The Child with Genitourinary Dysfunction
On completion of this chapter the reader will be able to:
 Describe the various factors that contribute to urinary tract infections in infants and children.
Describe the various factors that contribute to urinary tract infections in infants and children.
 Discuss the preoperative preparation of the child and parents when the child has a structural defect of the genitourinary tract.
Discuss the preoperative preparation of the child and parents when the child has a structural defect of the genitourinary tract.
 Demonstrate an understanding of the causes and mechanisms of edema formation in nephrotic syndrome.
Demonstrate an understanding of the causes and mechanisms of edema formation in nephrotic syndrome.
 Outline a nursing care plan for a child with nephrotic syndrome.
Outline a nursing care plan for a child with nephrotic syndrome.
 Compare the child with minimal-change nephrotic syndrome and the child with acute glomerulonephritis in terms of clinical manifestations and nursing care.
Compare the child with minimal-change nephrotic syndrome and the child with acute glomerulonephritis in terms of clinical manifestations and nursing care.
 Contrast the causes, complications, and management of acute and chronic renal failure.
Contrast the causes, complications, and management of acute and chronic renal failure.
GENITOURINARY DYSFUNCTION
Assessment of kidney and urinary tract integrity and diagnosis of renal or urinary tract disease are based on several evaluative tools. Physical examination, history taking, and observation of symptoms are the initial procedures. In suspected urinary tract diseases or disorders, further assessment by laboratory, radiologic, and other evaluative methods is carried out.
Clinical Manifestations
As in most disorders of childhood, the incidence and type of kidney or urinary tract dysfunction change with the age and maturation of the child. In addition, the presenting complaints and the significance of these complaints vary with maturation. For example, a complaint of enuresis has greater significance at age 8 years than at age 4. In the newborn, urinary tract disorders are associated with a number of obvious malformations of other body systems, including the curious and unexplained but frequent association between malformed or low-set ears and urinary tract anomalies.
Many of the clinical manifestations of renal disease are common to a variety of childhood disorders, but their presence is an indication to obtain further information from the child’s history, family history, and laboratory studies as part of a complete physical examination. Suspected renal disease can be further evaluated by means of radiographic studies and renal biopsy (Table 27-1).
Laboratory Tests
Both urine and blood studies contribute vital information for detection of renal problems. The single most important test is probably routine urinalysis. Specific urine and blood tests provide additional information. Because nurses are usually the persons who collect the specimens for examination and who often perform many of the screening tests, they should be familiar with the test, its function, and factors that can alter or distort the results of the test. The major urine and blood tests are outlined in Tables 27-2 and 27-3.
Nursing Care Management
Nursing responsibilities in assessment of genitourinary disorders or diseases begin with observation of the child for any manifestations that might indicate dysfunction. Many conditions have specific characteristics that distinguish them from other disorders. These are discussed as appropriate throughout the chapter.
The nurse is generally the one who is responsible for preparing infants, children, and parents for tests and for collection of urine and (sometimes) blood specimens for observation and laboratory analysis (see Preparation for Diagnostic and Therapeutic Procedures, and Collection of Specimens, Chapter 22). An important nursing responsibility is to maintain careful intake and output measurements and blood pressure on most children with genitourinary dysfunction and those who might be at risk for developing renal complications (e.g., children in shock, postoperative patients). For example, any significant degree of renal disease can diminish the glomerular filtration rate, a measure of the amount of plasma from which a given substance is totally cleared in 1 minute. A number of substances can be used, but the most useful clinical estimation of glomerular filtration is the clearance of creatinine, an end product of protein metabolism in muscle and a substance that is freely filtered by the glomerulus and secreted by renal tubular cells. The nurse’s responsibility in this test is collection of urine, usually a 12- or 24-hour specimen.
GENITOURINARY TRACT DISORDERS AND DEFECTS
Infection of the genitourinary tract is one of the most common conditions of childhood. Up to 10% of children will have a febrile urinary tract infection (UTI) during the first 2 years of life (Kanellopoulos, Salakos, Spiliopoulou, and others, 2006). Among febrile males, circumcision status is important in determining risk for UTI. Uncircumcised male infants younger than 3 months of age had the highest prevalence of UTI (20.1%) of any group, male or female (Shaikh, Morone, Bost, and others, 2008). Circumcision status should be assessed in male infants with unexplained fever. UTI may involve the urethra and bladder (lower urinary tract) or the ureters, renal pelvis, calyces, and renal parenchyma (upper urinary tract). Because it is often impossible to localize the infection, the broad designation UTI is applied to the presence of significant numbers of microorganisms anywhere within the urinary tract, except the distal third of the urethra, which is usually colonized with bacteria.
Classification
Infection of the urinary tract may be present with or without clinical symptoms. As a result, the site of infection is often difficult to pinpoint with any degree of accuracy. Various terms used to describe urinary tract disorders include:
Bacteriuria—Presence of bacteria in the urine
Asymptomatic bacteriuria—Significant bacteriuria (usually defined as >100,000 colony-forming units [CFUs]) with no evidence of clinical infection
Symptomatic bacteriuria—Bacteriuria accompanied by physical signs of UTI (dysuria, suprapubic discomfort, hematuria, fever)
Recurrent UTI—Repeated episode of bacteriuria or symptomatic UTI
Persistent UTI—Persistence of bacteriuria despite antibiotic treatment
Febrile UTI—Bacteriuria accompanied by fever and other physical signs of UTI; presence of a fever typically implies a pyelonephritis
Cystitis—Inflammation of the bladder
Urethritis—Inflammation of the urethra
Pyelonephritis—Inflammation of the upper urinary tract and kidneys
Urosepsis—Febrile UTI coexisting with systemic signs of bacterial illness; blood culture reveals presence of urinary pathogen
Etiology
A variety of organisms can be responsible for UTI. Escherichia coli (80% of cases) and other gram-negative enteric organisms are most frequently implicated; these organisms are usually found in the anal and perineal region. Other organisms associated with UTI include Proteus, Pseudomonas, Klebsiella, Staphylococcus aureus, Haemophilus, and coagulase-negative Staphylococcus organisms. Several factors contribute to the development of UTI in childhood.
Anatomic and Physical Factors.: The structure of the lower urinary tract is believed to account for the increased incidence of bacteriuria in females (Rosenthal, 2004). The short urethra, which measures about 2 cm (0.75 inch) in young girls and 4 cm (1.6 inches) in mature women, provides a ready pathway for invasion of organisms. In addition, the closure of the urethra at the end of micturition may return contaminated bacteria to the bladder. The longer male urethra (as long as 20 cm [8 inches] in an adult) and the antibacterial properties of prostatic secretions inhibit the entry and growth of pathogens.
The single most important host factor influencing the occurrence of UTI is urinary stasis. Ordinarily, urine is sterile, but at 37° C (98.6° F) it provides an excellent culture medium. Under normal conditions the act of completely and repeatedly emptying the bladder flushes away any organisms before they have an opportunity to multiply and invade surrounding tissue. However, urine that remains in the bladder allows bacteria from the urethra to rapidly become established in the rich medium. Incomplete bladder emptying (stasis) may result from reflux (see Vesicoureteral Reflux, p. 955), anatomic abnormalities (especially those involving the ureters), dysfunction of the voiding mechanism, or extrinsic ureteral or bladder compression that may be caused by constipation. The key to preventing UTI is to maintain adequate blood supply to the bladder wall by avoidance of overdistention and high bladder pressure.
Altered Urine and Bladder Chemistry.: Several mechanical and chemical characteristics of the urine and bladder mucosa help maintain urinary sterility. An increased fluid intake promotes flushing of the normal bladder and lowers the concentration of organisms in the infected bladder. Diuresis also seems to enhance the antibacterial properties of the renal medulla.
Most pathogens favor an alkaline medium. Normally, urine is slightly acidic with a median pH of 6.0. A urine pH of about 5 hampers but does not eliminate bacterial multiplication. Much has been reported about the use of cranberry products to increase urine acidity in an effort to prevent UTI. Studies done in adults offer limited evidence for the value of cranberry products in promoting urinary tract health (Jepson, Mihaljevic, and Craig, 2004; Bailey, Dalton, Daugherty, and others, 2007). Further research that controls for type of cranberry product used, dosing regimens, and patient selection based on age and underlying medical condition is required to clarify unanswered questions before recommendations can be made regarding the use of this supplement, especially in the pediatric population.
Diagnostic Evaluation
The clinical manifestations of UTI depend on the child’s age (Box 27-1). Diagnosis of UTI is confirmed by detection of bacteriuria in urine culture, but urine collection is often difficult, especially in infants and very small children. Several factors may alter a urine specimen, and contamination of a specimen by organisms from sources other than the urine, such as perineal and perianal flora in bag specimens, is the most frequent cause of false-positive results. Unless the specimen is a first morning sample, a recent high fluid intake may indicate a falsely low organism count. Therefore children should not be encouraged to drink large volumes of water in an attempt to obtain a specimen quickly.
More accurate estimates of bacterial content are obtained from suprapubic aspiration (in children younger than 2 years of age) and properly performed bladder catheterization (as long as the first few milliliters are excluded from collection). The specimen should be taken directly to the laboratory for immediate culture.
Tests to detect bacteriuria are being used with increased frequency in screening for UTI. The dipstick tests for leukocyte esterase or nitrite are quick and inexpensive methods for detecting infection before obtaining final culture results.
Localization of the infection site may involve more specific tests, including percutaneous kidney taps and bladder washout procedures. Other tests such as ultrasonography, voiding cystourethrogram (VCUG), intravenous pyelogram (IVP), and DMSA (dimercaptosuccinic acid) scan may be performed after the infection subsides to identify anatomic abnormalities contributing to the development of infection and existing kidney changes from recurrent infection.
Therapeutic Management
The objectives of treatment of children with UTI are (1) to eliminate current infection, (2) to identify contributing factors to reduce the risk of recurrence, (3) to prevent systemic spread of the infection, and (4) to preserve renal function. Antibiotic therapy should be initiated on the basis of identification of the pathogen, the child’s history of antibiotic use, and the location of the infection. Several antimicrobial drugs are available for treating UTI, but all of them can occasionally be ineffective because of resistance of organisms. Common antiinfective agents used for UTI include the penicillins, sulfonamide (including trimethoprim and sulfisoxazole in combination), the cephalosporins, and nitrofurantoin.
If anatomic defects such as primary reflux or bladder neck obstruction are present, surgical correction of these abnormalities may be necessary to prevent recurrent infection. Follow-up study is an important component of medical management, since the relapse rate is high and infection tends to recur 1 to 2 months after termination of treatment. The aim of therapy and careful follow-up is to reduce the chance of renal scarring. However, recurrent infection of the urinary bladder predisposes the individual to transient episodes of vesicoureteral reflux (VUR).
Vesicoureteral Reflux.: VUR refers to the abnormal retrograde flow of bladder urine into the ureters. During voiding, urine is swept up the ureters and then flows back into the empty bladder, where it acts as a reservoir for bacterial growth until the next void. Primary reflux results from congenitally abnormal insertion of ureters into the bladder; secondary reflux occurs as a result of an acquired condition.
It is not clear that reflux necessarily causes infections. What is clear is that reflux is more likely to be associated with recurring kidney infections rather than simple bladder infections (cystitis). In the presence of reflux, infected urine (bacteria) from the bladder has access to the kidney, resulting in kidney infections (pyelonephritis). These children are usually very symptomatic with high fevers, vomiting, and chills. Reflux, when associated with UTI, is the most common cause of renal scarring in children. Renal scarring may occur with the first episode of febrile UTI. Reflux in the presence of sterile urine does not cause renal damage. Therefore the most important concept in managing VUR is preventing bacteria from reaching the kidneys. VUR is managed conservatively with daily low-dose antibiotic therapy. A urine culture should be done every 2 to 3 months and any time the child has a fever. This method of management requires a motivated, reliable, and cooperative family. Many children will outgrow the reflux over a period of years. An annual VCUG is done to assess the status of the reflux.
For children with mild to moderate reflux, a minimally invasive endoscopic option (subtrigonal injection, or STING) is an alternative to daily antibiotics or open surgical intervention. A bulking agent—dextranomer-hyaluronic acid polymer (Deflux)–is injected into the mucous membrane of the ureter, making retrograde flow of urine more difficult. Overall cure rates relate to degree of reflux and range from 72% to 84% (Kirsch, Perez-Brayfield, and Scherz, 2003; Lavelle, Conlin, and Skoog, 2005).
Indications for open surgical intervention include significant anatomic abnormality at the ureterovesical junction, recurrent UTIs, severe forms of VUR, noncompliance with medical therapy, intolerance to antibiotics, and VUR after puberty in females.
Prognosis.: With prompt and adequate treatment at the time of diagnosis, the long-term prognosis for UTI is usually excellent. However, the hazard of progressive renal injury is greatest when infection occurs in young children (especially those younger than 2 years of age) and is associated with congenital renal malformations and reflux. Therefore early diagnosis of children at risk is particularly important.
Nursing Care Management
Nurses should instruct parents to observe regularly for clues suggesting UTI. Unfortunately, the signs of UTI are not as evident as those of upper respiratory tract infection. Therefore many cases go undetected because no one thought to investigate this very common problem.
Because infants and young children often are unable to express their feelings and sensations verbally, it is difficult to detect discomfort they may be experiencing from dysuria. A careful history regarding voiding habits, stooling pattern, and episodes of unexplained irritability may assist in detecting less obvious cases of UTI. Consequently, parents should be cautioned to observe for specific clues of UTI in suspected cases.
When infection is suspected, collecting an appropriate specimen is essential. It is the nurse’s responsibility to take every precaution to obtain acceptable clean-voided specimens to avoid the use of other, more invasive collecting procedures except when absolutely indicated. Because of the unreliability of a specimen obtained via a urine collection bag, suprapubic aspiration of urine or sterile catheterization should be done in the infant or young child who is seen with fever.
Frequently, additional tests are performed to detect anatomic defects. Children are prepared for these tests as appropriate for their age. This includes an explanation of the procedure, its purpose, and what the children will experience (see Preparation for Diagnostic and Therapeutic Procedures, Chapter 22). Sometimes a simple description of the urinary system is helpful. Especially for preschool children, the nurse must clarify that the urinary tract is separate from any sexual function and that the test is for a problem that they did not cause. Children may associate blame for perceived wrongdoing (e.g., masturbation) or unacceptable thoughts with the reason for the illness or the tests. For children younger than 3 to 4 years of age, the procedure can be explained on a doll. For those who are older, a simple drawing of the bladder, urethra, ureters, and kidneys makes the procedure more understandable.
Handling actual equipment when feasible can be helpful in allaying anxiety in children of all ages. Anticipatory instruction on distraction techniques such as deep breathing, storytelling, and imagery may help the child relax and be more cooperative during the actual procedures. If surgery is indicated, facts and understanding of the procedure will help decrease the child’s fear and anxiety concerning more extensive medical-surgical intervention.
Because antibacterial drugs are indicated in UTI, the nurse advises parents of proper dosage and administration. When antiseptics such as nitrofurantoin are used for prolonged therapy to maintain urine sterility, parents need an explanation of the drug’s continued necessity when no signs of infection are present. For all children an adequate or increased fluid intake is encouraged.
Prevention.: Prevention is the most important goal in both primary and recurrent infection, and most preventive measures are simple hygienic habits that should be a routine part of daily care (see Nursing Care Guidelines box). For example, parents are taught to cleanse their infant’s genital areas from front to back to avoid contaminating the urethral area with fecal organisms. Girls are taught to wipe from front to back after voiding or defecating. Children should void as soon as they feel the urge (see Critical Thinking Exercise).
Sexually active adolescent females are advised to urinate as soon as possible after they have intercourse to flush out bacteria introduced during the activity. Children who have recurrent UTIs or neurogenic bladder are frequently maintained on daily low-dose antibiotics. Giving the dose at bedtime allows the drug to remain in the bladder overnight. The nurse should reinforce the importance of compliance to parents and older children.
OBSTRUCTIVE UROPATHY
Structural or functional abnormalities of the urinary system that obstruct the normal flow of urine can produce renal disorders. When there is interference with urine flow, the backup of urine above the obstruction causes hydronephrosis (dilation of the renal pelvis from distention) with eventual pressure destruction of renal parenchyma, although the dilating ureters form a reservoir that reduces the effect on the kidneys for a long time.
Obstruction may be congenital or acquired, unilateral or bilateral, complete or incomplete, with acute or chronic manifestations. The obstruction can occur at any level of the upper or lower urinary tract (Fig. 27-1). Partial obstruction may not be symptomatic unless there is a water or solute diuresis. Boys are affected more frequently than girls, and malformations should be suspected when patients have some other congenital defects (e.g., prune belly syndrome, chromosomal anomalies, anorectal malformations, defects of the pinna of the ear).
Damage to distal nephrons in chronic uropathy alters the ability to concentrate urine, contributing to increased urine flow and metabolic acidosis occurring from decreased excretion of acid secondary to impaired ability of the distal nephron to secrete hydrogen ions. Partial obstruction results in progressive loss of renal function as a result of irreversible damage to the nephrons. Pooled urine serves as a medium for bacterial growth; therefore UTIs further increase the extent of renal damage.
Early diagnosis and surgical correction or procedures that divert the flow of urine to bypass the obstruction, such as placement of a temporary percutaneous nephrostomy tube or cutaneous ureterostomy, are essential to prevent progressive renal damage. Medical complications of acute or chronic renal failure or infection are managed as described for those disorders.
Nursing Care Management
Nursing goals in urinary tract obstruction include helping to identify cases, assisting with diagnostic procedures, and caring for children with complications (described elsewhere). Preparing parents and children for procedures is a major nursing responsibility. Preparation for urinary diversion procedures is of special importance (see Preparation for Diagnostic and Therapeutic Procedures, Chapter 22).
Parents and children need emotional support and counseling during the lengthy management of these disorders. Many children are discharged with ureteral drainage systems in place that must be protected from damage, and the danger of infection is a constant concern. Parents are taught to care for the equipment and recognize the signs of possible obstruction or infection within the system. Maintaining adequate urine flow is imperative. Fluids should be encouraged. The tube should be observed frequently for indications of obstruction resulting from sediment, small blood clots, or kinking. The physician should inspect any drainage from around the tube.
Children with external diversional systems need psychologic support and guidance, especially as they reach adolescence and body image concerns assume more prominence. Those with progressive renal deterioration may face the prospect of dialysis or transplantation and the emotions that accompany these procedures.
EXTERNAL DEFECTS
Defects of the external genitourinary tract are serious conditions primarily because of the psychologic impact on the child. Satisfactory surgical repair is successful for the more common disorders and is carried out or initiated as early as possible. The major anomalies of the lower genitourinary tract, their description, and their management are outlined in Table 27-4.
Psychologic Problems Related to Genital Surgery
Surgery involving sexual organs can be particularly disruptive to children, especially preschoolers fearing punishment, retaliation, body mutilation, or castration. Some of the problems of hospitalization, separation, and anxiety can be eased by hospital practices that are sensitive to the child’s needs (see Chapter 21).
A child’s body image is largely derived as a result of feedback from the primary caregivers, and parental anxiety regarding an acceptable physical appearance and adequate future sexual competency is readily communicated to an affected child. Therefore children with birth defects are at risk for developing a distorted body image that reflects the caregiver’s subtly communicated evaluation of their bodies. The trend toward repair of visible genital defects is based in large part on these psychologic variables. The earlier a repair can be achieved, the more likely it is that the child will develop a normal body image.
During the years from 3 to 6, the phallic-oedipal period, children show a strong interest and concern about the genital area, sex differences, and genital normality or its lack. It is also a time when children are frightened of what they perceive to be threats to their body and bodily function. They also view any untoward happening as a punishment for real or imagined wrongdoing or unacceptable sexual feelings, such as masturbation, sex play, or erotic feelings. Surgical repair is recommended before these fears and anxieties develop.
After extensive review of the emotional, cognitive, and body-image problems that may occur in children undergoing surgical reconstruction of a genital deformity, Kass (1996) recommended that surgery be accomplished between the ages of 6 and 15 months to minimize the psychologic effects of surgery and anesthesia.
Nursing Care Management
Preparing children and their families for diagnostic and surgical procedures (see Preparation for Diagnostic and Therapeutic Procedures, Chapter 22) and for home care is a major nursing function. Most postoperative care involves care of the surgical site. Tub baths are discouraged for 1 week after simple surgeries. The surgical site is kept clean and otherwise protected from infection and is inspected for signs of infection. Dressings, if any, are inspected regularly. More complex surgeries require additional care and observation (e.g., catheter care for urethral reconstruction and care of urinary diversion stomas and collection devices).
Some older children’s activities, such as pushing, lifting, playing with straddle toys or in sandboxes, swimming, and rough activities, may be restricted after some types of surgical repairs. Precise restrictions depend on the specific type of surgery. Activities of infants and toddlers are not limited.
In most cases the results of surgery are satisfactory. However, in some of the more severe defects, such as exstrophy and those that require stomas, additional emotional interventions may be needed. A major concern of parents and children is related to surgery affecting the genitalia directly. Concerns about penis size, appearance of the genitalia, potential ability to procreate, and rejection by peers (especially the opposite sex) are potential fears that require psychologic adjustment, particularly during adolescence.
GLOMERULAR DISEASE
Nephrotic syndrome is a clinical state that includes massive proteinuria, hypoalbuminemia, hyperlipidemia, and edema. The disorder can occur as (1) a primary disease known as idiopathic nephrosis, childhood nephrosis, or minimal-change nephrotic syndrome (MCNS); (2) a secondary disorder that occurs as a clinical manifestation after or in association with glomerular damage that has a known or presumed cause; or (3) a congenital form inherited as an autosomal recessive disorder. The disorder is characterized by increased glomerular permeability to plasma protein, which results in massive urinary protein loss. The glomerulus is responsible for the initial step in the formation of urine, and the filtration rate depends on an intact glomerular membrane. This discussion is devoted to MCNS because it constitutes 80% of nephrotic syndrome cases.
Pathophysiology
The onset of MCNS can occur at any age but predominantly occurs in children between 2 and 7 years of age. It is rare in children younger than 6 months of age, uncommon in infants younger than 1 year of age, and unusual after the age of 8. Patients with MCNS are twice as likely to be male.
The pathogenesis of MCNS is not understood. There may be a metabolic, biochemical, physiochemical, or immune-mediated disturbance that causes the basement membrane of the glomeruli to become increasingly permeable to protein, but the cause and mechanisms are only speculative.
The glomerular membrane, normally impermeable to albumin and other proteins, becomes permeable to proteins, especially albumin, which leak through the membrane and are lost in urine (hyperalbuminuria). This reduces the serum albumin level (hypoalbuminemia), decreasing the colloidal osmotic pressure in the capillaries. As a result, the vascular hydrostatic pressure exceeds the pull of the colloidal osmotic pressure, causing fluid to accumulate in the interstitial spaces (edema) and body cavities, particularly in the abdominal cavity (ascites). The shift of fluid from the plasma to the interstitial spaces reduces the vascular fluid volume (hypovolemia), which in turn stimulates the renin-angiotensin system and the secretion of antidiuretic hormone and aldosterone. Tubular reabsorption of sodium and water is increased in an attempt to increase intravascular volume. The elevation of serum lipids is not fully understood. The sequence of events in nephrotic syndrome is diagrammed in Fig. 27-2.
Diagnostic Evaluation
The disease is suspected on the basis of clinical manifestations (Box 27-2), especially when weight gain in a previously well child increases slowly over days or weeks. The generalized edema may develop rapidly or gradually but eventually prompts the family to seek medical attention. Parents usually give a history of the child being well but steadily gaining weight, appearing edematous, and then becoming anorexic, irritable, and less active.
The diagnosis of MCNS is suspected on the basis of the history and clinical manifestations (edema, proteinuria, hypoalbuminemia, and hypercholesterolemia in the absence of hematuria and hypertension) in children between the ages of 2 and 8 years. The hallmark of MCNS is massive proteinuria (higher than 3+ on urine dipstick). Hyaline casts, oval fat bodies, and a few red blood cells can be found in the urine of some affected children, although there is seldom gross hematuria. The glomerular filtration rate is usually normal or high.
Total serum protein concentration is low, with the serum albumin significantly reduced and plasma lipids elevated. Hemoglobin and hematocrit are usually normal or elevated as a result of hemoconcentration. The platelet count may be elevated. Serum sodium concentration may be low. If the patient does not respond to a 4- to 8-week course of steroids, a renal biopsy may be needed to distinguish between other types of nephrotic syndrome. The biopsy results of children with MCNS are remarkable for effacement of the foot processes of the epithelial cells lining the basement membrane, but otherwise the kidney tissue is normal.
Therapeutic Management
Objectives of therapeutic management include (1) reducing excretion of urinary protein, (2) reducing fluid retention in the tissues, (3) preventing infection, and (4) minimizing complications related to therapies. Dietary restrictions include a low-salt diet and, in more severe cases, fluid restriction. If complications of edema develop, diuretic therapy may be initiated to provide temporary relief from edema. Sometimes infusions of 25% albumin are used. Acute infections are treated with appropriate antibiotics.
Corticosteroids are the first line of therapy for MCNS. The starting dosage for prednisone is usually 2 mg/kg body weight/day, in one or more divided doses. In most children this response occurs within 7 to 21 days. The medication is then tapered over a period of several months and eventually stopped if the child remains asymptomatic. About two thirds of children with MCNS have a relapse, heralded first by increased urine protein. Relapses can be diagnosed early if parents are taught routine home monitoring of urine protein by dipstick. Relapses are treated with a repeated course of high-dose steroid therapy. Side effects of the steroids include weight gain, rounding of the face, behavior changes, and increased appetite. Long-term therapy may result in hirsutism, growth retardation, cataracts, hypertension, gastrointestinal bleeding, bone demineralization, infection, and hyperglycemia. Children who do not respond to steroid therapy, those who have frequent relapses, and those in whom the side effects threaten their growth and general health may be considered for a course of therapy using other immunosuppressant medications (cyclophosphamide, chlorambucil, or cyclosporine).
MCNS episodes, both the first episode and relapse, often happen in conjunction with a viral or bacterial infection. Relapses can also be triggered by allergies and immunizations. Relapses in children with MCNS may continue over many years.
Complications of nephrotic syndrome include infection, circulatory insufficiency secondary to hypovolemia, and thromboembolism. Infections that may be seen in children with nephrotic syndrome include peritonitis, cellulitis, and pneumonia and require prompt recognition and vigorous treatment with appropriate antibiotic therapy.
Prognosis.: The prognosis for ultimate recovery in most cases is good. It is a self-limiting disease, and in children who respond to steroid therapy the tendency to relapse decreases with time. With early detection and prompt implementation of therapy to eradicate proteinuria, progressive basement membrane damage is minimized, so that when the tendency to relapse is past, renal function is usually normal or near normal. It is estimated that approximately 80% of affected children have this favorable prognosis.
Nursing Care Management
Continuous monitoring of fluid retention or excretion is an important nursing function. Strict intake and output records are essential but may be difficult to obtain from very young children. Application of collection bags is irritating to edematous skin that is readily subject to breakdown. Applying diapers or weighing wet pads may be necessary.
Other methods of monitoring progress include urine examination for albumin, daily weight, and measurement of abdominal girth. Assessment of edema (e.g., increased or decreased swelling around the eyes and dependent areas), the degree of pitting, and the color and texture of skin are part of nursing care. Vital signs are monitored to detect any early signs of complications such as shock or an infective process.
Infection is a constant source of danger to edematous children and those receiving corticosteroid therapy. These children are particularly vulnerable to upper respiratory tract infection; therefore they must be kept warm and dry, active, and protected from contact with infected individuals (e.g., roommates, visitors, and personnel). Vital signs are monitored to detect any early signs of an infective process.
Loss of appetite accompanying active nephrosis creates a perplexing problem for nurses. During this time the combined efforts of nurse, dietitian, parents, and child are needed to formulate a nutritionally adequate and attractive diet. Salt is usually restricted (but not eliminated) during the edema phase and while the child is on steroid therapy. Fluid restriction (if prescribed) is limited to short-term use during massive edema. Every effort should be made to serve attractive meals with preferred foods and a minimum of fuss, but it usually requires considerable ingenuity to entice the child to eat (see Feeding the Sick Child, Chapter 22).
Children usually adjust activities according to their tolerance level. However, they may require guidance in selecting play activities. Suitable recreational and diversional activities are an important part of their care. Irritability and mood swings that accompany steroid therapy are not unusual in these children and may create an additional challenge for the nurse and family.
Family Support and Home Care.: Continuous support of the child and family is one of the major nursing considerations. Many children are treated at home during relapses. Parents are taught to detect signs of relapse and to call for changes in treatment at the earliest indications. Unless the edema and proteinuria are severe or the parents, for some reason, are unable to care for the ill child, home care is preferred. Parents are instructed in testing urine for albumin, administering medications, and providing general care. Parents are also instructed regarding avoiding contact with infected playmates, but the child should attend school.
The prolonged course of the relapsing form of nephrotic syndrome is taxing to both the child and the family. The up-and-down course of remissions and exacerbations with periodic disruption of family life by hospitalization places a severe strain on the child and the family, both psychologically and financially. Reassurance regarding this characteristic of the course of the disease, with emphasis on the importance of long-term care, needs to be provided to parents and children to gain their cooperation. A satisfactory response is more likely when relapses are detected and therapy is instituted early, and remissions are prolonged when instructions are carried out faithfully. Continuous support of the child and family is one of the major nursing considerations (see Chapter 18).
ACUTE GLOMERULONEPHRITIS
Acute glomerulonephritis (AGN) may be a primary event or a manifestation of a systemic disorder that can range from minimal to severe. Common features include oliguria, edema, hypertension and circulatory congestion, hematuria, and proteinuria. Most cases are postinfectious and have been associated with pneumococcal, streptococcal, and viral infections. Acute poststreptococcal glomerulonephritis (APSGN) is the most common of the postinfectious renal diseases in childhood and the one for which a cause can be established in the majority of cases. APSGN can occur at any age but affects primarily early school-age children, with a peak age of onset of 6 to 7 years. It is uncommon in children younger than 2 years of age, and males outnumber females 2:1.
Etiology
APSGN is an immune-complex disease that occurs after an antecedent streptococcal infection with certain strains of the group A β-hemolytic streptococcus. Most streptococcal infections do not cause APSGN. A latent period of 10 to 21 days occurs between the streptococcal infection and the onset of clinical manifestations. Disease secondary to streptococcal pharyngitis is more common in the winter or spring, but when APSGN is associated with pyoderma (principally impetigo), it may be more prevalent in later summer or early fall, especially in warmer climates. Second episodes of AGN are rare.
Pathophysiology
The pathophysiology of APSGN is still uncertain. Immune complexes are deposited in the glomerular basement membrane. The glomeruli become edematous and infiltrated with polymorphonuclear leukocytes, which occlude the capillary lumen. The resulting decrease in plasma filtration results in an excessive accumulation of water and retention of sodium that expands plasma and interstitial fluid volumes, leading to circulatory congestion and edema. The cause of the hypertension associated with AGN cannot be completely explained by fluid retention. Excess renin may also be produced.
Diagnostic Evaluation
Typically, affected children are in good health until they experience the streptococcal infection. In some instances they have a history of only a mild cold or no previous infection at all. The onset of nephritis appears after an average latency period of about 10 days (Box 27-3). Because the child appears to be well during the latency period, parents do not recognize the association. The edema is relatively moderate and may not be appreciated by someone unfamiliar with the child’s normal appearance.
Urinalysis during the acute phase characteristically shows hematuria and proteinuria. Proteinuria generally parallels the hematuria and may be 3+ or 4+ in the presence of gross hematuria. Gross discoloration of the urine reflects red blood cell and hemoglobin content. Microscopic examination of the sediment shows many red blood cells, leukocytes, epithelial cells, and granular and red blood cell casts. Bacteria are not seen.
Azotemia that results from impaired glomerular filtration is reflected in elevated blood urea nitrogen (BUN) and creatinine levels in at least 50% of cases. Occasionally proteinuria is excessive and the patient may have nephrotic syndrome (i.e., hypoproteinemia and hyperlipidemia).
Cultures of the pharynx are rarely positive for streptococci, since the renal disease occurs weeks after the infection.
Some serologic tests are necessary to make the diagnosis of AGN. Circulating serum antibodies to streptococci indicate the presence of a previous infection. The antistreptolysin O (ASO) titer is the most familiar and readily available test for streptococcal infection. Other antibodies that may aid in diagnosis are elevated antihyaluronidase (AHase), antideoxyribonuclease B (ADNase-B), and streptozyme.
All patients with APSGN have reduced serum complement (C3) activity in the early stages of the disease. Rising C3 levels are used as a guide to indicate improvement of the disease and should be normal in almost all patients 8 weeks after the disease onset.
Studies that may be useful include chest x-ray examination, which generally shows cardiac enlargement, pulmonary congestion, or pleural effusion during the edematous phase of acute disease. Renal biopsy for diagnostic purposes is seldom required but may be useful in the diagnosis of atypical cases.
Therapeutic Management
Management consists of general supportive measures and early recognition and treatment of complications. Children who have normal blood pressure and a satisfactory urinary output can generally be treated at home. Those with substantial edema, hypertension, gross hematuria, or significant oliguria should be hospitalized because of the unpredictability of complications.
Dietary restrictions depend on the stage and severity of the disease, especially the extent of edema. Moderate sodium restriction and even fluid restriction may be instituted for children with hypertension and edema. Foods with substantial amounts of potassium are generally restricted during the period of oliguria.
Regular measurement of vital signs, body weight, and intake and output is essential to monitor the progress of the disease and to detect complications that may appear at any time during the course of the disease. A record of daily weight is the most useful means for assessing fluid balance. Rarely, children with AGN will develop acute renal failure (ARF) with oliguria that significantly alters the fluid and electrolyte balance (resulting in hyperkalemia, acidosis, hypocalcemia, and/or hyperphosphatemia). These children require careful management. Peritoneal dialysis or hemodialysis is seldom needed.
Acute hypertension must be anticipated and identified early. Blood pressure measurements are taken every 4 to 6 hours. A variety of antihypertensive medications and diuretics are used to control hypertension. Antibiotic therapy is indicated only for those children with evidence of persistent streptococcal infections. It is used to prevent transmission of nephritogenic streptococci to other family members.
Prognosis.: Almost all children correctly diagnosed as having APSGN recover completely, and specific immunity is conferred, so that subsequent recurrences are uncommon. Some of these children have been reported to develop chronic disease, but most of these cases are now believed to be different glomerular diseases misdiagnosed as poststreptococcal disease.
Nursing Care Management
Nursing care of the child with glomerulonephritis involves careful assessment of the disease status, with regular monitoring of vital signs (including frequent measurement of blood pressure), fluid balance, and behavior.
Vital signs provide clues to the severity of the disease and early signs of complications. They are carefully measured, and any deviations are reported and recorded. The volume and character of urine are noted, and the child is weighed daily. Children with restricted fluid intake, especially those who are not severely edematous or those who have lost weight, are observed for signs of dehydration.
Assessment of the child’s appearance for signs of cerebral complications is an important nursing function, since the severity of the acute phase is variable and unpredictable. The child with edema, hypertension, and gross hematuria may be subject to complications, and anticipatory preparations such as seizure precautions and intravenous (IV) equipment are included in the nursing care plan.
For most children a regular diet is allowed, but it should contain no added salt. Foods high in sodium and salted treats are eliminated, and parents and friends are advised not to bring snacks such as potato chips or pretzels. However, the total amount of salt ingested is usually less than prescribed because of the child’s poor appetite. Fluid restriction, if prescribed, is more difficult, and the amount permitted should be evenly divided throughout the waking hours. Meal preparation and service require special attention, since the child is indifferent to meals during the acute phase. Again, collaboration with parents and the dietitian and special consideration for food preferences facilitate meal planning.
During the acute phase children are generally content to lie in bed. As they begin to feel better and their symptoms subside, they will want to be up and about. Activities should be planned to allow for frequent rest periods and avoidance of fatigue. Children who have mild edema and no hypertension, as well as convalescent children who are being treated at home, need follow-up care. Parents are instructed regarding general measures, including diet and prevention of infection.
Health supervision is continued, with weekly, followed by monthly, visits for evaluation and urinalysis. Parent education and support in preparation for discharge and home care include education in home management and the need for follow-up care and health supervision.
MISCELLANEOUS RENAL DISORDERS
Hemolytic uremic syndrome (HUS) is an uncommon, acute renal disease that occurs primarily in infants and small children between the ages of 6 months and 5 years. HUS is one of the most frequent causes of acquired ARF in children (Davis and Avner, 2004). The clinical features of the disease include acquired hemolytic anemia, thrombocytopenia, renal injury, and central nervous system symptoms. The etiology of HUS is thought to be associated with bacterial toxins, chemicals, and viruses. The appearance of the disease has been associated with Rickettsia organisms, viruses (especially coxsackievirus, echovirus, and adenovirus), Escherichia coli, pneumococci, shigellae, and salmonellae and may represent an unusual response to these infections. Multiple cases of HUS caused by enteric infection of the E. coli O157:H7 serotype have been traced to undercooked meat, especially ground beef. Other sources are unpasteurized milk or fruit juice, especially apple; alfalfa sprouts; lettuce; and salami; drinking or swimming in sewage-contaminated water can also cause infection. The clinical presentation is usually a history of a prodromal illness (most often gastroenteritis or an upper respiratory tract infection) followed by the sudden onset of hemolysis and renal failure.
Pathophysiology
The primary site of injury appears to be the endothelial lining of the small glomerular arterioles, which become swollen and occluded with deposits of platelets and fibrin clots (intravascular coagulation). Red blood cells are damaged as they attempt to move through the partially occluded blood vessels. These damaged cells are removed by the spleen, causing acute hemolytic anemia. The platelet aggregation within the damaged blood vessels or the damage and removal of platelets produce the characteristic thrombocytopenia.
Diagnostic Evaluation
The triad of anemia, thrombocytopenia, and renal failure is sufficient for diagnosis (Box 27-4). Renal involvement is evidenced by proteinuria, hematuria, and urinary casts; BUN and serum creatinine levels are elevated. A low hemoglobin and hematocrit and a high reticulocyte count confirm the hemolytic nature of the anemia.
Therapeutic Management
The goals of therapy are early diagnosis and aggressive, supportive care of the ARF and hemolytic anemia. The most consistently effective treatment of HUS is hemodialysis or peritoneal dialysis, which is instituted in any child who has been anuric for 24 hours or who demonstrates oliguria with uremia or hypertension and seizures. Other treatments include use of pharmacologic agents, fresh-frozen plasma, and plasmapheresis. Blood transfusions with fresh, washed packed cells are administered for severe anemia but are used with caution to prevent circulatory overload from added volume.
Prognosis.: With prompt treatment the recovery rate is about 95%, but residual renal impairment ranges from 10% to 50%. Long-term complications include chronic renal failure, hypertension, and central nervous system disorders. Death is usually caused by residual renal impairment or central nervous system injury.
Nursing Care Management
Nursing care is the same as that provided in ARF and, for children with continued impairment, includes management of chronic disease. Because of the sudden and life-threatening nature of the disorder in a previously well child, parents are often ill prepared for the impact of hospitalization and treatment. Therefore support and understanding are especially important aspects of care.
WILMS TUMOR
Wilms tumor, or nephroblastoma, is the most common malignant renal and intraabdominal tumor of childhood. Its frequency is estimated to be 7.6 cases per million in Caucasian children less than 15 years of age (Dome, Perlman, Ritchey, and others, 2006). Wilms tumor occurs about three times more often in African-Americans than in East Asians in the United States. The peak age at diagnosis is approximately 3 years, and occurrence is slightly more frequent in boys than in girls. The majority of patients with Wilms tumor are diagnosed at younger than 5 years of age, with 1% to 2.5% having a familial origin. Unfortunately, there is no method of identifying gene carriers at this time.
Etiology
Wilms tumor probably arises from a malignant, undifferentiated cluster of primordial cells capable of initiating the regeneration of an abnormal structure. Its occurrence slightly favors the left kidney, which is advantageous because surgically this kidney is easier to manipulate and remove. In about 10% of cases both kidneys are involved. Studies have shown that development of Wilms tumor is frequently associated with aniridia, hemihypertrophy, Beckwith-Wiedemann syndrome, or genitourinary anomalies (Kline and Sevier, 2003; Dome, Perlman, Ritchey, and others, 2006).
Diagnostic Evaluation
In a child suspected of having Wilms tumor, special emphasis is placed on the history and physical examination for the presence of congenital anomalies, a family history of cancer, and signs of malignancy (e.g., weight loss, size of liver and spleen, indications of anemia, lymphadenopathy). Most children with Wilms tumor are brought to the practitioner because of abdominal swelling or an abdominal mass (Box 27-5). Specific tests include radiographic studies, including abdominal ultrasound and abdominal and chest computed tomography scan; hematologic studies; biochemical studies; and urinalysis. Studies to demonstrate the relationship of the tumor to the ipsilateral kidney and the presence of a normal functioning kidney on the contralateral side are essential. If a large tumor is present, an inferior venacavogram is necessary to demonstrate possible tumor involvement adjacent to the vena cava. A bone marrow aspiration may be performed to rule out metastasis, which is rare in children with Wilms tumor.
Therapeutic Management
Combined treatment with surgery and chemotherapy with or without radiation is based on the histologic pattern and clinical stage (Box 27-6).
Surgery is scheduled as soon as possible after confirmation of a renal mass, usually within 24 to 48 hours of admission. A large transabdominal incision is performed for optimal visualization of the abdominal cavity. The tumor, affected kidney, and adjacent adrenal gland are removed. Great care is taken to keep the encapsulated tumor intact, since rupture can seed cancer cells throughout the abdomen, lymph channel, and bloodstream. The contralateral kidney is carefully inspected for evidence of disease or dysfunction. Regional lymph nodes are inspected, and a biopsy is performed when indicated. Any involved structures, such as part of the colon, diaphragm, or vena cava, are removed. Metal clips are placed around the tumor site for exact marking during radiotherapy.
If both kidneys are involved, the child may be treated with radiotherapy or chemotherapy before surgery to decrease the size of the tumor, allowing more conservative surgery. It may be possible to perform a partial nephrectomy on the less affected kidney, with a total nephrectomy on the opposite side. When a transplant is feasible, such as from a twin, sibling, or parent, bilateral nephrectomy is considered as a last resort.
Postoperative radiotherapy is indicated for children with large tumors, metastasis, residual postoperative disease, unfavorable histologic characteristics, or recurrence. Chemotherapy is indicated for all stages. The most effective agents for treating Wilms tumor are actinomycin D (dactinomycin), vincristine, and adriamycin, with the addition of cyclophosphamide for unfavorable histology or advanced disease (Dome, Perlman, Ritchey, and others, 2006). The duration of therapy ranges from 6 to 15 months.
Prognosis.: Survival rates for Wilms tumor are the highest among all childhood cancers. Children with localized tumor (stages I and II) have a 90% chance of cure with multimodal therapy. Factors that favorably affect the success of further therapy include initial treatment with only vincristine and dactinomycin, relapse to the lungs only, relapse in the abdomen of a patient who received no prior abdominal irradiation, and relapse more than 12 months after diagnosis. Wilms tumor may recur, especially in the lungs. Both chemotherapy and radiotherapy can induce second malignancies, usually in areas that have been irradiated (Dome, Perlman, Ritchey, and others, 2006).
Nursing Care Management
Nursing care of the child with Wilms tumor is similar to that of children with other cancers treated with surgery, irradiation, and chemotherapy. However, there are some significant differences; these are discussed for each phase of nursing intervention.
Preoperative Care.: The preoperative period is one of swift diagnosis. The nurse faces the challenge of preparing the child and parents for all laboratory and operative procedures within 24 to 48 hours of admission. Because of the minimal preparatory time, explanations should be simple, repetitive, and focused on the child’s actual experiences. In addition to the usual preoperative observations, blood pressure is monitored, since hypertension from excess renin production is a possibility.
There are several special preoperative concerns, the most important of which is that the tumor is not palpated unless absolutely necessary because manipulation of the mass may cause dissemination of cancer cells to adjacent and distant sites.
Because radiotherapy and chemotherapy are usually begun immediately after surgery, parents need an explanation of what to expect, such as major benefits and side effects. The timing of the information should be considered to avoid overwhelming the family. Ideally, the nurse should be present during physician-parent conferences to answer questions as they arise. It is usually better to postpone telling the child about these side effects until after surgery. Alopecia, usually of most concern to older children, does not occur until approximately 2 weeks after the initial treatment regimen. Therefore the child can be prepared for the hair loss postoperatively.
Postoperative Care.: Despite the extensive surgical intervention necessary in many children with Wilms tumor, the recovery is usually rapid. The major nursing responsibilities are the same as those after any abdominal surgery (see Surgical Procedures, Chapter 22). Because these children are at risk for intestinal obstruction from vincristine-induced ileus, radiation-induced edema, and postsurgical adhesion formation, the nurse carefully monitors gastrointestinal activity, such as bowel movements, bowel sounds, distention, vomiting, and pain. The nurse also monitors blood pressure, urinary output, and signs of infection, as well as instituting pulmonary hygiene to prevent postoperative pulmonary complications.
Family Support.: The postoperative period is frequently difficult for parents. The shock of seeing their child immediately after surgery may be the first realization of the seriousness of the diagnosis. It also marks the confirmation of the stage of the tumor. During this period, the nurse should be with the parents to assure them of the child’s recovery after surgery and to assess the parent’s understanding of the total experience. They need an opportunity to express their feelings and need to be provided the same emotional care discussed in Chapter 18 for families who have a child with a life-threatening disorder.
Older children need an opportunity to deal with their feelings concerning the many procedures to which they have been subjected in rapid succession. Play therapy with dolls or puppets or through drawing can be extremely beneficial in helping them adjust. It is not unusual for children to feel angry because of the extent of surgery, the need for additional therapy, or the seriousness of the disorder.
RENAL FAILURE
Renal failure is the inability of the kidneys to excrete waste material, concentrate urine, and conserve electrolytes. It can occur suddenly (acute renal failure [ARF]) in response to inadequate perfusion, kidney disease, or urinary tract obstruction, or it can develop slowly (chronic renal failure [CRF]) as a result of longstanding kidney disease or an anomaly.
Azotemia and uremia are terms often used in relation to renal failure. Azotemia is the accumulation of nitrogenous waste within the blood. Uremia is a more advanced condition in which retention of nitrogenous products produces toxic symptoms. Azotemia is not life threatening, whereas uremia is a serious condition that often involves other body systems.
ACUTE RENAL FAILURE
ARF is said to exist when the kidneys suddenly are unable to regulate the volume and composition of urine appropriately in response to food and fluid intake and the needs of the organism. The principal feature of ARF is oliguria* associated with azotemia, metabolic acidosis, and diverse electrolyte disturbances. ARF is not common in childhood, but the outcome depends on the cause, associated findings, and prompt recognition and treatment.
The pathologic conditions that produce ARF caused by glomerulonephritis and HUS are discussed in relation to those disorders. ARF can also develop as a result of a large number of related or unrelated clinical conditions: poor renal perfusion; urinary tract obstruction; acute renal injury; or the final expression of chronic, irreversible renal disease. The most common cause in children is transient renal failure resulting from severe dehydration or other causes of poor perfusion that may respond to restoration of fluid volume.
Pathophysiology
ARF is usually reversible, but the deviations of physiologic function can be extreme, and mortality in the pediatric age-group remains high. There is severe reduction in the glomerular filtration rate, an elevated BUN level, and a significant reduction in renal blood flow.
The clinical course is variable and depends on the cause. In reversible ARF there is a period of severe oliguria, or a low-output phase, followed by an abrupt onset of diuresis, or a high-output phase, and then a gradual return to (or toward) normal urine volumes.
Diagnostic Evaluation
In many instances of ARF the infant or child is already critically ill with the precipitating disorder, and the explanation for development of oliguria may or may not be readily apparent (Box 27-7). When a previously well child develops ARF without obvious cause, a careful history is taken to reveal symptoms that may be related to glomerulonephritis, obstructive uropathy, or exposure to nephrotoxic chemicals (e.g., ingestion of heavy metals, inhalation of carbon tetrachloride or other organic solvents, or medications such as nonsteroidal antiinflammatory drugs [Krause, Cleper, Eisenstein, and others, 2005] known to be toxic to the kidneys). Significant laboratory measurements during renal shutdown that serve as a guide for therapy are BUN, serum creatinine, pH, sodium, potassium, and calcium.
Therapeutic Management
Treatment of ARF is directed toward (1) treatment of the underlying cause, (2) management of the complications of renal failure, and (3) provision of supportive therapy within the constraints imposed by the renal failure.
Treatment of poor perfusion resulting from dehydration consists of volume restoration, as described in Chapter 24 in treatment of dehydration. If oliguria persists after restoration of fluid volume or if the renal failure is caused by intrinsic renal damage, the physiologic and biochemical abnormalities that have resulted from kidney dysfunction must be corrected or controlled. Initially a Foley catheter is inserted to rule out urine retention, to collect available urine for analysis, and to monitor results of diuretic administration. The catheter may or may not be removed during the oliguric phase.
The amount of exogenous water provided should not exceed the amount needed to maintain zero water balance. It is calculated on the basis of estimated endogenous water formation and losses from sensible (primarily gastrointestinal) and insensible sources. No allotment is calculated for urine as long as oliguria persists.
When the output begins to increase, either spontaneously or in response to diuretic therapy, the intake of fluid, potassium, and sodium must be monitored and adequate replacement provided to prevent depletion and its consequences. Some patients pass enormous amounts of electrolyte-rich urine.
Complications.: The child with ARF has a tendency to develop water intoxication and hyponatremia, which makes it difficult to provide calories in sufficient amounts to meet the child’s needs and reduce tissue catabolism, metabolic acidosis, hyperkalemia, and uremia. If the child is able to tolerate oral foods, food sources high in concentrated carbohydrate and fat but low in protein, potassium, and sodium may be provided. However, many children have functional disturbances of the gastrointestinal tract, such as nausea and vomiting; therefore the IV route is generally preferred and usually consists of essential amino acids or a combination of essential and nonessential amino acids administered by the central venous route.
Control of water balance in these patients requires careful monitoring of feedback information, such as accurate intake and output, body weight, and electrolyte measurements. In general, during the oliguric phase, no sodium, chloride, or potassium is given unless there are other large, ongoing losses. Regular measurement of plasma electrolyte, pH, BUN, and creatinine levels is required to assess the adequacy of fluid therapy and to anticipate complications that require specific treatment.
Hyperkalemia is the most immediate threat to the life of the child with ARF. Hyperkalemia can be minimized and sometimes avoided by eliminating potassium from all food and fluid, by reducing tissue catabolism, and by correcting acidosis. Measures employed for the reduction of serum potassium levels are oral or rectal administration of an ion-exchange resin such as sodium polystyrene sulfonate (Kayexalate) and peritoneal dialysis or hemodialysis (p. 970). The resin produces its effect by exchange of its sodium for the potassium, thus binding potassium for removal from the body. This increased sodium concentration may contribute to fluid overload, hypertension, and cardiac failure. Dialysis removes potassium and other waste products from the serum by diffusion through a semipermeable membrane.
Hypertension is a frequent and serious complication of ARF, and to detect it early, blood pressure measurements are made every 4 to 6 hours. The most common cause of hypertension in ARF is overexpansion of extracellular fluid and plasma volume together with activation of the renin-angiotensin system. Hypertension is controlled with antihypertensive drugs. Other measures that may be used include limiting fluids and salt.
Anemia is frequently associated with ARF, but transfusion is not recommended unless the hemoglobin drops below 6 g/dl. Transfusions, if used, consist of fresh, packed red blood cells given slowly to reduce the likelihood of increasing blood volume, hypertension, and hyperkalemia.
Seizures occur often when renal failure progresses to uremia and are also related to hypertension, hyponatremia, and hypocalcemia. Treatment is directed to the specific cause when known. More obscure causes are managed with antiepileptic drugs.
Cardiac failure with pulmonary edema is almost always associated with hypervolemia. Treatment is directed toward reduction of fluid volume, with water and sodium restriction and administration of diuretics.
Prognosis.: The prognosis of ARF depends largely on the nature and severity of the causative factor or precipitating event and the promptness and competence of management. The outcome is least favorable in children with rapidly progressive nephritis and cortical necrosis. Children in whom ARF is a result of HUS or AGN may recover completely, but residual renal impairment or hypertension is more often the rule. Complete recovery is usually expected in children whose renal failure is a result of dehydration, nephrotoxins, or ischemia. ARF after cardiac surgery is less favorable. It is often impossible to assess the extent of recovery for several months.
Nursing Care Management
Meticulous attention to fluid intake and output is mandatory and includes all of the physical measurements discussed previously in relation to problems of fluid balance. Monitoring fluid balance and vital signs is a continuous process, and observers are constantly on the alert for signs of complications so that appropriate interventions can be implemented. Because these children require intensive observation and often specialized treatment, such as dialysis, they are usually admitted to an intensive care unit in which needed equipment and trained personnel are available (see Nursing Care Plan).
Limiting fluid intake requires ingenuity on the part of caregivers to cope with the child who is thirsty. Rationing the daily intake in small amounts of fluid served in containers that give the impression of larger volumes is one strategy. Older children who understand the rationale of fluid limits can help determine how their daily ration should be distributed.
Meeting nutritional needs is sometimes a problem; the child may be nauseated, and encouraging concentrated foods without fluids may be difficult. When nourishment is provided by the IV route, careful monitoring is essential to prevent fluid overload. In addition, nursing measures such as maintaining an optimal thermal environment, reducing any elevation of body temperature, and reducing restlessness and anxiety are employed to decrease the rate of tissue catabolism.
The nurse must be continually alert for changes in behavior that indicate the onset of complications. Infection from reduced resistance, anemia, and general morbidity is a constant threat. Fluid overload and electrolyte disturbances can precipitate cardiovascular complications such as hypertension and cardiac failure. Fluid and electrolyte imbalances, acidosis, and accumulation of nitrogenous waste products can produce neurologic involvement manifested by coma, seizures, or alterations in sensorium.
Although children with ARF are usually quite ill and voluntarily diminish their activity, infants may become restless and irritable, and children are often anxious and frightened. Frequent, painful, and stress-producing treatments and tests must be performed. A supportive, empathetic nurse can provide comfort and stability in a threatening and unnatural environment.
Family Support.: Providing support and reassurance to parents is among the major nursing responsibilities. The seriousness of ARF and its emergency nature are stressful to parents, and most feel some degree of guilt regarding the child’s condition, especially when the illness is a result of ingestion of a toxic substance, dehydration, or a genetic disease. They need reassurance and a sympathetic listener. They also need to be kept informed of the child’s progress and provided explanations regarding the therapeutic regimen. The equipment and the child’s behavior are sometimes frightening and anxiety provoking. Nurses can do much to help parents comprehend and deal with the stresses of the situation.
CHRONIC RENAL FAILURE
The kidneys are able to maintain the chemical composition of fluids within normal limits until more than 50% of functional renal capacity is destroyed by disease or injury. Chronic renal insufficiency or failure begins when the diseased kidneys can no longer maintain the normal chemical structure of body fluids under normal conditions. Progressive deterioration over months or years produces a variety of clinical and biochemical disturbances that eventually culminate in the clinical syndrome known as uremia.
A variety of diseases and disorders can result in CRF. The most frequent causes are congenital renal and urinary tract malformations, vesicoureteral reflux associated with recurrent UTI, chronic pyelonephritis, hereditary disorders, chronic glomerulonephritis, and glomerulonephropathy associated with systemic diseases such as anaphylactoid purpura and lupus erythematosus.
Pathophysiology
Early in the course of progressive nephrotic destruction, the child remains asymptomatic with only minimal biochemical abnormalities. Unless the presence of CRF is detected in the process of routine assessment, signs and symptoms that indicate advanced renal damage frequently emerge only late in the course of the disease. Midway in the disease process, as increasing numbers of nephrons are totally destroyed and most others are damaged to varying degrees, the few that remain intact are hypertrophied but functional. These few normal nephrons are able to make sufficient adjustments to stresses to maintain reasonable degrees of fluid and electrolyte balance. Definitive biochemical examination at this time will reveal restricted tolerance to excesses or restrictions. As the disease progresses to the end stage, because of a severe reduction in the number of functioning nephrons, the kidneys are no longer able to maintain fluid and electrolyte balance, and the features of uremic syndrome appear.
The accumulation of various biochemical substances in the blood, those that result from diminished renal function, produces complications such as the following:
Retention of waste products, especially the BUN and creatinine
Water and sodium retention, which contributes to edema and vascular congestion
Hyperkalemia of dangerous levels
Metabolic acidosis of a sustained nature because of continual hydrogen ion retention and bicarbonate loss
Calcium and phosphorus disturbances, resulting in altered bone metabolism, which in turn causes growth arrest or retardation, bone pain, and deformities known as renal osteodystrophy
Anemia caused by hematologic dysfunction, including shortened life span of red blood cells, impaired red blood cell production related to decreased production of erythropoietin, prolonged bleeding time, and nutritional anemia
Growth disturbance, probably caused by such factors as renal osteodystrophy, poor nutrition associated with dietary restrictions and loss of appetite, and biochemical abnormalities
Children with CRF seem to be more susceptible to infection, especially pneumonia, UTI, and septicemia, although the reason for this is unclear. These children become extraordinarily sensitive to changes in vascular volume that may cause pulmonary overload, central nervous system symptoms, hypertension, and cardiac failure.
Diagnostic Evaluation
The diagnosis of CRF is usually suspected on the basis of any number of clinical manifestations, a history of prior renal disease, or biochemical findings. The onset is usually gradual, and the initial signs and symptoms are vague and nonspecific (Box 27-8).
Laboratory and other diagnostic tools and tests are of value in assessing the extent of renal damage, biochemical disturbances, and related physical dysfunction (see Tables 27-1 to 27-3). Often they can help establish the nature of the underlying disease and differentiate between other disease processes and the pathologic consequences of renal dysfunction.
Therapeutic Management
In irreversible renal failure the goals of medical management are to (1) promote maximum renal function, (2) maintain body fluid and electrolyte balance within safe biochemical limits, (3) treat systemic complications, and (4) promote as active and normal a life as possible for the child for as long as possible. The child is allowed unrestricted activity and is allowed to set his or her own limits regarding rest and extent of exertion. School attendance is encouraged as long as the child is able. When the effort is too great, home tutoring is arranged.
Diet regulation is the most effective means, short of dialysis, for reducing the quantity of materials that require renal excretion. The goal of diet management in renal failure is to provide sufficient calories and protein for growth while limiting the excretory demands made on the kidney, to minimize metabolic bone disease (osteodystrophy), and to minimize fluid and electrolyte disturbances. Dietary protein intake is limited only to the reference daily intake (RDA) for the child’s age. Restriction of protein intake below the RDA is believed to negatively affect growth and neurodevelopment. Malnutrition may develop in patients with CRF even before they need dialysis (Nailescu, Kaskel, and Kaskel, 2004).
Sodium and water are not usually limited unless there is evidence of edema or hypertension, and potassium is not usually restricted. However, restrictions of any or all three may be imposed in later stages or at any time that abnormal serum concentrations are evident.
Dietary phosphorus is controlled through reduction of protein and milk intake to prevent or correct the calcium/phosphorus imbalance. Phosphorus levels can be further reduced by oral administration of calcium carbonate preparations or other phosphate-binding agents that combine with the phosphorus to decrease gastrointestinal absorption and thus the serum levels of phosphate. Treatment with 25-OH vitamin D is begun to increase calcium absorption and suppress elevated parathyroid hormone levels.
Metabolic acidosis is alleviated through administration of alkalizing agents such as sodium bicarbonate or a combination of sodium and potassium citrate.
Growth failure is one major consequence of CRF, especially in the preadolescent. These children grow poorly both before and after the initiation of hemodialysis. The use of recombinant human growth hormone to accelerate growth in children with growth retardation secondary to CRF has been successful (Mehls, Schaefer, Tönshoff, and others, 2002; Vimalachandra, Hodson, Willis, and others, 2006). Osseous deformities that result from renal osteodystrophy, especially those related to ambulation, are troublesome and require correction if they occur. Dental defects are common in children with CRF, and the earlier the onset of the disease, the more severe are the dental manifestations (including hypoplasia, hypomineralization, tooth discoloration, alteration in size and shape of teeth, malocclusion, and ulcerative stomatitis). Therefore regular dental care is especially important in these children.
Anemia in children with CRF is related to decreased production of erythropoietin. Recombinant human erythropoietin (rHuEPO) is being offered to these children as thrice-weekly or weekly subcutaneous injections and is replacing the need for frequent blood transfusions. The drug corrects the anemia and in turn increases appetite, activity, and general well-being in the children who receive it.
Hypertension of advanced renal disease may be managed initially by cautious use of a low-sodium diet, fluid restriction, and perhaps diuretics such as hydrochlorothiazide or furosemide. Severe hypertension requires the use of antihypertensive agents, singly or in combination.
Intercurrent infections are treated with appropriate antimicrobials at the first sign of infection; however, any drug eliminated through the kidneys is administered with caution. Other complications are treated symptomatically (e.g., central-acting antiemetics for nausea, antiepileptics for seizures, and diphenhydramine [Benadryl] for pruritus).
Once evidence of end-stage renal disease (ESRD) appears in a child, the disease runs its relentless course and results in death in a few weeks, unless waste products and toxins are removed from body fluids by dialysis or kidney transplantation. These techniques have been adapted for infants and small children and are implemented in most cases of renal failure after conservative management is no longer effective (see Technologic Management of Renal Failure, p. 970).
Prognosis.: Dialysis and transplantation are the only treatments currently available for children with ESRD. Although children may survive on dialysis, it is not an ideal long-term modality. Complications include infection of access sites, growth failure, and disruption of normal socialization. Many pediatric centers encourage families of children with ESRD to consider kidney transplantation. The North American Renal Transplantation in Children Report of the North American Pediatric Renal Trials and Collaborative Studies reports a graft survival of 90% at 1 year and 74% at 6 years for living donor kidneys, and 80% at 1 year and 58% at 6 years for cadaver kidneys (Benfield, McDonald, Bartosh, and others, 2003).
Posttransplant complications include infection, hypertension, steroid toxicity, hyperlipidemia, aseptic necrosis, malignancy, and growth retardation (Benfield, 2003). Long-term graft survival is not guaranteed, and many children require a second or third transplant. Successful kidney transplantation does improve rehabilitation of children with CRF, both educationally and psychologically. Increasing use of primary or preemptive kidney transplants is becoming the optimal form of renal replacement therapy, leading to substantial improvement in quality of life (Goldstein, Graham, Burwinkle, and others, 2006).
Nursing Care Management
The multiple complications of ESRD are managed according to evidence-based clinical practice guidelines such as the ESRD Clinical Performance Measures Project (Fadrowski, Frankenfield, Amaral, and others, 2007). However, progressive disease places a number of stresses on the child and family, including those of a potentially fatal illness (see Chapter 18). There is a continuing need for repeated examinations that often entail painful procedures, side effects, and frequent hospitalizations. Diet therapy becomes progressively more restricted and intense, and the child is required to take a variety of medications. Ever present in all aspects of the treatment regimen is the agonizing realization that without treatment, death is inevitable.
Some specific stresses related to ESRD and its treatment are predictable. When it first becomes apparent that ESRD is inevitable, both parents and child experience depression and anxiety. Acceptance is particularly difficult if renal failure progresses rapidly after diagnosis. Denial and disbelief are usually pronounced, especially among the parents. After renal failure is established and symptoms become progressively more distressing, the initiation of dialysis is usually perceived as a positive experience, and after experiencing initial concerns regarding the treatment, the child begins to feel better and parental anxiety is relieved for a time.
Initiating a dialysis regimen is a traumatic and anxiety-provoking experience for most children because it involves surgery for implantation of a graft, fistula, or peritoneal catheter. The initial experience with the dialysis procedure is frightening to most children. They need reassurance about the nature of the preparations for dialysis and the conduct of the treatment.
Both the graft and the fistula require needle insertions at each dialysis. The goal is to perform pain-free venipuncture. Using buffered lidocaine with a small-gauge needle (30 gauge) to anesthetize the area before venipuncture of the graft or fistula is one method. Using an anesthetizing topical preparation such as EMLA (eutectic mixture of local anesthetics [lidocaine and prilocaine]) 1 hour before venipuncture is another approach (see Pain Management, Chapter 7). External dual-lumen venous access devices eliminate the need for needles but are more prone to infection and other central line complications.
Adolescents, with their increased need for independence and their urge for rebellion, usually adapt less well than younger children. They resent the control and enforced dependence imposed by the rigorous and unrelenting therapy program. They resent being dependent on hemodialysis technology, their parents, and the professional staff. Depression or hostility is common in adolescents undergoing hemodialysis.
The availability of home peritoneal dialysis has offered a greater degree of freedom for persons undergoing long-term dialysis. The nurse is responsible for teaching the family about (1) the disease, its implications, and the therapeutic plan; (2) the possible psychologic effects of the disease and the treatment; and (3) the technical aspects of the procedure. The family learns to manage the various aspects of the dialysis procedure, how to maintain accurate records, and how to observe for signs of complications that need to be reported to the proper persons.
Body changes related to the disease process, such as pale or ashen skin color, growth retardation, and lack of sexual maturation, are stress provoking. Dietary restrictions are particularly burdensome for both children and parents. Children feel deprived when they are unable to eat foods previously enjoyed and that are unrestricted for other family members. Consequently, they may fail to cooperate. Diet restrictions may be interpreted as punishment. Some children, unable to understand fully the purpose of restrictions, will sneak forbidden food items at every opportunity. Allowing children, especially adolescents, maximum participation in and responsibility for their own treatment program is helpful.
After months or years of dialysis, the parents and child feel anxiety associated with the prognosis and continued pressures of the treatment. The relentless need for treatment interferes with family plans. The time spent in transportation to and from the dialysis unit and the time spent undergoing dialysis treatments cut into time for outside activities, including school. Graft and fistula problems, as well as peritoneal catheter exit site infections, may develop and present a common source of aggravation (see Family Focus box).
The possibility of kidney transplantation often provides hope for relief from the rigors of hemodialysis and peritoneal dialysis. Most children and families respond well to a kidney transplant, and most children can be successfully rehabilitated.
The National Kidney Foundation* and other agencies provide a number of services and information for families of children with renal disease.
TECHNOLOGIC MANAGEMENT OF RENAL FAILURE
Dialysis is the process of separating colloids and crystalline substances in solution by the difference in their rate of diffusion through a semipermeable membrane. Methods of dialysis currently available for clinical management of renal failure are peritoneal dialysis, wherein the abdominal cavity acts as a semipermeable membrane through which water and solutes of small molecular size move by osmosis and diffusion according to their respective concentrations on either side of the membrane, and hemodialysis, in which blood is circulated outside the body through artificial membranes that permit a similar passage of water and solutes. A third type of dialysis is hemofiltration, in which blood filtrate is circulated outside the body by hydrostatic pressure exerted across a semipermeable membrane with simultaneous infusion of a replacement solution. Types of hemofiltration include continuous venovenous hemofiltration, continuous venovenous hemodialysis, and continuous venovenous hemodiafiltration. These continuous renal replacement therapies are used in ARF, severe fluid overload, and inborn errors of metabolism or after bone marrow transplant (Goldstein, 2003).
Peritoneal dialysis is the preferred form of dialysis for infants, for children and parents who wish to remain independent, for families who live a long distance from the medical center, and for children who prefer fewer dietary restrictions and a gentler form of dialysis. Chronic peritoneal dialysis is most often performed at home. The two types of peritoneal dialysis are continuous ambulatory peritoneal dialysis and continuous cycling peritoneal dialysis. In both methods, commercially available sterile dialysis solution is instilled into the peritoneal cavity through a surgically implanted indwelling catheter tunneled subcutaneously and sutured into place. The warmed solution is allowed to enter the peritoneal cavity by gravity and remains a variable length of time according to the rate of solute removal and glucose absorption in individual patients. The care and management of the procedure are the responsibility of the parents of young children. Some centers have initiated use of home health nurses to give parents respite from care. Older children and adolescents can carry out the procedure themselves, which provides them with some control and less dependency. This is especially important for adolescents.
Hemodialysis requires the creation of a vascular access and the use of special dialysis equipment—the hemodialyzer, or so-called artificial kidney. Vascular access may be one of three types: fistulas, grafts, or external vascular access devices. An arteriovenous fistula is an access in which a vein and artery are connected surgically. The preferred site is the radial artery and a forearm vein that produces dilation and thickening of the superficial vessels of the forearm to provide easy access for repeated venipuncture. An alternative is the creation of a subcutaneous (internal) arteriovenous graft by anastomosing artery and vein, with a synthetic prosthetic graft for circulatory access. The most commonly used material is expanded polytetrafluoroethylene (ePTFE). Both the graft and the fistula require needle insertions with each dialysis treatment.
For external vascular access devices, percutaneous catheters are inserted in the femoral, subclavian, or internal jugular veins, even in very small children. A more permanent form of external access is available via a central catheter inserted surgically into the internal jugular vein. This catheter has a dual lumen, which allows a larger volume of blood flow with minimum recirculation. Catheters eliminate the need for skin punctures but may require some home care.
Hemodialysis is best suited to children who do not have someone in the family who is able to perform home peritoneal dialysis and to those who live close to a dialysis center. The procedure is usually performed three times per week for 4 to 6 hours, depending on the child’s size. Hemodialysis achieves rapid correction of fluid and electrolyte abnormalities but can cause problems in association with this rapid change, such as muscle cramping and hypotension. Disadvantages include school absence during dialysis and strict fluid and dietary restrictions between dialysis sessions. Boredom for the child and family is often a problem during dialysis, and planned activities should be introduced (Fig. 27-3).
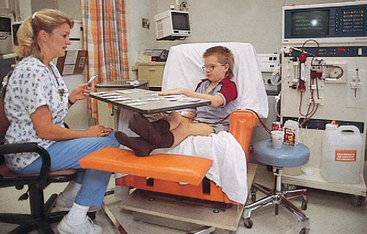
FIG. 27-3 Diversional activities help lessen the boredom children can experience during hemodialysis.
Most children show rapid clinical improvement with the implementation of dialysis, although it is directly related to the duration of uremia before dialysis and good nutrition. Growth rate and skeletal maturation improve, but recovery of normal growth is infrequent. In many cases, sexual development, although delayed, progresses to completion.
TRANSPLANTATION
Kidney transplantation is now an acceptable and effective means of therapy in the pediatric age-group. Although peritoneal dialysis and hemodialysis are life preserving, both require major alterations in lifestyle. Transplantation offers the opportunity for a relatively normal life and is the preferred form of treatment for children with ESRD. Primary or preemptive transplants maintain the greatest amount of normalcy in the family’s life.
Kidneys for transplant are available from two sources: a living related donor, usually a parent or a sibling, or a cadaver donor, wherein the family of a dead or brain-dead patient consents to donation of a healthy kidney. Retransplantation occurs frequently.
The primary goal in transplantation is the long-term survival of grafted tissue by securing tissue that is antigenically similar to that of the recipient and by suppressing the recipient’s immune mechanism. The immunosuppressant therapy of choice has been corticosteroids (prednisone) in conjunction with cyclosporine or tacrolimus and mycophenolate mofetil. Other therapies include antilymphoblast globulin or monoclonal antibodies. New immunosuppressant medications are rapidly coming into clinical trials and into use in large transplant centers. It is important for the nurse to learn about the medications used in the antirejection protocol(s) and their side effects. Because the immunosuppressant medications are taken indefinitely, transplant patients experience many side effects of the drugs, including hypertension, growth retardation, cataracts, risk of infection, obesity, characteristics of Cushing syndrome, and hirsutism (Smith, Nemeth, and McDonald, 2003).
Rejection of the transplanted kidney is the most common cause of transplant failure. Rejection is treated aggressively with immunosuppressant medications and can often be reversed. Some patients do not respond to treatment of acute rejection or develop chronic rejection and must eventually return to dialysis or undergo another kidney transplant.
REFERENCES
American Academy of Pediatrics. Task Force on Circumcision. Pediatrics. 1999;103(3):686–693.
Bailey, DT, Dalton, C, Daugherty, F, et al. Can a concentrated cranberry extract prevent recurrent urinary tract infections in women? A pilot study. Phytomedicine. 2007;14(4):237–241.
Benfield, MR. Current status of kidney transplant: update 2003. Pediatr Clin North Am. 2003;50(6):1301–1334.
Benfield, MR, McDonald, RA, Bartosh, S, et al. Changing trends in pediatric transplantation: 2001 annual report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant. 2003;7(4):321–335.
Davis, ID, Avner, ED. Conditions particularly associated with hematuria. In Behrman RE, Kliegman RM, Jenson HB, eds.: Nelson textbook of pediatrics, ed 17, Philadelphia: Saunders, 2004.
Dome, JS, Perlman, EJ, Ritchey, ML, et al. Renal tumors. In Pizzo PA, Poplack DP, eds.: Principles and practices of pediatric oncology, ed 5, Philadelphia: Lippincott, 2006.
Fadrowski, JJ, Frankenfield, D, Amaral, S, et al. Children on long-term dialysis in the United States: findings from the 2005 ESRD clinical performance measures project. Am J Kidney Dis. 2007;50(6):958–966.
Goldstein, SL. Overview of pediatric renal replacement therapy in acute renal failure. Artif Organs. 2003;27(9):781–785.
Goldstein, SL, Graham, N, Burwinkle, T, et al. Health-related quality of life in pediatric patients with ESRD. Pediatr Nephrol. 2006;21(6):846–850.
Jepson, RG, Mihaljevic, L, Craig, J, Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2004;2:CD001321.
Kanellopoulos, TA, Salakos, C, Spiliopoulou, I, et al. First urinary tract infection in neonates, infants and young children: a comparative study. Pediatr Nephrol. 2006;21(8):1131–1137.
Kass, E. Timing of elective surgery on the genitalia of male children with particular reference to the risks, benefits, and psychological effects of surgery and anesthesia. Pediatrics. 1996;97(4):590–594.
Kirsch, AJ, Perez-Brayfield, MR, Scherz, HC. Minimally invasive treatment of vesicoureteral reflux with endoscopic injection of dextranomer/hyaluronic acid copolymer: the Children’s Hospital of Atlanta experience. J Urol. 2003;170(1):211–215.
Kline, NE, Sevier, N. Solid tumors in children. J Pediatr Nurs. 2003;18(2):96–102.
Krause, I, Cleper, R, Eisenstein, B, et al. Acute renal failure, associated with non-steroidal anti-inflammatory drugs in healthy children. Pediatr Nephrol. 2005;20(9):1295–1298.
Lavelle, MT, Conlin, MJ, Skoog, SJ. Subureteral injection of Deflux for correction of reflux: analysis of factors predicting success. Urology. 2005;65(3):564–567.
Mehls, O, Schaefer, F, Tönshoff, B, et al. Effectiveness of growth hormone treatment in short children with chronic renal failure. J Pediatr. 2002;141(1):147–148.
Nailescu, C, Kaskel, PJ, Kaskel, FJ. Nutrition and metabolism. In Avner ED, Harmon WE, Niaudet P, eds.: Pediatric nephrology, ed 5, Philadelphia: Lippincott Williams & Wilkins, 2004.
National Kidney Foundation. The National Kidney Foundation kidney disease outcomes quality initiative (NKF KDOQI™). http://www.kidney.org/professionals/kdoqi. retrieved February 8, 2007, from
Rosenthal, M. Current concept in managing UTIs in children. Infect Dis Child. 2004;17(3):30–31.
Schaefer, F. Management of peritonitis in children receiving chronic peritoneal dialysis. Paediatr Drugs. 2003;5(5):315–325.
Shaikh, N, Morone, NE, Bost, JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302–308.
Smith, JM, Nemeth, TL, McDonald, RA. Current immunosuppressive agents: efficacy, side effects, and utilization. Pediatr Clin North Am. 2003;50(6):1283–1300.
Vimalachandra, D, Hodson, EM, Willis, NS, et al, Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev 2006;3:CD003264.
*The definition of oliguria varies extensively in the literature, from 1.8 to 4 dl/m2/24 hr.
*30 E. 33rd St., New York, NY 10016; (212) 889-2210, (800) 622-9010; http://www.kidney.org. In Canada: Kidney Foundation of Canada, 300-5165 Sherbrooke St. West, Montreal QC H4A 1T6; (514) 369-4806, (800) 361-7494; http://www.kidney.ca.
 IN TEXT
IN TEXT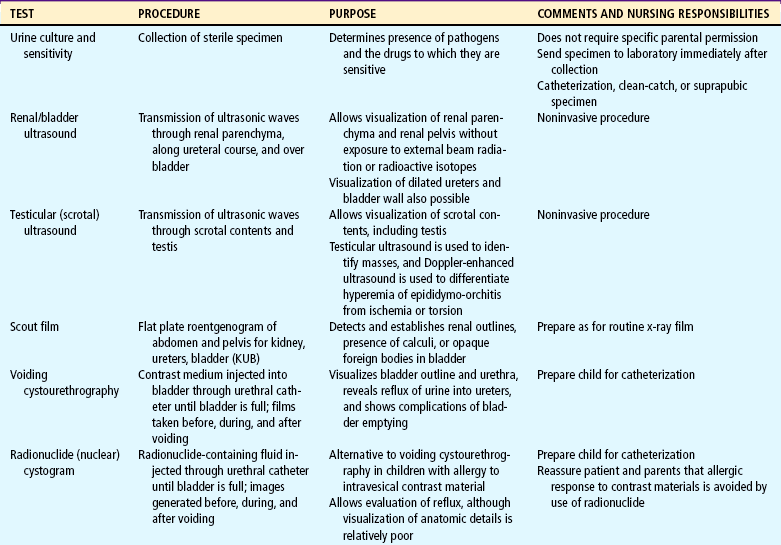
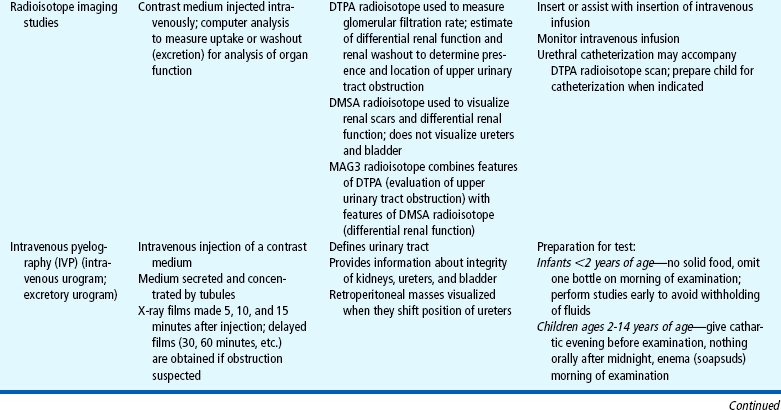
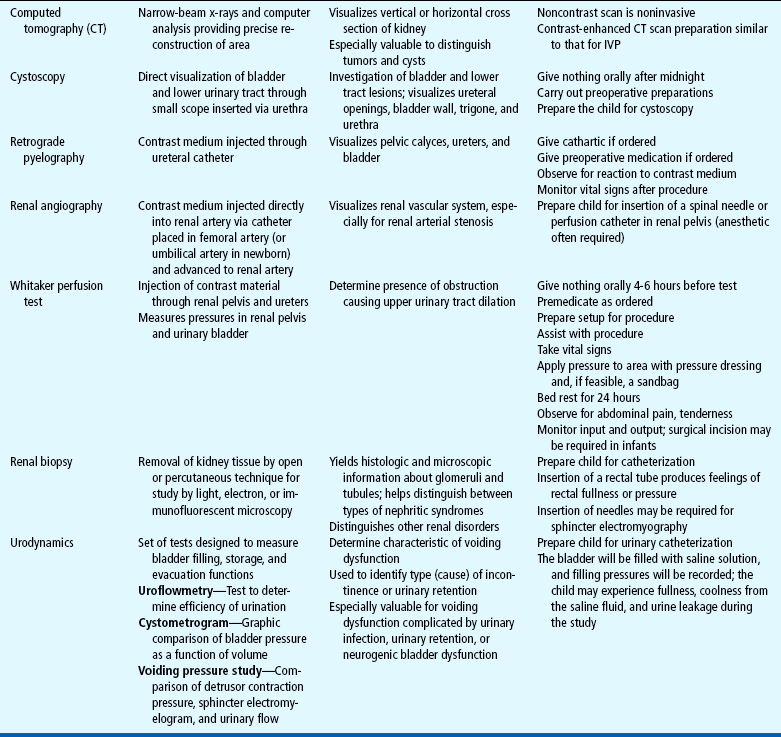
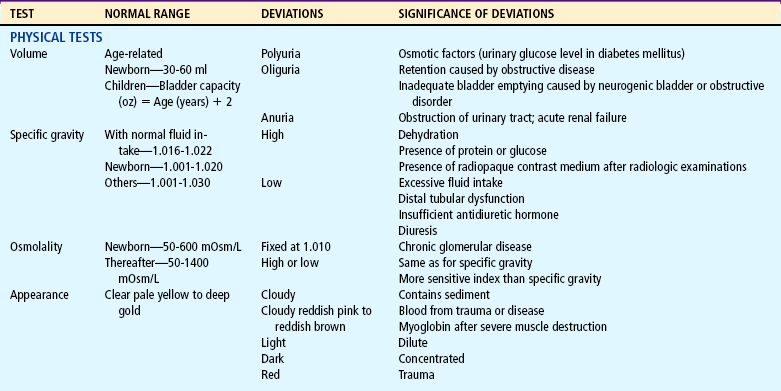
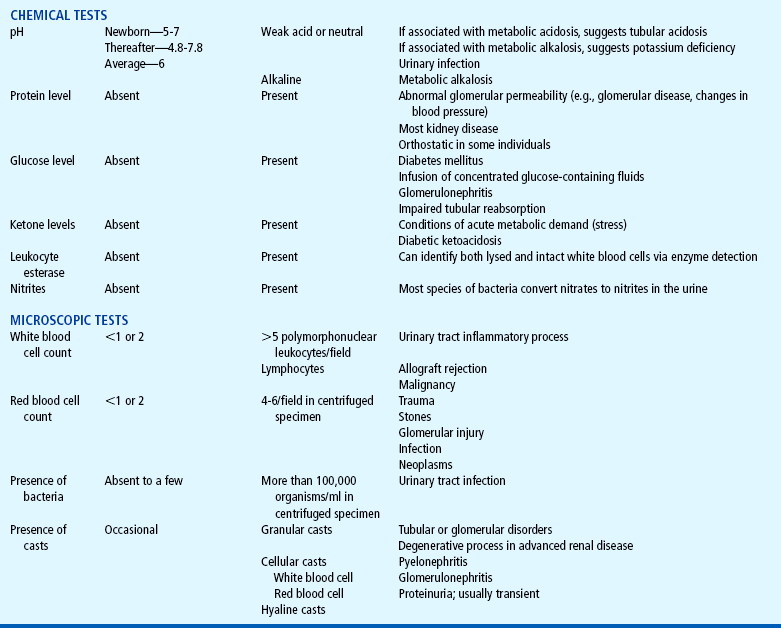
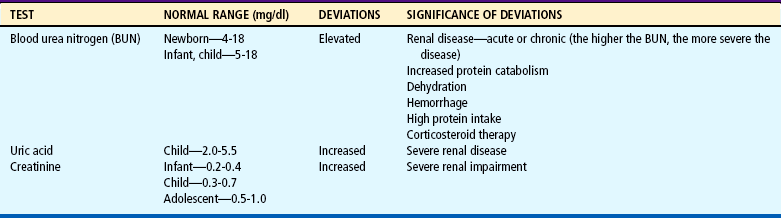
 CRITICAL THINKING EXERCISE
CRITICAL THINKING EXERCISE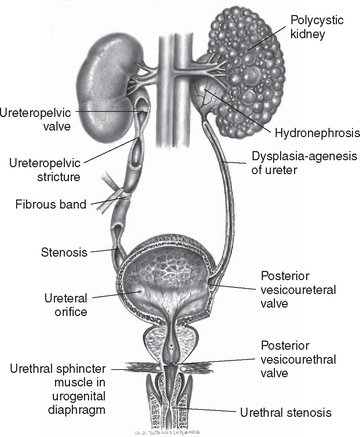
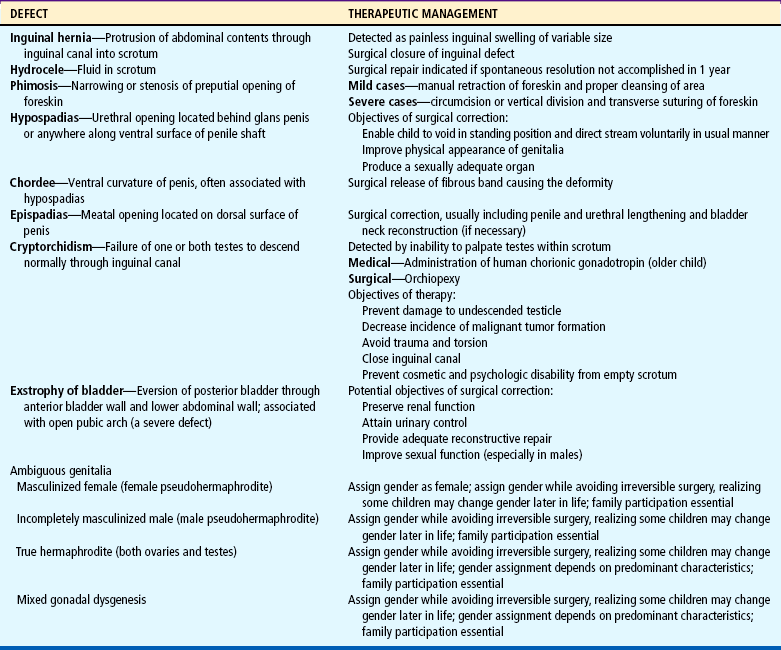
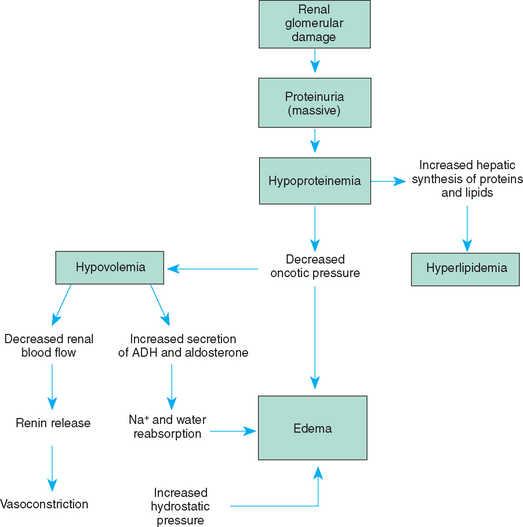
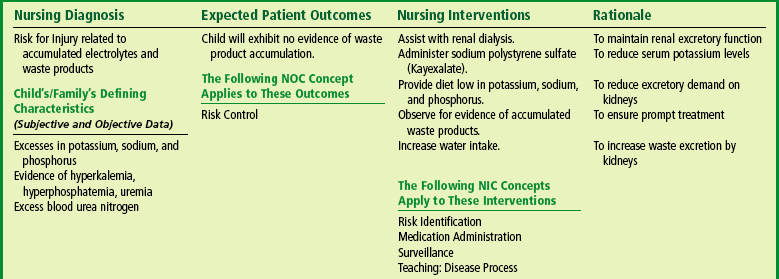
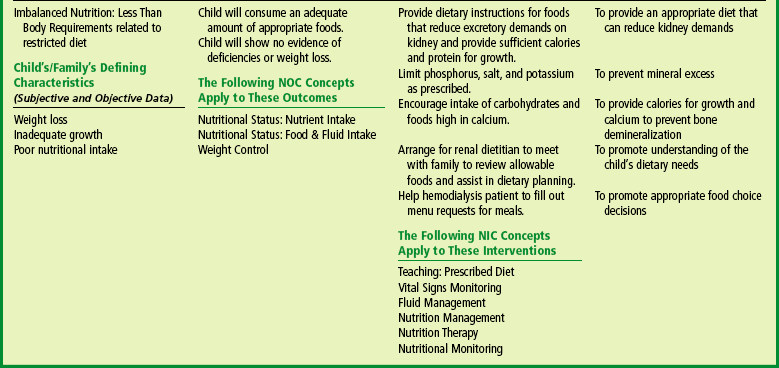
 FAMILY FOCUS
FAMILY FOCUS