
Gerhard Giebisch and Erich Windhager
Two separate but closely interrelated control systems regulate the volume and osmolality of the extracellular fluid (ECF). It is important to regulate the ECF volume to maintain blood pressure, which is essential for adequate tissue perfusion and function. The body regulates ECF volume by monitoring and adjusting the total body content of NaCl. It is important to regulate the extracellular osmolality because hypotonic or hypertonic osmolalities cause changes in cell volume (see Chapter 5) that seriously compromise cell function, especially in the central nervous system (CNS). The body regulates extracellular osmolality by monitoring and adjusting total body water content. These two homeostatic mechanisms—for ECF volume and osmolality—use different sensors, different hormonal transducers, and different effectors (Table 40-1). However, they have one thing in common: some of their effectors, although different, are located in the kidney. In the case of the ECF volume, the control system modulates the urinary excretion of Na+. In the case of osmolality, the control system modulates the urinary excretion of water.
Table 40-1 Comparison of the Systems Controlling Extracellular Fluid Volume and Osmolality

The maintenance of the ECF volume, or Na+ balance, depends on signals that reflect the adequacy of the circulation—the so-called effective circulating volume, discussed later. Low-and high-pressure baroreceptors send afferent signals to the brain (see Chapter 23), which translates this volume signal into several responses that can affect ECF over either the short term or the long term. The short-term effects (over a period of seconds to minutes) occur as the autonomic nervous system and humoral mechanisms modulate the heart and blood vessels to control blood pressure. The long-term effects (over a period of hours to days) consist of nervous, humoral, and hemodynamic mechanisms that modulate renal Na+ excretion. In the first part of this chapter, we discuss the entire feedback loop, of which Na+ excretion (see Chapter 35) is the effector.
Why is the Na+ content of the body the main determinant of the ECF volume? Na+, with its associated anions, Cl− and HCO−3, is the main osmotic constituent of the ECF volume; when Na salts move, water must follow. Because the body generally maintains ECF osmolality within narrow limits (e.g., ~290 mOsmol/kg or mOsm), it follows that whole-body Na+ content—which the kidneys control—must be the major determinant of the ECF volume. A simple example illustrates the point. If the kidney were to enhance the excretion of Na+ and its accompanying anions by 145 mEq each—the amount of solute normally present in 1 L of ECF—the kidneys would have to excrete an additional liter of urine to prevent a serious fall in osmolality. Alternatively, adding 145 mmol of “dry” NaCl to the ECF necessitates adding 1 L of water to the ECF; this addition can be accomplished by drinking water or by reducing renal excretion of solute-free water. Relatively small changes in Na+ excretion lead to marked alterations in the ECF volume. Thus, precise and sensitive control mechanisms are needed to safeguard and regulate the body’s content of Na+.
The maintenance of osmolality, or water balance, depends on receptors in the hypothalamus that detect changes in the plasma osmolality. These receptors send signals to areas of the brain that (1) control thirst and thus regulate water intake and (2) control the production of arginine vasopressin (AVP)—also known as antidiuretic hormone (ADH)—and thus regulate water excretion by the kidneys. We discuss renal water excretion in Chapter 37. In the second part of this chapter, we discuss the entire feedback loop, of which water excretion is merely the endpoint.
Why is the water content of the body the main determinant of osmolality? Total body osmolality is defined as the ratio of total body osmoles to total body water (see Chapter 5). Although the ECF-volume control system can regulate the amount of extracellular osmoles, it has little effect on total body osmoles. Total body osmoles are largely a function of the intracellular milieu because the intracellular compartment is larger than the ECF and its solute composition is highly regulated. Total body osmoles do not change substantially except during growth or during certain disease states, such as diabetes mellitus (in which excess glucose increases total body osmolality). Only by controlling water independent of Na+ control can the body control osmolality.
The two principal solutes in the ECF are Na+ and Cl−. Sodium, one of the most abundant ions in the body, totals ~58 mEq/kg body weight. Approximately 65% of the total Na+ is located in the ECF, and an additional 5% to 10% is found in the intracellular fluid. Extracellular and intracellular Na+, comprising 70% to 75% of the total body pool, is readily exchangeable, as defined by its ability to equilibrate rapidly with injected radioactive Na+. The remaining 25% to 30% of the body’s Na+ pool is bound as Na+ apatites in bone. The concentration of Na+ in the plasma and interstitial fluid typically ranges between 135 and 145 mM.
Chloride totals ~33 mEq/kg body weight. Approximately 85% is extracellular, and the remaining 15% is intracellular. Thus, all Cl− is readily exchangeable. The [Cl−] of plasma and interstitial fluid normally varies between 100 and 108 mM. Changes in total body Cl− are usually influenced by the same factors, and in the same direction, as changes in total body Na+. Exceptions arise during acid-base disturbances, when Cl− metabolism may change independently of Na+.
By definition, in the steady state, the total body content of water and electrolytes is constant. Focusing on Na+,

Under normal circumstances, extrarenal Na+ output is negligible. However, large fluid losses from the gastrointestinal tract (e.g., vomiting, diarrhea) or skin (e.g., excessive sweating, extensive burns) can represent substantial extrarenal Na+ losses. The kidney responds to such deficits by reducing renal Na+ excretion. Conversely, in conditions of excessive Na+ intake, the kidneys excrete the surfeit of Na+.
In contrast to many other renal mechanisms of electrolyte excretion, the renal excretion of Na+ depends on the amount of Na+ in the body and not on the Na+ concentration in ECF. Because the amount of Na+ is the product of ECF volume and the extracellular Na+ concentration, and because the osmoregulatory system keeps plasma osmolality constant within very narrow limits, it is actually the volume of ECF that acts as the signal for Na+ homeostasis.
Figure 40-1A demonstrates the renal response to an abrupt step increase and a step decrease in Na+ intake. A subject weighing 70 kg starts with an unusually low Na+ intake of 10 mmol/day, matched by an equally low urinary output. When the individual abruptly increases dietary Na+ intake from 10 to 150 mmol/day—and maintains it at this level for several days—urinary Na+ output also increases, but at first it lags behind intake. This initial period during which Na+ intake exceeds Na+ output is a state of positive Na+ balance. After ~5 days, urinary Na+ output rises to match dietary intake, after which total body Na+ does not increase further. In this example, we assume that the cumulative retention of Na+ amounts to 140 mmol.
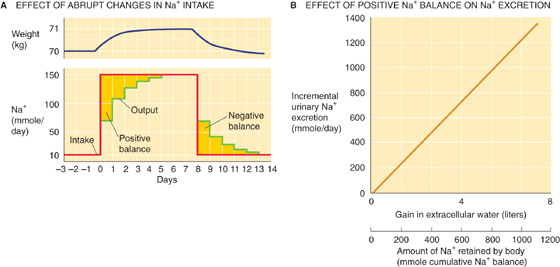
Figure 40-1 Na+ balance. In A, the red curve shows the time course of dietary Na+ intake, and the green curve shows Na+ excretion. The golden area between the two curves at the beginning of the experiment corresponds to the accumulated total body Na+ of 140 mmol. This additional Na+, dissolved in ~1 L of ECF, accounts for the 1-kg gain in body weight (blue curve). (B, Data from Walser M: Kidney Int 1985; 27:837-841.)
The abrupt increase in dietary Na+ initially elevates plasma osmolality, thus stimulating thirst and release of AVP. Because the subject has free access to water, and because the kidneys salvage water in response to AVP (see Chapter 38), the volume of solute-free water rises. This increase in free water not only prevents a rise in [Na+] and osmolality, but also it produces a weight gain that—in this example—is 1 kg (Fig. 40-1A). This weight gain corresponds, in our example, to the accumulation of 140 mmol of Na+ and the accompanying free water, which makes 1 L of isotonic saline. In the new steady state, only the extracellular compartment has increased in volume. Intracellular volume does not change because, in the end, no driving force exists for water to cross cell membranes (i.e., extracellular osmolality is normal). The slight expansion of the extracellular volume signals the kidney to increase its rate of Na+ excretion. The extracellular Na+ concentration is unchanged during this period and thus cannot be the signal to increase Na+ excretion.
When the subject in our example later reduces Na+ intake to the initial level of 10 mmol/day (Fig. 40-1A), Na+ excretion diminishes until the initial balanced state (input = output) is re-established once again. Immediately after the reduction in Na+ intake, Na+ is temporarily out of balance. This time, it is a period of negative Na+ balance, in which output exceeds input. During this period, the ECF volume falls by 1 L, and body weight returns to normal. The extracellular Na+ concentration is unchanged during this transient period.
Ingesting increasingly larger amounts of Na+ results in retaining progressively larger amounts in the steady state and thus accumulating progressively more ECF volume. Urinary Na+ excretion increases linearly with this rise in ECF volume or body weight; the slope in Figure 40-1B is idealized for the subject in Figure 40-1A. The control system that so tightly links urinary Na+ excretion to ECF volume is extremely sensitive. In our example (Fig. 40-1A)—a 70-kg individual with an initial ECF volume of 17 L—expanding ECF volume by 1 L, or ~6%, triggers a 15-fold increase in steady-state urinary Na+ excretion (i.e., from 10 mmol/day to 150 mmol/day in Fig. 40-1A). Physiologically normal individuals can be in Na+ balance on a nearly Na+-free diet (1 to 2 mmol/day) without overt signs of ECF volume depletion. Conversely, even on a high Na+ diet (200 mmol/day versus the “normal” ~100 mmol/day for a Western diet), clinical signs of ECF volume excess, such as edema, are absent.
Although we have referred to the overall expansion of the ECF volume as the signal for increased urinary Na+ excretion, this is an oversimplification. Only certain regions of the extracellular compartment are important for this signaling. For an expansion in ECF volume to stimulate Na+ excretion, the expansion must make itself evident in the part of the ECF compartment where the ECF-volume sensors are located.
The thoracic blood vessels appear to be the site of greatest importance. For example, in congestive heart failure, particularly when edema is extensive, the total extracellular volume is greatly increased. However, the low cardiac output fails to expand the thoracic blood vessels. As a result, Na+ reabsorption by the renal tubules remains high (i.e., urinary Na+ excretion is inappropriately low compared with Na+ intake) and thus exacerbates the systemic congestion.
Another example of the importance of the thoracic vessels for regulating renal Na+ excretion is the effect of gravity in modulating venous return. Urinary Na+ excretion is lowest when one is standing (when thoracic perfusion is lowest), higher when one is lying down (recumbency), and highest when immersing one’s self up to the chin for several hours in warm water. During immersion, the hydrostatic pressure of the water compresses the tissues—and thus the vessels, particularly the veins—in the extremities and abdomen and consequently enhances venous return to the thorax. The thoracic vessels are immune to this compression because their extravascular pressure (i.e., intrapleural pressure; see Chapter 27) is unaffected by the water. Thus, the enhanced venous return alone stimulates vascular sensors to increase Na+ excretion.
In the foregoing three examples of the effects of gravity, Na+ excretion varies widely even though the total ECF volume is the same. Thus, ECF volume per se is not the critical factor in regulating renal Na+ excretion. Recumbency—and, to a greater extent, water immersion—shifts blood into the thoracic vessels, increasing the so-called central blood volume (see Chapter 19). In contrast, the upright position depletes the intrathoracic blood volume. This example clearly demonstrates that only special portions within the ECF compartment play critical roles in the sensing of ECF volume.
The critical parameter that the body recognizes as an index of changes in Na+ content is the effective circulating volume. The effective circulating volume cannot be identified anatomically. Rather, it is a functional blood volume that reflects the extent of tissue perfusion in specific regions, as evidenced by the fullness or pressure within their blood vessels. Normally, changes in the effective circulating volume parallel those in total ECF volume. However, this relationship may be distorted in certain diseases. For example, in patients with congestive heart failure (see earlier), nephrotic syndrome, or liver cirrhosis, total ECF volume is grossly expanded (e.g., edema or ascites). In contrast, the effective circulating volume is low, resulting in Na+ retention.
Figure 40-2 summarizes the elements of the feedback loop that controls the effective circulating volume. As summarized in Table 40-2, sensors that monitor changes in effective circulating volume are baroreceptors located in both high-pressure and low-pressure areas of the circulation (see Chapter 23). Although most are located within the vascular tree of the thorax, additional baroreceptors are present in the kidney—particularly in the afferent arterioles (see Chapter 33)—CNS, and liver. These sensors generate four distinct hormonal or neural signals (Fig. 40-2, pathways 1 to 4).
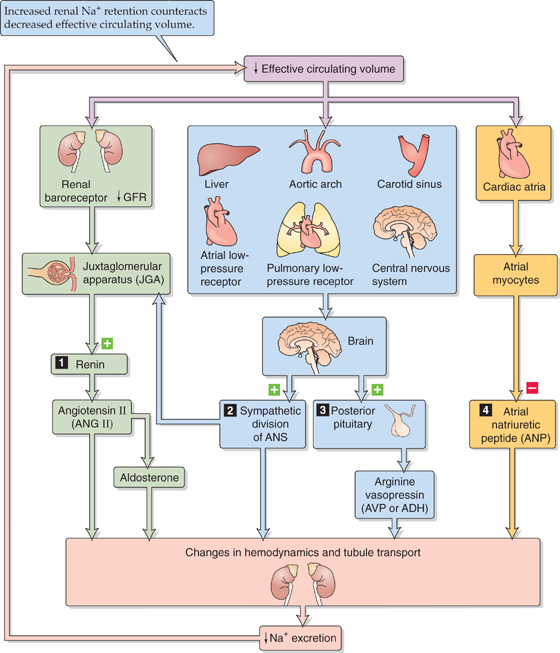
Figure 40-2 Feedback control of effective circulating volume. A low effective circulating volume triggers four parallel effector pathways (numbered 1 to 4) that act on the kidney, either by changing the hemodynamics or by changing Na+ transport by the renal tubule cells. ANS, autonomic nervous system.
Table 40-2 ECF Volume Receptors
“Central” Vascular Sensors |
Low-Pressure Sensors (very important) |
Cardiac atria |
Pulmonary vasculature |
High-Pressure Sensors (less important) |
Carotid sinus |
Aortic arch |
Juxtaglomerular apparatus (renal afferent arteriole) |
Sensors in the CNS (less important) |
Sensors in the Liver (less important) |
In the first pathway, a reduced effective circulating volume directly stimulates a hormonal effector pathway, the reninangiotensin-aldosterone system. The second and third effector pathways are neural. Baroreceptors detect decreases in effective circulating volume and communicate these through afferent neurons to the medulla of the brainstem. From the medulla, two types of efferent signals emerge that ultimately act on the kidney. In one, increased activity of the sympathetic division of the autonomic nervous system reduces renal blood flow and thus reduces Na+ excretion. In the other effector pathway, the posterior pituitary increases its secretion of AVP and thus conserves water. This second mechanism becomes active only after large declines in effective circulating volume. The final pathway is hormonal. Reduced effective circulating volume decreases the release of atrial natriuretic peptide (ANP), thus reducing Na+ excretion.
Volume Expansion and Contraction
When Na+ intake persists in the presence of impaired renal Na+ excretion (e.g., during renal failure), the body retains isosmotic fluid. The result is an expansion of plasma volume and of the interstitial fluid compartment. In the extreme, the interstitial volume increase can become so severe that the subepidermal tissues swell (e.g., around the ankles). When the physician presses with a finger against the skin, and then removes the finger, the finger imprint remains in the tissue (pitting edema). Not all cases of lower extremity edema reflect Na+ and fluid retention. Particularly in elderly persons, peripheral vascular insufficiency is a common cause and does not reflect total body fluid overload, but rather a local malfunction, usually of the veins. These patients should elevate their feet whenever possible and should wear compression stockings.
Fluid can also accumulate in certain transcellular spaces (see Chapter 5), such as the pleural cavity (pleural effusion) or the peritoneal cavity (ascites), conditions reflecting derangements of local Starling forces (see Chapter 20 for the box titled Interstitial Edema) that determine the fluid distribution between the plasma and the ECF. In cases of abnormal Na+ retention, a low-Na+ diet can partially correct the edema. Diuretics can also reduce volume overload, as long as the kidney retains sufficient function to respond to them. (See Note: Sensitivity of the Natriuretic Response to Increased Extracellular Fluid Volume)
An excessive loss of Na+ into the urine can be caused by disturbances of Na+ reabsorption along the nephron, thus leading to a dramatic shrinkage of the ECF volume. Because the plasma volume is part of the ECF volume, significant reductions can severely affect the circulation and can culminate in hypovolemic shock (see Chapter 25). Renal causes of reduced ECF volume include the prolonged use of powerful loop and osmotic diuretics (see Chapter 35) and various renal tubule malfunctions, including proximal renal tubular acidosis and the recovery phase following acute renal failure.
All four parallel effector pathways modulate renal Na+ excretion and correct the primary change in effective circulating blood volume. Thus, an increase in effective circulating volume promotes Na+ excretion (thus reducing ECF volume), whereas a decrease in effective circulating volume inhibits Na+ excretion (thus raising ECF volume). In addition to baroreceptors and the four parallel effector pathways, purely hemodynamic/physical mechanisms contribute to the regulation of Na+ excretion thus of effective circulating volume.
An important feature of renal Na+ excretion is the twoway redundancy of control mechanisms. First, efferent pathways may act in concert on a single effector within the kidney. For instance, both sympathetic input and hemodynamic/physical factors often act on proximal tubules. Second, one efferent pathway may act at different effector sites. For example, angiotensin II (ANG II) enhances Na+ retention directly by stimulating apical Na-H exchange in tubule cells (see Fig. 35-4) and indirectly by lowering renal plasma flow (see Chapter 34).
The next two sections focus on the four parallel effector pathways. In the third, we discuss how purely hemodynamic/physical factors modulate effective circulating volume.
The renin-angiotensin-aldosterone axis (Fig. 40-3) promotes Na+ retention through the actions of both ANG II and aldosterone. In Chapter 50, we discuss this axis in the context of the physiology of the adrenal cortex.
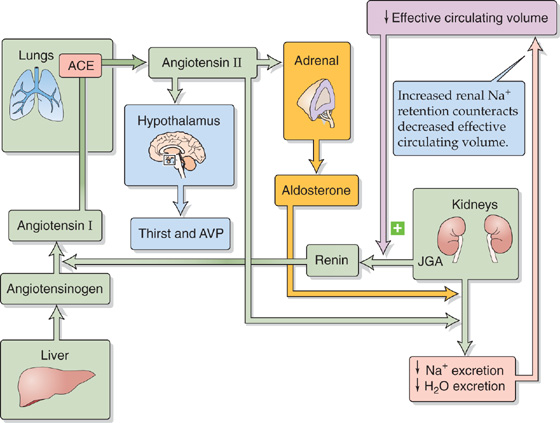
Figure 40-3 The renin-angiotensin-aldosterone axis.
Angiotensinogen, also known as renin substrate, is an α2-globulin that is synthesized by the liver and released into the systemic circulation. The liver contains only small stores of angiotensinogen. Another protein, renin, is produced and stored in distinctive granules by the granular cells of the renal juxtaglomerular apparatus (JGA; see Chapter 33). As discussed later, decreases in effective circulating volume stimulate these cells to release renin, which is a protease that cleaves a peptide bond near the C terminus of angiotensinogen and thereby releases the decapeptide ANG I. Angiotensin-converting enzyme (ACE) rapidly removes the two C-terminal amino acids from the physiologically inactive ANG I to form the physiologically active octapeptide ANG II. ACE is present on the luminal surface of vascular endothelia throughout the body and is abundantly present in the endothelium-rich lungs. ACE in the kidney—particularly in the endothelial cells of the afferent and efferent arterioles—can produce enough ANG II to exert local vascular effects. Thus, the kidney receives ANG II from two sources: (1) systemic ANG II comes from the general circulation and originates largely from the pulmonary region, and (2) local ANG II forms from the renal conversion of systemic ANG I. In addition, the proximal tubule secretes ANG II into its lumen and thus achieves intraluminal concentrations in excess of those in the general circulation. ANG II in the circulation has a short half-life (~2 min) because aminopeptidases further cleave it to the heptapeptide ANG III, which is still biologically active. (See Note: The Metabolism of the Angiotensins; Systemic versus Local Roles of the JGA)
The principal factor controlling plasma ANG II levels is renin release from JGA granular cells. A decrease in effective circulating volume manifests itself to the JGA—and thus stimulates renin release—in three ways (Fig. 40-2):
1. Decreased systemic blood pressure (sympathetic effect on JGA). A low effective circulating volume, sensed by baroreceptors located in the central arterial circulation (see Chapter 23), signals medullary control centers to increase sympathetic outflow to the JGA, thus increasing renin release. Renal denervation or β-adrenergic blocking drugs (e.g., propranolol) inhibit renin release.
2. Decreased NaCl concentration at macula densa (NaCl sensor). Independent of renal nerve activity (point 1) or renal perfusion pressure (point 3), decreased effective circulating volume decreases GFR and thus reduces luminal [NaCl] at the macula densa, thereby increasing renin release.
3. Decreased renal perfusion pressure (renal baroreceptor). Stretch receptors in the granular cells (see Chapter 33) of the afferent arterioles sense the decreased distention associated with low effective circulating volume. This decreased stretch lowers [Ca2+]i, thereby increasing renin release and initiating a cascade that tends to increase blood pressure. Conversely, increased distention (high extracellular volume) inhibits renin release.
The foregoing stimulation of renin release by a decrease in [Ca2+]i stands in contrast to most Ca2+-activated secretory processes, in which an increase in [Ca2+]i stimulates secretion (see Chapter 8). Another exception is the chief cell of the parathyroid gland, in which an increase in [Ca2+]i inhibits secretion of parathyroid hormone (see Chapter 52). (See Note: Renin Release from Granular Cells
[cAMP]i also appears to be a second messenger for renin release. Agents that activate adenylyl cyclase enhance renin secretion, presumably through protein kinase A. The question whether the effects of [cAMP]i and [Ca2+]i are independent or sequential remains open. (See Note: Other Factors That Activate Adenylyl Cyclase in Granular Cells)
Additional factors also modulate renin release. Prostaglandins E2 and I2 and endothelin all activate renin release. Agents that blunt renin release include ANG II (which represents a short feedback loop), AVP, thromboxane A2, high plasma levels of K+, and NO.
ANG II has several important actions, as follows:
1. Stimulation of aldosterone release from glomerulosa cells in the adrenal cortex (see Chapter 50). In turn, aldosterone promotes Na+ reabsorption in the collecting tubules and ducts (see Chapter 35).
2. Vasoconstriction of renal and other systemic vessels. ANG II increases Na+ reabsorption by altering renal hemodynamics, probably in two ways (Fig. 40-4). First, at high concentrations, ANG II constricts the efferent more than the afferent arterioles, thus increasing filtration fraction and reducing the hydrostatic pressure in the downstream peritubular capillaries. The increased filtration fraction also increases the protein concentration in the downstream blood and hence raises the colloid osmotic pressure of the peritubular capillaries. The changes in each of these two Starling forces favor the uptake of reabsorbate from peritubular interstitium into peritubular capillaries (see Chapter 35) and hence enhance the reabsorption of Na+ and fluid by the proximal tubule. Second, ANG II decreases medullary blood flow through vasa recta. Low blood flow decreases the medullary washout of NaCl and urea (see Chapter 38), a process that raises [urea] in the medullary interstitium and enhances Na+ reabsorption along the thin ascending limb of Henle’s loop (see Chapter 38).
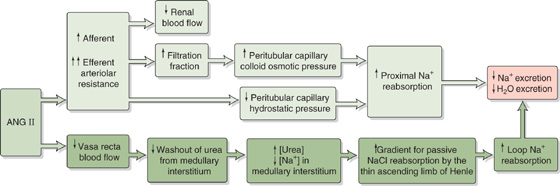
Figure 40-4 Hemodynamic actions of ANG II on Na+ reabsorption.
3. Enhanced tubuloglomerular feedback. ANG II raises the sensitivity and lowers the set point of the tubuloglomerular feedback mechanism (see Chapter 34), so that an increase in Na+ and fluid delivery to the macula densa elicits a more pronounced fall in the glomerular filtration rate (GFR).
4. Enhanced Na-H exchange. As noted in Chapter 34, ANG II promotes Na+ reabsorption in the proximal tubule, thick ascending limb (TAL), and initial collecting tubule (see Chapter 35).
5. Renal hypertrophy. ANG II induces hypertrophy of renal tubule cells.
6. Stimulated thirst and AVP release. ANG II acts on the hypothalamus, where it increases the sensation of thirst and stimulates secretion of AVP from the posterior pituitary, both of which increase total body free water. This ANG II effect represents an intersection between the systems for regulating effective circulating volume and osmolality.
Renal Sympathetic Nerve Activity The second of the four parallel effector pathways for the control of effective circulating volume is the sympathetic nervous system. As discussed in Chapter 35, enhanced activity of the renal sympathetic nerves has two direct effects on Na+ reabsorption: (1) increased renal vascular resistance and (2) increased Na+ reabsorption by tubule cells. In addition, increased sympathetic tone has an indirect effect—enhancing renin release from granular cells (see previous section). These multiple actions of sympathetic traffic to the kidney reduce GFR and enhance Na+ reabsorption, thereby increasing Na+ retention and increasing effective circulating volume.
In everyday life (i.e., the unstressed state), the role of sympathetic nerve activity in kidney function appears to be modest at best. However, sympathetic innervation may play a role during challenges to volume homeostasis. For example, low Na+ intake triggers reduced renal Na+ excretion; renal denervation blunts this response. Another example is hemorrhage, in which renal sympathetic nerves emerge as important participants in preserving ECF volume. Conversely, expansion of the intravascular volume increases renal Na+ excretion; renal denervation sharply reduces this response as well.
Arginine Vasopressin (Antidiuretic Hormone) As discussed in the next major section, the posterior pituitary releases AVP primarily in response to increases in extracellular osmolality. Indeed, AVP mainly increases distal nephron water permeability and thus promotes water retention (see Chapter 38). However, the posterior pituitary also releases AVP in response to large reductions in effective circulating volume (e.g., hemorrhage), and a secondary action of AVP—promoting Na+ retention (see Chapter 35)—is appropriate for this stimulus.
Renal Hypertension
In the 1930s, Goldblatt produced hypertension experimentally in unilaterally nephrectomized animals by placing a surgical clip around the renal artery of the remaining kidney (one-kidney Goldblatt hypertension). The constriction can be adjusted so that it does not result in renal ischemia, but only in a reduction of the perfusion pressure distal to the clip. This maneuver stimulates the renal baroreceptors and leads to a rapid increase in synthesis and secretion of renin from the clipped kidney. The renin release reaches a peak after 1 hour. As renin cleaves ANG I from angiotensinogen, systemic ANG I levels rise quickly. ACE, present mainly in the lungs but also in the kidneys, then rapidly converts ANG I into ANG II. Thus, within minutes of clamping the renal artery, one observes a sustained rise in systemic arterial pressure. The newly established stable elevation in systemic pressure then normalizes the pressure in the renal artery downstream from the constriction. From this time onward, circulating renin and ANG II levels decline toward normal over 5 to 7 days, whereas the systemic arterial pressure remains abnormally high. The early rise in blood pressure is the result of the renin-angiotensin vasoconstrictor mechanism, which is activated by the experimentally induced reduction in pressure and flow in the renal artery distal to the constriction. The later phase of systemic hypertension is the result of aldosterone release and of the retention of salt and water.
Unilateral partial clamping of a renal artery in an otherwise healthy animal also produces hypertension (two-kidney Goldblatt hypertension). As in the one-kidney model, the clipped kidney increases its synthesis and secretion of renin. Renin then causes ANG II levels to increase systemically and will, in addition to the effect on the clamped kidney, cause the nonclamped, contralateral kidney to retain salt and water. As in the one-kidney model, the resulting hypertension has an early vasoconstrictive phase and a delayed volume-dependent phase.
In both types of Goldblatt hypertension, administration of ACE inhibitors can lower arterial blood pressure. However, inhibiting the converting enzyme is therapeutically effective even after circulating renin and ANG II levels have normalized. Maintaining the hypertension must involve an increased intrarenal conversion of ANG I to ANG II (through renal ACE), with the ANG II enhancing proximal Na+ reabsorption. Indeed, direct measurements show that, even after circulating renin and ANG II levels have returned to normal, the intrarenal levels of ACE and ANG II are elevated.
These experimental models serve as paradigms for some forms of human hypertension, including renin-secreting tumors of the JGA and all cases of pathologic impairment of renal arterial blood supply. Thus, coarctation of the aorta, in which the aorta is constricted above the renal arteries but below the arteries to the head and upper extremities, invariably leads to hypertension. Renal hypertension also results from stenosis of a renal artery, caused, for example, by arteriosclerotic thickening of the vessel wall. However, the most common form of renal hypertension occurs, in parts of the kidney, when inflammatory or fibrotic lesions stenose preglomerular arteries or arterioles. Such local intrarenal vasoconstriction may lead to local ischemia, although some parts of the kidney remain entirely normal. This pattern therefore closely resembles the two-kidney Goldblatt hypertension model. In fact, high levels of ANG II may be encountered in such patients. The administration of ACE inhibitors is generally beneficial because they lower ANG II levels, systemically and within the kidney itself.
Atrial Natriuretic Peptide Of the four parallel effectors that correct a low effective circulating volume (Fig. 40-2), AVP is the only one that does so by decreasing its activity. As its name implies, ANP promotes natriuresis (i.e., Na+ excretion). Atrial myocytes synthesize and store ANP and release ANP in response to stretch (a low-pressure volume sensor) (see Chapter 23). Thus, reduced effective circulating volume inhibits ANP release and reduces Na+ excretion. ANP plays a role in the diuretic response to the redistribution of ECF and plasma volume into the thorax that occurs during water immersion and space flight (see Chapter 61).
Acting through a receptor guanylyl cyclase (see Chapter 3), ANP has many synergistic effects on renal hemodynamics and on transport by renal tubules that promote renal Na+ and water excretion. Although ANP directly inhibits Na+ transport in the inner medullary collecting duct, its major actions are hemodynamic—increased GFR, increased cortical and medullary blood flow, and decreased release of renin and AVP. Thus, a decrease in effective circulating volume leads to a fall in ANP release and a net decrease in Na+ and water excretion (see Chapter 35). (See Note: Renal Sites of Action of Atrial Natriuretic Peptide (ANP))
We have seen that expanding the effective circulating volume stimulates sensors that increase Na+ excretion through four parallel effector pathways (Fig. 40-2). However, the kidney can also modulate Na+ excretion in response to purely hemodynamic changes, as in the following two examples.
Large and Acute Decrease in Arterial Blood Pressure If glomerulotubular (GT) balance were perfect, decreasing the GFR would cause Na+ excretion to fall linearly (Fig. 40-5, blue line). However, acutely lowering GFR by partial clamping of the aorta causes a steep, nonlinear decrease in urinary Na+ excretion (Fig. 40-5, red curve). When GFR falls sufficiently, the kidneys excrete only traces of Na+ in a small volume of urine. This response primarily reflects the transport of the classical distal tubule, which continues to reabsorb Na+ at a high rate despite the decreased Na+ delivery (see Chapter 35).
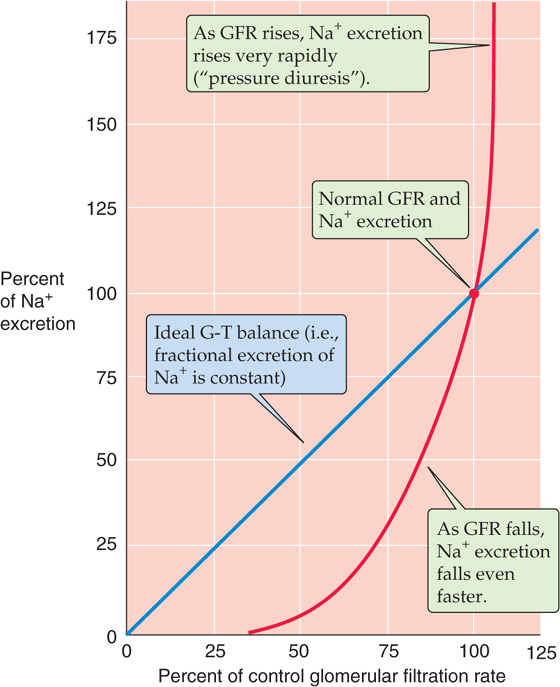
Figure 40-5 Effect of changes in GFR on urinary Na+ excretion. The blue line represents the ideal GT balance. The red curve summarizes data from dogs. The investigators reduced GFR by inflating a balloon in the aorta, above the level of the renal arteries. They increased GFR by compressing the carotid arteries, and thus increased blood pressure. (Data from Thompson DD, Pitts RF: Am J Physiol 1952; 168:490-499.)
Large Increase in Arterial Pressure In some cases, an increased effective circulating volume is accompanied by an increase in arterial pressure. An example is Liddle disease, a state of abnormally high distal Na+ reabsorption. The excess Na+ reabsorption leads to high blood pressure and compensatory pressure-induced natriuresis. One reason for this pressure diuresis is that hypertension increases GFR and raises the filtered load of Na+, which by itself would increase urinary Na+ excretion (Fig. 40-5, blue line). However, at least four other mechanisms contribute to the natriuresis (Fig. 40-5, red curve). First, the increased effective circulating volume inhibits the renin-angiotensin-aldosterone axis and thus reduces Na+ reabsorption (see Chapter 35). Second, the high blood pressure augments blood flow in the vasa recta, washes out medullary solutes and reduces interstitial hypertonicity in the medulla, and ultimately reduces passive Na+ reabsorption in the thin ascending limb (see Chapter 38). Third, an increase in arterial pressure leads, by an unknown mechanism, to prompt reduction in the number of apical Na-H exchangers in the proximal tubule. Normalizing the blood pressure rapidly reverses this effect. Finally, hypertension leads to increased pressure in the peritubular capillaries, thereby reducing proximal tubule reabsorption (physical factors; see Chapter 35). (See Note: Liddle Disease)
Water accounts for half or more of body weight (~60% in men and 50% in women) and is distributed between the intracellular fluid and ECF compartments (see Chapter 5). Changes in whole-body water content lead to changes in osmolality, to which the CNS is extremely sensitive. Osmolality deviations of ±15% lead to severe disturbances of CNS function. Thus, osmoregulation is critical.
Two elements control water content and thus whole-body osmolality: (1) the kidneys, which control water excretion (see Chapter 38), and (2) thirst mechanisms, which control the oral intake of water. These two effector mechanisms are part of negative feedback loops that begin within the hypothalamus. An increase in osmolality stimulates separate osmoreceptors to secrete AVP (which reduces renal excretion of free water) and to trigger thirst (which, if fulfilled, increases intake of free water). As a result, the two complementary feedback loops stabilize osmolality and thus [Na+].
An increase in the osmolality of the ECF is the primary signal for the secretion of AVP from the posterior pituitary gland. An elegant series of animal studies by Verney in the 1940s established that infusing a hyperosmotic NaCl solution into the carotid artery abruptly terminates an established water diuresis (Fig. 40-6A). Infusing hyperosmotic NaCl into the peripheral circulation has no effect because the hyperosmolar solution becomes diluted by the time it reaches the cerebral vessels. Therefore, the osmosensitive site is intracranial. Surgically removing the posterior pituitary abolishes the effect of infusing hyperosmotic NaCl into the carotid artery (Fig. 40-6B). However, injecting posterior pituitary extracts into the animal inhibits the diuresis, regardless of whether the posterior pituitary is intact. Later work showed that Verney’s posterior pituitary extract contained an “antidiuretic hormone”—now known to be AVP—that the posterior pituitary secretes in response to increased plasma osmolality. Ingesting large volumes of water causes plasma osmolality to fall, thus leading to reduced AVP secretion.
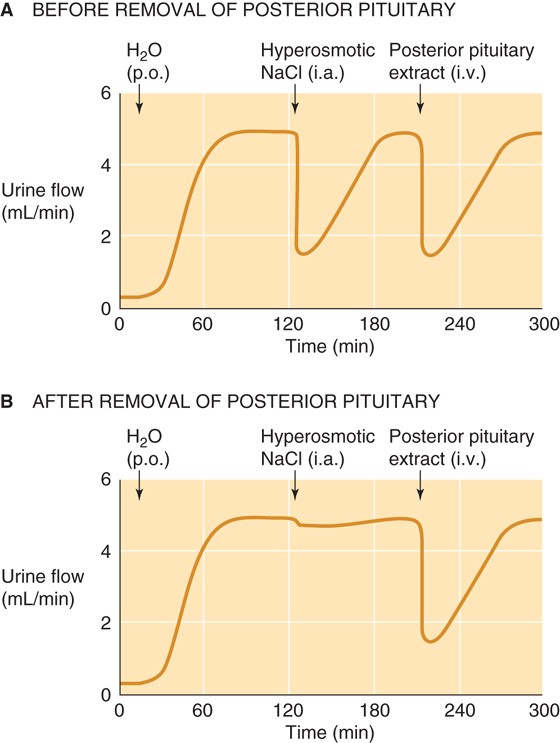
Figure 40-6 Sensing of blood osmolality in the dog brain. i.a., intra-arterial (carotid) injection; i.v., intravenous injection; p.o., per os (by mouth). (Data from Verney EG: Proc R Soc Lond B 1947;135:25-106.)
In healthy individuals, plasma osmolality is ~290 mOsm. The threshold for AVP release is somewhat lower, ~280 mOsm (Fig. 40-7, red curve). Increasing the osmolality by only 1% higher than this level is sufficient to produce a detectable increase in plasma [AVP], which rises steeply with further increases in osmolality. Thus, hyperosmolality leads to increased levels of AVP, which completes the feedback loop by causing the kidneys to retain water (see Chapter 38).
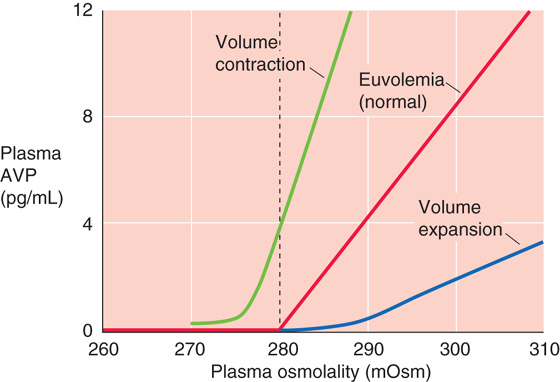
Figure 40-7 Dependence of AVP release on plasma osmolality. (Data from Robertson GL, Aycinena P, Zerbe RL: Am J Med 1982; 72:339-353.)
Although a change in plasma [NaCl] is usually responsible for a change in plasma osmolality, other solutes can do the same. For example, hypertonic mannitol resembles NaCl in stimulating AVP release. However, an equivalent increase in extracellular osmolality by urea has little effect on plasma AVP levels. The reason is that urea has a low effective osmolality or tonicity (see Chapter 5) and is thus ineffective in shrinking cells.
Osmoreceptors of the CNS appear to be located in two areas that breech the blood-brain barrier: the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO), two of the circumventricular organs (see Chapter 11). Specific neurons in these regions (Fig. 40-8) are able to sense changes in plasma osmolality. Elevated osmolality increases the activity of mechanosensitive cation channels located in the neuronal membrane and results in depolarization and thus an increased frequency of action potentials. Hypo-osmolality causes a striking decrease of frequency. The osmosensitive neurons project to large-diameter neurons in the supraoptic and paraventricular nuclei of the anterior hypothalamus (Fig. 40-8). These neurons synthesize AVP, package it into granules, and transport the granules along their axons to nerve terminals in the posterior lobe of the pituitary, which is part of the brain (see Chapter 47). When stimulated by the osmosensitive neurons, these magnocellular neurons release the stored AVP into the posterior pituitary—an area that also lacks a blood-brain barrier—and AVP enters the general circulation.
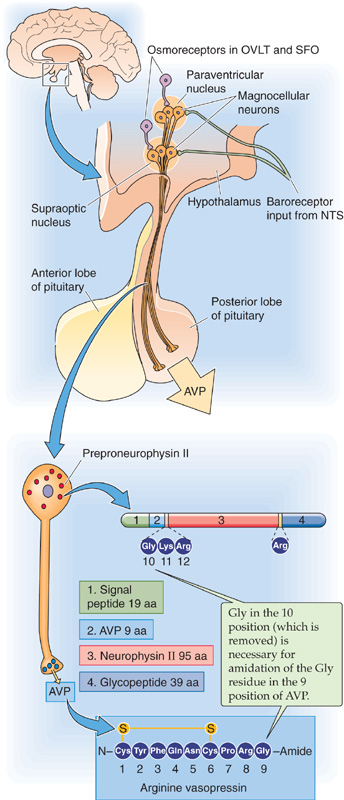
Figure 40-8 Control of AVP synthesis and release by osmoreceptors. Osmoreceptors are located in the OVLT and the SFO, two areas that breech the blood-brain barrier. Signals from atrial, low-pressure baroreceptors travel with the vagus nerve to the nucleus tractus solitarii (NTS); a second neuron carries the signal to the hypothalamus.
In humans and most mammals, the antidiuretic hormone is AVP, which is encoded by the messenger RNA for preproneurophysin II. After cleavage of the signal peptide, the resulting prohormone contains AVP, neurophysin II (NpII), and a glycopeptide. Cleavage of the prohormone within the secretory granule yields these three components. AVP has nine amino acids, with a disulfide bridge connecting two cysteine residues. Mutations of NpII impair AVP secretion, a finding suggesting that NpII assists in the processing or secretion of AVP.
Levels of circulating AVP depend on both the rate of AVP release from the posterior pituitary and the rate of AVP degradation. The major factor controlling AVP release is plasma osmolality. However, as discussed later, other factors also can modulate AVP secretion.
Two organs, the liver and the kidney, contribute to the breakdown of AVP and the rapid decline of AVP levels when secretion has ceased. The half-life of AVP in the circulation is 18 minutes. Diseases of the liver and kidney may impair AVP degradation and may thereby contribute to water retention. For example, the congestion of the liver and impairment of renal function that accompany heart failure can compromise AVP breakdown and can lead to inappropriately high circulating levels of AVP.
The second efferent pathway of the osmoregulatory system is thirst, which regulates the oral intake of water. Like the osmoreceptors that trigger AVP release, the osmoreceptors that trigger thirst are located in two circumventricular organs, the OVLT and the SFO. Also like the osmoreceptors that trigger AVP release, those that trigger thirst respond to the cell shrinkage that is caused by hyperosmolar solutions. However, these thirst osmoreceptor neurons are distinct from the adjacent AVP osmoreceptor neurons in the OVLT and SFO.
Hyperosmolality triggers two parallel feedback control mechanisms that have a common endpoint (Fig. 40-9): an increase in whole-body free water. In response to hyperosmolality, the AVP osmoreceptors in the hypothalamus trigger other neurons to release AVP. The result is the insertion of aquaporin 2 (AQP2) water channels in the collecting duct of the kidney, an increase in the reabsorption of water, and, therefore, a reduced excretion of free water. In response to hyperosmolality, the thirst osmoreceptors stimulate an appetite for water that leads to the increased intake of free water. The net effect is an increase in whole-body free water and, therefore, a reduction in osmolality.
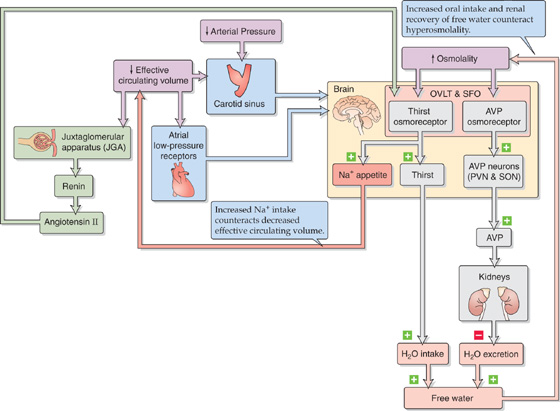
Figure 40-9 Feedback systems involved in the control of osmolality. PVN, paraventricular nucleus; SON, supraoptic nucleus of the hypothalamus.
Although an increase in plasma osmolality is the primary trigger for AVP release, several other stimuli increase AVP release, including a decrease in effective circulating volume or arterial pressure and pregnancy. Conversely, volume expansion diminishes AVP release.
Reduced Effective Circulating Volume As noted earlier in Chapter 23, a mere 1% rise in plasma osmolality stimulates AVP release by a detectable amount. However, fairly large reductions in effective circulating volume (5% to 10%) are required to stimulate AVP release of similar amounts. However, once the rather high threshold for nonosmotic release of AVP is exceeded, AVP release rises steeply with further volume depletion. The interaction between osmotic and volume stimuli on AVP release is illustrated in Figure 40-7, which shows that the effective circulating volume modifies the slope of the relationship between plasma AVP levels and osmolality, as well as the osmotic threshold for AVP release. At a fixed osmolality, volume contraction (Fig. 40-7, green curve) increases the rate of AVP release. Therefore, during volume depletion, low plasma osmolality that would normally inhibit the release of AVP allows AVP secretion to continue. This leftward shift of the osmolality threshold for AVP release is accompanied by an increased slope, reflecting an increased sensitivity of the osmoreceptors to changes in osmolality.
Figure 40-9 summarizes the three pathways by which decreased effective circulating volume and low arterial pressure enhance AVP release. First, a reduction in left atrial pressure—produced by volume depletion—through low-pressure receptors in the left atrium decreases the firing rate of vagal afferents (see Chapter 23). These afferents signal brainstem neurons and cause magnocellular neurons in the hypothalamus to release AVP (Fig. 40-8). Indeed, at constant osmolality, AVP secretion varies inversely with left atrial pressure. Second, low effective circulating volume triggers granular cells in the JGA to release renin. This leads to the formation of ANG II, which acts on receptors in the OVLT and the SFO to stimulate AVP release. Third and more important, a fall in the arterial pressure similarly causes high-pressure carotid sinus baroreceptors to stimulate AVP release (see Chapter 23).
Two clinical examples in which reduced effective circulating volume leads to increases in AVP are severe hemorrhagic shock and hypovolemic shock (e.g., shock resulting from excessive loss of ECF, as in cholera). In both cases, the water retention caused by AVP release accounts for the accompanying hyponatremia. In the first part of this chapter, we said that the appropriate renal response to decreased effective circulating volume is to retain Na+ (i.e., isotonic saline). Why is it that, in response to shock, the body also retains free water? Compared with isotonic saline, free water is less effective as an expander of the ECF volume (see Chapter 5). Nevertheless, in times of profound need, the body uses free water retention to help expand extracellular (and plasma) volume. Clearly, the body is willing to tolerate some hypo-osmolality of the body fluids as the price for maintaining an adequate blood volume.
A clinical example in which reduced effective circulating volume can lead to an inappropriate increase in AVP levels is congestive heart failure. In this situation, the water retention may be so severe that the patient develops hyponatremia (i.e., hypo-osmolality).
Volume Expansion In contrast to volume contraction, chronic volume expansion reduces AVP secretion, as a consequence of the rightward shift of the threshold to higher osmolalities and of a decline in the slope (Fig. 40-7, blue curve). In other words, volume expansion decreases the sensitivity of the central osmoreceptors to changes in plasma osmolality. A clinical example is hyperaldosteronism. With normal thirst and water excretion, the chronic Na+ retention resulting from the hyperaldosteronism would expand the ECF volume isotonically, thus leaving plasma [Na+] unchanged. However, because chronic volume expansion downregulates AVP release, the kidneys do not retain adequate water, and hypernatremia (i.e., elevated plasma [Na+]) and hyperosmolality result.
Pregnancy A leftward shift in the threshold for AVP release and thirst often occurs during pregnancy. These changes probably reflect the action of chorionic gonadotropin on the sensitivity of the osmoreceptors. Pregnancy is therefore often associated with a decrease of 8 to 10 mOsm in plasma osmolality. A similar but smaller change may also occur in the late phase of the menstrual cycle.
Diuretics reversibly inhibit Na+ reabsorption at specific sites along the nephron, increase the excretion of Na+ and water, create a state of negative Na+ balance, and thereby reduce the volume of ECF. Clinicians use diuretics to treat hypertension as well as edema (see Chapter 20 for the box titled Interstitial Edema) caused by heart failure, cirrhosis of the liver, or nephrotic syndrome. Common to these edematous diseases is an abnormal shift of ECF away from the effective circulating volume, thereby activating the feedback pathways. The results are Na+ retention and expansion of total extracellular volume. However, this expansion, which results in edema formation, falls short of correcting the underlying decrease in the effective circulating volume. The reason that most of this added extracellular volume remains ineffective—and does not restore the effective circulating volume—is not intuitive but reflects the underlying disorder that initiated the edema in the first place. Thus, treating these diseases requires generating a negative Na+ balance, which can often be achieved by rigid dietary Na+ restriction or the use of diuretics. Diuretics are also useful in treating hypertension. Even though the primary cause of the hypertension may not always be an increase in the effective circulating volume, enhanced Na+ excretion is frequently effective in lowering blood pressure.
Classification
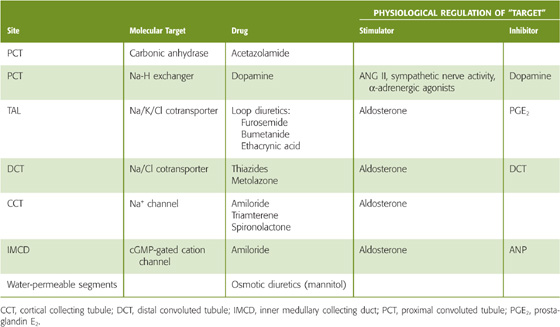
The site and mechanism of a diuretic’s action determine the magnitude and nature of the response (Table 40-3). Both chemically and functionally, diuretics are very heterogeneous. For example, acetazolamide produces diuresis by inhibiting carbonic anhydrase and thus the component of proximal tubule Na+ reabsorption that is coupled to HCO−3 reabsorption. The diuretic effect of hydrochlorothiazide is largely the result of its ability to inhibit Na/Cl cotransport in the distal convoluted tubule. Spironolactone (which resembles aldosterone) competitively inhibits mineralocorticoid receptors in principal cells of the initial and cortical collecting tubule. Mannitol (reduced fructose) is a powerful osmotic diuretic that reduces net Na+ transport along water-permeable nephron segments by retaining water in the lumen (see Chapter 35 for the box titled Osmotic Diuretics).
Table 40-3 Action of Diuretics
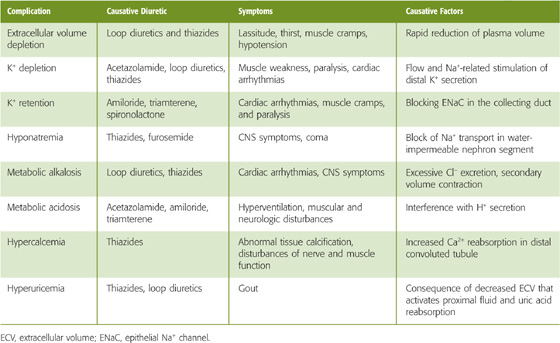
An ideal diuretic should promote the excretion of urine whose composition resembles that of the ECF. Such diuretics do not exist. In reality, diuretics not only inhibit the reabsorption of Na+ and its osmotically obligated water, but also interfere with the renal handling of Cl−, H+, K+, and Ca2+, as well as with urinary concentrating ability. Thus, many diuretics disturb the normal plasma electrolyte pattern. Table 40-4 summarizes the most frequent side effects of diuretic use on the electrolyte composition of the ECF. These electrolyte derangements are the predictable consequences of the mechanism of action of individual diuretics at specific tubule sites. (See Note: Secondary Effects of Diuretic Drugs)
Table 40-4 Complications of Diuretic Therapy
Delivery of Diuretics to Their Sites of Action
Diuretics generally inhibit transporters or channels at the apical membrane of tubule cells. How do the diuretics get there? Plasma proteins bind many diuretics so that the free concentration of the diuretic in plasma water may be fairly low. Thus, glomerular filtration may deliver only a modest amount to the tubule fluid. However, organic anion or organic cation transporters in the S3 segment of the proximal tubule can secrete diuretics and can thereby produce high luminal concentrations. For example, the basolateral organic anion transporter system that carries PAH also secretes the diuretics furosemide, ethacrynic acid, and spironolactone. The organic cation transporter secretes amiloride (see Chapter 36). The subsequent reabsorption of fluid in the loop of Henle and downstream nephron segments further concentrates diuretics in the tubule lumen. Not surprisingly, renal disease may compromise the delivery of diuretics. (See Note: Reduced Delivery of Diuretics in Renal Disease)
Response of Nephron Segments Downstream from a Diuretic’s Site of Action
The proximal tubule reabsorbs the largest fraction of filtered Na+; the loop of Henle, the distal convoluted tubule, and the collecting ducts retrieve smaller fractions. Thus, intuition could suggest that the proximal tubule would be the best target for diuretics. However, secondary effects in downstream nephron segments can substantially mitigate the primary effect of a diuretic. Inhibiting Na+ transport by the proximal tubule raises Na+ delivery to downstream segments and almost always stimulates Na+ reabsorption there (see Chapter 35). As a result of this downstream Na+ reclamation, the overall diuretic action of proximally acting diuretics (e.g., acetazolamide) is relatively weak.
A diuretic is most potent if it acts downstream of the proximal tubule, a condition met by loop diuretics, which inhibit Na+ transport along the TAL. Although the TAL normally reabsorbs only 15% to 25% of the filtered load of Na+, the reabsorptive capacity of the more distal nephron segments is limited. Thus, the loop diuretics are currently the most powerful diuretic agents. Because nephron segments distal to the TAL have only modest rates of Na+ reabsorption, diuretics that target these segments are not as potent as loop diuretics. Nevertheless, distally acting diuretics are important because their effects are long lasting and because they are K+ sparing (i.e., they tend to conserve body K+).
It is sometimes advantageous to use two diuretics that act at different sites along the nephron, to generate a synergistic effect. Thus, if a loop diuretic alone is providing inadequate diuresis, one could complement its action with acetazolamide, which can deliver a larger Na+ load to the inhibited loop.
Blunting of Diuretic Action with Long-Term Use
The prolonged administration of a diuretic may lead to sustained loss of body weight, but only transient natriuresis. Most of the decline in Na+ excretion occurs because the drug-induced fall in effective circulating volume triggers Na+ retention mediated by increased sympathetic outflow to the kidneys (which lowers GFR), increased secretion of aldosterone and AVP, and decreased secretion of ANP. Hypertrophy or increased activity of tubule segments downstream of the main site of action of the diuretic can also contribute to the diminished efficacy of the drug during long-term administration. (See Note: Blunting of Diuretic Action)
Other Factors Pain, nausea, and several drugs (e.g., morphine, nicotine, and high doses of barbiturates) stimulate AVP secretion. In contrast, alcohol and drugs that block the effect of morphine (opiate antagonists) inhibit AVP secretion and promote diuresis. Of great clinical importance is the hypersecretion of AVP that may occur postoperatively. In addition, ectopic metastases of some malignant tumors secrete large amounts of AVP. Such secretion of inappropriate amounts of “antidiuretic hormone” leads to pathologic retention of water with dilution of the plasma electrolytes, particularly Na+. If progressive and uncorrected, this condition may lead to life-threatening deterioration of cerebral function (see Chapter 38 for the box titled Syndrome of Inappropriate Antidiuretic Hormone Secretion).
Large decreases in effective circulating volume and blood pressure not only stimulate the release of AVP, they also profoundly stimulate the sensation of thirst. In fact, hemorrhage is one of the most powerful stimuli of hypovolemic thirst: “Thirst among the wounded on the battlefield is legendary” (Fitzsimons). Therefore, three distinct stimuli—hyperosmolality, profound volume contraction, and large decreases in blood pressure—lead to the sensation of thirst. Low effective circulating volume and low blood pressure stimulate thirst centers in the hypothalamus through the same pathways by which they stimulate AVP release (Fig. 40-9).
In addition to thirst, some of these hypothalamic areas are also involved in stimulating the desire to ingest salt (i.e., Na+ appetite). In Chapter 58, we discuss the role of the hypothalamus in the control of appetite.
Under physiological conditions, the body regulates plasma volume and plasma osmolality independently. However, as discussed earlier, this clear separation of defense mechanisms against volume and osmotic challenges breaks down when more dramatic derangements of fluid or salt metabolism occur. In general, the body defends volume at the expense of osmolality. Examples include severe reductions in absolute blood volume (e.g., hemorrhage) and decreases in effective circulating volume even when absolute ECF volume may be expanded (e.g., congestive heart failure, nephrotic syndrome, and liver cirrhosis). All are conditions that strongly stimulate both Na+ and water retaining mechanisms. However, hyponatremia can be the consequence.
An exception to the rule of defending volume over osmolality occurs during severe water loss (i.e., dehydration). In this case, the hyperosmolality that accompanies the dehydration maximally stimulates AVP secretion and thirst (Fig. 40-9). Of course, severe dehydration also reduces total body volume. However, this loss of free water occurs at the expense of both intracellular water (60%) and extracellular water (40%). Thus, dehydration does not put the effective circulating volume at as great a risk as the acute loss of an equivalent volume of blood. Because dehydration reduces effective circulating volume, you would think that the renin-angiotensin-aldosterone axis would lead to Na+ retention during dehydration. However, the opposite effect may occur, possibly because hyperosmolality makes the glomerulosa cells of the adrenal medulla less sensitive to ANG II, thereby reducing the release of aldosterone. Thus, the kidneys fail to retain Na+ appropriately. Accordingly, in severe dehydration, the net effect is an attempt to correct hyperosmolality by both water intake and retention, as well as by the loss of Na+ (i.e., natriuresis) that occurs because aldosterone levels are inappropriately low for the effective circulating volume. Therefore, in severe dehydration, the body violates the principle of defending volume over osmolality.
Books and Reviews
Bonny O, Rossier BC: Disturbances of Na/K balance: Pseudohypoaldosteronism revised. J Am Soc Nephrol 2002; 13:2399-2414.
Bourque CW, Oliet SHR: Osmoreceptors in the central nervous system. Annu Rev Physiol 1997; 59:601-619.
DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 1997; 77:75-197.
Fitzsimons JT. Angiotensin, thirst and sodium appetite. Physiol Rev 1998; 78:583-686.
Gutkowska J, Antunes-Rodrigues J, McCann SM: Atrial natriuretic peptide in brain and pituitary gland. Physiol Rev 1997; 77:465-515.
Navar LG, Zou L, Von Thun A, et al: Unraveling the mystery of Goldblatt hypertension. News Physiol Sci 1998; 13:170-176.
Rose BD, Rennke HG (eds): Renal Pathophysiology: The Essentials. Baltimore: Lippincott Williams & Wilkins; 1994.
Sterns RH: Fluid, electrolyte and acid-base disturbances. In Glassock RJ (ed): Nephrology Self-Assessment Program (NephSAP) 3, pp 187-238. Philadelphia: Lippincott Williams & Wilkins; 2004.
Journal Articles
Chou CL, Marsh DJ: Role of proximal convoluted tubule in pressure diuresis in the rat. Am J Physiol 1986; 251:F283-F289.
Mason WT: Supraoptic neurones of rat hypothalamus are osmosensitive. Nature 1980; 287:154-157.
Oliet SHR, Bourque CW: Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature 1993; 364:341-343.
Robertson GL, Aycinena P, Zerbe RL: Neurogenic disorders of osmoregulation. Am J Med 1982; 72:339-353.
Thompson DD, Pitts RF: Effects of alterations of renal arterial pressure on sodium and water excretion. Am J Physiol 1952; 168:490-499.
Walser M: Phenomenological analysis of renal regulation of sodium and potassium balance. Kidney Int 1985; 27:837-841.
Verney EG: The antidiuretic hormone and the factors which determine its release. Proc R Soc London B Biol Sci 1947; 135:25-106.
Yang LE, Maunsbach AB, Leong PKK, McDonough AA: Differential traffic of proximal tubule Na+ transporters during hypertension or PTH: NHE3 to base of microvilli vs. NaPi2 to endosomes. Am J Physiol 2004; 287:F896-F906.