Pediatric Anesthesia Systems and Equipment
PEDIATRIC ANESTHESIA EQUIPMENT
SUPRAGLOTTIC AIRWAYS IN PEDIATRIC PATIENTS
Fundamental Requirements of the Pediatric Breathing System
Mapleson D, E, and F System Development
Factors that Affect Delivered Tidal Volume
Miscellaneous Aspects of Mechanical Ventilation
CAPNOGRAPHY AND RESPIRATORY GAS ANALYSIS IN PEDIATRIC PATIENTS
Overview
The ultimate goal of the anesthesiologist during pediatric surgery is to provide the patient with a safe and smooth anesthetic along with a comfortable and uneventful recovery. A well-considered anesthetic plan for a pediatric patient must include a well-informed choice of appropriate equipment. Which breathing systems are appropriate for the pediatric patient? How do maturational changes in respiratory physiology affect the selection and use of equipment? Does an adult ventilator and breathing system function acceptably for the pediatric patient? Are monitors primarily designed for adults appropriate for pediatric patients? This chapter attempts to answer some of these questions.
A clear understanding of anesthetic equipment is important to the safe delivery of pediatric anesthesia. Approximately 40% of anesthetic incidents were attributed to equipment problems in one 11-year experience, which included both adult and pediatric patients.1 Standardization of equipment, improved monitoring, and the introduction of new anesthetic drugs have all improved the safety of pediatric anesthesia. Over time, the percentage of pediatric closed claims events attributable to respiratory events or to inadequate monitoring have declined.2
Physiology
Work of Breathing Under Anesthesia
Understanding the relevant aspects of pediatric respiratory physiology provides a rationale for the recommendations presented later in this chapter. Several factors may increase the work of breathing under anesthesia (Table 17-1). These factors can be broadly divided into two groups: first is the increased work of breathing that may be imposed by the anesthesia breathing system, including inspiratory and expiratory resistance of breathing circuits, circuit dead space, and rebreathing of carbon dioxide. Second are changes in the mechanics and in the control of breathing induced by general anesthesia. These include decreased total respiratory compliance, decreased lung volume, airway closure, and upper airway obstruction. Thus, during general anesthesia, many factors conspire to increase the demands placed on the respiratory system while simultaneously decreasing the patient’s ability to cope with these demands.
Table 17-2 compares some selected infant and adult respiratory physiologic values. In general, neonates and infants have less respiratory reserve than adults and may demonstrate an unpredictable response to any additional work of breathing through a circuit under general anesthesia. Patients breathing spontaneously with the Mapleson D, E, and F circuits will rebreathe alveolar gas if the fresh gas flow is less than 2.5 to 3 times the predicted minute ventilation.3 Under normal circumstances, most of these patients will increase their minute ventilation in response and thereby prevent significant hypercarbia. However, even some anesthetized adults are unable to respond to increases in inspired carbon dioxide by hyperventilation and thus become significantly hypercarbic.4
TABLE 17-2
Comparison of Selected Normal Respiratory Values in Infants and Adults
| Normal Value | Infant | Adult |
| Weight (kg) | 3.0 | 70 |
| Surface area (m2) | 0.2 | 1.8 |
| Surface area/weight (m2/kg) | 0.06 | 0.03 |
| Respiratory frequency (breaths/min) | 30-40 | 10-16 |
| Tidal volume (VT, mL/kg) | 7 | 7 |
| Dead space (VD, mL/kg) | 2.2 | 2.2 |
| VD/VT | 0.3 | 0.3 |
| Functional residual capacity (mL/kg) | 30 | 30 |
| Specific compliance (mL/cm H2O/mL) | 0.05 | 0.05 |
| Airways resistance (cm H2O/L/sec) | 25-30 | 1.6 |
| Work of breathing (J/L) | 0.5-0.7 | 0.5-0.7 |
| Alveolar ventilation (mL/kg/min) | 100-150 | 70 |
| Oxygen consumption (mL/kg/min) | 6 to 8 | 4 |
Olsson and Lindhal examined the response of spontaneously breathing anesthetized infants5 (nitrous oxide and 1% to 1.5% halothane) to an increase in inspired carbon dioxide. Patients younger than 6 months did not significantly increase their minute ventilation in response to the added carbon dioxide, whereas patients older than 6 months showed a markedly attenuated response to increased inspired carbon dioxide. When the halothane concentration was reduced to 0.8%, the ventilatory response to carbon dioxide returned in patients younger than 6 months; thus, inhalational anesthesia attenuates the ventilatory response to carbon dioxide in small infants.
General anesthesia also adversely affects infants’ ventilatory response to increased respiratory work in the forms of tubular resistance, dead space, and valvular resistance. For a given increase in apparatus dead space, the infant must increase minute ventilation to a proportionately greater degree to maintain normocarbia when compared with the older child.6 The neonate is prone to respiratory compromise in the face of prolonged increases in respiratory work. The newborn’s diaphragmatic muscle is immature, having a smaller proportion of fatigue-resistant high-oxidative muscle fibers.7 In addition, the infant diaphragm is less able to generate a maximal inspiratory force compared with the adult diaphragm.8
Pediatric Anesthesia Equipment
The face mask must be able to provide a good seal to the child’s face to facilitate effective positive-pressure ventilation, avoid entrainment of room air, and reduce operating room (OR) pollution. Other desirable face mask features include low dead space, increased patient comfort, and construction with transparent material that allows observation of patient color and the presence of secretions or vomitus (Fig. 17-1); in addition, transparent masks are less threatening to the patient.9 The ability to ventilate the patient by mask is of the utmost importance, but low dead space is also desirable to avoid retention of carbon dioxide and increased respiratory work. However, in practice, the functional dead space of most face masks is significantly less than the measured dead space because streaming of gas eliminates significant rebreathing.6,9
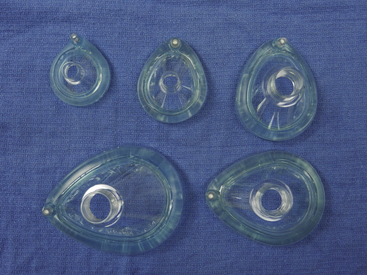
FIGURE 17-1 Transparent anesthesia face masks in various sizes, with syringe-adjustable air cushion.
Maintaining an airway with a face mask occasionally can be challenging in children because the anesthesiologist’s fingers can cause external compression of the compliant upper airway. Care should be taken to ensure that fingers do not stray from the mandible onto the soft tissues surrounding the airway. Mask anesthesia with spontaneous ventilation should probably not be used for prolonged procedures in small infants because infants are unable to tolerate the increased work of breathing and may respond with apnea or hypoventilation. Inhalational induction of anesthesia may be facilitated by the application of an odorant (e.g., lip gloss) to the inside of the face mask to disguise the smell of the potent volatile agents and the unpleasant plastic odor of the mask and breathing circuit.
Tracheal Tubes
Selection of the correct tracheal tube type and size, along with its accurate and secure positioning, is vitally important.
Cuffed Versus Uncuffed Endotracheal Tubes
The decision to use cuffed instead of uncuffed endotracheal tubes (ETTs) in pediatric patients depends on multiple factors. The use of cuffed tubes has historically been discouraged in children younger than 8 to 10 years. Most pediatric anesthesiologists have strong opinions on this matter, and evolving practice patterns are not always evidence driven. Although this topic generates vigorous debate, irrespective of whether a cuffed or uncuffed tube is selected, most experts would agree that safe use of any tube depends on meticulous attention to technical detail.
The vocal cords are the narrowest point of the pediatric airway, but they are more mobile that the cricoid cartilage, which completely surrounds the airway. Although the cricoid often is described as a “ring” of cartilage, the airway at this level has an ellipsoid cross-section.10-12 Intuitively, it would seem that the ellipsoid cross-sectional configuration of the airway makes it unlikely that a circular, cross-sectional tube can both provide an optimal airway seal and avoid local trauma to some part of the airway circumference, particularly the posterolateral walls of the larynx. However, the subglottic region is lined with loosely bound pseudostratified epithelium. Thus, trauma to this region, such as by intubation with a tube of too large a diameter, readily results in edema that decreases the lumen of the airway and increases resistance to gas flow. During laminar flow, resistance is inversely proportional to the fourth power of the radius. However, flow conditions in the upper airway are likely to be transitional or turbulent.13 Under these conditions, resistance is inversely proportional to the fifth power of the radius. A 1-mm ring of edema in the newborn will reduce the cross-sectional area of the airway by 75%. Under laminar flow conditions, resistance will increase 16-fold. By comparison, a 1-mm ring of edema in a healthy adult would have a less significant effect on airway resistance.14
Avoiding trauma to the vulnerable subglottic region is obviously critical. This applies whether the insult is generated by the shaft of an uncuffed tube or from an ETT cuff. Therefore, an important consideration of pediatric ETTs is their cuff design characteristics. Over time, cuff pressure-volume characteristics have led to the development of less traumatic high-volume low-pressure cuffs. However, recent attempts have been made to eliminate cuff contact with the subglottic region altogether by locating a shorter, ultrathin polyurethane cuff more distally on the shaft of the tube, thus producing a cuff-free subglottic zone.15 The more distal cuff location has forced the elimination of the Murphy eye. The intent of this design modification is to create a tracheal, rather than a cricoid, cuff seal. The cross-sectional configuration, presence of a nonrigid muscular posterior wall, and U-shaped cartilage make the trachea a more suitable site for cuff placement than the cricoid region. Modern high-volume, low-pressure, ultrathin polyurethane cuffs conform readily to the trachea and provide acceptable seals at lower pressures (<15 cm H2O).
We prefer to use cuffed ETTs in our practice. However, the use of cuffed tubes is predicated on the availability of tubes of the appropriate design and careful attention to leak pressure. Use of cuffed tubes must be accompanied by careful attention to cuff pressure and may require using a half-size smaller tube to provide space for the uninflated cuff. However, these limitations are offset by several advantages: first, use of a cuffed tube with appropriate inflation provides a reliable airway seal, reduces the risk of aspiration, and limits inspired gas leakage. Second, the improved seal also improves tidal volume measurement and the accuracy of airway gas samples. Third, contamination of the OR ambient atmosphere by anesthetic gas is reduced. Fourth, more consistent ventilation and better carbon dioxide management particularly benefits patients with congenital heart disease or pulmonary hypertension.
Uncuffed Tubes
The optimal size of an uncuffed tracheal tube is the largest size that will easily pass into the trachea without traumatizing the larynx. To determine the leak pressure after tracheal intubation, the reservoir bag should be slowly inflated; circuit pressure is measured while listening with a stethoscope, either at the mouth or in the sternal notch, for a leak. The optimal leak pressure has not been definitively determined, but the incidence of postextubation stridor seems to increase as the leak pressure increases, particularly to greater than 25 cm H2O. Most patients’ lungs can be adequately ventilated at leak pressures of 15 to 20 cm H2O. If the patient has lung disease or is undergoing an intrathoracic or high abdominal procedure, a higher leak pressure, such as 25 to 30 cm H2O, may be necessary to allow adequate ventilation in the face of decreased respiratory compliance. On occasion, an unacceptably large leak will be present with one size of tube but not with a tube 0.5 mm larger in diameter. Under these circumstances, a cuffed tube of the smaller size with the cuff deflated may produce an acceptable leak. Using a tube that is too small with a large leak may lead to difficulty ventilating the lungs and obtaining accurate end-tidal carbon dioxide measurements and, at least in theory, it increases the risk of aspiration. Suction catheters appropriate to all tube sizes must be immediately available to remove secretions from tracheal tubes.
Formulas to predict correct tracheal tube size are based on weight and age (Table 17-3). It is also good practice to also have tubes available sized 0.5 mm above and below the predicted size (Fig. 17-2).
TABLE 17-3
Determination of Endotracheal Tube Size and Depth of Insertion
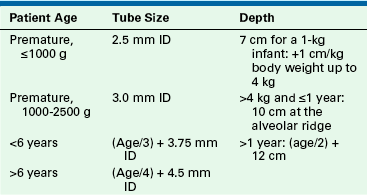
Tubes 0.5 mm ID larger and smaller than those calculated from these should be immediately available.
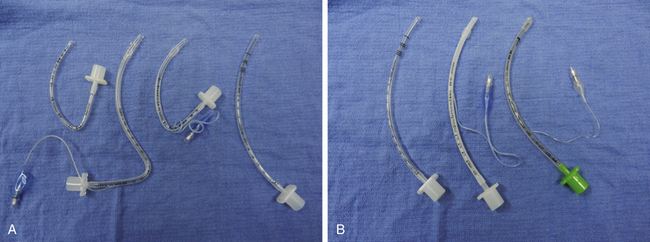
FIGURE 17-2 A selection of preformed and standard pediatric tracheal tubes. A, From left to right: uncuffed oral Ring-Adair-Elwyn tube (RAE; Mallinckrodt, St. Louis, MO), cuffed nasal RAE tube, cuffed oral RAE tube, standard oral uncuffed tube. B, Cuffed and uncuffed pediatric tracheal tubes.
The peak incidence of postextubation stridor is between 1 and 4 years of age.16 Factors that contribute to the development of postextubation stridor include a tight-fitting tracheal tube (i.e., a leak at >25 cm H2O), traumatic or multiple intubations, coughing with the tracheal tube in place during emergence from anesthesia, changing the patient’s head position while intubated, intubation lasting more than 1 hour, and operations in the neck region. Although recent upper respiratory tract infection was not initially thought to be a contributing factor, more recent data show that patients younger than 1 year have a 27-fold increased risk of postextubation stridor after upper respiratory tract infection.17 Patients with Down syndrome may have a narrow subglottic region and are thus prone to postextubation stridor; the clinician should consider using an ETT smaller than that predicted by the patient’s age or weight for such patients.
Selecting the Correct Insertion Depth
The term newborn trachea is approximately 4 to 5 cm long. Therefore, the margin of error for a tube located in the mid trachea is approximately 2 cm in either direction. A tube inserted too deeply will result in carinal irritation or endobronchial intubation, whereas too shallow an insertion depth may predispose to accidental extubation. A potential hazard of shallow tube insertion depth is that the cuff may be located in the subglottic, rather than the tracheal, region. To determine the correct insertion depth, gently advance the tube after intubation, while the chest is auscultated bilaterally, to determine the depth at which bronchial intubation occurs. The tube is then carefully withdrawn and secured at the appropriate depth. Of note, when the tube has a Murphy eye, it may be difficult to determine tube position by auscultation because sufficient gas may flow through the eye to produce contralateral breath sounds despite the tube tip being located in a mainstem bronchus.
In small infants, particularly those with lung disease, it is not always easy to determine by auscultation alone at what depth the tube should be secured. When accurate tube position is crucial (e.g., for repair of a tracheoesophageal fistula), a small-diameter flexible fiberoptic bronchoscope may be used to determine the tube location, and 1.8 mm (Olympus PF 18M; Olympus America, Center Valley, PA) and 2.2 mm (Olympus LF-P) outer diameter (OD) flexible fiberoptic bronchoscopes can help in tube positioning. The larger of these bronchoscopes has a directable distal tip. Neither has a suction channel, but both can be passed through a 2.5-mm internal diameter (ID) tracheal tube. If a suitable bronchoscope is unavailable, a chest radiograph may be a reasonable alternative.
Several manufacturers place intubation depth markers on their ETTs. These markers typically are one or more horizontal black bands located near the distal end of the tube. The intent is that when the marker is placed at the vocal cords, the distal tip of the ETT will be located in the mid trachea. Goel and colleagues18 examined intubation depth markers on several commonly used pediatric ETTs and noted a wide discrepancy in both the number and locations of the markers among manufacturers. Furthermore, a lack of consistency of markers was found among different-sized tubes from the same manufacturer. Thus it is important to be familiar with the location of the intubation depth markers on the tubes most frequently used at an institution. Optimal insertion depth is probably best achieved by using a combination of predictive formulas (see Table 17-3), intubation depth markers, and clinical examination.
Using the Oral RAE Tracheal Tube
The preformed Ring-Adair-Elwyn tube (RAE; Mallinckrodt, St. Louis, MO) (see Fig. 17-2) is often used during ear-nose-throat, plastic, and other head and neck procedures.19 The RAE tube has several advantages and potential pitfalls: it was originally designed for use during cleft lip and palate surgery at an institution where the surgeon preferred to leave the tube untaped so that its position could be adjusted during the procedure. The designers of the tube intended the distal tip of the tube to lie nearer to the carina than a conventional tube to reduce the likelihood of accidental extubation. Two Murphy eyes, located at the distal end of the tube, are intended to allow continued ventilation should endobronchial migration occur. The acute angle in the RAE tube, the part of the tube most likely to kink, is located outside the mouth and therefore is readily visible.
Although the above design features are useful, several potential hazards have been described. Seemingly appropriate positioning of the acute angle of an RAE tube at the lower lip does not guarantee that the distal tip of the tube is located correctly. The distal tips of 32% of RAE tubes positioned in this way were located at the carina or down a mainstem bronchus.20 In this situation, the Murphy eyes do not guarantee adequate ventilation during the procedure, but they may allow sufficient gas flow during manual ventilation and chest auscultation to falsely suggest acceptable tube position. It is recommended that a left precordial stethoscope be used to monitor tube position throughout the procedure, particularly during repositioning and mouth-gag insertion, to rapidly detect endobronchial migration. These tubes are labeled by the manufacturer with a suggested patient age range, but these recommendations should be interpreted with caution, as they may advocate a different-sized tube than that predicted by standard formulas for pediatric tracheal tubes.
It should be noted that, although unusual in current practice, occasional patients who require cleft lip and/or palate repair may be smaller than predicted by age, typically as a result of feeding difficulties or coexisting disease. In these patients it can be challenging to select an ETT size that is appropriate in both diameter and length.21 However, in the majority of patients with cleft lip and palate, standard formulas are acceptable predictors of ETT size and insertion depth.
Another obstacle to correct tube sizing is related to significant differences among manufacturers in the dimensions of oral preformed tubes. For example, when 4.5-mm ID uncuffed tubes from four manufacturers were measured, the distance from the bend to the tube tip ranged between 13.5 and 15 cm.22
Problems may occasionally be encountered when performing tracheal suctioning via an RAE tube, and passing a catheter beyond the acute angle in the tube can be difficult. Despite these potential problems, and with attention to the details noted above, anesthesiologists have found the RAE tube to be extremely useful during procedures of the head and neck.
Supraglottic Airways In Pediatric Patients
The laryngeal mask airway (LMA; LMA North America, San Diego, CA) revolutionized airway management for the practice of anesthesia (Fig. 17-3). The LMA provides a more secure airway than a face mask and pharyngeal airway but is less invasive than a tracheal tube. The success of the original LMA design has been underscored by the large number of supraglottic airway variants that have been developed and marketed by other manfacturers. The creation of a separate category of airway devices, the supraglottic airways, is also indicative of the importance of these devices to airway management. Although originally developed for the management of normal airways, the LMA has proven extremely useful for management of difficult airways.23,24
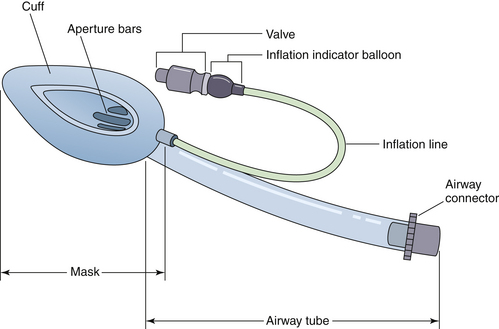
FIGURE 17-3 Parts of the Laryngeal Mask Airway (LMA North America, San Diego, CA). (Courtesy G. Sheplock, MD.)
Pediatric patients are especially suited for supraglottic airways, because upper airway obstruction during anesthesia is more likely in children than in adults. Children often require general anesthesia for short surgical or diagnostic procedures, and their small size can place the anesthesiologist at a considerable distance from the patient’s head and airway. Prior to the introduction of the LMA, tracheal intubation was often required for such procedures. Compared with tracheal intubation, advantages of the supraglottic airway include less hemodynamic response to insertion and, during emergence, a reduced risk of laryngeal complications such as croup; a decreased incidence of sore throat; and, quite possibly, less nausea and a faster discharge from the postanesthesia care unit (PACU).
Disadvantages of supraglottic airways in children are generally related to malposition of the device, excessive pressure on the oropharyngeal mucosa, hypoventilation, laryngospasm, and aspiration of gastric contents. A light plane of anesthesia may predispose to regurgitation or laryngospasm with a supraglottic airway in place.
Education and the Supraglottic Airway
Airway management during the administration of anesthesia requires precise positioning of the supraglottic airway to maximize ventilatory exchange and minimize the risk of hypoventilation, laryngospasm, and mechanical distortion of the upper airway. Instruction of supraglottic airway use to anesthesiology trainees must be methodical and controlled and must permit adequate experience for the trainee to understand the indications and contraindications for use.25 Fiberoptic examination of the supraglottic airway after insertion is invaluable for anyone learning to see position variation and how malposition may adversely affect airway function. Experience with different types of supraglottic airways is important for trainees because different institutions use different types.
Insertion Technique
The original insertion technique for LMA insertion has been modified by many users during the past 30 years. Ideal insertion of a supraglottic airway places the airway channel immediately above the glottic inlet, with the distal end resting in the upper esophageal sphincter and the upper border of the device bowl below the hyoid bone. The airway should slide along the hard and soft palates, between the tonsillar pillars, and into the hypopharynx. Insertion techniques for supraglottic airways with cuffs have been described with the cuff completely deflated, partially inflated, and completely inflated (Fig. 17-4). The potential problem with insertion with the cuff inflated is that the epiglottis will fold downward, and the device will be high in the hypopharynx, thereby increasing pressure on the base of the tongue and the lateral oropharyngeal wall. Insertion of a supraglottic airway upside-down and partially inflated, with rotation of the device after it enters the pharynx, has been advocated by many authors.26,27 Large inflation volumes may cause a greater incidence of sore throat and increase the risk of excessive pressure on the pharyngeal mucosa.
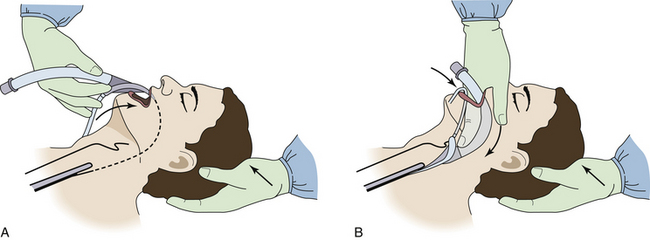
FIGURE 17-4 Insertion of the Laryngeal Mask Airway (LMA; LMA North America, San Diego, CA). A, During the initial part of the insertion, it is important that the LMA be directed in a maximally cephalad direction, with the wrist in complete flexion, so that the tip of the LMA follows the curvature of the hard and soft palates. B, The LMA is completely inserted when resistance is encountered by the guiding index finger, indicating that the tip of the cuff has reached the inferior recess of the hypopharynx. (Courtesy G. Sheplock, MD.)
An adequate depth of anesthesia must be attained prior to supraglottic airway insertion. Insertion of the device before attenuation of upper airway reflexes may provoke emesis or regurgitation. Satisfactory anesthesia can be achieved with both intravenous (IV) and inhaled agents.28,29
Types of Supraglottic Airways
Several manufacturers produce supraglottic airways, and product lines continue to evolve (Box 17-1). Many supraglottic airways do not undergo rigorous evaluation prior to clinical introduction, and potential complications do not become known until enough patient uses have been achieved. Consequently, some supraglottic airways have a very short market life. Most institutions now use single-use (disposable) supraglottic airways to minimize the risk of infectious disease transmission and to reduce processing costs.
Some supraglottic airways may be especially suited for a particular procedure, and it is unlikely that one supraglottic airway will fit all needs. Flexible bronchoscopy is easily performed through a supraglottic airway, but bronchoscope manipulation is easier through a silicone airway (e.g., classic reusable LMA).30 The pediatric anesthesiologist should be facile with several types of supraglottic airways to confront a wide array of patient airways.
Laryngeal Mask Airway
Since its introduction into clinical practice in the late 1980s, several models of the LMA have been developed (Box 17-2). In addition to the LMA Classic, single-use LMAs (LMA Unique), flexible LMAs, intubating LMAs (Fastrach), the ProSeal LMA, and Supreme LMA are currently available. The Fastrach LMA is noted for its ease of insertion and for the fact that it can serve as both a conduit for ventilation and tracheal intubation. The intubating LMA is available only in sizes 3, 4, and 5. All other models are available in smaller sizes. The LMA C Trach is an advanced model of the LMA Fastrach intubating LMA that has imaging capability of the larynx after insertion of the LMA. Although the C Trach has been most frequently used in adults, successful pediatric experience has been reported for children aged 9 to 17 years.31 The ProSeal LMA and the LMA Supreme are perhaps the most advanced of the LMAs and consist of a more anatomically designed bowl that results in a higher leak pressure without excessive pressure on the hypopharynx. A gastric conduit is built into the LMA Supreme and the ProSeal LMA and permits gastric decompression.
The medical literature regarding the LMA is extensive and documents its utility in pediatric patients with normal and difficult airways.
air-Q
The air-Q intubating laryngeal airway (Mercury Medical, Clearwater, FL) is a supraglottic airway that can be used by itself or as a conduit for tracheal intubation. It is available in sizes to fit a wide range of patients from neonates to large adults. One recent version, the ILA-SP, is self-pressurized. A study of 352 patients, aged newborn to 18 years, reported a mean leak pressure of 20.4 cm H2O, complications in 14 patients, and no cases of regurgitation or aspiration. Successful placement on the first attempt was performed in 95% of the patients.32 Tracheal intubation via the air-Q can be performed efficiently and effectively with guidance from a flexible fiberscope.33
Ambu Airway
There are five models of the Ambu laryngeal airway: the AuraFlex, AuraStraight, AuraOnce, Aura-I, and Aura40 (Ambu Inc., Ballerup, Denmark). The AuraFlex is available in sizes 2 through 6 with a small half size (2.5). All other models are available in sizes 1 through 6 with two small half sizes (1.5 and 2.5). The Aura40 is a curved reusable supraglottic airway, and the AuraOnce is a curved single-use supraglottic airway. Performance of the Ambu laryngeal airway compares favorably with other supraglottic airways, although few published studies have been done in pediatric patients.34
I-Gel
The I-Gel (Intersurgical, Liverpool, NY) is a supraglottic airway with a laryngeal cuff composed of a thermoplastic elastomer that produces a perilaryngal seal without an inflatable cuff. The purpose of this design is to provide an adequate laryngeal seal while minimizing the risk of pressure trauma to the hypoharyngeal tissue. A bite block and gastric drain port are also built into the device to permit decompression of the stomach. Reported experience with the I-Gel in children is limited but indicates a high insertion success rate and a mean leak pressure of 25 cm H2O.35 There are 7 sizes of the I-Gel that cover patients weighing 2 kg to more than 90 kg.
Pediatric Breathing Systems
The preterm infant can be adversely affected by hyperoxia.36,37 Consequently, no matter what breathing system is used, it is important to control the delivered oxygen concentration, which may be room air. The modern anesthesia machine can be used very successfully for most pediatric cases and has the great advantage of operator familiarity. However, it is very important that the machine have the capability of delivering air.
Fundamental Requirements of the Pediatric Breathing System
The pediatric breathing system should have a low resistance and low dead space, be efficient for use with both spontaneous and controlled ventilation, and be easy to humidify and scavenge (Box 17-3). If the circuit contains valves, they should offer minimal resistance to gas flow and must be reliable. In addition, the circuit should have a low compressible volume and be lightweight and compact. The circle system with carbon dioxide absorbent is commonly used for pediatric anesthesia, as is the Mapleson D and F systems for manual ventilation and in PACU settings.
Mapleson D, E, and F System Development
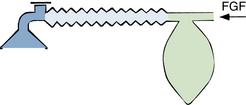
The Mapleson D, E, and F systems are direct descendants of the original T-piece system introduced in 1937 by Philip Ayre of Newcastle, England.38 The original Ayre T-piece has been greatly modified over the years and is rarely used today. All have the common feature of containing no valves that direct gas flow toward or away from the patient. The most commonly used configurations are the Mapleson D and F systems (Figs. 17-5 and 17-6). The Mapleson F system is a modification that is generally attributed to Jackson Rees, who added a reservoir bag with an adjustable valve to the expiratory limb.39 When used with the valve largely open, the movement of the reservoir bag allows monitoring of spontaneous ventilation. With the valve partially closed, manual compression of the bag allows controlled ventilation. The Mapleson D system has an expiratory valve located at the end of the expiratory limb and functions in a manner similar to the Mapleson F circuit. The volume of the expiratory limb should exceed the patient’s tidal volume. The Mapleson D and F circuits can be used with mechanical ventilation by removing the reservoir bag and connecting a ventilator.
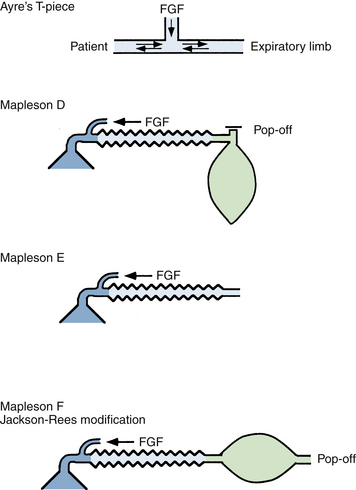
FIGURE 17-5 The Ayre T-piece and its direct descendants, the Mapleson D, E, and F circuits. FGF, fresh gas flow.
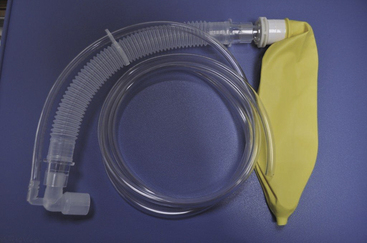
FIGURE 17-6 A portable, disposable Jackson-Rees circuit (Mapleson F) for use to transport an infant or to administer anesthesia. (Courtesy K. Premmer, MD.)
The absence of valves with the Mapleson systems can result in rebreathing of exhaled gases in a setting of decreased fresh gas flow (FGF). The use of capnometry to determine if there is rebreathing (PiCO2 >0) allows titration of FGF to eliminate rebreathing if desired. In general, an FGF of not less than 2.5 and up to 3 times the minute ventilation will usually eliminate rebreathing (Box 17-4).40 Neonates generally require an FGF of 3 L/min. FGF may need to be increased in the presence of a large tracheal tube leak. Significant FGF will be required in larger children and adults to prevent rebreathing, where peak inspiratory flows may exceed 20 L/min. FGF of this magnitude is both difficult to humidify and expensive. In general, a circle system is more efficient in terms of FGF requirements during anesthesia, particularly in older children and adults. A valved self-inflating breathing bag is appropriate for manual ventilation of larger children and adults when inhaled anesthetic delivery is not being used, particularly during patient transport.
The Bain circuit, described by Bain and Spoerel in 1972, is functionally identical to the Mapleson D system from which it is derived.41 In the Bain circuit, the FGF is carried in a smaller inner hose, contained within the expiratory limb, attached to the machine using a special adapter (Fig. 17-7). The outer expiratory limb ID is 22 mm and is 7 mm for the inner fresh gas tubing. FGF requirements for the Bain system are similar to those recommended for the Mapleson D and F systems.
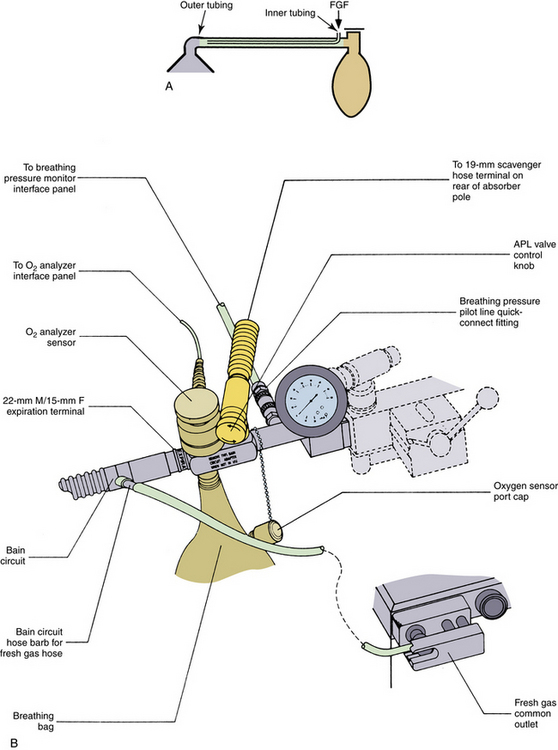
FIGURE 17-7 A, The Bain modification of the Mapleson D circuit. B, A Bain circuit adapter, which incorporates a bag mount, pressure gauge, adjustable pressure limiting (APL) valve, and a 19-mm scavenging connector. (Courtesy Dräger Medical, Telford, PA.)
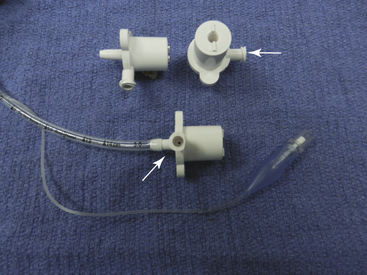
Before using the Bain circuit, it is important to verify that the fresh gas inner tube is not fractured or disconnected. In this situation, fresh gas will enter the expiratory limb near the reservoir bag, and a huge dead space will result.42 Pethick’s maneuver has been recommended to test the integrity of the circuit.43 First, the patient end of the circuit is occluded and the reservoir bag is filled. The patient end is then opened, and a high flow of oxygen is passed through the circuit using the oxygen flush valve. The high gas flow exiting from the patient end of an intact fresh gas hose will cause a reduction in pressure in the expiratory limb because of the Venturi effect, collapsing the reservoir bag. If the inner tube is disrupted, oxygen under pressure will enter the expiratory limb and distend the reservoir bag. This technique has been criticized because small leaks may go undetected, and the test itself may cause circuit disruption. Similarly, if the inner tube is intact but does not extend completely to the end of the expiratory limb, significant dead space will exist that may not be detected. An adapter has been described that tests the integrity of the inner and outer tubing separately (Fig. 17-8).44 A similar malfunction of a breathing system caused by disconnection of the central coaxial tubing can occur with a coaxial circuit used with a circle system.45
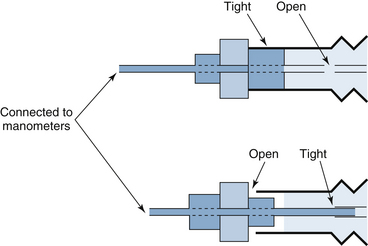
FIGURE 17-8 Bain circuit test adapter. One end of the device is connected to a manometer and a sphygmomanometer bulb to allow inflation and pressure measurement. The other end is inserted into the patient end of the Bain circuit. The specific calibers and lengths of the components of this device allow it to be inserted in one of two ways. In the top illustration, by occluding the outer lumen of the Bain circuit, the integrity of the entire circuit is determined. If the device is removed, reversed, and reinserted, the inner inspiratory limb can be isolated from the outer expiratory limb, and its integrity may be determined separately. (Modified from Berge JA, Gramstad L, Budd E: Safety testing the Bain circuit: a new test adaptor. Eur J Anaesthesiol 1991;8:309.)
There has been substantial investigation of the functional characteristics of Mapleson circuits.46 The near uniform use of capnography during anesthesia has greatly changed the clinical use of these systems and has largely supplanted previous work. Based on the capnogram, FGF can be adjusted as needed to ensure appropriate levels of rebreathing of exhaled gases.
Summary of Mapleson D and F Circuit Properties
The Mapleson D and F circuits are simple, lightweight, and have low resistance and dead space. Some means of humidification is necessary because of the high FGFs required by these circuits. Fresh gases entering the circuit can be passed through a heated humidifier, or a heat and moisture exchanger (HME) can be placed between the circuit and the tracheal tube. Despite the high FGFs required by these circuits, several scavenging systems have been found to be effective for both controlled and spontaneous ventilation.47 However, in larger patients, the clinician may prefer to use a Mapleson A or circle system during spontaneous ventilation. (Most newborns and small infants will not be breathing spontaneously while anesthetized for prolonged periods.) During spontaneous ventilation in infants and children, it is probably advisable to avoid rebreathing, in view of their unpredictable response to the increased work of breathing. During controlled ventilation, a certain amount of rebreathing is acceptable and may even be advantageous. Capnometry allows the monitoring of inspired carbon dioxide to detect rebreathing and has improved the anesthesiologist’s ability to accurately adjust FGFs when using these circuits.
Mapleson A (Magill) Circuit
The Mapleson A (Magill) circuit has the advantage of economy of FGF when used for the spontaneously breathing patient (Fig. 17-9). However, when used for controlled ventilation, this advantage is lost, and a Mapleson D or circle system is a better choice. For anything but the shortest duration of controlled ventilation, there are more appropriate alternatives to this system.
Circle Systems
The circle system is the most commonly used breathing circuit in the United States. Its main advantages are those of economy of anesthetic gas use, decreased environmental pollution, and conservation of heat and moisture. However, early experiences with the use of adult circle systems to anesthetize small children resulted in respiratory compromise in spontaneously breathing patients.48 High resistances of the valves, tubing, and soda lime and a relatively large apparatus dead space were the factors that led to early recommendations that the circle system not be used for children younger than 6 years.49 Since that time, developments in circuit design have led to the introduction of circle systems with smaller canister and tubing volumes, decreased compliance, and lower resistance valves and connectors. In contrast to the earlier experiences, recent clinical experience in pediatric patients has been extremely favorable. Compared with the Mapleson D or F circuit, the circle is more economical, offers better heat and moisture conservation, and is easier to scavenge.3 Although the resistance of circle systems has been found to be slightly higher than or similar to that of a Mapleson D or F system, it does not appear to significantly affect the minute ventilation or blood gas homeostasis of spontaneously breathing infants.50 To add some clinical perspective, the resistance to breathing of a circle system is significantly less than that of a size 3 or 3.5 mm tracheal tube. Hence, circle circuits appear to be acceptable for short-term spontaneous ventilation for healthy older infants.
Factors that Affect Delivered Tidal Volume
Several factors can make the patient’s actual delivered tidal volume differ significantly from that delivered into the circuit by the ventilator. These include compressible volume, tracheal tube leak, and augmentation of tidal volume by FGF. The volume delivered to the patient may be less than or greater than that expected by the anesthesiologist, but the importance of these factors can be reduced or eliminated using available technology. The use of end-tidal capnography provides a continuous monitor of ventilation with or without technology to address compressible volume and augmentation of tidal volume.
Compressible Volume
When a ventilator bellows delivers a tidal volume into a breathing circuit, a portion of that volume will not enter the patient’s lungs. This wasted ventilation is partly due to the compressible (compression) volume.51 The compressible volume is that volume loss attributable to the compliance of the circuit and the compression of the gases within the circuit. The loss of delivered volume as a result of distention of the circuit is a function of the circuit volume, circuit compliance, and peak inflation pressure. Disposable plastic circuits are more distensible than wire-reinforced circuits, and the nondisposable rubber circle system is more distensible than a nondisposable Mapleson D circuit.3 Breathing circuits can expand both in diameter and length when internally pressurized. The ventilator itself may also contribute to the compressible volume. An adult ventilator bellows usually has a significantly larger compressible volume than a pediatric bellows (4.5 mL/cm H2O vs. 1 to 2.5 mL/cm H2O).52 Volume loss as a result of compression of gases is a separate entity from that resulting from circuit distention and is purely a function of circuit volume and peak inflation pressure. Volume loss because of gas compression is significantly increased by including a humidifier chamber in the circuit.
Endotracheal Tube Leaks
The presence of a leak around the tracheal tube results in volume loss during inhalation and exhalation whenever the airway pressure is greater than the leak pressure of the tracheal tube. Maintaining constant minute ventilation in the presence of a variable tracheal tube leak can be difficult. In this situation, pressure-preset ventilation has some advantages (see also Chapter 6). An alternative approach is to use a cuffed tube, provided that the leak pressure is carefully adjusted and monitored as appropriate.
Tidal Volume Augmentation by Fresh Gas Flow
Depending on the type of anesthetic breathing system in use and its FGF requirements, the tidal volume delivered by a ventilator can be significantly augmented by continuous fresh gas inflow from the anesthesia machine.53,54 For example, when the FGF is 5 L/min and the inspiratory time is 0.6 seconds, the delivered tidal volume will be augmented by 50 mL (5000 mL/60 sec × 0.6 sec). The practical implication is that alterations in FGF may produce a change in tidal volume that may not be reflected by a change in the ventilator bellows displacement.55
The compliance of circle systems can be substantial compared with the compliance of smaller patients. Earlier mechanical ventilators used for the delivery of anesthesia did not compensate for the circuit compliance. In addition, the FGF could affect delivered volumes. Consequently, these earlier ventilators were problematic for use with smaller patients.
More recent anesthesia ventilators address many of the factors that influenced delivered tidal volumes during anesthesia.56 Investigation of anesthesia ventilators using test lungs demonstrates that more recent anesthesia machines that use ventilators that compensate for circuit compliance and FGF deliver very predictable tidal volumes, even with simulated lung compliances consistent with preterm infants.57,58 In contrast, older technology that does not compensate for these factors is considerably less accurate in volume delivery.
Compliance compensation and fresh gas decoupling are important advances for anesthesia ventilators. These and other changes, such as improved flow measurement, have eliminated many of the problems earlier generation anesthesia ventilators presented for use with pediatric patients. With these advances, delivered volumes are much more predictable and reliable. Current anesthesia ventilators offer additional forms of supported and controlled ventilation. As with any device, a good understanding of the operation of the ventilators is important for optimal utilization of the capabilities of the machines for clinical application.
Monitoring Ventilation
Adequacy of oxygenation and adequacy of carbon dioxide elimination are the most important endpoints to monitor during controlled ventilation. Tidal volumes, peak inflating pressures, gas delivery patterns, pressure-volume loops, markers of spontaneous respiratory effort, and capnography provide guides to assessing the appropriateness of patient ventilation.
Miscellaneous Aspects of Mechanical Ventilation
Positive End-Expiratory Pressure
During general anesthesia, several factors may decrease the patient’s functional residual capacity (FRC; Box 17-5). This drop in FRC can lead to airway closure, ventilation/perfusion (V/Q) mismatch, and hypoxemia.59 It has been demonstrated that the application of 5 cm H2O positive end-expiratory pressure (PEEP) can partially restore FRC and increase oxygenation.60,61 If PEEP is applied, airway pressure should be carefully monitored to allow the detection of excessive airway pressure. Ideally, airway pressure should be monitored as close to the tracheal tube as possible. In practice, airway pressure is usually monitored in the expiratory limb of the circuit. Of note, the application of PEEP may decrease the tidal volume delivered depending on the design of the ventilator.62 More recent anesthesia ventilators include electronically applied PEEP, which is intended to prevent inaccurate, improper, or unintended PEEP.56
Practical Hints for Ventilating Neonates and Infants
Although the healthy newborn has a normal spontaneous respiratory rate of approximately 40 breaths/min, in practice, most healthy newborns who undergo anesthesia are adequately ventilated at somewhat lower respiratory rates, such as 20 to 30 breaths/min.63
Some of the factors that determine the adequacy of mechanical ventilation of the neonate have been modeled mathematically.48 Although these data were derived several decades ago and modern anesthesia systems have since evolved significantly, the authors’ conclusions remain pertinent. The anesthesia circuit, ventilator bellows (if present), and humidifiers all have significant compliance. During inspiration a portion of the total flow delivered by the ventilator (Qt) is expended in distending the circuit (Qc), thus the inspiratory flow to the patient (Qp) will be reduced accordingly (Qt = Qc + Qp; Fig. 17-10). This explains in part why very small infants can be ventilated safely with ventilators that have minimum inspiratory flows that greatly exceed those of a spontaneously breathing infant.
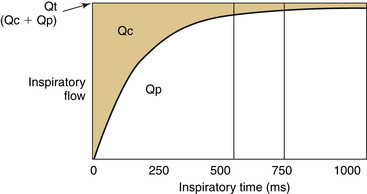
FIGURE 17-10 A schematic of the inspiratory flow pattern during constant flow ventilation. The total flow from the ventilator (Qt) is constant throughout inspiration. The proportion of flow spent in distending the anesthesia circuit (Qc) is large at the start of inspiration and decreases as inspiration continues. Thus longer inspiratory times may be more effective when the circuit compliance is high compared with that of the patient because the early part of inspiration is spent distending the circuit. Qp, inspiratory flow to the patient. (Modified from Epstein MAF, Epstein RA: Airway flow patterns during mechanical ventilation of infants: a mathematical model. IEEE Trans Biomed Eng 1979:26:299-306.)
The disparity between Qp and Qt is greatest at the start of inspiration, when the circuit pressure is low, and the circuit is most compliant. During the latter portion of the inspiration (after 360 ms), as the circuit becomes pressurized and less compliant, Qp approximates Qt (Qp/Qt = 0.9). Thus, as inspiratory time is prolonged, there may be a disproportionate increase in tidal volume. Conversely, if the inspiratory time is short, Qp will be small in relation to Qt for most of the inspiratory period. To complicate matters, at rapid respiratory rates, shorter inspiratory times (500 ms) may be necessary to allow sufficient time for exhalation. Therefore, when rapid ventilatory rates are used with short inspiratory times, significantly higher inspiratory flows and pressures may be required to ventilate the patient effectively. At lower respiratory rates with longer inspiratory times, a proportionately larger increase in the duration of the later, more effective period of inspiration occurs, and lower inspiratory flows may be sufficient. This may explain the apparently paradoxic deterioration in gas exchange occasionally observed in small infants when they are ventilated at rapid respiratory rates and the inspiratory time is shortened.
Manual Versus Mechanical Ventilation of Neonates and Infants
In the past it was often recommended that the neonate or small infant be ventilated by hand rather than mechanically.64 A potential advantage of manual ventilation is that it allows the rapid detection of changes in respiratory compliance by the “educated hand” of the pediatric anesthesiologist. Historically, manual ventilation was used because of the lack of both reliable respiratory monitoring and ventilators suitable for neonates. However, with the advent of pulse oximetry, capnography, routine monitoring of airway pressure, and reliable OR ventilators, the arguments for manual ventilation are now less compelling. Objective data suggest that even experienced pediatric anesthesiologists are unable to consistently detect significant changes in respiratory compliance (e.g., complete occlusion of the tracheal tube).65 Under ideal circumstances, experienced pediatric anesthesiologists (8 years) could detect complete ETT occlusion 83% of the time versus 64% for their less experienced colleagues (<2 years).66 Less dramatic changes in compliance and resistance than those that went undetected in these studies would probably produce clinically significant adverse changes in ventilation and oxygenation. Compliant reservoir bags combined with the relatively large compressible volume of many pediatric breathing circuits (compared with the neonate’s small tidal volume) will make manual detection of changes in the patient’s resistance and compliance unpredictable, particularly when higher FGFs are used (e.g., 6 L/min). Mechanical ventilation produces more consistent ventilation and frees the anesthesiologist’s hands to perform other essential tasks. The argument that the large compressible volume of the breathing circuit makes mechanical ventilation unpredictable in the neonate applies equally to manual ventilation of the neonate.67,68 When using volume-preset mechanical ventilation, the volume delivered to the circuit is constant. Although changes in the patient’s compliance may change the tidal volume delivered to the patient, changes in tidal volume should be accompanied by changes in airway pressure, thus enabling the detection of changes in the patient’s compliance. Manual ventilation has no advantage over mechanical ventilation for the detection of changes in the patient’s compliance, provided that mechanical ventilation is accompanied by continuous measurement of airway pressure, chest excursion, breath sounds, oxygen saturation, and end-tidal carbon dioxide concentration. However, in the case of acute adverse changes in respiratory function, it is still usual to revert to manual ventilation of the patient to confirm adequate lung inflation and allow breath-to-breath adjustment of ventilation.
Summary of Mechanical Ventilation of Infants and Children During Anesthesia
Most healthy children can be ventilated adequately with ventilators primarily designed for adults, provided they are used with appropriate respiratory monitoring. These adult devices have the advantage of being familiar to most anesthesiologists, and their operating characteristics are well understood. These devices are safest when used with age-appropriate, low-compliance/low-volume breathing circuits and when capnometry, oximetry, airway pressure, exhaled tidal volume, and auscultation of breath sounds are used to monitor ventilation. Although both pressure-limited ventilation and volume-controlled ventilation can be used safely, the operator should be aware that variable tube leaks, tube obstructions, and changes in pulmonary compliance and resistance may have differing presentations and effects depending on the mode of ventilation. Appropriately sized and adjusted cuffed tracheal tubes, along with anesthesia machines and ventilators that compensate for FGF and circuit compliance, eliminate many of these concerns and provide highly predictable volume ventilation for infants and older pediatric patients. Ventilators specifically designed for neonates, infants, and children are available that can be used in the OR (Fig. 17-11).
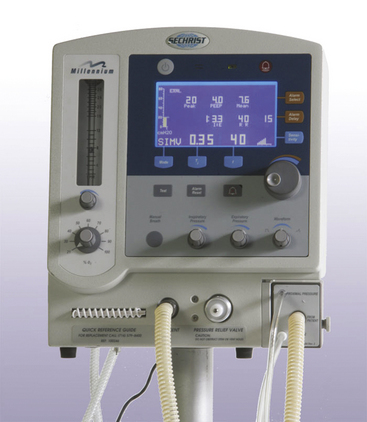
FIGURE 17-11 The Sechrist Millennium neonatal/infant/pediatric ventilator. The ventilator can operate in the SIMV/IMV, Assist-Control, and back-up ventilation continuous positive airway pressure modes. IMV, intermittent mechanical ventilation; SIMV, synchronized intermittent mechanical ventilation. (Courtesy Sechrist Industries, Anaheim, CA.)
High-Frequency Oscillatory Ventilation
Although high-frequency oscillatory ventilation (HFOV) is an established therapy in the neonatal and pediatric intensive care units (ICUs), the perioperative use of HFOV is less well described. As the complexity and number of procedures performed in sicker and smaller neonates increase, anesthesiologists will be even more exposed to patients who require this mode of ventilation. Perhaps the most common scenario is the neonate who is already receiving HFOV prior to patent ductus arteriosus ligation or exploratory laparotomy for necrotizing enterocolitis. However, anesthesiologists may occasionally encounter critically ill neonates who are receiving conventional ventilation, in whom HFOV represents a viable elective option for perioperative ventilation. Importantly, HFOV also represents an invaluable intraoperative rescue strategy that might be considered in certain circumstances.
The most important issues relating to the perioperative use of HFOV include the anesthesiologist’s relative unfamiliarity with this technology, the inability to monitor end-tidal carbon dioxide or to deliver volatile agents, and the inability to transport patients while delivering HFOV. Furthermore, it is possible that the neonate who requires HFOV is too sick to transport to the OR. Under these circumstances, the anesthesiologist will be working in the relatively unfamiliar environment of the neonatal intensive care unit (NICU).
On the other hand, although formal data are lacking, it is often the anesthesiologist’s experience when HFOV is used during thoracotomy for patent ductus arteriosus ligation that lung retraction appears to be relatively well tolerated, with less interference from ventilation with surgical exposure. Indeed, some anesthesiologists advocate the elective conversion to HFOV prior to this procedure.69 The use of HFOV has been reported during congenital diaphragmatic hernia repair, necrotizing enterocolitis, excision of pulmonary bullae, and surgery for congenital cystic adenomatoid malformations.70-72 The assistance of the neonatologist and the respiratory therapist can be helpful in adjusting HFOV settings and troubleshooting. A total IV anesthetic must be used, typically with an opioid-based technique. Because accurate end-tidal carbon dioxide measurement is not possible, transcutaneous or blood gas monitoring of carbon dioxide is needed. If the neonate requires transport prior to surgery and is already on HFOV, it is useful to test his or her ability to tolerate short periods of manual ventilation prior to leaving the NICU.
An in-depth discussion of HFOV weaning and adjustment strategies is beyond the scope of this chapter. However, the basic approach includes adjustment of the fraction of inspired oxygen (FiO2) and mean airway pressure to optimize oxygenation and manipulation of amplitude, usually referred to as ΔP, and optimizing inspiratory time to achieve the desired carbon dioxide elimination. A commonly monitored parameter of ventilation is the presence of abdominothoracic oscillation. Optimum alveolar recruitment is achieved when lung expansion to 8.5 to 9 ribs is observed on routine chest radiograph.
Humidification
The safe and precise function of the components of a modern anesthesia machine requires a supply of clean, dry anesthetic gases. However, the inhalation of dry, cold gases is suboptimal for the patient, and minimum levels of temperature and humidification have been recommended.73 The humidification of inspired gases is of particular importance during anesthesia in infants and children (see also Chapter 7). Available approaches include the active humidification of inspired gases, the use of HMEs, and minimizing FGF. Unfortunately, all have limitations in clinical applicability.
Beneficial Effects of Humidification
Beneficial effects of humidification include the reduction of heat and water loss from the respiratory tract, the prevention of airway damage, and the prevention of inspissation of secretions that could cause tracheal tube obstruction.
Reduction of Heat and Water Loss from the Respiratory Tract
During the course of normal breathing, cold, dry atmospheric air is inhaled, warmed to body temperature, and saturated with water vapor by the time it reaches the distal bronchi. Air fully saturated with water vapor at 37° C contains 44 mg/L of water (absolute humidity 44 mg/L, relative humidity 100%). To humidify inhaled gases, heat and water vapor are transferred from the mucosal lining of the airway. Under normal circumstances, most of the heat and water is returned to the respiratory mucosa during exhalation. If the upper airway is bypassed by a tracheal tube, significant heat and water loss will occur from the patient if inspired gases are not humidified. Because of the very low specific heat and thermal capacity of dry gas, it is difficult to provide sufficient energy to the patient to prevent intraoperative hypothermia without causing thermal injury to the airway. A 3-kg infant with a minute ventilation of 500 mL/min will expend 0.0035 kcal/min to raise the temperature of cold, dry inspired gases to body temperature. However, if in addition, another 0.012 kcal/min are required for the heat of vaporization to saturate the dry inspired gases with water vapor at body temperature,74 the total amount of energy expended will be 0.015 kcal/min. This amounts to approximately 10% to 20% of the total energy expenditure of the infant.
Prevention of Respiratory Tract Mucociliary Dysfunction
The inhalation of dry gases causes ciliary paralysis and decreases mucus flow, thus impeding mucociliary transport.75 Decreased humidity causes the viscosity of mucus to increase markedly, and greater than 50% relative humidity is required to allow continued flow of mucus.76 Humidification of inspired gases may reduce the incidence of postoperative pulmonary complications in adults,77 although similar data in pediatric patients are lacking.
Prevention of Tracheal Tube Obstruction
Given the small internal diameter of pediatric tracheal tubes, even a small amount of inspissated mucus within the lumen of the tube may produce significant airway obstruction. A small premature infant may require a 2.5-mm ID tracheal tube. These small patients have very little respiratory reserve and would be expected to benefit the most from humidification of inspired gases.
Methods of Providing Humidification in Pediatric Systems
Several methods are available to reduce heat and water loss from the airway during anesthesia. Even the tracheal tube itself may act as a low-efficiency passive HME, as the moisture in exhaled gases condenses within the tube and is then added to the inspired gas mixture. In the absence of either active or passive humidification, measurements of humidity at the distal end of the tube demonstrate that the tracheal tube alone will produce an inspired relative humidity of 30% at its distal end.78 However, this is less than the 50% relative humidity reported to be necessary to allow normal mucus flow.
Humidifiers in common use are either active, adding exogenous heat and water to the breathing system, or passive, conserving and allowing the rebreathing of the patient’s endogenous heat and moisture. Alternatively, the use of a circle system with low FGF will generate heat and humidity that can be inhaled by the patient. Each method of humidification has its specific advantages and disadvantages.
Passive Humidification
The simplest method of humidification is passive humidification, using an HME, although HMEs do not add heat or water to the inspired gas other than that exhaled by the patient in the preceding breaths. These devices are placed between the tracheal tube and the breathing system. The HME contains an exchange medium with a large surface area, and its ability to humidify inspired gas is highly dependent on the ambient conditions. When breathing room air (at 20° C with 50% relative humidity and 7 mg H2O/L absolute humidity), HMEs can produce a relative humidity of almost 100% in the inspired gas; however, when breathing cold, dry anesthetic gases (0 to 20° C, 0% relative humidity), the inspired gas relative humidity may be as low as 60% at 37° C. Improvements in the design of these devices have increased their efficiency.
Concerns about the use of these devices in pediatric anesthesia practice relate to the issues of dead space, resistance, and efficiency. Several HMEs have been developed specifically for use in infants (Fig. 17-12). The characteristics of one such device, the Mini Humid-Vent (Teleflex Medical, Research Triangle Park, NC) have been measured.79 Although the device has a dead space of 4.2 mL when measured in isolation, when added to the circuit, its dead space decreases to 2.7 mL because of the volume occupied by the circuit and tracheal tube connectors. The dead space is likely to be small in relation to the tidal volume (50 mL) of the pediatric patient, for whom this device is recommended. The resistance of the device at gas flows between 2 and 10 L/min approximates that of a 5.0-mm tracheal tube. At a gas flow of 10 L/min, the pressure drop across a 3.0-mm tracheal tube was increased from 9.6 cm H2O to 10.4 cm H2O by the addition of an HME. The resistance to flow across the HME was increased by 10% to 30% when humidified air was compared with dry air.
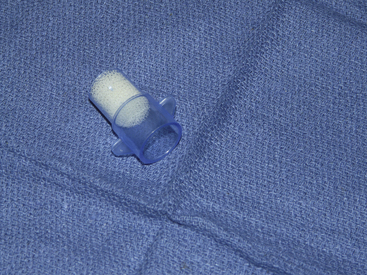
FIGURE 17-12 A low dead space, high-efficiency pediatric heat and moisture exchanger (Breathe Easy; Vital Signs, Totowa, NJ).
In contrast, Rodee and colleagues80 determined that the increased work of breathing imposed by some HMEs is significant. The Mini Humid-Vent was found to increase the work of breathing by up to 60% when dry, and even more when saturated. Investigation of the effect of a ClearTherm HME (ClearTherm Limited, Leicester, UK) found a 43% increase in the work of breathing.81 Those authors suggest that the HME be removed from the circuit during spontaneous ventilation. Bissonnette and colleagues82 compared the performance of an HME with that of active humidification in ventilated anesthetized children weighing between 5 and 30 kg and found that although passive humidification was less efficient than active humidification, it was significantly better than no humidification. Although the relative humidity of inspired gases with passive humidification was initially only 50%, after 80 minutes of anesthesia, the relative humidity of the inspired gases had increased to a level (80%) that was not significantly different from that of active humidification (90%). Passive humidification effectively reduced the mean temperature drop that occurred during surgery, from 0.75° C to 0.25° C. An investigation of two other HMEs concluded that the devices conserved airway humidity well but did not achieve desired levels of heat and humidity.83
HMEs can be effective in reducing heat loss during the perioperative period. Devices are manufactured whose resistance and dead space can be acceptable, especially considering that most infants and children undergoing surgery for an appreciable length of time will be ventilated rather than being allowed to breathe spontaneously. The humidification efficiency of HMEs is predicted to be decreased in the presence of a large leak around the tracheal tube because a variable proportion of the exhaled, heat- and moisture-rich gas will bypass the HME.
Active Humidification
Active humidification prevents energy and water loss from the respiratory tract. The temperature of the inspired gases is continuously monitored to avoid hyperthermia or thermal injury to the respiratory tract. Although active humidification may be more efficient than passive humidification, it is more cumbersome and costly. The presence of the humidifier chamber in the breathing system adds significant compressible volume to the circuit. The potential for circuit disconnection is increased because of the additional connections required for both the humidifier chamber and the temperature-monitoring equipment. Other potential hazards of humidifiers, particularly with ultrasonic nebulization, include overhydration and increased airway resistance. Ultrasonic nebulization adds small droplets (1 to 10 μm diameter) of unheated water to the airway in the form of an aerosol. Molecular humidification, such as with a heated water bath or HME, simply adds molecular water vapor to the airway (see also Chapters 7 and 26).
Active humidification using a heated water system has the advantages of being applicable to patients of all sizes, being highly efficient, and avoiding the problems of dead space and resistance encountered with HMEs. It has the disadvantages of additional compressive volume, higher cost, and increased circuit complexity. Thus active humidification can be used safely during both controlled and spontaneous ventilation.
Minimizing FGF reduces the amount of gas that must be humidified for optimal delivery. Although reducing the amount of FGF improves the level of humidification, the approach is inadequate in infants in the absence of active humidification and/or heat and moisture exchange.84
Capnography and Respiratory Gas Analysis In Pediatric Patients
Capnography is vitally important in the monitoring of ventilation during anesthesia (see also Chapter 10). Capnography is also used to confirm tracheal tube placement and to aid in the diagnosis of critical events that occur during anesthesia, including venous air embolism and malignant hyperthermia; however, it is important to be aware of the technical limitations in obtaining accurate capnometric data from small children (Table 17-4). Capnometers can be divided broadly into two groups, mainstream (flow-through) devices and sidestream (aspirating) devices (see also Chapter 8). Sidestream devices are more commonly used in the OR.
TABLE 17-4
Techniques to Improve Accuracy of End-Tidal Carbon Dioxide Monitoring in Infants and Children
| Technique | Advantage | Disadvantage |
| Distal sampling | Decreases dilution by FGF | Requires special sampling port (purchased or made) |
| Cuffed tube (absence of leak) | Decreases dilution by ambient air in oropharynx | Requires careful management to avoid tracheal injury |
| Sechrist ventilator∗ | Uses low FGF (250 mL/min) and simulates a nonrebreathing system by Venturi effect on respiration | Not commonly used in the operating room |
| Circle system | Valves isolate FGF to minimize dilution of sample | Valves increase work of breathing in infants who are spontaneously ventilating |
| Low FGF | Decreases dilution of expired sample | May increase rebreathing in a Mapleson D or Bain system |
| Lower sampling rates | Minimizes dilution by FGF | Decreases accuracy by increasing lag time and reducing response time, especially at rapid respiratory rates and low tidal volumes (does not record true CO2 peak) |
| Long I:E ratios (1:3.5) | Prevents rebreathing | Probably not significant |
| Controlled ventilation | Increases tidal volume relative to FGF | Prevents weaning from mechanical ventilation |
| Discontinuation of FGF during sampling | Prevents dilution of sample | Interrupts normal ventilation |
| Mainstream capnometer | Prevents plugging of the sampling catheter with secretions or humidity, decreases response time; minimally affected by FGF | Adds dead space and weight; patient must be tracheally intubated |
FGF, fresh gas flow; I:E, inspiratory/expiratory.
∗Sechrist Industries, Anaheim, CA.
Modified from Dubose R: Pediatric equipment and monitoring. In Bell C, Hughes C, Oh T, editors: The pediatric anesthesia handbook. St. Louis, 1991, Mosby–Year Book.
The accuracy of sidestream devices in pediatric patients, defined in this context as the degree to which end-tidal carbon dioxide tensions approximate arterial carbon dioxide tensions, has been investigated by several groups. Several factors appear to be important.
Gas Sampling Site
The site of gas sampling from the breathing circuit appears to be important in patients weighing less than 12 kg.85 When gas is sampled from the distal end of the tracheal tube with a sampling catheter, the accuracy of measurement was considerably improved over measurements made at the tracheal tube circuit connector (proximal sampling). On the other hand, distal versus proximal sampling may not affect the accuracy of measurement when a nonrebreathing circuit and ventilator with no fresh gas blowby is used, such as a Servo 900C system (Siemens, Munich, Germany).86 Gas need not be sampled from the distal tip of the tracheal tube but may be sampled with similar accuracy from the point of narrowing of the tracheal tube adapter (Fig. 17-13).87
Breathing System
When the Mapleson D circuit is used, end-tidal values are often decreased compared with arterial values. The relatively high FGF required in these circuits dilutes the end-expired carbon dioxide, thereby artifactually lowering the end-tidal CO2 reading. However, this “dilutional” effect is attenuated when certain ventilators are used, such as the Sechrist Infant Ventilator (Sechrist, Anaheim, CA).88
Mainstream Versus Sidestream Analysis
When mainstream capnometers have been compared with sidestream analyzers in children, they were found to be as accurate as distally sampling sidestream analyzers and considerably more accurate than proximally sampling sidestream analyzers.89 The sampling rate of sidestream capnometers affects their accuracy. High gas sampling rates (>250 mL/min) exaggerate the dilution effect by entraining fresh gas and diluting expired carbon dioxide. On the other hand, low sampling rates (<100 mL/min) can prolong the response time of the capnometer such that the ability to display a valid waveform at rapid respiratory rates is lost.90 Badgwell and colleagues91 have examined the effect of ventilation at rapid respiratory rates on the capnographic waveform and demonstrated significant artifactual elevation of the sidestream baseline attributable in part to longitudinal mixing of gas within the sampling line. No artifactual elevation was observed in flow-through capnograph waveform baselines. This study utilized multiplexed mass-spectrometer gas analysis with a sampling line that was approximately 50 m long. The authors predict that artifactual elevation of the baseline waveform would be less when using stand-alone sidestream monitors with shorter sampling lines.
Patient Factors
In general, the accuracy of sidestream measurements is reduced when used with small infants and in the presence of lung disease. With many neonatal lung diseases, measurements of end-tidal carbon dioxide do not accurately reflect arterial carbon dioxide.92 Also, patients with cyanotic heart disease who have reduced pulmonary blood flow or mixing lesions also tend to have reduced end-tidal carbon dioxide values compared with arterial measurements.93 Furthermore, for capnometry to be useful even as a monitor of trend in those patients, the arterial/end-tidal carbon dioxide difference must remain constant. Unfortunately, patients with cyanotic heart disease may have variations in shunt fraction, pulmonary blood flow, and dead space/tidal volume ratios during anesthesia and surgery. These variations will produce a variable arterial/end-tidal carbon dioxide difference, thereby reducing the utility of capnometry, even as a monitor of trend.
Although expired carbon dioxide monitoring may not always accurately reflect arterial carbon dioxide tension in children, it does appear to be very useful as a trend monitor in patients without cyanotic heart disease. Capnometry will continue to have a vital role in maintaining the safety of pediatric anesthesia.
Monitoring
Periodic blood pressure determination is an essential part of monitoring during anesthesia,94 and a variety of techniques and equipment are available to achieve this (see also Chapter 12). 95,96 All noninvasive forms of blood pressure determination depend in part on a snugly fitting cuff that contains an air-filled bladder that can be inflated to controlled pressures. The American Heart Association has developed recommendations for indirect blood pressure determination, including those for cuff sizes.95 The width of the inflatable bladder of the cuff should be 0.4 times the circumference of the extremity, and its length should be at least twice its width to cover at least 80% of the extremity circumference. Cuffs that are too narrow will result in an overestimation of the blood pressure, and cuffs that are too wide will underestimate the blood pressure.97,98 Given the same degree of mismatch, cuffs that are somewhat too wide will result in less error than cuffs that are too narrow.99 As a result, a series of blood pressure cuffs of various sizes must be available for the accurate, noninvasive determination of blood pressure in pediatric patients.
Ultrasound Detection of Arterial Flow
Doppler ultrasound detection of arterial flow is a useful method of determining systolic blood pressure even in small infants.100 One commonly used unit made by Parks Medical Electronics (Aloha, OR) is battery powered and has an ultrasonic detector and an emitter capable of identifying arterial blood flow and converting it into an audible sound; the return of arterial flow with the onset of systole is easily recognized and correlates well with both auscultatory assessment and intraarterial pressure measurement.100,101
Ultrasonic blood pressure determination has several advantages: it can be used even in hypovolemic patients, does not require a regular heart rate, and provides a method of beat-to-beat confirmation of cardiac contraction. Once the monitor has been positioned and secured, a blood pressure determination can be obtained rapidly with minimal effort. However, it has two primary limitations: it results in additional room noise and requires operator action to obtain each value.
Automated Noninvasive Systems
Most automated, noninvasive systems that measure blood pressure use an oscillometric method,102 which uses a rapid inflation of the blood pressure cuff, until the artery is occluded, followed by incremental reductions in cuff pressure. As arterial blood flow resumes, oscillations in the cuff pressure occur, indicating systole. Maximal oscillations appear to correlate with mean arterial pressure, and dampening of the oscillations indicates diastole.
Noninvasive blood pressure devices that use an oscillometric method are available from several manufacturers. The Dinamap unit (Device for Indirect Noninvasive Automated Mean Arterial Pressure, GE Healthcare, Waukesha, WI) was the first commercially available oscillometric device and has been investigated in a variety of clinical settings.96,102-107 Oscillometric devices provide a safe, easy method of periodic automated blood pressure determination in pediatric patients, including neonates, although accuracy may decrease in severely ill patients. The method does require several heartbeats to determine the blood pressure value. As a result, it cannot be applied when beat-to-beat information is needed, and it may fail when the heart rhythm is irregular.
Whatever method is used to determine the blood pressure, the value must be compared with the expected values for a patient of that age. Various tables of normal values are available in standard texts and other sources.95,108-111
Electrocardiographic Monitoring
The electrocardiogram (ECG) is an excellent noninvasive monitor of heart rate and rhythm (see also Chapter 13). Apart from sinus bradycardia and tachycardia, rhythm changes are not common in pediatric patients. Premature atrial and ventricular contractions, junctional rhythms, and respiratory variations are seen on occasion. Rhythm changes in pediatric patients generally relate to some aspect of the ventilatory, metabolic, anesthetic, or surgical management rather than to a primary cardiac event. They can generally be identified using standard limb lead electrode positions, although special leads may be helpful in some instances.112
Although the ECG is a very useful monitor of heart rate and rhythm, it can continue to be normal even in the presence of moderate to severe circulatory or ventilatory abnormalities. Indeed, the ECG can appear normal, at least briefly, without systemic perfusion. As a result, additional monitors must be used to supplement the ECG to assess the adequacy of systemic perfusion.
Pediatric ECG monitoring requires equipment capable of processing higher frequency signals.113 At a minimum, the equipment must be able to identify individual heartbeats and provide accurate rate analysis. Normal values for QRS amplitudes can vary depending on the frequency of sampling in digitized systems because slower sampling frequencies may miss the peak QRS amplitudes.114
Although myocardial ischemia is a rare event during uncomplicated pediatric anesthesia, it can occur in some circumstances.115 Kawasaki disease is an acute exanthematous condition usually seen in infants and children younger than 5 years.116 The illness is associated with coronary artery aneurysms in 20% to 25% of patients. These aneurysms generally resolve over time, but the lesions may become stenotic. Cardiac ischemia is well recognized in these patients, and appropriate ECG monitoring is indicated perioperatively.117 Other pediatric clinical situations in which cardiac ischemia may occur include surgery involving the coronary arteries, congenital coronary artery abnormalities, and systemic air embolism.
Pulse Oximetry
Hypoxemia is a major concern during any anesthesia procedure. Its incidence is increased during anesthesia administered to children aged 2 years and younger.118 Pulse oximetry provides a reliable method to detect hypoxemia prior to the time it is detected clinically (see also Chapter 11).119 Although pulse oximetry values may change several seconds after the arterial saturation changes,120 it is sufficiently rapid to detect changes in oxygen saturation as needed in clinical practice.
Pulse oximetry is applicable to all pediatric patients, including neonates.121,122 The probes must allow the emitter and detector portions of the probe to face each other across some pulsatile bed, and careful attention to probe placement is essential. Oximetry during anesthesia induction and emergence can also be difficult, because the probes may displace or dislodge with the patient’s movements. Fetal hemoglobin (HbF) does not affect pulse oximetry but does have an oxyhemoglobin dissociation curve to the left of normal adult hemoglobin.123 Common sources of inaccurate pulse oximetry values (SpO2) are summarized in Table 17-5.
TABLE 17-5
Common Sources of Inaccurate Saturations Obtained by Pulse Oximetry (SpO2)
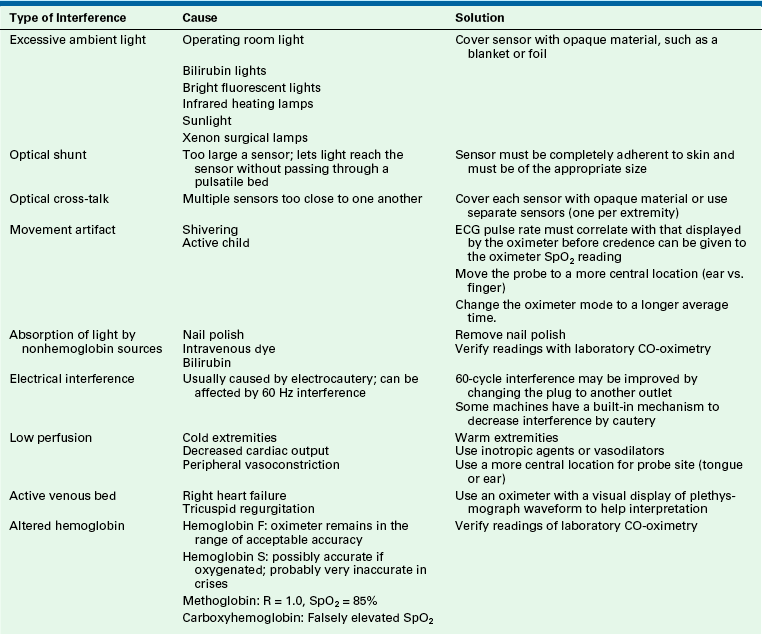
CO, carbon monoxide; ECG, electrocardiograph.
Modified from Dubose R: Pediatric equipment and monitoring. In Bell C, Hughes C, Oh T, editors: The pediatric anesthesia handbook. St Louis, 1991, Mosby–Year Book.
Unlike adult patients, hyperoxia is of concern in neonates with immature retinas because elevations in arterial oxygen tension are associated with the development of retinopathy of prematurity. The upper limit of desirable oxygenation varies from infant to infant depending on a variety of factors that include postconceptual age and the clinical stability of the infant. In infants with a patent ductus arteriosus, the pulse oximetry probe should be located at a site that indicates preductal saturation; this value will reflect the oxygenation state of blood supplying the brain and retina.124 The range of oxygen saturation of concern in hyperoxia occupies the flat portion of the oxyhemoglobin dissociation curve, where large changes in oxygen tension occur with small changes in hemoglobin saturation. Typical safe limits for SpO2 for the stable preterm infant are 85% to 93%.123 Preterm infants born at 24 to 27 weeks’ gestation have improved survival when managed with SpO2 of 91% to 95% compared with 85% to 89%.125 A single best range has not been established.123 Nonetheless, pulse oximetry is a valuable aid to the anesthesiologist caring for any patient, particularly the neonate, infant, and young child.
Tissue Oximetry
Tissue oximetry offers a noninvasive method to monitor areas of body perfusion.126,127 It uses near-infrared spectroscopy (NIRS) to assess tissue oxygenation.128 Both cerebral and somatic monitoring can provide the clinician with important information regarding patient condition,126,129 and multiple products are available from different manufacturers for this purpose. Technologic approaches to better address the infant patient have been introduced or are in process from multiple manufacturers.126 NIRS has been used as a routine monitor perioperative status of infants following stage 1 palliation of univentricular congenital heart disease.130 The role of NIRS in optimizing patient management continues to evolve.131-132
Temperature Monitoring and Maintenance
The normal human tightly regulates body temperature within a narrow range using defense mechanisms that prevent both hypothermia and hyperthermia.133 These defense mechanisms are more effective in adults compared with children, children compared with infants, and less premature infants compared with more premature infants. General anesthesia broadens the range of body temperature that must be reached before homeostatic mechanisms become active.134 Mild to moderate degrees of intraoperative hypothermia are common because of factors that include altered temperature homeostasis, cool environments, exposed skin surface, administration of cool fluids, increased evaporative losses, and redistribution of heat.135 Mild to moderate degrees of hypothermia can adversely affect outcome measures such as shivering, infection, platelet function and bleeding, cardiac events, drug metabolism, and PACU discharge time.136-138
The American Society of Anesthesiologists Monitoring Standards state that intraoperative temperature monitoring is indicated whenever a clinically significant change in body temperature is intended, anticipated, or suspected.94 In combination with the limited ability of the infant and young child to maintain normothermia during anesthesia, this strongly suggests that body temperature should be measured for the majority of young infants and in most pediatric patients undergoing anesthesia.
The approaches generally used to maintain the body temperature of nonanesthetized young infants—clothing, blankets, and heated and humidified enclosures—often cannot be applied in the OR setting. Warmed ORs can be used, but the degree of warmth necessary to maintain body temperature generally makes working conditions uncomfortable, particularly when wearing surgical gowns. Radiant warmers and the use of heated, humidified anesthetic gases can reduce heat loss to a limited degree.
Fluid warmers are useful in maintaining body temperature, particularly during procedures in which large volumes of either room temperature or 4° C blood or blood products are administered.139 However, at low fluid administration rates, warmed IV fluid tends to cool before reaching the patient. The Hotline fluid warming system (Smiths Medical, St. Paul, MN) uses a water-jacketed administration set able to deliver warm fluid to the end of the administration set at low and high flow rates more effectively than conventional dry warmers. This is advantageous with infants and young children, in whom the absolute rate of fluid administration is low, but the proportionate fluid administration rate to the patient is high.
In contrast to approaches of reducing heat loss, forced-air warmers can effectively add heat to patients. These warmers are manufactured with a variety of air dispersal products, allowing their adaptation for a variety of surgical procedures and patient sizes (Fig. 17-14).140-142
Ultrasonography
The term ultrasound refers to frequencies above the range of human hearing. For clinical application, frequencies between 2 and 15 MHz are typically used. An inverse relationship exists between the ability to identify smaller structures and the depth of penetration of ultrasound beams. Consequently, two or three probes with different frequencies provide both resolution of small superficial structures and penetration for deeper, larger structures. A good understanding of the basics of ultrasound and ultrasonography and of the appearance of structures with ultrasound are important to utilization of the technique in clinical practice,143 and ultrasonography is entering the curriculum of many medical schools.144
Since 2000, ultrasound equipment has become more portable and less expensive with greater capabilities. It has wide application across specialties in areas of procedural guidance, diagnosis, and screening.145 In anesthesiology, applications include vascular imaging and access, regional anesthesia, echocardiography, limited trauma examination, and assessment of bladder volume.146 Appropriate training, education, and experience, including the development of hand skills, is important to the clinical utilization of ultrasonography.146,147 Ultrasound-guided vascular cannulation and regional anesthesia have become widely adopted in pediatric anesthesia.148-152
References
1. James R.H. 1000 anaesthetic incidents: experience to date. Anaesthesia. 2003;58:856–863.
2. Jimenez N., Posner K.L., Cheney F.W., et al. An update on pediatric anesthesia liability: a closed claims analysis. Anesth Analg. 2007;104:147–153.
3. Rasch D.K., Bunegin L., Ledbetter J., et al. Comparison of circle absorber and Jackson-Rees systems for paediatric anaesthesia. Can J Anaesth. 1988;35:25–30.
4. Byrick R.J. Respiratory compensation during spontaneous ventilation with the Bain circuit. Can Anaes Soc J. 1980;27:96–105.
5. Olsson A.K., Lindhal S.G.E. Pulmonary ventilation, CO2 response and inspiratory drive in spontaneously breathing young infants during halothane anaesthesia. Acta Anaesthesiol Scand. 1986;30:431–437.
6. Charlton A.J., Lindhal S.G.E., Hatch D.J. Ventilatory responses of children to changes in dead space volume. Br J Anaesth. 1985;57:562–568.
7. Keens T.G., Bryan A.C., Levison H., et al. Developmental patterns of muscle fiber types in human ventilatory muscles. J Appl Physiol. 1978;44:909–913.
8. Scott C.B., Nickerson B.G., Sargent C.W., et al. Developmental pattern of maximal transdiaphragmatic pressure in infants during crying. Pediatr Res. 1983;17:707–709.
9. Fisher D.M. Anesthesia equipment for pediatrics. In: Gregory G.A., ed. Pediatric anesthesia. New York: Churchill Livingstone; 1990:470.
10. Litman R.S., Weissend E.E., Shibata D., Westesson P.L. Developmental changes of laryngeal dimensions in unparalyzed, sedated children. Anesth Analg. 2003;98:41–45.
11. Dalal P.G., Murray D., Messner A.H., et al. Pediatric laryngeal dimensions: an age-based analysis. Anesth Analg. 2009;108:1475–1479.
12. Motoyama E.K. The shape of the pediatric larynx: cylindrical or funnel shaped? Anesth Analg. 2009;108:1379–1381.
13. Lerman J. Gas flow in the upper airway: turbulent or laminar? Pediatr Anesth. 2009;19:1241.
14. Adewale L. Anatomy and assessment of the pediatric airway. Pediatr Anesth. 2009;19(Suppl 1):1–8.
15. Dullenkopf A., Gerber A.C., Weiss M. Fit and seal characteristics of a new paediatric tracheal tube with high volume–low pressure polyurethane cuff. Acta Anaesthesiol Scand. 2005;49:232–237.
16. Koka B.V., Jeon I.S., Andre J.M., et al. Post intubation croup in children. Anesth Analg. 1977;56:501–505.
17. Cohen M.M., Cameron C.B. Should you cancel the operation when a child has an upper respiratory tract infection? Anesth Analg. 1991;72:282–288.
18. Goel S.A., Lim S.L. The intubation depth marker: the confusion of the black line. Paediatr Anaesth. 2003;13:579–583.
19. Ring W.H., Adair J.C., Elwyn R.A. A new pediatric endotracheal tube. Anesth Analg. 1975;54:273–274.
20. Black A.E., Mackersie A.M. Accidental bronchial intubation with RAE tubes. Anaesthesia. 1991;46:42–43.
21. Kohjitani A., Iwase Y., Sugiyama K. Sizes and depths of endotracheal tubes for cleft lip and palate children undergoing primary cheiloplasty and palatoplasty. Paediatr Anaesth. 2008;18:845–851.
22. Sugiyama K., Shimomatsu K., Kohjitani A. Lengths of preformed pediatric orotracheal tubes for children with cleft palate. Pediatr Anesth. 2009;19:640–641.
23. Campo S.L., Denman W.T. The laryngeal mask airway: its role in the difficult airway. Int Anesthesiol Clin. 2000;38:29–45.
24. Walker R.W.M., Ellwood J. The management of difficult intubation in children. Paediatr Anaesth. 2009;19(Suppl 1):77–87.
25. Dierdorf S.F. Education in the use of the laryngeal mask airway. Int Anesthesiol Clin. 1998;36:19–28.
26. Ghai B., Wig J. Comparison of different techniques of laryngeal mask placement. Curr Opin Anaesthesiol. 2009;22:400–404.
27. Yun M.J., Hwang J.W., Park S.H., et al. The 90° rotation technique improves the ease of insertion of the ProSeal laryngeal mask airway in children. Can J Anesth. 2011;58:379–383.
28. Kol I.O., Egilmez H., Kaygusuz K., et al. Open-label, prospective, randomized comparison of propofol and sevoflurane for laryngeal mask anesthesia for magnetic resonance imaging in pediatric patients. Clin Ther. 2008;30:175–181.
29. Park H.J., Lee J.R., Kim C.S., et al. Remifentanil halves the EC50 of propofol for successful insertion of the laryngeal mask airway and laryngeal tube in pediatric patients. Anesth Analg. 2007;105:57–61.
30. Baker P.A., Brunette K.E.J., Byrnes C.A., Thompson J. A prospective randomized trial comparing supraglottic airways for flexible bronchoscopy in children. Pediatr Anesth. 2010;20:831–838.
31. Maurtua M.A., Finnegan P.S., DeBoer G. The use of the CTrach™ laryngeal mask airway in pediatric patients: a retrospective review of 25 cases. Can J Anaesth. 2011;58:409–410.
32. Jagannathan N., Sohn L.E., Mankoo R., et al. Prospective evaluation of the self-pressurized air-Q intubating laryngeal airway in children. Pediatr Anesth. 2011;21:673–680.
33. Jagannathan N., Kozlowski R.J., Sohn L.E., et al. A clinical evaluation of the intubating laryngeal airway as a conduit for tracheal intubation in children. Anesth Analg. 2011;112:176–182.
34. Theiler L.G., Kleine-Brueggeney M., Luepold B., et al. Performance of the pediatric-sized I-Gel compared with the Ambu AuraOnce laryngeal mask in anesthetized and ventilated children. Anesthesiology. 2011;115:102–110.
35. Beylacq L., Bordes M., Semjen F., Cros A M. The I-Gel, a single-use supraglottic airway device with a non-inflatable cuff and an esophageal vent: an observational study in children. Acta Anaesthiol Scand. 2009;53:376–379.
36. VanderWalt J. Oxygen—elixir of life or Trojan horse? Part 1: oxygen and neonatal resuscitation. Paediatr Anaesth. 2006;16:1107–1111.
37. VanderWalt J. Oxygen—elixir of life or Trojan horse? Part 2: oxygen and neonatal anesthesia. Paediatr Anesth. 2006;16:1205–1212.
38. Ayre P. Anaesthesia for intracranial operation: a new technique. Lancet. 1937;1:561–563.
39. Rees G.J. Anaesthesia in the newborn. Br Med J. 1950;1:1419.
40. Rose D.K., Byrick R.J., Froese A.B. Carbon dioxide elimination during spontaneous ventilation with a modified Mapleson D system: studies in a lung model. Can Anaesth Soc J. 1978;25:353–365.
41. Bain J.A., Spoerel W.E. A streamlined anaesthetic system. Can Anaesth Soc J. 1972;19:426–435.
42. Hannallah R., Rosales J.K. A hazard connected with the use of the Bain circuit: a case report. Can Anaesth Soc J. 1974;21:511–513.
43. Pethick S.L. (Letter to the editor). Can Anaesth Soc J. 1975;22:115.
44. Berge J.A., Gramstad L., Bodd E. Safety testing the Bain circuit: a new test adaptor. Eur J Anaesthesiol. 1991;8:309–310.
45. De Armendi A.J., Mayhew J.F., Cure J.A. Another cause of hypercapnia during induction of anesthesia. Pediatr Anes. 2010;20:1055.
46. Hillier S.C., McNiece W.L. Pediatric anesthesia systems and equipment. In: Ehrenwerth J., Eisenkraft J.B., eds. Anesthesia equipment: principles and applications. St. Louis: Mosby; 1993:537–564.
47. Hatch D.J., Miles R., Wagstaff M. An anaesthetic scavenging system for paediatric and adult use. Anaesthesia. 1980;35:496–499.
48. Adriani J., Griggs T. Rebreathing in pediatric anesthesia: recommendations and descriptions of improvements in apparatus. Anesthesiology. 1953;14:337–347.
49. Stephen C.R., Slater H.M. Agents and techniques employed in pediatric anesthesia. Anesth Analg. 1950;20:254.
50. Conterato J.P., Lindahl S.G.E., Meyer D.M., et al. Assessment of spontaneous ventilation in anesthetized children with use of a pediatric circle or a Jackson-Rees system. Anesth Analg. 1989;69:484–490.
51. Coté C.J., Petkau A.J., Ryan J.F., et al. Wasted ventilation measured with eight anesthetic circuits with and without inline humidification. Anesthesiology. 1983;59:442–446.
52. Binda R.E., Cook D.R., Fischer C.G. Advantages of infant ventilators over adapted adult ventilators in pediatrics. Anesth Analg. 1976;55:769–772.
53. Ghani G.A. Fresh gas flow affects minute volume during mechanical ventilation. (letter). Anesth Analg. 1984;63:619.
54. Scheller M.S., Jones B.L., Benumof J.L. The influence of fresh gas flow and I: E ratio on tidal volume and arterial PCO2 in mechanically ventilated surgical patients. J Cardiothorac Anesth. 1989;3:564–567.
55. Aldrete J.A., Castillo R.A., Bradley E.L. Changes in fresh gas flow affect the tidal volume delivered by anesthesia ventilators. Anesth Analg. 1986;65:S4. [abstract]
56. Stayer S., Olutoye O. Anesthesia ventilators: better options for children. Anesthesiol Clin North Am. 2005;23:677–691.
57. Bachiller P.R., McDonough J.M., Feldman J.M. Do new anesthesia ventilators deliver small tidal volumes accurately during volume-controlled ventilation? Anesth Analg. 2008;106:1392–1400.
58. Hjalmarson O., Sandberg K. Abnormal lung function in healthy preterm infants. Am J Respir Crit Care Med. 2002;165:83–87.
59. Mansell A., Bryan C., Levison H. Airway closure in children. J Appl Physiol. 1972;33:711–714.
60. Katayama M., Motoyama E.K. Respiratory mechanics in children under general anesthesia with and without PEEP. Anesthesiology. 1984;61:A514. (abstract)
61. Motoyama E.K., Brinkmeyer S.D., Mutich R.L., et al. Reduced FRC in anesthetized children: effects of low PEEP. Anesthesiology. 1983;57:A418. (abstract)
62. Mukkada T.J., Khathiwada S., Fernandez J., et al. Effect of positive end expiratory pressure on tidal volume. Anesthesiology. 1988;69:A269. (abstract)
63. Epstein M.A.F., Epstein R.A. Airway flow patterns during mechanical ventilation of infants: a mathematical model. IEEE Trans Biomed Eng. 1979;26:299–306.
64. Steward D.J. Pediatric anesthetic techniques and procedures. In Steward D.J., ed.: Manual of pediatric anesthesia, ed 2, New York: Churchill Livingstone, 1985. p 59
65. Spears R.S., Yeh A., Fisher D.M., et al. The “educated hand”: can anesthesiologists assess changes in neonatal pulmonary compliance manually? Anesthesiology. 1991;75:693–696.
66. Schily M., Koumoukelis H., Lerman J., et al. Can pediatric anesthesiologists detect an occluded endotracheal tube in neonates? Anesth Analg. 2001;93:66–70.
67. Picca S.M. Mechanical versus manual ventilation of the lungs of infants in the operating room [letter]. Anesthesiology. 1992;76:479.
68. Steward D.J. (Letter). Anesthesiology. 1992;76:479.
69. Tobias J.D., Burd R.S. Anesthetic management and high frequency oscillatory ventilation. Pediatr Anesth. 2001;11:483–487.
70. Miguet D., Claris O., Lapillonne A., et al. Preoperative stabilization using high-frequency oscillatory ventilation in the management of congenital diaphragmatic hernia. Crit Care Med. 1994;22:S77–S82.
71. Ratzenhofer-Komenda B., Prause G., Offner A., et al. Intraoperative application of high frequency ventilation on thoracic surgery. Acta Anaesth Scand Supp. 1996;109:149–153.
72. Aubin P., Vischoff D., Haig M., et al. Management of an infant with diffuse bullous pulmonary lesions using high-frequency oscillatory ventilation. Can J Anaesth. 1999;46:970–974.
73. Brock-Utne J.G. Humidification in paediatric anaesthesia. Pediatr Anesth. 2000;10:117–119.
74. Ryan J.F., Vacanti F.X. Temperature regulation. In: Ryan J.F., Todres I.D., Coté C.J., eds. A practice of anesthesia for infants and children. Orlando: Grune & Stratton; 1986:19–23.
75. Dalham T. Mucous flow and ciliary activity in the trachea of rats and rats exposed to respiratory irritant gases. Acta Physiol Scand. 1956;123:36.
76. Forbes A.R. Humidification and mucous flow in the intubated trachea. Br J Anaesth. 1973;45:118.
77. Chalon J., Patel C., Ali M., et al. Humidity and the anesthetized patient. Anesthesiology. 1979;50:195–198.
78. Bissonnette B., Sessler D.I., LaFlamme P. Passive and active inspired gas humidification in infants and children. Anesthesiology. 1989;71:350–354.
79. Jones B.R., Ozaki G.T., Benumof J.L., et al. Airway resistance caused by a pediatric heat and moisture exchanger. Anesthesiology. 1988;69:A786. (abstract)
80. Rodee W.D., Banner M.J., Gravenstein N. Variation in imposed work of breathing with heat and moisture exchangers. Anesth Analg. 1991;72:S226. (abstract)
81. Bell G.T., Martin K.M., Beaton S. Work of breathing in anesthetized infants increases when a breathing system filter is used. Pediatr Anesth. 2006;16:939–943.
82. Bissonnette B., Sessler D.I. Passive or active inspired gas humidification increases thermal steady state temperatures in anesthetized infants. Anesth Analg. 1989;69:783–787.
83. Luchetti M., Pigna A., Gentili A., Marraro G. Evaluation of the efficiency of heat and moisture exchangers during paediatric anaesthesia. Pediatr Anesth. 1999;9:39–45.
84. Hunter T., Lerman J., Bissonnette B. The temperature and humidity of inspired gases in infants using a pediatric circle system: effects of high- and low-flow anesthesia. Pediatr Anesth. 2005;15:750–754.
85. Badgwell J.M., McLeod M.E., Lerman J., et al. End-tidal pCO2 measurements sampled at the distal and proximal ends of the endotracheal tube in children. Anesth Analg. 1987;66:959–964.
86. Badgwell J.M., Heavner J.E., May W.S., et al. End-tidal pCO2 monitoring in infants and children ventilated with either a partial-rebreathing or a non-rebreathing circuit. Anesthesiology. 1987;66:405–410.
87. Halpern L., Bissonnette B. A new endotracheal tube connector for sampling end-tidal CO2 in infants. Anesthesiology. 1991;75:A930. (abstract)
88. Hillier S.C., Badgwell J.M., McLeod M.E., et al. Accuracy of end-tidal measurements using a sidestream capnometer in infants and children ventilated with the Sechrist infant ventilator. Can J Anaesth. 1990;37:318–321.
89. Gravenstein N. Capnometry in infants should not be done at lower sampling flow rates. J Clin Monit. 1989;5:63–64.
90. Hillier S.C., Lerman J. Mainstream vs. sidestream capnography in anesthetized infants and children. Anesthesiology. 1989;71:A357. (abstract)
91. Badgwell J.M., Kleinman S.E., Heavner J.E. Respiratory frequency and artifact affect the capnographic baseline in infants. Anesth Analg. 1993;77:708–712.
92. McEvedy B.A.B., McLeod M.E., Mulera M., et al. End-tidal, transcutaneous, and arterial pCO2 measurements in critically ill neonates: a comparative study. Anesthesiology. 1988;69:112–116.
93. Burrows F.A. Physiologic dead space, venous admixture, and the arterial to end-tidal carbon dioxide difference in infants and children undergoing cardiac surgery. Anesthesiology. 1989;70:219–225.
94. American Society of Anesthesiologists [ASA]. Standards for basic anesthetic monitoring, Approved by the ASA House of Delegates on October 21, 1986, as last amended October 20, 2010. http://www.asahq.org/For-Members/Standards-Guidelines-and-Statements.aspx. Accessed 2012 Feb 11 from
95. Pickering T.G., Hall J.E., Appel L.J., et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1. Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161.
96. Roguin A. Scipione Rova-Rocci and the men behind the mercury sphygmomanometer. Int J Clin Pract. 2006;60:73–79.
97. Kimble K.J., Darnall R.A., Jr., Yelderman M., et al. An automated oscillometric technique for estimating mean arterial pressure in critically ill newborns. Anesthesiology. 1981;54:423–425.
98. Okahata S., Kamiya T. Influencing factors on indirect measurement of blood pressure in children. Jpn Circ J. 1987;51:1400–1403.
99. Geddes L.A., Whistler S.J. The error in indirect blood pressure measurement with the incorrect size of cuff. Am Heart J. 1978;96:4–8.
100. Gordon L.S., Johnson P.R., Jr., Penido J.R., et al. Systolic and diastolic blood pressure measurements by transcutaneous Doppler ultrasound in premature infants in critical care nurseries and at closed-heart surgery. Anesth Analg. 1974;53:914–918.
101. Reder R.F., Dimich I., Cohen M.L., et al. Evaluating indirect blood pressure measurement techniques: a comparison of three systems in infants and children. Pediatrics. 1978;62:326–330.
102. Ramsey M. Blood pressure monitoring: automated oscillometric devices. J Clin Monit. 1991;7:56–67.
103. Dellagrammaticas H.D., Wilson A.J. Clinical evaluation of the Dinamap non-invasive blood pressure monitor in pre-term neonates. Clin Phys Physiol Meas. 1979;2:271–276.
104. Friesen R.H., Lichtor J.L. Indirect measurement of blood pressure in neonates and infants utilizing an automatic noninvasive oscillometric monitor. Anesth Analg. 1981;60:742–745.
105. Park M.K., Menard S.M. Normative oscillometric blood pressure values in the first 5 years in an office setting. Am J Dis Child. 1989;143:860–864.
106. Wong S.N., Sung R.Y.Z., Leung L.C.K. Validation of three oscillometric blood pressure devices against auscultatory mercury sphygmomanometer in children. Blood Press Monit. 2006;11:281–291.
107. Chiolero A., Paradis G., Lambert M. Accuracy of oscillometric devices in children and adults. Blood Press. 2010;19:254–259.
108. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. U.S. Department of Health and Human Services. NIH Publication No. 05–5267, revised May 2005.
109. Nielsen P.E., Clausen L.R., Olsen C.A., et al. Blood pressure measurement in childhood and adolescence: international recommendations and normal limits of blood pressure. Scand J Clin Lab Invest Suppl. 1989;192:7–12.
110. Kent A.L., Kecskes Z., Shadbolt B., et al. Blood pressure in the first year of life in healthy infants born at term. Pediatr Nephrol. 2007;22:1743–1749.
111. Nafiu O.O., Voepel-Lewis T., Morris M., et al. How do pediatric anesthesiologists define intraoperative hypotension? Paediatr Anaesth. 2009;19:1048–1053.
112. Greeley W.P., Kates R.A., Bushman G.A., et al. Intraoperative esophageal electrocardiography for dysrhythmia analysis and therapy in pediatric cardiac surgical patients. Anesthesiology. 1986;65:669–672.
113. Weinfurt P.T. Electrocardiographic monitoring: an overview. J Clin Monit. 1990;6:132–138.
114. Macfarlane P.A., Coleman E.N., Pomphrey E.O., et al. Normal limits of the high-fidelity pediatric ECG. J Electrocardiol. 1989;22(Supp l):162–168.
115. Bell C., Rimar S., Barash P. Intraoperative ST-segment changes consistent with myocardial ischemia in the neonate: a report of three cases. Anesthesiology. 1989;71:601–604.
116. Wood L.E., Tullor R.M.R. Kawasaki disease in children. Heart. 2009;95:787–792.
117. McNiece W.L., Krishna G. Kawasaki disease: a disease with anesthetic implications. Anesthesiology. 1983;58:269–271.
118. Coté C.J., Goldstein E.A., Coté M.A., et al. A single-blind study of pulse oximetry in children. Anesthesiology. 1988;68:184–188.
119. Coté C.J., Rolf N., Liu L.M.P., et al. A single-blind study of combined pulse oximetry and capnography in children. Anesthesiology. 1991;74:980–987.
120. Severinghaus J.W., Naifeh K.H. Accuracy of response of six pulse oximeters to profound hypoxia. Anesthesiology. 1987;67:551–558.
121. Deckardt R., Steward D.J. Noninvasive arterial hemoglobin oxygen saturation versus transcutaneous oxygen tension monitoring in the preterm infant. Crit Care Med. 1984;12:935–939.
122. Hay W.W., Jr., Brockway J.M., Eyzaguirre M. Neonatal pulse oximetry: accuracy and reliability. Pediatrics. 1989;83:717–722.
123. Fouzas S., Priftis K.N., Anthracopoulos M.B. Pulse oximetry in pediatric practice. Pediatrics. 2011;128:740–752.
124. Dimich I., Singh P.P., Adell A., et al. Evaluation of oxygen saturation monitoring by pulse oximetry in neonates. Can J Anaesth. 1991;38:985–988.
125. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely premature infants. N Engl J Med. 2010;362:1959–1969.
126. Mittnacht A.J.C. Near infrared spectroscopy in children at high risk of low perfusion. Cur Opinion Anesth. 2010;23:342–347.
127. Murkin J.M., Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–i13.
128. Chakravarti S., Srivastava S., Mittnacht A.J. Near infrared spectroscopy (NIRS) in children. Sem Cardiothorac Vasc Anesth. 2008;12:70–79.
129. Owens G.E., King K., Gurney J.G., Charpie J.R. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol. 2011;32:183–188.
130. Ghanayem N.S., Hoffman G.M., Mussatto K.A., et al. Perioperative monitoring in high-risk infants after stage 1 palliation of univentricular congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:857–863.
131. Grocott H.P. Avoid hypotension and hypoxia: an old anesthetic adage with renewed relevance from cerebral oximetry monitoring. Can J Anesth. 2011;58:697–702.
132. Kasman N., Brady K. Cerebral oximetry for pediatric anesthesia: why do intelligent clinicians disagree? Paediatr Anaesth. 2011;21:473–478.
133. Sessler D.I. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109:318–338.
134. Sessler D.I. Perioperative thermoregulation and heat balance. Ann N Y Acad Sci. 1997;813:757–777.
135. Sessler D.I. Perioperative heat balance. Anesthesiology. 2000;92:578–596.
136. Sessler D.I. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531–543.
137. Kurz A., Sessler D.I., Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–1215.
138. Frank S.M., Fleisher L.A., Breslow M.J., et al. Perioperative maintenance of normothermia reeuces the incidence of morbid cardiac events: a randomized clinical trial. JAMA. 1997;277:1127–1134.
139. Presson R.G., Jr., Bezruczko A.P., Hillier S.C., McNiece W.L. Evaluation of a new fluid warmer effective at low to moderate flow rates. Anesthesiology. 1993;78:974–980.
140. Giesbrecht G.G., Ducharme M.B., McGuire J.P. Comparison of forced-air patient warming systems for perioperative use. Anesthesiology. 1994;80:671–679.
141. Bräuer A., Quintel M. Forced-air warming: technology, physical background and practical aspects. Curr Opin Anaesthesiol. 2009;22:769–774.
142. Bräuer A., Bovenschulte H., Perl T., et al. What determines the efficacy of forced-air warming systems? A manikin evaluation with upper body blankets. Anesth Analg. 2009;108:192–198.
143. Marhofer P., Frickey N. Ultrasonographic guidance in pediatric regional anesthesia. Part 1: theoretical background. Paediatr Anaesth. 2006;16:1008–1018.
144. Rao S., VanHolsbeek L., Musial J.L., et al. A pilot study of comprehensive ultrasound education at the Wayne State University School of Medicine. J Ultrasound Med. 2008;27:745–749.
145. Moore C.L., Copel J.A. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–757.
146. Bodenham A.R. Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96:414–417.
147. Marhofer P., Willschke H., Kettner S. Imaging techniques for regional nerve blockade and vascular cannulation in children. Curr Opin Anaesthesiol. 2006;19:293–300.
148. Verghese S.T., McGill W.A., Patel R.I., et al. Ultrasound-guided internal jugular venous cannulation in infants. Anesthesiology. 1999;91:71–77.
149. Maecken T., Grau T. Ultrasound imaging in vascular access. Crit Care Med. 2007;35(Supp l):S178–S185.
150. Froehlich C.D., Rigby M.R., Rosenberg E.S., et al. Ultrasound-guided central venous catheter placement decreases complications and decreases placement attempts compared with the landmark technique in patients in a pediatric intensive care unit. Crit Care Med. 2009;37:1090–1096.
151. Tsui B.C.H., Suresh S. Ultrasound imaging for regional anesthesia in infants, children and adolescents: a review of current literature and its application in the practice of extremity and trunk blocks. Anesthesiology. 2010;112:473–492.
152. Tsui B.C.H., Suresh S. Ultrasound imaging for regional anesthesia in infants, children, and adolescents. Anesthesiology. 2010;12:719–728.