CHAPTER 17 Periodontal and Risk Assessment
 Describe healthy periodontium by clinical signs, histologic characteristics, and radiographic findings.
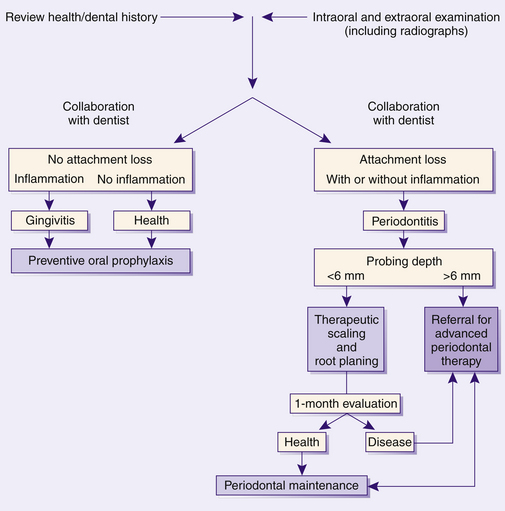
Describe healthy periodontium by clinical signs, histologic characteristics, and radiographic findings.RISK ASSESSMENT DEFINED1,2
Risk factors influence one's susceptibility to periodontitis. Assessment and analysis of risk factors provide information about a client's periodontal disease susceptibility beyond traditional clinical assessment parameters. Although specific pathogenic bacteria are the primary causative agents for periodontal diseases and are necessary for disease initiation, they are not sufficient to cause periodontal destruction. Many host, environmental, and systemic risk factors modify the body's response to bacterial pathogens in oral biofilm, resulting in great variability in individual susceptibility to periodontal disease. The number and type of client risk factors present modulate the onset, degree, and severity of periodontal disease (Box 17-1). Risk factor assessment is important because conditions associated with increased risk may affect treatment, client management, and outcomes. Risk factor assessment is based on information obtained through client interviews; the comprehensive health, dental, and pharmacologic history; and the clinical and radiographic examination.
RISK FACTORS
Risk factors are attributes or exposures that significantly increase the risk for onset and/or progression of a specific disease and affect treatment outcomes. Risk factors are categorized as follows:
Modifiable (Mutable) Risk Factors1-4
Smoking
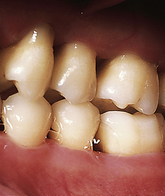
Tobacco smoking is one of the most significant risk factors for periodontal disease. Smokers have greater loss of attachment, bone loss, periodontal pocket depths, furcation involvement, dental calculus formation, and tooth loss than nonsmokers. Both surgical and nonsurgical interventions are less effective in those who smoke, and disease recurrence is more common than in nonsmokers. The effects of smoking on the periodontium are evidenced at an early age, usually beginning at age 20 to 30. Not only is the onset of disease earlier in smokers, but the disease progresses more rapidly.
The negative effects of smoking on the periodontium are linked with an altered host response and direct local (heat and chemical) damage to periodontal tissues. Immunosuppressive effects result from decreased salivary antibodies and impaired neutrophil functioning. Clinically, smokers exhibit gingiva that is thickened and fibrotic with rolled borders and minimal redness. Smoking masks gingival inflammation by reducing gingival blood flow as a result of constriction of blood vessels of the gingiva. Local damage may be related to direct thermal damage. Greater pocket depths and recession are found in the anterior areas and maxillary lingual sites owing to concentrated exposures of heat and toxins from tobacco smoke in these areas.
Length of time used and amount of smoking exposures are important assessment factors. A positive linear relationship exists between an increased amount of smoking and an increased loss of attachment (called a dose-response effect). For example, heavy smokers are five to seven times more likely to develop severe periodontitis when compared with individuals who have never smoked. Attachment loss is greater in heavy smokers when compared with individuals who are light smokers. Smoking cessation seems to elicit a positive response on the periodontium, but previous damage cannot be reversed. Research also links secondhand smoke exposure to increased oral bone loss. Dental hygienists incorporate smoking cessation strategies into care plans as appropriate (see Chapter 34).
Diabetes Mellitus
Diabetes mellitus (DM) is a strong risk factor for periodontal disease In type 1 and type 2 DM, greater gingivitis and periodontitis prevalence is observed. Prevalence increases significantly when the blood glucose is poorly controlled or uncontrolled. Persons who maintain good control have less attachment and bone loss than those with poor control, and they respond well to therapy. For persons older than age 40 who have diabetes, periodontal disease severity increases with years of disease duration.
With diabetes the increased susceptibility to periodontal infection is linked to immune dysfunction. Neutrophil chemotaxis and phagocytosis, impaired in diabetics, inhibits destruction of bacterial pathogens in the pocket, resulting in increased periodontal breakdown. (See the discussion of DM in Chapter 18Chapter 43.)
Specific Bacterial Pathogens (see Chapter 15)
Specific anaerobic, gram-negative bacteria must be in the gingival fluid for periodontal disease to occur. Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythensis are gram-negative bacteria strongly associated with the cause and progression of periodontitis (Box 17-2). These periodontopathic bacteria, also known as putative bacteria, can cause direct tissue damage resulting from the production of bacterial enzymes and toxins and play a major role in the immunopathologic processes that destroy periodontal tissues. Presence of one or more of the bacteria in oral biofilm does not predict that periodontal disease will occur. Other host and environmental factors must be involved.
Poor Oral Hygiene
Lack of oral hygiene has a strong association with periodontitis among all age groups and increases risk for periodontal breakdown. In contrast, good oral hygiene greatly reduces risk for periodontitis. Daily oral biofilm control in conjunction with regular professional care prevents attachment loss in most individuals. Lack of supragingival plaque biofilm control after professional treatment minimizes effective results and interferes with resolution of inflammation and periodontal disease.
Osteoporosis
Evidence indicates an association between alveolar bone loss and osteoporosis. Increased alveolar bone resorption, attachment loss, tooth loss, and edentulousness have been found in women with osteoporosis when compared with women without this condition. Estrogen deficiency also has been linked to decreases in alveolar bone density. For osteoporotic women who smoke, the risk for tooth loss is extremely high. Estrogen replacement therapy (ERT) may be beneficial in preventing tooth loss in women with osteoporosis and may lower gingival inflammation and frequency of attachment loss (see the discussion of osteoporosis in Chapters 18 and 53).
Human Immunodeficiency Virus and Acquired Immunodeficiency Syndrome
Human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) are suspected risk indicators for periodontal disease. Individuals infected with HIV may exhibit linear gingival erythema, characterized by acute gingival inflammation around the gingival margin that affects all teeth. There is a disproportionate amount of plaque present compared with the degree of inflammation. About 3% to 17% of persons with HIV disease have necrotizing ulcerative periodontitis, which results in severe, rapidly progressive periodontal destruction (see Chapter 45).
Stress
Epidemiologic evidence suggests that negative life experiences and psychologic factors likely contribute to enhanced susceptibility to periodontitis. Psychologic stress is associated with depression of the immune system; studies suggest a link between stress, poor coping skills, and periodontal attachment loss. Financial stress in adults with poor coping skills is a risk indicator for more severe periodontal disease. Individuals with adequate coping behaviors have less periodontal tissue destruction even with high financial stress than those individuals with inadequate coping skills. Research is ongoing to determine the link between psychologic stress and periodontal disease.
Bleeding on Probing
As a predictor of periodontal breakdown, bleeding on probing (BOP) alone has minimal value. Bleeding in itself is not a predictor of future attachment loss; however, bleeding on probing in combination with increasing pocket depths does increase risk for continued periodontal destruction. Repeated absence of BOP, especially on two or more occasions, generally indicates good periodontal health. Cessation of bleeding correlates with reduced gingival inflammation, repair of gingival connective tissue, and pocket reduction.
Medications
Risk assessment considers client medications (see Chapter 12). Although some medications, such as tetracycline and nonsteroidal antiinflammatory drugs, have a beneficial effect on the periodontium, others have a negative impact. Xerostomia is associated with more than 500 medications, including diuretics, antihistamines, antipsychotics, antihypertensives, and analgesics. Decreased salivary flow facilitates oral biofilm accumulation, especially at the cervical one third of the tooth, and diminishes resolution of gingival inflammation. Several categories of drugs cause drug-influenced gingival enlargement. Calcium channel blockers (e.g., nifedipine), immunosuppressive drugs (e.g., cyclosporine), and antiseizure drugs (e.g., phenytoin) have been implicated (Figure 17-1). Sex hormones (e.g., estrogen and progesterone) also have been reported to cause gingival enlargement. Gingival enlargement associated with these types of drugs and hormones is co-dependent on oral biofilm and generally can be minimized with good plaque control.
Local Contributing Factors
The dental hygienist identifies local contributing factors that may augment periodontal disease progression. Because of the biofilm's retentive nature, iatrogenic factors (those caused by the practitioner) can contribute to the initiation and progression of periodontal disease. Overhanging restorations (Figure 17-2), subgingival margin placement of crowns and restorations, orthodontic appliances, and removable partial dentures are examples of iatrogenic factors that may contribute to disease progression. Other factors include malpositioned teeth, oral jewelry, improper tooth contacts, size and shape of the root, calculus, mouth breathing, minimal amount of attached gingiva, and trauma such as toothbrush abrasion. Local contributing factors make oral biofilm removal difficult; the dental hygienist works with the dentist and client to modify these factors.
Nonmodifiable (Nonmutable) Risk Factors
History of Periodontitis
Presence and severity of existing periodontitis are strongly linked with future periodontal breakdown. Persons who have experienced previous episodes of periodontal disease are at a greater risk for future attachment loss than individuals who have not had periodontitis. Because individuals who have existing periodontitis are at great risk for continued periodontal destruction, frequent continued-care appointments are especially important to assist with maintenance of attachment levels.
Age
Aging is associated with enhanced susceptibility to periodontal disease; however, risk factors such as smoking and the presence of specific periodontal pathogens play a much larger role in disease susceptibility. Increased prevalence and severity of periodontal disease in the aging population do not result from changes associated with aging, as once thought. Rather, the cumulative effects of periodontal breakdown over a lifetime cause the increased periodontal disease observed with advancing age.
Gender and Race
Background characteristics that may increase the risk for periodontal disease are African American race and male gender. Studies report more bone loss, attachment loss, and tooth loss in men than in women even when oral hygiene, age, and socioeconomic status were considered. Other studies suggest that oral hygiene differences between men and women account for the differences in periodontal disease risk.
African Americans have greater susceptibility to both aggressive and chronic periodontitis, but socioeconomic class may be more of a factor than race itself. Research is needed to determine the degree to which periodontal disease susceptibility is a function of race and gender.
Genetic Disorders
Several genetic and inherited disorders are risk factors linked with a depressed immune system and increased periodontal disease susceptibility. Phagocyte dysfunction, cyclic neutropenia, acute monocytic leukemia, Papillon-Lefevre syndrome, Down syndrome, and Chediak-Higashi syndrome are highly associated with early-onset types of periodontal disease. (See a current pathology textbook for specific information on these diseases.)
Genetic Marker
An advance in risk factor assessment was the discovery of a genetic marker highly associated with severe periodontal disease. This discovery resulted in the development of a genetic susceptibility test for periodontal disease. Named the Periodontal Susceptibility Test (PST), this test analyzes DNA to identify specific variations in interleukin (IL)-1α and IL-1β. The test involves a noninvasive saliva sample. The sample is sent by mail; DNA analysis is completed by Kimball Genetics within 48 hours. A positive result is associated with increased susceptibility to chronic periodontal disease. Studies indicate that approximately 30% of the Caucasian population tests positive for this IL-1 gene type. A key regulator in the inflammatory process, IL-1 in high concentrations causes tissue destruction. Overproduction of IL-1 helps explain the more generalized and severe periodontal disease seen in many genotype-positive clients. The PST can identify potentially high-risk clients and therefore the need for more aggressive treatment and perhaps improved client compliance. It is important to note that the PST is not a diagnostic test but a prognostic test (e.g., some clients will test positive for the genotype and never develop severe periodontal disease; some clients who test negative will develop severe periodontal disease). Although genetic testing provides important information concerning the risk of periodontal disease in some populations, the best way to use this test clinically has not been determined. The multifactorial nature of periodontal disease must be considered when risk is assessed. The bacterial challenge, smoking, and other risk factors affect the degree to which IL-1 genotype status increases risk for periodontal breakdown. Genotype status is not as important as smoking or DM when risk for periodontitis is evaluated. More research is needed to clarify the role of IL-1 in all population types.
CLINICAL APPLICATION OF RISK ASSESSMENT
A risk assessment screening form assists in identifying risk factors, determining which are modifiable versus nonmodifiable, and planning evidence-based treatment interventions (Figure 17-3). Information to complete the risk assessment screening form is obtained through the comprehensive health and dental history and client interview.
A computerized risk assessment tool, available from PreViser Corporation, uses a numeric score from 1 to 5 to predict risk based on nine personal risk factors analyzed via a mathematical algorithm. Evidence suggests the system provides a valid, reliable predictor of periodontitis. This more scientific approach to risk assessment may prove beneficial for developing a plan of care based on risk.
Risk factors increase client susceptibility for periodontal breakdown; however, even one risk factor may substantially increase the client's degree of risk. The most significant risk factors are as follows:
In fact, heavy smokers (defined as ≥10 cigarettes per day) who also have a genotype-positive status are at the greatest risk for periodontal disease breakdown. Persons with these two risk factors are more than seven times more likely to lose teeth than those without these risk factors. After periodontal and risk assessment, suggestions for eliminating or modifying risk factors are addressed, for example, through consultation with a client's physician to determine if medications not associated with gingival enlargement can be substituted for those that are. Clients who smoke are counseled to quit or enroll in tobacco cessation programs based on their state of readiness to change; clients experiencing high levels of psychosocial stress could be provided with stress management strategies and counseled to relieve stress via healthy lifestyle behaviors, for example, exercise, a well-balanced diet, and adequate rest and sleep. Clients with osteoporosis could consult their physicians about use of weight-bearing exercise, bisphosphonate medications, and ERT. When risk factors are identified in the absence of periodontal disease, the client is educated about his or her increased susceptibility and encouraged to improve self-care, maintain frequent maintenance care, cease tobacco use, and reduce other risk factors as appropriate.
Clients with periodontitis and risk factors are treated aggressively (e.g., scheduled for 2- to 3-month periodontal maintenance care visits; referred for periodontal surgery earlier; and encouraged to follow a rigorous self-care program, including antimicrobial mouth rinse therapy, oral irrigation, systemic antibiotics, local controlled drug delivery, or subantimicrobial doses of doxycycline to control collagenase activity). Eliminating as many risk factors as possible is vital to long-term periodontal health.
Periodontal Assessment Instruments5,6
See the discussion of assessment and treatment instruments in Chapter 24.
Basic tools to assess clinical parameters include a good source of light, compressed air to dry the tissues, a mouth mirror, an explorer, a periodontal probe, and a current set of radiographs.
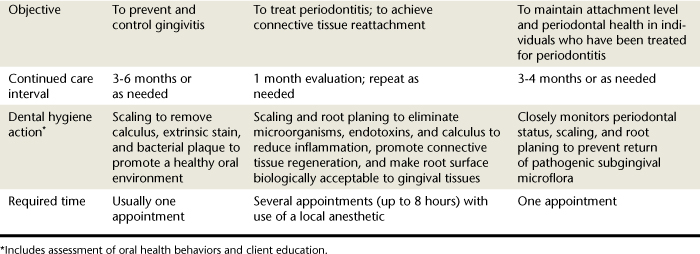
Many kinds of periodontal probes are available. All are calibrated in millimeters for use in assessing the health of the periodontium and may be made of plastic or metal. Figure 17-4 shows the Marquis probe with colored bands to indicate different measurement levels of 3, 6, 9, and 12 mm, and the Williams probe, which is calibrated with 3-, 5-, 7-, and 10-mm markings. When a probe is inserted into the space between the tooth and the gingiva, the calibrations show the depth of the space in millimeters (Figure 17-5). Probing depths are used to monitor periodontal health and disease. Figure 17-6 displays a plastic periodontal probe with interchangeable plastic tips with varying millimeter markings (see Chapter 24Figure 24-20Figure 24-21Figure 24-22Figure 24-23).
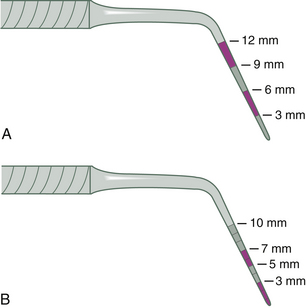
Figure 17-4 A, Marquis probe calibrated with color bands to indicate 3-, 6-, 9-, and 12-mm levels of penetration. B, Williams probe calibrated in 3-, 5-, 7-, and 10-mm increments.
Figure 17-5 Williams probe inserted into a gingival sulcus. Calibrations show a depth of 4 mm. (Probe readings are rounded up to the next highest millimeter. Here, the 3-mm mark is covered up, so the measurement is read “4 mm.”)
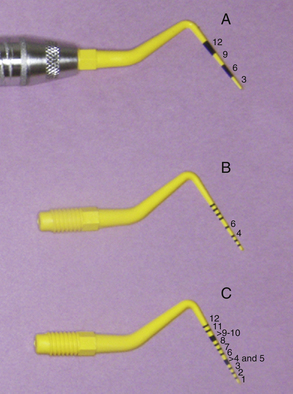
Figure 17-6 Plastic periodontal probe with three different types of interchangeable tips. A, Markings at every 3 mm. B, Markings at 1 to 10 mm with 4 and 6 absent. C, Markings at 1 to 12 mm with 4- to 5-mm band and 9- to 10-mm band.
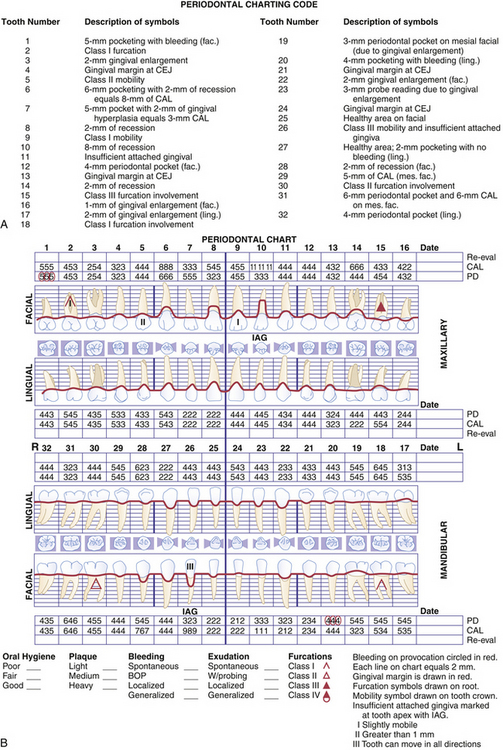
Computer-assisted, pressure-sensitive, and voice-activated probes are options to manual probing. Probing depths are entered using a computer keyboard and software (Figure 17-7). Pressure sensitive probes have the advantage of maintaining a standard probing force, typically 15 g, improving accuracy (Figure 17-8). In addition to probing depths, most computerized systems store and reveal information on attachment levels, recession, mobility, and furcation involvement. The resulting computer-generated chart (Figure 17-9) aids periodontal assessment and provides a visual tool for educating clients.

Figure 17-7 Computer-assisted probing device and setup.
(Courtesy Florida Probe Corporation, Gainesville, Florida.)
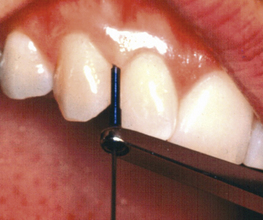
Figure 17-8 Pressure-sensitive periodontal probe.
(Courtesy Florida Probe Corporation, Gainesville, Florida.)
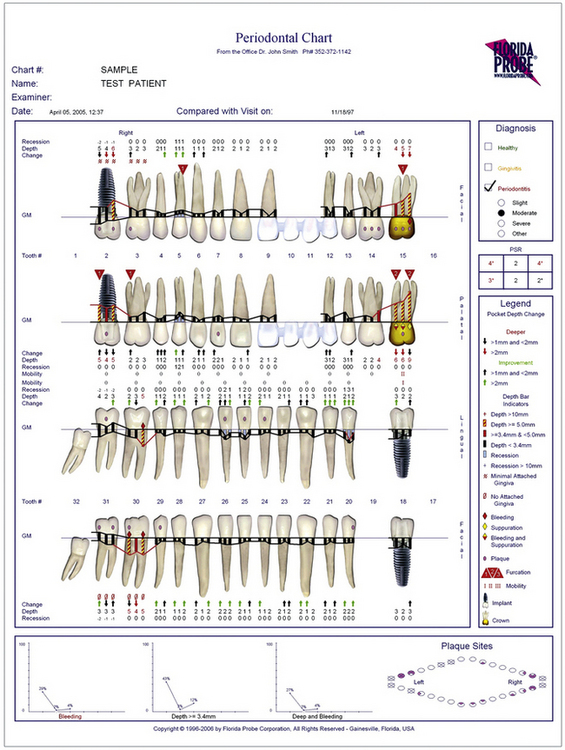
Figure 17-9 Computer-generated periodontal chart.
(Courtesy Florida Probe Corporation, Gainesville, Florida.)
A technologic advance in periodontal assessment is the periodontal endoscope, an illuminated fiberoptic instrument that provides high magnification views (24× to 48×) of the gingival sulcus. The instrument consists of a miniature camera attached to a tiny diagnostic explorer or probe. The probe is placed below the gum line, and subgingival images (periodontal pocket, root surface, bone, furcation areas, and so on) are immediately displayed on a chairside video screen. Use of this technology assists in visualizing and assessing the extent of the client's periodontal disease (see Chapter 24Figure 24-47Figure 24-48Figure 24-49Figure 24-50).
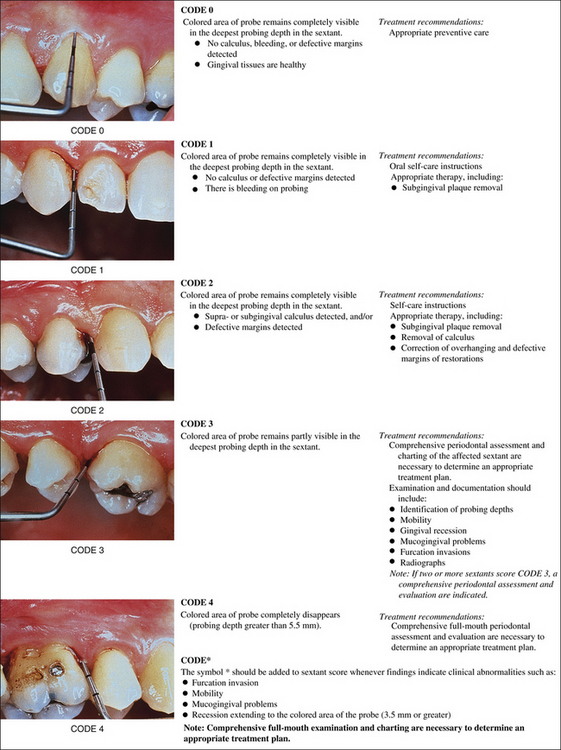
Periodontal Screening and Recording
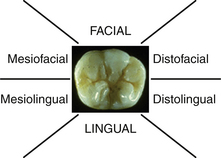
Periodontal Screening and Recording (PSR) is a method to screen clients for the presence of periodontal disease. This screening tool requires a specially designed probe that has a 0.5-mm ball tip and is color coded from 3.5 to 5.5 mm. The client's mouth is divided into six sextants, and each tooth is probed by walking the probe around the entire sulcus. At a minimum, six areas of the tooth are examined: mesiofacial, midfacial, distofacial, and the corresponding palatal and lingual areas. Only the highest score is recorded for each sextant according to the codes found in Figure 17-10. Clients found to be at high risk receive comprehensive periodontal examinations. All clients should receive a comprehensive periodontal examination annually.
Healthy Periodontium
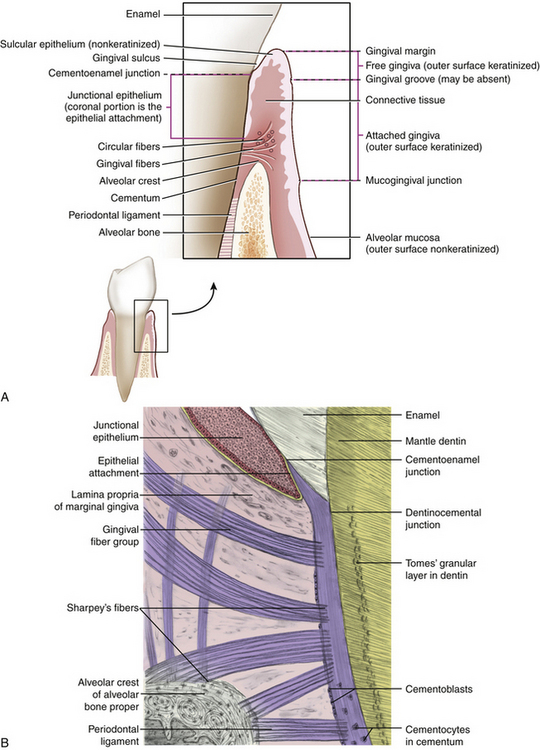
Healthy periodontium consists of four physical units: gingiva, periodontal ligament (PDL), alveolar process (supporting bone), and cementum.
Gingiva
Gingiva is masticatory oral mucosa that covers the alveolar process and surrounds the cervical portion of teeth. Histologically the gingiva has a protective layer of stratified squamous epithelium covering a dense, fibrous connective tissue. Gingiva is divided into four anatomic areas: the free or unattached gingiva; the gingival sulcus; the attached gingiva; and the interdental gingiva or interdental papilla (Figure 17-11).
Gingival tissue closest to the crown is the free or unattached gingiva. Free gingiva is not directly attached to the underlying alveolar bone. In healthy adult dentitions the free gingiva is located on the tooth enamel 0.5 to 2 mm coronal to the cementoenamel junction (CEJ) and fits tightly around each tooth. The edge of the free gingiva nearest the incisal or occlusal area of the tooth is the gingival margin or the gingival crest. The gingival margin marks the opening of the gingival sulcus.
Gingival Sulcus
The space between the free gingiva and the tooth is the gingival sulcus or gingival crevice. A healthy gingival sulcus generally measures 0.5 to 3 mm from the gingival margin to the base of the sulcus. Boundaries of the gingival sulcus are the sulcular epithelium and the tooth. Sulcular epithelium is the nonkeratinized continuation of the keratinized epithelium covering the marginal gingiva. Sulcular epithelium is clinically significant in that it is a semipermeable membrane, which in the presence of oral biofilm may allow bacterial endotoxins to penetrate underlying tissue.
Attached Gingiva
Free gingiva connects with the alveolar gingiva at the gingival groove. The attached or alveolar gingival, continuous with the free gingiva, is covered with stratified squamous epithelium. Free marginal gingiva joins to the attached gingiva at the gingival groove. This shallow groove is clinically visible in less than half of the population. Alveolar gingiva covers the crestal portion of the alveolar bone and the roof of the mouth. It is firmly attached to the alveolar bone, unlike the marginal gingiva, which has no attachment fibers. Mandibular facial and lingual attached gingiva and the maxillary facial attached gingiva are demarcated from the alveolar mucosa by the mucogingival junction (MGJ); the width of alveolar gingiva varies throughout the mouth (from 1 to 9 mm). The facial aspect of the maxillary anterior teeth has the widest alveolar gingiva. In general, at least 1 mm of alveolar gingiva is sufficient for gingival health.1 This 1-mm minimum width measurement has significance for planning educational and therapeutic interventions for persons with periodontal disease.
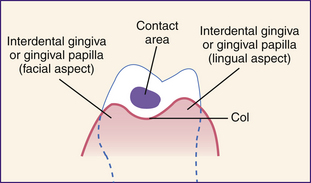
Gingival Papilla
An interdental or gingival papilla is located in the interdental space between two adjacent teeth (Figure 17-12). The tip and lateral borders of the interdental papilla are continuous with the marginal gingiva, and the center is composed of alveolar gingiva. The shape of the interdental papilla varies with the space or distance between two adjacent teeth. Given a wide space, the papilla is flat or saddle-shaped. If the interdental space is narrow, the papilla is pointed or pyramidal. When two teeth are in contact, the facial and lingual aspects of the papilla are connected by the col, a nonkeratinized area of interdental gingiva. Because the col is not keratinized, it is highly susceptible to disease. Most periodontal infections begin in the col.
Alveolar Mucosa
Alveolar mucosa is movable tissue loosely attached to underlying alveolar bone. Its surface appears smooth and shiny and is composed of thin, nonkeratinized epithelium. The alveolar mucosa is separated from the alveolar gingiva at the MGJ. The alveolar mucosa blends into the palatal gingiva in the maxilla so that no MGJ is distinguishable there. Alveolar mucosa is a darker shade of red than gingiva because of its richer blood supply.
Junctional Epithelium
Inside the gingival sulcus the sulcular epithelium attaches to the tooth at the coronal portion of the junctional epithelium (JE). The JE is a cufflike band of squamous epithelium that completely encircles the tooth. The apex, or base of the sulcus, is formed by the JE (see Figure 17-12). The epithelial attachment is the innermost part of the JE attached to the tooth by hemidesmosomes and the basement lamina. A hemidesmosome is half of a dense plate near the cell surface that forms a site of attachment between the JE and the surface of the tooth. Basement lamina is a thin layer of delicate, noncellular material underlying the epithelium, with the principal component being collagen.
Gingival Crevicular Fluid
Gingival crevicular fluid (GCF) is a serumlike fluid secreted from the underlying connective tissue into the sulcular space. Little or no fluid is found in the healthy gingival sulcus, but GCF has been found to flow after 1 day without oral biofilm control and increases with gingival inflammation. The GCF, part of the body's defense mechanism, transports antibodies and certain systemically administered drugs.
Clinical Appearance of Gingiva
In health and disease, gingiva has distinctive color, consistency, surface texture, contour, and size (Table 17-1 and Figure 17-13).
TABLE 17-1 Clinical Gingival Characteristics in Health and Disease
| Characteristic | Health | Disease |
|---|---|---|
| Color | Uniformly pale pink with or without generalized dark brown pigmentation | |
| Consistency | Firm, resilient | |
| Surface texture | ||
| Contour | ||
| Size | Free marginal gingiva is near CEJ and adheres closely to the tooth | |
| Probing depth | 0-4 mm; no apical migration of JE | More than 4 mm with or without apical migration of JE |
CEJ, Cementoenamel junction; JE, junctional epithelium.
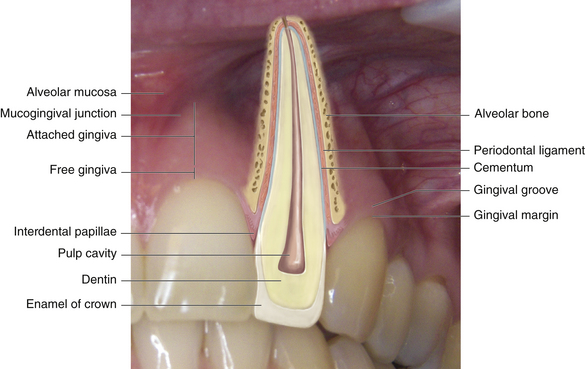
Figure 17-13 Anatomic relationship of normal gingiva. The gingival tissues: alveolar mucosa, mucogingival junction, attached gingiva, free gingiva, interdental papillae.
Healthy Gingiva
Gingival color varies according to degree of vascularity, amount of melanin pigmentation, degree of epithelial keratinization, and thickness of the epithelium. Pigment-containing cells in the basal layer of epithelium are commonly present in persons of color (Figure 17-14). Therefore some individuals normally have brown melanin pigmentation throughout the gingiva. Healthy attached gingiva is resilient, firm, and tightly bound to underlying bone by gingival fibers running between connective tissue and the alveolar periosteum.
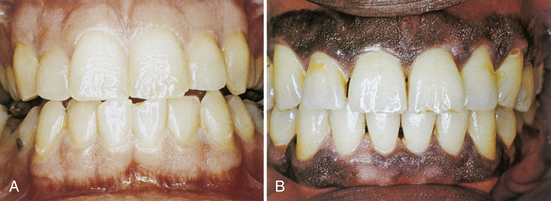
Figure 17-14 A, Clinically normal gingiva in light-skinned individual. B, Clinically normal pigmented gingiva in dark-skinned individual.
(From Glickman I, Smulow JB: Periodontal disease: clinical, radiographic, and histopathologic features, Philadelphia, 1974, Saunders.)
Healthy gingiva, when visually examined, air-dried, and probed, does not bleed or exude fluids. Healthy attached gingiva usually has an overall stippled texture that varies with individuals and areas of the mouth. The gingival margin in health is located 1 to 2 mm above the CEJ. The gingival contour in health follows the contour of the teeth. In addition, the contour, size, and shape of the gingiva depend on location, tooth size, and tooth alignment (Figure 17-15). Healthy gingiva does not feel hypersensitive to air or touch.
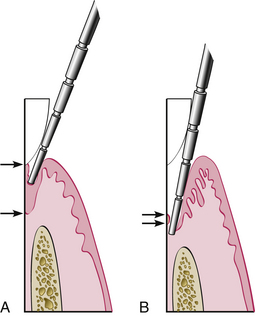
Figure 17-15 Some clinical parameters in health and in periodontitis. A, In a normal sulcus with a long junctional epithelium (JE) (between arrows), the probe penetrates about one third to half the length of the JE. B, In a periodontal pocket with a short JE (between arrows), the probe penetrates beyond the apical end of the JE.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Cementum
Cementum, a mineralized bonelike substance, covers the roots of teeth and provides attachment and anchorage for periodontal fibers. Cementum is usually a very thin cellular layer, not as hard as dentin, and it lacks blood vessels and nerves. In health, cementum is not exposed to the oral environment but is protected by the PDL.
Periodontal Ligament
The periodontal ligament is the fibrous connective tissue that surrounds and attaches the tooth roots to the alveolar bone. The width of the PDL, seen in radiographs only as a black (radiolucent) space, depends on age, stage of eruption, function of the tooth, and angle of the film. Collagen fibers of the ligament are inserted into the cementum and prevent tooth mobility by anchoring the tooth into its alveolar socket. PDL is connected to cementum and bone by collagen fibers called Sharpey's fibers. Functions of the PDL also include formation and maintenance of fibrous and calcified tissue, nutritional metabolite transport, and the sensory functions of pain and displacement sensitivity.
Alveolar Bone
Alveolar bone is composed of compact or cortical bone and of spongy bone that is marked by trabecular spaces seen on radiographs. Compact bone is the outside wall of the alveolar bone, where the PDL fibers are anchored and the rich vascular supply penetrates. Spongy bone is the interior of the alveolar bone. It increases and decreases in response to physical pressure, function, bacterial infection, and inflammation. The alveolar crest—the portion of the alveolar bone located between the teeth—varies in size and shape depending on tooth position.
DISEASED PERIODONTIUM
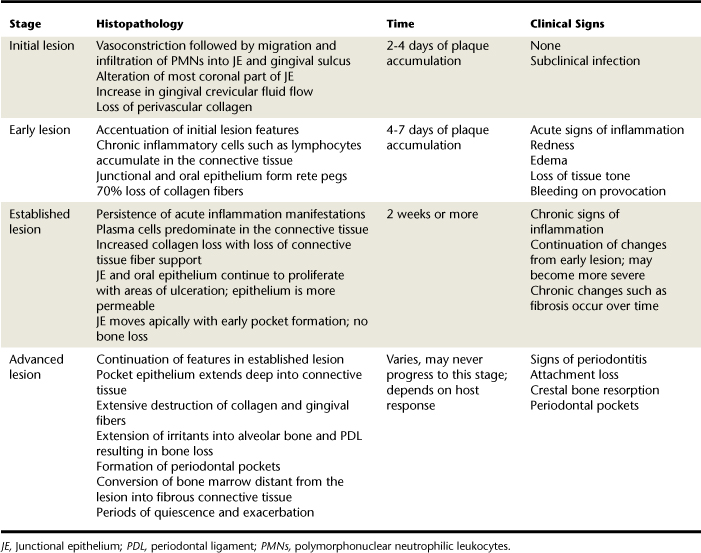
Histopathology of periodontal disease is explained in four stages (Table 17-2). Three of the stages describe a sequence of events resulting in gingivitis, and the last stage describes events resulting in periodontitis. Periodontal disease progression involves destruction of connective tissue attachment at the most apical portion of a periodontal pocket. Associated with this attachment loss is the apical downgrowth of the subgingival flora, apical migration of the JE, and alveolar bone resorption. Table 17-3 compares the clinical characteristics of gingivitis and periodontitis.
TABLE 17-3 Clinical Characteristics in Gingivitis and Periodontitis
| Characteristic | Gingivitis | Periodontitis |
|---|---|---|
| Gingival inflammation | Acute or chronic | Acute or chronic |
| Position of junctional epithelium | At the CEJ | Below the CEJ (attachment loss) |
| Position of gingival margin | Greater than 1-2 mm above the CEJ (gingival pocket) | Variable |
| Bleeding on probing | Present | May be present |
| Exudate | May be present | May be present |
| Furcation involvement | Absent | May be present |
| Tooth mobility | Absent | May be present |
| Bone loss | Absent | May be present |
CEJ, Cementoenamel junction.
Gingivitis
Understanding the characteristics of the healthy periodontium provides a foundation on which to recognize signs of disease and to make evidence-based decisions regarding care. The term periodontal disease includes both gingivitis and periodontitis. Gingivitis, inflammation of the gingival tissue, is a reversible bacterial infection confined to the gingiva. In gingivitis the free gingiva shows signs of inflammation, but there is no apical migration of the JE beyond the CEJ or bone loss (see Figure 17-11).
Although most forms of gingivitis are plaque-induced, host and systemic factors modify the clinical characteristics of the disease, hence the two classifications of gingival diseases: plaque-induced gingival diseases and non–plaque-induced gingival lesions (Boxes 17-3 and 17-4).
BOX 17-3 Classification of Periodontal Diseases and Conditions—Gingival Diseases
Adapted from Armitage GC: Development of a classification system for periodontal diseases and conditions, Ann Periodontol 4:1, 1999.
Dental Plaque–Induced Gingival Diseases∗
Non–Plaque-Induced Gingival Lesions
∗ Can occur on a periodontium with no attachment loss or on a periodontium with attachment loss that is not progressing.
BOX 17-4 Characteristics of Plaque-Induced Gingival Diseases
Data from Papapanou PN: Periodontal diseases: epidemiology, Ann Periodontol 1:1, 1996.
Dental Plaque-Induced Gingivitis
Dental plaque-induced gingivitis
Inflammation of the gingiva with plaque present at the gingival margin. Characterized by absence of attachment loss, clinical redness, bleeding on provocation, changes in contour, color, and consistency. No radiographic evidence of crestal bone loss. Local contributing factors may enhance susceptibility.
Plaque-Induced Gingival Diseases Modified by Systemic Factors
Endogenous sex steroid hormone gingival disease
Includes puberty-associated gingivitis, pregnancy-associated gingivitis, and menstrual cycle gingivitis; characterized by an exaggerated response to plaque, reflected by intense inflammation, redness, edema, and enlargement with absence of bone and attachment loss; in pregnancy may progress to a pyogenic granuloma (pregnancy tumor).
Diabetes mellitus–associated gingivitis
Found in children with poorly controlled type 1 diabetes mellitus. Characteristics similar to plaque-induced gingivitis, but severity is related to control of blood glucose levels rather than plaque control.
Hematologic (leukemic) gingival diseases
Swollen, glazed, and spongy gingival tissues that are red to deep purple in color; enlargement is first observed in the interdental papilla; plaque may exacerbate condition but is not necessary for it to occur.
Drug-influenced gingival enlargement
Occurs as a result of the use of phenytoin, cyclosporine, and calcium channel blockers such as nifedipine and verapamil. Onset is usually within 3 months of drug use and is more common in younger age groups. Characterized by an exaggerated response to plaque resulting in gingival overgrowth (most commonly occurring in the anterior area and beginning in the interdental papilla); found in gingiva with or without bone loss but is not associated with loss of attachment.
Dental plaque–induced gingivitis includes the following four main types of gingival diseases:
Periodontitis6
Periodontitis, inflammation of the periodontium, is an irreversible bacterial infection with inflammation extending from the gingiva into the connective tissue and alveolar bone that supports the teeth. In periodontitis there is apical migration of the JE with associated loss of attachment and destruction of alveolar bone. To classify periodontal disease, the practitioner decides if gingival disease or periodontitis is present. The American Academy of Periodontology classifies periodontitis into seven categories (Box 17-5).
BOX 17-5 Classification of Periodontal Diseases and Conditions—Periodontitis
Adapted from Armitage GC: Development of a classification system for periodontal diseases and conditions, Ann Periodontol 4:1, 1999.
∗ Can be further classified on the basis of extent and severity.
Describing the extent (localized or generalized) and severity (slight, moderate, or advanced) of sites affected can further identify disease types as follows:
Disease severity is determined by the amount of clinical attachment loss (Box 17-6) as follows:
BOX 17-6 Characteristics of Periodontitis
Chronic Periodontitis
Onset at any age but is most prevalent in adults. Characterized by inflammation of the supporting structures of the teeth, loss of clinical attachment resulting from destruction of the periodontal ligament and loss of adjacent bone. Prevalence and severity increase with age. The following levels of chronic periodontal classifications have been identified.
Slight or early periodontitis
Progression of gingival inflammation into the alveolar bone crest and early bone loss resulting in slight attachment loss of 1 to 2 mm with periodontal probing depths of 3 to 4 mm.
Moderate periodontitis
A more advanced state of the previous condition, with increased destruction of periodontal structures, clinical attachment loss up to 4 mm, moderate-to-deep pockets (5 to 7 mm), moderate bone loss, tooth mobility, and furcation involvement not exceeding Class I in molars.
Severe or advanced periodontitis
Further progression of periodontitis with severe destruction of the periodontal structures, clinical attachment loss over 5 mm, increased bone loss, increased pocket depth (usually 7 mm or greater), increased tooth mobility, and furcation involvement greater than Class I in molars.
Aggressive Periodontitis
Occurs before age 35 and is associated with rapid rate of progression of tissue destruction, host defense defects, and composition of subgingival flora. The following subclassifications have been identified.
Prepubertal periodontitis
Onset occurs between eruption of the primary teeth and puberty; occurs in localized forms usually not associated with a systemic disease and generalized forms usually accompanied by alteration of neutrophil functioning; clinically manifests as attachment loss around primary and or permanent teeth.
Juvenile periodontitis
Localized and generalized forms. Generalized form (GJP) occurs late in the teenage years with a variable microbial cause that may include Actinobacillus actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) and affects most teeth.
Localized form (LJP) is associated with less acute clinical signs of inflammation than would be expected based on the severity of destruction. The localized form is associated with bone and attachment loss confined mostly to permanent first molars and/or incisors. Age of onset is at or around puberty; associated with A. actinomycetemcomitans (Aa) and neutrophil dysfunction.
Necrotizing Periodontal Diseases
Necrotizing ulcerative gingivitis (NUG)
A gingival infection with complex causes (e.g., plaque, temporary depression of polymorphonuclear leukocyte functioning, stress, poor diet) characterized by sudden onset of pain, necrosis of the tips of the gingival papillae (punched out appearance), and bleeding. Secondary features include fetid breath and a pseudomembrane covering. Fusiform bacteria, Prevotella intermedia, and spirochetes have been associated with gingival lesions.
Necrotizing ulcerative periodontitis (NUP)
Characterized by necrosis of gingival tissues, periodontal ligament and alveolar bone. Associated with immune disorders such as human immunodeficiency virus (HIV) infection and individuals on immunosuppressive therapies; characteristics include severe and rapid periodontal destruction. Extensive necrosis of the soft tissue occurs simultaneously with alveolar bone loss resulting in a lack of deep pocket formation.
In general, chronic periodontitis is most prevalent in adults, with those affected averaging about 0.25 mm attachment loss per year. It thus progresses much more slowly than aggressive forms of periodontitis; however, progression rates vary widely. Periodontitis is cyclic in nature, with extended periods of quiescence or inactivity followed by short periods of exacerbation or activity. Connective tissue attachment loss during the active stage can vary from minor changes to extensive tissue loss. All forms of periodontal disease, however, are related to specific gram-negative anaerobic bacteria found in subgingival flora. The mere presence of these bacteria is not sufficient for periodontitis to occur because bacterial virulence and host susceptibility are critical contributing factors.
Immunopathology7
The following two distinct causative components cause periodontal destruction:
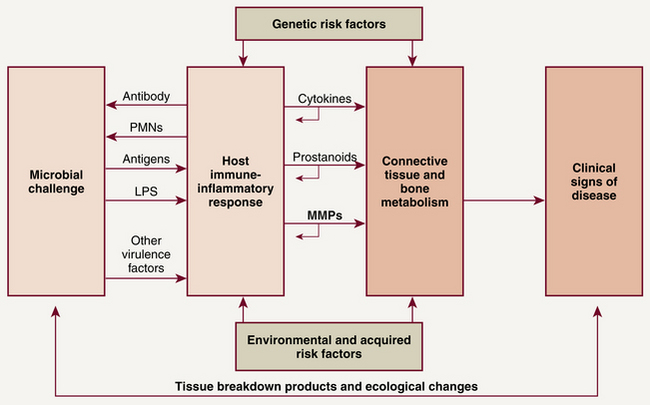
With adequate removal of oral biofilm and an intact host immune system, pathogen growth is held in check through neutrophil chemotaxis and phagocytosis. When biofilm is not adequately removed, it accumulates at the gingival margin; over several days, biofilm bacteria release byproducts and toxins such as lipopolysaccharides (LPSs) that penetrate the JE, connective tissue, and blood vessels. A host defense system imbalance, a result of bacterial virulence, altered host defense, or other periodontal risk factors, weakens the body's ability to fight the pathogens, and an overproduction of inflammatory mediators occurs. Instead of being protective, the overproduction of inflammatory mediators destroys periodontal tissues. The body responds to the bacteria and their byproducts by triggering the immune system and sending in B and T lymphocytes, macrophages, and plasma cells (Figure 17-16). LPS interacts with monocytes and macrophages to produce cytokines and inflammatory mediators such as IL-1, prostaglandin E2 (PGE2), tumor necrosis factor (TNF)–α, and matrix metalloproteinases (MMPs) that lead to periodontal destruction. IL-1 stimulates the synthesis of MMPs and PGE2. TNF-α, PGE2, and IL-1 mediate (enhance) bone resorption, thus promoting periodontal destruction. An overproduction of MMPs from the host's inflammatory reaction results in collagen destruction in the connective tissue of gingiva, PDL, and alveolar bone. Degradation of collagenous connective tissue and bone resorption results in the clinical manifestations of periodontal disease. Thus the host's own immunoinflammatory response is responsible for most of the tissue destruction associated with periodontal disease.
Signs of Gingival Disease8
Clinical Signs of Inflammation (Gingivitis) (Figure 17-17)
Inflammation begins in the epithelium of the col and of the marginal gingiva as a result of bacterial invasion or endotoxins. Endotoxins and enzymes from gram-negative bacteria break down epithelial intercellular substances, causing sulcular epithelium ulceration. This ulceration permits enzymes and toxins to penetrate further into the underlying connective tissue. Connective tissue inflammation results in dilation and increased permeability of capillaries, resulting in tissue redness, edema, bleeding, and an exudate—that is, the four characteristic signs of gingival or periodontal inflammation:
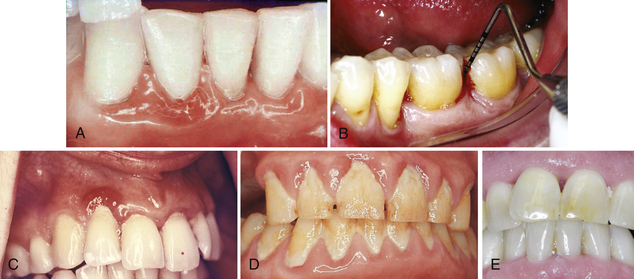
Figure 17-17 A, Edema, loss of stippling, and erythema associated with plaque-induced gingivitis. B, Inflamed tissue exhibiting bleeding on probing. C, Life Saver–like enlargement of the gingival margin with changes in color, contour, and consistency. D, Calculus, material alba, and oral biofilm contributing to gingival inflammation; note the lack of stippling, the rolled gingival margins, and the dark red color. E, Nodular, fibrotic tissue associated with chronic periodontal disease.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Oral tissue is assessed for these signs after it has been dried with compressed air.
Color Change
During assessment the first characteristic noted is gingival tissue color. Erythema (reddened gingiva), common in inflammation (see Figure 17-17A, B, and C), indicates an increase in the vascular supply as a result of the body's effort to defend itself against bacterial invasion or trauma (e.g., oral biofilm) or foreign objects (e.g., a popcorn shell). Bright red indicates acute gingival inflammation; blue or purple indicates venous congestion (cyanosis) in the connective tissue from chronic inflammation.
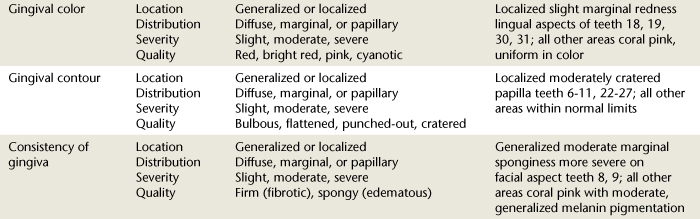
Dental hygienists monitor and record the slightest change in gingival color, contour, consistency, and texture, noting the location, distribution, severity, and quality of such changes (Table 17-4). Clients are informed of clinical findings and taught to monitor their gingival health. Using the hand mirror the dental hygienist points out gingival characteristics to the client and compares an inflamed gingival area of the mouth to one that is healthy in order to teach the client about his or her periodontal status.
Bleeding on Probing
Use of a periodontal probe to measure the depth of a healthy sulcus (one with an intact layer of sulcular epithelium) yields no bleeding. BOP is one of the earliest clinical signs of the presence of inflammation (see Figure 17-17, B).
 BOP predicts attachment loss only 30% of the time. Furthermore, fibrotic tissue that results from chronic inflammation as well as tissue in smokers may bleed very little or not at all.
BOP predicts attachment loss only 30% of the time. Furthermore, fibrotic tissue that results from chronic inflammation as well as tissue in smokers may bleed very little or not at all. BOP always signals the presence of gingival inflammation and has value in identifying clients at risk for periodontal disease progression.
BOP always signals the presence of gingival inflammation and has value in identifying clients at risk for periodontal disease progression. Gingival bleeding occurring at several sequential continued-care visits is associated with an increased risk for loss of attachment.
Gingival bleeding occurring at several sequential continued-care visits is associated with an increased risk for loss of attachment. Absence of bleeding is associated with lack of disease progression; however, the mere presence of bleeding does not predict periodontal breakdown.
Absence of bleeding is associated with lack of disease progression; however, the mere presence of bleeding does not predict periodontal breakdown. BOP is recorded in the client record and monitored. The dental hygienist explains to the client that BOP is caused by soft-tissue inflammation and gingival infection. Moreover, the hygienist points out that bleeding, or its absence, on brushing or interdental cleaning gives the individual a self-test for monitoring gingival health status at home.
BOP is recorded in the client record and monitored. The dental hygienist explains to the client that BOP is caused by soft-tissue inflammation and gingival infection. Moreover, the hygienist points out that bleeding, or its absence, on brushing or interdental cleaning gives the individual a self-test for monitoring gingival health status at home.Swelling or Edema
Microorganisms in oral biofilm produce harmful toxins, and enzymes increase permeability of blood vessels in the connective tissue underlying the gingival epithelium. Increased blood vessel permeability allows lymphocytes, plasma cells, and extracellular fluid to accumulate in gingival connective tissue. This accumulation results in enlarged, edematous tissue (see Figure 17-17, C) When there is no apical migration of the JE, the sulcus becomes deepened from gingival tissue edema, producing a gingival pocket, also called an artificially deepened sulcus or a pseudopocket because the marginal gingiva has moved coronally, not apically (Figure 17-18). Deeper periodontal structures are not involved, and there is no migration of the JE.
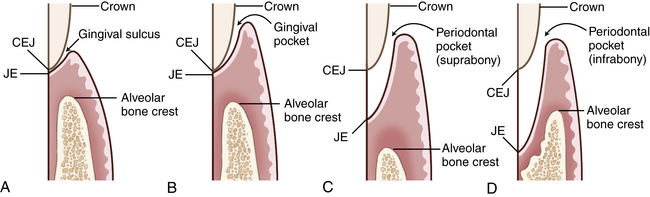
Figure 17-18 A, Comparison of the relationship of the junctional epithelium (JE) to the cementoenamel junction (CEJ) and alveolar bone in health. B, Gingival pocket. C, Suprabony periodontal pocket (periodontitis), JE above alveolar bone crest. D, Intrabony periodontal pocket (periodontitis), JE below alveolar bone crest.
A gingival pocket can be reversed to a healthy gingival sulcus by the client's daily plaque control regimen supplemented by professional mechanical therapy. When oral biofilm is controlled and calculus is removed, inflammation subsides; gingival enlargement decreases, with a resultant decrease in gingival pocket depth.
Changes in Texture and Contour
Swelling or edema produces gingival texture and contour changes (Figure 17-19, A to E). In gingivitis, gingival texture becomes shiny and smooth from loss of stippling and presence of edema. Contour changes occur from gingival enlargement, such that the position of the gingiva is high on the enamel, partly or nearly covering the anatomic crown. Marginal gingiva becomes rounded or rolled (rather than knife-edged or slightly rounded) and closely adapted to the tooth. In chronic inflammation, gingival surfaces may become nodular or fibrotic (see Figure 17-17, E).
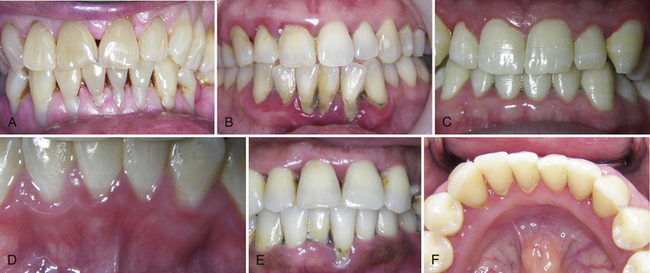
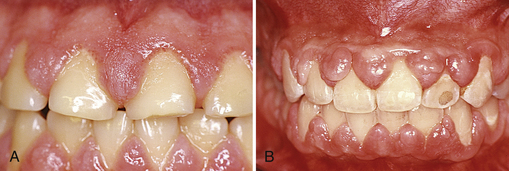
Figure 17-19 A, Significant recession of varying degrees throughout the mouth. Note composite restorations at cervical areas on the teeth along with the tobacco stain in mandibular interproximal areas. B, Severe inflammation in mandibular anterior tissues. Note blue color. Moderate erythema, edema, and loss of stippling through out. Note significant recession caused from calculus and oral biofilm in the mandibular anterior. C, Generalized marginal erythema with shiny, smooth enlarged gingival tissues. D, Plaque-induced gingivitis; interdental papillae has lost its knifelike shape and displays puffy, rolled borders with erythematous tissues. E, Loss of interdental papillae in anterior areas with significant recession on tooth 25. Note pigmented gingival tissues. F, Slight calculus in the mandibular anterior with slight inflammation of the gingival tissues.
Interdental Papillae Changes
While examining gingival color, texture, size, and shape, the clinician gives careful attention to the gingival papilla. When the col area is inflamed, epithelial and connective tissue layer degeneration can result in a blunted papilla, a split interdental papilla, or a cratered papilla (see Figures 17-17, B; 17-19, E; and 17-20). Such degradation usually indicates alveolar bone loss. Self-induced trauma from improper use of dental floss may cause laceration of the gingival papillae.
Exudate
GCF rarely is found in healthy gingiva but significantly increases in the presence of inflammation. GCF is measured by isolating a site, drying it with air, and inserting a small paper strip into the pocket or sulcus for 3 to 5 seconds. Electronic devices can measure the GCF volume of the paper strip, although the clinical value of such a test is still under investigation.
GCF is called suppuration when it is a clear serous liquid and purulent exudate when it contains living and dead polymorphonuclear neutrophilic leukocytes (PMNs), bacteria, necrotic tissue, and enzymes. When purulent exudate is present in the pocket, pus can be noticed during probing and expressed by applying pressure to the base of a pocket with one's finger and moving it coronally. Although purulent exudate is a dramatic sign of inflammation, it does not indicate the severity of inflammation or pocket depth. Some shallow and some deep pockets have pus formation, and some do not. The presence of pus is, however, a good indicator of active periodontal destruction. Suppuration correlates with specific attachment loss 2% to 30% of the time, so it is not a reliable indicator of active periodontal destruction. When suppuration or purulent exudate is observed, it is recorded for each area found.
Documentation of the Clinical Gingival Assessment (see Table 17-1)
When assessing the gingiva, changes in gingival color, consistency, surface texture, contour, and size are described with regard to the following:
The term healthy periodontium is appropriate for sites that are disease-free but have extensive attachment loss and recession resulting from previous episodes of periodontitis. For example, sites that have been successfully treated fall into this category. When successfully treated periodontitis sites become inflamed at the gingival margin, this condition is termed plaque-induced gingivitis on a reduced periodontium.
Signs of Disease Progression (Periodontitis)
Periodontal Pocket
Probing depth is the distance from the gingival margin to the base of the sulcus or pocket, as measured by the periodontal probe (Figure 17-21). Unlike a gingival pocket or pseudopocket, a periodontal pocket is a pathologically deepened sulcus caused by bacterial infection. When the coronal end of the JE (the surface that forms the actual sulcus or pocket bottom) comes in contact with oral biofilm, it detaches from the tooth. At the same time the apical end of the JE migrates apically, thus deepening the sulcus into a periodontal pocket. As inflammation causes apical migration of the JE, it also causes gradual alveolar bone resorption, which reduces the level of bone support for the tooth. Periodontal pockets are classified as follows (see Figure 17-18):
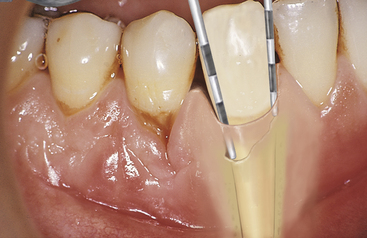
Figure 17-21 Probing depth and attachment loss measurement on same tooth using the Williams probe. Note that the probe on the left reveals a probe depth of 4 mm and a clinical attachment loss reading of 5 mm. The probe on the right reveals a probe reading within normal limits of 2 mm with no clinical attachment loss. Tooth 28 shows a good example of a gingival cleft.
(Adapted from Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
 Suprabony periodontal pocket when the JE has migrated below the CEJ but remains above the crest of the alveolar bone. Suprabony pockets are most commonly associated with horizontal bone loss.
Suprabony periodontal pocket when the JE has migrated below the CEJ but remains above the crest of the alveolar bone. Suprabony pockets are most commonly associated with horizontal bone loss. Intrabony periodontal pocket when the JE has migrated below the crest of the alveolar bone. Intrabony pockets are associated with vertical bone loss.
Intrabony periodontal pocket when the JE has migrated below the crest of the alveolar bone. Intrabony pockets are associated with vertical bone loss.Periodontal pockets may be present in the absence of clinical signs of gingival inflammation. Therefore clinical probing is the only accurate way to assess the gingiva for the presence of periodontal pockets. Because periodontal pockets can develop at any point around a tooth, the probe must be inserted around the entire circumference of the tooth. The deepest reading at each of the six tooth surfaces is the one that should be recorded on the client's periodontal charting form (Figure 17-22). The probe is walked along the pocket bottom and angled to keep the tip in contact with the tooth (Figure 17-23). If calculus is encountered, the probe is teased over the calculus, or the calculus is removed to allow insertion of the probe to the bottom of the pocket (Figure 17-24).
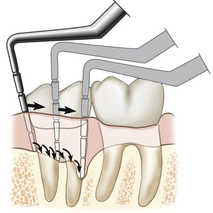
Figure 17-23 Facial view showing how the probe is moved around the tooth in short steps, reestablishing contact with the pocket bottom at each step.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
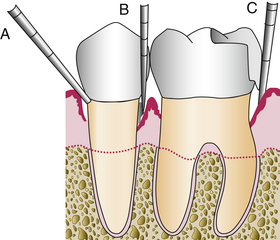
Figure 17-24 Periodontal probing limitations. A, Wrong angulation of probe. B, Probe blocked by calculus. C, Probe blocked by overhanging restoration.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
The interproximal area is the most difficult area for the client to clean and therefore is where periodontal pockets tend to form. To probe the interproximal area just apical to the contact, place the probe up against the interdental contact and tilt the probe mesially or distally as appropriate to keep the tip touching the tooth (Figure 17-25). Failure to tilt the probe enough to keep its tip in contact with the tooth surface is a common error and causes inaccurate interproximal probing depth readings (Figure 17-26). The interproximal tooth surfaces should be probed from both the facial and lingual sides of each tooth so that all of the epithelial attachment of the JE is explored (Figure 17-27; see Chapter 24, Procedure 24-2).
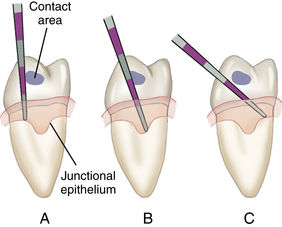
Figure 17-25 A, Incorrect technique for probing the interproximal area. B, Correct technique. C, Incorrect technique.
(Adapted from Perry D, Beemsterboer P, Carranza FA: Techniques and theory of periodontal instrumentation, Philadelphia, 1990, Saunders.)
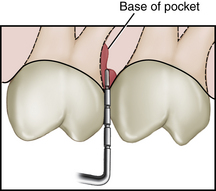
Figure 17-26 Failure to tilt the probe far enough to keep its end in contact with the tooth surface. Probe is resting on the pocket wall, resulting in an inaccurate probing depth measurement.
(Adapted from Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Gingival Recession
Gingival recession is a reduction of the height of the marginal gingiva to a location apical to the CEJ (see Figure 17-19, A and B). Recession signifies attachment loss. Causes of gingival recession are numerous. Chronic exposure to bacterial plaque, toothbrush abrasion, orthodontia, floss cuts, occlusal trauma, abfraction, root instrumentation, and tooth polishing with an abrasive prophylaxis paste or air polisher can result in JE migration and recession. Once the root surface is exposed by gingival recession, the connective tissue rarely reattaches because collagen breaks down when exposed to the oral environment, and cementoblasts grow only on root surface adjacent to the PDL. Areas of recession may be sensitive because the exposed cementum may be lost, exposing dentin. Exposed nerve endings in dentin may be stimulated mechanically (e.g., by toothbrushing), chemically (e.g., by acidic foods or bacterial plaque), or thermally (e.g., by cold air or food at extreme temperatures), producing sensitive teeth (see Chapter 38).
Noting areas of dentinal hypersensitivity on the client record provides information for planning care; for example, clients may require more time, a local anesthetic agent, or nitrous oxide–oxygen analgesia for effective tooth instrumentation (see Chapters 39 and 40).
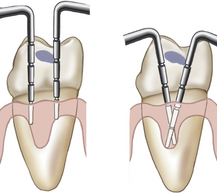
Clinical Attachment Level (Figure 17-28)
Clinical attachment level (CAL), the position of the attached periodontal tissues at the base of the pocket, is determined by comparing the distance from the CEJ to the base of the sulcus or pocket. Location of the gingival margin is important in determining the CAL, which includes both periodontal pocket depth and recession measurements. When the gingival margin coincides with the CEJ, the CAL and the pocket depth are equal. When the gingival margin is apical to the CEJ, the CAL is greater than the pocket depth and equal to the amount of visual recession plus the pocket depth.
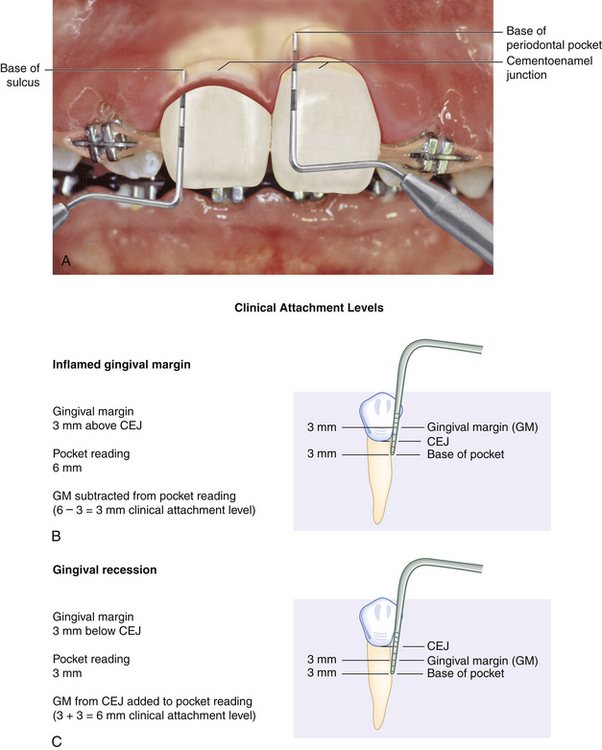
Figure 17-28 Measuring clinical attachment levels. A, On the maxillary right central incisor, the inflamed gingival margin hides the cementoenamel junction (CEJ), resulting in a 4-mm psuedopocket (gingival pocket). There is no clinical attachment loss and no bone loss. The base of the sulcus is in a normal relationship to the CEJ and alveolar bone. On the maxillary left central incisor, the gingival margin has receded 2 to 3 mm, exposing the CEJ, and bone loss is evident. There is clinical attachment loss of 6 mm and a 5-mm periodontal pocket. B, Gingival margin 3 mm above CEJ. C, Gingival margin 3 mm below CEJ (gingival recession).
(A, Adapted from Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
In cases of gingival inflammation or hypertrophy, when the gingival margin is on the enamel, the attachment loss is less than the pocket depth. The gingival margin placement above the CEJ must be measured and this reading subtracted from the periodontal probe reading to obtain the CAL. For example, if a client has generalized 6-mm probe readings but 2 mm of coronal movement of the gingival margin, the actual CAL is 4 mm. If this 2 mm is not subtracted from the probe reading, a realistic assessment of the CAL cannot be obtained. In this situation a client with only 4 mm of attachment loss might be misclassified as demonstrating a higher periodontal class than what is actually apparent. If a client has generalized 3 mm of recession and 3-mm pocket readings, the recession and the pocket readings are added together to obtain the actual CAL of 6 mm. If they were not added together, the client might be classified as having slight periodontal disease when 6-mm CAL would indicate severe disease.
Attachment loss over time (disease activity), not periodontal pocket readings, indicates actual progression of periodontal disease and is considered its defining feature. Consequently, regular documentation of attachment loss in the client record is important to track periodontal disease activity.
Relative Attachment Level
Relative attachment level (RAL), a record of past disease activity, is the measurement from a fixed reference point on a tooth (CEJ) or a stent to the JE. Such measurements are taken using a periodontal probe.
Furcation Involvement (Figures 17-29 and 17-30)
Furcation involvement (or loss of attachment between the roots of posterior teeth) is identified, classified, and monitored (Table 17-5). The client is informed about areas of furcation involvement and taught homecare techniques to manage these areas. The Nabers furcation probe is often used to detect and measure furcation involvement. Radiographs confirm but do not always reveal this condition. A separate notation indicating the use of the Nabers probe must be made or a serious misunderstanding may result, especially if the furcation reading is mixed up with mobility readings (see Chapter 24,Figure 24-24).
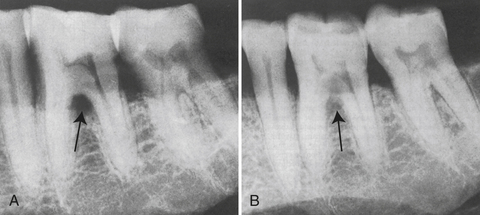
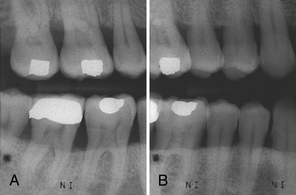
Figure 17-29 Furcation involvement. A, Triangular radiolucency in bifurcation area of mandibular first molar indicates furcation involvement. B, Same area, different angulation. The triangular radiolucency in the bifurcation of the first molar is obliterated, and involvement of the second bifurcation is apparent.
(A, From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
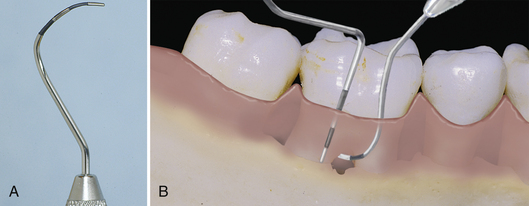
Figure 17-30 A, Nabers probe. B, Exploring with a periodontal probe (left) may not detect furcation involvement. Specially designed instruments (Nabers probe) (right) can enter furcation area.
(A, From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
TABLE 17-5 Classifications of Furcations
| Class | Description |
|---|---|
| Class I | Beginning involvement. Concavity of furcation can be detected with an explorer or probe, but it cannot be entered. Cannot be detected radiographically. |
| Class II | The clinician can enter the furcation from one aspect with a probe or explorer but cannot penetrate through to the opposite side. |
| Class III | Through-and-through involvement, but the furcation is still covered by soft tissue. A definite radiolucency in the furcation area on a radiograph is visible. |
| Class IV | A through-and-through furcation involvement that is not covered by soft tissue. Clinically it is open and exposed. |
Tooth Mobility
Tooth mobility is the degree to which a tooth is able to move in a horizontal or apical direction. Although caused by the loss of PDL and bone support in periodontitis, tooth mobility varies during the day according to diet and stress. Children, young adults, and some women exhibit more movement than other groups. Tooth mobility, which is not a cause of periodontal disease, may contribute to it. Therefore it is assessed along with attachment levels. To test for mobility the practitioner places an instrument handle on the lingual surface of the tooth and gently pushes on the facial surface with another instrument (e.g., a periodontal probe or mouth mirror). The feeling of movement is most acute at the contact points between two teeth. The classification of mobility should be recorded directly on the dental chart to allow comparative readings at successive appointments (Table 17-6).
TABLE 17-6 Classification of Mobility
| Class | Description |
|---|---|
| Class I | Tooth can be moved up to 1 mm in any direction. |
| Class II | Tooth can be moved >1 mm in any direction but is not depressible in socket. |
| Class III | Tooth can be moved in a buccolingual direction and is depressible in socket. |
Fremitus
Fremitus is the vibration or movement of the teeth when in contacting positions from the client's own occlusal forces. To assess fremitus the clinician places his or her index finger along the facial aspects of the cervical one third of each maxillary tooth, and the client is asked to tap the teeth together. Teeth that are displaced are then identified (Table 17-7).
TABLE 17-7 Classification of Fremitus
| Class | Description |
|---|---|
| Class I | Mild vibration or movement detected |
| Class II | Easily palpable vibration but no visible movement |
| Class III | Movement is clearly visible |
Occlusal Traumatism
Occlusal traumatism is a degenerative, noninflammatory periodontal condition resulting in the destruction of the periodontium because the supporting structures of the teeth cannot withstand the heavy forces. Excessive occlusal force occurs from bruxism, clenching, malocclusion, or iatrogenic factors such as a poorly made dental restoration or appliance.
Because clinical and radiographic signs and symptoms associated with occlusal traumatism (Box 17-7) often indicate other conditions, pulp vitality testing and evaluation of parafunctional habits is indicated. Figure 17-31 shows widening of the PDL, a primary radiographic sign of occlusal trauma.
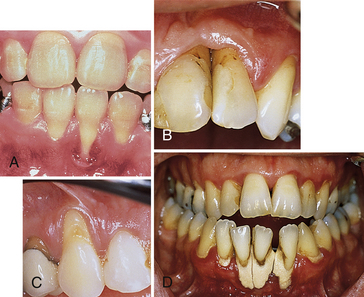
Figure 17-31 Mucogingival defects. A, Irregular gingival contours and recession with severe gingival inflammation. B, Gingival recession, crater formation, and chronic inflammation with fibrotic tissue. Bottom of the pocket is beyond mucogingival junction. C, Recession on maxillary canine with presence of shallow pocket and absence of attached gingiva. D, Advanced gingival recession and inflammation caused by heavy plaque and calculus accumulation.
(Courtesy Dr. Kenneth Marinak, Adjunct Clinical Instructor, Gene W. Hirschfeld School of Dental Hygiene, Old Dominion University, Norfolk, Virginia.)
Occlusal trauma causing destruction of the supporting structures may be primary or secondary.
 Primary occlusal traumatism is caused by excessive occlusal forces acting on an otherwise normal periodontium.
Primary occlusal traumatism is caused by excessive occlusal forces acting on an otherwise normal periodontium. Secondary occlusal traumatism is caused by excessive occlusal forces acting on an already diseased periodontium.
Secondary occlusal traumatism is caused by excessive occlusal forces acting on an already diseased periodontium.Occlusal trauma exacerbates periodontal disease because the supporting structures are already weakened. Elimination of the causative factors of gingivitis and periodontitis comes first, with occlusal therapy a secondary intervention by the dentist.
Mucogingival Conditions (Figure 17-32)
Deviations from the normal anatomic relationship between the gingival margin and the MGJ are termed mucogingival conditions.1 Recession, absence or reduction of attached gingiva, and probing depths that reach and extend beyond the MGJ resulting in no attached gingiva are common mucogingival conditions. When pockets extend up to or beyond this point, the area must be monitored closely for tooth loss potential because of reduced periodontium and vascular supply to this defect. Conscientious homecare and precise root planing are indicated. In the absence of pocket formation, gingival grafts may be performed by the dentist to cover root surfaces with a transplanted piece of gingival tissue from a donor site, such as the palate. In many cases the condition can be maintained nonsurgically. Frenectomy is a surgical technique to correct a high frenum attachment associated with pocket formation and mucogingival problems. It is usually performed in conjunction with pocket elimination methods.
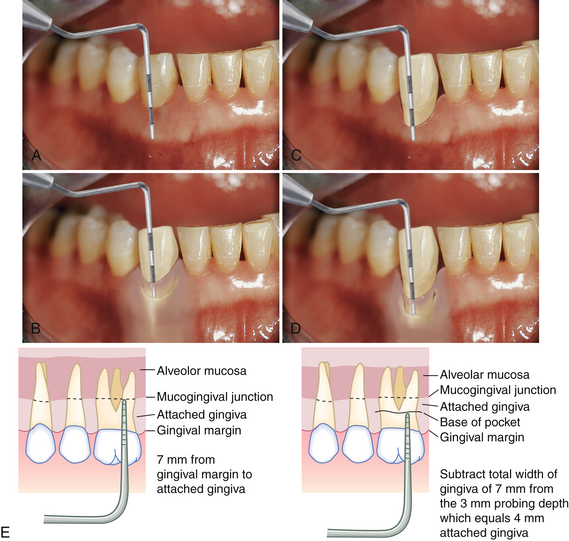
Figure 17-32 Measuring attached gingiva. A, Total width of attached gingiva is 6 mm. B, Depth of sulcus is 3 mm with no clinical attchment loss. Therefore there is still 3 mm of attached gingiva. C, Total width of attached gingiva is 3 mm. D, Depth of pocket is 3 mm, and there is 6 mm of clinical attachment loss. Therefore there is no attached gingiva. E, Diagram of how attached gingiva is determined.
Inadequately Attached Gingiva
Areas with a limited zone of attached gingiva, termed inadequately attached gingiva (IAG), are noted, shown to the client, and explained during the periodontal assessment. To measure the amount of attached gingiva, a periodontal probe is used to measure the total width of the gingiva from the free gingival margin to the MGJ. Next the periodontal pocket depth is obtained and subtracted from the total width of the gingiva (Figure 17-33). IAG is defined as less than 1 mm of keratinized attached gingiva. Areas with IAG are often sensitive, are difficult to maintain, and can develop into a mucogingival problem because the thin zone of attachment usually reflects a reduced blood supply and a potential for quick loss of supporting bone and connective tissue. Recession and high frena or muscle attachments may add to the reduction of alveolar mucosa. These chronic conditions must be recorded and monitored. Although good oral hygiene can maintain periodontal health with almost no alveolar gingiva, high frenum attachments or the use of the tooth as a crown and bridge abutment may indicate surgical intervention to widen the zone of attached gingiva (Procedure 17-1).
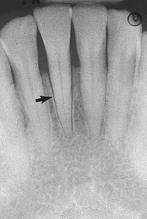
Figure 17-33 Radiograph showing widening of the periodontal ligament associated with occlusal trauma (arrow).
Procedure 17-1 PERIODONTAL CHARTING AND ASSESSMENT
Adapted from the process evaluation form used at the Gene W. Hirschfeld School of Dental Hygiene, Old Dominion University, Norfolk, Virginia.
STEPS
RADIOGRAPHIC ASSESSMENT9
Clinical Use of Radiographs
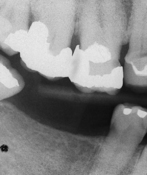
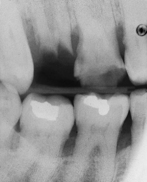
Periodontal assessment includes diagnostic radiographs. Good-quality radiographs are indispensable in assessing the amount of alveolar bone present as well as the pattern, location, and extent of alveolar bone loss. Radiographs are also helpful in identifying local causative factors involved in periodontal disease, such as calculus and bone loss (Figure 17-34), furcation involvement, and dental caries (Figure 17-35). Not all periodontal defects are visualized on radiographs because the image produced is a two-dimensional representation of a three-dimensional object. Radiographs indicate alveolar bone changes from past, not current, disease activity. In addition, soft-tissue changes are not reflected on radiographs. Because of these limitations, radiographs are always used in conjunction with a thorough clinical assessment.
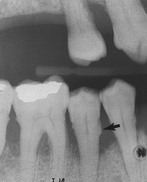
Figure 17-34 Radiograph showing vertical bone destruction, furcation involvement, and subgingival calculus (arrow).
Before any radiographic examination, a clinical examination and risk assessment of the client are conducted. Care is taken to consider health, dental, and pharmacologic histories; clinical assessment data; safety concerns; and radiographic history when exposing clients to radiation.
Selection criteria for client radiographic exposures are available from varying professional organizations including the American Dental Association. For most new clients with generalized signs or history of periodontal disease, a complete intraoral radiographic survey is recommended. When disease is localized, selected periapical or bitewing films should be exposed yearly. Dental office radiographic exposure policies that fail to recognize the individual's risk for oral diseases, but rather require annual or biannual radiographs for everyone, border on malpractice.
Selecting Types and Techniques
Periapical and/or vertical bitewing radiographs should be used to evaluate periodontal disease. Vertical bitewing radiographs are recommended over horizontal bitewings because moderate to severe bone loss cannot be adequately imaged on a horizontal bitewing film. When the long dimension of the film packet is positioned vertically instead of horizontally, the area of bone on the radiograph increases by more than 1 cm (Figure 17-36).
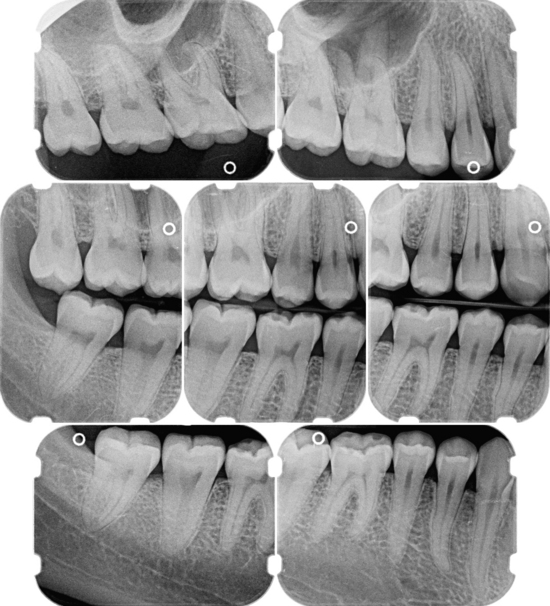
Figure 17-36 Vertical bitewing films can be used to cover a larger area of the alveolar bone.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
Panoramic projections are not recommended for evaluating periodontal disease. Magnification encountered with this type of image minimizes its usefulness in accurately detecting bone changes. The paralleling technique is recommended for periodontal disease assessment over the bisecting angle technique. The paralleling technique produces standardized films that are more anatomically correct, and crestal bone height appears more accurately. The bisecting angle technique can create a foreshortened image, resulting in a film that may show more or less bone than is actually present.
Radiographic Interpretation
Radiographically determining changes in the alveolar bone associated with periodontal disease is based on appearance of the crestal lamina dura (Figure 17-37). In health, crestal lamina dura appears radiographically as a continuous, radiopaque line running parallel to an imaginary line drawn between the CEJs of adjacent teeth. In health the difference between the normal alveolar bone crest and the CEJ can range from 0.4 to 2.9 mm. In general, however, a distance greater than 2 mm from CEJ to bone is considered evidence of disease. An early radiographic change associated with periodontal disease is a fuzziness or break in the continuity of the lamina dura at the mesial or distal aspect of the interdental area. This change results from a loss of crestal density. As inflammation spreads, a wedge-shaped widening of the PDL occurs, manifesting as a radiolucent area between the tooth and the crestal bone, known as triangulation. The V of the wedge of the triangle points apically. As inflammation spreads deeper into the connective tissue, bone degenerates with a subsequent reduction in bone height.
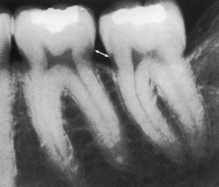
Figure 17-37 Crest of interdental septum normally parallel to a line drawn between the cementoenamel junction of the adjacent teeth (arrow). Note the radiopaque lamina dura around the roots and interdental septa.
(From Newman MG, Takei HH, Klokkevold PR, Carranza FA, eds: Carranza's clinical periodontology, ed 10, St Louis, 2006, Saunders.)
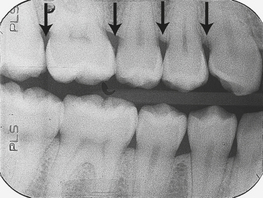
The pattern of bone loss is described as either horizontal or vertical. The CEJ of adjacent teeth can be used to determine bone loss. If teeth are erupted at varying levels or tilted, the lamina dura crest will be slanted to match the variation in crown level. Normal slanting may be confused with bone loss (Figure 17-38).
 When bone loss is parallel to the CEJ of adjacent teeth, horizontal bone loss is present (Figure 17-39).
When bone loss is parallel to the CEJ of adjacent teeth, horizontal bone loss is present (Figure 17-39). When bone height is oriented diagonally to the CEJ of adjacent teeth, vertical bone loss is present (see Figures 17-35 and 17-36).
When bone height is oriented diagonally to the CEJ of adjacent teeth, vertical bone loss is present (see Figures 17-35 and 17-36).Bone loss typically does not occur uniformly throughout the mouth; loss in one area may be more severe than in another. Distribution of bone loss is described as follows:
Bone loss severity is described as mild, moderate, or severe. Severity is assessed as a percentage loss of the normal amount of bone. To obtain percentage loss, the radiograph and probe are used to measure the total root length (from the CEJ to the root apex). Next, distance from the CEJ to alveolar crest is determined. The percentage of bone loss is a ratio of these two measurements (distance from the CEJ to alveolar crest divided by total root length). For example, a 6-mm distance from the CEJ to the crest of the bone with a 17-mm root length would equal a 25% bone loss (6 mm divided by 17 mm) (Table 17-8).
TABLE 17-8 Relationship between Periodontal Disease Severity and Radiographic Findings
| Disease | Radiographic Evidence |
|---|---|
| Gingivitis | No bone loss |
| Slight periodontitis | Less than 30% bone loss |
| Moderate periodontitis | 30%-50% bone loss |
| Severe periodontitis | More than 50% bone loss |
Furcation Involvement (see Figures 17-29 and 17-35)
Radiographs are used to detect alteration in furcations of multirooted teeth. When bone in a furcation is destroyed, it appears as a radiolucency in the furcal area. It is important to note that lack of radiolucency in the furcation does not mean that the disease has not spread to the area. Clinical examinations must always be implemented to ensure a true representation of furcation involvement. Exposing radiographs at differing angles may also assist in detecting furcation involvement.
Limitations of Radiographs
Radiographs reveal less severe bone loss than what is actually present, and early bone changes are not visible radiographically. Typically 30% of the bone mineral must be destroyed before it can be seen on a radiograph. Radiographs confirm clinical findings. For example, if the dental hygienist obtains probing depth readings of 2 to 3 mm but observes bone loss on the radiograph, probing depth measurements should be rechecked. In this case, radiographs provide a check of clinical findings for periodontal probing.
Radiographs are a serial record of the client's periodontal status, affording a basis for comparison with new findings, and show the history of disease progression, allowing the dental hygienist to monitor bone levels over time. As part of periodontal risk assessment, absence of bone loss is associated with a lower risk of future periodontal destruction. However, the presence of bone loss on a radiograph does not indicate that the client will experience continued destruction; rather it indicates an increased risk of future bone loss. See Box 17-8 for periodontal conditions observed on radiographs.
Standardized radiographs of like projections are most helpful in making longitudinal comparisons and providing objective documentation of clinical findings. For example, periapical projections of posterior teeth should not be compared with subsequent bitewing radiographs because an accurate comparison of the bone level cannot be made given two different projection angles. Periodontal probing depths and other clinical findings are subjective assessments, but radiographs present objective data that two or more clinicians can observe at the same time.
Limitations of radiographs in periodontal assessment are as follows:
 Projection factors such as cone-to-film distance, angulation, technique, and film positioning can distort or obscure radiographic images. For example, healthy alveolar bone is evenly located 1 mm apical to the tooth's CEJ, and the alveolar crest should parallel an imaginary line drawn from the CEJ of one tooth to the CEJ of the adjacent tooth. In the radiographic view, this bone position may be distorted by x-ray angulation, erroneously suggesting vertical bone loss.
Projection factors such as cone-to-film distance, angulation, technique, and film positioning can distort or obscure radiographic images. For example, healthy alveolar bone is evenly located 1 mm apical to the tooth's CEJ, and the alveolar crest should parallel an imaginary line drawn from the CEJ of one tooth to the CEJ of the adjacent tooth. In the radiographic view, this bone position may be distorted by x-ray angulation, erroneously suggesting vertical bone loss. Exposure errors such as cone cuts and imbalance of kilovolt peak (kVp) and milliamperage (mA) disguise anatomy and pathosis. Proper exposure uses the widest range of contrast (grays) so that minute changes in bone density and mineralized calculus are shown on the x-ray film. Increased kVp and lower mA produce this broad contrast range and reduce client exposure time, but this combination also lengthens processing time. Shortened processing time decreases contrast and reduces diagnostic information.
Exposure errors such as cone cuts and imbalance of kilovolt peak (kVp) and milliamperage (mA) disguise anatomy and pathosis. Proper exposure uses the widest range of contrast (grays) so that minute changes in bone density and mineralized calculus are shown on the x-ray film. Increased kVp and lower mA produce this broad contrast range and reduce client exposure time, but this combination also lengthens processing time. Shortened processing time decreases contrast and reduces diagnostic information. Facial and lingual supporting bone are obscured by the radiodense tooth structure. Therefore facial and lingual bone loss cannot be detected from radiographs.
Facial and lingual supporting bone are obscured by the radiodense tooth structure. Therefore facial and lingual bone loss cannot be detected from radiographs. Early interdental bone loss is not detectable on radiographs because horizontal alveolar bone loss may not be seen until 30% of the original bone height and density is lost. By the time bone loss is observed radiographically, it is so far advanced that it is easily detected clinically by probing.
Early interdental bone loss is not detectable on radiographs because horizontal alveolar bone loss may not be seen until 30% of the original bone height and density is lost. By the time bone loss is observed radiographically, it is so far advanced that it is easily detected clinically by probing. Bony interdental craters, resulting from vertical bone loss, are not well imaged because facial and lingual ridges of the teeth may be superimposed and because the dense facial and lingual walls of bone obscure the crater. Interdental craters, therefore, are detected only with the periodontal probe.
Bony interdental craters, resulting from vertical bone loss, are not well imaged because facial and lingual ridges of the teeth may be superimposed and because the dense facial and lingual walls of bone obscure the crater. Interdental craters, therefore, are detected only with the periodontal probe. Radiographs do not show soft tissues or connective tissue attachment and consequently cannot show soft-tissue changes. Pockets cannot be measured from radiographs except by using radiopaque markers, such as a periodontal probe or silver point placed at the depth of the sulcus before exposure.
Radiographs do not show soft tissues or connective tissue attachment and consequently cannot show soft-tissue changes. Pockets cannot be measured from radiographs except by using radiopaque markers, such as a periodontal probe or silver point placed at the depth of the sulcus before exposure. Although radiographic images such as a “motheaten” alveolar crest, discontinuous lamina dura, increased trabeculation, and thickened PDL space suggest periodontal abnormality, they are not indicators of periodontitis.
Although radiographic images such as a “motheaten” alveolar crest, discontinuous lamina dura, increased trabeculation, and thickened PDL space suggest periodontal abnormality, they are not indicators of periodontitis.Digital Radiography
Digital radiography in place of traditional film-based radiography is increasing; many practices are totally digital. The following two types of digital systems are available:
 The first system uses intraoral sensors and a radiation source to display radiographic images on a computer monitor after client exposure (Figure 17-40). A sensor, available in a variety of sizes, is positioned in the client's mouth in place of dental film and connected by a wire to the computer (Figure 17-41). This method requires no chemicals for developing, thus eliminating processing supplies and equipment and trips to the darkroom. Treatment is not interrupted for development of digital radiographic images as with the use of conventional radiographs. Major advantages of digital radiography are reduced client exposure to ionizing radiation by 60% to 80% when compared with E-speed, and film images are displayed on a computer monitor within seconds of a client's exposure. A direct digital imaging system requires a radiation source, typically a conventional dental x-ray unit with the timer modified, an intraoral sensor, and a computer monitor. For infection control the sensor is covered with a protective barrier before intraoral placement. Sensor-holding devices similar to traditional film-holding devices used for the paralleling technique are recommended (Figure 17-42).
The first system uses intraoral sensors and a radiation source to display radiographic images on a computer monitor after client exposure (Figure 17-40). A sensor, available in a variety of sizes, is positioned in the client's mouth in place of dental film and connected by a wire to the computer (Figure 17-41). This method requires no chemicals for developing, thus eliminating processing supplies and equipment and trips to the darkroom. Treatment is not interrupted for development of digital radiographic images as with the use of conventional radiographs. Major advantages of digital radiography are reduced client exposure to ionizing radiation by 60% to 80% when compared with E-speed, and film images are displayed on a computer monitor within seconds of a client's exposure. A direct digital imaging system requires a radiation source, typically a conventional dental x-ray unit with the timer modified, an intraoral sensor, and a computer monitor. For infection control the sensor is covered with a protective barrier before intraoral placement. Sensor-holding devices similar to traditional film-holding devices used for the paralleling technique are recommended (Figure 17-42). The second system uses phosphor (PSP) plates as detectors instead of sensors. PSP plates are placed and exposed similarly to regular x-ray film. After exposure the plates are placed on a scanner and scanned by a laser beam, and the radiographic image is displayed on the computer screen. Because darkroom use is still required, this method is not as time efficient but has the advantage of being less expensive than the intraoral sensor digital system.
The second system uses phosphor (PSP) plates as detectors instead of sensors. PSP plates are placed and exposed similarly to regular x-ray film. After exposure the plates are placed on a scanner and scanned by a laser beam, and the radiographic image is displayed on the computer screen. Because darkroom use is still required, this method is not as time efficient but has the advantage of being less expensive than the intraoral sensor digital system.
Figure 17-40 Digital dental radiography system.
(Courtesy Schick Technologies, Long Island City, New York.)
Figure 17-42 Full-mouth series of radiographs displayed on computer monitor using digital technology.
(Courtesy Schick Technologies, Long Island City, New York.)
Digital imaging has 256 colors of gray compared with the 16 to 25 shades of gray found with conventional dental x-ray film. Thus diagnosis is improved with enhanced image contrast. When digital imaging is used as part of periodontal assessment, diagnostic decision making is improved by the excellent images of the lamina dura, bone trabeculation, calculus, furcation involvement, and bone levels produced with minimal distortion (see Box 17-8).
Digital subtraction, another aspect of digital imaging, can improve diagnostic information by comparing a previously stored image with a current image. Subtraction radiography eliminates distracting background information. Radiographs, taken at different times, can be compared by subtracting one image from another. Similar areas of the two films cancel each other out, leaving a neutral gray appearance, whereas changes are highlighted. Areas of bone loss will appear darker gray, and areas of bone gain will be isolated as lighter gray.
By removing structures that do not exhibit change between radiographic examinations, differences are easily identified. Images can be manipulated to change and improve the contrast and enlarged for enhanced viewing. This image manipulation affords immediate opportunities to focus on client conditions and improve education, interaction, and compliance.
Digital radiography systems can be integrated with dental office software. Clinicians can electronically archive the dental images and transmit them via modem to insurance companies and specialists. Network-configured workstations allow radiographic images to be accessed by clinicians at any time from a variety of locations.
Disadvantages of digital radiography include startup costs; the bulky sensor, which may result in more client discomfort than with dental x-ray film; and the inability to heat-sterilize the sensor. Controversy surrounds the image quality and image manipulation, which may present legal concerns in lawsuits.
ASSESSMENT OF PERIODONTAL DISEASE ACTIVITY10
Periodontal disease progression is the pathologic process in which connective tissue attachment at the most apical portion of a periodontal pocket is destroyed. Related to attachment loss is the apical migration of the JE and resorption of alveolar bone. Progression of most forms of periodontitis appears to be associated with qualitative changes in the subgingival flora. Currently, no diagnostic tests reliably identify progressing or active periodontitis lesions other than longitudinal assessments of radiographs and probing attachment levels. In some circumstances supplemental testing of the GCF and subgingival microflora are performed, although the usefulness of this information in clinical practice has not been validated. More tests designed for this purpose will be marketed in the future; however, only valid data from well-controlled clinical trials justify their use.
Measurement of Attachment Loss
An increase in the distance measured from the CEJ to the base of the sulcus or pocket currently is the best measure for disease progression. Measurement error is related to the fact that the probe's penetration can vary with its thickness, the insertion force, and the degree of tissue inflammation. Also, it is difficult to position the periodontal probe in exactly the same position from one appointment to another. These limitations are minimized by using standardized equipment and techniques.
Clinical Signs of Inflammation
Redness, swelling, BOP, and suppuration have relatively good diagnostic value. Two long-term studies found that sites without BOP were almost certain not to show further loss of attachment. Of sites that did bleed on probing at four consecutive visits, 30% lost 2 mm or more of probing attachment. Whereas BOP may have some clinical value as an indicator of increased risk of progression, the continuous absence of BOP is a reliable indicator that periodontal health will be maintained.
Supplemental Diagnostic Tests
GCF flow increases with inflammation. The Periotron, a device that measures GCF, has been used in research but has minimal value clinically other than detecting the presence of fluid in the pocket. GCF contains disease markers, such as inflammatory cytokines (PGE2), enzymes (aspartate aminotransferase and alkaline phosphatase), and tissue breakdown products (proteinases) associated with periodontal disease progression. Tests to identify and quantify these markers in the GCF may prove useful in the future diagnosis of periodontitis owing to their association with active disease. One such test commercially available, the PerioGard Test, measures levels of aspartate aminotransferase in GCF. Yet to be determined is whether this test has any advantages over traditional clinical parameters in diagnosing periodontitis. Research is ongoing for valid, cost-effective diagnostic testing devices.
Microbiologic Assessment of Subgingival Plaque
Several bacteria have been identified as possible periodontopathogens (see Box 17-2). Which of these organisms, single or groups, is responsible for periodontitis progression remains unknown. Sufficient research exists to implicate these microorganisms as potential periodontopathogens, so methods have been devised to detect their presence in subgingival flora as a measure of disease activity. Such testing is limited, however, because the tests do not project progression of disease or identify specific types of periodontal diseases. They may be useful in identifying sites that harbor periodontal pathogens and hence need additional therapies.
In addition, microbiologic assessments may prove useful in high-risk clients when the presence of unusual microorganisms is suspected or in aggressive forms of periodontal disease when treatment may include antibiotics. But even with a complete understanding of the specific microbes responsible for the disease, treatment for it changes very little. Methods of microbiologic assessment include the following:
Microbiologic Cultural Analysis
Subgingival plaque is sampled and cultured in the laboratory to determine the presence of specific microorganisms—marker bacteria—associated with the progression of periodontitis (e.g., Aggregatibacter actinomycetemcomitans and P. gingivalis). The advantage of microbiologic testing is its ability to determine antibiotic susceptibility and resistance; however, this method is time-consuming and costly and relies on living anaerobic bacterial samples that must be specially handled to survive transport. Consequently, this test is not readily used in private practice settings.
Immunologic Methods
Antibodies specific for particular bacterial species are applied to plaque samples, and antibody-antigen reactions are detected by a variety of methods (e.g., direct and indirect immunofluorescence, rapid enzyme immunoassay, and latex agglutination tests). Although direct and indirect immunofluorescence is valuable as a research tool, it requires considerable expertise and expense to perform in evaluating plaque.
DNA Probes
Fragments of bacterial DNA are used in hybridization reactions to “probe” for complementary DNA in subgingival plaque samples. In-office tests, although available, cannot be used to determine antibiotic sensitivity. Only organisms for which tests are sensitive can be identified.
Bacterial Enzymatic Activity Test
Chemicals that indicate the presence of enzymes produced by periodontopathogens are applied to plaque samples to identify the presence of pathogenic bacteria by detecting their tissue-destructive enzymes. Such tests may have value in detecting relative levels of certain periodontopathogens; however, their use in practice is limited because they do not identify specific bacterial species and are unable to determine antimicrobial susceptibility.
PERIODONTAL INDICES
There are many ways of quantifying periodontal health. If the dental hygienist is to survey the prevalence of periodontal disease in a particular population (epidemiologic research), it is important to use indices used by other researchers so that outcomes can be compared. To assess a single individual's periodontal status for developing a care plan, however, a simple, cost-effective, and easily understood method is warranted (see Chapter 15, Box 15-1).
Indices Used in Client Care
Indices can motivate clients to improve their self-care behaviors and provide an easily understood numeric score for comparison between visits. Once scores are calculated over time, clients can identify changes in their periodontal health. The Gingival Index (GI) and the Plaque Index (PlI) are easy to use in clinical practice (Table 17-9 and see Chapter 15, Table 15-5).
TABLE 17-9 Periodontal Indices
CEJ, Cementoenamel junction; WHO, World Health Organization.
A limitation of indices is that each usually measures only one variable, and thus the GI (Silness and Loe) provides information about the presence and severity of gingival inflammation in a population at a given time, but it provides no information about the cause of the inflammation. In contrast, the PlI (Podshadley) provides information on location and thickness of plaque but does not provide information about inflammation (see Chapter 15, Table 15-5). Moreover, indices that measure the same variable often do not have the same focus. For example, the thickness of plaque is important in the Silness and Loe GI but not in the Turesky modification of the Quigley-Hein Index (see Table 17-9). Also, only a few teeth are evaluated with an index, perhaps missing a problem area in a client's mouth.
Indices Used in Research
Periodontal indices are used in epidemiology to quantify the prevalence and incidence of disease and oral debris in specific populations.
 Prevalence means the number of cases existing at a specific point in time per specified number of persons. For example, the statement “52% of 1328 college baseball athletes reported using dental floss daily” is a statement of floss use prevalence.
Prevalence means the number of cases existing at a specific point in time per specified number of persons. For example, the statement “52% of 1328 college baseball athletes reported using dental floss daily” is a statement of floss use prevalence. Incidence means the number of new cases or diseases per specified number of persons occurring in a specified period of time, typically 1 year. For example, the statement “50,000 new cases of periodontitis were diagnosed in the United States from 2009 to 2010” is a statement of incidence.
Incidence means the number of new cases or diseases per specified number of persons occurring in a specified period of time, typically 1 year. For example, the statement “50,000 new cases of periodontitis were diagnosed in the United States from 2009 to 2010” is a statement of incidence. Severity refers to how much destruction is present at one time. For instance, 5 mm CAL is a standard often used to indicate need for periodontal treatment.
Severity refers to how much destruction is present at one time. For instance, 5 mm CAL is a standard often used to indicate need for periodontal treatment.Periodontal and oral hygiene indices are also used in research to serve as outcome measures when testing the efficacy of approaches to care, such as when an antimicrobial toothpaste is tested to determine its effectiveness in decreasing gingivitis.
Popular periodontal indices used in research are listed in Table 17-9. Usually a subset of teeth described by Ramfjord is used in evaluating groups of people. Based on large-scale studies, Ramfjord determined that measurement of teeth 3, 9, 12, 19, 25, and 28 was representative of the entire dentition. These six teeth, the Ramfjord teeth, are used in many indices. When data are collected on a few representative teeth, the index is called “simplified.” Methods of substitution are always calibrated in the simplified index. In some studies, missing teeth are not counted; in others the researcher is required to substitute missing teeth with the next most distal tooth. Other indices require substitution by going mesially or to the contralateral tooth. More than one index is often needed, and examiners must be calibrated before using any oral index in research. With regard to probing depths, examiners are considered calibrated if each one's measurements are within ±1 mm of the others’. Some plaque indices require that disclosing solutions be applied and rinsed away after application, whereas others require no rinsing or no use of a disclosant. Whether in research or practice, the dental hygienist standardizes data-gathering procedures to enable comparable results.
Procedures vary with different indices. The Community Periodontal Index of Treatment Needs (CPITN) is of special interest because it provides information on periodontal status as well as treatment needs. A special periodontal probe with color-coded gradations, designed for this index, has a 0.5-mm ball tip to prevent severing of JE and to allow some tactile sensation as the clinician probes the tooth surface in the pocket. Shallow pockets, represented by reporting a sulcus less than a color-coded gradation from 3.5 to 5.5 mm, indicate that no special treatment is needed. Deeper pockets measuring within the color gradation require therapeutic scaling. The deepest pockets, where the color-coded gradation cannot be seen (more than 5.5 mm), require complex treatment, described as scaling and root planing under local anesthesia, with or without surgical exposure for access.
Sextants or the full mouth can be assessed by the CPITN, but in epidemiologic studies only 10 teeth are examined and only the worst score per sextant is recorded. This approach may underestimate the number of deep pockets in older adult populations that generally have many areas of attachment loss and may overestimate shallow pockets in younger age groups that have many healthy sulci. Other periodontal indices are shown in Table 17-9; oral hygiene indices can be found in Chapter 15, Table 15-5.
DOCUMENTATION AND RECORD KEEPING (SEE CHAPTER 10)
Client information collected throughout care is recorded at each appointment. Documentation allows the hygienist to monitor the client's personal oral hygiene efforts, healing, and ongoing health status. Data collected on periodontal and oral hygiene status facilitate assessment of the client's skin and mucous membrane integrity of the head and neck, a biologically sound and functional dentition, responsibility for oral health, and conceptualization and problem solving.
Legal and insurance regulations require thorough documentation of the client's periodontal and general health status at each visit. Documentation protocols are based on current information related to oral biofilm accumulation and the client's response (e.g., inflammation, attachment levels [probing depth and gingival recession], furcation involvement, tooth mobility, the width of alveolar gingiva, mucogingival problems, and bone loss determined from radiographs). Good records demonstrate the dental hygienist's awareness of the client's periodontal and general health status.
The record must provide a form for baseline documentation of data collected about the client. This form should be carefully organized before the client is seen, so that all required data are included and there is one standard location for the information. A well-organized record form eliminates searching for details or critical information, which signals inadequate record keeping to the client or to the healthcare professionals with whom the dental hygienist collaborates. At subsequent client visits, changes in the baseline conditions are further documented; data then are compared with baseline information. Diligent record keeping is key to tracking frequency of care, disease episodes, client response, and outcomes of care. Trend analysis is based on comparing ongoing findings with baseline data. Longitudinal evaluation is critical for providing optimal care, minimizing legal risks, and meeting third-party requirements for periodontal data on client needs and treatment outcomes. Moreover, objective notations of client perceptions, needs, and desires alert other personnel of special considerations and facilitate oral health education and continuity of high-quality, client-focused care.
Documentation
Periodontal status is monitored from appointment to appointment. Findings of inflammation, recession, pocket probe readings, aberrant tissue forms, bleeding, suppuration, minimum attached gingiva, tooth mobility, and furcation involvement are recorded. Initially, complete six-point probing measurements are recorded for each tooth; however, only changes can be recorded at subsequent visits. The practitioner determines improvement or disease progression by comparing assessment parameters and charting data from visit to visit. Comparison of notations facilitates diagnosis, care planning, and long-term monitoring.
The documentation form should list factors that may negatively affect outcomes of care. For example, the dental hygienist notes when gingival inflammation, disease progression, and healing may be affected by modifiable and nonmodifiable risk factors. Client noncompliance, tardiness, cancellations, and missed appointments are recorded to demonstrate that the client may be responsible for a less than satisfactory result (contributory negligence).
Record-Keeping Formats
Recorded findings provide a graphic display of the client's periodontal health status. Figure 17-43 is a chart showing gingival margin and probing measurements. Recession is visualized by the gingival margin. Other codes, such as for mobility, are added to the form, using the criteria described in Table 17-6, and bleeding is specified by circling probe readings in red.
DECISION-MAKING MATRIX
Figure 17-44 illustrates a decision-making matrix used in providing dental hygiene care. Decisions are the result of objective clinical and radiographic information collected and recorded during the assessment phase of care, the current research evidence base, and collaboration with the dentist and the client. Objective assessment data can be further evaluated in follow-up assessments.
The health, dental, pharmacologic, and personal history information influences choice of treatment modalities. For example, the host defense mechanisms and presence of systemic disease may compromise care results, as can nutritional status, substance use, medications, oral habits, occlusal trauma, oral appliances, and emotional factors. Orthodontic treatment often entails trauma to gingival tissue and compromises oral hygiene. Client motivation and degree of assumption of responsibility also affect self-care and therapeutic outcomes. Each situation needs to be assessed to identify the client's perception of his or her needs, the level of dexterity in oral biofilm control, and degree of anatomic access for professional and self-administered maintenance.
The levels of nonsurgical periodontal therapy that the dental hygienist provides are shown in Table 17-10 and explained in detail in Chapter 28. Data collected during periodontal assessment determine the level of care to be recommended to the client. If eliminating inflammation and arresting disease progression can be achieved by therapeutic scaling and root planing, then no further periodontal treatment is necessary. If, however, therapeutic scaling and root planing fail, then referral for periodontal surgery may be necessary.
CLIENT EDUCATION Tips
 Show clinical appearance of healthy gingiva, periodontal pocket readings, bleeding on probing, and other signs of disease; use client's radiographs for teaching.
Show clinical appearance of healthy gingiva, periodontal pocket readings, bleeding on probing, and other signs of disease; use client's radiographs for teaching.LEGAL, ETHICAL, AND SAFETY ISSUES
 Comprehensive periodontal assessment including risk assessment is a component of assessment and risk management.
Comprehensive periodontal assessment including risk assessment is a component of assessment and risk management. Documentation of assessment findings, at both baseline and all subsequent continued-care visits, is vital to evidenced-based decision making, care planning, care monitoring, referral, and evaluation of clinical outcomes.
Documentation of assessment findings, at both baseline and all subsequent continued-care visits, is vital to evidenced-based decision making, care planning, care monitoring, referral, and evaluation of clinical outcomes.KEY CONCEPTS
 Periodontal risk factors modulate periodontal disease susceptibility and influence the onset, progression, and severity of the disease.
Periodontal risk factors modulate periodontal disease susceptibility and influence the onset, progression, and severity of the disease. The most significant periodontal risk factors are smoking, poor oral hygiene, genetics, and diabetes mellitus.
The most significant periodontal risk factors are smoking, poor oral hygiene, genetics, and diabetes mellitus. The origin of periodontal disease is strongly linked to three periodontal pathogens: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythensis.
The origin of periodontal disease is strongly linked to three periodontal pathogens: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythensis. Clinical signs of inflammation, clinical attachment levels, probing depths, and radiographs are primary indicators for assessing periodontal health, diagnosing, and planning care.
Clinical signs of inflammation, clinical attachment levels, probing depths, and radiographs are primary indicators for assessing periodontal health, diagnosing, and planning care. Gingivitis is a reversible inflammatory condition of the gingiva characterized by no loss of clinical attachment and the presence of any of the following tissue changes: redness, edema, enlargement, spongy consistency, and bleeding on probing.
Gingivitis is a reversible inflammatory condition of the gingiva characterized by no loss of clinical attachment and the presence of any of the following tissue changes: redness, edema, enlargement, spongy consistency, and bleeding on probing. Although most forms of gingivitis are plaque-induced, host and systemic factors modify the clinical characteristics of the disease. Therefore gingival diseases are classified as dental plaque–induced gingival diseases or non–plaque-induced gingival lesions.
Although most forms of gingivitis are plaque-induced, host and systemic factors modify the clinical characteristics of the disease. Therefore gingival diseases are classified as dental plaque–induced gingival diseases or non–plaque-induced gingival lesions. Periodontitis is an inflammation of the supporting structures of the teeth and gingiva characterized by loss of clinical attachment as a result of the destruction of the periodontal ligament and alveolar bone. It exhibits periods of exacerbation (disease activity) and quiescence (inactivity).
Periodontitis is an inflammation of the supporting structures of the teeth and gingiva characterized by loss of clinical attachment as a result of the destruction of the periodontal ligament and alveolar bone. It exhibits periods of exacerbation (disease activity) and quiescence (inactivity). The host's immunoinflammatory and immunologic response to bacteria in oral biofilm is responsible for tissue destruction in periodontal disease.
The host's immunoinflammatory and immunologic response to bacteria in oral biofilm is responsible for tissue destruction in periodontal disease. Gingival bleeding occurring at several sequential continued-care visits is associated with an increased risk for periodontal destruction.
Gingival bleeding occurring at several sequential continued-care visits is associated with an increased risk for periodontal destruction. Because of limitations, radiographs must be used in conjunction with a thorough clinical assessment.
Because of limitations, radiographs must be used in conjunction with a thorough clinical assessment. Vertical bitewing radiographs are recommended over horizontal bitewings for evaluation of periodontitis.
Vertical bitewing radiographs are recommended over horizontal bitewings for evaluation of periodontitis.CRITICAL THINKING EXERCISES
Supplemental Examination Findings: Client had a full-mouth series of radiographs exposed 2 years ago. Clinical examination reveals pale whitish gingiva in the maxillary anterior and palatal areas and a whitish coat to the gingival margin in the posterior areas, with moderate enlargement. Heavy subgingival calculus is found throughout the mouth with generalized bleeding on probing. Pocket depths have increased from 2 years ago, when the deepest reading was 5 mm.
Periodontal assessment reveals generalized 6-mm pocket depths in all posterior areas, 7-mm pocket depths in the maxillary lingual area, and 4-mm pocket depths in all other areas; and 3-mm recession on teeth 6, 12, and 13 and 6-mm recession on 28. Use the case information to answer the following questions:
Refer to the Procedures Manual where rationales are provided for the steps outlined in the procedure presented in this chapter.
1. Al-Shammari K.F., Al-Khabbaz A.K., Al-Ansari J.M., et al. Risk Indicators for tooth loss due to periodontal disease. J Periodontol. 1910;76:2005.
2. Douglas C. Risk assessment and management of periodontal disease. J Am Dent Assoc. 2006;137:27S.
3. Peruzzo D.C., Bruno B.B., Ambrosano G.M., et al. A systemic review of stress and psychological factors as possible risk factors for periodontal diseases. J Periodontol. 2007;78:1491.
4. Slayton R. Genetics and environmental factors play important role in the risk for periodontal disease and edentulism. J Evid Based Dent Pract. 2006;3:238.
5. Ad Hoc Committee on Parameters of Care, American Academy of Periodontology: Parameter on comprehensive periodontal examination. J Periodontol. 2000;71(Suppl 5):847.
6. American Academy of Periodontology Research, Science and Therapy Committee. Diagnosis of periodontal diseases. J Periodontol. 2004;74:1237.
7. Dave S., Van Dyke T., Suzuki Z.J. Chronic inflammation in periodontal diseases: immunopathogenesis and treatment. Grand Rounds Oral Syst Med. 2007;2:10.
8. Armitage G. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1.
9. American Dental Association Council on Scientific Affairs. The use of dental radiographs; update and recommendations. J Am Dent Assoc. 2006;137:1304.
10. Burt B. Research Science and Therapy Committee: Position paper: epidemiology of periodontal diseases. J Periodontol. 2005;76:1406.
Visit the  website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..
website at http://evolve.elsevier.com/Darby/Hygiene for competency forms, suggested readings, glossary, and related websites..