Chapter 22 Promoting the safe administration of medicines
Introduction
Safe administration of medicines is of paramount importance to ensure patient/client safety. The legislation and professional guidance (Nursing and Midwifery Council [NMC] 2004a, 2004b) that should enable this are explored at the beginning of this chapter. The requirements for safe storage, ordering and prescribing of medicines in hospital and community settings are then reviewed.
In order to understand how drugs act, some pharmacological principles are explained and their implications for nursing practice are illustrated. Commonly used groups of drugs and their effects are listed. Adverse drug reactions, or side effects, and the safeguards that apply to newly marketed medicines are considered. Medications that come in several forms and are administered by a variety of routes are described later in this chapter.
This chapter discusses the essential checks that must be carried out before administering medicines and how to obtain valid consent. An overview of calculating drug doses is provided. The nursing skills needed to administer medication by several common routes are explained in detail using an evidence-based approach. Towards the end of the chapter polypharmacy and the nurse’s role in maximizing concordance and compliance are explored. This is important in maintaining patient/client safety and maximizing effective use of NHS financial resources. Finally, drug errors, the factors that may predispose to these incidents and how they are dealt with are considered.
Legislation concerning medicines
All medicines are potentially harmful and nurses must be fully aware of the importance of safe storage, ordering and prescribing of drugs, which are explained later in this section. The manufacture, safe storage, prescription and sales of medicines within the UK are subject to Acts of Parliament and guiding regulations with which every nurse should be familiar:
Additional legislation governs prescribing by appropriately qualified registered nurses. Nurses also need to be familiar with the professional guidance from the NMC.
The Medicines Act 1968
This act protects manufacturers, prescribers and recipients of medicines. It controls licensing, manufacturing and distribution of medicines, the registration of retail pharmacists, and identifies three classes of medicinal products:
The Act stipulated that only doctors, dentists and veterinary surgeons could prescribe medicines; however, later legislation has since extended prescribing to appropriately qualified registered nurses (RNs) (see below) and other healthcare professionals such as some pharmacists. The Act outlines:
Box 22.3 Good practice in relation to prescriptions
(Adapted from NMC 2004a)
The practitioner administering the medicine should be aware that:
Specific to community
Accepted abbreviations for drug routes
Notes: depending on local policy, abbreviations may also be written in upper case letters without punctuation, e.g. i.m., i.v., p.r., etc.
Units
SI units are normally used in prescriptions avoiding the use of decimal points:
International units (IU) are used for some preparations, e.g. heparin, insulin (see Table 22.1).
* This must not be abbreviated.
Many medicinal products not governed by legislation are widely available, e.g. homeopathic and herbal preparations. In addition, GSL medicines – often referred to as ‘over-the-counter drugs’, such as aspirin and medicines for indigestion – are widely believed to be safe. However, they can have serious side effects and may interact with each other and with prescribed medicines. Additionally, the use of alcohol and recreational drugs can have harmful effects and they too can interact with prescribed medication.
The Misuse of Drugs Act 1971
This Act identified controlled drugs that are likely to cause dependence and other harmful effects if misused. It aims to prevent the misuse of these drugs and protects public safety by controlling their importation, exportation, supply and possession. Controlled drugs are widely known as CDs and were previously known as ‘dangerous drugs of addiction’. The Act classifies CDs according to the harm they may cause if misused.
The Misuse of Drugs Regulations 1985
This divides controlled drugs into five schedules, which have specific requirements regarding their supply, possession, prescribing and record keeping. There is a legal requirement to keep a controlled drug register for drugs in Schedule 2, which includes the most addictive drugs used in practice such as morphine and pethidine. Further information can be found in the British National Formulary (BNF; see Useful websites, p. 651). Storage, ordering, prescribing and administration of CDs are described later.
Nurse prescribing
The Medicinal Products: Prescription by Nurses Act 1992 and The Health and Social Care Act 2001 contain the primary legislation that allows nurse prescribing and its subsequent extension to ‘non-medical prescribing’. Increasingly, RNs who have undertaken further specific training are prescribing many drugs in a range of settings.
Professional advice that affects nurses
In relation to the administration and prescribing of medicines, nurses are not only constrained by the legislation above but also by Guidelines for the Administration of Medicines (NMC 2004a). These outline nurses’ professional accountability (see Ch. 7) in relation to knowledge of drugs and their actions, and the safe administration of medicines. Nurses must also be familiar with the Code of Professional Conduct: Standards for Conduct, Performance and Ethics (NMC 2004b) and Guidelines for Records and Record Keeping (NMC 2005).
The NMC (2004a) recommend that only RNs, midwives and specialist community public health nurses should be involved in the administration of medicines. Practitioners must always be aware of local policy as it may vary regarding the number of practitioners involved. For example, in some placements the second checker will also require to be a registered practitioner whereas in others this may be a student nurse.
The Standards of Proficiency for Pre-registration Nursing Education (NMC 2004c) state that student nurses must be able to demonstrate competence in essential skills including administration of medicines. Student nurses undertaking administration of medicines must do so only under the direct supervision of a RN. The RN must countersign the signature of a student who administers any prescription (NMC 2004a).
Storage of medicines
In clinical settings all drugs, not just CDs and including GSL medicines, are ‘controlled’ by the legislation outlined above. Storage depends on the type of drug and the setting involved.
Storage of non-controlled medicines in hospitals and nursing homes
The Duthie report (Department of Health [DH] 1988) set out precautions concerning the storage of medicines in hospitals to safeguard staff and patients/clients. The nurse in charge of a clinical area is responsible for the safe storage of all medicines (Box 22.1). Safe storage requires that:
Storage of controlled drugs in hospitals and nursing homes
Because of their potential to cause harm if misused, there are specific legal requirements concerning the ordering, storage and dispensing of controlled drugs. Controlled drugs are the responsibility of the charge nurse. They are kept locked in an inner cupboard within a cupboard (Fig. 22.2). The keys for both cupboards are held in the sole custody of the charge nurse or a designated RN or other healthcare professional. The contents of the cupboard and the controlled drug register are checked regularly according to local policy. This may be at each change of shift, daily or weekly. The controlled drug register is kept as an accurate record of the contents of the controlled drugs cupboard. Once completed, the registers are kept in the clinical area for 2 years.
Storage of medicines in the home
The strict regulations used in hospitals cannot be maintained in people’s homes. A family member may collect any medicine if the person for whom the drug is prescribed is unable to do so. In general, patients/clients should be advised to:
Controlled drugs are dispensed from the pharmacy in the normal way. However, the pharmacist keeps a register of controlled drugs ordered, the amount delivered to the pharmacy and the volume or amount dispensed.
Ordering drugs in hospitals
This depends on the type of drug to be ordered.
Controlled drugs
In hospitals, qualified practitioners order controlled drugs in a specific way (Box 22.2).
Box 22.2 Ordering controlled drugs
Principles of prescribing
It is essential to ensure that the prescription meets the principles laid down in the Guidelines for the Administration of Medicines (NMC 2004a). In order to safeguard both the public and practitioners, specific guidelines govern the prescribing of medicines (Box 22.3). Drugs may be prescribed:
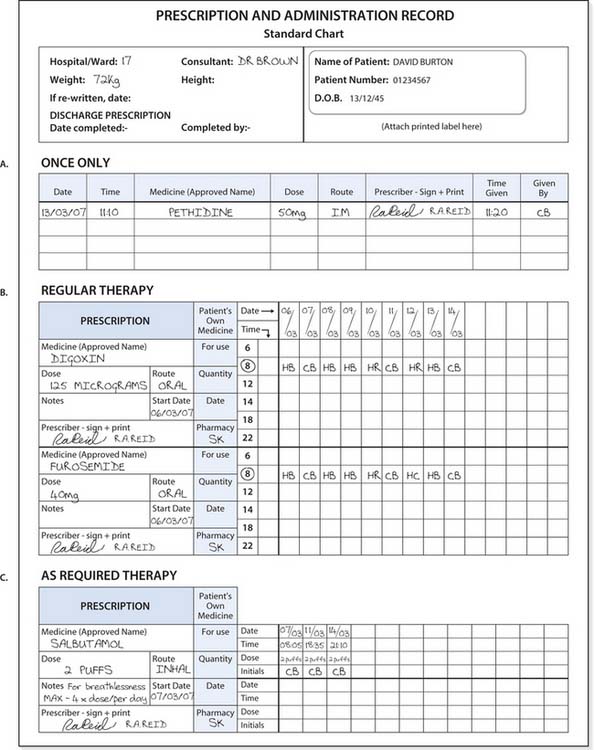
If the prescription does not meet the required standards for safe practice, the nurse should contact the prescriber to amend their prescription before proceeding further. An example of a prescription sheet is provided in Figure 22.3
Introduction to pharmacology
Pharmacology is the science of chemical substances, e.g. drugs, medications and other substances such as herbal and homeopathic preparations (see Ch. 10) that interact with the body. These interactions are divided into:
This section provides an overview of important processes; further information can be found in pharmacology textbooks (e.g. Downie et al 2003, Greenstein 2004).
Pharmacodynamics
Receptors on cell membranes often act as recognition sites for substances produced by the body to regulate or mediate specific functions. Substances that act on cell membrane receptors include hormones and neurotransmitters – chemicals that transmit nerve impulses across the tiny gaps (synapses) between nerve cells. Many drugs produce their effects because they are structurally similar to the naturally occurring substances that act on receptors (Fig. 22.4A). Drugs that act on receptor sites in a similar way to natural body substances are known as agonists, whereas those that prevent (block) their normal action are known as antagonists. Therefore, by attaching to specific receptor sites on target cells, some drugs act by stimulating or blocking the storage, manufacture or release of naturally produced substances.
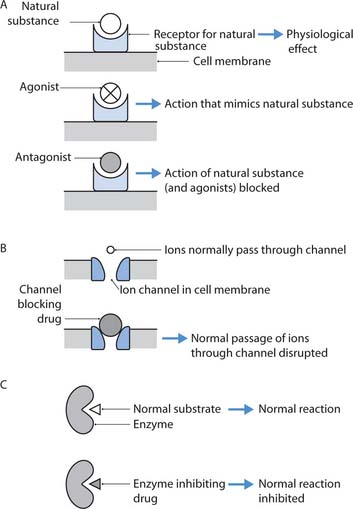
Fig. 22.4 Sites of drug action: A. Receptors. B. Ion channels. C. Enzymes
(adapted with permission from Rang et al 2003)
However, not all drugs act on receptor sites, e.g. antacids reduce indigestion by neutralizing gastric acid. Many other drugs act by inhibiting the actions of enzymes (Fig. 22.4B), e.g. non-steroidal anti-inflammatory drugs (NSAIDs), or by blocking ion channels in cell membranes (Fig. 22.4C), e.g. local anaesthetics.
Pharmacokinetics
Important processes influence plasma levels of a drug within the body, in particular absorption, distribution, metabolism and excretion (Fig. 22.5). Knowledge of pharmacokinetic principles is useful when considering factors that determine how much of a drug is needed to maintain appropriate (therapeutic) blood levels.
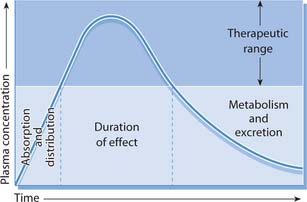
Fig. 22.5 Effects of absorption, distribution, metabolism and excretion on the plasma concentrations of an administered drug
(reproduced with permission from Downie et al 2003)
Absorption
The oral route is most commonly used for administration of drugs (see p. 639). In order to exert its action at the desired site, the drug must be absorbed from the digestive tract into the blood, which then travels through the liver before entering the systemic circulation (see ‘First pass metabolism’, below). The rate of absorption can be affected by several factors including the presence or absence of food in the stomach. It is recommended that some drugs are taken half an hour before meals, e.g. some antibiotics are absorbed more effectively from an empty stomach.
First pass metabolism
Drugs taken orally are usually absorbed in the small intestine and then transported to the liver via the hepatic portal vein before reaching the systemic circulation. Many drugs are broken down, or metabolized, as they pass through the liver and when this is extensive only a small amount enters the systemic circulation and even less reaches the site of action. This effect is called first pass metabolism.
Glyceryl trinitrate, which is given to provide very rapid relief from cardiac pain in angina, is almost completely broken down in the liver. It is administered sublingually (placed under the tongue) or as a spray to the oral mucosa. The oral mucosa has an extensive blood supply that facilitates rapid absorption and therefore the action of glyceryl trinitrate is also very rapid. The blood from the oral mucosa does not travel through the liver before entering the systemic circulation and therefore first pass metabolism is avoided.
Other drugs are given by injection or as transdermal patches (see p. 647) to avoid first pass metabolism. In people suffering from liver disorders, first pass metabolism is reduced, and therefore drug doses must be reduced to take this into account.
Distribution
After entering the bloodstream, the drug is transported throughout the body. In the capillaries drugs diffuse out of the bloodstream to reach their site(s) of action. Movement from the bloodstream into the tissues may be influenced by several factors such as the extent of plasma binding (see below), the quality and quantity of plasma proteins and blood flow. Where there is good systemic blood flow, drugs are transported more rapidly into the tissues. In some cardiac conditions, where the cardiac output is reduced (see Ch. 17), drug distribution will also be reduced. Many drugs cross the placenta, causing abnormal fetal development, and must therefore be avoided during pregnancy.
Plasma binding
Many drugs bind to plasma proteins in the blood. Some of the drug is bound and the remainder is unbound; however, only the unbound form is active. People who have a liver disorder or malnutrition have fewer plasma proteins available for binding and therefore more of the drug remains unbound and is available to act. In such situations, the dosage must be reduced to avoid excessive plasma levels, e.g. warfarin (see Table 22.1), which may cause severe bleeding.
Table 22.1 Common drug groups and their actions
| Group | Effect | Common examples (generic names) |
|---|---|---|
| Analgesics: | Reduce or prevent pain | |
| Opioids | Morphine | |
| Non-opioids (see also NSAIDs and Ch. 23) | Aspirin, paracetamol | |
| Antacids | Counteract gastric acidity in indigestion | Aluminium hydroxide, magnesium trisilicate |
| Antibiotics (antibacterials) | Kill bacteria (bactericidal) or arrest their growth (bacteriostatic) | Ampicillin, erythromycin, gentamicin |
| Anticoagulants | Prevent and/or break down blood clots in the circulation | Warfarin (oral), heparin (parenteral) |
| Anticonvulsants (antiepileptic) | Control and prevent seizures | Phenytoin, sodium valproate |
| Antidepressants: | Improve low mood | |
| Monoamine oxidase inhibitors (MAOIs) | Phenelzine | |
| Selective serotonin reuptake inhibitors (SSRIs) | Fluoxetine | |
| Tricyclic antidepressants | Amitriptyline | |
| Antidiarrhoeals | Reduce intestinal motility | Codeine phosphate, loperamide |
| Antiemetics | Alleviate nausea and/or vomiting | Metoclopramide, cyclizine |
| Antifungals | Combat fungal infection | Nystatin, fluconazole |
| Antihistamines | Treat and prevent allergic reactions | Chlorphenamine (chlorpheniramine) |
| Antihypertensives | Several groups that act in different ways to reduce high blood pressure, e.g.: | |
| Beta-blockers | Atenolol, propranolol | |
| Angiotensin-converting enzyme (ACE) inhibitors | Captopril, enalapril | |
| Antipsychotics (neuroleptics): | Alleviate symptoms of psychotic illness | |
| Typical | Haloperidol | |
| Atypical | Clozapine | |
| Antipyretics | Lower raised body temperature | Aspirin |
| Anxiolytics | Alleviate anxiety and related symptoms | Diazepam |
| Aperients, laxatives | Promote emptying/evacuation of the bowel | Lactulose, bisacodyl |
| Bronchodilators | Relax bronchial smooth muscle, thus increasing air entry to lungs | Salbutamol |
| Diuretics | Groups of drugs that increase production of urine | Bendroflumethiazide, furosemide (frusemide) |
| Hypoglycaemic agents (antidiabetic): | Lower raised blood glucose levels in diabetes mellitus | |
| Oral | ||
| Parenteral, usually given by subcutaneous injection (see p. 642) | Metformin, glipizide | |
| Insulin – short-, intermediate- and long- acting preparations | ||
| Hypnotics | Promote sleep | Zopiclone |
| Non-steroidal anti-inflammatory drugs (NSAIDs) | Reduce inflammation (see Ch. 23) | Ibuprofen, aspirin |
| Thrombolytics (‘clot busting’ drugs) | Disintegrate blood clots in, e.g., myocardial infarction, deep vein thrombosis (DVT), pulmonary embolism |
Metabolism
Metabolism includes processes that often involve specific enzymes which may break down the drug, combine it with another chemical (conjugation) or increase its solubility in water. In these states drugs are usually more active and can be easily eliminated by the kidneys. Some drugs are already water soluble and so do not require to be metabolized. Most drugs are metabolized in the liver.
Half-life
This is also referred to as T1/2 and is the time taken for the concentration of a drug in the bloodstream to fall by half of its original value. The half-life determines the length of time a drug is available within the body and the intensity of its action. The plasma concentration of a drug at one half-life is 50%, at two half-lives it is 25%, etc. By five half-lives most of the drug will have been eliminated from the body regardless of the dose or route of administration. The half-life is therefore used to determine the number of daily doses required for drug plasma levels to remain within the therapeutic range (see Fig. 22.5). It can also be useful when estimating how long it will take for a drug to be cleared from the body, e.g. in overdosage.
Excretion
The kidneys excrete most drugs from the body. The more water soluble the substance, the more easily it is excreted by the kidneys. People with kidney failure or impaired renal function may suffer toxic effects as elimination of drugs is reduced. Digoxin is a commonly used cardiac drug that can be toxic in older adults because kidney function is often reduced in this age group. The kidneys can reabsorb those drugs that are lipid soluble, making them available within the body for longer. Some drugs are excreted by the lungs or from the digestive tract.
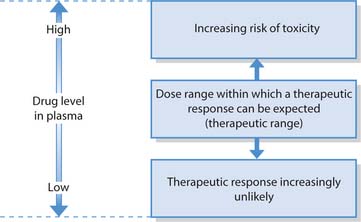
Therapeutic range
To achieve optimal concentrations at the target tissue the correct dose must be given. If this is too low drug action will be ineffective; if too much is administered it may produce side effects that might be toxic. The therapeutic range refers to the plasma levels of a drug that must be maintained so that it can exert its optimal response without producing side effects (Fig. 22.6). Some drugs have a narrow therapeutic range while for others this is wider. Nurses need to recognize the implications of this in relation to drug administration, i.e. if drugs are omitted or given at times other than those prescribed, plasma drug levels will not be maintained in the therapeutic range.
Fig. 22.7 Non-touch technique for dispensing oral medication
(reproduced with permission from Nicol et al 2004)
Measurement of plasma drug levels is carried out when drugs with a narrow therapeutic range are used, e.g. gentamicin (an antibiotic) causes irreversible kidney damage and hearing impairment when therapeutic levels are exceeded. Children, until their liver and kidneys are fully mature, older adults whose kidney and liver function may be declining and people with a liver or kidney disorder are at greatest risk of drug toxicity.
Adverse drug reactions
All medicines have the potential to cause harm including over-the-counter medication. Interactions increase with the number of drugs used, including homeopathic and herbal preparations, recreational drugs and alcohol. It is important, therefore, that nurses know about and recognize potential adverse effects of drugs they administer. Adverse drug reactions (ADRs) include any unwanted effects of drugs, which range from minor side effects to those that are harmful, serious and sometimes fatal. They can be classified into five groups, of which the two outlined below are the most common (DH 2001).
Type A
These are predictable, dose dependent and can be anticipated. They are related to the physiological effects of the drug, e.g. constipation that occurs in people receiving morphine.
Type B
These types are bizarre or idiosyncratic, unexpected and rare reactions, which are not dose related. However, they are generally severe, causing serious and sometimes fatal consequences such as severe allergic responses. Genetic, host and environmental factors are thought to contribute to their occurrence.
Surveillance for ADRs
Potential new drugs must undergo approved and staged clinical trials that report to the Committee on Safety of Medicines to ensure that they are as safe as possible before being granted a product licence. Once marketed, they become more widely available and the Committee on Safety of Medicines keeps them under surveillance so that the occurrence and incidence of ADRs can be monitored. Drugs under surveillance have the symbol  in their BNF listing (see Useful websites, p. 651).
in their BNF listing (see Useful websites, p. 651).
Healthcare practitioners, including nurses, and patients/relatives should report all adverse or unexpected reactions, however minor. Yellow cards, which can be found in the BNF, and online reporting are used to report ADRs so that they can be systematically evaluated and a drug withdrawn if there are safety concerns.
Naming of drugs and common groups
Drugs that have similar functions are classified into groups and there is more than one name for each drug. Some names reflect drug actions more clearly than others.
Naming of drugs
The recommended International Non-proprietary Name (rINN) of a drug is also referred to as the generic, non-proprietary or British Approved Name (BAN). This name may start with a lower case letter, e.g. ‘p’ in paracetamol.
The manufacturer gives the trade or proprietary name to a particular preparation. This is recognized by the symbol ® denoting its registered trademark, which follows its name on the packaging. Proprietary names start with a capital letter, e.g. ‘P’ in Panadol®, which is a trade name for paracetamol. The manufacturer who has registered a drug has exclusive rights to market it for up to 10 years, during which time they can recover the development costs. Different drug companies often manufacture similar or identical generic preparations and may give them similar names.
To avoid errors, and because manufacturers price drugs differently, the Department of Health (1999) recommended that drugs be prescribed by their generic name (e.g. paracetamol) and that trade names (e.g. Panadol®) should only be specified if a particular manufacturer’s drug has different effects. Lists of drugs can be found in the BNF.
Common groups of drugs and their actions
Common drug groups are listed in Table 22.1 together with their effects and examples. Nurses must understand how commonly used drugs in their practice area act and be familiar with their side effects and contraindications in order to maintain patient/client safety (Box 22.4). This information can be found in the BNF/Children’s BNF.
Drug groups, their actions and routes of administration
Under the supervision of your mentor, you are to be involved in drug administration. In order to do this safely, preparation is required.
Medicinal preparations and routes of administration
Medicines are manufactured in different forms for administration by particular routes. The different forms and their characteristics are summarized in Table 22.2. The prescribed route is determined by which will provide the optimal effect and minimize side effects, and the preparations available.
Table 22.2 Drug preparations for administration by specific routes
| Type | Characteristics |
|---|---|
| Oral preparations | |
| Tablets | Powdered substances compressed or moulded into solid forms |
| Many are covered or sugar-coated to assist swallowing or to prevent them dissolving in the stomach, which may result in release or disintegration of the drug causing gastric irritation | |
| Some tablets should be swallowed when fully dissolved in water and are labelled: soluble, dispersible or effervescent | |
| Capsules | Medication contained within a soluble shell, usually made of gelatin to aid swallowing and which carries the drug to the small intestine where it is absorbed |
| Lozenges | A solid form intended for sucking until fully dissolved; absorption is through the oral mucosa |
| Solutions | Medication is dissolved in a solvent, usually water |
| Suspensions | Contain solid particles that are dispersed in a liquid, not necessarily water |
| Syrup | A thick concentrated solution of medication that may include sugar (see Box 22.6) and flavouring |
| Emulsion | Minute globules of one liquid dispersed through another liquid |
| Preparations administered by other routes | |
| Pessaries | Moulded or compressed form of medication inserted into the vagina |
| Suppositories | Medicated solid bodies inserted into the rectum |
| Enemas | Medicated suspensions, oils or foam solutions administered into the rectum |
| Nebulized solutions | Minute liquid particles inhaled into the lungs as a vapour using a nebulizer (see Ch. 17) |
| Dry powder inhaler | A solid that is converted to minute particles, which are inhaled |
| Metered dose inhaler | A pressurized device that delivers a preset dose of tiny medicated particles into the lungs |
Routes of administration can be divided into two categories: systemic and local (Table 22.3). Following systemic administration, the drug circulates throughout the whole body. Use of local routes, e.g. inhalation or per vagina, involves giving lower doses directly to the site of action. As a result, the amount of the drug elsewhere in the body is relatively low and side effects are less likely. The term ‘parenteral’ includes all routes of administration except the oral route.
Table 22.3 Common routes of drug administration
| Route | Systemic/local | Site of administration |
|---|---|---|
| Oral | Systemic | Taken by mouth and swallowed, or given via a nasogastric tube (see Ch. 19) |
| Sublingual/buccal | Systemic | Sprayed into the mouth or allowed to dissolve under the tongue or in the cheek |
| Topical | Can be local and/or systemic | Applied onto the skin, e.g. ointments, creams (local, p. 647), transdermal patches (systemic, p. 647) or mucous membranes, e.g. eyes (local, p. 642), ears (local, p. 643), vagina (usually local, p. 644), rectum (may be either local or systemic, p. 644) |
| Inhalation | Local | Inhaled into the lungs, e.g. metered-dose inhalers (with or without a spacer device), nebulizers, steam inhalations |
| Injections: | Systemic | |
| Intradermal | Into the skin | |
| Subcutaneous (s.c.) | Into the subcutaneous tissue under the skin (see Fig. 22.9A) | |
| Intramuscular (i.m.) | Into a skeletal muscle (see Fig. 22.9B) | |
| Intravenous (i.v.) | Into a vein by a trained registered practitioner | |
| Intraosseous | Into a bone by a trained registered practitioner | |
| Intrathecal | Into the subarachnoid space (within the meninges) via a lumbar puncture by a trained registered practitioner |
Oral medication
Once swallowed, drugs are usually absorbed from the small intestine. Oral preparations include tablets, capsules and liquids (see Table 22.2). Some tablets are manufactured to exert their effect over long periods and are described as ‘slow release’ (SR) or ‘extended release’ (XR). Other tablets are covered in an enteric coating (e/c). For example, NSAIDs are potential gastric irritants and are enteric coated so that they travel through the stomach unchanged before entering the small intestine where they are absorbed, thus avoiding gastric irritation. People should therefore swallow these tablets whole and not crush or chew them, so that the enteric coating and/or extended release action is maintained. Crushing tablets increases the risk of adverse drug reactions and toxicity (Miller & Miller 2000). Bending (2001) cautions that when controlled release tablets are crushed the whole dose may be released within 5–15 minutes instead of over the 24-hour period intended. Manufacturers’ instructions warn against the crushing of tablets or opening of capsules as this is outwith the marketing licence (Wright 2003). When available, it is safer to use liquid preparations when swallowing tablets is difficult or impossible.
Sublingual and buccal routes
Some preparations put into the mouth are not intended for swallowing, including those for sublingual and buccal administration which avoids first pass metabolism, e.g. glyceryl trinitrate (see p. 633). Prochlorperazine (an antiemetic) can be administered via the buccal route. The tablet is placed between the upper lip and the gum to avoid swallowing the drug when nausea or vomiting is present.
Parenteral medication
Parenteral medication includes those given by any route other than the alimentary tract. This includes preparations that are:
Administering prescribed medication
Nurses should exercise professional judgement when administering medicines (NMC 2004a) and bear in mind that they are accountable when carrying out medical instructions (NMC 2004b; see also Ch. 7). Medicines must always be administered in accordance with legislation (see p. 627) and local policies. It is every nurse’s duty to be familiar with these policies and student nurses should refer to these in each new placement (NMC 2004a, NMC 2005). The importance of gaining consent and situations in which covert administration may be appropriate are considered below; calculation of drug doses is explained. The later parts of this section explain how nurses administer drugs by a number of routes.
Consent
It is important that healthcare professionals are aware of the individual’s right to refuse treatment, including medication, and must always respect their decisions (NMC 2004b). Adults must always be presumed to have the mental capacity (see Ch. 6) to consent to, or refuse, treatment, including taking medication, and no medication should be given without their agreement.
However, there are many situations when people may not be capable of providing valid consent. People with mental health problems, including dementia, or a learning disability may lack the capacity to make the decision whether or not to take medication (Box 22.5). When patients/clients are incapable of providing consent, or their wishes are contrary to their best interests (see Ch. 6), a registered practitioner should consult relevant people such as the family, carers or members of the multidisciplinary team (MDT). Assessment of capacity is primarily a matter for the treating clinicians but nurses as members of the MDT should be involved in such discussions.
Drug administration policies and obtaining consent
Drug administration policies may very between care settings.
For people detained under mental health legislation the principles of consent still apply to medications prescribed for other conditions. In other words, only medicines prescribed for mental health problems can be administered without the client’s consent.
The Children Act 1989 ensures that children’s wishes and feelings are taken into account, that they should always be consulted (subject to age and understanding) and kept informed about what is planned (see Ch. 6). The age of consent to medical treatment varies across the UK:
Covert administration of medicines
The United Kingdom Central Council (2001) states that the practice of covert, or ‘hidden’, administration of medicines may be only carried out in some specific instances. This should only be necessary or appropriate in the case of individuals who actively refuse medication and who are judged not to have the capacity to understand the consequences of their refusal. Treloar et al (2001) state that, in these cases, there is a duty to care and practitioners should be protected by transparent procedures which are framed in local policy. The following principles must always apply:
Calculation of drug doses
The units used for drug doses and accepted abbreviations are shown in Box 22.3. Most drugs are manufactured in a form that enables straightforward administration, and some are found in a variety of strengths. This helps to ensure that the prescribed dose can be provided accurately and easily calculated.
Accurate calculation of drug doses requires practice. Student nurses must always have their drug calculations checked by a RN.
Tablets and capsules
In order to calculate the number of tablets or capsules required, the nurse needs to know the dose prescribed and the strength of tablets/capsules available.
The following formula is then used:
For example: An adult is prescribed 1 g of paracetamol, for which 500 mg tablets are available.
If the calculation reveals that a tablet requires to be halved, a scored tablet is halved using a tablet splitter. Only scored tablets must be split to provide the correct dose and prevent potentially serious consequences. If the tablet cannot be halved, the medication should be withheld and advice should be sought from the prescriber and/or pharmacist.
Liquid preparations
In the case of liquid preparations the nurse needs to know the prescribed dose and the amount or weight of drug in a given amount of solution.
The following formula is used:
For example: An adult is prescribed 500 mg of penicillin. The stock is syrup containing 125 mg/5 mL.
Calculations based on body weight or surface area
These are used for some drugs in adults (e.g. chemotherapy treatment for cancer) and also in children.
Body weight
Body weight is often used for calculating doses for children and also for older adults. The following formula is used:
For example: A child weighing 16 kg is prescribed intravenous erythromycin for a severe infection. The dosage is 50 mg/kg/day in four doses.
In other words, a 200 mg injection would be administered every 6 hours.
Surface area
Doses are calculated according to body surface area, which is estimated in square metres (m2) using a nomogram (a graph that determines surface area from measurements of height and weight). This may be used for chemotherapy (drug treatment for cancers) in people of all ages. Drug doses are calculated using the following formula: surface area × prescribed dosage = dose required
For example: A child is prescribed cytarabine. The recommended dosage is 120 mg/m2 and their surface area is 0.5 m2.
(For more examples of drug calculations, see Gatford & Phillips 2002.)
Preparation for the administration of medications
Nurses must be familiar with both the procedure and the drugs(s) to be given in order to answer any questions the patient/client may ask about their medication and to administer it safely. It is important to explain to the patient/client how the drug works and how it is to be administered. Explanation may be either verbal, using language appropriate to the individual, and/or in writing, and must include the reason why the drug has been prescribed. Before receiving a new medicine, people may feel anxious about its administration and/or their reaction to it.
It is essential to ensure that any allergies are written in the medical/nursing notes and on the prescription sheet to ensure that people are not given drugs to which they are allergic.
There are several additional and specific precautions that must be adhered to when prescribing and administering drugs to children and also points of good practice (see Box 22.6).
Box 22.6 Administering medicines to children
Specific precautions for children
Points of good practice
Checks are carried out to ensure that the correct patient/client is given the right medication (correct dose via the right route and the correct preparation) at the right time and that the correct documentation is accurately completed afterwards. It is essential to adopt a systematic approach to ensure that medicines are administered safely (Box 22.7).
Principles of drug administration
Administration of oral medications
When possible the patient/client is assisted to sit upright as this makes swallowing easier. The person should be offered a glass of water, which moistens the mouth, prevents tablets or capsules sticking to the oral or oesophageal mucosa and aids their transport to the stomach. Principles for administering oral preparations are shown in Box 22.8. Some medications should be given at specific times. For example, nystatin pastilles given to treat an oral fungal infection should be taken after food as the drug would be removed by food and drink. Nurses administering medicines should be aware of specific instructions.
Administration of oral preparations
Injections
Injections are considered the appropriate route of administration when:
Injections can be given via the intradermal, subcutaneous, intramuscular, intravenous, intraosseous and intrathecal routes (see Table 22.3). Student nurses are normally only involved in administering injections by the subcutaneous and intramuscular routes.
Preparing and giving injections
Preparation of injections is carried out in accordance with local policies and the Good Practice Statement (Clinical Resource and Audit Group 2002). Box 22.9 outlines the steps in preparing or ‘drawing up’ injections. This section also considers intramuscular (i.m.) and subcutaneous (s.c.) injections, and the sites used. As i.m. injections are given into muscles and s.c. injections into the more superficial subcutaneous tissue, the depth of these injections is different.
Drawing up injections
Skin cleansing prior to injections
There is some controversy about the need to cleanse the skin prior to injections. The skin should be cleansed using an alcohol wipe only if local policy recommends this. Some local policies state that if the patient is physically clean, and the nurse has good hand hygiene and uses a non-touch technique during the procedure (see Ch. 15), skin cleansing with an alcohol-impregnated wipe is not required. If used, the skin should be cleansed for 30 seconds and then allowed to dry for another 30 seconds in order that adequate skin disinfection is achieved (Workman 1999).
The intramuscular route
Intramuscular injections are delivered into the muscles below the skin. Figure 22.9B shows the needle angle used to access the muscle layer. There are several sites that can be used for i.m. injections. The person’s general health and age are considered before deciding upon the most appropriate site. Older and emaciated people are likely to have less muscle than those who are young and active. The proposed site should be inspected for signs of swelling, inflammation, infection and skin lesions; affected areas should be avoided.
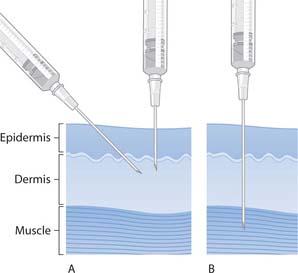
Fig. 22.9 Skin layers and needle insertion for injections: A. Subcutaneous. B. Intramuscular
(reproduced with permission from Downie et al 2003)
Deltoid muscle
The deltoid muscle is commonly used for vaccinations and for older children (Royal College of Paediatrics and Child Health 2002) (Fig. 22.10C).
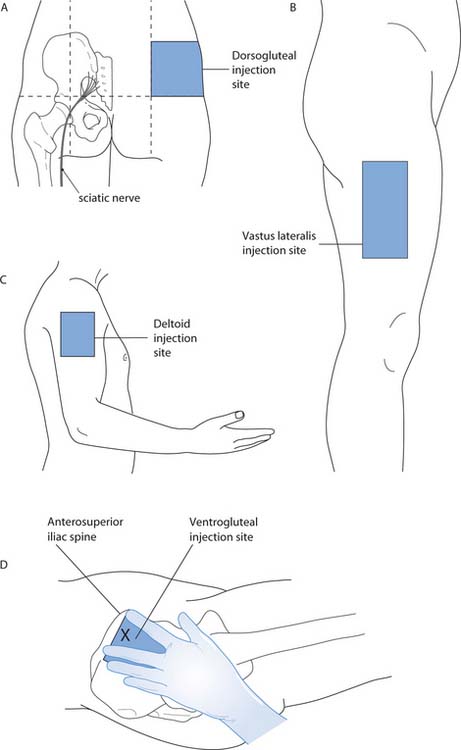
Fig. 22.10 Intramuscular injection sites
(A, B, C reproduced with permission of Nicol et al 2004; D reproduced with permission from Wong et al 2001)
Dorsogluteal site
The dorsogluteal site, also known as ‘the upper outer quadrant’ of the buttock, uses the gluteus maximus muscle (Fig. 22.10A). Studies have shown that there is relatively slow uptake of medication from this site (Rodger & King 2000). This is due to the large amount of adipose tissue located there, even in mildly obese patients, and means that the medication often ends up in the adipose tissue rather than the muscle (Workman 1999, Greenway & Hainsworth 2004). The nurse must therefore choose an appropriate length of needle depending on the size of the adult. There is also a risk of damaging the sciatic nerve if the site is not carefully located.
The Royal College of Paediatrics and Child Health (2002) do not advocate this site for children, except when a large volume of fluid is to be injected.
Ventrogluteal site
The ventrogluteal site accesses the gluteus medius muscle (Fig. 22.10D). Following an extensive literature review, Beyea and Nicoll (1995) promote the use of this site as it avoids potential sciatic nerve damage and the adipose tissue in the area is of relatively consistent thickness, thus ensuring the medication is administered into the muscle tissue (Greenway 2004). Workman (1999) suggests that a standard 21G (green) needle could be used in most adults of any size due to the consistent thickness of adipose tissue over this site.
Vastus lateralis muscle
This muscle is on the outer aspect of the thigh (Fig. 22.10B) and can be used for children, including infants (Royal College of Paediatrics and Child Health 2002). However Beyea and Nicoll (1996) suggest that, after 7 months of age, the ventrogluteal site should be the site of choice.
Rectus femoris
The rectus femoris is the anterior quadriceps muscle of the thigh. This is rarely used in adults, but can be easily accessed for self-administration or for infants (Workman 1999).
Administering intramuscular injections
The principles of administering i.m. injections are shown in Box 22.10.
Administration of intramuscular injections
Principles
The Z-track technique, formerly used exclusively for medications that stain the skin, is now widely recommended for all i.m. injections, as it is believed to reduce pain and leakage of medicine from the injection site. This technique involves gently pulling the skin and subcutaneous tissue so that it is no longer directly over the underlying muscle before carrying out the injection (Fig. 22.12) (Workman 1999). This is widely used for depot injections, commonly given to people with mental health problems.
Subcutaneous injections
These are given into the subcutaneous fat or connective tissue that lies between the muscles and the skin (see Fig. 22.9A). A short fine needle is used, e.g. 25G orange (Workman 1999). This route is suitable for drugs such as insulin that require slow and steady release. If a needle longer than 9 mm (25G orange) is used, an angle of 458 is recommended. When a shorter needle is required, e.g. for the administration of insulin, an angle of 908 is recommended (Workman 1999).
Some s.c. injections are pre-filled with the drug (such as heparin, given to prevent deep venous thrombosis), which means that there is no need to draw up the injection and therefore reduces the risk of needlestick injury. When using a shorter needle, it is not necessary to aspirate before injecting (Peragallo-Dittko 1997).
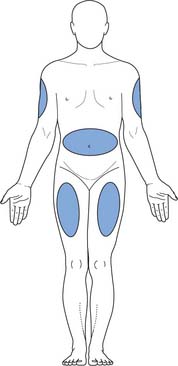
Figure 22.13 shows the sites that can be used for s.c. injections. People who require frequent s.c. injections, e.g. those with diabetes, should rotate injection sites and avoid using alcohol-impregnated wipes, which harden the skin. The principles of giving s.c. injections are shown in Box 22.11.
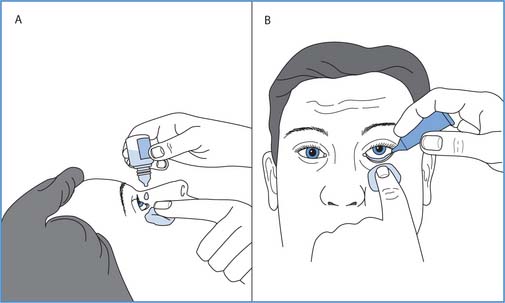
Fig. 22.14 Administration of eye medication: A. Drops. B. Ointment
(reproduced with permission from Nicol et al 2004)
Administering subcutaneous injections
Principles
Administration of medication into the eye
People who regularly receive eye drops or ointment may prefer to have the medication instilled either while lying down or sitting upright and their wishes should be taken into account. This may be for an eye condition (e.g. glaucoma), after eye surgery or in preparation for examination of the eye. Postoperatively it may be essential that the patient is lying down while eye drops are instilled. The patient/client is prepared to receive medication following the principles outlined on page 639. Box 22.12 lists the principles of administering eye medication.
Instillation of eye medication
Principles
For eye ointment
Afterwards, in either case
Administration of medicines into the ear
The patient is prepared to receive medication following the principles outlined on page 639. Ear drops may be instilled (Box 22.13, p. 646) to soften earwax prior to irrigation or syringing, or to relieve inflammatory or infective conditions of the outer ear.
Instillation of medication into the ear
Principles
Administration of medication into the nose
The patient is prepared to receive medication following the principles outlined on page 639. Box 22.14 lists the principles of administering nose drops.
Instillation of nasal drops
Principles
Rectal administration
Medication can be inserted into the rectum as suppositories and enemas (see Ch. 21). When this route is used for therapeutic drug administration (rather than for evacuation of the rectum), it must be explained that the medication should be retained, as many people associate suppositories and enemas with evacuation of the bowel. Ready access to lavatory facilities is necessary following administration of evacuant medication.
Administration of pessaries
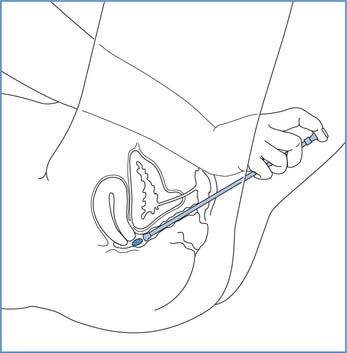
The patient should be prepared to receive medication following the principles outlined on page 639. Box 22.15 (p. 647) provides the principles of administering a pessary into the vagina. Nurses must remember that mostwomen find insertion of anything into the vagina very embarrassing and when possible the patient/client shouldbe encouraged to do this themselves. Nystatin pessaries may be prescribed for vaginal thrush (Candida albicans).
Administration of pessaries
Principles
Inhaled medication
Medications can be introduced directly into the airways for local action (e.g. bronchodilators to relieve bronchospasm, corticosteroids to reduce inflammation) in respiratory conditions such as asthma and chronic bronchitis. Action via this route is fast and high concentrations can be delivered. There are various types of inhaler devices and their use is explained in Chapter 17. These include:
Administration of topical medication
The patient should be prepared to receive medication following the principles outlined on page 639. Box 22.16 provides the principles involved in administering topical creams, ointments or lotions that are usually prescribed for skin conditions.
Administration of creams, ointments and lotions
Principles
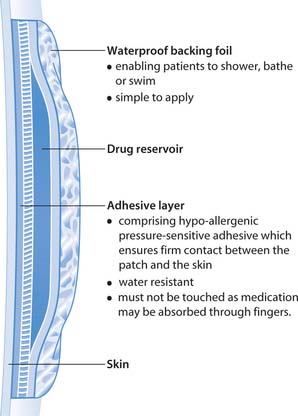
Transdermal patches
Transdermal patches are used to deliver medication such as hormones, opioids and nicotine replacement therapy (Fig. 22.16). In these situations topical application is used to provide systemic effects, usually for a prolonged period. The old patch is removed, the skin cleaned and a new patch is applied, usually to a different area. The skin is observed for redness, soreness and other signs of a reaction to either the drug or the adhesive. Patches are placed on non-hairy areas according to the prescription and the manufacturer’s instructions.
Post-drug administration measures
After administration of any medication, the nurse must always ensure that:
The nurse must ensure that the patient/client knows:
It should be noted that if the patient has been unable to swallow medicines, this should be reported immediately to the pharmacist and prescriber. A suitable alternative may be prescribed and dispensed.
Concordance, compliance and polypharmacy
The success of any medication regimen is most likely when it is completed according to the prescription. In this section, compliance, concordance and polypharmacy are considered.
Compliance
In order that medication can achieve its intended benefit it is important that people complete the prescribed course or continue to take it when a chronic condition is present. Compliance refers to the extent to which people follow health advice or other prescribed regimens, including drug treatment. It may be assumed that most people comply with prescribed drug treatment; however, this is often not the case and there are many reasons for this, including polypharmacy (see below) and difficulty in:
There may be complex emotional, motivational or physical reasons (such as difficulty in swallowing) why a person does not or cannot take their medication as prescribed. People may have religious or cultural beliefs about taking drugs or a specific treatment; for example, vegetarians and people who do not eat beef (e.g. Hindus) may refuse to take gelatin capsules. Some patients/clients have difficulty in remembering when to take the medicine or if they have taken it. Sensory and/or motor difficulties can make opening the packaging difficult or impossible. Others may stop taking their medication when their symptoms are alleviated or if side effects (actual or perceived) occur. Downie et al (2003) highlight that there is usually more than one reason behind non-compliance.
Bending (2002) found that approximately 50% of older people do not take their medication as directed. This not only incurs wastage in relation to the drugs and their costs to the NHS but also results in incomplete or inappropriate treatment.
Concordance
Because the term compliance is considered to suggest a degree of compulsion, it is beginning to be replaced by the term concordance, especially in mental health nursing. Concordance involves a partnership approach to treatment such as medicine taking where an agreement between the patient/client and the healthcare professional is negotiated in relation to the use of prescribed medication (DH 2001). This is a person-centred approach where an individual’s beliefs and wishes concerning their decision about medicine taking are paramount.
Polypharmacy
The Department of Health (2001) define polypharmacy as being prescribed four or more drugs. This is associated with more adverse drug reactions, predisposes to readmission of older adults following discharge from hospital and increases non-compliance. The Department of Health (2001) reviewed medicine-related aspects in the care of older adults and highlighted that medicine use increases with age:
Bending (2002) highlighted that up to 17% of all hospital admissions relate to ADRs, which illustrates the immensity of this problem.
People may forget to mention any over-the-counter medicines that they take regularly, assuming that they are not ‘real’ medications, and the nurse should therefore ask patients/clients about these products as their use is widespread.
Nurses should be aware that both under- and overmedication can arise from personal beliefs, forgetfulness, impatience, improvement in a person’s condition, lack of knowledge and/or misunderstanding about the drugs and that these should be considered when assessing compliance and monitoring response to treatment.
Improving compliance and concordance
Many people will require considerable support if compliance and concordance are to be achieved. Nurses must take the individual’s physical and psychological state into account when explaining, demonstrating and teaching people about medication. Relatives and carers may also become involved in education about drug treatment in, for example, children and people with a learning disability. Individuals who lack manual dexterity, e.g. due to arthritis, may require medications to be organized in a Dosette box or supplied in easy-to-open containers. For people with visual impairment large-print labels help with compliance. In Scotland, written instruction leaflets must be supplied with all medicines (Scottish Executive Health Department 2002). Overcoming the factors that may predispose to poor compliance/concordance forms the basis of enabling people to follow their drug treatment.
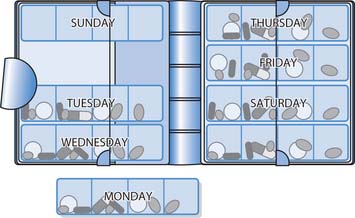
Dosette boxes
These contain sections for each day of the week, which are further divided to correspond with the times of day when the patient/client takes their medication, e.g. 8 am, 12 midday, 6 pm, 10 pm. Each daily column has a sliding lid that can be opened to expose only the drugs to be taken at a particular time (Fig. 22.17). Individuals, carers, pharmacists or other healthcare professionals can fill these boxes with the appropriate prescribed medications. There may, however, be a loss of efficacy if tablets are removed from blister packs and put into these boxes.
Self-medication
Deeks and Byatt (2000) reported that compliance may improve if patients/clients are encouraged to self-medicate. Self-medication has long been performed in the individual’s own home and now, increasingly, patients administer their own drugs in hospital. Since the introduction of lockable medicine cabinets (see Fig. 22.1B, p. 629) at each hospital bedside, self-administration hasbeen discussed widely (Scottish Executive Health Department 2002). However, successful self-administration and ultimately compliance relies not only on nurses providing adequate education and support but also patient cooperation (Nicol et al 2004).
Medication in older adults
Mary is 79 years old and was admitted to hospital in a confused state. Investigations revealed she had a chest infection. She takes regular medication for high blood pressure and arthritis and has been prescribed antibiotics for her chest infection.
Patient-centred drug administration
Many patients/clients successfully adhere to complex medication regimens at home, but in the past people almost universally gave up control of this aspect of their care when admitted to hospital. Today many clinical areas have introduced an individual-centred drug administration system where all a person’s drugs are stored locked in their bedside locker. These include their own drugs brought in on admission and/or new ones prescribed following admission.
In many clinical areas the nurse caring for a patient/client will administer their own drugs from the bedside locker according to the prescription sheet. This is a step towards complete individual-centred medicine administration where the patient/client administers their own medication from their bedside locker. One of the reasons behind this government-backed system is an effort to reduce the cost of medicines in both primary and secondary healthcare settings. Additionally, this system provides opportunities for patient education about drug treatment and assessment of people’s understanding and likely compliance after discharge.
Drug errors
Much has been done to make drug administration safe and yet errors do still sometimes occur. The extent of drug errors has been the subject of much attention from the government, hospital management, pharmacists and healthcare professionals, all of whom consider the safe administration of medicines to be an essential nursing skill.
Following studies into how errors occur, changes to drug administration policies have been made in an effort to make this as safe as possible. Student nurses are taught how to administer drugs safely in university, then supervised carrying this out in practice and later assessed as competent before gaining registration. Thereafter nurses are expected to continually update their knowledge of the drugs they administer. In spite of this, errors sometimes still occur and the consequences for a patient/client can be fatal. The consequences for nurses can result in disciplinary action, an investigation of professional misconduct, criminal charges or a civil case for negligence (see Ch. 6). Most importantly, however, being involved in drug errors significantly reduces their confidence as practitioners.
Minimizing drug errors
There are many models aimed at minimizing the occurrence of drug errors. Smetzer (2001) suggested a 10-step model to safeguard the recipient, the nurse (administrator), the pharmacist (dispenser) and the doctor (prescriber). She maintains that all involved should know:
The storage and distribution of drugs should be restricted and standardized, and any drug delivery systems should be assessed for safety and be user friendly. The care environment should be conducive to safe working practices that include nurses having appropriate education and sufficient practice before being assessed as competent to administer drugs safely. Smetzer (2001) advises that patients/clients should also be involved and become part of the safety net. In order to reduce drug errors, emphasis is placed on drug administration processes rather than the practitioners involved.
Many authors have considered factors that predispose to drug errors. These include omission of the drug, administration of an unauthorized drug, wrong dose given, wrong delivery route used, wrongly completed prescription forms (see Box 22.3), wrong time of administration, wrong preparation given and incorrect administration technique. Contributing factors identified in nurses and nursing practices that have been implicated in drug errors include:
Dealing with drug errors
When a drug error occurs it must be reported immediately. An incident form is completed and an investigation is undertaken (see Chs 6, 13). The NMC (2004a) recommend that this is undertaken by a multidisciplinary critical incident panel and staff should feel that they will be supported throughout the investigation. Information about reporting and developing a ‘no blame’ culture can be found on the National Patient Safety website (see p. 651).
| British National Formulary | www.bnf.org.uk/bnf |
| Available July 2006 | |
| British National Formulary for Children | http://bnfc.org/bnfc |
| Available July 2006 | |
| Medicines and Healthcare | www.mhra.gov.uk/home/idcplg?IdcService=S_GET_PAGE&nodeId= |
| Products Regulatory Agency | |
| Available July 2006 | |
| National Patient Safety Agency | www.npsa.nhs.uk |
| Available July 2006 |
Bending A. Hiding medicines. Primary Health Care. 2001;11(8):24-25.
Bending A. Just how do you make sure the medicine goes down. Nursing in Practice. March 2002:426-428.
Beyea S, Nicoll L. Administration of medications via the intramuscular route: an integrative review of the literature and research-based protocol for the procedure. Applied Nursing Research. 1995;8(1):23-33.
Beyea S, Nicoll L. Administering IM injections the right way. American Journal of Nursing. 1996;96(1):34-37.
Clinical Resource and Audit Group. 2002 Good practice statement for the preparation of injections in near-patient areas, including clinical and home environments. Online: www.show.scot.nhs.uk/crag/publications/inpa.pdf.
Deeks PA, Byatt K. Are patients who self-administer their medicines in hospital more satisfied with their care. Journal of Advanced Nursing. 2000;13(2):395-400.
Department of Health. Guidelines for the safe and secure handling of medicines (The Duthie Report). London: HMSO, 1988.
Department of Health. Review of prescribing: supply and administration of medicines. London: TSO, 1999.
Department of Health. Medicines and older people: implementing medicine-related aspects of the National Service Framework. London: TSO, 2001.
Downie G, Mackenzie J, Williams A. Pharmacology and medicines management for nurses, 3rd edn. Edinburgh: Churchill Livingstone, 2003.
Gatford JD, Phillips N. Nursing calculations, 6th edn. Edinburgh: Churchill Livingstone, 2002.
Greenstein B, editor. Trounce’s clinical pharmacology for nurses, 17th edn, Edinburgh: Churchill Livingstone, 2004.
Greenway K. Using the ventrogluteal site for intramuscular injection. Nursing Standard. 2004;18(25):39-42.
Greenway K, Hainsworth T. Is it right to be injecting the dorsogluteal site? NT practical procedures. Nursing Times. 2004;100(47):16.
Hopkins SJ, Kelly JC. Drugs and pharmacology for nurses, 13th edn. Edinburgh: Churchill Livingstone, 1999.
Miller D, Miller H. To crush or not to crush. Nursing. 2000;30(2):51. 52
National Institute for Health and Clinical Excellence. 2003 Infection control: prevention of healthcare-associated infections in primary and community care. Online: www.nice.org.uk/pdf/Infection_control_fullguideline.pdf.
NHS Education for Scotland. 2004 Healthcare associated infection. Online: www.space4.me.uk/hai/index.html.
Nicol M, Bavin C, S Bedford-Turner, et al. Essential nursing skills, 2nd edn. Edinburgh: Mosby, 2004.
Nursing and Midwifery Council. 2004a Guidelines for the administration of medicines. Online: http://www.nmc-uk.org/aFrameDisplay.aspx?DocumentID5610. Available July 2006.
Nursing and Midwifery Council. 2004b Code of professional conduct: standards for conduct, performance and ethics. Online: http://www.nmc-uk.org/aFrameDisplay.aspx?DocumentID5201. Available July 2006.
Nursing and Midwifery Council. 2004c Standards of proficiency for pre-registration nursing education. Online: http://www.nmc-uk.org/aFrameDisplay.aspx?DocumentID5328. Available July 2006.
Nursing and Midwifery Council. 2005 Guidelines for records and record keeping. Online: www.nmc-uk.org/aFrameDisplay.aspx?DocumentID5609.
Peragallo-Dittko V. Re-thinking subcutaneous injection technique. American Journal of Nursing. 1997;97(5):71-72.
Rang HP, Dale MM, Ritter J, Moore P. Pharmacology, 5th edn. Edinburgh: Churchill Livingstone, 2003.
Rodger MA, King L. Drawing up and administering intramuscular injections: a review of the literature. Journal of Advanced Nursing. 2000;31(3):574-582.
Royal College of Paediatrics and Child Health. 2002 Position statement on injection technique. Online: www.rcpch.ac.uk.
Scottish Executive Health Department. The right medicine: a strategy for pharmaceutical care in Scotland. Edinburgh: TSO, 2002.
Smetzer J. Take 10 giant steps to medication safety. Nursing. 2001;31(11):49-53.
Treloar A, Beats B, Philpot M. Concealing medication in patients’ food. Lancet. 2001;357(9249):62-64.
United Kingdom Central Council for Nursing, Midwifery and Health Visiting. 2001 UKCC position statement on the covert administration of medicines – disguising medicine in food and drink. Online: www.nmc-uk.org/aFrameDisplay.aspx?DocumentID5623.
Wong DL, M Hockenberry-Eaton. Wong’s essentials of pediatric nursing, 6th edn. St Louis: Mosby, 2001.
Workman B. Safe injection technique. Nursing Standard. 1999;13(39):47-53.
Wright DJ. Altering medication forms: what you should know. Nursing and Residential Care. 2003;5(8):372-375.
Chernecky CC et al. Drug calculations and drug administration. Philadelphia: Saunders, 2002.
Dougherty L, Lister S, editors. Drug administration: general principles. The Royal Marsden Hospital Manual of Clinical Nursing Procedures, 6th edn. Oxford: Blackwell. 2004:184-227.
Dunning G. The choice, application and review of topical treatments for skin conditions. Nursing Times. 2005;101(4):55-56.
Gabriel J, Dailly S, Keyley J. Needlestick and sharps injuries: avoiding the risk in clinical practice. Professional Nurse. 2004;20(1):25-26.
Hopkins SJ, Kelly JC. Drugs and pharmacology for nurses, 13th edn. Edinburgh: Churchill Livingstone, 1999.
Nicoll LH, Hesby A. Intramuscular injection: an integrative research review and guideline for evidence-based practice. Applied Nursing Research. 2002;15(3):149-162.
Pickering K. Nutrition: the administration of drugs via enteral feeding tubes. Nursing Times. 2003;99(46):46-47. 49
Small SP. Preventing sciatic nerve injury from intramuscular injections: literature review. Journal of Advanced Nursing. 2004;47(3):287-296.
Trim J. Clinical skills: a practical guide to working out drug calculations. British Journal of Nursing. 2004;13(10):602-606.
 REFLECTIVE PRACTICE
REFLECTIVE PRACTICE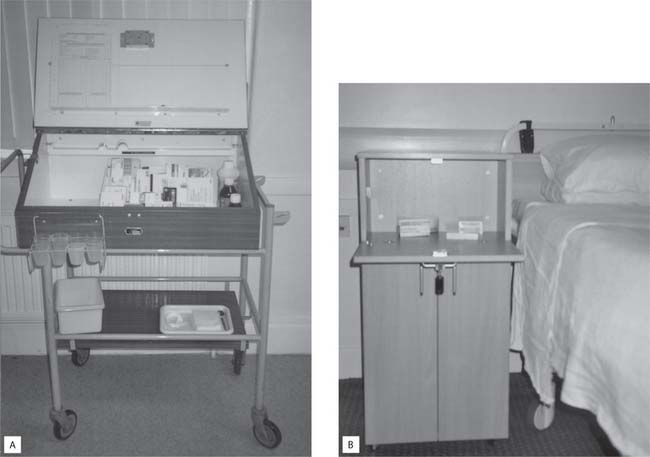

 CRITICAL THINKING
CRITICAL THINKING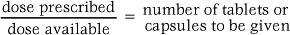
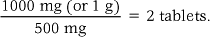
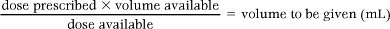
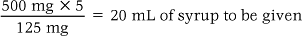
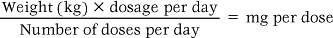
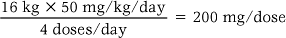
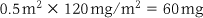
 NURSING SKILLS
NURSING SKILLS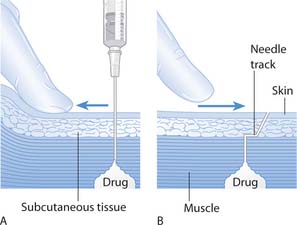

 HEALTH PROMOTION
HEALTH PROMOTION