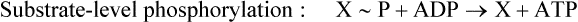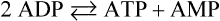Anaerobic Metabolism of Glucose in the Red Blood Cell
Introduction
Glycolysis is the central pathway of glucose metabolism in all cells
Glucose is the major carbohydrate on Earth, the backbone and monomer unit of cellulose and starch. It is also the only fuel that is used by all cells in our body. All of these cells, even the microbes in our intestines, begin the metabolism of glucose by a pathway termed glycolysis, i.e. carbohydrate (glyco) splitting (lysis). Glycolysis is catalyzed by soluble cytosolic enzymes and is the ubiquitous, central metabolic pathway for glucose metabolism. The erythrocyte, commonly known as the red blood cell (RBC), is unique among all cells in the body – it uses glucose and glycolysis as its sole source of energy. Thus, the RBC is a useful model for an introduction to glycolysis.
Pyruvate, a three-carbon carboxylic acid, is the end product of glycolysis; 2 moles of pyruvate are formed per mole of glucose
In cells with mitochondria and oxidative metabolism, pyruvate is converted completely into CO2 and H2O – glycolysis in this setting is termed aerobic glycolysis. In RBCs, which lack mitochondria and oxidative metabolism, pyruvate is reduced to lactic acid, a three-carbon hydroxyacid, the product of anaerobic glycolysis. Each mole of glucose yields 2 moles of lactate, which are then excreted into blood. Two molecules of lactic acid contain exactly the same number of carbons, hydrogens, and oxygens as one molecule of glucose (Fig. 12.1); however, there is sufficient free energy available from the cleavage and rearrangement of the glucose molecule to produce 2 moles of ATP per mole of glucose converted into lactate. The RBC uses most of this ATP to maintain electrochemical and ion gradients across its plasma membrane.
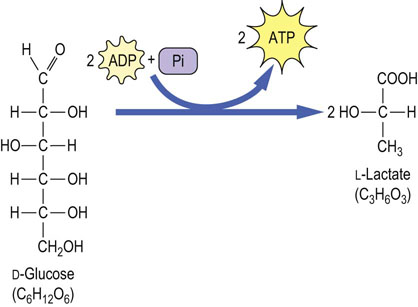
Fig. 12.1 Conversion of glucose to lactate during anaerobic glycolysis.
One mole of glucose is converted to 2 moles of lactate during anaerobic glycolysis. No oxygen is consumed, nor is CO2 produced in this pathway. There is a net yield of 2 mole of ATP per mole of glucose converted to lactate.
In the RBC, 10–20% of the glycolytic intermediate, 1,3-bisphosphoglycerate, is diverted to the synthesis of 2,3-bisphosphoglycerate (2,3-BPG), an allosteric regulator of the O2 affinity of Hb (Chapter 5). The pentose phosphate pathway, a shunt from glycolysis, accounts for about 10% of glucose metabolism in the red cell. In the red cell, this pathway has a special role in protection against oxidative stress, while in nucleated cells it also serves as a source of NADPH for biosynthetic reactions and pentoses for nucleic acid synthesis.
The erythrocyte
The erythocyte or red blood cell relies exclusively on blood glucose as a metabolic fuel
The erythrocyte, or red blood cell (RBC), represents 40–45% of blood volume and over 90% of the formed elements (erythrocytes, leukocytes, and platelets) in blood. The RBC is, both structurally and metabolically, the simplest cell in the body – the end product of the maturation of bone marrow reticulocytes. During its maturation, the RBC loses all its subcellular organelles. Without nuclei, it lacks the ability to synthesize DNA or RNA. Without ribosomes or an endoplasmic reticulum, it cannot synthesize or secrete protein. Because it cannot oxidize fats, a process requiring mitochondrial activity, the RBC relies exclusively on blood glucose as a fuel. Metabolism of glucose in the RBC is entirely anaerobic, consistent with the primary role of the RBC in oxygen transport and delivery, rather than its utilization.
Glycolysis
Overview
Pyruvate is the endproduct of anaerobic glycolysis
Glucose enters the RBC by facilitated diffusion, via the insulin-independent glucose transporter, GLUT-1. Glycolysis then proceeds through a series of phosphorylated intermediates, starting with the synthesis of glucose-6-phosphate (Glc-6-P). During this process, which involves 10 enzymatically catalyzed steps, two molecules of ATP are expended (investment stage) to build up a nearly symmetric intermediate, fructose-1,6-bisphosphate (Fru-1,6-BP), which is then cleaved (splitting stage) to two three-carbon triose phosphates. These are eventually converted into lactate, with production of ATP, during the yield stage of glycolysis. The yield stage includes both redox and phosphorylation reactions, leading to formation of four molecules of ATP during the conversion of the two triose phosphates into lactate. The outcome is a net 2 moles of ATP per mole of glucose converted into lactate.
Glycolysis is a relatively inefficient pathway for extracting energy from glucose: the yield of 2 moles of ATP per mole of glucose is only about 5% of the 30–32 ATP that are available by complete oxidation of glucose to CO2 and H2O by mitochondria in other tissues (Chapter 14).
One might ask why a 10-step pathway is required to convert glucose to lactate; couldn't it have been done in fewer steps or by cleavage of one carbon at a time? The answer, from a metabolic point of view, is that glycolysis is not an isolated pathway; most glycolytic intermediates serve as branch points to other metabolic pathways. In this way, the metabolism of glucose intersects with the metabolism of fats, proteins and nucleic acids, as well as other pathways of carbohydrate metabolism. Some of these metabolic interactions are shown in Figure 12.2.
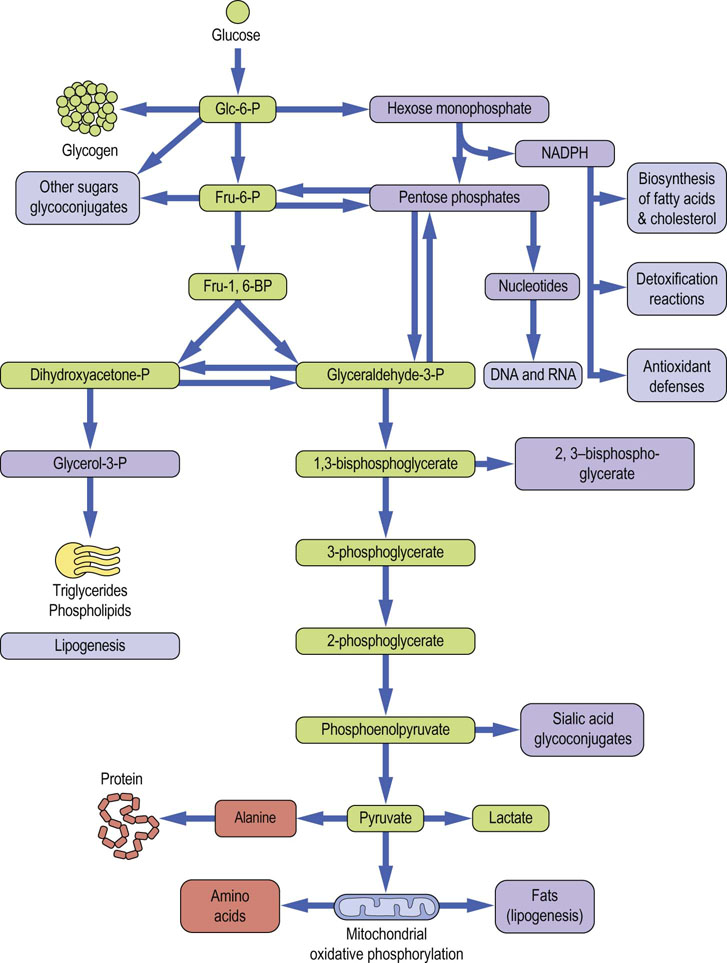
Fig. 12.2 Interactions between glycolysis and other metabolic pathways.
The green-colored boxes indicate intermediates involved in the pathway of glycolysis. Other boxes illustrate some of the metabolic interactions between glycolysis and other metabolic pathways in the cell. Not all of these pathways are active in the red cell, which has limited biosynthetic capacity and lacks mitochondria. Glc-6-P, glucose-6-phosphate; Fru-6-P, fructose-6-phosphate; Fru-1,6-BP, fructose-1,6-bisphosphate.
The investment stage of glycolysis
2 ATP are invested to prime the metabolism of glucose by glycolysis
Glucose-6-phosphate
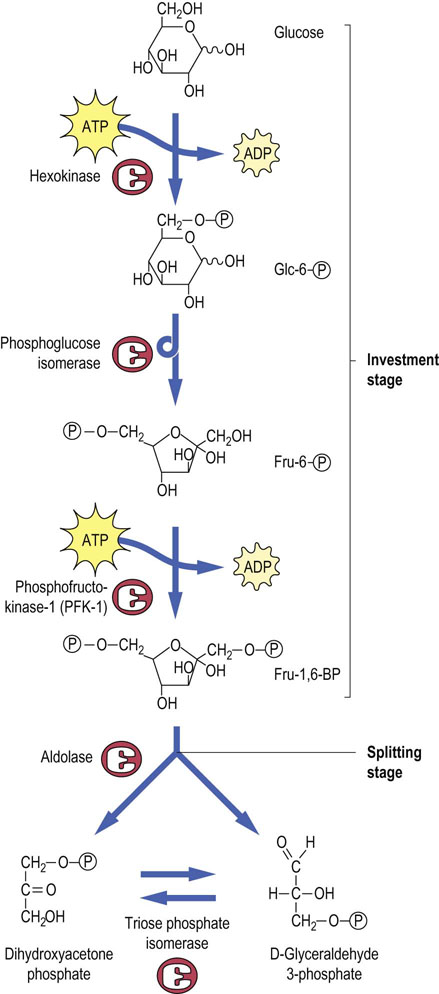
Glucose is taken up into the red cell via the facilitated transporter GLUT-1 (Chapter 8); this protein accounts for about 5% of total red cell membrane protein, so that glucose transport is not rate limiting for glycolysis. Thus, the steady state concentration of glucose in the RBC is only ~20% lower than that in plasma. The first step in the commitment of glucose to glycolysis is the phosphorylation of glucose to Glc-6-P, catalyzed by the enzyme hexokinase (Fig. 12.3, top). The formation of Glc-6-P from free glucose and inorganic phosphate is energetically unfavorable, so that a molecule of ATP must be expended or invested in the phosphorylation reaction; the hydrolysis of ATP is coupled to the synthesis of Glc-6-P. Glc-6-P is trapped in the RBC, along with other phosphorylated intermediates in glycolysis, because there are no transport systems for sugar phosphates in the plasma membranes of mammalian cells.
Fructose-6-phosphate
The second step in glycolysis is the conversion of Glc-6-P into Fru-6-P by phosphoglucose isomerase (Fig. 12.3, middle). Isomerases catalyze freely reversible equilibrium reactions, in this case an aldose–ketose interconversion. A second molecule of ATP is invested to phosphorylate Fru-6-P at the C-1 position; the reaction is catalyzed by phosphofructokinase-1 (PFK-1). The product, fructose 1,6-bisphosphate (Fru-1,6-BP), is a pseudosymmetric intermediate, with a phosphate ester on each end of the molecule. Like hexokinase, PFK-1 requires ATP as a substrate and catalyzes an essentially irreversible reaction. Both hexokinase and PFK-1 are important regulatory enzymes in glycolysis, but PFK-1 is the critical, commitment step. This reaction directs glucose to glycolysis, the only pathway for metabolism of Fru-1,6-BP.
The splitting stage of glycolysis
Fructose-1,6-BP is cleaved in the middle by a reverse aldol (aldolase) reaction
The aldolase reaction (Fig. 12.3, bottom) is a freely reversible equilibrium reaction, yielding two triose phosphates, dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, from the top and bottom halves of the Fru-1,6-BP molecule, respectively. Only the glyceraldehyde-3-phosphate continues through the yield stage of glycolysis, but triose phosphate isomerase catalyzes the interconversion of dihydroxyacetone phosphate and glyceraldehyde-3-phosphate, so that both halves of the glucose molecule are eventually metabolized to lactate.
The yield stage of glycolysis – synthesis of ATP by substrate-level phosphorylation
The yield stage of glycolysis produces 4 moles of ATP, yielding a net of 2 moles of ATP per mole of glucose converted into lactate
The synthesis of ATP during glycolysis is accomplished by kinases that catalyze substrate-level phosphorylation, a process in which a high-energy phosphate compound transfers its phosphate to ADP, yielding ATP.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
GAPDH catalyzes a redox reaction, forming a high energy acyl phosphate compound
To set the stage for substrate-level phosphorylation, the aldehyde group of glyceraldehyde-3-phosphate is oxidized to a carboxylic acid and the energy available from the oxidation reaction is used, in part, to trap a phosphate from the cytoplasmic pool as an acyl phosphate. This reaction is catalyzed by glyceraldehyde-3-phosphate dehydrogenase (GAPDH), yielding the high-energy compound (X~P), 1,3-bisphosphoglycerate (1,3-BPG). The coenzyme NAD+ is simultaneously reduced to NADH (Figs 12.4, 12.5).
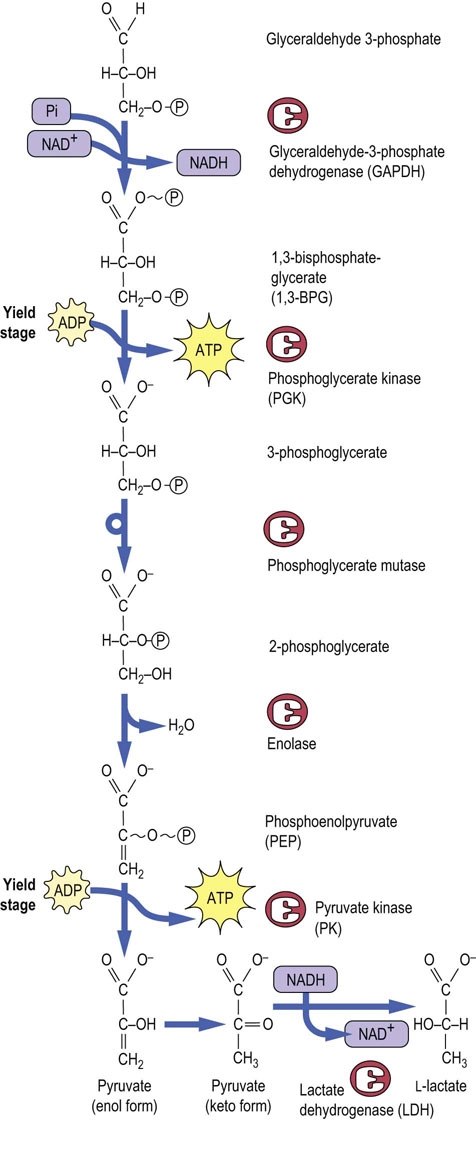
Fig. 12.4 The yield stage of glycolysis.
Substrate-level phosphorylation reactions catalyzed by phosphoglycerate kinase and pyruvate kinase produce ATP, using the high-energy compounds, 1,3-bisphosphoglycerate and phosphoenolpyruvate, respectively. Note that NADH produced during the glyceraldehyde-3-phosphate dehydrogenase reaction is recycled back to NAD+ during the lactate dehydrogenase reaction, permitting continued glycolysis in the presence of only catalytic amounts of NAD+.
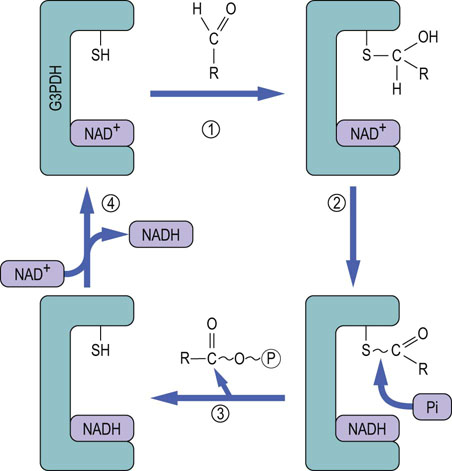
Fig. 12.5 Mechanism of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) reaction.
In Step 1, glyceraldehyde-3-P (RCHO) reacts with the active-site sulfhydryl group of GAPDH to form a thiohemiacetal adduct. In Step 2, the thiohemiacetal is oxidized to a thioester by NAD+, which is bound in the active site of the enzyme and is reduced to NADH. In Step 3, phosphate enters the active site and, in a phosphorylase reaction, cleaves the carbon–sulfur bond, displacing the 3-phosphoglycerate group, producing 1,3-bisphosphoglycerate and regenerating the sulfhydryl group. In Step 4, the enzyme exchanges NADH for NAD+, completing the catalytic cycle.
The GAPDH reaction provides an interesting illustration of the role of enzyme-bound intermediates in the formation of high-energy phosphates. How does the oxidation of an aldehyde and the reduction of NAD+ lead to the formation of an acyl phosphate bond in 1,3-BPG? How does the phosphate enter the picture, and become activated to a high-energy state? The inhibition of GAPDH by thiol reagents such as iodoacetamide, p-chloromecuribenzoate and N-ethylmaleimide pointed to involvement of an active-site sulfhydryl residue. The mechanism of action of this enzyme is described in Figure 12.5.
Substrate-level phosphorylation
Substrate-level phosphorylation produces ATP from another high energy phosphate compound
Phosphoglycerate kinase (PGK) catalyzes transfer of the phosphate group from the high-energy acyl phosphate of 1,3-BPG to ADP, forming ATP. This substrate-level phosphorylation reaction yields the first ATP produced in glycolysis. The remaining phosphate group in 3-phosphoglycerate is an ester phosphate and does not have enough energy to phosphorylate ADP, so a series of isomerization and dehydration reactions is enlisted to convert the ester phosphate into a high-energy enol phosphate. The first step is to shift the phosphate to C-2 of glycerate, converting 3-phosphoglycerate into 2-phosphoglycerate, catalyzed by the enzyme phosphoglycerate mutase (see Fig. 12.4). Mutases catalyze the transfer of functional groups within a molecule. Phosphoglycerate mutase has an active-site histidine residue, and a phospho-histidine adduct is formed as an enzyme-bound intermediate during the phosphate transfer reaction.
2-Phosphoglycerate then undergoes a dehydration reaction, catalyzed by enolase, a hydratase, to yield the high-energy phosphate compound, phosphoenolpyruvate (PEP). PEP is used by pyruvate kinase to phosphorylate ADP, yielding pyruvate and the second ATP, again by substrate-level phosphorylation. It seems strange that the high-energy phosphate bond in PEP can be formed from the low-energy phosphate compound 2-phosphoglycerate by a simple sequence of isomerization and dehydration reactions. However, the thermodynamic driving force for these reactions is probably derived from charge–charge repulsion between the phosphate and carboxylate groups of 2-phosphoglycerate and the isomerization of enolpyruvate to pyruvate following the phosphorylation reaction.
Phosphoglycerate kinase and pyruvate kinase catalyze substrate-level phosphorylation reactions
The ATP-generating reactions of glycolysis produce 2 moles of ATP per mole of triose phosphate, or a total of 4 moles of ATP per mole of Fru-1,6-BP. After adjustment for the ATP invested in the hexokinase and PFK-1 reactions, the net energy yield is 2 moles of ATP per mole of glucose converted into pyruvate.
Lactate dehydrogenase (LDH)
LDH regenerates NAD+ consumed in the GAPDH reaction, producing lactate, the end-product of anaerobic glycolysis
Two molecules of pyruvate have exactly the same number of carbons and oxygens as one molecule of glucose; however, there is a deficit of four hydrogens – each pyruvate has four hydrogens, a total of eight hydrogens for two pyruvates, compared with 12 in a molecule of glucose. The ‘missing’ four hydrogens remain in the form of the 2NADH and 2 H+ formed in the GAPDH reaction. Since NAD+ is present in only catalytic amounts in the cell and is an essential cofactor for glycolysis (and other reactions), there must be a mechanism for regeneration of NAD+ if glycolysis is to continue.
The oxidation of NADH is accomplished under anaerobic conditions by lactate dehydrogenase (LDH), which catalyzes reduction of pyruvate to lactate by NADH + H+ and regenerates NAD+. In mammals, all cells have LDH, and lactate is the end product of glycolysis under anaerobic conditions. Under aerobic conditions, mitochondria oxidize NADH to NAD+ and convert pyruvate to CO2 and H2O, so that lactate is not formed. Despite their capacity for oxidative metabolism, however, some cells may at times ‘go glycolytic’, forming lactate, e.g. in muscle during oxygen debt and in phagocytes in pus or in poorly perfused tissues. Most of the lactate excreted into blood is retrieved by the liver for use as a substrate for gluconeogenesis (Chapter 13).
Fermentation
Fermentation is a general term for anaerobic metabolism of glucose, usually applied to monocellular organisms
Some anaerobic bacteria, such as lactobacilli, produce lactate, while others have alternative pathways for anaerobic oxidation of NADH formed during glycolysis. During fermentation in yeast, the pathway of glycolysis is identical with that in the RBC, except that pyruvate is converted into ethanol (Fig. 12.6). The pyruvate is first decarboxylated by pyruvate decarboxylase to acetaldehyde, releasing CO2. The NADH produced in the GAPDH reaction is then reoxidized by alcohol dehydrogenase, regenerating NAD+ and producing ethanol. Ethanol is a toxic compound and most yeast die when the ethanol concentration in their medium reaches about 12%, which is the approximate concentration of alcohol in natural wines. Alcoholic beverages are a rich source of energy; alcohol yields ~7 kcal/g (29 kJ/g) by aerobic metabolism (Table 9.1), intermediate between carbohydrates and lipids. As a food, alcoholic beverages are more stable to long-term storage, compared to the fruits and vegetables from which they are produced. Beer, wine, cider and mead also provide varying amounts of vitamins, minerals, phytochemicals and xenobiotics.
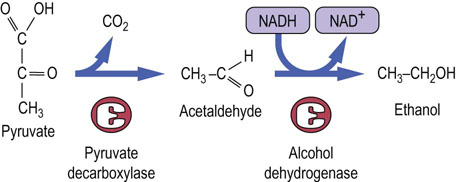
Fig. 12.6 Anaerobic glycolysis in yeast.
Formation of ethanol by anaerobic glycolysis during fermentation. Pyruvate is decarboxylated by pyruvate decarboxylase, yielding acetaldehyde and CO2. Alcohol dehydrogenase uses NADH to reduce acetaldehyde to ethanol, regenerating NAD+ for glycolysis.
Other fermented food products, which are estimated to account for a third of all foods eaten by humans worldwide, include pickles, sauerkraut, buttermilk, yogurt, sausage, some fish and meats, bread, cheese, and various sauces and condiments – even coffee and chocolate. The acidic environment produced during fermentation limits spoilage and growth of pathogenic microorganisms. Fermentation also pre-digests and increases the digestibility of foods, sometimes improves its nutritional value by adding bacteria-derived vitamins, and invariably contributes to the flavor and aroma of foodstuffs.
There are as many as 1000 species of anaerobic bacteria in our intestines. These enterobacteria thrive in a symbiotic relationship with man. They assist significantly in the digestion and extraction of energy from foodstuffs, are a source of biotin and vitamin K, provide protection against infection by pathogens, and promote gastrointestinal peristalsis. The species distribution also changes in response to the carbohydrate, fat and protein content of our diet.
Regulation of glycolysis in erythrocytes
Glycolysis is regulated allosterically at three kinase reactions
Hexokinase
RBCs consume glucose at a fairly steady rate. They are not physically active like muscle, and do not require energy for transport of O2 or CO2. Glycolysis in red cells appears to be regulated simply by the energy needs of the cell, primarily for maintenance of ion gradients. The balance between ATP consumption and production is controlled allosterically at three sites: the hexokinase, phosphofructokinase-1, and pyruvate kinase reactions (see Fig. 12.2). Based on measurements of the Vmax of the various enzymes in RBC lysates in vitro, hexokinase is present at the lowest activity of all glycolytic enzymes. Its maximal activity is about five times the rate of glucose consumption by the RBC, but it is subject to feedback (allosteric) inhibition by its product Glc-6-P. Hexokinase has 30% homology between its N- and C-terminal domains, the result of duplication and fusion of a primordial gene; binding of Glc-6-P to the N-terminal domain inhibits the activity of the enzyme and production of Glc-6-P at the active site in the C-terminal domain.
Phosphofructokinase-1 (PFK-1)
PFK-1 is the primary site of regulation of glycolysis
PFK-1 is the primary site of regulation of glycolysis,is the primary site of regulation of glycolysis,is the primary site of regulation of glycolysis, is the primary site of regulation of glycolysis, the flux of Fru-6-P to Fru-1,6-BP and, indirectly through the phosphoglucose isomerase reaction, the level of Glc-6-P and inhibition of hexokinase. Although present at 20 times higher activity than hexokinase, PFK-1 is strongly inhibited by ambient ATP, so that its activity varies with the energy status of the cell. Amazingly, ATP is both a substrate (see Fig. 12.3) and an allosteric inhibitor (Fig. 12.7) of PFK-1, a dual function that permits fine control over the activity of the enzyme.
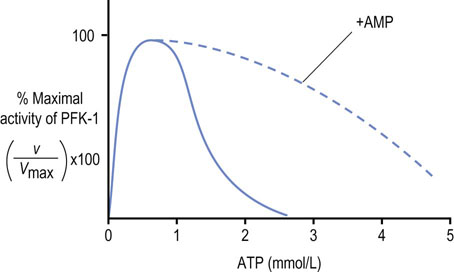
Fig. 12.7 Allosteric regulation of phosphofructokinase-1 (PFK-1) by ATP.
AMP is a potent activator of PFK-1 in the presence of ATP.
As shown in Figure 12.7, the concentration of ATP in the RBC (~2 mmol/L) normally suppresses the activity of PFK-1. AMP, which is present at much lower concentration (~0.05 mmol/L), relieves this inhibition. Because of their relative concentrations, a small fractional conversion of ATP to AMP in the RBC yields a large relative increase in AMP concentration, which activates PFK-1. ADP also relieves the inhibition of PFK-1 by ATP, but its concentration does not change as much with energy utilization. AMP (and ADP) not only relieves the inhibition of PFK-1 by ATP but also decreases the Km for the substrate Fru-6-P, further increasing the catalytic efficiency of the enzyme.
Through allosteric mechanisms, the activity of PFK-1 in the red cell is exquisitely sensitive to changes in the energy status of the cell, as measured by the relative concentrations of ATP, ADP and AMP. In effect, the overall activity of PFK-1, and thus the rate of glycolysis, depends on the cell's (AMP + ADP)/ATP concentration ratio. These products are interconvertible by the adenylate kinase reaction:
When ATP is consumed and ADP increases, AMP is formed by the adenylate kinase reaction. The increase in AMP concentrations relieves the inhibition of PFK-1 by ATP, activating glycolysis. The phosphorylation of ADP during glycolysis, and then of AMP by the adenylate kinase reaction, gradually restores the ATP concentration or energy charge of the cell and, as the AMP concentration declines, the rate of glycolysis decreases to a steady-state level. Glycolysis operates at a fairly constant rate in the red cell, where ATP consumption is steady, but the activity of this pathway changes rapidly in response to ATP utilization in muscle during exercise.
Pyruvate kinase (PK)
In addition to regulation by hexokinase and PFK-1, pyruvate kinase in liver is allosterically activated by Fru-1,6-BP, the product of the PFK-1 reaction. This process, known as feed-forward regulation, may be important in the RBC to limit the accumulation of chemically reactive triose phosphate intermediates in the cytosol.
Characteristics of regulatory enzymes
Each of the three enzymes involved in regulation of glycolysis – hexokinase, PFK-1, and pyruvate kinase – has the characteristic features of a regulatory enzyme: they are dimeric or tetrameric enzymes whose structure and activity are responsive to allosteric modulators; they are present at low Vmax in comparison with other enzymes in the pathway; and they catalyze irreversible reactions.
The regulation of glycolysis in liver, muscle, and other tissues is more complicated than in the RBC (Table 12.1) because of greater variability in the rate of fuel consumption and the interplay between carbohydrate and lipid metabolism during aerobic metabolism. In these tissues, the amount and activity of the regulatory enzymes are regulated by other allosteric effectors, by covalent modification, and by induction or repression of enzyme activity.
Synthesis of 2,3-bisphosphoglycerate (2,3-BPG)
2,3-BPG is a negative allosteric effector of the oxygen affinity of hemoglobin
2,3-Bisphosphoglycerate (Fig. 12.8) is an important by-product of glycolysis in the RBC, sometimes reaching 5 mmol/L concentration, which is comparable to the molar concentration of hemoglobin (Hb) in the RBC. 2,3-BPG is the major phosphorylated intermediate in the erythrocyte, present at even higher concentrations than ATP (1–2 mmol/L) or inorganic phosphate (1 mmol/L). 2,3-BPG is a negative allosteric effector of the O2 affinity of Hb. It decreases the O2 affinity of hemoglobin, promoting the release of O2 in peripheral tissue. The presence of 2,3-BPG in the RBC explains the observation that the O2 affinity of purified HbA is greater than that of whole RBCs. 2,3-BPG concentration increases in the RBC during adaptation to high altitude, in chronic obstructive pulmonary disease, and in anemia, promoting the release of O2 to tissues when the O2 tension and saturation of hemoglobin is decreased in the lung. Fetal Hb (HbF) is less sensitive than adult Hb (HbA) to the effects of 2,3-BPG; the higher oxygen affinity of HbF, even in the presence of 2,3-BPG, promotes efficient transfer of O2 across the placenta from HbA to HbF (see Chapter 5).
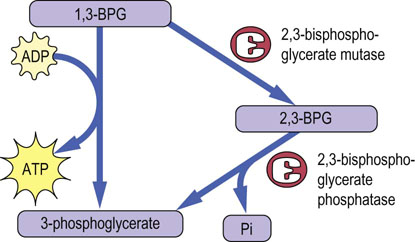
Fig. 12.8 Pathway for biosynthesis and degradation of 2,3-bisphosphoglycerate (2,3-BPG).
BPG mutase catalyzes the conversion of 1,3-BPG to 2,3-BPG. This same enzyme has bisphosphoglycerate phosphatase activity, so that it controls both the synthesis and hydrolysis of 2,3-BPG. Note that this pathway bypasses the phosphoglycerate kinase reaction, so that the overall yield of ATP per mole of glucose is decreased to zero.
The pentose phosphate pathway
Overview
The pentose phosphate pathway is the source of ribose phosphate for synthesis of RNA and DNA
The pentose phosphate pathway is a cytosolic pathway present in all cells, so named because it is the primary pathway for formation of pentose phosphates for synthesis of nucleotides for incorporation into DNA and RNA (see Chapter 30). This pathway branches from glycolysis at the level of Glc-6-P: thus, its alternative designation, the hexose monophosphate shunt. The pentose phosphate pathway is sometimes described as a shunt, rather than a pathway, because when pentoses are not needed for biosynthetic reactions, the pentose phosphate intermediates are recycled back into the mainstream of glycolysis by conversion into Fru-6-P and glyceraldehyde-3-phosphate. This rerouting is especially important in the RBC and in nondividing or quiescent cells, where there is limited need for synthesis of DNA and RNA.
NADPH is a major product of the pentose phosphate pathway in all cells
In tissues with active lipid biosynthesis, e.g. liver, adrenal cortex or lactating mammary glands, the NADPH is used in redox reactions required for biosynthesis of cholesterol, bile salts, steroid hormones and triglycerides. The liver also uses NADPH for hydroxylation reactions involved in the detoxification and excretion of drugs. The RBC has little biosynthetic activity, but still shunts about 10% of glucose through the pentose phosphate pathway, in this case almost exclusively for the production of NADPH. The NADPH is used primarily for the reduction of a cysteine-containing tripeptide, glutathione (GSH), an essential cofactor for antioxidant protection (Chapter 37).
The pentose phosphate pathway is divided into an irreversible redox stage, which yields both NADPH and pentose phosphates, and a reversible interconversion stage, in which excess pentose phosphates are converted into glycolytic intermediates. Both stages are important in the RBC, since it needs NADPH for reduction of glutathione, but has limited need for de novo synthesis of pentoses.
The redox stage of the pentose phosphate pathway – synthesis of NADPH
NADPH is synthesized by two dehydrogenases, in the first and third reactions of the pentose phosphate pathway (Fig. 12.9). In the first step of the pathway, the Glc-6-P dehydrogenase (G6PDH) reaction produces NADPH by oxidation of Glc-6-P to 6-phosphogluconic acid lactone, a cyclic sugar ester. The lactone is hydrolyzed to 6-phosphogluconic acid by lactonase. Oxidative decarboxylation of 6-phosphogluconate, catalyzed by 6-phosphogluconate dehydrogenase, then yields the ketose sugar, ribulose 5-phosphate, plus 1 mole of CO2, and the second mole of NADPH.
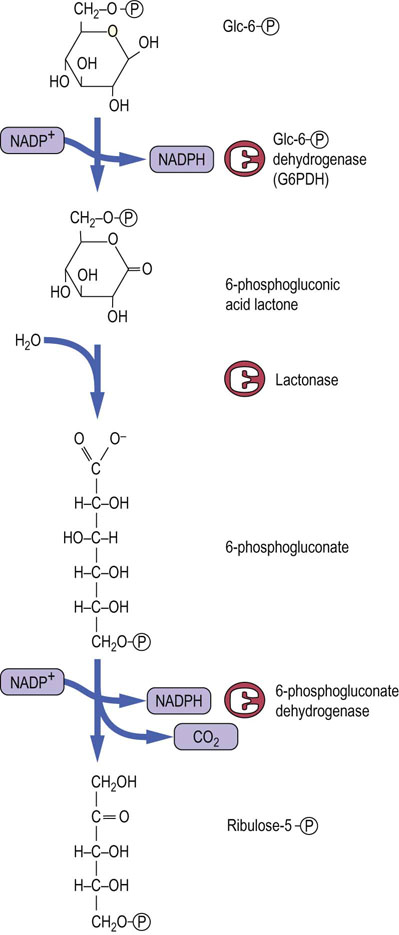
Fig. 12.9 The redox stage of the pentose phosphate pathway.
A sequence of three enzymes forms 2 moles of NADPH per mole of Glc-6-P, which is converted into ribulose-5-phosphate, with evolution of CO2.
G6PDH and 6-phosphogluconate dehydrogenase maintain a cytoplasmic ratio of NADPH/NADP+ ~ 100. Interestingly, because NAD+ is required for glycolysis, the ratio of NADH/NAD+ in the cytoplasm is nearly the inverse, less than 0.01. Although the total concentrations (oxidized plus reduced forms) of NAD(H) and NADP(H) in the RBC are similar (~25 µmol/L), the cell maintains these two redox systems with similar redox potentials at such different set-points in the same cell by isolating their metabolism through the specificity of cytoplasmic dehydrogenases. Glycolytic enzymes (GAPDH and LDH) use only NAD(H), while pentose phosphate pathway enzymes use only NADP(H). There are no enzymes in the RBC that catalyze the reduction of NAD+ by NADPH, so that high levels of both NAD+ and NADPH can exist simultaneously in the same compartment.
The interconversion stage of the pentose phosphate pathway
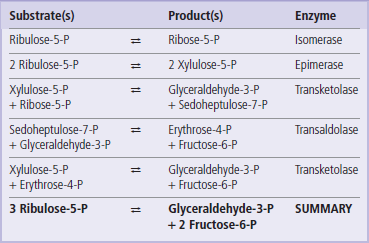
In cells with active nucleic acid synthesis, ribulose-5-phosphate from the 6-phosphoglucose dehydrogenase reaction is isomerized to ribose-5-phosphate for synthesis of ribo- and deoxyribonucleotides for RNA and DNA (Fig. 12.10). However, in nondividing cells, the pentose phosphates are routed back to glycolysis. This is accomplished by a series of equilibrium reactions in which 3 moles of ribulose-5-phosphate are converted into 2 moles of Fru-6-P and 1 mole of glyceraldehyde-3-phosphate. Certain restrictions are imposed on the interconversion reactions – they may be carried out only by transfer of two or three carbon units between sugar phosphates. Each reaction must also involve a ketose donor and an aldose receptor. Isomerases and epimerases convert ribulose-5-phosphate to the aldose- and ketose-phosphate substrates for the interconversion stage. Transketolase, a thiamine-dependent enzyme, catalyzes the two-carbon transfer reactions. Transaldolase acts similarly to the aldolase in glycolysis, except that the three-carbon unit is transferred to another sugar, rather than released as a free triose phosphate for glycolysis.
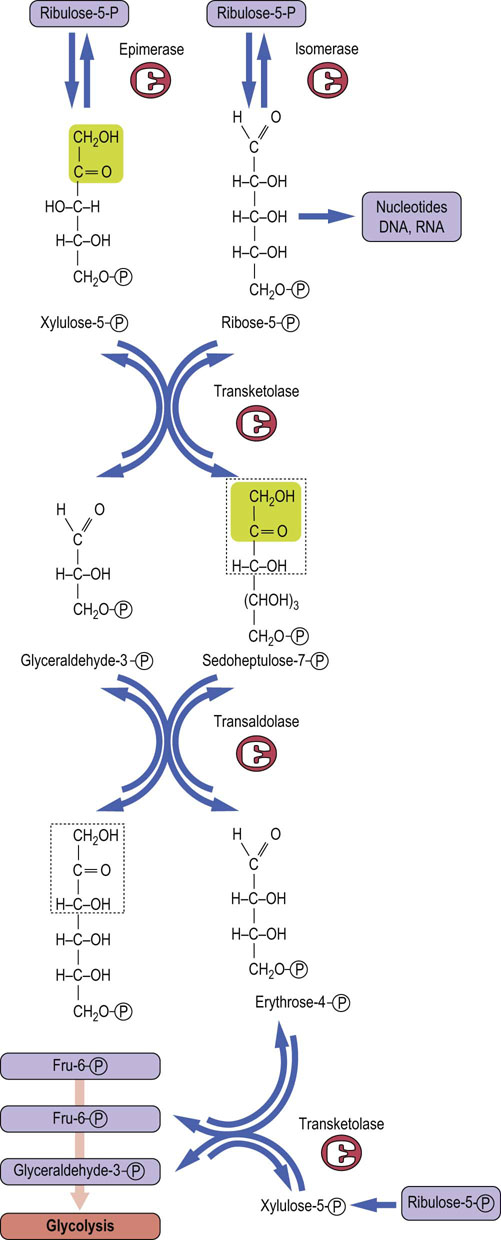
Fig. 12.10 The interconversion stage of the pentose phosphate pathway.
The carbon skeletons of three molecules of ribulose-5-phosphate are shuffled to form two molecules of Fru-6-P and one molecule of glyceraldehyde 3-phosphate, which enter into glycolysis.
As shown in Figure 12.10 and Table 12.2, two molecules of ribulose 5-phosphate, the first pentose product of the redox stage, are converted into separate products: one molecule is isomerized to the aldose sugar ribose-5-phosphate, and the other is epimerized to xylulose-5-phosphate. Transketolase then catalyzes transfer of two carbons from xylulose-5-phosphate to ribose-5-phosphate, yielding a seven-carbon ketose sugar, sedoheptulose-7-phosphate, and the three-carbon glyceraldehyde-3-phosphate. Transaldolase then catalyzes a three-carbon transfer between the two transketolase products, from sedoheptulose-7-phosphate to glyceraldehyde-3-phosphate, yielding the first glycolytic intermediate, Fru-6-P, and a residual erythrose-4-phosphate. A third molecule of xylulose-5-phosphate donates two carbons to erythrose-4-phosphate in a second transketolase reaction, yielding a second molecule of Fru-6-P and a molecule of glyceraldehyde-3-phosphate, both of which enter glycolysis.
Thus, three five-carbon sugar phosphates (ribulose-5-phosphate) formed in the redox stage of the pentose phosphate pathway are converted into one three-carbon (glyceraldehyde-3-phosphate) and two six-carbon (fructose-6-phosphate) intermediates for glycolysis. In the RBC, these glycolytic intermediates continue through glycolysis to lactate, illustrating that glucose is only temporarily shunted away from the mainstream of glycolysis.
Antioxidant function of the pentose phosphate pathway
The pentose phosphate pathway protects against oxidative damage in the red cell
Glutathione (GSH) is a tripeptide γ-glutamyl-cysteinyl-glycine (Fig. 12.11). It is present in cells at 2–5 mmol/L, 99% in the reduced (thiol) form, and is an essential coenzyme for protection of the cell against a range of oxidative and chemical insults (Chapter 37). Most of the NADPH formed in the red cell is used by glutathione reductase to maintain GSH in the reduced state. During its function as a coenzyme for antioxidant activities, GSH is oxidized to the disulfide form, GSSG, which is then regenerated by the action of glutathione reductase (Fig. 12.12).
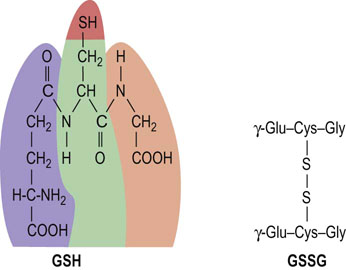
Fig. 12.11 Glutathione.
Structure of reduced glutathione (GSH) and oxidized glutathione (GSSG). Note the isopeptide bond between the γ-carboxyl, rather than the α-carboxyl, of glutamic acid and the α-amino group of cysteine.
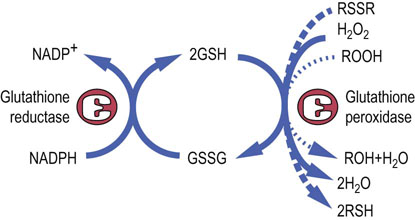
Fig. 12.12 Antioxidant activities of glutathione.
GSH is the coenzyme for glutathione peroxidases which detoxify hydrogen peroxide and organic (lipid) hydroperoxides. Hydrogen peroxide and lipid peroxides are formed spontaneously in the red cell, catalyzed by side reactions of heme iron during oxygen transport on hemoglobin (Chapter 37). GSH also reduces disulfide bonds in proteins (RSSR), formed during oxidative stress (Chapter 37), regenerating the native form of the protein (RSH).
GSH has a range of protective functions in the cell. Glutathione peroxidase (GPx) is found in all cells and uses GSH for detoxification of hydrogen peroxide and organic (lipid) peroxides in the cytosol and cell membranes (see Fig. 12.12). Because GPx contains a selenocysteine residue in its active site, selenium, which is required in trace amounts in the diet, is often described as an antioxidant nutrient (see Chapter 11).
GSH also acts as an intracellular sulfhydryl buffer, maintaining exposed –SH groups on proteins and enzymes in the reduced state. Under normal circumstances, when proteins are exposed to O2, their free sulfhydryl groups gradually oxidize to form disulfides, either intramolecularly or by intermolecular crosslinking with other protein molecules. In the red cell, GSH maintains the –SH groups of hemoglobin in the reduced state, inhibiting disulfide crosslinking of the protein.
Summary
This chapter describes two ancient metabolic pathways common to all cells in the body: glycolysis and the pentose phosphate pathway. The RBC, which lacks mitochondria and the capability for oxidative metabolism and obtains all of its ATP energy by glycolysis, is used as a model for introducing these pathways.
 Anaerobic glycolysis in the RBC provides a limited amount of ATP by conversion of the six-carbon sugar glucose to two molecules of the three-carbon hydroxyacid lactate.
Anaerobic glycolysis in the RBC provides a limited amount of ATP by conversion of the six-carbon sugar glucose to two molecules of the three-carbon hydroxyacid lactate.
 Through a series of sugar phosphate intermediates, glycolysis provides metabolites for branch points to numerous other metabolic pathways, including the pentose phosphate pathway.
Through a series of sugar phosphate intermediates, glycolysis provides metabolites for branch points to numerous other metabolic pathways, including the pentose phosphate pathway.
 The pentose phosphate pathway provides pentoses for synthesis of DNA and RNA in nucleated cells, and NADPH for biosynthetic reactions. NADPH is also required for maintenance of reduced glutathione, which is an essential cofactor for antioxidant defense systems that protect the cell against oxidative stress.
The pentose phosphate pathway provides pentoses for synthesis of DNA and RNA in nucleated cells, and NADPH for biosynthetic reactions. NADPH is also required for maintenance of reduced glutathione, which is an essential cofactor for antioxidant defense systems that protect the cell against oxidative stress.
Bar-Even, A, Flamholz, A, Noor, E, et al. Rethinking glycolysis: on the biochemical logic of metabolic pathways. Nat Chem Biol. 2012; 8:509–517.
Climent, F, Roset, F, Repiso, A, et al. Red cell glycolytic enzyme disorders caused by mutations: an update. Cardiovasc Hematol Disord Drug Targets. 2009; 9:95–106.
Granchi, C, Minutolo, F. Anticancer agents that counteract tumor glycolysis. Chem Med Chem. 2012; 7:1318–1350.
Katz, SE. The art of fermentation. White River Junction, VT: Chelsea Green Publishing; 2012.
Krol, DM, Nedley, MP. Dental caries: state of the science for the most common chronic disease of childhood. Adv Pediatr. 2007; 54:215–239.
Nicholson, JK, Holmes, E, Kinross, J, et al. Host-gut microbiota metabolic interactions. Science. 2012; 336:1262–1267.
Wamelink, MM, Struys, EA, Jakobs, C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008; 31:703–717.
Weissman, JS, Coyle, W. Stool transplants: ready for prime time? Curr Gastroenterol Rep. 2012; 14:313–316.
American Society of Hematology, case studies on anemia. http://teachingcases.hematology.org/.