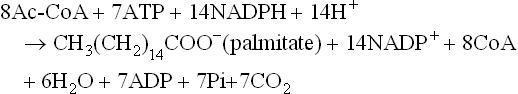Biosynthesis and Storage of Fatty Acids
Introduction
Most fatty acids required by humans are supplied in the diet; however, the pathway for their de novo synthesis (lipogenesis) from two-carbon compounds is present in many tissues such as liver, brain, kidney, mammary gland and adipose tissue. It is also highly active in many cancers. In general, the pathway of de novo synthesis is primarily active in situations of excess energy intake, particularly in the form of excess carbohydrate. In this situation, carbohydrate, and to a lesser extent amino acid precursors, are converted to fatty acids in the liver and stored as triacylglycerol (TAG, also known as triglyceride) in cellular lipid droplets. This may happen in many cell types but the adipocytes (and adipose tissue) are dedicated to store large quantities of TAG. In humans, adipose tissue may not be quantitatively the most important place of fatty acid synthesis: the main lipogenic organ is the liver.
The pathway for lipogenesis is not simply the reverse of oxidation of fatty acids (Chapter 15). Lipogenesis requires a completely different set of enzymes and is located in a different cellular compartment, the cytosol. Furthermore, it uses reduced nicotinamide adenine dinucleotide phosphate (NADPH) as a source of reductive power, as opposed to nicotinamide adenine dinucleotide (NAD+) required for β-oxidation.
The sterol regulatory element-binding proteins-1 (mainly SREBP 1c ; also SREBP1a) provides a master regulation of de novo lipogenesis through transcriptional control. The SREBP is an endoplasmic-reticulum-bound and membrane-sensing protein that undergoes proteolytic cleavage enabling its transport to the nucleus. In the nucleus, SREBP binds to specific DNA sequences (the sterol regulatory elements or SREs) located in the control regions of the genes that encode enzymes needed for lipogenesis.
Fatty acid synthesis
The preparatory stage: acetyl-CoA carboxylase
Carboxylation of acetyl-CoA to malonyl-CoA is the committed step of fatty acid synthesis
In the first stage of fatty acid biosynthesis, acetyl-CoA, mostly derived from carbohydrate metabolism, is converted to malonyl-CoA by the action of the enzyme acetyl-CoA carboxylase (Fig. 16.1). There are two forms of acetyl-CoA carboxylase (ACC1 and ACC2). ACC1 is located in the cytoplasm and committed to fatty acid synthesis, whereas ACC2 is in mitochondria where it regulates fatty acid oxidation (ACC2 inhibition results in increased lipid oxidation). ACC1 is a biotin-dependent enzyme with distinct enzymatic and a carrier protein function: its subunits serve as a biotin carboxylase, a transcarboxylase and biotin carboxyl carrier protein. The enzyme is synthesized in an inactive protomer form, each protomer containing all the above subunits, a molecule of biotin, and a regulatory allosteric site for the binding of citrate (a Krebs cycle metabolite) or palmitoyl-CoA (the end product of the fatty acid biosynthetic pathway). The reaction itself takes place in stages: first, there is the carboxylation of biotin, involving adenosine triphosphate (ATP), followed by the transfer of this carboxyl group to acetyl-CoA to produce the malonyl-CoA. At this stage, the free enzyme–biotin complex is released.
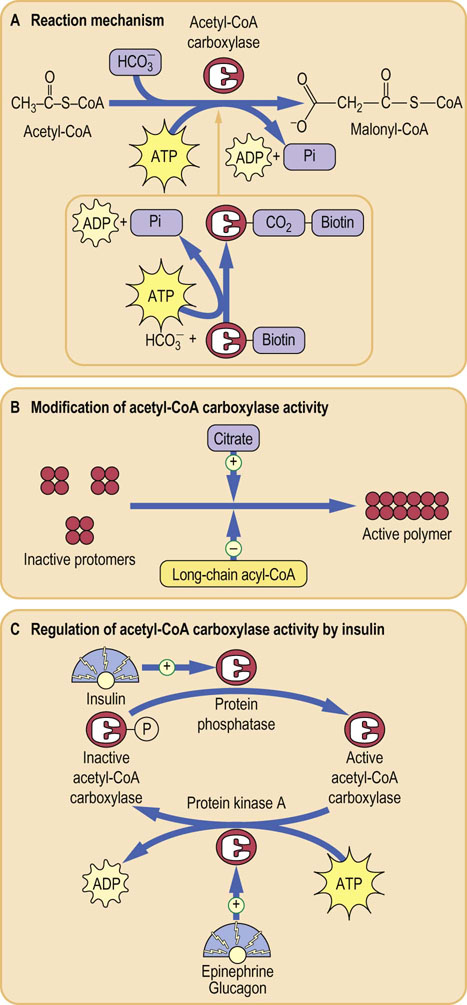
Fig. 16.1 Conversion of acetyl-CoA to malonyl-CoA.
(A) Reaction catalyzed by the acetyl-CoA carboxylase. The enzyme has the covalently attached biotin, which is carboxylated using a molecule of ATP. (B) Acetyl-CoA carboxylase requires the presence of citrate for polymerization to its active form. (C) The activity of acetyl-CoA carboxylase is regulated by a phosphorylation–dephosphorylation mechanism. This in turn is controlled by hormones that regulate fuel metabolism: insulin, glucagon and epinephrine.
This process allows the building up of fatty acid molecules with even numbers of carbon atoms. Propionyl-CoA is a substrate for the synthesis of fatty acids with an odd number of carbon atoms, but this is not seen in humans.
Acetyl-CoA carboxylase is subject to strict regulation
The protomers of acetyl-CoA carboxylase polymerize in the presence of citrate or isocitrate, producing the active form of the enzyme. The polymerization is inhibited by palmitoyl-CoA binding to the same allosteric site. The respective stimulatory and inhibitory effects of citrate and palmitoyl-CoA are entirely logical: under conditions of high citrate concentration, energy storage is desirable but when palmitoyl-CoA, the product of the pathway, accumulates, a decrease in the synthesis of fatty acids is appropriate. There is an additional control mechanism, independent of the citrate or palmitoyl-CoA, involving phosphorylation and dephosphorylation of the enzyme molecule. This involves hormone-dependent protein phosphatase/kinase (Fig. 16.1). Phosphorylation inhibits the enzyme and dephosphorylation activates it. Phosphorylation of the enzyme is promoted by glucagon or epinephrine and the dephosphorylation is promoted by the insulin, which is a lipogenic hormone. Phosphorylation is also dependent on the activation of the AMP-activated protein kinase (AMPK).
Dietary carbohydrate and fat intake also controls acetyl-CoA carboxylase
The carboxylation of acetyl-CoA to malonyl-CoA commits the pathway to fatty acid synthesis. This is why this enzyme is under such strict short-term control. Longer-term control is exerted by the induction or repression of enzyme synthesis affected by diet: synthesis of acetyl-CoA carboxylase is upregulated under conditions of high-carbohydrate/low-fat intake, whilst starvation or high-fat/low-carbohydrate intake leads to downregulation of synthesis of the enzyme.
Synthesizing a fatty acid chain: fatty acid synthase
The second major step in fatty acid synthesis also involves a multienzyme complex, the fatty acid synthase. This enzyme system is much more complex than acetyl-CoA carboxylase. The protein contains seven distinct enzyme activities and an acyl carrier protein (ACP). ACP, a highly conserved protein, replaces CoA as the entity that binds to the elongating fatty acid chain. The structure of this molecule is shown in Figure 16.2 and consists of a dimer of large identical polypeptides arranged head to tail. Each monomer contains all seven enzyme activities and the ACP. It also contains a long pantetheine group which acts as a flexible ‘arm’, making the molecule being synthesized available to different enzymes in the fatty acid synthetase complex. The function in fatty acid synthesis is shared between the two polypeptide chains.
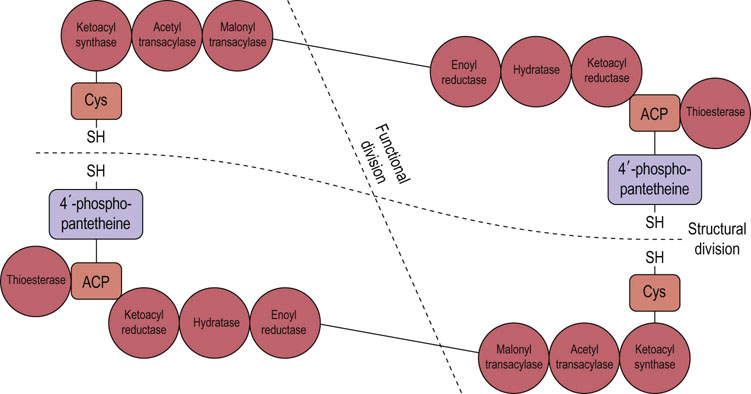
Fig. 16.2 Structure of fatty acid synthase.
Fatty acid synthase is a dimer consisting of two large subunits arranged head to tail. It contains seven distinct enzyme activities and an acylcarrier protein (ACP). Cys, cysteine.
Fatty acid synthase builds the fatty acid molecule up to 16-carbon length
The reaction proceeds after an initial priming of the cysteine (Cys-SH) group with acetyl-CoA, a reaction catalyzed by acetyl transacylase (Fig. 16.3). Then malonyl-CoA is transferred by malonyl transacylase to the -SH residue of the pantetheine group attached to the ACP in the other subunit. Next, 3-ketoacyl synthase (the condensing enzyme) catalyzes the reaction between the previously attached acetyl group and the malonyl residue, liberating CO2 and forming the 3-ketoacyl enzyme complex. This frees the cysteine residue on chain 1 that had been occupied by the acetyl-CoA. The 3-ketoacyl group subsequently undergoes sequential reduction, dehydration and again reduction to form a saturated acyl–enzyme complex. The next molecule of malonyl-CoA displaces the acyl group from the pantetheine-SH group to the now free cysteine group, and the reaction sequence is repeated through six more cycles (seven cycles altogether). Once the 16-carbon chain (palmitate) is formed, the saturated acyl–enzyme complex activates the thioesterase, releasing the molecule of palmitate from the enzyme complex. The two -SH sites are now free, allowing another cycle of palmitate synthesis to be initiated.
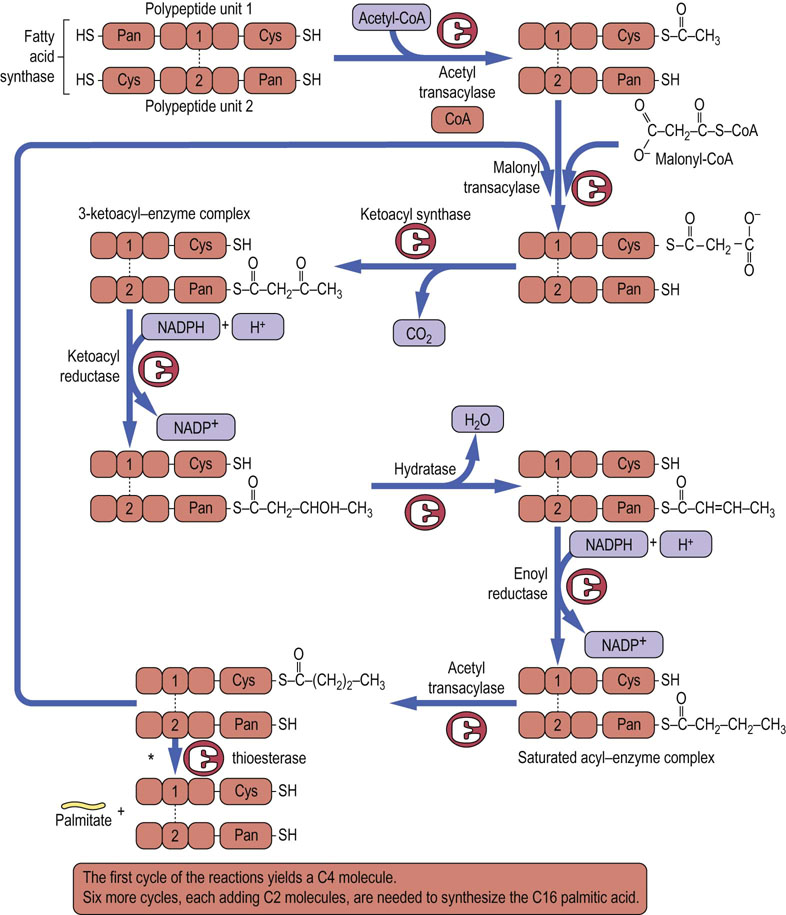
Fig. 16.3 Reactions catalyzed by fatty acid synthase.
The synthesis of a fatty acid chain is initiated by a molecule of malonyl-CoA (C3) which reacts with the first molecule of acetyl-CoA (C2); this produces a C4 molecule (1 carbon is lost as CO2 during condensation of malonyl-CoA and acetyl-CoA). There are six more cycles, each adding 2C to the fatty acid chain (seven cycles altogether), and the result is a 16-carbon molecule of palmitate. NADPH, reduced nicotinamide adenine dinucleotide phosphate; Pan, pantetheine.
*This reaction occurs once 16-carbon fatty acyl chain has been formed.
The synthesis of one palmitate molecule requires 8 molecules of acetyl-CoA, 7 ATP, 14 NADPH and 14 H+:
In common with the acetyl-CoA carboxylase system, fatty acid synthase is also regulated by the presence of phosphorylated sugars via an allosteric effect, and also by induction and repression of the enzyme.
The malate shuttle
Malate shuttle allows recruitment of two-carbon units from the mitochondrion to the cytoplasm
The primary molecule required for the synthesis of fatty acids is acetyl-CoA. However, acetyl-CoA is generated in the mitochondria and cannot freely cross the inner mitochondrial membrane. As said above, fatty acid biosynthesis occurs in the cytosol. The malate shuttle is a mechanism allowing the transfer of two-carbon units from the mitochondria to the cytosol: it involves the malate–citrate antiporter (Fig. 16.4). Pyruvate derived from glycolysis is decarboxylated to acetyl-CoA in the mitochondria; it subsequently reacts with oxaloacetate in the tricarboxylic acid (TCA) cycle (Chapter 14) to form citrate. Translocation of a molecule of citrate to the cytosol via the antiporter is accompanied by transfer of a molecule of malate to the mitochondrion. In the cytosol, citrate, in the presence of ATP and CoA, undergoes cleavage to acetyl-CoA and oxaloacetate by citrate lyase. This makes acetyl-CoA available for carboxylation to malonyl-CoA and for the synthesis of fatty acids. The synthesis of fatty acids is also linked to glucose metabolism through the pentose phosphate pathway, which is the main provider of NADPH required for lipogenesis. Fructose is specifically channeled through this pathway and is very lipogenic. Some NADPH is also generated by the NADP+-linked decarboxylation of malate to pyruvate by the malic enzyme.
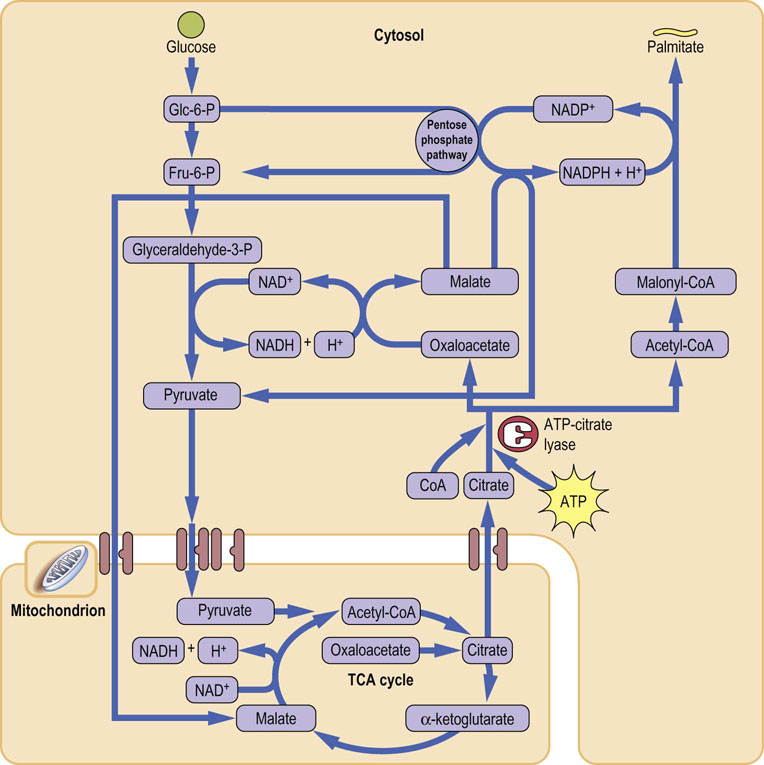
Fig. 16.4 The malate shuttle.
Acetyl-CoA is generated in the mitochondria and cannot cross the mitochondrial membrane. The malate shuttle facilitates the transport of two-carbon units from the mitochondria to cytoplasm. Citrate, synthesized from Acetyl-CoA and oxalate is transported out of the mitochondria. In the cytosol it is split back into Acetyl-CoA and oxalate. Oxalate is then converted to malate, which returns to the mitochondrion – thus, the ‘shuttle’. Acetyl-CoA is resynthesized in the cytoplasm and enters lipogenesis. Fru-6-P, fructose-6-phosphate; Glc-6-P, glucose-6-phosphate; NADH, reduced nicotinamide adenine dinucleotide. (See also Fig. 9.7.)
Fatty acid elongation
Elongation of a fatty acid chain beyond 16-carbon length requires another set of enzymes
Palmitate released from fatty acid synthase becomes a substrate for the synthesis of longer-chain fatty acids, with the exception of certain essential fatty acids (see below). Chain elongation occurs by the addition of further two-carbon fragments derived from malonyl-CoA (Fig. 16.5). This process occurs on the endoplasmic reticulum by the action of yet another multienzyme complex – fatty acid elongase. The reactions occurring during chain elongation are similar to those involved in fatty acid synthesis, except that the fatty acid is attached to CoA, rather than to the ACP. In fact, there are seven discrete fatty acid elongases with different tissue expressions and substrate specificities (ELOVL9-7; ELOVL stands for ‘elongation of very long chain fatty acids’).
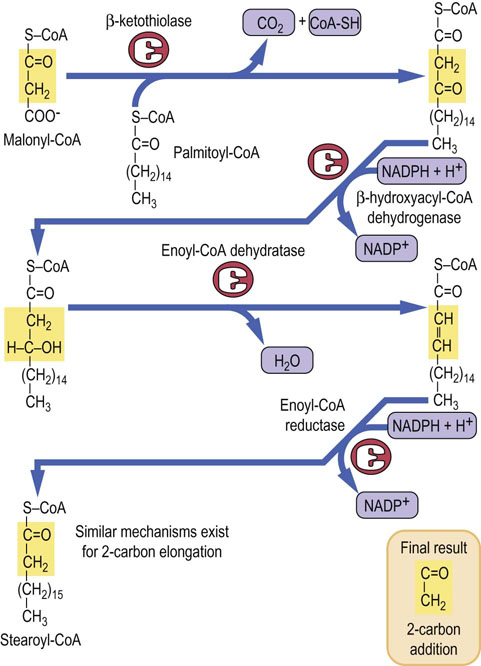
Fig. 16.5 Elongation of the fatty acids.
Fatty acid elongation occurs on the endoplasmic reticulum and is carried out by a multienzyme complex, fatty acid elongase.
The substrates for the cytosolic fatty acid elongase include saturated fatty acids with a chain length from 10-carbon upwards, and also unsaturated fatty acids. Very long-chain (22–24-carbon) fatty acids are produced in the brain, and elongation of stearoyl-CoA (C18) in the brain increases rapidly during myelination, producing fatty acids required for the synthesis of sphingolipids.
Fatty acids can also be elongated in the mitochondria, where yet another system is used: it is NADH-dependent and uses acetyl-CoA as a source of two-carbon fragments. It is simply the reverse of β-oxidation (Chapter 15) and the substrates for chain elongation are short- and medium-chain fatty acids containing fewer than 16 carbon atoms. During fasting and starvation, elongation of fatty acids is greatly reduced.
Desaturation of fatty acids
Desaturation reactions require molecular oxygen
The body has a requirement for mono- and polyunsaturated fatty acids, in addition to saturated fatty acids. Some of these need to be supplied in the diet; these two unsaturated fatty acids, linoleic and linolenic, are known as the essential fatty acids (EFAs). The desaturation system requires molecular oxygen, NADH, and cytochrome b5. The process of desaturation, like that of chain elongation, occurs on the endoplasmic reticulum and results in the oxidation of both the fatty acid and NADH (Fig. 16.6).
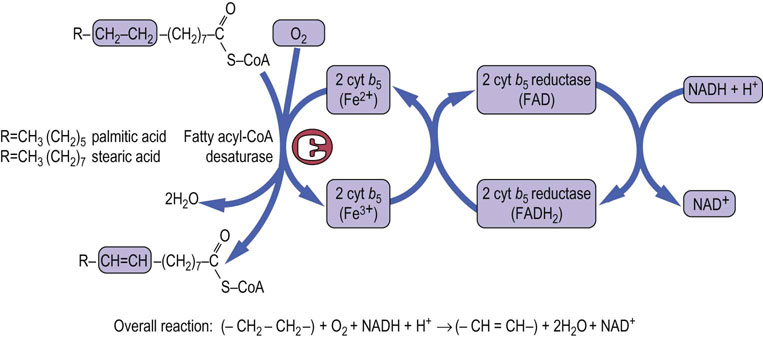
Fig. 16.6 Desaturation of fatty acids.
Desaturation of the fatty acids takes place in the endoplasmic reticulum. The reaction requires molecular oxygen, NADH2, FADH2 and cytochrome b5. cyt b5, cytochrome b5; FAD, flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide.
In man, the desaturase system is unable to introduce double bonds between carbon atoms beyond carbon-9 and the ω-(terminal methyl) carbon atom. Most desaturations occur between carbon atoms 9 and 10 (annotated as Δ9 desaturations), e.g. those with palmitic acid producing palmitoleic acid (C-16:1, Δ9), and those with stearic acid producing oleic acid (C-18:1, Δ9). This step is catalyzed by stearoyl-CoA desaturase (SCD).
Essential fatty acids
The ω-3 and ω-6 fatty acids (or their precursors) must be supplied with diet
As discussed above, the human desaturase is unable to introduce double bonds beyond C-9. On the other hand, two types of fatty acids – those having double bonds 3 carbons from the methyl end (ω-3 fatty acids) and 6 carbons from the methyl end (ω-6 fatty acids) – are required for the synthesis of eicosanoids (C-20 fatty acids), precursors of important molecules such as prostaglandins, thromboxanes and leukotrienes. Therefore, the ω-3 and ω-6 fatty acids (or their precursors) must be supplied in the diet. As it happens, they are obtained from dietary vegetable oils, and meat which contain the ω-6 fatty acid, linoleic acid (C-18:2, Δ9,12) and the ω-3 fatty acid, linolenic acid (C-18:3, Δ9,12,15). Linoleic acid is converted in a series of elongation and desaturation reactions to arachidonic acid (C-20:4, Δ5,8,11,14), the precursor for the synthesis of other eicosanoids in man. Elongation and desaturation of linolenic acid produce eicosapentaenoic acid (EPA; C-20:5, Δ5,8,11,14,17), which is a precursor of yet another series of eicosanoids (see Table 3.2). However, the elongation/desaturation of C-18:3, Δ9,12,15 to EPA occurs at a low rate; most of EPA in the human body derives from fish consumption.
Storage and transport of fatty acids: synthesis of triacylglycerols
Fatty acids derived from endogenous synthesis or from the diet are stored and transported as triacylglycerols
In both liver and adipose tissue, triacylglycerols (TAG) are produced by a pathway involving phosphatidic acid as an intermediate (Fig. 16.7). The source of glycerol phosphate is, however, different in the two tissues. Glycerol is the source of phosphatidic acid in the liver. However, in the adipose tissue, due to the lack of glycerol kinase, glucose is the indirect source of glycerol, with the glycolytic metabolite dihydroxy-acetone phosphate being its immediate precursor.
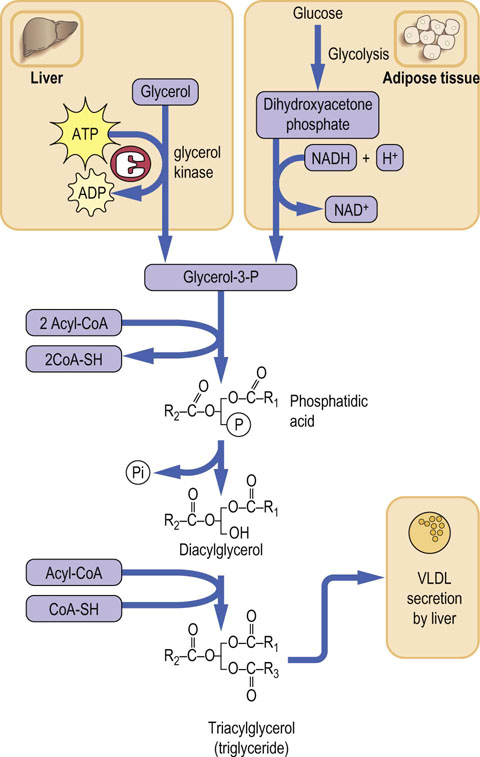
Fig. 16.7 Triacylglycerol synthesis.
Triacylglycerols (triglycerides) are synthesized in the liver and in adipose tissue. The source of glycerol-3-P is different in the two tissues. In the liver it is glycerol but adipose tissue has no glycerol kinase activity. There, glycerol-3-P is generated from the glycolytic intermediate, dihydroxyacetone phosphate. The central ‘backbone’ of the phosphatidic acid, diacylglycerol and triacylglycerol molecule shown in the figure consists of three carbon atoms saturated with hydrogens (compare Fig. 10.8 )
Triacylglycerols produced in the liver on the smooth endoplasmic reticulum can only be transiently stored
The liver has the unique capacity to off-load stored TAG by producing lipoprotein complexes with cholesterol, phospholipids and apolipoproteins (the latter also synthesized on the endoplasmic reticulum) for export in the form of very low-density lipoprotein (VLDL). The VLDL is then processed in the Golgi apparatus and released into the bloodstream for uptake by other tissues. In order to mobilize the transiently stored TAG, a lipolytic reaction occurs. (It results in the formation of diacylglycerols (DAG), which can then again enter the TAG synthetic pathway of VLDL assembly). The nature of this lipase is as yet not known, but is of significant medical interest due to the complications of fatty liver disease.
VLDL, once released into the bloodstream, is acted upon by lipoprotein lipase (LPL). This enzyme is found attached to the basement membrane glycoproteins of capillary endothelial cells and is active against both VLDL and chylomicrons (Chapter 18).
In the fed state, when adipose tissue is actively taking up fatty acids from the lipoproteins and storing them as TAG, the adipocytes synthesize LPL and secrete it into the capillaries of the adipose tissue. This increased synthesis and secretion of LPL is stimulated by insulin. Increased insulin levels also stimulate the uptake of glucose by adipose tissue and promote glycolysis. This has the net effect of producing increasing amounts of α-glycerophosphate, and it facilitates the synthesis of TAG within adipocytes. The skeletal muscle capillary bed also has LPL but it is inhibited by insulin. Instead, LPL is activated in skeletal muscle by its contractions.
Insulin is an important hormone in relation to fatty acid synthesis and storage. It promotes glucose uptake in both the liver and adipose tissue. In the liver, by increasing fructose-2,6-bisphosphate levels, it stimulates glycolysis, thus increasing pyruvate production. By stimulating dephosphorylation of pyruvate dehydrogenase complex and thus activating this enzyme, insulin promotes production of acetyl-CoA, stimulating the TCA cycle and increasing citrate levels, which, in turn, through stimulation of the acetyl-CoA carboxylase, increase the rate of fatty acid synthesis (see also Chapter 21).
Regulation of total body fat stores
Adipose tissue is an active endocrine organ
It has long been understood that increased energy intake without appropriate increase in energy expenditure is associated with obesity, which is characterized by increased adiposity, meaning both the number of adipocytes and their fat content. In this sense, the quantity of stored triacylglycerol is merely a consequence of energy balance. However, it is now clear that adipose tissue, far from being an inert storage reservoir, is hormonally active. Adipocytes produce hormones such as leptin, adiponectin and resistin (collectively known as adipokines), growth factors such as vascular endothelial growth factor, and proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6). Such hormonal signals, in particular leptin, may alter energy balance. This is further discussed in Chapter 22.
Summary
 Fatty acid synthesis and storage are essential components of body energy homeostasis.
Fatty acid synthesis and storage are essential components of body energy homeostasis.
 Fatty acid synthesis takes place in the cytosol. Its committed step is the reaction catalyzed by acetyl-CoA carboxylase.
Fatty acid synthesis takes place in the cytosol. Its committed step is the reaction catalyzed by acetyl-CoA carboxylase.
 Elongation of the fatty acid chain (up to the length of 16 carbon atoms) is carried out by the dimeric fatty acid synthase, which possesses several enzyme activities. Both acetyl-CoA carboxylase and fatty acid synthase are subject to a complex regulation.
Elongation of the fatty acid chain (up to the length of 16 carbon atoms) is carried out by the dimeric fatty acid synthase, which possesses several enzyme activities. Both acetyl-CoA carboxylase and fatty acid synthase are subject to a complex regulation.
 The malate shuttle facilitates the transfer of two-carbon units from the mitochondria to cytoplasm for use in fatty acid synthesis.
The malate shuttle facilitates the transfer of two-carbon units from the mitochondria to cytoplasm for use in fatty acid synthesis.
 The reducing power for fatty acid synthesis in the form of NADPH is supplied by the pentose phosphate pathway and also by the malate shuttle.
The reducing power for fatty acid synthesis in the form of NADPH is supplied by the pentose phosphate pathway and also by the malate shuttle.
 The essential unsaturated fatty acids are linoleic and linolenic acid. Linoleic acid is converted to arachidonic acid, which in turn serves as the precursor of prostaglandins.
The essential unsaturated fatty acids are linoleic and linolenic acid. Linoleic acid is converted to arachidonic acid, which in turn serves as the precursor of prostaglandins.
 Adiposity signals are provided by adipokines, particularly leptin. Insulin is also important in the regulation of food intake.
Adiposity signals are provided by adipokines, particularly leptin. Insulin is also important in the regulation of food intake.
Brown, MS, Ye, J, Rawson, RB, Goldstein, JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000; 100:391–398.
Guillou, H, Zadravec, D, Martin, PG, Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog Lipid Res. 2010; 49:186–199.
Gurr MI, Harwood JLK, Frayn KN, eds. Lipid biochemistry: an introduction. Oxford: Blackwell Science, 2008.