CHAPTER 11 B Cell Activation and Antibody Production
Humoral immunity is mediated by secreted antibodies, which are produced by cells of the B lymphocyte lineage. Two types of microbial antigens can induce robust antibody responses. First, multivalent antigens of microbial origin can activate B cells through the B cell receptor (BCR), often accompanied by signals provided by engagement of pattern recognition receptors on B cells by microbial products, but without T cell help. Second, microbial protein antigens can be presented by B cells to helper T cells, resulting in T-dependent responses in which helper T cells drive B cell activation. In both cases, antibodies are secreted and bind to the antigens of extracellular bacteria, viruses, and other microbes and function to neutralize and eliminate these pathogens. The elimination of different types of microbes requires several effector mechanisms that are mediated by distinct antibody isotypes. In general, antibodies produced with help from T cells bind more tightly to antigens and serve more diverse functions than antibodies produced without T cell help, and this is why antibodies against protein antigens (the stimulators of T cells) are the most effective mediators of humoral immunity. This chapter describes the molecular and cellular events of the humoral immune response, in particular the stimuli that induce B cell proliferation and differentiation and how these stimuli influence the type of antibody that is produced. The mechanisms by which antibodies eliminate microbes are described in Chapter 12.
General Features of Humoral Immune Responses
The earliest studies of adaptive immunity were devoted to analyses of serum antibodies produced in response to microbes, toxins, and model antigens. Much of our current understanding of adaptive immune responses and the cellular interactions that take place during such responses has evolved from studies of antibody production. We begin with a summary of some of the key features of B cell activation and antibody production.
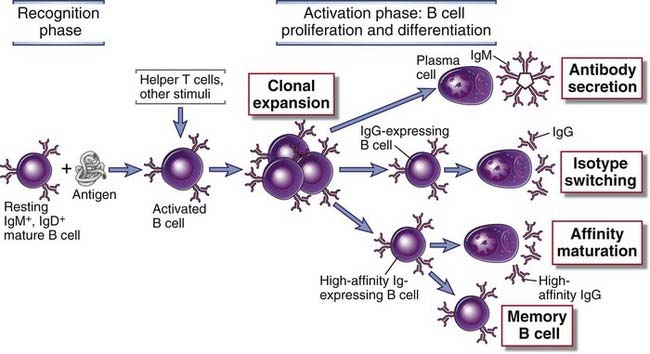
FIGURE 11–1 Phases of the humoral immune response.
The activation of B cells is initiated by specific recognition of antigens by the surface Ig receptors of the cells. Antigen and other stimuli, including helper T cells, stimulate the proliferation and differentiation of the specific B cell clone. Progeny of the clone may produce IgM or other Ig isotypes (e.g., IgG), may undergo affinity maturation, or may persist as memory cells.
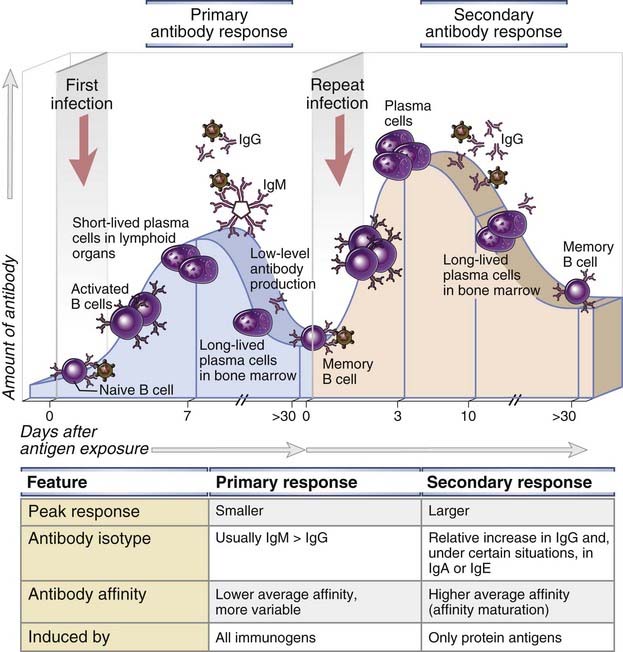
FIGURE 11–2 Primary and secondary humoral immune responses.
In a primary immune response, naive B cells are stimulated by antigen, become activated, and differentiate into antibody-secreting cells that produce antibodies specific for the eliciting antigen. Some of the antibody-secreting plasma cells migrate to and survive in the bone marrow, where they continue to produce antibodies for long periods. Long-lived memory B cells are also generated during the primary response. A secondary immune response is elicited when the same antigen stimulates these memory B cells, leading to more rapid proliferation and differentiation and production of greater quantities of specific antibody than are produced in the primary response. The principal characteristics of primary and secondary antibody responses are summarized in the table. These features are typical of T cell–dependent antibody responses to protein antigens.
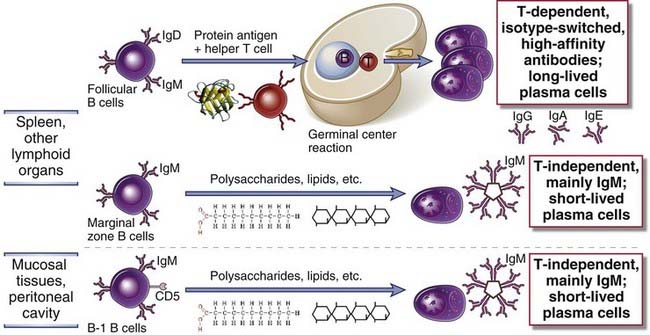
FIGURE 11–3 Distinct B cell subsets mediate different types of antibody responses.
Follicular B cells are recirculating cells that receive T cell help when they respond to protein antigens and thus initiate T-dependent antibody responses. These responses can lead to the formation of germinal centers, where class switching and somatic mutation of antibody gene occur, resulting in specialized high-affinity antibody responses. T-independent responses to multivalent antigens such as lipids, polysaccharides, and nucleic acids are mediated mainly by marginal zone B cells in the spleen and B-1 cells in mucosal sites. These functional distinctions between subsets are not absolute.
In the following sections, we will initially discuss the interaction of antigen with B cells and then the role of helper T cells in B cell responses to protein antigens and the mechanisms of isotype switching and affinity maturation. We will conclude with a discussion of T-independent antibody responses.
Antigen Recognition and Antigen-Induced B Cell Activation
To initiate antibody responses, antigens have to be captured and transported to the B cell areas of lymphoid organs. The antigens then initiate the process of B cell activation, often working in concert with other signals that are generated during innate immune responses triggered by microbes during infections or by adjuvants in vaccines. We next describe these early events in B cell activation.
Antigen Capture and Delivery to B Cells
Mature B lymphocytes migrate from one secondary lymphoid organ to the next in search of antigen. Naive B cells reside in and circulate through the follicles of peripheral lymphoid organs (the spleen, lymph nodes, and mucosal lymphoid tissues) in search of their cognate antigen (see Chapters 2 and 3). Most B cells enter follicles and are called follicular B cells or recirculating B cells. Entry into the follicles is guided by the chemokine CXCL13 secreted by follicular dendritic cells and other stromal cells in the follicle. CXCL13 binds to the CXCR5 chemokine receptor on recirculating naive B cells and attracts these cells into the follicles. As we discuss later, the same chemokine-receptor pair is also important during immune responses because it can attract a subset of activated T cells to the follicle. Naive follicular B cells survive for limited periods until they encounter antigen (see Chapter 2). Follicular B cell survival depends on signals from the BCR as well as on inputs received from a tumor necrosis factor (TNF) superfamily cytokine called BAFF (B cell–activating factor of the TNF family, also known as BLyS, for B lymphocyte stimulator), which provides maturation and survival signals through the BAFF receptor. BAFF and a related ligand, APRIL, can activate two other receptors, TACI and BCMA, which participate in later stages of B cell activation and differentiation (and will be discussed later). These cytokines are produced mainly by myeloid cells in lymphoid follicles and in the bone marrow.
Antigen may be delivered to naive B cells in lymphoid organs in different forms and by multiple routes. Antigens that enter by crossing an epithelial barrier as well as antigens in the circulation are capable of activating B cells and are brought to B cell zones by several mechanisms (Fig. 11-4).
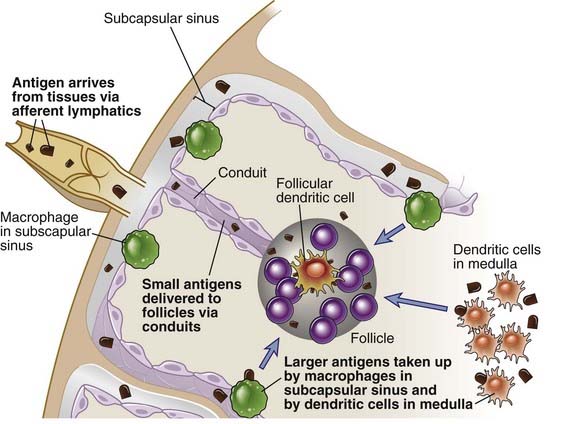
FIGURE 11–4 Pathways of antigen delivery to follicular B cells.
Antigen is delivered to B cells in follicles largely through afferent lymphatics that drain into the subcapsular sinus of the lymph node. Small antigens may reach the follicle through conduits. Larger antigens may be captured by subcapsular sinus macrophages and delivered to the follicle, or they may directly access dendritic cells in the medulla that may be involved in delivering antigen not only to the T cell zone but also to B cell–containing follicles.
In all these cases, the antigen that is presented to B cells is generally in its intact, native conformation and is not processed by antigen-presenting cells. This, of course, is one of the important distinctions between the forms of antigens recognized by B and T lymphocytes (see Chapter 6).
Activation of B Cells by Antigens and Other Signals
The activation of antigen-specific B lymphocytes is initiated by the binding of antigen to membrane Ig molecules, which, in conjunction with the associated Igα and Igβ proteins, make up the antigen receptor complex of mature B cells. The B lymphocyte antigen receptor, described in Chapter 7, serves two key roles in B cell activation. First, binding of antigen to the receptor delivers biochemical signals to the B cells that initiate the process of activation (see Chapter 7). Second, the receptor internalizes the bound antigen into endosomal vesicles, and if the antigen is a protein, it is processed into peptides that may be presented on the B cell surface for recognition by helper T cells. This antigen-presenting function of B cells will be considered later in the context of T-dependent B cell activation.
Although antigen recognition can initiate B cell responses, by itself it is usually inadequate to stimulate significant B cell proliferation and differentiation. For full responses to be induced, other stimuli cooperate with BCR engagement, including complement proteins, pattern recognition receptors, and, in the case of protein antigens, helper T cells (discussed later).
B cell activation is facilitated by the CR2/CD21 coreceptor on B cells, which recognizes complement fragments covalently attached to the antigen or that are part of immune complexes containing the antigen (Fig. 11-5). Complement activation is typically seen with microbes, which activate this system in the absence of antibodies by the alternative and lectin pathways, and in the presence of antibodies by the classical pathway (see Chapters 4 and 12). In all these situations, complement fragments are generated that bind to the microbes. One of these fragments, called C3d, is recognized by the complement receptor CR2 (also called CD21), which enhances the strength of BCR signaling and thus functions as a coreceptor for B cells (see Chapter 7). Some nonmicrobial polysaccharides also activate complement by the alternative or lectin pathway, and this is one reason that such antigens are able to induce antibody responses without T cell help.
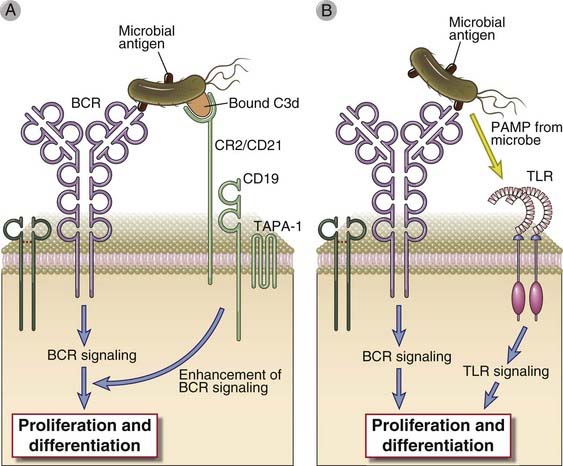
FIGURE 11–5 Role of CR2 and TLRs in B cell activation.
In immune responses to microbes, activation of B cells through the BCR may be enhanced by complement-coated antigen that can simultaneously ligate the BCR and complement receptor 2 (CR2) (A), and may also involve the contemporaneous activation of Toll-like receptors (TLRs) on B cells by molecules (so-called pathogen-associated molecular patterns [PAMPs]) derived from the microbe (B).
Microbial products engage Toll-like receptors on B cells, which also enhances B cell activation (see Fig. 11-5). Human B cells express several Toll-like receptors (TLRs), including TLR5, which recognizes bacterial flagellin; endosomal TLR7, which recognizes single-stranded RNA; and TLR9, which is specific for unmethylated CpG-rich DNA in endosomes (see Chapter 4). Murine B cells also express TLR4 on the cell surface. These pattern recognition receptors directly activate B cells. In addition, the activation of myeloid cells through pattern recognition receptors can promote B cell activation indirectly in two ways. Dendritic cells activated through TLRs contribute significantly to helper T cell activation (see Chapter 9). Myeloid cells activated by TLRs may secrete APRIL and BAFF, cytokines that can induce T-independent B cell responses.
Functional Responses of B Cells to Antigens
Distinct cellular events are induced by antigen-mediated cross-linking of the BCR by different types of antigens: multivalent antigens initiate B cell proliferation and differentiation, and protein antigens prepare B cells for subsequent interactions with helper T cells. Antigen receptor cross-linking by some antigens can stimulate several important changes in B cells (Fig. 11-6). The previously resting cells enter into the G1 stage of the cell cycle, and this is accompanied by increases in cell size, cytoplasmic RNA, and biosynthetic organelles such as ribosomes The survival of the stimulated B cells is enhanced as a result of the production of various antiapoptotic proteins, notably Bcl-2 (see Fig. 14-7, Chapter 14), and the cells may proliferate and secrete some antibody. Activation of B cells by antigen results in increased expression of class II major histocompatibility complex (MHC) molecules and B7 costimulators, because of which antigen-stimulated B cells are more efficient activators of helper T lymphocytes than are naive B cells. The expression of receptors for several T cell–derived cytokines is also increased, which enables antigen-stimulated B lymphocytes to respond to cytokines secreted by helperT cells. The expression of chemokine receptors may change, resulting in movement of the B cells out of the follicles.
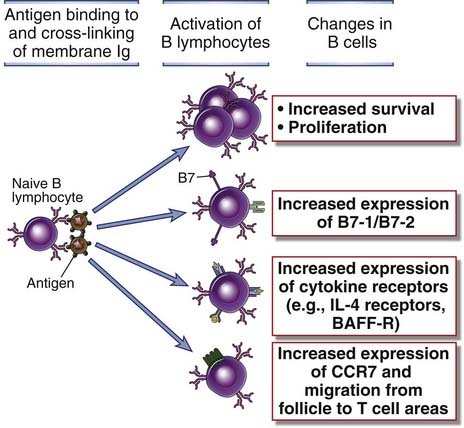
FIGURE 11–6 Functional responses induced by antigen-mediated cross-linking of the BCR complex.
Antigen-induced cross-linking of the B cell antigen receptor induces several cellular responses including proliferation, expression of new cell surface molecules including costimulators and cytokine receptors, and altered migration within the lymph node of the cells as a result of the expression of CCR7.
The importance of signaling by the BCR complex for the subsequent responses of the cells may vary with the nature of the antigen. Most T-independent antigens, such as polysaccharides, display multiple identical epitopes on each molecule or on a cell surface. Therefore, such multivalent antigens effectively cross-link many B cell antigen receptors and initiate responses even though they are not recognized by helper T lymphocytes. In contrast, many naturally occurring globular protein antigens possess only one copy of each epitope per molecule. Therefore, such protein antigens cannot simultaneously bind to and cross-link multiple Ig molecules, and their ability to activate the BCR is limited, so they do not typically induce signals that can drive B cell proliferation and differentiation. However, some protein antigens may be displayed as multivalent arrays on the surfaces of microbes or cells, or they may be multivalent because they are in aggregates. Protein antigens are also internalized by the BCR and processed and displayed to helper T cells, which are potent stimulators of B lymphocyte proliferation and differentiation. In fact, in T-dependent responses, a major function of membrane Ig may be not signaling but binding and internalization of the antigen for subsequent presentation to helper T cells. Therefore, protein antigens may only activate the BCR to enhance antigen presentation and the migration of antigen-specific B cells toward the T cell zone.
After specific B cells recognize antigens, the subsequent steps in humoral immune responses are very different in T-dependent and T-independent responses. We next describe the activation of B cells by protein antigens and helper T cells.
Helper T Cell–Dependent Antibody Responses to Protein Antigens
Antibody responses to protein antigens require recognition and processing of the antigen by B cells, followed by presentation of a peptide fragment of the antigen to helper T cells, leading to cooperation between the antigen-specific B and T lymphocytes. The helper function of T lymphocytes was discovered by experiments performed in the late 1960s, which showed that antibody responses required the presence of both bone marrow cells (now known to contain mature B lymphocytes) and thymus-derived cells (which were T lymphocytes). Subsequent experiments showed that only the bone marrow cells produced the antibody, but their activation required the thymic cells, which were called helper cells. These classic experimental studies were among the first formal proof of the importance of interactions between two completely different cell populations in the immune system. It took many years to establish that most helper T cells are CD4+CD8− lymphocytes that recognize peptide antigens presented by class II MHC molecules. One of the important accomplishments of immunology has been the elucidation of the mechanisms of T-B cell interactions and the actions of helper T cells in antibody responses.
The Sequence of Events During T Cell–Dependent Antibody Responses
Protein antigens are recognized by specific B and T lymphocytes in peripheral lymphoid organs, and the activated cell populations come together in these organs to initiate humoral immune responses (Fig. 11-7). The interaction between helper T cells and B lymphocytes is initiated by the recognition of protein antigens, and the sequence of events that drive B cell proliferation and differentiation is as follows:
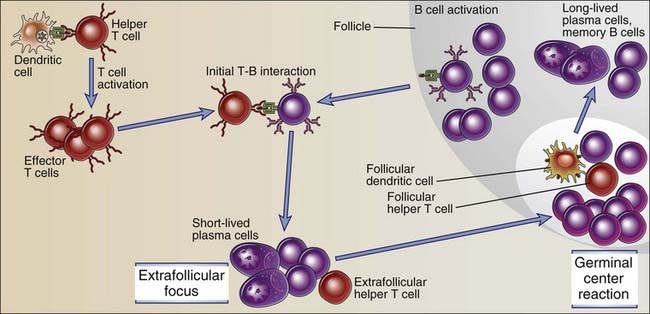
FIGURE 11–7 Sequence of events in humoral immune responses to T cell–dependent protein antigens.
Immune responses are initiated by the recognition of antigens by B cells and helper T cells. The activated lymphocytes migrate toward one another and interact, resulting in B cell proliferation and differentiation. Restimulation of B cells by helper T cells in extrafollicular sites leads to early isotype switching and short-lived plasma cell generation. The late events occur in germinal centers and include somatic mutation and the selection of high-affinity cells (affinity maturation), additional isotype switching, memory B cell generation, and the generation of long-lived plasma cells.
In the following sections, we describe each of these steps in detail.
Initial Activation and Migration of Helper T Cells and B Cells
The activation of specific B and T cells by antigen is essential for their functional interaction and brings them into proximity to enhance the possibility that the antigen-specific B and T cells will locate one another (Fig. 11-8). The frequency of naive B cells or T cells specific for a given epitope of an antigen is as low as 1 in 105 to 1 in 106 lymphocytes, and both populations have to be activated and the specific B and T cells have to find each other and physically interact to generate strong antibody responses. Helper T cells that have been activated by antigen and costimulation are induced to proliferate, express CD40L, and secrete cytokines. They also downregulate the chemokine receptor CCR7 and increase the expression of CXCR5 and as a result leave the T cell zone and migrate toward the follicle. As mentioned earlier, CXCL13, the ligand for CXCR5, is secreted by follicular dendritic cells and other follicular stromal cells, and it contributes to the migration of activated CD4+ T cells toward the follicle.
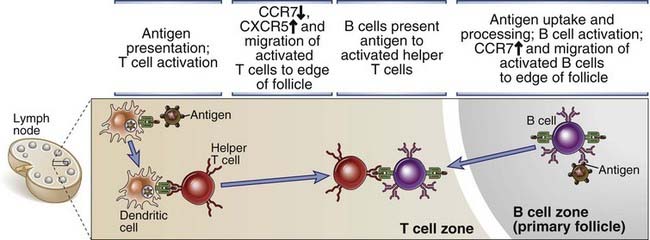
FIGURE 11–8 Migration of B cells and helper T cells and T-B interaction.
Antigen-activated helper T cells and B cells move toward one another in response to chemokine signals and make contact adjacent to the edge of primary follicles. In this location, the B cell presents antigen to the T cell, and the B cell receives activating signals from the T cell.
Although protein antigens, being monovalent, typically do not provide strong enough signals to induce much B cell proliferation and differentiation, they can activate B cells and initiate a number of events. BCR engagement by these antigens results in reduced cell surface expression of the chemokine receptor CXCR5 and increased expression of CCR7, which is normally expressed on T cells. As a result, activated B cells migrate toward the T cell zone drawn by a gradient of CCL19 and CCL21, the ligands for CCR7. B cells activated by protein antigens can also express CD69, which blocks surface expression of sphingosine 1-phosphate receptors, causing retention of activated B cells in lymph nodes (see Chapter 3). Protein antigens are endocytosed by the B cell and presented in a form that can be recognized by helper T cells, and this represents the next step in the process of T-dependent B cell activation.
Antigen Presentation by B Cells and the Hapten-Carrier Effect
Protein antigens that are recognized by specific B cell antigen receptors are endocytosed and delivered to a vesicular compartment for processing, leading to the association of a linear peptide derived from the protein with a class II MHC molecule that can bind and display the peptide (Fig. 11-9). This peptide is presented on the B cell surface to a helper T cell that was previously activated in the T cell zone when its TCR recognized an identical peptide presented by a dendritic cell that had encountered the same antigen. Because the BCR recognizes an epitope of the native protein with high affinity, specific B cells bind and present the antigen much more efficiently (i.e., at much lower concentrations) than do other B cells not specific for the antigen. This is why B cells specific for an antigen respond preferentially to that antigen, compared with other “bystander” cells. A protein antigen that elicits a T-dependent B cell response therefore makes use of at least two epitopes when activating specific B cells. A surface epitope on the native protein is recognized with high specificity by a B cell, and an internal linear peptide epitope is subsequently released from the protein, binds class II MHC molecules, and is recognized by helper T cells. The antibodies that are subsequently secreted are usually specific for conformational determinants of the native antigen because membrane Ig on B cells is capable of binding conformational epitopes of proteins, and the same Ig is secreted by plasma cells derived from those B cells. This feature of B cell antigen recognition determines the fine specificity of the antibody response and is independent of the fact that helper T cells recognize only linear epitopes of processed peptides. In fact, a single B lymphocyte specific for a native epitope may bind and endocytose a protein and present multiple different peptides complexed with class II MHC molecules to different helper T cells, but the resultant antibody response remains specific for the native protein.
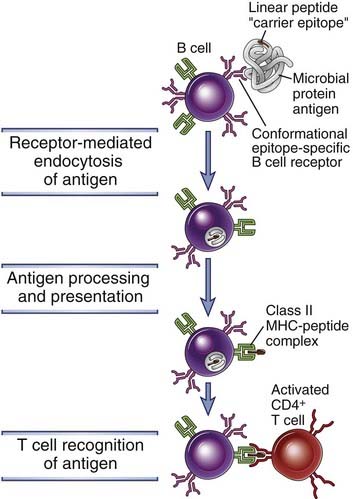
FIGURE 11–9 Antigen presentation on B cells to helper T cells.
Protein antigens bound to membrane Ig are endocytosed and processed, and peptide fragments are presented in association with class II MHC molecules. Helper T cells that were previously activated by dendritic cells recognize the MHC-peptide complexes on the B cells and then stimulate B cell responses. Activated B cells also express costimulators (not shown) that enhance helper T cell responses. In hapten-carrier responses, the protein is conjugated to a hapten (the B cell epitope) and is internalized by a hapten-specific B cell, which processes the antigen and presents the linear peptide (the T cell epitope, sometimes known as the carrier determinant) on class II MHC molecules to an activated helper T cell.
The principles outlined here for T-B cell collaboration help explain a phenomenon that is known as the hapten-carrier effect. Analysis of antibody responses to hapten-carrier conjugates was among the earliest approaches that demonstrated how antigen presentation by B lymphocytes contributes to the development of humoral immune responses. Haptens, such as dinitrophenol, are small chemicals that can be bound by specific antibodies but are not immunogenic by themselves. If, however, haptens are coupled to proteins, which serve as carriers, the conjugates are able to induce antibody responses against the haptens. There are three important characteristics of anti-hapten antibody responses to hapten-protein conjugates. First, such responses require both hapten-specific B cells and protein (carrier)–specific helper T cells. Second, to stimulate a response, the hapten and carrier portions have to be physically linked and cannot be administered separately. Third, the interaction is class II MHC restricted, that is, the helper T cells cooperate only with B lymphocytes that express class II MHC molecules that are identical to those that were involved in the initial activation of naive T cells by dendritic cells. All these features of antibody responses to hapten-protein conjugates can be explained by the antigen-presenting functions of B lymphocytes. Hapten-specific B cells bind the antigen through the hapten determinant, endocytose the hapten-carrier conjugate, and present peptides derived from the carrier protein to carrier-specific helper T lymphocytes (see Fig. 11-9). Thus, the two cooperating lymphocytes recognize different epitopes of the same complex antigen. The hapten is responsible for efficient carrier uptake, which explains why hapten and carrier must be physically linked. The requirement for MHC-associated antigen presentation for T cell activation accounts for the MHC restriction of T cell–B cell interactions.
The characteristics of humoral responses elucidated for hapten-carrier conjugates apply to all protein antigens in which one intrinsic determinant, usually a native conformational determinant, is recognized by B cells (and is therefore analogous to the hapten) and another determinant, in the form of a class II MHC–associated linear peptide, is recognized by helper T cells (and is analogous to the carrier that is the source of the peptide). The hapten-carrier effect is the basis for the development of conjugate vaccines, discussed later in the chapter.
Role of CD40L:CD40 Interaction in T-Dependent B Cell Activation
On activation, helper T cells express CD40 ligand (CD40L), which engages its receptor, CD40, on antigen-stimulated B cells at the T-B interface and induces subsequent proliferation and differentiation initially in extrafollicular foci and later in germinal centers (Fig. 11-10). CD40 is a member of the TNF receptor superfamily. Its ligand, CD40L (CD154), is a trimeric membrane protein that is homologous to TNF. CD40 is constitutively expressed on B cells, and CD40L is expressed on the surface of helper T cells after activation by antigen and costimulators. When these activated helper T cells interact physically with antigen-presenting B cells, CD40L recognizes CD40 on the B cell surface. CD40L binding to CD40 results in the conformational alteration of preformed CD40 trimers, and this induces the association of cytosolic proteins called TRAFs (TNF receptor–associated factors) with the cytoplasmic domain of CD40. The TRAFs recruited to CD40 initiate enzyme cascades that lead to the activation and nuclear translocation of transcription factors, including NF-κB and AP-1, which collectively stimulate B cell proliferation and increased synthesis and secretion of Ig. Similar signaling pathways are activated by TNF receptors (see Chapter 7). CD40-induced transcription factor induction is also crucial for subsequent germinal center formation and for the synthesis of activation-induced deaminase (AID), an enzyme that is required for somatic mutation and isotype switching, as will be discussed later. T cell–mediated dendritic cell and macrophage activation also involves the interaction of CD40L on activated helper T cells with CD40 on dendritic cells and macrophages (see Chapter 10). Thus, this pathway of contact-dependent cellular responses is a general mechanism for the activation of target cells by helper T lymphocytes and is not unique to antibody production.
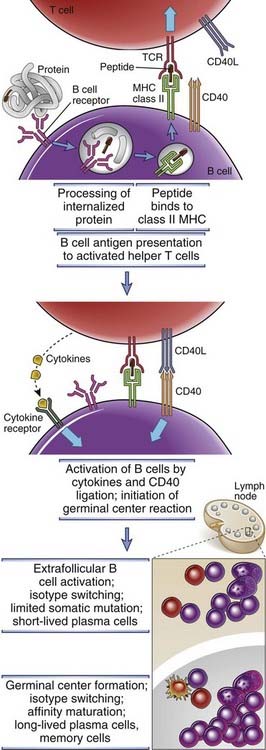
FIGURE 11–10 Mechanisms of helper T cell–mediated B cell activation.
Activated helper T cells that migrate toward the B cell zone express CD40L and their T cell receptors recognize peptide–class II MHC complexes on B cells that have been triggered by antigen and have in turn migrated to the interface between T and B cell zones. CD40L on the activated T helper cell then binds to CD40 on antigen-activated B cells and initiates B cell proliferation and differentiation. Cytokines bind to cytokine receptors on the B cells and also stimulate B cell responses. Two types of differentiation events may occur—the formation of extrafollicular foci and the induction of a germinal center B cell reaction.
Mutations in the CD40L gene result in a disease called the X-linked hyper-IgM syndrome, which is characterized by defects in antibody production, isotype switching, affinity maturation, and memory B cell generation in response to protein antigens, as well as deficient cell-mediated immunity (see Chapter 20). Similar abnormalities are seen in CD40 or CD40L gene knockout mice. Interestingly, a DNA virus called the Epstein-Barr virus (EBV) infects human B cells and induces their proliferation. This may lead to immortalization of the cells and the development of lymphomas. The cytoplasmic tail of a transforming protein of EBV called LMP1 (latent membrane protein 1) associates with the same TRAF molecules as does the cytoplasmic domain of CD40, and this apparently triggers B cell proliferation. Thus, EBV LMP1 is functionally homologous to a physiologic B cell signaling molecule, and EBV has apparently co-opted a normal pathway of B lymphocyte activation for its own purpose, which is to promote survival and proliferation of cells that the virus has infected.
In addition to CD40L on helper T cells activating B cells, helper T cells also secrete cytokines that contribute to B cell responses. The best defined roles of T cell–derived cytokines in humoral immune responses are in isotype switching, described later. Several cytokines have also been implicated in the early steps of B cell proliferation and differentiation, but it is not clear if any are actually essential for these responses.
The initial interaction of activated helper T cells with antigen-specific B cells at the edge of the follicle induces some proliferation and differentiation of B cells and leads to the formation of a collection of cells called an extrafollicular focus.
Extrafollicular B Cell Activation
After the initial interaction of B cells with helper T cells at the interface between the follicle and the T cell zone, subsequent activation of B cells by helper T cells can occur at two different locations, one outside the follicles and the other in the follicles, in germinal centers. The nature of the B cell response differs in these locations (Table 11-1). Extrafollicular foci of T-dependent B cell activation are generated relatively early in an immune response. Germinal centers, in which specialized follicular helper T (TFH) cells trigger B cells to undergo numerous changes, appear a few days later.
TABLE 11–1 Extrafollicular and Germinal Center B Cell Responses
| Feature | Follicular/Germinal Center | Extrafollicular |
|---|---|---|
| Localization | Secondary follicles | Medullary cords of lymph nodes and at junctions between T cell zone and red pulp of spleen |
| CD40 signals | Required | Required |
| Specialized T cell help | TFH cells in germinal center | Extrafollicular T helper cells |
| AID expression | Yes | Yes |
| Class switching | Yes | Yes |
| Somatic hypermutation | High rate | Low rate |
| Antibody affinity | High | Low |
| Terminally differentiated B cells | Long-lived plasma cells and memory cells | Short-lived plasma cells (life span of ~3 days) |
| Fate of plasma cells | Bone marrow or local MALT | Most die by apoptosis in secondary lymphoid tissues where they were produced |
| B cell transcription factors | Bcl-6 | Blimp-1 |
AID, activation-induced cytidine deaminase; Bcl-6, B cell lymphoma 6; Blimp-1, B lymphocyte–induced maturation protein 1; IL-21R, interleukin-21 receptor; MALT, mucosa-associated lymphoid tissue; TFH, follicular helper T cell.
Data from Vinusa CG, I Sanz, and MC Cook. Dysregulation of germinal centres in autoimmune disease. Nature Reviews Immunology 9:845-857, 2009.
B cells that are activated by helper T cells through CD40L in the extrafollicular foci that may undergo some degree of differentiation into plasma cells and isotype switching. Each such focus may produce 100 to 200 antibody-secreting plasma cells. In the spleen, extrafollicular foci develop in the outer portions of the T cell–rich periarteriolar lymphoid sheath (PALS) or between the T cell zone and the red pulp, and these collections of cells are also called PALS foci. Similar T-dependent foci are observed in the medullary cords of lymph nodes. Isotype switching first occurs in these extrafollicular foci. Some somatic hypermutation of Ig genes, which underlies the process of affinity maturation, also occurs, but this is much less than the magnitude of somatic hypermutation seen in germinal center responses described later. The circulating antibody-secreting cells, called plasmablasts, and tissue plasma cells that are generated in extrafollicular foci are mostly short-lived, and these cells do not acquire the ability to migrate to distant sites such as the bone marrow. The small amount of antibody produced in these foci may contribute to the formation of immune complexes (containing antigen, antibody, and perhaps complement) that are trapped by follicular dendritic cells in lymphoid follicles. It has been speculated that this deposition of immune complexes may be a necessary prelude to the release of chemokines from follicular dendritic cells that draw in a few (perhaps only one or two) activated B cells from the extrafollicular focus into the follicle to initiate the germinal center reaction.
The Germinal Center B Cell Reaction and the Function of Follicular Helper T Cells
The characteristic events of helper T cell–dependent antibody responses, including affinity maturation, isotype switching, generation of memory B cells, and long-lived plasma cell differentiation, occur primarily in the germinal centers of lymphoid follicles. Within 4 to 7 days after antigen exposure, some of the activated helper T cells that migrate to meet activated B cells are triggered by these B cells to differentiate into follicular helper T cells (TFH cells), which express high levels of the chemokine receptor CXCR5 and are drawn into lymphoid follicles by the ligand for CXCR5, which is produced only in follicles. These T cells are called follicular helper T (TFH) cells because they are the major CD4+ T cells present within follicles and they serve critical roles in the germinal center reaction. At the same time, a few activated B cells migrate back into the follicle and begin to proliferate rapidly, forming a lightly staining central region of the follicle called the germinal center (Figs. 11-11 and 11-12). Each fully formed germinal center contains cells derived from only one or a few antigen-specific B cell clones. Within the germinal center is a “dark zone” that is densely packed with rapidly proliferating B cells. The doubling time of these proliferating germinal center B cells, also called centroblasts, is estimated to be 6 to 12 hours, so that within 5 days, a single lymphocyte may give rise to almost 5000 progeny. The progeny of the proliferating B cells in the germinal center are smaller cells, sometimes called centrocytes, that undergo differentiation and selection processes in the “light zone,” described later. B cells in germinal centers express a transcriptional repressor known as Bcl-6 (for B cell lymphoma gene 6), whose role is described later when we consider the transcriptional regulation of B cell fate.
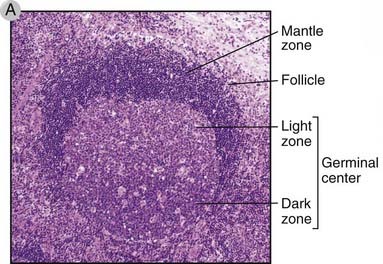
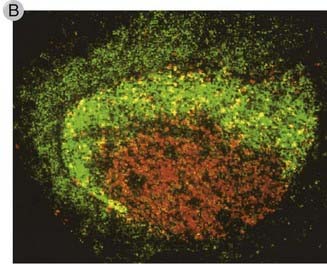
FIGURE 11–11 Germinal centers in secondary lymphoid organs.
A, Histology of a secondary follicle with a germinal center in a lymph node. The germinal center is contained within the follicle and includes a basal dark zone and an adjacent light zone. The mantle zone is the parent follicle within which the germinal center has formed.
(Courtesy of Dr. James Gulizia, Department of Pathology, Brigham and Women’s Hospital, Boston, Massachusetts.)
B, Cellular components of the germinal center. A secondary follicle has been stained with an anti-CD23 antibody (green), which brightly stains follicular dendritic cells in the light zone and dimly stains naive B cells in the mantle zone. Anti-Ki67 (red), which detects cycling cells, stains mitotically active B cell blasts in the dark zone.
(Modified from Liu YJ, GD Johnson, J Gordon, and IC MacLennan. Germinal centres in T-cell–dependent antibody responses. Immunology Today 13:17-21, Copyright 1992, with permission from Elsevier.)
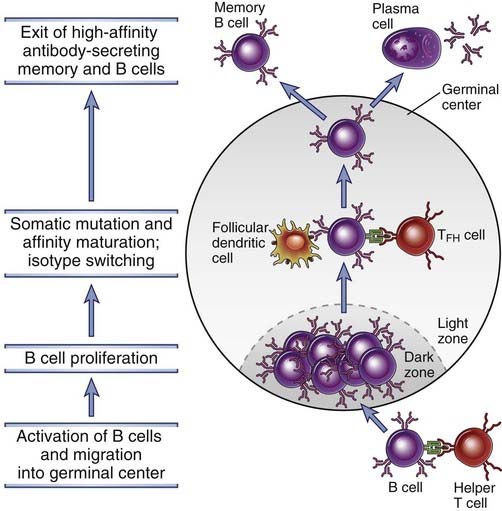
FIGURE 11–12 The germinal center reaction in a lymph node.
B cells that have been activated by T helper cells at the edge of a primary follicle migrate into the follicle and proliferate, forming the dark zone of the germinal center. Germinal center B cells undergo extensive isotype switching. Somatic hypermutation of Ig V genes occur in these B cells, and they migrate into the light zone, where they encounter follicular dendritic cells displaying antigen and TFH cells. B cells with the highest affinity Ig receptors are selected to survive, and they differentiate into antibody-secreting or memory B cells. The antibody-secreting cells leave and reside in the bone marrow as long-lived plasma cells, and the memory B cells enter the recirculating pool.
In addition to the chemokine receptor CXCR5, TFH cells are characterized by the expression of ICOS (inducible costimulator), the cytokine IL-21, and the transcription factor Bcl-6. TFH cells have a phenotype that makes them distinct from the TH1, TH2, TH17, and Treg subsets described in Chapters 9 and 10. It is possible that TFH cells can develop from naive CD4+ T cells or from polarized T cell subsets that retain developmental plasticity. The “signature” cytokine secreted by TFH cells is IL-21. It is required for germinal center development and also contributes to the generation of plasma cells in the germinal center reaction. In addition to IL-21, TFH cells secrete other cytokines, including IFN-γ and IL-4 (but at lower levels than differentiated TH1 and TH2 cells, respectively), and all these play important roles in isotype switching.
The mechanisms that drive the development of TFH cells from CD4+ cells and the mechanisms by which TFH cells activate B cells are not fully understood. A number of molecules on B cells and helper T cells are known to play key roles in these processes (Fig. 11-13). The costimulator ICOS, which is related to CD28 and is expressed on TFH cells, is essential for the germinal center reaction. The interaction of ICOS with ICOS ligand on B cells promotes the differentiation of T cells into TFH cells. The interactions between B cells and helper T cells are mediated by integrins and by members of the SLAM family of costimulators. A signaling molecule that associates with these SLAM family proteins in TFH cells is called SAP, and SAP signaling activates transcriptional regulators, particularly Bcl-6, that are required for TFH cell development. SAP is mutated in patients with a disease known as the X-linked lymphoproliferative syndrome, which is associated with defects in antibody and cytotoxic T cell responses (see Chapter 20). IL-21 secreted by TFH cells may facilitate germinal center B cell selection events and differentiation of activated B cells into plasmablasts. These helper T cells can secrete other cytokines that may be characteristic of TH1, TH2, and TH17 cells, and these cytokines can contribute to isotype switching. Germinal center formation is also dependent on CD40L:CD40 interactions. These may be critical for B cell proliferation, which is required for expansion of B cells in germinal centers, and also for isotype switching and affinity maturation. Therefore, germinal centers are defective in humans and in mice with genetic defects in T cell development or activation or with mutations of either CD40 or its ligand (see Chapter 20).
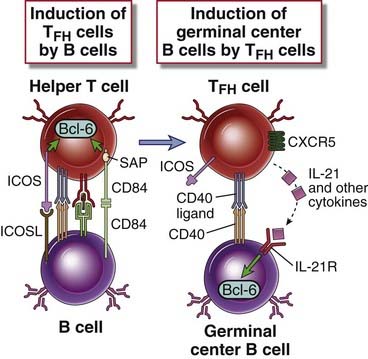
FIGURE 11–13 Molecular events in T follicular helper cell generation and function.
Activated B cells express ICOSL and signal to T helper cells. Triggering of ICOS and homotypic activation of SLAM family proteins on T cells result in differentiation into T follicular helper (TFH) cells. SLAM-associated protein (SAP) is a signaling molecule required for TFH cell differentiation. TFH cells express the Bcl-6 transcription factor, secrete IL-21 and other cytokines, and activate B cells in the germinal center reaction.
The architecture of lymphoid follicles and the germinal center reaction within follicles depend on the presence of follicular dendritic cells (FDCs). FDCs are found only in lymphoid follicles and express complement receptors (CR1, CR2, and CR3) and Fc receptors. These molecules are involved in displaying antigens for the selection of germinal center B cells, as described later. FDCs do not express class II MHC molecules and are not derived from progenitors in the bone marrow. In spite of their name, they are distinct from the class II MHC–expressing dendritic cells that capture antigens in tissues and transport them to lymphoid organs where they present peptides to T lymphocytes. The long cytoplasmic processes of FDCs form a meshwork around which germinal centers are formed. Proliferating B cells accumulate in the histologically identifiable dark zone of the germinal center, which has few FDCs. The small nondividing progeny of the B cells migrate to the adjacent light zone, where they come into close contact with the processes of the abundant FDCs and also form intimate contacts with TFH cells, and this is where subsequent selection events occur (see Fig. 11-12). The rim of naive B cells in the follicle, surrounding the germinal center, is called the mantle zone.
Heavy Chain Isotype (Class) Switching
In response to CD40 engagement and cytokines, some of the progeny of activated IgM- and IgD-expressing B cells undergo the process of heavy chain isotype (class) switching, leading to the production of antibodies with heavy chains of different classes, such as γ, α, and ε (Fig. 11-14). Isotype switching is observed in B cells in extrafollicular foci, driven by extrafollicular helper T cells, and much more in germinal centers, driven by TFH cells. The capacity of B cells to produce different antibody isotypes provides a remarkable plasticity in humoral immune responses by generating antibodies that perform distinct effector functions and are involved in defense against different types of infectious agents.
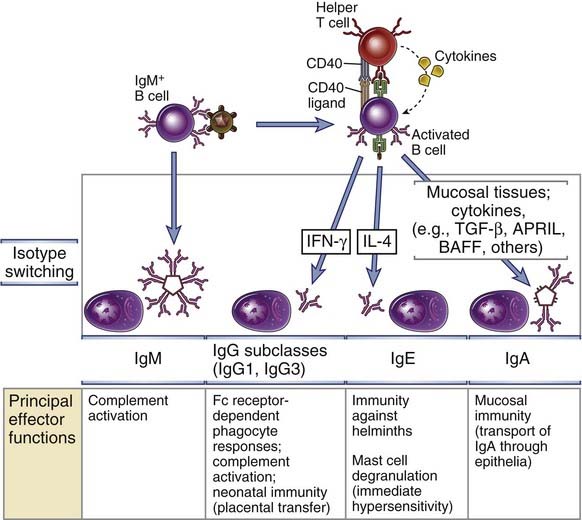
FIGURE 11–14 Ig heavy chain isotype switching.
B cells activated by helper T cell signals (CD40L, cytokines) undergo switching to different Ig isotypes, which mediate distinct effector functions. Selected examples of switched isotypes are shown. The role of IFN-γ in directing specific isotype switching events has been established only in rodents.
Isotype switching in response to different types of microbes is regulated by cytokines produced by the helper T cells that are activated by these microbes. For instance, the major protective humoral immune response to bacteria with polysaccharide-rich capsules consists of IgM antibodies, which bind to the bacteria, activate the complement system, and induce phagocytosis of the opsonized bacteria. Polysaccharide antigens, which do not elicit T cell help, stimulate mainly IgM antibodies, with little if any isotype switching to some IgG subclasses. The response to many viruses and bacteria involves production of IgG antibodies, which block entry of the microbes into host cells and also promote phagocytosis by macrophages. Viruses and many bacteria activate helper T cells of the TH1 subset, which produce the cytokine IFN-γ. In mice, IFN-γ is the main inducer of B cell switching to opsonizing and complement-fixing IgG subclasses; it is still not clear which cytokines serve this role in humans. The antibody response to many helminthic parasites is mainly IgE, which participates in eosinophil- and mast cell–mediated elimination of the helminths (see Chapters 12 and 15); IgE antibodies also mediate immediate hypersensitivity (allergic) reactions (see Chapter 19). Helminths activate the TH2 subset of helper T cells, which produces IL-4, the cytokine that induces switching to IgE. In the germinal center reaction, these cytokines might be produced not by classical TH1 and TH2 effector cells (which tend to migrate to peripheral sites of infection and inflammation) but by TFH cells that retain the capacity to produce TH1 or TH2 cytokines. In addition, B cells in different anatomic sites switch to different isotypes. Specifically, B cells in mucosal tissues switch to IgA, which is the antibody class that is most efficiently transported through epithelia into mucosal secretions, where it defends against microbes that try to enter through the epithelia (see Chapter 13). Switching to IgA is stimulated by transforming growth factor-β (TGF-β), which is produced by many cell types, including helper T cells, in mucosal and other tissues. Cytokines of the TNF family, BAFF and APRIL, also stimulate switching to IgA. Because these cytokines are produced by myeloid cells, they can stimulate IgA responses in the absence of T cell help. Some individuals who inherit mutant versions of the TACI gene, which encodes a receptor for these cytokines, have a selective deficiency of IgA production (see Chapter 20).
CD40 signals work together with cytokines to induce isotype switching. CD40 engagement induces the enzyme activation-induced deaminase (AID), which, as we shall see later, is crucial for both isotype switching and somatic mutation. The requirement for CD40 signaling and AID to promote isotype switching in B cells is well documented by analysis of mice and humans lacking CD40, its ligand, or AID. In all these cases, the antibody response to protein antigens is dominated by IgM antibodies, and there is limited switching to other isotypes. Cytokines, as described later, identify the specific Ig heavy chain loci that will participate in the switching process.
The molecular mechanism of isotype switching is a process called switch recombination, in which the rearranged VDJ exon that encodes an Ig heavy chain V domain recombines with a downstream C region gene and the intervening DNA is deleted. An overview of the process is provided in Figure 11-15. These DNA recombination events involve nucleotide sequences called switch regions, which are located in the introns between the J and C segments at the 5′ ends of each CH locus. Switch regions are 1 to 10 kilobases long, contain numerous tandem repeats of GC-rich DNA sequences, and are found upstream of every heavy chain gene. Upstream of each switch region is a small exon called the I exon (for initiator of transcription) preceded by an I region promoter. Signals from cytokines and CD40 induce transcription from a particular I region promoter reading through the I exon, switch region, and adjacent CH exons. These transcripts are known as germline transcripts. They do not encode specific proteins but are required for isotype switching to proceed. Germline transcripts are found at both the µ locus and the downstream heavy chain locus to which an activated B cell is being induced to switch. At each participating switch region, the germline transcript facilitates the generation of DNA double-stranded breaks, as described later. The DNA break in the upstream (µ) switch region is joined to the break in the downstream selected switch region. As a result, the rearranged VDJ exon just upstream of the µ switch region in the IgM-producing B cell recombines with the transcriptionally active downstream switch region. Cytokines determine which CH region will undergo germline transcription. For instance, IL-4 induces germline transcription through the Iε-Sε-Cε locus (see Fig. 11-15). This leads first to the production of germline ε transcripts in an IgM-expressing B cell and then to recombination of the Sµ switch region with the Sε switch region. The intervening DNA is lost, and the VDJ exon is thus brought adjacent to Cε. The end result is the production of IgE with the same V region as that of the original IgM produced by that B cell.
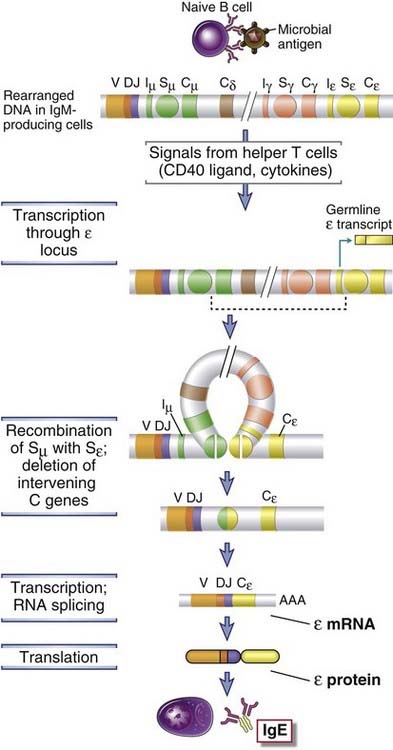
FIGURE 11–15 Mechanisms of heavy chain isotype switching.
In the absence of helper T cell signals, B cells produce IgM. When antigen-activated B cells encounter helper T cell signals (CD40L and, in this example, IL-4), the B cells undergo switching to other Ig isotypes (in this example, IgE). These stimuli initiate germline transcription through the Iε-Sε-Cε locus. The proximal CH genes are deleted in a circle of DNA, leading to recombination of the VDJ exon with the Cε gene. Switch regions are indicated by circles labeled Sµ or Sγ. Although a switch region is not shown for the δ gene, in humans a switch-like region upstream of the δ gene is functional. Iµ and Iε represent initiation site for germline transcription. (Note that there are multiple Cγ genes located between Cδ and Cε, but these are not shown.)
The key enzyme required for isotype switching (and somatic mutation, described later) is activation-induced deaminase (AID). Humans and knockout mice lacking this enzyme have profound defects in isotype switching and affinity maturation. AID expression is activated mainly by CD40 signals. The enzyme deaminates cytosines in single-stranded DNA templates, converting cytosine (C) residues to uracil (U) residues (Fig. 11-16). Switch regions are rich in G and C bases, and switch region transcripts tend to form stable DNA-RNA hybrids involving the coding (top) strand of DNA, thus freeing up the bottom or nontemplate strand, which forms an open single-stranded DNA loop called an R-loop. The R-loop is where a large number of C residues in the switch DNA sequence are converted to U residues by AID. An enzyme called uracil N-glycosylase removes the U residues, leaving abasic sites. The ApeI endonuclease and probably other endonucleases cleave these abasic sites, generating a nick at each position. Some nicks are generated on the upper strand as well in an AID-dependent manner, but it is less clear how that happens. Nicks on both strands contribute to double-stranded breaks both at the Sµ region and at the downstream switch locus that is involved in a particular isotype switch event. The existence of double-stranded breaks in two switch regions results in the deletion of the intervening DNA and joining together of the two broken switch regions by use of the machinery involved in double-stranded break repair by nonhomologous end joining. This machinery is also used to repair double-stranded breaks during V(D)J recombination (see Chapter 8).
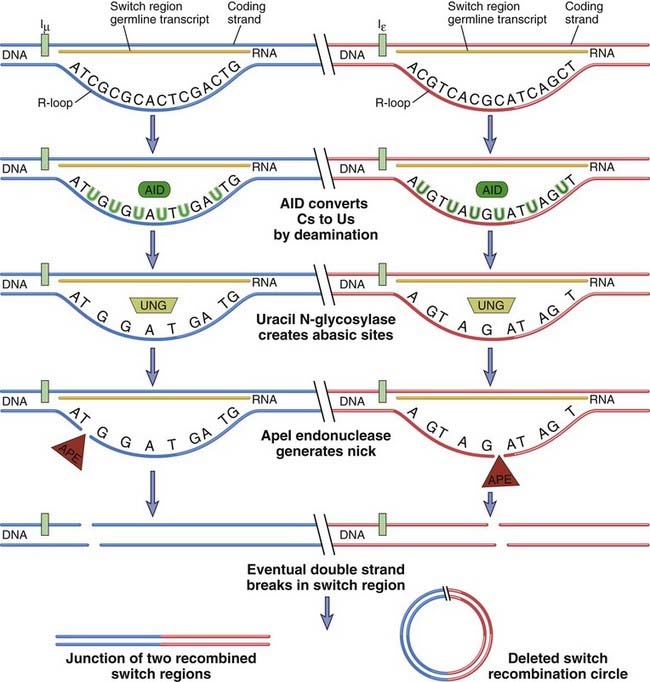
FIGURE 11–16 Mechanism by which AID and germline transcription collaborate to generate double-stranded breaks at switch regions.
Two different switch regions for µ and ε are shown. Germline transcripts form DNA-RNA hybrids in the switch region, freeing up the nontemplate strand as an R-loop of single-stranded DNA. This is a particularly good template for AID, which deaminates C residues to generate U residues in single-stranded DNA. Uracil N-glycosylase (UNG) removes U residues to generate abasic sites that can be sites of nick generation after the action of the ApeI endonuclease. Two nicks roughly opposite each other contribute to a double-stranded break. The mechanism of generation of the nick in the template strand is less well understood. Double-stranded breaks are made in each switch region, and these are recombined while the intervening DNA is deleted as a circle.
Affinity Maturation: Somatic Mutation of Ig Genes and Selection of High-Affinity B Cells
Affinity maturation is the process that leads to increased affinity of antibodies for a particular antigen as a T-dependent humoral response progresses and is the result of somatic mutation of Ig genes followed by selective survival of the B cells producing the antibodies with the highest affinities. The process of affinity maturation generates antibodies with an increasing capacity to bind antigens and thus to more efficiently bind to, neutralize, and eliminate microbes (Fig. 11-17). Helper T cells and CD40:CD40L interactions are required for somatic mutation to be initiated, and as a result, affinity maturation is observed only in antibody responses to T-dependent protein antigens. Some somatic mutation occurs in B cells in extrafollicular foci, but extensive somatic mutation occurs in germinal centers. As discussed earlier, the need for CD40 reflects the ability of this receptor to induce AID as well as extensive proliferation in B cells.
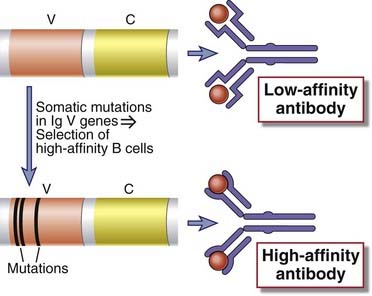
FIGURE 11–17 An overview of affinity maturation.
Early in the immune response, low-affinity antibodies are produced. During the germinal center reaction, somatic mutation of Ig V genes and selection of mutated B cells with high-affinity antigen receptors result in the production of antibodies with high affinity for antigen.
In proliferating germinal center B cells in the dark zone, Ig V genes undergo point mutations at an extremely high rate. This rate is estimated to be 1 in 103 V gene base pairs per cell division, which is about a thousand times higher than the spontaneous rate of mutation in other mammalian genes. (For this reason, mutation in Ig V genes is also called hypermutation.) The V genes of expressed heavy and light chains in each B cell contain a total of about 700 nucleotides; this implies that mutations will accumulate in expressed V regions at an average rate of almost one per cell division. Ig V gene mutations continue to occur in the progeny of individual B cells. As a result, any B cell clone can accumulate more and more mutations during its life in the germinal center. It is estimated that as a consequence of somatic mutations, the nucleotide sequences of IgG antibodies derived from one clone of B cells can diverge as much as 5% from the original germline sequence. This usually translates to up to 10 amino acid substitutions. Several features of these mutations are noteworthy. First, the mutations are clustered in the V regions, mostly in the antigen-binding complementarity-determining regions (Fig. 11-18). Second, there are far more mutations in IgG than in IgM antibodies. Third, the presence of mutations correlates with increasing affinities of the antibodies for the antigen that induced the response.
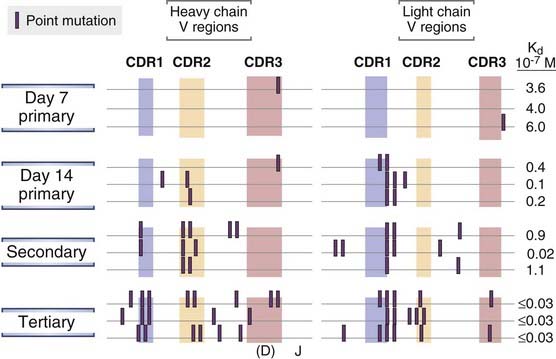
FIGURE 11–18 Somatic mutations in Ig V genes.
Hybridomas were produced from spleen cells of mice immunized 7 or 14 days previously with a hapten, oxazolone, coupled to a protein and from spleen cells obtained after secondary and tertiary immunizations with the same antigen. Hybridomas producing oxazolone-specific monoclonal antibodies were isolated, and the nucleotide sequences of the V genes encoding the Ig heavy and light chains were determined. Mutations in V genes increase with time after immunization and with repeated immunizations and are clustered in the complementarity-determining regions (CDRs). The location of CDR3 in the heavy chains is approximate. The affinities of the antibodies produced also tend to increase with more mutations, as indicated by the lower dissociation constants (Kd) for hapten binding.
(Modified from Berek C, and C Milstein. Mutation drift and repertoire shift in maturation of the immune response. Immunological Reviews 96:23-41, 1987, Blackwell Publishing.)
The mechanisms underlying somatic mutation in Ig genes are partially understood. It is clear that the rearranged Ig VDJ exon becomes highly susceptible to mutation, suggesting enhanced susceptibility of this region to DNA-binding factors that identify rearranged V regions for mutation. The enzyme AID, discussed before in the context of isotype switching, plays an essential role in affinity maturation. Its DNA deaminase activity converts C residues to U residues at hotspots for mutation. The U’s may be changed to T’s when DNA replication occurs, thus generating a common type of C to T mutation, or the U may be excised by uracil N-glycosylase, and the abasic site thus generated is repaired by an error-prone repair process, eventually generating all types of substitutions at each site of AID-induced cytidine deamination. These error-prone repair processes extend mutations to residues beyond the C residues that are targeted by AID.
Repeated stimulation by T cell–dependent protein antigens leads to increasing numbers of mutations in the Ig genes of antigen-specific germinal center B cells. Some of these mutations are likely to be useful because they will generate high-affinity antibodies. However, many of the mutations may result in a decline or even in a loss of antigen binding. Therefore, the next and crucial step in the process of affinity maturation is the selection of the most useful, high-affinity B cells.
B cells that bind antigens in germinal centers with high affinity are selected to survive (Fig. 11-19). The early response to antigen results in the production of antibodies, some of which form complexes with residual antigen and may activate complement. FDCs express receptors for the Fc portions of antibodies and for products of complement activation, including C3b and C3d. These receptors bind and display antigens that are complexed with antibodies and complement products. Antigen may also be displayed in free form in the germinal center. Meanwhile, germinal center B cells that have undergone somatic mutation migrate into the FDC-rich light zone of the germinal center. In germinal center B cells, IL-21 secreted by TFH cells induces the expression of proteins that induce apoptosis and reduces the expression of proteins that prevent apoptosis. Therefore, these B cells die by apoptosis unless they are rescued by recognition of antigen. B cells with high-affinity receptors for the antigen are best able to bind the antigen when it is present at low concentrations, and these B cells survive preferentially because of several mechanisms. First, antigen recognition by itself induces expression of antiapoptotic proteins of the Bcl-2 family. Second, high-affinity B cells will preferentially endocytose and present the antigen and interact stably with the limiting numbers of TFH cells in the germinal center. These helper T cells may use CD40L to promote the survival of the B cells they interact with. Third, some TFH cells express Fas ligand, which can recognize the death receptor Fas on germinal center B cells and deliver an apoptotic signal. High-affinity B cells, which are best able to recognize and respond to antigen, may activate endogenous inhibitors of Fas when their BCRs recognize antigen and thus be protected from death while low-affinity B cells are killed.
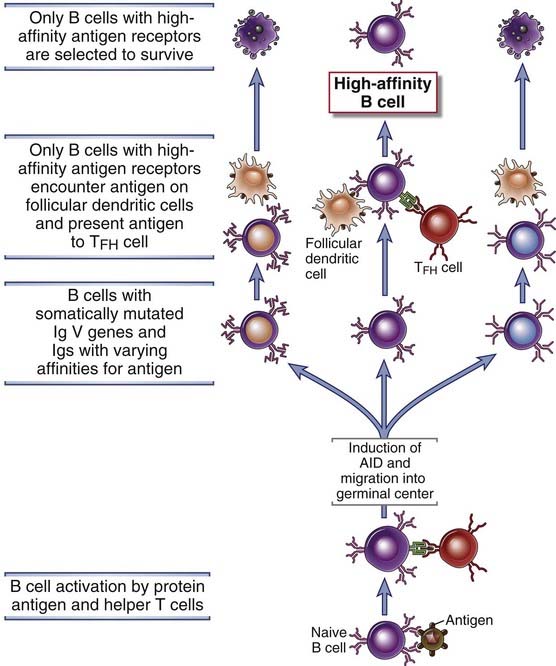
FIGURE 11–19 B cell selection in germinal centers.
Somatic mutation of V region genes in germinal center B cells generates antibodies with different affinities for antigen. Subsequently, binding of the B cells to antigen displayed on follicular dendritic cells is necessary to rescue the B cells from programmed cell death. B cells may also present antigen to germinal center TFH cells, which may promote B cell survival. The B cells with the highest affinity for antigen will have a selective advantage for survival as the amount of available antigen decreases during an immune response. This leads to an average increase in the affinity of antibodies for antigen as the humoral immune response progresses.
As more antibody is produced, more of the antigen is eliminated and less is available in the germinal centers. Therefore, the B cells that will be able to specifically bind this antigen and to be rescued from death need to express antigen receptors with higher and higher affinity for the antigen. As a result, as the antibody response to an antigen progresses, the B cells that are selected in germinal centers produce Ig of increasing affinity for the antigen. This selection process results in affinity maturation of the antibody response. Because somatic mutation also generates many B cells that do not express high-affinity receptors for antigen and cannot therefore be selected to survive, the germinal centers are sites of tremendous apoptosis.
Somatic mutation occurs in the basal dark zone of germinal centers in B cells called centroblasts, which contain nuclear AID, and these mutated cells may repeatedly cycle between the basal dark zone and the apical light zone, where they differentiate into morphologically distinct cells called centrocytes. Eventually, high-affinity centrocytes may be selected in the light zone by antigen, with help from TFH cells, and may undergo additional isotype switching. The selected cells then either differentiate into memory B cells or into high-affinity antibody-secreting plasma cells that exit the germinal center.
Given the extraordinary rate of mutation in germinal centers, it is not surprising that human lymphoid malignant neoplasms develop most often from B cells in this location because the DNA breaks associated with mutation and isotype switching set the stage for chromosomal translocations of various oncogenes into Ig gene loci, producing tumors of B cells (lymphomas). Germinal centers also assume an important role in the pathogenesis of autoimmunity because T cell tolerance mechanisms can be subverted if somatic mutation drives a B cell clone in the germinal center to become strongly self-reactive. In fact, it is known that dysregulation of B cell selection in germinal centers contributes to autoantibody production.
B Cell Differentiation into Antibody-Secreting Plasma Cells
Some of the progeny of the B cells that have proliferated in response to antigen and T cell help differentiate into antibody-secreting plasma cells. Plasma cells are morphologically distinct, terminally differentiated B cells committed to abundant antibody production (see Chapter 2). They are generated after the activation of B cells through signals from the BCR, CD40, TLRs, and other receptors including cytokine receptors. There are two types of plasma cells. Short-lived plasma cells are generated during T-independent responses and early during T cell–dependent responses in extrafollicular B cell foci, described earlier. These cells are generally found in secondary lymphoid organs and in peripheral nonlymphoid tissues. Long-lived plasma cells are generated in T-dependent germinal center responses to protein antigens. Signals from the B cell antigen receptor and IL-21 cooperate in the generation of plasma cells. Plasma cells (and their precursors, plasmablasts, which may be found in the circulation) generated in germinal centers acquire the ability to home to the bone marrow, where they are maintained by cytokines of the BAFF family which bind to a plasma cell membrane receptor called BCMA, thus allowing the plasma cells to survive for long periods, often as long as the life span of the host. Typically 2 to 3 weeks after immunization with a T cell–dependent antigen, the bone marrow becomes a major site of antibody production. Plasma cells in the bone marrow may continue to secrete antibodies for months or even years after the antigen is no longer present. These antibodies can provide immediate protection if the antigen is encountered later. It is estimated that almost half the antibody in the blood of a healthy adult is produced by long-lived plasma cells and is specific for antigens that were encountered in the past. Secreted antibodies enter the circulation and mucosal secretions, but mature plasma cells do not recirculate.
The differentiation of activated B cells into antibody-secreting plasma cells involves major structural alterations, especially of components of the endoplasmic reticulum and secretory pathway, and increased Ig production as well as a change in Ig heavy chains from the membrane to the secreted form. Although a plasma cell is derived from a B cell, it undergoes a remarkable transformation as it differentiates from an activated B cell to the plasmablast stage. Most strikingly, the cell enlarges dramatically, and the ratio of cytoplasm to nucleus also undergoes a striking increase. The endoplasmic reticulum becomes prominent, and the cell is transformed into a secretory cell that bears little or no resemblance to a B cell. The transcription factors that regulate the development of plasma cells are described later.
The change in Ig production from the membrane form (characteristic of B cells) to the secreted form (in plasma cells) is the result of changes in the carboxyl terminal of the Ig heavy chain (Fig. 11-20). For instance, in membrane µ, Cµ4 is followed by a short spacer, 26 hydrophobic transmembrane residues, and a cytoplasmic tail of three amino acids (lysine, valine, and lysine). In secreted IgM, on the other hand, the Cµ4 domain is followed by a tail piece containing polar amino acids. This transition from membrane to secreted Ig is caused by altered RNA processing of the heavy chain messenger RNA (mRNA). The primary RNA transcript in all IgM-producing B cells contains the rearranged VDJ cassette, the four Cµ exons coding for the constant (C) region domains, and the two exons encoding the transmembrane and cytoplasmic domains. Alternative processing of this transcript, which is regulated by RNA cleavage and the choice of polyadenylation sites, determines whether or not the transmembrane and cytoplasmic exons are included in the mature mRNA. If they are included, the µ chain produced contains the amino acids that make up the transmembrane and cytoplasmic segments and is therefore anchored in the lipid bilayer of the plasma membrane. If, on the other hand, the transmembrane segment is excluded from the µ chain, the carboxyl terminus consists of about 20 amino acids constituting the tail piece. Because this protein does not have a stretch of hydrophobic amino acids or a positively charged cytoplasmic tail, it cannot remain anchored in the endoplasmic reticulum membrane, resides initially in the luminal space of the secretory pathway, and is secreted. Thus, each B cell can synthesize both membrane and secreted Ig. Most of the Ig heavy chain mRNA in a plasma cell is cleaved at the upstream polyadenylation site, so most of this mRNA is of the secretory form. All CH genes contain similar membrane exons, and all heavy chains can be potentially expressed in membrane-bound and secreted forms. The secretory form of the δ heavy chain is rarely made, however, so that IgD is usually present only as a membrane-bound protein.
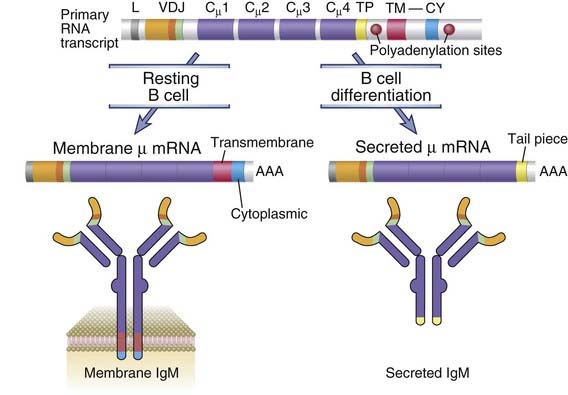
FIGURE 11–20 Production of membrane and secreted µ chains in B lymphocytes.
Alternative processing of a primary RNA transcript results in the formation of mRNA for the membrane or secreted form of the µ heavy chain. B cell differentiation results in an increasing fraction of the µ protein produced as the secreted form. TP, TM, and CY refer to tail piece, transmembrane, and cytoplasmic segments, respectively. Cµ1, Cµ2, Cµ3, and Cµ4 are four exons of the Cµ gene.
Generation of Memory B Cells and Secondary Humoral Immune Responses
Some of the antigen-activated B cells emerging from germinal centers acquire the ability to survive for long periods, apparently without continuing antigenic stimulation. These are memory cells, capable of mounting rapid responses to subsequent introduction of antigen. Because memory cells are generated mainly in germinal centers, they are seen in T-dependent immune responses and usually emerge in parallel with memory helper T cells. Memory B cells express high levels of the antiapoptotic protein Bcl-2, which contributes to the long life span of these cells. Some memory B cells may remain in the lymphoid organ where they were generated, whereas others exit germinal centers and recirculate between the blood and lymphoid organs. Memory cells typically bear high-affinity (mutated) antigen receptors and Ig molecules of switched isotypes more commonly than do naive B lymphocytes. The production of large quantities of isotype-switched, high-affinity antibodies is greatly accelerated after secondary exposure to antigens, and this can be attributed to the activation of memory cells in germinal centers.
Many of the features of secondary antibody responses to protein antigens, and their differences from primary responses (see Fig. 11-2), reflect the differences between responses of memory and naive B cells, respectively. Because memory B cell generation is optimal in the presence of T cell help, secondary responses are also best seen after exposure to T-dependent protein antigens. Thus, heavy chain class switching, which is typical of secondary responses, is stimulated by helper T cells. Affinity maturation, which increases with repeated antigenic stimulation, is also a consequence of helper T cell–induced B cell activation and somatic mutation. High-affinity antibodies are required to neutralize the infectivity of many microbes and the pathogenicity of microbial toxins, and memory B cells mount large, rapid and high-affinity responses to repeated infections. Therefore, effective vaccines against these microorganisms must induce both affinity maturation and memory B cell formation, and these events will occur only if the vaccines are able to activate helper T cells. This concept has been applied to the design of vaccines for some bacterial infections in which the target antigen is a capsular polysaccharide, which is incapable of stimulating T cells. In these cases, the polysaccharide is covalently linked to a foreign protein to form the equivalent of a hapten-carrier conjugate, which does activate helper T cells. Such vaccines, which are called conjugate vaccines, more readily induce high-affinity antibodies and memory than do polysaccharide vaccines without linked proteins. Such vaccines have proved particularly effective at inducing protective immunity in infants and young children, who are less able to make strong T-independent responses to polysaccharides than are adults.
Role of Transcriptional Regulators in Determining the Fate of Activated B Cells
The outcome of B cell differentiation is regulated by the induction and activation of different transcription factors. It is clear from the discussion so far that activated B cells can follow several fates. They can develop into short-lived or long-lived plasma cells, which secrete large amounts of antibodies, or into long-lived memory cells, which do not secrete antibodies but survive for prolonged periods and respond rapidly to antigen challenge. In Chapter 9, we discussed the concept that T cell fates are determined in large part by the expression of various transcriptional activators and repressors. The same general principle applies to the fates of activated B cells.
In germinal center B cells, signals delivered through CD40 and the IL-21 receptor induce the expression of a transcriptional repressor called Bcl-6. Bcl-6 antagonizes another repressor called Blimp-1, which is required for plasma cell development (see later) and thus prevents cells in the germinal center from differentiating into plasma cells during the massive proliferation that is characteristic of the germinal center reaction. B cells in extrafollicular foci express lower levels of Bcl-6 and more readily develop into short-lived plasma cells. Bcl-6 and other transcriptional regulators help orchestrate rapid cell cycle entry of germinal center B cells. They also make germinal center B cells sensitive to apoptosis by increasing levels of proapoptotic factors and reducing the levels of antiapoptotic proteins.
Two key steps in becoming a plasma cell are the loss of expression of a transcription factor, Pax-5, which is required for the development and maintenance of a mature B cell (see Chapter 8), and the repression of Bcl-6. During plasma cell differentiation, a transcriptional activator called IRF4 and a transcriptional repressor called Blimp-1 are induced. IRF4 and Blimp-1 together induce the expression and splicing of XBP-1, a transcription factor that plays a critical role in the unfolded protein response. XBP-1 may protect developing plasma cells from the injurious consequences of unfolded proteins (which are produced as a side effect of the massive increase in protein synthesis), or it may contribute to the maturation of plasma cells and the enhanced synthesis of Ig seen in these cells.
How exactly the decision is made for a given germinal center B cell to choose between becoming either a memory B cell or a long-lived plasma cell is unclear. Indeed, transcription factors that delineate memory B cell development remain to be identified. It appears, however, that some of the progeny of an antigen-stimulated B cell clone express low levels of IRF4, and these become functionally quiescent, self-renewing, long-lived memory cells. Whereas high levels of IRF4 lead to plasma cell differentiation, lower levels of IRF4 are insufficient to drive an activated B cell toward plasma cell differentiation and thus may be permissive for memory B cell generation.
Antibody Responses to T Cell–Independent Antigens
Many nonprotein antigens, such as polysaccharides and lipids, stimulate antibody production in the absence of helper T cells, and these antigens and the responses they elicit are termed thymus independent or T independent (TI). These antibody responses differ in several respects from responses to T cell–dependent protein antigens (Table 11-2). The antibodies that are produced in the absence of T cell help are generally of low affinity and consist mainly of IgM with limited isotype switching to some IgG subtypes and also to IgA.
TABLE 11–2 Properties of Thymus-Dependent and Thymus-Independent Antigens
| Thymus-Dependent Antigen | Thymus-Independent Antigen | |
|---|---|---|
| Chemical nature | Proteins | Polymeric antigens, especially polysaccharides; also glycolipids, nucleic acids |
| Features of Antibody Response | ||
| Isotype switching | Yes; IgG, IgE, and IgA | Little or no; may be some IgG and IgA |
| Affinity maturation | Yes | No |
| Secondary response (memory B cells) | Yes | Only seen with some antigens (e.g., polysaccharides) |
Nature of B Cells That Respond to T-Independent Antigens
The marginal zone and B-1 subsets of B cells are especially important for antibody responses to TI antigens. Whereas responses to T-dependent protein antigens are largely mediated by follicular B cells, other B cell subsets may be the primary responders to TI antigens (see Fig. 11-3). Marginal zone B cells are a distinct population of B cells that mainly respond to polysaccharides. After activation, these cells differentiate into short-lived plasma cells that produce mainly IgM. In humans these cells are also called IgM memory cells. B-1 cells represent another lineage of B cells that responds readily to TI antigens mainly in the peritoneum and in mucosal sites.
TI responses may be initiated in the spleen, bone marrow, peritoneal cavity, and mucosal sites. Macrophages located in the marginal zones surrounding lymphoid follicles in the spleen are particularly efficient at trapping polysaccharides when these antigens are injected intravenously. TI antigens may persist for prolonged periods on the surfaces of marginal zone macrophages, where they are recognized by specific B cells.
Mechanisms of T-Independent Antibody Responses
The most important TI antigens are polysaccharides, glycolipids, and nucleic acids, all of which induce specific antibody production in T cell–deficient animals. These antigens cannot be processed and presented in association with MHC molecules, and therefore they cannot be recognized by CD4+ helper T cells. Most TI antigens are multivalent, being composed of repeated identical antigenic epitopes. Such multivalent antigens may induce maximal cross-linking of the BCR complex on specific B cells, leading to activation without a requirement for cognate T cell help. In addition, many polysaccharides activate the complement system by the alternative pathway, generating C3d, which binds to the antigen and is recognized by CR2, thus augmenting B cell activation (see Fig. 11-5 and Chapter 7). Membrane proteins at a high density on a microbial surface may be functionally multivalent and may function in a T-independent as well as in a T-dependent manner. As mentioned earlier, TI responses may also be facilitated by additional signals derived from microbial products that activate TLRs on B cells.
Although TI responses typically show little isotype switching, some T-independent nonprotein antigens do induce Ig isotypes other than IgM. In humans, the dominant antibody class induced by pneumococcal capsular polysaccharide is IgG2. In mice engineered to lack CD40, IgE and many IgG subclasses are barely detectable in the serum, but levels of IgG3 (which resembles human IgG2) and IgA in the serum are reduced to only about half their normal levels. Cytokines produced by non-T cells may stimulate isotype switching in TI responses. As described earlier, in the absence of T cells, BAFF and APRIL produced by cells of myeloid origin, such as dendritic cells and macrophages, can induce the synthesis of AID in antigen-activated B cells through a receptor of the BAFF receptor family called TACI. This may be further facilitated by the activation of TLRs on these B cells. In addition, cytokines such as TGF-β that help mediate the IgA switch are secreted by many nonlymphoid cells at mucosal sites and may contribute to the generation of IgA antibodies directed against nonprotein antigens.
Functions of T-Independent Antibody Responses
The practical significance of TI antigens is that many bacterial cell wall polysaccharides belong to this category, and humoral immunity is the major mechanism of host defense against infections by such encapsulated bacteria. For this reason, individuals with congenital or acquired deficiencies of humoral immunity are especially susceptible to life-threatening infections with encapsulated bacteria, such as pneumococcus, meningococcus, and Haemophilus. In addition, TI antigens contribute to the generation of natural antibodies, which are present in the circulation of normal individuals and are apparently produced without overt exposure to pathogens. Most natural antibodies are low-affinity anticarbohydrate antibodies, postulated to be produced by B-1 peritoneal B cells stimulated by bacteria that colonize the gastrointestinal tract and by marginal zone B cells in the spleen. Antibodies to the A and B glycolipid blood group antigens are examples of these natural antibodies (see Chapter 16).
Despite their inability to specifically activate helper T cells, many polysaccharide vaccines, such as the pneumococcal vaccine, induce long-lived protective immunity. Rapid and large secondary responses typical of memory (but without much isotype switching or affinity maturation) do occur on secondary exposure to these carbohydrate antigens. The phenomenon of IgM memory has been clearly demonstrated in the mouse, and in both mice and adult humans, IgM memory B cells can be identified by the expression of specific cell surface markers. In humans, these memory-like cells express high levels of CD27 and IgM and IgD and they are also called marginal zone B cells.
Antibody Feedback: Regulation of Humoral Immune Responses by Fc Receptors
Secreted antibodies inhibit continuing B cell activation by forming antigen-antibody complexes that simultaneously bind to antigen receptors and inhibitory Fcγ receptors on antigen-specific B cells (Fig. 11-21). This is the explanation for a phenomenon called antibody feedback, which refers to the downregulation of antibody production by secreted IgG antibodies. IgG antibodies inhibit B cell activation by forming complexes with the antigen, and these complexes bind to a B cell receptor for the Fc portions of the IgG, called the Fcγ receptor II (FcγRIIB, or CD32). (The biology of Fc receptors is discussed in Chapter 12.) As discussed in Chapter 7, the cytoplasmic tail of FcγRIIB contains a six–amino acid (isoleucine-x-tyrosine-x-x-leucine) motif shared by other receptors in the immune system that mediate negative signals, including inhibitory receptors on NK cells. By analogy to ITAMs, this inhibitory motif is called an immunoreceptor tyrosine-based inhibition motif (ITIM). When the Fcγ receptor of B cells is engaged, the ITIM on the cytosolic tail of the receptor is phosphorylated on tyrosine residues, and it forms a docking site for the inositol 5-phosphatase SHIP (SH2 domain–containing inositol phosphatase). The recruited SHIP hydrolyses a phosphate on the signaling lipid intermediate phosphatidylinositol trisphosphate (PIP3) and inactivates this molecule. By this mechanism, engagement of FcγRII terminates the B cell response to antigen. The antigen-antibody complexes simultaneously interact with the antigen receptor (through the antigen) and with FcγRIIB (through the antibody), and this brings the inhibitory phosphatases close to the antigen receptors whose signaling is blocked. In addition to B cells, FcγRIIB binds and sends inhibitory signals to myeloid cells, including macrophages and dendritic cells, and perhaps also plasma cells.
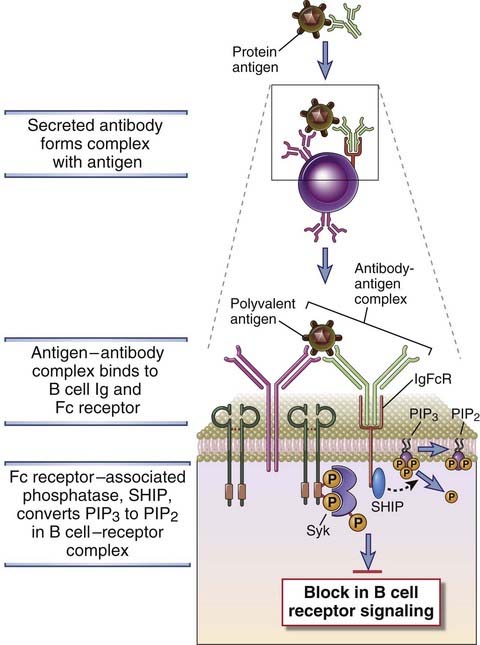
FIGURE 11–21 Regulation of B cell activation by FcγRIIB.
Antigen-antibody complexes can simultaneously bind to membrane Ig (through antigen) and the FcγRIIB receptor through the Fc portion of the antibody. As a consequence of this simultaneous ligation of receptors, phosphatases associated with the cytoplasmic tail of the FcγRIIB inhibit signaling by the BCR complex and block B cell activation.
Fc receptor–mediated antibody feedback is a physiologic control mechanism in humoral immune responses because it is triggered by secreted antibody and blocks further antibody production. We have stated earlier in the chapter that antibodies can also amplify antibody production by activating complement and generating C3d. It is not clear under which circumstances secreted antibodies provide complement-mediated amplification or Fc receptor–mediated inhibition. A likely scenario is that early in humoral immune responses, IgM antibodies (which activate complement but do not bind to the Fcγ receptor) are involved in amplification, whereas increasing production of IgG leads to feedback inhibition.
The importance of FcγRIIB-mediated inhibition is demonstrated by the uncontrolled antibody production seen in mice in which the gene encoding this receptor has been knocked out. A polymorphism in the FcγRIIB gene has been linked to susceptibility to the autoimmune disease systemic lupus erythematosus in humans.
B cells express another inhibitory receptor called CD22. CD22 is a sialic acid–binding lectin; its natural ligand is not known, and we do not know exactly how it is engaged during physiologic B cell responses. However, knockout mice lacking CD22 show greatly enhanced B cell activation. The cytoplasmic tail of this molecule contains ITIM tyrosine residues, which, when phosphorylated by the Src family kinase Lyn, bind the SH2 domain of the tyrosine phosphatase SHP-1. SHP-1 removes phosphates from the tyrosine residues of several enzymes and adaptor proteins involved in BCR signaling and thus abrogates B cell activation. A mouse strain called motheaten, which develops severe autoimmunity with uncontrolled B cell activation and autoantibody production, has a naturally occurring mutation in SHP-1. Conditional deletion of SHP-1 as well as the engineered loss of Lyn in B cells leads to a breakdown of peripheral B cell tolerance and the development of autoimmunity.
Summary
B Cell Subsets and B Cell Activation
Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nature Immunology. 2010;11:681-688.
Hardy RR. B-1 B cell development. Journal of Immunology. 2006;176:2749-2754.
Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609-619.
Martin F, Chan AC. B cell immunobiology in disease: evolving concept from the clinic. Annual Review of Immunology. 2006;24:467-496.
T Follicular Helper Cells and the Germinal Center Reaction
Crotty S. Follicular helper CD4 T cells. Annual Review of Immunology. 29, 2011.
Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature Immunology. 2010;11:114-120.
King C. New insights into the differentiation and function of T follicular helper cells. Nature Reviews Immunology. 2009;9:757-766.
McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annual Review of Immunology. 2005;23:487-513.
Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nature Reviews Immunology. 2006;6:741-750.
Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nature Reviews Immunology. 2009;9:845-857.
AID, Class Switching, and Somatic Mutation
Cerutti A. The regulation of IgA class switching. Nature Reviews Immunology. 2008;8:421-434.
Delker RK, Fugmann S, Papavasiliou FN. A coming-of-age story: activation-induced cytidine deaminase turns 10. Nature Immunology. 2009;10:1147-1153.
Liu M, Schatz DG. Balancing AID and DNA repair during somatic hypermutation. Trends in Immunology. 2009;30:173-181.
Neuberger MS. Antibody diversification by somatic mutation: from Burnet onwards. Immunology and Cell Biology. 2008;86:124-132.
Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The biochemistry of somatic hypermutation. Annual Review of Immunology. 2008;26:481-511.
Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annual Review of Immunology. 2008;26:261-292.