PROTECT FROM INFECTION AND INJURY
The most important practice for preventing cross-infection is thorough hand washing of all individuals involved in the infant’s care. Other procedures to prevent infection include eye care, umbilical care, bathing, and care of the circumcision. Artificial and long fingernails are discouraged for those working in neonatal care because the former have been implicated in the transmission of Pseudomonas organisms (Moolenaar, Crutcher, San Joaquin, and others, 2000) and Klebsiella pneumoniae (Gupta, Della-Latta, Todd, and others, 2004). Vitamin K is administered to protect against hemorrhage. In addition, several safety measures are practiced, particularly in terms of proper identification, and screening tests are used to detect various disorders.
Identification
Proper identification of the newborn is absolutely essential. The nurse must verify that identifying bands are securely fastened and verify the information (name, gender, mother’s admission number, date, and time of birth) against the birth records and the child’s actual gender. This identification process should take place optimally in the delivery room. Some institutions use methods of infant identification such as a color photograph kept in the medical record, storage of blood for DNA genotyping, or electronic surveillance systems for infant security. Footprinting or fingerprinting alone is not currently recommended for newborn identification (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007); however, the National Center for Missing and Exploited Children (NCMEC) does recommend the use of footprints as a form of identification in addition to a cord blood sample, which is stored until the day after discharge. Electronic tags that give off a radio frequency may also be used to prevent newborn abductions. A tag is placed on the newborn and removed at the time of discharge by hospital personnel.
A proactive hospital emergency plan should be implemented to prevent infant abduction and to respond promptly and effectively in the event one happens. A mock newborn abduction drill is an effective method that can be used to evaluate staff competence and response to the incident (Shogan, 2002). All hospital personnel should be educated regarding newborn abduction, preventive aspects, and methods to identify the potential risk of such an occurrence.
The nurse needs to discuss safety issues with the mother the first time the infant is brought to her. The NCMEC* has reported that 55% of infant abductions occur in the mother’s room (Rabun, 2000). A written copy of the safety instructions should also be given to the parent. Parents are instructed to look at identification badges of nurses and hospital personnel who come to take infants and not to relinquish their infants to anyone without proper identification. Mothers are also advised not to leave the infant alone in the crib while they shower or use the bathroom; rather, they should ask to have the infant observed by a health care worker if a family member is not present in the room. Parents and staff are encouraged to use a password system when the newborn is taken from the room as a routine security measure (Carroll, 2000). The nurse should document in the chart that these instructions were given and that appropriate identification band checks are routinely made throughout each shift. Nursing staff are also educated regarding the “typical” abductor profile and to be constantly aware of visitors with unusual behavior.
The typical profile of an abductor is a female between the ages of 15 and 44 who is often overweight and has low self-esteem; she may be emotionally disturbed because of the loss of her own child or inability to conceive and may have a strained relationship with her husband or partner. The typical abductor may also be seen visiting the newborn nursery or neonatal intensive care unit area before the abduction and may ask questions about the care of or the health of a specific newborn. The abductor may familiarize herself with the hospital routine and may also impersonate a health care worker. Parents are made aware of the fact that infant safety measures must be implemented in the home as well. Measures to prevent and decrease infant abduction after discharge to the home include avoiding the publication of birth announcements in the local newspaper and avoiding using yard decorations to announce a newborn’s arrival (Shogan, 2002).
Eye Care
Prophylactic eye treatment against ophthalmia neonatorum, infectious conjunctivitis of the newborn, includes the use of (1) silver nitrate (1%) solution, (2) erythromycin (0.5%) ophthalmic ointment or drops, or (3) tetracycline (1%) ophthalmic ointment or drops (preferably in single-dose ampules or tubes) (see Nursing Care Guidelines box). Chlamydia trachomatis is the major cause of ophthalmia neonatorum in the United States. Silver nitrate is effective against gonococcal conjunctivitis.
Topical antibiotics such as tetracycline and erythromycin, silver nitrate, and a 2.5% povidone-iodine solution (currently unavailable in commercial form in the United States) have not proved to be effective in the treatment of chlamydial conjunctivitis.
A 14-day course of oral erythromycin or an oral sulfonamide may be given for chlamydial conjunctivitis (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007). Administration of oral erythromycin in infants younger than 6 weeks old has been associated with infantile hypertrophic pyloric stenosis; therefore parents should be informed of the potential risks and signs of the illness (American Academy of Pediatrics, 2006b). Herpes simplex virus may also cause neonatal conjunctivitis; treatment in such cases involves the use of topical and systemic antiviral medications.
Because studies on maternal attachment emphasize that in the first hour of life a newborn has a greater ability to focus on coordinated movement than at any other time during the next several days and because eye contact is very important in the development of maternal-infant bonding, the routine administration of silver nitrate or topical ophthalmic antibiotics can be postponed for up to 1 hour in the United States and up to 2 hours in Canada. However, a checklist should be used to ensure that the drug is given.
Vitamin K Administration
Shortly after birth, vitamin K is administered as a single intramuscular dose of 0.5 to 1 mg to prevent hemorrhagic disease of the newborn, also called vitamin K deficiency bleeding (VKDB). Normally, vitamin K is synthesized by the intestinal flora. However, because the infant’s intestine is sterile at birth and because breast milk contains low levels of vitamin K, the supply is inadequate for at least the first 3 or 4 days. The major function of vitamin K is to catalyze the synthesis of prothrombin in the liver, which is needed for blood clotting. The vastus lateralis muscle is the traditionally recommended injection site, but the ventrogluteal (not dorsogluteal) muscle can be used.
Several countries have noted a resurgence in later onset of VKDB after practicing orally administered prophylaxis (American Academy of Pediatrics, 2003a). Current recommendations are that vitamin K be given to all newborns as a single intramuscular dose of 0.5 to 1.0 mg (American Academy of Pediatrics, 2003a). Additional study is needed on the efficacy, safety, and bioavailability of oral preparations and on the most effective dosing regimens to prevent VKDB.
Hepatitis B Vaccine Administration
To decrease the incidence of hepatitis B virus in children and its serious consequences, cirrhosis and liver cancer, in adulthood, the first of three doses of hepatitis B vaccine is recommended soon after birth and before hospital discharge for all newborns in the United States; this first dose may also be given at the practitioner’s discretion at the first well-newborn visit if the mother is hepatitis B surface antigen (HBsAg) negative. The injection is given in the vastus lateralis muscle, since this site is associated with a better immune response than is the dorsogluteal area (a muscle typically not used in infants in the United States) (see also Immunizations, Chapter 10). Giving the infant concentrated oral sucrose can reduce the pain of the injection (Stevens, Yamada, and Ohlsson, 2004).
Preterm infants who weigh less than 2000 g (4.4 pounds) and are born to HBsAg-negative women may be vaccinated at a chronologic age of 1 month if medically stable or at hospital discharge, if discharged before the infant reaches 1 month chronologic age (American Academy of Pediatrics, 2007). Infants born to HBsAg-positive mothers should be immunized within 12 hours after birth with hepatitis B vaccine and hepatitis B immune globulin (HBIG) at separate sites, regardless of gestational age or birth weight; the birth dose in such infants should not be counted in the series of three hepatitis B vaccines, and the full three-dose series should be administered starting at age 1 month (American Academy of Pediatrics, 2006b). An infant who weighs less than 2000 g and whose mother’s HBsAg status is unknown should receive the hepatitis B vaccine within 12 hours of birth; maternal status should be determined readily and, if unavailable, the infant should receive the dose of HBIG as soon as possible but within 7 days of birth. In Canada, hepatitis B vaccine is given to the newborn only if the mother is HBsAg positive at birth (see Immunizations, Chapter 10).
Newborn Screening for Disease
A number of genetic disorders can be detected in the newborn period. There is no national policy for such detection in the United States; therefore the extent of neonatal screening is determined by state laws and voluntary guidelines. Most states require screening for phenylketonuria (PKU), congenital hypothyroidism, galactosemia, and hemoglobin defects such as sickle cell disease (see Chapters 9 and 26); screening for congenital hearing loss is recommended at the same time as disease screening. Because concern has been voiced regarding the inconsistency among states in screening for genetic disorders based on cost, population demographics, resource availability, and political environment, the Task Force on Newborn Screening was formed by the American Academy of Pediatrics and other federal health care agencies to address this issue. A number of resolutions and policies have been developed to better address the issue of newborn screening (see American Academy of Pediatrics, 2000b).
The advent of tandem mass spectrometry has expanded newborn screening to include detection of disorders of fatty acid oxidation, amino acids, and organic acids. This technology uses a minimum amount of blood and can identify more than 20 different disorders in 2 minutes (Bryant, Horns, Longo, and others, 2004). Tandem mass spectrometry has improved sensitivity and specificity for the detection of such conditions as PKU and has a lower rate of false-positive results than other standardized testing methods.
The nurse’s responsibility is to educate parents regarding the importance of screening and to collect appropriate specimens at the recommended time (after 24 hours of age). With early newborn discharge before 24 hours, adequate screening for PKU requires a follow-up test within 2 weeks (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007). Accurate screening depends on high-quality blood spots on approved filter paper forms. The blood should completely saturate the filter paper spot on one side only. The paper should not be handled, placed on wet surfaces, or contaminated with any substance. (See Atraumatic Care box.)
The American Academy of Pediatrics and American College of Obstetricians and Gynecologists (2007) also recommend routine prenatal and perinatal human immunodeficiency virus (HIV) counseling and testing for all pregnant women and their newborns. Benefits of early identification of HIV-infected infants are early antiretroviral therapy and aggressive nutritional supplementation; appropriate changes in their immunization schedule; monitoring and evaluation of immunologic, neurologic, and neuropsychologic functions for possible changes caused by antiretroviral therapy; initiation of interventions for special educational needs; evaluation for the need of other therapies, such as immunoglobulin for the prevention of bacterial infections; tuberculosis screening and treatment; and management of communicable disease exposures. In addition, vertical transmission of HIV from mother to newborn may be reduced to 2% with a cesarean section before the rupture of membranes and onset of labor (American Academy of Pediatrics and American College of Obstetricians and Gynecologists, 2007). As a result of virologic diagnostic techniques such as HIV culture, polymerase chain reaction, and immune complex—dissociated p24 antigen, diagnosis of HIV infection can be made in 30% to 50% of infants at birth and in 100% of infants by 4 to 6 months of age. For information on additional diseases that may be screened in the newborn period, see Newborn Screening Fact Sheets (Kaye and American Association of Pediatrics Committee on Genetics, 2006).
Universal Newborn Hearing Screening
Approximately 1 to 6 per 1000 newborns may have significant hearing loss, which may go undetected until later in life. Such deficits may lead to subsequent speech and language delays, which could be treated with early detection. The Joint Committee on Infant Hearing, American Academy of Pediatrics (2000c), recommends that all birthing hospitals establish programs to screen all newborn infants before discharge for hearing loss by automated auditory brainstem response or transient evoked otoacoustic emissions. Newborns who fail the initial screening require documentation and referral for further testing by 1 month of age; newborns who do not receive initial screening before discharge should also be tested by 1 month. It is estimated that screening by high-risk factors alone fails to identify approximately 50% of all newborns with a congenital hearing loss. Guidelines for screening infants and older children for hearing loss have been published by the American Academy of Pediatrics (2003b).
Because approximately 50% of all causes of childhood hearing loss are attributed to genetic causes, molecular genetic tests on blood in all newborns has been proposed to detect infants with late-onset or prelingual hearing loss; in such infants hearing loss may not be detected by standard screening in early infancy and would not otherwise be identified until later in life (Morton and Nance, 2006).
Bathing
Bath time is an opportunity for the nurse to accomplish much more than general hygiene. It is an excellent time for observing the infant’s behavior, state of arousal, alertness, and muscular activity. Bathing is usually performed after the vital signs have stabilized, especially the temperature.
With the possibility of transmission of viruses such as hepatitis B virus and HIV via maternal blood and blood-stained amniotic fluid, the traditional timing of the newborn’s bath has been questioned. The newborn must be considered a potential contamination source until proved otherwise. As part of standard precautions, nurses should wear gloves when handling the newborn until blood and amniotic fluid are removed by bathing.
Studies indicate that healthy full-term newborns with a stable body temperature can be bathed as early as 1 hour of age without experiencing problems, provided that effective thermoregulation measures are taken after the bath (Penny-MacGillivray, 1996; Behring, Vezeau, and Fink, 2003; Varda and Behnke, 2000; Medves and O’Brien, 2004). Nurses are cautioned, however, to avoid instituting routine newborn bathing according to a rigid schedule; nursing interventions such as bathing should instead be based on individualized assessment and family interaction needs.
The bath time provides an opportunity for the nurse to involve the parents in the care of their child, to teach correct hygiene procedures, and to learn about their infant’s individual characteristics (Fig. 8-12). The appropriate types of bathing supplies and the need for safety in terms of water temperature and supervision of the infant at all times during the bath are stressed.
Parents are encouraged to examine their infant closely during bathing. Frequently, normal variations such as Epstein pearls, mongolian spots, or ‘stork bites’ cause parents much worry because they are unaware of the significance of such findings. Minor birth injuries may appear as major defects to them. Explaining how these occurred and when they will disappear reassures parents of their infant’s normalcy. Common variations are discussed further in Chapter 9.
One of the most important considerations in skin cleansing is preservation of the skin’s acid mantle, which is formed from the uppermost horny layer of the epidermis; sweat; superficial fat; metabolic products; and external substances such as amniotic fluid, microorganisms, and chemicals. The infant’s skin surface has a pH of about 5 soon after birth, and the bacteriostatic effects of this pH are significant. Consequently, only plain warm water or soap with appropriate pH should be used for the bath. Alkaline soaps, oils, powder, and lotions are not used because they alter the acid mantle, thus providing a medium for bacterial growth. Talcum powder has the added risk of aspiration if it is applied too close to the infant’s face. A safer alternative is a cornstarch-based powder (see also Diaper Dermatitis, Chapter 30).
Parents should be involved in a discussion regarding the newborn’s bath at home. It is recommended that for the first 2 weeks the infant be bathed no more than two or three times per week with a plain warm sponge bath. This practice will help maintain the integrity of the newborn’s skin and allow time for the umbilical cord to completely dry. Routine daily soap bathing for newborns is no longer recommended (Association of Women’s Health, Obstetric and Neonatal Nursing, 2007; Darmstadt and Dinulos, 2000).
Care of the Umbilicus
Because the umbilical stump is an excellent medium for bacterial growth, various methods of cord care are practiced to prevent infection. Common methods include the use of an antimicrobial agent such as bacitracin or triple dye, although some experts advocate the use of alcohol alone, soap and water, sterile water, povidone-iodine, or no treatment (natural healing). The use of antiseptic agents has been shown to prolong cord drying and separation (Zupan, Garner, and Omari, 2004). Studies regarding bacterial growth and colonization according to the cleansing method used have produced varied results (Janssen, Selwood, Dobson, and others, 2003; Golombek, Brill, and Salice, 2002; Dore, Buchan, Coulas, and others, 1998). A Cochrane Review of 21 studies found no significant difference between cords treated with antiseptics compared with dry cord care or placebo; there were no reported systemic infections or deaths, and a trend toward reduced colonization was found in cords treated with antiseptics (Zupan, Garner, and Omari, 2004). Recommendations for cord care by the Association of Women’s Health, Obstetric and Neonatal Nursing (2007) include cleaning the cord initially with sterile water or a neutral pH cleanser, then subsequently cleaning the cord with water. A one-time application of triple dye has been shown to be superior to alcohol, povidone-iodine, or topical antibiotics in reducing colonization or infection; the use of alcohol is associated with prolonged cord drying and separation (McConnell, Lee, Couillard, and others, 2004).
Nurses working in neonatal care must carefully evaluate the available studies and compare the risks and benefits regarding the method of cord care within their own population of newborns and families. Regardless of the method used, nurses must include cord care teaching in the discharge planning because it has been demonstrated to be a concern for parents following discharge to the home.
The diaper is folded in front below the cord to avoid irritation and wetness on the site. The area is kept free of urine and stool and cleansed daily with the cleanser of choice. Parents are instructed regarding stump deterioration and proper umbilical care. The stump deteriorates through the process of dry gangrene. Cord separation time is influenced by a number of factors, including type of cord care, type of delivery, and other perinatal events. The average cord separation time is 10 to 14 days.
It takes a few more weeks for the cord base to heal completely after cord separation. During this time, care consists of keeping the base clean and dry and observing for any signs of infection.
Circumcision
Circumcision, the surgical removal of the foreskin on the glans penis, may be done in the hospital or in an outpatient clinic, although it is not a common practice in most countries. In the United States, however, circumcision rates have increased significantly over time: 61.1% of boys born in the United States from 1997 to 2000 were circumcised, compared with 48.3% of boys born from 1988 to 1991 (Nelson, Dunn, Wan, and others, 2005). Despite the frequency of the procedure in the United States, there is still much controversy regarding the benefits and risks (Box 8-4). The American Academy of Pediatrics (1999) issued a circumcision policy statement stating that the medical benefits of male newborn circumcision are not sufficiently significant to recommend it as a routine procedure; this policy was reaffirmed in 2005 (American Academy of Pediatrics, 2005a). The Academy’s statement emphasizes parental autonomy to determine what is in the best interest of their male newborn. The policy encourages the physician to ensure that parents have been given accurate and unbiased information about the risks, benefits, and alternatives before making an informed choice and that they understand that circumcision is an elective procedure. In addition to examining the medical benefits of male newborn circumcision, the American Academy of Pediatrics recommends that procedural analgesia be provided if parents decide to have their male infant circumcised.
Nurses are in a unique position to help educate parents regarding the care of their newborns, and they must take responsibility for ensuring that each parent has accurate and unbiased information with which to make an informed decision regarding the appropriateness of the circumcision procedure for their newborn. Parents need to know the options for pain control, especially the choice of topical or injected anesthesia, and their option of observing the procedure. Nurses should also be proactive in advocating for circumcision analgesia.
The nurse should also use every nonpharmacologic intervention that can reduce the pain of this operative procedure (see Atraumatic Care box). Despite adequate scientific evidence that newborns feel and respond to pain, circumcisions are still being performed in the United States with either insufficient analgesia or no analgesia at all. Nurses can use the American Academy of Pediatrics’ policy statement to advocate more effectively for the use of optimal pain relief for circumcision.
Four types of anesthesia and analgesia are used in newborns undergoing circumcision: ring block, dorsal penile nerve block (DPNB), topical anesthetic such as EMLA (prilocaine-lidocaine) or LMX4 (4% lidocaine), and concentrated oral sucrose. Oral acetaminophen and comfort measures such as music, sucking on a pacifier, and soothing voices have not proved to be effective in reducing the pain of circumcision when used alone (Williamson, 1997); however, these may be used in addition to analgesia and anesthesia to decrease procedural pain.
The Cochrane group exploring pain relief for neonatal circumcision (Brady-Fryer, Wiebe, and Lander, 2007) found that DPNB was the most effective intervention for decreasing pain of circumcision. Studies exploring the use of several strategies concurrently, such as that conducted by Razmus, Dalton, and Wilson (2004), which included groups receiving both sucrose and ring block compared with ring block alone, have the most potential to clarify optimum strategies.
Circumcision should not be performed immediately after delivery because of the neonate’s unstabilized physiologic status and increased susceptibility to stress. Preoperative nursing care usually includes allowing the infant nothing by mouth before the procedure to prevent aspiration of vomitus (about 2 hours); however, the necessity of this practice has been questioned (Kraft, 2003). Additional measures include checking for a signed consent form and adequately restraining the infant, usually on a special board (Fig. 8-13) or physiologic circumcision restraint chair. All of the equipment used for the procedure, such as gloves, instruments, dressings, and draping towels, must be sterile.
The procedure involves freeing the foreskin from the glans penis by using a scalpel, Gomco or Mogen clamp (see Cultural Awareness box), or Plastibell. In the Gomco technique the foreskin is clamped, cut with a scalpel, and removed; the clamp crushes the nerve endings and blood vessels, promoting hemostasis. In the Plastibell procedure the foreskin is removed using a plastic ring and a string tied around the foreskin like a tourniquet. The excess foreskin is trimmed. In about 5 to 8 days the plastic ring separates and falls off.
After the procedure is completed, the infant is released from the restraints and comforted. If the parents were not present during the procedure, they are informed of the infant’s status and reunited with their son.
Care of the circumcised penis depends on the type of procedure performed. If a clamp (Gomco or Mogen) was used, a petrolatum gauze dressing may be applied loosely to prevent adherence to the diaper. If the Plastibell was applied, no special dressing is required. Because the area is tender, the diaper is applied loosely to prevent friction against the penis. The penis is evaluated for excessive bleeding in the first few hours after the procedure, and the first void is recorded.
Normally, on the second day a yellowish white exudate forms as part of the granulation process. This is not a sign of infection and is not forcibly removed. As healing progresses, the exudate disappears. Parents are educated to report any evidence of bleeding, unusual swelling, or absence of voiding to the practitioner.
PROVIDE OPTIMAL NUTRITION
Selection of a feeding method is one of the major decisions faced by parents. In general, there are three acceptable choices: human milk, commercially prepared whole cow’s milk formula, and modified evaporated cow’s milk. There are significant nutritional, economic, and psychologic advantages and differences among these methods. Nurses should be at the forefront in providing the parent(s) with accurate and unbiased information needed to make a conscientious informed decision regarding the feeding method.
Human Milk
Human milk is the best option for infant nutrition up to 1 year of age. Breast milk consists of a number of micronutrients that are called bioavailable, meaning these nutrients are available in quantities and qualities that make them easily digestible by the newborn’s intestine and absorbed for energy and growth. Breast milk offers a variety of immunologic properties that are found exclusively in human milk. Human milk has been shown to be effective in protecting the newborn against respiratory tract infections, gastrointestinal infections caused by enterococci, otitis media, numerous allergies, type 2 diabetes, and atopy.
The fat content of human milk is composed of lipids, triglycerides, and cholesterol; cholesterol is an essential element for brain growth. The function of these lipids is to allow optimal intestinal absorption of essential fatty acids and polyunsaturated fatty acids (PUFAs). Furthermore, lipids contribute approximately 50% of the total calories in human milk (Lawrence and Lawrence, 2005). Although the overall fat content in human milk is higher than in cow’s milk, it is used more efficiently by the infant.
The primary source of carbohydrate in human milk is lactose, which is present in higher concentrations (6.8 g/dl) than in cow’s milk–based formula (4.9 g/dl). The carbohydrates not only serve as a large portion of total calories in human milk, but they also have protective functions; the oligosaccharides in human milk stimulate the growth of Lactobacillus bifidus and prevent bacteria from adhering to epithelial surfaces. Additional carbohydrates found in human milk include glucose, galactose, and glucosamine.
Human milk also contains two proteins, whey (lactalbumin) and casein (curd), in a ratio of approximately 60:40 (vs 80:20 in most cow’s milk–based formula). This ratio in human milk makes it more digestible and produces the soft stools seen in breast-fed infants. Thus human milk has a laxative effect, and constipation is uncommon. The whey protein, lactoferrin, in human milk has iron-binding characteristics with bacteriostatic capabilities, particularly against gram-positive and gram-negative aerobes, anaerobes, and yeasts (Lawrence and Lawrence, 2005).
Lysozyme is found in large quantities in human milk and has bacteriostatic functions against gram-positive bacteria and Enterobacteriaceae. Human milk also contains numerous other host defense factors such as macrophages, granulocytes, and T and B lymphocytes. Casein in human milk greatly enhances the absorption of iron, thus preventing iron-dependent bacteria from proliferating in the gastrointestinal tract (Biancuzzo, 2003). Secretory IgA is found in high levels in colostrum, but levels gradually decline over the first 14 days of life. Secretory IgA prevents bacteria and viruses from invading the intestinal mucosa in breast-fed newborns, thus protecting from infection (Hanson and Korotkova, 2002). The whey protein is also believed to play an important role in preventing the development of certain allergies.
Several digestive enzymes also present in human milk include amylases, lipases, proteases, and ribonucleases, which enhance the digestion and absorption of various nutrients. The amounts of lipid- and water-soluble vitamins, electrolytes, minerals, and trace elements in human milk are sufficient for growth, development, and energy needs during the first 6 months of life. The one possible exception is vitamin D, which is found in varying amounts depending on the mother’s intake of vitamin D—fortified food and exposure to ultraviolet light. Therefore, to prevent vitamin-D—deficiency rickets, the American Academy of Pediatrics (2003d) now recommends that infants who are exclusively breast-fed or who are ingesting less than 500 ml/day of vitamin D—fortified formula be supplemented with 200 IU vitamin D (oral) per day.
Additional beneficial components of human milk include prostaglandins; epidermal growth factor; desoxyhexanoic acid (DHA); arachidonic acid (AA); taurine; cystine; carnitine; cytokine; interleukins; and natural hormones such as thyroid-releasing hormone, gonadotropin-releasing hormone, and prolactin. Studies have demonstrated that breastfeeding is associated with a decrease in the incidence of type 2 diabetes (Kue Young, Chateau, and Zhang, 2002; Young, Martens, Taback, and others, 2002), a decrease in the incidence of hospital admissions for respiratory tract illnesses in generally healthy infants (Bachrach, Schwarz, and Bachrach, 2003), and higher intelligence scores compared with cow’s milk–based formula—fed infants (Anderson, Johnston, and Remley, 1999). Some studies have demonstrated that breastfeeding has an analgesic effect on newborns during painful procedures such as heel puncture (Carbajal, Veerapen, Couderc, and others, 2003; Gray, Miller, Phillips, and others, 2002; Shah, Aliwalas, and Shah, 2006).
Breastfeeding
Human milk is the preferred form of nutrition for all infants. Healthy People 2010 has a goal to increase breastfeeding rates in the United States to 75% in early postpartum and to 50% for mothers who continue to breastfeed for at least 6 months (US Department of Health and Human Services, 2000). A survey by Ross Products (Ryan, Zhou, and Gaston, 2004) indicates that 70.1% of women breastfeed in early postpartum, and 33.2% continued to breastfeed for 6 months. Some have voiced concern that early discharge of new mothers from hospitals, more aggressive marketing of infant formulas to the public, and more employed mothers contributed to the decline of breastfeeding in the 1990s. In addition, some hospital practices intended to provide optimal maternal-newborn health may instead undermine breastfeeding. Early separation of mother and newborn, delays in initiating breastfeeding, provision of formula in the hospital and in discharge packs, conflicting information by health care workers, and formula coupons given at discharge have been implicated in the decline of breastfeeding after discharge. Rooming-in has correlated positively with successful breastfeeding, whereas the use of a pacifier has sometimes been associated with earlier weaning from breast to bottle.
A survey of breastfeeding mothers indicated that the determining factors for changing to bottle feeding included the mother’s perception of the father’s attitude toward breastfeeding and the mother’s uncertainty regarding the amount of milk the infant would receive (Arora, McJunkin, Wehrer, and others, 2000). These findings have important implications for involving fathers in education and discussion regarding breastfeeding before and during the pregnancy. Fathers may express concerns of feeling left out during the newborn period if they have little involvement other than diapering and holding the infant. Encouraging fathers regarding their positive role in supporting the mother in breastfeeding may enhance the mother-infant interaction and decrease her sense of helplessness and isolation.
The American Academy of Pediatrics (2005b) has reaffirmed its position exclusively recommending breastfeeding until at least 1 year of age as the best form of infant nutrition. The Academy also supports programs that enable women to continue breastfeeding after returning to work. In its support of breastfeeding practices, the Academy further discourages the advertisement of infant formula to breastfeeding mothers and distribution of formula discharge packs without the advice of a health care provider.
The Baby-Friendly Hospital Initiative (BFHI) is a joint effort of the World Health Organization and the United Nations Children’s Fund to encourage, promote, and support breastfeeding as the model for optimum infant nutrition. Ten research-supported practices were developed by BFHI as a guideline for maternity facilities worldwide to promote breastfeeding (Wright, Rice, and Wells, 1996; Kyenkya-Isabirye, 1992) (Box 8-5). Research indicates that Baby-Friendly designated hospitals in the United States have higher rates for breastfeeding initiation and exclusivity than hospitals that are not Baby-Friendly designates (Merewood, Mehta, Chamberlain, and others, 2005).
In addition to the physiologic qualities of human milk, the most outstanding psychologic benefit of breastfeeding is the close maternal-child relationship. The infant is nestled close to the mother’s skin, can hear the rhythm of her heartbeat, can feel the warmth of her body, and has a sense of peaceful security. The mother has a close feeling of union with her child and feels a sense of accomplishment and satisfaction as the infant sucks milk from her.
Human milk is the most economical form of feeding. It is always available, ready to serve at room temperature, and free of contamination. Although human milk is not sterile, healthy full-term infants can tolerate varying amounts of nonpathogenic and pathogenic organisms. The protection against infection can provide additional cost savings in terms of fewer medical visits and less time lost from work for the employed mother.
Breast-fed infants, especially beyond 2 to 3 months of age, tend to grow at a satisfactory but slower rate than bottle-fed infants.
Contraindications to breastfeeding include (Lawrence and Lawrence, 2005; American Academy of Pediatrics, 2005b):
 Maternal chemotherapy-antimetabolites and certain antineoplastic drugs
Maternal chemotherapy-antimetabolites and certain antineoplastic drugs
 Active tuberculosis not under treatment in mother
Active tuberculosis not under treatment in mother
 Maternal herpes simplex lesion on a breast
Maternal herpes simplex lesion on a breast
 Cytomegalovirus (CMV)—primary risk for preterm infants receiving CMV-infected donor milk, not for infected mother’s infant (who already has CMV)
Cytomegalovirus (CMV)—primary risk for preterm infants receiving CMV-infected donor milk, not for infected mother’s infant (who already has CMV)
 Maternal substance abuse (e.g., cocaine, methamphetamine, and marijuana) (Note: Maternal methadone treatment for substance abuse is not a contraindication to breastfeeding.)
Maternal substance abuse (e.g., cocaine, methamphetamine, and marijuana) (Note: Maternal methadone treatment for substance abuse is not a contraindication to breastfeeding.)
 Human T-cell leukemia virus types I and II
Human T-cell leukemia virus types I and II
 Mothers who are receiving diagnostic or radioactive isotopes or who have had exposure to radioactive materials (for as long as there is radioactivity in milk)
Mothers who are receiving diagnostic or radioactive isotopes or who have had exposure to radioactive materials (for as long as there is radioactivity in milk)
Mastitis is usually not a contraindication if the discomfort is tolerable.
A small number of medications are contraindicated for breastfeeding mothers. Consult a reference text such as Hale (2006). Some herbal products are presented as safe and effective alternatives to prescription or over-the-counter medications; certain herbal agents, called galactogogues, are reported to increase breast milk production. There is insufficient data to confirm or deny the assertion of increased milk production using galactogogues, and mothers are cautioned to seek advice from a practitioner to ensure that the herbal preparations will not harm the breastfeeding infant (Conover and Buehler, 2004).
Breastfeeding can be done with twins and other multiples. If both twins are full term, they can begin feeding immediately after birth (Fig. 8-14); late preterm (near-term) infants should be evaluated individually but may be put to breast if stable. Simultaneous feeding promotes the rapid production of milk needed for both infants and makes the milk that would normally be lost in the letdown reflex available to one of the twins. When only one infant is hungry, the mother should feed singly. She should also alternate breasts when feeding each infant and avoid favoring one breast for one infant. The suckling patterns of infants vary, and each infant needs the visual stimulation and exercise that alternating breasts provides.
A concern many mothers have is the perceived inconvenience of loss of freedom and independence. Being committed to feeding the infant every 2 to 3 hours can be overwhelming, especially to women with multiple responsibilities. Many women resume their careers shortly after their pregnancy and prefer to bottle-feed. Combining breastfeeding and employment is possible, and suggestions for the mother are discussed in Chapter 10. Although breastfeeding is the preferred form of infant feeding, mothers’ decisions regarding their preferences must be supported and respected.
Successful breastfeeding probably depends more on the mother’s desire to breastfeed, satisfaction with breastfeeding, and available support systems than on any other factors. Mothers need support, encouragement, and assistance during their postpartum hospital stay and at home to enhance their opportunities for success and satisfaction.
Three main criteria have been proposed as essential in promoting positive breastfeeding: absence of a rigid feeding schedule, correct positioning of the infant at the breast to achieve latch-on, and correct suckling technique. Correct suckling for breastfeeding is defined as a wide-open mouth, tongue under the areola, and expression of milk by effective alveolar compression (Fig. 8-15).
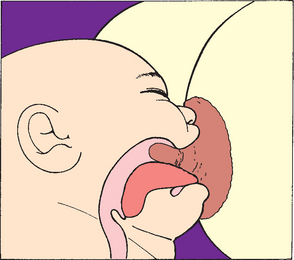
FIG. 8-15 The tongue is under the areola, with the tip of the nipple at the back of the wide-open mouth.
The following interventions promote breastfeeding:
• Frequent and early breastfeeding, especially during the first hour of life; immediate skin-to-skin contact; rooming-in; and feeding on demand
• Direct modeling of the importance of breastfeeding by health care providers, such as implementing demand nursing with no formula supplementation and decreased emphasis on infant formula products
• Increased information and support to mothers following discharge, especially phone follow-up
• Early breast pumping every 2 to 3 hours for 10 to 15 minutes bilaterally if the newborn is unable to nurse immediately (increases oxytocin production and thus milk production)
Nurses play a significant role in the breastfeeding decision and must make themselves available to families for guidance and support. Several excellent books and organizations, such as LaLeche League International,* are available as resources for professionals and breastfeeding mothers.
Bottle Feeding
Bottle feeding generally refers to the use of bottles for feeding commercial or evaporated milk formula rather than using the breast, although human milk may be expressed and fed with a bottle when necessary. Bottle feeding is an acceptable method of feeding. Nurses should not assume that new parents automatically know how to bottle feed their infant. Parents who choose bottle feeding also need support and assistance in meeting their infant’s needs.
Providing newborns with nutrition is only one aspect of the feeding. Holding them close to the body while rocking or cuddling them helps to ensure the emotional component of feeding. Like breast-fed infants, bottle-fed infants need to be held on alternate sides of the lap to expose them to different stimuli. The feeding should not be hurried. Even though they may suck vigorously for the first 5 minutes and seem to be satisfied, they should be allowed to continue sucking. Infants need at least 2 hours of sucking a day. If there are six feedings per day, then about 20 minutes of sucking at each feeding provides for oral gratification.
Propping the bottle is discouraged for the following reasons:
• It denies the infant the important component of close human contact.
• The infant may aspirate formula into the trachea and lungs while sleeping.
• It may facilitate the development of middle ear infections. If the infant lies flat and sucks, milk that has pooled in the pharynx becomes a suitable medium for bacterial growth. Bacteria then enters the eustachian tube, which leads to the middle ear, causing acute otitis media.
• It encourages continuous pooling of formula in the mouth, which can lead to nursing caries when the teeth erupt (see Chapter 12).
Commercially Prepared Formulas
The analysis of human and whole cow’s milk indicates that the latter is unsuitable for infant nutrition. Whole cow’s milk has a high protein content and low fat and lipid content, and there is evidence that it may cause intestinal bleeding and lead to iron deficiency anemia in infants. Questions have also been raised regarding the unmodified protein content of whole cow’s milk, which may trigger an undesired immune response and thus increase the incidence of allergies in children at an early age.
Commercially prepared formulas are cow’s milk based and have been modified to resemble the nutritional content of human milk. These formulas are altered from cow’s milk by removing butterfat, decreasing the protein content, and adding vegetable oil and carbohydrate. Some cow’s milk–based formulas have demineralized whey added to yield a whey/casein ratio of 60:40. The standard cow’s milk–based formulas, regardless of the commercial brand, have essentially the same compositions of vitamins, minerals, protein, carbohydrates, and essential amino acids, with minor variations such as the source of carbohydrate; nucleotides to enhance immune function; and long-chain polyunsaturated fatty acids (LCPUFAs), DHA and arachidonic acid (AA), which are thought to improve brain function (Georgieff, 2001). DHA and AA are both found in large quantities in human milk but until recently were not present in most infant formulas. Studies in full-term infants receiving supplements with DHA and AA have produced mixed results regarding brain function and visual acuity. Many studies report a variety of sources for LCPUFAs, including egg yolk lipid, phospholipids, and triglyceride. The evidence for supplementation of formula for preterm infants with LCPUFAs, however, has been more convincing, producing some transient improvement in visual acuity and general development (American Academy of Pediatrics, 2004b). There do not appear to be any adverse effects associated with LCPUFA supplementation in preterm infants with respect to the incidence of bronchopulmonary disease, necrotizing enterocolitis, or other conditions of prematurity (American Academy of Pediatrics, 2004b). The Food and Drug Administration (FDA) regulates the manufacture of infant formula in the United States to ensure product safety. Standard cow’s milk–based formulas are sold as low iron and iron fortified; however, only the iron-fortified formulas meet the requirements of infants (American Academy of Pediatrics, 2004b).
There are four main categories of commercially prepared infant formulas: (1) cow’s milk–based formulas, available in 20 kcal/floz as liquid (ready to feed), as powder (requires reconstitution with water), or as a concentrated liquid (requires dilution with water); (2) soy-based formulas, available commercially in ready-to-feed 20 kcal/floz powder and concentrated liquid forms, commonly used for children who are lactose or cow’s milk protein intolerant; (3) casein- or whey-hydrolysate formulas, commercially available in ready-to-feed and powder forms and used primarily for children who cannot tolerate or digest cow’s milk– or soy-based formulas; and (4) amino acid formulas.
The American Academy of Pediatrics (2004b) recommends the use of soy protein–based formulas for infants with galactosemia, hereditary lactase deficiency, documented IgE allergies caused by cow’s milk, and documented evidence of lactose intolerance. Soy protein—based formulas, however, have not been proved to be effective in preventing colic or allergy in healthy or high-risk infants (American Academy of Pediatrics, 2004b). Soy-based formulas, however, are not hypoallergenic, and a small percentage of the population may also be allergic to soy. Some researchers have speculated that exclusive use of soy formula in infants may adversely affect their endocrine, reproductive, and immune systems. This concern is related to isoflavones in soy and possible alteration in sexual maturity, immune response, and thyroid function (Greim, 2004; Chen and Rogan, 2004). Others report no long-term untoward effects from the ingestion of isoflavones in soy formula (Merritt and Jenks, 2004; Giampietro, Bruno, Furcolo, and others, 2004).
The casein- or whey-hydrolysate formulas are considered to be less antigenic than either cow’s milk–based or soy-based formulas. The protein hydrolysate formulas (casein and whey) are derived from cow’s milk–based formulas by a process of heat, filtration, and enzyme treatment designed to break the peptide chains into more digestible and hypoallergenic proteins. The hydrolysate formulas have the disadvantage of tasting bad; however, these may be made more palatable by adding a hypoallergenic flavoring such as Vari-Flavor. To reduce the incidence of cow’s milk allergy and atopic dermatitis in infants at high risk for these conditions, use of hydrolyzed formula must be combined with strict avoidance of solid food during the first 4 to 6 months of life (Host and Halken, 2004).
Neocate and EleCare are extensively hydrolyzed amino acid formulas, designed for infants who are sensitive to cow’s milk–based, soy-based, and partially hydrolyzed casein- and whey-based formulas. Both products are available in powder form. A wide variety of formulas are manufactured for infants and children with special needs; it is not within the scope of this text to discuss each one, but a formula company representative can provide product books that describe the purpose and content of each formula.
Follow-up formulas are marketed as a transitional formula for infants older than 6 months who are also eating solid foods. These generally contain a higher percentage of calories from protein and carbohydrate sources, a higher amount of iron and vitamins, and a lower amount of fat than standard cow’s milk–based formulas. Many nutrition experts and the Academy of Pediatrics (2004b), however, dispute the necessity of follow-up formulas if the infant is receiving an adequate amount of solid foods containing sufficient iron, vitamins, and minerals.
Preparation of Formula
Persons preparing infant formula wash their hands well and then wash all of the equipment used to prepare the formula with soap and water. Sterilizing bottles and nipples 5 minutes in boiling water may be required in certain situations where a hot-water dishwasher is not available. It is generally recommended that the tap water used to reconstitute powdered infant formula or to dilute concentrated liquid be brought to a rolling boil for 1 minute, then allowed to cool before use (American Academy of Pediatrics, 2004b). Using warm or hot tap water or placing the prepared formula bottle in the microwave is not adequate for preventing bacterial growth. Following the manufacturer’s instructions for preparing the formula is essential to ensure the infant receives adequate calories and fluid for adequate growth. Parents are cautioned not to alter the reconstitution or dilution of infant formula except under the specific directions of the primary practitioner. Powdered formula and concentrated formula are prepared and bottled immediately before each feeding. Warming the formula is optional, although many parents prefer to warm it before feeding. Any milk remaining in the bottle after the feeding is discarded because it is an excellent medium for bacterial growth. Opened cans of ready-to-feed or concentrated formula are covered and refrigerated immediately until the next feeding. Bottled water should not be considered sterile unless otherwise indicated; bottled water without fluoride should be avoided for mixing infant formula (Morin, 2007). Because of incidents involving contamination of powdered formula with Enterobacter sakazakii and subsequent infant death in a neonatal unit, it is now recommended that hospital formula preparation for newborns follow separate guidelines; these are discussed in Chapter 9.
Laws governing the labeling of infant formulas require that the directions for preparation and use of the formula include pictures and symbols for nonreading individuals. In addition, manufacturers are translating the directions into foreign languages, such as Spanish and Vietnamese, to prevent misunderstanding and errors in formula preparation.
Alternate Milk Products
In the United States few infants are fed evaporated milk formula, and its use is not recommended by the American Academy of Pediatrics (2004b). However, it has many advantages over whole milk. It is readily available in cans; needs no refrigeration if unopened; is less expensive than commercial formula; provides a softer, more digestible curd; and contains more lactalbumin and a higher calcium/phosphorus ratio. Disadvantages of evaporated milk for infant nutrition include low iron and vitamin C concentrations, excessive sodium and phosphorus, decreased vitamin A and D (except in fortified forms), and poorly digested fat. A common rule for preparing evaporated milk formula is diluting the 13-ounce can of milk with 19½ ounces of water and adding 3 tablespoons of sugar or commercially processed corn syrup.
Evaporated milk must not be confused with condensed milk, which is a form of evaporated milk with 45% more sugar. Because of its high carbohydrate concentration and disproportionately low fat and protein content, condensed milk is not used for infant feeding. Likewise, skim and low-fat milk must not be used for infant milk because they are deficient in caloric concentration, significantly increase the renal solute load and water demands, and deprive the body of essential fatty acids.
Goat’s milk is a poor source of iron and folic acid. It has an excessively high renal solute load as a result of its high protein content, making it unsuitable for infant nutrition (Hendriksz and Walter, 2004). Some parents believe that goat’s milk is less allergenic than other available milk sources and may feed it to their infant to reduce allergic milk reactions. However, infants allergic to cow’s milk have experienced anaphylaxis with their first exposure to goat’s milk (Pessler and Nejat, 2004). Raw, unpasteurized milk from any animal source is unacceptable for infant nutrition.
Feeding Schedules
Ideally, feeding schedules should be determined by the infant’s hunger. Demand feedings involve feeding infants when they signal readiness. Scheduled feedings are arranged at predetermined intervals. Some hospital routinely feed infants every 3 to 4 hours. Although this may be satisfactory for bottle-fed infants, it hinders the breastfeeding process. Breast-fed infants tend to be hungry every 2 to 3 hours because of the easy digestibility of the milk; therefore they should be fed on demand.
Supplemental feedings should not be offered to breast-fed infants before lactation is well established, since they may satiate the infant and may cause nipple preference (see Evidence-Based Practice box, p. 301). Supplemental water is not needed in breast-fed infants, even in hot climates (American Academy of Pediatrics, 2005b). Satiated infants suck less vigorously at the breast, and milk production depends on the breast being emptied at each feeding. If milk is allowed to accumulate in the ducts, causing breast engorgement, ischemia results, suppressing the activity of the acini, or milk-secreting cells. Consequently, milk production is reduced. In addition, the process of sucking from a bottle is different from breast nipple compression. The relatively inflexible rubber nipple prevents the tongue from its usual rhythmic action. Infants learn to put the tongue against the nipple holes to slow down the more rapid flow of fluid. When infants use these same tongue movements during breastfeeding, they may push the human nipple out of the mouth and may not grasp the areola properly.
Usually by 3 weeks of age lactation is well established. Bottle-fed infants retain about 2 to 3 ounces of formula at each feeding and are fed approximately six times a day. The quantity of formula consumed is based on the caloric need of 108 kcal/kg/day; therefore a newborn who weighs 3 kg requires 324 kcal/day. Because commercial formula has 20 kcal/oz, approximately 16 ounces (480 ml) will provide the daily caloric requirement. Breast-fed infants may feed as frequently as 10 to 12 times a day.
Feeding Behavior
Five behavioral stages occur during successful feeding. Recognizing these steps can assist nurses in identifying potential feeding problems caused by improper feeding techniques. Prefeeding behavior, such as crying or fussing, demonstrates the infant’s level of arousal and degree of hunger. To encourage the infant to grasp the breast properly, it is preferable to begin feeding during the quiet alert state, before the infant becomes upset. Approach behavior is indicated by sucking movements or the rooting reflex. Attachment behavior includes activities that occur from the time the infant receives the nipple and sucks (sometimes more pronounced during initial attempts at breastfeeding). Consummatory behavior consists of coordinated sucking and swallowing. Persistent gagging might indicate unsuccessful consummatory behavior. Satiety behavior is observed when infants let the parent know that they are satisfied, usually by falling asleep.
PROMOTE PARENT-INFANT BONDING (ATTACHMENT)
The process of parenting is based on a relationship between parent and infant. As more is learned of the complexity of neonates and of their potential for influencing and shaping their environments, particularly their interaction with significant others, it is apparent that promoting positive parent-child relationships necessitates an understanding of behavioral steps in attachment, variables that enhance or hinder this process, and methods of teaching parents to develop a stronger relationship with their children, especially by recognizing potential problems. (See also Assessment of Attachment Behaviors, p. 215.)
Infant Behavior
Nurses must appreciate the individuality and uniqueness of each infant. According to the individual temperament, the infant will change and shape the environment, which will influence future development. (See Patterns of Sleep and Activity, p. 214.) An infant who sleeps 20 hours a day will be exposed to fewer stimuli than one who sleeps 16 hours a day. In turn, each infant will likely elicit a different response from parents. The infant who is quiet, undemanding, and passive may receive much less attention than one who is responsive, alert, and active. Behavioral characteristics such as irritability and consolability can influence the ease of transition to parenthood and the parents’ perception of the infant.
Nurses can positively influence the attachment of parent and child. The first step is recognizing individual differences and explaining to parents that such characteristics are normal. For example, some people believe that infants sleep throughout the day, except for feedings. For some newborns this may be true, but for many it is not. Understanding that the infant’s wakefulness is part of a biologic rhythm and not a reflection of inadequate parenting can be crucial in promoting healthy parent-child relationships. Another aspect of helping parents concerns supplying guidelines on how to enhance the infant’s development during awake periods. Placing the child in a crib to stare at the same mobile every day is not exciting, but carrying the infant into each room as one does daily chores can be fascinating. A few suggestions can make life more stimulating for the infant and gratifying for the parents (Box 8-6).
Maternal Attachment
Research has suggested that there is a maternal sensitive period immediately and for a short time after birth when parents have a unique ability to attach to their infants (Klaus, Kennell, and Klaus, 1995). Mothers demonstrate a predictable and orderly pattern of behavior during the development of the attachment process. When mothers are presented with their nude infants, they begin to examine the infant with their fingertips, concentrating on touching the extremities, and then proceed to massage and encompass the trunk with their entire hands. Assuming the en face position, in which the mother’s and infant’s eyes meet in visual contact in the same vertical plane, is significant in the formation of affectional ties (Fig. 8-16). Although similar patterns of touching have been observed, additional studies demonstrate different patterns for mothers, as well as the same pattern for nonmaternal persons, such as male and female nurses. Consequently, nurses must exercise caution in interpreting behaviors such as touching.
Several studies have attempted to substantiate the long-term benefits of providing parents with opportunities to optimally bond with their infant during the initial postpartum period. Although there has been some evidence that increased parent-child contact encourages prolonged breastfeeding and may minimize the risks of parenting disorders, conclusions about the long-term effects of such early intervention on parenting and child development must be viewed cautiously. In addition, some authorities claim that the emphasis on bonding has been unjustified and may lead to guilt and fear in parents who did not have early contact with their infant. There is concern that the literal interpretation of “sensitive” or “critical” might imply that without early contact, optimum bonding cannot occur or, conversely, that early contact alone is sufficient to ensure competent parenting. Examples of cases where a strong maternal-infant attachment develops in less than ideal situations include adoption and the birth of a child with a congenital defect (Billings, 1995).
The nurse should stress to parents that, although early bonding is valuable, it does not represent an “all or none” phenomenon. Throughout the child’s life there will be multiple opportunities for development of parent-child attachment. Bonding is a complex process that develops gradually and is influenced by numerous factors, only one of which is the type of initial contact between the newborn and parent.
In a concept analysis of parent-infant attachment, Goulet, Bell, St-Cyr, and others (1998) describe attributes of parent-infant attachment as proximity, reciprocity, and commitment. Within these attributes are further dimensions, which include contact, emotional state, individualization, complementarity, sensitivity, centrality, and parent role exploration. The researchers describe the parent-infant attachment process as one that is complex and therefore cannot be evaluated simply by the observations of attitudes and behaviors of parents toward their infants (Goulet, Bell, St-Cyr, and others, 1998). Further research into the reciprocal relationships between infant and parent and the situational factors that influence such relationships are recommended.
One component of successful maternal attachment is the concept of reciprocity (Brazelton, 1974). As the mother responds to the infant, the infant must respond to the mother by some signal, such as sucking, cooing, eye contact, grasping, or molding (conforming to other’s body during close physical contact). The first step is initiation, in which interaction between infant and parent begins. Next is orientation, which establishes the partners’ expectations of each other during the interaction. Following orientation is acceleration of the attention cycle to a peak of excitement. The infant reaches out and coos, both arms jerk forward, the head moves backward, the eyes dilate, and the face brightens. After a short time, deceleration of the excitement and turning away occur, in which the infant’s eyes shift away from the parent’s and the child grasps his or her shirt. During this cycle of nonattention, repeated verbal or visual attempts to reinitiate the infant’s attention are ineffective. This deceleration and turning away probably prevents the infant from being overwhelmed by excessive stimuli. In a good interaction both partners have synchronized their attention-nonattention cycles. Parents or other caregivers who do not allow the infant to turn away and who continually attempt to maintain visual contact encourage the infant to turn off the attention cycle and thus prolong the nonattention phase.
Although this description of reciprocal interacting behavior is usually observed in the infant by 2 to 3 weeks of age, nurses can use this information to teach parents how to interact with their infant. Recognizing the attention vs nonattention cycles and understanding that the latter is not a rejection of the parent helps parents develop competence in parenting.
Paternal Engrossment
Fathers also show specific attachment behaviors to the newborn. This process of paternal engrossment, forming a sense of absorption, preoccupation, and interest in the infant, includes (1) visual awareness of the newborn, especially focusing on the beauty of the child; (2) tactile awareness, often expressed in a desire to hold the infant; (3) awareness of distinct characteristics with emphasis on those features of the infant that resemble the father; (4) perception of the infant as perfect; (5) development of a strong feeling of attraction to the child that leads to intense focusing of attention on the infant; (6) experiencing a feeling of extreme elation; and (7) feeling a sense of deep self-esteem and satisfaction. These responses are greatest during the early contacts with the infant and are intensified by the neonate’s normal reflex activity, especially the grasp reflex and visual alertness. In addition to behavioral reactions, fathers also demonstrate physiologic responses such as increased heart rate and BP during interactions with their newborns.
The process of engrossment has significant implications for nurses. It is imperative to recognize the importance of early father-infant contact in releasing these behaviors. Fathers need to be encouraged to express their positive feelings, especially if such emotions are contrary to any popular belief that fathers should remain stoic. If this is not clarified, fathers may feel confused and attempt to suppress the natural sensations of absorption, preoccupation, and interest in order to conform with societal expectations.
Mothers also need to be aware of the responses of the father toward the newborn, especially because one of the consequences of paternal preoccupation with the infant is less overt attention toward the mother. If both parents are able to share their feelings, each can appreciate the process of attachment toward their child and will avoid the unfortunate conflict of being insensitive and unaware of the other’s needs. In addition, a father who is encouraged to form a relationship with his newborn is less likely to feel excluded and abandoned after the family returns home and the mother directs her attention toward caring for the infant.
Ideally, the process of engrossment should be discussed with parents before the delivery, such as in prenatal classes, to reinforce the father’s awareness of his natural feelings toward the expected child. Focusing on the future experience of seeing, touching, and holding one’s newborn may also help expectant fathers become more comfortable in accepting their paternal feelings. This in turn can assist them in being more supportive toward the mother, especially as the labor and delivery draw near.
At the infant’s birth, the nurse can play a vital role in helping the father express engrossment by assessing the neonate in front of the couple; pointing out normal characteristics; encouraging identification through consistent referral to the child by name; encouraging the father to cuddle, hold, talk to, or feed the infant; and demonstrating whenever necessary the soothing powers of caressing, stroking, and rocking the child (Fig. 8-17). Fathers are encouraged to be with the mother during labor and delivery, to spend time alone with the mother and newborn after delivery, and to room-in with the mother and infant. Many birthing centers have adopted a family-centered focus, including sleeping accommodations that more closely resemble the home environment for the new mother and father.

FIG. 8-17 A desire to hold the infant and participate in caregiving activities is an indication of paternal engrossment.
Fathers, like mothers, may demonstrate attachment not only after the infant’s birth but during fetal life as well. Paternal attachment may proceed at a different pace than maternal attachment. Paternal preoccupation with events of labor and delivery and the spouse’s health may detract from paternal attachment (Anderson, 1996). Research has noted that, although fathers spend similar amounts of time in interaction with their newborns as do mothers, the nature of their interaction is different. Mothers and infants focus on face-to-face exchange and mutual gazing, co-vocalization, and affectionate touch. Fathers’ time with their infants includes quick peaks of high positive emotionality, including joint laughter and open exuberance. Interactions with fathers tend to center on physical games or games with an object focus, rather than on face-to-face signals (Feldman, 2007).
The nurse observes for the same indications of affection from the father as those expected in the mother, such as making visual contact in the en face position and embracing the infant close to the body. When present, such behaviors are reinforced. If such responses are not obvious, the nurse needs to assess the father’s feelings regarding this birth, cultural beliefs that may affect his expression of emotions, and other factors that influence his perception of the infant and the mother in order to facilitate a positive attachment during this critical period.
Siblings
Although the attachment process has been discussed almost exclusively in terms of the parents and infants, it is essential that nurses be aware of other family members, such as siblings and members of the extended family, who need preparation for the acceptance of this new child. Young children in particular need sensitive preparation for the birth to minimize sibling jealousy.
In support of family-centered care, there is an increasing trend to allow siblings to visit the mother on the postpartum unit and to hold the newborn (Fig. 8-18). Another trend has been the presence of siblings at childbirth. Unlike sibling visitation, the evidence supporting this practice has been controversial, yet the nature of truly providing family-centered care encompasses siblings, grandparents, and other significant persons who comprise the extended family unit (Tomlinson, Bryan, and Esau, 1996). The American Academy of Pediatrics and American College of Obstetricians and Gynecologists (2007) support the presence of siblings at childbirth and visitation of the newborn and mother; basic guidelines for infection control and adult supervision are also recommended.
Children exhibit different degrees of involvement in the birth process. Some reported benefits include children’s increased knowledge of the birth process, less regressive behavior after the birth, and more mothering and caregiving behavior toward the infant. Some practitioners add facilitated family bonding and assimilation of the newborn into the family as positive outcomes. Parents whose children attended the birth have echoed these same benefits and have expressed their desire to repeat the experience should another pregnancy occur. Despite these positive findings, opponents believe that allowing children to observe a delivery could lead to emotional difficulties, although there is no research to support this contention. As research mounts, birthing centers that allow siblings at the birth are developing more definitive guidelines, such as an age requirement of at least 4 to 5 years, the presence of a supportive person for the sibling only, and an adequate sequence of preparation in which parents explore all options for preparing their other children.
From observations during sibling visitation, there is evidence that sibling attachment occurs. However, the en face position is assumed much less often among the newborn and siblings than between mother and newborn, and when this position is used, it is brief. Siblings focus more on the head or face than on touching or talking to the infant. The siblings’ verbalizations are focused less on attracting the infant’s attention and more on addressing the mother about the newborn. Children who have established a prenatal relationship with the fetus have demonstrated more attachment behaviors, supporting the suggestion of encouraging prenatal acquaintance. Additional research is needed to establish theories on sibling bonding as have been constructed for parental bonding.
Multiple Births and Subsequent Children
A component of attachment that has special meaning for families with multiple births, monotropy refers to the principle that a person can become optimally attached to only one individual at a time. If a parent can form only one attachment at a time, how can all the siblings of a multiple birth receive optimum emotional care? Research on bonding and multiple births is still lacking despite the recent increase in multiple births, and even less is known about paternal engrossment and sibling attachment. In regard to maternal-twin bonding, the conclusions of different authors vary. Some report that mothers bond equally to each twin at the time of birth, even if one twin is ill. Others suggest that mothers of twins may take months or years to form individual attachments to each child, or even longer if the twins are identical.
Nurses can be instrumental in promoting bonding of multiple births. The most important principle is to assist the parents in recognizing the individuality of the children, especially in monozygotic (identical) twins. The mother should visit with each newborn, including a sick infant, as much as possible after birth. Rooming-in and breastfeeding are encouraged. Any characteristics that are unique to each child are emphasized, and each infant is called by name, rather than referring to “the twins.” Asking the family questions such as “How do you tell Sally and Amy apart?” and “In what ways are Sally and Amy different and similar?” helps point out their individual characteristics. Behaviors on the BNBAS can be used to illustrate these differences and to stress effective strategies for dealing with multiple personalities at the same time. Cobedding (bed sharing) of twins or other multiples may also be encouraged in the hospital and home, with the goal of maintaining the bond between siblings that was formed in utero (Fig. 8-19). Much research is focused on exploring the safety and benefits of the practice of cobedding (Hayward, Campbell-Yeo, Price, and others, 2007) (see also Sudden Infant Death Syndrome, Chapter 11); however, the cobedding of infant siblings differs from forms of cobedding that involve older siblings or adults. Other strategies for promoting individualism are discussed under Multiple Births in Chapter 3.
Another area of attachment that has received minimal attention is maternal bonding of multiparous mothers. Research suggests that there are several additional tasks to “taking on” a second child. These include:
• Promoting acceptance and approval of the second child
• Grieving and resolving the loss of an exclusive dyadic relationship with the first child
• Planning and coordinating family life to include a second child
• Reformulating a relationship with the first child
• Identifying with the second child by comparing this child with the first child in terms of physical and psychologic characteristics
• Assessing one’s affective capabilities in providing sufficient emotional support and nurturance simultaneously to two children
Employed mothers who have a second child report fewer concerns than with the first child regarding general aspects of separation from their child and the effect of separation on the child, but they have similar concerns regarding separation due to employment. It appears that, although experience may decrease some concerns, it may not minimize others.
PREPARE FOR DISCHARGE AND HOME CARE
With short postpartum hospital stay, as well as a trend toward mother-infant care, also called dyad or couplet care, discharge planning, referral, and home visits have become increasingly important components of comprehensive newborn care. First-time, as well as experienced, parents benefit from guidance and assistance with the infant’s care, such as breastfeeding or bottle feeding, and with the family’s integration of a new member, particularly sibling adjustment.
To assess and meet these needs, teaching must begin early, ideally before the birth. Not only is the postpartum stay sometimes very short (12 to 24 hours), but also mothers are in the taking-in phase, where they demonstrate passive and dependent behaviors. On the first postpartum day, as a result of fatigue and excitement, mothers may not be able to absorb large amounts of information. This time may need to be spent highlighting essential aspects of care, such as infant safety and feeding. Parents may also be given a list of mother and infant care topics as part of the nursing admission history to choose issues they wish to review. Teaching before discharge should focus on newborn feeding patterns, monitoring diapers for voiding and stooling, jaundice, and infant crying.
The American Academy of Pediatrics (2004a) has established guidelines for postpartum discharge before 48 hours of age (see Family-Centered Care box). The Academy emphasizes that the primary care physician rather than an insurance company should make the determination of appropriate discharge time.
Despite concerns expressed regarding early discharge and newborn rehospitalizations for dehydration, jaundice, breastfeeding failure, and other issues, studies in various parts of the United States and Canada continue to be reassuring. They show that rehospitalizations, emergency department visits, and infant morbidities have not significantly increased since early postpartum discharge was implemented (Gries, Phyall, and Barfield, 2000; Danielsen, Castles, Damberg, and others, 2000; Martens, Derksen, and Gupta, 2004). The American Academy of Pediatrics (2004a) recommends that newborns discharged early receive follow-up care within 48 hours either in the primary practitioner’s office or in the home.
Although many mothers and newborns may be safely discharged within 12 to 24 hours without detriment to their health, others require a longer stay. Follow-up home care within days (or even hours after discharge when minor problems are anticipated) appears to be the emerging trend in an effort to curtail hospital costs and provide adequate maternal-newborn care with minimal complications. (See Community Focus boxes.)
Despite the changing spectrum of well-newborn health care, the nurse’s role continues to be that of providing ongoing assessments of each mother-newborn dyad to ensure a safe transition to home and a successful adaptation into the family unit. The ultimate safety and success of early newborn discharge from hospital are contingent on using clear discharge criteria and having a high-quality early follow-up program (Radmacher, Massey, and Adamkin, 2002).
With family structures changing, it is essential that nurses identify the primary caregiver, which may not always be the mother but may be a father, grandparent, or baby-sitter. Depending on the family composition, the mother’s primary support system in the care of the newborn may not always be the traditional husband or male companion.
Nurses should not assume that terminology associated with mother-infant care is understood. Words relating to the anatomy (e.g., meconium, labia, edema, and genitalia) and to breastfeeding (e.g., areola, colostrum, and let-down reflex) may be unfamiliar to mothers. Mothers with other children do not necessarily understand more words, and younger, less educated moms may be at particular risk for not understanding teaching.
An essential area of discharge counseling is the safe transportation of the newborn home from the hospital. Ideally this information should also be provided before delivery to allow parents an opportunity to purchase a suitable infant car safety seat. When purchasing a car safety seat, parents should consider cost and convenience. The convertible-type seats are more expensive initially but cost less than two separate systems (infant-only model and infant-toddler convertible model). Convenience is a major factor, since a cumbersome restraint may be used less often or used improperly. Before buying a car safety seat, it is best to try out different models. For example, some types are too large for subcompact cars. Asking friends about the advantages and disadvantages of their restraints is helpful, but borrowing their car seat or purchasing a used one can be dangerous. Parents should use only a restraint that has directions for use and a certification label stating that it complies with federal motor vehicle safety standards (both should be on the seat). They should not use a restraint that has been involved in a crash. Some service clubs and hospitals have loan programs for restraints. Information about approved models and other aspects of car safety seat restraints is available from several organizations and sources.*
Parents are cautioned against placing an infant in the front seat of a car with a passenger-side air bag. Infants weighing less than 9 kg (20 pounds) or younger than 1 year should always be placed in a rear-facing child safety seat in the back seat of the car (American Academy of Pediatrics, 2002).
Although federal safety standards do not specify the minimum weight of an infant and the appropriate type of restraint, newborns weighing 2 kg (4.4 pounds) receive relatively good support in convertible seats with a seat back—to-crotch strap height of 14 cm (5.7 inches) or less. Rolled blankets or towels may be needed between the crotch and legs to prevent slouching and can be placed along the sides to minimize lateral movements. Placing the infant in a safety seat at a 45-degree angle will prevent slumping and airway obstruction (American Academy of Pediatrics, 2002). Seats with shields (large padded surfaces in front of the child) and armrests (found on some other models) are unacceptable because of their proximity to the infant’s face and neck. (For a discussion of appropriate car restraints for preterm infants, see p. 268, and for infants, see Motor Vehicle Injuries in Chapters 10 and 12.)
In the United States and Canada all states and provinces have mandated the use of child restraints. Therefore hospitals and birthing centers should have policies regarding the safe discharge of a newborn in a car safety seat and provisions for parents to learn to use the device correctly. In addition, hospital personnel should ensure that infants born before 37 weeks of gestation have a period of observation in the selected car seat to monitor for possible apnea, bradycardia, and oxygen desaturation (American Academy of Pediatrics, 2002). Parents are more likely to use a restraint correctly and consistently if the proper use of one is demonstrated and its necessity is stressed. Infants and children continue to be hurt and killed because car seat restraints are not installed properly.
 ATRAUMATIC CARE
ATRAUMATIC CARE
 CULTURAL AWARENESS
CULTURAL AWARENESS

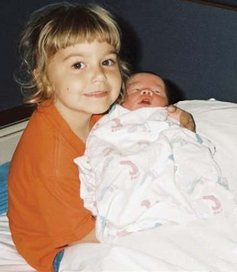
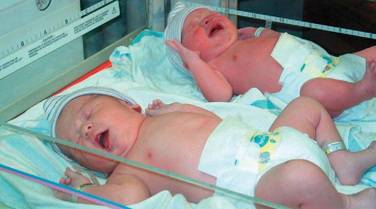
 FAMILY-CENTERED CARE
FAMILY-CENTERED CARE COMMUNITY FOCUS
COMMUNITY FOCUS