Screening the Chest, Breasts, and Ribs
Clients do not present in a physical therapy clinic with chest or breast pain as the primary symptom very often. The therapist is more likely to see the individual with an orthopedic or neurologic impairment who experiences chest or breast pain during exercise or during other intervention by the therapist.
In other situations, the client reports chest or breast pain as an additional symptom during the screening interview. The pain may occur along with (or alternating with) the presenting symptoms of jaw, neck, upper back, shoulder, breast, or arm pain. When chest pain is the primary complaint, it is often an atypical pain pattern (possibly in a young athlete) that has misled the client and/or the physician.1
On the other hand, it is also possible for clients to have primary chest pain from a human movement system impairment, particularly spinal referred pain.2 Symptoms persist or recur, often with months in between when the client is free of any symptoms. Countless medical tests are performed and repeated with referral to numerous specialists before a physical therapist is consulted (see Case Example 1-7).
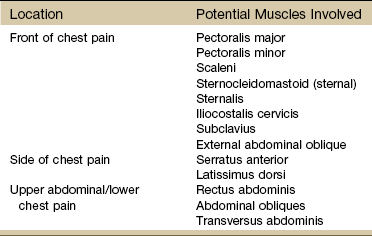
Finally, so many of today’s aging adults with movement system impairments have multiple medical comorbidities that the therapists must be able to identify signs and symptoms of systemic disease that can mimic neuromuscular or musculoskeletal dysfunction. Systemic or viscerogenic pain or symptoms that can be referred to the chest or breast include the cardiovascular, pulmonary, and upper gastrointestinal (GI) systems, as well as other causes such as cancer, anxiety, steroid use, and cocaine use (Table 17-1).3 Various neuromusculoskeletal (NMS) conditions, such as thoracic outlet syndrome, costochondritis, trigger points, and cervical spine disorders, can also affect the chest and breast.4
TABLE 17-1
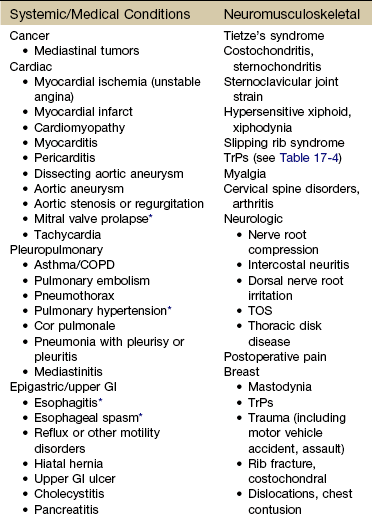

COPD, Chronic obstructive pulmonary disease; GI, gastrointestinal; TrPs, trigger points; TOS, thoracic outlet syndrome.
*Relieved by nitroglycerin because it relaxes smooth muscle.
When faced with chest pain, the therapist must know how to assess the situation quickly and decide if medical referral is required and whether medical attention is needed immediately. As experts in understanding and assessing the human movement system, we are the most capable health care professional when it comes to differentiating NMS from systemic origins of symptoms.
The therapist must especially know how and what to look for to screen for cancer, cancer recurrence, and/or the delayed effects of cancer treatment. Cancer can present as primary chest pain with or without accompanying neck, shoulder, and/or upper back pain/symptoms. Basic principles of cancer screening are presented in Chapter 13; specific clues related to the chest, breast, and ribs will be discussed in this chapter. Breast cancer is always a consideration with upper quadrant pain or dysfunction.
Using the Screening Model to Evaluate the Chest, Breasts, or Ribs
There are many causes of chest pain, both cardiac and noncardiac in origin (see Table 17-1). Two conditions may be present at the same time, each contributing to chest pain. For example, someone with cervicodorsal arthritis could also experience reflux esophagitis or coronary disease. Either or both of these conditions can contribute to chest pain.
Chest pain can be evaluated in one of two ways: cardiac versus noncardiac or systemic versus neuromusculoskeletal (NMS). Physicians and nurses assess chest pain from the first paradigm: cardiac versus noncardiac. The therapist must understand the basis for this screening method while also viewing each problem as potentially systemic versus NMS. Throughout the screening process, it is important to remember we are not medical cardiac specialists; we are just screening for systemic disease masquerading as NMS symptoms or dysfunction.
Paying attention to past medical history, recognizing unusual clinical presentation for a neuromuscular or musculoskeletal condition, and keeping in mind the clues to differentiating chest pain will help the therapist evaluate difficult cases.
Additionally, the woman with chest, breast, axillary, or shoulder pain of unknown origin at presentation must be questioned regarding breast self-examinations. Any recently discovered lumps or nodules must be examined by a physician. The client may need education regarding breast self-examination, and the physical therapist can provide this valuable information.5,6 Techniques of breast self-examination are commonly available in written form for the physical therapist or the client who is unfamiliar with these methods (see Appendix D-6).
Past Medical History
Although the past medical history (PMH) is important, it cannot be relied upon to confirm or rule out medical causes of chest pain. PMH does alert the therapist to an increased risk of systemic conditions that can masquerade as NMS disorders. Like risk factors, PMH varies according to each system affected or condition present and is reviewed individually in each section of this chapter.
Risk Factors
Any suspicious findings should be checked by a physician, especially in the case of the client with identified risk factors for cancer or heart disease. Identifying red-flag risk factors and PMH and then correlating this information with objective findings are important steps in the screening process.
Risk for cardiac-caused symptoms increases with advancing age, tobacco use, menopause (women), family history of hypertension or premature coronary artery disease, and high cholesterol. Risk factors associated with noncardiac conditions vary with each individual condition (e.g., infectious, rheumatologic, pulmonary, or other systemic causes).
Clinical Presentation
When the clinical presentation suggests further screening is needed, the therapist can follow the guide to pain assessment (see Chapter 3) and physical assessment for the upper quadrant as presented in Table 4-13. Assess vital signs and watch for trends in heart rate and blood pressure. Keep in mind that tachycardia may be a compensatory response to reduced cardiac output and bradycardia may be an indication of myocardial ischemia or (unreported) trauma.
The client’s general appearance, along with vital sign assessment, will offer some idea of the severity of the condition. Watch for uneven pulses from side to side, diminished or absent pulses, elevated blood pressure, or extreme hypotension. Auscultation for breath or lung sounds and chest percussion may provide additional cardiopulmonary clues.
Check to see if the pain can be reproduced or made worse by palpation or with pressure on the chest; and, of course, ask about associated symptoms such as nausea and shortness of breath. The key features that point to spinal referred pain are chest pain reproduced on movement (especially with resistive movement), tenderness and tightness of musculoskeletal structures at a spinal level supplying the painful area, and an absence or lack of symptoms suggestive of a nonmusculoskeletal cause.2
Chest Pain Patterns
From the previous discussion in Chapter 3, we know that there are at least three possible mechanisms for referred pain patterns to the soma from the viscera (embryologic development, multisegmental innervations, direct pressure on the diaphragm). Pain in the chest may be derived from the chest wall (dermatomes T1-12), the pleura, the trachea and main airways, the mediastinum (including the heart and esophagus), and the abdominal viscera. From an embryologic point of view, the lungs are derived from the same tissue as the gut, so problems can occur in both areas (lung or gut), causing chest pain and other related symptoms.
Certain chest pain patterns are more likely to point to a medical rather than musculoskeletal cause. For example, pain that is positional or reproduced by palpation is not as suspicious as pain that radiates to one or both shoulders or arms or that is precipitated by exertion. Physicians agree that the chest pain history by itself is not enough to rule out cardiac or other systemic origin of symptoms. In most cases, some diagnostic testing is needed.7
Chest pain associated with increased activity is a red flag for possible cardiovascular involvement. In such cases, the onset of pain is not immediate but rather occurs 5 to 10 minutes after activity begins. This is referred to as the “lag time” and is a screening clue used by the physical therapist to assess when chest pain may be caused by musculoskeletal dysfunction (immediate chest pain occurs with movement of the arms and/or trunk) or by possible vascular compromise (chest pain occurs 5 to 10 minutes after activity begins).
Parietal pain may appear as unilateral chest pain (rather than midline only) because at any given point the parietal peritoneum obtains innervation from only one side of the nervous system. It is usually not reproduced by palpation. Thoracic disk disease can also present as unilateral chest pain, requiring careful screening.8,9
The four types of pain discussed in Chapter 3 (cutaneous, deep somatic or parietal, visceral, and referred) also apply to the chest. Parietal (somatic) chest pain is the most common systemic chest discomfort encountered in a physical therapy practice. Parietal pain refers to pain generating from the wall of any cavity, such as the chest or pelvic cavity (see Fig. 6-5). Although the visceral pleura are insensitive to pain, the parietal pleura are well supplied with pain nerve endings. It is usually associated with infectious diseases but is also seen in pneumothorax, rib fractures, pulmonary embolism with infarction, and other systemic conditions.
Pain fibers, originating in the parietal pleura, are conveyed through the chest wall as fine twigs of the intercostal nerves. Irritation of these nerve fibers results in pain in the chest wall that is usually described as knifelike and is sharply localized close to the chest wall, occurring cutaneously (in the skin).
Pain from the thoracic viscera and true chest wall pain are both felt in the chest wall, but visceral pain is referred to the area supplied by the upper four thoracic nerve roots. Report of pain in the lower chest usually indicates local disease, but upper chest pain may be caused by disease located deeper in the chest.
There are few nerve endings (if any) in the visceral pleurae (linings of the various organs), such as the heart or lungs. The exception to this statement is in the area of the pericardium (sac enclosed around the entire heart), which is adjacent to the diaphragm (see Fig. 6-5). Extensive disease may develop within the body cavities without the occurrence of pain until the process extends to the parietal pleura. Neuritis (constant irritation of nerve endings) in the parietal pleura then produces the pain described in this section.
Pleural pain may be aggravated by any respiratory movement involving the diaphragm, such as sighing, deep breathing, coughing, sneezing, laughing, or the hiccups. It may be referred along the costal margins or into the upper abdominal quadrants. Palpation usually does not reproduce pleural pain; change in position does not relieve or exacerbate the pain. In some cases of pleurisy, the individual can point to the painful spot but deep breathing (not palpation) reproduces it.
Associated Signs and Symptoms
If the client has an underlying infectious or inflammatory process causing chest or breast pain or symptoms, there may be changes in vital signs and/or constitutional symptoms such as chills, night sweats, fever, upper respiratory symptoms, or GI distress.
Signs and symptoms associated with noncardiac causes of chest pain vary according to the underlying system involved. For example, cough, sputum production, and a recent history of upper respiratory infection may point to a pleuropulmonary origin of chest or breast pain. Anyone with persistent coughing or asthma can experience chest pain related to the strain of the chest wall muscles.
Chest or breast pain associated with GI disease is often food related in the presence of a history of peptic ulcer, gastroesophageal reflux disease (GERD), or gallbladder problems. Blood in the stool or vomitus, along with a history of chronic nonsteroidal antiinflammatory drug (NSAID) use, may point to a GI problem and so on.
Many of the conditions affecting the breast are not accompanied by other systemic signs and symptoms. Risk factors, client history, and clinical presentation provide the major clues as to a viscerogenic, systemic, or cancerous origin of chest and/or breast pain or symptoms.
Screening for Oncologic Causes of Chest or Rib Pain
Cancer can present as primary chest, neck, shoulder, and/or upper back pain and symptoms. A previous history of cancer of any kind is a major red flag (Case Example 17-1). Primary cancer affecting the chest with referred pain to the breast is not as common as cancer metastasized to the pulmonary system with subsequent pulmonary and chest/breast symptoms.
Clinical Presentation
The most common symptoms associated with metastases to the pulmonary system are pleural pain, dyspnea, and persistent cough. As with any visceral system, symptoms may not occur until the neoplasm is quite large or invasive because the lining surrounding the lungs has no pain perception. Symptoms first appear when the tumor is large enough to press on other nearby structures or against the chest wall. The presence of any skin changes, lesions, or masses should be documented using the information presented in Box 4-11. Skeletal pain from metastases to the bone or primary cancers such as multiple myeloma affecting the sternum can present much like costochondritis.
Skin Changes
Ask the client about any recent or current skin changes. Metastatic carcinoma can present with a cellulitic appearance on the anterior chest wall as a result of carcinoma of the lung (see Fig. 4-26). The skin lesion may be flat or raised and any color from brown to red or purple.
Liver impairment from cancer or any liver disease can also cause other skin changes, such as angiomas over the chest wall. An angioma is a benign tumor with blood (or lymph, as in lymphangioma) vessels. Spider angioma (also called spider nevus) is a form of telangiectasis, a permanently dilated group of superficial capillaries (or venules; see Fig. 9-3).
In the presence of skin lesions, ask about a recent history of infection of any kind, use of prescription drugs within the last 6 weeks, and previous history of cancer of any kind. Look for lymph node changes. Report all of these findings to the physician.
Palpable Mass
Occasionally, the therapist may palpate a painless sternal or chest wall mass when evaluating the head and neck region. Most mediastinal tumors are the result of a metastatic focus from a distant primary tumor and remain asymptomatic unless they compress mediastinal structures or invade the chest wall.
The primary tumor is usually a lymphoma (Hodgkin’s lymphoma in a young adult or non-Hodgkin’s lymphoma in a child or older adult; see Fig. 4-29), multiple myeloma (primarily observed in people over 60 years of age), or carcinoma of the breast, kidney, or thyroid.
When involvement of the chest wall and nerve roots results in pain, the pattern is more diffuse, with radiation of pain to the affected nerve roots (Case Example 17-2). Irritation of an intercostal nerve from rib metastasis produces burning pain that is unilateral and segmental in distribution. Sensory loss or hyperesthesia over the affected dermatomes may be noted.
Screening for Cardiovascular Causes of Chest, Breast, or Rib Pain
Cardiac-related chest pain may arise secondary to angina, myocardial infarction, pericarditis, endocarditis, mitral valve prolapse, or aortic aneurysm. Despite diagnostic advances, acute coronary syndromes and myocardial infarctions are missed in 2% to 10% of patients.7 There is no single element of chest pain history powerful enough to predict who is or who is not having a coronary-related incident. Medical referral is advised whenever there is any doubt; medical diagnostic testing is almost always required.7
Cardiac-related chest pain also can occur when there is normal coronary circulation, as in the case of clients with pernicious anemia. Affected clients may have chest pain or angina on physical exertion because of the lack of nutrition to the myocardium.
Risk Factors
Gender and age are nonmodifiable risk factors for chest pain caused by heart disease. The rate of coronary artery disease (CAD) is rising among women and falling among men. Men develop CAD at a younger age than women, but women make up for it after menopause. Many women know about the risk of breast cancer, but in truth, they are 10 times more likely to die of cardiovascular disease. While one in 30 women’s deaths is from breast cancer, one in 2.5 deaths is from heart disease.10
Women do not seem to do as well as men after taking medications to dissolve blood clots or after undergoing heart-related medical procedures. Of the women who survive a heart attack, 46% will be disabled by heart failure within 6 years.11 African-American women have a 70% higher death rate from CAD compared with Caucasian women.10 Whenever screening individuals who have chest pain, keep in mind that older men and women, menopausal women, and African-American women are at greatest risk for cardiovascular causes.
A common treatment for CAD after heart attack is angioplasty with insertion of a stent. A stent is a wire mesh tube that props open narrowed coronary arteries. Sometimes, the stent malfunctions or gets scarred over. Cardiologists have realized that such treatments, while effective at alleviating chest pain, do not reduce the risk of heart attacks for most people with stable angina.
When the client presents with chest pain, he or she often does not think it can be from the heart because there is a stent in place, but this may not be true. Anyone with a history of stent insertion presenting with chest pain should be assessed carefully. Take vital signs, and ask about associated signs and symptoms. Evaluate the effect of exercise on symptoms. For example, does the chest, neck, shoulder, or jaw pain start 3 to 5 minutes after exercise or activity? What is the effect on pain in the upper body when the individual is using just the lower extremities, such as walking on a treadmill or up a flight of stairs?
Other risk factors for CAD are listed in Table 6-3. Efforts are being made to determine evidence-based risk factors for low- versus high-risk chest pain of unknown origin. Predictive values for ischemia resulting in myocardial infarction or death include two or more episodes of chest pain typical of a heart attack in an adult 55 years old or older who has a family history of heart disease and/or a personal history of diabetes.12
Clinical Presentation
There are some well-known pain patterns specific to the heart and cardiac system. Sudden death can be the first sign of heart disease. In fact, according to the American Heart Association (AHA), 63% of women who died suddenly of cardiovascular disease had no previous symptoms. Sudden death is the first symptom for half of all men who have a heart attack. Cardiac arrest strikes immediately and without warning.
Cardiac Pain Patterns
Doctors and nurses often use “the three Ps” when screening for chest pain of a cardiac nature. The presence of any or all of these Ps suggests the client’s pain or symptoms are not caused by a myocardial infarction (MI):
• Pleuritic pain (exacerbation by deep breathing is more likely pulmonary in nature)
Cardiac pain patterns may differ for men and women. For many men, the most common report is a feeling of pressure or discomfort under the sternum (substernal), in the midchest region, or across the entire upper chest. It can feel like uncomfortable pressure, squeezing, fullness, or pain.
Pain may occur just in the jaw, upper neck, midback, or down the arm without chest pain or discomfort. Pain may also radiate from the chest to the neck, jaw, midback, or down the arm(s). Pain down the arm(s) affects the left arm most often in the pattern of the ulnar nerve distribution. Radiating pain down both arms is also possible.
For women, symptoms can be more subtle or atypical (Box 17-1). Chest pain or discomfort is less common in women but still a key feature for some. They often have prodromal symptoms (e.g., pain in the chest, pain in the shoulder or back, radiating pain or numbness in the arms, dyspnea, and fatigue) 12 months prior and up to 1 month before having a heart attack (see Table 6-4).13-15 Black women younger than 50 years are more likely to report frequent and intense prodromal symptoms.16
Fatigue, nausea, and lower abdominal pain may signal a heart attack. Many women pass these off as the flu or food poisoning. Other symptoms for women include a feeling of intense anxiety, isolated right biceps pain, or midthoracic pain. Heartburn; sudden shortness of breath or the inability to talk, move, or breathe; shoulder or arm pain; or ankle swelling or rapid weight gain are also common symptoms with MI.
Chest Pain Associated with Angina
The therapist should keep in mind that coronary disease may go unnoticed because the client has no anginal or infarct pain associated with ischemia. This situation occurs when collateral circulation is established to counteract the obstruction of the blood flow to the heart muscle. Anastomoses (connecting channels) between the branches of the right and left coronary arteries eliminate the person’s perception of pain until challenged by physical exertion or exercise in the physical therapy setting.
Chest pain caused by angina is often confused with heartburn or indigestion, hiatal hernia, esophageal spasm, or gallbladder disease, but the pain of these other conditions is not described as sharp or knifelike. The client often says the pain feels like “gas” or “heartburn” or “indigestion.” Referred pain from a trigger point in the external oblique abdominal muscle can cause a sensation of heartburn in the anterior chest wall (see Fig. 17-7).
Episodes of stable angina usually develop slowly and last 2 to 5 minutes. Discomfort may radiate to the neck, shoulders, or back (Case Example 17-3). Shortness of breath is common. Symptoms of angina may be similar to the pattern associated with a heart attack. One primary difference is duration. Angina lasts a limited time (a few minutes up to a half hour) and can be relieved by rest or nitroglycerin. When screening for angina, a lack of objective musculoskeletal findings is always a red flag:
• Active range of motion (AROM), such as trunk rotation, side bending, or shoulder motions, does not reproduce symptoms.
• Resisted motion does not reproduce symptoms (horizontal shoulder abduction/adduction).
The therapist should also watch for unstable angina in a client with known angina. Unlike stable angina, rest or nitroglycerin does not relieve symptoms associated with an MI, unless administered intravenously. Without intervention, symptoms of an MI may continue without stopping. A sudden change in the client’s typical anginal pain pattern suggests unstable angina. Pain that occurs without exertion, lasts longer than 10 minutes, or is not relieved by rest or nitroglycerin signals a higher risk for a heart attack. Immediate medical referral is required under these circumstances.
Screening for Pleuropulmonary Causes of Chest, Breast, or Rib Pain
Pulmonary chest pain usually results from obstruction, restriction, dilation, or distention of the large airways or large pulmonary artery walls. Specific diagnoses include pulmonary artery hypertension, pulmonary embolism, mediastinal emphysema, asthma, pleurisy, pneumonia, and pneumothorax. Pleuropulmonary disorders are discussed in detail in Chapter 7.
Past Medical History
A previous history of cancer of any kind, recent history of pulmonary infection, or recent accident or hospitalization may be significant. Look for other risk factors, such as age, smoking, prolonged immobility, immune system suppression (e.g., cancer chemotherapy, corticosteroids), and eating disorders (or malnutrition from some other cause).
Mechanical alterations related to the overload of respiratory muscles with chronic asthma can lead to chest pain from musculoskeletal dysfunction and alterations in muscle length and posture.17
Clinical Presentation
Pulmonary pain patterns differ slightly depending on the underlying pathology and the location of the disease. For example, tracheobronchial pain is referred to the anterior neck or chest at the same levels as the points of irritation in the air passages. Chest pain that tends to be sharply localized or that worsens with coughing, deep breathing, other respiratory movements or motion of the chest wall and that is relieved by maneuvers that limit the expansion of a particular part of the chest (e.g., autosplinting) is likely to be pleuritic in origin.
Symptoms that increase with deep breathing and activity or the presence of a productive cough with bloody or rust-colored sputum are red flags. The therapist should ask about new onset of wheezing at any time or difficulty breathing at night. Be careful when asking clients about changes in breathing patterns. It is not uncommon for the client to deny any shortness of breath.
Often, the reason for this is because the client has stopped doing anything that will bring on the symptoms. It may be necessary to ask what activities he or she can no longer do that were possible 6 weeks or 6 months ago. Symptoms that are relieved by sitting up are indicative of pulmonary impairment and must be evaluated more carefully.
Screening for Gastrointestinal Causes of Chest, Breast, or Rib Pain
GI causes of upper thorax pain are a result of epigastric or upper GI conditions. GERD (“heartburn” or esophagitis) accounts for a significant number of cases of noncardiac chest pain, in the young as well as older adults.3,18-20 Stomach acid or gastric juices from the stomach enter the esophagus, causing irritation to the protective lining of the lower esophagus. Whether the client is experiencing GERD or some other cause of chest pain, there is usually a telltale history or associated signs and symptoms to red flag the case.
Past Medical History
Watch for a history of alcoholism, cirrhosis, esophageal varices, and esophageal cancer or peptic ulcers. Any risk factors associated with these conditions are also red flags such as long-term use of NSAIDs as a cause of peptic ulcers or chronic alcohol use associated with cirrhosis of the liver.
Clinical Presentation
The GI system has a broad range of referred pain patterns based on embryologic development and multisegmental innervations, as discussed in Chapter 3. Upper GI and pancreatic problems are more likely than lower GI disease to cause chest pain. Chest pain referred from the upper GI tract can radiate from the chest posteriorly to the upper back or interscapular or subscapular regions from T10 to L2 (Fig. 17-1).
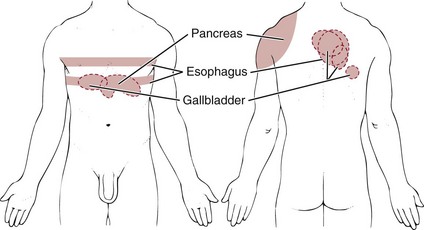
Fig. 17-1 Chest pain caused by gastrointestinal (GI) disease with referred pain to the shoulder and back. Upper GI problems can refer pain to the anterior chest with radiating pain to the thoracic spine at the same level. Look for accompanying GI symptoms and red-flag history.
Esophagus
Esophageal dysfunction will present with symptoms such as anterior neck and/or anterior chest pain, pain during swallowing (odynophagia), or difficulty swallowing (dysphagia) at the level of the lesion. Symptoms occur anywhere a lesion is present along the length of the esophagus. Early satiety, often with weight loss, is a common symptom with esophageal carcinoma.
Lesions of the upper esophagus may cause pain in the (anterior) neck, whereas lesions of the lower esophagus are more likely to be characterized by pain originating from the xiphoid process, radiating around the thorax to the middle of the back.
Chest pain with or without accompanying or alternating midthoracic back pain from an esophageal or other upper GI problem is usually red flagged by a suspicious history or cluster of associated signs and symptoms. The pain pattern associated with thoracic disk disease can be the same as for esophageal pathology. In the case of disk disease, there may be bowel and/or bladder changes and sometimes numbness and tingling in the upper extremities. The therapist should ask about a traumatic injury to the upper back region and conduct a neurologic screening examination to assess for this possibility as a cause of the symptoms.
Epigastric Pain
Epigastric pain is typically characterized by substernal or upper abdominal (just below the xiphoid process) discomfort (see Fig. 17-1). This may occur with radiation posteriorly to the back secondary to long-standing duodenal ulcers. Gastric duodenal peptic ulcer may occasionally cause pain in the lower chest rather than in the upper abdomen. Antacid and food often immediately relieve pain caused by an ulcer. Ulcer pain is not produced by effort and lasts longer than angina pectoris. The therapist will not be able to provoke or eliminate the client’s symptoms. Likewise, physical therapy intervention will not have any long-lasting effects unless the symptoms were caused by trigger points (TrPs).
Pain in the lower substernal area may arise as a result of reflux esophagitis (regurgitation of gastroduodenal secretions), a condition known as gastroesophageal reflux disease (GERD). It may be gripping, squeezing, or burning, described as “heartburn” or “indigestion.” Like that of angina pectoris, the discomfort of reflux esophagitis may be precipitated by recumbency or by meals; however, unlike angina, it is not precipitated by exercise and is relieved by antacids.
Hepatic and Pancreatic Systems
Epigastric pain or discomfort may occur in association with disorders of the liver, gallbladder, common bile duct, and pancreas, with referral of pain to the interscapular, subscapular, or middle/low back regions. This type of pain pattern can be mistaken for angina pectoris or myocardial infarction (e.g., hypotension occurring with pancreatitis produces a reduction of coronary blood flow with the production of angina pectoris).
Hepatic disorders may cause chest pain with radiation of pain to the shoulders and back. Cholecystitis (gallbladder inflammation) appears as discrete attacks of epigastric or right upper quadrant pain, usually associated with nausea, vomiting, and fever and chills. Dark urine and jaundice indicate that a stone has obstructed the common duct.
The pain has an abrupt onset, is either steady or intermittent, and is associated with tenderness to palpation in the right upper quadrant. The pain may be referred to the back and right scapular areas. A gallbladder problem can result in a sore tenth rib tip (right side anteriorly) as described in Chapter 9 (Case Example 17-4). Rarely, pain in the left upper quadrant and anterior chest can occur.
Acute pancreatitis causes pain in the upper part of the abdomen that radiates to the back (usually anywhere from T10 to L2) and may spread out over the lower chest. Fever and abdominal tenderness may develop.
Screening for Breast Conditions that Cause Chest or Breast Pain
Occasionally, a client may present with breast pain as the primary complaint, but most often the description is of shoulder or arm or neck or upper back pain. When asked if any symptoms occur elsewhere in the body, the client may mention breast pain (Case Example 17-5).
During examination of the upper quadrant, the therapist may observe suspicious or aberrant changes in the integument, breast, or surrounding soft tissues. The client may report discharge from the nipple. Discharge from both nipples is more likely to be from a benign condition; discharge from one nipple can be a sign of a precancerous or malignant condition.
Asking the client about history, risk factors, and the presence of other signs and symptoms is the next step (see Box 4-18). Knowing possible causes of breast pain can help guide the therapist during the screening interview (see Table 17-2).
Past Medical History
A past history of breast cancer, heart disease, recent birth, recent upper respiratory infection (URI), overuse, or trauma (including assault) may be significant for the client presenting with breast pain or symptoms. Any component of heart disease, such as hypertension, angina, myocardial infarction, and/or any heart procedure such as angioplasty, stent, or coronary artery bypass, is considered a red flag.
Any woman experiencing chest or breast pain should be asked about a personal history of previous breast surgeries, including mastectomy, breast reconstruction, or breast implantation or augmentation. A past history of breast cancer is a red flag even if the client has completed all treatment and has been cancer free for 5 years or more.
On the flip side, a past history of breast cancer in a client who presents with musculoskeletal symptoms with or without a history of trauma does not always mean cancer metastases. A complete evaluation with advanced imaging may be needed to uncover the true underlying etiology as in the reported case of fibular pain in a patient with a history of breast cancer that turned out to be an incomplete nondisplaced distal fibular stress fracture with no evidence of tumor or mass (Case Example 17-6).21
Breast cancer and cysts develop more frequently in individuals who have a family history of breast disease. A previous history of cancer is always cause to question the client further regarding the onset and pattern of current symptoms. This is especially true when a woman with a previous history of breast cancer or cancer of the reproductive system appears with shoulder, chest, hip, or sacroiliac pain of unknown cause.
If a client denies a previous history of cancer, the therapist should still ask whether that person has ever received chemotherapy or radiation therapy. It is surprising how often the answer to the question about a previous history of cancer is “no” but the answer to the question about prior treatment for cancer is “yes.”
Clinical Presentation
For the most part, breast pain (mastalgia), tenderness, and swelling are the result of monthly hormone fluctuations. Cyclical pain may get worse during perimenopause when hormone levels change erratically. These same symptoms may continue after menopause, especially in women who use hormone replacement therapy (HRT). Noncyclical breast pain is not linked to menstruation or hormonal fluctuations. It is unpredictable and may be constant or intermittent, affecting one or both breasts in a small area or the entire breast.
The typical referral pattern for breast pain is around the chest into the axilla, to the back at the level of the breast, and occasionally into the neck and posterior aspect of the shoulder girdle (Fig. 17-2). The pain may continue along the medial aspect of the ipsilateral arm to the fourth and fifth digits, mimicking pain of the ulnar nerve distribution.
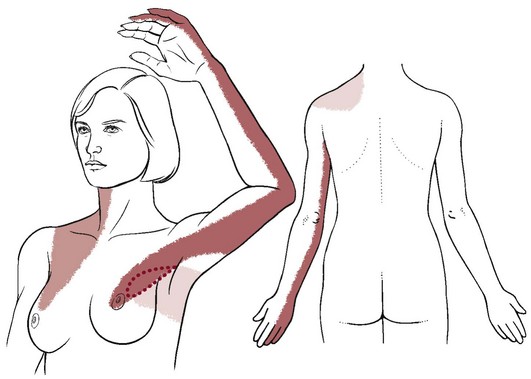
Fig. 17-2 Pain arising from the breast (mastalgia) can be referred into the axilla along the medial aspect of the arm. Referral pattern can also extend to the supraclavicular level and into the neck. Breast pain may be diffuse around the thorax through the intercostal nerves. Pain may be referred to the back and the posterior shoulder. Ask the client about the presence of lumps, nipple discharge, distended veins, or puckered or red skin (or any other skin changes).
Jarring or movement of the breasts and movement of the arms may aggravate this pain pattern. Pain in the upper inner arm may arise from outer quadrant breast tumors, but pain in the local chest wall may point to any pathologic condition of the breast.
Nipple discharge in women is common, especially in pregnant or lactating women, and does not always signal a serious underlying condition. It may occur as a result of some medications (e.g., estrogen-based drugs, tricyclic antidepressants, benzodiazepines, and others).
The fluid may be thin to thick in consistency and various colors (e.g., milky white, green, yellow, brown, or bloody). Any unusual nipple discharge should be evaluated by a medical doctor. Injury, hormonal imbalance, underactive thyroid, infection or abscess, or tumors are just a few possible causes of nipple discharge.
Causes of Breast Pain
There is a wide range of possible causes of breast pain, including both systemic or viscerogenic and NMS etiologies (Table 17-2). Not all conditions are life threatening or even require medical attention.
TABLE 17-2
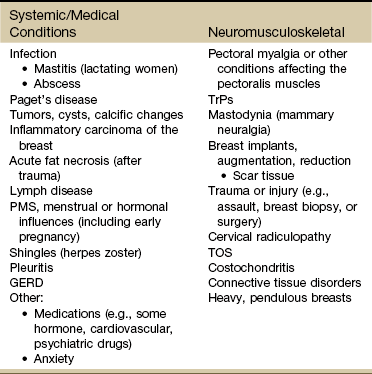
PMS, Premenstrual syndrome; GERD, gastroesophageal reflux disease; TrPs, trigger points; TOS, thoracic outlet syndrome.
Although it is more typical in women, both men and women can have chest, back, scapular, and shoulder pain referred by a pathologic condition of the breast. Only those conditions most likely to be seen in a physical therapist’s practice are included in this discussion.
Mastodynia
Mastodynia (irritation of the upper dorsal intercostal nerve) that causes chest pain is almost always associated with ovulatory cycles, especially premenstrually. The association between symptoms and menses may be discovered during the physical therapist’s interview when the client responds to Special Questions to Ask: Breast (see end of this chapter or Appendix B-7). The presentation is usually unilateral breast or chest pain and occurs initially at the premenstrual period and later more persistently throughout the menstrual cycle.
Mastitis
Mastitis is an inflammatory condition associated with lactation (breast feeding). Mammary duct obstruction causes the duct to become clogged. The breast becomes red, swollen, and painful. The involved breast area is often warm or even hot. Constitutional symptoms such as fever, chills, and flulike symptoms are common. Acute mastitis can occur in males (e.g., nipple chafing from jogging); the presentation is the same as for females.
Risk factors include previous history of mastitis; cracked, bleeding, painful nipples; and stress or fatigue. Bacteria can enter the breast through cracks in the nipple during trauma or nursing. Subsequent infection may lead to abscess formation. Obstructive and infectious mastitis are considered as two conditions on a continuum. Mastitis is often treated symptomatically, but the client should be encouraged to let her doctor know about any breast signs and symptoms present. Antibiotics may be needed in the case of a developing infection.
Benign Tumors and Cysts
Benign tumors and cysts were once lumped together and called “fibrocystic breast disease.” With additional research over the years, scientists have come to realize that a single label is not adequate for the variety of benign conditions possible, including fibroadenomas, cysts, and calcifications that can occur in the breast.
An unchanged lump of long duration (years) is more likely to be benign. Many lumps are hormonally induced cysts and resolve within two or three menstrual cycles. Cyclical breast cysts are less common after menopause.
Other conditions can include intraductal papillomas (wartlike growth inside the breast), fat necrosis (fat breaks down and clumps together), and mammary duct ectasia (ducts near the nipple become thin-walled and accumulate secretions). Some of these breast changes are a variation of the norm, and others are pathologic but nonmalignant. A medical diagnosis is needed to differentiate between these changes.
Paget’s Disease
Paget’s disease of the breast is a rare form of ductal carcinoma arising in the ducts near the nipple. The woman experiences itching, redness, and flaking of the nipple with occasional bleeding (Fig. 17-3). Paget’s disease of the breast is not related to Paget’s disease of the bone, except that the same physician (Dr. James Paget, a contemporary of Florence Nightingale, 1877) named both conditions after himself.
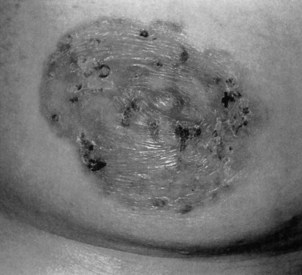
Fig. 17-3 Paget’s disease of the breast is a rare form of breast cancer affecting the nipple. It is characterized by a red (sometimes scaly) rash on the breast that often surrounds the nipple and areola, as seen in this photograph. Other presentations are possible such as a red pimple or sore on the nipple that does not heal. Symptoms are unilateral, and the breast may be sore, itch, or burn. Diagnosis is often delayed because the symptoms seem harmless or the condition is misdiagnosed as dermatitis. (From Callen JP, Jorizzo J, Greer KE, et al: Dermatological signs of internal disease, Philadelphia, 1988, Saunders.)
Breast Cancer
The breast is the second most common site of cancer in women (the skin is first). Cancer of the breast is second only to lung cancer as a cause of death from cancer among women. Male breast cancer is possible but rare, accounting for 1% of all new cases of breast cancer (2,140 cases in 2011 for men compared with 230,480 for women).22
Although the frequency of breast cancer in men is strikingly less than that in women, the disease in both sexes is remarkably similar in epidemiology, natural history, and response to treatment. Men with breast cancer are 5 to 10 years older than women at the time of diagnosis, with mean or median ages between 60 and 66 years. This apparent difference may occur because symptoms in men are ignored for a longer period and the disease is diagnosed at a more advanced state.
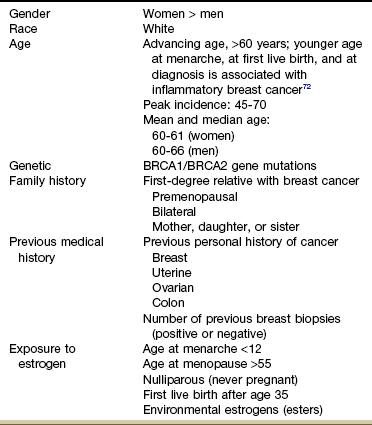
Risk Factors: Despite the discovery of a breast cancer gene (BRCA-1 and BRCA-2), researchers estimate that only 5% to 10% of breast cancers are a result of inherited genetic susceptibility. Normally, BRCA-1 and BRCA-2 help prevent cancer by making proteins that keep cells from growing abnormally. Inheriting either mutated gene from a parent does increase the risk of breast cancer.23 But a much larger proportion of cases are attributed to other factors, such as advancing age, race, smoking, obesity, physical inactivity, excess alcohol intake, exposure to ionizing radiation, and exposure to estrogens (Table 17-3).24
TABLE 17-3
Factors Associated With Breast Cancer
For a more detailed guide to risk factors for breast cancer, see the American Cancer Society’s document, What are the risk factors for breast cancer? Available at http://www.cancer.org/docroot/CRI/content/CRI_2_4_2x_what_are_the_risk_factors_for_breast_cancer_5.asp.
Women who received multiple fluoroscopies for tuberculosis or radiation treatment for mastitis during their adolescent or childbearing years are at increased risk for breast cancer as a result of exposure to ionizing radiation. In the past, irradiation was used for a variety of other medical conditions, including gynecomastia, thymic enlargement, eczema of the chest, chest burns, pulmonary tuberculosis, mediastinal lymphoma, and other cancers. Most of these clients are in their 70s now and at risk for cancer because of advancing age as well.
As a general principle, the risk of breast cancer is linked to a woman’s total lifelong exposure to estrogen. The increased incidence of estrogen-responsive tumors (tumors that are rich in estrogen receptors proliferate when exposed to estrogen) has been postulated to occur as a result of a variety of factors, such as prenatal and lifelong exposure to synthetic chemicals and environmental toxins, earlier age of menarche (first menstruation), improved nutrition in the United States, delayed and decreased childbearing, and longer average lifespan.
At the same time, it should be remembered that many women diagnosed with breast cancer have no identified risk factors. More than 70% of breast cancer cases are not explained by established risk factors.23,25 There is no history of breast cancer among female relatives in more than 90% of clients with breast cancer. However, first-degree relatives (mother, daughters, or sisters) of women with breast cancer have two to three times the risk of developing breast cancer than the general female population, and relatives of women with bilateral breast cancer have five times the normal risk.23
Risk factors for men are similar to those for women, but at least half of all cases do not have an identifiable risk factor. Risk factors for men include heredity, obesity, infertility, late onset of puberty, frequent chest x-ray examinations, history of testicular disorders (e.g., infection, injury, or undescended testes), and increasing age. Men who have several female relatives with breast cancer and those in families who have the BRCA-2 mutation have a greater risk potential.
The presence of any of these factors may become evident during the interview with the client and should alert the physical therapist to the potential for neuromusculoskeletal complaints from a systemic origin that would require a medical referral. There are several easy-to-use screening tools available. In addition to screening for current risk, clients should be given this information for future use (Box 17-2).
Clinical Presentation: Breast cancer may be asymptomatic in the early stages. The discovery of a breast lump with or without pain or tenderness is significant and must be investigated. Physical signs associated with advanced breast cancer have been summarized using the acronym BREAST: Breast mass, Retraction, Edema, Axillary mass, Scaly nipple, and Tender breast.26 Less common symptoms are breast pain; nipple discharge; nipple erosion, enlargement, itching, or redness; and generalized hardness, enlargement, or shrinking of the breast. Watery, serous, or bloody discharge from the nipple is an occasional early sign but is more often associated with benign disease.
Breast cancer usually consists of a nontender, firm, or hard lump with poorly delineated margins that is caused by local infiltration. Breast cancer in women has a predilection for the outer upper quadrant of the breast and the areola (nipple) area (Fig. 17-4) involving the breast tissue overlying the pectoral muscle. During palpation, breast tissue lumps move easily over the pectoral muscle, compared with a lump within the muscle tissue itself. Later signs of malignancy include fixation of the tumor to the skin or underlying muscle fascia.
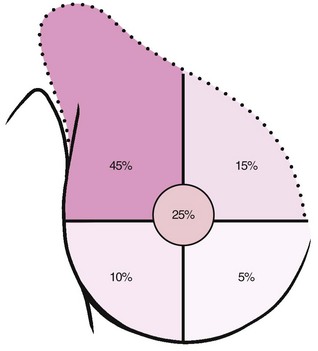
Fig. 17-4 Most breast cancer presents in the upper outer quadrant of the breast (45%) or around the nipple (25%). Metastases occur via the lymphatic system at the axillary lymph nodes to the bones (shoulder, hip, ribs, vertebrae) or central nervous system (brain, spinal cord). Breast cancer can also metastasize hematogenously to the lungs, pleural cavity, and liver.
Male breast cancer begins as a painless induration, retraction of the nipple, and an attached mass progressing to include lymphadenopathy and skin and chest wall lesions. A tumor of any size in male breast tissue is associated with skin fixation and ulceration and deep pectoral fixation more often than a tumor of similar size in female breast tissue is because of the small size of male breasts.
Clinical Breast Examination: Breast cancer mortality is reduced when women are screened by both clinical breast examination (CBE) and mammography. CBE alone detects 3% to 45% of diagnosed breast cancers that screening mammography misses. Studies show the sensitivity of CBE is 54% (test’s ability to determine a true positive) and specificity is 94% (test’s ability to determine a true negative).27 However, the rate of false-positive tests may be higher when CBE is performed without mammography.28
A previous edition of this text (Differential Diagnosis in Physical Therapy, edition 3) specifically stated, “breast examination is not within the scope of a physical therapist’s practice.” This practice is changing. As the number of cancer survivors increases in the United States, physical therapists treating postmastectomy women and clients of both genders with lymphedema are on the rise.
With direct and unrestricted access of consumers to physical therapists in many states, advanced skills have become necessary. For some clients, performing a CBE is an appropriate assessment tool in the screening process.29 The American Cancer Society (ACS) and National Cancer Institute (NCI) support the provision of cancer screening procedures by qualified health specialists. With additional training, physical therapists can qualify.29,30 Guidelines for CBE are provided in Appendix D-7.
Therapists who are trained to perform CBEs must make sure this examination is allowed according to the state practice act. In some states, it is allowed by exclusion, meaning it is not mentioned and therefore included. Discussion of the role of the physical therapist in primary care and cancer screening as it relates to integrating CBE into an upper quarter examination is available.29 A form for recording findings from the CBE is provided in Fig. 4-48 and Appendix C-10.
The physical therapist does not diagnose any kind of cancer, including breast cancer; only the pathologist diagnoses cancer. The therapist can identify aberrant soft tissue and refer the client for further evaluation. Early detection and intervention can reduce morbidity and mortality.
For the therapist who is not trained in CBE, the client should be questioned about the presence of any changes in breast tissue (e.g., lumps, distended veins, skin rash, open sores or lesions, or other skin changes) and nipple (e.g., rash or other skin changes, discharge, distortion). Visual inspection is also possible and may be very important postmastectomy (Fig. 17-5). Ask the client if he or she has noticed any changes in the scar. Continue by asking:
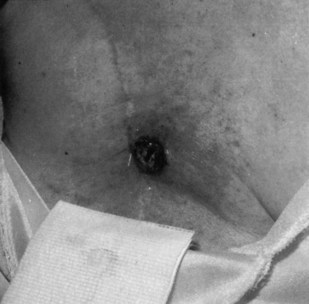
Fig. 17-5 This photo shows the chest of a woman who has had a right radical mastectomy. There is a metastatic nodule in the mastectomy scar as a result of local cancer recurrence. Breast cancer can occur (recur) if a mastectomy has been done. A closer look at the lesion suggests that the skin changes have been present for quite some time. Even in this black and white photo, the change in skin coloration is obvious in a large patch around the nodule. Anytime a woman with a past medical history of cancer develops neck, back, upper trapezius, or shoulder pain, or other symptoms, examining the site of the original cancer removal is a good idea. (From Callen JP, Jorizzo J, Greer KE, et al: Dermatological signs of internal disease, Philadelphia, 1988, Saunders.)
If the client declines or refuses, the therapist should follow up with counsel to perform self-inspection, emphasizing the need for continued CBEs and the importance of reporting any changes to the physician immediately.
Therapists have an important role in primary prevention and client education. The ACS offers recommendations for breast cancer screening. The therapist can encourage women (and men) to follow these guidelines (Box 17-3).
Lymph Node Assessment: Palpation of the underlying soft tissues (chest wall, axilla) and lymph nodes in the supraclavicular and axillary regions should be part of a screening exam in any client with chest pain (see Chapter 4 for description of lymph node palpation). Any report of palpable breast nodules, lumps, or changes in the appearance of the breast requires medical follow-up, especially when there is a personal or family history of breast disease.31
“Normal” lymph nodes are not palpable or visible, but not all palpable or visible lymph nodes are a sign of cancer. Infections, viruses, bacteria, allergies, and food intolerances can cause changes in the lymph nodes. Lymph nodes that are hard, immovable, irregular, and nontender raise the suspicion of cancer, especially in the presence of a previous history of cancer. The skin surface over a tumor may be red, warm, edematous, firm, and painful. There may be skin dimpling over the lesion, with attachment of the mass to surrounding tissues preventing normal mobilization of skin, fascia, and muscle.
In the past, therapists were taught that any changes in lymph nodes present for more than 1 month in more than one region were a red flag. This has changed with the increased understanding of cancer metastases via the lymphatic system and the potential for cancer recurrence. A physician must evaluate all suspicious lymph nodes.
Metastases: Metastases have been known to occur up to 25 years after the initial diagnosis of breast cancer. On the other hand, breast cancer can be a rapidly progressing, terminal disease. Approximately 40% of clients with stage II tumors experience relapse.
Knowledge of the usual metastatic patterns of breast cancer and the common complications can aid in early recognition and effective treatment. Because bone is the most frequent site of metastases from breast cancer in men and women, a past medical history of breast cancer is a major red flag in anyone presenting with new onset or persistent findings of NMS pain or dysfunction.
All distant visceral sites are potential sites of metastases. Other primary sites of involvement are lymph nodes, remaining breast tissue, lung, brain, central nervous system (CNS), and liver. Women with metastases to the liver or CNS have a poorer prognosis.
Spinal cord compression, usually from extradural metastases, may appear as back pain, leg weakness, and bowel/bladder symptoms. Rarely, an axillary mass, swelling of the arm, or bone pain from metastases may be the first symptom. Back or bone pain, jaundice, or weight loss may be the result of systemic metastases, but these symptoms are rarely seen on initial presentation.
Medical referral is advised before initiating treatment for anyone with a past history of cancer presenting with symptoms of unknown cause, especially without an identifiable movement system impairment.
A medical evaluation is still needed in light of new findings even if the client has been rechecked by a medical oncologist recently. It is better to err on the side of caution. Failure to recognize the need for medical referral can result in possible severe and irreversible consequences of any delay in diagnosis and therapy.32
Screening for Other Conditions as a Cause of Chest, Breast, or Rib Pain
Scar tissue or fibrosis from a previous breast surgery, such as reconstruction following mastectomy for breast cancer or augmentation or reduction mammoplasty for cosmetic reasons, is an important history to consider when assessing chest, breast, neck, or shoulder symptoms. Likewise, the client should be asked about a history of radiation to the chest, breast, or thorax.
Women who have silicone or saline implants for reconstruction following mastectomy for breast cancer are more likely to have complications including late complications33 (e.g., pain, capsular contracture, rupture, rippling, infection, hematoma, seroma) than those who receive implants for cosmetic reasons only.34,35 The rate of fibrosis and capsular contracture is significantly higher for irradiated breasts than for nonirradiated breasts.36,37
Studies show that ruptures are rare (0.4 %) in women who have breast implants after mastectomy; thick, tight scarring, implant malposition, and infection are more common.38,39
Other complications of breast implantation may include gel bleed, implant leaking, calcifications around the implant, chronic breast pain, prolonged wound healing, and formation of granulation tissue.
Anxiety
An anxiety state, or in its extreme form, panic attack, can cause chest or breast pain typical of a heart attack. The client experiences shortness of breath, perspiration, and pallor. It is the most common noncardiac cause of chest pain, accounting for half of all emergency department admissions each year for chest or breast pain (just ahead of chest pain caused by cocaine use).
Risk Factors
The first panic attack often follows a period of extreme stress, sometimes associated with being the victim of a crime or the loss of a job, partner, or close family member. The presence of another mental health disorder, such as depression or substance abuse (drugs or alcohol), increases the risk of developing panic disorder. There may be a familial component, but it is not clear if this is hereditary or environmental (learned behavior).40,41
Drugs such as over-the-counter (OTC) decongestants and cold remedies can trigger panic attacks. Excessive use of caffeine and stimulants, such as amphetamines and cocaine combined with a lack of sleep, can also trigger an attack. Menopause, quitting smoking, or caffeine withdrawal can also bring on new onset of panic attacks in someone who has never experienced this problem before. See Chapter 3 for further discussion.
Clinical Presentation
There are several types of chest or breast discomfort caused by anxiety. The pain may be sharp, intermittent, or stabbing and located in the region of the left breast. The area of pain is usually no larger than the tip of the finger but may be as large as the client’s hand. It is often associated with a local area of hyperesthesia of the chest wall. The client can point to it with one finger. It is not reproduced with palpation or activity. It is not changed or altered by a change in position.
Anxiety-related pain may be located precordially (region over the heart and lower part of the thorax) or retrosternally (behind the sternum). It may be of variable duration, lasting no longer than a second or for hours or days. This type of pain is unrelated to effort or exercise. Distinguishing this sensation from myocardial ischemia requires medical evaluation.
Discomfort in the upper portion of the chest, neck, and left arm, again unrelated to effort, may occur. There may be a sense of persistent weakness and unpleasant awareness of the heartbeat. In the past, radiation of chest discomfort to the neck or left arm was considered to be diagnostic of atherosclerotic coronary heart disease. More recently, stress testing and coronary arteriography have shown that chest discomfort of this type can occur in clients with normal coronary arteriograms.
Some individuals with anxiety-related chest pain may have a choking sensation in the throat caused by hysteria. There may be associated hyperventilation. Palpitation, claustrophobia, and occurrence of symptoms in crowded places are common.
Hyperventilation occurs in persons with and without heart disease and may be misleading. Such clients have numbness and tingling of the hands and lips and feel as if they are going to “pass out.” For a more detailed explanation of anxiety and its accompanying symptoms (e.g., hyperventilation), see Chapter 3.
Cocaine
Cocaine (also methamphetamine, known as crank, and phencyclidine [PCP]) is a stimulant that has profound effects on all organs systems of the body, especially cardiotoxic effects, including cocaine-dilated cardiomyopathy, angina, and left ventricular dysfunction. Injection or inhalation can precipitate MI, cardiac arrhythmias, and even sudden cardiac death.42-45
Chronic use of cocaine or any of its derivatives is the number-one cause of stroke in young people today. The incidence of stroke associated with substance use and abuse is increasing. Use of these stimulants also has an effect on anyone with a congenital cerebral aneurysm and can lead to rupture.
The physiologic stress of cocaine use on the heart accounts for an increasing number of heart transplants. Acute effects of cocaine include increased heart rate, blood pressure, and vasomotor tone.44,46 Cocaine remains the most common illicit drug–related cause of severe chest pain bringing the person to the emergency department.42,45 In fact, chest pain is the most common cocaine-related medical complaint.
Many people with chest pain have used cocaine within the last week but deny its use. The use of these substances is not uncommon in middle-aged and older adults of all socioeconomic backgrounds. The therapist should not neglect to ask clients about their use of substances because of preconceived ideas that only teenagers and young adults use drugs. Careful questioning (see Chapter 2; see also Appendix B-36) may assist the physical therapist in identifying a possible correlation between chest pain and cocaine use.
Anabolic-Androgenic Steroids
Anabolic steroids are synthetic derivatives of testosterone used to enhance athletic performance or cosmetically shape the body. Used in supraphysiologic doses (more than the body produces naturally), these drugs have a potent effect on the musculoskeletal system, including the heart, potentially altering cardiac cellular and physiologic function.47,48 Effects persist long after their use has been discontinued.49
The use of self-administered anabolic-androgenic steroids (AASs) is illegal but continues to increase dramatically among both athletes and nonathletes.48 It is used among preteens who do not compete in sports for cosmetic reasons. The goal is to advance to a more mature body build and enhance their looks. AASs do have medical uses and were added to prescribed controlled substances in 1990 under the control of the Drug Enforcement Administration.
In spite of stricter control of the manufacture and distribution of AASs, illegal supplies come from unlicensed sources all over the world. When dispensed without a regulating agency, the purity and processing of chemicals is unknown. The quality of black market supplies is a major concern. There is no guarantee that the products obtained are correctly labeled. Contents and dosage may be inaccurate. Some athletes are using injectable anabolic steroids intended for veterinary use only. There is a trend for self-administration of higher doses and for combining AAS with other potentially harmful drugs.50
Clinical Presentation
Any young adult with chest pain of unknown cause, possibly accompanied by dyspnea and elevated blood pressure and without clinical evidence of NMS involvement, may have a history of anabolic steroid use. Consider anabolic steroid use as a possibility in men and women presenting with chest pain in their early 20s who have used this type of steroid since age 11 or 12.
In the pediatric population, there is a risk of decreased or delayed bone growth. Tendon and muscle strains are common and take longer than normal to heal. Injuries that take longer than the expected physiologic time to heal are an important red flag. Delayed healing occurs because the soft tissues are working under the added strain of extra body mass.
The alert therapist may recognize the associated signs and symptoms accompanying chronic use of these steroids. Changes in personality are the most dramatic signs of steroid use. The user may become more aggressive or experience mood swings (hypomanic or manic symptoms) and psychologic delusions (e.g., believe he or she is indestructible; sometimes referred to as “steroid psychosis”). “Roid rages,” characterized by sudden outbursts of uncontrolled emotion, may be observed. Severe depression leading to suicide can occur with AAS withdrawal.48
The therapist who suspects a client may be using anabolic steroids should report findings to the physician or coach if one is involved. The therapist can begin by asking about the use of nutritional supplements or performance-enhancing agents. In the well-muscled male athlete, observe for common side effects of AAS such as acne, gynecomastia, and cutaneous striae in the deltopectoral region. Women who use AAS may exhibit muscular hypertrophy; male pattern baldness; excess hair growth on the face, breasts, and arms; and breast tissue atrophy.47 Asking about the presence of common side effects of AAS and testing for elevated blood pressure may provide an opportunity to ask if the client is using these chemicals.
Screening for Musculoskeletal Causes of Chest, Breast, or Rib Pain
It is estimated that half of all chest pain is of noncardiac nature and 20% to 25% of noncardiac chest pain has a musculoskeletal basis.51,52 Musculoskeletal causes of chest (wall) pain must be differentiated from pain of cardiac, pulmonary, epigastric, and breast origin (see Table 17-1) before physical therapy treatment begins. Careful history taking to identify red flag conditions differentiates those who require further investigation.
Movement system impairment is most often characterized by pain during specific postures, motion, or physical activities. Reproducing the pain by movement or palpation often directs the therapist in understanding the underlying problem.
Chest pain can occur as a result of cervical spine disorders because nerves originating as high as C3 and C4 can extend as far down as the nipple line. Pectoral, suprascapular, dorsal scapular, and long thoracic nerves originate in the lower cervical spine, and impingement of these nerves can cause chest pain.
Musculoskeletal disorders such as myalgia associated with muscle exertion, myofascial TrPs, costochondritis, osteomyelitis, or xiphoiditis can produce pain in the chest and arms. Compared with angina pectoris, the pain associated with these conditions may last for seconds or hours, and prompt relief does not occur with the ingestion of nitroglycerin.
Tietze’s syndrome, costochondritis, a hypersensitive xiphoid, and the slipping rib syndrome must be differentiated from problems involving the thoracic viscera, particularly those of the heart, great vessels, and mediastinum, as well as from illness originating in the head, neck, or abdomen.53
Rib pain (with or without neck, back, or chest pain or symptoms) must be evaluated for systemic versus musculoskeletal origins (Box 17-4). The same screening model used for all conditions can be applied.
Costochondritis
Costochondritis, also known as anterior chest wall syndrome, costosternal syndrome, and parasternal chondrodynia (pain in a cartilage), is used interchangeably with Tietze’s syndrome, although these two conditions are not the same. Costochondritis is more common than Tietze’s syndrome.
Although both disorders are characterized by inflammation of one or more costal cartilages (costochondral joints where the ribs join the sternum), costochondritis refers to pain in the costochondral articulations without swelling. This disorder can occur at almost any age but is observed most often in people older than 40. It tends to affect the second, third, fourth, and fifth costochondral joints; women are affected in 70% of all cases (Fig. 17-6). Other risk factors include trauma (e.g., driver striking steering wheel with chest during a motor vehicle accident, upper chest surgery, helmet tackle in sports, or other sports injury to the chest)54 or repetitive motion (e.g., grocery-store clerk lifting and scanning items, competitive swimming).55
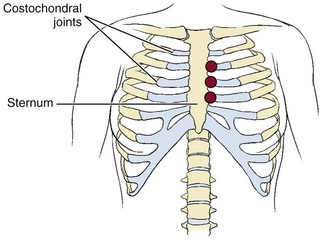
Fig. 17-6 Costochondritis is an inflammation of any of the costochondral joints (also called costal cartilages) where the rib joins the sternum. Sharp, stabbing, or aching pain can occur on either side of the sternum but tends to affect the left more often, even radiating down the left arm sometimes or to upper back. Many people mistake the symptoms for a heart attack. In most cases, symptoms occur at a single site involving the second or third costochondral joint, although any of the joints can be affected as shown.
Costochondritis is characterized by a sharp pain along the front edges of the sternum, especially on the left side with possible radiation to the arms, back, or shoulders, often misinterpreted as a heart attack.
In fact, it is estimated that one-third of all people who present at the emergency department with chest pain have costochondritis.56 The pain may radiate widely (to the arms, back, shoulders), stimulating intrathoracic or intraabdominal disease. It differs from a myocardial infarction because during a heart attack, the initial pain is usually in the center of the chest, under the sternum, not along the edges.
Costochondritis can be similar to muscular pain and (unlike cardiac-related pain) is elicited by palpatory pressure over the costochondral junctions. Occasionally, the affected individual will report a burning sensation in the breast(s) associated with this condition. Absence of associated signs and symptoms such as dyspnea, nausea, vomiting, and diaphoresis helps differentiate this condition from cardiac- or pulmonary-related chest pain.
Costochondritis may follow trauma or may be associated with systemic rheumatic disease. It can come and go (especially in conjunction with activities involving the upper extremities or rest) or persist for months. Inflammation of upper costal cartilages may cause chest pain, whereas inflammation of lower costal cartilages is more likely to cause abdominal or low back discomfort. In some cases, costochondritis is associated with an URI but may be a result of the stress of coughing rather than the body’s response to the virus.
Tietze’s Syndrome
Tietze’s syndrome (inflammation of a rib and its cartilage; costal chondritis) may be one possible cause of anterior chest wall pain, manifested by painful swelling of one or more costochondral articulations.
In most cases, the cause of Tietze’s syndrome is unknown. Other causes of sternal swelling may include an infectious process in the immunocompromised person resulting from tuberculosis, aspergillosis, brucellosis, staphylococcal infection, or pseudomonal disease producing sternal osteomyelitis. Onset is usually before 40 years of age, with a predilection for the second and third decades. However, it can occur in children.
Approximately 80% of clients have only single sites of involvement, most commonly the second or third costal cartilage (costochondral joint). Anterior chest pain may begin suddenly or gradually and may be associated with increased blood pressure, increased heart rate, and pain radiating down the left arm. Pain is aggravated by sneezing, coughing, deep inspirations, twisting motions of the trunk, horizontal shoulder abduction and adduction, or the “crowing rooster” movement of the upper extremities.
These symptoms may seem similar to those of a heart attack, but the raised blood pressure, reproduction of painful symptoms with palpation or pressure, and aggravating factors differentiate Tietze’s syndrome from myocardial infarction (Case Example 17-7). In rare cases, the individual has been diagnosed with Tietze’s syndrome only to find out later the precipitating cause was cancer (e.g., lymphoma, squamous cell carcinoma of the mediastinum).57,58 Tietze’s syndrome can also be confused with TrPs (pectoralis major, internal intercostalis), an often overlooked cause of the same symptoms.59
Hypersensitive Xiphoid
The hypersensitive xiphoid (xiphodynia) is tender to palpation, and local pressure may cause nausea and vomiting. This syndrome is manifested as epigastric pain, nausea, and vomiting.
Slipping Rib Syndrome
The slipping, or painful, rib syndrome (sometimes also referred to as the clicking rib syndrome) can present as chest pain and occurs most often when there is hypermobility of the lower ribs.53 In this condition, inadequacy or rupture of the interchondral fibrous attachments of the anterior ribs allows the costal cartilage tips to sublux, impinging on the intercostal nerves. This condition can occur alone or can be associated with a broader phenomenon such as myofascial pain syndrome.60
Rib syndrome can occur at any age, including during childhood61 but most commonly occurs during the middle-aged years. The physical therapist is usually able to identify readily a rib syndrome as the cause of chest pain after a careful musculoskeletal examination. In some cases, persistent upper abdominal and/or low thoracic pain occurs, leaving physicians, chiropractors, and therapists puzzled.62,63 A sonogram may be needed to make the diagnosis. Pain is made worse by slump sitting or side bending to the affected side. Reduction or elimination of symptoms following rib mobilization helps confirm the differential diagnosis.
Gallbladder impairment can also cause tenderness or soreness of the tip of the tenth rib on the right side. The affected individual may or may not have gallbladder symptoms. Because visceral and cutaneous fibers enter the spinal cord at the same level for the ribs and gallbladder, the nervous system may respond to the afferent input with sudomotor changes such as pruritus (itching of the skin) or a sore rib instead of gallbladder symptoms.
The clinical presentation appears as a biomechanical problem, such as a rib dysfunction, instead of nausea and food intolerances normally associated with gallbladder dysfunction. Symptoms will not be alleviated by physical therapy intervention, eventually sending the client back to his or her physician.
Trigger Points
The most common musculoskeletal cause of chest pain is TrPs, sometimes referred to as myofascial TrPs (MTrPs). TrPs (hypersensitive spots in the skeletal musculature or fascia) involving a variety of muscles (Table 17-4) may produce precordial pain (Fig. 17-7). Abdominal muscles have multiple referred pain patterns that may reach up into the chest or midback and produce heartburn or deep epigastric pain. Although these patterns strongly mimic cardiac pain, myofascial TrP pain shows a much wider variation in its response to daily activity than does angina pectoris to activity.59
TABLE 17-4
Modified from Travell JG, Simons DG: Myofascial pain and dysfunction: the trigger point manual, Baltimore, 1983, Williams & Wilkins, p 574.
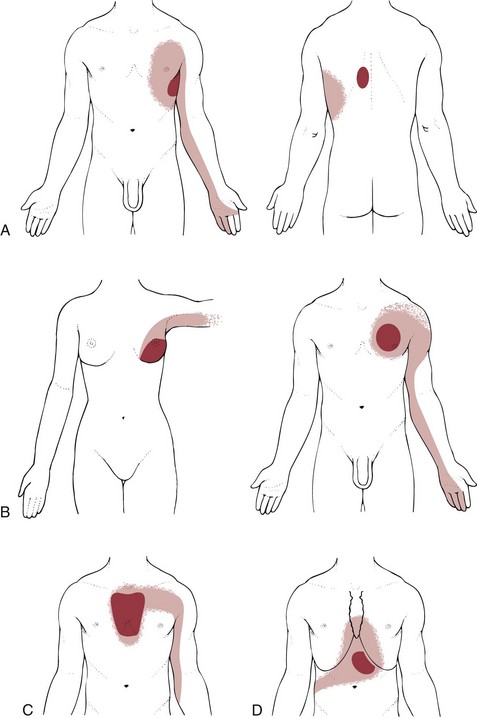
Fig. 17-7 A, Referred pain pattern from the left serratus anterior muscle. B, Left pectoralis major muscle: referred pain pattern in a woman and a man. C, Referred pain pattern from the left sternalis muscle. D, Referred pain from the external oblique abdominal muscle can cause “heartburn” in the anterior chest wall. Marathon runners may report chest pain mimicking a heart attack from this trigger point.
In addition to mimicking pain of a cardiac nature, TrPs can occur in response to cardiac disorders. A viscero-somatic response can occur when biochemical changes associated with visceral disease affect somatic structures innervated by the same spinal nerves. In such cases, the individual has a past history of visceral disease. TrPs accompanied by symptoms, such as vertigo, headache, visual changes, nausea, and syncope, are yellow flags warning of autonomic involvement not usually present with TrPs strictly from a somatic origin.
Chest pain that persists long after an acute MI may be due to myofascial TrPs. In acute MI, pain is commonly referred from the heart to the midregion of the pectoralis major and minor muscles (see discussion of viscero-somatic sources of pain in Chapter 3). The injury to the heart muscle initiates a viscero-somatic process that activates TrPs in the pectoral muscles.59
After recovery from the infarction, these self-perpetuating TrPs tend to persist in the chest wall. As with all myofascial syndromes, inactivation of the TrPs eliminates the client’s symptoms of chest pain. If the client’s symptoms are eliminated with TrP release, medical referral may not be required. However, communication with the physician is essential; the therapist is advised to document all findings and report them to the client’s primary care physician.
Past Medical History
There may be a history of URI with repeated forceful coughing. There is often a history of immobility (e.g., cast immobilization after fracture or injury). The therapist should also ask about muscle strain from lifting weights overhead, from pushups, and from prolonged, vigorous activity that requires forceful abdominal breathing, such as severe coughing, running a marathon, or repetitive bending and lifting.
Clinical Presentation
TrPs are reproduced with palpation or resisted motions. On examination, the physical therapist should palpate for tender points and taut bands of muscle tissue, squeeze the involved muscle, observe for increased pain with palpation, test for increased pain with resisted motion, and correlate symptoms with respiratory movements.
Chest pain from serratus anterior TrPs may occur at rest in severe cases. Clients with this myofascial syndrome may report that they are “short of breath” or that they are in pain when they take a deep breath. Serratus anterior TrPs on the left side of the chest can contribute to the pain associated with myocardial infarction. This pain is rarely aggravated by the usual tests for range of motion at the shoulder but may result from a strong effort to protract the scapula. Palpation reveals tender points that increase symptoms, and there is usually a palpable taut band present within the involved muscles.
One of the most extensive patterns of pain from irritable TrPs is the complex pattern from the anterior scalene muscle. This may produce ipsilateral sternal pain, anterior chest wall pain, breast pain, or pain along the vertebral border of the scapula, shoulder, and arm, radiating to the thumb and index finger.
Breast pain may be differentiated from the aching pain arising from the scalene or pectoral muscles by a history of upper extremity overuse usually associated with myalgia. Resistance to isometric movement of the upper extremities reproduces the symptoms of a myalgia but does not usually aggravate pain associated with breast tissue. Additionally, palpation of the underlying muscle reproduces the painful symptoms.
When active TrPs occur in the left pectoralis major muscle, the referred pain (anterior chest to the precordium and down the inner aspect of the arm) is easily confused with that of coronary insufficiency. Pacemakers placed superficially can cause pectoral trigger points. In the case of pacemaker-induced TrPs, the physical therapist can teach the client TrP self-treatment to carry out at home.
Myalgia
Myalgia, or muscular pain, can cause chest pain separate from TrP pain but with a similar etiologic basis of prolonged or repeated movement. As mentioned earlier, the physical therapy interview must include questions about recent URI with repeated forceful coughing and recent activities of a repetitive nature that could cause sore muscles (e.g., painting or washing walls; calisthenics, including pushups; or lifting heavy objects or weights).
Three tests must be used to confirm or rule out muscle as the source of symptoms: (1) palpation, (2) stretch, and (3) contraction. If the muscle is not sore or tender on palpation, stretch, or contraction, the source of the problem most likely lies somewhere else.
With true myalgia, squeezing the muscle belly will reproduce painful chest symptoms. The discomfort of myalgia is almost always described as aching and may range from mild to intense. Diaphragmatic irritation may be referred to the ipsilateral neck and shoulder, lower thorax, lumbar region, or upper abdomen as a muscular aching pain. Myalgia in the respiratory muscles is well localized, reproducible by palpation, and exacerbated by movement of the chest wall.
Rib Fractures
Periosteal (bone) pain associated with fractured ribs can cause sharp, localized pain at the level of the fracture with an increase in symptoms associated with trunk motions and respiratory movements, such as deep inspiration, laughing, sneezing, or coughing. The pain may be accompanied by a grating sensation during breathing. This localized pain pattern differs from bone pain associated with chronic disease affecting bone marrow and endosteum, which may result in poorly localized pain of varying degrees of severity.
Occult (hidden) rib fractures may occur, especially in a client with a chronic cough or someone who has had an explosive sneeze. Fractures may occur as a result of trauma (e.g., motor vehicle accident, assault), but painful symptoms may not be perceived at first if other injuries are more significant.
A history of long-term steroid use in the presence of rib pain of unknown cause should raise a red flag. Rib fractures must be confirmed by x-ray diagnosis. Rib pain without fracture may indicate bone tumor or disease affecting bone, such as multiple myeloma.
Cervical Spine Disorders
Cervicodorsal arthritis may produce chest pain that is seldom similar to that of angina pectoris. It is usually sharp and piercing but may be described as a deep, boring, dull discomfort. There is usually unilateral or bilateral chest pain with flexion or hyperextension of the upper spine or neck. The chest pain may radiate to the shoulder girdle and down the arms and is not related to exertion or exercise. Rest may not alleviate the symptoms, and prolonged recumbency makes the pain worse.
Diskogenic disease can also cause referred pain to the chest, but there is usually evidence of disk involvement observed with diagnostic imaging and the presence of neurologic symptoms (Case Example 17-8).
Screening for Neuromuscular or Neurologic Causes of Chest, Breast, or Rib Pain
There are several possible neurologic disorders that can cause chest and/or breast pain, including nerve root impingement or inflammation, herpes zoster (shingles), thoracic disk disease, postoperative neuralgia, and thoracic outlet syndrome (TOS; see Table 17-1).
Neurologic disorders such as intercostal neuritis and dorsal nerve root radiculitis or a neurovascular disorder such as thoracic outlet syndrome also can cause chest pain. The two most commonly recognized noncardiac causes of chest pain seen in the physical therapy clinic are herpes zoster (shingles) and TOS.
Intercostal Neuritis
Intercostal neuritis, such as herpes zoster or shingles produced by a viral infection of a dorsal nerve root, can cause neuritic chest wall pain, which can be differentiated from coronary pain.
Risk Factors
Shingles may occur or recur at any age, but there has been a recent increase in the number of cases in two distinct age groups: college-aged young adults and older adults (over 70 years). Health care experts suggest that stress is the key factor in the first group, and immune system failure is the key factor in the second group.
Anyone who is immunocompromised as a result of advancing age, underlying malignancy, organ transplantation, or acquired immunodeficiency syndrome (AIDS) is at risk for shingles. There is an increased incidence of herpes zoster in clients with lymphoma, tuberculosis, and leukemia, but it can be triggered by trauma or injection drugs or occur with no known cause.
Anyone in good health who had the chickenpox as a child is not at great risk for shingles. The risk of developing shingles increases for anyone who is immunocompromised for any reason or who has never had chickenpox.
Herpes zoster is a communicable disease and requires some type of isolation. Anyone in contact with the client before the outbreak of the skin lesions has already been exposed. Specific precautions depend on whether the disease is localized or disseminated and the condition of the client. Persons susceptible to chickenpox should avoid contact with the affected client and stay out of the client’s room.
Clinical Presentation
Herpes zoster is characterized by raised fluid-filled clusters of grouped vesicles that appear unilaterally along cranial or spinal nerve dermatomes 3 to 5 days after transmission of the virus (see Figs. 4-23 and 4-24). The affected individual experiences 1 to 2 days of pain, itching, and hyperesthesia before the outbreak of skin lesions.
The skin changes are referred to as “shingles” and are easily recognizable as they follow a dermatome anywhere on the body. The lesions do not cross the body midline as they follow nerve pathways, although nerves of both sides may be involved. The skin eruptions evolve into crusts on the skin and clear in about 2 weeks, unless the period between the pain and the eruption is longer than 2 days. Postherpetic neuralgia, with its burning and paroxysmal stabbing pain, may persist for long periods.
Neuritic pain occurs unrelated to effort and lasts longer (weeks, months, or years) than angina. The pain may be constant or intermittent and can vary from light burning to a deep visceral sensation. It may be associated with chills, fever, headache, and malaise. Symptoms are confined to the somatic distribution of the involved spinal nerve(s).
Dorsal Nerve Root Irritation
Dorsal nerve root irritation of the thoracic spine is another neuritic condition that can refer pain to the chest wall. This condition can be caused by infectious processes (e.g., radiculitis or inflammation of the spinal nerve root dural sheath; shingles can also fit in this category). However, the pain is more likely to be the result of mechanical irritation caused by spinal disease or deformity (e.g., bone spurs secondary to osteoarthritis or the presence of cervical ribs placing pressure on the brachial plexus).
The pain of dorsal nerve root irritation can appear as lateral or anterior chest wall pain with referral to one or both arms through the brachial plexus. Although it mimics the pain pattern of coronary heart disease, such pain is more superficial than cardiac pain. Like cardiac pain, dorsal nerve root irritation can be aggravated by exertion of only the upper extremities. However, unlike cardiac pain, exertion of the lower extremities has no exacerbating effect. It is usually accompanied by other neurologic signs such as muscle atrophy and numbness or tingling.
Thoracic Outlet Syndrome
Thoracic outlet syndrome (TOS) refers to compression of the neural and/or vascular structures that leave or pass over the superior rim of the thoracic cage (see Fig. 17-10). Various names have been given to this condition according to the presumed site of major neurovascular compression: First thoracic rib, cervical rib, scalenus anticus, costoclavicular, and hyperabduction syndromes.
Past Medical History
History of associated back pain may be the only significant past medical history. The presence of anatomic anomalies, such as an extra rib or unusual sternoclavicular and/or acromioclavicular angle, may be the only known history linked to the development of TOS.
Risk Factors
Symptoms may be related to occupational activities (e.g., carrying heavy loads, working with arms overhead), poor posture, sleeping with arms elevated over the head, or acute injuries such as cervical flexion/extension (whiplash). Athletes such as swimmers, volleyball players, tennis players, and baseball pitchers are also at increased risk for compression of the neurovascular structures. Most people become symptomatic in the third or fourth decade, and women (especially during pregnancy) are affected three times more often than are men.
Clinical Presentation
Chest/breast pain can occur (and may be the only symptom of TOS) as a result of cervical spine disorders, an underlying etiology in TOS. This is because spinal nerves originating as high as C3-4 can extend down as low as the nipple line.
The compressive forces associated with this problem usually affect the upper extremities in the ulnar nerve distribution but can result in episodic chest pain mimicking coronary heart disease. Neurogenic pain associated with TOS may be described as stabbing, cutting, burning, or electric. The pain is often unrelated to effort and lasts hours to days.64 Pectoralis minor syndrome has been identified as one cause of recurrent neurogenic TOS contributing to an estimated 75% or more of all cases.65
There may be radiating pain to the neck, shoulder, scapula, or axilla, but usually the superficial nature of the pain and associated changes in sensation and neurologic findings point to chest pain with an underlying neurologic cause (Table 17-5). Paresthesias (burning, pricking sensation) and hypoesthesia (abnormal decrease in sensitivity to stimulation) are common. Anesthesia and motor weakness are reported in about 10% of the cases.
TABLE 17-5
Assessing Symptoms of Thoracic Outlet Syndrome*
*Although no specific testing for thoracic outlet has proven valid in detecting upper extremity pain of a neurogenic origin, the use of these special tests may help identify patterns of positive objective findings to help characterize thoracic outlet syndrome.
When a vascular compressive component is involved, there may be more diffuse pain in the limb, with associated fatigue and weakness. With more severe arterial compromise, the client may describe coolness, pallor, cyanosis, or symptoms of Raynaud’s phenomenon. Although vascular in origin, these symptoms are differentiated from CAD by the local or regional presentation, affecting only a single extremity or only the upper extremities.
Palpation of the supraclavicular space may elicit tenderness or may define a prominence indicative of a cervical rib. The effect on pulse of the Adson or Halstead maneuvers (Fig. 17-8), the hyperabduction or Wright test (Fig. 17-9), and the costoclavicular test (exaggerated military attention posture) should be compared in both arms.

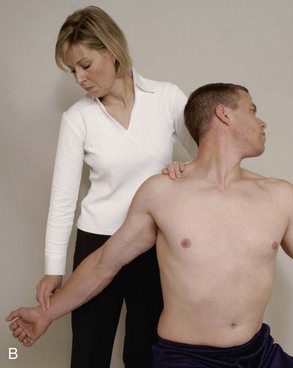
Fig. 17-8 Adson maneuver. The client begins in the sitting position with arms at his or her sides and face forward. The examiner takes a baseline, resting radial pulse rate for 1 minute. A, The client then turns his or her head toward the test arm. The head and neck are extended slightly while the examiner laterally rotates and extends the shoulder. The client is asked to take a deep breath and hold it. Reproduction of the symptoms is the best indication of thoracic outlet syndrome (TOS), but a disappearance of the pulse is considered a positive test. B, Halstead maneuver. Baseline radial pulse is obtained before the client hyperextends and rotates his head to the opposite side. The examiner applies a downward, traction force on the involved side. Once again, the test is considered positive for a vascular component of a TOS when there is a change in pulse rate or rhythm. (From Magee D: Orthopedic physical assessment, ed 5, Philadelphia, 2008, Saunders.)

Fig. 17-9 Modified Wright test, also known as the Allen test or maneuver. The hyperabduction test can help screen for vascular compromise in thoracic outlet syndrome (TOS). Start with the client’s arm resting at his or her side. Take the client’s resting radial pulse for a full minute. Make note of any irregular or skipped beats. Raise the client’s arm as shown with the client’s face turned away, and recheck the pulse. This test is used to detect compression in the costoclavicular space. Diminished or thready pulse or absence of the pulse is a positive sign for (vascular) TOS. In the standard test, the examiner waits up to 3 minutes before palpating to give time for an accurate assessment. In our experience, clients with a positive hyperabduction test almost always demonstrate early changes in symptoms, skin color, and skin temperature. Having the client take a breath and hold it may have an additional effect. Tests for other aspects of neurologic or vascular compromise are available.66,68 (From Magee D: Orthopedic physical assessment, ed 5, Philadelphia, 2008, Saunders.)
Despite the widespread use of these tests, the reliability remains unknown. Specificity reported ranges from 18% to 87%, but sensitivity has been documented at 94%.66 During assessment for vascular origin of symptoms, a change in pulse rate or rhythm is a positive test; however, because more than 50% of normal, asymptomatic individuals have pulse rate changes, it is better to reproduce the client’s symptoms as a true indicator of TOS.66,67
Other clinical tests are described in orthopedic assessment texts.66,68 With the use of special tests, patterns of positive objective findings may help characterize TOS as vascular, neural, or a combination of both (neurovascular).
Response to nerve blocks has not proved to be a predictable or reliable diagnostic or treatment approach to neurogenic TOS.69 Although no specific testing for thoracic outlet has proven valid in detecting upper extremity pain of a neurogenic origin, Table 17-5 may help guide the therapist in assessing for this condition.
Knowing what the tests are for can be helpful in guiding intervention. For example, Fig. 17-10 gives a visual representation of the effect of the hyperabduction test. A positive hyperabduction test may point to the need to restore normal function and movement of the pectoralis minor muscle.
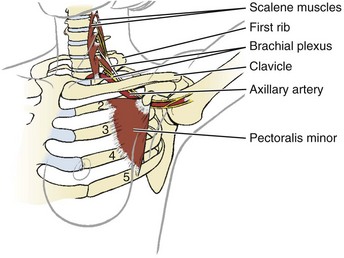
Fig. 17-10 The neurovascular bundle associated with TOS can become compressed by nearby soft tissue structures such as the pectoralis minor. This illustration shows why the hyperabduction test can alter the client’s pulse or reproduce symptoms. Effecting a change in the pectoralis minor may result in a change in the client’s symptoms and can be measured by a return of the normal pulse rate and rhythm in the hyperabducted position.
Likewise, if there is a neural component, assess for location (upper plexus versus lower plexus). Reproduction of pain or paresthesias with light pressure on the scalene, supraclavicular area, and pectoralis minor points to thoracic outlet rather than a cervical radiculopathy or more distal entrapment but several pathologic conditions can be present at the same time.70
TOS should be considered when persistent chest pain occurs in the presence of a normal coronary angiogram and normal esophageal function tests. Other conditions in the differential diagnosis include cervical degenerative disk disease or abnormality of the lung or chest wall.71
A vascular component to TOS may present with significant differences in blood pressure from side to side (a change of 10 mm Hg or more in diastolic is most likely). This does not mean that a medical referral is required immediately. Assess client age, PMH, and presence of comorbidities (e.g., known hypertension), and ask about any associated signs and symptoms that might point to heart disease as a cause of the underlying symptoms.
Physical therapy intervention can bring about a change in the soft tissue structures, putting pressure on the blood vessels in this area. In fact, blood pressure can be used as an outcome measure to document the effectiveness of the intervention. If blood pressure does not normalize and equalize from side to side, then medical referral may be required.
If there is a cluster of cardiac symptoms, especially in the presence of a significant history of hypertension or heart disease, medical referral may be required before initiating treatment. If the Review of Systems does not provide cause for concern, documentation and communication with the physician are still important while initiating a plan of care.
Physician Referral
Never dismiss chest pain as insignificant. Chest pain that falls into any of the categories in Table 6-5 requires medical evaluation. This table offers some helpful clues in matching client’s clinical presentation with the need for medical referral.
It may be impossible for a physician to differentiate anxiety from myocardial ischemia without further testing; such a differentiation is outside the scope of a physical therapist’s practice. The therapist must confine himself or herself to a screening process before conducting a differential diagnosis of movement system impairments.
The therapist is not making the differential diagnosis between angina, MI, mitral valve prolapse, or pericarditis. The therapist is screening for systemic or viscerogenic causes of chest, breast, shoulder or arm, jaw, or neck or upper back symptoms.
Knowing the chest and breast pain patterns and associated signs and symptoms of conditions that masquerade as NMS dysfunction will help the therapist recognize a condition requiring medical attention. Likewise, quickly recognizing red-flag signs and symptoms is important in providing early medical referral and intervention, preferably with improved outcomes for the client.
Guidelines for Immediate Medical Attention
• Sudden onset of acute chest pain with sudden dyspnea could be a life-threatening condition (e.g., pulmonary embolism, MI, ruptured abdominal aneurysm), especially in the presence of red-flag risk factors, personal medical history, and vital signs.
• A sudden change in the client’s typical anginal pain pattern suggests unstable angina. For the client with known angina, pain that occurs without exertion, lasts longer than 10 minutes, or is not relieved by rest or nitroglycerin signals a higher risk for a heart attack.
• The woman with chest, breast, axillary, or shoulder pain of unknown origin at presentation must be questioned regarding breast self-examinations. Any recently discovered breast lumps or nodules or lymph node changes must be examined by a physician.
Guidelines for Physician Referral
• No change is noted in uneven blood pressure from one arm to the other after intervention for a vascular TOS component.
• The therapist who suspects a client may be using anabolic steroids should report findings to the physician or coach if one is involved.
• Symptoms are unrelieved or unchanged by physical therapy intervention.
• Medical referral is advised before initiating treatment for anyone with a past history of cancer presenting with symptoms of unknown cause, especially without an identifiable movement system impairment.
Clues to Screening Chest, Breast, or Rib Pain
• History of repetitive motion; overuse; prolonged activity (e.g., marathon); long-term use of steroids, assault, or other trauma
• History of flu, trauma, URI, shingles (herpes zoster), recurrent pneumonia, chronic bronchitis, or emphysema
• History of breast cancer or any other cancer; history of chemotherapy or radiation therapy
• History of heart disease, hypertension, previous MI, heart transplantation, bypass surgery, or any other procedure affecting the chest/thorax (including breast reconstruction, implantation, or reduction)
• Prolonged use of cocaine or anabolic steroids
• Nocturnal pain, pain without precise movement aggravation, or pain that fails to respond to treatment
• Weight loss in the presence of immobility when weight gain would otherwise be expected
• Recent childbirth and/or lactation (breast feeding) (Pectoral myalgia, mastitis)
Risk Factors (see also Table 6-3)
Clinical Presentation
• Range of motion (e.g., trunk rotation of side bending, shoulder motions) does not reproduce symptoms (exception: intercostal tear caused by forceful coughing associated with diaphragmatic pleurisy).
• There is a lack of musculoskeletal objective findings; squeezing the underlying pectoral muscles does not reproduce symptoms; resisted motion (e.g., horizontal shoulder abduction or adduction) does not reproduce symptoms; heat and stretching do not reduce or eliminate the symptoms; pain or symptoms are not altered or eliminated with TrP therapy or other physical therapy intervention.
• Chest pain relieved by antacid (reflux esophagitis), rest from exertion or taking nitroglycerin (angina), recumbency (mitral valve prolapse), squatting (hypertrophic cardiomyopathy), passing gas (gas entrapment syndrome)
• Presence of painless sternal or chest wall mass or painless, hard lymph nodes
• Timing of symptoms in relation to physical or sexual activity (immediate, 5 to 10 minutes after engaging in activity, after activity ends). (Lag time is associated with angina; symptoms occurring immediately or after an activity may be a sign of TOS, asthma, myalgias, or TrPs.)
• Assess the effect of exertion; reproduction of chest, shoulder, or neck symptoms with exertion of only the lower extremities may be cardiovascular.
• Chest, neck, or shoulder pain that is aggravated by physical exertion, exposure to temperature changes, strong emotional reactions, or a large meal (coronary artery disease)
• Atypical chest pain associated with dyspnea, arrhythmias, and light-headedness or syncope
• Other signs and symptoms such as pallor, unexplained profuse perspiration, inability to talk, nausea, vomiting, sense of impending doom, or extreme anxiety
• Symptoms can be precipitated by working with arms overhead; the client becomes weak or short of breath 3 to 5 minutes after raising the arms above the heart.
Pleuropulmonary (see also Clues to Screening in Chapter 7):
• Autosplinting (lying on the involved side) quiets chest wall movements and reduces or eliminates chest or rib pain; symptoms are worse with recumbency (supine position).
• Pain is not reproduced by palpation.
• Assess for the three ps: Pleural pain, palpation, position (pleuritic pain exacerbated by respiratory movements, pain on palpation associated with musculoskeletal condition, pain with changes in neck, trunk, or shoulder position, indicating musculoskeletal origin).
• Musculoskeletal: Symptoms do not increase with pulmonary movements (unless there is an intercostal tear or rib dysfunction associated with forceful coughing from a concomitant pulmonary problem) but can be reproduced with palpation.
• Pleuropulmonary: Symptoms increase with pulmonary movements and cannot be reproduced with palpation (unless there is an intercostal tear or rib dysfunction associated with forceful coughing).
• Increased symptoms occur with recumbency (abdominal contents push up against diaphragm and in turn push against the parietal pleura).
• Increased chest pain with exercise or increased movement can also be a sign of asthma; ask about a personal or family history of asthma or allergies.
• Presence of associated signs and symptoms such as persistent cough, dyspnea (rest or exertional), or constitutional symptoms
• Chest pain with sudden drop in blood pressure or symptoms such as dizziness, dyspnea, vomiting, or unexplained sweating while standing or ambulating for the first time after surgery, an invasive medical procedure, assault, or accident involving the chest or thorax (Pneumothorax)
Gastrointestinal (Upper GI/Epigastric; see also Clues to Screening in Chapter 8):
• Effect of food on symptoms (better or worse); presence of GI symptoms, simultaneously or alternately with somatic symptoms
• Symptoms are relieved by antacids, food, passing gas, or assuming the upright position.
• Supine position aggravates symptoms (upper GI problem); symptoms are relieved by assuming an upright position.
• Symptoms radiate from the chest posteriorly to the upper back, interscapular, subscapular, or T10 to L2 areas.
• Symptoms are not reproduced or aggravated by effort or exertion.
• Presence of associated signs and symptoms such as nausea, vomiting, dark urine, jaundice, flatulence, indigestion, abdominal fullness or bloating, blood in stool, pain on swallowing
Breast (Alone or in Combination with Chest, Neck, or Shoulder Symptoms):
• Appearance (or report) of lump, nodule, discharge, skin puckering, or distended veins
• Jarring or movement of the breast tissue increases or reproduces the pain.
• Pain is palpable within the breast tissue.
• Assess for TrPs (sternalis, serratus anterior, pectoralis major; see Fig. 17-7); breast pain in the absence of TrPs or failure to respond to TrP therapy must be investigated further.
• Resisted isometric shoulder horizontal adduction or abduction does not reproduce breast pain.
• Breast pain is reproduced by exertion of the lower extremities (Cardiac).
• Association between painful symptoms and menstrual cycle (Ovulation or menses)
• Presence of aberrant or suspicious axillary or supraclavicular lymph nodes (e.g., large, firm, hard, or fixed)
• Skin dimpling especially with adherence of underlying tissue; ask about or visually inspect for:
Anxiety (see Table 3-9):
• Symptoms described using words typical of NMS origin (e.g., aching, burning, hot, scalding, searing, cutting, electric shock)
• Pain is superficial compared with pain of a cardiac or pleuropulmonary origin.
• Symptoms are confined to somatic or spinal nerve root distribution.
• History of associated back pain
• Positive hyperabduction test or other tests for TOS
• Presence of TrPs; elimination of TrPs reduces or eliminates symptoms (see Table 17-4 and Fig. 17-7)
• Symptoms are elicited easily by palpation (e.g., squeezing the pectoral muscle belly, palpating the chest wall, intercostal spaces, or costochondral junction).
• Symptoms are reproduced by resisted horizontal shoulder abduction, adduction, or other shoulder movements.
• Symptoms are relieved by heat and stretching.
• Soft tissues (tendon and muscle) take longer than the expected time to heal (Anabolic steroids).
• Costochondritis or Tietze’s syndrome may be accompanied by an increase in blood pressure but is usually palpable and aggravated by trunk movements.
• Presence of neurologic involvement (e.g., numbness, tingling, muscle atrophy); consider age and history of trauma or injury (Degenerative disk disease)
• Pain referred along peripheral nerve pathway (Dorsal nerve root irritation)
• Pain is unrelated to effort and lasts hours or weeks to months.
• Associated signs and symptoms: Numbness and tingling, muscle atrophy (Neurologic); rash, fever, chills, headache, malaise (Constitutional symptoms; neuritis or shingles)
1. Chest pain can be caused by trigger points of the:
2. During examination of a 42-year-old woman’s right axilla, you palpate a lump. Which characteristics most suggest the lump may be malignant?
3. A client complains of throbbing pain at the base of the anterior neck that radiates into the chest and interscapular areas and increases with exertion. What should you do first?
a. Monitor vital signs, and palpate pulses
b. Call the physician or 911 immediately
c. Continue with the exam; find out what relieves the pain
d. Ask about past medical history and associated signs and symptoms
4. A 55-year-old grocery store manager reports becoming extremely weak and breathless whenever stocking groceries on overhead shelves. What is the possible significance of this complaint?
5. Chest pain of a pleuritic nature can be distinguished by:
6. A 66-year-old woman has come to you with a report of anterior neck pain radiating down the left arm. Her past medical history is significant for chronic diabetes mellitus (insulin dependent), coronary artery disease, and peripheral vascular disease. About 6 weeks ago, she had an angioplasty with stent placement. Which test will help you differentiate a musculoskeletal cause from a cardiac cause of neck and arm pain?
7. You are evaluating a 30-year-old woman with left chest pain that starts just below the clavicle and extends down to the nipple line. The majority of test results point to thoracic outlet syndrome. Her blood pressure is 120/78 mm Hg on the right (sitting) and 125/100 on the left (sitting). She is in apparent good health with no history of surgeries or significant health problems. What plan of action would you recommend?
a. Refer her to a physician before initiating treatment.
b. Carry out a plan of care, and reassess after three sessions or 1 week, whichever comes first.
c. Document your findings, and contact the physician by phone or by fax while initiating treatment.
8. A 60-year-old woman with a history of left breast cancer (10 years postmastectomy) presents with pain in her midback. The pain is described as “sharp” and radiates around her chest to the sternum. She gets some relief from her pain by lying down. Her vital signs are normal, and there are no palpable or aberrant lymph nodes. She denies any changes in breast tissue on the right or the scar and soft tissue on the left. You do not have adequate training to perform a clinical breast examination, but the client agrees to visual inspection, which reveals nothing unusual. All other findings are within normal limits; you are unable to provoke or aggravate her symptoms. Neurologic screening examination is within normal limits. The client denies any history of trauma. What plan of action would you recommend?
a. Refer her to a physician before initiating treatment.
b. Carry out a plan of care, and reassess after three sessions or 1 week, whichever comes first.
c. Document your findings, and contact the physician by phone or by fax while initiating treatment.
9. You are working with a client in his home who had a total hip replacement 2 weeks ago. He describes chest pain with increased activity. Knowing what could cause this symptom will help guide you in asking appropriate screening questions. Can this be a symptom of:
10. Cardiac pain in women does not always follow classic patterns. Watch for this group of symptoms in women at risk:
References
1. Sik, EC. Atypical chest pain in athletes. Curr Sports Med Rep. 2009;8(2):52–58.
2. Harding, G, Yelland, M. Back, chest, and abdominal pain—is it spinal referred pain? Aust Fam Phys. 2007;36(6):422–429.
3. Lenfant, C. Chest pain of cardiac and noncardiac origin. Metabolism. 2010;59(suppl 1):S41–S46.
4. Bono, CM. An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J. 2011;11(1):64–72.
5. Lovelace-Chandler V, Bassar M, Dow D, et al: The role of physical therapists assisting women in skill development in performing breast self-examination. Poster presentation. Combined Sections Meeting, New Orleans, February 2005.
6. Goodman, CC, McGarvey, CL. The role of the physical therapist in primary care and cancer screening: integrating clinical breast examination (CBE) in the upper quarter examination. Rehabil Oncol. 2003;21(2):4–11.
7. Swap, C, Nagurney, JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary symptoms. JAMA. 2005;294(20):2623–2629.
8. Bruckner, FE, Greco, A, Leung, AW. Benign thoracic pain syndrome: role of magnetic resonance imaging in the detection and localization of thoracic disc disease. J R Soc Med. 1989;82:81–83.
9. Baranto, A. Acute chest pain in a top soccer player due to thoracic disc herniation. Spine. 2009;34(10):E359–362.
10. American Heart Association. Heart News. Available on-line at http://www.americanheart.org. [Accessed March 17, 2011].
11. Heart and stroke statistics. American Heart Association 2011. Available online at http://www.heart.org/HEARTORG/General/Heart-and-Stroke-Association-Statistics_UCM_319064_SubHomePage.jsp#. [Accessed March 17, 2011].
12. Sanchis, J. Identification of very low risk chest pain using clinical data in the emergency department. Int J Cardiol. 2011;150(3):260–263. [Epub ahead of print May 5, 2010].
13. Marrugat, J, et al. Mortality differences between men and women following first myocardial infarction. JAMA. 1998;280:1405–1409.
14. McSweeney, JC. Women’s early warning symptoms of acute myocardial infarction. Circulation. 2003;108(21):2619–2623.
15. Lovlien, M. Early warning signs of an acute myocardial infarction and their influence on symptoms during the acute phase, with comparisons by gender. Gend Med. 2009;6(3):444–453.
16. McSweeney, JC. Cluster analysis of women’s prodromal and acute myocardial infarction symptoms by race and other characteristics. J Cardiovasc Nurs. 2010;25(4):311–312.
17. Lunardi, AC. Musculoskeletal dysfunction and pain in adults with asthma. J Asthma. 2011;48(1):105–110.
18. Rathod, NR. Extra-oesophageal presentation of gastro-oesophageal reflux disease. J Indian Med Assoc. 2010;108(1):18–22.
19. Chait, MM. Gastoesophageal reflux disease: important considerations for the older patients. World J Gastrointest Endosc. 2010;2(12):388–396.
20. Seo, TH. Clinical distinct features of noncardiac chest pain in young patients. J Neurogastroenterol Motil. 2010;16(2):166–171.
21. Ryder, M, Deyle, GD. Differential diagnosis of fibular pain in a patient with a history of breast cancer. J Orthop Sports Phys Ther. 2009;39(3):230.
22. Siegel, R, Ward, E, Brawley, O, et al. Cancer statistics 2011. CA Cancer J Clin. 2011;61(4):212–236.
23. American Cancer Society (ACS). What are the risk factors for breast cancer? Available at http://www.cancer.org/docroot/CRI/content/CRI_2_4_2X_What_are_the_risk_factors_for_breast_cancer_5.asp. [Accessed March 18, 2011].
24. Travis, RC. Gene-environment interactions in 7610 women with breast cancer: prospective evidence from the Million women study. Lancet. 2010;375(9732):2143–2151.
25. Garfinkel, L. Current trends in breast cancer. CA Cancer J Clin. 1993;43(1):5–6.
26. Coleman, EA, Heard, JK. Clinical breast examination: an illustrated educational review and update. Clin Excell Nurse Pract. 2001;5:197–204.
27. Barton, MB, Harris, R, Fletcher, SW. Does this patient have breast cancer? The screening clinical breast examination: should it be done? How? JAMA. 1999;282:1270.
28. Chiarelli, AM. The contribution of clinical breast examination to the accuracy of breast screening. J Natl Cancer Inst. 2009;101(18):1236–1243.
29. Goodman CC, McGarvey CL: An introductory course to breast cancer and clinical breast examination for the physical therapist is available. (Charlie McGarvey, PT, MS and Catherine Goodman, MBA, PT present the course in various sites around the U.S. and upon request.)
30. A certified training program is also available through MammaCare Specialist. The program is offered to health care professionals at training centers in the United States. The course teaches proficient breast examination skills. For more information, contact. http://www.mammacare.com/index.php.
31. Schwartz, GF. Proceedings of the international consensus conference on breast cancer risk, genetics, and risk management. Breast J. 2009;15(1):4–16.
32. Rubin, RN. Woman with sharp back pain. Consultant. 1999;39(11):3065–3066.
33. Hall-Findlay, EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127(1):56–66.
34. Gabriel, SE, Woods, JE, O’Fallon, WM, et al. Complications leading to surgery after breast implantation. N Engl J Med. 1997;336(10):718–719.
35. Codner, MA. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127(3):1300–1310.
36. Benediktsson, K, Perback, L. Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: five years of monitoring of a prospective trial. J Plast Reconstr Aesthet Surg. 2006;59(1):27–34.
37. Lipa, JE. Pathogenesis of radiation-induced capsular contracture in tissue expander and implant breast reconstruction. Plast Reconstr Surg. 2010;125(2):437–445.
38. Henriksen, TF, Fryzek, JP, Holmich, LR, et al. Reconstructive breast implantation after mastectomy for breast cancer: clinical outcomes in a nationwide prospective cohort study. Arch Surg. 2005;140(12):1152–1159.
39. Scuderi, N. Multicenter study on breast reconstruction outcome using Becker implants. Aesthetic Plast Surg. 2011;35(1):66–72.
40. National Institute of Mental Health. Health Information—Anxiety Disorders. Available at http://www.nimh.nih.gov/. [Updated 3/16/11. Accessed March 18, 2011].
41. Rubio, M. Psychopathology risk and protective factors research program. National Institute of Mental Health (NIMH), 2009. Available online at http://www.nimh.nih.gov/about/organization/datr/adult-psychopathology-and-psychosocial-intervention-research-branch/psychopathology-risk-and-protective-factors-research-program.shtml. [Accessed March 18, 2011].
42. Velasquez, EM, Anand, RC, Newman, WP, et al. Cardiovascular complications associated with cocaine use. J La State Med Soc. 2004;156(6):302–310.
43. Bamberg, F. Presence and extent of coronary artery disease by cardiac computed tomography and risk for acute coronary syndrome in cocaine users among patients with chest pain. Am J Cardiol. 2009;103(5):620–625.
44. Milroy, CM, Parai, JL. The histopathology of drugs of abuse. Histopathology. Jan 25, 2011. [Epub ahead of print].
45. Schwartz, BG. Cardiovascular effects of cocaine. Circulation. 2010;122(24):2558–2569.
46. Pletcher, MJ, Kiefe, CI, Sidney, S, et al. Cocaine and coronary calcification in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am Heart J. 2005;150(5):921–926.
47. van Amsterdam, J. Adverse health effects of anabolic-androgenic steroids. Regul Toxicol Pharmacol. 2010;57(1):117–123.
48. Kanayama, G. Illicit anabolic-androgenic steroid use. Horm Behav. 2010;58(1):111–121.
49. Sullivan, ML, Martinez, CM, Gennis, P, et al. The cardiac toxicity of anabolic steroids. Prog Cardiovasc Dis. 1998;41(1):1–15.
50. Sanchez-Orio, M. Anabolic-androgenic steroids and liver injury. Liver Int. 2008;28(2):278–282.
51. Jensen, S. Musculoskeletal causes of chest pain. Aust Fam Phys. September 2001;30(9):834–839.
52. Cohen, SP. Noncardiac chest pain during war. Clin J Pain. 2011;27(1):19–26.
53. Stochkendahl, MJ. Chest pain in focal musculoskeletal disorders. Med Clin North Am. 2010;94(2):259–273.
54. Peterson, LL, Cavanaugh, DG. Two years of debilitating pain in a football spearing victim: slipping rib syndrome. Med Sci Sports Exerc. 2003;35(10):1634–1637.
55. Cubos, J. Chronic costochondritis in an adolescent competitive swimmer: a case report. J Can Chiropr Assoc. 2010;54(4):271–275.
56. Karnath, B. Chest pain: differentiating cardiac from noncardiac causes. Hospital Physician. 2000;36(4):24–38.
57. Thongngarm, T, Lemos, LB, Lawhon, N, et al. Malignant tumor with chest pain mimicking Tietze’s syndrome. Clin Rheumatol. 2001;20(4):276–278.
58. Fioravanti, A, Tofi, C, Volterrani, L, et al. Malignant lymphoma presenting as Tietze’s syndrome. Arthritis Rheum. 2003;49(5):737.
59. Simons, DG, Travell, JG, Simons, LS. Travell & Simons’ myofascial pain and dysfunction: the trigger point manual. Volume 1: Upper half of body, ed 2. Baltimore: Williams & Wilkins; 1999.
59a. Headley, BJ. When movement hurts: a self-help manual for treating trigger points. Minneapolis: Orthopedic Physical Therapy Products; 1997.
60. Hughes, KH. Painful rib syndrome: a variant of myofascial pain syndrome. AAOHN. 1998;46(3):115–120.
61. Saltzman, DA, Schmitz, ML, Smith, SD, et al. The slipping rib syndrome in children. Paediatr Anaesth. 2001;11(6):740–743.
62. Meuwly, JY, Wicky, S, Schnyder, P, et al. Slipping rib syndromes: a place for sonography in the diagnosis of a frequently overlooked cause of abdominal or low thoracic pain. J Ultrasound Med. 2002;21(3):339–343.
63. Udermann, BE, Cavanaugh, DG, Gibson, MH, et al. Slipping rib syndrome in a collegiate swimmer: a case report. J Athl Train. 2005;40(2):120–122.
64. Christo, PJ, McGreevy, K. Updated perspectives on neurogenic thoracic outlet syndrome. Curr Pain Headache Rep. 2011;15(1):14–21.
65. Sanders, RJ. The forgotten pectoralis minor syndrome: 100 operations for pectoralis minor syndrome alone or accompanied by neurogenic thoracic outlet syndrome. Ann Vasc Surg. 2010:701–708.
66. Dutton, M. Orthopaedic examination, evaluation, and intervention, ed 2. New York: McGraw-Hill; 2008.
67. Selke, FW, Kelly, TR. Thoracic outlet syndrome. Am J Surg. 1988;156:54–57.
68. Magee, D. Orthopedic physical assessment, ed 5. Philadelphia: Saunders; 2008.
69. Sanders, RJ. Recurrent neurogenic thoracic outlet syndrome stressing the importance of pectoralis minor syndrome. Vasc Endovascular Surg. 2011;45(1):33–38.
70. Yung, E. Screening for head, neck, and shoulder pathology in patients with upper extremity signs and symptoms. J Hand Ther. 2010;23(2):173–186.
71. Braun, RM. Thoracic outlet syndrome: a primer on objective methods of diagnosis. J Hand Surg. 2010;35A(9):1539–1541.
72. Robertson, FM. Inflammatory breast cancer. The disease, the biology, the treatment. CA Cancer J Clin. 2010;60(6):351–375.
73. Wolf, JM, Green, A. Influence of comorbidity on self-assessment instrument scores of patients with idiopathic adhesive capsulitis. J Bone Joint Surg. 2002;84A(7):1167–1173.
74. Smith, R, Athanasou, NA, Ostlere, SJ, et al. Pregnancy-associated osteoporosis. QJM. 1995;88:865–878.
75. Baitner, AC, Bernstein, AD, Jazrawi, AJ. Spontaneous rib fracture during pregnancy: a case report and review of the literature. Bull Hosp Jt Dis. 2000;59(3):163–165.
76. Boissonnault, WG, Boissonnault, JS. Transient osteoporosis of the hip associated with pregnancy. J Orthop Sports Phys Ther. 2001;31(7):359–367.
77. Michalakis, K. Pregnancy- and lactation-associated osteoporosis: a narrative minireview. Endocr Regul. 2011;45(1):43–47.
78. Kovacs, CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10(2):105–118.
79. Debnah, UK, Kishore, R, Black, RJ. Isolated acetabular osteoporosis in TOH in pregnancy: a case report. South Med J. 2005;98(11):1146–1148.
 pack/day for the last 2 months.
pack/day for the last 2 months.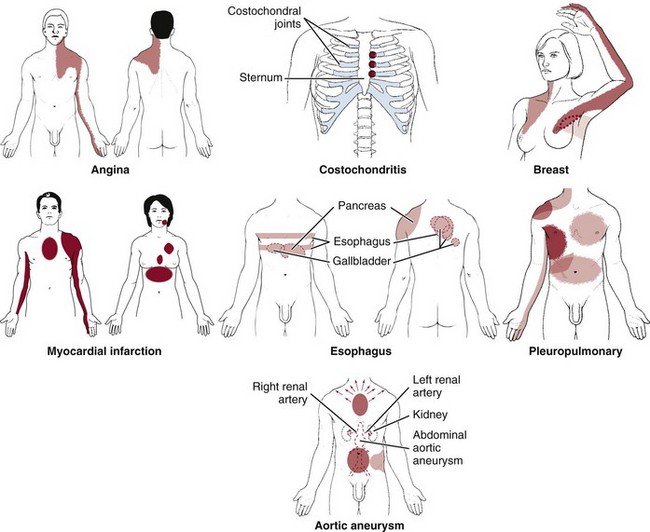
 Key Points to Remember
Key Points to Remember