Chapter 20 Drugs Affecting the Respiratory System
Normal Respiratory Physiology
Airway Caliber Changes
Nervous innervation to the smooth muscle of the respiratory tract is complex. The parasympathetic system provides the primary efferent innervation, with acetylcholine as the primary neurotransmitter.1,2 Its fibers are responsible for the baseline tone of mild bronchoconstriction that characterizes the normal respiratory tract through M3 muscarinic receptors. The effects are balanced by bronchodilation resulting from β2-receptor stimulation.3-5, In contrast, α-adrenergic stimulation can contribute to bronchoconstriction.2,4,6 A third, largely understood nervous system, referred to as the nonadrenergic, noncholinergic system or the purinergic system, also innervates bronchial smooth muscle.1,7 This system mediates bronchodilation by way of vagal stimulation. The afferent fibers of this system are probably irritant receptors, and although the neurotransmitter has not yet been conclusively identified, vasoactive intestinal peptide has been implicated in the cat.8,9 Malfunction of this system has been associated with bronchial hyperreactivity, which often characterizes asthma.7
The intracellular mechanisms that transmit signals from the nervous system to smooth muscle depend in part on changes in the intracellular concentration of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) (Figure 20-1). The effects of these two secondary messengers are reciprocal such that the increased intracellular concentration of one is associated with a decreased concentration of the other. Cyclic AMP-induced bronchodilation is decreased by α-adrenergic stimulation and increased by β2-receptor stimulation.3 In contrast, cGMP-induced bronchoconstriction is increased by stimulation of muscarinic (cholinergic) and, indirectly, histaminergic receptors (see Figure 20-1). The relative sensitivity of bronchial smooth muscle to histamine-induced and acetylcholine-induced bronchoconstriction varies with the location and species.5,10,11 Peripheral airways in dogs are more susceptible than in cats to acetylcholine; feline airways, in general, are more sensitive to acetylcholine and serotonin than histamine.12 Smooth muscle receptors are also susceptible to stimulation by a variety of chemical mediators (see Figure 20-1), which may also modulate cAMP and cGMP.13-15
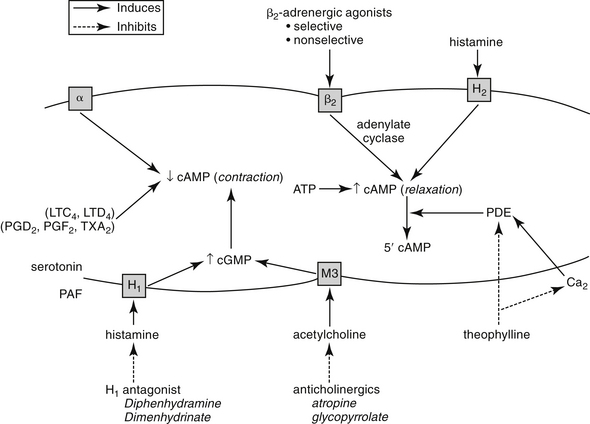
Figure 20-1 Factors determining bronchial smooth muscle tone. Reciprocal changes in cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) determine muscle tone. Contraction occurs when cAMP levels are decreased by events such as α-adrenergic stimulation or when cGMP levels increase in response to muscarinic receptor (M3) stimulation by acetylcholine or H1-receptor stimulation by histamine. Calcium (Ca2+) and several mediators can also induce bronchoconstriction. Increased cAMP levels induced by β-adrenergic or histamine (H2) receptor stimulation counteract muscle contraction. Inhibition of phosphodiesterase (PDE) also causes increase cAMP. Although the effects of most inflammatory mediators are best counteracted by preventing their release, several drugs may be used to antagonize smooth muscle contraction regardless of the etiology. LTC, Leukotriene C; LTD, leukotriene D; PAF, platelet-activating factor; PGD2, prostaglandin D2, PGF2, prostaglandin F2; TXA2, thromboxane.
KEY POINT 20-1
The normal state of the airway is one of bronchoconstriction, mediated by the parasympathetic system (via cGMP). However, this is offset by sympathetic (via cAMP) bronchodilation.
Control of bronchial smooth muscle tone is very complex and depends on input from sensory receptors. At least five types of sensory receptors have been identified in cat lungs, all of which can be classified as irritant (or mechanoreceptor), stretch, or J-receptors.7 All appear to be innervated by the parasympathetic system. Irritant receptors, located beneath the respiratory epithelium, occur in the upper airways2 and, in cats, as far peripherally as the alveoli.1 Physical, mechanical, or chemical stimulation of these receptors results in tachypnea, bronchoconstriction, and cough. As reviewed by Canning,7a rapidly adapting receptors (RARs) terminate in or below the epithelium primarily of intra-pulmonary, but to some degree, extra-pulmonary airways. They can adapt within 1 to 2 seconds to changes in airway mechanics and are subject to activation by multiple stimuli, including chemical mediators. In contrast, slowly adapting receptors (SARs) may be responsible for physiologic responses (i.e., termination of inspiration and initiation of expiration). SARS centrally inhibit respiration and decrease airway smooth muscle tone. However, they may facilitate cough. Cats have few SARS but many RARs.7a C-fibers represent the majority of fibers innervating the airways and lungs. They are physiologically similar to nociceptors of other tissues. They appear to synthesize neuropeptides (e.g., substance P) that influence the central and peripheral nerve terminals. Mediators stimulating C-fibers vary: in dogs, pulmonary subtypes do not (but bronchial subtypes do) respond to histamine, and in other animal models, they do not respond to serotonin or adenosine. C-fibers regulate airway defense reflexes, interacting with RAR, although the scientific description of these fibers is not clear. C-fibers and RARS interact with the spinal cord through central sensitization. Subsequent heightened reflex responsiveness may occur; tachykinins (including substance P) appear to play a role in hyperreflexia, an effect prevented by neurokinin receptor antagonists. Convergence of vagal afferents in the nucleus of the solitary tract may explain in part the close relationship between vomiting and coughing. Receptors also converge in the ventral respiratory column of the medulla. Airflow velocity appears to be the most critical factor determining stimulation of irritant receptors in the upper airways.1 Airway constriction sufficient to cause airflow velocity to exceed a specific threshold results in a vagally mediated cough reflex and bronchoconstriction. Airways can also be occluded by mucus and edema or by chemical mediators released during upper airway infections.7
In addition to the cough receptors, afferent nerves, efferent nerves and effector muscles, the cough reflex also consists of a poorly defined cough center.
Respiratory Defense Mechanisms
In addition to the cough and sneeze reflexes, two other systems provide the major defense of the respiratory tract against invading organisms or foreign materials: the mucociliary apparatus and the respiratory mononuclear phagocyte system.2 The mucociliary apparatus (Figure 20-2) is the first major defense and consists of the ciliary lining of the tracheobronchial tree and the fluid blanket surrounding the cilia. Nervous innervation to the cilia has not yet been identified. Although ciliary activity increases with β-adrenergic stimulation, this may simply reflect the sequelae of β-adrenergic stimulation on respiratory secretions.16 Two types of secretions form the fluid blanket of the respiratory tract.17 The cilia must be surrounded by a low-viscosity, watery medium to maintain their rhythmic beat. A more mucoid layer lies on top of the cilia and serves to trap foreign materials inspired with air. The synchronous motion of the cilia causes the cephalad movement of the mucous layer and any trapped materials.
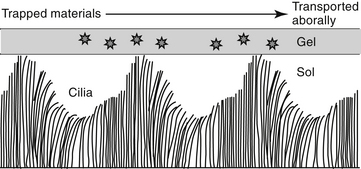
Figure 20-2 The mucociliary apparatus represents the first line of defense for pathogens entering the respiratory tract. Cilia are bathed in a water or sol layer. When the cilia beat in synchrony, the movements send forward (orally) the mucoid or gel layer that lies on top of the cilia. Materials trapped in this layer also move forward to be either swallowed or expectorated.
Changes in the viscoelastic properties of mucus such that it becomes either too watery or too rigid will result in mucus transport that is less than optimal.2 Mucus released by goblet cells results from direct irritation2 and is not amenable to pharmacologic manipulation. Surface goblet cells, which are uniquely prominent in feline bronchioles,18 increase in number with chronic disease. Submucosal glands of the bronchi secrete both a serous and a mucoid fluid. The secretions tend to be more fluid than that of the goblet cells, but the degree varies with the stimulus. The normal consistency of the combined secretions of the tracheobronchial tree is 95% water, 2% glycoprotein, 1% carbohydrate, and less than 1% lipid.2 Glycoproteins increase the viscosity of the secretions, providing protection and lubrication. Infection and chronic inflammatory diseases can have a profound effect on respiratory secretions. The glycoprotein component tends to be replaced by degradative products of inflammation such as DNA and actin.17 Goblet cell numbers increase with a subsequent increase in the viscosity of respiration secretion. Parasympathetic, cholinergic stimulation increases mucus secretion, whereas β-adrenergic stimulation causes secretion of mucus, electrolytes, and water.2,16
KEYPOINT 20-2
The importance of the mucocilary escalator to treatment of respiratory disorders should not be underestimated. Its viscosity must be balanced if secretions and their entrapped materials are to be properly cleared from the airways.
The second major component of the pulmonary defense system is the respiratory mononuclear phagocyte system. In cats, calves, pigs, sheep, and goats, this includes both alveolar macrophages and the pulmonary intravascular macrophages (PIMs).19 The PIMs are resident cells that are characterized by phagocytic properties and thus cause the release of inflammatory mediators. The clearance of blood-borne bacteria and particulate matter in these species is accomplished by PIMs rather than hepatic Kupffer cells and splenic macrophages as in most other species.19 The pharmacologic significance of the mononuclear phagocyte system reflects their role in inflammation (Figure 20-3; see also Figure 20-1). A number of preformed (e.g., histamine and serotonin) and in situ (e.g., prostaglandins, leukotrienes, and platelet-activating factor [PAF]) mediators are released by inflammation cells.13-15 Each is capable of inducing a variety of adverse effects that tend to decrease airway caliber size: edema, chemotaxis, increased mucus production, and bronchoconstriction (Table 20-1). The involvement of PIMs in both experimental and natural respiratory diseases of animals suggests that release of chemical mediators from these cells may be important in the pathogenesis of bronchial diseases.
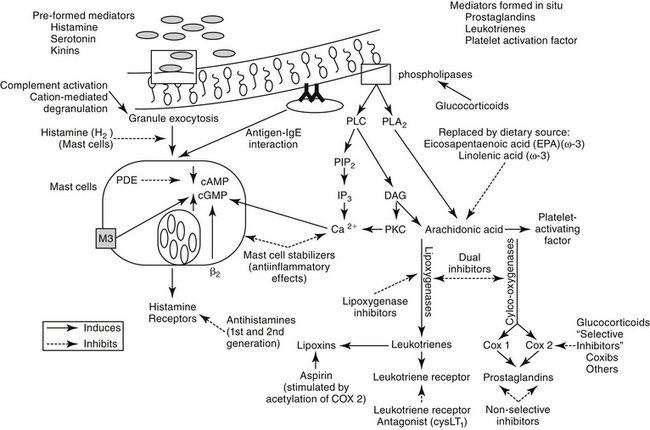
Figure 20-3 The formation of mediators important in the pathogenesis of respiratory disease. Leukocytes and other cells release arachidonic acid metabolites and platelet-activating factor (PAF) after activation of phospholipases by a variety of stimuli. Mast cell degranulation induced by both immune and nonimmune stimuli is also accompanied by arachidonic acid metabolism as well as by the release of preformed mediators that are stored in the granules. Intracellular mechanisms that induce mast cell degranulation include increased calcium (Ca2+), increased cyclic guanosine nucleotide (cGMP) mediated by muscarinic (M3) receptors, or decreased cyclic adenosine nucleotide (cAMP) mediated by α-adrenergic receptor stimulation. Drugs used to prevent mediator release include glucocorticoids, which are one of the few classes of drugs that can prevent activation of phospholipases (mediated by lipocortin) and thus release of arachidonic acid metabolites and PAF. Inhibition of prostaglandin synthesis by nonsteroidal antiinflammatory drugs may prove beneficial but also may lead to increased formation of leukotrienes by providing more arachidonic acid. Leukotriene actions can be blocked either by leukotriene receptor antagonists or by blockade of leukotriene receptors. Mast cell degranulation can be prevented by stimulation of β-adrenergic receptors, inhibition of calcium influx or phosphodiesterase (PDE), or prevention of M3 receptor stimulation. Drugs that block β-adrenergic receptors are contraindicated in most respiratory diseases. DAG, diacylglycerol; IP3, inositol triphosphate; PIP2, phosphatidylinositol; PKC, protein kinase C; PLC, phospholipase C; PLA2, phospholipase A2.
Surfactant
The primary function of surfactant is to decrease surface tension in alveoli, affecting lung mechanics and gas exchange. However, pulmonary surfactant also affects pulmonary defense mechanisms.20 Surfactant is a complex mixture of lipids and proteins synthesized and secreted by alveolar type II cells with relative consistency among mammalian species.20 In humans the proportion of components is predominantly phospholipids (80%) but includes proteins (12%) and other lipids (8%). The phospolipids principally comprise phosphatidylcholine (85%), the most important to reduction of surface tension being dipalmitoylated phosphatidylcholine (DPPC). Four surfactant-associated proteins (SP) are named A through D. Both SP-A and SP-D enhance phagycytosis of bacteria and viruses and regulate type II pneumocytes. The primary role of SP-B and SP-C is reduction of surface tension, particularly in terminal airways. A number of nonspecific host defense mechanisms have been attributed to surfactant.20 Enhanced stability of the alveolar lining film allows it to serve as a nonspecific barrier to microorganism adhesion and subsequent pulmonary invasion. Muciliary transport is improved by its viscoelastic and rheologic properties. Particle clearance is enhanced, in part through an apparently stimulatory effect on chloride ion transport. Antiinflammatory effects reflect superoxide dismutase– and catalase-mediated oxygen radical scavenging and reduced neutrophil production of superoxide.20,21 Direct antibacterial and antiviral properties have also been attributed to surfactant. Both SP-A and SP-D appear to provide a first line of defense by acting as collectins that target carbohydrate structures on a variety of pathogens. Alveolar macrophage response is increased by SP-A; SP-D enhances macrophage release of oxygen radicals. Cytokines (e.g., tumor necrosis factor [TNF]-α interleukin [IL]-1β, and IL-6) release is stimulated by SP-A.
Changes in surfactant have been associated with a number of respiratory diseases. In humans acute respiratory distress syndrome (ARDS) is associated with changes in the biochemical and biophysical characteristics of surfactant. As a result, surface tension increases, and the phospholipid, fatty acid, and protein profiles change. Serum proteins and inflammatory mediators directly inhibit or degrade surfactant. Abnormalities in the amount and both the phospholipid and the protein composition of surfactant have been associated with infectious diseases of the lungs. The ability of surfactant to suppress immune-mediated lung injury has been interpreted as a possible role of surfactant dysfunction in asthma. Elastase-induced dysfunction and impaired SP-A and -D also may contribute to the surface destruction associated with chronic obstructive pulmonary disease (COPD) and emphysema.20
Pathogenesis of Inflammatory Respiratory Diseases
Although it is not the only chronic disease of the respiratory tract, feline bronchial asthma has offered a model for understanding pathophysiology and identifying targets of drug therapy. The interaction of sensory receptors and mediators of bronchial tone is intricately balanced in the normal lung. A series of pathologic disturbances, however, severely disrupts the balance in bronchial asthma.
Asthma is a pathologic state of the lungs characterized by marked bronchoconstriction and inflammation.22-26 Although recent evidence is emerging that airway smooth muscle plays a role in asthma independent of inflammation,27 the latter is an important contributor to disease and warrants a continued focus for drug therapy. Airways become hypersensitive to selected mediators (e.g., histamine and cholinergic stimulants).5,10 The terms allergy and atopy are often used interchangeably; however, for this discussion allergy refers to an uncommitted biological response to an antigen. The pathophysiology of chronic allergic diseases is discussed in Chapter 31. Atopy refers to an allergic response characterized by immunoglobulin E (IgE)–mediated antibodies. Atopy generally reflects a genetic predisposition to IgE antibody production against environmental allergens. Most commonly, the allergic response is low grade and thus beneficial (removal of inciting allergen). However, in atopic patients the response, including IgE antibody production, is exaggerated. Asthma is one of several chronic atopic diseases characterized by an increasing incidence in human medicine; others include allergic rhinitis, atopic eczema, and inflammatory bowel disease.28 Inflammatory mediators are the major contributors of the pathophysiology in asthma (see Table 20-1).22,23,25,26,29,30 Studies in several species have shown that initial exposure to an allergen activate mast cells, macrophages, and other cells lining the airways, increasing mucosal epithelial permeability. Subsequent mediator release not only directly and intensely affects airway inflammation, contraction of smooth muscle, and capillary permeability but also initiates release of chemotactic factors, local infiltration of activated eosinophils, and activation of T cells.31
The critical role of T cells in the initiation and perpetuation of asthma has been well documented32 and is supported by response in asthmatics to both glucocorticoids and cyclosporine.32,33 The exaggerated response that characterizes the atopic patient appears to reflect an imbalance in Th1 versus Th2 helper cells. Researchers in human medicine have postulated that atopic individuals fail (possibly as infants) to transition from a Th2-primary response (mediated by ILs 4, 5, and 13) to an “allergy protective” Th1-mediated primary response. The Th1 response is dominated by cell-mediated immunity, and delayed hypersensitivity (cytotoxic T cells) is initiated through macrophage production of IL-12 followed by production of interferon (IFN)-γ by Th1 and natural killer cells. In normal infants the shift from Th2 to Th1 might be stimulated by exposure to a variety of antigens, including microbes with cytosine and guanosine nucleotides (CpG repeats). The hygiene hypothesis suggests that atopy develops because subjects are not sufficiently exposed to allergens at a young age, perhaps as a result of early use of antimicrobials or lack of exposure to allergens (e.g., children exposed to one another at day care, or environmental factors [e.g., farms]).28 The Th2 response that characterizes inflammatory disease is associated with increased production of IL-4, IL-5, and IL-13. The IL-4 from Th2 supports B-cell IgE synthesis. The Th2 cells have a particular influence on eosinophils through IL-5, which regulates both differentiation and bone marrow release (mature and immature cells), and the chemokine eotaxin.28 Subsequent accumulation of eosinophils in atopic tissue is influenced by continued recruitment by eotaxins, the presence of selective adhesion molecules, and delayed apotosis.28
KEYPOINT 20-4
The inflammation associated with chronic allergic respiratory disease may reflect an imbalance between Th1 and T h2 cells.
The role of the bone marrow in the pathophysiology of atopy increasingly is emerging as potential target, leading to a systemic rather than local approach to therapy.34 Signaling appears to occur between the lung and bone marrow, with IL-5 and/or eotaxin being the commonality.35 Increased IL-3 may also play a role in eosinophil stimulation, whereas IL-18, IL-12, and INF-γ are inhibitory toward eosinophils.28,36 In animal models of asthma, including the canine Ascaris model, allergen exposure is associated with an increase in bone marrow eosinophils and trafficking from the bone marrow to the lungs.37 Interestingly, cysteinyl leukotrienes may influence eosinophil differentiation (in the presence of IL-3 or IL-5); leukotriene-receptor antagonists are able (in vitro) to suppress eosinophil differentiation.37,38
Eosinophils contribute to mucosal damage through release of basic amines, cysteinyl leukotrienes, and PAF.28 Inhibitory muscarinic (M2) receptors are damaged, which facilitates persistent bronchoconstriction. Mediators are released following binding of allergens with the α chain of high-affinity IgE receptor (Fc€ RIα) on the cell. Regulation of IgE, which is found both on and in eosinophils, offers another potential target of therapy. IL-4 and IL-13 are the most important inducers of IgE production.28
As permeability increases in response to inflammation, histamine and other inflammatory mediators are better able to reach and stimulate inflammatory cells located in the submucosa. The continued release of mediators is associated with stimulation of afferent nerve endings in the mucosa and reflex cholinergic bronchoconstriction. Mediators also increase microvascular permeability, induce chemotaxis, and stimulate mucous secretion. The release of cytotoxic proteins and toxic oxygen radicals further damages the respiratory epithelium, and the bronchial tree becomes hypersensitive. Mediators can also inhibit mucociliary function.25 Mucus production increases as submucosal glands and goblet cells increase. The consistency of the mucus changes to become more viscous. Bronchial smooth muscle often hypertrophies and undergoes spasms.39 Airway obstruction in chronic disease reflects bronchoconstriction, bronchial wall edema, and accumulation of mucus and cells. As the disease progresses, airways eventually become plugged and ultimately collapse. Chronic inflammation leads to fibrosis, which contributes to the collapse, and air trapped within the alveoli can result in emphysema. Asthma is a disease of central and large as well as small (<2 mm) airways.40 Regarding the type of asthma previously considered “silent,” studies in humans have revealed the importance of a therapeutic focus on airways beyond the eighth or ninth generation of the bronchial tree. In humans asthma and allergic rhinitis commonly, but not always, co-exist,40 and allergens associated with rhinitis may be too large to penetrate the lower airways.41
The complex physiology of asthma is exemplified by the reparative role that eosinophils also have. Damage repair contributes to airway remodeling through release of growth factors and matrix metalloproteinases.
Inflammatory Mediators in the Respiratory Tract
Histamine
Histamine is a vasoactive amine stored in basophils and mast cells. Airway mast cells are located primarily beneath the epithelial basement membrane in dogs.24 Histamine produces a variety of effects (see Table 20-1) by interacting with specific receptors on target cells.12,42 At least four histamine receptors have been identified,5,29,43,44 two of which have been found in the trachea of the cat.5,6 Interaction with the H1 receptor causes an increase in intracellular calcium, and ultimately in cGMP (see Figure 20-3).29 Histamine also stimulates cholinergic receptors in the airway.24,29 Histamine causes constriction in both central and peripheral airways in dogs and cats.12,24 The effects of histamine so closely mimic the pathophysiology of early asthma that, for many years, histamine was considered the major cause of the syndrome.29 Lack of clinical response to H1-receptor antagonists, however, led to the realization that other factors are more important. In contrast to H1 receptors, stimulation of H2 receptors causes an increase in cAMP and bronchodilation.29 Thus antihistamine drugs that block H2 receptors may be contraindicated in asthma. Some studies have suggested that a defect in H2 receptors may contribute to airway hyperreactivity.29 Finally, recent evidence suggests that histamine regulates both Th1 and Th2 cells. Further, cells perpetuating the allergic response may be influenced by histamine through H4 receptors located on eosinophils, basophils, mast cells, and dendritic cells.43
Histamine contributes to bronchial occlusion by mechanisms other than bronchoconstriction. Mucous secretion is mediated via H2 receptors and by secretion of ions and water via H1 receptors.29 Microvascular leakage resulting from contraction of endothelial cells also follows H1-receptor stimulation.29 Histamine is chemotactic to inflammatory cells, particularly eosinophils and neutrophils. Interestingly, histamine stimulates T-lymphocyte suppressor cells by way of H2 receptors,5 a function that also may be depressed in human patients with asthma.29 Histamine also has a negative feedback effect on further histamine release mediated by IgE.29 Both of these latter effects are mediated by H2 receptors and would be inhibited by H2-receptor antagonists.5
Serotonin
Serotonin (5-hydroxytryptamine [5-HT]) is released during mast cell degranulation.29 Although serotonin does not appear to be an important mediator of human or canine bronchial asthma, both the central and peripheral airways in cats are very sensitive to its bronchoconstrictive effects after aerosolization or intravenous administration.12 Constriction may reflect interaction with serotonin receptors or enhanced release of acetylcholine. Serotonin may also cause profound vasoconstriction of the pulmonary vasculature and microvascular leakage.29
Prostaglandins and Leukotrienes
Prostaglandins (PGs) and leukotrienes (LTs) are eicosanoids that are formed when phospholipase A2 is activated in the cell membrane in response to a variety of stimuli (see Figure 20-3). Arachidonic acid (AA) is subsequently released from phospholipids and enters the cell. In the cell it is converted by cyclooxygenases to inflammatory, but unstable, cyclic endoperoxides. The actions of various synthetases and isomerases on the endoperoxides result in the final PG products, including PGE2, PGF2α, PGD2, prostacyclin (or PGI2), and thromboxane (TXA2). The amount of each PG produced in the lung varies with the cell type and species. The effects of the various PGs tend to balance one another. PGD2, PGF2α, and TXA2 cause bronchoconstriction, whereas PGE1 and, to a lesser extent PGI1, cause bronchodilation.1,29 Bronchoconstriction induced by PGD2 is about 30 times as potent as that induced by histamine.
Imbalances between PGs may be important in the pathogenesis of bronchial disease. Both PGD2 and TXA2 have been implicated in immediate bronchial airway hyperreactivity.29 Thromboxane A2 appears to be the predominant AA metabolite produced by feline lungs,45 although other PG mediators are also important.45
The role of LTs in atopy has been increasingly scrutinized in recent years. Lipoxygenases catalyze the conversion of AA to hydroperoxyeicosatetraenoic acid (HPETEs), which are further metabolized to several hydroxy acids (HETEs) and LTs (Chapter 29). Eosinophils have been known to be preferentially activated 5-lipoxygenase29; selective activation of lipoxygenase by antigenic challenge yields subsequent formation of the cysteinyl LTs (cystLTs: LTB4 C4, and D4), recognized as the components of slow reactive substance of anaphylaxis.29 However, both intermediate and end products of lipoxygenase are active in the respiratory tract (see Table 20-1).29 Among these mediators are some of the most potent inflammagens known. For example, bronchial smooth muscle contraction and microvascular permeability mediated by LTC4 and LTD4 is 100- to 1000-fold more potent than that induced by histamine. Both LTs are potent stimulators of mucous release in the dog but appear to be less potent in the cat.29 In humans airway allergen challenge increases LTs in urine and bronchoalveolar lavage (BAL) fluid with asthma and nasal secretions with allergic rhinitis.35 Increases in LTs in plasma, BAL fluid, or urine of challenged cats is controversial, although cats experimentally infected with dirofilariasis likewise exhibited increased plasma as well as BAL fluid cysLT concentrations (unpublished data). The cysteinyl LTs exert biologic effects through cysLT1 and cysLT2 receptors.35 Binding by LTs to eosinophilic cysL1 receptors (particularly LTD4) results in chemotaxis and prolonged survival, effects that are blocked by LT-receptor antagonists.35 Further evidence suggests that cysteinyl LTs are also involved in the initial systemic atopic response that is mediated from the bone marrow.35,46
Platelet-Activating Factor
PAF is also formed after activation of phospholipase A2 in cell membranes. It is a potent, dose-independent constrictor of human airways, and it is the most potent agent thus far discovered in causing airway microvascular leakage.22,23 PAF is also a potent chemotactant for platelets and eosinophils, both of which are a rich source of PAF. The effects of PAF may be mediated through LTs. PAF has been implicated as the cause of the sustained bronchial hyperresponsiveness that characterizes asthmatics.23 The role of PAF in feline and canine respiratory diseases has not been addressed. Eosinophils are, however, a major cell type associated with feline bronchial disease and some canine diseases, and it is likely that PAF is an important inflammatory mediator.
Reactive Oxygen Species
Reactive oxygen species (ROS) are constantly formed in the lung as part of pulmonary defense toward microorganisms and neoplasm.21 Free oxygen radicals, hydrogen peroxide and hypochlorous acid are among the species formed. Their formation is mediated, in part, by other inflammatory mediators such as TNF, IL-1, IL-6, and IL-8, and AA derivatives (PGs, LTs, and PAF). Hyperoxia will enhance generation of ROS.21 Normal cells are generally protected from ROS by a tightly controlled redox balance and formation of endogenous antioxidants; formation is enhanced by exposure to antioxidants. Those located primarily intraceullularly include catalase, superoxide dismutase, and glutathione redox compounds (glutathione, glutathione peroxidase, and glutathione reductase). Primarily extracellular antioxidants include fat-soluble compounds (vitamin E), water-soluble compounds (vitamin C, cysteine, reduced glutathione, taurine), and high-molecular-weight antioxidants (mucus and albumin).21
Neuropeptides
Several chemicals stimulate release of inflammatory neuropeptides from sensory neurons enervating smooth muscles. Cells stimulating neuropeptide release include macrophages, T-cells, eosinophils, and mast cells; example neuropeptides include substance P and, to a lesser degree, calcitonin gene-related peptide and neurokinin A.47 As with other inflammatory mediators, neuropeptides are associated with vasodilation, increased permeability and mucus production, as well as histamine release.
Cytokines
The role of cytokines in the coordination and persistence of chronic airway inflammation is integral and, not surprisingly, very complex. The integrated nature of their actions renders them simultaneously ideal as targets of drug therapy but potentially a source of adverse events should therapy be successful. Four cytokine classes have been proposed: lymphokines, chemokines, and proinflammatory and antiinflammatory cytokines (see Table 20-1). The role of lymphokines was largely addressed with the discussion of Th cell role in atopy. Initiation of Th2 cells is not clear, but appears to involve presentation of restricted antigens in the presence of IL-4 and IL-10. Perhaps the most notable lymphokine of asmtha is IL-5.48 Recruitment of eosinophils into the lungs—the hallmark of asthma—is IL-5 dependent. However, IL-5 also is integral to the differentiation, proliferation, and maturation of eosinophil progenitor cells in the bone marrow before recruitment into the lung.
Chemokines are cytokines that attract inflammatory cells, including eosinophils, monocytes, and T-lymphocytes. Two groups have been described based on proximity of cysteine residues to one another: CXC or α chemokines (separated by an amino-acid) and CC or β-chemokines (no separation; see Table 20-1). Chemokines exert their effects through rhodopsin-like G protein–coupled receptors (referred to as CXC-R or CC-R). Because certain cells express selected receptors, pharmacologic therapy increasingly will be designed to be selectively antiinflammatory.31 Among the cells influenced by chemokines are eosinophils, which in turn release more chemokines. Among the most notable drugs targeting proinflammatory chemokines, and particularly their transcription, are glucocorticoids (see Chapter 30). Newer therapies take advantage of the presence of antiinflammatory cytokines; indeed, glucocorticoids facilitate restoration of antiinflammatory cytokines such as IL-10.
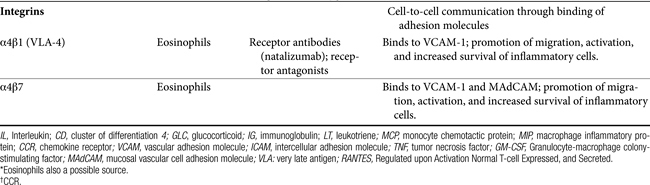
Communication Molecules: Adhesion Molecules and Integrins
Communication between cells is facilitated by adhesion molecules that interact, by way of ligand binding, with receptors on the surface of inflammatory cells known as integrins. Integrins are present on many cells, with receptor specificity occurring for selective cells. Integrins are absent on Th1 cells but are particularly prevalent on Th2 cells, mast cells, and eosinophils. Interaction between adhesion molecules and integrins promotes migration, activation, and increased survival of inflammatory cells. Drugs that target eosinophilic integrins (α4β1 and 7) offer a possible mechanism whereby selective control of inflammation may limit side effects.49
Drugs Used to Modulate the Respiratory Tract
The syndrome of chronic bronchial disease is best treated by breaking the inflammatory cycle while immediately relieving bronchoconstriction. Thus antiinflammatory drugs and bronchodilators represent the cornerstone of therapy for many bronchial diseases. Other categories of drugs that are effective for the management of respiratory diseases, particularly in small animals, include antitussives, respiratory stimulants, and decongestants.
Bronchodilators and Anti-Inflammatory Drugs
Because of a shared intracellular mechanism of action, most drugs that induce bronchodilation also reduce inflammation. Bronchodilators reverse airway smooth muscle contraction by increasing cAMP, decreasing cGMP, or decreasing calcium ion concentration (see Figure 20-1). In addition, these drugs also decrease mucosal edema and are antiinflammatory because they tend to prevent mediator release from inflammatory cells (see Figure 20-3). Rapidly acting bronchodilators include β-receptor agonists, methylxanthines, and cholinergic antagonists.
β-Receptor Agonists
β-receptor agonists (Figure 20-4) are the most effective bronchodilators because they act as functional antagonists of airway constriction, regardless of the stimulus.22,50,51 Few β-agonists generally have been sufficiently studied in animals to describe pharmacokinetics or pharmacodynamics.
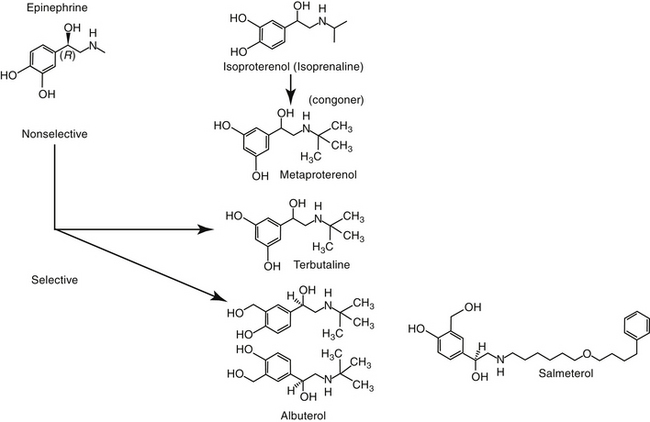
Figure 20-4 Structures of selected beta-adrenergic drugs used to induce bronchodilation. Epinephrine is the least and albuterol or terbutaline the most selective for β-2 receptors. Most β-adrenergic drugs are present as enantiomers, as is demonstrated here for albuterol.
Large numbers of β2-receptors are located on several cell types in the lung, including smooth muscle and inflammatory cells.3 The receptor is linked to a stimulatory guanine nucleotide-binding protein (G protein). The interaction between a β-agonist and receptor causes a conformational change in the receptor and subsequent activation of adenylyl cyclase on the inner cell membrane (see Figure 20-1).52 Adenylate cyclase converts adenosine triphosphate to cAMP, a second messenger for activation of specific protein kinases that ultimately activate enzymes responsible for airway smooth muscle relaxation. β-receptor agonists are most effective in states of bronchoconstriction. Additional effects of β-adrenergic receptors include increased mucociliary clearance, which reflects a decrease in fluid viscosity (presumed to reflect movement of chloride and water into the lumen) and an increase in ciliary beat frequency. Additionally, they inhibit cholinergic neurotransmission, enhance vascular integrity, and inhibit mediator release from mast cells, basophils, and other cells (see Figure 20-3).22,23,51,52 Eosinophil, but not lymphocyte, numbers appear to decrease, but long-term inflammation does not appear to be affected,52 probably because inflammatory cell β receptors are rapidly desensitized (discussed later). As such, long-term β-adrenergic use should be accompanied by antiinflammatory therapy.41
As with many membrane-associated receptors, high doses or repeated exposure to β-adrenergic agonists results in desensitization.51 The mechanism depends on the duration of therapy. Initial drug–receptor interaction causes phosphorylation, which interferes with G proteins. Longer exposure causes receptors to internalize such that they are not accessible by the drug; continued exposure causes downregulation of receptor mRNA such that the number of receptors is actually reduced.52 Reduction develops over several weeks before stabilizing. Desensitization may contribute to acute exacerbations of bronchoconstriction associated with long-acting β-adrenergics. Bronchodilation will be less in the desensitized airway necessitating increased treatment frequency. However, frequent use may mask clinical signs associated with uncontrolled inflammation and care must be taken that long-acting β-adrenergics are not used instead of antiinflammatory therapy. Despite these shortcomings, β-adrenergics are the most the most effective bronchodilators.
The β-adrenergic agonists (see Figure 20-4) can be given by a variety of routes. In humans inhalation is preferred because it is equally effective to parenteral administration and is safer. However, drug delivery to the peripheral airways must be addressed as much as possible.
Nonselective β-Agonists
The nonselective β-agonists (i.e., capable of both β1 and β2 stimulation) such as epinephrine, ephedrine, and isoproterenol are used for acute and chronic therapy of respiratory diseases. Epinephrine and isoproterenol can be administered parenterally to achieve rapid effects, and drugs that can be given orally for chronic therapy include isoproterenol and ephedrine.6 Both epinephrine and ephedrine cause α-adrenergic activity, which may cause vasoconstriction and systemic hypertension and may contribute to airway constriction.4 Nonselective β-agonists may cause adverse cardiac effects as a result of β1-receptor stimulation. Aerosolization reduces the adverse effects of nonselective β-adrenergic agonists by increasing β2 specificity, because only these β-receptors appear to line the airways.
β2-Selective Agonists
At appropriate doses, β-selective agonists are not generally associated with the undesirable effects of β1-adrenergic stimulation. The more commonly used β-agonists are categorized as to their duration of action: intermediate (3 to 6 hours) and long-acting (>12 hours). The two long-acting bronchodilators, salmeterol and formoterol, both have extended side chains, are highly lipophilic, and are characterized by high affinity for the β-2 receptors. However, the long duration of salmeterol reflects binding to a specific site within the receptor, whereas that for formoterol reflects gradual release from the cell membrane lipid.52 The long-acting nature of bitolterol reflects its pulmonary metabolism to an active metabolite. However, it has a rapid onset of action and generally is used for acute therapy.
KEY POINT 20-5
Beta-adrenergic agonists are such effective bronchodilators that they may mask signs of inflammation that indicate ongoing disease.
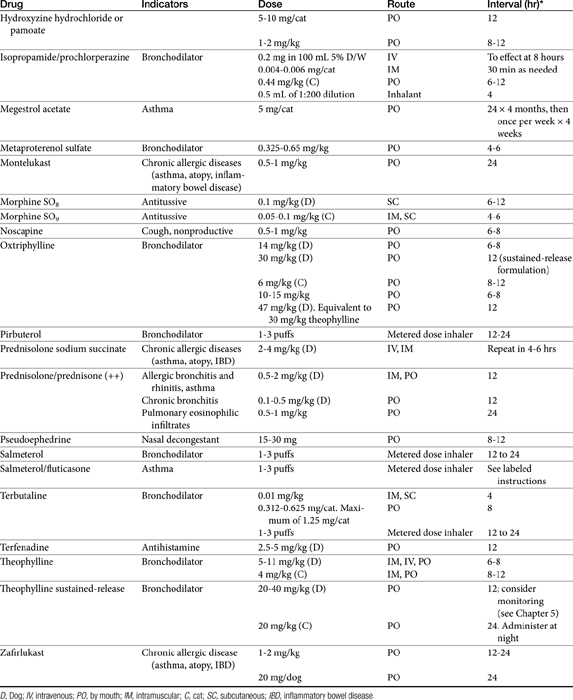
Short-acting drugs that have been used in animals include albuterol, metaprotereno (a derivative of isoproterenol), and its analog terbutaline.41 Metaproterenol is less β-2 selective than other agents and more apt to cause cardiac stimulation.52 Therefore repetitive use should be pursued only cautiously. Albuterol, metaproterenol and terbutaline are available as oral preparations; each has been used apparently safely in dogs. Rapid first-pass metabolism reduces systemic bioavailability after oral administration; as such, oral doses are greater than parenteral doses. These drugs, but particularly metaproterenol, can cause β1 side effects at high doses. Clenbuterol, also a β-2 selective agonist, is approved for use in horses. Although manufactured as the racemic mixture, pharmacologic effects are largely limited to the L-isomer.53 Tachycardia occurs in dogs at 0.4 mg/kg, which is only half of the therapeutic dose recommended in horses. Further, cardiac necrosis occurs at 2.5 mg/kg, which represents only a threefold increase over the equine therapeutic dose. Consequently, use of clenbuterol in dogs may not be prudent. Albuterol and isoetharine are examples of β2-selective agonists that have been administered by aerosolization to small animals.30 Salbutamol is frequently used as a rescue bronchodilator in humans.54
Inhalant Devices
With the advent of metered doses inhalers in the 1960s, beta-adrenergics became a common therapy for treatment of human asthma. Short-acting β2 agonists administered by aerosol (Figure 20-5) include albuterol and its R enantiomer levalbuterol, metaproteronol, pirbuterol, bitolterol, and terbutaline (see Figure 20-4). Longer-acting (in humans) beta-2 adrenergic drugs tend to be more lipophilic and thus remain in the presence of a receptor for a longer time. Examples include salmeterol and formoterol (see Figure 20-4).40,41,55 The drugs differ in their effect and use. Short-acting products are associated with rapid symptomatic relief in human asthmatics when used at appropriate doses. However, use at high doses has been associated with an increase in mortality in humans, leading to their recommended use on an “only as needed” basis.40,55 On the other hand, improvement in pulmonary function in humans was sustained with prolonged used of long-acting beta-adrenergics,55 and thus such use was not associated with a decrease in symptomatic relief afforded by short-acting drugs. The duration of onset of long-acting beta-adrenergics may be 1 or more hours. Although minimally effective by themselves for control of inflammation, long-acting beta-adrenergics appear to enhance responsiveness to glucocorticoids. Rebound hyperresponsiveness does not appear to occur with rapid discontinuation of the long-acting drugs. The development of tolerance (desensitization) has been discussed and is probably more likely with long-acting drugs.41 Because beta-adrenergics do not provide as much antiinflammatory control, their efficacy may be reduced in the presence of inflammation and combination therapy with an antiinflammatory drug (e.g., inhaled or systemic glucocorticoids), or use of theophylline may be indicated.40,55

Figure 20-5 An example of a device commercially available, intended as an adapter for inhalant metered dose devices marketed for human use.
Drugs that block β2-receptors such as propranolol are contraindicated in animals with bronchial disease. Inhalant devices are discussed more in depth in the following section.
Methylxanthine Derivatives
Pharmacologic Effects
Methylxanthines include caffeine, a potent respiratory stimulant, and theobromine, a recognized canine toxicant from chocolate and theophylline (Figure 20-6). Theophylline has been the cornerstone of long-term bronchodilatory therapy in animals, particularly dogs. Its mode of action has been attributed to nonspecific inhibition of phosphodiesterases (PDEs) and increased concentrations of cAMP and cGMP (see Figure 20-1).41,56 This mechanism has been controversial, however, because theophylline does not inhibit PDE at therapeutic concentrations. However, this may reflect the existence of PDE as various isoenzymes (at least 11 members to this superfamily, each located in different sites within the cell, some of which are inaccessible to drugs).22,23,41 Inhibition of PDE4 and PDE5 results in bronchodilation whereas inhibition of PDE4 contributes to its antiinflammatory effects.41 The nonselective action of theophylline results in bronchodilation and inhibition of inflammation. Theophylline also is a competitive antagonist at adenosine receptors. The inhibitory neurotransmitter adenosine, which induces bronchoconstriction and during hypoxia and inflammation. Another mechanism by which theophylline induces bronchodilation, however, may be through interference of calcium mobilization.22,23
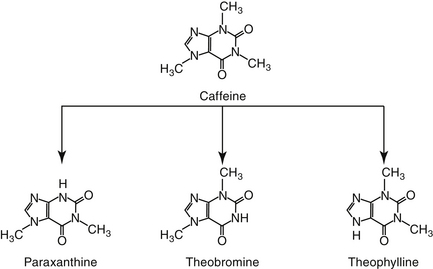
Figure 20-6 Caffeine, which is used as a respiratory stimulant in humans, is metabolized to other methylxanthines, theobromine, theophylline, and paraxanthine.
Compared to beta-2 agonists, theophylline is considered a weaker relaxant of airway smooth muscle.40 As with β-agonists, theophylline is equally effective in large and small airways. Theophylline has other effects in the respiratory system that are important to its clinical efficacy.22,23,56 In addition to its bronchodilatory effects, it inhibits mast cell degranulation and thus mediator release (see Figure 20-3)57; increases mucociliary clearance; and prevents microvascular leakage.58 Its antinflammatory effects appear to include modulation of cytokines, particularly of macrophage origin.59 Finally, antiinflammatory effects of theophylline may also reflect nuclear activation of histone deacetylase.41 Decreased TNF-α and IFN-γ and increased IL-10 (antiinflammatory) have been reported. Pentoxifylline has similar antiinflammatory effects; its IV administration is addressed in Chapter 29. Increased mucociliary clearance has been reported in dogs.60 In propofol-anesthetized cats (n = 6), no treatment effect could be detected in the mucociliary transport rate after oral administration of 25 mg/kg of a slow release (Slo-bid) product. The ability to detect a significant difference was not addressed. Further, the mucociliary the rate in untreated animals (22.2 ± 2.8 mm/min) was perceived to be maximal, leaving no room for theophylline-induced increase. The impact of propofol is not clear, and theophyllline concentrations were not measured to confirm adequate concentrations. Thus the lack of efficacy may not reflect theophylline as much as failed delivery or altered response.
In addition to its antiinflammatory effects, a major advantage of theophylline, compared with other bronchodilators, may be its increased strength of respiratory muscles and thus a decrease in the work associated with breathing.56,62,63 This may be important to animals with chronic bronchopulmonary disease.
Disposition
Theophylline is one of the few drugs active in the respiratory tract whose disposition has been studied in animals. Because theophylline is not water soluble, it can be given only orally. Salt preparations of theophylline are available for either oral or parenteral administration. Dosing of the various salt preparations must be based on the amount of active theophylline (Table 20-2). Aminophylline, an ethylenediamine salt, is 80% theophylline, whereas oxtriphylline is 65% theophylline, and glycinate and salicylate salts are only 50% theophylline. Regular aminophylline is well absorbed (bioavailability of at least 90%) after oral administration in both dogs and cats.64,65 In dogs peak plasma drug concentrations for the theophylline base (approximately 8 μg/mL after a dose of 9.4 mg/kg) occur 1.5 hours after oral administration.64 The volume of distribution (L/kg) and clearance (mL/min/kg) in dogs are, respectively, 0.59 ± 0.045 and 0.78 ± 0.13. The extrapolated peak concentration after intravenous administration of 11 mg/kg aminophylline (8.6 mg/kg theophylline equivalent) was 22.94 ± 5.8 μg/mL.66 In cats extrapolated concentrations after 10 mg/kg intravenous aminophylline approximated 10 μg/mL; volume of distribution (L/kg) and clearance (mL/min/kg) were 0.87 ± 0.07 and 0.87 ± 0.16, respectively.67 Interestingly, peak concentrations after intravenous or oral (sustained-release) products are higher in cats when dosed in the evening compared with the morning.68
More than 30 slow-release preparations exist for use in humans. Although several products have been studied in dogs and cats,68,69 their disposition is markedly variable and cannot be predicted on the basis of product name or description. Interchangability of products cannot be assumed, and monitoring is strongly encouraged to establish potential efficacy. For dogs, the rate of oral absorption of slow-release products is apparently faster than in humans. The extent of absorption varies with the preparation. Bioavailability of slow-release preparations varies from 30% (anhydrous theophylline 24-hour capsules; Theo-24, Searle Laboratories) to 76% (anhydrous theophylline tablets; TheoDur).69 The least variation among animals occurs for oxtriphylline enteric-coated capsules (Choledyl-SA Tablets; Parke-Davis) and a 12-hour capsular anhydrous theophylline (Slo-Bid Gyrocaps), which are approximately 60% bioavailable. The minimum effective concenteration recommended for humans (10 μg/mL) may not be reached by all slow-release products. Plasma drug concentrations during a 12-hour dosing interval varies, by almost 120% for the oxtriphylline product but only 48% for the anhydrous tablet (Theo-Dur), suggesting it may be the best product for use in dogs.69 However, neither Theo-Dur nor Slo-Bid Gyrocaps are currently available for use in the United States. Although the mean residence time of the slow-release preparation was significantly longer by 1 to 2 hours than that of the regular preparation in dogs, the clinical significance of this difference is questionable.69 More recently, Bach and coworkers66 described the disposition of a generic (Inwood Laboratories) extended-release theophylline tablet (Theochron) or capsule (TheoCap) in dogs (15.5 mg/kg orally; n = 6). The oral bioavailability (%) of the tablet and capsules was 98 ± 15.4 and 83.6 ± 18.5%, respectively. The Cmax (μg/mL) was 17.4 ± 4.9 and 12.2 ± 2.8 for the tablet and capsule, respectively, whereas the elimination half-life was 10.9 ± 3.6 and 12.7 ± 2.7. Drug concentrations remained within the recommended therapeutic range for 12 hours. The authors concluded that 10 mg/kg every 12 hours would result in theophylline concentrations in the therapeutic range.
KEY POINT 20-6
Oral bioavailability of slow-release theophyllines may markedly vary among products and patients, contributing to toxicity or therapeutic failure.
Cats also have been studied. Although it is not distributed to all body tissues, theophylline is characterized by a relatively large volume of distribution in dogs (0.7 to 0.8 L/kg) and a smaller volume in cats (0.41 L/kg).64,69,70 Unlike human beings, distribution of theophylline is not limited by binding to serum proteins in dogs; serum protein binding is less than 12%.71,72 Elimination of theophylline is not dose dependent in dose ranges of 3 to 15 mg/kg. Dye and coworkers68,70 studied two sustained-release theophylline products (TheoDur and Slo-Bid Gyrocaps, William H. Rorer, Inc) in the cat. Although both products were reasonably (>75%) bioavailable in the cat, neither product is currently available in the United States. A more recent study addressed the disposition of a generic (Inwood Laboratories) extended-release theophylline tablet (Theochron; 15 mg/kg) and capsule (TheoCap; 19 mg/kg) in cats (n = 6).67 The Cmax (μg/mL) of the tablet and capsule was 17.8 ± 3.4 and 15.8 ± 3.1, respectively. Although bioavailability of both products was 100% or greater, the elimination half-life for the tablet and capsule was 13.6 ± 3.9 hour and 18.3 ± 8.0, respectively, compared with 11.7 ± 1.8 hour for the intravenous preparation (aminophylline), suggesting that the drugs did not always act in an extended-release manner in cats. Nonetheless, drug concentrations remained within the recommended therapeutic range for 24 hours. These data suggested that once-daily administration in cats should be sufficient to maintain concentrations within the therapeutic range. (see Table 20-2). A longer dosing interval might be acceptable if evidence supports an antiinflammatory effect at even lower concentrations. Based on a chronopharmacokinetic study of these sustained-release products, dosing in the evening rather than in the morning appears to be associated with better bioavailability and less peak plasma theophylline concentration fluctuation.68 A disadvantage of the use of the slow-release products for small animals is the limited dose sizes available. The product cannot be divided for more accurate dosing without altering the kinetics of slow release.
Theophylline is metabolized by demethylation in the liver. Theobromine may be an active metabolite in some species. Different rates of metabolism result in variable clearance rates and drug elimination half-lives among animals, and doses consequently vary.72 For example, the elimination rate constant of theophylline is less in cats (0.089/hr)73 than dogs (0.12/hr), resulting in a longer half-life in the cat (7.8 hours) compared with the dog (5.7 hours),64,65 thus necessitating a smaller dose in cats.69 Theophylline concentrations can be affected—most commonly increased—by a number of drugs, including fluorinated quinolones,74 erythromycin and its congeners,75 and cimetidine.76
Adverse Reactions and Drug Interactions
Theophylline is associated with a wide range of adverse effects, including central nervous system excitation (manifested as restlessness, tremors, and seizures),72 gastrointestinal upset (nausea and vomiting), diuresis, and cardiac stimulation (e.g., tachycardia). In a retrospective study of theophylline toxicity in human patients, clinical signs included severe vomiting (89%), seizures (21%), and cardiac arrythmias (16%).77 Vomiting precluded treatment with oral charcoal but responded to metoclopramide. Therapy included mechanical ventilation, anticonvulsants or sedatives, and muscle relaxants. Because of the risk of toxicity, intravenous use should be limited to patients who have not responded to β-agonist therapy and are facing life-threatening disease. Compared with the salt preparations, theophylline is more irritating to the gastrointestinal tract than aminophylline.30,56 Rapid infusions or infusions of undiluted aminophylline can cause cardiac arrhythmias, hypotension, nausea, tremors, and acute respiratory failure.30
Theophylline may be involved in a number of drug interactions; hepatic metabolism and potentially serious adverse effects should lead to cautious use with other drugs. Inhibition of drug-metabolizing enzymes by fluorinated quinolones has been described in human medicine and for both enrofloxacin and marbofloxacin in animals.78 For enrofloxacin 5 mg/kg decreased theophyllline clearance by 43%79; a different study found a 26% decrease by marbofloxacin (5 mg/kg).78 This translates to an increase in Cmax by 31% for theophylline, sufficient to contribute to toxicity. Indeed, the author has measured peak theophylline concentrations above 70 μg/mL and an elimination half-life of 19 hours in a dog concomitantly given enrofloxacin at 5 mg/kg. Clinical signs of theophylline toxicity emerged within 24 hours of beginning enrofloxacin therapy. Theophylline–terbutaline interactions do not appear to be clinically relevant, as has been shown in humans,80 supporting their combined use for patients not responding to a single bronchodilator.
The application of therapeutic drug monitoring to guide therapy would assist in identifying the most appropriate dosing regimen, particularly when using slow-release preparations whose bioavailability might vary among patients, in patients receiving drug combinations that increase the risk of drug interactions, and in patients with hepatic dysfunction. Although a therapeutic range has not been established for small animals, the range recommended for humans (10 to 20 μg/mL) can be extrapolated until a more definitive range has been established. Dogs are apparently more tolerant of theophylline toxicity than are humans. In one study toxicity manifested as tachycardia, central nervous system stimulation (restlessness and excitement), and vomiting did not occur until plasma theophylline concentrations reached 37 to 60 μg/mL. Doses of 80 to 160 mg/kg of a sustained-release preparation were required to induce toxicity.81 In cats concentrations as high as 40 μg/mL do not induce adverse reactions, although salivation and vomiting are common after administration of more than 50 mg/kg, and seizures may occur at doses greater than 60 mg/kg.82
The side effects of theophylline are dose dependent and might be prevented to a large degree by appropriate dosing. Therapeutic drug monitoring should facilitate the design of proper dosing regimens to prevent toxicity.
Anticholinergic Drugs
Pharmacologic Effects
Anticholinergic drugs compete with acetylcholine at muscarinic receptor sites.83 In the respiratory tract, they reduce the sensitivity of irritant receptors and antagonize vagally mediated bronchoconstriction. The site of action of these drugs in the respiratory tract is controversial. In some studies bronchodilation is reported throughout the airways in asthmatic human patients and cats, whereas other investigators believe that the effects are confined to large airways.83 The route by which anticholinergic drugs are administered influences their bronchodilatory effects. Despite their effect on bronchial airways, the anticholinergic drugs have not proved clinically effective in the treatment of bronchial diseases in animals, and their use is limited to treatment of bronchoconstriction associated with organophosphate toxicity or in animals in status asthmaticus unresponsive to bronchodilator therapy. The lack of clinical efficacy of anticholinergics may reflect nonselective drug–receptor interaction.22,23 Thus far, three types of muscarinic receptors have been identified in airways. M3 receptors release acetylcholine, whereas M2 receptors block its release. Nonselective blockade of muscarinic receptors by atropine and ipratropium may actually potentiate acetylcholine release by antagonizing the effects of M2-receptor stimulation. Drugs specific for M3 receptors may ultimately lead to successful treatment of bronchial disease with anticholinergics drugs.22,23 As with beta-adrenergic drugs, use of inhaled anticholinergic drugs reduces systemic adverse effects.
Atropine
Aerosolized atropine, a prototype anticholinergic drug, affects predominantly the central airways, whereas both central and peripheral airways are affected if the drug is administered intravenously.22,23 Because atropine is highly specific for all muscarinic receptors, it causes a number of systemic side effects, including tachycardia, meiosis, and altered gastrointestinal and urinary tract function.64 In the respiratory tract, atropine reduces ciliary beat frequency, mucous secretion, and electrolyte and water flux into the trachea. The net effect is decreased mucociliary clearance, which is undesirable in patients with chronic lung disease.64 Aerosolization of atropine does not reduce the incidence of respiratory adverse reactions. Atropine is well absorbed (in humans) after oral administration. In humans atropine has proved most useful for treatment of chronic bronchitis and emphysema, diseases that are characterized by increased intrinsic vagal tone.83 Its adverse effects on respiratory secretions and ciliary activity, however, apparently negate its benefits to bronchial tone during long-term administration in animals. The primary indication for atropine in small animals is facilitation of bronchodilation in acutely dyspneic animals. It is the treatment of choice for life-threatening respiratory distress induced by anticholinesterases. A combination of atropine with either β-adrenergic agonists or glucocorticoids will cause better bronchodilation than either drug alone.83
Ipratropium Bromide
Ipratropium bromide is a synthetic anticholinergic that is pharmacodynamically superior to atropine. Although the two drugs are equipotent, ipratropium does not cross the blood–brain barrier. It is not well absorbed after aerosolization, which limits the likelihood of adverse effects. Ipratropium has been studied in the dog but not in the cat.83 Of the anticholinergic drugs studied in dogs, ipratropium appears to cause the greatest bronchodilation (twice as much as atropine) with the least change in salivation.83 When inhaled, it is more effective in preventing bronchoscopy-induced bronchoconstriction compared with intramuscular atropine.54 Unlike atropine, it does not alter mucociliary transport rates.
Ipratropium bromide is generally not promoted as a bronchodilator in dogs or cats. However, with the advent of inhalant devices, this may change. The combination of salbutamol (120 mg, 1 puff) and ipratropium bromide (20 mg, 2 puffs) was found to be superior to either drug alone in the prevention of BAL-induced bronchoconstriction in experimentally induced allergen sensitive, conscious cats (n = 18).54 Drugs were administered using a pressurized inhalant metered devices (pMDI). with a spacing chamber connected through an inspiratory valve to a face mask.
Glycopyrrolate
Glycopyrrolate can also be used as a bronchodilator in small animals. Although its onset of action is slower than that of atropine,30 its half-life is 4 to 6 hours compared with 1 to 2 hours for atropine. The potency after systemic therapy has apparently not been compared between the two drugs, although glycopyrrolate is twice as potent when aerosolized. The systemic side effects of glycopyrrolate are minimal.
Mast Cell Stabilizers
Drugs that stabilize mast cells are most effective in syndromes associated with marked mast cell activity. Included in this category may be newer antihistamines, discussed later. The stabilizing effects of β-adrenergic agonists, methylxanthines, and glucocorticoids (see Chapter 30) on inflammatory cells have been discussed.
Cromolyn
Although the mechanism of action of cromolyn is not certain, it appears to inhibit calcium influx into mast cells, thus preventing mast cell degranulation and the release of histamine and other inflammatory mediators (see Figure 20-3).22,23,84 At high concentrations, cromogylate inhibits IgE-triggered mediator release from mast cells.85 Some studies suggest that the activation of inflammatory cells other than the mast cells (e.g., macrophages, neutrophils, eosinophils) is also inhibited by cromogylate.28 Cromolyn is most useful as a preventive before activation of inflammatory cells. It is not significantly absorbed after oral administration and is characterized by a short half-life (=). Thus effective therapy depends on frequent aerosolization, which limits its utility in the treatment of small animal diseases. Currently, cromolyn is the safest drug used to manage asthma in humans.84 It is associated with only minor side effects, and its discovery has revolutionized the management of bronchial asthma in people. Because of its wide therapeutic window and its apparent efficacy in the control of many inflammatory cells, its use in the control of small animal bronchial disease warrants further investigation.
Calcium Antagonists
The efficacy of calcium antagonists in the management of asthma has yet to be identified.86 Their potential benefits include prevention of mediator release, smooth muscle contraction, vagus nerve conduction, and infiltration of inflammatory cells.86,87 Most studies indicate that calcium antagonists have only a modest effect on airway smooth muscle contraction. Their antiinflammatory effects may ultimately prove of greater benefit.
Drugs that Target Inflammatory Mediators
Glucocorticoids
Glucocorticoid use in dogs and cats is addressed in Chapter 30. In 1997 the National Heart, Lung, and Blood Institute Expert Panel Guidelines recommended control of mild persistent asthma with a single, long-term control medication with antiinflammatory properties.55 Accordingly, gluccocorticoids are the cornerstone of asthma therapy.88 The antiinflammatory effects of glucocorticoids reflect an inhibitory effect on essentially all phases of inflammation. Airway infiltration with eosinophils, mast cells, and basophils is decreased, although the effect on T cell population in the lungs is less clear. Glucocorticoids differentially downregulate Th2 cytokines, including IL-4, IL-5, and IL-13, but appear to upregulate Th1 cytokines, including IFN-γ and IL-12. Most recently, glucocorticoids have been demonstrated to reduce eotaxin and other eosinophil-associated chemokines but have little effect on IL-8.89 Glucocorticoids have little effect on cys-LTs.90 Glucocorticoids have a “permissive” effect on β-adrenergic receptors and help prevent desensitization, which may accompany therapy with long-acting drugs.
KEY POINT 20-7
The role of glucocorticoids as the cornerstone of inflammatory lung disease reflects both their antiinflammatory effects as well as their permissive effects of beta receptors mediating bronchodilation.
Glucocorticoid efficacy for treatment of respiratory inflammatory disease depends on therapeutic concentrations in and below the epithelium of all diseased airways. However, whereas systemic therapy might provide the most consistent exposure to diseased airways, it also provides the greatest exposure to tissues other than the lungs, leading to adverse effects. A number of approaches have been taken to minimize adverse effects of systemic glucocorticoids when used to treat asthma. Although dogs and cats appear to tolerate glucocorticoids better than humans, making these approaches less important, they nonetheless may be beneficial to animals. These include selective gene targeting, increased potency for the glucocorticoid receptors (allowing administration in specialized [inhalant] drug delivery systems), and development of drugs that undergo first-pass metabolism. Aerosol administration minimizes many side effects. The preferred route in humans with mild disease is low-dose inhaled glucocorticoids.55 Beclomethasone was among the first aerosol glucocorticoids developed for inhalant therapy.40 Corticoisteroids marketed as inhalant metered devices (MDIs; see later discussion) in the United States include beclomethasone dipropionate (Beclovent), triamcinolone acetonide (Azmacort), flunisolide (Aerobid), budesonide (Pulmicort), fluticasone propionate (Flovent), and mometasone (Asmanex) (Figure 20-7).
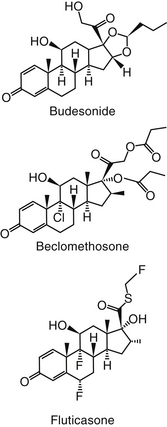
Figure 20-7 Structures of selected “soft” glucocorticoids used in inhalant devices for the topical treatment of respiratory inflammatory diseases. These drugs are very potent and often are characterized by first-pass metabolism such that swallowed drug will be minimally absorbed.
Aerosolized glucocorticoids, such as occurs with MDIs, result in local delivery of higher concentrations. Although side effects are minimized, up to 90% of an inhaled dose is still deposited on the oral mucosa or pharynx and swallowed in humans; a similar or greater proportion might be anticipated in animals. Thus multiple methods have emerged to reduce adversities without decreasing efficacy. The delivery device may influence adverse effects. Systemic side effects associated with deposition of glucocorticoids on the pharynx and central airways and local side effects in the upper airway (e.g., dysphonia in up to 50% of the patients) led to the inclusion of “spacers” that removed larger particles before they penetrated the pharynx.40
Corticosteroid delivery of MDIs also has been improved by the advent of hydrocarbon fluoroalkyl (HFA)–propelled MDI. Beclomethasone dipropionate delivery to peripheral airways increases from 5% to 15% for the chlorofluorocarbon -propelled preparation to 50% to 60% with the HFA propellant.91 Not only is total lung delivery increased, but the depth of penetration also is enhanced, a potentially critical improvement for controlling progression of the disease.
The glucocorticoid itself has been manipulated to decrease adversity. Corticosteroids marketed in MDI vary up to fivefold or more in potency. The relative potency of drugs marketed as MDI roughly follows the following order: monmetasone, which exceeds both fluticasone and budesonide, which, in turn, are 2 to 3 times more potent than beclomethasone; triamcinolone is the least potent of these drugs. While potency does allow administration of a small dose, it does not predict clinical efficacy of inhaled glucocorticoids.91 Despite sixfold differences in potencies among inhaled glucocorticoids, comparative clinical trials in humans have failed to demonstrate differences in efficacy when drugs are administered at equipotent dosages.91 Further, dose response curves for inhaled glucocorticoids tend to be flat, indicating that increasing doses is not likely to enhance efficacy.
Ultimately, differences in pharmaceutical (delivery) and pharmacokinetic properties largely determine variable responses to inhaled glucocorticoids. Inhaled corticosteroids generally are delivered as microcrystals, which must dissolve in the epithelial mucosal fluid. Crystals must be water soluble to ensure local delivery before the mucociliary tract removes the drug. However, alteration of dissolution times may also affect local delivery and thus local effects. For example, the dissolution time for budesonide is 6 minutes compared with beclomethasone dipropionate (5 hours) and fluticasone (8 hours). Lipophilicity of the drug enhances uptake and the duration of local effects. The addition of a halogen increases tissue retention compared with nonhalogenated drugs. Lipophilicity is greatest for beclomethasone and fluticasone followed by budesonide, with triamcinolone followed by dexamethasone and, finally, prednisolone as the least liphophilic. Not surprisingly, the most lipophilic of the drugs also is associated with the greatest number of side effects, including suppression of the hypothalamic pituitary adrenal axis. In humans, fluticasone is both the most potent and most lipophilic glucocorticoid. As such, it is characterized by the greatest evidence of systemic side effects. As such, recommendations for humans are that high-dose fluticasone propionate (>500 mg twice daily) be used only on the order of a physician and that the dose be titrated down to the lowest effective dose. Budesonide offers an example of a different type of manipulation that may allow longer dosing intervals while minimizing side effects. Because of its structure (a free C21 hydroxyl group) (see Chapter 30), excess intracellular budesonide complexes with long chain fatty acids. The complex is inactive but probably allows persistence of the drug at the site, much as a depot form would, with reversible esterification occuring as receptors are depleted of active drugs. Other drugs with a free C21 hydroxyl include triamcinolone, flunisolide, and ciclesonide, although long-chain fatty acid esterification has not been determined for them. Neither fluticasone, nor beclomethasone dipropionate, and probably mometasone, form fatty acid esters.91
Finally, although most devices require or allow twice- to four-times-daily dosing, those designed for once-daily administration also should enhance efficacy through improved compliance. Poor compliance with use of inhaled glucocorticoids in human patients also led to the development of combinations of steroids with long-acting β2 agonists (e.g., salmeterol/fluticasone or formoterol/budesonide). Although compliance has improved, concern has arisen that the β2 agonists will mask clinical signs that might otherwise indicate worsening of the disease.40 Indeed, recent studies of the efficacy of inhaled beclomethasone diproprionate found improvements in clinical signs to be short-lived, probably because the inhaled drug does not control inflammation well.40 Reduced airway caliber will further decrease efficacy by reducing drug delivery to the peripheral airways. Antiinflammatory therapy should target both large and small airways if inflammation is to be suppressed.40 Thus systemic therapy should be considered (either as sole therapy or in addition to systemic therapy) in animals with moderate to severe disease. The peak effect of inhaled glucocorticoids may not occur for 1 to 2 weeks after therapy has begun.
Inhaled glucocorticoids have been recommended for use in cats with asthma,39 although few studies have provided guidance. One abstract has reported a beneficial effect of flunisolide (250 μg/puff) but not zafirlukast (10 mg orally every 12 hours) in cats with experimental feline asthma.92 Reinero and coworkers93 compared the impact of inhaled flunisolide (250 μg puff twice daily) to that of oral prednisone (10 mg/day orally) and placebo on indices of inflammation and adrenal gland suppression in healthy cats (n = 6). No treatment effect was apparent with regard to serum immunoglobulin or cytokine activity. The inhaled glucocorticoid was associated with lower baseline cortisol compared with placebo and and lower cortisol after adrenocorticotropic hormone stimulation compared with oral and placebo therapy. A limitation of the study may have been the use of prednisone rather than prednisolone as is suggested by impact on cortisol concentrations, although oral therapy did impact both T and B cells. This study supports the potentially inappropriate use of prednisone in cats and suggests that topical flunisolide therapy may suppress the hypothalamic pituitary adrenal axis in cats with flunisolide.
Drugs that Target Leukotrienes
LTs are very potent causes of marked edema, inflammation, and bronchoconstriction.94 Their role in the pathophysiology of inflammation and their inhibition are discussed in Chapter 30. Their inhibition by glucocorticoids may be limited, leaving a void in therapy.90 The approval of drugs that specifically inhibit the formation of LTs or their actions have offered a new avenue of control of respiratory inflammatory disease (e.g., asthma) in human medicine. Two classes of drugs have focused on their impact on LTs: LT synthesis (5-lipoxygenase) inhibitors (zileuton) and LT-receptor antagonists (LRAs). The latter class has proved to be more effective (Figure 20-8). Currently, two LRAs are approved in the United States: zafirlukast, administered twice daily, and montelukast, administered once daily. The disposition of neither drug has been studied in dogs or cats; in humans the dosing intervals reflect a half-life of 5 and 10 hours, respectively.
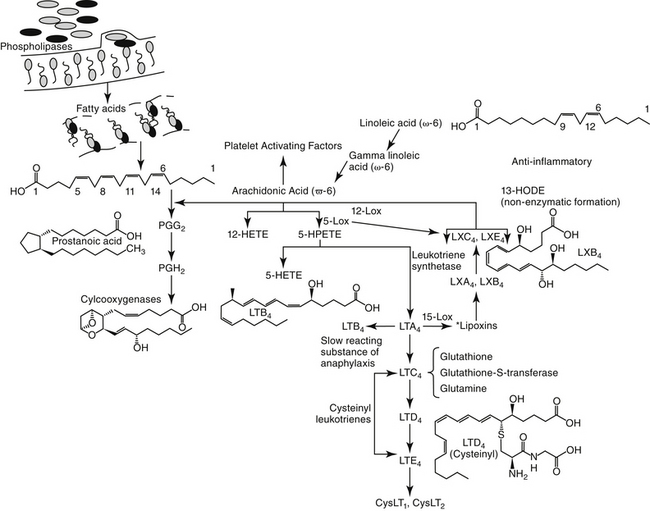
Figure 20-8 The arachidonic cascade and the formation of proinflammatory or antiinflalmmatory mediators catalyzed by lipoxygenases.
KEY POINT 20-8
Leukotriene receptor antagonists should be considered for both local and peripheral control of respiratory inflammation in mild cases, as adjunct therapy in poor responders, and in patients intolerant to glucocorticoids.
In human clinical trials, LRAs inhibit early- and late-phase bronchoconstriction and increased bronchial hyperresponsiveness in response to allergens; accumulation of inflammatory cells and mediators in bronchial lavage fluid; and acute bronchospasms stimulated by exercise, cold air, and aspirin.95 Their bronchodilatory effects are less than that of long-acting β-adrenergics, whereas the antiinflammatory effects are less than those of glucocorticoids.90 However, response does occur to single doses. In contrast to β-adrenergics, tolerance does not develop toward LRA effects. Their use has been associated with improvement of asthma either as sole therapy (instead of low-dose inhaled glucocorticoids) in mild to moderate asthma or as add-on therapy regardless of disease severity in glucocorticoid nonresponders.95 The LRAs are well tolerated, particularly compared with glucocorticoids. A subset of asthmatics treated with LRAs have developed Churg–Strauss syndrome, a state of systemic hypereosinophilia. However, its emergence may reflect decreased doses of glucocorticoids permitted once LRAs are begun rather than a toxic effect of LRAs.90
Recommendations regarding the role of LRAs in treatment of human asthma are dynamic. Currently, because they are less effective than glucocorticoids, their role as monotherapy is not recommended, even with mild disease. In contrast, LRAs have proved effective as monotherapy for treatment of allergic rhinitis in humans.90 For asthma, because of their unique mechanism of action, LRAs have been combined with a number of other drugs used to treat asthma. For example, glucocorticoids minimally affect cys-LT; as such, combination with LRAs would be expected to have an additive effect, as has been demonstrated when combined with inhaled glucocortiocids. The combination of LRAs and glucocorticoids generally is as effective as glucocorticoids combined with β-adrenergic agonists. The use of an LRA as part of triple therapy (i.e., with corticosteroids, β-adrenergics) is a reasonable, albeit understudied, therapeutic approach in patients not responding to dual therapy. Finally, LRAs also have been recommended in the treatment of aspirin-sensitive asthma (discussed later).
The role of LTs in the treatment of feline asthma was previously discussed. Therapy with LRAs has had little scientific support, although this should not preclude their use, particularly in animals that have not sufficiently responded to or cannot tolerate (e.g., diabetics) corticosteroids. Receptors for LTs have yet to be identified in the smooth muscle of airways in cats; further, LTs have not been identified in the urine or plasma of cats with experimentally induced asthma.92 However, LTs were associated with experimentally induced heartworm disease in cats.96 The recent approach to asthma as a systemic response mediated at the level of the bone marrow warrants consideration of the use of LRAs for treatment of feline asthma, particularly in nonresponders or patients that cannot tolerate glucocorticoids. Other potential indications would include control of inflammation in dogs in which glucocorticoids are contraindicated or pulmonary diseases characterized by eosinophilic infiltrate. Anecdotally, LRAs appear to be safe in both dogs and cats, although no study has confirmed their safety.
Nonsteroidal Antiinflammatory Drugs
The role of nonsteroidal antiinflammatory drugs (NSAIDs) in the treatment of respiratory inflammatory diseases requires definition.97,98 Both LTs and PGs are important in the pathophysiology of inflammatory diseases. Although NSAIDs effectively block PGs through inhibition of cyclooxygenase, they do not negatively affect lipoxygenase. Rather, in the aspirin-sensitive asthmatic (human) patient, aspirin and NSAIDs that selectively inhibit cyclooxygenase I shunt AA toward 5-lipoxygenas and the overproduction of cys-LTs.90 They have no effect on other chemical mediators of inflammation. Additionally, NSAIDs nonselectively block all PGs, including those that provide some protection during periods of bronchoconstriction.99 Some studies have shown that LT production increases in response to NSAID therapy, perhaps by providing more AA for lipoxygenase metabolism. Currently, labels include a contraindication for use in human patients with aspirin-induced asthma. However, although more studies are needed, thus far the newer NSAIDS generally are not associated with emergence of asthma.100
Currently, the use of NSAIDs for the treatment of respiratory diseases in small animals is limited to aspirin therapy as treatment for thromboembolism associated with heartworm disease.101,102 Aspirin is the preferred NSAID because at low doses it irreversibly inhibits TXA2, an important contributor to pulmonary arterial vasoconstriction that accompanies thromboembolism. Current efforts in NSAID research are oriented toward identifying drugs that successfully inhibit both arms of the AA metabolic cascade or specific PG or LT inhibitors. The use of selective TXA2 inhibitors for selected feline respiratory diseases is an example.45 Among the NSAIDs that might be considered, are the dual inhibitors, such as tepoxalin, which target both PGs and LTs.
Antihistamines
A number of nonantihistaminergic drugs act to ameliorate the effect of histamine (Figure 20-9). However, in general, histamine is not recognized to be a major contributor to clinical signs of asmtha based on the limited control of clinical signs afforded by antihistaminergic drugs. Those drugs that prevent histamine release may be of most benefit because they will also prevent release of other inflammatory mediators associated with asthma.
Figure 20-9 Structures of selected antihistaminergic drugs, leukotriene receptor antagonsists, and miscellaneous drugs used to treat disorders of the respiratory tract.
Drugs that interact with beta receptors and stimulate cAMP depress histamine release. Thus epinephrine can be used prior to or early during anaphylaxis to reduce histamine release. However, most of the histamine has already been released by the time treatment occurs, and in fact, most of the beneficial effects of epinephrine during anaphylaxis result from physiologic antagonism of histamine-induced bronchoconstriction. Glucocorticoids impair histamine release through their permissive effects on beta-adrenergic receptors. Additionally, glucocorticoids are lytic to neoplastic mast cells. The newer (but not older) antihistamines such as cetirizine or loratadine may inhibit histamine release from mast cells.103 These newer products have not been widely used in animals, although the disposition of several is discussed in Chapter 29.
The use of H1 blockers may be detrimental in animals with chronic disease because of their effects on airway secretions.29 The role of H2 receptors in bronchodilation, mucous secretion, and inflammation suggests that H2-receptor blockers should also be used with caution.5,29 Among the drugs for which anecdotal discussion suggests efficacy for treatment of tracheal collapse is doxepin, a tricyclic antidepressant that is also characterized by marked H1 antagonism. Efficacy may be related to antiinflammatory effects or some level of central nervous system depression.104 Alternatively, local anesthetic actions may be contributing to efficacy.105 Although not yet clinically available, drugs that specifically target H4 receptors may prove useful through control of signals mediating chronic allergic responses. Cyproheptadine is an antiserotinergic, antihistaminergic drug. Because feline airways are exquisitely sensitive to the constrictor effects of serotonin, this drug may prove particularly useful in cats either alone or as an adjunct to bronchodilators or glucocorticoids.106 The disposition of cyproheptadine has been described in cats (n = 6).107 It is characterized by 100% oral bioavailability, a volume of distribution of 1 ± 0.5 l/kg and an elimination half-life of 12.8 ± 9.9 hours, supporting a once- to twice-daily dosing interval. A single intravenous dose of 2 mg was well tolerated. An oral dose of 8 mg yielded a Cmax of 419 ± 99 ng/mL. Both the intravenous and oral dose were well tolerated. Studies following transdermal administration are pending.
Antitussives
The goal of antitussive therapy is to decrease the frequency and severity of cough without impairing mucociliary defenses (Figure 20-10). Whenever possible, the underlying cause should be identified and treated. Cough suppressants should be used cautiously and are contraindicated if the cough is productive.2 Irritants and perhaps chemoreceptors and stretch receptors initiate the cough reflex.2,73 Bronchoconstriction is probably the most frequent and important cough stimulus. The cough reflex can be blocked peripherally, either by facilitating removal of the irritant with mucolytics or expectorants or by blocking peripheral receptors to induce bronchodilation, or it can be blocked centrally at the cough center in the medulla (see Figure 20-4).2,108 Mediators targeted for treatment of cough might be opioid, serotonin (5-HT1A), nociceptin, or gamma amino butyric acid-B agonists and neurokinin (NK-1 or NK-2) or N-methyl-D-aspartate (NMDA) antagonists.7a-c
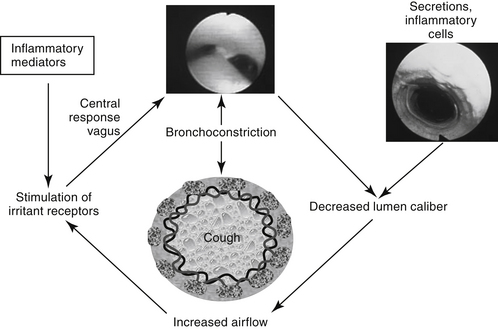
Figure 20-10 The most potent stimulus for the cough reflex is decreased airway caliber size. The subsequent increase in airflow velocity irritates stretch receptors. The vagus nerve serves as the afferent and efferent limbs of the cough reflex, which is mediated centrally by the respiratory center in the medulla. Accumulation of debris and inflammatory mediators can either irritate receptors or decrease airway luminal caliber. Cough is accompanied by bronchoconstriction, which can further exacerbate coughing.
KEY POINT 20-9
Cough might be reduced peripherally through therapies that increase airway caliber size. This includes bronchodilation or facilitating removal of intraluminal debris.
Centrally active antitussives are classified as narcotic and non-narcotic drugs.108,109
Opioid Antitussives
Opioid (narcotic) antitussives depress the cough center sensitivity to afferent stimuli. They can, however, be associated with strong sedative properties, as well as constipation, when administered chronically. Morphine, codeine, and hydrocodone are the narcotics most commonly used to control coughing. As class II drugs, each is subject to the Controlled Substances Act of 1970 and can be used for cough suppression in both dogs and cats. Other opioids may have antitussive actions but are generally not used for that indication. Examples include diphenoxylate, a drug to which powerful antitussive actions have been ascribed.
Codeine
Codeine is the prototype narcotic antitussive and one of the most effective drugs available to suppress the cough reflex. Codeine phosphate and codeine sulfate can be used either alone or in combination with peripheral cough suppressants or decongestants. Over-the-counter preparations are available for human use. Compared with morphine, codeine is equally effective as a cough suppressant but is less suppressing to other central centers and causes less constipation. Side effects of codeine include nausea and constipation. Codeine is also addressed in Chapter 28.
Hydrocodone
Hydrocodone is a more potent antitussive than codeine but causes less respiratory depression. It is probably the most commonly used antitussive for dogs. Hydrocodone bitartrate is a hydrolysis product of dihydrothebaine.
Butorphanol
Butorphanol tartrate is probably more commonly used as an analgesic. Reclassified as a Schedule IV drug, it is now subject to the Narcotics Act. It is approved for use as an antitussive for dogs. As an antitussive, it is 100 times more potent than codeine and 4 times more potent than morphine.110 In dogs, after subcutaneous administration, butorphanol concentrations peak at 1 hour. Mean half-life is 1.7 hours, with a duration of activity of 4 hours or more. Butorphanol is characterized by a wide safety margin. The LD50 in dogs after intramuscular administration is 20 mg/kg.111 Therapeutic concentrations cause minimal cardiac or respiratory depression. Side effects include sedation, which can be significant and desirable; nausea; some diarrhea; and appetite suppression. The narcotic agonist/antagonist butorphanol tartrate is a potent antitussive when given orally or parenterally in dogs and cats.
Non-Narcotic Antitussives
Non-narcotic antitussives commonly used in veterinary medicine include the narcotic agonist/antagonist butorphanol and dextromethorphan.
Dextromethorphan
Dextromethorphan hydrobromide (see Figure 20-9) is a semisynthetic derivative of opium. It is the d-isomer of the codeine analog of methorphan. Unlike its L-enantiomer, levomethorphan, it lacks narcotic, direct analgesic, or addictive properties. It is an acid receptor antagonist and as such is also discussed in Chapter 28. Antagonists of NMDA receptors are powerful antitussives in some species (including humans). Dextromethorphan’s metabolite dextrorphan also is active. Because sedation is unusual after its use, it is classified as non-narcotic. Its antitussive mechanism is not certain; only the D-isomer has antitussive activity, which is similar to codeine in potency. Dextromethorphan interacts with sigma receptors (previously thought to be opioid receptors). Its onset of action is rapid, being fully effective within 30 minutes after oral administration. It is metabolized by CYP2D6, a drug-metabolizing enzyme found to be deficient in a significant proportion of the human population. Dextromethorphan is commonly found in over-the-counter cough preparations. It can be used safely in cats. In humans it can release histamine and therefore is not often recommended for atopic children. Its use with selective serotonin uptake inhibitors and monoamine oxidase inhibitors is discouraged. Studies in humans have shown that the combination of dextromethorphan with a bronchodilator is superior to dextromethorphan alone for the control of cough.112
Noscapine
Noscapine is a nonaddictive opium alkaloid (benzylisoquinoline) that has antitussive effects similar to those of codeine.113 Its use for small animals appears to be limited.
Neurokinin Receptor Antagonists
The role of NK-antagonists in control of cough is being elucidated. Their appeal reflects, in part, the increasingly recognized role of tachykinins in mediating cough and hyper-reflexivity. A potential role of NK antagonists in control of asthma in humans was recognized in the 1990s, although their clinical role has not been recognized.113a Their role for control of cough is supported by a number of reviews.113a,b Although the antiemetic maropitant (see Chapter 19) has not been studied for treatment of cough in dogs, its anecdotal use is supported by response to other NK-1 antagonists.113b Cough (mechanical intrathoracic tracheal stimulation) was reduced by 52% in dogs receiving an experimental NK1 antagonist.
Peripheral Bronchodilators
Bronchodilators (previously discussed) are powerful peripheral antitussives because they relieve irritant receptor stimulation induced by mechanical deformation of the bronchial wall during bronchoconstriction. Ephedrine peripherally induces bronchodilation, and as both a bronchodilator and decongestant it is a common constituent of over-the-counter cough preparations. Theophylline and isoproterenol are also common ingredients found in some preparations. Other peripheral antitussives include mucokinetic agents and hydrating agents.108
Bronchodilators counteract irritant receptor stimulation induced by mechanical deformation of the bronchial wall during bronchoconstriction. Increased fluidity of secretions and ciliary action may facilitate removal of accumulated materials, thus decreasing airway caliber size, airflow velocity, and turbulence-induced receptor stimulation. As such, previously discussed bronchodilators can be effective peripheral antitussives. Several drugs classified as bronchodilators are found in over-the-counter cough preparations for their antitussive-effective effects. Ephedrine is both an α and a β agonist; additionally, it causes the release of norepinephrine from sympathetic neurons. It peripherally induces bronchodilation, and as both a bronchodilator and a decongestant, it is a common constituent of over-the-counter cough preparations. It is present in herbal agents as ma huang or ephedra. Ephedrine is a potent central nervous system and cardiac stimulant and can contribute to hypertension and dysuria as a result of its α-adrenergic effects. Theophylline and isoproterenol are also common ingredients found in some preparations. Other peripheral antitussives include mucokinetic agents and hydrating agents.108
Mucokinetics
Mucokinetic drugs facilitate the removal of secretions from the respiratory tree. They are indicated for conditions associated with viscous to inspissated pulmonary secretions such as are commonly associated with chronic bronchial diseases. Mucokinesis can be induced by drugs that improve ciliary activity (e.g., β-receptor agonists and methylxanthines) or by drugs that improve the mobility of bronchial secretions by changing viscosity. Viscosity of bronchial secretions can be decreased by hydration (e.g., sterile or bacteriostatic water or saline), increasing pH (e.g., sodium bicarbonate), increasing ionic strength (sodium bicarbonate and saline), or by rupturing sulfur (S-S) linkages in the mucus (e.g., acetylcysteine or iodine). Hydrating agents can be administered parenterally (i.e., isotonic crystalloids) or by aerosolization. Home aerosolization can be easily achieved with a humidifier or steamed bathroom or with a commercially available aerosolizer. The efficacy of aerosolization in liquefying airway secretions is controversial,26 with the greatest benefit occurring in the upper airways. Bland aerosols such as water and saline can actually be detrimental to mucociliary function.26 The efficacy of ionic solutions or alkaline solutions compared with that of water on enhanced mucous mobility is controversial.26
Bromhexine
Bromhexine is a mucolytic that acts at the level of the mucus-secreting gland, decreasing the formation of mucoid secretions. On the basis of product information, in humans it is rapidly absorbed after oral absorption but undergoes extensive first-pass metabolism, resulting in an oral bioavailability approximating 20%. It is highly bound to plasma proteins. Its distribution includes penetration of the blood–brain barrier. Metabolites are excreted in the urine mainly as metabolites, resulting in an elimination half-life of up to about 12 hours. Although characterized by low toxicity, the mucolytic effects will occur in other areas of the body. Accordingly, caution is recommended for use in patients with a history of gastrointestinal ulceration. The impact in the patient with a urinary tract infection is not known. A Cochrane review114 that focused on the efficacy of mucolytics in human patients with bronchiectasis found that bromhexine facilitated removal of mucus associated with infection, but the number of clinical trials was too few to allow meta-analysis.
Acetylcysteine
Acetylcysteine (N-acetyl-L-cystein) (NAC) (see Figure 20-9) is the N-acetyl derivative of the naturally occurring amino acid L- cysteine. It is the mucolytic drug most widely used by humans.26,115 Although it appears to be efficacious after aerosolization, oral administration has become the preferred route.115 In Europe the drug is available in solid and powder dosing forms. Unfortunately, only the solution, which is unpalatable and malodorous, is approved for use in the United States. The powder is available as a chemical reagent from several chemical companies and application to food or as a capsule might be considered.
KEY POINT 20-10
The beneficial effects of N-acetylcysteine, which include control of inflammation as well as mucokinesis, can be realized with oral as well as inhalant therapy.
Regardless of the route of administration, the mechanism of NAC reflects destruction of mucoprotein of the disulfide bonds by a free sulfhydryl group. The subsequent smaller molecules are less viscid and less able to efficiently bind to inflammatory debris. As a thiol compound, NAC is a radical scavenger, able to directly interact with oxidants such as hydrogen peroxide, hydroxyl radicals, and hypochlorous acid.21 In addition to these direct effects, as a cysteine, NAC appears to promotes cellular glutathione production, a critical intracellular scavenger of radicals.116 Increased intracellular glutathione appears to contribute to the control of inflammation.117 A number of animal studies indicate efficacy of NAC in the prevention and therapy of lung injury associated with oxygen radicals.116,118 These effects may be additive with dexamethasone.119 However, although NAC has an antiinflammatory effect, it has an apparent dose-dependent, paradoxical effect on polymorphonuclear cells. Respiratory burst is suppressed but phagocytosis is increased in humans receiving increasing doses of 6 to 18 g (60 to 180 mg/kg) of NAC by constant slow intravenous infusion.116 In contrast, at a low mucolytic dose (300 mg or approximately 4.5 mg/kg three times daily in humans), respiratory burst is increased. The impact of high doses can be beneficial in the presence of inflammatory disease or syndromes associated with ischemia/reperfusion or endothelial cell activation but detrimental in the presence of infectious diseases; lower doses might be recommended in the presence of infectious disease dependent on leukocyte activity. Acetylcysteine appears to induce respiratory tract secretions, probably by way of a gastropulmonary reflex. Effects on bronchial secretions appear to be clinically relevant. Acetylcysteine improved gas exchange in a study of dogs with experimentally induced methacholine bronchoconstriction.120 The effects of NAC in human patients with COPD is not conclusive, with study results ranging from no effect to clinical effect.
In humans acetylcysteine is rapidly absorbed from the gastrointestinal tract and extensively distributed to the liver, kidneys, and lungs, where it may accumulate. It is rapidly metabolized by the liver to the natural amino acids cysteine and cystine.115,121
The indications for oral acetylcysteine therapy for humans include toxic inhalants (including tobacco smoke), bronchitis, COPD, cystic fibrosis, asthma, tuberculosis, pneumonia and emphysema, and ARDS.117 Installation of a 10% to 20% solution has also been used to clean and treat chronic sinusitis.115 Physiotherapy will enhance the efficacy of acetylcysteine. For example, one study in humans with COPD (n = 523) could not establish a beneficial effect of NAC (4.5 mg/kg every 8 hours orally) for 3 years in prevention of deterioration of disease.122 However, a potential beneficial effect was found in those patients not receiving inhaled steroids. In contrast, Sutherland123 performed a meta-analysis from eight trials (randomized n = 2,214) and concluded that that NAC significantly reduced the odds of experiencing exacerbations, including in active smokers. This report also found a greater effect when persons using inhaled glucocorticoids were removed, suggesting that inhaled steroids attenuate the effect of NAC. Accordingly, the authors concluded that treatment with NAC may be beneficial in a subset of patients with COPD.
Acetylcysteine therapy is associated with few adverse affects. In humans doses as high as 500 mg/kg are well tolerated,121 although vomiting and anorexia can occur. Kao and coworkers124 retrospectively performed a meta-analysis to review the adverse events in humans receiving NAC intravenously for treatment of acetaminophen toxicity. Of the 187 patients receiving the drug, seven adverse events were reported, six of which were cutaneous and rapidly responded to antihistamines. The authors concluded that the rate of adversities was low. The median LD50 in dogs after oral use is 1 g/kg and parenterally 700 mg/kg. Because it is metabolized to sulfur-containing products, it should be used cautiously in animals suffering from liver disease characterized by hepatic encephalopathy. Aerosolization of NAC can cause reflex bronchoconstriction as a result of irritant receptor stimulation and should be preceded with bronchodilators.
No scientific support appears to exist for the use of NAC in animals with lung disease. Historical successful use focuses in treatment of acetaminophen toxicosis. The author has used these high doses (144 mg/kg intravenously followed by 70 mg/kg 12 hours later) in life-threatening pulmonic conditions, including pneumonia. Other conditions to consider include but are not necessarily limited to chronic bronchial diseases, chronic sinusitis, electrical-cord bites, and other respiratory syndromes associated with inflammation. Although NAC in the form of Mucomyst might be administered orally, an alternative method is procurement of a scientific-grade granule product (e.g., Spectrum Chemicals). Further, many health-food stores offer NAC in capsular form for reasonable prices, although the quality of the products may not be known.
Expectorants
Expectorants such as potassium iodide are common ingredients in over-the-counter cough preparations. Expectorants increase the fluidity of respiratory secretions through several mechanisms and are often used as adjuvants for the management of cough by facilitating removal of the inciting cause. Bronchial secretions are increased by vagal reflex after gastric mucosa irritation (iodide salts) and directly through sympathetic stimulation or by volatile oils that are partially eliminated by way of the respiratory tract. Although the combination of expectorants with antitussives in over-the-counter cough preparations may seem irrational, the antitussive drugs in these combination products do not appear to prevent stimulation of the cough reflex induced by liquefied secretions commonly included in over-the-counter cough preparations. Their mechanism of action is unknown, although they may be ineffective at the doses used in cough preparations.
Iodide Preparations
Potassium iodide is a saline expectorant capable of increasing secretions by 150%. Ethylenediamine dihydriodide, used as a nutritional source of iodine in cattle, may be useful for the treatment of mild respiratory diseases. Iodide preparations should not be used in pregnant or hyperthyroid animals or in milk-producing animals. Demulcent expectorants such as syrup are often used as the vehicle for cough medicaments but have no apparent expectorant value. They may, however, be useful for treatment of cough caused by pharyngeal irritation.
Stimulant Expectorants
Stimulant expectorants are used more commonly for coughing associated with chronic bronchial diseases. Guaiacol and its glyceryl ether guaifenesin (glyceryl guaiacolate) are wood tar derivatives. Neither the volume of viscosity nor respiratory secretions appear to change after treatment with guaifenesin, although airway particle clearance increases in bronchitic human patients.
Miscellaneous Expectorants
Among the chemicals that potentially contribute to COPD are DNA and actin.17 The ability of bovine pancreatic deoxyribonuclease (DNase) to decrease the viscosity of lung secretions led to its approval in the early 1960s. However, adverse reactions (probably to contaminating enzymes) limited its use. Recombinant technology allowed the development of human DNase (rhDNase; dornase alfa) which is used in the treatment of cystic fibrosis. Expiratory volume increases and the incident of clinical exacerbations is reduced when used (2.5 mg by aerosolization) on an alternate-day basis.17 rhDNase has been used for management of both acute and chronic disease, although time to response is longer in patients with the most severe disease. The effects of DNase may decrease with long-term therapy, and concerns have been raised that its effects may be merely cosmetic, masking clinical signs indicative of serious disease.17 The cost of rhDNase may preclude use in animals, particularly if clinical effects with long-term use are not definitive.
Aerosolized hypertonic (5.85%) saline (10 mL twice daily) also have demonstrated efficacy in human patients with cystic fibrosis. Postulated mechanisms focus on osmotic draw of fluid into the airway lumen or cleavage of mucin bonds, either of which decreases mucus viscosity. Bronchodilators should be administered before aerosolization to prevent bronchoconstriction.
Decongestants
The indications for decongestants include sinusitis of allergic or viral etiologies and reverse sneezing or other complications of postnasal drip. Information regarding the use of decongestants in animals is largely based on extrapolation from human patients for which allergic rhinitis and the common cold are the more common indications. Often decongestants are administered as a single drug combined with expectorants.
The two major categories of drugs used as decongestants are the histamine (H1) receptor antagonists (e.g., dimenhydrinate, diphenhydramine, chlorpheniramine, and hydroxyzine, as well as newer second-generation drugs such as loratadine and cetirizine) and the sympathomimetic drugs (i.e., α-adrenergic agonists) (such as ephedrine [EDE], pseudoephedrine [PSE], and phenylephrine [PNE]).125-127 These drugs can be given topically to avoid the systemic effects associated with oral therapy.
Stimulation of α2-receptors concentrated on precapillary arterioles results in vascular smooth muscle vasoconstriction. Blood flow to the nasal mucosal capillary bed is reduced; excess extracellular fluid associated with congestion and a “runny” nose is thus decreased. α-receptors are concentrated on the postcapillary venules; when stimulated, the venules act as capacitance vessels that reduce blood volume in the mucosa. Mucosal volume decreases, reducing congestion. Sympathomimetic drugs mimic norepinephrine. Direct-acting agents stimulate one (PNE: α1) or both types of α-receptors, depending on drug chemistry. Indirect-acting agents (PSE) displace norepinephrine from nerve terminals and sometimes block its reuptake, effectively increasing its action on postjunctional α-receptors. Some drugs (e.g., PSE, EDE) are both direct and indirect in their actions. Prolonged use of agents that act indirectly (e.g., EDE) may deplete storage granules, and the animal may become refractory to its effects. Alternatively, downregulation of receptors (tachyphylaxis) may result in refractoriness.126,127
Topical agents containing sympathomimetic drugs (i.e., nasal sprays) act within minutes, with minimal side effects. In contrast, rebound hyperemia is common, particularly with extended use of the drugs. The mechanism of rebound hyperemia is not clear but may result from secondary β-adrenergic effects, as β-receptors upregulate or desensitize α-receptors. Regardless of the cause, repeated contraction of the vasculature can result in ischemia and mucosal damage, perhaps as a result of loss of nutrition. Oral treatment of sympathomimetic drugs can be associated with a number of adverse reactions. Systemic vasoconstriction may cause hypertension; cardiac stimulation may result in tachycardia or reflex bradycardia. Stimulation of the central nervous system may also prove problematic, particularly with lipid-soluble agonists such as ephedrine. Stimulation of urinary sphincter α-receptors may result in urinary retention. Mydriasis may decrease aqueous humor exit and can prove detrimental in patients with glaucoma. Because of their effects on endocrine and other organs associated with metabolic function, these drugs should not be used in patients with metabolic disorders, including thyroid disease and diabetes mellitus. The relationship between plasma drug concentration and nasal decongestant efficacy with the α-agonists appears to be minimal, suggesting that topical therapy is as efficacious. In addition, oral administration of some drugs (e.g., PNE) is limited by first-pass metabolism, which prevents therapeutic concentrations of the drug from being reached. Thus topical therapy may be the preferred route for sympathomimetic drugs. Note, however, that in the United States PSE is an “old drug” and as such is exempt from Food and Drug Administration regulation, which includes various topical formulations. However, issues regarding substance abuse have led to a Schedule III status for PSE (along with EDE and phenylpropanolamine) with the Drug Enforcement Agency.
Antihistamines are effective for the treatment of allergic rhinitis in human patients. In this scenario, they relieve and prevent itching and rhinorrhea but not nasal congestion. Thus antihistamines are frequently combined with sympathomimetic drugs. The efficacy of these drugs for the treatment of symptoms related to the common cold (and, presumably, unknown microbial causes in animals) has not been proved. Sedation is the most common side effect of the first-generation antihistamines (diphenhydramine). Newer antihistamines (e.g., chlorpheniramine) are associated with minimal sedation. In contrast to other causes of rhinitis, topical decongestants may be more of a risk for patients with allergic rhinitis because of the risk of drug reaction (rhinitis medicamentosa). This side effect does not occur with topical therapy. Because the antihistamines are safer than the sympathomimetic drugs after oral administration, this may be the preferred route for antihistamines.125
Formulations of topical preparations can influence drug efficacy. Controlled-release polymers can decrease the rate of drug dissolution (and thus its ability to reach cellular targets). Although these differences may not be clinically relevant, it is important to realize that bioequivalency of the topical decongestant products containing older drugs may vary. The major disadvantage of topical agents is their short duration of action.
Respiratory Stimulants
Caffeine, as previously noted, is a respiratory stimulant often used to treat premature infants. It appears not to affect dogs and cats in that capacity. Doxapram (see Figure 20-9) is a respiratory stimulant that acts indirectly by stimulating chemoreceptors of the carotid arteries, which in turn stimulates the respiratory center. It may be helpful in counteracting the depressant effects of opioids or other drugs. In humans high doses may be associated with hypertension and tachycardia. The effect of doxapram (2.2 mg/kg intravenously) on laryngeal function was studied in healthy dogs (n = 30) preanesthetized with butorphanol and acepromazine followed by propofol (4 mg/kg intravenously) induction. Improved respiratory effort and laryngeal motion led the authors to suggest routine use of doxapram during laryngoscopy.128
Aerosolization as a Route of Drug Administration
Aerosolization of drugs (inhalation therapy) is characterized by several advantages. Higher drug concentrations in target tissues leads to lower doses, thus enhancing efficacy while minimizing toxicity (e.g., anticholinergics, glucocorticoids, and beta-adrenergics). Additionally, response to therapy may be more rapid than that to systemic therapy. Hepatic first-pass metabolism after oral administration is circumvented, which serves to prolong the pharmacologic effect of selected drugs (e.g., β-adrenergic agonists and beclomethasone). In human medicine, with the development of more effective inhalant devices, asthma is predominantly treated with inhalant medications. The primary indications for aerosolization in small animals also has been direct delivery of drugs to the respiratory tract and to facilitate liquefaction and mobilization of respiratory secretions. However, the use of human inhalant devices designed to treat asthma is generally accepted for cats, although use in dogs is less common.
The success of patient response to aerosolized drugs is more likely to reflect adequate drug delivery rather than drug efficacy. Three factors determine the amount of drug reaching the airways: anatomy, ventilation, and aerosol characteristics.129 The anatomy of the respiratory tract is designed to filter inhaled particles. Indeed, up to 90% of particles produced by pMDI are removed before they reach the airways in humans.129 The more tortuous the airways traversed by an aerosol, the smaller the percentage delivered. Canine and feline anatomy is likely to contribute substantially to deposition in the oral pharynx and upper airways.
Variations in tidal volume; airflow rates; and respiratory rate, depth, and pattern also will affect the amount of drug delivery. In humans breath holding is particularly important to drug delivery.129 With progression of chronic disease, or with moderate to severe acute disease, aerosol therapy may become less effective as the respiratory pattern becomes shallow and rapid. For stressed animals, tachypnea will further decrease depth of aerosol penetration decreases, with more drugs deposited in upper airways. The utility of aerosolization may be further limited because of stimulation of irritant receptors and reflex bronchoconstriction.26,130 Resistance by the animal to aerosolization may further exacerbate respiratory distress and thus affect the site of particle deposition.
Among the characteristics of the aerosol that will influence airway deposition are diameter, shape, electrical charge (for particles less than 1 μm in size), density, mass, hygroscopicity, and preparation type (i.e., solution versus suspension).2,113,129,131,132 The optimum particle size for particle (and drug) deposition in the trachea is 2 to 10 μm and in peripheral airways, 0.5 to 5.0 μm. Differences in methods, devices, diseases, and drugs have generated a number of contradictory reports regarding the best particle size for aerosolized drug delivery in diseased humans. Most studies suggest penetration of small airways is best accomplished with particles 1.5 μm or less in size, although others have found maximum improvement in lung function with an aerosol of 3 μm particles.129,131 Jet and ultrasonic nebulizers (described later) tend to generate heterogenous particles of that range from 1.2 to 6.9 and 3.7 to 10.5 μm in size, respectively. Spinning disk nebulizers produce particles that range from 1.3 to 30 μm. For inhalant devices, particle size generated for MDIs varies from 1 to 35 μm, but particle size is dependent on (inspiratory) flow for dried-powder inhaler (DPI) devices.129
Aerosol deposition also will be affected by the technique of delivery (i.e., mask versus endotracheal tube and nose versus mouth). Larger particles generated by aerosol devices will be impacted on masks, pharynx, and in upper airways (or endotracheal tubes). Indeed, oral absorption of particles is sufficient that side effects to inhaled glucocorticoids in humans tend to reflect systemic response to the drugs. In animals grooming may increase the risk of systemic side effects to drugs deposited on the face during mask administration. One method whereby drug delivery is enhanced, thus increasing efficacy and safety, is the device used for drug delivery. Modern aerosol therapy is administered through one of three devices: nebulizers, MDIs, and DPIs.131,133,134
Two basic types of nebulizers have been developed: jet and ultrasonic.131,133 The ultrasonic nebulizer is based on a high frequency (1-3 MHz) vibrating piezoelectric crystal that generates a fountain of liquid in the chamber. Droplet size decreases as vibration frequency increases. Jet nebulizers are based on the Bernoulli principle. Compressed gas (air or oxygen) is forced through a narrow orifice, creating a low-pressure area at the outlet of the adjacent liquid feed tube. Drug in solution is drawn from a fluid reservoir and shattered into droplets by the gas stream. Breath-enhanced jet nebulizers have an added valve system that directs inspired air to the well during inspiration. This second source of air optimizes the number and size of particles. Still, the amount of drug delivered by nebulizers to airways (in humans) can be as little as 10%. In humans adapters have been added that time drug release with the initial inspiratory effort, increasing the amount of drug delivery to airways to up to 69%; such adapters are not likely to be helpful in animals. Other factors particularly affecting nebulized drug delivery include solution viscosity, ionic strength, osmolarity, pH, and surface tension. Acidic and nonisotonic solutions increase the risk of bronchoconstriction, coughing, and irritation of the lung mucosa. High drug concentrations may also decrease the drug output; foaming is a particular problem with ultrasonic units.
A number of companies offer reasonably priced ($70 to $250 or above) portable compressor-based nebulizing units. Compressors that accompany the nebulizers can generate air at pressures (20 to 40 psi) and airflow velocities (7 to 10 mL) comparable to oxygen-based flow meters (e.g., Omron Healthcare Inc, www.omronhealthcare.com). Aerosol units are small enough to be placed in an aquarium of appropriate size or, for large dogs, can be adapted to a mask. For tracheostomized patients, infant or adult tracheostomy masks that deliver aerosol directly into the tracheostomy are available. Although aerosol units are generally reusable, attention must be made to keep units sterile; great care must be taken to ensure that nebulizers remain free of microorganisms between uses. Adherence to cleansing procedures after each use of nebulizing equipment should be strict. Cold sterilization agents should be effective against Pseudomonas spp. Manufacturers of reusable equipment recommend washing it in warm soapy water, rinsing it well, and soaking it (including the tubing) for 30 minutes in a solution of 1 part vinegar and 3 parts water. Disposable equipment should be replaced frequently; replacement after use in patients with infection is particularly encouraged.
The MDI has been described as a revolutionary change for aerosol therapy in humans; it is the most commonly used device. 88,133,134 The aerosol of an MDI is driven by a propellant. The more common older chlorofluorocarbons (CFCs) are being replaced by hydrofluoroalkanes (HFAs), which contain no chlorine and thus do not affect the ozone layer. The HFA-propelled MDIs also lack the “cold Freon” effect that causes human patients to fail to inspire completely. The drug in an MDI is emitted at a high velocity (>30 m/s) through a nozzle. However, as with nebulizers, only a small percentage of drug (10% to 20%) reaches peripheral airways, with most (in humans) being deposited in the oropharnyx. Surfactants enhance particle stability in the presence of CFC propellants; ethanol is similarly used for HFA propellants. For HFAs drugs are delivered as a solution rather than as a micronized suspension. As such, particles released by the device valve are much smaller; referred to as extra fine, the average size is 1.1 μm, compared with 3.5 to 4.0 μm in size for the CFC. The smaller size enhances deeper airway penetration.40 Indeed, the pattern of particle deposition with HFA propellants (60% deposited in small airways rather than the oropharynx) is reversed compared with that for CFC propellants.134 Spacer tubes, valved holding chambers, and mouthpiece extensions have been added to minimize the need for hand–breath–inhalation coordination, thus improving delivery. Spacers and holding chambers yield an aerosol that is more uniform in lung distribution and characterized by deeper penetration in peripheral airways. Breath-activated MDIs also have been developed, which, as with nebulizers, minimize the need for hand–breath–inhalation coordination. Despite these innovations, studies using radiolabeled aerosols in normal humans have documented that less than 20% (usually only 10%) of drug reaches the airways, even if the respiratory pattern is optimal.40,134 The DPI was designed to eliminate the need for hand–inhalant coordination necessary for the MDI. Drug aerosol of DPI is generated by air forced through powder. Particles are aggregated and too large for effective delivery. However, dispersal into smaller particles is accomplished by turbulent airflow. In contrast to MDIs, DPIs do not require spacers. A number of types of DPIs are available, ranging from single-dose devices loaded by the (human) patient to multiunit dose devices in a blister pack, blister strip, or reservoir. However, a major disadvantage of DPIs in veterinary medicine is the dependency of particle size on inspiratory effort. Each DPI is characterized by a different airflow resistance; finer particles require more resistance to air flow, which, in turn, requires greater inspiratory effort. Consequently, DPI application may be limited in animals. The use of nebulizers or inhalant devices in small animal patients is largely based on anecdotal reports. One study demonstrated that a radiopharmaceutical will be distributed to peripheral airways in cats using a simple face mask.135 To maximize the site of particle deposition, animals to be aerosolized should be pretreated with a β-adrenergic or methylxanthine bronchodilator 10 minutes before aerosolization, or (potentially less preferable) a bronchodilator should be included in the aerosolized medicament (e.g., 100 mg aminophylline). Care should be taken not to overhydrate and flood the respiratory tract. Treatments of approximately 30 to 45 minutes should be repeated every 4 to 12 hours. In humans aerosolization is a well-established route of administration for bronchodilators and antiinflammatories26,136 (Box 20-1) and is recommended for dogs for selected infectious tracheobronchitis.137 In veterinary patients aerosolization is more commonly used for administration of antimicrobials and mucolytics. Indications include asthma, chronic bronchial disease, and infections of both lower and upper airways. The dose of drug to be nebulized is generally not scientifically derived. A general approach would be dilution of the calculated systemic dose in a sufficient volume of saline necessary for a 30-minute aerosol. Drugs prepared by the manufacturer in irritating solutions (e.g., NAC) should be diluted. The choice of antibiotic to be aerosolized should be based on efficacy against the targeted organism. Because the amount of drug that reaches the site can not easily be quantitated and minimum inhibitory concentrations based on aerosolized drug have yet to be defined, the use of culture data based on the minimum inhibitory concentration (a plasma-based target) is a reasonable approach.
Box 20-1 Drugs Administered by Aerosolization∗∗
∗ In general, solutions can be made with injectable products (1 part) mixed with saline (9 parts). The concentration of specific drugs is noted in parentheses. The diluent for these drugs is saline, unless noted otherwise. Tris-EDTA might be used as a diluent when infections caused by Pseudomonas aeruginosa or other problematic gram-negative infections are being treated.
† Drug-induced bronchoconstriction may be severe.
† In combination with other bronchodilators.
Adaptations to inhalant devices for animals range from modifications in inhalant masks39 with a one-way valve that limits drug movement to inhalation to simple administration through an empty toilet paper roll “spacer” that facilitates adequate exposure during inspiration. Cats breathe six to seven times after the medication is dispensed from the inhaler. Despite innovations designed to enhance small airway penetration with inhalant devices, the smallest airways are likely to remain untreated. Additionally, aerosol therapy is limited to the epithelial surface of the airways. Finally, compliance in humans with inhaled therapy is poorer than with systemic therapy.40 As such, the use of inhalant devices should be accompanied by frequent thorough monitoring. Inhalant antimicrobial therapy should not replace systemic therapy. The factors decreasing small airway drug delivery in animals are likely to be greater than in humans. As such, scientific support is paramount to guide the appropriateness of and indications for inhalant bronchodilator and antiinflammatory therapy as a replacement for systemic therapy.
Drug Therapy of Specific Diseases of the Respiratory Tract
Treatements of infectious diseases of the respiratory tract are discussed in Chapter 9; diseases associated with chronic inflammation are also addressed in Chapter 19.
Therapy of Fungal Infections of the Nose
Nasal aspergillosis in dogs is difficult to treat and generally most successful if medical management is accompanied by surgical débridement. Topical therapy includes flushing the nasal mucosa with povidone–iodine solutions (10%) every 8 hours for 6 to 8 weeks after surgery; a 10% solution of clotrimazole in polyethylene glycol, instilled in nasal tubes and administered twice daily or in direct contact for 1 hour during surgical exploration; or enilconazole (10%) at 5 mg/kg instilled into nasal tubes twice daily for 7 to 14 days. Topical therapy should be accompanied by systemic therapy with itraconazole. Treatment of fungal infections is discussed in greater depth in Chapter 11.
Therapy of Disorders of the Trachea
Tracheitis
Resolution of underlying causes is important to successful control of the inflamed trachea. Noninfectious causes (e.g., exposure to smoke, cough) are more common than infectious causes. In addition to resolution of the underlying cause, drug therapy should be implemented to control symptoms. Cough can be controlled with peripheral or central antitussives or a combination thereof. Over-the-counter preparations that contain expectorants can prove helpful. Humidifying secretions (liquefaction of mucoid material) becomes increasingly important with chronic tracheitis and may include nebulization four to six times a day or exposure in a steam-filled bathroom for 15 to 20 minutes three times daily. Physical therapy (coupage) should be implemented after liquefaction of secretions. Short-term therapy with short-acting glucocorticoids may help break the cough cycle, although care must be taken that glucocorticoids do not exacerbate the underlying condition.
Antibiotics are indicated for infectious tracheitis/tracheobronchitis. Infectious tracheobronchitis in dogs (kennel cough) is a complex syndrome caused by multiple organisms, including viruses, bacteria, and mycoplasma. This syndrome is discussed with bronchial diseases.
Structural Disorders of the Trachea
Pharmacologic management of structural disorders of the trachea focuses on supportive therapy.
Hypoplastic trachea
Slight or moderate tracheal hypoplasia may respond to bronchodilator therapy. Recurrent infections (bacterial) should be anticipated because of a poorly functioning mucociliary tract. Although prophylactic antibiotic therapy is discouraged to avoid emergence of resistance, antibiotic therapy during active infection should be anticipated. Culture and susceptibility data may be particularly important for these patients because recurrent infections are more likely to occur in them than in animals with a normal trachea. Drugs that facilitate mucociliary clearance should be considered on a daily basis; these might include mucokinetic drugs. More serious episodes of respiratory compromise might benefit from NAC therapy administered by any route. The use of bronchodilators should be considered; although the tracheal diameter may not be affected, the effects of these drugs on peripheral airways can be beneficial. Supportive actions should also include weight control, avoidance of smoke and other environmental contaminants, and avoidance of actions or drugs that can compromise the immune response.
Tracheal collapse
Tracheal collapse as a cause of respiratory distress can progress to a life-threatening situation. Early therapy may help decrease or slow the progression of the syndrome in some animals, simply by decreasing damage to the trachea as a result of paroxysmal coughing. Tracheal rings in afflicted animals lose their ability to remain firm, leading to collapse. The characteristic “goose honk” cough is dry and chronic. Most commonly afflicting smaller breeds, tracheal collapse is often associated with chronic valvular (cardiac) disease, and it is important to differentiate between the two. Diagnosis requires proper radiographic examination and motion studies with either a fluoroscope or a bronchoscope.
Drug therapy targets control of the cough with bronchodilators and centrally acting antitussives. Severe coughs may require narcotic antitussives associated with sedation (a desirable characteristic in some patients) until the cough is controlled. Mucokinetic drugs may also be helpful. Short-term glucocorticoid therapy may be important to minimize the inflammatory response to damage induced by paroxysmal coughing. Nebulization may be helpful, but pretreatment with bronchodilators is probably important. The use of bronchodilators should be considered for their effects on peripheral airways. Digitalization reportedly has been beneficial in some patients that do not respond to other therapies.138
Proteoglycan content and size of tracheal cartilage matrix changes that accompany age have been demonstrated in a number of species, including humans.139,140 Hamaide and coworkers140 suggested that age-related changes may lead to compliance changes that accompany tracheal ring collapse.Trachea collapse appears to reflect loss of rigidity associated with decreased glucosamine glycan chondroitin sulfate. A number of old studies have demonstrated uptake of labeled glucosamine by cartilage rings under in vitro conditions, suggesting cartilage turnover may be sufficiently rapid that supplementation may be beneficial. Accordingly, supplementation in the form of injectable and oral products as is recommended for osteoarthritis is a reasonable approach for preventing or supporting other therapies for tracheal collapse. The role of glutamine and chondroitin sulfates in supporting damaged or healing cartilage in osteoarthritis is addressed in Chapter 29. Response, if it is to occur, will be prolonged in onset, depending on the rate of tracheal proteoglycan turnover.
Bronchial Diseases
Diagnosis of bronchial diseases should be based on physical examination, thoracic radiography, tracheal or bronchial wash, and bronchoscopy. Examination for structural defects, cytologic studies, and microbial cultures are among the diagnostic tools of use for bronchial diseases.
Canine Infectious Tracheobronchitis
Bordetella bronchiseptica is the bacterial organism most commonly associated with kennel cough. Viral organisms include canine parainfluenza, canine herpes, and canine distemper viruses. The clinical syndrome is characterized by a dry, hacking, paroxysmal cough in an otherwise healthy animal. Clinical signs of this highly contagious syndrome generally appear 3 to 5 days after exposure. Tracheal cytology should reveal neutrophils and bacteria. Therapy of uncomplicated cases is supportive. Antitussives, in relative order of efficacy (least to most), include dextromethorphan (antitussives), butorphanol, and hydrocodone. Hydrocodone may be associated with sedation, which may be beneficial in cases of paroxysmal coughing. Antimicrobial therapy in uncomplicated cases (lasting 7 to 10 days) is discouraged; indeed, most antimicrobials used empirically (e.g., amoxicillin) generally do not penetrate bronchial secretions in sufficient quantities to be effective.
In contrast, antibiotic therapy (in addition to other supportive therapy) is indicated for complicated infections or for dogs whose coughing persists after 2 weeks and for which evidence exists of secondary bacterial infection. Other indicators of complications include any evidence of infection occurring lower than the upper bronchi or systemic signs of illness. Because of the complicated nature, and particularly if the patient has received previous antimicrobials, selection of the appropriate antibiotic should be based on a properly collected culture at the site of infection (not a pharyngeal or laryngeal swab). Selection of an antimicrobial empirically is complicated by the possibility of mycoplasma as a causative agent. Selection of antimicrobials for treatment of respiratory tract infections is discussed in Chapter 8.
Therapies intended to support mucociliary function should be continued. Because coughing associated with kennel cough can be paroxysmal, a single treatment with a short-acting glucocorticoid might be considered to ameliorate some of the effects of inflammation. In the immunocompromised animal, however, this may lead to spread of infection.
Feline Bronchial Diseases
Feline bronchial diseases include feline bronchial asthma as well as acute and chronic bronchitis and emphysema. It is characterized by damage or hypertrophy (or both) of the airway epithelium; increased production of airway secretions; and spasms of bronchial smooth muscle, which itself may become hypertrophied.39 Causes of feline bronchial diseases have not been found, but a type I hypersensitivity reaction has been suspected as a cause of asthma. Initial contact between the allergen and bronchial mucosa may lead to the release of histamine and other mediators that allow penetration of the allergen into the submucosa. The resultant inflammatory response to the allergen leads to the characteristic disease. The source of inflammatory mediators includes essentially any cell of the respiratory tract, white blood cells, and platelets. Smooth muscle hypertrophy and increased mucus and inflammatory cell infiltrate (particularly eosinophils) characterize asthma and its clinical signs. Acute bronchitis is generally reversible and short in duration but can be life-threatening. Should airway inflammation persist, bronchitis may become chronic. Inflammation lasting 2 to 3 months can lead to deposition of fibrous tissue; these lesions tend to be irreversible. Emphysema can occur as a result of chronic bronchitis and is characterized by enlarged airspaces with destruction of bronchiolar and alveolar walls and airway collapse. Cough is the most consistent clinical sign of bronchial disease in cats. Respiratory distress may be absent or episodic, particularly in the presence of bronchial asthma.
Diagnosis should be based on thoracic radiographs, a complete blood count (which may reveal eosinophilia), tracheal or bronchial wash (particularly if bacterial or parasitic causes are suspected), and fecal examination (for parasitic causes). Cultures of Mycoplasma species should be performed whenever possible, particularly in nonresponders. Treatment should include environmental management. In particular, exposure to smoke (e.g., cigarette, fireplace) should be avoided; other potential environmental allergens include litter dust, perfumes, household cleaning products, deodorants, and insulation products. Asthma in cats has been classified according to the severity (mild, moderate, and severe) of clinical signs in order to facilitate the need for treatment.39 However, clinicians are urged not to become too complacent in their approach to the disease and to ensure that seemingly mild disease does not progress to a more severe state in the absence of therapy. The primary focus of therapy is control of inflammation. Control of inflammation in peripheral airways may be paramount to successful therapy.
Management of Acute Asthma
Acute respiratory distress resulting from bronchial disease should be handled as a medical emergency. Administration of drugs should be accompanied by oxygen therapy and rest. The hydration status of the patient should be assessed at presentation and corrected if indicated. Overzealous fluid therapy can prove detrimental, however, and should be avoided. Therapy should include bronchodilators, glucocorticoids (for their permissive effect on β-2 adrenergic receptors) and as necessary, anticholinergics.
Glucocorticoid therapy should be initiated in conjunction with bronchodilators in cats with status asthmaticus. The permissive effects of glucocorticoids are likely to improve response to bronchodilator therapy. Rapidly acting drugs such as prednisone sodium succinate should be administered at presentation and again at 4 to 6 hours.6,30 Prednisolone is preferred to prednisone; the latter should not be given orally (see Chapter 30). Alternatively, dexamethasone or dexamethasone phosphate may be administered because of its antiinflammatory potency. Oral bronchodilator and glucocorticoid therapy can begin when the patient is stabilized.
Among the bronchodilators, β2-adrenergic agonists are preferred (although doses have not been well established for animals), but nonselective agonists can be equally effective in critical cases. Parenteral rather than oral administration will ensure the most rapid onset of action, although use of a short-acting inhalant beta-adrenergic also might be considered (see the discussion of inhalant devices). Note that epinephrine has marked β1 (and α) effects and in the presence of hypoxemia can cause fatal cardiac arrhythmias. Aerosolization should not replace, but can be used in concert with, parenteral administration if the stress of aerosolization is not dangerous to severely dyspneic animals. Subcutaneous epinephrine can be administered at presentation and, if the patient responds, repeated every 30 minutes for several doses.30 Terbutaline can also be administered subcutaneously either instead of epinephrine or for animals that fail to respond to epinephrine. Aminophylline can be infused intravenously (2 to 5 mg/kg in 5% dextrose or saline) in animals that fail to respond to β-agonists.30 The addition of atropine or glycopyrrolate may facilitate bronchodilation. Exacerbation of hypoxia is a complication of bronchodilator therapy, particularly with theophylline, due to drug-induced pulmonary vasodilation, and the potential for ventilation-perfusion mismatching necessitates administration of humidified oxygen. The use of an anticholinergic (atropine preferred) also should be considered after therapy with beta-adrenergics and glucocorticoids.
Reinero and coworkers141 addressed the impact of flunisolide (inhaled glucocorticoid), prednisone, zafirlukast, and cyproheptadine along with placebo on inflammatory mediators in an experimental model of feline asthma (n = 6) using a randomized crossover design. The only significant changes detected were a decrease in the percentage of eosinophils in bronchoalveolar lavage fluid for both prednisone and flunisolide compared with control and the content of allergen- (Bermuda grass) specific IGE in serum in cats receiving oral glucocorticoids. The power of the study to detect significant differences and the use of prednisone were limitations of the study.
Long-term management of feline asthma
Response to glucocorticoids in the acute management of respiratory distress in cats may indicate a favorable response to long-term management. Glucocorticoids are the preferred drug, particularly in cats with moderate to severe disease, for control of inflammation. Prednisolone is the most commonly preferred maintenance drug, although triamcinolone is acceptable, prednisone is discouraged. Initially, doses should be as high as 2 to 3 mg/kg divided two to three times a day. A 2- to 3-week trial may be indicated to establish efficacy and need. Maintenance doses are likely to markedly vary among animals and should be slowly tapered to a minimum effective dose 1 to 2 weeks after therapy is started. Doses as little as 1.25 mg/cat every 72 hours may be sufficient in some animals. Glucocorticoid therapy should be maintained for a minimum of 2 months; complete cessation of therapy may not be possible in selected cases. Therapy should be continued for several weeks after cessation of signs to resolve residual and clinically inapparent small airway disease. Tracheal cytology may be helpful in identifying the continued need for antiinflammatory therapy both before and after therapy is discontinued. Repositol forms of glucocorticoids might be avoided because of the risk of exacerbation of disease.30A study in humans found the repositol form of methylprednisolone just as effective as a tapering regimen of oral glucocorticoids upon hospital discharge that followed acute management of asthma; however, the intent of the therapy was to slowly withdraw the drug with no continued therapy. Because remission of clinical signs appears to be more difficult in animals that have received these drugs, if therapy is anticipated to continue beyond the duration anticipated for reposital therapy, daily dosing should continue or care should be taken to avoid relapse. For animals for which daily glucocorticoids cannot be given consistently doses of 2 to 4 mg/kg can be given every 10 to 30 days to control clinical signs. In cases of exacerbation in patients receiving glucocorticoids, intermittent high doses of intravenously administered or aerosolized glucocorticoids, and particularly beclomethasone dipropionate, in conjunction with oral maintenance glucocorticoids can be used to treat animals whose disease worsens.30 Alternatively, megestrol acetate has been recommended instead of intermittent high doses of glucocorticoids in cats with refractory bronchial asthma.30
Addition of bronchodilator therapy should be considered for animals that do not respond sufficiently to glucocorticoid therapy. Intermittent use may help during periods of exacerbation of disease, although long-term therapy may be necessary for some animals. Bronchodilators may decrease the amount of glucocorticoids necessary to control clinical signs. Oral theophylline is the bronchodilator most commonly used for long-term bronchodilator therapy in dogs and cats,6,30 although terbutaline can be used as an alternative, particularly in animals refractory to theophylline. Alternating between theophylline and β-agonists may prevent the incidence of refractoriness owing to downregulation of β-receptors. Alternatively, the combination of the two should be considered, particularly in nonresponders; an additive response might be expected.142 Monitoring serum theophylline concentrations is encouraged, particularly in animals that do not respond sufficiently or in animals receiving long-acting theophylline products. Theophylline, particularly long-acting products, might be given to cats in the evening to maximize therapeutic efficacy.
The use of cyclosporine, cyproheptadine, and LRAs as antiinflammatories should be considered in cats that have not sufficiently responded to or cannot tolerate glucocorticoid or bronchodilator therapy. The treatment of asthma as a chronic inflammatory disease is also addressed in Chapter 31. Cats suffering from A. suum–induced airway reactivity had decreased reactivity and remodeling after receiving CsA; differences were noted with 24 hours of therapy.141b Schooley and coworkers143 examined the effect of cyproheptadine (8 mg orally twice daily) or cetirizine (5 mg orally twice daily) in cats (n = 9) with experimentally induced asthma (Bermuda grass allergen) using a randomized crossover design. Although no significant differences were detected, the percentage of eosinophils in bronchoalveolar lavage fluid was less in the cats treated with cyproheptadine (27 ± 16%) and cetirizine (31 ± 20%) compared with those in the placebo group (40 ± 22%).
The role of inhalant devices in treatment of feline asthma
Despite the paucity of well-designed, controlled clinical trials, the use of MDIs for the control of feline asthma is now a generally accepted method of administering glucocorticoids or bronchodilators to asthmatic cats.39 Studies are emerging. For example, response to orally administered prednisone, inhaled flunisolide, zafirlukast, or cyproheptadine was studied using a controlled crossover design in cats (n = 6) with experimentally induced (Bermuda grass allergen) asthma.141 Significant findings were limited to decreased eosinophils in bronchoalveolar lavage fluid for both glucocorticoid groups. On the basis of this study, response to inhaled glucocorticoids might be anticipated in the cat with spontaneous asthma, but the authors’ conclusions that inhaled glucocorticoids might serve as an alternative to oral glucocorticoids should be applied to long-term management only with caution.
Drug delivery in cats using inhalant devices is anecdotally described.39 Care must be taken to coordinate breath intake with drug administration, which is facilitated by the presence of spacer. In animals with mild disease, use of inhaled glucocorticoids (e.g., fluticasone propionate preferred, others include flunisolide, budesonide, and beclomethasone dipropionate) has been recommended twice daily.39 Inhaled short-acting beta-adrenergics (e.g., albuterol preferred, others include pirbuterol, bitolterol) are used as needed to control exacerbations or in animals for which clinical signs do not occur every day. However, client compliance should be ensured when using inhalant devices as primary therapy. Additionally, control of small airway inflammation may remain a concern. For animals with moderate disease (clinical signs have affected daily life, but cough and wheeze are not persistent), oral glucocorticoids should be added short term (e.g., 5 days twice daily followed by 5 days once daily). Systemic effects of inhaled glucocorticoids should preclude the need for tapering oral glucocorticoid doses further as they are discontinued. For severe disease both systemic and inhaled glucocorticoid therapy should be considered. Systemic therapy may include intravenous dexamethasone during an acute crisis. Inhaled bronchodilators should be continued as needed up to 4 times a day. For cases that continue to be refractory, the addition of cyproheptadine has generally found more support than LRAs.
The small number of feline studies precludes drawing conclusions regarding the use of other drugs (e.g., cyproheptadine, antihistamines, LRAs) or combination therapies for treatment as asthma. Their sole use should not be considered in animals with moderate or severe disease, and caution is recommended even for mild disease that might be insidiously progressive. Oral bronchodilator therapy also should be considered in animals with moderate to severe disease or in animals for which inhalant therapy is not reasonable or effective because of poor drug delivery or other reasons.
Canine Chronic Bronchitis
Acute bronchitis is not as likely to present as a life-threatening situation in dogs and refers primarily to duration of clinical signs. Inflammation that persists more than 2 months may cause permanent damage to airways and is referred to as chronic bronchitis.144 Bronchiectasis refers to irreversible dilation of the bronchi and can be a sequela of chronic bronchitis (inflammation) that does not resolve. The underlying cause is rarely identified but may include allergies; inhaled irritants (including cigarette smoke); viral, microbial, or parasitic infections; and heartworm disease. Bacterial infections are more difficult to diagnose, should be based on quantitative rather than qualitative assessment, and generally are not initiators of chronic disease.144 Foreign bodies are a less common cause. As with cats, eradication of the underlying cause is paramount to halting the progression and therapeutic success. Diagnostic aids are the same as discussed for cats. Medical management of chronic airway disease in dogs is frustrating and should be approached as a perpetuating, slowly progressive, noncurable disease. As such, resolution of identifiable inciting causes (including triggers such as cigarette smoke) and conditions that confound success should accompany drug therapy. Owners may have to adjust their tolerance levels. Additionally, therapy should be accompanied by weight loss and physical therapy (mild exercise or coupage to facilitate movement of respiratory secretions).
Allergic bronchitis is not a common or easy diagnosis in dogs. The canine respiratory tract is probably more resistant to antigenic stimulation as a cause of cough compared with that of cats (and humans). Parasitic infections including heartworm disease must be ruled out. If airway cytology supports an allergic response and the underlying cause has not been identified or yet eradicated, glucocorticoids are indicated. A minimum effective dose should be rapidly established. Long-term glucocorticoid therapy may not be indicated for dogs unless the disease is associated with eosinophilic or mononuclear infiltrates; this is particularly true for patients with bronchiectasis.
Medical management of chronic bronchitis in dogs must be modified for the individual patient. Exposure to irritants such as secondary cigarette smoke must be avoided. Bronchodilator therapy provides the mainstay of medical management of chronic bronchitis in many dogs. Ideally, bronchodilators will also facilitate movement of secretions and control inflammation. Drugs used for cats can be used for dogs; the major difference is in frequency of administration, which will be more common in dogs. Response generally is based on improvement in clinical signs; therapeutic monitoring is encouraged for theophylline products, particularly for dogs that do not sufficiently respond and in dogs receiving delayed-release products intended for human use. Both terbutaline and albuterol can be used for dogs and might be considered in combination with theophylline for nonresponders for which therapeutic concentrations of theophylline have been maximized or on an alternating basis with theophylline.
Control of inflammation may be facilitated by the use of NAC; additionally, its expectorant and mucolytic effects also should prove beneficial. LRAs should be considered as well. Glucocorticoids should be used only if cytologic examination indicates a large mononuclear or predominantly eosinophilic component to the inflammation. Use should be implemented only after appropriate quantitative culture techniques have failed to yield microbes in the presence inflammatory cytology. However, the role of Mycoplasma also must be ruled out. Ideally, inhaled glucocorticoids are preferable to systemic. However, their role is less well established for dogs than for cats.
The routine use of antimicrobials for the treatment of chronic diseases is and potentially contraindicated. Distinction between infection and colonization should be made whenever possible. The risk of causing a resistant infection in the presence of a chronic progressive disease suggests that antimicrobial selection be based on quantitative culture (> 2.7 × 103 colony-forming units/mL) based on a properly collected sample144 and be designed such that microorganisms (and particularly first-step mutants) are killed (rather than inhibited). High doses for a shorter duration of time are preferred to doses that target the minimum inhibitory concentration of the infecting organism. Drugs that penetrate bronchial secretions well are preferred in the presence of adequate susceptibility. Selection of the antimicrobial should be based on culture and susceptibility data. Cytologic findings should be used to guide the need for antimicrobial therapy; culture data are likely to enhance therapeutic success. Antimicrobial therapy should target Bordetella. The potential of infection with Mycoplasma should not be overlooked. A trial course of antimicrobials is indicated if cytologic findings are supportive of microbial infection; care should be taken to use an antimicrobial effective against Mycoplasma before microbial infection is ruled out. In general, when possible, drugs that accumulate in phagocytic cells should be considered when treating pulmonary conditions. A more comprehensive discussion of antimicrobial therapy for respiratory tract infections can be found in Chapter 8.
The role of antitussives in the treatment of diseases depends on the character of the cough. Inflammation and infection can result in mediator release and cough without an increase in bronchial secretions. Narcotic antitussives are generally indicated if the cough is nonproductive and is paroxysmal and irritating in of itself. Use of glucocorticoids is indicated if airway inflammation is the major contributing factor (as opposed to accumulation of airway secretions). In the case of productive cough, the use of expectorants or mucolytic antitussives may exacerbate cough as is intended if accumulated debris is to be removed. Hydration of respiratory secretions is critical to effective mucociliary transport function. As such, diuretics are contraindicated, and daily water intake must be maintained. Exposure to humidified air (i.e., humidifiers, vaporizers, or a visit to the bathroom during family member showers) is likely to facilitate liquefaction of respiratory secretions.
Pulmonary Diseases
As with other regions of the respiratory tract, causes of pulmonary diseases include viral, microbial, and parasitic infections; allergic (hypersensitivity) or immune-mediated diseases; and, although rare, nonspecific causes of interstitial lung disease. Malignancy of the lungs is discussed elsewhere. Supportive therapy should include bronchodilators and a means to maintain airway hydration (mucokinetics or mucolytics). NAC should be considered for both its antiinflammatory and mucolytic actions. Pentoxifylline also should be considered. Bronchodilators should not be used indiscriminately. Although they can contribute to both bronchial relaxation and controlled inflammation, they may also be associated with ventilation–perfusion mismatching. Diuretics are contraindicated unless vascular overload has led to pulmonary edema.
Oxygen is a consistent supportive therapy for the hypoxic animal; positive pressure ventilation is indicated for patients with poor pulmonary compliance. Physical therapy (coupage) is indicated in conditions associated with accumulation of respiratory secretions. Glucocorticoids are indicated for selected acute and chronic inflammatory conditions. Use in acute conditions generally is intended to minimize the acute inflammatory response; methylprednisolone is often recommended for immediate short-term therapy because of its ability to scavenge oxygen radicals.
Immune-Mediated Diseases
Diseases of the respiratory tract associated with eosinophilic infiltrates of the bronchi were discussed earlier with chronic causes of tracheobronchitis. Eosinophilic infiltrates that target lung parenchyma, referred to as pulmonary infiltrates with eosinophils, are associated with a spectrum of conditions ranging from mild diffuse infiltrates to granulomatous responses characterized by nodular masses that are visualized radiographically.
As with bronchial diseases, medical management of immune-mediated diseases should be accompanied by removal of any suspected allergen. Immunosuppressive doses of glucocorticoids are indicated for animals that do not respond to environmental changes. An exception is made for eosinophilic granulomatosis, for which cytotoxic drugs (cyclophosphamide) are indicated. For nongranulomatous disease, glucocorticoid therapy may need to be long term. Adherence to general principles of glucocorticoid use is indicated (i.e., tapering to a minimum effective dose, alternate-day therapy, and slow withdrawal). Granulomatosis, whether eosinophilic or lymphoid, is accompanied by a poor prognosis. Combination therapy should include cyclophosphamide (50 mg/m2 orally every 48 hours) and immunosuppressive doses (1 mg/kg every 12 hours) of prednisone. NAC and bronchodilators should be used as previously discussed.
Vascular Diseases
Pulmonary Hypertension
Because pulmonary hypertension is most commonly a secondary problem, treatment should focus on eradication of the underlying cause; its treatment is addressed in Chapter 14. Causes can be precapillary (alveolar hypoxia caused by lung disease or high altitude) or postcapillary (congenital heart disease with left to right shunting of blood or acquired heart disease). Dirofilariasis is probably the most common cause of pulmonary hypertension in dogs; bronchial asthma might be a cause in cats.
Pulmonary Edema
As in any tissue, excessive fluid accumulation in the lungs occurs as a result of increased hydrostatic pressure, decreased oncotic pressure, lymphatic blockage, or changes in vascular permeability. Increased hydrostatic pressure generally occurs as a result of volume (vascular) overload. In contrast to fluid dynamics in other tissues, hydrostatic pressure in the lungs is low, and lymphatic flow is high. Expansion of lymphatics, as well as fluid movement into the alveoli, can accommodate marked increases in capillary pressure. Thus capillary hydrostatic pressures must markedly increase for excessive fluid to accumulate in the lungs. Hypoalbuminemia is not a likely cause of pulmonary edema. Rather, vascular overload as a result of overcirculation is a common cause of pulmonary edema secondary to increased oncotic pressure. Left-sided heart failure is the most common cause of vascular overload. Regardless of the cause of pulmonary edema, oxygen therapy and actions that minimize stress and anxiety of the patient are indicated. Unless contraindicated, bronchodilators should be administered.
Diuretics are indicated for treatment of pulmonary edema associated with volume overload (increased hydrostatic pressure). Drugs that cause sodium and chloride excretion (i.e., furosemide) may be more effective, particularly in cases of sodium and water retention. Attention might be made to selection based on underlying cause (e.g., aldosterone antagonists in the presence of high aldosterone output). Diuretics are contraindicated for patients that are hypovolemic. Use in normovolemic animals should be cautious and the dose titrated to the minimum needed to control clinical signs associated with pulmonary edema. In life-threatening situations of pulmonary edema associated with volume overload, venous dilators can be used to increase the capacitance of the vascular system, thus “drawing” the increased volume into the veins, away from the heart and pulmonary system. Topically applied nitroglycerin or morphine sulfate (0.1 mg/kg intravenously as needed) can be used for this purpose. Morphine has the added advantage of sedating animals whose anxiety is contributing to hypoxia. Methylxanthines such as theophylline might be helpful in the short term because they also bronchodilate, and in the patient with heart failure they may improve contractility. They will also, however, increase oxygen demand by the heart, and their diuretic effects are short lived (2 to 3 days).
Pulmonary edema as a result of increased vascular permeability probably occurs more frequently than is anticipated. Any disorder that causes inflammation of the lungs will contribute to pulmonary edema of the lungs. The extreme manifestation of permeability-induced pulmonary edema is ARDS, which has been described in humans. The fluid contains protein that, as long as it is present, will continue to provide oncotic draw of fluid into the parenchyma. Pulmonary edema of this type is difficult to treat. Pulmonary wedge pressure is normal; vascular overload is not present. In this situation diuretics will serve to decrease fluid retention only at the cost of extracellular fluid volume and thus are not an effective treatment. Glucocorticoids might be indicated to decrease inflammation and support bronchodilation, although their use is controversial. Among the glucocorticoids, methylprednisolone should be considered because of its ability to scavenge oxygen radicals. Vasodilators might be used; the therapeutic intent of these drugs is not certain, but decreased delivery of blood to the lungs and a further decrease in wedge pressure may decrease movement of blood into the parenchyma. Vascular shunting and hypotension may, however, preclude their use. Newer therapies are likely to target mediators responsible for permeability, such as TNF or nitric oxide, or replace surfactant.
Miscellaneous
Aspiration Pneumonia
Clinical signs resulting from aspiration pneumonia may result from mechanical obstruction in small or large airways, and the inflammatory response to foreign materials (including gastric acid or other chemicals), bacterial infection is. Decreased pulmonary compliance and bronchoconstriction are likely to be a source of some of the clinical signs. Oxygen therapy, bronchodilatory therapy, and positive pressure ventilation are indicated, the latter particularly for patients with poor pulmonary compliance. Bronchoscopy can be used to guide removal of visible foreign material. Glucocorticoids might be used to minimize the inflammatory response during the initial phase of therapy; methylprednisolone and NAC might be considered to minimize oxygen radical damage. Immunosuppression probably negates the advantages of controlled inflammation after 48 hours of therapy. Among the drugs to be considered is pentoxifylline. Its early use (immediately after aspiration) as a loading dose followed by infusion resulted in 17% mortality versus 67% mortality in placebo-controlled rats.145 In another study involving rats and measurement of inflammatory mediators, pre-treatment was found to be superior to post-aspiration treatment, suggesting preventative therapy might be considered in patients at high risk for post-operative aspiration. 145 Routine antibiotic coverage should be avoided to minimize the risk of resistance until evidence of a bacterial component in the inflammatory process exists.
Near Drowning
Standard supportive therapy for near drowning includes oxygen, positive pressure ventilation, and therapy for shock. Bronchodilators may be of benefit. Using a rat model of hydrochloride-induced lung injury, pretreatment with pentoxifylline significantly reduced damage, with some benefit shown in animals whose treatment was withheld until post injury. Further, pentoxifylline administered as a continuous infusion significantly reduced lung injury in dogs in which near-drowning was experimentally induced.145a Therapies for treatment of inflammation associated with aspiration should be considered. Use of glucocorticoids is controversial; however, use of methylprednisolone is appealing because of its oxygen-scavenging abilities. NAC therapy may be useful for its oxygen radical–scavenging effects as well as other benefits. Short-term therapy may be of benefit. Use of antimicrobials should probably be reserved for evidence of infection. Supportive therapy should also target the advent of cerebral edema; an additional advantage of using methylprednisolone is minimizing oxygen radical damage in the event of cerebral hypoxia.
Smoke Inhalation
Oxygen therapy is critical for removal of carbon monoxide; the half-life of carboxyhemoglobin decreases from 4 to 0.5 hours in the presence of 100% oxygen. Other supportive therapy includes airway hydration (as needed), bronchodilators, and (if indicated) positive pressure ventilation, and drugs intended to control inflammation.147 Short-term administration of glucocorticoids (methylprednisolone preferred) may be of benefit to minimize inflammation and oxygen radical damage and to facilitate bronchodilation. However, pentoxifylline should also be considered. Using an ovine model, pentoxifylline administered continuously after induction of smoke inhalation injury was associated with less hypoxia and ventilation perfusion mismatching, pulmonary hypertension and markers of inflammation.147
Chylothorax
The pathophysiology of chylothorax, or the accumulation of lymphatic fluid in the pleural cavity, is not well understood. Beyond repetitive drainage, both surgical and medical management are limited in success. Rutin is a non-anticoagulant coumarin a flavone benzo-γ-pyrone plant fruit extract (bio-flavonoid) from the Brazilian fava d’anta (Dimorphandra). It acts to stimulate macrophage removal of proteins and thus removes the oncotic flux of fluid into tissues. It has been used to treat selected causes of peripheral limb edema associated with protein exudation in human patients. It is available in health food stores as a supplement. Response may take several weeks to months. There appear to be no toxicities associated with the drug. Several case reports cite partial resolution of chylous effusion in cats receiving 50 mg/kg orally every 8 hours.148-150 In humans, octrenotid is an octapeptide omatostatin-mimicking drug used to treat acromegaly. However, it also has been used (based on case reports) successfully with a medium chain triglyceride diet to control chylothorax associated with cardiac surgery.151
Diseases of the Pleura
A number of diseases are associated with pleural effusion, and successful management of the effusion largely depends on resolution of the underlying diseases. Thus pleural effusion resulting from cardiac failure, neoplastic disease, and other causes is treated by treatment of the cause; pleurodesis is used as needed to manage life-threatening effusion. Pleurodesis stimulated by lavage of irritating substances (with the intent of “closing” the pleural space by causing fibrosis) is strongly discouraged. An exception is made for empyema, which can be a primary disease.
Empyema
Empyema refers to the accumulation of infectious inflammatory material within the pleural space. Infection can be an extension of a primary pulmonary lesion,the result of direct penetrating trauma, or by way of a lymphatic or hematogenous route. Accumulation of inflammatory debris provides a continued colloidal draw of fluid into the cavity. Lymphatic obstruction by debris further worsens the ability of pleural fluid mechanics to resolve the accumulation. Thus chest drainage is critical to successful control. Microbiologic examination (including Gram stains initially pending culture and susceptibility data) should be the basis of initial antimicrobial selection. Subsequent daily cytologic studies with Gram staining should provide the basis for response to therapy. The fluid should be recultured if bacterial growth has not changed for 2 to 3 subsequent days or if the morphology of the organism changes. Note that absence of an organism on Gram stain does not necessarily indicate the absence of organisms at the site of infection. Because the incidence of anaerobic infections in empyema is high in dogs and cats, both aerobic and anaerobic cultures should be collected. Care must be taken to collect the anaerobic culture properly. Despite the presence of organisms on Gram stains, cultures often do not yield growth. Thus antimicrobial therapy often must be empirical.
Empirical therapy should include drugs effective against likely infecting organisms, including anerobic organisms (see Chapter 8).
1. Moses B.L., Spaulding G.L. Chronic bronchial disease of the cat. Vet Clin North Am Small Anim Pract. 1985;15:929-949.
2. Slonim N.F., Hamilton L.H. Development and functional anatomy of the bronchopulmonary system. In: Carson D., editor. Respiratory physiology. ed 5. St Louis: Mosby; 1987:27-47.
3. Scott J.S., Berney C.E., Derksen F.J., et al. α-Adrenergic receptor activity in ponies with recurrent obstructive pulmonary disease. Am J Vet Res. 1991;52:1416-1422.
4. Gustin P., Dhem A.R., Lekeux P., et al. Regulation of bronchomotor tone in conscious calves. J Vet Pharmacol Ther. 1989;12:58-64.
5. Chand N. Reactivity of isolated trachea, bronchus, and lung strip of cats to carbachol, 5-hydroxytryptamine and histamine: evidence for the existence of methylsergide-sensitive receptors. Br J Pharmacol. 1981;73:853-857.
6. Moise N.S., Spaulding G.L. Feline bronchial asthma: pathogenesis, pathophysiology, diagnostic, and therapeutic considerations. Compend Contin Educ Pract Vet. 1981;3:1091-1103.
7. Inque H., Masakazu I., Motohiko M., et al. Sensory receptors and reflex pathways of nonadrenergic inhibitory nervous system in feline airways. Am Rev Respir Dis. 1989;139:1175-1178.
7a. Canning BJ: Anatomy and neurophysiology of the cough reflex: ACCP evidence-based clinical practice guidelines, Chest 129(1Suppl):33S-47S, 2006.
7b. Bolser DC: Central mechanisms II: pharmacology of brainstem pathways, Handb Exp Pharmacol 187:203-217, 2009.
7c. Canning BJ, Chou YL: Cough sensors. I. Physiological and pharmacological properties of the afferent nerves regulating cough, Handb Exp Pharmacol 187:23-47, 2009.
8. Altiere R.J., Diamond L. Comparison of vasoactive intestinal peptide and isoproterenol relaxant effects in isolated cat airways. J Appl Physiol. 1984;56:986-992.
9. Altiere R.J., Szarek J.L., Diamond L. Neuronal control of relaxation in cat airway’s smooth muscle. J Appl Physiol. 1984;57:1536-1544.
10. Derksen F.J., Robinson N.E., Armstrong P.J., et al. Airway reactivity in ponies with recurrent airway obstruction (heaves). J Appl Physiol. 1985;58:598-604.
11. Downes H., Austin D.R., Parks C.M., et al. Comparison of drug responses in vivo and in vitro in airways of dogs with and without airway hyperresponsiveness. J Pharmacol Exp Ther. 1986;237:214-219.
12. Colebatch H.J.H., Olsen C.R., Nadel J.A. Effect of histamine, serotonin, and acetylcholine on the peripheral airways. J Appl Physiol. 1966;21:217-226.
13. Townley R.G., Hopp R.J., Agrawal D.K., et al. Platelet-activating factor and airway reactivity. J Allergy Clin Immun. 1989;83:997-1011.
14. Soler M., Mansour E., Fernandez A., et al. PAF-induced airway responses in sheep: effects of a PAF antagonist and nedocromil sodium. J Allergy Clin Immun. 1990;67:661-668.
15. Gray P.R., Derksen F.J., Robinson N.E., et al. The role of cyclooxygenase products in the acute airway obstruction and airway hyperreactivity of ponies with heaves. Am Rev Respir Dis. 1989;140:154-160.
16. Blair A.M., Woods A. The effects of isoprenaline, atropine, and disodium cromoglycate on ciliary motility and mucous flow in vivo measured in cats. Br J Pharmacol. 1969;35:P379-P380.
17. Suri R. The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis. BioDrugs. 2005;19(3):135-144.
18. Gallagher J.T., Kent P.W., Passatore M., et al. The composition of tracheal mucus and the nervous control of its secretion in the cat. Proc R Soc Lond. 1975;192:49-76.
19. Winkler G. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988;181:217-234.
20. Frerking I., Günther A., Seeger W., et al. Pulmonary surfactant: functions, abnormalities and therapeutic options. Intensive Care Med. 2001;27:1699-1717.
21. Gillissen A., Nowak D. Characterization of N-acetylcysteine and ambroxol in anti-oxidant therapy. Respir Med. 1998;92:609-623.
22. Barnes P.J. The drug therapy of asthma: directions for the 21st century. Agents Actions. 1988;23(Suppl):293-313.
23. Barnes P.J. Our changing understanding of asthma. Respir Med. 1989;83(Suppl):17-23.
24. Gold W.M., Meyers G.L., Dain D.S., et al. Changes in airway mast cells and histamine caused by antigen aerosol in allergic dogs. J Appl Physiol. 1977;43:271-275.
25. Norn S., Clementson P. Bronchial asthma: pathophysiological mechanisms and corticosteroids. Allergy. 1988;43:401-405.
26. Wanner A., Rao A. Clinical indications for and effects of bland, mucolytic, and antimicrobial aerosols. Am Rev Respir Dis. 1980;122:79-87.
27. An S.S., Bai T.R., Bates J.H., et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29(5):834-860.
28. Kay A.B., Walsh G.M., Moqbel R., et al. Disodium cromoglycate inhibits activation of human inflammatory cells in vitro. J Allergy Clin Immunol. 1987;80:1-8.
29. Barnes P.J., Chung K.F., Page C.P. Inflammatory mediators and asthma. Pharmacol Rev. 1988;40:49-84.
30. Bauer T. Pulmonary hypersensitivity disorders. In: Kirk R.W., editor. Current veterinary therapy IX. Philadelphia: Saunders; 1986:369-376.
31. Doshi U., Salat P., Parikh V. Cytokine modulators in asthma: clinical perspectives. Indian J Pharmacol. 2002;34:16-25.
32. Larché M., Robinson D.S., Kay A.B. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111(3):450-463.
33. Umetsu D.T., Akbari O. DeKruyff RH: Regulatory T cells control the development of allergic disease and asthma. J Allergy Clin Immunol. 2003;112:480-487.
34. Dorman S.C., Sehmi R., Gauvreau G.M., et al. Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004;169(5):565-572.
35. Braccioni F., Dorman S.C., O’Byrne P.M., et al. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic subjects. J Allergy Clin Immunol. 2002;110(1):96-101.
36. Sterk P.J., Hiemstra P.S. Eosinophil progenitors in sputum: throwing out the baby with the bath water? Am J Respir Crit Care Med. 2004;169:549-556.
37. Denburg J.A. The bone marrow and airway inflammation: evidence for allergy as a systemic disease. Clin Exp All Rev. 2003;3:23-27.
38. Denburg J.A. Haemopoietic mechanisms in nasal polyposis and asthma. Thorax. 2000;55(Suppl 2):S24-S25.
39. Padrid P. Feline asthma. Diagnosis and treatment. Vet Clin North Am Small Anim Pract. 2003;30(6):1279-1294.
40. Bjermer L. History and future perspectives of treating asthma as a systemic and small airways disease. Respir Med. 2001;95:703-719.
41. Undem B.J. Pharmacotherapy of asthma. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
42. Eiser N.M., Mills J., Snashall P.D., et al. The role of histamine receptors in asthma. Clin Sci. 1981;60:363-370.
43. Dunford P.J., O’Donnell N., Riley J.P., et al. The histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cells. J Immunol. 2006;176(11):7062-7070.
44. Skidgel R.A., Erdös E.G. Histamine, bradykinin, and their antagonists. In Brunton L.L., Lazo J.S., Parker K.L., editors: Goodman & Gilman’s the pharmacological basis of therapeutics, ed 11, New York: McGraw-Hill, 2006.
45. McNamara D.B., Harrington J.K., Bellan J.A., et al. Inhibition of pulmonary thromboxane A2 synthase activity and airway responses by CGS 13080. Mol Cell Biochem. 1989;85:29-41.
46. Inman M.D., Denbrug J.A., Parameswarn K., et al. The effect of cysteinyl leukotrienes on growth of eosinophil progenitors from peripheral blood and bone marrow of atopic. J Allergy Clin Immunol. 2002;110:96-101.
47. McKay I.R., Rosen F.S. Allergy and allergic disease. N Engl J Med. 2001;334:30-37.
48. Hogan J.B., Piktel D., Landreth K.S. IL-5 production by bone marrow stromal cells: implications for eosinophilia associated with asthma. J Allergy Clin Immunol. 2000;106:329-336.
49. Preventing migration of inflammatory cells. Dual integrin antagonists: a new approach to the treatment of asthma, 2001. Accessed October 26, 2009 at www.roche.com/pages/downloads/company/pdf/ om/pages/downloads/company/pdf/ rddpenzberg02 01e.pdf.
50. Daemen M.J.A.P., Smits J.F.M., Thijssen H.H.W., et al. Pharmacokinetic considerations in target-organ directed drug delivery. Trends Pharmacol Sci. 1988;9:138-141.
51. Reed M.T., Kelly H.W. Sympathomimetics for acute severe asthma: should only beta2-selective agonists be used? DICP Ann Pharmacother. 1990;24:868-873.
52. Nelson H.S. β-adrenergic bronchodilators. New Eng J Med. 1995;333(8):499-506.
53. Committee for Veterinary Medicinal Products. Clenbuterol Hydrochloride Summary Report. The European Agency for the Evaulatuion of Medicinal Products. Veterinary Medicine and Information Technology Unit. 2000.
54. Kirschvink N., Leemans J., Delvaoux F., et al. Bronchodilators in bronchoscopy-induced airflow limitation in allergen-sensitized cats. J Vet Intern Med. 2005;19:161-167.
55. Nelson H.S. Combination therapy of bronchial asthma. Allergy Asthma Proc. 2001;22(4):217-220.
56. Hendeles L., Weinberger M. Theophylline: a state of the art review. Pharmacotherapy. 1983;3:2-44.
57. Mizus I., Summer W., Farrkuhk I., et al. Isoproterenol or aminophylline attenuate pulmonary edema after acid lung injury. Am Rev Respir Dis. 1985;131:256-259.
58. Short C.E. Telazol: a new injectable anesthetic. Cornell Fel Health Cent Info Bull. 1987;2:1-3.
59. Mascali J.J., Cvietusa P., Negri J., et al. Anti-inflammatory effects of theophylline: modulation of cytokine production. Ann Allergy Asthma Immunol. 1996;77:34-38.
60. Sackner M.A. Effect of respiratory drugs on mucociliary clearance. Chest. 1978;73(6):958-966.
61. Dunn M.E., Taylor S.M. Effects of theophylline on tracheal mucociliary clearance rates in healthy cats. Am J Vet Res. 2002;63:1320-1322.
62. Murciano D., Aubier M., Lecocguic Y., et al. Effects of theophylline on diaphragmatic strength and fatigue in patients with chronic obstructive pulmonary disease. N Engl J Med. 1984;311:349-353.
63. Viires N., Aubier M., Murciano D., et al. Effects of aminophylline on diaphragmatic fatigue during acture respiratory failure. Am Rev Respir Dis. 1984;129:396-402.
64. McKiernan B.C., Davis C.A.N., Koritz G.D., et al. Pharmacokinetics studies of theophylline in dogs. J Vet Pharmacol Ther. 1981;4:103-110.
65. McKiernan B.C., Koritz G.D., Davis L.E., et al. Pharmacokinetics studies of theophylline in cats. J Vet Pharmacol Ther. 1983;6:99-104.
66. Bach J.E., Kukanich B., Papich M.G., et al. Evaluation of the bioavailability and pharmacokinetics of two extended-release theophylline formulations in dogs. J Am Vet Med Assoc. 2004;224(7):1113-1119.
67. Guenther-Yenke C.L., McKiernan B.C., Papich M.G., et al. Pharmacokinetics of an extended-release theophylline product in cats. J Am Vet Med Assoc. 2007;231(6):900-906.
68. Dye J.A., McKiernan B.C., Jones S.D. Sustained-release theophylline pharmacokinetics in cats. J Vet Pharmacol Ther. 1990;13:278-286.
69. Koritz G.D., McKiernan B.C., Davis C.A.N., et al. Bioavailability of four slow-release theophylline formulations in the beagle dog. J Vet Pharmacol Ther. 1986;9:293-302.
70. Dye J.A., McKiernan B.C., Neft Davis C.A., et al. Chronopharmacokinetics of theophylline in cats. J Vet Pharmacol Ther. 1989;12:133-140.
71. Munsiff I.J., Koritz G.D., McKiernan B.C., et al. Plasma protein binding of theophylline in dogs. J Vet Pharmacol Ther. 1988;11:112-114.
72. Larsson C.I., Kallings P., Persson S., et al. Pharmacokinetics and cardio-respiratory effects of oral theophylline in exercised horses. J Vet Pharmacol Ther. 1989;12:189-199.
73. McKiernan B.C. Principles of respiratory therapy. In: Kirk R.W., editor. Current veterinary therapy VIII: small animal practice. Philadelphia: Saunders; 1983:216-221.
74. Rybak M.J., Bowles S.K., Chandrasekar P.H., et al. Increased theophylline concentrations secondary to ciprofloxin. Drug Intell Clin Pharmacol. 1987;21:879-881.
75. Rodvold K.A. Clinical pharmacokinetics of clarithromycin. Clin Pharmacokinet. 1999;37:385-398.
76. Cremer K.F., Secor J., Speeg K.V. The effect of route of administration on the cimetidine-theophylline drug interaction. J Clin Pharmacol. 1989;29:451-456.
77. Henderson A., Wright D.M., Pond S.M. Management of theophylline overdose patients in the intensive care unit. Anaesth Intensive Care. 1992;20(1):56-62.
78. Hirt R.A., Teinfalt M., Dederichs D., et al. The effect of orally administered marbofloxacin on the pharmacokinetics of theophylline. J Vet Med A Physiol Pathol Clin Med. 2003;50(5):246-250.
79. Intorre L., Mengozzi G., Maccheroni M., et al. Enrofloxacin-theophylline interaction: influence of enrofloxacin on theophylline steady-state pharmacokinetics in the beagle dog. J Vet Pharmacol Ther. 1995;18(5):352-356.
80. Jonkman J.H., Borgstrom L., van der Boon W.J., et al. Theophylline-terbutaline, a steady state study on possible pharmacokinetic interactions with special reference to chronopharmacokinetic aspects. Br J Clin Pharmacol. 1988;26(3):285-293.
81. Munsiff I.J., McKiernan B.C., Davis C.A.N., et al. Determination of the acute oral toxicity of theophylline in conscious dogs. J Vet Pharmacol Ther. 1988;11:381-389.
82. Persson C.G.A., Ergefalt I. Seizure activity in animals given enprofylline and theophylline, two xanthines with partly different mechanisms of action. Arch Int Pharmacodyn. 1982;258:267-282.
83. Gross N.J., Skorodin M.S. Anticholinergic, antimuscarinic bronchodilators. Am Rev Respir Dis. 1984;129:856-870.
84. Murphy S., Kelly H.W. Cromolyn sodium: a review of mechanisms and clinical use in asthma. DICP Ann Pharmacother. 1987;21:22-35.
85. Holgate S.T. Reflections on the mechanisms of action of sodium cromoglycate (Intal) and the role of mast cells in asthma. Respir Med. 1989;83(Suppl):25-31.
86. Massey K.L., Hendeles L. Calcium antagonists in the management of asthma: breakthrough or ballyhoo? DICP Ann Pharmacother. 1987;21:505-508.
87. Creese B.R. Calcium ions, drug action and airways obstruction. Pharmacol Ther. 1983;20:357-375.
88. Gupta R., Jindals P.D., Kumar G. Corticosteroids: the mainstay in asthma. Bioorg Med Chem. 2004;12:6331-6342.
89. Hamid Q. Effects of steroids on inflammation and cytokine gene expression in airway inflammation. J Allergy Clin Immunol. 2003;112:636-638.
90. Currie G.P., Srivasta V.A., Dempsey O.J., et al. Therapeutic modulation of allergic airways disease with leukotriene receptor antagonists. Q J Med. 2005;98:171-182.
91. Kelly H.W. Pharmaceutical characteristics that influence the clinical efficacy of inhaled corticosteroids. Ann Allergy Asthma Immunol. 2003;91:326-334.
92. Norris CA: Research Abstract Program of the 21st Annual ACVIM Forum, #22 Charlotte, NC, June 4-7, 2003.
93. Reinero C.R., Brownlee L., Decile K.C., et al. Inhaled flunisolide suppresses the hypothalamic-pituitary-adrenocortical axis, but has minimal systemic immune effects in healthy cats. J Vet Intern Med. 2006;20(1):57-64.
94. Hui Y., Funk C.D. Cysteinyl leukotriene receptors. Biochem Pharmacol. 2002;64(11):1549-1557.
95. Tashkin D.P. The importance of control in asthma management: do leukotreine receptor antagonists meet patient and physician needs? Allergy Astham Proc. 2001;22(5):311-319.
97. Parratt J.R., Sturgess R.M. The effect of indomethacin on the cardiovascular and metabolic responses to E. coli endotoxin in the cat. Br J Pharmacol. 1974;50:177-183.
98. Wasserman M.A. Modulation of arachidonic acid metabolites as potential therapy of asthma. Agents Actions. 1988;23(Suppl):95-111.
99. Walker B.R., Voelkel N.F., Reeves J.T. Pulmonary pressor response after prostaglandin synthesis inhibition in conscious dogs. J Appl Physiol. 1982;52:705-709.
100. Bennett A. The importance of COX-2 inhibition for aspirin induced asthma. Thorax. 55(Suppl 2. 2000. S54-S56.
101. Keith J.C. Pulmonary thromboembolism during therapy of dirofilariasis with thiacetarsamide: modification with aspirin or prednisolone. Am J Vet Res. 1983;44:1278-1283.
102. Rawlings C.A., Keith J.C., Lewis R.E., et al. Aspirin and prednisolone modification of radiographic changes caused by adulticide treatment in dogs with heartworm infection. J Am Vet Med Assoc. 1983;183:131-132.
103. Chyrek-Borowska S., Siergiejko Z., Michalska I. The effects of a new generation of H1 antihistamines (cefirizine and loratadine) on histamine release and the bronchial response to histamine in atopic patients. J Invest Allergol Clin Immunol. 1995;5:103-107.
104. Stahl S.M. Selective histamine H1 antagonism: novel hypnotic and pharmacologic actions challenge classical notions of antihistamines. CNS Spectr. 2008;13(12):1027-1038.
105. Sudoh Y., Cahoon E.E., Gerner P., et al. Tricyclic antidepressants as long-acting local anesthetics. Pain. 2003;103(1-2):49-55.
106. Padrid P.A., Mitchell R.W., Ndukwu I.M., et al. Cyproheptadine-induced attenuation of type I immediate hypersensitivity reactions of airway smooth muscle from immune sensitized cats. Am J Vet Res. 1995;56:109-115.
107. Norris C.R., Boothe D.M., Esparza T., et al. Disposition of cyproheptadine in cats after intravenous or oral administration of a single dose. Am J Vet Res. 1998;59(1):79-81.
108. Roudebush P. Antitussive therapy in small companion animals. J Am Vet Med Assoc. 1982;180:1105-1107.
109. Irwin R.S., Curlye F.J., Bennett F.M. Appropriate use of antitussives and protussives. A practical review. Drugs. 1993;46:80-91.
110. Gingerich D.A., Rourke J.E., Strom P.W. Clinical efficacy of butorphanol injectable and tablets. Vet Med Small Anim Clin. 1983;78:179-182.
111. Christie G.J., Strom P.W., Rourke J.E. Butorphanol tartrate: a new antitussive agent for use in dogs. Vet Med Small Anim Clin. 1980;75:1559-1562.
112. Tukianinen H., Silvasti M., Flygare U., et al. The treatment of acute transient cough: a placebo-controlled comparison of dextromethorphan and dextromethorphan-beta2-sympathomimetic combination. Eur J Respir Dis. 1986;69:95-99.
113. Brain J.D. Factors influencing deposition of inhaled particles. Proc 3rd Annu Comp Respir Soc Symp. 1983;3:232.
113a. Ichinose M, Miura M, Yamauchi H, et al: A neurokinin 1-receptor antagonist improves exercise-induced airway narrowing in asthmatic patients, Am J Respir Crit Care Med 153(3):936-941, 1996.
113b. Chapman RW, House A, Liu F, Celly C, et al: Antitussive activity of the tachykinin NK1 receptor antagonist, CP-99994, in dogs, Eur J Pharmacol 485(1-3):329-332, 2004.
114. Crockett A., Cranston J.M., Alpers J.H., et al. Mucolytics for bronchiectasis. Cochrane Database of Systematic Reviews Issue 1. Art. No: CD001289. 2001.
115. Ziment I. Acetylcysteine: a drug that is much more than a mucokinetic. Biomed Pharmacother. 1988;42:513-520.
116. Heller A.R., Groth G., Heller S.C., et al. N-acetylcysteine reduces respiratory burst but augments neutrophil phagocytosis in intensive care unit patients. Crit Care Med. 2001;29(2):272-276.
117. Tirouvanziam R., Conrad C.K., Bottiglieri T., et al. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci U S A. 2006;103(12):4628-4633.
118. Cuzzocrea S., Mazzon E., Dugo L., et al. Protective effects of n-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the rat. FASEB J. 2001;15(7):1187-2000.
119. Rocksen D., Lilliehook B., Larsson R. Differential anti-inflammatory and anti-oxidative effects of dexamethasone and N-acetylcysteine in endotoxin-induced lung inflammation. Clin Exp Immunol. 2000;122(2):249-256.
120. Ueno O., Lee L.-N., Wagner P.D. Effect of N-acetylcysteine on gas exchange after methacholine challenge and isoprenaline inhalation in the dog. Eur Respir J. 1989;2:238-246.
121. Ziment I. Acetylcysteine: a drug with an interesting past and a fascinating future. Respiration. 1986;50(Suppl 1):26-30.
122. DeCramer M., Rutten-van Mölken M., Dekhuijzen P.N., et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365(9470):1552-1560.
123. Sutherland E.R., Crapo J.D., Bowler R.P. N-acetylcysteine and exacerbations of chronic obstructive pulmonary disease. COPD. 2006;3(4):195-202.
124. Kao L.W., Kirk M.A., Furbee R.B., et al. What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning? Ann Emerg Med. 2003;42(6):741-750.
125. Hendeles L. Selecting a decongestant. Pharmacotherapy. 1993;13:129S-134S.
126. Johnson D.A., Hricik J.G. The pharmacology of alpha-adrenergic decongestants. Pharmacotherapy. 1993;13:110S-115S.
127. Kanfer I., Dowse R., Vuma V. Pharmacokinetics of oral decongestants. Pharmacotherapy. 1993;13:116S-128S.
128. Miller C.J., McKinernan B.C., Pace J., et al. The effects of doxapram hydrochloride (Dopram-V) on laryngeal function in healthy dogs. J Vet Intern Med. 2002;16(5):524-528.
129. Thompson P.J. Drug delivery to the small airways. Am J Respir Crit Care Med. 1998;157:S199-S202.
130. Malik S.K., Jenkins D.E. Alterations in airway dynamics following inhalation of ultrasonic mist. Chest. 1972;62:660-664.
131. Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588-599.
132. Newhouse M.T., Ruffin R.E. Deposition and fate of aerosolized drugs. Chest. 1978;73(Suppl):936-943.
133. Labiris N.R., Dolovich M.B. Pulmonary drug delivery. Part II: the role of inhalant delivery devices and drug formulations in therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:600-612.
134. Dolovich M.B., Ahrens R.C., Hess D.R., et al. American College of Chest Physicians; American College of Asthma, Allergy, and Immunology. Device selection and outcomes of aerosol therapy: Evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335-371.
135. Schulman R.L., Crochik S.S., Kneller S.K., et al. Investigation of pulmonary deposition of a nebulized radiopharmaceutical agent in awake cats. Am J Vet Res. 2004;65(6):806-809.
136. Johnson C.E. Aerosol corticosteroids for the treatment of asthma. Drug Intell Clin Pharmacol. 1987;21:784-790.
137. Bemis D.A., Appel M.J.G. Aerosol, parenteral, and oral antibiotic treatment of Bordetella bronchiseptica infections in dogs. J Am Vet Med Assoc. 1977;170:1082-1086.
138. Ettinger S.J., Kantrowitz B. Diseases of the trachea. In Ettinger S.J., Feldman E., editors: Textbook of veterinary internal medicine, ed 6, St Louis: Saunders, 2005.
139. Roberts C.R., Pare P.D. Composition changes in human tracheal cartilage in growth and aging, including changes in proteoglycan structure. Am J Physiol. 1991;261(2 Pt 1):L92-101.
140. Hamaide A., Arnoczky S.P., Ciarelli M.J., et al. Effects of age and location on the biomechanical and biochemical properties of canine tracheal ring cartilage in dogs. Am J Vet Res. 1998;59(1):18-22.
141. Reinero C.R., Decline K.C., Byerly J.R., et al. Effects of drug treatment on inflammation and hyperreactivity of airways and on immune variables in cats with experimentally induced asthma. Am J Vet Res. 2005;66(7):1121-1127.
141a. Green S.S., Lamb G.C., Schmitt S., et al. Oral versus repository corticosteroid therapy after hospitalization for treatment of asthma. J Allergy Clin Immunol. 1995;95(1 Pt 1):15-22.
141b. Padrid PA, Cozzi P, Leff AR: Cyclosporin A inhibits airway reactivity and remodeling after chronic antigen challenge in cats, Am J Respir Crit Care Med 154:1812-1818, 1996.
142. Chow O.K., Fung K.P. Slow release terbutaline and theophylline for the long term therapy of children with asthma: a Latin square and factorial study of drug effects and interactions. Pediatrics. 1989;84:119-125.
143. Schooley E.K., McGee Turner J.B., Jiji R.D., et al. Effects of cyproheptadine and cetirizine on eosinophilic airway inflammation in cats with experimentally induced asthma. Am J Vet Res. 2007;68(11):1265-1271.
144. McKiernan B.C. Diagnosis and treatment of canine chronic bronchitis: twenty years of experience. Vet Clin N Amer Small Anim Pract. 2003;30(6):1267-1278.
145. Pawlik M.T., Schreyer A.G., Ittner K.P., et al. Early treatment with pentoxifylline reduces lung injury induced by acid aspiration in rats. Chest. 2005;127(2):613-621.
145a. Ji Q, Zhang L, Wang L, et al: Pentoxifylline reduces indirect lung injury of fresh water drowning in canis, Clin Chim Acta 365(1-2):221-229, 2006.
146. Oliveira-Júnior I.S., Maganhin C.C., Carbonel A.A., et al. Effects of pentoxifylline on TNF-alpha and lung histopathology in HCL-induced lung injury. Clinics (Sao Paulo). 2008;63(1):77-84.
147. Ogura H., Cioffi W.G., Okerberg C.V., et al. The effects of pentoxifylline on pulmonary function following smoke inhalation. J Surg Res. 1994;56(3):242-250.
148. Thompson MS, Cohn LA, Jordan RC: Use of rutin for medical management of idiopathic chylothorax in four cats, J Am Vet Med Assoc 1;215(3):345-348, 1999.
149. Gould L: The medical management of idiopathic chylothorax in a domestic long-haired cat, Can Vet J 45(1):51-54, 2004.
150. Kopko SH: The use of rutin in a cat with idiopathic chylothorax, Can Vet J 46(8):729-731, 2005.
151. Barbetakis N, Xenikakis T, Efstathiou A, et al: Successful octreotide treatment of chylothorax following coronary artery bypass grafting procedure. A case report and review of the literature, Hellenic J Cardiol 47(2):118-122, 2006.