CHAPTER 15 Oral Hygiene Assessment: Soft and Hard Deposits
ORAL HYGIENE ASSESSMENT
Oral hygiene is the degree to which the oral cavity is kept clean and free of soft and hard deposits by daily oral self-care or, when necessary, oral care provided by a caregiver. Before the dental hygienist can influence a client's oral health behavior, it is necessary to assess and document the client's current oral hygiene status. Oral hygiene assessment is the process of determining the following in the client:
 Amount of hard tooth deposits (extrinsic dental stain, dental calculus) and soft tooth deposits (food debris, materia alba, dental plaque biofilm)
Amount of hard tooth deposits (extrinsic dental stain, dental calculus) and soft tooth deposits (food debris, materia alba, dental plaque biofilm)Assessment Tools
Concepts for Oral Hygiene Assessment
Soft and hard dental deposits are assessed according to:
Assessment also involves evaluating the client's knowledge, skill, attitude, and motivation related to oral self-care. Table 15-1 describes the soft and hard deposits that accumulate in the oral cavity. Of these deposits, oral biofilm (bacterial plaque or dental plaque) is a risk factor for dental caries and periodontal diseases. Stain and calculus do not cause oral disease but rather provide irregular surfaces that retain bacterial plaque on teeth, dental appliances, and adjacent periodontal structures and have esthetic implications. The location, amount, and extent of oral biofilm, stain, and calculus, and to a lesser degree food debris and materia alba, are important variables to measure and record during baseline assessment and at continued-care intervals. Oral hygiene assessment findings must be understood by clients for the encouragement of self-care, prevention of oral disease, and health promotion.
TABLE 15-1 Soft and Hard Deposits Found in the Oral Cavity
| Term | Classification | Definition |
|---|---|---|
| Acquired pellicle and exogenous dental cuticle | Acellular, nonmineralized layer | An unstructured, homogenous film adhering to tooth surfaces, firm surfaces in the oral cavity, and old calculus; may be stained by tar products and tannin |
| Oral biofilm | Cellular, nonmineralized layer | A dense, transparent, nonmineralized, highly organized mass of bacterial colonies in a gel-like intermicrobial, enclosed matrix; a host-associated biofilm |
| Materia alba | Cellular, nonmineralized layer | Loose deposit of microorganisms, desquamated epithelial cells, and broken down food debris; white to yellowish-white in color; has cottage cheese–like appearanceCan be displaced with rinsing and water irrigation |
| Food debris | Cellular nonmineralized layer | Unstructured particles that remain in the mouth after eating and are removed with irrigation unless impacted between the teeth |
| Extrinsic stain | Cellular, may be mineralized or nonmineralized | Discolorations that accumulate on the external surface of the tooth via pellicle, plaque biofilm, or calculus; can be removed by power toothbrushing, scaling, and/or polishing |
| Supragingival calculus | Cellular, mineralized layer | Mineralized bacterial plaque permeated with moderately hard calcium phosphate crystals; superficially covered with bacterial plaque biofilm; usually white or yellowish-white in color but may be stained darker |
| Subgingival calculus | Cellular, mineralized layer | Mineralized bacterial plaque; adheres to tooth structure in gingival sulcus; organic matrix of bacteria permeated with hard calcium phosphate crystals;may be stained dark green to greenish-black; superficially covered with bacterial plaque biofilm |
Recognition of bacterial plaque as a biofilm (see section on oral biofilm) and as a major risk factor for dental caries, periodontal disease, and oral malodor is key to effective care planning. About 20% of the oral environment is occupied by teeth, the target for toothbrushing and interdental cleaning. The remaining 80% of the mouth includes the oral mucous membrane and specialized mucosa of the tongue. Pathogenic microorganisms can grow on all oral soft and hard surfaces and in saliva too. By understanding the bacterial load present in the oral cavity, mechanical and antimicrobial interventions can reduce oral biofilm in the entire mouth.
About 50% to 90% of the population exhibits some type of periodontal disease. When disease is present, usually there is a corresponding need for greater personal responsibility for oral health or knowledge about the pathogenicity of oral biofilm and its control. These facts underscore the importance of effective oral hygiene assessment and the corresponding client education plan that assessment results guide. Oral hygiene assessment allows the dental hygienist to determine the client's unmet human needs (e.g., need for responsibility for oral health, conceptualization and problem solving, protection from health risks), communicate these unmet needs to clients, and instruct them in effective self-care behaviors. Individualized oral hygiene instruction is important in motivating a client; no one wants the “one-size-fits-all brush-and-floss lecture.”
ORAL DEPOSITS
Oral Biofilm
A biofilm is a complex, highly organized, three-dimensional communal arrangement of microorganisms adhering to a surface where moisture and nutrients are available. Unlike free-floating (planktonic) bacteria, bacteria in a biofilm community are able to maximize nutrients, keep their community clean, communicate with one another when threatened, protect the community when under attack, and even relocate to start new biofilm communities. A host-associated biofilm, dental plaque (also known as microbial plaque, dental plaque, oral biofilm, dental plaque biofilm, bacterial plaque biofilm) is a dense, nonmineralized mass of bacterial colonies in a gel-like intermicrobial, enclosed matrix (slime layer) that is attached to a moist environmental surface (Figure 15-1, A and B). The biofilm lends other protective properties to the associated bacteria, including resistance to antibacterial agents such as chlorhexidine, gluconate, fixed combination of essential oils, cetylpyridinium chloride, and systemic antibiotics, and host defense mechanisms (immune system and inflammation). A network of slime layers of polysaccharides protects the biofilm bacteria from the host's immune system's defensive cells (neutrophils, leukocytes, macrophages, and lymphocytes) and antimicrobial and antibiotic agents. Bacteria within the biofilm adhere to one another and to tooth surfaces, dental appliances, restorations, the oral mucosa, the specialized mucosa of the tongue, and alveolar bone. Some bacteria are unattached and free floating (Figure 15-1, C).
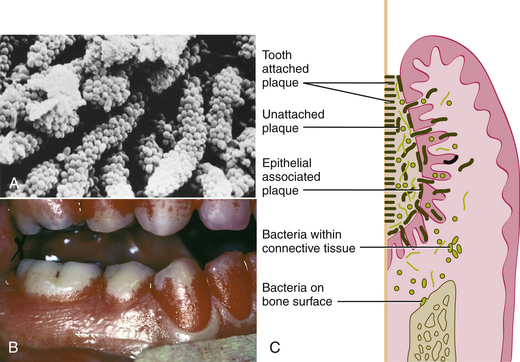
Figure 15-1 Oral biofilm. A, Long-standing supragingival plaque near the gingival margin demonstrates “corncob” arrangement. A central gram-negative filamentous core supports the outer coccal cells, which are firmly attached by interbacterial adherence or coaggregation. B, Disclosed supragingival plaque covering one third to two thirds of the clinical crown. C, Diagram depicting the plaque bacteria associated with tooth surface and periodontal tissues.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Dental caries and gingival and periodontal infections are caused by microorganisms in oral biofilms. Biofilm-enclosed bacteria benefit from the concentration and retention of metabolites that are produced by the bacteria, which enhances interactions among the species of bacteria. The structure of the plaque biofilm includes channels that use the motion of saliva within the oral cavity to extend bacterial colonization, receive nutrients, and transport bacterial wastes. The biofilm creates its own renewing source of lipopolysaccharide for long-term survival of microorganisms. There are loosely attached and unattached bacteria at the surface of the plaque biofilm (see Figure 15-1, C). Bacteria within the biofilm store sugars inside their cells and extend the time of their lactic acid production. This prolonged exposure to lactic acid causes the decalcification observed in dental caries. Because of these protective and self-sustaining properties of the biofilm, associated bacteria are likely to survive within the mouth, and oral diseases become chronic. Recognition of the self-sustaining nature of the biofilm community helps explain why periodontal disease is difficult to control and why periodontal pathogens resist antimicrobial agents, antibiotic therapies, and host-defense mechanisms (Figure 15-2).
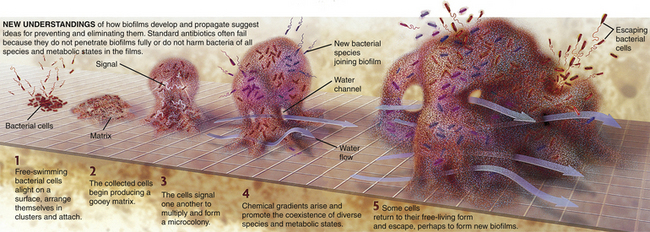
Figure 15-2 How biofilms form.
(Illustration by Keith Kasnot. Licensed for use, Philips Oral Healthcare, Inc, Andover, Massachusetts.)
Microorganisms within Oral Biofilm (Figure 15-3)
Supragingival Microorganisms
In healthy mouths, oral biofilm is mainly supragingival and confined to enamel surfaces and oral mucosa. Typically the bacteria associated with healthy dental plaque are aerobic gram-positive rods and cocci, with very few motile species. The bacterial species associated with periodontal health include Streptococcus mitis, Actinomyces species, and Streptococcus oralis (sanguis II). As undisturbed plaque matures, the bacterial population changes to a predominately gram-negative flora; this change in bacterial species brings signs of oral infection and inflammation.
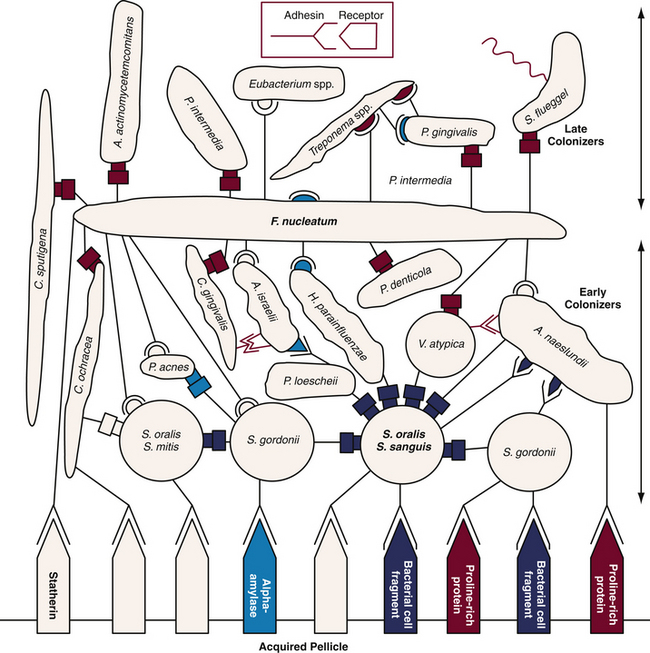
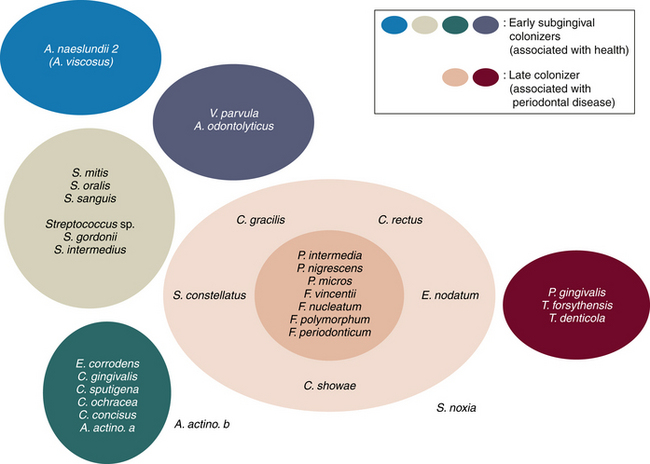
Figure 15-3 Diagrammatic representation of initial plaque formation by different bacterial species. Early colonizers bind to receptors in the acquired pellicle. Each adherent cell becomes the new surface or bridge for late colonizers.
(Adapted from Kolenbrandewr PE, London J: Adhere today, here tomorrow: oral bacterial adherence, J Bacteriol 175:3247, 1993.)
Subgingival Microorganisms
In dental plaque–induced gingival disease, there is an increase in both the quantity and quality of plaque. As supragingival plaque grows undisturbed, it extends subgingivally. Bacterial species associated with dental plaque–induced gingival disease include gram-negative spirochetes and motile rods such as Fusobacterium nucleatum, species of Prevotella and Treponema, and Campylobacter rectus. In advancing periodontal disease, plaque is characterized by a zone of gram-positive organisms attached to the tooth surface, and a loosely adherent zone of gram-negative species adjacent to the pocket wall. Bacteria associated with advancing periodontal disease are predominantly anaerobic and include Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythensis, and Peptostreptococcus micros. Color designations of plaque based on pathogenicity are shown in Figure 15-4. This color-coding has been used to differentiate bacterial complexes associated with health and disease severity. Early subgingival colonizers are in the blue, yellow, green, and purple complexes. Late colonizers, the orange and red complexes, are associated with mature subgingival plaque, periodontal pocketing, and clinical attachment loss.
Stages of Oral Biofilm Formation
Plaque formation occurs in four distinct stages: initial adherence, lag phase, rapid growth bacterial colonization, and steady state (see Figures 15-2 and 15-3). Within these stages, distinct changes take place within the overall biofilm.
Initial Adherence
The first stage is the deposition of salivary components (the acquired pellicle), a tenacious, insoluble, acellular protein film composed of glycoproteins found within saliva on oral surfaces. Although pellicle performs a protective function, acting as a barrier to acids, it also serves as the initial site of attachment for free-swimming (planktonic) bacteria beginning the first stage of biofilm development. Salivary proteins and peptides promote bacterial adhesion to oral surfaces. Immediately after cleansing of tooth surfaces, the pellicle begins to reform on exposed surfaces; within 1 hour, free-floating microorganisms attach to the acquired pellicle and begin sessile colonies. Gram-positive cocci are the first microorganisms to colonize the teeth. Early plaque, 1 to 2 days old, consists primarily of aerobic, gram-positive cocci such as Streptococcus mutans and Streptococcus sanguis. Primarily because of the methods of bacterial adhesion, plaque is not removed by oral irrigation; removal of the plaque biofilm requires mechanical action, such as toothbrushing and interdental cleaning with floss, a brush, or a wooden wedge.
Lag Phase
As the planktonic bacteria transition into sessile life, there is a lag in bacterial growth. Bacterial colonization occurs in stratified layers against the tooth surface. On days 2 through 4, filamentous forms grow on the surface of the coccal colonies and begin to infiltrate the sessile colonies, replacing the cocci.
Rapid Growth Bacterial Colonization
During rapid growth, adherent bacteria secrete extracellular polysaccharides to form the water-insoluble slime matrix. The matrix is composed of saliva and polysaccharides produced by the bacteria in response to dietary sucrose and fermentable carbohydrates. Protected by the matrix, microcolonies begin to form. The polysaccharides are sticky, and therefore further facilitate plaque adhesion. A client may experience this phenomenon as the “furry” or “filmy” feeling sometimes detected on the teeth; that furry feeling is the polysaccharides. In addition to providing a method of adherence for the bacterial colonies, these polysaccharides trap other nutrients, are a reserve food source for the bacteria, and contribute to the other protective functions of the biofilm. Additional varieties of bacteria coaggregate with the early colonizers, leading to structural stratification within the thickening of the biofilm.
By days 4 through 7, filamentous forms increase and rods and fusobacteria appear. Vibrios and spirochetes appear during days 7 through 14; overall the load of gram-negative anaerobic species increases, and white blood cells are found within the plaque. Clinically, signs of inflammation begin to be observed.
Steady State and Detachment
Initial formation is within distinct colonies that form from the indigenous oral microflora, but as the growth process continues an intermicrobial matrix (protective slime layer) connects the bacterial colonies.
The biofilm is now a fully functioning community of different species living symbiotically in “slime city.” Bacteria within the interior of the biofilm slow their growth or become static. Deep within the biofilm, bacteria show signs of death, disrupted cell walls and loss of cytoplasm, whereas bacteria near the surface remain intact. The toxic wastes of one species are the resources of another. Some surface bacteria detach and relocate to form new biofilm colonies. Crystals found in the interbacterial matrix may be initial calculus formation.
The plaque ages and undergoes a distinct change in population. By days 14 through 24, gingivitis is generally clinically evident, and the biofilm is composed of densely packed vibrios, spirochetes, and filamentous bacteria. As the biofilm colony matures, it blooms into a mushroom shape attached by a narrow base and incorporates channels that capitalize on the fluid movement present in the oral cavity. These fluid channels distribute nutrients, remove wastes, and allow for free-swimming bacteria to leave and begin new biofilm colonies. It is easy to appreciate the importance of thorough, daily mechanical plaque disruption and removal to inhibit the destructive processes of mature plaque. The longer the oral biofilm remains undisturbed, the greater its pathogenic potential for the host (see Figure 15-4). In response, the host's normal response to injury, inflammation, and foreign bodies, the immune response, is activated and eventually overresponds (Tables 15-2 and 15-3). It is the effects of the proinflammatory mediators and inability of the immune system to reach the site of injury or infection that causes the connective tissue and bone destruction in periodontal disease (see Chapter 17).
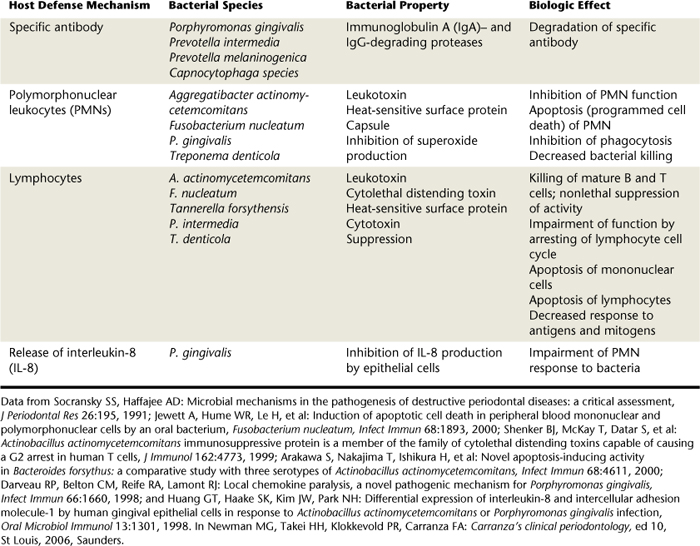
TABLE 15-3 Bacteria Enzymes Capable of Degrading Host Tissues
| Bacterial Enzyme | Species |
|---|---|
| Collagenase | |
| Trypsin-like enzyme | |
| Arylsulfatase | Campylobacter rectus |
| Neuraminidase | |
| Fibronectin-degrading enzyme | |
| Phospholipase A |
From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.
CLINICAL ASSESSMENT OF ORAL BIOFILM
Clinically, plaque manifests as a transparent film that begins to form within minutes after a surface has been cleaned. Although plaque can be difficult to visualize, it can be detected in several ways. It can be discriminated by direct vision, particularly if there are thick, furlike deposits of plaque or if it has acquired yellow, tan, or brown stains. Some people feel plaque as a fuzzy coating on their teeth, dental appliances, and tongue. Most commonly, however, the presence of plaque is assessed by passing a dental explorer over the tooth surface near the gingival margin to scrape up some plaque, making it easier to see, or by using disclosing agents.
Disclosing Agents
Disclosing agents, also known as disclosants, are used to make oral biofilm clinically visible (Figure 15-5). Available over the counter in liquid or tablet form, disclosants contain ingredients that temporarily stain plaque biofilm so that it can be observed and measured. Erythrosin dye, the most commonly employed agent, stains oral biofilm red. Two-tone disclosing agents that stain thicker plaque biofilm blue and thinner plaque red are also available. Ideally, disclosing agents should do the following:
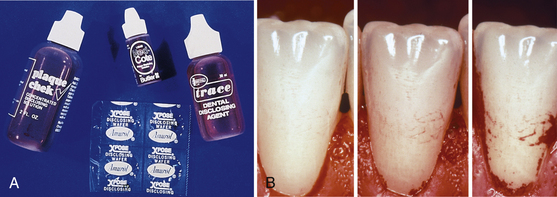
Figure 15-5 Use of disclosing agents to monitor oral biofilm on teeth. A, Examples of plaque biofilm disclosants. B, Clinical photos of the typical topography of plaque growth. Initial growth starts along the gingival margin and from the interdental space to extend farther in a coronal direction.
(B, From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Because disclosants can camouflage clinical signs of disease, disclosing agents should be applied after the oral and periodontal assessment and after the client sees the oral findings in his or her own mouth. The location of oral biofilm also should be seen by the client before disclosing deposits, so that the client understands the correlation among oral hygiene, infection, inflammation, and oral disease risks (see Chapters 16 and 17).
After performing the gingival assessment and instructing the client on the composition and detrimental effects of plaque biofilm, a non–petroleum-based lubricant can be applied to the lips and esthetic dental restorations to prevent them from staining. Petroleum-based products are not recommended because they break down the protective latex barrier of the clinician's gloves.
Disclosing techniques depends on the product used.
 Solutions are applied as a concentrate with a cotton swab or diluted with water in a cup for the client to use as an oral rinse.
Solutions are applied as a concentrate with a cotton swab or diluted with water in a cup for the client to use as an oral rinse.Clean tooth surfaces do not absorb the dye unless roughness is present (e.g., demineralization, hypocalcification, restorations, cementum). Acquired pellicle, plaque biofilm, debris, and calculus absorb the disclosing agent. This discriminate staining characteristic makes the disclosing agent an excellent oral hygiene aid because the client is able to use it at home for self-evaluation. Seeing, feeling, and smelling the oral biofilm deposits teaches and motivates individuals to improve and monitor their self-care effectiveness.
After application of the disclosant, excess is expectorated or suctioned from the mouth and the client is given a hand mirror to identify the stained deposits. The dental hygienist assists the client in identifying deposits and correlates findings with areas of gingival inflammation, periodontal disease parameters, and dental caries identified before staining. The client is then queried about what he or she wants to do or can do about the oral deposits. Mechanical and chemotherapeutic plaque control techniques are taught to improve oral hygiene and oral health. Instructions from the dental hygienist are followed by direct observation of the client's self-care technique. Each area of concern should be practiced because the client may need guidance adapting the toothbrush or interdental cleaner.
Assessment
Start assessment of oral biofilm by its location.
 Supragingivally, coronal to the free gingival margin on the clinical crown of the tooth, and subgingivally, apical to the margin of the free gingiva. Supragingival locations include the occlusal surfaces (most common in areas without opposing teeth), buccal or lingual fissures and pits, interproximal tooth surfaces, and free gingival margin.
Supragingivally, coronal to the free gingival margin on the clinical crown of the tooth, and subgingivally, apical to the margin of the free gingiva. Supragingival locations include the occlusal surfaces (most common in areas without opposing teeth), buccal or lingual fissures and pits, interproximal tooth surfaces, and free gingival margin. Subgingival plaque accumulates in the sulcus or periodontal pocket on all four aspects of the tooth (buccal, lingual, mesial, and distal interproximal spaces).
Subgingival plaque accumulates in the sulcus or periodontal pocket on all four aspects of the tooth (buccal, lingual, mesial, and distal interproximal spaces).Next, a determination is made about the amount of plaque present (e.g., is it light, moderate, or heavy?). Extent is an assessment about whether the plaque is generalized throughout the dentition or localized to several teeth. Oral biofilm is influenced by host mediating factors; therefore oral hygiene assessment includes the host's response to the plaque. In health there is a balance point between the plaque and the host where no irreparable damage occurs. If the biofilm bacteria cause tissue destruction that exceeds the reparative ability of the host, disease occurs. Quality of plaque is more important than quantity of plaque. Quality of the plaque (types of microorganisms present) and the client's host response to that bacterial challenge guide both clinician and client. For example, a client with a high plaque score in the lingual region of the mouth, plaque-free facial tooth surfaces, and healthy gingival tissue clearly requires instructions targeting the lingual areas, while reinforcing effective techniques in the facial area. A client with a small quantity of plaque accumulation but with severe gingival bleeding requires a different approach to care. The client's oral contributing factors influence the growth, retention, and removal of oral biofilm:
 Tight lingual frenum interferes with natural self-cleansing action of the tongue. Papillae on the tongue are conducive to oral biofilm growth (coated tongue).
Tight lingual frenum interferes with natural self-cleansing action of the tongue. Papillae on the tongue are conducive to oral biofilm growth (coated tongue). Faulty restorations with open or overhanging margins or poorly contoured surfaces readily harbor plaque.
Faulty restorations with open or overhanging margins or poorly contoured surfaces readily harbor plaque. Missing teeth contribute to plaque retention and inhibit the self-cleaning of occlusal surfaces during mastication.
Missing teeth contribute to plaque retention and inhibit the self-cleaning of occlusal surfaces during mastication. Malocclusions results in crowding and tipping of teeth, which can make plaque removal difficult or lead to traumatic occlusion, resulting in widened PDL spaces that lend themselves to greater plaque accumulation.
Malocclusions results in crowding and tipping of teeth, which can make plaque removal difficult or lead to traumatic occlusion, resulting in widened PDL spaces that lend themselves to greater plaque accumulation. Mouth breathing, with its drying effects on oral tissues, favors growth of oral biofilm in the absence of the bactericidal action of saliva. Ropey, viscous saliva is less self-cleansing than watery saliva.
Mouth breathing, with its drying effects on oral tissues, favors growth of oral biofilm in the absence of the bactericidal action of saliva. Ropey, viscous saliva is less self-cleansing than watery saliva.All of these factors influence the retention of bacterial plaque and can make oral plaque control challenging.
Tooth Stains
Tooth stain is a discolored accretion or area on a tooth contrasting with the rest of the tooth color (Figures 15-6 and 15-7). Stains are divided into intrinsic stains and extrinsic stains.
 Intrinsic stains are incorporated within the tooth structure itself and cannot be removed by scaling or polishing. Such stains result from alterations during the development of the tooth (embryonic to 6 years of age) associated with antibiotic use, fever, trauma, infection, and ingestion of high amounts of systemic fluoride. Examples include dental fluorosis (a mottled, opaque, or brownish discoloration caused by ingesting excessive amounts of fluoride during enamel formation) and tetracycline stain (a yellow, brown, gray, or orange discoloration within the substance of the tooth from ingestion of the antibiotic when the tooth is developing) (see Figure 15-6).
Intrinsic stains are incorporated within the tooth structure itself and cannot be removed by scaling or polishing. Such stains result from alterations during the development of the tooth (embryonic to 6 years of age) associated with antibiotic use, fever, trauma, infection, and ingestion of high amounts of systemic fluoride. Examples include dental fluorosis (a mottled, opaque, or brownish discoloration caused by ingesting excessive amounts of fluoride during enamel formation) and tetracycline stain (a yellow, brown, gray, or orange discoloration within the substance of the tooth from ingestion of the antibiotic when the tooth is developing) (see Figure 15-6). Extrinsic stains occur on the tooth surface and usually can be removed by coronal polishing or scaling. Method of attachment is the acquired pellicle; without pellicle, stains cannot adhere to the smooth enamel surfaces. Extrinsic stains develop because of the presence of chromogenic bacteria (color-producing bacteria); use of staining substances such as tobacco, red wine, tea, coffee, soda, blueberries, and some drugs; and exposure to metallic compounds (see Figure 15-7).
Extrinsic stains occur on the tooth surface and usually can be removed by coronal polishing or scaling. Method of attachment is the acquired pellicle; without pellicle, stains cannot adhere to the smooth enamel surfaces. Extrinsic stains develop because of the presence of chromogenic bacteria (color-producing bacteria); use of staining substances such as tobacco, red wine, tea, coffee, soda, blueberries, and some drugs; and exposure to metallic compounds (see Figure 15-7).
Figure 15-6 Intrinsic tooth stains. A, Dental fluorosis. B, Tetracycline stain.
(From Ibsen OAC, Phelan JA: Oral pathology for the dental hygienist, ed 5, St Louis, 2009, Saunders.)
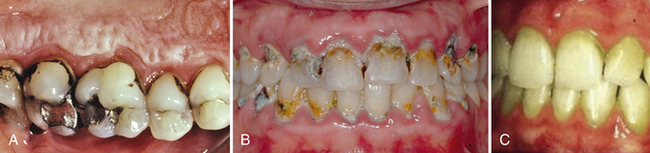
Figure 15-7 Extrinsic tooth stains. A, Tobacco stain. B, Orange stain in person with poor oral hygiene, severe periodontal disease, and rampant caries. C, Green stain.
(A, From Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders. B, Courtesy Dr. Thomas E. O'Connor, St Louis, Missouri; and Dr. Kevin Thorpe, St Louis, Missouri. C, From Scully C, Welbury R, Flaitz C, Paes de Almeida O: A color atlas of orofacial health and disease in children and adolescents, ed 2, Oxford, England, 2002, Taylor and Francis.)
Of the extrinsic stains, green stain is attributed to chromogenic bacteria, Penicillium and Aspergillus. Green stain, found in poor oral hygiene, occurs near the cervical third of the teeth. This stain can easily become incorporated within decalcified enamel. Orange stain, less common than other types of stains, is also associated with poor oral hygiene. This stain occurs frequently on anterior teeth and is believed to be due to the presence of chromogenic bacteria Serratia marcescens and Flavobacterium lutescens.
Chromogenic stain can usually be removed safely with 3% hydrogen peroxide to loosen and bleach the stain, followed by selective polishing and in-office fluoride therapy. If the area under the stain is decalcified, scaling is contraindicated owing to the risk of damaging demineralized tooth surface, and at-home fluoride may be prescribed to remineralize the tooth surface.
Sources of tooth stain can often be identified by the color of the stain and client self-reported information about lifestyle behavior, diet, work environment, and oral habits. Identification of the stain and its source assists in developing a specific care plan that facilitates stain control and a more esthetic appearance for clients. The client can often reduce stain formation with improved oral hygiene practices and appropriate over-the-counter product selection (e.g., whitening toothpaste, power toothbrushes, frequent tooth cleaning). Table 15-4 describes some common dental stains. See Chapter 27 for professional management of tooth stains.
TABLE 15-4 Types of Tooth Stains
| Type | Source | Clinical Approach |
|---|---|---|
| Extrinsic Stains | ||
| Green | Chromogenic bacteria and fungi (Penicilium and Aspergillus species) from poor oral hygiene; most often seen in children with enamel irregularities | Should not be scaled because of underlying demineralized enamel. Have client remove during toothbrush instruction or lightly polish; may use hydrogen peroxide to help with bleaching and removal. |
| Black stain | Iron in saliva; iron-containing oral solutions; Actinomyces species; industrial exposure to iron, manganese, and silver | Firmly scale because of calculus-like nature and selectively polish for complete removal. |
| Orange | Chromogenic bacteria (Serratia marcescens and Flavobacterium lutescens) from poor oral hygiene | Lightly scale and then polish selectively. |
| Brown stains | ||
| Topical medications | Stannous fluoride, chlorhexidine, or cetylpyridinium chloride mouth rinses | Lightly scale and then polish selectively. |
| Yellow | Oral biofilm | Have client remove during toothbrush instruction. |
| Blue-green stain | Mercury and lead dust | Lightly scale and then polish selectively. |
| Red-black stain | Chewing betel nut, betel leaf, and lime (pan); found in Western pacific and South Asian cultures | Firmly scale and then polish selectively. |
| Intrinsic Stains | ||
| Dental fluorosis (white-spotted to brown-pitted enamel) | Excessive fluoride ingestion during enamel development | Cannot be removed by scaling or selective polishing. |
| Hypocalcification (white spots on enamel) | High fever during enamel formation | Cannot be removed by scaling or selective polishing. |
| Demineralization (white or brown spots on enamel, may be smooth or rough) | Acid erosion of enamel caused by oral biofilm | Cannot be removed by scaling or polishing. Recommend daily 0.05% sodium fluoride rinses for remineralization. |
| Tetracycline (grayish brown discoloration) | Ingestion of tetracycline during tooth development | Cannot be removed by scaling or selective polishing. |
Brown stains can have multiple causes. Tobacco use causes dark brown, tenacious stains that can become intrinsic; tobacco stains do not necessarily correlate with the amount of tobacco used. Food stains may also be tan to brown and result from the ingestion of foods with tannins, such as red wine, sodas, coffee, tea, and certain fruits. Agents such as 0.12% chlorhexidine gluconate mouthrinse, cetylpyridinium chloride mouth rinse, and stannous fluoride dentifrice or mouth rinse may also impart a brown stain if used twice daily over 2 to 3 months. These stains, related to the substantivity of the product, may be somewhat difficult to remove and often require scaling in addition to selective polishing. Yellow stain is most commonly associated with heavy plaque accumulation and can often be removed by the client with improved toothbrushing techniques. Black stain (black-line stain) can occur in clients with meticulous oral hygiene. These stains are found on the tooth surface near the gingival margin and are associated with iron in the saliva. Middle-aged females with good oral hygiene are the most likely population to have black-line stain.
Dental Calculus
Dental calculus, commonly referred to as tartar, is oral biofilm that has been mineralized by calcium and phosphate salts from saliva. Although calculus is not the causative factor in periodontal infection, it facilitates the attachment and retention of plaque biofilm; therefore professional calculus removal is always indicated. The dental hygienist removes calculus so that teeth have biologically acceptable smooth surfaces. Like plaque, dental calculus is classified by its location (either supragingival or subgingival), degree (slight, moderate, heavy), and extent (localized or generalized).
Supragingival Calculus
Supragingival calculus, calculus above the free gingival margin, is most commonly located adjacent to the sublingual and parotid salivary gland ducts, resulting in calcified deposits on the mandibular anterior lingual surfaces and maxillary posterior facial surfaces of teeth (Figure 15-8, A). However, supragingival calculus can be found in any area of the mouth where there is poor oral hygiene or associated contributory factors such as kidney dialysis, use of 0.12% chlorhexidine mouth rinse, or genetic predisposition. Supragingival calculus is identified using direct visualization and compressed air. Generally the deposits are yellowish-white but may take on surface stains and appear dark yellow or light brown (see Figure 15-8, B). Drying the teeth with compressed air allows for a more accurate assessment, because as the calculus is dried it takes on a chalky-white appearance, making it easier to visualize. Supragingival calculus is moderately hard, bridging adjacent teeth or deposited on individual teeth.
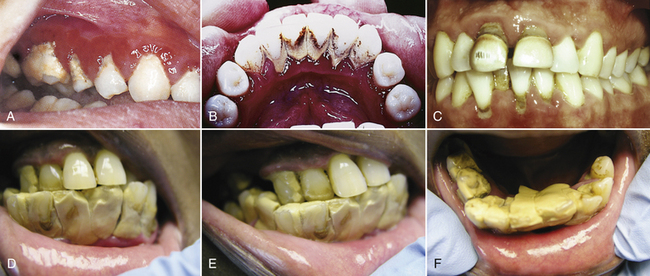
Figure 15-8 Dental calculus. A, Heavy calculus on molar and premolars in area opposite Stenson's duct. Note severe gingival inflammation and edema. B, Calculus superimposed with tobacco stains in relation to Wharton's ducts. C, Generalized supragingival and subgingival calculus and stain in a 31-year-old Caucasian man. D, E, and F show heavy supragingival calculus that is easy to identify.
(C, from Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders. D, E, and F, courtesy Fred Ochave, Virginia Beach, Virginia.)
Subgingival Calculus
Subgingival calculus is mineralized oral biofilm formed below the free gingival margin, often on the root surface. Unlike supragingival calculus, subgingival calculus is more likely to have a dark green–brown-black color owing to the absorption of blood pigments from the gingival sulcus or diseased periodontal pocket (see Figure 15-8, C). These deposits may be hard and tenacious and occasionally are visualized within the sulcus or pocket by deflecting the gingival margin with compressed air or seen through thin gingival tissues. The most accurate method of subgingival calculus detection is via subgingival exploration using a periodontal explorer; however, calculus can sometimes by detected during periodontal probing. The quantity of dental calculus is related to personal oral hygiene, diet, and individual biochemistry.
With transillumination, calculus can be observed as a dark, opaque, shadowlike area against the translucent proximal enamel. Heavy calculus deposits are easily identified, as in Figure 15-8, D, E, and F. Some deposits are mineralized to the extent that they become visible on radiographs (Figure 15-9; see Chapter 17).
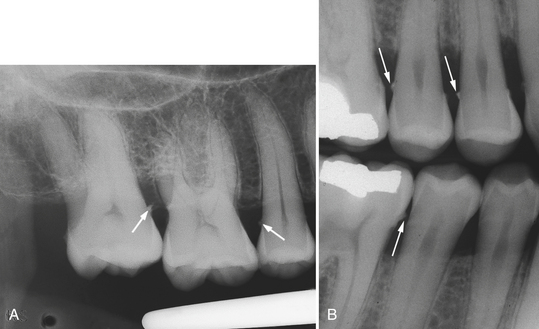
Figure 15-9 A, Periapical radiograph of teeth with subgingival calculus deposits on mesial surface of maxillary second molar and distal surface of second premolar. B, Vertical bitewing radiograph illustrating extensive subgingival calculus deposits as interproximal spurs (arrows).
(B, from Newman MG, Takei HH, Klokkevold PR, Carranza FA: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Subgingival calculus occurs most frequently in interproximal spaces, because these areas are the most difficult for a client to clean. Subgingival calculus may take many forms, including granular deposits, veneers or thin layers, and spurs or rings that extend around several surfaces of the root and have dimension. It is this change in tooth surface texture and dimension that the dental hygienist explores when assessing for subgingival calculus. Calculus may feel like a ledge or ring around a tooth, nodular, or smooth when it is layered in thin veneers.
Calculus Formation
Because calculus is calcified plaque biofilm, its formation follows the stages of biofilm formation (see section on stages of oral biofilm formation). Calculus forms and grows by the apposition of new layers of biofilm. Mineralization occurs in the intermicrobial matrix of the biofilm. The mineral source for supragingival calculus is saliva, gingival crevicular fluid, and inflammatory exudate. Crystals of hydroxyapatite, octocalcium phosphate, whitlockite, and brushite form in the intercellular matrix, on the surface of bacteria, and within the bacteria. About 10 days (rapid calculus formers) to 20 days (slow calculus formers) are required for oral biofilm to change to mineralized calculus, although the mineralization process can begin within 24 to 48 hours. Heavy calculus formers have higher salivary concentrations of calcium and phosphate than light formers. In contrast, light calculus formers have higher levels of pyrophosphate, a known inhibitor of calcification used in anticalculus dentifrice.
Calculus Composition
Calculus composition is similar in both supragingival and subgingival calculus.
Materia Alba and Food Debris
Materia alba (white material) is a loosely attached collection of oral debris, desquamated epithelial cells, leukocytes, salivary proteins and lipids, and bacteria that is seen as a whitish to yellowish to grayish mass on the teeth or overlying oral biofilm (Figure 15-10). Typically, it resembles small curds of cottage cheese. It is less adherent than oral biofilm and can be found in areas of poor oral hygiene.
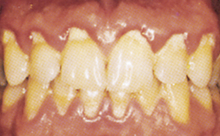
Figure 15-10 Materia alba generalized throughout the mouth, with heaviest accumulation near the gingiva. Note the plaque-induced gingivitis present.
Food debris is composed of remnants of food retained after a meal. Rinsing, use of an oral irrigator, and the self-cleansing action of the tongue and saliva can remove both materia alba and food debris. If present in great amounts, material alba and food debris accumulations impede the dental hygienist's ability to accurately assess the level of oral biofilm and calculus. However, presence of soft deposits might indicate inadequate oral hygiene knowledge and skill, infrequent oral self-care, poor manual dexterity, or low motivation level of the client. It is the bacteria in the material alba and the carboxylic acid in the food particles that can contribute to oral disease. Material alba and food debris supply nutrients to the oral biofilm and therefore should be removed regularly.
SKILL, MOTIVATION, AND COMPLIANCE
The client's ability to manage oral self-care must be assessed. A client may be capable of performing the necessary mechanical interventions but have little desire to do so, or the client may be highly motivated but have physical limitations that make self-care difficult. Some clients may be totally dependent on a caregiver for daily oral care. The dental hygienist assesses factors that limit the client's ability to perform daily self-care to make appropriate recommendations that meet individual needs. Assessment occurs through the following:
Procedure 15-1 ORAL DEPOSIT ASSESSMENT
STEPS
Once an accurate assessment is made and documented, and the client's readiness to change behavior is determined, the dental hygienist educates and motivates the client (or caregiver) in small steps aimed at changes that will support oral health.
As part of professional care, discussing characteristics of oral soft and hard deposits can serve as a useful motivator for clients having difficulty controlling oral biofilm. Visualization should be combined with the sensory feeling of biofilm on the teeth, the smell of biofilm, and the effects of biofilm (gingival bleeding and demineralization of tooth structure). Client knowledge of the biofilm provides a rationale for frequent professional subgingival root debridement, because biofilm in deep periodontal pockets cannot be reached by toothbrushes, interdental cleaners, and mouth rinses. Teaching the client about the resistant nature of the biofilm and the importance of disrupting and removing oral biofilm daily via mechanical and chemical measures remains the most effective means for its control (see Chapters 21, 22, and 29).
ORAL HYGIENE INDICES
To monitor oral hygiene of an individual or group over time, dental indices are used as quantitative measures of oral status (see Chapter 17, Table 17-9 for periodontal indices and Chapter 5757-257-357-457-557-6 for indices used with dental implant clients). A dental index is a data collection tool that allows the practitioner (or researcher) to convert specific clinical observations into numeric values that can be quantified, summarized, analyzed, and interpreted. Oral hygiene indices measure levels of oral hygiene to accomplish the following:
 Establish a baseline; monitor, over time, an individual's oral self-care progress; and motivate the client to achieve higher levels of oral wellness
Establish a baseline; monitor, over time, an individual's oral self-care progress; and motivate the client to achieve higher levels of oral wellness Establish a baseline, and monitor, over time, the oral health status of a target population in order to evaluate the effectiveness of a community-based program or intervention
Establish a baseline, and monitor, over time, the oral health status of a target population in order to evaluate the effectiveness of a community-based program or interventionThe index used must meet criteria for validity, reliability, and usability (Box 15-1).
Indices Used for Assessing Oral Deposits
Use of a standardized method of assessment can be valuable for motivating a client and documenting progress. The ability to show improvement is a powerful positive reinforcement tool that can help a client follow oral care recommendations. An index can also illustrate repeated neglect of a specific area of the mouth and thus guide a client in adhering to a self-care regimen. For maximum effectiveness an index performed with an individual should evaluate the entire dentition rather than a specific sample of teeth (for example the six Ramford index teeth: maxillary right and mandibular left first molars, maxillary left and mandibular right first premolars, and maxillary left and mandibular right central incisors), as is often done when conducting a randomized clinical trial. Even indices originally designed to measure a sample of teeth in a research subject's mouth can be adapted to measure all teeth present.
A simple plaque index is O'Leary's Plaque Control Record, illustrated in Figure 15-11 and described in Table 15-5. This index provides a method of recording plaque on the mesial, distal, facial, and lingual tooth surfaces at the gingival margin. Plaque observed is recorded by striking a dash through the appropriate surface or surfaces. After all teeth are examined and scored for plaque, the index is computed by dividing the number of plaque-containing surfaces by the total number of available surfaces. The resulting score is the percentage of tooth surfaces in the mouth with plaque. Use of the index over time allows clients to visualize and monitor their own plaque control progress and therefore facilitates client motivation to improve oral-self-care behaviors. This index can also be used to quantify stain in the same manner. Table 15-5 shows commonly used oral hygiene indices.
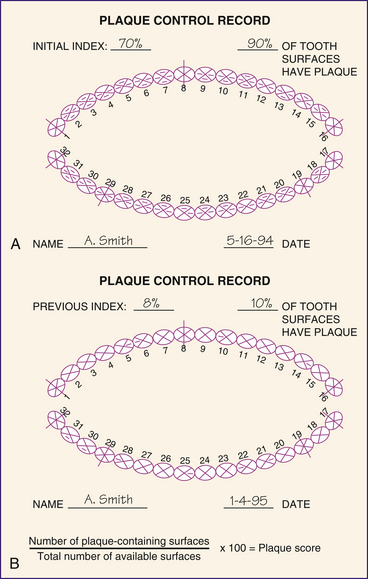
Figure 15-11 Plaque control record form. A, Seventy percent of tooth surfaces have plaque at initial appointment. B, Eight percent of tooth surfaces have plaque at a follow-up visit.
(Redrawn from O'Leary TJ, Drake RB, Naylor JE: The plaque control record, J Periodontol 48:38, 1972.)
TABLE 15-5 Oral Hygiene Indices
| Index and Purpose | Procedure for Use | Interpretation |
|---|---|---|
| Plaque Control Record | ||
|
• Best suited for use with an individual client for plaque visualization and oral hygiene motivation.
• All teeth are included in the assessment. Plaque present on four tooth surfaces is recorded: buccal, lingual, mesial, and distal.
• Apply plaque disclosing agent and rinse. Examine gingival margin for plaque, and record each surface with plaque with a slash (see Figure 15-11).
|
Scored as a percentage of tooth surfaces with plaque. Emphasizing plaque-free status can be a positive approach with many clients. | |
| Plaque-Free Score | ||
|
• Best suited for use with an individual client for plaque visualization and positive reinforcement of plaque control behaviors.
|
||
| Simplified Oral Hygiene Index (OHI-S) | ||
|
• Useful for either an individual client with poor oral hygiene or for a population-based assessment.
• Using the side of the tip of the periodontal probe or explorer, estimate oral debris and supragingival and subgingival calculus on the facial and lingual surfaces of the teeth.
• Select one tooth from each sextant with the greatest amount of debris or calculus, and score the facial and lingual surfaces using the following criteria:
Oral Debris Index (DI)
1 = Soft debris covering not more than one third of the tooth surface being examined, or the presence of extrinsic stains without debris, regardless of surface area covered
2 = Soft debris covering more than one third but not more than two thirds of the exposed tooth surface
1 = Supragingival calculus covering not more than one third of the exposed tooth surface being examined
Separately determine the DI and CI by totaling the scores and dividing the total by the number of sextants. Add the DI and CI to determine the OHI-S. |
An OHI-S is scored as follows: Individually, the DI-S and CI-S are scored as follows: | |
| Plaque Index (PI) | ||
|
• Useful for either an individual client who has significant plaque accumulation or a population-based assessment.
• Four gingival scoring units (mesial, distal, buccal, and lingual) are examined on the following teeth: 3, 9, 12, 19, 25, and 28.
• Amouth mirror, dental explorer, and air are used to score the above tooth surfaces for plaque using the following criteria:
1 = A film of plaque adhering to the free gingival margin and adjacent area of the tooth. The plaque may be recognized only after application of disclosing agent or by running the explorer across the tooth surface
2 = Moderate accumulation of soft deposits within the gingival pocket that can be seen with the naked eye or on the tooth and gingival margin
|
A PI is scored as follows: | |
| Patient Hygiene Performance (PHP) | ||
| The PHP is scored as follows: | ||
| Plaque Index (PI) | ||
|
• Useful for either an individual client who has significant plaque accumulation or a population-based assessment.
• Four gingival scoring units, mesial, distal, buccal, and lingual, are examined on the following teeth: numbers 3, 9, 12, 19, 25, and 28.
2 = Plaque present on all interproximal, facial, and lingual surfaces, but covers less than half of these surfaces
|
A PI is scored as a numerical expression ranging from 0-3: | |
| Calculus Index (CI) | ||
|
• Useful for either an individual client who has significant calculus accumulation or a population-based assessment.
• For teeth numbers 3, 9, 12, 19, 25, and 28, four surfaces (facial, lingual, mesial, and distal) are scored using the following criteria:
|
A CI is scored as a numerical expression ranging from 0-3: |
RECORD KEEPING AND DOCUMENTATION
Maintaining a record of a client's oral hygiene status is part of the assessment phase of care. Such records provide baseline reference for subsequent visits and a basis for making professional care and product recommendations. Documenting oral hygiene products used and previous instruction given to the client provides continuity of care and ensures that educational interventions are appropriate.
Comparing plaque scores at subsequent appointments facilitates client skill development and acceptance of oral hygiene recommendations. Documentation allows the clinician to expand the client's oral health knowledge, reinforce instructions, and encourage effective use of techniques and products. Clients expect a continuing conversation about their success with recommended oral products and devices, and an index that documents this information supports such interaction.
CLIENT EDUCATION TIPS
 Explain role of oral biofilm and host response in the development and control of gingival inflammation and periodontal disease progression.
Explain role of oral biofilm and host response in the development and control of gingival inflammation and periodontal disease progression. Explain bacterial plaque as a complex biofilm community that is self-sufficient, secure, and self-sustaining, rather than as a mere accumulation of planktonic bacteria.
Explain bacterial plaque as a complex biofilm community that is self-sufficient, secure, and self-sustaining, rather than as a mere accumulation of planktonic bacteria. Use disclosing agents, bleeding points, and the senses of smell and feel to identify oral areas that need self-care interventions.
Use disclosing agents, bleeding points, and the senses of smell and feel to identify oral areas that need self-care interventions.LEGAL, ETHICAL, AND SAFETY ISSUES
 Prophylactic antibiotic premedication is indicated for clients with highest risk of adverse outcomes resulting from infective endocarditis during invasive dental procedures.
Prophylactic antibiotic premedication is indicated for clients with highest risk of adverse outcomes resulting from infective endocarditis during invasive dental procedures.KEY CONCEPTS
 Oral hygiene assessment gives the clinician an accurate understanding of the client's oral hygiene status. Host response (inflammatory and immune responses) to the oral deposits present must be considered in the interpretation of oral hygiene assessment data.
Oral hygiene assessment gives the clinician an accurate understanding of the client's oral hygiene status. Host response (inflammatory and immune responses) to the oral deposits present must be considered in the interpretation of oral hygiene assessment data. Oral hygiene assessment yields information that can be used as a teaching tool to motivate the client to achieve or maintain oral health.
Oral hygiene assessment yields information that can be used as a teaching tool to motivate the client to achieve or maintain oral health. Assessment of soft and hard deposits, their origin, and their location is essential for dental hygiene diagnosis and care planning.
Assessment of soft and hard deposits, their origin, and their location is essential for dental hygiene diagnosis and care planning. Many factors contribute to the retention of oral biofilm, including stain, calculus, local predisposing factors, specialized mucosa of the tongue, saliva, and oral contributing factors.
Many factors contribute to the retention of oral biofilm, including stain, calculus, local predisposing factors, specialized mucosa of the tongue, saliva, and oral contributing factors. Oral biofilm creates its own renewing source of lipopolysaccharide for the long-term survival of microorganisms. Biofilm lends protective properties to the associated microorganisms, e.g., resistance to antibacterial and antibiotic agents such as chlorhexidine and systemic amoxicillin, respectively.
Oral biofilm creates its own renewing source of lipopolysaccharide for the long-term survival of microorganisms. Biofilm lends protective properties to the associated microorganisms, e.g., resistance to antibacterial and antibiotic agents such as chlorhexidine and systemic amoxicillin, respectively. About 20% of the oral cavity is occupied by teeth; about 80% includes the oral mucosa, specialized mucosa. Oral biofilm grow on all of these surfaces and in saliva.
About 20% of the oral cavity is occupied by teeth; about 80% includes the oral mucosa, specialized mucosa. Oral biofilm grow on all of these surfaces and in saliva. Mechanical removal and twice-daily use of an effective antimicrobial mouth rinse is the most effective method to control oral biofilm. Without disruption and removal of the oral biofilm daily and frequent periodontal maintenance therapy (see Chapter 28), antimicrobial and antibiotic therapies may not penetrate the resistant biofilm community.
Mechanical removal and twice-daily use of an effective antimicrobial mouth rinse is the most effective method to control oral biofilm. Without disruption and removal of the oral biofilm daily and frequent periodontal maintenance therapy (see Chapter 28), antimicrobial and antibiotic therapies may not penetrate the resistant biofilm community.CRITICAL THINKING EXERCISES
ACKNOWLEDGMENT
The authors acknowledge Gwen Essex for her past contributions to this chapter.
Refer to the Procedures Manual where rationales are provided for the steps outlined in the procedure presented in this chapter.
Visit the  website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites.
website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites.