The thyroid and control of metabolic rate
Thyroid function
The main functions of thyroid hormones are the control of metabolism, growth and development. The term ‘basal metabolism’ refers to the energy-utilising biochemical processes of the body at rest. Basal metabolic rate is controlled by thyroid hormones, which stimulate tissue oxygen consumption and regulate energy and heat production, mainly through an increase in the metabolism of fats, carbohydrates and proteins. Most of these functions are a result of thyroid hormones acting in combination with other hormones such as insulin. Thyroid hormones have a thermogenic action on brown fat in infants, increasing synthesis of uncoupling proteins that divert the energy released during lipolysis into heat rather than high-energy phosphate synthesis. They also promote gluconeogenesis, obtaining the substrate for glucose formation from amino acids in tissues such as muscle and bone. Thyroid hormones facilitate the development of the nervous system, somatic growth (synergistically with growth hormone) and puberty. They also regulate the synthesis of proteins involved in hepatic, cardiac, neurological and muscular functions. Thyroid hormones increase the body's sensitivity to catecholamines, and therefore to sympathetic nervous system activation, in particular enhancing the effects of β-adrenoceptor stimulation (Ch. 4).
There are two thyroid hormones: triiodothyronine (T3) and thyroxine (T4). T3 is mainly responsible for effects at a cellular level, while T4 is now considered to be a prohormone. T3 and T4 are synthesised in the thyroid gland (Fig. 41.1), where inorganic iodide is trapped with great avidity by an enzyme-dependent process. The iodide is then oxidised to iodine by thyroid peroxidase. Iodine is very reactive and attaches to tyrosine (as tyrosyl residues of the glycoprotein thyroglobulin) to form mono-iodotyrosine and di-iodotyrosine residues. Two di-iodinated tyrosine molecules are conjugated to form T4, and one di-iodinated molecule with one mono-iodinated tyrosine molecule conjugate to form T3 (Fig. 41.1). Proteolytic enzymes from thyroid lysosomes then degrade thyroglobulin and release thyroid hormone into the circulation.
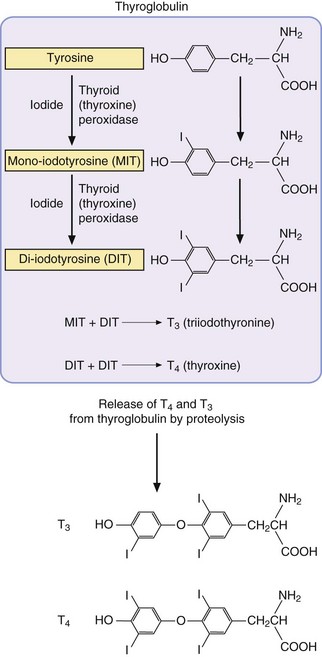
Fig. 41.1 The synthesis of thyroid hormones.
Iodide is oxidised to iodine by thyroid peroxidase and incorporated into tyrosine residues of thyroglobulin, the colloidal substance that fills the lumen of the thyroid follicles. Conjugation of mono-iodotyrosine (MIT) and di-iodotyrosine (DIT) residues into T3, or of two DIT residues to form T4, is followed by the release of T3 and T4 when thyroglobulin is proteolysed.
The synthesis and release of thyroid hormones are controlled by the anterior pituitary hormone thyrotropin (thyroid-stimulating hormone, TSH). This in turn is controlled by the hypothalamus, which secretes thyrotropin-releasing hormone (TRH). Circulating T3 and T4 exert negative feedback on both the hypothalamic and pituitary hormones (Fig. 41.2).
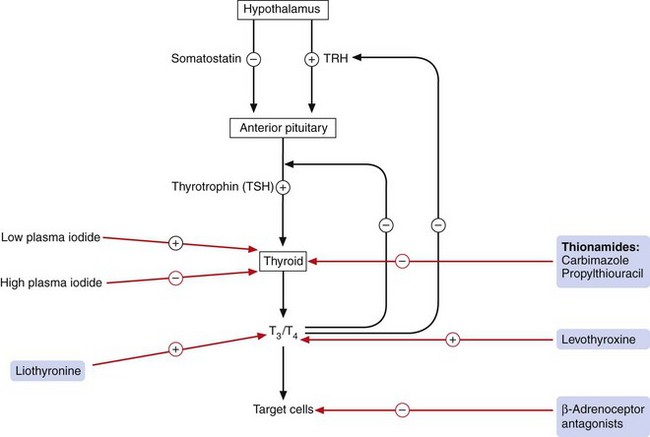
Fig. 41.2 Control of thyroid hormone synthesis and release.
Thyrotropin (thyroid-stimulating hormone, TSH) and thyrotropin-releasing hormone (TRH) are inhibited by circulating levels of T3 and T4. The sites of action of drugs acting on thyroid pathways are also shown.
The thyroid secretes mainly T4 and a small amount of T3. Circulating thyroid hormones are highly protein-bound, mostly to thyroxine-binding globulin (TBG). Less than 0.03% of T4 and less than 0.3% of T3 circulate unbound and only this free fraction of hormone is available to bind to specific intracellular receptors. Most T3 is derived from peripheral deiodination of T4 by iodothyronine deiodinase, which is found in the liver, kidney, brain and brown adipose tissue. About 35% of T4 is converted to T3, while about 40% is converted to reverse T3 (a metabolically inactive isomer of T3). T3 has a half-life in the circulation of about 1.5 days compared with about 7 days for T4. Elimination of T3 and T4 is by conjugation, mainly in the liver.
Thyroid hormones cross cell membranes via active transporters and bind to intracellular thyroid hormone receptors (or TRs) (Ch. 1), which belong to the superfamily of nuclear receptors. Thyroid hormone receptors usually repress target genes but, following thyroid hormone binding, the complex recruits co-activators and regulates gene transcription via thyroid response elements. Thyroid hormone receptors are expressed in most tissues, but there are three isoforms which differ in their tissue distribution and may mediate different effects of thyroid hormones. T3 also has non-genomic actions that include stimulation of cellular uptake of amino acids and glucose, and interactions with G-protein-coupled membrane receptors with activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase (MAPK) pathways.
Hyperthyroidism
The commonest form of hyperthyroidism (often, and interchangeably, called thyrotoxicosis) is Graves' disease, an autoimmune condition in which thyroid-stimulating immunoglobulin binds to thyrotropin (TSH) receptors on thyroid cells and initiates signal transduction. This is often accompanied by an immunologically mediated inflammatory reaction in the extrinsic muscles and fat of the orbit, causing swelling and the characteristic exophthalmos. Toxic multinodular goitre, thyroid adenomas (toxic or ‘hot’ nodules) and various forms of thyroiditis are much less common causes of hyperthyroidism. Rarely, the condition arises from excess production of thyrotropin or it can be induced by treatment with amiodarone (Ch. 8). Symptoms of hyperthyroidism include weight loss, palpitation, sweating, fatigue, nervousness, heat sensitivity and tremor. These are in part mediated by the action of excess thyroid hormone, and partly by excess sensitivity of tissues to β-adrenoceptor stimulation. Signs are often less marked in the elderly, who are more likely to present with atrial fibrillation that is resistant to treatment.
Drugs for treatment of hyperthyroidism
Mechanism of action: Thionamides inhibit thyroid peroxidase and, therefore, the synthesis of thyroid hormone (Fig. 41.2). The long half-life of T4 means that changes in the rate of synthesis take 4–6 weeks to lower circulating T4 and T3 concentrations to within the normal range. These drugs also appear to have an immunosuppressant effect in individuals with autoimmune thyroid disease. They reduce the levels of thyroid-stimulating immunoglobulin, although the clinical importance of this is uncertain. Large doses of propylthiouracil also decrease peripheral conversion of T4 to T3.
Pharmacokinetics: Carbimazole is converted by first-pass metabolism to the active derivative methimazole. Propylthiouracil has about one-tenth of the activity of methimazole and a shorter half-life; it is usually reserved for individuals intolerant to carbimazole. Both drugs accumulate in the thyroid, which extends their duration of action.
 Gastrointestinal upset (especially nausea and epigastric discomfort), headache, arthralgia and pruritic rashes are common in the first 8 weeks of treatment.
Gastrointestinal upset (especially nausea and epigastric discomfort), headache, arthralgia and pruritic rashes are common in the first 8 weeks of treatment.
 Allergic reactions, including vasculitis, a lupus-like syndrome, myopathy, cholestatic jaundice and nephritis. Some cross-sensitivity occurs between carbimazole and propylthiouracil.
Allergic reactions, including vasculitis, a lupus-like syndrome, myopathy, cholestatic jaundice and nephritis. Some cross-sensitivity occurs between carbimazole and propylthiouracil.
 Bone marrow suppression, especially agranulocytosis, is an important unwanted effect and is more common with propylthiouracil than with carbimazole. A severe sore throat with fever is often the presenting complaint, and the occurrence of this, or any other infection, should be immediately reported to a doctor. The onset of agranulocytosis is sudden, and probably immunologically mediated, so that routine blood counts are unhelpful for monitoring. The blood count usually recovers about 3 weeks after drug withdrawal.
Bone marrow suppression, especially agranulocytosis, is an important unwanted effect and is more common with propylthiouracil than with carbimazole. A severe sore throat with fever is often the presenting complaint, and the occurrence of this, or any other infection, should be immediately reported to a doctor. The onset of agranulocytosis is sudden, and probably immunologically mediated, so that routine blood counts are unhelpful for monitoring. The blood count usually recovers about 3 weeks after drug withdrawal.
 Placental transfer of the active metabolite of carbimazole can produce neonatal hypothyroidism, but propylthiouracil does not transfer in large enough quantities to cause problems. However, in Graves' disease the thyroid-stimulating antibody crosses the placenta and causes fetal thyrotoxicosis; therefore, carbimazole is the treatment of choice for maternal Graves' disease. Carbimazole is secreted in breast milk but rarely produces hypothyroidism in the infant.
Placental transfer of the active metabolite of carbimazole can produce neonatal hypothyroidism, but propylthiouracil does not transfer in large enough quantities to cause problems. However, in Graves' disease the thyroid-stimulating antibody crosses the placenta and causes fetal thyrotoxicosis; therefore, carbimazole is the treatment of choice for maternal Graves' disease. Carbimazole is secreted in breast milk but rarely produces hypothyroidism in the infant.
Management of hyperthyroidism
Carbimazole is the drug of choice for Graves' hyperthyroidism, and will usually decrease the thyroid hormone concentration to normal levels over 4–8 weeks. It is usual to start treatment with a high dosage unless the thyrotoxicosis is mild, when smaller initial doses may be more appropriate. Once the thyroid hormone concentration is normal, the dosage is then gradually reduced every 4–6 weeks to reach the lowest possible dose that controls the serum T4. Initially treatment should be continued for 12–18 months, after which the dose can be tapered or treatment withdrawn. Occasionally, a block–replace regimen is used, giving a high dosage of carbimazole in conjunction with thyroxine replacement for 6–12 months. This maintains normal thyroid function regardless of the dose of carbimazole.
A β-adrenoceptor antagonist (especially propranolol because of its non-selective action; Ch. 5) is particularly useful for symptomatic relief from tremor, anxiety or palpitation during the early period of treatment with carbimazole. It has immediate effects on symptoms but does not alter the rate of thyroid hormone synthesis or secretion.
Exophthalmos associated with Graves' disease usually responds poorly to treatment with antithyroid drugs, since it is caused by TSH receptor antibody. Severe thyroid eye disease can be helped by treatment with oral prednisolone if antithyroid treatment is not improving the condition.
Approximately 50% of people with Graves' disease have a single episode that is cured by drug treatment (spontaneous remission). Those who relapse will usually do so within 6 months, and thereafter repeat relapses are common. Most are then offered definitive treatment by either a subtotal thyroidectomy (for a large goitre) or a therapeutic dose of radioactive iodine (131I).
Radioiodine can be used as first-line treatment for Graves' disease or for relapse after antithyroid drug treatment. Radioiodine can make thyroid ophthalmopathy worse, but this can be prevented by treatment with a corticosteroid such as prednisolone for 2–3 months. Before radioiodine treatment it is often recommended that the thyrotoxicosis should be stabilised with carbimazole. This reduces the risk of exacerbation of hyperthyroidism from radiation thyroiditis immediately after isotope treatment. However, antithyroid drug treatment must be stopped 3–5 days before radioactive iodine is given or it will prevent uptake of the radioiodine by the thyroid cells. A β-adrenoceptor antagonist can be useful in this period to prevent symptomatic relapse. Carbimazole can be restarted 2–4 days after radioiodine, to cover the period of up to 8 weeks before radioiodine is fully effective. Between 10 and 20% of individuals will require a second dose of radioiodine to achieve euthyroid status. Permanent hypothyroidism can occur following radioiodine treatment. The incidence of hypothyroidism is related to the initial dose of radioactivity up to 1 year after treatment; thereafter, the risk is 2–3% annually. The theoretical increase in risk of cancer or leukaemia following radioiodine treatment has not been substantiated in clinical studies.
Surgery in Graves' disease is used if there is a poor response to antithyroid drugs, a very large goitre, for coexisting thyroid malignancy or if the person expresses a preference for this treatment. Before surgery, carbimazole is usually used to achieve a euthyroid state. If the thyrotoxicosis is drug-resistant then oral potassium iodide can be used for up to 2 weeks before surgery to inhibit thyroxine synthesis and release and to reduce the vascularity of the hyperplastic thyroid gland. Hypothyroidism, often delayed by several months or years, is common after surgery.
Toxic nodular goitre
Radioiodine is also used for toxic multinodular goitre. A solitary toxic thyroid nodule can be removed surgically, but radioactive iodine is extremely effective, because the isotope is taken up only by the abnormal tissue (the remainder is suppressed by the absence of thyrotropin in the circulation). Carbimazole is unsuitable as sole treatment for these conditions, since spontaneous remission does not occur. However, some elderly people may choose to continue treatment with carbimazole for life.
Amiodarone-induced thyrotoxicosis
Treatment of amiodarone-induced thyrotoxicosis (Ch. 8) depends on the clinical subtype. Type 1 is provoked in people with an underlying multinodular goitre by the iodide contained in the drug, and responds to antithyroid drug treatment. Type 2 is an inflammatory thyrotoxicosis that arises from a direct toxic effect of the drug on the gland, and responds well to treatment with a corticosteroid.
Hypothyroidism
Hypothyroidism is usually caused by primary thyroid failure, and the low circulating T4 concentration is accompanied by a raised plasma thyrotropin (TSH) concentration. Autoimmune thyroiditis is the commonest cause, but hypothyroidism is occasionally congenital or can follow treatment for hyperthyroidism by surgery or radioiodine. Rarely, hypothyroidism can be secondary to pituitary or hypothalamic failure, when the circulating TSH concentrations will be low. Drug therapy with lithium (Ch. 22) or amiodarone (Ch. 8) can produce hypothyroidism.
Typical symptoms of hypothyroidism in an adult are non-specific and include lethargy, slowing of mental processes, cold intolerance, dry skin, hoarseness, weight gain, constipation and menorrhagia. Severe hypothyroidism (myxoedema) produces marked coarsening of the facial appearance and may ultimately lead to a hypothermic, comatose state. In children, hypothyroidism stunts mental and physical development, resulting in a condition known as cretinism.
Management of hypothyroidism
Standard treatment is with oral levothyroxine (T4). Although its absorption is incomplete and variable, sufficient T3 will be formed by peripheral deiodination of T4. The proportion of circulating T3 is usually lower than normal, so circulating levels of T4 will often need to be higher than those in healthy individuals in order to obtain a satisfactory response. In some people, particularly those with ischaemic heart disease, a rapid increase in metabolic activity with levothyroxine replacement can cause excessive cardiac stimulation, and therefore levothyroxine should be introduced gradually in those at risk of cardiac complications. In others, the anticipated weight-related maintenance dose can be given from the start. Because of its long half-life, steady-state plasma concentrations of levothyroxine will not be achieved with constant dosage for 4–5 weeks. The adequacy of levothyroxine replacement therapy is best monitored by measurement of the serum TSH concentration 4–6 weeks after a change in levothyroxine dose. The TSH concentration should be in the lower third of the normal range, and then the plasma T4 will usually be slightly high or in the upper part of the normal range. Once the dose of levothyroxine is correct an annual check of serum TSH is sufficient, unless there are symptoms suggesting hypo- or hyperthyroidism. When the hypothyroidism is caused by drug treatment the precipitating drug can be continued while levothyroxine is given. Problems with thyroid-replacement preparations are uncommon unless excessive doses are used, but allergic reactions have been reported, and transient scalp hair loss can occur in the first few weeks of treatment.
Some drugs interfere with the absorption of levothyroxine from the gut. These include iron, calcium carbonate, mineral supplements, colestyramine (Ch. 48) and sucralfate (Ch. 33). The metabolism of levothyroxine is accelerated by the concurrent use of the hepatic enzyme-inducing drugs phenobarbital, phenytoin, carbamazepine (Ch. 23) and rifampicin (Ch. 51). The therapeutic response to levothyroxine may be impaired in all of these situations.
Liothyronine (T3) is usually reserved for intravenous use in severe hypothyroidism (myxoedema coma), when its potency, more rapid effect and shorter half-life allow more rapid attainment of a therapeutic blood concentration. However, even in this situation a large dose of levothyroxine has been successfully used, and may be associated with a lower mortality. An oral formulation of liothyronine is also available for rapid response in severe hypothyroid states.
True/false questions
1. Secretion of triiodothyronine (T3) and thyroxine (T4) is controlled by anterior pituitary and hypothalamic hormones.
2. Circulating T3 and T4 are highly bound to plasma albumin.
3. T4 has a long residence time in the body.
4. At target cells, T4 is converted to T3, which then binds to specific nuclear receptors.
5. Hyperthyroidism will be made worse by iodine administration.
6. Severe hypothyroidism causes cretinism in children.
7. Therapy with 131I can cause hypothyroidism.
8. Propylthiouracil is the drug of choice for Graves' disease.
One-best-answer (OBA) questions
1. Choose the incorrect statement below about the treatment of thyrotoxicosis with carbimazole.
A Carbimazole takes several weeks to reduce circulating T4 and T3 to normal concentrations.
C Carbimazole may cause bone marrow suppression.
D Carbimazole inhibits the stimulant action of thyrotropin on the thyroid.
2. Choose the correct statement below about hypothyroidism and its treatment.
A Low circulating T4 levels in hypothyroidism are accompanied by low levels of thyrotropin.
B Hepatic enzyme-inducing drugs reduce the response to levothyroxine.
C During regular dosing, steady-state plasma levels of levothyroxine are reached within 7 days.
D No precautions are required when prescribing levothyroxine in a person with hypothyroidism who also has ischaemic heart disease.
E Oxygen consumption in metabolically active tissues is unaffected by levothyroxine.
Case-based questions
A 45-year-old man suffered from weight loss, palpitations, tremor, anxiety and sweating, plus eyelid retraction and orbital and ocular inflammation. Blood tests showed increased levels of free and bound T3 and T4 and suppressed TSH. A diagnosis of Graves' thyrotoxicosis was made. An electrocardiogram showed atrial fibrillation.
B What other blood tests could be performed to confirm this diagnosis?
C How could the symptoms be controlled?
D What drug could be given to control the hyperthyroidism?
E With treatment, he became euthyroid, but relapsed in the following year. A decision was made to treat him with 131I.
What treatment should be given before administering the 131I and what are the reasons for this?
1. True. T3 and T4 synthesis and release are controlled by thyrotropin-releasing hormone (TRH) and somatostatin from the hypothalamus, by thyrotropin (thyrotropin-stimulating hormone, TSH) from the anterior pituitary, and by plasma iodide.
2. False. T3 and T4 in the plasma are largely bound to thyroxine-binding globulin (TBG), which should not be confused with thyroglobulin in the thyroid gland.
3. True. Thyroxine has a half-life of about 7 days.
4. True. The complex of T3 and thyroid hormone receptor (TR) activates gene transcription and protein synthesis.
5. False. Iodine is converted to iodide and inhibits T3 and T4 release.
6. True. Severe hypothyroidism causes myxoedema in adults and cretinism in children.
7. True. Permanent hypothyroidism can occur after radioiodine treatment.
8. False. Propylthiouracil is usually reserved for people intolerant to carbimazole.
9. True. Oral levothyroxine is the standard treatment.
10. True. Liothyronine (T3) is more potent and has a more rapid effect than levothyroxine, so is given intravenously in severe hypothyroidism (myxoedema coma)
OBA answers
1. Answer D is the incorrect statement.
A Correct. The long half-life of T4 (about 7 days) means that carbimazole takes 5–6 weeks to reduce thyroid hormone levels to normal.
B Correct. The active metabolite of carbimazole is methimazole.
C Correct. Bone marrow suppression is a serious unwanted effect of carbimazole; it can be indicated by infection, especially fever and a severe sore throat.
D Incorrect. Carbimazole inhibits the liberation of iodine by thyroid peroxidase.
E Correct. Carbimazole accumulates in the thyroid and is given only once daily.
A Incorrect. If T4 levels were low, then thyrotropin levels would be raised, as the negative-feedback effect of T4 on thyrotropin release would be reduced.
B Correct. Hepatic metabolism of levothyroxine is accelerated by cytochrome P450 inducers such as phenobarbitone and phenytoin.
C Incorrect. The half-life of levothyroxine is 6–7 days; therefore, the steady state would not be reached until 5–7 weeks of administration.
D Incorrect. Special care is required in ischaemic heart disease as a rapid increase in metabolic activity can cause excessive heart stimulation.
E Incorrect. Levothyroxine stimulates oxygen consumption in metabolically active tissues.
Case-based answers
A Graves' disease is an autoimmune disease in which antibodies to TSH are generated which bind to and activate TSH receptors in the thyroid, promoting thyroid hormone release.
B Thyrotropin (TSH) concentration could be measured. It will be low, due to the negative feedback effect of elevated T3 and T4.
C Drugs of choice for controlling symptoms are β-adrenoceptor antagonists, although they do not improve fatigue and muscle weakness. Propranolol should also control a high ventricular rate due to the atrial fibrillation (Ch. 8) and anticoagulation with warfarin to prevent thromboembolism, which has an increased incidence in people with both atrial fibrillation and thyrotoxicosis.
D Carbimazole is the drug of choice, given in a high dose, reducing over 4–6 weeks.
E The clinical state should be stabilised with carbimazole and a β-adrenoceptor antagonist. Carbimazole is stopped 3–4 days before radioiodine is given, as it can prevent the uptake of iodine by thyroid cells.
F It can take several months for the maximum benefit of 131I to occur.