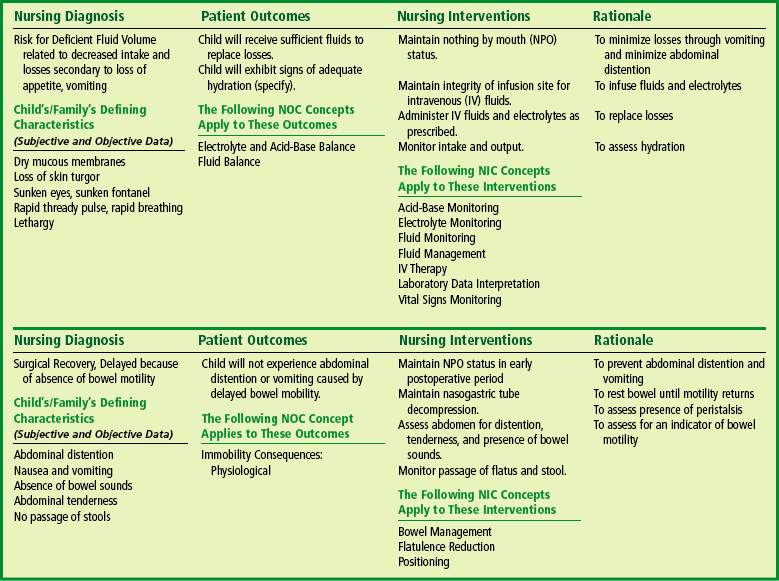
The Child with Gastrointestinal Dysfunction
On completion of this chapter the reader will be able to:
 Describe the characteristics of infants that affect their ability to adapt to fluid loss or gain.
Describe the characteristics of infants that affect their ability to adapt to fluid loss or gain.
 Formulate a care plan for the infant with acute diarrhea.
Formulate a care plan for the infant with acute diarrhea.
 Compare and contrast the inflammatory diseases of the gastrointestinal tract.
Compare and contrast the inflammatory diseases of the gastrointestinal tract.
 Describe the nursing care of the child with hepatitis.
Describe the nursing care of the child with hepatitis.
 Formulate a plan for teaching parents preoperative and postoperative care of the child with a cleft lip or palate.
Formulate a plan for teaching parents preoperative and postoperative care of the child with a cleft lip or palate.
 Formulate a care plan for the child with an obstructive disorder.
Formulate a care plan for the child with an obstructive disorder.
 Identify nutritional therapies for the child with a malabsorption syndrome.
Identify nutritional therapies for the child with a malabsorption syndrome.
GASTROINTESTINAL DYSFUNCTION
The extensive surface area of the gastrointestinal (GI) tract and its digestive function represent the major means of exchange between the human organism and the environment. Inflammatory and malabsorptive disorders impair the functional integrity of the GI tract. In addition, the infant’s intestine is extremely vulnerable to infection. Acute infectious diarrhea causes significant alterations in fluid and electrolyte balance in both infants and children.
Numerous clinical observations provide clues to specific GI problems (Box 24-1). In any disorder that involves GI losses of large amounts of fluid, dehydration poses a serious threat to life and demands immediate attention.
DEHYDRATION
Dehydration is a common body fluid disturbance in infants and children and occurs whenever the total output of fluid exceeds the total intake, regardless of the cause. Dehydration may result from a number of diseases that cause insensible losses through the skin and respiratory tract, through increased renal excretion, and through the GI tract. Although dehydration can result from lack of oral intake (especially in elevated environmental temperatures), more often it is a result of abnormal losses, such as those that occur in vomiting or diarrhea, when oral intake only partially compensates for the abnormal losses. Other significant causes of dehydration are diabetic ketoacidosis and extensive burns.
Water Balance in Infants
Compared with older children and adults, infants and young children have a greater need for water and are more vulnerable to alterations in fluid and electrolyte balance. Infants have a greater fluid intake and output relative to size. Water and electrolyte disturbances occur more frequently and more rapidly, and infants and children adjust less promptly to these alterations.
The fluid compartments in the infant vary significantly from those in the adult, primarily because of an expanded extracellular compartment. The extracellular fluid (ECF) compartment constitutes more than half the total body water at birth and has a greater relative content of extracellular sodium and chloride. The infant loses a large amount of fluid at birth and maintains a larger amount of ECF than the adult until about 2 years of age. This contributes to greater and more rapid water loss during this age period.
Fluid losses create compartment deficits that are reflected throughout the duration of dehydration. In general, approximately 60% of fluid is lost from the ECF, and the remaining 40% comes from the intracellular fluid (ICF). The amount of fluid lost from the ECF increases with acute illness and decreases with chronic loss.
Fluid losses vary with age and are divided into insensible, urinary, and fecal losses. Approximately two thirds of insensible losses occur through the skin; the remaining one third is lost through the respiratory tract. Heat and humidity, body temperature, and respiratory rate influence insensible fluid loss. Infants and children have a greater tendency to become highly febrile than do adults. Fever increases insensible water loss approximately 7 ml/kg/24 hr for each degree rise in temperature above 37.2° C (99° F). Fever and increased surface area relative to volume are factors that contribute to greater insensible fluid losses in young patients.
Body Surface Area.: The infant’s relatively greater body surface area (BSA) allows larger quantities of fluid to be lost in insensible perspiration through the skin. It is estimated that the BSA of the preterm neonate is five times greater, and that of the newborn is two to three times greater, than that of the older child or adult. The proportionately longer GI tract in infancy is another source of fluid loss, especially from diarrhea.
Basal Metabolic Rate.: The rate of metabolism in infancy is significantly higher than in adulthood because of the larger BSA in relation to the mass of active tissue. Consequently, there is a greater production of metabolic wastes that must be excreted by the kidneys. Any condition that increases metabolism causes greater heat production, insensible fluid loss, and an increased need for water for excretion. The basal metabolic rate in infants and children is higher to support growth.
Kidney Function.: The kidneys of the infant are functionally immature at birth and are inefficient in excreting waste products of metabolism. Of particular importance for fluid balance is the inability of the infant’s kidneys to concentrate or dilute urine, to conserve or excrete sodium, and to acidify urine. The infant is less able to handle large quantities of solute-free water than is the older child, and infants are more likely to become dehydrated when given concentrated formulas or overhydrated when given excessive water or dilute formula.
Fluid Requirements.: Infants ingest and excrete a greater amount of fluid per kilogram of body weight than do older children. Because electrolytes are excreted with water and the infant has limited ability for conservation, maintenance requirements include both water and electrolytes. The daily exchange of ECF in the infant is greatly increased over that of older children, which leaves the infant little fluid volume reserve in dehydrated states. Fluid requirements depend on hydration status, size, environmental factors, and underlying disease. Box 24-2 lists daily maintenance fluid requirements.
Types of Dehydration
The pathophysiology of dehydration is understood by recognizing that the distribution of water between the ECF and ICF spaces depends on active transport of potassium into and sodium out of cells by energy-requiring processes. Sodium is the chief solute in ECF and is the primary determinant of ECF volume. Potassium is primarily intracellular. When ECF volume is reduced in acute dehydration, the total body sodium content is almost always reduced as well, regardless of serum sodium measurements. Replacement of fluid volume should therefore be accompanied by sodium repletion. Sodium depletion in diarrhea occurs in two ways: out of the body in stool and into the ICF compartment to replace potassium to maintain electrical equilibrium.
Dehydration is classified into three categories on the basis of osmolality and depends primarily on the serum sodium concentration: (1) isotonic, (2) hypotonic, and (3) hypertonic.
Isotonic (isosmotic or isonatremic) dehydration, the primary form of dehydration in children, occurs in conditions in which electrolyte and water deficits are present in approximately balanced proportions. Water and salt are lost in approximately equal amounts. The observable fluid losses are not necessarily isotonic, since losses from other avenues make adjustments so that the sum of all losses, or the net loss, is isotonic. There is no osmotic force between the ICF and the ECF, so the major loss is sustained from the ECF compartment. This significantly reduces the plasma volume and the circulating blood volume, which affects the skin, muscles, and kidneys. Shock is the greatest threat to life, and the child with isotonic dehydration displays symptoms characteristic of hypovolemic shock. Plasma sodium remains within normal limits, between 130 and 150 mEq/L.
Hypotonic (hyposmotic or hyponatremic) dehydration occurs when the electrolyte deficit exceeds the water deficit, leaving the serum hypotonic. Because ICF is more concentrated than ECF in hypotonic dehydration, water moves from the ECF to the ICF to establish osmotic equilibrium. This movement further increases the ECF volume loss, and shock is a frequent finding. Because there is a greater proportional loss of ECF in hypotonic dehydration, the physical signs tend to be more severe with smaller fluid losses than with isotonic or hypertonic dehydration. Serum sodium concentration is less than 130 mEq/L.
Hypertonic (hyperosmotic or hypernatremic) dehydration results from water loss in excess of electrolyte loss and is usually caused by a proportionately larger loss of water or a larger intake of electrolytes. This type of dehydration is the most dangerous and requires more specific fluid therapy. Hypertonic diarrhea may occur in infants who are given fluids by mouth that contain large amounts of solute, or in children who receive high-protein nasogastric (NG) tube feedings that place an excessive solute load on the kidneys. In hypertonic dehydration, fluid shifts from the lesser concentration of the ICF to the ECF. Plasma sodium concentration is greater than 150 mEq/L.
Because the ECF volume is proportionately larger, hypertonic dehydration consists of a greater degree of water loss for the same intensity of physical signs. Shock is less apparent. However, neurologic disturbances, including alterations in consciousness, poor ability to focus attention, lethargy, increased muscle tone with hyperreflexia, and hyperirritability to stimuli, are more likely to occur. Cerebral changes are serious and may result in permanent damage.
Diagnostic Evaluation
Diagnosis of the type and degree of dehydration is necessary to develop an effective plan of therapy. The degree of dehydration has been described as a percentage: 3% to 5% (mild), 6% to 9% (moderate), or more than 10% (severe) (American Academy of Pediatrics, 1996). Water constitutes only 60% to 70% of the infant’s weight. However, adipose tissue contains little water and is highly variable in individual infants and children. A more accurate means of describing dehydration is to reflect acute loss (over 48 hours or less) in milliliters per kilogram of body weight. For example, a loss of 50 ml/kg is considered to be a mild fluid loss, whereas a loss of 100 ml/kg produces severe dehydration. Weight is the most important determinant of the percent of total body fluid loss in infants and younger children. However, often the preillness weight is unknown. Other predictors of fluid loss include a changing level of consciousness (irritability to lethargy), response to stimuli, decreased skin elasticity and turgor, prolonged capillary refill, increased heart rate, and sunken eyes and fontanels. Clinical signs provide clues to the extent of dehydration (Table 24-1). Using multiple predictors increases the sensitivity of assessing the fluid deficit, and early studies have shown a reasonably high degree of agreement between experienced observers in assessment of the level of dehydration. Objective signs of dehydration are present at a fluid deficit of less than 5%. Any two of the following signs—capillary refill of 2 seconds, absent tears, dry mucous membranes, and an ill general appearance—are predictors of a deficit of at least 5%. Generally, three or more clinical findings are present at a deficit of 5% to 9%, and four or more findings are found with a deficit of 10% or more (Steiner, DeWalt, Byerley, and others, 2004). Shock, tachycardia, and very low blood pressure are common features of severe depletion of ECF volume (see Shock, Chapter 25).
TABLE 24-1
Evaluating Extent of Dehydration
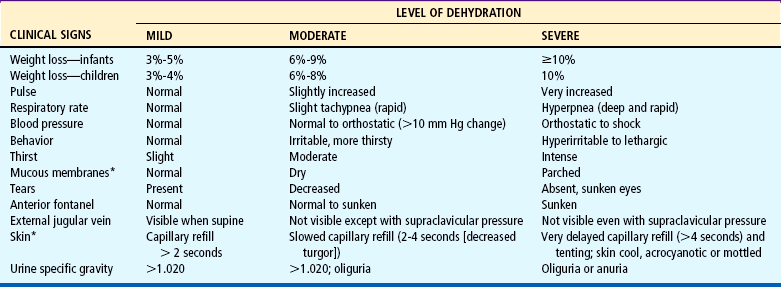
*These signs are less prominent in patients who have hypernatremia.
Data from Jospe N, Forbes G: Fluids and electrolytes—clinical aspects, Pediatr Rev 17(11):395-403, 1996; and Steiner MJ, DeWalt DA, Byerley JS: Is this child dehydrated? JAMA 291(22):2746-2754, 2004.
Nursing Care Management
Nursing observation and intervention are essential for detection and therapeutic management of dehydration. A variety of circumstances cause fluid losses in infants, and changes can take place quickly. An important nursing responsibility is observation for signs of dehydration. Nursing assessment should begin with observation of general appearance and proceed to more specific observations. Conditions in which dehydration may develop quickly include diarrhea; vomiting; sweating; fever; disorders such as diabetes, renal disease, and cardiac anomalies; administration of certain drugs (such as diuretics and steroids); and trauma (major surgery, burns, and other extensive injury).
Intake and Output.: Accurate measurements of fluid intake and output are vital to the assessment of dehydration. This includes oral and parenteral intake and losses from urine, stools, vomiting, fistulas, NG suction, sweat, and wound drainage:
Urine—Frequency, color, consistency, and volume (when weighing diapers, approximately 1 g wet diaper weight equals 1 ml urine)
Stools—Frequency, volume, and consistency
Vomitus—Volume, frequency, and type
Sweating—Can be only estimated from frequency of clothing and linen changes
In addition to fluid intake and output, the following observations assist in assessment of dehydration:
Vital signs—Temperature (normal, elevated, or lowered depending on degree of dehydration), pulse (tachycardia), respirations (hyperpnea), and blood pressure (hypotension)
Skin—Color, temperature, turgor, presence or absence of edema, and capillary refill
Mucous membranes—Moisture, color, and presence and consistency of secretions
Body weight—Decreased in relation to degree of dehydration
For nursing interventions, see discussion under specific disorders.
DISORDERS OF MOTILITY
Diarrhea is a symptom that results from disorders involving digestive, absorptive, and secretory functions. Diarrhea is caused by abnormal intestinal water and electrolyte transport. Worldwide, there are an estimated 1.3 billion episodes of diarrhea each year. Approximately 24% of all deaths in children living in developing countries are related to diarrhea and dehydration. Most children living in developed countries who have gastroenteritis have mild forms. However, in the United States, approximately 200,000 children younger than age 5 are hospitalized and approximately 200 children younger than 5 years die of diarrhea and dehydration each year (Malek, Curns, Holman, and others, 2006; Staat, 2006).
Diarrheal disturbances involve the stomach and intestines (gastroenteritis), the small intestine (enteritis), the colon (colitis), or the colon and intestines (enterocolitis). Diarrhea is classified as acute or chronic.
Acute diarrhea, a leading cause of illness in children younger than 5 years of age, is defined as a sudden increase in frequency and a change in consistency of stools, often caused by an infectious agent in the GI tract. It may be associated with upper respiratory or urinary tract infections, antibiotic therapy, or laxative use. Acute diarrhea is usually self-limited (less than 14 days’ duration) and subsides without specific treatment if dehydration does not occur. Acute infectious diarrhea (infectious gastroenteritis) is caused by a variety of viral, bacterial, and parasitic pathogens (Table 24-2).
TABLE 24-2
Infectious Causes of Acute Diarrhea
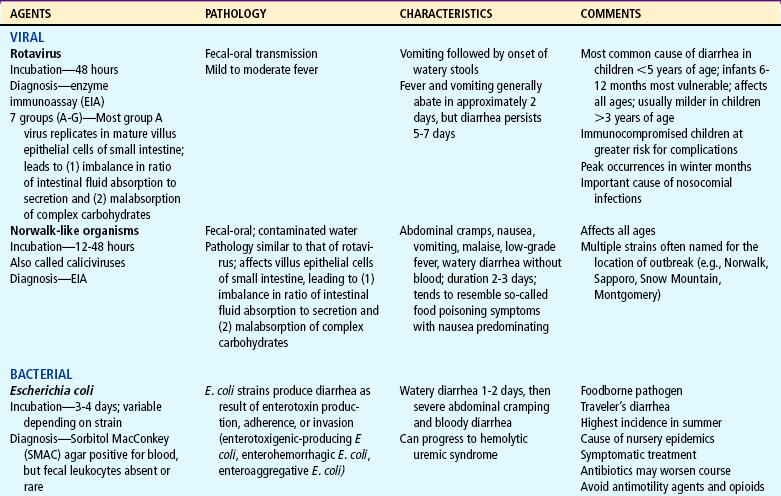
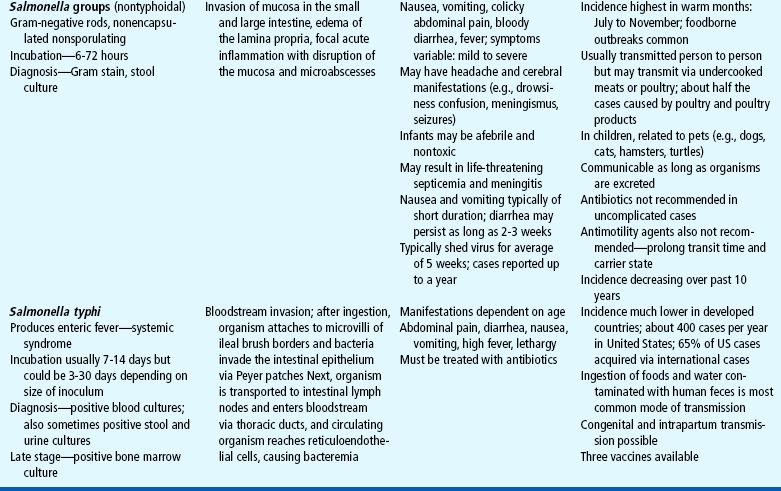
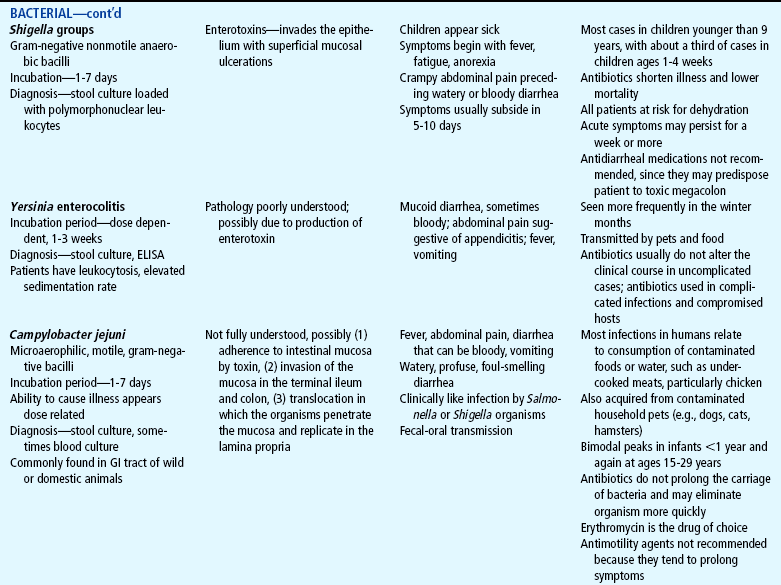

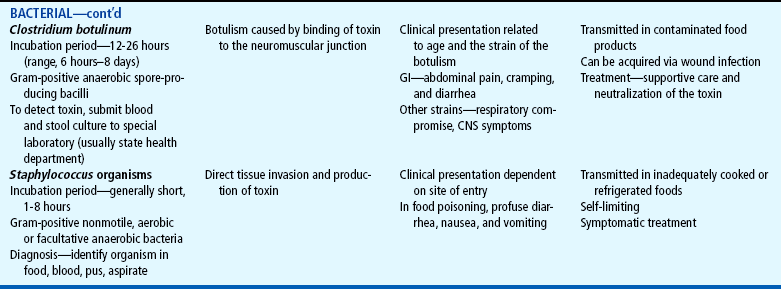
CNS, Central nervous system; ELISA, enzyme-linked immunosorbent assay; GI, gastrointestinal.
Chronic diarrhea is defined as an increase in stool frequency and increased water content with a duration of more than 14 days. It is often caused by chronic conditions such as malabsorption syndromes, inflammatory bowel disease (IBD), immunodeficiency, food allergy, lactose intolerance, or chronic nonspecific diarrhea, or as a result of inadequate management of acute diarrhea.
Intractable diarrhea of infancy is a syndrome that occurs in the first few months of life, persists for longer than 2 weeks with no recognized pathogens, and is refractory to treatment. The most common cause is acute infectious diarrhea that was not managed adequately.
Chronic nonspecific diarrhea (CNSD), also known as irritable colon of childhood and toddlers’ diarrhea, is a common cause of chronic diarrhea in children 6 to 54 months of age. These children have loose stools, often with undigested food particles, and diarrhea greater than 2 weeks’ duration. Children with CNSD grow normally and have no evidence of malnutrition, no blood in their stool, and no enteric infection. Dietary indiscretions and food sensitivities have been linked to chronic diarrhea. The excessive intake of juices and artificial sweeteners such as sorbitol, a substance found in many commercially prepared beverages and foods, may be a factor.
Etiology
Most pathogens that cause diarrhea are spread by the fecal-oral route through contaminated food or water or are spread from person to person where there is close contact (e.g., daycare centers). Lack of clean water, crowding, poor hygiene, nutritional deficiency, and poor sanitation are major risk factors, especially for bacterial or parasitic pathogens. The increased frequency and severity of diarrheal disease in infants is also related to age-specific alterations in susceptibility to pathogens. For example, the immune system of infants has not been exposed to many pathogens and has not acquired protective antibodies. Worldwide, the most common causes of acute gastroenteritis are infectious agents, viruses, bacteria, and parasites. In developed nations, viruses, primarily rotavirus, cause 70% to 80% of infectious diarrhea.
Rotavirus is the most important cause of serious gastroenteritis among children and a significant nosocomial (hospital-acquired) pathogen, accounting for 55,000 to 70,000 hospitalizations annually (Staat, 2006). Rotavirus disease is most severe in children 3 to 24 months of age. Children younger than 3 months of age have some protection from the disease because of maternally acquired antibodies. Approximately 25% of severe cases of rotavirus occur in older children (Coffin, 2001).
Salmonella, Shigella, and Campylobacter organisms are the most frequently isolated bacterial pathogens. Salmonella has the highest occurrence in infants; Giardia and Shigella have the highest incidence among toddlers. Shigella infection is uncommon in the United States, accounting for less than 5% of diarrheal illnesses in infants and toddlers. Campylobacter infection has a bimodal presentation (highest in children less than 12 months of age with a second rise in incidence at age 15 to 19 years). Giardia and Cryptosporidium organisms are parasites. Giardia infection represents 15% of nondysenteric illness in the United States; Cryptosporidium infection is often associated with outbreaks in young children in daycare centers. Plesiomonas and Yersinia are also parasites that are frequently responsible for causing diarrhea that lasts more than 10 days in a previously healthy adolescent. (See also Intestinal Parasitic Diseases, Chapter 14.)
Antibiotic administration is frequently associated with diarrhea because antibiotics alter the normal intestinal flora, resulting in an overgrowth of other bacteria such as Clostridium difficile. National rates of C. difficile have more than doubled since 2000 (Richards, 2006). Antibiotic-associated diarrhea can also be caused by Salmonella organisms, Clostridium porringers type A, and Staphylococcus aureus pathogens (Jabbar and Wright, 2003).
Pathophysiology
Invasion of the GI tract by pathogens results in increased intestinal secretion as a result of enterotoxins, cytotoxic mediators, or decreased intestinal absorption secondary to intestinal damage or inflammation. Enteric pathogens attach to the mucosal cells and form a cuplike pedestal on which the bacteria rest. The pathogenesis of the diarrhea depends on whether the organism remains attached to the cell surface, resulting in a secretory toxin (noninvasive, toxin-producing, noninflammatory type diarrhea), or penetrates the mucosa (systemic diarrhea). Noninflammatory diarrhea is the most common diarrheal illness, resulting from the action of enterotoxin that is released after attachment to the mucosa (Ramaswamy and Jacobson, 2001). The most serious and immediate physiologic disturbances associated with severe diarrheal disease are (1) dehydration, (2) acid-base imbalance with acidosis, and (3) shock that occurs when dehydration progresses to the point that circulatory status is seriously impaired.
Diagnostic Evaluation
Evaluation of the child with acute gastroenteritis begins with a careful history that seeks to discover the possible cause of diarrhea, to assess the severity of symptoms and the risk of complications, and to elicit information about current symptoms indicating other treatable illnesses that could be causing the diarrhea. The history should include questions about recent travel, exposure to untreated drinking or washing water sources, contact with animals or birds, daycare center attendance, recent treatment with antibiotics, or recent diet changes. History questions should also explore the presence or absence of other symptoms such as fever and vomiting, frequency and character of stools (e.g., watery, bloody), urinary output, dietary habits, and recent food intake.
Extensive laboratory evaluation is not indicated in children who have uncomplicated diarrhea and no evidence of dehydration, since most diarrheal illnesses are self-limiting. Laboratory tests are indicated for children who are severely dehydrated and receiving intravenous (IV) therapy. Watery, explosive stools suggest glucose intolerance; foul-smelling, greasy, bulky stools suggest fat malabsorption. Diarrhea that develops after the introduction of cow’s milk, fruits, or cereal may be related to enzyme deficiency or protein intolerance. Neutrophils or red blood cells in the stool indicate bacterial gastroenteritis or IBD. The presence of eosinophils suggests protein intolerance or parasitic infection. Stool cultures should be performed only when blood, mucus, or polymorphonuclear leukocytes are present in the stool, when symptoms are severe, when there is a history of travel to a developing country, and when a specific pathogen is suspected. Gross blood or occult blood may indicate pathogens such as Shigella, Campylobacter, or hemorrhagic Escherichia coli strains. An enzyme-linked immunosorbent assay (ELISA) may be used to confirm the presence of rotavirus or Giardia organisms. If there is a history of recent antibiotic use, the stool should be tested for C. difficile toxin. When bacterial and viral cultures are negative and when diarrhea persists for more than a few days, stools should be examined for ova and parasites. A stool specimen with a pH of less than 6 and the presence of reducing substances may indicate carbohydrate malabsorption or secondary lactase deficiency. Stool electrolyte measurements may help identify children with secretory diarrhea.
Urine specific gravity should be determined if dehydration is suspected. A complete blood count (CBC), serum electrolytes, creatinine, and blood urea nitrogen (BUN) should be obtained in the child who requires hospitalization. The hemoglobin, hematocrit, creatinine, and BUN levels are usually elevated in acute diarrhea and should normalize with rehydration.
Therapeutic Management
The major goals in the management of acute diarrhea include (1) assessment of fluid and electrolyte imbalance, (2) rehydration, (3) maintenance fluid therapy, and (4) reintroduction of an adequate diet. Infants and children with acute diarrhea and dehydration should be treated first with oral rehydration therapy (ORT). ORT is one of the major worldwide health care advances. It is more effective, safer, less painful, and less costly than IV rehydration. The American Academy of Pediatrics, World Health Organization, and Centers for Disease Control and Prevention all recommend ORT as the treatment of choice for most cases of dehydration caused by diarrhea (American Academy of Pediatrics, 2001). Oral rehydration solutions (ORSs) enhance and promote the reabsorption of sodium and water, and studies indicate that these solutions greatly reduce vomiting, volume loss from diarrhea, and the duration of the illness. ORSs, including reduced osmolarity ORS, are available in the United States as commercially prepared solutions and are successful in treating the majority of infants with dehydration. Guidelines for rehydration recommended by the American Academy of Pediatrics are included in Box 24-3.
After rehydration, ORS may be used during maintenance fluid therapy by alternating the solution with a low-sodium fluid such as water, breast milk, lactose-free formula, or half-strength lactose-containing formula. In older children ORS can be given and a regular diet continued. Ongoing stool losses should be replaced on a 1:1 basis with ORS. If the stool volume is not known, approximately 10 ml/kg (4 to 8 ounces) of ORS should be given for each diarrheal stool.
Solutions for oral hydration are useful in most cases of dehydration, and vomiting is not a contraindication. A child who is vomiting should be given an ORS at frequent intervals and in small amounts. For young children the caregiver may give the fluid with a spoon or small syringe in 5- to 10-ml increments every 1 to 5 minutes. An ORS may also be given via NG or gastrostomy tube infusion. Infants without clinical signs of dehydration do not need ORT. They should, however, receive the same fluids recommended for infants with signs of dehydration in the maintenance phase and for ongoing stool losses. The use of probiotics reduces the risk of antibiotic-associated diarrhea in children by 56% (Szajewska, Ruszcynski, and Radzikowski, 2006).
Prevention
Human-bovine reassortant rotavirus vaccine (RotaTeq) became available in 2006, and live-attenuated human rotavirus vaccine (Rotarix) will be available soon for the prevention of rotavirus. Infants should receive three doses of RotaTeq oral vaccine at 2, 4, and 6 months of age. Two doses of Rotarix will induce protective immunity (Marshall, 2006).
Early reintroduction of nutrients is desirable and is gaining more widespread acceptance. Continued feeding or early reintroduction of a normal diet has no adverse effects and actually lessens the severity and duration of the illness and improves weight gain when compared with the gradual reintroduction of foods (Zangwill, 2006). Infants who are breastfeeding should continue to do so, and ORS should be used to replace ongoing losses in these infants. Formula-fed infants should resume their formula; if it is not tolerated, a lactose-free formula may be used for a few days. In older children a regular diet, including milk, can generally be offered after rehydration has been achieved. In toddlers there is no contraindication to continuing soft or pureed foods. A diet of easily digestible foods such as cereals, cooked vegetables, and meats is adequate for the older child.
In cases of severe dehydration and shock, IV fluids are initiated whenever the child is unable to ingest sufficient amounts of fluid and electrolytes to (1) meet ongoing daily physiologic losses, (2) replace previous deficits, and (3) replace ongoing abnormal losses. Patients who usually require IV fluids are those with severe dehydration, those with uncontrollable vomiting, those who are unable to drink for any reason (e.g., extreme fatigue, coma), and those with severe gastric distention.
The IV solution is selected on the basis of what is known regarding the probable type and cause of the dehydration—usually a saline solution containing 5% dextrose in water. Sodium bicarbonate may be added, since acidosis is usually associated with severe dehydration. Although the initial phase of fluid replacement is rapid in both isotonic and hypotonic dehydration, rapid fluid replacement is contraindicated in hypertonic dehydration because of the risk of water intoxication, especially in the brain cells.
After the severe effects of dehydration are under control, specific diagnostic and therapeutic measures are begun to detect and treat the cause of the diarrhea. Because of the self-limiting nature of vomiting and its tendency to improve when dehydration is corrected, the use of antiemetic agents is not recommended. The use of antibiotic therapy in children with acute gastroenteritis is controversial. Antibiotics may shorten the course of some diarrheal illnesses (e.g., those caused by Shigella organisms). However, most bacterial diarrheas are self-limiting, and the diarrhea often resolves before the causative organism can be determined. Antibiotics may prolong the carrier period for bacteria such as Salmonella. Antibiotics may be considered, however, in patients with immunosuppression, severe symptoms, or persistent disease or in patients who have had transplantation (Jabbar and Wright, 2003) (see Intestinal Parasitic Diseases, Chapter 14).
Nursing Care Management
The management of most cases of acute diarrhea takes place in the home with education of the caregiver. Caregivers are taught to monitor for signs of dehydration (especially the number of wet diapers or voidings) and the amount of fluids taken by mouth, and to assess the frequency and amount of stool losses. Education relating to ORT, including the administration of maintenance fluids and replacement of ongoing losses, is important (see Critical Thinking Exercise). ORS should be administered in small quantities at frequent intervals. Vomiting is not a contraindication to ORT unless it is severe. Information concerning the introduction of a normal diet is essential. Parents need to know that a slightly higher stool output initially occurs with continuation of a normal diet and with ongoing replacement of stool losses. The benefits of a better nutritional outcome with fewer complications and a shorter duration of illness outweigh the potential increase in stool frequency. Parents’ concerns should be addressed to ensure adherence to the treatment plan.
If the child with acute diarrhea and dehydration is hospitalized, an accurate weight must be obtained, as well as careful monitoring of intake and output. The child may be placed on parenteral fluid therapy with nothing by mouth (NPO) for 12 to 48 hours. Monitoring the IV infusion is an important nursing function. The nurse must ensure that the correct fluid and electrolyte concentration is infused, that the flow rate is adjusted to deliver the desired volume in a given time, and that the IV site is maintained.
Accurate measurement of output is essential to determine whether renal blood flow is sufficient to permit the addition of potassium to the IV fluids. The nurse is responsible for examination of stools and collection of specimens for laboratory examination (see Collection of Specimens, Chapter 22). Care should be taken when obtaining and transporting stools to prevent possible spread of infection. A clean tongue depressor can be used to obtain specimens for laboratory examination or as an applicator for transfer to a culture medium. Stool specimens should be transported to the laboratory in appropriate containers and media according to hospital policy.
Diarrheal stools are highly irritating to the skin, and extra care is needed to protect the skin of the diaper region from excoriation (see Diaper Dermatitis, Chapter 30). Rectal temperatures are avoided because they stimulate the bowel, increasing passage of stool.
Support for the child and family involves the same care and consideration given all hospitalized children (see Chapter 21). Parents are kept informed of the child’s progress and instructed in the use of frequent and proper hand washing and the disposal of soiled diapers, clothes, and bed linen. Everyone caring for the child must be aware of “clean” areas and “dirty” areas, especially in the hospital, where the sink in the child’s room is used for many purposes. Soiled diapers and linen should be discarded in receptacles close to the bedside. To remind caregivers to keep diapers and other soiled articles away from clean areas, place signs identifying “clean” (e.g., bed table) and “dirty” (e.g., sink, bathroom) areas. List the articles that may be stored in each area on these signs.
Prevention.: The best intervention for diarrhea is prevention. The fecal-oral route spreads most infections, and parents need information about preventive measures such as personal hygiene, protection of the water supply from contamination, and careful food preparation.
Meticulous attention to perianal hygiene, disposal of soiled diapers, proper hand washing, and isolation of infected persons also minimizes the transmission of infection (see Infection Control, Chapter 22).
Parents need information about preventing diarrhea while traveling. They are cautioned against giving their children adult medications that are used to prevent traveler’s diarrhea. Until vaccines or other prophylactic measures are proved to be safe for children, the best measure during travel to areas where water may be contaminated is to allow children to drink only bottled water and carbonated beverages (from the container through a straw supplied from home). Tap water, ice, unpasteurized dairy products, raw vegetables, unpeeled fruits, meats, and seafood should also be avoided.
The expected outcomes are described in the Nursing Process Box.
CONSTIPATION
Constipation is an alteration in the frequency, consistency, or ease of passing stool. Parents often define constipation as passing less than three stools per week. It may also be defined as painful bowel movements, which are often blood streaked, or include the retention of stool, with or without soiling, even with a stool frequency of more than three stools per week (Loening-Baucke and Pashankar, 2006). The frequency of bowel movements, however, is not considered a diagnostic criterion because it varies widely among children. Having extremely long intervals between defecation is termed obstipation. Constipation with fecal soiling is referred to as encopresis.
Constipation may arise secondary to a variety of organic disorders or in association with a wide range of systemic disorders. Structural disorders of the intestine, such as strictures, ectopic anus, and Hirschsprung disease (HD), may be associated with constipation. Systemic disorders associated with constipation include hypothyroidism, hypercalcemia resulting from hyperparathyroidism or vitamin D excess, and chronic lead poisoning. Constipation may be associated with drugs such as antacids, diuretics, antiepileptics, antihistamines, opioids, and iron supplementation. Spinal cord lesions may be associated with loss of rectal tone and sensation. Affected children are prone to chronic fecal retention and overflow incontinence.
The majority of children have idiopathic or functional constipation, since no underlying cause can be identified. Chronic constipation may occur as a result of environmental or psychosocial factors, or a combination of both. Transient illness, withholding and avoidance secondary to painful or negative experiences with stooling, and dietary intake with decreased fluid and fiber all play a role in the etiology of constipation.
Newborn Period
Normally the newborn infant passes a first meconium stool within 24 to 36 hours of birth. Any infant who does not do so should be assessed for evidence of intestinal atresia or stenosis, HD, hypothyroidism, meconium plugs, or meconium ileus. Meconium plugs are caused by meconium that has reduced water content and are usually evacuated after digital examination but may require irrigations with a hypertonic solution or contrast medium.
Meconium ileus, the initial manifestation of cystic fibrosis, is the luminal obstruction of the distal small intestine by abnormal meconium. Treatment is the same as for a meconium plug; early surgical intervention may be needed to evacuate the small intestine.
Infancy
The onset of constipation frequently occurs during infancy and may result from organic causes such as HD, hypothyroidism, and strictures. It is important to differentiate these conditions from functional constipation. Constipation in infancy is often related to dietary practices. It is less common in breast-fed infants, who have softer stools than bottle-fed infants. Breast-fed infants may also have decreased stools because of more complete use of breast milk with little residue. When constipation occurs with a change from human milk or modified cow’s milk to whole cow’s milk, simple measures such as adding or increasing the amount of cereal, vegetables, and fruit in the infant’s diet usually corrects the problem. When a bottle-fed infant passes a hard stool that results in an anal fissure, stool-withholding behaviors may develop in response to pain on defecation (see Critical Thinking Exercise).
Childhood
Most constipation in early childhood is due to environmental changes or normal development when a child begins to attain control over bodily functions. A child who has experienced discomfort during bowel movements may deliberately try to withhold stool. Over time, the rectum accommodates to the accumulation of stool, and the urge to defecate passes. When the bowel contents are ultimately evacuated, the accumulated feces are passed with pain, thus reinforcing the desire to withhold stool.
Constipation in school-age children may represent an ongoing problem or a first-time event. The onset of constipation at this age is often the result of environmental changes, stresses, and changes in toileting patterns. A common cause of new-onset constipation at school entry is fear of using the school bathrooms, which are noted for their lack of privacy. Early and hurried departure for school immediately after breakfast may also impede bathroom use.
The management of simple constipation consists of a plan to promote regular bowel movements. Often this is as simple as changing the diet to provide more fiber and fluids, eliminating foods known to be constipating, and establishing a bowel routine that allows for regular passage of stool. Stool-softening agents such as docusate or lactulose may also be helpful. Polyethylene glycol (PEG) 3350 without electrolytes (Miralax) is a chemically inert polymer that has been introduced as a new laxative in recent years. It is tolerated well by children because it can be mixed in a beverage of choice (Loening-Baucke and Pashankar, 2006). If other symptoms such as vomiting, abdominal distention, or pain and evidence of growth failure are associated with the constipation, the condition should be investigated further.
Nursing Care Management
Constipation tends to be self-perpetuating. A child who has difficulty or discomfort when attempting to evacuate the bowels has a tendency to retain the bowel contents, and this may initiate a vicious circle. Nursing assessment begins with an accurate history of bowel habits; diet; events associated with the onset of constipation; drugs or other substances that the child may be taking; and the consistency, color, frequency, and other characteristics of the stool. If there is no evidence of a pathologic condition, the major task is to educate the parents regarding normal stool patterns and to participate in the education and treatment of the child.
Dietary modifications are essential in preventing constipation. During infancy, simply increasing the carbohydrate (sugar or corn syrup) in the infant’s formula will often relieve the problem. During childhood the diet should contain increased amounts of fiber and fluid. Parents benefit from guidance in selecting foods that facilitate bowel movements (Box 24-4). They need reassurance concerning the benign nature of the condition. It is also important to discuss their attitudes and expectations regarding toilet habits.
When constipation persists despite dietary intervention, more aggressive management may be necessary. It is important to differentiate an acute episode of constipation from chronic functional constipation, which can result from chronic stool-withholding behavior. As the rectal vault becomes distended over time, further complications such as fecal impaction and encopresis may develop (see Chapter 17).
HIRSCHSPRUNG DISEASE
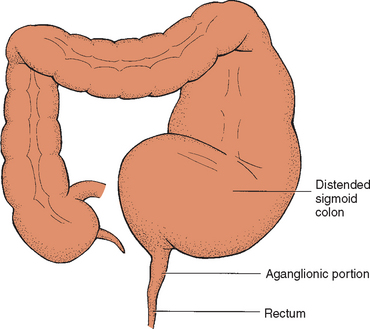
HD (congenital aganglionic megacolon) is a mechanical obstruction caused by inadequate motility of part of the intestine. It accounts for about one fourth of all cases of neonatal obstruction, although it may not be diagnosed until later in infancy or childhood. The incidence is 1 in 5000 live births (Dasgupta and Langer, 2004). It is four times more common in males than in females and may follow a familial pattern in about 10% of cases. HD is usually an isolated birth defect, but it has been associated with other syndromes, including Down syndrome. Mutations in the RET proto-oncogene have been found in 17% to 38% of children with short-segment HD and in 70% to 80% of those with long-segment involvement (Dasgupta and Langer, 2004). Depending on its presentation, it may be an acute, life-threatening, or chronic condition.
Pathophysiology
HD is a developmental disorder of the enteric nervous system that is characterized by the absence of ganglion cells, originating in from the neural crest, in both the Auerbach myenteric and Meissner submucosal plexuses of the distal intestine. The length of the aganglionic distal bowel depends on the timing of the arrest in craniocaudal migration of ganglion cells. The aganglionic bowel is chronically contracted. This results in absent peristalsis in the affected bowel and the development of a functional intestinal obstruction (Fig. 24-1) (Dasgupta and Langer, 2004). Intestinal distention and ischemia may also occur as a result of distention of the bowel wall, which contributes to the development of enterocolitis (inflammation of the small bowel and colon). Enterocolitis is characterized by fever, abdominal distention, and diarrhea that may be severe and lead to life-threatening dehydration or sepsis (Dasgupta and Langer, 2004).
Diagnostic Evaluation
Most children with HD are diagnosed in the first few months of life. Clinical manifestations vary according to the age when symptoms are recognized and the presence of complications, such as enterocolitis (Box 24-5). A neonate usually is seen with distended abdomen, feeding intolerance with bilious vomiting, and delay in the passage of meconium. Typically, 95% of normal term infants pass meconium in the first 24 hours of life, whereas less than 10% of infants with HD do so. In older children, a careful history is helpful. Radiographs, an unprepped barium enema, and anorectal manometric examinations assist in the differential diagnosis, which is confirmed by a full-thickness rectal biopsy demonstrating the absence of ganglion cells in the myenteric and submucosal plexuses.
Therapeutic Management
Treatment is primarily surgery to remove the aganglionic portion of the bowel to relieve obstruction and restore normal bowel motility and function of the internal anal sphincter. If the bowel is not significantly distended, this is accomplished in one surgery. However, in most cases two stages are required. First, a temporary ostomy is created proximal to the aganglionic segment to relieve obstruction and allow the normally enervated and dilated bowel to return to its normal size. Complete corrective surgery is performed later. The various surgical procedures that can be performed are the Swenson, Duhamel, Boley, and Soave procedures. The Soave endorectal pull-through procedure, one of the most frequently used procedures, consists of pulling the end of the normal bowel through the muscular sleeve of the rectum, from which the aganglionic mucosa has been removed. The ostomy is usually closed at the time of the pull-through procedure.
Prognosis.: Most children with HD require surgery rather than medical therapy. Once the child is stabilized with fluid and electrolyte replacement, if needed, the temporary colostomy is performed and has a high rate of success. After the later pull-through procedure, anal stricture and incontinence are potential complications, requiring further therapy, including dilation or bowel-retraining therapy.
Nursing Care Management
The nursing concerns depend on the child’s age and the type of treatment. If the disorder is diagnosed during the neonatal period, the main objectives are (1) to help the parents adjust to a congenital defect in their child, (2) to foster infant-parent bonding, (3) to prepare them for the medical-surgical intervention, and (4) to assist them in colostomy care after discharge.
Preoperative Care.: The child’s preoperative care depends on the age and clinical condition. A child who is malnourished may not be able to withstand surgery until his or her physical status improves. Often this involves symptomatic treatment with enemas; a low-fiber, high-calorie, and high-protein diet; and in severe situations the use of total parenteral nutrition (TPN).
Physical preoperative preparation includes the same measures that are common to any surgery (see Surgical Procedures, Chapter 22). In the newborn, whose bowel is sterile, no additional preparation is necessary. However, in other children, preparation for the pull-through procedure involves emptying the bowel with repeated saline enemas and decreasing bacterial flora with oral or systemic antibiotics and colonic irrigations using antibiotic solution. Enterocolitis is the most serious complication of HD. Emergency preoperative care includes frequent monitoring of vital signs and blood pressure for signs of shock; monitoring fluid and electrolyte replacements, as well as plasma or other blood derivatives; and observing for symptoms of bowel perforation, such as fever, increasing abdominal distention, vomiting, increased tenderness, irritability, dyspnea, and cyanosis.
Because progressive distention of the abdomen is a serious sign, the nurse measures abdominal circumference with a paper tape measure, usually at the level of the umbilicus or at the widest part of the abdomen. The point of measurement is marked with a pen to ensure reliability of subsequent measurements. Abdominal measurement can be obtained with the vital sign measurements and is recorded in serial order so that any change is obvious. To reduce stress to the acutely ill child when frequent measurements of abdominal circumference are needed, the tape measure can be left in place beneath the child rather than removed each time.
The child’s age dictates the type and extent of psychologic preparation. Because a colostomy is usually performed, the child who is of preschool age is told about the procedure in concrete terms with the use of visual aids (see Chapter 22). It is important to time explanations appropriately to prevent the anxiety and confusion that could result from too much information. It is also important to stress to parents and older children that the colostomy for HD is temporary, unless so much bowel is involved that a permanent ileostomy must be performed. In most instances the extent of bowel resection is known before surgery, although the nurse should be aware of cases when doubt exists concerning repair. The nurse should remember that although a temporary colostomy is favorable in terms of future health and adjustment, it requires additional surgery, which may be stressful to parents and children.
Postoperative Care.: Postoperative care is the same as that for any child or infant with abdominal surgery (see Surgical Procedures, Chapter 22). When a colostomy is part of the corrective procedure, stomal care is a major nursing task (see Ostomies, Chapter 22). To prevent contamination of an infant’s abdominal wound with urine, the diaper should be pinned below the dressing. Sometimes a Foley catheter is used in the immediate postoperative period to divert the flow of urine away from the abdomen.
Discharge Care.: After surgery, parents need instruction concerning colostomy care. Even a preschooler can be included in the care by handing articles to the parent, rolling up the colostomy pouch after it is emptied, or applying barrier preparations to the surrounding skin. Although the diagnosis of HD is less frequent in school-age children or adolescents, children this age can often be involved in colostomy care to the point of total responsibility.
Some institutions and communities have enterostomal therapists who provide expert assistance in planning home care. If families require financial assistance and psychologic support, referral to a social worker, home health care agency, or community health nurse provides continuity of care.
VOMITING
Vomiting is the forceful ejection of gastric contents through the mouth. It is a well-defined, complex, coordinated process that is under central nervous system control and is often accompanied by nausea and retching. Vomiting may be divided into two categories: nonbilious and bilious. Some small intestinal reflux is common in all vomiting. In nonbilious vomiting, the majority of bile drains into the more distal portions of the intestine. If an obstruction is present, nonbilious vomiting suggests a more proximal obstruction. Bilious vomiting implies a disorder of motility or distal physical blockage. Causes of nonbilious vomiting include infectious, inflammatory, metabolic or endocrinologic, neurologic, and psychologic causes and obstructive lesions. Causes of bilious vomiting include intestinal atresia and stenosis, malrotation with or without volvulus, ileus, intussusceptions, intestinal duplication, mass lesions, incarcerated inguinal hernia, and appendicitis. Vomiting may also be associated with other processes, including acute infectious diseases, increased intracranial pressure, toxic ingestions, food intolerances and allergies, mechanical obstruction of the GI tract, metabolic disorders, and psychogenic problems. Vomiting is common in childhood, is usually self-limiting, and requires no specific treatment. However, complications may occur, including dehydration and electrolyte disturbances, malnutrition, aspiration, and Mallory-Weiss syndrome (small tears in the distal esophageal mucosa).
Therapeutic Management
Management is directed toward detection and treatment of the cause of the vomiting and prevention of complications from the loss of fluid. Fluids are administered in the same manner and in a similar electrolyte composition to those administered for diarrhea. Although most children respond to these measures, antiemetic drugs may be needed. Antiemetics such as ondansetron (Zofran) and trimethobenzamide (Tigan) block receptors in the chemoreceptor trigger zone; others such as metoclopramide (Reglan) enhance gastroduodenal peristalsis; still others such as promethazine (Phenergan) compete for H1-receptor sites. For children who are prone to motion sickness, it is helpful to administer an appropriate dose of dimenhydrinate (Dramamine) before a trip.
Nursing Care Management
The major focus of nursing care is observation and reporting of vomiting behavior and associated symptoms and the implementation of measures to reduce the vomiting. Accurate assessment of the type of vomiting, the appearance of the vomitus, and the child’s behavior in association with the vomiting helps to establish a diagnosis.
Nursing interventions are determined by the cause of the vomiting. When the vomiting is a manifestation of improper feeding methods, establishing proper techniques through teaching and example will usually correct the situation. If vomiting is believed to be an indication of obstruction, food is usually withheld or special feeding techniques are implemented. In situations in which vomiting is related to concurrent infection, dietary indiscretion, or emotional factors, efforts are directed toward maintaining hydration or preventing dehydration.
The thirst mechanism is the most sensitive guide to fluid needs, and ad libitum administration of a glucose-electrolyte solution to an alert child will restore water and electrolytes satisfactorily. It is important to include carbohydrate to spare body protein and avoid ketosis resulting from exhaustion of glycogen stores. Small, frequent feedings of fluids or foods are preferred. After vomiting has stopped, more liberal amounts of fluids are offered, followed by gradual resumption of the regular diet.
The vomiting infant or child is positioned on the side or semireclining to prevent aspiration and observed for evidence of dehydration. It is important to emphasize the need for the child to brush the teeth or rinse the mouth after vomiting to dilute hydrochloric acid that comes in contact with the teeth. A flavored mouthwash or tooth brushing will freshen the mouth. Careful monitoring of fluid and electrolyte status is necessary to prevent an electrolyte disturbance.
GASTROESOPHAGEAL REFLUX
Gastroesophageal reflux (GER) is defined as the transfer of gastric contents into the esophagus. This phenomenon is physiologic, occurring throughout the day, most frequently after meals and at night; therefore it is important to differentiate GER from gastroesophageal reflux disease (GERD). GERD represents symptoms or tissue damage that result from GER. Approximately 50% of infants less than 2 months old are reported to have GER (Suwandhi, Ton, and Schwarz, 2006). This “physiologic” GER usually resolves spontaneously by 1 year of age. GER becomes a disease when complications such as failure to thrive, bleeding, or dysphagia develop. GERD is associated with respiratory symptoms, including apnea, bronchospasm, laryngospasm, and pneumonia (Zeiter and Hyams, 1999). Heartburn is also a frequent symptom in children who are able to describe it (Box 24-6). Certain conditions predispose children to a high prevalence of GERD, including neurologic impairment, hiatal hernia, repaired esophageal atresia, and morbid obesity (Suwandhi, Ton, and Schwarz, 2006).
Sandifer syndrome is an uncommon condition, usually occurring in young children, characterized by repetitive stretching and arching of the head and neck that can be mistaken for a seizure. This maneuver likely represents a physiologic neuromuscular response attempting to prevent acid refluxate from reaching the upper portion of the esophagus (Cavataio and Guandalini, 2005).
Pathophysiology
Although the pathogenesis of GER is multifactorial, its primary causative mechanism likely involves inappropriate transient relaxation of the lower esophageal sphincter (LES) (Suwandhi, Ton, and Schwarz, 2006). Factors that increase abdominal pressure such as coughing and sneezing, scoliosis, and overeating may contribute to GERD. Esophageal symptoms are caused by inflammation from the acid in the gastric refluxate, whereas reactive airway disease may result from stimulation of airway reflexes by the acid refluxate.
Diagnostic Evaluation
The history and physical examination are usually sufficiently reliable to establish the diagnosis of GER. However, the upper GI series is helpful in evaluating the presence of anatomic abnormalities (e.g., pyloric stenosis, malrotation, annular pancreas, hiatal hernia, esophageal stricture). The 24-hour intraesophageal pH monitoring study is the gold standard in the diagnosis of GER (Suwandhi, Ton, and Schwarz, 2006). Endoscopy with biopsy may be helpful to assess the presence and severity of esophagitis, strictures, and Barrett esophagus and to exclude other disorders such as Crohn disease. Scintigraphy detects radioactive substances in the esophagus after a feeding of the compound and assesses gastric emptying. It can differentiate between aspiration of gastric contents from reflux vs aspiration from poor oropharyngeal muscle coordination.
Therapeutic Management
Therapeutic management of GER depends on its severity. No therapy is needed for the infant who is thriving and has no respiratory complications. Avoidance of certain foods that exacerbate acid reflux (e.g., caffeine, citrus, tomatoes, alcohol, peppermint, spicy or fried foods), lifestyle modifications in children (e.g., weight control if indicated; small, more frequent meals; smoking cessation), and feeding maneuvers in infants (e.g., thickened feedings, upright positioning) can improve mild GER symptoms. Thickened feedings do not improve pH scores on 24-hour intraesophageal monitoring but may decrease the number of vomiting episodes. Feedings thickened with 1 teaspoon to 1 tablespoon of rice cereal per ounce of formula may be recommended. This may benefit infants who are underweight as a result of GERD. Constant NG feedings may be necessary for the infant with severe reflux and failure to thrive until surgery can be performed. Elevating the head of the bed 30 degrees or placing the infant in an infant seat elevated 30 degrees for 1 hour after feedings may decrease GER. Prone positioning of infants also decreases episodes of GER but is recommended only with extreme caution when the risk of GERD complications exceeds the risk of sudden infant death syndrome (Cavataio and Guandalini, 2005). The American Academy of Pediatrics recommends supine positioning for sleep (see Chapter 11). If the prone position is used, parents need to be cautioned to avoid soft bedding.
Pharmacologic therapy may be used to treat infants and children with GERD. Both H2-receptor antagonists (cimetidine [Tagamet], ranitidine [Zantac], or famotidine [Pepcid]) and proton pump inhibitors (PPIs; esomeprazole [Nexium], lansoprazole [Prevacid], omeprazole [Prilosec], pantoprazole [Protonix], and rabeprazole [Aciphex]) reduce gastric hydrochloric acid secretion and may stimulate some increase in LES tone. Use of available prokinetic drugs (e.g., bethanechol [Urecholine] and metoclopramide) remains controversial. Careful analyses of published data have failed to demonstrate clinical efficacy in modifying the natural history or therapeutic outcomes of GER in childhood (Suwandhi, Ton, and Schwartz, 2006).
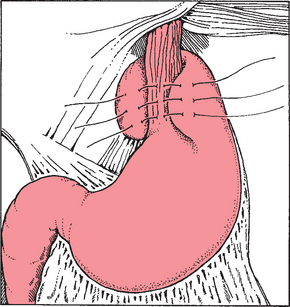
Surgical management of GER is reserved for children with severe complications such as recurrent aspiration pneumonia, apnea, severe esophagitis, or failure to thrive, and for children who have failed to respond to medical therapy. The Nissen fundoplication (Fig. 24-2) is the most common surgical procedure (Christian and Buyske, 2005). This surgery involves passage of the gastric fundus behind the esophagus to encircle the distal esophagus. The most recent surgical advance is the introduction of the laparoscopic Nissen fundoplication (Jackson, Gleiber, Askar, and others, 2001). Complications following fundoplication include breakdown of the wrap, small bowel obstruction, gas-bloat syndrome, infection, retching, and dumping syndrome (Rudolph, Mazur, Liptak, and others, 2001).
Nursing Care Management
Nursing care is directed at (1) identifying children with symptoms; (2) educating parents regarding home care, including feeding, positioning, and medications; and (3) if appropriate, providing care for the child undergoing surgical repair (see Surgical Procedures, Chapter 22). Early in the treatment program, parents should be reassured that most infants and children outgrow GER and often conservative lifestyle changes are sufficient. Parents need support and reassurance to implement lifestyle changes. Although it is not known if lifestyle changes bring additional benefit to patients receiving pharmacologic interventions, some changes may be helpful. To help parents cope with the inconvenience of dealing with a child who spits up frequently, simple measures such as using bibs and protective cloths during and after feedings are beneficial. Older children and adolescents need to know that caffeine, chocolate, and spicy foods may weaken the LES and aggravate symptoms. Exposure to tobacco and alcohol are also associated with GER. Obesity increases abdominal pressure, and weight management may reduce GER symptoms. When medical management is necessary, parents need information about the medications and their potential side effects. Prokinetic medications must be given before feedings. Medications for acid control must be timed to provide coverage and given regularly and as ordered.
FUNCTIONAL ABDOMINAL PAIN DISORDERS
Functional abdominal pain disorders include functional abdominal pain (FAP), irritable bowel syndrome (IBS), and abdominal migraine (Saps and Li, 2006). FAP is defined as nearly continuous abdominal pain in school-aged children or adolescents with only occasional relation of pain to eating, menses, or defecation. This pain may be accompanied by dizziness, headache, nausea, and vomiting. IBS is defined as abdominal pain associated with bowel movements. Patients may experience diarrhea, constipation, mucus in stools, straining, urgency, and/or a sense of incomplete evacuation. As many as 16% of all high school students and 6% of all middle school students report symptoms consistent with IBS (Saps, Sztainberg, DiLorenzo, and others, 2005). Abdominal migraine is characterized by discrete, paroxysmal episodes of severe abdominal pain between which the child is completely normal (Li and Balint, 2000). The child may have an aura and photophobia.
Etiology
There is a higher seasonal prevalence of abdominal pain symptoms during winter months, suggesting that school-related stressors and daylight hours may play a role. Also, an increased prevalence has been found among first-degree relatives.
Pathophysiology
Although the pathogenesis of functional abdominal pain disorders is incompletely understood, most investigators agree on a multifactorial etiology and the presence of an altered brain-gut interaction (Saps and Li, 2006). Children with FAP exhibit generalized visceral hyperanalgesia, whereas those with IBS exhibit rectal hyperanalgesia (DiLorenzo, Youssef, Sigurdsson, and others, 2001). All may have abnormal GI motility.
Diagnostic Evaluation
Exclusion of an organic condition can usually be accomplished through screening diagnostic tests such as CBC; erythrocyte sedimentation rate (ESR); liver transaminases; celiac antibodies; urinalysis; and stool examination for blood, ova, or parasites (Saps and Li, 2006).
Therapeutic Management
Alleviating symptoms is the main goal when treating children with functional abdominal pain disorders because complete eradication of pain often is impossible. Children may experience reduced pain with the use of anticholinergics, tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), or serotonin receptor modulators. Children with FAP may benefit from a smooth muscle relaxant. Children with constipation-dominant IBS may find relief by increasing fiber combined with a laxative as necessary, whereas those with diarrhea-dominant IBS may experience relief by increasing fiber and adding an antidiarrheal medication as necessary. Antimigraine therapy is often used to prevent recurrences of abdominal migraine or to abort attacks.
Nursing Care Management
It is important to validate the child’s symptoms by stating that the pain is “real,” not just “in the head” (Saps and Li, 2006). In addition to medications, children with functional abdominal pain disorders may benefit from relaxation training. The goals are to return the child to functional status, attending school and extracurricular activities.
INFLAMMATORY DISORDERS
Appendicitis, inflammation of the vermiform appendix (blind sac at the end of the cecum), is the most common cause of emergency abdominal surgery in childhood. In the United States, 60,000 to 80,000 cases are diagnosed each year. The average age of children with appendicitis is 10 years, with boys and girls equally affected before puberty. Classically, the first symptom of appendicitis is periumbilical pain, followed by nausea, right lower quadrant pain, and later vomiting with fever (Kwok, Kim, and Gorelick, 2004). Perforation of the appendix can occur within approximately 48 hours of the initial complaint of pain. At the time of initial presentation, about one third of all cases involve an already perforated appendix. Complications from appendiceal perforation include major abscess, phlegmon, enterocutaneous fistula, peritonitis, and partial bowel obstruction (Kwok, Kim, and Gorelick, 2004). A phlegmon is an acute suppurative inflammation of subcutaneous connective tissue that spreads.
Etiology
The cause of appendicitis is obstruction of the lumen of the appendix, usually by hardened fecal material (fecalith). Swollen lymphoid tissue, frequently occurring after a viral infection, can also obstruct the appendix. Another rare cause of obstruction is a parasite such as Enterobius vermicularis, or pinworms, which can obstruct the appendiceal lumen.
Pathophysiology
With acute obstruction, the outflow of mucus secretions is blocked and pressure builds within the lumen, resulting in compression of blood vessels. The resulting ischemia is followed by ulceration of the epithelial lining and bacterial invasion. Subsequent necrosis causes perforation or rupture with fecal and bacterial contamination of the peritoneal cavity. The resulting inflammation spreads rapidly throughout the abdomen (peritonitis), especially in young children, who are unable to localize infection. Progressive peritoneal inflammation results in functional intestinal obstruction of the small bowel (ileus) because intense GI reflexes severely inhibit bowel motility. Because the peritoneum represents a major portion of total body surface, the loss of ECF to the peritoneal cavity leads to electrolyte imbalance and hypovolemic shock.
Diagnostic Evaluation
Diagnosis is not always straightforward. Fever, vomiting, abdominal pain, and an elevated white blood count are associated with appendicitis but are also seen in IBD, pelvic inflammatory disease, gastroenteritis, urinary tract infection, right lower lobe pneumonia, mesenteric adenitis, Meckel diverticulum, and intussusception. Prolonged symptoms and delayed diagnosis often occur in younger children, in whom the risk of perforation is greatest because of their inability to verbalize their complaints.
The diagnosis is based primarily on the history and physical examination (Box 24-7). Pain, the cardinal feature, is initially generalized (usually periumbilical); however, it usually descends to the lower right quadrant. The most intense site of pain may be at McBurney point, located at a point midway between the anterior superior iliac crest and the umbilicus. Rebound tenderness is not a reliable sign and is extremely painful to the child. Referred pain, elicited by light percussion around the perimeter of the abdomen, indicates peritoneal irritation. Movement, such as riding over bumps in an automobile or gurney, aggravates the pain. In addition to pain, significant clinical manifestations include fever, a change in behavior, anorexia, and vomiting.
Laboratory studies usually include a CBC; urinalysis (to rule out a urinary tract infection); and, in adolescent females, serum human chorionic gonadotropin (to rule out an ectopic pregnancy). A white blood cell (WBC) count greater than 10,000/mm3 and a C-reactive protein (CRP) are common but are not necessarily specific for appendicitis. An elevated percentage of bands (often referred to as “a shift to the left”) may indicate an inflammatory process. CRP is an acute-phase reactant that rises within 12 hours of the onset of infection.
Computed tomography (CT) scan has become the imaging technique of choice, although ultrasound may also be helpful in diagnosing appendicitis. A CT scan is considered positive in the presence of enlarged appendiceal diameter; appendiceal wall thickening; and periappendiceal inflammatory changes, including fat streaks, phlegmon, fluid collection, and extraluminal gas (Kaiser, Frenchner, and Jorulf, 2002).
Therapeutic Management
Treatment of appendicitis before perforation includes rehydration, antibiotics, and surgical removal of the appendix (appendectomy). Laparoscopic surgery is now commonly used to treat nonperforated acute appendicitis (Holcomb, 2001). Recovery is rapid, and, if no complications occur, the hospital stay is short.
Ruptured Appendix.: Management of the child diagnosed with peritonitis caused by a ruptured appendix often begins preoperatively with IV administration of fluid and electrolytes, systemic antibiotics, and NG suction. Postoperative management includes IV fluids, continued administration of antibiotics, and NG suction for abdominal decompression until intestinal activity returns. Sometimes surgeons close the wound after irrigation of the peritoneal cavity. Other times, they leave the wound open (delayed closure) to prevent wound infection. A Penrose drain may be used to permit transperitoneal drainage.
Prognosis.: Complications are uncommon after a simple appendectomy. The mortality rate for perforating appendicitis has improved from nearly certain death a century ago to 1% or less at present (Strahlman, 2001). Early recognition of the illness is essential to prevent complications.
Nursing Care Management
Because abdominal pain is the most common childhood complaint with appendicitis, it is important to assess the severity of pain (see Pain Assessment, Chapter 7). One of the most reliable estimates is the degree of change in behavior. The younger, nonverbal child will assume a rigid, motionless, side-lying posture with the knees flexed on the abdomen, and there is decreased range of motion of the right hip. Older children may exhibit all of these behaviors while complaining of abdominal pain. They can always indicate a point at which the pain is worse than at any other location.
Postoperative Care.: Postoperative care for the nonperforated appendix is the same as for most abdominal procedures. Care of the child with a ruptured appendix and peritonitis involves more complex care, and the course of recovery is considerably longer (usually 7 to 10 days of hospitalization). The child is maintained on IV fluids, NPO, and the NG tube is kept on low continuous gastric decompression until there is evidence of intestinal activity. Listening for bowel sounds and observing for other signs of bowel activity (e.g., passage of stool) are part of the routine assessment. Management of IV therapy is the same as for any child receiving fluids and parenteral antibiotics. A drain is often placed in the wound during surgery, and frequent dressing changes with meticulous skin care are essential to prevent excoriation of the area surrounding the surgical site. Wound care includes irrigation with antibacterial solution.
Management of pain from the incision and repeated dressing changes and irrigations are an essential part of the child’s care. Psychologic care of the child and parents is similar to that used in other emergency situations (see Emergency Admission, Chapter 21). Parents and older children need to express their feelings and concerns regarding the events surrounding the illness and hospitalization. The nurse can provide education and psychosocial support to promote adequate coping and alleviate anxiety for both the child and the family (see Nursing Care Plan).
MECKEL DIVERTICULUM
Meckel diverticulum is a remnant of the fetal omphalomesenteric duct that connects the yolk sac with the primitive midgut during fetal life. Normally this structure is obliterated by the fifth to seventh week of gestation, when the placenta replaces the yolk sac as the source of nutrition for the fetus (Sagar, Kumar, and Shah, 2006). Failure of obliteration may result in an omphalomesenteric fistula, a fibrous band connecting the small intestine to the umbilicus, known as Meckel diverticulum.
Meckel diverticulum is a true diverticulum because it arises from the antimesenteric border of the small intestine and contains all layers of the intestinal wall with a separate blood supply from the vitelline artery. The diverticulum is usually found within 100 cm (40 inches) of the ileocecal valve and averages 1 to 10 cm (0.4 to 4 inches) in length.
Meckel diverticulum is the most common congenital malformation of the GI tract and is present in 2% to 4% of the population (Sagar, Kumar, and Shah, 2006). Its occurrence in males and females is equal, but the incidence of complications is three to four times greater in males. Most symptomatic cases are seen in childhood. Patients requiring surgery are generally less than 10 years of age, and about 50% are less than 2 years of age (Sagar, Kumar, and Shah, 2006).
Pathophysiology
The symptomatic complications of Meckel diverticulum are ulceration, bleeding, intussusception, intestinal obstruction, diverticulitis, and perforation; bleeding is the most common problem in children. Gastric mucosa is the most common ectopic tissue found in Meckel diverticulum. Bleeding is caused by peptic ulceration or perforation because of the unbuffered acidic gastric secretion. Several mechanisms can cause obstruction. Intussusception may be led by the diverticulum. Obstruction may also be caused by entanglement of the small intestine around a fibrous cord, trapping of a loop of intestine under the band, incarceration within a hernia sac, or volvulus of the intestinal segment containing the diverticulum. Diverticulitis occurs when peptic ulceration or obstruction leads to inflammation.
Diagnostic Evaluation
Diagnosis is usually based on the history, physical examination, and a specialized radiographic study. The most common clinical presentation in children includes painless rectal bleeding, abdominal pain, or signs of intestinal obstruction (Box 24-8). Bleeding, which may be mild or profuse, often appears as dark red or “currant jelly” stools; bleeding may be significant enough to cause hypotension. The Meckel scan, a technetium-99m pertechnate scan, detects the presence of gastric mucosa with an overall diagnostic accuracy of 90%. Blood studies are performed to screen for bleeding disorders and anemia.
Therapeutic Management
The standard treatment is surgical removal of the diverticulum. When severe hemorrhage increases the surgical risk, interventions to correct hypovolemic shock, such as blood replacement, IV fluids, and oxygen, may be necessary. Antibiotics may be used preoperatively to control infection. If intestinal obstruction has occurred, appropriate preoperative measures are used to reverse electrolyte imbalances and prevent abdominal distention.
Nursing Care Management
Nursing objectives are similar to those for any child undergoing surgery (see Chapter 22). Because the onset of this condition is often rapid, parents require psychologic support. The massive intestinal bleeding that can accompany a Meckel diverticulum is traumatic to both the child and the parents and may significantly affect their emotional reaction to hospitalization and surgery.
Specific preoperative considerations with intestinal bleeding include (1) frequent monitoring of vital signs and blood pressure for shock, (2) keeping the child on bed rest, and (3) recording the approximate amount of blood lost in stools. In the absence of frank hemorrhage, the nurse tests the stools for occult blood. Postoperatively, the child requires IV fluids and an NG tube for the decompression and evacuation of gastric contents.
INFLAMMATORY BOWEL DISEASE
Inflammatory bowel disease (IBD) is a term that is used for two forms of chronic intestinal inflammation: ulcerative colitis (UC) and Crohn disease (CD). Although UC and CD have similar epidemiologic, immunologic, and clinical features, there are important differences (Table 24-3).
TABLE 24-3
Clinical Manifestations of Inflammatory Bowel Diseases
| CHARACTERISTICS | ULCERATIVE COLITIS | CROHN DISEASE |
| Rectal bleeding | Common | Uncommon |
| Diarrhea | Often severe | Moderate to severe |
| Pain | Less frequent | Common |
| Anorexia | Mild or moderate | May be severe |
| Weight loss | Moderate | May be severe |
| Growth retardation | Usually mild | May be severe |
| Anal and perianal lesions | Rare | Common |
| Fistulas and strictures | Rare | Common |
| Rashes | Mild | Mild |
| Joint pain | Mild to moderate | Mild to moderate |
GI symptoms, extraintestinal and systemic inflammatory responses, and exacerbations and remissions without complete resolution characterize these diseases. Growth failure, particularly common in CD, is an important problem unique to the pediatric population. CD is more disabling, has more serious complications, and has less effective medical and surgical treatment than UC. Because UC is confined to the colon, theoretically it may be cured with a colectomy. Over the past 30 years, the incidence of CD has risen, whereas the incidence of UC in children has remained stable (Silbermintz and Markowitz, 2006). The incidence of UC in children has been estimated as 3.5 new cases per 100,000 per year; the incidence of CD is 3.11 per 100,000 per year (Jackson and Grand, 1999).
Etiology
Despite decades of research, the etiology of IBD is not completely understood, and there is no known cure. There is evidence to indicate a multifactorial etiology. Research is focused on theories of defective immunoregulation of the inflammatory response to bacteria or viruses in the GI tract in individuals with a genetic predisposition (Silbermintz and Markowitz, 2006). In CD the chronic immune process is characterized by a T helper 1 cytokine profile, whereas in UC the response is more humoral and mediated by T helper 2 cells (Silbermintz and Markowitz, 2006). An epidemiologic study by Kugathasan, Judd, Hoffman, and others (2003) found an equal distribution of IBD among all racial and ethnic groups, contrasting with earlier studies that suggested IBD typically affects Caucasians (especially Ashkenazi Jews) more than those of Asian or African decent. The Kugathasan study also did not demonstrate a higher risk for developing IBD in urban populations, as had been reported in earlier studies.
There is an influence of genetics in the development of CD. Several CD susceptibility genes have now been identified, including NOD2/CARD15 on chromosome 16 associated with ileal disease, IBD5 on chromosome 5, and IBD6 on chromosome 6 (Kugathasan, Collins, Maresso, and others, 2004; Ahmad, Armuzzi, Bunce, and others, 2002). The influence of genetics in UC appears to be smaller than in CD; however, the MDRI gene has been associated with UC (Schwab, Schaeffeler, Marx, and others, 2003). Environmental factors appear to play a role in the development of IBD. Breastfeeding decreases the risk of developing CD, whereas infantile diarrhea seems to increase the risk.
Pathophysiology of Ulcerative Colitis
The inflammation is limited to the colon and rectum, with the distal colon and rectum the most severely affected. Inflammation affects the mucosa and submucosa and involves continuous segments along the length of the bowel with varying degrees of ulceration, bleeding, and edema. The presentation may be mild, moderate, or severe, depending on the extent of mucosal inflammation and systemic symptoms. Children with UC usually are seen with diarrhea, rectal bleeding, and abdominal pain, often associated with tenesmus and urgency (Silbermintz and Markowitz, 2006). Thickening of the bowel wall and fibrosis are unusual, but longstanding disease can result in shortening of the colon and strictures. Extraintestinal manifestations are less common in UC than in CD. Toxic megacolon is the most dangerous form of severe colitis.
Pathophysiology of Crohn Disease
The chronic inflammatory process of CD involves any part of the GI tract from the mouth to the anus but most often affects the terminal ileum. The disease involves all layers of the bowel wall (transmural) in a discontinuous fashion, meaning that between areas of intact mucosa, there are areas of affected mucosa (skip lesions). The most common symptoms are abdominal pain, diarrhea, and decrease in appetite resulting in weight loss. Perianal disease, including skin tags, fistulas, and abscesses, occur in CD. Fever, growth delay, and delayed sexual development are seen commonly (Silbermintz and Markowitz, 2006). Mild GI symptoms, poor growth, and extraintestinal manifestations may be present for several years before overt GI symptoms occur. The inflammation may result in ulcerations; fibrosis; adhesions; stiffening of the bowel wall; stricture formation; and fistulas to other loops of bowel, bladder, vagina, or skin. Extraintestinal manifestations include erythema nodosum, pyoderma gangrenosum, arthralgia and arthritis, uveitis and episcleritis, sclerosing cholangitis, autoimmune hepatitis, nephrolithiasis, and pneumonitis (Silbermintz and Markowitz, 2006).
Diagnostic Evaluation
The diagnosis of UC and CD is derived from the history, physical examination, laboratory evaluation, and other diagnostic procedures. Laboratory tests include a CBC to evaluate anemia and an ESR or CRP to assess the systemic reaction to the inflammatory process. Levels of total protein, albumin, iron, zinc, magnesium, vitamin B12, and fat-soluble vitamins may be low in children with CD. Stools are examined for blood, leukocytes, and infectious organisms. A serologic panel is often used in combination with clinical findings to diagnose IBD and to differentiate between CD and UC. In IBD, autoantibodies called antineutrophil cytoplasmic antibodies (ANCAs) may be detected in the blood. The perinuclear antineutrophil cytoplasmic antibody (pANCA) is associated with UC. Approximately 60% of children with UC and 10% of those with CD are pANCA-positive. Anti–Saccharomyces cerevisiae antibodies (ASCA) and anti–outer membrane porin of E. coli (anti-OmpC) have been found in up to 60% of children with CD (Ruemmele, Targan, Levy, and others, 1998).
In patients with CD, an upper GI series with small bowel follow-through assists in assessing the existence, location, and extent of disease. Upper endoscopy and colonoscopy with biopsies are an integral part of diagnosing IBD. Endoscopy allows direct visualization of the surface of the GI tract so that the extent of inflammation and narrowing can be evaluated. CT and ultrasound also may be used to identify bowel wall inflammation, intraabdominal abscesses, and fistulas. CD lesions may pierce the walls of the small intestine and colon, creating tracts called fistulas between the intestine and adjacent structures such as the bladder, anus, vagina, or skin.
Therapeutic Management
The goals of therapy are to (1) control the inflammatory process to reduce or eliminate the symptoms, (2) obtain long-term remission, (3) promote normal growth and development, and (4) allow as normal a lifestyle as possible. Treatment is individualized and managed according to the type and the severity of the disease, its location, and the response to therapy.
Medical Treatment.: The goal of any treatment regimen is first to induce remission of acute symptoms and then to maintain remission over time. 5-Aminosalicylates (5-ASAs) are effective in the induction and maintenance of remission in mild to moderate UC. Mesalamine, olsalazine, and balsalazide are now preferred over sulfasalazine because of reduced side effects (headache, nausea, vomiting, neutropenia, and oligospermia). Suppository and enema preparations of mesalamine are used to treat left-sided colitis. These drugs decrease inflammation by inhibiting prostaglandin synthesis. 5-ASAs can be used to induce remission in mild CD. Corticosteroids, such as prednisone and prednisolone, are indicated in induction therapy in children with moderate to severe UC and CD. These drugs inhibit the production of adhesion molecules, cytokines, and leukotrienes. Although these drugs reduce the acute symptoms of IBD, they have side effects that relate to long-term use, including growth suppression (adrenal suppression), weight gain, and decreased bone density (Baron, 2002). High doses of IV corticosteroids may be administered in acute episodes and tapered according to clinical response. Budesonide, a synthetic corticosteroid, is designed for controlled release in the ileum and is indicated for ileal and right-sided colitis; budesonide has fewer side effects than predinsone and prednisolone (Silbermintz and Markowitz, 2006). Rectal steroid therapy (enemas and foam-based preparations) are available for both induction and maintenance therapy in left-sided colitis.
Immunomodulators, such as azathioprine and its metabolite 6-mercaptopurine (6-MP), are used to induce and maintain remission in children with IBD who are steroid resistant or steroid dependent and in treating chronic draining fistulas. They block the synthesis of purine, thus inhibiting the ability of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) to hinder lymphocyte function, especially that of T cells. Side effects include infection, pancreatitis, hepatitis, bone marrow toxicity, arthralgia, and malignancy. Methotrexate has also been shown to be useful in inducing and maintaining remission in CD patients unresponsive to standard therapies. Cyclosporine and tacrolimus have both been shown to be effective in inducing remission in severe steroid-dependent UC. 6-MP or azathioprine is then used to maintain remission. Patients on immunomodulating medications require regular monitoring of their CBC and differential to assess for changes that reflect suppression of the immune system, since many of the side effects can be prevented or managed by dose reduction or discontinuation of medication.
Antibiotics, such as metronidazole and ciprofloxacin, may be used as an adjunctive therapy to treat complications such as perianal disease or small bowel bacterial overgrowth in CD. Side effects of these drugs are peripheral neuropathy, nausea, and a metallic taste.
Biologic therapies act to regulate inflammatory and antiinflammatory cytokines. Tumor necrosis factor-alpha (TNF-α) is believed to influence active inflammation. Infliximab (Remicade) is a chimeric human-murine monoclonal antibody to TNF-α that is administered intravenously. The U.S. Food and Drug Administration (FDA) has approved it for inducing and maintaining remission in adult CD patients with moderate to severe disease who have not responded sufficiently to conventional therapy. Children appear to respond to infliximab similarly to adults. Infliximab is approved for the treatment of fistulas in CD and for systemic manifestations of IBD such as ankylosing spondylitis, pyoderma gangrenosum, and chronic uveitis. Recently infliximab was approved by the FDA for the treatment of UC unresponsive to steroids. Many patients relapse if treatment is stopped; therefore long-term maintenance therapy with infliximab every 8 weeks may be required. Patients generally require concurrent maintenance with 6-MP or methotrexate to avoid the development of antibodies to infliximab, which are associated with infusion reactions and loss of efficacy. Approximately 5% of patients have acute allergic reactions to infliximab and require premedication with prednisone and diphenhydramine before infusion to prevent reactions. Long intervals between infusions may predispose patients to serum sickness (an immune response causing fever, muscle pain, arthritis, and hives) (Baron, 2002). Severe complications of this drug include lupuslike syndrome or lymphoma. Adalimumab (Humira) was introduced in 2007 for adults with IBD who have lost response or are intolerant to infliximab. Tuberculosis and other opportunistic infections have been observed in patients receiving adalimumab (Abbott Laboratories, 2007).
Nutritional Support.: Nutritional support is important in the treatment of IBD. Growth failure is a common serious complication, especially in CD. Growth failure is characterized by weight loss, alteration in body composition, retarded height, and delayed sexual maturation. Malnutrition causes the growth failure, and its etiology is multifactorial. Malnutrition occurs as a result of inadequate dietary intake, excessive GI losses, malabsorption, drug-nutrient interaction, and increased nutritional requirements. Inadequate dietary intake occurs with anorexia and episodes of increased disease activity. Excessive loss of nutrients (protein, blood, electrolytes, and minerals) occurs secondary to intestinal inflammation and diarrhea. Carbohydrate, lactose, fat, vitamin, and mineral malabsorption, as well as vitamin B12 and folic acid deficiencies, occur with disease episodes and with drug administration and when the terminal ileum is resected. Finally, nutritional requirements are increased with inflammation, fever, fistulas, and periods of rapid growth (e.g., adolescence).
The goals of nutritional support include (1) correction of nutrient deficits and replacement of ongoing losses, (2) provision of adequate energy and protein for healing, and (3) provision of adequate nutrients to promote normal growth. Nutritional support includes both enteral and parenteral nutrition. A well-balanced, high-protein, high-calorie diet is recommended for children whose symptoms do not prohibit an adequate oral intake. There is little evidence that avoiding specific foods influences the severity of the disease. Supplementation with multivitamins, iron, and folic acid is recommended.
Special enteral formulas, given either by mouth or continuous NG infusion (often at night), may be required. Elemental formulas are completely absorbed in the small intestine with almost no residue. A diet consisting only of elemental formula not only improves nutritional status but also induces disease remission, either without steroids or with a diminished dosage of steroids required. An elemental diet is a safe and potentially effective primary therapy for patients with CD. Unfortunately, remission is not sustained when NG feedings are discontinued unless maintenance medications are added to the treatment regimen.
TPN has also improved nutritional status in patients with IBD. Short-term remissions have been achieved after TPN, although complete bowel rest has not reduced inflammation or added to the benefits of improved nutrition by TPN. Nutritional support is less likely to induce a remission in UC than in CD. Improvement of nutritional status is important, however, in preventing deterioration of the patient’s health status and in preparing the patient for surgery.
Surgical Treatment.: Surgery is indicated for UC when medical and nutritional therapies fail to prevent complications. Surgical options include a subtotal colectomy and ileostomy that leaves a rectal stump as a blind pouch. A reservoir pouch is created in the configuration of a J or S to help improve continence postoperatively. An ileoanal pull-through preserves the normal pathway for defecation. Pouchitis, an inflammation of the surgically created pouch, is the most common late complication of this procedure and had been reported to occur in up to 50% of cases. Metronidazole and ciprofloxacin are effective in treating pouchitis (Alexander, Sarigol, DiFiore, and others, 2003). In many cases UC can be cured with a total colectomy.
Surgery may be required in children with CD when complications cannot be controlled by medical and nutritional therapy. Segmental intestinal resections are performed for small bowel obstructions, strictures, or fistulas. Partial colonic resection is not curative, and the disease often recurs.
Prognosis.: IBD is a chronic disease. Relatively long periods of quiescent disease may follow exacerbations. The outcome of the disease is influenced by the regions and severity of involvement, as well as by appropriate therapeutic management. Malnutrition, growth failure, and bleeding are serious complications. The overall prognosis for UC is good.
The development of colorectal cancer (CRC) is a long-term complication of IBD. In UC, the cumulative incidence of CRC is 2.5% after 20 years, increasing to 10.8% after 30 years (Rutter, Saunders, Wilkinson, and others, 2006). Surveillance colonoscopy with multiple biopsies should begin approximately 10 years after diagnosis of UC or Crohn colitis and continue every 1 to 2 years (Rubin and Kavitt, 2006). Removal of the diseased colon prevents development of CRC. In CD, however, surgical removal of the affected colon does not prevent cancer from developing elsewhere in the GI tract.
Nursing Care Management
The nursing considerations in the management of IBD extend beyond the immediate period of hospitalization. These interventions involve continued guidance of families in terms of (1) managing diet; (2) coping with factors that increase stress and emotional lability; (3) adjusting to a disease of remissions and exacerbations; and (4) when indicated, preparing the child and parents for the possibility of diversionary bowel surgery.
Because nutritional support is an essential part of therapy, encouraging the anorectic child to consume sufficient quantities of food is often a challenge. Successful interventions include involving the child in meal planning; encouraging small, frequent meals or snacks rather than three large meals a day; serving meals around medication schedules when diarrhea, mouth pain, and intestinal spasm are controlled; and preparing high-protein, high-calorie foods such as eggnog, milkshakes, cream soups, puddings, or custard (if lactose is tolerated) (see Feeding the Sick Child, Chapter 22). Foods that aggravate the condition are avoided. Using bran or a high-fiber diet for active IBD is questionable. Bran, even in small amounts, has been shown to worsen the patient’s condition. Occasionally the occurrence of aphthous stomatitis further complicates adherence to dietary management. Mouth care before eating and the selection of bland foods help relieve the discomfort of mouth sores.
When NG feedings or TPN is indicated, nurses play an important role in explaining the purpose and the expected outcomes of this therapy. The nurse should acknowledge the anxieties of the child and family members and give them adequate time to demonstrate the skills necessary to continue the therapy at home if needed (see Critical Thinking Exercise).
The importance of continued drug therapy despite remission of symptoms must be stressed to the child and family members. Failure to adhere to the pharmacologic regimen can result in exacerbation of the disease (see Compliance, Chapter 22). Unfortunately, exacerbation of IBD can occur even if the child and family are compliant with the treatment regimen; this is difficult for the child and family to cope with.
Family Support.: The nurse should attend to the emotional components of the disease and assess any sources of stress. Frequently, the nurse can help children adjust to problems of growth retardation, delayed sexual maturation, dietary restrictions, feelings of being “different” or “sickly,” inability to compete with peers, and necessary absence from school during exacerbations of the illness (see Impact of Chronic Illness or Disability on the Child, Chapter 18).
If a permanent colectomy-ileostomy is required, the nurse can teach the child and family how to care for the ileostomy. The nurse can also emphasize the positive aspects of the surgery, particularly accelerated growth and sexual development, permanent recovery, and the eliminated risk of colonic cancer in UC, as well as the normality of life despite bowel diversion. Introducing the child and parents to other ostomy patients, especially those who are the same age, can be effective in fostering eventual acceptance. Whenever possible, continent ostomies should be offered as options to the child, although they are not performed in all centers in the United States.
Because of the chronic and often lifelong nature of the disease, families benefit from the educational services provided by organizations such as the Crohn’s and Colitis Foundation of America (CCFA).* If diversionary bowel surgery is indicated, United Ostomy Associations of America† and the Wound, Ostomy and Continence Nurses Society‡ are available to assist with ileostomy care and provide important psychologic support through their self-help groups. Adolescents often benefit by participating in peer-support groups, which are sponsored by the CCFA.
 IN TEXT
IN TEXT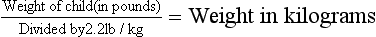
 CRITICAL THINKING EXERCISE
CRITICAL THINKING EXERCISE