Nutrition in Infancy
During the first 2 years of life, which are characterized by rapid physical and social growth and development, many changes occur that affect feeding and nutrient intake. The adequacy of infants’ nutrient intakes affects their interaction with their environment. Healthy, well-nourished infants have the energy to respond to and learn from the stimuli in their environment and to interact with their parents and caregivers in a manner that encourages bonding and attachment.
Physiologic Development
The length of gestation, the mother’s prepregnancy weight, and the mother’s weight gain during gestation determine an infant’s birth weight. After birth, the growth of an infant is influenced by genetics and nourishment. Most infants who are genetically determined to be larger reach their growth channel, a curve of weight and length or height gain throughout the period of growth, at between 3 and 6 months of age. However, many infants born at or below the tenth percentile for length may not reach their genetically appropriate growth channel until 1 year of age; this is called catch-up growth. Infants who are larger at birth and who are genetically determined to be smaller grow at their fetal rate for several months and often do not reach their growth channel until 13 months of age. This phenomenon during the first year of life is called lag-down growth.
Infants lose approximately 6% of their body weight during the first few days of life, but their birth weight is usually regained by the seventh to tenth day. Growth thereafter proceeds at a rapid but decelerating rate. Infants usually double their birth weight by 4 to 6 months of age and triple it by the age of 1 year. The amount of weight gained by the infant during the second year approximates the birth weight. Infants increase their length by 50% during the first year of life and double it by 4 years. Total body fat increases rapidly during the first 9 months, after which the rate of fat gain tapers off throughout the rest of childhood. Total body water decreases throughout infancy from 70% at birth to 60% at 1 year. The decrease is almost all in extracellular water, which declines from 42% at birth to 32% at 1 year of age.
The stomach capacity of infants increases from a range of 10 to 20 mL at birth to 200 mL by 1 year, enabling infants to consume more food at a given time and at less frequent intervals as they grow older. During the first weeks of life, gastric acidity decreases and for the first few months remains lower than that of older infants and adults. The rate of emptying is relatively slow, depending on the size and composition of the meal.
Fat absorption varies in the neonate. Human milk fat is well absorbed, but butterfat is poorly absorbed, with fecal excretions of 20% to 48%. The fat combinations in commercially prepared infant formula are well absorbed. The infant’s lingual and gastric lipases hydrolyze short- and medium-chain fatty acids in the stomach. Gastric lipase also hydrolyzes long-chain fatty acids and is important in initiating the digestion of triglycerides in the stomach. Most long-chain triglycerides pass unhydrolyzed into the small intestine, where they are broken down by pancreatic lipase. The bile salt–stimulated lipase present in human milk is stimulated by the infant’s bile salts and hydrolyzes the triglycerides in the small intestine into free fatty acids and glycerol. Bile salts, which are effective emulsifiers when combined with monoglycerides, fatty acids, and lecithin, aid in the intestinal digestion of fat.
The activities of the enzymes responsible for the digestion of disaccharides—maltase, isomaltase, and sucrase—reach adult levels by 28 to 32 weeks’ gestation. Lactase activity (responsible for digesting the disaccharide in milk) reaches adult levels by birth. Pancreatic amylase, which digests starch, continues to remain low during the first 6 months after birth. If the infant consumes starch before this time, increased activity of salivary amylase and digestion in the colon usually compensate.
The neonate has functional but physiologically immature kidneys that increase in size and concentrating capacity in the early weeks of life. The kidneys double in weight by 6 months and triple in weight by 1 year of age. The last renal tubule is estimated to form between the eighth fetal month and the end of the first postnatal month. The glomerular tuft is covered by a much thicker layer of cells throughout neonatal life than at any later time, which may explain why the glomerular filtration rate is lower during the first 9 months of life than it is in later childhood and adulthood. In the neonatal period the ability to form acid, urine, and concentrate solutes is often limited. The renal concentrating capacity at birth may be limited to as little as 700 mOsm/L in some infants. Others have the concentrating capacity of adults (1200 to 1400 mOsm/L). By 6 weeks, most infants can concentrate urine at adult levels. Renal function in a normal newborn infant is rarely a concern; however, difficulties may arise in infants with diarrhea or those who are fed formula that is too concentrated (Butte et al., 2004).
Nutrient Requirements
Nutrient needs of infants reflect rates of growth, energy expended in activity, basal metabolic needs, and the interaction of the nutrients consumed. Balance studies have defined minimum acceptable levels of intakes for a few nutrients, but for most nutrients the suggested intakes have been extrapolated from the intakes of normal, thriving infants consuming human milk. The dietary reference intakes (DRIs) for infants are shown in the inside front cover.
Energy
Full-term infants who are breast-fed to satiety and infants who are fed a standard 20-kcal/oz formula generally adjust their intake to meet their energy needs when caregivers are sensitive to the infants’ hunger and satiety cues. An effective method for determining the adequacy of an infant’s energy intake is to carefully monitor gains in weight, length, head circumference, and weight-for-length for age and plot these data on the World Health Organization (WHO) growth charts shown in Appendix Tables 9, 10, 13, and 14. It is important to recognize that during the first year a catch-up or lag-down period in growth may occur.
If infants begin to experience a decrease in their rate of weight gain, do not gain weight, or lose weight, their energy and nutrient intake should be monitored carefully. If the rate of growth in length decreases or ceases, potential malnutrition, an undetected disease, or both should be investigated thoroughly. If the weight gain proceeds at a much more rapid rate than growth in length, the energy concentration of the formula, the quantity of formula consumed, and the amount and type of semisolid and table foods offered should be evaluated. The activity level of the infant should also be assessed. Infants who are at the highest end of the growth charts for weight-for-length, or who grow rapidly in infancy, tend to be at greater risk for obesity later in life.
Protein
Protein is needed for tissue replacement, deposition of lean body mass, and growth (Rodriguez, 2005). Protein requirements during the rapid growth of infancy are higher per kilogram of weight than those for older children or adults. Recommendations for protein intake are based on the composition of human milk, and it is assumed that the efficiency of human milk use is 100%.
Infants require a larger percentage of total amino acids as essential amino acids than do adults. Histidine seems to be an essential amino acid for infants, but not for adults. Tyrosine, cystine, and taurine may be essential for premature infants.
Human milk or infant formula provides the major portion of protein during the first year of life. The amount of protein in human milk is adequate for the first 6 months of life, even though the amount of protein in human milk is considerably less than in infant formula. From 6 to 12 months of age the diet should be supplemented with additional sources of high-quality protein such as yogurt, strained meats, or cereal mixed with formula or human milk.
Infants may not receive adequate protein if their formula is excessively diluted for a prolonged period, as may be done to treat diarrhea after an enteric illness, or they have multiple food allergies causing their intake to be restricted (see Chapter 27).
Lipids
The current recommendation for infants younger than 1 year of age is to consume a minimum of 30 g of fat per day. This quantity is present in human milk and all infant formulas. Significantly lower fat intakes (e.g., with skim-milk feedings) may result in an inadequate total energy intake. An infant may try to correct the energy deficit by increasing the volume of milk ingested but usually cannot make up the entire deficit this way.
Human milk contains a generous amount of the essential fatty acids linoleic acid and α-linolenic acid, as well as the longer-chain derivatives arachidonic acid (ARA) (C20:4ω-6) and docosahexaenoic acid (DHA) (C22:6ω-3). Infant formulas are supplemented with linoleic acid and α-linolenic acid, from which ARA and DHA are derived. Increasingly, many formulas are also supplemented with ARA and DHA.
Linoleic acid, which is essential for growth and dermal integrity, should provide 3% of the infant’s total energy intake, or 4.4 g/day for infants younger than 6 months of age and 4.6 g/day for infants 7 months to 1 year of age. In human milk 5% of the kilocalories and 10% in most infant formulas are derived from linoleic acid. Smaller amounts of α-linolenic acid, a precursor of the ω-3 fatty acids DHA and eicosapentaenoic acid (EPA), should be included. The current recommendation is 0.5 g/day during the first year of life.
Because DHA can be formed by desaturation of α-linolenic acid, the importance of dietary DHA intake is uncertain. The concentration of DHA in human milk varies, depending on the amount of DHA in the mother’s diet. DHA and ARA are the major ω-3 and ω-6 long-chain polyunsaturated fatty acids (LCPUFAs) of neural tissues, and DHA is the major fatty acid of the photoreceptor membranes of the retina. Some studies suggest that supplementation of DHA and ARA positively affects visual acuity and psychomotor development, especially in premature infants. However, other studies have shown no differences in development. The American Academy of Pediatrics (AAP) has not taken an official stand on the addition of LCPUFAs to infant formula; however, they are now added to most infant formulas.
Carbohydrates
Carbohydrates should supply 30% to 60% of the energy intake during infancy. Approximately 40% of the energy in human milk and 40% to 50% of the energy in infant formulas is derived from lactose or other carbohydrates. Although rare, some infants cannot tolerate lactose and require a modified formula in their diet (see Chapters 29 and 44).
Botulism in infancy is caused by the ingestion of Clostridium botulinum spores, which germinate and produce toxin in the bowel lumen. The carbohydrates honey and corn syrup, occasionally used in home-prepared foods, have been identified as the only food sources of these spores in infants’ diets. The spores are extremely resistant to heat treatment and are not destroyed by current methods of processing. Thus honey and corn syrup should not be fed to infants younger than 1 year of age because they have not yet developed the immunity required to resist botulism spore development.
Water
The water requirement for infants is determined by the amount lost from the skin and lungs and in the feces and urine, in addition to a small amount needed for growth. The recommended total water intake for infants, based on the DRIs, is 0.7 L/day for infants up to 6 months and 0.8 L/day for infants 7 to 12 months of age. Note that total water includes all water contained in food, beverages, and drinking water. Water requirements per kilogram of body weight are shown in Table 17-1.
TABLE 17-1
Water Requirements of Infants and Children
| Age | Water Requirement (mL/kg/day) |
| 10 days | 125-150 |
| 3 mo | 140-160 |
| 6 mo | 130-155 |
| 1 yr | 120-135 |
| 2 yr | 115-125 |
| 6 yr | 90-100 |
| 10 yr | 70-85 |
| 14 yr | 50-60 |
From Barness LA: Nutrition and nutritional disorders. In Behrman RE, Kliegman RM: Nelson textbook of pediatrics, ed 17, Philadelphia, 2003, Saunders.
Because the renal concentrating capacity of young infants may be less than that of older children and adults, they may be vulnerable to developing a water imbalance. Under ordinary conditions, human milk and formula that is properly prepared supply adequate amounts of water. However, when formula is boiled, the water evaporates and the solutes become concentrated; therefore boiled milk or formula is inappropriate for infants. In very hot, humid environments, infants may require additional water. When losses of water are high (e.g., vomiting and diarrhea), infants should be monitored carefully for fluid and electrolyte imbalances.
Water deficits result in hypernatremic dehydration and its associated neurologic consequences (e.g., seizures, vascular damage). Hypernatremic dehydration has been reported in breast-fed infants who lose greater than 10% of their birth weight in the first few days of life (Leven and Macdonald, 2008). Because of the potential for hypernatremic dehydration, careful monitoring of volume of intake, daily weights, and hydration status (e.g., number of wet diapers) in all newborns is warranted.
Water intoxication results in hyponatremia, restlessness, nausea, vomiting, diarrhea, and polyuria or oliguria; seizures can also result. This condition may occur when water is provided as a replacement for milk, the formula is excessively diluted, or bottled water is used instead of an electrolyte solution in the treatment of diarrhea.
Minerals
Breast-fed infants retain approximately two thirds of their calcium intake. The recommended adequate intake (AI), the mean intake, is based on calcium intakes in healthy breast-fed infants. The AI for infants 0 to 6 months of age is 200 mg/day; for infants 7 to 12 months of age the AI is 260 mg/day; formulas are enhanced accordingly.
Fluoride
The importance of fluoride in preventing dental caries has been well documented. However, excessive fluoride may cause dental fluorosis, ranging from fine white lines to entirely chalky teeth (see Chapter 26). To prevent fluorosis, the tolerable upper intake level for fluoride has been set at 0.7 mg/day for infants up to 6 months and 0.9 mg/day for infants 7 to 12 months of age.
Human milk is very low in fluoride. Commercially prepared infant cereals, wet pack cereals, and fruit juice produced with fluoridated water are significant sources of fluoride in infancy. Fluoride supplementation is not recommended for infants younger than 6 months of age. After tooth eruption it is recommended that fluoridated water be offered several times per day to breast-fed infants, those who receive cow’s milk, and those fed formulas made with water that contains less than 0.3 mg of fluoride/L (American Academy of Pediatrics [AAP], 2009).
Iron
Full-term infants are considered to have adequate stores of iron for growth up to a doubling of their birth weight. This occurs at approximately 4 months of age in full-term infants and much earlier in prematurely born infants. Recommended intakes of iron increase according to age, growth rate, and iron stores. At 4 to 6 months of age, infants who are fed only human milk are at risk for developing a negative iron balance and may deplete their reserves by 6 to 9 months. Iron in human milk is highly bioavailable; however, breast-fed infants should receive an additional source of iron by 4 to 6 months of age (AAP, 2005). Iron-fortified cereals and infant formula are common food sources. Cow’s milk is a poor source of iron and should not be given before 12 months of age. The AAP recommends iron supplementation of 1 mg/kg/day starting at 4 months of age and continuing until appropriate complementary foods have been introduced (Baker and Greer, 2010).
Iron deficiency and iron deficiency anemia are common health concerns for the older infant. The prevalence of iron deficiency in children 9 months to 3 years of age who are living in the United States and the United Kingdom and are primarily among low socioeconomic and minority groups is higher than the 10% in the general population, and has been estimated at 30% (Eden, 2005).
Monitoring iron status is important because of the long-term cognitive effects of iron deficiency in infancy (Eden, 2005). Low hemoglobin concentrations at 8 months of age correlate with impaired motor development at 18 months of age (Sherriff et al., 2001). In addition, children with chronic iron deficiency in infancy demonstrate long-term developmental deficits and behavioral issues in early adolescence.
Zinc
Newborn infants are immediately dependent on a dietary source of zinc. Zinc is better absorbed from human milk than from infant formula. Human milk and infant formulas provide adequate zinc (0.3 to 0.5 mg/100 kcal) for the first year of life. Other foods (e.g., meats, cereals) should provide most of the zinc required during the second year. Infants who are zinc-deficient can exhibit growth retardation (Cole and Lifshitz, 2008).
Vitamins
Milk from lactating mothers who follow a strict vegan diet may be deficient in vitamin B12, especially if the mother followed the regimen for a long time before and during her pregnancy. Vitamin B12 deficiency has also been diagnosed in infants breast-fed by mothers with pernicious anemia (Weiss et al., 2004) (see Chapter 32).
Vitamin D
Human milk derived from an adequately fed, lactating mother supplies all the vitamins the term infant needs except for vitamin D; human milk contains approximately only 20 international units (IU)/L (0.5 mcg cholecalciferol) of vitamin D. For the prevention of rickets and vitamin D deficiency, the AAP recommends a minimum vitamin D intake of 400 IU per day shortly after birth for all infants. All breast-fed infants need a vitamin D supplement of 400 IU per day. Formula-fed infants who consume less than 1000 mL of formula per day also need supplementation (Wagner and Greer, 2008). There appears to be a higher risk of rickets among young, breast-fed infants and children with dark skin (Weisberg et al., 2004). Because a variety of environmental and family lifestyle factors can affect both sunlight exposure and absorption of vitamin D, the AAP recommendations to provide supplemental vitamin D are appropriate for all infants. Supplementation up to 800 IU of vitamin D per day may be needed for infants at higher risk, such as premature infants, dark-skinned infants and children, and those who reside in northern latitudes or at higher altitudes (Misra et el., 2008).
The Food and Drug Administration (FDA) states that some droppers that come with liquid vitamin D supplements could hold more than the 400 IU per day recommended by the AAP. Thus it is important that parents or caregivers provide only the recommended amount. Excessive vitamin D can cause nausea and vomiting, loss of appetite, excessive thirst, frequent urination, constipation, abdominal pain, muscle weakness, muscle and joint aches, confusion, fatigue or damage to kidneys.
Vitamin K
The vitamin K requirements of the neonate need special attention. Deficiency may result in bleeding or hemorrhagic disease of the newborn. This condition is more common in breast-fed infants than in other infants because human milk contains only 2.5 mcg/L of vitamin K, whereas cow’s milk–based formulas contain approximately 20 times this amount. All infant formulas contain a minimum of 4 mcg of vitamin K per 100 kcal of formula. The AI for infants is 2 mcg/day during the first 6 months and 2.5 mcg/day during the second 6 months of life. This can be supplied by mature breast milk, although perhaps not during the first week of life. For breast-fed infants vitamin K supplementation is necessary during that time to considerably decrease the risk for hemorrhagic disease. Most hospitals require that infants receive an injection of vitamin K as a prophylactic measure shortly after birth.
Supplementation
Vitamin and mineral supplements should be prescribed only after careful evaluation of the infant’s intake. Commercially prepared infant formulas are fortified with all necessary vitamins and minerals; therefore formula-fed infants rarely need supplements. Breast-fed infants need additional vitamin D supplementation shortly after birth and iron by 4 to 6 months of age (see Focus On: Vitamin and Mineral Supplementation Recommendations for Full-Term Infants). Chapter 43 discusses the feeding of premature or high-risk infants and their special needs.
Milk
Human milk is unquestionably the food of choice for the infant. Its composition is designed to provide the necessary energy and nutrients in appropriate amounts. It contains specific and nonspecific immune factors that support and strengthen the immature immune system of the newborn and thus protect the body against infections. Human milk also helps prevent diarrhea and otitis media (AAP, 2005). Allergic reactions to human milk protein are rare. Moreover, the closeness of the mother and infant during breast-feeding facilitates attachment and bonding (see Chapter 16) and breast milk provides nutritional benefits (e.g., optimal nourishment in an easily digestible and bioavailable form), decreases infant morbidity, provides maternal health benefits (e.g., lactation amenorrhea, maternal weight loss, some cancer protection), and has economic and environmental benefits (American Dietetic Association [ADA], 2009).
During the first few days of life, a breast-feeding infant receives colostrum, a yellow, transparent fluid that meets the infant’s needs during the first week. It contains less fat and carbohydrate but more protein and greater concentrations of sodium, potassium, and chloride than mature milk. It is also an excellent source of immunologic substances.
Note that breast feeding may not be appropriate for mothers with certain infections or those who are taking medications that may have untoward effects on the infant. For example, a mother who is infected with human immunodeficiency virus can transmit the infection to the infant and a mother using psychotropic drugs or other pharmacologic drugs may pass the medication to the infant through her breast milk (AAP, 2005). See Chapter 16.
The American Dietetic Association (ADA) and the AAP support exclusive breast feeding (EBF) for the first 6 months of life and then breast feeding supplemented by complementary foods until at least 12 months (AAP, 2005; ADA, 2009). It is important to note the ages of the infants in these recommendations; adding other foods at too young of an age decreases breast-milk intake and increases early weaning. Healthy Children 2020 objectives propose to support breast-feeding among mothers of newborn infants (see Focus On: Healthy Children 2020 Objectives: Nourishment of Infants).
Composition of Human and Cow’s Milk
The composition of human milk is different from that of cow’s milk; for this reason, unmodified cow’s milk is not recommended for infants until at least 1 year of age. Both provide 20 kcal/oz; however, the nutrient sources of the energy are different. Protein provides 6% to 7% of the energy in human milk and 20% of the energy in cow’s milk. Human milk is 60% whey proteins (mainly lactalbumins) and 40% casein; by contrast, cow’s milk is 20% whey proteins and 80% casein. Casein forms a tough, hard-to-digest curd in the infant’s stomach, whereas lactalbumin in human milk forms soft, flocculent, easy-to-digest curds. Taurine and cystine are present in higher concentrations in human milk than in cow’s milk; these amino acids may be essential for premature infants. Lactose provides 42% of the energy in human milk and only 30% of the energy in cow’s milk.
Lipids provide 50% of the energy in human and whole cow’s milk. Linoleic acid, an essential fatty acid, provides 4% of the energy in human milk and only 1% in cow’s milk. The cholesterol content of human milk is 10 to 20 mg/dL compared with 10 to 15 mg/dL in whole cow’s milk. Less fat is absorbed from cow’s milk than from human milk; a lipase in human milk is stimulated by bile salts and contributes significantly to the hydrolysis of milk triglycerides.
All of the water-soluble vitamins in human milk reflect maternal intake. Cow’s milk contains adequate quantities of the B-complex vitamins, but little vitamin C. Human milk and supplemented cow’s milk provide sufficient vitamin A. Human milk is a richer source of vitamin E than cow’s milk.
The quantity of iron in human and cow’s milk is small (0.3 mg/L). Approximately 50% of the iron in human milk is absorbed, whereas less than 1% of the iron in cow’s milk is absorbed. The bioavailability of zinc in human milk is higher than in cow’s milk. Cow’s milk contains three times as much calcium and six times as much phosphorus as human milk, and its fluoride concentration is twice that of human milk.
The much higher protein and ash content of cow’s milk results in a higher renal solute load, or amount of nitrogenous waste and minerals that must be excreted by the kidney. The sodium and potassium concentrations in human milk are about one third those in cow’s milk, contributing to the lower renal solute load of human milk. The osmolality of human milk averages 300 mOsm/kg, whereas that of cow’s milk is 400 mOsm/kg.
Antiinfective Factors
Human milk and colostrum contain antibodies and antiinfective factors that are not present in infant formulas. Secretory immunoglobulin A (sIgA), the predominant immunoglobulin in human milk, plays a role in protecting the infant’s immature gut from infection. Breast feeding should be maintained until the infant is at least 3 months of age to obtain this benefit.
The iron-binding protein lactoferrin in human milk deprives bacteria of iron and thus slows their growth. Lysozymes, which are bacteriolytic enzymes found in human milk, destroy the cell membranes of bacteria after the peroxides and ascorbic acid that are also present in human milk have inactivated them. Human milk enhances the growth of the bacterium Lactobacillus bifidus, which produces an acidic gastrointestinal (GI) environment that interferes with the growth of certain pathogenic organisms. Because of these antiinfective factors, the incidence of infections is lower in breast-fed infants than in formula-fed infants.
Colonization with nonpathogenic microbiota is important for infant health and may affect health in later life. By the time a mother weans her infant, the baby’s GI tract has established its normal flora. This ecosystem in early life is influenced by such factors as mode of birth, environment, diet, and use of antibiotics (Marques et al., 2010). The role and safety of supplemental probiotic use is under study.
Formulas
Infants that are not breastfed are usually fed a formula based on cow’s milk or a soy product. Many mothers may also choose to offer a combination of breast milk and formula feedings. Those infants who have special requirements receive specially designed products.
Commercial formulas made from heat-treated nonfat milk or a soy product and supplemented with vegetables fats, vitamins, and minerals are formulated to approximate, as closely as possible, the composition of human milk. They provide the necessary nutrients in an easily absorbed form. The manufacture of infant formulas is regulated by the FDA through the Infant Formula Act (Nutrient Requirements for Infant Formulas, 1985). By law, infant formulas are required to have a nutrient level that is consistent with these guidelines (Table 17-2). Refer to individual manufacturers’ websites to obtain the most accurate information and compare the composition of various infant formulas and feeding products.
TABLE 17-2
Nutrient Levels in Infant Formulas As Specified by the Infant Formula Act
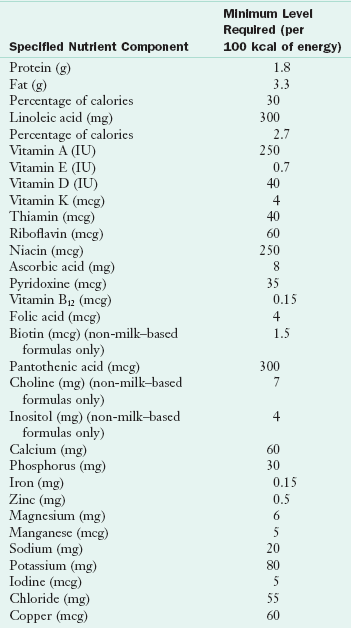
From Nutrient requirements for infant formulas, Final Rule (21 CFR 107), Fed Reg 50:45106, 1985.
Formulas are also available for older infants and toddlers. However, typically “older infant” formulas are unnecessary unless toddlers are not receiving adequate amounts of infant or table foods.
Efforts are ongoing to manufacture infant formulas that closely approximate human milk. Recent additions to infant formulas include ARA, DHA, prebiotics, and probiotics. No current documentation shows that the growth or development of formula-fed infants is compromised when they consume formulas without these additives. The declining prevalence of anemia in infants is credited to the use of iron-fortified formula. The AAP recommends iron-fortified formulas for all formula-fed infants. The widespread theory that iron-fortified formula may cause constipation, loose stools, colic (severe abdominal pain), and spitting up has not been confirmed by clinical studies (AAP, 1999).
Various products are available for infants who cannot tolerate the protein in cow’s milk–based formulas. Soy-based infant formulas are recommended for (1) infants in vegetarian families, and (2) infants with galactosemia or hereditary primary lactase deficiency. Soy formulas are not recommended for children known to have protein allergies because many infants who are allergic to cow’s milk protein also develop allergies to soy protein (Bhatia and Greer, 2008) (see Chapter 27).
Infants who cannot tolerate cow’s milk–based or soy products can be fed formulas made from a casein hydrolysate, which is casein that has been split into smaller components by treatment with acid, alkali, or enzymes. These formulas do not contain lactose. For infants who have severe food protein intolerances and cannot tolerate hydrolysate formulas, free amino acid–based formulas are available. Other formulas are available for children with problems such as malabsorption or metabolic disorders (e.g., phenylketonuria) (see Chapters 18 and 44).
Soy-based formulas are under regular scrutiny. Full-term infants ingesting soy formulas grow and absorb minerals as well as infants fed cow’s milk–based formulas, but they are exposed to several thousand times higher levels of phytoestrogens and isoflavones. The biologic effect of these elevated isoflavone levels on long-term infant development is not yet clear (National Toxicology Program, 2010). Soy-based formulas are not recommended for preterm infants because of the increased risk for osteopenia (Bhatia and Greer, 2008).
The protein in soy infant formula is soy protein isolate supplemented with L-methionine, L-carnitine, and taurine. Contaminants include phytates, which bind minerals and niacin; and protease inhibitors, which have antitrypsin, antichymotrypsin, and antielastin properties. Aluminum from mineral salts is found in soy infant formulas at concentrations of 600-1300 ng/mL, levels that exceed aluminum concentrations in human milk of 4-65 ng/mL (Bhatia and Greer, 2008).
Whole Cow’s Milk
Some parents may choose to transition their infant from formula to fresh cow’s milk before 1 year of age. However, the AAP Committee on Nutrition has concluded that infants should not be fed whole cow’s milk during the first year of life (AAP, 2009). Infants who are fed whole cow’s milk have been found to have lower intakes of iron, linoleic acid, and vitamin E, and excessive intakes of sodium, potassium, and protein. Cow’s milk may cause a small amount of GI blood loss.
Low-fat (1% to 2%) and nonfat milk are also inappropriate for infants during the first 12 months of life. The infants may ingest excessive amounts of protein in large volumes of milk in an effort to meet their energy needs, and the decreased amount of essential fatty acids may be insufficient for preventing deficiency (AAP, 2009). In addition, substitute or imitation milks such as rice, oat, or nut milks are inappropriate and should not be fed to infants unless they are properly supplemented.
Formula Preparation
Commercial infant formulas are available in ready-to-feed forms that require no preparation, as concentrates prepared by mixing with equal parts of water, and in powder form that is designed to be mixed with 2 oz of water per level scoop of powder.
Infant formulas should be prepared in a clean environment. All equipment, including bottles, nipples, mixers, and the top of the can of formula, should be washed thoroughly. Formula may be prepared for up to a 24-hour period and refrigerated. Formula for each feeding should be warmed in a hot water bath. Microwave heating is not recommended because of the risk of burns from formula that is too hot or unevenly heated. Any formula warmed and not consumed at that feeding should be discarded.
Food
Dry infant cereals are fortified with electrolytically reduced iron, which is iron that has been fractionated into small particles for improved absorption. Four level tablespoons of cereal provide approximately 5 mg of iron or approximately half the amount the infant requires. Therefore infant cereal is usually the first food added to the infant’s diet.
Strained and “junior” vegetables and fruits provide carbohydrates and vitamins A and C. Vitamin C is added to numerous jarred fruits and all fruit juices. In addition, tapioca is added to several of the jarred fruits. Milk is added to the creamed vegetables, and wheat is incorporated into the mixed vegetables.
Most strained and junior meats are prepared with water. Strained meats, which have the highest energy density of any of the commercial baby foods, are an excellent source of high-quality protein and heme iron.
Numerous dessert items are also available such as puddings and fruit desserts. The nutrient composition of these products varies, but all contain excess energy in the form of sugar and modified cornstarch or tapioca starch. Most infants do not need this excess energy.
Various commercially prepared foods and organically grown products are available for infants. See Chapter 10 for a discussion of organic foods. These products vary widely in their nutrient value. Foods for infants should be thoughtfully selected to meet their nutritional and developmental needs.
Mothers who would like to make their own infant food can easily do so by following the directions in Box 17-1. Home-prepared foods are generally more concentrated in nutrients than commercially prepared foods because less water is used. Salt and sugar should not be added to foods prepared for infants.
Feeding
Because milk from a mother with an adequate diet is uniquely designed to meet the needs of the human infant, breast feeding for the first 6 months of life is strongly recommended. Most chronic medical conditions do not contraindicate breast-feeding.
A mother should be encouraged to nurse her infant immediately after birth. Those who care for and counsel parents during the first postpartum days should acquaint themselves with ways in which they can be supportive of breast-feeding. Ideally, counseling and preparation for breast feeding starts in the last few months or weeks of pregnancy (see Chapter 16).
Regardless of whether infants are breast-fed or formula fed, they should be held and cuddled during feedings. Once a feeding rhythm has been established, infants become fussy or cry to indicate they are hungry, whereas they often smile and fall asleep when they are satisfied (Table 17-3). Infants, not adults, should establish the feeding schedules. Initially, most infants feed every 2 to 3 hours; by 2 months of age most feed every 4 hours. By 3 to 4 months of age infants have usually matured enough to allow the mother to omit night feedings.
TABLE 17-3
| Age (Weeks) | Behavior |
| 4-12 | Draws head away from the nipple |
| Falls asleep | |
| When nipple is reinserted, closes lips tightly | |
| Bites nipple, purses lips, or smiles and lets go | |
| 16-24 | Releases nipple and withdraws head |
| Fusses or cries | |
| Obstructs mouth with hands | |
| Pays more attention to surroundings | |
| Bites nipple | |
| 28-36 | Changes posture |
| Keeps mouth tightly closed | |
| Shakes head as if to say “no” | |
| Plays with utensils | |
| Uses hands more actively | |
| Throws utensils | |
| 40-52 | See behaviors listed for previous age range |
| Sputters with tongue and lips | |
| Hands bottle or cup to mother |
From Pipes PL: Health care professionals. In Garwood G, Fewell R, editors: Educating handicapped infants, Rockville, Md, 1982, Aspen Systems.
Bisphenol A (BPA) is a chemical present in many hard plastic bottles, such as baby bottles and reusable cups, and metal food and beverage containers, including canned liquid infant formula. Concern has been raised about the potential effects of BPA on the brain, behavior, and prostate gland in fetuses, infants, and young children. Studies are ongoing; however, in the interim the FDA recommends taking reasonable steps to reduce human exposure to BPA. The major U.S. manufacturers have stopped producing BPA-containing bottles and infant feeding cups for the U.S. market. The FDA is facilitating the development of alternatives to BPA for the linings of infant formula cans and supporting efforts to replace or minimize BPA in other food can linings (Food and Drug Administration [FDA], 2010).
Development of Feeding Skills
At birth, infants coordinate sucking, swallowing, and breathing, and are prepared to suckle liquids from the breast or bottle, but are not able to handle foods with texture. During the first year, typical infants develop head control, the ability to move into and sustain a sitting posture, and the ability to grasp, first with a palmar grasp and then with a refined pincer grasp (Figure 17-1). They develop mature sucking and rotary chewing abilities and progress from being fed to feeding themselves using their fingers. In the second year, they learn to feed themselves independently with a spoon (Figure 17-2).

FIGURE 17-1 Development of feeding skills in infants and toddlers. A, This 7-month-old child shows the beginnings of involvement with feeding by anticipating the spoon. B, This 9-month-old girl is using a refined pincer grasp to pick up her food. C, This 19-month-old boy is beginning to use his spoon independently, although he is not yet able to rotate his wrist to keep food on it.
Addition of Semisolid Foods
Developmental readiness and nutrient needs are the criteria that determine appropriate times for the addition of various foods. During the first 4 months of life, the infant attains head and neck control, and oral motor patterns progress from a suck to a suckling to the beginnings of a mature sucking pattern. Puréed foods introduced during this phase are consumed in the same manner as are liquids, with each suckle being followed by a tongue-thrust swallow. Table 17-4 lists developmental landmarks and their indications for semisolid and table food introduction.
TABLE 17-4
Feeding Behaviors: Developmental Landmarks During the First 2 Years of Life
| Developmental Landmarks | Change Indicated | Examples of Appropriate Foods |
| Tongue laterally transfers food in the mouth Shows voluntary and independent movements of the tongue and lips Sitting posture can be sustained Shows beginning of chewing movements (up and down movements of the jaw) |
Introduction of soft, mashed table food | Tuna fish; mashed potatoes; well-cooked, mashed vegetables; ground meats in gravy and sauces; soft, diced fruit such as bananas, peaches, and pears; flavored yogurt |
| Reaches for and grasps objects with palmar grasp Brings hand to mouth |
Finger feeding (large pieces of food) | Oven-dried toast, teething biscuits; cheese sticks (should be soluble in the mouth to prevent choking) |
| Voluntarily releases food (refined digital [pincer] grasp) | Finger feeding (small pieces of food) | Bits of cottage cheese, dry cereal, peas, and other bite-size vegetables; small pieces of meat |
| Shows rotary chewing pattern | Introduction of food of varied textures from family menu | Well-cooked, chopped meats and casseroles; cooked vegetables and canned fruit (not mashed); toast; potatoes; macaroni, spaghetti; peeled ripe fruit |
| Approximates lips to rim of the cup | Introduction of cup for sipping liquids | |
| Understands relationship of container and its contents | Beginning of self-feeding (though messiness should be expected) | Food that when scooped adheres to the spoon, such as applesauce, cooked cereal, mashed potatoes, cottage cheese, yogurt |
| Shows increased movements of the jaw Shows development of ulnar deviation of the wrist |
More skilled at cup and spoon feeding | Chopped fibrous meats, such as roast and steak; raw vegetables and fruit (introduced gradually) |
| Walks alone | May seek food and obtain food independently | Foods of high nutritional value |
| Names food, expresses preferences; prefers unmixed foods Goes on food jags Appetite appears to decrease |
Balanced food choices, with child permitted to develop food preferences (parents should not be concerned that these preferences will last forever) |
Modified from Trahms CM, Pipes P: Nutrition in infancy and childhood, ed 6, New York, 1997, McGraw-Hill.
Between 4 and 6 months of age, when the mature sucking movement is refined and munching movements (up-and-down chopping motions) begin, the introduction of strained foods is appropriate. Infant cereal is usually introduced first. To support developmental progress, cereal is offered to the infant from a spoon, not combined with formula in a bottle. Thereafter various commercially or home-prepared foods may be offered. The sequence in which these foods are introduced is not important; however, it is important that one single ingredient food (e.g., peaches, not peach cobbler, which has many ingredients) be introduced at a time. Introducing a single new food at a time at 2- to 7-day intervals enables parents to identify any allergic responses or food intolerances (Butte et al., 2004). Introducing vegetables before fruits may increase vegetable acceptance.
Infants demonstrate their acceptance of new foods by slowly increasing the variety and quantity of solids they accept. Breast-fed infants seem to accept greater quantities than do formula-fed infants. Parents who thoughtfully offer a variety of nourishing foods are more likely to provide a well-balanced diet and help their children learn to accept more flavors.
As oral-motor maturation proceeds, an infant’s rotary chewing ability develops, indicating a readiness for more textured foods such as well-cooked mashed vegetables, casseroles, and pasta from the family menu. Learning to grasp—with the palmar grasp, then with an inferior pincer grasp, and finally with the refined pincer grasp—indicates a readiness for finger foods such as oven-dried toast, arrowroot biscuits, or cheese sticks (see Figure 17-1). Table 17-5 presents recommendations for adding foods to an infant’s diet. Foods with skins or rinds and foods that stick to the roof of the mouth (e.g., hot dogs, grapes, bread with peanut butter) may cause choking and should not be offered to young infants.
TABLE 17-5
Suggested Ages for the Introduction of Juice, Semisolid Foods, and Table Foods
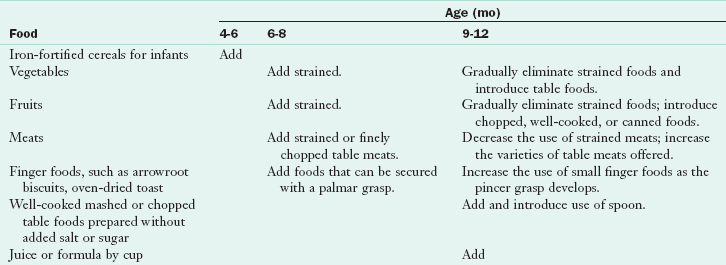
Modified from Trahms CM, Pipes P: Nutrition in infancy and childhood, ed 6, New York, 1997, McGraw-Hill.
During the last quarter of the first year, infants can approximate their lips to the rim of the cup and can drink if the cup is held for them. During the second year they gain the ability to rotate their wrists and elevate their elbows, thus allowing them to hold the cup themselves and manage a spoon. They are very messy eaters at first, but by 2 years of age most typical children skillfully feed themselves (see Figure 17-2).
Weaning from Breast or Bottle to Cup
The introduction of solids into an infant’s diet begins the weaning process in which the infant transitions from a diet of only breast milk or formula to a more varied one. Weaning should proceed gradually and be based on the infant’s rate of growth and developmental skills. Weaning foods should be carefully chosen to complement the nutrient needs of the infant, promote appropriate nutrient intake, and maintain growth.
Many infants begin the process of weaning with the introduction of the cup at approximately 6 to 9 months of age and complete the process when they are able to ingest an adequate amount of milk or formula from a cup at 18 to 24 months of age. Parents of infants who are breast-fed may choose to transition the infant directly to a cup or have an intermittent transition to a bottle before the cup is introduced.
Early Childhood Caries
Early childhood caries (ECC), or “baby bottle tooth decay,” is the most common chronic disease of childhood. ECC is a pattern of tooth decay that involves the upper anterior and sometimes lower posterior teeth. ECC is common among infants and children who are allowed to bathe their teeth in sugar (sucrose or lactose) throughout the day and night. If infants are given sugar-sweetened beverages or fruit juice in a bottle during the day or at bedtime after teeth have erupted, the risk of dental caries increases (see Chapter 26).
To promote dental health, infants should be fed and burped and then put to bed without milk, juice, or food. Juice should not be introduced into the diet before 6 months of age. Juice should be limited to 4 to 6 oz/day for infants and young children and offered to children only from a cup (AAP, 2007). Parents and caregivers can be taught effective oral health practices for infants, not only by dentists but by other paraprofessionals (MacIntosh et al., 2010).
Feeding Older Infants
As maturation proceeds and the rate of growth slows down, infants’ interest in and approach to food changes. Between 9 and 18 months of age most reduce their breast-milk or formula intake. They can become finicky about what and how much they eat.
In the weaning stage infants have to learn many manipulative skills, including the ability to chew and swallow solid food and use utensils. They learn to tolerate various textures and flavors of food, eat with their fingers, and then feed themselves with a utensil. Very young children should be encouraged to feed themselves. See Clinical Insight: A New Look at the Food Practices of Infants and Toddlers.
At the beginning of a meal, children are hungry and should be allowed to feed themselves; when they become tired, they can be helped quietly. Emphasis on table manners and the fine points of eating should be delayed until they have the necessary maturity and developmental readiness for such training.
The food should be in a form that is easy to handle and eat. Meat should be cut into bite-size pieces. Potatoes and vegetables should be mashed so that they can be eaten easily with a spoon. Raw fruits and vegetables should be in sizes that can be picked up easily. In addition, the utensils should be small and manageable. Cups should be easy to hold, and dishes should be designed so that they do not tip over easily.
Type of Food
In general, children prefer simple, uncomplicated foods. Food from the family meal can be adapted for the child and served in child-size portions. Children younger than 6 years of age usually prefer mild-flavored foods. Because a young child’s stomach is small, a snack may be required between meals. Fruit, cheese, crackers, dry cereal, fruit juices, and milk contribute nutrients and energy. Children ages 2 to 6 years often prefer raw instead of cooked vegetables and fruits.
Infants should be offered foods that vary in texture and flavor. Infants who are accustomed to many kinds of foods are less likely to limit their variety of food choices later. To add variety to an infant’s diet, vegetables and fruits can be added to cereal feedings. It is important to offer various foods and not allow the infant to continue consuming a diet consisting of one or two favorite foods. Older infants generally reject unfamiliar foods the first time they are offered. When parents continue to offer small portions of these foods without comment, infants become familiar with them and often accept them. It is important that fruit juice does not replace more nutrient-dense foods. If excessive amounts of juice are consumed, children may fail to thrive.
Serving Size
The size of a serving of food offered to a child is very important. At 1 year of age infants eat one third to one half the amount an adult normally consumes. This proportion increases to one half an adult portion by the time the child reaches 3 years of age and increases to about two thirds by 6 years of age. Young children should not be served a large plateful of food; the size of the plate and the amount should be in proportion to their age. A tablespoon (not a heaping tablespoon) of each food for each year of age is a good guide to follow. Serving less food than parents think or hope will be eaten helps children eat successfully and happily. They will ask for more food if their appetite is not satisfied.
Forced Feeding
Children should not be forced to eat; instead, the cause for the unwillingness to eat should be determined. A typical, healthy child eats without coaxing. Children may refuse food because they are too inactive to be hungry or too active and overtired. To avoid both overfeeding and underfeeding, parents should be responsive to the cues for hunger and satiety offered by the infant. A child who is fed snacks or given a bottle too close to mealtime (within 90 minutes) is not hungry for the meal and may refuse it (Butte et al., 2004).
Parents who support the development of self-feeding skills respond to the infant’s need for assistance and offer encouragement for self-feeding; they also allow the infant to initiate and guide feeding interactions without excessive pressure on the infant for neatness in self-feeding or amount of food consumed. If a child refuses to eat, the family meal should be completed without comment, and the plate should be removed. This procedure is usually harder on the parent than on the child. At the next mealtime, the child will be hungry enough to enjoy the food presented.
Eating Environment
Young children should eat their meals at the family table; it gives them an opportunity to learn table manners while enjoying meals with a family group. Sharing the family fare strengthens ties and makes mealtime pleasant. However, if the family meal is delayed, the children should receive their meal at the usual time. When children eat with the family, everyone must be careful not to make unfavorable comments about any food. Children are great imitators of the people they admire; thus, if the father or older siblings make disparaging remarks about squash, for example, young children are likely to do the same.
The Bright Futures materials and guidelines (www.brightfutures.org/nutrition/) provide information and support to families as they guide their children to healthy eating habits and nourishment.
American Academy of Pediatrics
Bright Futures: Nutrition in Practice
www.brightfutures.org/nutrition/
Healthy People 2020: Objectives for Improving Health
University of Washington Assuring Pediatric Nutrition in the Hospital and Community
References
American Academy of Pediatrics (AAP), Committee on Nutrition. Iron fortification of infant formulas. Pediatrics. 1999;104:119.
American Academy of Pediatrics (AAP), Committee on Nutrition. The use and misuse of fruit juice in pediatrics. Pediatrics. 2001;107:1210. [(reaffirmed Feb 2007)].
American Academy of Pediatrics (AAP), Committee on Nutrition. Pediatric nutrition handbook, ed 6. Elk Grove Village, IL: The Academy; 2009.
American Academy of Pediatrics (AAP), Section on Breastfeeding. breastfeeding and the use of human milk. Pediatrics. 2005;115:496.
American Dietetic Association. Position of the American Dietetic Association: promoting and supporting breastfeeding. J Am Diet Assoc. 2009;109:1926.
Baker, RD, Greer, FR, American Academy of Pediatrics, Committee on Nutrition. diagnosis and prevention of iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040.
Bhatia, J, Greer, F, American Academy of Pediatrics, Committee on Nutrition. use of soy protein–based formulas in infant feeding. Pediatrics. 2008;121:1062.
Butte, N, et al. The Start Healthy feeding guidelines for infants and toddlers. J Am Diet Assoc. 2004;104:442.
Carruth, BR, et al. Developmental milestones and self-feeding behaviors in infants and toddlers. J Am Diet Assoc. 2004;104:S51.
Carruth, BR, et al. Prevalence of picky eaters among infants and toddlers and their caregivers’ decisions about offering a new food. J Am Diet Assoc. 2004;104:S57.
Cole, CR, Lifshitz, F. Zinc nutrition and growth retardation. Pediatr Endocrinol Rev. 2008;5:889.
Eden, AN. Iron deficiency and impaired cognition in toddlers: an underestimated and undertreated problem. Pediatr Drugs. 2005;7:347.
Food and Drug Administration (FDA). Update on bisphenol A for use in food contact applications. http://www.fda.gov/NewsEvents/%20PublicHealthFocus/ucm197739.htm, 2010. [Accessed from].
Fox, MK, et al. Feeding infants and toddlers study: what foods are infants and toddlers eating? J Am Diet Assoc. 2004;104:S22.
Fox, MK, et al. Relationship between portion size and energy intake among infants and toddlers: evidence of self-regulation. J Am Diet Assoc. 2006;106:S77.
Leven, LV, MacDonald, PD. Reducing the incidence of neonatal hypernatraemic dehydration. Arch Dis Child. 2008;93:811.
Marques, TM, et al. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol. 2010;21(2):149.
MacIntosh, AC, et al. The impact of community workshops on improving early childhood oral health knowledge. Pediatric Dent. 2010;32:110.
Misra, M, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398.
National Toxicology Program (NTP). Draft NTP brief on soy infant formula. Washington, DC: US Department of Health and Human Services; 16 March 2010.
1985. Nutrient Requirements for Infant Formulas, Final Rule (21 CFR 107). Fed Reg. 1985;50:45106.
Rodriguez, NR. Optimal quantity and composition of protein for growing children. J Am Coll Nutr. 2005;24:150S.
Sherriff, A, et al. Should infants be screened for anemia? A prospective study investigating the ratio between hemoglobin at 8, 12, and 18 months and development at 18 months. Arch Dis Child. 2001;84:480.
Skinner, JD, et al. Meal and snack patterns of infants and toddlers. J Am Diet Assoc. 2004;104:S65.
Wagner, CL, Greer, FR, American Academy of Pediatrics, Section on Breastfeeding; American Academy of Pediatrics, Committee on Nutrition. prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122:1142.
Weisberg, P, et al. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004;80:1697S.
Weiss, R, et al. Severe vitamin B12 deficiency in an infant associated with a maternal deficiency and a strict vegetarian diet. J Pediatr Hematol Oncol. 2004;26:270.
