Health Promotion of the Infant and Family
PROMOTING OPTIMAL GROWTH AND DEVELOPMENT
Psychosocial development: developing a sense of trust (erikson)
Cognitive development: sensorimotor phase (piaget)
Coping with Concerns Related to Normal Growth and Development
On completion of this chapter the reader will be able to:
 Identify the major biologic, psychosocial, cognitive, and social developments during the first year.
Identify the major biologic, psychosocial, cognitive, and social developments during the first year.
 Relate parent-child attachment, separation anxiety, and stranger fear to developmental achievements during infancy.
Relate parent-child attachment, separation anxiety, and stranger fear to developmental achievements during infancy.
 Provide anticipatory guidance to parents regarding common parental concerns during infancy.
Provide anticipatory guidance to parents regarding common parental concerns during infancy.
 Provide parents with feeding recommendations for infants.
Provide parents with feeding recommendations for infants.
 Outline immunization requirements during infancy.
Outline immunization requirements during infancy.
 List general contraindications, precautions, and administration routes for childhood immunizations.
List general contraindications, precautions, and administration routes for childhood immunizations.
 Provide anticipatory guidance to parents regarding injury prevention based on the infant’s developmental achievements.
Provide anticipatory guidance to parents regarding injury prevention based on the infant’s developmental achievements.
PROMOTING OPTIMAL GROWTH AND DEVELOPMENT
At no other time in life are physical changes and developmental achievements as dramatic as during infancy. All major body systems undergo progressive maturation, and there is concurrent development of skills that increasingly allow infants to respond to and cope with the environment. Acquisition of these fine and gross motor skills occurs in an orderly head-to-toe and center-to-periphery (cephalocaudal-proximodistal) sequence.
Proportional Changes
During the first year, especially the initial 6 months, growth is very rapid. Infants gain 150 to 210 g (about 5 to 7 ounces) weekly until approximately age 5 to 6 months, when the birth weight has at least doubled. An average weight for a 6-month-old child is 7.3 kg (16 pounds). Weight gain slows during the second 6 months. By 1 year of age the infant’s birth weight has tripled, for an average weight of 9.75 kg (21.5 pounds). Infants who are breast-fed beyond 4 to 6 months of age typically gain less weight than those who are bottle-fed, yet head circumference is more than adequate. Dewey (2001) attributes the decreased weight gain seen in breast-fed infants to self-regulation of energy intake. This self-regulation of intake with breastfeeding (vs formula feeding) is believed to have further significance in the development of childhood obesity and subsequent cardiovascular disease (Grummer-Strawn, Mei, and Centers for Disease Control and Prevention, 2004; Schack-Nielsen and Michaelsen, 2006).
Height increases by 2.5 cm (1 inch) a month during the first 6 months and also slows during the second 6 months. Increases in length occur in sudden spurts, rather than in a slow, gradual pattern. Average height is 65 cm (25.5 inches) at 6 months and 74 cm (29 inches) at 12 months. By 1 year the birth length has increased by almost 50%. This increase occurs mainly in the trunk, rather than in the legs and contributes to the characteristic physique of the infant.
Head growth is also rapid. During the first 6 months head circumference increases approximately 1.5 cm (0.6 inch) per month, but the rate of growth declines to only 0.5 cm (0.2 inch) monthly during the second 6 months. The average size is 43 cm (17 inches) at 6 months and 46 cm (18 inches) at 12 months. By 1 year, head size has increased by almost 33%. Closure of the cranial sutures occurs, with the posterior fontanel fusing by 6 to 8 weeks of age and the anterior fontanel closing by 12 to 18 months of age (average 14 months).
Expanding head size reflects the growth and differentiation of the nervous system. By the end of the first year the brain has increased in weight about two and one half times. Maturation of the brain is exhibited in the dramatic developmental achievements of infancy (see Table 10-2). Primitive reflexes are replaced by voluntary, purposeful movement, and new reflexes that influence motor development appear.
TABLE 10-2
Growth and Development During Infancy
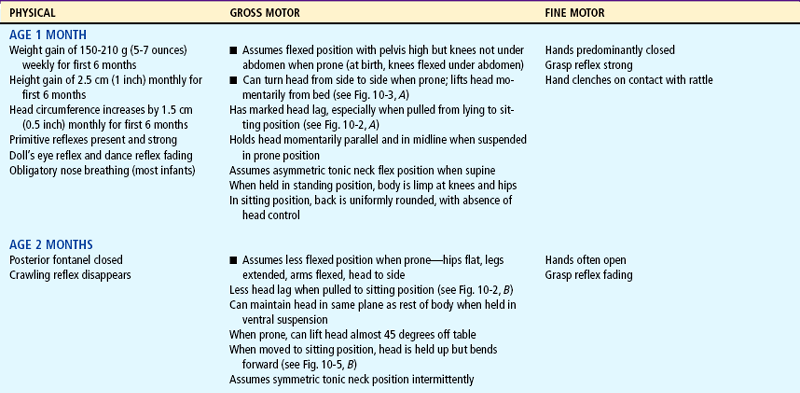

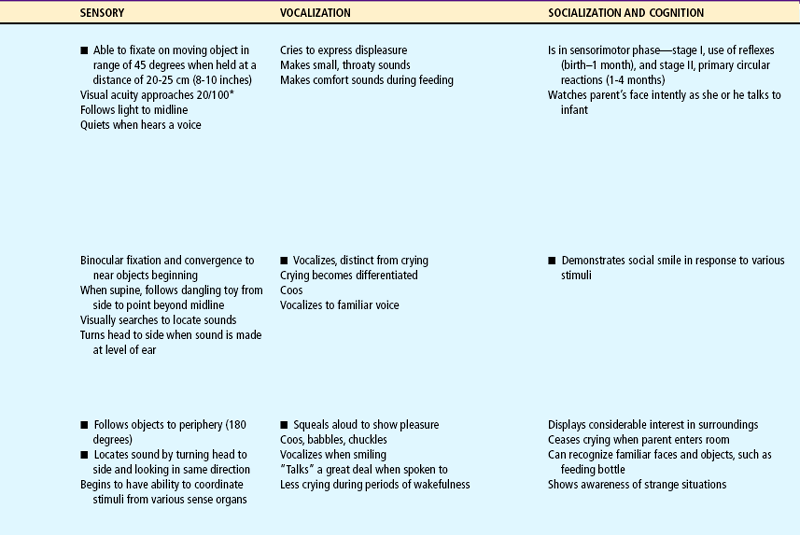
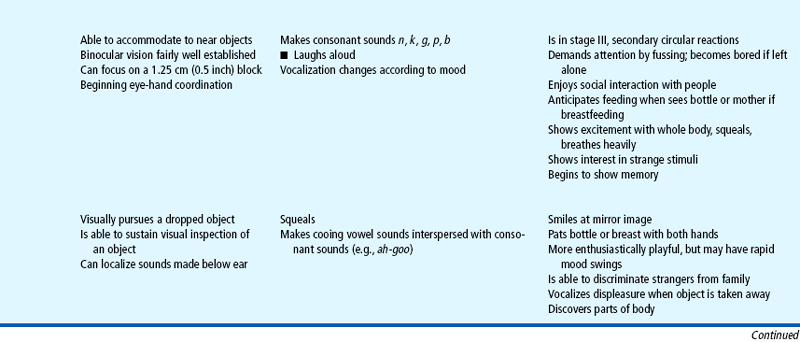
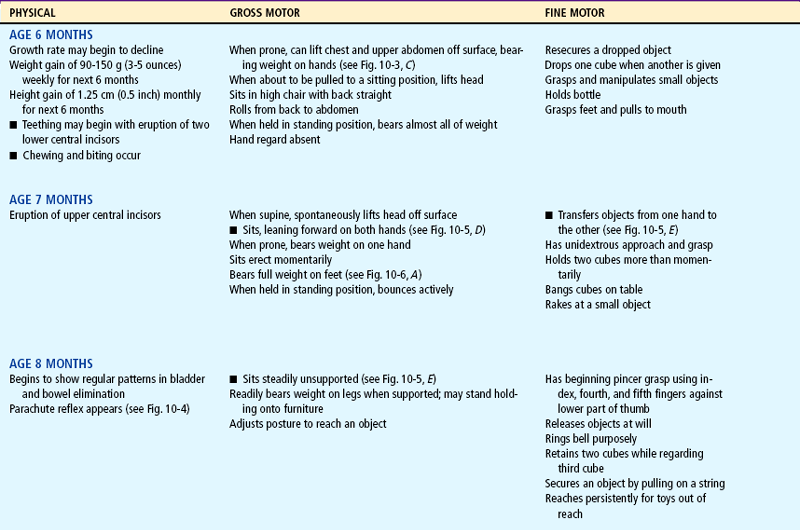

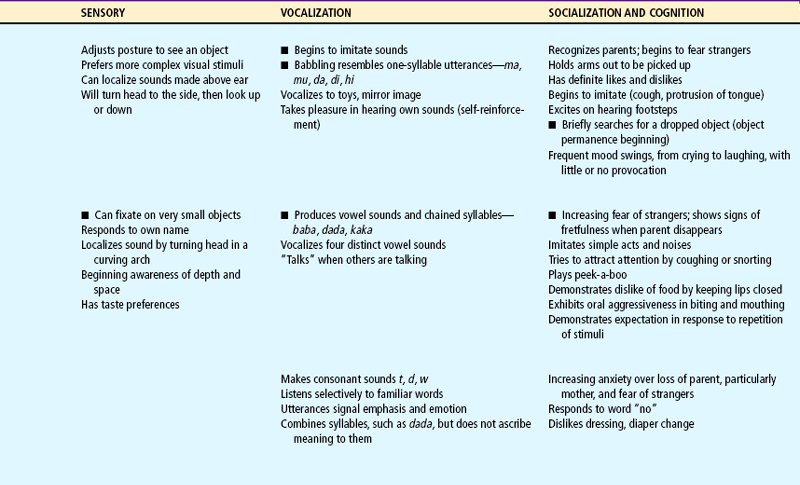
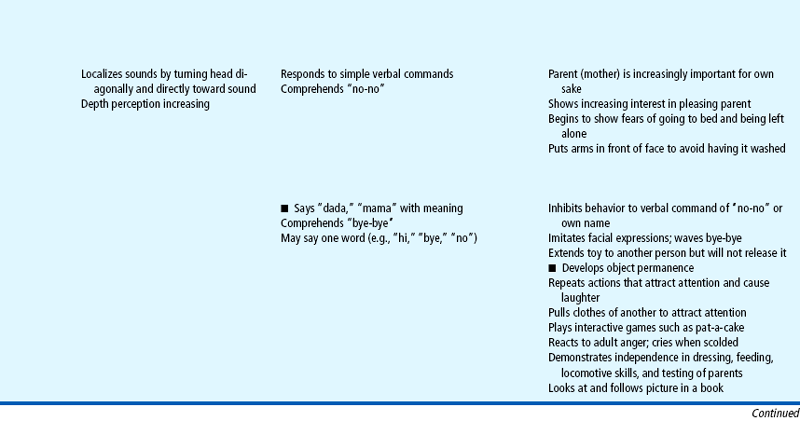

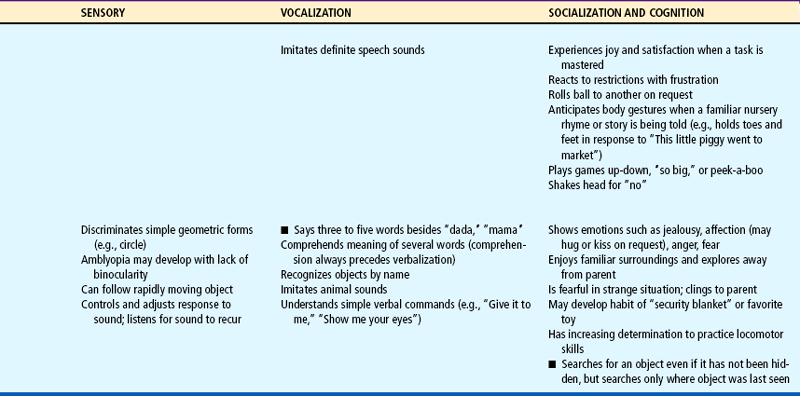
 Milestones that represent essential integrative aspects of development that lay the foundation for the achievement of more advanced skills.
Milestones that represent essential integrative aspects of development that lay the foundation for the achievement of more advanced skills.
*Degree of visual acuity varies according to vision measurement procedure used.
The chest assumes a more adult contour, with the lateral diameter becoming larger than the anteroposterior diameter. The chest circumference approximately equals the head circumference by the end of the first year. The heart grows less rapidly than does the rest of the body. Its weight is usually doubled by 1 year of age in comparison with body weight, which triples during the same period. The size of the heart is still large in relation to the chest cavity; its width is approximately 55% of the chest width.
Maturation of Systems
Other organ systems also change and grow during infancy. The respiratory rate slows somewhat (see inside back cover) and is relatively stable. Respiratory movements continue to be abdominal. Several factors predispose the infant to more severe and acute respiratory problems than older children. The close proximity of the trachea to the bronchi and its branching structures rapidly transmits an infectious agent from one anatomic location to another. The short, straight eustachian tube closely communicates with the ear, allowing infection to ascend from the pharynx to the middle ear. In addition, the inability of the immune system to produce immunoglobulin A (IgA) in the mucosal lining provides less protection against infection in infancy than during later childhood.
The heart rate slows (see inside back cover), and the rhythm is often sinus arrhythmia (rate increases with inspiration and decreases with expiration). Blood pressure also changes during infancy (see inside back cover). Systolic pressure rises during the first 2 months as a result of the increasing ability of the left ventricle to pump blood into the systemic circulation. Diastolic pressure decreases during the first 3 months, then gradually rises to values close to those at birth. Fluctuations in blood pressure occur during varying states of activity and emotion.
Significant hematopoietic changes occur during the first year (see Appendix C). Fetal hemoglobin (HgbF) is present for the first 5 months, with adult hemoglobin steadily increasing through the first half of infancy. Fetal hemoglobin results in a shortened survival of red blood cells and thus a decreased number of red blood cells. A common result at 2 to 3 months of age is physiologic anemia. High levels of fetal hemoglobin are thought to depress the production of erythropoietin, a hormone released by the kidney that stimulates red blood cell production.
Maternally derived iron stores are present for the first 5 to 6 months and gradually diminish, which also accounts for lowered hemoglobin levels toward the end of the first 6 months. The occurrence of physiologic anemia is not affected by an adequate supply of iron. However, when erythropoiesis is stimulated, iron supplies are necessary for the formation of hemoglobin.
The digestive processes are relatively immature at birth. Although term newborn infants have some limitations in digestive function, studies indicate that human milk has properties that partially compensate for decreased digestive enzymatic activity, thus enabling the breast-fed infant to receive optimal nutrition during the first several months of life. Saliva is secreted in small amounts, but the majority of the digestive processes do not begin functioning until age 3 months, when drooling is common because of the poorly coordinated swallowing reflex. The enzyme amylase (also called ptyalin) is present in small amounts but usually has little effect on the foodstuffs because of the small amount of time the food stays in the mouth. Gastric digestion in the stomach consists primarily of the action of hydrochloric acid and rennin, an enzyme that acts specifically on the casein in milk to cause the formation of curds—coagulated semisolid particles of milk. The curds cause the milk to be retained in the stomach long enough for digestion to occur.
Digestion also takes place in the duodenum, where pancreatic enzymes and bile begin to break down protein and fat. Secretion of the pancreatic enzyme amylase, which is needed for digestion of complex carbohydrates, is deficient until about the fourth to sixth month of life. Lipase is also limited, and infants do not achieve adult levels of fat absorption until 4 to 5 months of age. Trypsin is secreted in sufficient quantities to catabolize protein into polypeptides and some amino acids.
The immaturity of the digestive processes is evident in the appearance of stools. During infancy, solid foods (e.g., peas, carrots, corn, raisins) are passed incompletely broken down in the feces. An excess quantity of fiber easily disposes the child to loose, bulky stools.
During infancy the stomach enlarges to accommodate a greater volume of food. By the end of the first year the infant is able to tolerate three meals a day and an evening bottle and may have one or two bowel movements daily. However, with any type of gastric irritation the infant is vulnerable to diarrhea, vomiting, and dehydration (see Chapter 24).
The liver is the most immature of all the gastrointestinal organs throughout infancy. The ability to conjugate bilirubin and secrete bile is achieved after the first couple of weeks of life. However, the capacities for gluconeogenesis, formation of plasma protein and ketones, storage of vitamins, and deaminization of amino acids remain relatively immature for the first year of life.
Maturation of the suckling, sucking, and swallowing reflexes and the eruption of teeth (see Teething, p. 342) parallel the changes in the gastrointestinal tract and prepare the infant for the introduction of solid foods.
The immunologic system undergoes numerous changes during the first year. The full-term newborn receives significant amounts of maternal immunoglobulin G (IgG), which, for approximately 3 months, confers immunity against antigens to which the mother was exposed. During this time the infant begins to synthesize IgG; approximately 40% of adult levels are reached by 1 year of age. Significant amounts of immunoglobulin M (IgM) are produced at birth, and adult levels are reached by 9 months of age. Secretory IgA is not present at birth but is found in saliva and tears by 2 to 5 weeks. IgA is present in large amounts in colostrum; IgA confers protection to the mucous membranes of the gastrointestinal tract (Lawrence and Lawrence, 2005) against many bacteria, such as Escherichia coli, and viruses such as rubella, poliovirus, and the enteroviruses. The development of the mucosa-associated lymphoid tissue (MALT) occurs during infancy; in part this system is believed to prevent colonization and passage of bacteria across the infant’s mucosal barrier (Lawrence and Lawrence, 2005). The function and quantity of T lymphocytes, lymphokines, interferon-γ, interleukins, tumor necrosis factor-α, and complement are reduced in early infancy, thus preventing optimal response to certain bacteria and viruses. The production of IgA and immunoglobulins D and E (IgD and IgE) is much more gradual, and maximum levels are not attained until early childhood.
There is evidence that vernix caseosa, a white oily substance that coats the term infant’s body and is often found in abundance in creases of the axilla and groin, has innate immunologic properties that serve to protect the newborn from infection (Narendran and Hoath, 2006). The epidermis of the full-term infant undergoes maturation during the first month of life; the newborn’s skin acts as a barrier to infection, assists in thermal regulation, and prevents transepidermal water loss in term infants.
During infancy, thermoregulation becomes more efficient; the ability of the skin to contract and of muscles to shiver in response to cold increases. The peripheral capillaries respond to changes in ambient temperature to regulate heat loss. The capillaries constrict in response to cold, conserving core body temperature and decreasing potential evaporative heat loss from the skin surface. The capillaries dilate in response to heat, decreasing internal body temperature through evaporation, conduction, and convection. Shivering (thermogenesis) causes the muscles and muscle fibers to contract, generating metabolic heat, which is distributed throughout the body. Increased adipose tissue during the first 6 months insulates the body against heat loss.
A shift in the total body fluid occurs; at birth 75% of the term infant’s body weight is water, and there is an excess of extracellular fluid. As the percentage of body water decreases, so does the amount of extracellular fluid—from 40% at term to 20% in adulthood. The high proportion of extracellular fluid, which is composed of blood plasma, interstitial fluid, and lymph, predisposes the infant to a more rapid loss of total body fluid and, consequently, dehydration.
The immaturity of the renal structures also predisposes the infant to dehydration and electrolyte imbalance. Complete maturity of the kidney occurs during the latter half of the second year, when the cuboidal epithelium of the glomeruli becomes flattened. Before this time the filtration capacity of the glomeruli is reduced. Urine is voided frequently and has a low specific gravity (1.000 to 1.010).
Auditory acuity is at adult levels during infancy. Visual acuity begins to improve, and binocular fixation is established. Binocularity, or the fixation of two ocular images into one cerebral picture (fusion), begins to develop by 6 weeks of age and should be well established by age 4 months. Depth perception (stereopsis) begins to develop by age 7 to 9 months but may not be fully mature until 2 or 3 years of age, thus increasing the infant’s and younger toddler’s risk of falling.
Fine Motor Development
Fine motor behavior includes the use of the hands and fingers in the prehension (grasp) of an object. Grasping occurs during the first 2 to 3 months as a reflex and gradually becomes voluntary. At 1 month of age the hands are predominantly closed, and by 3 months they are mostly open. By this time infants demonstrate a desire to grasp an object, but they “grasp” it more with the eyes than with the hands. If a rattle is placed in the hand, the infant will actively hold onto it. By 4 months of age the infant regards both a small pellet and the hands and then looks from the object to the hands and back again. By 5 months the infant is able to voluntarily grasp an object.
By 6 months of age infants have increased manipulative skill. They hold their bottle, grasp their feet and pull them to their mouth, and feed themselves a cracker. By 7 months they transfer objects from one hand to the other, use one hand for grasping, and hold a cube in each hand simultaneously. They enjoy banging objects and will explore the movable parts of a toy.
Gradually the palmar grasp (using the whole hand) is replaced by a pincer grasp (using the thumb and index finger). By 8 to 10 months of age the infant uses a crude pincer grasp and by 11 months has progressed to a neat pincer grasp (Fig. 10-1). By 10 months of age the pincer grasp is sufficiently established to enable infants to pick up a raisin and other finger foods. They can deliberately let go of an object and will offer it to someone. By 11 months they put objects into a container and like to remove them. By age 1 year, infants try to build a tower of two blocks but fail.
Gross Motor Development
Head Control.: The full-term newborn can momentarily hold the head in midline and parallel when the body is suspended ventrally and can lift and turn the head from side to side when prone (see Fig. 8-7). This is not the case when the infant is lying prone on a pillow or soft surface; infants do not have the head control to lift their head out of the depression of the object and therefore risk suffocation in the prone position early in infancy (see Sudden Infant Death Syndrome, Chapter 11). Marked head lag is evident when the infant is pulled from a lying to a sitting position. By 3 months of age infants can hold their head well beyond the plane of the body. By 4 months of age infants can lift the head and front portion of the chest approximately 90 degrees above the table, bearing their weight on the forearms. Only slight head lag is evident when the infant is pulled from a lying to a sitting position, and by 4 to 6 months head control is well established (Figs. 10-2 and 10-3).
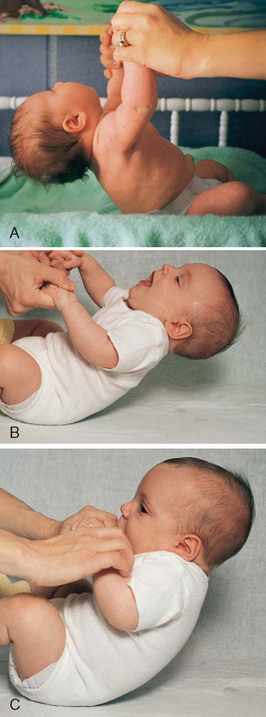
FIG. 10-2 Head control while pulled to sitting position. A, Complete head lag at 1 month. B, Partial head lag at 2 months. C, Almost no head lag at 4 months.
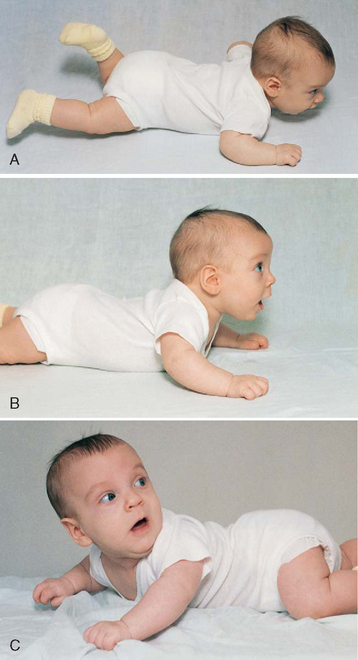
FIG. 10-3 Head control while prone. A, Infant momentarily lifts head at 1 month. B, Infant lifts head and chest 90 degrees and bears weight on forearms at 4 months. C, Infant lifts head, chest, and upper abdomen and can bear weight on hands at 6 months. Note how this position facilitates turning from abdomen to back.
Rolling Over.: Newborns may roll over accidentally because of their rounded back. The ability to willfully turn from the abdomen to the back occurs around 5 months, and the ability to turn from the back to the abdomen occurs at approximately 6 months. Infants put to sleep on their side may easily roll over to a prone (face-down) position, thus placing them at higher risk for sudden infant death syndrome (SIDS). It is therefore important to place infants in a supine position for sleep. While the infant is awake, a prone position is acceptable to enhance achievement of milestones such as head control, crawling, creeping, and turning over. It is noteworthy that the parachute reflex (Fig. 10-4), a protective response to falling, appears at approximately 7 months.
Sitting.: The ability to sit follows progressive head control and straightening of the back (Fig. 10-5). For the first 2 to 3 months the back is uniformly rounded. The convex cervical curve forms at approximately 3 to 4 months of age, when head control is established. The convex lumbar curve appears when the child begins to sit, at about age 4 months. As the spinal column straightens, the infant can be propped in a sitting position. By age 7 months infants can sit alone, leaning forward on their hands for support. By age 8 months they can sit well while unsupported and begin to explore their surroundings in this position rather than in a lying position. By 10 months they can maneuver from a prone to a sitting position.
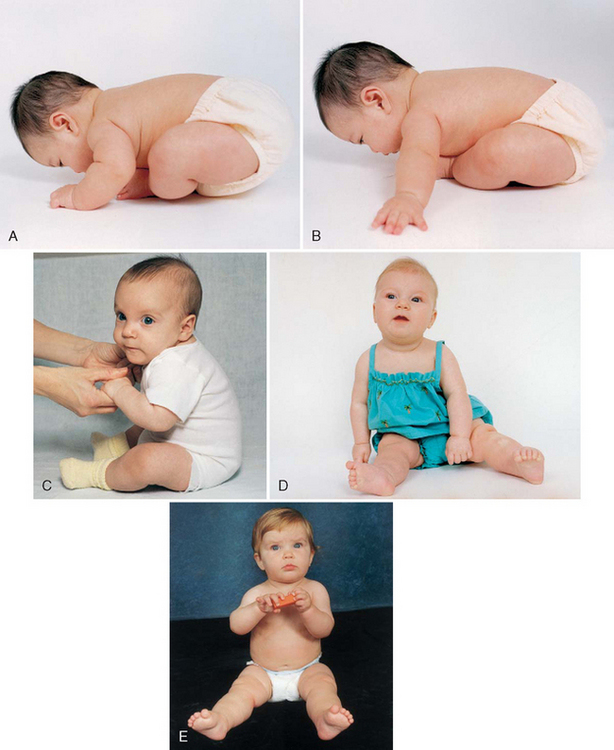
FIG. 10-5 Development of sitting. A, Back is completely rounded, and infant has no ability to sit upright at 1 month. B, At 2 months, infant exhibits more control; back is still rounded, but infant can try to pull up with some head control. C, Back is rounded only in lumbar area, and infant is able to sit erect with good head control at 4 months. D, Infant can sit alond, leaning on hands for support, at 7 months. E, Infant sits without support at 8 months. Note the transferring of objects that occurs at 7 months. (B, D, and E photos by Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
Locomotion.: Locomotion involves acquiring the ability to bear weight, propel forward on all four extremities, stand upright with support, cruise by holding onto furniture, and finally, walk alone (Fig. 10-6). Following a cephalocaudal pattern, infants 4 to 6 months old have increasing coordination in their arms. Initial locomotion results in infants propelling themselves backward by pushing with the arms. By 6 to 7 months of age they are able to bear all their weight on their legs with assistance. Crawling (propelling forward with belly on floor) progresses to creeping on hands and knees (with belly off floor) by 9 months. At this time they stand while holding onto furniture and can pull themselves to the standing position, but they are unable to maneuver back down except by falling. By 11 months they walk while holding onto furniture or with both hands held, and by age 1 year they may be able to walk with one hand held. A number of infants attempt their first independent steps by their first birthday.
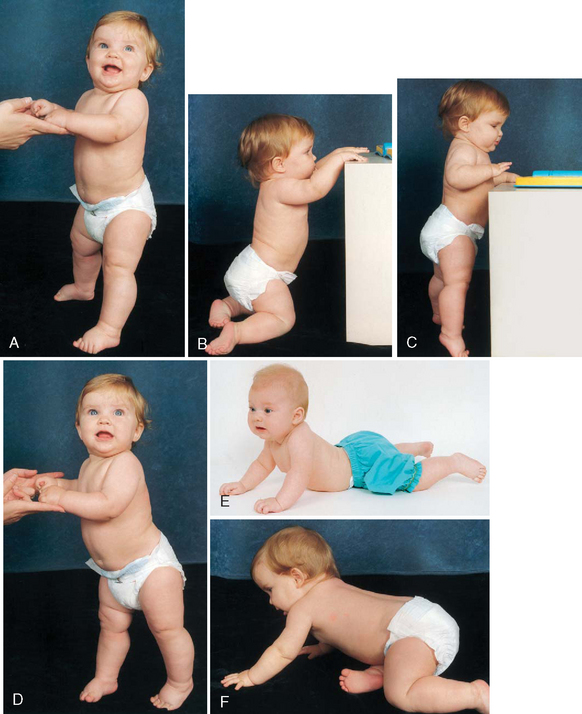
FIG. 10-6 Development of locomotion. A, Infant bears full weight on feet by 7 months. B, Infant can maneuver from sitting to kneeling position. C, Infant can stand holding onto furniture at 9 months. D, While standing, infant takes deliberate step at 10 months. E, Infant crawls with abdomen on floor and pulls self forward at about 7 months, and then, F, creeps on hands and knees at 9 months. (Photos by Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
PSYCHOSOCIAL DEVELOPMENT: DEVELOPING A SENSE OF TRUST (ERIKSON)
Erikson’s phase I (birth to 1 year) is concerned with acquiring a sense of trust while overcoming a sense of mistrust. The trust that develops is a trust of self, of others, and of the world. Infants “trust” that their feeding, comfort, stimulation, and caring needs will be met. The crucial element for the achievement of this task is the quality of both the parent (caregiver)–child relationship and the care the infant receives. The provision of food, warmth, and shelter by itself is inadequate for the development of a strong sense of self. The infant and parent must jointly learn to satisfactorily meet their needs for mutual regulation of frustration to occur. When this synchrony fails to develop, mistrust is the eventual outcome.
Failure to learn delayed gratification leads to mistrust. Mistrust can result from either too much or too little frustration. If parents always meet their children’s needs before the children signal their readiness, infants will never learn to test their ability to control the environment. If the delay is prolonged, infants will experience constant frustration and eventually mistrust others in their efforts to satisfy them. Therefore consistency of care is essential.
The trust acquired in infancy provides the foundation for all succeeding phases. Trust allows infants a feeling of physical comfort and security, which assists them in experiencing unfamiliar, unknown situations with a minimum of fear. Erikson has divided the first year of life into two oral-social stages. During the first 3 to 4 months, food intake is the most important social activity in which the infant engages. The newborn can tolerate little frustration or delay of gratification. Primary narcissism (total concern for oneself) is at its height.
However, as bodily processes such as vision, motor movements, and vocalization become better controlled, infants use more advanced behaviors to interact with others. For example, rather than cry, infants may put their arms up to signify a desire to be held. The next social modality involves a mode of reaching out to others through grasping. Grasping is initially reflexive, but even as a reflex it has a powerful social meaning for the parents. The reciprocal response to the infant’s grasping is the parents’ holding on and touching. There is pleasurable tactile stimulation for both the child and the parents.
Tactile stimulation is extremely important in the total process of acquiring trust. The degree of mothering skill, the quantity of food, or the length of sucking does not determine the quality of the experience. Rather, it is the total nature of the quality of the interpersonal relationship that influences the infant’s formulation of trust.
During the second stage the more active and aggressive modality of biting occurs. Infants learn that they can hold onto what is their own and can more fully control their environment. During this stage infants may be confronted with one of their first conflicts. If they are breastfeeding, they quickly learn that biting causes the mother to become upset and withdraw the breast. Yet biting also brings internal relief from teething discomfort and a sense of power or control.
This conflict may be solved in a variety of ways. The mother may wean the infant from the breast and begin bottle feeding, or the infant may learn to bite substitute nipples, such as a pacifier, and retain pleasurable breastfeeding. The successful resolution of this conflict strengthens the mother-child relationship because it occurs at a time when infants are recognizing the mother as the most significant person in their life.
COGNITIVE DEVELOPMENT: SENSORIMOTOR PHASE (PIAGET)
The theory most commonly used to explain cognition, or the ability to know, is that of Piaget. The period from birth to 24 months is termed the sensorimotor phase and is composed of six stages; however, because this discussion is concerned with ages birth to 12 months, only the first four stages are discussed. The last two stages occur during the toddler period of 12 to 24 months and are discussed in Chapter 12.
During the sensorimotor phase, infants progress from reflexive behaviors to simple repetitive acts to imitative activity. Three crucial events take place during this phase. The first event involves separation, in which infants learn to separate themselves from other objects in the environment. They realize that others besides themselves control the environment and that certain readjustments must take place for mutual satisfaction to occur. This coincides with Erikson’s concept of the formation of trust.
The second major accomplishment is achieving the concept of object permanence, or the realization that objects that leave the visual field still exist. A typical example of the development of object permanence is when infants are able to pursue objects they observe being hidden under a pillow or behind a chair (Fig. 10-7). This skill develops at approximately 9 to 10 months of age, which corresponds to the time of increased locomotion skills.

FIG. 10-7 Nine-month-old is able to find hidden object under pillow. (Photo by Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
The last major intellectual achievement of this period is the ability to use symbols, or mental representation. The use of symbols allows the infant to think of an object or situation without actually experiencing it. The recognition of symbols is the beginning of the understanding of time and space.
The first stage, from birth to 1 month, is identified by the infant’s use of reflexes. At birth the infant’s individuality and temperament are expressed through the physiologic reflexes of sucking, rooting, grasping, and crying. The repetitious nature of the reflexes is the beginning of associations between an act and a sequential response. When infants cry because they are hungry, a nipple is put in the mouth, and they suck, feel satisfaction, and sleep. They are assimilating this experience while perceiving auditory, tactile, and visual cues. This experience of perceiving certain patterns, or “ordering,” provides a foundation for the subsequent stages.
The second stage, primary circular reactions, marks the beginning of the replacement of reflexive behavior with voluntary acts. During the period from 1 to 4 months, activities such as sucking or grasping become deliberate acts that elicit certain responses. The beginning of accommodation is evident. Infants incorporate and adapt their reactions to the environment and recognize the stimulus that produced a response. Previously they would cry until the nipple was brought to the mouth. Now they associate the nipple with the sound of the parent’s voice. They accommodate this new piece of information and adapt by ceasing to cry when they hear the voice—before receiving the nipple. What is taking place is realization of causality and recognition of an orderly sequence of events. The environment is taken in with all of the senses and with whatever motor ability is present.
The secondary circular reactions stage is a continuation of primary circular reactions and lasts until 8 months of age. In this stage the primary circular reactions are repeated and prolonged for the response that results. Grasping and holding now become shaking, banging, and pulling. Shaking is performed to hear a noise, not solely for the pleasure of shaking. The quality and quantity of an act become evident. “More” or “less” shaking produces different responses. Causality, time, deliberate intention, and separateness from the environment begin to develop.
Three new processes of human behavior occur. Imitation requires the differentiation of selected acts from several events. By the second half of the first year, infants can imitate sounds and simple gestures. Play becomes evident as they take pleasure in performing an act after they have mastered it. Much of infants’ waking hours are absorbed in sensorimotor play. Affect (outward manifestation of emotion and feeling) is seen as infants begin to develop a sense of permanence. During the first 6 months infants believe that an object exists only for as long as they can visually perceive it. In other words: out of sight, out of mind. Affect to external objects is evident when the object continues to be present or remembered even though it is beyond the range of perception. Object permanence is a critical component of parent-child attachment and is seen in the development of stranger anxiety at 6 to 8 months of age (see p. 332).
During the fourth sensorimotor stage, coordination of secondary schemas and their application to new situations, infants use previous behavioral achievements primarily as the foundation for adding new intellectual skills to their expanding repertoire. This stage is largely transitional. Increasing motor skills allow for greater exploration of the environment. They begin to discover that hiding an object does not mean that it is gone, but that removing an obstacle will reveal the object. This marks the beginning of intellectual reasoning. Furthermore, they can experience an event by observing it, and they begin to associate symbols with events (e.g., “bye-bye” with “Mommy or Daddy goes to work”), but the classification is purely their own. In this stage they learn from the object itself; this is in contrast to the second stage, in which infants learn from the type of interaction between objects or individuals. Intentionality is further developed in that infants now actively attempt to remove a barrier to the desired (or undesired) action (see Fig. 10-7). If something is in their way, they attempt to climb over it or push it away. Previously an obstacle would cause them to give up any further attempt to achieve the desired goal.
DEVELOPMENT OF BODY IMAGE
The development of body image parallels sensorimotor development. Infants’ kinesthetic and tactile experiences are the first perceptions of their body, and the mouth is the principal area of pleasurable sensations. Other parts of the body are primarily objects of pleasure—the hands and fingers to suck and the feet to play with. As physical needs are met, they feel comfort and satisfaction with their body. Messages conveyed by the caregivers reinforce these feelings. For example, when infants smile, they receive emotional satisfaction from others who smile back.
Achieving the concept of object permanence is basic to the development of self-image. By the end of the first year infants recognize that they are distinct from their parents. At the same time, they have increasing interest in their image, especially in the mirror (Fig. 10-8). As motor skills develop, they learn that parts of the body are useful; for example, the hands bring objects to the mouth, and the legs help them move to different locations. All these achievements transmit messages to them about themselves. Therefore it is important to transmit positive messages to infants about their bodies.
SOCIAL DEVELOPMENT
Infants’ social development is initially influenced by their reflexive behavior, such as the grasp, and eventually depends primarily on the interaction between them and the principal caregivers. Attachment to the parent is increasingly evident during the second half of the first year. In addition, tremendous strides are made in communication and personal-social behavior. Whereas crying and reflexive behavior are methods to meet one’s needs in the neonatal period, the social smile is an early step in social communication. This has a profound effect on family members and is a tremendous stimulus for evoking continued responses from others. By 4 months infants laugh aloud.
Play is a major socializing agent and provides stimulation needed to learn from and interact with the environment. By age 6 months infants are very personable. They play games such as peek-a-boo when their head is hidden in a towel, they signal their desire to be picked up by extending their arms, and they show displeasure when a toy is removed or their face is washed.
Attachment
The importance of human physical contact to infants cannot be overemphasized. Parenting is not an instinctual ability but a learned, acquired process. The attachment of parent and child, which often begins before birth and assumes even more importance at birth (see Chapter 8), continues during the first year (Fig. 10-9). In the following discussion of attachment, the term mother is used in the broad context of the consistent caregiver with whom the child relates more than anyone else. However, with society’s changing social climate and sex-role stereotypes, this person may well be the father or a grandparent. Studies on paternal-infant attachment demonstrate that stages similar to maternal attachment occur and that fathers are more involved in child care when mothers are employed (although mothers continue to do the majority of infant care). Additional research has shown that inexperienced, first-time fathers are as capable as experienced fathers of developing a close attachment with their infants. Studies of fathers of high-risk infants demonstrate that fathers experience feelings of love and affection toward their offspring during the newborn period; fathers in one study verbalized more positive feelings of love and affection toward the newborn when they were able to have close physical contact such as holding the child (Sullivan, 1999). Children who had insecure attachment to their teenage mothers were found to have a strong attachment to the grandmother who was also a primary caretaker (Patterson, 1997). With many single-parent families in existence, a grandmother (or other significant caretaker) may become the primary caretaker. It is important for nurses to recognize that infant-parent attachments may be present or absent in situations wherein caretaker roles are less well defined by those involved.

FIG. 10-9 Infancy is an important time for attachment to significant others. (Photo by Paul Vincent Kuntz, Texas Children’s Hospital, Houston.)
Attachment progresses during infancy, with the child assuming an increasingly significant role. Two components of cognitive development are required for attachment: (1) the ability to discriminate the mother from other individuals, and (2) the achievement of object permanence. Both these processes prepare the infant for an equally important aspect of attachment: separation from the parent. Separation-individuation should occur as a harmonious, parallel process with emotional attachment.
During the formation of attachment to the parent, the infant progresses through four distinct but overlapping stages. For the first few weeks infants respond indiscriminately to anyone. Beginning at approximately 8 to 12 weeks of age, they cry, smile, and vocalize more to the mother than to anyone else but continue to respond to others, whether familiar or not. At approximately 6 months of age, infants show a distinct preference for the mother. They follow her more, cry when she leaves, enjoy playing with her more, and feel most secure in her arms. About 1 month after showing attachment to the mother, many infants begin attaching to other members of the family, most often the father.
Infants acquire other developmental behaviors that influence the attachment process. These include:
 Differential crying, smiling, and vocalization (more to the mother than to anyone else)
Differential crying, smiling, and vocalization (more to the mother than to anyone else)
 Visual-motor orientation (looking more at the mother, even if she is not close)
Visual-motor orientation (looking more at the mother, even if she is not close)
 Crying when the mother leaves the room
Crying when the mother leaves the room
 Approaching through locomotion (crawling, creeping, or walking)
Approaching through locomotion (crawling, creeping, or walking)
 Clinging (especially in the presence of a stranger)
Clinging (especially in the presence of a stranger)
 Exploring away from the mother while using her as a secure base
Exploring away from the mother while using her as a secure base
Reactive attachment disorder (RAD) is a psychologic and developmental problem that stems from maladaptive or absent attachment between the infant and parent and may persist into childhood and even adulthood (Wilson, 2001; Zeanah and Fox, 2004). Two different patterns of RAD have emerged: the emotionally withdrawn–inhibited pattern and an indiscriminate-disinhibited pattern (Zeanah and Fox, 2004). Signs of RAD are usually seen before the age of 5 years in infants who had insecure attachments to the mother or other primary caretaker (American Psychiatric Association, 2002). The child may manifest behaviors such as not being cuddly with parents, failing to make eye contact with significant others, having poor impulse control, and being destructive to self and others. Maltreated and orphaned children often are diagnosed with this complex disorder. Without early intervention, some of these children fail to develop a conscience and suffer from an antisocial personality disorder that may lead to criminal acts.
Separation Anxiety.: Between ages 4 and 8 months the infant progresses through the first stage of separation-individuation and begins to have some awareness of self and mother as separate beings. At the same time, object permanence is developing, and the infant is aware that the parent can be absent. Therefore separation anxiety develops and is manifested through a predictable sequence of behaviors.
During the early second half of the first year, infants protest when placed in their crib, and a short time later they object when the mother leaves the room. Infants may not notice the mother’s absence if they are absorbed in an activity. However, when they realize her absence, they protest. From this point on, they become alert to her activities and whereabouts. By 11 to 12 months they are able to anticipate her imminent departure by watching her behaviors, and they begin to protest before she leaves. At this point many parents learn to postpone alerting the child to their departure until just before leaving.
Stranger Fear.: As infants demonstrate attachment to one person, they correspondingly exhibit less friendliness to others. Between ages 6 and 8 months, fear of strangers and stranger anxiety become prominent and are related to infants’ ability to discriminate between familiar and unfamiliar people. Behaviors such as clinging to the parent, crying, and turning away from the stranger are common.
Language Development
The infant’s first means of verbal communication is crying. Crying as a biologic sign conveys a message of urgency and signals displeasure, such as hunger. However, crying is also a social event that affects the development of the parent-infant relationship—either by its absence, which usually has a positive effect on parents, or its presence, which may evoke a negative response or persuade parents to minister to the child’s physical or emotional needs.
In the first few weeks of life, crying has a reflexive quality and is mostly related to physiologic needs. Infants cry for 1 to 1½ hours a day up to 3 weeks of age and then build up to 2, and even 4, hours by 6 weeks. Crying tends to decrease by 12 weeks. It is thought that the increase in crying for no apparent reason during the first few months may be related to the discharge of energy and the maturational changes in the central nervous system. At the end of the first year, infants cry for attention, from fear (especially stranger fear), and from frustration, usually in response to their developing but inadequate motor skills.
Vocalizations heard during crying eventually become syllables and words (e.g., the “mama” heard during vigorous crying). Infants vocalize as early as 5 to 6 weeks of age by making small throaty sounds. By 2 months they make single vowel sounds such as ah, eh, and uh. By 3 to 4 months the consonants n, k, g, p, and b are added, and the infants coo, gurgle, and laugh aloud. By 8 months they imitate sounds, add the consonants t, d, and w, and combine syllables (e.g., “dada”), but they do not ascribe meaning to the word until 10 to 11 months of age (see Family Focus box). By 9 to 10 months they comprehend the meaning of the word “no” and obey simple commands. By age 1 year they can say three to five words with meaning. Because language development is based on expressive skills (ability to make thoughts, ideas, and desires known to others) and receptive skills (ability to understand the words being spoken), it is important that infants are exposed to expressive speech and that infants with delays in achieving milestones are carefully evaluated for potential hearing loss. (See Universal Newborn Hearing Screening, Chapter 8.)
Play
Play during infancy represents the various social modalities observed during cognitive development. The activity of infants is primarily narcissistic and revolves around their own body. As discussed under Development of Body Image (p. 330), body parts are primarily objects of play and pleasure.
During the first year, play becomes more sophisticated and interdependent. From birth to 3 months, infants’ responses to the environment are global and largely undifferentiated. Play is dependent; pleasure is demonstrated by a quieting attitude (1 month), a smile (2 months), or a squeal (3 months). From 3 to 6 months, infants show more discriminate interest in stimuli and begin to play alone with a rattle or a soft stuffed toy or with someone else. There is much more interaction during play. By 4 months of age they laugh aloud, show preference for certain toys, and become excited when food or a favorite object is brought to them. They recognize an image in a mirror, smile at it, and vocalize to it.
By 6 months to 1 year, play involves sensorimotor skills. Actual games such as peek-a-boo and pat-a-cake are played. Verbal repetition and imitation of simple gestures occur in response to demonstration. Play is much more selective, not only in terms of specific toys, but also in terms of “playmates.” Although play is solitary or one sided, infants choose with whom they will interact. At 6 to 8 months they usually refuse to play with strangers. Parents are definite favorites, and infants know how to attract their attention. At 6 months they extend the arms to be picked up, at 7 months cough to make their presence known, at 10 months pull the parent’s clothing, and at 12 months call them by name. This represents a tremendous advance from the newborn who signaled biologic needs by crying to express displeasure.
Stimulation is as important for psychosocial growth as food is for physical growth. Knowledge of developmental milestones allows nurses to guide parents regarding proper play for infants. It is not sufficient to place a mobile over a crib and toys in a playpen for a child’s optimum social, emotional, and intellectual development. Play must provide interpersonal contact and recreational and educational stimulation. Infants need to be played with, not merely allowed to play. Although the type of play infants engage in is called solitary, this is a figurative, not literal, term to denote one-sided play. The type of toys given to the child is much less important than the quality of personal interaction that occurs.
Table 10-1 lists play activities appropriate for the developmental level of the infant in view of motor, language, and personal-social achievements. Although the activities are grouped according to the major mode of stimulation provided, there is overlap in many instances. In addition, play activities suggested for one age-group may be appropriate for older infants but inappropriate for younger infants.
TEMPERAMENT
The infant’s temperament or behavioral style influences the type of interaction that occurs between the child and parents, especially the mother, and other family members (see Temperament, Chapter 5). In assessing a child’s temperament, it is the parents’ perception of the child and the degree of fit between their expectations and the child’s actual temperament that are important. The more dissonance, or lack of harmony, between the child’s temperament and the parent’s ability to accept and deal with the behavior, the more risk for subsequent parent-child conflicts.
Although most behavioral researchers agree there is a strong biologic component to temperament, researchers also suggest that temperament may be modified by the environment, particularly the family (Wilson, White, Cobb, and others, 2000). Family interaction with the infant is perceived as a circular process wherein each family member affects the others and the family as a unit. With these concepts in mind, the nurse has an important role in helping the family understand the infant’s temperament as it relates to family dynamics and the eventual well-being of the child and family unit (Wilson, White, Cobb, and others, 2000).
The Revised Infant Temperament Questionnaire (RITQ) can be used as a screening tool with parents (Carey and McDevitt, 1978). The questionnaire focuses on nine temperament variables, but the 95 questions relate specifically to activities such as sleep, feeding, play, diapering, and dressing. The scores from the RITQ help identify the child’s temperamental style. Use of the RITQ is well accepted by parents and should be accompanied by an adequate explanation of the results. In discussing the results, it is best to avoid descriptors such as “difficult”; instead, infants can be described in terms of characteristics such as “intense” or “less predictable.” The Early Infancy Temperament Questionnaire is a 76-item parent questionnaire that was adapted from the RITQ to specifically evaluate temperament characteristics of infants 1 to 4 months old, whereas the RITQ is best suited for infants 4 months old and older (Medoff-Cooper, Carey, and McDevitt, 1993).
With knowledge of the infant’s temperament, nurses are better able to (1) provide parents with background information that will help them see their child in a better perspective, (2) offer a more organized picture of their child’s behavior and possibly reveal distortions in their perceptions of the behavior, and (3) guide parents regarding appropriate childrearing techniques.
Childrearing Practices Related to Temperament
Most parents realize that their infant is born with unique characteristics, and few parents of difficult infants need to be told of the challenge of caring for them. However, few parents are aware of the significance of the temperamental characteristics and of constructive approaches to dealing with them. The following are examples of interventions that promote more positive parenting of infants with different temperament styles.*
“Difficult” children may respond better to scheduled feedings and structured caregiving routines than to demand feedings and frequent changes in daily routines. These children sleep less and may need more structured approaches to bedtime to prevent bedtime problems. “Highly distractible” children may require additional soothing measures such as swinging, rocking, or being carried in a pack that the parent wears across the chest or back. Children with “high activity” levels require vigilant watching, and parents need to take extra precautions in safeguarding the home. These children benefit from increased opportunities for gross motor activity to constructively channel their energy.
The child who is “slow to warm up” may demonstrate more stranger fear than other children and may require gradual and frequent preparation for new situations, such as substitute child care. Even the “easy child” can present problems in that the parents may need reminders to feed the child who sleeps for prolonged intervals and rarely cries. They may need to “retrain” the child because of the ease of developing habits such as keeping the child up late or sleeping with the youngster, which may later become troublesome.
Appropriate counseling based on awareness of the child’s temperament can greatly enhance the quality of interaction between parents and infant. Even just letting parents know that “difficult” traits are innate can relieve feelings of guilt and incompetence.
Because of the complexity of the developmental process during the first 12 months, Table 10-2 is presented to help organize and clarify the data already discussed. Although all milestones are important, some represent essential integrative aspects of development that lay the foundation for achievement of more advanced skills. These essential milestones are designated by a square ( ) in the table. The table represents the average monthly age at which various skills are attained. It must be remembered that, although the sequence is the same, the rate will vary among children.
) in the table. The table represents the average monthly age at which various skills are attained. It must be remembered that, although the sequence is the same, the rate will vary among children.
COPING WITH CONCERNS RELATED TO NORMAL GROWTH AND DEVELOPMENT
A number of fears can appear during infancy. However, the fear that causes parents the most concern is fear related to strangers and separation. Although erroneously interpreted by some as a sign of undesirable, antisocial behavior, stranger fear and separation anxiety are important components of a strong, healthy parent-child attachment. Nevertheless, this period can present difficulties for the parent and child. Parents may be more confined to the home because the infant violently protests having baby-sitters. To accustom the infant to new people, parents are encouraged to have close friends or relatives visit often. This provides other persons with whom the child is comfortable and can give parents time for themselves.
Infants also need opportunities to safely experience strangers. Usually toward the end of the first year, infants begin to venture away from the parent and demonstrate curiosity about strangers. If allowed to explore at their own rate, many infants eventually “warm up.” If parents hold the child away from their face, the infant can observe while maintaining close physical contact.
The best approach for the stranger (including nurses) is to talk softly; meet the child at eye level (to appear smaller); maintain a safe distance from the infant; and avoid sudden, intrusive gestures, such as holding the arms out and smiling broadly.
Parents also may wonder whether they should encourage the child’s clinging, dependent behavior, especially if there is pressure from others who view this as “spoiling” (see following discussion). Parents need to be reassured that such behavior is healthy, desirable, and necessary for the child’s optimal emotional development. If parents can reassure the infant of their presence, the infant will learn to realize that they are still there even if not physically present. Talking to infants when leaving the room, allowing them to hear one’s voice on the telephone, and using transitional objects (e.g., a favorite blanket or toy) reassures them of the parent’s continued presence.
Alternate Child Care Arrangements
For many parents, especially working mothers, locating safe and competent child care facilities for the infant is an increasingly difficult problem—one that is compounded by the number of mothers working outside the home. Over the past 40 years there have been variable shifts in child care arrangements; whereas the majority of children are cared for in group centers or other settings, there are increasingly more children being cared for in home settings.
The basic types of care are in-home care, either in the parents’ or caregivers’ home (family daycare), and center-based care, usually in a daycare center. In-home care may consist of a full-time baby-sitter who lives in the home, a full-time baby-sitter who comes to the home, cooperative arrangements such as exchange baby-sitting, or family daycare. A licensed small family childcare home typically provides care and protection for up to six children for part of a 24-hour day and does not include informal arrangements such as exchange baby-sitting or caregivers in the child’s own home. The six children may include the family daycare provider’s own children younger than 5 years of age living in the home. Large family childcare homes may provide care for eight to twelve children. Unfortunately, many family daycare homes operate without a license and may care for large numbers of infants without adequate staff and facilities.
Child center-based care usually refers to a licensed daycare facility that provides care for six or more children, for 6 or more hours in a 24-hour day. Work-based group care is another option that is becoming increasingly popular as employers recognize the benefit of providing high-quality and convenient child care to their employees. Sick-child care may also be available for times when the youngster is ill. Such programs are often located in community hospitals or in work settings.
A major nursing responsibility is guiding parents in locating suitable facilities that have a well-qualified staff. State licensing agencies can help parents identify daycare centers that accept children of specific age-groups and are convenient to home and work. Their records are available to the public and provide reports from the health, safety, and fire departments; periodic evaluations from the licensing agency; complaints filed against the center; and qualification of the center’s employees. State-licensed programs are supposed to abide by established standards, which represent the minimum requirements and safeguards. However, enforcement of the standards is sometimes inadequate.
Early childhood programs may also belong to a voluntary accreditation system sponsored by National Association for the Education of Young Children, which serves as a model for optimum care.* References from other parents are also helpful, provided that they have investigated the center carefully and have remained involved with the agency’s activities.
Guidelines for selecting child care facilities are discussed under Preschool and Kindergarten Experience, Chapter 13. The same conscientious attention should be applied to locating competent baby-sitters. References from other employers are essential, and there is no substitute for observing the interaction between the individual and the child. Although very young infants need little if any preparation for the introduction of a new caregiver, older infants may benefit from a gradual placement to reduce stranger anxiety. At all times the parent should have the right to visit the child, and regular conferences should be established to review the child’s progress.
One of the areas that is increasingly important in selecting child care is the center’s health practices; however, parents often do not check the center for health and safety features. Evidence shows that children, especially those under age 3 years in daycare centers, have more illnesses—especially diarrhea, otitis media, respiratory tract infections (especially if the caregiver smokes), hepatitis A, meningitis, and cytomegalovirus—than children cared for in their home. The strongest predictor of risk of illness is the number of unrelated children in the room. Proactive infection control measures and education of staff have been effective in reducing the incidence of upper respiratory tract infections, diarrhea, and rotavirus. It has been reported that families who have children in out-of-home child care lose an estimated 13 days of work per year as a result of infections (Brady, 2005). Parents should inquire about the center’s policy regarding the attendance and care of sick children.
Limit Setting and Discipline
As infants’ motor skills advance and mobility increases, parents are faced with the need to set safe limits to protect the child and establish a positive and supportive parent-child relationship (see Nurse’s Role in Injury Prevention, p. 372). Although there are numerous disciplinary techniques, some are more appropriate for this age than others. An effective approach used in disciplining a child is the use of time-out. The basic principles are the same as those discussed in Chapter 3, except that the place for time-out needs to be commensurate with the child’s abilities. For example, the playpen is better for most infants than a chair. Although parents may be concerned about instituting discipline during infancy, it is important to stress that the earlier effective disciplinary methods are employed, the easier it is to continue these approaches.
Parents must recognize the infant’s cognitive and behavioral limitations; adequate protection from hazards must be implemented because infants and toddlers do not understand a cause-and-effect relationship between dangerous objects and physical harm. Additionally, parents may need reassurance that their infant’s behavior is exploratory in nature, not oppositional (at this age) and primarily centered on the infant’s basic needs of warmth, love, food, security, and comfort. Parents may verbalize that comforting the infant too much or meeting its needs will result in a spoiled child; there is no substantial evidence that meeting the infant’s basic needs will result in such behaviors later in life. Children will innately test limits and explore during the exploratory phase of growth; instead of discouraging exploration, parents should provide safe alternatives, put dangerous household items away, and give children consistent discipline and nurturing.
Effective teaching for injury prevention optimally begins in infancy by helping parents understand the nature of their child’s normal development. It must be reiterated continually that infants cry because a need is not being met, not to intentionally irritate an adult. The fussy or irritable infant is a potential victim of shaken baby syndrome (or other bodily harm), since adults and caretakers may not understand the nature of the infant’s crying.
Thumb Sucking and Use of a Pacifier
Sucking is the infant’s chief pleasure and may not be entirely satisfied by breastfeeding or bottle feeding. It is such a strong need that infants who are deprived of sucking, such as those with a cleft lip repair, will suck on their tongue. Some newborns are born with sucking blisters on their hands from in utero sucking activity. The benefits of nonnutritive sucking in preterm infants include increased weight gain, decreased length of stay, and improved pain management (Pinelli and Symington, 2000; Pinelli, Symington, and Ciliska, 2002). There is currently no evidence that pacifier use and nonnutritive sucking in preterm infants has any effect on the initiation and length of breastfeeding. Nonnutritive sucking should not be withheld from preterm infants, especially when performed in conjunction with the use of concentrated sucrose for pain management.
Pacifier use, particularly in the early days after birth and in the birth hospital, has gained considerable attention in the scientific literature. Some experts now state that “nontherapeutic” pacifier use should be discouraged, since there are no known benefits to its use other than for nonnutritive sucking and for managing pain.
Biancuzzo (2003) suggests that it cannot be stated with absolute certainty that pacifier use is bad in every situation but warns there is potential harm in the use of pacifiers based on available evidence. Furthermore, she admonishes health care workers to be informed regarding potential harm in pacifier use and to inform parents of the potential. Lawrence and Lawrence (2005), as well as other experts in breastfeeding, recommend that health care workers not introduce pacifiers to breast-fed infants unless at the request of the parent. Pacifier use is not recommended as part of the Baby-Friendly Hospital Initiative. (See Box 8-5, p. 230.)
A review of studies by the Joanna Briggs Institute (2005) found an association between pacifier use in infancy and a reduction in breastfeeding and exclusive breastfeeding. However, the authors concluded that pacifier use did not cause a reduction in breastfeeding; rather it was a “marker for socioeconomic, demographic, psychosocial and cultural factors that determine pacifier use and breastfeeding.” In addition, the researchers examined studies related to pacifier use and prevention of SIDS; infants put to sleep with a pacifier had a reduced risk of SIDS. Because of the limited number of studies correlating pacifier use and increased risk of infections or dental malocclusion, the authors were unable to make any recommendations for or against pacifier use in relation to these practices (Joanna Briggs Institute, 2005).
The American Academy of Pediatrics (2005) recommends limited pacifier use in infants, citing the strong evidence for pacifier use and its protective effect in SIDS reduction; the exact mechanism involved in the protection for SIDS is not known. Still, pacifier use should not replace actual feeding or suckling; prohibiting pacifier use will not absolutely ensure an increase in the length of breastfeeding; and there should be an emphasis on allowing the infant to control the pace, frequency, and termination of feeding rather than allowing the pacifier (or anything else) to become the focus of the interaction.
To decrease dependence on nonnutritive sucking in young infants, sucking pleasure can be increased by prolonging feeding time. Also, the parent’s excessive use of the pacifier to calm the child should be explored. It is not unusual for parents to place a pacifier in the infant’s mouth as soon as crying begins, thus reinforcing a pattern of distress-relief.
The effect of continual pacifier use on early speech and language development is unknown, but the pacifier may decrease the child’s desire to imitate sounds and affect intelligibility. Parents need to be alerted that continual dependence on a pacifier may influence social and speech development.
If the child uses a pacifier, safety considerations in purchasing one must be stressed. Parents should be cautioned against altering a pacifier, thus making it more dangerous (see Aspiration of Foreign Objects, p. 365).
During infancy and early childhood there is no need to restrain nonnutritive sucking of the fingers. Malocclusion may occur if thumb sucking persists past 4 to 6 years of age, or when the permanent teeth erupt. Pacifiers may be perceived by some parents as less damaging because they are discarded by 2 to 3 years of age, whereas thumb sucking may persist well into school-age years. Both pacifier use and thumb sucking may also have significant cultural variations.
Thumb sucking reaches its peak at age 18 to 20 months and is most prevalent when the child is hungry, tired, or feeling insecure. Persistent thumb sucking in a listless, apathetic child always warrants investigation. It may be a sign of an emotional problem between parent and child or of boredom, isolation, and lack of stimulation.
Teething
One of the more difficult periods in the infant’s (and parents’) life is the eruption of the deciduous (primary) teeth, often referred to as teething. The age of tooth eruption shows considerable variation among children, but the order of their appearance is fairly regular and predictable (Fig. 10-10). The first primary teeth to erupt are the lower central incisors, which appear at approximately 6 to 10 months of age (average 8 months). These are followed closely by the upper central incisors.
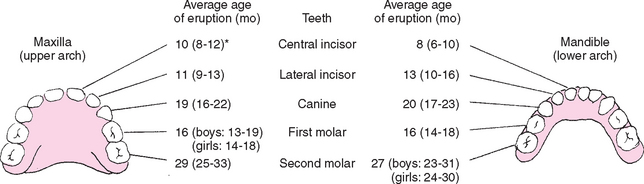
FIG. 10-10 Sequence of eruption of primary teeth. *Range represents ±1 standard deviation, or 67% of subjects studied. (Data from McDonald RE, Avery DR: Dentistry for the child and adolescent, ed 6, St Louis, 1994, Mosby.)
Teething is a physiologic process; some discomfort is common as the crown of the tooth breaks through the periodontal membrane. Some children show minimum evidence of teething, such as drooling, swollen gums, increased finger sucking, or biting on hard objects. Others are very irritable, have difficulty sleeping, and refuse to eat. Generally, signs of illness such as fever, vomiting, or diarrhea are not symptoms of teething but of illness and may warrant further investigation (Macknin, Piedmonte, Jacobs, and others, 2000). Anderson (2004) suggests that frequent waking periods are related to environmental, behavioral, or developmental changes rather than teething. The author also cautions parents (and health care workers) to not overdiagnose teething; the ill-appearing child or child with a temperature over 38° C (100.4° F) should be evaluated by the practitioner.
Because teething pain is a result of inflammation, cold is soothing. Giving the child a frozen teething ring or an ice cube wrapped in a washcloth helps relieve the inflammation. Several nonprescription topical anesthetic ointments are available, such as Baby Ora-Jel, although parents and health care workers should be aware of the risks of using topical anesthetic products (absorption rates vary in infants) (Anderson, 2004). The active ingredient in most of them is benzocaine. If such products are used, parents are advised to apply them correctly. In the event of persistent irritability that affects sleeping and feeding, systemic analgesics such as acetaminophen or ibuprofen can be given (if age appropriate) for no more than 3 days (Anderson, 2004); however, parents should know that this is a temporary measure and should contact the practitioner if symptoms persist or if the child’s condition changes.
Infant Shoes
Many parents are unaware of the types of shoes that are appropriate for the older infant and buy expensive infant shoes because of misleading advertising claims. Inflexible shoes that have hard soles can be detrimental by delaying walking, aggravating intoeing or outtoeing, and impeding the development of supportive foot muscles. Therefore counseling parents regarding footwear should begin when infants are 6 months old—well before they are walking.
It is helpful to begin by explaining to parents that changes in the feet occur during infancy and early childhood as locomotion and weight bearing progress. At birth the feet are flat because the arches are protected by fat pads on the soles. As the bones in the arches develop, the pads disappear and the feet begin to assume a mature shape. A normal arch is determined by proper alignment of all the bones and development of the surrounding musculature, not by the height of the arch.
When children begin walking, the main reason for shoes is protection. To provide protection, the shoe should retain its fit; be made of durable material with a smooth interior and few construction seams to irritate the skin; and be soft and flexible, especially in the toe area. A high-top shoe is not necessary for support but may be helpful in keeping the foot in the shoe. A good infant-toddler shoe is one that can easily be bent in half by squeezing the heel and toe with the thumb and index finger (Wall, 2000).
A good shoe conforms to the anatomic shape of the foot, with a rounded toe and sufficient toe room. During weight bearing there should be the space of at least half the width of the thumbnail, or 1.25 cm (0.5 inch), between the end of the longest toe and the shoe. Roomy and square-toed socks allow for proper growth and alignment. Inexpensive but well-constructed athletic shoes or soft-leather moccasin-type shoes are suggested as adequate footgear for walking infants.
Even if the shoes are fitted properly, frequent changes are needed to accommodate the infant’s rapidly growing feet. Shoe size changes at approximately 3-month intervals between 12 and 36 months; during this time the child’s foot should be measured every 3 months. Curled toes when shoes are removed and redness and irritation of the skin on the bottom of the toes indicate the need for a larger size.
PROMOTING OPTIMAL HEALTH DURING INFANCY
Ideally, discussion of optimal nutrition should begin prenatally with a discussion regarding maternal intake of adequate nutrition in the form of a balanced diet and adequate amounts of protein, vitamins, and minerals, all of which have an impact on the growing fetus. Nurses should encourage and provide information for parents to discuss the options of breastfeeding or bottle feeding the infant well in advance of the delivery date. The choice for either is highly individual and is discussed in Chapter 8. This section is primarily concerned with infant nutrition during the months when growth needs and developmental milestones ready the child for the introduction of solid foods.
Despite adequate availability of optimal nutrient sources, concern remains that infants are not being fed appropriately. These practices may have far-reaching, long-term health consequences, affecting infants and, as they grow, children and adults. Certain chronic health conditions have been linked to feeding practices in infancy. Nurses must continue to be proactive in teaching parents about what constitutes appropriate infant nutrition and nutritional habits, which provide the opportunity to grow and develop into a healthy child and adult.
Health care professionals have recently become more aware of the use of complementary and alternative medical therapies in children that may not be as beneficial as touted in various media sources. One concern is children’s intake of megavitamins and herbs; parents may assume that the word natural in reference to ingredients means the product is safe, when this may not be the case. One report recently cited the home administration of star anise tea to treat colic as the cause of adverse neurologic reactions in seven infants (Ize-Ludlow, Ragone, Bruck, and others, 2004). It is important for nurses to be aware of the effects, availability, and practice of complementary therapies and to be able to cogently discuss their use with parents (Loman, 2003; Niggemann and Grüber, 2003).
The First 6 Months
Human milk is the most desirable complete diet for the infant during the first 6 months. The normal infant receiving breast milk from a well-nourished mother usually requires no specific vitamin or mineral supplements, with a few exceptions. The American Academy of Pediatrics (2003) recommends that all infants (including those exclusively breast-fed) receive a daily supplement of 200 IU of vitamin D beginning in the first 2 months of life to prevent rickets and vitamin D deficiency. If the infant is being exclusively breast-fed after 4 to 6 months (when fetal iron stores are depleted), iron supplementation, which may be accomplished with iron-fortified cereal, is recommended to offset the decrease in iron available in human milk at this time and to enhance erythropoiesis. Infants who are breast-fed or bottle-fed do not require additional fluids, especially water or juice, during the first 4 months of life. Excessive intake of water in infants may result in water intoxication, failure to thrive, and hyponatremia.
Employed mothers can continue breastfeeding with guidance and encouragement. Mothers are encouraged to set realistic goals for employment and breastfeeding, with accurate information regarding the costs, risks, and benefits of available feeding options. Barriers encountered by working breastfeeding mothers include lack of employer or co-worker support, unavailable or inadequate facilities for pumping and storing milk, and insufficient time allowed to pump during work (Rojjanasrirat, 2004). Important themes that emerged in the study by Rojjanasrirat (2004) of working breastfeeding mothers included support (emotional, informational, and instrumental), attitude, and psychologic distress.
Many mothers may find that a program of breast pumping when away from home and bottle feeding the infant the expressed milk with or without formula supplementation is successful. Expressed breast milk may be stored in the refrigerator (4° C [39° F]) without danger of bacterial contamination for up to 5 days (Lawrence and Lawrence, 2005). Although feeding the infant at home may occur on a demand basis, pumping milk away from home may be needed every 3 to 4 hours to maintain adequate supply. Breast milk may be expressed by hand or pump (manual or electric) and stored in an appropriate air-tight glass or plastic container. Expressed breast milk may be frozen (−18° C [0° F] or lower) for up to 12 months (depending on the type of freezer used), but care should be taken to prevent freezer burn (see Appendix P, protocol no. 8, in Lawrence and Lawrence, 2005, for further guidelines on storing and freezing human milk). Health care workers and new mothers may find the booklet “Working and Breastfeeding—Can You Do It? Yes, You Can!” (available in English and Spanish) by Johnson & Johnson helpful.*
In addition to efficient breast pumping, mothers also need child care by a trusted individual or agency and support and assistance from significant others. As with all breastfeeding mothers, these women must have proper nutrition and rest for adequate lactation. Maternal fatigue is considered the biggest threat to successful breastfeeding in employed mothers.
An acceptable alternative to breastfeeding is commercial iron-fortified formula. Like human milk, it supplies all nutrients needed by the infant for the first 6 months. Unmodified whole cow’s milk, low-fat cow’s milk, skim milk, other animal milks, and imitation milks are not acceptable as a major source of nutrition for infants because of their limited digestibility, increased risk of contamination, and lack of components needed for appropriate growth. Whole milk can cause iron deficiency anemia in infants, possibly as a result of occult gastrointestinal blood loss. Pasteurized whole cow’s milk is deficient in iron, zinc, and vitamin C and has a high renal solute load, which makes it undesirable for infants less than 12 months of age (American Academy of Pediatrics, 2004).
The amount of formula per feeding and the number of feedings per day vary among infants. Infants being fed on demand usually determine their own feeding schedule, but some infants may need a more planned schedule based on average feeding patterns to ensure sufficient nutrients. In general, the number of feedings decreases from six at 1 month of age to four or five at 6 months. Regardless of the number of feedings, the total amount of formula ingested will usually level off at about 32 ounces (946 ml) per day. Water supplementation is not recommended for healthy infants because it may lead to water intoxication (American Academy of Pediatrics, 2004).
The addition of solid foods before 4 to 6 months of age is not recommended. During the early months solid foods are not compatible with the ability of the gastrointestinal tract and infant’s nutritional needs. Feeding solids to young infants exposes them to food antigens that may produce food protein allergy. There is now ample evidence that early introduction of foods other than maternal milk in the first 6 months of life predisposes children to an increased risk for food allergy development; foods known to be allergenic (e.g., peanuts, eggs, fish, seafood) should be introduced later than 12 months according to the child’s risk for atopy (Fiocchi, Assa’ad, Bahna, and others, 2006).
Developmentally, infants are not ready for solid food. The extrusion (protrusion) reflex is strong and often causes them to push food out of the mouth. Infants instinctively suck when given food. Because of their limited motor abilities, infants are unable to deliberately push food away or avoid feeding. Therefore early introduction of solids is a type of forced feeding that may lead to excessive weight gain and increased predisposition to allergies and iron deficiency anemia. Parents should be cautioned concerning the excessive use of juices and nonnutritive drinks such as fruit-flavored drinks or carbonated beverages (soda or pop) during this period. Many juices and nonnutritive drinks, although readily available to consumers, do not provide sufficient and appropriate caloric intake for infants less than 12 months of age; such drinks may replace the nutrients in breast milk or formula and lead to growth or health problems. Fruit juices are not required in the first 6 months; there are no studies demonstrating benefits of giving fruit juice to infants.
The Second 6 Months
During the second half of the first year, human milk or formula should optimally continue to be the primary source of nutrition. Fluoride supplementation should begin, depending on the infant’s intake of fluoride (in formula mixed with tap water or bottled water [containing fluoride] as appropriate) (see Dental Health, p. 349). If breastfeeding is discontinued, a commercial iron-fortified formula should be substituted. Follow-up or transition formulas marketed for older infants offer no special advantages over other infant formulas and provide excessive protein (American Academy of Pediatrics, 2004).
The major change in feeding habits is the addition of solid foods to the infant’s diet. Physiologically and developmentally, the infant 4 to 6 months of age is in a transition period. By this time the gastrointestinal tract has matured sufficiently to handle more complex nutrients and is less sensitive to potentially allergenic foods. Tooth eruption is beginning and facilitates biting and chewing. The extrusion reflex has disappeared, and swallowing is more coordinated to allow the infant to accept solids easily. Head control is well developed, which permits infants to sit with support and purposely turn the head away to communicate lack of interest in food. Voluntary grasping and improved eye-hand coordination gradually allow infants to pick up finger foods and feed themselves. Their increasing sense of independence is evident in their desire to hold the bottle and try to “help” during feeding.
Selection and Preparation of Solid Foods
The choice of solid foods to introduce first is variable but should meet the reasons for feeding solids, such as supplying nutrients not found in formula or breast milk. Iron-fortified infant cereal is generally introduced first because of its high iron content (7 mg/3 Tbsp of prepared dry cereal). Commercially prepared ready-to-serve dry cereals for infants include rice, barley, oatmeal, and high-protein cereals; rice is usually suggested as an initial food because of its easy digestibility and low allergenic potential. Cereals such as cream of farina are not used, since infant commercial cereals are a better source of iron. Some of the commercial baby cereals are combined with fruit. There is little nutritional benefit from these preparations, and they are more expensive. New foods should be added one at a time; therefore parents should avoid cereal combinations when beginning a new grain.
Infant cereal (iron fortified) may be mixed with expressed breast milk or water until whole milk is given. After 6 months of age, small amounts of fruit juices can be mixed with the dry cereal; the vitamin C content of the juice enhances the absorption of iron in the cereal. Because of their benefit as a source of iron, infant cereals should be continued until the child is 18 months of age.
Fruit juice can be offered from a cup for its rich source of vitamin C and as a substitute for milk for one feeding a day. Large quantities of certain juices (e.g., apple, pear, prune, sweet cherry, peach, grape) are avoided because they may cause abdominal pain, diarrhea, or bloating in some children. Avoid fruit-flavored drinks, which may be marketed as juices but contain high concentrations of complex sugars. White grape juice (no more than 5 oz/day) is reported to be better absorbed and safe for infants this age without causing gastrointestinal distress (Calamaro, 2000). Some researchers have found fruit juice, particularly apple juice, to exacerbate colic and diarrhea, possibly because of carbohydrate malabsorption (Duro, Rising, Cedillo, and others, 2002; Moukarzel, Lesicka, and Ament, 2002). The American Academy of Pediatrics (2001b, 2006a) recommends that fruit juice intake not exceed 4 to 6 ounces per day and that juices not be given to infants less than 4 to 6 months old. Because vitamin C is naturally destroyed by heat, juice is not warmed. Juice containers are always kept covered and refrigerated to prevent further vitamin loss.
The addition of other foods is arbitrary. A common sequence is to introduce strained fruits followed by vegetables and, finally, meats; however, some clinicians prefer to add vegetables before fruit. If foods are introduced early, citrus fruits, meats, and eggs are delayed until after 6 months of age because of their potential to result in allergy. At 6 months, foods such as a cracker or zwieback can be offered as finger and teething foods. By 8 to 9 months, junior foods and nutritious finger foods such as firmly cooked vegetable, raw pieces of fruit, or cheese can be given. By 1 year, well-cooked table foods are served.
The introduction of solid foods into the infant’s diet at this age is primarily for taste and chewing experience. The majority of the infant’s caloric needs are derived from the primary milk source (human or formula); therefore solids should not be perceived as a substitute for milk until the child is older than 12 months. Portion sizes may vary according to the infant’s taste. In general, 1 tablespoon per year of age (i.e., ½ to ¾ tablespoon for most infants under 12 months) is adequate for most infants. In most cases 2 tablespoons may be served, but because of the infant’s focus on the texture and feel of the food, smaller amounts will be consumed. Another reason for smaller portions is the concern over feeding habits in early childhood and obesity; early feeding of smaller portions may help prevent the “clean your plate” or “eat all your food or you can’t get down from the table” concepts, which are known to contribute to overeating in later life. The addition of solid foods to the exclusively breast-fed infant’s diet does not significantly increase overall caloric intake or weight gain (Dewey, 2001).
Commercially prepared baby foods are the most common type of food served to infants in the United States. They are convenient but sometimes contain added salt or sugar and can be relatively expensive. An alternative is to prepare baby foods at home, which is a simple and inexpensive process. Fruits and vegetables can be steamed in a small amount of water and pureed in a blender or food processor. Many of them, such as ripe banana, can be mashed fine with a fork. Fruits such as apples or pears require little or no water in the cooking process. Vegetables such as carrots, potatoes, or string beans require additional water in the cooking and blending process.
Parents are cautioned to avoid reliance on foods and supplements marketed as iron- or vitamin-fortified as primary sources of minerals. Instead encourage parents to offer the child a variety of fruits, vegetables, and whole grains, including those known to naturally be rich in iron (Fox, Reidy, Novak, and others, 2006).
Introduction of Solid Foods
When the spoon is first introduced, infants often push it away and appear dissatisfied. Some patience and skill are required to overcome this initial response. Food that is placed on the front of the tongue and pushed out is simply scooped up and refed. As infants become accustomed to the spoon, they will more eagerly accept the food and eventually open the mouth in anticipation (or keep it closed in dislike). Because the first introduction of food is a new experience, spoon feeding should be attempted after ingestion of some breast milk or formula to associate this activity with a pleasurable and satisfying experience. Trying to introduce a food after the entire milk feeding is generally useless because the infant is satiated and has no inclination to try something new.
After several spoon feedings, food can be introduced at the beginning of a meal. It is best to introduce many foods during the first year, when the infant is more likely to eat them because of a hearty appetite resulting from a rapid growth rate. During the toddler years eating becomes less of an adventure, and strong food preferences emerge.
One food item is introduced at intervals of 4 to 7 days to allow for identification of food allergies. New foods are fed in small amounts, from 1 teaspoon to a few tablespoons. As the amount of solid food increases, the quantity of milk is decreased to less than 1 L/day to prevent overfeeding.
Because feeding is a learning process, as well as a means of nutrition, new foods are given alone to allow the child to learn new tastes and textures. Food should not be mixed in the bottle and fed through a nipple with a large hole. This deprives the child of the pleasure of learning new tastes and developing a discriminating palate. It can also cause problems with poor chewing of food later in life because of lack of experience. Guidelines for the introduction of new foods are given in the Family-Centered Care box.
Introducing solid foods can be an exciting time for parent and child. Most infants are good eaters and enjoy eating from a spoon and later feeding themselves. However, the transition from “parent doing it” to “baby doing it” can be a trying experience, particularly for those who value a clean house or who view cleaning up the mess as a waste of time. Finger foods such as soft fruits or vegetables are just as good as playthings as food; they can be squeezed, smeared, squashed, and thoroughly painted on oneself, others, and the environment. However, all of this is part of learning, and mastery follows many accidents.
Weaning
Defined as the process of giving up one method of feeding for another, weaning usually refers to relinquishing the breast or bottle for a cup. In Western societies this is generally regarded as a major task for infants and is often seen as a potentially traumatic experience. It is psychologically significant because the infant is required to give up a major source of oral pleasure and gratification.
There is no one time for weaning that is best for every child, but generally most infants show signs of readiness during the second half of the first year. They have learned that good things come from a spoon. It is recommended that weaning be accomplished with the infant’s needs as a guide (Lawrence and Lawrence, 2005). Their increasing desire for freedom of movement may lessen their desire to be held close for feedings. They are acquiring more control over their actions and can easily manipulate a cup to their lips (even if it is held upside down!). Imitation becomes a powerful motivator by age 8 or 9 months, and they enjoy using a cup or glass like others do.
Weaning should be gradual by replacing one bottle or breastfeeding session at a time. The nighttime feeding is usually the last feeding to be discontinued. It is advisable to never allow a child to take a bottle of milk to bed—this is a major cause of nursing caries in deciduous teeth. If breastfeeding is terminated before 5 or 6 months of age, weaning should be to a bottle to provide for the infant’s continued sucking needs. If discontinued later, weaning can be directly to a cup, especially by age 12 to 14 months. Any sweet liquid, such as fruit juice, should be given in a cup, and not at bedtime.
SLEEP AND ACTIVITY
Sleep patterns vary among infants, with active infants typically sleeping less than placid children. Generally, by 3 to 4 months of age most infants have developed a nocturnal pattern of sleep that lasts 9 to 11 hours. The total daily sleep is approximately 15 hours. The number of naps per day varies, but infants may take one or two naps by the end of the first year. Breast-fed infants usually sleep for shorter periods, with more frequent waking, especially during the night, compared with bottle-fed infants (Quillin and Glenn, 2004); average total sleep for 4-week-old infants in this study was 14 hours. Because of the trend toward breastfeeding, sleep norms such as those previously described, which were based primarily on bottle-fed infants, may not be relevant.
Most infants are naturally active and need no encouragement to be mobile. Problems can arise when devices such as playpens, strollers, commercial swings, and mobile walkers are used excessively. These items restrict movement and prevent infants from exploring and developing gross motor skills. Contrary to popular belief, mobile walkers do not enhance coordination and are dangerous if tipped over or placed near stairs. The American Academy of Pediatrics (2001a) recommended a ban on the sale of infant walkers because of the large number of injuries. Newer models of infant walkers have been designed to decrease infant injuries (see Falls, p. 370).
Sleep Problems
A number of sleep problems are identified in small children. The two major categories are the dyssomnias: the child has trouble either falling or staying asleep at night, or has difficulty staying awake during the day. The second category, parasomnias, are characterized as confusional arousals, sleep-walking, sleep terrors, nightmares, and rhythmic movement disorders; these typically occur in children 3 to 8 years old (Dahl, 1998) and decline in incidence as the child matures (Davis, Parker, and Montgomery, 2004). This discussion focuses on minor sleep issues in infants such as refusal to go to sleep or frequent waking during the night (Table 10-3). Other sleep disturbances such as obstructive sleep apnea and sleep terrors are discussed elsewhere in this text.
TABLE 10-3
Selected Sleep Disturbances During Infancy and Early Childhood
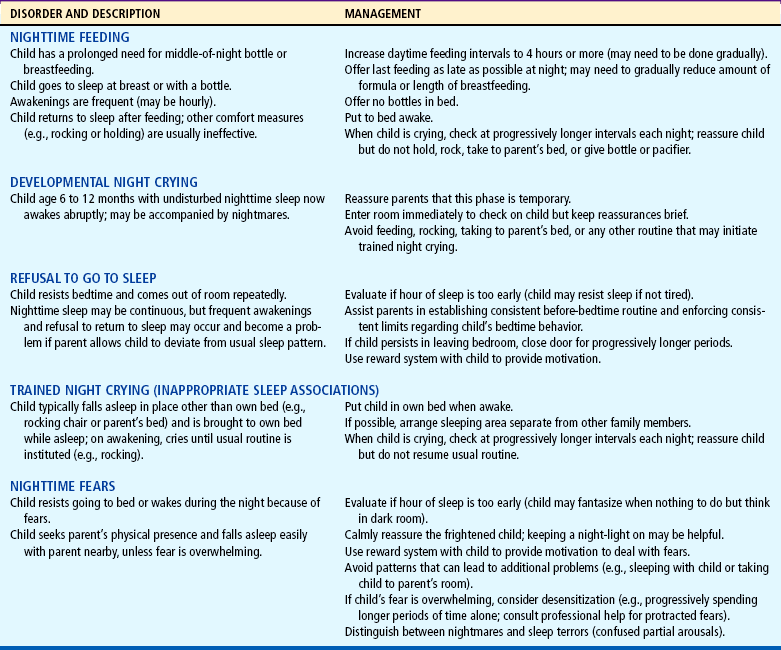
Modified from Ferber R: Behavioral “insomnia” in the child, Psychiatr Clin North Am 10(4):641–653, 1987.
Concerns regarding sleep are common during infancy (see Cultural Awareness box). Sometimes these concerns are as basic as parents’ questioning whether the infant needs additional sleep. In this case it is best to investigate the reason for their concern, stressing the individual needs of each child. Infants who are active during wakeful periods and growing normally are sleeping a sufficient amount of time.
Sleep problems in early infancy have also been positively correlated with higher maternal depression scores (Hiscock and Wake, 2001; Hawkins-Walsh, 2003) and poorer general and mental health in both mother and father (Martin, Hiscock, Hardy, and others, 2007); therefore nurses must discuss infant sleep problems with the mother (and family) in addition to other developmental aspects of newborn care.
When a sleeping problem is presented, a careful assessment is essential. Charting sleep habits both before and after interventions is also an important strategy. Questions regarding the frequency and duration of waking, the usual bedtime routine, the number of nighttime feedings, the perceived problem (e.g., how much disruption the behavior generates), and the attempted interventions are important in planning effective approaches designed for the specific sleep problem. A common suggestion given for any type of sleep problem, “Let the child cry until he or she falls asleep,” is very difficult to implement and is inappropriate for certain conditions. After the parents relent and console the child, they have only reinforced the crying.
Another approach to night crying is known as graduated extinction . This involves letting the child cry for progressively longer times between brief parental interventions that consist only of reassurance—not rocking, holding, or using a bottle or pacifier. For example, the parents may check on the child every 5 minutes (of crying) during the first night and progressively extend this interval by 5 minutes on successive nights (Ferber and Kryger, 1995).
Families who cannot tolerate unexpected crying spells while everyone else is asleep can try the two-step approach. Graduated extinction is used during naps and at bedtime until the parents retire. If the child cries during the night, the parents use comforting measures. However, after the child is partially trained, step 2 is initiated—the use of graduated extinction at all times.
Children who learn to fall asleep on their own at bedtime have longer sustained sleep periods than those who fall asleep with a parent present (Davis, Parker, and Montgomery, 2004). In addition, comforting children outside their own bed at night once they awaken was associated with poor sleep consolidation. Feeding the 5-month-old after awakening at night has been associated with fewer consecutive sleep hours (Touchette, Petit, Paquet, and others, 2005). The authors of this study recommend parental presence at bedtime until the child is drowsy, then placing the child in his or her own bed for a night’s sleep.
The best way to prevent sleep problems is to encourage parents to establish bedtime rituals that do not foster problematic patterns. One of the most constructive is placing infants awake in their own crib. When infants are accustomed to falling asleep somewhere else, such as in their parent’s arms, and then being transferred to their crib, they awaken in unfamiliar surroundings and are unable to fall asleep until the routine is repeated. Also, the bed should be used for sleeping only—not as a playpen. It is advisable not to hang playthings over or on the bed; in this way the child associates the bed with sleep, not with activity. Although the interventions described previously and in Table 10-3 are usually successful, it is much easier to prevent the problem with appropriate counseling during the early months of the infant’s life.
DENTAL HEALTH
Good infant dental hygiene begins with appropriate maternal dental health prior to and during the pregnancy and counseling during early infancy regarding dietary intake for the promotion of optimum oral hygiene (Douglass, Douglass, and Silk, 2004). Parents are counseled early regarding the risk of feeding practices that increase the risk of poor dental health. Some of these, as previously mentioned, include avoiding propping the milk bottle, giving the milk bottle in the bed, or giving fruit juices in a bottle, especially before 6 months of age.
Once the primary teeth erupt, cleaning should begin. The teeth and gums are initially cleaned by wiping with a damp cloth; toothbrushing is too harsh for the tender gingiva. The caregiver can stabilize the infant by cradling the child with one arm and using the free hand to cleanse the teeth. Oral hygiene can be made pleasant by singing or talking to the infant. It is recommended that the infant have an oral health examination by 6 months of age from a qualified pediatric health practitioner. Infants at high risk for dental caries should be seen by a dentist between 6 months and 1 year of age (American Academy of Pediatric Dentistry, 2006). It is generally recommended that a small, soft-bristled toothbrush be used as more teeth erupt and the infant adjusts to the routine of cleaning. Water is preferred to toothpaste, which the infant will swallow (and if the toothpaste is fluoridated, the infant will ingest excessive amounts of fluoride).
Fluoride, an essential mineral for building caries-resistant teeth, is needed beginning at 6 months of age if the infant does not receive water with adequate fluoride content. The American Academy of Pediatric Dentistry recommends fluoride supplementation begin at 6 months. The fluoride dosage has been decreased from earlier recommendations because of an increased occurrence of dental fluorosis from excessive fluoride ingestion. The latest recommendation is to give children 6 months to 3 years of age 0.25 mg of fluoride daily if water fluoride content is less than 0.3 ppm (parts per million) (American Academy of Pediatric Dentistry, 2006). If bottled water is used to reconstitute powdered or concentrated formula, it should either be fluoride free or contain low levels of fluoride (American Dental Association, 2007).
Dietary considerations are also important because habits begun during infancy tend to continue into later years. Foods with concentrated sugar are used sparingly (if at all) in the infant’s diet. The practice of coating pacifiers with honey or using commercially available hard-candy pacifiers is discouraged. Besides being cariogenic, honey also may cause infant botulism, and parts of the candy pacifier can be aspirated (see Aspiration of Foreign Objects, p. 365). Parents need to be counseled regarding the detrimental effects of frequent and prolonged bottle feeding or breastfeeding during sleep, when the sweet milk or other fluid, such as juice, bathes the teeth, producing nursing caries. In addition, carbonated beverages should be avoided in infancy. (See also Chapter 12 for a more extensive discussion of dental care, including nursing caries.)
IMMUNIZATIONS
One of the most dramatic advances in pediatrics has been the decline of infectious diseases during the twentieth century because of the widespread use of immunization for preventable diseases. Although many of the immunizations can be given to individuals of any age, the recommended primary schedule begins during infancy and, with the exception of boosters, is completed during early childhood. Therefore health promotion during infancy includes a discussion of childhood immunizations for diphtheria, tetanus, and pertussis (DTaP, using acellular pertussis); poliovirus; measles, mumps, and rubella (MMR); Haemophilus influenzae type b (Hib); hepatitis B virus (HepB); hepatitis A virus (HepA); meningococcal; pneumococcal conjugate vaccine (PCV); influenza; rotavirus vaccine; and chickenpox (varicella). Selected vaccines generally reserved for children considered at high risk for the disease are discussed here and as appropriate throughout the text. (See also Communicable Diseases, Chapter 14, for a discussion of several of the diseases for which vaccines are available.)
Schedule for Immunizations
In the United States two organizations—the Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention, and the Committee on Infectious Diseases of the American Academy of Pediatrics—govern the recommendations for immunization policies and procedures. In Canada, recommendations are from the National Advisory Committee on Immunization under the authority of the minister of health and Public Health Agency of Canada. The policies of each committee are recommendations, not rules, and they change as a result of advances in the field of immunology. Nurses need to keep informed of the latest advances and changes in policy (see Community Focus box).
In the United States the recommended age for beginning primary immunizations of infants is within 2 weeks of birth or, in special circumstances, at birth (Fig. 10-11, A). Infants born preterm should receive the full dose of each vaccine at the appropriate chronologic age. Recommended schedules for children not immunized during infancy are included in Fig. 10-12. Table 10-4 describes Canadian immunization schedules for children.
TABLE 10-4
Recommended Immunization Schedules: Canada, 2006
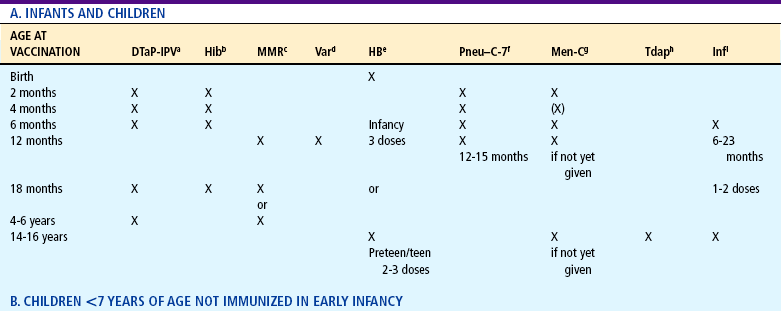
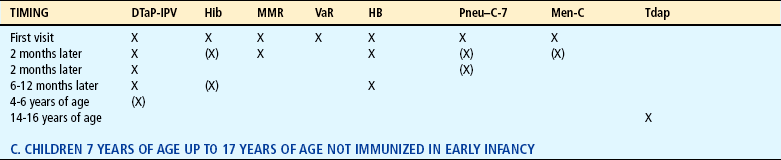
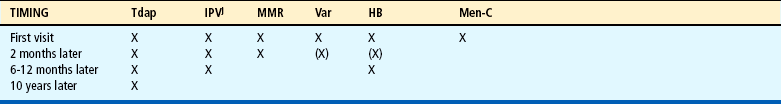
aDiphtheria, tetanus, acellular pertussis, and inactivated polio virus vaccine (DTaP-IPV): DTaP-IPV (±Hib) vaccine is the preferred vaccine for all doses in the vaccination series, including completion of the series in children who have received ≥1 dose of DPT (whole cell) vaccine (e.g., recent immigrants). In schedules A and B, the 4- to 6-year dose can be omitted if the fourth dose was given after the fourth birthday.
bHaemophilus influenzae type b conjugate vaccine (Hib): The Hib schedule shown is for the Haemophilus b capsular polysaccharide–polyribosylribitol phosphate (PRP) conjugated to tetanus toxoid (PRP-T). For catch up, the number of doses depends on the age at which the schedule is begun. Not usually required past age 5 years.
cMeasles, mumps, and rubella vaccine (MMR): A second dose of MMR is recommended for children at least 1 month after the first dose for the purpose of better measles protection. For convenience, options include giving it with the next scheduled vaccination at 18 months of age or at school entry (4–6 years) (depending on the provincial/territorial policy) or at any intervening age that is practical. In the catch-up schedule (B), the first dose should not be given until the child is ≥12 months old. MMR should be given to all susceptible adolescents and adults.
dVaricella vaccine (Var): Children aged 12 months to 12 years should receive 1 dose of varicella vaccine. Susceptible individuals ≥13 years of age should receive 2 doses at least 28 days apart.
eHepatitis B vaccine (HB): Hepatitis B vaccine can be routinely given to infants or preadolescents, depending on the provincial/territorial policy. For infants born to chronic carrier mothers, the first dose should be given at birth (with hepatitis B immunoglobulin); otherwise, the first dose can be given at 2 months of age to fit more conveniently with other routine infant immunization visits. The second dose should be administered at least 1 month after the first dose, and the third at least 2 months after the second dose, but these may fit more conveniently into the 4- and 6-month immunization visits. A 2-dose schedule for adolescents is an option.
fPneumococcal conjugate vaccine–7-valent (Pneu-C–7): Recommended for all children ≤2 years of age. The recommended schedule depends on the age of the child when vaccination is begun.
gMeningococcal C conjugate vaccine (Men-C): Recommended for children ≤5 years of age, adolescents, and young adults. The recommended schedule depends on the age of the individual, and the conjugate vaccine used. At least 1 dose in the primary infant series should be given after 5 months of age. If the provincial/territorial policy is to give Men-C to persons ≥12 months of age, 1 dose is sufficient.
hDiphtheria, tetanus, acellular pertussis vaccine—adult/adolescent formulation (Tdap): A combined adsorbed “adult type” preparation for use in people ≥7 years of age; contains less diphtheria toxoid and pertussis antigens than preparations given to younger children and is less likely to cause reactions in older people.
iInfluenza vaccine (Inf): Recommended for all children 6–23 months of age and all persons ≥65 years of age. Previously unvaccinated children <9 years of age require 2 doses of the current season’s vaccine with an interval of ≥4 weeks. The second dose within the same season is not required if the child received ≥1 doses of influenza vaccine during the previous influenza season.
jInactivated polio virus (IPV)
Modified from Public Health Agency of Canada: Canadian immunization guide, ed 7, Ottawa, Ontario, Canada, 2006, The Agency. Parentheses imply that these doses may not be required, depending on the age of the child or adult


FIG. 10-11 Recommended immunization schedules for persons aged 0 to 6 years (A) and 7 to 18 years (B)—United States, 2008. (From Centers for Disease Control and Prevention: Recommended immunization schedules for persons aged 0-18 years-United States, 2008. MMWR 56[51 and 52]:Q1-Q4, 2007.) Recommended immunization schedules for persons aged 0 to 6 years (A) and 7 to 18 years (B)—United States, 2008. (From Centers for Disease Control and Prevention: Recommended immunization schedules for persons aged 0-18 years-United States, 2008. MMWR 56[51 and 52]:Q1-Q4, 2007.)
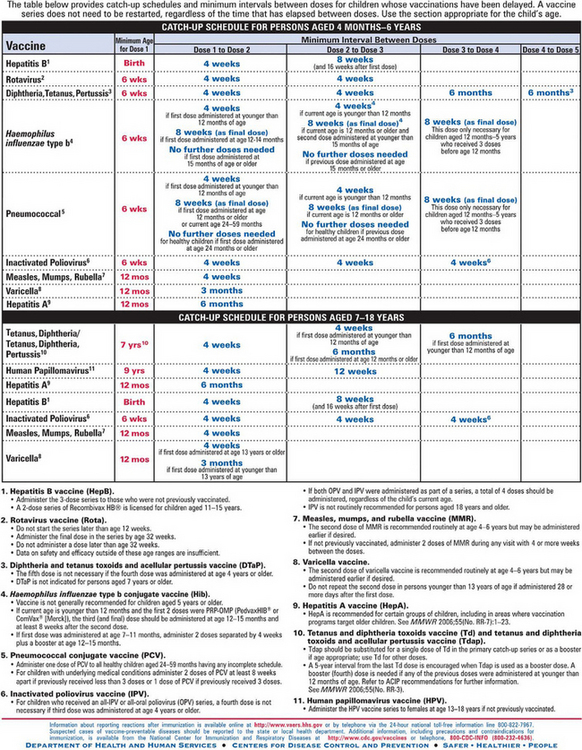
FIG. 10-12 Catch-up immunization schedule for persons aged 4 months to 18 years who start late or who are more than 1 month behind. (From Centers for Disease Control and Prevention: Recommended immunization schedules for persons aged 0-18 years—United States, 2008. MMWR 56[51 and 52]:Q1-Q4, 2007.)
Children who began primary immunizations at the recommended age but fail to receive all of the doses do not need to begin the series again but instead receive only the missed doses. For situations in which there is doubt that the child will return for immunizations according to the optimum schedule, any of the recommended vaccines can be administered simultaneously. Parenteral vaccines are given in separate syringes in different injection sites (American Academy of Pediatrics, 2006b). The product brand names, routes of administration, and manufacturers for the principal childhood vaccinations are listed in Table 10-7.
TABLE 10-7
Product Brand Names for Principal Childhood Vaccine Types
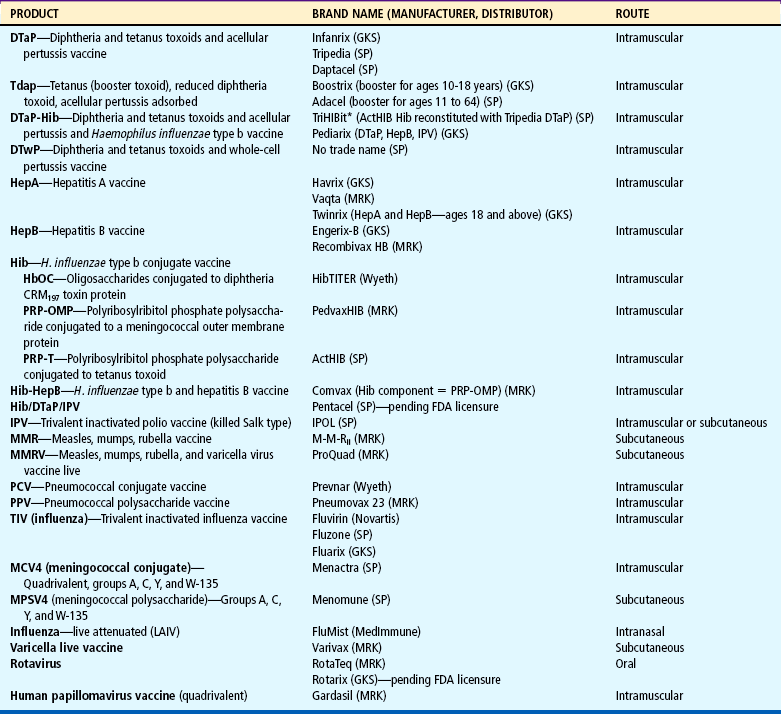
GKS, GlaxoKlineSmith; MRK, Merck & Co.; SP, SanofiPasteur.
*TriHIBit is licensed only for the fourth dose in the vaccination series, recommended at ages 15–18 months.
Modified from American Academy of Pediatrics: Combination vaccines for childhood immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP), Pediatrics 103(5):1072, 1999; American Academy of Pediatrics, Committee on Infectious Diseases, Pickering L, editor: Red book: 2006 report of the Committee on Infectious Diseases, ed 27, Elk Grove Village, Ill, 2006, The Academy; Centers for Disease Control and Prevention: Quadrivalent human papillomavirus vaccine, MMWR 56(RR-2):1–24, 2007; and US Food and Drug Administration: Biological license application supplement: noteworthy approvals. In FDA News, Rockville, Md, Feb 3, 2006, The Administration, retrieved Jan 2, 2008 from http://www.fda.gov/cber/appr2007/2007Lsup.htm.
Recommendations for Routine Immunizations
Hepatitis A Virus (HAV). HAV has been recognized as a significant child health problem, particularly in communities where widespread childhood hepatitis A immunization is not recommended. HAV is spread by the fecal-oral route and from person-to-person contact, by ingestion of contaminated food or water, and rarely by blood transfusion. The illness has an abrupt onset, with fever, malaise, anorexia, nausea, abdominal discomfort, dark urine, and jaundice being the most common clinical signs of infection. In children under 6 years of age the disease may be asymptomatic, and jaundice is rarely evident.
HAV vaccine is now recommended for all children beginning at age 1 year (i.e., 12 months to 23 months); the second dose in the two-dose series may be administered no sooner than 6 months after the first dose. Since the implementation of widespread childhood HepA vaccination, infection rates among children ages 5 to 14 years have declined significantly (Centers for Disease Control and Prevention, 2006a). For further information see footnote 9 in Fig. 10-11, A.
Hepatitis B Virus (HBV).: HBV is an important pediatric disease because HBV infections that occur during childhood and adolescence can lead to fatal consequences from cirrhosis or liver cancer during adulthood. Up to 90% of infants infected perinatally and 25% to 50% of children infected before age 5 years become HBV carriers. In addition, the incidence of HBV infection increases rapidly during adolescence (American Academy of Pediatrics, 2006b). Past immunization strategies targeted several high-risk groups, including health care workers and others in contact with blood and body fluids, recipients of certain blood products (such as those with hemophilia), heterosexuals with multiple partners, sexually active homosexual and bisexual men, intravenous drug abusers, immigrants from countries in which HBV is widespread, and children born to mothers who are hepatitis B surface antigen (HBsAg)–positive.
To improve immunization rates, current recommendations include vaccinations for all newborns. Both full-term and preterm infants born to mothers whose HBsAg status is positive should receive HepB, 0.5 ml, within 12 hours of birth. If the mother’s HBsAg status is unknown, her blood should be drawn for HBsAg status determination; if she is positive, the infant should receive hepatitis B immune globulin (HBIG), 0.5 ml, as soon as possible but no later than age 1 week. Because the immune response to HepB is not optimum in newborns weighing less than 2000 g (4.4 pounds), the first HepB dose should be given to such infants at 1 month, as long as the mother’s HBsAg status is negative (American Academy of Pediatrics, 2006b). Only the single-antigen (monovalent) HepB should be given as the first dose (see also Fig. 10-11, A, footnote 1).
In the late 1990s HepB contained small amounts of mercury (thimerosal) as a preservative, which generated concern regarding possible mercury poisoning in infants and led to a subsequent decrease in hepatitis B immunization rates in newborns. However, a preservative-free HepB (Recombivax HB, pediatric-adolescent formulation) is available, and the Centers for Disease Control and Prevention (2005a) strongly recommends that HepB immunization occur in newborns before discharge from the birth hospital. To date, studies have not found any association between thimerosal in vaccines and neurologic developmental disorders such as autism spectrum disorder (Parker, Schwartz, Todd, and others, 2004; Heron, Golding, and ALSPAC Study Team, 2004). The American Academy of Pediatrics (2006b) also encourages immunization of all children by age 11 years.
The vaccine is given intramuscularly in the vastus lateralis muscle in newborns or in the deltoid muscle for toddlers and children. Regardless of age, the dorsogluteal site is avoided because it has been associated with low antibody seroconversion rates, indicating a reduced immune response (Zuckerman, Cockcroft, and Zuckerman, 1992). No data exist regarding the seroconversion when the ventrogluteal site is used. The vaccine can be safely administered simultaneously at a separate site with DTaP, MMR, and Hib vaccines.
Diphtheria.: Although cases of diphtheria are rarely seen in the United States, the disease can result in significant morbidity. Respiratory manifestations include respiratory nasopharyngitis or obstructive laryngotracheitis with upper airway obstruction. The cutaneous manifestations of the disease include vaginal, otic, conjunctival, or cutaneous lesions, which are primarily seen in the tropics (American Academy of Pediatrics, 2006b). Diphtheria vaccine is commonly administered (1) in combination with tetanus and pertussis vaccines (DTaP) or DTaP and Hib vaccines for children younger than 7 years of age, (2) in combination with a conjugate Hib vaccine (see Fig. 10-11, A, footnote 4), (3) in a combined vaccine with tetanus (DT) for children younger than 7 years of age who have some contraindication to receiving pertussis vaccine, or (4) as a single antigen when combined antigen preparations are not indicated.
Although the diphtheria vaccine does not produce absolute immunity, protective antitoxin persists for 10 years or more when given according to the recommended schedule, and boosters are given every 10 years for life (see discussion below for adolescent diphtheria and acellular pertussis and tetanus toxoid recommendation). Several vaccines contain diphtheria toxoid (Hib, meningococcal, pneumococcal), but this does not confer immunity to the disease.
Tetanus.: Tetanus vaccine comes in three forms: tetanus toxoid, tetanus immunoglobulin (TIG) (human), and tetanus antitoxin (equine antitoxin). However, tetanus antitoxin is no longer available in the United States. Tetanus toxoid is used for routine primary immunization, usually in one of the combinations listed for diphtheria, and provides protective antitoxin levels for approximately 10 years.
Tetanus and diphtheria toxoids along with acellular pertussis vaccine (Tdap–adolescent formulation) is now recommended for children 11 to 12 years who have completed the recommended DTaP/DTP vaccine series yet have not received the tetanus (Td) booster dose. Adolescents who are 13 to 18 years of age and have not received the Td/Tdap booster should receive a single Tdap booster provided the routine DTaP/DTP childhood immunization series has been previously received. It is recommended that subsequent Td boosters be administered every 10 years (American Academy of Pediatrics, 2006b) (see Fig. 10-11, B, footnote 1). Boostrix (Tdap) is currently licensed for children 10 to 18 years of age, whereas Adacel (Tdap) is licensed for individuals 11 to 64 years of age.
For wound management, passive immunity is available with TIG. In persons with a history of two previous doses of tetanus toxoid, a booster dose of the toxoid can be given. Separate syringes and different sites are used when tetanus toxoid and TIG are given concurrently. Table 10-5 summarizes the recommended procedure for tetanus prophylaxis in wound management.
TABLE 10-5
Guide to Tetanus Prophylaxis in Routine Wound Management
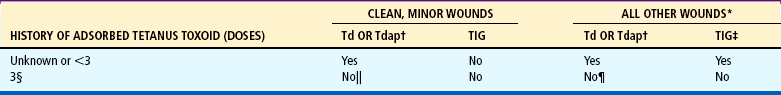
Td, Tetanus, diphtheria; Tdap, booster tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis; TIG, tetanus immune globulin.
*Such as, but not limited to, wounds contaminated with dirt, feces, soil, and saliva; puncture wounds; avulsions; and wounds resulting from missiles, crushing, burns, and frostbite.
†Tdap is preferred to Td for adolescents who never have received Tdap. Td is preferred to tetanus toxoid (TT) for adolescents who received Tdap previously or when Tdap is not available.
‡Intravenous immune globulin should be used when TIG is not available.
§If only three doses of fluid toxoid have been received, then a fourth dose of toxoid, preferably an adsorbed toxoid, should be given.
 Yes, if 10 years since last dose.
Yes, if 10 years since last dose.
¶Yes, if 5 years since last dose. (More frequent boosters are not needed and can accentuate side effects.)
Data from American Academy of Pediatrics, Committee on Infectious Diseases, Pickering L, editor: Red book: 2006 report of the Committee on Infectious Diseases, ed 27, Elk Grove Village, Ill, 2006, The Academy.
For children over 7 years who require wound prophylaxis, tetanus immunization may be accomplished by administering Td (adult-type diphtheria and tetanus toxoids). In the event that TIG is not available, the equine antitoxin (not available in the United States) may be administered after appropriate testing for sensitivity; the antitoxin is administered in a separate syringe and at a separate intramuscular site if given concurrently with tetanus toxoid.
Pertussis.: Pertussis vaccine is recommended for all children 6 weeks through 6 years of age (up to the seventh birthday) who have no neurologic contraindications to its use. Concerns over outbreaks of the disease in the past decade have prompted discussion about vaccinating infants and adults; many cases of pertussis have been seen in children less than 6 months or persons over 7 years, both categories for which pertussis vaccination was not previously recommended (Centers for Disease Control and Prevention, 2005c). The tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap) is now recommended at age 11 to 12 years for children who have completed the DTaP/DTP childhood series; the Tdap is also recommended for adolescents 13 to 18 years who have not received a tetanus booster (Td) or Tdap dose and have completed the childhood DTaP/DTP series (American Academy of Pediatrics, 2006b).
Currently, two forms of pertussis vaccine are available in the United States. The whole-cell pertussis vaccine is prepared from inactivated cells of Bordetella pertussis and contains multiple antigens. In contrast, the acellular pertussis vaccine contains one or more immunogens derived from B. pertussis. The highly purified acellular vaccine is associated with fewer local and systemic reactions than those occurring with the whole-cell vaccine in children of similar age. The acellular pertussis vaccine is recommended by the American Academy of Pediatrics (2006b) for the first three immunizations and is usually given at 2, 4, and 6 months of age with diphtheria and tetanus (DTaP). Four forms of acellular pertussis vaccine are currently licensed for use in infants: Tripedia, Daptacel, Pediarix, and Infanrix (diphtheria, tetanus toxoid, and acellular pertussis conjugate). TriHIBit may be used for the fourth dose after three doses of DTaP and after a primary series of any Hib vaccine. Either the acellular or whole-cell vaccine may be given for the fourth and fifth doses, but the acellular is preferred. It is also recommended that the first three DTaP vaccinations be from the same manufacturer; the fourth dose may be from a different manufacturer. The child who has received one or more whole-cell vaccines may complete the series of five with the acellular vaccine.
Health care workers under age 65 who have potential exposure to children or adults with pertussis should be given the Tdap booster Adacel every 10 years; in addition, health care workers should take the necessary protective precautions against droplet contamination (wear procedural or surgical masks and practice hand washing). The diagnosis of pertussis may be missed or delayed in unvaccinated infants, who often are seen with respiratory distress and apnea without the typical cough (Centers for Disease Control and Prevention, 2005c). Additional guidelines for prevention and treatment of pertussis among health care workers and close contacts are found in the Centers for Disease Control and Prevention report (2006b).
Polio.: An all-IPV (inactivated poliovirus vaccine) schedule for routine childhood polio vaccination is now recommended for children in the United States. All children should receive four doses of IPV at 2 months, 4 months, 6 to 18 months, and 4 to 6 years of age (American Academy of Pediatrics, 2006b).
The change from the exclusive use of oral polio vaccine (OPV) to the exclusive use of IPV is related to the rare risk of vaccine-associated polio paralysis (VAPP) from OPV. The exclusive use of IPV eliminates the risk of VAPP but is associated with an increased number of injections and increased cost. Since IPV usage was instituted in the United States in 2000, no new cases of VAPP have occurred. Pediarix is a combination vaccine containing DTaP, hepatitis B, and IPV; this may be used as the primary immunization beginning at 2 months of age (American Academy of Pediatrics, 2006b).
Measles.: The measles (rubeola) vaccine is given at 12 to 15 months of age. During the course of measles outbreaks, the vaccine can be given any time after 6 months of age, followed by a second inoculation after age 12 months. The second measles immunization is recommended at 4 to 6 years of age (at school entry) but may be given earlier provided that 4 weeks have lapsed since the administration of the previous dose. Revaccination should occur by 11 to 12 years of age if the measles vaccine was not administered at school entry (4 to 6 years). Any child who is vaccinated before 12 months of age should receive two additional doses beginning at 12 to 15 months and separated by at least 4 weeks (American Academy of Pediatrics, 2006b). Revaccination should include all individuals born after 1956 who have not received two doses of measles vaccine after 12 months of age. Individuals born before this date are thought to be immune from exposure to natural measles virus. Because of the continuing occurrence of measles in older children and young adults, potentially susceptible adolescents and young adults should be identified and immunized if two doses of measles vaccine have not been administered previously or the person had a confirmed case of the illness.
Mumps.: Mumps virus vaccine is recommended for children at 12 to 15 months of age and is typically given in combination with measles and rubella. It should not be administered to infants younger than 12 months because persisting maternal antibodies can interfere with the immune response. Because of continued occurrence of the disease, especially in children 10 to 19 years of age, mumps immunization is recommended for all individuals born after 1957 who may be susceptible to mumps (i.e., those who have no history of having had the disease or vaccine and who have no laboratory evidence of immunity).
Rubella.: Rubella is a relatively mild infection in children, but in a pregnant woman the actual infection presents serious risks to the developing fetus. Therefore the aim of rubella immunization is actually protection of the unborn child rather than the recipient of the immunization.
Rubella immunization is recommended for all children at 12 to 15 months of age and is administered in a combined form with measles and mumps vaccine. Increased emphasis should also be placed on vaccinating all unimmunized prepubertal children and susceptible adolescents and adult women in the childbearing age-group. Because the live attenuated virus may cross the placenta and theoretically present a risk to the developing fetus, rubella vaccine is currently not given to any pregnant woman.
Haemophilus influenzae Type b.: Hib conjugate vaccines protect against a number of serious infections caused by Hib, especially bacterial meningitis, epiglottitis, bacterial pneumonia, septic arthritis, and sepsis (Hib is not associated with the viruses that cause influenza, or “flu”). Hib vaccines that are currently available include PedvaxHIB and Comvax, which are combination vaccines; and HibTITER and ActHIB (see Table 10-7). These conjugate vaccines connect Hib to a nontoxic form of another organism, such as meningococcal protein or diphtheria protein. There is no antibody response to these nontoxic proteins, but they significantly improve the antibody response to Hib, especially in infants. The use of combination vaccines provides equivalent immunogenicity and decreases the number of injections an infant receives; however, it is important that they be given to the appropriate-age child.
When possible, the Hib conjugate vaccine used at the first vaccination should be used for all subsequent vaccinations in the primary series. The DTaP/Hib combination vaccine (TriHIBit) should not be used for the first three doses at 2, 4, or 6 months but may be used as a booster after any Hib vaccine. All Hib vaccines are administered by intramuscular injection using a separate syringe and at a site separate from any concurrent vaccinations.
Varicella.: Administration of the cell-free live-attenuated varicella vaccine (Varivax) is recommended for any susceptible child (who lacks proof of varicella vaccination or a reliable history of varicella infection). The first dose of varicella vaccine is recommended for children ages 12 to 15 months; to ensure adequate protection, a second varicella vaccine is recommended for children at 4 to 6 years of age (American Academy of Pediatrics, 2007).
A single dose of 0.5 ml should be given by subcutaneous injection. Children 13 years of age or older who are susceptible should receive two doses administered at least 4 weeks apart (American Academy of Pediatrics, 2006b). The vaccine should be kept frozen in the lyophilic form (stable particles that readily go into solution) and used within 30 minutes of being reconstituted to ensure viral potency. Varicella vaccine may be administered simultaneously with MMR. However, separate syringes and injection sites should be used. If they are not administered simultaneously, the interval between administration of varicella vaccine and MMR should be at least 1 month. Varicella vaccine may also be given simultaneously with DTaP, IPV, HepB, or Hib (American Academy of Pediatrics, 2006b).
The varicella vaccine is reported to be effective in preventing varicella for at least 11 years (American Academy of Pediatrics, 2006b).
Pneumococcal.: A seven-valent Streptococcus pneumoniae conjugate vaccine (PCV7, or Prevnar) has been used for children under 2 years of age since 2000. Streptococcal pneumococci are responsible for a number of bacterial infections in children under 2 years, which may cause serious morbidity and mortality. Among these are generalized infections such as septicemia and meningitis or localized infections such as otitis media, sinusitis, and pneumonia. These illnesses are particularly problematic in children who attend daycare facilities (the incidence in daycare children is two or three times higher than in children not attending out-of-home daycare) and in those who are immunocompromised.
The vaccine is administered at 2, 4, and 6 months, with a fourth dose at 12 to 15 months of age; children 7 to 11 months old may receive three doses as long as they are 6 to 8 weeks apart and a fourth dose at 12 to 15 months; children 12 to 23 months who have not been immunized with the pneumococcal vaccine may be given two doses, 6 to 8 weeks apart. PCV7 is also recommended for all children under 24 months and in older children (24 to 59 months) with sickle cell disease; functional or anatomic asplenia; nephrotic syndrome or chronic renal failure; conditions associated with immunosuppression, such as solid organ transplantation, drug therapy, or cytoreduction therapy (including long-term systemic corticosteroid therapy); diabetes mellitus; cochlear implants; congenital immunodeficiency; human immunodeficiency virus (HIV) infection; cerebrospinal fluid leaks; chronic cardiovascular disease (e.g., congestive heart failure or cardiomyopathy); chronic pulmonary disease (e.g., emphysema or cystic fibrosis, but not asthma); chronic liver disease (e.g., cirrhosis); or exposure to living environments or social settings in which the risk of invasive pneumococcal disease or its complications is very high (e.g., Alaskan Native, African-American, and certain Native American populations) (American Academy of Pediatrics, 2006b). Low-birth-weight infants (≤1500 g [3.3 pounds]) should receive the vaccine when they reach a chronologic age of 6 to 8 weeks regardless of calculated gestational age. The PCV7 vaccine may be administered in conjunction with all other immunizations in a separate syringe and at a separate intramuscular site.
The PPV (pneumococcal polysaccharide [23-valent] vaccine) is not recommended for children younger than 24 months who do not have one of the high-risk conditions described previously. One dose of PPV is recommended in children older than 23 months who have one of the high-risk conditions after primary immunization with PCV7. (See American Academy of Pediatrics, 2006b, Tables 3–48 and 3–49 for PCV7 and PPV schedule.)
Influenza.: The influenza vaccine is now recommended annually for children 6 months and older. The vaccine should be administered annually to children 5 years of age and older with risk factors (including asthma, cardiac disease, HIV, diabetes, and sickle cell disease), health care workers, and other persons in close contact (including household members) with persons in groups at high risk. It is also recommended that close contacts in households with children ages 0 to 5 months receive the trivalent inactivated influenza vaccine because of the high rate of influenza-related hospitalization. Influenza vaccine (trivalent inactivated influenza vaccine) may be given to any healthy children 6 to 59 months old, since this age-group is at particular risk for influenza-related hospitalizations. Children who have a reported anaphylactic hypersensitivity to eggs should not receive the vaccine.
The vaccine is administered in early fall before the flu season begins and is repeated yearly for ongoing protection. The intramuscular vaccine is administered as two separate doses 2 weeks apart in first-time recipients under the age of 8 years. The dose is 0.25 ml for children ages 3 months to 35 months and 0.5 ml for children 3 years and above. The vaccine may be given simultaneously with other vaccines but at a separate site (American Academy of Pediatrics, 2006b). The vaccine is administered yearly because different strains of influenza are used each year in the manufacture of the vaccine.
FluMist, a live attenuated influenza vaccine, is an acceptable alternative to the intramuscular trivalent vaccine in specific age-groups. The vaccine is given nasally as two doses 4 weeks apart in healthy persons ages 2 to 49 years.
Meningococcal.: Invasive meningococcal disease continues to be the cause of high morbidity in children in the United States. Infants younger than 1 year of age are particularly susceptible, yet the highest fatalities occur in adolescents (approximately 20%). There is also evidence that the risk of meningococcal infections is high in college freshmen living in dormitories. Meningococcal infections are also responsible for significant morbidities, including limb or digit amputation, skin scarring, hearing loss, and neurologic disabilities (American Academy of Pediatrics, 2006b).
Neisseria meningitidis is the leading cause of bacterial meningitis in the United States. It is now recommended that children receive the quadrivalent conjugate vaccine MCV4 (Menactra) at the 11- to 12-year visit. Menactra was recently approved for use in children 2 to 10 years of age.
The vaccine protects against meningococcal disease caused by serogroups A, C, Y, and W-135 and should also be administered to adolescents at high school entry or 15 years of age (whichever comes first) who have not been previously immunized for meningococcal disease, college freshmen living in dormitories, and pediatric patients 11 years and older who are at increased risk of meningococcal disease (adolescents with asplenia or complement deficiencies and HIV). The MCV4 vaccine is also recommended for adolescents ages 11 to 18 years traveling to countries where meningococcal infections are prevalent. MCV4 is administered as an intramuscular injection (0.5 ml) and may be administered in conjunction with other vaccines in a separate syringe and at a separate site.
There have been recent concerns regarding reports of an association between Menactra and cases of Guillain-Barré syndrome in vaccinated persons ages 11 to 19 years of age; onset of symptoms occurred within 2 to 23 days of vaccination. A preliminary survey by the Centers for Disease Control and Prevention (2006c) indicates there are insufficient data to change the 2005 recommendation for adolescents, college freshmen residing in dormitories, and other high-risk populations.
The meningococcal vaccine Menomune (A, C, Y, W-135; MPSV4), which has been available since the early 1980s, may be used for children 2 to 10 years of age and MCV4 for children over 10 years. The MCV4 is recommended over the MPSV4 for children aged 2 to 10 years who are at increased risk for meningitis (American Academy of Pediatrics, 2008). MPSV4 may be administered subcutaneously conjointly with other vaccines in a separate syringe at a separate site. It is unknown whether future recommendations for booster immunization with MCV4 will be necessary (American Academy of Pediatrics, 2006b).
Immunization with MCV4 is contraindicated in persons with hypersensitivity to any components of the vaccine, including diphtheria toxoid, and to rubber latex (part of vial stopper).
 IN TEXT
IN TEXT

 FAMILY FOCUS
FAMILY FOCUS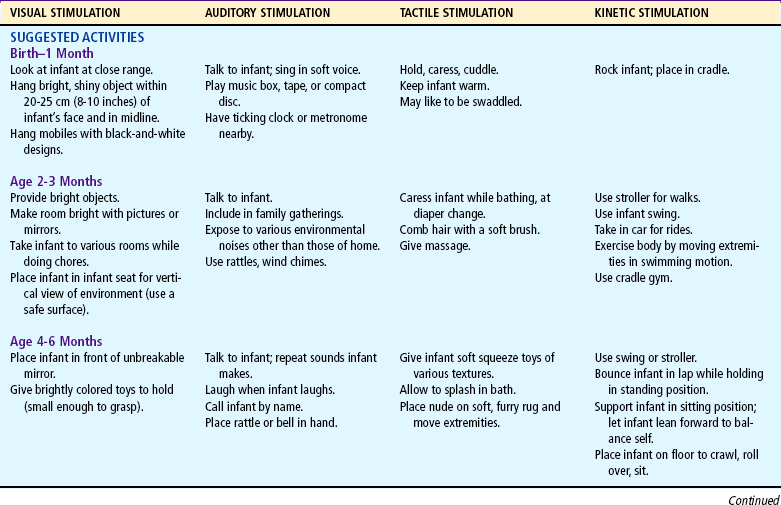
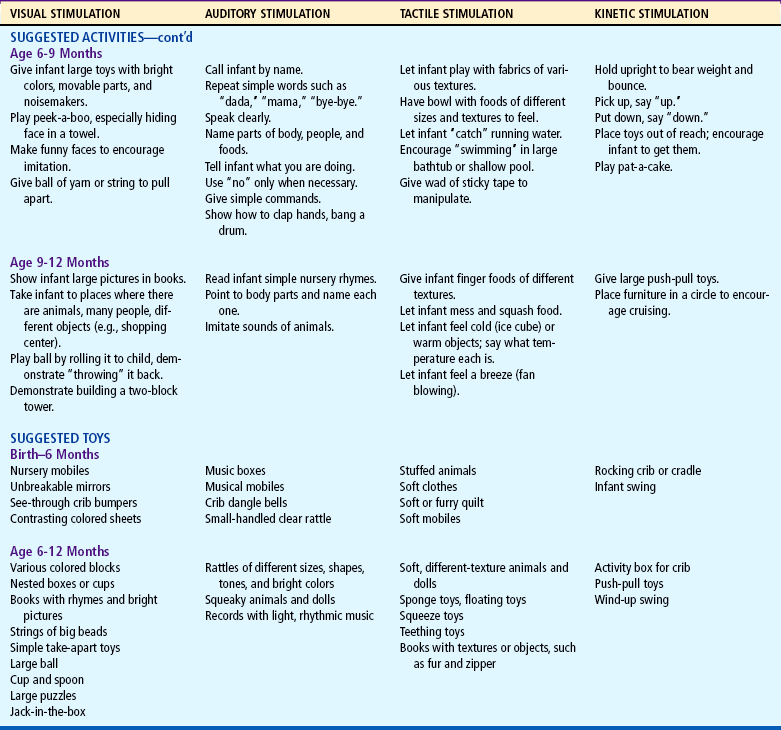
 FAMILY-CENTERED CARE
FAMILY-CENTERED CARE CULTURAL AWARENESS
CULTURAL AWARENESS COMMUNITY FOCUS
COMMUNITY FOCUS