Ascites, Hemoperitoneum, and Other Peritoneal Fluids
Free fluid in the abdomen can be diagnosed by a number of imaging modalities. Imaging cannot accurately determine the exact composition of intraabdominal fluid, though the cause is sometimes evident from history, examination, other clinical findings, or other imaging findings. For example, fluid within the abdomen may be suspected to be blood when solid abdominal organ injury is discovered or when an abdominal aortic aneurysm is diagnosed. However, a differential diagnosis for abdominal fluid, including simple ascites, infectious ascites, malignant ascites, blood, and urine, should be considered. Often, direct sampling of the fluid is necessary for specific diagnosis. Imaging of peritoneal fluids is demonstrated in Figures 9-129 through 9-133.
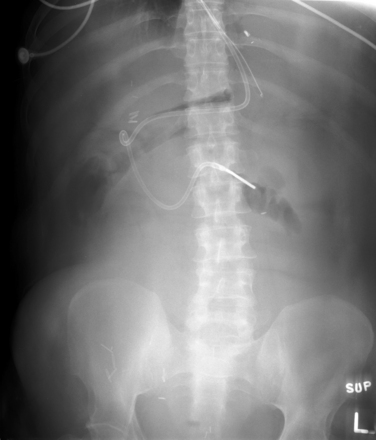
X-ray is a poor modality for diagnosis of ascites. However, ascites can produce x-ray findings suggesting diffuse abdominal fluid. Typical findings include a diffuse hazy appearance, centralization of bowel gas (caused by bowel floating in the peritoneal fluid), and a paucity of bowel gas. These findings are nonspecific and relatively insensitive. Other diagnoses are not excluded by these x-ray findings, and the presence of ascites is not confirmed. Note the paucity of bowel gas and hazy appearance in this x-ray. Bowel obstruction can give a similar appearance as gas is expelled distal to the point of obstruction. This is a supine x-ray, so air–fluid levels would not be expected to be seen. The patient has a feeding tube terminating in the distal duodenum. Compare this x-ray with the ultrasound and CT from the same patient in Figure 9-130 through 9-132.
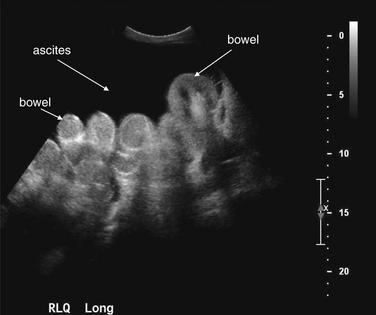
Figure 9-130 Ascites, ultrasound.
Ultrasound is useful for detection of ascites. Simple fluids such as ascites are excellent sound transmission media, reflecting almost no sound waves. As a consequence, they appear quite hypoechoic (black) on ultrasound. This view of the right lower quadrant in the same patient as Figure 9-129 shows loops of bowel surrounded by fluid. During the ultrasound, the bowel loops would be seen to undergo peristalsis and drift back and forth in the ascitic fluid with patient movement. Ultrasound cannot distinguish the composition of the fluid—ascites, liquid blood, liquid bile, urine, and infectious fluids have a similar appearance, with a few exceptions. Blood may coagulate and form septations within the fluid collection. Infectious fluids also frequently form loculated fluid collections that may be recognized on ultrasound, although the exact composition cannot be determined.
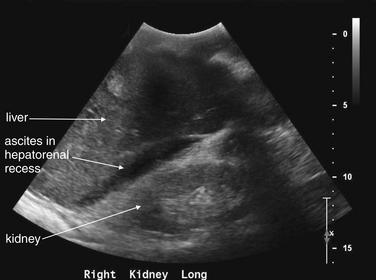
Figure 9-131 Ascites, ultrasound.
Ultrasound is excellent for detection of ascites. In this view of the right upper quadrant from the same patient as Figures 9-129 and 9-130, fluid is visible in Morison’s pouch (hepatorenal recess), separating the liver and right kidney. Ascites has settled in this location because it is the most dependent portion of the abdomen in a supine patient. Were this a trauma patient, this fluid could represent blood and this view would constitute a positive Focused Assessment with Sonography for Trauma (FAST). Ultrasound cannot determine the composition of the fluid directly, because simple fluids such as ascites, urine, and liquid blood look alike.
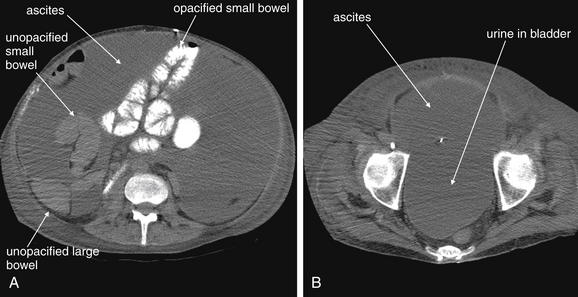
Figure 9-132 Ascites, CT with oral contrast, soft-tissue window.
Free fluid in the abdomen can be recognized on CT without the administration of contrast. Depending on its composition (ascites, blood, bile, intestinal contents, urine, or pus), its density may vary somewhat. Simple ascites and urine have densities similar to water, zero Hounsfield units (HU). Blood varies in density (see Table 9-25).The density of fluid can be measured to estimate the likely composition; however, this can be unreliable. For example, mixing of two types of fluid (e.g., blood and ascites) can occur, or blood of varying hematocrits may have varying densities. In this patient, massive ascites were present after a liver and kidney transplant. No intravenous contrast was given. Oral contrast was given but did not fill all of the small bowel (A). The ascites are a low-density (dark gray) fluid, slightly less dense than unopacified small bowel, which is also fluid-filled. Urine in the bladder is slightly less dense than the ascitic fluid and appears slightly darker as a consequence (B).
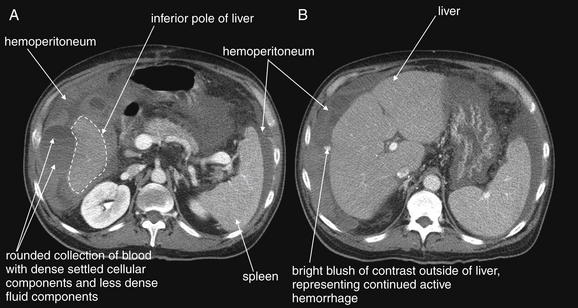
Figure 9-133 Hemoperitoneum, CT with IV contrast, soft-tissue window.
This 55-year-old liver transplant patient presented with hypotension developing during a liver biopsy. Bedside ultrasound showed free fluid, which could have represented existing ascites or hemoperitoneum. Peritoneal aspiration showed frank blood, and the patient was treated with emergency angiographic embolization of a bleeding vessel. A, B, The axial CT images were obtained after that procedure to determine whether hemostasis had been achieved. The images show complex fluid of multiple densities surrounding the liver and spleen, representing blood in various stages of coagulation. A rounded area with a fluid–fluid level is visible, representing blood with a hematocrit level resulting from settling of cellular components. B, Injected contrast material is seen next to the liver, representing continued hemorrhage at the moment of CT.
X-ray of Peritoneal Fluid
X-ray is insensitive and nonspecific for the detection of abdominal fluid. Large amounts of fluid may give the entire abdomen a ground-glass appearance. In the case of large ascites or other fluid, the small bowel may be suspended within the fluid and assume a central location in the abdomen (see Figure 9-129). Overall, ultrasound and CT provide such superior diagnostic capabilities that x-ray should not be ordered for this indication.
Ultrasound of Peritoneal Fluid
With ultrasound, abdominal fluid appears black (anechoic) and usually homogeneous (see Figures 9-130 and 9-131). Blood may be suspected based on a more heterogeneous appearance, with anechoic and echoic components (representing liquid and coagulated blood, respectively). Sometimes fibrinous strands can be seen with ultrasound, adhered to other structures such as bowel. The use of ultrasound to detect traumatic hemoperitoneum is discussed in Chapter 10. Simple ascites, infected ascites as in spontaneous bacterial peritonitis, and fluids such as bile or urine leaking from their respective ductal systems have a similar anechoic appearance. Consequently, when the cause of the fluid is important to diagnosis and treatment, sampling of the fluid should be performed, rather than relying upon imaging criteria. Some recent studies have examined the use of injected vascular contrast agents to allow detection of hemoperitoneum with active bleeding (discussed in more detail in Chapter 11, and briefly in Chapter 10).310
Large quantities of free abdominal fluid may be immediately visible from any location using ultrasound. More subtle free fluid can be difficult to detect. The hepatorenal space is a dependent region that can trap small amounts of fluid and should be inspected, as in the FAST examination (discussed in more detail in Chapter 10). Fluid appears as a dark stripe between the liver and the kidney. The rectovaginal space can be inspected as well, using a midline sagittal position of the ultrasound probe. Fluid appears as a dark stripe separating the posterior wall of the vagina and the anterior wall of the rectum (discussed in more detail in Chapter 12).
Computed Tomography of Peritoneal Fluids
CT provides additional information about the density of peritoneal fluid, as well as offering a panoramic view of other abdominal organs with possibly related pathology. On a soft-tissue window, ascites appears intermediate gray, with a density usually between 0 and +30 Hounsfield units (see Figure 9-132).311 Water has a density of 0 Hounsfield units, so it comes as little surprise that fluids such as urine and simple ascites may be indistinguishable with routine CT. CT techniques to assess for urine leak are discussed in Chapter 12. Dense fluid (~+40 Hounsfield units) detected by CT is more likely to be pathologic, including blood.311-312 Hemoperitoneum has a widely variable CT appearance (Table 9-25; see Figure 9-133).311
Table 9-25 CT Appearance of Hemoperitoneum
| Blood Product | Hounsfield Density |
|---|---|
| Fresh unclotted blood | 30-45 Hounsfield units |
| Clotted blood | 60-100 Hounsfield units |
| Serum (after clotting) | 0-20 Hounsfield units |
| Fresh blood with “hematocrit effect” | Settling of cellular component can create a fluid–fluid level, with the more dependent and higher-density layer being cells |
Adapted from Gayer G, Hertz M, Manor H, et al: Dense ascites: CT manifestations and clinical implications. Emerg Radiol 10:262-267, 2004.
Free abdominal fluid requires no contrast agents for diagnosis with CT. However, contrast agents can sometimes reveal the source of fluid. For example, injected contrast agents may leak into the peritoneal cavity from blood vessels, revealing existing abdominal fluid to be blood. On delayed CT images, injected contrast agents may be concentrated and excreted by the kidneys and can then leak into the peritoneal cavity or retroperitoneum—suggesting existing fluid in these locations to be urine.311 Oral contrast agents may leak through bowel perforations, although in reality this occurs rarely and oral contrast agents have been shown to have rare value in diagnosis of conditions such as traumatic bowel injury. Infectious fluids may have variable densities, from near zero HU (e.g., spontaneous bacterial peritonitis) to near solid-organ density in the case of thick purulent material. Enhancement of ascites on delayed images after administration of IV contrast has been described but is nonspecific, occurring in both malignancies and benign conditions.311 Like ultrasound, CT cannot always discriminate among fluids as different as ascites, blood, bile, and urine, so sampling of fluid may be necessary to determine its composition.
Large amounts of abdominal fluid are usually readily visible using CT, because they are distributed throughout the abdomen. Bowel may float within abdominal fluid and assume a centralized location. Smaller amounts of free fluid are more subtle. Dependent portions of the abdomen and pelvis should be examined for free fluid. Morison’s pouch (the hepatorenal space) and the pouch of Douglas (rectovaginal space) should be inspected. Fluid between loops of bowel in the abdomen and pelvis may have an irregular contour, because it occupies any potential space. The paracolic gutters may also contain free fluid.
Magnetic Resonance Imaging of Peritoneal Fluid
MRI can be used to assess abdominal fluid, although it is rarely indicated in the emergency department because of the diagnostic accuracy of ultrasound and CT. MRI can reveal peritoneal or ascites enhancement, suggesting infectious peritonitis or malignant ascites.313 Nonetheless, sampling of fluid is usually required to determine the exact cause, so the advantage of MRI is limited.
Foreign Body Ingestion or Insertion
Orally ingested, rectally inserted, or living enteric (parasitic) foreign bodies can be detected by a variety of imaging modalities, as shown in Figures 9-134 through 9-139. Foreign bodies from penetrating trauma and medical devices can also be identified.
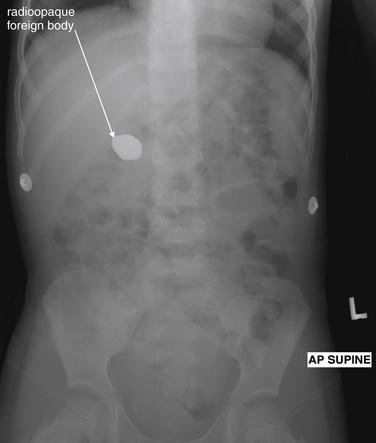
Figure 9-134 Ingested foreign body, x-ray.
This 3-year-old patient swallowed a single magnetic stone measuring 2.5 × 1.5 cm. It was allowed to pass spontaneously and did so without complication. X-rays of the abdomen have little utility in most scenarios. Ingested foreign body is one exception, where x-ray may confirm ingestion, allow characterization of size and shape, and allow tracking of the progress of the foreign body through the gastrointestinal track.
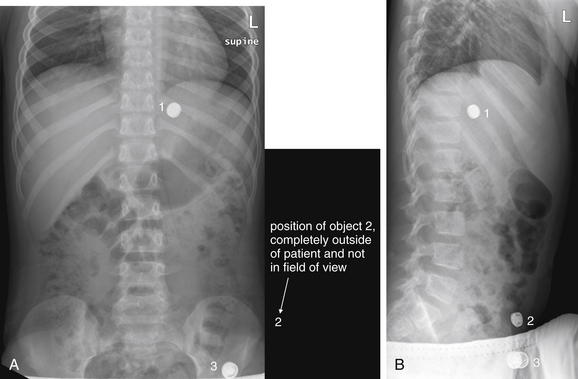
Figure 9-135 Ingested foreign body, x-ray.
This 7-year-old child was “washing” a button battery in her mouth in an effort to fix her electric toy when she reported accidentally swallowing the battery. Parents were uncertain whether any ingestion had occurred. The anterior–posterior (AP) supine view (A) reveals two radiopaque objects; the lateral view (B) reveals three. Did the patient ingest multiple batteries? Only object 1 is projected in a consistent location overlying the abdomen on both views and is intraabdominal. The other two objects are external to the patient. Object 3 on the lateral corresponds to the caudad object on the AP view. Object 2 on the lateral view is so far lateral that it is not visible on the AP view. The patient underwent upper endoscopy but the battery had moved beyond the pylorus and could not be retrieved. Plain x-rays obtained 24 hours later confirmed passage of the battery.
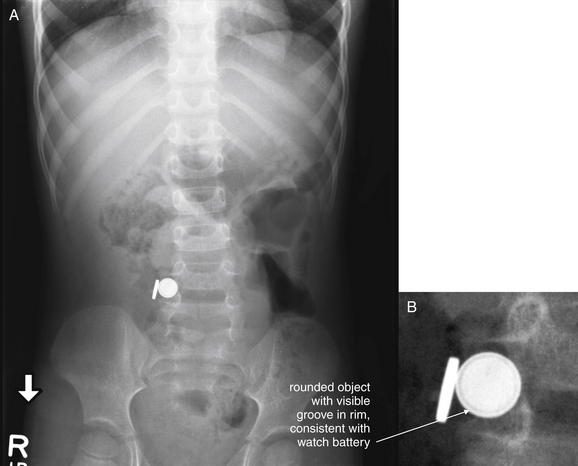
Figure 9-136 Ingested battery, x-ray.
This 3-year-old patient presented with vomiting. He initially was thought to have a viral illness, but he continued to be symptomatic over 23 hours of emergency department observation and developed substantial leukocytosis. This abdominal x-ray revealed the surprise finding of two foreign bodies, one corresponding in size and shape to a lithium watch battery (note the slightly lucent band around the rim). A, Anterior–posterior upright x-ray. B, Close-up from A.
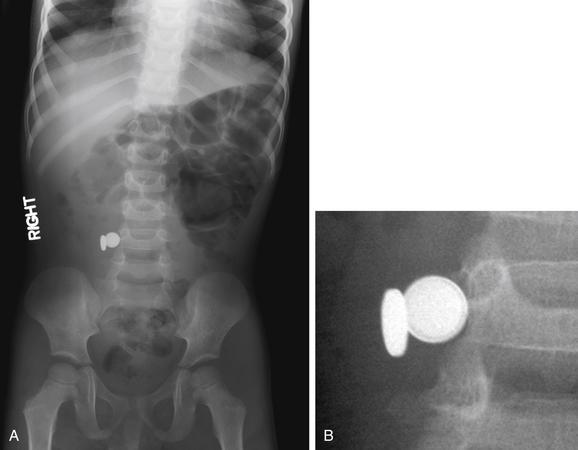
Figure 9-137 Ingested battery, x-ray (obtained 3 days after the x-ray in Figure 9-136).
This 3-year-old patient was found to have ingested two objects, one suspected to be a watch battery. X-rays over the course of 3 days showed no significant change in the location of the ingested foreign bodies—and peculiarly, they remained touching each other. A, Anterior–posterior supine x-ray. B, Close-up from A.
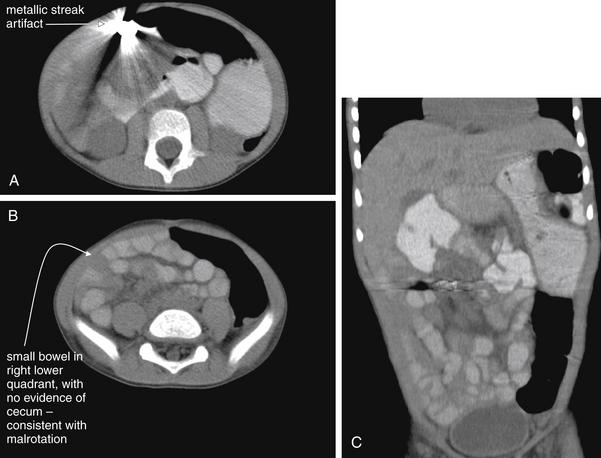
Figure 9-138 Ingested battery, CT with oral contrast.
Same patient as Figures 9-136 and 9-137. CT was performed to further evaluate the location of the ingested foreign bodies but provided little additional detail because of metallic streak artifact. Unexpectedly, it revealed malrotation, with the small bowel in the right abdomen. A, B, Axial images. C, Coronal CT reconstruction clarifying the malrotation. The small bowel is seen occupying predominantly the right abdomen, whereas the large bowel occupies the left. Laparotomy was performed to repair the malrotation and retrieve the foreign bodies, which were found to be a battery and a magnet, magnetically attracted to one another in two adjacent loops of small bowel.
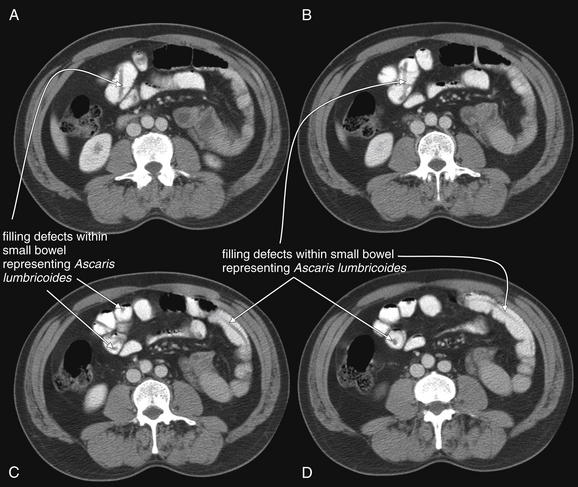
Figure 9-139 Intestinal foreign body—Ascaris lumbricoides.
Oral contrast can assist in detection of gastrointestinal (GI) foreign bodies. Traditionally, an upper gastrointestinal series would be performed to identify an intraluminal foreign body, which appears as a filling defect in the column of enteral contrast. In this case, the patient underwent abdominal CT for suspected appendicitis. The diagnosis was instead infection with the GI macroparasite A. lumbricoides, a giant roundworm. These worms appear as tubular filling defects within the small bowel when seen in long section and as circular filling defects when seen in short-axis cross section. Without oral contrast, these worms would have a similar density to surrounding intestinal contents and would not be identified. A. lumbricoides infection is the one of the most common parasitic infections worldwide, with an estimated prevalence of 1.25 billion cases. In the United States, the infection is rarer but may have a prevalence as high as 4 million cases, concentrated in the southern states. The parasite is endemic in tropical regions worldwide and should be considered as a cause of abdominal pain in immigrants and returning travelers. Ascaris infections can be complicated by small-bowel obstruction, appendicitis, and biliary ductal obstruction.
X-ray of Abdominal Foreign Bodies
Metal, stone, and glass are usually visible on plain x-ray—in contrast to a common misconception that only leaded glass is visible on x-ray (see Figures 9-134 through 9-137).314 Ingested bone may be visible on x-ray, depending on the density. Small or poorly calcified bones such as fish bones are often not visible on x-ray.315 X-ray can also reveal complications such as perforation or obstruction, with the caveat that the sensitivity of x-ray is limited (as discussed earlier in this chapter). Lower-density materials including plastic, meat, and wood are often invisible on x-ray. One small study found that ingested cocaine packages were invisible on x-ray in 40% of cases.316
Two orthogonal x-ray views are required for localization of a foreign body. An object located posterior to the patient, for example, in a skin fold or trapped in clothing, can appear to be within the patient on a single frontal projection. A lateral view can confirm the location within or outside of the patient (see Figure 9-135).
Contrast Studies of Gastrointestinal Foreign Bodies
Fluoroscopic GI studies using enteral contrast agents (e.g., Gastrografin swallow esophagography, small-bowel series, or barium enema) can detect low-density intraluminal foreign bodies not seen with standard x-ray. The contrast agent surrounds the foreign body, creating a recognizable void or filling defect.
Computed Tomography of Abdominal Foreign Bodies
When an ingested or intraabdominal foreign body is not visible on x-ray, CT offers an alternative that can localize the object three-dimensionally. CT can reveal foreign bodies with a range of densities, including plastics and organic food material. However, low-density material may be impossible to distinguish from normally occurring food materials in the digestive tract. Enteral contrast agents can outline a foreign body, making it more evident. Macroscopic intestinal parasites can be detected by this same mechanism (see Figure 9-139).317 Although CT easily detects dense materials such as metal, stone, and glass, these may create metallic streak artifact that can make precise identification of the object and exact determination of its location impossible (see Figure 9-138). Therefore x-ray can actually be superior to CT for identification and localization of dense objects.
Ultrasound of Abdominal Foreign Bodies
Ultrasound can detect some ingested objects in the small bowel; in one study, 60% of ingested cocaine packages were identified with ultrasound.316 However, ultrasound is often nondiagnostic because of the presence of bowel gas that interrupts and scatters the ultrasound beam.
Adrenal Gland Pathology
Adrenal gland pathology is rarely the indication for abdominal CT in the emergency department, but CT can provide information about pathologic processes affecting these glands. The normal adrenal glands are thin, lambda-shaped structures located in the retroperitoneum at the cephalad pole of each kidney. Adrenal masses or hemorrhage can distort or obscure these structures. Figures 9-140 through 9-143 demonstrate normal adrenal glands and adrenal pathology.
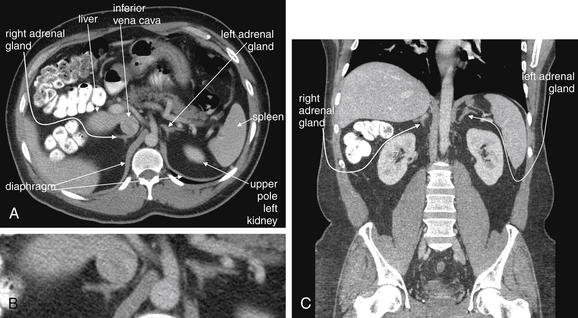
Figure 9-140 Normal adrenal glands, CT with IV and oral contrast.
The normal adrenal glands are located superior to the kidneys, tucked into the recess below the diaphragm on each side of the midline. They commonly have a thin lambda (λ) shape in both axial and coronal cross section. Depending on the amount of retroperitoneal fat, they may be separated from the adjacent kidney by several centimeters in both an anteroposterior and a cephalad–caudad direction. In thin patients, they may be immediately adjacent to the kidney, liver (right), spleen (left), and other retroperitoneal structures, such as the inferior vena cava. We look at several examples of normal adrenal glands to demonstrate the range of their normal size, shape, and location. A (B, close-up), In this patient, the adrenal glands are separated from adjacent solid organs by a moderate amount of retroperitoneal fat. The right adrenal gland abuts the inferior vena cava. C, In a coronal view from the same patient, the right adrenal gland lies close to the liver.
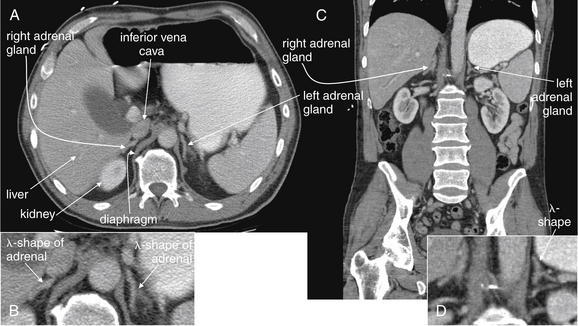
Figure 9-141 Normal adrenal glands, CT with IV and oral contrast.
This patient is thinner than the previous patient and has less retroperitoneal fat. The adrenal glands are even more closely abutting adjacent structures, though a thin layer of fat separates them from the surrounding organs. Without this layer of fat, they would be virtually indistinguishable because they share the tissue density of liver, contrasted inferior vena cava, and spleen. Note the lambda (λ) shape of the adrenals. Realize that this cross-sectional shape may vary from patient to patient and slice to slice. Some cross sections may appear more V shaped. Look for enlargement beyond this thin wispy shape, which may signal adrenal mass or hemorrhage. A, Axial view. B, Close-up from A. C, Coronal image. D, Close-up from C.
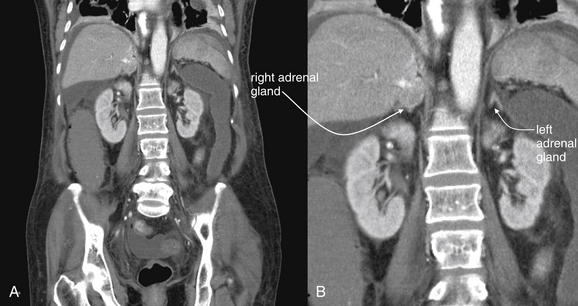
Figure 9-142 Adrenal adenoma, CT with IV contrast.
This CT shows a rounded mass in the expected position of the right adrenal gland. At first glance, it may look like a portion of the liver, but note the normal thin lambda-shaped (λ) left adrenal. Scrolling through the entire CT, you would readily note the absence of a normal right adrenal gland. Technically, this adrenal mass cannot be classified as an adenoma based on the CT, although this is the most likely diagnosis. A, Coronal image. B, Close-up.
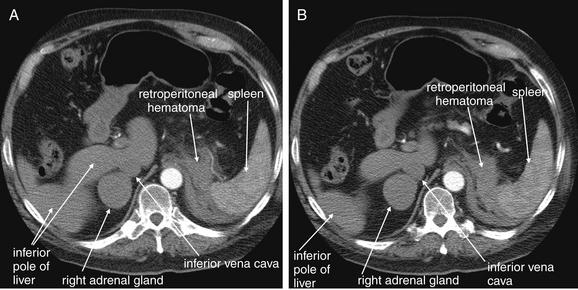
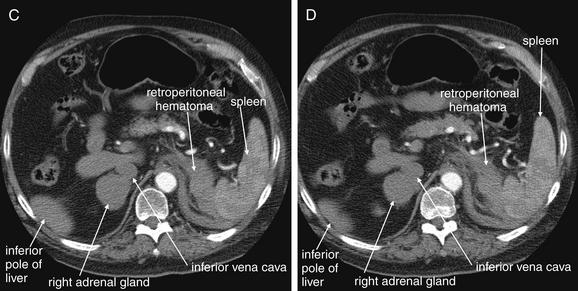
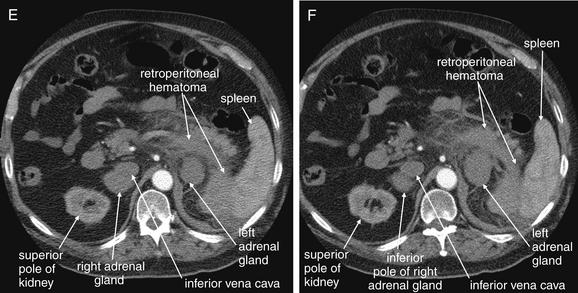
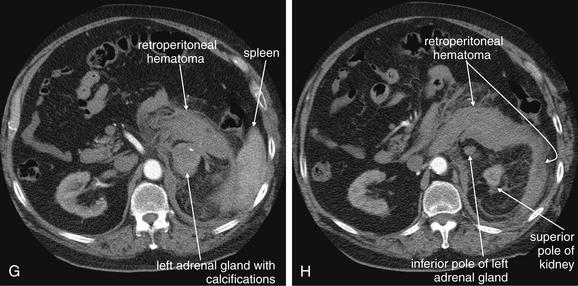
Figure 9-143 Adrenal hemorrhage, CT with IV contrast.
This 72-year-old male presented with acute flank pain, diaphoresis, and hypotension. He was being treated with coumadin for deep venous thrombosis. The leading diagnosis was catastrophic aortic pathology—rupture or dissection. CT without contrast, followed by IV-contrasted CT, was performed. A through H, Sequential axial images from CT with IV contrast. Noncontrast CT showed a normal-caliber aorta with significant retroperitoneal hemorrhage present, extending from the level of the spleen to the iliac bifurcation. The apparent source was the left adrenal gland. CT with IV contrast confirms no aortic dissection and no active extravasation of IV contrast. The patient was noted to have bilaterally enlarged adrenal glands on CT, and his cortisol level was subsequently found to be low. He remained hypotensive disproportionate to his apparent blood loss, even after transfusion. His blood pressure improved with steroid therapy for apparent Addison’s disease (adrenal insufficiency) resulting from adrenal hemorrhage. This case raises several interesting points. First, given the concern for aortic catastrophe, clinical management, including volume resuscitation and surgical consultation, takes precedence over imaging. Figure 9-143, cont’dBedside imaging with ultrasound to evaluate for aortic aneurysm or free peritoneal fluid is a wise initial option. Oral contrast plays no role in the diagnosis of aortic pathology and would result in unacceptable delay in this case. Aortic rupture can be diagnosed without IV contrast; thus noncontrast CT should be performed immediately if a question exists about the patient’s renal function or allergies to contrast. IV contrast should be administered to evaluate for aortic dissection or active extravasation of contrast if the noncontrast CT does not provide all desired diagnostic information. In this patient, noncontrast CT demonstrated a large retroperitoneal hematoma, ruled out aortic aneurysm, and appeared to identify the source of hemorrhage as the left adrenal gland. IV contrast confirmed the absence of aortic dissection and demonstrated no active bleeding, an important finding for management, because active retroperitoneal bleeding might require angiographic embolization for hemostasis. How can the adrenal glands be recognized on CT? Emergency physicians may pay little attention to the adrenal glands on CT—they are a rare source of emergency pathology, and the normal glands are inconspicuous. In this case, knowledge of normal anatomy and process of elimination guides the diagnosis. The normal location of the adrenal glands is just cephalad and anterior to the kidneys on each side. The adrenals are often separated slightly from the kidneys by retroperitoneal fat. Look closely at the series of images. A, Cephalad and anterior to the right kidney, a rounded structure is seen, right of the spine and abutting the inferior vena cava. Because the CT was obtained during an arterial phase of injection, to maximize contrast enhancement of the aorta, the vena cava does not contain contrast, and the adjacent adrenal gland is easily mistaken for the inferior vena cava. The right adrenal gland might also initially be mistaken for the superior pole of the right kidney, but the kidney comes into view as a separate structure several slices caudad (C). Reviewing these images, you’ll realize that the normal lambda (λ)-shaped adrenal glands cannot be found—instead, rounded structures have replaced them, suggesting adrenal mass or internal hemorrhage within the adrenal glands.
Esophageal Abnormalities
Esophageal abnormalities are often assessed by direct visualization with upper endoscopy. However, in some cases, diagnostic imaging can provide information not readily available from endoscopy or when endoscopy is contraindicated.
X-ray of Esophageal Abnormalities
Plain x-ray provides limited information about the esophagus. Radiodense esophageal foreign bodies may be seen, as described earlier. Frank perforation of the esophagus may be evident from air within the mediastinum (pneumomediastinum), discussed in more detail in Chapter 5.318 Low-density esophageal foreign bodies such as impacted bread or meat are not usually visible on x-ray, though an air–fluid level in the esophagus may be seen in some cases.319
Barium Versus Gastrografin Swallow Studies for Esophageal Abnormalities
When esophageal injury is suspected, as in the case of recurrent vomiting or hematemesis, swallowed foreign body (see Chapter 4, Figures 4-35 and 4-36), or penetrating chest trauma, an esophagram can demonstrate partial- or full-thickness injury. A radiodense contrast material is ingested, and residual contrast retained within mucosal tears or extravasating into the mediastinum can be seen. Gastrografin is recommended as the initial contrast agent, because it is relatively nontoxic to the mediastinum when a full-thickness esophageal injury is present. However, Gastrografin is low in viscosity, resulting in less material being retained in small esophageal defects. If Gastrografin study is normal but injury is still suspected, higher-viscosity barium sulfate can be used to achieve higher sensitivity. Barium is more toxic than Gastrografin when full-thickness tears are present, so it is not the suggested first-line contrast agent.320-321 Ingested contrast agents can make subsequent endoscopy more difficult.322
Computed Tomography of Esophageal Abnormalities
CT is not commonly used for assessment of esophageal injury in the emergency department. However, CT can demonstrate abnormalities including thickening of the esophagus at sites of injury, mediastinal air and fluid in cases of esophageal perforation, and foreign body.322-323 Oral contrast agents usually are not retained well within the esophagus in the supine position used for CT, unless complete esophageal obstruction is present—but they may leak from the esophagus in cases of perforation. In a small prospective study comparing swallow fluoroscopy, CT with oral contrast, and endoscopy, CT and swallow fluoroscopy were comparable, detecting 88% of leaks.324
Gastrointestinal Hemorrhage
Imaging plays little role in the diagnosis of upper GI hemorrhage, because lesions within the stomach and proximal small bowel lie within reach of an endoscope for both diagnosis and treatment. Bleeding from the middle and lower intestine presents a greater endoscopic challenge, particularly in emergency situations without preceding bowel cleansing. Three imaging modalities present diagnostic solutions: nuclear scintigraphy, mesenteric angiography, and CT mesenteric angiography.
Nuclear Scintigraphy for Gastrointestinal Hemorrhage
Nuclear scintigraphy allows detection of relatively slow GI bleeding. The technique can identify bleeding anywhere within the GI tract, although its primary use is for suspected lower GI bleeding. In this test, red blood cells are harvested from the patient, tagged with a radionuclide (technetium-99m), and reinfused. Alternatively, a technetium-99m sulfur colloid may be injected.325 A gamma camera positioned outside of the patient detects scintigraphic emission. A series of images are taken over time, demonstrating accumulation of the radionuclide. A normal image shows counts concentrated over the aorta and iliac arteries, with a low background resulting from blood flow in other organs. When blood carrying the radionuclide tracer leaks into the intestinal lumen because of GI hemorrhage, the gamma camera records abnormally high signal outside of normal blood vessels and organs. The intensity of the counts increases over subsequent images if bleeding is ongoing. The location of the signal may appear to migrate distally over the time course of the images, caused by continued peristalsis (Figures 9-144 and 9-145).
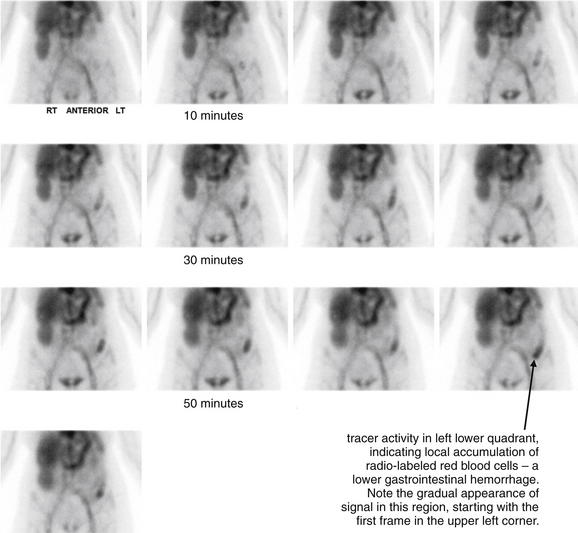
Figure 9-144 Gastrointestinal hemorrhage, tagged red blood cell study.
This 67-year-old female presented with several episodes of bright red blood per rectum at home. In the emergency department, she had another significant episode of rectal bleeding. In addition, over 10 hours of observation, her hematocrit fell from 37 to 26. Tagged red blood cell study was performed, with images generated at 5-minute intervals. Tracer activity is visible in the left lower quadrant within 10 minutes and becomes progressively more evident in the region of the descending colon. The patient subsequently underwent mesenteric angiography that was negative for active bleeding. Why the disparate test results? The patient undoubtedly was actively bleeding during the tagged red blood cell study. However, by the time of the angiogram, bleeding had spontaneously stopped. Mesenteric angiography is performed over a briefer period—extravasation of injected contrast (indicating active bleeding) is only witnessed if bleeding is occurring during the moment after contrast injection, when the fluoroscope is turned on. If bleeding is intermittent, angiography can fail to detect even moderate bleeding.
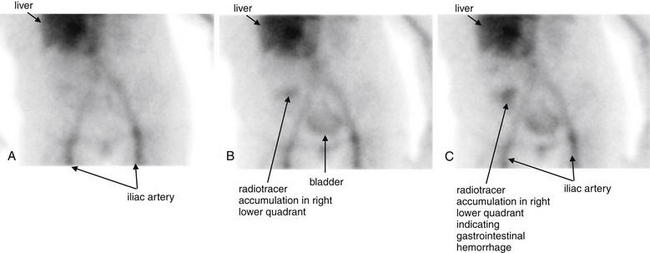
Figure 9-145 Gastrointestinal hemorrhage, tagged red blood cell study.
This 58-year-old presented with bright red blood per rectum. A technetium-99m tagged red blood cell study was performed. A, Acquired 10 minutes after injection of the labeled red cells. B, Acquired 45 minutes after injection. C, Acquired 55 minutes after injection. A focus of radiotracer activity is seen gradually accumulating in the right lower quadrant, consistent with hemorrhage within the cecum. Normal tracer is also seen in the region of the liver (because this is a vascular organ), in the iliac arteries, and in the urinary bladder. Compare this with the angiogram in Figure 9-146.
Nuclear scintigraphy is performed over approximately 1 hour, with advantages and disadvantages. An advantage of the technique is relatively high sensitivity for even slow rates of bleeding, as low as 0.1 to 0.4 mL per minute.326 Intermittent bleeding may also be detected by this technique. Paradoxically, this high sensitivity can also be a disadvantage, because slow bleeding detected by nuclear scintigraphy may have ceased spontaneously by the time an intervention is undertaken to stop bleeding. In one study, almost half of patients with positive scintigraphy had no bleeding detected by angiography.327 Nontherapeutic invasive procedures may result, although the rate of positive angiography is more than twice as high in patients who have been screened for active bleeding first using scintigraphy.327 Another disadvantage of scintigraphy is the need to remove a potentially unstable patient from the emergency department for a relatively extended period. However, positive scintigraphy can accurately predict the site of bleeding, which is advantageous in patients with continued bleeding requiring surgical resection.328
Mesenteric Angiography for Gastrointestinal Hemorrhage
Mesenteric angiography can be performed to assess the source of GI bleeding and to treat ongoing hemorrhage. In this technique, the major mesenteric arteries are sequentially cannulated using a catheter introduced through the femoral artery. Contrast is injected into each mesenteric branch, and extravasation of contrast is observed if active bleeding is present (Figure 9-146). Small branches of mesenteric arteries can be cannulated and embolized to terminate bleeding (discussed in more detail in Chapter 16). Advantages of this technique include a therapeutic ability, which is not seen with nuclear scintigraphy. This is an invasive procedure with risks including arterial injury and contrast nephropathy or allergy. The technique requires the services of an interventional radiologist, not routinely available at all hours even in large medical centers. Mesenteric angiography is less sensitive than nuclear scintigraphy, because bleeding must be ongoing at the time of contrast injection and imaging to be detected. Overall, the sensitivity of digital subtraction angiography for active bleeding is approximately 60%, with 100% specificity.329 The disparity between the relatively high sensitivity of nuclear scintigraphy and the lower sensitivity of mesenteric angiography means that many positive nuclear scintigraphy studies (showing bleeding) are followed by negative and nontherapeutic mesenteric angiograms, because bleeding may have stopped spontaneously by the time angiography is performed.
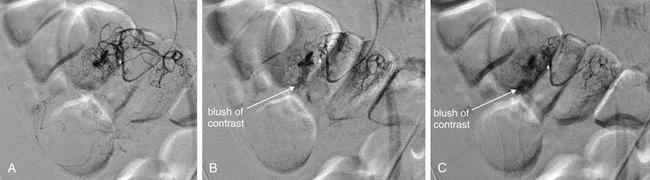
Figure 9-146 Gastrointestinal (GI) hemorrhage imaged with mesenteric angiography.
A, B, C, Close-up fluoroscopic images of the cecum during angiography, separated in time by a few seconds. This patient presented with rectal bleeding and underwent a tagged red blood cell study showing radiotracer accumulation in the right lower quadrant (Figure 9-145). Mesenteric angiography was performed and shows active extravasation of contrast in the region of the cecum arising from a branch of the ileocolic artery. This was selectively embolized with a combination of particles and coils. Selective arteriography following embolization showed no further active extravasation of contrast. These images show sequential frames from a digital subtraction angiogram, cropped to show just the region of the cecum. A faint blush of contrast is seen developing over a few seconds, representing active GI hemorrhage.
Computed Tomography Angiography for Gastrointestinal Hemorrhage
CTA for detection of GI bleeding is a relatively newly described technique.330-339 CTA relies on similar principles to those of standard catheter angiography. If bleeding is actively occurring at the time of CT, injected contrast will be seen in the intestinal lumen. No oral contrast is needed for this technique; in fact, it should not be administered, because positive enteral contrast disguises extravasating injected contrast. Advantages of the technique include speed and availability, because it uses standard CT equipment widely available in many hospitals. In some cases, not only the location but also the cause of the hemorrhage can be determined.336 A potentially unstable patient must be removed only briefly from the emergency department for the procedure. A disadvantage of the technique is that intermittent or slow bleeding may not be detected, because bleeding must be ongoing during the brief duration of the CT (seconds) to be detected. However, intermittent bleeding not detected by CT may not require angiographic embolization. Another disadvantage is that the patient is exposed to iodinated contrast with its attendant risks. Because the technique has no therapeutic option, a positive CT must be followed by a standard mesenteric angiogram for treatment, requiring a second exposure to iodinated contrast. Despite this, some authors have proposed that patients be screened with CT before undergoing invasive angiography to increase the therapeutic yield of angiography.
Abdominal Trauma
X-rays play relatively little role in evaluation of abdominal and retroperitoneal blunt mechanism injuries, although chest and pelvic x-rays are routinely obtained for evaluation of associated thoracic and pelvic injuries, discussed in detail in ∗∗∗∗Chapters 6 and 13, respectively. Abdominal and chest x-rays are often used in the evaluation of penetrating abdominal trauma. Ultrasound plays a useful bedside role in blunt trauma to identify hemoperitoneum or hemopericardium in unstable patients. CT scan is today the key imaging modality for patients with blunt abdominal trauma not requiring immediate operative therapy. It plays a growing role in patients with penetrating abdominal and flank injuries, allowing some to be managed nonoperatively. Abdominal trauma imaging is discussed in detail in Chapter 10.
Summary
Abdominal imaging remains an area of intense utilization and research. X-ray is of little value for most emergency abdominal conditions. Ultrasound provides important clinical information about many disorders, particularly right upper quadrant and pelvic pathology, without radiation exposure. CT provides detailed information about a multitude of disorders, but controversy remains about the necessity for contrast agents and the clinical benefit to patients from the information obtained.
1. Pitts S.R., Niska R.W., Xu J., Burt C.W. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl Health Stat Report. 2008:1-38.
2. Broder J., Warshauer D.M. Increasing utilization of computed tomography in the adult emergency department, 2000-2005. Emerg Radiol. 2006;13:25-30.
2a. American College of Radiology: ACR Practice Guideline for the Performance of Abdominal Radiography. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/dx/gastro/abdominal_radiography.aspx. Accessed March 2, 2011.
3. Megibow A.J., Babb J.S., Hecht E.M., et al. Evaluation of bowel distention and bowel wall appearance by using neutral oral contrast agent for multi-detector row CT. Radiology. 2006;238:87-95.
4. Hamlin D.J., Burgener F.A. Positive and negative contrast agents in CT evaluation of the abdomen and pelvis. J Comput Tomogr. 1981;5:82-90.
5. Holmes J.F., Offerman S.R., Chang C.H., et al. Performance of helical computed tomography without oral contrast for the detection of gastrointestinal injuries. Ann Emerg Med. 2004;43:120-128.
6. Stafford R.E., McGonigal M.D., Weigelt J.A., Johnson T.J. Oral contrast solution and computed tomography for blunt abdominal trauma: A randomized study. Arch Surg. 1999;134:622-626. discussion 626-627
6a. American College of Radiology: ACR appropriateness criteria: Suspected Small-Bowel Obstruction. 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/SuspectedSmallBowelObstructionDoc15.aspx.
7. Rao P.M., Rhea J.T., Novelline R.A., et al. Helical CT with only colonic contrast material for diagnosing diverticulitis: Prospective evaluation of 150 patients. AJR Am J Roentgenol. 1998;170:1445-1449.
8. RT Image. CT data sheet index: 2010. (Accessed at http://www.rt-image.com/datasheet/index.cfm?chartID=63&action=1.)
9. Personal communication with Department of Radiology, North Carolina State University College of Veterinary Medicine. 2008.
10. Behrendt F.F., Bruners P., Keil S., et al. Impact of different vein catheter sizes for mechanical power injection in CT: In vitro evaluation with use of a circulation phantom. Cardiovasc Intervent Radiol. 2009;32:25-31.
11. Morcos S.K., Thomsen H.S. Adverse reactions to iodinated contrast media. Eur Radiol. 2001;11:1267-1275.
12. American College of Radiology: ACR Manual on Contrast Media, version 7. 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual/FullManual.aspx.
13. McCullough P.A., Adam A., Becker C.R., et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98:5K-13K.
14. Band R.A., Gaieski D.F., Mills A.M., et al. Discordance between serum creatinine and creatinine clearance for identification of ED patients with abdominal pain at risk for contrast-induced nephropathy. Am J Emerg Med. 2007;25:268-272.
15. Trivedi H.S., Moore H., Nasr S., et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29-C34.
16. Krasuski R.A., Beard B.M., Geoghagan J.D., Thompson C.M., Guidera S.A. Optimal timing of hydration to erase contrast-associated nephropathy: The OTHER CAN study. J Invasive Cardiol. 2003;15:699-702.
17. Solomon R., Werner C., Mann D., et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416-1420.
18. Merten G.J., Burgess W.P., Gray L.V., et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: A randomized controlled trial. JAMA. 2004;291:2328-2334.
19. Brar S.S., Shen A.Y., Jorgensen M.B., et al. Sodium bicarbonate vs. sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: A randomized trial. JAMA. 2008;300:1038-1046.
20. Kay J., Chow W.H., Chan T.M., et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: A randomized controlled trial. JAMA. 2003;289:553-558.
21. Baker C.S., Wragg A., Kumar S., et al. A rapid protocol for the prevention of contrast-induced renal dysfunction: The RAPPID study. J Am Coll Cardiol. 2003;41:2114-2118.
22. Orlandini F., Boini S., Iochum-Duchamps S., et al. Assessment of the use of a saline chaser to reduce the volume of contrast medium in abdominal CT. AJR Am J Roentgenol. 2006;187:511-515.
23. Dorio P.J., Lee F.T.Jr., Henseler K.P., et al. Using a saline chaser to decrease contrast media in abdominal CT. AJR Am J Roentgenol. 2003;180:929-934.
24. Takao H., Nojo T., Ohtomo K. Use of a saline chaser in abdominal computed tomography: A systematic review. Clin Imaging. 2009;33:261-266.
25. Atwell T.D., Lteif A.N., Brown D.L., et al. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol. 2008;191:268-271.
26. Johnson T.R., Krauss B., Sedlmair M., et al. Material differentiation by dual energy CT: Initial experience. Eur Radiol. 2007;17:1510-1517.
27. Garra G., Singer A.J., Bamber D., et al. Pretreatment of patients requiring oral contrast abdominal computed tomography with antiemetics: A randomized controlled trial of efficacy. Ann Emerg Med. 2009;53:528-533.
28. Basak S., Nazarian L.N., Wechsler R.J., et al. Is unenhanced CT sufficient for evaluation of acute abdominal pain? Clin Imaging. 2002;26:405-407.
29. Tack D., Bohy P., Perlot I., et al. Suspected acute colon diverticulitis: Imaging with low-dose unenhanced multi-detector row CT. Radiology. 2005;237:189-196.
30. Lee S.Y., Coughlin B., Wolfe J.M., et al. Prospective comparison of helical CT of the abdomen and pelvis without and with oral contrast in assessing acute abdominal pain in adult emergency department patients. Emerg Radiol. 2006;12:150-157.
31. Stuhlfaut J.W., Soto J.A., Lucey B.C., et al. Blunt abdominal trauma: Performance of CT without oral contrast material. Radiology. 2004;233:689-694.
32. Anderson B.A., Salem L., Flum D.R. A systematic review of whether oral contrast is necessary for the computed tomography diagnosis of appendicitis in adults. Am J Surg. 2005;190:474-478.
33. Spitzer A.L., Thoeni R.F., Barcia A.M., et al. Early nonenhanced abdominal computed tomography can predict mortality in severe acute pancreatitis. J Gastrointest Surg. 2005;9:928-933.
34. Atri M., McGregor C., McInnes M., et al. Multidetector helical CT in the evaluation of acute small bowel obstruction: Comparison of non-enhanced (no oral, rectal or IV contrast) and IV enhanced CT. Eur J Radiol. 2009;71:135-140.
35. Choi H.S., Choi B.W., Choe K.O., et al. Pitfalls, artifacts, and remedies in multi-detector row CT coronary angiography. Radiographics. 2004;24:787-800.
36. Feldman M.K., Katyal S., Blackwood M.S. US artifacts. Radiographics. 2009;29:1179-1189.
37. Levine M.S., Scheiner J.D., Rubesin S.E., et al. Diagnosis of pneumoperitoneum on supine abdominal radiographs. AJR Am J Roentgenol. 1991;156:731-735.
38. Markowitz S.K., Ziter F.M.Jr. The lateral chest film and pneumoperitoneum. Ann Emerg Med. 1986;15:425-427.
39. Woodring J.H., Heiser M.J. Detection of pneumoperitoneum on chest radiographs: Comparison of upright lateral and posteroanterior projections. AJR Am J Roentgenol. 1995;165:45-47.
40. Miller R.E., Becker G.J., Slabaugh R.D. Detection of pneumoperitoneum: Optimum body position and respiratory phase. AJR Am J Roentgenol. 1980;135:487-490.
41. Miller R.E., Nelson S.W. The roentgenologic demonstration of tiny amounts of free intraperitoneal gas: Experimental and clinical studies. Am J Roentgenol Radium Ther Nucl Med. 1971;112:574-585.
42. Maull K.I., Reath D.B. Pneumogastrography in the diagnosis of perforated peptic ulcer. Am J Surg. 1984;148:340-345.
43. Lee C.W., Yip A.W., Lam K.H. Pneumogastrogram in the diagnosis of perforated peptic ulcer. Aust N Z J Surg. 1993;63:459-461.
44. Lo B.M. Radiographic look-alikes: Distinguishing between pneumoperitoneum and pseudopneumoperitoneum. J Emerg Med. 2010;38:36-39.
45. Earls J.P., Dachman A.H., Colon E., et al. Prevalence and duration of postoperative pneumoperitoneum: Sensitivity of CT vs. left lateral decubitus radiography. AJR Am J Roentgenol. 1993;161:781-785.
46. Pinto A., Scaglione M., Giovine S., et al. Comparison between the site of multislice CT signs of gastrointestinal perforation and the site of perforation detected at surgery in forty perforated patients. Radiol Med. 2004;108:208-217.
47. Stapakis J.C., Thickman D. Diagnosis of pneumoperitoneum: Abdominal CT vs. upright chest film. J Comput Assist Tomogr. 1992;16:713-716.
48. Chen S.C., Wang H.P., Chen W.J., et al. Selective use of ultrasonography for the detection of pneumoperitoneum. Acad Emerg Med. 2002;9:643-645.
49. Chen S.C., Yen Z.S., Wang H.P., et al. Ultrasonography is superior to plain radiography in the diagnosis of pneumoperitoneum. Br J Surg. 2002;89:351-354.
50. Braccini G., Lamacchia M., Boraschi P., et al. Ultrasound versus plain film in the detection of pneumoperitoneum. Abdom Imaging. 1996;21:404-412.
51. Moriwaki Y., Sugiyama M., Toyoda H., et al. Ultrasonography for the diagnosis of intraperitoneal free air in chest–abdominal–pelvic blunt trauma and critical acute abdominal pain. Arch Surg. 2009;144:137-141. discussion 42
52. Miller R.E., Becker G.J., Slabaugh R.D. Nonsurgical pneumoperitoneum. Gastrointest Radiol. 1981;6:73-74.
53. Cho K.C., Baker S.R. Extraluminal air: Diagnosis and significance. Radiol Clin North Am. 1994;32:829-844.
54. Millitz K., Moote D.J., Sparrow R.K., et al. Pneumoperitoneum after laparoscopic cholecystectomy: Frequency and duration as seen on upright chest radiographs. AJR Am J Roentgenol. 1994;163:837-839.
55. Nielsen K.T., Lund L., Larsen L.P., Knudsen P. Duration of postoperative pneumoperitoneum. Eur J Surg. 1997;163:501-503.
56. Ba-Ssalamah A., Prokop M., Uffmann M., et al. Dedicated multidetector CT of the stomach: Spectrum of diseases. Radiographics. 2003;23:625-644.
57. van der Schouw Y.T., van der Velden M.T., Hitge-Boetes C., et al. Diagnosis of hypertrophic pyloric stenosis: Value of sonography when used in conjunction with clinical findings and laboratory data. AJR Am J Roentgenol. 1994;163:905-909.
58. Hernanz-Schulman M., Sells L.L., Ambrosino M.M., et al. Hypertrophic pyloric stenosis in the infant without a palpable olive: Accuracy of sonographic diagnosis. Radiology. 1994;193:771-776.
59. McKenna D.A., Meehan C.P., Alhajeri A.N., et al. The use of MRI to demonstrate small bowel obstruction during pregnancy. Br J Radiol. 2007;80:E11-E14.
60. Golden D.A., Gefter W.B., Gohel V.K. Digital examination of the rectum as a source of rectal gas. Radiology. 1981;141:618.
61. Harlow C.L., Stears R.L., Zeligman B.E., et al. Diagnosis of bowel obstruction on plain abdominal radiographs: Significance of air-fluid levels at different heights in the same loop of bowel. AJR Am J Roentgenol. 1993;161:291-295.
62. Fukuya T., Hawes D.R., Lu C.C., et al. CT diagnosis of small-bowel obstruction: Efficacy in 60 patients. AJR Am J Roentgenol. 1992;158:765-769. discussion 771-772
63. Gazelle G.S., Goldberg M.A., Wittenberg J., et al. Efficacy of CT in distinguishing small-bowel obstruction from other causes of small-bowel dilatation. AJR Am J Roentgenol. 1994;162:43-47.
64. Aufort S., Charra L., Lesnik A., et al. Multidetector CT of bowel obstruction: Value of post-processing. Eur Radiol. 2005;15:2323-2329.
65. Nicolaou S., Kai B., Ho S., et al. Imaging of acute small-bowel obstruction. AJR Am J Roentgenol. 2005;185:1036-1044.
66. Furukawa A., Yamasaki M., Furuichi K., et al. Helical CT in the diagnosis of small bowel obstruction. Radiographics. 2001;21:341-355.
67. Mayo-Smith W.W., Wittenberg J., Bennett G.L., et al. The CT small bowel faeces sign: Description and clinical significance. Clin Radiol. 1995;50:765-767.
68. Silva A.C., Pimenta M., Guimaraes L.S. Small bowel obstruction: What to look for. Radiographics. 2009;29:423-439.
69. O’Daly B.J., Ridgway P.F., Keenan N., et al. Detected peritoneal fluid in small bowel obstruction is associated with the need for surgical intervention. Can J Surg. 2009;52:201-206.
70. Balthazar E.J., Bauman J.S., Megibow A.J. CT diagnosis of closed loop obstruction. J Comput Assist Tomogr. 1985;9:953-955.
71. Balthazar E.J., Birnbaum B.A., Megibow A.J., et al. Closed-loop and strangulating intestinal obstruction: CT signs. Radiology. 1992;185:769-775.
72. Ha H.K., Kim J.S., Lee M.S., et al. Differentiation of simple and strangulated small-bowel obstructions: Usefulness of known CT criteria. Radiology. 1997;204:507-512.
73. Frager D., Baer J.W., Medwid S.W., et al. Detection of intestinal ischemia in patients with acute small-bowel obstruction due to adhesions or hernia: Efficacy of CT. AJR Am J Roentgenol. 1996;166:67-71.
74. American College of Radiology: ACR appropriateness criteria: Suspected Small-Bowel Obstruction. 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/SuspectedSmallBowelObstructionDoc15.aspx.
75. Balthazar E.J., Liebeskind M.E., Macari M. Intestinal ischemia in patients in whom small bowel obstruction is suspected: Evaluation of accuracy, limitations, and clinical implications of CT in diagnosis. Radiology. 1997;205:519-522.
75a. Balthazar E.J., Birnbaum B.A., Megibow A.J., et al. Closed-loop and strangulating intestinal obstruction: CT signs. Radiology. 1992;185(3):769-775.
76. Zalcman M., Sy M., Donckier V., et al. Helical CT signs in the diagnosis of intestinal ischemia in small-bowel obstruction. AJR Am J Roentgenol. 2000;175:1601-1607.
77. Mallo R.D., Salem L., Lalani T., Flum D.R. Computed tomography diagnosis of ischemia and complete obstruction in small bowel obstruction: A systematic review. J Gastrointest Surg. 2005;9:690-694.
78. Sheedy S.P., Earnest F.T., Fletcher J.G., et al. CT of small-bowel ischemia associated with obstruction in emergency department patients: Diagnostic performance evaluation. Radiology. 2006;241:729-736.
79. Jang K.M., Min K., Kim M.J., et al. Diagnostic performance of CT in the detection of intestinal ischemia associated with small-bowel obstruction using maximal attenuation of region of interest. AJR Am J Roentgenol. 2010;194:957-963.
80. Frager D., Medwid S.W., Baer J.W., et al. CT of small-bowel obstruction: Value in establishing the diagnosis and determining the degree and cause. AJR Am J Roentgenol. 1994;162:37-41.
81. Filippone A., Cianci R., Storto M.L. Bowel obstruction: Comparison between multidetector-row CT axial and coronal planes. Abdom Imaging. 2007;32:310-316.
82. Colon M.J., Telem D.A., Wong D., Divino C.M. The relevance of transition zones on computed tomography in the management of small bowel obstruction. Surgery. 2010;147:373-377.
83. Maglinte D.D., Reyes B.L., Harmon B.H., et al. Reliability and role of plain film radiography and CT in the diagnosis of small-bowel obstruction. AJR Am J Roentgenol. 1996;167:1451-1455.
84. Shrake P.D., Rex D.K., Lappas J.C., Maglinte D.D. Radiographic evaluation of suspected small bowel obstruction. Am J Gastroenterol. 1991;86:175-178.
85. Megibow A.J., Balthazar E.J., Cho K.C., et al. Bowel obstruction: Evaluation with CT. Radiology. 1991;180:313-318.
86. Suri S., Gupta S., Sudhakar P.J., et al. Comparative evaluation of plain films, ultrasound and CT in the diagnosis of intestinal obstruction. Acta Radiol. 1999;40:422-428.
87. Maglinte D.D., Gage S.N., Harmon B.H., et al. Obstruction of the small intestine: Accuracy and role of CT in diagnosis. Radiology. 1993;188:61-64.
88. Ko Y.T., Lim J.H., Lee D.H., et al. Small bowel obstruction: Sonographic evaluation. Radiology. 1993;188:649-653.
89. Ogata M., Mateer J.R., Condon R.E. Prospective evaluation of abdominal sonography for the diagnosis of bowel obstruction. Ann Surg. 1996;223:237-241.
90. Czechowski J. Conventional radiography and ultrasonography in the diagnosis of small bowel obstruction and strangulation. Acta Radiol. 1996;37:186-189.
91. Hata J., Kamada T., Haruma K., Kusunoki H. Evaluation of bowel ischemia with contrast-enhanced US: Initial experience. Radiology. 2005;236:712-715.
92. Gorey T.F., O’Sullivan M. Prognostic factors in extensive mesenteric ischaemia. Ann R Coll Surg Engl. 1988;70:191-194.
93. Bozlar U., Ugurel M.S., Ustunsoz B., Coskun U. CT angiographic demonstration of a mesenteric vessel “whirlpool” in intestinal malrotation and midgut volvulus: A case report. Korean J Radiol. 2008;9:466-469.
94. Sizemore A.W., Rabbani K.Z., Ladd A., Applegate K.E. Diagnostic performance of the upper gastrointestinal series in the evaluation of children with clinically suspected malrotation. Pediatr Radiol. 2008;38:518-528.
95. Henesch S.M., Nance M.L., Jaramillo D.M. Enhanced CT perfusion cut-off sign in midgut volvulus. Pediatr Radiol. 2006;36:355-357.
96. Aidlen J., Anupindi S.A., Jaramillo D., Doody D.P. Malrotation with midgut volvulus: CT findings of bowel infarction. Pediatr Radiol. 2005;35:529-531.
97. Ai V.H., Lam W.W., Cheng W., Chan F.L. CT appearance of midgut volvulus with malrotation in a young infant. Clin Radiol. 1999;54:687-689.
98. Inoue Y., Nakamura H., Mizumoto S., Akashi H. 3D angiography with helical CT in midgut volvulus. J Comput Assist Tomogr. 1995;19:663-665.
99. Orzech N., Navarro O.M., Langer J.C. Is ultrasonography a good screening test for intestinal malrotation? J Pediatr Surg. 2006;41:1005-1009.
100. Sundaram B., Miller C.N., Cohan R.H., et al. Can CT features be used to diagnose surgical adult bowel intussusceptions? AJR Am J Roentgenol. 2009;193:471-478.
101. Sargent M.A., Babyn P., Alton D.J. Plain abdominal radiography in suspected intussusception: A reassessment. Pediatr Radiol. 1994;24:17-20.
102. Smith D.S., Bonadio W.A., Losek J.D., et al. The role of abdominal x-rays in the diagnosis and management of intussusception. Pediatr Emerg Care. 1992;8:325-327.
103. Morrison J., Lucas N., Gravel J. The role of abdominal radiography in the diagnosis of intussusception when interpreted by pediatric emergency physicians. J Pediatr. 2009;155:556-559.
104. Hryhorczuk A.L., Strouse P.J. Validation of US as a first-line diagnostic test for assessment of pediatric ileocolic intussusception. Pediatr Radiol. 2009;39:1075-1079.
105. Chamroonrat W., Cheng G., Servaes S., Zhuang H. Intussusception incidentally detected by FDG-PET/CT in a pediatric lymphoma patient. Ann Nucl Med. 2010.
106. Cox T.D., Winters W.D., Weinberger E. CT of intussusception in the pediatric patient: Diagnosis and pitfalls. Pediatr Radiol. 1996;26:26-32.
107. Shiels W.E.II, Maves C.K., Hedlund G.L., Kirks D.R. Air enema for diagnosis and reduction of intussusception: Clinical experience and pressure correlates. Radiology. 1991;181:169-172.
108. Lui K.W., Wong H.F., Cheung Y.C., et al. Air enema for diagnosis and reduction of intussusception in children: Clinical experience and fluoroscopy time correlation. J Pediatr Surg. 2001;36:479-481.
109. Al-Jazaeri A., Yazbeck S., Filiatrault D., et al. Utility of hospital admission after successful enema reduction of ileocolic intussusception. J Pediatr Surg. 2006;41:1010-1013.
110. Markogiannakis H., Messaris E., Dardamanis D., et al. Acute mechanical bowel obstruction: Clinical presentation, etiology, management and outcome. World J Gastroenterol. 2007;13:432-437.
111. Taourel P., Kessler N., Lesnik A., et al. Helical CT of large bowel obstruction. Abdom Imaging. 2003;28:267-275.
112. Wiest P., Roth P. Fundamentals of Emergency Radiology. Philadelphia: WB Saunders; 1996.
113. Chapman A.H., McNamara M., Porter G. The acute contrast enema in suspected large bowel obstruction: Value and technique. Clin Radiol. 1992;46:273-278.
114. Jacob S.E., Lee S.H., Hill J. The demise of the instant/unprepared contrast enema in large bowel obstruction. Colorectal Dis. 2008;10:729-731.
115. Stewart J., Finan P.J., Courtney D.F., Brennan T.G. Does a water soluble contrast enema assist in the management of acute large bowel obstruction: A prospective study of 117 cases. Br J Surg. 1984;71:799-801.
116. Sinha R., Verma R. Multidetector row computed tomography in bowel obstruction. Part 2. Large bowel obstruction. Clin Radiol. 2005;60:1068-1075.
117. Cartlidge D., Seenath M. Acute pseudo-obstruction of the large bowel with caecal perforation following normal vaginal delivery: A case report. J Med Case Reports. 2010;4:123.
118. Gilchrist A.M., Mills J.O., Russell C.G. Acute large-bowel pseudo-obstruction. Clin Radiol. 1985;36:401-404.
119. Juchems M.S., Hoffmann M.H., Schmidt S.A., et al. Bowel preparation for CT-colonography: Comparison of two different cleansing protocols. Eur J Radiol. 2006;60:460-464.
120. Borden Z.S., Pickhardt P.J., Kim D.H., et al. Bowel preparation for CT colonography: Blinded comparison of magnesium citrate and sodium phosphate for catharsis. Radiology. 2010;254:138-144.
121. Pickhardt P.J., Choi J.H. Electronic cleansing and stool tagging in CT colonography: Advantages and pitfalls with primary three-dimensional evaluation. AJR Am J Roentgenol. 2003;181:799-805.
122. Anderson J.R., Mills J.O. Caecal volvulus: A frequently missed diagnosis? Clin Radiol. 1984;35:65-69.
123. Burrell H.C., Baker D.M., Wardrop P., Evans A.J. Significant plain film findings in sigmoid volvulus. Clin Radiol. 1994;49:317-319.
124. Moore C.J., Corl F.M., Fishman E.K. CT of cecal volvulus: Unraveling the image. AJR Am J Roentgenol. 2001;177:95-98.
125. Frank A.J., Goffner L.B., Fruauff A.A., Losada R.A. Cecal volvulus: The CT whirl sign. Abdom Imaging. 1993;18:288-289.
126. Catalano O. Computed tomographic appearance of sigmoid volvulus. Abdom Imaging. 1996;21:314-317.
127. Shaff M.I., Himmelfarb E., Sacks G.A., et al. The whirl sign: A CT finding in volvulus of the large bowel. J Comput Assist Tomogr. 1985;9:410.
128. Frager D., Rovno H.D., Baer J.W., et al. Prospective evaluation of colonic obstruction with computed tomography. Abdom Imaging. 1998;23:141-146.
129. Beattie G.C., Peters R.T., Guy S., Mendelson R.M. Computed tomography in the assessment of suspected large bowel obstruction. ANZ J Surg. 2007;77:160-165.
130. Olutola P.S. Plain film radiographic diagnosis of acute appendicitis: An evaluation of the signs. Can Assoc Radiol J. 1988;39:254-256.
131. Bakhda R.K., McNair M.M. Useful radiological signs in acute appendicitis in children. Clin Radiol. 1977;28:193-196.
132. Rao P.M., Rhea J.T., Rao J.A., Conn A.K. Plain abdominal radiography in clinically suspected appendicitis: Diagnostic yield, resource use, and comparison with CT. Am J Emerg Med. 1999;17:325-328.
133. Fedyshin P., Kelvin F.M., Rice R.P. Nonspecificity of barium enema findings in acute appendicitis. AJR Am J Roentgenol. 1984;143:99-102.
134. American College of Radiology: ACR appropriateness criteria: Right Lower Quadrant Pain—Suspected Appendicitis. 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/RightLowerQuadrantPainDoc12.aspx.
135. Doria A.S., Moineddin R., Kellenberger C.J., et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
136. Choi D., Park H., Lee Y.R., et al. The most useful findings for diagnosing acute appendicitis on contrast-enhanced helical CT. Acta Radiol. 2003;44:574-582.
137. Rao P.M., Rhea J.T., Novelline R.A. Sensitivity and specificity of the individual CT signs of appendicitis: Experience with 200 helical appendiceal CT examinations. J Comput Assist Tomogr. 1997;21:686-692.
138. Oto A., Ernst R.D., Mileski W.J., et al. Localization of appendix with MDCT and influence of findings on choice of appendectomy incision. AJR Am J Roentgenol. 2006;187:987-990.
139. Yu S.H., Kim C.B., Park J.W., et al. Ultrasonography in the diagnosis of appendicitis: Evaluation by meta-analysis. Korean J Radiol. 2005;6:267-277.
140. Terasawa T., Blackmore C.C., Bent S., Kohlwes R.J. Systematic review: Computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med. 2004;141:537-546.
141. van Randen A., Bipat S., Zwinderman A.H., et al. Acute appendicitis: Meta-analysis of diagnostic performance of CT and graded compression US related to prevalence of disease. Radiology. 2008;249:97-106.
142. Garcia Pena B.M., Mandl K.D., Kraus S.J., et al. Ultrasonography and limited computed tomography in the diagnosis and management of appendicitis in children. JAMA. 1999;282:1041-1046.
143. Pedrosa I., Levine D., Eyvazzadeh A.D., et al. MR imaging evaluation of acute appendicitis in pregnancy. Radiology. 2006;238:891-899.
144. Pedrosa I., Lafornara M., Pandharipande P.V., et al. Pregnant patients suspected of having acute appendicitis: Effect of MR imaging on negative laparotomy rate and appendiceal perforation rate. Radiology. 2009;250:749-757.
145. Israel G.M., Malguria N., McCarthy S., et al. MRI vs. ultrasound for suspected appendicitis during pregnancy. J Magn Reson Imaging. 2008;28:428-433.
146. Cobben L.P., Groot I., Haans L., et al. MRI for clinically suspected appendicitis during pregnancy. AJR Am J Roentgenol. 2004;183:671-675.
147. Chabanova E., Balslev I., Achiam M., et al. Unenhanced MR imaging in adults with clinically suspected acute appendicitis. Eur J Radiol. 2010.
148. Basaran A., Basaran M. Diagnosis of acute appendicitis during pregnancy: A systematic review. Obstet Gynecol Surv. 2009;64:481-488. quiz 99
149. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
150. McKay R., Shepherd J. The use of the clinical scoring system by Alvarado in the decision to perform computed tomography for acute appendicitis in the ED. Am J Emerg Med. 2007;25:489-493.
151. Kim K., Rhee J.E., Lee C.C., et al. Impact of helical computed tomography in clinically evident appendicitis. Emerg Med J. 2008;25:477-481.
152. Bhatt M., Joseph L., Ducharme F.M., et al. Prospective validation of the pediatric appendicitis score in a Canadian pediatric emergency department. Acad Emerg Med. 2009;16:591-596.
153. Birkhahn R.H., Briggs M., Datillo P.A., et al. Classifying patients suspected of appendicitis with regard to likelihood. Am J Surg. 2006;191:497-502.
154. Lewis F.R., Holcroft J.W., Boey J., Dunphy E. Appendicitis: A critical review of diagnosis and treatment in 1,000 cases. Arch Surg. 1975;110:677-684.
155. Coursey C.A., Nelson R.C., Patel M.B., et al. Making the diagnosis of acute appendicitis: Do more preoperative CT scans mean fewer negative appendectomies? A 10-year study. Radiology. 2010;254:460-468.
156. Balthazar E.J., Rofsky N.M., Zucker R. Appendicitis: The impact of computed tomography imaging on negative appendectomy and perforation rates. Am J Gastroenterol. 1998;93:768-771.
157. Rao P.M., Rhea J.T., Rattner D.W., et al. Introduction of appendiceal CT: Impact on negative appendectomy and appendiceal perforation rates. Ann Surg. 1999;229:344-349.
158. Karakas S.P., Guelfguat M., Leonidas J.C., et al. Acute appendicitis in children: Comparison of clinical diagnosis with ultrasound and CT imaging. Pediatr Radiol. 2000;30:94-98.
159. Applegate K.E., Sivit C.J., Salvator A.E., et al. Effect of cross-sectional imaging on negative appendectomy and perforation rates in children. Radiology. 2001;220:103-107.
160. McDonald G.P., Pendarvis D.P., Wilmoth R., Daley B.J. Influence of preoperative computed tomography on patients undergoing appendectomy. Am Surg. 2001;67:1017-1021.
161. Bendeck S.E., Nino-Murcia M., Berry G.J., Jeffrey R.B.Jr. Imaging for suspected appendicitis: Negative appendectomy and perforation rates. Radiology. 2002;225:131-136.
162. Fuchs J.R., Schlamberg J.S., Shortsleeve M.J., Schuler J.G. Impact of abdominal CT imaging on the management of appendicitis: An update. J Surg Res. 2002;106:131-136.
163. Partrick D.A., Janik J.E., Janik J.S., et al. Increased CT scan utilization does not improve the diagnostic accuracy of appendicitis in children. J Pediatr Surg. 2003;38:659-662.
164. Torbati S.S., Guss D.A. Impact of helical computed tomography on the outcomes of emergency department patients with suspected appendicitis. Acad Emerg Med. 2003;10:823-829.
165. Antevil J., Rivera L., Langenberg B., Brown C.V. The influence of age and gender on the utility of computed tomography to diagnose acute appendicitis. Am Surg. 2004;70:850-853.
166. Vadeboncoeur T.F., Heister R.R., Behling C.A., Guss D.A. Impact of helical computed tomography on the rate of negative appendicitis. Am J Emerg Med. 2006;24:43-47.
167. Chooi W.K., Brown J.A., Zetler P., et al. Imaging of acute appendicitis and its impact on negative appendectomy and perforation rates: The St. Paul’s experience. Can Assoc Radiol J. 2007;58:220-224.
168. Riesenman P.J., Riesenman K.P., Stone T.J., et al. Nonfocused enhanced CT evaluation of acute appendicitis increases length of stay in the emergency department but does not increase perforation rate. Am Surg. 2008;74:488-492. discussion 492-3
169. Hong J.J., Cohn S.M., Ekeh A.P., et al. A prospective randomized study of clinical assessment versus computed tomography for the diagnosis of acute appendicitis. Surg Infect (Larchmt). 2003;4:231-239.
170. Lee C.C., Golub R., Singer A.J., et al. Routine versus selective abdominal computed tomography scan in the evaluation of right lower quadrant pain: A randomized controlled trial. Acad Emerg Med. 2007;14:117-122.
171. Lopez P.P., Cohn S.M., Popkin C.A., et al. The use of a computed tomography scan to rule out appendicitis in women of childbearing age is as accurate as clinical examination: A prospective randomized trial. Am Surg. 2007;73:1232-1236.
172. Iwahashi N., Kitagawa Y., Mayumi T., Kohno H. Intravenous contrast–enhanced computed tomography in the diagnosis of acute appendicitis. World J Surg. 2005;29:83-87.
173. Mun S., Ernst R.D., Chen K., et al. Rapid CT diagnosis of acute appendicitis with IV contrast material. Emerg Radiol. 2006;12:99-102.
174. Anderson S.W., Soto J.A., Lucey B.C., et al. Abdominal 64-MDCT for suspected appendicitis: The use of oral and IV contrast material versus IV contrast material only. AJR Am J Roentgenol. 2009;193:1282-1288.
175. Keyzer C., Cullus P., Tack D., et al. MDCT for suspected acute appendicitis in adults: Impact of oral and IV contrast media at standard-dose and simulated low-dose techniques. AJR Am J Roentgenol. 2009;193:1272-1281.
176. Seo H., Lee K.H., Kim H.J., et al. Diagnosis of acute appendicitis with sliding slab ray-sum interpretation of low-dose unenhanced CT and standard-dose IV contrast–enhanced CT scans. AJR Am J Roentgenol. 2009;193:96-105.
177. Hlibczuk V., Dattaro J.A., Jin Z., et al. Diagnostic accuracy of noncontrast computed tomography for appendicitis in adults: A systematic review. Ann Emerg Med. 2009.
178. Kaiser S., Finnbogason T., Jorulf H.K., et al. Suspected appendicitis in children: Diagnosis with contrast-enhanced versus nonenhanced helical CT. Radiology. 2004;231:427-433.
179. Grayson D.E., Wettlaufer J.R., Dalrymple N.C., Keesling C.A. Appendiceal CT in pediatric patients: Relationship of visualization to amount of peritoneal fat. AJR Am J Roentgenol. 2001;176:497-500.
180. Benjaminov O., Atri M., Hamilton P., Rappaport D. Frequency of visualization and thickness of normal appendix at nonenhanced helical CT. Radiology. 2002;225:400-406.
181. Ganguli S., Raptopoulos V., Komlos F., et al. Right lower quadrant pain: Value of the nonvisualized appendix in patients at multidetector CT. Radiology. 2006;241:175-180.
182. Nikolaidis P., Hwang C.M., Miller F.H., Papanicolaou N. The nonvisualized appendix: Incidence of acute appendicitis when secondary inflammatory changes are absent. AJR Am J Roentgenol. 2004;183:889-892.
183. Garcia K., Hernanz-Schulman M., Bennett D.L., et al. Suspected appendicitis in children: Diagnostic importance of normal abdominopelvic CT findings with nonvisualized appendix. Radiology. 2009;250:531-537.
184. Daly C.P., Cohan R.H., Francis I.R., et al. Incidence of acute appendicitis in patients with equivocal CT findings. AJR Am J Roentgenol. 2005;184:1813-1820.
185. Brenner D.J., Hall E.J. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284.
186. Kung H.C., Hoyert D.L., Xu J., Murphy S.L. Deaths: Final data for 2005. Natl Vital Stat Rep. 2008;56:1-120.
187. Brenner D., Elliston C., Hall E., Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296.
188. Shetty M.K., Garrett N.M., Carpenter W.S., et al. Abdominal computed tomography during pregnancy for suspected appendicitis: A 5-year experience at a maternity hospital. Semin Ultrasound CT MR. 2010;31:8-13.
189. Ratnapalan S., Bentur Y., Koren G. “Doctor, will that x-ray harm my unborn child? CMAJ. 2008;179:1293-1296.
190. Ratnapalan S., Bona N., Chandra K., Koren G. Physicians’ perceptions of teratogenic risk associated with radiography and CT during early pregnancy. AJR Am J Roentgenol. 2004;182:1107-1109.
191. Chen M.M., Coakley F.V., Kaimal A., Laros R.K.Jr. Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol. 2008;112:333-340.
192. Rao P.M., Rhea J.T., Novelline R.A., et al. Helical CT technique for the diagnosis of appendicitis: Prospective evaluation of a focused appendix CT examination. Radiology. 1997;202:139-144.
193. Rhea J.T., Rao P.M., Novelline R.A., McCabe C.J. A focused appendiceal CT technique to reduce the cost of caring for patients with clinically suspected appendicitis. AJR Am J Roentgenol. 1997;169:113-118.
194. Fefferman N.R., Roche K.J., Pinkney L.P., et al. Suspected appendicitis in children: Focused CT technique for evaluation. Radiology. 2001;220:691-695.
195. Mullins M.E., Kircher M.F., Ryan D.P., et al. Evaluation of suspected appendicitis in children using limited helical CT and colonic contrast material. AJR Am J Roentgenol. 2001;176:37-41.
196. Broder J.S., Hollingsworth C.L., Miller C.M., et al. Prospective double-blinded study of abdominal–pelvic computed tomography guided by the region of tenderness: Estimation of detection of acute pathology and radiation exposure reduction. Ann Emerg Med. 2010.
197. Lowe L.H., Perez R.Jr., Scheker L.E., et al. Appendicitis and alternate diagnoses in children: Findings on unenhanced limited helical CT. Pediatr Radiol. 2001;31:569-577.
198. Osada H., Ohno H., Watanabe W., et al. Multidetector computed tomography diagnosis of primary and secondary epiploic appendagitis. Radiat Med. 2008;26:582-586.
199. Ozdemir S., Gulpinar K., Leventoglu S., et al. Torsion of the primary epiploic appendagitis: A case series and review of the literature. Am J Surg. 2010;199:453-458.
200. Hiller N., Berelowitz D., Hadas-Halpern I. Primary epiploic appendagitis: Clinical and radiological manifestations. Isr Med Assoc J. 2000;2:896-898.
201. Leclercq P, Dorthu L. Epiploic appendagitis. CMAJ 2010.
202. Lucey B.C., Stuhlfaut J.W., Soto J.A. Mesenteric lymph nodes seen at imaging: Causes and significance. Radiographics. 2005;25:351-365.
203. Lucey B.C., Stuhlfaut J.W., Soto J.A. Mesenteric lymph nodes: Detection and significance on MDCT. AJR Am J Roentgenol. 2005;184:41-44.
204. Prince R.L. Evidence for an aetiological role for adenovirus type 7 in the mesenteric adenitis syndrome. Med J Aust. 1979;2:56-57.
205. Macari M., Hines J., Balthazar E., Megibow A. Mesenteric adenitis: CT diagnosis of primary versus secondary causes, incidence, and clinical significance in pediatric and adult patients. AJR Am J Roentgenol. 2002;178:853-858.
206. Rao P.M., Rhea J.T., Novelline R.A. CT diagnosis of mesenteric adenitis. Radiology. 1997;202:145-149.
207. Jacobs D.O. Clinical practice: Diverticulitis, N Engl J Med. 2007;357:2057-2066.
208. American College of Radiology. ACR appropriateness criteria: Left lower quadrant pain, 2008. (Accessed at http://www.acr.org/secondarymainmenucategories/quality_safety/app_criteria.aspx.)
209. Lameris W., van Randen A., van Gulik T.M., et al. A clinical decision rule to establish the diagnosis of acute diverticulitis at the emergency department. Dis Colon Rectum. 2010;53:896-904.
210. Toorenvliet B.R., Bakker R.F., Breslau P.J., et al. Colonic diverticulitis: A prospective analysis of diagnostic accuracy and clinical decision-making. Colorectal Dis. 2010;12:179-186.
211. Ambrosetti P. Acute diverticulitis of the left colon: Value of the initial CT and timing of elective colectomy. J Gastrointest Surg. 2008;12:1318-1320.
212. Lameris W., van Randen A., Bipat S., et al. Graded compression ultrasonography and computed tomography in acute colonic diverticulitis: Meta-analysis of test accuracy. Eur Radiol. 2008;18:2498-2511.
213. Ambrosetti P., Becker C., Terrier F. Colonic diverticulitis: Impact of imaging on surgical management—A prospective study of 542 patients. Eur Radiol. 2002;12:1145-1149.
214. Liljegren G., Chabok A., Wickbom M., et al. Acute colonic diverticulitis: A systematic review of diagnostic accuracy. Colorectal Dis. 2007;9:480-488.
215. Buckley O., Geoghegan T., O’Riordain D.S., et al. Computed tomography in the imaging of colonic diverticulitis. Clin Radiol. 2004;59:977-983.
216. Lohrmann C., Ghanem N., Pache G., et al. CT in acute perforated sigmoid diverticulitis. Eur J Radiol. 2005;56:78-83.
217. Kaewlai R., Nazinitsky K.J. Acute colonic diverticulitis in a community-based hospital: CT evaluation in 138 patients. Emerg Radiol. 2007;13:171-179.
218. Cho K.C., Morehouse H.T., Alterman D.D., Thornhill B.A. Sigmoid diverticulitis: Diagnostic role of CT—Comparison with barium enema studies. Radiology. 1990;176:111-115.
219. Ambrosetti P., Robert J., Witzig J.A., et al. Incidence, outcome, and proposed management of isolated abscesses complicating acute left-sided colonic diverticulitis: A prospective study of 140 patients. Dis Colon Rectum. 1992;35:1072-1076.
220. Ambrosetti P., Robert J., Witzig J.A., et al. Prognostic factors from computed tomography in acute left colonic diverticulitis. Br J Surg. 1992;79:117-119.
221. Ambrosetti P., Grossholz M., Becker C., et al. Computed tomography in acute left colonic diverticulitis. Br J Surg. 1997;84:532-534.
222. Bernini A., Spencer M.P., Wong W.D., et al. Computed tomography-guided percutaneous abscess drainage in intestinal disease: Factors associated with outcome. Dis Colon Rectum. 1997;40:1009-1013.
223. Siewert B., Tye G., Kruskal J., et al. Impact of CT-guided drainage in the treatment of diverticular abscesses: Size matters. AJR Am J Roentgenol. 2006;186:680-686.
224. Singh B., May K., Coltart I., et al. The long-term results of percutaneous drainage of diverticular abscess. Ann R Coll Surg Engl. 2008;90:297-301.
225. Sparks F.C., Strauss E.B., Corey J.M. Percutaneous drainage of a diverticular abscess can make colostomy unnecessary in selected cases. Conn Med. 1990;54:305-307.
226. Stabile B.E., Puccio E., vanSonnenberg E., Neff C.C. Preoperative percutaneous drainage of diverticular abscesses. Am J Surg. 1990;159:99-104. discussion
227. Goh V., Halligan S., Taylor S.A., et al. Differentiation between diverticulitis and colorectal cancer: Quantitative CT perfusion measurements versus morphologic criteria—initial experience. Radiology. 2007;242:456-462.
228. Pradel J.A., Adell J.F., Taourel P., et al. Acute colonic diverticulitis: Prospective comparative evaluation with US and CT. Radiology. 1997;205:503-512.
229. Schwerk W.B., Schwarz S., Rothmund M. Sonography in acute colonic diverticulitis: A prospective study. Dis Colon Rectum. 1992;35:1077-1084.
230. Verbanck J., Lambrecht S., Rutgeerts L., et al. Can sonography diagnose acute colonic diverticulitis in patients with acute intestinal inflammation? A prospective study. J Clin Ultrasound. 1989;17:661-666.
231. Hollerweger A., Macheiner P., Rettenbacher T., et al. Colonic diverticulitis: Diagnostic value and appearance of inflamed diverticula-sonographic evaluation. Eur Radiol. 2001;11:1956-1963.
232. Ajaj W., Ruehm S.G., Lauenstein T., et al. Dark-lumen magnetic resonance colonography in patients with suspected sigmoid diverticulitis: A feasibility study. Eur Radiol. 2005;15:2316-2322.
233. Heverhagen J.T., Zielke A., Ishaque N., et al. Acute colonic diverticulitis: Visualization in magnetic resonance imaging. Magn Reson Imaging. 2001;19:1275-1277.
234. Huprich J.E., Fletcher J.G. CT enterography: Principles, technique and utility in Crohn’s disease. Eur J Radiol. 2009;69:393-397.
235. Horsthuis K., Bipat S., Bennink R.J., Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: Meta-analysis of prospective studies. Radiology. 2008;247:64-79.
236. Boudiaf M., Jaff A., Soyer P., et al. Small-bowel diseases: Prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338-344.
237. Hara A.K., Alam S., Heigh R.I., et al. Using CT enterography to monitor Crohn’s disease activity: A preliminary study. AJR Am J Roentgenol. 2008;190:1512-1516.
238. Higgins P.D., Caoili E., Zimmermann M., et al. Computed tomographic enterography adds information to clinical management in small bowel Crohn’s disease. Inflamm Bowel Dis. 2007;13:262-268.
239. Bodily K.D., Fletcher J.G., Solem C.A., et al. Crohn disease: Mural attenuation and thickness at contrast-enhanced CT enterography—Correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505-516.
240. Amitai M.M., Arazi-Kleinman T., Avidan B., et al. Fat halo sign in the bowel wall of patients with Crohn’s disease. Clin Radiol. 2007;62:994-997.
241. Harisinghani M.G., Wittenberg J., Lee W., et al. Bowel wall fat halo sign in patients without intestinal disease. AJR Am J Roentgenol. 2003;181:781-784.
242. Horton K.M., Corl F.M., Fishman E.K. CT evaluation of the colon: Inflammatory disease. Radiographics. 2000;20:399-418.
243. Fishman E.K., Kavuru M., Jones B., et al. Pseudomembranous colitis: CT evaluation of 26 cases. Radiology. 1991;180:57-60.
244. Imbriaco M., Balthazar E.J. Toxic megacolon: Role of CT in evaluation and detection of complications. Clin Imaging. 2001;25:349-354.
245. Vas W.G., Seelig R., Mahanta B., et al. Neutropenic colitis: Evaluation with computed tomography. J Comput Tomogr. 1988;12:211-215.
246. Frick M.P., Maile C.W., Crass J.R., et al. Computed tomography of neutropenic colitis. AJR Am J Roentgenol. 1984;143:763-765.
247. Rothrock S.G., Gorrhuis H., Howard R.M. Efficacy of plain abdominal radiography in patients with biliary tract disease. J Emerg Med. 1990;8:271-275.
248. Trowbridge R.L., Rutkowski N.K., Shojania K.G. Does this patient have acute cholecystitis? JAMA. 2003;289:80-86.
249. Gruber P.J., Silverman R.A., Gottesfeld S., Flaster E. Presence of fever and leukocytosis in acute cholecystitis. Ann Emerg Med. 1996;28:273-277.
250. Huffman J.L., Schenker S. Acute acalculous cholecystitis: A review. Clin Gastroenterol Hepatol. 2010;8:15-22.
251. American College of Radiology: ACR appropriateness criteria: Right upper quadrant pain. 2010. (Accessed at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/RightUpperQuadrantPainDoc13.aspx.)
252. Stephen A.E., Berger D.L. Carcinoma in the porcelain gallbladder: A relationship revisited. Surgery. 2001;129:699-703.
253. Bortoff G.A., Chen M.Y., Ott D.J., et al. Gallbladder stones: Imaging and intervention. Radiographics. 2000;20:751-766.
254. Rosenthal S.J., Cox G.G., Wetzel L.H., Batnitzky S. Pitfalls and differential diagnosis in biliary sonography. Radiographics. 1990;10:285-311.
255. Wegener M., Borsch G., Schneider J., et al. Gallbladder wall thickening: A frequent finding in various nonbiliary disorders—A prospective ultrasonographic study. J Clin Ultrasound. 1987;15:307-312.
256. Bree R.L. Further observations on the usefulness of the sonographic Murphy sign in the evaluation of suspected acute cholecystitis. J Clin Ultrasound. 1995;23:169-172.
257. Shea J.A., Berlin J.A., Escarce J.J., et al. Revised estimates of diagnostic test sensitivity and specificity in suspected biliary tract disease. Arch Intern Med. 1994;154:2573-2581.
258. Watson A., Better N., Kalff V., et al. Cholecystokinin (CCK)–HIDA scintigraphy in patients with suspected gall-bladder dysfunction. Australas Radiol. 1994;38:30-33.
259. Fink-Bennett D., Balon H., Robbins T., Tsai D. Morphine-augmented cholescintigraphy: Its efficacy in detecting acute cholecystitis. J Nucl Med. 1991;32:1231-1233.
260. Mirvis S.E., Vainright J.R., Nelson A.W., et al. The diagnosis of acute acalculous cholecystitis: A comparison of sonography, scintigraphy, and CT. AJR Am J Roentgenol. 1986;147:1171-1175.
261. Fidler J., Paulson E.K., Layfield L. CT evaluation of acute cholecystitis: Findings and usefulness in diagnosis. AJR Am J Roentgenol. 1996;166:1085-1088.
262. Paulson E.K. Acute cholecystitis: CT findings, Semin Ultrasound CT MR. 2000;21:56-63.
263. Singh A.K., Sagar P. Gangrenous cholecystitis: Prediction with CT imaging. Abdom Imaging. 2005;30:218-221.
264. Hanninen E.L., Pech M., Jonas S., et al. Magnetic resonance imaging including magnetic resonance cholangiopancreatography for tumor localization and therapy planning in malignant hilar obstructions. Acta Radiol. 2005;46:462-470.
265. Hakansson K., Leander P., Ekberg O., Hakansson H.O. MR imaging in clinically suspected acute cholecystitis: A comparison with ultrasonography. Acta Radiol. 2000;41:322-328.
266. Loud P.A., Semelka R.C., Kettritz U., et al. MRI of acute cholecystitis: Comparison with the normal gallbladder and other entities. Magn Reson Imaging. 1996;14:349-355.
267. Bachar G.N., Cohen M., Belenky A., et al. Effect of aging on the adult extrahepatic bile duct: A sonographic study. J Ultrasound Med. 2003;22:879-882. quiz 83-5
268. Soto J.A. Bile duct stones: Diagnosis with MR cholangiography and helical CT. Semin Ultrasound CT MR. 1999;20:304-316.
269. Soto J.A., Alvarez O., Lopera J.E., et al. Biliary obstruction: Findings at MR cholangiography and cross-sectional MR imaging. Radiographics. 2000;20:353-366.
270. Watanabe Y., Nagayama M., Okumura A., et al. MR imaging of acute biliary disorders. Radiographics. 2007;27:477-495.
271. Yeh B.M., Liu P.S., Soto J.A., et al. MR imaging and CT of the biliary tract. Radiographics. 2009;29:1669-1688.
272. Soto J.A., Alvarez O., Munera F., et al. Diagnosing bile duct stones: Comparison of unenhanced helical CT, oral contrast–enhanced CT cholangiography, and MR cholangiography. AJR Am J Roentgenol. 2000;175:1127-1134.
273. Hoeffel C., Azizi L., Lewin M., et al. Normal and pathologic features of the postoperative biliary tract at 3D MR cholangiopancreatography and MR imaging. Radiographics. 2006;26:1603-1620.
274. Anderson S.W., Lucey B.C., Varghese J.C., Soto J.A. Accuracy of MDCT in the diagnosis of choledocholithiasis. AJR Am J Roentgenol. 2006;187:174-180.
275. Ahmetoglu A., Kosucu P., Kul S., et al. MDCT cholangiography with volume rendering for the assessment of patients with biliary obstruction. AJR Am J Roentgenol. 2004;183:1327-1332.
276. Anderson S.W., Rho E., Soto J.A. Detection of biliary duct narrowing and choledocholithiasis: Accuracy of portal venous phase multidetector CT. Radiology. 2008;247:418-427.
277. Kim H.J., Park D.I., Park J.H., et al. Multidetector computed tomography cholangiography with multiplanar reformation for the assessment of patients with biliary obstruction. J Gastroenterol Hepatol. 2007;22:400-405.
278. Balthazar E.J., Birnbaum B.A., Naidich M. Acute cholangitis: CT evaluation. J Comput Assist Tomogr. 1993;17:283-289.
279. Lee N.K., Kim S., Lee J.W., et al. Discrimination of suppurative cholangitis from nonsuppurative cholangitis with computed tomography (CT). Eur J Radiol. 2009;69:528-535.
280. Lee N.K., Kim S., Lee J.W., et al. Biliary MR imaging with Gd-EOB-DTPA and its clinical applications. Radiographics. 2009;29:1707-1724.
281. Seale M.K., Catalano O.A., Saini S., et al. Hepatobiliary-specific MR contrast agents: Role in imaging the liver and biliary tree. Radiographics. 2009;29:1725-1748.
282. American College of Radiology: ACR appropriateness criteria: Acute Pancreatitis. 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/AcutePancreatitisDoc2.aspx.
283. Davis S., Parbhoo S.P., Gibson M.J. The plain abdominal radiograph in acute pancreatitis. Clin Radiol. 1980;31:87-93.
284. Paulson E.K., Vitellas K.M., Keogan M.T., et al. Acute pancreatitis complicated by gland necrosis: Spectrum of findings on contrast-enhanced CT. AJR Am J Roentgenol. 1999;172:609-613.
285. Johnson C.D., Stephens D.H., Sarr M.G. CT of acute pancreatitis: Correlation between lack of contrast enhancement and pancreatic necrosis. AJR Am J Roentgenol. 1991;156:93-95.
286. Kivisaari L., Somer K., Standertskjold-Nordenstam C.G., et al. A new method for the diagnosis of acute hemorrhagic-necrotizing pancreatitis using contrast-enhanced CT. Gastrointest Radiol. 1984;9:27-30.
287. Vitellas K.M., Paulson E.K., Enns R.A., et al. Pancreatitis complicated by gland necrosis: Evolution of findings on contrast-enhanced CT. J Comput Assist Tomogr. 1999;23:898-905.
288. Balthazar E.J. CT diagnosis and staging of acute pancreatitis. Radiol Clin North Am. 1989;27:19-37.
289. Balthazar E.J. Staging of acute pancreatitis. Radiol Clin North Am. 2002;40:1199-1209.
290. Balthazar E.J. Acute pancreatitis: Assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603-613.
291. Foitzik T., Bassi D.G., Fernandez-del Castillo C., et al. Intravenous contrast medium impairs oxygenation of the pancreas in acute necrotizing pancreatitis in the rat. Arch Surg. 1994;129:706-711.
292. McMenamin D.A., Gates L.K.Jr. A retrospective analysis of the effect of contrast-enhanced CT on the outcome of acute pancreatitis. Am J Gastroenterol. 1996;91:1384-1387.
293. Ranson J.H., Rifkind K.M., Roses D.F., et al. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974;139:69-81.
294. Balthazar E.J., Ranson J.H., Naidich D.P., et al. Acute pancreatitis: Prognostic value of CT. Radiology. 1985;156:767-772.
295. Balthazar E.J., Robinson D.L., Megibow A.J., Ranson J.H. Acute pancreatitis: Value of CT in establishing prognosis. Radiology. 1990;174:331-336.
296. Clavien P.A., Hauser H., Meyer P., Rohner A. Value of contrast-enhanced computerized tomography in the early diagnosis and prognosis of acute pancreatitis: A prospective study of 202 patients. Am J Surg. 1988;155:457-466.
297. London N.J., Neoptolemos J.P., Lavelle J., et al. Contrast-enhanced abdominal computed tomography scanning and prediction of severity of acute pancreatitis: A prospective study. Br J Surg. 1989;76:268-272.
298. Basterra G., Alvarez M., Marcaide A., et al. Acute pancreatitis: Evaluation of the prognostic criteria of the latest Balthazar tomographic classification. Rev Esp Enferm Dig. 1999;91:433-438.
299. Corfield A.P., Cooper M.J., Williamson R.C., et al. Prediction of severity in acute pancreatitis: Prospective comparison of three prognostic indices. Lancet. 1985;2:403-407.
300. Ju S., Chen F., Liu S., et al. Value of CT and clinical criteria in assessment of patients with acute pancreatitis. Eur J Radiol. 2006;57:102-107.
301. van den Biezenbos A.R., Kruyt P.M., Bosscha K., et al. Added value of CT criteria compared to the clinical SAP score in patients with acute pancreatitis. Abdom Imaging. 1998;23:622-626.
302. Rickes S., Uhle C., Kahl S., et al. Echo enhanced ultrasound: A new valid initial imaging approach for severe acute pancreatitis. Gut. 2006;55:74-78.
303. Lecesne R., Taourel P., Bret P.M., et al. Acute pancreatitis: Interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727-735.
304. Ward J., Chalmers A.G., Guthrie A.J., et al. T2-weighted and dynamic enhanced MRI in acute pancreatitis: Comparison with contrast enhanced CT. Clin Radiol. 1997;52:109-114.
305. American College of Radiology: ACR appropriateness criteria: Palpable Abdominal Mass. 2011. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/PalpableAbdominalMassDoc10.aspx.
306. American College of Radiology: ACR appropriateness criteria: Acute abdominal pain and fever or suspected abdominal abscess. 2008. (Accessed at http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/AcuteAbdominalPainandFeverorSuspectedAbdominalAbscessDoc1.aspx.)
307. Guillaumin E., Jeffrey R.B.Jr., Shea W.J., et al. Perirectal inflammatory disease: CT findings. Radiology. 1986;161:153-157.
308. Kaminski A., Liu I.L., Applebaum H., et al. Routine interval appendectomy is not justified after initial nonoperative treatment of acute appendicitis. Arch Surg. 2005;140:897-901.
309. American College of Radiology: ACR appropriateness criteria: Liver Lesion—Initial Characterization. 2010. Available at: http://www.acr.org/SecondaryMainMenuCategories/quality_safety/app_criteria/pdf/ExpertPanelonGastrointestinalImaging/LiverLesionCharacterizationDoc9.aspx.
310. Catalano O., Cusati B., Nunziata A., Siani A. Active abdominal bleeding: Contrast-enhanced sonography. Abdom Imaging. 2006;31:9-16.
311. Gayer G., Hertz M., Manor H., et al. Dense ascites: CT manifestations and clinical implications. Emerg Radiol. 2004;10:262-267.
312. Drasin T.E., Anderson S.W., Asandra A., et al. MDCT evaluation of blunt abdominal trauma: Clinical significance of free intraperitoneal fluid in males with absence of identifiable injury. AJR Am J Roentgenol. 2008;191:1821-1826.
313. Kanematsu M., Hoshi H., Murakami T., et al. Spontaneous bacterial peritonitis in cirrhosis: Enhancement of ascites on delayed MR imaging. Radiat Med. 1997;15:185-187.
314. Felman A.H. Detection of glass in soft tissue by x-ray. Pediatrics. 1970;45:478-480.
315. Goh B.K., Tan Y.M., Lin S.E., et al. CT in the preoperative diagnosis of fish bone perforation of the gastrointestinal tract. AJR Am J Roentgenol. 2006;187:710-714.
316. Hierholzer J., Cordes M., Tantow H., et al. Drug smuggling by ingested cocaine-filled packages: Conventional x-ray and ultrasound. Abdom Imaging. 1995;20:333-338.
317. Hommeyer S.C., Hamill G.S., Johnson J.A. CT diagnosis of intestinal ascariasis. Abdom Imaging. 1995;20:315-316.
318. Panzini L., Burrell M.I., Traube M. Instrumental esophageal perforation: Chest film findings. Am J Gastroenterol. 1994;89:367-370.
319. Stark P., Thordarson S., McKinney M. Manifestations of esophageal disease on plain chest radiographs. AJR Am J Roentgenol. 1990;155:729-734.
320. James A.E.Jr., Montali R.J., Chaffee V., et al. Barium or Gastrografin: Which contrast media for diagnosis of esophageal tears? Gastroenterology. 1975;68:1103-1113.
321. Brick S.H., Caroline D.F., Lev-Toaff A.S., et al. Esophageal disruption: Evaluation with iohexol esophagography. Radiology. 1988;169:141-143.
322. Marco De Lucas E., Sadaba P., Lastra Garcia-Baron P., et al. Value of helical computed tomography in the management of upper esophageal foreign bodies. Acta Radiol. 2004;45:369-374.
323. Young C.A., Menias C.O., Bhalla S., Prasad S.R. CT features of esophageal emergencies. Radiographics. 2008;28:1541-1553.
324. Hogan B.A., Winter D.C., Broe D., et al. Prospective trial comparing contrast swallow, computed tomography and endoscopy to identify anastomotic leak following oesophagogastric surgery. Surg Endosc. 2008;22:767-771.
325. Ponzo F., Zhuang H., Liu F.M., et al. Tc-99m sulfur colloid and Tc-99m tagged red blood cell methods are comparable for detecting lower gastrointestinal bleeding in clinical practice. Clin Nucl Med. 2002;27:405-409.
326. Smith R., Copely D.J., Bolen F.H. 99mTc RBC scintigraphy: Correlation of gastrointestinal bleeding rates with scintigraphic findings. AJR Am J Roentgenol. 1987;148:869-874.
327. Gunderman R., Leef J., Ong K., et al. Scintigraphic screening prior to visceral arteriography in acute lower gastrointestinal bleeding. J Nucl Med. 1998;39:1081-1083.
328. Suzman M.S., Talmor M., Jennis R., et al. Accurate localization and surgical management of active lower gastrointestinal hemorrhage with technetium-labeled erythrocyte scintigraphy. Ann Surg. 1996;224:29-36.
329. Defreyne L., Uder M., Vanlangenhove P., et al. Angiography for acute lower gastrointestinal hemorrhage: Efficacy of cut film compared with digital subtraction techniques. J Vasc Interv Radiol. 2003;14:313-322.
330. Ernst O., Bulois P., Saint-Drenant S., et al. Helical CT in acute lower gastrointestinal bleeding. Eur Radiol. 2003;13:114-117.
331. Yamaguchi T., Yoshikawa K. Enhanced CT for initial localization of active lower gastrointestinal bleeding. Abdom Imaging. 2003;28:634-636.
332. Rajan R., Dhar P., Praseedom R.K., et al. Role of contrast CT in acute lower gastrointestinal bleeding. Dig Surg. 2004;21:293-296.
333. Duchesne J., Jacome T., Serou M., et al. CT-angiography for the detection of a lower gastrointestinal bleeding source. Am Surg. 2005;71:392-397.
334. Lim C.M., Shridhar I., Tan L., Cheah W.K. Contrast CT in localization of acute lower gastrointestinal bleeding. Asian J Surg. 2006;29:92-94.
335. Sabharwal R., Vladica P., Chou R., Law W.P. Helical CT in the diagnosis of acute lower gastrointestinal haemorrhage. Eur J Radiol. 2006;58:273-279.
336. Scheffel H., Pfammatter T., Wildi S., et al. Acute gastrointestinal bleeding: Detection of source and etiology with multi-detector-row CT. Eur Radiol. 2007;17:1555-1565.
337. Jaeckle T., Stuber G., Hoffmann M.H., et al. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol. 2008;18:1406-1413.
338. Zink S.I., Ohki S.K., Stein B., et al. Noninvasive evaluation of active lower gastrointestinal bleeding: Comparison between contrast-enhanced MDCT and 99mTc-labeled RBC scintigraphy. AJR Am J Roentgenol. 2008;191:1107-1114.
339. Lee S., Welman C.J., Ramsay D. Investigation of acute lower gastrointestinal bleeding with 16- and 64-slice multidetector CT. J Med Imaging Radiat Oncol. 2009;53:56-63.