35 Bone metabolism
Overview
In this chapter, we consider first the cellular and biochemical processes involved in bone remodelling, and the various hormones and other mediators that regulate these processes. We then describe the drugs used to treat disorders of bone and finally deal with the new agents in the pipeline.
Introduction
The human skeleton undergoes a continuous process of remodelling throughout life—some bone being resorbed and new bone being laid down resulting in the complete skeleton being replaced every 10 years. With advancing age, there is an increasing possibility of structural deterioration and decreased bone mass (osteoporosis). This constitutes a major health problem throughout the world, and there are, in addition, various other conditions that can lead to pathological changes in bone that require therapy. In the past decade, there have been significant advances in the understanding of bone biology, which have already led to new drugs, progress that will no doubt continue.
Bone Structure and Composition
The human skeleton consists of 80% cortical bone and 20% trabecular bone. Cortical bone is the dense, compact outer part, and trabecular bone the inner meshwork. The former predominates in the shafts of long bones, the latter in the vertebrae, the epiphyses of long bones and the iliac crest. Trabecular bone, having a large surface area, is metabolically more active and more affected by factors that lead to bone loss (see below).
The main minerals in bone are calcium and phosphates. More than 99% of the calcium in the body is in the skeleton, mostly as crystalline hydroxyapatite but some as non-crystalline phosphates and carbonates; together, these make up half the bone mass.
The main cells in bone homeostasis are osteoblasts, osteoclasts and osteocytes.
Osteoid is the organic matrix of bone and its principal component is collagen. But there are also other components such as proteoglycans, osteocalcin and various phosphoproteins, one of which, osteonectin, binds to both calcium and collagen and thus links these two major constituents of bone matrix.
Calcium phosphate crystals in the form of hydroxyapatite [Ca10(PO4)6(OH)2] are deposited in the osteoid, converting it into hard bone matrix.
In addition to its structural function, bone plays a major role in overall calcium homeostasis in the body.
Bone Remodelling
There has been substantial progress in our understanding of bone remodelling in recent years (see reviews by Boyce & Xing, 2008; Gallagher, 2008; Deal, 2009; and Wright et al., 2009.)
The process of remodelling involves the following:
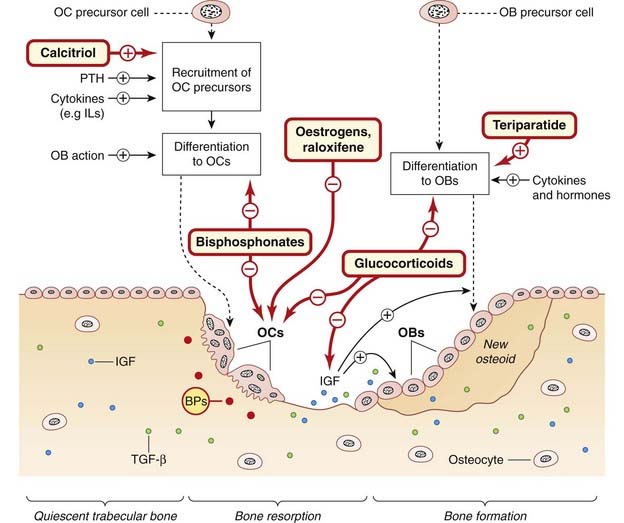
Fig. 35.1 The bone-remodelling cycle and the action of hormones, cytokines and drugs.
Quiescent trabecular bone. Cytokines such as insulin-like growth factor (IGF) and transforming growth factor (TGF)-β, shown as dots, are embedded in the bone matrix. Bone resorption. Osteoclast (OC) precursor cells, recruited by cytokines and hormones, are activated by osteoblasts (OBs) to form mobile multinuclear OCs (see Fig. 35.2) that move along the bone surface, resorbing bone and releasing the embedded cytokines. Bone formation. The released cytokines recruit OBs, which lay down osteoid and embed cytokines IGF and TGF-β in it. Some OBs also become embedded, forming terminal osteocytes (now known not to be inert). The osteoid then becomes mineralised, and lining cells cover the area (not shown). Oestrogens cause apoptosis (programmed cell death) of OCs. Note that pharmacological concentrations of glucocorticoids have the effects specified above, but physiological concentrations are required for OB differentiation. BPs, embedded bisphosphonates—these are ingested by OCs when bone is resorbed (not shown); IL, interleukin; PTH, parathyroid hormone.
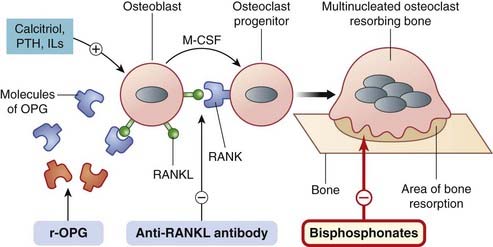
Fig. 35.2 Schematic diagram of the role of the osteoblast and cytokines in the differentiation and activation of the osteoclast and the action of drugs thereon.
The osteoblast is stimulated by calcitriol, parathyroid hormone (PTH) and cytokines (not shown) to express a surface ligand, the RANK ligand (RANKL). RANKL expression is increased by various interleukins, PTH, tumour necrosis factor (TNF)-α and glucocorticoids. RANKL interacts with a receptor on the osteoclast—an osteoclast differentiation and activation receptor termed RANK (receptor activator of nuclear factor kappa B). This, with cytokines (e.g. macrophage colony-stimulating factor, MCSF) released by the osteoblast, causes differentiation and activation of the osteoclast progenitors to form mature osteoclasts (not shown). Fusion of osteoclasts occurs to give giant multinucleated bone-resorbing cells, which are polarised with a ruffled border on the bone-resorbing side (shown). Bisphosphonates inhibit bone resorption by osteoclasts. Anti-RANKL antibodies (e.g. denosumab) bind RANKL and prevent the RANK–RANKL interaction. The osteoblast also releases ‘decoy’ molecules of osteoprotegerin (OPG), which can bind RANKL and prevent activation of the RANK receptor. Recombinant OPG (r-OPG)—which has this effect—is in clinical trial. Drugs used clinically are in red-bordered boxes, those in development in blue boxes.
Diet, drugs and physical factors (exercise, loading) also affect remodelling. Bone loss—of 0.5–1% per year—starts in the 35–40 age group in both sexes. The rate accelerates by as much as 10-fold during the menopause in women or with castration in men, and then gradually settles at 1–3% per year. The loss during the menopause is due to increased osteoclast activity and affects mainly trabecular bone; the later loss in both sexes with increasing age is due to decreased osteoblast numbers and affects mainly cortical bone.
The Action of Cells and Cytokines
A cycle of remodelling starts with recruitment of the cells that give rise to osteoclast precursors and the subsequent differentiation of these to mature multinucleated osteoclasts induced by cytokines (Fig. 35.1). The osteoclasts adhere to an area of trabecular bone, developing a ruffled border at the attachment site. They move along the bone, digging a pit by secreting hydrogen ions and proteolytic enzymes, mainly cathepsin K. This process gradually liberates cytokines such as insulin-like growth factor (IGF)-1 and transforming growth factor (TGF)-β, which have been embedded in the osteoid (Fig. 35.1); these in turn recruit and activate successive teams of osteoblasts that have been stimulated to develop from precursor cells and are awaiting the call to duty (see Fig. 35.1 and below). The osteoblasts invade the site, synthesising and secreting the organic matrix of bone, the osteoid, and secreting IGF-1 and TGF-β (which become embedded in the osteoid; see above). Some osteoblasts become embedded in the osteoid, forming terminal osteocytes; others interact with and activate osteoclast precursors—and we are back to the beginning of the cycle.
Cytokines involved in bone remodelling other than IGF-1 and TGF-β include other members of the TGF-β family, such as the bone morphogenic proteins (BMPs), a range of interleukins, various hormones and members of the tumour necrosis factor (TNF) family. A member of this last family—a ligand for a receptor on the osteoclast precursor cell—is of particular importance. The receptor is termed (wait for it—biological terminology has fallen over its own feet here) RANK, which stands for receptor activator of nuclear factor kappa B (NFκB), NFκB being the principal transcription factor involved in osteoclast differentiation and activation. And the ligand is termed, unsurprisingly, RANK ligand (RANKL).
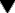 The osteoblast synthesises and releases a molecule termed osteoprotegerin (OPG), identical with RANK, which functions as a decoy receptor. In a sibling-undermining process by the two cells (osteoblast and osteoclast precursor), OPG can bind to RANKL1 (generated by the very cell that OPG itself is generated by) and inhibit RANKL’s binding to the functional receptor, RANK, on the osteoclast precursor cell (Fig. 35.2). The ratio of RANKL to OPG is critical in the formation and activity of osteoclasts and thus the optimal functioning of the RANK, RANKL, OPG system is fundamental to bone remodelling (reviewed by Boyce & Xing, 2008; Wright et al., 2009).
The osteoblast synthesises and releases a molecule termed osteoprotegerin (OPG), identical with RANK, which functions as a decoy receptor. In a sibling-undermining process by the two cells (osteoblast and osteoclast precursor), OPG can bind to RANKL1 (generated by the very cell that OPG itself is generated by) and inhibit RANKL’s binding to the functional receptor, RANK, on the osteoclast precursor cell (Fig. 35.2). The ratio of RANKL to OPG is critical in the formation and activity of osteoclasts and thus the optimal functioning of the RANK, RANKL, OPG system is fundamental to bone remodelling (reviewed by Boyce & Xing, 2008; Wright et al., 2009).
The Turnover of Bone Minerals
The main bone minerals are calcium and phosphates.
Calcium Metabolism
The daily turnover of bone minerals during remodelling involves about 700 mg of calcium. Calcium has numerous roles in physiological functioning. Intracellular Ca2+ is part of the signal transduction mechanism of many cells (see Ch. 4), so the concentration of Ca2+ in the extracellular fluid and the plasma, normally about 2.5 mmol/l, needs to be controlled with great precision. The plasma Ca2+ concentration is regulated by interactions between PTH and various forms of vitamin D (Figs 35.3 and 35.4); calcitonin also plays a part.
Fig. 35.3 The main factors involved in maintaining the concentration of Ca2+ in the plasma and the action of drugs.
The calcium receptor on the parathyroid cell is a G-protein-coupled receptor. Calcifediol and calcitriol are metabolites of vitamin D3 and constitute the ‘hormones’ 25-hydroxy-vitamin D3 and 1,25-dihydroxy-vitamin D3, respectively. Endogenous calcitonin, secreted by the thyroid, inhibits Ca2+ mobilisation from bone and decreases its resorption in the kidney, thus reducing blood Ca2+. Calcitonin is also used therapeutically in osteoporosis.
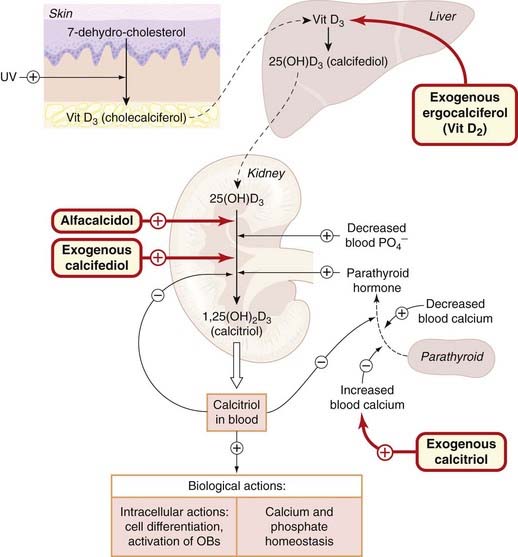
Fig. 35.4 Summary of the actions of the vitamin D endocrine system and the action of drugs.
Exogenous ergocalciferol, vitamin (Vit) D2 (formed in plants by ultraviolet, UV, light), is converted to the corresponding D2 metabolites in liver and kidney, as is the D2 analogue dihydrotachysterol (not shown). Alfacalcidol (1α-hydroxycholecalciferol) is 25-hydroxylated to calcitriol in the liver. OB, osteoblast.
Calcium absorption in the intestine involves a Ca2+-binding protein whose synthesis is regulated by calcitriol (see Fig. 35.3). It is probable that the overall calcium content of the body is regulated largely by this absorption mechanism, because urinary Ca2+ excretion normally remains more or less constant. However, with high blood Ca2+ concentrations urinary excretion increases, and with low blood concentrations urinary excretion can be reduced by PTH and calcitriol, both of which enhance Ca2+ reabsorption in the renal tubules (Fig. 35.3).
Phosphate Metabolism
Phosphates are important constituents of bone, and are also critically important in the structure and function of all the cells of the body. They are constituents of nucleic acids, provide energy in the form of ATP, and control—through phosphorylation—the actitivity of many functional proteins. They also have roles as intracellular buffers and in the excretion of hydrogen ions in the kidney.
Phosphate absorption is an energy-requiring process regulated by calcitriol. Phosphate deposition in bone, as hydroxyapatite, depends on the plasma concentration of PTH, which, with calcitriol, mobilises both Ca2+ and phosphate from the bone matrix. Phosphate is excreted by the kidney; here PTH inhibits reabsorption and thus increases excretion.
Bone remodelling ![]()
Hormones Involved in Bone Metabolism and Remodelling
The main hormones involved in bone metabolism and remodelling are parathyroid hormone (PTH), members of the vitamin D family, oestrogens and calcitonin. Glucocorticoids and thyroid hormone also affect bone.
Parathyroid Hormone
Parathyroid hormone, which consists of a single-chain polypeptide of 84 amino acids, is an important physiological regulator of Ca2+ metabolism. It acts on PTH receptors in various tissues (bone, kidney, gastrointestinal tract) to maintain the plasma Ca2+ concentration. It mobilises Ca2+ from bone, promotes its reabsorption by the kidney and stimulates the synthesis of calcitriol, which in turn increases Ca2+ absorption from the intestine and synergises with PTH in mobilising bone Ca2+ (Figs 35.3 and 35.4). PTH promotes phosphate excretion, and thus its net effect is to increase the concentration of Ca2+ in the plasma and lower that of phosphate.
The mobilisation of Ca2+ from bone by PTH is mediated, at least in part, by stimulation of the recruitment and activation of osteoclasts. Pathological oversecretion of PTH (hyperparathyroidism) inhibits osteoblast activity (not shown in Fig. 35.1). But given therapeutically in a low intermittent dose, PTH and fragments of PTH paradoxically stimulate osteoblast activity and enhance bone formation.
Parathyroid hormone is synthesised in the cells of the parathyroid glands and stored in vesicles. The principal factor controlling secretion is the concentration of ionised calcium in the plasma, low plasma Ca2+ stimulating secretion, high plasma Ca2+ decreasing it by binding to and activating a Ca2+-sensing G-protein-coupled surface receptor (see Ch. 3, Figure 35.3). (For reviews, see Stewart, 2004; Deal, 2009.)
Vitamin D
Vitamin D (calciferol) consists of a group of lipophilic prehormones that are converted in the body into a number of biologically active metabolites that function as true hormones, circulating in the blood and regulating the activities of various cell types (see Reichel et al., 1989). Their main action, mediated by nuclear receptors of the steroid receptor superfamily (see Ch. 3), is the maintenance of plasma Ca2+ by increasing Ca2+ absorption in the intestine, mobilising Ca2+ from bone and decreasing its renal excretion (see Fig. 35.3). In humans, there are two sources of vitamin D:
Cholecalciferol is converted to calcifediol (25-hydroxy-vitamin D3) in the liver, and this is converted to a series of other metabolites of varying activity in the kidney, the most potent of which is calcitriol (1,25-dihydroxy-vitamin D3); see Fig. 35.4).
The synthesis of calcitriol from calcifediol is regulated by PTH, and is also influenced by the phosphate concentration in the plasma and by the calcitriol concentration itself through a negative feedback mechanism (Fig. 35.4). Receptors for calcitriol are ubiquitous, and calcitriol is important in the functioning of many cell types.
The main actions of calcitriol are the stimulation of absorption of Ca2+ and phosphate in the intestine, and the mobilisation of Ca2+ from bone, but it also increases Ca2+ reabsorption in the kidney tubules (Fig. 35.3). Its effect on bone involves promotion of maturation of osteoclasts and indirect stimulation of their activity (Figs 35.1 and 35.3). It decreases collagen synthesis by osteoblasts. However, the effect on bone is complex and not confined to mobilising Ca2+, because in clinical vitamin D deficiency (see below), in which the mineralisation of bone is impaired, administration of vitamin D restores bone formation. One explanation may lie in the fact that calcitriol stimulates synthesis of osteocalcin, the Ca2+-binding protein of bone matrix.
Oestrogens
During reproductive life in the female, oestrogens have an important role in maintenance of bone integrity, acting on both osteoblasts and osteoclasts. They inhibit the cytokines that recruit osteoclasts and oppose the bone-resorbing, Ca2+-mobilising action of PTH. They increase osteoblast proliferation, augment the production of TGF-β and bone morphogenic proteins, and inhibit apoptosis (see Ch. 5). Withdrawal of oestrogen, as happens at the menopause, can (and usually does) lead to osteoporosis.
Calcitonin
Calcitonin is a peptide hormone secreted by the specialised ‘C’ cells found in the thyroid follicles (see Ch. 33).
The main action of calcitonin is on bone; it inhibits bone resorption by binding to a specific receptor on osteoclasts, inhibiting their action. In the kidney, it decreases the reabsorption of both Ca2+ and phosphate in the proximal tubules. Its overall effect is to decrease the plasma Ca2+ concentration (Fig. 35.3).
Secretion is determined mainly by the plasma Ca2+ concentration.
Other Hormones
Physiological concentrations of glucocorticoids are required for osteoblast differentiation. Excessive pharmacological concentrations inhibit bone formation by inhibiting osteoblast differentiation and activity, and may stimulate osteoclast action—leading to osteoporosis, which is a feature of Cushing’s syndrome (Fig. 32.7) and an important adverse effect of glucocorticoid administration (Ch. 32).
Thyroxine stimulates osteoclast action, reducing bone density and liberating Ca2+. Osteoporosis occurs in association with thyrotoxicosis, and care must be taken not to use excessive thyroxine dosage for treating hypothyroidism (see Ch. 33).
Parathyroid, vitamin D and bone mineral homeostasis ![]()
Disorders of Bone
The reduction of bone mass with distortion of the microarchitecture is termed osteoporosis; a reduction in the mineral content is termed osteopenia. Osteoporotic bone fractures easily after minimal trauma. The commonest causes of osteoporosis are postmenopausal deficiency of oestrogen and age-related deterioration in bone homeostasis. It is calculated that, in England and Wales, one in two women and one in five men over the age of 50 will have a fracture due to osteoporosis (van Staa et al., 2001), while in the USA a 50-year-old woman is estimated to have a 40% lifetime risk of an osteoporotic fracture (Strewler, 2005). Osteoporosis can also occur secondary to conditions such as rheumatoid arthritis, and can result from other factors, such as excessive thyroxine or glucocorticoid administration.
Because life expectancy has increased significantly in the developed world, osteoporosis is now regarded as being of epidemic proportions, and has become an important public health problem, affecting about 75 million people in the USA, Japan and Europe. Drugs that can be used in prevention and treatment are being vigorously sought and substantial progress has been made in recent years.
Other diseases of bone requiring drug therapy are osteomalacia and rickets (the juvenile form of osteomalacia), in which there are defects in bone mineralisation due to vitamin D deficiency, and Paget’s disease, in which there is distortion of the processes of bone resorption and remodelling.
Drugs Used in Bone Disorders
Two types of agent are currently used for treatment of osteoporosis:
Strontium ranelate has both actions.
Rickets and osteomalacia, nutritionally induced deficiencies in bone mass, result from vitamin D deficiency and are treated with vitamin D preparations.
Bisphosphonates
Bisphosphonates (Fig. 35.5) are enzyme-resistant analogues of pyrophosphate, a normal constituent of tissue fluids that accumulates in bone, and has a role in regulating bone resorption. Bisphosphonates inhibit bone resorption by an action mainly on the osteoclasts. They form tight complexes with calcium in the bone matrix, and are released slowly as bone is resorbed by the osteoclasts, which are thus exposed to high concentrations of the drugs.
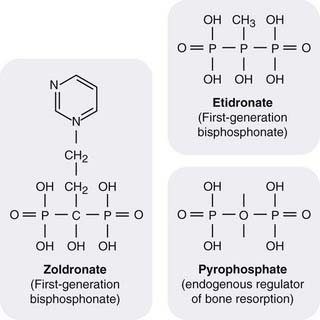
Fig. 35.5 Structure of bisphosphonates.
Replacement of the oxygen atom in pyrophosphate renders the compounds enzyme resistant. Addition of an N-containing side chain alters the mechanism of action (see text) and greatly increases potency.
Mechanism of action
In terms of their molecular mechanism of action, the bisphosphonates can be grouped into two classes:
Pharmacokinetic aspects
Bisphosphonates are usually given orally and are poorly absorbed. They may be given intravenously in malignancy. About 50% of a dose accumulates at sites of bone mineralisation, where it remains, potentially for months or years, until the bone is resorbed. The free drug is excreted unchanged by the kidney.
Absorption is impaired by food, particularly milk, so the drugs must be taken on an empty stomach.
Unwanted effects include gastrointestinal disturbances including peptic ulcers and oesophagitis. Bone pain occurs occasionally. Given intravenously, some bisphosphonates (in particular zoledronate) can lead to osteonecrosis of the jaw.
Clinical use
Alendronate and risedronate are given orally for prophylaxis and treatment of osteoporosis. Etidronate is an alternative. Clodronate is used in patients with malignant disease involving bone and pamidronate is given by intravenous infusion to treat hypercalcaemia of malignancy or for Paget’s disease. Ibandronate is given intravenously every 3–4 weeks in patients with breast cancer metastatic to bone or every 3 months to treat postmenopausal osteoporosis. Zoledronate, which is given as an intravenous infusion, is used for advanced malignancy involving bone, for Paget’s disease and selected cases of osteoporosis (postmenopausal or in men) when it is administered once a year (see clinical box.)
Bisphosphonates ![]()
Clinical uses of bisphosphonates ![]()
Oestrogens and Related Compounds
The decline in oestrogen levels is a major factor in postmenopausal osteoporosis, and there is evidence that giving oestrogen as hormone replacement therapy (HRT; see Ch. 34) can ameliorate this condition. But HRT has actions on many systems, and newer non-hormonal agents (e.g. raloxifene, see Ch. 34) have now been developed that exhibit agonist actions on some tissues and antagonist actions on others. These are termed selective oestrogen receptor modulators (SERMs). The most important of these is raloxifene (Ch. 34).
Raloxifene
Raloxifene is a SERM that has agonist activity on bone, stimulating osteoblasts and inhibiting osteoclasts. It also has agonist actions on the cardiovascular system, and antagonist activity on mammary tissue and the uterus.
It is well absorbed in the gastrointestinal tract, and undergoes extensive first-pass metabolism in the liver to give the glucuronide—resulting in only about 2% bioavailability. Colestyramine (Ch. 23), given with it, reduces the enterohepatic cycling of raloxifene by 60%.
Raloxifene is widely distributed in the tissues, and is converted to an active metabolite in liver, lungs, bone, spleen, uterus and kidney. Its half-life averages 32 h. It is excreted mainly in the faeces.
Unwanted effects include hot flushes, leg cramps, flu-like symptoms and peripheral oedema. Less common are thrombophlebitis and thromboembolism. Other rarer adverse effects are thrombocytopenia, gastrointestinal disturbances, rashes, raised blood pressure and arterial thromboembolism. It is not recommended for primary prevention of osteoporotic fractures, but is one alternative to a bisphosphonate for secondary prevention in postmenopausal women who cannot tolerate a bisphosphonate.
Parathyroid Hormone and Teriparatide
PTH and fragments of PTH given in small doses paradoxically stimulate osteoblast activity and enhance bone formation, and are used to treat selected patients with osteoporosis. The main compound currently used is teriparatide—the peptide fragment (1–34) of recombinant PTH. A new peptide analogue (ostabolin—cyclic PTH1–35, which increases bone mass with less effect on plasma calcium concentration than PTH) is in development.
Teriparatide has anabolic effects on bone. It reverses osteoporosis by stimulating new bone formation (Yasothan & Santwana, 2008). It increases bone mass, structural integrity and bone strength by increasing the number of osteoblasts and by activating those osteoblasts already in bone. It also reduces osteoblast apoptosis.
It acts on the G-protein-coupled receptor PTH1 in the membrane of target cells, and its effects are mediated through adenylyl cyclase, phospholipases A, C and D, and increases in intracellular Ca2+ and cyclic AMP (see Brixen et al., 2004; Deal, 2009).
Teriparatide is given subcutaneously once daily. It is well tolerated, and serious adverse effects are few. Nausea, dizziness, headache and arthralgias can occur. Mild hypercalcaemia, transient orthostatic hypotension and leg cramps have been reported.
Teriparatide is used to treat osteoporosis. There is controversy as to whether or not this drug should be given sequentially or in combination with one of the bisphosphonates (Heaney & Recker, 2005); however, a bisphosphonate should be given at the end of a course of teriparatide to prevent bone loss due to teriparatide withdrawal.
Strontium Ranelate
Strontium (given as the ranelate salt) inhibits bone resorption and also stimulates bone formation. In recent trials, it has been shown to be effective in preventing vertebral and non-vertebral fractures in older women (see Fogelman & Blake, 2005). It is approved in the UK and recommended by the National Institute for Health and Clinical Excellence as an alternative to a bisphosphonate in primary or secondary prevention of osteoporotic fractures, when a bisphosphonate is not tolerated, although some authors consider it to be first-line treatment for osteoporosis because of its positive risk–benefit ratio (Reginster et al., 2009).
The precise mechanism of action is not clear. Like calcium it is absorbed from the intestine, incorporated into bone and excreted via the kidney. Strontium atoms are adsorbed onto the hydroxyapatite crystals, but eventually they exchange for calcium in the bone minerals and remain in the bone for many years.
The drug is well tolerated; a low incidence of nausea and diarrhoea is reported.
Vitamin D Preparations
Vitamin D preparations are used in the treatment of vitamin D deficiencies, bone problems associated with renal failure and hypoparathyroidism—acute hypoparathyroidism necessitating the use of intravenous calcium and injectable vitamin D preparations.
The main vitamin D preparation used clinically is ergocalciferol. Other preparations are alfacalcidol and calcitriol. All can be given orally and are well absorbed from the intestine. Vitamin D preparations are fat soluble, and bile salts are necessary for absorption. Injectable forms are available. A vitamin D analogue with less potential to cause hypercalcaemia is the vitamin D sterol, paracalcitol (Salusky, 2005).
Given orally, vitamin D is bound to a specific α-globulin in the blood. The plasma half-life is about 22 h, but vitamin D can be found in the fat for many months. The main route of elimination is in the faeces.
The clinical uses of vitamin D preparations are given in the box.
Excessive intake of vitamin D causes hypercalcaemia. If hypercalcaemia persists, calcium salts are deposited in the kidney and urine, causing renal failure and kidney stones.
Clinical uses of vitamin D ![]()
Plasma Ca2+ levels should be monitored during therapy with vitamin D.
Calcitonin
The main preparation available for clinical use (see the clinical box) is salcatonin (synthetic salmon calcitonin). Synthetic human calcitonin is now also available. Calcitonin is given by subcutaneous or intramuscular injection, and there may be a local inflammatory action at the injection site. It can also be given intranasally. Its plasma half-life is 4–12 min, but its action lasts for several hours.
Unwanted effects include nausea and vomiting. Facial flushing may occur, as may a tingling sensation in the hands and an unpleasant taste in the mouth.
Calcium Salts
Calcium salts used therapeutically include calcium gluconate and calcium lactate, given orally. Calcium gluconate is also used for intravenous injection in emergency treatment of hyperkalaemia (Ch. 28); intramuscular injection is not used, because it causes local necrosis.
Calcium carbonate, an antacid, is usually poorly absorbed in the gut, but there is concern about possible systemic absorption with the potential to cause arterial calcification.
Unwanted effects: oral calcium salts can cause gastrointestinal disturbance. Intravenous administration requires care, especially in patients on cardiac glycosides (see Ch. 21).
The clinical uses of the calcium salts are given in the clinical box.
Clinical uses of calcium salts ![]()
Calcimimetic Compounds
Calcimimetics enhance the sensitivity of the parathyroid Ca2+-sensing receptor to the concentration of blood Ca2+. The effect is to decrease the secretion of PTH and reduce the serum Ca2+ concentration. There are two types of calcimimetics:
Potential New Therapies
The recent substantial increase in the understanding of bone remodelling (Deal, 2009; Yasothan & Kar, 2008) has opened possible therapeutic approaches that may yield new drugs for clinical use in the foreseeable future. These include:
Other promising targets are discussed by Deal (2009).
References and Further Reading
Bone disorders and bone remodelling
Boyce B.F., Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys.. 2008;473:139-146. (Good review of the role of the RANK/RANKL/OPG in osteoclast formation and the transcription factors involved)
Compston J. Clinical and therapeutic aspects of osteoporosis. Eur. J. Radiol.. 2009;71:388-391. (Clear brief coverage of epidemiology, clinical aspects, pathogenesis and drugs currently used)
Deal C. Potential new drug targets for osteoporosis. Nat. Clin. Pract. Rheumatol.. 2009;5:174-180. (Outstanding review; good diagrams)
Deftos L.J. Treatment of Paget’s disease—taming the wild osteoclast. N. Engl. J. Med.. 2005;353:872-875. (Editorial covering the use of OPG and zoledronic acid for Paget’s disease; excellent diagram. See also article by Cundy et al. in the same issue, pp. 918–923)
Gallagher J.C. Advances in bone biology and new treatments for bone loss. Maturitas. 2008;20:65-69. (Article on preventing bone loss by targeting the RANK/RANKL/OPG system with denosumab)
Heaney R.P., Recker R.R. Combination and sequential therapy for osteoporosis. N. Engl. J. Med.. 2005;353:624-625. (Editorial)
Horowitz M.C., Xi Y., Wilson K., Kacena M.A. Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands. Cytokine Growth Factor Rev.. 2001;12:9-18. (Worthwhile minireview, good diagram)
Khosla K., Westendorf J.J., Oursler M.J. Building bone to reverse osteoporosis and repair fractures. J. Clin. Invest.. 2008;118:421-428. (Good review; covers the role of Wnt signalling and sclerostin secretion)
Reichel H., Koeftler H.P., Norman A.W. The role of the vitamin D endocrine system in health and disease. N. Engl. J. Med.. 1989;320:980-991. (Outstanding comprehensive early review)
Reid R. Anti-resorptive therapies for osteoporosis. Semin. Cell Dev. Biol.. 2008;19:5473-5478. (Excellent review of the actions of current and novel anti-resorptive drugs)
Roodman G.D. Mechanisms of bone metastasis. N. Engl. J. Med.. 2004;350:1655-1664. (Has excellent section on bone remodelling, with good diagrams)
Salusky I.B. Are new vitamin D analogues in renal bone disease superior to calcitriol? Pediatr. Nephrol.. 2005;20:393-398.
Stewart J.F. Translational implications of the parathyroid calcium receptor. N. Engl. J. Med.. 2004;351:324-326. (Succinct article with useful diagram)
Tolar J., Teitelbaum S.L., Orchard P.J. Osteopetrosis. N. Engl. J. Med.. 2004;351:2839-2849. (Very good review; has excellent section on the biology of osteoclasts, with very good diagrams)
van Staa T.P., Dennison E.M., Leufkens H.G., Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522.
Wright H.L., McCarthy H.S., Middleton J., Marshall M.J. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr. Rev. Musculoskelet. Med.. 2009;2:56-64. (Synopsis of the structures of RANK, RANKL and OPG, and the intracellular RANK/RANKL signalling pathways with a review of diseases linked to their malfunction)
Drugs used to treat bone disorders
Brennan T.C., Rybchyn M.S., Green W., et al. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol.. 2009;57:1291-1300. (A study in human cells showing that strontium ranelate acts at least in part by activating the calcium-sensing receptor)
Brixen K.T., Christensen P.M., Ejersted C., Langdahl B.L. Teriparatide (biosynthetic human parathyroid hormone 1–34): a new paradigm in the treatment of osteoporosis. Basic Clin. Pharmacol. Toxicol.. 2004;94:260-270. (A minireview of the action, mechanism of action, clinical studies and adverse effects)
Clemett D., Spenser C.M. Raloxifene: a review of its use in postmenopausal osteoporosis. Drugs. 2000;60:379-411. (Comprehensive review covering the mechanism of action, pharmacology, pharmacokinetic aspects, therapeutic use and adverse effects of raloxifene)
Cummings S.R., San Martin J., McClung M.R., et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med.. 2009;361:818-820. (‘Freedom Trial’ with 239 collaborators. Denosumab was effective in reducing fracture risk in women with osteoporosis)
Fogelman I., Blake G.M. Strontium ranelate for the treatment of osteoporosis. Br. Med. J.. 2005;330:1400-1401. (Crisp editorial analysis)
Khosla K. Increasing options for the treatment of osteoporosis. N. Engl. J. Med.. 2009;361:818-820. (Useful editorial)
Nemeth E.F., Heaton W.H., Miller M., et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J. Pharmacol. Exp. Ther.. 2004;398:627-635. (Detailed study of pharmacokinetic aspects and the pharmacological action of cinacalcet hydrochloride)
Peacock M., Bilezikian J.P., Klassen P.S., et al. Cinacalcet hydrochloride maintains long-term normocalcaemia in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab.. 2005;90:135-141.
Quattrocchi E., Kourlas H. New drugs. Teriparatide: a review. Clin. Ther.. 2004;26:841-854. (Excellent review)
Reginster J.Y., Deroisy R., Neuprez A., et al. Strontium ranelate: new data on fracture prevention and mechanisms of action. Curr. Osteoporos. Rep.. 2009;7:96-102. (Stresses that in 5-year studies this drug has proved efficacious in both decreasing bone reabsorption and stimulating bone formation, and has a positive risk–benefit ratio)
Rogers M.J. New insights into the mechanisms of action of the bisphosphonates. Curr. Pharm. Des.. 2003;9:2643-2658. (Covers the different mechanisms of action of the simple bisphosphonates and the nitrogen-containing bisphosphonates)
Strewler G.J. Decimal point—osteoporosis therapy at the 10-year mark. N. Engl. J. Med.. 2005;350:1172-1174. (Crisp article concentrating mainly on bisphosphonates; excellent diagram of bisphosphonate action)
Whyte M.P. The long and the short of bone therapy. N. Engl. J. Med.. 2006;354:860-863. (Succinct article on the present status of and future possibilities for bone therapy. Excellent diagram)
Yasothan U., Kar S. Osteoporosis: overview and pipeline. Nat. Rev. Drug. Discov.. 2008;7:725-726.
Yasothan U., Santwana K. From the analyst’s couch. Osteoporosis: overview and pipeline. Nat. Rev. Drug. Discov.. 2008;7:725-726. (Crisp outline of current antiosteoporosis drugs with table of new drugs in phase I and phase II development)
1RANKL is also sometimes confusingly termed OPG ligand.