CHAPTER 11 SUPPLEMENT A Gingival Diseases in Childhood
Periodontal disease in adults is partly precipitated by gingival inflammation in the formative years of childhood and early adolescence. The nondestructive gingival inflammation of childhood without appropriate intervention may progress to the more significant periodontal diseases seen in the adult population.
After first reviewing anatomic and physiologic changes in the periodontium and dentition, this chapter presents the gingival changes associated with childhood and adolescence. Periodontal diseases in these early periods of life are presented in the chapters dealing with the respective diseases (see Chapters 16, 17, 18, and 27).
Periodontium of the Primary Dentition
The normal gingiva of the primary dentition is somewhat different from that found in adults. The tissues are pale pink but to a lesser degree than the attached gingiva of adults because the thinness of the keratinized layer causes the underlying vessels in children to be more visible.36 Stippling appears at about 3 years of age and has been reported in 56% of children between ages 3 and 10 years, with little difference between maxillary and mandibular arches or males and females throughout childhood10 (Supplement A Figure 11-1).
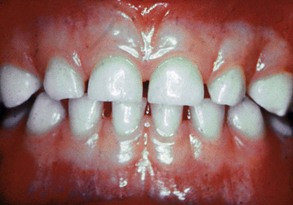
Supplement A Figure 11-1 Normal gingival of a 5-year-old child showing light stippling and flattened interproximal gingival in areas of physiologic spacing.
The interdental gingiva is broad buccolingually and narrow mesiodistally, consistent with the morphology of the primary dentition. Its structure and composition are similar to adult gingiva.
Gingival sulcular depth is shallower in the primary dentition than in the permanent dentition. Probing depths range from 1 to 2 mm, with an increase in depth from anterior to posterior.8,20,49
The attached gingiva varies in width anteroposteriorly, with a range of 3 to 6 mm. On the buccal surfaces, the width decreases from anterior to posterior, with some data indicating a narrowing over the canines (Supplement A Figure 11-2). The lingual attached gingiva shows an inverse relationship, with an increase in width from anterior to posterior.20 The gingival width normally increases with age as children transition from primary to permanent dentition.5,8,14,49 Interestingly, the junctional epithelium is thicker in the primary dentition than in the permanent dentition,9 which is a phenomenon thought to reduce the permeability of the epithelium to bacterial toxins.
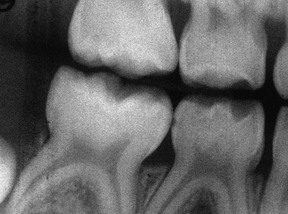
Radiographically, the lamina dura is prominent in the primary dentition, with a wider periodontal space than in the permanent dentition. The marrow spaces of the bone are larger, and the crests of the interdental bony septa are flat, with bony crests within 1 to 2 mm of the cementoenamel junction21 (Supplement A Figure 11-3).
Supplement A Figure 11-2 Normal gingiva demonstrating width of the attached gingiva, as illustrated by the pigmentation that occurs only in the attached gingival area. This 6-year-old African-American youngster has erupting lower central incisors. As the incisors erupt, the attached gingiva will widen as the alveolus grows.
Periodontal Changes Associated with Normal Development
Significant changes occur in the periodontium as the dentition changes from primary to permanent teeth. Most of the changes are associated with eruption and are physiologic in nature. These changes should be distinguished from gingival disease, which may occur simultaneously.
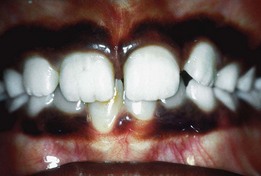
Before eruption of a primary or permanent tooth, the gingiva reveals a bulge that is firm and pink or blanched because of the underlying tooth crown (Supplement A Figure 11-4). Occasionally, an eruption cyst, which may be filled with blood and generally presents as a bluish or deep red enlargement of the gingiva over the erupting tooth, may be evident. The most common sites are the primary lower incisors and the permanent first molars. Many resolve without treatment but may be marsupialized if they are painful or interfere with occlusion.13
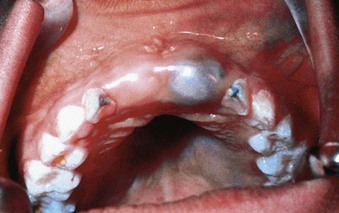
Supplement A Figure 11-4 On the right side, the central incisor bulge is evident, a normal finding at this stage of eruption. On the left side, an eruption cyst is evident. These cysts may exhibit the blue color illustrated here or may be hemorrhagic in nature.
As a tooth erupts, the gingival margin and sulcus develop. At this point the margin is rounded, edematous, and reddened. During the period of active tooth eruption, it is normal for the marginal gingiva surrounding partially erupted teeth to appear prominent; this is most evident in the maxillary anterior region. The prominence is caused by the height of contour of the erupting tooth and mild inflammation from mastication. Poor oral hygiene can contribute to the development of significant gingivitis in unprotected gingival areas.
External trauma, occlusal trauma, or developmental disturbances may result in ankylosis of primary teeth and occasionally of permanent teeth. These teeth appear to be intruded or sunken relative to adjacent teeth. Ankylosed primary teeth may interfere with alveolar bone growth around erupting permanent teeth and may prevent or delay their timely emergence into the oral cavity. Ankylosis of primary first molars often resolves spontaneously as the succedaneous premolar erupts; however, a significant number of ankylosed second primary molars will prevent the eruption of the underlying premolar and should be managed appropriately before the completion of root development of the premolar.
Like tooth eruption, the process of tooth exfoliation involves changes in the periodontium. The depth of the gingival sulcus increases as the junctional epithelium migrates down the resorbing root of an exfoliating tooth.7,9 During this process, there may be changes in the permeability and integrity of the junctional epithelium, making the exfoliating tooth more susceptible to inflammation.9
Normal functional occlusal forces may traumatize the remaining supporting periodontal tissues of an exfoliating primary tooth or its succedaneous replacement.22 During the process of exfoliation, teeth may change position, possibly leading to changes in occlusion. Malalignment caused by spacing and changes in skeletal relationships related to erupting teeth also may contribute to significant trauma of periodontal structures.
Microscopically, minor traumatic changes may demonstrate compression, ischemia, and hyalinization of the periodontal ligament.26,42 With more severe injury, crushing and necrosis of the periodontal ligament may occur. In most patients, these injuries are spontaneously resolved as teeth exfoliate, erupt, and align through the normal growth and development processes.
Relation of Periodontal Status to Malocclusion
Data indicate an association between abnormal tooth position and gingivitis.17 Crowding in the mixed dentition can often make plaque and food removal more difficult, leading to an increased incidence of gingivitis (Supplement A Figures 11-5 and 11-6). Severe changes may include gingival enlargement, discoloration, occasional ulceration, and the formation of deep pockets or pseudopockets. Generally, gingival health can be restored by orthodontic correction, but failure to align teeth does not necessarily have an effect on periodontal disease later in life.17
Mucogingival Problems
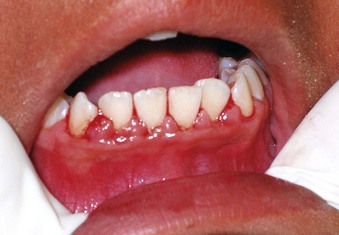
The prevalence of mucogingival problems and recession in children ranges from 1% to 19%, depending on the criteria used to assess the condition.30 Evidence suggests that some mucogingival problems may start in the primary dentition as a consequence of developmental aberrations in eruption and deficiencies in the thickness of the periodontium.36,37 Frenum pull may also be a factor in the development of mucogingival problems.45,50 In the mixed dentition, recession is most often found on the facial aspect of mandibular permanent incisors secondary to rotations or labial positioning related to space problems (Supplement A Figure 11-7). Although erupting permanent lower incisors often show minimal attached gingiva, gingival width often increases as the teeth erupt and stimulate bone development14 (see Supplement A Figure 11-2).
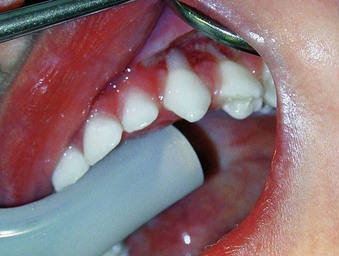
Supplement A Figure 11-7 Labial stripping secondary to a class II growth pattern and lower incisor crowding. The labially erupting lower incisor demonstrates an associated gingivitis from poor oral hygiene. Immediate orthodontic treatment should be undertaken followed by appropriate grafting, if necessary.
The maxillary canine region is also prone to localized gingival recession. Late-erupting canines in a crowded dentition may be displaced buccally, erupting into or near unattached gingiva or mucosa, and increasing the risk of insufficient gingival tissue width and recession. Recession also may be associated with an anterior open bite secondary to labial inclination of the teeth.33 Orthodontic treatment and realignment may be necessary to protect the integrity of the attached gingiva.
Mucogingival problems also can result from factitious habits or excessive toothbrushing either by a parent or child (Supplement A Figure 11-8). Because the width of the attached gingiva increases with age, any of these problems may resolve spontaneously, suggesting a cautious approach to treatment with judicious monitoring instead of immediate surgical intervention.14,46
Gingival Diseases of Childhood
Plaque-Induced Gingival Disease
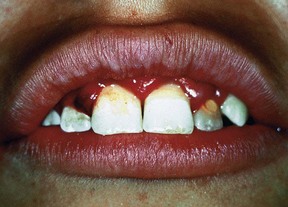
Gingivitis is extremely common in children and adolescents, affecting up to 70% of children older than seven years of age.15,40 Inflammation is generally limited to the marginal gingiva (Supplement A Figure 11-9), with undetectable loss of bone or connective tissue attachment in most cases. Although gingivitis does not always progress to periodontitis, management of the gingival disease in children is important because periodontitis is always preceded by gingivitis.40
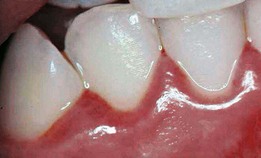
Supplement A Figure 11-9 Chronic marginal gingivitis secondary to poor oral hygiene. Note the erythematous and edematous tissues of the gingival margin.
In children, as in adults, the primary cause of gingivitis is dental plaque, which is related to poor oral hygiene. The relationship between plaque and the gingival index, however, is weak and remains unclear.7,34,35 Although gingivitis is highly prevalent in children, its severity generally is less intense than in adults.9 Similar oral hygiene conditions generally produce less severe forms of disease in children compared to adults.40
As children age, their tendency to develop gingivitis increases.9 The prevalence of disease is lowest during the preschool years and increases throughout childhood, peaking during puberty. Increases in gingivitis do not fully correlate with the amount of plaque, suggesting the influence of other factors.
The most prevalent type of gingival disease in childhood is chronic marginal gingivitis (see Supplement A Figure 11-9). The gingival tissues exhibit changes in color, size, consistency, and surface texture similar to chronic inflammation in the adult. Red, linear inflammation is accompanied by underlying chronic changes, including swelling, increased vascularization, and hyperplasia. Bleeding and increased pocket depth are not found as often in children as in adults but may be observed if severe gingival hypertrophy or hyperplasia occur.7,40 Chronic gingivitis in children is characterized by loss of collagen in the area around the junctional epithelium and an infiltrate consisting mostly of lymphocytes, with small numbers of polymorphonuclear leukocytes, plasma cells, monocytes, and mast cells. Lesions generally have relatively few plasma cells and resemble the early nondestructive, nonprogressive lesions seen in adults.
Gingivitis in children also differs from adult gingivitis in that the response is dominated by T lymphocytes, with few B lymphocytes and plasma cells in the infiltrate. This difference could explain why gingivitis in children rarely progresses to periodontitis.29,31,32,35,47
Gingival histology in children also demonstrates other unique features that may contribute to a decreased tendency to progress to severe gingivitis. The junctional epithelium of the primary dentition tends to be thicker than in the permanent dentition,9 which is thought to reduce the permeability of the gingival structures to bacterial toxins that initiate the inflammatory response.
Calculus deposits are uncommon in infants and toddlers, but may increase with age. About 9% of 4- to 6-year-old children exhibit calculus deposits. By age 7 to 9 years, 18% of children present with calculus deposits, and by age 10 to 15 years, 33% to 43% have some calculus formation. Within the category of special needs patients, children with cystic fibrosis have a higher incidence of number of calculus deposits, which may be caused by increased calcium and phosphate concentrations in their saliva.54 Children fed exclusively with gastric or nasogastric tubes show significant calculus buildup secondary to lack of function and increased oral pH.
Because the intensity of gingival disease increases as a child develops into adulthood, it is important to understand the microbiology of disease, which is discussed more fully in Chapter 23. Interestingly, the composition of the oral microflora also changes as the child matures.9 Yang et al analyzed samples of dental plaque in children and reported that 71% of 18- to 48-month-old children were infected with at least one periodontal pathogen. Sixty-eight percent were infected with Porphyromonas gingivalis and 20% exhibited Bacteroides forsythus (Tannerella forsythia).55 A moderate correlation also has been found between B. forsythus in children and periodontal disease in their mothers. B. forsythus also has been associated with gingival bleeding in children.
In a similar study, 60% of children between 2 and 18 years of age had detectable levels of P. gingivalis in their plaque and 75% showed similar levels of Actinobacillus actinomycetemcomitans. The presence of P. gingivalis was most strongly associated with the progression of gingivitis and the onset of periodontitis in healthy children.39
Experimental gingivitis models in children have demonstrated increased subgingival levels of Actinomyces, Capnocytophaga, Leptotrichia and Selenomonas15—pathogens that generally are not seen in adult gingivitis—thereby raising interest in their potential role in the etiology of childhood gingivitis.
Gingivitis associated with tooth eruption is so common that the term eruption gingivitis has come into common use. Tooth eruption per se does not cause gingivitis; however, inflammation associated with plaque accumulation around erupting teeth, perhaps secondary to discomfort caused by brushing these friable areas, may contribute to gingivitis.9 The gingiva around erupting teeth may appear reddened because gingival margins have not yet keratinized fully and sulcus development is incomplete (Supplement A Figure 11-10).
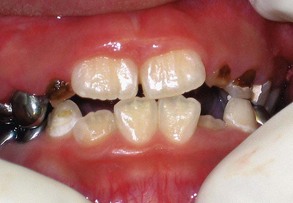
Supplement A Figure 11-10 Eruption gingivitis complicated with severe marginal gingivitis secondary to the illustrated poor oral hygiene.
Exfoliating and severely carious primary teeth often contribute to gingivitis caused by plaque accumulation secondary to pain during brushing or food impaction in areas of tooth destruction. As a normal part of exfoliation, the junctional epithelium migrates under the resorbing tooth, increasing pocket depth and potentially creating a niche for pathogenic bacteria.9 The discomfort of chewing on severely infected teeth often leads to unilateral chewing on the unaffected side.
As mentioned previously, the incidence of marginal gingivitis increases as a child matures, peaking at 9 to 14 years of age, then decreasing slightly after puberty.9 Gingival disease that behaves in such a manner is often referred to as pubertal (or puberty) gingivitis. Chapters 9 and 27 continue the discussion of this condition.
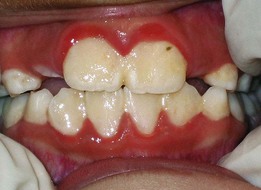
The most frequent manifestation of puberty gingivitis is bleeding and inflammation in interproximal areas. Inflammatory gingival enlargement may also be noted in both males and females and generally subsides after puberty38 (Supplement A Figure 11-11).
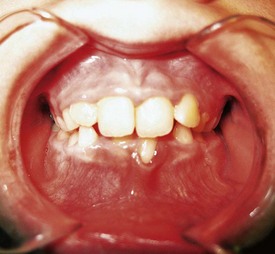
Supplement A Figure 11-11 Pubertal gingival inflammation and enlargement secondary to poor oral hygiene and hormonal influences. Little or no calculus was found when the child was scaled. This most often clears with improved oral hygiene and natural stabilization of estrogen and testosterone levels.
The altered gingival response during this developmental stage is thought to be the result of hormonal changes that magnify the vascular and inflammatory response to dental plaque9,40 and modify reactions of dental plaque microbes.19
Drug-Induced Gingival Enlargement
Gingival enlargement, discussed in Chapter 9, may result from the use of certain drugs. Cyclosporine, phenytoin, and calcium channel blockers—drugs used to treat conditions encountered in childhood—result in a higher prevalence of gingival enlargement. Although complicated by the plaque levels along the gingival margin, this form of gingival disease has features that are not typical of chronic marginal gingivitis.40
Gingival Changes Related to Orthodontic Appliances
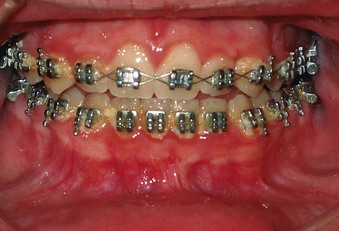
Gingival enlargement can be related to the presence of fixed orthodontic appliances, which complicate plaque removal (Supplement A Figure 11-12). Gingival changes can occur within 1 to 2 months of appliance placement, are generally transient, and only rarely produce long-term damage to periodontal tissues.17 The fact that most orthodontic treatment is provided to individuals during puberty when they are subject to the inflammatory changes associated with puberty gingivitis may exacerbate the observed effect.
Mouth breathing and lip incompetence, or an open mouth posture, are often associated with increased plaque and gingival inflammation.17 The area of inflammation is often limited to the gingiva of the maxillary incisors. There is often a clear line of demarcation in which the gingiva is uncovered by the lip (Supplement A Figure 11-13).
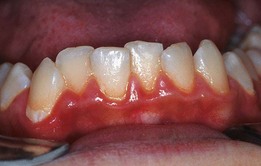
Supplement A Figure 11-13 Patient with gingivitis secondary to compulsive mouth breathing secondary to allergic rhinitis. This is a typical gingival response to chronic desiccation in adolescent patients. This was originally diagnosed as “allergic gingivitis” by the patient’s pediatrician because it subsided when he gave the patient antihistamines. Of course, the antihistamines simply allowed him to breathe through his nose so he could keep his mouth closed.
Non–Plaque-Induced Gingival Lesions
Intraoral soft tissue lesions may be encountered in the pediatric population as in the adult population. The six most common pediatric intraoral lesions are primary herpetic gingivostomatitis, recurrent herpes simplex, recurrent aphthous stomatitis, candidiasis, angular cheilitis, and geographic tongue.40 Most of these lesions present without significant difference between the pediatric and adult population. Two have specific pediatric considerations.
Primary Herpetic Gingivostomatitis
Primary herpetic gingivostomatitis is an acute-onset viral infection that occurs early in childhood, with a heightened incidence from 1 to 3 years of age (see Chapter 10). In children with primary herpetic infections, 99% are symptom-free or have symptoms that are attributed to teething. The remaining 1% can develop significant gingival inflammation and ulceration of the lips and mucous membranes24,40 (Supplement A Figure 11-14).
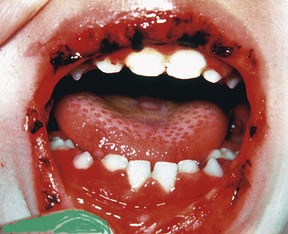
Supplement A Figure 11-14 Acute herpetic gingivostomatitis in an 18-month-old child. Several active lesions still exist on the tongue. The gingiva demonstrates the typical red, swollen appearance associated with the herpes virus. The infection is mostly limited to the attached gingiva, tongue, palate, and lips. It is most important to control hydration with bland, nonacetic fluids. Hospitalization may be necessary for rehydration in severe cases.
Candidiasis results from an overgrowth of Candida albicans, usually after a course of antibiotics or as a result of congenital or acquired immunodeficiencies. It is far less common in the child than the adult and is rarely associated with a healthy child.12
Periodontal Diseases of Childhood
Although gingivitis is considered to be “nearly universal” in children over the age of 7 years,15,40 frank periodontal disease with loss of periodontal attachment and supporting bone is far less common in the pediatric population than in adults.15 The incidence of disease begins increasing between age 12 to 17 years, but the prevalence of severe attachment loss involving multiple teeth remains low at 0.2% to 0.5%.15 When comparing the different presentations of periodontal disease, chronic periodontitis has been shown to be more prevalent in adults and aggressive periodontitis is more common in children and adolescents.15
See Chapters 16, 17, and 18 for detailed descriptions of the different types of periodontal disease.
Aggressive Periodontitis
Aggressive periodontitis is addressed in greater detail in Chapter 18. Because of the relatively early presentation of disease, which occurs around the time of puberty, former classifications include mention of developmental stage: early onset periodontitis, prepubertal periodontitis, and juvenile periodontitis.15,40 The currently accepted designation of aggressive periodontitis may be further broken down into two forms: localized or generalized.
Localized aggressive periodontitis is defined as “interproximal attachment loss on at least two permanent first molars and incisors, with attachment loss on no more than two teeth other than first molars and incisors.”15
In young individuals, localized aggressive periodontitis is more common than the generalized form. Prevalence of the localized form has been reported to range from 0.1% to 15%, with most studies estimating less than 1%. Black and Hispanic individuals are reported to have a higher prevalence2,15; some studies suggest a higher prevalence in Asian children as well.2,48 Of relevance to the pediatric population is the finding that the classic presentation of localized aggressive periodontitis may be preceded by signs of bone loss around teeth in the primary dentition.15
The generalized form of aggressive periodontitis, which is defined as a “generalized interproximal attachment loss, including at least three teeth that are not first molars and incisors” is rare in children. The onset of this form of periodontitis generally occurs after the initiation of adolescence. The general prevalence is 0.13% in 14 to 17 year olds15; however, individuals with Down syndrome demonstrate a higher prevalence.3,14,43 A purported genetic influence in the overall disease process suggests that any signs of disease in a child with a family history of generalized aggressive form of this disease should be evaluated.
Several studies have suggested the involvement of A. actinomycetemcomitans2,28,48 and P. gingivalis2 in the pathogenesis of aggressive periodontitis, with the former found at higher levels in children with the localized form and the latter at higher levels in the generalized form. Both of these pathogens are relatively rare in healthy children, with a prevalence of 4.8%, but are elevated in children with periodontitis, with a reported prevalence of 20%.41
Chronic Periodontitis
Chronic periodontitis, formerly known as adult periodontitis or chronic adult periodontitis, is one of the most prevalent forms of periodontitis. It is characterized by a “slow to moderate rate of progression that may include periods of rapid destruction.”15 Although the disease can appear in children and adolescent populations secondary to retained plaque and calculus, it is far less prevalent than in adults.15
Similar to the adult version that is discussed in greater detail in Chapter 16, chronic periodontitis may occur in children in the localized form in which less than 30% of dentition is affected, as well the general form, in which more than 30% of the dentition is affected.
Although the microbiology of this disease is discussed in Chapters 16 and 23, it is important to note that recent studies suggest a familial transmission of certain bacteria associated with chronic periodontitis. Strains such as T. forsythensis, P. intermedia, and P. nigrescens are found more often in the children of individuals who have been shown to harbor these types.53 Both F. nucleatum and/or P. gingivalis have been noted at significant levels in children of similarly affected parents.11,25 Levels of these strains have been observed to increase with age, suggesting that P. gingivalis and B. forsythus might serve as early markers in the screening of periodontal disease.11,28,51 Thus, even though chronic periodontitis may not be highly prevalent in children, early colonization may underscore the importance of early detection, particularly for those at elevated risk for adult forms of the disease.
Gingival Manifestation of Systemic Disease in Children
Systemic diseases resulting in periodontitis occur more frequently in children than adults.2 Chapter 27 discusses some of the general systemic diseases and disorders that impact periodontal health. Many diseases, however, are expressed differently in children than in adults and therefore merit special mention.
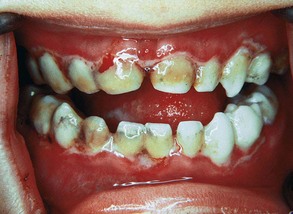
Acute necrotizing gingivitis (Supplement A Figure 11-15) is very rarely seen except in cases of primary or secondary immune suppression, Down syndrome, or severe malnutrition.16,23,43 The breath is fetid, and the child complains of pain and discomfort when eating (see Chapter 10).
Endocrine Disorders and Hormonal Changes
Diabetes Mellitus
Type 1 or insulin-dependent diabetes mellitus occurs more frequently in children and young adults than type 2 or non–insulin-dependent diabetes mellitus. As in the diabetic adult, gingival inflammation and periodontitis are more prevalent in affected children than in unaffected individuals.40,44 Clinical consequences include premature tooth loss and impaired immune response to the oral flora. The severity of periodontal disease is worse in children with poor metabolic control.
Although destructive changes are rather rare in healthy children, periodontal destruction can be observed in diabetic children, usually appearing around the time of puberty and becoming progressively worse as children mature into adulthood. Disease prevention and fastidious oral hygiene measures should be highly promoted.27
Hematologic Disorders and Immune Deficiencies
Leukemias
Leukemia is the most common type of cancer in children. Acute lymphocytic leukemia accounts for the majority of cases in children under the 7 years of age. Leukemia must be considered in the differential diagnosis for children who present with the hallmark features of acute gingival enlargement, ulceration, bleeding, and infection.1
Leukocyte (Neutrophil) Disorders
As mentioned in Chapter 27, neutrophil disorders impair defenses against infections, making afflicted individuals susceptible to severe periodontal destruction. Many neutrophil disorders are genetic, including some forms of neutropenia, Chediak-Higashi syndrome, leukocyte adhesion deficiency, and Papillon-Lefèvre syndrome. Therefore diagnosis of the systemic disorder generally will have occurred before any signs of periodontal destruction appear. Since periodontal changes are difficult to reverse in children with neutrophil disorders, disease management includes oral hygiene measures, mechanical debridement, antimicrobial therapy, and supportive care for resultant tissue destruction or tooth loss. Treatment success is unpredictable as a result of the impact of systemic disease.15
Congenital Anomalies
Down syndrome is another congenital condition that would be diagnosed before expression of periodontal disease. Afflicted individuals experience a high prevalence of severe aggressive periodontitis in early adulthood. The disease process is thought to be related to some kind of host susceptibility resulting in an exaggerated immune-inflammatory response rather than a reaction to a specific causative microbe.3,4,16,52
Oral Mucosa in Childhood Diseases
Some childhood diseases present alterations or lesions of the oral mucosa and underlying tissues such as rubeola (rubella, measles), varicella (chickenpox), diphtheria, and scarlatina (scarlet fever). For a discussion of these diseases, the reader is referred to texts on oral and pediatric pathology.
Therapeutic Considerations in the Pediatric Patient
The diagnostic process for pediatric patients follows the general outline described in Chapter 30. Medical and dental histories should be recorded for each child, with parents serving as primary historians. However, differences between the primary and permanent dentition and aspects related to development warrant some differences in clinical practices involving children.
Periodontal indices do not need to be recorded in the primary dentition unless a child exhibits signs of aggressive periodontitis or other unusual disease processes. More explicit periodontal assessments should begin in the mixed dentition, when children have permanent incisors and first molars. Rather than recording full-mouth probing depths, clinicians may elect to focus on selected teeth. For example, a rudimentary assessment of teeth 3, 8, 14, 19, 24, and 30 has been suggested,17 noting gingival health, bleeding on probing, and/or the presence of calculus. This quick screen is generally sufficient for children up to the age of 11 years. Between ages 12 to 19 years, when most individuals have a full permanent dentition, clinicians also should note pocket depths greater than 4 mm. By this stage of dental development, full-mouth pocket-depth probings may be warranted based on general indicators of each patient’s gingival health or risk of disease.
In-office professional plaque control procedures can vary in accordance with a patient’s stage of development. As noted previously, calculus deposits are uncommon in infants and toddlers. Supragingival plaque removal using simple rubber-cup coronal polishing or a toothbrush is usually sufficient in the primary dentition.17 If calculus deposits are evident, selective supragingival scaling may be performed. As the permanent teeth erupt, the prevalence of calculus deposits increases, often necessitating targeted subgingival scaling in addition to supragingival plaque removal.17
Chapter 44 discusses plaque control for periodontal patients. In children, however, the dynamic process of developing manual dexterity impacts the ability of a child to perform expected procedures. Each child requires an individualized home care program based on his or her ability to actually perform the requested activities. For young children, plaque control should be a shared responsibility between children and parents. Instruction in plaque control should be delivered to parents and children in language and terms that both understand.
For children younger than 7 years of age, parents should be asked to assist in toothbrushing.17 Children may be encouraged to take a turn brushing their teeth using a simple scrub technique. However, parents should also take a turn to ensure proper removal of plaque. By 7 years of age, children generally possess the manual dexterity to brush their teeth on their own and may only require limited adult supervision.17 More refined brushing techniques can be introduced during adolescence.
Mechanical toothbrushes with rotary heads have been shown to be effective for plaque removal.17 Use of these devices can be encouraged as soon as children are able to tolerate the vibrating sensation, as many children initially dislike the feeling of the rotary movement. Mechanical toothbrushes are especially recommended for physically challenged children and individuals with fixed orthodontic appliances.40
Flossing is usually not indicated for children in the primary dentition stage, since most children have interdental spacing throughout most of their arches. However, as interdental contacts develop, flossing should be added to the home care routine. Studies have demonstrated both a decrease in gingival bleeding and quantity of microbes associated with periodontal disease when tooth and tongue brushing is combined with flossing.6,18 Again, limitations in manual dexterity may necessitate parental assistance with flossing during the mixed dentition stage. Adolescents with sufficient manual dexterity can be expected to floss on their own.17
Antimicrobial mouth rinses for chemical plaque control are not indicated in very young children because of the risk of ingestion of chemical agents.17 However, rinses may be indicated for older children who demonstrate the ability to expectorate after rinsing.
Summary
1 Abdullah BH, Yahya HI, Kummoona RK, Hilmi FA, Mirza KB. Gingival fine needle aspiration cytology in acute leukemia. J Oral Pathol Med. 2002;31:55-58.
2 Albandar JM, Rams TE. Risk factors for periodontitis in children and young persons. Periodontol 2000. 2002;29:207-222.
3 Amano A, Kishima T, Kimura S, et al. Periodontopathic bacteria in children with Down syndrome. J Periodontol. 2000;71:249-255.
4 Amano A, Kishima T, Akiyama S, Nakagawa I, Hamada S, Morisaki I. Relationship of periodontopathic bacteria with early-onset periodontitis in Down’s syndrome. J Periodontol. 2001;72:368-373.
5 Andlin-Sobocki A. Changes of facial gingival dimensions in children. A 2-year longitudinal study. J Clin Periodontol. 1993;20:212-218.
6 Biesbrock A, Corby PM, Bartizek R, et al. Assessment of treatment responses to dental flossing in twins. J Periodontol. 2006;77:1386-1391.
7 Bimstein E, Lustmann J, Soskolne WA. A clinical and histometric study of gingivitis associated with the human deciduous dentition. J Periodontol. 1985;56:293-296.
8 Bimstein E, Eidelman E. Morphological changes in the attached and keratinized gingiva and gingival sulcus in the mixed dentition period. A 5-year longitudinal study. J Clin Periodontol. 1988;15:175-179.
9 Bimstein E, Matsson L. Growth and development considerations in the diagnosis of gingivitis and periodontitis in children. Pediatr Dent. 1999;21:186-191.
10 Bimstein E, Peretz B, Holan G. Prevalence of gingival stippling in children. J Clin Pediatr Dent. 2003;27:163-165.
11 Bimstein E, Sapir S, Houri-Haddad Y, Dibart S, Van Dyke TE, Shapira L. The relationship between Porphyromonas gingivalis infection and local and systemic factors in children. J Periodontol. 2004;75:1371-1376.
12 Blyth CC, Chen SC, Slavin MA, et al. Not just littler adults: candidemia epidemiology, molecular characterization and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360-1368.
13 Bodner L, Goldstein J, Sarnat H. Eruption cysts: a clinical report of 24 new cases. J Clin Pediatr Dent. 2004;28:183-186.
14 Bosnjak A, Jorgic-Srdjak K, Maricevic T, Plancak D. The width of clinically-defined keratinized gingiva in the mixed dentition. ASDC J Dent Child. 2002;69:266-270. 234
15 Califano JV, Research S, Therapy Committee American Academy of P. Position paper: periodontal diseases of children and adolescents. J Periodontol. 2003;74:1696-1704.
16 Cichon P, Crawford L, Grimm WD. Early-onset periodontitis associated with Down’s syndrome–clinical interventional study. Ann Periodontol. 1998;3:370-380.
17 Clerehugh V, Tugnait A. Diagnosis and management of periodontal diseases in children and adolescents. Periodontol 2000. 2001;26:146-168.
18 Corby PM, Biesbrock A, Bartizek R, et al. Treatment outcomes of dental flossing in twins: molecular analysis of the interproximal microflora. J Periodontol. 2008;79:1426-1433.
19 Demir T, Orbak R, Tezel A, Canakc V, Kaya H. The changes in the T-lymphocyte subsets in a population of Turkish children with puberty gingivitis. Int J Paediatr Dent. 2009;19:206-212.
20 Gomes-Filho IS, Miranda DA, Trindade SC, et al. Relationship among gender, race, age, gingival width, and probing depth in primary teeth. J Periodontol. 2006;77:1032-1042.
21 Griffen AL. Periodontal Problems in Children and Adolescents. In: Pinkham JR, Cassamassimo PS, Field HW, editors. Pediatric Dentistry in Infancy through Adolescence. ed 4. Philadelphia, PA: Saunders; 1999:414-422.
22 Grimmer EA. Trauma in an erupting premolar. Journal of Dental Research. 1939;18:267.
23 Jimenez M, Ramos J, Garrington G, Baer PN. The familial occurrence of acute necrotizing gingivitis in children in Colombia, South America. J Periodontol. 1969;40:414-416.
24 King DL, Steinhauer W, Garcia-Godoy F, Elkins CJ. Herpetic gingivostomatitis and teething difficulty in infants. Pediatr Dent. 1992;14:82-85.
25 Kobayashi N, Ishihara K, Sugihara N, Kusumoto M, Yakushiji M, Okuda K. Colonization pattern of periodontal bacteria in Japanese children and their mothers. J Periodontal Res. 2008;43:156-161.
26 Kronfeld R, Weinmann J. Traumatic changes in the periodontal tissues of deciduous teeth. Journal of Dental Research. 1940;19:441.
27 Lalla E, Cheng B, Lal S, et al. Periodontal changes in children and adolescents with diabetes: a case-control study. Diabetes Care. 2006;29:295-299.
28 Lamell CW, Griffen AL, McClellan DL, Leys EJ. Acquisition and colonization stability of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in children. J Clin Microbiol. 2000;38:1196-1199.
29 Lindhe J, Liljenberg B, Listgarten M. Some microbiological and histopathological features of periodontal disease in man. J Periodontol. 1980;51:264-269.
30 Loe H, Anerud A, Boysen H. The natural history of periodontal disease in man: prevalence, severity, and extent of gingival recession. J Periodontol. 1992;63:489-495.
31 Longhurst P, Johnson NW, Hopps RM. Differences in lymphocyte and plasma cell densities in inflamed gingiva from adults and young children. J Periodontol. 1977;48:705-710.
32 Longhurst P, Gillett R, Johnson NW. Electron microscope quantitation of inflammatory infiltrates in childhood gingivits. J Periodontal Res. 1980;15:255-266.
33 Machtei EE, Zubery Y, Bimstein E, Becker A. Anterior open bite and gingival recession in children and adolescents. Int Dent J. 1990;40:369-373.
34 Mackler SB, Crawford JJ. Plaque development and gingivitis in the primary dentition. J Periodontol. 1973;44:18-24.
35 Matsson L. Development of gingivitis in pre-school children and young adults. A comparative experimental study. J Clin Periodontol. 1978;5:24-34.
36 Maynard JGJr, Ochsenbein C. Mucogingival problems, prevalence and therapy in children. J Periodontol. 1975;46:543-552.
37 Maynard JGJr, Wilson RD. Diagnosis and management of mucogingival problems in children. Dent Clin North Am. 1980;24:683-703.
38 Modeer T, Wondimu B. Periodontal diseases in children and adolescents. Dent Clin North Am. 2000;44:633-658.
39 Morinushi T, Lopatin DE, Van Poperin N, Ueda Y. The relationship between gingivitis and colonization by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in children. J Periodontol. 2000;71:403-409.
40 Oh TJ, Eber R, Wang HL. Periodontal diseases in the child and adolescent. J Clin Periodontol. 2002;29:400-410.
41 Okada M, Hayashi F, Nagasaka N. Detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in dental plaque samples from children 2 to 12 years of age. J Clin Periodontol. 2000;27:763-768.
42 Orban B, Weinmann J. Signs of traumatic occlusion in average human jaws. Journal of Dental Research. 1933;13:216.
43 Pindborg JJ, Bhat M, Devanath KR, Narayana HR, Ramachandra S. Occurrence of acute necrotizing gingivitis in South Indian children. J Periodontol. 1966;37:14-19.
44 Pinson M, Hoffman WH, Garnick JJ, Litaker MS. Periodontal disease and type I diabetes mellitus in children and adolescents. J Clin Periodontol. 1995;22:118-123.
45 Powell RN, McEniery TM. A longitudinal study of isolated gingival recession in the mandibular central incisor region of children aged 6-8 years. J Clin Periodontol. 1982;9:357-364.
46 Saario M, Ainamo A, Mattila K, Ainamo J. The width of radiologically-defined attached gingiva over permanent teeth in children. J Clin Periodontol. 1994;21:666-669.
47 Seymour GJ, Crouch MS, Powell RN, et al. The identification of lymphoid cell subpopulations in sections of human lymphoid tissue and gingivitis in children using monoclonal antibodies. J Periodontal Res. 1982;17:247-256.
48 Sjodin B, Arnrup K, Matsson L, Wranne L, Carlsson J, Hanstrom L. Periodontal and systemic findings in children with marginal bone loss in the primary dentition. J Clin Periodontol. 1995;22:214-224.
49 Srivastava B, Chandra S, Jaiswal JN, Saimbi CS, Srivastava D. Cross-sectional study to evaluate variations in attached gingiva and gingival sulcus in the three periods of dentition. J Clin Pediatr Dent. 1990;15:17-24.
50 Stoner JE, Mazdyasna S. Gingival recession in the lower incisor region of 15-year-old subjects. J Periodontol. 1980;51:74-76.
51 Suda R, Kurihara C, Kurihara M, Sato T, Lai CH, Hasegawa K. Determination of eight selected periodontal pathogens in the subgingival plaque of maxillary first molars in Japanese school children aged 8-11 years. J Periodontal Res. 2003;38:28-35.
52 Sznajder N, Carraro JJ, Otero E, Carranza FAJr. Clinical periodontal findings in trisomy 21 (mongolism). J Periodontal Res. 1968;3:1-5.
53 Umeda M, Miwa Z, Takeuchi Y, et al. The distribution of periodontopathic bacteria among Japanese children and their parents. J Periodontal Res. 2004;39:398-404.
54 Wotman S, Mercadante J, Mandel ID, Goldman RS, Denning C. The occurrence of calculus in normal children, children with cystic fibrosis, and children with asthma. J Periodontol. 1973;44:278-280.
55 Yang EY, Tanner AC, Milgrom P, et al. Periodontal pathogen detection in gingiva/tooth and tongue flora samples from 18- to 48-month-old children and periodontal status of their mothers. Oral Microbiol Immunol. 2002;17:55-59.
Suggested Readings
Califano JV, Research Science and Therapy Committee American Academy of Periodontology. Position paper: periodontal diseases of children and adolescents. J Periodontol. 2003;74:1696-1704.
Clerehugh V, Tugnait A. Diagnosis and management of periodontal diseases in children and adolescents. Periodontol 2000. 2001;26:146-168.
Griffen AL. Periodontal problems in children and adolescents. In Pinkham JR, Cassamassimo PS, Field HW, editors: Pediatric dentistry in infancy through adolescence, ed 4, Philadelphia: Saunders, 1999.
Oh TJ, Eber R, Wang HL. Periodontal diseases in the child and adolescent. J Clin Periodontol. 2002;29:400-410.