Screening for Cardiovascular Disease
The cardiovascular system consists of the heart, capillaries, veins, and lymphatics and functions in coordination with the pulmonary system to circulate oxygenated blood through the arterial system to all cells. This system then collects deoxygenated blood from the venous system and delivers it to the lungs for reoxygenation (Fig. 6-1).
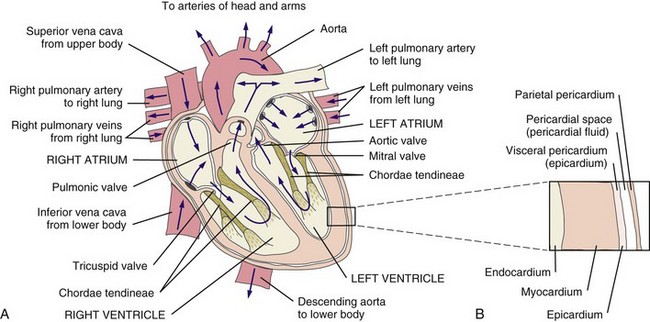
Fig. 6-1 Structure and circulation of the heart. Blood entering the left atrium from the right and left pulmonary veins flows into the left ventricle. The left ventricle pumps blood into the systemic circulation through the aorta. From the systemic circulation, blood returns to the heart through the superior and inferior venae cavae. From there the right ventricle pumps blood into the lungs through the right and left pulmonary arteries. A thick layer of connective tissue called the septum separates the left and right chambers of the heart. The top of the heart (atria) is also separated from the bottom of the heart (ventricles) by connective tissue, which does not conduct electrical activity and serves as an electrical barrier or insulator. (Redrawn from Black JM, Hawks JH: Luckmann and Sorenson’s medical-surgical nursing, ed 8, Philadelphia, 2009, WB Saunders.)
Heart disease remains the leading cause of death in industrialized nations. In the United States alone, cardiovascular disease (CVD) is responsible for approximately one million deaths each year. One in three Americans has some form of cardiovascular disease. The American Heart Association (AHA) reports that about half of all deaths from heart disease are sudden and unexpected.1
Known risk factors include advancing age, hypertension, obesity, sedentary lifestyle, excessive alcohol consumption, oral contraceptive use (over age 35, combined with smoking), first-generation family history, tobacco use (including exposure to second-hand smoke), abnormal cholesterol levels, and race (e.g., African Americans, Mexican Americans, Native Americans, and Pacific Islanders are at greater risk).
Fortunately, during the last two decades cardiovascular research has greatly increased our understanding of the structure and function of the cardiovascular system in health and disease. Despite the formidable statistics regarding the prevalence of CVD, during the last 15 years a steady decline in mortality from cardiovascular disorders has been witnessed. Effective application of the increased knowledge regarding CVD and its risk factors will assist health care professionals to educate clients in achieving and maintaining cardiovascular health.
Information about heart disease is changing rapidly. Part of the therapist’s intervention includes patient/client education. The therapist can access up-to-date information at many useful websites (Box 6-1).
Signs and Symptoms of Cardiovascular Disease
Cardinal symptoms of cardiac disease usually include chest, neck and/or arm pain or discomfort, palpitation, dyspnea, syncope (fainting), fatigue, cough, diaphoresis, and cyanosis. Edema and leg pain (claudication) are the most common symptoms of the vascular component of a cardiovascular pathologic condition. Symptoms of cardiovascular involvement should also be reviewed by system (Table 6-1).
TABLE 6-1
Cardiovascular Signs and Symptoms by System
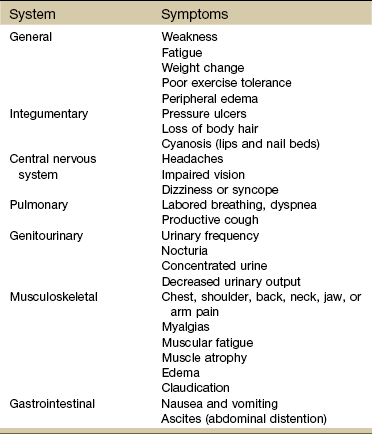
Modified from Goodman CC, Fuller K: Pathology: implications for the physical therapist, ed 3, Philadelphia, 2009, WB Saunders.
Chest Pain or Discomfort
Chest pain or discomfort is a common presenting symptom of cardiovascular disease and must be evaluated carefully. Chest pain may be cardiac or noncardiac in origin and may radiate to the neck, jaw, upper trapezius muscle, upper back, shoulder, or arms (most commonly the left arm).
Radiating pain down the arm follows the pattern of ulnar nerve distribution. Pain of cardiac origin can be experienced in the somatic areas because the heart is supplied by the C3 to T4 spinal segments, referring visceral pain to the corresponding somatic area (see Fig. 3-3). For example, the heart and the diaphragm, supplied by the C5-6 spinal segment, can refer pain to the shoulder (see Figs. 3-4 and 3-5).
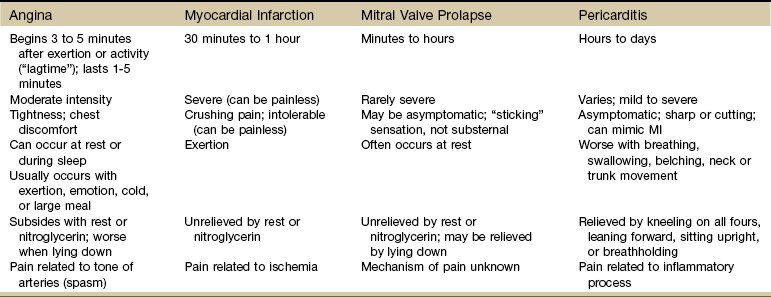
Cardiac-related chest pain may arise secondary to angina, myocardial infarction (MI), pericarditis, endocarditis, mitral valve prolapse, or dissecting aortic aneurysm. Location and description (frequency, intensity, and duration) vary according to the underlying pathologic condition (see each individual condition).
Cardiac chest pain is often accompanied by associated signs and symptoms such as nausea, vomiting, diaphoresis, dyspnea, fatigue, pallor, or syncope. These associated signs and symptoms provide the therapist with red flags to identify musculoskeletal symptoms of a systemic origin.
Cardiac chest pain or discomfort can also occur when the coronary circulation is normal, as in the case of clients with anemia, causing lack of oxygenation of the myocardium (heart muscle) during physical exertion.
Noncardiac chest pain can be caused by an extensive list of disorders requiring screening for medical disease. For example, cervical disk disease and arthritic changes can mimic atypical chest pain. Chest pain that is attributed to anxiety, trigger points, cocaine use, and other noncardiac causes is discussed in Chapter 17.
Palpitation
Palpitation, the presence of an irregular heartbeat, may also be referred to as arrhythmia or dysrhythmia, which may be caused by a relatively benign condition (e.g., mitral valve prolapse, “athlete’s heart,” caffeine, anxiety, exercise) or a severe condition (e.g., coronary artery disease, cardiomyopathy, complete heart block, ventricular aneurysm, atrioventricular valve disease, mitral or aortic stenosis).
The sensation of palpitations has been described as a bump, pound, jump, flop, flutter, or racing sensation of the heart. Associated symptoms may include lightheadedness or syncope. Palpated pulse may feel rapid or irregular, as if the heart “skipped” a beat.
Occasionally, a client will report “fluttering” sensations in the neck. Generally, unless accompanied by other symptoms, these sensations in the neck are caused by anxiety, random muscle fasciculation, or minor muscle strain or overuse.
Palpitations can be considered physiologic (i.e., when less than six occur per minute, this may be considered within normal function of the heart). However, palpitation lasting for hours or occurring in association with pain, shortness of breath, fainting, or severe lightheadedness requires medical evaluation. Palpitation in any person with a history of unexplained sudden death in the family requires medical referral.
Clients describing “palpitations” or similar phenomena may not be experiencing symptoms of heart disease. Palpitations may occur as a result of an overactive thyroid, secondary to caffeine sensitivity, as a side effect of some medications, and with the use of drugs such as cocaine. Encourage the client to report any such symptoms to the physician if this information has not already been brought to the physician’s attention.
Dyspnea
Dyspnea, also referred to as breathlessness or shortness of breath, can be cardiovascular in origin, but it may also occur secondary to a pulmonary pathologic condition (see also Chapter 7), fever, certain medications, allergies, poor physical conditioning, or obesity. Early onset of dyspnea may be described as having to breathe too much or as an uncomfortable feeling during breathing after exercise or exertion.
Shortness of breath with mild exertion (dyspnea on exertion [DOE]), when caused by an impaired left ventricle that is unable to contract completely, results in the lung’s inability to empty itself of blood. Pulmonary congestion and shortness of breath then occur. With severe compromise of the cardiovascular or pulmonary systems, dyspnea may occur at rest.
The severity of dyspnea is determined by the extent of disease. Thus the more severe the heart disease is, the easier it is to bring on dyspnea. Extreme dyspnea includes paroxysmal nocturnal dyspnea (PND) and orthopnea (breathlessness that is relieved by sitting upright with pillows used to prop the trunk and head).
PND and sudden, unexplained episodes of shortness of breath frequently accompany congestive heart failure (CHF). During the day the effects of gravity in the upright position and the shunting of excessive fluid to the lower extremities permit more effective ventilation and perfusion of the lungs, keeping the lungs relatively fluid free, depending on the degree of CHF. PND awakens the person sleeping in the recumbent position because the amount of blood returning to the heart and lungs from the lower extremities increases in this position.
Anyone who cannot climb a single flight of stairs without feeling moderately to severely winded or who awakens at night or experiences shortness of breath when lying down should be evaluated by a physician. Anyone with known cardiac involvement who develops progressively worse dyspnea must also notify the physician of these changes.
Dyspnea relieved by specific breathing patterns (e.g., pursed-lip breathing) or by specific body position (e.g., leaning forward on the arms to lock the shoulder girdle) is more likely to be pulmonary than cardiac in origin. Because breathlessness can be a terrifying experience for many persons, any activity that provokes the sensation is avoided, thus quickly reducing functional activities.
Cardiac Syncope
Cardiac syncope (fainting) or more mild lightheadedness can be caused by reduced oxygen delivery to the brain. Cardiac conditions resulting in syncope include arrhythmias, orthostatic hypotension, poor ventricular function, coronary artery disease, and vertebral artery insufficiency.
Lightheadedness that results from orthostatic hypotension (sudden drop in blood pressure [BP]) may occur with any quick change in a prolonged position (e.g., going from a supine position to an upright posture or standing up from a sitting position) or physical exertion involving increased abdominal pressure (e.g., straining with a bowel movement, lifting). Any client with aortic stenosis is likely to experience lightheadedness as a result of these activities.
Noncardiac conditions, such as anxiety and emotional stress, can cause hyperventilation and subsequent lightheadedness (vasovagal syncope). Side effects, such as orthostatic hypotension, may also occur during the period of initiation and regulation of cardiac medications (e.g., vasodilators).
Syncope that occurs without any warning period of lightheadedness, dizziness, or nausea may be a sign of heart valve or arrhythmia problems. Since sudden death can thus occur, medical referral is recommended for any unexplained syncope, especially in the presence of heart or circulatory problems or if the client has any risk factors for heart attack or stroke.
Examination of the cervical spine may include vertebral artery tests for compression of the vertebral arteries.2-5 If signs of eye nystagmus, changes in pupil size, or visual disturbances and symptoms of dizziness or lightheadedness occur, care must be taken concerning any treatment that follows. It has been suggested, however, that other factors, such as individual sensitivity to extreme head positions, age, and vestibular responsiveness, could affect the results of these tests.6
The test may be contraindicated in individuals with cervical spine fusion, Down syndrome (due to cervical hypermobility and/or instability), or other cervical spine instabilities. Although there is controversy and uncertainty about the safety and accuracy of vertebral artery tests, long-term complications have not been reported as a result of administering these tests.7,8 For a video of how to perform this test, go to www.youtube.com and type in “Gans video vertebral artery test.”
Fatigue
Fatigue provoked by minimal exertion indicates a lack of energy, which may be cardiac in origin (e.g., coronary artery disease, aortic valve dysfunction, cardiomyopathy, or myocarditis) or may occur secondary to a neurologic, muscular, metabolic, or pulmonary pathologic condition. Often, fatigue of a cardiac nature is accompanied by associated symptoms such as dyspnea, chest pain, palpitations, or headache.
Fatigue that goes beyond expectations during or after exercise, especially in a client with a known cardiac condition, must be closely monitored. It should be remembered that beta-blockers prescribed for cardiac problems can also cause unusual fatigue symptoms.
For the client experiencing fatigue without a prior diagnosis of heart disease, monitoring vital signs may indicate a failure of the BP to rise with increasing workloads. Such a situation may indicate cardiac output that is inadequate in meeting the demands of exercise. However, poor exercise tolerance is often the result of deconditioning, especially in the older adult population. Further testing (e.g., exercise treadmill test) may be helpful in determining whether fatigue is cardiac-induced.
Cough
Cough (see also Chapter 7) is usually associated with pulmonary conditions, but it may occur as a pulmonary complication of a cardiovascular pathologic complex. Left ventricular dysfunction, including mitral valve dysfunction resulting from pulmonary edema or left ventricular CHF, may result in a cough when aggravated by exercise, metabolic stress, supine position, or PND. The cough is often hacking and may produce large amounts of frothy, blood-tinged sputum. In the case of CHF, cough develops because a large amount of fluid is trapped in the pulmonary tree, irritating the lung mucosa.
Cyanosis
Cyanosis is a bluish discoloration of the lips and nail beds of the fingers and toes that accompanies inadequate blood oxygen levels (reduced amounts of hemoglobin). Although cyanosis can accompany hematologic or central nervous system disorders, most often, visible cyanosis accompanies cardiac and pulmonary problems.
Edema
Edema in the form of a 3-pound or greater weight gain or a gradual, continuous gain over several days that results in swelling of the ankles, abdomen, and hands combined with shortness of breath, fatigue, and dizziness may be red-flag symptoms of CHF.
Other accompanying symptoms may include jugular vein distention (JVD; see Fig. 4-44) and cyanosis (of lips and appendages). Right upper quadrant pain described as a constant aching or sharp pain may occur secondary to an enlarged liver in this condition.
Right heart failure and subsequent edema can also occur secondary to cardiac surgery, venous valve incompetence or obstruction, cardiac valve stenosis, coronary artery disease, or mitral valve dysfunction.
Noncardiac causes of edema may include pulmonary hypertension, kidney dysfunction, cirrhosis, burns, infection, lymphatic obstruction, use of nonsteroidal antiinflammatory drugs (NSAIDs), or allergic reaction.
When edema and other accompanying symptoms persist despite rest, medical referral is required. Edema of a cardiac origin may require electrocardiogram (ECG) monitoring during exercise or activity (the physician may not want the client stressed when extensive ECG changes are present), whereas edema of peripheral origin requires treatment of the underlying etiologic complex.
Claudication
Claudication or leg pain occurs with peripheral vascular disease (PVD; arterial or venous), often occurring simultaneously with coronary artery disease. Claudication can be more functionally debilitating than other associated symptoms, such as angina or dyspnea, and may occur in addition to these other symptoms. The presence of pitting edema along with leg pain is usually associated with vascular disease.
Other noncardiac causes of leg pain (e.g., sciatica, pseudoclaudication, anterior compartment syndrome, gout, peripheral neuropathy) must be differentiated from pain associated with peripheral vascular disease. Low back pain associated with pseudoclaudication often indicates spinal stenosis. The discomfort associated with pseudoclaudication is frequently bilateral and improves with rest or flexion of the lumbar spine (see also Chapter 14).
Vascular claudication may occur in the absence of physical findings but is usually accompanied by skin discoloration and trophic changes (e.g., thin, dry, hairless skin) in the presence of vascular disease. Core temperature, peripheral pulses, and skin temperature should be assessed. Cool skin is more indicative of vascular obstruction; warm to hot skin may indicate inflammation or infection. Abrupt onset of ischemic rest pain or sudden worsening of intermittent claudication may be due to thromboembolism and must be reported to the physician immediately.
If people with intermittent claudication have normal-appearing skin at rest, exercising the extremity to the point of claudication usually produces marked pallor in the skin over the distal third of the extremity. This postexercise cutaneous ischemia occurs in both upper and lower extremities and is due to selective shunting of the available blood to the exercised muscle and away from the more distal parts of the extremity.
Vital Signs
The therapist may see signs of cardiac dysfunction as abnormal responses of heart rate and BP during exercise. The therapist must remain alert to a heart rate that is either too high or too low during exercise, an irregular pulse rate, a systolic BP that does not rise progressively as the work level increases, a systolic BP that falls during exercise, or a change in diastolic pressure greater than 15 to 20 mm Hg (Case Example 6-1).
Monitor vital signs in anyone with known heart disease. Some BP lowering medications can keep a client’s heart rate from exceeding 90 bpm. For these individuals the therapist can monitor heart rate, but use perceived rate of exertion (PRE) as a gauge of exercise intensity. See Chapter 4 for more specific information about vital sign assessment.
Cardiac Pathophysiology
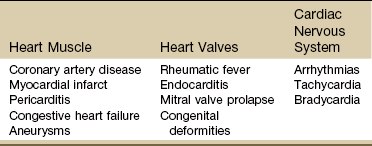
Three components of cardiac disease are discussed, including diseases affecting the heart muscle, diseases affecting heart valves, and defects of the cardiac nervous system (Table 6-2).
Conditions Affecting the Heart Muscle
In most cases, a cardiopulmonary pathologic condition can be traced to at least one of three processes:
Any combination of these can cause chest, neck, back, and/or shoulder pain. Frequently, these conditions occur sequentially. For example, an underlying obstruction, such as pulmonary embolus, leads to congestion, and subsequent dilation of the vessels blocked by the embolus.
The most common cardiovascular conditions to mimic musculoskeletal dysfunction are angina, MI, pericarditis, and dissecting aortic aneurysm. Other cardiovascular diseases are not included in this text because they are rare or because they do not mimic musculoskeletal symptoms.
Degenerative heart disease refers to the changes in the heart and blood supply to the heart and major blood vessels that occur with aging. As the population ages, degenerative heart disease becomes the most prevalent form of cardiovascular disease. Degenerative heart disease is also called atherosclerotic cardiovascular disease, arteriosclerotic cardiovascular disease, coronary heart disease (CHD), and coronary artery disease (CAD).
Hyperlipidemia
Hyperlipidemia refers to a group of metabolic abnormalities resulting in combinations of elevated serum total cholesterol (hypercholesterolemia), elevated low-density lipoproteins, elevated triglycerides (hypertriglyceridemia) and decreased high-density lipoproteins. These abnormalities are the primary risk factors for atherosclerosis and coronary artery disease.9-11
Statin medications (e.g., Zocor, Lipitor, Crestor, Lescol, Mevacor, Pravachol) are used to reduce low-density lipoprotein (LDL) cholesterol. While statins are generally well tolerated, there is a wide body of medical literature that associates the adverse reaction of myalgia and the more serious reaction of rhabdomyolysis with statin medications.11,12 If detected early, statin-related symptoms can be reversible with reduction of dose, selection of another statin, or cessation of statin use.13-15
Screening for Side Effects of Statins: Myalgia and myopathy (including respiratory myopathy) are the most common myotoxic event associated with statins; joint pain is also reported.16,17 The incidence of myotoxic events appears to be dose-dependent. Rates of adverse events from statins vary in the literature from 5% up to 18%18 but with an increasing number of people taking these medications, physical therapists can expect to see a rise in the prevalence of this condition among the older age group.19,20
Monitoring for elevated serum liver enzymes and creatine kinase are significant laboratory indicators of muscle and liver impairment.21 Symptoms of mild myalgia (muscle ache or weakness without increased creatine kinase [CK] levels), myositis (muscle symptoms with increased CK levels), or frank rhabdomyolysis (muscle symptoms with marked CK elevation; more than 10 times the normal upper limit) range from 1% to 7%.11
Muscle soreness after exercise that is caused by statin use may go undetected even by the therapist. Awareness of potential risk factors and monitoring in anyone taking statins and any of these additional risk factors is advised. Muscular symptoms are more common in older individuals (Case Example 6-2).11,22 Other risk factors include23:
• Age over 80 (women more than men)
• Drinks excessive grapefruit juice daily (more than 1 quart/day)
• Use of other medications (e.g., cyclosporine, some antibiotics, verapamil, human immunodeficiency virus [HIV] protease inhibitors, some antidepressants)23
Muscle aches and pain, unexplained fever, nausea, vomiting and dark urine can potentially be signs of myositis and should be referred to a physician immediately. Risk for statin-induced myositis is highest in people with liver disease, acute infection, and hypothyroidism. Severe statin-induced myopathy can lead to rhabdomyolysis (enzyme leakage, muscle cell destruction, and elevated CK levels). Rhabdomyolysis is associated with impaired renal and liver function. Screening for liver impairment (see Chapter 9) in people taking statins is an important part of assessing for rhabdomyolysis (see further discussion of rhabdomyolysis in Chapter 9).24
The therapist will need to perform clinical tests and measures to differentiate exercise-related muscle fatigue and soreness from statin-induced symptoms. For example, exercise-induced muscle fatigue and soreness should be limited to the muscles exercised and resolve within 24 to 48 hours. Statin-related weakness may involve muscles not recently exercised and may progress or fail to show signs of improvement even after several days of rest.25 Dynamometer testing can be used as a valid and reliable indicator of change in muscle strength.26,27
Strength testing, combined with client history, risk factors, and performance on the Stair-Climbing Test and Six-Minute Walk test, may prove to be an adequate means of assessment to detect meaningful declines in functional status; baseline measures are important.25
Coronary Artery Disease
The heart muscle must have an adequate blood supply to contract properly. As mentioned, the coronary arteries carry oxygen and blood to the myocardium. When a coronary artery becomes narrowed or blocked, the area of the heart muscle supplied by that artery becomes ischemic and injured, and infarction may result.
The major disorders caused by insufficient blood supply to the myocardium are angina pectoris and MI. These disorders are collectively known as coronary artery disease (CAD), also called coronary heart disease or ischemic heart disease. CAD includes atherosclerosis (fatty buildup), thrombus (blood clot), and spasm (intermittent constriction).
CAD results from a person’s complex genetic makeup and interactions with the environment, including nutrition, activity levels, and history of smoking. Susceptibility to CVD may be explained by genetic factors, and it is likely that an “atherosclerosis gene” or “heart attack gene” will be identified.28 The therapeutic use of drugs that act by modifying gene transcription is a well-established practice in the treatment of CAD and essential hypertension.29,30
Atherosclerosis: Atherosclerosis is the disease process often called arteriosclerosis or hardening of the arteries. It is a progressive process that begins in childhood. It can occur in any artery in the body, but it is most common in medium-sized arteries such as those of the heart, brain, kidneys, and legs. Starting in childhood, the arteries begin to fill with a fatty substance, or lipids such as triglycerides and cholesterol, which then calcify or harden (Fig. 6-2).
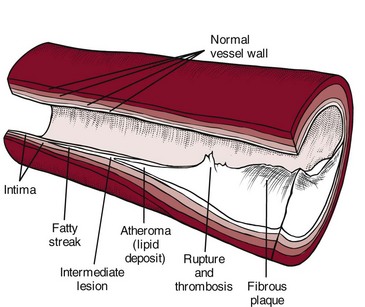
Fig. 6-2 Hardening of the arteries. Atherosclerosis begins with an injury to the endothelial lining of the artery (intimal layer) that makes the vessel permeable to circulating lipoproteins. Penetration of lipoproteins into the smooth muscle cells of the intima produces “fatty streaks.” A fibrous plaque large enough to decrease blood flow through the artery develops. Calcification with rupture or hemorrhage of the fibrous plaque is the final advanced stage. Thrombosis (stationary blood clot) may occur, further occluding the lumen of the blood vessel.
This filler, called plaque, is made up of fats, calcium, and fibrous scar tissue, and lines the usually supple arterial walls, progressively narrowing the arteries. These arteries carry blood rich in oxygen to the myocardium (middle layer of the heart consisting of the heart muscle), but the atherosclerotic process leads to ischemia and to necrosis of the heart muscle. Necrotic tissue gradually forms a scar, but before scar formation, the weakened area is susceptible to aneurysm development.
When fully developed, plaque can cause bleeding, clot formation, and distortion or rupture of a blood vessel (Fig. 6-3). Heart attacks and strokes are the most sudden and often fatal signs of the disease.
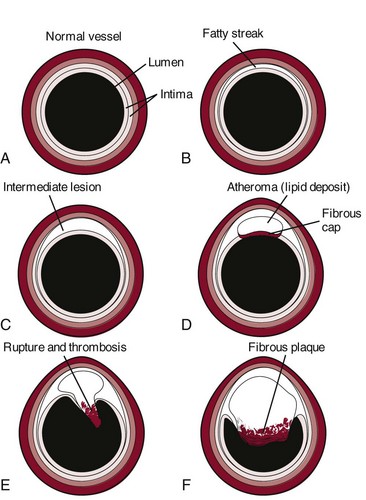
Fig. 6-3 Updated model of atherosclerosis. New technology using intravascular ultrasound shows the whole atherosclerotic plaque and has changed the way we view things. The traditional model held that an atherosclerotic plaque in the blood vessel, particularly a coronary blood vessel, kept growing inward and obstructing flow until it closed off and caused a heart attack. This is not entirely correct. It is more accurate to say that in the normal vessel (A), penetration of lipoproteins into the smooth muscle cells of the intima produces fatty streaks (B) and the start of a coronary lesion forms. C and D, The coronary lesion grows outward first in a compensatory manner to maintain the open lumen. This is called positive remodeling, as the blood vessel tries to maintain an open lumen until it can do so no more. E, Only then does the plaque (atheroma) begin to build up, gradually pressing inward into the lumen with obstruction of blood flow and possible rupture and thrombosis, potentially leading to myocardial infarction (MI) or stroke. F, Vascular disease today is considered a disease of the wall. Some researchers like to say the disease is in the donut, not the hole of a donut, and that is a new concept.102
Thrombus: When plaque builds up on the artery walls, the blood flow is slowed and a clot (thrombus) may form on the plaque. When a vessel becomes blocked with a clot, it is called thrombosis. Coronary thrombosis refers to the formation of a clot in one of the coronary arteries, usually causing a heart attack.
Spasm: Sudden constriction of a coronary artery is called a spasm; blood flow to that part of the heart is cut off or decreased. A brief spasm may cause mild symptoms that never return. A prolonged spasm may cause heart damage such as an infarct. This process can occur in healthy persons who have no cardiac history, as well as in those who have known atherosclerosis. Chemicals like nicotine and cocaine may lead to coronary artery spasm; other possible factors include anxiety and cold air.
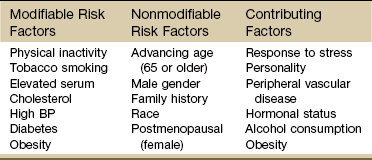
Risk Factors: In 1948 the United States government decided to investigate the etiology, incidence, and pathology of CAD by studying the residents of Framingham, Massachusetts, a typical small town in the United States. Over the next multiple decades, various aspects of lifestyle, health, and disease were studied.
The research revealed important modifiable and nonmodifiable risk factors associated with death caused by CAD. Since that time, an additional category, contributing factors, has been added (Table 6-3). The AHA has also developed a validated health-risk appraisal instrument to assess individual risk of heart attack, stroke, and diabetes (see Box 6-1).
TABLE 6-3
Risk Factors for Coronary Artery Disease
AHA Scientific Statements on Prevention of Coronary Heart Disease and Stroke, 2010. Available on-line at www.heart.org (Risk Factors and Coronary Heart Disease). Accessed Sept. 1, 2010.
More recent research has identified other possible risk factors for and predictors of cardiac events, especially for those persons who have already had a heart attack. These additional risk factors include:
1. Exposure to bacteria such as Chlamydia pneumoniae, Porphyromonas gingivalis, and Cytomegalovirus organisms31-33
2. Excess levels of homocysteine, an amino acid by-product of food rich in proteins
3. High levels of α-lipoprotein, a close cousin of LDL that transports fat throughout the body
4. High levels of fibrinogen, a protein that binds together platelet cells in blood clots34
5. Large amounts of C-reactive protein (CRP), a specialized protein necessary for repair of tissue injury.35,36
6. The presence of troponin T, a regulatory protein that helps heart muscle contract37
7. The presence of diagonal earlobe creases (under continued investigation)38-42
8. Past history of cancer treatment (cardiotoxic chemotherapeutic agents, trunk radiation)43
Therapists can assist clients in assessing their 10-year risk for heart attack using a risk assessment tool from the National Cholesterol Education program available at http://hin.nhlbi.nih.gov/atpiii/calculator.asp?usertype=prof.
Women and Heart Disease: Many women know about the risk of breast cancer, but in truth, they are 10 times more likely to die of cardiovascular disease. While 1 in 30 deaths is from breast cancer, 1 in 2.5 deaths are from heart disease.44
Women do not seem to do as well as men after taking medications to dissolve blood clots or after undergoing heart-related medical procedures. Of the women who survive a heart attack, 46% will be disabled by heart failure within 6 years.45
In general, the rate of CAD is rising among women and falling among men. Men develop CAD at a younger age than women, but women make up for it after menopause. African-American women have a 70% higher death rate from CAD than white women. So whenever screening chest pain, keep in mind the demographics: older men and women, menopausal women, and black women are at greatest risk.
Diabetes alone poses a greater risk than any other factor in predicting cardiovascular problems in women. Women with diabetes are seven times more likely to have cardiovascular complications and about half of them will die of CAD.46
Studies have shown that women and men actually differ in the symptoms of CAD and in the manner in which acute MI can present. Women experience symptoms of CAD, which are more subtle and are “atypical” compared to the traditional symptoms such as angina and chest pain.
One of the most important primary signs of CAD in women is unexplained, severe episodic fatigue and weakness associated with decreased ability to carry out normal activities of daily living (ADLs). Because fatigue, weakness, and trouble sleeping are general types of symptoms, they are not as easily associated with cardiovascular events and are many times missed by health care providers in screening for heart disease.47
Symptoms of weakness, fatigue and sleeping difficulty, and nausea have been reported as a common occurrence as much as a month prior to the development of acute MI in women (see Table 6-4). The classic pain of CAD is usually substernal chest pain characterized by a crushing, heavy, squeezing sensation commonly occurring during emotion or exertion. The pain of CAD in women, however, may vary greatly from that in men (see further discussion on MI in this chapter).
Risk reduction in women focuses on lifestyle changes such as smoking cessation; low fat, low cholesterol diet; increased intake of omega-3 fatty acids; increased fruit, vegetable, whole grain intake; salt and alcohol limitation; and increased exercise and weight loss. If the woman has diabetes, strict glucose control is extremely important.48
Clinical Signs and Symptoms: Atherosclerosis, by itself, does not necessarily produce symptoms. For manifestations to develop, there must be a critical deficit in blood supply to the heart in proportion to the demands of the myocardium for oxygen and nutrients (supply and demand imbalance). When atherosclerosis develops slowly, collateral circulation develops to meet the heart’s demands. Often, symptoms of CAD do not appear until the lumen of the coronary artery narrows by 75% (see also Hypertension).
Although the arteries are rarely completely blocked, the deposits of plaque are often extensive enough to restrict blood flow to the heart, especially during exercise in a clinical practice when there is a need to deliver more oxygen-carrying blood to the heart. Like other muscles, the heart, when deprived of oxygen, may ache, causing chest pain or discomfort referred to as angina.
CAD is a progressive disorder, especially if left untreated. If the blood flow is entirely disrupted, usually by a clot that has formed in the obstructed region, some of the tissue that is supplied by the vessel can die, and a heart attack or even sudden cardiac death results.
When tissue loss is extensive enough to disrupt the electrical impulses that stimulate the heart’s contractions, heart failure, chronic arrhythmias, and conduction disturbances may develop.
Angina
Acute pain in the chest, called angina pectoris, results from the imbalance between cardiac workload and oxygen supply to myocardial tissue. Angina is a symptom of obstructed or decreased blood supply to the heart muscle primarily from a condition called atherosclerosis.
Atherosclerosis is now recognized as an inflammatory condition affecting the coronary arteries as well as the peripheral vessels. It is often accompanied by hypertension and signs of PVD. Although the primary cause of angina is CAD, angina can occur in individuals with normal coronary arteries and with other conditions affecting the supply/demand balance.
As vessels become lined with atherosclerotic plaque, symptoms of inadequate blood supply develop in the tissues supplied by these vessels. A growing mass of plaque in the vessel collects platelets, fibrin, and cellular debris. Platelet aggregations are known to release prostaglandin capable of causing vessel spasm. This in turn promotes platelet aggregation, and a vicious spasm/pain cycle begins.
The present theory of heart pain suggests that pain occurs as a result of an accumulation of metabolites within an ischemic segment of the myocardium. The transient ischemia of angina or the prolonged, necrotic ischemia of an MI sets off pain impulses secondary to rapid accumulation of these metabolites in the heart muscle.
The imbalance between cardiac workload and oxygen supply can develop as a result of disorders of the coronary vessels, disorders of circulation, increased demands on output of the heart, or damaged myocardium unable to utilize oxygen properly.
Types of Anginal Pain: There are a number of types of anginal pain, including chronic stable angina (also referred to as walk-through angina), resting angina (angina decubitus), unstable angina, nocturnal angina, atypical angina, new-onset angina, and Prinzmetal’s or “variant” angina.
Chronic stable angina occurs at a predictable level of physical or emotional stress and responds promptly to rest or to nitroglycerin. No pain occurs at rest, and the location, duration, intensity, and frequency of chest pain are consistent over time.
Resting angina, or angina decubitus, is chest pain that occurs at rest in the supine position and frequently at the same time every day. The pain is neither brought on by exercise nor relieved by rest.
Unstable angina, also known as crescendo angina, preinfarction angina, or progressive angina, is an abrupt change in the intensity and frequency of symptoms or decreased threshold of stimulus, such as the onset of chest pain while at rest. The most common trigger of unstable angina is the bursting of a cholesterol-filled plaque in the lining of a coronary artery. A blood clot forms at that site, partially blocking blood flow. The duration of these attacks is longer than the usual 1 to 5 minutes; they may last for up to 20 to 30 minutes and can progress into a full-blown heart attack. Pain or discomfort unrelieved by rest or nitroglycerin signals a higher risk for MI. Such changes in the pattern of angina require immediate medical follow up by the client’s physician.
Nocturnal angina may awaken a person from sleep with the same sensation experienced during exertion. During sleep this exertion is usually caused by dreams. This type of angina may be associated with underlying CHF.
Atypical angina refers to unusual symptoms (e.g., toothache or earache) related to physical or emotional exertion. These symptoms subside with rest or nitroglycerin. New-onset angina describes angina that has developed for the first time within the last 60 days.
Prinzmetal’s (variant) angina produces symptoms similar to those of typical angina but is caused by abnormal or involuntary coronary artery spasm rather than directly by a build-up of plaque from atherosclerosis. These spasms periodically squeeze arteries shut and keep the blood from reaching the heart. About two thirds of people with Prinzmetal’s have severe coronary atherosclerosis in at least one major vessel. The spasm usually occurs very close to the blockage.
This form of angina typically occurs at rest, especially in the early hours of the morning, and can be difficult to induce by exercise. It is cyclic and frequently occurs at the same time each day. In postmenopausal women who are not undergoing hormone replacement therapy, the reduction in estrogen may cause coronary arteries to spasm, resulting in vasospastic (Prinzmetal’s) angina.
Clinical Signs and Symptoms: The client may indicate the location of the symptoms by placing a clenched fist against the sternum. Angina radiates most commonly to the left shoulder and down the inside of the arm to the fingers; but it can also refer pain to the neck, jaw, teeth, upper back, possibly down the right arm, and occasionally to the abdomen (see Fig. 6-8).
Recognizing heart pain in women is more difficult because the symptoms are less reliable and often do not follow the classic pattern described earlier. Many women describe the pain in ways consistent with unstable angina, suggesting that they first become aware of their chest discomfort or have it diagnosed only after it reaches more advanced stages.
Some experience a sensation similar to inhaling cold air, rather than the more typical shortness of breath. Other women complain only of weakness and lethargy, and some have noted isolated pain in the midthoracic spine or throbbing and aching in the right biceps muscle (Fig. 6-4).
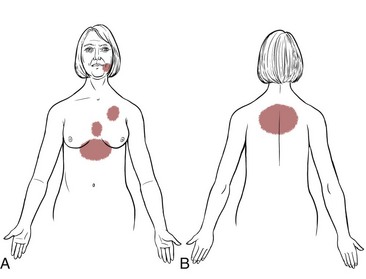
Fig. 6-4 Pain patterns associated with angina in women may differ from patterns in men. Many presenting symptoms are subjective such as extreme fatigue, lethargy, breathlessness, or weakness. Unusual patterns (e.g., temporomandibular joint [TMJ] pain) and failure to seek medical diagnosis may delay treatment with less optimal results. More classic pain patterns as shown in Fig. 6-8 are also possible.
Pain associated with the angina and MI occurring along the inner aspect of the arm and corresponding to the ulnar nerve distribution results from common connections between the cardiac and brachial plexuses.
Cardiac pain referred to the jaw occurs through internuncial (neurons connecting other neurons) fibers from cervical spinal cord posterior horns to the spinal nucleus of the trigeminal nerve. Abdominal pain produced by referred cardiac pain is more difficult to explain and may be due to the overflow of segmental levels to which visceral afferent nerve pathways flow (see Fig. 3-3). This overflow increases the chances that final common pain pathways between the chest and the abdomen may occur.
The sensation of angina is described as squeezing, burning, pressing, choking, aching, or bursting. Chest pain can be brought on by a wide variety of noncardiac causes (see discussion of chest pain in Chapter 17).
In particular, angina is often confused with heartburn or indigestion, hiatal hernia, esophageal spasm, or gallbladder disease, but the pain of these other conditions is not described as sharp or knifelike.
The client often says the pain feels like “gas” or “heartburn” or “indigestion.” Referred pain from a trigger point in the external oblique abdominal muscle can cause a sensation of heartburn in the anterior chest wall (see Fig. 17-7, D). A physician must make the differentiation between angina and heartburn, hiatal hernia, and gallbladder disease. The therapist can assess for trigger points; relief of symptoms with elimination of trigger points is an important diagnostic finding.
Severity is usually mild or moderate. Rarely is the pain described as severe. The five-grade angina scale ranks angina as:
| Grade 0 | No angina |
| Grade 1 | Light, barely noticeable |
| Grade 2 | Moderately bothersome |
| Grade 3 | Severe, very uncomfortable |
| Grade 4 | Most pain ever experienced |
As to location, 80% to 90% of clients experience the pain as retrosternal or slightly to the left of the sternum. The duration of angina as a direct result of myocardial ischemia is typically 1 to 3 minutes and no longer than 3 to 5 minutes. However, attacks precipitated by a heavy meal or extreme anger may last 15 to 20 minutes. Angina is relieved by rest or nitroglycerin (a coronary artery vasodilator).
People who have had coronary artery stents placed can experience angina if an occlusion occurs above, below, or within the stent. Anyone with a stent who has chest pain should be immediately sent for referral to a physician.
Severity of pain is not a good prognostic indicator; some persons with severe discomfort live for many years, whereas others with mild symptoms may die suddenly. If the pain is not relieved by rest or up to 3 nitroglycerin tablets (taken one at a time at 5-minute intervals) in 10 to 15 minutes, the physician should be notified and the client taken to a cardiac care unit.
The client should take his or her own nitroglycerin. The therapist should not dispense medication but may assist the client in taking this medication. Nitroglycerin dilates the coronary arteries and improves collateral cardiac circulation, thus providing an increase in oxygen to the heart muscle and a decrease in symptoms of angina.
When screening for chest pain, a lack of objective musculoskeletal findings is always a red flag:
Myocardial Infarction
Myocardial infarction (MI), also known as a heart attack, coronary occlusion, or a “coronary,” is the development of ischemia and necrosis of myocardial tissue. It results from a sudden decrease in coronary perfusion or an increase in myocardial oxygen demand without adequate blood supply. If the requirements for blood are not eased (e.g., by decreased activity), the heart attempts to continue meeting the increased demands for oxygen with an inadequate blood supply, which leads to an MI. Myocardial tissue death is usually preceded by a sudden occlusion of one or more of the major coronary arteries.
The myocardium receives its blood supply from the two large coronary arteries and their branches. Occlusion of one or more of these blood vessels (coronary occlusion) is one of the major causes of MI. The occlusion may result from the formation of a clot that develops suddenly when an atheromatous plaque ruptures through the sublayers of a blood vessel, or when the narrow, roughened inner lining of a sclerosed artery leads to complete thrombosis.
Although coronary thrombosis is the most common cause of infarction, many interrelated factors may be responsible, including coronary artery spasm, platelet aggregation and embolism, thrombus secondary to rheumatic heart disease, endocarditis, aortic stenosis, a thrombus on a prosthetic mitral or aortic valve, or a dislodged calcium plaque from a calcified aortic or mitral valve.
Coronary blood flow is affected by the tonus (tone) of the coronary arteries. Arteries “clogged” by plaque formation become rigid, and resultant spasm may be provoked by cold and by exercise, which explains the adverse effect of both factors on clients with angina.
Clinical Signs and Symptoms: There are some well-known pain patterns specific to the heart and cardiac system. Sudden death can be the first sign of heart disease. In fact, according to the AHA, 63% of women who died suddenly of cardiovascular disease had no previous symptoms. Sudden death is the first symptom for half of all men who have a heart attack.
The onset of an infarct may be characterized by severe fatigue for several days before the infarct. The likelihood of having a heart attack in the morning hours is 40% higher than during the rest of the day.49 The morning is when the body’s clotting system is more active, BP surges, heart rate increases, and there may be reduced blood flow to the heart.
Additionally, the levels and activity of stress hormones (e.g., catecholamines), which can induce vasoconstriction, increase in the morning. Combined with these factors are the increased mental and physical stresses that typically occur after waking. The shift worker would experience this same phenomenon in the evening or on arising.
Persons who have MIs may not experience any pain and may be unaware that damage is occurring to the heart muscle as a result of prolonged ischemia. The presence of silent infarction (SI) increases with advancing age, especially SI without a history of CAD.
Cardiac Arrest: Researchers expect the number of Americans living with angina to grow as new treatments improve survival after heart attacks.44 Failure to recognize prodromal symptoms in men or women may account for many cases of sudden cardiac death.50
Cardiac arrest strikes immediately and without warning. Signs of sudden cardiac arrest include44:
• Sudden loss of responsiveness. No response to gentle shaking.
• No normal breathing. The client does not take a normal breath when you check for several seconds.
If cardiac arrest occurs, call for emergency help and begin cardiopulmonary resuscitation (CPR) immediately, unless the client has a do not resuscitate (DNR) on file. Use an automated external defibrillator (AED) if available and appropriate.
Classic Warning Signs of Myocardial Infarction: Those who do have warning signs of MI may have severe unrelenting chest pain described as “crushing pain” lasting 30 or more minutes that is not alleviated by rest or by nitroglycerin. This chest pain may radiate to the arms, throat, and back, persisting for hours (see Fig. 6-9).
Other symptoms include pallor, profuse perspiration, and possibly nausea and vomiting. The pain of an MI may be misinterpreted as indigestion because of the nausea and vomiting. Nausea may be the only prodromal symptom. A medical evaluation may be difficult because many clients have coexisting hiatal hernia, peptic ulcer, or gallbladder disease.
Cardiac pain patterns may differ for men and women. For many men, the most common report is a feeling of pressure or discomfort under the sternum (substernal), in the mid-chest region, or across the entire upper chest. It can feel like uncomfortable pressure, squeezing, fullness, or pain.
Pain may occur just in the jaw, upper neck, mid-back, or down the arm without chest pain or discomfort. Pain may also radiate from the chest to the neck, jaw, mid-back or down the arm(s). Pain down the arm(s) affects the left arm most often in the pattern of the ulnar nerve distribution. Radiating pain down both arms is also possible.
An MI may occur during exertion, exercise, or exposure to extremes of temperature, or it may occur while the person is at rest. A subtle variation on ischemia during exertion is an important one for the therapist.
The onset of angina and a subsequent MI is known to be precipitated when working with the arms extended over the head. Oxygen requirements of the heart are greater during arm work compared to leg work at the same workload level. If the person becomes weak or short of breath while in this position, ischemia or infarction may be the cause of the pain and associated symptoms; workload should be cut in half to prevent cardiac ischemia.
Because the infarction process may take up to 6 hours to complete, restoration of adequate myocardial perfusion is important if significant necrosis is to be limited. Deaths generally result from severe arrhythmias, cardiogenic shock, CHF, rupture of the heart, and recurrent MI.
Warning Signs of Myocardial Infarction in Women: For women, symptoms can be more subtle or “atypical.” Chest pain or discomfort is less common in women but still a key feature for some. Women describe heaviness, squeezing, or pain in the left side of the chest, abdomen, mid-back (thoracic), shoulder, or arm with no mid-chest symptoms.51
They often have prodromal symptoms up to 1 month before having a heart attack (Table 6-4).50,52
TABLE 6-4
Heart Attack Symptoms in Women
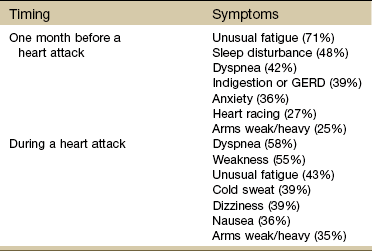
GERD, Gastroesophageal reflux disease.
From McSweeney JC: Women’s early warning symptoms of acute myocardial infarction, Circulation 108(21):2619-2623, 2003.
Fatigue, nausea, and lower abdominal pain may signal a heart attack. Many women pass these off as the flu or food poisoning. Other symptoms for women include a feeling of intense anxiety, isolated right biceps pain, mid-thoracic pain, or heartburn; sudden shortness of breath, or the inability to talk, move, or breathe; shoulder or arm pain; or ankle swelling or rapid weight gain.
In addition, she may describe palpitations or pain that is sharp and fleeting. Antacids may relieve it rather than rest or nitroglycerin. Women having an acute MI have also described a pain in the jaw, neck, shoulder, back, or ear and a feeling of intense anxiety, nausea, or shortness of breath. Many women do not associate these symptoms with having a heart attack, and they may do nothing to seek help.51
Pericarditis
Pericarditis is an inflammation of the pericardium, the saclike covering of the heart. Specifically, it affects the parietal pericardium (fluidlike membrane between the fibrous pericardium and the epicardium) and the visceral (epicardium) pericardium (Fig. 6-5).
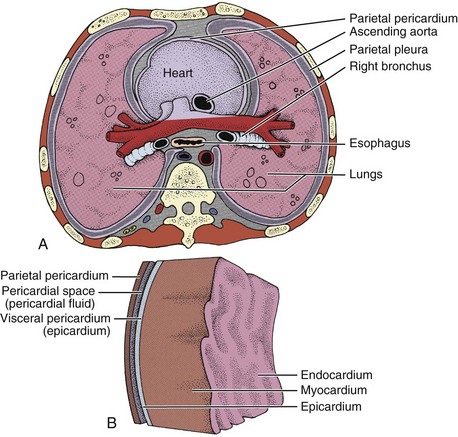
Fig. 6-5 The heart and associated layers of membranes. A, Cross-section through the thorax just above the heart, emphasizing the lining of the cavity that contains the lungs (parietal pleura) and the lining of the cavity that contains the heart (parietal pericardium). B, Sagittal view of the layers of the heart.
This inflammatory process may develop either as a primary condition or secondary to a number of diseases and conditions (e.g., influenza, HIV infection, tuberculosis, cancer, kidney failure, hypothyroidism, autoimmune disorders). Myocardial injury or trauma such as a heart attack, chest injury, chest radiation, or cardiac surgery can cause pericarditis. Very often the cause is unknown, resulting in a diagnosis of idiopathic pericarditis.
Pericarditis may be acute or chronic (recurring); it is not known why pericarditis may be a single illness in some persons and recurrent in others. Chronic or recurring pericarditis is accompanied by a pericardium that is rigid, thickened, and scarred.
Previous infection may be mild or asymptomatic with postinfectious onset of pain occurring 1 to 3 weeks later. Because this condition can occur in any age group, a history of recent pericarditis in the presence of new onset of chest, neck, or left shoulder pain is important.
Clinical Signs and Symptoms: At first, pericarditis may have no external signs or symptoms. The symptoms of acute pericarditis vary with the cause but usually include chest pain and dyspnea, an increase in the pulse rate, and a rise in temperature. Malaise and myalgia may occur.
Over time, the inflammatory process may result in an accumulation of fluid in the pericardial sac, preventing the heart from expanding fully. The inflamed pericardium may cause pain when it rubs against the heart. Chest pain from pericarditis (see Fig. 6-10) closely mimics that of an MI since it is substernal, is associated with cough, and may radiate to the left shoulder or supraclavicular area. It can be differentiated from MI by the pattern of relieving and aggravating factors (Table 6-5).
For example, the pain of an MI is unaffected by position, breathing, or movement, whereas the pain associated with pericarditis may be relieved by kneeling on all fours, leaning forward, or sitting upright. Pericardial chest pain is often worse with breathing, swallowing, belching, or neck or trunk movements, especially sidebending or rotation. The pain tends to be sharp or cutting and may recur in intermittent bursts that are usually precipitated by a change in body position. Pericarditis pain may diminish if the breath is held.
Congestive Heart Failure or Heart Failure
Heart failure, also called cardiac decompensation and cardiac insufficiency, can be defined as a physiologic state in which the heart is unable to pump enough blood to meet the metabolic needs of the body (determined as oxygen consumption) at rest or during exercise, even though filling pressures are adequate.
The heart fails when, because of intrinsic disease or structural defects, it cannot handle a normal blood volume, or in the absence of disease cannot tolerate a sudden expansion in blood volume (e.g., exercise). Heart failure is not a disease itself; instead, the term denotes a group of manifestations related to inadequate pump performance from either the cardiac valves or the myocardium.
Whatever the cause, when the heart fails to propel blood forward normally, congestion occurs in the pulmonary circulation as blood accumulates in the lungs. The right ventricle, which is not yet affected by congestive heart disease, continues to pump more blood into the lungs. The immediate result is shortness of breath and, if the process continues, actual flooding of the air spaces of the lungs with fluid seeping from the distended blood vessels. This last phenomenon is called pulmonary congestion or pulmonary edema.
Because a properly functioning heart depends on both ventricles, failure of one ventricle almost always leads to failure of the other ventricle. This is called ventricular interdependence. Right-sided ventricular failure (right-sided heart failure) causes congestion of the peripheral tissues and viscera. The liver may enlarge, the ankles may swell, and the client develops ascites (fluid accumulates in the abdomen).
Some clients have preexisting mild-to-moderate heart disease with no evidence of CHF. However, when the heart undergoes undue stress or deterioration from risk factors, compensatory mechanisms may be inadequate and the heart fails.
Conditions (risk factors) that precipitate or exacerbate heart failure include hypertension, CAD, cardiomyopathy, heart valve abnormalities, arrhythmia, fever, infection, anemia, thyroid disorders, pregnancy, Paget’s disease, nutritional deficiency (e.g., thiamine deficiency secondary to alcoholism), pulmonary disease, spinal cord injury, and hypervolemia from poor renal function.
Medications are frequently implicated in the development of CHF. Examples include cardiovascular drugs, antibiotics, central nervous system drugs (e.g., sedatives, hypnotics, antidepressants, narcotic analgesics), and antiinflammatory drugs (both nonsteroidal and steroidal).
Chemotherapy used with a variety of different types of cancer (including childhood cancers) has also been linked with increased risk of cardiovascular disease and congestive heart failure.43 There may be a significant delay between treatment and the development of left ventricular dysfunction. Signs and symptoms of heart failure in young adults are unexpected. Consider cancer treatment in children who were treated successfully for cancer years ago a warning flag.
Clinical Signs and Symptoms: The incidence of CHF increases with advancing age. Because of the increasing age of the U.S. population and newer medications and technologies that have increased survival at the expense of increased cardiovascular morbidity, the population affected by CHF is markedly increasing. In view of this increase, many individuals with a wide variety of heart and lung diseases will very likely develop CHF at some time during their lives, manifesting itself as pulmonary congestion or edema.53
Left Ventricular Failure: Failure of the left ventricle causes either pulmonary congestion or a disturbance in the respiratory control mechanisms. These problems in turn precipitate respiratory distress. The degree of distress varies with the client’s position, activity, and level of stress.
However, many persons with severely impaired ventricular performance may have few or no symptoms, particularly if heart failure has developed gradually. Breathlessness, exhaustion, and lower extremity edema are the most common signs and symptoms of CHF.
Dyspnea is subjective and does not always correlate with the extent of heart failure. To some degree, exertional dyspnea occurs in all clients. The increased fluid in the tissue spaces causes dyspnea, at first on effort and then at rest, by stimulation of stretch receptors in the lung and chest wall and by the increased work of breathing with stiff lungs.
Paroxysmal nocturnal dyspnea (PND) resembles the frightening sensation of suffocation. The client suddenly awakens with the feeling of severe suffocation. Once the client is in the upright position, relief from the attack may not occur for 30 minutes or longer.
Orthopnea is a more advanced stage of dyspnea. The client often assumes a “three-point position,” sitting up with both hands on the knees and leaning forward. Orthopnea develops because the supine position increases the amount of blood returning from the lower extremities to the heart and lungs. This gravitational redistribution of blood increases pulmonary congestion and dyspnea. The client learns to avoid respiratory distress at night by supporting the head and thorax on pillows. In severe heart failure, the client may resort to sleeping upright in a chair.
Cough is a common symptom of left ventricular failure and is often hacking, producing large amounts of frothy, blood-tinged sputum. The client coughs because a large amount of fluid is trapped in the pulmonary tree, irritating the lung mucosa.
Pulmonary edema may develop when rapidly rising pulmonary capillary pressure causes fluid to move into the alveoli, resulting in extreme breathlessness, anxiety, frothy sputum, nasal flaring, use of accessory breathing muscles, tachypnea, noisy and wet breathing, and diaphoresis.
Cerebral hypoxia may occur as a result of a decrease in cardiac output, causing inadequate brain perfusion. Depressed cerebral function can cause anxiety, irritability, restlessness, confusion, impaired memory, bad dreams, and insomnia.
Fatigue and muscular cramping or weakness is often associated with left ventricular failure (Case Example 6-3). Inadequate cardiac output leads to hypoxic tissue and slowed removal of metabolic wastes, which in turn causes the client to tire easily. A common report is feeling tired after an activity or type of exertion that was easily accomplished previously. Disturbances in sleep and rest patterns may aggravate fatigue.
The therapist must view this symptom in relation to the bigger picture. Is this someone who is taking diuretics? Is the diuretic a potassium-sparing drug? When does the muscle cramping occur? For example, muscle cramping and fatigue after working out in the garden under a hot sun may be related to fluid loss, dehydration, and exertion, whereas cramping that wakes the person up at night unrelated to exertion (including disturbing dreams) may indicate a different type of electrolyte imbalance.
Nocturia (urination at night) develops as a result of renal changes that can occur in both right- and left-sided heart failure (but more evident in left-sided failure). During the day the affected individual is upright and blood flow is away from the kidneys with reduced formation of urine. At night, urine formation increases as blood flow to the kidneys improves.
Nocturia may interfere with effective sleep patterns, contributing to the fatigue associated with CHF. As cardiac output falls, decreased renal blood flow may result in oliguria (reduced urine output), which is a late sign of heart failure.
Right Ventricular Failure: Failure of the right ventricle may occur in response to left-sided CHF or as a result of pulmonary embolism (see cor pulmonale in Chapter 7). Right ventricular failure results in peripheral edema and venous congestion of the organs.
For example, as the liver becomes congested with venous blood, it becomes enlarged and abdominal pain occurs. If this occurs rapidly, stretching of the capsule surrounding the liver causes severe discomfort. The client may notice either a constant aching or a sharp pain in the right upper quadrant.
Dependent edema is one of the early signs of right ventricular failure. Edema is usually symmetric and occurs in the dependent parts of the body, where venous pressure is the highest. In ambulatory individuals, edema begins in the feet and ankles and ascends the lower legs. It is most noticeable at the end of a day and often decreases after a night’s rest.
Many people experiencing this type of edema assume that it is a normal sign of aging and fail to report it to their physician. In the recumbent person, pitting edema may develop in the presacral area and, as it worsens, progress to the genital area and medial thighs (Case Example 6-4).
Cyanosis of the nail beds appears as venous congestion reduces peripheral blood flow. Clients with CHF often feel anxious, frightened, and depressed. Fears may be expressed as frightening nightmares, insomnia, acute anxiety states, depression, or withdrawal from reality.
Diastolic Heart Failure: Diastolic heart failure describes a condition in which the left ventricle stiffens and hypertrophies. Open space inside the ventricle can become restricted by the thickened ventricle walls. The stiff heart muscle loses some of its flexibility. During diastole (when the muscle fibers relax and stretch), the chambers of the heart expand and fill with blood. Restrictions from a bulky heart muscle due to overwork or other causes make it more difficult for the muscle to relax between beats and thus unable to fill completely. This is different from systolic heart failure in which the left ventricle becomes weak and flabby. Although diastolic heart failure appears to be a “new” type of heart failure, it has been a factor in many people’s health. Newer diagnostic technology has made it possible to differentiate diastolic from systolic heart failure. Both types of heart failure have the same end result: loss of blood supply (and oxygen) to the organs and tissues.
Risk factors for diastolic heart failure include advancing age, high BP, coronary artery disease (atherosclerosis), cardiac muscle damage from a previous heart attack, and valve dysfunction. Other medical conditions, such as diabetes, anemia, and thyroid disease, can also increase the risk of developing diastolic heart failure.
Clinical presentation is similar to systolic heart failure. A low ejection fraction (less than 35%) with symptoms suggests systolic heart failure, whereas a normal ejection fraction with symptoms is more typical with diastolic heart failure. Ejection fraction is the percentage of blood in the filled left ventricle that is pumped out during a contraction.
Aneurysm54
An aneurysm is an abnormal dilatation (commonly a saclike formation) in the wall of an artery, a vein, or the heart. Aneurysms occur when the vessel or heart wall becomes weakened from trauma, congenital vascular disease, infection, or atherosclerosis. This section could also be discussed under peripheral vascular diseases because aneurysms of arterial blood vessels can result in some form of PVD.
Aneurysms really fall under the broader category of thoracic-aortic disease (TAD), including aortic aneurysm and aortic dissection. Aneurysms can also be designated either venous or arterial and described according to the specific vessel in which they develop. Thoracic aneurysms usually involve the ascending, transverse, or descending portion of the aorta from the heart to the top of the diaphragm; abdominal aneurysms generally involve the aorta below the diaphragm between the renal arteries and the iliac branches; peripheral arterial aneurysms affect the femoral and popliteal arteries.54
Thoracic and Peripheral Arterial Aneurysms: A dissecting aneurysm (most often a thoracic aneurysm) occurs when a tear develops in the inner lining of the aortic wall. The inner and outer layers peel apart, creating an extra channel or “false vessel.” Small tears may do no harm but aneurysms divert blood from organs and tissues and can result in heart attack, stroke, kidney damage, or other problems. Thoracic aneurysms occur most frequently in hypertensive men between the ages of 40 and 70 years. Marked elevation of BP may facilitate rapid disruption and final rupture (a break in all three layers of the aortic wall) when a small tear in the intima has occurred. Following a rupture, massive internal hemorrhage occurs as blood flows from the aorta into the chest.
The most common site for peripheral arterial aneurysms is the popliteal space in the lower extremities. Popliteal aneurysms cause ischemic symptoms in the lower limbs and an easily palpable pulse of larger amplitude. An enlarged area behind the knee may be present, seldom with discomfort.
Abdominal Aortic Aneurysms: An aneurysm is an abnormal dilation in a weak or diseased arterial wall causing a saclike protrusion. Aneurysms can occur anywhere in any blood vessel, but the two most common places are the aorta and cerebral vascular system. The aneurysm may be dissecting, which means a tear has occurred between two layers of the intima and blood is flowing between these two layers rather than through the lumen.
Abdominal aortic aneurysms (AAAs) occur about four times more often than thoracic aneurysms. The natural course of an untreated AAA is expansion and rupture in one of several places, including the peritoneal cavity, the mesentery, behind the peritoneum, into the inferior vena cava, or into the duodenum or rectum.
The most common site for an AAA is just below the kidney (immediately below the takeoff of the renal arteries), with referred pain to the thoracolumbar junction (see Fig. 6-11). Aneurysms can be caused by:
Risk Factors: The therapist should look for a history of smoking,55-57 known congenital heart disease (e.g., bicuspid aortic valve), surgery to replace or repair an aortic valve before age 70, recent infection, diagnosis of CAD (atherosclerosis), and some genetic conditions such as Marfan syndrome, Loeys-Dietz syndrome, Turner syndrome, or vascular Ehlers-Danlos syndrome. Many seniors are keeping active and fit by participating in activities at the gym, at home, or elsewhere that involve lifting weights. There is an increased risk of aneurysm in older adults, especially for these active clients. AAAs can be exacerbated by anticoagulant therapy (another risk factor).
The therapist may be prescribing progressive resistive exercises that can have an adverse effect in an older adult with any of these etiologies (Case Example 6-5). Monitoring vital signs is important among exercising senior adults. Teaching proper breathing and abdominal support without using a Valsalva maneuver is important in any exercise program, but especially for those clients at increased risk for aortic aneurysm.
The U.S. Preventive Services Task Force now recommends one-time ultrasonographic screening for abdominal aortic aneurysm for men ages 65 to 75 who presently smoke or who have smoked in the past. No recommendation is made for or against men who have never smoked. Routine screening for women is not advised.58
Clients who have had orthopedic surgery involving anterior spinal procedures of any kind (e.g., spinal fusion, spinal fusion with cages, artificial disk replacement) are at risk for trauma to the aorta (rather than aortic aneurysm) from damage to blood vessels moved out of the way during surgery. Internal bleeding can result in a distended abdomen, changes in BP, changes in stool (e.g., melena, bloody diarrhea), and possible back and/or shoulder pain.
Clinical Signs and Symptoms: Most AAAs are asymptomatic59; discovery occurs on physical or x-ray examination of the abdomen or lower spine for some other reason.60
The most common symptom is awareness of a pulsating mass in the abdomen, with or without pain, followed by abdominal pain and back pain. The therapist is most likely to observe rapid onset of severe neck or back pain (Case Example 6-6).
The client may report feeling a heartbeat in the abdomen or stomach when lying down. Back pain may be the only presenting feature. Groin (scrotal), buttock, and/or flank pain may be experienced because of increasing pressure on other structures.
The pain is usually described as sharp, intense, severe, or knifelike in the abdomen, chest or anywhere in the back (including the sacrum). Pain may radiate to the chest, neck, between the scapulae, or to the posterior thighs.
The location of the symptoms is determined by the location of the aneurysm. Most aortic aneurysms (95%) occur just below the renal arteries. Extreme pain described as “tearing” or “ripping” may be felt at the base of the neck along the back, particularly in the interscapular area, while dissection proceeds over the aortic arch and into the descending aorta. Symptoms are not relieved by a change in position.
The physical therapist can palpate the width of the arterial pulses; these pulses (e.g., aortic, femoral) should be uniform in width from the midline outward on either side (see Fig. 4-55). The normal aortic pulse width is between 2.5 and 4.0 cm (some sources say the width must be no more than 3.0 cm; others list 4.0). Average pulse width is 2.5 cm or about 1.2 inches wide.61,62
According to the longitudinal community-based Framingham Heart Study, aortic root diameter increases with age in both men and women but is larger in men at any given age. Each 10-year increase in age is associated with a larger aortic root (by 0.89 mm in men and 0.68 mm in women) after adjustment for body size and BP. A 5-kg/m2 increase in body mass index (BMI) was associated with a larger aortic root (by 0.78 mm in men and 0.51 mm in women) after adjustment for age and BP. Each 10-mm Hg increase in pulse pressure is related to age-related increase in stiffness and resultant increase in aortic pressure63,64 and a smaller aortic root (by 0.19 mm in men and 0.08 mm in women) after adjustment for age and body size.65 Cadaver studies also show significant reduction in tensile strength and stretch after age 30.66
As the aorta increases in diameter from an expanding aneurysm, the pulse width expands as well. The sensitivity of detection with abdominal palpation increases with the increasing diameter and has been reported as high as 82% with a diameter of 5 cm or more.67 Sensitivity and specificity for detecting an abdominal aortic aneurysm with palpation have also been reported as 28% and 97%, respectively, for a definite pulsatile mass.68 The risk of rupture approaches 25% for AAAs that are 6.0 cm (2.4 inches).61
Palpation is followed by auscultation for bruits (abnormal blowing or swishing sounds heard on auscultation of the arteries). Sensitivity for femoral bruit as a screening tool for detecting an abdominal aortic aneurysm has been reported as 17% with specificity of 87%. Abdominal bruit has an 11% sensitivity and 95% specificity.68 Without a careful assessment, smaller diameter aneurysms may escape clinical detection.69
The abdominal aorta passes posterior to the diaphragm (aortic hiatus) at the level of the T12 vertebral body and bifurcates at the level of the L4 vertebral body to form the right and left common iliac arteries. Watch for a widening of the pulse width before reaching the umbilicus. The pulse width expands normally at the aortic bifurcation, usually observed just below the umbilicus. Ninety-five percent of all AAAs occur just below the renal arteries.
Systolic BP below 100 mm Hg and pulse rate over 100 beats per minute may indicate signs of shock. Other symptoms may include ecchymoses in the flank and perianal area; severe and sudden pain in the abdomen, paravertebral area, or flank; and lightheadedness and nausea with sudden hypotension.
The therapist may observe cold, pulseless lower extremities and/or BP differences (more than 10 mm Hg) between the arms. Consistent with the model for a screening examination the therapist must look for screening clues in the history, pain patterns, and associated signs and symptoms. Knowledge of the clinical signs and symptoms of impending rupture or actual rupture of the aortic aneurysm is important.
If a client (usually a postoperative inpatient) has internal bleeding (rather than an aneurysm) from complications of anterior spinal surgery the therapist may note
The client’s recent history of anterior spinal surgery accompanied by any of these symptoms is enough to notify nursing or medical staff of these observations. Monitoring postoperative vital signs in these clients is essential.
Conditions Affecting the Heart Valves
The second category of heart problems includes those that occur secondary to impairment of the valves caused by disease (e.g., rheumatic fever or coronary thrombosis), congenital deformity, or infection such as endocarditis. Three types of valve deformities may affect aortic, mitral, tricuspid, or pulmonic valves: stenosis, insufficiency, or prolapse.
Stenosis is a narrowing or constriction that prevents the valve from opening fully, and may be caused by growths, scars, or abnormal deposits on the leaflets. Insufficiency (also referred to as regurgitation) occurs when the valve does not close properly and causes blood to flow back into the heart chamber. Prolapse affects only the mitral valve and occurs when enlarged valve leaflets bulge backward into the left atrium.
These valve conditions increase the workload of the heart and require the heart to pump harder to force blood through a stenosed valve or to maintain adequate flow if blood is seeping back. Further complications for individuals with a malfunctioning valve may occur secondary to a bacterial infection of the valves (endocarditis).
Persons affected by diseases of the heart valves may be asymptomatic, and extensive auscultation with a stethoscope and diagnostic study may be required to differentiate one condition from another. In its early symptomatic stages cardiac valvular disease causes the person to become fatigued easily. As stenosis or insufficiency progresses, the main symptom of heart failure (breathlessness or dyspnea) appears.
Rheumatic Fever
Rheumatic fever is an infection caused by streptococcal bacteria that can be fatal or may lead to rheumatic heart disease, a chronic condition caused by scarring and deformity of the heart valves. It is called rheumatic fever because two of the most common symptoms are fever and joint pain.
The infection generally starts with strep throat in children between the ages of 5 and 15 years and damages the heart in approximately 50% of cases. Rheumatic fever produces a diffuse, proliferative, and exudative inflammatory process.
The aggressive use of specific antibiotics in the United States had effectively removed rheumatic fever as the primary cause of valvular damage. However, in 1985 a series of epidemics of rheumatic fever occurred in several widely diverse geographic regions of the continental United States. Currently, the prevalence and incidence of cases have not approximated the 1985 record, but they have remained above baseline levels.70
Clinical Signs and Symptoms: The most typical clinical profile of a child or young adult with acute rheumatic fever is an initial cold or sore throat followed 2 or 3 weeks later by sudden or gradual onset of painful migratory joint symptoms in the knees, shoulders, feet, ankles, elbows, fingers, or neck. Fever of 37.2° C to 39.4° C (99° F to 103° F) and palpitations and fatigue are also present. Malaise, weakness, weight loss, and anorexia may accompany the fever.
The migratory arthralgias may last only 24 hours, or they may persist for several weeks. Joints that are sore and hot and contain fluid completely resolve, followed by acute synovitis, heat, synovial space tenderness, swelling, and effusion present in a different area the next day. The persistence of swelling, heat, and synovitis in a single joint or joints for more than 2 to 3 weeks is extremely unusual in acute rheumatic fever.
In the acute full-blown sequelae, shortness of breath and increasing nocturnal cough will also occur. A rash on the skin of the limbs or trunk is present in fewer than 2% of clients with acute rheumatic fever. Subcutaneous nodules over the extensor surfaces of the arms, heels, knees, or back of the head may occur.
All layers of the heart (epicardium, endocardium, myocardium, and pericardium) may be involved, and the heart valves are affected by this inflammatory reaction. The most characteristic and potentially dangerous anatomic lesion of rheumatic inflammation is the gross effect on cardiac valves, most commonly the mitral and aortic valves. If untreated, as many as 25% of clients will have mitral valvular disease 25 to 30 years later.
Rheumatic chorea (also called chorea or St. Vitus’ dance) may occur 1 to 3 months after the strep infection and always is noted after polyarthritis. Chorea in a child, teenager, or young adult is almost always a manifestation of acute rheumatic fever. Other uncommon causes of chorea are systemic lupus erythematosus, thyrotoxicosis, and cerebrovascular accident (CVA), but these are unlikely in a child.
The client develops rapid, purposeless, nonrepetitive movements that may involve all muscles except the eyes. This chorea may last for 1 week or several months or may persist for several years without permanent impairment of the central nervous system.
Initial episodes of rheumatic fever last months in children and weeks in adults. Twenty percent of children have recurrences within 5 years. Recurrences are uncommon after 5 years of good health and are rare after 21 years of age.
Endocarditis
Bacterial endocarditis, another common heart infection, causes inflammation of the cardiac endothelium (layer of cells lining the cavities of the heart) and damages the tricuspid, aortic, or mitral valve.
This infection may be caused by bacteria entering the bloodstream from a remote part of the body (e.g., skin infection, oral cavity), or it may occur as a result of abnormal growths on the closure lines of previously damaged valves or artificial valves. These growths called vegetations consist of collagen fibers and may separate from the valve, embolize, and cause infarction in the myocardium, kidney, brain, spleen, abdomen, or extremities.
Risk Factors: In addition to clients with previous valvular damage, injection drug users and postcardiac surgical clients are at high risk for developing endocarditis. Congenital heart disease and degenerative heart disease, such as calcific aortic stenosis, may also cause bacterial endocarditis. The prosthetic cardiac valve (valve replacement) has become more important as a predisposing factor for endocarditis because cardiac surgery is performed on a much larger scale than in the past.
This infection is often the consequence of invasive diagnostic procedures, such as renal shunts and urinary catheters, long-term indwelling catheters, or dental treatment (because of the increased opportunities for normal oral micro-organisms to gain entrance to the circulatory system by way of highly vascularized oral structures). Individuals who are susceptible may take antibiotics as a precaution before undergoing any of these procedures.
Clinical Signs and Symptoms: A significant number of clients (up to 45%) with bacterial endocarditis initially have musculoskeletal symptoms, including arthralgia, arthritis, low back pain, and myalgias. Half these clients will have only musculoskeletal symptoms without other signs of endocarditis.
The early onset of joint pain and myalgia is more likely if the client is older and has had a previously diagnosed heart murmur. Musculoskeletal problems make up a significant part of the clinical picture of infective endocarditis diagnosed in an injection drug user.
The most common musculoskeletal symptom in clients with bacterial endocarditis is arthralgia, generally in the proximal joints. The shoulder is the most commonly affected site, followed (in declining incidence) by the knee, hip, wrist, ankle, metatarsophalangeal, and metacarpophalangeal joints, and acromioclavicular joints.
Most endocarditis clients with arthralgias have only one or two painful joints, although some may have pain in several joints. Painful symptoms begin suddenly in one or two joints, accompanied by warmth, tenderness, and redness. Symmetric arthralgia in the knees or ankles may lead to a diagnosis of rheumatoid arthritis. One helpful clue: as a rule, morning stiffness is not as prevalent in clients with endocarditis as in those with rheumatoid arthritis or polymyalgia rheumatica.
Osteoarticular infections are diagnosed infrequently and most commonly in association with injection drug use. Most commonly affected sites include the vertebrae, the wrist, the sternoclavicular joints, and the sacroiliac joints. Often, multiple joint involvement occurs.71,72
Endocarditis may produce destructive changes in the sacroiliac joint, probably as a result of seeding the joint by septic emboli. The pain will be localized over the sacroiliac joint, and the physician will use x-rays and bone scans to verify this diagnosis.
Almost one third of clients with bacterial endocarditis have low back pain; in many clients it is the principal musculoskeletal symptom reported. Back pain is accompanied by decreased ROM and spinal tenderness. Pain may affect only one side, and it may be limited to the paraspinal muscles.
Endocarditis-induced low back pain may be very similar to that associated with a herniated lumbar disk; it radiates to the leg and may be accentuated by raising the leg or by sneezing. The key difference is that neurologic deficits are usually absent in clients with bacterial endocarditis.
Widespread diffuse myalgias may occur during periods of fever, but these are not appreciably different from the general myalgia seen in clients with other febrile illnesses. More commonly, myalgia will be restricted to the calf or thigh. Bilateral or unilateral leg myalgias occur in approximately 10% to 15% of all clients with bacterial endocarditis.
The cause of back pain and leg myalgia associated with bacterial endocarditis has not been determined. Some suggest that concurrent aseptic meningitis may contribute to both leg and back pain. Others suggest that leg pain is related to emboli that break off from the infected cardiac valves. The latter theory is supported by biopsy evidence of muscle necrosis or vasculitis in clients with bacterial endocarditis.
Rarely, other musculoskeletal symptoms, such as osteomyelitis, nail clubbing, tendinitis, hypertrophic osteoarthropathy, bone infarcts, and ischemic bone necrosis, may occur.
Lupus Carditis
Systemic lupus erythematosus (SLE) is a multisystem clinical illness associated with the release of a broad spectrum of autoantibodies into the circulation (see Chapter 12). The inflammatory process mediated by the immune response can target the heart and vasculature of the client with SLE.
Except for pericarditis, clinically significant cardiac disease directly associated with SLE is relatively infrequent, but because of the musculoskeletal involvement, it may be of major importance for the therapist. Primary lupus cardiac involvement may include pericarditis, myocarditis, endocarditis, or a combination of the three.
Pericarditis is the most common cardiac lesion associated with SLE, appearing with the characteristic substernal chest pain that varies with posture, becoming worse in recumbency and improving with sitting or bending forward. Myocarditis may occur and is strongly associated with skeletal myositis in SLE.
Congenital Valvular Defects
Congenital malformations of the heart occur in approximately 1 of every 100 infants (1%) born in the United States.73 The most common defects include (Fig. 6-6):
• Ventricular or atrial septal defect (hole between the ventricles or atria)
• Tetralogy of Fallot (combination of four defects)
• Patent ductus arteriosus (shunt caused by an opening between the aorta and the pulmonary artery)
• Congenital stenosis of the pulmonary, aortic, and tricuspid valves
These congenital defects require surgical correction and may be part of the client’s past medical history. They are not conditions that are likely to mimic musculoskeletal lesions and are therefore not covered in detail in this text.
Congenital cardiovascular abnormalities, which are usually asymptomatic and often undiagnosed during life, are the main cause of sudden death in athletes. Aortic stenosis, hypertrophic cardiomyopathy, Marfan syndrome, congenital coronary artery anomalies, and ruptured aorta are the most commonly reported causes of sudden death during the practice of a sports activity.74,75
Family history of any of these conditions, premature sudden unexpected syncope, or family member death is an indication for a thorough cardiovascular evaluation of the athlete before participation in sports.76
Mitral Valve Prolapse: Echocardiographic studies have advanced our knowledge of mitral valve prolapse (MVP) in the last 2 decades. A more precise definition of MVP has resulted in a more accurate estimate of prevalence rate (2% to 3%).77 This is equally distributed between men and women,78,79 although men seem to have a higher incidence of complications.
MVP is characterized by mitral leaflet thickness with increased extensibility, decreased stiffness, and decreased strength compared to normal valves. This structural variation has many other names, including floppy valve syndrome, Barlow’s syndrome, and click-murmur syndrome.
MVP appears to be due to connective tissue abnormalities in the valve leaflets or in response to abnormalities in left ventricular cavity geometry.80 Normally, when the lower part of the heart contracts, the mitral valve remains firm and allows no blood to leak back into the upper chambers. In MVP the structural changes in the mitral valve allows one part of the valve, the leaflet, to billow back into the upper chamber during contraction of the ventricle.
One or both of the valve leaflets may bulge into the left atrium during ventricular systole. This protrusion can often be heard through a stethoscope as a sound known as a “click.” Leaking of blood backward through the mitral valve can also be heard and is referred to as a heart murmur.
Risk Factors: MVP is a benign condition in isolation; however, it can be associated with a number of other conditions, especially the heritable connective tissue disorders such as Ehlers-Danlos syndrome, Marfan syndrome, and osteogenesis imperfecta. Other risk factors include endocarditis, myocarditis, atherosclerosis, SLE, muscular dystrophy, acromegaly, and cardiac sarcoidosis.
Clinical Signs and Symptoms: Two thirds of the individuals with MVP experience no symptoms. Approximately one third experience occasional symptoms that are mildly to moderately uncomfortable—enough to interfere with the person’s ability to enjoy an unrestricted life. Only about 1% suffer severe symptoms and lifestyle restrictions.
Almost all the symptoms of MVP syndrome are due to an imbalance in the autonomic nervous system, called dysautonomia. Frequently, when there is a slight variation in structure of the heart valve, there is also a slight variation in the function or balance of the autonomic nervous system (ANS).80 This description in the autonomic innervation of the heart may account for the high incidence of MVP in fibromyalgia, a condition known to be associated with dysregulation or dysautonomia of the ANS.
Symptoms include profound fatigue that cannot be correlated with exercise or stress, cold hands and feet, shortness of breath, chest pain, and heart palpitations. The most common triad of symptoms associated with MVP is fatigue, palpitations, and dyspnea (Fig. 6-7). Frequently occurring musculoskeletal findings in clients with MVP include joint hypermobility, temporomandibular joint (TMJ) syndrome, and myalgias.
Although the fatigue that accompanies MVP is not related to exertion, deconditioning from prolonged inactivity may develop, further complicating the picture. Chest pain associated with MVP can be severe, but it differs from pain associated with MI (see Table 6-5). When there is an imbalance in the ANS, which controls contraction and relaxation of the chest wall muscles (the muscles of breathing), there may be inadequate relaxation between respirations. Over time these chest wall muscles go into spasm, resulting in chest pain.
It is important that the therapist evaluate the client with chest pain for trigger points. If palpation of the chest reproduces symptoms, especially radiating pain, deactivation of trigger points must be carried out followed by a reevaluation as part of the screening process for pain of a cardiac origin.
MVP is not life-threatening but may be lifestyle threatening for the small number of persons (rare) who have more severe structural problems that may progress to the point at which surgical replacement of the valve is required. Sudden death is a recognized risk for cases of severe mitral regurgitation. To prevent infective endocarditis, the client may be given antibiotics prophylactically before any invasive procedures.
MVP is included in this section because of its increasing prevalence in the physical therapy client population. At presentation, usually the client with MVP has some other unrelated primary (musculoskeletal) diagnosis. During physical therapy intervention, the symptomatic MVP client may experience symptoms associated with MVP and require assurance or education regarding exercise and MVP.
Most individuals with MVP do not have to restrict their activity level or lifestyle; regular exercise is encouraged. Clients with mitral regurgitation (backward flow of blood into the left atrium) during exercise, but not at rest, have a higher rate of complications, such as heart failure, syncope, and progressive mitral regurgitation, requiring further medical treatment.80
Monitoring vital signs and observing or asking about additional signs and symptoms is important. Caution is advised in the use of weight training for the MVP client. Gradual buildup using lightweights and increased repetitions is recommended.
Conditions Affecting the Cardiac Nervous System
The third component of cardiac disease is caused by failure of the heart’s nervous system to conduct normal electrical impulses. The heart has its own intrinsic conduction system that allows the orderly depolarization of cardiac muscle tissue. Arrhythmias, also called dysrhythmias, are disorders of the heart rate and rhythm caused by disturbances in the conduction system.
Arrhythmias may cause the heart to beat too quickly (tachycardia), too slowly (bradycardia), or with extra beats and fibrillations. Arrhythmias can lead to dramatic changes in circulatory dynamics such as hypotension, heart failure, and shock.
Clients who are neurologically unstable owing to recent CVA, head trauma, spinal cord injury, or other central nervous system insult often exhibit new arrhythmias during the period of instability (Case Example 6-7). These may be due to elevation of intracranial pressure, and once this has been controlled and returned to normal range, arrhythmias usually disappear.
Arrhythmias also may be triggered by environmental factors, abnormal thyroid function, and some medications. Clients who have preexisting arrhythmias, CAD, or CHF, may progress from “transient arrhythmias” to arrhythmias that do not disappear, with the potential for serious complications. Dizziness and loss of consciousness may occur when the arrhythmia results in a serious reduction in cardiac output, owing to loss of brain perfusion (not caused by transient ischemic attacks, as is often suspected).
The therapist should monitor pulse carefully before, during, and after exercise when working with any client who has had a stroke. Any pulse irregularities not already documented should be reported to the physician immediately and an ECG should be done to determine the nature of the irregularity.
The therapist may be the first health care professional to identify an arrhythmia that appears during exercise. In the early recovery period, the therapist should monitor for these arrhythmias by taking the client’s pulse. Arrhythmias should be reported to the physician (Case Example 6-8).
Fibrillation
The sinoatrial (SA) node (or cardiac pacemaker) initiates and paces the heartbeat. During an MI, damaged heart muscle cells, deprived of oxygen, can release small electrical impulses that may disrupt the heart’s normal conduction pathway. These fibrillation impulses can occur in the atria or the ventricles.
If the heart attack develops suddenly into ventricular fibrillation, a potentially lethal arrhythmia, it can result in sudden death. Similarly, a heart damaged by CAD (with or without previous infarcts) can go into ventricular fibrillation. Ventricular fibrillation usually requires resuscitation and emergency electrical countershock (defibrillation) as life saving measures.
Atrial fibrillation (AF) is the most common cardiac dysrhythmia and although not immediately lethal, it can increase the risk of heart failure and stroke. It is characterized by a total disorganization of atrial activity without effective atrial contraction. The upper chambers of the heart contract in an unsynchronized pattern, causing the atrium to quiver rather than to contract and often causing blood to pool, which allows for clots to form. These clots can break loose and travel to the brain, causing a stroke.
Individuals with transient episodes (in and out of AF) or developing new onset of AF are at greater risk of clot migration than those who have a long-term history, especially if they are already on blood thinners. Medical consult is more imperative for anyone who has not previously been identified as having AF and/or who is not receiving anticoagulants.
The therapist can easily and quickly screen individuals at risk and teach them to screen themselves by checking the pulse for the telltale signs of an irregular heartbeat. A regular heartbeat is characterized by a series of even and continuous pulsations, whereas an irregular heartbeat often feels like an extra or missed beat. To help determine the steadiness of the heartbeat, the therapist or individual keeps time by tapping the foot.
Risk Factors: Persons at risk for fibrillation who require screening include those who have had a previous heart attack or a history that includes high BP, CHF, digitalis toxicity, pericarditis, or rheumatic mitral stenosis.
Other factors that can overstimulate the sinus node include emotional stress, excessive production of thyroid hormone (hyperthyroidism), alcohol and caffeine consumption, and high fevers. In many instances, particularly in younger persons, there is no apparent cause.
Recent studies have shown that presence of the organism Helicobacter pylori in the stomach is associated with persistent AF. In addition, high concentrations of CRP, which confirm the presence of systemic inflammation, are present in people with AF. A potential noncardiovascular disease that predisposes to AF may be chronic gastritis caused by chronic H. pylori infection.81
Clinical Signs and Symptoms: Symptoms of fibrillation vary, depending on the functional state of the heart and the location of the fibrillation. Fibrillation may exist without symptoms. The affected individual is usually aware of the irregular heart action and reports feeling “palpitations.” Careful questioning may be required to pinpoint the exact description of sensations reported by the client.
Some individuals experience the symptoms of inadequate blood flow and low oxygen levels, such as dizziness, chest pain, and fainting. Chronic AF may cause CHF, which is often experienced as shortness of breath during exercise and fluid accumulation in the feet and legs.
More than six palpitations occurring in a minute or prolonged, repeated palpitations, especially if accompanied by chest pain, dyspnea, fainting, or other associated signs and symptoms, should be reported to the physician.
Sinus Tachycardia
Sinus tachycardia, defined as an abnormally rapid heart rate, usually taken to be more than 100 bpm, is the normal physiologic response to such stressors as fever, hypotension, thyrotoxicosis, anemia, anxiety, exertion, hypovolemia, pulmonary emboli, myocardial ischemia, CHF, and shock.
Sinus tachycardia is usually of no physiologic significance; however, in clients with organic myocardial disease, the result may be reduced cardiac output, CHF, or arrhythmias. Because heart rate is a major determinant of oxygen requirements, angina or perhaps an increase in the size of an infarction may accompany persistent tachycardia in clients with CAD.
Clinical Signs and Symptoms: The symptoms of tachycardia vary from one person to another and may range from an increased pulse to a group of symptoms that would restrict normal activity of the client. Anxiety and apprehension may occur, depending on the pain threshold and emotional reaction of the client.
Sinus Bradycardia
In sinus bradycardia, impulses travel down the same pathway as in sinus rhythm, but the sinus node discharges at a rate less than 60 bpm. Bradycardia may be normal in athletes or young adults and is therefore asymptomatic.
In most cases, sinus bradycardia is a benign arrhythmia and may actually be beneficial by producing a longer period of diastole and increased ventricular filling. In some clients who have acute MI, it reduces oxygen demands and may help minimize the size of the infarction.
Eye surgery, meningitis, intracranial tumors, cervical and mediastinal tumors, and certain disease states (e.g., MI, myxedema, obstructive jaundice, and cardiac fibrosis) may produce sinus bradycardia.
Clinical Signs and Symptoms: Syncope may be preceded by sudden onset of weakness, sweating, nausea, pallor, vomiting, and distortion or dimming of vision. Signs and symptoms remit promptly when the client is placed in the horizontal position.
Physician referral for sinus bradycardia is needed only when symptoms, such as chest pain, dyspnea, lightheadedness, or hypotension, occur.