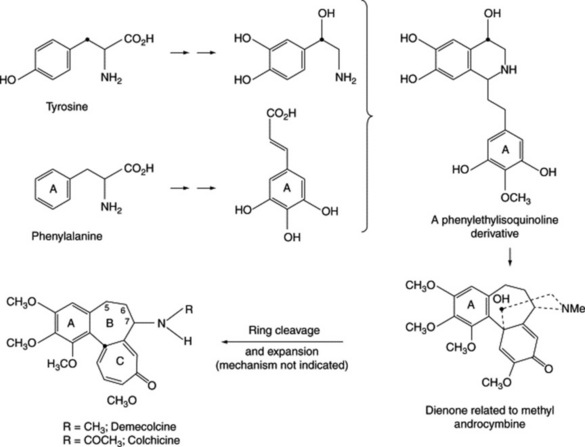
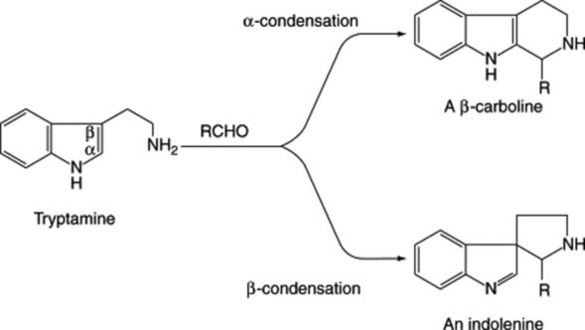
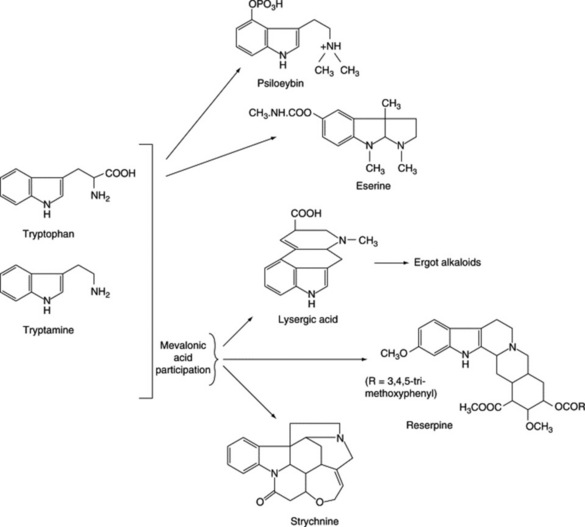
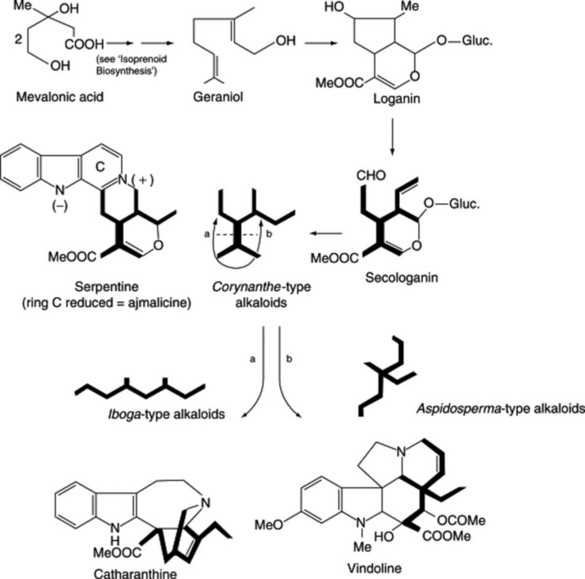
ERGOT AND ERGOT ALKALOIDS
Ergot (Ergot of Rye) is the dried sclerotium of a fungus, Claviceps purpurea Tulasne (Clavicipitaceae), arising in the ovary of the rye, Secale cereale. Controlled field cultivation on rye is the main source of the crude drug. The most important producers are Czechoslovakia, Hungary, Switzerland and former Yugoslavia. With modern farming the supply of ‘natural’ ergot is decreasing and fields of rye are devoted to its cultivation. Different selected strains of C. purpurea are used for the production of the alkaloids ergotamine, ergocristine, or ergocornine and ergokryptine. Commercially, ergot of rye is becoming less important and by 1994 UK dealers were trading mainly in ergot of wheat.
History
There is considerable doubt as to whether ergot and ergotism were known to the ancients, and it is impossible to say whether the ‘ignis sacer’ of the Romans referred to ergotism. The outbreaks of ‘ignis St Antonii’, or St Antony’s fire, which occurred during the Middle Ages, do, however, appear to have been of ergot origin. Outbreaks of ergotism occurred in Germany in 1581, 1587 and 1596 and at intervals in Europe until recent years. Ergotism was never common in England, probably owing to the fact that rye is little grown, and the only serious outbreak recorded, which took place in 1762, was caused by wheat.
World-wide, sporadic reports of ergot poisoning still appear in the literature and in 1992 an analysis of rye flour sold in Canada showed that low-level contamination by the fungus still exists; of 128 samples tested 118 proved positive for ergot alkaloids at concentrations of 70–414 ng g−1 whereas with wheat flour the incidence and levels were much lower.
The obstetric use of ergot was known in the sixteenth century, but the drug was not widely employed until the nineteenth century. It was first introduced into the London Pharmacopoeia of 1836. The fungoid origin of ergot was recognized by Münchausen in 1764, while the life history of the fungus was worked out and the name Claviceps purpurea given to it by Tulasne in 1853.
Life history and collection
The fungus C. purpurea and other species such as C. microcephala Wallr., C. nigricans Tul. and C. paspali produce ergots on many members of the Gramineae (including the genera Triticum, Avena, Festuca, Poa, Lolium, Molinia and Nardus) and Cyperaceae (including the genera Scirpus and Ampelodesma). Many of these ergots appear to be extremely toxic and to produce typical ergotism.
For the life-cycle and illustrations of the fungus, see earlier editions.
Macroscopical characters
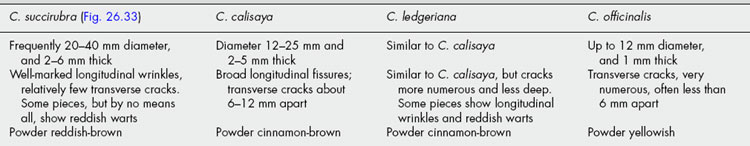
The drug consists almost entirely of sclerotia, the amount of other organic matter being generally limited to not more than 1%. Each sclerotium is about 1.0–4 cm long and 2–7 mm broad; fusiform in shape and usually slightly curved. The outer surface, which is of a dark, violet-black colour, is often longitudinally furrowed and may bear small transverse cracks. Ergot breaks with a short fracture and shows within the thin, dark outer layer a whitish or pinkish-white central zone of pseudoparenchyma in which darker lines radiating from the centre may be visible. Ergot has a characteristic odour and an unpleasant taste.
Powdered ergot when treated with sodium hydroxide solution develops a strong odour of trimethylamine. In filtered ultraviolet light it has a strong reddish colour by means of which its presence in flour may be detected.
Microscopical characters
Ergot shows an outer zone of purplish-brown, rectangular cells, which are often more or less obliterated. The pseudoparenchyma consists of oval or rounded cells containing fixed oil and protein, and possessing highly refractive walls which give a reaction for chitin. Cellulose and lignin are absent.
Constituents
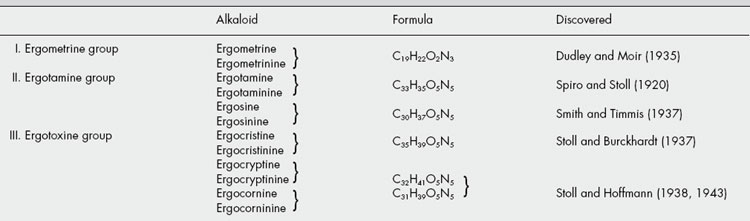
The ergot alkaloids (ergolines) can be divided into two classes: (1) the clavine-type alkaloids, which are derivatives of 6,8-dimethylergoline and have been extensively studied in cultures of the mycelium of the ergot fungus; and (2) the lysergic acid derivatives, which are peptide alkaloids. It is the latter class that contains the pharmacologically active alkaloids that characterize the ergot sclerotium (ergot). Each active alkaloid occurs with an inactive isomer involving isolysergic acid; the inactive isomers are not formed initially in the sclerotium but tend to accumulate as a result of unsuitable processing and poor or long storage. These alkaloids have been studied over many years and were not easy to characterize. Thus ‘ergotoxine’, which since its isolation in 1906 (by Barger and Carr and independently by Kraft) had been accepted as a pure substance, and in the form of ergotoxine ethanosulphonate was formerly used as a standard, was shown to be a mixture of the three alkaloids ergocristine, ergocornine and ergocryptine.
Six pairs of alkaloids predominate in the sclerotium and fall into either the water-soluble ergometrine (or ergonovine) group or the water-insoluble ergotamine and ‘ergotoxine’ groups. Table 26.6 gives the morephysiologically active member of each pair first. Alkaloids of groups II and III are polypeptides in which lysergic acid or isolysergic acid is linked to other amino acids. In the ergometrine alkaloids lysergic acid or its isomer is linked to an amino alcohol. Ergometrine was synthesized by Stoll and Hofmann in 1943. Other, new, peptide alkaloids have been isolated from submerged cultures of C. purpurea and from the field-growing fungus (L. Cvak et al., Phytochemistry, 1996, 42, 231; 1997, 44, 365).
Among the less important constituents of ergot may be mentioned histamine, tyramine and other amines and amino acids; acetylcholine; colouring matters; sterols (ergosterol and fungisterol); and about 30% fat. The cell walls are chitinous.
Variation in alkaloid constituents. Not only are chemical races very evident in C. purpurea with respect to alkaloid production but also the host plant is not without influence. Thus a new commercial strain of ergot adapted from a wild grass (Anthraxon lancifolius) to rye gave sclerotia containing 0.5% total alkaloids involving ergometrine (33%), ergotamine (17.6%), ergocornine (18.7%) and ergocryptine (22.7%). However, sclerotia produced on the grass as a result of natural infection did not contain ergometrine (K. K. Janardhanan et al., Planta Med., 1982, 44, 166). The application of specific amino acids to maturing sclerotia can also be used to influence the type of alkaloids produced (a technique also used with saprophytic cultures).
For recent studies and references on the investigation of the alkaloid gene cluster in C. purpurea see T. Haarmann et al., Phytochemistry, 2005, 66, 1312.
Alkaloid production in artificial cultures
The artificial culture of the ergot fungus has received considerable attention, and, obviously, large-scale submerged fermentation with selected strains to give alkaloids of choice has commercial possibilities. Abe’s initial work in Japan showed that submerged cultures did not produce the typical alkaloids associated with the sclerotium but, rather, a series of new non-peptide bases (clavines) which unfortunately possessed no significant pharmacological action. Attempts were made by many workers to influence alkaloid production by modification of the culture medium and the fungus strain. As a result of successful experiments in 1960, the commercial manufacture of simple lysergic acid derivatives by fermentative growth of a strain of Claviceps paspali became feasible. The alkaloids produced are converted to lysergic acid which is used for the part-synthesis of ergometrine and related alkaloids. Other strains are now available which produce the peptide alkaloids in culture; not only can different chemical races of the fungus be used to produce specific groups of alkaloids but synthesis can also be directed by the addition of certain amino acids or their analogues to the fermentation liquid. In this way new unnatural alkaloids can be produced.
Flieger et al. (J. Nat. Prod., 1989, 52, 1003) found that with submerged cultures in the postproduction stage both the alkaloid concentration and the composition of the alkaloid mixtures underwent dramatic changes including the production of two new alkaloids, 8-hydroxyergine and 8-hydroxyerginine. (For papers pertaining to the isolation of new and unnatural alkaloids from submerged cultures of C. purpurea see N. C. Perellino et al., J. Nat. Prod., 1992, 55, 424; 1993, 56, 489.)
Biosynthesis
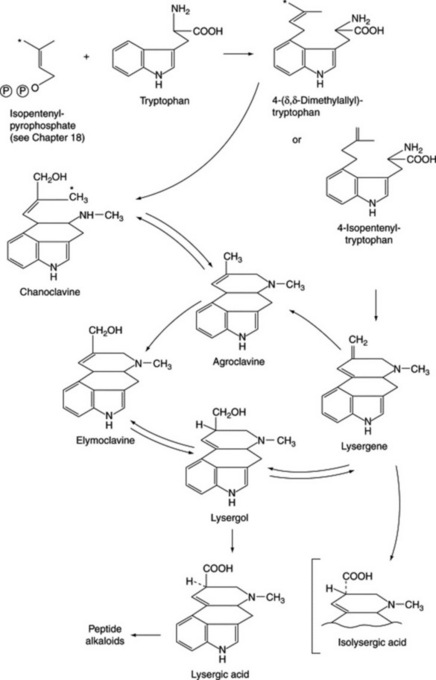
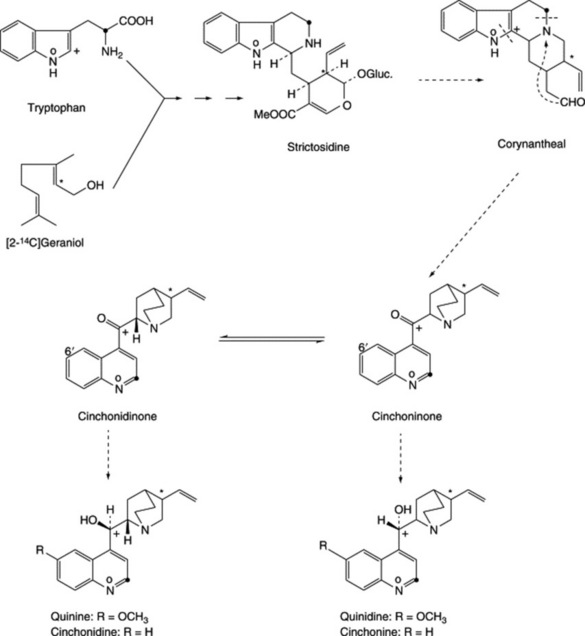
The majority of biosynthetic studies were at first directed to the clavine alkaloids, which could be easily produced in cultures but, until recently, their biological relationship to the lysergic acid derivatives remained obscure. The ergoline nucleus is derived from tryptophan and mevalonate, and current work has involved elucidating the biosynthetic relationship between the various clavine alkaloids, determining which of these intermediates is the true natural precursor of lysergic acid, and studying the initial hydrogen elimination from the C-4 of mevalonate to yield the stereo-configuration of chanoclavine-I. Possible biosynthetic routes for lysergic acid involving two isomeric intermediates are given in Fig. 26.29. A major problem has been not so much in discovering which reactions the fungus can effect when supplied with a given substrate as which route is actually involved in its normal metabolism. As a result of work with cell-free systems, Abe assigned a primary role to 4-isopentenyltryptophan and lysergene rather than to the dimethylallyl compound, agroclavine or chanoclavine. Later work by Gröger et al. (Planta Med., 1980, 40, 109) appeared to favour a scheme involving the latter compounds and this has now been generally substantiated with the intermediates involved with the ring closure between dimethylallyltryptophan and chanoclavine having been investigated (A. P. Kozikowski et al., J. Amer. Chem. Soc., 1993, 115, 2482).
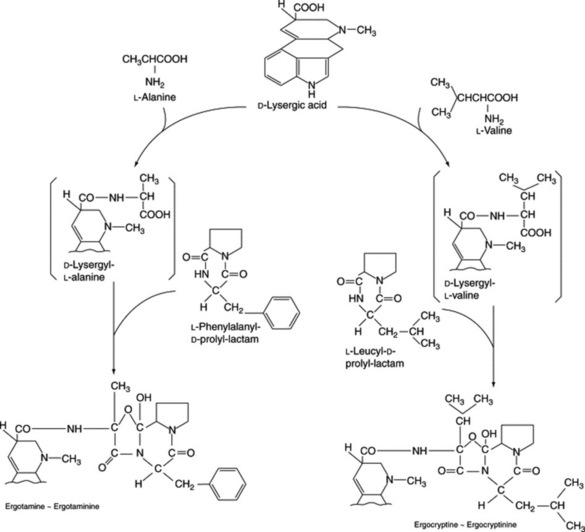
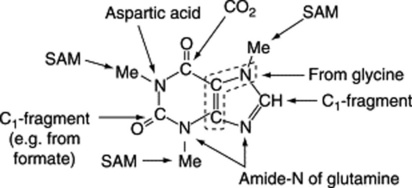
Work on the origin of the nitrogen of the peptide portions of the ergot alkaloids indicates that appropriate amino acids are specifically incorporated. Abe’s scheme is shown in Fig. 26.30 and he obtained intact incorporation of the units shown, in certain strains, but workers in Germany were unable to confirm these results. As has been mentioned earlier, unnatural amino acids can also be incorporated into the alkaloids.
Varieties
Commercial ergot varies considerably in activity from batch to batch, and the differences cannot be fully explained by differences in storage; further, it is often found that inferior-looking ergot is highly active. Such variations are apparently due to the fact that there are a number of different chemical races of C. purpurea and in cultivating ergot by modern methods it is obviously important to prepare the spore-cultures used for infecting the rye from a race of the fungus known to develop ergots having a high content of the requiredalkaloids. Cultivated ergot may contain up to 0.5% of total alkaloids, and 0.15% is a minimum commercial value. In addition, there may be a minimal requirement for water-soluble alkaloids. The alkaloids can be determined by colorimetry, as they give a blue colour with a solution of p-dimethylaminobenzaldehyde.
Substitutes
Ergot of wheat is now imported into Britain and has been used medicinally in France. The sclerotia are shorter and thicker than those of rye. Instances of ergot on barley and rye in Britain, and on wheat and rye in the USA and Canada have been reported.
Ergot of oats has been used medicinally in Algiers. The sclerotia are black in colour, 10–12 mm long and 3–4 mm diameter.
Ergot of diss, which is produced on the Algerian reed Ampelodesma tenax, has appeared in commerce and is said to be highly active. The sclerotia may attain as much as 9 cm in length and are spirally twisted.
Storage
Ergot is particularly liable to attack by insects, moulds and bacteria. After collection it should be thoroughly dried, kept entire, and stored in a cool, dry place. If powdered and not immediately defatted, the activity decreases, but if defatted and carefully stored in an air-tight container, it will remain active for a long period. However, as indicated above, under certain conditions, loss of activity arises by the conversion of the pharmacologically important alkaloids to inactive isomers. Any sample of ergot which shows worm holes or a considerable amount of insect debris will almost certainly deteriorate further on storage.
Uses
Although whole ergot preparations were traditionally used in labour to assist delivery and to reduce post-partum haemorrhage, ergot itself has been largely replaced in the pharmacopoeias by the isolated alkaloids. Only ergometrine produces an oxytocic (literally ‘quick delivery’) effect, ergotoxine and ergotamine having quite a different action. Ergometrine is soluble in water or in dilute alcohol. It is often known, particularly in the USA, as ergonovine. Ergotamine and the semisynthetic dihydroergotamine salts are employed as specific analgesics for the treatment of migraine. Lysergic acid diethylamide (LSD-25), prepared by partial synthesis from lysergic acid, is a potent specific psychotomimetic.
Calabar bean and physostigmine
Calabar beans (Ordeal beans) are the dried ripe seeds of Physostigma venenosum (Leguminosae), a perennial woody climber found on the banks of streams in West Africa. The plant bears typical papilionaceous flowers, and legumes about 15 cm long, each containing two or three seeds.
History
The seeds were formerly used by the west African tribes as an ‘ordeal poison’. They were first known in England in 1840. The myotic effect of the drug was noted in 1862 by Fraser, and physostigmine was isolated in 1864 by Jobst and Hesse.
Characters
Calabar beans have a somewhat flattened, reniform shape. They are 15–30 mm long, 10–15 mm wide and up to 15 mm thick. The seeds are extremely hard. The dark brown testa is smooth, except in the neighbourhood of the grooved hilum, which runs the whole length of the convex side and round one end, where it is somewhat wrinkled. On either side of the groove is a well-marked ridge and in the groove itself are the greyish, papery remains of the funiculus. A transverse section shows a large central cavity and two, very hard, concavo-convex cotyledons.
Constituents
The seeds contain the alkaloids physostigmine or eserine, eseramine, isophysostigmine, physovenine, geneserine, N-8- norphysostigmine, calabatine and calabacine. The structure of geneserine, long regarded as an N-oxide, has been revised to include the oxygen in a ring system. The chief alkaloid, physostigmine, is present to the extent of about 0.15%. It is derived from tryptophan (see Fig. 26.26). On exposure to air it oxidizes into a red compound, rubreserine, and should therefore be protected from air and light. Both physostigmine salicylate and sulphate are included in the BP/EP. The former of the two is more stable and non-deliquescent. For both salts there is a colorimetic test for the elimination of eseridine and a non-aqueous titration assay.
Uses
Physostigmine salicylate is used for contracting the pupil of the eye, often to combat the effect of mydriatics. It has also been investigated as an intravenous injection for reversing the effects of a number of sedatives. With Alzheimer’s disease it has shown some evidence of inducing a slight improvement in intellectual and cognitive performance (Pharm. J., 1992, 249, 376) but galanthamine (q.v.) may prove superior. Physovenine has the same order of activity but that of eseramine is much lower.
Nux vomica
Nux vomica consists of the dried, ripe seeds of Strychnos nux-vomica (Loganiaceae), a tree 10–13 m high with a distribution including Ceylon, India, East Bengal, Burma, Thailand, Laos, Cambodia and S. Vietnam. The drug is mainly collected in India and exported from Mumbai (Bombay), Madras, Cochin, Cocanada and Calcutta.
History
Nux vomica was known in Europe in the sixteenth century and was sold in England in the time of Parkinson (1640), mainly for poisoning animals. Strychnine was isolated in 1817 and brucine in 1819.
Collection and preparation
The fruit is a berry about the size of a small orange. When ripe it has a rather hard orange-yellow epicarp and a white, pulpy, interior in which 1–5 seeds are embedded. The seeds are washed free from pulp and dried. They are exported in small sacks, known as ‘pockets’, holding about 18–25 kg.
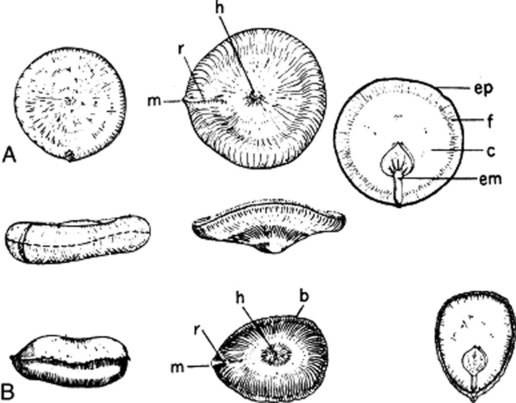
Macroscopical characters
Nux vomica seeds are extremely hard and should be boiled in water for at least an hour in order to soften them sufficiently for dissection. The seeds are greenish-grey, disc-shaped, 10–30 mm diameter and 4–6 mm thick. Most of the seeds are nearly flat and regular in shape, but a few are irregularly bent and somewhat oval in outline. The edge is rounded or acute. The testa is covered with silky, closely appressed, radiating hairs. In the centre of one of the flattened sides is a distinct hilum, and a small prominence on the circumference marks the position of the micropyle, which is joined to the hilum by a radial ridge. To examine further, a boiled seed should be cut transversely and another one opened like an oyster by inserting the blade of a small knife or scalpel at a point on the circumference opposite the micropyle. The small embryo with two cordate cotyledons and a cylindrical radicle, the latter directed towards the micropyle, will be seen embedded in a grey, horny endosperm (Fig. 26.31). In the centre of the seed is a slit-like cavity. The seeds are odourless when dry; but if soaked in water and left for a day or two, they develop a very unpleasant odour. They have a very bitter taste.
Microscopical characters
A radial section shows a very thin testa consisting of collapsed parenchyma and an epidermal layer of very characteristic lignified hairs (Fig. 41.7M). The latter have a very large, thick-walled base with slit-like pits. Surface irregularities in the bases of the hairs cause them to interlock with one another. The upper portions of the hairs are set at almost a right angle to the bases and all radiate out towards the margin of the seed, giving the testa its characteristic silky appearance. On the ridge connecting hilum and micropyle, however, the hairs are irregularly arranged. The upper part of the wallof the hair is composed of about 10 longitudinal ridge-like thickenings united by a thin wall so that the lignified ribs readily separate from one another on powdering. The lumen is circular in the upper part, but in the base has branches corresponding with the oblique pits in the wall. Fragments of testa, removed from a soaked seed, may be disintegrated by treatment with 50% nitric acid and a little potassium chlorate; the hairs can then be separated.
The endosperm consists of large, thick-walled cells, which are nonlignified and yield galactose and mannose on hydrolysis. When mounted in solution of iodine, they show well-marked protoplasmic threads (plasmodesma) passing through the walls (see Fig. 42.1G) and an oily plasma containing a few aleurone grains and the alkaloids strychnine and brucine. Strychnine is most abundant in the inner part of the endosperm and brucine in the outer layers. The presence of strychnine is shown by mounting a section in a solution of ammonium vanadate in sulphuric acid, when a violet colour is produced; of brucine, by mounting in nitric acid, when a crimson colour is observed.
The length of lignified ribs of the hairs per milligram of nux vomica seed has been used for the determination of the content of seeds in veterinary medicines, see ‘Quantitative Microscopy’.
Constituents
Nux vomica usually contains about 1.8–5.3% of the indole alkaloids strychnine and brucine. Strychnine (formula Fig. 26.26) is physiologically much more active than brucine and the seeds are therefore assayed for strychnine and not for total alkaloids. They usually contain about 1.23% of strychnine and about 1.55% of brucine. Minor related alkaloids include α-colubrine, β-colubrine, icajine, 3-methoxyicajine, protostrychnine, vomicine, novacine, N-oxystrychnine, pseudo-strychnine and isostrychnine.
For a review (188 refs) of recent studies concerning the synthesis of strychnine, see M. Shibasaki and T. Ohshima, The Alkaloids, 2006, 64, 103.
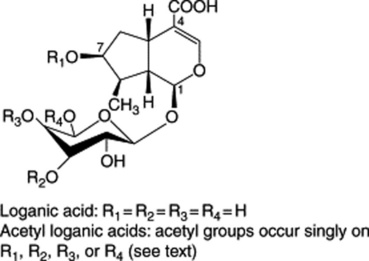
Iridoids of the seeds include the glycoside loganin (Fig. 26.27), loganic acid and 7-O-acetyl loganic acid together with three new iridoids, 6′-O-acetyl loganic acid, 4′-O-acetyl loganic acid and 3′-O- acetyl loganic acid (X. Zhang et al., Phytochemistry, 2003, 64, 1341).
The seeds also contain chlorogenic acid (see Fig. 19.5) and about 3% of fixed oil.
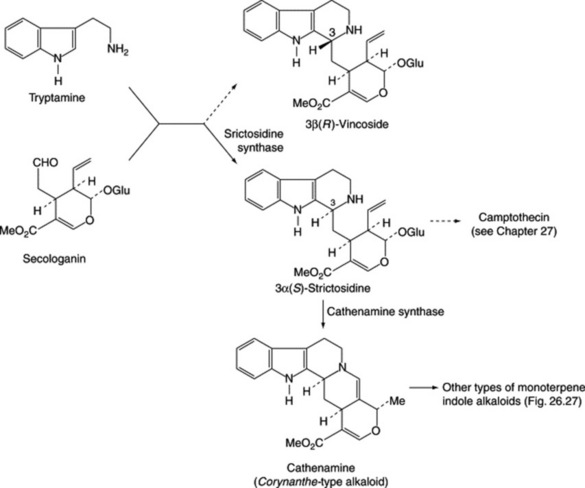
Loganin, although present only in small amounts in the seed, occurs to the extent of about 5% in the fruit pulp together with secologanin; these compounds are intermediates in the biogenesis of the strychnine-type alkaloids (Fig. 26.27).
Seasonal variations in alkaloid content of S. nux-vomica have been studied (K. H. C. Baser and N. G. Bisset, Phytochemistry, 1982, 21, 1423).
Uses
The action of the whole drug closely resembles that of strychnine. The alkaloid was formerly used as a circulatory stimulant in such cases as surgical shock, but its use is now more limited to that of a respiratory stimulant in certain cases of poisoning. Like other bitters, strychnine improves the appetite and digestion, but it has been considerably misused as a ‘general tonic’. Nux vomica is used in Chinese medicine for much the same purposes as in Western medicine and the seeds are usually processed to reduce their toxicity. Heat-treatment of the seeds reduces the normal levels of the principal alkaloids and the amounts of isostrychnine, isobrucine, strychnine N-oxide and brucine N-oxide are increased (B.-C. Cai et al., Chem. Pharm. Bull., 1990, 38, 1295).
Allied drugs
The genus Strychnos continues to attract considerable attention. (For extensive reviews on the taxonomy, chemistry and ethnobotany of the American, African and Asian species see N. G. Bisset et al., Lloydia, 33, 201; 34, 1; 35, 95, 193; 36, 179; 37, 62; 39, 263; also Bisset’s review on alkaloids of the Loganiaceae in Indole and Biogenetically Related Alkaloids, 1980 (eds J. D. Phillipson and M. K. Zenk) p. 27, London: Academic Press; and J. Quetin-Leclercq et al., J. Ethnopharm., 1990, 28, 1, review with c. 150 refs.)
Ignatius beans are the seeds of Strychnos ignatii, a plant occurring in the Philippines, Vietnam and elsewhere. The fruits are larger than those of nux vomica and may contain as many as 30 seeds. These are about 25 mm long, dark grey in colour and irregularly ovoid in shape. The structure closely resembles that of nux vomica, but the testa, which bears irregularly arranged greyish hairs, is easily rubbed off and is almost entirely absent in the commercial drug. The seeds contain about 2.5–3.0% of total alkaloids, of which about 46–62% is strychnine. They are mainly used for the preparation of strychnine and brucine. The seeds of S. ignatii from Java (S. tieute) contain 1.5% strychnine and no brucine and from Hainen (S. hainanensis) mainly brucine with little strychnine.
In addition to the seeds, other parts of the plants of Strychnos spp. may contain alkaloids including strychnine (B. De Datta and N. G. Bisset, Planta Med., 1990, 56, 133; G. Massiot et al., Phytochemistry, 1992, 31, 2873).
S. potatorum, from India, and S. nux-blanda, from Burma, have been substituted for nux vomica; although they contain no strychnine or brucine, seeds of the former have been reported to contain the alkaloid diaboline and its acetyl derivative, triterpenes and sterols. They are best distinguished by means of the ammonium vanadate reagent. The seeds of S. potatorum are used in India for clearing water, whence the specific name. They will also flocculate heavy metal contaminants in water and are capable of mopping up radioactive isotopes from nuclear waste. The protein responsible for this property has now been isolated (Pharm. J., 1994, 252, 238). The tannins present in the seeds are suggested as the possible active constituents associated with the folklore treatment of chronic diarrhoea (S. Biswas et al., Fitoterapia, 2002, 73, 43).
Gelsemium
Gelsemium consists of the dried rhizomes and roots of the American yellow jasmine, Gelsemium sempervirens (G. nitidum) family Loganiaceae, indigenous to southern USA. It is a climbing plant and produces scented yellow flowers; it should not be confused with the yellow-flowering jasmine (Jasminum nudiflorum, family Oleaceae) cultivated as an ornamental in Europe.
The drug occurs in cylindrical pieces 3–20 cm long and 3–30 mm diameter. The outer cork cells of the rhizome are reddish-brown and the inner ones yellowish. As growth takes place, the outer cork cells crack and the inner cork shows itself as a yellowish-brown reticulation. The roots are somewhat smaller than the rhizome and have a uniform yellowish-brown cork. Gelsemium breaks with an irregular splintery fracture. It has a slightly aromatic odour and a bitter taste. A transverse section of the rhizome shows a thick cork, a cortex containing groups of sclerenchyma, a dense wood, internal as well as external phloem and a small pith. The roots, on the other hand, have no sclerenchyma in the cortex and no pith.
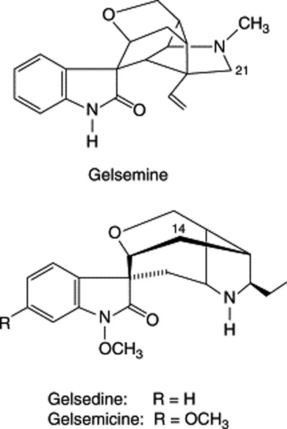
Gelsemium contains extremely toxic alkaloids of unique skeletal type. Gelsemine is the principal alkaloid and is the one most studied although it is not as toxic as gelsemicine. Other oxindole bases characterized are sempervirine, 1-methoxy- and 21-oxo-gelsemine, 14- hydroxygelsemicine, gelsedine and 14-hydroxy-gelsedine. Three new alkaloids of the gelsidine type, together with an iridoid, have been reported by M. Kitajima et al., Chem. Pharm. Bull., 2003, 51, 1211.
In a review (129 refs) H. Takayama and S. Sakai list 45 alkaloids derived from G. elegans, G. sempervirens and G. rankinii; they are classified into five groups according to structure (The Alkaloids, 1997, 49, 1).
Scopoletin is responsible for the blue fluorescence of the broken drug in ultraviolet light. Iridoids and glucoiridoids have been isolated from the aerial parts.
Gelsemium is used (BHP, 1983) in the treatment of trigeminal neuralgia and migraine, but its use requires great care, as dangerous side-effects may develop. It has been studied for its anticancer properties.
G. elegans is used in Oriental folk medicine for much the same purposes as G. nitidum. For information on new and known alkaloids of the leaves and stems of this species see Y.-K. Xu et al., J. Nat. Prod., 2006, 69, 1347.
Rauwolfia (Rauvolfia)
Rauwolfia consists of the dried rhizome and roots of Rauwolfia serpentina (Rauvolfia serpentina), Apocynaceae, a small shrub found in India, Pakistan, Burma, Thailand and Java. The geographical source appears to influence the alkaloidal content, and manufacturers tend to prefer drug obtained from India or Pakistan. Reserpine, the most important constituent, is contained in many other species of Rauwolfia (see ‘African Rauwolfia’ below); it is included in the BP/EP.
History
Although used in India from time immemorial, it was not until 1942 that favourable reports were published of the use of the drug in powdered form. Since then research workers have studied the pharmacognosy, chemistry, pharmacology and clinical uses of many species of Rauwolfia and of the alkaloids obtained from them.
Collection and preparation
The drug is collected mainly from wild plants, but cultivation of the drug will probably increase as wild plants become more scarce; in parts of India collectors are required to leave some root from each plant in the ground for future growth. Nevertheless, and coupled with the low seed viability, the plant is regarded as an endangered species in India. Consequently, the potential for the regeneration of plants from cell cultures and the possible utilization of nodal culture has received some attention (see C. M. Ruyter et al., Planta Med., 1991, 57, 328; N. Sharma and K. P. S. Chandel, Plant Cell Rep., 1992, 11, 200).
As other species of Rauwolfia are found in India, care is needed to identify the correct plant. When first imported, many commercial samples were found to be adulterated; this was due in many cases to lack of knowledge, and substitution of, or adulteration with, other species has become much rarer in recent years. After collection the drug is cut transversely into convenient-sized pieces and dried.
Macroscopical characters
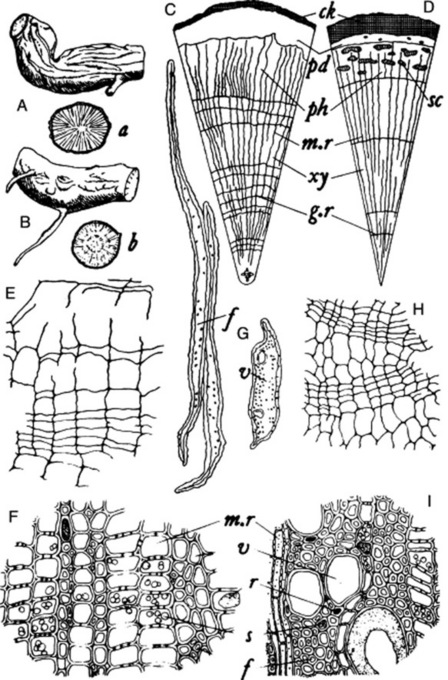
The first detailed description of the drug was made by Wallis and Rohatgi in 1949. It usually occurs in cylindrical or slightly tapering, tortuous pieces about 2–10 cm long and 5–22 mm in diameter (Fig. 26.32A). The roots are rarely branched and rootlets, 0.5–1 mm in diameter, are rare. Pieces of rhizome closely resemble the root but may be identified by a small central pith; they occasionally have attached to them small pieces of aerial stem.
The outer surface is greyish-yellow, light brown or brown with slight wrinkles (young pieces) or longitudinal ridges (older pieces); occasional circular scars of rootlets. In this species the bark exfoliates readily, particularly in the older pieces, and may leave patches of exposed wood. The drug breaks readily with a short fracture. The smoothed transverse surface shows a narrow, yellowish-brown bark and a dense pale yellow wood, which occupies about three-quarters of the diameter. Both bark and wood contain abundant starch. Some commercial samples show mould. The recently dried drug has a slight odour which seems to decrease with age. Taste, bitter.
Microscopical characters
The cork is stratified into about two to eight zones (Fig. 26.32E), which consist of smaller and radially narrower suberized but unlignified cells alternating with larger radially broader cells which are lignified. In many pieces much of the cork is exfoliated, and for section cutting it may be best to select pieces with little exfoliation, separate these from the wood and cut sections of the bark and wood separately. Most of the cells of the secondary cortex are parenchymatous and contain starch; isolated latex cells may occur in this region, particularly in the Dehra Dun variety. The phloem is narrow and consists mainly of parenchyma with scattered sieve tissue. Sclerenchyma is absent (distinction from many other species such as R. tetraphylla (R. canescens), R. micrantha, R. densiflora, R. perakensis and R. vomitoria; see Fig. 26.32 C, D). Most of the parenchymatous cells of the bark contain starch grains, and others prisms or conglomerate crystals of calcium oxalate.
The xylem is entirely lignified and usually shows three to six annual rings. The medullary rays, which are one to five cells wide, contain starch and alternate with the rays of the secondary xylem, which consist of vessels, fibres and xylem parenchyma. Compared with many other species of Rauwolfia, the vessels of R. serpentina are small (up to 57 μm) and are less numerous than in most of the likely adulterants. The starch grains are larger in the wood than in the bark and measure from 5–8 to 12–20 μm.
Constituents
Rauwolfia contains at least 40 alkaloids, which total some 0.7–2.4%. Other substances present include phytosterols, fatty acids, unsaturated alcohols and sugars.
In 1931 Siddiqui and Siddiqui isolated ajmaline (rauwolfine), ajmalinine, ajmalicine, serpentine and serpentinine. The chief therapeutically important alkaloids are reserpine (isolated in 1952; formula Fig. 26.26) and rescinnamine (isolated in 1954). These are esters derived from methyl reserpate and trimethoxybenzoic acid in the case of reserpine and trimethoxycinnamic acid in the case of rescinnamine. New alkaloids continue to be isolated; recently, five anhydronium bases (e.g. 3,4,5,6-tetradehydroyohimbine) for the first time (O. Wachsmuth and R. Matusch, Phytochemistry, 2002, 61, 705) and five new indole alkaloids together with a new iridoid glycoside, 7-epiloganin, a new sucrose derivative and 20 known compounds (A. Itoh et al., J. Nat. Prod., 2005, 68, 848).
Cell and root cultures
R. serpentina cell suspension cultures have proved an important tool in the elucidation of monoterpenoid indole alkaloid biogenesis and in this connection the significance, and isolation, of strictosidine synthase has been considered. By the use of cell cultures Stöckigt in 1988 was able to clarify a 10-step biosynthetic pathway from strictosidine to the typical rauwolfia alkaloid, ajmaline. His group has also shown that the principal alkaloid of cell suspension cultures of R. serpentina is raucaffricine occurring in amounts of up to 1–6 g l−1 in the nutrient medium, representing 2.3% of the dried cells, a value some 67 times higher than found for the roots. Ajmalicine, an antiarrhythmic drug, is also produced to the extent of 0.6% (cell dry wt.) together with over 30 different monoterpenoid alkaloids in trace amounts including five glucoalkaloids. Addition of high levels of ajmaline to the cell culture medium promoted the formation of a new group of alkaloids, the raumaclines, not found in the roots (for further details see S. Endress et al., Phytochemistry, 1993, 32, 725 and references cited therein).
Hairy root cultures of R. serpentina, produced by Agrobacterium rhizogenes transformation, synthesized ajmaline (0.045% dry wt.) and serpentine (0.007% dry wt.) (B. D. Benjamin et al., Phytochemistry, 1994, 35, 381); minor alkaloids have also been recorded (H. Falkenhagen et al., Can. J. Chem., 1993, 71, 2201). R. verticillata hairy roots have been shown to produce reserpine and aimaline. Working on hairy root cultures, Stockigt’s group has reported on the isolation of three new monoterpenoid indole alkaloids of the sarpagine group along with 16 known compounds together with the first natural occurrence (cf. cell cultures above), of the rare raumacline type alkaloids (Y. Sheludko et al., J. Nat. Prod., 2002, 65, 1006; Planta Medica 2002, 68, 435).
Two monoterpenoid indole alkaloids and four β-carbolines have been isolated from cultured hybrid cells of Rauwolfia serpentina and Rhazya stricta, not all of the compounds being found in the parent plants (N. Aimi et al., Chem. Pharm. Bull., 1996, 44, 1637).
Standardization
An assay for total alkaloids is not a true measure of therapeutic activity, since only some of the alkaloids have the desired pharmacological action. The BPC 1988 and USP/NF 1995 determine the reserpine-like alkaloids by utilizing the colour reaction between an acid solution of reserpine (and rescinnamine) and sodium nitrite solution.
Allied drugs
The 110 Rauwolfia species classified by Pichon in 1947 were reduced by Woodson in 1957 to 86. Some of these occur in more than one geographic area but their approximate geographic areas are as follows: Central and South America 34, Africa 20, Far East 24, India and Burma 7, Hawaii, New Guinea and New Caledonia 6. A large number of these species have been examined for reserpine and related alkaloids.
In the identification of the roots of species of Rauwolfia useful characters to be seen in transverse sections are: cork (whether stratified or lignified); cortex and phloem (presence or absence of sclereids or fibres); wood (relative number, distribution and size of vessels). As the species vary from herbs to large trees, the roots vary considerably in size. Some samples of drug contain aerial stems, which usually contain less reserpine than the roots and have unlignified pericyclic fibres.
R. tetraphylla (R. canescens, R. hirsuta) is a species of wide distribution—tropical South America, the Caribbean, India, Australia (Queensland). The root was at one time occasionally substituted for R. serpentina and could be recognized by its non-stratified cork, and sclereid groups in the phloem. It has served as a commercial source of reserpine and the alkaloid deserpidine, possibly particularly important as R. serpentina is now classed as an endangered species. Micropropagation protocols have been described for in vitro mass multiplication of the plant (D. Sarma et al., Planta Medica, 1999, 65, 277).
R. nitida is a West Indian species from the root-bark of which 33 indole alkaloids have been isolated.
Uses
Rauwolfia preparations and reserpine are used in the management of essential hypertension and in certain neuropsychiatric disorders. Ajmaline, which has pharmacological properties similar to those of quinidine, is marketed in Japan for the treatment of cardiac arrhythmias.
An estimated 3500 kg of ajmalicine is isolated annually from either Rauwolfia or Catharanthus spp. by pharmaceutical industries for the treatment of circulatory diseases.
Conflicting reports on the possible involvement of the rauwolfia alkaloids in breast cancer engendered a natural hesitation in their use. A report in the Lancet (1976) suggested that the alkaloids do not initiate the carcinogenic process but that they promote breast cancer from previously initiated cells.
African Rauwolfia (African Rauvolfia)
African rauwolfia consists of the dried roots of Rauwolfia vomitoria Afz. The plant is a bush or tree widely distributed in tropical Africa from the west coast to Mozambique. It is the most important African rauwolfia for the commercial preparation of reserpine.
Collection and preparation
As the tree may attain a height of 10 m, the roots are larger than those of R. serpentina. Before drying they are cut transversely, but are rarely sliced longitudinally. Roots up to 5 cm or more in diameter are sometimes found but the commercial drug usually consists of much smaller pieces. Occasional shipments have been made consisting of the bark only.
Macroscopical characters
The first detailed description of the drug was published by Evans in 1956. It occurs in cylindrical or flattened pieces, usually 0.15–1.5 cm diameter and up to 30 cm long. The roots taper slightly and are occasionally branched. The outer surface is greyish-brown, longitudinally furrowed or rubbed smooth, since the outer cork easily flakes off. Pieces do not break easily, but the fracture is short in the bark and splintery in the wood. The smoothed transverse surface shows a narrow brown bark and a buff or yellowish, finely radiate wood. Odourless; taste, bitter. Pieces of rootstock with attached stem-bases are sometimes found in the drug.
Microscopical characters
The drug is easily distinguished from R. serpentina by the groups of sclereids in the bark arranged in up to five discontinuous bands and by the large vessels of the wood which are up to 180 mu;m in diameter (Fig. 26.32 D and I).
Constituents
African rauwolfia contains reserpine and rescin-namine and alkaloids of the same type such as reserpoxidine and seredine. Many other alkaloids such as ajmaline, alstonine and yohimbine are also present. Court’s group (see Planta Med., 1982, 45, 105) isolated 42 indole alkaloids from the stem-bark and identified 39. The major alkaloids were heteroyohimbines (especially reserpiline) and Na-demethyldihydroindoles. The interrelationship of these alkaloids with those in the root of the plant is discussed in the same paper. New indole alkaloids from R. vomitoria extracts have continued to be reported.
Allied drugs
Other African rauwolfias containing reserpine are R. caffra (R. natalensis), R. mombasiana, R. oreogiton, R. obscura, R. cumminsii, R. volkensii and R. rosea. Court and coworkers have made a systematic study of the microscopy and chemistry of African species (for a report see W. E. Court, Planta Med., 1983, 48, 228). The roots of R. caffra closely resemble those of R. vomitoria but the cork has not the same tendency to flake off. In sections the main difference is that in R. vomitoria there are alternating lignified and unlignified cork cells, while in R. caffra all the cork cells are lignified. R. caffra contains the alkaloid raucaffricine—one of relatively few examples of monoterpenoid indole glucoalkaloids within the group. R. mombasiana differs from R. vomitoria in the structure of the wood of the root; R. rosea, R. volkensii and R. obscura lack sclereid development.
Alstonia barks
Several Alstonia species (Apocynaceae) have been used in the past as antimalarials, and the barks of the Indian Alstonia scholaris and the Australian A. constricta were included in the 1914 edition of the British Pharmacopoeia. At least 11 species are known to contain alkaloids, such as alstonine, alstoniline, cillastonine and echitamine. Interest in them was again awakened by the isolation in 1955 of reserpine in moderate yield from the root-bark of A. constricta; reserpine has since been isolated from A. venenata. For a report on the isolation of a new indole alkaloid and a new glycosidic indole alkaloid from the trunk bark of Indonesian Alstonia scholaris, see A. A. Salim et al., J. Nat. Prod., 2004, 67, 1591. Descriptions of Alstonia barks will be found in the older editions of reference books (e.g. the 22nd edition of the USD, p. 1227).
Yohimbe bark
Yohimbe bark is derived from Pausinystalia yohimbe (Rubiaceae), a tree growing in the Cameroon Republic. It occurs in flat or slightly quilled pieces up to 75 cm long and 2 cm thick. The grey-brown cork has furrows and cracks and patches of lichen. The inner surface is reddish-brown and striated. Taste, bitter. It contains the indole alkaloid yohimbine, which is structurally related to reserpine.
The bark is well-recognized for its aphrodisiac property and yohimbine is effective in the symptomatic treatment of erectile dysfunction, producing fewer side-effects than invasive treatments (M. H. Pittler, Fortschritte der Medizin, 1998, 116, 32).
Aspidosperma barks
The large genus Aspidosperma (Apocynaceae) contains many alkaloid-containing South American trees. The alkaloids are of various indole types formed by a number of different biogenetic pathways. Among the many investigated are yohimbine, which is structurally related to reserpine, and aspidospermine, which has the same general structure as vindoline (see Fig. 26.27). The Aspidosperma barks are therefore potential sources of alkaloids, because the trees are large and the barks would be commercially available cheaply and in almost unlimited quantities.
Catharanthus roseus
For an account of this important plant, see Chapter 27.
Mitragyna leaves
The genus Mitragyna (Rubiaceae) occurs in West and East Africa, India and S.E. Asia. More than 30 different alkaloids have been characterized, and the majority of these are indole or oxindole structures with an open or closed ring E; they exist in various isomeric forms. One alkaloid, mitragynine, isolated from Mitragyna speciosa has analgesic and antitussive properties similar to those of codeine. Shellard and coworkers published extensively on the genus during the 1960s and 1970s (for more recent work on M. speciosa from the same Department see P. J. Houghton et al., Phytochemistry, 1991, 30, 347). The mis-use of mitragyna as an hallucinogen is considered in Chapter 39.
Uncaria species
In addition to Uncaria gambir, the source of catechu (q.v.), the genus is notable for its alkaloids, which resemble those of Mitragyna. Uncaria hooks, the dried climbing hooks and stems of U. sinensis, have sedative and antispasmodic properties. They are used in Chinese medicine for the relief of headaches and dizziness caused by hypertension and for the treatment of convulsions in children. The drug contains indole alkaloids, e.g. rhyncophylline and indole alkaloid glycosides which exhibit a long-lasting hypotensive effect (K. Endo et al., Planta Med., 1983, 49, 188; S. Kawazoe et al., ibid., 1991, 57, 47).
Uncaria rhynchophylla, a species also used in Chinese medicine, is reported to contain various alkaloids including rhynchophylline, corynoxeine, corynantheine and among others, hirsutine which exhibit antihypertensive, neuroprotective and vasodilator effects; (+)-catechin and (−)-epicatechin have been isolated for the first time from this species (W.-C. Hou et al., J. Ethnopharmacology 2005, 100, 216). Other research suggests this species to be an effective anxiolytic agent acting via the serotonergic nervous system (K. W. Jung et al., J. Ethnopharmacology, 2006, 108, 193).
U. tomentosa, one of two species found in S. America, features in the traditional medicine of Peru. It produces similar hooks to U. sinensis being known locally by the Spanish as ‘una degato’ (tomcat’s claw).
In a number of reports on this species, M. Kitajima et al., have recorded a new glucoindole akaloid, 3,4-dehydro-5-carboxystrictosodine, various triterpenes including nor-triterpene glycosides and cincholic acid glycosides (see Chem. Pharm. Bull., 2004, 52, 1258 and references cited therein). The plant has a potential immunostimulant action and has been examined for its pharmacological and toxicological properties (K. Keplinger et al., J. Ethnopharmacology, 1999, 64, 23; I. Lemaire et al., J. Ethnopharmacology, 64, 109). It is being used traditionally to treat a large number of conditions, including cancer (R. Pilarski et al., J. Ethnopharmacology, 2006, 104, 18; L. De Martino et al., J. Ethnopharmacology, 2006, 107, 91; G. Gonçalves et al., Phytochemistry, 2005, 66, 89).