Health Problems During Infancy
http://evolve.elsevier.com/wong/ncic
Anaphylaxis, Ch. 29
Apnea of Prematurity, Ch. 10
Asthma, Ch. 32
Autistic Spectrum Disorders (Autism), Ch. 24
Breast-Feeding Problems, Ch. 8
Candidiasis, Ch. 9
Cardiopulmonary Resuscitation, Ch. 31
The Child with Cognitive, Sensory, or Communication Impairment, Ch. 24
Diarrhea, Ch. 29
Disorders Affecting the Skin, Ch. 18
Family-Centered Care of the Child with Chronic Illness or Disability, Ch. 22
Feeding Resistance, Ch. 10
Gastroesophageal Reflux, Ch. 33
Iron Deficiency Anemia, Ch. 35
Nutritional Assessment, Ch. 6
Nutritional Disturbances
Although true vitamin deficiencies are rare in the United States, subclinical deficiencies are commonly seen in population subgroups in which either maternal or child dietary intake is imbalanced and contains inadequate amounts of vitamins. Vitamin D–deficiency rickets, once rarely seen because of the widespread commercial availability of vitamin D–fortified milk, increased before the turn of the century. Populations at risk include:
• Children who are exclusively breast-fed by mothers with an inadequate intake of vitamin D or are exclusively breast-fed longer than 6 months without adequate maternal vitamin D intake or supplementation
• Children with dark skin pigmentation who are exposed to minimal sunlight because of socioeconomic, religious, or cultural beliefs or housing in urban areas with high levels of pollution
• Children with diets that are low in sources of vitamin D and calcium
• Individuals who use milk products not supplemented with vitamin D (e.g., yogurt,* raw cow’s milk) as the primary source of milk
The American Academy of Pediatrics (2008) now recommends that infants who are exclusively breast-fed should receive 400 international units of vitamin D beginning shortly after birth to prevent rickets and vitamin D deficiency. Vitamin D supplementation should continue until the infant is consuming at least 1 L/day (or 1 quart/day) of vitamin D–fortified formula (American Academy of Pediatrics, 2008). Non-breast-fed infants who are taking less than 1 L/day of vitamin D–fortified formula should also receive a daily vitamin D supplement of 400 international units. Inadequate maternal ingestion of cobalamin (vitamin B12) may contribute to infant neurologic impairment when exclusive breast-feeding (past 6 months) is the only source of the infant’s nutrition (Centers for Disease Control and Prevention, 2003).
Children may also be at risk secondary to disorders or their treatment. For example, vitamin deficiencies of the fat-soluble vitamins A and D may occur in malabsorptive disorders. Preterm infants may develop rickets in the second month of life as a result of inadequate intake of vitamin D, calcium, and phosphorus. Children receiving high doses of salicylates may have impaired vitamin C storage. Environmental tobacco smoke exposure has been implicated in decreased concentrations of ascorbate in children; therefore increased intake of sources of vitamin C should be encouraged even in children minimally exposed to environmental tobacco smoke (Preston, Rodriguez, and Rivera, 2006; Preston, Rodriguez, Rivera, et al, 2003). Children with chronic illnesses resulting in anorexia, decreased food intake, or possible nutrient malabsorption as a result of multiple medications should be carefully evaluated for adequate vitamin and mineral intake in some form (parenteral or enteral).
Children with sickle cell disease are reported to have suboptimal intakes (according to dietary reference intake [DRI] recommendations) of vitamins E and D, folate, calcium, and fiber, which decrease significantly with increasing age. Poor dietary intake was a significant factor in the study’s findings (Kawchak, Schall, Zemel, et al, 2007).
Vitamin A deficiency has been reported with increased morbidity and mortality in children with measles. However, a Cochrane review of studies wherein a single dose of vitamin A was administered to children with measles found no decrease in mortality. Children with measles under the age of 2 years who received two doses of vitamin A (200,000 international units) on consecutive days did have decreased mortality rates and a reduced rate of pneumonia-specific mortality (Huiming, Chaomin, and Meng, 2005). Complications from diarrhea and infections are often increased in infants and children with vitamin A deficiency. Although scurvy (caused by a deficiency of vitamin C) is rare in developed countries, cases have been reported in children who were fed an organic diet deficient in vegetables and fruits (Burk and Molodow, 2007).
An excessive dose of a vitamin is generally defined as 10 or more times the recommended dietary allowance (RDA), although the fat-soluble vitamins, especially A and D, tend to cause toxic reactions at lower doses. With the addition of vitamins to commercially prepared foods, the potential for hypervitaminosis has increased, especially when combined with the excessive use of vitamin supplements. Hypervitaminosis of A and D presents the greatest problems, since these fat-soluble vitamins are stored in the body. High intakes of vitamin A have been linked to physeal growth arrest, which can lead to osteoporosis, fracture, and metaphyseal irregularity (Saltzman and King, 2007). Vitamin D is the most likely of all vitamins to cause toxic reactions in relatively small overdoses. The water-soluble vitamins, primarily niacin, B6, and C, can also cause toxicity. Poor outcomes in infants (e.g., fatal hypermagnesemia) have been associated with megavitamin therapy with high doses of magnesium oxide (McGuire, Kulkarni, and Baden, 2000), and severe anemia and thrombocytopenia have resulted from megadoses of vitamin A (Perrotta, Nobili, Rossi, et al, 2002).
One vitamin supplement that is recommended for all women of childbearing age is a daily dose of 0.4 mg of folic acid, the usual RDA. Folic acid taken before conception and during early pregnancy can reduce the risk of neural tube defects such as spina bifida by as much as 70%. Drugs such as oral contraceptives and antidepressants may decrease folic acid absorption; thus adolescent females taking such medications should consider supplementation. (See Spina Bifida and Myelodysplasia, Chapter 11.) General nursing care management is discussed on p. 524.
Complementary and Alternative Medicine
The misuse or overuse of vitamins as a part of complementary and alternative medicine (CAM) places some children at risk for health problems. One survey found that a relatively small group of parents routinely gave their children megavitamin therapy; however, the researcher recommends further research to ascertain a realistic number of children using multivitamin preparations (Loman, 2003). Sawni, Ragothaman, Thomas, and colleagues (2007) noted that of persons reportedly using CAM, the most common CAM remedies reportedly used in children seen in the emergency department for other health problems were home or folk remedies (59%), herbs (41%), prayer for healing (14%), and massage therapy (10%). A survey in a Women, Infants, and Children (WIC) clinic found that child herbal use was common, especially among Hispanic children attending the clinic. Some of the herbs used by the children in the survey (St. John’s wort, dong quai, and kava) have questionable safety (Lohse, Stotts, and Priebe, 2006).
There is concern among health care workers that terms often used to market supplements such as megavitamins may mislead parents regarding the actual benefits (or harm) of such therapies. The intention herein is not to discredit the use of CAM such as vitamin supplements; rather, it is to ensure safety and efficacy in children and to avoid inadvertent harm. The use of various herbal therapies, or intake of herbs, is also becoming more popular; many of these supplements have been a part of medicine since early days and are beneficial in some cases.
The use of herbs by lactating mothers to increase breast milk supply is reportedly increasing. The galactogogues fenugreek, blessed thistle, fennel, and chaste tree have been purported to increase maternal milk supply; however, few studies support the efficacy or safety of these herbs in breast-feeding infants. Fenugreek has been the most widely studied, yet it may have adverse effects such as colic and diarrhea in breast-feeding infants (Conover and Buehler, 2004; Lawrence and Lawrence, 2005). For a discussion of galactogogues, including those mentioned previously, see Appendix P in Lawrence and Lawrence (2005).
Herbs known to have adverse effects in children include ephedra, comfrey, and pennyroyal; some herbs may not be harmful taken alone but may counteract or potentiate prescription medications when taken concurrently (Loman, 2003). Parents should be fully informed of the use of herbs to ensure they confer more benefit than potential harm. Health care workers also need to be knowledgeable about the benefits or potential harm in herbs so that they can appropriately counsel parents and address their concerns. Little research has been performed in children on many over-the-counter herbal medicines, yet some herbs are known to cause harm in children (Kemper and Gardiner, 2007; Lanski, Greenwald, Perkins, et al, 2003; Loman, 2003). Parents should be cautioned not to exceed the upper limits of vitamin intake according to the new DRI (see p. 524).*
Mineral Disturbances
A number of minerals are essential nutrients. The macrominerals are those with daily requirements greater than 100 mg, including calcium, phosphorus, magnesium, sodium, potassium, chloride, and sulfur. Microminerals, or trace elements, have daily requirements of less than 100 mg and include several essential minerals and those whose exact role in nutrition is still unclear. The greatest concern with minerals is deficiency, especially iron deficiency anemia. (See Chapter 35.) However, other minerals that may be inadequate in children’s diets, even with supplementation, include calcium, phosphorus, magnesium, and zinc. Low levels of zinc can cause nutritional growth failure (failure to thrive).
The regulation of mineral balance in the body is a complex process. Dietary extremes of mineral intake can cause a number of mineral-mineral interactions that could result in unexpected deficiencies or excesses. For example, excessive amounts of one mineral, such as zinc, can result in a deficiency of another mineral, such as copper, even if sufficient amounts of copper are ingested. This is thought to be a result of competition in the process of absorption because of (1) displacement of one mineral by another on the molecule necessary for their uptake from the lumen in the intestinal cell or (2) competition for pathways through the intestinal wall or into the bloodstream. Therefore megadose therapy with one mineral may not cause adverse effects from an excess but rather from a deficiency in a competing mineral.
Deficiencies can also occur when various substances in the diet interact with minerals. For example, iron, zinc, and calcium can form insoluble complexes with phytates or oxalates (substances found in plant proteins), which impairs the bioavailability of the mineral. This type of interaction is important in vegetarian diets because plant foods, such as soy, are high in phytates. Contrary to popular opinion, spinach is not a rich source of iron or calcium because of its high oxalate content.
Children with certain illnesses are at greater risk for growth failure, especially in relation to bone mineral deficiency as a result of the treatment of the disease, decreased nutrient intake, or decreased absorption of necessary minerals. Those at risk for such deficiencies include children who are receiving or have received radiation and chemotherapy for cancer; children with human immunodeficiency virus (HIV), sickle cell disease, cystic fibrosis, gastrointestinal (GI) malabsorption, or nephrosis; and very low–birth-weight (VLBW) preterm infants.
Box 13-1 lists factors that affect iron absorption. General nursing care management is discussed below, and specific interventions are discussed in the table.
Vegetarian Diets
Vegetarian diets have become increasingly popular in the United States because people are concerned about hypertension; cholesterol; obesity; cardiovascular disease; the animal rights movement; and cancer of the stomach, intestine, and colon. In one survey, adolescent vegetarians were more likely than nonvegetarians to meet the Healthy People 2010 objectives for overall nutrient consumption (Perry, McGuire, Neumark-Sztainer, et al, 2002). According to the Vegetarian Resource Group (Stahler, 2005), approximately 3% of children ages 8 to 18 years in the United States are vegetarians. The American Dietetic Association and Dietitians of Canada (2003) issued a statement endorsing vegetarian diets for adults and children; the statement further notes that well-planned vegetarian diets are adequate for all stages of the life cycle and promote normal growth. Children and adolescents on vegetarian diets have the potential for lifelong healthy diets and have been shown to have lower intakes of cholesterol, saturated fat, and total fat and higher intakes of fruits, fiber, and vegetables than nonvegetarians (American Dietetic Association and Dietitians of Canada, 2003).
The major types of vegetarianism are:
Lacto-ovo vegetarians, who exclude meat from their diet but consume dairy products and rarely fish
Lactovegetarians, who exclude meat and eggs but drink milk
Pure vegetarians (vegans), who eliminate any food of animal origin, including milk and eggs
Macrobiotics, who are even more restrictive than pure vegetarians, allowing only a few types of fruits, vegetables, and legumes
Semivegetarians, who consume a lacto-ovo vegetarian diet with some fish and poultry (an increasingly popular form of vegetarianism that poses little or no nutritional risk to infants unless dietary fat and cholesterol intake are severely restricted)
Many individuals who are concerned about healthy diets subscribe to vegetarian diets that may not be typified by these categories. Therefore during nutritional assessment it is necessary to clearly list exactly what the diet includes and excludes.*
The major deficiencies that may occur in the stricter vegan diets are inadequate protein for growth; inadequate calories for energy and growth; poor digestibility of many of the bulky natural, unprocessed foods, especially for infants; and deficiencies of vitamin B6, niacin, riboflavin, vitamin D, iron, calcium, and zinc. Strict vegan diets also require supplements of vitamin B12 and vitamin D. Vitamin D is essential if exposure to sunlight is inadequate (<5 to 15 min/day on the hands, arms, and face of light-skinned persons; slightly more in darker pigmented individuals) or in persons who are dark-skinned or who live in northern latitudes or cloudy or smoky areas. A multivitamin-mineral supplement can be given to avoid many of these deficiencies in children who are not consuming 100% of the RDA of vitamins and minerals (Dunham and Kollar, 2006) (see Cultural Competence box).
Children on strict vegetarian and macrobiotic diets should be evaluated for iron deficiency anemia, zinc deficiency, and rickets; this may occur as a result of consuming plant foods such as unrefined cereals, which impair the absorption of iron, calcium, and zinc. Other factors that affect iron absorption are listed in Box 13-1.
Nursing Care Management
Evaluation of adequacy of nutrient intake is the initial nursing goal and requires assessment based on a dietary history and physical examination for signs of deficiency or excess. Once assessment data are collected, this information is evaluated against standard intakes to identify areas of concern. (See Nutritional Assessment, Chapter 6.)
The DRIs† (see also Chapter 6) are quantitative estimates of nutrient requirements for planning and evaluating diets for healthy infants and are made of four categories, including estimated average (EA) requirements for age and gender categories, tolerable upper-limit (TUL) nutrient intakes that are associated with a low risk of adverse effects, adequate intakes (AIs) of nutrients, and new standard RDAs. The guidelines present information about lifestyle factors that may affect nutrient function, such as caffeine intake and exercise, and about how the nutrient may be related to chronic disease. DRIs currently published include calcium, magnesium, phosphorus, vitamin D, and fluoride. Additional groups of nutrients include folate and other B vitamins, dietary antioxidants, micronutrients, macronutrients, trace elements, electrolytes, and food components such as dietary fiber. The comprehensive set of guidelines covers nutrient needs across the life span, including infancy. An important factor in the development of the DRIs that affects children, particularly infants 0 to 6 months, is that the AIs are based on the nutrient intake of term, healthy, breast-fed infants (by well-nourished mothers), which now represents the gold standard for infant nutrition in this age-group. This represents a major change in infant nutrition recommendations; specific needs to meet the nutrient requirements for formula-fed infants were not included in DRI reports (Devaney and Barr, 2002; Institute of Medicine, 2000).
The American Heart Association Dietary Guidelines, patterned after the 2005 Dietary Guidelines for Americans, may also be used to encourage healthy dietary intakes designed to decrease obesity and cardiovascular risk factors and subsequent cardiovascular disease, which is now known to occur in both young children and adults (Gidding, Dennison, Birch, et al, 2005; Gidding, Lichtenstein, Faith, et al, 2009). The American Heart Association guidelines have been endorsed by the American Academy of Pediatrics (2006), but it is important to note that these guidelines are for children ages 2 years and older. The guidelines encourage a variety of fruits, vegetables, whole grains, and low-fat dairy and nonfat dairy products, in addition to fish, beans, and lean meat.
In 2011 the United States Department of Agriculture replaced the Food Guide Pyramid (MyPyramid) with MyPlate (http://www.choosemyplate.gov/) (Figure 6-4). This colorful plate shows the 5 main food groups—fruits, grains, vegetables, protein, and dairy—with the intended purpose to involve children and their families in making appropriate food choices for meals and decrease the incidence of overweight and obesity in the United States. MyPlate provides an online interactive feature that allows the individual to select (click on) an individual food group and see choices for foods in that group. Approximate serving sizes are suggested and vegetarian substitutions are also provided.
A vegetarian food pyramid (rainbow for Canadian vegetarians) developed by the American Dietetic Association and Dietitians of Canada includes guidelines for meeting the minimum recommendations for nutrients, including protein, iron, zinc, calcium, vitamin D, riboflavin, and iodine. The new food guide can be adapted to different types of vegetarian diets according to specific needs (American Dietetic Association and Dietitians of Canada, 2003; Messina, Melina, and Mangels, 2003).
Achieving a nutritionally adequate vegetarian diet is not difficult (except with the strictest diets), but it requires careful planning and knowledge of nutrient sources. For children the lacto-ovo vegetarian diet is nutritionally adequate; however, the vegan diet requires supplementation with vitamins D and B12 for children ages 2 to 12 years. Infants should be breast-fed for the first 6 months and preferably for 1 year, be introduced to some solid foods after about 4 to 6 months, and receive iron-fortified cereal for at least 18 months. Vitamin B12 supplementation of breast-feeding infants is recommended if the mother’s intake of the vitamin is inadequate and she is not taking vitamin supplements (Dunham and Kollar, 2006). Solids may be introduced to vegetarian infants using the same guidelines as for other children (see p. 492). The American Dietetic Association and Dietitians of Canada (2003) and American Academy of Pediatrics (2009) recommend iron supplementation in infants exclusively breast-fed after 4 to 6 months by vegetarian mothers and no dietary fat restrictions in vegetarian children younger than 2 years. The use of vitamin C juices (in moderate amounts, not as a milk substitute) with foods high in iron further improves iron absorption. If cow’s or human milk or commercial infant formula is not given, fortified soy formula is recommended. A variety of foods should be introduced during the early years to ensure a well-balanced intake. A registered dietitian is a good resource for assisting with meal planning for vegetarian infants and children.
To ensure sufficient protein in the diet, foods with incomplete proteins (those that do not have all the essential amino acids) must be eaten at the same meal with other foods that supply the missing amino acids. The three basic combinations of foods consumed by vegetarians that generally provide the appropriate amounts of essential amino acids are:
1. Grains (cereal, rice, pasta) and legumes (beans, peas, lentils, peanuts)
Additional dietary considerations for young children are found in Chapters 12 and 14.
The nurse has an important role in evaluating the food intake of infants, children, and adolescents and may serve as a resource for parents; the overall nutritional goal should be to provide the best sources of vitamin and mineral intake through food intake rather than rely on supplemental vitamins, which may not always be as well absorbed as food products.
Based on current recommendations, the infant who is exclusively breast-feeding for the first 4 to 6 months (the latter is preferred) should receive a vitamin D supplement of 400 international units; at approximately 6 months the breast-fed infant should begin receiving an iron supplement (elemental iron, 1 mg/kg/day), or iron requirements may be met with iron-fortified cereal (average of 2 servings of 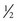 oz of dry cereal per serving) (American Academy of Pediatrics, 2009). Fruit juice is not necessary for adequate growth in infants but may be given after 6 months in amounts not to exceed 4 oz/day. Low iron formula is not recommended for infants.
oz of dry cereal per serving) (American Academy of Pediatrics, 2009). Fruit juice is not necessary for adequate growth in infants but may be given after 6 months in amounts not to exceed 4 oz/day. Low iron formula is not recommended for infants.
Protein-Energy Malnutrition (Severe Childhood Undernutrition)
Malnutrition continues to be a major health problem in the world today, particularly in children under 5 years of age. However, lack of food is not always the primary cause for malnutrition. In many developing and underdeveloped nations, diarrhea (gastroenteritis) is a major factor. Additional factors are bottle-feeding (in poor sanitary conditions), inadequate knowledge of proper child care practices, parental illiteracy, economic and political factors, climate conditions, cultural and religious food preferences, and simply the lack of adequate food. Müller and Krawinkel (2005) point out that poverty is the underlying cause of malnutrition. The most extreme forms of malnutrition, or protein-energy malnutrition (PEM), are kwashiorkor and marasmus. Some authorities suggest that severe malnutrition encompasses more than protein energy deficits and thus prefer the term severe childhood undernutrition (SCU) (Heird, 2007). Entities such as the World Health Organization continue to use the term protein energy malnutrition. SCU may also be subdivided into edematous (kwashiorkor) and nonedematous (marasmus) types (Heird, 2007).
In the United States milder forms of PEM are seen as a result of primary malnutrition, although the classic cases of marasmus and kwashiorkor may also occur. Unlike in developing countries, where the main reason for PEM is inadequate food, in the United States PEM occurs despite ample dietary supplies (see Growth Failure [Failure to Thrive], p. 534). PEM may also be seen in persons with chronic health problems such as cystic fibrosis, renal dialysis, cancer, and GI malabsorption; in the elderly who have chronic malnutrition; or in persons with acute illnesses such as prolonged, untreated anorexia nervosa. Kwashiorkor has been reported in the United States in children fed only a rice beverage diet (Rice Dream) and few solid foods (Katz, Mahlberg, Honig, et al, 2005). The rice drink contains 0.13 g of protein per ounce (compared with the 0.5 g found in human milk and infant formulas) and is an inadequate source of nutrition for children. Other reported cases of kwashiorkor in developed countries involved infants who were fed nonstandard infant diets such as flour water, corn porridge, molasses, and nondairy creamer (Katz, Mahlberg, Honig, et al, 2005). Kwashiorkor has also been reported in the United States when infants have been fed inappropriate food as a result of parental (caretaker) nutritional ignorance, a perceived cow’s milk–based formula intolerance, family social chaos, or cow’s milk intolerance (Liu, Howard, Mancini, et al, 2001). Therefore it is important that health care workers not assume that PEM cannot occur in developed countries; a comprehensive dietary history should be obtained in any child with clinical features resembling PEM.
Kwashiorkor
Kwashiorkor has been defined as primarily a deficiency of protein with an adequate supply of calories. A diet consisting mainly of starch grains or tubers provides adequate calories in the form of carbohydrates but an inadequate amount of high-quality proteins. Some evidence, however, supports a multifactorial etiology, including cultural, psychologic, and infective factors that may interact to place the child at risk for kwashiorkor. Penny (2003) suggests that kwashiorkor may result from the interplay of nutrient deprivation and infectious or environmental stresses, which produces an imbalanced response to such insults. Kwashiorkor often occurs subsequent to an infectious outbreak of measles and dysentery. There is further evidence that oxidative stress occurs in children with kwashiorkor, resulting in free radical damage, which may precipitate cellular changes, resulting in edema and muscle wasting (Penny, 2003). The role of the essential fatty acid arachidonic acid in lipid metabolism, altered leukotriene production, and oxidative stress in kwashiorkor has yet to be fully understood, but arachidonic acid seems to have an interactive role in its development (Penny, 2003).
Taken from the Ga language (Ghana), the word kwashiorkor means “the sickness the older child gets when the next baby is born” and aptly describes the syndrome that develops in the first child, usually between 1 and 4 years of age, when weaned from the breast after the second child is born.
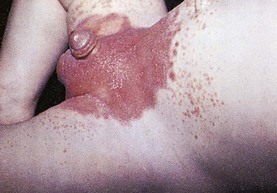
The child with kwashiorkor has thin, wasted extremities and a prominent abdomen from edema (ascites). The edema often masks severe muscular atrophy, making the child appear less debilitated than he or she actually is (Fig. 13-1). The skin is scaly and dry and has areas of depigmentation. Several dermatoses may be evident, partly resulting from the vitamin deficiencies. Permanent blindness often results from the severe lack of vitamin A. Mineral deficiencies are common, especially iron, calcium, and zinc. Acute zinc deficiency is a common complication of severe PEM and results in skin rashes, loss of hair, impaired immune response and susceptibility to infections, digestive problems, night blindness, changes in affective behavior, defective wound healing, and impaired growth. Its depressant effect on appetite further limits food intake. The hair is thin, dry, coarse, and dull. Depigmentation is common, and patchy alopecia may occur.

Fig. 13-1 Kwashiorkor. The infant shows generalized edema, seen in the puffiness of the face, arms, and legs. (From Kumar V, Abbas A, Fausto N, et al: Robbins basic pathology, ed 8, Philadelphia, 2007, Saunders.)
Diarrhea (persistent diarrhea malnutrition syndrome) commonly occurs from a lowered resistance to infection and further complicates the electrolyte imbalance. Low levels of cytokines (protein cells involved in the primary response to infection) have been reported in children with kwashiorkor, suggesting that such children have a blunted immune response to infection. A large number of deaths in children with kwashiorkor occur in those who develop HIV infection. GI disturbances such as fatty infiltration of the liver and atrophy of the acini cells of the pancreas occur. Anemia is also a common finding in malnourished children. Protein deficiency increases the child’s susceptibility to infection, which eventually results in death. Fatal deterioration may be caused by diarrhea and infection or by circulatory failure.
Marasmus
Marasmus results from general malnutrition of both calories and protein. It is common in underdeveloped countries during times of drought, especially in cultures where adults eat first; the remaining food is often insufficient in quality and quantity for the children.
Marasmus is usually a syndrome of physical and emotional deprivation and is not confined to geographic areas where food supplies are inadequate. It may be seen in children with growth failure in whom the cause is not solely nutritional but primarily emotional. Marasmus may be seen in infants as young as 3 months of age if breast-feeding is not successful and there are no suitable alternatives. Marasmic kwashiorkor is a form of PEM in which clinical findings of both kwashiorkor and marasmus are evident; the child has edema, severe wasting, and stunted growth. In marasmic kwashiorkor the child suffers from inadequate nutrient intake and superimposed infection. Fluid and electrolyte disturbances, hypothermia, and hypoglycemia are associated with a poor prognosis.
Marasmus is characterized by gradual wasting and atrophy of body tissues, especially of subcutaneous fat. The child appears to be very old, with loose and wrinkled skin, unlike the child with kwashiorkor, who appears more rounded from the edema. Fat metabolism is less impaired than in kwashiorkor; thus deficiency of fat-soluble vitamins is usually minimal or absent. In general, the clinical manifestations of marasmus are similar to those seen in kwashiorkor with the following exceptions: with marasmus there is no edema from hypoalbuminemia or sodium retention, which contributes to a severely emaciated appearance; no dermatoses caused by vitamin deficiencies; little or no depigmentation of hair or skin; moderately normal fat metabolism and lipid absorption; and smaller head size and slower recovery after treatment.
The child is fretful, apathetic, withdrawn, and so lethargic that prostration frequently occurs. Intercurrent infection with debilitating diseases such as tuberculosis, parasitosis, HIV, and dysentery is common.
Therapeutic Management
The treatment of PEM includes providing a diet with high-quality proteins, carbohydrates, vitamins, and minerals. When PEM occurs as a result of persistent diarrhea, three management goals are identified:
1. Rehydration with an oral rehydration solution that also replaces electrolytes
2. Administration of medications such as antibiotics and antidiarrheals
3. Provision of adequate nutrition by either breast-feeding or a proper weaning diet
Local protocols are used in developing countries to deal with PEM. Penny (2003) proposes a three-phase treatment protocol: (1) acute or initial phase in the first 2 to 10 days, involving initiation of treatment for oral rehydration, diarrhea, and intestinal parasites; prevention of hypoglycemia and hypothermia; and subsequent dietary management; (2) recovery or rehabilitation (2 to 6 weeks), focusing on increasing dietary intake and weight gain; and (3) follow-up phase, focusing on care after discharge in an outpatient setting to prevent relapse and promote weight gain, provide developmental stimulation, and evaluate cognitive and motor deficits. In the acute phase care is taken to prevent fluid overload; the child is observed closely for signs of food or fluid intolerance. The refeeding syndrome may occur if intake progresses too rapidly; cardiac failure may cause sudden death in the child who has been malnourished and refed too rapidly.
Vitamin and mineral supplementation are required in most cases of PEM; vitamin A, zinc, and copper are recommended; iron supplementation is not recommended until the child is able to tolerate a steady food source. In addition, the child is observed for signs of skin breakdown, which should be treated to prevent infection. Breast-feeding is encouraged if the mother and child are able to do so effectively; in some cases partial supplementation with a modified cow’s milk–based formula may be necessary (Penny, 2003).
The World Health Organization (2006) issued a statement recognizing the importance of breast-feeding for the first 6 months in developing countries where HIV is prevalent among childbearing women and children. The World Health Organization recognizes that appropriate sources of food and water for infants may not be available once the 6 months are concluded and that the risk for malnutrition is greater among such children than the theoretical risk of HIV. However, the organization does recommend that breast-feeding continue after 6 months with the introduction of complementary foods, provided these are safe for child consumption. In severely malnourished children a modest energy food source is given initially, followed by a high-protein and energy food source; severely malnourished children will not tolerate a high-energy and high-protein source initially. A number of food sources may be provided to treat PEM. They include oral rehydration solutions (ReSoMal), amino acid–based elemental food, and ready-to-feed foods that do not require the addition of water (to minimize contaminated water consumption); parenteral and oral antibiotics are often part of the standard treatment for PEM (Ciliberto, Sandige, Ndehka, et al, 2005; Amadi, Mwiya, Chomba, et al, 2005) (see Cultural Competence box).
Nursing Care Management
Because PEM appears early in childhood, primarily in children 6 months to 2 years of age, and is associated with early weaning, low-protein diet, delayed introduction of complementary foods, and frequent infections (Müller and Krawinkel, 2005), it is essential that nursing care focus on prevention of PEM through parent education about feeding practices during this crucial period. Breast-feeding is the optimal method of feeding for the first 6 months. The immune properties naturally found in breast milk not only nourish the infant but also help prevent opportunistic infections, which may contribute to PEM. Providing for essential physiologic needs, such as appropriate nutrient intake, protection from infection, adequate hydration, skin care, and restoration of physiologic integrity, is paramount. Additional nursing care focuses on education about and administration of childhood vaccinations to prevent illness, promotion of nutrition and well-being for the lactating mother, encouragement and participation in well-child visits for infants and toddlers, appropriate food sources for children being weaned from the breast, and education regarding sanitation practices to prevent childhood GI diseases.
Poor skin integrity further increases the chance of infections, hypothermia, water loss, and skin breakdown. Tube feedings may be required in infants too weak to breast- or bottle-feed. Oral rehydration with an approved oral rehydration solution is commonly used in cases of PEM in which diarrhea and infection are not immediately life threatening.
One approach that has gained acceptance for treating childhood malnutrition in developing countries is the home-based use of ready-to-use therapeutic food (RUTF). The RUTF is a paste based on peanut butter and dried skim milk with vitamins and minerals; it requires no mixing with water or milk. The packaged RUTF can be stored without refrigeration. Studies have demonstrated improved survival rates in malnourished children (Amthor, Cole, and Manary, 2009; Ciliberto, Sandige, Ndehka, et al, 2005). Some of the reported advantages of home-based treatment include that children are not exposed to hospital-acquired infections and may receive the RUTF from village health aides (Kapil, 2009).
It is imperative that nurses be at the forefront in educating and reinforcing healthy nutrition habits in parents of small children to prevent malnutrition. Because children with marasmus may suffer from emotional starvation as well, care should be consistent with care of the child with growth failure (p. 534).
The World Health Organization has published guidelines for the treatment and management of children with severe malnutrition (Ashworth, Khanum, Jackson, et al, 2003). These guidelines include a two-phase program with a 10-step guide to treating the child with malnutrition.
Food Sensitivity
Food sensitivity is a general term that includes any type of adverse reaction to food or food additives. The terms food sensitivity, hypersensitivity, allergy, and intolerance are often used interchangeably. The American Academy of Allergy, Asthma and Immunology further suggests defining food-induced reactions according to the following: adverse food reactions, food hypersensitivity (allergy), food anaphylaxis, food intolerance, food idiosyncrasy, food toxicity or poisoning, anaphylactoid reaction to food, pharmacologic food reaction, and metabolic food reaction (American Academy of Pediatrics, 2009). Approximately 6% of children may experience food allergic reactions in the first 3 years of life; 1.5% will have an allergy to eggs and 0.6% to peanuts (Sampson and Leung, 2007a).
The clinical manifestations of food hypersensitivity may be divided as follows (American Academy of Pediatrics, 2009):
Systemic—Anaphylactic, growth failure
GI—Abdominal pain, vomiting, cramping, diarrhea
Food hypersensitivities usually occur either as an immunoglobulin E (IgE)–mediated or non–IgE-mediated immune response; some toxic reactions may occur as a result of a toxin found within the food (Sampson, 2004). Food allergy is caused by exposure to allergens, usually proteins (but not the smaller amino acids) that are capable of inducing IgE antibody formation (sensitization) when ingested. Sensitization refers to the initial exposure of an individual to an allergen, resulting in an immune response; subsequent exposure induces a much stronger response that is clinically apparent. Consequently, food hypersensitivity typically occurs after the food has been ingested one or more times.
Oral allergy syndrome occurs when a food allergen (commonly fruits and vegetables) is ingested and there is subsequent edema and pruritus involving the lips, tongue, palate, and throat. Recovery from symptoms is usually rapid. Immediate GI hypersensitivity is an IgE-mediated reaction to a food allergen; reactions include nausea, abdominal pain, cramping, diarrhea, vomiting, anaphylaxis, or all of these. Additional food hypersensitivities seen in young children include allergic eosinophilic esophagitis, allergic eosinophilic gastroenteritis, food protein–induced proctocolitis, and food protein–induced enteropathy (milk [cow or soy] protein intolerance, celiac disease).
Food hypersensitivity may also be classified according to the interval between ingestion and the manifestation of symptoms: immediate (within minutes to hours) or delayed (2 to 48 hours) (American Academy of Pediatrics, 2009).
Allergens can produce an allergic response when inhaled or injected, but these routes rarely apply to food allergens. (See also Asthma, Chapter 32.) The most common food allergens in children are eggs, cow’s milk, and peanuts (Sampson and Leung, 2007a); soy, wheat, corn, tree nuts, shellfish, and fish allergies are more common in adults (Sicherer, 2003; Sampson, 2004) (Box 13-2).
Food allergies can occur at any time but are common during infancy, since the immature intestinal tract is more permeable to proteins than the mature intestinal tract, thus increasing the likelihood of an immune response. Allergies in general demonstrate a genetic component: children who have one parent with allergy have a 50% or greater risk of developing allergy; children who have both parents with allergy have up to a 100% risk of developing allergy. Allergy with a hereditary tendency is referred to as atopy. Some infants with atopy can be identified at birth from elevated levels of IgE in cord blood.
Deaths have been reported in children who suffered an anaphylactic reaction to food. Onset of the reactions occurred shortly after ingestion (5 to 30 minutes). In most of the children the reactions did not begin with skin signs, such as hives, red rash, and flushing, but rather mimicked an acute asthma attack (wheezing, decreased air movement in airways, dyspnea). Watch children with food anaphylaxis closely because a biphasic response has been recorded in a number of cases in which there is an immediate response, apparent recovery, then acute recurrence of symptoms (Simons, 2009). Children with extremely sensitive food allergies should wear a medical identification bracelet and have an injectable epinephrine cartridge (EpiPen) readily available. (See Anaphylaxis, Chapter 29.) Any child with a history of food allergy or previous severe reaction to food should have a written emergency treatment plan, as well as an EpiPen and liquid diphenhydramine (Benadryl) or cetirizine (Zyrtec) (Sampson and Leung, 2007b).
Educate parents, teachers, and daycare workers regarding signs and symptoms of food hypersensitivity reactions. People with food sensitivity should avoid unfamiliar foods and restaurants that do not disclose food ingredients. New labeling guidelines require that food additives such as spices and flavoring be clearly labeled on commercially sold, store-bought foods. Hidden ingredients in prepared foods are also a potential source of food hypersensitivity.
Children with a history of food allergy may spend a considerable amount of time in daycare; therefore persons working in daycare centers and other children’s settings need to be properly educated regarding recognition and management of severe anaphylactic reactions (Bansal, Marsh, Patel, et al, 2005).
Although the reason is unknown, many children “outgrow” their food allergies. About 80% of all infants who are intolerant to cow’s milk usually develop tolerance by the fourth birthday (Sampson, 2004). More than half (60%) of infants have an IgE-mediated reaction to cow’s milk, and 25% will retain sensitivity until the second decade of their life. About 35% develop other food allergies (Sampson, 2004). Children who are allergic to more than one food may develop tolerance to each food at a different time. The most common allergens, such as peanuts, are outgrown less readily than other food allergens. Because of the tendency to lose the hypersensitivity, reintroduce allergenic foods into the diet after a period of abstinence (usually ≥1 year) to evaluate whether the food can be safely added to the diet. Foods that are associated with severe anaphylactic reactions, however, continue to present a lifelong risk and must be avoided.
The only way to positively establish the diagnosis of food allergy is by eliminating the suspected food from the diet for approximately 10 to 14 days (for IgE-mediated allergy; longer for cell-mediated disorder), then following with a food challenge. Food challenges should only be performed by an allergist who has the appropriate equipment and training to manage anaphylaxis (Sampson and Leung, 2007a).
Because children with food allergies (usually two or more) are at risk for inadequate nutrient intake and growth failure, it is recommended that they have an annual nutritional assessment to prevent such problems (Christie, Hine, Parker, et al, 2002).
Breast-feeding is now considered a primary strategy for avoiding atopy in families with known food sensitivities; however, there is no evidence that maternal avoidance (during pregnancy or lactation) of cow’s milk protein or other dietary products known to cause food hypersensitivity will prevent atopy in children (American Academy of Pediatrics, 2009). Researchers indicate that delaying the introduction of highly allergenic foods past 4 to 6 months may not be as protective for atopy as previously believed (Greer, Sicherer, Burks, et al, 2008). Likewise studies have shown that soy formula does not prevent allergic disease in infants and children (American Academy of Pediatrics, 2009). The strategies listed in the Nursing Care Guidelines box are those recommended by most authorities for infants with a family history of atopy.*
Cow’s Milk Allergy
Cow’s milk allergy (CMA) is a multifaceted disorder representing adverse systemic and local GI reactions to cow’s milk protein. Approximately 2.5% of infants develop cow’s milk hypersensitivity; 80% of those children may outgrow the hypersensitivity by 4 years of age (Sampson, 2004). Recent studies reveal that milk allergy may persist and some children may not be able to tolerate milk until they are 16 years of age (American Academy of Pediatrics, 2009). (This discussion centers on cow’s milk protein contained in commercial infant formulas; whole milk is not recommended for infants <12 months of age.) The hypersensitivity may be manifested within the first 4 months of life through a variety of signs and symptoms that may appear within 45 minutes of milk ingestion or after several days (Box 13-3). The diagnosis may initially be made from the history, although the history alone is not diagnostic. The timing and diversity of clinical manifestations vary greatly. For example, CMA may be manifested as colic (see p. 532), diarrhea, vomiting, GI bleeding, gastroesophageal reflux, chronic constipation, or sleeplessness in an otherwise healthy infant.
Diagnostic Evaluation: A number of diagnostic tests may be performed, including stool analysis for blood, eosinophils, and leukocytes (both frank and occult bleeding can occur from the colitis); serum IgE levels; skin-prick or scratch testing; and radioallergosorbent test (RAST) (measures IgE antibodies to specific allergens in serum by radioimmunoassay). Both skin testing and RAST may help identity the offending food, but the results are not always conclusive. No single diagnostic test is considered definitive for the diagnosis (American Academy of Pediatrics, 2009).
The most definitive diagnostic strategy is elimination of milk in the diet, followed by challenge testing after improvement of symptoms. A clinical diagnosis is made when symptoms improve after removal of milk from the diet and two or more challenge tests produce symptoms (Ewing and Allen, 2005). Challenge testing involves reintroducing small quantities of milk in the diet to detect resurgence of symptoms; at times it involves the use of a placebo so that the parent is unaware of (or “blind” to) the timing of allergen ingestion. A double-blind placebo-controlled food challenge is the gold standard for diagnosing food allergies such as CMA, yet it may not be used often for diagnosing CMA because of the expense, time involved, and risk for further exposure and anaphylactic reaction (Ewing and Allen, 2005). Careful observation of the child is required during a challenge test because of the possibility of anaphylactic reaction.
Therapeutic Management: Treatment of CMA is elimination of cow’s milk–based formula and all other dairy products. For infants fed cow’s milk formula, this primarily involves changing the formula to a casein hydrolysate milk formula (Pregestimil, Nutramigen, or Alimentum), in which the protein has been broken down into its amino acids through enzymatic hydrolysis. Although the American Academy of Pediatrics (2009) recommends the use of extensively hydrolyzed formulas for CMA, many practitioners may start a soy formula instead because of the expense of the hydrolyzed formulas. Approximately 50% of infants who are sensitive to cow’s milk protein also demonstrate sensitivity to soy, yet soy is less expensive than protein hydrolysate formula. Other choices for children who are intolerant to cow’s milk–based formula are the amino acid–based formulas Neocate or EleCare, yet their cost is a major consideration. Goat’s milk is not an acceptable substitute because it cross-reacts with cow’s milk protein, is deficient in folic acid, and is unsuitable as the only source of calories. Anaphylactic reaction to goat’s milk has been noted in an infant who was also allergic to cow’s milk (Pessler and Nejat, 2004). Infants usually remain on the milk-free diet for 12 months, after which time small quantities of milk are reintroduced.
Children who have CMA may tolerate extensively heated cow’s milk (Nowak-Wegrzyn, Bloom, Sicherer, et al, 2008).
Nursing Care Management: The principal nursing objectives are identification of potential CMA and appropriate counseling of parents regarding substitute formulas. Parents often interpret GI symptoms such as spitting up and a loose stool or fussiness as an indication that the infant is allergic to cow’s milk and switch the infant to a variety of formulas in an attempt to resolve the problem.
Parents need much reassurance regarding the needs of nonverbal infants with such an array of symptoms. Endless nights of lost sleep and a crying infant may promote feelings of parenting inadequacy and role conflict, thus aggravating the situation. Nurses can reassure parents that many of these symptoms are common and the reasons are often never found, yet the child does achieve appropriate growth and development. Report acute symptoms to the practitioner for further evaluation. Parents need reassurance that the infant will receive complete nutrition from the new formula and will suffer no ill effects from the absence of cow’s milk.
Once solid foods are started, parents need guidance in avoiding milk product (see Box 13-3). Carefully reading all food labels helps avoid exposure to prepared foods containing milk products.
Lactose Intolerance
Lactose intolerance refers to at least four different entities that involve a deficiency of the enzyme lactase, which is needed for the hydrolysis or digestion of lactose in the small intestine; lactose is hydrolyzed into glucose and galactose. Congenital lactase deficiency occurs soon after birth after the newborn has consumed lactose-containing milk (human milk or commercial formula). This inborn error of metabolism involves the complete absence or severely reduced presence of lactase. It is rare and requires lifelong lactose-free or extremely reduced lactose diet.
Primary lactase deficiency, sometimes referred to as late-onset lactase deficiency, is the most common type of lactose intolerance and is manifested usually after 4 or 5 years of age, although the time of onset varies. Ethnic groups with a high incidence of lactase deficiency include Asians, southern Europeans, Arabs, Israelis, and African-Americans, whereas Scandinavians tend to have the lowest. Lactose malabsorption manifests as lactose intolerance and is characterized by an imbalance between the ability of lactase to hydrolyze the ingested lactose and the amount of lactose ingested (Heyman and American Academy of Pediatrics, 2006).
Secondary lactase deficiency may occur secondary to damage of the intestinal lumen, which decreases or destroys the enzyme lactase. Cystic fibrosis; sprue; celiac disease; kwashiorkor; or infections such as giardiasis, HIV, or rotavirus may cause temporary or permanent lactose intolerance.
Developmental lactase deficiency refers to the relative lactase deficiency observed in preterm infants of less than 34 weeks of gestation (Heyman and American Academy of Pediatrics, 2006).
The primary symptoms of lactose intolerance include abdominal pain, bloating, flatulence, and diarrhea after the ingestion of lactose. The onset of symptoms occurs within 30 minutes to several hours of lactose consumption. Lactose intolerance is often perceived as an allergy; and in several studies with reports of acute GI symptoms ascribed to lactose intolerance, measurement of lactase activity is normal (Goldberg, Folta, and Must, 2002).
Lactose intolerance may be diagnosed on the basis of the history and improvement with a lactose-reduced diet. The breath hydrogen test is used to positively diagnose the condition. Breath samples in lactose-deficient individuals yield a higher percentage of hydrogen (≥20 ppm above baseline). In infants lactose malabsorption may be diagnosed by evaluating fecal pH and reducing substances; fecal pH in infants is usually lower than in older children, but an acidic pH may indicate malabsorption (Heyman and American Academy of Pediatrics, 2006).
Treatment of lactose intolerance involves elimination of offending dairy products; however, some advocate decreasing amounts of dairy products rather than total elimination, especially in small children (Heyman and American Academy of Pediatrics, 2006; Goldberg, Folta, and Must, 2002). In infants lactose-free or low-lactose formula offers no special advantages over lactose-containing formula, except in the severely malnourished (Heyman and American Academy of Pediatrics, 2006).
One concern is that dairy avoidance in children and adolescents with lactose intolerance contributes to reduced bone mineral density and osteoporosis (Sibley, 2004). There is evidence that dietary lactose enhances calcium absorption and that lactose-free diets may negatively affect bone mineralization (Heyman and American Academy of Pediatrics, 2006). Individuals with lactose maldigestion who do not experience lactose intolerance symptoms should continue to consume small amounts of dairy products with meals to prevent reduced bone mass density and subsequent osteoporosis (Sibley, 2004). In one study a decreased intake of calcium-containing dairy foods in early adolescent girls with perceived lactose intolerance resulted in lower spinal bone mineral content than in girls without perceived milk intolerance; the researchers cautioned that decreased dairy intake in persons with lactose intolerance may increase the risk of osteoporosis in later life (Matlik, Savaiano, McCabe, et al, 2007). One option to meet calcium intake needs of adolescents with dairy restriction due to lactose intolerance is to consume one half to one and a half servings of calcium-fortified citrus juice per day; vitamin D supplementation and increased physical activity are also recommended to increase bone health (Gao, Wilde, Lichtenstein, et al, 2006). There is evidence that probiotics (food preparations containing microorganisms such as Lactobacillus, which alter the GI microflora and thus are beneficial to the host) improve lactose intolerance when live cultures are fermented in dairy products (de Vrese and Schrezenmeir, 2008; Zeisel and Erickson, 2003). The positive attributes of probiotics for those with lactose maldigestion include delayed GI transit (slower than milk), positive effects on intestinal and colonic microflora, and a reduction of maldigestion symptoms.
Most people are able to tolerate small amounts of lactose even in the presence of deficient lactase activity (Heyman and American Academy of Pediatrics, 2006) and should be encouraged to continue their intake of dairy products in small amounts to obtain much-needed nutrients. Milk taken at meals may be better tolerated than when taken alone (see Family-Centered Care box). Pretreated milk (with microbial-derived lactase) is reported to be effective in improving lactose absorption. Because dairy products are a major source of calcium and vitamin D, supplementation of these nutrients is needed to prevent deficiency. Yogurt contains inactive lactase enzyme, which is activated by the temperature and pH of the duodenum; this lactase activity substitutes for the lack of endogenous lactase. Fresh, plain yogurt may be tolerated better than frozen or flavored yogurt; hard cheeses, lactase-treated dairy products, and lactase tablets taken with dairy products are also viable options. An important distinction between lactose intolerance and food hypersensitivity is that lactose intolerance does not manifest as an anaphylactic-type reaction.
Nursing Care Management: Nursing care is similar to the interventions discussed for CMA: explaining the dietary restrictions to the family; identifying alternate sources of calcium such as yogurt and calcium supplementation; explaining the importance of supplementation; and discussing sources of lactose, especially hidden sources such as its use as a bulk agent in certain medications, and ways of controlling the symptoms (see Family-Centered Care box). Parents are advised to check with the pharmacist regarding this possibility when obtaining medication.*
Conditions Related to Feeding
A common cause of feeding problems is improper feeding technique. A satisfactory feeding requires a number of mechanical skills, such as placing the infant to the breast properly (see Breast-Feeding, Chapter 8); holding the bottle at an angle that allows fluid, not air, to flow into the nipple; understanding and responding to the infant’s cues for burping or satiation; and holding the infant during feeding, rather than propping the bottle. A number of other problems can also occur singly or in combination, such as:
• Feeding too much or too little food
• Feeding too often, especially during the night, or too infrequently
• Selecting inappropriate foods for the infant’s physiologic and motor development
Although such feeding problems are more common for first-time inexperienced parents, they can also occur with seasoned parents who are unprepared for an infant who has different needs or exhibits less clear cues of hunger or satiation. Improper feeding may also occur in caretakers (parents) who are too immature to understand the infant’s need for human contact during feeding and thus misinterpret hunger and satiation cues. It also occurs in parents who have an infant with a difficult temperament (see Temperament, Chapter 12) and in parents who may be impaired as a result of recreational drugs such as methamphetamines (see Drug-Exposed Infants, Chapter 10). Improper feeding is also a potential concern in family situations where an older child is left to care for a younger sibling when there is social disruption, dysfunction, and no responsible adult to intervene.
Most of these feeding problems are easily corrected with reassurance, guidance, and demonstration. Early assessment is essential to prevent complex problems from developing between parent and child at mealtime.
Regurgitation and “Spitting Up”
The return of small amounts of food after a feeding is common during infancy. Do not confuse this with actual vomiting, which can be associated with a number of disturbances that may be insignificant or serious. It is usually benign, although persistent regurgitation necessitates medical evaluation to rule out gastroesophageal reflux. For clarification, the following terms are defined:
Regurgitation—Return of undigested food from the stomach, usually accompanied by burping
Spitting up—Dribbling of unswallowed formula from the infant’s mouth immediately after a feeding
The nurse should explain the normal occurrence of regurgitation or spitting up to parents, especially those who are unduly concerned about it. Regurgitation can be reduced by some simple measures, such as frequent burping during and after feeding, minimum handling during and after feeding, and positioning the child on the right side with the head slightly elevated after feeding. The inconvenience of spitting up can be managed with absorbent bibs on the infant and protective cloths on the parent.
Sometimes frequent dribbling of formula causes excoriation of the corners of the mouth, chin, and neck. Keeping the area dry promotes healing but can be difficult to maintain.
Colic (Paroxysmal Abdominal Pain)
![]() Colic is reported to occur in 15% to 40% of all infants (Morin, 2009), yet has no particular affinity in regard to the gender, race, or socioeconomic status (Ellett, 2003). An organic cause may be identified in less than 5% of infants seen by physicians because of excessive crying (Roberts, Ostapchuk, and O’Brien, 2004). The condition is generally described as abdominal pain or cramping that is manifested by loud crying and drawing the legs up to the abdomen. Other definitions include variables such as duration of cry greater than 3 hours a day, occurring more than 3 days per week, and for more than 3 weeks and parental dissatisfaction with the child’s behavior. Some studies report an increase in symptoms (fussiness and crying) in the late afternoon or evening (Morin, 2009); however, in some infants the onset of symptoms occurs at another time. Colic is more common in young infants under the age of 3 months than in older infants, and infants with difficult temperaments are more likely to be colicky.
Colic is reported to occur in 15% to 40% of all infants (Morin, 2009), yet has no particular affinity in regard to the gender, race, or socioeconomic status (Ellett, 2003). An organic cause may be identified in less than 5% of infants seen by physicians because of excessive crying (Roberts, Ostapchuk, and O’Brien, 2004). The condition is generally described as abdominal pain or cramping that is manifested by loud crying and drawing the legs up to the abdomen. Other definitions include variables such as duration of cry greater than 3 hours a day, occurring more than 3 days per week, and for more than 3 weeks and parental dissatisfaction with the child’s behavior. Some studies report an increase in symptoms (fussiness and crying) in the late afternoon or evening (Morin, 2009); however, in some infants the onset of symptoms occurs at another time. Colic is more common in young infants under the age of 3 months than in older infants, and infants with difficult temperaments are more likely to be colicky.
![]() Case Study—Health Problems of Infants
Case Study—Health Problems of Infants
Despite the obvious behavioral indications of pain, the infant with colic gains weight and usually thrives. There is no evidence of a residual effect of colic on older children, except perhaps a strained parent-child relationship in some cases. In other words, infants who are colicky grow up to be normal children and adults. Colic is self-limiting and in most cases resolves as the infant matures, generally around 12 to 16 weeks of age (Lobo, Kotzer, Keefe, et al, 2004; O’Connor, 2009).
Etiology
Among the theories investigated as potential causes are too rapid feeding, overeating, swallowing excessive air, improper feeding technique (especially in positioning and burping), and emotional stress or tension between parent and child. Although all these may occur, there is no evidence that one factor is consistently present. Infants with CMA symptoms have a high rate of colic (44%), and eliminating cow’s milk products from the infant’s diet can reduce the symptoms. However, there is considerable controversy about the role of allergy and colic because there does not appear to be an increased incidence of atopy in infants with colic (Sicherer, 2003).
Parental smoking, strained parent-infant interaction, lactase deficiency, difficult infant temperament, difficulty regulating emotions, overstimulation, central nervous system immaturity, and neurochemical dysregulation in the brain have also been proposed as potential causes of colic (Ellett, 2003; Neu and Robinson, 2003). A positive association between consumption of fruit juices (carbohydrate malabsorption) and colic has been demonstrated in some cases (Duro, Rising, Cedillo, et al, 2002). Some experts have suggested that gastroesophageal reflux is a cause of colic, but studies have not supported this theory (St. James-Roberts, 2008). The consensus of many experts who study colic is that it is multifactorial and that no single treatment for every colicky infant will be effective in alleviating the symptoms.
Therapeutic Management
Management of colic should begin with an investigation of possible organic causes, such as CMA, intussusception, or other GI problem. If a sensitivity to cow’s milk is strongly suspected, a trial substitution of another formula such as an extensively hydrolyzed (Nutramigen, Alimentum, Pregestimil), whey hydrolysate, or amino acid (Neocate, EleCare) formula is warranted. Soy formulas are usually avoided because of the possibility of sensitivity to soy protein as well (American Academy of Pediatrics, 2009). Oral administration of Lactobacillus reuteri to colicky breast-fed infants decreased symptoms within 1 week of initiation in one small study (Savino, Pelle, Palumeri, et al, 2007).
When no specific inciting agent can be found, the supportive measures discussed under Nursing Care Management are employed.
The use of drugs, including sedatives, antispasmodics, antihistamines, and antiflatulents, is sometimes recommended. The most commonly used sedatives are phenobarbital, hydroxyzine hydrochloride (Atarax), and chloral hydrate. Simethicone (Mylicon) may also help allay the symptoms of colic. However, in most controlled studies none of these drugs completely reduced the symptoms of colic. Herbal (chamomile) tea offered at the onset of crying and up to three times daily has proved effective in relieving the symptoms of colic in some infants (Weizman, Alkrinawi, Goldfarb, et al, 1993); however, parents are to be cautioned regarding the unknown safety of this treatment (Crotteau, Wright, and Eglash, 2006). Behavioral interventions have not proved effective at reducing symptoms of colic but have helped parents deal with the crying infant in a more positive manner. The addition of lactase to infant formula has produced mixed results as far as abatement of overall symptoms.
One study found that a combination of interventions—massage, herbal tea, sucrose solution, and hydrolyzed formula—decreased crying in reported colicky infants; the administration of the hydrolyzed formula achieved best results, whereas massage was least effective at reducing crying (Arikan, Alp, Gozum, et al, 2008).
An extensive review of a wide variety of interventions for colic indicates there are no specific safe remedies to alleviate symptoms of colic in every infant. Dietary changes, such as eliminating cow’s milk protein from the lactating mother’s diet, and behavioral interventions were shown to be effective in helping parents reduce stimulation and respond to the infant’s crying, yet these interventions are perceived only as moderately effective (Joanna Briggs Institute, 2008). Administering sucrose was effective at reducing crying in colicky infants for a short period (3 to 30 minutes) (Joanna Briggs Institute, 2008).
The literature on colic contains many behavioral remedies for treatment; however, none has proved entirely effective at reducing the symptoms of colic in all infants treated. Studies on infant massage for relieving the symptoms of colic demonstrated no advantage, and the practice is not recommended (Roberts, Ostapchuk, and O’Brien, 2004). The Internet offers a variety of remedies for colic. Parents should be aware of the sources of information and use home remedies such as herbal teas or natural remedies touted for colic relief cautiously.
A survey of pediatric nurse practitioners (PNPs) and pediatricians managing infants with colic found that the PNPs were more likely to suggest behavioral or environmental modification strategies, whereas the pediatricians predominantly suggested pharmacologic interventions or formula changes (Lobo, Kotzer, Keefe, et al, 2004). The study emphasized the lack of understanding and consensus regarding the etiology and management of infant colic.
Nursing Care Management
The initial step in managing colic is to take a thorough, detailed history of the usual daily events. Areas that should be stressed include (1) the infant’s diet; (2) the diet of the breast-feeding mother; (3) the time of day when crying occurs; (4) the relationship of crying to feeding time; (5) the presence of specific family members during crying and habits of family members, such as smoking; (6) activity of the mother or usual caregiver before, during, and after crying; (7) characteristics of the cry (duration, intensity); (8) measures used to relieve crying and their effectiveness; and (9) the infant’s stooling, voiding, and sleeping patterns. Of special emphasis is a careful assessment of the feeding process via demonstration by the parent.
If cow’s milk sensitivity is suspected, breast-feeding mothers should follow a milk-free diet (see Box 13-3) for a minimum of 3 to 5 days in an attempt to reduce the infant’s symptoms. Caution mothers that some nondairy creamers may contain calcium caseinate, a cow’s milk protein. If a milk-free diet is helpful, lactating mothers may need calcium supplements to meet the body’s requirement. Bottle-fed infants may improve with the same dietary modifications as for the child with CMA (see p. 530).
One important nursing intervention (before or after organic cause has been eliminated) is reassuring both parents they are not doing anything wrong and that the infant is not experiencing any physical or emotional harm. Parents, especially mothers, become easily frustrated with the infant’s crying and perceive this as a sign that something is horribly wrong. Additionally, colicky infants may be at increased risk for being shaken by the caregiver and experiencing traumatic brain injury. A survey of fathers of colicky infants revealed that professional assistance was limited. The fathers described the experience of having a colicky infant as like falling into an abyss from which they had to climb with the assistance of family and friends, thus reinforcing the importance of empathetic nurses (Ellett, Appleton, and Sloan, 2009). An empathetic, gentle, and reassuring attitude, in addition to suggestions for treatment, will help allay parents’ anxieties, which are usually exacerbated by loss of sleep and preoccupation over the infant’s welfare. Colic disappears spontaneously, usually by 3 to 4 months of age, although guarantees should never be given, since it may continue for much longer. Other support persons and extended family members may be enlisted to support the parents during this difficult time.*
When no specific organic or behavioral cause can be identified, it is preferable to determine the time of the onset of crying and attempt to manipulate the circumstances associated with it. For example, some infants have episodes of colic around the family’s dinner time, when all household members are home and often tired and busy. The overstimulating, tense atmosphere may upset the infant. Changing the evening routine, such as encouraging someone other than the mother or primary caregiver to prepare dinner, preparing dinner earlier in the day, and feeding the infant in a quieter area of the house, may help. Other approaches for relieving colic are listed in the Family-Centered Care box and shown in Fig. 13-2. Encourage parents to try as many of these approaches as possible, since not all are effective for every infant.

Fig. 13-2 The “colic carry” may be comforting to an infant with colic. (Courtesy Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
One author suggests that a problem-solving discussion with the parents, in addition to acknowledgment that the infant has colic, is an optimal strategy for helping parents manage the infant with colic until a cure is found (Ellett, 2003). Nurses must also be aware that once colic symptoms are resolved, family function may be negatively impacted by residual feelings and emotions experienced during the acute phase of the colic (Ellett, Schuff, and Davis, 2005). Practical parental support interventions include the provision of a colic hotline (mother-to–nurse practitioner or nurse) and nurse-managed colic support groups (Ellett, Schuff, and Davis, 2005).
Growth Failure (Failure to Thrive)
Growth failure, or failure to thrive (FTT), is a sign of inadequate growth resulting from inability to obtain or use calories required for growth. FTT has no universal definition, although one of the more common criteria is a weight (and sometimes height) that falls below the 5th percentile for the child’s age. Another definition of FTT includes a weight for age (and height) z value of less than −2.0 (a z value is a standard deviation value that represents anthropometric data normalizing for sex and age with greater precision than growth percentile curves [Markowitz, Watkins, and Duggan, 2008]). A third way to define FTT is a weight curve that crosses more than 2 percentile lines on the National Center for Health Statistics growth charts after previous achievement of a stable growth pattern. Growth measurements alone are not used to diagnose children with FTT. Rather, the finding of a pattern of persistent deviation from established growth parameters is cause for concern. In addition to lack of consensus on the precise definition of FTT, there are those who advocate for a change in terminology; thus terms such as growth failure and pediatric undernutrition are used in the literature for FTT (Locklin, 2005).
Some experts suggest that the previously used classifications of organic FTT and nonorganic FTT are too simplistic because most cases of growth failure have mixed causes; they suggest that FTT be classified according to pathophysiology in the following categories (Krugman and Dubowitz, 2003):
Inadequate caloric intake—Incorrect formula preparation, neglect, food fads, excessive juice consumption, poverty, behavioral problems affecting eating, or central nervous system problems affecting intake
Inadequate absorption—Cystic fibrosis, celiac disease, vitamin or mineral deficiencies, biliary atresia, or hepatic disease
Increased metabolism—Hyperthyroidism, congenital heart defects, or chronic immunodeficiency
Defective utilization—Genetic anomaly such as trisomy 21 or 18, congenital infection, or metabolic storage diseases
The cause of growth failure is often multifactorial and involves a combination of infant organic disease, dysfunctional parenting behaviors, subtle neurologic or behavioral problems, and disturbed parent-child interactions (Block, Krebs, and Committee on Child Abuse and Neglect and Committee on Nutrition [American Academy of Pediatrics], 2005).
Infants who are born preterm and with VLBW or extremely low birth weight (ELBW), as well as those with intrauterine growth restriction, are often referred for growth failure within the first 2 years of life because they typically do not grow physically at the same rate as term cohorts, even after discharge from the acute care facility. Catch-up growth has been shown to be much more difficult to achieve in ELBW and VLBW infants. As children, former VLBW and ELBW infants are more likely to have small stature and demonstrate lower cognitive and academic achievement scores than term cohorts (Casey, Whiteside-Mansell, Barrett, et al, 2006).
Other factors that can lead to inadequate caloric intake in infancy include poverty, health or childrearing beliefs such as fad diets, inadequate nutritional knowledge, family stress, feeding resistance, and insufficient breast milk intake.
Diagnostic Evaluation
Diagnosis is initially made from evidence of growth failure. If FTT is recent, the weight, but not the height, is below accepted standards (usually the 5th percentile); if FTT is longstanding, both weight and height are low, indicating chronic malnutrition. Perhaps as important as anthropometric measurements are a complete health and dietary history (including perinatal history), physical examination for evidence of organic causes, developmental assessment, and family assessment. A dietary intake history, either a 24-hour food intake or a history of food consumed over a 3- to 5-day period, is also essential. In addition, explore the child’s activity level, parental height, perceived food allergies, and dietary restrictions. An assessment of household organization and mealtime behaviors and rituals is important in the collection of pertinent data. It is often helpful to obtain the growth patterns of the affected child’s parents and siblings; these can be compared with norm-referenced standards to evaluate the child’s growth (Markowitz, Watkins, and Duggan, 2008). An assessment of the home environment and child-parent interaction may be helpful as well. Other tests (lead toxicity, anemia, stool-reducing substances, occult blood, ova and parasites, alkaline phosphatase, and zinc levels) are selected only as indicated to rule out organic problems. In most cases laboratory studies are of little diagnostic value (American Academy of Pediatrics, 2009). To prevent the overuse of diagnostic procedures, consider FTT early in the differential diagnosis. To avoid the social stigma of FTT during the early investigative phase, some health care workers use the term growth delay (or failure) until the actual cause is established.
Therapeutic Management
The primary management of FTT is aimed at reversing the cause of the growth failure. If malnutrition is severe, the initial treatment is directed at reversing the malnutrition. The goal is to provide sufficient calories to support “catch-up” growth—a rate of growth greater than the expected rate for age. The following formula may be used to calculate required caloric intake:
In this formula ideal weight for height is the median weight for the child’s height based on the current National Center for Health Statistics weight-for-height growth charts.
In addition to adding caloric density to feedings, the child may require multivitamin supplements and dietary supplementation with high-calorie foods and drinks. Any coexisting medical problems are treated.
In most cases of FTT an interdisciplinary team of physician, nurse, dietitian, child life specialist, occupational therapist, pediatric feeding specialist, and social worker or mental health professional is needed to deal with the multiple problems. Make efforts to relieve any additional stresses on the family by offering referrals to welfare agencies or supplemental food programs. In some cases family therapy may be required. Temporary placement in a foster home may relieve the family’s stress, protect the child, and allow the child some stability if insurmountable obstacles are preventing appropriate family function. Behavior modification aimed at mealtime rituals (or lack thereof) and family social time may be required. Hospitalization admission is indicated for (1) evidence (anthropometric) of severe acute malnutrition, (2) child abuse or neglect, (3) significant dehydration, (4) caretaker substance abuse or psychosis, (5) outpatient management that does not result in weight gain, and (6) serious intercurrent infection (American Academy of Pediatrics, 2009; Block, Krebs, and Committee on Child Abuse and Neglect and Committee on Nutrition [American Academy of Pediatrics], 2005).
Prognosis
The prognosis for FTT is related to the cause. If the parents have simply not understood the infant’s needs, teaching may remedy the child’s limited caloric intake and permanently reverse the growth failure. Inadequate or infrequent feeding periods by the infant’s primary caretaker, in conjunction with family disorganization, are often observed to be the cause of FTT.
Few long-term studies provide data on the prognosis for children with FTT; however, some studies indicate that children who had FTT as infants had shorter heights, lower weights, and lower scores on measures of psychomotor development than peers (Black, Dubowitz, Krishnakumar, et al, 2007; Rudolf and Logan, 2005). Factors related to poor prognosis are severe feeding resistance, lack of awareness in and cooperation from the parent(s), low family income, low maternal educational level, adolescent mother, preterm birth, intrauterine growth restriction, and early age of onset of FTT. Because later cognitive and motor function is affected by malnourishment in infancy, many of these children are below normal in intellectual development, have poorer language development and less well-developed reading skills, attain lower social maturity, and have a higher incidence of behavioral disturbances (Markowitz, Watkins, and Duggan, 2008). Such findings indicate that a long-term plan and follow-up care are needed for the optimum development of these children.
Nursing Care Management
Caring for the child with FTT presents many nursing challenges, whether treatment takes place in the hospital, clinic, or home. Providing a positive feeding environment, teaching the parent successful feeding strategies, and supporting the child and family are essential components of care.
Nurses play a critical role in the diagnosis of FTT through their assessment of the child, parents, and family interactions. Knowledge of the characteristics of children with FTT and their families is essential in helping identify these children and hastening the confirmation of a diagnosis. Accurate assessment of initial weight and height and daily weight, as well as recording of all food intake, is mandatory. The nurse documents the child’s feeding behavior and the parent-child interaction during feeding, other caregiving activities, and play. One available feeding observation instrument is the Nursing Child Assessment Satellite Training (NCAST) Feeding Scale, which is designed to assess the feeding interaction of infants up to 12 months of age (Barnard, Hammond, Booth, et al, 1993).* (See Nutritional Assessment, Chapter 6.)
The nurse should assess the approximate developmental age on admission by administering an appropriate developmental test. Only after objective measurements are available is a care plan for stimulation outlined. The nursing admission history and ongoing assessment should also focus on the following characteristics that have been identified in many of these children and their parents.
The Child: Besides showing signs of malnutrition and delayed social development, children with FTT may exhibit altered behavioral interactions. They may display intense interest in inanimate objects, such as a toy, but much less interest in social interactions. They are often watchful of people at a distance but become increasingly distressed as others come closer. They may dislike being touched or held and avoid face-to-face contact. However, when held, they protest briefly on being put down and are apathetic when left alone.
Children with growth failure may have a history of difficult feeding, vomiting, sleep disturbance, and excessive irritability. Patterns such as crying during feedings; vomiting; hoarding food in the mouth; ruminating after feeding; refusing to switch from liquids to solids; and displaying aversion behavior, such as turning from food or spitting food, become attention-seeking mechanisms to prolong the attention received at mealtime. In some cases the child may use feeding as a control mechanism in a poorly organized or chaotic family situation; parents may allow the child to dictate the norms for behavior and feeding because of inexperience with parenting or poor parenting role models. Thus refusing to eat or only eating high sugar foods may be the child’s norm. In such cases family therapy is essential to reverse the trend and assist the parents and child in understanding each others’ roles.
The Parents: Some parents are at increased risk for attachment problems because of (1) isolation and social crisis; (2) inadequate support systems, such as for teenage and single mothers; and (3) poor parenting role models as a child. Other factors that should be considered are lack of education; physical and mental health problems such as physical and sexual abuse, depression, or drug dependence; immaturity, especially in adolescent parents; and lack of commitment to parenting, such as giving priority to entertainment or employment. Often these parents and their families are under stress and in multiple chronic emotional, social, and financial crises.
Because part of the difficulty between parent and child is dissatisfaction and frustration, the child should have a primary core of nurses (Fig. 13-3). The nurses caring for the child can learn to perceive the child’s cues and reverse the cycle of dissatisfaction, especially in the area of feeding. Depending on the cause of FTT, children may be treated on an outpatient basis.
Because many of these children are responding to stimuli that have led to the negative feeding patterns, the first goal is to structure the feeding environment to encourage eating. Initially staff members and a feeding specialist may need to feed these children to thoroughly assess the difficulties encountered during the feeding process and to devise strategies that eliminate or minimize such problems. General guidelines for the feeding process are outlined in the Nursing Care Guidelines box.
Nutritional Management
Four primary goals in the nutritional management of FTT are to (1) correct nutritional deficiencies and achieve ideal weight for height, (2) allow for catch-up growth, (3) restore optimum body composition, and (4) educate the parents or primary caregivers regarding the child’s nutritional requirements and appropriate feeding methods (Corrales and Utter, 2005; Maggioni and Lifshitz, 1995). For infants, 24 kcal/oz formulas may be provided to increase caloric intake; older children (1 to 6 years) may benefit from a 30 kcal/oz formula (American Academy of Pediatrics, 2009). Other carbohydrate additives include fortified rice cereal and vegetable oil. Because vitamin and mineral deficiencies may occur, multivitamin supplementation, including zinc and iron, is recommended. For toddlers, a high-calorie milk drink such as PediaSure may be used to increase caloric intake. Carefully monitor for signs of intolerance to the formula. Usually only in extreme cases of malnourishment are tube feedings or intravenous therapy required.
Because maladaptive feeding practices often contribute to growth failure, give parents specific step-by-step directions for formula preparation, as well as a written schedule of feeding times. Avoid juices in children with growth failure until adequate weight gain has been achieved with appropriate milk sources; thereafter give no more than 4 oz/day of juice.
Behavior-modification techniques may be used with older infants and toddlers to interrupt poor feeding patterns. Feeding times may actually involve “struggles of will” in cases of maladaptive feedings that result in FTT. These behaviors are different from the occasional toddler behavior of food refusal, which is primarily developmental, not pathologic. The association of appropriate food with good or bad behaviors and consequent rewards may be part of the complex problem. In severe cases of malnourishment, tube feedings or intravenous therapy may be required.
In addition to attending to the child’s physical needs, the interdisciplinary team must plan care for appropriate developmental stimulation. After an approximate developmental age is established, a planned program of play is begun. Ideally a child life specialist is involved to implement and supervise the stimulation program. Every effort is made to teach the parent how to play and interact with the child.
Nursing care of these children involves a “family systems” approach. In other words, for the entire family to become healthy, each member must be helped to change. Care of the parents is aimed at helping them improve their self-esteem by acquiring positive, successful parenting skills. Initially this necessitates providing an environment in which they feel welcomed and accepted. Depending on the cause of FTT, many children are treated on an outpatient basis.
Skin Disorders
Diaper dermatitis is common in infants and one of several acute inflammatory skin disorders caused either directly or indirectly by wearing diapers. Diaper dermatitis is a form of irritant contact dermatitis, which may also involve secondary bacterial or yeast infection. The peak age for diaper dermatitis is 9 to 12 months, and the incidence is greater in bottle-fed infants than in breast-fed infants. Prevalence rates vary among sources; Noonan, Quigley, and Curley (2006) reported a prevalence rate of 17% in one hospital. Others report prevalence rates as high as 42% (McLane, Bookout, McCord, et al, 2004).
Pathophysiology and Clinical Manifestations
Diaper dermatitis is caused by prolonged and repetitive contact with an irritant, principally urine, feces, soaps, detergents, ointments, and friction. Although the obvious irritant in the majority of incidences is urine and feces, the specific components that contribute to irritation include a combination of factors (Fig. 13-4).
Prolonged contact of the skin with diaper wetness affects several skin properties. Continuous moisture exposure enhances permeability to exogenous materials, produces maceration, increases susceptibility to friction damage, increases transepidermal permeability, and increases microbial counts. Irritant exposure also affects the epidermal barrier structure and function, further increasing permeability and inflammation. Therefore healthy skin, specifically the stratum corneum, becomes less resistant to potential irritants.
Although ammonia was once thought to cause diaper rash because of the association between the strong odor on diapers and dermatitis, ammonia alone is not sufficient. The irritant quality of urine is related to an increase in pH from the breakdown of urea in the presence of fecal urease. The increased pH promotes the activity of fecal enzymes, principally proteases and lipases, which act as irritants. Fecal enzymes also increase the permeability of skin to bile salts, another potential irritant in feces. Researchers believe the decreased incidence of diaper dermatitis in breast-fed infants is related to this interaction between pH and fecal enzymes, since feces from breast-fed infants have lower fecal enzyme activity and lower pH.
The eruption of diaper dermatitis occurs primarily on convex surfaces or in the folds (intertriginous areas), and the lesions can represent a variety of types and configurations. Eruptions commonly involve the skin in most intimate contact with the diaper (e.g., the convex surfaces of buttocks, inner thighs, mons pubis, and scrotum). However, lesions not involving the folds are likely to be caused by chemical irritants, especially from urine and feces (Fig. 13-5). Other causes are detergents or soaps from inadequately rinsed cloth diapers or the chemicals (alcohol) in disposable wipes. Dyes in disposable diapers have also been cited as causing diaper dermatitis.
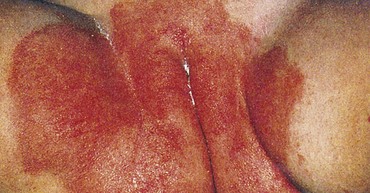
Fig. 13-5 Irritant diaper dermatitis. Note sharply demarcated edges. (From Habif TP: Clinical dermatology: a color guide to diagnosis and therapy, ed 3, St Louis, 1996, Mosby.)
Perianal involvement is usually the result of chemical irritation from feces, especially diarrheal stools. Candida albicans infection produces bright red, confluent lesions with raised borders and often with satellite lesions (Fig. 13-6). The infected area usually includes the folds and is painful. Risk factors for development of Candida infection are an altered immune status and antibiotic therapy.
Therapeutic Management
Treatment is primarily aimed at prevention of diaper dermatitis. A number of interventions are discussed under Nursing Care Management. For inflammations that do not respond to these interventions, topical glucocorticoid preparations are sometimes required. If steroids are prescribed, their use is limited to low-potency preparations, such as a 0.5% or 1% hydrocortisone cream. Potential side effects include striae, epidermal atrophy, suppression of the pituitary-adrenal axis, cessation of longitudinal growth, and Cushing syndrome from chronic use. Topical antifungals are used to treat candidal infections and include clotrimazole, miconazole, ketoconazole, and nystatin ointment. When C. albicans is present elsewhere, oral administration of a fungicide is advised because the GI tract is usually the source of infection. (See Candidiasis, Chapter 9.)
Nursing Care Management
Nursing interventions are aimed at altering the three factors considered to produce dermatitis: wetness (hydration), pH, and fecal irritants. The most significant factor amenable to intervention is the moist environment created in the diaper area. Changing the diaper as soon as it becomes wet eliminates a large part of the problem, and removing the diaper to expose intact skin to air facilitates drying as long as fecal contamination of the skin does not occur, in which case the skin is again exposed to possible injury. However, avoid rubbing or washing the skin frequently (unless fecal matter is present), since frequent washing or rubbing may also disrupt the barrier function of the skin. Many commercial diaper wipes contain alcohol and other products that are detrimental to the natural skin barrier and should be avoided.
The use of a hair dryer or heat lamp is not recommended because they can cause burns. Also, on denuded skin, dry heat delays healing. Instead, occlusive (barrier) ointments or dressings are applied to provide a moist healing environment for open wounds and to protect the skin from further irritation. (See Ostomies, Chapter 27.) Recommended barrier ointments include white petrolatum ointment, Aquaphor (contains lanolin), and zinc oxide. A protective barrier such as zinc oxide prevents skin injury and allows the skin to heal. In the event of diaper dermatitis caused by fungal and contact irritants, a layer of antifungal powder (nystatin [Mycostatin] powder) or cream under a zinc oxide–based skin barrier ointment may be used, but providers must take care to avoid mixing the two preparations together. A combination of products may provide better results for some children; combining a protective powder such as Stomahesive Protective Powder with karaya or cornstarch powder may be helpful (Borkowski, 2004). Some diaper pastes called butt balm or cream may be helpful with some cases of diaper dermatitis, but parents should check to ensure that the ingredients are safe for use in this age-group. Also, such pastes are often expensive (Borkowski, 2004). A Cavilon No-Sting Barrier Film may be used on infants older than 30 days (Baharestani, 2007). Other products include iLEX Skin Protectant Paste and Sureprep No-Sting Protective Barrier Wipe. Once a barrier paste has been applied, it is recommended that the paste not be removed when soiling occurs because this further disrupts the integrity of the skin; instead wipe off the stool or contaminated portion only, leaving as much barrier paste intact as possible.
A 1 : 1 mixture of iLEX paste and petroleum jelly applied liberally to the affected area is reported to be very effective in healing diaper dermatitis within 24 to 48 hours; as noted above, wipe off only excess stool, leaving as much paste in place as possible, and then reapply more as necessary (Hodge, 2009).
Diaper construction has a significant impact on the incidence and severity of diaper dermatitis. Superabsorbent disposable diapers reduce diaper dermatitis because they contain an absorbent gelling material that binds water tightly to decrease skin wetness, maintains pH control by providing a buffering capacity, and decreases skin irritation by preventing mixing of urine and feces in the diaper (Visscher, 2009). The improved containment of urine and feces is also an important factor in decreasing contamination of the environment, such as in daycare centers, and spread of disease.
Another advance in diapers is the addition of an inner layer or top sheet that is impregnated with petrolatum or zinc oxide. The liner transfers the petrolatum to the skin, where it acts as a barrier to moisture and irritants. Guidelines for controlling diaper rash are presented in the Family-Centered Care box.
Some caregivers may choose to apply a powder that may contain either talc or cornstarch. A common misconception about using cornstarch on skin is that it promotes the growth of C. albicans. Both cornstarch and talc do not support growth of the fungi under conditions normally found in the diaper area; however, the use of powders in the hospital nursery is not recommended (Association of Women’s Health, Obstetric and Neonatal Nurses, 2007).
An objective assessment of diaper dermatitis may be performed using the scale in Box 13-4.
Seborrheic Dermatitis
Seborrheic dermatitis is a chronic, recurrent, inflammatory reaction of the skin that occurs most commonly on the scalp (cradle cap) but may involve the eyelids (blepharitis), external ear canal (otitis externa), nasolabial folds, and inguinal region. The cause is unknown, although it is more common in early infancy when sebum production is increased and is thought to be linked to the overgrowth of Malassezia yeast (O’Connor, McLaughlin, and Ham, 2008).
Seborrheic dermatitis in infants has historically been identified as occurring as a result of poor personal care and hygiene; however, such is not the case. The condition may also occur in adolescence but is localized to the scalp and intertriginous areas; pruritus is more common when the condition occurs in adolescents (Morelli, 2007). The lesions are characteristically thick, adherent, yellowish, scaly, oily patches that may or may not be mildly pruritic. If pruritus is present, the infant may be irritable. Unlike AD, seborrheic dermatitis is not associated with a positive family history for allergy, is common in infants shortly after birth, and is also common after puberty. Diagnosis is made primarily by the appearance and location of the crusts or scales.
Nursing Care Management
When seborrheic lesions are present, direct the treatment mainly at removing the crusts. White petrolatum may be applied to the scalp to assist with the removal of the scaly patches; another remedy involves soaking the scalp several hours in vegetable oil then removing the scales (O’Connor, McLaughlin, and Ham, 2008). Tar-containing shampoos are more expensive but may be effective. Teach parents the appropriate procedure to clean the scalp, which may necessitate a demonstration. Shampooing should be done daily with a mild soap or commercial baby shampoo. Medicated shampoos are usually not needed, but an antiseborrheic shampoo containing sulfur and salicylic acid may be used. The shampoo is applied to the scalp and allowed to remain on until the crusts are softened, and then the scalp is thoroughly rinsed. Using a fine-tooth comb or a soft facial brush after shampooing helps remove the loosened crusts from the strands of hair. If shampoos are not effective, an antifungal cream (ketoconazole) or shampoo may be helpful.
Atopic Dermatitis (Eczema)
Atopic dermatitis (AD), or eczema, is a chronic inflammatory skin condition that usually begins during infancy and is associated with allergy with a hereditary tendency (atopy). AD occurs as a result of complex interactions between genetic host factors, infectious and environmental agents, defects in skin barrier function, and immunologic inflammatory responses, which result in chronic skin inflammation (Leung, 2007; Wasserbauer and Ballow, 2009). AD affects approximately 10% to 20% of children worldwide (Leung, 2007). AD is commonly referred to as the “rash that itches” because of the intense pruritus. Because the disease often begins within the first 6 months of life, this discussion is restricted to the infantile form of AD.
The diagnosis of AD is based on a combination of history and morphologic findings (Box 13-5 and Fig. 13-7). Children with the disease have a lower threshold for cutaneous itching, and some authorities believe the dermatologic manifestations appear subsequent to scratching from the intense pruritus. Lesions often disappear if the scratching is stopped.
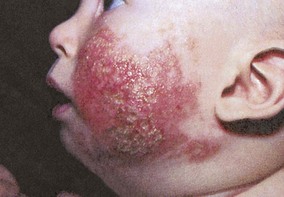
Fig. 13-7 Atopic dermatitis with oozing and crusting of lesions. (From Weston WL, Lane AT, Morelli JG: Color textbook of pediatric dermatology, ed 4, St Louis, 2007, Mosby.)
The majority of children with AD have a family history of eczema, asthma, food allergies, or allergic rhinitis, which strongly supports a genetic predisposition. In addition, approximately 50% of children with AD subsequently develop asthma (Boguniewicz, 2005). The cause is unknown but may be related to an immune reaction with abnormal function of the skin, including alterations in perspiration, peripheral vascular function, and heat tolerance. Patients with AD have dry skin and evidence of increased transepidermal water loss; a defect in the ceramide cells, which help retain water and provide a barrier function; and increased colonization of the skin with Staphylococcus aureus (Boguniewicz, 2005). The chronic disease is better in humid climates and worse in fall and winter, when homes are heated and environmental humidity is lower. The disorder can be controlled but not cured. House dust mites, certain foods, mold, and animal hair may play a role in the etiology of AD. Many children with AD have elevated toxic-specific IgE levels, and a T-cell dysfunction is currently believed to be a major factor in the development of AD (Boguniewicz, 2005). In addition, some evidence suggests that abnormally low levels of the protein filaggrin may have a role in altering the protective barrier function of the skin, thus increasing transepidermal water loss and increasing inflammation (Ong and Boguniewicz, 2008). Leung (2007) describes two types of AD: atopic, which is IgE mediated and affects the majority of children with AD (70% to 80%), and nonatopic eczema, which is not associated with IgE-mediated sensitization. Approximately 30% to 40% of children with moderate AD have IgE-mediated food reactions (Ong and Boguniewicz, 2008). Therefore it is suggested that those with a family history of atopy follow feeding guidelines in early infancy discussed earlier in this chapter.
Therapeutic Management
The major goals of management are to (1) hydrate the skin, (2) relieve pruritus, (3) reduce flare-ups or inflammation by avoiding triggers, (4) prevent and control infection, and (5) live as near as normal a childhood as possible. Most of the general measures for managing AD serve to reduce pruritus and other aspects of the disease. General management includes:
There are a number of ways to enhance skin hydration and prevent dry, flaky skin, depending on the child’s skin characteristics and individual needs. A tepid bath with a mild soap (Dove or Neutrogena), no soap, or an emulsifying oil, followed immediately by application of an emollient (within 3 minutes), assists in preventing moisture loss. Avoid bubble baths and harsh soaps. The bath may need to be repeated once or twice daily, depending on the child’s status. Excessive bathing without emollient application only dries out the skin. Some lotions are not effective, and emollients should be chosen carefully to prevent excessive skin drying. Aquaphor, Cetaphil, and Eucerin are acceptable for skin hydration. A nighttime bath, followed by emollient application and dressing in soft cotton pajamas, may alleviate much of the nighttime pruritus.
Moderate or severe pruritus is usually relieved by administration of oral antihistamine drugs (hydroxyzine or diphenhydramine), with the amount tailored to the individual child. Nonsedating antihistamines such as loratadine (Claritin) or fexofenadine (Allegra) may be preferred for daytime pruritus relief. Because pruritus increases at night, a mildly sedating antihistamine may be needed.
Topical corticosteroids are now considered first-line treatment for AD (Leung, 2007; Williams, 2005). Occasional flare-ups require the use of topical steroids to diminish inflammation. Low-, moderate-, or high-potency topical corticosteroids are prescribed, depending on the degree of involvement, the body area to be treated, the child’s age, potential for local side effects (striae, skin atrophy, and pigment changes), and the type of vehicle to be used (e.g., cream, lotion, ointment). Medical management of secondary skin infections with systemic antibiotics is an important part of the treatment of AD. Coal tar preparations may also be used to hydrate the skin yet are considered cumbersome because they stain clothing.
Two calcineurin inhibitors (immunomodulators) used in children with AD are tacrolimus and pimecrolimus (Elidel). Tacrolimus is available in two ointment strengths (0.03% and 0.1%); these have been approved for use in children 2 years of age and older. Pimecrolimus is available in a 1% cream that has no systemic accumulation or effects; this drug has been approved for children ages 2 years and older.
Acute flare-ups may require the use of wet wraps. One method is to apply a light coat of topical corticosteroid, then wrap the child in cool wet towels for 10 minutes (warm towels slightly in the winter to prevent heat loss). Once the towels are removed, the steroid ointment is reapplied, followed by a moisturizer. Be careful, however, not to use excessive wet wraps, since these may cause skin maceration and secondary infections.
Controversy exists regarding prevention of AD by limiting the exposure of infants at risk to allergens both prenatally and postnatally. Studies have shown a decrease in atopic eczema in infants at risk for atopy whose mothers breast-fed at least 4 months; avoiding highly allergenic foods during lactation may or may not help reduce the incidence of atopy. Infants who are not breast-fed and who are at risk for AD may benefit from extensively hydrolyzed formula (Greer, Sicherer, Burks, et al, 2008). Although conclusive evidence for preventive strategies is lacking, the precautions in the Nursing Care Guidelines box on p. 530 may be recommended.
Nursing Care Management
The child with AD presents a nursing challenge. Controlling the intense pruritus is imperative if the disorder is to be successfully managed, since scratching leads to the formation of new lesions and may cause secondary infection. In addition to the medical regimen, other measures can prevent or minimize the scratching. Keep fingernails and toenails short and clean, and file them frequently to prevent sharp edges. Gloves or cotton socks can be placed over the hands and pinned to shirtsleeves.
Also, eliminate conditions that increase itching when possible. Remove woolen clothes or blankets, rough fabrics, and furry stuffed animals. Because heat and humidity cause perspiration, which intensifies the itching, proper dress for climatic conditions is essential. Pruritus is often precipitated by exposure to the irritant effects of certain components of common products such as soaps, detergents, fabric softeners, perfumes, diaper wipes, and powders. Most children experience less itching when soft cotton fabrics are worn next to the skin. Avoid exposure to latex products, such as gloves and balloons. Launder clothes and sheets in a mild liquid detergent and rinse them thoroughly in clear water (without fabric softeners or antistatic chemicals); use a second rinse cycle to further reduce residual detergent. The use of skin cleansers with minimal defatting activity and a neutral pH is preferred over the usual soaps.
Preventing infection is usually accomplished by preventing scratching. Maintain personal hygiene as described previously. Give baths as prescribed, keeping the water tepid, and avoid soaps (except as indicated), bubble baths, oils, and powders. Skinfolds and diaper areas need frequent cleansing with plain water. A room humidifier or vaporizer may benefit children with extremely dry skin. The lesions are examined for signs of infection—usually honey-colored crusts with surrounding erythema. Report any signs of infection to the practitioner.
Wet soaks or compresses are applied as needed, and medications for pruritus or infection are administered as directed. The nurse gives the family explicit written instructions on the preparation and use of soaks, special baths, and topical medications, including the order of application if more than one is prescribed. If children have difficulty remaining still for a 10- or 15-minute soak, bath, or dressing application, perform these at nap time or when the child is watching television, listening to a story, or playing with tub toys.
No particular diet is recommended for children with AD. When a hypoallergenic diet is prescribed, parents need help in understanding the reason for the diet and guidelines for avoiding hyperallergenic foods (see Box 13-2). Because hypoallergenic diets take time before visible effects are apparent, parents need reassurance that results may not be seen immediately. If airborne allergens also worsen the eczema, the family is counseled regarding measures to “allergy proof” the home. (See Asthma, Chapter 32.)
Family Support*: The nurse can assure parents that the lesions will not produce scarring (unless secondarily infected) and that the disease is not contagious. However, the child will be subject to repeated exacerbations and remissions. Spontaneous and permanent remission takes place at approximately 5 years of age in most children, though they may have an occasional relapse in adolescence or adulthood (Leung, 2007).
During periods of acute exacerbation, when the physical problems may seem insurmountable, the emotional stress becomes intense for family members. They need time to discuss negative feelings and to be reassured that these feelings are expected, normal, acceptable, and healthy, provided they have an emotional outlet to dissipate pent-up energy. During acute phases, efforts aimed at relieving anxiety in both parents and child have a beneficial emotional and physical effect because stress tends to aggravate the severity of the condition.
Disorders of Unknown Etiology
Sudden infant death syndrome (SIDS) is defined as the sudden death of an infant younger than 1 year of age that remains unexplained after a complete postmortem examination, including an investigation of the death scene and a review of the case history. Since 1992, the incidence of SIDS in the United States has decreased by 53% to an all-time low of 0.57 per 1000 live births in 2002 (American Academy of Pediatrics, 2005). The dramatic decrease is attributed to the Back to Sleep campaign.† SIDS is the third leading cause of infant deaths (birth to 12 months) and the first leading cause of postneonatal deaths (between 1 and 12 months). SIDS claimed the lives of 2162 infants in the United States in 2003 (Heron and Smith, 2007).
The SIDS rate remained fairly static between 1999 and 2001. This has been attributed to improved death scene investigation and determination of non-SIDS causes of postneonatal mortality. In addition, there is speculation that deaths attributed to SIDS during the period of 1992 to 2001 may have been a result of other causes (American Academy of Pediatrics, 2005). Table 13-1 summarizes the major epidemiologic characteristics of SIDS.
TABLE 13-1
EPIDEMIOLOGY OF SUDDEN INFANT DEATH SYNDROME

*Although a rare event, simultaneous death of twins from SIDS can occur.
Data from American Academy of Pediatrics, Task Force on Sudden Infant Death Syndrome: The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk, Pediatrics 116(5):1245-1255, 2005; American Academy of Pediatrics, Task Force on Infant Sleep Position and Sudden Infant Death Syndrome: Changing concepts of sudden infant death syndrome: implications for infant sleeping environment and sleep position, Pediatrics 105(3):650-656, 2000.
There has been much debate over the term SIDS, yet the definition noted above remains for the time being. Other terms have been developed to explain sudden deaths in infants. Sudden unexpected early neonatal death (SUEND) and sudden unexpected death in infancy (SUDI) share similar features but differ in regards to the timing of death: SUDI is considered a death in the postneonatal period, whereas SUEND occurs in the first week of life.
Etiology
Numerous theories have been proposed regarding the etiology of SIDS; however, the cause remains unknown. One compelling hypothesis is that SIDS is related to a brainstem abnormality in the neurologic regulation of cardiorespiratory control. This maldevelopment affects arousal and physiologic responses to a life-threatening challenge during sleep (American Academy of Pediatrics, 2005). Abnormalities include prolonged sleep apnea, increased frequency of brief inspiratory pauses, excessive periodic breathing, and impaired arousal responsiveness to increased carbon dioxide or decreased oxygen. However, sleep apnea is not the cause of SIDS. The vast majority of infants with apnea do not die, and only a minority of SIDS victims have documented apparent life-threatening events (ALTEs) (see Apnea and Apparent Life-Threatening Events, p. 546). Numerous studies indicate that there is no association between SIDS and any childhood vaccine.
A genetic predisposition to SIDS has been postulated as a cause. In one study a genetic mutation on chromosome 6q 22.1-22.31 was positively linked to a syndrome of SIDS and dysgenesis of the testis (Puffenberger, Hu-Lince, Parod, et al, 2004).
Recently there has been increased interest in infection and inflammation as a possible cause of SIDS (Blood-Siegfried, 2009; Highet, 2008; Mitchell, 2009).
Maternal smoking during pregnancy has emerged in numerous epidemiologic studies as a major factor in SIDS, and tobacco smoke in the infant’s environment after birth has also been shown to have a possible relationship to the incidence of SIDS (American Academy of Pediatrics, 2005). Data show that exposure to tobacco smoke increased an infant’s risk for SIDS 1.9 times over infants not exposed; 59% of SIDS deaths in smoke-exposed infants were attributed to maternal smoking (Anderson, Johnson, and Batal, 2005). It has been postulated that 12% of all SIDS deaths could be prevented with prenatal maternal smoking cessation (Pollack, 2001). One mechanism that has been proposed as a link between maternal smoking and SIDS is a decrease in the ability to arouse to auditory stimuli in infants of mothers who smoked prenatally (Franco, Groswasser, Hassid, et al, 1999). Exposure to maternal smoking has recently been positively correlated with decreased arousal potential in infants (Richardson, Walker, and Horne, 2009). Increased nicotine concentrations in lung tissue were found in children who died from SIDS compared with a group of control children (McMartin, Platt, Hackman, et al, 2002).
Cosleeping, or an infant sharing a bed with an adult or older child on a noninfant bed, has been reported to have a positive association with SIDS. One survey found a high association between infant deaths, nonstandard beds (sofa, day bed), and bed sharing; a large percentage of infants were found dead on their backs when bed sharing, suggesting suffocation (Unger, Kemp, Wilkins, et al, 2003). A study from Scotland indicates that the risk for SIDS when bed sharing is significantly increased for infants less than 11 weeks of age (Tappin, Ecob, and Brooke, 2005). Vennemann, Bajanowski, Brinkmann, and colleagues (2009) identified infant sleeping in the house of a friend or relative and sleeping in the family living room as significant risk factors for SIDS. Other studies correlated higher incidences of SIDS and infant cosleeping with maternal smoking, cosleeping with multiple family members, sleeping on a couch, use of a pillow in the infant’s bed, maternal overweight, soft bedding, and unintentional asphyxiation resulting from adult intoxication (overlaying) (American Academy of Pediatrics, 2000, 2005; Blair, Sidebotham, Evason-Coombe, et al, 2009; Hauck, Herman, Donovan, et al, 2003; Carroll-Pankhurst and Mortimer, 2001; Li, Zhang, Zielke, et al, 2009; Person, Lavezzi, and Wolf, 2002; McGarvey, McDonnell, Chong, et al, 2003).
A study by Hauck, Herman, Donovan, and colleagues (2003) found that bed sharing and SIDS correlated positively only in cases where the infant was sleeping with someone other than the parent; a high number of SIDS cases involved sleeping on a sofa. There are reports of a greater incidence of SIDS among African-American and non-Caucasian infants. In a population-based study Hauck, Herman, Donovan, and colleagues (2003) found that SIDS occurred more frequently among African-American infants. The prone sleep position was associated with twice (2.4% odds ratio) the rate of SIDS compared with infants placed nonprone to sleep. It has been suggested that the higher SIDS deaths among non-Caucasian infants is related to a higher incidence of prone sleep positioning (American Academy of Pediatrics, 2005; Hauck, Herman, Donovan, et al, 2003).
Cosleeping with infants in the age range when most SIDS deaths occur has not been shown to be preventive. The latest recommendation for cosleeping from the American Academy of Pediatrics (2005) is that the infant’s crib or bassinette be placed in close proximity to the mother’s bed and that the infant be placed in the adult bed only for breast-feeding, then placed to sleep in his or her own crib once the feeding session is completed.
Mesich (2005) notes that the current scientific literature fails to provide definitive guidance regarding mother-infant sleeping together in relation to safety or nonsafety; certain sleep environments (prone sleeping, tobacco smoke exposure, soft bedding, noninfant bed surface, use of certain drugs by cosleeper, and thermal stress), however, are known to increase the risk for SIDS.
Studies from countries other than the United States link sleep habits with an increased risk of SIDS. Prone sleeping may cause oropharyngeal obstruction or affect thermal balance or arousal state. One study found that healthy full-term infants had significantly impaired arousal from active and quiet sleep states when sleeping prone (Horne, Ferens, Watts, et al, 2001). Rebreathing of carbon dioxide by infants in the prone position is also a possible cause for SIDS. Infants sleeping prone and on soft bedding may not be able to move their heads to the side, thus increasing the risk of suffocation and lethal rebreathing. Evidence from other countries and the United States shows an increased incidence of SIDS in infants placed in a side-lying position; thus the side-lying position is no longer recommended for infants sleeping at home, daycare, or hospitals (unless medically indicated). Most preterm infants being discharged from the hospital should be placed in a supine sleeping position unless special factors predispose them to airway obstruction.
One postulated cause of SIDS has been a prolonged Q-T interval; however, there has been no strong evidence to support this as a cause of SIDS or universal testing of newborns for prolonged Q-T interval (American Academy of Pediatrics, 2005).
Soft bedding such as waterbeds, sheepskins, beanbags, pillows, or quilts should be avoided for infant sleeping surfaces. Bedding items such as stuffed animals or toys should be removed from the crib while the infant is asleep. Head covering by a blanket has also been found to be a risk factor for SIDS, thus supporting the recommendation to avoid extra bed linens or other items (Mitchell, Thompson, Becroft, et al, 2008).
One study indicated that breast-feeding during the first 16 weeks of life decreased the likelihood of SIDS (Alm, Wennergren, Norvenius, et al, 2002). Some studies have found pacifier use in infants to be a protective factor against the occurrence of SIDS; the data for pacifier use in infants in the first year of life are said to be more compelling than data linking pacifier use to the development of dental complications and the inhibition of breast-feeding (American Academy of Pediatrics, 2005). Therefore the American Academy of Pediatrics recommends using a pacifier at naptime and bedtime, using a pacifier only if the infant is breast-feeding successfully, not using a sweetened coating on the pacifier, and avoiding forcing the infant to use the pacifier.
The American Academy of Pediatrics (2005) recommends that healthy infants be placed to sleep in the supine (on the back) position. Since the Back to Sleep campaign in 1992 advocating nonprone sleeping for infants, an increased incidence of positional plagiocephaly has been observed. (See Chapter 11 and Fig. 11-9.) It is recommended that an infant’s head position be alternated during sleep time to prevent plagiocephaly. Infants may be placed prone during awake periods to prevent positional plagiocephaly and to encourage development of upper shoulder girdle strength (American Academy of Pediatrics, 2005).
Although the cause of SIDS is unknown, autopsies reveal consistent pathologic findings, such as pulmonary edema and intrathoracic hemorrhages that confirm the diagnosis. Consequently, autopsies should be performed on all infants suspected of dying of SIDS, and findings should be shared with the parents as soon as possible after the death. Postmortem findings in SIDS and accidental suffocation or intentional suffocation such as in Munchausen syndrome by proxy (see Child Maltreatment, Chapter 16) are practically the same. Individuals with less experience and training in performing autopsies, such as coroners instead of medical examiners, may not correctly identify some deaths as SIDS. Therefore mortality statistics can vary in different regions.
Infants at Risk for SIDS
Certain groups of children are at increased risk for SIDS:
Factors that are often listed as being protective against SIDS include:
No diagnostic tests exist to predict which infants, including those in the above groups, will survive, and home monitoring is no guarantee of survival. Whether subsequent siblings of one SIDS infant are at increased risk for SIDS is unclear. Even if the risk is increased, families have a 99% chance that their subsequent child will not die of SIDS. A review of sibling deaths attributed to SIDS in England failed to ascertain a precise risk of recurrence; previous studies suggested a recurrence risk range of 1.7 to 10.1, yet the researchers concluded the studies had too many methodologic flaws to draw any firm conclusions (Bacon, Hall, Stephenson, et al, 2008). Others report that recurrence risks for a SIDS death in a family with a previous infant SIDS death range from 2% to 6% (American Academy of Pediatrics, 2005). Home monitoring is not recommended for this group of children, but it is often used by practitioners and may even be requested by parents (American Academy of Pediatrics, 2005). Monitoring is best initiated on an individual basis.
Nursing Care Management
Nurses have a vital role in preventing SIDS by educating families about the risk of prone sleeping position in infants from birth to 6 months of age, the use of appropriate bedding surfaces, the association with maternal smoking, and the dangers of cosleeping on noninfant surfaces with adults or other children. Additionally nurses have an important role in modeling behaviors for parents to foster practices that decrease the risk of SIDS: placing infants in a supine sleeping position in the hospital and using a pacifier at nap and bedtime. Data indicate that a small percentage of nurses still place healthy infants in a side-lying position in the hospital (Bullock, Mickey, Green, et al, 2004; Thompson, 2005). Statistics for infants being placed in a prone sleeping position in the United States decreased from 70% in 1992 to 13% in 2004 (American Academy of Pediatrics, 2005). One study of neonatal intensive care nurses indicated that 52% routinely provided discharge instructions that promote supine sleep positions at home; common nonsupine positions recommended by the nurses included either supine or side or exclusive side-lying sleep position (Aris, Stevens, Lemura, et al, 2006). Nurses must be proactive in further decreasing the incidence of SIDS; postpartum discharge planning, newborn discharges, follow-up home visits, well-baby clinic visits, and immunization visits provide excellent opportunities to educate parents in these matters.
Many health care workers are concerned that infants placed on the back to sleep will aspirate emesis or mucus, yet studies fail to show an increase in infant deaths, spitting up during sleep, aspiration, asphyxia, or respiratory failure as a result of supine sleep positioning (Malloy, 2002; Tablizo, Jacinto, Parsley, et al, 2007).
Loss of a child from SIDS presents several crises with which the parents must cope. In addition to grief and mourning the death of their child, the parents must face a tragedy that was sudden, unexpected, and unexplained. The psychologic intervention for the family must deal with these additional variables. This discussion focuses primarily on the objectives of care for families experiencing SIDS, rather than on the process of grief and mourning, which is explored in Chapter 23.
Research findings have important implications for practices that may reduce the risk of SIDS, such as avoiding smoking during pregnancy and near the infant; using the supine sleeping position; avoiding soft, moldable mattresses, blankets, and pillows; avoiding bed sharing; breast-feeding; and avoiding overheating during sleep. The infant’s head position should be varied to prevent flattening of the skull (positional plagiocephaly).
The first persons to arrive at the scene may be the police and emergency medical service personnel. They should handle the situation by asking few questions; giving no indication of wrongdoing, abuse, or neglect; making sensitive judgments concerning any resuscitation efforts for the child; and comforting the family members as much as possible. A compassionate, sensitive approach to the family during the first few minutes can help spare them some of the overwhelming guilt and anguish that commonly follow this type of death.
The medical examiner or coroner may go to the home or place of death and make the death pronouncement; until then the sleep environment should remain as it was when the infant was initially found (Koehler, 2008). If the infant is not pronounced at the scene, he or she may be transported to the emergency department to be pronounced dead by a physician. Usually there is no attempt at resuscitation in the emergency department. While they are in the emergency department, parents are asked only factual questions, such as when they found the infant, how he or she looked, and whom they called for help. The nurse avoids any remarks that may suggest responsibility, such as “Why didn’t you go in earlier?” “Didn’t you hear the infant cry out?” “Was the head buried in a blanket?” or “Were the siblings jealous of this child?” It is the coroner’s responsibility to document these findings at the scene rather than have parents recount the experience in the emergency department (Koehler, 2008). Parents may also express feelings of guilt about administering cardiopulmonary resuscitation (CPR) correctly or the timing of CPR in relation to finding the infant.
At this time the physician should initiate the discussion of an autopsy, often with the nurse being present to support the family. The physician or medical examiner, depending on the circumstances, emphasizes that a diagnosis cannot be confirmed until the postmortem examination is completed. Nurses may balk at the idea of requesting an autopsy because of the parents’ emotional state; however, an autopsy may clear up possible misconceptions regarding the death. Instructions about the autopsy and funeral arrangements may need to be repeated or put in writing. If the mother was breast-feeding, she needs information about abrupt discontinuation of lactation. The nurse or physician should contact the primary care practitioner for the infant and the mother to avoid any miscommunications or telephone calls at a later date inquiring about the child’s health status.
A review of 60 studies shows that parents experiencing perinatal death perceive health care workers’ responses as having a significant impact on the parents’ grieving process; parents perceived the behavior of many health care workers as thoughtless or insensitive. The findings suggest that nurses and physicians would benefit from more bereavement training (Gold, 2007).
An important aspect of compassionate care for these parents is allowing them to say good-bye to their child. These are the parents’ last moments with their child, and they should be as quiet, meaningful, peaceful, and undisturbed as possible. Encourage parents to hold their infant before leaving the emergency department. Because the parents leave the hospital without their infant, it is helpful to accompany them to the car or arrange for someone else to take them home. A debriefing session may help health care workers who dealt with the family and deceased infant to cope with emotions that are often engendered when a SIDS victim is brought into the acute care facility. Comprehensive guidelines have been published for health professionals involved in SIDS investigations to assist the family and at the same time to determine that the infant’s death was not the result of other factors such as child maltreatment (American Academy of Pediatrics, 2001).
When the parents return home, a competent, qualified professional should visit them as soon after the death as possible. They should receive printed material that contains excellent information about SIDS (available from the national organizations*).
During the initial visit help the parents gain an intellectual understanding of the condition. The nursing objectives are to assess what the parents have been told about SIDS; what they think happened; and how they explained this to the other siblings, family members, and friends. One question that the nurse will never be able to answer and therefore should not attempt to is, “Why did this happen to our baby?” or “Who is responsible for this tragedy?” These and other questions may linger in the parents’ minds for months or even years.
When the unexpected death of a child occurs, it is not uncommon for one parent to blame the other for the child’s death. Parents may also experience guilt over the child’s death; if they had checked earlier, the child might still be alive. It is important that the nurse assist parents in working through these feelings to prevent marital disruption in addition to the loss of the loved child.
Some parents are able to discuss their feelings openly, and the nurse supports this coping skill. However, others may be reluctant to express their grief, and the nurse can encourage the expression of emotions by asking about crying and feeling sad, angry, or guilty. This is an attempt to provoke a display of emotion, not just an admission of a feeling. During this session, help the parents explore their usual coping mechanisms and, if these are ineffectual, to investigate new approaches. For example, one parent may refrain from discussing the death for fear of upsetting the other parent, but each may need to hear how the other feels.
Ideally, the number of visits and plans for subsequent intervention need to be flexible. Parents facing the question of a subsequent child will need support. Both the birth of a subsequent child and the survival of that child, especially past the age of death of the previous child, are important transitional stages for parents.
Apnea and Apparent Life-Threatening Events
Apnea is defined as a cessation of breathing for 20 seconds. Apnea of infancy is defined as an unexplained respiratory pause of 20 seconds or more, or pauses of less than 20 seconds that are accompanied by pallor, cyanosis, bradycardia, or hypotension in the term infant. The latter is distinct from apnea of prematurity, which is the cessation of breathing longer than 20 seconds, or any period if accompanied by bradycardia and cyanosis; it is not associated with any predisposing conditions (Dudell and Stoll, 2007). An ALTE, formerly referred to as aborted SIDS death or near-miss SIDS, generally refers to an event that is sudden and frightening to the observer, in which the infant exhibits a combination of apnea, change in color (pallor, cyanosis, redness), change in muscle tone (usually hypotonia), choking, gagging, or coughing, and which usually involves a significant intervention and even CPR by the caregiver who witnesses the event (National Institutes of Health Consensus Development Conference, 1987). The definition of ALTE may include apnea, but ALTE may occur without apnea (Silvestri and Weese-Mayer, 2003). It is erroneous to characterize ALTE as a near-miss SIDS incident (Adams, Good, and Defranco, 2009).
Apnea during infancy can be a symptom of any one of many disorders—including sepsis, seizures or other neurologic disorder, upper or lower airway infection or abnormality, gastroesophageal reflux, hypoglycemia or other metabolic problems, and impaired regulation of breathing during sleep or feeding—or a result of intentional harm by an adult caregiver. Delayed ventilatory responses to hypercapnia and hypoxia were observed in one study of 69 infants with apnea of infancy (Katz-Salamon, 2004). Abusive head injury has been reported in a small percentage (2.5%) of children with ALTE (Altman, Brand, Forman, et al, 2003). Intentional suffocation and Munchausen syndrome by proxy cases have also been reported with ALTE (Hall and Zalman, 2005). However, in about half the cases of ALTE no cause is identified.
Infants with a history of ALTEs may be at increased risk for SIDS, but these children constitute only approximately 7% to 12% of all SIDS victims. Most infants with ALTE are less than 6 months of age, and although there has been a significant decrease in SIDS since 1992, the incidence of ALTE has not changed (Hall and Zalman, 2005). A diagnosis of apnea of infancy or idiopathic ALTE is often made when no cause is found.
One European study found that infants with ALTE demonstrated behavioral abnormalities in the first weeks of life that included episodes of cyanosis, repeated episodes of apnea, pallor, and difficulty feeding (Kiechl-Kohlendorfer, Hof, Peglow, et al, 2005). Others have noted that a significant number of ALTEs in their emergency department were associated with accidental poisoning; over-the-counter medications were identified in toxicology screens (Pitetti, Whitman, and Zaylor, 2008).
Results from the Collaborative Home Infant Monitoring Evaluation study found that apnea and bradycardia occurred at conventional and extreme alarm thresholds in all groups of infants studied: siblings of SIDS infants, infants with ALTEs, symptomatic (of apnea and bradycardia) and asymptomatic preterm infants weighing less than 1750 g (3.8 lb) at birth, and healthy term infants. The researchers concluded that many infants in each of these groups experience apnea and bradycardia yet do not die (Jobe, 2001; Ramanathan, Corwin, Hunt, et al, 2001). Furthermore, it was reported that apnea does not appear to be an immediate precursor to SIDS and that cardiorespiratory monitoring is not an effective tool for identifying infants at greater risk for SIDS (American Academy of Pediatrics, 2003). CHIME data indicate that infants with ALTE did not have some of the typical characteristics associated with SIDS infants; these include fewer infants with low birth weight and who are small for gestational age at birth, fewer teenage pregnancies, and a younger infant age at the time of ALTE. The researchers concluded that despite some similar characteristics between ALTE and SIDS, the differences warrant a separate focus on ALTE events (Esani, Hodgman, Ehsani, et al, 2008).
Diagnostic Evaluation
An essential component of the diagnostic process includes a detailed description of the event—who witnessed the event, where the infant was during the event, and what, if any, activities were involved (such as during or after a feeding, riding in a car seat restraint, presence of siblings or any minor children, what clothing the infant was wearing). In addition, a prenatal and postnatal history must be obtained. A short period of observation in the emergency department may be appropriate to observe the infant’s respiratory pattern and response to feeding. A careful evaluation of the preterm infant in the car restraint currently in use is essential; upper airway occlusion and subsequent apnea and cyanosis may occur if the infant is not positioned properly. Reported diagnoses in infants with ALTE include a neurologic event such as a seizure (30% of cases seen); GI problem, including gastroesophageal reflux (50%); respiratory conditions (20%); and metabolic conditions, cardiac anomaly, or child abuse (each <5%). In some cases, multiple diagnoses may be made (Hall and Zalman, 2005).
In the event that an underlying diagnosis such as those mentioned previously is not established, home monitoring may be recommended. The most commonly used monitoring is continuous recording of cardiorespiratory patterns (cardiopneumogram, or pneumocardiogram). Four-channel pneumocardiograms (or multichannel pneumogram) monitor heart rate, respirations (chest impedance), nasal airflow, and oxygen saturation. A more sophisticated test, polysomnography (sleep study), also records brain waves, eye and body movements, esophageal manometry, and end-tidal carbon dioxide measurements. However, none of these tests can predict risk. Some children with normal results may still have subsequent apneic episodes.
Therapeutic Management
The treatment of the infant with an ALTE depends on the underlying condition (see above). Treatment of recurrent apnea (without an underlying organic problem) usually involves continuous home monitoring of cardiorespiratory rhythms and in some cases the use of methylxanthines (respiratory stimulant drugs, such as theophylline or caffeine). The decision to discontinue the monitoring is based on the infant’s clinical condition. A general guideline for discontinuation is when infants with ALTEs have gone 2 or 3 months without significant numbers of episodes requiring intervention.
Newer home apnea monitors allow download of information that assists the practitioner in deciding when to discontinue home monitoring. It is imperative to remember, however, that the home apnea monitor will not predict or prevent SIDS deaths. Furthermore, impedance-based monitors detect chest wall movement and will not detect obstructive apnea unless the episode involves significant bradycardia (see Family-Centered Care box).
Nursing Care Management
The diagnosis of an ALTE engenders great anxiety and concern in parents, and the institution of home monitoring presents additional physical and emotional burdens. Parents of infants on home apnea monitors report experiencing emotional distress, especially depression and hostility, during the first few weeks after hospital discharge (Abendroth, Moser, Dracup, et al, 1999). For parents of a SIDS victim who have a new infant on home apnea monitoring, the anxiety is compounded by the uncertainty of the future of the living child and grief for the lost child. Home apnea monitoring may offer some predictability and control over the current child’s survival through the period of uncertainty.
If home monitoring is required, the nurse can be a major source of support to the family in terms of education about the equipment; education regarding observation of the infant’s status; and instructions regarding immediate intervention during apneic episodes, including CPR. Several reports indicate that the first week to month after discharge is the most stressful for parents, particularly when the rate of false alarms is high (Bennett, 2002). To help the family cope with the numerous procedures they must learn, adequate preparation before discharge and written instructions are essential. In the first few weeks after discharge, parents may benefit by having a practitioner readily available to answer questions regarding false alarms and for other technical assistance (Abendroth, Moser, Dracup, et al, 1999).
Several types of home monitors are available and are set up by either a home monitor equipment company or home health staff. Nurses, especially those involved in the care at home, must become familiar with the equipment, including its advantages and disadvantages. Safety is a major concern because monitors can cause electrical burns and electrocution. The following precautions are recommended:
• Remove leads from infant when not attached to monitor.
• Unplug power cord from electrical outlet when cord is not plugged into monitor.
• Use safety covers on electrical outlets to discourage children from inserting objects into a socket.
Siblings should also be supervised when near the infant and taught that the monitor is not a toy. Other safety practices include informing local utility and rescue squads of the home monitoring in case of an emergency. Telephone numbers for these services should be posted near all telephones in the home.
Caregivers need detailed information regarding proper attachment of the electrodes to the infant’s chest with impedance monitors that detect chest movement. The electrodes are placed in the midaxillary line, at a space one or two fingerbreadths below the nipple. For home use, electrodes attached to a belt that is placed around the child’s trunk are preferred (Fig. 13-8). The belt is positioned so that the electrodes contact the skin in the same area. Monitors may have memory chips that allow for event recording, which can be an effective tool in evaluating the use of the monitor, events immediately before and after the ALTE, and reported frequency of alarms.
Fig. 13-8 Electrode placement for apnea monitoring. In small infants, one fingerbreadth may be used.
Monitors are effective only if they are used. They do not prevent death but alert the caregiver to the ALTE in time to intervene. The need to use the monitor and to respond appropriately to alarms must be stressed. Noncompliance can result in the infant’s death.
Family Support
Many of the stresses observed during the monitoring period are characteristic of those of families with chronically ill children. The child with an apnea or cardiorespiratory monitor may have additional health care needs such as a gastrostomy, tracheostomy, ostomy, and myriad medications or treatments that exacerbate the parents’ stress. Parents report increased stress, including concern for the child’s survival, fear of incompetence in assuming home responsibility, inadequate respite care, lack of time for other children and spouse, social isolation from friends and extended family, constant work, and fatigue. The monitored child is at risk for vulnerable child syndrome, which may lead to lack of parental separation and preferential treatment, causing further family disruption (Bennett, 2002). To deal with these potential effects, nurses need to employ the same interventions as those discussed for children with chronic illness and be aware of the need for referral when difficulties are suspected.
To lessen the continuous responsibility of monitoring, other family members, such as grandparents, should be taught how to manipulate the equipment, read and interpret the signals, and administer CPR. They are encouraged to stay with the infant for regular periods to allow parents respite. Support groups of other families who have successfully completed monitoring can also be of benefit. Because reliable baby-sitters are difficult to locate, support group members or nursing students may be potential sources of qualified caregivers.
Key Points
• Common nutritional disturbances of infancy include vitamin and mineral disturbances, some types of vegetarian diets, childhood malnutrition, and food hypersensitivity or intolerance.
• Mineral disturbances may be caused by mineral-mineral interactions and mineral-diet interactions.
• Nurses should counsel parents to provide the RDA of vitamins and minerals through appropriate foods instead of depending on supplements.
• Nutrient consumption varies among vegetarians; therefore a detailed dietary assessment is essential for planning AIs, particularly in children and pregnant and lactating women.
• PEM (SCU) may occur as a complication of social unrest when the child lacks food as a result of an underlying disease, a fad diet, lack of parental education about infant nutrition, inappropriate management of food allergy, incorrect preparation of formula, or poor food storage and handling.
• Food intolerance encompasses food allergies and food sensitivities during infancy, the most serious of which is CMA.
• Food hypersensitivity may cause a severe anaphylactic reaction in some children; a ready-to-administer dose of intramuscular epinephrine should be carried at all times by such children.
• Common feeding difficulties in the infant include regurgitation, spitting up, and colic.
• Treatment of colic may involve change in feeding practices, correction of a stressful environment, and support of the parent. Medications may or may not relieve some of the symptoms of colic.
• Behavioral interventions aimed at helping parents deal with the colicky infant may be more helpful than changing feeding practices or medications.
• Common skin disorders of infancy are diaper dermatitis, seborrheic dermatitis, and AD.
• Growth failure, or FTT, may occur in children who have a chronic illness, or it may occur in a family environment wherein healthy infant feeding practices are poorly managed or understood. FTT is not always associated with a pattern of disturbed maternal-infant relationship.
• SIDS is the third leading cause of infant death in the United States.
• Factors that place the infant at high risk for SIDS include prone sleeping position, soft bedding, sleeping in a noninfant bed with an adult or older child, and maternal prenatal smoking.
• The primary nursing responsibility in care associated with sudden infant death is educating the family of newborns about the risks for SIDS, modeling appropriate behaviors in the hospital such as placing the infant in a supine sleep position, and providing emotional support of the family that has experienced a SIDS loss.
• Infants with ALTEs are carefully evaluated for clues to the underlying cause.
• Home apnea or cardiorespiratory monitors do not prevent SIDS.
References
Abendroth, D, Moser, DK, Dracup, K, et al. Do apnea monitors decrease emotional distress in parents of infants at high risk for cardiopulmonary arrest? J Pediatr Health Care. 1999;13(2):50–57.
Adams, SM, Good, MW, Defranco, GM. Sudden infant death syndrome. Am Fam Physician. 2009;79(10):870–874.
Alm, B, Wennergren, G, Norvenius, SG, et al. Breast-feeding and the sudden infant death syndrome in Scandinavia, 1992-1995. Arch Dis Child. 2002;86(6):400–402.
Altman, RL, Brand, DA, Forman, S, et al. Abusive head injury as a cause of apparent life-threatening events in infancy. Arch Pediatr Adolesc Med. 2003;157(10):1011–1015.
Amadi, B, Mwiya, M, Chomba, E, et al. Improved nutritional recovery on an elemental diet in Zambian children with persistent diarrhoea and malnutrition. J Trop Pediatr. 2005;51(1):5–10.
American Academy of Pediatrics. Pediatric nutrition handbook, ed 6. Elk Grove Village, Ill: The Academy; 2009.
American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1148.
American Academy of Pediatrics. Dietary recommendations for children and adolescents: a guide for practitioners. Pediatrics. 2006;117(2):544–559.
American Academy of Pediatrics. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116(5):1245–1255.
American Academy of Pediatrics, Committee on Fetus and Newborn. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics. 2003;111(4):914–917.
American Academy of Pediatrics, Committee on Child Abuse and Neglect. Distinguishing sudden infant death syndrome from child abuse fatalities. Pediatrics. 2001;107(2):437–441.
American Academy of Pediatrics, Task Force on Infant Sleep Position and Sudden Infant Death Syndrome. Changing concepts of sudden infant death syndrome: implications for infant sleeping environment and sleep position. Pediatrics. 2000;105(3):650–656.
American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: vegetarian diets. J Am Diet Assoc. 2003;103(6):748–765.
Amthor, RE, Cole, SM, Manary, MJ. The use of home-based therapy with ready-to-use therapeutic food to treat malnutrition in a rural area during a food crisis. J Am Diet Assoc. 2009;109(3):464–467.
Anderson, ME, Johnson, DC, Batal, HA. Sudden infant death syndrome and prenatal maternal smoking: rising attributed risk in the Back to Sleep era. BMC Med. 2005;3(1):4.
Arikan, D, Alp, H, Gozum, S, et al. Effectiveness of massage, sucrose solution, herbal tea or hydrolyzed formula in the treatment of infantile colic. J Clin Nurs. 2008;17(13):1754–1761.
Aris, C, Stevens, TP, Lemura, C, et al. NICU nurses’ knowledge and discharge teaching related to infant sleep position and risk of SIDS. Adv Neonatal Care. 2006;6(5):281–294.
Ashworth, A, Khanum, S, Jackson, A, et al, Guidelines for the inpatient treatment of severely malnourished children. Geneva: World Health Organization; 2003. available at www.who.int/nutrition/publications/severemalnutrition/9241546093_eng.pdf [(accessed July 2009)].
Association of Women’s Health, Obstetric and Neonatal Nurses. Neonatal skin care: evidence-based clinical practice guideline,, ed 2. Washington, DC: The Association; 2007.
Bacon, CJ, Hall, DB, Stephenson, TJ, et al. How common is repeat sudden infant death syndrome? Arch Dis Child. 2008;93(4):323–326.
Baharestani, MM. An overview of neonatal and pediatric wound care knowledge and considerations. Ostomy Wound Manage. 2007;53(6):34–55.
Bansal, PJ, Marsh, R, Patel, B, et al. Recognition, evaluation, and treatment of anaphylaxis in the child care setting. Ann Allergy Asthma Immunol. 2005;94(1):55–59.
Barnard, K, Hammond, MA, Booth, CL, et al. Measurement and meaning of parent-child interaction. In: Morrison, F, Lord, C, Keating, D, eds. Applied developmental psychology, vol 3. New York: Academic Press; 1993.
Bennett, AD. Home apnea monitoring for infants: a discussion of primary care issues. Adv Nurs Pract. 2002;10(3):48–53.
Black, MM, Dubowitz, H, Krishnakumar, A, et al. Early intervention and recovery among children with failure to thrive: follow-up at age 8. Pediatrics. 2007;120(1):59–69.
Blair, PS, Sidebotham, P, Evason-Coombe, C, et al. Hazardous cosleeping environments and risk factors amenable to change: case-control study of SIDS in south west England. BMJ. 2009;339:b3446.
Block, RW, Krebs, NF, Committee on Child Abuse and Neglect and Committee on Nutrition (American Academy of Pediatrics). Failure to thrive as a manifestation of child neglect. Pediatrics. 2005;116(5):1234–1237.
Blood-Siegfried, J. The role of infection with inflammation in sudden infant death syndrome. Immunopharmacol Immunotoxicol. 2009;31(4):516–523.
Boguniewicz, M. Atopic dermatitis: beyond the itch that rashes. Immunol Allergy Clin North Am. 2005;25(2):333–351.
Borkowski, S. Diaper rash care and management. Pediatr Nurs. 2004;30(6):467–470.
Bullock, LF, Mickey, K, Green, J, et al. Are nurses acting as role models for the prevention of SIDS? MCN. 2004;29(3):172–177.
Burk, CJ, Molodow, R. Infantile scurvy: an old diagnosis revisited with a modern dietary twist. Am J Clin Dermatol. 2007;8(2):103–106.
Carroll-Pankhurst, C, Mortimer, EA. Sudden infant death syndrome, bedsharing, parental weight, and age of death. Pediatrics. 2001;107(3):530–536.
Casey, PH, Whiteside-Mansell, L, Barrett, K, et al. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics. 2006;118(3):1078–1086.
Centers for Disease Control and Prevention. Neurologic impairment in children associated with maternal dietary deficiency of cobalamin—Georgia, 2001. MMWR. 2003;52(4):61–64.
Christie, L, Hine, RJ, Parker, JG, et al. Food allergies in children affect nutrient intake and growth. J Am Diet Assoc. 2002;102(11):1648–1651.
Ciliberto, MA, Sandige, H, Ndekha, MJ, et al. Comparison of a home-based therapy with ready-to-use therapeutic food with standard therapy in the treatment of malnourished Malawin children: a controlled, clinical effectiveness trial. Am J Clin Nutr. 2005;81(4):864–870.
Conover, E, Buehler, BA. Use of herbal agents by breastfeeding women may affect infants. Pediatr Ann. 2004;33(4):235–240.
Corrales, KM, Utter, SL. Growth failure. In Samour PQ, King K, eds.: Handbook of pediatric nutrition, ed 3, Sudbury, Mass: Jones & Bartlett, 2005.
Crotteau, CA, Wright, ST, Eglash, A. What is the best treatment for infants with colic? J Fam Pract. 2006;55(7):634–636.
Devaney, BL, Barr, SI. DRI, EAR, RDA, AI, UL: making sense of this alphabet soup. Pediatr Basics. 2002;97(Winter):2–9.
de Vrese, M, Schrezenmeir, J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66.
Dudell, GG, Stoll, BJ. Respiratory tract disorders. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Dunham, L, Kollar, L. Vegetarian eating for children and adolescents. J Pediatr Health Care. 2006;20(1):27–34.
Duro, D, Rising, R, Cedillo, M, et al. Association between infantile colic and carbohydrate malabsorption from fruit juices in infancy. Pediatrics. 2002;109(5):797–805.
Ellett, MLC. What is known about colic? Gastroenterol Nurs. 2003;26(2):60–65.
Ellett, ML, Appleton, MM, Sloan, RS. Out of the abyss of colic: a view through the father’s eyes. MCN. 2009;34(3):164–171.
Ellett, M, Schuff, A, Davis, JB. Parental perceptions of the lasting effects of infant colic. MCN. 2005;30(1):127–132.
Esani, N, Hodgman, JE, Ehsani, N, et al. Apparent life-threatening events and sudden infant death syndrome: comparison of risk factors. J Pediatr. 2008;152(3):A2.
Ewing, WM, Allen, PJ. The diagnosis and management of cow’s milk protein intolerance in the primary care setting. Pediatr Nurs. 2005;31(6):486–492.
Franco, P, Groswasser, J, Hassid, S, et al. Prenatal exposure to cigarette smoking is associated with a decrease in arousal in infants. J Pediatr. 1999;135(1):34–38.
Gao, X, Wilde, PE, Lichtenstein, AH, et al. Meeting adequate intake for dietary calcium without dairy foods in adolescents aged 9 to 18 years (National Health and Nutrition Examination Survey 2001-2002). J Am Diet Assoc. 2006;106(11):1759–1765.
Gidding, SS, Dennison, BA, Birch, LL, et al. Dietary recommendations for children and adolescents: a guide for practitioners: consensus statement from the American Heart Association. Circulation. 2005;112(13):2061–2075.
Gidding, SS, Lichtenstein, AH, Faith, MS, et al. Implementing American Heart Association pediatric and adult nutrition guidelines. Circulation. 2009;119(8):1161–1175.
Gold, KJ. Navigating care after a baby dies: a systematic review of parent experiences with health providers. J Perinatol. 2007;27(4):230–237.
Goldberg, JP, Folta, SC, Must, A. Milk: can a “good” food be so bad? Pediatrics. 2002;110(4):826–831.
Greer, FR, Sicherer, SH, Burks, AW, et al. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121(1):183–191.
Hall, KL, Zalman, B. Evaluation and management of apparent life threatening events in children. Am Fam Physician. 2005;71(12):2301–2308.
Hauck, FR, Herman, SM, Donovan, M, et al. Sleep environment and the risk of sudden infant death syndrome in an urban population: the Chicago Infant Mortality Study. Pediatrics. 2003;111(5):1207–1214.
Heird, WC. Food insecurity, hunger, and undernutrition. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Heron, M, Hoyert, DL, Murphy, SL, et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134.
Heron, MP, Smith, BL. Deaths: leading causes for 2003. Natl Vital Stat Rep. 2007;55(10):1–92.
Heyman, MB, American Academy of Pediatrics, Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118(3):1279–1286.
Highet, AR. An infectious aetiology of sudden infant death syndrome. J Appl Microbiol. 2008;105(3):625–635.
Hodge D: Diaper dermatitis: iLEX and Vaseline, Personal Communication, Nashville, Tenn, December 29, 2009, Vanderbilt Children’s Hospital.
Horne, RS, Ferens, D, Watts, AM, et al. The prone sleeping position impairs arousability in term infants. J Pediatr. 2001;138(6):793–795.
Huiming Y, Chaomin W, Meng M: Vitamin A for treating measles in children, Cochrane Database Syst Rev (4):CD001479, 2005.
Institute of Medicine, Food and Nutrition Board. Dietary reference intakes: applications in dietary assessment. Washington, DC: National Academy Press; 2000.
Joanna Briggs Institute, Best practice: the effectiveness of interventions for infant colic. Best Practice Information Sheet 2008;12(6):1–4. available at www.joannabriggs.edu.au/pdf/BPIScolic.pdf [(accessed July 20, 2009)].
Jobe, AH. What do home monitors contribute to the SIDS problem? (editorial). JAMA. 2001;285(17):2244–2245.
Kapil, U. Ready to use therapeutic food (RUTF) in the management of severe acute malnutrition in India. Indian Pediatr. 2009;46(5):381–382.
Katz, KA, Mahlberg, MA, Honig, PJ, et al. Rice nightmare: kwashiorkor in two Philadelphia-area infants fed Rice Dream beverage. J Am Acad Dermatol. 2005;52(5 Suppl 1):S69–S72.
Katz-Salamon, M. Delayed chemoreceptor responses in infants with apnoea. Arch Dis Child. 2004;89(3):261–266.
Kawchak, DA, Schall, JI, Zemel, BS, et al. Adequacy of dietary intake declines with age in children with sickle cell disease. J Am Diet Assoc. 2007;107(5):843–848.
Kemper, KJ, Gardiner, P. Herbal medicines. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Kiechl-Kohlendorfer, U, Hof, D, Peglow, UP, et al. Epidemiology of apparent life threatening events. Arch Dis Child. 2005;90(3):297–300.
Koehler, SA. Sudden infant death syndrome deaths: the role of forensic nurses. J Forensic Nurs. 2008;4(3):141–142.
Krugman, SD, Dubowitz, H. Failure to thrive. Am Fam Physician. 2003;68(5):879–886.
Lanski, SL, Greenwald, M, Perkins, A, et al. Herbal therapy in a pediatric emergency department population: expect the unexpected. Pediatrics. 2003;111(5 Pt 1):981–985.
Lawrence, RA, Lawrence, RM. Breastfeeding: a guide for the medical profession, ed 6. Philadelphia: Saunders; 2005.
Leung, DYM. Atopic dermatitis (atopic eczema). In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Li, L, Zhang, Y, Zielke, RH, et al. Observations on increased accidental asphyxia deaths in infancy while cosleeping in the state of Maryland. Am J Forensic Med Pathol. 2009;30(4):318–321.
Liu, T, Howard, RM, Mancini, AJ, et al. Kwashiorkor in the United States: fad diets, perceived and true milk allergy, and nutritional ignorance. Arch Dermatol. 2001;137(5):630–636.
Lobo, ML, Kotzer, AM, Keefe, MR, et al. Current beliefs and management strategies for treating infant colic. J Pediatr Health Care. 2004;18(3):115–122.
Locklin, M. The redefinition of failure to thrive from a case study perspective. Pediatr Nurs. 2005;31(6):474–479. [495].
Lohse, B, Stotts, JL, Priebe, JR. Survey of herbal use by Kansas and Wisconsin WIC participants reveals moderate, appropriate use and identifies herbal education needs. J Am Diet Assoc. 2006;106(2):227–237.
Loman, DG. The use of complementary and alternative health care practices among children. J Pediatr Health Care. 2003;17(2):58–63.
Maggioni, A, Lifshitz, F. Nutritional management of failure to thrive. Pediatr Clin North Am. 1995;42(4):791–810.
Malloy, MH. Trends in postneonatal aspiration deaths and reclassification of sudden infant death syndrome: impact of the “Back to Sleep” program. Pediatrics. 2002;109(4):661–665.
Markowitz, R, Watkins, JB, Duggan, C. Failure to thrive: malnutrition in the pediatric outpatient setting. In Markowitz R, Watkins JB, Duggan C, eds.: Nutrition in pediatrics, ed 4, Hamilton, Ontario: BC Decker, 2008.
Matlik, L, Savaiano, D, McCabe, G, et al. Perceived milk intolerance is related to bone mineral content in 10- to 13-year- old female adolescents. Pediatrics. 2007;120(3):e669–e677.
McGarvey, C, McDonnell, M, Chong, A, et al. Factors relating to the infant’s last sleep environment in sudden infant death syndrome in the Republic of Ireland. Arch Dis Child. 2003;88(12):1058–1064.
McGuire, JK, Kulkarni, MS, Baden, HP. Fatal hypermagnesemia in a child treated with megavitamin/megamineral therapy. Pediatrics. 2000;105(2):414.
McLane, KM, Bookout, K, McCord, S, et al. The 2003 national pediatric pressure ulcer and skin breakdown prevalence survey: a multisite study. J Wound Ostomy Continence Nurs. 2004;31(4):168–178.
McMartin, KI, Platt, MS, Hackman, R, et al. Lung tissue concentrations of nicotine in sudden infant death syndrome. J Pediatr. 2002;140(2):205–209.
Mesich, HM. Mother-infant co-sleeping: understanding the debate and maximizing infant safety. MCN. 2005;30(1):30–37.
Messina, V, Melina, V, Mangels, AR. A new food guide for North American vegetarians. J Am Diet Assoc. 2003;103(6):771–775.
Mitchell, EA. What is the mechanism of SIDS? Clues from epidemiology. Dev Psychobiol. 2009;51(3):215–222.
Mitchell, EA, Thompson, JM, Becroft, DM, et al. Head covering and the risk for SIDS: findings from the New Zealand and German SIDS case-control studies. Pediatrics. 2008;121(6):e1478–e1483.
Morelli, J. Eczematous disorders. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Morin, K. The challenge of colic in infants. MCN. 2009;34(3):192.
Müller, O, Krawinkel, M. Malnutrition and health in developing countries. CMAJ. 2005;173(3):279–286.
1987. National Institutes of Health Consensus Development Conference on Infantile Apnea and Home Monitoring, Sept 29 to Oct 1, 1986. Pediatrics. 1987;79(2):292–299.
Neu, M, Robinson, JA. Infants with colic: their childhood characteristics. J Pediatr Nurs. 2003;18(1):12–20.
Noonan, C, Quigley, S, Curley, MAQ. Skin integrity in hospitalized infants and children: a prevalence survey. J Pediatr Nurs. 2006;21(6):445–453.
Nowak-Wegrzyn, A, Bloom, KA, Sicherer, SH, et al. Tolerance to extensively heated milk in children with cow’s milk allergy. J Allergy Clin Immunol. 2008;122(2):342–347.
O’Connor, NR. Infant formula. Am Family Physician. 2009;79(7):565–570.
O’Connor, NR, McLaughlin, MR, Ham, P. Newborn skin, part I, Common problems. Am Fam Physician. 2008;77(1):47–52.
Ong, PY, Boguniewicz, M. Atopic dermatitis. Primary Care. 2008;35(1):105–117.
Penny, ME. Protein-energy malnutrition: pathophysiology, clinical consequences, and treatment. In Walker WA, Watkins JB, Duggan C, eds.: Nutrition in pediatrics, ed 3, Hamilton, Ontario: BC Decker, 2003.
Perrotta, S, Nobili, B, Rossi, F, et al. Infant hypervitaminosis A causes severe anemia and thrombocytopenia: evidence of a retinol-dependent bone marrow cell growth inhibition. Blood. 2002;99(6):2017–2022.
Perry, CL, McGuire, MT, Neumark-Sztainer, D, et al. Adolescent vegetarians: how well do their dietary patterns meet the healthy people 2010 objectives? Arch Pediatr Adolesc Med. 2002;156(5):426–427.
Person, TL, Lavezzi, WA, Wolf, BC. Cosleeping and sudden unexpected death in infancy. Arch Pathol Lab Med. 2002;126(3):343–345.
Pessler, F, Nejat, M. Anaphylactic reaction to goat’s milk in a cow’s milk–allergic infant. Pediatr Allergy Immunol. 2004;15(2):183–185.
Pitetti, RD, Whitman, E, Zaylor, A. Accidental and nonaccidental poisonings as a cause of apparent life-threatening events in infants. Pediatrics. 2008;122(2):e359–e362.
Pollack, HA. Sudden infant death syndrome, maternal smoking during pregnancy and effectiveness of smoking cessation intervention. Am J Pub Health. 2001;91(3):432–436.
Preston, AM, Rodriguez, C, Rivera, CE. Plasma ascorbate in a population of children: influence of age, gender, vitamin C intake, and smoke exposure. P R Health Sci J. 2006;25(2):137–142.
Preston, AM, Rodriguez, C, Rivera, CE, et al. Influence of environmental tobacco smoke on vitamin C status in children. Am J Clin Nutr. 2003;77(1):167–172.
Puffenberger, EG, Hu-Lince, D, Parod, JM, et al. Mapping of sudden infant death with dysgenesis of the testis syndrome (SIDDT) by a SNP genome scan and identification of TSPYL loss of function. Proc Natl Acad Sci USA. 2004;101(32):11,689–11,694.
Ramanathan, R, Corwin, MJ, Hunt, DE, et al. Cardiorespiratory events recorded on home monitors: comparison of healthy infants with those at increased risk for SIDS. JAMA. 2001;285(17):2199–2243.
Richardson, HL, Walker, AM, Horne, RS. Maternal smoking impairs arousal patterns in sleeping infants. Sleep. 2009;32(4):515–521.
Roberts, DM, Ostapchuk, M, O’Brien, J. Infantile colic. Am Fam Physician. 2004;70(4):735–740. [741-742].
Rudolf, MCJ, Logan, S. What is the long term outcome for children who fail to thrive? A systematic review. Arch Dis Child. 2005;90(9):925–931.
Saltzman, MD, King, EC. Central physeal arrests as a manifestation of hypervitaminosis A. J Pediatr Orthop. 2007;27(3):351–353.
Sampson, HA. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805–819.
Sampson, HA, Leung, DYM. Adverse reactions to foods. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Sampson, HA, Leung, DYM. Anaphylaxis. In Kliegman RM, Behrman RE, Jenson HB, et al, eds.: Nelson textbook of pediatrics, ed 18, Philadelphia: Saunders, 2007.
Savino, F, Pelle, E, Palumeri, E, et al. Lactobacillus reuteri (American type culture collection strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics. 2007;119(1):e124–e130.
Sawni, A, Ragothaman, R, Thomas, RL, et al. The use of complementary/alternative therapies among children attending an urban pediatric emergency department. Clin Pediatr. 2007;46(1):36–41.
Sibley, E. Carbohydrate intolerance. Curr Opin Gastroenterol. 2004;20(2):162–167.
Sicherer, SH. Clinical aspects of gastrointestinal food allergy in children. Pediatrics. 2003;111(6 Pt 3):1609–1616.
Silvestri, JM, Weese-Mayer, DE. Disorders of respiratory control: apnea and SIDS. In Rudolph CD, Rudolph AM, Hostetter MK, eds.: Rudolph’s pediatrics, ed 21, New York: McGraw-Hill, 2003.
Simons, FE. Anaphylaxis: recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124(4):625–636.
Stahler, C, How many youth are vegetarian? Vegetarian J 2005;4:1–2. available at www.vrg.org/journal/vj2005issue4/vj2005issue4youth.htm [(accessed July 3, 2009)].
St. James-Roberts, I. Infant crying and sleeping: helping parents to prevent and manage problems. Primary Care. 2008;35(3):547–567.
Tablizo, MA, Jacinto, P, Parsley, D, et al. Supine sleeping position does not cause clinical aspiration in neonates in hospital newborn nurseries. Arch Pediatr Adolesc Med. 2007;161(5):507–510.
Tappin, D, Ecob, R, Brooke, H. Bedsharing, roomsharing, and sudden infant death syndrome in Scotland: a case-control study. J Pediatr. 2005;147(1):32–37.
Thompson, DG. Safe sleep practices for hospitalized infants. Pediatr Nurs. 2005;31(5):400–403. [409].
Unger, B, Kemp, JS, Wilkins, D, et al. Racial disparity and modifiable risk factors among infants dying suddenly and unexpectedly. Pediatrics. 2003;111(2):e127–e131.
Vennemann, MM, Bajanowski, T, Brinkmann, B, et al. Sleep environment risk factors for sudden infant death syndrome: the German Sudden Infant Death Syndrome Study. Pediatrics. 2009;123(4):1162–1170.
Visscher, MO, Recent advances in diaper dermatitis: etiology and treatment. Pediatr Health 2009;3(1):81–98. available at www.medscape.com/viewarticle/589066_print [(accessed April 14, 2009)].
Wang, J, Sampson, HA. Food anaphylaxis. Clin Exp Allergy. 2007;37(5):651–660.
Wasserbauer, N, Ballow, M. Atopic dermatitis. Am J Med. 2009;122(2):121–125.
Weizman, Z, Alkrinawi, S, Goldfarb, D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122(4):650–652.
Williams, HC. Atopic dermatitis. N Engl J Med. 2005;352(22):2314–2324.
World Health Organization. HIV and infant feeding update. Geneva: The Organization; 2006.
Zeisel, SH, Erickson, KE. Dietary supplements (nutraceuticals. In Walker WA, Watkins JB, Duggan C, eds.: Nutrition in pediatrics, ed 3, Hamilton, Ontario: BC Decker, 2003.
*Yogurt does not contain adequate amounts of vitamins A and D yet is an acceptable source of calcium and phosphorus.
*Helpful websites for health care and consumer information concerning herbs are National Center for Complementary and Alternative Medicine, www.nccam.nih.gov; American Botanical Council, www.herbalgram.org; and Herb Research Foundation, www.herbs.org.
*Further information regarding vegetarian diets may be found at the Vegetarian Resource Group, PO Box 1463, Baltimore, MD 21203; 410-366-8343; www.vrg.org.
†For information on the DRIs go to the Institute of Medicine website, www.iom.edu, and click on the link for Food and Nutrition; or call 202-334-2352.
*Further information for parents of infants with food allergies is available from the American Academy of Allergy, Asthma and Immunology, 555 E. Wells St., Suite 1100, Milwaukee, WI 53202; 414-272-6071; www.aaaai.org. Additional helpful websites for information on food allergy include MedlinePlus (sponsored by U.S. National Library of Medicine and National Institutes of Health), http://medlineplus.gov; Food Allergy and Anaphylaxis Network, 800-929-4040, www.foodallergy.org; National Institute of Allergy and Infectious Diseases, www3.niaid.nih.gov; and www.allergicchild.com.
*Parents may find updated resources on lactose intolerance at www.allallergy.net.
*Parents may find helpful resources at www.colicnet.com. Additional resources for parents include the following: Jones S, Ziedrich L, Thompson M: Crying Baby, Sleepless Nights: Why Your Baby Is Crying and What You Can Do About It, Cambridge, Mass, 1992, Harvard Common Press; and Sears W, Sears M: The Fussy Baby Book: Parenting Your High-Need Child from Birth to Five, New York, 1996, Little Brown.
*Training is required to use the feeding scale. For information, contact NCAST Programs, University of Washington, PO Box 357920, Seattle, WA 98195; 206-543-8528; e-mail: ncast@u.washington.edu; www.ncast.org.
*Parents may also find helpful information at the American Academy of Dermatology, 866-503-7546; www.aad.org; and for pamphlets www.aad.org/public/publications/pamphlets/skin_eczema.html; and the National Eczema Association, 800-818-7546; www.nationaleczema.org.
†Back to Sleep materials may be ordered by contacting NICHD Information Resource Center, Back to Sleep, PO Box 3006, Rockville, MD 20847; 800-370-2943; fax: 866-760-5947; www.nichd.nih.gov/sids.
*American SIDS Institute, 509 Augusta Drive, Marietta, GA 30067; 800-232-SIDS, 770-426-8746; www.sids.org; First Candle, 1314 Bedford Ave., Suite 210, Baltimore, MD 21208; 800-221-7437; www.sidsalliance.org; National Sudden and Unexpected Infant/Child Death and Pregnancy Loss Resource Center, Georgetown University, Box 571272, Washington, DC 20057-1272; 866-866-7437, 202-687-7466; www.sidscenter.org.