers have opposite absolute configurations. Other components of the oil which find industrial uses are β-phellandrene, δ-3-carene (a major component of Indian and ‘Russian’ turpentines), limonene, p-cymene, longifoline and estragol.
Oil of turpentine is now rarely given internally. Externally it is used as a counterirritant and rubefacient. For inhalation, terebene is usually preferred. Terebene is prepared from oil of turpentine by the action of cold sulphuric acid, which converts the pinene into the optically inactive (±)- limonene (dipentene). Now, most turpentine is processed to give its various constituents which find use in the manufacture of fragrances, flavours, vitamins, insecticides, etc.
PINUS PINASTER TYPE TURPENTINE OIL
This oil, official in the European and British Pharmacopoeias 2007, is obtained by steam distillation of the oleoresin from Pinus pinaster Aiton and rectified. The French oils from this species are strongly laevorotatory (limits −40° to −28°), cf. Oil of Turpentine BP. The principal constituents and official limits are α-pinene (70–85%), β-pinene (11–20%) and limonene (1–7%). Other components in small amounts are camphene, car-3-ene, β-myrcene, longifolene, β-caryophyllene and caryophyllene oxide. Over 40 compounds have been reported in P. pinaster oil.
Standards relevant to the quality of turpentine oils are refractive index, relative density, residue on evaporation, optical rotation, acid value and peroxide value. Tests for fixed oils and resinified oil together with solubility in alcohol are also important.
Turpentine oils are used medicinally for their rubifacient acitivity.
Canada turpentine
Canada turpentine, or ‘Canada balsam’ as it is often incorrectly called, is an oleoresin obtained from the stem of Abies balsamea (Pinaceae), the balsam fir. It is collected in eastern Canada and in the State of Maine in the USA. The oleoresin in the bark occurs in schizogenous ducts and large cavities. As the cavities fill with secretion, blister-like swellings develop on the trunk, and it is from these that the oleoresin is collected.
Canada turpentine when fresh is a pale-yellow liquid with a slight, greenish fluorescence and is of honey-like consistency. It has a pleasant, terebinthinate odour and a somewhat bitter and acrid taste. On exposure to air, Canada turpentine becomes more viscous and finally forms a glass-like varnish, a property which rendered it suitable as a microscopic mountant and as a cement for lenses. It contains volatile oil (23–24%) and a number of terpenoid acids.
Pumilio pine oil
A distillation of the fresh leaves of the pumilio pine, Pinus mugo var. pumilio (Pinaceae) yields the BP (1980) oil. It is produced in Eastern Europe.
The oil has an agreeable odour and contains principally terpenes and sesquiterpenes, with up to 10% bornyl acetate (BP 1980 limits 4–10% of ester). It may be distinguished from other similar oils by the above ester content and its weight per millilitre value. It is used as a decongestant inhalant, in the preparation of compound thymol glycerin, and as a constituent of zinc undecenoate dusting-powder.
Savin tops
These are the young shoots of Juniperus sabina (Cupressaceae), an evergreen shrub about 2–6 m high. It grows wild in the mountains of Austria, Switzerland, Italy, France and Spain. The leaves are imbricated, sessile, more or less adnate to the stem and usually opposite and decussate. The shape and size of the leaves differ very considerably on different parts of the plant. Each leaf has a depression on its dorsal surface, below which is a large oil gland in the mesophyll. This oil gland is oval in young leaves but more elongated in old ones. Savin contains a volatile oil (1–3%) which is a powerful irritant both internally and externally. It contains the terpene alcohol sabinol and its acetate. Other constituents are podophyllotoxin (0.2%), coumarins and savinin. Many diterpenoids with various skeletal structures have been reported among the non-volatile constituents of a hexane fraction of the berries of this plant. (For reports see A. San Feliciano et al., Phytochemistry, 1991, 30, 695; Fitoterapia, 1991, 62, 435.)
Oil of cade
Oil of cade is obtained by the destructive distillation of the woody portions of Juniperus oxycedrus (Cupressaceae). It is prepared in Portugal, Spain and former Yugoslavia.
The distillate is allowed to stand for at least 15–20 days when a layer constituting oil of cade may be separated.
Oil of cade is a reddish-brown or blackish, oily liquid. Odour, empyreumatic; taste, aromatic, bitter and acrid. The chief constituents are sesquiterpenes (e.g. cadinene) and phenolic compounds (guaiacol, ethyl guaiacol and cresol).
The oil composition of the leaves of J. oxycedrus resembles that of J. communis (below). The oil is of variable composition; based on geographical location, subspecies and varieties, T. Dob et al., (Pharm. Biol., 2006, 1, 1) suggest a classification of the oil based on four chemotypes: α-pinene, limonene, sabinene and trans-pinocarveol.
Oil of cade has been used for veterinary purposes for centuries, and has been prescribed for skin diseases.
JUNIPER BERRIES AND OIL
Juniper berries are the dried ripe fruits of Juniperus communis (Cupressaceae), an evergreen shrub or small tree. They are collected in former Yugoslavia, Italy, Hungary, Poland, Thuringia, Sweden and other countries. Generally speaking, the berries from the more southern countries contain the most oil.
In Tuscany the collection of the berries is very much a family industry. Bushes are beaten to remove the ripe fruits and the product is roughly cleaned before drying. Importers may further remove extraneous material by a winnowing process involving warm air. Any green berries are removed and the remaining fruits graded.
The female cones consist of scales arranged in whorls of three. The berry-like fruit takes 2 years to ripen, eventually becoming a deep purple colour and having a bluish-grey bloom. On drying, the berries become somewhat darker and shrivel slightly. They are about 3–10 mm in diameter. The apex shows a triradiate mark and depression indicating the sutures of the three fleshy scales. At the base there are usually six, small, pointed bracts arranged in two whorls, but occasionally three or four such whorls are found.
A transverse section of the fruit shows a thin outer skin or epicarp, a yellowish-brown, pulpy mesocarp and three seeds. The seeds lie close together in the centre of the fruit and are hard and woody. Partly embedded in the hard testa of each seed are large oleoresin glands. These usually number from four to eight on the outer side of the seed, and one or two on the inner. The drug has a pleasant, somewhat terebinthinate odour, and a sweetish taste.
The main constituents are volatile oil (about 0.5–1.5%), invert sugar (about 33%) and resin; the BP/EP specifies a minimum essential oil content of 1.0% with reference to the anhydrous drug.
The aerial parts of this species and its varieties have been examined for water-soluble constituents resulting in the isolation of various phenylpropanoid, neolignan and flavonoid glycosides. Megastigmane glycosides and a new monoterpene glucoside have recently been reported (T. Nakanishi et al., Chem. Pharm. Biol., 2005, 53, 783). For a phytochemical and genetic survey of the species, see N. Filipowicz et al., Planta Med., 2006, 72, 850.
Juniper berries are used for the preparation of oil of juniper and in making certain varieties of gin. The oil has diuretic and antiseptic properties. It has been reported that commercial oils vary in composition and prolonged intake of some may cause kidney damage. These side-effects are correlated with a high terpene hydrocarbon content and a low proportion of terpinen-4-ol.
Oil of Juniper BP/EP
This is obtained from the non-fermented berry cones of Juniperus communis L. by steam distillation. The oil contains over 60 constituents, although over 100 compounds have been detected in oil from wild berries collected in Greece. Principal components and official limits are α-pinene (20–50%), β-myrcene (1–35%), limonene (2–12%), β-pinene (1–12%), terpinen-4-ol (0.5–10%), sabinene (less than 20%) and β-caryophyllene (less than 7.0%). Other components not quantitatively specified are cadinene, camphene and various alcohols and esters.
The above figures demonstrate the possible variable composition of Juniper oil. For commercial oils in general this variation can be great and, as reported by P. Bradley (BHPC, Vol. 2, 2006), such oils are rarely prepared from a uniform source and may involve the distillate from fermented berries after their use in the manufacture of gin or the use of unripe berries, needles and wood of the plant.
Juniper oil is traditionally used for its diuretic, carminative and antirheumatic properties. Side-effects of some oils have been attributed to a relatively high proportion of terpene hydrocarbons and a low proportion of terpinen-4-ol.
BITTER ORANGE PEEL
Bitter orange peel is the dried outer part of the pericarp of the ripe or nearly ripe fruit known as the bitter, Seville or Bigarade orange. In botanical characteristics the tree is not unlike the sweet orange and both are regarded as subspecies or varieties of Citrus aurantium L. (Rutaceae).These are named, respectively, C. aurantium var. amara-L. and C. aurantium var. sinensis L. (C. sinensis (L.) Osbeck.). The bitter orange is not as widely cultivated as the sweet orange and European supplies come from southern Spain (Seville and Malaga), Sicily (Messina and Palermo), Tripoli via Malta and the West Indies. The dried bitter peel is official in the BP/EP.
History
The bitter orange tree appears to have been introduced from northern India into eastern Africa, Arabia and Syria, whence it was brought to Europe by either the Arabs or Crusaders about AD 1200. The sweet orange was not known in Europe until the fifteenth century and appears to be of Chinese origin.
Collection and preparation
Orange peel may be prepared in the Mediterranean countries or in England. The peel should be removed with as little of white ‘zest’ as possible. Hand-cut, English dried peel is most esteemed. The peel may be removed in four ‘quarters’, or in a spiral band. It is also found in thin strips, similar to those found in marmalade, cut by machines. The so-called Maltese is of this type, which is known as ‘gelatin-cut’. Fine slicing causes the rupture of a large number of oil glands and some loss in aroma.
Characters
The colour of the dried peel is somewhat variable, but frequently reddish-brown in the spiral form and greenish-brown in the ‘quarters’. The other surface is rugged, being somewhat raised over the oil glands, which are clearly seen in sections with the naked eye. The inner surface bears a small amount of white ‘zest’. Fragrant odour; aromatic and very bitter taste.
Microscopic examination shows a small-celled epidermis with characteristic stomata; parenchyma containing prismatic crystals of calcium oxalate 20–45 mm long, or sphaerocrystalline masses of hesperidin; small anastomosing vascular bundles; and large oil-filled cavities usually arranged in two irregular rows.
Constituents
Dried bitter orange peel contains not less than 2.0% of volatile oil, vitamin C and the flavonoid glycosides hesperidin and neohesperidin. The latter, present to the extent of 5–14% in the unripe peel, gradually disappears on ripening.
Citrus glycosides and limonoids
Citrus fruits contain a large number of flavanone glycosides. The best-known of these, hesperidin (see Fig. 21.18), was first isolated in 1828. It is present in oranges, both bitter and sweet, and in lemons. See also ‘Flavone and Related Flavonoid Glycosides’ and ‘Hesperidin and Rutin’. An isomer of hesperidin, neohesperidin, is present in certain samples of Seville oranges. Naringin, present in some Seville oranges, is the chief flavonoid constituent of the grapefruit. Coniferin (Table 21.1) has been reported in C. sinensis and may add to the effects of limonin and naringin.
The bioproduction of neohesperidin and naringin in callus cultures of C. aurantium has been demonstrated (J. A. del Río et al., Plant Cell Rep., 1992, 11, 592).
Sweet orange peel
The peel of the sweet orange is thinner than that of the bitter, more yellowish in colour and less rough, and the taste, though pungent and aromatic, lacks the extreme bitterness of the Seville peel. As studied in Valencia orange peel, the colour originates from a complex mixture of carbonyl carotenoids, the principal components being violeoxanthin (9-cis-violaxanthin), di-cis-violaxanthin and all-trans-violaxanthin, together with a number of other carotenoids.
ORANGE OILS
The volatile oil from the orange may be extracted by methods other than by distillation (see ‘Lemon Oils’ for details of methods). That from the bitter orange is known as Essence de Bigarde and that from the sweet orange is called Essence de Portugal. The latter is official in the BP/EP and is obtained by mechanical expression of the fresh peel; although chemically almost identical with the bitter orange oil, it does not have the bitter taste or odour of the latter. These oils contain the terpene (+)-limonene and smaller quantities of citral, citronellal, methyl anthranilate, etc. In 1988, 62 components from the steam-distilled oil of Libyan fresh orange peel were identified. Sixteen of the identified compounds had not previously been reported as orange volatiles (A. J. MacLeod et al., Phytochemistry, 1988, 27, 2185). Brazil and the USA are the largest producers of sweet orange oil.
Terpeneless orange oil
By removing about 95% of the terpenes by vacuum distillation a terpeneless oil of orange may be obtained. One part of the terpeneless oil is equivalent to about 15 parts of the sweet orange oil. The BP/EP oil is required to contain not less than 18% of aldehydes calculated as decanal.
BITTER ORANGE FLOWER OIL
This oil, also known as Oil of Neroli, official in the BP/EP is prepared by steam distillation from fresh flowers of the bitter orange. An alcoholic solution of the oil has a violet-blue fluorescence arising from the small content (0.1–1.0%) of methyl anthranilate which is also responsible for the characteristic odour of the oil. Other constituents, with BP/EP permitted ranges, are trans-nerolidol (1.0–5.0%), geranylacetate (1.0–5.0%), α-terpineol (2.0–5.5%), linalyl acetate (2.0–15.0%), linalol (28.0–44.0%), limonene (9.0–18.0%), β-pinene (7.0–17.0%). There is a TLC test for the absence of bergapten, present should the oil be adulterated with that from the bitter peel.
In Britain the oil was traditionally used for the making of concentrated orange-flower water, syrup of orange flowers and Cologne spirit. It is used in aromatherapy.
LEMON PEEL
Lemon peel (Limonis Cortex) is obtained from the fruit of Citrus limon (L.) Burm. f. (Rutaceae), a small tree, 3–5 m high, cultivated in the countries bordering the Mediterranean and elsewhere (see ‘Lemon Oils’). The lemon is of Indian origin and appears to have been unknown in Europe until the twelfth century. Numerous varieties and hybrids (particularly with C. medica Risso) are cultivated. Dried lemon peel is official in the BP/EP.
Collection and preparation
Lemons are collected in January, August and November, before their green colour changes to yellow. They are exported in cases containing from 200 to 360 fruits. The smaller fruits, which would not have a ready sale, are used in the preparation of oil of lemon. The peel is removed with a sharp knife in the form of a spiral band.
Characters
Dried lemon peel occurs in spiral bands up to 2 cm wide and 2–3 mm thick. Some pieces bear the apex of the fruit, which has a nipple-like appearance. The outer surface is rough and yellow; the inner surface is pulpy and white. Odour, strong and characteristic; taste, aromatic and bitter. The anatomical structure closely resembles that of orange peel (q.v.).
LEMON OILS
Lemons are widely cultivated and the volatile oil is prepared around the Mediterranean, North and South America, in Australia and in parts of Africa. Lemon and other Citrus oils are best extracted by means other than distillation. The definition of pharmaceutical lemon oil given in the British Pharmacopoeia states that it is obtained by suitable mechanical means, without the aid of heat, from the fresh peel of C. limon (L.) Burm. f.
Once the oil has been separated from the peel, it can be distilled without deterioration in quality, and some expressed oil of lemon is fractionally distilled to make terpeneless oil of lemon (q.v.). Distillation direct from the peel is quite different, and, although much oil is prepared from the peel by steam distillation, this is inferior and does not comply with the definition given above. Distilled oil of lemon is cheaper than that prepared by expression and large quantities of it are made and used for non-pharmaceutical purposes.
History
Both expressed and distilled oils of lemon were sold in Paris as early as 1692. The sponge process as used in Sicily was described by Barrett in 1892. Machines were first introduced for oil of lemon production in 1920 and by 1930 about half the Italian oil was produced by their aid. New machines are being frequently introduced, and although some hand-expressed oil is still made (e.g. for eau de cologne, which requires the highest quality), pharmaceutical oil is now machine made.
Preparation
Oil of lemon is only one of several products made from lemons. In addition to dried peel, much lemon peel is candied with sugar. The pulp of the fruit yields on expression lemon juice, which may be canned or used for the preparation of citric acid and citrates. Pulp residues are used for pectin manufacture and as cattle food.
The following processes are used for the production of oil:
Hand methods
As these are no longer applicable to pharmaceutical oils they will not be described here and the reader is referred to earlier editions of this book for accounts of the spugna or sponge process, the scorzetta process, and the écuelle à piquer process.
Machine processes
The quality of machine-produced oil is rather inferior to the best hand-pressed. The machines are designed to set free the oil by puncture, rasping or cutting and by imitating the gentle squeezing action of the sponge method. The superiority of sponge-pressed oil appears to be due to the fact that there is virtually no contact between the oil and the inner white part of the peel (albedo). Deterioration in odour results from enzyme action in the finely divided albedo and is likely to be most pronounced when the machines penetrate deeply into the peel and when the resulting finely divided albedo and the water used for spraying are in contact with the oil for any length of time.
For the reasons given above, mincing of the whole fruit or peel followed by expression is unsatisfactory. Machines such as the pelatric which abrade the fruit surface are rather better, because they give less admixture of oil and albedo. The sfumatrice machines (squeezing machines) as first introduced, imitate more closely the hand method, since they exert a gentle pressing action on the peel passing on a stainless-steel band against stationary protrusions. Spray water is used to remove the oil, which is separated by a centrifuge. The new sfumatrice machines are a modification of the above in which fine knives cut into the outer peel (flavido), but partly also in the albedo. After treatment in these the peel shows ‘almost invisible criss-cross cuts’. The newest machines extract the oil more completely than the older ones and therefore give a substantially higher yield.
Constituents
Lemon oil contains terpenes (about 94% mainly (+)- limonene), sesquiterpenes, aldehydes (citral, about 3.4–3.6%, and citronellal) and esters (about 1% geranyl acetate). Limonene (see Fig. 22.2) is a liquid, b.p. 175°C. Citral (see Fig. 22.2) or geranial, a liquid, b.p. 230°C, is the aldehyde corresponding to the alcohol geraniol. Lemon oil shows a marked tendency to resinify and should be protected from the action of air and light as much as possible. It has been shown by the use of GC and TLC that the oil obtained directly from the oil glands of the rind by capillary insertion differs from the fresh and stored commercial oils in composition. Principal reactions which cause these changes are oxidations of monoterpenes, aldehydes and esters, peroxide formation, polymerizations and isomerizations (e.g. limonene → α-terpinene).
Adulteration
Oil of lemon was at one time frequently adulterated with oil of turpentine, but analysts now have to contend with more scientific methods of adulteration. These include the addition of terpenes obtained in the preparation of ‘terpeneless oil of lemon’ and the addition of the cheaper distilled oil of lemon. The value of the oil is judged to some extent on the citral content, but a normal citral content alone is not a sure indication of purity, since citral may be added from a cheaper source such as oil of lemon-grass, which contains 75–85% of this aldehyde. It will be gathered that a careful examination of the oil by both physical and chemical methods is necessary, as exemplified by the standards and tests given in the BP.
Terpeneless lemon oil
Terpeneless Lemon Oil of the BP is prepared by concentrating lemon oil in vacuo until most of the terpenes have been removed, or by solvent partition. The concentrate is the terpeneless oil, which has a citral content of 40–50%. Terpeneless lemon oil is equivalent in flavour to about 10–15 times its volume of lemon oil; Lemon Spirit BP is a 10% solution in ethanol (96%) and it is also an ingredient of Compound Orange Spirit BP.
Buchu leaf
The name ‘buchu’ is applied to the leaves of several species of Barosma (Agathosma) (Rutaceae) grown in South Africa. The leaf, official in the BHP, is obtained from Barosma betulina (Thunb). Bartl. & Wendl. and known in English commerce as ‘short’ or ‘round’ buchu. The leaves of Barosma crenulata (oval buchu) and B. serratifolia (long buchu) are also used.
The leaves of B. betulina, B. crenulata and B. serratifolia are all small, shortly petiolate, green to greenish-yellow in colour, and supplied with numerous oil glands which are readily visible on holding them to the light.
Round or short buchu consists of the leaves and a small percentage of the stems, fruits and flowers of Barosma betulina. The leaves are 12–20 mm long and 4–25 mm broad. They are rhomboid-obovate in shape, with a blunt and recurved apex. The margin is dentate in the upper two-thirds of the leaf and serrate towards the base. A large oil gland is situated at the base of each marginal indentation and at the apex, while numerous smaller ones are scattered throughout the lamina. The leaves when dry are brittle and coriaceous, but on moistening become cartilaginous or mucilaginous. Odour and taste, strong and characteristic. Reddish-brown fragments of stems, up to about 5 cm, brown fruits with five carpels and flowers with five whitish petals are usually present, but an excessive amount of these must be regarded as an adulteration.
Oval buchu is obtained from Barosma crenulata Hooker. The leaves, which are accompanied by a certain amount of stem, are 15–30 mm long and 7–10 mm broad. The shape is more or less oval; the apex is blunt but not recurved and possesses a terminal oil gland; marginal serration very minute. (For a report see E. Wollenweber and E. H. Graven, Fitoterapia, 1992, 62, 86.)
Long buchu is obtained from Barosma serratifolia Willd. The leaves are 12–40 mm long and 4–10 mm broad, and linear lanceolate in shape; the apex is truncate and possesses a terminal oil gland; the margin is serrate.
Buchu leaves contain volatile oil, diosmin (see Fig. 42.1B), mucilage, resin and calcium oxalate. In addition to the principal components pulegone, menthone, isomenthone and limonene the oil, in which over 120 components have been identified, contains diosphenol or buchu camphor to which the diuretic activity of the drug has been ascribed. The characteristic odour of the oil has been ascribed to sulphur compounds and p-menthane-8-thio-3-one has been characterized; it is present in quantities of up to 0.5% of the oil and is probably derived from (−)-pulegone. Buchu is still occasionally used as a diuretic and urinary antiseptic and is considered effective by herbal practitioners. For a recent review see A. Moola and A. M. Viljoen, J. Ethnopharm., 2008, 119, 413.
NUTMEG AND NUTMEG OIL
Nutmegs are the dried kernels of the seeds of Myristica fragrans (Myristicaceae), an evergreen tree about 10–20 m high, indigenous to the Molucca Islands. The plant is now widely cultivated not only in Indonesia and Malaysia (Molucca Islands, Sumatra, Java and Penang), but also in Ceylon and the West Indies (Grenada). Current world demand for nutmegs stands at about 10 000 tonnes per annum of which about 75% originates from Indonesia and 15% from Grenada.
History
Nutmegs and mace appear to have been first introduced into the Levant by the Arabs in the middle of the twelfth century and by the end of that century were found in northern Europe. The native country of the nutmeg (the Molucca or Spice Islands) was known to Arabian writers of the thirteenth century, and the Banda Islands, a group of the Moluccas where the plant is very abundant, were discovered by the Portuguese in 1512. The Portuguese, after holding the spice trade for about a century, lost it to the Dutch, who maintained a complete monopoly by destroying the trees in neighbouring islands and preventing the export of living seeds. The ordinary drying process destroys the vitality of the seeds, but they were also soaked in milk of lime for many weeks and were seldom sold until they were several years old. The Spice Islands were occupied by the English for a few years (1796–1802), during which period the opportunity was taken to start cultivation in Penang and Sumatra. Until the trees so planted reached maturity the effect of the Dutch restriction was still felt, and in 1806 the import price of mace in London was as high as £10 kg−1.
Cultivation of the spice was subsequently introduced to the West Indies and during the Second World War production of nutmegs in Grenada was expanded enormously. In 1955 a hurricane destroyed 90% of the trees but the industry has now recovered and nutmegs remain the island’s main commodity export.
Cultivation, collection and preparation
Nutmeg trees can be grown from fresh seed sown in the shell. The seeds germinate in about 5 weeks, and when the young plants are about 6 months old, they are transplanted to the fields. When the sex can be determined (5–8 years), the male trees are reduced to about 10% of the total. This method leads to irregularly spaced trees in the plantation and now in Grenada vegetative propagation of the female trees is performed by marcotting or air layering. In this, female shoots are split but not detached, and by the use of hormone powder and suitable packing of the wound, are induced to root. This takes 4–18 months after which the rooted shoots are detached and brought on before planting out. A success rate of over 40% for rooting is now obtainable. Another technique which has been used to increase the number of female trees is the employment of approach grafts. The trees bear fruit from their eighth or ninth year and continue to fruit well for about 20–30 years. The peach-like fruit splits when ripe, exposing the seed with its lobed, red arillus. The plant fruits almost continuously and two or three crops are collected annually. In the East the fruits are collected by hand or by means of a hooked stick, but in Grenada the fruits are allowed to fall to the ground. The orange-yellow pericarp which is about 12.5 mm thick, is usually removed on the spot. Later the arillus is picked off and constitutes, when dried, mace. From mature plantations the annual yield per acre is about 250–500 kg of nutmeg and about 50–100 kg of mace. The nutmegs are dried in the shells, the procedure differing according to local conditions but usually taking about 3–6 weeks. In Malaya sun-drying is used to some extent, but the seeds require adequate cover at night or in wet weather. Large quantities are dried in ovens and in brick buildings. In the latter the seeds are placed on trays over low charcoal fires, being turned and gradually moved nearer to the fires during the process. When drying is completed, the kernel rattles within the brittle testa, which constitutes about one-quarter of the weight of the seed. The testa is cracked by means of a wooden truncheon, mallet or special machine, and the nutmeg extracted. However, machines are liable to cause bruises, and cracking by hand is preferable. The liming of nutmegs to reduce insect attack is now less commonly practised than in the past. After cracking, the nutmegs are now usually graded abroad into sizes represented by numbers per unit weight. Elongated nutmegs, which fetch a lower price, and small or damaged ones are kept separate. Nutmegs are exported in barrels or cases containing about 50 kg.
Macroscopical characters
Nutmegs are broadly oval in outline, 2–3 cm long and about 2 cm broad. If not heavily limed, the surface is of a brown or greyish-brown colour and is reticulately furrowed. At one end is a light-coloured patch with brown lines radiating from the hilum, which is surrounded by a raised ring. From this an ill-defined furrow (imprint of the raphe) runs to the chalaza, at the opposite end of the kernel, where there is a small dark depression. Odour, strong and aromatic; taste, pungent and slightly bitter.
A longitudinal section (Fig. 22.9C) has a lustrous, marbled appearance. The outer tissue, which consists of dark brown perisperm, penetrates the light brown endosperm, the infoldings branching and giving rise to the marbled appearance. The perisperm possesses fibrovascular bundles, the position of which is indicated by the reticulate furrows found on the outer surface.
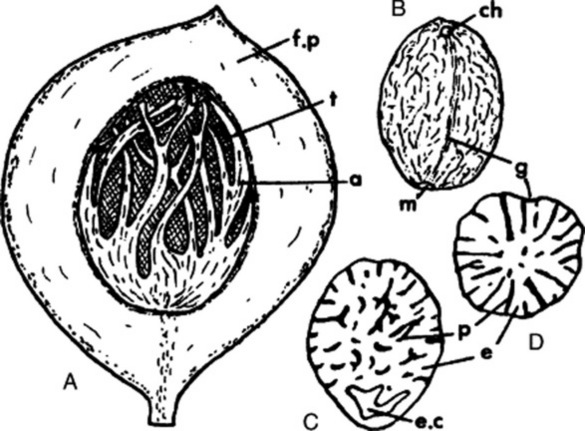
Fig. 22.9 Myristica fragrans. A, Fruit with half of the pericarp removed; B, nutmeg (dried kernel); C, longitudinal section nutmeg; D, transverse section nutmeg (all × 1). a, Aril (mace); ch, chalaza; e, endosperm; e.c, cavity left by embryo; f.p, fleshy pericarp; g, groove marking line of raphe; m, micropyle region; p, perisperm; t, testa.
Microscopical characters
The outer perisperm cells are radially flattened and have brownish contents, insoluble in potassium hydroxide or chloral hydrate. A few of the cells contain prismatic or disc-shaped crystals, thought to consist of potassium acid tartrate. The inner perisperm shows numerous extensive lamellae, corresponding to the furrows on the surface, and penetrating into the endosperm. These lamellae are composed of parenchymatous cells with thin brown walls and of oval oil cells, and show in their outer part vascular strands composed of lignified spiral vessels. The endosperm is composed of parenchymatous cells, with thin brown cell walls, and containing simple or 2–10 compound starch grains (individual grains up to 22 μm in diameter, globular or irregular in shape, with sometimes a slit-like hilum); aleurone grains, the larger of which show a well-defined crystalloid; and feathery crystals of fat. A few tannin cells, containing tannin and starch, occur scattered in the endosperm.
Allied drugs
Papua nutmegs are derived from M. argentea, a tree grown in New Guinea. They are often taken to Macassar and enter commerce as Macassar, Papua, long or wild nutmegs. They have a uniform, scurfy surface, little odour and a disagreeable taste.
Bombay nutmegs are derived from M. malabarica, grown in India. They are very long and narrow and lack the characteristic aroma of the genuine drug.
Mace
Common mace or Banda mace consists of the dried arillus or arillode of M. fragrans. This, when fresh, is of a bright red colour and is removed either by the finger or a knife. When removed entire, it forms‘double blade’ mace, but if in two pieces, it is known as ‘single blade’ mace. After flattening by treading under the feet or pressing between boards the mace is slowly dried. The volatile oil of mace resembles that of nutmeg, the major phenolic compounds isolated being dehydrodiisoeugenol and 5′-methoxydehydrodiisoeugenol, both of which have a significant antibacterial action. In recent years a series of lignans and neolignans has been isolated from mace; see Table 21.7 for the formula of macelignan.
Bombay mace is a regular article of commerce, although almost valueless as a spice. It is dark red in colour, is lacking in aroma and yields about 30% of extractive to light petroleum (genuine mace yields about 3.5%). Papua mace is distinguished by its shape, dull brownish surface, lack of aroma and acrid taste.
NUTMEG OIL
Nutmeg oil is distilled from the kernels imported into Europe and the USA, and is produced in Indonesia (about 120 tons annually), Sri Lanka (30 tons) and India (5 tons). It contains (BP/EP limits as determined by gas chromatography), α-pinene (15–26%), β-pinene (13–18%), sabinine (14–29%), myristicin (5–12%), limonene (2–7%), γ-terpinene (2–6%), terpinen-4-ol (2–6%), car-3-ene (0.5–2%), safrole (2.5% maximum). Other minor constituents include elemicin and isoelemicin, eugenol, methyleugenol, methoxyeugenol, methylisoeugenol and isoeugenol.
There are differences in optical rotation, refractive index, weight per millilitre and solubility in alcohol between the West Indian and East Indian oils. Myristicin (formula Chapter 39), is 4-allyl-6-methoxy-1,2-methylenedioxybenzene. It is crystalline and, owing to its high boiling point, is mainly found in the last portions of the distillate. Myristicin is toxic to human beings and large doses of nutmeg or its oil may cause convulsions. Workers in Canada and Japan have isolated a considerable number of dimeric phenyl-propanoids from the seed; the units include isoeugenol, elemicin and myristicin. Similar dimeric compounds, shown to cause significant changes in hepatic enzyme systems, have been isolated from mace oil (W. S. Woo et al., Phytochemistry, 1987, 26, 1542).
Calamus
Calamus or sweet flag consists of the rhizome of Acorus calamus (Araceae), which occurs in commerce both peeled and unpeeled. The perennial plant is common on the banks of streams. Originating in Asia, it is now widely distributed in Asia, Europe and North America. The subcylindrical rhizome is up to 20 cm long and 2 cm diameter; longitudinally furrowed on the upper surface and with conspicuous root scars on the lower surface.
Calamus contains 2–4% of volatile oil containing a number of sesquiterpenes and asarone, a compound related to myristicin (see ‘Nutmeg and Nutmeg Oil’). Calamus has been official in many pharmacopoeias, and although still used in some regions, is now mainly used as a source of calamus oil, which is employed in perfumery. The composition of the oil from 2n, 3n and 4n varieties differs and the β-asarone content increases with ploidy. As the phenylpropane derivatives have been shown to be carcinogenic in animal tests, Keller and Stahl (Planta Med., 1983, 47, 71; 1985, p. 6) recommend the selection of races for pharmaceutical use. The oil from the rhizome of the American 2n race contains no β-asarone but consists of shyobunones and acorones, which are also components of the pharmaceutically used oils. GC-MS studies combined with gene sequencing have also been employed for the identification of a 2n β-asarone-free race (C. M. Bertea et al., Phytochemistry, 2005, 66, 507).
Chemotypes of A. calamus having differences in essential oil composition have been DNA profiled (N. Sugimoto et al., Biol. Pharm. Bull., 1999, 22, 481).
A number of sesquiterpenes based on the cadinane, acorane and eudesmane skeletons have been isolated from A. calamus and some of these are strong germination inhibitors of lettuce seeds. Such secondary metabolites are called allelochemicals, well-known examples being the nagilactones of Podocarpus nagi (K. Nawamaki and M. Kuroyanagi, Phytochemistry, 1996, 43, 1175).
For details of the main constituents of calamus and the acetylcholinesterase inhibitory activity of the oil, see P. K. Mukhergee et al., Planta Medica, 2007, 73, 191.
Mukherjee PK, Kumar V, Mal M, Houghton PJ. Acorus calamus: scientific validation of Ayurvedic tradition from natural resources. Pharmaceutical Biology. A review (ca 130 refs) exploring the various constituents and pharmacological activities of the drug. 2007;45(8):651-666.
Motley TJ. Ethnobotany of sweet flag (Acorus calamus). Economic Botany. 1994;48(4):397-412. A comprehensive review with over 160 references
CINNAMON AND CINNAMON OIL
The BP/EP states that ‘cinnamon is the dried bark of the shoots grown on cut stock of Cinnamomum zeylanicum Blume, freed from the outer cork and underlying parenchyma.’ However, Kostermans (see Bibliographia Lauracearum, 1964) indicates the plant to be more correctly named C. verum Presl. (Lauraceae), of which there are two varieties, better called subspecies, one (var. subcordata Nees) with ovate, subcordate leaves, the other (var. vulgare Nees, now properly called var. verum) with oblong or elliptic leaves pointed at both ends; both produce a good drug. Many other ‘varieties’ (about 23) have been described and exist wild in Sri Lanka and southern India; most of these, however, on current taxonomic grounds, represent other species. The tree is also cultivated in the Seychelles, Madagascar, Martinique, Cayenne, Jamaica and Brazil. Ceylon cinnamon is the commonest variety on the English market, but good-quality Seychelles drug, which closely resembles the product from Sri Lanka, is also available.
The British and Americans do not give the same meaning to the words ‘cinnamon’ and ‘oil of cinnamon’. In Britain cinnamon and oil of cinnamon are derived as above. In the USA, however, ‘Cinnamon NF’ is Saigon cinnamon and ‘Oil of Cinnamon NF’ is the oil which British call Cassia Oil, which is derived from Cassia bark (q.v.).
History
Cassia bark was known to the Chinese in 2700 BC but it is not until the thirteenth century that any reference is found to the collection of cinnamon in Ceylon. Ceylon was occupied by the Portuguese in 1536, the Dutch in 1656, and the English East India Company in 1796. Cinnamon cultivation was started by the Dutch in 1770 and they exercised a strict monopoly comparable with their monopoly of nutmegs. This was continued until the monopoly of the English East India Company was abolished in 1833.
Cultivation, collection and preparation
In Sri Lanka about 26 000 acres are devoted to cinnamon plantations. Most of the plantations are small and are situated in the southern or western provinces. Sri Lanka and the Seychelles both export large quantities of cinnamon leaf oil.
The production of the characteristic compound quills of the inner bark is a multistage process and was fully described and illustrated in earlier editions of this book. Briefly, the cinnamon plants are grown from seed and coppiced almost to the ground when 2 or 3 years old. About five or six shoots are allowed to grow from the stump and are kept vertical by pruning. After about 18 months of growth, and when some 3 m long and 2 cm diameter, shoots are harvested, trimmed and, following a few hours ‘fermentation’, they have the bark removed with a non-ferrous knife. The peeled bark is then stretched over a suitable stick and the outer cork and cortex scraped off with a curved scraper. Individual pieces of scraped bark are then placed one inside the other and built up to a length of about 42 in (c. 106 cm). The compound quills are dried on wooden frames in the open air without exposure to direct sunlight and then finally sorted into grades and made into compact bales of about 45 kg.
The traditional grades of cinnamon are designated: 00000, 0000, 000, 00, 0, 1, 2, 3, 4, quillings, featherings, chips. Most commercial material corresponds to Nos. 1–4 grades. Quillings and featheringsconsist of small pieces, the latter often containing some outer bark; they are used for grinding and for oil distillation. Chips consist mainly of outer pieces of bark, and the oil derived from them has a lower specific gravity and a lower aldehyde content than that from the inner bark.
Macroscopical characters
Cinnamon is imported in large bundles about 1 m in length. Retailers generally receive their supplies in shorter lengths known as ‘cigar lengths’. The drug consists of single or double compound quills about 6–10 mm diameter and of varying length (Fig. 22.10). In the different grades the thickness of each piece of bark varies considerably, but in good-quality cinnamon it is usually not more than about 0.5 mm, while the number of pieces of bark forming the compound quill varies from about 10 to 40. The external surface of each piece is yellowish-brown and shows longitudinal shining, wavy lines (pericyclic fibres) and occasional scars and holes (indicating the positions of leaves or twigs). The inner surface is somewhat darker and longitudinally striated. The bark breaks with a short, splintery fracture. Odour is fragrant; taste, warm, sweet and aromatic.
Fig. 22.10 Cinnamon. A, Compound double quill (× 0.5); B, transverse section (×50); C, elements of the powder (×200). ck, Cork cells; cr, acicular crystals of calcium oxalate; l.f, laminated fracture of compound quill; m.r, medullary ray; o.c, oil cells; o.t, remains of outer tissues; p.f, pericyclic fibres; ph.f, phloem fibres; r.ck, residual patches of cork; s, scar of twig; sc, sclereids; sc.l, sclereid layer of pericycle; s.q, transverse surface of compound quill; st, starch granules.
Microscopical characters
Transverse sections of cinnamon (Fig. 22.10) show under the microscope a complete absence of epidermis and cork. Shrivelled remains of cortex occur in patches. The outer limit of the bark is marked by a pericycle composed of a continuous ring of three to four layers of sclereids with small groups of pericyclic fibres embedded in it at intervals. The latter produce the lighter-coloured, wavy, longitudinal lines on the outside of the commercial bark. The sclereids (Fig. 22.10) have thickened lignified walls, showing well-defined pit-canals. The thickening on the outer walls is often less pronounced than on the radial and inner tangential walls. The lumen is clearly visible and sometimes contains starch. The pericyclic fibres range from 1000 to 2500 μm long and have strongly thickened lignified walls showing stratification and pit-canals. Primary phloem cannot be distinguished. The secondary phloem is composed of phloem parenchyma containing oil and mucilage cells; phloem fibres; and medullary rays. The sieve-tube tissue, embedded in the phloemparenchyma, is often obliterated. The phloem parenchyma is composed of thin-walled cells, with yellowish-brown walls, and contains starch in compound and simple grains, the latter not exceeding 10 μm diameter (those of Cinnamomum cassia often exceed this figure) and numerous acicular crystals of calcium oxalate about 5–8 μm long. Some of the phloem parenchyma cells contain tannin. The secretion cells, containing volatile oil or mucilage, are two or three times the diameter of the phloem fibres, and are axially elongated. The phloem fibres, which occur isolated or in tangential rows, are more abundant towards the inner part of the bark. They are usually less than 30 μm in diameter (those of C. cassia measure 30–40 μm in diameter) and have a length of 200–600 μm. The thick lignified walls show stratification. The secondary phloem is divided up by the radial medullary rays, which are uni- or biseriate near the cambium but become broader towards the outside by tangential growth of the cells. The rays are 7–14 cells high. The medullary ray cells are radially elongated, thin-walled with yellow–brown cell contents containing numerous acicular crystals of calcium oxalate. For illustrations of the powdered elements, see Fig. 22.10.
CINNAMON OIL
Oil of cinnamon contains about 60–75% w/w of trans-cinnamic aldehyde, C6H5CH=CHCHO. Genuine oils also contain 4–10% of phenols (chiefly eugenol), hydrocarbons (pinene, phellandrene and caryophyllene), and small quantities of ketones, alcohols and esters; GLC shows the presence of many compounds and limits for specific constituents are given in the Pharmacopoeia. Oil distilled from fresh bark samples collected in Ghana by Angmor et al. (Planta Med., 1979, 35, 342) contained a high proportion of cinnamyl acetate, but by a protracted preparation of the drug which simulated the commercial preparation this ester was largely converted into aldehyde. Phenylalanine has been shown to be a precursor of both cinnamic aldehyde and eugenol in the living plant, but the metabolic interrelationships between the aromatic compounds appear complex.
The oil is liable to adulteration with cinnamon leaf oil and with oil of cassia. Oil of cassia contains about 80–95% of aldehydes and a similar test with ferric chloride gives a brown colour. Oil from the root-bark contains much camphor and other monoterpenes but negligible phenylpropanes.
Allied drugs
Cayenne cinnamon consists of the bark of cultivated plants of Cinnamomum zeylanicum grown in French Guiana, Brazil and some of the islands of the West Indies. It is generally obtained from older branches than the Ceylon drug and appears to be inferior to it in quality. It is not used to any extent in Britain.
C. loureirii is commercially important in Vietnam and grows in the mountainous districts of Annam. The plant, which is closely related to C. cassia is also found in China and Japan. It resembles cassia bark more closely than cinnamon, and occurs in quills up to 30 cm long, 4 cm wide and 0.5–7.0 mm thick. The outer surface is greyish-brown, warty and ridged. The odour is coarser than that of Ceylon cinnamon and the taste sweeter.
CEYLON CINNAMON LEAF OIL
The oil is obtained by steam distillation of the leaves of Cinnamomum verum J. S. Presl. It is a commercial article, and some twenty times the amount of the bark oil is produced. It contains 70–95% of eugenol (BP/EP limits 70–85%) giving the oil a clove-like odour. An alcoholic solution of the oil gives with ferric chloride solution a blue colour; other components of the oil, which are limited individually within the range 1.0–7% and determined by gas chromatography, are cineole, linalol, β-caryophyllene, safrole, trans-cinnamic aldehyde, cinnamyl acetate, eugenol and coumarin. Other standard specifications are relative density, refractive index and optical rotation.
The high eugenol content gives the oil antiseptic and anaesthetic properties; it is mainly employed for the extraction of eugenol and in the cosmetic industry in carnation-type perfumes.
Cassia, Chinese cinnamon or cassia lignea
Various barks have been imported under the name of ‘cassia’. That known in the London market as Chinese cassia lignea is derived from C. cassia Blume, a tree cultivated in the south-eastern provinces of China (Jiangxi and Guangdong). When about 6 years old, the bark is removed from the older branches, the twigs and leaves being used for distillation. The cork and cortex are partly removed by planing, the bark tied into bundles and exported in boxes, via Guangzhou and Hong Kong.
Cassia bark occurs in channelled pieces or single quills up to 40 cm long, 1–2 cm wide and 1–3 mm thick. The outer surface is darker than that of Ceylon cinnamon and, owing to careless planing, shows patches of grey cork. The odour is coarser than that of cinnamon and the taste more astringent.
Transverse sections resemble cinnamon as far as the inner part of the bark is concerned, except that the starch grains and phloem fibres are somewhat larger. However, the utility of the fibre size for distinguishing the two barks has been questioned owing to the limited sample numbers used in the original investigation. Outside the sclerenchymatous ring is the cortex and cork layer.
Cassia bark has been reported to contain about 10% mucilage, whereas Ceylon and Seychelles cinnamon samples contained 1.6–2.9%. TLC tests have also been used for distinguishing the barks. Such distinctions could well arise from the fact that the Ceylon cinnamon is an inner bark, whereas with cassia bark outer cortex and cork are present.
Cassia yields 1–2% of volatile oil which, when pure, contains no eugenol but rarely less than 85% of cinnamic aldehyde; other components are cinnamyl acetate, phenylpropyl acetate and numerous trace constituents. 2′-Hydroxycinnamaldehyde has recently been isolated from the stem bark (B.-M. Kwon et al., Planta Medica, 1996, 62, 183). The oil is included in the USP/NF under the name of Cinnamon Oil and is required to contain not less than 80% by volume of aldehydes. Large quantities of oil are distilled from the leaves and twigs as well as from the bark. Although inferior in flavour to the oil of C. zeylanicum, it is cheaper and is described in many pharmacopoeias.
Considerable advances in the chemistry of the non-volatile components of cassia bark have been made by Japanese researchers and have demonstrated the pharmacological activities of these substances. In a number of papers Nohara et al. (see Phytochemistry, 1985, 24, 1849 and references therein) have reported the isolation of a series of diterpenes from a fraction of the bark showing antiallergic activity. Aqueous extracts have been shown to have antiulcerogenic activity (T. Akira et al., Planta Med., 1986, p. 440).
In 1986 Morimonto et al. characterized a number of compounds of the tannin complex (Chem. Pharm. Bull., 34, 633, 643) as below. Three flavan-3-ol glucosides were identified as (–epicatechin 3-O-, 8-C- and 6-C-β-D-glucopyranosides respectively. Three oligomeric procyanidins (named cinnamatannins A2, A3, A4) were tetra-, penta- and hexameric compounds respectively, consisting exclusively of (−)-epicatechin units linked linearly through C-4–C-8 bonds (see formula). Free (−)-epicatechin and procyanidins were present, the latter occurring also in dimeric form and as C-glucosides.
An arabinoxylan which activates the reticuloendothelial system was described in 1989; this neutral polysaccharide, named cinnaman AX, contains L-arabinose and D-xylose in the respective molar ratio of 4:3. Other pharmacologically similarly-acting polysaccharides have been reported in Panax and Saposhnikovia.
Callus and suspension cultures of Cinnamomum cassia produce large amounts of condensed tannins; the precursors (−)-epicatechin and-procyanidins B2, B4 and C1 have been isolated from callus cultures (see K. Yazaki and T. Okuda, Phytochemistry, 1990, 29, 1559).
Cassia bark is an important drug of Oriental medicine.
Cassia ‘buds’, perhaps inappropriately named, are the dried immature fruits of C. cassia. They yield about 20% of volatile oil having a cinnamaldehyde content of around 80%.
Java or Indonesian cinnamon is derived from C. burmanii Blume, and is used in Holland. The tree is found in Sumatra, through Java to Timor. It may be distinguished from ordinary cinnamon when in powder by the presence of tabular crystals of calcium oxalate. The oil contains about 75% of cinnamic aldehyde.
Oliver bark or black sassafras is obtained from the so-called Brisbane ‘white sassafras’ tree, C. oliveri, a native of Queensland. It is used locally as a cinnamon substitute. The bark is easily distinguished from the drugs mentioned above. It occurs in flat strips about 20 cm long, 4 cm wide and 1 cm thick. The outer surface is brownish and warty, and bears patches of greyish cork. It yields about 1–2.4% of volatile oil.
TEA-TREE OIL
The clear, colourless to pale-yellow oil is obtained by distillation from the leaves and terminal branches of Melaleuca alternifolia (Maiden and Betch) Cheel, family Myrtaceae, and other species of Melaleuca including M. linariifolia Smith and M. dissitiflora F. Mueller. These species are closely related to M. leucadendron (q.v.) and occur wild in New South Wales, Australia, where they constitute a well-known article of traditional aboriginal medicine.
Cyclic monoterpenes constitute the principal components of the oil, for which the BP/EP sets specified limits: terpinen-4-ol (30.0% minimum), γ-terpinene (10–28%), p-cymene (0.5–12.0%), α-terpinene (5–13%), cineole (less than 15.0%), other components generally present in smaller amounts for which limits are given include α-pinene, sabinene, limonene, terpinolene, aromadendrene and α-terpineol. These compounds are determined by gas chromatography using a reference solution for calculation. Other pharmacopoeial tests are relative density, refractive index, optical rotation and TLC.
In recent years the popularity of tea-tree preparations has increased enormously to include antiseptic creams for skin treatment, inhalations and pastilles for throat infections. A recent report (D. V. Henley et al., New Engl. J. Med., 2007, 356, 479) records that natural lavender and tea-tree oils in moisturisers can cause breast enlargement in prepubertal boys. Laboratory tests on breast cells have shown that the oils activate the female oestrogen receptor and suppress male hormones.
NATURAL CAMPHOR
Natural camphor is a white, dextrorotatory ketone, C10H16O (see Fig. 18.17), obtained from the wood of Cinnamomum camphora (Lauraceae), a tree which is widely grown in Taiwan, Japan and south China; it is also produced commercially in India and Georgia. Synthetic camphor, which is optically inactive, is prepared from turpentine and would probably have completely replaced the natural product had it not been for other important byproducts of the industry. Monographs for both the natural and synthetic camphor are included in the BP/EP.
Preparation
The best yield of camphor is obtained from old trees. The wood is cut into chips and treated with steam, when a solid sublimate of camphor and liquid volatile oil pass into the receiver. The volatile oil is treated to yield more camphor and much of the residual camphor oil is used as a source of safrole. The impure camphor is treated with lime and charcoal and resublimed into large chambers. It collects in the form of ‘flowers of camphor’, which can be made into the familiar blocks by hydraulic pressure. Camphor can also be prepared from suitable leaves of the tree and their use is helping to reduce the complete destruction of camphor tree forests.
Synthetic camphor is largely prepared from American turpentine. By the action of hydrogen chloride the pinene is converted into bornyl chloride which, on treatment with sodium acetate, yields isobornyl acetate. Hydrolysis of this is to isoborneol and subsequent oxidation gives camphor.
Tests for identity and purity of natural camphor are important to eliminate synthetic racemic material, excess camphor oil, and camphors from inappropriate natural sources. These tests include melting point (175–179°C), specific optical rotation (+40.0 to +43.0), acidity and limit of halogens (particularly chlorides arising from the synthesis of racemic camphor), gas chromatographic detection of extraneous material arising from the synthesis of camphor and other sources including α- and β-pinene, cineole, fenchone, fenchol and borneol.
Characters
Camphor occurs in small, colourless crystals or in transparent fibrous blocks. It has a characteristic odour and a pungent, aromatic taste, which is followed by a sensation of cold. It volatilizes at ordinary temperatures, forming an encrustation on the walls of the vessel in which it is kept.
Camphor oil (see above) contains, in addition to camphor, safrole, borneol, heliotropin, vanillin and terpineol, a number of sesquiterpene alcohols. Oil of Pakistan origin has been shown to contain 25 monoterpenoids, and four chemotypes with respect to oil composition have been recorded for Vietnamese material.
Canella bark
Canella bark is the dried rossed bark of Canella alba (C. winterana) (Canellaceae), a small tree growing in the Bahamas and Florida. It occurs in quills or channelled pieces up to 5 cm long and 5 mm thick. It contains about 1% of volatile oil and as a condiment goes under the names of ‘white cinnamon’ or ‘wild cinnamon’. The oil contains monoterpenes, eugenol and myristicin.
In 1978 a novel antimicrobial sesquiterpene dialdehyde (canellal) was reported from the bark and more recently 3-methoxy-4,5-methylenedioxycinnamaldehyde. Cannellal also has insect antifeedant, antifungal and cytoxic properties. For work on the isolation of drimane sesquiterpenes and other compounds, see D. Kioy et al., J. Nat. Prod., 1989, 52, 174; 1990, 53, 1372 and M. S. Al-Said et al., Phytochemistry, 1990, 29, 975.
Oil of Cajuput
Oil of Cajuput is a volatile oil distilled from the fresh leaves of Melaleuca leucadendron L. and other species of Melaleuca (Myrtaeceae) and rectified by steam distillation. The plants are evergreen shrubs or trees found in the East Indies and Australia. Most of the oil is produced in the islands of Bouru and Banda. It has a pleasant, camphoraceous odour and a bitter aromatic taste. It contains about 50–60% of cineole, terpineol and its acetate, terpenes and sesquiterpenes.
Medicinally, the oil is used both internally and externally as a stimulant and for the treatment of various parasitic conditions. It finds considerable use in India and the Far East.
Pimento or allspice
Pimento (Jamaica or Clove Pepper) is the dried nearly ripe fruit of Pimenta dioica (Myrtaceae), an evergreen tree grown in the West Indies (Jamaica, Cuba, Trinidad etc.) and central America.
The fruits are collected before they are quite ripe, as they otherwise lose much of their aroma and become filled with a sweet pulp; they are normally sun-dried but artificial drying has been recommended.
The pimento flower and fruit closely resemble those of the clove. The biocular ovary, however, develops two seeds, whereas only one is produced in the clove. Pimento fruits are globular and 4–7 mm in diameter. At the apex of the fruit are four small calyx teeth surrounding a short style (cf. clove fruit, Fig. 22.12). The pericarp is reddish-brown, rough and woody, and about 1 mm thick. Sections show numerous oil glands in the pericarp. Each of the two loculi contains a single planoconvex seed. Pimento has a characteristic aromatic odour and taste.
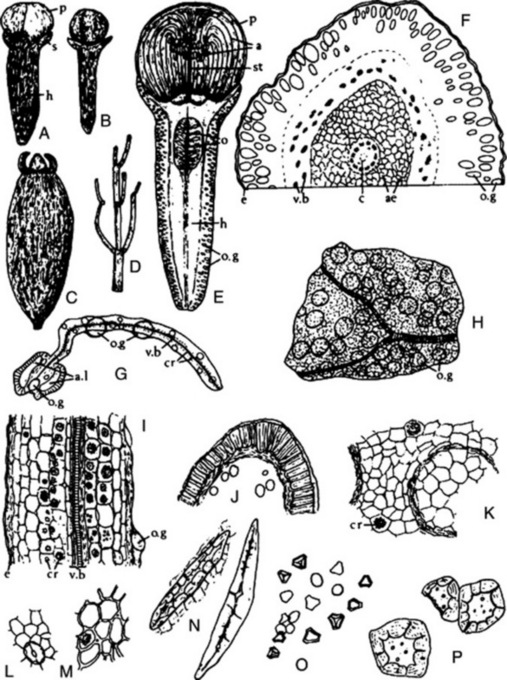
Fig. 22.12 Syzygium aromaticum. A, Penang clove; B, Zanzibar clove; C, fruit (mother clove), (all ×2); D, clove stalk (×1); E, clove cut longitudinally (×5); F, transverse section of hypanthium; G, portion of anther (both ×15); H, surface view of petal (×50); I–P, elements of powdered clove (all ×200); I, portion of anther filament; J, fibrous wall of anther lobe and immature pollen; K, fragment of hypanthium showing portions of oil glands; L, epidermal cells and stoma of hypanthium; M, parenchyma of hypanthium; N, phloem fibres; O, pollen grains; P, sclereids from clove stalk. a, Stamens; ae, aerenchyma; a.I, anther lobes; c, columella; cr, cluster crystal of calcium oxalate; e, epidermis; h, hypanthium; o, ovules; o.g, oil gland; p, imbricated petal; s, sepal; st, style; v.b, vascular bundle.
Pimento fruits yield about 3–4.5% of volatile oil which, estimated for eugenol by the method used for oil of cloves, shows a phenol content of 65–80%. The oil also contains cineole, (−)-phellandrene and caryophyllene; in all some 44 compounds have been identified.
CLOVE AND CLOVE OIL
Cloves are the dried flower buds of Syzygium aromaticum (Eugenia caryophyllus) (Myrtaceae), a tree 10–20 m high which is indigenous to the Molucca or Clove Islands. It is cultivated in Zanzibar and in the neighbouring island of Pemba, which together, for many years, produced more than three-quarters of the world’s supply of cloves. However, the industry has deteriorated in these areas and the principal producers are now Madagascar, Indonesia and Brazil. Smaller quantities are grown in Sri Lanka and Tanzania.
History
Cloves were used in China as early as 266 BC and by the fourth century they were known in Europe, although very expensive. The Spice Islands were occupied by the Portuguese at the beginning of the sixteenth century, but they were expelled by the Dutch in 1605. As in the case of nutmegs, the Dutch made every effort to secure a monopoly, destroying all the trees in their native islands (Ternate, Tidor, Mortir, Makiyan and Bachian) and cultivating them only in a group of small islands, of which Amboyna is the largest. In 1770, however, the French succeeded in introducing clove trees into Mauritius, and cultivation was afterwards taken up in Sumatra (1803), Penang, Cayenne, Madagascar, Zanzibar (1818), Pemba and elsewhere.
Collection and preparation
The flower buds are collected when their lower part turns from green to crimson. In Zanzibar and Pemba collection takes place twice yearly, between August and December. The inflorescences are collected from movable platforms. The cloves are dried in the open air on mats and separated from their peduncles, the latter forming a separate article of commerce known as ‘clove stalks’ (Fig. 22.12D). If left too long on the tree, the buds open and the petals fall, leaving ‘blown cloves’; later the fruits (Fig. 22.12C) known as ‘mother cloves’ are produced. A small proportion of these, usually at a stage intermediate between that of a clove and a fully ripe fruit, are frequently found in the drug. Cloves are imported in bales covered with matting made from strips of coconut leaves.
Macroscopical characters
Cloves are 10–17.5 mm long (cf. A and B in Fig. 22.12). The Penang and Amboyna varieties are the largest and plumpest and are most esteemed, but they are in such demand in the East that relatively small quantities of them reach Europe; they are used principally for the making of pomanders. The Zanzibar variety, however, is of good quality, although smaller and leaner than the Penang and of a blackish-brown rather than a reddish-brown colour.
The ‘stalk’ of the clove consists of a cylindrical hypanthium or swelling of the torus, above which is a bilocular ovary containing numerous ovules attached to axile placentae. The ‘head’ consists of four slightly projecting calyx teeth; four membranous, imbricated petals, and numerous incurved stamens around a large style (Fig. 22.12E).
Cloves have a strong, fragrant and spicy odour and a pungent, aromatic taste. When indented with the fingernail, they readily exude oil. Cloves sink in freshly boiled and cooled water (distinction from cloves which have been exhausted of volatile oil).
Microscopical characters
The hypanthium, in the region below the ovary, shows in transverse section (Fig. 22.12) a heavy cuticularized epidermis in which occur stomata, slightly raised above the surface and showing well-defined substomatal spaces. Within this is a zone of roughly radially arranged parenchymatous cells containing numerous schizolysigenous oil glands arranged in two or three more or less intermixed layers. The oil glands are ellipsoidal in shape, with the long axis radial, and show an epithelium composed of two or three layers of flattened cells. The contents of the oil glands are soluble in alcohol and are blackened by treatment with alcoholic ferric chloride or osmic acid. The ground mass of parenchyma also gives the blackening with ferric chloride. Cluster crystals of calcium oxalate (5–25 μm in diameter) occur in many of the parenchymatous cells. Within the oil gland layer is a zone of cells with somewhat thickened walls, embedding a ring of bicollateral vascular bundles. The ground tissue of this zone contains cluster crystals of calcium oxalate. The meristeles are enclosed in an incomplete ring of lignified fibres; the xylem is composed of 3–5 lignified spiral vessels. Within the ring of vascular bundles is a zone of aerenchyma composed of air spaces separated by lamellae one cell thick, which supports the central columella. The ground tissue of the columella is parenchymatous and is particularly rich in calcium oxalate clusters. In the outer region of the columella is a ring of some 17 small vascular bundles.
The hypanthium, in the region of the ovary, shows epidermis, oil gland layer and ring of bicollateral bundles. Within this is a zone of cells with very strongly thickened cellulose walls, limited internally by an inner epidermis forming the wall of the ovary. The dissepiment of the ovary is parenchymatous; the placentae are rich in cluster crystals and contain vascular bundles. If sections of the hypanthium are mounted in a concentrated solution of potassium hydroxide, acicular and radiately aggregate crystals separate, owing to the presence of the phenol eugenol in the oil.
The sepals and petals have a simplified leaf structure. The mesophyll parenchyma contains calcium oxalate and embeds numerous oil glands. The epidermis of the sepals shows stomata. The epidermis of the petals is devoid of stomata and is composed of very irregular cells.
The stamens are composed of filament, connective and anther. The filament shows an epidermis of longitudinal elongated cells, a ground mass of parenchyma embedding numerous oil glands and a single vascular strand enclosed in a sheath of crystal cells. The vascular strand is continuous into the connective, which is terminated by an oil gland. The fibrous layer of the anther wall is composed of cells showing spiral bands of lignified thickening. The pollen grains are triangular in outline and 15–20 μm diameter. The style and stigma yield similar characters to those of the filament. See Fig. 22.12 for the illustrated characters of the powder.
Starch, prisms of calcium oxalate and lignified sclereids are absent from a powder consisting of the flower buds only. Clove stalks contain lignified sclereids (Fig. 22.12P) and reticulately thickened xylem vessels. Clove fruits (‘anthophylli’, ‘mother cloves’) contain starch. As there is a permissible pharmacopoeial limit (not more than 6%) for these structures in the drug, a few sclereids and starch grains may therefore be found in the powder. There are also limits for deteriorated cloves ( 2.0%) and other foreign matter (
2.0%) and other foreign matter (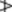 0.5%).
0.5%).
Clove oil
Oil distilled in Britain and the US usually requires no purification, but oil distilled abroad (e.g. in Madagascar) is, when imported, usually wet and discoloured by the presence of metallic salts. The latter type of oil is always rectified and may be sold with different eugenol contents. Oil of cloves is a colourless or pale yellow liquid, which is slightly heavier than water (relative density 1.047–1.060). It is soluble in from one to two volumes of alcohol (70%).
Clove oil contains 84–95% of phenols (eugenol with about 3% of acetyleugenol), sesquiterpenes (α- and β-caryophyllenes) and small quantities of esters, ketones and alcohols. Some 28 compounds have been reported in the oil and this rather low number, compared with many other oils, is due to the lack of monoterpene derivatives. The phenols can be estimated by absorption with solution of potassium hydroxide in a graduated flask, as described in the BP (1989). The current BP/EP employs GC using internal standards for the determination of eugenol (limits 75–88%), β-caryophyllene (5–14%), acetyl eugenol (4–15%) and other components. Those oils which have a relatively low phenol content are known in commerce as ‘opt’ and are the ones mainly used in pharmacy, while the ‘strong’ oils are used in the manufacture of vanillin.
Oil of clove, like other essential oils, should be stored in well-filled, airtight containers, protected from light and heat. It is used as a flavouring agent, stimulant, aromatic and antiseptic.
Clove stem oil is produced in Tanzania and in Madagascar; it is used mainly in the flavouring and perfumery industries. Clove leaf oil is distilled in Madagascar, Tanzania and in Indonesia, and is used for the isolation of eugenol.
EUCALYPTUS LEAF
Eucalyptus leaf of the EP and BP consists of whole or cut dried leaves of the older branches of Eucalyptus globulus Labill. Eucalyptus trees possess two kinds of leaves, those on young plants being cordate and sessile whereas those on mature trees which constitute the official drug are petiolate and scimitar-shaped. The dried leaves are greyish-brown in colour, coriaceous in texture and have lateral veins which anastomose near the margin. Secretory oil glands are visible in leaves held to the light. Microscopy shows epidermal cells with thick cuticle, anisocytic stomata, together with mesophyll having schizogenous oil glands and prisms and cluster crystals of calcium oxalate.
The leaves are required to contain not less than 2.0% v/w of essential oil and have limits of 3% for dark and brown leaves, 5% for stems and 2% for other foreign matter. Other significant components of the leaves are phloroglucinol-sesquiterpene coupled compounds named macrocarpals, which show antibacterial activity against oral pathogenic microorganisms and inhibition of glycosyltransferase activity. Such substances could have potential in the maintenance of oral hygiene. For further details see K. Osawa and H. Yasuda, J. Nat. Prod., 1996, 59, 823.
EUCALYPTUS OIL
Oil of eucalytus is distilled from the fresh leaves of various species of Eucalyptus (Myrtaceae) and rectified. Eucalyptus oils are produced in Portugal, South Africa, Spain, China, Brazil, Australia, India and Paraguay.
Only a certain number of species produce oils suitable for medicinal use. The chief requirements are a high cineole content and the absence of appreciable quantities of phellandrene and aldehydes (for formulae see Fig. 22.2). Suitable oils are derived from E. polybractea, E. smithii, E. globulus and E. australiana. In the case of the latter species the oil used in pharmacy is that collected during the first hour of the distillation, that which passes over subsequently being used for mineral separation. ‘Citron-scented’ eucalyptus oil, which is derived from E. citriodora, is used in perfumery and contains a high proportion of the aldehyde citronellal.
Characters
Oil of eucalyptus is a colourless or pale yellow liquid. It has an aromatic and camphoraceous odour; a pungent, camphoraceous taste, which is followed by a sensation of cold. It is required to contain not less than 70.0% of cineole. 1,8-Cineole and o-cresol form a solid complex and the crystallizing temperature of this forms the basis for the official assay of the oil. The Pharmacopoeia also includes a TLC identification test, and tests which limit the content of aldehydes and phellandrene; these eliminate oils containing citronellal and the so-called industrial eucalyptus oils.
GINGER
Ginger (Zingiber) is the scraped or unscraped rhizome of Zingiber officinale (Zingiberaceae). The BP drug is known in commerce as ‘unbleached ginger’. Z. officinale, a reed-like plant, is grown in many parts of the world, including Jamaica, China, India and Africa. Jamaica ginger, once the traditional pharmaceutical ginger, has been largely replaced by other sources.
History
Ginger has been cultivated in India from the earliest times; the plant is unknown in the wild state. The spice was used by the Greeks and Romans, and was a common article of European commerce in the Middle Ages. It was well known in England in the eleventh century. Ginger was introduced into Jamaica and other West Indian islands by the Spaniards, and a considerable quantity of the drug was sent from the West Indies to Spain as early as 1547.
Cultivation and preparation
Ginger grows well at subtropical temperatures where the rainfall is at least 1.98 m per annum. As the plant is sterile, it is grown by vegetative means. Selected pieces of rhizome (‘seed pieces’ or ‘setts’) each bearing a bud are planted inholes or trenches during March or April, preferably in a well-drained clayey loam. The procedures resemble potato cultivation. Mulching or manuring is necessary as the plant rapidly exhausts the soil of nutrients. When the stems wither, about December or January, the rhizomes are ready for collection. For the scraped drug, after removal of soil the rhizomes are killed by boiling water. They are then carefully peeled, thoroughly washed and then dried in the sun on mats or barbecues. During drying they are turned from time to time and protected during any damp weather. This first drying usually takes about 5 or 6 days. To obtain a whiter product the ginger is again moistened and dried for a further two days, when it is ready for export. It should not be limed.
With some gingers little or no cork is removed (‘coated’ or ‘unscraped’ gingers) and these grades are now also included in the BP/EP; these are sometimes whitened by dusting with calcium carbonate or lime but are eliminated by the BP ash value (not more than 6.0%).
Macroscopical characters
The dried scraped drug (Fig. 22.13) shows little resemblance to the fresh rhizome, owing to loss in weight and shrinkage. It occurs in sympodially branched pieces known as ‘hands’ or ‘races’. These are 7–15 cm long, 1–1.5 cm broad and laterally compressed. The branches arise obliquely from the rhizome, are about 1–3 cm long and terminate in depressed scars or in undeveloped buds. The outer surface is buff-coloured and longitudinally striated or fibrous; it shows no sign of cork. The drug breaks with a short fracture, the fibres of the fibrovascular bundles often projecting from the broken surface. It has an agreeable aromatic odour and a pungent taste.
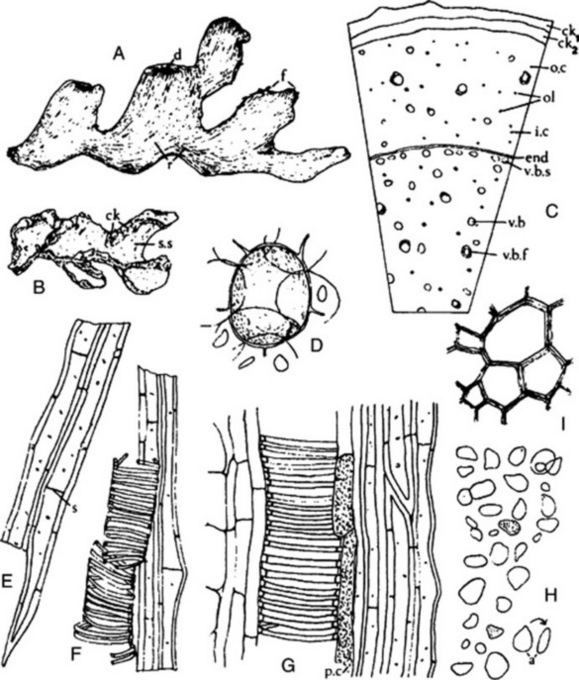
Fig. 22.13 Ginger. A, Peeled Jamaican rhizome; B, partially peeled African root (both ×0.75); C, diagrammatic transverse section of unpeeled rhizome (×15); D, oleoresin cell with adjacent parenchyma; E, portions of septate fibres; F, G, portions of septate fibres with attached vessels; H, starch; I, cork cells in surface view from unpeeled drug (all ×200). a, Starch granule, side- and end-aspects; ck, cork; ck1, irregularly arranged cells of outer cork; ck2, radially arranged cork cells; d, depressed scar; end, endodermis; f, projecting fibres; i.c, inner cortex; ol, oleoresin cells; o.c, flattened cells of outer cortex; p.c, pigment cell; r, ridges produced by vascular bundles; s, septum; s.s, scraped surface; v.b, vascular bundle; v.f.b, vascular bundle with fibrous sheath; v.b.s, ring of small vascular bundles.
In transverse section a lens shows the cortex, a dark line (the pericycle and endodermis, the latter without starch) and the stele with numerous scattered fibrovascular bundles. Similar bundles also occur in the cortex. The bundles appear as greyish points, the smaller yellowish points which can also be seen being secretion cells.
The unscraped rhizome resembles the above in structure but is more or less covered by brownish layers of cork with conspicuous ridges; the cork readily exfoliates from the lateral surfaces but persists between the branches.
Microscopical characters
The unpeeled rhizome, in transverse section (Fig. 22.13C), shows a zone of cork tissue, differentiated into an outer zone of irregularly arranged cells produced by suberization of the cortical cells without division and an inner zone of cells arranged in radial rows and produced by tangential division of the cortical cells. No cork cambium is differentiated. Within the cork is a broad cortex, differentiated into an outer zone of flattened parenchyma and an inner zone of normal parenchyma. The cortical cells contain abundant starch grains. These are almost entirely simple, ovoid or sack-shaped, are 5–15–30–60 μm long and have a markedly eccentric hilum (Fig. 22.13H). Scattered in the cortex are numerous oil cells, with suberized walls enclosing yellow–brown oleoresin. The inner cortical zone usually contains about three rings of collateral, closed vascular bundles. The larger bundles are enclosed in a sheath of septate, non-lignified fibres. Each vascular bundle contains phloem, showing well-marked sieve-tubes and a xylem composed of 1–14 vessels with annular, spiral or reticulate thickening. These vessels do not give a marked lignin reaction with phloroglucinol and hydrochloric acid. Axially elongated secretion cells with dark contents occasionally accompany the vessels. The inner limit of the cortex is marked by a single-layered endodermis free from starch. The outermost layer of the stele is marked by a single-layered pericycle. The vascular bundles of the stele resemble those of the cortex, and are, except for a ring of small bundles immediately within the pericycle, scattered as is typical of monocotyledonous stems. The ground mass of the stele is composed of parenchyma resembling the cortical parenchyma and containing much starch, and numerous oil cells. Cork cells are absent from the scraped drug. (For illustrations of the above features see Fig. 22.13D–H.)
Constituents
Ginger contains about 1–2% of volatile oil (BP/EP 1.5%); for the assay, liquid paraffin (10 drops) or other antifoaming agent may be added to the distillation flask. The rhizomes also contain 5–8% of resinous matter, starch and mucilage. Oil of ginger, to which the drug mainly owes its aroma, contains a mixture of over 50 constituents, consisting of monoterpenes (β-phellandrene, (+)-camphene, cineole, citral and borneol), sesquiterpene hydrocarbons (zingiberene, β-bisabolene, (E,E)-α-farnesene, β-sesquiphellandrene and ar-curcumene) and the sesquiterpene alcohol zingiberol. Over 50 volatile constituents of fresh organically grown ginger (fresh Chinese white and Japanese yellow varieties) have been recorded (S. D. Jolad et al., Phytochemistry, 2004, 65, 1937).
1.5%); for the assay, liquid paraffin (10 drops) or other antifoaming agent may be added to the distillation flask. The rhizomes also contain 5–8% of resinous matter, starch and mucilage. Oil of ginger, to which the drug mainly owes its aroma, contains a mixture of over 50 constituents, consisting of monoterpenes (β-phellandrene, (+)-camphene, cineole, citral and borneol), sesquiterpene hydrocarbons (zingiberene, β-bisabolene, (E,E)-α-farnesene, β-sesquiphellandrene and ar-curcumene) and the sesquiterpene alcohol zingiberol. Over 50 volatile constituents of fresh organically grown ginger (fresh Chinese white and Japanese yellow varieties) have been recorded (S. D. Jolad et al., Phytochemistry, 2004, 65, 1937).
The pungency of ginger is due to gingerol, an oily liquid consisting of homologous phenols. The principal one of these is [6]-gingerol (i.e. where n = 4). It is formed in the plant from phenylalanine, malonate and hexanoate (see Denniff et al., J. Chem. Soc. Perkin, I, 1980, 2637).
Smaller amounts of gingerols with other chain-lengths are also present. Similarly, [6]-gingerdiol is accompanied by four analogues which were isolated as minor components of the rhizome by Kikuzaki et al. from the less-polar fractions of the dichloromethane extracts (Phytochemistry, 1992, 31, 1783). The same group also characterized (Phytochemistry, 1991, 30, 3647; 1996, 43, 273) a number of diaryl-heptanoids and a further seven have since been reported from Chinese Z. officinalis (J. Ma et al., Phytochemistry, 2004, 65, 1137). These diarylheptanoids are similar to the curcuminoids present in greater quantity in turmeric (q.v.). A number of diarylheptanones – gingerenones A, B, C and isogingerenone B have been investigated by Endo and colleagues (Phytochemistry, 1990, 29, 797). Other minor components are methylgingediol, gingediacetates, methylgingediacetates and a C20-dialdehyde.
The pungency of gingerol is destroyed by boiling with 2% potassium hydroxide. Boiling with baryta water decomposes it with formation of a phenolic ketone called zingerone and aliphatic aldehydes (mainly normal heptaldehyde). Zingerone also occurs in the rhizome and, like gingerol, is pungent but possesses in addition a sweet odour. Its pungency is destroyed by prolonged contact with 5% sodium hydroxide. Shogaols, components of the oil, represent compounds formed by loss of water from the gingerols and were not thought to be present in the fresh rhizome. However, T.-S. Wu et al. (Phytochemistry, 1998, 48, 1889) have now isolated three new dehydroshogaols from fresh roots purchased from a market in Taiwan. For formulae of ginger constituents see Figure 22.14.
Varieties
The plant which yields the official ginger is grown in many tropical countries, including India (Cochin, Calicut and Bengal), Africa (Nigeria, Sierra Leone), China, the East Indies, Cochin China, Australia and Florida. The chief varieties in English commerce are the Chinese, Nigerian, Cochin and African.
A number of commercial varieties of root, oleoresin and essential oil are available, seemingly derived from Z. officinale; whether these arise from different chemical races, from differences in cultivation and harvesting techniques or from different climatic conditions is not clear. They vary considerably in sensory characters. Australian oils are characterized by a ‘lemon, citrus-like’ odour. Oil from Fiji has a high citral content and a relatively high content of 1,8-cineole similar to Japanese oil.
Nigerian ginger
The best Nigerian closely resembles the Jamaica drug, but can be distinguished from it in the whole condition by its somewhat darker colour, its smaller size and that it is rather less deeply scraped. Nigerian ginger has a more pungent taste and rather less aroma than Jamaican. It yields less volatile oil (about 0.7–1%).
Cochin ginger
This is grown in southern India and is imported via Bombay or Madras. It occurs in both coated and scraped forms. The coated variety bears on the upper and lower surfaces a wrinkled reddish-grey cork which readily exfoliates. The lateral surfaces are without cork but are decidedly darker than the surface of the Jamaican drug. Pieces may be found of almost exactly the same size and shape as the Jamaican, but on the whole the pieces are smaller and the branches somewhat thicker. Cochin ginger is more starchy and breaks with a shorter fracture than the official; it is equally pungent but less agreeably aromatic. Calicut ginger closely resembles the Cochin, but the latter is usually regarded as the better grade.
Chinese ginger
This is produced in large quantity as various grades; it is sliced as opposed to split and the peeled drug is reported to be of Jamaican quality. It is often the principal variety available in the UK.
African ginger
This is typically smaller and darker than the Cochin. It is ‘coated’, a brown cork extending over a greater area than in the Cochin. The relatively small exposed portions of cortex on the lateral sides are grey to blackish in colour. It lacks the fine aroma of the Jamaica drug, although exceeding it in pungency. Bombay ginger resembles the African.
Allied drugs
Japanese ginger is derived from Z. mioga. The volatile oil which it contains differs in physical properties from that of the official species and gives the drug a bergamot-like odour. The taste is less pungent than that of Z. officinale and the starch grains are compound and less eccentric.
Preserved ginger consists of young undried rhizomes which are preserved by boiling in syrup. The West Indian variety is made from the official plant, but that from China is said to be obtained from the greater galangal, Alpinia galanga (Zingiberaceae).
Galangal rhizome, now little used in England although employed on the Continent, is derived from the lesser galangal, A. officinarum.
Adulteration
Most of the likely vegetable adulterants can be detected by a routine microscopical examination. Powdered ginger may have been prepared from ‘wormy’ drug, and so attention should be paid to the absence of insect fragments.
Adulteration may also take the form of the addition of ‘spent ginger’ which has been exhausted in the preparation of essence. This may be detected by the official standards for alcohol-soluble extractive, water-soluble extractive, total ash and water-soluble ash.
Exhausted ginger and, more particularly, ginger galenicals may have their pungency increased by the addition of capsicum or grains of paradise. The suspected liquid, or a tincture prepared from the suspected powder, is heated in a water-bath with caustic alkali. The liquid is then evaporated, and the residue acidified with hydrochloric acid and shaken with ether. Some of the ethereal solution evaporated on a watch-glass gives a residue which is not markedly pungent to taste. This test depends on the fact that gingerol is more readily decomposed by alkalis than are capsaicin or paradol.
Uses and actions
Ginger is used as a carminative and stimulant. A US study by Mowrey and Clayson (Lancet, 1982, 1, 655) indicated that powdered ginger may be a more effective antiemetic than dimenhydrinate (Dramamine). The authors suggested that it may ameliorate the effects of motion sickness in the gastrointestinal tract itself, in contrast to antihistamines, which act centrally. Other reports claim that ginger is effective in the control of excessive and uncontrolled vomiting occurring in the first trimester of pregnancy and that it might provide a cheap antiemetic adjunct to cancer therapy (S. S. Sharma et al., J. Ethnopharmacology, 1997, 57, 93).
A considerable number of pharmacological studies involving the digestive, central nervous and cardiovascular systems have been reported for the isolated constituents of ginger. These activities include the potent inhibitory actions of the gingerols against prostaglandin synthetase which correspond with the anti-inflammatory and antiplatelet aggregation properties of the drug. These compounds, together with [6]-shogaol, also produce enhanced gastrointestinal activity with effects on bile secretion. The C20-dial mentioned previously has a cholesterol-biosynthesis inhibitory activity in animal preparations and is assumed to be a HMG-CoA reductase inhibitor (M. Tanabe et al., Chem. Pharm. Bull., 1993, 41, 710). The sesquiterpene hydrocarbons have also been associated with the antiulcer activity of the drug. A strong antibacterial and antifungal action has been demonstrated for a number of the rhizome constituents.
Turmeric
Turmeric (Curcuma) is the dried rhizome of Curcuma longa (Zingiberaceae), cultivated in India, West Pakistan, China and Malaya. It contains constituents similar to those of ginger and is described in Chapter 29 under ‘Antihepatotoxic Drugs’.
For a review of the principal pharmacological activities of turmeric (anti-inflammatory, hepatoprotective, antimicrobial, wound healing, anticancer, antitumour, antiviral) see R. C. Srimal, Fitoterapia, 1997, 68,483.A radioprotective effect ascribed to free radical scavenging and electron/hydrogen donation has been demonstrated in mice (D. Choudhary et al., J. Ethnopharmacology, 1999, 64, 1) and an assay-guided fractionation of an ethanolic extract has furnished three DPPH free-radical scavenging diaryheptanoids: curcumin, demethoxycurcumin and bisdemethoxycurcumin (DPPH = 1,1-diphenyl-2-picrylhydrazyl). See E. K. Song et al., Planta Med., 2001, 67, 876.
CARDAMOM FRUIT AND CARDAMOM OIL
Cardamom consists of the dried, nearly ripe fruits of Elettaria cardamomum Maton var. minuscula Burkill (Zingiberaceae). The seeds, the part used medicinally and as a spice, are directed to be kept in the fruits until required for use. This prevents loss of volatile oil and helps one to distinguish the fruits from those of E. cardamomum var. major (unofficial long wild native cardamoms) and from the fruits of other genera of the same family. However, to cut costs on transport much seed is now imported in sealed tins. Cardamom is expensive, its price among other common spices being exceeded only by those of saffron and vanilla.
Principal producers are Sri Lanka, southern India and Guatemala.
History
Cardamoms are mentioned in the early Sanskrit writings of Susruta, but it is difficult to say with any certainty when they first appeared in Europe. Immense quantities are still used in Hindu festivals. Both Amomum and Cardamomum appear in a list of Indian spices liable to duty at Alexandria, about AD 176–180. The Portuguese navigator Barbosa (1514) appears to have been the first to mention the source of our official drug as the Malabar coast.
Production, collection and preparation
Although wild plants are found in India and Sri Lanka, cardamoms are mainly obtained from cultivated plants. Propagation is by seedlings or vegetatively but the latter gives problems owing to possible infection by mosaic or katte virus. The plant is reed-like, 4 m or more high, and bears long leaves arising from the rhizome. As the capsular fruits on the same raceme ripen at different times and it is important to collect them when nearly ripe and before they split to shed their seeds, it is best to cut off each fruit at the correct stage with a pair of short-bladed scissors. Pickers can, by this method, collect about 5 kg of fruit per day, although collecting all the fruits on one raceme together is naturally quicker. In Sri Lanka and India flowering and fruiting continues for practically the whole year but most of the crop is collected from October to December.
The fruits are dried slowly, either outdoors or in a curing house. Too-rapid drying is to be avoided, as it causes the fruits to split and shed their seeds. Sometimes the capsules are remoistened and further exposed to the sun but this sun-bleaching, although improving the appearance, also increases the number of split fruits. Bleaching may also be done by placing trays of the fruit over burning sulphur. Bleached fruits appear to have become less common and there is now an increased proportion of the unbleached Alleppy and Ceylon greens. The green curing procedure is also used in Guatemala and it has been claimed that enhanced colour retention is obtained by soaking the fruits for 10 min in 2% sodium carbonate solution before drying.
The capsules have the remains of the calyx at the apex and a stalk at the base. These may be removed either by hand-clipping or by machines. The fruits are then graded by means of sieves into ‘longs’, ‘mediums’, ‘shorts’ and ‘tiny’. If they have been sulphur-bleached, they are aired in the open before being packed for export.
Macroscopical characters
The cardamom fruit is an inferior, ovoid or oblong capsule, about 1–2 cm long. The size, shape and surface vary in the different commercial varieties and grades (see below). The apex is shortly beaked and may show floral remains, while the base is rounded and shows the remains of the stalk. Internally the capsule is three-celled, a double row of seeds attached to axial placentas occurring in each cell. In good samples the seeds form about 70% of the total weight. The seeds in each loculus are tightly pressed together and usually separate in a single mass.
Each seed is about 4 mm long and 3 mm broad and somewhat angular. The colour varies from a dark reddish-brown in fully ripe seeds to a much paler colour in the unripe ones. The testa is transversely wrinkled and is covered by a membranous aril. A groove on one side of the seed indicates the position of the raphe and a depression at one end of the hilum. Cardamom seeds have a strongly aromatic odour and a pleasantly aromatic, although somewhat pungent, taste.
Seeds cut longitudinally and transversely and stained with iodine show the aril, testa, perisperm (containing starch) and the endosperm and embryo (both free from starch), as illustrated in Fig. 22.15.
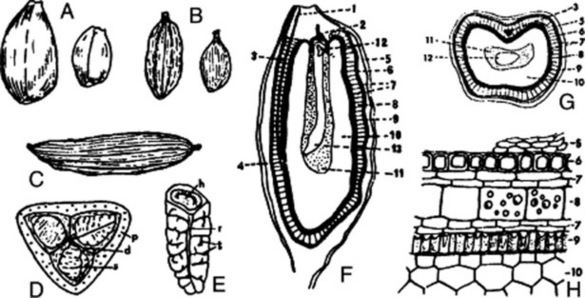
Fig. 22.15 Cardamom fruits and seeds. A, Mysore; B, Alleppy green; C, long, wild native (all × 1); D, transverse section of fruit (×1.5); E, whole seed (about × 4); F, longitudinal section of seed; G, transverse section of seed; H, arrangement of cells in transverse section of seed coat. d, Dissepiment of fruit; p, pericarp; r, raphe; s, seed; t, wrinkled testa; 1, funicle; 2, operculum or embryonic cap; 3, raphe; 4, chalaza; 5, arillus; 6, epidermis of testa; 7, parenchyma layers of the testa; 8, oil cell layer; 9, sclerenchymatous layer of testa; 10, perisperm; 11, endosperm; 12, embryo; 13, haustorium.
Varieties
Mysore fruits have a cream or pale buff colour and a nearly smooth surface. Malabar are usually smaller and have a rather darker and less smooth pericarp. Mangalore resemble the Malabar but are usually more globular and have a rougher pericarp; they occur both bleached and semi-bleached. Alleppy fruits are narrower than the above varieties, have a markedly striated pericarp and vary in colour from greenish-buff to green. Ceylon greens resemble Alleppy, but are generally greener and more elongated. The seeds of the above varieties are almost indistinguishable from one another, and also from the seeds of the long wild native cardamom (see below under ‘Allied drugs’.)
Microscopical characters
Sections of the seed (Fig. 22.15) show a very thin membranous arillus, enveloping the seed and composed of several layers of collapsed cells, yellow in colour and containing oil. The brownish testa is composed of the following layers. (1) An outer epidermis consisting of a single layer of cells rectangular in transverse section, longitudinally elongated and with prosenchymatous end walls in surface view; light yellow in colour and having slightly thick end walls. (2) A single or double layer of parenchymatous cells, elongated at right angles to the long axis of the overlying epidermal cells (see Fig. 22.15H). (3) A single layer of large parenchymatous cells containing volatile oil; in the region of the raphe there are two layers of oil cells separated by the raphe meristele. (4) Several layers of small flattened parenchymatous cells, their structure often partially obliterated. (5) An inner epidermis of sclerenchymatous cells, radially elongated, with anticlinal and inner walls very strongly thickened and reddish-brown in colour. Lumen bowl-shaped and containing a module of silica (see Fig. 22.15H). The operculum or embryonic cap is composed of two or three layers of these sclerenchymatous cells, continuous with those of the inner epidermis. The micropyle is a narrow canal passing through the operculum. Within the testa is a well-developed perisperm composed of parenchymatous cells packed with minute globular starch grains, 4 μm diameter and containing in the centre of each cell a small prismatic crystal of calcium oxalate. The perisperm encloses the endosperm and embryo, both composed of thin-walled cells rich in protein.
Cardamom pericarps or husks which have been used for the adulteration of powdered drugs may be identified in the form of powder by the pitted fibres and spiral vessels of the fibrovascular bundles and by the abundant, empty parenchymatous cells.
Constituents
Samples of cardamom seed yield 2.8–6.2% (BP not less than 4.0%) of volatile oil and also contain abundant starch (up to 50.0%), fixed oil (1–10%) and calcium oxalate.
Cardamom oil
The oil is distilled in relatively limited quantities in Sri Lanka, India and Guatemala with an estimated global production of some 4 tonnes in 1984. The oil contains a high proportion of terpinyl acetate and cineole and smaller quantities of other monoterpenes, including alcohols and esters. Over 40 compounds have been identified in the oils of Elettaria species. The BP requires an ester value of 90–156 and an optical rotation of +20° to +40°. The loss of oil from seeds kept in the pericarp is small but a loss of 30% in 8 months takes place when the seeds are separated from the fruits. Gas chromatography has shown oils from different varieties of cardamom to have qualitatively the same composition, but variations in the proportions of individual components are evident.
Allied drugs
The long wild native cardamoms of Sri Lanka (Fig. 22.15C) are derived from E. cardamomum var. major Thwaites. They are much more elongated than the official variety, sometimes attaining a length of about 4 cm. The pericarps are dark brown and coarsely striated. The oil distilled from them is used in liqueurs.
‘Amomum’, of the Indian Pharmaceutical Codex, consists of the ripe or nearly ripe seeds of Amomum aromaticum or A. subulatum. The former, obtained from Bengal and Assam, is known as Bengal Cardamom; the latter, obtained from Nepal, Bengal, Sikkim and Assam, as Nepal or Greater Cardamom.
No other similar drugs, unless we include grains of paradise (see below), are imported in any quantity or with any regularity; the following is a list of allied species the seeds of which somewhat resemble those of the true cardamom: A. cardamomum, the round or cluster cardamom of Siam and Java; A. xanthioides, the bastard or wild Siamese cardamom; A. maximum, a Javanese plant; Aframomum korarima, the Korarima or Abyssinian cardamom; A. mala, the East African cardamom; A. hanburii and A. daniellii, Cameroon cardamoms; A. angustifolium, Madagascar cardamom; Costus speciosus, Chinese cardamom. The antiplasmodial activity of A. zambesiacum seeds has been investigated and five new labdane diterpenoids and five known ones isolated (M. Kenmogne et al., Phytochemistry, 2006, 67, 433).
Uses
The principal uses of cardamom are as a flavouring agent in curries and cake. Large quantities are used in Scandinavia and Germany and, with a large proportion of Asiatics in the population, consumption has increased in Britain. Some is used in the manufacture of liqueurs and a relatively small amount in pharmacy, chiefly in the form of Compound Tincture of Cardamom.
Biological activities demonstrated for cardamom include antimicrobial, anti-inflammatory, analgesic, antispasmodic and, recently, gastroprotective (A. Jamal et al., J. Ethnopharmacol., 2006, 103, 149).
Grains of paradise
This spice, also known as Guinea grains or melegueta pepper, has been an article of commerce from very early times. It consists of the seeds of the West African reed-like herb Aframomum melegueta (Zingiberaceae), which has many of the characters of cardamom (q.v.).
The seeds are hard, reddish-brown, about 3 mm long and of a flattened pyramidal shape. The testa is papillose. Internally the structure resembles that of a cardamom seed. They have an aromatic odour and a pungent taste. The aroma is due to about 0.5% of volatile oil which contains principally β-caryophyllene, α-humulene and their epoxides. The pungency arises from paradol, a substance related to gingerol, and from small quantities of shogaol and gingerol. For the detection of paradol in ginger galenicals, see ‘Ginger’. The essential oils from the seeds of A. melegueta and other Aframomum spp. from the Cameroon have been analysed by GC-MS (C. Menut et al., Flavour Fragrance J., 1991, 6, 183). The seeds are used in alcoholic liquors and to some extent in veterinary medicine. Excessive consumption of the seeds can lead to ocular toxicity (S. A. Igwe et al., J. Ethnopharmacology, 1999, 65, 203).
CHAMOMILE FLOWERS
Roman Chamomile Flowers are the expanded flower-heads of Chamaemelum nobile (L.) All (Anthemis nobilis L.) (Compositae), collected from cultivated plants and dried. Chamomiles are cultivated in the south of England and in Belgium, France, Germany, Hungary, Poland, former Yugoslavia, Bulgaria, Egypt and Argentina. As a result of long cultivation most of the tubular florets present in the wild plant have become ligulate, and it is these ‘double’ or ‘semi-double’ flower-heads which form the commercial drug. They are included in the BP/EP.
History
Owing to the large number of similar composite plants, it has proved impossible to trace the drug in classical writings. The double variety was certainly known in the eighteenth century.
Collection
The flowers are collected in dry weather and carefully dried. The crop is often damaged by wet weather and the discoloured flowers then obtained fetch a much lower price than those having a good colour.
Characters
Each dried flower-head (Fig. 22.16A) is hemispherical and about 12–20 mm in diameter (the BP imposes a 3% limit on small or blemished heads). The florets are of a white to pale buff colour, the outer ones hiding the involucre of bracts. A few hermaphrodite, tubular florets are usually found near the apex of the solid receptacle (see Fig. 22.16B). A transition between the typical tubular florets and typical ligulate ones is often seen. The ligulate florets show three teeth (or occasionally two), the centre one being most developed. There are four principal veins. The corolla is contracted near its base into a tube from which a bifid style projects. The ovary is inferior and devoid of pappus. Each floret arises in the axil of a thin membranous bract or palea which has a blunt apex. At the base of the receptacle is an involucre consisting of two or three rows of oblong bracts which have membranous margins.
Fig. 22.16 A, Cultivated Roman chamomile; B, the same cut longitudinally; C, German chamomile; D, a ligulate floret of same; E, German chamomile cut longitudinally. 1, Tubular floret; 2, ligulate floret; 3, palea; 4, receptacle; 5, bract of involucre. (B after Greenish, remainder after Gilg.)
Chamomiles have a strong, aromatic odour and a bitter taste. The BP/EP includes a TLC test for identity and requires the drug to contain not less than 0.7% of volatile oil and not more than 10.0% water.
Constituents
Chamomiles contain 0.4–1.0% of volatile oil which is blue when freshly distilled owing to the presence of azulene. Other components of the oil are n-butyl angelate (principal), isoamyl angelate, 3-phenylpropyl isobutyrate, tridecanal, pentadecanal and terpenes. Chamomiles also contain sesquiterpene lactones of the germacranolide type, hydroperoxides, dihydroxycinnamic acid and apigenin (a trihydroxy flavone) and luteolin both free and as glucosides. (For the isolation of other constituents see A. Carnat et al., Fitoterapia, 2004, 75, 32.
MATRICARIA FLOWERS
Matricaria flowers (German or Hungarian chamomile flowers) are the dried flower-heads of Matricaria recutita L. (Chamomilla recutita (L.) Rausch.) (Compositae). The plant is a native to and is cultivated in southern and eastern Europe; Argentina and Egypt are also producers. It is official in the EP and described in the BP.
The capitulum when spread out, is 10–17 mm in diameter and consists of a receptacle, an involucre, 12–20 marginal ligulate florets and numerous central tubular florets. Unlike chamomile flowers, matricaria possesses a hollow receptacle which is devoid of paleae (see Fig. 22.16C–E). Broken flowers are limited to 25%. The drug has a pleasant aromatic odour.
Constituents
The flower-heads are required to contain not less than 0.4% of a blue volatile oil; this consists mainly of the sesquiterpenes α-bisabolol, chamazulene and farnesene. Chamazulene itself does not occur in the plant but is formed from a sesquiterpene lactone (matricin) during steam distillation.
Flavones and coumarins (e.g. herniarin) are present and the dried ligulate florets contain 7–9% of apigenin glucosides (the 7-glucoside and a mixture of acetates as determined by 13C-NMR analysis) and 0.3–0.5% free apigenin, which may arise by post-harvest hydrolysis of the glucosides. In this respect, V. Švehlíková et al. (Phytochemistry, 2004, 65, 2323) have studied the isolation, identification and stability of apigenin-7-O-glucoside in the white florets.A number of chemotypes depending on the proportions of bisabolol, bisabolol oxides and farnesene in the oil have been described. Most Turkish varieties of M. chamomilla yield yellow oils containing no chamazulene; 2n and 4n races have been studied for their respective coumarin variations (A. Pastirová et al., Pharm. Biol., 2005, 43, 205). For an article listing the many known constituents of matricaria flowers see A. Ahmad and L. N. Nisra, Int. J. Pharmacognosy, 1997, 35, 121.
Uses
Matricaria flowers are mainly used on the Continent of Europe and in the USA for their anti-inflammatory and spasmolytic properties. The ulcer-protective properties of German chamomile have been ascribed to bisabolol-type constituents, on which considerable pharmacological work has been reported. Four optically active isomers of bisabolol are possible; extracts for pharmaceutical use should be prepared only from clearly defined types containing the active constituents.
Allied drug
Tanacetum parthenium (L.) Schultz-Bip.; Chrysanthemum parthenium (L.) Bernh., or feverfew flowers may be single or double. The receptacle is flatter than that of the Roman chamomile and may or may not bear paleae. If the latter are present, they are acute and less membranous than those of the chamomile. The whole flowering tops are usually sold. Feverfew herb yields 0.07–0.4% of volatile oil. It is used in herbal medicine; for details concerning its content of sesquiterpene lactones see Chapter 24.
MATRICARIA OIL
Matricaria oil is that steam-distilled from the fresh or dried flower heads or flowering tops of Matricaria recutita L. Resulting from the chemotypes mentioned above two types of oil are described in the pharmacopoeia—one rich in bisabolol oxides and the other rich in (−)-α-bisabolol. These compounds, together with chamazulene, are determined by gas chromatography.
These oils are blue in colour and have a characteristic odour.
YARROW
Yarrow (millefolium, milfoil) is described in the BP/EP, BHP and a number of continental pharmacopoeias. It is also the subject of German Commission E and ESCOP monographs. The drug consists of the dried flowering tops of Achillea millefolium L. (Compositae), an extremely diverse aggregate species with varying chromosome numbers and differences in oil composition. The British Herbal Compendium, Vol. 1, 1992 points out that work reported under ‘Achilleum millefolium’ may refer to A. millefolium sensu stricto or any number of other species which have been more recently and narrowly defined.
Yarrow is native to Europe and Western Asia but is now widespread in most temperate regions including N. America; commercial supplies come largely from south-eastern Europe, although it is also collected in other European countries including the UK.
Characters
The flowers occur in characteristically dense terminal corymbs about 3–5 cm in diameter and composed of capitula 3–5 cm in diameter. Each capitulum possesses an involucre of bracts, usually with five white to reddish ligulate ray florets, and 3–20 tubular disk florets. The fruits are achenes. The powdered material contains numerous elements, not only from the flower but also from stems and leaves. These include the typical Compositae pollen grains, leaf epidermis with anomocytic stomata and glandular and clothing trichomes again typical of the Compositae. Fuller details are given in the BP and EP.
Constituents
The pharmacopoeia requires an essential oil content of not less than 0.2% and not less than 0.002% of proazulenes calculated as chamazulene (Fig. 22.17). The tetraploid form of the plant (A. millifolium L., ssp. collina Becker) appears the most suitable as it produces considerable chamazulene as a component of the oil whereas the widespread hexaploid species (A. millefolium L., ssp. millefolium) lacks this guaianolide sesquiterpene. Germacranolide- and eudesmanolide-type sesquiterpenes are also constituents of the oil together with caryophyllene, sabinene, α- and β-pinene, borneol, bornyl acetate, camphor and small quantities of thujone. The proazulenes are determined by measurement of the absorbance (608 nm) of the oil (in xylene) obtained by distillation from the herb.
Other isolates from yarrow include sesquiterpene lactones (achillin, achillicin, etc.), flavonoids (apigenin, luteolin, quercetin and their 7-O-glycosides), alkaloids (betonicine, stachydrine, trigonelline) and various acetylenes, coumarins, triterpenes, sterols and plant acids.
V. K. Agnihotri et al. (Planta Med., 2005, 71, 280) studied plants from two different high-altitude (1600 m) populations propagated under uniform environmental conditions at a lower altitude (300 m); the populations represented two ecotypes, a 1:8-cineole type and a borneol type, which differed in oil content and in composition of mono- and sesqui-terpenes.
Uses
Yarrow is used, as is chamomile and matricaria, to treat various skin conditions and digestive disorders. Its pharmacological actions can arise from various groups of compounds—anti-inflammatory (chamazulene and prochamazulenes, apigenin, salicyclic acid), haemostatic (betonicine), spasmolytic (flavonoids).
Chandler RF, Hooper SN, Harvey HJ. Ethnobotany and phytochemistry of yarrow, Achillea millefolium, Compositae. Economic Botany. 1982;36:203-223.
Hofmann L, Fritz D, Nitz S, Kollmannberger H, Drawert F. Essential oil composition of three polyploids in the Achillea millefolium ‘complex’. Phytochemistry. 1992;31:537-542.
WORMWOOD
Wormwood is essentially the dried leaves and flowering tops of Artemisia absinthium L., Compositae, widely distributed in Europe and the New World and recorded as a household remedy from biblical times. It is now included in the EP, BP, BHP 1983 and a number of European pharmacopoeias. There are official requirements for its volatile oil content and bitterness. The principal producers are the former USSR, Bulgaria, former Yugoslavia, Hungary and Poland; it is also cultivated in the USA and elsewhere.
The plant is a subshrub with deeply dissected leaves. The insignificant globose flowers form loose panicles and consist mainly of tubular florets and a few yellow ray florets. The leaves and grooved stems are covered with silky hairs.
Characteristic features of the microscopy are the T-shaped trichomes (see Fig. 42.3I) on both leaf epidermi; these have uniseriate stalks of up to three cells and long tapering unicellular heads. There are numerous unicellular long, twisted trichomes and secretory trichomes with biseriate two-celled stalks and heads of two to four cells. The stomata are of the anomyocytic type. Numerous spherical pollen grains, 30 µm in diameter with three pores and a spiny exine, are seen in the powdered drug.
The drug has an aromatic odour and is intensely bitter. The active constituents are the bitter substances and essential oil. Bitter substances (0.15–0.4%) consist of sesquiterpene lactones, principally the dimeric guaianolide absinthin (0.20–0.28%), artabsin, artabsinolides A, B, and C and others. They are evaluated in the BP by the organoleptic test for ‘bitterness value’ using a quinine hydrochloride solution for comparison. The essential oil (BP requirement not less than 0.2%) is variable in composition according to geographical source and chemotype with any one of p-thujone, trans-sabinyl acetate, cis-epoxyocimene and chrysanthenyl acetate forming over 40% of the mixture; also present are other sesquiterpenes and monoterpenes.
Over the years many medicinal properties have been ascribed to wormwood. It is considered of value for promoting the appetite, for its strengthening effect in the treatment of colds and influenza, for gall bladder and menstrual problems and for the expulsion of round worms. Thujone is toxic, making the cultivation of low-thujune chemotypes desirable. The herb is also used in the making of liqueurs.
LOVAGE
Lovage is the whole or cut dried rhizome and root of Levisticum officinale (Ligusticum levisticum), family Umbelliferae. The official drug should contain not less than 0.4% essential oil for the whole drug and not less than 0.3% for that in the cut condition calculated with reference to the anhydrous drug.
The plant is native to southern Europe, western Asia and the Orient but has for a long time been cultivated elsewhere; it is produced commercially in the Balkans, Germany, Holland, Poland and the USA.
In habit, lovage is a tall, aromatic perennial herb with bipinnate, cauline leaves coarsely toothed at the apex and greenish-yellow flowers. The rhizomes and roots are obtained from plants 2 to 3 years old and when split, cut and dried are in pieces up to 5 cm in diameter for the rhizomes and up to 25 cm in length for the roots. Externally the drug is greyish-brown in colour and longitudinally furrowed; a transverse section of the roots shows a thick yellowish-white bark separated from a brownish-yellow radiate wood by a dark line. Oil-containing structures are visible in the outer regions of the transverse section. Microscopic characters of the powdered drug include polygonal or rounded cork cells as seen in surface view, considerable parenchyma, reticulately thickened vessels, fragments of secretory cells and single and compound starch granules.
The drug contains up to 1.0% of volatile oil, the characteristic odoriferous components being alkyl phthalides of which 3-butylphthalide (c. 32%), ligustilide (c. 24%) and ligusticum lactone are principal components. Terpenes include α- and β-pinene, α- and β-phellandrene, α- and β-terpinene, camphene, myrcene, etc. Other constituents are various coumarins and plant acids. The BP includes a TLC examination as a test for identity and for the absence of angelica root.
Lovage has been used for centuries as a herbal remedy. It has carminative, diuretic and antimicrobial properties making it useful for the treatment of dyspepsia, cystitis and as a mouthwash for tonsillitis. Herbalists usually prescribe it in admixture with other drugs.
Tansy
Tansy (Tanacetum vulgare (L.); Chrysanthemum vulgare (L.) Bernh.) (Compositae) is used as an anthelminthic in herbal medicine but its poisonous properties are well appreciated. The herb contains about 0.2–0.6% volatile oil containing around 70% of thujone. Many sesquiterpene lactones have been isolated from the flowers and herb together with flavones. Numerous chemical races, originating from different geographical areas, are known and involve both the oil constituents and the sesquiterpenes. (For a series of reports involving three other species of Tanacetum see O. O. Thomas, Fitoterapia, 1989, 60, 138, 231, 329 and references cited therein. With regard to the anti-inflammatory properties of the herb, C. A. Williams et al. (Phytochemistry, 1999, 51, 417) have compared the flavonoids of T. vulgare and feverfew, and revised some flavonoid formulae.)
Sandalwood oil
Sandalwood oil is obtained from the heartwood of Santalum album (Santalaceae), an evergreen tree 8–12 m in height which is widely distributed in India and the Malay Archipelago.
Supplies are mainly derived from Indonesia and southern India where the trees are systematically cultivated and the cutting is controlled. The volatile oil is contained in all the elements of the wood, medullary ray cells, vessels, wood fibres and wood parenchyma. The oil contains about 90–97% of sesquiterpene alcohols, distinguished for purpose of analysis as ‘santalol’. This consists of α-santalol (b.p. 300–301°C) and β-santalol (b.p. 170–171°C). The hydrocarbon fraction contains about nine components. C. G. Jones et al. (Phytochemistry, 2006, 67, 2463) have discussed the biosynthesis of sandalwood oil sesquiterpenes chemotaxonomically with respect to the co-occurrence patterns of the four types studied: (1) α- and β-santalenes and bergamotene, (2) γ- and β-curcumene, (3) β-bisabolene and α-bisabol, and (4) four unidentified sesquiterpenes.
Recent reports suggest that the oil is being adulterated with polyethylene glycols. The oil is now mainly used in perfumery; a possible chemoprotective action on liver carcinogenesis in mice has been demonstrated (S. Banerjee et al., Cancer Lett., 1993, 68, 105). Bioassay-guided fractionations coupled with NMR structural determinations have shown that of eleven sesquiterpenes, (Z)-α-santalol and (Z)-β-santalol have strong anti-Helicobacter pylori activities against a clarithromycin resistant strain. (T. Ochi et al., J. Nat. Prod., 2005, 68, 819).
Australian sandalwood oil is prepared by distillation and rectification from the wood of Eucarya spicata, a small tree growing in Western Australia. It contains sesquiterpene alcohols.
RESINS, GUM-RESINS AND SIMILAR SUBSTANCES
The term ‘resin’ is applied to more or less solid, amorphous products of complex chemical nature. On heating they soften and finally melt. They are insoluble in water and usually insoluble in petroleum spirit but dissolve more or less completely in alcohol, chloroform and ether. Chemically, resins are complex mixtures of resin acids, resin alcohols (resinols), resin phenols (resinotannols), esters and chemically inert compounds known as resenes. The chemical structures of many of these compounds have now been elucidated.
Resins, as described above, are often associated with volatile oils (oleoresins), with gums (gum-resins) or with oil and gum (oleo-gum-resins). However, no hard and fast distinction can be made between these groups, as products such as mastic and ammoniacum, which are usually considered as a resin and a gum-resin, respectively, both contain volatile oil. Resins may also be combined in a glycosidal manner with sugars, as in the Convolvulaceae.
The term ‘balsam’ is often wrongly applied to oleoresins such as Canada turpentine and copaiba, and should be reserved for such substances as balsam of Peru, balsam of Tolu and storax, which contain a high proportion of aromatic balsamic acids (see Chapter 19). These balsams, if containing free acids, are partially soluble in hot water, owing to the solubility of benzoic and cinnamic acids, while the aromatic esters and resins are insoluble. Benzoin is perhaps best described as a balsamic resin.
The above products are usually contained in schizogenous or schizolysigenous ducts or cavities. They are often preformed in the plant (i.e. they are normally physiological products), but the yield is usually increased by injury (e.g. in the case of Pinus). Many products (e.g. benzoin and balsam of Tolu) are not formed by the plant until it has been injured: that is, they are of pathological origin. The gums which are often associated with resins and volatile oils usually resemble acacia gum in chemical nature and in the fact that they are often accompanied by oxidase enzymes. While resins are usually produced in ducts or cavities, they may be found in other positions—for example, in the resin cells of bloodroot, in the elements of the heartwood of guaiacum, in the external glands of Indian hemp, in the internal glands of male fern or in the glands on the surface of the lac insect.
MYRRH
Myrrh (Arabian or Somali Myrrh) is an oleo-gum resin, obtained from the stem of various species of Commiphora (Burseraceae), growing in north-east Africa and Arabia. British texts have traditionally given the principal source as C. molmol but Tucker (Econ. Bot., 1986, 40, 425) states that the chief source today is C. myrrha. The EP and BP definition cites Commiphora molmol Engler and/or other species of Commiphora. Two other species, C. abyssinica and C. schimperi, both of which may attain a height of 10 m, grow in Arabia and Abyssinia. The drug is chiefly collected in Somaliland and Ethiopia.
History
Products of the myrrh type were well known to the ancients under the names of bola, bal or bol. The drug is still known to the Indian traders as ‘heerabol’, while the Somalis call it ‘mulmul’ or ‘ogo’. The name ‘myrrh’ is probably derived from the Arabic and Hebrew word mur, which means bitter. Many references occur in the Old Testament, but the product was apparently that derived from C. erthyaea var. glabrescens, which is known to the Somalis as ‘habbak hadi’, and commercially as perfumed bdellium or bissabol.
Guban myrrh, which is produced from the trees of the Somali coast area known as the Guban, is rather oily and is regarded as inferior to the more powdery ‘ogo’ produced further inland.
Collection
Almost all members of the Burseraceae possess in the phloem oleoresin canals, which are formed schizogenously and may afterwards unite with one another to form schizolysigenous cavities. This occurs in the species Commiphora. Much of the secretion is obtained by spontaneous exudation from the cracks and fissures which commonly form in the bark, and some is obtained from incisions made by the Somalis. The yellowish-white, viscous fluid soon hardens in the great heat to reddish-brown masses, which are collected by the Somalis. As bdelliums and gums are collected at the same time, these frequently find their way into the drug and have subsequently to be picked out.
Characters
Myrrh occurs in somewhat irregular tears or masses weighing up to about 250 g. The surface is reddish-brown or reddish-yellow in colour and powdery. The drug fractures and powders readily, the freshly exposed surface being of a rich brown colour and oily. Whitish marks are sometimes seen and thin splinters are translucent. Myrrh has an aromatic odour and an aromatic, bitter and acrid taste.
Myrrh forms a yellowish emulsion when triturated with water. When extracted with alcohol (90%), as in the preparation of Tincture of Myrrh, a whitish mass of gum and impurities remains. The BP alcohol-insoluble matter should not exceed 70%. Lump myrrh usually yields not more than 5% of ash, but the commercial powdered drug frequently yields more. It may be distinguished from perfumed bdellium and similar products by allowing an ethereal extract of the drug to evaporate to dryness and passing the vapour of bromine over the resinous film produced. A violet colour is given by genuine myrrh but not by bdellium. TLC and visualization with ultraviolet light at 365 nm is used by the BP as an identification test and also to establish the absence of C. mukul, an inferior bdellium product.
Constituents
Myrrh contains 7–17% of volatile oil, 25–40% of resin, 57–61% of ‘gum’ and some 3–4% of impurities.
The volatile oil contains terpenes, sesquiterpenes, esters, cuminic aldehyde and eugenol. The sesquiterpene fraction (Fig. 22.18) contains furanosesquiterpenes including furanogermacranes, furanoguaianes and furanoeudesmanes (N. Zhu et al., J. Nat. Prod., 2001, 64, 1460). Furaneudesma-1,3-diene and curzarene have morphine-like properties and act on the CNS opioid receptors; furanodiene-6-one and methoxy furanoguaia-9-ene-8-one show antibacterial and antifungal activity against standard strains of pathogenic species (P. Dolara et al., Nature, 1996, 379, 29; Planta Medica, 2000, 66, 356). The oil, which is distilled outside the countries of origin, readily resinifies and then gives a violet colour with bromine.
The chemistry of the resins is complex and not fully elucidated. The larger ether-soluble portion contains α-, β- and γ-commiphoric acids, the esters of another resin acid and two phenolic resins. The smaller ether-insoluble fraction contains α- and β-heerabomyrrholic acids. The crude alcohol-insoluble matter (‘gum’) contains about 18% of protein and 64% of carbohydrate containing galactose, arabinose and glucuronic acid. This gum is associated with an oxidase enzyme.
Allied drugs
Four different varieties of ‘bdellium’ were recognized by Holmes. Of these, perfumed or scented bdellium or bissabol is probably derived from C. erythaea var. glabrescens. It resembles soft myrrh in appearance but is easily distinguished from it by the more aromatic odour and by the fact that it does not give a violet colour with the bromine test. Hotai bdellium or gum hotai is opaque and odourless; it contains a saponin and is used for washing the hair. The resin of C. confusa collected in Kenya contains dammarane triterpenoids, as does C. kua (L. O. A. Manguro et al., Chem. Pharm. Bull., 2003, 51, 479, 483).
Uses
Myrrh is used in incense and perfumes. Like many other resins, it has local stimulant and antiseptic properties. It is chiefly employed in medicine in the form of a mouth-wash or gargle for its astringent effect on mucous membranes. A number of its traditional and historic uses have received experimental support.
Olibanum
Olibanum (Frankincense) is an oleo-gum-resin obtained by incision from the bark of Boswellia carterii, B. frereana and other species of Boswellia (Burseraceae), small trees indigenous to north-eastern Africa and Arabia. The drug occurs in more or less ovoid tears, 5–25 mm long, which are sometimes stuck together. The surface is dusty and of a yellowish, bluish or greenish tint. Fracture, brittle; inner surface, waxy and semitranslucent. Odour is characteristic, especially when burned; taste, slightly bitter. The drug contains 3–8% of volatile oil consisting of numerous terpenes (e.g. p-cymene) and sesquiterpenes, about 60–70% of resin, and 27–35% of gum. In 1956 the gum was found to contain two polysaccharides; one consisting of units of galactose and arabinose and the other of galactose and galacturonic acid. Modern methods of analysis have allowed the taxonomic identity of diverse frankincense products to be determined; examples are incense mixtures, traditional medicines and archaeological specimens (S. Hamm et al., Phytochemistry, 2005, 66, 1399).Olibanum is used in incense and fumigating preparations. Formerly, it was considered a stimulant and has been used in China for the treatment of leprosy. With animal models, Duwiejua et al. (Planta Medica, 1993, 59, 12) have reported a positive anti-inflammatory activity for the drug.
Asafoetida
Asafoetida is an oleo-gum-resin obtained by incision from the living rhizome and root of Ferula foetida Regel, F. rubricaulis Boiss., and other species of Ferula (Umbelliferae), plants about 3 m in height. The drug is collected in Iran, Pakistan and Afghanistan.
Collection and preparation
The collection of asafoetida involves removal of the stem and the cutting of successive slices from the vertical rootstock. After each slice is removed, oleo-gum-resin exudes and, when sufficiently hardened, is collected. The product is packed in tin-lined cases for export.
Characters
Asafoetida occurs in two principal forms.
Tears. These are rounded or flattened and about 5–30 mm diameter. They are greyish-white, dull yellow or reddish-brown in colour, some specimens acquiring the latter colour with age, while others remain greyish or yellowish.
Mass. This consists of similar tears to those described above agglutinated into masses and usually mixed with fruits, fragments of root, earth and other impurities. Mass asafoetida is the commonest commercial form.
Asafoetida has a strong, alliaceous odour and a bitter, acrid and alliaceous taste. It should yield not more than 50% of matter insoluble in alcohol (90%) and not more than 15% of ash.
Constituents
Asafoetida consists of volatile oil, resin, gum and impurities. The oil has a particularly evil smell and contains sulphur compounds of the formulae C7H14S2, C16H20S2, C8H16S2, C10H18S2, C7H14S3, and C8H16S3; some of these show pesticidal activity. The flavour is largely due to R-2-butyl-1-propenyl disulphide (a mixture of E and Z isomers), 1-(1-methylthiopropenyl)-1-propenyl disulphide and 2-butyl-3-methylthioallyl disulphide (both as mixtures of diastereoisomers). The drug also contains a complex mixture of sesquiterpene umbelliferyl ethers mostly with a monocyclic or bicyclic terpenoid moiety; more recently (G. Appendino et al., Phytochemistry, 1994, 35, 183) three new sesquiterpene coumarin ethers have been isolated. Also present are asaresinol ferulate and free ferulic acid. For selected formulae see Fig. 22.19. The drug contains no free umbelliferone (distinction from galbanum). However, on boiling it with hydrochloric acid and filtering into ammonia, a blue fluorescence is produced owing to the formation of umbelliferone. Ferulic acid is closely related to umbellic acid and umbelliferone (both of which occur in galbanum).
Allied drugs
Galbanum and ammoniacum are oleo-gum-resins obtained, respectively, from Ferula galbaniflua and Dorema ammoniacum. Galbanum contains, besides umbelliferone, a number of umbelliferone ethers; also gum and up to 30% of volatile oil containing numerous mono- and sesquiterpenes, azulenes and sulphur-containing esters. Ammoniacum, listed in the BHP, contains free salicylic acid but no umbelliferone. The major phenolic constituent is ammoresinol; Appendino et al. (Helv. Chim. Acta, 1991, 74, 495) isolated an epimeric mixture of prenylated chromandiones termed ammodoremin. The volatile oil (c. 0.5%) contains various terpenoids with ferulene as the major component. The demonstration of the broad spectrum and antimicrobial activity of ammoniacum has supported its traditional use for chest infections (M. Rajani et al., Pharm. Biol., 2002, 40, 534).
Damiana
Damiana consists of the dried leaves of Turnera diffusa var. aphrodisiaca (Turneraceae), and probably other species of Turnera. The drug is collected in Bolivia and Mexico. The leaves are yellowish-green to green in colour, broadly lanceolate, shortly petiolate, and 10–25 cm long; margin with 3–6 teeth on each side; veins pinnate and prominent on the lower surface. The drug usually contains some of the reddish-brown, cylindrical twigs, flowers and spherical fruits. Damiana has an aromatic odour and taste. It contains 0.5–1.0% of volatile oil, from which thymol, α-copaene, δ-cadinene and calamenene have been isolated; in addition, a brown amorphous substance, damianin, resins and gum.
It would appear that in Mexico the wild populations of the plant are threatened by over-collection, and cultivation is recommended using micropropagation, the latter having now been shown to be a commercial feasibility (L. Alcaraz-Meléndez et al., Plant Cell Rep., 1994, 13, 679.)
Damiana is traditionally used in Mexico and Southern USA to revive libido where subconscious causative factors are involved. Elixir of Damiana and Saw Palmetto or other admixtures are used as an aphrodisiac for men.
For a review of the genus Turnera (93 references), see S. Kumar et al., Pharm. Biol., 2005, 43, 383.
Copaiba
Copaiba is an oleoresin obtained from the trunks of various species of Copaifera (Leguminosae) and contains at least 24 sesquiterpene hydrocarbons and a number of diterpenes. It was formerly used as a urinary antiseptic but has now been almost completely replaced by antibiotics and other drugs.
Eriodictyon leaf
Eriodictyon or Yerba Santa consists of the dried leaf of Eriodictyon californicum (Hydrophyllaceae), a low evergreen shrub of the hills and mountains of California and northern Mexico.
The leaves usually occur in fragments; when entire, they are lanceolate, 5–15 cm long and 1–3 cm wide. The apex is acute; the base slightly tapering into a short petiole. The margin is irregularly serrate or crenate-dentate. The upper surface is yellowish-brown to greenish-brown and covered with a glistening resin. The lower surface is greenish-grey to yellowish-grey, conspicuously reticulate, with greenish-yellow or brown veins, and minutely tomentose (cottony) between the reticulations. The leaves are thick and brittle. They have an aromatic odour and a balsamic bitter taste, which becomes sweetish and slightly acrid.
Eriodictyon contains volatile oil, resin, eriodictyol (see ‘Hesperidin’ and ‘Eriodictyol’), homoeriodictyol, chrysoeriodictyol, xanthoeriodictyol, eriodonol, eriodictyonic acid and ericolin.
Yerba Santa is employed in the USA for the preparation of a fluid extract and Aromatic Eriodictyon Syrup, which is used to mask the taste of bitter and otherwise disagreeable medicines, particularly quinine. American Indians smoked or chewed the leaves as a cure for asthma. Some herbalists consider it an excellent expectorant. Externally it can be used for the treatment of bruises, insect bites, etc.
Gamboge
Gamboge is a gum-resin obtained from Garcinia hanburii (Guttiferae), a tree indigenous to South-East Asia.
Gamboge is a typical gum-resin, and when triturated with water, it forms a yellow emulsion. Good gamboge contains 70–80% of resin (gambogic acid) and 15–20% of water-soluble gum with which is associated an oxidase enzyme. Gamboge acts as a purgative but is now little used in human medicine. It is used as a pigment. For a report on its bioactivity, see A. Panthong et al., J. Ethnopharmacol., 2007, 111, 335.
MASTIC
Mastic is a resin or, more correctly, an oleoresin containing little oil, obtained from various cultivated varieties of Pistacia lentiscus L. (Anacardiaceae); the BP/EP specifies var. latifolius Coss; another quoted in the literature is var. chia from the Greek island of Chios, which is the principal exporter.
The plant is an evergreen shrub and tapping is limited by law to the period 15 July–15 October. The base of the shrub is cleared of weeds, flattened and covered with a special white soil to receive some of the flow. The stem and larger branches are then wounded by means of a gouge-like instrument which makes an incision about 2 cm long and 3 mm deep. Each plant is tapped repeatedly for about 5 or 6 weeks, receiving in all about 200–300 wounds. A special tool is used for removing the tears which harden on the plant and the flat plates of mastic which collect on the ground. These are graded by the collector and regraded, washed and dried in a central depot before being exported in wooden boxes. Chios exports about 250 000 kg annually.
Mastic occurs in yellow or greenish-yellow rounded or pear-shaped tears about 3 mm diameter. The shape of the tears is sufficient to distinguish them from those of sandarac. The tears are brittle but become plastic when chewed. Odour, slightly balsamic; taste, mildly terebinthinate.
The resin component of mastic is a complex mixture. It contains tri-, tetra- and penta-cyclic triterpene acids and alcohols (for a report see F.-J. Marner et al., Phytochemistry, 1991, 30, 3790). About 2% of volatile oil is also present, the pharmacopoeial minimum being 1%; over 60 compounds have been reported from mastic and up to 250 recorded in plant oils. The principal components appear to be the monoterpene hydrocarbons α-pinene, β-myrcene and camphene. Four neutral novel triterpenoids and ten triterpenoid acids have now been characterized, see V. P. Papageorgiou et al., J. Chromat. A, 1997,769, 263. The acid value of about 50 (BP, 1980, not more than 70) distinguishes it from East Indian or Bombay mastic, which has an acid value of more than 100.
Mastic is used in the preparation of Compound Mastic Paint and as a microscopical mountant. In Greece and the Middle East mastic has been used for centuries as a protective agent for the stomach, and investigations at the University of Nottingham Medical School indicated success in the treatment of gastric ulcers. Research has shown that mastic will kill Helicobacter pylori at concentrations of 0.06 mg/ml (see F. U. Huwez et al., New Engl. J. Med., 1998, 339, 1946). Further studies were planned with patient volunteers infected with H. pylori (Pharm. J., 2000, 264, 459).
Sandarac
Sandarac is a resin obtained from the stem of Tetraclinis articulata (Cupressaceae), a tree 6–12 m high, which is found in North and North-west Africa and in Spain.
Sandarac occurs in small tears about 0.5–1.5 cm in length. These usually have an elongated, stalactic or cylindrical shape, globular or pear-shaped tears being relatively rare. The surface is covered with a yellowish dust, but the interior is more or less transparent, and if the tears are held up to the light, small insects can frequently be seen embedded in them. The drug is easily powdered and when chewed remains gritty, showing no tendency to form a plastic mass (distinction from mastic). The drug has a faint, terebinthinate odour, and a somewhat bitter taste.
Sandarac resin consists of sandarocopimaric acid (inactive pimaric acid), sandaracinic acid, sandaracinolic acid and sandaracoresene. The drug also contains a bitter principle and 0.26–1.3% of volatile oil.
Grindelia herb
Grindelia or gum plant consists of the dried leaves and flowering tops of Grindelia camporum (G. robusta), G. humilis and G. squarrosa (Compositae), collected in south-western USA. The plants are herbaceous with cylindrical stems, sessile or amplexicaul leaves, and resinous flower-heads each surrounded by an involucre of linear-lanceolate bracts. Odour, balsamic; taste, aromatic and bitter.
In the wild 2n and 4n forms occur and selection of the latter for cultivation should produce higher yields of resin (J. L. McLaughlin et al., Econ. Bot., 1986, 40, 155).
Grindelia contains about 20% of resin, which contains a large number of labdane diterpene acids termed grindelanes and methyl esters (see B. A. Timmermann et al., Phytochemistry, 1985, 24, 1031; M. Adinolfi et al., ibid., 1988, 27, 1878). The plants yield about 0.2% of a volatile oil containing over 100 components. Oil composition from the different species varies quantitatively with bornyl acetate and alpha;-pinene the major components of the monoterpenoid fraction (see G. Kaltenbach et al., Planta Medica, 1991, 57, (Suppl. 2), A82). For a report on the oil content, and its antioxidant activity, of plants raised experimentally in Central Italy, see D. Fraternale et al., Fitoterapia, 2007, 78, 443.
The herb has been used for the treatment of bronchitis and asthma, but is now mainly employed in the form of a lotion for dermatitis produced by the poison ivy, Rhus toxicodendron (Anacardiaceae). Some grindelanes have been shown to have antifeeding deterrent activity towards aphids.
Guaiacum resin
Guaiacum resin is obtained from the heartwood of Guaiacum officinale and G. sanctum (Zygophyllaceae), small evergreen trees found in the dry coastal regions of tropical America. Guaiacum officinale is found on the coast of Venezuela and Colombia and in the West Indies, while G. sanctum occurs in Cuba, Haiti, the Bahamas and Florida. Little is now found in commerce.
Guaiacum resin occurs in large blocks or rounded tears about 2–3 cm diameter. The freshly fractured surface is brown and glassy. The powder is greyish but becomes green on exposure. Taste, somewhat acrid; odour, when warmed, aromatic. When free from woody debris, guaiacum is soluble in alcohol, chloroform and solutions of alkalis. An alcoholic solution gives a deep blue colour (guaiac-blue) on the addition of oxidizing agents such as ferric chloride. This colour is destroyed by reducing agents. Colophony, the most likely adulterant, may be detected by the cupric acetate test.
Some of the main resinous constituents are lignans. These are phenolic compounds having a C18 structure formed from two C6–C3 units (Table 21.7). Guaiaretic acid, which forms about 10% of guaiacum resin, is a diaryl butane.
The flowers, fruit and bark of the tree contain triterpenoid and nortriterpenoid saponins.
For use as a reagent the resin as extracted from the wood by means of chloroform is said to be the most sensitive. An alcoholic solution is used for the detection of blood stains, cyanogenetic glycosides, oxidase and peroxidase enzymes.
Guaiacum resin, included in the BHP (Vol. 1, 1990) is indicated for the treatment of chronic rheumatic conditions. It is a permitted food additive in the USA and in Europe.
COLOPHONY
Colophony (rosin) is the resin remaining in the still after removal of the volatile turpentine oil from the oleoresin of species of Pinus (see Turpentine Oil). Generally, the resin obtained from trees during their first year of tapping is of a lighter colour than that obtained subsequently. Traditionally, some 17 grades of rosin have been recognized, extending from the almost black wood rosin (B&F grades) through paler colours to the window glass (WG), water white (WW) and extra white (X) grades. For a detailed account of the oleoresin collection (cup and gutter method) and preparation of the rosin, see the 15th edition of this book.
Characters
The colophony described in the BP/EP occurs in translucent glassy masses of a pale yellow or amber colour. It is brittle and easily powdered. It fuses gradually at about 100°C, and at a higher temperature burns with a smoky flame, leaving not more than about 0.1% of ash. Colophony is insoluble in water but soluble in alcohol, ether, benzene and carbon disulphide.
Chemical tests
Constituents
Colophony contains resin acids (about 90%), neutral inert substances formerly known as resenes and esters of fatty acids.The exact composition varies with biological source, preparation, age and method of storage.
The resin acids are isomeric diterpene acids. It will be noted that colophony has a high acid value of 150–180.
Before distillation the resin contains large amounts of (+)- and (−)-pimaric acids. During distillation the (+)-pimaric acid is stable but the (−)-pimaric acid undergoes isomeric change into abietic acid, the major constituent of the commercial resin (see formula, p. 264). On heating at 300°C abietic acid undergoes further molecular rearrangement to produce some neo-abietic acid. The commercial ‘abietic acid’ is prepared by digesting colophony with weak alcohol.
The abietane acids have been considerably investigated over recent years; they are obtained chiefly from colophony but are also present in other conifers of the Araucariaceae, Cupressaceae, Pinaceae and Podocarpaceae. Their activities are mainly antimicrobial, antiulcer and cardiovascular; some have filmogenic, surfactant and antifeedant properties.
Uses
The amount of colophony used in pharmacy for the preparation of zinc oxide and other adhesive plasters, ointments, etc., is relatively small. Much rosin is artificially modified by hydrogenation or polymerization; products involving its use include paper size, adhesives, printing inks, rubber, linoleum, thermoplastic floor tiles and surface coatings.
Ipomoea
Ipomoea (Orizaba Jalap, Mexican Scammony Root) is the dried root of Ipomoea orizabensis (Convolvulaceae), a convolvulaceous twining plant with a fusiform root about 60 cm long. The drug is collected in the Mexican State of Orizaba and is exported from Vera Cruz.
Orizaba was originally imported as a substitute or adulterant of jalap or its resin (‘jalapin’). However, the resin is more soluble in ether than is jalap resin and more closely resembles that obtained from the root of Convolvulus scammonia, which was the original source of scammony resin.
Whole roots of ipomoea are rarely imported, and the drug usually consists of transverse or oblique slices about 3–10 cm wide and 2–4 cm thick.
The outer surface is covered with a greyish-brown, wrinkled cork. The transverse surface is greyish or brownish and shows about 3–6 concentric rings of fibrovascular bundles. The parenchymatous tissue of both bark and stele resembles that of jalap in containing starch and calcium oxalate. Like jalap, the section shows numerous scattered secretion cells with resinous contents. Odour, slight; taste, faintly acrid.
Ipomoea, when extracted with alcohol (90%), yields about 10–20% of a complex resinous mixture, of which about 65% is soluble in ether. The chief constituents of ipomoea resin are the methyl pentosides and other glycosides of jalapinolic acid and its methyl ester; these are the orizabins. Also isolated are the scammonins, the structures of which are indicated below. For details of these resin glycosides see B. Hernández-Carlos et al., J. Nat. Prod., 1999, 62, 1096. Also present are sitosterol and other phytosterol glycosides.
Ipomoea is mainly used for the preparation of ipomoea resin. It resembles jalap in medicinal properties.
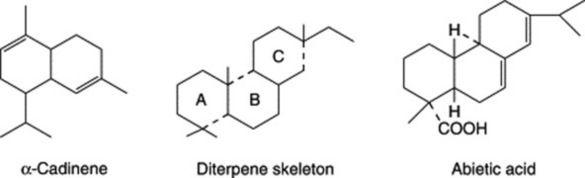
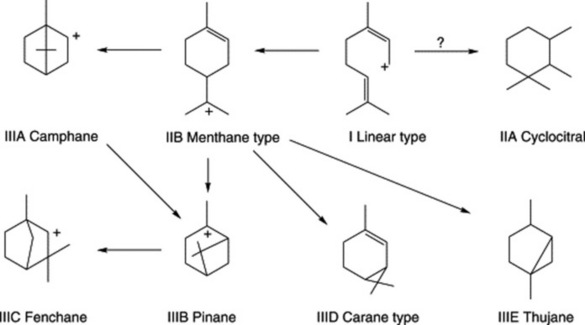
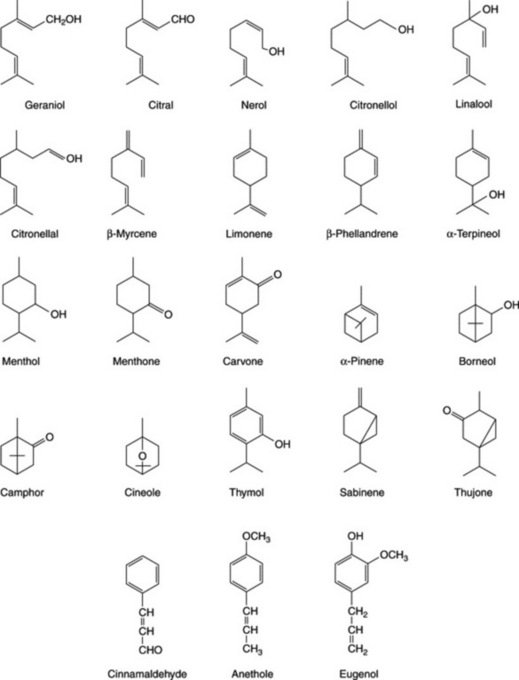
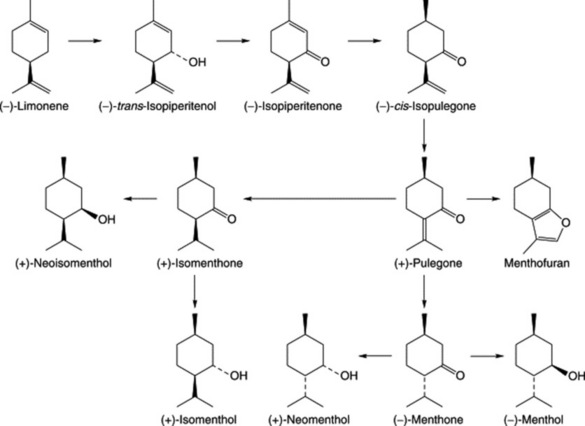
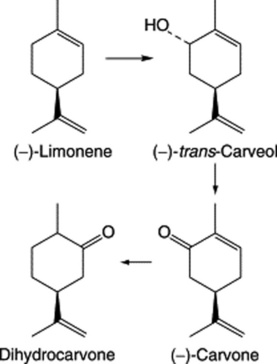
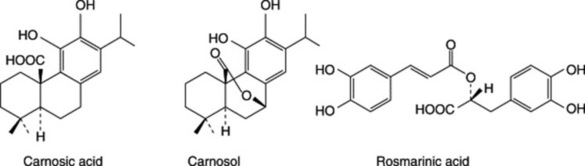
 2.0%), which is almost identical with that obtained from star-anise fruits. A number of water-soluble constituents have been isolated from the fruits including various glucosides, see E. Fujimatu et al., Phytochemistry, 2003, 63 (5).
2.0%), which is almost identical with that obtained from star-anise fruits. A number of water-soluble constituents have been isolated from the fruits including various glucosides, see E. Fujimatu et al., Phytochemistry, 2003, 63 (5).