36 CANCER
INTRODUCTION
Cancer is a common disease of adults in Australia and New Zealand, and the risk of developing cancer increases markedly with age. Since the 1970s, intensive research has led to a much improved understanding of this complex and often frightening disease. We now understand that cancer is a collection of many different diseases, all caused by an accumulation of genetic alterations. Environmental and heredity factors interact to modify both the risk of an individual developing cancer and their response to treatment. Our increased understanding of the pathophysiology of cancer has contributed to the development of a number of effective treatment options. Although it was previously thought that cancer affected mainly the elderly, diagnosis of cancer in some younger and middle-aged celebrities has contributed to an increasing awareness that cancer affects younger people too. For example, the following celebrities have been diagnosed with cancer over recent years: Delta Goodrem (singer) diagnosed with Hodgkin’s lymphoma at age 18; Lance Armstrong (cyclist) diagnosed with testicular cancer at age 25; Kylie Minogue (singer) diagnosed with breast cancer at age 36; and Patrick Swayze (actor) diagnosed with pancreatic cancer at age 56.
In this chapter, we define what types of growth cancers there are and then look at how cells can actually become cancerous. We focus on the most significant cancers in Australia and New Zealand. In terms of the cancers with the highest incidence (the number of new cases diagnosed each year) and the highest mortality (causes of death), those that impact on the greatest number of people are lung cancer, colorectal cancer, breast cancer, prostate cancer, melanoma and pancreatic cancer.
CANCER IS A CHRONIC DISEASE
The 5-year survival rate is used to indicate the percentage of people who are alive 5 years after their initial diagnosis of cancer. When data from all cancer types are combined, the latest information indicates that approximately 60% of Australians diagnosed with cancer are still alive after 5 years (see Figure 36-1). The improved survival rates are largely a result of improved screening and treatment options. So, whereas once cancer was seen as a death sentence, it is now seen as a chronic disease that many people will live with for many years. Cancers that are treated effectively may go into remission (no current evidence of cancer), but then reappear years later in relapse (reappearance of cancer). Thus the patient with cancer may not be ‘cured’ completely, but usually requires regular monitoring and awareness for signs of reappearance. Although difficult to measure, it is likely that cancer patients now have an improved quality of life too due to the improvements in treatment options.
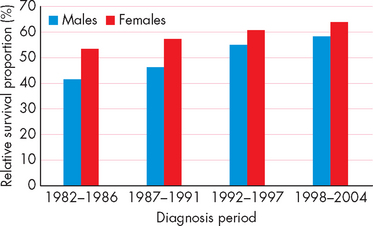
FIGURE 36-1 Increased 5-year survival rate for all cancers, Australia.
This shows that a greater percentage of people with cancer are now alive 5 years after being diagnosed.
Source: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview. Cancer series no. 46. Catalogue no. CAN 42. Canberra: AIHW; 2008.
CANCER CHARACTERISTICS AND TERMINOLOGY
What is cancer?
Cancer is an uncontrolled proliferation (growth and multiplication) of cells that can arise from virtually any cell type in the body. The term cancer comes from the Greek word for crab, karkinoma, which the physician Hippocrates used to describe the appendage-like projections extending from tumours. It refers to any malignant tumour or neoplasm that grows rapidly (and has other defining characteristics, as described in the next section; see Figure 36-2). Not all tumours or neoplasms are cancerous, however — for example, a benign growth or hypertrophy of an organ (such as skeletal muscle in response to training) is not cancer. Yet it is important to recognise that benign neoplasms may cause life-threatening symptoms if they enlarge in critical locations. For example, a benign tumour at the base of the skull may cause devastating symptoms by compressing the adjacent normal brainstem.
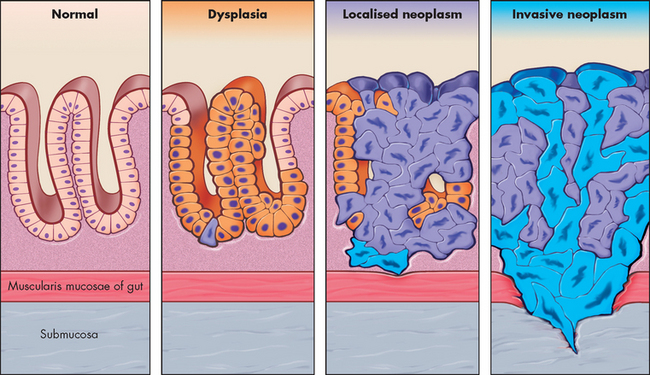
FIGURE 36-2 The progression of dysplasia to neoplasm.
A sequence of cellular and tissue changes progressing from dysplasia to localised neoplasia and then to invasive neoplasia is seen often in the development of cancer. In this diagram, as in real life, distinguishing between dysplasia and localised neoplasia is difficult. Loss of normal tissue architecture signifies the development of neoplasia. The localised neoplasms are most commonly found in the squamous epithelium of the uterine cervix, the epidermis of sun-exposed skin, and colonic and gastric mucosa after longstanding inflammation. The altered cell turnover during inflammation probably allows local environmental factors to cause genetic abnormalities leading to neoplasia.
Source: Based on Stevens A, Lowe J. Pathology: illustrated review in color. 2nd edn. Edinburgh: Mosby; 2000.
Benign tumours are not called cancers. However, some benign tumours can progress to cancer. They are usually well-encapsulated, the cells and tissues appear normal in structure, and they do not spread to regional lymph nodes or distant locations. Benign tumours are generally named according to the tissues from which they arise, with the suffix -oma. For example, adenoma is a benign growth of glandular tissue.
Malignant tumours are distinguished from benign tumours by their more rapid growth rates and alterations in microscopic appearance, such as loss of cell differentiation and absence of normal tissue organisation. Malignant tumours are not enclosed in a capsule; can undergo invasion into blood vessels, lymphatics and surrounding structures; and can spread to distant locations (metastasis). One of the hallmarks of malignant cells is anaplasia (loss of cell differentiation), nuclear irregularities and loss of normal tissue structure.
Table 36-1 illustrates some key differences between benign and malignant tumours. We explore these concepts later in the chapter when discussing how cancers spread.
Table 36-1 A COMPARISON OF THE FEATURES OF BENIGN AND MALIGNANT CANCERS
| BENIGN TUMOURS | MALIGNANT TUMOURS |
|---|---|
| Grow slowly | Grow rapidly |
| Are well-differentiated; look like the tissue from which they arose | Are poorly differentiated; do not look like the tissue from which they arose |
| Have a well-defined capsule | Are not encapsulated |
| Are not invasive | Invade local structures and tissues |
| Do not metastasise | Spread distantly through bloodstream and lymphatics |
| Have a good prognosis; often not life-threatening | Have a poor prognosis; usually become life-threatening |
Cancer names
Cancers are named according to the cell type of origin (see Table 36-2). Cancers arising in epithelial tissue are called carcinomas and those arising from ductal or glandular epithelium are named adenocarcinomas. Hence, a malignant tumour arising from breast glandular tissue is known as a mammary adenocarcinoma. Cancers of lymphatic tissue are called lymphomas, whereas cancers of blood-forming cells are known as leukaemias. In addition, there are other cancers named for historical reasons (e.g. Hodgkin’s disease) that do not follow this naming convention. It is worth noting the differences in the names of cancers as these will be commonly referred to in clinical practice.
Table 36-2 EXAMPLES OF TUMOUR NOMENCLATURE
| CELL/TISSUE | ORIGIN |
|---|---|
| Carcinomas | Arise from endothelial and epithelial tissues, such as hepatocellular carcinoma |
| Sarcomas | Arise from connective tissues, such as osteogenic sarcoma |
| Adenoma | Benign tumour arising from glandular or ductal epithelium |
| Adenocarcinomas | Carcinomas arising from glandular or ductal epithelium, such as mammary adenocarcinoma |
| Terato- | Arise from germ cells (teratocarcinoma) |
THE GENETIC BASIS OF CANCER
Cancer is a genetic condition — it arises due to abnormalities or mutations in genes. (See Chapter 5 for an introduction to genes and their functioning.) The reasons why genes become abnormal is a current topic of extensive research. Sometimes genes become abnormal for reasons that seem to be unpredictable (genetic or inherited causes) and other times they are altered by factors that have influenced the cell, such as the toxic effects of cigarette smoke (environmental causes). The delicate balance between a genetic predisposition to cancer and environmental and lifestyle contributions to gene alterations that lead to cancer is not well understood, but there is certainly evidence that both genes and the environment are involved. We explore some environmental and lifestyle factors; first, we look at genetic alterations.
Types of gene mutations in cancer
Alteration of progrowth and antigrowth signals
Different genetic mutations are possible and it is likely that a number of cellular control pathways must be altered for a cell to become fully malignant.1 First, cancer cells must have mutations that enable them to proliferate — to achieve this, some cancers secrete growth factors that stimulate their own growth, a process known as autocrine stimulation (see Figure 36-3A). Other cancers have an increase in growth factor receptors; for example, in breast cancer, the epidermal growth factor (erbB2) receptor is up-regulated, which means that even when the epidermal growth factor is at very low levels, the cell still receives the signal to grow (see Figure 36-3B).
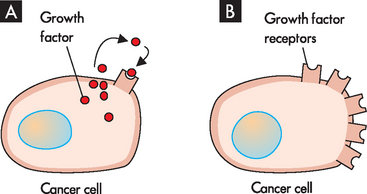
FIGURE 36-3 Cancer cell growth.
Cancer cells can support their own growth by either A, secreting growth factors (known as autocrine stimulation) or B, increasing the numbers of growth factor receptors.
Cells have a mechanism that induces them to self-destruct when growth is excessive. This self-destruct mechanism, called apoptosis, is a way of ensuring that abnormal cells are destroyed to preserve the health of the body (see Chapter 4). But apoptosis is actually disabled in advanced cancers. The most common mutations that allow cancer cells to resist apoptosis occur in the p53 gene (see below). The cancer cells are ‘immortal’, meaning they can avoid apoptosis. A structure known as a telomere is located at the end of each chromosome and, in a normal cell, these shorten slightly after each cycle of cell division. As the telomeres become shorter, they are unable to protect the ends of the chromosomes; as a result, the chromosomes fragment and the cell dies. Cancer cells are able to continue to restore their telomeres (by the enzyme telomerase), thereby protecting their chromosomes and allowing them to divide over and over (see Box 36-1).2–4 4 6
As normal cells divide, their telomeres become shortened. With repeated cell division, shorter telomeres make the chromosomes prone to fragmenting and dying. Because most normal cells do not divide frequently, they do not require regeneration of telomeres. Cancer cells are different, as they divide rapidly, losing parts of the telomere each time. In order for the chromosomes to survive this process, they need to be regenerated, so cancer cells have the telomerase enzyme to repair their telomeres, thereby maintaining the health of the chromosomes and allowing the cancer cells to live. Researchers Elizabeth Blackburn, Carol Greider and Jack Szostak were awarded a Nobel Prize for research in this field in 2009.
Source: http://nobelprize.org/nobel_prizes/medicine/laureates/2009.
Mutations to genes: oncogenes and tumour-suppressor genes
What types of mutations actually occur in cancer? Oncogenes are mutant genes that promote cell proliferation and the development of cancer. Conversely, tumour-suppressor genes encode proteins that in their normal state suppress the development of cancer. Mutations or abnormalities in the DNA of these genes result in cancer (see Figure 36-4).
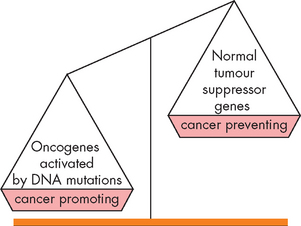
FIGURE 36-4 An imbalance in cancer-promoting oncogenes and cancer-preventing tumour-suppressor genes leads to cancer.
When oncogenes are activated this can lead to uncontrolled cell growth and replication.
Several types of genetic events can activate oncogenes. Perhaps the most common are small changes in DNA (known as point mutations), the alteration of one or a few components of the gene, which can have profound effects on the activity of proteins. This causes an oncogene to become more active, thereby increasing the development of cancer. Oncogenes can also contribute to the development of cancer by chromosome amplifications, whereby a small piece of a chromosome is duplicated over and over again, so that instead of the normal two copies of a gene, there are tens or even hundreds of copies. This results in increased expression of an oncogene or even drug-resistant genes. For instance, the epidermal growth factor receptor erbB2 is amplified in 20% of breast cancers5 — an increase in the amount of receptors for this growth factor allows the growth of the cancerous cell to be stimulated (see Figure 36-3B).
Tumour-suppressor genes are genes whose major function is to slow the cell cycle, inhibit proliferation from growth signals and stop cell division when cells are damaged. Examples of abnormal conditions where loss of tumour suppressor function leads to cancers are given in Table 36-3. One of the best known examples of a tumour-suppressor gene is the p53 gene, which causes apoptosis of cancerous cells, thereby preventing the cancerous cell from dividing. Abnormalities in the p53 gene are often inherited and changes in this important protective gene are strongly linked to the development of cancer (see Figure 36-5).
Table 36-3 FAMILIAL CANCER SYNDROMES CAUSED BY TUMOUR-SUPPRESSOR GENE FUNCTION LOSS
| SYNDROME | GENE |
|---|---|
| Familial melanoma | p16INK4a, CDK4 |
| Familial adenomatous polyposis (colorectal cancer) | APC |
| Breast cancer | BRCA1, BRCA2 |
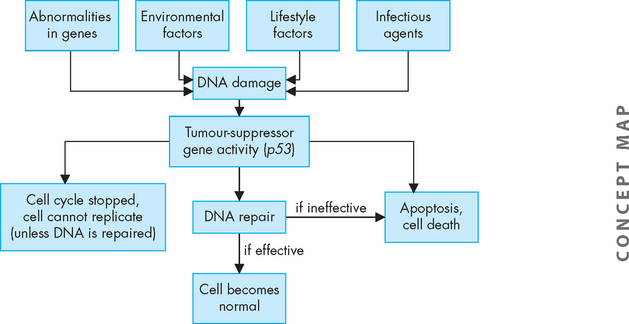
FIGURE 36-5 Damage to DNA may occur due to genetic abnormalities or environmental and lifestyle factors.
In response to damaged DNA, the tumour suppressor gene p53 can stop the cell cycle, cause cell apoptosis or control DNA repair.
Although oncogenes can become activated to contribute to cancer development by a single mutation, tumour-suppressor genes must be inactivated to enable cancer to occur (see Figure 36-6). There are two chromosomal copies, or alleles, of each gene, one from each parent. It therefore takes two ‘hits’ to inactivate both copies of a tumour-suppressor gene. The first copy of a tumour suppressor is often inactivated by point mutations. If the remaining gene is mutated, then another step towards cancer occurs (see Figure 36-7).
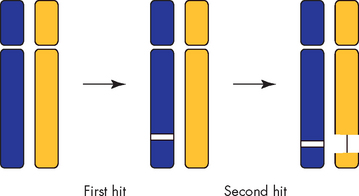
FIGURE 36-6 Two distinct hits are required to inactivate a tumour-suppressor gene.
Tumour-suppressor genes are often inactivated by a mutation (first hit) followed by complete loss of an entire region of chromosome encompassing the remaining normal allele (second hit).
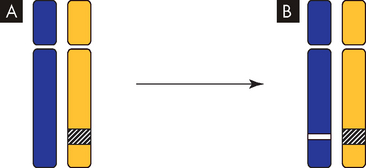
FIGURE 36-7 Silencing of tumour-suppressor genes.
A One allele is inactivated. In this example, the first copy of a gene is turned off by gene silencing without mutation. B Mutation of the other allele results in no functional protein production. In this example, the remaining normal gene is inactivated by mutation.
Caretaker genes encode proteins that are involved in repairing damaged DNA — DNA damage may occur due to errors in DNA replication or mutations due to environmental and lifestyle causes (see later). Loss of caretaker gene function leads to increased mutation rates (see Figure 36-8A). Inherited mutations can also disrupt the caretaker gene function. For example, hereditary nonpolyposis colorectal cancer (HNPCC, particularly with genetic mutations in MLH1 and MSH2) is an inherited defect in DNA repair and affected individuals have a high rate of colon cancer.6
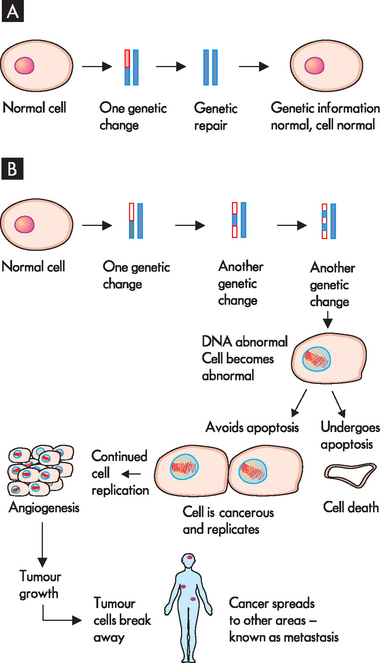
FIGURE 36-8 Development of cancer.
A A normal cell can have one or two minor genetic changes and actually repair the DNA using caretaker genes to return to normal. B A number of genetic mutations lead to the development of an abnormal cell. If the cell undergoes apoptosis, it is removed. However, cells that can avoid apoptosis can replicate, forming a large tumour growth; this can stimulate angiogenesis, as well as spreading to other body areas by metastasis.
Genetics and cancer-prone families
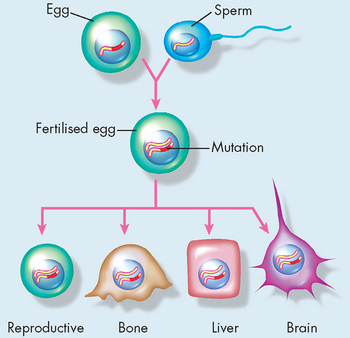
Most of the genetic alterations that cause cancer occur during the lifetime of the individual. The frequency of these events can be altered by exposure to mutagens — that is, agents causing mutations — and by defects in DNA repair that increase the rate of mutations. Because these genetic events occur in the individual’s mature body cells, as opposed to the germ cells (i.e. eggs or sperm), they are not transmitted to future generations. Even though they are genetic events, they are not inherited! It is possible, however, for cancer-predisposing mutations to occur in germline cells (cells that produce gametes; see Figure 36-9). Mutations present in germline cells result in the transmission of cancer-causing genes from one generation to the next, producing families with a high incidence of specific cancers. These inherited mutations that predispose to cancer are almost invariably in tumour-suppressor and caretaker genes (see Table 36-3), and hence protection from cancer is lessened.
Although rare, such ‘cancer-prone families’ demonstrate that inheritance of a mutated gene can cause cancer. In these families, inheritance of one mutant allele predisposes to a specific form of cancer: individuals who inherit the germline mutant allele are quite likely to develop the tumour, as they have only one allele (from the other parent) that is normal. Examples of cancers that can be inherited are inherited breast cancer (altered BRCA1 gene) and familial adenomatous polyposis coli (FAP, mutation of the APC gene; see Figure 36-10). Characterisation of cancer-causing genes and other genetic factors helps identify individuals prone to developing cancer. Individuals known to carry mutations in tumour-suppressor genes, such as women with BRCA1 mutation, are targeted for cancer screening to facilitate early cancer detection and therapy.7
Cancer growth rates
Cancer is predominantly a disease of ageing (see Figure 36-11). The incidence of cancer — that is, the percentage of individuals who develop cancer — increases dramatically with age. Cancers may take many years to develop from the initial stages until they are diagnosed and therefore it is more likely that they will occur in older age groups. Also, genetic changes and exposure to environmental risks increase over time. When sufficient genetic mutations occur, cancer develops. For example, an individual mutation may occur in a single cell such that it may acquire a characteristic of cancer — say, increased growth rate. That cell may then quickly divide and become an early-stage tumour. In this example, because the gene was altered, the cellular process changed (namely, increased growth rate), which resulted in the development of cancer.
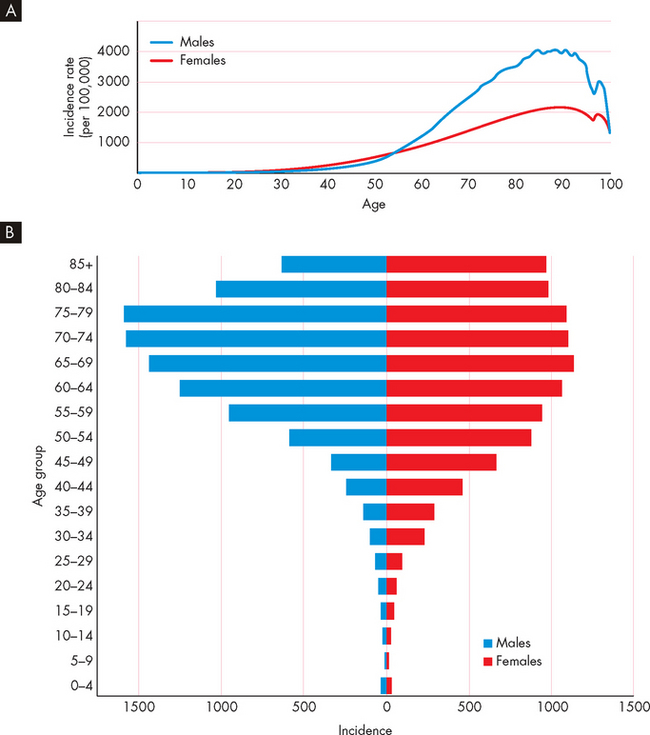
FIGURE 36-11 The total incidence of cancer increases with age.
A The incidence of cancer in Australian males and females over a 5-year period. B Note that New Zealand cancer data is comparable.
Source: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview. Cancer series no. 46. Catalogue no. CAN 42. Canberra: AIHW; 2008; New Zealand Health Information Service. Cancer: new registrations and deaths, 2004. Wellington: Ministry of Health; 2007.
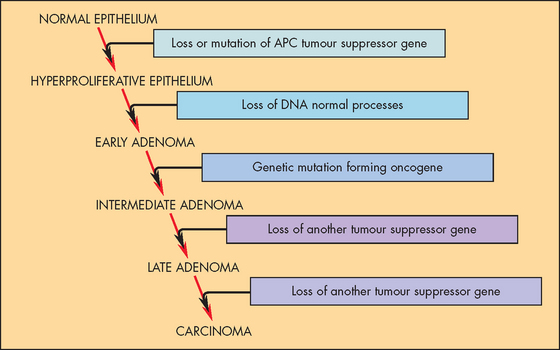
One organ in which this relationship of genetic effects and clinical progression has been especially well studied is the colon.8 The colon is accessible to inspection with a colonoscope (a flexible tube with a light and camera), so neoplastic growths of varying size can readily be detected and removed. Intestinal polyps are benign neoplasms and are the first stage in the development of colon cancer. Small polyps tend to have only a few genetic mutations, while large polyps have more mutations. This strongly supports the notion that several genetic changes are required for the development of cancer.
Cancer growth, spread and metastasis
After the genetic changes occur that allow cancer to commence, the cancer cell replicates. This one cancer cell divides to become two cancer cells, each of which then grows rapidly and divides to produce more cancerous cells. In this way, the original cancer cell can form a large number of cancer cells, which may continue growing and dividing to become life-threatening to the individual (see Figure 36-8).
Cancer cells have two important properties: autonomy and anaplasia. Autonomy refers to the cancer cell’s independence from normal cellular controls, such as its ability to avoid apoptosis. Anaplasia is the loss of differentiation, which normally gives the cell its specialised organisation and functions. In particular, anaplasia is characterised by an increase in the size of the nucleus due to rapid DNA replication and cell division. The normal function of the cell is lost as the cell becomes cancerous. For example, a benign bone tumour retains the ability to make bone, whereas in a malignant bone tumour, new bone formation is rare.
Cancers spread locally as the increasing number of cells occupies more room. This leaves less space for normal cells and contributes to loss of normal tissue function. In order for cancers to enlarge, they need an adequate blood supply to deliver oxygen and nutrients (see Figure 36-12). The process of angiogenesis is the development of new blood vessels, which occurs normally during growth such as in childhood or with increased muscle size from training. Angiogenesis is also necessary for continued cancer growth. The growth of small cancers is limited as they lack the ability to grow new blood vessels, but more advanced cancers secrete substances such as vascular endothelial growth factor that stimulate angiogenesis. Some drugs inhibit the process of angiogenesis, thereby preventing further capillary growth and limiting the cancer enlargement. For example, bevacizumab inactivates vascular endothelial growth factor and is used in combination with other drugs for colorectal cancer, non-small-cell lung cancer and breast cancer to limit the cancer growth.9,10
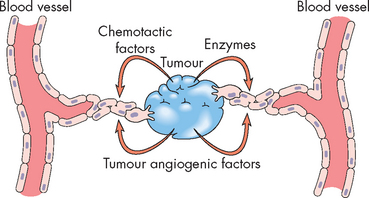
FIGURE 36-12 Tumour-induced angiogenesis.
Malignant tumours, especially those in metastatic sites, induce the formation of blood vessels, which serve as routes for the transport of nutrients into the tumour. The approved drug bevacizumab blocks vascular endothelial growth factor.
Source: Damjanov I. Pathology for the health professions. 3rd edn. St Louis: Saunders; 2006.
Metastasis is the spread of cancer cells from the site of the original tumour to distant tissues and organs throughout the body. The original site of the cancer is known as the primary tumour. Metastasis causes the development of secondary cancers (see Figure 36-13). In some cases the primary cancer has already metastasised to other body tissues prior to diagnosis; this makes it very difficult to identify the primary site, and the individual may be described as having cancer of unknown origin.
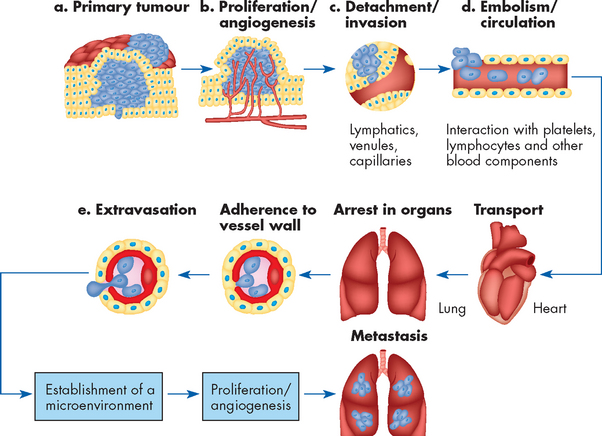
FIGURE 36-13 The multistep nature of metastasis.
Source: Fidler IT. The pathogenesis of cancer metastasis. Nat Rev Ca 2003; 3:453–458.
Metastasis is a defining characteristic of cancer, contributes significantly to the pain and suffering caused by cancer and is the major cause of death from cancer. While localised, low-stage cancers can often be cured by a combination of surgery, chemotherapy and radiation, these same therapies are frequently ineffective against cancer that has metastasised. For example, in women with breast cancer that has not reached the lymph nodes, the 5-year survival rate in Australia is 97%. However, in those whose cancer has spread to the lymph nodes, the 5-year survival rate is lowered to 80%.11 Not all types of cancers undergo metastasis and those that do metastasise are usually more aggressive, with a less promising patient prognosis.
Cancer cells can spread through vascular and lymphatic pathways, as well as through tissues. The blood vessels and lymphatic vessels within tumours offer malignant cells direct access into the blood and lymph circulation. Because the cancers often spread through the local lymph nodes, these are often assessed for possible metastasis of cancer (see ‘Diagnosis and evaluation of cancer’ below). The most common sites of metastasis are the liver, bone, lungs and brain (see Figure 36-14), although particular cancers metastasise to predictable areas: breast cancer often spreads through the blood to bones but rarely to the kidneys or spleen, whereas lymphomas often spread to the spleen but uncommonly spread to bone (lung cancer metastasis is shown in Figure 36-15). This tissue selectivity is probably due to specific interactions between the cancer cells and specific receptors on the small blood vessels in different organs (see Table 36-4).
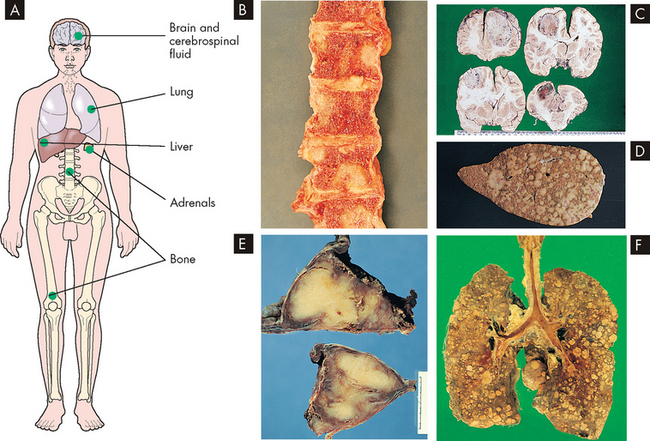
FIGURE 36-14 The main sites of blood-borne metastasis.
A Sites of haematogenous metastasis. B Metastasis in bone. C Metastasis in the brain. D Metastasis in the liver. E Metastasis in the adrenals. F Metastasis in the lungs. Blood-borne tumour metastasis leads to growth of secondary tumours in several main sites. The macroscopic appearances of bone metastasis are shown in B, where lesions are seen in the vertebrae. Numerous metastases from a neoplasm of the stomach are seen in the brain in C. The liver is the most common site for metastases from tumours in the gastrointestinal tract, as seen in D, which arose from a colonic neoplasm. In E, metastatic tumour has replaced both adrenal glands, as is commonly seen with spread from lung and breast tumours. The lung, F, is the most common site for blood-borne metastases from tumours outside the spinal tract, particularly mesenchymal tumours.
Source: Stevens A, Lowe J. Pathology: illustrated review in color. 2nd edn. Edinburgh: Mosby; 2000.
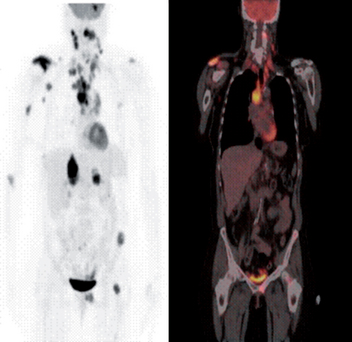
FIGURE 36-15 Metastatic non-small-cell lung cancer (NSCLC).
This 54-year-old woman had a NSCLC resected from the left upper lobe. Five years later, these studies were obtained. The positron emission tomography (PET) scan shows metastatic lesions in the brain, right shoulder, mediastinal and cervical lymph nodes, as well as the liver, left pelvis and proximal femur. (Left) PET whole body image. (Right) Whole body PET/CT scan image of the same patient. The pattern of spread is most likely from the primary tumour to the large mediastinal lymph nodes, followed by lymphatic spread to the cervical nodes.
Source: Images courtesy of John Hoffman, MD, Huntsman Cancer Institute.
Table 36-4 COMMON SITES OF METASTASIS
| PRIMARY TUMOUR | MAJOR ANATOMICAL PATHWAY | COMMON SITE OF DISTANT METASTASIS |
|---|---|---|
| Lung | Pulmonary vein, left ventricle | Multiple organs, including brain |
| Colorectal | Mesenteric lymphatics, portal venous system | Liver |
| Inferior vena cava, right ventricle, pulmonary artery | Lungs | |
| Prostate | Regional lymphatics and veins | Bones (especially lumbar spine), liver |
| Breast | Axillary, transpectoral and internal mammary lymphatics | Bone, lungs, brain, liver |
| Melanoma | Regional lymphatics | In-transit lymphatics, lungs, liver, brain, gastrointestinal tract |
It is also difficult to conclude immediately if surgical removal of a cancer has been an effective treatment, as some cells may have broken away prior to surgical removal of the cancer: the presence of microscopic levels of cells which have metastasised elsewhere in the body cannot be detected. Therefore, months may need to pass to allow the potential development of metastatic growth to a detectable size before the surgery can be considered a success.
CANCER, IMMUNITY, INFLAMMATION AND INFECTION
Cancer and the immune system
The immune system reacts to infection and tissue damage; it also recognises non-self and foreign antigens (see Chapter 12). The immune system plays a complex role in the development and progression of cancer. It has been suggested that immune surveillance might recognise some early cancers as ‘foreign’ and suppress or eliminate them before they can develop. Components of the immune system that are particularly involved in protection from cancer are cytotoxic T lymphocytes and natural killer cells, as well as macrophages, which perform phagocytosis of foreign substances. A generalised inflammatory response is also part of the process of defending the body from a foreign agent. The immune system defences are probably effective against a very small number of cancer cells, but are unable to destroy large growths, and immune processes are likely to be altered with cancer. As a consequence, the immune system will continue to be activated during proliferation of cancer in an attempt to rid the body of the foreign substance, although unfortunately the cancer will not be effectively destroyed and removed.
Cytokines are chemical signals that are released by immune cells to signal between immune system processes. Cytokines that are particularly associated with the response to cancer are:
 interleukin-1 (IL-1) from macrophages (a type of antigen presenting cell) to induce the inflammatory response
interleukin-1 (IL-1) from macrophages (a type of antigen presenting cell) to induce the inflammatory responseAn increased knowledge of the details of how the immune system works in targeting cancer may contribute to improved cancer treatments in the future. In developed cancers, it appears that the normal role of these cytokines is altered through complex mechanisms.
Defects of the immune system, due to HIV infection or immunosuppressant drugs, can substantially increase the incidence of some cancers, such as lymphomas and herpes virus-caused cervical cancer.12,13 However, patients receiving immunosuppression after an organ transplant have little or no increase in the most prevalent cancers, such as breast, prostate and colon cancers, strongly suggesting that immune surveillance is not important in preventing all types of cancers.
Chronic inflammation
Chronic inflammation is a form of immune response that has been recognised since the 1860s as an important contributing factor to the development of cancer.14,15 For example, people with ulcerative colitis (chronic inflammation of the large intestine) have a substantially higher risk of developing colon cancer; and chronic inflammation of the liver as a result of chronic viral hepatitis B or C markedly increases the risk of liver cancer.
The reasons for the inflammation leading to cancer are complex. After injury, inflammatory cells release cytokines and growth factors that stimulate local cell proliferation, vascular growth and wound healing. In chronic inflammation, these factors combine to promote continued proliferation (see Figure 36-16). In addition, inflammatory cells release other substances that can both promote mutations and block the cellular response to DNA damage. Notably, increased abundance of the enzyme cyclo-oxygenase 2 (COX-2), which generates prostaglandins during acute inflammation, has been associated with colon and other cancers. Non-steroidal anti-inflammatory drugs, such as aspirin and ibuprofen, inhibit COX-2, prevent the formation of inflammatory mediators and protect against colon cancer development.
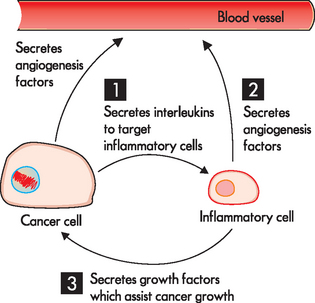
FIGURE 36-16 The intimate association of inflammation and cancer.
Cancers attract inflammatory cells, and can then (1) secrete cytokines, which allows the cancer cells to regulate the inflammatory cell function. The inflammatory cell response is (2) secretion of angiogenesis factors (in addition to the cancer cell also secreting substances to promote angiogenesis) and (3) secretion of growth factors for the cancer. These responses of the inflammatory cell can contribute to the success of the cancer growing.
Thus, the role of the immune system in cancer is complex. The concept that the immune system protects us against cancer is true for a limited number of cancers, but mainly when only a small number of cancer cells are present. Chronic activation of the immune system also promotes cancer growth, angiogenesis and cancer progression — this is the subject of current research.
Viral causes of cancer
A number of viruses have been associated with cancer.16,17 Hepatitis B and C viruses (which affect the liver) and human papillomavirus (HPV — which affects the cervix) are common viral causes of cancer in Australia and New Zealand; cancers of the liver and cervix account for about 80% of virus-linked cancers. The initial acute infection with hepatitis B or C is not associated with hepatic cancer; instead, it is the chronic viral hepatitis that markedly increases cancer risk. Widespread use of the hepatitis B vaccine is expected to significantly decrease the incidence of chronic hepatitis B and hence hepatocellular carcinoma. Healthcare workers are immunised with hepatitis B vaccine prior to commencing work with patients.
Approximately 80% of all cervical cancer is due to infection with specific subtypes of HPV. Although there are dozens of types of HPV, only a few are associated with cervical cancer: the majority of HPV infections cause only minor problems such as genital warts.18 HPV is spread primarily through genital contact (oral, touching or sexual intercourse); therefore, condoms are not necessarily protective. The HPV viral DNA becomes accidentally integrated into the infected cervical basal cell chromosome and directs the production of viral oncogenes. Early oncogenic HPV infection is readily detected by Papanicolaou (Pap) smear, an examination of cervical epithelial cells. Early detection of abnormal cells in a Pap smear often leads to the detection of cervical carcinoma while it is still localised and can be easily and effectively treated. In Australia and New Zealand, where routine Pap smears are common, cervical cancer is rare. Vaccines against HPV (Gardasil™) have proven effective in preventing infection and have the potential to significantly reduce cancer mortality (see Box 36-2).19
Box 36-2 CERVICAL CANCER VACCINATION
The cervical cancer vaccine protects from cancer by providing protection from a number of strains of the human papillomavirus. The technology used to create the vaccine was developed by Professor Ian Frazer’s research team in Brisbane. After 15 years, the vaccine was ready, which led to Prof Frazer being named Australian of the Year in 2006. The vaccine, known as Gardasil™, was added to the National Immunisation Program in Australia in 2007 and New Zealand in 2009. The goal is to offer protection from this virus before teenage girls become sexually active. However, the vaccine does not protect against all types of cervical cancer, so regular Pap smear testing is still recommended.
Source: Department of Health and Ageing. Australian government funding of Gardasil. Fact sheet. Updated November 2006. Available at www.health.gov.au; Cervical cancer prevention and human papillomavirus (HPV) vaccine. Fact sheet for young women, parents and caregivers. Available at www.health.gov.au.
Bacterial causes of cancer
Helicobacter pylori (H. pylori) is a bacterium that infects the lining of the stomach and duodenum of approximately 15% of the Australian population.20 H. pylori is responsible for the majority of cases of gastric carcinomas.21–23 4 6 The mechanisms proposed for H. pylori–associated tumour development include alterations of the gastric epithelial cell cycle, direct mutations to the DNA, alterations in growth factor secretion and cytokines and decreased gastric secretion. Research is currently underway into potential screening for H. pylori, which may be considered in Australia in future years.24,25
GENE–ENVIRONMENT INTERACTION
Environmental factors can cause genetic mutations. There are environmental agents that have carcinogenic properties — substances that cause cancer are known as carcinogens. For example, evidence shows that cigarette smoke contains many carcinogenic substances, which cause lung cancer (and other cancers too). Many specific risk factors for cancer are known, the most significant being smoking, radiation, obesity, some viruses and H. pylori bacteria.26,27
One consideration of the role of genetics in cancer is the differing incidence of cancers between different countries. Breast cancer, for example, is prevalent in Australia and New Zealand, but relatively rare among women in developing countries. The difficulty lies in determining whether these differences between populations are attributable to lifestyle factors or genetics, or both. In one study of breast cancer in women who have immigrated to Australia, the rate of breast cancer was found to be similar to that in their homeland for women who had not long arrived, but for those who had spent years living in Australia the cancer rate was more like that of the rest of the Australian population — this emphasises the importance of the environment, as presumably the migrants ended up adopting a more Australian diet and lifestyle.28 These observations strongly implicate environmental and lifestyle factors in the development of cancers. However, because some individuals within the same environment develop cancer and others do not, cancer risk seems to depend on an interaction between genetic or inherited factors and environmental agents.
It is interesting to consider the importance placed on genetics when searching for ways to treat and prevent diseases such as cancers. There was great anticipation at the time that when the sequencing (or coding) of the full human genome (both copies of all 23 chromosomes) was discovered it would greatly assist in advancing our knowledge of genetics and therefore assist our management of diseases. However, there is increasing evidence that the environmental and lifestyle factors to which our genes are exposed are critical in the prevention and development of cancer (see Box 36-3).
Box 36-3 GENETICS CAUSING CANCER?
After sequencing his own genome, pioneer genomic researcher Craig Venter remarked at a leadership for the twenty-first century conference:
‘Human biology is actually far more complicated than we imagine. Everybody talks about the genes that they received from their mother and father, for this trait or the other. But in reality, those genes have very little impact on life outcomes. Our biology is way too complicated for that and deals with hundreds of thousands of independent factors. Genes are absolutely not our fate. They can give us useful information about the increased risk of a disease, but in most cases they will not determine the actual cause of the disease, or the actual incidence of somebody getting it. Most biology will come from the complex interactions from all the proteins and cells working with environmental factors, not driven directly by the genetic code.’
This statement is very important because looking to the human genome for solutions to most chronic illnesses, including the diagnosis, prevention and treatment of cancer, is overemphasised in today’s world.
Source: Anand P et al. Cancer is a preventable disease that requires major lifestyle changes. Pharmaceutical Research 2008; 25:2097–2116.
Factors that increase the risk of cancer
Cigarette smoking
Cigarette smoke is carcinogenic and remains the most important cause of cancer. The risk is greatest in those who begin to smoke when young and continue throughout life.26 Tobacco smoking is responsible for approximately 20% of all cancer deaths in Australia.29 Tobacco use is associated primarily with squamous and small cell carcinomas of the lung and pulmonary adenocarcinomas. It is also linked to other cancers of the lower urinary tract (renal, penis and bladder), upper respiratory and digestive tracts (oral cavity, pharynx, larynx, nasal cavities, paranasal sinuses, oesophagus and stomach), liver, kidney, pancreas, cervix and myeloid leukaemia.30 In addition, smoking contributes to mortality due to other diseases such as cardiovascular disease; in total it accounts for an astonishing 4–5 million deaths per year worldwide.30
Passive smoking occurs when someone who is not smoking inhales cigarette smoke. It is the combination of smoke burning at the end of a cigarette and the smoke exhaled by the smoker. More than 4000 chemicals have been identified in tobacco smoke, 60 of which are considered carcinogenic.31 Passive smoking is a particular issue for children who live with smokers. In an Australian study, Aboriginal children who were hospitalised for lower respiratory infection or infectious gastroenteritis were surveyed and almost two-thirds lived with at least one smoker and almost half of these children had a regular cough32 — this illustrates the damage of smoking to children of a young age and strongly indicates the need to avoid smoking near children.
The Australian government has recently released some preliminary health goals for 2020, including a target to reduce the smoking rate to less than 10% of the population.33 However, it has been calculated that if current rates of starting and quitting smoking continue, 14% of the population will still be smoking in 2020.34 If this rate is to be reduced to less than 10%, clearly more people need to quit and fewer people need to start smoking. Those in blue-collar occupations such as manual labour have particularly high smoking rates, as do those in lower socioeconomic groups;35,36 and people in rural areas and those with financial difficulties may be less likely to succeed in quitting smoking.37,38 These groups may represent targets for anti-smoking education measures.
Measures that prevent young people from starting smoking would substantially avoid the future burden of disease. Therefore, a public health approach is needed that prevents young people from starting smoking and helps others to stop smoking. A study conducted in 2009 of students aged 12–17 years in Australia showed that although smoking was more common among Indigenous students than among non-Indigenous students, the overall prevalence for this age group is lower now than it was 10 years ago.39 In 2006, Australia was one of the first countries to introduce graphical, pathological images of the consequences of smoking on cigarette packets. A survey of smokers in a central business district showed that 38% felt motivated to quit by the packaging.40 Other measures are still needed, however, to assist in decreasing the prevalence of smoking.
The use of cannabis (marijuana) is often seen as a less-harmful option to tobacco smoking. There is some evidence that cannabis is carcinogenic, indicating that it has an increased risk of cancer compared with tobacco smoke, but other data suggest that it is less carcinogenic than tobacco smoke.41–44 42 43 44 For now, the safety of cannabis use regarding lung cancer development is unclear.
Dietary factors
Understanding dietary factors that increase the risk for cancer is challenging. It is complex because of reasons including the variety of foods consumed, the many constituents of foods and changes in the patterns of food consumed over many years. Furthermore, if dietary carcinogens are ingested, the liver may be able to metabolise many of them before they reach the circulation. Cancer risks in the elderly may also depend as much on diet in early life as on current eating practices.26,45
Dietary sources of potentially toxic carcinogenic substances include compounds produced in the cooking of protein, particularly meats. The greatest levels are found in well-done, chargrilled beef. Naturally occurring carcinogens are associated with aflatoxin (produced by mould), which can contaminate corn, peanuts and rice stored in hot, humid environments.46
Abundant research since the 1980s has shown that rates for various cancers correlate fairly consistently with certain dietary factors, but opinions differ on the strength of the evidence.26,47,48 The Western dietary pattern, common in Australia and New Zealand, is generally described as being high-fat/low-fibre and includes a high intake of red and processed meats, refined grains, sweets, desserts and high-fat dairy products. This pattern is opposed to a prudent pattern of fruits, vegetables, whole grains, low-fat dairy products, fish and poultry. The Western diet is certainly related to cancers. For example, there is a 30–40% increase in colon cancer in those who consume high amounts of red and processed meat,49 and high levels of dietary fats are associated with many cancers, including breast,50 prostate51 and pancreatic cancers.52
Obesity
The prevalence of overweight and obesity in Australia and New Zealand is increasing (refer to Chapter 35). Compared with people whose body mass index (BMI) is normal, men and women with substantial obesity (BMI ≥ 40.0) have significant increases in cancer mortality; people with lesser degrees of obesity have lesser increases in cancer mortality. Men with a higher BMI have higher rates of death from oesophageal, stomach, colorectal, liver, gallbladder, pancreatic, prostate and kidney cancers and non-Hodgkin’s lymphoma, multiple myeloma and leukaemia.53 Women with a higher BMI have greater morbidity from colorectal, liver, gallbladder, pancreatic, breast, uterine, cervical, ovarian and kidney cancers and from non-Hodgkin’s lymphoma and multiple myeloma. It is estimated that obesity accounts for 14–20% of all cancer deaths. The impact of obesity on the development of cancer may become more noticeable in the future, as not only is the prevalence of obesity increasing, but also those who are obese now may still be in the early asymptomatic stages of cancer development.
Adipose tissue is active endocrine and metabolic tissue that can have profound effects on the physiology of other tissues (see Chapter 35). In response to signals from other organs, adipose cells respond by increasing or decreasing the rate of triglyceride breakdown and the release of free fatty acids to the blood as fuel for skeletal muscle and other tissues. Abdominal visceral adipose cells are more metabolically active and release larger amounts of free fatty acids compared with adipose cells elsewhere in the body and so abdominal fat is of higher significance.
Increased release of free fatty acids and TNF-α by adipose tissue gives rise to insulin resistance — a state characterised by the reduced metabolic response of tissues (muscle, liver, adipose) to insulin and to compensatory hyperinsulinaemia (high blood insulin).54 Chronically increased insulin levels have been related to the pathogenesis of colon, breast, pancreatic and endometrial cancers (see Figure 36-17).55
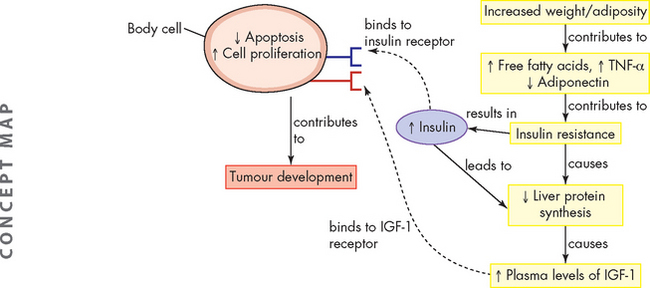
FIGURE 36-17 Energy balance, lipid metabolism, insulin sensitivity and tumour development.
High levels of insulin and insulin-like growth factor-1 (IGF-1) arising from increased levels of free fatty acids; tumour necrosis factor-alpha (TNF-α) induced by obesity leads to changes in cellular responses. These include an increase in cell proliferation and reductions in apoptosis. These effects can promote tumour development.
Adipose cells also produce various steroid-hormone-metabolising enzymes and are an important source of circulating oestrogens in postmenopausal women. In addition, insulin promotes the production of the sex hormones, including oestrogens, progesterone and androgens. Adiposity-induced alterations in blood levels of sex steroids could explain the relationship between excess weight and the risks of breast cancer (postmenopausal) and endometrial cancer (both premenopausal and postmenopausal).54 Among men, prostate carcinogenesis is thought to be related to androgen production and possibly oestrogen as well (see Chapter 32).
Alcohol consumption
Chronic alcohol consumption is a strong risk factor for cancer of the oral cavity, pharynx, larynx, oesophagus and liver. Cirrhosis resulting from alcohol intake increases the risk of liver cancer and alcohol also contributes to breast cancer and colorectal cancer. Alcohol interacts with cigarette smoke, increasing the risk of malignancies, particularly those of the head and neck which are anatomically close to regions where cigarette smoke and alcohol enter the body. In addition, inherited genetic factors such as altered DNA repair, metabolism of carcinogens and control of the cell cycle put some individuals at increased risk.56 Mechanisms that may promote carcinogenic action include the presence of carcinogens in alcoholic beverages, nutritional deficiencies (e.g. vitamins A and C, folate, riboflavin and iron) which give rise to altered cell and tissue structure and function, and decreased immune responsiveness.57 In addition, alcoholic liver injury may affect important mechanisms of chemical detoxification — a critical function of the liver — thereby increasing cancer risk. The relationship between cancer risk and alcohol consumption is difficult to determine because of problems in accurately measuring the amount of alcohol ingested and in defining other behavioural habits, such as smoking, that further complicate the clinical picture.
Ultraviolet radiation
Exposure to ultraviolet (UV) radiation is mainly from sunlight and it causes skin cancers. With further depletion of the ozone layer, people will be exposed to higher intensities of UV radiation. The degree of damage in skin depends on the intensity of exposure, the wavelength content (there are two different wavelengths, UVA and UVB) and the depth of penetration. UV radiation is known to cause specific gene mutations.
The relationship between sun exposure and the risk of melanoma (a malignant pigmented mole) remains complex. UV radiation exposure is the most significant factor for the development of melanoma. Although non-melanoma skin cancers are related to cumulative exposure to UV radiation, melanoma is related to episodes of intense, intermittent exposure (a history of sunburn).58 Sunburn reflects an overdose of UV light, triggering inflammation with an increase in cytokine production. Melanomas more commonly occur in areas less continuously exposed to sunlight, like the trunks in men and the back of the legs in women. Family history (including relevance of genetic factors), skin type and the density of moles are important in determining the risk of developing melanoma. Traits associated with a high risk of melanoma are light-coloured hair, eyes and skin, an inability to tan and a tendency to freckle and sunburn.59
The non-melanoma skin cancers are basal cell carcinomas and squamous cell carcinomas. Basal cell carcinomas commonly occur on areas of the body that receive the greatest sun exposure, such as the head and neck. Individuals with these tumours generally have light complexions, light eyes and fair hair. They tend to sunburn rather than tan and live in areas of high sunlight exposure. Squamous cell carcinomas are found more commonly in men who work outdoors. These tumours are distributed over the head, neck and exposed areas of the upper extremities. There has not been consistent population-wide reporting of these cancers in Australia and New Zealand, and hence statistics on cancers usually only include the melanoma skin cancers.
Occupational hazards
A substantial percentage of cancers of the upper respiratory passages, lung and bladder are attributed to occupational factors.60 One notable occupational factor is asbestos, which increases the risk of mesothelioma and lung cancer. Asbestos was used in homes and buildings built before the 1970s to insulate ceiling tiles, flooring and pipe covers. The dust and fibres from the asbestos contribute to mesothelioma; if left undisturbed as sheets in housing structures asbestos appears to be safe. The epidemic of mesothelioma in building workers born after 1940 did not become apparent until the 1990s (due to the long latency). Cancers of the bladder and leukaemia are also linked to particular industries. Studies of occupational exposure to diesel exhaust indicate an increased risk of lung cancer.61 Disentangling data related to lung cancer and occupational risks is complex, especially in combination with active and passive smoking and the interplay of other environmental factors and genetic mutations.
Air pollution
A person inhales thousands of litres of air in one day, so even modest contamination of the atmosphere can result in inhalation of appreciable doses of pollutants. Contaminants include outdoor and indoor air pollutants, such as a wide variety of chemicals (e.g. industrial emissions, chloroform and formaldehyde).62 Living close to certain industries is a recognised cancer risk factor.
A significant indoor air pollutant is radon gas. Radon is a natural radioactive gas that occurs in rock and soil; it can become trapped in houses and gives rise to products known to be carcinogenic to humans. The most hazardous houses can be modified to prevent further radon contamination. Exposure levels are greater from underground mines than from houses, and radon increases the risk of lung cancer in underground miners whether they smoke or not.
Ionising radiation
Much of the knowledge of the effects of ionising radiation on cancer stems from the Hiroshima and Nagasaki atomic bomb exposures in 1945. These caused acute leukaemias in adults and children and increased frequencies of other cancers including thyroid, breast, lung, stomach, colon, oesophageal and urinary tract cancers and multiple myeloma. These risks are apparently not heritable — offspring of both atomic bombs and cancer survivors do not have an increased risk of malformations or cancer.63–65 4 6 Nuclear weapon testing in Australia in the 1950s exposed thousands of Australians to radiation; however, although no direct relationship between cancer mortality and radiation could be shown, other factors such as smoking and exposure to asbestos complicated the findings.66
Exposure to ionising radiation includes emissions from X-rays, radioisotopes (chemicals that emit radiation) and other radioactive sources. Health risks include not only neoplastic diseases, but also inherited mutations that may affect the incidence of diseases in future generations. The cancer risk at lower doses has been the subject of controversy for decades. Two opposing hypotheses have emerged: (1) there is no dose of radiation considered safe and the use of radiation must always be considered on the basis of risk versus benefit; and (2) the health risks of low doses used to assist in medical diagnosis are not now measurable and may be nonexistent.67
Biological consequences of exposure to ionising radiation include cell death and gene mutations (see Figure 36-18). Genes are constantly challenged by factors including DNA replication and cell division, intracellular and extracellular environmental stresses, exposure to toxic chemical agents and background radiation. Although cells have complex mechanisms for trying to maintain genetic stability, failure to repair the DNA can result in alterations in cell proliferation, contributing to cancer.
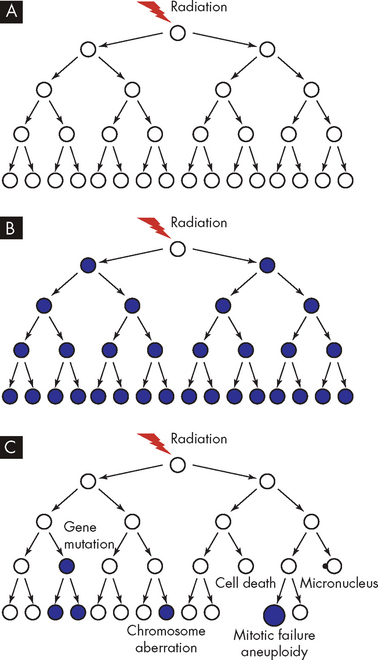
FIGURE 36-18 Models of the responses of clonogenic cells to ionising radiation.
Mutations or chromosomal aberrations are shown as filled circles and apparently normal cells as open circles. A If a cell faithfully repairs DNA damage, then its clonal descendants will appear normal. B If a cell is directly mutated by radiation, then all its descendants will express the same mutation. C Radiation-induced genomic instability is characterised by nonclonal effects in descendant cells.
Source: Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene 2003; 22(45):7058–7069.
Electromagnetic fields
Health risks associated with electromagnetic fields are controversial. Electromagnetic fields are a type of non-ionising, low-frequency radiation, generated from microwaves, radar, electricity and radio waves, fluorescent lights, computers and other electrical equipment. Currently, the evidence supporting an association between electromagnetic fields and cancer is inconclusive.66 Importantly, in one recent study of more than 5000 women in Norway, there was a link between residential electromagnetic field exposure from high-voltage power lines and a 60% increased risk of breast cancer.68 Therefore, electromagnetic fields remain a possible carcinogen.
The controversy about potential health hazards associated with exposure to electromagnetic fields has been stimulated by the recent rapid increased use of mobile telecommunication devices and emissions from mobile phone towers. An association between non-Hodgkin’s lymphoma and the use of mobile and cordless telephones has been found with 5-year or greater use.69 In addition, a study on the use of mobile and cordless phones found a significantly increased risk for high-grade brain tumours (astrocytoma — see Chapter 9).70 Brain tumour risk increased with increasing numbers of hours of phone use. On the other hand, a Swedish study, which included a large number of long-term users, did not find any risk increases for either short- or long-term exposure.71
In order to survey the possible links between electromagnetic fields and childhood cancer, a small number of Melbourne homes were tested and higher than expected magnetic levels were found in rooms where children spent substantial amounts of time72 — although this does not confirm a role in developing childhood cancers. Thus the evidence from various studies does not consistently show a link between electromagnetic fields and cancer, but neither do the results establish the absence of any hazard.
Cancer prevention
Cancer is fundamentally genetic. Tumours occur when certain changes, or mutations, occur in genes. The frequency and consequences of these genetic changes can, however, be altered by environmental factors. For example, certain chemicals can cause genetic mutations and induce tumour development. In addition, environmental factors may increase the growth of genetically altered cells without directly causing new mutations. Thus, environmental factors interacting with genes play an important role in cancer development and progression. Two important means of decreasing the risk of cancers are undertaking adequate physical activity and improving dietary factors.
Physical activity
Physical activity reduces the risk of breast and colon cancers and may reduce the risk of other cancers. Several biological mechanisms causing this effect have been proposed and include the benefits of physical activity in decreasing insulin levels, decreasing obesity, altering inflammatory mediators and decreasing circulating levels of oestrogens and androgens.73,74 For colon cancer, physical activity increases gut motility, which reduces the amount of time that the intestinal lining is exposed to potential mutagens (agents that cause mutations).75 For breast cancer, vigorous physical activity may decrease exposure of breast tissue to ovarian hormones and insulin. For example, after 12 months of moderate-intensity exercise, postmenopausal women had significantly decreased serum oestrogens.76 Decreased tissue exposure to growth factors may also improve outcomes for those with prostate cancer. Physical activity helps prevent type 2 diabetes that has been associated with a risk of cancer of the colon and pancreas, as well as improving cancer-related fatigue.76–78 4 6
Many questions are unanswered regarding the frequency, intensity and duration of exercise. Much of the literature suggests that between 3.5 and 4 hours of vigorous activity per week is necessary to optimise protection for colon cancer.74 There is probably an increased protection with more exercise for colon cancer and breast cancer, and 30–60 minutes per day of moderate- to vigorous-intensity activity is proposed to decrease breast cancer risk.79
Dietary factors
A wide range of substances, many from fruits and vegetables, have been shown to be useful in decreasing the risk of cancers (see Figure 36-19).80,81 According to one recent study: ‘Modification of diet alone by increasing vegetable and fruit intake could prevent 20% or more of all cases of cancer.’82
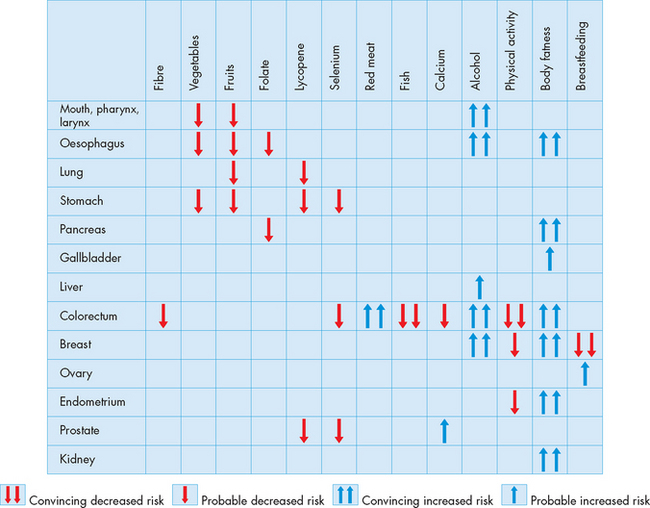
FIGURE 36-19 A summary of cancer prevention through dietary and lifestyle modifications.
Source: Based on Molokhia EA, Perkins A. Preventing cancer. Prim Care Clin Office Pract 2008; 35:609–623.
Antioxidants have become well known — these substances can protect against damage to DNA caused by reactive oxygen species (see Chapter 4). Antioxidants are just one of many types of substances that have shown a protective effect from cancer. Although the high fibre content of fruits and vegetables is likely to be a major contributor to cancer protection, a variety of specific substances with anti-carcinogenic activity found in various fruits and vegetables are protective, including carotenoids such as lycopene (found in pink and red fruits like watermelon and tomatoes), flavonoids (found in vegetables such as cauliflower, cabbage and spinach), selenium and some vitamins. Other substances with anti-carcinogenic activity include polyphenols found in tea and resveratrol found in red wine and grape skins.80–82 4 6
The possible components of a cancer-prevention diet
Some foods increase the risk of cancer, others decrease the risk. Observing the following dietary guidelines might reduce the risk of cancer.
*Influences cancer risk in a complex way; lower levels (≤ 1000 mg/day may) confer protection.
DIAGNOSIS AND EVALUATION OF CANCER
Cancer can be first diagnosed by a number of processes. Some cancers are detected at early stages by screening procedures such as skin inspection, blood tests and routine colonoscopy, whereas some people simply feel the lump of a more advanced cancer. Other cancers come to attention because of symptoms due to the cancer location — for example, benign tumours in the brain may cause neurological disturbances despite being quite small. Still other cancers such as malignant cancers in the abdomen (e.g. pancreatic or liver cancers) may not be detected until they are quite advanced.
Symptoms can be caused by tumour size, by the tumour pressing on a nearby vital structure (e.g. pressure on nerves may cause pain, and erosion of bone can lead to pathological fractures) or by loss of function of an organ. For example, individuals with leukaemia seek medical attention when their healthy bone marrow has been replaced by leukaemia cells; as a result of this decreased bone marrow function, pallor and fatigue result from anaemia, bleeding is caused by low platelet counts and infection is related to loss of white blood cells. Sometimes cancers are detected when symptoms are caused by metastasis rather than the primary tumour.
After establishing the initial diagnosis of a particular cancer, adequate evaluation and cancer staging allows predictions about how the cancer will behave over time, including where it might spread and how likely it is to respond to treatment.
Tumour markers
Tumour markers are substances produced by cancer cells that are detectable in the blood, spinal fluid or urine — for diseases that have a blood-based tumour marker, there is indeed a blood test for cancer. Tumour markers include a variety of substances such as hormones, enzymes, genes and antigens. For example, prostate tumours secrete prostate-specific antigen (PSA) into the blood. Important tumour markers are shown in Table 36-5. These markers can be used to: (1) screen and identify individuals at high risk for cancer; (2) help diagnose the specific type of tumour; and most often (3) follow the clinical course of the cancer. For example, a falling PSA after therapy for prostate cancer indicates successful treatment and a later rise in PSA may indicate a recurrence.
Table 36-5 IMPORTANT TUMOUR MARKERS
| MARKER | MAIN CANCER TYPE |
|---|---|
| Prostate-specific antigen (PSA) | Prostate cancer |
| Carcinoembryonic antigen (CEA) | Colorectal cancer, breast cancer |
| CA 15-3 | Breast cancer |
| Neuron-specific enolase | Lung cancer |
| α-fetoprotein (AFP) | Hepatic cancer |
| CA 19-9 | Pancreatic cancer, colorectal cancer |
| Human chorionic gonadotropin (hCG) | Several tumours (note: also a marker of pregnancy) |
| CA 125 | Ovarian cancer |
A significant problem in diagnosing cancer using tumour markers is that markers may be produced by non-cancerous tissues. As an example, human chorionic gonadotropin (hCG) is increased with some tumours, but it is also produced as a normal process during pregnancy. In addition, some markers are elevated in more than one cancer type, so additional investigations are necessary. For example, carcinoembryonic antigen (CEA) is elevated in the blood by both colorectal cancer and breast cancer. Therefore, the presence of a tumour marker may suggest a particular cancer, but it is not used alone as a diagnostic test. The need to identify specific tumour markers remains a high priority because the early detection of cancer often improves the treatment outcome.
Evaluation
The location of cancers can be observed using X-ray, CT or MRI scans; the use of such scans depends on the organs involved. These scans can also be used to ascertain sites of metastasis (see Figure 36-20). The diagnosis of cancer requires a pathologist to examine a tissue biopsy from the patient. Tissue can be obtained by diverse means, including brushings (e.g. Pap smear), fine needle aspirations (e.g. of a breast mass), core needle or open biopsies that sample a small part of a mass, or complete excision of a mass (e.g. for melanoma). The pathologist uses microscopy to determine whether the tissue is benign or malignant and may use more sophisticated tests to provide additional information.
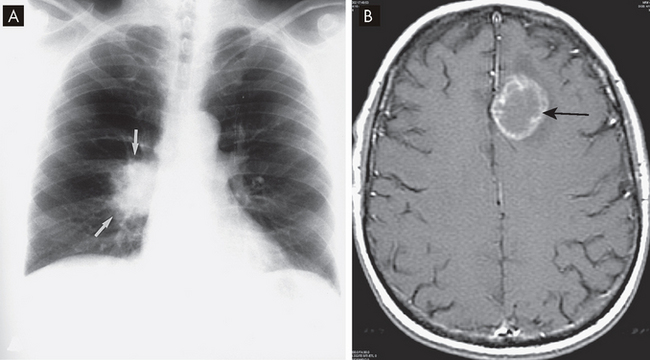
FIGURE 36-20 Imaging of lung cancer.
A Chest X-ray showing the cancer as an ill-defined mass (arrows). B In a patient with small-cell lung cancer, a cranial MRI shows a cerebellar metastasis (arrow).
Source: A Mettler FA. Essentials of radiology. 2nd edn. Philadelphia: Saunders; 2005. B Haaga JR. CT and MRI of the whole body. 5th edn. St Louis: Mosby; 2008.
Sentinel nodes are a set of lymph nodes that are first to receive drainage from any given anatomical location. Presumably, cancer metastasises to these nodes before other nodes. Sentinel node biopsy is widely used in the initial staging of cancer and surgical treatments. Also, a dye or radioactive tracer may be injected into the primary tumour — for example, a breast cancer or melanoma — and then allowed to be taken up by the regional lymph nodes draining the tumour to provide more accurate staging.
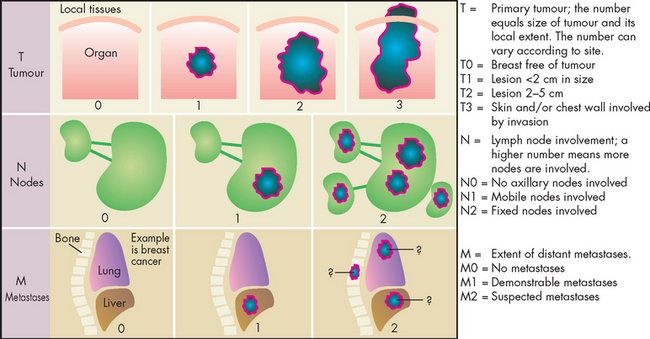
Clinical staging
The information obtained from the pathology diagnosis is combined with clinical information to determine the extent of the cancer and allows the cancer to be classified accorded to different stages. Staging the cancer is an important component of diagnosis and treatment planning. Diverse schemes are employed for staging different tumours, but a general classification using the TNM system is used for many cancers. This defines the tumour size (T), involvement of lymphatic nodes (N) and degree of metastasis (M). An example of the TNM system used for breast cancer is shown in Figure 36-21.
Information from the TNM system can be summarised to a simple four-stage system:
In general, the earlier the stage, the more amenable the cancer is to treatment and the better the prognosis for the patient. Note that the specific guidelines for staging can vary considerably with different types of cancers.
CLINICAL MANIFESTATIONS OF CANCER
There are many clinical manifestations of cancer. The type of sign or symptom is often dependent on the location and the type of tumour. For instance, a patient with a brain tumour is likely to experience headaches due to the location of the cancer, while a patient with leukaemia is likely to experience anaemia. However, cancer symptoms are often common, especially if the cancer is advanced and the patient is terminally ill. In addition, complications of treatment can contribute to the clinical manifestations. Chemotherapy often causes nausea and vomiting and can cause disturbances to eating and therefore nutrition and energy balance. The most common clinical manifestations of cancer include infection, anaemia, pain, fatigue, cachexia and anorexia. The reason for the similarities of these between cancers is complex, but many of the symptoms experienced by individuals with cancer may be mediated by cytokines acting on the peripheral and central nervous systems (see Figure 36-22).
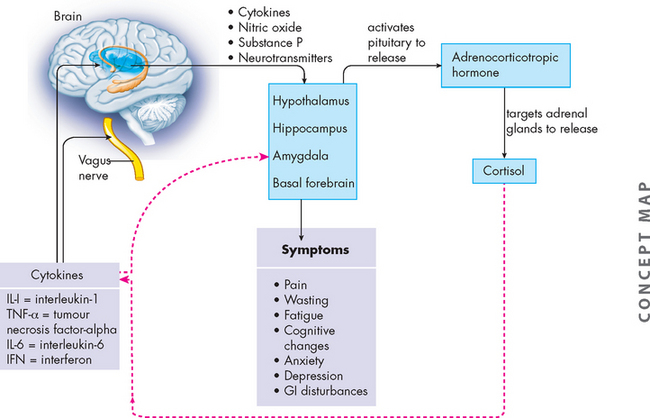
FIGURE 36-22 Theoretical framework for cytokine-induced cancer symptoms.
Pro-inflammatory cytokines and chemokines (IL-1, TNF-α, IL-6, IFN) (solid black lines) are released by immune cells. They exert their effect on peripheral nerves and the brain. Neurotransmitter responses by the brain are affected. The hypothalamic-pituitary-adrenal axis is activated with increased release of corticosteroids, which provide feedback (dotted red lines) to decrease cytokine production.
Source: Based on Cleeland CS et al. Actytokine-immunologic model of cancer symptoms. Cancer 2003; 97(11):2919–2925.
Infection, anaemia and thrombocytopenia
Cancers and cancer treatments can suppress the function of the bone marrow. This can lead to insufficient production of blood cell types — deficiency in red blood cells leads to anaemia, insufficient platelets (thrombocytes) causes thrombocytopenia and deficiency in white cells (leucocytes) leads to leucopenia (see Figure 36-23). Of greatest significance to the patient with cancer is the risk of infection due to inadequate levels of white blood cells.
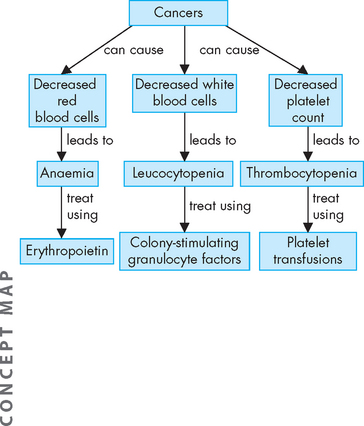
FIGURE 36-23 The effects of cancer on bone marrow production of blood cells.
Note that cancer treatments often cause similar suppression of bone marrow function.
Infection is the most significant cause of complications and death in those with malignant disease. When the neutrophil or lymphocyte count falls, the risk of infection increases, and individuals with cancer have reduced immunological functions. Direct tumour invasion of the bone marrow causes an overall leucopenia, and chemotherapy and radiotherapy are also damaging to the bone marrow. This deficiency in functional white blood cells leaves the patient vulnerable to infection. Other factors that predispose individuals with cancer to infection are summarised in Table 36-6. Surgery can lower resistance to infection because removal of large quantities of tissue, together with haemorrhage and poor tissue perfusion, creates favourable sites for infection. Hospital-acquired infections increase because of indwelling medical devices, inadequate wound care and the introduction of microorganisms from visitors and other persons (see Chapter 14). Patients may be treated with granulocyte colony-stimulating factors (GCSF) such as filgrastim to increase the count of neutrophils (see Figure 36-23) and those who exhibit slight elevations in body temperature are immediately treated with prophylactic antibiotics. In particular, the mouth is prone to infection and children with cancer undergo a regimen of mouth care; for example, nystatin anti-fungal treatment is used several times a day to prevent and treat infection.
Table 36-6 FACTORS PREDISPOSING INDIVIDUALS WITH CANCER TO INFECTION
| FACTOR | BASIS |
|---|---|
| Age | |
| Tumour | |
| Leukaemias | |
| Lymphomas | |
| Treatment surgery |
Source: Donovan MI, Girton SF. Cancer care nursing. 2nd edn. New York: Appleton-Century-Crofts; 1984; Murphy GP, Lawrence W, Lenhard RE. Clinical oncology. 2nd edn. New York: American Cancer Society; 1994.
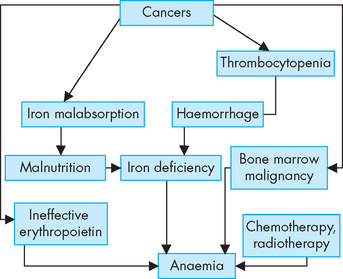
Anaemia is commonly associated with malignancy and may result from chronic bleeding resulting in iron deficiency, malabsorption of iron, severe malnutrition (and anorexia) or medical therapies (see Figure 36-24). If the malignancy is actually in the bone marrow, then normal blood cell production will also be decreased. The release of cytokines during cancer may suppress the action of erythropoietin to stimulate bone marrow production of red blood cells.83 The pharmacological administration of erythropoietin can significantly improve the anaemia and the quality of life for these individuals.84
Thrombocytopenia is a low platelet count and is a major cause of haemorrhage in persons with cancer. It usually results from chemotherapy or bone marrow involvement by the malignancy. Haemorrhage, including any which are undetected, can cause loss of iron, which in turn worsens the anaemia.
Pain
Usually little or no pain is associated with the early stages of malignant disease, but pain occurs in 60–80% of those individuals who are terminally ill with cancer. Pain is strongly influenced by fear, anxiety, sleep loss, fatigue and overall physical deterioration, and the way that pain is perceived and its impact are individual.
General mechanisms that cause pain associated with cancer include pressure, obstruction, stretching, tissue destruction and inflammation. The pain may be directly related to the malignancy or it can result from other problems, such as infection. Bone metastasis causes pain that may be referred away from the involved bone and manifested as back pain. Tumours can obstruct and distend the bowel causing pain. Brain tumours, in particular, have little space to grow without compressing nerves between the tumour and the cranium (nerves within the meninges), thereby causing pain (interestingly, the brain tissue itself does not have pain receptors). The mouth, which is often the site of ulcerative lesions resulting from cancer, can become infected and painful.
The priority of treatment is to control pain rapidly and then to continue evaluating and preventing the recurrence of pain. Combinations of traditional analgesics (opiates such as morphine), novel agents and delivery systems, and attention to a person’s psychological responses, including depression and sleep disturbances, are included in pain management (see Chapter 7).
Fatigue
Fatigue is persistent tiredness and is the most frequently reported symptom of cancer and cancer treatment. Most cancer patients, whether receiving treatment or not, will report fatigue at some time during their illness. It can be caused by a multitude of factors. Fatigue may result from sleep disturbances, changes in cytokines and neurotransmitters, numerous psychosocial factors (such as grief and depression), level of activity and nutritional status.
Similar to pain, fatigue is a subjective clinical manifestation, which means that individuals will experience similar situations differently. For instance, two patients may have the same type of cancer and be receiving the same chemotherapy but report differing levels of fatigue. One patient may have less family support and therefore be more fatigued due to physically having to perform more activities of daily living, such as cooking. Moreover, the differences between these two patients may have a biological origin and arise due to changes in the levels of cytokines. Therefore, fatigue can be induced from several different causes and combinations of physiological, treatment and lifestyle conditions.
Individuals with cancer describe fatigue as tiredness, weakness, lack of energy, exhaustion, lethargy, inability to concentrate, depression, sleepiness, boredom, lack of motivation and decreased mental status. Also, fatigue may be experienced by individuals with cancer in an absence of associated conditions, which indicates that the origins of fatigue are not fully understood.85
Cachexia
Cachexia is severe tissue wasting (extensive weight loss) and emaciation (loss of fat stores and muscle causing an individual to appear excessively thin) that can occur with cancer (see Figure 36-25). It contributes to a decreased quality of life, as well as being one of the most common causes of death among individuals with cancer.86 Anorexia associated with cancer can result from taste alterations. An unusual and frustrating component of cancer care is the person’s early satiety — that is, sense of being full after only a few mouthfuls of food. One of the most significant pro-inflammatory involved cytokines is the activated macrophage-produced TNF-α, also called cachectin because of its role in the cachexia syndrome. TNF plays an important role in the defence against viral, bacterial and parasitic infections; in autoimmune responses; and in the selective destruction of malignant cells.88 Its overproduction, however, may be detrimental to the host as well as via the cytokines mentioned earlier. Clinical practice to date has mostly focused on increasing kilojoule intake to slow weight loss, although research into the mechanisms of cachexia may lead to improved treatments. Anorexia may contribute to cachexia by processes including hormones (such as leptin), cytokines and the hypothalamus, which is the brain region involved in appetite regulation.
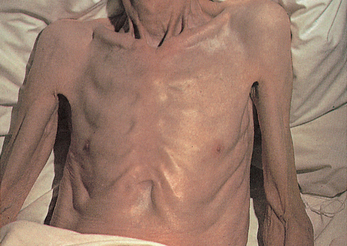
This severe form of malnutrition results in wasting and extensive loss of adipose tissue.
Source: Kamal A, Brockelhurst JC. Color atlas of geriatric medicine. 2nd edn. St Louis: Mosby; 1991.
Progressive weight loss may still occur even with normal food intake. Weight loss can be massive: up to 80% of adipose and skeletal muscle tissue mass.87 Altered glucose metabolism causes a syndrome resembling diabetes mellitus — hyperglycaemia, hyperinsulinaemia and insulin resistance. These disturbances cause the breakdown of proteins (such as muscle protein) to increase blood glucose levels. In starvation, protein is usually spared to protect vital structures, but in cancer protein is broken down to meet energy needs — hence, skeletal muscle loss occurs.
Paraneoplastic syndromes
Paraneoplastic syndromes are symptoms that cannot be explained by the local or distant spread of the tumour or by the effects of hormones released from the tumour. About 10% of individuals with malignancy are affected. Although infrequent, paraneoplastic syndromes are significant because they may be the earliest symptoms of an unknown cancer and therefore can prompt the person to seek medical attention. In affected individuals they may also represent serious and life-threatening problems and can mimic progression and therefore interfere with appropriate treatment. As an example, small-cell carcinoma of the lung can secrete antidiuretic hormone and the effects of elevated levels of this hormone may be an early indication of cancer (see Chapter 11).
CANCER TREATMENTS
The main treatment options for cancer are chemotherapy, hormone therapy, immunotherapy, radiotherapy and surgery. It is usual to use more than one cancer treatment to allow for more effective destruction of the cancer while causing less harm to healthy cells (see Table 36-7).
Table 36-7 EXAMPLES OF TREATMENT FOR SITE-SPECIFIC CANCERS
| USUAL TREATMENT | SITE |
|---|---|
| Chemotherapy | |
| Hormone therapy | |
| Immunotherapy | |
| Radiation | |
| Surgery |
Chemotherapy
Chemotherapy refers to relatively non-selective cytotoxic drugs that target vital cellular or metabolic processes critical to both malignant and normal cell growth and replication. To be curative, chemotherapy must eradicate enough tumour cells so that the body’s own immune system can eradicate any remaining cells.
Combination chemotherapy is the synergistic use of several agents, each of which individually has an effect against a certain cancer. This is to avoid drug resistance associated with using just one drug, which interestingly can occur even in previously untreated tumours (see Figure 36-26). In addition, combination chemotherapy provides a means of targeting cancer throughout the cell cycle (refer to Chapter 5 to review the cell cycle). For instance, some chemotherapeutic agents act against specific phases of the cell cycle, whereas others work generally across the cell cycle. Therefore, cancers can be targeted when undergoing different phases of growth. Another benefit of using lower doses of each drug in a combined manner is that the harmful effects to normal cells may be reduced.
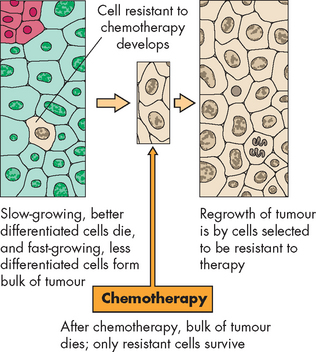
FIGURE 36-26 Chemotherapy and resistant cells.
A cell resistant to single-agent chemotherapy can develop from the pool of fast-growing tumour cells. Combination therapy helps prevent the development of resistant cells.
Source: Stevens A, Lowe J. Pathology: illustrated review in color. 2nd edn. Edinburgh: Mosby; 2000.
One chemotherapeutic agent, fluorouracil (5-fluorouracil), interferes with DNA synthesis and has been used for many years in cancer treatments. It is a well-known example of a drug used in combination chemotherapy. As a whole range of new cancer treatment drugs are becoming available, it is hoped that they may be more effective or more specific to the cancer and less toxic to normal cells. However, fluorouracil continues to be used in combination with other drugs for cancers such as breast cancer, colorectal cancer and oesophageal cancer.89
The principle of dose intensity implies that there is a direct relationship between the dose of a chemotherapeutic agent and killing of tumour cells. Often, even a small increase in dosage can significantly enhance the anti-tumour effect.90,91 Use of higher doses of chemotherapeutic agents is limited by the increasing toxicities associated with their use as defined by the therapeutic index of the drug (see Figure 36-27). The therapeutic index — that is, the relative effective dose needed to kill cancer cells as compared to the dose that would be harmful to normal cells — is generally quite low and is one of the limiting factors in the escalation of chemotherapy use.
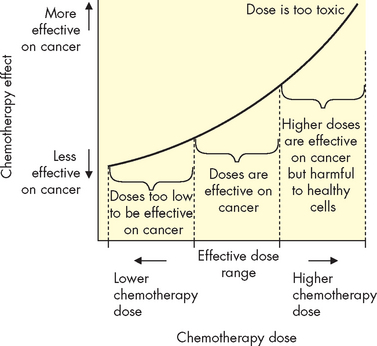
FIGURE 36-27 The relationship between doses of chemotherapy drugs and their effects on the cancer.
Although very high doses are more effective on destroying cancer cells, a large number of healthy cells are also destroyed, and therefore these doses are too high to be tolerated.
Adjuvant chemotherapy refers to the use of these drugs after local treatment or removal of the primary tumour. It is in this context that chemotherapy has proven most useful — for individuals who have minimal cancer remaining but who are at high risk for metastasis. Chemotherapy prevents the growth of micrometastatic deposits that are not clinically detectable at diagnosis, because they may consist of only small numbers of cells. A variation of this approach, termed neoadjuvant chemotherapy (or primary chemotherapy), is the early use of agents before definitive local control surgery or irradiation to decrease initial tumour size.92 This approach allows for less-extensive local control measures, as well as the opportunity to begin treatment early for micrometastatic disease.
Several classes of chemotherapeutic agents are used concurrently to treat different types of tumours. The mechanism by which each drug acts to eradicate tumour cells depends largely on its effect on the cell cycle. Cancer cells may be classed as belonging to different compartments, depending on which part of the cell cycle they are in:
 compartment B: cells that are currently resting in the G0 phase, which means that they may return to active cell division at some time again
compartment B: cells that are currently resting in the G0 phase, which means that they may return to active cell division at some time againCancer cells in compartment C are of low concern, as they will not contribute to enlargement of the cancer. Somewhat surprisingly, only a small percentage of cancerous cells may be in compartment A in a tumour that has already undergone major growth. If cancer cells are in compartment A, they are easily targeted by chemotherapy while undergoing rapid cell division. It is actually cells in compartment B, which are temporarily resting, that are of concern, as they may divide again at any time, but chemotherapy drugs are not particularly effective at destroying these cells while they are resting.
Hormonal therapy
Many tumours are hormone-dependent, particularly those associated with the reproductive organs, most notably the breast, prostate and ovaries. These cancers may be targeted by using drugs that interact with the hormone receptors. For example, tamoxifen is an oestrogen-blocking drug used in breast cancer, which prevents the cells from receiving hormonal stimulation by oestrogen. Interestingly, some other hormonal medications are actually receptor agonists, which bind to the receptor causing the growth effects of the hormone. This stimulates the cancer cells to undergo more rapid growth and thus become vulnerable to other cancer treatments — this has the effect of stimulating cells to come out of compartment B and into compartment A where they can be destroyed by chemotherapy. Table 36-8 lists the commonly used hormonal agents and their primary indications.
Table 36-8 COMMON HORMONAL AGENTS AND TYPES OF TUMOURS
| AGENTS | TYPES OF TUMOURS |
|---|---|
| Corticosteroids | |
| Androgens | Breast cancer |
| Oestrogens | |
| Anti-oestrogens | |
| Anti-androgens | Prostate cancer |
Immunotherapy
Because cancer is a dynamic disease in which the transformed cells in a tumour mass can adapt to changes in their environment, a single form of cancer therapy that is effective against all types of cancer may not be possible. In this regard, immunotherapy (immunological treatment) holds promise.
Immunotherapy is a specific method of treatment used to eliminate cancer cells without damaging normal tissues. The immune system recognises antigens and is highly regulated; thus, theoretically anti-tumour immune rejection responses can selectively eliminate cancer cells while sparing normal tissues. Immune memory cells are long-lived and can provide extended protection against the emergence of recurrent primary tumour cells and metastatic cancer cells. Numerous immunological mechanisms are able to cause rejection of various types of cancer. Research efforts in anti-cancer immunotherapies focus on characterising the immunological properties of various tumour-specific antigens and developing methods to selectively enhance tumour rejection immune responses, including the development of new vaccines and monoclonal antibodies.
Development of tumour-specific vaccines has been most notable with malignant melanoma. Melanoma-specific antigens are genetically manipulated in an attempt to develop a T cell-based immune response against the tumour. Although early clinical results show promise in decreasing tumour size in small numbers of individuals tested, the ideal means of maximally stimulating the immune system is yet to be developed.
Monoclonal antibodies are antibodies that are produced to bind to specific antigens on the surface of cancerous cells, thereby leading to their destruction. The principles of antibody–antigen interactions are similar to those that occur with antibodies of the immune system (see Figure 36-28). This newer type of drug has been most successful against haematological and lymphatic malignancies. For example, B-cell neoplasms (previously known as non-Hodgkin’s lymphomas) have been successfully targeted with rituximab, which binds to antigens on the B lymphocytes.93 Trastuzumab (Herceptin) recently became available in Australia and New Zealand; it binds with the human epidermal growth factor receptor-2 (erbB2) antigen, which is increased in some types of breast cancer, and also inhibits cell replication of cancerous cells with this antigen. Cetuximab (Erbitux) binds to the epidermal growth factor receptor on cancerous cells of the colon, causing them to undergo apoptosis.10 The majority of these antibodies are used in combination with other standard cytotoxic chemotherapy agents. A current focus of research involves conjugated antibodies, in which radioisotopes (chemicals that emit radiation) or toxins are attached to the antibodies, thus delivering very specific doses of radiation or toxic agents to involved tissues.94–96 4 6
Radiation
The goals of ionising radiation are (1) to eradicate the cancer without producing excessive toxicity during treatment and (2) to avoid damage to normal structures. Ionising radiation damages DNA and components of the microenvironment. Cellular compartments with rapidly renewing cells are, in general, more radiosensitive. Radiation therapy may be administered either externally, or internally via an implant (such as brachytherapy, commonly used for prostate cancer).
Surgery
Surgery may be completely curative, whereby the tumour is removed with an adequate margin of normal tissue, thus alleviating the need for additional therapy. The margin around the cancer (which appears to be healthy) is removed to limit the chances of cells that have broken away remaining in the body. An example of curative surgery is colon cancer that is detected early: the individual will have that section of the colon removed (called a colectomy) and, if the tumour is localised, an adequate margin of non-cancerous tissue will be removed too, thereby clearing the individual of the cancer.
However, if the tumour cannot be completely excised because of fear of causing undue morbidity, debulking surgery may be performed in which the majority of the tumour is removed, thereby allowing for increased success of adjuvant chemotherapy or irradiation. Debulking surgery is not often associated with cure and the individual may require multiple debulking surgeries, especially if the tumour is capable of rapid growth.
Surgery may also take on a palliative role when cure is not possible, allowing for the relief of current symptoms or the prevention or delaying of anticipated symptoms as the tumour grows. Surgery is also indicated for benign tumours that could progress into malignant tumours. Premalignant or localised tumours of epithelial tissues, such as the skin, mouth and cervix, are therefore removed.
Complementary and alternative cancer treatments
Many patients seek alternative options to the mainstream conventional cancer treatments that are currently available. Cancer patients often have a strong feeling of helplessness and despair and the need to explore other therapies can be strong. Accordingly, there is a tendency for some patients to try alternative treatments, also referred to as complementary medicines — the options for cancer treatment include dietary modifications, acupuncture, aromatherapy, homeopathy and herbal treatments.97 Although many of these treatments have been viewed sceptically by the medical profession, some cancer patients see a reduction in symptoms using treatments such as acupuncture.
Briefly, there are two main issues of concern regarding the use of alternative medicines. First, many cancer patients are using alternative medicines without informing their doctor: approximately half of all Australians using such medicines do not inform their doctor about their use of alternative medications — these alternative medicines may in fact interfere with their mainstream treatment. Second, and related to the first point, some of these complementary and alternative medicines may be potentially harmful: many patients are unaware that these medicines do not undergo the same strict testing procedures that are required for conventional medicines.98 Similarly, complementary medicines are not usually tested for interactions with other drugs such as chemotherapy agents. Interestingly, a study of male cancer patients found that scientific evidence was of low concern regarding their potential use of alternative medicines — of more concern in their decision whether to use these medications were issues such as personal stories of those who had benefited from using them.99
Side effects of cancer treatments
To many individuals, the side effects and complications of cancer therapy can be quite troublesome, often rivalling the diagnosis of cancer itself. Special care needs to be directed towards addressing and alleviating these effects because individual compliance with therapy is directly linked to a person’s perception of discomfort and treatment-related complications. Effective education regarding expected side effects and treatments to alleviate them is required.
With the exception of surgery, most side effects can be attributed to the relatively nonspecific nature of cancer therapy — the targeting of the rapidly growing cell. Therefore organ systems consisting of rapidly dividing cells are targeted as well as the cancer. Knowing which systems will be affected allows prediction of some of the more common general therapy-related side effects.
Gastrointestinal tract
The entire gastrointestinal tract relies on rapidly growing cells to produce an effective barrier to trauma and infection and to provide an absorptive surface for nutrients. Both chemotherapy and radiation therapy may cause decreased cell turnover, thereby leading to oral ulcers (stomatitis), malabsorption and diarrhoea. The disruption of barrier defences also increases the risk for infection, especially invasion by a person’s own normal flora in the digestive system. Therapy-induced nausea, thought to be caused by a direct action of chemotherapeutic agents on the central nervous system’s vomiting centre, historically has been a major obstacle in therapy.
Aggressive antiemetic therapy (to decrease nausea and vomiting) — including the centrally acting serotonin antagonists (or 5-hydroxytryptamine 3 antagonists) such as ondansetron — has allowed better tolerance of the highly emetogenic treatments (drugs that bring on vomiting). Other popular antiemetics include steroids and phenothiazines. Analgesia often includes opiate agents, vital in treating severe cases of mucosal lesions. Supplemental nutrition through enteral routes (directly into the intestines) or parenteral routes (injections that puncture the skin and mucosa, often intravenous) may be needed to combat malnutrition. Good oral care and close attention to hygiene may help prevent complications arising from mucosal membrane breakdown.
Bone marrow
Chemotherapy is the usual offending agent causing bone marrow suppression, although radiation therapy also may contribute to suppression, especially if the two therapies are used together. The timing of suppression can often be predicted based on what agent is used. All three cell lines are usually affected — red blood cells, white blood cells and platelets (refer to Figure 36-23). The anaemia caused by red cell suppression may contribute to the generalised fatigue of the person with cancer and may require transfusion depending on the severity of the anaemia or other co-morbid medical conditions. Decreased platelet numbers may increase a person’s tendency to spontaneously bleed and require transfusion as well. Perhaps the most potentially serious side effect is that of white blood cell suppression (neutropenia), creating for the person who already has weakened host immune defences an even greater risk of infection. The risk of infection increases with both greater degrees of and prolonged durations of neutropenia. This infection risk mandates immediate evaluation of fever and initiation of antibiotic medication until the infection is resolved.
Red blood cell and platelet transfusions are routinely used in supportive care of marrow suppression; white cell transfusions are not. The use of parenterally administered medications include granulocyte colony-stimulating factor to aid in recovery of white blood cells, erythropoietin to stimulate red cell production and thrombopoietin to stimulate platelet recovery. Bone marrow transplants are also used.
Hair and skin
Alopecia (hair loss) results from chemotherapy effects on hair follicles, as these cells of the hair are rapidly dividing and therefore vulnerable to chemotherapy. Alopecia is usually temporary, although hair may grow back with a different texture initially. Not all chemotherapeutic agents cause alopecia.
Decreased renewal rates of the epidermal layers in the skin may lead to skin breakdown and dryness, altering the normal barrier protection against infection. Radiation therapy may cause skin erythema (redness) and contribute to breakdown.
Reproductive tract
Radiation therapy and chemotherapy may affect the gametes, leading to varying degrees of decreased fertility and premature menopause. These effects are dose and age dependent, with the pre-pubertal gonad thought to be more resistant to damage. The potential for harm is also dependent on the agent used, with the alkylating category of chemotherapy drugs carrying the greatest risk. The gametes (oocytes and sperm) are particularly vulnerable as they are rapidly dividing. Craniospinal irradiation for central nervous system tumours may also affect the hypothalamus or pituitary gland, with subsequent secondary gonadal failure because of lack of production of gonadotropin-releasing hormone, luteinising hormone and follicle-stimulating hormone. The potential for reproductive harm should be addressed before therapy, if possible, with provisions made for sperm or embryo banking.
CANCERS OF GREATEST SIGNIFICANCE IN AUSTRALIA AND NEW ZEALAND
The cancers with the highest incidence rates are almost identical between Australia and New Zealand (see Figure 36-29): prostate and breast cancers (female breast only), colorectal cancer, melanoma and lung cancer. The cancers with the highest mortality rates include lung, prostate and breast, colorectal and pancreatic cancers (see Figure 36-30). Importantly, Australian data also show a category of ‘unknown’; this indicates that the primary cancer site could not be shown, which probably means the cancer was advanced and had undergone metastasis prior to it being diagnosed. Data on unknown cancer types are not presented for New Zealand.
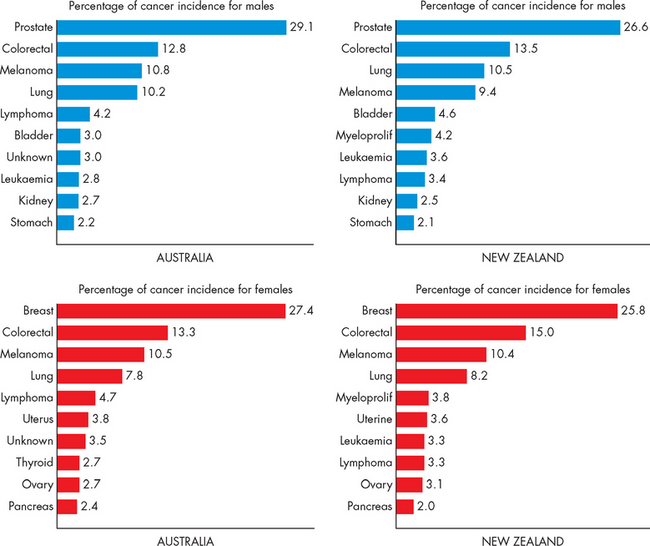
FIGURE 36-29 The 10 cancers with the highest incidence, Australia and New Zealand.
Cancer incidence is shown as a percentage of total cancer incidence by sex and country. Lung cancer includes cancer of the lung, bronchus and trachea. Lymphoma includes Hodgkin’s and non-Hodgkin’s in Australia, and non-Hodgkin’s in New Zealand. Colorectal cancer in New Zealand includes anal cancer, but this is not included in the Australian data. In either case, the inclusion is a very small number of cases and would have little effect on the data.
Source: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview. Cancer series no. 46. Catalogue no. CAN 42. Canberra: AIHW; 2008; New Zealand Health Information Service. Cancer: new registrations and deaths, 2004. Wellington: Ministry of Health; 2007.
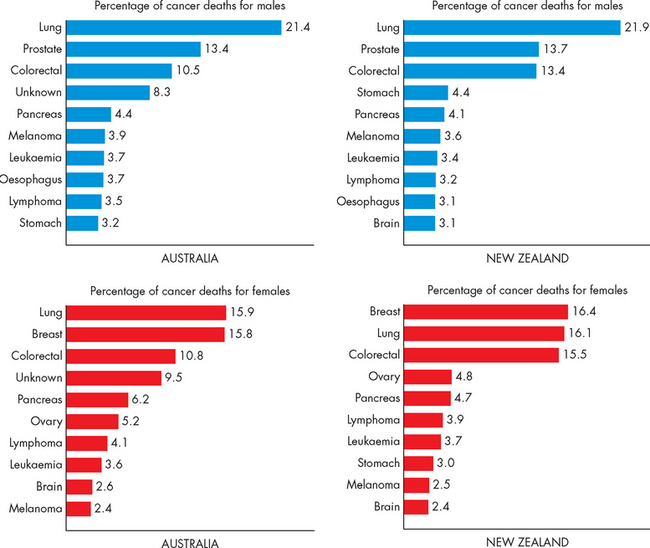
FIGURE 36-30 The 10 cancers with highest mortality, Australia and New Zealand.
Cancer incidence is shown as a percentage of total cancer incidence for sex and country. Lung cancer includes cancer of the lung, bronchus and trachea. Lymphoma includes Hodgkin’s and non-Hodgkin’s in Australia, and non-Hodgkin’s in New Zealand. Colorectal cancer in New Zealand includes anal cancer, but this is not included in the Australian data. In either case, the inclusion is a very small number of cases and would have little effect on the data.
Source: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview. Cancer series no. 46. Catalogue no. CAN 42. Canberra: AIHW; 2008; New Zealand Health Information Service. Cancer: new registrations and deaths, 2004. Wellington: Ministry of Health; 2007.
Although cancer can affect individuals at any stage in their lifetime, the majority of cases of cancer occur in the aged population: the risk of being diagnosed with cancer increases dramatically after the age of 60 years. In addition, the cancers that cause the highest mortality are more prevalent in the aged population (see Figure 36-11).
The cancers discussed in this section are compared, statistics incorporated and screening programs reviewed. See earlier chapters for detailed explanations of each of these cancers.
The incidence and mortality rates of various cancers
Breast and prostate cancers
Interestingly, prostate cancer has a higher incidence rate than breast cancer. Nevertheless, cancers of the prostate and breast are clearly the most prevalent cancers in males and females, respectively. Rates of death due to breast cancer are slightly higher than rates of death due to prostate cancer in both countries — this may be partly explained by the larger number of male deaths attributed to smoking, which thereby reduce the percentages of deaths attributed to other cancers for men. The actual number of deaths (rather than percentages) shows that in Australia more men die of prostate cancer than women die of breast cancer, while in New Zealand more women die of breast cancer than men die of prostate cancer.
Over recent years, there has been a surge in public awareness of breast cancer. This is partly the result of a number of high-profile women being diagnosed with breast cancer, including Kylie Minogue (singer), Belinda Emmett (actress), Jane McGrath (wife of cricketer Glenn), Anastacia (singer), Melissa Etheridge (singer) and Jools Topp (singer). In addition, there has been extensive cooperation between celebrities and companies that are promoting breast cancer awareness and fundraising for research, which has increased the number of women attending mammography screening.100
One of the distinct differences between treatment options for prostate cancer and breast cancer has to do with the individual’s age at diagnosis. Breast cancer is most often diagnosed in younger women, who are more able to tolerate the more aggressive cancer treatments. However, breast cancer is perceived to be more devastating to younger women, who are of reproductive age or have young families, and therefore the impact of this disease on young women is considered high. On the other hand, prostate cancer is most often diagnosed in older men, which is unfortunate in that many older people do not tolerate cancer treatments well and therefore they may not be able to receive as much life-saving treatment as younger people. In addition, those being diagnosed at an older age are likely to have other diseases and disorders as well, which can complicate cancer treatment options. For example, some chemotherapeutic agents are contraindicated in those with heart disease, which is common in the elderly.
Colorectal cancer
In both countries colorectal cancer is the second most prevalent cancer in males and females and is the third highest cause of death from cancer. Colorectal cancer is perhaps thought by some people to affect mainly males, but it actually causes more female deaths than male deaths in New Zealand. Colorectal cancer statistics show that it remains one of our most significant cancers and both Australia and New Zealand are in the stages of introducing a national bowel cancer screening program (see Chapter 27). Blood in the faeces can be highly indicative of colorectal cancer, and so screening consists of placing a faecal sample on a reagent strip to detect the presence of blood. This screening tool is non-invasive and may be received well by the general population. In addition, there are already guidelines in place for regular screening colonoscopies for individuals at greater risk due to their family history.
Melanoma
Broadly, there are two different types of skin cancers: non-melanoma skin cancers and melanomas. Non-melanoma skin cancers are the most common type and comprise basal cell carcinomas (very common, with a slow growth rate, rarely spread and rarely fatal, almost all cases are treated well by surgical removal) and squamous cell carcinomas (less common, but with a faster growth rate, and can spread and be fatal). Actinic keratosis is an early stage of squamous cell carcinoma.101 Melanomas are the least common but most aggressive type of skin cancer: they spread to other organs easily, can be resistant to treatments and are most likely to be fatal. When all types of cancers are considered, skin cancers become the most common cancers in our community. However, because non-melanoma cancers are not required to be reported, they are usually absent from health statistics.
Although melanoma is the third most prevalent cancer for males and females of both countries, fortunately it is not associated with a high mortality rate, particularly for females. Melanomas can be detected earlier than many other cancers as they are visible to the individual. Both countries have conducted public awareness campaigns to promote regular skin checking to facilitate early detection. Once detected, melanomas can be removed and this usually provides a longer survival time. In general, females tend to seek medical attention earlier than males, and earlier removal of melanomas may explain the lower mortality rates in females. Although the incidence of melanoma is currently high, it is anticipated that this rate will decline in the future as generations of children become exposed to a culture of sun protection from a young age.
Lung cancer
Lung cancer is approximately the fourth most prevalent cancer among males and females and so is diagnosed relatively frequently. However, deaths due to lung cancer place it first or second in the mortality figures, so the death rate for lung cancer is high compared with the incidence rate. The death rate is currently substantially higher in males than females for both Australia and New Zealand. This may be explained by previous trends in smoking rates during the 1970s and 1980s, when smoking rates for males were higher than for females (although rates for males were declining, rates of smoking for females were increasing). Due to the time it takes for lung cancer to develop after smoking has commenced, we are now beginning to see a corresponding decline in the number of male deaths from lung cancer, and expect the number of female deaths to rise in future years due to the current increase in the number of female smokers.
The role of cancer screening
Australia and New Zealand have both introduced national screening programs for cervical cancer and breast cancer, and both are in the process of introducing a colorectal screening program. The main goal of national cancer screening programs is to facilitate early detection for a large percentage of the population, thereby allowing early treatments, which are more effective than treatments of advanced cancers. With all cancers, early detection is the best option for adequate treatment and survival, particularly if the cancer is found before it has grown considerably or undergone metastasis. In most cases, cancers that are identified early using a screening program are not associated with any symptoms and therefore, had the person not participated in the screening, the cancer would have developed undetected.
Cervical cancer
Cervical cancer is described as a preventable cancer, because early detection can identify cells that are abnormal and still pre-cancerous, enabling them to be treated before they develop into cancer.102–104 4 6 Interestingly, although both Australia and New Zealand have national screening programs for cervical cancer, this cancer does not appear in the top 10 cancers in terms of incidence or mortality rates. Does this mean that screening is not necessary? Certainly not! In fact, the declining incidence and mortality rates for this cancer are evidence that the screening programs are effective and should be continued. The incidence and mortality rates for cervical cancer are shown in Figure 36-31. Cervical cancer constitutes only 1.7% of the total incidence of cancer for females and 1.3% of the cancer mortality. For the Pap smear test, a cell sample obtained from the cervix is examined by pathology. The goal of screening is to identify early changes in cells, thereby allowing effective early treatment, and the evidence indicates that this has been successful. A national cervical cancer screening program was implemented in New Zealand in 1990 and in Australia in 1991.105,106
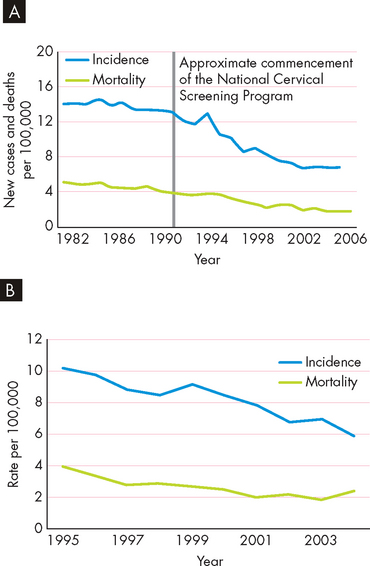
FIGURE 36-31 The incidence and mortality rates for cervical cancer in women in (A) Australia and (B) New Zealand.
Source: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview. Cancer series no. 46. Catalogue no. CAN 42. Canberra: AIHW; 2008; New Zealand Health Information Service. Cancer: new registrations and deaths, 2004. Wellington: Ministry of Health; 2007.
In both countries, Indigenous women are more prone to cervical cancer than non-Indigenous women. The rate of mortality from cervical cancer is approximately five times higher for Aboriginal and Torres Strait Islander women than for other Australian women.106 Although data on the participation of these women in the national screening program are not provided, it is likely that those who are screened receive earlier treatment and have lower mortality rates. Similarly, in New Zealand, the rates of cervical cancer incidence and mortality are approximately twice as high in Maori women as in non-Maori women.107
Breast cancer
A national breast screening program commenced in Australia in 1994 and in New Zealand in 1998. The main target audience for each program is women aged 50–69, although older women and those in their 40s are also eligible.108 Rates of participation in the Australian program are significantly lower for Aboriginal and Torres Strait Islander women than for non-Indigenous women.11
The screening program uses a mammogram to detect a mass long before it develops sufficiently to cause symptoms (see Figure 36-32). The average age of death due to breast cancer has been increasing and is now almost 68 years old.29
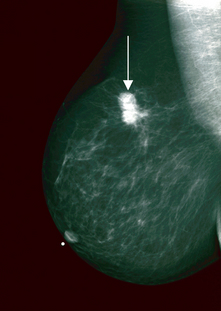
FIGURE 36-32 A mammogram of the right breast shows cancer (arrow).
Source: Abeloff MD. Abeloff’s clinical oncology. 4th edn. Philadelphia: Elsevier; 2008.
The high incidence and mortality rates of breast cancer are shown in Figure 36-33. Interestingly, Indigenous women actually have a lower incidence of breast cancer than non-Indigenous women.11 It is possible that for Aboriginal and Torres Strait Islander women having children at a younger age along with having a higher number of pregnancies may protect them from breast cancer (see Chapter 32).11 However, despite the lower incidence rate, the mortality rate is the same for Aboriginal and non-Indigenous women.11 The 5-year survival rate is 82% for non-Indigenous women, but only 65% for Aboriginal women.11 In New Zealand, the incidence rate is almost identical between Maori and non-Maori women, but the mortality is actually higher for Maori women.107
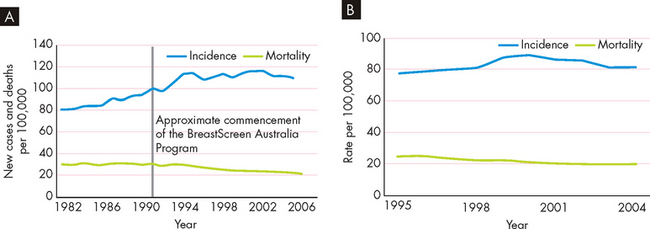
FIGURE 36-33 The incidence and mortality rates for breast cancer in women in (A) Australia and (B) New Zealand.
Source: Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer in Australia: an overview. Cancer series no. 46. Catalogue no. CAN 42. Canberra: AIHW; 2008; New Zealand Health Information Service. Cancer: new registrations and deaths, 2004. Wellington: Ministry of Health; 2007.
Colorectal cancer
The incidence rate of colorectal cancer is relatively constant in Australia and New Zealand and the mortality rate may actually be declining slightly. In New Zealand, both the incidence rate and the mortality rate are substantially lower for Maori than non-Maori males and females.107 In both countries a national colorectal cancer screening program is in the process of being introduced and it is hoped that such a program will lead to a decline in mortality due to colorectal cancer.
Melanoma
Currently in Australia and New Zealand there is no recommendation for a population-wide regular skin screening program. However, individuals at risk are advised to check their own skin and seek medical attention where appropriate. An example of a malignant melanoma is shown in Figure 36-34. Figure 36-35 shows that overall there appears to be an increase in both the incidence rate and the mortality rate associated with melanoma.
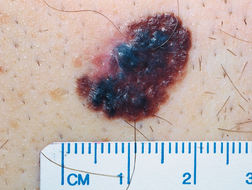
FIGURE 36-34 A malignant melanoma.
Source: James WD. Andrews’ diseases of the skin: clinical dermatology. 10th edn. Philadelphia: Saunders; 2005.
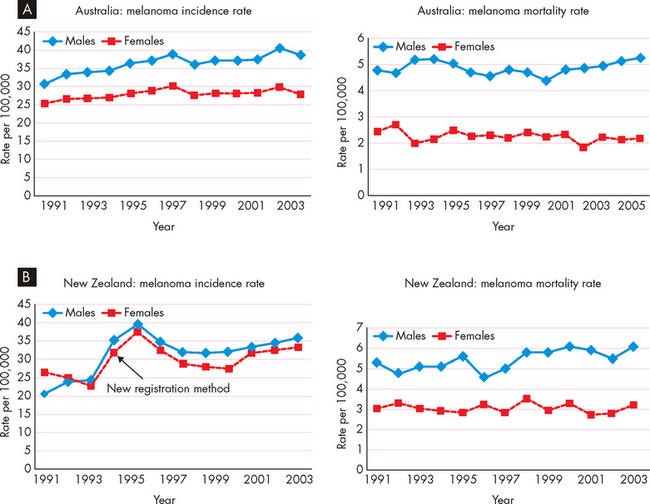
FIGURE 36-35 The incidence and mortality rates of melanoma in (A) Australia and (B) New Zealand.
Source: Australian Cancer Network Melanoma Guidelines Revision Working Party. Clinical practice guidelines for the management of melanoma in Australia and New Zealand. Sydney: Cancer Council Australia and Australian Cancer Network; 2008.
Prostate cancer
The mortality rate from prostate cancer is shown in Figure 36-36. There is no national screening program for prostate cancer in Australia or New Zealand (see Box 36-4). On an individual basis a blood test may be recommended so that the presence of prostate cancer can be indicated using the tumour marker PSA (prostate-specific antigen). This is then confirmed using imaging (see Figure 36-37).
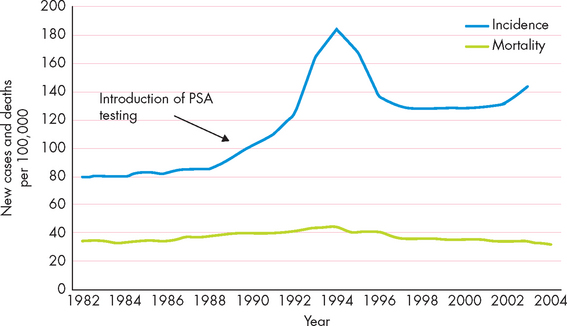
FIGURE 36-36 Mortality and incidence rates due to prostate cancer in Australia.
Source: Australian Institute of Health and Welfare & Australasian Association of Cancer Registries. Cancer in Australia: an overview, 2006. Cancer series no 37. Cat no CAN32. Canberra: AIHW; 2007.
Box 36-4 PROSTATE CANCER SCREENING
One difficulty in screening for prostate cancer using the PSA marker is that it is not always conclusive: it is possible to have prostate cancer without having increased PSA. Furthermore, if PSA is elevated, the presence of any cancer needs to be confirmed using a biopsy. The patient will then need to consider cancer treatment options; the complications of such treatments include impotence, urinary incontinence and faecal incontinence, as well as the mental and emotional toll on the patient — this can impact substantially on quality of life. In some cases, prostate cancer does not cause significant alterations in health or mortality, so is early screening for the population actually necessary? Prior to the development of any national prostate cancer screening program, extensive research is needed into the potential benefits and consequences of screening for the individual, as well as the feasibility and benefits for the healthcare system. For now, the decision to test for PSA is entirely up to the individual, with emphasis placed on testing for those who are in high-risk categories.
Source: Cancer Council Australia. National Cancer Prevention Policy, 2007–09. Screening to detect cancer early: prostate cancer. Available at www.cancer.org.au, accessed November 2009.
CANCER ACROSS THE LIFE SPAN
The most common cancers diagnosed at each point in the life span are shown in Figure 36-38. For young boys and girls in Australia and New Zealand, leukaemia is the main cancer (discussed below). In the mid-teens through to young adulthood for girls, melanoma is the most common cancer; in the mid-teens for boys, melanoma is equal with testicular cancer in Australia and testicular cancer has the highest incidence in New Zealand. Melanoma becomes the most common cancer for men from their mid- to late 20s through to middle-age; prostate cancer then has the highest incidence through the remaining ages for men. For females, breast cancer is the most commonly diagnosed cancer from early 30s right through to the mid-70s, when colorectal cancer becomes most common.
FIGURE 36-38 The cancers with the highest incidence across the life span.
A Males, Australia. B Males, New Zealand. C Females, Australia. D Females New Zealand. In A, two cancers are shown in the same age group as their incidence is equal. It is remarkable how similar the data are for the same sexes in the two countries. Also interesting is that the incidence of colorectal cancer is highest in females, yet it still attracts the misconception that it is predominantly a male cancer. Cancers of the prostate and breast (female) clearly have the highest incidence over most periods of life. A high incidence of leukaemia in childhood and melanoma in early adulthood is also common.
CHILDHOOD CANCER
Cancer in children is rare. The most common type of childhood cancer in Australia is leukaemia: more than 200 children aged up to 14 years old are diagnosed with leukaemia each year.109 The main type of leukaemia in childhood is acute lymphoblastic leukaemia (ALL), which differs from the most common leukaemias in adulthood, namely chronic lymphocytic leukaemia (CLL) and acute myeloid leukaemia (AML).110 Carcinomas (arising from epithelial tissue) rarely occur in children because these cancers most commonly result from environmental carcinogens and require a long period of time between exposure to the carcinogen and the appearance of the carcinoma. Carcinomas begin to increase in incidence between the ages of 15 and 19 years, becoming the most common cancer type seen after adolescence.
Aetiology of childhood leukaemia
As in adult cancer, the causes of cancer in childhood are largely unknown. Some factors that may contribute to the development of childhood leukaemia include genetics, as well as exposure to intense radiation, chemicals and the human T cell leukaemia virus.111 One autosomal recessive disorder associated with paediatric malignancies is Fanconi anaemia: children with Fanconi anaemia have a high risk of developing acute myeloid leukaemia.112 Interestingly, a child who has a twin who develops leukaemia has a higher risk of developing acute lymphoblastic leukaemia. This association is not true when considering other types of affected first-degree relatives.113
Prognosis of childhood leukaemia
Today, childhood cancer should not be considered as an inevitably fatal illness. Since the mid-1980s, the 5-year survival rate for childhood leukaemia has increased from 65% to 83% (see Figure 36-39). Most of these children are still alive for at least the next few years, as the 10-year survival rate is 79%.114 This improvement in the survival rate is due to significant advances in treatment. Overall, children have a more favourable prognosis than adults. Children appear to be more responsive to therapies and more tolerant of the immediate side effects of treatment. However, survivors of childhood cancer are at increased risk of developing a second malignancy later in life. This risk may be associated with a variety of factors, including previous chemotherapy or radiotherapy, genetic factors and the type of the primary cancer.115 The frequency of relapse of acute lymphatic leukaemia in children is only 25%.116
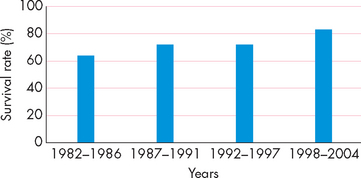
FIGURE 36-39 Trends in the 5-year survival rate for childhood leukaemia.
Source: Australian Institute of Health and Welfare, Cancer Australia and Australasian Association of Cancer Registries. Cancer survival and prevalence in Australia: cancers diagnosed from 1982 to 2004. Cancer Series no. 42. Catalogue no. CAN 38. Canberra: AIHW; 2008.
Because childhood cancer is viewed as a chronic disease instead of a fatal illness, the focus of treatment is on quality of life. Even those cancers that cannot be cured generally can be treated, resulting in significantly improved quality of life. Although children may be cured, they still face residual and late effects of their treatment (see the box, ‘Health alert: late effects of childhood cancer’). These late effects are more significant in children than in adults because treatment given in childhood occurs in a physically immature, growing individual. Late effects that need further study include physical impairments, reproductive dysfunction, soft-tissue and bone atrophy, learning disabilities, secondary cancers and psychological sequelae. More needs to be learned about the genetic factors associated with childhood malignancies and about the genetic consequences of treatment.
Late effects of childhood cancer
Because of significant advances in cancer treatment, more than 80% of children treated for cancer survive for 5 years or more — an increase of almost 45% since the early 1960s. However, it has become apparent that the effects of childhood cancer treatment may affect a child’s health later in life. This is known as the late effects of childhood cancer. Late effects result from the injury that cancer treatment causes to healthy cells in the body and may occur as a result of surgery, radiation therapy, chemotherapy or bone marrow transplant. Late effects may impair the brain, heart, lungs, breasts, muscle and bone, vision and hearing, growth and development, and fertility. One of the most serious late effects is the development of a second malignancy later in life. Each child receiving cancer therapy is unique. Late effects vary and depend largely on the type and doses of therapy received. The very young child may be at the greatest risk. Regular follow-up examinations are extremely important during and after treatment. Health providers involved in the care of childhood cancer survivors watch for short- and long-term effects of treatment, as well as signs of recurrent disease.
Source: Bhatia S, Blatt J, Meadows A. Late effects of childhood cancer and its treatment. In: Pizzo PA, Poplack DG (eds). Principles and practice of pediatric oncology. 5th edn. Philadelphia: Lippincott Williams & Wilkins; 2006
Cancer is a chronic disease
Cancer characteristics and terminology
The genetic basis of cancer
 Cancers arise from alterations in genes. These may be triggered by the genes themselves, or by external influences such as the environment.
Cancers arise from alterations in genes. These may be triggered by the genes themselves, or by external influences such as the environment. Oncogenes promote the development of cancer, while tumour-suppressor genes limit the development of cancer.
Oncogenes promote the development of cancer, while tumour-suppressor genes limit the development of cancer.Cancer, immunity, inflammation and infection
 The immune system may be effective in removing very early stages of cancer in the form of small numbers of isolated cells.
The immune system may be effective in removing very early stages of cancer in the form of small numbers of isolated cells.Gene–environment interaction
 Cigarette smoking is one of the main environmental factors that causes cancer, and includes passive smoking.
Cigarette smoking is one of the main environmental factors that causes cancer, and includes passive smoking. Dietary factors associated with the modern Western diet include high-fat and low-fibre intake, which can increase the likelihood of developing cancers.
Dietary factors associated with the modern Western diet include high-fat and low-fibre intake, which can increase the likelihood of developing cancers. Obesity is linked with increased mortality of many cancers, and may account for 20% of cancer deaths. Adipose cells are metabolically active, and produce and respond to substances including hormones.
Obesity is linked with increased mortality of many cancers, and may account for 20% of cancer deaths. Adipose cells are metabolically active, and produce and respond to substances including hormones. Alcohol consumption is linked to cancers of the upper body; alcoholic cirrhosis is associated with liver, breast and colorectal cancers.
Alcohol consumption is linked to cancers of the upper body; alcoholic cirrhosis is associated with liver, breast and colorectal cancers. Other environmental factors associated with cancers include occupational hazards, air pollution, ionising radiation and possibly electromagnetic fields.
Other environmental factors associated with cancers include occupational hazards, air pollution, ionising radiation and possibly electromagnetic fields.Diagnosis and evaluation of cancer
 Detection of tumour markers in body fluids can assist in the diagnosis of cancer; however, in many cases cancer symptoms are nonspecific so further investigations are necessary.
Detection of tumour markers in body fluids can assist in the diagnosis of cancer; however, in many cases cancer symptoms are nonspecific so further investigations are necessary.Clinical manifestations of cancer
Cancer treatments
 Chemotherapy is the use of drugs that interfere with cellular processes usually associated with cell division to destroy cancer cells.
Chemotherapy is the use of drugs that interfere with cellular processes usually associated with cell division to destroy cancer cells. Combination chemotherapy involves using more than one chemotherapy agent at the same time, while adjuvant chemotherapy involves using drugs after another treatment (such as surgical removal). Use of more than one cancer treatment method usually has more promising results.
Combination chemotherapy involves using more than one chemotherapy agent at the same time, while adjuvant chemotherapy involves using drugs after another treatment (such as surgical removal). Use of more than one cancer treatment method usually has more promising results. Hormonal therapy is the use of drugs that interact with hormones and their receptors involved in the hormone-dependent cancers.
Hormonal therapy is the use of drugs that interact with hormones and their receptors involved in the hormone-dependent cancers. Immunotherapy involves the immune system rejecting the cancer; an example includes the early-stage development of a vaccine against melanoma.
Immunotherapy involves the immune system rejecting the cancer; an example includes the early-stage development of a vaccine against melanoma. Radiation therapy can eliminate cancer cells or weaken them to be more vulnerable to destruction by another treatment method.
Radiation therapy can eliminate cancer cells or weaken them to be more vulnerable to destruction by another treatment method. Complementary and alternative treatments are used by many cancer patients; it is important that the medical team is aware of these, due to their possible interactions with mainstream treatments.
Complementary and alternative treatments are used by many cancer patients; it is important that the medical team is aware of these, due to their possible interactions with mainstream treatments. Gastrointestinal side effects include profound nausea and vomiting, as well as oral ulcers and diarrhoea.
Gastrointestinal side effects include profound nausea and vomiting, as well as oral ulcers and diarrhoea.Cancers of greatest significance in Australia and New Zealand
 The incidence of lung cancer is high, but the mortality rate is even higher: lung cancer features either first or second on lists of cancer mortality statistics. Lung cancer is particularly slow to develop and so the current mortality rate probably reflects the higher smoking rates seen in individuals several decades ago.
The incidence of lung cancer is high, but the mortality rate is even higher: lung cancer features either first or second on lists of cancer mortality statistics. Lung cancer is particularly slow to develop and so the current mortality rate probably reflects the higher smoking rates seen in individuals several decades ago. Breast and prostate cancers have similar incidence and mortality rates for females and males, respectively. However, breast cancers are usually diagnosed in younger individuals than prostate cancers.
Breast and prostate cancers have similar incidence and mortality rates for females and males, respectively. However, breast cancers are usually diagnosed in younger individuals than prostate cancers. Melanomas are the least common skin cancers, but the most aggressive. Although there is a high incidence of skin cancers, the slightly lower mortality rate may reflect the ability to notice changes in the skin and seek medical attention.
Melanomas are the least common skin cancers, but the most aggressive. Although there is a high incidence of skin cancers, the slightly lower mortality rate may reflect the ability to notice changes in the skin and seek medical attention. National screening programs are available for cervical cancer and breast cancer in both Australia and New Zealand. A program for colorectal cancer is being phased in for Australia and is planned for New Zealand. No national screening programs are currently in place for either skin cancer or prostate cancer.
National screening programs are available for cervical cancer and breast cancer in both Australia and New Zealand. A program for colorectal cancer is being phased in for Australia and is planned for New Zealand. No national screening programs are currently in place for either skin cancer or prostate cancer.Cancer across the life span
Mavis is an 88-year-old grandmother. She has been going for regular mammograms and recently a small growth was detected. A biopsy revealed that it was an early-stage benign tumour and could be removed relatively easily with surgery. With Mavis’ whole family now having an increased focus on this condition, her 29-year-old granddaughter, Talia, decided to start checking her own breasts and unexpectedly she found a lump. Talia promptly visited her doctor, and subsequent investigations revealed that she has a malignant growth that fortunately has been detected early. The cancer is mainly located in a very small lump, although there is evidence that it is invading the surrounding tissue.