CHAPTER 3 Leukocyte Migration into Tissues
A unique property of the immune system that distinguishes it from all other tissue systems in the body is the constant and highly regulated movement of its major cellular components through the blood, into tissues, and often back into the blood again. This movement accomplishes three main functions (Fig. 3-1):
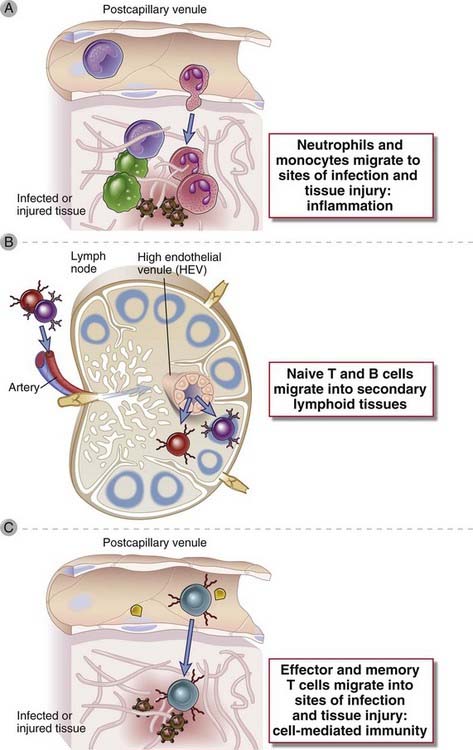
FIGURE 3–1 The main functions served by leukocyte migration from blood into tissues.
A, Neutrophils and monocytes that arise in the bone marrow are recruited into tissue sites of infection or injury, where they eliminate infectious pathogens, clear dead tissues, and repair the damage. B, Naive lymphocytes that arise in bone marrow or thymus home to secondary lymphoid organs, such as lymph nodes (or spleen, not shown), where they become activated by antigens and differentiate into effector lymphocytes. C, Effector lymphocytes arising in secondary lymphoid organs migrate into tissue sites of infection, where they participate in microbial defense.
The migration of a particular type of leukocyte into a restricted type of tissue, or a tissue with an ongoing infection or injury, is often called leukocyte homing, and the general process of leukocyte movement from blood into tissues is called recruitment. The migration of leukocytes to tissues follows several general principles.
Leukocyte recruitment from the blood into tissues depends first on adhesion of the leukocytes to the endothelial lining of postcapillary venules and then movement through the endothelium and underlying basement membrane into the extravascular tissue. This is a multistep process in which each step is orchestrated by different types of molecules, including chemokines and adhesion molecules. The same basic process occurs for different types of leukocytes (neutrophils, monocytes, and naive and effector lymphocytes) homing to different types of tissues (secondary lymphoid organs, infected tissues), although the specific chemokines and adhesion molecules vary in ways that result in different migration properties for each cell type. Before describing the process, we discuss the properties and functions of the adhesion molecules and chemokines that are involved in leukocyte recruitment.
Adhesion Molecules on Leukocytes and Endothelial Cells Involved in Leukocyte Recruitment
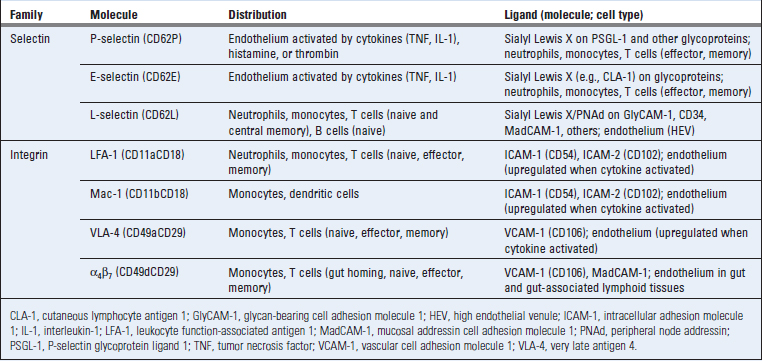
The migration of leukocytes from the blood into tissues involves adhesion between the circulating leukocytes and vascular endothelial cells as a prelude to the movement of the leukocytes out of the vessels into the tissues. This adhesion is mediated by two classes of molecules, called selectins and integrins, and their ligands. The expression of these molecules varies among different types of leukocytes and in blood vessels at different locations. We next describe the major selectins and integrins and their ligands and their roles in leukocyte recruitment into tissues.
Selectins and Selectin Ligands
Selectins are plasma membrane carbohydrate-binding adhesion molecules that mediate an initial step of low-affinity adhesion of circulating leukocytes to endothelial cells lining postcapillary venules (Table 3-1). The extracellular domains of selectins are similar to C-type lectins, so called because they bind carbohydrate structures (the definition of lectins) in a calcium-dependent manner. Selectins and their ligands are expressed on leukocytes and endothelial cells.
Two types of selectins are expressed by endothelial cells, called P-selectin (CD62P) and E-selectin (CD62E). P-selectin, so called because it was first found in platelets, is stored in cytoplasmic granules of endothelial cells and is rapidly redistributed to the surface in response to microbial products, cytokines, histamine from mast cells, and thrombin generated during blood coagulation. E-selectin is synthesized and expressed on the endothelial cell surface within 1 to 2 hours in response to the cytokines interleukin-1 (IL-1) and tumor necrosis factor (TNF) and microbial products such as lipopolysaccharide (LPS). We will discuss IL-1, TNF, and LPS in our discussion of inflammation in Chapter 4.
The ligands on leukocytes that bind to E-selectin and P-selectin on endothelial cells are complex sialylated carbohydrate groups related to the Lewis X or Lewis A family, present on various surface glycoproteins of granulocytes, monocytes, and some previously activated effector and memory T cells. The best defined of these is the tetrasaccharide sialyl Lewis X (sLeX). A leukocyte membrane glycoprotein called P-selectin glycoprotein ligand 1 (PSGL-1) is post-translationally modified to display the carbohydrate ligands for P-selectin. Several different molecules may display the carbohydrate ligands for E-selectin, including the glycoproteins PSGL-1 and E-selectin ligand 1 and some glycolipids.
A third selectin, called L-selectin (CD62L), is expressed on leukocytes but not on endothelial cells. The ligands for L-selectin are sialomucins displayed on high endothelial venules, collectively called peripheral node addressin (PNAd). A major recognition determinant that L-selectin binds to on these sialomucins is sialyl 6-sulfo Lewis X. The expression of these ligands is increased by cytokine activation of endothelial cells. L-selectin on neutrophils serves to bind these cells to endothelial cells that are activated by IL-1, TNF, and other cytokines produced at sites of inflammation. In adaptive immunity, L-selectin is important for naive T lymphocytes to home into lymph nodes through high endothelial venules. Leukocytes express L-selectin and the carbohydrate ligands for P-selectin and E-selectin at the tips of their microvilli, facilitating interactions with molecules on the endothelial cell surface.
Integrins and Integrin Ligands
Integrins are heterodimeric cell surface proteins composed of two noncovalently linked polypeptide chains that mediate adhesion of cells to other cells or to extracellular matrix, through specific binding interactions with various ligands. There are more than 30 different integrins, all with the same basic structure, containing one of more than 15 types of α chains and one of seven types of β chains. The extracellular globular heads of both chains contribute to interchain linking and to divalent cation-dependent ligand binding. The cytoplasmic domains of the integrins interact with cytoskeletal components (including vinculin, talin, actin, α-actinin, and tropomyosin). The name of this family of proteins derives from the idea that they coordinate (i.e., integrate) signals generated when they bind extracellular ligands with cytoskeleton-dependent motility, shape change, and phagocytic responses.
In the immune system, the most important integrins are two that are expressed on leukocytes, called LFA-1 (leukocyte function-associated antigen 1, more precisely named β2αL or CD11aCD18) and VLA-4 (very late antigen 4, or β1α4, or CD49dCD29) (see Table 3-1). One important ligand for LFA-1 is intercellular adhesion molecule 1 (ICAM-1, CD54), a membrane glycoprotein expressed on cytokine-activated endothelial cells and on a variety of other cell types, including lymphocytes, dendritic cells, macrophages, fibroblasts, and keratinocytes. The extracellular portion of ICAM-1 is composed of globular domains that share some sequence homology and tertiary structural features of domains found in immunoglobulin (Ig) molecules and are called Ig domains. (Many proteins in the immune system contain Ig domains and belong to the Ig superfamily, which is discussed in more detail in Chapter 5.) LFA-1 binding to ICAM-1 is important for leukocyte-endothelial interactions (discussed later) and T cell interactions with antigen-presenting cells (see Chapter 6). Two other Ig superfamily ligands for LFA-1 are ICAM-2, which is expressed on endothelial cells, and ICAM-3, which is expressed on lymphocytes. VLA-4 binds to vascular cell adhesion molecule 1 (VCAM-1, CD106), an Ig superfamily protein expressed on cytokine-activated endothelial cells in some tissues, and this interaction is important for leukocyte recruitment into inflammatory sites. Other integrins also play roles in innate and adaptive immune responses. For example, Mac-1 (β2αm, CD11bCD18) on circulating monocytes binds to ICAM-1 and mediates adhesion to endothelium. Mac-1 also functions as a complement receptor, binding particles opsonized with a product of complement activation called the inactivated C3b (iC3b) fragment, and thereby enhances phagocytosis of microbes.
An important feature of integrins is their ability to respond to intracellular signals by rapidly increasing their affinity for their ligands (Fig. 3-2). This is referred to as activation and occurs in response to signals generated from chemokine binding to chemokine receptors and in lymphocytes by intracellular signals generated when antigen binds to antigen receptors. The process of changes in the binding functions of the extracellular domain of integrins induced by intracellular signals is called inside-out signaling. Chemokine- and antigen receptor–induced inside-out signaling involves GTP-binding proteins (described in more detail later), eventually leading to the association of RAP family molecules and cytoskeleton-interacting proteins with the cytoplasmic tails of the integrin proteins. The resulting affinity changes are a consequence of conformational changes in the extracellular domains. In the low-affinity state, the stalks of the extracellular domains of each integrin subunit appear to be bent over, and the ligand-binding globular heads are close to the membrane. In response to alterations in the cytoplasmic tail, the stalks extend in switchblade fashion, bringing the globular heads away from the membrane to a position where they more effectively interact with their ligands (see Fig. 3-2).
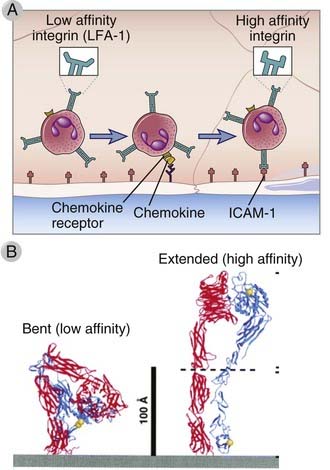
FIGURE 3–2 Integrin activation.
A, The integrins on blood leukocytes are normally in a low-affinity state. If a leukocyte comes close to endothelial cells, such as when selectin-dependent rolling of leukocytes occurs, then chemokines displayed on the endothelial surface can bind chemokine receptors on the leukocyte. Chemokine receptor signaling then occurs, which activates the leukocyte integrins, increasing their affinity for their ligands on the endothelial cells. B, Ribbon diagrams are shown of bent and extended conformations of a leukocyte integrin, corresponding to low- and high-affinity states, respectively.
(B From Takagi J, and TA Springer. Integrin activation and structural rearrangement. Immunological Reviews 186:141-163, 2002.)
Chemokines also induce membrane clustering of integrins. This results in increased avidity of integrin interactions with ligands on the endothelial cells, and therefore tighter binding of the leukocytes to the endothelium.
Chemokines and Chemokine Receptors
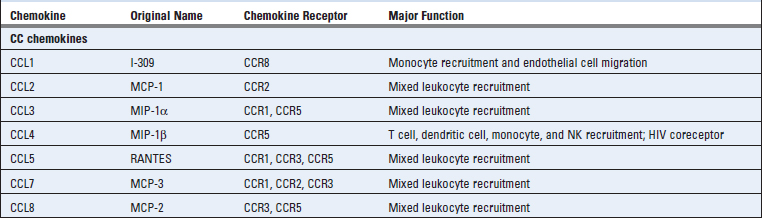
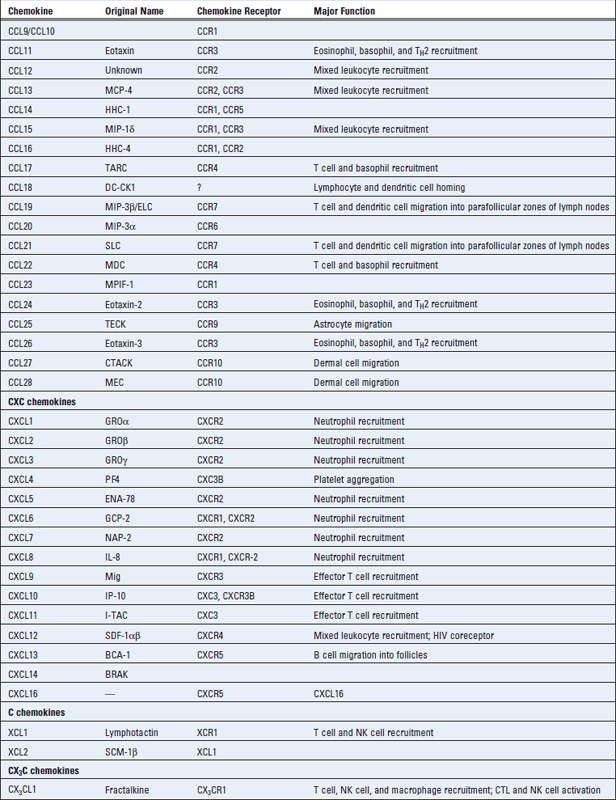
Chemokines are a large family of structurally homologous cytokines that stimulate leukocyte movement and regulate the migration of leukocytes from the blood to tissues. The name chemokine is a contraction of “chemotactic cytokine.” We have already referred to the role of chemokines in the organization of lymphoid tissue and now we will describe the general properties of this family of cytokines and summarize their multiple roles in innate and adaptive immunity. Table 3-2 summarizes the major features of individual chemokines and their receptors.
Chemokine Structure, Production, and Receptors
There are about 50 human chemokines, all of which are 8- to 12-kD polypeptides that contain two internal disulfide loops. The chemokines are classified into four families on the basis of the number and location of N-terminal cysteine residues. The two major families are the CC (also called β) chemokines, in which the cysteine residues are adjacent, and the CXC (or α) family, in which these residues are separated by one amino acid. These differences correlate with organization of the subfamilies into separate gene clusters. A small number of chemokines have a single cysteine (C family) or two cysteines separated by three amino acids (CX3C). Chemokines were originally named on the basis of how they were identified and what responses they triggered. More recently, a standard nomenclature, based in part on which receptors the chemokines bind to (see Table 3-2), is being used. Although there are exceptions, most of the CC chemokines and their receptors mediate recruitment of neutrophils and lymphocytes, and most of the CXC chemokines and their receptors recruit monocytes and lymphocytes.
The chemokines of the CC and CXC subfamilies are produced by leukocytes and by several types of tissue cells, such as endothelial cells, epithelial cells, and fibroblasts. In many of these cells, secretion of chemokines is induced by recognition of microbes through various cell receptors of the innate immune system discussed in Chapter 4. In addition, inflammatory cytokines, mainly TNF and IL-1, induce chemokine production. Several CC chemokines are also produced by antigen-stimulated T cells, providing a link between adaptive immunity and recruitment of inflammatory leukocytes.
The receptors for chemokines belong to the seven-transmembrane, guanosine triphosphate (GTP)–binding (G) protein–coupled receptor (GPCR) superfamily. These receptors initiate intracellular responses through associated trimeric G proteins. In a resting cell, the receptor-associated G proteins form a stable inactive complex containing guanosine diphosphate (GDP) bound to Gα subunits. Occupancy of the receptor by ligand results in an exchange of GTP for GDP. The GTP-bound form of the G protein activates numerous cellular enzymes, including an isoform of phosphatidylinositol-specific phospholipase C that functions to increase intracellular calcium and activate protein kinase C. The G proteins stimulate cytoskeletal changes and polymerization of actin and myosin filaments, resulting in increased cell motility. These signals also change the conformation of cell surface integrins and increase the affinity of the integrins for their ligands. Chemokine receptors may be rapidly downregulated by exposure to the chemokine, and this is a likely mechanism for termination of responses.
Different combinations of more than 17 different chemokine receptors are expressed on different types of leukocytes, which results in distinct patterns of migration of the leukocytes. There are 10 distinct receptors for CC chemokines (called CCR1 through CCR10), six for CXC chemokines (called CXCR1 through CXCR6), and one for CX3CL1 (called CX3CR1) (see Table 3-2). Chemokine receptors are expressed on all leukocytes, with the greatest number and diversity seen on T cells. The receptors exhibit overlapping specificity for chemokines within each family, and the pattern of cellular expression of the receptors determines which cell types respond to which chemokines. Certain chemokine receptors, notably CCR5 and CXCR4, act as coreceptors for the human immunodeficiency virus (HIV) (see Chapter 20). Some activated T lymphocytes secrete chemokines that bind to CCR5 and block infection with HIV by competing with the virus.
Biologic Actions of Chemokines
Some chemokines are produced by leukocytes and other cells in response to external stimuli and are involved in inflammatory reactions, and other chemokines are produced constitutively in tissues and play a role in tissue organization. Chemokines were discovered on the basis of their activity as leukocyte chemoattractants, and this action is the main basis of their functional roles.
Leukocyte-Endothelial Interactions and Leukocyte Extravasation
Selectins, integrins, and chemokines work in concert to govern the leukocyte-endothelial interactions that are required for migration of leukocytes into tissues (Fig. 3-3). Studies of these interactions under flow conditions in vitro, and in vivo, by use of intravital microscopic techniques, have established a sequence of events common to migration of most leukocytes into most tissues. These events include the following.
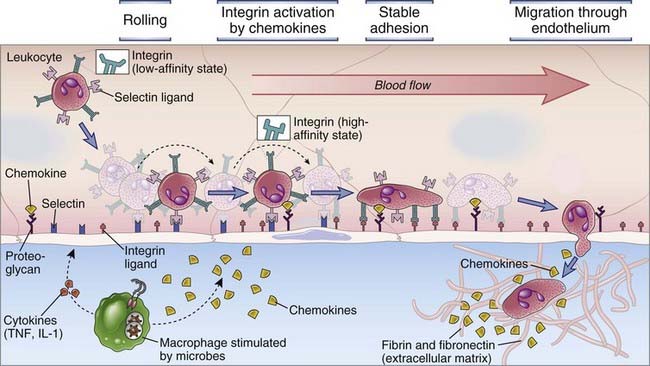
FIGURE 3–3 Multistep leukocyte-endothelial interactions mediating leukocyte recruitment into tissues.
At sites of infection, macrophages that have encountered microbes produce cytokines (such as TNF and IL-1) that activate the endothelial cells of nearby venules to produce selectins, ligands for integrins, and chemokines. Selectins mediate weak tethering and rolling of blood leukocytes on the endothelium, and the shear force of blood flow causes the leukocytes to roll along the endothelial surface. Chemokines produced in the surrounding infected tissues or by the endothelial cells are displayed on the endothelial surface and bind to receptors on the rolling leukocytes, which results in activation of the leukocyte integrins to a high-affinity binding state. The activated integrins bind to their Ig superfamily ligands on the endothelial cells, and this mediates firm adhesion of leukocytes. The leukocytes then crawl to junctions between endothelial cells and migrate through the venular wall. Neutrophils, monocytes, and T lymphocytes use essentially the same mechanisms to migrate out of the blood.
There is specificity in this process of leukocyte migration based on the expression of distinct combinations of adhesion molecule and chemokine receptors on neutrophils, monocytes, and different subsets of lymphocytes, as we will discuss in more detail later.
Evidence for the essential role of selectins, integrins, and chemokines in leukocyte migration has come from gene knockout mice and rare human diseases caused by gene mutations. For example, mice lacking fucosyltransferases, which are enzymes required to synthesize the carbohydrate ligands that bind to selectins, have marked defects in leukocyte migration and immune responses. Humans who lack one of the enzymes needed to express the carbohydrate ligands for E-selectin and P-selectin on neutrophils have similar problems, resulting in a syndrome called type 2 leukocyte adhesion deficiency (LAD-2) (see Chapter 20). Similarly, an autosomal recessive inherited deficiency in the CD18 gene, which encodes the β subunit of LFA-1 and Mac-1, is the cause of an immune deficiency disease called type 1 leukocyte adhesion deficiency (LAD-1). These disorders are characterized by recurrent bacterial and fungal infections, lack of neutrophil accumulation at sites of infection, and defects in adherence-dependent lymphocyte functions. Rare human mutations in the signaling pathways linking chemokine receptors to integrin activation also result in impaired leukocyte adhesion and recruitment into tissues and therefore ineffective leukocyte defense against infections.
Migration of Neutrophils and Monocytes to Sites of Infection or Tissue Injury
After maturing in the bone marrow, neutrophils and monocytes enter the blood and circulate throughout the body. Although these cells can perform some phagocytic functions within the blood, their main functions, including phagocytosis of microbes and dead tissue cells, take place in extravascular sites of infection virtually anywhere in the body.
Blood neutrophils and monocytes are recruited to tissue sites of infection and injury by a selectin-, integrin-, and chemokine-dependent multistep process, which follows the basic sequence common to the migration of all leukocytes into tissues, discussed before. Cytokines (TNF and IL-1) secreted during the innate immune response to microbes induce the expression of adhesion molecules (selectins and integrin ligands) on endothelial cells and the local production of chemokines. Neutrophils and monocytes in the circulation bind to these adhesion molecules and respond to the chemokines, resulting in recruitment of the leukocytes into the tissues.
Neutrophils and monocytes express distinct sets of adhesion molecules and chemokine receptors and therefore migrate into different inflammatory sites or into the same inflammatory site at different times. As we will discuss in detail in Chapter 4, neutrophils are the first type of leukocyte to be recruited from the blood into a site of infection or tissue injury. Monocyte recruitment follows hours later and continues, perhaps for days, after neutrophil recruitment stops. Furthermore, in some inflammatory sites, neutrophils are not recruited at all, but monocytes are. These different migratory behaviors reflect variations in expression of adhesion molecules and chemokine receptors on neutrophils and monocytes and the fact that different chemokines are expressed in different sites or at different times in the same site. Both monocytes and neutrophils express L-selectin and P- and E-selectin ligands and use all three selectins to mediate initial rolling interactions with cytokine-activated endothelial cells. Neutrophils express the integrins LFA-1 and Mac-1, which, on activation, bind to endothelial ICAM-1 and mediate stable arrest of the cells on the vessel wall. Monocytes express the integrins LFA-1 and VLA-4, which bind to endothelial ICAM-1 and VCAM-1, causing stable arrest of these leukocytes.
The chemokine receptors expressed on neutrophils and monocytes are also different, which is probably the major determinant of the divergent migratory behavior of each cell type. Neutrophils express CXCR1 and CXCR2, which bind GRO family chemokines including CXCL8 (IL-8), the major chemokine supporting neutrophil migration into tissues (see Table 3-2). Thus, early neutrophil recruitment reflects early and abundant CXCL8 production by tissue resident macrophages in response to infections. There are at least two populations of monocytes in the blood, and in both humans and mice, the populations are defined, in part, by chemokine receptor expression. Inflammatory monocytes, which are the main type recruited to inflammatory sites, express CCR2 in both mice and humans. This receptor binds several chemokines, but the most important one for monocyte recruitment is CCL2 (MCP-1). Thus, monocyte recruitment occurs when resident tissue cells express CCL2 in response to infection. The other population of monocytes, sometimes called nonclassical, lacks CCR2 but expresses CX3CR1. The ligand for this receptor, CX3CL1, is expressed both in soluble form and as a membrane-bound molecule that can support adhesion of monocytes to endothelium.
Once neutrophils enter inflammatory sites, they perform various effector functions, which we will describe in Chapter 4, and die within hours. Monocytes become macrophages in the tissues and perform their effector functions during the course of many days to weeks. Some macrophages may migrate into lymph nodes through draining lymphatics.
Migration and Recirculation of T Lymphocytes
Lymphocytes are continuously moving through the blood stream, lymphatics, secondary lymphoid tissues, and peripheral nonlymphoid tissues, and functionally distinct populations of lymphocytes show different trafficking patterns through these sites (Fig. 3-4). When a mature naive T cell emerges from the thymus and enters the blood, it homes to lymph nodes, spleen, or mucosal lymphoid tissues and migrates into the T cell zones of these secondary lymphoid tissues. If the T cell does not recognize antigen in these sites, it remains naive and leaves the nodes or mucosal tissue through lymphatics and eventually drains back into the blood stream. Naive T cells leave the spleen directly through the circulation. Once back in the blood, a naive T cell repeats its homing to other secondary lymph nodes. This traffic pattern of naive lymphocytes, called lymphocyte recirculation, maximizes the chances that the limited number of naive lymphocytes emerging from the thymus that are specific for a particular foreign antigen will encounter the antigen if it shows up anywhere in the body. Lymphocytes that have recognized and become activated by antigen proliferate and differentiate to produce thousands of effector and memory cells within secondary lymphoid tissues. The effector and memory lymphocytes may move back into the blood stream and then migrate into sites of infection or inflammation in peripheral (nonlymphoid) tissues. Some effector lymphocyte subsets preferentially migrate into a particular tissue, such as skin or gut. The process by which particular populations of lymphocytes selectively enter lymph nodes or particular tissues but not others is called lymphocyte homing. The existence of different homing patterns ensures that different subsets of lymphocytes are delivered to the tissue microenvironments where they are required to combat different types of microbes and not, wastefully, to places where they would serve no purpose. In the following section, we describe the mechanisms and pathways of lymphocyte recirculation and homing. Our discussion emphasizes T cells because much more is known about their movement through tissues than is known about B cell recirculation.
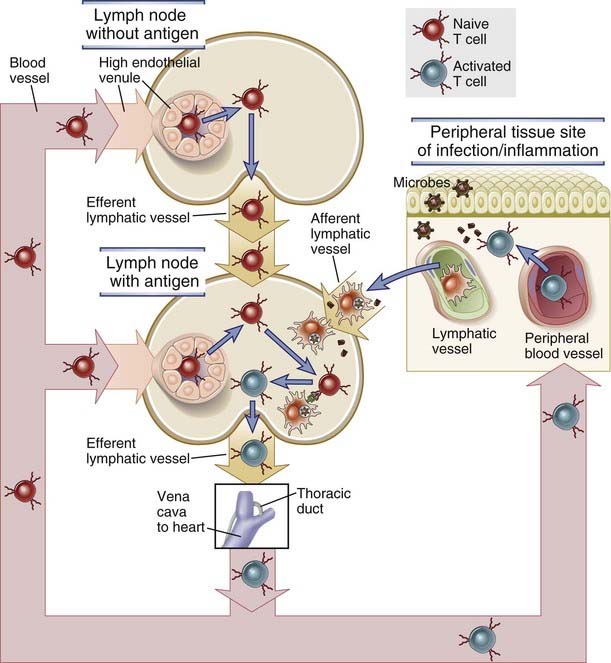
FIGURE 3–4 Pathways of T lymphocyte recirculation.
Naive T cells preferentially leave the blood and enter lymph nodes across the high endothelial venules. Dendritic cells bearing antigen enter the lymph node through lymphatic vessels. If the T cells recognize antigen, they are activated, and they return to the circulation through the efferent lymphatics and the thoracic duct, which empties into the superior vena cava, then into the heart, and ultimately into the arterial circulation. Effector and memory T cells preferentially leave the blood and enter peripheral tissues through venules at sites of inflammation. Recirculation through peripheral lymphoid organs other than lymph nodes is not shown.
Recirculation of Naive T Lymphocytes between Blood and Secondary Lymphoid Organs
T lymphocyte recirculation depends on mechanisms that control entry of naive T cells from the blood into lymph nodes as well as molecular signals that control when naive T cells exit the nodes. We will discuss these two mechanisms separately.
Migration of Naive T Cells into Lymph Nodes
The homing mechanisms that bring naive T cells into lymph nodes are very efficient, resulting in a net flux of lymphocytes through lymph nodes of up to 25 × 109 cells each day. Each lymphocyte goes through one node once a day on average. Peripheral tissue inflammation, which usually accompanies infections, causes a significant increase of blood flow into lymph nodes and consequently an increase in T cell influx into lymph nodes draining the site of inflammation. At the same time, egress of the T cells into efferent lymphatics is transiently reduced by mechanisms we will discuss later, so that T cells stay in lymph nodes that drain sites of inflammation longer than in other lymph nodes. Antigens are concentrated in the secondary lymphoid organs, including lymph nodes, mucosal lymphoid tissues, and spleen, where they are presented by mature dendritic cells, the type of antigen-presenting cell that is best able to initiate responses of naive T cells (see Chapter 6). Thus, movement and transient retention of naive T cells in the secondary lymphoid organs maximizes the chances of specific encounter with antigen and initiation of an adaptive immune response.
Homing of naive T cells into lymph nodes and mucosa-associated lymphoid tissues occurs through specialized postcapillary venules called high endothelial venules (HEVs) located in the T cell zones. Naive T lymphocytes are delivered to secondary lymphoid tissues through arterial blood flow, and they leave the circulation and migrate into the stroma of lymph nodes through HEVs. These vessels are lined by plump endothelial cells and not the flat endothelial cells that are typical of other venules (Fig. 3-5). HEVs are also present in mucosal lymphoid tissues, such as Peyer’s patches in the gut, but not in the spleen. The endothelial cells of HEVs are specialized to display certain adhesion molecules and chemokines on their surfaces, discussed later, which support the selective homing of only certain populations of lymphocytes. Certain cytokines, such as lymphotoxin, are required for HEV development. In fact, HEVs may develop in extralymphoid sites of chronic inflammation where such cytokines are produced for prolonged periods.
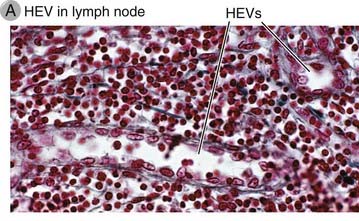
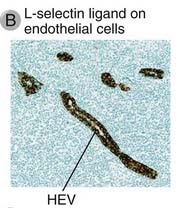
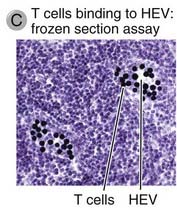
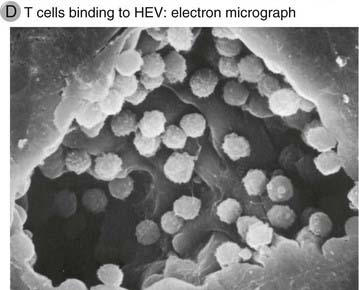
FIGURE 3–5 High endothelial venules.
A, Light micrograph of an HEV in a lymph node illustrating the tall endothelial cells.
(Courtesy of Dr. Steve Rosen, Department of Anatomy, University of California, San Francisco.)
B, Expression of L-selectin ligand on HEVs, stained with a specific antibody by the immunoperoxidase technique. (The location of the antibody is revealed by a brown reaction product of peroxidase, which is coupled to the antibody; see Appendix IV for details.) The HEVs are abundant in the T cell zone of the lymph node.
(Courtesy of Drs. Steve Rosen and Akio Kikuta, Department of Anatomy, University of California, San Francisco.)
C, A binding assay in which lymphocytes are incubated with frozen sections of a lymph node. The lymphocytes (stained dark blue) bind selectively to HEVs.
(Courtesy of Dr. Steve Rosen, Department of Anatomy, University of California, San Francisco.)
D, Scanning electron micrograph of an HEV with lymphocytes attached to the luminal surface of the endothelial cells.
(Courtesy of J. Emerson and T. Yednock, University of California, San Francisco, School of Medicine. From Rosen SD, and LM Stoolman. Potential role of cell surface lectin in lymphocyte recirculation. In Olden K, and J Parent [eds]. Vertebrate Lectins. Van Nostrand Reinhold, New York, 1987.)
Naive T cell migration out of the blood through the HEVs into the lymph node parenchyma is a multistep process consisting of selectin-mediated rolling of the cells, chemokine-induced integrin activation, integrin-mediated firm adhesion, and transmigration through the vessel wall (Fig. 3-6). This process is similar to the migration of other leukocytes, described earlier. The adhesion molecules expressed on lymphocytes are often called homing receptors, and the adhesion molecules that the homing receptors bind to on the endothelial cells are called addressins. The sequential events involved in homing of naive T cells to lymph nodes and the molecules involved are the following.
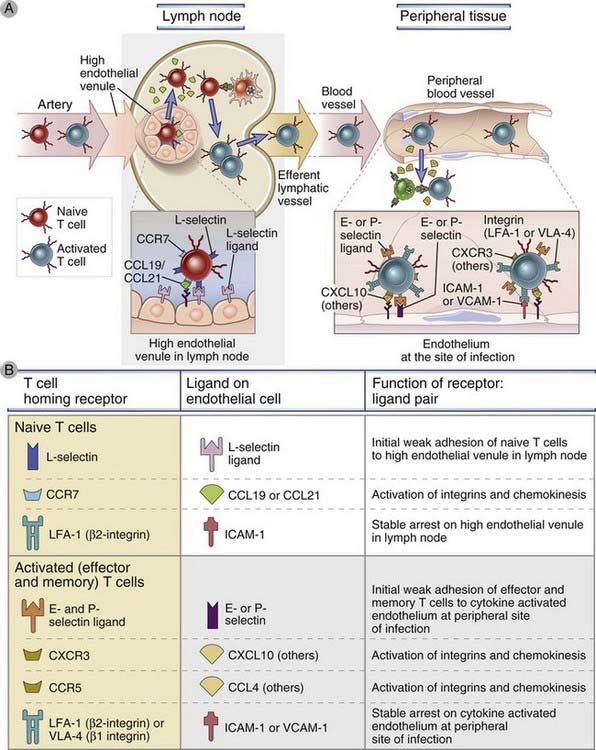
FIGURE 3–6 Migration of naive and effector T lymphocytes.
A, Naive T lymphocytes home to lymph nodes as a result of L-selectin binding to its ligand on high endothelial venules, which are present only in lymph nodes, and as a result of binding chemokines (CCL19 and CCL21) displayed on the surface of the high endothelial venule. Activated T lymphocytes, including effector cells, home to sites of infection in peripheral tissues, and this migration is mediated by E-selectin and P-selectin, integrins, and chemokines that are produced at sites of infection. Additional chemokines and chemokine receptors, besides the ones shown, are involved in effector/memory T cell migration. B, The adhesion molecules, chemokines, and chemokine receptors involved in naive and effector/memory T cell migration are described.
The important role for L-selectin and chemokines in naive T cell homing to secondary lymphoid tissues is supported by many different experimental observations. Lymphocytes from L-selectin knockout mice do not bind to peripheral lymph node HEVs, and the mice have a marked reduction in the number of lymphocytes in peripheral lymph nodes. There are very few naive T cells in the lymph nodes of mice with genetic deficiencies in CCL19 and CCL21, or CCR7, but the B cell content of these lymph nodes is relatively normal.
Exit of Naive T Cells from Lymph Nodes
Naive T cells that have homed into lymph nodes but fail to recognize antigen and to become activated will eventually return to the blood stream. This return to the blood completes one recirculation loop and provides the naive T cells another chance to enter secondary lymphoid tissues and search for the antigens they can recognize. The major route of reentry into the blood is through the efferent lymphatics, perhaps via other lymph nodes in the same chain, and then through the lymphatic vasculature to the thoracic or right lymphatic duct, and finally into the superior vena cava or right subclavian vein.
The exit of naive T cells from lymph nodes is dependent on a lipid chemoattractant called sphingosine 1-phosphate (S1P), which binds to a signaling receptor on T cells called sphingosine 1-phosphate receptor 1 (S1PR1) (Fig. 3-7). S1P is present at relatively high concentrations in the blood and lymph compared with tissues. This concentration gradient is maintained because an S1P-degrading enzyme, S1P lyase, is ubiquitously present in tissues, so the tissue concentration of the lipid is less than in the lymph and blood. S1PR1 is a G protein–coupled receptor. Signals generated by S1P binding to S1PR1 stimulate directed movement of the naive T cells along the S1P concentration gradient out of the lymph node parenchyma. Circulating naive T cells have very little surface S1PR1 because the high blood concentration of S1P causes internalization of the receptor. Once a naive T cell enters a lymph node, where S1P concentrations are low, it may take several hours for the surface S1P1R to be re-expressed. This allows time for a naive T cell to interact with antigen-presenting cells before it is directed down the S1P concentration gradient into the efferent lymphatic. S1P and the S1P1R are also required for mature naive T cell egress from the thymus, migration of activated T cells out of lymph nodes, and migration of antibody-secreting B cells from secondary lymphoid organs.
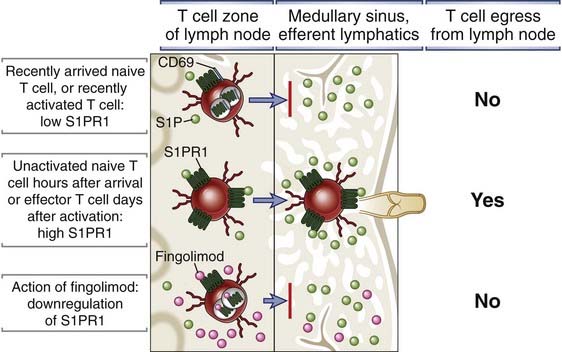
FIGURE 3–7 Mechanism of egress of lymphocytes from lymphoid organs.
T cell egress from thymus and lymph node requires the expression of a signaling receptor called S1PR1 that binds to the chemoattractant lipid sphingosine 1-phosphate (S1P). S1P concentrations in blood and lymph are much higher than in lymphoid tissues because of the action of the S1P-degrading enzyme S1P lyase in the tissues. Circulating naive T cells have low levels of S1PR1 because the receptor is internalized after binding S1P in the blood. Therefore, naive T cells that have recently entered a lymph node cannot sense the S1P concentration gradient between the T cell zone of the node and the lymph in the medullary sinus and efferent lymphatics, and these T cells cannot exit the node. After activation of a naive T cell by antigen, S1PR1 is not re-expressed for several days, and the activated cells will also not leave the node. After several hours for naive T cells or days for activated and differentiated effector T cells, S1PR1 is re-expressed, and these cells can then sense the S1P gradient and exit the node. The immunosuppressive drug fingolimod is an agonist of S1PR1 that binds S1PR1 and causes its downmodulation, and is not degraded by S1P lyase. Therefore, this drug interferes with the sensing of the S1P concentration gradient and will block exit of naive and effector cells from the lymph node and reentry into the circulation.
Our understanding of the role of S1P and S1PR1 in T cell trafficking is based in large part on studies of the effects of a drug called fingolimod (FTY720), which binds to S1P1R and causes its down-modulation from the cell surface. Fingolimod blocks T cell egress from lymphoid organs and thereby acts as an immunosuppressive drug. It is now approved for the treatment of multiple sclerosis, an autoimmune disease of the central nervous system, and there is great interest in use of fingolimod and other drugs with a similar mechanism of action to treat various autoimmune diseases or graft rejection. Additional experimental evidence for the central role of S1P in naive T cell trafficking comes from studies of mice with genetic ablation of S1PR1. In these mice, there is failure of T cells to leave the thymus and populate secondary lymphoid organs. If naive T cells from S1PR1 knockout are injected into the circulation of other mice, the cells enter lymph nodes but are unable to exit.
Recirculation of T Cells through Other Lymphoid Tissues
Naive T cell homing into gut-associated lymphoid tissues, including Peyer’s patches and mesenteric lymph nodes, is fundamentally similar to homing to other lymph nodes and relies on interactions of the T cells with HEVs, which are mediated by selectins, integrins, and chemokines. One particular feature of naive T cell homing to mesenteric lymph nodes and Peyer’s patches is the contribution of an Ig superfamily molecule called MadCAM-1 (mucosal addressin cell adhesion molecule 1), which is expressed on HEVs in these sites but not typically elsewhere in the body. Naive T cells express two ligands that bind to MadCAM-1, L-selectin and an integrin called α4β7, and both contribute to the rolling step of naive T cell homing into gut-associated lymphoid tissues.
Naive T cell migration into the spleen is not as finely regulated as homing into lymph nodes. The spleen does not contain HEVs, and it appears that naive T cells are delivered to the marginal zone and red pulp sinuses by passive mechanisms that do not involve selectins, integrins, or chemokines. However, CCR7-binding chemokines do participate in directing the naive T cells into the white pulp. Even though homing of naive T cells to the spleen appears to be less tightly regulated than homing into lymph nodes, the rate of lymphocyte passage through the spleen is very high, about half the total circulating lymphocyte population every 24 hours.
Migration of Effector T Lymphocytes to Sites of Infection
Effector T cells that have been generated by antigen-induced activation of naive T cells exit secondary lymphoid tissues through lymphatic drainage and return to the circulating blood. Many of the protective, antimicrobial functions of effector T cells must be performed locally at sites of infections, and therefore these cells must be able to leave lymphoid tissues. During differentiation of naive T cells into effector cells, which occurs in the peripheral lymphoid organs, the cells undergo a change in expression of chemokine receptors, S1PR1, and adhesion molecules, which determine the migratory behavior of these cells. The expression of S1PR1 is suppressed for several days after antigen-mediated activation of naive T cells, and therefore the ability of the cells to leave the lymphoid tissue in response to an S1P gradient is impaired. This suppression of S1PR1 is controlled in part by cytokines called type I interferons that are expressed during innate immune responses to infections, as we will discuss. Antigenic stimulation and interferons together increase the expression of a T cell membrane protein called CD69, which binds to S1PR1 and blocks its cell surface expression. Thus, the activated T cell becomes transiently insensitive to the S1P gradient. This allows the antigen-activated T cells to remain in the lymphoid organ and undergo clonal expansion and differentiation into effector T cells, a process that takes several days. When differentiation into effector cells is complete, the cells re-express S1PR1 and therefore become responsive to the concentration gradient of S1P, which is low in the lymphoid tissue and high in the draining lymph. CCR7 expression is also greatly reduced in the effector T cells, and therefore these cells are not constrained to remain in the T cell zones where the CCR7 ligands CCL19 and CCL20 are produced. These changes in S1PR1 and CCR7 expression favor egress of the effector T cells out of the lymphoid tissue into efferent lymphatics and subsequent return to the circulating blood. L-selectin expression, which is required for entry of naive T cells into secondary lymphoid tissues, is also reduced in recently differentiated effector T cells. Therefore, two essential molecules needed for reentry of T cells into secondary lymphoid organs through HEV (CCR7 and L-selectin) are lacking on the effector T cells, which prevents these cells from reentering lymphoid tissues and keeps them available for migration into infected tissues.
Circulating effector T cells preferentially home to peripheral tissue sites of infection by a selectin-, integrin-, and chemokine-dependent multistep process (see Fig. 3-6). As with neutrophils and monocytes, selective recruitment of effector T cells into sites of infection, but not into healthy tissues, is initially dependent on the innate immune response to microbes, leading to cytokine-induced expression of E-selectin, P-selectin, and integrin ligands on postcapillary venular endothelial cells, and local production of various chemokines, which are displayed on the endothelial lining of postcapillary venules. Effector T cells in the circulation express selectin ligands, integrins, and chemokine receptors that bind to the types of selectins, integrin ligands, and chemokines, respectively, that are induced by innate immune responses. The net result is enhanced T cell adhesion to endothelium and transmigration through the venule wall. Because naive T cells do not express ligands for E-selectin and P-selectin nor the chemokine receptors that bind inflammatory chemokines, they are not recruited efficiently to these sites of infection (see Fig. 3-6). Antigen-induced activation of the effector T cells in the inflamed tissues and the continued presence of chemokines keep the integrins on these cells in high-affinity states, and this favors retention of the effector T cells at these sites. Most effector cells that enter a site of infection eventually die at these sites, after performing their effector functions.
Different subsets of effector T cells exist, each with distinct functions, and these subsets have different although often overlapping patterns of migration. Effector T cells include CD8+ cytotoxic T cells and CD4+ helper T cells. Helper T cells include TH1, TH2, and TH17 subsets, each of which expresses different types of cytokines and protects against different types of microbes. The characteristics and functions of these subsets will be discussed in detail in Chapters 9 and 10. For now, it is important to know that the migration of each subset is different. This is because the array of chemokine receptors and adhesion molecules expressed by each subset differs in ways that result in preferential recruitment of each subset into inflammatory sites elicited by different types of infections.
Some effector cells have a propensity to migrate to particular types of tissues. This selective migration capacity is acquired during the differentiation of the effector T cells from naive precursors in secondary lymphoid tissues. By enabling distinct groups of effector T cells to migrate to different sites, the adaptive immune system directs cells with specialized effector functions to the locations where they are best suited to deal with particular types of infections. The clearest examples of populations of effector T cells that specifically home to different tissues are skin-homing and gut-homing T cells. Skin-homing effector T cells express a carbohydrate ligand for E-selectin called CLA-1 (cutaneous lymphocyte antigen 1) and the CCR4 and CCR10 chemokine receptors, which bind CCL17 and CCL27, chemokines that are commonly expressed in inflamed skin. Gut-homing effector T cells express the α4β7 integrin, which binds to MadCAM-1 on gut endothelial cells, and CCR9, which binds to CCL25, a chemokine expressed in inflamed bowel. Remarkably, these distinct migratory phenotypes of skin- and gut-homing effector T cells may be induced by distinct signals delivered to naive T cells at the time of antigen presentation by dendritic cells in either subcutaneous lymph nodes or gut-associated lymphoid tissues, respectively. Although the molecular basis for this imprinting of migratory phenotype is not known, there is evidence that dendritic cells in Peyer’s patches produce retinoic acid, which promotes the expression of α4β7 and CCR9 by responding T cells. Similarly, dendritic cells in the skin draining lymph nodes produce vitamin D, which instructs T cells to express CLA-1, CCR4, and CCR10. Other T cells express an integrin called CD103 (αEβ7) that can bind to E-cadherin molecules on epithelial cells, allowing T cells to maintain residence as intraepithelial lymphocytes in both skin and gut. We will discuss tissue-specific lymphocyte homing further in Chapter 13.
Memory T Cell Migration
Memory T cells are heterogeneous in their patterns of expression of adhesion molecules and chemokine receptors and in their propensity to migrate to different tissues. Because the ways of identifying memory T cells are still imperfect (see Chapter 2), the distinction between effector and memory T cells in experimental studies and humans is often not precise. Two subsets of memory T cells, namely, central memory and effector memory T cells, were initially identified on the basis of differences in CCR7 and L-selectin expression. Central memory T cells were defined as human CD45RO+ blood T cells that express high levels of CCR7 and L-selectin; effector memory T cells were defined as CD45RO+ blood T cells that express low levels of CCR7 and L-selectin but express other chemokine receptors that bind inflammatory chemokines. These phenotypes suggest that central memory T cells home to secondary lymphoid organs, whereas effector memory T cells home to peripheral tissues. Although central and effector memory T cell populations can also be detected in mice, experimental homing studies have indicated that CCR7 expression is not a definitive marker to distinguish central and effector memory T cell subpopulations. Nonetheless, it is clear that some memory T cells either remain in or tend to home to secondary lymphoid organs, whereas others migrate into peripheral tissues, especially mucosal tissues. In general, the peripheral tissue–homing effector memory T cells respond to antigenic stimulation by rapidly producing effector cytokines, whereas the lymphoid tissue–based central memory cells tend to proliferate more (providing a pool of cells for recall responses) and provide helper functions for B cells.
Migration of B Lymphocytes
Naive B cells use the same basic mechanisms as do naive T cells to home to secondary lymphoid tissues throughout the body, which enhances their likelihood of responding to microbial antigens in different sites. Immature B cells leave the bone marrow through the blood and enter the red pulp of the spleen, migrate to the periphery of the white pulp, and then, as they mature further, move into the white pulp in response to a chemokine called CXCL13, which binds to the chemokine receptor CXCR5 expressed by the B cell. Once the maturation is completed within the white pulp, naive follicular B cells reenter the circulation and home to lymph nodes and mucosal lymphoid tissues. Homing of naive B cells from the blood into lymph nodes involves rolling interactions on HEVs, chemokine activation of integrins, and stable arrest, as described earlier for naive T cells. Once naive B cells enter the stroma of secondary lymphoid organs, they migrate into follicles, the site where they may encounter antigen and become activated. This migration of naive B cells into follicles is mediated by CXCL13, which is produced in follicles and binds to the CXCR5 receptor on naive B cells. Homing of naive B cells into Peyer’s patches involves CXCR5 and the integrin α4β7, which binds to MadCAM-1. During the course of B cell responses to protein antigens, B cells and helper T cells must directly interact, and this is made possible by highly regulated movements of both cell types within the secondary lymphoid organs. These local migratory events, and the chemokines that orchestrate them, will be discussed in detail in Chapter 11.
Egress of B cells from secondary lymphoid organs depends on S1P1. This has been most clearly shown for differentiated antibody-secreting B cells, which leave the secondary lymphoid organs in which they were generated from naive B cells by antigen activation and home to bone marrow or tissue sites. S1PR1-deficient antibody-secreting cells have diminished ability to home from spleen to bone marrow or to form gut-associated lymphoid tissues. Presumably, naive B cells that have entered secondary lymphoid tissues but do not become activated by antigen reenter the circulation, like naive T cells do, but how this process is controlled is not clear.
Subsets of B cells committed to producing particular types of antibodies migrate from secondary lymphoid organs into specific tissues. As we will describe in later chapters, different populations of activated B cells may secrete different types of antibodies, called isotypes, each of which performs a distinct set of effector functions. Many antibody-producing plasma cells migrate to the bone marrow, where they secrete antibodies for long periods. Most bone marrow–homing plasma cells produce IgG antibodies, which are then distributed throughout the body through the blood stream. B cells within mucosa-associated lymphoid tissues usually become committed to expression of the IgA isotype of antibody, and these committed cells may home specifically to epithelium-lined mucosal tissues. This homing pattern, combined with the local differentiation within the mucosa of B cells into IgA-secreting plasma cells, serves to optimize IgA responses to mucosal infections. As we will discuss in more detail in Chapter 13, IgA is efficiently excreted into the lumen of tissues lined by mucosal epithelia, such as the gut and respiratory tract. The mechanisms by which different B cell populations migrate to different tissues are, not surprisingly, similar to the mechanisms we described for tissue-specific migration of effector T cells and depend on expression of distinct combinations of adhesion molecules and chemokine receptors on each B cell subset. For example, bone marrow–homing IgG-secreting plasma cells express VLA-4 and CXCR4, which bind respectively to VCAM-1 and CXCL12 expressed on bone marrow sinusoidal endothelial cells. In contrast, mucosa-homing IgA-secreting plasma cells express α4β7, CCR9, and CCR10, which bind respectively to MadCAM-1, CCL25, and CCL28, expressed on mucosal endothelial cells. IgG-secreting B cells are also recruited to chronic inflammatory sites in various tissues, and this pattern of homing can be attributed to CXCR3 and VLA-4 on these B cells binding to VCAM-1, CXCL9, and CXCL10, which are often found on the endothelial surface at sites of chronic inflammation.
Summary
Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nature Reviews Immunology. 2005;5:546-559.
Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7:678-689.
Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature Immunology. 2008;9:970-980.
Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nature Immunology. 2008;9:949-952.
Lymphocyte Migration through Lymphoid Tissues
Bajénoff M, Egen JG, Qi H, Huang AY, Castellino F, Germain RN. Highways, byways and breadcrumbs: directing lymphocyte traffic in the lymph node. Trends in Immunology. 2007;28:346-352.
Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annual Review of Immunology. 2005;23:127-159.
Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annual Review of Immunology. 2004;22:891-928.
Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nature Immunology. 2008;9:981-987.
von Andrian UH, Mackay CR. T-cell function and migration: two sides of the same coin. New England Journal of Medicine. 2000;343:1020-1034.