Vitamins and Minerals
Introduction
Vitamins and trace elements are micronutrients essential for metabolism
They form prosthetic groups of enzymes, or serve as their cofactors. They participate in metabolism of carbohydrates, fat and proteins. Some vitamins (A and D) act as hormones. Both vitamins and trace metals are also important for cell growth, proliferation and differentiation, and many of them affect immune phenomena. The requirement for vitamins depends, to some extent, on the macronutrient intake (Chapter 22).
Deficiency of a micronutrient may develop because of inadequate intake, poor absorption from the intestinal tract, inefficient utilization or increased loss, or increased demand. Such deficiencies of micronutrients lead to specific clinical syndromes. They may develop as a component of general malnutrition, may themselves be a cause of illness, or may develop during periods of increased demand such as pregnancy or the adolescent growth spurt. In old age, deficiencies may be associated with less efficient intestinal absorption (Chapter 10). They may also occur as complications of gastrointestinal surgery. Multiple micronutrient deficiencies are much more common than single ones.
Trace metals – and some vitamins, are toxic in excess
Trace elements such as cadmium, mercury, and aluminum find their way into the food chain and are cytotoxic. Essential trace elements, e.g. copper and manganese, may also be toxic in excess. For evaluation of trace element toxicity, tissues other than blood may need to be analyzed before a diagnosis of metal poisoning can be made.
Vitamins are divided into fat-soluble and water-soluble vitamins
Fat-soluble vitamins are A, D, E, and K, and water-soluble vitamins are B1, B2, B3, B5, B6, B12, folate, biotin and vitamin C.
Assessment of micronutrient status is difficult for several reasons
Measurements of plasma concentrations of vitamins are inappropriate in the case of water-soluble ones, because these levels relate to the recent intake and do not reflect the overall body status. In such cases, the measurement of activities of enzymes associated with particular vitamins has been more appropriate. This is usually carried out as a stimulation test: the activity of an enzyme is measured in the absence and in the presence of a tested vitamin. A deficit is recognized if the enzyme activity is stimulated in the presence of added vitamin.
Finally, a decrease in a concentration of a nutrient in blood or plasma does not necessarily indicate a deficiency; it could be simply reflecting a metabolic response to stress or a change in physiologic state such as pregnancy.
Fat-soluble vitamins: A, D, E, K
Fat-soluble vitamins are stored in tissues
Fat-soluble vitamins are associated with body fat and are often stored in tissues, with circulating concentrations being kept relatively constant. For example, vitamin A is stored in the liver and is transported in plasma by specific binding proteins. Fat-soluble vitamins are not as readily absorbed from the diet as are water-soluble vitamins but, on the other hand, ample amounts are stored in tissues. With the exception of vitamin K they do not act as coenzymes: vitamins A and D behave like hormones. Vitamins A and D, but not vitamin E or K, can be toxic in excess.
Vitamin A
‘Vitamin A’ is a generic term for retinol, retinal and retinoic acid, all of which are found in animals. Retinal and retinoic acid are the active forms of vitamin A. The term ‘retinoids’ has been used to define these substances as well as other synthetic compounds associated with vitamin A-like activity.
The provitamin of vitamin A is a plant pigment β-carotene and other carotenoids. β-Carotene is converted in the small bowel to all-trans retinal by the action of the β-carotene dioxygenase. Further metabolism in the enterocytes produces retinol and retinoic acid, which are transported to the liver for storage (Fig. 11.1). β-Carotene is water soluble and is found in plant food. Good sources of β-carotene are dark-green and yellow vegetables and tomatoes.
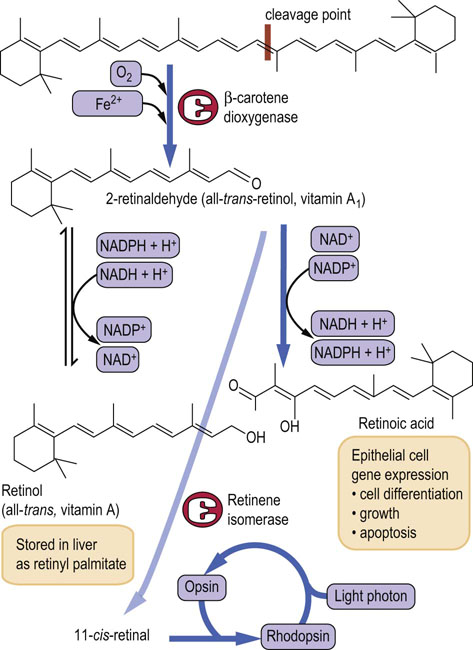
Fig. 11.1 Structure, metabolism and function of vitamin A.
Conversion of retinaldehyde to retinoic acid is irreversible. (See also Chapter 40.) Reproduced from Dominiczak MH. Medical Biochemistry Flash Cards. London: Elsevier, 2012, Card 39.
All dietary forms of vitamin A are converted to retinol
All dietary forms of vitamin A are converted to retinol. Conversion of carotenoids to vitamin A is rarely 100% efficient and the potency of foods is described in retinol activity equivalents (RAE); 1 µg of retinol is equivalent to 12 µg β-carotene, or 24 µg of other carotenes. Retinol in turn can be converted to retinal and retinoic acid. Liver, fish oil, egg yolk, butter, and milk are good sources of preformed retinol and retinoic acid.
Vitamin A is stored in the liver and needs to be transported to its sites of action
Vitamin A is stored in the liver in the form of retinol and retinyl esters (retinol palmitate), bound to the cytosolic retinol-binding proteins (CRBP). The stores in the liver comprise approximately 1 year's supply. Retinol is secreted from the liver bound to serum retinol-binding protein (RBP). Retinoic acid is thought to be transported to cells bound to either albumin or to a specific retinoic acid-binding protein (RABP). Retinol is taken up by cells via a membrane receptor.
Retinoic acid is a signaling molecule. It interacts with ligand-activated transcription factors, known as the nuclear retinoid receptors. The retinoid acid receptors (RARs) bind all-trans- and 9-cis-retinoic acid, while the so-called rexinoid receptors (RXRs) bind the 9-cis isomer only. These receptors can form heterodimers. RXR-type receptors can also interact with other nuclear receptors such as those for vitamin D3, thyroid hormones or peroxisome proliferator-activated receptors (PPARs). Retinoic acid also has a role in the growth and differentiation of cells, including the ones in the central nervous system.
Vitamin A deficiency presents as night blindness
Vitamin A is a component of the visual pigment rhodopsin, which is found in the rod cells of the retina and is formed by the binding of 11-cis-retinal to the apoprotein opsin. When rhodopsin is exposed to light, it is bleached; the retinal dissociates and is isomerized and reduced to all-trans-retinol (Fig. 11.1). This reaction is accompanied by a conformational change and elicits a nerve impulse perceived by the brain as light. The rod cells are responsible for vision in poor light.
Vitamin A deficiency presents as defective night vision or night blindness (it is the most common symptom of vitamin A deficiency in children and pregnant women). Since vitamin A affects growth and differentiation of epithelial cells, its deficiency produces defective epithelialization and keratomalacia – corneal softening and opacity. Severe vitamin A deficiency leads to permanent blindness. Vitamin A deficiency is the commonest cause of blindness in the world.
Subclinical deficiency may also lead to increased susceptibility to infections. Severe vitamin A deficiency occurs mostly in the developing world but it is also fairly common in patients with severe liver disease or fat malabsorption (e.g. cystic fibrosis).
Pregnant and lactating women are also prone to vitamin A deficiency. The most vulnerable group are premature infants and, in the developing countries, breastfed children of mothers who themselves are vitamin A deficient.
Vitamin A is toxic in excess
Vitamin A is toxic in excess, with symptoms including increased intracranial pressure, headaches, double vision, dizziness, bone and joint pains, hair loss, dermatitis, hepatosplenomegaly, and diarrhea and vomiting. It is virtually impossible to develop vitamin A toxicity by ingesting normal foods; however, toxicity may result from the use of vitamin A supplements. Increased intake of vitamin A is also associated with teratogenicity and it should be avoided during pregnancy.
Vitamin D
Vitamin D (calciol) is a hormone. It is a group of closely related sterols produced by the action of ultraviolet light (wavelength 290–310 nm) on provitamins (ergosterol in plants and 7-dehydrocholesterol in animals). 7-Dehydrocholesterol is synthesized in the liver and is found in the skin. The products of the photolytic reaction are ergocalciferol (vitamin D2) and cholecalciferol (vitamin D3). They are equipotent and both are converted to a series of hydroxylated derivatives, in the liver to 25-hydroxycholecalciferol (25(OH)D3; calcidiol) and then in the kidney, to the active compound 1α-,25-dihydroxycholecalciferol (1,25(OH)2D3; calcitriol). Vitamin D metabolism and action are described in Chapter 26.
Vitamin D also influences genes involved in cell proliferation, differentiation and apoptosis. It modulates growth, participates in immune function, and is anti-inflammatory.
Vitamin D is the only vitamin that is not usually required in the diet
It is only under conditions of inadequate exposure to sunlight that dietary intake of vitamin D is required. Most of the intake is via milk and other fortified foodstuffs. Fish oil, beef, egg yolks and liver are also rich in vitamin D. The requirements are greater in winter due to lower exposure to sunlight.
Deficiency of vitamin D produces rickets in children and osteomalacia in adults
Vitamin D deficiency may be caused by insufficient exposure to sunlight or increased metabolism of vitamin D due to low calcium intake or absorption. Deficiency may also develop in kidney disease, fat malabsorption (in cystic fibrosis, Crohn's disease and after gastric bypass).
Rickets is characterized by soft pliable bones due to defective mineralization secondary to calcium deficiency. The characteristic bowing of the leg bones and the formation of the ‘rickety rosary’ around costochondral junctions result. In the adult, demineralization of preexisting bones takes place, increasing susceptibility to fractures. Vitamin D deficiency is also characterized by low circulating concentrations of calcium and an increased serum alkaline phosphatase activity (Chapter 26).
Vitamin D is toxic in excess
Vitamin D excess causes enhanced calcium absorption and bone reabsorption, leading to hypercalcemia and metastatic calcium deposition. The symptoms are anorexia, weight loss and polyuria, There is also a tendency to develop kidney stones because of the hypercalciuria secondary to hypercalcemia.
Vitamin E
Dietary vitamin E is a mixture of several compounds, called tocopherols. Ninety percent of vitamin E present in human tissues is in the form of the natural isomer, α-tocopherol (Fig. 11.2). It is involved in the immune function, and also in cellular signaling and gene expression. α-Tocopherol inhibits the activity of protein kinase C (PKC) and affects cell adhesion as well as arachidonic acid metabolism. In European folklore, vitamin E has been associated with fertility and sexual activity. This is certainly true in other animal species where vitamin E plays a role in sperm production and egg implantation, but it is not the case in man. It is absorbed from the diet in the small intestine with lipids, and there is no specific transport protein. It is packed into the chylomicrons and in the circulation it is associated with lipoproteins.
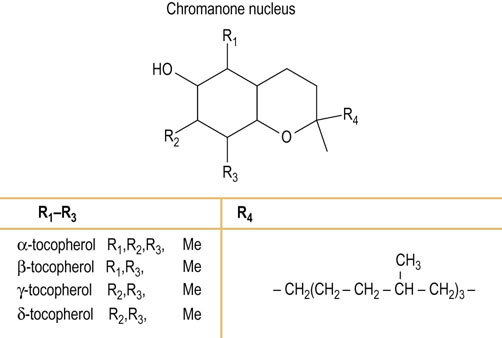
Fig. 11.2 Structure of vitamin E family (tocopherols).
R1–R3 can be methylated in a variety of combinations. R4 is a polyisoprenoid chain. Me, methyl.
Vitamin E is a membrane antioxidant
Vitamin E is the most abundant natural antioxidant and, owing to its lipid solubility, it is associated with all lipid-containing structures: membranes, lipoproteins and fat deposits (Fig. 37.9). It protects lipids from oxidation by the reactive oxygen species.
The richest sources of naturally occurring vitamin E are vegetable oils, nuts and also green leafy vegetables.
Fat malabsorption reduces vitamin E absorption
Apart from fat malabsorption, abetalipoproteinemia may also cause vitamin E deficiency. Deficiency may develop as a result of low vitamin E intake in pregnancy and newborn infants (mostly in preterm infants fed with formula milk with low vitamin E content). Deficiency of vitamin E in premature infants causes hemolytic anemia, thrombocytosis and edema and also peripheral neuropathy, myopathy and ataxia. There is little evidence in support of vitamin E toxicity.
Vitamin K
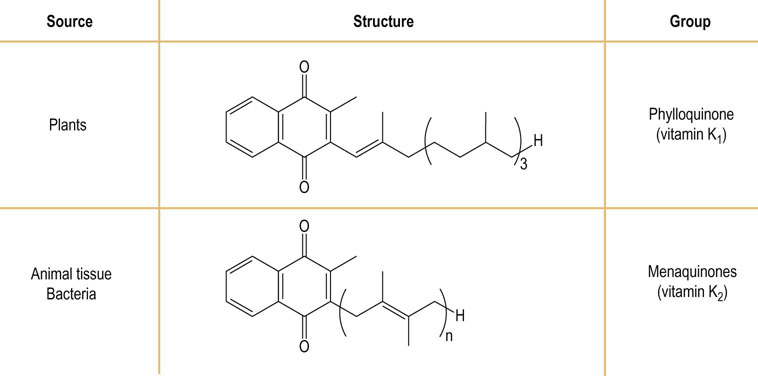
Vitamin K is a group of compounds, varying in the number of isoprenoid units in their side chain. Vitamin K circulates as phylloquinone (vitamin K1) and its hepatic stores are in the form of manaquinones (vitamin K2). The structure, nomenclature and sources of the vitamins K are outlined in Figure 11.3. Absorption of vitamin K depends on the ability to absorb fat.
Vitamin K is necessary for blood clotting
Vitamin K is required for post-translational modification of coagulation factors (factors II, VII, IX, and X; Fig. 7.3). All these proteins are synthesized by the liver as inactive precursors and are activated by the carboxylation of specific glutamic acid (Gla) residues by a vitamin K-dependent enzyme (Fig. 11.4). Prothrombin (factor II) contains 10 of these carboxylated residues and all are required for this protein's specific chelation of Ca2+ ions during its function in the coagulation process. Recently, other proteins containing vitamin K-dependent Gla residues, such as osteocalcin, have been identified in tissues.
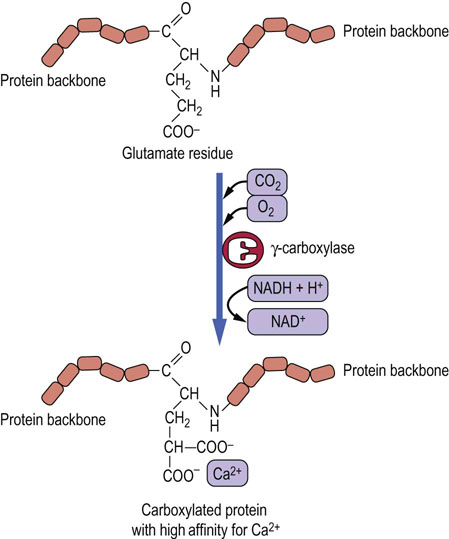
Fig. 11.4 Vitamin K-mediated carboxylation of glutamate residues.
The carboxylated residues are required for Ca2+ chelation.
Vitamin K is widely distributed in nature: its dietary sources are green leafy vegetables, fruits, dairy products, vegetable oils and cereals. Vitamin K is also produced by the intestinal microflora.
Vitamin K deficiency causes bleeding disorders
Vitamin K supply by the intestinal microflora virtually ensures that dietary deficiency does not occur in man, except for the newborn infants. Rarely, deficiency may develop in those with liver disease or fat malabsorption. The deficiency is associated with bleeding disorders.
Premature infants are especially at risk of deficiency and may develop the hemorrhagic disease of the newborn
Placental transfer of vitamin K to the fetus is inefficient. Immediately after birth the circulating concentration decreases. Normally, it recovers on absorption of food but this might be delayed in preterm infants. In addition, the gut of the newborn is sterile, and therefore for several days after birth there is no source of vitamin K.
Inhibitors of vitamin K action are valuable antithrombotic drugs
Specific inhibitors of vitamin K-dependent carboxylation are used in the treatment of thrombosis-related diseases, e.g. in patients with deep vein thrombosis and pulmonary thromboembolism, or those with atrial fibrillation who are at risk of thrombosis. These are the drugs of the dicoumarin group, e.g. warfarin, which inhibit the action of vitamin K (Chapter 7). Warfarin is also used as rat poison and vitamin K is the antidote for human poisoning by this agent.
Water-soluble vitamins B, C
With the exception of vitamin B12 the body has no storage capacity for water-soluble vitamins. As a consequence, they must be regularly supplied in the diet. Any excess is excreted in the urine. In contrast to the fat-soluble vitamins, there is no toxicity associated with B vitamins.
B-complex vitamins
B-complex vitamins are essential for normal metabolism and serve as coenzymes in many reactions in carbohydrate, fat and protein metabolism. The greater the caloric intake, the larger the requirement for B vitamins. Increased energy supply, in particular from carbohydrates, requires increased amounts of B vitamins. Diseases associated with high caloric requirement require greater intake of thiamine and other B vitamins. Therefore, beri-beri (see below) might develop on a high-carbohydrate diet.
Thiamine (vitamin B1)
Thiamine is essential for carboxylation reactions
Thiamine is important for carbohydrate metabolism. In its active form, thiamine pyrophosphate (TPP), it is a coenzyme of pyruvate dehydrogenase (the E1 enzyme in the PDH complex, Chapter 14). It participates in a similar reaction of oxidative decarboxylation of α-ketoglutarate and also in the metabolism of branched chain amino acids. It is also a coenzyme for transketolase in the pentose phosphate pathway (Chapter 12), and it is important in the production of hydrochloric acid in the stomach.
Beriberi was the first discovered ‘deficiency disease’
Severe thiamine deficiency results in beriberi, either ‘dry’ (without fluid retention) or ‘wet’ (associated with cardiac failure with edema). Beriberi is characterized primarily by neuromuscular symptoms, and occurs in populations relying exclusively on polished rice for food. The signs and symptoms of deficiency may also be seen in the elderly or in low-income groups with poor diet.
Thiamine deficiency is associated with alcoholism
Thiamine depletion can occur quickly (within approximately 14 days), Early symptoms are loss of appetite, constipation and nausea. They may progress to depression, peripheral neuropathy and unsteadiness. Further deterioration results in mental confusion (loss of short-term memory), ataxia and loss of eye coordination. This combination, often seen in alcoholic patients, is the Wernicke–Korsakoff psychosis. Wet beri-beri is particularly associated with alcoholism.
The tests used to assess the thiamine status include its direct measurement by high-pressure liquid chromatography and the ‘classic’ measurement of erythrocyte transketolase activity.
Riboflavin (vitamin B2)
Riboflavin is associated with oxidoreductases
Riboflavin is attached to the sugar alcohol ribitol. The molecule is colored, fluorescent and decomposes in visible light but is heat stable. It is found in the oxidoreductases such as the flavin mononucleotide (FMN) and the flavin adenine dinucleotide (FAD), and is required for the energy metabolism of both carbohydrates and lipids (Chapter 9).
Lack of riboflavin in the diet causes a deficiency syndrome of inflammation of the corners of the mouth (angular stomatitis), the tongue (glossitis) and scaly dermatitis. Photophobia may also develop. Owing to its light sensitivity, riboflavin deficiency may occur in newborn infants with jaundice, who are treated by phototherapy. Hypothyroidism is also known to affect the conversion of riboflavin to FMN and FAD.
To determine the riboflavin status, erythrocyte glutathione reductase activity is measured.
Niacin (vitamin B3)
Niacin is required for NAD+ and NADP+ synthesis
Niacin is a generic name for nicotinic acid or nicotinamide, both of which are essential nutrients. Niacin is active as part of the coenzyme nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+), both of which participate in oxidoreductase-catalyzed reactions. The active form of the vitamin required for the synthesis of NAD+ and NADP+ is nicotinate, and therefore nicotinamide must be deamidated before becoming available for synthesis of these coenzymes. Niacin can be synthesized from tryptophan and, hence, in the truest sense, is not a vitamin. The conversion is, however, very inefficient and cannot supply sufficient amounts of niacin. In addition, the conversion requires thiamine, pyridoxine, and riboflavin, and on marginal diets such synthesis would be problematic. The requirement for niacin is also related to energy expenditure.
Severe niacin deficiency produces dermatitis, diarrhea and dementia
Niacin deficiency initially produces a superficial glossitis but may progress to pellagra, which is characterized by dermatitis, sunburn-like skin lesions in areas of body exposed to sunlight and to pressure, and also by diarrhea, and dementia. Untreated pellagra is fatal. Certain drugs, e.g. the antituberculosis drug isoniazid, predispose to niacin deficiency. In the modern world pellagra is a medical curiosity.
On the other hand, very high doses of niacin can cause hepatotoxicity, which is reversible on withdrawal.
Pyridoxine (vitamin B6)
Pyridoxine is important for amino acid metabolism
Vitamin B6 is a mixture of pyridoxine (an alcohol), pyridoxal (an aldehyde), pyridoxamine, and their 5′-phosphate esters. Pyridoxine is the major form of vitamin B6 in the diet, and pyridoxal phosphate is its active form. It is absorbed in the jejunum. During absorption some hydrolysis of the phosphates occurs. Most tissues, however, contain pyridoxal kinase, which resynthesizes the phosphorylated forms required for the synthesis, catabolism and interconversion of amino acids (Chapter 19).
Pyridoxine requirements increase with high protein intake
Pyridoxal phosphate and pyridoxamine are involved in over 100 reactions in carbohydrate (including the glycogen phosphorylase reaction), lipid, and particularly amino acid metabolism, and the metabolism of one-carbon units (see Fig. 19.2). Pyridoxine is required for the synthesis of the neurotransmitters serotonin and noradrenaline (Chapter 41.1), and the synthesis of sphingosine, a component of sphingomyelin and sphingolipids (Chapter 28). It is also required for the synthesis of heme (Chapter 30) and it influences immune function. Because of its role in amino acid metabolism, vitamin B6 requirements increase with protein intake.
Vitamin B6 is present in a wide variety of foods such as fish, beef, liver, poultry, and also potatoes and fruits (but not citrus fruits).
Vitamin B6 deficiency causes neurologic symptoms and anemia
Vitamin B6 deficiency in its mild form causes irritability, nervousness and depression, progressing in severe deficiency to peripheral neuropathy, convulsions and coma. Severe deficiency is also associated with a sideroblastic anemia (anemia characterized by the presence of nucleated red blood cells with iron granules). Dermatitis, cheliosis and glossitis also occur. Decreased levels are observed in alcoholism, obesity, and in malabsorption states (Crohn's disease, celiac disease and ulcerative colitis), as well as in the end-stage renal disease and in autoimmune conditions.
The drug isoniazid, by binding to pyridoxine, and the oral contraceptive pill, by increasing the synthesis of enzymes requiring the vitamin, may precipitate deficiency. The debate concerning the contraceptive pill continues but it is generally accepted that there is an increased requirement for pyridoxine.
Assessment of pyridoxine status is based on the measurement of erythrocyte aspartate aminotransferase.
Biotin
Biotin is important for carboxylation reactions
Biotin serves as a coenzyme in multienzyme complexes involved in carboxylation reactions (Fig. 14.4). It is important in lipogenesis, gluconeogenesis, and in the catabolism of the branched-chain amino acids.
Biotin is normally synthesized by the intestinal flora and this meets most of the body requirements.
Symptoms of biotin deficiency include depression, hallucinations, muscle pain and dermatitis. Children with multiple decarboxylase deficiency also develop immunodeficiency disease. Consumption of raw eggs can cause biotin deficiency because the egg-white protein avidin combines with biotin, preventing its absorption.
Vitamin B12
Vitamin B12 is part of the heme structure
Vitamin B12 (cobalamin) has a complex ring structure similar to the porphyrin of heme (Chapter 30) but is hydrogenated to a greater extent. The iron at the center of the heme ring is replaced by a cobalt ion (Co3+). This is the only known function of cobalt in the body. In addition, a dimethylbenzimidazole ring is also part of the active molecule (it is essential for the chelation of the cobalt ion; Fig. 11.5). and in methionine synthesis
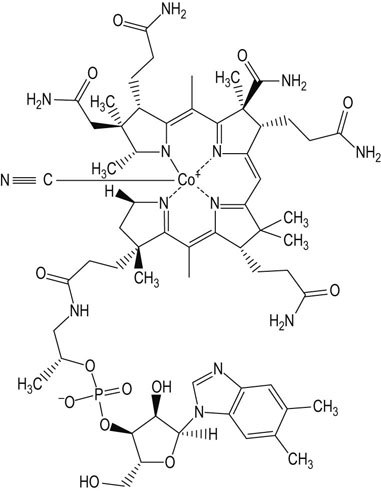
Fig. 11.5 Vitamin B12.
There is a cyano-group (CN) attached to the cobalt: this is an artifact of extraction but it is also the most stable form of the vitamin and indeed is the commercially available product. The cyano group does require removal for conversion to the active form of the vitamin.
Vitamin B12 participates in the nucleic acid synthesis, in the production of erythrocytes, and also in the recycling of folates. Together with folate and vitamin B6 it controls the homocysteine metabolism, where it is a cofactor for methionine synthase, which converts homocysteine to methionine. It participates in the synthesis of the methyl donor molecule, the S-adenosylmethionine. Vitamin B12 is required in only one further reaction, that of L-methylmalonyl-CoA mutase, which converts methylmalonyl-CoA to succinyl-CoA. The coenzyme form of the vitamin in this case is 5′-deoxy-adenosyl cobalamin.
Vitamin B12 requires the intrinsic factor for its absorption
There is a specific mechanisms exist for absorption and transport of cobalamin (Fig. 11.6). Cobalamin is released from foods by a gastric protease and HCl. It is bound to the intrinsic factor secreted by the parietal cells in the stomach, and is absorbed in the distal ileum by receptor-mediated endocytosis. Vitamin B12 is excreted in the bile, and there is a marked enterohepatic circulation.

Fig. 11.6 Digestion, absorption and transport of vitamin B12.
Simple diffusion of free vitamin B12 across the intestinal membrane accounts for 3% of transported vitamin, and complexing with intrinsic factor (IF) accounts for 97%. Vitamin B12 derivatives are released from food by peptic digestion in the stomach and bind to IF secreted by the parietal cells of the gastric mucosa. The IF–B12 complex binds to specific receptor sites on the ileal mucosa. The rate-limiting factor for vitamin B12 absorption is the number of ileal receptor sites. Other transport proteins, transcobalamin I, II and III (TC I, II and III) and R-proteins, are involved in the delivery or storage of the cobalamins. The latter are secreted by the salivary glands and gastric mucosa.
Vitamin B12 is only present in animal products
Vitamin B12 is synthesized solely by bacteria. It is absent from all plants but is concentrated in the livers of animals in three forms: methylcobalamin, adenosylcobalamin, and hydroxycobalamin. It is found only in animal products such as fish, dairy products, meats, and particularly organ meats such as liver and kidney. In the past, liver was used in the treatment of deficiency states. Fortified breakfast cereals also contain the vitamin. Vegans are therefore at risk of developing a dietary deficiency of vitamin B12.
Vitamin B12 deficiency causes pernicious anemia
Vitamin B12 deficiency is characterized by anemia, fatigue, constipation, weight loss, diarrhea and neurological symptoms such as numbness and tingling, loss of balance, confusion, mood disturbances and dementia. Deficiency can occur through several mechanisms. The most common one is pernicious anemia, an autoimmune condition that results in gastric atrophy and the lack of the intrinsic factor, which prevents the vitamin absorption in the terminal ileum. Pernicious anemia affects 1–3% of older adults. The intrinsic factor deficiency can also be caused by gastric surgery and by bariatric (weight loss) surgery. A similar situation, albeit caused through a different mechanism, arises upon surgical removal of the ileum, for instance in Crohn's disease (Chapter 10).
Deficiency may also be caused by hypochlorhydria associated with age (Table 11.1).
The function of vitamin B12 needs to be considered together with folate
Functions of vitamin B12 and folate are interrelated, and deficiency of either produces the same signs and symptoms. The reaction, which involves both these vitamins is the conversion of homocysteine to methionine (Fig. 11.7).
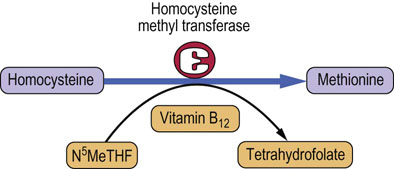
Fig. 11.7 The ‘tetrahydrolate trap’.
Vitamin B12 and folate are involved in the conversion of homocysteine to methionine. An absence of vitamin B12 inhibits the reaction and leads to the build-up of N5-methyltetrahydrofolate (N5MeTHF).
Megaloblastic anemia characteristic of vitamin B12 deficiency is probably due to a secondary deficiency of reduced folate, and a consequence of the accumulation of N5-methyltetrahydrofolate. A neurologic presentation can also develop in the absence of anemia. This is known as subacute combined degeneration of the cord and is probably secondary to a relative deficiency of methionine in the cord.
Deficiency of vitamin B12 results in the accumulation of methylmalonic acid and homocysteine, and consequent methylmalonic aciduria and homocystinuria.
Folic acid
Folic acid derivatives are important in single carbon transfer reactions. They are necessary for the synthesis of DNA
Folic acid (pteroyl-L-glutamic acid) exists in a number of derivatives collectively known as folates. It participates in single carbon transfer reactions such as methylation (important in both metabolism and regulation of gene expression), and in synthetic pathways of choline, serine, glycine and methionine. Folic acid is also necessary for the synthesis of purines and pyrimidine thymine, and thus for the synthesis of nucleic acids. Polymorphisms associated with variants of 5,10-methylenetetrahydrofolate reductase gene, the key enzyme in folate metabolism, are associated with conditions such as colon cancer, spina bifida and adult acute lymphocytic leukemia.
Folic acid is physiologically inactive until reduced to dihydrofolic acid. Its main forms are tetrahydrofolate, 5-methyl tetrahydrofolate (N5MeTHF), and N10-formyltetrahydrofolate-polyglutamate derived from N5MeTHF predominant in fresh food. Before polyglutamates can be absorbed, they must be hydrolyzed by glutamyl hydrolase in the small intestine. The main circulating form of folate is the monoglutamate-N5-THF.
Folic acid is present in liver, yeast, and green leafy vegetables (spinach) and fruits, including citrus fruits. Its sources are also folic acid enriched cereals and grains.
It can be measured by high-performance liquid chromatography (HPLC).
Structural analogues of folate are used as antibiotics and anticancer drugs
Not surprisingly, rapidly dividing cells have high requirements for folate since it is necessary for the synthesis of purines and pyrimidine thymine, all required for DNA synthesis (Chapter 31). Structural analogues of folate exhibit selective toxicity towards rapidly growing cells such as bacteria and cancer cells. This is the principle behind the development of drugs known as the folic acid antagonists, which are used as antibiotics (e.g. trimethoprim) and anticancer agents (methotrexate).
Folate deficiency is one of the commonest vitamin deficiencies
Causes of folate deficiency include inadequate intake, impaired absorption, impaired metabolism, and increased demand.
The most common examples of increased demand are pregnancy and lactation. Folic acid requirements increase greatly as the blood volume and number of erythrocytes increase in pregnancy. By the third trimester of pregnancy folic acid requirements double. Megaloblastic anemia in pregnancy (other than multiple pregnancy) is rare. However, folate deficiency increases the risk of neural tube defects. low birth weight and premature birth. In infants it results in a decreased growth rate. Other causes of folate deficiency are alcoholism, malabsorption, dialysis and liver disease. Folate deficiencies are seen in the elderly as a result of poor diet and poor absorption.
Folate deficiency in adults causes megaloblastic anemia
Failure to synthesize methionine and nucleic acids in folate deficiency states accounts for the signs and symptoms of megaloblastic anemia, i.e. the presence of enlarged blast cells in the bone marrow. Macrocytic erythrocytes have fragile membranes and a tendency to hemolyze: a macrocytic anemia exists in association with a megaloblastic bone marrow. The hematologic abnormalities cannot be distinguished from vitamin B12 deficiency (see below). The neurologic changes are also similar. Deficiency of folate also contributes to hyperhomocysteinemia. Many symptoms such as loss of appetite, diarrhea and weakness are nonspecific.
Adequate intake of folate around conception is essential
Common practice is to provide folate supplements during pregnancy. The supplementation during the periconception period (definitions of that period are variable: the one used in clinical studies is 4 weeks before and 8 weeks after conception) prevents spina bifida, as the closure of the neural tube occurs between 22 and 28 days after conception.
Vitamin B12 must be supplemented during folate treatment
Folate supplementation without B12 supplements can mask symptoms but lead to neurologic damage.
Giving folate alone in a case of vitamin B12 deficiency aggravates the neuropathy. Therefore, if supplementation is required during investigation of the cause of megaloblastic anemia, after blood and bone marrow specimens have been taken to confirm the diagnosis, folate needs to be given together with vitamin B12.
Vitamin C
Vitamin C serves as a reducing agent. Its active form is ascorbic acid, which is oxidized during the transfer of reducing equivalents, yielding dehydroascorbic acid. The synthetic pathway and structure of vitamin C are shown in Figure 11.8 and its antioxidant activity is illustrated in Figure 37.8. It participates in the regeneration of another antioxidant vitamin – α-tocopherol. Vitamin C takes part in the synthesis of collagen and epinephrine, in steroidogenesis, degradation of tyrosine, formation of bile acids and also in the synthesis of L-carnitine and neurotransmitters. It improves absorption of nonheme iron, and participates in bone mineral metabolism. Its prime function is to maintain metal cofactors in their lower valence state, e.g. Fe2+ and Cu2+. In the synthesis of collagen it is required specifically for the hydroxylation of proline (Chapter 27).
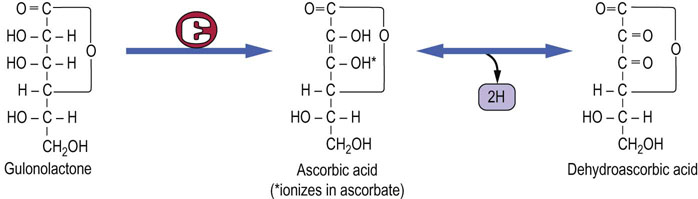
Fig. 11.8 Structure and synthesis of vitamin C (ascorbic acid).
Note that the enzyme that converts gulonolactone to ascorbic acid is absent in man and higher primate.
Vitamin C is absorbed in the intestine by a carrier-mediated, sodium-dependent transporter. It is reabsorbed in renal proximal tubules. Progressively more vitamin C is excreted in urine as intake increases.
Humans cannot synthesize ascorbic acid; therefore it is an essential nutrient
Vitamin C is labile: it is easily destroyed by oxygen, metal ions, increased pH, heat and light. Citrus, soft fruits, tomatoes and peppers are rich sources of vitamin C.
Vitamin C deficiency causes scurvy and compromises immune function
Vitamin C deficiency causes defective collagen synthesis. Scurvy is characterized by capillary fragility causing subcutaneous and other hemorrhages, muscle weakness, soft, swollen, bleeding gums, loosening of teeth, poor wound healing and anemia. There is fatigue, malaise and depression. Inability to maintain bone matrix in association with demineralization results in osteoporosis.
Vitamin C deficiency resulting in the full clinical picture of scurvy is now rare, except in older individuals. Milder forms of vitamin C deficiency are more common, and their manifestation includes easy bruising and the formation of petechiae (small, pinpoint hemorrhages under the skin). Immune function is also compromised. This reduction in immunocompetence has been the basis for providing megadoses of the vitamin to prevent the common cold and also for its role in cancer prevention. No clear evidence exists, however, to substantiate these claims first made by Linus Pauling in the 1970s. Vitamin C is certainly required for normal leukocyte function, and leukocyte vitamin C levels drop precipitously during stress caused by either trauma or infection. Elderly individuals are at increased risk of deficiency as are smokers and infants fed evaporated or boiled milk.
There is no evidence that vitamin C taken in excess is toxic. Theoretically, since it is metabolized to oxalate, there is a risk of the development of renal oxalate stones in susceptible individuals. However, this has not been substantiated in practice.
Dietary supplementation of vitamins
Supplementation of some vitamins results in a clear health benefit. This includes supplementation of folic acid to women who are pregnant or are planning pregnancy, to prevent neural tube defects. Vitamin D provision to people living in areas of low sunlight has also been beneficial.
Benefits of vitamin supplementation in cancer and cardiovascular disease are uncertain
Because supplementation of folic acid and vitamin B6 and B12 lowers plasma homocysteine concentration, it has been suggested that it could be beneficial for the prevention of cardiovascular disease. There also were suggestions that supplementation of vitamins A, C and E is protective against cancer. Some observational studies suggested that the supplementation of vitamins C and E could also be useful in the prevention of cardiovascular disease. However, prospective studies of this yielded controversial results. The recommendations of the US Preventive Services Task Force published in 2003 say that ‘current evidence is insufficient to recommend for or against the use of supplements of vitamins A, C, or E, multivitamins with folic acid, or antioxidant combinations for the prevention of cancer or cardiovascular disease’. Note that these recommendations do not apply to people with nutritional deficiencies, pregnant and lactating women, children, elderly persons, and people with chronic illnesses.
Vitamin supplementation can be harmful
As mentioned above, high-dose vitamin supplementation may be harmful: the example is the reduction of bone mineral density, hepatotoxicity, and teratogenicity associated with high doses of vitamin A. Supplementation of β-carotene to smokers was also harmful, resulting in an increase in lung cancer mortality.
Fruit and vegetables are the best sources of vitamins
In clinical studies, vitamins have been supplemented in a pure form, rather than as complete foodstuffs, and it might be that this is why the benefit of supplementation was not evident. Clearly, there are benefits of eating diets rich in vegetables and fruit, which are the most important sources of vitamins. There is no reason to discourage people from taking vitamin supplements apart from proven instances of toxicity.
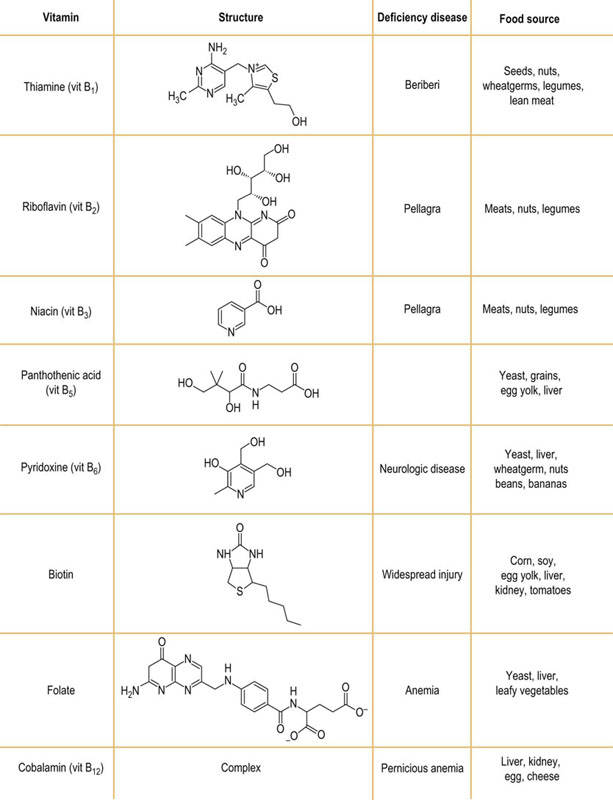
The deficiency states of vitamins B and folate are summarized in Figure 11.9. Patients often present with multiple deficiencies; a deficiency of a single B vitamin is rare.
Minerals
Major minerals present in the human body are sodium, potassium, chloride, calcium, phosphate, and magnesium
The daily body requirements for minerals range from gram (sodium, calcium, chloride, phosphorus) through milligram (iron, iodine, magnesium, manganese, molybdenum) to microgram (zinc, copper, selenium, other trace elements) amounts. Many are essential for normal biological function.
Sodium and chloride are important for the maintenance of osmolality of the extracellular fluid and cell volume (Chapter 24). Sodium participates in electrophysiologic phenomena and, together with potassium, is essential for maintaining transmembrane potential and impulse transmission (Chapter 8). Potassium is the main intracellular cation. Potassium is contained in vegetables and fruit, particularly bananas, and in fruit juices. Dietary potassium intake needs to be limited in renal disease because of its impaired excretion and a consequent tendency to hyperkalemia (Chapter 24). Importantly, both hyperkalemia and hypokalemia may lead to life-threatening arrhythmias.
 Magnesium functions as cofactor for many enzymes and is also important in the maintenance of membrane electrical potential. Its role is linked to that of potassium and calcium. It is important for skeletal development and for the maintenance of electrical potential in nerve and muscle membranes. It is also a cofactor for ATP-requiring enzymes, and is important for the replication of DNA, and for RNA synthesis. Magnesium deficiency develops in starvation and malabsorption, may be due to the loss from the gastrointestinal tract in diarrhea and vomiting, and sometimes occurs as a result of diuretic treatment and surgical procedures on the gastrointestinal tract. It is also associated with acute pancreatitis and alcoholism. Hypomagnesemia is often accompanied by hypocalcemia and hypokalemia. Magnesium deficiency leads to muscle weakness and cardiac arrhythmias.
Magnesium functions as cofactor for many enzymes and is also important in the maintenance of membrane electrical potential. Its role is linked to that of potassium and calcium. It is important for skeletal development and for the maintenance of electrical potential in nerve and muscle membranes. It is also a cofactor for ATP-requiring enzymes, and is important for the replication of DNA, and for RNA synthesis. Magnesium deficiency develops in starvation and malabsorption, may be due to the loss from the gastrointestinal tract in diarrhea and vomiting, and sometimes occurs as a result of diuretic treatment and surgical procedures on the gastrointestinal tract. It is also associated with acute pancreatitis and alcoholism. Hypomagnesemia is often accompanied by hypocalcemia and hypokalemia. Magnesium deficiency leads to muscle weakness and cardiac arrhythmias.
 Calcium and phosphate are essential for bone metabolism, and for secretory processes and cellular signaling (Chapter 26). Calcium is present in milk and milk products, and in some vegetables. Phosphates are abundant in plant and animal cells.
Calcium and phosphate are essential for bone metabolism, and for secretory processes and cellular signaling (Chapter 26). Calcium is present in milk and milk products, and in some vegetables. Phosphates are abundant in plant and animal cells.
 Iodine is essential for the synthesis of thyroid hormones (Chapter 39). The iodine content of food depends on the composition of the soil where it is grown. Marine fish and shellfish have the highest content. It is also present in freshwater fish, meat and dairy products, as well as in legumes, vegetables and fruit.
Iodine is essential for the synthesis of thyroid hormones (Chapter 39). The iodine content of food depends on the composition of the soil where it is grown. Marine fish and shellfish have the highest content. It is also present in freshwater fish, meat and dairy products, as well as in legumes, vegetables and fruit.
 Fluoride influences the structure of the bone and teeth enamel. In many areas fluoride is added to water to prevent teeth decay. Excess leads to teeth discoloration and fragility of bones.
Fluoride influences the structure of the bone and teeth enamel. In many areas fluoride is added to water to prevent teeth decay. Excess leads to teeth discoloration and fragility of bones.
Iron
Iron is important in the transfer of molecular oxygen
Iron is a component of heme in hemoglobin and myoglobin (Chapter 5). Cytochromes a, b, and c also contain iron (Chapter 9). Altogether, there are 3–4 g of iron in the body. Seventy-five percent of body iron is in hemoglobin and myoglobin, and 25% is stored in tissues such as bone marrow, liver, and reticuloendothelial system.
Dietary sources of iron include organ meats, poultry and fish and oysters, and also egg yolks, dried beans, dried figs and dates, and some green vegetables.
Iron is transported in plasma bound to transferrin
Iron is absorbed in the upper small intestine. Meat and ascorbic acid increase its absorption, and vegetable fiber inhibits it. It is transported in blood bound to transferrin and is stored as ferritin and hemosiderin. Transferrin is normally about 30% saturated with iron. Iron is lost through the skin and through the gastrointestinal tract.
Dietary iron is in the ferric (Fe3+) form. It is reduced in the gastrointestinal tract to divalent Fe2+ by ascorbate and a ferrireductase enzyme located in the intestinal brush border. Fe2+ is transported into the cells by a divalent metal transporter (which also transports most trace metals). The iron pool within the enterocyte is controlled by the iron regulatory proteins.
Erythrocyte content of iron affects its absorption from the intestine
If erythrocytes are iron rich, the iron is stored in the enterocytes incorporated into ferritin. Otherwise, it is transported through the basolateral membrane, where one of the transport-facilitating proteins, ferroxidase, also called hephaestin, oxidizes Fe2+ to Fe3+, which is then bound to transferrin in plasma. Transferrin is taken up in the bone marrow by erythrocyte precursors cells in a receptor-dependent manner. Within the cells, iron is released, again reduced to Fe2+, and transported to the mitochondria for incorporation into heme in the Fe2+ form. After destruction of the old erythrocytes by macrophages in the reticuloendothelial system, the iron is released as Fe2+, re-oxidized to Fe3+, and loaded back onto transferrin. The outline of iron metabolism is shown in Figure 11.10.
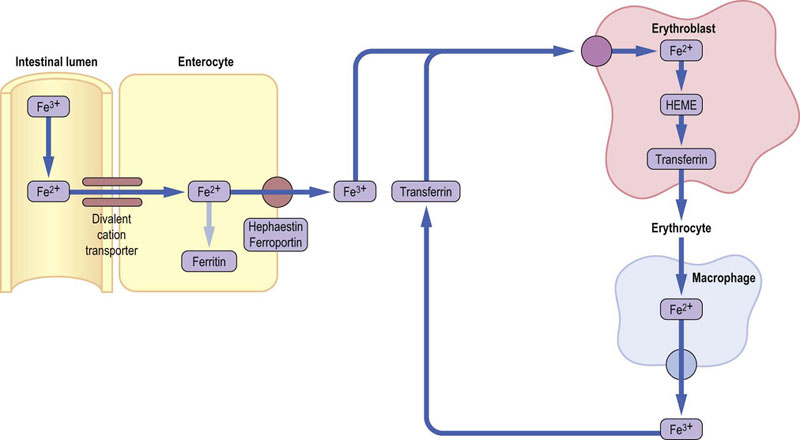
Fig.11.10 Iron metabolism.
Dietary iron is absorbed in the intestine and either stored in enterocytes as ferritin or transported out to plasma. Hephaestin and ferroportin are iron transporters located in the basolateral membrane of ethe enterocyte. In plasma, iron remains bound to transferrin. It is taken up by cells such as erythroblasts, through the mediation of the membrane transferrin receptor. In erythroblasts, iron is incorporated into heme, and then hemoglobin. Old erythrocytes are degraded by macrophages in the reticuloendothelial system. Liberated iron is released from cells and recycled bound to transferrin. Note that the dietary iron is in the ferric (Fe3+) form. This is reduced to ferrous ion (Fe2+) at the intestinal brush border. The transported form of iron is ferric again, but the form incorporated into heme is ferrous.
Iron deficiency causes anemia
Requirement for iron increases during growth and pregnancy. Iron deficiency results in defective erythropoiesis and in normocytic or microcytic (small erythrocytes) hypochromic anemia. This is most likely to develop in infants and adolescents, in pregnant and menstruating women, and also in the elderly. Iron deficiency most often develops as a result of abnormal blood loss, and therefore persons who present with iron deficiency anemia always need to be investigated for causes of bleeding, particularly from the gastrointestinal tract.
Assessment of iron status includes the measurements of transferrin and ferritin in plasma, hematologic variables, and the bone marrow smear.
Humans do not have a mechanism to excrete iron and free iron is toxic.
Trace elements
Zinc
Zinc is a component of numerous (approximately 100) enzymes associated with carbohydrate and energy metabolism, protein synthesis and degradation, and nucleic acid synthesis
It plays a role in cellular transport and protection from oxidative damage, as well as immune function, cell division and growth. Spermatogenesis is also zinc-dependent. Zinc plays a role in maintaining exocrine and endocrine pancreatic function. Its effects are most obviously seen in the maintenance of skin integrity and in wound healing.
Zinc from shares transport mechanisms with copper and iron the gut
On absorption, zinc is bound to metallothioneins, a family of cysteine-rich proteins, which also bind other divalent metal ions such as copper. Synthesis of metallothioneins is dependent on the amount of trace metals present in the diet. Increased synthesis is part of the metabolic response to trauma and results in a reduction of serum zinc concentration.
Zinc deficiency is common
Increased losses of zinc occur in patients with major burns and in those with renal damage. Zinc loss in renal disease is due to its association with plasma albumin, and it accompanies urinary protein loss. Substantial amounts of zinc may also be lost during dialysis. During intravenous feeding, failure to replace it may produce symptomatic deficiency.
The sources of zinc include oysters (highest content), red meat, poultry, beans and nuts. Note that phytates bind zinc. Zinc is not stored in the body.
Zinc deficiency might be a result of malabsorption associated with gastrointestinal GI surgery, short bowel syndrome, Crohn's disease, and ulcerative colitis, and may occur in liver and kidney disease. Chronic illnesses such as diabetes, malignancy and chronic diarrhea also lead to deficiency. Pregnant women and alcoholics are prone to deficiency.
Zinc deficiency affects growth, skin integrity and wound healing
In children zinc deficiency is characterized by growth retardation, skin lesions, and impairment of immune function and sexual development. A specific inherited defect in the absorption of zinc from the gut was identified in the 1970s; it was termed acrodermatitis enteropathica and presented with severe skin lesions, diarrhea and loss of hair (alopecia). Zinc deficiency also leads to impairment in taste and smell and to delayed wound healing.
Zinc is probably the least toxic of the trace metals but increased oral intake interferes with copper absorption, and may lead to copper deficiency and anemia.
Zinc supplements are used in the treatment of diarrhea in children
Zinc supplementation was shown to reduce the severity and duration of diarrhea in children in developing countries, and prevent further episodes of diarrhea. Therefore, zinc supplements are now recommended by WHO/UNICEF along with the oral rehydration treatment.
Measurement of serum zinc concentration is the usual method of assessing zinc status. However, many conditions and environmental factors affect its concentration in plasma, including inflammation, stress, cancer, smoking, steroid administration and hemolysis.
Copper
Copper scavenges superoxide and other reactive oxygen species
Copper is associated with oxygenase enzymes including cytochrome oxidase and superoxide dismutase (the latter also requires zinc). One of the main roles of copper, especially in superoxide dismutase but also in association with the plasma copper-carrying protein ceruloplasmin, is the scavenging of superoxide and other reactive oxygen species. Copper is also required for the crosslinking of collagen, being an essential component of lysyl oxidase.
Pathways of copper metabolism are shared with other metals
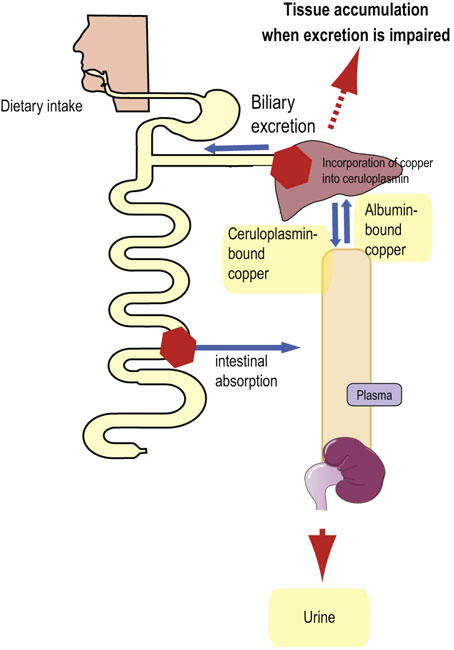
Absorption of copper from the gut is, similarly to zinc, associated with metallothionein. Copper availability in the diet is less affected by dietary constituents than zinc, although high fiber intake reduces availability by complexing copper. In plasma, the absorbed copper is bound to albumin. Copper–albumin complex is quickly taken up by the liver. Within the hepatocyte, copper is associated with intracellular metallothioneins which are also capable of binding zinc and cadmium. Copper is transported within the hepatocyte to sites of protein synthesis by a chaperone protein and it is incorporated into apoceruloplasmin. The incorporation is catalyzed by an ATPase called ATP7B. Ceruloplasmin is then released into circulation. The only mechanism of copper excretion is elimination in bile (Fig. 11.11).
Rare copper deficiency leads to an anemia; skin and hair may also be affected
Rare copper deficiency is most likely to occur from reduced intake, or excess loss, e.g. during renal dialysis. Deficiency manifests itself as a microcytic hypochromic anemia (small pale erythrocytes) resistant to iron therapy. There is also a reduction in the number of leukocytes in the blood (neutropenia) and degeneration of vascular tissue with bleeding, due to defects in the synthesis of elastin and collagen. In severe deficiency, skin depigmentation and alteration in hair structure also occur. A very rare Menkes' syndrome results from copper depletion caused by a deficiency of the intestinal ATP7B ATPase.
Copper excess causes liver cirrhosis
When taken orally, copper is generally nontoxic. However, in large doses, it accumulates in tissues. Chronic excessive intake results in liver cirrhosis. Acute toxicity is manifested by marked hemolysis and damage to both liver and brain cells. The latter is seen in the autosomal dominant inherited metabolic defect, Wilson's disease, where the liver's capacity to synthesize ceruloplasmin is compromised. The cause is mutations in the gene coding for the ATP7B ATPase. This results in a reduced incorporation of copper into ceruloplasmin, and in its cellular accumulation. Excess of apoceruloplasmin is degraded. Copper accumulates in tissues such as the brain and cornea. Patients present with neurologic symptoms or liver cirrhosis and have typical Kaiser–Fleischer rings in the cornea. There is also a low concentration of ceruloplasmin and high urinary copper excretion (see box on p. 88).
Selenium
Selenium is present in all cells as amino acids seleno-methionine and selenocysteine
Selenium is a component of selenoproteins, which contain the amino acid selenocysteine. The antioxidant enzyme glutathione peroxidase is a selenoprotein, as are the iodothyronine deiodinases, enzymes that produces triiodothyronine (T3) and reverse T3 (rT3). Thioredoxin reductases that participate in cell proliferation apoptosis and DNA synthesis also contain selenocysteine.
Selenium affects functions of the immune system, including stimulation of differentiation of T cells and proliferation of activated T lymphocytes, as well as increase in natural killer cell activity. It also plays a role in spermatogenesis.
Selenium is absorbed in the small intestine. It remains protein bound in circulation, and is excreted in urine. Selenoprotein P possesses 10 selenocysteine residues and it transports selenium in plasma from the liver to, primarily, the brain, testis and kidney. In the brain it binds to a membrane receptor apoER2, which belongs to the family of lipoprotein receptors.
Selenium is present in diet as selenomethionine and selenocysteine. Brazil nuts are its richest source. Its dietary sources also include organ meats, fish (tuna) and shellfish, and cereals. Its content in plant-derived food depends on the content of the soil.
Selenium status may influence the risk of many chronic conditions
Low selenium is associated with decline in immune function and with cognitive problems. Low concentration has been observed in individuals with epileptic seizures and also in preeclampsia. Deficiency of selenium can also develop during total parenteral nutrition. There is a rare selenium-responsive cardiomyopathy (Keshan disease), which is endemic in China in areas of very low selenium intake. Selenium deficiency may result in chronic muscle pain, abnormal nail beds, and cardiomyopathy. Excess of selenium, on the other hand, leads to liver cirrhosis, splenomegaly, gastrointestinal bleeding and depression.
Increased intake of selenium might be required during lactation. Several studies indicate beneficial effect of selenium on the risk of lung, prostate, bladder and other cancers. Single nucleotide polymorphisms in selenoprotein genes were shown to be important in determining risk of conditions such as various cancers, pre-eclampsia and possibly cardiovascular disease.
Currently it seems that while people with low selenium concentration may benefit from supplementation, supplementing it to those with normal or high values can actually be harmful.
Other metals
Numerous other trace metals are required for normal biological function: for example, manganese, molybdenum, vanadium, nickel and cadmium. Some, similar to zinc and copper, form prosthetic groups of enzymes. These include molybdenum (xanthine oxidase) and manganese (superoxide dismutase and pyruvate carboxylase) Chromium has been associated with glucose tolerance.
Many of these metals were previously thought to be toxic; indeed, their environmental excess does result in toxicity such as the renal toxicity observed in shipyard workers exposed to cadmium over long periods of time. As techniques for separation and analysis develop, other metals and other functions of known essential minerals will become known. This will lead to a better understanding of the epidemiology of certain diseases that may have, at least in part, an environmental etiology.
Summary
 Vitamins function mostly as cofactors to enzymes.
Vitamins function mostly as cofactors to enzymes.
 Fat-soluble vitamins can be stored in the adipose tissue, but there usually is only a short-term supply of the water-soluble vitamins.
Fat-soluble vitamins can be stored in the adipose tissue, but there usually is only a short-term supply of the water-soluble vitamins.
 Dietary micronutrient deficiencies are most likely to occur in susceptible groups with increased demand, or in people unable to maintain sufficient intake. Children, pregnant women, the elderly, alcoholics and low-income groups are particularly vulnerable.
Dietary micronutrient deficiencies are most likely to occur in susceptible groups with increased demand, or in people unable to maintain sufficient intake. Children, pregnant women, the elderly, alcoholics and low-income groups are particularly vulnerable.
 Gastrointestinal disease and gastrointestinal surgery are potential causes of micronutrient deficiencies.
Gastrointestinal disease and gastrointestinal surgery are potential causes of micronutrient deficiencies.
 Vitamin and trace metal supplements are particularly important in patients who remain on artificial diets and on parenteral nutrition.
Vitamin and trace metal supplements are particularly important in patients who remain on artificial diets and on parenteral nutrition.
 While there are controversies regarding some vitamin supplementation, the intake of fruit and vegetables as sources of micronutrients is unequivocally recommended.
While there are controversies regarding some vitamin supplementation, the intake of fruit and vegetables as sources of micronutrients is unequivocally recommended.
Asplund, K. Antioxidant vitamins in the prevention of cardiovascular disease: a systematic review. J Int Med. 2002; 251:372–392.
El-Youssef, M. Wilson disease. Mayo Clin Proc. 2003; 78:1126–1136.
Fairfield, KM, Fletcher, RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA. 2002; 287:3116–3126.
Fletcher, RH, Fairfield, KM. Vitamins for chronic disease prevention in adults: clinical applications. JAMA. 2002; 287:3127–3129.
Fisher Walker, CL, Black, RE. Zinc treatment for serious infections in young infants. Lancet. 2012; 379:2031–2033.
Lucock, M. Is folic acid the ultimate functional food component for disease prevention? BMJ. 2004; 328:211–214.
Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. Dietary reference values for food energy and nutrients for the United Kingdom. London: TSO; 2003.
Rayman, M. Selenium and human health. Lancet. 2012; 379:1256–1268.
Schneider, BD, Leibold, EA. Regulation of mammalian iron homeostasis. Curr Opin Clin Nutr Metab Care. 2000; 3:267–273.
Bender, DA. Introduction to nutrition and metabolism, 4th ed. Boca Raton, FL: CRC Press; 2008.
FAO Corporate Document Repository. Human vitamin and mineral requirements. http://www.fao.org/docrep/004/Y2809E/y2809e01.htm#TopOfPage.
Fact sheets concerning vitamins and trace elements are available from the Office of Dietary Supplements, National Institutes of Health, USA. http://ods.od.nih.gov/factsheets/list-all/.
UNICEF. Nutrition in emergencies. www.unicef.org/nutrition/training/2.1/5.html.