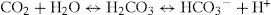Chapter 28 Drugs used in respiratory disorders
The respiratory system maintains the exchange of oxygen and carbon dioxide between the lungs and cells, and regulates the pH of body fluids. This chapter provides a review of the relevant anatomy and physiology, and describes how drugs are administered by inhalation. The various drugs used for effects in the respiratory tract are discussed: medical gases (oxygen and carbon dioxide), respiratory stimulants and depressants, drugs affecting mucus and surfactant secretions, anti-asthma medications (bronchodilators, symptom controllers and anti-inflammatory agents) and drugs used in the management of chronic obstructive pulmonary disease, respiratory tract infections and conditions affecting the nose.
key abbreviations
cAMP cyclic 3,5-adenosine monophosphate
COAD chronic obstructive airways disease
COPD chronic obstructive pulmonary disease
FEV1 forced expiratory volume in one second
M3 muscarinic type 3 (receptors)
PaCO2 partial pressure of carbon dioxide in arterial blood
PaO2 partial pressure of oxygen in arterial blood
Key background
The respiratory system
THE respiratory system includes all structures involved in the movement and exchange of oxygen and carbon dioxide: that is, the nose, airway passages, lungs, nasal cavities, pharynx, larynx, trachea, bronchi, bronchioles, pulmonary lobules with their alveoli, the diaphragm and all muscles concerned with respiration itself (Figures 28-1 and 28-6).

Figure 28-1 Tracheobronchial tree and bronchial smooth muscle. A Diagram of tracheobronchial tree. B Longitudinal section of inner lining of airway.
An adequate, uninterrupted supply of oxygen is essential for life; oxygen is supplied to the body through the process of respiration. ‘Respiration’ is loosely used to describe three distinct but interrelated processes:
Parts of the respiratory system also participate in warming, filtering and moistening the air taken in as well as in the senses of smell and taste, produce sounds and assist in control of pH, in removal of foreign bodies and mucus, in immune system defence mechanisms, in inactivation of many biogenic amines and autacoids and in temperature regulation.
Airway efficiency is determined by the following factors:
Any alteration in these factors affects the ease with which air flows through the air passages, or mucus is effectively cleared. Congenital anomalies, injuries, allergies or disease cause airflow resistance if these factors are abnormally affected. For example, resistance occurs if there is stenosis, or narrowing, of any portion of the respiratory tract, a loss of cilia that ordinarily sweep out foreign substances, any thick or tenacious secretions, loss of elasticity or the presence of inhaled foreign objects.
Respiratory tract secretions
Mucus
Mucus secreted by the goblet cells and bronchial glands located in the submucosa of the tracheobronchial tree moistens and lubricates the branching tubular airways, shown in Figure 28-1B. The mucus glands are under vagal (parasympathetic) control and can be stimulated by irritant agents or aerosol drugs to release their contents into the lumen of the airways.
The products of the goblet cells and bronchial glands form the sol–gel film that comprises the mucociliary blanket bathing the ciliated epithelium of the tracheobronchial tree. The process of moving mucus up the tracheobronchial tree towards the larynx is called mucociliary transport (or mucokinesis). In some obstructive pulmonary diseases, mucus secretion is greatly increased, making it difficult for the cilia to transport secretions along the airway. Adequate humidity should be maintained to prevent thickening of the respiratory secretions (see Clinical Interest Box 28-1).
Clinical interest Box 28-1 Cystic fibrosis and gene therapy
The disease cystic fibrosis (CF), an inherited autosomal–recessive condition, involves abnormally thick mucus secretions in many organs (including lungs, sweat glands, pancreas and liver) due to abnormal chloride transport, sodium hyperabsorption, deregulation of calcium homeostasis and an enhanced inflammatory response. Life expectancy used to be very short, but is now more than 30 years.
Most patients suffer from severe respiratory infections due to impaired mucociliary transport. Standard treatment involves use of antibiotics for bacterial infections, enzymes and mucolytics to reduce mucus viscosity, physiotherapy and exercise to clear mucopurulent secretions, bronchodilators, oxygen, antiinflammatory agents and nutritional support.
The gene for CF was identified in 1989 and its product, the CF transmembrane conductance regulator (CFTR), has been studied intensively; this membrane protein in epithelial cells is defective in CF patients. The aim is to develop methods to transfer the gene into cells of the airways of CF patients, so that they can express the CFTR protein and so improve chloride and sodium conductance. Clinically useful therapy has been carried out in some pa tients; however, the barrier to effective gene therapy is finding vectors that successfully transfer the gene to the appropriate cells, without inflammatory effects induced by the vector virus.
The respiratory tract is considered particularly feasible as a target for gene therapy because of the ease of access by viral and other vectors. In addition to CF, potential indications for gene therapy include α1-antitrypsin deficiency, acute transplant rejection and acute lung injury.
Other new methods to improve CFTR protein function include drugs aimed at suppressing premature termination of the synthesis of the protein, stabilising the protein structure, activating the protein or enhancing normal chloride channel functions. Miglustat, a drug currently in phase II trials, has so far shown promise in restoring the activity of CFTR and stabilising diseased cells.
Source: Atkinson 2008; Kerem 2005.
Pulmonary surfactant
Pulmonary surfactant (i.e. surface-active agent) is a phosphatidylcholine–apoprotein lipoprotein mixture secreted from alveolar epithelial cells and present in the secretions in the alveoli. Surfactant reduces surface tension in the lung, stabilises the alveoli and improves lung mechanics.
Synthesis and secretion of surfactant is low in the fetus until immediately before birth, when a surge in maternal glucocorticoids triggers surfactant release. Infants born preterm are at risk of respiratory distress syndrome due to immature mechanisms for producing surfactant, and are likely to suffer rapid shallow breathing, hypoxaemia and acidosis unless treated with synthetic surfactant. Surfactant can be purified from animal lung sources, synthesised in the laboratory or produced by genetic engineering techniques in bacterial cell cultures. Two forms used in Australia are beractant (a modified bovine product) and poractant alfa (derived from pigs’ lungs). The products are supplied as solutions for intratracheal administration; they are normally used only in neonatal intensive care units.
Treatment of premature infants suffering respiratory distress with exogenous surfactant instilled into the trachea is remarkably effective; surfactant reduces dependence on a ventilator, reduces risk of pneumothorax, increases oxygenation and has improved survival rates of premature babies from 30% in the 1970s to 90% today. Concomitant nasal positive airways pressure (PAP) enhances survival. If premature birth is anticipated, glucocorticoids given prophylactically to the mother can enhance fetal lung maturation and synthesis of surfactant.
Bronchial smooth muscle
Efferent nerve supply, mediators and receptors
The airway, or tracheobronchial tree, is innervated by the autonomic nervous system (see Chapters 11–12). The tone of the bronchial smooth muscle (arranged along the length of the tubular tree in a spiral pattern) is influenced by the balance maintained between parasympathetic and sympathetic stimuli during rest. The muscle tone is normally determined by tonic vagal activity, i.e. it is normally mildly contracted.
Activation of the parasympathetic pathway (vagus nerve) releases acetylcholine (ACh), which stimulates muscarinic type 3 (M3) receptors in bronchial smooth muscle and glands. Stimulation of the M3 receptors and coupling with a G-protein increases the activity of the enzyme phospholipase C, thereby increasing the rate of formation of second messengers IP3 and DAG. This leads to secretion from mucus glands and contraction of bronchial smooth muscle, which result in bronchoconstriction, a narrowing of the lumen of the bronchial airway.
In contrast, there is little sympathetic innervation of the human airways; however, general stimulation of sympathetic pathways releases the catecholamines adrenaline and noradrenaline from the adrenal medulla into the circulation. Their action on the β2-adrenoceptor sites on bronchial smooth muscle cells produces bronchodilation by means of smooth muscle relaxation, inhibits mediator release from mast cells and increases mucociliary clearance. All these effects improve ventilation of the lungs. The transduction mechanism whereby catecholamines induce smooth muscle relaxation is via adenylate cyclase, ATP and cyclic AMP (cAMP; see Figure 28-4). Cyclic AMP is inactivated by an enzyme, phosphodiesterase, which metabolises it to the inactive 5́-AMP, resulting in a fall in the cAMP level. If the action of phosphodiesterase is inhibited (e.g. by a xanthine drug such as theophylline), the cAMP level remains elevated and the smooth muscle relaxed. Few α-adrenoceptors are present on the bronchial smooth muscle, and their stimulation results in only mild bronchoconstriction.
Thus bronchodilation is induced by sympathetic stimulation, circulating catecholamines or by blocking the parasympathetic effects in the airways. Another important natural bronchodilator substance is the mediator nitric oxide, which can be readily formed in the airways by the nitric oxide synthase enzymes. Bronchoconstriction is induced by mimicking the actions of acetylcholine, by blocking β2-adrenoceptors or by releasing cytokines from mast cells. Excitatory neuropeptides, including substance P and neurokinin A, also cause bronchoconstriction when released during inflammation or chemical irritation.
Control of respiration
Central control
Respiration is normally under involuntary central and autonomic control. The basic rhythm for respiration1 is initiated and maintained by the central pattern generator located in the medulla oblongata (see Figure 14-2). Signals from the spinal cord, the cerebral cortex and midbrain, the pons and vagal afferents from the lungs can all modify the rhythm of respiration, contribute to the normal pattern of respiration and facilitate 15–20-fold increases in oxygen use during vigorous exercise. Voluntary influence and control of breathing, however, are possible via connections between the cerebral cortex and motor neurons that control respiration.
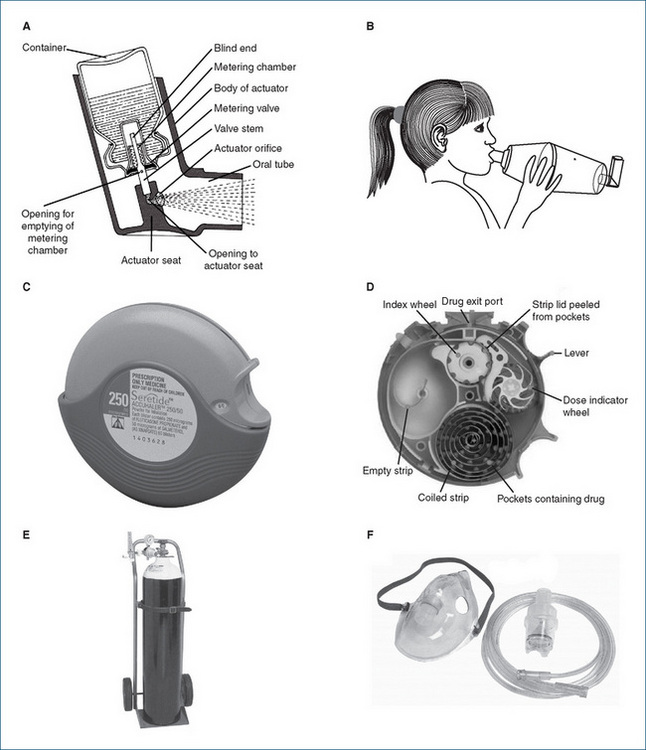
Figure 28-2 Devices for drug administration by inhalation. A Metered-dose inhaler (MDI, or ‘puffer’) shown in cross-section. B MDI in combination with a large-volume spacer. C Accuhaler™. D Accuhaler; cross-section. E Gas cylinder on trolley, with regulator and flow meter. F Adult nebuliser bowl, tubing and mask. A, C and D: courtesy GlaxoSmithKline, Australia, used with permission; E and F: photographs courtesy of BOC Gases Australia Ltd, reproduced with permission.
Peripheral control
Humoral regulation of respiration is achieved primarily through changes in the concentrations of oxygen, carbon dioxide or hydrogen ions in body fluids; carbon dioxide is the chief respiratory stimulant. An increase in the carbon dioxide tension of the blood (hypercapnia) directly stimulates the inspiratory and expiratory centres, which increases both the rate and depth of breathing. This results in hyperventilation, which enhances removal of carbon dioxide from the lungs to keep the carbon dioxide tension of the blood constant.
Small changes in arterial oxygen concentration usually have little if any direct effect on the respiratory centre, but if the arterial oxygen concentration falls below about 60% of normal (hypoxaemia), the chemoreceptors in the carotid and aortic bodies are stimulated and in turn stimulate the respiratory centre to increase alveolar ventilation. This mechanism operates primarily under abnormal conditions such as chronic obstructive pulmonary disease or exposure to high altitude.
Control of pH
The acid–base balance of the body is largely determined by pH homeostatic mechanisms in the kidneys and lungs. Respiration is effective in regulating the pH of the blood by controlling the carbon dioxide tension of the blood. Bicarbonate ions 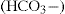 and proteins in the blood function as buffer systems, according to the equation
and proteins in the blood function as buffer systems, according to the equation
which shows the combination of carbon dioxide with water to form carbonic acid which dissociates to bicarbonate and hydrogen ions. This reaction is catalysed by carbonic anhydrase enzymes, widely distributed in all tissues, especially in red blood cells and epithelia. When the carbon dioxide content of the blood increases, there is an increase in the formation of carbonic acid in the blood, resulting in respiratory acidosis. Conversely, a decrease in the carbon dioxide content of the blood results in alkalosis.
Aerosol therapy
Aerosols
Aerosol therapy is a form of inhaled, topical pulmonary drug administration. An aerosol is a suspension of fine liquid or solid particles dispersed in a gas or in solution. Aerosols are most commonly inhaled, and can also be administered to the skin (as topical sprays) or to body cavities (ear, nose, rectum, vagina). After inhalation, some of the particles are deposited in the respiratory tract; however, the remainder tends to be swallowed, depending on droplet size (see section below). Inhalation may be via steam, from a nasal spray or with devices such as metereddose inhalers (MDI), spacers, face masks and nebulisers (see Figure 28-2). Dry powder inhalers (DPI) are also available. Liquid or solid particles range in size from about 0.005 to 50 μm in diameter.
Aerosol therapy has many advantages:
Inhaled aerosols can promote bronchodilation, pulmonary decongestion, loosening of secretions, topical application of corticosteroids and other drugs and moistening of inspired air.
Drug delivery by inhalation
Metered-dose inhalers
Metered-dose inhalers (MDI), invented over 50 years ago, are small hand-held ‘puffers’ containing multiple doses of the active drug, mixed with a dispersing agent and a propellant, in a canister. The canister is shaken and then the top is depressed (while inhaling) to deliver an accurate dose of the aerosol along with the inert propellant gas, usually a hydrofluoralkane (previously chlorofluorocarbons [CFCs] were used; while biologically inert, these were infamous for their deleterious effects on the Earth’s ozone layer).
Effective use of MDIs requires good hand–breath coordination, which may be difficult for young children; breath-activated MDIs, spacers and face masks help improve drug administration (see Clinical Interest Box 28-2). Mouthpieces of inhalers may need regular cleaning; manufacturers’ instructions for specific devices should be followed.
Clinical interest Box 28-2 Puffers and other inhaler devices
Because of the importance of correct use of inhaler devices to maximise drug delivery to the airways, patients need to be shown and reminded of the best way to use inhalers, how to know when they are getting empty and how to clean them. Patient information sheets are available to demonstrate the correct techniques. With ‘puffers’ the technique is as follows:
The technique for handling different styles of inhalers varies, but the inhaling drug—breath holding—exhaling method is similar. The plastic casing should be cleaned at least once a week; cleaning techniques for devices should be checked from information supplied with the inhaler. The simplest way to determine when a puffer is running out of drug is to count and keep a record of the number of times it has been used.
Children sometimes find it easier to use a puffer with a spacer (Figure 28–2B), which reduces the amount of drug deposited in the mouth and throat. With very young children, a small volume spacer or nebuliser and face mask may be useful.
Dry powder inhalers
These are similar to MDIs, except that the drug is delivered as finely divided particles rather than in aerosol solution. Examples are the ‘Accuhaler’, a compact device with a foil strip inside containing doses of finely powdered drug (Figure 28-2D), and the ‘Turbuhaler’, in which the drug is loaded as a capsule that is broken open when the base is rotated, releasing the active drug.
Nebulisers
Nebulisers (‘pumps’; Figure 28-2F) use compressed air or oxygen, or ultrasonic energy, to produce a fine mist of drug in aerosol form from a solution. They are useful for delivering large doses over long periods, especially in severe asthma attacks. The aerosol drug or solution may irritate facial skin.
Droplet size
The effectiveness of aerosol therapy depends on the number and size of droplets that can be suspended in an inhaled aerosol. Large droplets of more than 40 μm in diameter will be deposited primarily in the upper airway (mouth, pharynx, trachea and main bronchi). This may be useful for keeping large airways (nose and trachea) moist and for loosening secretions. Medium-sized droplets (8–15 μm in diameter) will be deposited primarily in the bronchioles and bronchi. Smaller droplets (2–4 μm in diameter) are more likely to reach the periphery of the lungs—the alveolar ducts and sacs. (For comparison, tiny fibres of asbestos dust, which after long-term exposure and inhalation can deposit in the lungs and set up chronic inflammatory responses leading to asbestosis and eventually bronchogenic carcinoma and mesothelioma, are 0.1–1.5 μm in diameter.) Particles smaller than about 0.6 μm are unlikely to be deposited, and will be exhaled.
Inhaled drugs
Drugs administered by inhalation are generally intended for local effects only. However, the lung is an absorptive organ (think: oxygen) and thus is a route of access for drugs to enter the systemic circulation. Absorption is generally rapid because of the highly vascular pulmonary capillary system, but also depends on the inhaled drug’s lipid solubility, the aerosol particle size and pulmonary function. For example, after inhaled general anaesthetic agents enter the airways, they are absorbed into the pulmonary capillaries (because they are lipid-soluble). They then circulate rapidly towards the brain, readily cross the blood–brain barrier and then (within seconds) act to depress nerve cell functions.
Studies have shown that a considerable proportion of an inhaled drug dose is swallowed, and is thus likely to produce systemic effects or may be digested or metabolised rapidly. Bronchodilator β2-agonist aerosols do produce systemic effects such as tremor and tachycardia because the drug stimulates cardiac β1-adrenoceptors after absorption into the bloodstream. Other potential problems from aerosol administration include oral infections after corticosteroid inhalation, dental caries from acidic drugs or ocular effects from corneal deposition of drugs if the aerosol mist reaches the eyes.
It is important to be aware of the proper recommendations for drug administration when two or more inhalation aerosols are prescribed together. For example, if a corticosteroid or mast-cell stabiliser puffer is prescribed to be administered as well as a bronchodilator puffer, the bronchodilator should be administered five minutes before the other drug to promote bronchodilation and maximise inhalation of the second aerosol.
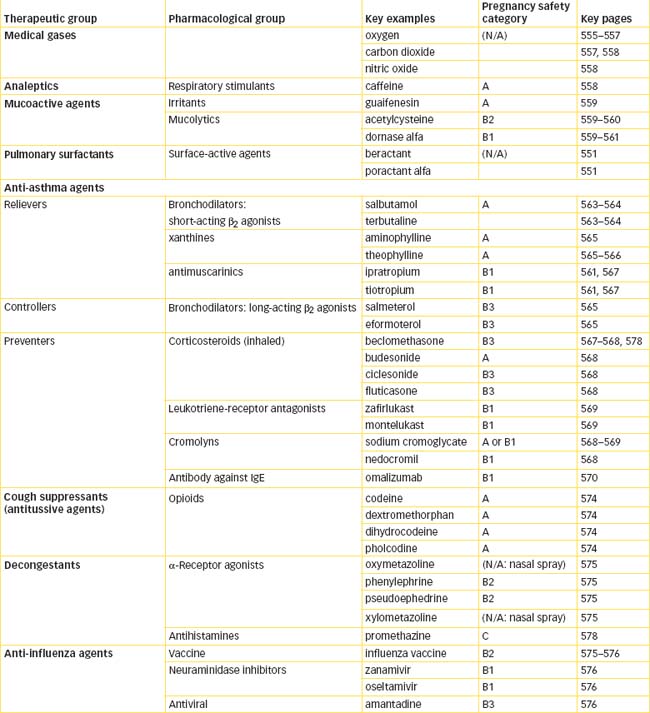
Medical gases
Oxygen
Oxygen is a gas that is essential for life; it is colourless, odourless and tasteless. Inspired air normally contains 20.9% oxygen which, at an atmospheric pressure of 760 mmHg, exerts a partial pressure (Po2) or tension of 159 mmHg. The partial pressure of oxygen in arterial blood 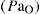 is normally greater than 80 mmHg.
is normally greater than 80 mmHg.
Oxygen must be continuously supplied to all cells. Of all the tissues affected by hypoxia (inadequate cellular oxygen), the brain is most susceptible to hypoxia: an acute reduction of the 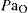 level to 50 mmHg decre ases mental functioning, emotional stability and fine mus cular coordination. Further reduction leads to impaired judgement, decreased pain perception, impairment of muscular coordination and eventually unconsciousness and irreversible damage. When circulatory stress exists, blood flow to the brain, kidneys and heart tends to be preserved at the expense of other less vital organs.
level to 50 mmHg decre ases mental functioning, emotional stability and fine mus cular coordination. Further reduction leads to impaired judgement, decreased pain perception, impairment of muscular coordination and eventually unconsciousness and irreversible damage. When circulatory stress exists, blood flow to the brain, kidneys and heart tends to be preserved at the expense of other less vital organs.
Indications for oxygen therapy
While essential for life, oxygen is also potentially toxic (see ‘Adverse effects’, below). It should be administered in appropriate dosage regimens (% concentration, flow rate and duration) and with careful monitoring of blood gas concentrations. Oxygen is used chiefly to treat hypoxia and hypoxaemia (oxygen deficiency in arterial blood). A 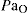 of less than 50 mmHg in an acutely ill patient (not adapted to chronic low
of less than 50 mmHg in an acutely ill patient (not adapted to chronic low 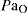 levels) indicates tissue hypoxia.
levels) indicates tissue hypoxia.
The most common form of hypoxia necessitating oxygen treatment is hypoxic hypoxia, produced by conditions causing a decrease in Po2, e.g. airway obstruction, hypoventilation or high altitude. Oxygen is also used as a carrier gas in virtually all general anaesthetic techniques, and in treatment of cyanosis, chest wounds, shock, severe haemorrhage, cardiac or respiratory arrest, coronary artery occlusion and in neonatal resuscitation.
The effectiveness of oxygen administration depends on the carbon dioxide content of the blood, as a high carbon dioxide level is the main stimulant to respiration. Highconcentration oxygen therapy (50%–90%) in the hospital situation is used in acute conditions associated with a normal or low 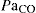 , such as in pulmonary embolism or oedema, myocardial infarction or status asthmaticus (acute severe asthma). People with chronic obstructive pulmonary disease (COPD), however, are subject to hypercapnia (high
, such as in pulmonary embolism or oedema, myocardial infarction or status asthmaticus (acute severe asthma). People with chronic obstructive pulmonary disease (COPD), however, are subject to hypercapnia (high 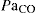 ) with low
) with low 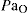 . Their medullary centres are relatively insensitive to stimulation by carbon dioxide; rather, the low
. Their medullary centres are relatively insensitive to stimulation by carbon dioxide; rather, the low 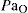 serves as a stimulant to respiration. Oxygen concentration (25%) and flow rates (1–2 L/min) are therefore kept low for patients with COPD; however, the guiding principle is that hypoxaemia is more dangerous than hypercapnia, so adequate oxygen levels must always be maintained.
serves as a stimulant to respiration. Oxygen concentration (25%) and flow rates (1–2 L/min) are therefore kept low for patients with COPD; however, the guiding principle is that hypoxaemia is more dangerous than hypercapnia, so adequate oxygen levels must always be maintained.
Administration
Most of the oxygen administered in hospitals for therapy is provided from a central source, where it is stored as a gas or liquid oxygen. Compressed oxygen is marketed in steel cylinders fitted with reducing valves for the delivery of the gas. The regulators and fittings are non-interchangeable, to minimise the risk of inadvertent administration of the wrong gas (see Clinical Interest Box 28-3 and Figure 28-2E); in Australia, oxygen cylinders are white with a white shoulder. Because the gas is under considerable pressure and is potentially explosive, the tanks must be handled and stored carefully. Oxygen cylinders may also be supplied to homes (domiciliary oxygen therapy) of patients with severe persistent hypoxaemia, e.g. due to chronic bronchitis, emphysema, pulmonary hypertension or cancer affecting the lungs. Oxygen-rich air may also be supplied by an oxygen concentrator, a small mobile floorstanding electrically powered machine that removes nitrogen from room air. Oxygen is administered by inhalation via catheters, nasal cannulae or masks.
Clinical interest Box 28-3 Medical gases
Medical gases are supplied in a great range of container sizes, from small portable aluminium cylinders (about 200 L capacity) to steel cylinders of compressed gases (several thousand litres capacity), through to systems of tanks and plumbed-in gas lines servicing hospitals and research institutions. The new colour coding being adopted in Australia for medical gases is as follows:
| Gas | Colours | Uses |
| Air, compressed | white cylinder, black & white shoulder | Breathing apparatus; carrier gas for anaesthesia; driving surgical air tools |
| Carbogen (usually 5% CO2 in oxygen) | white cylinder, green-grey & white shoulder | Respiratory stimulant; oxygenation of isolated tissues in physiological and pharmacological research |
| Carbon dioxide | white cylinder, grey-green shoulder | Respiratory stimulant; in anaesthesia; in cryosurgery; to facilitate vasodilation |
| Helium | white cylinder, brown shoulder | Vehicle gas; gaining access to obstructed airways; in magnetic resonance imaging machines; in balloons |
| Nitrous oxide | white cylinder, ultramarine blue shoulder | Analgesia and anaesthesia (with oxygen); vehicle gas in anaesthesia; in cryosurgery |
| Entonox (50% oxygen, 50% nitrous oxide) | white cylinder, ultramarine blue & white shoulder | Self-administered anaesthetic in obstetrics, first aid, dentistry, doctors’ surgeries, ambulances etc |
| Oxygen, compressed | white cylinder, white shoulder | Respiratory therapy; carrier gas in anaesthesia; resuscitation; high altitude and underwater breathing; hyperbaric chambers |
Note: The New Zealand manufacturer refers to green/grey as ‘French grey’ and ultramarine blue as ‘royal blue’.
Equipment used to handle and administer gases includes regulators and flow meters, carry bags, trolleys, oxygen concentrators and conserving devices, pressure gauges, masks, cylinder backpacks, suction units, cannulae, tubing and connectors.
Sources: BOC Gases Group 2008; BOC website: www.boc.com.au.
Hyperbaric oxygen
Hyperbaric oxygen (oxygen supplied at a pressure of 3–4 times normal) has been used in the treatment of various conditions, such as infections caused by Clostridium welchii, the anaerobic bacillus that produces gas gangrene. Increased oxygen pressure in the tissue may exert an inhibitory effect on enzyme systems of anaerobic microorganisms.
Hyperbaric oxygen has also been used in circulatory disturbances such as air or gas embolism, decompression sickness, carbon monoxide or cyanide poisoning, acute traumatic ischaemia, crush injury and compartment syndrome, and also in compromised (ischaemic) skin grafts and flaps, radiation necrosis, refractory osteo myelitis and to enhance healing in problem wounds.
Adverse effects of oxygen
While oxygen is essential for life in aerobic organisms including humans, it has also been described as a toxic mutagenic gas; aerobic organisms including humans survive because they have evolved antioxidant defences against oxygen. Exposure to 80%–100% oxygen for a prolonged period can cause an inflammatory response with subsequent destruction of the alveolo-capillary membrane of the respiratory tract. Toxicity is often difficult to recognise but the most common symptoms are substernal distress (ache or burning sensation behind the sternum), respiratory distress with decreased vital capacity, nausea, vomiting, restlessness, tremors, twitching, paraesthesias, convulsions and a dry, hacking cough. Excessive oxygen supplied to preterm infants to treat respiratory distress syndrome can cause blindness (Clinical Interest Box 28-4).
Clinical interest Box 28-4 Oxygen administration in the premature infant
Health-care professionals caring for premature infants must be constantly aware of the danger of retinopathy of prematurity (retrolental fibroplasia). This is a vascular proliferative disorder of the retina that occurs in some premature infants who have been administered high concentrations of oxygen after birth to treat respiratory distress of the newborn.
Excessive oxygen constricts the developing retinal vessels in the eye, suppressing normal vascularisation. On return to normal oxygen levels, the tissue becomes relatively hypoxic, blood vessels proliferate, endothelial cells become disorganised and there can be destruction of the immature retina, resulting in blindness.
The pathogenesis of retrolental fibroplasia was discovered gradually from the 1940s to the 1970s, after medical ‘detective’ work in America, England and Australia. Dr Kate Campbell, a Melbourne paediatrician, contributed by showing that the incidence was highest in premature babies nursed in neonatal units equipped with ‘oxygen-cots’ that could provide high levels of oxygen, thus associating the blindness with oxygen toxicity.
Careful monitoring of arterial blood gases is therefore essential, and the oxygen concentration of inspired air should be kept between 30% and 40%. Some incubators are equipped with a safety valve that automatically releases any excess oxygen outside the chamber. More recent advances in treatment include cryotherapy, laser photocoagulation and surgical vitrectomies.
Based on: Howell & Ford 1986.
Oxygen free radicals
Free radicals are chemical species containing one or more unpaired electrons that readily participate in oxidation–reduction reactions. Reactive oxygen species (ROS) include the superoxide radical (O2•––) and hydroxyl radical (•OH). These oxygen free radicals are formed in many biochemical reactions in the body, e.g. by enzymes such as peroxidases, xanthine oxidase and nitric oxide synthase, and in the electron transport chain.
Oxygen free radicals have been implicated in many pathological processes, in particular in causing oxidative stress, in which there is imbalance between ROS and levels of antioxidant defences. This may lead either to adaptation or to cell injury and cell death. Oxygen free radicals are implicated in the pathogenesis of post-ischaemic reperfusion injury and many of the processes of ageing and carcinogenesis, in radiation-induced damage, vitamin E deficiency, atherosclerosis, rheumatoid arthritis, diabetes, inflammatory bowel disease and hypertension and in some types of adverse drug reactions. To protect against ROS toxicity, mitochondria have evolved defence mechanisms including the enzymes superoxide dismutase and catalase.
There is clear evidence that a diet high in antioxidants protects against many of the major diseases of older age, such as ischaemic heart disease and many cancers. The antioxidant vitamins E (tocopherols) and C (ascorbic acid) and α-lipoic acid are protective, and a diet rich in fruit, vegetables, nuts, beans and lentils is encouraged.
Carbon dioxide
Carbon dioxide (CO2) is a colourless, odourless gas that is heavier than air; normal air contains only 0.04% CO2. Inhalation of 3%–5% CO2 for a short period increases both rate and depth of respiration unless the respiratory centre is depressed by drugs or disease. As a pharmacological agent, it affects respiration, circulation and the central nervous system (CNS).
Carbon dioxide stimulates cells of the sympathetic nervous system, the respiratory centre and the peripheral chemoreceptors. When CO2 increases the rate and force of respiration, venous return to the heart is usually enhanced as a result of decreased peripheral resistance; there is improved rate and force of myocardial contraction and less likelihood of myocardial irritability and arrhythmias.
Too much CO2 in inhaled air (>7%) may cause:
Indications
Indications for clinical use of CO2 are:
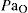 is important
is importantOther uses
Carbon dioxide in solution (as carbonated ‘fizzy’ drinks) stimulates the absorption of liquids by mucous membranes and hence rapidly relieves thirst (and hastens the absorption of alcohol). Solid CO2 (‘dry ice’, at –78 °C) has a destructive action on tissues; in cryotherapy it is applied directly to warts and other skin lesions to destroy them.
A mixture of CO2 (usually 5%) in oxygen, known as Carbogen, is used in many pharmacological and physiological experiments to oxygenate isolated tissues (see Clinical Interest Box 28-3).
Carbon dioxide has also been used in the treatment of intractable hiccups: stimulation of the respiratory centre causes large contractions of the diaphragm, which suppresses spasmodic contractions.
Carbon dioxide levels are also altered, indirectly, by drugs that inhibit the enzyme carbonic anhydrase (see above, under ‘Control of pH’). These drugs, such as acetazolamide and dorzolamide, produce a metabolic acidosis by inhibiting carbonic anhydrase in the kidney, the ciliary processes of the eye and in other tissues, thereby causing loss of bicarbonate and an alkaline diuresis. The acidosis has a stimulatory effect. Carbonic anhydrase inhibitors also have membrane-stabilising and antiepileptic properties, produce drowsiness in high doses and lower intraocular pressure by inhibiting bicarbonate synthesis; hence they are useful in glaucoma (see Chapter 31 and Table 31-4).
Administration and toxicity
Carbon dioxide is kept in metal cylinders (with a grey-green shoulder). When it is used for medical purposes it is administered in combination with oxygen. A 5%–10% concentration of CO2 delivered through a tight-fitting face mask is inhaled by the patient until the depth of respiration is increased. A simpler way of administering CO2 is to allow the patient to hyperventilate with a paper bag held over the face; re-inhaling expired air causes the CO2 content to be continually increased. Administration should be stopped as soon as the desired effects on the patient’s respiration have been obtained.
Signs of CO2 overdosage are dyspnoea, breath-holding, markedly increased chest and abdominal movements, nausea and raised systolic blood pressure. Administration should be discontinued when these symptoms appear. Prolonged administration of 5% CO2 may produce severe CNS depression; a 10% concentration can lead to loss of consciousness within 10 minutes.
Other gases
Other gases used medically include nitrous oxide (as an analgesic), nitric oxide (as a vasodilator) and helium (to assist oxygen flow)—see Clinical Interest Box 28-3.
Nitric oxide
A new use for the gas nitric oxide (NO) has been implemented in the last decade: to improve tissue oxygenation in neonates suffering hypoxic respiratory failure resulting from meconium aspiration or pulmonary hypertension. Nitric oxide is a mediator generated locally in tissues, with many physiological actions including vasodilation—see Figure 23-1. When administered as a gas (maximum 20 ppm in nitrogen), NO dilates the blood vessels in the lungs, and thus enhances oxygenation and helps overcome hypoxia. While administration of NO reduces the need for extracorporeal membrane oxygenation of the babies, there are many adverse effects (including formation of methaemoglobin, hypotension and haematuria), and overall survival of very premature babies is not markedly increased. It is only approved for use in neonates of over 34 weeks gestation; however, it is also used frequently in adults in intensive care units.
Respiratory stimulants and depressants
Respiratory stimulants: analeptics
Direct respiratory stimulants come under the broader classification of CNS stimulants and are referred to as analeptics (see Chapter 19). These drugs act directly on the respiratory and vasomotor centres in the medulla to increase respiratory rate and tidal exchange, and also raise the blood pressure. Although these drugs are available for stimulating respiration, they may in large doses cause convulsions, CNS depression and respiratory paralysis.
The only drug routinely used as a respiratory stimulant now is caffeine, given PO or IV to treat respiratory distress and apnoea in preterm infants—see Drug Monograph 19-2.
Reflex respiratory stimulants
Aromatic ammonia spirit and the natural compounds camphor, menthol and thujone (a constituent of absinthe) are given by inhalation for their actions as reflex respiratory stimulants. In cases of fainting, they may be administered by inhaling the vapours (‘smelling salts’). Reflex stimulation of the medullary centre occurs through peripheral irritation of sensory nerve receptors in the pharynx, oesophagus and stomach. The rate and depth of respiration are then increased through afferent messages to the respiratory control centres; reflex stimulation of the vasomotor centre results in a rise in blood pressure.
Respiratory depressants
The most important drugs causing respiratory depression as an adverse reaction are the opioid analgesics, such as morphine. These agents depress the sensitivity of the respiratory centre to CO2, thereby making breathing slower and more shallow and lessening the irritability of the respiratory centre. Respiratory depression, however, is seldom desirable or necessary, although it is sometimes unavoidable. It is also an adverse effect of many otherwise useful CNS depressant drugs, including the benzodiazepines, barbiturates, antihistamines and alcohol.
Occasionally, an opioid such as pholcodine is administered as an antitussive for a painful or harmful cough, and may also inhibit the rate and depth of respiration (see later section on cough suppressants, and Drug Monograph 28-5).
Drugs affecting secretions and mucociliary transport
Expectorants
Sputum (or phlegm) is an abnormal viscous secretion of the lower respiratory tree. It consists mainly of mucus, a mucopolysaccharide–glycoprotein material continually produced by the cells in the mucous membrane. It may also contain leucocytes, bacteria and DNA derived from the breakdown of mucosal cells, which are responsible for the characteristic thickness and yellow colour of sputum. Expectorants are drugs that aid in the removal (swallowing or spitting out) of sputum from the bronchial passages.
Respiratory disorders such as chronic bronchitis lead to significant impairment of the mucus clearance process, with mucus plugging of airways and alveoli (Figure 28-3) and pathogenic colonisation by microorganisms in the lower respiratory tract. These changes lead to overproduction of thick, tenacious sputum. The advantage provided by expectorant and mucolytic drugs is that they alter the consistency of the sputum, either by diluting thickened secretions (diluents, irritants) or by chemically breaking down mucus (mucolytics), promoting the eventual expectoration, or spitting out, of these secretions.
Diluents
Water and saline solutions
The agent most commonly used to dilute respiratory secretions is water, administered by ultrasonic nebuliser or, more traditionally, by inhaling steam over a basin of boiling water. Small amounts of water deposited on the gel layer of the respiratory tree appear to reduce the adhesive characteristics and general viscosity of the gelatinous substances found in this layer. Usually large amounts of water are needed to liquefy the respiratory secretions. (For patients receiving restricted fluid intake, water absorbed through the inhalation route must be added to the intake record.)
Normal saline (0.9% sodium chloride) is an isotonic solution that exerts the same osmotic pressure as plasma fluids. Therapy by nebulisation is well tolerated, resulting in hydration of respiratory secretions. Inhalation of hypotonic solution (e.g. 0.45% sodium chloride) may provide deeper penetration into the more distal airways (whereas inhalation of hypertonic solution, 1.8% sodium chloride, stimulates a productive cough).
Irritant expectorants
Older compounds promoted as expectorants are thought to act by an irritant action on the mucous membranes, which increases the secretion of mucus from bronchial secretory cells, facilitating ciliary action and productive coughing and soothing and lubricating dry tissues. Such substances include the natural compounds ipecacuanha, squill, guaifenesin, iodides, senega, ammonia and volatile oils (lemon, eucalyptus, tea-tree etc). While they contribute much to the colour, flavour, smell and placebo effect of many old-fashioned over-the-counter (OTC) cough mixtures (see later section), there is little objective evidence of any pharmacological efficacy. In higher doses these compounds also have direct and irritant emetic actions.
Mucolytic drugs
Acetylcysteine and bromhexine
Mucolytic drugs exert a disintegrating effect on mucus, facilitating removal of mucus or other exudates from the lung, bronchi or trachea by postural drainage, coughing, spitting or swallowing. The more commonly used mucolytics are acetylcysteine (see Drug Monograph 28-1), which splits disulfide bonds in mucoproteins, and bromhexine, thought to improve mucus flow by enhancing the hydrolysing activity of lysosomal enzymes, but there is little hard evidence of clinical efficacy for either compound, except in reducing exacerbations and disability in patients with COPD.
Drug monograph 28-1 Acetylcysteine
Acetylcysteine reduces the viscosity and stickiness of purulent and non-purulent pulmonary secretions by splitting disulfide bonds in mucoprotein molecules.
Indications
Acetylcysteine is administered by intratracheal tube or nebuliser to reduce thick or abnormal mucus in bronchopulmonary disease, in atelectasis caused by a mucus obstruction and in tracheostomy care. Note that acetylcysteine is also indicated as a specific antidote to paracetamol poisoning, by providing cysteine groups for glutathione synthesis (see Clinical Interest Box 15-9).
Pharmacokinetics
When inhaled or instilled directly via an intratracheal catheter, acetylcysteine produces rapid local effects on the mucus in the lungs. The peak response from inhalation occurs within 5–10 minutes. Acetylcysteine is metabolised in the liver.
Adverse effects
These include nausea, mouth ulcers and respiratory difficulties including broncho spasm. No significant drug interaction has been reported.
Dornase alfa
Dornase alfa is a prescribed respiratory inhalant product with some proven mucolytic efficacy, administered to increase expectoration in cystic fibrosis (see Clinical Interest Box 28-1). It is recombinant human deoxyribonuclease, a DNA-degrading enzyme that digests extracellular DNA released from degenerating neutrophils and cellular debris in purulent sputum, thus improving pulmonary function and reducing the risk of respiratory tract infections common in CF. Its use has resulted in a decrease in the incidence of respiratory infections, hospitalisations and medical costs but it is expensive, so continued treatment needs to be justified by a proven benefit.
The enzyme solution is inhaled via a nebuliser—usually one 2.5 mg ampoule/day regularly for 6–12 months. Inhaled enzyme acts locally in the respiratory tract and is not absorbed into the systemic circulation. Significant improvement in pulmonary function may be seen within 3–7 days and a decrease in respiratory infections within weeks to several months. Adverse reactions include chest pain, sore throat, laryngitis, skin rash and conjunctivitis. No significant drug interaction has been reported.
Muscarinic antagonists (antimuscarinics)
Acetylcholine muscarinic M3 receptors, present on bronchial smooth muscle cells and gland cells, mediate contraction of smooth muscle (bronchoconstriction) and stimulation of bronchial secretions (see Figure 28-3). Thus one of the many pharmacological effects of muscarinicreceptor antagonists (antimuscarinic drugs) such as atropine is inhibition of bronchial secretions. Salivary, lacrimal and sweat gland secretions are also inhibited, leading to the common ‘atropinic’ effect of dry mouth (see Drug Monograph 11-2).
A muscarinic antagonist such as ipratropium or tiotropium is sometimes used in bronchial asthma as a bronchodilator. Potential adverse effects include inhibition of bronchial secretion and mucociliary transport and accumulation of thickened secretions; however, as there is often excessive mucus production in asthma, the effects tend to cancel out. Tiotropium is indicated in COPD to improve exercise capacity and reduce morbidity and mortality.
Drug treatment of asthma
Pathophysiology of asthma
Asthma is a chronic inflammatory disease of the airways that affects over 300 million people worldwide. In asthma, the passage of air into and out of the lungs is obstructed because of reversible bronchoconstriction, chronic inflammation of the epithelium of the airways and increased mucus secretion; there is airway hypersensitivity to a variety of stimuli. Asthma affects more than 10% of Australians (Clinical Interest Box 28-5) and 15% of New Zealanders (Clinical Interest Box 28-6); approximately half of all cases occur during childhood.
Clinical interest Box 28-5 Asthma in the australian community
Sources: National Asthma Campaign (www.nationalasthma.org.au) and Asthma Victoria (www.asthma.org.au) [28 August 2009].
Clinical interest Box 28-6 Asthma in new zealand and pacific island countries
The prevalence of asthma in New Zealand is one of the highest in the world. According to the New Zealand Health Survey 2002–2003, one in five adults aged 15–44 years had been diagnosed with asthma. The prevalence was highest in the 15–24-year age group, and was about four times higher among European/other ethnic groups than among Pacific and Asian ethnic groups, with no significant difference between males and females.
During the 1970s and 1980s, there was an ‘epidemic’ of asthmarelated deaths and hospital admissions in New Zealand, prompting urgent study of possible causes. Since 1989, there has been a decline in these statistics, reflecting changes in management and treatment. In 1990, Sears et al reported that regular inhalation of fenoterol was associated with deterio ration of asthma control. Two later case-controlled studies supported the hypothesis that inhaled fenoterol increased the risk of death in patients with severe asthma. The drug was withdrawn in New Zealand. Interestingly, an evalu ation of international data on medication sales in countries such as Australia, Belgium, Austria and Germany did not point to a relation between asthma mortality and bronchodilator β2 agonists in general or fenoterol in particular.
The effectiveness of a 6-month Maori rural community-based asthma self-management program involving a ‘credit card’ asthma self-management plan was assessed during the 6 years after the formal end of the program. The program participants still had reduced asthma morbidity 6 years after the program had ended, but these benefits were less than those measured at 2 years. It appeared that underrecognition and undertreatment of asthma with the appropriate amounts of inhaled steroids were major factors contributing to asthma morbidity. Continued reinforcement of the self-management skills seemed to be an essential component of any follow-up to an asthma selfmanagement program.
International studies comparing prevalence of wheezing in childhood asthma in various Pacific Island countries have recently shown considerable variation: Tokelau Islands (19.7%), Tonga (16.2%), Niue (12.7%), French Polynesia (11.3%), Cook Islands (10.6%), Fiji Islands (10.4%), New Caledonia (8.2%) and Samoa (5.8%). Prevalence levels were considerably lower than those in Pacific children in New Zealand (31%), which suggested that children who migrate experience an altered risk of asthma as a result of exposure to a new environment during childhood.
Adapted from: Sears et al 1990; D’Souza et al 2000; Foliaki et al 2007.
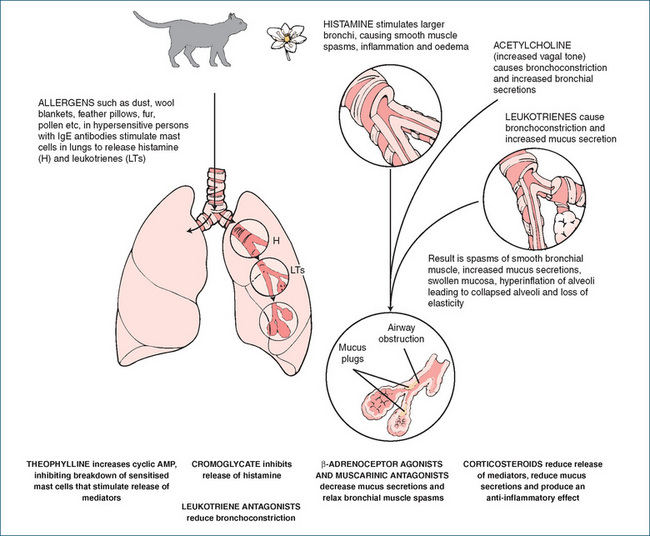
Airways inflammation
Asthma was earlier thought to be a disease mainly of impaired autonomic control of airways lumen diameter, but it is now recognised that many physiological mediators are involved in the pathogenesis of an asthma attack, including leukotrienes, interleukins, histamine,2 prostaglandins (PGs) and other cytokines and nitric oxide, as well as autonomic neurotransmitters. The early phase of an acute attack involves vasodilation and increased capillary permeability, with infiltration of bronchial mucosa by white blood cells. Numerous immune cell types are involved, particularly mast cells, eosinophils, macrophages, and Th2 and CD4+ lymphocytes. Activation of these cells leads to release of pro-inflammatory mediators and cytokines, notably nuclear factor κB, interleukin-2, −4, −5 and −13 and tumour necrosis factor-α, as well as immunoglobulins (IgEs).
This inflammatory process involves vascular leakage, contraction of bronchial smooth muscle (bronchoconstriction), inflammatory cell infiltration, increased oedema and mucus production, impaired mucociliary function and eventually thickening of airway walls, airway hyper-reactivity and irreversible airways obstruction (see Figure 28-3). The late-phase (chronic) response involves inflammation, proliferation of fibroblasts and fibrosis, oedema of the airway mucosa, necrosis of bronchial epithelial cells and airway wall remodelling, with increased collagen deposition. Expiration is particularly impaired, leading to air trapping, hypoxaemia and a raised 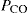 . The principal signs and symptoms are wheezing and cough, dyspnoea (difficult breathing), chest tightness, tachycardia, fatigue, sweating and anxiety. If bronchoconstriction is not reversed, status asthmaticus occurs, with respiratory acidosis and possibly life-threatening respiratory failure.
. The principal signs and symptoms are wheezing and cough, dyspnoea (difficult breathing), chest tightness, tachycardia, fatigue, sweating and anxiety. If bronchoconstriction is not reversed, status asthmaticus occurs, with respiratory acidosis and possibly life-threatening respiratory failure.
Allergic asthma
In most asthma patients there is an allergic component mediated by IgEs. Extrinsic (atopic, allergic) asthma is triggered by factors not normally in the body, including allergens such as pollens, house dust mites, animal fur, moulds or proteins in foods such as eggs; some drugs including penicillins and aspirin can also precipitate allergic asthma. There is some evidence that ‘Westernisation’ of environments (reduced infant infections, reduced exposure to some allergens and increased use of antibiotics) is associated with increased risk of childhood asthma. Other common triggers are drugs that cause bronchoconstriction including β-blockers (Clinical Interest Box 28-7), chemicals such as sulfites used as preservatives, exercise (breathing cold air is thought to be the stimulus), emotional stress, respiratory infections and environmental pollutants including cigarette smoke. All people with asthma are hypersensitive to bronchoconstrictor agents, including acetylcholine and PGF2-α. In ‘intrinsic asthma’ there is no identified causative agent.
Drugs used in asthma
Not surprisingly, many types of drugs are used to inhibit the pathological effects of the various mediators; the major groups are:
Figure 28-4 gives an overview of the effects of antiasthma medications and the primary sites of action for these drugs.
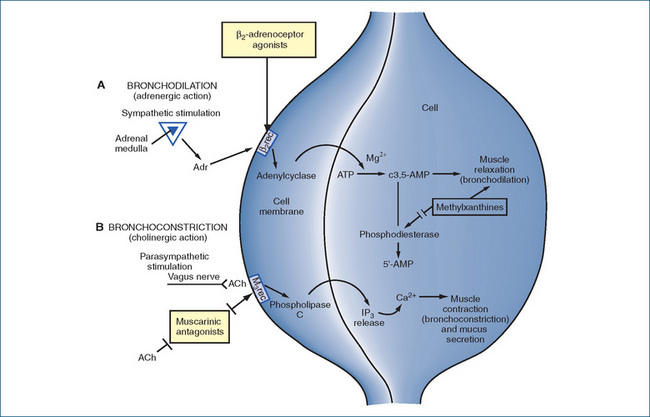
Figure 28-4 Typical mechanisms of action of drugs on bronchial smooth muscle A Bronchodilation pathway. B Bronchoconstriction pathway. ACh = acetylcholine; Adr = adrenaline; β2rec = β2−adrenoreceptor; IP = inositol phosphate; M3rec = M3 muscarinic receptor
There is a large degree of variability in the response of asthma patients to bronchodilators, inhaled corticosteroids and leukotriene modifiers. Some of the variability is attributed to genetic variation, and many variants of single nucleotide polymorphisms have been identified that alter airways responsiveness and lead to exacerbations (see Lima et al [2009]).
The choice of drugs depends on patient factors, aetiological factors, drug factors such as adverse drug reactions and classified severity of asthma. Triggering factors should be avoided if possible. Asthma is classified as mild, moderate or severe, according to the frequency and severity of patients’ asthma attacks during the previous 3 years, because this information is useful when considering step-wise pharmacological treatment (see later and Figure 28-5):
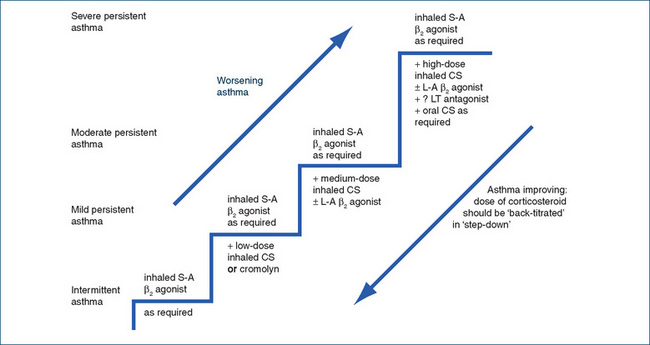
Figure 28-5 Stepwise maintenance of asthma in adults. CS = corticosteroid; LT = leukotriene; L-A = long-acting; S-A = short-acting.
Persistent: >3–4 attacks/week; moderate: asthma not controlled by low-dose inhaled CS + β2 agonist.
The overall management of asthma is summarised later in this chapter, after discussion of the groups of drugs commonly used in this condition.
Bronchodilator drugs
History
Bronchodilator drugs are used to treat pulmonary diseases such as asthma, chronic bronchitis and emphysema. They have been used in respiratory medicine for over 5000 years: ephedrine, a sympathomimetic amine closely related structurally to adrenaline, is an alkaloid obtained from the plant Ephedra sinica and was introduced from traditional Chinese medicine into Western medicine in 1923. Ephedrine has a predominantly indirect action, via release of noradrenaline from adrenergic nerve terminals; thus it has effects on both α- and β-adrenoceptors. The effects mediated by α-adrenoceptors (especially vaso constriction and hypertension) and β1-adrenoceptors (especially cardiac stimulation) count as adverse drug reactions in the context of asthma therapy, so much research effort has gone into development of specific β2-adrenoceptor agonist bronchodilators.
β-adrenoceptor agonists
The classification of adrenoceptors and the effects of agents that stimulate or block specific receptors are discussed in Chapter 12 and summarised in Table 12-1; reviewing these will help in understanding the mechanisms and actions of sympathomimetic bronchodilators.
Mechanism of action
Activation of β2-adrenoceptors in bronchial smooth muscle leads to increased formation of cAMP, enhancement of calcium extrusion from the cell and binding of intracellular calcium, which lowers the concentration of intracellular calcium and thus strongly relaxes the bronchial smooth muscle (Figure 28-4). Agonist actions on β2-adrenoceptors in the uterus can cause relaxation of uterine smooth muscle; the drugs have been used to delay threatened miscarriage (see Chapter 38).
Administration
The optimal route of administration of β2 agonists is by inhalation; ‘puffers’ and ‘pumps’ deliver low doses of drug directly to the airway smooth muscle and have rapid and relatively specific effects. Some inhaled drug is inevitably deposited in the oropharynx and swallowed; it may be absorbed into the systemic circulation and cause adverse reactions in other tissues. In rare cases, drugs administered by inhalation may cause bronchospasm, and propellants may induce cardiac arrhythmias or allergic reactions. In emergencies systemic administration may be required: adrenaline, salbutamol and terbutaline can be administered by injection. Salbutamol is also available in a syrup, especially useful for children.
Adverse effects
While agonists with specific actions on β2-adrenoceptors are available, it needs to be remembered that any substrate specificity in pharmacology is relative, and such agonists may stimulate also α and β1 receptors, especially in high doses, so adverse effects in the cardiovascular system (tachycardia), skeletal muscle (tremor) and CNS (anxiety) may occur. The reverse is also true: β1-adrenoceptor antagonists (β-blockers) used in cardiovascular disease may have potentially life-threatening bronchoconstrictor (β2 antagonism) effects in people with asthma (see the case study in Clinical Interest Box 28-7).
‘Epidemics’ of deaths from asthma occurred in the 1960s in Britain (attributed to over-the-counter preparations of high-dose isoprenaline) and in the 1980s in New Zealand (attributed to high doses of fenoterol, see Clinical Interest Box 28-6). Studies of morbidity have suggested that nonselective β-agonists may downregulate receptors, leading to the development of tolerance to the bronchodilator effects of these agents, which encourages overuse and exacerbates adverse effects arising from the cardiac and vascular actions. In addition, people with partic ular polymorphisms of the β2-adrenoceptor appear to experience reduced lung function and increased asthma exacerbations.
Short-acting β2 agonists (relievers)
Short-acting β2 agonists are fast-acting bronchodilators, and hence are used as relievers in first-line treatment for acute relief of asthma symptoms; salbutamol (well known as Ventolin) and terbutaline are described in Drug Monograph 28-2. They are also used for symptom relief in chronic obstructive pulmonary disease and during allergic reactions, and to prevent exercise-induced asthma (see Clinical Interest Box 28-12: Asthma, athletes and anabolics). Over-dependence on β2 agonists may indicate that other aspects of asthma management, including prophylactic use of anti-inflammatory drugs and monitoring of FEV1, are not optimal.
Drug monograph 28-2 Salbutamol and terbutaline
Indications
Short-acting β2-agonist bronchodilators are indicated for symptomatic relief of acute asthma and protection against exercise-induced asthma, and for symptomatic relief of bronchospasm in COPD and allergic reactions.
Pharmacokinetics
Onset of action by inhalation is rapid, within 5–15 minutes, with peak effect within 1–2 hours and duration of action of 3–6 hours. Salbutamol is metabolised in the liver and excreted in the kidneys, whereas terbutaline is excreted largely unchanged. Small amounts of either drug that have been swallowed are rapidly metabolised.
Drug interactions
Concomitant therapy with other sympathomimetic amines will cause excessive sympathetic stimulation (tremor, tachycardia). β-Blockers antagonise the effects of β2 agonists and may precipitate asthma, so they are contraindicated. Hypokalaemia resulting from β2-agonist therapy may be potentiated by xanthine derivatives, steroids or diuretics; potassium levels should be monitored. Antidepressant drugs may potentiate cardiovascular effects.
Adverse effects
These include tremor, palpitations, anxiety, restlessness, headaches, muscle cramps, hyperglycaemia, tachycardia, an unusual taste in the mouth and hyperactivity in children. Symptoms of overdose are those of excessive α- or β1-adrenoceptor stimulation, e.g. hypertension or palpitations.
Precautions
Precautions are needed in patients with cardiovascular disease, diabetes or hyperthyroidism. Excessive use of bronchodilator aerosols by patients, or lack of response, may indicate worsening asthma control. Both drugs are safe in pregnancy, breastfeeding and in the elderly.
Dosage and administration
The adult bronchodilator dose is 1–2 inhalations (100 mcg salbutamol, 500 mcg terbutaline), with the second inhalation at least 1 minute after the first, then again every 4–6 hours. Both drugs can also be administered by nebuliser, orally or parenterally in acute severe attacks.
Long-acting β2 agonists (controllers)
Long-acting β2 agonists (LABAs) commonly used include salmeterol and eformoterol; they have half-lives in the range 6–12 hours and are administered once or twice daily by metered-dose or dry-powder inhaler They are known as symptom controllers and are used in conjunction with inhaled corticosteroids (preventers) in maintenance treatment of asthma or COPD.
Clinical interest Box 28-7 Not in the script: a case of drug-induced asthma
Mr Bloggs, a busy businessman from Bentleigh, was in a hurry—couldn’t wait for an examination, just wanted a prescription for a salbutamol puffer for his asthma. The doctor obliged, against his better judgement, and Bloggs went off.
Three months later Bloggs was back—this time for a repeat prescription for his blood pressure pills. But this time the doctor insisted that Bloggs needed to be examined and it became apparent that he was a red-faced, obese, alcoholic, wheezing smoker. He complained he’d had asthma on and off for 2 years since his blood pressure tablets were changed.
Closer examination of medical records showed that 2 years previously, Bloggs’ antihypertensive medication had been changed from methyldopa to metoprolol. When questioned further, Bloggs admitted he had had asthma when young, but not since adolescence. It became clear that the β-blocker had caused bronchoconstriction, exacerbating his quiescent asthma.
Adapted from: Murtagh 1992; used with permission.
There is some controversy about the safety of LABAs: some studies show that the use of LABAs leads to increased risk of exacerbations of asthma, especially if LABAs are used alone (i.e. without inhaled corticosteroids). There may be a genetic component to this increased susceptibility.
Xanthine derivatives
The xanthine group of drugs includes caffeine, theophylline and theobromine; all are methylxanthines. Beverages from the extracts of plants containing these alkaloids have been used by humans since ancient times (the social use of the xanthines as coffee, tea, cocoa and cola drinks is discussed in Chapter 21). Xanthine derivatives relax smooth muscle (particularly bronchial muscle), stimulate cardiac muscle and the CNS (hence their social use) and also produce diuresis, probably through combined actions of increased renal perfusion and increased sodium and chloride ion excretion. Although caffeine is present in some preparations prescribed for migraine and in some OTC products taken to reduce mental fatigue, the main medical use of these natural products and their synthetic analogues is as bronchodilators.
The most active bronchodilator is theophylline (Drug Monograph 28-3), which is sometimes used as one of its derivatives, aminophylline (a more soluble but highly alkaline ethylene-diamine-theophylline derivative, given IV). Optimal clinical use of theophylline compounds can be difficult because of the variable pharmacokinetic parameters, narrow therapeutic index and many drug interactions (Drug Interactions 28-1), so their use in asthma is declining. However, there is evidence that low-dose theophylline has useful anti-inflammatory effects and may increase responsiveness to corticosteroids in patients resistant to steroids.
Drug monograph 28-3 Theophylline
Theophylline is the prototype of the xanthine derivative bronchodilators. It is most commonly prescribed as controlledrelease tablets for maintenance treatment of poorly-controlled moderate–severe asthma and COPD.
Pharmacokinetics
The rate of absorption and therapeutic effects of theophylline products, especially slow-release products, can vary even if they have the same strength and active ingredient. The different dosage forms do not have proven bioequivalence and so should not be substituted. Half-life varies markedly depending on age, concurrent illness and smoking status
Sustained-release (SR) tablets are formulated to opti mise absorption: most provide a bioavailability of 100%, with peak levels 4–6 hours after dosage. Absorption is little altered by food. Protein binding is moderate (50%–70%) and theophylline distributes across the placen ta (Pregnancy Category A) and into breast milk. Liver metabolism produces various uric acid and xanthine derivatives (some with low activity), which are excreted via the kidneys.
Plasma levels
Theophylline has a narrow thera peutic window: trough plasma levels are between 10 and 20 mg/L; however, thera peutic responses are variable and close supervision is necessary.
Drug interactions
There are many drug interactions with theophylline (summarised in Drug Interactions 28-1); reference books should be consulted for specific drugs and therapy monitored closely.
Adverse effects
These are dose-dependent and are related to the other main actions of xanthines (CNS and cardiac stimulation and diuresis), and include nausea, headache, insomnia, increased anxiety, vomiting, gastro-oesophageal reflux and increased urination. Tachycardia and convulsions may appear at high plasma levels (>30 mg/L); toxicity may however occur even at therapeutic levels.
Precautions
Use with caution in patients with fever, gastrointestinal or cardiovascular disorders, thyroid or liver dysfunction and in the elderly.
Dosage and administration
The dosage of theophylline preparations should be adjusted to maintain a serum concentration of 10–20 mcg/mL (= mg/L; see previous comments on serum levels). Doses are increased gradually over several days while monitoring for adverse effects, e.g. 10 mg/kg/day for 3 days, then 13 mg/kg/day for 3 days, then 16 mg/kg/day for 3 days, at which stage plasma concentration should be measured. Sustained-release tablets are taken every 12 hours and steady state is achieved after about 4 days; SR preparations should never be chewed or crushed.
Drug interactions 28-1 Theophylline
| Drug | Possible effects and management |
| Aciclovir, allopurinol, cimetidine, cipro- and norfloxacin, disulfiram, fluvoxamine, interferon alpha, macrolide antibiotics | Theophylline concentration may be increased; its dose may need to be reduced |
| Phenobarbitone, phenytoin, rifampicin, ritonavir, sucralfate | Theophylline concentration may be decreased; its dose may need to be increased |
| β2-Agonists or diuretics | Theophylline can potentiate hypokalaemia caused by these drugs |
| Lithium, macrolides, pancuronium, phenytoin | Theophylline may decrease concentration of or response to these drugs—hence dose of the other drug may need to be increased |
Mechanisms of action
Despite their long history and worldwide social and medical use, the mechanism of action of the xanthine derivatives is not well understood. The simple mechanism long held to account for their bronchodilator effect is inhibition of phosphodiesterase (the enzyme that metabolises cAMP), leading to increased intracellular levels of cAMP, smooth muscle relaxation and bronchodilation (see Figure 28-4). However the concentrations of theophylline required to inhibit the enzyme in vitro are much greater than therapeutic levels. Other mechanisms proposed include inhibition of cyclic GMP phosphodiesterase and competitive antagonism of adenosine (which activates adenylate cyclase, and has cardiac-depressant, bronchoconstrictor, pro-inflammatory and platelet-aggregation-suppressant effects) at adenosine receptors. In the treatment of asthma, theophylline derivatives act as bronchodilators, inhibit the late (inflammatory) phase of asthma and directly stimulate the medullary respiratory centre.
Pharmacokinetics
Theophylline is notorious for having very variable pharmacokinetic parameters:
Antimuscarinics
Antimuscarinic agents (anticholinergics) produce broncho dilation by blocking vagal tone and parasympathetic reflexes mediating bronchoconstriction; however, they may also decrease secretions and make them hard to expectorate. Typical ‘atropinic side effects’ are dry mouth and throat, urinary retention and constipation; antimuscarinic agents can exacerbate glaucoma or prostatic hypertrophy.
Quaternary ammonium compounds (i.e. those containing a positively-charged nitrogen atom) are unlikely to be absorbed from the gastrointestinal tract or to cross the blood–brain barrier, hence will have fewer adverse effects than do tertiary amines such as atropine, hyoscine and dicyclomine. Thus the atropinic agents ipratropium and tiotropium are effective when inhaled for respiratory actions, and have fewer side effects than does atropine.
Many remedies used to treat colds contain drugs with anticholinergic effects; many drugs (e.g. antihistamines, antidepressants, antipsychotics) have atropinic adverse effects.
Ipratropium and tiotropium
The atropinic drugs ipratropium and tiotropium have useful bronchodilator actions after inhalation; they may be used as maintenance treatment in severe asthma and COPD (see later discussion). Ipratropium is available as metered-dose inhaler or nebuliser and may be used 3–4 times daily, and tiotropium as a dry-powder inhaler, inhaled once daily. They should not be used for relief of symptoms, but as adjunctive therapy with corticosteroids. Interactions with other drugs with anticholinergic effects are common and combinations should be avoided.
Prophylactic anti-asthma drugs
These drugs are collectively known as preventers; they include the corticosteroids, cromolyns (mast-cell stabilisers) and newer drugs that prevent inflammatory responses.
Corticosteroids
Corticosteroids are used in chronic asthma to decrease airway obstruction. The adrenal cortex hormones are discussed in detail in Chapter 35; the actions useful in asthma are the glucocorticoid effects, i.e. anti-inflammatory and immunosuppressant effects. Appreciation of the great value of prophylactic use of inhaled corticosteroids in preventing the late-phase inflammatory response and decreasing bronchial hyper-reactivity has recently revolutionised management of asthma—see Figure 28-5 for step-wise adjustment of dosage in asthma management.
Mechanism of action
Corticosteroids act by entering the cytoplasm of cells, where they bind to specific glucocorticoid receptors, then translocate into the nucleus, where they bind to response elements in the target genes and bring about induction or repression of gene transcription. Their exact mechanism in asthma is still poorly understood but involves:
Overall, glucocorticoids reduce both the early and late (proliferative) stages of the inflammatory response. They are indicated prophylactically in maintenance treatment of severe asthma and COPD, and also in acute asthma and croup. The maximum improvement in pulmonary function may take 1–4 weeks.
Adverse effects
Prior to the development of inhaled steroids, daily administration of systemic (oral) therapy provided great therapeutic benefits, but the high incidence of adverse effects led to the use of the alternate-day schedule of treatment. Then chemical modifications of the steroid molecule produced compounds with enhanced absorption after inhalation and reduced risk of systemic adverse effects (see below). The products available now are beclomethasone (Drug Monograph 28-4), budesonide, fluticasone and ciclesonide. Budesonide is available via both dry-powder inhaler and nebuliser. The more recent fluticasone and ciclesonide may be less likely to cause systemic adverse effects. To prevent oral fungal infections, patients are advised to rinse the mouth out with water after use of a corticosteroid inhaler.
Drug monograph 28-4 Beclomethasone inhaled
Indications
Inhaled corticosteroids are indicated for maintenance treatment and prophylaxis in persistent asthma.
Pharmacokinetics
A considerable proportion (up to 80%) of an inhaled dose of beclomethasone is likely to be swallowed, then absorbed from the intestinal tract. Peak plasma concentrations are reached 3–5 hours after administration; the drug is subject to metabolism in the liver and excretion in faeces and urine.
Adverse effects Local adverse effects include dysphonia (changed voice), oropharyngeal candidiasis (oral thrush) and allergic reactions; systemic effects are rare.
Warnings and precautions
Oral deposition of drug (and hence oral infections and systemic absorption) can be reduced by use of a spacer and by rinsing the mouth and throat after each dose. Correct inhaler technique is important. The drug is not useful for acute asthma attacks, as it is not a bronchodilator. If prescribed with an inhaled bronchodilator, the β2 agonist or antimuscarinic should be inhaled (to open the airways) before the corticosteroid. Dosage should not be reduced or stopped unless advised. The drug is safe during pregnancy and breastfeeding.
Dosage and administration
Dosage starts at levels likely to be effective, then is reduced to the mini mum dose that controls symptoms and then is ‘stepped down’ by 25% every 3 months if possible. Dosage may be doubled if asthma worsens or respiratory tract infection occurs. Typical adult dosage is 50–200 mcg twice daily to a maximum of 400 mcg twice daily, but may be up to 2000 mcg daily in severe persistent asthma.
Systemic administration and effects
Systemic corticosteroids are still used (e.g. short courses of prednisolone given orally) when inhaled medications (corticosteroids, β2 agonists, antimuscarinics) and oral theophylline cannot adequately control asthma. In emergencies, corticosteroids may be administered parenterally (e.g. IV hydrocortisone, dexamethasone). Daily doses above 10 mg oral prednisolone (or 1–1.5 mg inhaled beclomethasone) can cause systemic adverse effects, including adrenal suppression and growth suppression; altered deposition of muscle, fat, skin, hair and bone; ocular changes, infections, mineralocorticoid effects and psychological disturbances (see Chapter 35). Frequent use of inhaled corticosteroids may lead to a doserelated decrease in bone mineral density (BMD), with the increased risk of osteoporosis, so postmenopausal women taking inhaled steroids are advised to have their BMD monitored every 2 years.
Combination therapy
Combined inhalers containing both a long-acting β2 -agonist symptom controller and a corticosteroid preventer are available, for example salmeterol plus fluticasone or eformoterol plus budesonide. The combination is indicated for regular treatment of asthma when use of both drugs is appropriate, not for relief of acute symptoms. The pharmacokinetic parameters of each drug appear to be unaffected by coadministration, and the adverse reactions, precautions and interactions are as for each component drug. The advantages are: convenience of using only one inhaler, cost reduction, better control of asthma and regular use of a low-dose steroid.
Cromolyns (mast-cell stabilisers)
Cromoglycate (Clinical Interest Box 28-8) and nedocromil are examples of cromolyns or mast-cell stabilisers; these drugs are anti-inflammatory agents that inhibit the release of histamine, leukotrienes and other mediators of inflammation from mast cells and macrophages.
Clinical interest Box 28-8 Cromoglycate: A most unusual drug
Sodium cromoglycate, also known as cromolyn sodium and as [di]sodium cromoglycate, has many unusual properties:
Mechanism of action
The mechanism is not clear: they are said to stabilise mast cells but may also act by blocking chloride channels, suppressing activation of sensory nerves, desensitising neuronal reflexes and inhibiting release of cytokines. Inhaled before an attack, the overall effect of these drugs is to inhibit bronchoconstriction and reduce bronchial hyperreactivity. Neither drug has any bronchodilator effect, nor do they have any effect on any inflammatory mediators already released in the body.
Other drug groups
Leukotriene-receptor antagonists
The first two drugs released in the category of leukotrienereceptor antagonists are montelukast and zafirlukast.
Mechanism of action
These drugs block receptors for the cysteinyl leukotrienes (C4, D4 and E4; Figure 32-3), which are components of slow-reacting substance of anaphylaxis (SRS-A), thought to be a mediator of inflammation in both early and late phases of asthma. They also inhibit other pro-inflammatory cytokines and can therefore reduce the inflammation, mucus secretion and bronchoconstriction associated with asthma. As this drug group is relatively new, clinical experience is not yet extensive; they are useful as ‘add-on’ therapy for patients inadequately controlled with inhaled corticosteroids where they may allow a reduction in dosage of inhaled corticosteroid. Improvement in asthma symptoms should be noted within a few days. The drugs are not indicated for reversal of bronchospasm in acute asthma attacks, but have additive effects to β2-agonists.
Pharmacokinetics
Administered orally, both drugs are rapidly absorbed and highly bound to plasma proteins. They have a rapid onset of action and are metabolised in the liver with metabolites excreted primarily in faeces. Leukotriene-receptor antagonists may be given with other anti-asthma medications.
New drugs for asthma
New drugs are constantly being developed and tested for use in asthma, particularly as more details of the actions of mediators of the inflammatory response are determined. Some currently in development include antagonists of cytokines (e.g. anti-interleukin-4, −5 and −13 agents), antagonists of cell adhesion molecules, anti-TNF-α agents (e.g. etanercept or infliximab), antagonists of platelet activating factor (disappointing thus far), neurokininreceptor antagonists, antibodies against IgE receptors, specific inhibitors of PDE-4 (roflumilast), inhibitors of p38 mitogen-activated protein kinases, antisense oligonucleotides targeted against chemokine receptors, macrolide antibiotics with anti-inflammatory actions (e.g. clarithromycin), adenosine A2B antagonists, inhaled antioxidants and the antigout drug colchicine, which decreases the late-phase inflammatory response (see Adcock et al [2008]; Hanania [2008]).
Patients’ responses to anti-asthma drugs can vary greatly, suggesting pharmacogenetic differences in the metabolising enzymes or receptors involved. Studies are being carried out into variant polymorphisms of the genes for β2-receptors, M2- or M3-receptors, glucocorticoid receptors or cys-leukotriene receptors, which may assist understanding of how people respond to standard drugs.
Omalizumab
Mast-cell activation is also reduced by omalizumab, a recombinant humanised monoclonal antibody that complexes with free IgE antigens to prevent their binding to mast cells and the subsequent cascade of inflammation. The drug has a slow onset and long duration of action after SC injection; it effectively reduces IgE concentrations and asthma symptoms in patients with allergic asthma, allowing reduction or cessation of steroid dosage. Interestingly, the drug itself can cause an anaphylactic reaction. Its clinical use has been associated with an increased rate of malignancies, presumably because of reduced immune responsiveness.
5-lipoxygenase inhibitors
A group of drugs under clinical trial act by inhibition of the enzyme 5-lipoxygenase, thus preventing synthesis of the leukotrienes from arachidonic acid. (This mechanism is analogous to the inhibition of cyclo-oxygenase by non-steroidal anti-inflammatory drugs, preventing the synthesis of prostaglandins.) Zileuton, the first of these drugs approved in the USA, thus interferes with the formation of leukotrienes that cause mucus plugs and constriction of bronchial airways. The late inflammatory response is also impaired; similar drugs are being tested in prevention of cancer.
Overview of asthma management
Sub-optimal therapy
Despite the availability of several groups of drugs for the treatment of asthma, many of which date back thousands of years, asthma therapy is frequently not optimal, and there is still an unacceptably high level of mortality and morbidity from asthma. In particular, studies in children with asthma show that only 40% have well-controlled asthma. Possible reasons suggested for unsuccessful therapy include:
Stepwise management
In recent years, guidelines have been prepared and published by groups of specialist physicians to encourage accurate and appropriate evidence-based treatment. The basis for the guidelines is stepwise management, with treatment stepped up to stronger drugs as necessary to achieve good control of symptoms, and cautious stepping down after improvement and review of therapy. An example of the stepwise approach for management of adult asthma is shown in Figure 28-5.
Before treatment of chronic asthma begins, severity needs to be assessed and classified, and trigger factors identified and managed. Treatment is tailored to suit the patient and the severity. Inhaled corticosteroids are started at a dose sufficient to be effective, as monitored by peak-flow meter readings, then reduced to the minimum required to maintain control. Antibacterials are reserved for specific infections, antihistamines are rarely useful and sedatives are contraindicated because of agitation from dyspnoea; Clinical Interest Box 28-9 includes a summary of drugs used in asthma.
Clinical interest Box 28-9 Therapeutic tips for asthma
Specific stepwise guidelines are available for childhood asthma, home management of acute asthma and asthma in accident and emergency departments. Acute asthma is a life-threatening situation and may require systemic corticosteroids, adrenaline, aminophylline, oxygen, nebulised bronchodilators and close monitoring of lung function, blood gases and CNS function.
Asthma action plans
Individualised written action plans should be devised for each patient, including education and self-management aspects so that patients know how to recognise their own symptoms, start and step-up treatment and promptly reach medical attention. Action plans may need to take into account factors such as closeness to hospital help, as rural patients may face added risk factors (isolation, lack of support networks and seasonal high levels of environmental allergens).
A six-point action plan (proposed by several Australian and New Zealand groups through the National Asthma Campaign) advises patients and carers to:
Special plans are developed for exercise-induced asthma, athletes (see Clinical Interest Box 28-10), infants, the elderly, travellers, occupational asthma and for asthma in pregnancy (good asthma control is the priority, to maintain the health of the mother and fetus). The Australian ‘GP Asthma Initiative’ promotes the ‘Asthma cycle of care’ plan as the best-practice model for managing asthma (see www.health.gov.au under keyword ‘asthma’).
Clinical interest Box 28-10 Athletes, asthma and anabolics
The World Anti-Doping Agency (WADA) has the responsibility to control the use of drugs in sport, in order to keep sport drugfree (see Chapter 49). WADA annually publishes lists of drugs that are prohibited, restricted (i.e. allowed only in some sports) or monitored. The main drug groups that are prohibited are anabolic agents (steroids and β2 agonists), various hormones and diuretics and other masking agents. Methods of blood doping or sample manipulation are also prohibited. However, athletes with medical conditions, whether acute or chronic, have the right to take drugs for treatment. This poses particular problems with asthma, as it is such a common condition and the main drugs used (β2 agonists and corticosteroids) are normally banned in sport.
The β2 agonists are prohibited because they have anabolic actions as well as smooth muscle relaxant and bronchodilator effects: they can increase lean (muscle) mass and reduce body fat. While there is no evidence that they enhance sporting performance, they are often abused by athletes looking for an extra boost to their chances of winning. The ban is relaxed for two β2 agonists (salbutamol, salmeterol) which are permitted to be used via inhalation by athletes who have previously registered with their sporting authority as being asthmatic (and had the diagnosis confirmed by respiratory tests, hence needing access to the bronchodilators for therapeutic purposes), and been granted a Therapeutic Use Exemption. Even so, if the drug level exceeds a specified maximum concentration, the onus is on the athlete to prove that the adverse result was due to a therapeutic use. Inhaled corticosteroid use also requires an approved Therapeutic Use Exemption.
During the Sydney Olympic Games (2000), it was suspected that many more athletes registered as being asthmatic than would have been expected. However, for the Beijing Olympics in 2008, it was generally agreed that the reported prevalence of asthma in athletes reflected the known prevalence of asthma in each country. The policy of requiring athletes to demonstrate the diagnosis of asthma before being approved to inhale β2 agonists will continue.
Source: Fitch et al 2008.
Drug treatment of chronic obstructive pulmonary disease (COPD)
COPD, also known as chronic obstructive airways disease (COAD) or as chronic airways limitation (CAL), is a disorder characterised by airflow obstruction that is not fully reversible. COPD is often associated with cough, emphysema, airway damage, excessive mucus and sputum production and recurrent respiratory infections, so drugs used in respiratory tract infections may be indicated.
COPD includes three important disorders: chronic bronchitis, emphysema and chronic asthma with fixed airflow obstruction. Asthma (characterised by reversible airways narrowing) can occur with COPD. COPD typically affects middle-aged and older people. Cigarette smoking is the major aetiological factor, and stopping smoking is the only measure that slows progression of COPD. Inherited conditions such as deficiency of the α1-antitrypsin enzyme predispose to alveolar collapse and exacerbate problems caused by smoking. Dyspnoea (difficult, laboured breathing) develops insidiously over many years and the FEV1 is typically reduced to less than 70% of the forced vital capacity. Cardiac disease and disordered breathing during sleep (sleep apnoea) are also common with COPD and need to be effectively treated. Management involves cessation of smoking, oxygen for hypoxaemia, lung-reduction surgery for patients with emphysema and immunisations, plus drug therapy.
Drug therapy of COPD
While there is no successful drug therapy that reverses the decline in lung function, drugs may relieve symptoms, prevent or treat exacerbations and improve quality of life. The drugs used in COPD and in acute exacerbations are all considered in other sections; a brief summary of their use in COPD follows.
Bronchodilators
Bronchodilators may be administered by puffer with a spacer or by nebuliser; long-acting drugs such as salmeterol and tiotropium (and similar combinations) are especially effective at reducing symptoms and increasing exercise capacity. A recent study showed that tiotropium may reduce COPD mortality.
Inhaled corticosteroids
Inhaled corticosteroids (or short oral course if there is associated asthma) can slow the decline in respiratory function and reduce mortality; however, there is increased risk of pneumonia.
Oxygen
‘Domiciliary oxygen’, i.e. long-term continuous oxygen therapy in the home, for at least 15 hours per day, reduces mortality in patients with severe hypoxia. There is less evidence of benefit from intermittent ambulatory oxygen therapy.
Other treatments
Other drugs used in COPD include theophylline, mucolytic agents and antibiotics (specific to the current pathogen). Patients should have pneumococcal vaccination and annual influenza vaccination. Respiratory rehabilitation, exercise programs and weight reduction are also effective and improve quality of life. Support networks with a multidisciplinary team of health professionals and self-management plans are important. Severe disease unresponsive to treatment may require lung volume reduction surgery or lung transplantation; in the terminal situation, palliative care should be considered (see Abramson et al [2007]).
Cessation of smoking
However, the most important measure to improve COPD is smoking cessation. Smoking is described as the largest single preventable cause of death and disability in Australia. Smoking causes respiratory disease, particularly lung cancer, and increases the risks of cardiovascular, cerebrovascular and peripheral vascular diseases (see Clinical Interest Box 28-11). ‘Quit Smoking’ programs involve assessing dependency, providing education and support, monitoring of compliance, complementary therapies and pharmacological assistance with antidepressants and nicotine replacement therapy as patches, gum or inhaler. (The pharmacological effects of nicotine are discussed in Chapter 11, and the social use and abuse of nicotine in Chapter 21.)
Clinical interest Box 28-11 Smoking
Cigarette smoke is an aerosol, consisting of tarry particles suspended in a complex mixture of organic and inorganic gases, containing more than 4000 different known chemicals.
The gas phase (smoke) from burning cigarettes contains carbon monoxide, hydrogen cyanide, oxides of nitrogen, ammonia, volatile aldehydes and vapours including benzene, acetone, acrolein and vinyl chloride.
The particulate phase of cigarette smoke contains nicotine (about 0.2–3.5 mg) plus many other potentially toxic chemicals including tar, metals and carcinogenic hydrocarbons such as benzpyrene and nitrosamines.
The decreased respiratory efficiency of smokers is attributed to toxic effects of nicotine (causing broncho constriction), carbon monoxide (reducing oxygen-carrying capacity of haemoglobin) and irritants (increasing mucus secretion and destroying cilia and alveolar sacs).
Many components of smoke are free radical species, some highly stable, including reactive oxygen and nitrogen species. These are responsible for much of the damage produced by smoking, including lipid peroxidation, lung damage and COPD.
(See also sections on tobacco and nicotine in Chapter 21.)
Bronchiectasis
Bronchiectasis—chronic necrotising infection of the bronchi and bronchioles, with purulent sputum—most commonly is due to CF, but can also occur in pneumonia and other respiratory diseases. Drugs useful in CF, such as inhaled hypertonic saline or mannitol, are being trialled in bronchiectasis generally, along with antibiotics, inhaled corticosteroids, vaccinations (pneumococcal, influenza) and physiotherapy (see McLean [2008]).
Drugs used in respiratory tract infections
Treatment of colds and influenza
Most upper respiratory tract infections (URTIs) are caused by viruses, for which there are no safe, specific antiviral agents available. The recommended treatment is therefore symptomatic, as for the common cold.
Viral infections are notorious for lowering the body’s immune defences and predisposing the patient to subsequent secondary bacterial infections, which may be dangerous in patients with other chronic conditions such as asthma, COPD or rheumatic heart disease. These patients may be prescribed antibiotics appropriate for the specific infecting bacteria, following advice in the current Antibiotic Guidelines (Antibiotic Expert Group 2006). For example, for Streptococcus pyogenes pharyngitis (‘strep sore throat’), phenoxymethylpenicillin or roxithromycin is recommended and for acute bacterial sinusitis, amoxycillin or doxycycline.
Common cold (coryza)
The viruses most commonly responsible for the common cold are a group of RNA rhinoviruses, which are spread by contact and by droplets, often via the conjunctiva. The virus multiplies, mainly in the cells lining the nostrils, and causes inflammation of the nose and throat, hence the common symptoms of redness and watery secretions of the nose, eyes and throat.
Development of vaccines against the common cold has been largely unsuccessful because of the many different and variable antigenic types of rhinoviruses. The Australian National Prescribing Service has mounted a campaign to discourage patients (and parents) from expecting to be prescribed antibiotics for colds; the punch-line is ‘Antibiotics won’t help a common cold; common sense will’ (see www.nps.org.au/“the cold hard facts”). Treatment of the common cold is thus mainly symptomatic, with decongestants, antiseptics, expectorants, aspirin-like antipyretic analgesics and rest. Antihistamines, whisky or brandy and ‘hot toddies’ have no proven efficacy in colds, other than as sedatives or placebos. (See Clinical Interest Box 28-12 for complementary and alternative therapies in respiratory disorders.)
Clinical interest Box 28-12 Complementary and alternative therapies in respiratory disorders
There are good pharmacological rationales for many of the traditional, complementary and alternative medi cine (CAM) treatments for asthma: garlic and horseradish contain several anti-allergy and moistening sulfur compounds; coffee and tea contain xanthine bronchodilators; saltpetre (potassium nitrate) is a smooth muscle relaxant; the herb Ephedra sinica (ma huang) from traditional Chinese medicine contains the broncho dilator drug ephedrine; and New Zealand green-lipped mussel has antiinflammatory actions.
Many Australian native plants have long been used by Indigenous Australians in symptomatic treatment of cough and respiratory tract congestion, either by inhalation or drinking a decoction (tea) containing aromatic or essential plant oils and cineoles which have mucolytic and decongestant properties. These are present in eucalypt species, especially the Tasmanian blue gum (Eucalyptus globulus), and in the liniment tree (Melaleuca symphocarpa), lemon grasses (Cymbopogon) and river mint (Mentha australia).
Volatile scented oils, discussed previously under ‘Expector ants’, are present in many cough and cold ‘cures’, and are sold in many alternative therapy outlets. They may be inhaled in mists or sprays or vaporised over a candle. One popular OTC ointment, Vicks Vaporub, formulated to be applied topically to the nasal passages or chest or inhaled via steam, contains oils of menthol, camphor, thymol, eucalyptus, turpentine, nutmeg and cedarleaf.
Echinacea extracts are the best-sellers in the herbal industry, particularly for treatment of coughs and colds and some inflammatory conditions. Clinical trials suggest that echinacea extracts are sometimes useful, possibly by stimulating phagocyte activity in the non-specific immune system.
Fijian plants traditionally used to treat respiratory disorders include dilo (Calophyllum inophyllum) for TB, weleti (Carica papaya, pawpaw) for asthma, kalakalabucidamu (Acalypha wilkesiana) for pleurisy and uci flowers (Euodia hortensis) as an inhalation for chest colds.
Other CAM methods claimed to have benefit in asthma include:
Adapted from: Spencer & Jacobs 1999; Braun & Cohen 2007; Cambie & Ash 1994.
Vitamin C has long been recommended for the common cold, sometimes in massive doses (>1 g/day). Meta-analyses of trials of the effects of vitamin C overall indicate that vitamin C has no preventive effect in well-nourished persons, but may slightly decrease the duration of colds and may lower the incidence of colds in people with low dietary vitamin C intake and those predisposed to respiratory tract infections.
‘Cough mixtures’
Cough is a protective reflex by which a sudden blast of compressed air from the bronchial tubes expels irritating, infective or obstructive material. The newly described ‘cough receptors’ are sensory nerves (part of the vagal afferents) with terminals in airway walls, which are activated by various stimuli including chemical irritants, inflammatory mediators, intraluminal material and mechanical stimulation to airway epithelium. Common causes of cough are URTIs, pollutants including cigarette smoke, asthma, COPD, gastro-oesophageal reflux and adverse reactions to angiotensin-converting enzyme (ACE) inhibitor drugs (see Clinical Interest Box 23-5).
Cough may also occur in more serious disorders such as bronchiectasis, lung cancer or heart failure. Such underlying disorders should be treated, rather than simply suppressing the cough with an antitussive agent (cough suppressant).
As well as a cough suppressant, ‘cough mixtures’ may include (with varying degrees of efficacy):
The combination of an antitussive (cough suppressant) and an expectorant (cough stimulant) in a cough mixture is illogical and should be avoided. If there is a component of bronchial hyper-reactivity to the cough, an inhaled cromolyn or inhaled corticosteroid (e.g. beclomethasone) may be effective, as in asthma. There is little evidence of any efficacy greater than demulcent (soothing) or placebo effect for OTC cough preparations in children with acute cough.3 Cough and cold remedies for children under 2 years of age have recently been rescheduled to Prescription-Only (S4) due to lack of evidence of efficacy, reports of adverse events and accidental overdoses. It is recommended that parents be advised to use simple remedies for their coughing children such as rest, hydration and analgesics (see Sung and Cranswick [2009]).
Cough suppressants
Prescribing of cough suppressants is usually reserved for a non-productive (dry, hacking) cough that is inadequately controlled or unresponsive to over-the-counter medications. Treatment of cough is secondary to treatment of the underlying disorder, i.e. the therapeutic objective is to decrease the intensity and frequency of the cough yet permit adequate elimination of tracheobronchial secretions and exudates. For example, cough secondary to gastrooesophageal reflux may be reduced with histamine H2-receptor antagonists and proton pump inhibitors.
Opioid antitussive drugs
Opiates such as morphine potently suppress the cough reflex by direct depression of the medullary cough centre, a mechanism unrelated to their analgesic or respiratory depressant actions. They are widely used and many products containing them are available over the counter (see also Chapters 15 and 21).
Adverse effects
Their clinical usefulness as antitussives, however, is limited by adverse effects: they inhibit the ciliary activity of the respiratory mucous membrane, may cause bronchial constriction in patients with allergies or asthma and cause drowsiness, drug dependence and constipation. Codeine, pholcodine, dextromethorphan and dihydrocodeine (Drug Monograph 28-5) exhibit less pronounced antitussive effects than morphine but they also have fewer adverse effects.
Drug monograph 28-5 Codeine and pholcodine
Indications
The opioid cough suppressants such as codeine are indicated for the symptomatic treatment of non-productive cough by their depressant actions on the medullary cough centre. They are generally formulated as oral liquids known as linctuses or ‘cough mixtures’.
Pharmacokinetics
After oral administration, the onset of action is rapid, with duration of action of 6–8 hours. Codeine is normally metabolised to morphine and norcodeine, and the metabolites are excreted by the kidneys. Pholcodine undergoes 2-compartment distribution and extensive liver metabolism and has a very long elimination half-life, with metabolites and parent drug excreted in urine.
Drug interactions
Opioids have additive effects with other CNS depressants, including antihistamines and alcohol, and concurrent use should be avoided.
Adverse effects
These include drowsiness, respiratory depression, nausea and vomiting and consti pation. Adverse effects in the CNS, including dependence and withdrawal, are more likely with codeine. Excessive constipation tends to limit the use of codeine.
Warnings and contraindications
Patients should be warned of the risk of drowsiness and should not drive or operate machinery if affected. Avoid use in respiratory failure or asthma, in children or if the patient has a productive cough.
Dosage and administration
The dosage of co deine for adults is 15–30 mg 3–4 times daily, and for pholcodine 10–15 mg 3–4 times daily. (For com pari son, the analgesic dose of codeine in a Panadeine Forte tab let is 30 mg codeine phosphate.) Dosage should be reduced in renal or hepatic impairment and in the elderly.
Decongestants
Vasoconstriction in mucous membranes, leading to decongestion, may be achieved by the topical application of sympathomimetic amine decongestants such as xylometazoline (Drug Monograph 28-6), oxymetazoline, phenylephrine and pseudoephedrine, which stimulate α1- adrenoceptors. This is the main clinical use of such drugs which, if administered systemically (as pseudoephedrine frequently is), cause generalised sympathetic effects, including hypertension and smooth muscle contraction.
Drug monograph 28-6 Xylometazoline nasal spray
Xylometazoline and oxymetazoline are examples of sympathomimetic amines commonly administered for decongestant effects as nasal sprays, cough mixtures, capsules or tablets and as eye-drops. Tolerance can occur rapidly to their α-receptor-mediated vasoconstrictor actions.
Indications
Decongestants are indicated for symptomatic relief of congestion (inflammation and excess secretions) and red eyes associated with acute rhinitis, common cold and sinusitis. Intranasal administration also assists intranasal examination.
Pharmacokinetics
Topically applied vasoconstrictors act rapidly, e.g. in the eyes or nose. Plasma levels of xylometazoline after intranasal administration are below the limits of detection, hence pharmacokinetic parameters cannot be determined.
Adverse effects
Transient stinging and throat dryness may be felt. Systemic sympathomimetic effects can occur, such as headache and insomnia, so decongestants should not be taken by patients with hypertension.
Warnings and contraindications
Patients should be advised that prolonged use (4–5 days) of nasal vasoconstrictor sprays can cause rebound nasal congestion, and also that the drug may become less effective because of the development of tolerance—it is not simply that a cold is becoming worse. Sympathomimetics should not be used by patients taking antidepressants, because of potentiation of effects.
Decongestion may also be achieved by blocking muscarinic receptors, which mediate increased respiratory secretions, with atropinic drugs such as ipratropium. However, the preferred treatment for ‘blocked nose’, especially in infants and children, is simply isotonic (0.9%) saline solution, as nasal drops or spray.
Influenza
Influenza is a common respiratory viral infection occurring during most winters, sporadically or in epidemics. Systemic symptoms of headache, myalgia, fever and chills occur 1–2 days before the respiratory symptoms (sore throat, cough, nasal obstruction). In an influenza epidemic, the incidence in crowded groups can reach as high as 70%, and mortality due to secondary bacterial infection is high among elderly and compromised patients.
Vaccination
As with the rhinoviruses that cause the common cold, the influenza virus shows great antigenic variation, with frequent mutations. Influenza vaccine (Drug Monograph 28-7) for active immunisation must be prepared regularly against strains currently in circulation. To a certain extent, those living in the Southern Hemisphere are fortunate in that influenza strains usually cause flu epidemics in the southern winter six months after their appearance in the Northern Hemisphere, so vaccines can be prepared in time.
Drug monograph 28-7 Influenza vaccine
Influenza vaccines are prepared from viral cultures that have been inactivated, purified and preserved.
Indications
Administration of the current vaccine before winter induces antibodies against viral surface antigens and proteins, and provides protection against infection. Current Australian National Health and Medical Research Council recommendations are that the following groups be vaccinated annually:
Adverse effects
Mild localised reactions, fever and malaise have been reported; very rarely, neurological reactions occur.
Vaccines are manufactured to conform with annual requirements of the Australian or New Zealand Ministries of Health; recommendations as to who should receive vaccination vary from time to time. For example, safe and effective vaccines have been developed to immunise against the H1N1 strain of ‘swine flu’ that became a pandemic in 2009. Vaccination is strongly recommended especially for pregnant women, parents and guardians of young infants, health workers and community care workers, Indigenous Australians, people with underlying chronic conditions and those who are severely obese.
Treatment
Otherwise healthy people are advised to treat flu symptoms with rest, increased fluid intake and paracetamol for fever; patients at high risk of complications may be prescribed anti-flu drugs to minimise complications.
Amantadine is an antiviral drug that specifically inhibits the replication of the A2 (Asian) strain influenza virus. The dose is 100 mg orally twice daily for treatment in high-risk individuals and also prophylactically until 10 days after vaccination. There are many potential adverse drug reactions and precautions relating to the use of this drug, and dosage schedules are complicated, especially in the elderly and in renal failure. Many strains of influenza A virus have become resistant. Interestingly, amantadine is also used as an antiparkinsonian agent, as it appears to have indirect dopamine-receptor agonist actions and blocks receptors for acetylcholine and NMDA. Overdosage symptoms include neuromuscular disturbances and symptoms of acute psychosis.
Zanamivir and oseltamivir are neuraminidase inhibitors for treating infections due to influenza viruses A and B (see Drug Monograph 28-8 and Clinical Interest Box 4-5).
Drug monograph 28-8 Zanamivir (relenza)
Zanamivir is an Australian-developed drug introduced in 1999 which alleviates and reduces the duration of symptoms of influenza virus A and B infection. The mechanism of action of zanamivir is via selective inhibition of the viral surface enzyme neuraminidase; the effect is to inhibit replication and shedding of virus from respiratory epithelium, thus reducing severity and duration of symptoms. The drug is administered by oral inhalation of dry powder for 5 days. It reduces the time to alleviation of symptoms by about 2.5 days. A similar drug, oseltamivir, can be taken orally; resistance to the latter is increasing.
Indications
Zanamivir is indicated for treatment of influenza A and B viral infections in children 5 years and over and in adults. It can be used prophylactically (1 inhalation/day), but annual flu vaccination is recommended.
Pharmacokinetics
After oral inhalation of the powder, the drug is distributed widely in the oropharynx and lung, with 10%–20% being absorbed systemically. It is excreted unchanged in the urine, with a half-life of 2.5–5 hours after oral inhalation.
Other respiratory tract infections
Note: Antimicrobial agents are considered in detail in Unit 14, ‘Drugs affecting microorganisms’.
Pneumonia
Pneumonia is a condition of inflammation of the lower respiratory passages (bronchioles) and alveoli arising from infections, irritation or toxic material. The clinical features include fever and chills, shortness of breath and coughing of green or blood-stained sputum. It is most common at the extremes of life and is frequently the specified cause of death in frail elderly persons (‘the old man’s friend’). Other significant risk factors include underlying cardiorespiratory disorders and immunodeficiency states. A wide variety of pathogenic organisms may be involved. Communityacquired pneumonia is most commonly caused by Streptococcus pneumoniae and there is often occupational exposure, e.g. Mycoplasma pneumoniae in health-care institutions and Legionella pneumophila from air-conditioning systems. Pneumococcal vaccines are available, and are recommended for elderly people and those who are immunocompromised or at particular risk of contracting pneumococcal pneumonia.
Management is designed to preserve life and maintain lung function. It may entail resuscitative methods, drug therapy, postural drainage and physiotherapy, monitoring of blood gases and radiographic changes and detection of associated lung diseases and complications.
Antibiotic therapy
Antimicrobial therapy depends on identifying the pathogenic organisms (usually bacteria) and careful selection of the appropriate antibiotics; for example, Antibiotic Guidelines recommends oral amoxycillin plus roxithromycin or doxycycline for mild community-acquired pneumonia, whereas severe pneumonia infections with Legionella species (Legionnaire’s disease) require parenteral gentamicin plus erythromycin IV or ciprofloxacin IV.
The spectrum of potential pathogens causing hospitalacquired pneumonia differs from that in the community, and ill patients are often immunocompromised, so recommendations for antibiotic therapy vary. Organisms are emerging with significant levels of resistance to antibiotics, and current local prescribing guidelines must be followed rigorously.
Tuberculosis (TB)
Infection with Mycobacterium tuberculosis may be clinically manifest primarily in the lungs or in other organs, including lymph nodes, skin or bones. Pulmonary TB is the commonest form (around 75%); it is infectious and is a notifiable disease in Australia, which has a very low prevalence (7 per 100,000 population in 2006). It is estimated that one-third of the world’s population is infected with TB.
Because many strains of the causative organism have developed resistance to previously effective antibiotics, multi-drug regimens are necessary in all cases for several months. Pretreatment screening (of visual acuity, renal function, liver function) is essential and compliance and treatment must be monitored. Standard short-course therapy consists of combination chemotherapy, with rifampicin, isoniazid, pyrazinamide and ethambutol. Regimens with daily, twice-weekly or thrice-weekly doses for 2–6 months have been devised, with direct observation of administration of doses to maximise compliance (see Chapter 46). This strategy leads to cure in about 95% of cases; actual local recommendations for doses and regimens depend on local resistance patterns.
Croup
Croup is an acute syndrome of hoarse voice, barking cough and noisy breathing usually occurring at night. It is one of the more common respiratory tract infections of childhood. Most cases have a viral aetiology. Corticosteroids, either systemic or inhaled, are effective in reducing symptoms of airway obstruction and respiratory distress, and also reduce time spent in hospital.
Whooping cough
Pertussis (or whooping cough) is a highly contagious respiratory infection caused by the bacterium Bordetella pertussis. While infants (who have not been vaccinated) are most at risk of life-threatening disease, the peak incidence now occurs in people aged over 15 years. Antibiotics (especially macrolides like erythromycin) may reduce the infectious period, but have little effect on duration or severity of the disease. Antibiotic prophylaxis for household contacts reduces the risk of spread among infants. Booster vaccinations are recommended for adolescents and adults, at about 10-year intervals (see Marchant [2009]).
Drugs affecting the nose
While drugs administered by inhalation are commonly inhaled through the mouth (e.g. bronchodilators in treatment of asthma), they may also be administered by nasal inhalation for local effects (e.g. vasoconstrictors for decongestion), for systemic effects (e.g. inhaled general anaesthetic gases) and for effects on closely located tissues (e.g. desmopressin nasal spray for effects on the pituitary gland; see Figure 28-6 and Chapter 33).
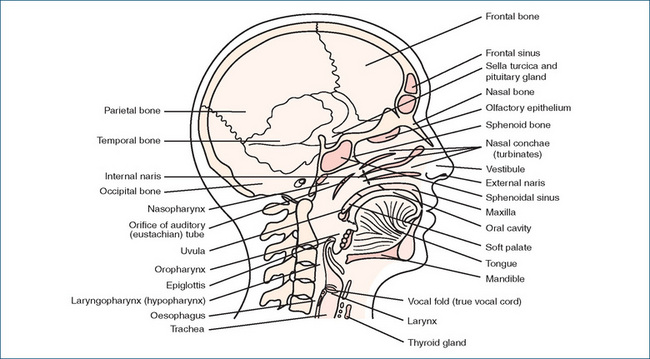
Figure 28-6 Sagittal section of head and neck showing the locations of the respiratory structures; note also the location of the pituitary gland, cradled in the sella turcica above the sphenoidal sinus.
Rhinitis and nasal obstruction
Obstruction to free air flow in the nose occurs very commonly as a feature of the common cold. It is due to rhinitis, or inflammation of the mucous membrane lining the nose. Overactivity of the mucus glands causes excessive mucus production and a watery discharge (rhinorrhoea, ‘runny nose’). The condition may be due to viral infection, impaired nervous system control of blood vessels in the membrane (vasomotor rhinitis), hypersensitivity reactions (allergic rhinitis), hypertrophic or atrophic changes, drug adverse effects or dietary factors.
Allergic rhinitis
Allergic rhinitis, or ‘hay fever’, is an atopic disorder (type 1 hypersensitivity reaction) mediated by IgE antibodies. Sensitised mast cells and basophils release autacoids, including histamine, serotonin, prostaglandins and leukotrienes, which mediate the inflammatory and immune responses. The prevalence of allergic rhinitis is highest in children and young adults (20%). There is a strong association with asthma. Some inhaled allergens (e.g. grass or flower pollens and some moulds) cause seasonal symptoms (e.g. ‘spring fever’), whereas other allergens (e.g. house dust mites, animal fur and some foods) cause chronic or perennial rhinitis. Specific allergens can be identified by skin testing or in-vitro testing. Common symptoms include sneezing; nasal congestion and hypersecretion; itchy nose, palate and eyes; blocked ears and irritated pharynx.
Management programs include attempts to identify and reduce exposure to specific allergens or environments, immunotherapy against antigens and symptomatic drug treatment.
Nasal corticosteroids
The first-line treatment for rhinitis and nasal polyposis is now topical corticosteroids, administered intranasally by nasal spray or drops; several nasal formulations are available including for beclomethasone, budesonide and mometasone. Nasal administration markedly reduces risk of systemic adverse effects, and reduces inflammation and mucus production. They can be used as needed or regularly. The main adverse effects are local: stinging, nose-bleeding, itching and sore throat. Patient adherence to treatment (compliance) is necessary.
H1-receptor antagonists (antihistamines)
The other common drugs of choice in treating allergic rhinitis are the antihistamines (H1-receptor antagonists). The many actions of histamine as an autacoid are discussed in Chapter 47 and the clinical uses of histamine-receptor antagonists in Chapters 29 and 47. H1-antihistamines are indicated for the treatment of allergies and may also be useful for their antiemetic, sedative, antimuscarinic and antitussive actions.
Mechanism of action
In allergic rhinitis, the useful action of H1-receptor antagonists is the blocking of histamine-induced vasodilation, thus decreasing capillary permeability, erythema and oedema. While released histamine normally contracts bronchial smooth muscle (bronchoconstriction), H1-antihistamines are not useful bronchodilators and are not effective in asthma, as more powerful mediators (SRS-A, ACh, leukotrienes) are involved in allergic asthma.
Adverse effects
The older H1-antihistamines such as promethazine and diphenhydramine have powerful sedative effects,4 which accounts for the common warning on packs of tablets ‘Do not drive or operate machinery after taking this drug’. The newer, second-generation H1-antihistamines, such as loratadine and fexofenadine, were developed to minimise this adverse effect. They are referred to as being ‘less sedating’, as they may still cross the blood–brain barrier and have some sedative effects. The sedating antihistamines may be useful at night for hay-fever sufferers. The sedative effect common to older antihistamines is additive with that of other CNS depressants, including alcohol.
H1-antihistamines are indicated for allergic rhinitis and are given orally (e.g. promethazine 10–25 mg 2–3 times daily) or intranasally (e.g. levocabastine or azelastine nasal sprays, sprayed into each nostril twice daily).
Other intranasal drugs for allergic rhinitis
Many drugs are formulated as nasal sprays or drops to be used topically in rhinitis; these have been considered earlier in this chapter and include:
Sinusitis
Sinusitis, or inflammation of the mucous membranes lin ing the bone cavities (sinuses) of the face (see Figure 28-6), usually results from infection. Clinical features include feeling of fullness or pain in the forehead or cheeks, fever and nasal congestion. Bacterial infection causes purulent discharge. Specific antibiotics are prescribed if the infecting organism is bacterial: amoxycillin, doxycycline or cefaclor are usually suitable. Treatment with antipyretic analgesics (e.g. paracetamol), saline irrigations and decongestants helps relieve symptoms.
Key points
 The respiratory system comprises the structures involved in the passage of gases from the nose, via the conducting airways (trachea, bronchi, bronchioles), to the respiratory bronchioles and alveoli in the lungs.
The respiratory system comprises the structures involved in the passage of gases from the nose, via the conducting airways (trachea, bronchi, bronchioles), to the respiratory bronchioles and alveoli in the lungs. The primary function of the lungs is gas exchange: oxygen is transported from the air to the blood in pulmonary capillaries and carbon dioxide is removed from the blood and exhaled.
The primary function of the lungs is gas exchange: oxygen is transported from the air to the blood in pulmonary capillaries and carbon dioxide is removed from the blood and exhaled. The main processes involved are pulmonary ventilation (bulk movement of air); gas transport across pulmonary, capillary and red cell membranes; and cellular respiration, providing oxygen for metabolic functions in body tissues and cells.
The main processes involved are pulmonary ventilation (bulk movement of air); gas transport across pulmonary, capillary and red cell membranes; and cellular respiration, providing oxygen for metabolic functions in body tissues and cells. Other functions of the respiratory system include regulation of blood pH; defence mechanisms, including removal of foreign particles; participation in speech, taste and smell; and metabolic functions.
Other functions of the respiratory system include regulation of blood pH; defence mechanisms, including removal of foreign particles; participation in speech, taste and smell; and metabolic functions. Respiratory tract secretions, produced by goblet cells and bronchial glands, form a protective mucociliary blanket and provide surfactant functions.
Respiratory tract secretions, produced by goblet cells and bronchial glands, form a protective mucociliary blanket and provide surfactant functions. Pulmonary surfactant (natural or synthetic) can be administered to infants with respiratory distress syndrome.
Pulmonary surfactant (natural or synthetic) can be administered to infants with respiratory distress syndrome. Bronchial smooth muscle is innervated by the parasympathetic and sympathetic nervous systems, mediating bronchoconstriction (via ACh M3 receptors) and bronchodilation (via β2-adrenoceptors), respectively.
Bronchial smooth muscle is innervated by the parasympathetic and sympathetic nervous systems, mediating bronchoconstriction (via ACh M3 receptors) and bronchodilation (via β2-adrenoceptors), respectively. Constriction of the airways can also be produced by neuropeptides and cytokine mediators released during inflammatory responses.
Constriction of the airways can also be produced by neuropeptides and cytokine mediators released during inflammatory responses. The main buffering capacity of the blood is provided by the bicarbonate–carbonic acid–carbon dioxide equilibrium, which is catalysed by carbonic anhydrase enzymes.
The main buffering capacity of the blood is provided by the bicarbonate–carbonic acid–carbon dioxide equilibrium, which is catalysed by carbonic anhydrase enzymes. Drugs can be administered locally to the airways as aerosols or fine powders by means of sprays, inhalers and nebulisers.
Drugs can be administered locally to the airways as aerosols or fine powders by means of sprays, inhalers and nebulisers. Advantages of inhaled aerosol or fine powder administration include rapid local effect, low dose and few systemic adverse effects.
Advantages of inhaled aerosol or fine powder administration include rapid local effect, low dose and few systemic adverse effects. Oxygen is a therapeutic gas that is essential to sustaining life and is used in many clinical situations, especially to treat hypoxia. Oxygen toxicity is a potential problem.
Oxygen is a therapeutic gas that is essential to sustaining life and is used in many clinical situations, especially to treat hypoxia. Oxygen toxicity is a potential problem. Carbon dioxide gas is used for its effects on respiration, circulation and the CNS; in low concentrations it is a respiratory stimulant. Other medical gases include nitrous oxide, helium and nitric oxide.
Carbon dioxide gas is used for its effects on respiration, circulation and the CNS; in low concentrations it is a respiratory stimulant. Other medical gases include nitrous oxide, helium and nitric oxide. Patients with abnormal or excessive respiratory tract secretions often need drugs to remove respiratory tract secretions. Mucolytics such as acetylcysteine may break down and reduce the viscosity of sputum, while expectorants aid in the removal of sputum.
Patients with abnormal or excessive respiratory tract secretions often need drugs to remove respiratory tract secretions. Mucolytics such as acetylcysteine may break down and reduce the viscosity of sputum, while expectorants aid in the removal of sputum. Asthma is a major cause of morbidity and mortality in the community. Management plans for asthma involve educating the patient; regular monitoring of lung function, progress and compliance; avoiding trigger factors; and stepwise use of various anti-asthma drugs.
Asthma is a major cause of morbidity and mortality in the community. Management plans for asthma involve educating the patient; regular monitoring of lung function, progress and compliance; avoiding trigger factors; and stepwise use of various anti-asthma drugs. Chronic obstructive pulmonary disease is best prevented by cessation of smoking; drugs used in treatment include bronchodilators, inhaled corticosteroids and oxygen.
Chronic obstructive pulmonary disease is best prevented by cessation of smoking; drugs used in treatment include bronchodilators, inhaled corticosteroids and oxygen. Viral respiratory tract infections (RTIs: cold, influenza, croup) are treated largely symptomatically, with antipyretic agents and decongestants.
Viral respiratory tract infections (RTIs: cold, influenza, croup) are treated largely symptomatically, with antipyretic agents and decongestants.Review exercises
References and further reading
Abramson M., Glasgow N., McDonald C. Managing chronic obstructive pulmonary disease. Australian Prescriber. 2007;30(3):64-66.
Adcock I.M., Caramori G., Chung K.F. New targets for drug development in asthma. Lancet. 2008;372(9643):1073-1087.
Antibiotic Expert Group. Therapeutic Guidelines Antibiotic, version 13. Melbourne: Therapeutic Guidelines Limited; 2006.
Atkinson T.J. Cystic fibrosis, vector-mediated gene therapy, and relevance of toll-like receptors: a review of problems, progress and possibilities. Current Gene Therapy. 2008;8(3):201-207.
Australian Medicines Handbook 2010. Adelaide: AMH, 2010.
BOC Medical. Medical Products and Services. Sydney: BOC Limited Aust; 2008.
Braun L., Cohen M. Herbs and Natural Supplements: An Evidence-Based Guide, 2nd edn. Sydney: Elsevier Mosby; 2007.
Cambie R.C., Ash J. Fijian Medicinal Plants. Australia: CSIRO; 1994.
Celli B.R. Update on the management of COPD. Chest. 2008;133:1451-1462.
D’Souza W.J., et al. Asthma morbidity 6 years after an effective asthma self-management programme in a Maori community. European Respiratory Journal. 2000;15(3):464-469.
Fitch K.D., Sue-Chu M., Anderson S.D., et al. Asthma and the elite athlete: summary of the International Olympic Committee’s consensus conference, Lausanne, Switzerland, January 22-24, 2008. Journal of Allergy and Clinical Immunology. 2008;122(2):254-260.
Foliaki S., Annesi-Maesano I., Daniel R., et al. Prevalence of symptoms of childhood asthma, allergic rhinoconjunctivitis and eczema in the Pacific: the International Study of Asthma and Allergies in Childhood (ISAAC). Allergy. 2007;62(3):259-264.
Gaga M., Zervas E., Grivas S., Castro M., Chanez P. Evaluation and management of severe asthma. Current Medicinal Chemistry. 2007;14(9):1049-1059.
Gillespie M.B., Osguthorpe J.D. Pharmacologic management of chronic rhinosinusitis, alone or with nasal polyposis. Current Asthma and Allergy Reports. 2004;4(6):478-485.
Hall I.P. Pharmacogenetics of asthma. Chest. 2006;130:1873-1878.
Hanania N.A. Targeting airway inflammation in asthma: current and future therapies. Chest. 2008;133(4):989-998.
Holley A.D., Boots R.J. Management of acute severe and near-fatal asthma. Emergency Medicine Australasia. 2009;21(4):259-268.
Holt S., Pearce N. Asthma in New Zealand: myths and realities. New Zealand Medical Journal. 2000;113(1103):39-41.
Howell M., Ford P. The Ghost Disease and Twelve Other Stories of Detective Work in the Medical Field. Harmondsworth: Penguin; 1986.
Hu W., Katelaris C.H., Kemp A.S. Allergic rhinitis: practical management strategies. Australian Family Physician. 2008;37(4):214-220.
Kerem E. Pharmacological induction of CFTR function in patients with cystic fibrosis: mutation-specific therapy. Pediatric Pulmonology. 2005;40(3):183-196.
Kuitert L.M., Watson D. Antileukotrienes as adjunctive therapy in acute asthma. Drugs. 2007;67(12):1665-1670.
Lima J.J., Blake K.V., Tantisira K.G., Weiss S.T. Pharmacogenetics of asthma. Current Opinion in Pulmonary Medicine. 2009;15(1):57-62.
McLean A. Bronchiectasis: a new look at an old adversary. Australian Prescriber. 2008;31(3):77-79.
Marchant J. Managing pertussis in adults. Australian Prescriber. 2009;32(2):36-38.
Murtagh J. Cautionary Tales: Authentic Case Histories from Medical Practice. Sydney: McGraw-Hill; 1992.
National Asthma Council. Asthma Management Handbook 2006. South Melbourne: National Asthma Campaign, 2006.(downloadable from website, see below).
Poff C.D., Balazy M. Drugs that target lipoxygenases and leukotrienes as emerging therapies for asthma and cancer. Current Drug Targets. 2004;3(1):19-33.
Respiratory Expert Group. Therapeutic Guidelines Respiratory, version 4. Melbourne: Therapeutic Guidelines Limited; 2009.
Schroeder K., Fahey T. Over-the-counter medications for acute cough in children and adults in ambulatory settings. Cochrane Database of Systematic Reviews. 2004:CD001831.
Sears M.R., Taylor D.R., Print C.G., et al. Regular inhaled betaagonist treatment in bronchial asthma. Lancet. 1990;336:1391-1396.
Spencer J.W., Jacobs J.J. Complementary/Alternative Medicine: An Evidence-Based Approach. St Louis: Mosby; 1999.
Sung V., Cranswick N. Cough and cold remedies for children. Australian Prescriber. 2009;32(5):122-124.
Suresh G.K., Soll R.F. Overview of surfactant replacement trials. Journal of Perinatology. 2005;25(Suppl 2):S40-S44.
Virchow J.C., Crompton G.K., Dal Negro R., et al. Importance of inhaler devices in the management of airway disease. Respiratory Medicine. 2008;102(1):10-19. Jan
Willson D.F., Chess P.R., Notter R.H. Surfactant for pediatric acute lung injury. Pediatric Clinics of North America. 2008;55(3):545-575.
Winterbourn C.C. Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Biology. 2008;4(5):278-286.
Worsnop C. Combination inhalers for asthma. Australian Prescriber. 2005;28(2):26-28.
For specific New Zealand drugs, check Medsafe website: www.medsafe.govt.nz.
Asthma Foundation of Victoria: www.asthma.org.au
National Asthma Council website: www.nationalasthma.org.au/html/home
National Prescribing Service campaign for optimising treatment of common cold: www.gottacold.com
World Anti-doping Agency: www.wada-ama.org/en/
World Health Organization: the five elements of DOTS: www.who.int/tb/dots/whatisdots/en/index.html
More weblinks at: http://evolve.elsevier.com/AU/Bryant/pharmacology/