Screening for Pulmonary Disease
For the client with neck, shoulder, or back pain at presentation, it may be necessary to consider the possibility of a pulmonary cause requiring medical referral. The most common pulmonary conditions to mimic those of the musculoskeletal system include pneumonia, pulmonary embolism, pleurisy, pneumothorax, and pulmonary arterial hypertension.
As always, using the past medical history, risk factor assessment, and clinical presentation, as well as asking about the presence of any associated signs and symptoms guide the screening process. In the case of pleuropulmonary disorders, the client’s recent personal medical history may include a previous or recurrent upper respiratory infection or pneumonia.
Pneumothorax may be preceded by trauma, overexertion, or recent scuba diving. Each pulmonary condition will have its own unique risk factors that can predispose clients to a specific respiratory disease or illness.
A previous history of cancer, especially primary lung cancer or cancers that metastasize to the lungs (e.g., breast, bone), is a red flag and a risk factor for cancer recurrence. Risk factor assessment also helps identify increased risk for other respiratory conditions or illnesses that can present as a primary musculoskeletal problem.
The material in this chapter will assist the therapist in treating both the client with a known pulmonary problem and the client with musculoskeletal signs and symptoms that may have an underlying systemic basis (Case Example 7-1).
Signs and Symptoms of Pulmonary Disorders
Signs and symptoms of pulmonary disorders can be many and varied; the most common symptoms associated with pulmonary disorders are cough and dyspnea. Other manifestations include chest pain, abnormal sputum, hemoptysis, cyanosis, digital clubbing, altered breathing patterns, and chest pain.
Cough
As a physiologic response, cough occurs frequently in healthy people, but a persistent dry cough may be caused by a tumor, congestion, or hypersensitive airways (allergies). A productive cough with purulent sputum (yellow or green in color) may indicate infection, whereas a productive cough with nonpurulent sputum (clear or white) is nonspecific and indicates airway irritation. Rust colored sputum may be a sign of pneumonia and should also be investigated. Hemoptysis (coughing and spitting blood) indicates a pathologic condition—infection, inflammation, abscess, tumor, or infarction.
Dyspnea
Shortness of breath (SOB), or dyspnea, usually indicates hypoxemia but can be associated with emotional states, particularly fear and anxiety. Dyspnea is usually caused by diffuse and extensive rather than focal pulmonary disease; pulmonary embolism is the exception. Factors contributing to the sensation of dyspnea include increased work of breathing (WOB), respiratory muscle fatigue, increased systemic metabolic demands, and decreased respiratory reserve capacity. Dyspnea when the person is lying down is called orthopnea and is caused by redistribution of body water. Fluid shift leads to increased fluid in the lung, which interferes with gas exchange and leads to orthopnea. In supine and prone, the abdominal contents also exert pressure on the diaphragm, increasing the WOB and often limiting vital capacity.
The therapist must be careful when screening for dyspnea or SOB, either with exertion or while at rest. If a client denies compromised breathing, look for functional changes as the client accommodates for difficulty breathing by reducing activity or exertion.
Cyanosis
The presence of cyanosis, a bluish color of the skin and mucous membranes, depends on the oxygen saturation of arterial blood and the total amount of circulating hemoglobin. It may be observed as a bluish discoloration in the oral mucous membranes, lips, and conjunctivae and pale (white) or blue nail beds and nose.
Clubbing (see Chapter 4)
Thickening and widening of the terminal phalanges of the fingers and toes result in a painless clublike appearance recognized by the loss of the angle between the nail and the nail bed (see Figs. 4-36 and 4-37). Conditions that chronically interfere with tissue perfusion and nutrition may cause clubbing, including cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD), lung cancer, bronchiectasis, pulmonary fibrosis, congenital heart disease, and lung abscess. Most of the time, clubbing is due to pulmonary disease and resultant hypoxia (diminished availability of blood to the body tissues), but clubbing can be a sign of heart disease, peripheral vascular disease, and disorders of the liver and gastrointestinal tract.
Altered Breathing Patterns
Changes in the rate, depth, regularity, and effort of breathing occur in response to any condition affecting the pulmonary system. Breathing patterns can vary, depending on the neuromuscular or neurologic disease or trauma (Box 7-1). Breathing pattern abnormalities seen with head trauma, brain abscess, diaphragmatic paralysis of chest wall muscles and thorax (e.g., generalized myopathy or neuropathy), heat stroke, spinal meningitis, and encephalitis can include apneustic breathing, ataxic breathing, or Cheyne-Stokes respiration (CSR).
Apneustic breathing (gasping inspiration with short expiration) localizes damage to the midpons and is most commonly a result of a basilar artery infarct. Ataxic, or Biot’s, breathing (irregular pattern of deep and shallow breaths with abrupt pauses) is caused by disruption of the respiratory rhythm generator in the medulla.
CSR may be evident in the well older adult, as well as in compromised clients. The most common cause of CSR is severe congestive heart failure (CHF), but it can also occur with renal failure, meningitis, drug overdose, and increased intracranial pressure. It may be a normal breathing pattern in infants and older persons during sleep.
Exercise may induce pleural pain, coughing, hemoptysis, SOB, and/or other abnormal changes in breathing patterns. When asked if the client is ever short of breath, the individual may say “no” because he or she has reduced activity levels to avoid dyspnea (see Appendix B-12).
Pulmonary Pain Patterns
The most common sites for referred pain from the pulmonary system are the chest, ribs, upper trapezius, shoulder, and thoracic spine. The first symptoms may not appear until the client’s respiratory system is stressed by the addition of exercise during physical therapy. On the other hand, the client may present with what appears to be primary musculoskeletal pain in any one of those areas. Auscultation may reveal the first signs of pulmonary distress (see Chapter 4 for screening examination by auscultation).
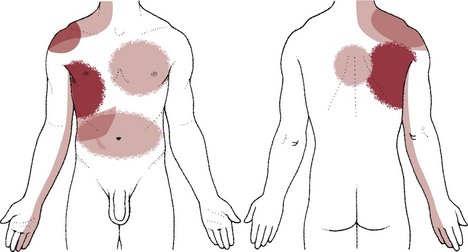
Pulmonary pain patterns are usually localized in the substernal or chest region over involved lung fields that may include the anterior chest, side, or back (Fig. 7-1). However, pulmonary pain can radiate to the neck, upper trapezius muscle, costal margins, thoracic back, scapulae, or shoulder. Shoulder pain may radiate along the medial aspect of the arm, mimicking other neuromuscular causes of neck or shoulder pain (see Fig. 7-10).
Fig. 7-1 Pulmonary pain patterns are localized over involved lung fields affecting the anterior chest, side, or back. Radiating pain can also cause neck, shoulder, upper trapezius, rib, and/or scapular pain. A, Anterior chest. B, Posterior chest. The posterior chest is comprised primarily of lower lung lobes. The upper lobes occupy a small area from T1 to T3 or T4. (From Jarvis C: Physical examination and assessment, ed 5, Philadelphia, 2007, WB Saunders.)
Pulmonary pain usually increases with inspiratory movements, such as laughing, coughing, sneezing, or deep breathing, and the client notes the presence of associated symptoms, such as dyspnea (exertional or at rest), persistent cough, fever, and chills. Palpation and resisted movements will not reproduce the symptoms, which may get worse with recumbency, especially at night or while sleeping.
The thoracic cavity is lined with pleura, or serous membrane. One surface of the pleura lines the inside of the rib cage (parietal) and the other surface covers the lungs (visceral). The parietal pleura is sensitive to painful stimulation, but the visceral pleura is insensitive to pain. Extensive disease may occur in the lung without occurrence of pain until the process extends to the parietal pleura. This explains why pathology of the lungs may be painless until obstruction or inflammation is enough to press on the parietal pleura.
Pleural irritation then results in sharp, localized pain that is aggravated by any respiratory movement. Clients usually note that the pain is alleviated by autosplinting, that is, lying on the affected side, which diminishes the movement of that side of the chest (see further discussion of pleural pain in this chapter).1
Tracheobronchial Pain
Within the pulmonary system, the trachea and large bronchi are innervated by the vagus trunks, whereas the finer bronchi and lung parenchyma appear to be free of pain innervation. Tracheobronchial pain is referred to sites in the neck or anterior chest at the same levels as the points of irritation in the air passages (Fig. 7-2). This irritation may be caused by inflammatory lesions, irritating foreign materials, or cancerous tumors.
Pleural Pain
When the disease progresses enough to extend to the parietal pleura, pleural irritation occurs and results in sharp, localized pain that is aggravated by any respiratory movement. Clients usually note that the pain is alleviated by lying on the affected side, which diminishes the movement of that side of the chest called autosplinting.
Debate continues concerning the mechanism by which pain occurs in the parietal membrane. It has been long thought that friction between the two pleural surfaces (when the membranes are irritated and covered with fibrinous exudate) causes sharp pain. Other theories suggest that intercostal muscle spasm resulting from pleurisy or stretching of the parietal pleura causes this pain.
Pleural pain is present in pulmonary diseases such as pleurisy, pneumonia, pulmonary infarct (when it extends to the pleural surface, thus causing pleurisy), tumor (when it invades the parietal pleura), and pneumothorax. Tumor, especially bronchogenic carcinoma, may be accompanied by severe, continuous pain when the tumor tissue, extending to the parietal pleura through the lung, constantly irritates the pain nerve endings in the pleura.
Diaphragmatic Pleural Pain
The diaphragmatic pleura receives dual pain innervation through the phrenic and intercostal nerves. Damage to the phrenic nerve produces paralysis of the corresponding half of the diaphragm. The phrenic nerves are sensory and motor from both surfaces of the diaphragm.
Stimulation of the peripheral portions of the diaphragmatic pleura results in sharp pain felt along the costal margins, which can be referred to the lumbar region by the lower thoracic somatic nerves. Stimulation of the central portion of the diaphragmatic pleura results in sharp pain referred to the upper trapezius muscle and shoulder on the ipsilateral side of the stimulation (see Figs. 3-4 and 3-5).
Pain of cardiac and diaphragmatic origin is often experienced in the shoulder because the heart and diaphragm are supplied by the C5-6 spinal segment, and the visceral pain is referred to the corresponding somatic area.
Diaphragmatic pleurisy secondary to pneumonia is common and refers sharp pain along the costal margins or upper trapezius, which is aggravated by any diaphragmatic motion, such as coughing, laughing, or deep breathing.
There may be tenderness to palpation along the costal margins, and sharp pain occurs when the client is asked to take a deep breath. A change in position (sidebending or rotation of the trunk) does not reproduce the symptoms, which would be the case with a true intercostal lesion or tear.
Forceful, repeated coughing can result in an intercostal lesion in the presence of referred intercostal pain from diaphragmatic pleurisy, which can make differentiation between these two entities impossible without a medical referral and further diagnostic testing.
Pulmonary Physiology
The primary function of the respiratory system is to provide oxygen to and to remove carbon dioxide from cells in the body. The act of breathing, in which the oxygen and carbon dioxide exchange occurs, involves the two interrelated processes of ventilation and respiration.
Ventilation is the movement of air from outside the body to the alveoli of the lungs. Respiration is the process of oxygen uptake and carbon dioxide elimination between the body and the outside environment.
Breathing is an automatic process by which sensors detect changes in the levels of carbon dioxide and continuously direct data to the medulla. The medulla then directs respiratory muscles that adjust ventilation. Breathing patterns can be altered voluntarily when this automatic response is overridden by conscious thought.
The major sensors mentioned here are the central chemoreceptors (located near the medulla) and the peripheral sensors (located in the carotid body and aortic arch). The central chemoreceptors respond to increases in carbon dioxide and decreases in pH in cerebrospinal fluid.
As carbon dioxide increases, the medulla signals a response to increase respiration. The peripheral chemoreceptor system responds to low arterial blood oxygen and is believed to function only in pathologic situations such as when there are chronically elevated carbon dioxide levels (e.g., COPD).
Acid-Base Regulation
The proper balance of acids and bases in the body is essential to life. This balance is very complex and must be kept within the narrow parameters of a pH of 7.35 to 7.45 in the extracellular fluid. This number (or pH value) represents the hydrogen ion concentration in body fluid.
A reading of less than 7.35 is considered acidosis, and a reading greater than 7.45 is called alkalosis. Life cannot be sustained if the pH values are less than 7 or greater than 7.8.
Living human cells are extremely sensitive to alterations in body fluid pH (hydrogen ion concentration); thus various mechanisms are in operation to keep the pH at a relatively constant level.
Acid-base regulatory mechanisms include chemical buffer systems, the respiratory system, and the renal system. These systems interact very closely to maintain a normal acid-base ratio of 20 parts of bicarbonate to 1 part of carbonic acid and thus to maintain normal body fluid pH.
The blood test used most often to measure the effectiveness of ventilation and oxygen transport is the arterial blood gas (ABG) test (Table 7-1). The measurement of arterial blood gases is important in the diagnosis and treatment of ventilation, oxygen transport, and acid-base problems.
TABLE 7-1
Arterial Blood Gas Values*
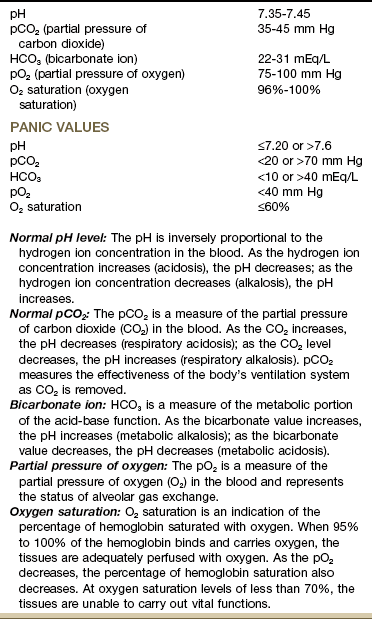
*Modified from Chernecky C, Berger B: Laboratory tests and diagnostic procedures, ed 5, Philadelphia, 2008, WB Saunders.
The ABG test measures the amount of dissolved oxygen and carbon dioxide in arterial blood and indicates acid-base status by measurement of the arterial blood pH. In simple terms a low pH reflects increased acid buildup, and a high pH reflects an increased base buildup.
Acid buildup occurs when there is an ineffective removal of carbon dioxide from the lungs or when there is excess acid production from the tissues of the body. These problems are corrected by adjusting the ventilation or buffering the acid with bicarbonate.
Pulmonary Pathophysiology
Any condition that decreases pulmonary ventilation increases the retention and concentration of carbon dioxide (CO2), hydrogen, and carbonic acid; this results in an increase in the amount of circulating hydrogen and is called respiratory acidosis.
If ventilation is severely compromised, CO2 levels become extremely high and respiration is depressed even further, causing hypoxia as well.
During respiratory acidosis, potassium moves out of cells into the extracellular fluid to exchange with circulating hydrogen. This results in hyperkalemia (abnormally high potassium concentration in the blood) and cardiac changes that can cause cardiac arrest.
Respiratory acidosis can result from pathologic conditions that decrease the efficiency of the respiratory system. These pathologies can include damage to the medulla, which controls respiration, obstruction of airways (e.g., neoplasm, foreign bodies, pulmonary disease such as COPD, pneumonia), loss of lung surface ventilation (e.g., pneumothorax, pulmonary fibrosis), weakness of respiratory muscles (e.g., poliomyelitis, spinal cord injury, Guillain-Barré syndrome), or overdose of respiratory depressant drugs.
As hypoxia becomes more severe, diaphoresis, shallow rapid breathing, restlessness, and cyanosis may appear. Cardiac arrhythmias may also be present as the potassium level in the blood serum rises.
Treatment is directed at restoration of efficient ventilation. If the respiratory depression and acidosis are severe, injection of intravenous sodium bicarbonate and use of a mechanical ventilator may be necessary. Any client with symptoms of inadequate ventilation or CO2 retention needs immediate medical referral.
Respiratory Alkalosis
Increased respiratory rate and depth decrease the amount of available CO2 and hydrogen and create a condition of increased pH, or alkalosis. When pulmonary ventilation is increased, CO2 and hydrogen are eliminated from the body too quickly and are not available to buffer the increasingly alkaline environment.
Respiratory alkalosis is usually due to hyperventilation. Rapid, deep respirations are often caused by neurogenic or psychogenic problems, including anxiety, pain, and cerebral trauma or lesions. Other causes can be related to conditions that greatly increase metabolism (e.g., hyperthyroidism) or overventilation of clients who are using a mechanical ventilator.
If the alkalosis becomes more severe, muscular tetany and convulsions can occur. Cardiac arrhythmias caused by serum potassium loss through the kidneys may also occur. The kidneys keep hydrogen in exchange for potassium.
Treatment of respiratory alkalosis includes reassurance, assistance in slowing breathing and facilitating relaxation, sedation, pain control, CO2 administration, and use of a rebreathing device such as a rebreathing mask or paper bag. A rebreathing device allows the client to inhale and “rebreathe” the exhaled CO2.
Respiratory alkalosis related to hyperventilation is a relatively common condition and might be present more often in the physical therapy setting than respiratory acidosis. Pain and anxiety are common causes of hyperventilation, and treatment needs to be focused toward reduction of both of these interrelated elements. If hyperventilation continues in the absence of pain or anxiety, serious systemic problems may be the cause, and immediate physician referral is necessary.
If either respiratory acidosis or alkalosis persists for hours to days in a chronic and not life-threatening manner, the kidneys then begin to assist in the restoration of normal body fluid pH by selective excretion or retention of hydrogen ions or bicarbonate. This process is called renal compensation. When the kidneys compensate effectively, blood pH values are within normal limits (7.35 to 7.45) even though the underlying problem may still cause the respiratory imbalance.
Chronic Obstructive Pulmonary Disease
COPD, also called chronic obstructive lung disease (COLD), refers to a number of disorders that have in common abnormal airway structures resulting in obstruction of air in and out of the lungs. The most important of these disorders are obstructive bronchitis, emphysema, and asthma.
Although bronchitis, emphysema, and asthma may occur in a “pure form,” they most commonly coexist. For example, adult subjects with active asthma are as much as 12 times more likely to acquire COPD over time than subjects with no active asthma.2-5
COPD is a leading cause of morbidity and mortality among cigarette smokers. Other factors predisposing to COPD include air pollution; occupational exposure to aerosol pesticides, irritating dusts or gases, or art materials (e.g., paint, glass, ceramics, sculpture); hereditary factors; infection; allergies; aging; and potentially harmful drugs and chemicals.6
COPD rarely occurs in nonsmokers; however, only a minority of cigarette smokers develop symptomatic disease, suggesting that genetic factors or other underlying predisposition may contribute to the development of COPD.7
In all forms of COPD, narrowing of the airways obstructs airflow to and from the lungs (Table 7-2). This narrowing impairs ventilation by trapping air in the bronchioles and alveoli. The obstruction increases the resistance to airflow. The severity of symptoms depends on how much of the lungs have been damaged or destroyed.
TABLE 7-2
Respiratory Diseases: Summary of Differences
| Disease | Primary Area Affected | Results |
| Bronchitis | Membrane lining bronchial tubes | Inflammation of lining |
| Bronchiectasis | Bronchial tubes (bronchi or air passages) | Bronchial dilation with inflammation |
| Pneumonia | Alveoli (air sacs) | Causative agent invades alveoli with resultant outpouring from lung capillaries into air spaces and continued healing process |
| Emphysema | Air spaces beyond terminal bronchioles (small airways) | Breakdown of alveolar walls; air spaces enlarged |
| Asthma | Bronchioles (small airways) | Bronchioles obstructed by muscle spasm, swelling of mucosa, thick secretions |
| Cystic fibrosis | Bronchioles | Bronchioles become obstructed and obliterated. Later, larger airways become involved. Plugs of mucus cling to airway walls, leading to bronchitis, bronchiectasis, atelectasis, pneumonia, or pulmonary abscess |
Trapped air hinders normal gas exchange and causes distention of the alveoli. Other mechanisms of COPD vary with each form of the disease. In the healthy adult the bottom margin of the respiratory diaphragm sits at T9 when the lungs are at rest. Taking a deep breath expands the diaphragm (and lungs) inferiorly to T11. For the client with COPD the lower lung lobes are already at T11 when the lungs are at rest from overexpansion as a result of alveoli distention and hyperinflation.
COPD develops earlier in life than is usually recognized, making it the most underdiagnosed and undertreated pulmonary disease. Smoking cessation is the only intervention shown to slow decline in lung function. Identifying risk factors and recognizing early signs and symptoms of COPD increases the affected individual’s chances of reduced morbidity through early intervention.6
Acute: Acute bronchitis is an inflammation of the trachea and bronchi (tracheobronchial tree) that is self-limiting and of short duration with few pulmonary signs. This condition may result from chemical irritation (e.g., smoke, fumes, gas) or may occur with viral infections such as influenza, measles, chickenpox, or whooping cough.
These predisposing conditions may become apparent during the subjective examination (i.e., Personal/Family History form or the Physical Therapy Interview). Although bronchitis is usually mild, it can become complicated in older clients and clients with chronic lung or heart disease. Pneumonia is a critical complication.
Chronic: Chronic bronchitis is a condition associated with prolonged exposure to nonspecific bronchial irritants and is accompanied by mucus hypersecretion and structural changes in the bronchi (large air passages leading into the lungs). This irritation of the tissue usually results from exposure to cigarette smoke or long-term inhalation of dust or air pollution and causes hypertrophy of mucus-producing cells in the bronchi.
In bronchitis, partial or complete blockage of the airways from mucus secretions causes insufficient oxygenation in the alveoli (Fig. 7-3). The swollen mucous membrane and thick sputum obstruct the airways, causing wheezing, and the client develops a cough to clear the airways. The clinical definition of a person with chronic bronchitis is anyone who coughs for at least 3 months per year for 2 consecutive years without having had a precipitating disease.
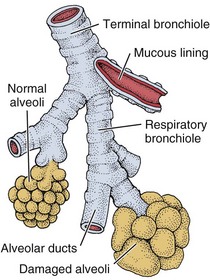
Fig. 7-3 Chronic bronchitis may lead to the formation of misshapen or large alveolar sacs with reduced space for oxygen and carbon dioxide exchange. The client may develop cyanosis and pulmonary edema.
To confirm that the condition is chronic bronchitis, tests are performed to determine whether the airways are obstructed and to exclude other diseases that may cause similar symptoms such as silicosis, tuberculosis, or a tumor in the upper airway. Sputum samples will be analyzed, and lung function tests may be performed.
Treatment is aimed at keeping the airways as clear as possible. Smokers are encouraged and helped to stop smoking. A combination of drugs may be prescribed to relieve the symptoms, including bronchodilators to open the obstructed airways and to thin the obstructive mucus so that it can be coughed up more easily.
Chronic bronchitis may develop slowly over a period of years, but it will not go away if untreated. Eventually, the bronchial walls thicken, and the number of mucous glands increases. The client is increasingly susceptible to respiratory infections, during which the bronchial tissue becomes inflamed and the mucus becomes even thicker and more profuse.
Chronic bronchitis can be incapacitating and lead to more serious and potentially fatal lung disease. Influenza and pneumococcal vaccines are recommended for these clients.
Bronchiectasis: Bronchiectasis is a form of obstructive lung disease that is actually a type of bronchitis. It is a progressive and chronic pulmonary condition that occurs after infections such as childhood pneumonia or CF.
Although bronchiectasis was once a common disease because of measles, pertussis, tuberculosis, and poorly treated bacterial pneumonias, the prevalence of bronchiectasis has diminished greatly since the introduction of antibiotics. It is characterized by abnormal and permanent dilatation of the large air passages leading into the lungs (bronchi) and by destruction of bronchial walls.
Bronchiectasis is caused by repeated damage to bronchial walls. The resultant destruction and bronchial dilatation reduce bronchial wall movement so that secretions cannot be removed effectively from the lungs, and the person is predisposed to frequent respiratory infections.
This vicious cycle of bacterial infection and inflammation of the bronchial wall leads to loss of ventilation and irreversible lung damage. Advanced bronchiectasis may cause pneumonia, cor pulmonale, or right-sided ventricular failure.
All pulmonary irritants, especially cigarette smoke, should be avoided. Postural drainage, adequate hydration, good nutrition, and bronchodilator therapy in bronchospasm are important components in treatment. Antibiotics are used during disease exacerbations (e.g., increased cough, purulent sputum, hemoptysis, malaise, and weight loss). The use of immunomodulatory therapy to alter the host response directly and thereby reduce tissue damage is under investigation.8,9
Emphysema: Emphysema may develop in a person after a long history of chronic bronchitis in which the alveolar walls are destroyed, leading to permanent overdistention of the air spaces and loss of normal elastic tension in the lung tissue.
Air passages are obstructed as a result of these changes (rather than as a result of mucus production, as in chronic bronchitis). Difficult expiration in emphysema is due to the destruction of the walls (septa) between the alveoli, partial airway collapse, and loss of elastic recoil.
As the alveoli and septa collapse, pockets of air form between the alveolar spaces (called blebs) and within the lung parenchyma (called bullae). This process leads to increased ventilatory “dead space,” or areas that do not participate in gas or blood exchange. The WOB is increased because there is less functional lung tissue to exchange oxygen and CO2. Emphysema also causes destruction of the pulmonary capillaries, further decreasing oxygen perfusion and ventilation.
In advanced emphysema, oxygen therapy is usually necessary to treat the progressive hypoxemia that occurs as the disease worsens. Oxygen therapy is carefully titrated and monitored to maintain venous oxygen saturation levels at or slightly above 90%. Too much oxygen can depress the respiratory drive of a person with emphysema.
The drive to breathe in a healthy person results from an increase in the arterial carbon dioxide level (pCO2). In the normal adult, increased CO2 levels stimulate chemoreceptors in the brainstem to increase the respiratory rate. With some chronic lung disorders these central chemoreceptors may become desensitized to pCO2 changes resulting in a dependence on the peripheral chemoreceptors to detect a fall in arterial oxygen levels (pO2) to stimulate the respiratory drive. Therefore too much oxygen delivered as a treatment can depress the respiratory drive in those individuals with COPD who have a dampening of the CO2 drive.
Monitoring respiratory rate, level of oxygen administered by nasal canula, and oxygen saturation levels is very important in this client population. Some pulmonologists agree that supplemental oxygen levels can be increased during activity without compromising the individual because they will “blow it (carbon dioxide) off” anyway. To our knowledge, there is no evidence yet to support this clinical practice.
Types of Emphysema: There are three types of emphysema. Centrilobular emphysema (Fig. 7-4), the most common type, destroys the bronchioles, usually in the upper lung regions. Inflammation develops in the bronchioles, but usually the alveolar sac remains intact.
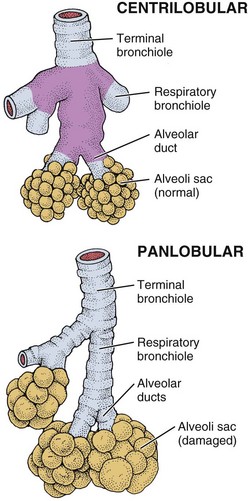
Fig. 7-4 Emphysema traps air in the lungs so that expelling air becomes increasingly difficult. Centrilobular emphysema affects the upper airways and produces destructive changes in the bronchioles. Panlobular emphysema affects the lower airways and is more diffusely scattered throughout the alveoli.
Panlobular emphysema destroys the more distal alveolar walls, most commonly involving the lower lung. This destruction of alveolar walls may occur secondary to infection or to irritants (most commonly, cigarette smoke). These two forms of emphysema, collectively called centriacinar emphysema, occur most often in smokers.
Paraseptal (or panacinar) emphysema destroys the alveoli in the lower lobes of the lungs, resulting in isolated blebs along the lung periphery. Paraseptal emphysema is believed to be the likely cause of spontaneous pneumothorax.
Clinical Signs and Symptoms: The irreversible destruction reduces elasticity of the lung and increases the effort to exhale trapped air, causing marked dyspnea on exertion later progressing to dyspnea at rest. Cough is uncommon.
The client is often thin, has tachypnea with prolonged expiration, and uses the accessory muscles for respiration. The client often leans forward with the arms braced on the knees to support the shoulders and chest for breathing. The combined effects of trapped air and alveolar distention change the size and shape of the client’s chest, causing a barrel chest and increased expiratory effort.
As the disease progresses, there is a loss of surface area available for gas exchange. In the final stages of emphysema, cardiac complications, especially enlargement and dilatation of the right ventricle, may develop. The overloaded heart reaches its limit of muscular compensation and begins to fail (cor pulmonale).
The most important factor in the treatment of emphysema is smoking cessation. The main goals for the client with emphysema are to improve oxygenation and decrease CO2 retention.
Pursed-lip breathing causes resistance to outflow at the lips, which in turn maintains intrabronchial pressure and improves the mixing of gases in the lungs. This type of breathing should be encouraged to help the client get rid of the stale air trapped in the lungs.
Exercise has not been shown to improve pulmonary function but is used to enhance cardiovascular fitness and train skeletal muscles to function more effectively. Routine progressive walking is the most common form of exercise.
Lung volume reduction surgery is available for some individuals and improves not only lung function and exercise performance, but also activities of daily function and quality of life.10,11
Inflammatory/Infectious Disease
Asthma is a reversible obstructive lung disease caused by increased reaction of the airways to various stimuli. It is a chronic inflammatory condition with acute exacerbations that can be life-threatening if not properly managed. Our understanding of asthma has changed dramatically over the last decade.
Asthma was once viewed as a bronchoconstrictive disorder in which the airways narrowed, causing wheezing and breathing difficulties. Treatment with bronchodilators to open airways was the primary focus. Scientific evidence now supports the idea that asthma is primarily an inflammatory disorder in which the constriction of airways is a symptom of the underlying inflammation.
Asthma and other atopic disorders are the result of complex interactions between genetic predisposition and multiple environmental influences. The marked increase in asthma prevalence in the last 3 decades suggests environmental factors as a key contributor in the process of allergic sensitization.12
Fifteen million persons of all ages are affected by asthma in the United States. This represents a 61% increase over the last 15 years with a 45% increase in mortality during the last decade. Women are affected more than men, accounting for about 60% of the nearly 18 million cases of adult asthma. Hormones are thought to be a possible cause for this increase in incidence in women.13
Immune Sensitization and Inflammation
There are two major components to asthma. When the immune system becomes sensitized to an allergen, usually through heavy exposure in early life, an inflammatory cascade occurs, extending beyond the upper airways into the lungs.
The lungs become hyperreactive, responding to allergens and other irritants in an exaggerated way. This hyperresponsiveness causes the muscles of the airways to constrict, making breathing more difficult (Fig. 7-5). The second component is inflammation, which causes the air passages to swell and the cells lining the passages to produce excess mucus, further impairing breathing.
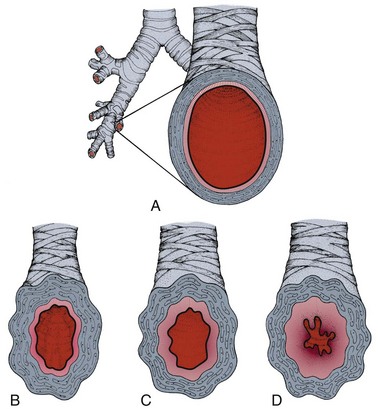
Fig. 7-5 Airway changes with asthma. A, Normal bronchus: cross-section of a normal bronchus (mucous membrane in color). Healthy bronchioles accommodate a constant flow of air when open and relaxed. B, Asthma: airway inflammation begins. The smooth muscle surrounding the bronchus contracts and causes narrowing of the airway, called bronchospasm. C, The airway tissue swells; this edema of the mucous membrane further narrows airways. D, Mucus is produced, further compromising airflow.
Asthma may be categorized as conventional asthma, occupational asthma, or exercise-induced asthma (EIA), but the underlying pathophysiologic complex remains the same. Since the triggers or allergens vary, each person reacts differently. SOB, wheezing, tightness in the chest, and cough are the most commonly reported symptoms, but other symptoms may also occur.
Clinical Signs and Symptoms
Anytime a client experiences SOB, wheezing, and cough and comments, “I’m more out of shape than I thought,” the therapist should ask about a past medical history of asthma and review the list of symptoms with the client. Therapists working with known asthmatic clients should encourage them to maintain hydration by drinking fluids to prevent mucous plugs from hardening and to take prescribed medications.
EIA or hyperventilation-induced asthma potentially can be prevented by exercising in a moist, humid environment and by grading exercise according to client tolerance using diaphragmatic breathing. Any type of sustained running or cycling or activity in the cold is more likely to precipitate EIA (Box 7-2).
Complications: Status asthmaticus is a severe, life-threatening complication of asthma. With severe bronchospasm the workload of breathing increases five to ten times, which can lead to acute cor pulmonale. When air is trapped, a severe paradoxic pulse develops as venous return is obstructed. This condition is seen as a blood pressure drop of more than 10 mm Hg during inspiration.
Pneumothorax can develop. If status asthmaticus continues, hypoxemia worsens and acidosis begins. If the condition is untreated or not reversed, respiratory or cardiac arrest will occur. An acute asthma episode may constitute a medical emergency.
Medical treatment for the underlying inflammation and resulting airway obstruction is with antiinflammatory agents and bronchodilators to prevent, interrupt, or terminate ongoing inflammatory reactions in the airways. A new class of antiinflammatory agents known as leukotriene modifiers works by blocking the activity of chemicals called leukotrienes, which are involved in airway inflammation. Reducing, eliminating, and avoiding allergens or triggers are important in self-care (see Box 7-2).
Pneumonia
Pneumonia is an inflammation of the lungs and can be caused by (1) aspiration of food, fluids, or vomitus; (2) inhalation of toxic or caustic chemicals, smoke, dust, or gases; or (3) a bacterial, viral, or mycoplasmal infection. It may be primary or secondary (a complication of another disease); it often follows influenza.
The common feature of all types of pneumonia is an inflammatory pulmonary response to the offending organism or agent. This response may involve one or both lungs at the level of the lobe (lobar pneumonia) or more distally beginning in the terminal bronchioles and alveoli (bronchopneumonia). Bronchopneumonia is seen more frequently than lobar pneumonia and is common in clients postoperatively and in clients with chronic bronchitis, particularly when these two situations coexist.
There are three main types of pneumonia: hospital-acquired pneumonia (HAP; also known as nosocomial pneumonia), ventilator-associated pneumonia (VAP), and community-acquired pneumonia (CAP). By definition, HAP occurs 48 hours or more after hospital admission; when HAP develops in a mechanically ventilated patient after endotracheal intubation, it becomes VAP.
Risk Factors
Infectious agents responsible for pneumonia are typically present in the upper respiratory tract and cause no harm unless resistance is lowered severely by some other factor such as smoking, a severe cold, disease, alcoholism, or generally poor health (e.g., poorly controlled diabetes, chronic renal problems, compromised immune function).
Older or bedridden clients are particularly at risk because of physical inactivity and immobility. Limited mobility causes normal secretions to pool in the airways and facilitates bacterial growth. Other risk factors predisposing a client to pneumonia are listed in Box 7-3.
Pneumocystis carinii is a protozoan organism that rarely causes pneumonia in healthy individuals. Pneumocystis carinii pneumonia (PCP) has been the most common life-threatening opportunistic infection in persons with acquired immunodeficiency syndrome (AIDS). PCP also has been shown to be the first indicator of conversion from human immunodeficiency virus (HIV) infection to the designation of AIDS.
Clinical Signs and Symptoms
The onset of all pneumonias is generally marked by any of the following: fever, chills, sweats, pleuritic chest pain, cough, sputum production, hemoptysis, dyspnea, headache, or fatigue. PCP causes a dry, hacking cough without sputum production.
The older client can have full-blown pneumonia and may appear with altered mental status (especially confusion) rather than fever or respiratory symptoms because of the changes in temperature regulation as we age. Anytime an older person has shoulder pain and confusion at presentation, consider the possibility of diaphragmatic impingement by an underlying lung pathologic condition (Case Example 7-2).
The clinical manifestations of PCP are slow to develop; they include fever, tachypnea, tachycardia, dyspnea, nonproductive cough, and hypoxemia. A diffuse, bilateral pattern of alveolar infiltration is apparent on chest radiograph.
Hospitalization may be required for the immunocompromised client. Otherwise, if the client has an intact defense system and good general health, recuperation can take place at home with rest and supportive treatment. In the hospital, rigorous handwashing by medical personnel is essential for reducing the transmission of infectious agents.
Tuberculosis
Tuberculosis (TB) is a bacterial infectious disease transmitted by the gram-positive, acid-fast bacillus Mycobacterium tuberculosis. Despite improved methods of detection and treatment, TB remains a worldwide health problem with increasing spread of a highly drug-resistant strain of TB present in almost every state in the United States.
Before the development of anti-TB drugs in the late 1940s, TB was the leading cause of death in the United States. Drug therapy, along with improvements in public health and general living standards, resulted in a marked decline in incidence. However, recent influxes of immigrants from developing Third World nations, rising homeless populations, and the emergence of HIV led to an increase in reported cases in the mid1980s, reversing a 40-year period of decline.
Risk Factors
Although TB can affect anyone, certain segments of the population have an increased risk of contracting the disease (Box 7-4). The mycobacterium is usually spread by airborne droplet nuclei, which are produced when actively infected persons sneeze, speak, sing, or cough.
Once released into the atmosphere, the organisms are dispersed and can be inhaled by a susceptible host. Brief exposure to a few bacilli rarely causes an infection. More commonly, it is spread with repeated close contact with an infected person.
Drug-resistant strains of TB have developed when the full course of treatment, lasting 6 to 9 months, is not completed. Once the infected person feels better and stops taking the prescribed medication, a new drug-resistant strain is passed along. Incomplete treatment among inner-city residents and the homeless presents a major factor in the failure to eradicate TB.
Drug-resistant strains are also developing globally. Areas of the world with increased rates of drug-resistant disease include countries of the former Soviet Union (e.g., Estonia, Kazakhstan, Latvia, Lithuania, Uzbekistan) and in Central Asia.
Families who adopt internationally should be aware of potential TB infection in children from some of the high-risk areas of the world. The vaccine BCG (bacille Calmette-Guerin) has been used in many foreign countries to attempt to prevent serious dissemination of TB infection in those countries.
The value of BCG is controversial, since the protection it confers is short term. The Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics strongly recommend that history of BCG vaccination in a child from a high-risk part of the world should usually be ignored and all children adopted internationally should be skin tested for TB and treated if the disease is latent or active.14
TB most often involves the lungs, but extrapulmonary TB (XPTB) can also occur in the kidneys, bone growth plates, lymph nodes, and meninges and can be disseminated throughout the body.
Widespread dissemination throughout the body is termed miliary tuberculosis and is more common in people 50 years or older and very young children with unstable or underdeveloped immune systems.
On rare occasions, TB will affect the hip joints and vertebrae, resulting in severe, arthritis-like damage, possibly even avascular necrosis of the hip. Tuberculosis of the spine, referred to as Pott’s disease, is rare but can result in compression fracture of the vertebrae.
Pyogenic vertebral osteomyelitis can be caused by atypical organisms such as tuberculosis. As with other pyogenic infection, back pain is the most common symptom, but it is less severe than in other infections. Individuals from high-risk areas of the world, high-risk living conditions, and the immunocompromised and malnourished should be considered suspect for this condition.15
Clinical Signs and Symptoms
Clinical signs and symptoms are absent in the early stages of TB. Many cases are found incidentally when routine chest radiographs are made for other reasons. When systemic manifestations of active disease initially appear, the clinical signs and symptoms listed here may appear.
Tuberculin skin testing is done to determine whether the body’s immune response has been activated by the presence of the bacillus. A positive reaction develops 3 to 10 weeks after the initial infection. A positive skin test reaction indicates the presence of a tuberculous infection but does not show whether the infection is dormant or is causing a clinical illness.
Chest x-ray films and sputum cultures are done as a follow-up to positive skin tests. All cases of active disease are treated, and certain cases of inactive disease are treated prophylactically.
Systemic Sclerosis Lung Disease
Systemic sclerosis (SS), or scleroderma, is a restrictive lung disease of unknown etiologic origin characterized by inflammation and fibrosis of many organs (see Chapter 12). Fibrosis affecting the skin and the visceral organs is the hallmark of SS.
The lungs, highly vascularized and composed of abundant connective tissue, are a frequent target organ, ranking second only to the esophagus in visceral involvement.
The most common pulmonary manifestation of SS is interstitial fibrosis, which is clinically apparent in more than 50% of cases. Autopsy results suggest a prevalence of 75%, indicating the insensitivity of traditional tests such as pulmonary function tests and chest radiographs.
Clinical Signs and Symptoms
As discussed in Chapter 12, skin changes associated with SS generally precede visceral alterations. Dyspnea on exertion and nonproductive cough are the most common clinical findings associated with SS. Rarely, these symptoms precede the occurrence of cutaneous changes of scleroderma.
Clubbing of the nails rarely occurs in SS because of the nearly universal presence of sclerodactyly (hardening and shrinking of connective tissues of fingers and toes). Peripheral edema may develop secondary to cor pulmonale, which occurs as the pulmonary fibrosis becomes advanced.
Pulmonary manifestations in systemic sclerosis include:
• Common: Interstitial pneumonitis and fibrosis and pulmonary vascular disease
• Less common: Pleural disease, aspiration pneumonia, pneumothorax, neoplasm, pneumoconiosis, pulmonary hemorrhage, and drug-induced pneumonitis
Pleural effusions may appear with orthopnea, edema, and paroxysmal nocturnal dyspnea if CHF occurs. Cystic changes in the parenchyma may progress to form pneumatoceles (thin-walled air-containing cysts) that may rupture spontaneously and produce a pneumothorax. Clients with SS have an increased incidence of lung cancer. Hemoptysis is often the first signal of pulmonary malignancy in individuals with SS.
The course of SS is unpredictable, from a mild, protracted course to rapid respiratory failure and death. Treatment of pulmonary complications, pulmonary hypertension, and interstitial lung disease remains difficult.
Neoplastic Disease
Lung Cancer (Bronchogenic Carcinoma)
Lung cancer is malignancy in the epithelium of the respiratory tract. At least a dozen different cell types of tumors are included under the classification of lung cancer.
Clinically, lung cancers are grouped into two divisions: small cell lung cancer (15% of all lung cancers) and non–small cell lung cancer. The four major types of lung cancer include small cell carcinoma (oat cell carcinoma), and the subtypes of non–small cell lung cancer (e.g., squamous cell carcinoma [25% to 30%], adenocarcinoma [40%], and large cell carcinoma [10% to 15%]).
Since the mid1950s, lung cancer has been the most common cause of death from cancer in men. In 1987, lung cancer surpassed breast cancer to become the leading cause of cancer death in women in the United States. It is now the second most commonly diagnosed cancer in both men and women and remains the number one cause of death in both groups.15a Lung cancer affects some races more than others; blacks have higher incidence and mortality rates than do whites.16
The rate of lung cancer among women in the United States may be declining for some age groups. This decline is expected to continue through at least the year 2025. Sustaining that trend will require continued reductions in the number of female children who start to smoke and continued smoking cessation among addicted female smokers.17
Risk Factors: Smoking is the major risk factor for lung cancer, accounting for 82% of deaths caused by lung cancer.18 Other risk factors are listed in Box 7-5. Compared with nonsmokers, heavy smokers (i.e., those who smoke more than 25 cigarettes a day) have a twentyfold greater risk of developing cancer.
States with strong anti-tobacco programs (e.g., Arizona, California) have the fewest current smokers, the most people who have quit smoking in some age groups, and the greatest drop in the death rate from lung cancer.19 Quitting smoking lowers the risk, but the decrease is gradual and does not approach that of a nonsmoker.
The risk of lung cancer is increased in the smoker who is exposed to other carcinogenic agents such as radon, asbestos, and chemical carcinogens. Internationally, the incidence of lung cancer is growing with industrialized nations having the highest rates.20
The increase of lung cancer mortality in the last decades can be entirely attributed to the trend of tobacco consumption. However, there is a lag time of many years between beginning smoking and the clinical manifestation of cancer. The therapist can have a key role in the prevention of lung cancer through risk-factor assessment and client education (see Chapter 2). Personal risk of lung cancer can be assessed at www.yourdiseaserisk.wustl.edu.
Metastases: Metastatic spread of pulmonary tumors is usually to the long bones, vertebral column (especially the thoracic vertebrae), liver, and adrenal glands. Brain metastasis is also common, occurring in as many as 50% of cases.
Local metastases by direct extension may involve the chest wall, pleura, pulmonary parenchyma, or bronchi. Further local tumor growth may erode the first and second ribs and associated vertebrae, causing bone pain and paravertebral pain associated with involvement of sympathetic nerve ganglia.
The respiratory system is a common site for complications associated with cancer and cancer therapy. Several factors can lead to pulmonary complications. Immunosuppression caused by the underlying disease or the cancer therapy can lead to infectious disease.
In addition, the lungs contain an enormous capillary bed through which flows the entire venous circulation, making it a common site of metastasis from other primary cancers and pulmonary emboli. Carcinomas of the kidney, breast, pancreas, colon, and uterus are especially likely to metastasize to the lungs.
Clinical Signs and Symptoms: Clinical signs and symptoms of lung cancer often remain silent until the disease process is at an advanced stage. In many instances, lung cancer may mimic other pulmonary conditions or may initially appear as chest, shoulder, or arm pain (Case Example 7-3).
Chest pain is a vague aching, and depending on the type of cancer, the client may have pleuritic pain on inspiration that limits lung expansion. Anorexia and weight loss occur in many clients with lung cancer and can be a symptom of advanced disease.21
Hemoptysis (coughing or spitting up blood) may occur secondary to ulceration of blood vessels. Wheezing occurs when the tumor obstructs the bronchus. Dyspnea, either unexplained or out of proportion, is a red flag indicating the need for medical screening, as is unexplainable weight loss accompanied by dyspnea.
Centrally located tumors cause increased cough, dyspnea, and diffuse chest pain that can be referred to the shoulder, scapulae, and upper back. This pain is the result of peribronchial or perivascular nerve involvement.
Other symptoms may include postobstructive pneumonia with fever, chills, malaise, anorexia, hemoptysis, and fecal breath odor (secondary to infection within a necrotic tumor mass). If these tumors extend to the pericardium, the client may develop a sudden onset of arrhythmia (tachycardia or atrial fibrillation), weakness, anxiety, and dyspnea.
Peripheral tumors are most often asymptomatic until the tumor extends through visceral and parietal pleura to the chest wall. Irritation of the nerves causes localized sharp, pleuritic pain that is aggravated by inspiration.
Metastases to the mediastinum (tissue and organs between the sternum and the vertebrae, including the heart and its large vessels; trachea; esophagus; thymus; lymph nodes) may cause hoarseness or dysphagia secondary to vocal cord paralysis as a result of entrapment or local compression of the laryngeal nerve.
Apical (Pancoast’s) tumors of the lung apex do not usually cause symptoms while confined to the pulmonary parenchyma. They can extend into surrounding structures and frequently involve the eighth cervical and first thoracic nerves within the brachial plexus.
A constellation of symptoms referred to as Pancoast’s syndrome present in the distribution of the C8, T1, and T2 dermatomes, mimicking thoracic outlet syndrome.22 Tumor invasion of any anatomic structures of the lower trunks of the brachial plexus and/or the C8 and T1 nerve roots can result in significant disability and loss of hand function.22 Extension of the tumor into the paravertebral sympathetic nerves results in Horner’s syndrome, which consists of enophthalmos (backward displacement of the eye), ptosis (drooping eyelid), and miosis (pupil constriction).
The most common initial symptom is sharp (often posterior) shoulder pain produced by invasion of the brachial plexus and/or extension of the tumor into the parietal pleura, endothoracic fascia, first and second ribs, or vertebral bodies. There may be pain in the axilla and subscapular areas on the affected side (Case Example 7-4).
Pain may radiate up to the head and neck, across the chest, and/or down the medial aspect of the arm and hand (ulnar nerve distribution) (Fig. 7-6). There may be subsequent atrophy of the upper extremity muscles with weakness of the muscles of the hand.
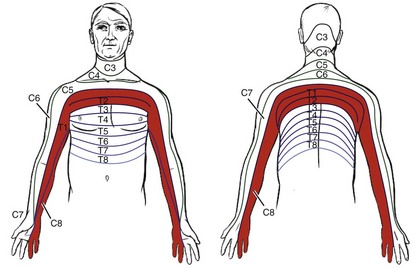
Fig. 7-6 Pancoast’s tumors can present with changes in cutaneous dermatomal innervation. The shaded areas show the dermatomes affected when the superior (apical) sulcus tumors associated with Pancoast’s syndrome invade the brachial plexus. Direct extension to the brachial plexus involving C8 and T1 result in symptoms affecting the C8, T1, and T2 dermatomes.
Pulmonary symptoms, such as cough, hemoptysis, and dyspnea, are uncommon until late in the disease. Affected individuals are often treated for presumed cervical osteoarthritis or shoulder bursitis resulting in delay of diagnosis. The pain eventually progresses to become severe and constant, eventually resulting in more thorough testing and accurate diagnosis.23 The onset of pulmonary symptoms in any client with neck, shoulder, and/or arm pain should be a red flag symptom for the therapist.
Trigger points of the serratus anterior muscle (see Fig. 17-7) also mimic the distribution of pain caused by the eighth cervical nerve root compression. Trigger points can be ruled out by palpation and lack of neurologic deficits and may be confirmed by elimination with appropriate physical therapy intervention.
Paraneoplastic syndromes (remote effects of a malignancy; see explanation in Chapter 13) occur in 10% to 20% of lung cancer clients and represent a feature of advanced disease. These usually result from the secretion of hormones by the tumor acting on target organs, producing a variety of symptoms. Occasionally, symptoms of paraneoplastic syndrome occur before detection of the primary lung tumor.
As mentioned earlier, brain metastasis is common, occurring in as much as 50% of cases. About 10% of all individuals with lung cancer have central nervous system (CNS) involvement at the time of diagnosis. CNS symptoms, such as muscle weakness, muscle atrophy, loss of lower extremity sensation, and localized or radicular back pain, may be associated with lung cancer and must be investigated by a physician to establish a medical diagnosis.
Other clinical symptoms of brain metastasis resulting from increased intracranial pressure may include headache, nausea, vomiting, malaise, anorexia, weakness, and alterations in mental processes. Localized motor or sensory deficits occur, depending on the location of lesions (see Chapter 13).
Metastasis to the spinal cord produces signs and symptoms of cord compression (see Table 13-5 and Appendix A-2), including back pain (localized or radicular), muscle weakness, loss of lower extremity sensation, bowel and bladder incontinence, and diminished or absent lower extremity reflexes (unilateral or bilateral).
Genetic Disease of the Lung
Cystic fibrosis (CF) is an inherited disease of the exocrine (“outward-secreting”) glands primarily affecting the digestive and respiratory systems.
This disease is the most common genetic disease in the United States, inherited as a recessive trait: both parents must be carriers, each having a defective copy of the CF gene. Each time two carriers conceive a child, there is a 25% chance that the child will have CF, a 50% chance that the child will be a carrier, and a 25% chance that the child will be a noncarrier. In the United States, 5% of the population, or 12 million people, carry a single copy of the CF gene.
Because cysts and scar tissue on the pancreas were observed during autopsy when the disease was first being differentiated from other conditions, it was given the name cystic fibrosis of the pancreas. Although this term describes a secondary rather than primary characteristic, it has been retained.
In 1989, scientists isolated the CF gene located on chromosome 7. In healthy people a protein called CF transmembrane conductance regulator (CFTR) provides a channel by which chloride (a component of salt) can pass in and out of cells.
Persons with CF have a defective copy of the gene that normally enables cells to construct that channel. As a result, salt accumulates in the cells lining the lungs and digestive tissues, making the surrounding mucus abnormally thick and sticky. These secretions, which obstruct ducts in the pancreas, liver, and lungs, and abnormal secretion of sweat and saliva are the two main features of CF.
Usually, CF manifests itself in early childhood, but there are some individuals who have a variant form of the disease in which symptoms can appear during adolescence or adulthood. Symptoms tend to be milder and sweat chloride concentration may be normal.24
Obstruction of the bronchioles by mucous plugs and trapped air predisposes the client to infection, which starts a destructive cycle of increased mucus production with increased bronchial obstruction, infection, and inflammation with eventual destruction of lung tissue.
Clinical Signs and Symptoms
Pulmonary involvement is the most common and severe manifestation of CF. Obstruction of the airways leads to a state of hyperinflation and bronchiectasis. In time, fibrosis develops, and restrictive lung disease is superimposed on the obstructive disease.
Over time, pulmonary obstruction leads to chronic hypoxia, hypercapnia, and acidosis. Pneumothorax, pulmonary hypertension, and eventually cor pulmonale may develop. These are very poor prognostic indicators in adults. The course of CF varies from one client to another depending on the degree of pulmonary involvement.
Advances in treatment, including aerosolized antibiotics, mucus thinning agents, antiinflammatory agents, chest physical therapy, enzyme supplements, and nutrition programs, have extended the average life expectancy for CF sufferers into their early 20s, with maximal survival estimated at 30 to 40 years.
Because the genetic abnormality has been identified, considerable progress has been made in the development of gene therapy and preventive gene transfer for this disease.25-27 Lung transplantation in older childhood and adolescence is a possible treatment option based on rapidly declining lung function.28
Occupational Lung Diseases
Lung diseases are among the most common occupational health problems. They are caused by the inhalation of various chemicals, dusts, and other particulate matter present in certain work settings. Not everyone exposed to occupational inhalants will develop lung disease. Prolonged exposure combined with smoking increases the risk of developing occupational lung disease and increases the severity of these diseases.29
During the interview process, the therapist will ask questions about occupational and smoking history to identify the possibility of an underlying pulmonary pathologic condition (see Chapter 2 and Appendix B-14).
The most commonly encountered occupational lung diseases are occupational lung cancer, occupational asthma (also known as work-related asthma), asbestosis, mesothelioma, and byssinosis (brown lung disease). Other less common occupational lung diseases include hypersensitivity pneumonitis, acute respiratory irritation, and pneumoconiosis (black lung disease, silicosis).
The greatest number of occupational agents causing asthma are those with known or suspected allergic properties such as plant and animal proteins (e.g., wheat, flour, cotton, flax, and grain mites). Exposures within the workplace can aggravate preexisting asthma.30
Asbestosis and mesothelioma occur as a result of asbestos exposure. Asbestos is the name of a group of naturally occurring minerals that separate into strong, very fine fibers. The fibers are heat-resistant and extremely durable, which are qualities that made asbestos useful in construction and industry.
Scarring of the lung tissue occurs in asbestosis as a result of exposure to the microscopic fibers of asbestos. Under certain circumstances, fibers can be released and pose a health risk such as lung cancer from inhaling the fibers. Mesothelioma is an otherwise rare cancer of the chest lining caused by asbestos exposure.
Byssinosis (brown lung disease) caused by dusts from hemp, flax, and cotton processing, results in chronic obstruction of the small airways impairing lung function. Textile workers are at greatest risk of disability from byssinosis.
Hypersensitivity pneumonitis, or allergic alveolitis, is most commonly due to the inhalation of organic antigens of fungal, bacterial, or animal origin. Acute respiratory irritation results from the inhalation of chemicals such as ammonia, chlorine, and nitrogen oxides in the form of gases, aerosols, or particulate matter. If such irritants reach the lower airways, alveolar damage and pulmonary edema can result. Although the effects of these acute irritants are usually short-lived, some may cause chronic alveolar damage or airway obstruction.
Pneumoconioses, or “the dust diseases,” result from inhalation of minerals, notably silica, coal dust, or asbestos. These diseases are most commonly seen in miners, construction workers, sandblasters, potters, and foundry and quarry workers. Occupational exposure to dust, fumes, or gases (including diesel) increases mortality due to COPD, even among workers who have never smoked.31
Pneumoconioses usually develop gradually over a period of years, eventually leading to diffuse pulmonary fibrosis, which diminishes lung capacity and produces restrictive lung disease.31
Home Remodeling
Home remodeling projects in the United States have increased dramatically in the last decade. Whether it is a do-it-yourself project or the occupants remain in the home during remodeling, problems can occur from dust inhalation and exposure to hazardous materials such as lead, asbestos, and creosote. Creosote is toxic (inhaled as fumes) and is a skin and eye irritant.
Lead poisoning is a serious problem in home remodeling projects throughout the United States. Special precautions to avoid lead poisoning must be followed if the home was built prior to 1978.
Lead poisoning can occur from inhaling paint dust (the result of sanding or scraping painted surfaces) and lead can be found in soil (children come into contact during play). Both sources of poisoning are common problems associated with remodeling projects.
Anyone presenting with a constellation of integumentary, musculoskeletal, and/or neurologic symptoms accompanied by pulmonary involvement should be asked about the possibility of recent home remodeling projects and exposure to any of these materials.
Clinical Signs and Symptoms
Early symptoms of occupational-related lung disease depend on the specific exposure but may include noninflammatory joint pain, myalgia, cough, and dyspnea on exertion.
Chest pain, productive cough, and dyspnea at rest develop as the condition progresses. The therapist needs to be alert for the combination of significant arthralgias and myalgias with associated respiratory symptoms, accompanied by a past occupational and smoking history (see Appendix B-14).
Pleuropulmonary Disorders
Pulmonary Embolism and Deep Venous Thrombosis
Pulmonary embolism (PE) involves pulmonary vascular obstruction by a displaced thrombus (blood clot), an air bubble, a fat globule, a clump of bacteria, amniotic fluid, vegetations on heart valves that develop with endocarditis, or other particulate matter. Once dislodged, the obstruction travels to the blood vessels supplying the lungs, causing SOB, tachypnea (very rapid breathing), tachycardia, and chest pain.
The most common cause of PE is deep venous thrombosis (DVT) originating in the proximal deep venous system of the lower legs. The embolism causes an area of blockage, which then results in a localized area of ischemia known as a pulmonary infarct. The infarct may be caused by small emboli that extend to the lung surface (pleura) and result in acute pleuritic chest pain.
Risk Factors
Three major risk factors linked with DVT are blood stasis (e.g., immobilization because of bed rest, such as with burn clients, obstetric and gynecologic clients, and older or obese populations), endothelial injury (secondary to neoplasm, surgical procedures, trauma, or fractures of the legs or pelvis), and hypercoagulable states (see Box 6-2).
Other people at increased risk for DVT and PE include those with CHF, trauma, surgery (especially hip, knee, and prostate surgery), age over 50 years, previous history of thromboembolism, malignant disease, infection, diabetes mellitus, inactivity or obesity, pregnancy, clotting abnormalities, and oral contraceptive use (see Chapter 6).
Prevention
Given the mortality of PE and the difficulties involved in its clinical diagnosis, prevention of DVT and PE is critical. A careful review of the Personal/Family History form (outpatient) or hospital medical chart (inpatient) may alert the therapist to the presence of factors that predispose a client to have a PE. Risk factor assessment is an important part of screening and prevention.
Although frequent changing of position, exercise, and early ambulation are necessary to prevent thrombosis and embolism, sudden and extreme movements should be avoided. Under no circumstances should the legs be massaged to relieve “muscle cramps,” especially when the pain is located in the calf and the client has not been up and about.
Restrictive clothing and prolonged sitting or standing should be avoided. Elevating the legs should be accomplished with caution to avoid severe flexion of the hips, which will slow blood flow and increase the risk of new thrombi.
Deep Venous Thrombosis (see also Chapter 6)
Signs and symptoms of DVT include tenderness, leg pain, swelling (a difference in leg circumference of 1.4 cm in men and 1.2 cm in women is significant), and warmth. One may also see subcutaneous venous distention, discoloration, a palpable cord, and/or pain upon placement of a blood pressure cuff around the calf (considerable pain with the cuff inflated to 160 to 180 mm Hg).
Homans’ sign is an unreliable test to diagnose DVT. The therapist should be aware that using the Homans’ test to assess for DVT is no longer recommended or supported by evidence.32-35 Homans’ sign is elicited by gentle squeezing of the affected calf or slow dorsiflexion of the foot on the affected side to elicit deep calf pain. In theory, the inflamed nerves in the veins within the muscle are compressed or stretched causing deep calf pain. Only about half of all clients with DVT experience pain and Homans’ sign.
Unfortunately, at least half the cases of DVT are asymptomatic,34 and in up to one-third of all clients with apparent clinical appearance of DVT, there is no DVT demonstrable. A more sensitive and specific tool for predicting DVT is the Autar DVT Scale (see Table 4-11).
Pulmonary Embolism
Signs and symptoms of PE are nonspecific and vary greatly, depending on the extent to which the lung is involved, the size of the clot, and the general condition of the client.
Clinical presentation does not differ between younger and older persons. Dyspnea, pleuritic chest pain, and cough are the most common symptoms reported. Pleuritic pain is caused by an inflammatory reaction of the lung parenchyma or by pulmonary infarction or ischemia caused by obstruction of small pulmonary arterial branches.
Typical pleuritic chest pain is sudden in onset and aggravated by breathing. The client may also report hemoptysis, apprehension, tachypnea, and fever (temperature as high as 39.5° C, or 103.5° F). The presence of hemoptysis indicates that the infarction or areas of atelectasis have produced alveolar damage.
The therapist can use the simplified Wells criteria for clinical assessment of pulmonary embolism when assessing the need for referral for possible PE (Table 7-3). Previously, the modified Wells rule for assessing clinical probability for exclusion of pulmonary embolism was used with 1 to 3 points assigned for the variables listed.36 Since that time, studies assigning only 1 point to the original variables have shown the simplified Wells rule to be a simpler, yet valid assessment tool37-39; prospective validation remains necessary.
TABLE 7-3
Simplified Wells Criteria for the Clinical Assessment of Pulmonary Embolism
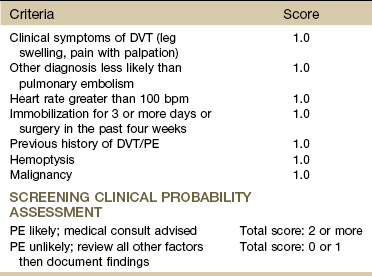
DVT, Deep venous thrombosis; PE, pulmonary embolism.
Data from Douma RA: Validity and clinical utility of the simplified Wells rule for assessing clinical probability for the exclusion of pulmonary embolism, Thromb Haemost 101(1):197-200, 2009, and Gibson NS: Further validation and simplification of the Wells clinical decision rule in pulmonary embolism, Thromb Haemost 99:229-234, 2008.
The Wells criteria also outline factors that constitute the PE rule-out criteria (PERC). Combining information from the list below with the PE score can help the therapist recognize low versus high priority for medical consultation.36 The following parameters reduce the likelihood of a pulmonary embolism:
• Heart rate less than 100 bpm
• Oxyhemoglobin saturation equal to or greater than 95%
• No surgery or trauma requiring hospitalization within the past 4 weeks
The PERC approach has a high negative predictive value and sensitivity when combined with a low probability of PE using the Wells criteria, but a low positive predictive value and specificity. In other words, low-risk patients who have all of the PERC are highly unlikely to have PE, but the absence of one or more of the PERC does not mean that a PE exists.40
Cor Pulmonale
When a PE has been sufficiently massive to obstruct 60% to 75% of the pulmonary circulation, the client may have central chest pain, and acute cor pulmonale occurs. Cor pulmonale is a serious cardiac condition and an emergency situation arising from a sudden dilatation of the right ventricle as a result of PE.
As cor pulmonale progresses, edema, and other signs of right-sided heart failure develop. Symptoms are similar to those of CHF from other causes: dyspnea, edema of the lower extremities, distention of the veins of the neck, and liver distention. The hematocrit is increased as the body attempts to compensate for impaired circulation by producing more erythrocytes.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a condition of vasoconstriction of the pulmonary arterial vascular bed. PAH is medically defined as a mean pulmonary artery pressure of 25 mm Hg or more with a pulmonary capillary wedge pressure of 15 mm Hg or less (measured by cardiac catheterization).41
It can be either primary (rare), occurring three times more often in women in their 30s and 40s compared with men, or secondary, occurring as a result of other clinical conditions such as PE, chronic lung disease, sickle-cell disease, Graves’ disease, polycythemia, collagen vascular disease, portal hypertension, heart abnormalities, and sleep apnea. Along with thromboemboli, tumors can also obstruct pulmonary circulation. Either type is probably a combination of genetic and environmental factors.41,42
Normally, the pulmonary circulation has a low resistance and can accommodate large increases in blood flow during exertion. When pulmonary arterial vasoconstriction occurs and pulmonary arterial pressures rise above normal, the condition becomes self-perpetuating inducing further vasoconstriction in the pulmonary vasculature, structural abnormalities, and eventual right-sided heart failure (cor pulmonale).
Clinical Signs and Symptoms
There may not be any symptoms in the early stages of PAH. Onset of symptoms can be very subtle and difficult to recognize initially, especially in secondary PAH since underlying lung disease is usually present. PAH may present as progressive dyspnea (present on exertion first and later developing at rest). Dull retrosternal chest pain, fatigue, and dizziness on exertion are common and often mimic angina pectoris.41,42
The right ventricle must pump very hard against a narrowed, resistant pulmonary vascular bed, thus resulting in pump failure. The right ventricle enlarges in its effort to overcome abnormally high PA pressure. Ascites (increased abdominal girth) is a common visible sign. With auscultation there may be an accentuated pulmonic component of S2 caused by the increased force of pulmonary valve closure in the presence of PAH. There may be a pulmonary regurgitation murmur as well.41
Pleurisy
Pleurisy is an inflammation of the pleura (serous membrane enveloping the lungs) and is caused by infection, injury, or tumor. The membranous pleura that encases each lung consists of two close-fitting layers: the visceral layer encasing the lungs and the parietal layer lining the inner chest wall. A lubricating fluid lies between these two layers.
If the fluid content remains unchanged by the disease, the pleurisy is said to be dry. If the fluid increases abnormally, it is a wet pleurisy or pleurisy with effusion (pleural effusion). If the wet pleurisy becomes infected with formation of pus, the condition is known as purulent pleurisy or empyema.
Pleurisy may occur as a result of many factors, including pneumonia, tuberculosis, lung abscess, influenza, systemic lupus erythematosus (SLE), rheumatoid arthritis, and pulmonary infarction. Any of these conditions is actually a risk factor for the development of pleurisy, especially in the aging adult population.
Pleurisy, with or without effusion associated with SLE, may be accompanied by acute pleuritic pain and dysfunction of the diaphragm.
Clinical Signs and Symptoms
The chest pain is sudden and may vary from vague discomfort to an intense stabbing or knifelike sensation in the chest. The pain is aggravated by breathing, coughing, laughing, or other similar movements associated with deep inspiration.
The visceral pleura is insensitive; pain results from inflammation of the parietal pleura. Because the latter is innervated by the intercostal nerves, chest pain is usually felt over the site of the pleuritis, but pain may be referred to the lower chest wall, abdomen, neck, upper trapezius muscle, and shoulder because of irritation of the central diaphragmatic pleura (Fig. 7-7).
Pneumothorax
Pneumothorax, or free air in the pleural cavity between the visceral and parietal pleurae, may occur secondary to pulmonary disease (e.g., when an emphysematous bulla or other weakened area on the lung ruptures) or as a result of trauma and subsequent perforation of the chest wall. Other risk factors include scuba diving and overexertion.
Pneumothorax is not uncommon after surgery or after an invasive medical procedure involving the chest or thorax. Air may enter the pleural space directly through a hole in the chest wall (open pneumothorax) or diaphragm. Pneumothorax associated with surgical management of patent ductus arteriosus (PDA) in neonates has been reported.43
Air may escape into the pleural space from a puncture or tear in an internal respiratory structure (e.g., bronchus, bronchioles, or alveoli). This form of pneumothorax is called closed or spontaneous pneumothorax.
Pneumothorax associated with scuba diving occurs as a result of arterial gas embolism (AGE). AGE is caused by pulmonary overinflation if the breathing gas cannot be exhaled adequately during the ascent. Inert gas bubbles cause impairment of pulmonary functions due to hypoxia.44
Extraalveolar air (pulmonary barotrauma) from scuba diving can be overlooked, resulting in serious neurologic sequelae. Scuba diving is contraindicated in anyone with asthma, hypertension, coronary heart disease, diabetes, or a history of pneumothorax.
Spontaneous pneumothorax occasionally affects the exercising individual and occurs without preceding trauma or infection. In a healthy individual, abrupt onset of dyspnea raises the suspicion of a spontaneous pneumothorax. Peak incidence for this type of pneumothorax is in adults between 20 and 40 years. Spontaneous pneumothorax in term newborn infants is significantly more likely in males with higher birth weights and with vacuum delivery.45
Idiopathic spontaneous pneumothorax (SP) is the result of leakage of air from the lung parenchyma through a ruptured visceral pleura into the pleural cavity. This rupture may be caused by an increased pressure difference between parenchymal airspace and pleural cavity. Another theory is that peripheral airway inflammation leads to obstruction with airtrapping in the lung parenchyma, which precedes spontaneous pneumothorax.46
Clinical Signs and Symptoms
Symptoms of pneumothorax, whether occurring spontaneously or as a result of injury or trauma, vary, depending on the size and location of the pneumothorax and on the extent of lung disease. When air enters the pleural cavity, the lung collapses, producing dyspnea and a shift of tissues and organs to the unaffected side.
The client may have severe pain in the upper and lateral thoracic wall, which is aggravated by any movement and by the cough and dyspnea that accompany it. The pain may be referred to the ipsilateral shoulder (corresponding shoulder on the same side as the pneumothorax), across the chest, or over the abdomen (Fig. 7-8). The client may be most comfortable when sitting in an upright position.
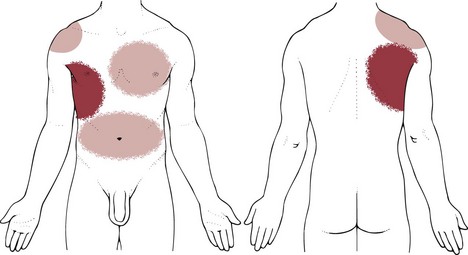
Fig. 7-8 Possible pain patterns associated with pneumothorax: upper and lateral thoracic wall with referral to the ipsilateral shoulder, across the chest, or over the abdomen.
Other symptoms may include a fall in blood pressure, a weak and rapid pulse, and cessation of normal respiratory movements on the affected side of the chest (Case Example 7-5).
Physician Referral
It is more common for a therapist to be treating a client with a previously diagnosed musculoskeletal problem who now has chronic, recurrent pulmonary symptoms than to be the primary evaluator and health care provider of a client with pulmonary symptoms.
In either case, the therapist needs to know what further questions to ask and which of the client’s responses represent serious symptoms that require medical follow-up.
Shoulder or back pain can be referred from diseases of the diaphragmatic or parietal pleura or secondary to metastatic lung cancer. When clients have chest pain, they usually fall into two categories: those who demonstrate chest pain associated with pulmonary symptoms and those who have true musculoskeletal problems, such as intercostal strains and tears, myofascial trigger points, fractured ribs, or myalgias secondary to overuse.
Clients with chronic, persistent cough, whether that cough is productive or dry and hacking, may develop sharp, localized intercostal pain similar to pleuritic pain. Both intercostal and pleuritic pain are aggravated by respiratory movements, such as laughing, coughing, deep breathing, or sneezing. Clients who have intercostal pain secondary to insidious trauma or repetitive movements, such as coughing, can benefit from physical therapy.
For the client with asthma, it is important to maintain contact with the physician if the client develops signs of asthma or any bronchial activity during exercise. The physician must be informed to help alter the dosage or the medications to maintain optimal physical performance.
The therapist will want to screen for medical disease through a series of questions to elicit the presence of associated systemic (pulmonary) signs and symptoms. Aggravating and relieving factors may provide further clues that can assist in making treatment or referral decision.
In all these situations, the referral of a client to a physician is based on the family/personal history of pulmonary disease, the presence of pulmonary symptoms of a systemic nature, or the absence of substantiating objective findings indicating a musculoskeletal lesion.
Guidelines for Immediate Medical Attention
• Abrupt onset of dyspnea accompanied by weak and rapid pulse and fall in blood pressure (pneumothorax), especially following motor vehicle accident, chest injury, or other traumatic event
• Chest, rib, or shoulder pain with neurologic symptoms following recent recreational or competitive scuba diving
• Client with symptoms of inadequate ventilation or CO2 retention (see Respiratory Acidosis)
• Any red flag signs and symptoms in a client with a previous history of cancer, especially lung cancer
Guidelines for Physician Referral
• Shoulder pain aggravated by respiratory movements; have the client hold his or her breath and reassess symptoms; any reduction or elimination of symptoms with breath holding or the Valsalva maneuver suggests pulmonary or cardiac source of symptoms.
• Shoulder pain that is aggravated by supine positioning; pain that is worse when lying down and improves when sitting up or leaning forward is often pleuritic in origin; abdominal contents push up against diaphragm and, in turn, against the parietal pleura.
• Shoulder or chest (thorax) pain that subsides with autosplinting (lying on the painful side).
• For the client with asthma: Signs of asthma or bronchial activity during exercise.
• Weak and rapid pulse accompanied by fall in blood pressure (pneumothorax).
• Presence of associated signs and symptoms such as persistent cough, dyspnea (rest or exertional), or constitutional symptoms (see Box 1-3).
Clues to Screening for Pulmonary Disease
These clues will help the therapist in the decision-making process:
• History of cigarette smoking for many years.
• Past medical history of breast, prostate, kidney, pancreas, colon, or uterine cancer.
• Recent history of upper respiratory infection, especially when followed by noninflammatory joint pain of unknown cause.
• Musculoskeletal pain exacerbated by respiratory movements (e.g., deep breathing, coughing, laughing).
• Respiratory movements are diminished or absent on one side (pneumothorax).
• Dyspnea (unexplained or out of proportion), especially when accompanied by unexplained weight loss.
• Unable to localize pain by palpation.
• Pain does not change with spinal motions (e.g., no change in symptoms with side bending, rotation, flexion, or extension).
• Pain does not change with alterations in position (possible exceptions: sitting upright is preferred with pneumothorax; symptoms may be worse at night with recumbency, sitting upright eases or relieves symptoms).
• Symptoms are increased with recumbency (lying supine shifts the contents of the abdominal cavity in an upward direction, thereby placing pressure on the diaphragm and in turn, the lungs, referring pain from a lower lung pathologic condition).
• Presence of associated signs and symptoms, especially persistent cough, hemoptysis, dyspnea, and constitutional symptoms, most commonly sore throat, fever, and chills.
• Autosplinting decreases pain.
• Elimination of trigger points resolves symptoms, confirming a musculoskeletal problem (or conversely, trigger point therapy does NOT resolve symptoms, raising a red flag for further examination and evaluation).
• Range of motion does not reproduce symptoms* (e.g., trunk rotation, trunk side bending, shoulder motions).
• Anytime an older person has shoulder pain and confusion at presentation, consider the possibility of diaphragmatic impingement by an underlying lung pathologic condition, especially pneumonia.
1. If a client reports that the shoulder/upper trapezius muscle pain increases with deep breathing, how can you assess whether this results from a pulmonary or musculoskeletal cause?
2. Neurologic symptoms such as muscle weakness or muscle atrophy may be the first indication of:
3. Back pain with radiating numbness and tingling down the leg past the knee does not occur as a result of:
4. Pain associated with pleuropulmonary disorders can radiate to the:
5. The presence of a persistent dry cough (no sputum or phlegm produced) has no clinical significance to the therapist. True or false?
6. Dyspnea associated with emphysema is the result of:
7. What is the significance of autosplinting?
8. Which symptom has greater significance: dyspnea at rest or exertional dyspnea?
9. The presence of pain and anxiety in a client can often lead to hyperventilation. When a client hyperventilates, the arterial concentration of carbon dioxide will do which of the following?
10. Common symptoms of respiratory acidosis would be most closely represented by which of the following descriptions?
References
1. Scharf, SM. History and physical examination. In Baum GL, Wolinsky E, eds.: Textbook of pulmonary diseases, ed 5, Boston: Little, Brown, 1989.
2. Guerra, S. Overlap of asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2005;11(1):7–13.
3. Zuskin, E. Respiratory function in pesticide workers. J Occup Environ Med. 2008;50(11):1299–1305.
4. Zuskin, E. Occupational health hazards of artists. Acta Dermatovenerol Croat. 2007;15(3):167–177.
5. Schachter, EN. Gender and respiratory findings in workers occupationally exposed to organic aerosols: a meta analysis of 12 cross-sectional studies. Environ Health. 2009;12(8):1–9.
6. Pauwels, RA, Rabe, KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet. 2004;364(9434):613–620.
7. Meyers, DA, Larj, MJ, Lange, L. Genetics of asthma and COPD. Similar results for different phenotypes. Chest. 2004;126(2 Suppl):105S–110S.
8. Amsden, GW. Anti-inflammatory effects of macrolides: an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. 2005;55(1):10–21.
9. Rubin, BK, Henke, MO. Immunomodulatory activity and effectiveness of macrolides in chronic airway disease. Chest. 2004;125(2 Suppl):70S–78S.
10. Goto, Y, Kurosawa, H, Mori, N, et al. Improved activities of daily living, psychological state and health-related quality of life for 12 months following lung volume reduction surgery in patients with severe emphysema. Respirology. 2004;9(3):337–344.
11. Trow, TK. Lung-volume reduction surgery for severe emphysema: appraisal of its current status. Curr Opin Pulm Med. 2004;10(2):128–132.
12. Upham, JW, Holt, PG. Environment and development of atopy. Curr Opin Allergy Clin Immunol. 2005;5(2):167–172.
13. Asthma in older women. Harvard Women’s Health Watch. 2003;11(3):5–6.
14. Wisniewski, A. Chronic bronchitis and emphysema: clearing the air. Nursing 2003. 2003;33(5):44–49.
15. Tay, B, Deckey, J, Hu, S. Spinal infections. J Am Acad Orthop Surg. 2002;10(3):188–197.
15a. Jemal, A. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
16. Fairley, TL. Racial/ethnic disparities and geographic differences in lung cancer incidence. MMWR. 2010;59(44):1434–1438.
17. Jemal, A, Ward, E, Thun, MJ. Contemporary lung cancer trends among U.S. women. Cancer Epidemiol Biomarkers Prev. 2005;14(3):582–585.
18. American Lung Association. Trends in tobacco use. Feb 2010. Available at http://www.lungusa.org/finding-cures/our-research/trend-reports/Tobacco-Trend-Report.pdf. [Posted on October 27, 2010. Accessed January 28, 2011].
19. Jemal, A. Lung cancer trends in young adults: an early indicator of progress in tobacco control (United States). Cancer Causes Control. 2003;14(6):579–585.
20. Porello, P. Neoplasms, Lung. eMedicine. Available at www.emedicine.com/emerg/topic335.htm. [Accessed January 28, 2011].
21. Kreamer, K. Getting the lowdown on lung cancer. Nursing 2003. 2003;33(11):36–42.
22. Davis, GA. Pancoast tumors. Neurosurg Clin N Am. 2008;19:545–557.
23. Arcasoy, SM, Jett, JR, Schild, SE. Pancoast’s syndrome and superior (pulmonary) sulcus tumors. UpToDate Patient Information. Sponsored by the American Society of General Internal Medicine and the American College of Rheumatology. Available at http://patients.uptodate.com/topic.asp?file=lung_ca/12055. [(version 13.2), Updated September 2010. Accessed January 28, 2011].
24. Donaldson, SH. Update on pathogenesis of cystic fibrosis lung disease. Curr Opin Pulm Med. 2003;9(6):486–491.
25. Mitomo, K. Toward gene therapy for cystic fibrosis using lentivirus pseudotyped with Sendai virus envelopes. Mol Ther. 2010;18(6):1173–1182.
26. Ostedgaard, LS, Rokhlina, T, Karp, PH, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A. 2005;102(8):2952–2957.
27. Sloane, PA. Cystic fibrosis transmembrane conductance regulator protein repair as a therapeutic strategy in cystic fibrosis. Curr Opin Pulm Med. 2010;16(6):591–597.
28. Rosenbluth, DB, Wilson, K, Ferkol, T, et al. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest. 2004;126(2):412–419.
29. American Lung Association. Occupational lung disease fact sheet. January 2004. Available at http://www.lungusa.org/. [Accessed January 28, 2011].
30. National Institute for Occupational Safety and Health: Chapter 2: Respiratory diseases. worker health chartbook, Pub No. 2004–146, 2004.
31. Bergdahl, IA, Toren, K, Eriksson, K, et al. Increased mortality in COPD among construction workers exposed to inorganic dust. Eur Respir J. 2004;23(3):402–406.
32. O’Donnell, T, Abbott, W, Athanasoulis, C, et al. Diagnosis of deep venous thrombosis in the outpatient by venography. Surg Gynecol Obstet. 1980;150:69–74.
33. Molloy, W, English, J, O’Dwyer, R, et al. Clinical findings in the diagnosis of proximal deep venous thrombosis. Ir Med J. 1982;75:119–120.
34. Delis, KT. Incidence, natural history, and risk factors of deep vein thrombosis in elective knee arthroscopy. Thromb Haemost. 2001;86(3):817–821.
35. Shah, NB. 82-year-old man with bilateral leg swelling. Mayo Clinic Proc. 2010;85(9):859–862.
36. van Belle, A, Buller, HR, Huisman, MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295:172.
37. Wells, PS. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the simplified D-dimer. Thromb Haemost. 2000;83:416–430.
38. Douma, RA. Validity and clinical utility of the simplified Wells rule for assessing clinical probability for the exclusion of pulmonary embolism. Thromb Haemost. 2009;101(1):197–200.
39. Gibson, NS. Further validation and simplification of the Wells clinical decision rule in pulmonary embolism. Thromb Haemost. 2008;99(6):1134–1136.
40. Wolf, SJ. Assessment of the pulmonary embolism rule-out criteria rule for evaluation of suspected pulmonary embolism in the emergency department. Am J Emerg Med. 2008;26:181.
41. Badesch, DB. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131(6):1917–1928.
42. Pulmonary Hypertension Association. What is pulmonary hypertension? Available at http://www.phassociation.org. [Accessed December 21, 2010].
43. Jog, SM, Patole, SK. Diaphragmatic paralysis in extremely low birthweight neonates: is waiting for spontaneous recovery justified? J Paediatr Child Health. 2002;38(1):101–103.
44. Taylor, DM, O’Toole, KS, Ryan, CM. Experienced, recreational scuba divers in Australia continue to dive despite medical contraindications. Wilderness Environ Med. 2002;13(3):187–193.
45. Al Tawil, K, Abu-Ekteish, FM, Tamimi, O, et al. Symptomatic spontaneous pneumothorax in term newborn infants. Pediatr Pulmonol. 2004;37(5):443–446.
46. Smit, HJ, Golding, RP, Schramel, FM, et al. Lung density measurements in spontaneous pneumothorax demonstrate airtrapping. Chest. 2004;125(6):2083–2090.
47. Racial disparities in tuberculosis, selected Southeastern States, 1991-2002. MMWR. 2004;53(25):556–559.
48. Carmona, L, Hernández-Garcia, C, Vadillo, C, et al. Increased risk of tuberculosis in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1436–1439.
*There are two possible exceptions to this guideline. Painful symptoms from an intercostal tear (secondary to forceful coughing caused by diaphragmatic pleurisy) will be reproduced by trunk side bending to the opposite side and trunk rotation to one or both sides. In such a case there is an underlying pulmonary pathologic condition, and a musculoskeletal component. Pleuritic pain can also be reproduced by trunk movements, but the therapist will be unable to localize the pain on palpation.
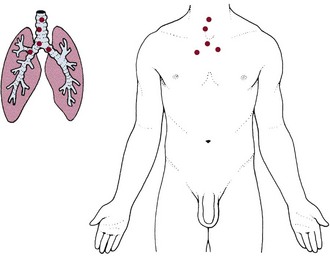
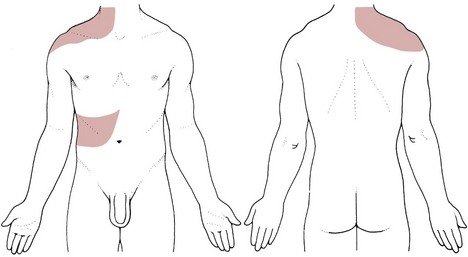
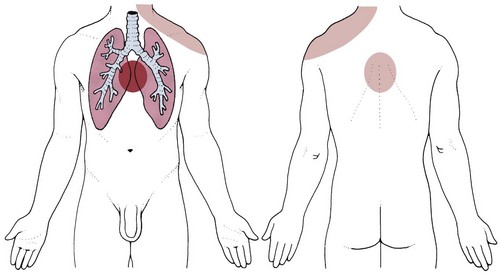

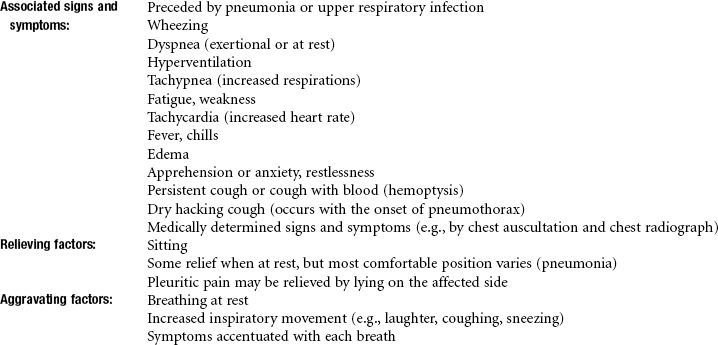

 Key Points to Remember
Key Points to Remember