Chapter 31 Drugs Affecting Thrombosis and Haemostasis
Disorders that affect vision markedly impair a person’s ability to function independently, and require early detection and treatment. The common route of administration of drugs for ocular effects is topical, via eye-drops or ointments. Drugs can be absorbed across the cornea and can have systemic effects.
Drugs are used to treat ocular conditions, such as glaucomas, infections, infl ammations and muscular dysfunction, and to assist in ocular examination, diagnosis and surgery. Adverse reactions may be manifest in the eyes after ocular or systemic administration of drugs.
Key abbreviations
Key background: anatomy and physiology
Ocular anatomy and physiology
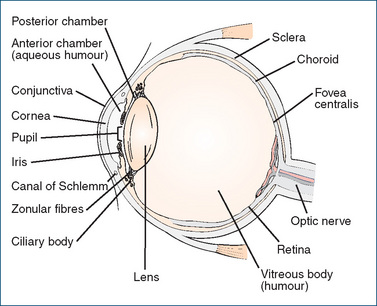
THE eye is the receptor organ for one of the most delicate and valuable senses—vision; the eyeball is protected in a deep depression of the skull called the orbit. The eyeball has three layers (see Figure 31-1): the protective external layer, the sclera (made up of the white outer coat, which is continuous with the cornea); the uvea, i.e. the middle layer (containing the choroid, iris and ciliary body); and the light-sensitive retina.
The anterior covering of the eye is the cornea, which is normally transparent, allowing light to enter the eye. The cornea is avascular (has no blood vessels) and receives its nutrition from the aqueous humour, and its oxygen supply by diffusion from the air and surrounding structures. The surface consists of a thin layer of epithelial cells, which are resistant to infection unless damaged. The cornea is also supplied with sensory fibres that elicit pain whenever the corneal epithelium is damaged. Seriously injured corneal tissue is replaced by scar tissue, which is usually not transparent and hence results in impaired vision. Increased intraocular pressure (IOP) also results in loss of transparency of the cornea.
The sclera, which is continuous with the cornea, is non-transparent; it is the white fibrous envelope of the eye (the ‘white of the eye’). The conjunctiva is the mucous membrane lining the anterior part of the sclera and the inner surfaces of each eyelid.
The iris gives the eye its brown, blue, grey, green or hazel colour. It surrounds the pupil; the sphincter and dilator muscles in the iris alter pupil size. Pupil constriction normally occurs in bright light or when the eye is focusing on nearby objects, whereas pupil dilation occurs in dim light or when the eye is focusing on distant objects.
The lens, situated behind the iris, is a transparent mass of uniformly arranged fibres encased in a thin elastic capsule. Its protein concentration is higher than that of any other tissue of the body. The zonular fibres around its edge, which connect with the ciliary body, help to change the shape of the lens to ensure sharp focussing. Accommodation for near vision occurs readily in young people, but with age the lens becomes more rigid; the ability to focus on close objects is then lost (presbyopia). With age, the lens may also lose its transparency and become opaque (cataract), leading to blindness unless treated.
The part of the eye in front of the lens is the anterior segment, of which the front part between the cornea and iris is the anterior chamber, and the part between the iris and the lens is the posterior chamber; the anterior segment is filled with aqueous humour, a fluid similar in composition to blood plasma. Aqueous humour, formed continually by the ciliary body, bathes and nourishes the lens, iris and posterior surface of the cornea. After formation it flows forward between the lens and the iris and drains, through a trabecular meshwork called the canal of Schlemm located near the junction of the cornea and sclera, into the venous system of the eye (see Figures 31-1 and 31-2). This process is known as uveoscleral outflow (or clearance); an equilibrium between formation and outflow of aqueous humour is critical for maintaining a constant intraocular pressure.

Figure 31-2 Main structures of the eye and enlargements of the canal of Schlemm, showing aqueous humour flow. A Normal. B Closedangle glaucoma. C Open-angle glaucoma. Note: The ‘angle’ is the angle of the anterior chamber, effectively the angle between the iris and the cornea; it is the main region of drainage of the aqueous chamber.
Behind the lens is the vitreous humour, comprising about 80% of the eye volume. This is a gel-like viscous fluid–collagen matrix, which bathes the retina. Vitreous humour forms in the embryo and normally lasts the person’s lifetime.
The retina contains nerve endings and the rods and cones that function as visual sensory receptors. It is connected to the brain by the optic nerve, which leaves the orbit through a bony canal in the posterior wall. Trauma or inflammation may cause the retina to separate from the pigmented epithelium, leading to retinal detachment and loss of vision; this is an optical emergency.
Eyelashes, eyelids, blinking and tears all protect the eye. Whenever a foreign body touches the eyelashes, a bilateral blink reflex occurs to prevent the foreign substance from entering the eye. Blinking keeps the corneal surface free of mucus and spreads the lacrimal fluid evenly over the cornea. Blepharospasm, a dystonia characterised by involuntary sustained or spasmodic contractions of muscles leading to eyelid blinking or twitching, is presumed to be due to irritation of the facial nerve by an artery or tumour; it is difficult to treat.
Tears are secreted by lacrimal glands at the rate of about 1–2 μL/min and contain lysozyme, a mucolytic enzyme with bactericidal action; tears have lubrication and cleansing actions, and provide the cornea with a good optical surface. Tear fluid is lost by evaporation and by draining into two small nasolacrimal ducts (the lacrimal canaliculi) at the inner corners of the upper and lower eyelids, and thence eventually into the throat and the systemic circulation. Through these ducts, drugs applied topically to the eye can reach the systemic circulation and cause systemic effects (and may be tasted).
Autonomic innervation of ocular tissues
Many drugs used in the eye act by effects on autonomic pathways and responses; hence, it is important to review autonomic innervation of ocular tissues and the relevant neurotransmitters and receptors involved (see Unit 3, ‘Peripheral nervous system’, Table 11-1, and Table 31-1).
Table 31-1 Effects of autonomic stimulation on ocular tissues*
| OCULAR TISSUE | SYMPATHETIC | PARASYMPATHETIC |
| Smooth muscle of iris | Dilator pupillae (radial muscle) (α1) causes mydriasis | Sphincter pupillae (circular muscle) (M3) causes miosis and regulation of IOP |
| Ciliary muscle (adjusts curvature of lens) | Relaxation (β2) causes focus for distant vision | Contraction causes accommodation for near vision and increases filtration angle, so drains aqueous humour; paralysis causes cycloplegia |
| Lacrimal gland | Secretion of tears | |
| Blood vessels | Vasoconstriction (α1) decreases formation of aqueous humour | |
| Muscle of upper lid | Contraction (α1) widens eyes |
* The main effects of stimulation of autonomic pathways to ocular tissues are shown; sympathetic effects are mediated by actions of noradrenaline on α- or β-adrenoceptors, and parasympathetic effects are mediated by acetylcholine actions on muscarinic (M) receptors.
The sphincter muscle, which encircles the pupil, is parasympathetically innervated; contraction, either alone or with relaxation of the dilator muscle, causes constriction of the pupil, or miosis. The dilator muscle, which runs radially from the pupil to the periphery of the iris, is sympathetically innervated; contraction of the dilator muscle or relaxation of the sphincter muscle causes dilation of the pupil, or mydriasis.
Accommodation for near vision depends on two factors: (1) ciliary muscle contraction and (2) the ability of the lens to assume a more biconvex shape when tension on the ligaments is relaxed. The ciliary muscle is innervated by parasympathetic fibres; normally the eye is accommodated for near vision, with the pupil constricted in response to contraction of the sphincter muscle and the zonular fibres relaxed. In the unaccommodated eye (i.e. focused for distant vision), the ciliary muscle is relaxed, the zonular fibres are taut and the pupil dilates, resulting in sharp distant vision and blurred near vision.
Ocular administration of drugs
Drugs for eye disorders
Main ocular drug groups
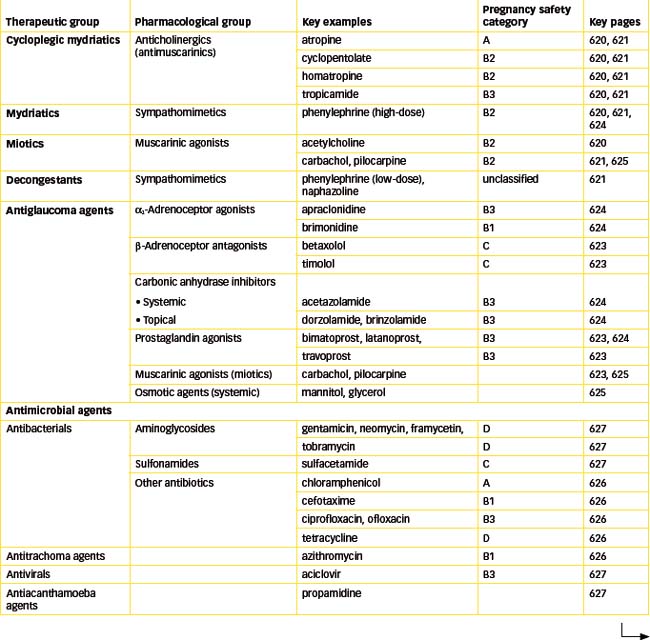
Drugs used to treat eye disorders can be divided into the following major groups: mydriatic and cycloplegic agents; drugs used to treat glaucoma; antimicrobial agents; antiinflammatory and antiallergic agents; and ocular local anaesthetics (LAs). In addition, there are many miscellaneous agents used in the eye, such as preparations used during ophthalmic surgery, diagnostic agents to stain damaged tissue and botulinum toxin, which is used to paralyse muscle. (Most of these drugs are used systemically in other conditions, so their pharmacology is covered in detail in other chapters.)
Routes of administration
Drugs intended to treat eye conditions may be administered in three ways: systemically, by injection to the eye or topically to the eye. Examples of systemic administration of drugs to act in the eye include oral or intravenous administration of acetazolamide to treat glaucoma or of systemic antibiotics in eye infections. In some acute serious conditions, drugs may be administered by direct injection to the eye, either by a periocular route (subconjunctival or retrobulbar), e.g. antibiotics, corticosteroids or LAs; or by the intravitreal route (into the vitreous humour), e.g. antibiotics for a severe infection. An LA may be required first to relieve the pain of the main injection.
Ocular administration
Most commonly, drugs intended to act in the eye are administered topically to the eye, usually directly onto the conjunctival surface as eye-drops or ointment. Ocular administration requires some dexterity and may be difficult with children.
Some general points to note with respect to ocular administration of drugs are:
Ocular formulations
There are many eye formulations available, including eyedrops, eye ointments, eye lotions and irrigating solutions and inserts. The formulation of ocular preparations is a specialised branch of pharmaceutics, as the preparation must be sterile (see Clinical Interest Box 31-1), buffered to body pH and isotonic with body solutions, so that it is nonirritant, and stable in solution. Consequently, ocular formulations may contain buffers and pH adjusters (typically phosphates or borates), preservatives (benzalkonium chloride), antioxidants, agents to increase viscosity (such as polyvinyl alcohol or hypromellose) and salts, all of which may affect tissues or cause allergies.
Clinical interest Box 31-1 Sterility of ocular formulations
Some of the eye tissues are relatively or completely avascular—notably the cornea and lens, hence their transparency—so they are at severe risk if infection or trauma occurs, as they have no immediate blood supply to provide immune defences. Thus it is important that all drugs administered to the eye, particularly during operations or when there is tissue damage, be provided in a sterile form and be maintained non-contaminated as far as possible. Most formulations of eye preparations contain preservatives to minimise bacterial growth; these preservatives can themselves impair healing or cause allergies.
The shelf-life of eye formulations is usually stated to be a maximum of 28 days after opening (in the home situation); in a clinic situation, 7 days is more appropriate. Every effort must be made to avoid contaminating the preparation by touching the tip of the dropper bottle or ointment tube onto any surface, including the conjunctival surface or the bench-top.
A new technique in ocular formulations involves the use of vehicles that are solutions of polymers that form a gel in situ when applied to the eye, and hence prolong contact time with the cornea and increase ocular bioavailability and enhance effects. The sol-to-gel transition is triggered by a change when the dose form is applied to the eye, e.g. a change in temperature, pH or ionic strength (see review by Mundada and Avari [2009]).
Eye-drops
Eye-drops are drugs formulated in aqueous or oily solutions, dispensed in a small dropper bottle (usually 10–15-mL capacity) such that a small drop can be instilled into the conjunctival sac. Aqueous (watery) drops generally provide for quick absorption and effect, have a brief duration of action and produce little interference with eye examination. The response may be variable because of spillage or blinking away of the drop, and systemic effects are possible after absorption, without the drug having passed through the liver first. Oily drops are less common, as they interfere more with vision, but provide longer retention time on the cornea, are more stable and are less likely to cause systemic toxicity. Some eye-drops are thickened as gel formulations, which also have prolonged contact times.
The technique of administering eye-drops is important: the person should be instructed to wash the hands, shake the bottle gently, pull down the lower lid, instil one drop, then press on the inner corner of the eyelid for 3 minutes to minimise systemic absorption of the drug. If another eye-drop preparation is also being used, wait at least 3 minutes before instilling the next drop (see review by Steiner [2008]).
There is no point in trying to add more than one drop to the eye, because the average volume of a drop from a dropper bottle is about 25–50 μL so it will overfill the conjunctival sac, which is estimated to have a capacity of 10 μL. Excess solution will simply overflow onto the cheek or drain or be blinked away.
To minimise the risk of contamination of formulations and consequent growth of microorganisms in solutions, some eye-drops are also dispensed in single-dose containers (e.g. the Minim brand in Australia). These come as a tiny pack (0.5 mL) for one use only and are then discarded. The advantages are that the drop is always sterile, the solution need contain no preservative, there is a cost saving because there is less waste to be discarded, and there is no risk of cross-contamination between eyes or patients. Drugs available in single-dose eye-drop packs include sodium chloride, prednisolone, LA (amethocaine, oxybuprocaine, lignocaine), antibiotics (chloramphenicol, gentamicin), mydriatics (atropine, tropicamide, phenyl ephrine, cyclopentolate), pilocarpine, fluorescein and some lubricants and contact lens products.
Strength of eye-drops
The strength of drug solutions in eye-drops is often expressed in percentage terms rather than as milligrams per millilitre or in molar units. Pilocarpine eye-drops, for example, are available in strengths ranging from 0.5% to 6%. In this context, ‘%’ refers to % weight in volume (% w/v), i.e. the grams in weight of the solid dissolved in 100 mL of solution. Thus, 1% means 1 g/100 mL, which equates to 10 mg/mL. For some more modern formulations, the strength may be quoted in mg/mL, e.g. dorzolamide eye-drops are formulated as a 20 mg/mL solution.
Eye ointments and gels
Ointments are semisolid preparations intended for topical application to the skin or mucous membranes; the active drug is incorporated into an oily vehicle and is thinly spread on the surface to which it is applied. Eye ointments are supplied in small (e.g. 5 g) tubes and have short shelf-lives due to the risk of contamination and infection: they should be discarded after 1 month (at home) or 1 week (in a practice or clinic).
The disadvantages of eye ointments are that they can be difficult to self-administer, they can cause blurred vision or interfere with ocular examination, and they cannot be used with contact lenses, as the greasy base forms an oily film over the lens. As with eye-drops, good hygiene is important to minimise contamination.
Gels are thick liquid or semisolid suspensions, usually aqueous (like a jelly) rather than oily; they are less likely to blur vision than are ointments, yet retain drug in contact with the corneal surface longer than do watery eye-drops. Timolol has been formulated in a gel eye-drop form, suitable for once-daily administration.
Other ocular formulations
Eye lotions or irrigating solutions are used to wash foreign materials from the eye. They should be sterile, at pH 6.6–9, and isotonic; in an emergency, 0.9% sodium chloride (normal saline) can be used. Some specific formulations are available, e.g. a solution of dihydrogen sodium versenate to be used if the eyes are splashed with lime (calcium oxide, a caustic powder). Buffered solutions of sodium hyaluronate and of salts are used during intraocular surgery.
Ocular inserts impregnated with drug can be applied to the conjunctival surface to encourage absorption of drug across the cornea. Such devices include lamellae (dissolvable discs), hydrophilic contact lenses and crescent-shaped polymer inserts that float below the pupil. They may be difficult to insert and remove, and may appear to become lost (temporarily) in the conjunctival sac. Absorbable gelatin implants are available for use in ocular surgery.
Iontophoresis is a technique in which the drug solution is placed in an eyecup bearing an electrode; when held up against the eye the current ‘drives’ the drug in by altering its ionisation and enhancing absorption.
Autonomic drugs in the eye
Many important ocular drugs act by mechanisms involving the autonomic nervous system (ANS)—see ‘Key background’ section above. Two conditions affecting the eye, Horner’s syndrome and Adie’s tonic pupil, demonstrate autonomic effects in the eye (see Clinical Interest Box 31-2).
Clinical interest Box 31-2 Sterility of ocular formulations
Autonomic effects in the eye are well demonstrated in two classic pathological conditions of the eye, Horner’s syndrome and Adie’s tonic pupil, and the tests used to diagnose them.
Horner’s syndrome occurs in any oculo-sympathetic paralysis, i.e. interruption to sympathetic supply to the orbit; the lesion may be central, preganglionic or postganglionic. Common causes are trauma (especially in young people), tumours (in older people), infections, poliomyelitis, stroke and aneurysms. The signs are those of impaired autonomic innervation, including slow re-dilation of the pupil in dim light, miosis and ptosis (drooping of the eyelid). Diagnosis is by the cocaine test: slow or absent pupil dilation in response to cocaine (an indirect sympathomimetic agent). Management may be surgical, medical or neurological, depending on the aetiology.
Adie’s tonic pupil syndrome is due to a unilateral lesion (often in the ciliary ganglion) with damage to postganglionic parasympathetic innervation to the constrictor pupillae and ciliary muscle. This leads to absent or slow responses to bright light or to near-vision effort. Diagnosis is by the pilocarpine test: the ‘denervated’ iris sphincter is hypersensitive to cholinergic agonists.
Ocular uses of autonomic drugs
Drugs acting on the autonomic nervous system are frequently used in ocular conditions:
Mydriatic and cycloplegic agents
Mydriatics
Mydriatics are drugs that cause pupil dilation (mydriasis). They are primarily used to facilitate examination of the peripheral lens and retina in the diagnosis of ophthalmic disorders and to prevent or break down posterior synechiae (adhesions) in iridocyclitis. Mydriasis can be achieved either by blocking acetylcholine (ACh) effects on muscarinic (AChM) receptors or by enhancing noradrenaline effects on α1-adrenoceptors. The main clinical differences are that sympathomimetics are less likely to raise IOP and do not cause cycloplegia, so the pupillary light reflex is retained. Frequently, an antimuscarinic and an adrenergic agent are given together to cause faster and more complete mydriasis. The effects of these agents depend on the patient’s age, race and colour of iris. Mydriatic agents evoke less of a response in people with heavily pigmented (dark) irises than in those with lighter-pigmented (blue) eyes, because the drug binds to melanin.
Anticholinergics (antimuscarinics, atropinic agents)
Anticholinergic agents reversibly block AChM receptors on iris sphincter muscle and ciliary muscle, producing mydriasis and cycloplegia (paralysis of ciliary muscle). They are indicated to relieve ocular pain by relaxing inflamed intraocular muscles in inflammations, such as uveitis and keratitis, and for relaxation of ciliary muscles for accurate measurement of refractive errors, which permits proper lens determination for eyeglasses.
Contraction of the iris sphincter can lead to an increase in IOP, hence glaucoma may be precipitated. Other adverse effects include increased glare (due to paralysed response to light), blurred vision and stinging (which may be relieved by prior administration of an LA eye-drop). Patients should be advised to wear dark glasses afterwards to reduce glare.
Systemic effects that may follow absorption via the nasolacrimal ducts include classical ‘atropinic’ effects: dryness of the mouth, tachycardia, decreased gastrointestinal tract functions and ataxia. Anticholinergic eye-drops should be used cautiously in patients with head injury or glaucoma, and in children.
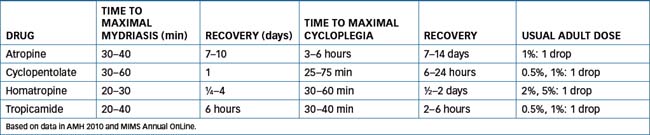
Commonly used anticholinergic agents include atropine, tropicamide and cyclopentolate (see Table 31-2 for the pharmacokinetics and dosing of anticholinergic agents). Note that atropine eye-drops cause prolonged mydriasis and cycloplegia (for 7–14 days) and are too strong for routine use—see Clinical Interest Box 1-5 for a case of accidental toxicity with atropine eye-drops. Many other drugs have antimuscarinic effects and hence affect eyes. Examples of systemic drugs with atropinic effects include some antihistamines, phenothiazines, antiparkinson agents and antidepressants (see Australian Medicines Handbook, 2010Australian Medicines Handbook 2010, Appendix B, Table B-2).
Sympathomimetics (adrenergic agonists)
Topical sympathomimetic agents mimic (directly acting) or potentiate (indirectly acting) the α1-receptor-mediated actions of noradrenaline on the dilator muscle of the iris. This results in mydriasis, vasoconstriction and decreased congestion of conjunctival blood vessels, a decrease in aqueous humour formation and increase in outflow, and relaxation of the ciliary muscle. Sympathomimetics do not affect accommodation or the pupillary light reflex.
Adrenergic drugs are used to produce mydriasis for ocular examination, to treat wide-angle glaucoma and glaucoma secondary to uveitis, and to relieve congestion and hyperaemia (red eyes). They are contraindicated in the treatment of narrow-angle glaucoma or abraded cornea because dilation of the pupil will further restrict ocular fluid outflow, which may cause an acute attack of glaucoma. As mydriatics, they are generally used as adjuncts with the anticholinergic mydriatics.
Serious systemic effects from these drugs are unusual, but can include brow ache, sweating, tremors and confusion; adverse effects are likely to be greater in children and in the elderly. Systemic absorption is a concern in patients with cardiovascular disease because tachycardia and elevated blood pressure can occur. Adverse drug interactions may occur with monoamine oxidase inhibitors and with α- or β-adrenoceptor antagonists.
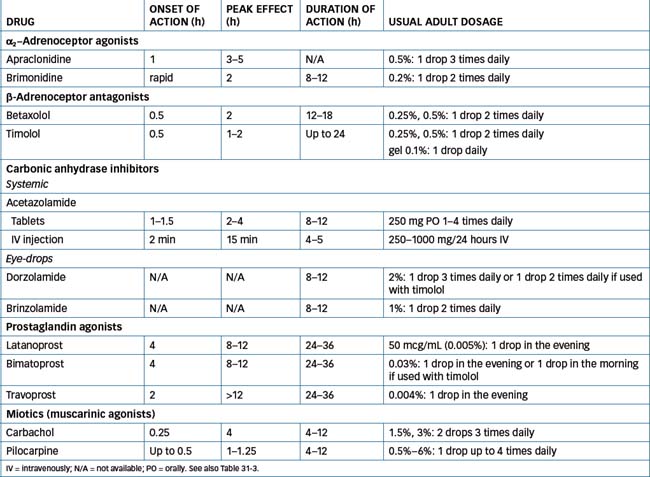
The main adrenergic drugs used in ophthalmology include: phenylephrine, naphazoline and tetrahydrozoline (mild agents used as vasoconstrictors); and new α2-agonists used in glaucoma, apraclonidine and brimonidine. Table 31-3 lists adrenergic ophthalmic drugs with their uses and usual adult dosages; note that the strengths of the solutions, and hence the dosages, vary widely depending on use (antiglaucoma, mydriatic or vasoconstrictor).
Table 31-3 Ocular adrenergic agents
| DRUG | USES | USUAL ADULT DOSAGE |
| Naphazoline | V | 0.01–0.1%: 1 drop every 3–4 hours as necessary |
| Phenylephrine | IOP, M | 2.5%, 10%: 1 drop as necessary |
| V | 0.12%: 1 or 2 drops every 3–4 hours as necessary |
|
| Tetrahydrozoline | V | 0.05%: 1 or 2 drops up to 4 times daily |
| Brimonidine | IOP | 0.2%: 1 drop 2 times daily |
| Apraclonidine | IOP | 0.5%: 1 drop 3 times daily |
IOP = reduction in intraocular pressure; M = mydriasis; V = vasoconstriction.
Cycloplegics
Cycloplegic agents are drugs that paralyse ciliary muscle, causing loss of accommodation. As explained earlier, cycloplegia is invariably accompanied by mydriasis, as it is induced by antimuscarinic agents, whereas mydriasis induced by adrenergic agonists is not accompanied by cycloplegia.
Cycloplegic agents are the antimuscarinics, i.e. atropine, homatropine, cyclopentolate and tropicamide; see again Table 31-2. Note that the same peripheral and central anticholinergic effects can occur; these agents are used only with great caution in children (particularly those with blue eyes, or people with disorders of the central nervous system [CNS]) due to the risk of central adverse effects.
Miotic agents
Miotics are drugs that constrict the pupil, i.e. cause miosis. Their clinical uses are to treat glaucoma (see later discussion); they have also been used to reverse mydriatic effects. Because the main autonomic tone in the eye is parasympathetic, with constriction of pupils and accommodation for near vision, parasympathomimetic drugs act as miotics; however, they are likely to cause blurring of vision and spasm of accommodation. Drugs can enhance parasympathetic effects by acting either as direct agonists on ACh muscarinic receptors, or by increasing the amount of ACh available to act, as do anticholinesterases. The long-acting, ‘irreversible’ anticholinesterase ecothiopate (formerly known as phos pholine iodide) was previously available in eye-drop formulation for use as a miotic and in treatment of glaucoma and squint; it has since been deleted.
Muscarinic agonists
Muscarinic agonists stimulate muscarinic receptors in the circular muscle of the iris, causing contraction and thus pupil constriction. ACh itself can be used; however, it is subject to very rapid hydrolysis and inactivation by cholinesterase enzymes, so has a very brief action. It is occasionally used by injection into the anterior chamber for rapid miosis during surgery. Carbachol, a potent ACh ‘look-alike’ that is more stable to metabolism, is a quaternary amine (charged), so is poorly absorbed across the cornea and has fewer systemic or CNS effects. It is used for rapid miosis during surgery at a strength of 0.01% (injections) and in open-angle glaucoma.
Pilocarpine is a natural compound from various Pilocarpus species plants. Its vasodilator properties were described by Langley in 1875; it mimics the effects of ACh in the parasympathetic nervous system. Pilocarpine is an uncharged molecule and so is well absorbed and likely to have CNS adverse effects. It has little effect on the ciliary muscle and thus does not markedly affect accommodation. It is an effective miotic, enhancing outflow of aqueous humour and decreasing IOP, hence its usefulness in glaucoma. To allow careful titration of doses and effects, pilocarpine eye-drops are available in a wide range of strengths (0.5%, 1%, 2%, 3%, 4% and 6%).
Ocular decongestants
Drugs that are vasoconstrictors have useful ‘decongestant’ effects in the eye (and nose: see Drug Monograph 28-6). These drugs are sympathomimetics, and their mechanism of action is as α-adrenoceptor agonists. By causing vasoconstriction, they reduce hyperaemia and fluid exudation, hence they reduce reddening and signs of inflammation. Vasoconstriction may also decrease the absorption of other drugs into the bloodstream.
Examples of drugs used as ocular decongestants are phenylephrine, naphazoline and tetrahydrozoline. Note that much lower doses are used for vasoconstrictor and decongestant effects than are required for mydriasis (see Table 31-3). Decongestants are often formulated as eye-drops together with another drug, such as:
Drugs for glaucoma
Glaucomas
 Glaucomas are a group of optic neuropathies involv ing damage to the optic nerve, changes in visual fields and abnormally elevated intraocular pressure (IOP) (>21 mmHg); this is sometimes referred to as ‘ocular hypertension’ (strictly speaking, hypertensive retinopathy). The raised IOP may result from excessive production of aqueous humour or diminished ocular fluid outflow, and is the third most common cause of blindness worldwide. Although it is primarily a disease of middle age, occurring in about 2% of all people aged 40 or over, it has also been diagnosed in younger adults and children. It is estimated that about a third of the population has a familial tendency to raised IOP and is predisposed to glaucoma.
Glaucomas are a group of optic neuropathies involv ing damage to the optic nerve, changes in visual fields and abnormally elevated intraocular pressure (IOP) (>21 mmHg); this is sometimes referred to as ‘ocular hypertension’ (strictly speaking, hypertensive retinopathy). The raised IOP may result from excessive production of aqueous humour or diminished ocular fluid outflow, and is the third most common cause of blindness worldwide. Although it is primarily a disease of middle age, occurring in about 2% of all people aged 40 or over, it has also been diagnosed in younger adults and children. It is estimated that about a third of the population has a familial tendency to raised IOP and is predisposed to glaucoma.
Primary glaucoma includes closed-angle (acute congestive) glaucoma and open-angle (chronic simple, or wide-angle) glaucoma (Figure 31-2). Primary open-angle glaucoma (POAG) is the more common, occurring in about 90% of individuals with primary glaucoma, and in 2%–3% of the population aged over 70 years. It is a chronic, familial condition, with gradual insidious onset.
Acute closed-angle glaucoma is due to a physiological or anatomical predisposition to mechanical blockage of the trabecular network. This is a rare acute optical emergency, with severe pain and a rapid rise in IOP, threatening vision. Emergency drug therapy with IV acetazolamide or mannitol, oral glycerol or topical pilocarpine is needed to control the acute attack, followed usually by surgery, such as iridectomy, filtration surgery or laser trabeculoplasty.
Secondary glaucoma may result from previous eye disease or cataract extraction, or may be secondary to inflammation (uveitis), trauma, tumour, adhesions (iritis) or drugs (corticosteroids, mydriatics, vasodilators, phenothiazines, antidepressants or antimuscarinics). Therapy for secondary glaucoma requires attention first to the primary cause and avoidance of precipitating factors, if possible, then antiglaucoma drugs.
Drug treatment of glaucomas
The main medications used to treat glaucoma include β-adrenoceptor antagonists (β-blockers), prostaglandin agonists, carbonic anhydrase (CA) inhibitors, miotics (cholin ergics) and sympathomimetics (see Tables 31-3 and 31-4); selection of a drug is determined largely by the require ments and individual response of the patient.
A suggested step-wise treatment approach to POAG is as follows:
Some combination preparations are now available, such as eye-drops containing timolol plus dorzolamide or timolol plus latanoprost. The target IOP is 30% below the original baseline reading. Patients with dark eyes may need higher doses, and it is important to teach patients to minimise systemic absorption of drugs by pressing on the nasolacrimal ducts for 2–3 minutes after administration of drops.
Prostaglandin agonists (prostanoid-receptor agonists)
Latanoprost, bimatoprost and travoprost are synthetic prostaglandin F2 agonists approved to treat POAG. They reduce IOP by increasing aqueous humour outflow; the precise mechanism of action is not yet well clarified (see Drug Monograph 31-1). They have overtaken β-blockers as drugs of first choice in treatment of glaucoma.
Drug monograph 31-1 Latanoprost
Latanoprost is a prostaglandin F2αanalogue, which reduces intraocular pressure (IOP) by enhancing uveoscleral outflow of aqueous humour. It can reduce IOP by 27%–34%; no tolerance develops over at least 4 years.
INDICATIONS Latanoprost is indicated for patients with openangle glaucoma, to reduce IOP and prevent the risk of optic nerve damage.
PHARMACOKINETICS Latanoprost is administered as eyedrops. Its onset of action is 3–4 hours, maximum effect occurs in 8–12 hours, and duration of action is >24 hours. It is a prodrug, an ester that is hydrolysed to the active form during passage through the cornea; it is distributed to the anterior segment of the eye, conjunctiva and eyelids. Following ocular administration, approximately 45% of the administered dose is absorbed systemically; it is metabolised in the liver to inactive metabolites, which are excreted in the urine. The elimination half-life is approximately 17 minutes.
DRUG INTERACTIONS There are additive effects with timolol and other β-blockers, and the drug can be used effectively as adjunct therapy with most other antiglaucoma agents. Eye-drops containing NSAIDs can reduce the effects of a prostaglandin eye-drop.
ADVERSE REACTIONS The most common adverse reactions are stinging, blurred vision, conjunctivitis, red eye, itching and eye pain and a bitter taste. An unusual side effect is change in iris colour: people with hazel or yellow-brown eyes are particularly susceptible to darkening of the iris; this effect, if it occurs, usually starts within 8 months of commencing therapy. Latanoprost should be used with caution in patients with, or susceptible to, asthma or macular oedema.
WARNINGS AND CONTRAINDICATIONS Patients should be warned of the possible change in eye colour, especially if the drug is being applied only to one eye. It is contraindicated if there is known hypersensitivity to any ingredient; thus far there are few data on use in children or during pregnancy or lactation. In people with a history of herpes simplex keratitis there may be a recurrence.
DOSAGE AND ADMINISTRATION One drop of latanoprost solution (50 mcg/mL) is administered to the eye daily, preferably in the evening; pressure should be applied to the tear duct to minimise systemic absorption. It is also available in a combination formulation with timolol (5 mg/mL) for use if either drug alone does not provide adequate reduction in IOP.
Adverse reactions include blurred vision, burning and stinging, itching, photophobia and conjunctival hyper aemia. The drugs may also permanently increase pigmentation (brown) of the iris. Their long duration of action enhances patient compliance.
Ocular β-adrenoceptor antagonists
The β-blockers used in glaucoma are betaxolol and timolol, as 0.25% and 0.5% drops. The exact mechanism of action for these drugs in glaucoma is unknown; however, it is suggested that they block β-receptor-mediated stimulation of ciliary epithelium, leading to impaired aqueous humour formation. The advantages of β-blockers are their safety, their duration of action (meaning they need be given in only one or two doses per day) and their lack of effect on pupil size or accommodation.
Betaxolol, a cardioselective (β1) blocking agent, is indicated for the treatment of POAG and ocular hypertension, and may be preferred for patients with airways disease, as it is less likely than non-cardioselective β-blockers to cause bronchoconstriction and asthma (see Chapter 12).
Adverse reactions are primarily local: burning, stinging or eye irritation. Rare effects include visual disturbances, pruritus or allergic reaction. Systemic absorption can lead to adverse effects including hypotension, asthma and depression; precautions need to be taken in patients with asthma or diabetes, and in the elderly and children.
Table 31-4 lists ocular β-blocking agents, times of action and dosing information.
Sympathomimetic agents (α-receptor agonists)
The sympathomimetic agents have been discussed earlier as mydriatics and decongestants and their characteristics are summarised in Table 31-3. When used in glaucoma, it appears that the α-receptor stimulation increases aqueous humour outflow via vasoconstriction and may also suppress aqueous humour formation. These agents are indicated for the treatment of POAG. The older directlyacting agents are adrenaline and phenylephrine.
Newer sympathomimetic agents are the α2-receptor selective agonists apraclonidine and brimonidine; these are related to the antihypertensive agent clonidine. They are indicated as adjunct therapy when IOP is not controlled with other agents. Apraclonidine is indicated for short-term use only, for up to 3 months. Adverse reactions are rarely troublesome; they include eye irritation, headache and mydriasis. Effects of systemic absorption are those of adrenoceptor stimulation: palpitations, hypertension, tremors and light-headedness.
Carbonic anhydrase inhibitors
The enzyme carbonic anhydrase catalyses the interconversion of bicarbonate with carbon dioxide and water; its actions are necessary for the secretion of aqueous humour. Drugs that inhibit this enzyme are used as mild diuretic agents, to treat epilepsy and raised intracranial pressure, and in glaucoma.
The most commonly used systemic CA inhibitor is acetazolamide. It is administered in glaucoma emergencies; given PO or IV it lowers IOP by decreasing the aqueous production to about half of its baseline measurement. Some new CA inhibitors are available as topical eye-drops (see Table 31-4): dorzolamide and brinzolamide; the latter has a high affinity for the ocular enzyme and can be used twice daily. Currently they are recommended for short-term use only.
After ocular administration, some drug is absorbed systemically and binds to the CA enzyme in red blood cells, with a very long elimination half-life. However, no significant adverse effects on electrolyte concentrations have been noted. Important drug interactions can occur from systemically administered CA inhibitors, due to alkalinisation of the urine and hence decreased excretion of basic drugs such as amphetamines, ephedrine and quinidine. High-dose aspirin, or renal impairment, can increase the toxicity of systemic acetazolamide.
Cholinergic agents (miotics)
Miotics contract the circular muscle of the iris, thus relieving obstruction to outflow of aqueous humour and reducing IOP in glaucoma. These drugs have been discussed earlier (see ‘Miotic agents’). They are used much less commonly these days, as more specific drugs have been developed.
Adverse reactions to cholinergic agents such as pilocarpine and carbachol include visual blurring, eye irritation, myopia and headache. Miosis also makes it difficult to adjust quickly to changes in illumination; this may be serious in elderly people. Systemic effects include symptoms of parasympathetic stimulation, such as salivation, nausea, vomiting, diarrhoea, precipitation of asthmatic attacks and a fall in blood pressure.
Other agents
Osmotic agents
Osmotic agents are hyperosmotic solutions of chemicals that remain in the bloodstream and raise plasma osmotic pressure (OP); they are given intravenously or orally to reduce IOP. The rationale for their use in glaucoma is that these agents generally do not cross the blood–aqueous humour barrier into the anterior chamber of the eye. Consequently, the plasma OP exceeds IOP, leading to dehydration of the vitreous body and decreased formation and increased resorption of aqueous humour. Examples are mannitol 10% or 20% solution IV, which is rapid and effective, and glycerol 50% solution for oral administration. They are used in emergency treatment of acute glaucoma, before surgery for cataract and sometimes to reduce intracranial pressure.
Others
Other drugs that can decrease IOP include marijuana (Δ9-tetrahydrocannabinol; see Chapter 21), tranquillisers, phenytoin and digoxin. These effects, however, are not sufficiently specific or safe for the drugs to be used in glaucoma.
New treatments currently being trialled for glaucoma include drugs for ‘neuroprotection’, such as:
Antimicrobial agents
Because the eye (conjunctival surface) is open to the atmosphere and maintained in a moist condition, it is very prone to infection. Some parts of the eye are avascular, hence the body’s natural defences cannot readily function there, and severe infections may damage the eye and impair vision. Thus eye infections require prompt treatment with antimicrobial agents; solutions (eye-drops) are preferred formulations because ointment bases tend to interfere with healing.
Ocular infections
The common routes of transmission of infection to the eye include:
Common pathogens
Common ocular pathogens include bacteria (especially Staphylococcus aureus, streptococci and pneumococci), viruses (adenovirus, herpes simplex virus), Chlamydia and protozoa (acanthamoebae). Diagnosis of infections may be difficult to differentiate from severe inflammation, as the signs and symptoms (pain, reddening, swelling, heat and loss of function) are similar. Conjunctivitis, for example, may be of infectious or inflammatory aetiology.
Common ocular infections
Some of the common ocular infections treated with antimicrobials are described briefly below.
Conjunctivitis is an acute inflammation of the conjunctiva resulting from bacterial or viral infection, or of allergic or irritative origin. Symptoms include redness and burning of the eye, lacrimation, itching and at times photophobia. Conjunctivitis is usually self-limiting. The eye should be protected from light. In severe cases, antibiotic eye-drops or ointment may be required. Gonococcal conjunctivitis in neonates is sight-threatening and requires IV antibiotics.
Blepharitis (inflammation of the eyelids) may result from bacterial or viral infection, dandruff-type inflammation or allergy; symptoms are crusting, irritation of the eye and red and oedematous lid margins. For seborrhoeic (dandruff-type) blepharitis, treatment is to wash lids gently with a mild soap (e.g. ‘baby shampoo’ or ‘baby soap’) or sodium bicarbonate solution. If the infection is staphylococcal, the lids are cleansed, then antibiotic eye ointment (tetracycline or chloramphenicol) is applied.
Hordeolum (stye) is an acute localised infection of the eyelash follicles and the glands of the anterior lid margin, resulting in the formation of a small abscess or cyst, usually effectively treated by drainage. An internal hordeolum may also require oral antistaphylococcal antibiotics, e.g. dicloxacillin.
Keratitis is corneal inflammation caused by bacterial or viral infection. Adenoviral keratoconjunctivitis is very contagious, but usually resolves simply. Herpes simplex keratitis, however, may cause corneal ulcers and scarring and requires treatment with an ocular antiviral agent (aciclovir)
Infection with Acanthamoeba (a protozoon) can occur in wearers of soft contact lenses, from contaminated water and solutions. It causes redness, pain and photophobia, and can lead to corneal breakdown, scarring and loss of vision. Consequently, it requires early diagnosis and aggressive antimicrobial therapy, e.g. with an antiamoebic agent (propamidine) and an antibacterial (neomycin) and possibly an antifungal to prevent secondary infections.
Trachoma is an infection caused by the organism Chlamydia trachomatis, an intracellular microorganism, which in the eye produces keratoconjunctivitis. It can also infect the genital tract and cause sexually transmitted disease and sterility. Trachoma is a serious world health problem (estimated to affect 500 million people) and is the major cause of preventable blindness. There is a high incidence in hot dry areas with poor hygiene and crowded living conditions; it is a major public health problem in northern parts of Australia. It is common in children. Although it may appear mild, chronic infection can lead to visual loss in middle age; hence the importance of early detection, good public health education and effective compliance with therapy. Treatment is with one dose of oral azithromycin (1 g adult dose).
Toxoplasmosis is an infection with the unicellular organism Toxoplasma gondii; it is commonly contracted before birth or from domestic cats. If the eye is affected, posterior uveitis can lead to loss of sight. Immunocompromised patients are particularly at risk. Treatment is combination therapy with specific antimicrobials (such as clindamycin or pyrimethamine) plus corticosteroids to limit the damaging inflammatory response.
Ocular antimicrobial chemotherapy
Selection of an antimicrobial for ocular infection is based on clinical experience, the nature and sensitivity of the organisms most commonly causing the condition, the disease itself, the sensitivity and response of the patient and laboratory results. Prophylactic use of anti-infective agents in general is useless, wasteful and potentially dangerous due to the risk of resistance developing in microorganisms. Topical application of anti-infective agents can also interfere with the normal flora of the eye and encourage growth of other organisms.
Most antimicrobial agents do not easily penetrate the eye when applied. Some drugs, however, will penetrate the inflamed eye when the blood–aqueous humour barrier is impaired by injury or inflammation. Topically applied antiinfective agents can cause sensitivity reactions (stinging, itching and dermatitis) and an unpleasant taste following nasolacrimal drainage. Individuals sensitised to one drug may show cross-reactions to chemically related drugs (e.g. penicillins and cefalosporins).
The ideal properties of antimicrobials are that they should have the appropriate spectrum of antimicrobial activity; should have long-lasting, non-toxic actions; should not interfere with vision or healing; and should be available in sterile, single-dose containers. Antimicrobials used locally (whether in the eye or on the skin) should be different from those used systemically; there is then less likelihood of inducing resistance in the organisms to the actions of the drug, or of possible sensitisation in the person to systemic antimicrobial drugs. In addition, drugs that are too toxic systemically can often be safely used locally. For local administration to the eye, these agents are administered topically or by ocular injection. In Australia, some topical antibiotics (ciprofloxacin, gentamicin and tobramycin) are preferentially reserved for ophthalmologists’ use, either to reduce resistance or because the drugs are potentially toxic. (Antimicrobial drugs are considered in detail in Unit 14, where their mechanisms of action and typical antimicrobial spectra of activity are discussed.)
Antibacterial agents used in ocular infections
Antibacterial antibiotics used in the eye include chloramphenicol, tetracycline, aminoglycosides (gentamicin, framycetin and tobramycin) and quinolones (ciprofloxacin and ofloxacin). Previously, combination preparations such as ‘triple antibiotic’ ophthalmic ointment and drops were available, containing various combinations of neomycin, polymyxin B and gramicidin with or without a corticosteroid; these preparations have been discontinued in Australia and New Zealand. Other antibacterials for ocular use are propamidine and sulfacetamide.
In severe eye infections, it may be necessary to administer antimicrobial agents systemically. Drugs are selected specifically for the organism cultured, e.g. cefotaxime or benzylpenicillin for gonococcal ophthalmia (in parents and newborn) or azithromycin for trachoma.
Chloramphenicol
A broad-spectrum bacteriostatic agent, chloramphenicol prevents peptide bond formation and protein synthesis in a wide variety of Gram-positive and Gram-negative organisms, and is a useful drug for ocular infections. Burning and stinging on administration have been reported. Irreversible aplastic anaemia has not been reported with topical chloramphenicol (as it has with oral administration).
Aminoglycosides
Aminoglycosides (neomycin, gentamicin, framycetin and tobramycin) are used against a wide variety of Gram-negative organisms, including Proteus and Klebsiella organ isms and Escherichia coli. Gentamicin and tobramycin are also active against Pseudomonas infections. Typical formulations are:
They are applied as an ointment 2 or 3 times daily or as 1 drop of solution every 4 hours.
The aminoglycosides are considerably safer when applied topically than systemically. Adverse reactions include ocular toxicity and hypersensitivity, including lid itching, swelling and conjunctival erythema. When topical aminoglycosides are used concurrently with systemic aminoglycosides, the total plasma concentration will be increased and should be monitored, as systemic toxicity (renal damage, ototoxicity and impaired neuromuscular transmission) may occur from excessive use.
Sulfonamides
Sulfacetamide sodium is a sulfonamide and, as such, blocks the synthesis of folic acid in susceptible bacterial organisms. The action of sulfonamides, however, is reduced by the presence of purulent exudate (pus), so lid exudate should be removed before the drug is instilled. Sulfacetamide sodium is irritant, and its use is not recommended. It is still available OTC in an eye-drop formulation (Pharmacy-Only, S3).
Ocular antiviral agent
The only ophthalmic antiviral preparation available currently in Australia is aciclovir ointment, 30 mg/g, indicated for treatment of herpes simplex keratitis. The dose is 1-cm ribbon of the aciclovir ointment added to the lower conjunctival sac, five times daily. Aciclovir is well absorbed through the cornea, and effective concentrations penetrate into the aqueous humour. Adverse reactions include transient stinging, sensitivity reactions and, occasionally, reversible superficial corneal damage. Although antiviral agents are potentially teratogenic, aciclovir use during pregnancy is considered safe (Pregnancy Safety Category B3).
Antiseptics
Many antiseptics that were used to treat infections of the eye before the advent of antibiotics are now obsolete. Inorganic mercuric salts such as yellow mercuric oxide have been used, but Golden Eye Ointment (1%) has recently been deleted.
Anti-amoeba agents
Propamidine is an old drug with a new use. It was previously used in ointments and creams as a mild antiseptic and skin disinfectant, effective against skin flora including S. aureus and some streptococci and clostridia. Propamidine and its dibromo- derivative have been found to be effective topically against acanthamoeba, an infection transmitted via tap water to contact lens solutions and the eyes of wearers, with potentially sightthreatening consequences. Propamidine eye-drops (0.1%) and dibromopropamidine eye ointment (0.15%) are available for the treatment of acanthamoeba keratitis and for mild acute conjunctivitis.
Anti-inflammatory and antiallergy agents
Inflammation of the eye
Inflammation of the eyes, with reddening, tearing, itching and mild pain, is relatively common and may accompany infections, mechanical damage or allergies, or occur as an ocular adverse effect of systemic medications. Inflammatory conditions include uveitis (intraocular inflammation), episcleritis and scleritis; these range in severity from common and mild to severe vision-threatening conditions. Treatment is with cold compresses, decongestant drops, mydriatics, oral or ocular non-steroidal anti-inflammatory drugs (NSAIDs) and steroid drops if more severe. Zinc sulfate is included in some formulations to aid healing.
A major potential risk involving inflammation of the eye is postoperative inflammation, which can occur, for example, after cataract surgery or after eye trauma, and lead to the formation of adhesions, which can threaten vision. To prevent adhesions, a decreasing course of topical corticosteroids is administered with antibiotic cover, e.g. 1 drop four times per day in the first week, reducing to 1 drop/day in the 4th week. (Inflammatory mediators and anti-inflammatory drugs are covered in detail in Chapter 47.)
Ocular corticosteroids
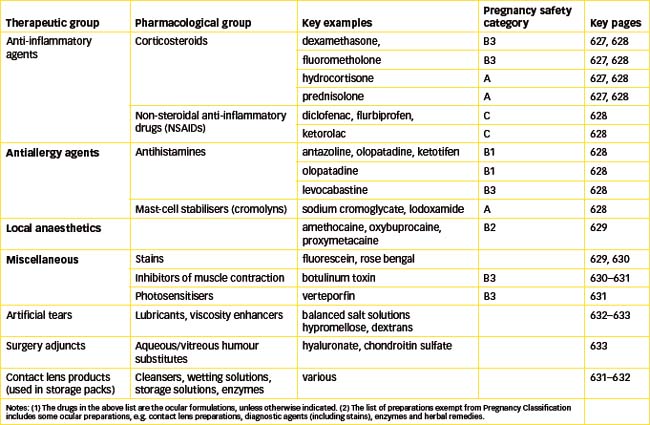
Corticosteroids inhibit the inflammatory cascade and the functions of fibroblasts and keratocytes (see Chapter 47). Their anti-inflammatory and immunosuppressant effects are useful in many ocular conditions, including inflammations and allergies of the conjunctiva, cornea and anterior segment of the eye, such as contact dermatitis, allergic blepharitis and conjunctivitis, vernal conjunctivitis, keratitis, iritis and iridocyclitis, posterior uveitis, scleritis and optic neuritis. Corticosteroids are now also being trialled in posterior segment diseases, such as agerelated macular degeneration, diabetic retinopathy and macular oedema, because of their angiostatic actions in reducing growth of new blood vessels and their reduction in vas cular permeability. Many corticosteroids are available for ophthalmic use as topical solutions, suspensions or ointments. They include dexamethasone, fluorometholone, hydrocortisone and prednisolone, available in varying strengths and sometimes in combination with antibiotics (see Table 31-5). Modifications in formulation can produce large differences in intraocular drug concentration.
Table 31-5 Potency of ocular corticosteroids
| STEROID DROPS | POTENCY | RELATIVE TENDENCY TO RAISE INTRAOCULAR PRESSURE |
| Hydrocortisone | Low | ++ |
| Prednisolone sodium phosphate | Mid | +++ |
| Fluorometholone | Mid–high | +++ |
| Prednisolone acetate | High | ++++ |
| Dexamethasone | High | ++++ |
After Australian Medicines Handbook, 2010.
Adverse reactions include burning, lacrimation, visual disturbances, eye pain, headaches, enlarged pupils, raised IOP and glaucoma, impaired corneal healing, rebound inflammation and opportunistic infections (see Clinical Interest Box 31-3). More rarely, corneal damage, refractive changes and cataracts can occur: these should be reported to the prescriber. Corticosteroids are contraindicated in ocular infections and glaucoma.
Clinical interest Box 31-3 Sterility of ocular formulations
An early classic textbook of ocular pharmacology, discussing ‘The Ten Commandments of Eye Care’, listed as the first commandment: ‘Thou shalt not use cortisone’. Despite this, the text went on to describe the many ocular inflammatory conditions in which steroid eye-drops and ointments give great relief, including allergic conjunctivitis and acute iritis.
The point was well made, however, that steroids can result in corneal perforation in the presence of viral infection; can predispose to devastating bacterial and fungal infections, especially when there is a foreign body; and, when used chronically, can induce blindness from cataract or glaucoma. It is now recommended that ocular steroids not be prescribed without the close supervision of an ophthal mologist to monitor the corneal epithelium and IOP.
See review by McGee et al (2002).
Non-steroidal anti-inflammatory drugs
The NSAIDs are now available in formulations for ocular use (early drugs like aspirin were too irritant to the cornea). Diclofenac (0.1%), flurbiprofen (0.03%) or ketorolac drops (0.5%) are used in inflammatory conditions. They have the following indications:
These agents, if absorbed, may produce systemic effects.
Because they have the potential to cause increased bleeding, their use should be monitored closely in patients who are known to have bleeding tendencies. The most common adverse reaction reported is transient burning or stinging on application. Other minor symptoms of ocular irritation have also been reported, such as itching, redness and allergic reactions.
Ocular antiallergic agents
Allergic reactions of the eyelid and conjunctiva can lead to oedema, erythema, itching, crusting and contact dermatitis. Typical allergens are pollens, dust, bites and stings, food, cosmetics, jewellery, animals and chemicals. Drugs that are known to cause ocular allergies include some antibiotics, preservatives, topical antihistamines (a paradoxical effect) and timolol.
Hay fever is often associated with extreme itching of the eyes, with blurring of vision and development of papillae (small projections of tissue from the conjunctiva). Prophylactic cromoglycate may be preventive; treatment is with antihistamines or prednisolone (tapering the dose over 1–2 weeks).
Ocular antihistamines
Treatment of ocular allergies is first to eliminate the allergen (if possible); then cooling, saline lotions and oral NSAIDs may bring relief. Topical treatment is with eyedrops, e.g. antihistamines (those blocking H1 receptors) such as azelastine, levocabastine 0.05%, antazoline 0.5%, pheniramine 0.3%, ketotifen 0.025% or olopatadine 0.1%. If allergy is severe, corticosteroids such as prednisolone 1% may be required.
Note that the combination of a sympathomimetic decongestant with an antihistamine often leads to rebound conjunctivitis, with exacerbation of symptoms.
Mast-cell stabilisers (cromolyns)
Drugs such as sodium cromoglycate 2% (cromolyn sodium) and lodoxamide 0.1% inhibit degranulation of sensitised mast cells occurring after exposure to a specific antigen; this prevents the mediators of inflammation from producing their effects. The antihistamines ketotifen and olopatadine also stabilise mast cells. The drugs are used for allergic eye disorders (vernal and allergic keratoconjunctivitis, papillary conjunctivitis and keratitis) that have symptoms of itching, tearing, redness and discharge. They have a delayed onset of action (see Clinical Interest Box 28-8) so treatment should be started 1 month before the hay-fever season.
Adverse reactions include stinging and burning sensation in the eyes. Concomitant use of corticosteroids may be necessary. For adults and children (over 4 years), 1 drop is instilled in each affected eye 4–6 times a day at regular intervals; the clinical effects may not be felt for some days.
Local anaesthetics
Actions and indications
Local anaesthetics (LAs) temporarily block nerve conduction by reducing membrane permeability to sodium. The first nerves blocked are small unmyelinated fibres, which carry the sensation of pain, hence these ‘membranestabilising agents’ are relatively selective at inhibiting transmission of pain impulses (see general discussion of LAs in Chapter 14). LAs can be applied topically to the eye as drops; these will temporarily anaesthetise the conjunctival and corneal epithelium and provide short-term ocular anaesthesia. LA solutions can also be injected subcutaneously or by retrobulbar technique, or around the pathway of specific nerves for nerve blocks, e.g. of the orbital or frontal nerve. A vasoconstrictor (adrenaline) can be added to the LA solution to localise the drug in the tissue into which it has been injected and prolong its actions.
The indications for use of LAs are for minor surgery and surgery in which the cooperation of the patient is required; thus, they are particularly useful for ophthalmic surgery and to relieve pain associated with other ocular procedures and drug administrations. They are also useful in foreign body removal, contact lens fitting, removal of sutures, some diagnostic procedures, painful irritations, in relieving stinging of other drops and in tonometry (measurement of IOP) and gonioscopy (examination of the interior of the eye).
Ocular LAs usually increase the penetration of other drugs (eye-drops) applied around the same time and commonly cause stinging and sometimes allergies. It has been recommended that a patient never be given LA drops to take home, as the person may overuse the drops without realising that the eye’s normal protective reflexes (blinking, tear production) may be abolished, leading to risk of impaired healing and possibly ulceration.
Ocular local anaesthetics
The ideal properties of an ocular LA are that it should have:
The LAs available for ocular administration as eye-drops are amethocaine, oxybuprocaine and proxymetacaine. They have onset of action within 10–20 seconds and duration of action of about 20 minutes. One of the most commonly used is proxymetacaine (known as proparacaine in the USA); it has the advantages of remaining stable in solution, with rapid onset of action and short duration, while causing minimal mydriasis, irritation or other adverse reactions. Adverse reactions from excessive use can include allergic contact dermatitis, pupillary dilation, cycloplegia and damage to cornea and conjunctiva. It is more toxic if it enters the systemic circulation. Lignocaine is added to fluorescein eye-drops and paper strips, to reduce stinging (see ‘Stains’, below).
Other ophthalmic preparations
Diagnostic aids: stains
Stains are diagnostic agents that rapidly provide useful information due to their differential staining characteristics on cells and cell constituents. The ideal properties of an ocular stain are that:
The two stains used in the eye are fluorescein and rose bengal.
Fluorescein
Fluorescein is a non-toxic, orange-red, water-soluble dye that fluoresces even when very dilute and colours the tear film. Normal corneal epithelium is impermeable and hence is not stained; however, areas of abrasion or desquamation, which have a higher pH, show up intensely green. Thus when fluorescein is applied to the cornea, it permits detection of corneal epithelial defects caused by injury or infection. Fluorescein is very commonly used for tonometry (measurement of IOP), to show corneal abrasions, in location of a foreign body, in detection of retinopathy, in fitting hard contact lenses and to test whether the nasolacrimal drainage system is open.
Fluorescein solutions readily support growth of Pseudomonas colonies; however, the usual preservatives are incompatible with the dye. The dye is formulated in single-dose packages as eye-drops (1%, 2%) and as drugimpregnated paper strips (see Drug Monograph 31-2). Drops combining fluorescein with lignocaine are also available, to reduce the stinging caused by fluorescein.
Drug monograph 31-2 Fluorescein strips
Fluorescein sodium is an orange-red dye, soluble in water and alcohol, which stains lesions of the cornea.
INDICATIONS It is used in diagnosis of eye damage and in tonometry and fitting of contact lenses.
ADMINISTRATION/PHARMACOKINETICS The fluorescein is impregnated into paper strips, which are provided dry and sterile. After the individually wrapped strip has been carefully opened, the coloured tip is moistened with 1–2 drops of sterile saline solution then touched to the conjunctiva. The patient blinks to distribute the fluorescein solution. There should be minimal systemic absorption of the drug, although there may be some staining of adjacent tissues (lids, tears, cheek).
ADVERSE REACTIONS, INTERACTIONS There are no significant adverse reactions or drug interactions with normal clinical use. (With the lignocaine–fluorescein combination drops, there may be hypersensitivity reactions to the LA component, and precautions need to be taken to protect the anaesthetised eye.)
DOSAGE AND ADMINISTRATION The paper strips contain 1 mg of fluorescein; after moistening, sufficient dye is applied to stain the required area; excess solution is wiped away or washed off with sterile saline solution.
Intravenous injection of a sterile solution of fluorescein is used in ophthalmic angiography to examine the fundus, vasculature of the iris and aqueous flow, and to determine time for blood circulation in the eye. Possible adverse reactions after IV injection include nausea, headache, abdominal distress, vomiting, hypotension, hypersensitivity reactions and anaphylaxis.
Rose bengal
Rose bengal is a reddish-brown fluorescein derivative (disodium tetrachlorotetraiodofluorescein). It is used as an ocular stain and has been used as a food dye. It stains dead cells in the cornea and conjunctiva, and is useful in diagnosis of dry eyes and infections and in detection of minute foreign bodies. Rose bengal drops can cause severe stinging of the eyes.
Botulinum toxin
Botulinum toxin type A is a purified fraction of toxin from Clostridium botulinum, the organism that commonly causes food poisoning (botulism) from poorly cooked or preserved food. It is one of the most poisonous biological toxins known. The toxin blocks neuromuscular transmission by binding to membrane receptors on cholinergic nerve terminals and entering the cell, where peptidase enzymes in the toxin then cleave proteins involved in exocytosis and thus specifically inhibit ACh release (see Chapters 11 and 13, and Figure 13-2). It is used to treat muscle spasticity in many dystonic conditions, including blepharospasm (involuntary blinking) and strabismus (squint) in the eye, cerebral palsy, torticollis, hemifacial spasm, migraine and tension headaches, and in cosmetic surgery to tighten facial muscles (see Drug Monograph 31-3; and review by Scheinberg [2009]).
Drug monograph 31-3 Botulinum toxin
The toxin is purified from a culture of the organism Clostridium botulinum and is prepared as a dried complex of the highmolecular- weight toxin protein plus a haemagglutinin and albumin; it is reconstituted before use with sterile saline. When injected IM or SC, the toxin causes localised ‘chemical denervation’ and muscle paralysis, leading to muscle atrophy. The paralysis is slowly reversible over a period of months; the duration of action is 6 weeks to 6 months.
INDICATIONS In blepharospasm, botulinum toxin decreases excessive abnormal contractions of the injected muscle; care must be taken to avoid injecting the lower lid. In strabismus, the toxin causes atrophic lengthening of the injected muscle and hence can be used to relieve squint.
PHARMACOKINETICS Studies in animals have shown that the toxin diffuses slowly from the injected muscle and is metabolised and excreted over a period of 1–2 days. It has a high affinity for cholinergic nerve terminals and is transported in a retrograde manner back along the axons.
ADVERSE REACTIONS Adverse reactions from ocular injections include rashes, swelling, ptosis, pain and diplopia. Antibody production may lead to decreased effectiveness of the toxin. Muscle weakness is an expected effect. There have been rare fatalities associated with dysphagia or cardiovascular reactions. Overdosage can lead to difficulty in swallowing and muscle paralysis; long-term medical supervision is required.
DRUG INTERACTIONS Botulinum toxin can interact with any other drugs that interfere with neuromuscular transmission, including aminoglycoside antibiotics and skeletal muscle relaxants.
WARNINGS AND CONTRAINDICATIONS Anaphylactic reactions to the foreign protein can occur, and impaired ability to blink can lead to corneal damage. Long-term studies in pregnant or lactating women have not yet been carried out, so the drug is contraindicated in these people. It is contraindicated in patients with myasthenia gravis.
DOSAGE AND ADMINISTRATION As treatment with botulinum toxin is highly specialised, it is recommended that physicians be especially trained in the procedures. The dose is expressed in units of activity as measured by biological assay; in this case, a unit of activity is defined as the calculated median lethal intraperitoneal dose in mice. The human dose may range up to 360 units over any 2-month period, depending on the muscle(s) being injected and the technique used. The effect of the toxin wears off after 3–4 months. (There are two brands of botulinum toxin in Australia; prescribers are warned that they are not therapeutically bioequivalent.)
Drugs for macular degeneration
 Macular degeneration is the condition in elderly people where the most sensitive part of the retina degenerates, new blood vessels are formed and central vision is lost. It is the leading cause of irreversible blindness in Australia. This has previously been treatable only by laser burns to seal the leaks into the retina.
Macular degeneration is the condition in elderly people where the most sensitive part of the retina degenerates, new blood vessels are formed and central vision is lost. It is the leading cause of irreversible blindness in Australia. This has previously been treatable only by laser burns to seal the leaks into the retina.
Verteporfin, a photosentiser
Many chemical compounds can act as photosensitisers, i.e. the molecules absorb energy from electromagnetic radiation or light and form activated metabolites, such as oxygen free radicals, which damage cell constituents. Examples of photosensitisers are the drug groups sulfonamides and phenothiazines and the natural compounds porphyrins, which are products of haemoglobin biosynthesis and metabolism. This process can be exploited in photodynamic therapy, in which a photosensitiser plus light energy can be directed to ablate specific lesions.
Verteporfin is a porphyrin-type molecule that is being used as a photosensitiser in the treatment of macular degeneration. It is a dark green-black chemical that is provided as a powder to be reconstituted then administered as an IV infusion over 10 minutes. At 15 minutes, nonthermal red light from a laser source is focused on the macular lesion (e.g. neovasculature) for about 80 seconds (photodynamic therapy). Verteporfin is activated by the light to form reactive oxygen free radicals, which cause local damage and vessel occlusion. It is indicated in treatment of age-related macular degeneration and choroidal neovascularisation due to other macular diseases. Adverse reactions include loss of visual acuity, field defects, haemorrhages, cataract, blepharitis and pain at the infusion site.
Ranibizumab
Ranibizumab is a monoclonal antibody fragment against vascular endothelial growth factor A; following intravitreal injection, it inhibits growth of new blood vessels under the macula of the retina. Monthly injections (only one eye each visit) have been shown to reduce loss of vision compared to controls. Potential adverse events include infection and raised intraocular pressure.
Contact lens products
Types of contact lenses
Contact lenses are classified as hard (including ‘rigid gas-permeable’ lenses) or soft; some are now disposable after one day’s wear, to obviate need for cleaning and risk of contamination and infection.
Hard lenses are generally manufactured from polymethylmethacrylate, and rigid gas-permeable lenses (permeable to oxygen) are made from silicone resins. Hard (and rigid) lenses are less comfortable for wearers in the initial adaptation period, but have the advantages of being more durable, less adsorbent (hence drugs and other chemicals are less likely to bind) and better optically.
Soft contact lenses are made from materials such as hydrogels and silicone elastomers, and all contain more than 80% water, hence their softness. They have the advantages of being more comfortable, requiring a shorter adaptation period and allowing prolonged extended wear; more than 90% of new contact lens fits are with soft lenses. However, there are potential problems of chemicals (even systemically administered drugs) binding to the lenses and staining them, and of microbial growth due to the high water content.
Both types of lenses can be used for bifocals or extended wear. If a person with contact lenses is prescribed eye-drops, it is recommended that the drops be instilled before the lenses are inserted in the morning and again after the lenses are removed in the evening. Oily drops or eye ointments should not be used because they may contaminate the lenses and obscure vision.
Products for use with contact lenses
Many products (in fact, a bewildering array in most pharmacies) are available for care of contact lenses. These products must be selected carefully as they are not interchangeable between soft and hard lenses. As with all products intended for use in the eye, solutions should be sterile (initially), non-harmful to the lens or eye, simple to use and should have a reasonable shelf-life. Likely pathogens in solutions include E. coli, S. aureus, Pseudomonas aeruginosa, Serratia, H. influenzae, fungi and acanthamoebae from tap water. Contact lens wearers are advised never to use saliva or tap water to clean their lenses; boiled water or sterile saline solution is preferred.
A typical routine is that, after the lens is removed in the evening, it is cleansed by gentle rubbing with a few drops of cleaning solution, then rinsed and stored in a case in storage solution. Before insertion next morning it may be rinsed again. Wetting solutions and ‘comfort drops’ facilitate insertion and wearing; enzymatic solutions are used occasionally to remove deposits. Combination solutions for cleaning, wetting and storage are available and simplify lens care. Multipurpose one-bottle lens care systems have improved compliance and lens care.
Cleaning solutions
These loosen and remove debris from the lens and may include detergents, surfactants or hydrogen peroxide. Typical bactericidal disinfectants are benzalkonium chloride, chlorhexidine or EDTA (ethylenediamine tetraacetic acid). Enzymatic cleaning solutions are reconstituted from tablets containing dried enzymes (non-specific lipases and/or proteolytic enzymes), which actively remove deposits of fat or protein that have built up on the lenses. The lenses are soaked in the enzymatic solution overnight, on an occasional basis.
Over-the-counter eye products
This is an area of medicine where people often ‘selfmedicate’ with products that can be bought over the counter (OTC). Many of the ocular drugs already discussed are available OTC, including:
Clinical interest Box 31-4 Complementary and alternative therapies in ocular medicine
Many natural and alternative therapies have been tried in chronic ocular conditions, some with good pharmacological rationale. Extracts of hamamelis and tamarindus plants are included in some lubricant drops, and berberine for antiinflammatory effects. Overall, there is little evidence for efficacy of herbal preparations in ocular diseases.
In cataracts, antioxidants such as lipoic acid, vitamins E and C and selenium are used as nutrients to increase glutathione concentrations within the lens. Other nutrients and herbs that may benefit cataract patients are vitamin A and carotenes such as lutein and lycopene, riboflavin, folic acid, melatonin and bilberry. However, evidence does not support use to prevent cataracts in healthy individuals.
Diabetic cataracts are caused by raised concentrations of polyols in the lens, such as sorbitol formed from high concentrations of glucose by aldose reductase; natural aldose reductase inhibitors include flavonoids such as quercetin. In glaucoma, vitamin C and glucosamines may improve glycosaminoglycan metabolism; high-dose vitamin C has an osmotic effect; Ginkgo biloba improves circulation; topical forskolin (from Coleus forskohlii) lowers IOP; IM Salvia injections improve vision; and various other nutrients and vitamins have been tried.
To protect against age-related macular degeneration, the carotenoids lutein and lycopene have been tried, as have Gingko biloba and zinc.
Dietary supplements and herbal medicines can cause severe adverse reactions, and one of the first signs of potential toxicity is in the visual system, as patients become rapidly aware of impaired vision. In most instances stopping the supplement allows resolution of ocular symptoms.
Sources: Head 2001; Braun & Cohen 2007; West et al 2006; Fraunfelder 2005 inter alia.
Some are available in supermarkets, but most are scheduled Pharmacy-Only (S2) or Pharmacist-Only (S3), so that a pharmacist is able to give professional advice and counselling as needed.
Artificial tear solutions and lubricants
Eyes can become excessively dry in conditions of hot winds, dry air-conditioning or heating, or due to inadequate tear production; the medical term for the condition is keratoconjunctivitis sicca: dry eyes. It is common in older adults and contact lens users and in dry areas. It can also occur as an adverse drug reaction. Lack of adequate tears causes a burning, scratchy sensation.
Lubricants or artificial tears are used to provide moisture and lubrication in diseases in which tear production is deficient, to lubricate artificial eyes and moisten contact lenses, to remove debris and to protect the cornea during procedures on the eye. These agents may also be incorporated in ophthalmic preparations to prolong the contact time of topically applied drugs.
Such products may include a balanced salt solution (BSS; equivalent to 0.9% sodium chloride), buffers to adjust pH (especially boric acid/sodium borate) and preservatives to reduce microbial growth. Agents to increase viscosity and extend eye contact time may also be present, such as hypromellose and carmellose (methylcellulose derivatives), propylene glycol, carbomers (polyacrylic acids), dextrans (polysaccharides), polyvinyl alcohol (PVA: a resin), glycerol, mannitol, povidone (polyvinylpyrrolidone) and triglycerides. Similar chemicals are also used in some contact lens solutions and blood volume expanders. These products are usually administered three or four times a day.
Ointment preparations are also used as ocular lubricants. They will help to protect the eye and lubricate the eye, e.g. during and after eye surgery. They are particularly valuable for patients who have an impaired blink reflex and for night-time use.
Irrigating solutions
The sterile isotonic external irrigating solutions are used in tonometry, fluorescein procedures and removal of foreign material, and to cleanse and soothe the eyes of patients wearing hard contact lenses. These products do not require a prescription and are available as drops, irrigations and eye-washes.
Miscellaneous products used in ocular surgery
Systemic diseases and drugs affecting the eye
Systemic diseases affecting the eye
Many systemic diseases can affect the eye; in general, the primary condition is treated first, then specific treatment for the ocular manifestations may not be required. Some of the major conditions commonly affecting the eye, and the drugs treating them, are described briefly below.
Cardiovascular diseases
Hypertension can cause damage to retinal arteries, with constriction, sclerosis, fibrosis and necrosis, leading to haemorrhages, papilloedema and loss of vision. Pharmacological treatment is with antihypertensive agents. Atheroma in the internal carotid artery may embolise to the retinal circulation and cause transient or permanent blindness. Treatment with antihyperlipidaemic drugs can reduce the risk of atheroma and embolism. Advanced congestive heart failure causes cerebral hypoxia and reduced blood supply to the eye. Pharmacological treatment is with drugs, including cardiac glycosides, which have important ocular adverse effects such as impaired vision and change in colour vision (see below).
Endocrine disorders
Hyperthyroidism, especially Graves’ disease, leads to major pathological changes in the eyes, and thyrotoxicosis may cause exophthalmos, orbital pain, photophobia, ocular muscle weakness and blurred vision. Treatment is by thyroid surgery or with antithyroid drugs such as carbimazole.
Ocular manifestations of diabetes mellitus include retinopathy, haemorrhages, detachment and oedema; morbidity is decreased by optimal control of diabetes, hypertension and hyperlipidaemia. Pharmacological treatment is with insulin (type 1 diabetes) or oral hypoglycaemic drugs (type 2). Photocoagulation of vessels or tissue relieves retinal oedema and haemorrhages.
Collagen diseases
Rheumatoid arthritis, systemic lupus erythematosus and Sjögren’s syndrome may cause many ocular manifestations, e.g. dry eyes, scleritis, pain, uveitis, corneal opacity and retinopathy. Pharmacological treatment to the eye is with steroid drops and artificial tears; systemic steroids, NSAIDs and chloroquines are given as anti-inflammatory agents. Ocular adverse drug reactions from steroids and chloroquine require monitoring. Temporal arteritis, especially of the ophthalmic artery or central retinal artery, can cause sudden unilateral vision loss. Treatment is with steroidal anti-inflammatory agents.
Muscular diseases
Myasthenia gravis involves autoimmune reactions to ACh receptors at the neuromuscular junction; in more than 90% of cases, the ocular muscles are the first affected, with ptosis (the usual first sign) and diplopia. The specific diagnostic test is a positive improvement in ptosis in response to an IV dose of edrophonium (an anticholinesterase drug). Pharmacological treatment is with anticholinesterases (neostigmine, pyridostigmine), which raise the concentration of ACh to act at remaining functional ACh receptors.
Systemic drugs affecting the eye
With respect to the eye and adverse drug reactions (ADRs), there are several possible scenarios:
Table 31-6 Ocular adverse effects induced by some systemic medications
| DRUG | POSSIBLE OCULAR ADVERSE EFFECT INDUCED |
| Allopurinol | Retinal haemorrhage, exudative lesions |
| Anticholinergics | Dry eyes, mydriasis, glaucoma |
| Anticholinesterases | Cataracts |
| Antidepressants | Glaucoma |
| Aspirin | Allergic dermatitis, including keratitis and conjunctivitis |
| Barbiturates | Nystagmus |
| Busulfan | Cataracts |
| Cannabis, marijuana | Nystagmus, conjunctivitis, double vision, red eyes |
| Chloral hydrate | Eyelid oedema, conjunctivitis, miosis |
| Chloroquine | Lenticular and corneal opacity, retinopathy |
| Clomiphene citrate | Blurred vision, light flashes |
| Clonidine | Miosis |
| CNS depressants | Impaired vision, nystagmus, diplopia |
| Corticosteroids | Cataracts, raised IOP, papilloedema |
| Diazoxide | Oculogyric crisis |
| Digitalis glycosides | Scotomas, optic neuritis, changes in colour vision |
| Ethambutol | Optic neuritis |
| Ethanol | Nystagmus |
| Glyceryl trinitrate | Transient elevation in IOP |
| Guanethidine | Miosis, ptosis, blurred vision |
| Hydralazine | Lacrimation, blurred vision |
| Ibuprofen | Altered colour vision, blurred vision |
| Indomethacin | Mydriasis, retinopathy |
| Isoniazid | Optic neuritis |
| Lithium carbonate | Exophthalmos |
| Methanol | Retinopathy, blindness |
| Oestrogens | Vessel occlusion |
| Opiates | Miosis, nystagmus |
| Oxygen | Retrolental hyperplasia, blindness (in infants) |
| Phenothiazines | Corneal and conjunctival deposits, cataracts, retinopathy, oculogyric crisis |
| Phenytoin | Nystagmus |
| Quinine | Blurring of vision, optic neuritis, blindness (reversible) |
| Thiazide diuretics | Acute transient myopia, yellow colouring of vision |
| Vincristine | Ptosis, paresis of extraocular muscles |
| Vitamin A overdose or toxicity | Papilloedema, increased IOP |
| Vitamin D toxicity | Calcium deposits in cornea |
Table 31-7 Ophthalmic drugs: adverse systemic effects
| OPHTHALMIC DRUG | REPORTED ADVERSE EFFECT |
| Antimicrobial agents | |
| Antibiotics | Secondary infections, drug resistance |
| Sulfacetamide | Stevens–Johnson syndrome, systemic lupus erythematosus |
| Anticholinergic drugs | |
| Atropine | Tachycardia, elevated temperature, fever, delirium |
| Cyclopentolate | Convulsions, hallucinations |
| Antiglaucoma medications | |
| β-Blocking agents (timolol) | Bradycardia, syncope, low blood pressure, asthmatic attack, congestive heart failure, hallucinations, loss of appetite, headaches, nausea, weakness, depression |
| Parasympathomimetics (pilocarpine) | Nausea, stomach pain, increased sweating, salivation, tremors, bradycardia, lightheadedness |
| Adrenergic medications | |
| Phenylephrine (10%) | Hypertension, cerebral haemorrhage, arrhythmias, myocardial infarction |
Because of the potential for ADRs and drug interactions, patients being treated for ocular conditions should be asked about their drug therapy, including prescription drugs, over-the-counter drugs and complementary and alternative therapies. In particular, important parameters to consider are the drug’s name, formulation, therapeutic index, dose, duration of therapy and the ‘use-by’ date of the formulation (usually 28 days after opening). Patient parameters include age, sex, eye health, eye colour, whether contact lenses are worn, tendency to allergies and other medical conditions (see Clinical Interest Box 31-5).
Clinical interest Box 31-5 Complementary and alternative therapies in ocular medicine
The active ingredient in eye-drops can be absorbed systemically and may cause systemic ADRs, as evidenced by the following case study.
Violet was a 65-year-old former nurse with a history of mild hypertension, migraine and back pain; her medications were a thiazide diuretic daily and paracetamol as required. She presented with headaches resembling migraine, and as her blood pressure was elevated, a cardioselective β-blocker (metoprolol) was prescribed.
The headaches and hypertension were relieved, but Violet complained of severe nocturnal coughing. Examination revealed no abnormality, so a cough linctus was prescribed, but proved ineffective. Auscultation of her chest revealed wheezing, at which Violet commented that she had not suffered asthma since a child.
At this stage, it became apparent that the β-blocker had precipitated iatrogenic (drug-induced) asthma, so it was withdrawn and the hypertension treated with other drugs. The nocturnal cough persisted, responding to oral cortico steroids. A consultant specialist physician added more drugs and physiotherapy to the treatment regimen.
When Violet requested a referral to her ophthalmologist for review of her glaucoma, the missing piece of the jigsaw was found: unbeknown to her general practitioner, Violet was using timolol eye-drops prescribed by her eye specialist for glaucoma!
Adapted from: Murtagh 1992; used with permission.
The most common ocular ADRs are decreased tolerance to contact lenses, dry eyes, stinging or irritation from eye-drops, development of cataract, diplopia, retinopathy, corneal irritation or conjunctivitis, raised IOP and impaired accommodation with or without mydriasis. Many of the important ADRs have been described in the context of particular groups of drugs and are summarised in Tables 31-6 and 31-7. In the next section, drugs causing particular ocular pathologies or impairment of vision are grouped together. Formulation additives such as preservatives can also cause adverse ocular effects.
Drugs causing retinopathies
Ethanol
The ocular effects of alcohol, such as nystagmus (rapid jerky eye movements), are reversible. The closely related alcohol, methanol (as in methylated spirits), however, is highly toxic to the retina—as little as 10 g methanol can cause blindness (see Box 21Clinical Interest Box 21-9). The toxicity is due to the metabolite formaldehyde (formalin), which inhibits cellular respiration and glycolysis in the retina, whereas the analogous metabolite of ethanol, acetaldehyde, is much less toxic.
Chloroquine and related antimalarial and anti-inflammatory agents
Chronic high doses of these drugs accumulate in the pigmentary epithelium of the retina, where they impair protein synthesis and vitamin A metabolism and can lead to irreversible damage. The maximum safe dose is considered to be about 250 mg per day for 1 year. All patients should be monitored for early detection of retinal changes.
Other drugs
Oxygen in high concentrations is retinotoxic in newborns. Neonates, especially premature infants with respiratory distress syndrome, often need high levels of oxygen administered to prevent hypoxia; however, this can lead to permanent blindness (see Clinical Interest Box 28-4). Thus the levels of oxygen provided to these vulnerable infants must be restricted.
Phenothiazine antipsychotics (antischizophrenic neuroleptic agents) can bind to melanin and also cause lens deposits; high doses are retinotoxic. Other drugs that can cause retinal damage include digoxin, corticosteroids, chloramphenicol, cocaine and interferon.
Drugs causing development of cataracts
Many organic chemicals can induce development of cataracts (i.e. opacity in the crystalline lens), including:
Other ocular adverse drug reactions
Photosensitivity
Photosensitivity is a hypersensitivity reaction in which UV light energy stimulates production of a hapten–protein complex between the drug and a natural protein, leading to damage to lysosomes and a photoallergy or phototoxicity, which may be manifest as severe sunburn or skin eruptions. The drugs most commonly implicated are the sulfonamides, tetracyclines, phenothiazines and thiazide diuretics. Note that mydriatics also increase the sensitivity of the eye to light.
Photosensitivity can also be a symptom of porphyria. This property of porphyrins is exploited in the use of verteporfin to occlude retinal blood vessels in the treatment of macular degeneration (described earlier).
Excessive tear formation
Lacrimators (better or more infamously known as ‘tear gases’) are chemicals that cause intense corneal and conjunctival irritation and pain, and hence induce reflex tear secretion and eyelid spasm. They are used as crowd controllers, ‘harassing agents’ and war gases, as the excessive tear production tends to inhibit people’s interest in other activities. These agents also irritate other mucous membranes and induce coughing and nausea. If used in confined spaces, their toxicity can cause blindness and death. Many are highly reactive organic chemicals with cyano groups (carbon and nitrogen linked in a triple bond). Others include bromoacetone, acrolein (a compound produced from overheated cooking fats) and the organic sulfides present in onions and garlic.
Key points
 Drugs are administered to the eye (as eye-drops, ointments or via inserts) for local effects in the eye after absorption across the cornea. Some systemic absorption can occur via the nasolacrimal ducts.
Drugs are administered to the eye (as eye-drops, ointments or via inserts) for local effects in the eye after absorption across the cornea. Some systemic absorption can occur via the nasolacrimal ducts. Autonomic effects in the eye are important: sympathetic innervation leads to mydriasis, focus for distant vision and vasoconstriction, whereas parasympathetic effects include miosis, reduced IOP, accommodation for near vision and secretion of tears.
Autonomic effects in the eye are important: sympathetic innervation leads to mydriasis, focus for distant vision and vasoconstriction, whereas parasympathetic effects include miosis, reduced IOP, accommodation for near vision and secretion of tears. Mydriatric agents dilate the pupil and are used to facilitate ocular examination. Drugs with mydriatic actions are anticholinergics, such as atropine (which also cause cycloplegia), and α-adrenergic agonists, such as phenylephrine; the latter drugs also have useful vasoconstrictor and decongestant effects in the eye.
Mydriatric agents dilate the pupil and are used to facilitate ocular examination. Drugs with mydriatic actions are anticholinergics, such as atropine (which also cause cycloplegia), and α-adrenergic agonists, such as phenylephrine; the latter drugs also have useful vasoconstrictor and decongestant effects in the eye. Miotic agents reduce pupil size and reduce IOP. Miosis is caused by muscarinic agonists (carbachol, pilocarpine) and anticholinesterases.
Miotic agents reduce pupil size and reduce IOP. Miosis is caused by muscarinic agonists (carbachol, pilocarpine) and anticholinesterases. Glaucomas are a group of conditions associated with raised IOP, which can threaten vision. Ocular administration of β-blockers, carbonic anhydrase inhibitors, α-agonists, prostaglandin agonists or miotics is used to reduce IOP.
Glaucomas are a group of conditions associated with raised IOP, which can threaten vision. Ocular administration of β-blockers, carbonic anhydrase inhibitors, α-agonists, prostaglandin agonists or miotics is used to reduce IOP. Ocular infections can be caused by bacteria, viruses, protozoa or Chlamydia. Serious infections threaten vision and require treatment with antimicrobials specific to the pathogenic organism. Antimicrobials that are not commonly used systemically are preferred for topical use, including chloramphenicol, neomycin, aciclovir, propamidine and azithromycin.
Ocular infections can be caused by bacteria, viruses, protozoa or Chlamydia. Serious infections threaten vision and require treatment with antimicrobials specific to the pathogenic organism. Antimicrobials that are not commonly used systemically are preferred for topical use, including chloramphenicol, neomycin, aciclovir, propamidine and azithromycin. Minor ocular inflammations may be self-limiting; however, severe or chronic inflammations can cause scarring or retinal detachment and require treatment with antiinflammatory agents, such as corticosteroids or NSAIDs.
Minor ocular inflammations may be self-limiting; however, severe or chronic inflammations can cause scarring or retinal detachment and require treatment with antiinflammatory agents, such as corticosteroids or NSAIDs. Allergic reactions in the eye are treated with antihistamines (e.g. levocabastine) or mast-cell stabilisers (sodium cromoglycate).
Allergic reactions in the eye are treated with antihistamines (e.g. levocabastine) or mast-cell stabilisers (sodium cromoglycate). Local anaesthetics are used in the eye for ophthalmic surgery, to facilitate examinations and procedures and to treat pain. Proxymetacaine is particularly effective in the eye.
Local anaesthetics are used in the eye for ophthalmic surgery, to facilitate examinations and procedures and to treat pain. Proxymetacaine is particularly effective in the eye. The stains fluorescein and rose bengal are administered topically to show up areas of abrasion and cell damage.
The stains fluorescein and rose bengal are administered topically to show up areas of abrasion and cell damage. Botulinum toxin, a long-acting skeletal muscle paralysing agent, is used in blepharospasm and strabismus.
Botulinum toxin, a long-acting skeletal muscle paralysing agent, is used in blepharospasm and strabismus. Verteporfin is a new agent utilised in photodynamic therapy to occlude abnormal blood vessels in macular degeneration.
Verteporfin is a new agent utilised in photodynamic therapy to occlude abnormal blood vessels in macular degeneration. Contact lenses (hard or rigid gas-permeable, or soft) require meticulous handling and regular care (unless disposable). Solutions for cleansing, soaking, wetting and enzymatic digestion of deposits are available.
Contact lenses (hard or rigid gas-permeable, or soft) require meticulous handling and regular care (unless disposable). Solutions for cleansing, soaking, wetting and enzymatic digestion of deposits are available. Other drugs used in the eye include artificial tear solutions, products used to facilitate eye surgery and various complementary and alternative therapies (antioxidants, vitamins and herbal remedies).
Other drugs used in the eye include artificial tear solutions, products used to facilitate eye surgery and various complementary and alternative therapies (antioxidants, vitamins and herbal remedies). The eye can be affected in many systemic diseases, particularly cardiovascular, endocrine and musculoskeletal conditions. The primary disease needs to be treated first, to minimise ocular complications.
The eye can be affected in many systemic diseases, particularly cardiovascular, endocrine and musculoskeletal conditions. The primary disease needs to be treated first, to minimise ocular complications. Many adverse drug reactions occur in the eye, such as ocular irritation, retinopathy, cataract, raised IOP and glaucoma and photosensitivity, from both ocular and systemically administered drugs. Agents that commonly cause ocular effects are methanol, chloroquine, corticosteroids, phenothiazines, sulfonamides and oxygen in newborns. ‘Tear gases’ are used purposely to induce ocular irritation and pain.
Many adverse drug reactions occur in the eye, such as ocular irritation, retinopathy, cataract, raised IOP and glaucoma and photosensitivity, from both ocular and systemically administered drugs. Agents that commonly cause ocular effects are methanol, chloroquine, corticosteroids, phenothiazines, sulfonamides and oxygen in newborns. ‘Tear gases’ are used purposely to induce ocular irritation and pain.Review exercises
References and further reading
Antibiotic Expert Group. Therapeutic Guidelines Antibiotic, version 13. Melbourne: Therapeutic Guidelines Limited; 2006.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
Bartlett J.D., Jaanus S.D. Clinical Ocular Pharmacology, 4th edn. Boston: Butterworth–Heinemann; 2001.
Braun L., Cohen M. Herbs and Natural Supplements: An Evidence-Based Guide, 2nd edn. Sydney: Elsevier Mosby; 2007.
Ciulla T.A., Walker J.D., Fong D.S., Criswell M.H. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Current Opinion in Ophthalmology. 2004;15(3):211-220.
Fraunfelder F.T. Ocular side effects associated with dietary supplements and herbal medicines. Drugs of Today. 2005;41(8):537-545.
Fraunfelder F.T., Fraunfelder F.W., Chambers W.A. Clinical Ocular Toxicology: Drug-Induced Ocular Side-Effects. USA: Elsevier Saunders; 2008.
Head K.A. Natural therapies for ocular disorders, part two: cataracts and glaucoma. Alternative Medicine Review. 2001;6:141-166.
Mann J. Murder, Magic and Medicine. Oxford: Oxford University Press; 1992.
McGee C.N., Dean S., Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Safety. 2002;25(1):33-55.
Mozaffarieh M., Flammer J. Is there more to glaucoma treatment than lowering IOP? Survey of Ophthalmology. 2007;52(Suppl 2):S174-S179.
Mundada A.S., Avari J.G. In situ gelling polymers in ocular drug delivery systems: a review. Critical Reviews in Therapeutic Drug Carrier Systems. 2009;26(1):85-118.
Murtagh J. Cautionary Tales: Authentic Case Histories from Medical Practice. Sydney: McGraw-Hill; 1992.
Scheinberg A. Clinical use of botulinum toxin. Australian Prescriber. 2009;32(2):39-42.
Spencer J.W., Jacobs J.J. Complementary/Alternative Medicine: An Evidence-Based Approach. St Louis: Mosby; 1999.
Steiner M. On the correct use of eye drops. Australian Prescriber. 2008;31(1):16-17.
West A.L., Oren G.A., Moroi S.E. Evidence for the use of nutritional supplements and herbal medicines in common eye diseases. American Journal of Ophthalmology. 2006;141(1):157-166.
Whitson J.T. Glaucoma: a review of adjunctive therapy and new management strategies. Expert Opinion on Pharmacotherapy. 2007;8(18):3237-3249.
MIMS OnLine: www.mims.com.au/
For specific New Zealand drugs, check Medsafe website: www.medsafe.gvt.nz
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology