Vitamin K Administration
Shortly after birth, vitamin K is administered to prevent hemorrhagic disease of the newborn. (See Chapter 9.) Normally, the intestinal flora synthesizes vitamin K. However, because the infant’s intestine is presumably sterile at birth and because breast milk contains low levels of vitamin K, the supply is inadequate for at least the first 3 to 4 days. The major function of vitamin K is to catalyze the synthesis of prothrombin in the liver, which is needed for blood clotting. The vastus lateralis muscle is the traditionally recommended injection site, but the ventrogluteal (not dorsogluteal) muscle can be used.
Several countries have noted a resurgence in later onset of vitamin K deficiency bleeding (VKDB) after practicing orally administered prophylaxis (American Academy of Pediatrics, 2003c). Current recommendations are that vitamin K be given to all newborns as a single intramuscular dose of 0.5 to 1.0 mg (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007). Additional study is needed on the efficacy, safety, and bioavailability of oral preparations and on the most effective dosing regimens to prevent VKDB (see Research Focus box).
Hepatitis B Vaccine Administration
To decrease the incidence of hepatitis B virus (HBV) in children and its serious consequences (cirrhosis and liver cancer) in adulthood, the first of three doses of HBV vaccine is recommended between birth and 2 months of age for all newborns born to hepatitis B surface antigen (HBsAg)–negative mothers. The injection is given in the vastus lateralis muscle, since this site is associated with a better immune response than the dorsogluteal area (although the dorsogluteal muscle typically is not used in infants in the United States) (American Academy of Pediatrics, 2009b). (See Immunizations, Chapter 12.) Giving the infant concentrated oral sucrose can reduce the pain of the injection (Stevens, Yamada, and Ohlsson, 2004). Preterm infants born to HBsAg-negative mothers should be vaccinated as early as 30 days of age regardless of gestational age or birth weight. Preterm infants weighing less than 2000 g (4.4 lb) who are ready to be released from hospital should receive hepatitis B vaccine just before hospital discharge. Infants born to HBsAg-positive mothers should be immunized within 12 hours after birth with HBV vaccine and hepatitis B immune globulin at separate sites, regardless of gestational age or birth weight (American Academy of Pediatrics, 2009b).
Newborn Screening for Disease
Blood sampling can detect a large number of congenital disorders in the newborn period so that early intervention can take place to decrease the long-term effects and cost of not treating such conditions. Currently no national policy regulates newborn screening; therefore the extent of screening has been largely determined by state laws and individual practice. All states now mandate screening tests for phenylketonuria (PKU) and congenital hypothyroidism (see Chapters 9 and 35); many states also have programs that include screening for sickle cell disease and galactosemia. Because of concern regarding the inconsistency among states in screening for such conditions based on cost, population demographics, resource availability, and political environment, the Task Force on Newborn Screening was formed by the American Academy of Pediatrics and other federal health care agencies and has developed a number of resolutions and policies to better address the issue of newborn screening (American Academy of Pediatrics, 2008b).
The advent of tandem mass spectrometry has expanded newborn screening to include detection of disorders of fatty acid oxidation, amino acids, and organic acids. This technology uses a minimum amount of blood and can identify more than 40 different disorders in 2 minutes (Bryant, Horns, Longo, et al, 2004). Tandem mass spectrometry has improved sensitivity and specificity for the detection of such conditions as hyperphenylalaninemia (PKU) and has a lower rate of false-positive results than other standardized testing methods.
The nurse’s responsibility is to educate parents regarding the importance of screening and to collect appropriate specimens at the recommended time (after 24 hours of age or after the introduction of feedings; hospitalized infants must be screened before 7 days of age). With early newborn discharge before 24 hours, adequate screening for PKU requires a follow-up test within 2 weeks (Kaye and American Academy of Pediatrics, 2006). Accurate screening depends on high-quality blood spots on approved filter paper forms. The blood should completely saturate the filter paper spot on one side only. The paper should not be handled, placed on wet surfaces, or contaminated with any substance (see Atraumatic Care box). The American Academy of Pediatrics (2008a) recommends routine prenatal and perinatal human immunodeficiency virus (HIV) counseling and testing for all pregnant women. Benefits of early identification of HIV-infected infants include:
• Early antiretroviral therapy and aggressive nutritional supplementation
• Appropriate changes in their immunization schedule
• Monitoring and evaluation of immunologic, neurologic, and neuropsychologic functions for possible changes caused by antiretroviral therapy
• Initiation of special educational services
• Evaluation of the need for other therapies, such as immunoglobulin for the prevention of bacterial infections
Cesarean section, performed before the rupture of membranes or the onset of labor, may prevent mother-to-child transmission of HIV in optimally treated women and is associated with a 50% or more reduction in the risk of mother-to-child transmission among HIV-infected women who are either not receiving antiretroviral therapy or are receiving minimal therapy. For infants whose mother’s HIV status is unknown, rapid HIV antibody testing provides information within 12 hours of the infant’s birth. Antiretroviral prophylaxis is started as soon as possible, pending completion of confirmatory HIV testing. Breast-feeding is delayed until confirmatory testing is done. If the test is negative, prophylaxis is stopped and breast-feeding may start. If the test is positive, infants should be treated with antiretroviral prophylaxis for 6 weeks, and the mother should not breast-feed (American Academy of Pediatrics, 2008a). HIV-exposed infants who test negative initially should undergo further testing at 1 to 2 months and at 4 to 6 months of age to exclude or identify HIV infection (Havens, Mofenson, and Committee on Pediatric AIDS, 2009). For information on several diseases that may be included in newborn screening, see American Academy of Pediatrics’ Introduction to the Newborn Screening Fact Sheets (Kaye and American Academy of Pediatrics, 2006).
Universal Newborn Hearing Screening
It has been estimated that screening children for hearing loss by risk factors alone fails to identify approximately 50% of all newborns with a congenital hearing loss. Furthermore, infants who are hard of hearing or deaf, yet receive intervention before the age of 6 months, maintain appropriate language development matching their cognitive abilities through the age of 5 years (Yoshinaga-Itano, Sedey, Coulter, et al, 1998). For these reasons the American Academy of Pediatrics Joint Committee on Infant Hearing (2007b) recommends universal hearing screening of all newborns before discharge from the birthing hospital. Infants may be screened for hearing loss by auditory brainstem response or evoked otoacoustic emissions. Newborns who fail the initial screening require referral for outpatient retesting and intervention by 1 month of age; newborns who do not receive initial screening before discharge should also be tested by 1 month (American Academy of Pediatrics, 2007b; Connolly, Carron, and Roark, 2005). A subsequent audiologic assessment should be performed at least once by 24 to 36 months of age if the infant has any hearing risk factors despite passing the newborn hearing screening (American Academy of Pediatrics, 2009c).
Bathing
Bath time is an opportunity for the nurse to accomplish much more than general hygiene. It is an excellent time for observing the infant’s behavior, state of arousal, alertness, and muscular activity. Bathing is usually performed after the vital signs, especially the temperature, have stabilized.
With the possibility of transmission of HBV and HIV via maternal blood and blood-stained amniotic fluid, the traditional timing of the newborn’s bath has been questioned. Studies indicate that healthy full-term newborns with a stable body temperature can be bathed as early as 1 hour of age without experiencing problems, provided that effective thermoregulation measures are taken after the bath (Penny-MacGillivray, 1996; Behring, Vezeau, and Fink, 2003; Varda and Behnke, 2000; Medves and O’Brien, 2004). Take caution, however, to avoid instituting routine newborn bathing according to a rigid schedule; nursing interventions such as bathing should instead be based on individualized assessment and family interaction needs.
Because of the possibility of blood and body fluid contagions, as part of Standard Precautions, nurses should wear gloves when handling the newborn until blood and amniotic fluid are removed by bathing.
The bath time provides an opportunity for the nurse to involve the parents in the care of their child, to teach correct hygiene procedures, and to help them learn about their infant’s individual characteristics (Fig. 8-11). The bath may also be used to help parents learn and better understand their newborn’s behavioral characteristics using the BNBAS. The nurse stresses appropriate bathing supplies and the need for safety in terms of water temperature and supervision of the infant at all times during the bath.
Encourage parents to examine every finger and toe of their infant during bathing. Frequently normal variations such as milia, erythema toxicum (rash), or “stork bites” worry parents who are unaware of the insignificance of such findings. Minor birth injuries may appear as major defects to them. Explaining how these occurred and when they will disappear reassures parents of their infant’s normalcy. Chapter 9 discusses common variations.
One of the most important considerations in skin cleansing is preservation of the skin’s “acid mantle,” which is formed by the uppermost horny layer of the epidermis; sweat; superficial fat; metabolic products; and external substances such as amniotic fluid, microorganisms, and chemicals. The infant’s skin surface has a pH of about 5 soon after birth, and the bacteriostatic effects of this pH are significant. In addition, newborn skin is covered with host-defense proteins, such as lysozyme and lactoferrin, which contribute importantly to a newborn’s defense against bacterial infections (Walker, Akinbi, Meinzen-Derr, et al, 2008). Consequently, use only plain warm water for routine bathing. If a cleanser is needed, Dove (fragrance free) has a neutral pH and is mild. Alkaline soaps, oils, powder, and lotions are not used because they alter the acid mantle, thus providing a medium for bacterial growth. Talcum powder has the added risk of aspiration if it is applied close to the infant’s face. Corn starch powder may also cause respiratory problems and aspiration. (See Diaper Dermatitis, Chapter 13.)
Parents should be involved in a discussion regarding the newborn’s bath at home. It is recommended that for the first 2 to 4 weeks the infant be bathed no more than two or three times per week with a plain warm sponge bath. This practice will help maintain the integrity of the newborn’s skin and allow time for the umbilical cord to dry completely. Routine daily bathing for newborns is no longer recommended.
Cleansing should proceed in the cephalocaudal (head-to-toe) direction. Vigorous rubbing to remove vernix is unnecessary and may cause more harm than good. A diaper is applied after the bath, and the infant is clothed appropriately to prevent heat loss.
The nurse should discuss the choice of cloth or disposable diapers with parents. Disposable diapers are the most convenient, although a diaper service eliminates the need to shop for replacement diapers. Disposable diapers with absorbent gelling material have benefits related to preserving healthy skin; preventing diaper dermatitis, especially beyond the neonatal period (see Chapter 13); and controlling contamination of the environment because of their better containment of urine and feces.
Care of the Umbilicus
Because the umbilical stump is an excellent medium for bacterial growth, various methods of cord care have been practiced to prevent infection. Some methods popular in the past include the use of an antimicrobial agent such as bacitracin or triple dye, or agents such as alcohol or povidone. Many studies report that the use of antiseptic agents prolongs cord drying and separation (Zupan, Garner, and Omari, 2004; Dore, Buchan, Coulas, et al, 1998). Although studies regarding bacterial growth and colonization according to the cleansing method used have produced varied results (Janssen, Selwood, Dobson, et al, 2003; Golombek, Brill, and Salice, 2002; Dore, Buchan, Coulas, et al, 1998), a Cochrane Review of 21 studies found no significant difference between cords treated with antiseptics compared with dry cord care or placebo; there were no reported systemic infections or deaths, and a trend towards reduced colonization was found in cords treated with antiseptics (Zupan, Garner, and Omari, 2004). Current recommendations for cord care by the Association of Women’s Health, Obstetric and Neonatal Nurses (2007) includes cleaning the cord initially with sterile water or a neutral pH cleanser, then subsequently cleaning the cord with water.
Nurses working in neonatal care must carefully evaluate the available studies and compare the risks and benefits regarding the method of cord care within their own population of newborns and families. Particularly in the developing world, infants may encounter increased risk of potentially life-threatening sepsis; thus antimicrobial treatment may be appropriate in some settings (Mullany, Darmstadt, Katz, et al, 2009). A recent randomized controlled study showed that use of a newer antimicrobial for cord care, chlorhexidine powder, resulted in faster cord separation time and fewer complications than dry cord care (Kapellen, Gebauer, Brosteanu, et al, 2009). Regardless of the method used, nurses must teach parents about the importance of observation and monitoring of the cord, in addition to cord care methods, in discharge planning.
The diaper is placed below the cord to avoid irritation and wetness on the site. Parents are instructed regarding stump deterioration and proper umbilical care. The stump deteriorates through the process of dry gangrene. Cord separation time is influenced by a number of factors, including type of cord care, type of delivery, and other perinatal events. The average cord separation time is 5 to 15 days. It takes a few more weeks for the cord base to heal completely after cord separation. During this time, care consists of keeping the base clean and dry and observing for any signs of infection.
With early hospital discharge, newborns may be discharged before it is safe to remove the cord clamp. Teach the parent how to safely remove the clamp once the newborn is at least 24 hours old and no oozing from the cord is evident.
Circumcision
![]() Circumcision, the surgical removal of the foreskin on the glans penis, is usually done in the hospital, although it is not a common practice in most countries. In the United States, however, circumcision rates have increased significantly over time: 61.1% of U.S. boys born from 1997 to 2000 were circumcised, compared with 48.3% of U.S. boys born from 1988 to 1991 (Nelson, Dunn, Wan, et al, 2005). Despite the frequency of the procedure in the United States, there is still much controversy regarding the benefits and risks (Box 8-5). One study that received considerable criticism demonstrated a ninefold increase in urinary tract infections in uncircumcised boys during the first year of life (Bartman, 2001; Schoen, Colby, and Ray, 2000). Other researchers have responded that such infections are best prevented by practicing good hygiene, rather than advocating an invasive procedure such as circumcision (Kinkade, Meadows, and Gracia-Trujillo, 2005).
Circumcision, the surgical removal of the foreskin on the glans penis, is usually done in the hospital, although it is not a common practice in most countries. In the United States, however, circumcision rates have increased significantly over time: 61.1% of U.S. boys born from 1997 to 2000 were circumcised, compared with 48.3% of U.S. boys born from 1988 to 1991 (Nelson, Dunn, Wan, et al, 2005). Despite the frequency of the procedure in the United States, there is still much controversy regarding the benefits and risks (Box 8-5). One study that received considerable criticism demonstrated a ninefold increase in urinary tract infections in uncircumcised boys during the first year of life (Bartman, 2001; Schoen, Colby, and Ray, 2000). Other researchers have responded that such infections are best prevented by practicing good hygiene, rather than advocating an invasive procedure such as circumcision (Kinkade, Meadows, and Gracia-Trujillo, 2005).
![]() Critical Thinking Exercise—Circumcision
Critical Thinking Exercise—Circumcision
Recently, research has explored the possible link between circumcision and reduced transmission of communicable illnesses such as human papillomavirus (HPV) and HIV in later life. Several researchers report that circumcision is associated with reduced likelihood of transmission of HPV in men known to be exposed (Lu, Wu, Nielson, et al, 2009; Nielson, Schiaffino, Dunne, et al, 2009). Warner, Ghanem, Newman, and colleagues (2009) report that circumcision is associated with substantially reduced transmission of HIV in men known to be exposed. There is concern, however, regarding findings from such observational studies, since there is no opportunity for control of confounding variables (factors other than circumcision that explain the results). A Cochrane Review cautioned against adoption of circumcision as a public health measure until stronger prospective studies demonstrate a clear link between lack of circumcision and disease transmission (Siegfried, Muller, Volmink, et al, 2003). Since that caution, some prospective studies have reported reduced risk of HIV transmission after adult circumcision in high-risk groups (Bailey, Moses, Parker, et al, 2007; Gray, Kigozi, Serwadda, et al, 2007). More research is needed to better understand any possible link between neonatal circumcision and subsequent risk of sexually transmitted infection.
The American Academy of Pediatrics (1999) issued a circumcision policy statement stating that the medical benefits of male newborn circumcision are not sufficiently significant to recommend it as a routine procedure. The academy statement emphasizes parental autonomy to determine what is in the best interest of their newborn boy. The policy encourages the physician to ensure that parents have been given accurate and unbiased information about the risks, benefits, and alternatives before making an informed choice and that they understand that circumcision is an elective procedure. In addition to examining the medical benefits of newborn circumcision, the academy recommended that if parents decide to have their male infant circumcised, procedural analgesia should be provided.
This policy statement has direct implications for nurses caring for newborns and their families. First, because nurses are in a unique position to educate parents regarding the care of their newborns, they must take responsibility for ensuring that each parent has accurate and unbiased information on which to make an informed decision. Parents need to know the options for pain control, especially the choice of topical or injected anesthesia, and their option of observing the procedure.*
Second, the nurse should use nonpharmacologic interventions as an adjunct to reduce the pain of this operative procedure (see Atraumatic Care box). Despite adequate scientific evidence that newborns feel and respond to pain, circumcisions are still performed in the United States with either insufficient analgesia or no analgesia at all. Nurses can use the academy’s policy statement to advocate more effectively for the use of optimum pain relief during circumcision.
Four types of anesthesia and analgesia are used in newborns undergoing circumcision: ring block, dorsal penile nerve block (DPNB), topical anesthetic such as EMLA (prilocaine-lidocaine), and oral sucrose. Oral acetaminophen and comfort measures such as music, sucking on a pacifier, and soothing voices have not proved to be effective in reducing the pain of circumcision when used alone (Williamson, 1997).
A Cochrane Review (Brady-Fryer, Wiebe, and Lander, 2004) reports that DPNB is the most frequently studied intervention and the most effective method of preventing circumcision pain. Ring block is also effective in reducing pain and is reported to be technically easier and potentially safer because it eliminates the risk of injecting lidocaine into the dorsal vessels. The topical application of EMLA to the penis before circumcision has also been helpful in reducing operative pain (Taddio, Ohlsson, and Ohlsson, 2000). An occlusive dressing must be placed over the cream, which must be applied approximately 1 hour before the procedure. Although this preparation may be perceived as complicated and requiring too much advance notice, it is important to remember that most newborns are kept NPO (nothing by mouth) for 1 to 2 hours before the procedure to prevent aspiration. In clinical practice, however, the issues of difficulty in application and time required to reach maximum effect may result in EMLA being used less often as an anesthetic (Brady-Fryer, Wiebe, and Lander, 2004). The use of EMLA cream for neonatal circumcision has not been associated with methemoglobinemia, a serious but rare complication associated with prilocaine. A localized rash has been associated with EMLA when used for circumcision.
A nonpharmacologic strategy for providing pain relief for circumcision is the use of intraoral sucrose. The administration of a concentrated dose of oral sucrose and nonnutritive sucking have proved more effective than no treatment in decreasing procedural pain (venipuncture, heel stick, circumcision) in full-term and preterm infants in many studies (Herschel, Khoshnood, Ellman, et al, 1998; Stevens, Taddio, Ohlsson, et al, 1997; Stevens, Johnston, Franck, et al, 1999). A Cochrane Review (Stevens, Yamada, and Ohlsson, 2004) concludes that oral sucrose is safe and effective for reducing procedural pain for single painful events, although the optimum dose for maximum pain relief is not yet known. Research addressing the use of sucrose as an adjunct to other nonpharmacologic and pharmacologic pain management methods is needed. Many nurses use intraoral sucrose to reduce the pain associated with procedures such as circumcision, venipuncture, and heel stick.
The Cochrane group exploring pain relief for neonatal circumcision (Brady-Fryer, Wiebe, and Lander, 2004) recommends that future research focus on benefits of infants receiving two or more active interventions for pain relief, and states that a placebo or no-treatment group is no longer acceptable. Studies exploring the use of several strategies concurrently, such as that conducted by Razmus, Dalton, and Wilson (2004), which included groups receiving both sucrose and ring block compared with ring block alone, have the most potential to clarify optimum strategies.
A recent Cochrane Review on pain relief for boys undergoing circumcision emphasized the need for postoperative pain relief (Cyna and Middleton, 2008). The authors advocate anticipating and controlling postoperative pain both to enhance patient comfort and to decrease crying and agitation that may increase the risk of postoperative bleeding.
Circumcision is usually performed in the nursery. It should not be performed immediately after delivery because of the neonate’s unstable physiologic status and increased susceptibility to stress. Preoperative nursing care includes allowing the infant nothing by mouth before the procedure to prevent aspiration of vomitus (about 1 to 2 hours); however, the necessity of this practice has been challenged (Kraft, 2003). Additional measures include the surgical time-out, checking for a signed consent form, and adequately restraining the infant, usually on a special board (Fig. 8-12) or physiologic circumcision restraint chair. The circumcision chair is padded and allows free movement of the newborn’s extremities without compromising the surgical field. In addition, the chair allows the infant to sit at a 30- to 45-degree angle, and it is adjustable to accommodate smaller newborns (Stang, Snellman, Condon, et al, 1997). All the equipment used for the procedure, such as gloves, instruments, dressings, and draping towels, must be sterile.

Fig. 8-12 Proper positioning of infant in Circumstraint. (Courtesy Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
The procedure involves freeing the foreskin from the glans penis by using a scalpel, Gomco or Mogen clamp (see Cultural Competence box), or Plastibell. In the Gomco technique the foreskin is clamped, cut with a scalpel, and removed; the clamp crushes the nerve endings and blood vessels, promoting hemostasis. In the Plastibell procedure the foreskin is removed using a plastic ring and a string tied around the foreskin like a tourniquet. The excess foreskin is trimmed. In about 5 to 8 days the plastic ring separates and falls off.
Once the procedure is completed, the infant is released from the restraints and comforted. If the parents were not present during the procedure, they are informed of the infant’s status and reunited with their son.
Care of the circumcision depends on the type of procedure. If a clamp (Gomco or Mogen) was used, a petrolatum gauze dressing may be applied loosely to prevent adherence to the diaper. If the Plastibell was applied, no special dressing is required. Because the area is tender, the diaper is applied loosely to prevent friction against the penis. The circumcision is evaluated for excessive bleeding in the first few hours after the procedure, and the first void is recorded. A recommended standard is to evaluate the site every 30 minutes for at least 2 hours and then at least every 2 hours thereafter (Williamson, 1997).
Normally, on the second day a yellowish white exudate forms as part of the granulation process. This is not a sign of infection and is not forcibly removed. As healing progresses, the exudate disappears. Parents are educated to report any evidence of bleeding, unusual swelling, or absence of voiding to the practitioner.


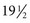 oz of water and adding 3 tbsp of sugar or commercially processed corn syrup.
oz of water and adding 3 tbsp of sugar or commercially processed corn syrup.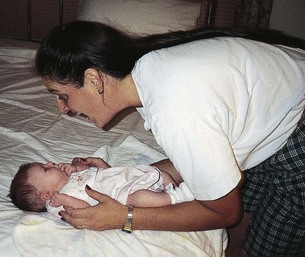


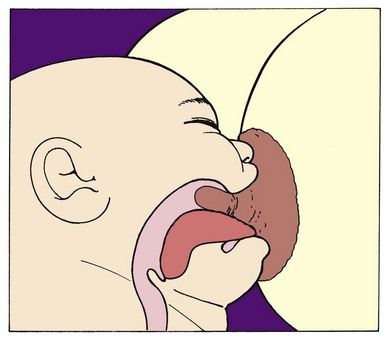
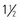 to 3 hours daily. Swallowing should be audible.
to 3 hours daily. Swallowing should be audible.