Other types of lung tumours
Other types of primary lung tumours include mesotheliomas, sarcomas, lymphomas and bronchial adenomas. Mesothelioma is caused by exposure to asbestos and there is a relatively high incidence of this form of lung cancer in Australasia due to the widespread use of asbestos in homes and public buildings built before 1982. Bronchial adenomas are small tumours that arise from the lower trachea or major bronchi and are considered malignant because they are locally invasive and frequently metastasise. Clinical manifestations of bronchial adenomas include haemoptysis, persistent cough, localised obstructive wheezing and pneumonia. Bronchial adenomas can usually be treated successfully with surgical resection.
The lungs are a common site for secondary metastases and are more often affected by metastatic growth than by primary lung tumours. The pulmonary capillaries, with their extensive network, are ideal sites for tumour emboli. In addition, the lungs have an extensive lymphatic network. The primary malignancies that spread to the lungs often originate in the GI or genitourinary (GU) tracts and in the breast. General symptoms of lung metastases are chest pain and non-productive cough.
Benign tumours of the lung are generally classified as mesenchymal. Their occurrence is rare and they have the potential to become malignant. The most common mesenchymal tumours are chondromas, which arise in the bronchial cartilage, and leiomyomas, which are myomas of smooth, non-striated muscle fibres.
Hamartomas of the lung are also a common benign tumour. These tumours, composed of fibrous tissue, fat and blood vessels, are congenital malformations of the connective tissue of the bronchiolar walls. Hamartomas are slow-growing tumours.
CHEST TRAUMA AND THORACIC INJURIES
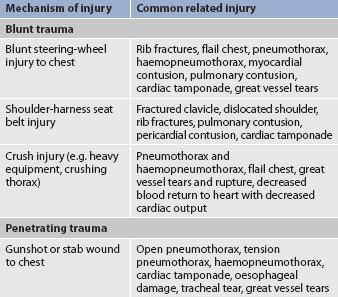
Thoracic injuries are the primary cause of death in 20–25% of all trauma victims, and injury to the chest wall is found in 45% of thoracic trauma victims.61 Traumatic injuries fall into two major categories: (1) blunt trauma; and (2) penetrating trauma.
Blunt trauma occurs when the body is struck by a blunt object, such as a steering wheel. The external injury may appear minor but the impact may cause severe, life-threatening internal injuries, such as a ruptured spleen. Contrecoup trauma, a type of blunt trauma, is caused by the impact of parts of the body against other objects. This type of injury differs from blunt trauma primarily in the velocity of the impact. Internal organs are rapidly forced back and forth within the bony structures that surround them so that internal injury is sustained not only on the side of the impact but also on the opposite side, where the organ or organs hit bony structures. If the velocity of impact is great enough, organs and blood vessels can literally be torn from their points of origin. Many head injuries are caused by contrecoup trauma. Penetrating trauma occurs when a foreign body impales or passes through the body tissues (e.g. gunshot wounds, stabbings).
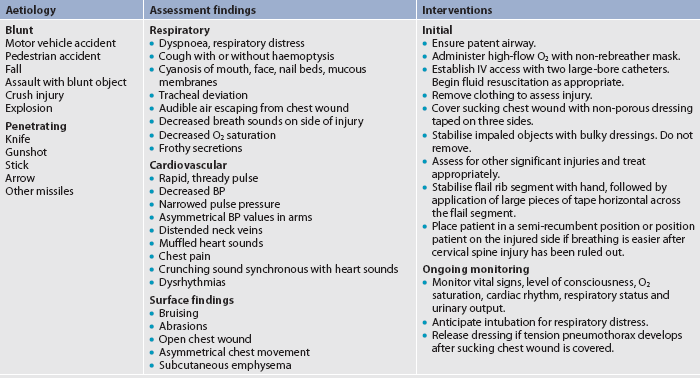
Table 27-12 describes selected traumatic injuries as they relate to the categories of trauma and the mechanism of injury. Emergency care of the patient with a chest injury is presented in Table 27-13.
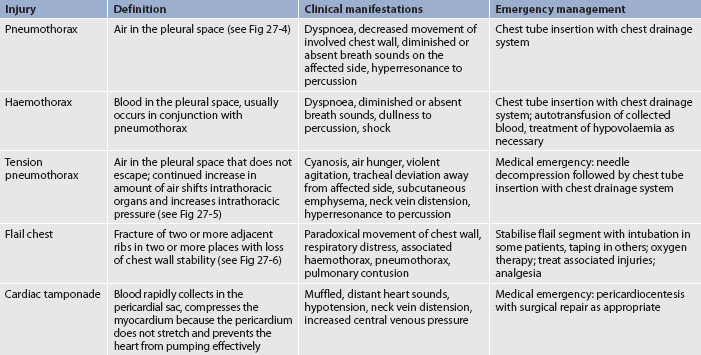
Thoracic injuries range from simple rib fractures to life-threatening tears of the aorta, vena cava and other major vessels. The most common thoracic emergencies and their management are described in Table 27-14.
Pneumothorax
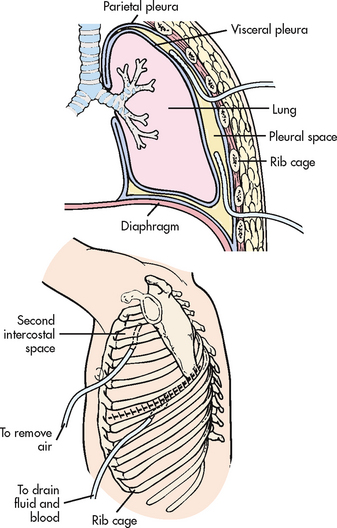
A pneumothorax is air in the pleural space. There is an associated complete or partial collapse of a lung due to the accumulation of air in the pleural space. This condition should be suspected after any blunt trauma to the chest wall.
TYPES OF PNEUMOTHORAX
Closed pneumothorax
Closed pneumothorax has no associated external wound. The most common form is a spontaneous pneumothorax, which is accumulation of air in the pleural space without an apparent antecedent event. It is caused by rupture of small blisters on the visceral pleural space. The cause of the blisters is unknown. This condition occurs most commonly in underweight male cigarette smokers between 20 and 40 years of age. There is a tendency for this condition to recur.
Open pneumothorax
Open pneumothorax occurs when air enters the pleural space through an opening in the chest wall (see Fig 27-4, B). Examples include stab or gunshot wounds and surgical thoracotomies. A penetrating chest wound is often referred to as a sucking chest wound.
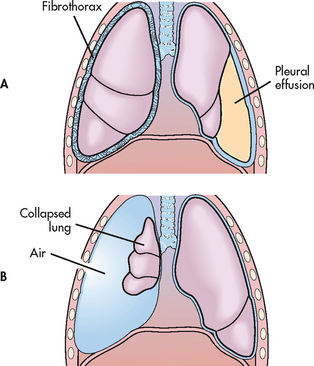
Figure 27-4 Disorders of the pleura. A, Fibrothorax resulting from an organisation of inflammatory exudate and pleural effusion. B, Open pneumothorax resulting from collapse of the lung due to disruption of the chest wall and outside air entering.
An open pneumothorax should be covered with a vented dressing. (A vented dressing is one secured on three sides with the fourth side left untapped.) This allows air to escape from the vent and decreases the likelihood of tension pneumothorax developing. If the object that caused the open chest wound is still in place, it should not be removed until a doctor is present. The impaled object should be stabilised with a bulky dressing.
Tension pneumothorax
Tension pneumothorax is a pneumothorax with rapid accumulation of air in the pleural space causing severely high intra-pleural pressures with resultant tension on the heart and great vessels. It may result from either an open or a closed pneumothorax (see Fig 27-5). In an open chest wound, a flap may act as a one-way valve; thus air can enter on inspiration but cannot escape. The intrathoracic pressure increases, the lung collapses and the mediastinum shifts towards the unaffected side, which is subsequently compressed. As the pressure increases, cardiac output is altered because of decreased venous return and compression of the vena cava and aorta. Tension pneumothorax can occur with mechanical ventilation and resuscitative efforts. It can also occur if chest tubes are clamped or become blocked in a patient with a pneumothorax. Unclamping the tube or relief of the obstruction will remedy this situation.
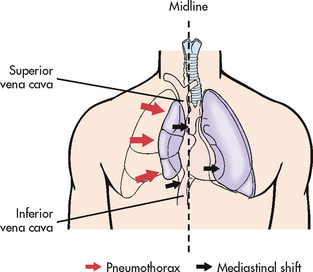
Figure 27-5 Tension pneumothorax. As pleural pressure on the affected side increases, mediastinal displacement ensues with resultant respiratory and cardiovascular compromise.
Tension pneumothorax is a medical emergency, with both the respiratory and the circulatory systems affected. If the tension in the pleural space is not relieved, the patient is likely to die from inadequate cardiac output and/or marked hypoxaemia. After further training, some nurses and paramedics are now inserting large-bore needles and chest tubes into the chest wall to release the trapped air.
Haemothorax
Haemothorax is an accumulation of blood in the intrapleural space. It is frequently found in association with open pneumothorax and is then called a haemopneumothorax. Causes of haemothorax include chest trauma, lung malignancy, complications of anticoagulant therapy, pulmonary embolus and tearing of pleural adhesions or vessels.
Chylothorax
Chylothorax is lymphatic fluid in the pleural space due to a leak in the thoracic duct. Causes include trauma, surgical procedures and malignancy. The thoracic duct is disrupted and the chylous fluid, milky white with high lipid content, fills the pleural space. Total lymphatic flow through the thoracic duct is 1500–2400 mL/day. Fifty per cent of cases will heal with conservative treatment (chest drainage, bowel rest and total parenteral nutrition). Surgery and pleurodesis are options if conservative therapy fails. Pleurodesis involves the artificial production of adhesions between the parietal and visceral pleura, usually done with a chemical sclerosing agent.
CLINICAL MANIFESTATIONS
If the pneumothorax is small, mild tachycardia and dyspnoea may be the only manifestations. If the pneumothorax is large, respiratory distress may be present, including shallow, rapid respirations, dyspnoea and air hunger. Chest pain and a cough with or without haemoptysis may be present. On auscultation there are no breath sounds over the affected area and hyperresonance may be present. A chest X-ray shows the presence of pneumothorax.
If a tension pneumothorax develops, severe respiratory distress, tachycardia and hypotension occur. Mediastinal displacement occurs and the trachea may shift to the unaffected side.
MULTIDISCIPLINARY CARE
Treatment depends on the severity of the pneumothorax and the nature of the underlying disease. If the patient is stable and the amount of air and fluid accumulated in the intrapleural space is minimal, no treatment may be needed as the pneumothorax resolves spontaneously. If the amount of air or fluid is minimal, the pleural space can also be aspirated with a large-bore needle. As a lifesaving measure, needle venting (using a large-bore needle) of the pleural space may be used. A small flutter valve such as the Heimlich or Pneumostat may also be used to evacuate air from the pleural space. These valves are portable, lightweight one-way flutter valve devices similar to a water-seal. The most definitive and common form of treatment of pneumothorax and haemothorax is to insert a chest tube and connect it to underwater-seal drainage. Repeated spontaneous pneumothorax may need to be treated surgically by a partial pleurectomy, stapling or pleurodesis to promote adherence of the pleurae to one another.
Fractured ribs
Rib fractures are the most common type of chest injury resulting from trauma. Ribs 5 to 10 are most commonly fractured because they are the least protected by chest muscles. If a fractured rib is splintered or displaced, it may damage the pleura and lungs.
Clinical manifestations of fractured ribs include pain (especially on inspiration) at the site of injury. The individual splints the affected area and takes shallow breaths to try to decrease the pain. Because the individual is reluctant to take deep breaths, atelectasis may develop because of decreased ventilation.
The main goal in treatment is to decrease pain so that the patient can breathe adequately to promote good chest expansion. Intercostal nerve blocks with local anaesthesia may be used to provide pain relief. The effect of the anaesthesia lasts for a period of hours to days. It must be repeated as necessary to provide pain relief. Strapping the chest with tape or using a binder is not common practice because these measures reduce lung expansion and predispose the individual to atelectasis. Opioid drug therapy must be individualised and used with caution because these drugs can depress respirations.
Flail chest
Flail chest results from multiple rib fractures, causing instability of the chest wall (see Fig 27-6). The diagnosis of flail chest is made on the basis of fracture of two or more ribs, in two or more separate locations, causing an unstable segment.62 The flail segment usually involves anterior (sternal separation) or lateral rib fractures. The chest wall cannot provide the bony structure necessary to maintain bellows action and ventilation. The affected (flail) area moves paradoxically to the intact portion of the chest during respiration. During inspiration the affected portion is sucked in and during expiration it bulges out. This paradoxical chest movement prevents adequate ventilation of the lung in the injured area. The underlying lung may or may not have a serious injury. Associated pain and any lung injury, giving rise to loss of compliance, will contribute to an alteration in breathing patterns and lead to hypoxaemia.
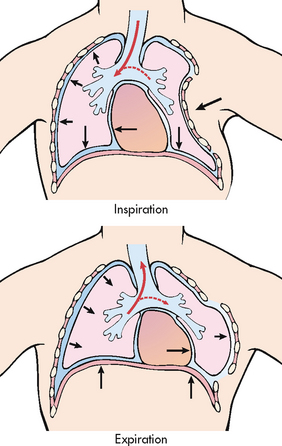
Figure 27-6 Flail chest produces paradoxical respiration. On inspiration the flail section sinks in with the mediastinal shift to the uninjured side. On expiration the flail section bulges outwards with the mediastinal shift to the injured side.
A flail chest is usually apparent on visual examination of the unconscious patient. The patient manifests rapid, shallow respirations and tachycardia. A flail chest may not be initially apparent in the conscious patient as a result of splinting of the chest wall. The patient moves air poorly and movement of the thorax is asymmetrical and uncoordinated. Palpation of abnormal respiratory movements, crepitus of the rib, chest X-ray and ABGs assist in the diagnosis.
Initial therapy consists of adequate ventilation, humidified oxygen and careful administration of resuscitation fluids. The definitive therapy is to re-expand the lung and ensure adequate oxygenation. Although many patients can be managed without the use of mechanical ventilation, a short period of intubation and ventilation may be necessary until the diagnosis of the lung injury is complete.
Positive end-expiratory pressure (PEEP) used with mechanical ventilation to improve oxygenation will maintain positive pressure in the lungs throughout the respiratory cycle. Mechanical ventilation is discussed in Chapter 67. The lung parenchyma and fractured ribs will heal with time.
Chest tubes and pleural drainage
The purpose of chest tubes and pleural drainage is to remove air and fluid from the pleural space and to restore normal intrapleural pressure so that the lungs can re-expand. Small accumulations of air or fluid in the pleural space may not require removal by thoracentesis or chest-tube insertion. Instead, the air and fluid may be reabsorbed over time.
Under normal conditions, intrapleural pressure is below atmospheric pressure (approximately 4–5 cm H2O below atmospheric pressure during expiration and approximately 8–10 cm H2O below atmospheric pressure during inspiration). (Intrapleural pressure and the intrapleural space are described in Ch 25.) If intrapleural pressure becomes equal to atmospheric pressure, the lungs will collapse (pneumothorax). Air can enter the intrapleural space by a variety of mechanisms, including traumatic chest injury (e.g. gunshot wound, fractured rib), thoracotomy and spontaneous pneumothorax. Excess fluid accumulation can occur in the pleural space as a result of impaired lymphatic drainage (e.g. from malignancy) or changes in the colloid osmotic pressure (e.g. in CHF). Empyema is purulent pleural fluid, which may be associated with lung abscesses or pneumonia.
CHEST TUBE INSERTION
Chest tubes can be inserted in the emergency department (ED), at the patient’s bedside or in the operating room (OR), depending on the situation. In the OR the chest tube is inserted using a thoracotomy incision. In the ED or at the bedside the patient is placed in a sitting position or is lying down with the affected side elevated. The area is prepared with antiseptic solution and the site is infiltrated with a local anaesthetic agent. After a small incision is made, one or two chest tubes are inserted into the pleural space. One catheter is placed anterior through the second intercostal space to remove air (see Fig 27-7); the other is placed posterior through the eighth or ninth intercostal space to drain fluid and blood. The fourth or fifth anterior or mid-axillary line can also be used because the anterior approach requires dissection of the pectoral muscles. In this instance the tube is directed apically for air evacuation and inferiorly and posterior for fluid removal.
The tubes are sutured to the chest wall and the puncture wound is covered with an airtight dressing. During insertion, the tubes are kept clamped. After the tubes are in place in the pleural space, they are connected to drainage tubing and a closed or underwater pleural drainage system and the clamp is removed. Each tube may be connected to a separate drainage system and suction. More commonly, a Y-connector is used to attach both chest tubes to the same drainage system.
PLEURAL DRAINAGE
Most pleural drainage systems have three basic compartments, each with its own separate function. In early drainage systems the three compartments were bottles, hence the name three-bottle system. Modern products incorporate these same basic concepts in their disposable plastic chest drainage systems (see Fig 27-8).

Figure 27-8 Chest drainage units. Both units have three chambers: (1) collection chamber; (2) water-seal chamber; (3) suction control chamber. The suction control chamber requires a connection to a wall suction source that is dialled up higher than the prescribed suction for the suction to work. A, Water suction: this unit uses water in the suction control chamber to control the wall suction pressure. B, Dry suction: this unit controls wall suction by using a regulator control dial.
The first compartment, or collection chamber, receives fluid and air from the chest cavity. The fluid stays in this chamber while the air vents to the second compartment. The second compartment, called the water-seal chamber, contains water, which acts as a one-way valve. The incoming air enters from the collection chamber and bubbles up through the water. (The water acts as a one-way valve to prevent backflow of air into the patient from the system.) The air then exits the water seal and enters the suction chamber. Initial bubbling of air is seen in this chamber when a pneumothorax is evacuated. Intermittent bubbling can also be seen during exhalation, coughing or sneezing due to an increase in the patient’s intrathoracic pressure. In this chamber fluctuations, or ‘swinging’, will be seen that reflect the pressures in the pleural space. If swinging is not seen, either the lungs may not have re-expanded or there may be a kink or obstruction in the tubing.
The third compartment, the suction control chamber, applies controlled suction to the chest drainage system. The classic suction control chamber uses tubing with one end submerged in a column of water and the other end vented to the atmosphere (see Fig 27-8). It is typically filled with 20 cm of water. When the negative pressure generated by the suction source exceeds 20 cm, the air from the atmosphere enters the chamber through a vent and begins bubbling up through the water. As a result, excess pressure is relieved. The amount of suction applied is regulated by the depth of the suction control tube in the water and not by the amount of suction applied to the system. An increase in suction does not result in an increase in negative pressure to the system because any excess suction merely draws in air through the vented tubing. The suction is usually ordered to be 20 cm H2O, but it varies according to the underlying condition being treated.63
Two types of suction control chambers are available on the market: wet and dry. The wet suction control chamber system is the classic system outlined above. Bubbling is one way to tell that suction is functioning. To start the suction, the vacuum source is turned up until gentle bubbling appears. Turning the vacuum source higher just makes the bubbling more vigorous and the water evaporate faster.63 Even at gentle bubbling, water evaporates in this chamber and water must be added periodically. The dry suction control chamber system contains no water. It uses either a restrictive device or a regulator to control the desired negative pressure; this is internal in the chest drainage system. The dry system has a visual alert that indicates whether the suction is working, so bubbling is not seen in the third chamber. To increase the suction pressures, the dial is turned on the drainage system. Increasing the vacuum suction source will not increase the pressure.
A variety of commercial disposable plastic chest drainage systems are available. The Pleurevac unit was one of the first disposable systems released. Another system in use in Australia and New Zealand is the Atrium Chest Drainage Unit. The manufacturer’s suggestions for use are included with the equipment. Portable chest drainage units can be used to allow the patient more mobility. Such units (see Fig 27-9) allow for a maximum of 500 mL drainage, offer a dry-seal system to prevent air leaks and can be used for long-term drainage. If a patient is discharged home with this device, patient and family teaching includes how to use the system and empty the drainage chamber.64
Flutter valves
Another device that may be used to evacuate air from the pleural space is the flutter valve, such as the Heimlich valve or the Pneumostat. This device consists of a rubber flutter one-way valve within a rigid plastic tube. It is attached to the external end of the chest tube. The valve opens whenever the pressure is greater than atmospheric pressure and closes when the reverse occurs. The flutter valve functions like a water seal and is usually used for emergency transport or in special home-care situations.
Small chest tubes
Small chest tubes (‘pigtail’ catheters) are used in selected patients because they are less traumatic. They drain air and fluid equally as well as large-bore chest tubes.65 The drains may be straight catheters or ‘pigtail’ catheters (curled at the distal end to look like a pig’s tail). Curled catheters are considered to be less traumatic than straight catheters. These catheters, if occluded, can be irrigated by the doctor or specialist nurse using sterile water. Clinical pleurodesis can also be performed through this catheter. This system is not suitable for trauma or for drainage of blood.
Both the small-bore chest tube and the flutter valve should be used with caution in patients on mechanical ventilators as there is the potential for rapid accumulation of air and the development of a tension pneumothorax.66
 NURSING MANAGEMENT: CHEST DRAINAGE
NURSING MANAGEMENT: CHEST DRAINAGE
Some general guidelines for nursing care of the patient with chest tubes and water-seal drainage systems are presented in Box 27-7. The traditional practice of routine milking and/or stripping of chest tubes to maintain patency is no longer necessary. Drainage and blood are not likely to clot inside chest tubes because the tubes are defibrinogenated. Additionally, the newer chest tubes are made with a coating that makes them non-thrombogenic. Clinical unit protocol and the doctor’s preference should be ascertained before the initiation of stripping or milking. The nurse should remember that these procedures can cause the patient to experience pain and that dislodgement of the tube may occur if the tube is not stabilised above the area being stripped.
BOX 27-7 Clinical guidelines for care of the patient with chest tubes and water-seal drainage
Drainage system
1. Keep all tubing as straight as possible and coiled loosely below chest level. Do not let the patient lie on it.
2. Keep all connections between chest tubes, drainage tubing and the drainage collector tight and tape at connections.
3. Observe for air bubbling in the water-seal chamber and fluctuations (swinging).
If no swinging is observed (rising with inspiration and falling with expiration in the spontaneously breathing patient), the drainage system is blocked, the lungs are re-expanded or the system is attached to suction.
If bubbling increases, there may be an air leak in the drainage system or a leak from the patient (bronchopleural leak).
4. If the chest tube is connected to suction, disconnect from wall suction to check for swinging.
5. Bubbling in the water seal may occur intermittently.
When bubbling is continuous and constant, the source of the air leak may be determined by momentarily clamping the tubing at successively distal points, starting at the patient’s chest site and ending at the drainage set, until the bubbling ceases.
When bubbling ceases, the leak is above the clamp. Retaping tubing connections, replacing the drainage apparatus or securing the chest tube with air occlusive dressing may be necessary to correct the air leak.
6. Keep the water-seal chamber at the appropriate water level by adding sterile water as needed due to evaporation of water.
7. High fluid levels in the water seal indicate residual negative pressure.
The chest system may need to be vented by using the high negativity release valve available on the drainage system to release residual pressure from the system.
Do not lower the water-seal column when wall suction is not operating or when the patient is on gravity drainage.
Patient’s clinical status
1. Monitor the patient’s clinical status. Take and document vital signs, auscultate lungs, observe chest wall. Document pain level.
2. Assess every shift for manifestations of reaccumulation of air and fluid in the chest (↓ or absent breath sounds), significant bleeding (>100 mL/h), chest drainage site infection (drainage, erythema, fever, WBC) or poor wound healing. Notify doctor for management plan. Evaluate for subcutaneous emphysema at the chest tube site.
3. Encourage the patient to breathe deeply periodically to facilitate lung expansion and encourage range-of-motion exercises to the shoulder on the affected side. Incentive spirometry every hour while awake may be necessary to prevent atelectasis or pneumonia.
4. Chest tubes are not routinely clamped. They are not clamped for transport. A doctor may request clamping for 24 h to evaluate for reaccumulation of fluid or air prior to discontinuing the chest tube.
Chest drainage
1. Never elevate the drainage system to the level of the patient’s chest because this will cause fluid to drain back into the lungs. Secure the unit to the drainage stand. If the drainage chambers are full, change the system. Do not try to empty it.
2. Mark the time of measurement and the fluid level on the drainage bottle according to the prescribed orders. Marking intervals may range from once per hour to every 8 h. Any change in the quantity or characteristics of drainage (e.g. clear yellow to bloody) should be reported to the doctor and recorded. Notify the doctor if >100 mL/h drainage.
3. Monitor the fluid drainage and evacuate no more than 1000–1200 mL of pleural fluid from the pleural space at one time to prevent rebound hypotension or re-expansion pulmonary oedema.
4. Check the position of the chest drainage container. If the drainage system is overturned and the water seal is disrupted, return it to an upright position and encourage the patient to take a few deep breaths, followed by forced exhalations and cough manoeuvres.
5. If the drainage system breaks, place the distal end of the chest tubing connection in a sterile water container at a 2 cm level as an emergency water seal.
6. Do not strip or milk chest tubes routinely as this dangerously increases pleural pressures.
Stripping: Pinch the tubing close to the chest with one hand and using a thumb and forefinger, compress and slide the fingers down towards the receptacle; release the pressure on the tube and repeat stripping action down the tube.
Milking: Alternately fold or squeeze and then release the drainage tubing. Milk only if drainage and evidence of clots/obstruction. Take 15 cm strips of the chest tube and squeeze and release, starting close to the chest and repeating down the tube distally.
Monitoring wet versus dry suction chest drainage systems
Suction control chamber in wet suction system
1. Keep the suction control chamber at the appropriate water level by adding sterile water as needed due to evaporation.
2. After filling the suction control chamber to the ordered suction amount (generally 20 cm water suction), connect the suction tubing to the wall suction.
3. Set the wall suction regulator higher than the ordered suction amount until bubbling is seen in the suction control chamber (generally 80–120 mmHg). Vigorous bubbling is not necessary and will cause quicker evaporation of the water in the chamber. Use gentle bubbling.
4. The suction control chamber should have constant bubbling. This indicates the suction is working and functioning.
5. If there is no bubbling seen in the suction control chamber, this indicates: (1) no suction/suction loss; (2) suction is not high enough; (3) pleural air leak so large that suction is not high enough to evacuate it.
Suction control chamber in dry suction system (atrium dry system)
1. After connecting the patient to the system, turn the dial on the chest drainage system to the amount ordered (generally 20 cm pressure), connect the suction tubing to the wall suction source and increase the suction until all of the orange float valve is seen in the window.
2. The orange float valve is a visual indicator of suction. The orange bellows needs to be extended to the arrow (delta mark) or further. Wall suction must be set from 80 to 120 mmHg. There is no water, bubbling or evaporation. This system can be set up to 40 cm suction if needed for a patient.
3. If suction is to be decreased, turn the dial down. There will still be high negative pressure in the system, as evidenced by high water-seal manometer pressure and it needs to be vented by using the high negativity release valve.
Chest tube dressings
1. Change the dressing when wet; change routinely every 2 days unless ordered more frequently by the doctor.
2. Remove old dressing carefully to avoid removing unsecured chest tube. Evaluate the site and culture the site if necessary.
3. Clean the site with sterile normal saline. Apply sterile gauze and tape to secure the dressing. Vaseline gauze may be used around the tube to prevent air leak. Date the dressing and document the dressing change.
Obtaining a sample from the chest tube
1. Form a loop in the tubing in an area to get the most recently drained fluid.
2. Swab an area of the tubing with alcohol and allow to air dry.
3. Insert a 20-gauge or smaller syringe needle at an angle and aspirate the sample; do not puncture the other side of the tubing.
4. Place the sample in an appropriate, labelled container and send to the laboratory.
Clamping of chest tubes during transport or when the tube is accidentally disconnected is no longer advocated. The danger of rapid accumulation of air in the pleural space causing tension pneumothorax is far greater than that of a small amount of atmospheric air entering the pleural space. Chest tubes may be momentarily clamped to change the drainage apparatus or to check for air leaks. Clamping for more than a few moments is indicated only in assessing how the patient will tolerate chest tube removal. It is done to simulate chest tube removal and identify whether there will be negative clinical problems with tube removal. Generally this is done 4–6 hours before the tube is removed and the patient is monitored closely. If a chest tube becomes disconnected, the most important intervention is re-establishment of the water-seal system immediately and attachment of a new drainage system as soon as possible. In some hospitals, when disconnection occurs, the chest tube is immersed in sterile water (about 2 cm) until the system can be re-established. It is important for the nurse to know the clinical unit protocol, the individual clinical situation (whether an air leak exists) and the doctor’s preference before resorting to prolonged chest tube clamping.
Chest tube malposition is the most common complication. To evaluate whether the chest drainage is successful, the nurse routinely monitors the patient, observing for swinging in the underwater-seal chamber, listening for breath sounds over the lung fields and measuring the amount of fluid drainage. Re-expansion pulmonary oedema can occur after rapid expansion of a collapsed lung in patients with a pneumothorax or after evacuation of large volumes of pleural fluid (>1–1.5 L). A vasovagal response with symptomatic hypotension can occur from too rapid removal of fluid.
Infection at the skin site is also a concern. Meticulous sterile technique during dressing changes can reduce the incidence of infected sites. Other complications include: (1) pneumonia from not taking deep breaths, from not using incentive spirometers or by splinting on the affected side; and (2) shoulder disuse (‘frozen shoulder’) from lack of range-of-motion exercises. Poor patient compliance or lack of patient teaching can contribute to these complications. Nurses can have a tremendous impact in preventing such complications.67
CHEST TUBE REMOVAL
The patient with chest tubes will have chest X-rays to follow the course of lung expansion. The chest tubes are removed when the lungs are re-expanded and fluid drainage has ceased. Generally, suction is removed and the patient is placed on gravity drainage for a period of time before the tubes are removed. The tube is removed aseptically by: cutting the sutures; applying a sterile petroleum jelly gauze dressing; having the patient take a deep breath, exhale and bear down (the Valsalva manoeuvre); then removing the tube. Pain medication is generally given before removal. The site is covered with an airtight dressing, the pleura self-seal and the wound is healed in several days. A chest X-ray is done after chest tube removal to evaluate for pneumothorax and/or re-accumulation of fluid. The wound should be observed for drainage and should be reinforced if necessary. The patient should be observed for respiratory distress, which may signify a recurrent or new pneumothorax.
Chest surgery
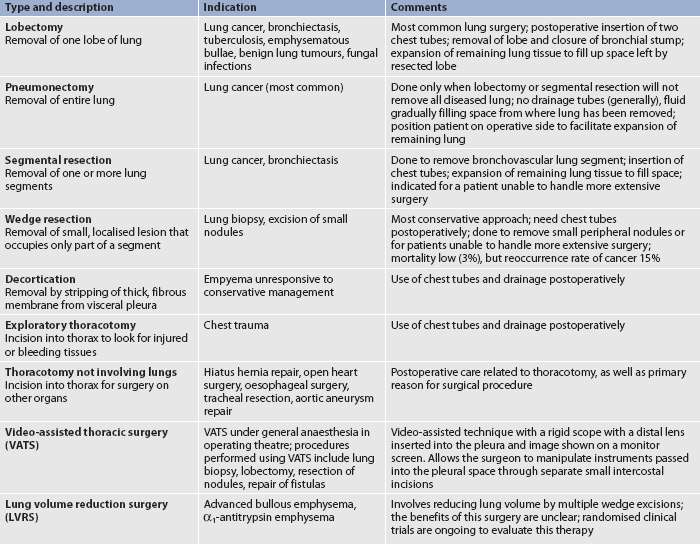
Chest surgery is performed for a variety of reasons, some of which are unrelated to primary lung problems. For example, a thoracotomy is performed for heart and oesophageal surgery. Types of chest surgery are compared in Table 27-15.
PREOPERATIVE CARE
Before chest surgery, baseline data are obtained on the respiratory and cardiovascular systems. Diagnostic studies performed include pulmonary function studies, chest X-rays, electrocardiogram (ECG), ABGs, serum urea, serum creatinine, blood glucose, serum electrolytes and FBC. Additional studies of cardiac function, such as cardiac catheterisation, may be done for the patient who is to undergo a pneumonectomy. A careful physical assessment of the lungs, including percussion and auscultation, should be done. This will allow the nurse to compare preoperative and postoperative findings.
The patient should be encouraged to stop smoking before surgery to decrease secretions and increase oxygen saturation. In the anxious period before surgery this is not an easy thing for the habitual smoker to do. Chest physiotherapy may be indicated to help clear the lungs of accumulated secretions; this is especially indicated for the patient with a lung abscess or bronchiectasis.
Preoperative teaching should include exercises for effective deep breathing and incentive spirometry. If the patient practises these techniques before surgery, the techniques will be easier to perform postoperatively. The nurse should explain to the patient that adequate medication will be given to reduce the pain and should help the patient to splint the incision with a pillow to facilitate deep breathing.
For most types of chest surgery, chest tubes are inserted and connected to water-sealed drainage systems. The purpose of these tubes should be explained to the patient. In addition, oxygen is frequently given for the first 24 hours, or as required after surgery. Range-of-motion exercises on the surgical side similar to those for the mastectomy patient should be taught (see Ch 51).
The thought of losing part of a vital organ is frequently frightening. The patient should be reassured that the lungs have a large degree of functional reserve, and that even after the removal of one lung, there is enough lung tissue to maintain adequate oxygenation.
The nurse should be available to deal with the questions asked by the patient and family, facilitating the expression of concerns, feelings and questions. (General preoperative care and teaching are discussed in Ch 17.)
SURGICAL THERAPY
Thoracotomy (surgical opening into the thoracic cavity) is considered major surgery because the incision is large, cutting into bone, muscle and cartilage. The two types of thoracic incision are median sternotomy and lateral thoracotomy. Median sternotomy, performed by splitting the sternum, is primarily used for surgery involving the heart. There are two types of lateral thoracotomy: posterolateral and anterolateral. Posterolateral thoracotomy is used for most surgeries involving the lung. The incision is made from the anterior axillary line below the nipple level posteriorly at the fourth, fifth or sixth intercostal space. It is rarely necessary to remove the ribs. Strong mechanical retractors are used to gain access to the lung. The anterolateral incision is made in the fourth or fifth intercostal space from the sternal border to the midaxillary line. This procedure is commonly used for surgery or trauma victims, mediastinal operations and wedge resections of the upper and middle lobes of the lung.
The extensiveness of the thoracotomy incision often results in severe pain for the patient after surgery. Because muscles have been severed, the patient is reluctant to move the shoulder and arm on the surgical side. Chest tubes are placed in the pleural space except in pneumonectomy surgery. In a pneumonectomy the space from which the lung was removed gradually fills with serosanguineous fluid.
Thorascopic surgery
Thorascopic surgery (endoscopic thoracotomy) is a procedure that in many cases can avoid the impact of a full thoracotomy. The procedure usually involves three to four 2.5-cm incisions made on the chest to allow the thorascope (a special fibreoptic camera) and instruments to be inserted and manipulated. Video-assisted thorascopes improve visualisation because the surgeon can view the thoracic cavity from the video monitor. Thorascopy can be used to diagnose and treat a variety of conditions of the lungs, pleura and mediastinum.
The candidate for this type of procedure should not have a prior history of conventional thoracic surgery because the probability of adhesion formation makes access more difficult. The patient whose lesions are in the lung periphery or the mediastinum is a better candidate because of better accessibility. The patient considered for thorascopic surgery should have sufficient pulmonary function preoperatively to allow the surgeon to perform conventional thoracotomy if complications occur. Complications that may occur are bleeding, diaphragmatic perforation, air emboli, persistent pleural air leaks and tension pneumothorax.
There are many benefits of thorascopic surgery when compared with a conventional thoracotomy. These include less adhesion formation, minimal blood loss, less time under anaesthesia, shorter length of hospitalisation, faster recovery, less pain and no need for postoperative rehabilitation therapy because of minimal disruption to the thoracic structures.
At the end of the procedure chest tubes are placed through one of the incisions. The incisions are closed with sutures or a wound-approximating adhesive bandage. Nursing assessment and care postoperatively include monitoring respiratory status and lung re-expansion with the chest tubes and checking the incisions for drainage or dehiscence. The most common complication is prolonged air leak. A return to prior activities should be encouraged as quickly as possible. The hospital stay averages from 1 to 5 days, depending on the type of surgery.
POSTOPERATIVE CARE
Specific measures related to patient care after a thoracotomy are presented in NCP 27-2. The specific follow-up care depends on the type of surgical procedure. General postoperative care is discussed in Chapter 19.
RESTRICTIVE RESPIRATORY DISORDERS
Restrictive respiratory disorders are characterised by decreased compliance of the lungs or chest wall, or both. This is in contrast to obstructive respiratory disorders, which are characterised by increased resistance to airflow. Pulmonary function tests are the best means to use in differentiating between restrictive and obstructive respiratory disorders (see Table 27-16). Mixed obstructive and restrictive respiratory disorders are often manifested. For example, a patient may have both chronic bronchitis (an obstructive problem) and pulmonary fibrosis (a restrictive problem).
Restrictive problems are generally categorised into extrapulmonary disorders and intrapulmonary disorders. Extrapulmonary causes of restrictive lung disease include disorders involving the central nervous system, neuromuscular system and chest wall. In these disorders the lung tissue is normal. Table 27-17 lists some of the extrapulmonary disorders. Intrapulmonary causes of restrictive lung disease involve the pleura or lung tissue. Table 27-18 lists some of the intrapulmonary disorders.
TABLE 27-18 Intrapulmonary causes of restrictive lung disease

ARDS, acute respiratory distress syndrome; TB, tuberculosis.
* see Chapter 67 for clinical manifestations and management.
Pleural effusion
TYPES
The pleural space lies between the lung and chest wall and normally contains a very thin layer of fluid. Pleural effusion is a collection of fluid in the pleural space (see Fig 27-4, A). It is not a disease but rather a sign of a serious disease. Pleural effusion is frequently classified as transudative or exudative according to whether the protein content of the effusion is low or high, respectively.68 A transudate occurs primarily in non-inflammatory conditions and is an accumulation of protein-poor, cell-poor fluid. A transudative pleural effusion (also called a hydrothorax) is caused by: (1) increased hydrostatic pressure found in CHF, which is the most common cause of pleural effusion; or (2) decreased oncotic pressure (from hypoalbuminaemia) found in chronic liver or renal disease. In these situations, fluid movement is facilitated out of the capillaries and into the pleural space.
An exudate is an accumulation of fluid and cells in an area of inflammation. An exudative pleural effusion results from an increased capillary permeability characteristic of the inflammatory reaction. This type of effusion occurs secondary to conditions such as pulmonary malignancies, pulmonary infections, pulmonary embolisation and GI disease (e.g. pancreatic disease, oesophageal perforation).
A sample of pleural fluid can be obtained from the pleural space by thoracentesis to determine the type of pleural effusion. Exudates have a high protein content and the fluid is generally dark yellow or amber.69 Transudates have a low protein content or contain no protein and the fluid is clear or pale yellow. The fluid can also be analysed for red and white blood cells, malignant cells, bacteria and glucose.
An empyema is a pleural effusion that contains pus. It is caused by conditions such as pneumonia, TB, lung abscess and infection of surgical wounds of the chest. A complication of empyema is fibrothorax, in which there is fibrous fusion of the visceral and parietal pleurae (see Fig 27-4, A).
CLINICAL MANIFESTATIONS
Common clinical manifestations of pleural effusion are progressive dyspnoea and decreased movement of the chest wall on the affected side. There may be pleuritic pain from the underlying disease. Physical examination of the chest will indicate dullness to percussion and absent or decreased breath sounds over the affected area. The chest X-ray will indicate an abnormality if the effusion is more than 250 mL. Manifestations of empyema include the manifestations of pleural effusion, as well as fever, night sweats, cough and weight loss. A thoracentesis reveals an exudate containing thick, purulent material.
THORACENTESIS
If the cause of the pleural effusion is not known, a diagnostic thoracentesis is needed to obtain pleural fluid for analysis (see Fig 25-17). If the degree of pleural effusion is severe enough to impair breathing, a therapeutic thoracentesis is done to remove fluid for analysis.
A thoracentesis is performed by having the patient sit on the edge of the bed and lean forwards over a bedside table. The puncture site is determined by chest X-ray and percussion of the chest is used to assess the maximum degree of dullness. The skin is cleaned with an antiseptic solution and anaesthetised locally. The thoracentesis needle is inserted into the intercostal space. Fluid can be aspirated with a syringe or tubing can be connected to allow fluid to drain into a sterile collecting bottle. After the fluid is removed, the needle is withdrawn and a bandage is applied over the insertion site.
Usually only 1000–1200 mL of pleural fluid is removed at one time. Because high volumes are removed, rapid removal can result in hypotension, hypoxaemia or pulmonary oedema.70 A follow-up chest X-ray should be done to detect a possible pneumothorax that could have been induced by perforation of the visceral pleura. During and after the procedure the patient should be observed for any manifestations of respiratory distress.
MULTIDISCIPLINARY CARE
The main goal of management of pleural effusions is to treat the underlying cause. For example, adequate treatment of CHF with diuretics and sodium restriction will result in decreased pleural effusions. The treatment of pleural effusions secondary to malignant disease represents a more difficult problem. These types of pleural effusions are frequently recurrent and accumulate quickly after thoracentesis. Chemical pleurodesis may be used to sclerose the pleural space and prevent re-accumulation of effusion fluid. Although doxycycline and bleomycin have been used for sclerosing with good results, talc appears to be the most effective agent for pleurodesis.68 Thoracoscopy can be used to perform talc pleurodesis after inspection of the pleural space. After instillation of the sclerosing agent, patients are usually instructed to rotate their positions to spread the agent uniformly throughout the pleural space. The decision to rotate the patient from side to side to back depends on the surgeon’s preference and the patient’s ability to tolerate turning.71 Chest tubes are left in place after pleurodesis until fluid drainage is greater than 150 mL/day and no air leaks are noted. A more rapid completion of the pleurodesis procedure in less than 24 hours is reported in the literature with good results and this limited admission for symptomatic malignant effusions may become more frequent.72
Treatment of empyema is directed at drainage of the pleural space via thoracentesis or a closed thoracotomy tube. Appropriate antibiotic therapy is also needed to eradicate the causative organism. If a fibrothorax results from the empyema and causes severe pulmonary restriction, a decortication surgical procedure is done in which the pleural membranes are separated.
Pleurisy
Pleurisy (pleuritis) is an inflammation of the pleura. The most common causes are pneumonia, TB, chest trauma, pulmonary infarctions and neoplasms. The inflammation usually subsides with adequate treatment of the primary disease. Pleurisy can be classified as fibrinous (dry) with fibrinous deposits on the pleural surface or serofibrinous (wet) with increased production of pleural fluid that may result in pleural effusion.
The pain of pleurisy is typically abrupt and sharp in onset and is aggravated by inspiration. The patient’s breathing is shallow and rapid to avoid unnecessary movement of the pleura and chest wall. A pleural friction rub may occur, which is the sound over areas where inflamed visceral pleura and parietal pleura rub over one another during inspiration. This sound is usually loudest at peak inspiration but can be heard during exhalation as well.
Treatment of pleurisy is aimed at treating the underlying disease and providing pain relief. Taking analgesics and lying on or splinting the affected side may provide some relief. The patient should be taught to splint the rib cage when coughing. Intercostal nerve blocks may be done if the pain is severe.
Atelectasis
Atelectasis is a condition of the lungs characterised by collapsed, airless alveoli. The most common cause of atelectasis is airway obstruction that results from retained exudates and secretions. This is frequently observed in the postoperative patient. Normally the pores of Kohn (see Fig 25-1) provide for collateral passage of air from one alveolus to another. Deep inspiration is necessary to open the pores effectively. For this reason, deep-breathing exercises are important in preventing atelectasis in high-risk patients (e.g. postoperative, immobilised patients). Pulmonary fibrosis can occur as a complication of chronic atelectasis. (The prevention and treatment of atelectasis are discussed in Ch 19.)
INTERSTITIAL LUNG DISEASES
Many acute and chronic lung disorders with variable degrees of pulmonary inflammation and fibrosis are collectively referred to as interstitial lung diseases (ILDs). ILDs have been difficult to classify because more than 200 known diseases have diffuse lung involvement, either as the primary condition or as a significant part of a multi-organ process, as may occur in connective tissue disorders (e.g. systemic lupus erythematosus, rheumatoid arthritis).
Among the ILDs of known cause, the largest group comprises occupational and environmental exposure, especially the inhalation of dusts and various fumes or gases. The most common ILDs of unknown aetiology are idiopathic pulmonary fibrosis and sarcoidosis.
Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is characterised by scar tissue in the connective tissue of the lungs as a sequela to inflammation or irritation. A common risk factor for IPF is environmental or occupational inhalation of organic and inorganic substances. Other risk factors include cigarette smoking and a history of chronic aspiration. There also may be genetic risk factor.
Clinical manifestations of IPF include exertional dyspnoea, non-productive cough and inspirational crackles with or without clubbing. Chest X-ray shows changes characteristic of IPF. Pulmonary function tests show a typical pattern characteristic of restrictive lung disease (see Table 27-16).
The clinical course is variable, with a 5-year survival rate of 30–50% after diagnosis. Treatment includes corticosteroids, cytotoxic agents (azathioprine, cyclophosphamide) and anti-fibrotic agents (colchicine). However, there is no good evidence that any of these treatments improves survival or quality of life. Lung transplantation is an option that should be considered for those who meet the criteria. (Lung transplantation is discussed on p 669.)
Sarcoidosis
Sarcoidosis is a chronic, multisystem granulomatous disease of unknown cause that primarily affects the lungs. The disease may also involve the skin, eyes, liver, kidneys, heart and lymph nodes. Seen worldwide, it can be familial and is more prevalent among people of African descent, although Japanese people show the highest rate of cardiac sarcoid-attributed death.73 The disease is often acute or subacute and self-limiting, but in many individuals it is chronic with remissions and exacerbations. Marked pulmonary fibrosis can be present with severe restrictive lung disease. Cor pulmonale and bronchiectasis can develop in the advanced stages.
There is no specific treatment for sarcoidosis. Often the disease is self-limiting and the patient gets well without treatment. Corticosteroids have been used to relieve symptoms and suppress the acute inflammation. A trial of methotrexate may be considered if the patient does not respond to or cannot tolerate corticosteroid therapy. If this is ineffective or not tolerated, cyclophosphamide or azathioprine may be initiated. Non-steroidal anti-inflammatory agents, such as ibuprofen, may help decrease acute inflammation or relieve symptoms but are not a treatment for sarcoidosis. Disease progression is monitored by pulmonary function tests, chest X-ray and CT scan.73
Pulmonary oedema
Pulmonary oedema is an abnormal accumulation of fluid in the alveoli and interstitial spaces of the lungs. It is a complication of various heart and lung diseases (see Box 27-8). It is considered a medical emergency and may be life-threatening.
BOX 27-8 Causes of pulmonary oedema
• Overhydration with intravenous fluids
• Hypoalbuminaemia: nephrotic syndrome, hepatic disease, nutritional disorders
• Altered capillary permeability of lungs: inhaled toxins, inflammation (e.g. pneumonia), severe hypoxia, near-drowning
• Malignancies of the lymph system
• Respiratory distress syndrome (e.g. O2 toxicity)
• Unknown causes: neurogenic condition, narcotic overdose, re-expansion pulmonary oedema, high altitude
Normally, there is a balance between the hydrostatic and oncotic pressures in the pulmonary capillaries. If the hydrostatic pressure increases or the colloid oncotic pressure decreases, the net effect will be fluid leaving the pulmonary capillaries and entering the interstitial space. This stage is referred to as interstitial oedema. At this stage the lymphatics can usually drain away the excess fluid. If fluid continues to leak from the pulmonary capillaries it will enter the alveoli. This stage is referred to as alveolar oedema. Pulmonary oedema interferes with gas exchange by causing an alteration in the diffusing pathway between the alveoli and the pulmonary capillaries.
The most common cause of pulmonary oedema is left-ventricular heart failure. (The clinical manifestations and management of pulmonary oedema are described in Ch 34.) Chronic forms of pulmonary oedema are not common. The condition can be asymptomatic for a long period of time while changes occur, resulting in pulmonary fibrosis. An early manifestation of this condition may be paroxysmal nocturnal dyspnoea as a result of increased hydrostatic pressure in the lungs in the recumbent position.
Pulmonary embolism
A pulmonary embolism is the blockage of pulmonary arteries by a thrombus or, less commonly, fat or air emboli, or tumour tissue. The word embolus derives from a Greek word meaning ‘plug’ or ‘stopper’. A pulmonary embolus (PE) consists of material that gains access to the venous system and then to the pulmonary circulation. The material eventually reaches a section of the pulmonary arterial vessels where it lodges, thus obstructing perfusion (see Fig 27-10). The result of the thromboembolic occlusion is complete or partial occlusion of the pulmonary arterial blood flow to parts of the lung. Thus the lung tissue distal to the embolus is ventilated but not perfused. As the pressure increases in the pulmonary vasculature, pulmonary hypertension may result.
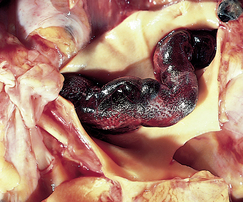
Figure 27-10 Large embolus from the femoral vein lying in the main left and right pulmonary arteries.
Source: Kumar V, Abbas AK. Robbins & Cotran pathologic basis of disease. 7th edn. Philadelphia: Saunders; 2005.
The most common source of the thrombus is the deep veins of the legs. The thrombus breaks loose and travels as an embolus until it lodges in the pulmonary vasculature. Other sites of origin include the right side of the heart (especially with atrial fibrillation), the upper extremities (rare) and the pelvic veins (especially after surgery or childbirth). Lethal PEs most commonly originate in the femoral or iliac veins. Emboli are mobile clots that generally do not stop moving until they lodge at a narrowed part of the circulatory system. The lungs are an ideal location for emboli to lodge because of their extensive arterial and capillary network. The lower lobes are most frequently affected because they have a higher blood flow than the other lobes.
Thrombi in the deep veins can dislodge spontaneously. However, a more common mechanism is jarring of the thrombus by mechanical forces, such as sudden standing and changes in the rate of blood flow, such as those that occur with the Valsalva manoeuvre. The majority of patients with PE due to deep vein thrombosis (DVT) have no leg symptoms at the time of diagnosis.74
The most common risk factors for PE are immobilisation, surgery within the last 3 months, stroke, a history of DVT and malignancy. Patients hospitalised with heart failure also have a higher relative risk of PE than those without heart failure.75 The highest rate of DVT is seen in spinal cord injury patients (67–100%).76
The vast majority of those who die from PE do so because of failure of diagnosis.77 Without treatment, the mortality rate is 30%; with treatment, it is 2–8%.74 Patients undergoing surgery are at high risk of PE and so should receive preventative measures, as PE is one of the most common causes of preventable death in hospitalised patients.77
CLINICAL MANIFESTATIONS
The signs and symptoms of PE are generally subtle and non-specific, making diagnosis difficult. The classic triad of dyspnoea, chest pain and haemoptysis occurs in only about 20% of patients. The most common manifestations of PE are anxiety and the sudden onset of unexplained dyspnoea, tachypnoea or tachycardia. A mild-to-moderate hypoxaemia with a low PaCO2 is a common finding. Other manifestations are cough, pleuritic chest pain, haemoptysis, crackles, fever, accentuation of the pulmonic heart sound and sudden change in mental status as a result of hypoxaemia.
Massive emboli may produce sudden collapse of the patient with shock, pallor, severe dyspnoea, hypoxaemia and crushing chest pain. However, some patients with massive PE do not have pain. The pulse is rapid and weak, the blood pressure is low and an ECG indicates right ventricular strain. When rapid obstruction of 50% or more of the pulmonary vascular bed occurs, acute cor pulmonale may result because the right ventricle can no longer pump blood into the lungs.77 The mortality rate of persons with massive PE and shock is approximately 33%.
Medium-sized emboli often cause pleuritic chest pain, dyspnoea, slight fever and a productive cough with blood-streaked sputum. A physical examination may reveal tachycardia and a pleural friction rub. Small emboli frequently are undetected or produce vague, transient symptoms. The exception to this is the patient with underlying cardiopulmonary disease in whom even small or medium-sized emboli may result in severe cardiopulmonary compromise. However, repeated small emboli gradually cause a reduction in the capillary bed and eventual pulmonary hypertension. An ECG and chest X-ray may indicate right ventricular hypertrophy secondary to pulmonary hypertension.
COMPLICATIONS
Pulmonary infarction (death of lung tissue) is most likely when any of the following factors are present: (1) occlusion of a large- or medium-sized pulmonary vessel (greater than 2 mm in diameter); (2) insufficient collateral blood flow from the bronchial circulation; or (3) pre-existing lung disease. Infarction results in alveolar necrosis and haemorrhage. Occasionally the infarcted tissue becomes infected and an abscess may develop. Concomitant pleural effusion is frequently found.
Pulmonary hypertension occurs when more than 50% of the area of the normal pulmonary bed is compromised. Pulmonary hypertension also results from hypoxaemia. As a single event an embolus does not cause pulmonary hypertension unless it is massive. However, recurrent small- to medium-sized emboli may result in chronic pulmonary hypertension. Pulmonary hypertension eventually results in dilation and hypertrophy of the right ventricle. Depending on the degree of pulmonary hypertension and its rate of development, outcomes can vary, with some patients dying within months of the diagnosis and others living for decades.
DIAGNOSTIC STUDIES
A ventilation–perfusion lung scan is the most frequently used test to aid in the diagnosis of PE. The lung scan has two components and is most accurate when both are performed:
1. Perfusion scanning involves IV injection of a radioisotope. A scanning device detects the adequacy of the pulmonary circulation.
2. Ventilation scanning involves inhalation of a radioactive gas such as xenon. Scanning reflects the distribution of gas through the lung. The ventilation component requires the cooperation of the patient and may be impossible to perform in the critically ill patient, particularly if the patient is intubated.
D-dimer testing may be recommended when a PE is initially suspected. D-dimer is a degradation product rarely found in healthy individuals. However, levels of D-dimer are elevated in any condition involving degradation of fibrin (infection, cancer, surgery, heart failure). They are elevated eightfold in venous thromboembolism. When D-dimer levels are normal (less than 500 μg/L), it is highly unlikely that the patient has a PE. Thus a normal or near-normal D-dimer level can rule out a PE. In the event that the D-dimer levels are elevated, a non-invasive venous study (see Box 27-9) is indicated to look for a DVT as the likely source of a PE. If a DVT is located by venous ultrasound, the index of suspicion for PE is very high and anticoagulant treatment should be initiated immediately.
BOX 27-9 Acute pulmonary embolism
MULTIDISCIPLINARY CARE
Diagnostic studies
History and physical examination
FBC count with WBC differential
Collaborative therapy
Supplemental oxygen, intubation may be necessary
IV for medications and fluid replacement
Continuous IV heparin for acute treatments
Warfarin for long-term therapy
Monitoring of PTT and INR levels
Thrombolytic agent may be considered
Pulmonary embolectomy in life-threatening situation
ABGs, arterial blood gases; BNP, B-type natriuretic peptide; CT, computed tomography; ECG, electrocardiogram; FBC, full blood count; INR, international normalised ratio; IV, intravenous; PTT, partial thromboplastin time; WBC, white blood cell.
Patients with an elevated D-dimer level but normal venous ultrasound need a lung scan or spiral CT. If the lung scan is inconclusive, pulmonary angiography is recommended. Pulmonary angiography is an invasive procedure that involves the insertion of a catheter through the antecubital or femoral vein, advancement to the pulmonary artery and injection of contrast medium. This allows visualisation of the pulmonary vascular system and location of the embolus.
The use of CT scans has revolutionised the diagnosis of PE, with CT pulmonary angiography being a widely used radiological investigation for suspected PE. A spiral (or helical) CT scan, a non-invasive diagnostic test, may also be used to diagnose PE. Conventional CT scans rotate a frame 360° in one direction, stop, make an image (called a slice) and then spin back in the opposite direction to make another slice after again stopping. However, the spiral CT scan is able to continuously rotate while obtaining slices and does not have to start and stop between each slice. This allows visualisation of entire anatomical regions such as the lungs. The computer reconstructs the data to allow for a three-dimensional picture of the area being imaged and assist in emboli visualisation.
ABG analysis is important, but not diagnostic. The PaO2 is low because of inadequate oxygenation secondary to an occluded pulmonary vasculature. The PaCO2 is usually low because of hyperventilation. The pH remains normal unless respiratory alkalosis develops as a result of prolonged hyperventilation or to compensate for lactic acidosis caused by shock. There are usually abnormal findings reported on chest X-ray (atelectasis, pleural effusion) and ECG (ST segment and T wave changes), but they are not diagnostic for PE. Serum troponin levels are elevated in 30–50% of patients with PE and, although not diagnostic, they are predictive of an adverse prognosis.74 Serum B-type natriuretic peptide (BNP) levels, while not diagnostic, may be helpful in identifying the severity of the clinical course.
MULTIDISCIPLINARY CARE
When the diagnosis of PE has been made, treatment should be instituted immediately (see Box 27-9). The objectives of treatment are to: (1) prevent further growth or multiplication of thrombi in the lower extremities; (2) prevent embolisation from the upper or lower extremities to the pulmonary vascular system; and (3) provide cardiopulmonary support if indicated.
Conservative therapy
Supportive therapy for the patient’s cardiopulmonary status varies according to the severity of the PE. The administration of supplemental O2 by mask or cannula may be adequate for some patients. O2 is given in a concentration determined by ABG analysis. In some situations, endotracheal intubation and mechanical ventilation may be needed to maintain adequate oxygenation. Respiratory measures such as turning, coughing and deep breathing are necessary to prevent or treat atelectasis. If shock is present, vasopressor agents may be necessary to support systemic circulation (see Ch 66). If heart failure is present, diuretics are used (see Ch 34). Pain resulting from pleural irritation or reduced coronary blood flow is treated with narcotics, usually morphine.
Drug therapy
Properly managed anticoagulant therapy is effective in the treatment of many patients with PE. Although unfractionated heparin traditionally has been used for PE, low-molecular-weight heparin (e.g. dalteparin sodium) is now the agent of choice for initial treatment of PE. Warfarin is used for ongoing anticoagulation and is typically administered for 3–6 months. Factor Xa inhibitors and direct thrombin inhibitors are also being used in the treatment of PE.
The dosage of heparin may need to be adjusted, to ensure a therapeutic activated partial thromboplastin time (APTT), while warfarin dose is determined by the international normalised ratio (INR). Frequent changes and titrations of heparin doses are needed initially in order to obtain a therapeutic APTT level, although with low-molecular-weight heparin this is less likely. The heparin works to prevent future clots, but does not dissolve existing clots. Anticoagulant therapy may be contraindicated if the patient has blood dyscrasias, hepatic dysfunction causing alteration in the clotting mechanism, injury to the intestine, overt bleeding and a history of haemorrhagic stroke or neurological conditions.
Fibrinolytic agents, such as reteplase or altepase, dissolve the PE and the source of the thrombus in the pelvis or deep leg veins, thereby decreasing the likelihood of recurrent emboli. Indications for thrombolytic therapy in PE include haemodynamic instability and right ventricular dysfunction. (Fibrinolytic therapy is discussed in Ch 33.)
Surgical therapy
If the degree of pulmonary arterial obstruction is severe and the patient does not respond to conservative therapy, an immediate embolectomy may be indicated. Pulmonary embolectomy, a rare procedure, has a 50% mortality rate. Preoperative pulmonary angiography is necessary to identify and locate the site of the embolus. When a pulmonary embolectomy is performed, the patient also has placement of a vena cava filter.
To prevent further pulmonary embolisation, an inferior vena cava (IVC) filter may be warranted. This device prevents migration of large clots into the pulmonary system, is easily and safely placed percutaneously, is biocompatible and does not require the patient to be anticoagulated. It can be used for patients who have an absolute contraindication to anticoagulant therapy. In addition, it may be used as a prophylactic measure for patients at high risk of PE (e.g. spinal cord injury, cor pulmonale). The complications associated with this device are rare and include misplacement, migration and perforation. The spinal cord injury patient with an IVC filter cannot have an assisted cough (‘quad cough’) procedure to mobilise secretions since the procedure can displace the filter.
 NURSING MANAGEMENT: PULMONARY EMBOLISM
NURSING MANAGEMENT: PULMONARY EMBOLISM
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Nursing measures aimed at prevention of pulmonary embolism parallel those for prophylaxis of DVT (see Ch 38).
 Acute intervention
Acute intervention
The prognosis of a patient with PE is good if therapy is promptly instituted. The patient should be kept on bed rest in a semi-recumbent position to facilitate breathing. An IV line should be maintained for medications and fluid therapy. The nurse should know the side effects of medications and observe for them. Oxygen therapy should be administered as ordered. Careful monitoring of vital signs, cardiac dysrhythmia monitoring, pulse oximetry, ABGs and lung sounds is critical to assess the patient’s status. Laboratory results are monitored to assure normal ranges of APTT and INR. Nursing care includes assessing for the complications of anticoagulant therapy (e.g. bleeding, haematomas, bruising) and PE (e.g. atelectasis, pneumonia). The nursing care plan includes interventions related to immobility and fall precautions.
The patient is usually anxious because of pain, a sense of doom, an inability to breathe and a fear of death. The nurse should carefully explain the situation and provide emotional support and reassurance to help relieve the patient’s anxiety.
The patient affected by thromboembolic processes may require psychological and emotional support. In addition to the thromboembolic problems, the patient may have an underlying chronic illness requiring long-term treatment. To provide supportive therapy, the nurse must understand and differentiate between the various problems caused by the underlying disease and those related to thromboembolic disease. Patient teaching regarding long-term anticoagulant therapy is critical. The anticoagulant therapy continues for at least 12 weeks to 6 months; patients with recurrent emboli are treated indefinitely. Warfarin blood levels are initially sampled monthly and the patient may have follow-up appointments at a nurse-managed anticoagulation clinic to monitor their medication and adjust dosages.
Long-term management is similar to that for the patient with DVT (see the discussion of DVT in Ch 38). Discharge planning is aimed at limiting progression of the condition and preventing complications and recurrence. The nurse must reinforce the need for the patient to return to the healthcare provider for regular follow-up examinations.
PULMONARY HYPERTENSION
Pulmonary hypertension is elevated pulmonary pressure resulting from an increase in pulmonary vascular resistance to blood flow through small arteries and arterioles. A 60–70% reduction in the pulmonary vascular bed is required before pulmonary hypertension develops. Pulmonary hypertension can occur as a primary disease (primary pulmonary arterial hypertension) or as a complication of a large number of respiratory and cardiac disorders (secondary pulmonary hypertension).
Primary pulmonary arterial hypertension
Primary pulmonary arterial hypertension (PPAH) is a rare disease whose exact cause is unknown (see Fig 27-11). PPAH is characterised by a mean pulmonary arterial pressure greater than 25 mmHg at rest or greater than 30 mmHg with exercise, in the absence of a demonstrable cause. PPAH is associated with a poor prognosis because diagnosis is often late and there is little definitive therapy.
AETIOLOGY AND PATHOPHYSIOLOGY
The exact aetiology of PPAH is unknown. PPAH has been linked to the use of fenfluramine, which was used as an appetite suppressant to treat obesity but has now been withdrawn from the market. PPAH affects more women than men. It may have a genetic component as the incidence is higher in certain families.
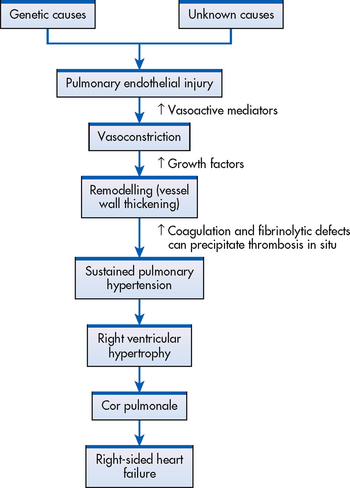
Normally the pulmonary circulation is characterised by low resistance and low pressure. In pulmonary hypertension the pulmonary pressures are elevated. Until recently the pathophysiology of PPAH was poorly understood. Recently it was discovered that a key mechanism involved in PPAH is a deficient release of vasodilator mediators from the pulmonary epithelium with a resultant cascade of injury (see Fig 27-11). Vasoconstriction, remodelling of the walls of the pulmonary vessels and thrombosis in situ are the three elements that combine to cause the increased vascular resistance.78 The remodelling process is a complex set of events involving endothelial cell injury that results in intimal/medial wall thickening.
CLINICAL MANIFESTATIONS
Classic symptoms of pulmonary hypertension are dyspnoea on exertion and fatigue. Exertional chest pain, dizziness and exertional syncope are other symptoms. These symptoms are related to the inability of cardiac output to increase in response to increased oxygen demand. Eventually as the disease progresses, dyspnoea occurs at rest. Pulmonary hypertension increases the workload of the right ventricle and causes right ventricular hypertrophy (a condition called cor pulmonale; see below) and eventually heart failure. A chest X-ray generally shows enlarged central pulmonary arteries and clear lung fields. An enlarged right heart may be seen. Echocardiogram usually reveals right ventricular hypertrophy.
MULTIDISCIPLINARY CARE
Diagnostic evaluation includes ECG, chest X-ray and echocardiogram. Right cardiac catheterisation is the definitive test to measure pulmonary artery pressures and can be performed as an outpatient procedure.79 Additional tests may be done to exclude secondary factors. Early recognition of pulmonary hypertension is essential to interrupt the self-perpetuation cycle responsible for the progression of this problem (see Fig 27-11).
Although there is no cure for PPAH, treatment can relieve symptoms, increase quality of life and prolong life. Diuretic therapy relieves dyspnoea and peripheral oedema and may be useful in reducing right ventricular volume overload. Anticoagulation therapy is recommended for patients with severe pulmonary hypertension to prevent in situ thrombus formation and venous thrombosis. Warfarin is given to keep the INR in the 2–3 range. Hypoxia is a potent pulmonary vasoconstrictor and use of low-flow oxygen provides symptomatic relief. The goal is to keep oxygen saturation at or above 90%.
Vasodilator therapy is used to reduce right ventricular overload by dilating pulmonary vessels and reversing remodelling. Many patients with pulmonary hypertension can be effectively managed with calcium channel blocker therapy, such as nifedipine and diltiazem.
Prostacyclins, such as epoprostenol, which promote pulmonary vasodilation and reduce pulmonary vascular resistance, have revolutionised the management of PPAH. Continuous prostacyclin therapy has been shown to give significant improvement in clinical symptoms and long-term survival.80 It is now the treatment of choice for selected patients unresponsive to calcium channel blockers. Although the patient and family teaching needed is extensive, the patient on continuous prostacyclin therapy can be successfully managed with a collaborative healthcare team. The administration of continuous prostacyclin therapy requires the placement of an indwelling central line catheter and continuous infusion pump. The patient and family must be trained to use the portable intravenous infusion pump, mix medications, manage the central line and monitor for complications (see Fig 27-12). The half-life of the drug is less than 6 minutes. If the central line is disrupted, stopped or dislodged for any reason, clinical deterioration from abrupt withdrawal of prostacyclin can occur. This is a serious event with potential rebound pulmonary hypertension and clinical deterioration within minutes. The major problems are infections related to vascular access and broken central lines.

Figure 27-12 A patient with pulmonary hypertension who is on continuous epoprostenol infusion is being taught how to use the portable infusion pump.
Treprostinil, another prostacyclin, is used as a continuous subcutaneous injection. It causes vasodilation of the pulmonary arterial system and inhibits platelet aggregation. The subcutaneous route has drawbacks including needles that could dislodge and infusion site pain and reactions. This drug is stable at room temperature and has a longer half-life than epoprostenol. It may be a reasonable alternative to epoprostenol for some patients.
Bosentan, a form of prostacyclin, is the only oral vasodilator currently available for the treatment of pulmonary hypertension. It is an active endothelin receptor antagonist and works by blocking the hormone endothelin, which causes blood vessels to constrict. Monthly liver function tests are needed since there is a risk of hepatotoxicity. Sildenafil (better known as Viagra) is an oral phosphodiesterase inhibitor that prolongs the vasodilatory effect of nitric oxide and appears to be as effective in decreasing pulmonary vascular resistance.81 Further research is needed with this agent.
Iloprost (an inhaled form of prostacyclin in an aerosolised preparation) is used in Europe and Australasia. Beraprost (an oral formulation of prostacyclin) is undergoing clinical trials for the treatment of early-stage pulmonary hypertension.
Surgical interventions for pulmonary hypertension include atrial septostomy (AS), pulmonary thromboendarterectomy (PTE) and lung transplantation.82 AS involves the creation of an intraatrial right-to-left shunt to decompress the right ventricle. It is indicated for a select group of patients awaiting lung transplantation. PTE may provide a potential cure for those patients suffering from chronic thromboembolic pulmonary hypertension. It is recommended only for patients with operable sites where the emboli can be surgically removed by embolectomy.
Lung transplantation is recommended for those patients who do not respond to prostacyclins and who progress to severe right-sided heart failure. Recurrence of the disease has not been reported in individuals who have undergone transplantation. Patient education and support for pulmonary hypertension can be found on the Pulmonary Hypertension Association’s website (see Resources on p 672).
Secondary pulmonary hypertension
Secondary pulmonary hypertension (SPH) occurs when a primary disease causes a chronic increase in pulmonary artery pressures. The specific primary disease pathology may result in anatomical or vascular changes causing the pulmonary hypertension. Anatomical changes causing increased vascular resistance include: (1) loss of capillaries as a result of alveolar wall damage (e.g. COPD); (2) stiffening of the pulmonary vasculature (e.g. pulmonary fibrosis connective tissue disorders); and (3) obstruction of blood flow (chronic emboli).
Vasomotor increases in pulmonary vascular resistance are found in conditions characterised by alveolar hypoxia. Hypoxia causes localised vasoconstriction and shunting of blood away from poorly ventilated alveoli. Alveolar hypoxia can be caused by a wide variety of conditions (e.g. Pickwickian syndrome, kyphoscoliosis, neuromuscular disease). It is possible to have a combination of anatomical restriction and vasomotor constriction. This is found in patients with longstanding chronic bronchitis who have chronic hypoxia in addition to loss of lung tissue.
The symptoms can reflect the underlying disease, but some are directly attributable to SPH, including dyspnoea, fatigue, lethargy and chest pain. The initial physical findings can include right ventricular hypertrophy and signs of right ventricular failure (increased pulmonic heart sound, right-sided fourth heart sound, peripheral oedema, hepatomegaly). Diagnosis of SPH is similar to PPAH.
Treatment of pulmonary hypertension caused primarily by pulmonary or cardiac disorders consists mainly of treating the underlying disorder, such as COPD or pulmonary emboli. When irreversible pulmonary vascular damage has occurred, therapies used for PPAH are initiated. Epoprostenol is used in the management of SPH. More studies are ongoing into effective therapies for SPH.83
Cor pulmonale
Cor pulmonale is enlargement of the right ventricle secondary to diseases of the lung, thorax or pulmonary circulation. Pulmonary hypertension is usually a pre-existing condition in the individual with cor pulmonale. Cor pulmonale may be present with or without overt cardiac failure. The most common cause of cor pulmonale is COPD. Almost any disorder that affects the respiratory system can cause cor pulmonale. The aetiology and pathogenesis of pulmonary hypertension and cor pulmonale are outlined in Figure 27-11.
CLINICAL MANIFESTATIONS
Clinical manifestations of cor pulmonale include dyspnoea, chronic productive cough, wheezing respirations, retrosternal or substernal pain and fatigue. Chronic hypoxaemia leads to polycythaemia and increased total blood volume and viscosity of the blood. (Polycythaemia is often present in cor pulmonale secondary to COPD.) Compensatory mechanisms that are secondary to hypoxaemia can aggravate the pulmonary hypertension. Episodes of cor pulmonale in a person with underlying chronic respiratory problems are frequently triggered by an acute respiratory tract infection.
If heart failure accompanies cor pulmonale, additional manifestations, such as peripheral oedema, weight gain, distended neck veins, full, bounding pulse and enlarged liver will also be found. (Heart failure is discussed in Ch 34.) A chest X-ray will show an enlarged right ventricle and pulmonary artery.
MULTIDISCIPLINARY CARE
The primary management of cor pulmonale is directed at treating the underlying pulmonary problem that precipitated the heart problem (see Box 27-10). Long-term low-flow oxygen therapy is used to correct the hypoxaemia and reduce vasoconstriction in chronic states of respiratory disorders. If fluid, electrolyte and acid–base imbalances are present, they must be corrected. Diuretics and a low-sodium diet will help decrease the plasma volume and the load on the heart. Bronchodilator therapy is indicated if the underlying respiratory problem is due to an obstructive disorder. Digoxin may be used if there is left-sided heart failure. Digoxin use is controversial, but studies have confirmed a modest effect of digoxin on the failing right ventricle in chronic cor pulmonale.84 Other treatments include those used for pulmonary hypertension, such as vasodilator therapy, calcium channel blockers and anticoagulants. Theophylline and terbutaline may help reduce dyspnoea, possibly due to myocardial contractility and acute decompensation of cor pulmonale.84 Phlebotomy may be needed in the patient with severe polycythaemia (haematocrit >0.55) because a reduction in volume is associated with a resultant decrease in pulmonary pressures. When medical treatment fails, lung transplantation is an option for some patients.
Chronic management of cor pulmonale resulting from COPD is similar to that described for COPD (see Ch 28). Exercise, small frequent meals and continuous low-flow oxygen during sleep may allow the patient to feel better and be more active.
Lung transplantation
Lung transplantation has evolved as a viable therapy for patients with end-stage lung disease. Improved selection criteria, technical advances and better methods of immunosuppression have resulted in improved survival rates. A variety of pulmonary disorders are potentially treatable with some type of lung transplantation. Various transplant options are available, including single lung transplant, bilateral lung transplant and heart–lung transplant.85
Patients being considered for a lung transplant need to undergo extensive evaluation due to the enormous commitment required of them pre- and post-transplant.86 Candidates for lung transplantation should not have a malignancy or recent history of malignancy (within the last 2 years), renal or liver insufficiency or HIV. The average wait for a lung transplant in Australia and New Zealand is greater than 1 year. The candidate and the family undergo psychological screening to determine their ability to cope with a postoperative regimen that requires strict adherence to immunosuppressive therapy, continuous monitoring for early signs of infection and prompt reporting of manifestations of infection for medical evaluation. Many transplant centres require preoperative outpatient pulmonary rehabilitation to maximise physical conditioning.
Postoperatively, infection is the leading cause of morbidity and mortality. Viral infection with CMV and herpes simplex virus occurs frequently. CMV is a leading cause of mortality, usually seen 4–8 weeks postoperatively. Bacterial pathogens and fungal infections are seen. An empirical antibiotic regimen is routine perioperatively for potential pathogens isolated from the donor or recipient. Pulmonary clearance measures, including bronchodilators, chest physiotherapy and deep-breathing and coughing techniques, are mandatory to minimise potential complications. Maintenance of fluid balance is vital in the postoperative phase.
Immunosuppressive therapy usually includes a triple-drug regimen of cyclosporin, azathioprine and prednisone. However, because of an array of potential adverse effects and drug interactions, there are limitations to immunosuppressive therapy. Drug levels are monitored on a regular basis. Immunosuppressive drugs are discussed in Chapter 13 and Table 13-13.
Acute rejection can be seen as soon as 5–7 days after surgery. It is characterised by low-grade fever, fatigue and oxygen desaturation with exercise. Accurate diagnosis is by transtracheal biopsy. Treatment is bolus corticosteroids, which results in complete remission of symptoms.
Bronchiolitis obliterans (an obstructive airways disease causing progressive occlusion) is considered to represent chronic rejection in lung transplant patients. The onset is often subacute, with gradual onset of progressive obstructive airflow defect, including cough, dyspnoea and recurrent lower respiratory tract infection. Treatment involves optimum maintenance immunosuppression.
Discharge planning begins in the preoperative phase because regaining functional capacity is the primary goal of lung transplantation.87 Prior to discharge the patient needs to be able to perform self-care activities, including medication management and activities of daily living, and accurately identify when to call the transplant team. Patients are placed in an outpatient rehabilitation program to improve physical endurance. The use of home spirometry has been useful in monitoring trends in lung function. Patients are taught to keep logs of medications, laboratory results and spirometry.88 After discharge, patients are followed by the transplant team for transplant-related issues. Patients return to their primary care team for their health maintenance and routine illnesses. As transplant procedures become more frequent, transplant patients will return to hospitals for other routine procedures. Coordination of care between the transplant team, primary care team and inpatient teams is essential for ongoing successful management of these patients. Over the past decade, lung transplantation has become an increasingly important mode of therapy for patients with a variety of end-stage lung diseases.
The patient with aspiration pneumonia
CASE STUDY
Patient profile
Sam Turkera, a 27-year-old Polynesian man, was admitted to the hospital because of an uncontrollable fever. He was transferred from a long-term care facility. He has a history of a gunshot wound to his left chest. Following a cardiac arrest after the accident he developed hypoxic encephalopathy. He has a tracheostomy and gastrostomy tube. He has a history of methicillin-resistant Staphylococcus aureus (MRSA) in his sputum.
Objective data
Physical examination
• Thin, cachectic Polynesian man in moderate respiratory distress
• Unresponsive to voice, touch or painful stimuli
• Temperature: 40°C; heart rate: 120 beats/min; respiratory rate: 30 breaths/min; O2 saturation: 90%
• Chest auscultation revealed crackles and scattered rhonchi in the left upper lobe
Diagnostic studies
• White blood cell count: 18 × 109/L
• Sputum specimen: thick, green, foul smelling; cultures pending
• Blood gas analyses: pH 7.29; PaO2 10.6 kPa (80 mmHg); PaCO2 5.3 kPa (40 mmHg); bicarbonate 16 mmol/L
• Stool culture positive for Clostridium difficile
• Chest X-ray: infiltrate in left upper lobe; no pleural effusions noted
CRITICAL THINKING QUESTIONS
1. What types of infectious disease precautions should be taken related to this patient’s hospitalisation?
2. What clinical manifestations of aspiration pneumonia does this patient exhibit? Explain their pathophysiological bases.
3. What antibiotic medication is likely to be prescribed?
4. What is this patient’s oxygenation status and metabolic state?
5. What other clinical issues must be addressed in his plan of care?
6. What family interventions would you initiate?
7. Based on the assessment data presented, write one or more appropriate nursing diagnoses. What are the collaborative problems?
1. What effective measures can the nurse institute to increase patient compliance with long-term anti-tuberculosis medication?
2. Indicate which strategy to use when helping a patient who is unwilling to quit smoking.
3. What position should a patient assume following lung surgery for comfort and maximum oxygenation?
4. Is there a significant impact on the quality of life and survival of pulmonary hypertension patients on epoprostenol therapy?
5. Does a patient-focused approach for the lung transplant patient meet the family’s needs? Why or why not?
1. In assessing a patient with pneumococcal pneumonia, the nurse recognises that clinical manifestations of this condition include:
2. An appropriate nursing intervention for a patient with pneumonia with the nursing diagnosis of ineffective airway clearance related to thick secretions and fatigue would be to:
3. A patient with tuberculosis (TB) has a nursing diagnosis of non-compliance. The nurse recognises that the most common aetiological factor for this diagnosis in patients with TB is:
4. A patient has been receiving high-dose corticosteroids and broad-spectrum antibiotics for treatment of serious trauma and infection. The nurse plans care for the patient knowing that the patient is most susceptible to:
5. A primary goal for the patient with bronchiectasis is that the patient will:
6. A common pathophysiological characteristic of many types of pneumoconiosis is:
7. The type of lung cancer generally associated with the best prognosis because it is potentially surgically resectable is:
8. The nurse identifies a flail chest in a trauma patient when:
9. The nurse notes swinging of the water level in the tube submerged in the water-seal chamber for a patient with closed chest-tube drainage. The nurse should:
10. A nursing measure that should be instituted after a pneumonectomy is:
11. Guillain-Barré syndrome causes respiratory problems primarily by:
12. A patient with COPD asks why the heart is affected by the respiratory disease. The nurse’s response is based on the knowledge that cor pulmonale is characterised by:
13. In responding to a patient with emphysema who asks about the possibility of a lung transplant, the nurse knows that lung transplantation may be contraindicated in patients:
1 World Health Organization (WHO). Top 10 causes of death. Available at www.who.int/mediacentre/factsheets/fs310/en/index.html. accessed 29 March 2011.
2 Australian Institute of Health & Welfare (AIHW). Australia’s health, 2010. Australia’s health series no. 12. Cat. no. AUS 122. Canberra: AIHW, 2010.
3 New Zealand Ministry of Health. Mortality statistics. Available at www.moh.govt.nz/moh.nsf/Files/mortdemo/$file/mort-06to09-prov-may2010.pdf, 2006–2009. accessed 29 March 2011.
4 World Health Organization (WHO). Tuberculosis. Fact sheet no.104. Available at www.who.int/mediacentre/factsheets/fs104/en/, March 2010. accessed 29 March 2011.
5 Worrall G. Acute bronchitis. Can Fam Physician. 2008;54:238.
6 Niederman MS. Antibiotic therapy of acute exacerbations of chronic bronchitis. Semin Respir Infect. 2000;15(1):59–70.
7 Maxwell DJ, McIntosh KA, Pulver LK, et al. Empiric management of community-acquired pneumonia in Australian emergency departments. Med J Aust. 2005;183(10):520–524.
8 Scott G, Scott H, Turley M, et al. Economic cost of community-acquired pneumonia in New Zealand adults. NZ Med J. 2004;117(1198):U933.
9 Chambers S, Laing R, Murdoch D, et al. Māori have a much higher incidence of community-acquired pneumonia and pneumococcal disease than non-Māori: findings from two New Zealand hospitals. NZ Med J. 2006;119(1234):U1978.
10 American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–1754.
11 Charles PG, Johnson PD, Grayson ML. Conundrums in community-acquired pneumonia. Med J Aust. 2006;185(3):131–132.
12 Boots RJ, Lipman J, Bellomo R, et al. Disease risk and mortality prediction in intensive care patients with pneumonia. Australia and New Zealand Practice in Intensive Care (ANZPIC II). Anaesth Intensive Care. 2005;33(1):101–111.
13 Royal Australian College of General Practitioners, Pharmaceutical Society of Australia, Australasian Society of Clinical and Experimental Pharmacologists and Toxicologists. Australian medicines handbook. Adelaide: AMH, 2006.
14 Shuey KM. Persistent fever and cough with nonspecific lower lung lobe consolidation. Clin J Oncol Nurs. 2003;7(4):463–466.
15 Seale H, MacIntyre CR, Gidding HF, et al. National serosurvey of cytomegalovirus in Australia. Clin Vaccine Immunol. 2006;13(11):1181–1184.
16 Centers for Disease Control and Prevention. Influenza antiviral medications: summary for clinicians. Available at www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. accessed 26 March 2011.
17 Menzies R, McIntyre P. Vaccine preventable diseases and vaccination policy for indigenous populations. Epidemiol Rev. 2006;28:71–80.
18 Mandell LA, Bartlett JG, Dowell SC, et al. Update of practice guidelines for the management of community-acquired pneumonia for immunocompetent adults. Clin Infect Dis. 2003;37(11):1405–1433.
19 Guppy MPB, Mickan SM, Del Mar CB. Advising patients to increase fluid intake for treating acute respiratory infections. Cochrane Database Syst Rev. (4):2005. CD004419.
20 Laux L, Herbert C. Decreasing ventilator-associated pneumonia: getting on board. Crit Care Nurs Q. 2006;29(3):253–258.
21 Fink JB. Positioning versus postural drainage. Respir Care. 2002;47(7):769–777.
22 World Health Organization (WHO). Global tuberculosis control report. Available at http://whqlibdoc.who.int/publications/2010/9789241564069eng.pdf, 2010. accessed 27 March 2011.
23 Simpson G, Clark P, Knight T. Changing patterns of tuberculosis in Far North Queensland. Med J Aust. 2006;184(5):252.
24 Communicable Diseases Australia. National notifiable diseases surveillance system. Available at www9.health.gov.au/cda/Source/Rpt_3.cfm. accessed 27 March 2011.
25 Das D, et al. Why the tuberculosis incidence rate is not falling in New Zealand. NZ Med J. 2006;119(1243):1–11.
26 Lauzardo M, Ashkin D. Phthisiology at the dawn of the new century. A review of tuberculosis and the prospects for its elimination. Chest. 2000;117(5):1455–1473.
27 Frieden TR, Sterling TR, Munsiff SS, et al. Tuberculosis. Lancet. 2003;362(9387):887–899.
28 State Government of Victoria. Management, prevention and control of tuberculosis: guidelines for healthcare providers. Available at www.health.vic.gov.au/ideas/diseases/tb_mgmt_guide.htm, 2002–2005. accessed 27 March 2011.
29 Ferrara G, Losi M, D’Amico R, et al. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with mycobacterium tuberculosis: a prospective study. Lancet. 2006;367(9519):1328–1334.
30 Taggart EW, Hill HR, Ruegner RG, et al. Evaluation of an in vitro assay for gamma interferon production in response to mycobacterium tuberculosis infections. Clin Diagn Lab Immunol. 2004;11(6):1089–1093.
31 Centers for Disease Control and Prevention. Public health dispatch update: fatal and severe liver injuries associated with rifampin and pyrazinamide treatment for latent tuberculosis infection, MMWR. 2002;51(44):998–999. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5144a4.htm accessed 27 March 2011.
32 Neff M, ATS, CDC, IDSA. Update recommendations on the treatment of tuberculosis. Am Fam Physician. 2003;68(9):1854. 1857–1858, 1861–1862.
33 Rengasamy A, Zhuang Z, Berryann R. Respiratory protection against bioaerosols: literature review and research needs. Am J Infect Control. 2004;32(6):345–354.
34 Falkinham JO, 3rd. Mycobacterial aerosols and respiratory disease. Emerg Infect Dis. 2003;9(7):763–767.
35 Chen KY, Ko SC, Hsueh PR, et al. Pulmonary fungal infection: emphasis on microbiological spectra, patient outcome, and prognostic factors. Chest. 2001;120(1):177–184.
36 Ahya VN, Tino G. Bronchiectasis: new perspectives. J Respir Dis. 2001;22(4):252–261.
37 Moloney E, O’Sullivan S, Hogan T, et al. Airway dehydration: a therapeutic target in asthma? Chest. 2002;121(6):1806–1811.
38 Kuschner WG, Stark P. Occupational lung disease: part 2. Postgrad Med. 2003;113(4):81–88.
39 National Center of Infectious Diseases. All about hantaviruses. Available at www.cdc.gov/ncidod/diseases/hanta/hps/. accessed 27 March 2011.
40 Australian Institute of Health & Welfare (AIHW). Occupational asthma in Australia. AIHW bulletin no. 59. Available at www.aihw.gov.au/publication-detail/?id=6442468087, May 2008. accessed 27 March 2011.
41 Australian Institute of Health & Welfare (AIHW). Cancer incidence projections for Australia, 2002–2011. Canberra: AIHW; 2005. Available at www.aihw.gov.au/publications/can/cipa02-11/cipa02-11-c02.pdf accessed 27 March 2011.
42 Garrett J. Lung cancer management concerns in New Zealand. NZ Med J. 2004;117(1196):U931.
43 Centers for Disease Control and Prevention. Women and smoking: a report of the Surgeon General. Available at www.cdc.gov/tobacco/data_statistics/sgr/sgr_2001/index.htm, 2001. accessed 27 March 2011.
44 Mayor S. NICE issues guidance for diagnosis and treatment of lung cancer. Br Med J. 2005;330(7489):439.
45 US Department of Health & Human Services. Treating tobacco use and dependence: 2008 update. Available at www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. accessed 27 March 2011.
46 Larsson ML, Frisk M, Hallstrom J, et al. Environmental tobacco smoke exposure during childhood is associated with increased prevalence of asthma in adults. Chest. 2001;120(30):711–717.
47 Kreamer KM. Getting the lowdown on lung cancer. Nursing. 2003;33(11):36–42.
48 Australian Institute of Health & Welfare (AIHW). Why is cancer control a national health priorities area? Available at www.aihw.gov.au/nhpa/cancer/index.cfm#why_cancer. accessed 27 March 2011.
49 Mahon SM. Screening for lung cancer: what is the evidence? Clin J Oncol Nurs. 2004;8(2):188–189.
50 Machado RF, Laskowski D, Deffenderfer O, et al. Detection of lung cancer by sensor array analyses of exhaled breath. Am J Respir Crit Care Med. 2005;171:1286–1291.
51 Inzeo D, Haughney A. Laser therapy in the management of lung cancer. Clin J Oncol Nurs. 2004;8(1):94–95.
52 National Comprehensive Cancer Network. Non-small cell lung cancer: clinical practice guidelines in oncology. V1. Available at www.nccn.org/professionals/physician_gls/default.asp, 2007. accessed 28 March 2011.
53 Walker S. Updates in small cell lung cancer treatment. Clin J Oncol Nurs. 2003;7(5):563–568.
54 National Comprehensive Cancer Network. Small cell lung cancer: clinical practice guidelines in oncology, V1. Available at www.nccn.org/professionals/physician_gls/default.asp, 2007. accessed 28 March 2011.
55 Young JM, Ward JE. Implementing guidelines for smoking cessation advice in Australian general practice: opinions, current practices, readiness to change and perceived barriers. Fam Pract. 2001;18(1):14–20.
56 Goolsby MJ. Treating tobacco use and dependence. J Am Acad Nurse Pract. 2001;13(3):101–105.
57 Prochazka AV. New developments in smoking cessation. Chest. 2000;117(4 suppl 1):169–175.
58 Meraviglia MG. The effects of spirituality on well-being of people with lung cancer. Oncol Nurs Forum. 2004;31(1):89–94.
59 Zhang AY, Siminoff LA. The role of the family in treatment decision making by patients with cancer. Oncol Nurs Forum. 2003;30(6):1022–1028.
60 Whyte RE, Watson HE, McIntosh J. Nurses’ opportunistic interventions with patients in relation to smoking. J Adv Nurs. 2006;55(5):568–577.
61 Yeo TP. Long-term sequelae following blunt thoracic trauma. Orthop Nurs. 2001;20(5):35–47.
62 Yamamoto L, Schroeder C, Morley D, et al. Thoracic trauma: the deadly dozen. Crit Care Nurs Q. 2005;28(1):22–40.
63 Lazzara D. Eliminate the air of mystery from chest tubes. Nursing. 2002;32(6):36–43.
64 Andrs K. Drainage to go. Learn how to set up and maintain this portable drainage system so your patient can become more independent. Nursing. 2004;34(5):54–55.
65 Charnock Y, Evans D. Nursing management of chest drains: a systematic review. Aust Crit Care. 2001;14(4):156–160.
66 Behnia MM, Garrett K. Association of tension pneumothorax with use of small-bore chest tubes in patients receiving mechanical ventilation. Crit Care Nurse. 2004;24(1):64–65.
67 Lancey RA, Gaca C, Vander Salm TJ. The use of smaller, more flexible chest drains following open heart surgery: an initial evaluation. Chest. 2001;119(1):19–24.
68 Hayes DD. Stemming the tide of pleural effusions. Nurs Manag. 2001;32(10):30–34.
69 Allibone L. Assessment and management of patients with pleural effusions. Nurs Stand. 2006;20(22):55–64.
70 Gabbay E, Reed A, Williams TJ. Assessment and treatment of pulmonary arterial hypertension: an Australian perspective in 2006. Intern Med J. 2007;37(1):38–48.
71 Roman M, Weinstein A, Macaluso S. Primary spontaneous pneumothorax. Medsurg Nurs. 2003;12(3):161–169.
72 Spiegler PA, Hurewitz AN, Groth ML. Rapid pleurodesis for malignant pleural effusions. Chest. 2003;123(6):1895–1898.
73 Demedts M, Wells AU, Anto JM, et al. Interstitial lung diseases: an epidemiological overview. Eur Respir J. 2001;32:S2–S16.
74 Roderick P, Ferris G, Wilson K, et al. Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextra and regional anaesthesia as thromboprophylaxis. Health Technol Assess. 2005;9(49):iii–iv. ix–x, 1–78.
75 Beemath A, Stein PD, Skaf E, et al. Risk of venous thromboembolism in patients hospitalized with heart failure. Am J Cardiol. 2006;98(6):793–795.
76 Yang JC. Prevention and treatment of deep vein thrombosis and pulmonary embolism in critically ill patients. Crit Care Nurs Q. 2005;28(1):72–79.
77 Dalen JE. Pulmonary embolism: what have we learned since Virchow? Chest. 2002;122(5):1801–1817.
78 Berkowitz DS, Coyne NG. Understanding primary pulmonary hypertension. Crit Care Nurs Q. 2003;26(1):28–34.
79 Adiutori DM. Primary pulmonary hypertension: a review for advanced practice nurses. Medsurg Nurs. 2000;9(5):255–264.
80 Dandel M, Lehmkuhl HB, Hetzer R. Advances in the medical treatment of pulmonary hypertension. Kidney Blood Press Res. 2005;28(5–6):311–324.
81 Kuroda T, Hirota H, Masaki M, et al. Sildenafil as adjunct therapy to high-dose epoprostenol in a patient with pulmonary veno-occlusive disease. Heart Lung Circ. 2006;15(2):139–142.
82 Doyle RL, McCrory D, Channick RN, et al. Surgical treatments/interventions for pulmonary arterial hypertension. Chest. 2004;126(1 suppl):63–71.
83 Carbone R, Bossone E, Bottino G, et al. Secondary pulmonary hypertension—diagnosis and management. Eur Rev Med Pharmacol Sci. 2005;9(6):331–342.
84 Budev MM, Arroliga AC, Wiedemann HP, et al. Cor pulmonale: an overview. Semin Respir Crit Care Med. 2003;24(3):233–244.
85 Fischer S, Meyer K, Tessmann R, et al. Outcome following single vs bilateral lung transplantation in recipients 60 years of age and older. Transplant Proc. 2005;37(2):1369–1370.
86 Orens JB, Estenne M, Arcasoy S, et al. International guidelines for the selection of lung transplant candidates: 2006 update—a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006;25(7):745–755.
87 Sager JS, Kotloff RM, Ahya VN, et al. Association of clinical risk factors with functional status following lung transplantation. Am J Transplant. 2006;6(9):2191–2201.
88 Petty M. Lung and heart-lung transplantation: implications for nursing care when hospitalized outside the transplant center. Medsurg Nurs. 2003;12(4):250–259.
American Lung Association. www.lungusa.org
American Society of Clinical Oncology. www.asco.org
Asbestos Diseases Foundation of Australia Inc. www.adfa.org.au
Australian Lung Foundation. www.lungnet.org.au
Australian Respiratory Council. www.thearc.org.au
Cancer Council of Australia. www.cancer.org.au
Cancer Society of New Zealand. www.cancernz.org.nz
Centers for Disease Control and Prevention, National Center for Health Statistics (US). www.cdc.gov/nchs/fastats/Default.htm
CURB-65. www.watag.org.au/watag/docs/CAP%20CURB-65%20Guidelines.pdf
Memorial Sloan-Kettering Cancer Center. www.mskcc.org/PredictionTools/LungCancer
Pulmonary Hypertension Association. www.phassociation.org
Quit Now National Tobacco Campaign. www.quitnow.info.au
Thoracic Society of Australia and New Zealand. www.thoracic.org.au
Tobacco Information and Prevention Source, National Center for Chronic Disease Prevention and Health Promotion (US). www.cdc.gov/tobacco
World Health Organization Global Tuberculosis Program. www.who.int/gtb