Screening for Cancer
A 56-year-old man has come to you for an evaluation without a referral. He has not seen any type of physician for at least 3 years. He is seeking an examination at the insistence of his wife, who has noticed that his collar size has increased two sizes in the last year and that his neck looks “puffy.” He has no complaints of any kind (including pain or discomfort), and he denies any known trauma; however, his wife insists that he has limited ability in turning his head when backing the car out of the driveway.
• What questions would be appropriate for your first physical therapy interview with this client?
• What test procedures will you carry out during the first session?
• If you suggest to this man that he should see his physician, how would you make that recommendation? (See the Case Study at the end of the chapter.)
A large part of the screening process is identifying red flag histories and red flag signs and symptoms. Advancing age and previous history of any kind of cancer are two of the most important risk factors for cancer. Following the screening model presented in Chapters 1 and 2, the therapist will use past medical history, clinical presentation, and associated signs and symptoms as the basic tools to screen for cancer.
The client history with interview is the number one tool for cancer screening. Take the client’s history, looking for the presence of any risk factors for cancer. Cancer in its early stages is often asymptomatic. Survival rates are increased with early detection and screening, making this element of client management extremely important.
Keep in mind that some cancers, such as malignant melanoma (skin cancer), do not have a highly effective treatment. Early detection and referral can make a life and death difference in the final outcome. Morbidity can be reduced and quality of life and function improved with early intervention.
Whether primary cancer, cancer that has recurred locally, or cancer that has metastasized, clinical manifestations can mimic neuromuscular or musculoskeletal dysfunction. The therapist’s task is to identify abnormal tissue, not diagnose the lesion.
Cancer Statistics
Cancer accounts for more deaths than heart disease in the United States in persons under the age of 85 years. There are more than 1.5 million new cases of cancer in the United States each year; more than half a million will die from cancer this year. One in four deaths in the United States is attributed to cancer.1
Predicting lifetime risk of cancer is based on present rates of cancer. Using today’s epidemiologic data, 44% of all men and 38% of all women will develop cancer at some time in their lifetime.1 It is estimated that by 2030, 20% of the U.S. population will be 65 years old or older, accounting for 70% of all cancers and 85% of all cancer-related mortality.2
In the past, certain types of cancer were invariably fatal. Today, however, death rates continue to decline for most cancers, and there continues to be a reported reduced mortality from cancer. The percentage of people who have survived longer than 5 years after cancer diagnosis has increased over the past 2 decades.3
Fig. 13-1 summarizes current U.S. figures for cancer incidence and deaths by site and sex. Although prostate and breast cancers are the most common malignancies in men and women, respectively, the cancer that most commonly causes death is lung cancer.1
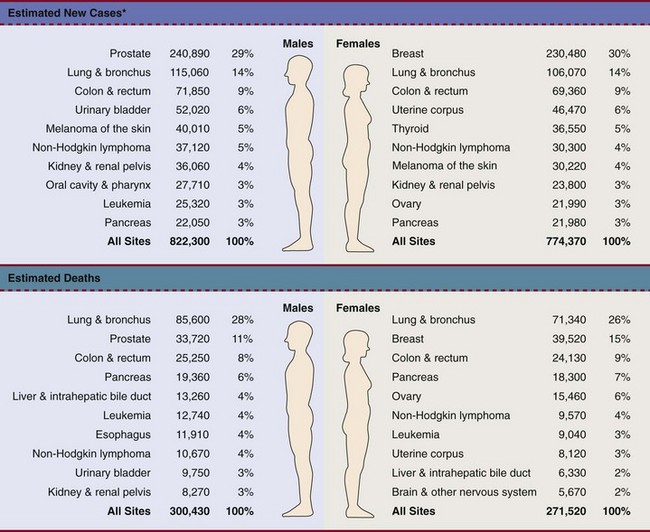
Fig. 13-1 Estimated new cases of cancer and cancer deaths by site for men and women.
*Estimates are rounded to the nearest 10 and exclude basal and squamous cell skin cancers and in situ carcinoma except urinary bladder. (From Siegel R, Ward E, Brawley O, et al: Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths, CA Cancer J Clin 61:212-236, 2011. © 2011 American Cancer Society.)
Carcinoma in situ is not included in the statistics related to invasive carcinoma or sarcoma as reported by the American Cancer Society (ACS) or the National Cancer Institute (NCI). Carcinoma in situ is considered a premalignant cancer that is localized to the organ of origin. As noted, it is reported separately and primarily relative to breast and skin cancer. Carcinoma in situ of the breast accounts for about 46,000 new cases every year, and in situ melanoma accounts for about 50,000 new cases annually.1
Cancer Cure and Recurrence
Cancer is considered cured or in remission when evidence of the disease cannot be found in the individual’s body. Early diagnosis and aggressive intervention help people obtain a cure. In general, individuals with no evidence of cancer are considered to have the same life expectancy as those who never had cancer. However, late physical and psychosocial complications of disease and treatment are being recognized.
Cancer recurrence or a new cancer can occur in some individuals with a previous personal history of cancer. Causes of cancer recurrence can include inadequate surgical margin, skip metastases, tumor thrombus, and lymph node metastasis.
Additionally, many of the antineoplastic strategies (e.g., chemotherapy, hormone therapy, radiation therapy) mutate cells further and can initiate or stimulate new malignant tumors. Besides second malignancies, these treatments can come with many unintended long-term problems and adverse consequences (e.g., fibrosis, occlusive coronary artery disease and cardiotoxicity, impaired motion and strength). The therapist should consider it a red flag any time a client has a previous history of cancer or cancer treatment.
Childhood Cancers
Cancer is the second leading cause of death in children between the ages of 1 and 14, with accidents remaining the most frequent cause of death in this age group. The most frequently occurring cancers in children are leukemia (primarily, acute lymphocytic leukemia), brain and other nervous system cancers, soft tissue sarcomas, non-Hodgkin lymphoma, and renal (Wilms’) tumor.1
Survival rates for childhood cancer have increased to 81% now because of improvements in treatment for many types of cancer over the past 2 decades. The result is an increasing population of long-term cancer survivors (or “overcomers” as some “survivors” prefer to call themselves). Currently, 1 in 900 young adults is a childhood cancer survivor. Long-term health problems related to the effects of cancer therapy are a major focus of this population group.4
It has been shown that, to varying degrees, long-term survivors of childhood cancer are at risk of developing second cancers and of experiencing organ dysfunction, such as cardiomyopathy, joint dysfunction, reduced growth and development, decreased fertility, and early death.5
The degree of risk of late effects may be influenced by various treatment-related factors such as the intensity, duration, and timing of therapy. Individual characteristics, such as the type of cancer diagnosis, the person’s sex, age at time of intervention, and genetic factors as indicated by, for example, family history of cancer, may also play a role in cancer recurrence and late effects of treatment.5,6
Risk Factor Assessment
Risk factor assessment is a part of the cancer prevention model. Every health care professional has a role and a responsibility to help clients identify risk factors for disease. Knowing the various risk factors for different kinds of cancers is an important part of the medical screening process. Educating clients about their risk factors is a key element in risk factor reduction.
A new branch of medicine called preventive oncology has developed to address this important area. Preventive oncology or chemoprevention includes primary and secondary prevention. Chemoprevention is based on the hypothesis that certain nontoxic chemicals (e.g., retinoids, cyclooxygenase-2 [COX-2] inhibitors, hormonal agents) can be given preventatively to interrupt the biological processes involved in carcinogenesis and thus reduce its incidence. Currently, many clinical trials and studies have been devoted to the idea of chemical prevention of cancer development.7
Therapists can have an active role in both primary and secondary prevention through screening and education. Primary prevention involves stopping the processes that lead to the formation of cancer in the first place. According to the Guide to Physical Therapist Practice (the Guide),8 physical therapists are involved in primary prevention by “preventing a target condition in a susceptible or potentially susceptible population through such specific measures as general health promotion efforts.”8 Risk factor assessment and risk reduction fall under this category.
Secondary prevention involves regular screening for early detection of cancer and the prevention of progression of known premalignant lesions such as skin and colon lesions. This does not prevent cancer but improves the outcome. The Guide outlines the physical therapist’s role in secondary prevention as “decreasing duration of illness, severity of disease, and number of sequelae through early diagnosis and prompt intervention.”8
Another way to look at this is through the use of screening and surveillance. Screening is a method for detecting disease or body dysfunction before an individual would normally seek medical care. Medical screening tests are usually administered to individuals who do not have current symptoms but who may be at high risk for certain adverse health outcomes.
Surveillance is the analysis of health information to look for problems that may be occurring in the workplace that require targeted prevention. Surveillance has often used screening results from groups of individuals to look for abnormal trends in health status.
Known Risk Factors for Cancer
Certain risk factors have been identified as linked to cancer in general (Table 13-1). More than half of all cancer deaths in the United States could be prevented if Americans adopted a healthier lifestyle and made better use of available screening tests.9
TABLE 13-1
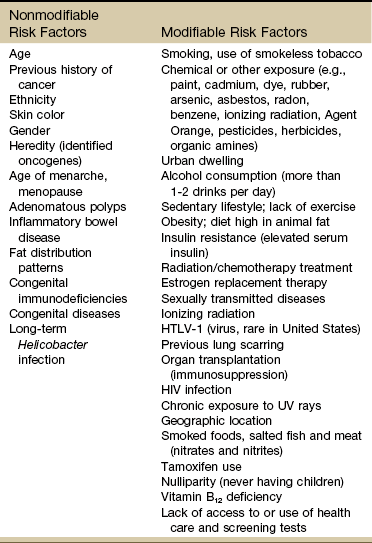
HTLV-1, Human T-lymphotropic virus type 1; HIV, human immunodeficiency virus; UV, ultraviolet.
According to the NCI, environmental factors, whether linked to lifestyle issues such as smoking and diet or exposure to carcinogens in the air and water, are thought to be linked to an estimated 80% to 90% of cancer cases.10
Some of the most common risk factors for cancer include the following:
Age
The majority of cancer incidence and mortality occurs in individuals aged 65 and older.11 With estimates from the U.S. Census Bureau predicting a rapid rise in the number of people 65 years old and older, the therapist must pay close attention to the client’s age, especially in correlation with a personal or family history of cancer. Many cancers, such as prostate, colon, ovarian, and some chronic leukemias, have increased incidence in older adults. The incidence of cancer doubles after 25 years of age and increases with every 5-year increase in age until the mid-80s, when cancer incidence and mortality reach a plateau and even decline slightly.
Other cancers occur within very narrow age ranges. Testicular cancer is found in men from about 20 to 40 years of age. Breast cancer shows a sharp increase after age 45. Ovarian cancer is more common in women older than 55. A number of cancers, such as Ewing sarcoma, acute leukemia, Wilms’ tumor, and retinoblastoma, occur mainly in childhood.
Screening for age is discussed more completely in Chapter 2. Please refer to this section for information on screening for this red flag/risk factor.
Ethnicity
Racial/ethnic minorities account for a disproportionate number of newly diagnosed cancers. African Americans have a 10% higher incidence rate than whites and a 30% higher death rate from all cancers combined than whites.1,12
African Americans have the highest mortality and worst survival of any population, and diagnosis occurs at a later stage.1 The statistics have gotten worse over the past 20 years, and studies have shown that equal treatment yields equal outcomes among individuals with equal disease.13,14
Compared with the general population, African Americans die of cancer at a 40% greater rate (they are 30% more likely to die from heart disease). The Institute of Medicine (IOM) document, Unequal Treatment, Confronting Racial and Ethnic Disparities in Health Care, suggests that care providers may be part of the problem.14
In terms of risk assessment, therapists must keep these figures in mind when examining and evaluating clients of African-American descent. For any ethnic group, the therapist is advised to be aware of cancer and disease demographics and epidemiology for that particular group.
Cancer statistics for Hispanic Americans compared with non-Hispanic Americans are becoming more available.1,15,16 There are lower rates of incidence and mortality from the four major cancer killers (breast, prostate, lung, colorectal) but a higher incidence and mortality from cancer with an infectious etiology (stomach, liver, uterine cervix, gallbladder).
The use of cancer screening tests and early detection has been increasing among this group. Mammography among Hispanic women exceeds the national average, but screening for colorectal, cervical, and prostate cancers is below average.
Cancer statistics and epidemiology in this group are problematic. Hispanic people originate from 23 different countries and have enormous diversity among themselves. They are the poorest minority group and have the highest uninsured rate of all groups. The uninsured are less likely to get preventive care such as cancer screening.
The most common cancer among Hispanic women is breast cancer; second is lung cancer. Men are more likely to have prostate cancer but die more often of lung cancer. Hispanics have twice the incidence rate and a 70% higher death rate from liver cancer compared with non-Hispanics. This type of cancer is on the rise in Hispanic women. Cancer is typically diagnosed in Hispanics at a later stage than in non-Hispanic white Americans. Consequently, they have lower cure rates.
Therapists can offer health care education and cancer screening to this unique group of people. This will be increasingly common in our practice as our health care delivery system moves from an illness-based system to a health promotion–based system. For all groups, high-quality prevention and early detection and intervention can reduce cancer incidence and mortality.3 Screening for ethnicity is discussed more completely in Chapter 2. Please refer to this section for information on screening for this important red flag/risk factor.
Family History and Genetics
Family history is often an important factor in the development of some cancers. This usually includes only first generation family members, including parents, siblings, and children.
Hereditary cancer syndromes account for approximately 5% of breast, ovarian, and colon cancers. Both clients and providers are becoming aware of the potential therapeutic advantages of early identification of hereditary cancer risk.16a
The hereditary syndromes most frequently identified are hereditary breast and ovarian cancer (HBOC) syndrome due to mutations in BRCA1 and BRCA2 genes; hereditary colon cancer (HCC), specifically, familial adenomatous polyposis (FAP); and hereditary nonpolyposis colorectal cancer.
The small percentage of people who may be suspected of a hereditary cancer syndrome can be screened regarding personal and family medical history. Critical details, such as the cancer site and age at diagnosis, are needed for risk assessment. The following are some basic hallmarks of families who could have a hereditary cancer syndrome17:
• Diagnosis of cancer in two or more relatives in a family
• Diagnosis of cancer in a family member under the age of 50
• Occurrence of the same type of cancer in several members of a family
• Occurrence of more than one type of cancer in one person
• Occurrence of a rare type of cancer in one or more members of a family18
Environment and Lifestyle Factors
It is now apparent that, although genetic predisposition varies, the two key factors determining whether people develop cancer are environment and lifestyle. The most important way to reduce cancer risk is to avoid cancer-causing agents.
Obesity, diet, sedentary lifestyle, sexual practices, and the use of tobacco, alcohol, and/or other drugs make up the largest percentage of modifiable risk factors for cancer. Current data support the findings that obesity, inappropriate diet, and excess weight cause around one-third of all cancer deaths (Box 13-1).19,20 Increased body weight and obesity (as measured by body mass index [BMI], an approximation of body adiposity) are associated with increased death rates for all cancers and for cancers at specific sites, especially when combined with a sedentary lifestyle.21-25
Overweight and obesity may account for 20% of all cancer deaths in U.S. women and 14% in U.S. men.26 It is estimated that 90,000 cancer deaths could be prevented each year if Americans maintained a healthy body weight.21
Excess body weight increases amounts of circulating hormones, such as estrogens, androgens, and insulin,27,28 all of which are associated with cellular and tumor growth. It has also been shown that physical activity reduces the risk of breast and colon cancers and may reduce the risk of several other types of cancer by decreasing excess body weight and by actually decreasing the circulation of some of the growth-related hormones.29
In 1999, the American Institute for Cancer Research (AICR) estimated that at least 20% of all cancers could be prevented if everyone ate at least 5 (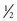 cup) servings of fruits and vegetables each day.29 Numerous resources on nutrition and its influence in preventing and treating cancer are available.30-32
cup) servings of fruits and vegetables each day.29 Numerous resources on nutrition and its influence in preventing and treating cancer are available.30-32
Dietary guidelines were updated to 9 servings a day (equal to 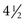 cups) for overall health and chemoprevention in January 2005 by the U.S. Department of Health and Human Services (HHS) in the publication Dietary Guidelines for Americans 2005. The Guidelines provide authoritative advice for people 2 years of age and older about how good dietary habits can promote health and reduce risks of major chronic diseases. The numbers of avoidable cancers through avoidance of excess weight are substantial.33
cups) for overall health and chemoprevention in January 2005 by the U.S. Department of Health and Human Services (HHS) in the publication Dietary Guidelines for Americans 2005. The Guidelines provide authoritative advice for people 2 years of age and older about how good dietary habits can promote health and reduce risks of major chronic diseases. The numbers of avoidable cancers through avoidance of excess weight are substantial.33
Specific factors associated with individual cancer types are known in some cases. For example, inadequate hydration is known to increase the risks of colon and bladder cancers. Alcohol consumption is linked with breast, head or neck, and gastrointestinal (GI) cancers. High dietary animal fat intake and tobacco use increase prostate cancer risk. Current smoking is an additive risk factor when combined with obesity for esophageal squamous cell carcinoma and lung and pancreatic cancers.33 Adenomatous polyps in the colon are known precursors of colorectal cancer.
Sexually Transmitted Infections: Sexually transmitted diseases (STDs) or sexually transmitted infections (STIs) have been positively identified as a risk factor for cancer. Not all STIs are linked with cancer, but studies have confirmed that human papillomavirus (HPV) is the primary cause of cervical cancer (see Fig. 4-20). With current technology, high-risk HPV DNA can be detected in cervical specimens.34
HPV is the leading viral STI in the United States. More than 100 types of HPV have been identified; more than 30 types are transmitted sexually35: 23 infect the cervix and 13 types are associated with cancer (men and women). Infection with one of these viruses does not predict cancer, but the risk of cancer is increased.
In 1970, 1 of every 300 Americans had an STI. Today, 3 million teenagers contract STIs every year; STIs affect about 1 in 4 teens who are sexually active and 50% to 75% of sexually active adults will develop HPV sometime in their lives.36 Nearly 50% of African-American teenagers have genital herpes.37 For every unwed adolescent who gets pregnant this year, 10 teenagers will get an STI.38,39
Tobacco Use: Tobacco and tobacco products are known carcinogens, not just for lung cancer but also for leukemia and cancers of the cervix, kidney, pancreas, stomach, bladder, esophagus, and oropharyngeal and laryngeal structures. This includes second-hand smoke, pipes, cigars, cigarettes, and chewing (smokeless) tobacco. Combining tobacco with caffeine and/or alcohol brings on additional problems. More people die from tobacco use than from use of alcohol and all the other addictive agents combined.
In any physical therapy practice, clients should be screened for the use of tobacco products (see Fig. 2-2). Client education includes a review of the physiologic effects of tobacco (see Table 2-3). For a more complete discussion of screening for tobacco use, see Chapter 2.
If the client indicates a desire to quit smoking or using tobacco, the therapist must be prepared to help him or her explore options for smoking cessation. Pamphlets and other reading material should be available for any client interested in tobacco cessation. Referral to medical doctors who specialize in smoking cessation may be appropriate for some clients.
Occupation and Local Environment: Well-defined problems occur in people engaging in specific occupations, especially involving exposure to chemicals and gases. Exposure to carcinogens in the air and water and on our food sources may be linked to cancer. Isolated cases of excessive copy toner dust linked with lung cancer have been reported.40,41
Reactions can be delayed up to 30 years, making client history an extremely important tool in identifying potential risk factors. People may or may not even remember past exposures to chemicals or gases. Some may not be aware of childhood exposures. Taking a work or military history may be important (see Chapter 2 and Appendix B-14).
The industrial chemicals people are exposed to vary across the country and will depend on where the individual has lived or where the client lives now. Each state in the United States has its own unique environmental issues. For example, in Montana, there has been a significant chlorine spill, exposure to agricultural chemicals, vermiculite mining, and many other forms of mining.
In New York, Love Canal was the focus of concern in the 1980s and 1990s, when the effects of hazardous wastes dumped in the area were discovered. Alaskan oil spills, air pollution in Los Angeles, and hazardous and radioactive nuclear waste in Washington state burial grounds are a few more examples.
In Utah, Nevada, and Arizona, the Radiation Exposure Compensation Act (RECA) was passed by Congress in 1990 after studies showed a possible link between hundreds of above-ground nuclear tests in the late 1950s and early 1960s and various cancers and primary organ diseases.42-45 Groundwater wells at old open-pit copper mines in various states have tested positive for uranium up to 40 times higher than legal limits. Hundreds of active wells tap into groundwater within 5 miles of these sites.
Wherever the therapist practices, it is important to be aware of local environmental issues and the impact these may have on people in the vicinity.
Ionizing Radiation: Exposure to ionizing radiation is potentially harmful. Ionizing radiation is the result of electromagnetic waves entering the body and acting on neutral atoms or molecules with sufficient force to remove electrons, creating an ion. The most common sources of ionizing radiation exposure in humans are accidental environmental exposure and medical, therapeutic, or diagnostic irradiation.
Nonionizing radiation is electromagnetic radiation that includes radio waves, microwaves, infrared light, and visible light. Nonionizing radiation does not have enough energy to ionize (i.e., break up) atoms. Electronic devices, such as laser scanners, high-intensity lamps, and electronic antitheft surveillance devices, expose the human to nonionizing radiation. There is not a proven link between exposure to nonionizing radiation and cancer, but there is considerable speculation that long-term exposure to electromagnetic fields may be correlated with the development of various illnesses and diseases.
Some studies have reported the possibility of increased cancer risks, especially of leukemia and brain cancer, for electrical workers and others whose jobs require them to be around electrical equipment. Additional risk factors, however, such as exposure to cancer-initiating agents, may also be involved.46
Some researchers have looked at possible associations between electromagnetic exposure and breast cancer, miscarriages, depression, suicides, Alzheimer’s disease, and amyotrophic lateral sclerosis (ALS, or Lou Gehrig disease), but the general scientific consensus is that the evidence is not yet conclusive.46
Ultraviolet radiation (UVR), sometimes also called UV light, is invisible electromagnetic radiation of the same nature as visible light but having shorter wavelengths and higher energies. The main source of natural UVR is the sun. UVR is conventionally divided into three bands in order of increasing energy: UVA, UVB, and UVC.
In the electromagnetic spectrum, UVR extends between the blue end of the visible spectrum and low-energy x-rays, straddling the boundary between ionizing and nonionizing radiation (which is conventionally set at 100 nm). Because of the different wavelengths and energies, each of the three bands has distinct effects on biologic tissue.
The highest-energy band, UVC, can damage DNA and other molecules and is used in hospitals for sterilization. UVC is rapidly attenuated in air, and therefore it is not found in ground-level solar radiation. Exposure to UVC, however, can take place close to sources such as welding arcs or germicidal lamps.
UVB is the most effective UV band in causing tanning and sunburn (erythema), and it can affect the immune system. UVA penetrates deeper in the skin because of its longer wavelength and plays a role in skin photoaging. UVA can also affect the immune system. Exposure to UVA and UVB has been implicated in the development of skin cancer.
Tanning lamps emit mostly UVA radiation with a few percentage content of UVB. Use of tanning lamps and beds can lead to significant exposure to UVA radiation. The risk of melanoma reportedly increases 75% when the use of tanning devices starts before age 30.47,48
The greater the frequency and intensity of exposure, the greater the risk. The risk is even higher for individuals using high-intensity or high-pressure devices.48 Despite known negative health effects from the use of indoor tanning devices, this practice is still very popular in the United States and Europe. Therapists have a role in client education, especially concerning reducing modifiable risk factors such as outdoor exposure to the sun without protection, exposure to sun lamps, and indoor tanning.49
Military Workers: Survivors of recent wars who have been exposed to chemical agents may be at risk for the development of soft tissue sarcoma, non-Hodgkin’s lymphoma, Hodgkin’s disease, respiratory and prostate cancers, skin diseases, and many more problems in themselves and their offspring.
Three million Americans served in the armed forces in Vietnam during the 1960s and early 1970s. Large quantities of defoliant agents, such as Agent Orange, were used to remove forest cover, destroy crops, and clear vegetation from around U.S. military bases.
At least half of the 3 million Americans in Vietnam were there during the heaviest spraying. Many of our military personnel were exposed to this toxic substance. Exposure could occur through inhalation, ingestion, and skin or eye absorption.
In early 2003, the military acknowledged that exposure to Agent Orange is associated with chronic lymphocytic leukemia among surviving veterans. There is also sufficient evidence of an association between Agent Orange and soft tissue sarcoma and non-Hodgkin’s lymphoma.50-52
Taking an environmental, occupational, or military history may be appropriate when a client has a history of asthma, allergies, or autoimmune disease, along with puzzling, nonspecific symptoms such as myalgias, arthralgias, headaches, back pain, sleep disturbance, loss of appetite, loss of sexual interest, and recurrent upper respiratory symptoms.
The affected individual often presents with an unusual combination of multiorgan signs and symptoms. A medical diagnosis of chronic fatigue syndrome, fibromyalgia, or another more nonspecific disorder is a yellow flag. When and how to take the history and how to interpret the findings are discussed in Chapter 2. The mnemonic CH2OPD2 (Community, Home, Hobbies, Occupation, Personal habits, Diet, and Drugs) can be used as a tool to identify a client’s history of exposure to potentially toxic environmental contaminants.53
Risk Factors for Cancer Recurrence
As cancer survivors live longer, the chance of recurring cancer increases. Positive lymph nodes, tumor size greater than 2 cm, and a high-grade histopathologic designation increase a client’s risk of cancer recurrence. Recurrence can occur at the original location of the first cancer, in local or distant lymph nodes, or in metastatic sites such as the bone or lung tissues.
Each type of cancer has its own risk factors for cancer recurrence. For example, increased numbers of positive lymph nodes and negative estrogen/progesterone receptor (ER/PGR) status for breast cancer survivors are risk factors (Case Example 13-1). A positive ER/PGR status lowers the risk of breast cancer recurrence because it allows the woman to receive treatment for prevention of recurrence according to age and stage of cancer.
Cancer Prevention
Cancer prevention begins with risk factor assessment and risk reduction. The key to cancer prevention lies in minimizing as many of the individual modifiable risk factors as possible. The AICR estimates that recommended diets, together with maintenance of physical activity and appropriate body mass, can in time reduce cancer incidence by 30% to 40%. At current rates, on a global basis, this represents 3 to 4 million cases of cancer per year that could be prevented by dietary and associated means.20
There are some simple steps to take in starting this process. The first is to assess personal/family health history. Note any cancers present in first-generation family members. Some helpful tools are available for assessing cancer risk. The Harvard School of Public Health offers an interactive tool to estimate an individual’s risk of cancer and offers cancer prevention strategies. Anyone can benefit from it, but accuracy is greatest for adults over age 40 who have never had any type of cancer. It is available at the Harvard Center for Cancer Prevention website at www.yourcancerrisk.harvard.edu.
Cancer screening is available and widely recommended for the following types of cancer: colorectal, breast, cervix (women), and prostate (men). Early detection at a localized stage is linked with less morbidity and lower mortality.
For example, 90% of colon cancer cases and deaths can be prevented. The ACS provides a summary of risk factors and early detection screening tests for many types of cancer, including colon cancer. (This information is available at www.cancer.org/Healthy/InformationforHealthCareProfessionals/ColonMDClinicansInformationSource/index, on the ACS website.) The Gail Model Risk Assessment Tool can also be used to assess personal risk for breast cancer. It is available at www.halls.md/breast/riskcom.htm (Breast Cancer Risk [Gail Model] Calculation Methods).
As health care educators, therapists can make use of this information to promote cancer prevention for themselves, their families, and their clients.
Genomics and Cancer Prevention
With the advent of the Genome Project, the sequencing of all genes in humans is nearly completed. Along with this discovery has come the development of a new biology of genetics called genomics. Understanding gene–environment interaction will be a major focus of genomics-based public health.54
There are many known or suspected carcinogens that increase an individual’s risk of cancer. Different people respond to carcinogens differently. It is still not clear why one person develops cancer and another does not when both have the same risk factors.
Toxicogenomics, the development of molecular signatures for the effects of specific hazardous chemical agents, will bring to our understanding ways to track multiple sources of the same agent, multiple media and pathways of exposures, multiple effects or risks from the same agent, and multiple agents that cause similar effects.54
Defects may occur in one or more genes. Damage may occur in genes that involve the metabolism of carcinogens or in genes that deal with the DNA-repair process.55 An important discovery in the area of gene identification related to cancer suppression is discovery of the p53 tumor-suppressor gene.56 The p53 gene encodes a protein with cancer-inhibiting properties. Loss of p53 activity predisposes cells to become unstable and more likely to take on mutations. Mutation of the p53 gene is the most common genetic alteration in human cancers.57
It is possible that genetic defects combined with lifestyle or environmental factors may contribute to the development of cancer. For example, there is a known increase in risk of breast cancer in American women born after 1940 who have BRCA1 or BRCA2 mutation. This suggests that changes in the environment or lifestyle increased the risk already conferred by these genes.58
Air pollution is moderately associated with increased lung cancer, but when combined with exposure to tobacco smoke, the risk increases dramatically. About 50% of all people lack the GSTM1 metabolic gene that can detoxify tobacco smoke and air pollution. People who have this genetic defect and who have heavy exposure to pollution may have a higher risk of developing lung cancer.55
Once it is understood how genes and the environment work to contribute to cancer development, this knowledge can be applied to intervention. Anyone with genes that lead to a higher risk of cancer may benefit from chemoprevention.
Major Types of Cancer
There are three major types of cancer: carcinoma, sarcoma, and bloodborne cancers such as lymphoma and the leukemias.
Carcinoma is a malignant tumor that comprises epithelial tissue and accounts for 85% of all cancers. Carcinomas affect structures such as the skin, large intestine, stomach, breast, and lungs. These can be fast-growing tumors because they are derived from the epithelial lining of the organ, which grows rapidly and replaces itself frequently.
Carcinomas spread by invading local tissues and by metastasis. Generally, carcinomas tend to metastasize via the lymphatics, whereas sarcomas are more likely to metastasize hematogenously.
Sarcoma is a fleshy growth and refers to a large variety of tumors arising in the connective tissues that are grouped together because of similarities in pathologic appearance and clinical presentation.
Tissues affected include connective tissue such as bone and cartilage (discussed subsequently under Bone Tumors), muscle, fibrous tissue, fat, and synovium. The different types of sarcomas are named for the specific tissues affected (e.g., fibrosarcomas are tumors of the fibrous connective tissue; osteosarcomas are tumors of the bone; and chondrosarcomas are tumors arising in cartilage) (Table 13-2).
TABLE 13-2
Classification of Soft Tissue and Bone Tumors
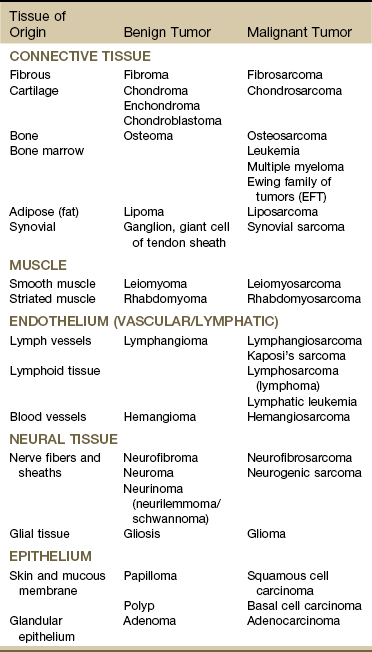
Data from Purtilo DT, Purtilo RB: A survey of human disease, ed 2, Boston, 1989, Little, Brown; Phipps W, et al: Medical-surgical nursing: concepts and clinical practice, ed 4, St. Louis, 1990, Mosby.
As a general category, sarcoma differs from carcinoma in the origin of cells composing the tumor (Table 13-3). As mentioned, sarcomas arise in connective tissue (embryologic mesoderm), whereas carcinomas arise in epithelial tissue (embryologic ectoderm) (i.e., cellular structures covering or lining surfaces of body cavities, small vessels, or visceral organs).
Cancers of the blood and lymph system arise from the bone marrow and include leukemia, multiple myeloma, and lymphoma. These cancers are characterized by the uncontrolled growth of blood cells. Metastasis is hematogenous.
Resources
Although there are many websites related to cancer, we recommend what the physicians use: the well-known and respected National Comprehensive Cancer Network (NCCN). The NCCN Clinical Practice Guidelines in Oncology are recognized by clinicians around the world as the standard for oncology care. The NCCN now has consumer versions of its clinical practice guidelines (www.nccn.com).
Other reliable sites include Abramson Cancer Center of the University of Pennsylvania at www.oncolink.org or www.oncolink.com and the National Cancer Institute (www.cancer.gov/). For self-assessment of cancer (and other diseases), go to www.yourdiseaserisk.wustl.edu.
Metastases
Neoplasms are divided into three categories: benign, invasive, and metastatic. Benign neoplasms are noncancerous tumors that are localized, encapsulated, slow growing, and unable to move or metastasize to other sites.
Invasive carcinoma is a malignant cancer that has invaded surrounding tissue. The spread of cancer cells from the primary site to secondary sites is called metastasis. A regional metastasis is the local arrest, growth, and development of a malignant lesion to regional lymph nodes.
A distal or distant metastasis is the distant arrest, growth, and development of a malignant lesion to another organ (e.g., lung, liver, brain). Within the categories of invasive and metastatic tumors, four large subcategories of malignancy have been identified and classified according to the cell type of origin (see Table 13-3).
For the therapist, primary cancers arising from specific body structures are not as likely to present with musculoskeletal signs and symptoms. It is more likely that recurrence of a previously treated cancer will have metastasized from another part of the body (secondary neoplasm) with subsequent bone, joint, or muscular presentation.
Metastatic spread can occur as late as 15 to 20 years after initial diagnosis and medical intervention. As many as 70% of people who die of cancer have been shown on autopsy to have spinal metastases; up to 14% exhibit clinical symptomatic disease before death.59 For these reasons, the therapist must take care to conduct a screening interview during the examination, including past medical history of cancer or cancer treatment (e.g., chemotherapy, radiation).
Use the personal/family history form (see Fig. 2-2) in Chapter 2 to assess for a personal or first-degree family history of cancer. When asked about a past medical history of cancer, clients may say “No,” even in the presence of a personal history of cancer. This is especially common in those clients who have reached and/or passed the 5-year survival mark.
Always link these two questions together:
Mechanisms and Modes of Metastases
Cancer cells can spread throughout the body through the bloodstream (hematogenous or vascular dissemination), via the lymphatic system, or by direct extension into neighboring tissue or body cavities (Fig. 13-2). Once a primary tumor is initiated and starts to move by local invasion, tumor angiogenesis occurs (blood vessels from surrounding tissue grow into the solid tumor). Tumor cells then invade host blood vessels and are discharged into the venous drainage.
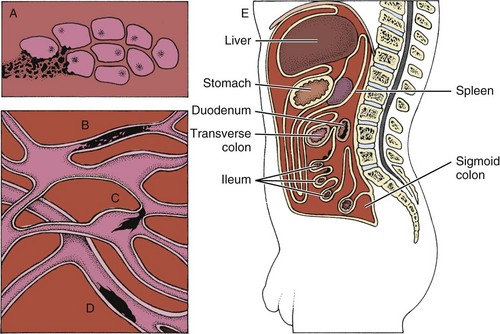
Fig. 13-2 Some modes of dissemination of cancer. A, Direct extension into neighboring tissue. B, Permeation along lymphatic vessels. C, Embolism via lymphatic vessels to the lymph nodes. D, Embolism via blood vessels (hematogenous spread). E, Invasion of a body cavity by diffusion. (Modified from Monahan et al, editors: Phipps’ medical-surgical nursing: health and illness perspective, ed 8, St. Louis, 2007, Mosby.)
Many individuals develop multiple sites of metastatic disease because of the potential of cancers to spread. A metastatic colony is the end result of a complicated series of tumor–host interactions called the metastatic cascade.
Metastasis requires a good deal of coordination between cancer cells and the body. Fortunately, many early metastases die in transit for a number of reasons such as blood vessel turbulence and genes that normally suppress growth of micrometastases in new environments. Even so, some metastatic cells do survive and move on to other sites. At secondary sites, the malignant cells continue to reproduce, and new tumors or lesions develop.
Some clients with newly diagnosed cancers have clinically detectable metastases; remaining clients who are clinically free of metastases may harbor occult metastases.
The usual mode of spread and eventual location of metastases vary with the type of cancer and the tissue from which the cancer arises. Early clinical observations led to the idea that carcinomas spread by the lymphatic route and mesenchymal tumors, such as melanoma, spread through the bloodstream. We now know that both the lymphatic and vascular systems have many interconnections that allow tumor cells to pass from one system to the other.
During invasion, tumor cells can easily penetrate small lymphatic vessels and are then transported via the lymph. Tumor emboli may be trapped in the first draining lymph node, or they may bypass these regional lymph nodes (RLNs) to form noncontinuous and distant nodal metastases called “skip metastasis.”
The relatively high incidence of anatomic skip metastasis can be attributed to aberrant distribution of lymph nodes.60 Multiple interconnections between the lymphatic and hematogenous systems may also allow transport of tumor cells via the arterial or venous blood supply, bypassing some lymph nodes while reaching other more distant nodes.61
Patterns of blood flow, regional venous drainage, and lymphatic channels determine the distribution pattern of most metastases. For example, breast cancer spreads via the lymphatics and via the vertebral venous system to bones in the shoulder, hip, ribs, vertebrae, lungs, and liver.
Primary bone cancer, such as osteogenic sarcoma, initially metastasizes via blood to the lungs. Prostate cancer spreads via lymphatics to pelvic and vertebral bones, sometimes appearing as low back and/or pelvic pain radiating down the leg. The more common cancers and their metastatic pathways are provided in Table 13-4.
TABLE 13-4
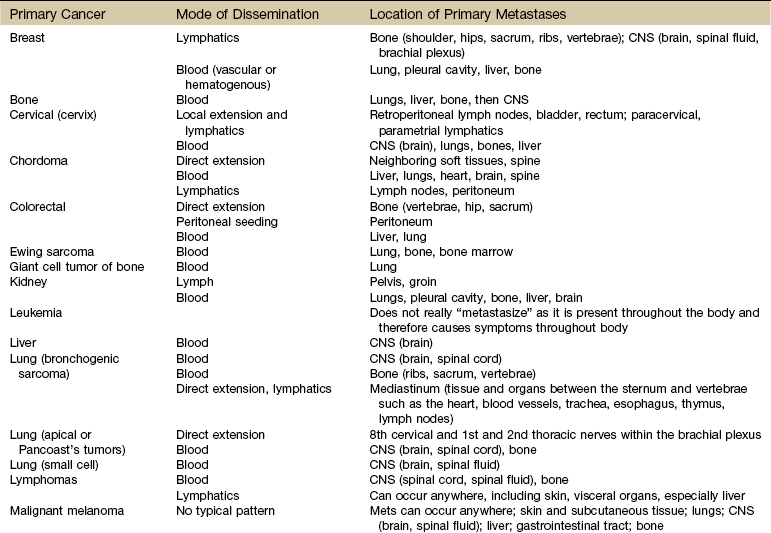
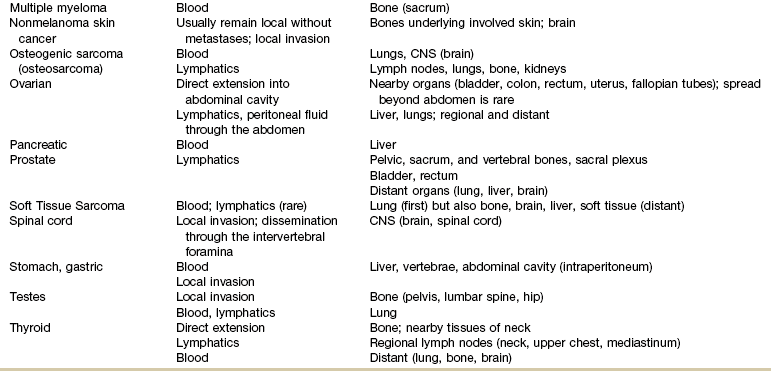
CNS, Central nervous system; Mets, metastases.
Use this table to identify the most likely location of symptoms associated with cancer recurrence/metastases. If the new symptoms match areas of common metastases for the client’s primary cancer, further screening and/or consult or referral are indicated.
The high proportion of bone metastases in breast, prostate, and lung cancers is an example of selective movement of tumor cells to a specific organ. For example, in breast cancer, it is thought that the continuous remodeling of bone by osteoclasts and osteoblasts predisposes bone to metastatic lesions.62,63 For some cancers, such as malignant melanoma, no typical pattern exists, and metastases may occur anywhere.
Increased tumor contact with the circulatory system provides tumors with a mechanism to enter the general circulation and colonize at distant sites. Both vascular endothelial growth factor (VEGF) and fibroblast growth factor stimulate proliferation of vascular cells and even allow the newly formed blood vessels to be easily invaded by the cancer cells that are closely adjacent to them.64 Resection of tumors without clear margins has the potential to provide remaining tumor cells with a means of metastasizing as new blood vessels form during the healing process.
Benign Mechanical Transport
Mechanical transport rather than metastasis may be another mechanism of cancer spread. Two potential modes of benign mechanical transport (BMT) have been detected: lymphatic transport of epithelial cells displaced by biopsy of the primary tumor and breast massage–assisted sentinel lymph node (SLN) localization.65
Samples of malignant tissues must be very carefully excised by surgeons who are expert in the biopsy of malignant tissues.66,67 The risk of local recurrence is increased when intralesional curettage alone is performed.68 Recurrence along the surgical pathway has been reported for some tumors following needle biopsy.69,70 It is hypothesized that this recurrence is the result of intraoperative seeding. Poorly planned biopsies or incomplete tumor resection increases the risk of local recurrence and metastasis. The biopsy tract should be excised when complete tumor removal occurs.71
A second mode of BMT may be the pre-SLN breast massage used to facilitate the localization of SLNs during breast cancer staging. Mechanical transport of epithelial cells to SLNs has been verified. The significance of small epithelial clusters in SLN is unknown; further research is needed before changes are recommended for biopsy and SLN-localizing practices.65
The bottom line for the therapist is this: Anyone who has had a recent biopsy (within the past 6 months) must be followed carefully for any signs of local cancer recurrence.
Clinical Manifestations of Malignancy
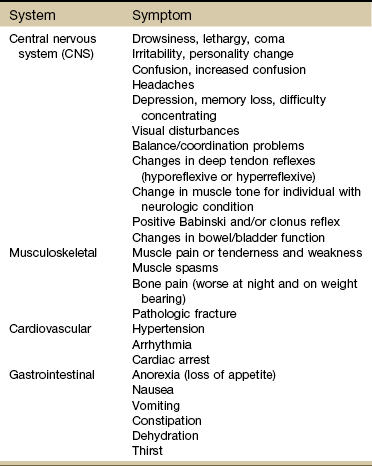
The therapist may be the first to see clinical manifestations of primary cancer but is more likely to see signs and symptoms of cancer recurrence or cancer metastasis. In general, the five most common sites of cancer metastasis are bone, lymph nodes, lung, liver, and brain. However, the therapist is most likely to observe signs and symptoms affecting one of the following systems:
Each of these systems has a core group of most commonly observed signs and symptoms that will be discussed throughout this section (Table 13-5).
TABLE 13-5
Signs and Symptoms of Metastases*
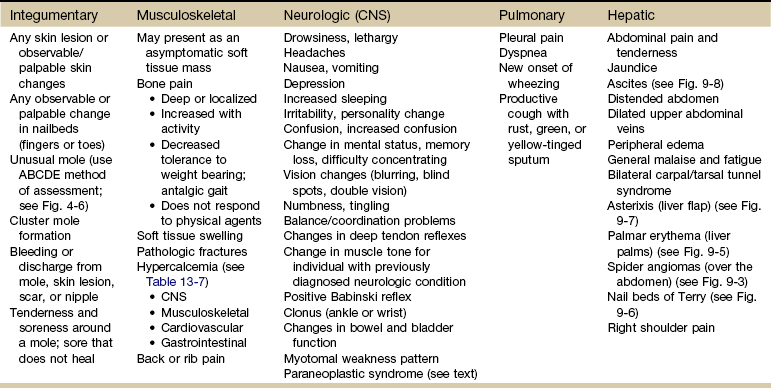
CNS, Central nervous system; ABCDE, asymmetry, border, color, diameter, evolving.
*Seen most often in a physical therapy practice.
Early Warning Signs
For many years, the ACS has publicized the seven warning signs of cancer, the appearance of which could indicate the presence of cancer and the need for a medical evaluation. The mnemonic in Box 13-2 is often used as a helpful reminder of these warning signs.
Other early warning signs can include rapid, unintentional weight loss in a short period of time (e.g., 10% of the person’s body weight in 2 weeks), unusual changes in vital signs, frequent infections (e.g., respiratory or urinary), and night pain. Bleeding is an important sign of cancer, but a cancer is generally well established by the time bleeding occurs. Bleeding develops secondary to ulcerations in the central areas of the tumor or by pressure on or rupture of local blood vessels. As the tumor continues to grow, it may enlarge beyond its capacity to obtain necessary nutrients, resulting in revitalization of portions of the tumor.
This process of invading and compressing local tissue, shutting off blood supply to normal cells, is called necrosis. Tissue necrosis leads ultimately to secondary infection, severe hemorrhage, and the development of pain when regional sensory nerves become involved. Other symptoms can include pathologic fractures, anemia, and thrombus formation.
Awareness of these signals is useful, but it is generally agreed that these symptoms do not always reflect early curable cancer nor does this list include all possible signs for the different types of cancer.
Lumps, Lesions, and Lymph Nodes
The therapist should take special note of “T,” which is thickening or lump in breast or elsewhere. Clients often point out a subcutaneous lesion (often a benign lipoma) and ask us to identify what it is. Baseline examination of a lump or lesion is important. Palpation of skin lesions and lymph nodes is presented in Chapter 4.
Whenever examining a lump or lesion, one should use the mnemonic in Box 4-11 to document and report findings on location, size, shape, consistency, mobility or fixation, and signs of tenderness (see Fig. 4-48). A clinically detectable tumor the size of a small pea already contains billions of cells. Most therapists will be able to palpate a lesion below the skin when it is half that size.61
Review Appendix B-21 for appropriate follow-up questions. Keep in mind that the therapist cannot know what the underlying pathology may be when lymph nodes are palpable and questionable. Performing a baseline assessment and reporting the findings are important outcomes of the assessment.
In previous editions of this text, it was noted that any changes in lymph nodes present for longer than 1 month in more than one location was a red flag. This recommendation is no longer appropriate. The recommendation today, based on an increased understanding of cancer metastases via the lymphatic system and the potential for cancer recurrence, is that all suspicious lymph nodes should be evaluated by a physician (Case Example 13-2).
Supraclavicular lymph nodes that are easily palpable on examination may indicate possible metastatic disease. Any lymph nodes that are hard, immovable, and nontender raise the suspicion of cancer, especially in the presence of a previous history of cancer.
Keep in mind that lymph nodes can fluctuate over the course of 10 to 14 days. When making the medical referral, look for a cluster of signs and symptoms, recent trauma (including recent biopsy), or a past history of chronic fatigue syndrome, mononucleosis, and allergies. Record and report all findings.
Proximal Muscle Weakness
For the therapist, idiopathic proximal muscle weakness may be an early sign of cancer (Fig. 13-3). This syndrome of proximal muscle weakness is referred to as carcinomatous neuromyopathy. It is accompanied by changes in two or more deep tendon reflexes (ankle jerk usually remains intact). Muscle weakness may occur secondary to hypercalcemia, which occurs as an indirect humoral effect on bone (see the later discussion on Paraneoplastic Syndrome in this chapter). Clients with advanced cancer, multiple myeloma, or breast or lung cancer are affected most often by hypercalcemia.
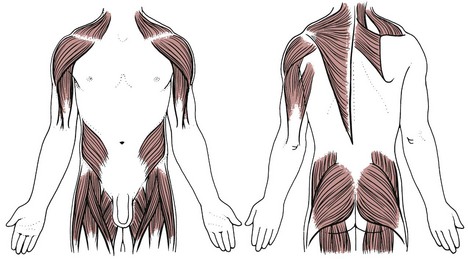
Fig. 13-3 Proximal muscle weakness can be observed clinically as a positive Trendelenburg test (usually present bilaterally) and abnormal manual muscle testing. It can also be observed functionally when the client has difficulty getting up from sitting or climbing stairs. As the weakness progresses, the client may have trouble getting into and out of a vehicle and/or the bathtub. Respiratory muscle weakness may be seen as shortness of breath or reported as altered activity to avoid dyspnea.
Screening for muscle weakness is not always a straightforward process. Sometimes, questions must be directed toward function to find out this information. If a client is asked whether he or she has any muscle weakness, difficulty getting up from sitting, trouble climbing stairs, or shortness of breath, the answer may very well be “No” on all accounts. Consider using the following flow of questions:
Pain
Pain is rarely an early warning sign of cancer, even in the presence of unexplained bleeding. Night pain that is constant and intense (often rated 7 or higher on the Numeric Rating Scale; see Fig. 3-6) is a red flag symptom of primary or recurring cancer. But not all people with musculoskeletal cancers experience night pain.72
Pain is usually the result of destruction of tissue or pressure on tissue due to the presence of a tumor or lesion. The lesion or lesions must be of significant size or location to create pressure and/or occlusion of normal structures; pain will be dependent on the area of the body affected.
Acute and chronic cancer-related pain syndromes can occur in association with diagnostic and therapeutic interventions such as bone marrow biopsy, lumbar puncture, colonoscopy, percutaneous biopsy, and thoracentesis. Chemotherapy and radiation toxicities can result in painful peripheral neuropathies.
Likewise, many different chronic pain syndromes (e.g., tumor-related radiculopathy, phantom breast pain, postsurgical pelvic or abdominal pain, burning perineum syndrome, postradiation pain syndrome) can occur as a result of tumors or cancer therapy. See further discussion under Oncologic Pain in this chapter.
Change in One or More Deep Tendon Reflexes
When a neurologic screening examination is performed, testing of deep tendon reflexes (DTRs) is usually included. Some individuals have very brisk reflexes under normal circumstances; others are much more hyporeflexive.
Tumors (whether benign or malignant) can also press on the spinal nerve root, mimicking a disk problem. A lesion that is small enough can put just enough pressure to irritate the nerve root, resulting in a hyperreflexive DTR. A large tumor can obliterate the reflex arc, resulting in diminished or absent reflexes.
Either way, changes in DTRs must be considered a red flag sign (possibly of cancer) that should be documented and further investigated. For example, a hyporesponsive patellar tendon reflex that is unchanged with distraction or repeated testing and is accompanied by back, hip, or thigh pain, along with a past history of prostate cancer, presents a different clinical picture altogether. Guidelines for assessing reflexes are discussed in Chapter 4.
Integumentary Manifestations
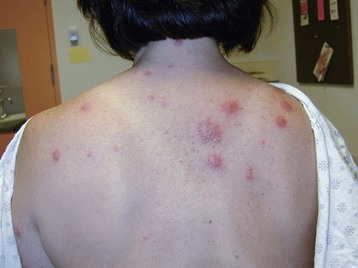
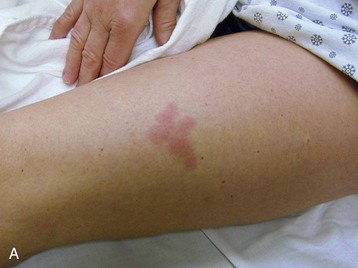
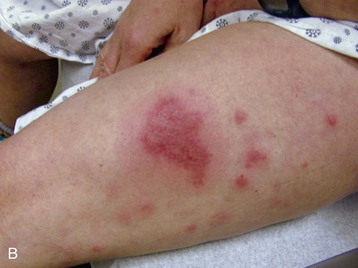
Internal cancers can invade the skin through vascular dissemination or direct extension. Metastases to the skin may be the first sign of malignancy, especially for breast or upper respiratory tract cancer. Integumentary carcinomatous metastases often present as asymmetrical, firm, skin-colored, red, purple, or blue nodules near the site of the primary tumor (see Fig. 4-26).
Distant cutaneous metastasis can result from lymphoma (see Fig. 4-29), multiple myeloma (see Fig. 4-27), and stomach/colon, ovarian, pancreatic, kidney, and breast cancer (Case Example 13-3). The scalp is a common site for such lesions (see Fig. 4-28), which are sometimes accompanied by hair loss called alopecia neoplastica.
The integumentary screening examination, including assessment of common skin lesions and nailbed assessment, is presented in Chapter 4. Cancer-related skin lesions (e.g., pinch purpura, renal nodule, local cancer recurrence, Kaposi’s sarcoma, xanthomas) are also included in Chapter 4.
During observation and inspection, the therapist should be alert to any potential signs of primary skin cancer or integumentary metastases. When a suspicious skin lesion is noted, the therapist should conduct a risk factor assessment and ask three questions:
• How long have you had this area of skin discoloration/mole/spot (use whatever brief description seems most appropriate)?
No matter what the therapist’s own cultural background, as a health care professional, his or her responsibility to screen skin lesions is clear. How questions are posed is just as important as what is said.
The therapist may want to introduce the subject by saying that as health care professionals, we are trained to observe many body parts (skin, joints, posture, and so on). You notice that the client has an unusual mole (or rash or whatever has been observed), and you wonder whether this is something that has been there for years. Has it changed in the past 6 weeks to 6 months? Has the client ever shown it to the doctor?
A client with a past medical history of cancer now presenting with a suspicious skin lesion that has not been evaluated by the physician must be advised to have this evaluated as soon as possible.
For any client with a previous history of cancer with surgical removal, it is always a good idea to look at the surgical site(s) for any sign of local cancer recurrence. Start by asking the client if he or she has noticed any changes in the scar. Continue by asking the following:
Any suspicious scab or tissue granulation, redness, or discoloration must be noted (photographed, if possible) (see Figs. 4-9 and 17-5). Again, three screening questions apply in this situation. The therapist has a responsibility to report these findings to the appropriate health care professional and to make every effort to ensure client compliance with follow-up.
Skin Cancers
Skin cancers are the most common of all types of cancer and are usually classified as nonmelanoma skin cancer (NMSC) or melanoma. Most skin cancers are classified as nonmelanoma and are slow growing, easy to recognize, and responsive to intervention, if found early. Nonmelanoma skin cancers are further classified as basal cell or squamous cell, depending on the tissue affected. They rarely metastasize to other parts of the body and have a nearly 100% rate of cure.
Melanoma, the most serious of the skin cancers, has a 96% 5-year survival rate if localized, but only a 13% 5-year survival if it is invasive or has spread to other parts of the body. More than 77% of cancer deaths result from invasive melanoma.
The primary warning sign for melanoma is a flat, colored, irregularly shaped lesion that can be mottled with light brown to black colors. It may turn various shades of red, blue, or white or crust on the surface and bleed. A changing mole, the appearance of a new mole, or a mole that is different or growing requires prompt medical attention.73 The Skin Cancer Foundation advocates use of the ABCD (asymmetry, border irregularity, color variegation, and a diameter of 6 mm or greater) method of early detection of melanoma and dysplastic (abnormal in size or shape) moles (see discussion, Chapter 4 and Fig. 4-6).
Actinic keratosis is a premalignant form of skin cancer (see Fig. 4-7). With actinic keratosis, overexposure to sunlight results in abnormal cell growth, causing a well-defined, crusty patch or bump on sun-exposed parts of the body.
Clients often point out skin lesions or ask the therapist about various lumps and bumps. In addition, the therapist may observe changes in skin, skin lesions, or aberrant tissue during the visual inspection and palpation portion of the examination (see Chapter 4) that need further medical investigation. Mortality is reduced when lesions are found early and treated promptly. Therapists can and should be a part of the screening process for skin cancer.
The cause of skin cancer is well known. Prolonged or intermittent exposure to UVR from the sun, especially when it results in sunburn and blistering, damages DNA. The majority of all NMSCs occur on parts of the body unprotected by clothing (i.e., face, neck, forearms, and backs of hands) and in persons who have received considerable exposure to sunlight.
Risk Factor Assessment: All adults, regardless of skin tone and hair color, are at risk for skin cancer; however, some people are at much greater risk than others (Box 13-3). In general, individuals with red, blonde, or light brown hair with light complexion and maybe freckles, many of Celtic or Scandinavian origin, are most susceptible; persons of African or Asian origin are least susceptible.
The most severely affected people usually have a history of long-term occupational or recreational sun exposure. Australia and New Zealand have the highest incidence of melanoma in the world. New Zealand has nearly 5 times the amount of skin cancer that occurs in the United States.
Melanoma occurs in every part of the North American continent. In the United States, the five states with the highest predicted incidence of new cases are California, Florida, Texas, New York, and Pennsylvania. Men are more likely than women to develop nonmelanoma and melanoma skin cancers. The rate of melanoma is 10 times higher for whites than blacks because blacks have the protective effects of skin pigment.74
Many older adults assume that skin changes are a “normal” sign of aging and do not see a physician when lesions first appear. Early detection and referral is always the key to a better prognosis. In asking the three important questions, the therapist plays an instrumental part in the cancer screening process.
An increased incidence of skin cancers has been noted after solid organ transplantation, especially liver, heart, and kidney transplants. Squamous and basal cell carcinomas are 250 and 10 times more frequent, respectively, in transplant recipients compared with the general population.75 Skin cancers developing in transplant recipients are more aggressive, making early detection and intervention imperative. Renal transplant recipients have a cumulative increase that corresponds with the number of years posttransplantation (e.g., 7% after 1 year of immunosuppression, 45% after 11 years, 70% after 20 years).75
Basal Cell Carcinoma: Basal cell carcinoma involves the bottom layer of the epidermis and occurs mainly on any hair-bearing area exposed to the sun (e.g., face, neck, head, ears, hands). Occasionally, basal cell carcinoma may appear on the trunk, especially the upper back and chest. These lesions grow slowly, attaining a size of 1 to 2 cm in diameter, often after years of growth. Metastases almost never occur, but neglected lesions may ulcerate and produce great destruction, ultimately invading vital structures.
Squamous Cell Carcinoma: Squamous cell carcinoma arises from the top of the epidermis and is found on areas often exposed to the sun, which are typically the rim of the ear, the face, the lips and mouth, and the dorsa of the hands. These lesions appear as small, red, hard nodules with a smooth or warty surface. The central portion may be scaly, ulcerated, or crusted. Premalignant lesions include sun-damaged skin or dysplasias (whitish-discolored areas), scars, radiation-induced keratosis, actinic keratosis (rough, scaly spots), and chronic ulcers.
Metastases are uncommon but are much more likely to occur in lesions arising in chronic leg ulcers, burn scars, and areas of prior x-ray exposure. Although these tumors do not usually metastasize, they are potentially dangerous. They may infiltrate surrounding structures and metastasize to lymph nodes and eventually to distant sites, including bone, brain, and lungs, to become fatal. Invasive tumors are firm and increase in elevation and diameter. The surface may be granular and may bleed easily.
Malignant Melanoma: Malignant melanoma (MM) is the most serious form of skin cancer. It arises from pigmented cells in the skin called melanocytes. In contrast to basal and squamous cell carcinomas, the majority of MMs appear to be associated with the intensity rather than the duration of sunlight exposure.
The average lifetime risk of developing invasive melanoma is 1 in 58 (a 2000% increase from 1930).1 An individual’s risk is much greater if any of the risk factors listed in Box 13-3 are present.
Melanoma can appear anywhere on the body, not just on sun-exposed areas. The clinical characteristics of early malignant melanoma are similar, regardless of anatomic site. Unlike benign pigmented lesions, which are generally round and symmetric, the shape of an early MM is often asymmetric.
Whereas benign pigmented lesions tend to have regular margins, the borders of early MM are often irregular (see Fig. 4-6). Round, symmetric skin lesions such as common moles, freckles, and birthmarks are considered “normal.” If an existing mole or other skin lesion starts to change and a line drawn down the middle shows two different halves, medical evaluation is needed.
Compared with benign pigmented lesions, which are more uniform in color, MMs are usually variegated, ranging from various hues of tan and brown to black, sometimes intermingled with red and white. The diameters of MM are often 6 mm or larger when first identified.
The most common sites of distant metastasis associated with MM are the skin and subcutaneous tissue, lungs, and surrounding visceral pleura, although any anatomic site may be involved. In-transit metastases (unique malignancies that have spread from the primary tumor but may not have reached the regional lymph nodes) typically develop multiple bulky tumors on an arm or leg. Often, these tumors cause pain, swelling, bleeding, ulceration, and decreased mobility.76
Other signs that may be important include irritation and itching; tenderness, soreness, or new moles developing around the mole in question; or a sore that keeps crusting and does not heal within 6 weeks. Benign moles tend to be flat, hairless, round or oval, and less than 6 mm in diameter. Pigmentation is generally even. Although there may be color variations, especially in shades of brown, benign moles, freckles, “liver spots,” and other benign skin changes are usually of a single color (most often, a single shade of brown or tan). A single lesion with more than one shade of black, brown, or blue may be a sign of malignant melanoma.
Adolescents frequently have nevi with irregular borders, multiple shades of pigment, or both. Most are normal variations of benign nevi, but a physician should examine any lesion that arouses clinical suspicion or is of concern to the client.
If any of these signs and symptoms is present in a client whose skin lesion has not been examined by a physician, a medical referral is recommended. If the client is planning a follow-up visit with the physician within the next 2 to 4 weeks, the client is advised to point out the mole or skin changes at that time. If no appointment is pending, the client is encouraged to make a specific visit either to the family/personal physician or to a dermatologist.
Resources: The Skin Cancer Foundation (www.skincancer.org) has many public education materials available to help the therapist identify suspicious skin lesions. In addition to its website, the Skin Cancer Foundation has posters, brochures, videos, and other materials available for use in the clinic. It is highly recommended that these types of education materials be available in waiting rooms as part of a nationwide primary prevention program.
Other websites, such as the Melanoma Education Foundation at www.skincheck.com and the Melanoma Foundation of the University of Sydney, Australia at http://melanomafoundation.com.au/, provide additional photos of suspicious lesions with additional screening guidelines. For information on ratings of sunscreen sold in the United States, see the Environmental Awareness Group’s Sunscreen Guide at www.ewg.org/2010sunscreen.
The therapist must become as familiar as possible with what suspicious skin aberrations may look like in order to refer as early as possible. Prognosis in melanoma is directly related to the depth of the neoplasm. Melanoma typically start growing horizontally within the epidermis (in situ) but then become invasive as tumor cells penetrate into the dermis. The vertical depth of the melanoma correlates with prognosis.77 That is why early detection and referral is so important. See also the discussion on Examining a Mass or Skin Lesion in Chapter 4.
Pulmonary Manifestations
Pulmonary metastases are the most common of all metastatic tumors because venous drainage of most areas of the body passes through the superior and inferior venae cavae into the heart, making the lungs the first organ to filter malignant cells. Primary bone tumors (e.g., osteogenic sarcoma) metastasize first to the lungs.
Pleural pain and dyspnea may be the first two symptoms experienced by the person (Case Example 13-4). When either or both of these pulmonary symptoms occur, look for increased symptoms with deep breathing and activity. Ask about a productive cough with bloody or rust-colored sputum. Ask about new onset of wheezing at any time or difficulty breathing at night. Symptoms that are relieved by sitting up are indicative of pulmonary impairment and must be reported to the physician.
Symptoms may not occur until tumor cells have expanded and become large enough or invasive enough to reach the parietal pleura, where pain fibers are stimulated. The lining surrounding the lungs allows no pain perception, so it is not until the tumor is large enough to press on other nearby structures or against the chest wall that symptoms may first appear.
Lung cancer is the most common primary tumor to metastasize to the brain. Tumor cells from the lung embolizing via the pulmonary veins and carotid artery can result in metastases to the central nervous system (CNS). Anyone with a history of lung cancer should be screened for neurologic involvement. In any individual, any neurologic sign may be the presentation of a silent lung tumor.78
Neurologic Manifestations
As just mentioned, cancer metastasis to the CNS is a common problem. Secondary metastases to the brain are 10 times more common than primary brain tumors. In all, 20% to 25% of individuals with primary sites outside of the CNS will develop brain metastases.79 The most common primary cancers with metastases to the brain are lung, colon, kidney, skin (melanoma), and breast cancer (Case Example 13-5).
Tumor cells can easily embolize via the pulmonary veins and carotid artery to the brain. The blood–brain barrier does not prevent invasion of the brain parenchyma by circulating metastatic cells. Metastatic brain tumors can increase intracranial pressure, obstruct the normal flow of cerebrospinal fluid, change mentation, and reduce sensory and motor function.
Whether the pressure-causing lesion is a primary cancer of the brain or spinal cord or whether it is a cancer that has metastasized to the CNS, clinical signs and symptoms of pressure will be the same because in both cases, the same system is affected.
Primary tumors can also cause peripheral nervous system (PNS) problems when tumors compress, impinge, or infiltrate any of the nerve plexuses. No matter where neural compression occurs, the primary sign is unrelenting pain (worse at night) followed by development of weakness. Watch for focal sensory disturbances or weakness in the distribution of the affected plexus or spinal cord segment involved. Brachial plexopathy most commonly occurs in carcinoma of the breast and lung; lumbosacral plexopathy is most common with colorectal and gynecologic tumors, sarcomas, and lymphomas.80,81
Clinical Signs and Symptoms
Brain tumors can be asymptomatic. When symptoms do occur, they are usually general or focal symptoms, depending on the size and location of the lesion. For example, if a tumor is growing in the motor cortex, the client may develop isolated extremity weakness or hemiparesis. If the tumor is developing in the cerebellum, coordination may be affected with ataxia as an observable sign.
Two of the most common clinical manifestations of brain tumor are headache and personality change, but personality change is often attributed to depression, delaying the diagnosis of brain tumor. Tumors that affect the frontal lobes are most likely to produce personality changes. Seizures occur in approximately one-third of persons with metastatic brain tumors.
Headaches occur in 30% to 50% of persons with brain tumors and are usually bioccipital or bifrontal. They are usually intermittent and of increasing duration and may be intensified by a change in posture or by straining.
The headache is characteristically worse on awakening because of differences in CNS drainage in the supine and prone positions; it usually disappears soon after the person arises. It may be intensified or precipitated by any activity that increases intracranial pressure, such as straining during a bowel movement, stooping, lifting heavy objects, or coughing.
Often, the pain can be relieved by taking aspirin, acetaminophen, or other moderate painkillers. Vomiting with or without nausea (unrelated to food) occurs in about 25% to 30% of people with brain tumors and often accompanies headaches when there is an increase in intracranial pressure. If the tumor invades the meninges, the headaches will be more severe.
Focal manifestations of a space-occupying brain lesion are caused by the local compression or destruction of the brain tissue, as well as by compression secondary to edema. Papilledema (edema and hyperemia of the optic disc) may be the first sign of intracranial tumors. Visual changes do not occur until prolonged papilledema causes optic atrophy.
Nerve and Cord Compression
Symptoms of nerve and/or cord compression may occur when tumors invade and impinge directly on the spinal cord, thecal sac, or nerve root.82 Severe destructive osteolytic lesions of the vertebral bodies from metastases can lead to pathologic fracture, fragility, and subsequent deformity of one or more vertebral bodies. Bone collapse can occur spontaneously or following trivial injury, sometimes with bone fragments adding to the compression.83
Compressive pathologies affecting the spinal cord and nerve roots affect 5% to 10% of all people with cancer.82 The thoracic spine is affected most often (70% of all cases), usually secondary to metastatic lung and breast cancer. Twenty per cent develop in the lumbosacral region as a result of metastases from prostate and gastrointestinal cancers or melanoma. A small number of cases (10%) arise in the cervical region of the vertebral column.84,85
Other (more rare) cancer-related causes of spinal cord compression include radiation myelopathy, malignant plexopathy, and paraneoplastic disorders. Chronic progressive radiation myelopathy can occur in anyone who has received irradiation to the spine or nearby structures. Localized spinal cord dysfunction within the area of the radiation port occurs with numbness and upper motor neuron findings.86
Whether from a primary cord tumor or a metastasis, compression of the cord can be the first symptom of cancer. Prostate, lung, and breast cancers are the most common tumors to metastasize to the spine, leading to epidural spinal cord compression, but lymphoma, multiple myeloma, and carcinomas of the colon or kidney and sarcomas can also result in spinal cord and nerve root compression.87
Individuals with lymphoma or retroperitoneal tumors may suffer cord compression from tumors that grow through the intervertebral foramen and compress the cord without involving the vertebra.87 Cord compression is becoming increasingly common, as individuals affected by cancer survive longer with medical treatment.
Signs and Symptoms of Cord Compression: Spinal cord compression with resultant quadriplegia, paraplegia, and possible death is the most common pathologic feature of all tumors within the spinal column. Pain and sensory symptoms usually occur in the body below the level of the tumor but not necessarily at predictable levels. For example, 54% of individuals with T1-T6 compression have lumbosacral pain, and a similar number with lumbosacral compression have thoracic pain.88
The location of the metastasis is proportionate to the volume or mass of bone in each region: 60% of metastases occur in the thoracic spine, 30% in the lumbosacral spine, and 10% in the cervical spine.89,90 Compression at the level of the cauda equina is relatively rare (0.7%).91 The therapist must observe carefully for subtle objective neurologic deficit (e.g., decreased sensory function, decreased but useful motor function, change in reflexes) that might otherwise be interpreted as side effects of medication.59
Breast and lung cancers typically cause thoracic lesions, whereas colon and pelvic carcinomas are more likely to affect the lumbosacral spine. In up to one-third of affected individuals, spinal cord compression occurs at multiple sites.87
Early characteristics of spinal cord compression include pain, sensory loss, muscle weakness, and muscle atrophy. Back pain at the level of the spinal cord lesion occurs in up to 95% of cases, presenting hours to months before the compression is diagnosed.
Pain is caused by the expanding tumor in the bone, bone collapse, and/or nerve damage. Pain is usually described as sharp, shooting, deep, or burning and may be aggravated by lying down, weight bearing, bending, sneezing, or coughing.87
Discomfort may occur as thoracolumbar back pain in a beltlike distribution; the pain may extend to the groin or the legs. The pain may be constant or intermittent and occurs most often at rest; pain occurring at night can awaken an individual from sleep; the person reports that it is impossible to go back to sleep.
Symptoms of severe pain preceding the onset of motor weakness generally correlate with epidural compression, whereas muscle weakness and bowel/bladder sphincter dysfunction with very little pain indicates intramedullary metastasis.92
Weakness in an individual with cancer may be incorrectly attributed to fatigue, anemia, pain medication, or metabolic derangement. The therapist must remain alert to any subtle signs and symptoms of spinal cord compression as the underlying etiology and report these to the physician immediately.84,86
Over half of individuals present with sensory changes, either starting in the toes and moving caudally in a stocking-like pattern to the level of the lesion or starting 1 to 5 levels below the level of the actual cord compression.89
Less commonly, chest or abdominal pain may occur, caused by nerve root compression from epidural tumor(s). Progressive cord compression is manifested by spastic weakness below the level of the lesion, decreased sensation, and increased weakness. Bowel and bladder dysfunction are late findings.
Cauda Equina Syndrome: Cauda equina syndrome is defined as a constellation of symptoms that result from damage to the cauda equina, the portion of the nervous system below the conus medullaris (i.e., lumbar and sacral spinal nerves descending from the conus medullaris). Although tumors are the focus here, other causes of cauda equina syndrome include acute lumbar disk herniation, spinal stenosis, spinal infection, epidural hematoma, and spinal fracture or dislocation. This syndrome involves peripheral nerves (sensory and motor) within the spinal canal and thecal sac.93
Individuals with cauda equina syndrome present differently from those with spinal cord compression. The three most common symptoms of cauda equina syndrome include saddle anesthesia, bowel or bladder dysfunction, and lower extremity weakness.94
Diminished sensation over the buttocks and posterior-superior thighs is also common.
Decreased anal (rectal) sphincter tone, urinary retention, and overflow incontinence occur in 60% to 80% of patients at the time of diagnosis. About half of clients need urinary catheters.89
Individuals with cauda equina syndrome caused by neoplasm may present with a long history of back pain and paresthesias; urinary difficulties are very common.95 The presentation may mimic a diskogenic source, causing a delay in diagnosis, especially in the young adult with a primary tumor. Individuals with metastatic tumors are older with a previous history of cancer.93
Associated signs and symptoms of primary or metastatic tumors causing cauda equina syndrome may include abnormal weight loss, hematuria, hemoptysis, melena, and/or constipation. The medical diagnosis of cauda equina syndrome is not always straightforward. Abnormal rectal tone may be delayed in individuals presenting with cauda equina syndrome. This is because sensory nerves are smaller and more sensitive than motor nerves; even so, some people present with abnormal rectal tone (motor) without saddle anesthesia.96
Peripheral Neuropathy: Peripheral neuropathy with loss of vibratory sense, proprioception, and deep tendon reflexes is most often chemotherapy-related (e.g., cisplatin, Taxol, vincristine). Numbness, tingling, and burning pain in the hands and feet and loss of balance and difficulties with mobility are common with this problem.97 It is important to differentiate the type and etiology of peripheral neuropathy before planning treatment intervention.
For example, chemotherapy-induced peripheral neuropathy (CIPN) may not be as likely to respond to lymph drainage and compression bandaging, whereas good results have been seen when this treatment intervention is used for weakness and paresthesias from lymphedema-induced nerve compression (e.g., breast cancer, ovarian cancer, testicular cancer).
In other words, resolution of neuropathy symptoms utilizing principles of manual lymphatic drainage may confirm subclinical lymphedema as the major etiologic factor in some clients.
Paraneoplastic Syndromes
Other neurologic problems occur frequently in individuals with cancer. These may be nonmetastatic and associated with cancer-related opportunistic infections, metabolic disturbances, vascular complications, treatment neurotoxicity, and paraneoplastic syndromes.
When tumors produce signs and symptoms at a distance from the tumor or its metastasized sites, these “remote effects” of malignancy are collectively referred to as paraneoplastic syndromes. This can be the first sign of malignancy and may show up months (even years) before the cancer is detected. They are usually caused by one of three phenomena:
The causes of these syndromes are not well understood. In contrast to the hormone syndromes in which the cancer directly produces a substance that circulates within blood to produce symptoms, the neurologic syndromes are a group of syndromes mediated by the immune response.
Tumors involved in this type of syndrome stimulate the production of immunologically active nervous system proteins. These immune responses are frequently associated with antineuronal antibodies that can be used as diagnostic markers of paraneoplastic disorders. As a result of these immune responses, discrete or multifocal areas of nervous system degeneration can occur, causing diverse symptoms and deficits.98
These are not direct effects of either the tumor or its metastases. Cancer cells can acquire new cellular functions uncharacteristic of the originating tissue. Many of these syndromes involve ectopic hormone production by tumor cells. These hormones are distributed by the circulation and act on target organs at a site other than the location of the tumor. Some tumor cells secrete biochemically active substances that can also cause metabolic abnormalities.
The reported frequency of paraneoplastic syndromes ranges from 10% to 15% to 2% to 20% of malignancies. However, these could be underestimates. Neurologic paraneoplastic syndromes are estimated to occur in fewer than 1% of patients with cancer.99
The neuromusculoskeletal system is often affected, and the clinical presentation is unusual. The clinical manifestation of paraneoplastic syndrome depends on the tumor effects. The therapist is often the first health care professional to see and/or recognize the incongruence of the signs and symptoms.
In fact, the presentation may confound the medical staff. When the client fails to respond to palliative treatment, physical therapy is recommended. The alert therapist will recognize the unusual presentation and will follow up with a screening examination.
The paraneoplastic syndromes are of considerable importance because they may accompany relatively limited neoplastic growth and provide an early clue to the presence of certain types of cancer (e.g., osteoarthropathy caused by bronchogenic carcinoma, hypercalcemia from osteolytic skeletal metastases). The most common cancer associated with paraneoplastic syndromes is small cell cancer of the lungs (produces adrenocorticotrophic hormone [ACTH] and causes Cushing’s syndrome).
Clinical Signs and Symptoms of Paraneoplastic Syndromes: Clinical findings of paraneoplastic syndromes may resemble those of primary endocrine, metabolic, hematologic, or neuromuscular disorders. Depending on which system is compromised, symptoms can include rheumatologic, renal, GI, vascular, hematologic, cutaneous, metabolic, endocrine, neurologic, and/or neuromuscular physical findings.100-102
For example, the Lambert-Eaton myasthenic syndrome (LEMS), often secondary to small cell lung carcinoma, results in muscle weakness when autoantibodies directed against the presynaptic calcium channels at the neuromuscular junction cause impaired release of acetylcholine from presynaptic nerve terminals. The clinical presentation is distinct from myasthenia gravis (MG), with lower limb muscle fatigability and autonomic symptoms appearing first in the paraneoplastic form.103
Gradual, progressive muscle weakness during a period of weeks to months (especially of the pelvic girdle muscles) may occur. Proximal muscles are most likely to be involved (see Fig. 13-3). The weakness does stabilize. Reflexes of the involved extremities are present but diminished. The weakness often improves, and deep tendon reflexes may return with exercise.
In clients who develop myopathies, such as dermatomyositis (DM) or polymyositis (PM), the myositis may precede, follow, or arise concurrently with the malignancy. No particular type of cancer has been found to predominate in such cases, but the clients affected are generally older and respond poorly to medical treatment for the myositis.
The course of the paraneoplastic syndrome usually parallels that of the tumor. Therefore effective medical intervention (rather than physical therapy) should result in resolution of the syndrome. A paraneoplastic syndrome may be the first sign of a malignancy or recurrence of cancer that may be cured if detected early. Paraneoplastic syndromes with musculoskeletal manifestations are listed in Table 13-6.
TABLE 13-6
Paraneoplastic Syndromes Having Musculoskeletal Manifestations
Data from Santacroce L: Paraneoplastic syndromes, eMedicine Specialties, 2010. Available online at www.emedicine.com/med/TOPIC1747.HTM. Accessed March 7, 2011.
Even such nonspecific symptoms as anorexia, malaise, weight loss, and fever are truly neoplastic and are probably due to the production of specific factors by the tumor itself. For example, anorexia is a common symptom in clients with cancer that is attributed to tumor production of the protein tumor necrosis factor (TNF), also called cachectin. Fever may be seen in clients with cancer in the absence of infection when it is produced by tumor induction of pyrogen formation by host white cells or by direct tumor production of a pyrogen.
Rheumatologic Manifestations: Cancer can be associated with arthritis and can present as a paraneoplastic syndrome called carcinoma polyarthritis. Paraneoplastic rheumatic disorders of this type are induced by the malignancy through hormones, peptides, autocrine and paracrine mediators, antibodies, and cytotoxic lymphocytes.104 Polyarthritis has been reported in adults ages 43 to 80 years of age when associated with solid tumors and in individuals from 12 to 65 years old with hematologic malignancies.105
Cancer-associated rheumatic syndromes are characterized by a relatively short interval between the appearance of the rheumatic disorder and diagnosis of its associated neoplasm (usually less than 2 years).104
Rheumatic disorders caused by cancer have been associated most often with breast and lung cancers. Palmar fasciitis and polyarthritis have been reported in association with metastatic ovarian carcinoma (Case Example 13-6).106 Even though cancer polyarthritis is a fairly uncommon occurrence, the therapist is more likely than most other health care professionals to see this. Timely recognition can reduce morbidity and mortality.
The medical diagnosis can be missed or delayed without careful evaluation. Sometimes, the diagnosis of polymyalgia rheumatica is made in error. Anyone with a sudden onset of rheumatic disease that is seronegative and monarticular and occurs in the presence of a past history of cancer may be demonstrating signs of metastatic cancer or an occult malignancy.
Rheumatologic complaints have a sudden onset and may spare the small joints of the hands and wrists. Clinical features of carcinoma polyarthritis primarily affect asymmetric joints of the lower extremities, often the result of metastasis to the joint or periarticular bone.
Other rheumatologic conditions and muscular disorders can be associated with malignancy (Box 13-4). These conditions often disappear after successful treatment of the underlying malignancy.105,107
There is also a link between longstanding rheumatic disorders and cancer. The risk of malignant transformation during the course of chronic rheumatic disorders, including rheumatoid arthritis, Sjögren’s syndrome, and systemic sclerosis is well-known. The underlying mechanism is likely the result of immune dysregulation.108
Digital Clubbing: Digital clubbing is another possible sign of paraneoplastic syndrome, especially when associated with pulmonary malignancy (see Fig. 4-36). Clubbing of the fingers and toes is seen most often with chronic conditions such as congenital heart disease with cyanosis, cystic fibrosis, or chronic obstructive pulmonary disease (COPD). It can also develop with paraneoplastic syndromes and within 10 days of acute systemic illness such as acute pulmonary abscess, heart disease, and ulcerative colitis.
Digital clubbing occurs in the distal phalanx and causes the ends of the digits to become round and wide, like “little clubs.” The thumb and index finger are affected first and can be assessed by the Schamroth method (see Fig. 4-37).
Look for recent onset of other signs and symptoms (e.g., pulmonary, hepatic, cardiac, gastrointestinal). For example, digital clubbing accompanied by a recent, unexplained weight loss, hemoptysis, and a significant smoking history may be a red flag sign associated with lung cancer.
Skeletal Manifestations
Primary bone cancer is uncommon; primary cancers of the musculoskeletal system are discussed later in this chapter. The skeleton is, however, the most common organ affected by metastatic cancer. Tumors arising from the breast, prostate, thyroid, lung, and kidney possess a special propensity to spread to bone.
Tumor cells commonly metastasize to the most heavily vascularized parts of the skeleton, particularly the red bone marrow of the axial skeleton and the proximal ends of the long bones (humerus and femur), and the vertebral column, pelvis, and ribs (Box 13-5).
Occasionally, a growing bone mass is the first sign of disease. Diagnosis is made by x-ray study and surgical biopsy, requiring immediate attention to suspicious symptoms by referral to the client’s physician.
Local swelling can be detected when the lesion protrudes beyond the normal confines of the bone. The swelling of a benign lesion is usually firm and nontender. In the presence of a rapidly growing malignant neoplasm, however, the swelling is more diffuse and frequently tender (Fig. 13-6).
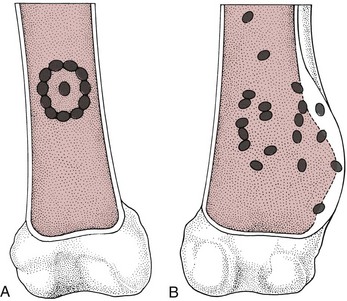
Fig. 13-6 A, Benign bone tumors have a characteristic sclerotic rim around the periphery of the lesion. The lesion is usually well defined, and there is no evidence of erosion of the cortex or a soft tissue mass. B, Malignant bone tumors can have lytic or sclerotic components. It is frequently difficult to know the extent of the lesion within the bone because there is no well-defined sclerotic rim around the tumor. The destructive process is diffuse within the medullary cavity of the bone, and the tumor may break through the cortex of the bone, producing Codman’s triangle. Frequently, an associated soft tissue mass is present. Medical differential diagnosis of this lesion is between an osteogenic sarcoma and a chondrosarcoma.
The overlying skin may be warm because of the highly vascularized nature of neoplasms. If the lesion is close to a joint, function in that joint may be disturbed, with painful and restricted range of motion.
Bone Pain
Bone pain, resulting from structural damage, rate of bone resorption, periosteal irritation, and nerve entrapment, is the most common complication of metastatic disease to the skeletal system. A history of sudden onset of severe pain usually indicates the complication of a pathologic fracture (a break in an already weakened bone). Pathologic fractures are the result of metastatic disease of primary cancers most often affecting the lung, prostate, and breast.
Pathologic fractures tend to affect the vertebral body at both the thoracic and lumbar levels. Kyphotic deformity can occur with compression of the cord or cauda equina (see further discussion on Cauda Equina Syndrome in this chapter).
Bone pain is usually deep, intractable, and poorly localized, sometimes described as burning or aching and accompanied by episodes of stabbing discomfort (Case Example 13-7). The pain may be cyclic and progressive until it becomes constant. The pain is made worse by activity, especially weight bearing. The pain is often associated with trauma during a game or exercise and may be dismissed in children as “growing pains.”
It is often worse at night, awakening the person; neither sleep nor lying down provides relief. Pain at night that is unrelieved by rest or change in position is a red flag. Assessing night pain is discussed in detail in Chapter 3 in Night Pain and Cancer (see Box 3-7).
Beware of the client who reports disproportionate (excessive) pain relief with aspirin, as this may be a sign of a particular bone cancer called osteoid osteoma. Pain subsiding with aspirin (contains salicylates) is the hallmark of this entity. Salicylates inhibit the prostaglandins that are produced by osteoid osteomas.
Bone pain associated with skeletal metastases can often be reproduced with a heel strike when an undiagnosed fracture is present in the lower extremities. Watch for pain on weight bearing with a positive heel strike test or reproduced symptoms when hopping on one leg (in the younger client; this is not a likely test to use in the older adult). Perform translational/rotational tests for stress fracture.
The pain does not respond to physical agents or physical therapy intervention. Sometimes, the client has some relief after the first few sessions of physical therapy, but pain returns and may even be worse than before. The therapist may think the chosen intervention has been unsuccessful and is at fault. Consider it a red flag whenever a client fails to improve or improves and then gets worse. Further investigation and screening is advised under these circumstances.
Pain may occur around joints because of mechanical, chemical, or bony change; pain and the rate of bone resorption appear to be linked. There is often disturbance of the highly innervated periosteum, giving bone pain its neurogenic-like qualities, especially its unrelenting, intractable quality.
Fracture
Pathologic fractures (e.g., vertebrae, long bones) occur in half of all people with osteolytic metastases. In fact, this may be the presenting sign of bone cancer. An injury with subsequent medical evaluation reveals the fracture and the cancer simultaneously.
Back Pain
Neoplastic disease can cause backache, particularly in older adults, or shoulder pain in the presence of breast cancer. Although primary neoplasms of the spine are rare, myeloma and metastatic disease are more common. Malignancy as a cause of low back pain in primary care clients accounts for only 1% of the affected population.109
In anyone with a known cancer, the onset of back pain could suggest spinal metastases. An insidious onset of waist-level or midback pain that becomes progressively more severe and more persistent often occurs. The pain is usually unrelieved by lying down and frequently becomes worse at night. Unexplained weight loss with severe back pain aggravated by rest may point to metastatic carcinoma of the spine. Other bone-related cancers, such as multiple myeloma, can cause severe, unremitting backaches that are present at rest and become worse when lying down.
Cancer causing low back pain can be ruled out with 100% sensitivity if the client is less than 50 years old, has not experienced unexplained or unintentional weight loss, has never had cancer before, and has responded to the physical therapist’s intervention.110,111 Referral to a spine specialist may not be needed if radiographs have not been taken yet and/or laboratory tests have not been ordered to complete a simple screening strategy.110,112 If the therapist is not in a setting that allows these next steps to be generated from within the department, then referral may be advised.
Hypercalcemia from Skeletal Metastases
Hypercalcemia (greater than normal amounts of calcium in the blood) occurs frequently in clients with metastatic bone disease who have osteolytic lesions (Fig. 13-7). Normal serum calcium levels range between 8.2 and 10.2 mg/dL. Mild hypercalcemia occurs when this level drops to around 12 mg/dL; severe hypercalcemia is defined by serum calcium at 14 mg/dL or more.
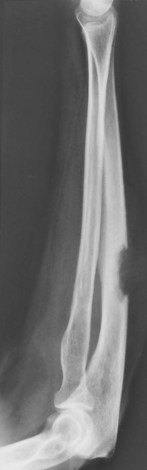
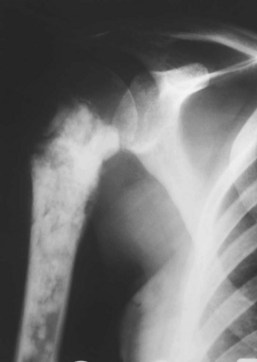
Fig. 13-7 Lytic versus blastic bone. In the top x-ray, you will see a lytic bone lesion from breast cancer. Notice how the bone has distinct punched out segments. This is characteristic of a lytic lesion. On the bottom, you will see blastic bone lesions from osteosarcoma. The blastic form of bone cancer is a more diffuse pattern of degeneration. (From Dorfman HD, Czerniak B: Bone tumors, St. Louis, 1998, Mosby.)
Hypercalcemia is very common in cases of breast cancer and myeloma, primarily because of an increase in bone resorption, which is caused in turn by tumor cell production of parathyroid hormone–related protein that stimulates osteoclastic bone resorption.90,113
Other tumors associated with hypercalcemia may include carcinomas of the lung (most commonly, small cell lung cancers), squamous cell carcinoma of the head and neck, renal cell cancer, prostate cancer, lymphoma and leukemia, thyroid cancer, and parathyroid carcinoma (rare). In most cases, hypercalcemia is an indication of progression of disease. Hypercalcemia associated with metastatic breast cancer involving bone may occur with hormone therapy.
Hypercalcemia is characterized by musculoskeletal, nervous system, cardiovascular/pulmonary, and GI symptoms (Table 13-7). The therapist may see the first signs and symptoms of hypercalcemia in the musculoskeletal system but should watch for others as well.
Signs and symptoms of CNS-related hypercalcemia are similar to other causes of CNS problems and include confusion, drowsiness, lethargy, headache, depression, and irritability. Hypercalcemia can also affect the GI system. The most common hypercalcemia-induced GI signs and symptoms are anorexia, nausea, vomiting, constipation, dehydration, and thirst.
Finally, in the clinical practice of a therapist, hypercalcemia secondary to bone cancer or metastases to the bone may affect the cardiac system. These clients are usually inpatients or are known to have cancer. Hypertension may be the only outward sign of hypercalcemia-induced cardiovascular changes. Vital sign assessment may help identify early signs of cardiac involvement. However, cardiac arrest may present as the first sign of a problem.
Bisphosphonates (bone resorption inhibitors, such as Fosamax [alendronate], Actonel [risedronate], Evista [raloxifene], and Miacalcin [calcitonin-salmon]) are drugs used to control hypercalcemia and limit or prevent bone loss. In emergent or predictable situations, intravenous use of bisphosphonates (e.g., pamidronate, zoledronic acid) can be used to stabilize and/or prevent hypercalcemia. With their use, health care professionals expect to see fewer cases of hypercalcemia than in the past. These drugs also reduce bone pain, delay skeletally related events (SREs), reduce the number of pathologic fractures, and in some cases, prolong survival.
Hepatic Manifestations
Liver metastases are among the most ominous signs of advanced cancer. The liver filters blood coming in from the GI tract, making it a primary metastatic site for tumors of the stomach, colorectum, and pancreas.
Symptoms observed in a physical therapy practice include bilateral carpal/tarsal tunnel syndrome, possibly accompanied by abdominal pain and tenderness with general malaise and fatigue. Right upper quadrant pain with possible referral to the right shoulder may also occur with or without carpal tunnel syndrome (see Table 13-5).
Carpal Tunnel Syndrome
Carpal tunnel syndrome (CTS) can be caused by a wide range of both neuromusculoskeletal and systemic conditions and illnesses (see Table 11-2). Whenever anyone presents with bilateral symptoms of any kind, it is considered a “red flag” symptom. In Chapter 2 of this text, we discussed the various bilateral symptoms the therapist might encounter in a clinical practice.
A common systemic cause of CTS involves the hepatic system (see Chapter 9 for an explanation). Briefly, liver dysfunction results in increased serum ammonia and urea levels. When these toxins are no longer absorbed into the portal vein and removed from the body, they pass directly to the brain.
Ammonia transported to the brain reacts with glutamate (an excitatory neurotransmitter), producing glutamine. The reduction of brain glutamate impairs neurotransmission. This leads to altered CNS metabolism and function. As blood ammonia levels rise, many unusual compounds (e.g., octopamine) form and serve as false neurotransmitters in the CNS. Asterixis (also known as liver flap) and numbness/tingling occur as a result of this ammonia abnormality, causing intrinsic nerve pathology. This can be misinterpreted as CTS (or tarsal tunnel syndrome) (Case Example 13-8).
When screening for bilateral CTS as a result of liver impairment, always ask about the presence of similar symptoms in the feet. Look for a history of alcoholism, cirrhosis, previous cancer, other liver disease, and the use of statins (cholesterol-lowering drugs such as simvastatin [Zocor] and atorvastatin calcium [Lipitor]; liver damage occurs in some people taking these medications).
Ask about the presence of other GI signs and symptoms. A client presenting with shoulder or upper back pain may not think nausea and abdominal bloating are related in any way to the symptoms in the wrists and hands. Perform a quick liver screen and look for signs of liver disease (see Box 9-1 or Appendix B-4).
Oncologic Pain
As mentioned earlier, pain is rarely an early warning sign of cancer and is uncommon in some cancers such as leukemia. However, pain occurs in 60% to 80% of clients with solid tumors.
This pain syndrome has multiple causes, and the therapist must always keep in mind common patterns of referred pain (see Chapter 3; see also Table 3-8). Some pain is caused by pressure on peripheral nerves or displacement of these nerves. Pain may also result from interference with blood supply or from blockage within hollow organs.
A common cause of cancer pain is metastasis of cancer to the bone. This type of pain can occur as a result of pathologic fracture with resultant muscle spasms; if the spine is involved, nerves may be affected. Pain may also result from iatrogenic causes such as surgery, radiation therapy, and chemotherapy. Immobility and inflammation also can lead to pain.
Signs and Symptoms Associated with Levels of Pain
The severity of pain varies from one client to another, but certain signs and symptoms are characteristic of particular levels of pain. For example, in mild-to-moderate superficial pain, a sympathetic nervous system response is usually elicited with hypertension, tachycardia, and tachypnea (rapid, shallow breathing).
In severe or visceral pain, a parasympathetic nervous system response is more characteristic, with hypotension, bradycardia, nausea, vomiting, tachypnea, weakness, or fainting. Depression and anxiety may increase the client’s perception of pain, requiring additional psychologic and emotional support.
Biologic Mechanisms
Five biologic mechanisms have been implicated in the development of chronic cancer pain. The characteristics of the pain depend on tissue structure, as well as on the mechanisms involved.
Bone Destruction
Bone destruction secondary to infiltration by malignant cells or resulting from metastatic lesions is the first and most common of the biologic mechanisms causing chronic cancer pain. Bone metastases cause increased release of prostaglandins and subsequent bone breakdown and resorption.
The client’s pain threshold is reduced through sensitization of free nerve endings. Bone pain may be mild to intense. Maladaptive outcomes of bone destruction may include sharp, continuous pain that increases on movement or ambulation. The rich supply of nerves and tension or pressure on the sensitive periosteum or endosteum may cause bone pain.
Other factors contributing to the intense discomfort reported by clients include limited space for relief of pressure, altered local metabolism, weakening of the bone structure, and pathologic fractures ranging in size from microscopic to large.
Visceral Obstruction
Obstruction of a hollow visceral organ and ducts, such as the bowel, stomach, or ureters, is a second physiologic factor in the development of chronic cancer pain.
Viscus obstruction is most often due to the obstruction of an organ lumen by tumor growth. In the GI or genitourinary (GU) tract, obstruction results in either a severe, colicky, crampy pain or true visceral pain that is dull, diffuse, boring, and poorly localized.
If a vein, artery, or lymphatic channel is obstructed, venous engorgement, arterial ischemia, or edema, respectively, will result. In these cases, pain is described as dull, diffuse, burning, and aching. Obstruction of the ducts leading from the gallbladder and pancreas is common in cancer of these organs, although jaundice is more frequently an earlier symptom than pain. Cancer of the throat or esophagus can obstruct these organs, leading to difficulties in eating or speaking.
Nerve Compression
Infiltration or compression of peripheral nerves is the third physiologic factor that produces chronic cancer pain and discomfort. Pressure on nerves from adjacent tumor masses and microscopic infiltration of nerves by tumor cells result in continuous, sharp, stabbing pain that generally follows the pattern of nerve distribution. The invading cells affect the conduction of impulses by the nervous system and sometimes result in constant, dull, poorly localized pain and altered sensation.
Blockage of the blood in arteries and veins, again both by pressure from tumor masses nearby and by infiltration, can decrease oxygen and nutrient supply to tissues. This deficiency can be perceived as pain that is similar in origin and character to cardiac pain or angina pectoris, which is chest pain from an insufficient supply of oxygen to the heart. Hyperesthesia or paresthesia may result.
Skin or Tissue Distention
Infiltration or distention of the integument (skin) or tissue is the fourth physiologic phenomenon resulting in chronic, severe cancer pain. This type of pain is secondary to the painful stretching of skin or tissue because of underlying tumor growth. This stretching produces severe, dull, aching, and localized pain, with severity of pain increasing concurrently with increase in tumor size.
Pain associated with headache secondary to brain tumor is thought to be due to traction on pain-sensitive intracranial structures.
Tissue Inflammation, Infection, and Necrosis
Inflammation, infection, and necrosis of tissue may be the fifth and final cause of cancer pain. Inflammation, with its accompanying symptoms of redness, edema, pain, heat, and loss of function, may progress to infection, necrosis, and sloughing of tissue.
If the inflammatory process alone is present, the pain is characterized by a sensitive tenderness. If, however, necrosis and tissue sloughing have occurred, pain may be excruciating.
Side Effects of Cancer Treatment
Conventional cancer treatment has many side effects because the goal of treatment is to remove or to kill certain tissues. In any situation, healthy tissue also is usually sacrificed. It is not always possible to differentiate between cancer recurrence and the acute or long-term effects of cancer treatment. For this reason, knowledge of both immediate and delayed side effects of cancer treatment is helpful.
For many years, three basic modalities of cancer treatment have been used, either alone or in combination: surgery, radiation therapy, and chemotherapy. In recent years, immunotherapies involving the use of cells of the immune system to prompt a tumor-killing response have been developed. Immunotherapy may be most effective when combined with conventional treatments, such as chemotherapy and radiation, to improve the success of treatment and decrease the side effects of conventional modalities.
The pharmaceuticals used in chemotherapy are cytotoxic (destructive) and are designed to kill dividing cells selectively by blocking the ability of DNA and RNA to reproduce and by lysing cell membranes. All types of rapidly dividing cells, not just the cancer cells, are affected. Damage to otherwise healthy tissue, such as bone marrow, hair follicles, mucosal cells in the mouth, digestive tract, and reproductive system, not just to cancer cells, is the cause of most side effects.
In addition, a combination of drugs (each causing cell death through different pharmacologic mechanisms) is traditionally used for greater efficacy in the systemic treatment of some cancers (e.g., breast cancer). Hence, an overlap of toxicities may result in greater side effects.
Common Physical Effects
The effects of treatment for cancer can be debilitating physiologically, physically, and psychologically. Common physical side effects include bone marrow suppression, severe mucositis, mouth sores, nausea and vomiting, fluid retention, pulmonary edema, cough, headache, CNS effects, peripheral neuropathies, malaise, fatigue, dyspnea, and loss of hair. Emotional and psychologic side effects are present but less evident (Table 13-8).
TABLE 13-8
Side Effects of Cancer Treatment
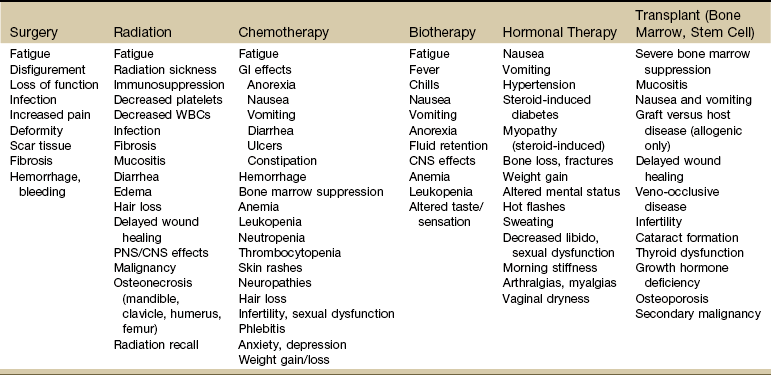
GI, Gastrointestinal; WBC, white blood cell; PNS, peripheral nervous system; CNS, central nervous system.
Adapted from Goodman CC, Fuller KS: Pathology: implications for the physical therapist, ed 3, Philadelphia, 2009, WB Saunders.
Bone marrow suppression (myelosuppression) is a common and serious side effect of many chemotherapeutic agents and can be a side effect of radiation therapy in some instances. This condition may lead to significant decreases in production of white blood cells (leukopenia), red blood cells (anemia), and, in some cases, platelets (thrombocytopenia).
Leukopenia (neutropenia) and resultant opportunistic infections have been shown to result in dose reductions, treatment delays, and hospitalizations. People at risk for leukopenia are taught infection prevention techniques and are often supportively or emergently treated with injections of colony-stimulating factors, such as granulocyte colony-stimulating factor (GCSF) or a newer version colony-stimulating factor, pegfilgrastim (Neulasta), to stimulate increased production of needed white blood cells.114
Another relatively common bone marrow treatment toxicity is anemia. A drop in the production of red blood cells and in associated hemoglobin levels causes a loss of oxygenation to many body tissues and results in the many associated symptoms of anemia such as severe fatigue, muscle weakness, dizziness, dyspnea, pallor, and tachycardia.
Red blood cell transfusions and/or the use of injectable epoetin alfa (Epogen), the recombinant form of human erythropoietin, or darbepoetin (Aranesp), a newer version of Epogen, is very useful in the treatment of anemia.
Closely related to anemia is fatigue. Cancer-related fatigue is a frequent, difficult, and often debilitating problem. It differs from fatigue of healthy people because it happens independently of rest and activity patterns.115 Factors contributing to fatigue can include many physical and emotional components of cancer such as anemia, poor nutrition, infection, low thyroid output, tumor breakdown by products, depression, pain, and medications.
Fatigue has been identified as a major determinant of perceived quality of life; it may be temporary, may persist throughout the episode of care, and may even continue many months after treatment has concluded. Adequate hydration, exercise, dietary measures, and treatment of anemia and depression, and addressing barriers to exercise are all measures used to help in the treatment of patients with cancer-related fatigue.116-120
Aggressive chemotherapeutic agents and chest irradiation can cause cardiopulmonary dysfunction, especially in the treatment of Hodgkin’s disease and breast and lung cancers. High-dose radiation can result in pericardial fibrosis (scarring of the pericardium) and constrictive pericarditis (inflammation of the pericardium). These conditions are usually asymptomatic until the client starts to exercise, and then, exertional dyspnea is the first symptom.
Other causes of dyspnea include deconditioning, anemia, peripheral arterial disease, and increased physiologic demand for oxygen because of fever or infection. During radiation therapy, the client may be more tired than usual. Resting throughout exercise is important, as are adequate nutrition and hydration.
The skin in the irradiated area may become red or dry and should be exposed to the air but protected from the sun and from tight clothing. Gels, lotions, oils, or other topical agents should not be used over the irradiated skin without a physician’s approval. Clients may have other side effects, depending on the areas treated. For example, radiation to the lower back may cause nausea, vomiting, or diarrhea because the lower digestive tract is exposed to the radiation.
Radiation recall is a severe skin reaction that can occur when certain chemotherapy drugs (e.g., Actinomycin, doxorubicin, methotrexate, fluorouracil, hydroxyurea, paclitaxel, liposomal doxorubicin) are given during or soon after radiation treatment.
The skin reaction appears like a severe sunburn or rash on the area of skin where the radiation was previously administered. It can appear weeks to months after the last dose of radiation. It is very important that this reaction be immediately reported because symptoms may be severe enough that chemotherapy must be delayed until the skin has healed.
Bone necrosis and demineralization (radiation osteonecrosis) can also result from radiation therapy and are usually not reversible. Individuals with this problem have an increased likelihood of pathologic fractures and need to be carefully handled by the therapist. Any activities, including weightbearing activities and range of motion, should be addressed prior to the initiation of therapeutic exercise.121
Monitoring Laboratory Values
It is very important to review hematologic values in clients receiving these treatment modalities before any type of vigorous physical therapy is initiated. A guideline still used by some physical therapy exercise programs is the Winningham Contraindications for Aerobic Exercise. According to these guidelines, aerobic exercise is contraindicated in chemotherapy clients when laboratory values are as follows122:
| Platelet count | <50,000/mm3 |
| Hemoglobin | <10g/dL |
| White blood cell count | <3000/mm3 |
| Absolute granulocytes | <2500/mm3 |
These guideline values have not been tested under today’s patient or treatment parameters and may not be considered valid or reliable. More specific exercise guidelines for all diseases are available.123 All treatment facilities (and even individual physicians within the center) establish their own parameters and protocols. Many centers use hemoglobin levels exclusively because hematocrit is linked with hydration and may not provide the information needed. It does not appear that there is an “industry standard” for these measures.
In an outpatient setting without the benefit of laboratory values for guidance, the therapist is advised to use vital signs as discussed in Chapter 3, along with rate of perceived exertion (RPE) during exercise. Observe for clinical signs and symptoms of infection and fever, thrombocytopenia, deep vein thrombosis, dehydration, and electrolyte imbalance.
Late and Long-Term Physical Effects
Today, more than 10 million individuals treated for various types of cancers are surviving disease-free (but not “free of their disease”) for many years following surgery, chemotherapy, immunotherapy, stem cell transplantation, and/or radiation. Many treatments are not highly specific and put normal cells, organs, and systems (especially the nervous system) at risk. Adjunctive medications, such as corticosteroids, antiepileptic medications, immunosuppressive agents, opioids, hypnotics, and antiemetics, contribute to impaired cognition.
Late effects from treatment, recurrence, secondary malignancies, and issues related to body image and quality of life are often part of the survivorship experience.124,125 Any body system can be affected with long-term sequelae determined by the specific treatment received (Box 13-6). Therapists are increasingly becoming a part of the rehabilitation of cancer survivors and will need to be aware of the potential issues faced by cancer survivors.126
These may include fatigue, lymphedema, endocrine and fertility effects in male survivors, reproductive and hormonal changes in women, sleep disturbances, impaired cognitive function, osteoporosis, spiritual issues, financial problems, poor quality of life, pain, disfigurement, neuropathy, sexuality and body image, and dental changes. There can be functional decline, poor quality of life, and psychosocial stress and difficulty coping.127
Primary muscle shortening and secondary loss of muscle activity may produce movement disorders. Radiation-induced changes in vascular networks resulting in ischemia may affect muscle contractility. Magnetic resonance imaging (MRI) studies have shown radiation-induced muscle morbidity in cervical, prostate, and breast cancers.128
The controversial term chemobrain is often used to describe mental fogginess experienced by some people during the course of chemotherapy. But this type of cognitive function is reported to persist well after treatment has ended.129 Memory lapses, difficulty concentrating or staying focused on a task, trouble remembering details (e.g., names, dates, phone numbers), and difficulty with word retrieval are just a few examples of the specific experiences represented by chemobrain. Depression, insomnia, and difficulty doing two things at once (e.g., talking on the phone and cleaning or cooking while carrying on a conversation) are also typical of people with chemobrain.129,130